Haematology, Oncology and Palliative Care Directorate.
|
|
|
- Bryce Greer
- 5 years ago
- Views:
Transcription
1 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile Chemotherapy Unit. Treatment from the appropriate cycle is indicated with suitability This list should be used as a guideline and the suitability of different regimens must also be based on the patient s Performance Status, suitability for treatment and demographics as well as whether treatment can feasibly be administered on an appropriate day. Any patient who has requested use of the scalp cooler will need to be treated at MPH. COLOUR KEY SUITABLE RESTRICTED INDIVIDUAL PATIENT BASIS NOT SUITABLE BRAIN CANCER BREAST CANCER Abraxane/Gemcitabine 8 hour expiry on Abraxane, difficult to make up 2 1 hour 30 mins Carboplatin (Single Agent) 3 weekly 3 1 hour 30 mins Carboplatin (Single Agent) - weekly 3 Docetaxel (Taxotere ) 3-weekly 3 1 hour 30 mins Docetaxel (Taxotere ) weekly 3 1 hour 30 mins EC 2 1 hour 30 mins Epirubicin (Single Agent) 3-weekly 2 1 hour Epirubicin (Single Agent) weekly 2 1 hour Eribulin 2 4 mins FEC 2 2 hours Paclitaxel + Cyclophosphamide (TC) Paclitaxel (Taxol equiv.) 3-weekly Paclitaxel (Taxol equiv.) weekly 3 2 hours Paclitaxel Albumin (Abraxane ) Drug only has an 8-hour expiry Pamidronate 2 For 1 or 2 hour infusion only (30mg 4 mins, 90mg 2 hours) Trastuzumab (Herceptin ) IV need to be given in the morning. Alternatively vials 2 could be made up on the bus using an aseptic technique (1 hour) Trastuzumab (Herceptin ) SC 2 30 mins Trastuzumab (Herceptin ) + Docetaxel 3 need to be given in the morning. (1hour 30 mins) Trastuzumab (Herceptin ) + Docetaxel + 3 Peruzumab need to be given in the morning. (3hours) Trastuzumab (Herceptin ) + Pertuzumab 3 need to be given in the morning. (3hours) Kadcyla 3 Vinorelbine 2 need to be given in the morning (4 mins) Zoledronate 2 30 mins COLORECTAL CANCER 2 1 Page 1 of
2 -FU (-Fluorouracil) bolus 2 4 mins -FU pump change + Capecitabine 2 + Irinotecan + Capecitabine Cetuximab + Oxaliplatin + Capecitabine Cetuximab + Oxaliplatin + MdG Irinotecan Irinotecan + MdG (FOLFIRI) Irinotecan + MdG (FOLFIRI) + Cetuximab + Irinotecan + MdG Mitomycin-C + Capecitabine 2 need to be given in the morning. (4mins) Mitomycin-C & -FU Infusion Mod de Gramont (MdG) 2 3 hours Oxaliplatin + Capecitabine (XELOX) Oxaliplatin + Capecitabine (XELOX) + Oxaliplatin + MdG (FOLFOX) Oxaliplatin + MdG (FOLFOX) + Panitumumab 3 2hours 30 mins GYNAECOLOGICAL CANCER Carboplatin 3 1 hour 30 mins Carboplatin & Gemcitabine 3 Suitable from #2 Day 8 (2hours) Carboplatin & Paclitaxel (Taxol) Cisplatin Cisplatin + Doxorubicin (MMMT) Cisplatin + -FU (4 days) Cisplatin + Topotecan Doxorubicin Liposomal (Caelyx ) Only has a 24hr expiry Gemcitabine (Day 8) 2 1 hour Paclitaxel (Taxol equiv.) 3-weekly Paclitaxel (Taxol equiv.) weekly 3 2 hours HAEMATOLOGICAL DISORDERS Venofer 3 2 hours Vincristine (single agent) IV Infusion (ITP) 2 4 mins HEAD & NECK CANCER Cisplatin, Doxorubicin & -FU (TPF) Docetaxel 3 1 hour 30 mins Cisplatin + -FU ( 4 / 7 ) IMMUNOGLOBULINS Flebogammadif 3 Prolonged infusion but possible to be given on bus (4 hours) Privagen 3 Prolonged infusion but possible to be given on bus (4 hours) Vigam Impractical to give on bus as infusion is given slower than other IVIGs LEUKAEMIA ADE Alemtuzumab (MabThera ) Arsenic Trioxide Azacytidine Drug only has a 24-hour expiry, cold chain must be maintained Cladribine Day 3 Difficult as Treatment is given over or 7 days 2 1 Page 2 of
