Development of a Cost-Effective Test System for Measurement of Vestibular Evoked Myogenic Potentials (VEMPs)
|
|
|
- Ronald Flynn
- 6 years ago
- Views:
Transcription
1 Development of a Cost-Effective Test System for Measurement of Vestibular Evoked Myogenic Potentials (VEMPs) Nor Haniza Abdul Wahat B. Audio (Hons), MSc. Audiology This thesis is presented for the degree of Doctor of Philosophy of The University of Western Australia. Physiology School of Biomedical, Biomolecular and Chemical Sciences 2010
2 Abstract The present study aimed at developing a cost-effective, portable, robust and easily adaptable cervical vestibular evoked myogenic potentials (cvemps) and ocular vestibular evoked myogenic potentials (ovemps) recording system and protocol as a diagnostic tool and screening test for vestibular dysfunction. We modified both the standard recording electrode montages and the method of vestibular acoustic and mechanical stimulation to produce our process (introducing the palm-pulse and finger-tap methods). For cvemps, we recruited ten healthy adult subjects (seven for palm-pulse and three for finger-tap) and also two bilateral vestibular loss subjects for palm-pulse alone. For the finger-tap ovemps, we recruited ten healthy adults and five pathological subjects diagnosed with unilateral vestibular schwannoma. It was initially hypothesized that our electrode montages would produce similar responses as previously used montages. We also hypothesized that our longer acoustic 0.5 ms click stimulus would evoke similar VEMPs responses in the same way as the 0.1 ms click, if not better. We hypothesized that our methods of recording cvemps and ovemps would produce reliable and true vestibular responses, uncontaminated by reflex responses from the neurons of the facial skin. We also hypothesized that our skull-tap process would evoke vestibular responses through their entire range of input-output function. Following the work of others, we also hypothesized that our palm-pulse and finger-tap stimuli would produce whole-head accelerations because of its directed impulse to the skull, and that our directed skull-tap stimulus to explicit stimulation sites on the skull would evoke responses from different hair cell groups of the left and right otolith organs. We found that our electrode montages produced acoustic cvemps and ovemps very similar to others who used different electrodes montages. We also found that our acoustic 0.5 ms click stimulus was better than the 0.1 ms click, because not only did it give similar acoustic VEMPs responses, but it also provided five times greater low-frequency stimulation to the vestibular organs and less harmful effects to the cochlea. The control studies for both cvemps and ovemps presented in this report also proved that our recorded cvemps and ovemps were true vestibular responses, uncontaminated by any artifacts or other facial reflexes (e.g. blinks). They could be modified by slow rocking of the head or postural changes, and were masked by random vibration to the face and nose. We also found that our palm-pulse stimulus was too strong, and unsuitable in eliciting responses from the otolith, because most of the recorded responses from the normals were saturated and from other non-vestibular sources (probably neck reflexes), because the responses from our pathological subjects were similar to those of normals. On the other hand, our finger-tap technique elicited strong ovemp s responses from the vestibular system alone, down to threshold level (i.e. the softest tap) and beyond maximal levels (i.e. the strongest tap). Responses presented in this report also suggest that, the head moves in a very complex manner and not just accelerate as a single entity, the dominant stimulus mechanism was local deformation of the ipsilateral skull, and with possibly resonance or vibration of the skull. Overall, it is not yet possible to determine the specific hair cell groups that are stimulated by finger-taps at different stimulation sites on the skull. We suggest that dominant local deformation of the skull causes local acceleration of the ipsilateral hair cells (multiple sets of them), as well as less strong stimulation of the contralateral hair cells by whole-head translation. The complex anatomical structure of the otolith organs and the possible variability of anatomy and unitary muscle action potential waveforms also complicated the determination of the stimulated hair cells groups. Overall, we have successfully developed software and hardware that are inexpensive and mobile that produces
3 reliable vestibular responses, and we have also established a simple and rapid test protocol for both the cvemps and ovemps.
4 Acknowledgements First, I would like to express my sincere thanks and gratitude to my supervisor, Dr Robert Patuzzi. He has given me constant support and help during all the years that I ve known him. He has been a great inspiration to me and taught me on how to see things in a broader horizon and also to think analytically. I can t really thank him enough for all his advices and help. I would also like to thank Professor Don Robertson, my co-supervisor, for all his inputs on the research. Thank you to Dr Normani Zakaria, Dr Daniel Brown, Dr Helen Goulios, Ms Nashrah Maamor, Dr Andrew Gerrett and Dr Kumar for all the friendship through out the years in the Physiology building. I would also like to thank Dr Eda and Dr Razak, as there were together with me in Perth since day one. Also, my sincere thank to my good friend, Padillah. Finally, I want to thank my family. To my parents, Hj Abdul Wahat and Hjh Zainabu, who are always there for me. For coming over all the way to Malaysia to just be with me when I needed them most, for providing me the best moral support and understanding that any daughter could ask for. To my only sister, Dr Wahiza, for the encouragement that she has given me all these years. Her success is an inspiration to me. And last but not least, a special thank to my husband, Amran, for his moral support and love. Both of us have learnt a lot during my journey in completing my study, and I strongly believed that our love and trust have grown stronger than before.
5 Statement of candidate contribution The present study investigated on the development of the cost-effective VEMPs. The LabView program that was used to develop our personalized software was developed solely by Dr Robert Patuzzi. The external portable amplifier was also created by Dr Robert Patuzzi, while the rest of the hardware used for both the cvemps and ovemps was developed by the present author, with the advice from Dr Robert Patuzzi. All the experiments in normal and pathological subjects were solely conducted by the present author. The control experiments was also conducted by the present author, with occasional advice from Dr Robert Patuzzi.
6 Table of Contents Abstract Acknowledgements Statement of candidate contribution Table of Contents Abbreviations List of Figures List of Tables References Appendix page 1. General Background of the Vestibular System and General Thesis 1-18 Introduction 1.1 The anatomy and physiology of the human s vestibular 2 system 1.2 The vestibular hair cells (sensory organs) Central and motor output of the vestibular system Vestibular reflexes Vestibulo-ocular reflex (VOR) Vestibulo-spinal reflexes (VSR) Vestibulo-collic reflex (VCR) Vestibular function The vestibular test battery General thesis introduction Software and Hardware Used During Experimentation and Data Collection 2.1 Introduction Software developed and used for experimentation and 20 data collection 2.3 Hardware for experimentation and data collection Acoustic stimuli for cvemps and ovemps 29 and data collection Stimuli for palm-pulse cvemps and finger-tap 30 cvemps and ovemps Data acquisition and EMG recordings Calibration Acoustic calibration for the ER3A 31 inserts earphones Piezo-electric transducer calibration Summary 40
7 3. cvemp and ovemp Testing Development and General Methods Introduction Initial skull-tap cvemps stimulator s development Initial skull-tap stimulation trials Using the tester s palm as stimulator Tendon hammer Pendulum-like stimulator Finger-taps Three-dimensional (3D) 50 accelerometry 3.3 Summary for the skull-taps stimulators' investigation Acoustic click stimulation Investigating technical aspects effecting the cvemps 51 and ovemps Filter Effect cvemps electrodes montage ovemps electrodes montage Control studies to verify vestibular origin Trigeminal responses on the cvemps Materials and Methods Electrical stimulation and recording 59 of the response Results Summary trigeminal responses on 68 the cvemp responses Blink response and the ovemp General test methodology General recording preparation Muscle activation Acoustic stimulations Skull-taps Stimulation sites Cervical vestibular evoked myogenic potentials (cvemps) Introduction Background of cvemps Review of different VEMPs stimulation methods Details of click acoustic stimulation Short tone bursts acoustic stimulation Galvanic vestibular stimulation Bone conduction vestibular stimulation Details of skull-tap cvemps Summary of skull-tap study Electrode placement of the cvemps 89
8 4.3.7 EMG monitoring and activation of SCM muscle 90 in cvemps Acoustic versus skull-tap cvemps Material and Methods Subjects General recording preparation SCM muscle activation Acoustic cvemps Palm-pulse cvemps Finger-tap cvemps Results in normal subjects Click cvemps Palm-pulse cvemps Response latencies Response amplitudes Finger-tap cvemps Number of response averages Details of the finger-tap cvemps EMG activation levels on finger-tap 121 cvemps Control measurements Discussion on the results obtained in normal subjects Responses of acoustic cvemps Rational for the tap sites for the palm-pulse and 129 finger-tap cvemps The palm-pulse cvemps The finger-tap cvemps Bilateral vestibular loss subjects Results and discussion from bilateral losses 134 subjects 5. Ocular vestibular evoked myogenic potentials (ovemps) Introduction Extraocular muscles and its relations to the vestibular 140 system 5.3 Historical background of ovemps Acoustic ovemps in normals Skull-tap ovemps in normals ovemps responses in neurectomy patients Electrode placement and eye gaze in ovemps Materials and methods Subjects General recording preparation Extraocular muscle activation Acoustic ovemps 151
9 5.4.5 Finger-tap ovemps Control measurements Results in normal subjects Acoustic click ovemps in healthy subjects Finger-tap ovemps Number of response averages Details of the finger-tap ovemps Control measures Discussion on the results obtained in normal subjects Responses of acoustic ovemps Responses of the finger-tap ovemps Origin of the peaks of the ovemps responses Unilateral vestibular neurectomy subjects Results from unilateral neurectomy subjects Acoustic click ovemps Finger-tap ovemps Discussion on the results obtained from the unilateral 200 neurectomy subjects Acoustic ovemps Finger-tap responses in neurectomy subjects Summary Conclusions Introduction and review of initial hypothesis Our electrode montage during the cvemps and 208 ovemps recordings would also give similar responses as others who used different electrode montages Our developed acoustic stimuli (i.e. 0.5 ms click 210 stimulus) would be able to evoke the VEMPs responses in the same way as the more established acoustic stimuli (i.e. 0.1 ms click stimulus), if not better The recorded cvemps and ovemps responses 211 represent the true vestibular responses The palm-pulse and the finger-taps would be 215 able to evoke the vestibular responses, down to the threshold level and up to maximal responses The skull-taps would produce whole-head 215 accelerations because the finger-taps caused a directed impulse to the skull All of the hair cell groups could be stimulated 217 specifically by tapping the skull at different stimulation sites from different directions
10 7. References Appendix Appendix Appendix Appendix Appendix Appendix Appendix 6 251
11 Abbreviations AC alternating current arb. arbitrary CCR cervicocollic reflex Ch channel contra contralateral COR cervico-ocular reflex CSR cervicospinal reflex CT computed axial tomography cvemps cervical vestibular evoked myogenic potentials db Decibels db nhl Decibel normal hearing level DC direct current EAC external auditory canal EAM external auditory meatus ECG electrocardiography EMG electromyogenic ENG electronystagmography GP general practitioner GVS galvanic vestibular stimulation HC hair cell IAM internal auditory meatus ipsi ipsilateral kg kilogram LARP Left Anterior-Right Posterior canal ma milliampere ma milliampere MAS mastoid mm millimeter MRI magnetic resonance imaging ms millisecond n negative peak nhl normal hearing level No nose bridge ovemps ocular vestibular evoked myogenic potentials p positive peak pp peak-to-peak PTA pure tone audiometry RALP rms SCC SCM T3 T4 VCR VEMPs VNG VOR vs. VSR Right Anterior-Left Posterior Canal root mean square semicircular canal sternocleidomastoid 2 cm above left pinna 2 cm above right pinna vestibulo-collic reflex vestibular evoked myogenic responses video-nystagmography vestibulo-ocular reflex versus vestibulo-spinal reflex
12 Glossary LARP and RALP Zygomatic arch Both terms are used in describing angular axis directions for the SCCs. During LARP stimulation, the anterior canal on the left and the posterior canal on the right will be stimulated. During RALP stimulation, the opposite happened i.e. the anterior canal on the right and the posterior canal on the left will be stimulated. Also known as the check bone as it forms the prominence of the cheek.
13 Figures 1.1 The anatomical layout of the vestibular system in the temporal 3 bone 1.2 Micro-CT scan of the utricular macular Micro-CT scan of the saccular macular Organizations of the vestibular system and the orientation of 8 the vestibular hair cells 1.5 Block diagram illustrating the organization of the vestibular 12 system 1.6 VOR diagram A MVST diagram B LVST diagram Front panel for the custom-designed software Set tab window for the custom-designed software An example for the live recordings waveforms during palmpulse 25 stimulations 2.4 Raw and auto-normalized waveforms from a single subject 27 during the unilateral left SCM activation 2.5 Raw and auto-normalized waveforms from a single subject 28 during ovemps recordings 2.6 Block diagram for the hardware used for the acoustic cvemps 29 and ovemps stimuli production and recordings 2.7 Block diagram for the hardware used for palm-pulse and fingertaps 30 stimuli production and recordings 2.8 Block diagram for the 0.5 ms rarefaction click calibration Growth functions and correlations between responses from the 34 finger sensor and the accelerometer on the head 2.10 Averaged waveforms from piezo-electric accelerometers on either the median finger and on the head surface Trigger and responses waveforms during palm-pulse 44 stimulations 3.2 Response waveforms during right SCM muscle activation with 47 tendon hammer stimulation 3.3 Trigger and responses waveforms from a pendulum-like 49 stimulation 3.4 cvemps waveforms during online adjustment of the filter 52 bandwidth and filter order 3.5 Raw and auto-normalized cvemps responses following 0.5 ms 54 click stimulation with the used of ours and others electrode montage 3.6 Raw and auto-normalized ovemps responses following 0.5 ms 56 click stimulation with the used of ours and others electrode montage 3.7 The trigeminal nerve and its three branches Block diagram during electrical stimulation and recording of 60 the responses 3.9 Responses of the right SCM muscle after electrical stimulation at right D8 62
14 3.10 Responses of the right SCM muscle after electrical stimulation at right cheek 3.11 Responses of the right SCM muscle after electrical stimulation at forehead centre 3.12 Blink and maximal ovemp responses with finger-taps at different stimulation sites 3.13 Stimulation sites, electrode montages and stimulations for our cvemps and ovemps protocol Ours and others cvemps electrode montages Directions of RALP and LARP from above a subject's head A Changes in cvemps peak latencies with increment of tap 100 intensity at the midline tap stimulation sites during bilateral SCM activation 4.3B Changes in cvemps peak latencies with increment of tap 102 intensity at the side tap stimulation sites during bilateral SCM activation 4.4 Changes in cvemps peak latencies with increment of tap 103 intensity during unilateral left SCM activation 4.5 Changes in cvemps peak latencies with increment of tap 104 intensity during unilateral right SCM activation 4.6A Tap intensity versus peak amplitudes for bilateral SCM at 106 midline stimulation sites 4.6B Tap intensity versus peak amplitudes for bilateral SCM at T3 108 and T4 stimulation sites 4.7 Tap intensity versus peak amplitudes for unilateral left SCM 109 activation 4.8 Tap intensity versus peak amplitudes for unilateral right SCM 110 activation 4.9 SCM responses after RALP tap with activated left SCM 112 muscles 4.10 cvemp responses from three healthy subjects following fingertap 115 stimulation during bilateral SCM activation 4.11 Overlaid waveforms responses from left and right SCM 118 following bilateral SCM activation 4.12 cvemp responses from three healthy subjects following fingertap 122 stimulation during unilateral left SCM activation 4.13 cvemp responses from three healthy subjects following fingertap 124 stimulation during unilateral right SCM activation 4.14 Raw and auto-normalized SCM responses during unilateral 125 right activation at three different EMG activation levels 4.15 Masked cvemp responses after application of a vibrator to the 127 nose 4.16 cvemp responses following different body posture at three 128 different stimulation sites 4.17 cvemp acoustic responses from two bilateral vestibular loss 136 subjects 4.18 Palm-pulse responses from two bilateral vestibular loss subjects 137 at midline site taps 4.19 Palm-pulse responses from two bilateral vestibular loss subjects at T3 and T4 site taps 138
15 5.1 Extraocular muscles on the left eye from both the lateral and 141 frontal view 5.2 Growth of trigger signal and ovemps intensity functions from 153 a subject following Oz finger-taps 5.3 Effect of averaging ovemps responses at right mastoid 158 stimulation sites 5.4 Sample data illustrating the growth of averaged ovemps from 162 one healthy subject 5.5 Dot plots of the normalized peak-to-peak ovemps amplitudes 164 from three healthy subjects 5.6 Dot plots of the ovemps peak-to-peak latencies from three 166 healthy subjects 5.7A Tap responses from the left and right eyes from four healthy 168 subjects at midline stimulation sites 5.7B Tap responses from the left and right eyes from four healthy 169 subjects at side stimulation sites 5.8 High quality ovemps responses by taps at various stimulation 171 sites from a single subject 5.9 Detailed midline taps from a single subject from both eyes ovemps responses from ten healthy subjects represented by 173 the dot plots 5.11 Masked ovemps responses from T4 and Oz taps from both 177 eyes 5.12 T4 and T3 ovemps responses versus pinna flick ovemps responses produced by dynamic rocking of the head 179 to the left and right 5.14 Acoustic ovemps responses from three unilateral neurectomy 190 subjects 5.15 Grand average ovemps waveforms from ten healthy subjects at the maximal stimulation A ovemps responses from Subject 1 and 2 overlying the grand 197 average responses from the healthy subjects 5.16B ovemps responses from Subject 3 and 3 overlying the grand 198 average responses from the healthy subjects
16 Table 3.1 Peak latencies following right D8 electrical stimulation Peak latencies following right cheek electrical stimulation Peak latencies following forehead centre electrical stimulation Peak-to-peak amplitudes and latencies with unilateral SCM activation and with ipsilateral 0.5 ms acoustic clicks stimulation from normal subjects 4.2 Relative auto-normalized amplitude values for the midline, side and angular taps following bilateral muscles activation 4.3 Latency values for the midline, side and angular taps following bilateral muscles activation Extraocular muscles and their insertion to the eye The involvement of the extraocular muscles during movements of 143 the eyes 5.3 Peak-to-peak amplitudes and latencies of the ovemps after 156 monaural 0.5 ms acoustic clicks stimulation from normal subjects 5.4 Mean and standard error of mean for the amplitudes and latencies of 174 the ovemps responses from ten healthy subjects 5.5 Peak amplitudes and latencies values for acoustic ovemps in three 191 unilateral neurectomy subjects 5.6 Grand average amplitudes and latencies for acoustic ovemps from ten healthy subjects 192
17 Chapter 1 General Background of the Vestibular System and General Thesis Introduction
18 1. General Background of the Vestibular System 1.1 The anatomy and physiology of the human s vestibular system In this part of the report, a brief overview of the anatomy and physiology of the human s vestibular system is presented. As known, the vestibular system is housed together with the cochlea in the inner ear. This system, which is comprised of five receptors on each side of the temporal bone, is anatomically aligned to form mirror-images of each other. The semicircular canals (SCCs) and the otolith organs complement each other physiologically and functionally. The symmetrical structure of the left and right SCCs allows them to work together as conjugate pairs to harmonize or complement each other. Each horizontal (or lateral) canal pairs with the opposite canal of the same plane, and working as a push-pull system. The anterior (or superior) canal is paired with the posterior (or inferior) canal of the opposite side, and vise versa (Figure 1.1 A). Della Santina et al., (2005) had proven through the use of 3-D CT scan technology that the human canals are angled equally (the angle between the anterior and horizontal SCC was approximately 90.6 while the angle between the horizontal and posterior SCC was 90.4 ). Even though anatomically the layout of these canals is simple because they are mirror images of each other, Furman et al. (2003) have reported that head movements in any particular directions will stimulate one or more canals. On the other hand, Honrubia et al. (1999) believed that due to the distinctive position of these canals, any whereabouts of the head would at least stimulate two canals, but most often all three. Thus, we could say that these canals are stimulated in a complex and indirect manner. The anterior canal is inclined 30 from the horizontal plane (Jacobson et al., 1999), and slanted 45 with respect to the coronal plane (Goldberg et al., 2000). From Figure 1.1 B, we could see that the anterior canal lies on the roof of one of the otolith organs, (i.e. the utricle) while the posterior canal is angled downward in the opposite direction, and positioned laterally behind the utricle. Each of these canals has its own sensory organs, which is positioned at the dilated end of each canal. These dilated portions are called the ampullae 1. The utricle and the saccule together form the otolith organs on each side of the temporal bone. The utricle is oriented in the horizontal plane, and lies in the elliptical 2 recess on the medial wall of the vestibule. On the other hand the saccule, 1 More explanation about the sensory organs of the SCCs can be found in Section The utricle appears as an oval depression on the roof on the medial wall of the vestibule. 2
19 A A Anterior canal plane Horizontal semicircular canal Anterior semicircular canal Temporal bone 90 Posterior semicircular canal Foramen magnum Posterior canal plane (from Irvine, 2006) B B Crista within ampulla of semicircular duct Anterior Vestibular branch of vestibulocochlear (VIII) nerve Posterior Lateral vestibulocochlear (VIII) nerve Facial (VII) nerve Cochlear branch of vestibulocochlear (VIII) nerve Scala tympani Cochlear duct Macula within utricle Scala vestibuli Macula within saccule Ductus reuniens Cochlea Lateral Vestibular membrane Cochlear duct Medial (from Irvine, 2006) Figure 1.1: A) The anatomical layout of the left and right SCCs housed in the temporal bone. These canals work together as conjugate pairs in a push-pull system. The anterior canal is paired with the posterior canal of the opposite side and vise versa. The horizontal canal pairs with the opposite canal of the same plane. B) The utricle and saccule of the otolith organs position in relation to the SCCs. The sensory organs of the otolith are housed within the maculae, while the sensory organs of the SCCs are housed within the ampullae. The membranous labyrinth of the saccule has a closed connection with the cochlea via the ductus reuniens. Both the cochlea and the vestibular organs are innervated by the vestibulocochlear component of the VIII nerve. 3
20 which lies in the spherical 3 recess on the medial wall, is more vertically aligned. Earlier researches by Igarashi (1967), Sasaki (1970) and Takagi et al. (1988) have described that the otolith organs were just a plane or the formation of a few planes. On the other hand, Naganuma et al. (2001; 2003) in their three-dimensional analysis of the human saccular macula and utricular macula revealed that both the saccular and utricular maculae in humans are a curved structure of an ellipsoid, and not just a simple plane. These findings were later supported by the animal model studies conducted by Uzun-Coruhlu et al. (2007a), who have confirmed that the utricular and saccular maculae are curved structures and not just a simple plane. Similar with the SCCs, the otolith organs also have their own set of sensory organs, which is situated in the maculae of the saccule and the utricle 4. As early as 1966, Spoendlin has reported that the otolith organs are attached on a rigid and non-compliant bony surface. This was the general accepted belief for years because the investigations of the morphology and physiology details of the otolith organs are difficult as they are embedded in the very hard petrous part of the temporal bone (Lindeman, 1969). In an attempt to establish a new anatomical model of the inner ear, in particular the utricle and saccule of the otolith organs, Uzun-Coruhlu et al. (2007b) utilized a new visualization method i.e. the X-ray micro-tomography procedures which enabled more detailed 2D and 3D visualizations. Their study was initially based on a guinea pig model and once it was established, the method was applied to the human model. They discovered that the utricular maculae in both species are attached to the non-compliant bony surface at the anterior region only, while the posterior region is essentially floating in fluid. This is because the posterior part is attached to a plate of supporting tissue which in turn is attached to the membranous wall of the labyrinth (Figure 1.2 A and B). On the other hand, they found that the saccular maculae are attached to the curved bony surface of the temporal bone and this finding agrees with other earlier findings (Figure 1.3 A and B). These recent anatomical findings by Uzun-Coruhlu et al. (2007b) proposed to us that the response of the utricular macular to a particular force involved a more complex deflection of the basement membrane, and not just simple and direct linear forces. Thus, interpretation of the utricular macular responses should be interpreted 3 The saccule appears as a rounded depression on the medial wall. 4 More explanation of the maculae of the utricle and saccule are in Section
21 A 0.5 mm B utricular macular anterior anterior posterior posterior bone medial lateral (adapted from Uzun-Coruhlu, 2007b) 100 µm Figure 1.2: Micro-CT scan of the utricular macular. A) The region within the box in the large panel on the left illustrates the location of the utricular macula of a human while the insets on the left side represents every 4 th successive section (which represents a distance of µm) of the utricular macula. It is clear from here that the utricular macula is only attached to the bone (shown as grey in this figure) at the anterior most portion. B) This digitally extracted 3D images of the utricular macular of a guinea pig clearly proved that the utricular macular is attached to bony surface only at the anterior region (Uzun-Coruhlu et al., 2007b). 5
22 A 0.5 mm B saccular macular posterior anterior bone 100 µm (adapted from Uzun-Coruhlu, 2007b) Figure 1.3: Micro-CT scan of the saccular macular. A) The region within the box in the large panel on the left illustrates the location of the saccular macula of a human while the insets on the left side represents every 4 th successive section (which represents a distance of µm) of the saccular macula. It is clear from here that the saccular macula is attached to the non-compliant bony surface throughout its entire surface (shown as grey in this figure). B) This digitally extracted 3D images of the saccular macular of a guinea pig clearly proved that the whole structure is attached to the hard bony surface (Uzun-Coruhlu et al., 2007b). 6
23 with caution as a linear stimulation would not only involve responses from the individual sensory cells but also from the deflections of the entire basement membrane of the utricular macular. We know that both the cochlea and the vestibular system have very close connections, via the inner ear fluids and also the blood supply. From Figure 1.1 B, note that the membranous labyrinths of the saccule are interconnected with the cochlea via a duct, named the ductus reuniens. Additionally, both the hearing and balance organs are innervated by the vestibulocochlear component of the VIII nerve. However, this nerve is split into two different branches to supply the hearing and balance organs individually (i.e. the cochlear branch and the vestibular branch). From 1.1 B, both the cochlear and vestibular branches enter the vestibule and the cochlea through the internal auditory meatus 5. The anterior and horizontal SCCs, together with the utricle are innervated by the superior branch of the VIII nerve, while the inferior branch supplies the posterior SCC and the saccule. Due to the close communication between the cochlea and the vestibular system, it is common that diseases or disorders affecting the cochlea also affect the vestibular system. 1.2 The vestibular hair cells (sensory organs) The vestibular hair cells are located in the ampullae of the SCCs and the maculae of the otolith organs. Wersäll (1956) and Lindeman (1969) in their early studies in the guinea pig SCCs and otolith organs found that there are two types of hair cells in the vestibular system, namely Type I and Type II hair cells. Later researchers confirmed that both Type I and Type II hair cells are found throughout the sensory organs of the SCCs and otolith organs in the mammals (Goldberg et al., (2000); Lysakowski et al., 2004). The Type I hair cells are larger, and flaskshaped, while Type II hair cells are cylindrical-shaped (Figure 1.4 A and B). The Type I hair cells are more inclined to be in the centre region of the SCCs ampullae and the otolith organs maculae. This means that for the maculae of the utricle and saccule, the Type I hair cells are located near the striola region. On the other hand, the Type II hair cells are present in the peripheral region of the sensory organs. From Figure 1.4 C, we see that the orientation of the hair cells in the utricle and saccule are different, and they are more polarized than the SCCs. The direction 5 Note that the facial (VII) nerve is also in a close connection with the VIII nerve and it enters the vestibule and the cochlea through the internal auditory meatus. 7
24 A A crista crista ampulla cupula hair bundles nerve fiber hair cells utricle macula saccule enlargement of macula otoconia Otolith stereocilia kinocilium Type II hair Type I cell hair cell supporting nerve fiber basement cells membrane Type I B enlargement of crista enlargement of macula (from Encyclopaedia Britannica Online, 2009) Type II Kinocilium Stereocilia C Striola Posterior Superior Superior Anterior Medial Striola Ampulla of the SCC Anterior Lateral Inferior Posterior Saccule Utricle Maculae of the otolith organs (from Fitzpatrick, 2001) Figure 1.4 8
25 Figure 1.4: Organizations of the vestibular system. A) The sensory ends of the SCCs (also known as crista) are located in the dilated end of each canal, known as ampullae. While for the utricle and saccule, their sensory cells are contain within the maculae. The hair cells in the SCC s ampullae are embedded by gelatinous diaphragm (cupula) which expands from the floor of the ampulla till the roof end. The hair cells of the utricle and saccule are housed in the maculae with otoconia (calcium carbonate crystals) sitting on top of them. Both the cupula and the otoconia act as weight to the hair cells. B) The Type I hair cells are flask-shaped and larger, while the Type II are cylindrical-shaped. The hair cells are arranged in rows with increasing length (the tallest cilia named kinocilium, while the rest named stereocilia). C) The orientation of the hair cells in the otolith organs are different than the SCCs because they have a more polarized structure. The arrows indicate the most sensitive direction for individual hair cells on the surface of the ampulla and maculae. At the centre of each macula is a region called striola where it divides the hair cells in two different groups. It is where the orientation of the hair cells changed abruptly (Lindeman, 1969; Rosenhall, 1972). 9
26 of the arrows in the Figure indicates the most sensitive direction for the individual hair cells. The hair cells of the utricle are directed towards the striola, which causes them to be most sensitive to horizontal movements. On the contrary, the saccular hair cells are directed away from the striola and cause them to be most responsive to vertical movements. Any stimulation of head movements in any particular direction will cause the hair bundle to move in the opposite direction owing to the inertia of the otoconia. It is well known that the SCCs are responsive to angular acceleration of the head. In contrast, the otolith organs detect movements in relation to gravity, the pitch (X axes) and roll change (Y axes) and linear acceleration of the head. The utricle is solely responsible for detecting motion in the horizontal plane (i.e. leftright movement) while the saccule is responsible for the vertical movement (i.e. up-down movement). It is also known that both the utricle and saccule are responsive to movement in the naso-occipital plane or forward-backward movement (Furman et al., 2003). Also, because of their ellipsoid curved structure, the utricle and saccule in humans are thought to be more sensitive, and capable of identifying a much wider range of linear acceleration (Naganuma et al., 2001; Naganuma et al., 2003). This suggest to us that any particular linear stimulation not only capable in stimulating a particular group of otolithic hair cells but the stimulation could stimulate several groups simultaneously and in utricular macular, the stimulation could also caused deflection of the entire basement membrane (Uzun-Coruhlu et al., 2007b). Despite all these facts, Goldberg et al., (2000) recently showed that the hair cells are even more complex, and responded differently to the same stimulus. Lysakowski et al., (2004) showed that the Type I hair cells are innervated by irregularly firing neurons, while the Type II are innervated by regularly firing neurons. In the presence of a stimulus, the irregular neurons (from Type I hair cells) response at the onset, while the regular neurons (from Type II hair cells) response in a sustained manner. These observations are important because the irregular afferents project mainly to the vestibulo-spinal system, while the regular afferents to the oculomotor system 6. However, Curthoys (2007) has also suggested that because of the complexity of the anatomy of the otolith organs and the SCCs, there is high possibility of considerable overlap between the projections of the irregular and regular afferents to both the vestibulospinal and oculomotor system. But, this statement has yet to be investigated. 6 The vestibulo-spinal system and the vestibulo-ocular system are discussed in Section
