Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
|
|
|
- Sylvia Gregory
- 5 years ago
- Views:
Transcription
1 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical devices from Swemac. These instructions apply to all instruments that are sold by Swemac for reuse. These instructions also apply to single use devices that are delivered non-sterile. The methods have been developed with standard equipment and in accordance to applicable standards common for hospitals worldwide. Swemac_Cleaning_004_EN Dated
2 Recommended cleaning, disinfection and sterilization instructions Cleaning is the single most important step in preparing a device prior to use. Effective cleaning must be carried out to achieve proper disinfection/sterilization. Thorough cleaning and rinsing are vital to reprocessing reusable medical devices. Also, thorough rinsing is important for the removal of any residual cleaning agents from the medical devices. The purpose of cleaning is to remove all adherent visible soil and to reduce the number of particulates, microorganisms, and pyrogens. The recommended cleaning instructions in this document include both manual and automatic washing/disinfection procedures
3 Swemac Innovation AB Cobolgatan 1 SE Linköping, Sweden Phone Fax info@swemac.com The following orthopedic products are applicable: Re-sterilization of reusable instruments Implants delivered non-sterilie Reusable instruments delivered non-sterile To the attention of the operating doctor and surgical, cleaning personnel. Directory 1. Cleaning and sterilization 1.1 Reprocessing cleaning, disinfection and sterilization 2. Cleaning and disinfection 2.1. Pre-treatment for implants and instruments 2.2. Automated cleaning and disinfection 2.3 Procedure for implants and instruments 3 Manual cleaning and disinfection 3.1 Ultra sonic cleaning 4. Control 5. Maintenance (for instruments) 6. Packaging 7. Sterilization 8. Storage 9. Material resistance 10. Reusability 11. Specific remarks 12. Warnings and precautions 1. Cleaning and disinfection 1.1 Processing (cleaning, disinfection and sterilization) 0413 All implants and instruments delivered non-sterile have to be cleaned, disinfected and sterilized prior to use. All non-sterile implants must be processesd in a validated cleaning and disinfectionprocess after they have been removed from the protective packaging. Use scissores to open the PE-foil packaging to avoid leavings of plastic on the product. An effective cleaning and disinfection is a vital requirement for an effective sterilization of the implants and instruments. If implants had already been in contacted with patients or they had been contaminated with blood or other bodily fluids, it is not allowed to reuse them. The user is responsible to ensure that implants and instruments are sterile prior to use. Cleaning shall be performed in a validated cleaning process in accordance to ISO Sterilization and re-sterilization shall be performed in accordance to ISO Cleaning and disinfection If possible, an automated procedure (disinfector) should be used for cleaning and disinfection of the instruments. A manual procedure even in case of application of a ultrasonic bath should only be used if an automated procedure is not available. In this case, the significantly lower efficiency and reproducibility of a manual procedure has to be considered. If a manual cleaning and disinfection process is used a vital that a thorough inspection is conducted to ensure that suitable result have been achived. The process shall be validated. 2.1 Pre-treatment for instruments Remove coarse impurities of the instruments directly after application (within a maximum of 2 h after use). For this use only running water or a disinfectant solution. The disinfectant should be aldehyde-free (otherwise fixation of blood impurities), possess a fundamentally approved efficiency (for example VAH/DGHM or FDA approval or CE marking), be suitable for the disinfection of instruments and be compatible with the instruments (see chapter Material resistance). Lay instruments down with care to avoid damages. Do not put instruments in physiological saline solution as a longer contact could create corrosion. For manual removal of impurities only a soft brush or a clean soft cloth has to be used, in no case metal brushes or steel wool. Please consider, that the disinfectant used in the pre-treatment step serves only the personal`s safety and cannot under any curcumstances replace the disinfection step in the validated cleaning process. Pre-treatment for implants Implants which already had contact with patients should not be re-used Automated cleaning and disinfection (Disinfector/Washer-Disinfector) Pay attention to following points during selection of the disinfector: - Fundamentally approved efficiency of the disinfector (for example CE marking according to EN ISO or DGHM or FDA approval). - Possibility for an approved program for thermal disinfection (A0 value > 3000 or in case of older devices - at least 5 min at 90 C; in case of chemical disinfection danger of remnants of the disinfectant on the instruments). - Fundamental suitability of the program for instruments as well a sufficient rinsing steps in the program. - Postrinsing only with sterile or low contaminated water (max. 10 germs/ml, max endotoxin units/ml), for example purified/highly purified water. - Only use of filtered air for drying. - Periodical maintenance and check/calibration of the disinfector. - Instruments with joints shall be layed down in open state. - Instruments with hollows or cannulated instruments have to be thoroughly rinsed inside. Standard 134 Prion 134 Standard º C (273º F) for 3 minutes* 134º C (273º F) for 18 minutes* 121 C (250 F) for 20 minutes*. *These times does not include the air removal, penetration or drying times. Consult the manufacturer of the sterilization equipment for instruction on loading and configuration parameters. Flash sterilization is not recommended. Flash sterilization is only allowed in a case of emergency and shall be preformed in accordance to ANSI/AAMI ST37: 1996 Flash Sterilization- Steam Sterilization of Patient Care Items for Immediate Use. Additionally, please pay attention to the legal provisions valid for your country as well as to the hygienic instructions of the doctor s practice or of the hospital. This applies particularly to the different guidelines regarding the inactivation of prions. Please pay attention to the specific product- and process requirements in case of choosing the manual cleaning and disinfection process.