3 (2 hours 30 mins) Cytarabine IV 2 2 hours Cytarabine Subcutaneous 2 30 mins Daunorubicin + Clofarabine Daunorubicin + Cytarabine (DA) FC (IV) 2 Difficult as Treatment is given over 3 or days FLA(G) FLA-IDA Fludarabine (IV) Days 1- Day 3 Difficult as Treatment is given over 3 or days Intrathecal Methotrexate / Cytarabine MACE MCP 2 4 mins MiDAC Vincristine (single agent) IV bolus 3 4 mins LUNG CANCER & MESOTHELIOMA Carboplatin (iv) + Etoposide (po) 3 2 hours 30 mins Carboplatin + Gemcitabine 3 2 hours Carboplatin + Pemetrexed (Alimta ) 3 need to be given in the morning. (2hours) Carboplatin + Vinorelbine 3 need to be given in the morning. (2hour 30mins) Cisplatin + Etoposide Cisplatin + Pemetrexed (Alimta ) Cisplatin + Vinorelbine Docetaxel (Taxotere ) 3 1 hour 30 mins Etoposide IV 2 1 hour 1 mins Pamidronate 2 (30mg 4 mins, 90mg 2 hours) Paclitaxel + Gemcitabine Paclitaxel + Pemetrexed (Alimta ) Pemetrexed only has a 24hr expiry Pemetrexed 2 Refer to Healthcare@Home if patient agrees (1 hour) Vinorelbine 2 need to be given in the morning (4 mins) Zolendronate 2 30 mins LYMPHOMA ABVD Not recommended as dacarbazine only has a 24hr expiry and is usually made on day of infusion in view of risk of reaction. BEAM Bendamustine 2 ChlVEP 2 1 hour ChlVPP 2 1 hour CNOP 2 CHOP 2 CODOX-M Difficult as Treatment is given over 2 days although should be possible to administer on a (1 hour 30 mins) 2 1 Page 3 of
4 COP / CVP 2 Cyclophosphamide PBSC DHAP FC (Fludaradine + Cyclophosphamide) IV 2 Fludarabine (IV) 2 FMD (IV) 2 FMD (po) 2 Gemcitabine 2 1 hour Intrathecal Methotrexate / Cytarabine 2 Difficult as Treatment is given over 3 or days Difficult as Treatment is given over days although should be possible to administer on a IVAC MCP / MCD 2 Methylprednisolone IV Stat 2 1 hour Pixantrone 2 R-CEP 3 3 hours 1 mins R-CHOP 3 3 hours 30 mins R-CNOP 3 3 hours 1 mins R-CODOX-M R-CVP 3 3 hours 1 mins R-DHAP R-FC (Fludaradine + Cyclophosphamide) PO 3 3 hours 1 mins R-FC (Fludaradine + Cyclophosphamide) IV Day 2 Difficult as Treatment is given over 3 or days R-MCP 3 3 hours 1 mins Rituximab (single agent) IV 3 3 hours Rituximab SC 3 30 mins (maintenance Ritux to be referred to Healthcare at home if patient agrees) R-MCP 3 3 hours 1 mins MULTIPLE MYELOMA Bortezomib (Velcade ) - Weekly 2 need to be given in the morning. Refer to Healthcare@Home if patient agrees (30 mins) Bortezomib (Velcade ) D1,4,8 & 11 2 Refer to Healthcare@Home if patient agrees. Drug only has a 24-hour expiry so treatment would need to be given in the morning. Caution if having on MCU some doses will need to be given in BDU (30 mins) Melphalan IV Expiry of drug only 2-3 hours Pamidronate 2 (30mg 4 mins, 90mg 2 hours) Zolendronate 2 30 mins RENAL CELL CARCINOMA SARCOMA Cisplatin + Doxorubicin Docetaxel (Taxotere ) + Gemcitabine 3 Doxorubicin (Single Agent) 2 1 hour SKIN CANCER CVD (Cisplatin, Vinblastine & Docetaxel) Dacarbazine (DTIC) Ipilimumab Pembrolizumab 1 Page 4 of
5 Vinblastine Weekly 2 UPPER GASTROINTESTINAL CANCER Abraxane/Gemcitabine Abraxane 8 hour expiry, difficult to make up Cetuximab 2 3 hours Cisplatin + -FU (4-day infusion) Cisplatin + -FU (Continuous) Docetaxel, Cisplatin and -Fluorouracil (TPF) ECarboF 3 Possible with walkmed infusor (2hours) ECisF ECX ECX + Gemcitabine 2 1 hour Octreotide LAR 2 Ranucirumab 2 need to be given in the morning. (2 hours 30 mins) UNKNOWN PRIMARY UROLOGICAL CANCER Cabazitaxel 3 CarboGem 3 Can be given #2 Day 8 (2hours) CarboMV 3 CisMV Cisplatin + -FU ( 4 / 7 ) Docetaxel (Taxotere ) + Prednisolone 3 Mitoxantrone + Prednisolone 2 4 mins Pamidronate 2 Zolendronate Page of