27 1.3 Central and motor output of the vestibular system From Section 1.1 and 1.2 above, we know that the vestibular sensory organs are polarized and are excited in a very systematic but complex manner. From the peripheral organs, information regarding any head movements is conveyed by the vestibular component of the eighth nerve to the brainstem level. The vestibular nerve synapses on cells in the vestibular nuclei (the superior, inferior, medial and lateral nuclei) and also in the cerebellum. The cerebellum is unique in the sense of its function in the vestibular system. Although it is not involved directly in the vestibular reflexes, it is a major receiver for all of the output from the vestibular nucleus complex. Thus, removal of the cerebellum causes impairment of the vestibular reflexes, because these reflexes become ineffective and uncalibrated (Honrubia et al., 1997). The vestibular nuclei not only receive information from the vestibular periphery, but also from the hearing, vision and somatic divisions of the sensory organs. The motor output of the vestibular system influences the motor output of the eye (i.e. the extraocular muscles of the eyes), and also influences the spatial orientation, the whole body and head, and neck stability in relation to ones environment. Figure 1.5 illustrates the organization of the vestibular system, from its sensory input to the central processing, and finally the output to the motor system. 1.4 Vestibular reflexes The integration of all the sensory organs, central and motor outputs of the vestibular system enables the vestibular system to have its own reflexes [i.e. the vestibulo-ocular reflex (VOR), vestibulo-spinal reflexes (VSR) and the vestibulocollic reflex (VCR)], as summarized below Vestibulo-ocular reflex (VOR) The VOR is a mechanism that enables the eyes to stay on target even though the head is moving. It functions to stabilize the eyes in response to both angular (Aw et al., 1996; Paige, 1989; Suzuki et al., 1964) and linear head disturbances (Angelaki, 1998; Lichtenberg et al.,1982; Schwarz et al.,1989). This mechanism is possible because of the capability of the eyes to move in an almost equal and opposite direction to the head movement. From Figure 1.6, the head is 11
28 Visual Vestibular Primary processor (vestibular nuclear complex) Motor neurons Eye movements Positional movements Proprioceptive Adaptive processor (cerebellum) (adapted from Hain et al., 2007) Figure 1.5: Block diagram illustrating the organization of the vestibular system. Refer Section 1.3 for more explanation. Left eye lr mr Right eye VI III vn mlf 8th Left horizontal canal Right horizontal canal (from Desmond, 2004) Figure 1.6: Diagram of the VOR which is vital in maintaining stable vision during head movements. The SCCs components compensates for the angular VOR during head rotation, while the otoliths components compensates for the linear VOR during head translation (Hain et al., 2007). 12
29 moving to the right, and owing to VOR, the eyes are turning to the left. The VOR reflexes start by the excitation on the right horizontal canal (indicated by the dark black line), which travels via the VIII nerve to the vestibular nucleus (VN) in the brainstem. It then crosses to the contralateral abducens nucleus of the VI nerve and travels along to the contralateral lateral rectus (LR) of the left eye. At the same time, the ipsilateral medial rectus (MR) muscle is also excited via the same excitation which crossed through the medial lateral fasciculus (MLF). Concurrently, the inhibition mechanisms apply on the left horizontal canal, with similar nerve relays (shown by the grey line in Figure 1.4). Besides these extraocular muscles, there are also other extraocular muscles (i.e. the superior and inferior rectus, and the superior and inferior oblique, which also play vital role in the VOR 7 ) Vestibulo-spinal reflexes (VSR) Besides the VOR, the VSR also plays a major role in our position sense in space because it enables us to control our head and limb position as well as our body or trunk stability in space. From Figure 1.7 A and B, the VSR has two descending tracts from the brainstem [i.e. the medial vestibulo-spinal tract (MVST) and the lateral vestibulo-spinal tract (LVST)]. The MVST has a bilateral pathway (but its ipsilateral projections are more pronounced), and it innervates the cervical and the upper thoracic spinal cord, while the LVST influences the ipsilateral limb muscles as it course through the uncrossed pathways through the entire spinal cord (Highstein et al., 2006; Schor et al., 1988; Wilson et al., 1979) Vestibulo-collic reflex (VCR) This reflex functions to neutralize the movement sensed by the SCCs and the otolith as this reflex acts on the neck muscle to stabilize the head (i.e. it acts as the righting reflex). Its pathways have yet to be understood, but Pozzo et al. (1994) suggest that the VCR is mediated through the otolith organs and the MVST. 7 More about the extraocular muscles and its functions can be found in Chapter 5. 13
30 A Lateral Vestibular nuclei Superior Medial Inferior Vestibular nerve Vestibular ganglion Posterior SCC Horizontal SCC Level 4 Level 1 From hair cells in the SCCs, utricle and saccule Medial vestibulospinal tract (MVST) Anterior SCC Cervical Thoracic Lumbar (adapted from Resources, 2006) Figure 1.7 A: The VSR which acts to stabilize the body. A) The MVST has a bilateral pathway with a more pronounced ipsilateral projection (refer Section for more explanation). 14
31 B Vestibular nuclei Superior Lateral Medial Inferior Lateral vestibular nucleus Vestibular nerve Posterior SCC Vestibular ganglion Horizontal SCC Level 4 Level 1 From hair cells in the SCCs, utricle and saccule Lateral vestibulospinal tract (LVST) Anterior SCC Cervical Thoracic LVST Lumbar (LVST) in the ventral funiculus uncrossed (adapted from Resources, 2006) Figure 1.7 B: The VSR which acts to stabilize the body. B) The LVST with its ipsilateral pathway (refer Section for more explanation). 15
32 1.5 Vestibular function Generally, the SCCs detect angular acceleration, while the otolith organs detect linear acceleration and gravity. As a whole, the vestibular system helps us to navigate ourselves in space (i.e. it helps us to maintain our balance or equilibrium, and our well being in our surroundings). It plays an important role in controlling our posture. Without doubt, the system deals with a complicated task, which involves the vestibular sensory organs interpreting and sending signals to the brain, providing the position sense of the body in relation with the body motions. The vestibular system is also responsible for gaze stability, despite head and body motion. All this is possible via its reflexes (i.e. the VOR, VSR and the VCR). 1.6 The vestibular test battery Ideas regarding the vestibular function have been developed since the late eighteenth century. Scientists such as Erasmus Darwin, Pierre Flourens, J.E. Purkynê and Hermann von Helmholtz are examples of leaders in the early thinking about the vestibular and ocular motor function (Furman, 1999). The common vestibular test battery is comprised of a number of tests (i.e. electronystagmography (ENG) or video-nystagmography (VNG), rotational or Bárány chair and posturography). All of these tests mainly concentrate on the diagnosis of the SCCs, specifically the horizontal canal. For years, the caloric and rotational chairs (of the ENG or VNG) have been accepted as the sole representative of the physiologic health of the entire membranous labyrinth, despite the fact that these tests only diagnose the physiology of the horizontal canal (Desmond, 2004a; Desmond, 2004b; Jacobson et al., 1997). Not only that, but the ENG and VNG are also time-consuming and costly. Despite the limitation of this conventional vestibular assessment, they have been used for years as the main vestibular test battery. The evaluation of the otolith organs have not been examined until recently, after the discovery of the vestibular evoked myogenic potentials (VEMPs) of the neck muscle, specifically from the sternocleidomastoid muscle (SCM) of the neck following the acoustic stimulation by Colebatch et al. (1992; 1994). This test was later termed the cervical vestibular evoked myogenic potentials (cvemps). It has been suggested by many researchers that recording of sound-evoked cvemps may supplement the current test battery by providing diagnostic information about saccular or inferior vestibular nerve function (Akin et al., 2004; 2003; Colebatch et 16
33 al., 1994; Zhou et al., 2004). Following the use of the acoustic stimulation on cvemps, Halmagyi et al. (1995) introduced the technique of tapping on the midline of the head, just above the hairline by using the clinical reflex hammer. Since then, Rosengren et al. (2005) have pioneered the technique of evoking responses from the utricle and/or assessing the function of the superior vestibular nerve. They used bone conducted sound during that initial test. Other researchers have used different methods and at the same time measured the responses from the utricle. 1.7 General thesis introduction Almost all of the established vestibular test battery is expensive, cumbersome and lack flexibility. We started our research with the aim of developing the VEMP test as a cost-effective diagnostic tool, and as a screening test. With this aim in mind, we decided to take a totally different approach to that used by others. This required developing our own software and hardware, and developing a test protocol for the screening and diagnostic purposes. After we had successfully developed and familiarized ourselves with the software and hardware, we started the development of the test protocol with the use of palm-pulses, followed by finger-taps as stimuli in normals and pathological adult subjects. We also developed different acoustic stimuli. We initially hypothesized that: 1. Our electrode montage during the cvemps and ovemps recordings would also give similar responses as others who used different electrode montage. 2. Our developed acoustic stimuli (i.e. 0.5 ms click stimulus) would be able to evoke the VEMPs responses in the same way as the more established acoustic stimuli (i.e. 0.1 ms click stimulus), if not better. 3. The recorded cvemps and ovemps responses represent the true vestibular responses. 4. The palm-pulse and the finger-taps would be able to evoke the vestibular responses, down to the threshold level and up to maximal responses. 17
34 5. The skull-taps would produce whole-head accelerations because the finger-taps caused a directed impulse to the skull. 6. All of the hair cell groups could be stimulated specifically by tapping the skull at different stimulation sites from different directions. We believe that with the success of our developed VEMP test, it can be used as a screening test that is reliable, portable, versatile and not cumbersome. Because the system is cost-effective and very cheap in comparable with other commercially available test instruments, we hope that our system will be further developed and will be used for teaching purposes in teaching labs, as well as it can be used by other professionals (e.g. audiologist, neurologist and physiotherapist) clinically. 18
35 Chapter 2 Software and Hardware Used During Experimentation and Data Collection
36 2. Software and Hardware Used During Experimentation and Data Collection 2.1 Introduction This chapter describes the main software and hardware developed for the recording of both cervical vestibular evoked myogenic potentials (cvemps) and ocular vestibular evoked myogenic potentials (ovemps). We also describe the calibration process carried out during the software and hardware development. 2.2 Software developed and used for experimentation and data collection One data acquisition programme was custom-designed by Dr. Robert Patuzzi, using LabVIEW software version 7.0 (National Instruments Corp., TX, USA) 1. Laboratory Virtual Instrumentation Engineering Workbench or LabVIEW is a programme which uses graphical language named G. LabVIEW incorporates a set of programmes called virtual instruments (VIs), and each has its own block diagram, front panel and connector panel. Before running, parameters were written into a Microsoft Notepad text file selected on the software front panel. These parameters served as defaults [at section (a) of Figure 2.1] and set tabs (Figure 2.2) of the front panel. The electromyographic (EMG) signals were pre-set as Channel 4 (Ch 4) and Channel 5 (Ch 5), amplified by 10, and bandpass filtered (second-order Hz). The trigger pulse stimulus 2, which was set at Ch 3, for palm-pulse cvemps and fingertap ovemps was amplified by 4 and also bandpass filtered in the software between Hz. The acoustic stimulus trigger pulse for both cvemps and ovemps had an amplification of 1.0 and no filtering was applied. The default settings were the same for all experiments, unless specified otherwise. The amplitude and timing of the first and dominant peak of each trigger pulse from the stimulus was detected by the software, and all EMG activity and trigger waveforms were time-shifted equally, with the peak of each trigger pulse defined as zero time. All waveforms were displayed in real time. The screen capture of a typical front panel of the programme used for all the experiments is shown in Figure 2.1, while an example of an online recording front panel is shown in Figure 2.3. The front panel of the 1 The software was named Impulse Testing Multi Averager version 253 (ImpulseTestingMultiAv253.vi) 2 Piezo-electric sensor was used as stimulus trigger for both palm-pulse cvemps and finger-tap cvemps and ovemps, which enabled the tester to vary the stimulus strength. 0.5 ms click acoustic stimulus was used for both acoustic cvemps and ovemps, which was discussed in later Section
37 software was divided into eight different sections (as shown in Figure 2.1). Some points of interests from the eight sections are: (a) An input tab which features the defaults parameters of the recordings. Here, the electromyographic (EMG) and stimulus recording channels were chosen and pre-set at alternating current (AC) mode before each testing session. The filters bandwidths were set as stated earlier. Note also the set tab, which also features the remaining recording parameters (shown in Figure 2.2). (b) Raw tab with a display panel, which provides visual feedback for the live and recorded EMG. The threshold value 3 for the stimulus was pre-set at 0.16 g units (with 1 g = 9.8m/sec²). (c) The software also allows the testing to be done via manual or automatic mode. This was done by checking the autoplot button (abbreviated as autoplt on the front panel). (d) Nine different display panels for the recorded waveforms, which function as visual feedback during testing. For both palm-pulse cvemps and finger-tap cvemps and ovemps, if the manual mode was selected the software averaged the incoming recorded waveforms to a particular waveform buffer regardless of tap strength. The resultant waveforms were displayed in the chosen display panel. If the automatic mode was selected, the software automatically sorted the incoming waveforms to different waveform buffers based on the measured tap strength. This process yielded nine waveforms, based on the pre-programmed threshold ratios, which represent the stimulation strength intensity series (explained in a later section). As for acoustic cvemps and ovemps, the software was always set at manual mode because only one stimulus intensity was employed, while for palm-pulse cvemps and finger-tap cvemps and ovemps it was always set at automatic mode unless otherwise specified. (e) x- and y-axis scale bar dialogue box for the nine display panels in (d). This dialogue box enabled manipulation of live recorded waveforms latencies and amplitude [x-axis was in milliseconds (ms) for and y-axis was in Volts (V)]. (f) Scale bar dialogue box to manipulate the amplitudes and latencies for the live recorded waveforms displays in (g). This scale bar enabled the 3 Similar stimulus pre-set threshold value was employed for acoustics as well as palm-pulse and finger-taps measurements. 21
38 tester to either re-group or resize the waveforms from the two channels during live recordings. (g) Display window for two selected channels (i.e. Ch A and Ch B). (h) EMG meters or bar chart to monitor the online activation of the recorded muscles as root mean square (RMS) EMG. The meters act as a self-monitoring mechanism that was able to assist the subjects in keeping the SCM muscle activation consistent. The remaining default parameters appeared on the set tab of the front panel (shown in Figure 2.2). The pre-trigger and post-trigger accept were set at 50 ms and 900 ms, respectively, and the waveform acquisition and averaging was done over a 200 ms post-peak window, with a second-order filter. As mentioned earlier, two channels (Ch 4 and Ch 5) were set as the recording EMG channels (set as Ch A and Ch B dialogue box), while the other two remaining channels available were not used unless stated otherwise. The maximum threshold ratios (abbreviated as maxthr in the dialogue box) were set at 2.0. The threshold ratios dialogue box was set from to 1.0. This enabled manipulation of the pre-programmed intensity series for the palm-pulse and finger-tap stimuli. Other tabs available on this window were not used. The averaged waveform responses were later saved, and automatically loaded to a Microsoft Excel Workbook file. An example of a live recorded front panel is shown in Figure 2.3. In this Figure, left unilateral sternocleidomastoid (SCM) muscle activation was induced from a subject (indicated by the higher EMG meter on the left). This live recorded front panel allowed the tester to monitor the waveforms during recording and simultaneously manipulate the recorded results online. 22
39 (a) (d) (f) (c) (b) 0.16 (g) (h) (e) Figure 2.1: Front panel for the Impulse Testing Multi Averager version 253 (Impulse TestingMultiAv253.vi) custom-designed by Dr. Robert Patuzzi using LabVIEW software version 7.0 (National Instruments Corp., TX, USA). (a) Input tab with the channel (Ch), gain and bandpass filter functions. The software was designed with eight Ch in total (Ch 0- Ch7), with Ch 0 at the top panel and Ch 7 at the bottom panel. In this Figure, the gain for Ch 4 and Ch 5 were set at 10, with the filter bandwidth in between (10-600) Hz. The trigger stimulus Ch was set to Ch 3, with the gain set at 4, and filter bandwidth in between ( ) Hz.; (b) was the raw tab, with the waveforms display panel; (c) Autoplot or manual selections; (d) Nine display panels for the live recorded waveforms; (e) Scale bar for the display panels in (d). This scale bar enabled manipulation of the latency (ms) and amplitude (V) display; (f) Scale bar for display panel in (g); (g) Display panel that allowed both Ch 4 and Ch 5 to be overlapped and compared during live recordings, (h) EMG meters to monitor live EMG activation during recordings. There are two meters there that enabled both channels to be monitored simultaneously. 23
40 Figure 2.2: Set tab window for the front panel for the Impulse Testing Multi Averager version 253 (Impulse TestingMultiAv253.vi). The pre-trigger and posttrigger accept were set at 50 ms and 900 ms respectively. The waveforms acquisition and averaging was done over 200 ms window. The filter order chosen was 2. The threshold ratios were set between These ratios were actually the ratios applied for the palm-pulse or finger-tap strength during the automatic recordings. With these ratios, the recorded responses were slotted into different display windows on the front panel, as well as the results were slotted in the Excel worksheet. The maximum threshold was set at 2.0 g. 24
41 (c) (a) (d) (e) (b) 0.16 Figure 2.3: An example of live recordings waveforms, taken during palm-pulse stimulations, during unilateral sternocleidomastoid (SCM) muscle recordings. (a) Live recorded waveforms from Ch 4 and Ch5 and a waveform from Ch 3 i.e. the trigger stimulus waveform; (b) raw EMG waveforms (c) Nine display panels with the live recorded waveforms form Ch 4, Ch5 and Ch 3; (d) Display panel which allowed tester to overlap both Ch 4 and Ch 5 simultaneously. In this Figure, only Ch 4 waveforms were selected to be displayed; (e) EMG meters to monitor Ch 4 and Ch 5. 25
42 Two versions of the waveforms were loaded by the software (i.e. the raw waveforms and also the auto-normalized waveforms). The auto-normalized waveforms were actually the average of all the incoming raw waveforms at that average EMG tone over each 200 ms data epoch (this means that the autonormalized waveforms unit is actually in probability unit). The ability of the software to produce the auto-normalized waveforms partially compensated for any variations in muscle tone over the test period. This technique was very useful, especially during the cvemp recordings from the SCM, because the responses from the SCM muscles were often influenced by the muscle activation. The autonormalized application was also useful in reducing the variations in the ocular muscle tone during the eye gaze of the ovemp recordings. However, we found that there are no obvious and significant differences in the raw and autonormalized versions of the ovemps responses. The auto-normalized waveforms were stored along with the raw waveforms, and later automatically loaded to the Excel Workbook file. Examples of the raw versus the auto-normalized responses are shown in Figure 2.4 and Figure 2.5. From Figure 2.4, finger-tap cvemps were obtained from a subject while he was instructed to activate his left SCM muscle by turning his head to the right side. From this example, we can see that the autonormalized waveform from the left SCM was bigger than the raw waveform. On the other hand, there was no difference between the raw and auto-normalized waveforms for the right SCM. This is because only the left SCM was activated during recordings 4. The ability of the software in producing the auto-normalized responses from the raw responses is an advantage because during cvemps recordings, the variations in the SCM muscle tone was being corrected and this is evident from the example given in Figure 2.4. Thus, unless indicated, for the entire cvemps responses in this report, we analysed the auto-normalized responses and not the raw responses. On the other hand, we found that there is no significant difference between the raw and auto-normalized responses for the ovemps recordings. This is evident from the example given in Figure 2.5, where the amplitude of the waveforms for both the raw and auto-normalized responses is almost similar for both eyes. Thus, unless indicated, all the ovemps responses analysed in this report is actually the raw responses, and not the auto-normalized waveforms. 4 More detailed explanation and more sample data of the cvemp responses were discussed in Chapter 4. 26
43 Figure 2.4: One sample of data (with two replicates) from a single subject illustrating the differences between the raw and auto-normalized waveforms (n=160) from the left and right SCM, during the unilateral left SCM activation. Vertical dashed lines marked the stimulus peak which is at 0 ms. As predicted, the activated left SCM have bigger and more prominent responses than the inactivated right SCM. Note that the left auto-normalized waveforms have bigger amplitudes compared to the raw left waveforms, while for both right SCM raw and autonormalized waveforms, there was no difference in the waveforms amplitudes. This is because only the left SCM muscle was activated during the recordings. The bigger left SCM auto-normalized responses actually represent the corrected responses of the raw waveforms from any variations of the SCM muscles activation during recordings. 27
44 Figure 2.5: One sample of data (with two replicates) from a single subject illustrating the differences between the raw and auto-normalized responses (n= 50) from the right and left eye during ovemps recordings. The subject was instructed to gaze upward during recordings. It is obvious from this Figure that there are no significant differences in the raw and auto-normalized waveforms for both eyes. 28
45 2.3 Hardware for experimentation and data collection Acoustic stimuli for cvemps and ovemps and data collection The soundcard of a personal laptop computer was used to produce the output for the acoustic stimulus (0.5 ms monophasic clicks) 5 that was presynthesized as wave files using Sound Forge 4.5. These acoustic stimuli were then amplified by an external battery-operated amplifier (T-Amp), before being presented at 130 db SPL using EARTONE 3A insert earphones from EAR Auditory Systems to the left and right ears, separately. This portable amplifier was chosen because it was light and small (approximately 300g without the batteries). It was capable of supplying an output power of about 9 watt/channel with 4 AA batteries. The block diagram for the hardware used is shown in Figure 2.6. sound in amplifier in a box power in sound out Laptop T-amp trigger in power out trigger out insert earphones wire electrodes Figure 2.6: Block diagram for the hardware used for acoustic cvemps and ovemps stimuli production and recordings. (a) E-A-RTONE 3A insert earphones from E-A-R Auditory Systems (b) Battery-operated tripath amplifier (T-Amp) (c) A custom-built multi-channel AC amplifier established using a National Instruments USB6009 Data Acquisition (DAQ) system. This amplifier was enclosed in the box. 5 The reason of why 0.5 ms clicks was chosen as the acoustic stimulus was discussed in detail in Chapter 3. 29
46 2.3.2 Stimuli for palm-pulse cvemps and finger-tap cvemps and ovemps An inexpensive piezo-electric transducer (Figure 2.7) with a dimension of (in mm) 30Ø x 5H was used to synchronize averaging of responses, and to measure the timing and magnitude of the palm-pulse and finger-taps. The response from the piezo actually measures the deceleration of the tapping finger as it hit the skull, which was a function of the velocity of the hand, stiffness of the finger at the time of impact, and the mass and stiffness of the subject s head to a small extent (the head is approximately immobile). A detailed explanation on how the palm-pulse and finger-taps were delivered during recordings was presented in Chapter 3, Chapter 4 and Chapter 5. power out amplifier in a box Laptop power in piezo-electric wire electrodes Figure 2.7: Block diagram for the hardware used for palm-pulse and finger-taps stimuli production and recordings. (a) piezo-electric transducer (b) A custom-built multi-channel AC amplifier established using a National Instruments USB6009 Data Acquisition (DAQ) system. This amplifier was enclosed in the box. 30
47 2.3.3 Data acquisition and EMG recordings For the purpose of data acquisition and EMG recordings, a custom-built multi-channel alternating current (AC) amplifier (developed by Dr. Robert Patuzzi) was created using a National Instruments USB6009 Data Acquisition (DAQ) system. This USB was chosen because it was compatible with the LabVIEW software (Section 2.2). This custom-built amplifier provided 1000 times amplification to the incoming waveforms, and also allowed multi-channel simultaneous waveform acquisition and averaging, which was done at 5 khz sampling rate over a 200 ms window). As mentioned earlier in Section 2.2, the EMG signals were bandpass filtered in software (second order Hz), while the trigger stimulus was bandpass filtered between Hz. The amplitude and timing of the first and dominant peak of each trigger pulse from the piezo-electric transducer was determined by the software, and all EMG activity and trigger waveforms were time-shifted equally, with the peak of each trigger pulse defined as zero time Calibration Calibration was done for the 0.5 ms click stimulus, piezo-electric transducer and also the EMG levels. Besides the calibration processes that were done, we also conducted routine biological check for the software and hardware employed during testing. Troubleshooting was performed to check for any faulty parts. Once all the equipment was in optimum condition, the test began Acoustic calibration for the ER3A inserts earphones The block diagram for the acoustic calibration for the ER3A insert earphones are shown in Figure 2.8. The calibration was done using the 0.5 ms click generated by the sound card of the personal laptop (as explained in Section 2.3.1). The click stimuli were channelled via insert tip to the 2cc coupler, which was then coupled to the B&K condenser microphone. This condenser microphone, was connected to a pre-amplifier (which provided a 40 db gain to boost the acoustic output level), measured the output of the acoustic click in db SPL. Finally the condenser microphone was coupled to an oscilloscope. The db SPL level of the 0.5 ms click was then calibrated via this oscilloscope. 31
48 sound in amplifier in a box power in sound out Laptop insert earphones T-amp trigger in power out trigger out 2cc coupler condenser microphone oscilloscope 40 db gain pre-amplifier Figure 2.8: The block diagram for the 0.5 ms rarefaction click calibration. The stimuli were generated by the laptop sound card and further amplified by the T- amp. The insert tip of the ER3A insert earphone was coupled to the 2cc coupler, and later this coupler then coupled to the B&K condenser microphone. A 40 db gain was provided by a pre-amplifier connected to the condenser microphone. 32
49 Piezo-electric transducer calibration Figure 2.9 and Figure 2.10 presents averaged waveforms obtained simultaneously from two identical piezo-electric accelerometers. One was placed on the median finger of the tester and measured the impulse delivered by the finger to the subject s head (based on the deceleration of the finger). The other was placed on either the right mastoid (orthogonal to its surface) or just forward of T4 (allowing taps to be delivered at T4 itself). The response from these sensors was amplified with the bioamplifier (x100), software-filtered with a second-order bandpass filter (1 Hz to 600 Hz), and averaged using the positive-going peak of the finger signal to trigger the averaging (positive peak defined as 20 ms time). The responses were obtained with moderate finger taps to the head surface at the sites indicated, either at a nominally constant intensity (as judged by the tester and finger-sensor output), or over the intensity range used clinically (when in autoplot mode). Two important issues are apparent from these and similar waveforms not reported here. First, the response from the finger sensor was an accurate and reliable measure of the magnitude and timing of the mechanical impulse delivered by the taps and, second, the response from the finger sensor was a good indicator of the magnitude and timing of the acceleration of the head surface (although there were differences in the waveforms at the finger and around the head, as discussed below). As for the reliability of the response from the finger sensor, the waveforms showed little sign of smearing in time, in that the averaged waveforms were almost identical to individual (unaveraged) waveforms, and were very similar (apart from magnitude), regardless of the intensity of the taps or the tap site. The magnitude of the response from the finger sensor was also proportional to the transient movement at the skull surface. This proportionality can be seen in Figure 2.9 E, where the peak-to-peak amplitude of the head acceleration is plotted against the peak-to-peak amplitude of the signal from the finger sensor. The acceleration at the head surface was measured at either T4 (with taps delivered to the right mastoid; black circles), or at the right mastoid (with taps delivered to T4; open circles). Two replicate intensity functions were obtained for each, and data from both replicates were pooled. Most obviously, the stimulus (the finger signal) and the response (the head signal) were proportional over the entire range of tap 33
50 >1.6 > time (ms) time (ms) >1.6 > strength time (ms) time (ms) acceleration (arb. units) acceleration (arb. units) acceleration (arb. units) strength strength strength 2 head acceleration vs. finger tap strength T4 response with right mastoid tap R 2 = R 2 = finger tap strength (arb. units) acceleration (arb. units) acceleration (arb. units) A. C. T4 accelerometer with right mastoid tap B. T4 accelerometer with right mastoid tap Right mastoid accelerometer with T4 tap Right mastoid accelerometer with T4 tap D. E. Right mastoid response with T4 tap Figure 2.9: Growth functions and correlations between responses from the fingersensor and the accelerometer on the head. In this case two replicates were obtained for each monitoring site (T4 for A. and B. or right mastoid for C. and D.). The finger sensor signal in each case is shown in grey, and the head sensor signal is shown in black.note that the signal from the finger-sensor was a very reliable indicator of both the timing and magnitude of the head acceleration, as indicated by the maintenance of the accelerometer waveforms with increases in Figure 2.9 tap strength and in the excellent correlation between the finger-sensor signal and the signals from the head accelerometers (panel E. showing correlations between the peak-to-peak amplitude of the finger and head signals of about R 2 =
51 Figure 2.9: Growth functions and correlations between responses from the finger sensor and the accelerometer on the head. In this case, two replicates were obtained for each monitoring site (T4 for A and B or right mastoid for C and D). The finger sensor signal in each case is shown in grey, and the head sensor signal is shown in black. Note that the signal from the finger sensor was a very reliable indicator of both the timing and magnitude of the head acceleration, as indicated by the maintenance of the accelerometer waveforms with increases in tap strength and in the excellent correlation between the finger sensor signal and the signals from the head accelerometers (panel E showing correlations between the peak-to-peak amplitude of the finger and head signals of about R² = ). 35
52 intensity used clinically, with a very high correlation between the two signals (R2= for both sets of data). Another point emphasized by these recordings is that, when tapping the head at various sites (e.g. No, Fz, Cz, Oz, T4, T3, left mastoid, right mastoid), in most cases the whole head moved as one, with almost identical accelerations measured at each site, apart from the differences due to the direction of the sensor relative the direction of the tap. The output from the sensor followed the expected cosine rule in its sensitivity, with accelerations along its sensitive access (orthogonal to its disk shape) producing large responses, but accelerations at right angles to this axis producing no signal. Apart from this expected variation in magnitude with sensor angle relative to tap direction, there was another difference between the waveforms measured at different sites around the head. The acceleration transients at the head surface were much larger (and sometimes more complex) when measured close to the tap site. This is illustrated in Figure 2.10, where acceleration waveforms at two sites (at T4 for A and B, or at the right mastoid for C and D) for taps at various sites are compared. Clearly the measured waveforms were very similar (apart from the expected variation in magnitude and polarity with the relative angle of the tap and the sensor), except when the tap was close to the accelerometer (waveforms marked with asterisks). The simplest explanation for this observation is that the head did not just move as a single entity when tapped, but also deformed locally near the tap site, just as a tennis ball deforms when hit with a racquet. That the time-course of the wave shapes recorded near the tap site was different from those measured at distant sites suggests that the skull surface not only deformed locally, but responded in a complex way, possibly resonating (compare the simple biphasic wave shapes recorded when the tap site was distant, with the multi-peaked waveform when the tap was near the recording site. While we have not investigated the details of this head deformation, it is clearly important in understanding the mode of stimulation of the vestibular system with finger-taps (or any impulse), because the local transient deformation may stimulate the hair cells in a manner different from any whole-head acceleration. There seem to be at least three possible stimulation mechanisms: whole-head translation, local-head deformation producing local accelerations of hair cells different on each side of the head, and acoustic shock waves or (transverse) travelling waves that travel around the skull and reach all vestibular hair cells, 36
