Dow Haltermann Custom Processing The World of Hydrogen Cyanide Chemistry
|
|
|
- Solomon Moses Jordan
- 6 years ago
- Views:
Transcription
1 Dow altermann Custom Processing The World of ydrogen Cyanide Chemistry Sharing Your Vision esourcing Your Success
2 Specialists in ydrogen Cyanide Chemistry With Dow s acquisition of the ampshire Chemical Corporation in 1998, and the subsequent formation of Dow altermann Custom Processing, you now have access to over 40 years of experience in hydrogen cyanide chemistry and a worldwide leadership position in custom manufacturing and toll synthesis projects. Dow altermann Custom Processing serves the diverse needs of the pharmaceutical, agrochemical, water treatment, polymer, and photographic industries with an extensive family of intermediates and derivatives based on chemistry. Dow altermann Custom Processing has the unique ability to handle both liquid and sodium cyanide. This unique flexibility is not offered by most custom manufacturers who provide cyanation capabilities. With the ability to handle both and sodium cyanide, we can manufacture a variety of intermediates in both aqueous and non-aqueous reaction systems. Liquid is a unique and versatile building block that often leads to simplified reaction pathways and high purity products that require less purification and minimizes the need to remove salt by-products. The production of chiral intermediates and products is becoming increasingly more important in today s society, especially in the pharmaceutical and agricultural markets. Liquid can be used, with a variety of enzymes, at moderate ps, to produce novel chiral molecules. hydantoins -amino acids cyanohydrins Dow altermann Custom Processing -keto acids amino nitriles Sharing Your Vision... esourcing Your Success -hydroxy acids nitriles amino nitriles Dow altermann Custom Processing, a business unit of The Dow Chemical Company, is exclusively dedicated to providing world-class product and process development and contract manufacturing services to the specialty and fine chemicals industries. Key processes include distillation, batch and continuous fractionation, advanced separations, batch reactions, continuous reactions, and multi-step organic chemistry. Markets served include household and personal care products, electronics, agricultural chemicals, petrochemicals, mining, photographic chemicals, flavors and fragrances, coatings, textiles, and oleochemicals. To learn more, visit 2
3 Look to Dow altermann Custom Processing for these Toll Synthesis and Custom Manufacturing Capabilities... More than 40 years of experience handling liquid and sodium cyanide An extensive family of intermediates and derivatives Fast and reliable development and production of specialty intermediates esearch and development support Process development and optimization Complete analytical services Full scale-up support in the U.S. and Europe Flexible multi-purpose production facilities Large commercial capacity in two dedicated plants Schotten-Baumann acylation expertise Supported by The Dow Chemical Company s global resources The world of hydrogen cyanide chemistry offers many possibilities... Amino Amides: Glycinamide Cl, Cycloleucine amide Cl Acylated Amino Acids: Acyl Glutamates, Acyl Sarcosinates, -Lauroylethylenediamine-,, -triacetic acid (LED3A) Amino Acids: Glycine, Sarcosine, Amino Isobutyric Acid, Amino Cyclohexane Carboxylic Acid, Cycloleucine Amino Esters: Methyl Glycinate Cl, Ethyl Glycinate Cl, Methyl Sarcosinate Cl, Ethyl Sarcosinate Cl Amino itriles: Acetone Amino itrile, Cyclohexanone Amino itrile, (Dimethylamino) acetonitrile, Methyl Ethyl Ketone Amino itrile, Methyl Isobutyl Ketone Amino itrile Amino Polycarboxylic Acids: Ethylenediamine-,, -triacetic acid (ED3A), Iminodiacetic Acid Chiral Molecules: ()-Mandelic acid, ()-2- Chloromandelic acid, D-(4-ydroxyphenyl)glycine, D-(4-ydroxybutyl)glycine Cyanohydrins: Acetone Cyanohydrin, Methyl Ethyl Ketone Cyanohydrin, Mandelonitrile ydantoins: 5,5-Dimethylhydantoin, 5-Ethyl-5-methylhydantoin, 5-Phenylhydantoin, 5-(4-ydroxybutyl)hydantoin -ydroxy Acids: -ydroxyisobutyric Acid, Mandelic Acid -Keto Acids: Phenylpyruvic Acid, 3-Methyl-2- oxobutanoic Acid, 4-Methyl-2-oxopentanoic Acid itriles: 1,6-Dicyanohexane, Isophorone itrile Miscellaneous: Pantolactone, 5,5-Dimethyl-2, 4-oxazolidinedione, 2-ydroxyisobutyramide, Methyl Mandelate 3