4 Pay attention to following points during selection of the cleaning detergent: - Fundamental suitability for the cleaning of implants to be made of metal or plastic material and of instruments to be made of steel or plastic material. - Additional application in case of non-application of a thermal disinfection of a suitable disinfectant with approved efficiency (for example VAH/DGHM or FDA approval or CE marking) compatible to the used cleaning detergent. - Compatibility of the used detergents with the instruments (see chapter Material resistance). Pay attention to the instructions of the detergent manufacturers regarding concentration and soaking time. 6. Dry and pack the instruments immediately after the removal. 7. Check the instruments. 3.1 Ultrasonic cleaning Ultrasonic-cleaning is part of the manual cleaning process as a mechanical help to remove insisting contamination before or after the automatic process. 2. Soak the disassembled instruments for the given soaking time in the cleaning solution so that the instruments are sufficiently covered. 3. Remove the instruments of the cleaning solution and postrinse them at least three times with water. 2.3 Procedure for implants: Note! Implants which have had contact with patient are not allowed to be re-sterilized. 1. Place the implants in the disinfector. Make sure that the implants do not have contact with eachother. 2. Start the program. 3. Remove the implants from the disinfector after end of the program. 4. Check and package the implants immediately after the removal (see chapters Control, Maintenance and Packaging if necessary after additional post-drying at a clean place). Procedure for instruments: 2. Transfer the disassembled instruments in the disinfector. Make sure that the implants do not have contact with eachother. 3. Start the program. 4. Remove the instruments of the disinfector after end of the program. 5. Canullated instruments must be controlled thoroughly to ensure sufficient cleaning has been achived. 6. Check and package the implants immediately after the removal (see chapters Control, Maintenance and Packaging if necessary after additional post-drying at a clean place). 3. Manual cleaning and disinfection Pay attention to following points during selection of the cleaning and disinfection detergents: - Fundamental suitability for cleaning and disinfection of metal implants and instruments made of metal or plastic material. - In case of application of an ultrasonic bath: suitability of the cleaning detergent for ultrasonic cleaning (no foam development). - Application of a disinfectant with approved efficiency (for example VAH/DGHM or FDA approval or CE marking) compatible with the used cleaning detergent. - Compatibility of the used detergents with the implants and instruments (see chapter Material resistance). Combined cleaning/disinfection detergents should not be used. Pay attention to the instructions of the detergent manufacturers regarding concentration and soaking time. Please use only freshly prepared solutions as well as only sterile or low contaminated water (max. 10 germs/ml) as well as low endotoxin contaminated water (max endotoxin units/ml), for example purified/highly purified water, and filtered air for drying, respectively. Basics for use of an ultrasonic-bath : - The bath shall be filled in respect of the manufacturer codes. - A suited detergent or combined cleaning/disinfection solution shall be added to the bath. - Concentration, temperatures and exposure time in respect of the manufacturers codes shall be adapted to the cleaning detergent. - Temperatures between 40 and 50 C help the cleaning effects, higher temperatures (> 50 C) may create blood traces. - Instruments with hinges shall be treated in open state. Post rinse the instruments carefully after the ultrasonic bath. You may use clean water (if possible de- salted), to remove traces of cleaning and disinfection detergents. Manual disinfection 1. Soak the disassembled instruments for the given soaking time in the disinfectant solution so that the instruments are sufficiently covered. Pay attention that there is no contact between the instruments. 2. Then, remove the instruments of the cleaning solution and postrinse them at least three times with water. 