53 but possibly stimulate them differently because of attenuations around the head or local acoustic compliances determining the effective stimulus strength. That local deformation did stimulate nearby vestibular hair cells via a mechanism different from whole-head acceleration seems clear from the ovemp waveforms reported later. For example, the ovemp waveforms produced by tapping at T4 were quite different (inverted and shifted in latency) from those obtained by tapping at the right mastoid a few centimetres away, despite the similar whole-head acceleration produced (as judged by the acceleration measured on the other side of the head at T3 or the left mastoid; Figure 2.10 C and D). Some of these differences may be due to a local rotation of the vestibular structures on the side of the head being tapped, with the rotation being different depending on whether the tap was delivered at T4 or on the right mastoid (possibly in opposite directions). Having said that, whole-head rotation does not seem to be the dominant stimulus, because tapping at the midline at No (nose bridge) and Fz (forehead) produced similar ovemp waveforms, even though they produce opposite rotations (nose down for No and nose up for Fz). Similarly, Oz taps that were forward directed and produced a nose-down rotation produced a different waveform again. The point here is that local deformation dominates whole-head translation, which dominates whole-head rotation. Importantly, similar results (head movements) would be obtained from any mechanical impulse, whether it came from a tendon hammer, a finger, a palm or an electromagnetic Minishaker. The complexity in the skull s response comes from its own mechanical properties (local compliance, damping and densities) which determine its local mechanical response. As long as the delivered impulse is brief relative to the skull s own natural response (i.e. the spectrum of the skull s natural response consists of lower frequency components than those in the stimulus impulse), then the skull determines the response waveform, not the stimulus. In this case the skull s response would be similar, regardless of the source of the stimulus impulse (as long as the impulse was delivered over a similar surface area). It is also relevant that the fine details of the delivered impulse (e.g. duration or precise waveform) does not determine the acceleration of the whole head, because the head s inertia is so large that its movement is a dramatically low-pass filtered version of the impulse stimulus. This is particularly true of the whole-head movement, and less so for the local surface deformations, which are likely to be more rapid, and therefore more responsive to the details of stimulus impulse. 37
54 A. B. T4 accelerometer responses No Fz T4 accelerometer responses No Fz acceleration (arb. units) * Cz Oz T4 T3 Left mastoid Right mastoid acceleration (arb. units) * Cz Oz T4 T3 Left mastoid Right mastoid sham sham tap site tap site time (ms) C. Right mastoid accelerometer responses D. No Fz time (ms) Right mastoid accelerometer responses No Fz acceleration (arb. units) * Cz Oz T4 T3 Left mastoid Right mastoid acceleration (arb. units) * Cz Oz T4 T3 Left mastoid Right mastoid sham sham tap site tap site time (ms) time (ms) Figure
55 Figure 2.10: Averaged waveforms from piezo-electric accelerometers on either the median finger (light grey waveforms in all panels, monitoring finger tap stimulus) and on the head surface (black waveforms, at T4 for A and B, or the right mastoid for C and D). Tap site for each waveform is shown: nose No, forehead Fz, crown Cz, back of head Oz, right T4, left T3, left mastoid Lm, right mastoid Rm, or sham with tapping on the tester's knee. The head-response waveforms were similar, except for their amplitude (which depended on angle of head-surface and accelerometer relative to the stimulus axis), and in the case where the tap site was very close to the monitoring site (waveform shown as grey dashed waveforms and asterisk). In this case the response of the head surface was generally larger and more complex than the responses when the tap site and monitoring site were apart. This suggests that the head surface was deforming at the time of the tap. The tap intensity in this case was kept approximately constant by the tester (which was confirmed by the similar magnitudes for the finger signal in each case). Note that right mastoid accelerometer was at abut 45 to the midline and detected lateral and front-back movement. 39
56 Overall, these results (and many more like them not reported here) indicate clearly that those tap sites that are remote from the vestibular end-organs are likely to produce the most homogenous whole-head accelerations, so that we can be confident that both the left and right vestibular organs receive the same acceleration stimulus. Conversely, those taps or impulses delivered close to the vestibular organs are likely to produce the most complex and localized stimulus to the nearby vestibular hair cells. While in some cases this local stimulus may make it difficult to determine which vestibular hair cells are stimulated (and how), there may be great advantages to being able to stimulate the left and right components of the vestibular system independently (just as unilateral caloric irrigation has advantages over bilateral Barany test) or air conduction hearing tests can be more local than bone conduction tests. 2.4 Summary We have successfully developed cost-effective software and hardware to be employed in the testing protocols for the cvemps and ovemps. Both the software and hardware were proven to be not only cost-effective, but also able to produce reliable and high-quality stimuli and responses rapidly and conveniently. 40
57 Chapter 3 cvemp and ovemp Testing Development and General Methods
58 3. cvemp and ovemp Testing Development and General Methods 3.1 Introduction The acoustic cvemp test was the first to be reported in 1992 and 1994 by Colebatch and co-workers, followed by the skull-tap cervical vestibular evoked myogenic potentials (cvemps), using the clinical reflex hammer by Halmagyi et al. (1992). Recently in 2005, just after we commenced our study, Rosengren et al. (2005) successfully evoked (extra)-ocular vestibular evoked myogenic potentials (ovemps) with bone-conducted tone burst stimulation of the skull. Having that in mind, our main objective during the cvemp and ovemp test development was to find other stimulation methods for both the skull-taps and acoustic VEMPs that were efficient, versatile, reliable and cost-effective. Second, we wanted to study other appropriate stimulation sites on the subjects head that could provide responses from the otolith organs and or the semicircular canals (SCCs). Third, our objective was also to solve any technical problems to allow cost-effective testing. Finally, we wanted to determine whether there were other physiological or neurological factors that could contaminate or cause artifacts in the cvemps and/or ovemps, like non-vestibular sources. Our experiments started with the investigation of cvemps. We then concentrated on the investigation of ovemp techniques. In this Chapter, we describe the development of both methods (i.e. the cvemps and ovemps), because their developments were interrelated. 3.2 Initial skull-tap cvemps stimulator s development Our first investigation of the skull-tap cvemps used the tester s palm, followed by a tendon hammer, and lastly a pendulum-like stimulator. These stimuli to the subjects heads were measured using a piezo-electric transducer (section 2.3.2). A commercial three-dimensional accelerometer was also used. Much of the technical development of cvemp measurement was conducted in two healthy adult subjects (one female aged 30 years old, and one male aged 48 years old) at The University of Western Australia. All protocols were approved by The University of Western Australia Human Research Ethics Committee (RA/4/1/1496). Surface EMG activity was recorded using standard adult ECG foam electrodes (Conmed Corporation). These particular electrodes were chosen because they were highly adhesive, and able to comply with the skin surface. Only minimal 42
59 skin preparation was required. For all these initial experiments, both the active and indifferent electrodes were placed on the right neck of the subjects, unless otherwise specified. The active electrodes were placed on the upper two thirds of the SCM muscle, the indifferent electrode was placed on the lower part of the SCM muscle (i.e. at the sternoclavicular joint), just above the sternum, while the reference electrode was placed on the right sternum. The tests were conducted in the laboratory initially using a portable computer and MacLab/4e ADInstruments running Scope V for Windows software (ADInstruments, N.S.W., Australia). The responses were averaged over 1280 samples over a 100 ms time base. Care was taken to minimize electrical interference and ensure stability of electrophysiological recordings Initial skull-tap stimulation trials Using the tester s palm as stimulator The first experiment used the heel of the tester s right palm in a tapping manner. The piezo-electric transducer was placed at the back of the right wrist and secured using a wrist band. The subjects heads were then tapped at various stimulation sites. During these experiments, the seated subjects were instructed to either activate or relax their right SCM muscle. This was done by instructing the subject to keep their head facing forward (the muscle was not activated) or turn their neck to the left (so the right SCM muscle was activated). The reason for this was because we were interested in investigating the differences in the responses with the right SCM muscle activated and relaxed, and also to see how different stimulation sites influenced the responses. From these experiments (Figure 3.1), we learnt that: a) The piezo-electric transducer was able to measure the deceleration at impact of the palm as it landed on the subject s head. The trigger waveforms were consistent and reproducible for the same stimulation site, which is the trigger waveforms were reproducible with the right SCM muscle activated or relaxed. It was also capable of measuring the differences in the deceleration on the different sites on the subject s head. This was evident when we changed the stimulation sites (e.g. from the top of the right ear to the top centre of the head). 43
60 Trigger Stimulator: Palm palm SCM Response A X palm B C palm palm D + 0.5V 10 ms + 100µV 10 ms Figure
61 Figure 3.1: Trigger and response waveforms obtained during palm-pulse stimulation on the (A) forehead, (B) top centre of the head, (C) back centre and (D) top of the right ear [20 averages (n=20) per waveform]. The experiments were conducted during right SCM muscle activation (black waveforms) and nonactivation (grey waveforms). The trigger's waveforms were reproducible during SCM activation and relaxation, but they were different between different sites. Different stimulation sites also gave different responses and activation of the right SCM muscle resulted in bigger and more prominent responses than the nonactivated muscle. These recordings were done using Maclab. 45
62 b) Different stimulation sites gave different responses (i.e. the SCM muscle responses were directionally sensitive). From the response waveforms in Figure 3.1, it was clear that stimulation on the top of the right ear gave much earlier responses when compared to the later responses from stimulation on the top centre of the head. c) The responses were different with the SCM muscle activated or relaxed. Stimulation above the right ear gave much earlier responses when the right SCM muscle was activated, compared to when it was relaxed. Relaxation of the right SCM muscle gave flat responses when palmpulses were delivered to the top centre of the head. d) From these trials, we also learnt that it was important for the tester to keep reminding the subject to activate the SCM muscle consistently during the experiment Tendon hammer The second investigation used a bulky tendon hammer routinely used in undergraduate teaching, incorporating a spring switch that generated a trigger pulse (Figure 3.2). From this we learnt that the tendon hammer stimulation produced different responses when tapped on different sites on the subject s head. But our two subjects reported that the taps caused discomfort and pain, especially when the taps were delivered to the left and right side of the head. Additionally, we found that the tendon hammer used required too strong a trigger (it was based on movement of a spring) and it could not be used to evoke the responses at the threshold level. We decided to terminate the use of the tendon hammer stimulation. 46
63 Stimulator: Tendon hammer A tendon hammer µv 10 ms B tendon hammer C X tendon hammer Figure 3.2: The response waveforms obtained during right SCM muscle activation with tendon hammer stimulation to the (A) top of the right ear, (B) top of the left ear and (C) top centre of the head. It was obvious that different stimulation sites from the tendon hammer gave different responses. 47
64 Pendulum-like stimulator The third and final approach used during the initial development of cvemp measurement and software involved the application of a pendulum-like stimulator. We decided to try the pendulum because it might provide a softer stimulation compared to the tendon hammer, but at the same time it may give reliable responses which could be easily calibrated. The pendulum stimulator was built using a semi-elastic band stretched along a square frame of wood. This square frame pivoted at the ceiling towards the subject s head (Figure 3.3 A). A piezoelectric sensor was attached to this frame. Before the trials, the subject was instructed to stand in front of the pendulum. During stimulations, the tester swung the pendulum upward 45 degrees from its stationary position. From these experiments, we found that the responses were sluggish, because the stimulus itself was too slow and broad as it landed on the stimulation sites (Figure 3.3 B). The pendulum-like stimulator was also not sufficiently versatile, because it was incapable of stimulating the site on top of the ear on each side of the head accurately, and could not stimulate the centre top of the head. Overall the stimulus was too strong, too slow, and lacked versatility. As a result the technique was abandoned Finger-taps We developed the use of the finger-tap skull-taps after we had obtained the complete results from the used of the palm-pulse in normals, as well as from pathological subjects. After analyzing the recorded responses from the normals, and the pathological subjects, we decided to abort the palm-pulse used as stimulator, again because the stimulus was too intense (more in depth discussion on this in Chapter 4). Finally, we decided to use finger-taps as the stimulation because it was not only cost-effective and reliable, but also the taps could delivered down to threshold levels but was clearly more intense than the commercial bone conductor stimuli reported by others and avoided electrical stimulus artifacts. More examples of the finger-tap stimuli and responses are discussed in depth in Chapter 5. The finger-tap stimulus was applied during the ovemp protocol, and also during the final part of our experiments on the cvemps in normal subjects. 48
65 A ceiling pivoting wooden frame semi-elastic band piezo-electric transducer B Trigger Stimulator: 'Pendulum' Responses 0.5 V µV 10 ms 10 ms Figure 3.3: (A) The trigger's and responses waveforms from a pendulum-like stimulation directed to the top of the right ear and forehead during SCM muscle activation (black waveforms) and relaxation (grey waveforms). The pendulum-like triggering waveforms were consistent in between trials. However, the responses to the right ear stimulations were very poor and sluggish, as compared to better quality responses after the forehead stimulation. (B) Pendulum-like stimulator that was used during the experiment. 49
66 Three-dimensional (3D) accelerometry Besides using the one-dimensional (1D) piezo-electric sensor, initially we also used a 3D accelerometer to measure the head accelerations. The 3D accelerometer was glued to a head band, and during the experiment the subject wore it on the forehead. The 3D accelerometer required a +5V power supply. We decided to abort its use because we found that it caused a large stimulus artifact, which was visible and contaminated the recorded responses from our subjects. The detailed responses from the 3D accelerometry are not shown in this report although it was used to calibrate the piezo-sensor. 3.3 Summary for the skull-taps stimulators investigation In summary, in our initial trials during software and hardware development, we trialed four stimulus methods: palm-pulse, tendon hammer, pendulum and finger-taps. All but the finger-taps were abandoned because they were too intense, too sluggish or lacked versatility. The finger-taps, however, seemed to offer the greatest versatility and reproducibility over an appropriate range of stimulus intensity. 3.4 Acoustic click stimulation The development of our click stimuli was done after the successful development of our custom-made software (Chapter 2, Section 2.2). We have used longer 0.5 ms monophasic clicks, although previous researchers, Rosengren et al. (2005) and Chihara et al. (2007) used 0.1 ms rarefaction clicks, and Chihara et al. (2007) and Todd et al. (2007) used 500 Hz tone bursts. We opted for the longer click stimuli because they provided a five-fold greater low-frequency stimulus to the otolith organ than the 0.1 ms rarefaction clicks with the same peak sound level (limited by law to 140 db SPL peak), and they had more chance of producing a monophasic acoustic drive to the vestibular hair cells than 500 Hz tone bursts (Todd et al., 2008 found that the 500 Hz tone bursts produced alternating excitatory and inhibitory drive to the hair cells which would confuse any understanding of the hair cells involved and the neural pathways. Because the otolith organ may have a mechanical corner frequency (and possibly a mechanical resonance) near 500 Hz (Goldberg and Fernandez, 1975), the high-frequency spectral components in a 0.1 ms click above 500 Hz play little or no role in stimulating the otolith organ, but increase the danger of trauma to nearby cochlear 50
67 hair cells. Fortunately, prolonging the clicks from 0.1 ms to 0.5 ms to increase the vestibular drive produced no significant latency shifts in the VEMP responses. These 0.5 ms clicks were synthesized as wave files using Sound Forge 4.5, were output from the soundcard of a personal laptop computer, and were amplified by an external battery-operated audio amplifier, before being presented at 130 db SPL peak, using ER3A insert earphones to the left and right ears separately. 3.5 Investigating technical aspects effecting the cvemps and ovemps Both the filter effect and the electrode montage of the cvemp recordings were investigated further Filter Effect After the initial software development (Chapter 2), we investigated the effect of filter bandwidth on the cvemp responses. This was done on a healthy, adult female subject. She was stimulated only at Fz, while both SCM muscles were activated 1. She was only stimulated at the maximal stimulation level (i.e. g 2.0 g) to improve reproducibility. The filter bandwidth and order were adjusted online (i.e. during the cvemp recordings). Both the low-pass and high-pass filters together with the filter order were adjusted to preserve the EMG responses and avoid any over-filtering in the recorded responses. With the filter order set at 2, the low-pass corner frequencies of (0.1, 1.0 and 10.0) Hz and the high-pass corner frequencies of (300, 600 and 900) Hz was adjusted, one at a time. After obtaining the responses with the filter order set at 2, we increased the filter order to 4. From Figure 3.4 (a), we found that all responses gave almost identical responses for the first three peaks, however, we found that during the 2 nd order application, 0.1 and 1.0 Hz low-pass filter had too much lowfrequency noise in the recorded responses (the 10.0 Hz low-pass filter produced a much quieter and cleaner response). On the other hand, although we found that the responses from the 4 th order filter [Figure 3.4 (b)] were also not contaminated with low-frequencies noise, the recorded responses were distorted (over filtered), eliminating some components of the electrophysiological responses. Finally, we 1 The electrode montage and testing protocol employed on this subject was similar with other subjects recruited during the palm-pulse cvemp testing (Chapter 4). 51
68 A 2nd order filter function Hz Hz Hz Hz Hz Hz Hz Hz Hz B 4th order filter function Hz Hz Hz Hz Hz Hz Hz Hz Hz 50 µv + 10 ms 50 µv + 10 ms + 50 µv 10 ms Figure 3.4: cvemps waveforms following palm-pulse stimulation, at the Fz stimulation site from the left SCM muscle during bilateral SCM muscle activation. Both the low-pass filter and high-pass filter were adjusted online. (A) The cvemps waveforms recorded with the 2nd order filter function and (B) The cvemps waveforms recorded with a 4th order filter function. Not much difference was noted following the adjustment of the filter bandwidth and filter order. Hence, we decided to use the 2nd order filter for all the cvemps and ovemps recordings. 52
69 were left with the 10Hz low-pass corner frequency responses paired with 300, 600 and 900 Hz high-pass corner frequencies. We found that the 600 Hz high-pass corner frequency gave optimal responses, because 300 Hz caused the responses to be over-filtered. As a result, we chose the 10 Hz-600 Hz filter bandwidth for all experiments involving the cvemps cvemps electrodes montage During the initial skull-tap cvemp development (Section 3.2), we employed the electrode montage used by other earlier researchers (i.e. the active electrodes were placed on the upper two thirds of the SCM muscle, the indifferent electrode was placed on the lower part of the SCM muscle (i.e. at the sternoclavicular joint, just above the sternum), while the reference electrode was placed on the right sternum (refer to Figure 4.1, Chapter 4). We later found that placing the indifferent electrodes at the lower part of the SCM muscle caused discomfort to subjects with chest hair. Similar problems were faced when the reference electrode was placed on the sternum. Thus, we decided to investigate the use of a different electrode montage. During our investigation, we compared our new electrode montage with the ones used by others. We still placed the active electrode on the upper two thirds of the SCM muscle, but placed the indifferent electrode on the upper tendon of the muscle (approximately cm below the mastoid bone), while the reference electrode was placed on the nape of the neck. We conducted the test on two healthy adult subjects. The test was only done using monaural 0.5 ms click stimuli, with ipsilateral right SCM activation. From Figure 3.5, we found that the responses from both subjects were similar with the two different electrode montages. Thus, we decided to use our montage, rather than the ones used by others to avoid discomfort to subjects. 53
70 Right SCM muscle Subject 1 Raw Our montage Auto-normalized Other's montage Subject 2 Our montage Other's montage µv 50 units 10 ms 10 ms Figure 3.5: Raw and auto-normalized acoustic 0.5 ms click cvemps responses (n=200) obtained from two healthy subjects (one male and one female, aged years old). These responses were obtained with monaural stimulation during ipsilateral right SCM activation. The vertical dashed lines mark the peak of the trigger stimulus (0 ms). Note that both subjects produced approximately similar responses with the used of ours and other s electrode montage. 54
71 3.5.3 ovemps electrodes montage Besides developing the electrode montage for the cvemps (Section above), we also developed our own electrode montage for the ovemps. Earlier researchers had come to the conclusion that active electrodes positioned inferior to the eye gave the largest acoustic ovemp responses. However, with the active electrodes placed inferior to the eye, different researchers have employed different ways for placing the indifferent electrodes (i.e. placing the electrodes either on the earlobes, or even 2-3 cm inferiorly to the active electrodes) 2. Thus, we decided to investigate the best and most suitable position for the placement of our indifferent electrodes. As the investigation for our cvemps electrode montage, we conducted the test on three healthy adult subjects using monaural 0.5 ms click stimuli. We compared the subjects responses with our electrode montage and others. During the investigation, the active electrodes were always placed on the orbital margin immediately below both eyes. For our montage, we placed only one common indifferent electrode on the philtrum under the nose. We later repeated the same testing by placing two indifferent electrodes placed 2-3 cm inferior to the active electrodes under the right and left eyes. Each recording was done twice. We found that our electrode montage and the ones used by others gave insignificant differences in the responses recorded (here we only showed one sample waveforms responses on the right eyes from one subject in Figure 3.6, with left ear acoustic stimulation). The latency, amplitude and morphology of the recorded responses were almost similar. Thus, we chose to only use one common indifferent electrode placed on the philtrum because this is more cost and time effective, rather than using two indifferent electrodes. 2 Please see Section 5.3.4, Chapter 5 for more in depth discussion on the past researches for the investigation of the ovemps electrode placement. 55
72 Subject 1 Raw Right eye Our montage Auto-normalized Other's montage Subject 2 Our montage Figure 3.6: Raw and auto-normalized acoustic ovemps responses from the right eyes (n=200) obtained with 0.5 ms click stimulation to the left ears from one healthy adult. During recording, the subjects were asked to gaze upward and to relax their facial area (more explanation on the testing methods can be found in Chapter 5). As Figure 3.5, the vertical line mark the peak of the trigger stimulus at Other's montage 0 ms µv 10 ms + 10 units 10 ms 56
73 3.6 Control studies to verify vestibular origin Trigeminal responses on the cvemps To investigate possible non-vestibular sources of artifact, electrical stimulation of certain facial areas was studied because electrical stimulation of the skin on the human face elicits motor responses in the neck, as well as in the facial muscles (Sartucci et al., 1986). Years later, Browne et al. (1993) reported the possibility of functional coupling of the trigeminal nerve and the cervical systems. Di Lazzaro et al. (1995) has also reported that the electrical stimulation on the branches of the trigeminal nerve (especially the infraorbital branch) at low current strength produces excitatory postsynaptic potentials (EPSPs) in motoneurons innervating the dorsal neck muscles, and inhibitory postsynaptic potentials (IPSPs) in motoneurons innervating the SCM and the trapezius muscles. The trigeminal nerve is responsible for sensation in the face, as well as motor functions. It is the largest cranial nerve and has three major branches (ophthalmic, maxillary and mandibular nerve which innervate different areas on the face; Leong, 1988; Moore et al., 2010a; Moore et al., 2010b; Ongerboer de Visser, 1983). The ophthalmic and maxillary nerves are purely sensory, while the mandibular nerve has both sensory and motor functions. From Figure 3.7, it is clear that the ophthalmic nerve carries sensory information from the scalp and forehead area, including the upper eyelid, the conjunctiva and the cornea of the eye, and also the nose. The mandibular nerve carries sensory information from the lower part of the lip, teeth and gum, the chin and jaw, and also the anterior 2/3 of the tongue. The maxillary nerve is responsible for the sensory information from the upper part of the lip, teeth and gums, the lower eyelid, and the cheek. This series of experiments sought to determine the latencies of different trigeminal nerve branches on different facial areas in response to electrical stimulation associated with the activation of the SCM muscle. We hoped to differentiate the true vestibular responses to palm-pulse and finger-tap stimulation from any responses due to skin stimulation of the trigeminal nerves. Moreover, we wanted to determine whether taps at the intensities used for vestibular testing produced any trigeminal responses. 57
74 Figure 3.7: The trigeminal nerve and its three branches from both the anterior and lateral view (adapted from Moore et al., 2010b) 58
75 Materials and Methods Four healthy adult subjects (three males, one female; age range years) with no history or disorders involving the central or peripheral nervous system participated in these experiments. All were informed of the aims and procedures of the study and gave their consent. All subjects read and signed an informed consent form which had been approved by The University of Western Australia Human Research Ethics Committee (Ethics Approval Project no. RA/4/1/1496) (Appendix 1). Prior to the actual recording, subjects were given a few practice sessions to turn their neck to the left while the strength of the SCM muscle contraction was monitored. This was important to ensure the consistency of the level of right SCM muscle activation during recording. When the experimentation started, the SCM muscle was activated unilaterally (i.e. subjects were instructed to turn their neck to the left to have optimum activation of the right SCM muscle). Subjects were constantly reminded to keep their SCM muscle contraction at a steady level, and the level of activity was monitored on the front panel of our custom-made software, via the personal laptop. Our electrode montage (as described earlier) was used during these recordings Electrical stimulation and recording of the response A block diagram of the equipment used is shown in Figure 3.8 (A). Electrical stimuli were delivered via two small electrodes (1-cm-diameter tip, 1-cm interelectrode distance). Precautions were taken to avoid the electrodes being shorted by electrode gel. Both electrodes were then coupled to an EMG stimulator box which was driven by a MacLab/4e ADInstruments running Scope V for Windows software (ADInstruments, N.S.W., Australia). Waveform acquisition and averaging were accomplished through a personal laptop running our custom-made software (Section 2.2 Chapter 2). Electrical stimuli (0 ms delay, 2.5 ms duration) as a trigger to averaging were bandpass filtered ( Hz), and averaged (512 samples) using a sampling rate of 4 khz and a 100 ms time base. The electrical stimulus strength was described by the subjects as moderately painful, but tolerable. All subjects were stimulated by current pulses between 5 ma to 25 ma at different stimulation sites on the face area (Figure 3.8 B). EMG activity was amplified 1000 times (by the NI USB 6009), bandpass filtered in software ( Hz) and averaged (usually 59
76 B D8 Forehead SCM Indifferent Active A NI USB 6009 Stimulating electrodes Cheek EMG stimulator box Reference Laptop MacLab/4e ADInstrument Desktop computer running Scope V Figure 3.8: Block diagram of the recording and the stimulation system. (A) The active electrodes were placed on the upper two thirds of the SCM muscle, the indifferent electrode was placed on the SCM muscle tendon, while the reference electrode was on the sternum. All the electrodes were placed on the right side of the subjects. (B) The subjects were stimulated only on their right side of the face, i.e. centre of the forehead, meaty part of the cheek and D8 (approximately 5 cm anterior to the pinna). 60
77 n=100) using a sampling rate of 5 khz, and averaging was done over a 200 ms window. The software recording parameters were the same as described earlier in Section 2.2, Chapter Results Figure 3.9 shows SCM muscle responses obtained from four different subjects following electrical stimulation on the right D8. The responses in this Figure were obtained following different electrical stimulation strengths (between 15 and 20 ma). It is clear from Figure 3.9 that electrical stimulation at right D8 produced early latencies with negative deflection first (N1) followed by positive deflection (P1). There were slight intrasubject differences in the latencies (Table 3.1 A), as well as intersubjects differences (Table 3.1 B). These results will be discussed later. Following electrical stimulation on the right D8, the same four subjects were then stimulated on their right cheek (electrical stimulation between 15 and 20 ma). This was important to determine whether innervation of different branches of the trigeminal nerve following electrical stimulation gave similar or dissimilar responses to the recorded vestibular responses. This will be discussed in a later section. Right cheek stimulation produced a positive peak first, followed by a negative peak (Figure 3.10). It was also clear that right cheek stimulation produced later mean peak latencies as shown in Table 3.2 B compared to right D8 (Table 3.1 B). To further determine the peak latencies of different branches of the trigeminal nerve following electrical stimulation, the center forehead of three subjects was stimulated (10 to 15 ma). As apparent from Figure 3.11, early positive peaks were present in the three subjects, as opposed to a much later first positive peak (P1) following stimulation to the right cheek. All the peak latencies in response to center forehead stimulation were shorter than the responses to the right D8 and right cheek. Note that there were also large peak latency differences between the subjects (Table 3.3 A). 61
78 Electrical stimulation at right D8 N1 N2 N2 P1 P2 N1 N2 P1 P2 N1 N2 P1 P2 N1 N2 P1 P2 - Figure 3.9: The responses (n=100) of the right SCM muscle following stimulation on the right D8 stimulation site from four subjects. The vertical lines on the left marked the time of the application of the stimulus. The arrow indicates the electrical pulse stimulus. Two consecutive responses from the same subject are superimposed to show the repeatability of the responses. As in this and subsequent figures, downward deflection of the traces is marked as positive while the upward deflection is marked as negative. 62
79 A Subject Waveform Peak latency (ms) N1 P1 N2 P2 Subject Subject Subject Subject B Mean peak latency (ms) N1 P1 N2 P ± ± ± ± 4.5 Table 3.1: (A) Peak latencies (ms) obtained from four subjects following right D8 electrical stimulation. Each subject had two consecutive runs as to ensure the repeatability of the responses (waveform 1 and 2). (B) Mean (± SD) values were given for the peak latencies of the responses. 63
80 Electrical stimulation at right cheek N1 P1 P2 N1 P1 P2 N1 P1 P2 N1 P1 P2 Figure 3.10: As in Figure 3.9, the responses (n=100) of the right SCM muscle following electrical stimulation on the right cheek from four subjects. 64
81 A Subject Waveform Peak latency (ms) P1 N1 P2 S S S S B Mean peak latency (ms) P1 N1 P ± ± ± 7.2 Table 3.2: (A) Peak latencies (ms) obtained from four subjects following right cheek electrical stimulation. Each subject had two consecutive runs as to ensure the repeatability of the responses (waveform 1 and 2). (B) Mean (± SD) values are given for the peak latencies of the responses. 65
82 Subject 1 Electrical Electrical stimulation stimulation at at forehead right centre N1 n1 cheek Subject 2 P1 p1 N1 n1 P2 p2 p1 P1 p2 P2 Subject 3 N1 n1 p1 P1 p2 P2 50 µv - 10 ms Figure 3.11: As in Figure 3.9 and Figure 3.10, the responses (n=100) of the right SCM muscle following electrical stimulation on the forehead centre. 66
83 A Subject Waveform Peak latency (ms) P1 N1 P2 Subject Subject Subject B P1 N1 P2 Mean peak latency (ms) 22.9 ± ± ± 16.8 Table 3.3 (A): Peak latencies (ms) obtained from three subjects following forehead centre electrical stimulation. Each subject had two consecutive runs as to ensure the repeatability of the responses (waveform 1 and 2). (B) Mean (± SD) values are given for the peak latencies of the responses. 67
84 Summary trigeminal responses on the cvemp responses After analyzing the responses from our volunteer subjects following electrical stimulation, we concluded that these responses were different from the cvemp responses recorded after the palm-pulse and finger-tap stimulation (Chapter 4). We conclude that our cvemps were not contaminated by the responses from the trigeminal nerve and its pathways. Because the trigeminal responses occurred between ms after the electrical stimulation and the taps used were not painful (far from it), so there is little chance that the cvemps reported here are nociceptive responses Blink response and the ovemp The blink reflex was first described by Overand in 1896, who reported that slight taps on the forehead using a stethoscope caused ipsilateral twitch on the lower eyelid, and also slight taps on the midline of the forehead caused twitching on both sides of the eye (Ongerboer de Visser, 1983; Torres, 2002). Years later, Weingrow (1933) reported that the blink reflex could be elicited from many areas of the face (Rushworth, 1962). The exact mechanism of blink reflex remained unclear until Kugelberg (1952) completed an EMG examination of the blink reflex. He confirmed that the blink reflex consists of two components, the R1 and R2. The early R1 component occurred at a latency of about ms, and it only happened ipsilateral to the side of stimulation. While the more prolonged responses, the R2 occurred bilaterally (i.e. ipsilateral and contralateral to stimulation), at a latency of about 21 ms to 43 ms. The R2 is considered to be nociceptive (defense) reflex, and it protects the eyeball from external harmful stimuli (Torres, 2002). In 2002, Bour et al. (2000) studied the EMG recordings of the orbicularis oculi muscles during spontaneous, voluntary and reflex blinking in a group of healthy adult subjects. They reported that the R1 component happened ipsilaterally at a latency of about 15 ms. These findings are in total agreement with the ones reported earlier. To confirm that the observed ovemp responses to the finger-taps were the result of vestibular stimulation, and were not artifactual or produced by the blink reflexes, we conducted a control study in eight adult subjects (four male, and four female, aged between years old). All subjects read and signed an informed consent form which had been approved by The University of Western Australia Human Research Ethics Committee (Ethics Approval Project no. RA/4/1/1496) (Appendix 2). During these recordings, the same electrode montage as discussed in 68