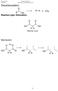
4 ur Product Families Dow altermann Custom Processing s extensive familiarity with hydrogen cyanide chemistry and its many reaction products makes us a uniquely qualified resource for helping you achieve optimum production efficiency and economy. Following are brief descriptions of our hydrogen cyanide product families. -Amino Acids Acylated Amino Acids 1. / '' 2 ' C 2 2 glycine C 2 ' 2. a / C 2 sarcosine '' -Amino acids are prepared by reacting either sodium cyanide (Bersworth Chemistry) or hydrogen cyanide (Singer Chemistry) with an aldehyde or a ketone and an amine. Singer Chemistry provides the advantage of higher purity products due to isolation of the intermediate amino nitrile. -Amino acids and aminopoly-carboxylic acids (chelating agents) are commonly used as pharmaceutical, nutraceutical, and agricultural intermediates, as well as common components in cleaning, personal care, and photographic formulations. C 2 Me ' base C2 acyl sarosinates Cl acyl glutamates C 2 C2 -Amino acids are acylated using the Schotten- Baumann reaction, a core technology for Dow. Acylated amino acids are used in a variety of applications including personal care, cleaning, polymer, as well as pharmaceutical and agricultural intermediates. ' C 2 C 2 2 C C 2 2 C C 2 iminodiacetic acid C a 2 C a 2 C C 2 a LED3A 2 ethylenediamine triacetic acid trisodium salt acyl cycloleucine amide 4
5 -Amino Esters and Amides 2 CI '' ' CI solvent 2 3 -Amino esters and -amino amides can be prepared from the corresponding carboxylic acids, acid chlorides, or acid anhydrides. A more direct pathway is from the amino nitrile eliminating the additional steps leading to the carboxylic acid derivatives described above. -Amino esters and -amino amides are commonly used as pharmaceutical and agricultural intermediates. 3 C 2 '' ' ' - Cl 2 - Cl Amino itriles 2 ' '' 2 acetophenone amino nitrile acetone amino nitrile 2 ' '' Amino nitriles are readily prepared from the reaction of aldehydes or ketones with hydrogen cyanide and anhydrous ammonia in the presence of a base. Amino nitriles are precursors to -amino acids, -amino amides, and -amino esters. Amino nitriles are also commonly used as precursors to azo bis initiators via oxidative coupling. 2 3 C 2 Et cyclopentanone amino nitrile ethyl glycinate glycinamide hydrochloride 3 C 2 Me glycinonitrile dimethylaminoacetonitrile methyl phenylglycinate 3 2 cycloleucine amide C 2 Et ethyl sarcosinate 5
6 ()-pantolactone Chiral Molecules ' ()-oxynitrilase itrilase 2 C 2 L-6-hydroxynorleucine ' C 2 4 Chiral molecules are critical intermediates in a majority of the new pharmaceutical and agricultural products developed today. Three enzymes, oxynitrilase, hydantoinase, and nitrilase are useful in preparing chiral cyanohydrins, amino acids, and hydroxy acids. All of these specialties are useful intermediates leading to a variety of chiral molecules. Dow has used oxynitrilase enzymes commercially and is developing pathways leading to new chiral molecules using both of these enzymes. Cyanohydrins ' glycolonitrile base Cyanohydrins are common intermediates leading to a variety of molecules including amino nitriles, -hydroxy acids, amides, esters,, -unsaturated nitriles, acids, and esters. Cyanohydrins are readily prepared by reacting aldehydes and ketones with hydrogen cyanide in the presence of a base. The simplest cyanohydrin is glycolonitrile and it is frequently used to prepare aminopolycarboxylic acids (chelating agents). Acetone cyanohydrin, another common cyanohydrin, is a key intermediate in the preparation of methacrylic acid. Chiral cyanohydrins can be prepared using chemical resolving agents and with oxynitrilase enzymes leading to a variety of chiral entities. ' mandelonitrile acetone cyanohydrin 6
