Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis
|
|
|
- Arlene Glenn
- 5 years ago
- Views:
Transcription
1 ARTICLE DOI: 1.138/s OPEN Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis Dalia E. Gaddis 1, Lindsey E. Padgett 1, Runpei Wu 1, Chantel McSkimming 2, Veronica Romines 1, Angela M. Taylor 2, Coleen A. McNamara 2, Mitchell Kronenberg 3, Shane Crotty 4,, Michael J. Thomas 6, Mary G. Sorci-Thomas 6,7 & Catherine C. Hedrick ():,; Regulatory T (Treg) cells contribute to the anti-inflammatory response during atherogenesis. Here we show that during atherogenesis Treg cells lose Foxp3 expression and their immunosuppressive function, leading to the conversion of a fraction of these cells into T follicular helper (Tfh) cells. We show that Tfh cells are pro-atherogenic and that their depletion reduces atherosclerosis. Mechanistically, the conversion of Treg cells to Tfh cells correlates with reduced expression of IL-2Rα and pstat levels and increased expression of IL-6Rα. In vitro, incubation of naive T cells with oxldl prevents their differentiation into Treg cells. Furthermore, injection of lipid-free Apolipoprotein AI (ApoAI) into ApoE / mice reduces intracellular cholesterol levels in Treg cells and prevents their conversion into Tfh cells. Together our results suggest that ApoAI, the main protein in high-density lipoprotein particles, modulates the cellular fate of Treg cells and thus influences the immune response during atherosclerosis. 1 Division of Inflammation Biology, La Jolla Institute for Allergy and Immunology, 942 Athena Circle, La Jolla, CA 9237, USA. 2 Cardiovascular Research Center and Division of Cardiology, University of Virginia, 41 Lane Road, Charlottesville, VA 2298, USA. 3 Division of Developmental Immunology, La Jolla Institute for Allergy and Immunology, 942 Athena Circle, La Jolla, CA 9237, USA. 4 Division of Vaccine Discovery, La Jolla Institute for Allergy and Immunology, 942 Athena Circle, La Jolla, CA 9237, USA. Division of Infectious Diseases, Department of Medicine, UCSD School of Medicine, 9 Gilman Drive, La Jolla, CA 9293, USA. 6 Department of Pharmacology and Toxicology, Medical College of Wisconsin, 871 Watertown Plank Rd, Milwaukee, WI 3226, USA. 7 Department of Medicine, Division of Endocrinology, Medical College of Wisconsin, 92W. Wisconsin Ave., Milwaukee, WI 3226, USA. Correspondence and requests for materials should be addressed to C.C.H. ( Hedrick@lji.org) NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
2 ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s Regulatory T cells (Treg) play an important role during atherosclerosis development. Depletion of Treg exacerbates atherosclerosis in mouse models, while the transfer of Treg prevents disease progression 1 4. IL-1 and TGFβ also inhibit atherosclerosis development 7. Treg are a dynamic cell population that are reduced in the aorta of mice fed an atherogenic diet, and can increase when mice are then switched to a regular chow diet 8. Treg can lose Foxp3 and convert into other CD4 T cell subsets 9 11, indicating the Treg conversion in inflammatory conditions. A recent study by Butcher et al. has shown that Treg can convert to IFNγ + CD4 T cells in older ApoE / mice 12. Whether Treg conversion is limited to IFNγ + cells or can extend to other pathogenic T cell subsets during atherogenesis, and understanding the factors that govern this conversion need to be determined. Apolipoprotein AI (ApoAI) is the major structural protein of plasma HDL. Without ApoAI, plasma HDL concentrations are dramatically reduced 13. ApoAI is made by hepatocytes and before its release into the plasma interacts on the plasma membrane with ABCA1 to acquire phospholipids and cholesterol to form nascent HDL or pre-hdl particles ABCA The formation of pre- HDL promotes cholesterol efflux from cells, and thereby stimulates the process of reverse cholesterol transport. Because of ApoAI s inherent ability to form cholesterol-rich nascent HDL particles, its anti-inflammatory properties have been associated with changes in lipid raft composition, which can modulate immune cell signaling and proliferation 17,18. The anti-inflammatory role of ApoAI is documented in multiple inflammatory conditions, including lupus 19,Alzheimer s disease 2 and dermatitis 21. ApoAI can also decrease the maturation of dendritic cells in a way that dampens T cell activation 22, suggesting that ApoAI can also indirectly influence T cell responses during inflammation. The relationship between ApoAI and Treg is poorly understood. A study by Wilhelm et al. showed that administration of ApoAI to ApoAI / LDLR / mice resulted in a decrease in T effector to Treg ratios in the skin draining lymph nodes, and reduced the number of skin-infiltrating T cells in these mice 23. Can ApoAI influence Treg plasticity during atherogenesis? If yes, what are the mechanisms involved? In this study, we sought to determine the fate of Treg during atherogenesis and how ApoAI affected this process. Collectively, our results show novel findings regarding Treg plasticity and their conversion to T follicular helper cells during atherogenesis and indicate a role for ApoAI in regulating this Treg conversion, shedding light on a collaborative effort between cholesterol metabolism and Treg homeostasis that dampens pro-atherogenic immune responses. Results ExTreg cells convert to Tfh cells during atherogenesis. Inorder to be able to track Treg during atherosclerosis and since Foxp3 is the marker that defines Treg, we needed to create a mouse model that allowed us to track Treg despite Foxp3 expression, on the assumption that Treg may lose Foxp3 expression during atherogenesis. Thus, we developed a novel Treg lineage tracker mouse model; Foxp3-IRES-YFP-Cre-Rosa26-loxp-td-RFP-loxp-ApoE / (LT-ApoE / ) and studied these mice in the context of atherosclerosis. In this mouse model, Treg express YFP and Cre recombinase under the control of an IRES element that follows the expression of the Foxp3 fusion gene. Cre recombinase deletes the loxp sites that flank RFP, marking Treg red as well. In this mouse model, current Treg cells, which express Foxp3, are both yellow and red. If Treg lose Foxp3 expression, they become an, where they lose YFP expression but retain RFP expression (Fig. 1a). The original Foxp3-IRES-YFP-Cre mice were described in Rubtsov et al. 24.Usingflow cytometry, we can identify and track both current and cells in the aorta and lymphoid tissues in vivo and can determine the fate of Treg during atherogenesis. When LT-ApoE / mice were fed a western diet for 1 weeks, we observed an increase in the number of CD4 + T cells in the aorta and most of these cells were activated, CD62L lo cells (Fig. 1b), suggesting that in our mouse model there is CD4 T cell participation during atherosclerosis development. We found an increase (~2 fold) in the percentages of cells (RFP + YFP CD4 T cells) (Fig. 1c)intheaortaofthesemice(SeeSupplementary Fig. 1 for gating strategy). There were no significant changes in the percentages of current Treg (RFP + YFP + CD4 T cells) in the aortas (Fig. 1c). However, this increase in did result in a significant change in the ratio of current: cells in (Fig. 1d), indicating alterations of Treg homeostasis in atherosclerosis. To determine if these retained any Foxp3 expression or suppressive function, we sorted current and cells based on YFP and RFP expression and measured both Foxp3 expression and suppressive capacity of the Treg populations. As expected, cells lacked expression of Foxp3 mrna (Fig. 1e), as well as Foxp3 protein levels (Fig. 1f;similartolevelsofexpressioninTCRβ negative cells) compared to current Treg. In addition, had no suppressive capacity compared to current Treg when cultured with effector CD4 T cells in a standard Treg suppression assay (Fig. 1g). When comparing both current and cells in western diet-fed, atherosclerotic mice, we found that had lower expression of CD2 (IL-2Rα), as well as increased PD1 expression. There were no differences in expression of Nrp1, CD62L and CD44 between the two cell populations (Fig. 1h and Supplementary Fig. 2). These results suggested that and current Treg possess different capacities in their response to IL-2 cytokine and/or their susceptibility to T cell exhaustion. Our next goal was to study the population in more detail to determine if they were phenotypically converting into a different CD4 T cell subset. We investigated the expression of transcription factors related to CD4 T cell function and cytokines present in current and cells. We found that cells expressed lower levels of Prdm1 and higher expression of Bcl6, Il21, and IFNγ compared to current Tregs (Fig. 2a). Prdm1 encodes Blimp1. Since low Blimp1 expression, accompanied by Bcl6, PD1 (Fig. 1g and Supplementary Fig. 2) and IL-21 expression, are defining features of T follicular helper cells (Tfh) 2, thus these results indicates that a portion of cells (RFP + YFP ) are converting to Tfh and Th-1 cells during atherosclerosis development. We next examined the distribution of Th-1, Tfh and Th-17 cell subsets within the population in the of LT-ApoE / mice fed a western diet. We found that the population is composed of memory, IFNγ + and Tfh cells (Fig. 2b and Supplementary Fig. 3a) and that the cell numbers of these subsets were increased in atherosclerosis. In addition, these subsets represented ~12 2% of their respective total cell population showing the relevance of these populations. We tested for Th-17 cells that originated from cells, but found that less than.% of total cells produced IL-17 (Fig. 2b and Supplementary Fig. 3a). We also did not observe a significant increase in Th-17 between chow and western diet-fed mice (Fig. 2b and Supplementary Fig. 3a), suggesting that the role of Th-17 was negligible in our model. The role of IFNγ in atherosclerosis has been extensively studied as has Treg:Th-1 plasticity 12. However, the role of Tfh cells has not been previously demonstrated in atherosclerosis. Therefore, we focused our studies on exploring the causes and consequences of Treg:Tfh plasticity during atherogenesis. As such, we analyzed the cells (RFP + YFP ) for activated effector cells that express CXCR, PD1 and Bcl6 (Tfh are defined as 2 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
3 RFP NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE a RFP + YFP ( lacks Foxp3) RFP YFP RFP + YFP + (current Treg Foxp3 + ) b CD4 T cells effector T cells (CD62L Io ) d Current Treg/ ratio RFP YFP CD4 + T cells c YFP Percent of CD4 T cells (RFP + YFP ) current Treg (RFP + YFP + ) (RFP + YFP ) current Treg (RFP + YFP + ) e Relative expression h 1 1 Foxp3 Current Treg f Current Treg TCRβ ve Foxp3 MFI Foxp3 protein Current Treg g Percent of dividing effector CD4 + T cells Total proliferating cells 1: 1:1 2:1 4:1 8:1 Teff:Treg Current Treg Effectors only CD2 CD62L Nrp1 Current Treg CD44 PD1 Fig. 1 ExTreg cells are increased during atherogenesis. a Schematic diagram with a representative flow cytometry plot of the Treg lineage tracker-apoe / (LT-ApoE / ) mouse model used to track Treg development during atherosclerosis. The diagram shows two population of Treg; current Treg (RFP + YFP + ) cells, which express Foxp3, and (RFP + YFP ) cells, which previously expressed Foxp3. b d LT-ApoE / mice were fed a western diet for 1 weeks. Bar graphs compare the numbers of total CD4 T cells and effector CD62L lo cells (b), the percentages and numbers of and current Treg (c) in the aorta, and the ratio of current Treg to in the aorta and (d) of western fed-diet to chow controls. c Representative flow cytometry plots and graphs showing the percentages of and current Treg in the aorta of the above mice. (e) Current and cells were sorted from the of LT- ApoE / mice and mrna levels for Foxp3 were examined in the extracted RNA and normalized to β-actin. f Foxp3 protein expression levels in current Treg, cells and TCRβ negative cells. Representative flow cytometry histograms and bar graphs show the MFI of Foxp3 in the two cell populations. g Treg suppression assay using sorted current and from spleens and peripheral LNs from LT-ApoE / mice. Sorted current and cells were cultured with CD4 depleted feeder cells from spleens of B6 mice and increasing numbers of naive effector CD4.1 CD4 T cells (CD2 CD44 lo CD62L hi ) that were labeled with Cell Trace Violet (CTV). Cells were stimulated with soluble αcd3 for 4 days, harvested and effector cells (CD4 + CD4.1 + ) were examined for the dilution of CTV by flow cytometry. h Flow cytometry histograms showing expression pattern of CD2 (IL-2Rα), CD62L, Nrp1, CD44 and PD1 on current Treg and cells. Results are expressed as mean ± s.e.m. from two independent experiments (n = 14) (b d), a representative of two independent experiments (n = 3) (e, g), and a representative of two independent experiments (n = 11) (f). Statistical significant differences were at P <., P <.1, P <.1, and P <.1 (unpaired Student s t-test) NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