85 Section was employed. In some subjects, the blinks were evoked most efficiently by tapping a rectangular rubber block (a pencil eraser) placed on the glabella above the nose. The most efficient stimulus was not orthogonal tapping on the either the skin or the block, but was a downwardly-directed tap on the upper surface of the block, producing maximum shearing of the skin of the glabella 3 and forehead. In some subjects blinks were triggered by finger-tapping the closed eyelids separately. Importantly, as shown in Figure 3.12, the blink EMG was clearly different from the ovemps evoked by finger-taps to the different sites on the skull (which is on the nose bridge, back of the head, right side above pinna and the right mastoid) 4. The blink responses had different latencies and wave shapes and, when present (evoked intentionally as described above), they were much larger than the ovemps and clearly distinguishable from them. None of the inflections in the normal ovemp waveforms could be attributed to them. 3 Glabella is the space or the smooth area between the eyebrows and above the nose. 4 More in depth explanation of the ovemps method and the stimulation sites for the taps is discussed in Chapter 5 of this report. 69
86 No Oz T4 mas sham blinks 20 µv Figure 3.12: Comparison of two maximal ovemp replicates with finger-taps at (top to bottom) the nose bridge (No), back of head (Oz), right side above pinna (T4) and the right mastoid (mas). The bottom black trace is a sham trace obtained by averaging 50 responses obtained by tapping the subject s elbow. The bottom traces (grey) are 10 raw blink responses from the same individual obtained by a sharp downward tap to a rubber block on the glabella. Each vertical dashed line shows a 10 ms time step. 70
87 3.7 General test methodology General recording preparation Before recording, the skin was cleaned briskly with 75% ethanol on gauze. A contact resistance less than 5kΩ was typical. For the cvemps recordings, large circular Cleartrace electrode from Conmed, with extra electrode gel were used. The active electrodes were placed on the upper two thirds on both the left and right muscle of the SCM, while the indifferent electrodes were placed on the upper tendon of the muscle (approximately cm below the mastoid bone). The reference electrode was placed on the nape of the neck (Figure 3.13 A). While from Figure 3.13 B, for ovemps, the extraocular potentials were measured using narrow self-adhesive electrodes (Ag/AgCl 3M Red Dot electrodes halved along their long axis), with the active electrodes on the orbital margin immediately below both eyes, and with one common indifferent electrode on the philtrum 5 under the nose (earlier tests indicated that there was no difference when two independent indifferent electrodes were used beneath each eye). A large circular Cleartrace electrode from Conmed was used for the reference electrode, which was placed on the prepared nape of the neck with extra electrode gel. 5 Philtrum is a midline groove extending from the nose to the upper lip. 71
88 A Cz T3/4 B Fz indifferent electrodes No Oz active electrodes indifferent electrode active electrode mastoid left/ right sternocleidomastoid muscle C D Figure 3.13: All the stimulations sites for both cvemps and ovemps were shown in A, except for LARP and RALP. Electrode montage for ovemps are shown in A and for cvemps are in B. Note that the reference electrode for cvemps, which was positioned on the nape of the neck was not shown in this Figure. C Showed the tester s right palm with the piezo secured using a hand band on the palm s heel. The position of the tester s right middle finger, with the piezo secured on it was shown in D. 72
89 3.7.2 Muscles activation For both the cvemps and ovemps, all subjects were tested while they were sitting in an upright position. We decided to carry out testing while all the subjects sitting upright (unless otherwise specified), because we wanted to provide a testing option for testing environments that do not have hospital beds. Also, considering that some of our later volunteers were elderly subjects, and they did complain of having backache when lying flat on the bed, this option was favoured. All subjects were given instructions and a practice session before commencing tests. For cvemps, they were instructed either to activate their left or right SCM muscle one at a time (unilateral activation) or both muscles at the same time (bilateral activation). To assist them in activating their SCM muscles, they were also asked to look at the bio-feedback meters (located at the front panel of the laptop). During the unilateral SCM activation, they were asked to turn their head outward laterally, facing the shoulder on the same side of the muscle. While for bilateral activation, they were given a block of Styrofoam to press against. During the muscle activation, they were instructed to press against it as hard as possible, while self-monitoring the activation through the bio-feedback meters. ovemp subjects were instructed to relax their facial muscles during recordings, because active movement produced unwanted EMG interference. While sitting upright, they were asked to ensure that their head was kept still and facing forward, while fixing their gaze at a ceiling spot 1 m above and 0.5 m to the front of their head. To help them, we marked a spot on the ceiling, approximately ½ a meter in front of them, and reminded them to gaze at that particular spot at all times during recording Acoustic stimulations For all volunteer subjects for both cvemps and ovemps, they were stimulated monaurally using 0.5 ms monophasic clicks presented at 130 db SPL. The stimuli were presented using ER3A insert earphones. We only conducted ipsilateral activation testing for the acoustic VEMPs, because we wanted to ensure that they had normal acoustic VEMP responses. 73
90 3.7.4 Skull-taps Our skull-tap protocol consisted of the palm-pulse and also the finger-tap stimulation. The palm-pulse stimulation was used for cvemps only, while the finger-tap stimulation was used for both cvemps and ovemps. The stimulation was delivered at about ½ second intervals, partially randomized to minimize the chance of subjects anticipating the stimulus and synchronizing to it. For the palm-pulse stimulation, the piezo-electric transducer was placed on the heel of the right tester s palm, secured in placed with a wrist band (Figure 3.13 C), while for the finger-taps the piezo was placed on the tester s right middle finger (Figure 3.13 D). To ensure the consistency of each palm-pulse and finger-tap, they were delivered orthogonal to the skull at each stimulation site Stimulation sites After we were confident that the trigeminal responses did not cause any artifactual responses in both the cvemps and ovemps, we used different tap sites on the skull. The named tap sites were based on The International Electrode (placement) System (Jasper, 1958; Steinmetz et al., 1989). For both cvemps and ovemps, the stimulation sites included mid hairline (Fz), vertex (Cz), back center (Oz), on the skull 2 cm above the left and right pinna (T3 and T4, respectively). While Left Anterior-Right Posterior canal (LARP) and Right Anterior-Left Posterior canal (RALP) sites directions were done for finger-taps cvemps only (Figure 4.2, Chapter 4). Additional tap sites for ovemps were the nose bridge (No, just below the inion), and finally orthogonal to the lower bony portion of the left and right mastoids, just behind the pinna (Figure 3.13 A). During testing, the tester randomized stimulation presentation to minimize the chance of subjects anticipating the stimulus. 74
91 Chapter 4 Cervical Vestibular Evoked Myogenic Potentials (cvemps)
92 4. Cervical vestibular evoked myogenic potentials (cvemps) 4.1 Introduction In this chapter, the background of the cervical vestibular evoked myogenic potential (cvemp) and its relationship to the vestibular organs are reviewed. The section focuses on our cvemp experiments, which used three different forms of stimulation: acoustic stimulation, the heel of the tester s right palm (the palm-pulse stimulus), and the tester s median finger (the finger-tap stimulus). These experiments were carried out after our initial control experiments which showed that the responses to stimulation on the subject s head were not of trigeminal nerve origin (as discussed in Chapter 3). Our experiments started with the application of palm-pulse cvemps in normals, followed by the same stimulus in a small number of bilateral vestibular loss subjects. Based on the development of the better finger-tap stimulus, we aborted the use of the palm stimulus, and only later (i.e. after the ovemp experiments, described in Chapter 5), we conducted the finger-tap cvemps experiment in normals. Thus, the cvemps in this study was only conducted as a small scale study compared to the ovemps. However, the information obtained on our earlier study on cvemps (through the use of palm-pulse) was used to develop the technique for the finger-taps. 4.2 Background of cvemps Earlier neurophysiologic studies have indicated that the vestibular system (i.e. the saccule in particular) is sensitive to sound. In non-mammals, the saccule has been found to be responsive to sound in goldfish (Furukawa, 1981; Furukawa et al., 1967), teleost fishes (Popper et al., 1973), the American toad (Moffat et al., 1976) and also in pigeons (Witt et al., 1984). While for mammals, research has shown that in squirrel monkeys (Young et al., 1977), cats (McCue et al., 1994; Murofushi et al., 1995; Rapoport et al., 1977; Uchino et al., 1997) and guinea pigs (Curthoys et al., 2006; Murofushi et al., 1995), the vestibular neurons are responsive to acoustic stimulation with frequencies and latencies similar to human hearing. Bickford et al. (1964) have recorded responses to acoustic clicks with the active electrode placed just below the inion. They concluded that it was myogenic in origin, and they proposed that the responses were the results of the activation of the vestibular apparatus, rather than cochlea, because it was present in patients with sensorineural hearing loss. Years later, other groups of researchers (Cody et 76
93 al., 1969; Townsend et al., 1971) reported the inion response to acoustic stimulation in other vestibular loss subjects, and they proposed that the inion response was of vestibular origin, in particular from the saccule. In the following part of this chapter, the vestibular evoked myogenic responses (VEMPs) conducted in past years are reviewed. 4.3 Review of different VEMPs stimulation methods Different stimulation methods have been used in VEMP testing (i.e. air conduction acoustic stimulation using clicks or tone bursts, galvanic, bone conduction, and also skull tap). For this report, we only describe briefly the galvanic, bone conduction and acoustic tone bursts stimulation, because we did not use these methods in our test protocol Details of click acoustic stimulation After the initial discovery of the vestibular responses to acoustic stimulation (Bickford et al., 1964; Cody et al., 1969; Townsend et al., 1971), years later in 1992 and 1994, Colebatch et al. tried to apply the same principles in evoking responses from the vestibular system using acoustic stimulation, but they used the sternocleidomastoid (SCM) muscles (i.e. one of the neck muscles) instead of the inion for the placement of the active electrode. These recordings were named cervical vestibular evoked myogenic potentials (cvemps). Using 0.1 ms rarefaction click stimulation presented at 140 or 145 db SPL, the stimuli were delivered via headphones. Active electrodes were placed over the upper half of the SCM, with the indifferent over the upper sternum (Figure 4.1). Their subjects were ten normal adults and eight patients (three with profound sensorineural hearing loss and another five with selective vestibular nerve section). Most of them were tested while seated and their neck muscles were activated by pressing against a padded bar in front of them, but some of the recordings were made while the subjects were lying supine and both left and right neck muscles activated when their heads were slightly raised (both methods involved bilateral activation of the SCM muscle). Some subjects turned their head to one side (this method involved unilateral muscle activation). They successfully recorded early positive-negative peaks (p13- n23) on both sides in all of their normal subjects. They also recorded later peaks (n34-p44), but these peaks were not present in all normals. Colebatch et al. (1994) also reported that in one of their normal subjects, the responses were 77
94 our indifferent electrode X sternocleidomastoid muscle active electrode (adapted from Moore et al.) others indifferent electrode Figure 4.1: An example of electrode montages used for cvemps that was first introduced by Colebatch et al. (1992, 1994) and later by others (marked as the black circles). The active electrode was placed on the upper half (i.e. about two third) of the SCM muscle, while the indifferent electrode was placed on the sternoclavicular joint, just above the sternum. We placed our active electrode on different position i.e. on the upper tendon of the SCM muscle (approximately cm below the mastoid bone, marked with X ). The neck Figure was adapted from Moore et al. (2010), page
95 abolished during an upper respiratory tract infection, but recording done later, after recovery, revealed that the responses had returned to normal. Subsequent to Colebatch and co-workers success, many other researchers applied the same principles of click cvemps in their research in normals, as well as pathological subjects (Huang et al., 2005; Lim et al., 1995; Murofushi et al., 1998; Murofushi et al., 2001; Takeichi et al., 2001; Welgampola et al., 2005; Wu et al., 1999). Despite the application of monaural acoustic clicks, Bhagat (2006) and Wang et al. (2003) applied binaural click stimulation in their experiments. They reported that binaural clicks generated shorter latencies positive peaks followed by negative peaks compared with monaural click stimulation, on both sides of the SCM 1. Animal data have suggested an otolithic origin, especially a saccular one, based on the experiments showing that the sacculus was the only part of the vestibular apparatus in mammals that was activated by sound (i.e. some of the vestibular neurons that responded to tilt were sound-sensitive, whereas the vestibular neurons that responded to angular accelerations were not; Murofushi et al., 1995; Young et al., 1977). The idea of a saccular origin for the sound-sensitive vestibular neurons was also supported by patient data, which demonstrated a close relationship between cvemps and the functional integrity on the inferior vestibular nerve, which innervates the major part of the sacculus (Murofushi et al., 1998). Colebatch et al. (1994) have recorded the p13-n23 peak responses in the affected ear in three of their profound sensorineural hearing loss subjects. Furthermore, in their selected unilateral vestibular nerve section subjects, the p13-n23 peaks were absent on the side of the nerve section. In one male patient, the recordings were made prior to and after three months of the nerve section. They found that the previously present p13-n23 peaks were abolished after the operation (Colebatch et al., 1992; Colebatch et al., 1994). Interestingly, the later peaks (n34-p44) were still recorded from the operated ear in all the unilateral vestibular nerve section subjects. Because of this, they proposed that the later peaks depended on the integrity of the cochlea and the nerve innervating it. Since the initial report of cvemps by Colebatch et al. (1994), many other researchers have also reported that acoustic cvemps were only recorded following 1 In this entire report, the differences between monaural and binaural click stimulation are not investigated and discussed further. 79
96 monaural acoustic stimulation (i.e. the early peaks p13-n23 responses were always present on the ipsilateral side, and these early peaks were not recorded on the contralateral side of the stimulation). On the other hand, infrequent recording of the later peaks, n34-p44 were also reported, and those later peaks were reported to be present bilaterally (i.e. on both ipsilateral and contralateral SCM side), even during unilateral stimulation. With this, those researchers proposed that the p13- n23 peaks originated mainly from the ipsilateral side of the intact labyrinth, and also depended on the integrity of the inferior vestibular nerve. While for the later bilateral peaks, it was suggested that they are nonvestibular and probably cochlear in origin (Murofushi et al., 2001; Welgampola et al., 2005; Wu et al., 2003; Young et al., 2003). On the contrary, some researchers choose not to discuss further about these later peaks because they are not consistently recorded from the subjects (Zhou et al., 2004). The initial hypothesis of the acoustic cvemps pathways was made by Colebatch et al. (1994). They proposed the approximate neural conduction and synaptic transmission times based on their findings in the normal subjects, as well as the findings from guinea pigs (Didier et al., 1989) and cats (Precht et al., 1965). Colebatch et al. (1994) hypothesized that once the saccule was stimulated by the sound stimulus, the neural response transverses via the inferior branch of the VIIIth nerve (i.e. the vestibulocochlear nerves branch that innervates the saccule) to the lateral (Deiter s) nucleus of the vestibular nucleus at the brainstem level. The impulses then terminate in the ipsilateral LVST. Later, Todd et al. (2000) included the MVST as part of the pathways, as the impulses are sent to the neck muscles via that route. At present, the click cvemps have been accepted and widely applied clinically, and in most vestibular clinics it is now used as part of the vestibular test battery. However, air-conducted click stimulation is not applicable in conductive hearing loss patients, because it depends on a high level of stimulation (i.e db SPL). Colebatch et al. (1994) have found that in one of their subjects with an upper respiratory tract infection, the cvemps responses were completely abolished, but returned to normal level after the patient recovered. However, the problems in conductive hearing loss patients can be overcome by the application of bone conduction stimuli (Welgampola et al., 2003). Click cvemps have also been applied in Tullio phenomenon patients (i.e. patients with hypersensitivity to low-frequency sound). Brantberg et al. (1999) and 80
97 Colebatch et al. (1998) have reported that the thresholds to click stimulation have been pathologically reduced in their tested patients (i.e. patients were hypersensitive). This was expected because, in Tullio patients, it was common to have an extra third window (i.e. the opening on the roof of the superior SCC, which reduces the impedance of the saccule to the stapes stimulus, allowing greater endolymph displacement). The acoustic cvemps have also been clinically applied in patients with vestibular schwannoma. The vestibular schwannoma or acoustic neuroma involves the vestibular portion of the VIIIth cranial nerve and it arises from Schwann cells. Epidemiological studies have shown that this neuroma happens in more than 90% of cerebellopontine angle tumors (Chen et al., 2002). The vestibular schwannoma could either grow in the superior or the inferior branch of the VIIIth nerve, and by and large it grows only ipsilaterally. However, the neuroma can grow bilaterally in cases of neurofibromatosis type 2 2. Colebatch et al. (1992, 1994) reported that in their selected unilateral vestibular nerve section subjects, the p13-n23 peaks were absent on the side of the nerve section. In one male patient, the recordings were made prior to and three months after the nerve section. They found that the previously present p13-n23 peaks were abolished after the operation. Similar findings were found by Iwasaki et al. (2005), Patko et al. (2003), Takeichi et al. (2001) and Tsutsumi et al. (2000; 2001). On the other hand, acoustic cvemps were reportedly absent bilaterally in patients with bilateral vestibular schwannoma due to neurofibromatosis type 2 (Halmagyi et al., 2007) Short tone bursts acoustic stimulation Ever since the success of Colebatch and co-workers (1992; 1994) in describing the p13-n23 in normals using 0.1 ms click stimulation, many other researchers have tried to apply the same principle of testing, but using short tone bursts as the stimulus (Akin et al., 2003; Murofushi et al., 1999; Wang et al., 2004; Welgampola et al., 2001). They have found that 500 Hz and 1000 Hz short tone bursts, with 7 ms duration and repetition rates of about 5/sec was the best tone bursts. In this report, we do not discuss the application of short tone bursts in cvemps in great detail. 2 Neurofibromatosis type 2 is a congenital disorder which affects the central nervous system resulting in the growth of tumors on the affected nerve tissues. 81
98 4.3.3 Galvanic vestibular stimulation Galvanic vestibular stimulation (GVS) is the process of applying a small non-traumatic electrical direct current (DC) across the head to stimulate the vestibular receptors directly (the current is typically less than 3 ma, and can be simply produced by 3 Volts or two standard AA batteries). Clearly it is not a natural stimulus, but has the advantages that it can stimulate the vestibular system alone, without exciting other sensory systems, and without interfering with wholebody function (Day, 1999). GVS produces stereotypical postural and ocular responses (a lean of a standing subject, and a slight eye twist; Cass et al., 1996; Wardman et al., 2002; Wardman et al., 2003). GVS of the vestibular system has a long history in both animal and human investigations. In 1820, Purkinje provoked vertigo by DC electrical stimulation to the mastoid bone, and later Bárány experimented with GVS in humans (Cass et al., 1996). Despite long experience with GVS, its clinical potential has been largely unrealized, especially because detection of retrocochlear pathology was more efficiently and accurately performed using brainstem auditory evoked potentials, computer-aided tomography (CT), or magnetic resonance imaging (MRI). Although the clinical application of GVS has been limited, it has been applied extensively as a research tool. Studies of the effects of GVS on postural sway have proliferated (Cass et al., 1996; Fitzpatrick et al., 2004; Fitzpatrick et al., 1999; Marsden et al., 2002). The presence of postural effects suggests an action of GVS on otolith pathways, but it is still uncertain whether GVS acts only on a subpopulation of the vestibular afferent fibers (such as those innervating the otolith organs), or vestibular afferent fibers generally. When only the otolith organs are stimulated simple static eye torsion results, while stimulation of the SCCs mimics sustained head rotation, and evokes nystagmus (a saw tooth pattern of eye movement with many slow rotations, each followed by a fast saccadic reset). As mentioned earlier, in 1820, Purkinje described the effects of GVS on eye movements and posture. He reported horizontal nystagmus, and concluded that GVS mainly affected the SCC function. Based on animal studies, Wardman et al.(2002) have concluded that vestibular afferents from the otolith organs and SCC are affected similarly by GVS, while Watson et al. (1998b) measured evoked GVS with high DC currents (up to 5 ma) and found maintained torsion of both eyes in healthy humans without nystagmus, suggesting only otolith stimulation without 82
99 semicircular canal stimulation. Additionally, Watson et al. (1998a) using galvanic stimulation illustrated bilateral responses after unilateral galvanic stimulation over the mastoid process (with positive 13 ms and negative 23 ms on the ipsilateral side, and inverted responses on the contralateral side). Zink et al. (1997) suggested that lower currents ( ma) seemed to elicit predominantly tonic otolith effects (eye torsion without nystagmus) Bone conduction vestibular stimulation Bone conduction stimulation, using tone bursts stimuli can also be used to elicit cvemps. It has advantages over air conducted acoustic stimulation because it by-passes the middle ear conductive mechanisms, allowing its application in patients with middle ear problems (Miyamoto et al., 2006; Welgampola et al., 2003). Todd et al. (2003) and Welgampola et al. (2003) have investigated the properties and clinical use of bone conducted cvemps. They have found that the optimal stimulus frequency for the bone conduction cvemps was less than 300 Hz, because the cvemp amplitudes were largest at 250 Hz. It was also found that the bone conduction cvemps have a much lower threshold (i.e. only about db nhl, probably because the more efficient bone conduction stimulation bypasses the air conducted pathways and directly stimulated the saccule) as compared to the air conduction tone bursts (Welgampola et al., 2003; i.e. only about db nhl). These findings have made the bone conduction cvemps a useful alternative in clinical applications, especially in patients with conductive hearing losses, because in these patients it was not possible to obtain the cvemp responses using the air conducted acoustic stimulation Details of skull-tap cvemps After the initial development of acoustic click cvemps by Colebatch et al. (1992; 1994), Halmagyi et al. suggested a new way of evoking the SCM muscle potentials using a clinical reflex hammer fitted with an inertial trigger switch as a testing tool. Twenty normal adults and twenty other patients with different pathologies 83
100 volunteered in the research. They applied manual skull-taps on the subjects Fz 3 with their heads elevated while in the supine position 4. In healthy adult subjects, they found that there was a positive potential at 9.8 ms (denoted p 9.8), followed by a negative potential at 16.5 ms (denoted n 16.5). The average peak-to-peak amplitude was large (i.e. 243 µv), which was larger than responses found in cvemps responses evoked by clicks (Colebatch et al., 1992; Colebatch et al., 1994; 2006; Murofushi et al., 1998; Murofushi et al., 1999; Ochi et al., 2001; Sheykholeslami et al., 2001; Welgampola et al., 2005; Wu et al., 1999). On the other hand, in all their unilateral vestibular neurectomy subjects, they found that these two initial peaks were abolished on the side of the neurectomy. Contrary to that, they also found that these initial peaks were preserved on all their severe conductive hearing loss and sensorineural hearing loss subjects. The results with conductive and sensorineural hearing loss were consistent with the earlier acoustic cvemps findings found by Colebatch et al. (1994) and Welgampola et al. (2001, 2005), so the skull-tap responses were also thought to be from vestibular afferents. Halmagyi et al. (1995) also found additional waves after the two initial positive, p, and negative, n, peaks in normal subjects, as well as in the unilateral neurectomy subjects, however they did not accept that the later peaks were of vestibular origin, because often the averaged 16.3 ms negative peaks were not differentiated from the later peaks. To explain the reason why the peak-to-peak amplitude in the normal subjects in response to reflex hammer taps were so large compared to the acoustic click-evoked responses, Halmagyi et al. (1995) suggested that: 1. additional vestibular afferents were activated during the tap stimulation, and 2. the reflex hammer skull-tap offered a more effective stimulation method than clicks, and stimulated the same vestibular afferents (i.e. same pathways were activated as during acoustic click stimulation, but with skull-tap these afferents were activated in a more effective manner). Three years later in 1998, Iida attempted the same method of testing as Halmagyi and co-workers (1995) in three groups of adult subjects: twenty normals, three patients with central and six with peripheral vestibular lesions (in whom caloric responses were absent). Iida (1998) reported an average positive peak at 3 Fz is located on the very high forehead of a human. A labeled figure on the Fz position can be found in Chapter 3, page The method of testing while subjects were lying in supine position offers both SCM muscles on the right and left to be activated simultaneously. This means that both right and left vestibular system are evoked at the same time (as explained earlier in Section 4.3.1). 84
101 about 12 ms, followed by a negative peak at 17 ms in both the SCM muscles. These two early peaks were followed by additional peaks, however they did not discuss the possible causes of the later peaks. In their central lesion subjects, they found that the responses were the same as in their healthy subjects on the unaffected side, while different responses were evident on the affected side. They reported that the initial responses were totally eliminated in a patient with thalamic 5 infarction 6, while for both brainstem infarction and internuclear ophthalmoplegia 7, the initial peak latencies were elongated. In addition, in their tested peripheral vestibular lesion subjects, the responses were absent on the affected side (in patients with vestibulopathy and vestibular neuritis), while one patient with vestibular neuritis had longer latency responses on the affected side than the averaged responses from normals. They also found that the later peaks were not eliminated in normals or patients. Iida s (1998) findings were in total agreement with those of Halmagyi et al. (1995). They proposed that the skull-tap stimulation on the very high forehead (i.e. Fz) was not as efficient as click-evoked stimulation in obtaining accurate responses from the saccule. Halmagyi et al. (1995) had the view that tapping the skull involved complex compression waves traveling through the skull (i.e. with the involvement of bone conduction mechanisms). Furthermore, findings from their patients suggested that skull-taps were also influenced by both central and peripheral vestibular lesions. As a result, they suggested the involvement of a direct pathway with the innervations via the lateral nucleus (Deiters afferents), the thalamus and the LVST, because most vestibulocollic neurons are monosynaptically excited from the ipsilateral labyrinth (Rapoport et al., 1977). Iida (1998) also proposed a possible cause of the 12 ms delay in their normals. They proposed the possibility of other pathways besides the accepted direct pathways mentioned earlier that were responsible for the 12 ms delay. This was based on earlier studies by Shinoda et al. (1988), who suggested a central conduction delay of about 0.5 ms from the midpons to the neck motor neurons, and another 5.2 ms peripheral conduction delay from the neck motor nucleus to the SCM. Additionally, Iida (1998) also assumed that the direct pathway from the vestibular apparatus to the neck motor neurons via the brainstem 5 In an adult human brain, the thalamus is situated in the diencephalon part of the brain i.e. at the most forward part of the brainstem and it is veiled from sight by the cerebral hemispheres (Drake et al., 2005). 6 Infarction is defined as death of tissue (Weisbrodt et al., 1992). 7 Internuclear ophthalmoplegia is paralysis of one or more of the ocular or eye muscles due to disorders in between the biological nuclei (Oyster, 1999). 85
102 must have at least two synaptic delays. On top of that, they proposed another 1 ms conduction delay from the otolith organs to the midpons. Taking into account all of these suggested central and peripheral delays, the total conduction delay was approximately 7.9 ms. Although Halmagyi et al. (1995) and Iida (1998) successfully introduced the skull-tap technique and the click cvemp by tapping only on the midline of the skull, they were yet to prove laterality of the vestibular organ s responses. As a result, in 2002 Brantberg et al. attempted to clarify the role of stimulus direction and laterality on the skull-tap cvemps. They tested thirteen healthy adult subjects and also five patients with severe unilateral vestibular function (two of them were post-operative patients for removal of unilateral acoustic neuroma, while the remaining patients were treated using gentamicin due to Meniere s Syndrome). These patients had absent caloric responses from the affected ear post-operatively. Like the earlier research groups, Brantberg et al. (2002) tested all of the volunteered subjects using the clinical reflex hammer, while they were lying supine. Besides tapping on the midline forehead, this time the subjects were also tapped laterally, orthogonally above each ear on each side of the skull. Midline stimulation from the normals evoked similar responses as those reported by Halmagyi et al. (1995) and Iida (1998) (i.e. early positive peaks followed by negative peaks on both sides of the SCM). Nevertheless, tapping above the left ear elicited an average positive peak at 10.6 ms, followed by a negative 18.4 ms peak on the contralateral right SCM, and a first negative 8.8 ms followed by 17.3 peaks on the ipsilateral left SCM. As a result, tapping above the right ear produced opposite or inverted polarity peaks to the ones measured during the left ear tapping. Brantberg et al. (2002) concluded that tapping on the lateral side of the skull in normal subjects produced coordinated contraction-relaxation responses (i.e. peaks with opposite or inverted polarity on the same side, where the stimuli were conveyed), while on the opposite SCM, standard peaks were observed as with the usual acoustic cvemps obtained by other earlier researchers. Subsequently, they also found that the averaged responses with lateral taps were almost double the responses from midline-taps (approximately 180 µv versus 100 µv on right SCM and 150 µv versus 90 µv on the left SCM). In all of their unilateral vestibular loss subjects, Brantberg et al. (2002) reported that midline skull-taps generated positive-negative peaks only on the healthy side, and negativepositive peaks on the lesioned side. This was different from their findings in 86
103 normals. Analogous responses were also recorded when the pathological subjects were evoked laterally on the lesioned side. Contrary to that, lateral taps on the healthy side produced no responses on the healthy side, but small positive-negative peaks were visible on the opposite lesioned side. From these response patterns, Brantberg et al. (2002) had successfully demonstrated the direction-dependency in both normals and in the unilateral vestibular loss subjects. They also proposed the possibility of contralateral labyrinth activation, because tapping on the lesioned side still produced consistent negative-positive peaks as in normals, but surprisingly this did not happen when tapping on the healthy side. Earlier on, Watson et al. (1998a) using galvanic stimulation had illustrated bilateral responses after unilateral galvanic stimulation over the mastoid process (with positive 13 ms and negative 23 ms on the ipsilateral side, and inverted responses on the contralateral side) 8. As a result, Brantberg et al. (2002) also proposed that the single vestibular organ or labyrinth affected both SCM muscles (in this case, they suggested that a single contralateral labyrinth influenced both left and right SCM responses). A few years later, Brantberg et al. (2008, 2009) took a step further by applying an automated electro-mechanical tapper on the skull. This tapper enabled them to tap on the skull with a more consistent and controlled stimulus intensity than the clinical reflex hammer used previously (Brantberg et al., 2002; Halmagyi et al., 1995; Iida, 1998). In both studies, Brantberg et al. (2008, 2009) tested ten healthy adult subjects, and another ten subjects diagnosed with unilateral loss of vestibular function. While they were lying supine, the subjects were tapped on the midline (forehead, vertex and occiput) sites (2008), and also on each side of the skull, about 2 cm above each outer ear canal (2009). Consistent with the earlier findings, the grand averaged responses in normals evoked positive 13 ms peaks followed by negative 23 ms peaks on both SCM for all three midline stimulation sites, but the data suggested that the vertex responses were somewhat in between the forehead and occiput responses (the researchers averaged the forehead grand mean response with the occiput response to produce a virtual vertex response. This virtual resultant curve was similar to the vertex response observed). The amplitude from forehead taps was 121 ± 43 and 143 ± 73 µv on the right and left SCM, vertex was 107 ± 52 and 121 ± 74 µv on right and left SCM, and lastly at the occiput was 84 ± 34 and 97 ± 45 µv on right and left SCM. For the unilateral loss 8 See section for details. 87