7 ydantoins ' 3 / C 2 ' itriles X base ydantoins are prepared using the well-known Bucherer-Bergs reaction, which involves the reaction of an aldehyde or a ketone with hydrogen cyanide, ammonia, and carbon dioxide. Dimethylhydantoin (DM) is a key intermediate in the preparation of brominated and chlorinated biocides. Substituted hydantoins are used as intermediates to many pharmaceuticals and can be hydrolyzed to form -substituted amino acids. ptically active unnatural amino acids can be prepared using D-hydantoinase enzymes. hydantoin Ph phenylhydantoin C base C itriles are prepared via nucleophilic substitutions of alky halides using an alkaline form of hydrogen cyanide. Michael Additions are common methods for the preparation of nitriles by the addition of hydrogen cyanide to activated double bonds in the presence of an alkaline catalyst. Substituted amines, prepared by the reduction of alkyl and aryl nitriles, are used in a variety of pharmaceutical, agricultural, and industrial applications. 1,6-dicyanohexane isophorone nitrile 5-(4-hydroxybutyl)hydantoin 5,5-dimethylhydantoin DMDM 7
8 To Learn More... ydrogen cyanide chemistry from Dow altermann Custom Processing offers a world of processing possibilities for a diverse range of chemical components and final products. We welcome your inquiries regarding our custom and toll synthesis capabilities and how we might put them to work for your process or application. Visit us on the web at r call In the United States and Canada: In Europe: Dedicated manufacturing facilities in Deer Park, Texas, and Seal Sands, UK TICE: o freedom from any patent owned by Seller or others is to be inferred. Because use conditions and applicable laws may differ from one location to another and may change with time, Customer is responsible for determining whether products and the information in this document are appropriate for Customer s use and for ensuring that Customer s workplace and disposal practices are in compliance with applicable laws and other governmental enactments. Seller assumes no obligation or liability for the information in this document. WAATIES AE GIVE; ALL IMPLIED WAATIES F MECATABILITY FITESS F A PATICULA PUPSE AE EXPESSLY EXCLUDED. * *Trademark of The Dow Chemical Company Form o AMS
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
10. CARBOXYLIC ACIDS AND THEIR DERIVATIVES 10.1 Nomenclature of Carboxylic Acids 10.2 Physical Properties of Carboxylic Acids 10.
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
Lecture 20. Herman Emil Fischer Nobel Prize 1902 Sugars, Esters and Purines. April 4, Chemistry 328N
 Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
H 3 C OCH 3 3 C N(CH 3 ) 2 H 3 C H H 3 C CH 3. ketone. pk a = 9 H H. 1,3-keto ester pk a = 11
 hapter 21: Ester Enolates 21.1: Ester α ydrogens and Their pk a s. The α-protons of s are less acidic that ketones and aldehydes. Typical pk a s of carbonyl compounds (α-protons): aldehydes 17 ketones
hapter 21: Ester Enolates 21.1: Ester α ydrogens and Their pk a s. The α-protons of s are less acidic that ketones and aldehydes. Typical pk a s of carbonyl compounds (α-protons): aldehydes 17 ketones
R O R' Acid anhydride. Acid halide. Carboxylic acid. Ester O O O O. Nitrile Acyl phosphate Thioester. Amide
 Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Carboxylic Acid Derivatives
 arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