4 CXCR CXCR ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s a Relative expression Treg Prdm Treg Bcl Treg IL Treg IFNγ b Tfh from IFN γ from IL-17 from NS c 17.7 Tfh from Tfh from Spleen Tfh from PD1. d Bcl6 Spleen CXCR + Bcl6 + CXCR + Bcl Fig. 2 A portion of population is converting to T follicular helper cells (Tfh) and Th-1 cells. a The expression of Prdm1, Bcl6, Il21 and IFNγ genes by RTqPCR from RNA extracted from current and cells sorted from from chow and western diet-fed LT-ApoE / mice. The data were analyzed using the 2 ΔΔCt method and it was normalized to β-actin. b Distribution of different T cell subsets by cell numbers within the population. Graphs show the number of Tfh, Th1 and Th17 cells within the population in the. c Representative flow cytometry plots showing the percentages of Tfh (CXCR + PD1 + ) cells in of above mice. Graphs show numbers of Tfh cells in the aorta, and spleens of above mice. d Representative flow cytometry plots showing the percentages of Tfh (CXCR + Bcl6 + ) cells in of above mice. Graphs show numbers of Tfh cells in the and spleens of above mice. Results are expressed as mean ± s.e.m. from two independent experiments (n = 3) (a), and mean ± s.e. m from one experiment (n = 7 (chow) and n = 8 ()) (b), and mean ± s.e.m from one experiment (n = 7 for aorta and spleen, and n = 4 for ) (c), and n = 4(d). Statistical significant differences were at P <., P <.1, and P <.1 (two-way ANOVA followed by Tukey s multiple comparisons test) (a) and (unpaired Student s t-test) (b d) acxcr + PD1 + Bcl6 + CD62L lo CD44 hi CD4 + T cell) (See Supplementary Fig. 1 for gating strategy). In atherosclerosis, we saw increases of 2 4 fold in both percentages and numbers of Tfh cells derived from cells in the aortas, and spleens of western diet-fed mice (Fig. 2c, d). Thus, a significant portion of Treg cells converts to Tfh cells during atherosclerosis development. 4 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
5 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE a of Isotype ExTreg αicosl of b Tfh from Isotype ExTreg αicosl of CXCR + PD1 + (Tfh) Tfh from Isotype αicosl Isotype αicosl Spleen Tfh from Isotype αicosl c Number of germinal center (Fas + GL7 + ) B cells current Treg NS Isotype αicosl d Number of current Treg Isotype αicosl. 1 6 Spleen current Treg Isotype NS αicosl e Isotype αicosl Aortic roots Neutral lipid area (μm 2 ) Isotype αicosl Fig. 3 Blocking ICOSL reduces Tfh cells and atherosclerosis. a e LT-ApoE / mice were fed a western diet for 9 weeks and then were injected with antibody against ICOSL or isotype i.p. (1 μg per mouse, twice per week) for 6 weeks. Graphs show the numbers of (a), Tfh cells (b) in aorta, and spleen, germinal center B cells in (c) and current Treg cells in aorta and spleen (d). e Oil Red O stain of aortic roots from the above mice. Images are representative section from each group of mice. Each individual dot in the graph is the average of at least 4 sections per aorta per mouse. Scale bars = 1 μm. Results are expressed as mean ± s.e.m. from two independent experiments (For aorta, n = 6 (isotype) and n = 1 (αicosl). For and spleen, n = 8 (isotype) and n = 1 (αicosl). For aortic roots, n = 8 (isotype) and n = 11 (αicosl). Statistical significant differences were at P <., P <.1, and P <.1 (unpaired Student s t-test) Blocking Tfh differentiation reduces generation. Next, we wanted to determine if blocking Tfh differentiation would affect the population during atherosclerosis progression. ICOS/ICOSL signaling is critical for the differentiation and maintenance of Tfh cells 3. ICOSL signals to CD4 T cells can be provided by DCs or B cells, and can be blocked with an α-icosl mab 3. LT-ApoE / mice were fed a western diet for 1 weeks and given α-icosl or isotype antibody twice a week for the last 6 weeks. We found significant reductions in numbers, of and 2-fold in the aorta and s, respectively, when mice were treated with α-icosl antibody (Fig. 3a). This was accompanied by a 1-fold reduction in the numbers of Tfh cells derived from in the aorta, s and spleen (Fig. 3b), as well as a significant decrease in germinal center B cells (Fig. 3c). We observed no differences in current Treg percentages and numbers in αicosl antibody-treated mice (Fig. 3d). In addition, to their loss of Tfh cells, αicosl antibody-treated mice showed a 3% reduction in atherosclerosis as measured by Oil Red O staining of aortic roots (Fig. 3e). These results suggested that blocking the interactions that generate Tfh cells is beneficial in reducing the amounts of Tfh generated during atherogenesis, and suggests that Tfh may be pro-atherogenic. Tfh cells are pro-atherogenic. Because the neutralization of ICOS/ICOSL interactions could impact other cell populations, and to identify whether Tfh cells are indeed directly proatherogenic, we further validated our ICOSL blocking experiments using mice that are selectively deficient in Bcl6 in CD4 T cells (Bcl6 fl/fl CD4-Cre + ) 31. Bcl6 is the master regulator transcription factor for Tfh cell differentiation, and the lack of this transcription factor in CD4 T cells results in loss of Tfh cells Bone marrow cells from Bcl6 fl/fl CD4-Cre + and corresponding WT, B6, controls, was used to reconstitute LdLr / mice to generate an atherosclerosis-susceptible animal model that specifically lacked Bcl6 expression in CD4 + T cells. Following reconstitution, these mice were fed an atherogenic diet for 1 weeks. The Bcl6 fl/fl CD4-Cre + Ldlr / recipients lacked Tfh cells in aorta, and spleens (Fig. 4a), but had normal Treg percentages compared to control mice (Fig. 4b). Bcl6 fl/fl CD4-Cre + Ldlr / NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
6 CXCR acxcr ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s B6 Bcl6 fl/fl CD4Cre PD Percent of CXCR + Bcl6 + (Tfh) B6 Bcl6 fl/fl CD4Cre + of CXCR + Bcl6 + (Tfh) B6 Bcl6 fl/fl CD4Cre + of CXCR + PD1 + (Tfh) B6 Bcl6 fl/fl CD4Cre Spleen B6 Bcl6 fl/fl CD4Cre + Bcl6 b Percent of Treg (CD2 + Foxp3 + ) NS B6 Bcl6 fl/fl CD4Cre + c B6 Bcl6 fl/fl CD4Cre + Neutral lipid area (μm 2 ) B6 Aortic roots Bcl6 fl/fl CD4Cre + d B6 Bcl6 fl/fl CD4Cre + Percent of aortic lesions B6 En face Bcl6 fl/fl CD4Cre + Fig. 4 Tfh cells are pro-atherogenic. a d B6 or Bcl6 fl/fl CD4-Cre + BM chimera mice on Ldlr / background were fed high cholesterol diet for 1 weeks. a b Flow cytometry plots and graphs showing the percentages and numbers of Tfh cells in, aorta and spleen (a) and percentages of Treg cells (b) in the. c Oil Red O stain of the aortic roots from the above mice. Images are representative sections from each group of mice. The amount of red pixels in each section was determined and the corresponding area was calculated. Each individual dot in the graph is the average of at least 4 sections per aorta per mouse. Scale Bars = 1 µm. d En face Oil Red O staining in the aorta of the above mice. Graph shows quantification of the lesion area as a percentage of the total aortic surface area. Results are expressed as mean ± s.e.m. from one experiment (n = 12 (B6) and n = 14 (Bcl6 fl/fl CD4Cre + )(a b), n = 8 (B6) and n = 13 (Bcl6 fl/fl CD4Cre + ) for and spleen, n = 7 (B6) and n = 6(Bcl6 fl/fl /CD4Cre + ) for aorta (c), and n = 1 (B6) and n = 13 (Bcl6 fl/fl CD4Cre + )(d)). Statistical significant differences were at P <., P <.1, and P <.1 (unpaired Student s t-test). NS none significant mice exhibited 2% and 3% reductions of atherosclerosis development as determined by Oil red O staining in aortic roots (Fig. 4c) and in entire aortae (Fig. 4d), respectively. Thus, Tfh cells in aorta are directly pro-atherogenic. To determine if our results were applicable to humans, we measured IL-21, a major Tfh cytokine, in the plasma of human subjects with coronary artery disease (See Supplementary Table 1 for subjects characteristics) and the percentage of Foxp3 in Treg (CD4 + CD2 + CD127 ). There was a significant inverse correlation between IL-21 plasma levels and Foxp3 expression in Treg cells (Supplementary Fig. 4), validating that similar to mice, in humans Treg and Tfh subsets counterbalanced each other. Treg and Tfh cells differentially express IL-2Rα. IL-2 plays an important role in the homeostasis of Foxp3 expression and the maintenance of the Treg cells 3. Constitutively high IL- 2Rα expression is a canonical feature of Treg cells. Conversely, IL- 2 is an extremely potent inhibitor of Tfh differentiation, mediated by altering expression of Bcl6 and Blimp-1 in a STAT- 6 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
7 CD2 CD2 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE a - CD2 Current Treg Tfh from IL-2R α expression (CD2 MFI) Current Treg Tfh from b pstat 2 ry Ab. only Unst. Unst. St. St. Fold difference over unstimulated pstat Treg c - CD13 Current Treg Tfh from IL-6R α expression (CD13 MFI) Current Treg Tfh from d pg/ml IL-6 e Media Foxp3 OxLDL Percent of induced Treg cells Media OxLDL f IL-2R α expression (CD2 MFI) 1 1 Media OxLDL IL-6R α expression (CD13 MFI) Media OxLDL g pg/ml Media IL-2 oxldl α CD3/CD28 +TGF β h Relative expression 1 1 Media OxLDL αcd3/cd28 Foxp3 αcd3/cd28 +TGF β i Media OxLDL OxLDL + IL Foxp3 Percent of induced Treg cells Media NS OxLDL OxLDL + IL-2 Fig. IL-2 and IL-6 receptors expression is altered between current Treg and Tfh cells. a Flow cytometry histograms and graphs of mean fluorescence intensity of IL-2Rα (CD2) expression on current Treg, and Tfh cells from LT-ApoE / mice on western diet. b Histogram plots and differential expression of phosphorylated STAT by phosphoflow in current Treg cells in the western diet-fed and chow controls LT-ApoE / following IL-2 stimulation. Graph shows the fold change between unstimulated and IL-2 stimulated samples. c Flow cytometry histograms and graph of mean fluorescence intensity of IL-6Rα (CD13) expression on current Treg, and Tfh from LT-ApoE / mice on western diet. d Plasma levels of IL-6 in the mice described above. e i Naive CD4 T cells from ApoE / mice were isolated and stimulated with αcd3/cd28 and TGFβ to induce Treg in vitro with or without the addition of oxldl or IL-2. e Flow cytometry plots and graphs showing the percentages of induced Treg following 4 days in culture. f Expression of IL-2Rα (CD2) and IL-6Rα (CD13) on total CD4 T cells in culture following 4 days of stimulation. h g Supernatant (h) and RNA (g) were harvested from the above cultures for the assessment of IL-2 by ELISA (h) and Foxp3 mrna by qrt-pcr (g). i Flow cytometry plots and graphs showing the percentage of induced Treg with the addition of exogenous IL-2. Results are expressed as the mean ± s.e.m. from one of two independent experiments (n = 7) (a, c), from one of two independent experiments (n = 4 (chow) and 6 ()) (b), from three independent experiments (n = 14 (chow) and 18 ()) (d), from one of two independent experiments (n = 3) (e g) and (n = 6) (h), and one of two experiments (n = 4) (i). Statistical significant differences were at P <., P <.1, P <.1, and P <.1 (one-way ANOVA) (a, c, i) and (Unpaired Student s t-test) (b, d h) NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