104 subjects, the healthy sides were similar to the ones obtained in normals, but the peaks were inverted (negative peak followed by positive peak) on the lesioned side. There was also a significant difference in the stimulation sites between the healthy sides and the lesioned sides, with the occiput being significantly different from the other two sites. Brantberg et al. (2008) also subtracted the lesioned side cvemp from the healthy side responses, giving them similar responses regardless, of the sites tapped. They proposed that two different components were responsible for these findings. First, a sound (or vibration) component, and a second acceleration component. The sound component was most likely from the sacculus, which was known to be responsible for the early positive peak (Colebatch et al., 1994; Young et al., 1977). Later, Kushiro et al. (1999) suggested that the saccule projections were predominantly to the ipsilateral SCM motor neurons only. The acceleration component was said to be due to the utricular component, which was bilaterally projected to both SCM motoneurons (Kushiro et al., 1999), which caused the normal responses on the healthy side in the unilateral loss subjects and inverted responses in the lesioned side. Brantberg et al. (2008) also proposed that the anterior or superior semicircular canals might be responsible for the acceleration component, based on the earlier findings by Uchino et al. (1990). Lateral taps by Brantberg et al. (2009) in normal subjects were similar to Brantberg et al. (2002), where tapping on the right side gave approximately positive (inhibitory) responses at 13 ms, followed by negative (excitatory) responses at 23 ms on the contralateral left SCM. Similar responses were observed during left side tapping. Brantberg et al. (2009) also recorded similar responses in the unilateral loss subjects as their research in 2002, irrespective of the different stimulation method. With the aim of explaining these recorded responses, the researchers attempted to separate the left and right responses by deriving the difference between the left and right response and averaging the subtraction results. They believed that these remaining responses could have explained the underlying mechanism. The same approach was applied to their unilateral vestibular loss subjects. They hypothesized that the subtraction responses between the healthy side and the lesioned side would be similar to the subtraction responses from normals. However, their hypothesis was proven wrong, because the subtracted responses between the two groups were different. They then proposed that subtracting the responses between the left and right responses in the normals is not as straightforward as subtracting the responses in the vestibular loss responses. 88
105 In normals, the responses from the ipsilateral SCM muscle from both sides, which contributed to the early positive-negative peak, are still present, and this could have disguised the subtraction process, but in the unilateral vestibular loss responses, the subtraction process was more direct, because there were only responses remaining from one healthy side Summary of skull-tap study Halmagyi et al. (1995) had introduced the skull-tap cvemp stimulation method in an attempt to supplement the acoustic cvemp testing. This skull-tap stimulation proved to have an advantage over the acoustic stimulation, because it was not affected by conductive and sensorineural hearing losses. Thus, the skulltap stimulation could be applied clinically to diagnose purely vestibular pathologies, without the influence of the middle ear. Although the midline skulltap cvemps stimulation had an advantage over acoustic stimulation, it was still unable to determine the separate responses from each left and right vestibular labyrinth or different hair cell components. As a result, Brantberg et al. (2002) used the side-tap or the laterally directed simulation on each side of the head. They reported that in normal subjects, tapping on one side gave inverted cvemp responses on the tapping side (i.e. negative peak followed by positive peak), while normal cvemp on the contralateral side (i.e. positive peak followed by negative peak). While in their unilateral vestibular loss patients, they reported that the responses were also inverted on the lesioned side, but not on the healthy ear. From these findings, they suggested that laterally directed taps possibly elicited responses from the contralateral labyrinth. Years later, Brantberg et al. (2008, 2009) employed automated delivery of the skull-taps, and proposed two different mechanisms which might be responsible for the skull-tap stimulation. Their first mechanism is that there might be a local component, which is the vibrational response of the skull which does not depend on the stimulation site. Their second mechanism is the whole-head acceleration, or the translation of the head which is dependent on the stimulation site. They suggested that the combination of these two mechanisms resulted in the complex recorded responses. They also suggested that if the responses from these two mechanisms are separable, one might be able to determine the specific stimulated labyrinth, and possibly the individual hair cell groups within that labyrinth. 89
106 4.3.6 Electrode placement of the cvemps Early experiments with acoustic stimulation were made with the active electrodes placed at the inion (Bickford et al., 1964 and later Cody et al., 1969). However, in 1992 and 1994, Colebatch et al. changed the recording site, by placing the active electrodes on the upper half of the SCM muscle. The changes were made because they proposed that by choosing the SCM as the specific recording site would allow greater certainty rather than the midline recording site (i.e. inion). With the changes made, they successfully obtained the p13-n23 responses with acoustic stimulation in all their healthy subjects. Seven years later, Sheykholeslami et al. (2001) investigated the effect of the SCM electrode location on the cvemps. Using 500 Hz tone bursts on 15 normal adults, the active EMG electrodes were placed at four different positions: on the upper part of the SCM at the level of mandibular angle, the middle part of the SCM, the lower part of the SCM (i.e. just above the clavicle), and the fourth position was immediately above the sternum. The indifferent electrode was placed on the upper sternum. Their findings confirmed the results obtained by Colebatch et al. (1992, 1994), because they found that the middle part of the SCM was the optimal location because it gave the most consistent p13-n23 biphasic responses, with large peak-to-peak amplitudes. Later researchers of acoustic cvemps have also adopted this electrode montage in their studies (as in Figure 4.1) (Akin et al., 2003; Beyea et al., 2008; Cheng et al., 2003; Huang et al., 2005; Huang et al., 2006; Ochi et al., 2003; Welgampola et al., 2001; Welgampola et al., 2005). The same electrode montage was also used for the skull-tap cvemps (Brantberg et al., 2002; Brantberg et al., 2004a; Brantberg et al., 2003; Brantberg et al., 2009; Halmagyi et al., 1995; Halmagyi et al., 2008; Iida, 1998) EMG monitoring and activation of SCM muscle in cvemps The degree of tonic muscle contraction during cvemp recordings is linearly correlated with the cvemp amplitude (Colebatch et al., 1994). Thus, it is vital for accurate monitoring of the EMG levels during cvemp recordings. Some researchers employed a simple and direct approach (i.e. by providing selfmonitoring visual feedback for the subjects; Akin et al., 2001; Colebatch et al., 1994; Murofushi et al., 1999) to maintain the EMG levels, while others calculated the post-recording corrected VEMP amplitudes (Brantberg et al., 2004b). 90
107 As previously described, cvemps depend on the strength of the activated muscle tone. Earlier researchers either recruited the cvemp responses bilaterally (i.e. subjects were lying supine and their heads were raised during the SCM activation), or unilaterally (i.e. the subjects were sitting upright with their heads rotated laterally to one side, parallel with the shoulder). Despite the fact that the bilateral SCM activation via the head elevation method shortened the total testing time (meaning less muscle fatigue; Wang et al., 2003), false-positive responses were often encountered in subjects who were unable to sustain SCM muscle activation with the head elevation method (Wang et al., 2006). In 2006, Wang et al. studied the effect of head elevation versus rotation with monaural 500 Hz short tone bursts stimulation. In their study, all subjects were tested while they were lying supine, but during the elevation, a pillow was placed under their head to elevate the neck about 30 degrees from the horizontal plane. During the rotation trials, no pillow was placed under the subjects head, and they were instructed to rotate their heads sideways towards one shoulder. Comparison between the responses in those two different trials were made. They reported that the head rotation method gave lower response rates with smaller amplitudes than the head elevation method. Nonetheless, they suggested that the head rotation method served as an option in clinical settings where patients were unable to sustain the bilateral SCM activation via the head elevation method, and this was important in reducing false-positive diagnosis Acoustic versus skull-tap cvemps Earlier in Section 4.3.5, we discussed the findings of Brantberg et al. (2002, 2008, 2009), who evoked the responses from volunteer subjects using a clinical reflex hammer at the midline, and from the lateral side of the skull. Additionally, their responses were also evoked acoustically, using binaural click stimulation. Their findings in normal subjects using binaural click stimulation were entirely in agreement with Bhagat (2006) and Wang et al. (2003) (see Section 4.3.1), where binaural clicks generated positive peaks with shorter latencies followed by negative peaks on both sides of the SCM (in this case they recorded approximately 11 ms positive peak and 19 ms negative peaks). These findings were similar to the midline skull-tap responses. However, in contrast to the lateral skull-taps, initial positive peaks only occurred on the side of the SCM contralateral to where the lateral-taps took place, while on the same or ipsilateral side of the SCM, initial 91
108 negative peaks were recorded. Brantberg et al. (2002) also reported that the amplitude for the midline and lateral skull-taps were almost double (about 100 µv) and triple (about µv) the size of the acoustic responses (about 50 µv). Furthermore, in all their unilateral vestibular loss subjects, normal (positivenegative) click responses were recorded on the healthy side, and no responses were observed on the lesioned side. In comparison, as discussed in Section 4.3.5, positive-negative peaks were visible on the healthy side during midline-taps and lateral-taps on the lesioned side, and inverted peaks were visible on the other side. Small positive-negative peaks were also recorded on the opposite lesioned side with tapping on the healthy side, and no responses at all were seen on the same healthy side. Earlier researchers using click-evoked cvemps have suggested that the saccule was probably responsible for the neural firing which generated inhibitory postsynaptic potentials in the ipsilateral SCM motor neurons (Kushiro et al., 1999; Sheykholesami et al., 2002; Townsend et al., 1971; Young et al., 1977). Furthermore, the utricular nerve was shown to evoke excitatory postsynaptic potentials in both ipsilateral and contralateral SCM motor neurons (Kushiro et al., 1999). 4.4 Material and Methods We started our cvemp experiments with acoustic stimulation in the healthy adult subjects. The acoustic cvemps were conducted as a routine test in all of our subjects. Only monaural stimulation with unilateral SCM activation was used in this study. This was because it was an established test protocol, and is widely used in the vestibular clinical settings. Subsequent to that, four subjects were tested using the tester s palm as the stimulator (the palm-pulse cvemps). Before collecting data from many more healthy subjects, we decided to apply the palm-pulse stimulation in vestibular pathology subjects to check whether it was abolished (and therefore vestibular in origin). After analyzing the results from both the normals and the pathological subjects, we decided to abort the use of the palmpulse, because it was too intense (more explanation can be found in the Results and Discussion Sections). We ended our cvemp experiments by recording their responses in three healthy adults to finger-tap stimulation (the finger-tap cvemps). Only three subjects were recruited for this purpose. The finger-tap cvemp experiments were carried out when we had already obtained reliable ocular vestibular evoked 92
109 myogenic potentials (ovemps) 9 to finger taps. We also conducted two control measurements in these three subjects to make sure that the finger-tap cvemps were not contaminated by other responses. The first control experiment involved the use of a hand-held vibrator that was positioned on various sites on the head to mask the finger-tap responses. The second experiment involved the subjects being tested while they were sitting upright (our normal test position) and while supine (a position normally used by others). The assumption was that skin-evoked responses would not be masked by the vibrator, and that only vestibular responses would be position-dependent. All the acoustic, palm-pulse and finger-tap protocols in the normal and pathological subjects were conducted with subjects sitting in an upright position. As discussed earlier in Chapter 3, this was because we were anticipating a test environment where there would be no hospital beds. We wanted to provide an option for different testing conditions (because most of the earlier researches were conducted with the subjects lying supine on hospital beds). Secondly, because the upright position is the usual position for otolith function. Also, we were anticipating problems that may have encountered by the elderly and pathological subjects, who might have backache. We found that sitting upright was more robust for them, compared to lying flat on their back, especially during the bilateral SCM muscles test routine. Their failure to successfully activate both SCM muscles as a result may cause false-positive responses. The palm-pulse responses were obtained with the pulse strength varied by the tester from the pre-set threshold value of 0.16 g units (1 g = 9.8m/sec²), up to a maximum of about 2.0 g level. The whole cvemp input-output function was obtained with palm-pulse stimulation, while the finger-taps were used at maximal levels only. Finally, we only describe the methodology briefly, because similar methods are presented in Chapter Subjects A total of seven healthy adult subjects (three males, four females; aged between years) with no known history of hearing or vestibular problems participated in this part of the research. We also recruited two vestibular pathology subjects with diagnosed bilateral loss (aged 38 and 68 years old). All subjects were 9 The details for the ovemps can be found in Chapter 5 of this report. 93
110 informed of the aims and procedures of this study (Appendix 3 and 4) and gave their consent (Appendix 5). They had read and signed a consent form, which had been approved by The University of Western Australia Human Research Ethics Committee (Ethics Approval Project no. RA/4/1/1496) General recording preparation Before recording, the skin was cleaned briskly with 75% ethanol on gauze. A contact resistance less than 5kΩ was typical. All SCM potentials were measured using a large circular Cleartrace electrode from Conmed, with extra electrode gel. The active electrodes were placed on the upper two thirds on both the left and right muscle of the SCM, while the indifferent electrodes were placed on the upper tendon of the muscle (approximately cm below the mastoid bone). The reference electrode was placed on the nape of the neck. This electrode montage was used because we wanted to avoid any inconvenience in subjects with chest hair. In Section in Chapter 3 we showed that there was no significant latency shift and amplitude difference between our preferred montage and the montage used by others (i.e. with the reference electrodes placed on the lower tendon of the SCM muscles) SCM muscle activation All subjects were tested while they were sitting in an upright position. Prior to testing, subjects were given instructions and a practice session. They were instructed either to activate their left or right SCM muscle one at a time (unilateral activation), or both muscles at the same time (bilateral activation). The acoustic stimulation recordings only involved unilateral activation, while the palm-pulse recordings involved both the unilateral and bilateral activation. The final part of the recordings, which involved the finger-tap cvemp protocol, was only conducted with the bilateral and unilateral activation of the muscles Acoustic cvemps All seven healthy adult subjects including two pathological subjects participated in the acoustic recordings. We used longer monophasic 0.5 ms clicks 10 as the stimulus, rather than the typical 0.1 ms clicks or 500 Hz tone bursts. The More explanations on the reason why we used the 0.5 ms clicks, rather than the usual 0.1 ms clicks were explained in details in Chapter 3, Section
111 ms clicks were synthesized as wave files, using Sound Forge 4.5, were output from the soundcard of a personal laptop computer, and were amplified by an external battery-operated audio amplifier, before being presented at 130 db SPL peak. The acoustic stimuli were presented using ER3A insert earphones to the left and right ears separately Palm-pulse cvemps Four adult healthy subjects (three females and one male; aged between years) and two vestibular pathology subjects (aged 38 and 68 years old) participated for this experiment. The palm-pulse stimulation was delivered on five different tap sites around the subjects head: at the midline sites (Fz, Cz and Oz) and lateral sites (T3 left and T4 right) during bilateral SCM muscles activation and four tap sites: all midline sites and T3 left during left SCM activation and T4 right during right SCM activation Finger-tap cvemps For this final part of the cvemp protocol, only three adult healthy subjects (two males and one female; aged between years) were recruited. The recordings were done only with bilateral and unilateral activation of the SCM muscles. The stimulation sites were the same as the palm-pulse cvemps, but for the finger-tap protocol, we added more stimulation sites: on both the left and right side of the mastoid bone, and also the angular taps at the Left Anterior-Right Posterior canal (LARP) axis and Right Anterior-Left Posterior canal (RALP) axis directions 11 (Figure 4.2). From Figure 4.2, we see that the LARP canal axis actually corresponds to the RALP plane, and the RALP canal axis corresponds to the LARP plane (Halmagyi et al., 2004). We decided to include these angular taps in our finger-tap cvemps in the normal subjects, because both the saccular and utricular maculae in humans are not just a simple plane, but they are actually curved structures (Naganuma et al., 2001; 2003) The LARP and RALP terms are used as an expression for the SCCs which were involved during the head impulse testing for the VOR 12 More explanations of these can be found in Chapter 1. 95
112 x axis RALP axis y axis LARP axis (from Halmagyi et al., 2004) Figure 4.2: The graphical view from above a subject s head for the Left Anterior - Right Posterior canal (LARP) and Right Anterior Left Posterior canal (RALP) axis. As defined here, the RALP axis is within the LARP plane and vise versa. These descriptions on the LARP and RALP axis, which corresponds to the RALP and LARP planes, were used in the head impulse test in the vestibular clinics. 96
113 4.5 Results in normal subjects In describing the various peaks of the cvemps, we have adopted two different systems of naming the peaks. First, by using a lower case letter in describing the approximate latency (in ms) of the positive (p) and negative (n) peaks (e.g. a positive peak at 15 ms was described as p15, and a negative peak at 25 ms was described as n25). This approach has been used by many previous authors. Secondly, we have also adopted another system, using uppercase letters for these same peaks when describing the amplitudes and also the latencies on the basis of their sequential order along the various cvemp waveforms (e.g. P1, P2, P3 etc.). As mentioned earlier, the latencies and amplitudes for palm-pulse cvemps were obtained in four healthy adult subjects. While the finger-tap cvemps were obtained in three different healthy adult subjects. For both stimulations, the recordings were done for both bilateral and ipsilateral activation of the SCM muscles. For palm-pulse and finger-tap stimuli, the responses were subdivided into midline-sites (No, Fz, Cz and Oz) and lateral- or side-sites (T3, T4). Additional to that, for finger-tap cvemps, both left and right side of the mastoid, and also the LARP and RALP directions were stimulated. The input-output functions were recorded for the palm-pulse stimulation (between the pre-set threshold value and the maximum), while the finger-tap responses were recorded only with maximal stimulation. This was because the palm-pulse protocol was conducted first, and at that early stage we wanted to investigate the whole range of possible responses from the stimulation. On the other hand, the finger-tap protocol was conducted last (i.e. at the end of the whole series of experiments), after we had an understanding of the sensitivity and saturation of the ovemp responses (as discussed later in Chapter 5). Note that all acoustic, palm-pulse and finger-tap responses discussed below were autonormalized, unless stated otherwise Click cvemps In all seven healthy subjects, monaural 0.5 ms acoustic clicks were presented to the left and right ear, subsequent to the ipsilateral left and right unilateral SCM muscle activation. We recorded biphasic positive-negative peaks in the activated muscle (data summarized in Table 1.1). Stimulation on the ipsilateral left SCM activation resulted in an early positive peak, 14.7 ± 0.7 (mean ± SE), 97
114 followed by a later negative peak, 24.3 ± 1.1. Ipsilateral right SCM activation also resulted in an early positive peak, 12.9 ± 0.2, followed by a later negative peak, 21.8 ± 0.8. There was no significant difference between the positive and negative peak latencies at the left and right SCM (p > 0.05). We recorded bigger P1N1 peak-to-peak (pp) amplitudes on the right SCM (i.e ± 18.6) units pp compared to the left SCM (i.e ± 19.9) units pp, however there was no significant difference (p > 0.05). All values were obtained from the autonormalized waveforms responses. Left SCM Right SCM P1N1 amplitude (units pp) ± ± 18.6 Mean P1 latency (ms) 14.7 ± ± 0.2 Mean N1 latency (ms) 24.3 ± ± 0.8 n = 7 n = 7 Table 4.1 Peak-to-peak amplitudes and latencies with unilateral SCM activation and with ipsilateral 0.5 ms acoustic clicks, obtained in seven normal healthy subjects (n=7). These values were obtained from the auto-normalized waveforms, which is the probability units values Palm-pulse cvemps Response latencies Figure 4.3 A and B, Figure 4.4 and 4.5 show average latency data from four healthy adult subjects, where the change in latency with tap strength is plotted for the five tap sites: midline sites (Fz, Cz and Oz) and lateral sites (T3 left and T4 right) during bilateral SCM muscles activation and four tap sites: all midline sites and T3 left during left SCM activation and T4 right during right SCM activation. On the right side of each Figure is a sample of the auto-normalized waveform obtained from one subject at the maximal tap strength. The asterisk (*) indicates that only one subject had that particular response at that particular peak latency at the specified tap strength. For bilateral SCM muscle activation (Figure 4.3 A), all four subjects gave early inhibition peaks (P1) followed by excitation peaks (N1) at both left and right muscles with all midline stimulation sites. Note that the grey area on the right SCM muscle responses following Fz taps stimulation. We have marked this area to highlight two missing peaks, which interestingly happened at the mid latency region of both inhibition (positive, P) and excitation (negative, N) peaks. Despite these two missing peaks, the rest of the peaks for both left and 98
115 right SCMs were similar. The possible reason for the missing peaks in all four subjects were: (i) when the palm-pulse stimulation landed on the subjects head, it might have pushed the heads in a certain direction that caused the two peaks to be missing (ii) the acceleration of the heads were not equal for both the left and right SCM muscles as the palm-pulse landed on the heads. That was why only the right SCM muscles have the missing peaks, and not the left SCM muscles. From Figure 4.3 B, left T3 side-taps gave an early N1 peak on the left SCM and an early P1 peak on the right SCM, and right T4 tap gave an early P1 peak on the left muscle and an early N1 peak on the right muscle. It was obvious that side-taps recruited early excitation peaks on the ipsilaterally stimulated and activated SCM (left T3 tap gave early N1 peak from left muscle and right T4 tap gave early N1 peak from right muscle). Figure 4.4 and 4.5 present latencies for unilateral left and right SCM muscle activation only. For the midline-tap stimulation, both left and right muscle activation gave early P1 peaks on the activated muscles, contrary to the inhibited sides, which gave early N1 peaks, except for the Cz tap which also gave early P1 peaks. Left T3 taps gave an early P1 peak in the left activated muscles, and N1 from the right inhibited muscle (Figure 4.4). These responses were opposite to the one obtained from both muscles during bilateral activation after T3 taps. Contrary to unilateral left activation, right SCM activation gave early N1 peaks on the unilateral right muscle activation after T4 side taps, and early P1 peaks on the inhibited left muscle. These responses were consistent with the responses obtained from the right muscle during bilateral muscle activation for T4 side-taps. We also note that for the unilateral activation (Figure 4.4 and Figure 4.5), most of the later peaks were only observed in a single subject (marked with the * signed). 99
116 Peak latencies vs. tap intensity for bilateral activation Figure 4.3 A Figure 4.3 A 100
117 Figure 4.3 A: Changes in latencies of the various cvemps peaks recorded at the left and right SCM muscles as shown with an increase in strength of taps at the midline stimulation sites during bilateral SCM activation. Upward is increasing tap strength (evenly spaced from 0.08 to 2 g). On the right side of this Figure is a sample of an auto-normalized waveform obtained from one subjects at the maximal tap strength. The grey area following Fz stimulation marked the two missing peaks on the right SCM. The black waveforms represent the left SCM muscles, while grey waveforms represent the right SCM muscles. Error bars represents +/- one standard error. 101
118 Peak latencies vs. tap intensity for bilateral activation Figure 4.3 B: As in the earlier Figure, this Figure represents the changes in the latencies at the side tap stimulation sides during bilateral SCM activation. Figure 4.3 B: As in the earlier Figure, this Figure represents the changes in the latencies at the side tap stimulation sides during bilateral SCM activation. 102
119 Peak latencies vs. tap intensity for unilateral left SCM activation Figure 4.4: As in the earlier two Figures, this Figure represents the changes in the latencies during unilateral left SCM activation. 103
120 Peak latencies vs. tap intensity for unilateral right SCM activation Figure 4.5: As in the earlier three Figures, this Figure represents the changes in the latencies during unilateral right SCM activation Response amplitudes 104
121 Figure 4.6 A and B to Figure 4.8 present the relative auto-normalized amplitudes, corresponding to the latency data from the subjects shown in Figure 4.3 A and B, Figure 4.4 and 4.5. Maximal responses following Fz taps were used as a definition of 100% amplitude. All peak amplitudes have been normalized in each subject using a single P1N1 peak-to-peak amplitude for each muscle during bilateral SCM activation. For the unilateral left and right activation, the single P1N1 from the activated muscle from Fz tap responses and N1P1 from the inhibited muscles were used for the normalization. These peaks for the Fz stimulation were chosen because we found that they were present in all tested subjects, and because Fz was the stimulation site used by others (Brantberg et al., 2002; Brantberg et al., 2008; Halmagyi et al., 1995; Iida, 1998). Normalized amplitudes were employed to minimize variability due to electrode placements and/or SCM muscle activation. With the increment of the tap strength, one might expect that the cvemps responses would grow in proportion with the increment of the tap strength, but this was not the case, as seen from Figure 4.6 A and B, 4.7 and 4.8. All of the peaks did grow, but saturated at low tap intensities for almost all the stimulation tap sites. Note that not all tested healthy subjects had all peaks. Before recruiting more healthy subjects, we applied the palm-pulse stimulation method on vestibular loss subjects. Detailed results from the pathological subjects are discussed in Section below. Based on the finding from the normals and subjects with pathologies, we discontinued the use of palmpulse stimulation. The reasons for this are discussed below in Section
122 Tap intensity vs. peak amplitudes for bilateral SCM activation Figure 4.6 A 106
123 Figure 4.6 A: Normalized amplitude functions at the midline stimulation sites corresponding to the latency data from the subjects shown in Figure 4.3 A. Maximal responses following Fz taps were used as a definition of 100% amplitude. All peak amplitudes have been normalized in each subject using a single P1N1 peak-to-peak amplitude for each muscle during bilateral SCM activation. 107
124 Tap intensity vs. peak amplitudes for bilateral SCM activation Figure 4.6 B: Normalized amplitude functions at the side (T3 and T4) stimulation sites corresponding to the latency data from the subjects shown in Figure 4.3 B. Note that for the missing peaks, the label is in grey. 108
125 Tap intensity vs. peak amplitudes for unilateral left SCM activation Figure 4.7: Normalized amplitude functions corresponding to the latency data from the subjects shown in Figure 4.4. Note that for the missing peaks, the label is in grey. 109
126 Tap intensity vs. peak amplitudes for unilateral right SCM activation Figure 4.8: Normalized amplitude functions corresponding to the latency data from the subjects shown in Figure 4.5. Note that for the missing peaks, the label is in grey. 110
127 4.5.3 Finger-tap cvemps As stated in the Introduction (Section 4.1), the finger-tap cvemps were investigated last during this study, after we had found finger-taps to be more promising and more consistent than palm-pulse stimulation, and following ovemp recording from the extraocular muscles (described in detailed in Chapter 5) Number of response averages To investigate the number of averages needed to give reliable and consistent responses, we conducted a trial in one healthy adult subject. The recordings were obtained with left SCM muscle activation and with finger-taps applied on the RALP site. The responses were observable with a minimum of 20 averages (n = 20) up to a maximum of 200 averages (n = 200). Two replicate responses were obtained to show that these responses were reliable and repeatable. Clearly from Figure 4.9, 100 averages (n = 100) were more than sufficient for reliable results. 111
128 RALP tap with activated left SCM Figure 4.9: Responses obtained from a single subject, after RALP tap with activated left SCM. Different number of averages was used during recording and two responses were obtained for each From Figure 4.10, we knew that RALP stimulation during left SCM activation recruited responses from the left SCM muscle. Note that these are auto-normalizes waveforms, and the un-normalized waveforms from the activated left SCM were about ten times larger than the right SCM responses. 112
129 Details of the finger-tap cvemps We have recorded finger-tap cvemps in three healthy adult subjects with bilateral SCM muscles activation, and also left and right unilateral activation. Initially we took the simplest approach (looking at the subjects responses with bilateral SCM muscle activation), because we anticipated that these responses would be simpler than the unilateral activation responses. We also anticipated that both muscles on the left and right side should produce approximately similar responses. From Figure 4.10, the bilateral finger-tap cvemps responses had multiple peaks, with slightly different wave-shapes and latencies. All responses were obtained twice. These replicate responses proved that the finger-tap cvemps were repeatable in each subject. We initially supposed that Fz taps (which was the stimulation site used by earlier researchers) would give the strongest and most reliable responses, but this was not the case. From Figure 4.10, Oz midline-taps seemed to give strongest responses in all three subjects (with bigger amplitudes), when compared to Fz and Cz taps. We also found that, compared with Fz taps, the Cz responses were strong in Subjects 1 and 2, but not in Subject 3. To support this statement, we provide the detailed relative auto-normalized amplitudes for these responses in Table 4.2. It was obvious that Oz taps gave auto-normalized amplitudes on both sides that were bigger than Fz and Cz taps. Figure 4.11 presents the corresponding responses of Figure 4.10, but this time the responses were overlaid to give a straightforward comparison between the two left and right activated muscles. Black waveforms (and also the black stimulation site labels) represent the left SCM muscle, while grey waveforms (and grey stimulation site labels) represent the right SCM muscle. Midline-taps responses gave similar responses from both muscles, with early inhibition P1N1 peaks for Fz and Oz taps, but excitation N1P1 peaks for Cz taps. From Table 4.3, we could see that the N1P1 latencies of both muscles peaked earlier than both the Fz and Oz responses. Note from these Tables that some peaks were only obtained from a single subject (in this case, no standard error of mean was available). For side-taps, we expected that the ipsilateral stimulation (i.e. left T3 stimulation on the left SCM and right T4 stimulation on the right SCM) might produce mirror image responses, and this would apply for the contralateral stimulation. This was also expected for right mastoid and left mastoid responses. Thus, these pairs were overlaid for the left and right muscles (Figure 4.11). 113
130 Bilateral SCM activation Figure 4.10 Figure
131 Figure 4.10: Averaged cvemp responses (n=50 to 80) at the left and right SCM muscles following bilateral muscle activation from three subjects (S3-S3) in response to maximal taps (1.0 to 2.0 g) at the sites indicated (two replicates waveforms shown). All traces share the same scale. Vertical dashed lines mark the peak of the trigger stimulus (0 ms). Responses obtained from (A) midline taps [Fz, Cz, Oz], (B) side taps [T3, T4, left mastoid (Lt mas), right mastoid (Rt mas)] and (C) angular taps (LARP, RALP). 115
132 116 Table 4.2: Detailed relative auto-normalized amplitudes for the midline, side and angular taps following bilateral muscles activations. These amplitudes responses are the corresponding values for the waveforms amplitudes in Figure 4.10.