1/3/2011. Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Carboxylic Acid Derivatives Reading Study Problems Key Concepts and Skills Lecture Topics: Structures and reactivity of carboxylic acid derivatives
 Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Identifying Functional Groups. (Chapter 2 in the Klein text)
 Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Chapter 20: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
Defoaming Surfactants
 TERGITOL L Series Biodegradable Surfactants TERGITOL L-61 E, L-62 E, L-64 E, L-81 E Defoaming Surfactants Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow. These surfactants
TERGITOL L Series Biodegradable Surfactants TERGITOL L-61 E, L-62 E, L-64 E, L-81 E Defoaming Surfactants Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow. These surfactants
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Chapter 19: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution 19.1: Nomenclature of Carboxylic Acid Derivatives (please read)
 problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
Carboxylic acid derivatives
 Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
ACUMER 4161 Phosphinopolycarboxylic Acid Scale Inhibitor and Dispersant
 Technical Data Sheet ACUMER 4161 Phosphinopolycarboxylic Acid Scale Inhibitor and Dispersant Description ACUMER 4161 (PCA) polymer combines features of both phosphonates and polyacrylates to deliver a
Technical Data Sheet ACUMER 4161 Phosphinopolycarboxylic Acid Scale Inhibitor and Dispersant Description ACUMER 4161 (PCA) polymer combines features of both phosphonates and polyacrylates to deliver a
Lecture Notes Chemistry Mukund P. Sibi Lecture 31 Reactions at the Alpha-Carbon of Carbonyl Compounds
 Lecture Notes hemistry 342-2008 Mukund P. Sibi eactions at the Alpha-arbon of arbonyl ompounds Enolates are nucleophilic and undergo reaction with electrophiles. For example, one can do halogenation under
Lecture Notes hemistry 342-2008 Mukund P. Sibi eactions at the Alpha-arbon of arbonyl ompounds Enolates are nucleophilic and undergo reaction with electrophiles. For example, one can do halogenation under
Carboxylic Acids and Nitriles. Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry
 Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Coupling Agents and Surfactants for the Preparation of Aqueous Solutions of DOWANOL EPh Glycol Ether
 Coupling Agents and Surfactants for the Preparation of Aqueous Solutions of DOWANOL EPh Glycol Ether Introduction Challenge DOWANOL EPh glycol ether is an aromatic, slow-evaporating glycol ether with applications
Coupling Agents and Surfactants for the Preparation of Aqueous Solutions of DOWANOL EPh Glycol Ether Introduction Challenge DOWANOL EPh glycol ether is an aromatic, slow-evaporating glycol ether with applications
DOWFAX. Nonionic Surfactants DOW POLYGLYCOLS. High-performance polyglycols for demanding applications
 DOWFAX Nonionic Surfactants DOW POLYGLYCOLS High-performance polyglycols for demanding applications DOW Polyglycols Focused on Your Success Dow strength in polyglycols brings customers many benefits The
DOWFAX Nonionic Surfactants DOW POLYGLYCOLS High-performance polyglycols for demanding applications DOW Polyglycols Focused on Your Success Dow strength in polyglycols brings customers many benefits The
Functional Derivatives of Carboxylic Acids
 Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
NORKOOL DESITHERM Desiccant
 NORKOOL DESITHERM Desiccant Introducing a New Premium Desiccant Designed to Fight Corrosion 2 Looking for a high-performance triethylene glycol (TEG) for a natural gas dehydration system? Search no further.
NORKOOL DESITHERM Desiccant Introducing a New Premium Desiccant Designed to Fight Corrosion 2 Looking for a high-performance triethylene glycol (TEG) for a natural gas dehydration system? Search no further.