8 RFP ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s a /ApoAI YFP Percent of CD4 T cells (RFP + YFP ) /ApoAI Percent of CD4 T cells Current Treg (RFP + YFP + ) /ApoAI b (RFP + YFP ) Current Treg (RFP + YFP + ) (RFP + YFP ) Current Treg (RFP + YFP + ) Percent of CD4 T cells /ApoAI Percent of CD4 T cells /ApoAI of /ApoAI of current Treg /ApoAI c /ApoAI Aortic roots Neutral lipid area (μm 2 ) /ApoAI Fig. 6 ApoAI administration reduces during atherogenesis. (a, b) LT-ApoE / mice were fed western diet and after 6 weeks, human lipid free ApoAI ( μg per mouse)(or BSA as control) were given subcutaneously 3 times per week for 9 weeks. Mice were sacrificed after 1 weeks and aorta and were harvested for analysis. Flow cytometry plots and graphs showing the percentages and/or numbers of RFP + YFP () and RFP + YFP + (current Treg) in the aorta (a) and (b). Results are expressed as the mean ± s.e.m. from four independent experiments (n = 29 (), n = 27 () and n = 26 (/ApoAI)). c Aortic roots from the above mice groups were sectioned and stained with Oil Red O. Images are representative sections from each group of mice. The amount of red pixels in each section was determined and the corresponding area was calculated. Each individual dot in the graph is the average of 8 sections per aorta per mouse. Scale bars = 1 μm. Results are expressed as the mean ± s.e.m. from three independent experiments (n = 1 ( and ) and n = 9 (/ApoAI)). Statistical significant differences were at P <., P <.1, and P <.1 (one-way ANOVA) dependent manner Our results showed ~ 1-fold reduction in IL-2Rα expression between current and cells (Fig. 1h and Supplementary Fig. 2); therefore, we examined whether IL- 2Rα expression was different between current Treg and Tfh cells in mice fed a western diet. Comparison of IL-2Rα expression on current Treg, and Tfh cells in of western diet-fed mice revealed that IL-2Rα expression was reduced dramatically (~ 2-fold) in Tfh compared to current Treg cells (Fig. a). In addition, current Treg from western diet-fed mice displayed reduced ability to phosphorylate STAT upon IL-2 stimulation compared to current Treg from chow diet-fed mice (Fig. b). IL-6 plays a key role in early Tfh differentiation through the induction of Bcl Comparison of IL-6Rα expression on current Treg, and Tfh cells showed a stepwise increase in IL-6Rα expression (Fig. c). IL-2 and IL-6 can counterregulate expression of IL-6 receptors and IL-2 receptors 44. Since plasma IL-6 is increased in western diet-fed mice (Fig. d), these results suggest that alteration of IL-2 and IL-6 receptor expression in current Treg cells during atherogenesis reduces the ability of Treg cells to receive signals from IL-2, thus, weakening the maintenance of Treg cells, and stimulate Tfh cell generation through the upregulation of IL-6 receptor. 8 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
9 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE a CXCR PD1 Tfh from /ApoAI Number of CXCR + PD1 + (Tfh) Tfh from / ApoAI Tfh from / ApoAI Spleen Tfh from / ApoAI b Number of CD19 + B cells / ApoAI Number of germinal center (Fas + GL7 + ) B cells / ApoAI Number of plasma cells (CD138 + IgD ) 1 1 / ApoAI c of CD11b + cells NS / ApoAI CD68 + area (μm 2 ) Aortic roots NS / ApoAI d IFN γ + from e Total IFN γ + CD4 T cells NS NS / ApoAI. / ApoAI Fig. 7 ApoAI selectively prevents the conversion of into Tfh cells and reduces B cells in the aorta during atherogenesis. a Flow cytometry plots and graphs showing percentages and numbers of Tfh cells (CD44 hi CD62L lo CXCR + PD1 + ) from (CD4 + RFP + YFP ) in the aorta, and spleen in LT- ApoE / western diet-fed mice with and without ApoAI treatment or chow controls. b Graphs showing numbers of B cells (CD19 + ), germinal center B cells (CD19 + Fas + GL7 + ) and plasma cells (CD19 + CD138 + IgD ) in the aorta of the mice above. c Graphs showing numbers of CD11b + cells in the aorta by flow cytometry and area of CD68 + in aortic roots by immunofluorescence of the mice above. d, e Graphs showing numbers of IFNγ + from, and IFNγ + from total CD4 T cells (d, e, respectively) in the of the above mice, following stimulation with PMA/ionomycin for h. Results are expressed as mean ± s.e.m from three independent experiments (n = 19 (), n = 16 ( and /ApoAI)) (a), mean ± s.e.m from two independent experiments (n = 1) (b), mean ± s.e.m from one experiment (n = 6 ( and ), n = 7 (/ApoAI)) (c) and n = 7(d e). Statistical significant differences were at P <., P <.1, and P <.1 (one-way ANOVA) Since low-density lipoprotein (LDL) becomes oxidized in the vessel wall, and contributes to plaque formation during atherogenesis 4,46, we next isolated naive T cells from ApoE / mice and induced Treg differentiation in vitro with TGFβ with or without addition of oxldl to determine if oxldl directly modulated IL-2 and IL-6 receptors on Treg cells. OxLDL reduced itreg formation (Fig. e), which was accompanied by a decrease in IL-2Rα and an increase in IL-6Rα expression (Fig. f). In addition, T cells treated with oxldl produced ~ 3% less IL-2 in culture (Fig. g) and had ~ 3% lower Foxp3 expression (Fig. h), observed as early as 12 h following stimulation (Supplementary Fig. ). Addition of exogenous IL-2 to cultures along with oxldl increased the percentages of itreg to levels comparable to without oxldl (Fig. i), thus showing that the reduction in itreg with oxldl is due to suboptimal IL-2 levels. These results demonstrate that oxldl can alter the levels of IL-2 and IL-6 receptor on Treg cells in a manner that impairs Treg homeostasis and favors Tfh cell generation. ApoAI reduces generation during atherogenesis. Elevated ApoAI expression in vivo has been associated with reduced Tfh cells 19 and preservation of Treg cells 23. We, therefore, tested whether treatment of atherogenic mice with ApoAI could reduce the generation of and Tfh cells. LT-ApoE / mice were fed a western diet for 6 weeks and then given subcutaneous injections of human lipid free ApoAI or BSA (as control) three times per week for an additional 9 weeks. At the end of the 1 weeks, we determined the fate of Treg cells in aorta and. ApoAI administration reduced the population in the aorta NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
10 CXCR CD2 ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s c a IL-2R α expression (CD2 MFI) Current Treg /ApoAI /ApoAI IL-6R α expression (CD13 MFI) Current Treg /ApoAI b pg/ml Plasma IL-6 levels /ApoAI d Tfh (CXCR + Bcl6 + ) Media OxLDL OxLDL + ApoAI Foxp3 Percent of induced Treg cells Media OxLDL OxLDL + ApoAI Isotype for Bcl6 /ApoAI Bcl6 Fig. 8 ApoAI administration increases IL-2Rα expression and reduces IL-6 production and Bcl6 expression during atherogenesis. a Expression of IL-2Rα (CD2) and IL-6Rα (CD13) on current Treg in the aorta of LT-ApoE / western diet-fed mice with and without ApoAI treatment or chow controls. b Plasma levels of IL-6 in the mice described above. c Flow cytometry plots and graphs showing the percentages and numbers of CXCR + Bcl6 + Tfh in cells of above mice. Plots are gated on CD44 + RFP + YFP CD4 T cells. d Naive CD4 T cells from ApoE / mice were isolated and stimulated with αcd3/cd28 and TGFβ to induce Treg in vitro with or without the addition of oxldl or ApoAI. Flow cytometry plots and graphs showing the percentages of induced Treg following 4 days in culture. Results are expressed as the mean ± s.e.m. from one of two independent experiments (n = 6 ( and / ApoAI), n = 7 ()) (a), mean ± s.e.m. from 3 different experiments (n = 18 (), n = 17 () and n = 13 (/ApoAI)) (b), the mean ± s.e.m. from two independent experiments (n = 11 ( and ) and n = 1 (/ApoAI) (c), and the mean ± s.e.m. from one of two experiments (n = 4) (d). Statistical significant differences were at P <., P <.1 and P <.1 (one-way ANOVA) of western diet-fed mice to almost background chow levels (Fig. 6a). Similarly, in, ApoAI administration to LT-ApoE / mice reduced the percentages and numbers of (Fig. 6b). This was accompanied by an increase in the percentages of current Treg in the compared to the chow control (Fig. 6b). However, ApoAI had no effect on the total number of Tfh cells (Supplementary Fig. 7b), suggesting that ApoAI prevents the conversion of Treg to during atherosclerosis progression, rather than affecting the total Tfh differentiation program. ApoAI administration also reduced the atherosclerotic burden in these mice. Mice that were fed a western diet showed increased atherosclerosis, while those treated with ApoAI showed a significant 2% reduction in neutral lipid staining in aortic roots (Fig. 6c). To determine if these findings were relevant in human subjects, we measured ApoAI levels and Treg percentages in blood of human subjects. We found a positive correlation between the levels of ApoAI and the percentages of Treg in blood (Supplementary Fig. 6), further confirming that in mice and in humans, higher levels of ApoAI resulted in an increase in Treg. ApoAI prevents the conversion of to Tfh cells. Since ApoAI reduced cells generation during atherogenesis, we next determined if ApoAI affected Tfh cell generation from cells. Administration of ApoAI abolished the increase in Tfh cells in the aortas of western diet-fed mice to baseline levels found in chow diet-fed mice (Fig. 7a). We saw similar results in the and the spleens of LT-ApoE / mice (Fig. 7a). Upon examining B cell populations in the aorta, ApoAI administration reduced the total number of CD19 + B cells, germinal center B cells (CD19 + Fas + GL7 + ) and plasma cells (CD19 + CD138 + IgD ) to levels similar to baseline chow levels (Fig. 7b) (See Supplementary Fig. 8 for gating strategy for B cells in aorta). To exclude the possibility that the observed changes in cell numbers were not merely due to the anti-inflammatory properties of ApoAI, we examined macrophages in the aorta of LT-ApoE / mice that received ApoAI and we found no reduction in the total numbers of CD11b + cells in the aorta by flow cytometry or by immunofluorescence staining for CD68 + cells (Fig. 7c). We also did not observe a reduction in IFNγ + (Fig. 7d) or total IFNγ + CD4 T cells (Fig. 7e) upon ApoAI administration. In addition, there was no change in total IL-17 + CD4 T cells with ApoAI administration (Supplementary Fig. 9). These results suggested that ApoAI was specifically affecting the to Tfh cell conversion and not other cell types that may be contributing to atherosclerosis development. ApoAI increases IL-2Rα on Treg cells. We next determined if ApoAI administration could affect IL-2Rα and IL-6Rα expression by current Treg cells. ApoAI infusion reversed the decrease in IL- 2Rα expression on current Treg cells in western diet-fed mice (Fig. 8a), indicating that ApoAI can reverse the defect in IL-2 signaling induced by western diet. This was accompanied by a 1 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
11 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE decrease in the expression of IL-6Rα on current Treg cells (Fig. 8a) and a reduction in plasma IL-6 levels (Fig. 8b). Administration of ApoAI also reduced the expression of Bcl6 in Tfh cells (Fig. 8c). Furthermore, addition of ApoAI together with oxldl during in vitro Treg induction reverses the oxldl impairment of Treg differentiation (Fig. 8d). Our results suggest that ApoAI reduces the to Tfh generation through maintenance of IL-2R expression on Treg cells and reduction of IL-6, resulting in reduced Bcl6 expression, correcting the imbalance in these cytokine receptors and transcription factors that drive the Treg: Tfh conversion during western diet feeding. ApoAI reduces cellular cholesterol levels in Treg. Cholesterolis an important component in membrane lipid rafts where IL-2 receptor is enriched 47. Thus, we wanted to determine if the changes in IL-2R expression with western diet and ApoAI administration were due to changes in cholesterol levels in current Treg. First, we measured the levels of plasma cholesterol and HDL in our model. No differences in total plasma cholesterol or HDL were observed between the BSA and ApoAI-treated western diet-fed mice (Fig. 9a), showing that it is not merely a function of ApoAI changing the plasma cholesterol or HDL levels. This is consistent with prior studies showing that ApoAI infusion results in transient or low changes in plasma HDL concentration 21,48.Uponmeasuringthe intracellular cholesterol levels of current Treg, we found an increase in the ratio of esterified cholesterol to total cholesterol (EC/TC) in current Treg from western diet fed mice (Fig. 9b), which was reduced by treatment of mice with ApoAI. These results suggested that with western diet feeding, there was a disruption in cellular cholesterol homeostasis, resulting in the accumulation of esterified cholesterol in Treg. The intracellular cholesterol of Treg was reduced with ApoAI administration (Fig. 9b). Since ApoAI couples with the cholesterol transporter ABCA1 to efflux cellular cholesterol and it has been shown that ApoAI can regulate ABCA1 expression 49, we next checked for levels of ABCA1 expression. Treatment with ApoAI significantly increased the levels of ABCA1 mrna above baseline levels (Fig. 9c). This finding suggests that ApoAI may alter intracellular cholesterol levels by increasing the levels of ABCA1 and thus, increasing efflux through ABCA1 in Treg. Thus, it is likely that the changes in IL-2R and the stability of the Treg population could be at least partly due to changes in intracellular cholesterol levels, which are reversed by the administration of ApoAI due to its ability to facilitate cholesterol efflux cholesterol from cells. Discussion The main objectives of this study were two-fold: (i) to determine the fate of Treg cells during atherosclerosis development, and (ii) to determine how ApoAI affects Treg cell plasticity during atherogenesis. Here, we show two novel findings. First, during