133 Table 4.3: Detailed latencies for the midline, side and angular taps following bilateral muscles activations. These amplitudes responses are the corresponding values for the waveforms latencies in Figure
134 Bilateral SCM activation with overlapped left and right waveforms Figure
135 Figure 4.11: Corresponding responses of Figure 4.10, but in this Figure the responses were overlaid to give a straightforward comparison between the two left and right activated muscles. Black waveforms (and black site labels) represent the left SCM muscle, while grey waveforms (and grey site labels) represent right SCM muscle. 119
136 We also paired these for Table 4.2 and Table 4.3 for easier comparison. T3 and T4 ipsilateral stimulation evoked excitation (N1P1) for both muscles, but contralateral stimulation evoked inhibition (P1N1) for both muscles. Later peaks for both muscles after T3 and T4 stimulations were not identical in all three subjects. On the other hand, left and right mastoid stimulation produced quite similar responses from both muscles in Subject 2, but not for the other two subjects. For angular taps, the LARP and RALP directions may have been the natural axes for the hair cells. This appeared so because the recorded responses are quite similar on both muscles, except for Subject 3 s RALP response on the right SCM (Figure 4.11). As described earlier, the LARP tap s direction actually corresponded to the RALP plane, and vice versa (Figure 4.2). We would suggest that both the angular stimulation sites (i.e. the LARP and RALP) may have recruited responses from both the SCCs and the otoliths. This was because the angular taps caused the subjects head to be slightly tilted up and down ( torsional movement), and at the same time the head was accelerated linearly. During this angular stimulation, we proposed that the saccule would not have primary role in the recorded responses, but the utricles might have been the primary organs for the evoked responses. This was because the utricle with its complex anatomy structure (it has ellipsoidal curve-like structure) was in close proximity with the SCCs, i.e. approximately sitting below the anterior canal and in front of the utricle. Thus, with the torsional and linear acceleration during the LARP and RALP, the utricle could have been most stimulated. Also, in 2001 and 2003, Naganuma et al. reported that the otolith organs were more capable of identifying a much wider range for linear accelerations. However, at this stage we could not determine the exact group of the sensory organs (i.e. the hair cells) which were involved during this stimulation. As previously discussed in Chapter 1, Section 1.2, Type I hair cells project mainly to the vestibulo-spinal system, while Type II hair cells project mostly to the oculomotor system. However, we believe that there is considerable overlap between these two, because of the complexity of the otolith structure. This argument is also supported by Curthoys (2007). Figure 4.12 and 4.13 show responses obtained from unilateral left and right SCM activation. We hypothesized that the unilaterally activated muscle would produce more prominent peaks with better repeatability. As expected, midline-taps evoked stronger peaks from the ipsilaterally activated muscle compared with the non-activated muscle. This was also true for the side-tap and angular-tap 120
137 stimulation. However, in agreement with the bilateral activation (Figure 4.10), Subject 3 gave poor responses for ipsilateral LARP and RALP during both left and right unilateral SCM activation. Note that these Figures (Figure 4.10 to Figure 4.13) were not grand averages, but were the auto-normalized responses obtained twice for each subject (with n = 100 for each response) EMG activation levels on finger-tap cvemps We were also interested to see the effect of different EMG activation levels on the evoked responses. Figure 4.14 shows raw and auto-normalized responses from two healthy subjects with left T3 taps with bilateral and unilateral right SCM activation. The unilateral right activation was at three different EMG activation levels (100%, 50% and 25% activation based on the left and right rms EMG meters on the software) 13. Note that prior to recording, the subjects had practice sessions to determine the level of their EMG activity. From this Figure, it was obvious that Subject 1 gave approximately identical waveforms with both bilateral and unilateral activation on the activated right SCM. However, 100% unilateral right activation gave much bigger responses than those with bilateral activation. We could not make the same comparison with Subject 2, because the morphology of the evoked waveforms were different with bilateral and unilateral activation. However, note that the raw responses from both subjects had obvious differences (i.e. the raw amplitude of the response were bigger at 100% activation, and the raw amplitude reduced to half at 50% and about a quarter at 25% activation). However, the auto-normalized responses were approximately the same, despite the changes in the EMG activation level during the unilateral right SCM activation. 13 Details on how these meters worked were thoroughly explained in Chapter
138 Unilateral left SCM activation Figure
139 Figure 4.12: Responses obtained from unilateral left SCM activation. Averaged cvemp responses (n=50 to 80) obtained from three subjects (S3-S3) in response to maximal taps (1.0 to 2.0 g) at the sites indicated (two replicates waveforms shown). All traces share the same scale. Vertical dashed lines mark the peak of the trigger stimulus (0 ms). Responses obtained from (A) midline taps [Fz, Cz, Oz], (B) side taps [T3, T4, left mastoid (Lt mas), right mastoid (Rt mas)] and (C) angular taps (LARP, RALP). 123
140 Unilateral right SCM activation Figure 4.13: Similar with Figure 4.12, but in this Figure, the responses were obtained following unilateral right SCM activation. 124
141 Subject 1 Left SCM muscle Right SCM muscle Raw Auto-normalized Raw Auto-normalized Subject 2 Figure 4.14: Raw and auto-normalized responses (n=50 to 100) from two healthy subjects (S1 and S2) following left T3 taps with bilateral and unilateral right SCM activation (two replicates waveforms shown). The unilateral right activation was at three different EMG activation levels (100%, 50% and 25% activation based on the left and right rms EMG meters on the software). Vertical dashed lines marl the peak of the trigger stimulus (0 ms). 125
142 Control measurements To confirm that the observed responses to the finger-taps were the result of vestibular stimulation, we carried out two different controls. First, we used a handheld vibrator to mask responses by vibrating the head and, second, we used different head positions in an attempt to mechanically bias the vestibular hair cells. The first experiment with nose vibration was done as an attempt to mask the finger-tap cvemps assuming that non-vestibular responses would be unaffected. The recordings were done in two healthy adult subjects. From Figure 4.15, the black waveforms represent the cvemps obtained at Oz, T3, T4, and left and right mastoid stimulation sites, with either right or left unilateral SCM muscles activation, but no vibration masking. In this Figure, only the auto-normalized waveforms are shown. It was obvious that all the cvemp peaks on both SCM muscles were totally masked with the head vibrator, and for all stimulation sites. Thus, we conclude that, despite the differences in the waveform shapes in the subjects (as discussed earlier), the observed and recorded responses were a true vestibular response. During the second experiment, a subject was tested while he was in our normal testing position (i.e. sitting upright), and later in the supine position (i.e. the normal position used by earlier researchers). Finger-taps were delivered at Fz, right T4 and left T3 stimulation sites during bilateral SCM activation. From Figure 4.16, we see that the responses were similar between the raw and auto-normalized responses, and that responses at supine positions after Fz and right T4 taps were bigger compared to during upright positions. However, the recorded responses were quite similar for both positions with left T3 taps. This suggests that some cvemps responses to some stimuli can be biased by postural changes. This again suggests a vestibular origin and emphasized the importance of posture during clinical tests. 126
143 Figure 4.15: Masked responses obtained from two subjects (n = 100). Black waveforms represent the control cvemps' responses, while the grey responses are during masking responses produced by applying a vibrator to the nose (two replicates obtained for each response). Positive polarity is up. Vertical dashed lines mark the peak of the trigger stimulus (0 ms). 127
144 128 Figure 4.16: Changes with posture in raw and auto-normalized responses from a single subject (n = 100) obtained with bilateral SCM activation (two replicates obtained for each response). Positive polarity is up. Vertical dashed lines mark the peak of the trigger stimulus (0 ms).
145 4.6 Discussion on the results obtained in normal subjects Responses of acoustic cvemps We used the acoustic cvemps in our study as a routine test to ensure that all the participating healthy adult subjects had some vestibular function. As expected, all normal volunteers showed the p13-n23 peaks, as reported by earlier researchers, and there were no significant differences between the left and right SCM responses Rational for the tap sites for the palm-pulse and finger-tap cvemps During the development of our cvemp protocol (as discussed in Chapter 3), we hypothesized that tapping each subject s head at different tap sites (i.e. midline, side or lateral, mastoid left and right, LARP and RALP), would give us a direct and clear answer about which particular hair cell groups were evoked during the stimulation. We also hypothesized that the same testing protocol on the vestibular pathological subjects would provide a better understanding about the nature of the evoked responses (e.g. absent peaks). By stimulating at different sites on the skull, we initially presumed that different stimulation sites would give clear and different responses, and that these responses would suggest the specific hair cells involved The palm-pulse cvemps We had first attempted the skull-tap stimulation by placing the piezoelectric transducer on the tester s wrist and by systematically tapping on the specific sites around the subjects heads with the tester s palm. From Section 4.5.2, we found that the palm-pulse stimulus was too intense. It caused significant changes in the latencies obtained (indicated by the large standard error of mean bars in Figure 4.3 A and B, Figure 4.4 and 4.5) and the corresponding autonormalized amplitudes were saturated for the lowest tap strength (Figure 4.6 A and B, 4.7 and 4.8). We hoped that these significant changes and the large standard errors in the latencies could be reduced by recruiting more healthy subjects to participate in the study. However, after considering the saturated amplitudes obtained from normals, the confusing bilateral results obtained from our pathological subjects, and the more delicate and controllable finger-tap technique, we decided to discontinue the palm-pulse stimulation altogether. 129
146 4.6.4 The finger-tap cvemps We had demonstrated that only 100 averages (n = 100) were needed to produce reliable and repeatable finger-tap cvemps (Figure 4.9). Thus approximately 1.5 to 2.0 minutes were needed to tap at each site (because the taps were delivered at about ½ second intervals). This meant that our subjects were not subjected to any fatigue due to a long testing time. Furthermore, the shorter testing time should mean cost effectiveness compared to other stimulation methods. We also observed that there were multiple peaks in the finger-tap responses for all stimulation sites, with both bilateral and unilateral SCM activation (Figure 4.10 to 4.13). From the midline-taps, we observed that the Oz responses gave more prominent responses than Fz and Cz sites. Both Fz and Oz gave initial positive inhibition responses, while Cz gave negative excitation responses. Our responses were not consistent with those of Brantberg et al. (2008) whose data suggested that the vertex responses were somewhat in between the forehead and occiput responses, with all three stimulation sites giving initial positive inhibition responses (these researchers averaged the forehead grand mean response with the occiput response to estimate a virtual vertex response). The virtual response they obtained was similar to their recorded vertex responses (i.e. with similar latencies and morphology with the first positive peak followed by second negative peak). Our side-tap responses at T3 and T4 agree with those of Brantberg et al. (2009). Additionally, we also recorded mirror-image response from the left SCM with ipsilateral side-taps compared with the right SCM with right ipsilateral T4 taps suggesting the possibility of crossed-innervations. All the early peaks approximate each other (Figure 4.11). We also recorded some possible prepotential responses on the left SCM with left mastoid taps in all three subjects, and also on the right SCM in Subjects 2 and 3. These pre-potential responses were also recorded on the right SCM with RALP stimulation in Subject 1, and with LARP stimulation in Subject 2. This pre-potential occurred at the very early latencies (approximately positive or negative 3 to 5 ms). We propose that this early pre-potential might be a stationary potential or far-field potential (Brown, 2006). A trigger artifact was not the cause of the early activity because our trigger occurred much earlier, and no artifact was visible at these latencies in sham traces. We also note that the later peaks for all responses with side-taps stimulation were different, even though the earlier two peaks were similar. We suggest that the similar peaks were recruited from the same group of hair cells and the same neural circuits. 130
147 However, this same mechanism and hair cell group did not seem to apply to the later peaks. The differences in the later peaks might suggest that (a) the same group of hair cells is stimulated, but they act with different latencies due to a different inertia of the hair cells; (b) there were other additional neural circuits which correspond to different threshold levels of the tap strength, and/or the firing rate of the neurons from the evoked hair cell groups could also be different; (c) as mentioned earlier, each human is different, in skull thickness and in their symmetry in the SCM muscles; (d) the SCM muscles may have their own unique muscle action potential that caused the difference in the recorded responses, not only between the subjects, but also the difference between the left and right muscle of the same subjects (e) our stimulation was not along the natural axes of the stimulated hair cells, and therefore did not provide one dominant mode of stimulation. Interestingly, we recorded bigger responses with the right unilateral activation method (i.e. subjects sitting upright, with the neck rotated laterally toward one shoulder) than with the bilateral activation (i.e. subjects sitting upright, while activating both muscles simultaneously by pressing against a block of Styrofoam in the first tested subject. Our findings in Subject 1 were not similar to the earlier findings of Wang et al. (2006), who reported that bigger amplitudes and higher response rates were recorded with bilateral SCM activation, as compared to unilateral head rotation. However, Wang and co-workers (2006) conducted their tests with all their subjects lying supine, and not in our upright position. Thus, we cannot make a direct comparison. The totally masked VEMP responses with the hand-held vibrator suggest that the cvemps observed were true vestibular responses, and were not trigeminal reflexes or due to any other skin reflex response 14, or even neck reflexes. If the recorded multiple peaks were from these other origins, it is unlikely that they would be masked so effectively by nose vibration. We also conducted postural bias testing on one subject (Figure 4.16), and recorded some bigger peaks when the subject was lying supine than when in our normal upright position. From Chapter 1, we know that the utricular maculae and saccular maculae of the otolith organs have different sets of receptor hair cells with their own set of complex responses. These hair cells have different firing patterns, 14 We have conducted control experiments using electrical stimulations during the development of cvemps (Chapter 3). 131
148 different response threshold levels, and also different polarized directions (saccular hair cells depolarized directions are facing away from the striola, while for the utricular hair cells are facing towards the striola) 15. Animal studies (Lysakowski et al., 2004) also show that neurons from Type I hair cells project predominantly to the vestibular spinal system, and suggest that the hair cells around the striola were probably responsible for our observed biased responses. However, Curthoys (2007) has suggested that there is considerable overlap between the Type I and Type II hair cells. Thus, the contribution of Type II hair cells must also be considered. We also know that the utricle is responsible for detecting vertical static stimulation, while the saccule is responsible for detecting horizontal static stimulation (see Chapter 1). This is because of their anatomical orientation in the temporal bone. As a result, we also propose that when the subject is sitting upright, most of the utricular hair cells dominate the responses, however when the subject was in the supine position, most of the saccular hair cells dominate. However, based on the findings of Naganuma et al. (2001, 2003), otolith organs are not simply flat in the vertical and horizontal planes. Their three-dimensional analysis of the human saccular macula and utricular macular has revealed that both the saccular and utricular maculae in humans are a curved-like structure of an ellipsoid. Thus, we propose that there is probably redundancy in responses between both the otolith organs. Recently Fujimoto et al., (2009) used tone bursts to see the effect of the SCC on cvemps. They found that there were functional interactions between the saccule and the posterior semicircular canal. They suggested that both anterior and posterior SCC have connections with the ipsilateral and contralateral SCM, while the saccule has only ipsilateral SCM connections. This suggests to us that at some point, the anterior and posterior canals might also be contributing to the multiple peaks observed in our study, but the primary cause of the evoked responses is mainly from the otolith organ. This is supported by Kushiro et al. (1999), who proved that the utricular nerve evoked excitatory post-synaptic potentials in both ipsilateral and contralateral SCM motor neurons. 15 The polarized directions of the hair cells in the otolith organs were shown in Figure 1.4 of Chapter
149 4.7 Bilateral vestibular loss subjects Besides conducting the cvemp testing on normal healthy subjects, we also conducted our testing on the bilateral vestibular loss subjects. Some of these subjects were diagnosed as having a vestibular loss due to the effect of the ototoxic drugs. Ototoxic drugs or ototoxins as defined by the Dictionary of Audiology are pharmaceutically known to be toxic to the inner ear structures (i.e. both cochlear and vestibular organs; Mendel et al., 1999). Among these drugs are aminoglycosides, which function as antibiotics against certain types of bacteria, or are also used during therapy treatment. The aminoglycosides include gentamicin, kanamycin, streptomycin and amikacin, and they are known to cause vestibular hypofunction bilaterally (i.e. loss of both left and right vestibular function). They have been reported to cause sudden irreversible loss of both vestibular functions (Fetter, 2007). We recruited two subjects (one male and one female), aged 38 and 68 years old, diagnosed with bilateral vestibular loss to participate in our study. Below are the details about the background of these subjects. (a) Patient 1: WW Our first subject, WW, aged 38 years old, was born in He was presented with severe bilateral vestibular loss with gait ataxia and oscillopsia due to the administration of gentamicin for his heart problem. He has no hearing problem and no other central nervous system problems. On vestibular examination, caloric and rotational chairs tests confirmed severe bilateral impairment of the horizontal SCC function. His horizontal and vertical vestibulo-ocular reflexes on head impulse test were also impaired. He attended therapy sessions to help him overcome his vestibular loss, and now he was coping very well. (b) Patient 2: LS LS was diagnosed of having bilateral vestibular loss with vestibular insufficiency. She notices ataxia and oscillopsia when walking. LS, aged 68 years old during the testing, who was born in 1941, had all of her vestibular loss problems developed in 1967, following treatment with streptomycin for five days, for Tuberculosis. She has bidirectional impairment of horizontal and vertical vestibulo-ocular reflexes on head impulse test. She has severely impaired 133
150 horizontal SCC function on caloric and rotational testing. However both her left and right hearing were fine. She is now physically very active and had adapted very well to her bilateral vestibular loss Results and discussion from bilateral losses subjects Similar to our normals, both of our bilateral vestibular losses subjects were monaurally tested with the 0.5 ms clicks, presented at 130 db SPL, while they were sitting upright, and head rotated to one side facing one shoulder. As expected, no acoustic cvemps were visible on both the left and right SCM muscles, for both subjects (Figure 4.17). The palm-pulse cvemps were recorded from both of them, while they were sitting upright, and they were instructed to activate both SCM muscles simultaneously. Only the bilateral activation protocol was conducted on them, due to the time that we had with them. Also, we wanted to avoid the possibility of muscle fatigue from both of them. Two replicates were obtained for each tap sites, with averages, n = 160. Initially, we would expect that no responses (i.e. flat waveforms) would be recorded from both of our bilateral vestibular loss s subjects (Figure 4.18 and Figure 4.19). However, we found that both of them had approximately similar responses recorded from our normals (i.e. with approximately similar peaks for the wave responses). The results from our normals from the bilateral SCM activation in midline taps (the sample waveforms from one subject in Figure 4.3 A) were similar to the bilateral loss subjects responses with midline palm-pulses (Figure 4.18). Similar responses were also recorded in normals and our bilateral loss subjects with the side tap palm-pulses (Figure 4.3 B and Figure 4.19), despite the fact that they had no vestibular function on both sides. Both of our diagnosed bilateral vestibular loss subjects presented with histories of administration of ototoxic drugs (i.e. gentamicin and streptomycin) because of their general health conditions. Ototoxic drugs may cause irreversible damage to both vestibular organs. Also, from their medical records, other vestibular tests conducted earlier proved that they had severe and or absent vestibular functions on both sides. These facts were supported by our acoustic click cvemps results, which indicated that there were no responses from both sides (which indicated the absent of saccular functions on the left and right side). We would therefore expect that both of them would give no responses to palm-pulse stimulation, or at least the responses recorded would be different from our normals. This proved wrong. 134
151 Earlier, we had noted from our normals that the palm-pulse stimulation recruited saturated responses almost at the softest pulse strength. Taking into account the recorded responses from these vestibular loss subjects, we decided to abort the use of the palm-pulse as the stimulus, because we believed that it was too intense and probably accelerated the head and neck sufficiently that it evoked a stretch-reflex from the neck that produced VEMP-like responses, even in bilateral loss subjects. So, we decided that only finger-taps stimulation would be applied for the remaining of our experiments (i.e. for our ovemps testing protocol in Chapter 5). 135
152 Acoustic responses from bilateral vestibular loss subjects Bilateral vestibular loss acoustic responses Figure 4.17: Acoustic responses (n=80 to 100) from two bilateral vestibular loss subjects, from both the left and right SCM. There is no responses at all Figure from 4.17: both sides. Acoustic Positive responses polarity (n=80 is up. to 100) from two bilateral vestibular loss subjects, from both the left and right SCM. There is no responses at all from both sides. Positive polarity is up. 136
153 Bilateral SCM activation from bilateral vestibular loss subjects Midline taps Figure 4.18: Palm-pulse responses (n=80 to 100) from two bilateral vestibular loss subjects, from both the left and right SCM following bilateral activation at midline site taps (Fz, Cz and Oz). These responses were obtained with increasing tap strength (equivalent to head accelerations evenly spaced between 0.08 to 2.0 g). Vertical dashed lines mark the peak of the trigger stimulus (0 ms). Positive polarity is up. 137
154 Bilateral SCM activation from bilateral vestibular loss subjects Side taps Figure 4.19: As with Figure 4.18, the responses in this Figure was obtained at side taps (left T3 and right T4). These responses were also obtained with increasing tap strength (equivalent to head accelerations evenly spaced between 0.08 to 2.0 g). Vertical dashed lines mark the peak of the trigger stimulus (0 ms). Positive polarity is up. 138
155 Chapter 5 Ocular Vestibular Evoked Myogenic Potentials (ovemps)
156 5. Ocular vestibular evoked myogenic potentials (ovemps) 5.1 Introduction In earlier chapters, we have discussed the relationship between the cvemps and the vestibular system. In this chapter, the background of the ocular vestibular evoked myogenic potentials (ovemps) is reviewed. After our relatively unsuccessful results with the palm-pulse cvemps 1, we decided to develop a new method of stimulation, able to evoke otolith responses from threshold up to maximal responses (unlike the palm-pulses that were too intense). For all ovemp tests, all normals and the pathological subjects were tested using the acoustic ovemps first, followed by finger-tap stimulation. Similar test protocols as described earlier in Chapter 3 were employed, unless otherwise specified. 5.2 Extraocular muscles and its relations to the vestibular system There are six extraocular muscles of the human eye (i.e. four rectus muscles and two oblique muscles; Figures 5.1 A and B). Clearly from Figure 5.1 B, the four rectus muscles are arranged approximately as horizontal and vertical pairs. The horizontal pair is formed by the medial and lateral rectus, while the vertical pair is formed by the superior and medial rectus. All of these rectus muscles have an anterior insertion. A third pair is formed by the superior and inferior oblique muscles, and these muscles are present with a posterior insertion (Table 5.1). All of these muscles course from the apex of the orbit, from an elliptical band of tendon known as the annulus of Zinn to the anterior part of the eye orbit, except for the inferior oblique muscle. The inferior oblique originates near the floor of the orbit on the anterior part of the medial wall. Even though the superior oblique muscle originates from the annulus of Zinn, it has a more complex path, coursing through a ring-like rigid tendon pulley, named the trochlea (Figure 5.1 A). Four of the six muscles are innervated by the oculomotor nerve (Cranial nerve III), while the superior oblique is innervated by the trochlear nerve (cranial nerve IV), and the lateral rectus is innervated by the abducens nerve (cranial nerve VI). 1 We have report earlier in Chapter 4 that the palm-pulse responses from both the normals and pathologies were not a success and we finally aborted the use of the palm-pulse as our test stimulus. 140
157 trochlea superior oblique superior rectus annulus of Zinn lateral rectus medial rectus inferior rectus inferior oblique (from Montgomery, 2009) superior rectus lateral rectus tendon of superior oblique medial rectus inferior oblique inferior rectus Figure 5.1: (A) The insertion of the (from six Oyster, extraocular 1999) muscles on the left eye from the lateral view and (B) the insertions of the muscles from the frontal view. 141
158 Extraocular muscles Insertion to the eye i. medial rectus (MR) anterior, medial surface ii. lateral rectus (LR) anterior, lateral surface iii. superior rectus (SR) anterior, superior surface iv. inferior rectus (IR) anterior, inferior surface v. superior oblique (SO) posterior, superior and lateral surface vi. inferior oblique (IO) posterior, inferior and lateral surface Table 5.1: The six extraocular muscles of the human eye and their insertion in relation to the eye [adapted from Feldon et al. (1987), Montgomery (2009) and (Oyster, 1999)]. The rectus horizontal and vertical pairs are responsible for the rotation of the eye in the horizontal plane (around the vertical axis), and rotations upward and downward, while the oblique muscles primarily rotate the top of the eye toward or away from the nose. Generally, the actions of the extraocular muscles depend on the position of the eye at a particular time. The primary muscle or the agonist moves the eye in a given direction, and works together with the synergist muscles i.e. the muscle in the same eye that increase the effectiveness of the movement made by the agonist. Additionally the antagonist muscle is the muscle in the same eye that acts in the opposite direction to the agonist muscle. Table 5.2 summarizes the extraocular muscle movements and their specific antagonist and synergist role. 142
159 Muscle movements Agonist Synergist 1. Adduction (moving the eye inward, i.e. towards the nose) 2. Abduction (moving the eye outward, i.e. away from the nose) 3. Elevation or supraduction (moving the eye upward) 4. Depression or infraduction (moving the eye downward) 5. Intorsion or incycloduction (rotates the top of the eye towards the nose) 6. Extorsion or excycloduction (rotates the top of the eye away from the nose) MR LR SR IR SO IO SR, IR SO, IO Table 5.2: The movements of the eyes and the involvement of the extraocular muscles as the agonist and synergist during the movements [adapted from Montgomery (2009)]. IO SO SR IR Earlier in Chapter 1, we described that the extraocular muscles work together to execute the task during the VOR. The capability of these muscles to accomplish the task is vital, as it helps the VOR to stabilize the eyes in response to both angular (Aw et al., 1996) and linear disturbances (Angelaki, 1998). Data obtained through electrical stimulation in cats has suggested that there are polysynaptic circuits from the utricular nerve to the inferior oblique muscle (Uchino et al., 1994; Uchino et al., 1996). Years later, Isu et al. (2000) electrically stimulated the saccular nerve in cats, and found that there was a very weak neural connection between the sacculo-ocular system when compared to the utriculoocular connections. Data from Cohen et al. (1964) also suggested that electrically stimulated ampullary nerves in the SCCs of cats also evoked eye and head movements parallel to the plane of the activated canal. It was clear from these observations that the utricle and the SCCs were linked to the extraocular muscles. The question now arose whether all of the sensory hair cells in the utricle and/or SCC were equally stimulated during these trials using different mode of stimulation? To answer this question, Curthoys et al. (2006) used a bone conduction vibrator on 346 neurons, and found that the irregular otolithic neurons were the most preferentially activated by the vibrator. 143
160 Most of the activated afferents were in the superior division of the eight nerve, and were probably from the utricle. On the other hand, they also reported that both the regular and irregular SCC neurons were insensitive to the vibrations, and the regularly firing otolithic afferents also gave very few and poor responses. As described in Chapter 1, the irregular neurons from Type I hair cells were located on the central areas of the otolith and the SCC, and they projected predominantly to the vestibulo-spinal system. Regularly firing neurons from Type II hair cells project mostly to the vestibular oculomotor system. However, Chen-Huang (1997) has argued that because of the complexity of the anatomy of the otoliths, there is a considerable overlap between the Type I and Type II hair cells, and one cannot easily separate the physiological functions of these sensory cells. 5.3 Historical background of ovemps More than forty years ago, Geisler et al. (1958) found the presence of responses of cortical origin following click stimulation. In 1964, Bickford et al. demonstrated other responses that were myogenic in origin. Since then, many researchers have shown high interest in finding out more about the myogenic responses following acoustic stimulation. In the last few years it has been observed that loud clicks evoke short-latency ipsilateral positive-negative (p13-n23) EMG responses from the tonically contracted sternocleidomastoid muscle (SCM) muscle (Colebatch et al., 1992; Colebatch et al., 1994; Murofushi et al., 1995; Murofushi et al., 1997). It now seems clear that this cervical muscle response is of vestibular origin. Animal data has suggested an otolithic origin, especially a saccular one, based on experiments showing that the sacculus was the only part of the vestibular apparatus in mammals activated by sound (i.e. some of the vestibular neurons that responded to tilt were sound-sensitive, whereas the vestibular neurons that responded to angular accelerations were not; Murofushi et al., 1995; Young et al., 1977). The idea of a saccular origin for the sound-sensitive vestibular neurons was also supported by patient data, which has demonstrated a close relationship between cervical vestibular evoked myogenic potentials (cvemps) and the functional integrity of the inferior vestibular nerve, which innervates the major part of the sacculus (Akin et al., 2004; Colebatch et al., 1992; Colebatch et al., 1994; Murofushi et al., 1995; Murofushi et al., 1997; Zhou et al., 2004). It is now known that cvemps evoked by acoustic clicks or tone-bursts are a robust and reproducible screening test of saccular function. cvemps have been clinically 144
161 applied as a test on their own, as well as part of a test battery in diagnosing retrocochlear pathology and vestibulopathy Acoustic ovemps in normals Following the success of cvemps, recently Rosengren et al. (2005) successfully established responses from the ocular muscles using 500 Hz, 8 ms bone-conducted tone-burst stimulation. The stimuli were delivered with the bone conductor placed behind each ear. These responses, later termed extraocular vestibular evoked myogenic potentials (ovemps) recruited negative, short-latency extraocular potentials beneath each left and right eye in the tested healthy adult subjects. EMG recordings were made from eight active electrodes positions (i.e. at the superior, inferior, medial and lateral position to each eye, using one common indifferent electrode positioned on the sternum or C7). Subjects were also asked to gaze in different positions. They found that there was no significant difference in the ovemps responses by the different in the stimulation side (i.e. left or right stimulations), or the side of the recorded eye or even the ipsilateral or contralateral stimulation. However, significant responses were recorded according to the difference in the active electrodes positions. They found that the largest responses were obtained from the inferior electrodes, with the superomedial gaze. Two years later, Chihara et al. (2007) investigated the presence of ovemps using two different acoustic stimuli i.e. 500 Hz short tone burst and also 0.1 ms click, both presented at 135 db SPL. Active electrodes were placed inferior to each eye, with the indifferent electrodes placed 1-2 cm below. The normal subjects were asked to look in five different directions (i.e. superolateral, superomedial, inferolateral, inferomedial and also straight ahead). They found that the 500 Hz tone burst stimulation gave the highest prevalence (i.e. 90% in the contralateral eye, and only 45% in the ipsilateral eye). On the other hand, the 0.1 acoustic clicks gave 50% prevalence in the contralateral eye and 0% in the ipsilateral eye. They also reported that the tone burst gave about 11 ms negative peaks, compared to about 9 ms peak from using the clicks. Because the largest ovemps were recorded from the contralateral eye to the side of the acoustic stimulation, Chihara et al. (2007) believed that the ovemps were mediated by a crossed pathway (unlike the cvemps, where it was believed to be mediated ipsilaterally). This was based on 145