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Infrared Spectroscopy
 Carbonyl Compounds Cl H H N 2 1810 cm -1 (band 1) 1800 cm -1 1760 cm -1 both present (band 2) 1735 cm -1 1725 cm -1 1715 cm -1 1710 cm -1 1690 cm -1 Inductive Effects esonance Effects stronger bond W W
Carbonyl Compounds Cl H H N 2 1810 cm -1 (band 1) 1800 cm -1 1760 cm -1 both present (band 2) 1735 cm -1 1725 cm -1 1715 cm -1 1710 cm -1 1690 cm -1 Inductive Effects esonance Effects stronger bond W W
Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves
 Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
Paper 9: ORGANIC CHEMISTRY-III (Reaction Mechanism-2) Module17: Reduction by Metal hydrides Part-II CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
ACUMER 4300 Polymer. Scale Inhibitor and Dispersant for Calcium Carbonate and Calcium Sulfate Control
 Technical Data Sheet Polymer Scale Inhibitor and Dispersant for Calcium Carbonate and Calcium Sulfate Control Description Benefits is a high performance scale inhibitor, specifically developed to prevent
Technical Data Sheet Polymer Scale Inhibitor and Dispersant for Calcium Carbonate and Calcium Sulfate Control Description Benefits is a high performance scale inhibitor, specifically developed to prevent
Carboxylic Acids and Their Derivatives. Chapter 17. Carboxylic Acids and Their Derivatives
 Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Carboxylic Acids, Esters and Acyl Chlorides
 R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
Lecture 19. Nucleophilic Acyl Substitution Y - + X - Y X R C X. April 2, Chemistry 328N
 Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
Esters of Carboxylic Acids These are derivatives of carboxylic acids where the hydroxyl group is replaced by an alkoxy group.
 Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
Lab 6: Reactions of Organic Compounds and Qualitative Analysis
 Lab 6: eactions of rganic Compounds and Qualitative Analysis bjectives: - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties.
Lab 6: eactions of rganic Compounds and Qualitative Analysis bjectives: - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties.
Carboxylic Acids and Their Derivatives
 arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
ORGANIC SYNTHESIS VIA ENOLATES
 1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
PROCESS ECONOMICS PROGRAM
 Abstract..-. PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Mm10 Park, California 9402E Process Economics Program Report No. 11C METHACRYLIC ACID AND METHACRYLIC ESTERS (September 1984) For the last fifteen
Abstract..-. PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Mm10 Park, California 9402E Process Economics Program Report No. 11C METHACRYLIC ACID AND METHACRYLIC ESTERS (September 1984) For the last fifteen
Esterification. Preparation of β-d-glucose pentaacetate. Dr. Zerong Wang at UHCL. Table of contents
 Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
DERIVATIVES OF CARBOXYLIC ACIDS
 13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
Fatty acid product guide
 Fatty acid product guide Kraton Corporation s Sylfat and Century fatty acids are useful in a wide range of industrial applications. These fatty acids (tall oil fatty acid, monomer acid, stearic acid and
Fatty acid product guide Kraton Corporation s Sylfat and Century fatty acids are useful in a wide range of industrial applications. These fatty acids (tall oil fatty acid, monomer acid, stearic acid and
13. Carboxylic Acids (text )
 2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
ACRYLAMIDE AND POLYACRYLAMIDE
 Report No. 99 ACRYLAMIDE AND POLYACRYLAMIDE by YEN-CHEN YEN June 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK. CALIFORNIA CONTENTS 1 INTRODUCTION......,..................
Report No. 99 ACRYLAMIDE AND POLYACRYLAMIDE by YEN-CHEN YEN June 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK. CALIFORNIA CONTENTS 1 INTRODUCTION......,..................