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
CCC Chemotherapy Protocols V9.0
 CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Appendix 2. Adjuvant Regimens. AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2
 Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
Appendix 2 Adjuvant Regimens AC doxorubin 60 mg/m 2 every 3 weeks x 4 cycles Cyclophosphamide 600 mg/m 2 CMF IV cyclophosphamide 600 mg/m 2 days 1 & 8 every 4 weeks methotrexate 40 mg/m 2 for 6 cycles
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
Standard Regimens for Haematology
 Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
FREEDOM OF INFORMATION SOUTH EAST SCOTLAND CANCER NETWORK
 Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Regimens highlighted in red contain an expensive drug that is not currently publicly funded for the regimen and treatment intent.
 Palliative Breast Cancer Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for breast cancer used in the palliative setting. It is expected that the prescribing oncologist
Palliative Breast Cancer Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for breast cancer used in the palliative setting. It is expected that the prescribing oncologist
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens. Low Grade Lymphoma
 Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
Guidelines for the Management of Extravasation (Version 5 May 2012)
 Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto
 Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
OPCS Classification of Interventions and Procedures Version 4.6 (April 2011)
 Chemotherapy Regimens Clinical Coding Guidance OPCS-4.6 Version 1.0 Programme Sub Programme Data Standards & Products Clinical Classifications Document Record ID Key NPFIT-SHR-SHI-0318.01 Programme Director
Chemotherapy Regimens Clinical Coding Guidance OPCS-4.6 Version 1.0 Programme Sub Programme Data Standards & Products Clinical Classifications Document Record ID Key NPFIT-SHR-SHI-0318.01 Programme Director
Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens. High Grade Lymphoma
 Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for high grade and Burkitt s lymphoma used in the
Adjuvant/Curative/Neo-adjuvant High Grade and Burkitt s Lymphoma Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for high grade and Burkitt s lymphoma used in the
General Authorization Criteria for ALL Agents and Indications:
 Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Guidelines for the Management of Extravasation
 Guidelines for the Management of Extravasation (Version 5.5-04 Nov 2016) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT
Guidelines for the Management of Extravasation (Version 5.5-04 Nov 2016) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT
Chapter. Contents Breast Cancer Adjuvant Epirubicin weekly. Docetaxel Copy No:
 Chapter 2: Breast Cancer Contents Chapter 2: Breast Cancer... 1 Breast Cancer... 2 Adjuvant...... 2 Epi-CMF... 2 FEC / docetaxel... 3 FEC100... 4 AC/EC/TC... 4 (neo) adjuvant... 5... 5 HER2 positive: TCarboH...
Chapter 2: Breast Cancer Contents Chapter 2: Breast Cancer... 1 Breast Cancer... 2 Adjuvant...... 2 Epi-CMF... 2 FEC / docetaxel... 3 FEC100... 4 AC/EC/TC... 4 (neo) adjuvant... 5... 5 HER2 positive: TCarboH...