atherosclerosis development, a portion of Treg cells switch phenotype into pro-atherogenic Tfh cells. Second, ApoAI prevents Treg to Tfh cell conversion during atherosclerosis. Our results show that in atherosclerosis, a portion of Treg cells converts to Tfh cells, which are responsible for increasing tertiary lymphoid structures in the aorta, promoting B cell-mediated antibody production 2. The concept of Treg plasticity has been well-documented (reviewed in refs. 3,4 ). T cell differentiation depends largely on the extent of antigenic stimulation, the cytokine milieu, and fluctuations in the metabolic and nutrient levels to which the cells are exposed. Treg conversion into inflammatory and pathogenic Th1 or Th17 has been reported in autoimmune diseases and lethal infections (reviewed in ). In mouse models of type I diabetes, as well as in type I diabetic patients, exfoxp3 or Foxp3 expressing cells produce IFNγ 9,11. Isolated Treg can convert to Tfh cells in Peyer s patches of mice 6. A recent report has shown that in old ApoE / mice, IFNγ + Treg exist in the aorta and lack suppressive functions 12. To our knowledge, this is the first report to show the conversion of Treg into Tfh cells during atherosclerosis. Tfh cells play critical roles in germinal center formation and B cell activation, both of which are important aspects of atherosclerotic disease pathogenesis 1,2.Itis understood how naive T cells become Tfh cells 2, however, whether Treg: Tfh cells plasticity occurs is controversial 7 9. Here, we show that increasing intracellular cholesterol levels, either by western diet feeding or oxldl uptake, reduces the expression and signaling of IL-2R, a potent stabilizer of Treg 3. IL-2 and pstat are also potent inhibitors of Tfh differentiation 36,37, suggesting that cholesterol-mediated IL-2 signaling attenuation, together with increased IL-6R and Bcl6 expression, initiate Tfh differentiation in context of atherosclerosis. When sufficient concentrations of ApoAI are available, ApoAI likely prevents this conversion process by regulating cholesterol levels in Treg cells and sustaining IL-2 receptor expression. Other studies have alluded to the pro-atherogenic role of Tfh cells in atherogenesis. Clement et al. have shown regulatory CD8 T cells can reduce Tfh-B cell activation and limit atherogenesis 1. More recently, a study by Ziad Mallat s group elegantly showed that marginal zone B cells limited Tfh cells and decreased atherosclerosis development 6. In our experiments, we first show that the lack of Tfh cells via anti-icosl blockade results in reduction of atherosclerosis. ICOS signaling is preferentially required for Tfh cell differentiation but does impact additional cell types. Thus, to prove a direct role of Tfh cells in atherogenesis, we used mice that possessed CD4 T cells that were conditionally-deficient in Bcl6, the lineage transcription factor of Tfh cells. In these mice, we also saw reduced atherosclerosis burden. Thus, we show definitively using two independent methods in vivo that Tfh cells are pro-atherogenic and the regulation of this population can control atherosclerosis progression. The protective anti-inflammatory role of lipid-free ApoAI, phospholipid-bound ApoAI (i.e., recombinant HDL) and ApoAI mimetics has been previously documented in atherosclerosis and other inflammatory conditions 17,19,2,23,61. Recombinant HDL doses used in humans reflect the current dogma that plasma HDL concentrations must increase in order to show efficacy in reducing arterial cholesterol burden. However, animal model studies have shown significant peripheral cholesterol transport following subcutaneous injections of low dose lipid-free human ApoAI that either results in minor or transient increases in plasma HDL concentrations 21,48. Previously, we have shown that ApoAI administration reduced cholesterol-mediated T cell expansion and increased Treg to effector CD4 T cell ratios in skin draining LNs in Ldlr / ApoAI / mice, reversing the autoimmune phenotype seen in these mice 23. In this current study, we observe no changes in plasma HDL concentration in western diet-fed ApoAI treated mice compared to BSA controls, yet we observe a significant impact of ApoAI on Treg homeostasis. ApoAI changed the intracellular cholesterol levels as monitored by the esterified cholesterol to total cholesterol ratio. Macrophages transduced with human ApoAI led to a reduction in atherosclerosis and dermatitis without affecting plasma HDL levels 21, further proving that by changing the cellular cholesterol content, ApoAI affects the cellular inflammatory state and, hence, the outcome of inflammatory diseases. Here, we also provide evidence that ApoAI affects Treg plasticity and conversion to Tfh cells. Our results are in agreement with a study showing that ApoAI transgenic mice have reduced Tfh cells and germinal center B cell generation in a Lupus model 19. Since ApoAI reduces dendritic cell differentiation and maturation 22, we hypothesize that ApoAI influences Treg maintenance both directly through modulation of cholesterol content as we show here, and indirectly, through NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
12 ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s a Total plasma cholesterol (mg/dl) b Ratio Total plasma cholesterol /ApoAI Current Treg EC/TC /ApoAI dendritic cell inhibition. That said, the athero-protective properties of ApoAI can not be directly linked to the reduction of Tfh cells as other anti-inflammatory properties of ApoAI may play a role. Further studies are needed to tease out if the ApoAI effect on Tfh cells can directly impact atherosclerosis. One question that arises from our study is whether ApoAI administration is sufficient to rescue T cells from the massive loads of cholesterol in our system. While cells, like endothelial cells have mechanism(s) that regulate their cholesterol intake and they never become foam cells (i.e., cholesterol-ester loaded), other cells are unable to effectively regulate the amount of cholesterol due to extensive scavenger receptors and readily become foam cells (reviewed in ref. 62 ). Regardless, the amount of cholesterol per cell needed to elicit dramatic changes in cellular signaling and activation mediated by elevated microdomain cholesterol composition is relatively small. This was recently demonstrated by Kaul et al., 17. In addition, the efficacy of subcutaneous injections of lipid-free ApoAI has been previously shown in other reports 23,63. Human and mouse ApoAI have different primary structures but they both interact with ABCA1 to yield nascent HDL particles which eventually become spherical mature plasma HDL. Mouse plasma HDL is homogenously sized while human plasma HDL tends to be more heterogeneous sized and the structure is likely the basis for this difference 64. Differences in ApoAI structure relative to cholesterol efflux and nascent HDL formation may explain small differences in efflux, but to focus on bulk plasma HDL size and composition is not the mechanism by which lipid-free ApoAI suppresses atherosclerosis or alters T cell HDL (mg/dl) c Relative expression ABCA1 /ApoAI HDL /ApoAI Fig. 9 ApoAI administration reduces the cholesterol content in current Treg cells through increasing ABCA1 expression. a Plasma from LT-ApoE / western diet-fed mice with and without ApoAI treatment or chow controls were tested for total cholesterol and HDL. b Sorted current Treg (RFP + YFP + ) cells from of the above mice were analyzed for the amounts of intracellular free and total cholesterol using mass spectroscopy. The amount of esterified cholesterol was calculated followed by the ratio of esterified to total cholesterol. c The mrna expression of ABCA1 by RTqPCR from RNA extracted from current Treg sorted from the of chow and western diet-fed LT-ApoE / mice, with and without ApoAI treatment. The data were analyzed using the 2 ΔΔCt method and it was normalized to β-actin. Results are expressed as the mean ± s.e.m from one experiment (n = 7) (a, b), and mean ± s.e.m from one of two independent experiments (n = 3) (c). Statistical significant differences were at P <., P <.1 and P <.1 (one-way ANOVA) plasticity. Rather, lipid-free ApoAI stimulates ABCA1 removal of excess cellular cholesterol via the formation of nascent HDL particles, thereby suppressing signaling that would otherwise lead to activation and/or recruitment of immune cells to the atherosclerosis plaque. We have previously published that 1% of the subcutaneously injected ApoAI enters plasma in lipidated form 23. We believe that mouse ApoAI would work equally well as human ApoAI as they both work well in vitro to stimulate efflux. We do not know if both forms of ApoAI are found in plaque. Since atherosclerotic plaques in humans have been shown to harbor large amounts of serum albumin and other abundant plasma proteins that diffuse into the oxidative environment of the plaque where they become modified and degraded. We would assume both human and mouse ApoAI would be found in nearly equal amounts in plaque respectively, although plasma HDL cholesterol concentrations in mice is higher (~6 mg dl -1 ) than in humans (~4 mg dl -1 ) and diffusion into plaques appear to be driven through mass action. A recent study from our laboratory showed that ABCG1, another cholesterol transporter, regulated Treg development through modulation of cholesterol levels and mtor activation 6. The results from both studies suggest that Treg require an optimal level of plasma membrane cholesterol for their development and/or maintenance, and that slight changes in cholesterol levels, affect Treg homeostasis. In this study, our results indicate that cholesterol levels can play a role regulating IL-2 signaling, a critical factor for the maintenance of Treg. Minor changes in plasma membrane lipids can either enhance or abolish IL-2 signaling events 47,66. Could accumulation of too much cholesterol in Treg be detrimental for their proper function and maintenance? The increase in and their conversion to Tfh cells in LT-ApoE / mice fed a western diet, as well as our in vitro Treg induction in presence of oxldl, would provide evidence that this is true. We hypothesize that cholesterol accumulation in Treg from western diet-fed mice increases lipid micro-domains, decreasing Treg maintenance and allowing for their conversion to Tfh cells, a process that is reversed by ApoAI, in part mediated by its role in increasing reverse cholesterol transport. Our results show a negative correlation between plasma levels of IL-21 and Foxp3 and a positive correlation between plasma ApoAI and Treg and in human samples obtained from patients with coronary artery disease, indicating that our findings in atherosclerotic mouse models have relevance in humans. Tfh cells and tertiary lymphoid structures have been found in human atherosclerotic aneurysmal arteries 1. IL-21 and IL-21R positively correlate with inflammatory factors in human adipose tissue, suggesting a potential role for IL-21 in fat tissue 67. Treg cells are found to be defective in patients with coronary artery disease 68. Our studies suggest that increasing ApoAI levels in humans could potentially reduce the generation of Tfh cells and increase Treg maintenance in such a way that helps reduce inflammation and the overall outcome of plaque deposition and atherosclerosis. Here, we provide mechanistic insights into how ApoAI may be protective by regulating Treg:Tfh conversion during disease progression. What is not clear is whether increasing HDL concentrations by pathways that do not directly involve ABCA1 (i.e., decreased catabolism, aqueous diffusion of cellular cholesterol to HDL), would be as efficacious as increasing lipid-free or even lipid-poor ApoA-I levels. Overall, this study uncovers how Treg cells convert into Tfh cells under atherogenic conditions and how ApoAI influences Treg plasticity by altering cholesterol content in these cells. We believe our study opens a new avenue for the future use of lipidfree ApoAI to modulate Treg:Tfh responses that can benefit and be applicable to atherosclerosis, as well as to other inflammatory diseases. 12 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