Product Safety Assessment VenPure Borohydride Products
 Product Safety Assessment VenPure Borohydride Products Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product
Product Safety Assessment VenPure Borohydride Products Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product
Carboxylic Acids and Carboxylic Acid Deriva3ves. Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on)
 Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Loudon Chapter 21 Review: Carboxylic Acid Derivatives Jacquie Richardson, CU Boulder Last updated 3/20/2018
 Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
TEREPHTHALICACID DIMETHYLTEREPHTHALATE SUPPLEMENT
 Report No. 9-A TEREPHTHALICACID DIMETHYLTEREPHTHALATE SUPPLEMENT AND A. contributions by SHIGEYOSHI by LLOYD M. ELKIN TAKAOKA January 1967 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH
Report No. 9-A TEREPHTHALICACID DIMETHYLTEREPHTHALATE SUPPLEMENT AND A. contributions by SHIGEYOSHI by LLOYD M. ELKIN TAKAOKA January 1967 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH
BUTYROLACTAMandLAUROLACTAM
 Report No. 118 BUTYROLACTAMandLAUROLACTAM by ROBERT H. SCHWAAR and TOHRU YAMADA September 1977 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK, CALIFORNIA For
Report No. 118 BUTYROLACTAMandLAUROLACTAM by ROBERT H. SCHWAAR and TOHRU YAMADA September 1977 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK, CALIFORNIA For
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Organic Chemistry. Chapter 23. Hill, Petrucci, McCreary & Perry 4 th. Ed. Alkane to Substituent Group methane CH 4 methyl CH 3
 hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
PROCESS ECONOMICS PROGRAM
 PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Mmlo Park, California 94025 Abstract Process Economics Program Report No. 1lE METRACRYLIC ACID AND METHACRYLIC ESTERS (July 1980) Continued growth in markets
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Mmlo Park, California 94025 Abstract Process Economics Program Report No. 1lE METRACRYLIC ACID AND METHACRYLIC ESTERS (July 1980) Continued growth in markets
ORGANIC AND BIOORGANIC CHEMISTRY
 P. GERGELY Department os Medical Chemistry Medical and Health Science Center University of Debrecen ORGANIC AND BIOORGANIC CHEMISTRY FÓR MEDICAL STUDENTS THIRD EDITION University of Debrecen Medical and
P. GERGELY Department os Medical Chemistry Medical and Health Science Center University of Debrecen ORGANIC AND BIOORGANIC CHEMISTRY FÓR MEDICAL STUDENTS THIRD EDITION University of Debrecen Medical and
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
Ch. 21: CARBOXYLIC ACID DERIVATIVES AND NUCLEOPHILIC ACYL SUBSTITUTION REACTIONS Nomenclature of Carboxylic Acid Derivatives:
 h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
METHACRYLIC ACID METHACRYLIC ESTERS
 Report No. 11 A METHACRYLIC ACID METHACRYLIC ESTERS Supplement A by DONALD C. THOMAS Contributions by Brij M. Sood and Leslie A. Carmichael May 1974 A private report by the PROCESS ECONOMICS PROGRAM STANFORD
Report No. 11 A METHACRYLIC ACID METHACRYLIC ESTERS Supplement A by DONALD C. THOMAS Contributions by Brij M. Sood and Leslie A. Carmichael May 1974 A private report by the PROCESS ECONOMICS PROGRAM STANFORD
Topic 4.5 COMPOUNDS CONTAINING THE CARBONYL GROUP. Aldehydes and Ketones Carboxylic Acids and their Salts Esters Acyl Chlorides and Acid Anhydrides
 Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol
 Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol Select a Topic: Names Product Overview Manufacture of Product Product Description Product Uses Exposure Potential Health Information
Product Safety Assessment Propoxylated/Ethoxylated Ethylenediamine Polyol Select a Topic: Names Product Overview Manufacture of Product Product Description Product Uses Exposure Potential Health Information
Product Safety Assessment DOW Borohydride Bleaching Products
 Product Safety Assessment DOW Borohydride Bleaching Products Product Safety Assessment documents are available at www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of
Product Safety Assessment DOW Borohydride Bleaching Products Product Safety Assessment documents are available at www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Product Safety Assessment DOW Triethylene Glycol
 Product Safety Assessment DOW Triethylene Glycol Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product Product
Product Safety Assessment DOW Triethylene Glycol Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview Manufacture of Product Product
Page 2. Q1.Which one of the following is not a correct statement about vitamin C, shown below? It is a cyclic ester.
 Q1.Which one of the following is not a correct statement about vitamin, shown below? It is a cyclic ester. It can form a carboxylic acid on oxidation. It decolourises a solution of bromine in water. It
Q1.Which one of the following is not a correct statement about vitamin, shown below? It is a cyclic ester. It can form a carboxylic acid on oxidation. It decolourises a solution of bromine in water. It
Product Safety Assessment Toluenediamine Propoxylated/Ethoxylated Polyols
 Product Safety Assessment Toluenediamine Propoxylated/Ethoxylated Polyols Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview
Product Safety Assessment Toluenediamine Propoxylated/Ethoxylated Polyols Product Safety Assessment documents are available at: www.dow.com/productsafety/finder/. Select a Topic: Names Product Overview
Level 3 Chemistry, 2007
 Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
Lab 5: Reactions of Organic Compounds and Qualitative Analysis
 Lab 5: eactions of rganic Compounds and Qualitative Analysis bjectives: - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties.