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Pharmacy Prior Authorization Colony Stimulating Factor (CSF)/Myeloid Growth Factor (MGF) Clinical Guideline
 Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
Guidelines for prevention and management of CHEMOTHERAPY EXTRAVASATION
 Guidelines for prevention and management of CHEMOTHERAPY EXTRAVASATION Please note that this policy refers to the management of extravasation of cytotoxic chemotherapy only. Refer to local Trust policies
Guidelines for prevention and management of CHEMOTHERAPY EXTRAVASATION Please note that this policy refers to the management of extravasation of cytotoxic chemotherapy only. Refer to local Trust policies
Disease Site Sub-Disease Site Intent Regimen Code Regimen Details Status (as of October 17, 2017) Breast Not Applicable Adjuvant / Curative
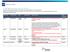 ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
Job title: Consultant Pharmacist/Advanced Practice Pharmacist
 Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Type Days HRG Procurement 1 Cost and Volume Delivery 1 Day case. Weekly for 30 weeks. 1-14inc Capecitabine 1250mg/m² twice daily
 Colorectal - Adjuvant Standard adjuvant therapy for Duke's C Day(s) Drug Dose Route Comments and high risk Duke's B Colorectal cancer 5-FU/FA Weekly 1 Folinic acid 50mg IV IV Bolus injection via fast running
Colorectal - Adjuvant Standard adjuvant therapy for Duke's C Day(s) Drug Dose Route Comments and high risk Duke's B Colorectal cancer 5-FU/FA Weekly 1 Folinic acid 50mg IV IV Bolus injection via fast running
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Roche setting the standards of cancer care Oncology Event for Investors, June 19
 Roche setting the standards of cancer care Oncology Event for Investors, June 19 Kapil Dhingra, VP Medical Science Developing a drug to the standard of care Superior clinical benefit, resources and time
Roche setting the standards of cancer care Oncology Event for Investors, June 19 Kapil Dhingra, VP Medical Science Developing a drug to the standard of care Superior clinical benefit, resources and time
Pharmacy Prior Authorization Colony Stimulating Factors Clinical Guideline. Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF)
 Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
Neulasta (peg-filgrastim; G-CSF) Neupogen (filgrastim; G-CSF) Neulasta Onpro (peg-filgrastim; G-CSF) Leukine (sargramostim; GM-CSF) General Authorization Criteria for ALL Agents and Indications: Prescribed
CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT
 CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT OF ETRAVASATION Policy for the management of extravasation Contents Page number Introduction 3 Definition 3 Prevention 3 Follow up 6 Signs and symptoms
CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT OF ETRAVASATION Policy for the management of extravasation Contents Page number Introduction 3 Definition 3 Prevention 3 Follow up 6 Signs and symptoms
Medical Therapies in Ovarian Cancer The Arabic Perspectives. Mezghani Bassem -Tunisia
 Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
Proposing Trastuzumab as an Essential Medicine to Treat Cancer: Insight on Methodologies, Processes and Outcomes. Lorenzo Moja
 Proposing Trastuzumab as an Essential Medicine to Treat Cancer: Insight on Methodologies, Processes and Outcomes Lorenzo Moja Essential Medicines List Secretariat Essential Medicines and Health Products
Proposing Trastuzumab as an Essential Medicine to Treat Cancer: Insight on Methodologies, Processes and Outcomes Lorenzo Moja Essential Medicines List Secretariat Essential Medicines and Health Products
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Trust Guideline for Prevention and Control of Chemotherapy and Radiotherapy Induced Nausea and Vomiting in Adults
 A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Cancer drug approvals for paediatric indications (n=43)
 Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Contents Trials. Dose guidelines. Calvert formula Issue Date: January Author: No: Copy. Pagee 1 of 21. Issue.