13 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE Methods Mice. The original breeding pairs of B6, CD4.1, ApoE /, and LDLR / mice were purchased from The Jackson Laboratories (Bar Harbor, Maine; Stock numbers 664, 214, 22, and 227, respectively). The original Foxp3-IRES- YFP-CRE-Rosa26-dt-RFP (Treg lineage tracker) was a kind gift from Mitchell Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA) (LJI). Bcl6 fl/fl mice 69 crossed to CD4-Cre + mice were bred at LJI 31. Treg Lineage tracker mice were crossed in our laboratory to ApoE / mice to create the LT-ApoE / mice. Mice were used for experiments at 6-12 weeks of age. Mice of both sexes were used and all mice were randomly selected for different treatment groups. For most experiments, investigators were blinded from the group allocation when analyzing data. For atherosclerosis studies, LT-ApoE / mice were fed a Western Diet (42% from fat,.2% from cholesterol) (Harlan, #TD 88137, Placentia, CA). LDLR / mice were fed a high-cholesterol diet (1.2% cholesterol and 4% calories from fat) (Research Diets, D1218C). All other mice including chow controls were fed a standard chow diet containing % cholesterol and % calories from fat (Pico lab, #3, Saint Louis, MO). All mice were bred and housed in microisolator cages in a pathogen free animal facility of LJI. All experiments followed guidelines of the LJI Animal Care and Use Committee and the approval of the use of rodents was obtained from LJI according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Flow cytometry. Peri-aortic LNs () or spleens from the mice were passed through a 4 μm cell strainers and washed with ice cold PBS. For spleens, RBCs were lysed with 1 RBCs lysis buffer (Biolegend, San Diego, CA). e were explanted after perfusion with ice cold PBS, cut and were digested with DNAse I, Collagenase type I, Collagenase type XI and Hyaluronidase type I as previously described 6, then they were passed through 4 μm filters. Cells were incubated with RPMI media with 1% FCS to restore expression of surface markers for at least 3 min at 37 C. All staining protocols were done on ice for 3 min unless otherwise indicated. Samples were stained with Live Dead fixable dye (Life Technologies, Carlsbad, CA). Samples were acquired using LSRII (BD, Bioscience, San Diego, CA) and data were analyzed using Flowjo Ver 9.8 and 1..8 (Tree Star, Ashland, OR). For surface staining, LT-ApoE / lymphocytes were stained with antibodies against CD4 (clone RM4-4,.6 μg per test, ebioscience), TCRβ (clone H7-97, 1 μg per test, ebioscience), CD2 (clone PC61,.6 μg per test, ebioscience) and/or CD62L (clone MEL-14,.12 μg per test, ebioscience), CD13 (IL-6Rα; clone KGP13,.12 μg per test, ebioscience), Nrp1 (clone N43-7,.1 μg per test, MBL international), and CD4 (clone 3-F11; for,.12 μg per test, ebioscience), in cold FACS buffer (2% BSA,.1% sodium azide in PBS). YFP and RFP were detected without further staining unless otherwise indicated. For suppression assay, cells were stained with antibodies against CD4, TCRβ, CD2 and CD4.1 (clone A2; for suppression assay,. μg per test, ebioscience). For Tfh detection, cells were stained initially with CXCR-Biotin (clone 2G8,. μg per test, BD bioscience), followed by fluorescent-labeled Streptavidin together with the previous antibodies and CD44 (clone IM7,.12 μg per test, Biolegend), CD62L, PD1 (clone J43,.2 μg per test, ebioscience) and CD19 (clone 6D,. μg per test, Biolegend). For B cells, cells were stained with antibodies against CD19, Fas (clone 1A7,. μg per test, ebioscience), IgD (clone 11-26c,.2 μg per test, Biolegend), GL7 (clone GL-7,. μg per test, ebioscience), CD138 (clone 281-2,. μg per test, Biolegend) and CD4, CD8 (clone 3-6.7,.2 μg per test, ebioscience), CD11c (clone N418,.6 μg per test, ebioscience), Gr-1 (clone RB6-8C,.3 μg per test, ebioscience) and F4/8 (clone BM8,.12 μg per test, ebioscience) (for dump gate). For macrophages, CD11b (clone M1/7,. μg per test, ebioscience) was used. For Bcl6 Expression, cells were stained for Tfh as above. Cells were fixed and permeabilized and stained with antibodies against Bcl6 (clone K112-91, μl per test, BD Bioscience). For Treg staining, cells were surface stained with antibodies against CD4, TCRβ and CD2. Cells were fixed and permeabilized with Foxp3 staining buffer kit (Ebioscience). Cells were then stained with antibody against Foxp3 (clone FJK-16S,.12 μg per test, ebioscience) in permeabilization buffer. For intracellular cytokine staining, cells from were stimulated in RPMI- 1% FCS media with PMA and Ionomycin ( ng ml 1 and 1 μgml 1, respectively) (Sigma Aldrich) for h in the presence of GolgiPlug (BD Biosciences) at 1 μlml 1. Cells were then stained for CD4, TCRβ, CD44, CD19, PD1, and CXCR as described above. Cells were fixed for 1 min with 2% freshly prepared paraformaldehyde (Sigma-Aldrich) and then permeabilized as described above. Cells were then stained with antibodies against IFNγ (clone XMG-1.2,. μg per test, BD bioscience), IL-17 (clone ebio1787,.12 μg per test, ebioscience), Foxp3, and rabbit-rfp (polyclonal,.2 μg per test, Rockland, Limerick, PA) followed by a stain for donkey anti-rabbit-pe (.12 μg per test, Biolegend) for the detection of RFP. For pstat, spleen cells were stimulated with recombinant human IL-2 (1 Uml 1 ) (Peprotech, Rocky Hill, NJ) for 4 min at 37 C. Cells were surface stained as previously described. Cells were washed and fixed with 1 Lyse/Fix buffer (BD Biosciences) for 1 min at 37 C. Cells were washed and chilled on ice and permeabilized with pre-chilled BD phosphoflow Perm Buffer III for 3 min (BD Biosciences). Cells were washed and stained for Foxp3 and rabbit pstat (D47E7, 1:2, Cell Signaling Technologies, Beverly, MA) followed by anti-rabbit-af647 (.12 μg per test, Biolegend). Suppression assay. Current and cells were sorted based on expression of YFP and RFP. Naive effector cells (CD4 + CD2 CD44 lo CD62L hi ) cells were sorted from spleens and peripheral LNs of CD4.1 mice and were labeled with cell trace violet (CTV) (Life Technologies) according to manufacturer s instruction. Cell sorting was done using FACSAria (BD Biosciences). Feeder cells were prepared from spleens of B6 mice and CD4 T cells were depleted using CD4 + magnetic beads (Miltenyi Biotec, Auburn, CA) and then were irradiated with 3 rads. Feeders were cultured with naive effector T cells at a ratio of 3:1 in U-shaped 96 well plates. Sorted current or cells were added at a ratio of 1:1, 1:2, 1:4, 1:8 or not added for effectors only wells. All wells, except for unstimulated wells, were stimulated with soluble αcd3 antibody at 2. μgml 1. All cells were cultured in RPMI media supplemented with 1% FCS, L-glutamine, penicillin and streptomycin, β-mercaptoethanol. Cells were harvested 4 days following stimulation and the percentage of effector CD4.1 cells that diluted CTV was determined. In vitro Treg induction. Naive CD4 T cells were sorted from spleens and lymph nodes of ApoE / as described earlier or using Easy Sep TM Naive T cell isolation kit (STEMCELL Technologies, Inc., Vancouver, Canada). Equal numbers of cells in serum free CST OpTmizer T cell expansion media (Life Technologies) supplemented with L-glutamine, penicillin and streptomycin and β-mercaptoethanol were incubated in αcd3 coated plates at 2 μgml 1 concentration. Soluble αcd28 (1 μgml 1 ) and TGFβ (2 ng ml 1 ) were added to the wells. For oxldl conditions, 1 μgml 1 of human oxldl (KalenBiomed, Montgomery Village, MD) was added to the culture. For IL-2 conditions, cells were stimulated with recombinant human IL-2 (1 U ml 1 ). For in vitro ApoAI conditions, 2 μgml 1 final concentration was used. Cells were incubated for 36 h (IL-2 detection or Foxp3 mrna) and for 4 days (for induced Treg staining). Staining for Treg was done as indicated earlier. Purification of Human plasma ApoAI and administration in LT-ApoE / mice. Human ApoAI was purified as previously described 23. ApoAI concentration was adjusted to 2. mgml 1 with saline. BSA (Sigma, St. Louis, MO) was prepared at the same concentration and filter sterilized to serve as control. After feeding LT- ApoE / mice a western diet for 6 weeks, mice were injected subcutaneously with μg of ApoAI or BSA. mice also received BSA as controls. In vivo ICOSL blocking. LT-ApoE / mice were fed a western diet for 1 weeks. For the last 6 weeks, mice were injected intraperitoneal with 1 μg of anti-icosl (clone HK.3) or isotype control (Rat IgG2a) (BioXcell, West Lebanon, NH) twice a week, similar to Choi et al. 3. Mice were sacrificed and Tfh populations were assessed in aorta, peri-aortic LNs and spleen as described earlier. Bcl6 deficient LDLR / chimera generation. Femurs from B6, (WT; control) and Bcl6 fl/fl- CD4Cre + mice were centrifuged to collect the bone marrow cells in PBS. Recipient LDLR / mice were irradiated with rads twice, 4 h apart. Approximately bone marrow cells were injected retro-orbitally into recipient mice. Mice were allowed to reconstitute for 6 weeks before they were fed a high-cholesterol diet for 1 weeks. Atherosclerosis quantification. Aortic roots were dissected and snap frozen in OCT over dry ice. Serial frozen sections were cut at 1 μm thickness and stained with Oil Red O (for neutral lipids) and in some cases counterstained with hematoxylin (for nuclei) using standard procedure. At least four sections per aortic root per mouse were stained and scanned with Zeiss AxioScan Z1 slide scanner at 2 magnification. The area of red pixels was determined by Image-Pro Analyzer software 7..1 and the pixel area values were converted to μm 2. For en face staining, mouse aortae were collected and immersed in paraformaldehyde and stained with Oil Red O, open longitudinally and pinned as previously 6. Images were scanned and the percentage of lesion surface area was determined with Photoshop software. Immunofluorescence staining of CD68 + cell in aortic roots. Aortic roots described above where stained for macrophages using immunofluorescence using purified rat anti-cd68 (clone FA-11, μgml 1, Biolegend), following by goat antirat-af68 (4 μgml 1, ThermoFisher) and counterstained with phalloidin AF647 for actin (ThermoFisher) and Hoechst for nuclei (ThermoFisher). Isotype control sections were used to set thresholds. Images were acquired using Zeiss AxioScan Z1 slide scanner and analyze with Image J software. 4 sections were used per mouse and the average area of all four sections was used to calculate the CD68 + area. Plasma cholesterol and HDL levels. The lipoprotein cholesterol distribution was determined in the plasma of LT-ApoE / mice following fasting for 4 h by liquid chromatography (FPLC) as previously described 23. Mass spectroscopy for intracellular lipids. To determine the cholesterol content in the cells, current Treg (RFP + YFP + ) from LT-ApoE / chow or western diet fed, NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
14 ARTICLE NATURE COMMUNICATIONS DOI: 1.138/s with and without administration of ApoAI, were sorted from peri-aortic LNs, washed and cell pellets were frozen at 8 C till analyzed. Cholesterol was extracted from the cells and the lipids extracts were analyzed by mass spectroscopy as previously described 7. Quantitative real time PCR. Total cellular RNA from sorted current or from LT-ApoE / mice was extracted by Trizol (Life Technologies) followed by RNA purification using Direct-zol TM RNA miniprep (Zymo Research, Irvine, CA) per manufacturer s instructions. RNA purity and quantity was determined by nanodrop spectrophotometer (Thermo Scientific) and equal amounts of RNA was used to synthesis cdna using iscript cdna synthesis kit (Bio-Rad). mrna expression was measured in real time quantitative PCR using TaqMan Gene Expression system and predesigned TaqMan primers for Foxp3, Blimp1, Bcl6, Il21, IFNγ, ABCA1, and β-actin (Applied Biosytems). Data were analyzed and presented on the basis of relative expression method. The 2 -ΔΔCT method was used with β- actin as a housekeeping gene. ELISA. Plasma samples were collected from LT-ApoE / mice using cardiac puncture and EDTA coated syringes. Samples were centrifuged to remove RBCs and frozen at 8 C till analyzed. Supernatants from in vitro Treg induction were collected following 36 h of stimulation and frozen at 8 C till analyzed. Both mouse IL-6 and IL-2 ELISAs were performed using Ready-Set-Go ELISA kits (ebioscience) according to manufacturer s instruction. Human samples. PBMCs and plasma sample were isolated from blood of donors at the Cardiac Catheterization laboratory in University of Virginia, Charlottesville. Enrolled participants included males and females of different ages (for human subjects characteristics, see Supplementary Table 1) and informed consent was obtained from all subjects. Studies were performed with the approval of the Institutional Review Board of Health Science Research at the University of Virginia. Blood was drawn into vacutainer EDTA (BD Bioscience), spun and the top plasma layer was collected and frozen at 8 C until analyzed. Thawed plasma samples were tested for the levels of ApoAI and IL-21 using human ApoAI (ABCAM, Cambridge, MA) and IL-21 ELISA (ebioscience) kits, respectively, according to manufacturer s instruction. Peripheral blood mononuclear cells (PBMC) were isolated from blood that was depleted from plasma with Ficoll-Paque PLUS (GE Healthcare) and SepMate tubes (STEMCELL Technologies, Inc.). For Treg percentages, Treg were identified as CD4 + CD2 + CD127 [and/or Foxp3 + ] cells by flow cytometry. Antibodies used were anti-cd4 (clone OKT4; 1 test per sample, Tonbo Bioscience), anti-cd2 (clone BC96; 1 test per sample, Tonbo Bioscience), anti-cd127 (clone A19D, 1 test per sample, Biolegend), and anti-foxp3 (clone PCH11,. μg per test ebioscience). Statistical analyses. All results are expressed as mean ± s.e.m. Results were analyzed by either unpaired two-tailed Student s t-test, one-way analysis of variance (one way ANOVA), or two-way ANOVA, followed by Tukey s multiple comparisons test. Our sample size was based on previous studies, consulting with biostatisticians, and using nquery Advisor 6. software. Via this analysis, we found that 9% power with 1% type 1 error can be achieved with 8 mice per group for atherosclerosis quantification. For in vitro and qrt-pcr studies, no statistical method was used to determine sample size. No exclusion was performed. The data seemed to be normally distributed with similar s.d. and error observations between experimental groups and controls. A P value of <. is considered to be significant. Statistical analysis was performed using GraphPad Prism software version 6 (GraphPad Software, Inc.). Linear regression and Pearson correlation coefficient (r) were used to determine the statistical significance and correlation in the human studies. Data availability. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. Received: 19 November 216 Accepted: 19 February 218 References 1. Klingenberg, R. et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 123, (213). 2. Gotsman, I. et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation 114, (26). 3. Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, (26). 4. Mor, A. et al. Role of naturally occurring CD4+CD2+ regulatory T cells in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, (27).. Mallat, Z. et al. Protective role of interleukin-1 in atherosclerosis. Circ. Res. 8, e17 e24 (1999). 6. Robertson, A.-K. L. et al. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 112, (23). 7. Mallat, Z. et al. Inhibition of transforming growth factor- signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89, (21). 8. Maganto-Garcia, E., Tarrio, M. L., Grabie, N., Bu, D. X. & Lichtman, A. H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124, (211). 9. McClymont, S. A. et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 186, (211). 1. Sujino, T. et al. Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science 32, (216). 11. Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 1, 1 17 (29). 12. Butcher, M. J. et al. Atherosclerosis-driven treg plasticity results in formation of a dysfunctional subset of plastic IFNgamma +Th1/Tregs. Circ. Res. 119, (216). 13. Lux, S. E., Levy, R. I., Gotto, A. M. & Fredrickson, D. S. Studies on the protein defect in Tangier disease. Isolation and characterization of an abnormal high density lipoprotein. J. Clin. Invest. 1, (1972). 14. Sorci-Thomas, M. G. et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoa-i monomers. J. Lipid Res. 3, (212). 1. Lewis, G. F. & Rader, D. J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96, (2). 16. Linsel-Nitschke, P. & Tall, A. R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug. Discov. 4,193 2 (2). 17. Kaul, S. et al. Lipid-free apolipoprotein a-i reduces progression of atherosclerosis by mobilizing microdomain cholesterol and attenuating the number of CD131 expressing cells: monitoring cholesterol homeostasis using the cellular ester to total cholesterol ratio. J. Am. Heart Assoc., e441 (216). 18. Sorci-Thomas, M. G. & Thomas, M. J. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler. Thromb. Vasc. Biol. 32, (212). 19. Black, L. L. et al. Cholesterol-independent suppression of lymphocyte activation, autoimmunity, and glomerulonephritis by apolipoprotein A-I in normocholesterolemic Lupus-Prone mice. J. Immunol. 19, (21). 2. Lewis, T. L. et al. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J. Biol. Chem. 28, (21). 21. Tavori, H. et al. Macrophage apoai protects against dyslipidemia-induced dermatitis and atherosclerosis without affecting HDL. J. Lipid Res. 6, (21). 22. Kim, K. D. et al. Apolipoprotein A-I induces IL-1 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem. Biophys. Res. Commun. 338, (2). 23. Wilhelm, A. J. et al. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr-/-, ApoA-I-/- mice. J. Biol. Chem. 28, (21). 24. Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-1 limits inflammation at environmental interfaces. Immunity 28, 46 8 (28). 2. Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 41, (214). 26. Gupta, S. et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, (1997). 27. Whitman, S. C., Ravisankar, P., Elam, H. & Daugherty, A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E-/- mice. Am. J. Pathol. 17, (2). 28. Frostegard, J. et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 14, (1999). 29. Buono, C. et al. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 23, (23). 3. Choi, Y. S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, (211). 31. Nance, J. P., Belanger, S., Johnston, R. J., Takemori, T. & Crotty, S. Cutting edge: T follicular helper cell differentiation is defective in the absence of Bcl6 BTB repressor domain function. J. Immunol. 194, (21). 32. Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 32, 11 1 (29). 14 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
15 NATURE COMMUNICATIONS DOI: 1.138/s ARTICLE 33. Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 32, (29). 34. Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, (29). 3. Chen, Q., Kim, Y. C., Laurence, A., Punkosdy, G. A. & Shevach, E. M. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+T cells in vivo. J. Immunol. 186, (211). 36. Johnston, R. J., Choi, Y. S., Diamond, J. A., Yang, J. A. & Crotty, S. STAT is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 29, (212). 37. Ballesteros-Tato, A. et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, (212). 38. Pepper, M., Pagan, A. J., Igyarto, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 3, 83 9 (211). 39. Oestreich, K. J., Mohn, S. E. & Weinmann, A. S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 13, (212). 4. Choi, Y. S. et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 19, (213). 41. Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 19, (213). 42. Karnowski, A. et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J. Exp. Med. 29, (212). 43. Eto, D. et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 6, e17739 (211). 44. Liao, W., Lin, J. X., Wang, L., Li, P. & Leonard, W. J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 12, 1 9 (211). 4. Podrez, E. A. et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277, (22). 46. Ravandi, A. et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J. Am. Coll. Cardiol. 63, (214). 47. Marmor, M. D. & Julius, M. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood 98, (21). 48. Sorci-Thomas, M. G. & Thomas, M. J. Why targeting HDL should work as a therapeutic tool, but has not. J. Cardiovasc. Pharmacol. 62, (213). 49. Arakawa, R. & Yokoyama, S. Helical apolipoproteins stabilize ATP binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277, (22).. Mohanta, S. K. et al. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ. Res. 114, (214). 1. Clement, M. et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 6 7 (21). 2. Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 27, (21). 3. Slack, M., Wang, T. & Wang, R. T cell metabolic reprogramming and plasticity. Mol. Immunol. 68, 7 12 (21). 4. Zhou, X., Bailey-Bucktrout, S., Jeker, L. T. & Bluestone, J. A. Plasticity of CD4 (+) FoxP3(+) T cells. Curr. Opin. Immunol. 21, (29).. Hoeppli, R. E., Wu, D., Cook, L. & Levings, M. K. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front. Immunol. 6, 61 (21). 6. Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3 +T cells in gut Peyer s patches. Science 323, (29). 7. Crotty, S. Do memory CD4 T cells keep their cell-type programming: plasticity versus fate commitment? complexities of interpretation due to the heterogeneity of memory CD4 T cells, including T follicular helper cells. Cold Spring Harb. Perspect. Biol. 1, a3212 (217). 8. Sallusto, F. et al. Cells keep their cell-type programming: plasticity versus fate commitment? t-cell heterogeneity, plasticity, and selection in humans. Cold Spring Harb Perspect. Biol. 1, a29421 (217). 9. Johnson, J. L., Vahedi, G. Do memory CD4 T cells keep their cell-type programming: plasticity versus fate commitment? epigenome: a dynamic vehicle for transmitting and recording cytokine signaling. Cold Spring Harb. Perspect. Biol. 1, a28779 (217). 6. Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, (217). 61. Smith, J. D. Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr. Opin. Investig. Drugs 11, (21). 62. Sorci-Thomas, M. G. & Thomas, M. J. Microdomains, inflammation, and atherosclerosis. Circ. Res. 118, (216). 63. Gao, M. et al. Regulation of high-density lipoprotein on hematopoietic stem/ progenitor cells in atherosclerosis requires scavenger receptor type BI expression. Arterioscler. Thromb. Vasc. Biol. 34, (214). 64. Reschly, E. J. et al. Apolipoprotein A-Ialpha -helices 7 and 8 modulate high density lipoprotein subclass distribution. J. Biol. Chem. 277, (22). 6. Cheng, H. Y. et al. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Invest. 126, (216). 66. Goebel, J. et al. Atorvastatin affects interleukin-2 signaling by altering the lipid raft enrichment of the interleukin-2 receptor beta chain. J. Investig. Med 3, (2). 67. Fabrizi, M. et al. IL-21 is a major negative regulator of IRF4-dependent lipolysis affecting Tregs in adipose tissue and systemic insulin sensitivity. Diabetes 63, (214). 68. Potekhina, A. V. et al. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis 238, (21). 69. Kaji, T. et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J. Exp. Med. 29, (212). 7. Wilhelm, A. J. et al. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler. Thromb. Vasc. Biol. 29, (29). Acknowledgements We thank D. Yoakum for assistance with the mice colony management and mice injections, LJI Flow core facility for sorting, M. Chadwell and A. Lamberth for technical assistance with Oil red O and CD68 staining of aortic roots, Z. Mikulski and S. McArdle for assistance with slide scanning and microscopy, and J. Hu and M. Murai for discussions. This project was supported by the American Heart Association (13POST to D.E.G.), (14GRNT229 to M.J.T), American Diabetes Association (#7-12-MN-13 to D.E.G. and C.C.H.), and the National Institutes of Health (NHLBI P1 HL798 to C.C.H, C.A.M, and A.M.T, R1 HL to C.C.H. and M.S. T., R1 HL to M.S.T, P1 AI89624 to M.K., R1 AI7243 to S.C., T32 AI12279 to L.E.P., and S1 RR A1 to LJI flow cytometry core). Author contributions D.E.G designed, performed, and analyzed the experiments. R.W. and C.M. performed the experiments. L.E.P. performed and analyzed the experiments. V.R. analyzed data. A.M.T., C.A.M., M.S.T., and C.C.H. designed the experiments. S.C. provided mice, helpful discussion, and edited the manuscript. M.K. provided mice and edited the manuscript. M.J. T. helped with mass spectroscopy experiments. D.E.G., M.S.T., and C.C.H. wrote the manuscript. Additional information Supplementary Information accompanies this paper at Competing interests: The authors declare no competing interests. Reprints and permission information is available online at reprintsandpermissions/ Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit licenses/by/4./. The Author(s) 218 NATURE COMMUNICATIONS (218) 9:19 DOI: 1.138/s
16 Supplementary Information Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis Gaddis et al.
17 FSC YFP Live SSC 99.1 CD4 8.1 CD4 Effector memory CXCR + CD62L CD4+ CD4 YFP RFP () - FSC 4.39 CD19 RFP current Treg 6.44 SSC (A) CD4+TCRβ+ Singlets FSC (A) CD4+CD SSC (W) 98. TCRβ SSC 33. Singlets Live Dead dye Lymphocytes FSC (W) Total Cells Tfh CD44 PD1 Supplementary Figure 1: Gating Strategy of current Treg, and Tfh from cells in the aorta of western diet-fed LT-ApoE-/- mice. Flow cytometry plots showing gating strategy for current Treg, and Tfh from cells in the aorta of -fed LT-ApoE-/- mice. Similar gating strategy was performed for and spleens excluding the CD4 staining. Results show a representative mouse.