Lab 5: eactions of rganic Compounds and Qualitative Analysis bjectives: - To better understand several chemical reactions. - To identify an unknown chemical by testing its chemical and physical properties.
Oregon State University
 H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
Lecture'11:'February'21,'2013 Reac&ons*of*Deriva&ves*( )
 CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
Chem 263 B6 Notes March 30, 2006 Demo-In-Class: O
 hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
Alkenes. Question Paper 1. Chemistry (0620/0971) Cambridge International Examinations (CIE) Topic. Organic chemistry Sub-Topic. Alkenes.
 or more awesome resources, visit us at www.savemyexams.co.uk/ lkenes Question Paper 1 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic Organic chemistry
or more awesome resources, visit us at www.savemyexams.co.uk/ lkenes Question Paper 1 Level IGSE Subject hemistry (0620/0971) Exam oard ambridge International Examinations (IE) Topic Organic chemistry
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Carbonyl Chemistry VI + C O C. 1pm In Geology Room 112. Exam is Monday 11am-1pm. Chemistry /06/02
 arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
Chapter 21. Carboxylic Acid Derivatives. and Nucleophilic Acyl Substitution. Reactions. - many carboxylic acid derivatives are known:
 hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
CORROSION INHIBITORS CATALOGUE
 CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
Oxidizing Alcohols. Questions. Prediction. Analysis. Safety Precautions. Materials. Conclusions. Procedure. 74 MHR Unit 1 Organic Chemistry
 xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
POLYBUTYLENE TEREPHTHALATE AND BUTANEDIOL PROCESS ECONOMICS PROGRAM. Report No. 96A. Supplement. by LLOYD M. ELKIN. November 1977
 Report No. 96A POLYBUTYLENE TEREPHTHALATE AND BUTANEDIOL Supplement A by LLOYD M. ELKIN and YU-REN CHIN November 1977 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO
Report No. 96A POLYBUTYLENE TEREPHTHALATE AND BUTANEDIOL Supplement A by LLOYD M. ELKIN and YU-REN CHIN November 1977 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO
Organic Chemistry. AQA Chemistry topic 7
 rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Connecting our chemistries creating shared values
 AkzoNobel Surface Chemistry Connecting our chemistries creating shared values Intermediates 2 AkzoNobel Surface Chemistry AkzoNobel Surface Chemistry 3 Global manufacturing footprint World-class technology
AkzoNobel Surface Chemistry Connecting our chemistries creating shared values Intermediates 2 AkzoNobel Surface Chemistry AkzoNobel Surface Chemistry 3 Global manufacturing footprint World-class technology
SENTRY TM POLYOX Water Soluble Resins
 SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
A carboxylic acid is an organic compound that contains a carboxyl group, COOH
 1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
REACTIONS OF CARBOXYLIC ACID DERIVATIVES WITH NUCLEOPHILES A. Reactions of Acid Chlorides with Nucleophiles
 1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
Supporting Information
 Supporting Information Cooper et al. 10.1073/pnas.1105715108 SI Text Subsequent Chemistry of Keto Acids/Amides/Nitriles (Fig. 2A). The presence of a carbonyl (ketone) group in the keto acids may have lead
Supporting Information Cooper et al. 10.1073/pnas.1105715108 SI Text Subsequent Chemistry of Keto Acids/Amides/Nitriles (Fig. 2A). The presence of a carbonyl (ketone) group in the keto acids may have lead
ACUSOL 820 Rheology Modifier/Stabilizer
 Technical Data Sheet Description Applications is a Hydrophobically modified Alkali Soluble acrylic polymer Emulsion (HASE) with unusually high aqueous thickening and stabilising efficiency. When neutralized
Technical Data Sheet Description Applications is a Hydrophobically modified Alkali Soluble acrylic polymer Emulsion (HASE) with unusually high aqueous thickening and stabilising efficiency. When neutralized