 Chapter 1: General information Contents Chapter 1: General information... 1 General observations... 2 Protocol Additions 2013... 2 Cancer Drugs Fund... 2 Off Protocol Treatment Policy... 2 Trials... 2
Chapter 1: General information Contents Chapter 1: General information... 1 General observations... 2 Protocol Additions 2013... 2 Cancer Drugs Fund... 2 Off Protocol Treatment Policy... 2 Trials... 2
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment
 Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment Julie Kennerly-Shah, PharmD, MS, MHA, BCPS May 30, 2018 The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer
Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment Julie Kennerly-Shah, PharmD, MS, MHA, BCPS May 30, 2018 The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Vasco OP Underglove Sterile Surgical and Protective Gloves
 data sheet EN 374:2003 EN 374:2003 AQL 0.65 B. Braun Melsungen AG confirms that Vasco OP Underglove gloves comply with the following standards and directives: EC CERTIFICATES AND Applied standards Medical
data sheet EN 374:2003 EN 374:2003 AQL 0.65 B. Braun Melsungen AG confirms that Vasco OP Underglove gloves comply with the following standards and directives: EC CERTIFICATES AND Applied standards Medical
CancerPACT Cancer Patients Alliance for Clinical Trials
 TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
For Health Professionals Who Care For Cancer Patients
 April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Bladder Cancer (Urothelial) Pathways
 Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Transitioning Inpatient Chemotherapy to the Outpatient Setting
 Transitioning Inpatient Chemotherapy to the Outpatient Setting A L I M C B R I D E, P H A R M D, M S, B C P S, B C O P C L I N I C A L C O O R D I N A T O R, T H E U N I V E R S I T Y O F A R I Z O N A
Transitioning Inpatient Chemotherapy to the Outpatient Setting A L I M C B R I D E, P H A R M D, M S, B C P S, B C O P C L I N I C A L C O O R D I N A T O R, T H E U N I V E R S I T Y O F A R I Z O N A
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
TRANSPARENCY COMMITTEE OPINION. 15 February 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 February 2006 Taxotere 20 mg, concentrate and solvent for solution for infusion B/1 vial of Taxotere and 1 vial
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 February 2006 Taxotere 20 mg, concentrate and solvent for solution for infusion B/1 vial of Taxotere and 1 vial
DRUG EXTRAVASATION. Vesicants. Irritants
 DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
Bladder Cancer Pathways (Urothelial)
 Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi )
 09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
For Health Professionals Who Care For Cancer Patients
 March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
Title Developed By. Implementation Contact person(s) Review Date Group Responsible. NICaN Policy: Management of Chemotherapy Extravasation; Version 3
 Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients
 Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
亞東紀念醫院 Breast Cancer 化學治療處方集
 亞東紀念醫院 Breast Cancer 化學治療處方集 2008-08 制定 最近修改日期 :2015-01 CMF Breast cancer 化學治療處方參考集 Adjuvant Classic CMF Cyclophosphamide 100mg/m2 PO qd; D1-D14 Methotrexate 40mg/m 2 in N/S 100 ml IV drip 30 mins; D1,
亞東紀念醫院 Breast Cancer 化學治療處方集 2008-08 制定 最近修改日期 :2015-01 CMF Breast cancer 化學治療處方參考集 Adjuvant Classic CMF Cyclophosphamide 100mg/m2 PO qd; D1-D14 Methotrexate 40mg/m 2 in N/S 100 ml IV drip 30 mins; D1,
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Patient 1: Patient 2:
 Appendix A Compiled by Dr. Raymond Ngeh and Dr. Robert Luk Clinical notes and PET/CT scan images of eleven patients: 1. Middle age woman has cancer of the pancreas in the body of the gland. After just
Appendix A Compiled by Dr. Raymond Ngeh and Dr. Robert Luk Clinical notes and PET/CT scan images of eleven patients: 1. Middle age woman has cancer of the pancreas in the body of the gland. After just
ST-QBP: Systemic Treatment Quality-Based Program (formerly STFM) DF: Drug Formulary GYNECOLOGICAL SKIN HEMATOLOGY ST- QBP
 Updates from October 17, 2017 Please note that the following are regimen updates applicable to webpage documents and/or Drug Formulary s regimen monographs, as indicated by checkmarks. : Systemic Treatment
Updates from October 17, 2017 Please note that the following are regimen updates applicable to webpage documents and/or Drug Formulary s regimen monographs, as indicated by checkmarks. : Systemic Treatment
NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria
 NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria The information in this document is a summary of the funding criteria for the New Drug Funding
NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria The information in this document is a summary of the funding criteria for the New Drug Funding