18 CD2 CD62L Nrp1 2 2 CD2 MFI CD62L MFI 1 1 Nrp1 MFI CD44 8 PD1 Current Treg CD44 MFI 1 PD1 MFI Supplementary Figure 2: Expression of surface markers on current and cells. Current and cells from western diet-fed LT-ApoE -/- mice were evaluated for the expression of CD2, CD62L, Nrp1, CD44 and PD1. Graphs show the mean fluorescence intensity of each molecule on both cell subsets. Results are expressed as the mean ± s.e.m from one experiment (n=4-7). Statistical significant differences were at P <., P <.1 and P <.1 (Unpaired Student s t-test).
19 CD4 T cells Tfh from 11.3 RFP Percent of Tfh cells a LT-ApoE-/- 1 Memory D 1 1 C ho w W D Percent of + cells IFN.4 IFN from 9.29 CXCR 2.6 CD4 IFNγ 7.33 C Foxp3 W ho w PD RFP 1.6 IL-17 from Percent of IL-17+ cells CD4 T cells LT-ApoE C ho w -/-. D CD44 W IL Foxp3 1 D D 1 W IL-17+ % of total IL ho w 1 PD1 C 1 W W ho C D 2 ho w 2 C 1 IFN + % of total IFN + Percent of Th1 of total Th1 CD4 T cells w Percent of Tfh of total Tfh cells 1 CD44 Percent of Th17 of total Th17 CD4 T cells IL-17 Tfh % of total Tfh 13.1 CXCR.44 b Memory 3.3 CD4 IFNγ 1.4 Supplementary Figure 3: Distribution of the different T cell subsets within the population. from LT-ApoE-/- mice on western diet and chow controls were examined for the distribution of the different T cell subsets within the population. (a) Flow plots and graphs show the percentages of memory, Th-1, Th-17 and Tfh cells within the population. (b) Graphs showing the percentages of each population within the total population of cells. Results are expressed as mean ± s.e.m. from one experiment (n=7 (chow) and n=8 ()). Statistical significant differences were at P <., and P <.1 (unpaired Student s t-test).
20 1 IL-21-Foxp3 correlation in human patients IL-21 (pg/ml) 1 Gaddis et al. Figure 4 P =.38 r = Percent of Foxp3 in Treg (CD4+CD2+CD127-) Supplementary Figure 4: In CAD patients, IL-21 levels inversely correlates with Foxp3 expression. Graph shows a negative correlation between plasma levels of IL-21 and Foxp3 expression on Treg from PBMCs of human patients. Correlation was determined using linear regression and the results were statistically significant at P =.38.
21 1 Relative expression1 CD3/CD28 Foxp3 12 hours CD3/CD28 +TGF Relative expression CD3/CD28 Foxp3 24 hours CD3/CD28 +TGF Media OxLDL Supplementary Figure : OxLDL reduces Foxp3 mrna expression. Naïve CD4 T cells from ApoE -/- mice were isolated and stimulated with αcd3/cd28 and TGFβ to induce Treg in vitro with or without the addition of oxldl. RNA was harvested at 12 and 24 hours post-stimulation and the levels of Foxp3 mrna were detected by qrt-pcr. Results are expressed as the mean ± s.e.m. from one experiment (n=3). Statistical significant differences were at P <. and P <.1 (Unpaired Student s t test).
22 Treg percent (CD2+CD127-) of CD ApoAI-Treg correlation in human blood ApoAI (mg/ml) P =.19 r =.38 Supplementary Figure 6: In CAD patients, ApoAI levels positively correlates with Treg percentages in blood. PBMC and plasma samples from human patients were assessed for the percentages of Treg and levels of plasma ApoAI. A positive correlation of the percentages of Treg cells and ApoAI plasma levels was determined using linear regression and the results were statistically significant at P =.19.
23 a of current Treg Spleen Current Treg (RFP + YFP + ) /ApoAI b of total Tfh cells Total Tfh /ApoAI Total Tfh /ApoAI Spleen Total Tfh /ApoAI Supplementary Figure 7: Treg and total Tfh cells number in ApoAI treated LT-ApoE -/- mice. of Treg cells in the spleen (a) and total Tfh cells in the aorta, and spleen (b) of LT-ApoE -/- western diet-fed mice with and without ApoAI treatment or chow controls. Results are expressed as the mean ± s.e.m. from four independent experiments (n=29 (), n=27 () and n=26 (/ApoAI) (a), and from three independent experiments (n=19 (), n=16 ( & /ApoAI)) (b). Statistical significant differences were at P <., P <.1, P <.1 (one-way Anova).
24 Live Dead dye Singlets SSC (W) FSC (W) 36.9 Lymphocytes FSC FSC (A) SSC (A) Singlets 99. FSC 7.92 CD4+ Live SSC Germinal Center B cells CD4/CD8/F4/8/ Gr-1/CD11c GL7 CD19+Dump- FAS CD138 SSC Total Cells 3. Plasma Cells CD19 CD4 IgD Supplementary Figure 8: Gating Strategy of germinal center B cells and plasma cells in aorta. Flow cytometry plots showing gating strategy for germinal center B cells and plasma cells in the aorta of -fed LT-ApoE-/- mice. Similar gating strategy was performed for and spleens excluding the CD4 staining. Results show a representative mouse.
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the
 Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
Supplementary Information:
 Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured
 Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplementary Figure 1. Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Nature Immunology: doi: /ni.
 Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Akt and mtor pathways differentially regulate the development of natural and inducible. T H 17 cells
 Akt and mtor pathways differentially regulate the development of natural and inducible T H 17 cells Jiyeon S Kim, Tammarah Sklarz, Lauren Banks, Mercy Gohil, Adam T Waickman, Nicolas Skuli, Bryan L Krock,
Akt and mtor pathways differentially regulate the development of natural and inducible T H 17 cells Jiyeon S Kim, Tammarah Sklarz, Lauren Banks, Mercy Gohil, Adam T Waickman, Nicolas Skuli, Bryan L Krock,
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
Supplemental Materials
 Supplemental Materials Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability Qi Wang 1, Jianwei He 1, Dallas B. Flies 2, Liqun Luo
Supplemental Materials Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability Qi Wang 1, Jianwei He 1, Dallas B. Flies 2, Liqun Luo
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Supplemental Information. CD4 + CD25 + Foxp3 + Regulatory T Cells Promote. Th17 Cells In Vitro and Enhance Host Resistance
 Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
SUPPLEMENTARY FIGURE 1
 SUPPLEMENTARY FIGURE 1 A LN Cell count (1 ) 1 3 1 CD+ 1 1 CDL lo CD hi 1 CD+FoxP3+ 1 1 1 7 3 3 3 % of cells 9 7 7 % of cells CD+ 3 1 % of cells CDL lo CD hi 1 1 % of CD+ cells CD+FoxP3+ 3 1 % of CD+ T
SUPPLEMENTARY FIGURE 1 A LN Cell count (1 ) 1 3 1 CD+ 1 1 CDL lo CD hi 1 CD+FoxP3+ 1 1 1 7 3 3 3 % of cells 9 7 7 % of cells CD+ 3 1 % of cells CDL lo CD hi 1 1 % of CD+ cells CD+FoxP3+ 3 1 % of CD+ T
Eosinophils are required. for the maintenance of plasma cells in the bone marrow
 Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Supplementary Figure Legends. group) and analyzed for Siglec-G expression utilizing a monoclonal antibody to Siglec-G (clone SH2.1).
 Supplementary Figure Legends Supplemental Figure : Naïve T cells express Siglec-G. Splenocytes were isolated from WT B or Siglec-G -/- animals that have not been transplanted (n= per group) and analyzed
Supplementary Figure Legends Supplemental Figure : Naïve T cells express Siglec-G. Splenocytes were isolated from WT B or Siglec-G -/- animals that have not been transplanted (n= per group) and analyzed
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was
 Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Krishnamoorthy et al.,
 Krishnamoorthy et al., c d e ND ND Supplementary Figure 1 RSV-induces inflammation even in the asence of allergen. Tolerized pups were either infected with RSV or not. The mice were sacrificed a week following
Krishnamoorthy et al., c d e ND ND Supplementary Figure 1 RSV-induces inflammation even in the asence of allergen. Tolerized pups were either infected with RSV or not. The mice were sacrificed a week following
Effector T Cells and
 1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
B cell activation and antibody production. Abul K. Abbas UCSF
 1 B cell activation and antibody production Abul K. Abbas UCSF 2 Lecture outline B cell activation; the role of helper T cells in antibody production Therapeutic targeting of B cells 3 Principles of humoral
1 B cell activation and antibody production Abul K. Abbas UCSF 2 Lecture outline B cell activation; the role of helper T cells in antibody production Therapeutic targeting of B cells 3 Principles of humoral
IMMUNOLOGICAL MEMORY. CD4 T Follicular Helper Cells. Memory CD8 T Cell Differentiation
 IMMUNOLOGICAL MEMORY CD4 T Follicular Helper Cells Memory CD8 T Cell Differentiation CD4 T Cell Differentiation Bcl-6 T-bet GATA-3 ROR t Foxp3 CD4 T follicular helper (Tfh) cells FUNCTION Provide essential
IMMUNOLOGICAL MEMORY CD4 T Follicular Helper Cells Memory CD8 T Cell Differentiation CD4 T Cell Differentiation Bcl-6 T-bet GATA-3 ROR t Foxp3 CD4 T follicular helper (Tfh) cells FUNCTION Provide essential
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies
 NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
Supplemental Materials for. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to. FTY720 during neuroinflammation
 Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Examples of questions for Cellular Immunology/Cellular Biology and Immunology
 Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy. Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L.
 Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c)
 1 Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day
1 Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day
D CD8 T cell number (x10 6 )
 IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Immune Cells in Atherosclerosis Regulatory vs Inflammatory T cells Göran K Hansson
 Immune Cells in Atherosclerosis Regulatory vs Inflammatory T cells Göran K Hansson Center for Molecular Medicine Karolinska Institute Stockholm, Sweden DECLARATION OF CONFLICT OF INTEREST Goran Hansson
Immune Cells in Atherosclerosis Regulatory vs Inflammatory T cells Göran K Hansson Center for Molecular Medicine Karolinska Institute Stockholm, Sweden DECLARATION OF CONFLICT OF INTEREST Goran Hansson
Nature Immunology: doi: /ni Supplementary Figure 1. Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice.
 Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
B220 CD4 CD8. Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN
 B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
ONLINE SUPPLEMENT MATERIAL. CD70 limits atherosclerosis and promotes macrophage function.