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Revision Sheet Final Exam Term
 Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 12 A, B, C Required Materials: Chapter: 22 Section: 1,2,3,4 (Textbook pg. 669-697) Chapter: 23 Section: 1,2 (Textbook pg. 707-715)
Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 12 A, B, C Required Materials: Chapter: 22 Section: 1,2,3,4 (Textbook pg. 669-697) Chapter: 23 Section: 1,2 (Textbook pg. 707-715)
Direct Compression. With the right ingredients it s a simple, cost-effective manufacturing process
 Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
Chapter 20 Carboxylic Acids. Introduction
 hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
TEREPHTHALIC ACID AND DIMETHYL TEREPHTHALATE
 Report No. 9C Supplement TEREPHTHALIC ACID AND DIMETHYL TEREPHTHALATE and FUMIHIKO by LLOYD M. ELKIN YAMAGUCHI August 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE
Report No. 9C Supplement TEREPHTHALIC ACID AND DIMETHYL TEREPHTHALATE and FUMIHIKO by LLOYD M. ELKIN YAMAGUCHI August 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE
DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin
 Product Information DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin Active Matter: 1,3-Dimethylol-5,5-dimethylhydantoin H 3 C CH 3 O HOCH
Product Information DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin Active Matter: 1,3-Dimethylol-5,5-dimethylhydantoin H 3 C CH 3 O HOCH
Carboxylic Acids and Derivatives. Decarboxylation R H + CO 2. R OH Reaction type: Elimination. H H Malonic acid. Mechanism:
 rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
CLEAR+STABLE The clear choice for food and beverage stability
 CLEAR+STABLE The clear choice for food and beverage stability Dow Pharma & Food Solutions is dedicated to supporting the food and drink industry with a range of food ingredients designed to cater for the
CLEAR+STABLE The clear choice for food and beverage stability Dow Pharma & Food Solutions is dedicated to supporting the food and drink industry with a range of food ingredients designed to cater for the
Abstract. Process Economics Program Report No. 168 NONIONIC SURFACTANTS. (March 1984)
 Abstract Process Economics Program Report No. 168 SRI INTERNATIONAL Menlo Park, California 94025 NONIONIC SURFACTANTS (March 1984) This report covers the technology and costs of manufacturing three types
Abstract Process Economics Program Report No. 168 SRI INTERNATIONAL Menlo Park, California 94025 NONIONIC SURFACTANTS (March 1984) This report covers the technology and costs of manufacturing three types
E c o S m o o t h TM. S a t i n. F o r m u l a t i o n G u i d e l i n e s
 E c o S m o o t h TM S a t i n F o r m u l a t i o n G u i d e l i n e s Formulating Guidelines for EcoSmooth Satin Conditioning Polymer in 2-in-1 Conditioning Shampoo and Body Wash Use EcoSmooth Satin
E c o S m o o t h TM S a t i n F o r m u l a t i o n G u i d e l i n e s Formulating Guidelines for EcoSmooth Satin Conditioning Polymer in 2-in-1 Conditioning Shampoo and Body Wash Use EcoSmooth Satin
Committed to Environment, Health, & Safety
 Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
SELECTRA-SIL DERIVATIZING REAGENTS
 Purpose of Derivatization: Derivatization is performed for two significant reasons. The first of which is to reduce the polarity and enhance the volatility of high molecular weight polar drugs, making
Purpose of Derivatization: Derivatization is performed for two significant reasons. The first of which is to reduce the polarity and enhance the volatility of high molecular weight polar drugs, making
Nu: - Addition or Nu: - Acyl Substitution?
 12. Apply nucleophilic addition and elimination concepts to nucleophilic acyl substution reactions of acids and derivatives (focus on esters and amides) In Class problems: 1. The reactive site of aldehydes,
12. Apply nucleophilic addition and elimination concepts to nucleophilic acyl substution reactions of acids and derivatives (focus on esters and amides) In Class problems: 1. The reactive site of aldehydes,