162 the findings by Chan et al. (1977), who found that the sacculo-ocular pathway was crossed via the Deiters nucleus in cats 2. Todd et al. (2007) investigated the ovemps further as to make sure that the ovemps were not the possibility of the eyes movement. They used both air and bone conduction sound using a single cycle of a 500 Hz sine wave. Their healthy adult subjects were tested while lying supine with their gaze directed straight ahead at a target about 2 m to the front. They used a bipolar electrode montage with two pairs of electrodes placed superior to both eyes, and another two pairs placed inferiorly. They found that the recorded potentials were consistent with the modulation of the extraocular muscles, because the recorded potentials were not morphologically correlated with the eye movements or with differing recording potentials Skull-tap ovemps in normals After the success of the early researchers in producing acoustic ovemps, Iwasaki et al. (2007) pioneered skull-tap testing of the ovemps by using the clinical reflex hammer, a bone vibrator (B-71 bone oscillator), and also the Bruel and Kjaer Mini-shaker to deliver taps at Fz. Their healthy tested subjects were instructed to lay supine, while looking up straight ahead at about 25 degrees to a small fixation dot. The active electrodes were placed 1 cm below each eye, with the indifferent electrodes positioned 3 cm below the centre of each lower eyelid. They successfully recorded short negative peak at about 10 ms, followed by a positive peak at about 14 ms. A year later, Iwasaki et al. (2008a) repeated the same experiments in healthy adults subjects. He reported that the B-71 bone oscillator was not adequate to elicit reliable n10 responses at Fz, compared to the use of the clinical reflex hammer and the Mini-shaker. They also reported that the n10 responses increased in latency while its amplitude decreased as the subjects age increased. Besides the n10, they also recorded other later peaks, but decided to ignore these peaks because they argued that these later peaks could be due to a jaw reflex, blink reflex, cervico-ocular reflex, or even brainstem reflexes. Todd et al. (2008) also investigated ovemps responses in ten healthy adults using a hand-held vibrator or Minishaker, placed in the horizontal plane to the skull (i.e. at the left and right mastoid). The Minishaker was held against the 2 The sensory cells in the saccule of the cats were electrically stimulated. 146
163 head by the experimenter with about 1-2 kg force. From the three different electrode montages employed (lateral, superior and inferior electrode pairs), they found that the inferior electrode pairs, specifically the left inferior electrode, gave the largest responses with either upwards or rightwards gaze. They recorded n11 and p16 in the ipsilateral eye and n15 in the contralateral eye to the stimulus from inferior electrodes with upward gaze. Besides measuring the ovemps responses, they also investigated the accelerometry (i.e. the head acceleration in four other subjects). This was done using accelerometers, placed in five different positions on the skull. They found that stimulation on one side of the mastoid caused a similar magnitude of acceleration on both sides of the mastoid, but for other accelerometer sites, smaller accelerations were measured. They explained that these findings were consistent with the movement of the head as the whole head was accelerating in the interaural plane during the mastoid vibration. They proposed that because the Minishaker was placed on each side of the mastoid, which caused transtemporal stimulation on each side of the head, the utricular afferents were likely to be activated ovemps responses in neurectomy patients The success of acoustic ovemp testing has produced an interest of many researches to apply the test in pathological cases. Rosengren et al. (2005) have recorded the ovemps potentials in a patient with Miller-Fisher syndrome, and found that the responses were delayed compared to normals. On the other hand, they also found that the responses were larger in patients with superior canal dehiscence than in normals (Rosengren et al., 2005; Rosengren et al., 2008). Besides that, Chihara et al. (2007) and Govender et al. (2009) applied acoustic ovemps in vestibular neuritis patients, where they found that the ovemps responses were rarely produced on the affected side. Curthoys et al. (2009) on the other hand evoked the ovemps responses in a bigger group of vestibular neuritis patients (they tested 138 patients with superior vestibular neuritis) by using boneconducted vibration. With the vibrator placed at Fz, they concluded that the n10 responses of the ovemps primarily indicate the function of the contralateral utricular macula. In this report, we are not describing the application of ovemp test in other pathologies, other than vestibular schwannoma, in great detail. Rosengren et al. (2005), Iwasaki et al. (2007), Iwasaki et al. (2008b) and Iwasaki et al., (2009) were among the few researchers who recorded ovemps 147
164 responses in unilateral neurectomy subjects. Rosengren et al. (2005) presented 0.2 ms click acoustic stimulation and bone-conducted short tone burst stimulation to a single left vestibular neurectomy subject. The tone bursts were delivered using a standard clinical bone-conductor (B-71 Radioear Corporation). No potentials were recorded from the operated side, while negative potentials with mean peak amplitudes of about 2.2 µv and latencies of 13.1 ms were found from both eyes after the acoustic stimulation. While for the bone-conducted stimulus, they recorded responses from both the left and right sides. However, stimulation on the operated side resulted in a very small response (0.8 µv) as opposed to the nonoperated side (1.3 µv). They concluded that the small responses from the operated side might be the end result of cross-over stimulation from the healthy side to the neurectomy side through the skull. Two years later, Iwasaki et al. (2007) tested eight unilateral vestibular neurectomy subjects by tapping the heads at the forehead (Fz). They used three different stimulation techniques: clinical reflex hammer, B-71 bone oscillator (Radioear, New Eagle, PA) and Bruel and Kjaer Mini-shaker They found that in contrast to their healthy subjects (from whom they recorded analogous early initial responses at 10 ms from both left and right eyes), none of their neurectomy subjects had responses from the eyes contralateral to the operated side. Because they had clear responses from the eyes contralateral to the healthy side, and none at all from the contralateral eyes of the operated side, they strongly believed that unilateral vestibular neurectomy cases were the best cases to exemplify clear laterality of the functioning labyrinth. They also concluded that B-71 bone oscillators are not suitable in testing the vestibular labyrinth at the forehead (Fz) because they recorded poor and unreliable responses from all of their healthy subjects and also from the healthy sides of their neurectomy subjects. This finding was in disagreement with Rosengren et al (2005), who used the bone oscillator at the left and right mastoids. A year later, Iwasaki et al. (2008b) expanded their research by testing eleven patients with unilateral vestibular neurectomy following the diagnosis of vestibular schwannoma. These subjects were stimulated with either a reflex tendon hammer, or a Mini-shaker using the 500 Hz short tone burst or condensation or rarefaction pulses. They found that some of these neurectomy subjects still had very small early excitation responses at 10 ms (n10) below the eyes contralateral to the operated side. All of the recorded responses from these neurectomy subjects 148
165 were later computed as asymmetry ratios (percentage ratios of the differences between larger responses and the smaller responses). Iwasaki et al. (2008b) then compared these results with their healthy subjects asymmetry ratios (Iwasaki et al., 2008a). Because some of the neurectomy subjects still had very small responses, this contributed to the asymmetry ratios that were not 100% between the operated and non-operated side. However, Iwasaki et al. (2008b) concluded that the asymmetry ratios were a good indicator of the laterality of the loss. They also concluded that the small responses seen from the opposite eyes of the operated side may be due to some vestibular fibers remaining untouched during the operation. Just recently, Iwasaki et al. (2009) expanded their ovemp research by testing thirty six patients with unilateral vestibular schwannoma, and these patients were tested using a bone-vibrator to evoke the ovemps, acoustic stimulation to evoke cvemps, and with also caloric testing. These three approaches were taken because they were interested in determining which part of the vestibular nerve (i.e. either the superior or the inferior part of the nerve) were affected. This was because the utricular macula was thought to be most responsible for the ovemps, while the cvemps were the results of saccular macula stimulation. The superior vestibular nerve innervated the utricle and the horizontal canal, and the inferior branch innervated the saccule. They found that the consistency of the results between the ovemps and the caloric test responses were significantly higher than the ovemp and cvemps responses. They concluded that the ovemps responses to the bonevibrator indicated the function and integrity of the superior branch of the vestibular nerve Electrode placement and eye gaze in ovemps As described in earlier Section, many researchers have adopted different electrode montages and direction of eye gaze during the investigation of the ovemps responses. Rosengren et al. (2005) have employed eight active electrodes positioned at superior, medial, lateral and inferior to each eye with the indifferent electrodes positioned on the earlobes. Todd et al. (2007, 2008) have used six active electrodes placed superior, lateral and inferior to the eye, with the indifferent electrodes placed 2-3 cm superiorly, laterally and inferiorly to each of the active electrodes. On the other hand, Chihara et al. (2007) and Iwasaki et al. (2007) have positioned both the active and indifferent electrodes inferior to the eye, but with the indifferent electrodes placed 2-3 cm lower than the active electrodes. Despite 149
166 the differences in the electrode montages and the directions of eye gaze, all have come to the conclusion that the active electrodes placed inferior to the eye, and the eye gaze looking up or superomedial, gave the largest amplitudes of ovemps responses. 5.4 Materials and methods We started our ovemp experiments with unilateral acoustic stimulation in all of our subjects. As mentioned earlier in Chapter 4, the acoustic cvemps are a standard protocol in most vestibular clinics, and so we routinely conducted the acoustic ovemp test first, before conducting any other tests. All of the tests for ovemps on both of our healthy and pathological subjects were conducted while they were sitting in an upright position 3. For the finger-tap ovemps, the responses were obtained with the pulse strength varied from the pre-set threshold value of g units (1 g = 9.8m/sec²), up to the maximum of 2.0 g level. This pre-set threshold value was set to half the value lower than the ones employed during our cvemp test (i.e g units), because: (a) The finger-taps were a much softer stimuli compared to the palm-pulse (b) We wanted to trigger the ovemps responses with stimuli down to the threshold level Subjects Ten healthy adult subjects (four males, six female; age between years) and five diagnosed with unilateral vestibular schwannoma (aged between years) volunteered for the ovemp test. The healthy subjects had no history or disorders involving hearing or vestibular function. All of them were informed of the aims and procedures of the study, and gave their consent (Appendix 5 and Appendix 6). All subjects read and signed an informed consent form which had been approved by The University of Western Australia Human Research Ethics Committee (Ethics Approval Project no. RA/4/1/1496). 3 The reason why we were interested in conducting the test while our subjects were sitting upright was explained earlier in Chapter 3 and Chapter
167 5.4.2 General recording preparation Before the procedure started, the general skin preparation for each subject took place. To optimize the electrode contact with the skin, the skin area of interest for the electrodes placement were cleaned and rubbed briskly with 75% ethanol, using gauze. A contact resistance of 5kΩ was typical. In all subjects, extraocular potentials were measured using Ag/AgCl 3M Red Dot electrodes (halved along their long axis), with the active electrodes on the orbital margin immediately below both eyes, and with one common indifferent electrode on the philtrum under the nose 4 (as described in Chapter 3, Figure 3.13 A). A large circular Cleartrace electrode from Conmed was used for the reference electrode, which was placed on the prepared nape of the neck with extra electrode gel Extraocular muscle activation Before testing, subjects were instructed to look upward at the marked spot on the ceiling (situated 1 m above and 0.5 m to the front of their head). They were constantly reminded to keep on fixating on the marked spot during the recording Acoustic ovemps All ten healthy adult subjects and five unilateral neurectomy subjects were tested using the 0.5 ms clicks, presented at 130 db SPL, using ER3A insert earphones to the left and right ears separately. Even though past researchers had used different acoustic stimuli to record ovemps (among them were Rosengren et al. (2005), and Chihara et al. (2007), who used 0.1 ms clicks and Chihara et al. (2007) and Todd et al. (2007) who used 500 Hz tone bursts), we decided to use 0.5 ms clicks because this stimulus had a better chance of producing monophasic drive to the vestibular sensory cells, and also provided greater low-frequency stimulus to the otolith organs (as discussed in detail in Chapter 3). 4 Our earlier tests indicated that there was no significant difference in using two independent indifferent electrodes beneath each eye or just one common indifferent electrode on the philtrum (Chapter 3, Section 3.5.3). 151
168 5.4.5 Finger-tap ovemps Finger-taps were applied on various sites on each subject s head and facial area. The named stimulation spots were based on The International Electrode (placement) System (Jasper, 1958; Steinmetz et al., 1989). The same protocol for finger-taps (as described earlier in Chapter 3 and Chapter 4) were applied. All of our healthy and pathological subjects were tapped at all of these spots unless otherwise specified: the nose bridge (No) just below the inion, mid hairline (Fz), vertex (Cz), back centre (Oz), on the skull 2 cm above the left and right pinna (T3 and T4, relatively), and finally on the left and right mastoid (as shown earlier in Figure 3.13 A in Chapter 3). The sequence of the finger tap spots were randomized between subjects. A piezo-electric sensor was placed on the tester s right middle finger (as shown in Figure 3.13 D), and used to measure the relative finger-tap intensity and provide a trigger pulse (Figure 5.2). The finger-tap strength was varied by the tester from the pre-set threshold value (0.082 g) to a maximum of 2.0 g (unless otherwise specified). To ensure the consistency of each finger-tap when landing on the tap spots, the tester always made sure that the directions of the taps were orthogonal to each location. The finger-taps were delivered at a rate of about 0.5 Hz. The tester was also connected to the subject s reference electrode to avoid any electrostatic artifact. Prior to the recording, subjects were given instructions and a practice session. While sitting upright, they were asked to ensure that their head was kept still and facing forward, while at the same time they had to fixate both eyes at a marked ceiling spot situated about 1 m above and 0.5 m to the front of their head. They were also asked to relax their facial muscles, because active movement of the facial area produced unwanted EMG responses. Subjects were also reminded to keep their eyes fixated at the marked spot during the recording time. 152
169 + 10 µv 10 ms Figure 5.2: Growth of trigger signal (n=50, black waveforms) and ovemps (grey waveforms) intensity functions obtained from a subject following Oz finger-taps over a complete 200 ms data epoch. The ovemps responses were obtained with increasing Oz tap strength (equivalent to head accelerations evenly spaced between g and 2 g; gaze upward). The scale bar in the above figure represents the scale for the ovemps responses, while for the trigger vertical scale is in arbitrary units. The vertical dashed line mark the peak of the trigger stimulus (0 ms). Note that the ovemps saturated as the tap strength increased, but peak latencies changed a little. 153
170 5.4.6 Control measurements As described in Chapter 3, we conducted some control measures (i.e. trials to elicit blinks) to make sure that the finger-tap responses were the result of otolith stimulation, and not from any other artefactual sources 5. Additionally, after obtaining the ovemps responses from the normals, we also conducted more control measurements on some of the normal subjects, to make sure that the responses obtained were entirely from the otolith organs, and not due to other sources. The control measurements that were conducted were: a) Masking the finger-tap responses by using a hand-held vibrator Two healthy adult subjects were recruited for this purposed. During the trial, the same protocol for the finger-tap testing was employed on the subjects, and the only different was that during the finger-tap stimulation, a hand-held vibrator was placed at different positions on the subjects head. b) Finger flick to the ear We also flicked the pinna and the distended eyebrow without skull contact using the finger, to ensure that the recorded ovemps responses were purely of vestibular origin, and were not contaminated by other factors. c) Bias experiment During this trial, the subjects were asked to rock their body to the left and right, and also to the front and back. The rest of the test protocol was the same as that described earlier. 5.5 Results in normal subjects In describing the many ovemp peaks, we have adopted the same naming system as we had used for the cvemps. The approximate millisecond latency of the positive (p) and negative (n) peaks were provided as a subscript to a lower case letter (e.g. n11, p23, etc.). In addition, when referring to these same peaks on the basis of their sequential order along the various ovemp waveforms we have used subscripts after capital letters (e.g. N1, N2, N3 etc.). The ovemp responses obtained from the ten healthy adults were subdivided into the midline sites (No, Fz, Cz and Oz) and also the lateral side of the head (T3, T4, left and right mastoid) unless otherwise specified. These responses were 5 After obtaining those controlled results, it was then that we finalized with the VEMPs testing protocol. 154
171 obtained for the whole range of the input-output function (i.e. from the pre-set threshold value g to a maximum of 2.0 g), while for the control measurements, the trials were done only at the maximal level (i.e. 2.0 g). Unlike cvemps, for ovemps we used the raw waveforms to analyse all the ovemp responses because we noticed that there was no significant difference between the raw and the auto-normalized waveforms of the ovemps (as discussed earlier in Chapter 3). However, for the finger-tap responses, all peak amplitudes have also been normalized in each subject using a single N2P2 peak-to-peak amplitude for a maximal Fz tap, as a definition of an amplitude of 100%. The N2P2 for Fz stimulation site was chosen because we found that these two peaks were consistent and strong for all healthy subjects, and because Fz is a stimulation site used by others (Halmagyi et al., 1995; Iwasaki et al., 2007; Iwasaki et al., 2008a; Iwasaki et al., 2008b) Acoustic click ovemps in healthy subjects In all ten healthy subjects, short latency negative-positive (N1P1) biphasic responses were obtained from the contralateral eyes following monaural 0.5 ms acoustic click stimulation to the left and right ear. Contralateral eye recording with left ear stimulation resulted in an early negative (N1) peak, 9.8 ± 0.3 ms (mean ± SE), followed by a later positive (P1) peak, 13.9 ± 0.4 ms. Right ear stimulation resulted in 10.0 ± 0.2 ms for n1 and 14.4 ± 0.3 ms for p1 from the contralateral eye (Table 5.3). However, there was no significant difference between left and right ear acoustic stimulation in the contralateral eye (p > 0.05). Only 6 and 5 subjects (for left and right ear stimulation) gave N1P1 responses on the ipsilateral eye recordings. For the left ear acoustic stimulation, the N1P1 peak-to-peak amplitude was doubled in the contralateral eye relative to the ipsilateral eye (5.7 ± 1.2 µv and 2.4 ± 0.5 µv, respectively). This was also similar for the right ear stimulation, which gave 6.0 ± 1.3 µv and 4.0 ± 1.4 µv at the contralateral and ipsilateral eyes, respectively. There was a significant difference (p < 0.05) between contralateral and ipsilateral eye recordings for both left and right ear stimulation. There was no significant difference (p > 0.05) between contralateral eye recordings for right and left ears. 155
172 Left ear stimulation Right ear stimulation Contra eye Ipsi eye Contra eye Ipsi eye N1P1 amplitude (µvpp) 5.7 ± ± ± ± 1.4 Mean N1 latency (ms) 9.8 ± ± ± ± 1.0 Mean P1 latency (ms) 13.9 ± ± ± ± 1.2 n = 10 n = 6 n = 10 n = 5 Table 5.3: Peak-to-peak amplitudes and latencies for the ovemps responses, obtained after the monaural 0.5 ms clicks in ten normal healthy subjects. These values were from the raw waveforms responses of the subjects. Note that all ten subjects gave responses on the contralateral (contra) eye for both the left and right ear stimulation, and not for the ipsilateral (ipsi) eye. 156
173 5.5.2 Finger-tap ovemps The latencies and amplitudes for finger-tap ovemps were obtained in ten healthy adult subjects (20 eyes and 20 ears). The responses were subdivided into midline-tap stimulation (nose-bridge, Fz, Cz and Oz) and side-tap stimulation (T3, T4 and left and right side of the mastoid). Figure 5.2 shows the amplitude and timing of the first and dominant peak of each trigger pulse from the piezo-electric transducer. This peak was determined by the software, and all EMG activity and trigger waveforms were time-shifted equally, with the peak of each trigger pulse defined as zero time. As seen in Figure 5.2, each trigger was a simple and well defined pulse with an initial dominant peak of about 4 ms wide at its base, and with sufficient signal-to-noise ratio that each pulse s peak amplitude and timing could be accurately analyzed (to within a small fraction of a millisecond), even for tap levels below ovemp threshold. Note that all of the ovemps waveforms are presented as the raw or the non-normalized waveforms of the responses Number of response averages The ovemp responses also dominated the EMG activity (at least for maximal stimulation i.e. g 2.0 and adequate activation of the relevant eye muscles), so that they were often clearly visible in the raw EMG waveforms (Figure 5.3, lowest waveform). As a result, very little averaging was required. Figure 5.3 shows an example of the averaging of a maximal ovemp evoked by right mastoid stimulation. Clearly the ovemp dominated the EMG trace, so that only 12 averages or so were sufficient to produce reproducible maximal responses. We routinely average between 30 and 100 responses, with 100 averages used when recording ovemps near threshold. As a result of this good signal-to-noise ratio and the efficiency of the software, a replicated intensity series as shown in Figure 5.4 took only about 4 minutes to obtain. Note also the similarity between the single raw ovemp waveform (bottom trace of Figure 5.3) and the highly averaged ovemp waveform (upper trace of Figure 5.3), indicating clearly that there was very little temporal smearing of the averaged waveform, due to either jitter in the triggering process, or in latency variations in the response itself. 157
174 Figure 5.3: Example of averaging maximal right mastoid ovemps responses (each vertical dashed line shows 10 ms time step; number of waveforms averaged as shown, with two replicates overlaid, except for n = 1. The errors indicate the peak of the trigger stimulus at 0 ms. Clearly the ovemp dominated the EMG, so that little averaging is required. We routinely averaged between 30 and 100 responses. 158
175 Details of the finger-tap ovemps Figure 5.4 shows one representative data set from one subject, showing the growth of ovemps at the right (grey) and left (black) eyes with tap strength for eight stimulus sites. For clarity, single waveforms are shown without replicates. Clearly, the softest taps were near or below ovemp threshold (almost flat waveforms), but as tap strength increased (from lowest to highest traces), the amplitudes of the ovemp peaks grew in a nonlinear (saturating) manner, with multiple positive and negative peaks with different latencies. Figure 5.5 showed the derived intensity functions for the main ovemp peaks, obtained with the finger-tap strengths between < g 2.0. Because full intensity functions were not available from every subject at every site, we have averaged the data from three subjects who provided the most complete data sets 6. To emphasize the relative amplitudes of the response peaks, and to minimize variability due to electrode placements and/or eye muscle tone (gaze strength), all peak amplitudes have also been normalized in each subject using a single N2P2 peak-to-peak amplitude for a maximal Fz tap, as a definition of an amplitude of 100%. The N2P2 for Fz stimulation site was chosen because we found that these two peaks were consistent and strong for all healthy subjects, and because Fz is a stimulation site used by others (Halmagyi et al., 1995; Iwasaki et al., 2007; Iwasaki et al., 2008a; Iwasaki et al., 2008b). Note that from Figure 5.5, all the peaks for Fz sites (on the left and right eye) saturated, while for Cz, only the two early peaks saturated. While for No and Oz, the peaks seemed to grow with the increased in the taps strength. As for the T3 left and T4 right side taps, stimulating one side gave smaller peak responses on the opposite side (for e.g. tapping on the T3 left gave smaller peak responses on the right eye and the same goes for the T4 right tap). This did not happen to the mastoid tap. Tapping either on the left or the right mastoid gave approximately the same peak on both eyes. Analogous latency data for the same subjects are shown in Figure 5.6, where the changed in latency with tap strength was plotted for the eight tap sites (averaged across the 3 subjects used for the amplitude data of Figure 5.5). The main point here is that most peak latencies did not change significantly with tap 6 During No stimulation, only 3 subjects gave uncontaminated responses in both eyes, while another 3 had responses at one eye. The others were obscured by either artifact or a blink. For the remaining 7 tap sites, some subjects did not have all peaks. The numbers of subjects responses for each peak are indicated in each figure. 159
176 strength (except for some later peaks for the sites), so that the peak latencies of the maximal responses were representative of the latencies at lower tap strengths. From the results obtained from Figure 5.5 and Figure 5.6, we were interested to see the similarities and differences across subjects at the maximal responses (i.e. g 2.0). Figure 5.7 A and B illustrates the responses obtained from four subjects (Subjects 1 to 4) following taps at maximal strength. It was apparent for the midline-taps (No, Fz, Cz and Oz) that the responses at the right and left eyes were very similar, although there were slight differences between the responses across the four subjects, and even between eyes in single subjects. Other authors have presented grand average waveforms across populations that may disguise this variability, which we suspect may be due to differences in the neurology of the underlying hair cells and neural circuits, rather than being due to variability in our stimulation and recording procedures. Clearly, unlike midline-taps, side-taps gave asymmetric responses, similar to the ovemps evoked by impulse stimuli described by others (Todd et al., 2008). Taps from the left side (T3 and left mastoid) resulted in responses that were approximately opposite in polarity to the responses from the opposite side (T4 and right mastoid; Figures 5.7), as described by others, but we believe that this apparent inversion of the ovemp responses may be misleading in some cases. For example, while the responses at a particular eye from mastoid taps delivered from opposite sides of the head, were not simply inverted relative to each other, and nor were the T3/4 responses relative to the mastoid taps on the same side. This is emphasized in Figure 5.8 which presents high quality ovemp waveforms evoked by taps at the various stimulus sites (both midlines and side taps from a single subject, at maximal stimulation). Most responses seem consistent with a complex response waveform simply shifted in latency, but sometimes inverted (e.g. changing from an excitatory to an inhibitory response). The fact that significant latency shifts exist between different responses suggests that the stimulus to the otoliths was not a simple (scalar) shock wave through the bone. Figure 5.9 represents quality data also from a single subject, but the responses were only evoked at the midline. The detailed midline responses from both Figures indicated that the difference in the responses was not due to a simple linear acceleration of the head. These complex responses from both the side and midline taps suggests to us that the responses were not produced by a single set of receptors, but was due to a directed or vectorial mechanical impulse to different sets of vestibular hair cells 160
177 driving different ocular muscles through different neural circuits with different delays. It should be clear that each waveform was different, and that the responses were close to symmetric for midline taps, but were asymmetric for side-taps, but the responses at each eye with side-taps were not simply inverted with respect to each other, rather they had onset latencies that were shifted. To emphasize that most of the evoked wave shapes were similar, but shifted in latency and in some cases inverted, the Oz waveform (shown grey in each panel) has been scaled vertically and shifted in latency to underlie each of the other responses (dotted waveforms for the right eye and black waveforms for the left eye). This will be discussed later (Figure 5.8). In summary, Figure 5.10 represent the means and standard errors of the mean (SE) for the absolute amplitudes and latencies from all healthy subjects. Although there were positive-negative biphasic responses at much later latencies, we have only included the first three positive (P1, P2 and P3) and three negative peaks (N1, N2 and N3), because these were more consistent when compared to the later peaks. All midline stimulation sites gave initial excitatory (negative) peaks, followed by later inhibitory (positive) peaks at both the left and right eyes. On the other hand, T3 and T4 stimulation gave early negative peaks on the ipsilateral eye (e.g. T3 tap gave early negative peak on left eye). This was followed by later positive peaks at the contralateral eye. In contrast, for left and right mastoid stimulation, early negative peaks were present at the contralateral eye (e.g. left mastoid tap gave early negative peak on the right eye). Later positive peaks were present from the ipsilateral eye. Nose bridge taps gave the largest peak-to-peak amplitudes. All of the N1 peaks were present as early as 8.0 ms, and all positive and negative peaks were present earlier than 40 ms, except for responses from the left eye for a T3 tap. To supplement this Figure, Table 5.4 gave the detailed mean for the amplitudes and latencies for the ten subjects responses. 161
178 Figure µv 162
Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear.
 Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Ear. Utricle & saccule in the vestibule Connected to each other and to the endolymphatic sac by a utriculosaccular duct
 Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY. Professor.Dr. M.K.Rajasekar MS., DLO.,
 VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY Professor.Dr. M.K.Rajasekar MS., DLO., Life is hard for those who don t have a VOR During a walk I found too much motion in my visual picture of the surroundings
VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY Professor.Dr. M.K.Rajasekar MS., DLO., Life is hard for those who don t have a VOR During a walk I found too much motion in my visual picture of the surroundings
VEMP: Vestibular Evoked Myogenic Potential
 VEMP is a neurophysiological assessment technique used to determine the function of the otolithic organs (utricle and saccule) of the inner ear. It complements the information provided by other forms of
VEMP is a neurophysiological assessment technique used to determine the function of the otolithic organs (utricle and saccule) of the inner ear. It complements the information provided by other forms of
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Vestibular Physiology Richard M. Costanzo, Ph.D.
 Vestibular Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the structure and function of the vestibular organs.
Vestibular Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the structure and function of the vestibular organs.
University of Connecticut Schools of Medicine and Dental Medicine Systems Neuroscience Meds Vestibular System
 University of Connecticut Schools of Medicine and Dental Medicine Systems Neuroscience Meds 371 2007-08 Vestibular System S. Kuwada Reading: Purves et al. (2008, 4 th edition), Neuroscience, Chapter 14.
University of Connecticut Schools of Medicine and Dental Medicine Systems Neuroscience Meds 371 2007-08 Vestibular System S. Kuwada Reading: Purves et al. (2008, 4 th edition), Neuroscience, Chapter 14.
Vestibular Function and Anatomy. UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD
 Vestibular Function and Anatomy UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD System of balance Membranous and bony labyrinth embedded in petrous bone 5 distinct end organs 3 semicircular
Vestibular Function and Anatomy UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD System of balance Membranous and bony labyrinth embedded in petrous bone 5 distinct end organs 3 semicircular
The Physiology of the Senses Lecture 10 - Balance
 The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates from the labyrinth... 2 The auditory and vestibular systems have a common
The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates from the labyrinth... 2 The auditory and vestibular systems have a common
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vestibular_function_testing 5/2017 N/A 10/2017 5/2017 Description of Procedure or Service Dizziness, vertigo,
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vestibular_function_testing 5/2017 N/A 10/2017 5/2017 Description of Procedure or Service Dizziness, vertigo,
Vestibular System. Dian Yu, class of 2016
 Vestibular System Dian Yu, class of 2016 Objectives 1. Describe the functions of the vestibular system: What is it? How do you stimulate it? What are the consequences of stimulation? 2. Describe the vestibular
Vestibular System Dian Yu, class of 2016 Objectives 1. Describe the functions of the vestibular system: What is it? How do you stimulate it? What are the consequences of stimulation? 2. Describe the vestibular
Current Perspectives in Balance Assessment. Topics for Today. How are we doing? 3/5/2010. Scott K. Griffiths, Ph.D. March 26, 2010
 Current Perspectives in Balance Assessment Scott K. Griffiths, Ph.D. March 26, 2010 Topics for Today Evaluating the Dizzy Patient looking back, looking ahead The (Not So) New Kids on the Block: VEMPs,
Current Perspectives in Balance Assessment Scott K. Griffiths, Ph.D. March 26, 2010 Topics for Today Evaluating the Dizzy Patient looking back, looking ahead The (Not So) New Kids on the Block: VEMPs,
Bony and membranous labyrinth. Vestibular system. János Hanics M.D.