 ONLINE SUPPLEMENT MATERIAL CD7 limits atherosclerosis and promotes macrophage function. Holger Winkels* 1,2, Svenja Meiler* 1,2, Esther Smeets* 2, Dirk Lievens 1, David Engel 3, Charlotte Spitz 1, Christina
ONLINE SUPPLEMENT MATERIAL CD7 limits atherosclerosis and promotes macrophage function. Holger Winkels* 1,2, Svenja Meiler* 1,2, Esther Smeets* 2, Dirk Lievens 1, David Engel 3, Charlotte Spitz 1, Christina
SUPPLEMENT Supplementary Figure 1: (A) (B)
 SUPPLEMENT Supplementary Figure 1: CD4 + naïve effector T cells (CD4 effector) were labeled with CFSE, stimulated with α-cd2/cd3/cd28 coated beads (at 2 beads/cell) and cultured alone or cocultured with
SUPPLEMENT Supplementary Figure 1: CD4 + naïve effector T cells (CD4 effector) were labeled with CFSE, stimulated with α-cd2/cd3/cd28 coated beads (at 2 beads/cell) and cultured alone or cocultured with
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity
 NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases. Abul K. Abbas UCSF
 Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Supplementary. presence of the. (c) mrna expression. Error. in naive or
 Figure 1. (a) Naive CD4 + T cells were activated in the presence of the indicated cytokines for 3 days. Enpp2 mrna expression was measured by qrt-pcrhr, infected with (b, c) Naive CD4 + T cells were activated
Figure 1. (a) Naive CD4 + T cells were activated in the presence of the indicated cytokines for 3 days. Enpp2 mrna expression was measured by qrt-pcrhr, infected with (b, c) Naive CD4 + T cells were activated
Supplemental Information. Checkpoint Blockade Immunotherapy. Induces Dynamic Changes. in PD-1 CD8 + Tumor-Infiltrating T Cells
 Immunity, Volume 50 Supplemental Information Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1 CD8 + Tumor-Infiltrating T Cells Sema Kurtulus, Asaf Madi, Giulia Escobar, Max Klapholz, Jackson
Immunity, Volume 50 Supplemental Information Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1 CD8 + Tumor-Infiltrating T Cells Sema Kurtulus, Asaf Madi, Giulia Escobar, Max Klapholz, Jackson
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic
 Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
Tolerance 2. Regulatory T cells; why tolerance fails. Abul K. Abbas UCSF. FOCiS
 1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
1 Tolerance 2. Regulatory T cells; why tolerance fails Abul K. Abbas UCSF FOCiS 2 Lecture outline Regulatory T cells: functions and clinical relevance Pathogenesis of autoimmunity: why selftolerance fails
W/T Itgam -/- F4/80 CD115. F4/80 hi CD115 + F4/80 + CD115 +
 F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells
 CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation
 Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation Jurg Rohrer, PhD Director, R&D BD Biosciences 23-11773-00 For Research Use Only. Not for use in diagnostic or therapeutic
Simultaneous correlation of cytokine production with Treg and Th17 cell proliferation Jurg Rohrer, PhD Director, R&D BD Biosciences 23-11773-00 For Research Use Only. Not for use in diagnostic or therapeutic
Supplementary Information. Tissue-wide immunity against Leishmania. through collective production of nitric oxide
 Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with
 Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
SUPPLEMENTARY INFORMATION
 Supplementary Text Results In vitro activation experiments established that treatment with anti-cd3 plus anti- CD28 was sufficient to promote EBI2 transcript on CD4 T cells (Extended Data Fig. 1d, e).
Supplementary Text Results In vitro activation experiments established that treatment with anti-cd3 plus anti- CD28 was sufficient to promote EBI2 transcript on CD4 T cells (Extended Data Fig. 1d, e).
Nature Immunology: doi: /ni Supplementary Figure 1. Id2 and Id3 define polyclonal T H 1 and T FH cell subsets.
 Supplementary Figure 1 Id2 and Id3 define polyclonal T H 1 and T FH cell subsets. Id2 YFP/+ (a) or Id3 GFP/+ (b) mice were analyzed 7 days after LCMV infection. T H 1 (SLAM + CXCR5 or CXCR5 PD-1 ), T FH
Supplementary Figure 1 Id2 and Id3 define polyclonal T H 1 and T FH cell subsets. Id2 YFP/+ (a) or Id3 GFP/+ (b) mice were analyzed 7 days after LCMV infection. T H 1 (SLAM + CXCR5 or CXCR5 PD-1 ), T FH
CD4 + T cells recovered in Rag2 / recipient ( 10 5 ) Heart Lung Pancreas
 a CD4 + T cells recovered in Rag2 / recipient ( 1 5 ) Heart Lung Pancreas.5 1 2 4 6 2 4 6 Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas b Heart Lung Pancreas Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas
a CD4 + T cells recovered in Rag2 / recipient ( 1 5 ) Heart Lung Pancreas.5 1 2 4 6 2 4 6 Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas b Heart Lung Pancreas Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas
ILC1 and ILC3 isolation and culture Following cell sorting, we confirmed that the recovered cells belonged to the ILC1, ILC2 and
 Supplementary Methods and isolation and culture Following cell sorting, we confirmed that the recovered cells belonged to the, ILC2 and subsets. For this purpose we performed intracellular flow cytometry
Supplementary Methods and isolation and culture Following cell sorting, we confirmed that the recovered cells belonged to the, ILC2 and subsets. For this purpose we performed intracellular flow cytometry
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-10. Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald,
 Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Spleen. mlns. E Spleen 4.1. mlns. Spleen. mlns. Mock 17. Mock CD8 HIV-1 CD38 HLA-DR. Ki67. Spleen. Spleen. mlns. Cheng et al. Fig.
 C D E F Mock 17 Mock 4.1 CD38 57 CD8 23.7 HLA-DR Ki67 G H I Cheng et al. Fig.S1 Supplementary Figure 1. persistent infection leads to human T cell depletion and hyper-immune activation. Humanized mice
C D E F Mock 17 Mock 4.1 CD38 57 CD8 23.7 HLA-DR Ki67 G H I Cheng et al. Fig.S1 Supplementary Figure 1. persistent infection leads to human T cell depletion and hyper-immune activation. Humanized mice
Supplementary Figure 1
 d f a IL7 b IL GATA RORγt h HDM IL IL7 PBS Ilra R7 PBS HDM Ilra R7 HDM Foxp Foxp Ilra R7 HDM HDM Ilra R7 HDM. 9..79. CD + FOXP + T reg cell CD + FOXP T conv cell PBS Ilra R7 PBS HDM Ilra R7 HDM CD + FOXP
d f a IL7 b IL GATA RORγt h HDM IL IL7 PBS Ilra R7 PBS HDM Ilra R7 HDM Foxp Foxp Ilra R7 HDM HDM Ilra R7 HDM. 9..79. CD + FOXP + T reg cell CD + FOXP T conv cell PBS Ilra R7 PBS HDM Ilra R7 HDM CD + FOXP
Alessandra Franco MD PhD UCSD School of Medicine Department of Pediatrics Division of Allergy Immunology and Rheumatology
 Immunodominant peptides derived from the heavy constant region of IgG1 stimulate natural regulatory T cells: identification of pan- HLA binders for clinical translation Alessandra Franco MD PhD UCSD School
Immunodominant peptides derived from the heavy constant region of IgG1 stimulate natural regulatory T cells: identification of pan- HLA binders for clinical translation Alessandra Franco MD PhD UCSD School
Supplementary Figures. T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ
 Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figure 1.
 Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway.
 MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
Gene+c fate mapping. x loxp. Foxp3 3 UTR ROSA26 RFP IRES GFP CRE. STOP loxp. Stable Foxp3 expression. Foxp3 expression in new Treg.
 1 Introduc+on (CD4 + CD25 + Foxp3 + )are indispensable for immune homeostasis. Muta+ons in Foxp3 gene leads to fatal autoimmune disorder. Condi+onal dele+on of Foxp3 reprograms cells into pathogenic Th
1 Introduc+on (CD4 + CD25 + Foxp3 + )are indispensable for immune homeostasis. Muta+ons in Foxp3 gene leads to fatal autoimmune disorder. Condi+onal dele+on of Foxp3 reprograms cells into pathogenic Th
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
TCR, MHC and coreceptors
 Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D.
 Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Appendix Figure S1 A B C D E F G H
 ppendix Figure S1 C D E F G H ppendix Figure S1. RT and chemotherapy alter PD-L1 expression in PDC cells. Flow cytometric analysis of PD-L1 expression in () KPC and () Pan02 cells following treatment with
ppendix Figure S1 C D E F G H ppendix Figure S1. RT and chemotherapy alter PD-L1 expression in PDC cells. Flow cytometric analysis of PD-L1 expression in () KPC and () Pan02 cells following treatment with
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
VISTA, a novel immune checkpoint protein ligand that suppresses anti-tumor tumor T cell responses. Li Wang. Dartmouth Medical School
 VISTA, a novel immune checkpoint protein ligand that suppresses anti-tumor tumor T cell responses Li Wang Dartmouth Medical School The B7 Immunoglobulin Super-Family immune regulators APC T cell Co-stimulatory:
VISTA, a novel immune checkpoint protein ligand that suppresses anti-tumor tumor T cell responses Li Wang Dartmouth Medical School The B7 Immunoglobulin Super-Family immune regulators APC T cell Co-stimulatory:
activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows
 Supplemental Data Supplemental Figure 1 compares CXCR4 expression in untreated CD8 + T cells, following activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows the
Supplemental Data Supplemental Figure 1 compares CXCR4 expression in untreated CD8 + T cells, following activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows the
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the T H 17 cell it reg cell balance Rajatava Basu 1,5, Sarah K.
SUPPLEMENTARY MATERIAL IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the T H 17 cell it reg cell balance Rajatava Basu 1,5, Sarah K.
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Supplementary Fig. 1 No relative growth advantage of Foxp3 negative cells.
 Supplementary Fig. 1 Supplementary Figure S1: No relative growth advantage of Foxp3 negative cells. itreg were induced from WT (A) or FIR (B) CD4 + T cells. FIR itregs were then removed from the TCR signal
Supplementary Fig. 1 Supplementary Figure S1: No relative growth advantage of Foxp3 negative cells. itreg were induced from WT (A) or FIR (B) CD4 + T cells. FIR itregs were then removed from the TCR signal
Peli1 negatively regulates T-cell activation and prevents autoimmunity
 Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/8/352/352ra110/dc1 Supplementary Materials for Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy
www.sciencetranslationalmedicine.org/cgi/content/full/8/352/352ra110/dc1 Supplementary Materials for Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy
Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ
 Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
PD-L1 hi B cells are critical regulators of humoral immunity
 Received 3 Oct 214 Accepted 1 Dec 214 Published 22 Jan 215 DOI: 1.138/ncomms6997 B cells are critical regulators of humoral immunity Adnan R. Khan 1, Emily Hams 1, Achilleas Floudas 1, Tim Sparwasser 2,
Received 3 Oct 214 Accepted 1 Dec 214 Published 22 Jan 215 DOI: 1.138/ncomms6997 B cells are critical regulators of humoral immunity Adnan R. Khan 1, Emily Hams 1, Achilleas Floudas 1, Tim Sparwasser 2,
Nature Medicine doi: /nm.3150
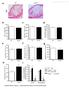 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/3/114/ra23/dc1 Supplementary Materials for Regulation of Zap70 Expression During Thymocyte Development Enables Temporal Separation of CD4 and CD8 Repertoire Selection
www.sciencesignaling.org/cgi/content/full/3/114/ra23/dc1 Supplementary Materials for Regulation of Zap70 Expression During Thymocyte Development Enables Temporal Separation of CD4 and CD8 Repertoire Selection
IL-6Rα IL-6RαT-KO KO. IL-6Rα f/f bp. f/f 628 bp deleted 368 bp. 500 bp
 STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel
 Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA
 AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27
 Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Novel Reduction of PCSK9 Expression: Mechanistic Insights into the Anti-Atherosclerotic & Hypolipidemic Effects of Heat Shock Protein 27 Ed O Brien, Jean-Claude Bakala-N Goma, Chunhua Shi Cumming School
Taylor Yohe. Project Advisor: Dr. Martha A. Belury. Department of Human Nutrition at the Ohio State University
 Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Atherosclerosis Development and the Inflammatory Response of Hepatocytes to Sesame Oil Supplementation Taylor Yohe Project Advisor: Dr. Martha A. Belury Department of Human Nutrition at the Ohio State
Immune Regulation and Tolerance
 Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder I Nakaya, Kousik
 SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
Follicular Lymphoma. ced3 APOPTOSIS. *In the nematode Caenorhabditis elegans 131 of the organism's 1031 cells die during development.
 Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Follicular Lymphoma 1. Characterized by t(14:18) translocation 2. Ig heavy