 Bony and membranous labyrinth. Vestibular system. János Hanics M.D. The position of the inner ear The labyrinthes of the inner ear - Continuous cavity system in the petrous part of temporal bone - Cavity
Bony and membranous labyrinth. Vestibular system. János Hanics M.D. The position of the inner ear The labyrinthes of the inner ear - Continuous cavity system in the petrous part of temporal bone - Cavity
Chapter 15 Hearing & Equilibrium
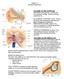 Chapter 15 Hearing & Equilibrium ANATOMY OF THE OUTER EAR EAR PINNA is the outer ear it is thin skin covering elastic cartilage. It directs incoming sound waves to the EXTERNAL AUDITORY CANAL, which is
Chapter 15 Hearing & Equilibrium ANATOMY OF THE OUTER EAR EAR PINNA is the outer ear it is thin skin covering elastic cartilage. It directs incoming sound waves to the EXTERNAL AUDITORY CANAL, which is
Anatomy of the Ear Region. External ear Middle ear Internal ear
 Ear Lecture Objectives Make a list of structures making the external, middle, and internal ear. Discuss the features of the external auditory meatus and tympanic membrane. Describe the shape, position,
Ear Lecture Objectives Make a list of structures making the external, middle, and internal ear. Discuss the features of the external auditory meatus and tympanic membrane. Describe the shape, position,
The Vestibular System
 The Vestibular System Vestibular and Auditory Sensory Organs Bill Yates, Ph.D. Depts. Otolaryngology & Neuroscience University of Pittsburgh Organization of Sensory Epithelium Displacement of Stereocilia
The Vestibular System Vestibular and Auditory Sensory Organs Bill Yates, Ph.D. Depts. Otolaryngology & Neuroscience University of Pittsburgh Organization of Sensory Epithelium Displacement of Stereocilia
The Physiology of the Senses Lecture 10 - Balance
 The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates in the labyrinth.... 2 The vestibular system has two parts.... 3 The Anatomy
The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates in the labyrinth.... 2 The vestibular system has two parts.... 3 The Anatomy
Otoconia: Calcium carbonate crystals Gelatinous mass. Cilia. Hair cells. Vestibular nerve. Vestibular ganglion
 VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
A&P 1. Ear, Hearing & Equilibrium Lab. Basic Concepts. These notes follow Carl s Talk at the beginning of lab
 A&P 1 Ear, Hearing & Equilibrium Lab Basic Concepts These notes follow Carl s Talk at the beginning of lab In this "Lab Exercise Guide", we will be looking at the basics of hearing and equilibrium. NOTE:
A&P 1 Ear, Hearing & Equilibrium Lab Basic Concepts These notes follow Carl s Talk at the beginning of lab In this "Lab Exercise Guide", we will be looking at the basics of hearing and equilibrium. NOTE:
VESTIBULAR LABYRINTHS comprising of 3 semicircular canals, saccule, utricle VESTIBULAR NERVE with the sup. & inf. vestibular nerves VESTIBULAR
 VESTIBULAR LABYRINTHS comprising of 3 semicircular canals, saccule, utricle VESTIBULAR NERVE with the sup. & inf. vestibular nerves VESTIBULAR NUCLEUS BRAINSTEM CEREBELLUM VESTIBULAR CORTEX EYES SPINAL
VESTIBULAR LABYRINTHS comprising of 3 semicircular canals, saccule, utricle VESTIBULAR NERVE with the sup. & inf. vestibular nerves VESTIBULAR NUCLEUS BRAINSTEM CEREBELLUM VESTIBULAR CORTEX EYES SPINAL
A&P 1. Ear, Hearing & Equilibrium Lab. Basic Concepts. Pre-lab Exercises
 A&P 1 Ear, Hearing & Equilibrium Lab Basic Concepts Pre-lab Exercises In this "Lab Exercise Guide", we will be looking at the basics of hearing and equilibrium. NOTE: these notes do not follow the order
A&P 1 Ear, Hearing & Equilibrium Lab Basic Concepts Pre-lab Exercises In this "Lab Exercise Guide", we will be looking at the basics of hearing and equilibrium. NOTE: these notes do not follow the order
THE VESTIBULAR APPRATUS AND PATHWAY
 Dental Neuroanatomy February 23, 2012 Suzanne Stensaas, Ph.D. Reading: Waxman Chapter 17 Also pp 105-108 on control of eye movments Computer Resources: HyperBrain Ch. 8 Vestibulospinal Pathway Quiz http://library.med.utah.edu/kw/animations/hyperbrain/pathways/
Dental Neuroanatomy February 23, 2012 Suzanne Stensaas, Ph.D. Reading: Waxman Chapter 17 Also pp 105-108 on control of eye movments Computer Resources: HyperBrain Ch. 8 Vestibulospinal Pathway Quiz http://library.med.utah.edu/kw/animations/hyperbrain/pathways/
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute
 Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute 412-647-9614 byates@pitt.edu What is the Vestibular System? The vestibular system is the sensory system,
Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute 412-647-9614 byates@pitt.edu What is the Vestibular System? The vestibular system is the sensory system,
Video Head Impulse Testing
 Authored by: David J. Coffin, Au.D. e3 Gordon Stowe Chicago Chicago, Illinois The video Head Impulse Test (vhit) is a relatively new test that provides diagnostic and functional information about the vestibular
Authored by: David J. Coffin, Au.D. e3 Gordon Stowe Chicago Chicago, Illinois The video Head Impulse Test (vhit) is a relatively new test that provides diagnostic and functional information about the vestibular
Vestibular-Evoked Myogenic Potentials as a Test of Otolith Function
 Original Paper Med Principles Pract 2002;11:136 140 Received: April 10, 2001 Accepted: March 17, 2002 Vestibular-Evoked Myogenic Potentials as a Test of Otolith Function Khalid Al-Sebeih a Anthony Zeitouni
Original Paper Med Principles Pract 2002;11:136 140 Received: April 10, 2001 Accepted: March 17, 2002 Vestibular-Evoked Myogenic Potentials as a Test of Otolith Function Khalid Al-Sebeih a Anthony Zeitouni
AUDITORY STEADY STATE RESPONSE (ASSR)
 AUDITORY STEADY STATE RESPONSE (ASSR) Introduction A far-field evoked auditory potential test Principle Similarity to ABR o Sound stimulus converted to electrical impulse pathway EE COLI recording via
AUDITORY STEADY STATE RESPONSE (ASSR) Introduction A far-field evoked auditory potential test Principle Similarity to ABR o Sound stimulus converted to electrical impulse pathway EE COLI recording via
New approaches to VEMP measurement
 New approaches to VEMP measurement Steve Bell, Hearing and Balance Centre, ISVR, University of Southampton Collaborators: Neil Todd, Jennifer Parker, Mike Griffin Motivation It is desirable to have good
New approaches to VEMP measurement Steve Bell, Hearing and Balance Centre, ISVR, University of Southampton Collaborators: Neil Todd, Jennifer Parker, Mike Griffin Motivation It is desirable to have good
DOWNLOAD OR READ : VESTIBULAR EVOKED MYOGENIC POTENTIAL ITS BASICS AND CLINICAL APPLICATIONS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : VESTIBULAR EVOKED MYOGENIC POTENTIAL ITS BASICS AND CLINICAL APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 vestibular evoked myogenic potential its basics and clinical applications
DOWNLOAD OR READ : VESTIBULAR EVOKED MYOGENIC POTENTIAL ITS BASICS AND CLINICAL APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 vestibular evoked myogenic potential its basics and clinical applications
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Vestibular/Auditory Systems
 Vestibular/Auditory Systems Jay Zenner on February 3, 2012 Dental Neuroanatomy Scott Rogers Office: SOM 2C132 Boney Labyrinth Vestibular Apparatus Two Major Divisions Cochlea (anterior) VIII VII Semicircular
Vestibular/Auditory Systems Jay Zenner on February 3, 2012 Dental Neuroanatomy Scott Rogers Office: SOM 2C132 Boney Labyrinth Vestibular Apparatus Two Major Divisions Cochlea (anterior) VIII VII Semicircular
VESTIBULAR SYSTEM. Deficits cause: Vertigo. Falling Tilting Nystagmus Nausea, vomiting
 VESTIBULAR SYSTEM Objectives: Understand the functions of the vestibular system: What is it? How do you stimulate it? What are the consequences of stimulation? Describe the vestibular apparatus, the 2
VESTIBULAR SYSTEM Objectives: Understand the functions of the vestibular system: What is it? How do you stimulate it? What are the consequences of stimulation? Describe the vestibular apparatus, the 2
Extraocular Muscles and Ocular Motor Control of Eye Movements
 Extraocular Muscles and Ocular Motor Control of Eye Movements Linda K. McLoon PhD mcloo001@umn.edu Department of Ophthalmology and Visual Neurosciences Your Eyes Are Constantly Moving. Yarbus, 1967 Eye
Extraocular Muscles and Ocular Motor Control of Eye Movements Linda K. McLoon PhD mcloo001@umn.edu Department of Ophthalmology and Visual Neurosciences Your Eyes Are Constantly Moving. Yarbus, 1967 Eye
Window to an Unusual Vestibular Disorder By Mark Parker
 WELCOME BACK to an ongoing series that challenges the audiologist to identify a diagnosis for a case study based on a listing and explanation of the nonaudiology and audiology test battery. It is important
WELCOME BACK to an ongoing series that challenges the audiologist to identify a diagnosis for a case study based on a listing and explanation of the nonaudiology and audiology test battery. It is important
Vestibular testing: what patients can expect
 American Hearing Research Foundation Symposium on Dizziness & Balance Disorders April 6, 2013 Vestibular testing: what patients can expect Marcello Cherchi, MD PhD Assistant Professor of Neurology Northwestern
American Hearing Research Foundation Symposium on Dizziness & Balance Disorders April 6, 2013 Vestibular testing: what patients can expect Marcello Cherchi, MD PhD Assistant Professor of Neurology Northwestern
Medical Coverage Policy Vestibular Function Tests
 Medical Coverage Policy Vestibular Function Tests EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 04 18 2017 OVERVIEW Dizziness, vertigo, and balance impairments can arise from a loss of vestibular function.
Medical Coverage Policy Vestibular Function Tests EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 04 18 2017 OVERVIEW Dizziness, vertigo, and balance impairments can arise from a loss of vestibular function.
Three-Dimensional Eye-Movement Responses to Surface Galvanic Vestibular Stimulation in Normal Subjects and in Patients
 Three-Dimensional Eye-Movement Responses to Surface Galvanic Vestibular Stimulation in Normal Subjects and in Patients A Comparison H.G. MACDOUGALL, a A.E. BRIZUELA, a I.S. CURTHOYS, a AND G.M. HALMAGYI
Three-Dimensional Eye-Movement Responses to Surface Galvanic Vestibular Stimulation in Normal Subjects and in Patients A Comparison H.G. MACDOUGALL, a A.E. BRIZUELA, a I.S. CURTHOYS, a AND G.M. HALMAGYI
Gathering information the sensory systems; Vision
 Visual System Gathering information the sensory systems; Vision The retina is the light-sensitive receptor layer at the back of the eye. - Light passes through the cornea, the aqueous chamber, the lens,
Visual System Gathering information the sensory systems; Vision The retina is the light-sensitive receptor layer at the back of the eye. - Light passes through the cornea, the aqueous chamber, the lens,
VESTIBULAR FUNCTION TESTING
 VESTIBULAR FUNCTION TESTING Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
VESTIBULAR FUNCTION TESTING Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Vestibular physiology
 Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
THE COCHLEA AND AUDITORY PATHWAY
 Dental Neuroanatomy Suzanne S. Stensaas, PhD February 23, 2012 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea
Dental Neuroanatomy Suzanne S. Stensaas, PhD February 23, 2012 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea
AUDITORY APPARATUS. Mr. P Mazengenya. Tel 72204
 AUDITORY APPARATUS Mr. P Mazengenya Tel 72204 Describe the anatomical features of the external ear Describe the tympanic membrane (ear drum) Describe the walls of the middle ear Outline the structures
AUDITORY APPARATUS Mr. P Mazengenya Tel 72204 Describe the anatomical features of the external ear Describe the tympanic membrane (ear drum) Describe the walls of the middle ear Outline the structures
latest development in advanced testing the vestibular function
 latest development in advanced testing the vestibular function how to explore the vestibular function in detail Herman Kingma ENT Department Maastricht University Medical Centre The Netherlands how I do
latest development in advanced testing the vestibular function how to explore the vestibular function in detail Herman Kingma ENT Department Maastricht University Medical Centre The Netherlands how I do
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Protocol. Vestibular Function Testing. Medical Benefit Effective Date: 10/01/17 Next Review Date: 05/18 Preauthorization No Review Dates: 05/17
 Protocol Vestibular Function Testing (201104) Medical Benefit Effective Date: 10/01/17 Next Review Date: 05/18 Preauthorization No Review Dates: 05/17 Preauthorization is not required. The following protocol
Protocol Vestibular Function Testing (201104) Medical Benefit Effective Date: 10/01/17 Next Review Date: 05/18 Preauthorization No Review Dates: 05/17 Preauthorization is not required. The following protocol
Update on the Clinical Utility of Vestibular Evoked Myogenic Potentials
 Update on the Clinical Utility of Vestibular Evoked Myogenic Potentials Faith W. Akin, Ph.D. and Owen D. Murnane, Ph.D. Vestibular/Balance Laboratory VA Medical Center, Mountain Home, TN Department of
Update on the Clinical Utility of Vestibular Evoked Myogenic Potentials Faith W. Akin, Ph.D. and Owen D. Murnane, Ph.D. Vestibular/Balance Laboratory VA Medical Center, Mountain Home, TN Department of
Effects of varying linear acceleration on the vestibularevoked myogenic potential (VEMP)
 Effects of varying linear acceleration on the vestibularevoked myogenic potential (VEMP) David Solomon University of Pennsylvania Vinay Singh Romesh Khumbani Adam Jenkins LRY: We need to study the saccule
Effects of varying linear acceleration on the vestibularevoked myogenic potential (VEMP) David Solomon University of Pennsylvania Vinay Singh Romesh Khumbani Adam Jenkins LRY: We need to study the saccule
THE EAR AND HEARING Be sure you have read and understand Chapter 16 before beginning this lab. INTRODUCTION: hair cells outer ear tympanic membrane
 BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** THE EAR AND HEARING ******************************************************************************************************
BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** THE EAR AND HEARING ******************************************************************************************************
Test-retest Reliability of Ocular Vestibular Myogenic Potential in Healthy Pilots G Meng 1, C Shan 1, L Han 1, SJ Xie 2 ABSTRACT
 Test-retest Reliability of Ocular Vestibular Myogenic Potential in Healthy Pilots G Meng 1, C Shan 1, L Han 1, SJ Xie 2 ABSTRACT Background: Vestibular function is essential to pilots. But for now there
Test-retest Reliability of Ocular Vestibular Myogenic Potential in Healthy Pilots G Meng 1, C Shan 1, L Han 1, SJ Xie 2 ABSTRACT Background: Vestibular function is essential to pilots. But for now there
Hearing and Balance 1
 Hearing and Balance 1 Slide 3 Sound is produced by vibration of an object which produces alternating waves of pressure and rarefaction, for example this tuning fork. Slide 4 Two characteristics of sound
Hearing and Balance 1 Slide 3 Sound is produced by vibration of an object which produces alternating waves of pressure and rarefaction, for example this tuning fork. Slide 4 Two characteristics of sound
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
Sensory system. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Sensory system Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Sensory receptors Detect stimuli Classified by structure Origin Distribution Modality Structural Classification naked nerve endings
Sensory system Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Sensory receptors Detect stimuli Classified by structure Origin Distribution Modality Structural Classification naked nerve endings
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
For this lab you will use parts of Exercise #18 in your Wise lab manual. Please be sure to read those sections before coming to lab
 Bio 322 Human Anatomy Objectives for the laboratory exercise The Eye and Ear Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 17 For this lab you will use parts
Bio 322 Human Anatomy Objectives for the laboratory exercise The Eye and Ear Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 17 For this lab you will use parts
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
COGS 107B Week 2. Hyun Ji Friday 4:00-4:50pm
 COGS 107B Week 2 Hyun Ji Friday 4:00-4:50pm Lecture 3: Proprioception Principles: The Neuron Doctrine and The Law of Dynamic Polarization Proprioception Joint-protecting reflexes (ex. Knee jerk reflex)
COGS 107B Week 2 Hyun Ji Friday 4:00-4:50pm Lecture 3: Proprioception Principles: The Neuron Doctrine and The Law of Dynamic Polarization Proprioception Joint-protecting reflexes (ex. Knee jerk reflex)
Cranial Nerve VIII (The Vestibulo-Cochlear Nerve)
 Cranial Nerve VIII (The Vestibulo-Cochlear Nerve) Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives
Cranial Nerve VIII (The Vestibulo-Cochlear Nerve) Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives
Sasan Dabiri, MD, Assistant Professor
 Sasan Dabiri, MD, Assistant Professor Department of Otorhinolaryngology Head & Neck Surgery Amir A lam hospital Tehran University of Medical Sciences October 2015 Outlines Anatomy of Vestibular System
Sasan Dabiri, MD, Assistant Professor Department of Otorhinolaryngology Head & Neck Surgery Amir A lam hospital Tehran University of Medical Sciences October 2015 Outlines Anatomy of Vestibular System
THE COCHLEA AND AUDITORY PATHWAY
 Dental Neuroanatomy Suzanne S. Stensaas, PhD April 14, 2010 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea HyperBrain
Dental Neuroanatomy Suzanne S. Stensaas, PhD April 14, 2010 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea HyperBrain
Anatomy and Physiology of Hearing
 Anatomy and Physiology of Hearing The Human Ear Temporal Bone Found on each side of the skull and contains the organs for hearing and balance Divided into four major portions: - squamous - mastoid - tympanic
Anatomy and Physiology of Hearing The Human Ear Temporal Bone Found on each side of the skull and contains the organs for hearing and balance Divided into four major portions: - squamous - mastoid - tympanic
Major Anatomic Components of the Orbit
 Major Anatomic Components of the Orbit 1. Osseous Framework 2. Globe 3. Optic nerve and sheath 4. Extraocular muscles Bony Orbit Seven Bones Frontal bone Zygomatic bone Maxillary bone Ethmoid bone Sphenoid
Major Anatomic Components of the Orbit 1. Osseous Framework 2. Globe 3. Optic nerve and sheath 4. Extraocular muscles Bony Orbit Seven Bones Frontal bone Zygomatic bone Maxillary bone Ethmoid bone Sphenoid
Cervical reflex Giovanni Ralli. Dipartimento di Organi di Senso, Università di Roma La Sapienza
 Cervical reflex Giovanni Ralli Dipartimento di Organi di Senso, Università di Roma La Sapienza The development of the neck in vertebrates allows the individual to rotate the head independently of the trunk
Cervical reflex Giovanni Ralli Dipartimento di Organi di Senso, Università di Roma La Sapienza The development of the neck in vertebrates allows the individual to rotate the head independently of the trunk
Chapter 18 Senses SENSORY RECEPTION 10/21/2011. Sensory Receptors and Sensations. Sensory Receptors and Sensations. Sensory Receptors and Sensations
 SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Ocular Vestibular Evoked Myogenic Potentials to Air Conducted Tone Bursts in Patients with Unilateral Definite Ménière s Disease
 Int. Adv. Otol. 2013; 9:(2) 180-185 ORIGINAL AERICLE Ocular Vestibular Evoked Myogenic Potentials to Air Conducted Tone Bursts in Patients with Unilateral Definite Ménière s Disease Mohamed M Abdeltawwab
Int. Adv. Otol. 2013; 9:(2) 180-185 ORIGINAL AERICLE Ocular Vestibular Evoked Myogenic Potentials to Air Conducted Tone Bursts in Patients with Unilateral Definite Ménière s Disease Mohamed M Abdeltawwab
Hearing. By: Jimmy, Dana, and Karissa
 Hearing By: Jimmy, Dana, and Karissa Anatomy - The ear is divided up into three parts - Sound enters in through the outer ear and passes into the middle where the vibrations are received and sent to the
Hearing By: Jimmy, Dana, and Karissa Anatomy - The ear is divided up into three parts - Sound enters in through the outer ear and passes into the middle where the vibrations are received and sent to the
Quick Guide - eabr with Eclipse
 What is eabr? Quick Guide - eabr with Eclipse An electrical Auditory Brainstem Response (eabr) is a measurement of the ABR using an electrical stimulus. Instead of a traditional acoustic stimulus the cochlear
What is eabr? Quick Guide - eabr with Eclipse An electrical Auditory Brainstem Response (eabr) is a measurement of the ABR using an electrical stimulus. Instead of a traditional acoustic stimulus the cochlear
A NORMATIVE STUDY ON AIR AND BONE CONDUCTION OCULAR VESTIBULAR EVOKED MYOGENIC POTENTIALS. Ho Sen Kee
 A NORMATIVE STUDY ON AIR AND BONE CONDUCTION OCULAR VESTIBULAR EVOKED MYOGENIC POTENTIALS Ho Sen Kee INTRODUCTION Dizziness and imbalance are two of the most frequent complains amongst the elderly population.
A NORMATIVE STUDY ON AIR AND BONE CONDUCTION OCULAR VESTIBULAR EVOKED MYOGENIC POTENTIALS Ho Sen Kee INTRODUCTION Dizziness and imbalance are two of the most frequent complains amongst the elderly population.
Auditory and vestibular system
 Auditory and vestibular system Sensory organs on the inner ear inner ear: audition (exteroceptor) and vestibular apparatus (proprioceptor) bony and membranous labyrinths within the temporal bone (os temporale)
Auditory and vestibular system Sensory organs on the inner ear inner ear: audition (exteroceptor) and vestibular apparatus (proprioceptor) bony and membranous labyrinths within the temporal bone (os temporale)
TEMPLATES FOR COMPREHENSIVE BALANCE EVALUATION REPORTS. David Domoracki PhD Cleveland Louis Stokes VA Medical Center
 TEMPLATES FOR COMPREHENSIVE BALANCE EVALUATION REPORTS David Domoracki PhD Cleveland Louis Stokes VA Medical Center The following templates are in outline form. I designed them so that the IRM local network
TEMPLATES FOR COMPREHENSIVE BALANCE EVALUATION REPORTS David Domoracki PhD Cleveland Louis Stokes VA Medical Center The following templates are in outline form. I designed them so that the IRM local network
Waseem Abu Obeida. Muhammad Abid. Loai Al-zghoul
 4 Waseem Abu Obeida Muhammad Abid Loai Al-zghoul 1 P a g e We knew that we have two types of deafness; one that resulted from damage to the neuronal part beginning from the hair cells up to any part of
4 Waseem Abu Obeida Muhammad Abid Loai Al-zghoul 1 P a g e We knew that we have two types of deafness; one that resulted from damage to the neuronal part beginning from the hair cells up to any part of
SPECIAL SENSES: THE AUDITORY SYSTEM
 SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
Hot Topics in Vestibular Research. Neil Todd, Manchester, UK
 Hot Topics in Vestibular Research Neil Todd, Manchester, UK Over the last decade or so the employment of selective acoustic and inertial stimulation methods has led to a number of important advances in
Hot Topics in Vestibular Research Neil Todd, Manchester, UK Over the last decade or so the employment of selective acoustic and inertial stimulation methods has led to a number of important advances in
THE EAR Dr. Lily V. Hughes, Audiologist
 WHY AM I HERE? HEARING & THE BRAIN THE EAR Dr. Lily V. Hughes, Audiologist Fairbanks Hearing & Balance Center at the ENT Clinic 1 out of every 5 adults has hearing loss. That s more than 48 million people
WHY AM I HERE? HEARING & THE BRAIN THE EAR Dr. Lily V. Hughes, Audiologist Fairbanks Hearing & Balance Center at the ENT Clinic 1 out of every 5 adults has hearing loss. That s more than 48 million people
Sensory- motor control of head-neck musculature By
 IMPERIAL COLLEGE Sensory- motor control of head-neck musculature By Sofia Nousi (CID:00670442) Thesis submitted for the degree of Doctor of Philosophy Academic department of Neuro-Otology, Division of
IMPERIAL COLLEGE Sensory- motor control of head-neck musculature By Sofia Nousi (CID:00670442) Thesis submitted for the degree of Doctor of Philosophy Academic department of Neuro-Otology, Division of
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
The Ear. Dr. Heba Kalbouneh Assistant Professor of Anatomy and Histology
 The Ear Dr. Heba Kalbouneh Assistant Professor of Anatomy and Histology The Ear The ear consists of the external ear; the middle ear (tympanic cavity); and the internal ear (labyrinth), which contains
The Ear Dr. Heba Kalbouneh Assistant Professor of Anatomy and Histology The Ear The ear consists of the external ear; the middle ear (tympanic cavity); and the internal ear (labyrinth), which contains
Taste buds Gustatory cells extend taste hairs through a narrow taste pore
 The Special Senses Objectives Describe the sensory organs of smell, and olfaction. Identify the accessory and internal structures of the eye, and explain their function. Explain how light stimulates the
The Special Senses Objectives Describe the sensory organs of smell, and olfaction. Identify the accessory and internal structures of the eye, and explain their function. Explain how light stimulates the
cortical and brain stem control of motor function
 cortical and brain stem control of motor function cortical and brain stem control of motor function most voluntary movements initiated by the cerebral cortex are achieved when the cortex activates patterns
cortical and brain stem control of motor function cortical and brain stem control of motor function most voluntary movements initiated by the cerebral cortex are achieved when the cortex activates patterns
C h a p t e r PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 15 The Nervous System: The Brain and Cranial Nerves PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing
C h a p t e r 15 The Nervous System: The Brain and Cranial Nerves PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing
Nervous System. Student Learning Objectives:
 Nervous System Student Learning Objectives: Identify the primary parts of the neuron Identify the major structures of the central nervous system Identify the major structures of the peripheral nervous
Nervous System Student Learning Objectives: Identify the primary parts of the neuron Identify the major structures of the central nervous system Identify the major structures of the peripheral nervous
Best practices for ocular and cervical VEMP tests
 Washington University School of Medicine Digital Commons@Becker Independent Studies and Capstones Program in Audiology and Communication Sciences 2015 Best practices for ocular and cervical VEMP tests
Washington University School of Medicine Digital Commons@Becker Independent Studies and Capstones Program in Audiology and Communication Sciences 2015 Best practices for ocular and cervical VEMP tests
Control of eye movement
 Control of eye movement Third Nerve Palsy Eye down and out Trochlear Nerve Palsy Note: Right eye Instead of intorsion and depression action of superior oblique See extorsion and elevation Observe how
Control of eye movement Third Nerve Palsy Eye down and out Trochlear Nerve Palsy Note: Right eye Instead of intorsion and depression action of superior oblique See extorsion and elevation Observe how
III. Comprehensive. Efficient. Auditory EP Testing
 V I III Comprehensive. Efficient. Auditory EP Testing Fast, Flexible and User-Friendly Efficient workflow = Focus on the patient The intuitive software and streamlined interpretation with normative data
V I III Comprehensive. Efficient. Auditory EP Testing Fast, Flexible and User-Friendly Efficient workflow = Focus on the patient The intuitive software and streamlined interpretation with normative data
The cochlea: auditory sense. The cochlea: auditory sense
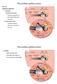 Inner ear apparatus 1- Vestibule macula and sacculus sensing acceleration of the head and direction of gravity 2- Semicircular canals mainly for sensing direction of rotation of the head 1 3- cochlea in
Inner ear apparatus 1- Vestibule macula and sacculus sensing acceleration of the head and direction of gravity 2- Semicircular canals mainly for sensing direction of rotation of the head 1 3- cochlea in
Senses and Sense Organs
 Senses and Sense Organs SENSORY SYSTEMS Human experience is effected by both internal and external stimuli. Humans are able to distinguish among many different types of stimuli by means of a highly developed
Senses and Sense Organs SENSORY SYSTEMS Human experience is effected by both internal and external stimuli. Humans are able to distinguish among many different types of stimuli by means of a highly developed
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
The Senses. Chapter 10 7/8/11. Introduction
 Chapter 10 The Senses Introduction A. Sensory receptors detect changes in the environment and stimulate neurons to send nerve impulses to the brain. B. A sensation is formed based on the sensory input.
Chapter 10 The Senses Introduction A. Sensory receptors detect changes in the environment and stimulate neurons to send nerve impulses to the brain. B. A sensation is formed based on the sensory input.
Anatomy of the ear: Lymphatics
 Anatomy of the ear: 1. External ear which consist of auricle and external auditory canal. The auricle has a framework of cartilage except the lobule, the skin is closely adherent to perichonderium at the
Anatomy of the ear: 1. External ear which consist of auricle and external auditory canal. The auricle has a framework of cartilage except the lobule, the skin is closely adherent to perichonderium at the
Presentation On SENSATION. Prof- Mrs.Kuldeep Kaur
 Presentation On SENSATION Prof- Mrs.Kuldeep Kaur INTRODUCTION:- Sensation is a specialty area within Psychology that works at understanding how are senses work and how we perceive stimuli in the environment.
Presentation On SENSATION Prof- Mrs.Kuldeep Kaur INTRODUCTION:- Sensation is a specialty area within Psychology that works at understanding how are senses work and how we perceive stimuli in the environment.
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
General Sensory Pathways of the Face Area, Taste Pathways and Hearing Pathways
 General Sensory Pathways of the Face Area, Taste Pathways and Hearing Pathways Lecture Objectives Describe pathways for general sensations (pain, temperature, touch and proprioception) from the face area.
General Sensory Pathways of the Face Area, Taste Pathways and Hearing Pathways Lecture Objectives Describe pathways for general sensations (pain, temperature, touch and proprioception) from the face area.
Dr. Sami Zaqout Faculty of Medicine IUG
 Auricle External Ear External auditory meatus The Ear Middle Ear (Tympanic Cavity) Auditory ossicles Internal Ear (Labyrinth) Bony labyrinth Membranous labyrinth External Ear Auricle External auditory
Auricle External Ear External auditory meatus The Ear Middle Ear (Tympanic Cavity) Auditory ossicles Internal Ear (Labyrinth) Bony labyrinth Membranous labyrinth External Ear Auricle External auditory
Special Senses. Mechanoreception Electroreception Chemoreception Others
 Special Senses Mechanoreception Electroreception Chemoreception Others Recall our receptor types Chemically regulated: Respond to particular chemicals Voltage regulated: respond to changing membrane potential
Special Senses Mechanoreception Electroreception Chemoreception Others Recall our receptor types Chemically regulated: Respond to particular chemicals Voltage regulated: respond to changing membrane potential