3. Dry and pack the instruments immediately after the removal (see 6 Packaging) if necessary after additional postdrying at a clean place). For instruments with cannulations and inner spaces make sure that they are free inside and in full contact with the solution. Make sure that airbubbles can araise by moving and holding the instruments so that a complete contact of all surfaces is provided. Check the inner room of the instruments after cleaning and make sure that nothing blocks inside. Instruments with blocking residues have to be treated again. When a second treatment failed, exchange the instruments. In case of higher contamination, a frequent changing of the solution is recommended to avoid corrosion. The implementation of the manual cleaning process - Put dirty implants and instruments in (fully de-salted) water by adding a blood-chasing additive for at least 10 min. - Submerge and wash by hand in water with added neutralizing additive. - Brush with a soft nylon-brush under attention to hinges, threads, hollow parts and other hardly to attempt zones - Is the instrument perforated, brush the inside with a soft nylonbrush - Afterwards rinse carefully under clean, rinsing water - Dry carefully the inner- and outerside to avoid corrosion. Use a clean, soft tissue. A clean air pression could be used to try inner holes. Manual cleaning: 4. Control 2. Soak the disassembled instruments for the given soaking time in Check all implants and instruments after cleaning or the cleaning solution so that the instruments are sufficiently covered. cleaning/disinfection, respectively, on corrosion, damaged surfaces, 3. Clean by ultrasonic treatment (3.1) or careful brushing with a soft brush. and impurities. Do not further use damaged implants or instruments 4. If the instrument is cannulated, clean it thoroughly with a soft brush. (limitation of the numbers of re-use cycles see 9 Reusability). 5. Remove the instruments of the cleaning solution and postrinse them at Still dirty instruments are to be cleaned and disinfected again. least three times with water. After completion of washing and disinfection program remove all products from the machine so that by residual moisture no corrosion can attack
5 5. Maintenance and remounting of instruments Assemble disassembled instruments again (see specific instructions). If possible do not use instrument oils. In case of application use only instrument oils (white oil) admitted to steam sterilization considering the maximum possible sterilization temperature and with approved biocompatibility. 6. Packaging Please insert the cleaned and disinfected instruments in the corresponding sterilization trays and pack them in single-use sterilization packagings (single or double packaging) and/or sterilization containers, which fulfill the following requirements: - EN ISO/ANSI AAMI ISO Suitable for steam sterilization (temperature resistance up to at least 137 C (279 F), sufficient steam permeability). - Sufficient protection of the instruments as well as of the sterilization packaging to mechanical damage. - Regular maintenance according to the instructions of the manufacturer (sterilization container). 7. Sterilization The sterilization process shall be validated in accordance to ISO Do not use any other sterilization process than the once described in this document. Steam sterilization - Fractionated vacuum procedure or gravity procedure 1 (with sufficient product drying). - Steam sterilizer according EN 13060/EN Validated according to EN ISO Maximum sterilization temperature 134 C (273 F; plus tolerance according to EN ISO Sterilization time (exposure time at the sterilization temperature) at least 20 min at 121 C (250 F) or 3 min 2 at 134 C (270 F)/134 C (273 F). 1 The less effective gravity procedure must not be used in case of availability of the fractionated vacuum procedure. In case of application of the gravity procedure significantly longer sterilization times and a product and procedure specific validation under responsibility of the user are required. 2 respectively 18 min (inactivation of prions) The flash sterilization procedure must not be used. Do not use dry heat sterilization, radiation sterilization, formaldehyde and ethylene oxide sterilization, as well as plasma sterilization. 8. Storage Please store the instruments after sterilization in the sterilization packagings at a dry and dust-free place. Avoid direct sunlight. The products of Swemac Innovation AB are not restricted to a certain durability. The products shall not be stored for long periods as the package could be damaged. In these cases the safety of the products can not be guaranteed. 9. Material resistance Please take care that the listed substances are not ingredients of the cleaning or disinfection detergent: - Organic, mineral, and oxidizing acids (minimum admitted phvalue5.5) - Strong lyes (maximum admitted ph-value 8.5, neutral/enzymatic cleaner recommended) - Organic solvents (for example: acetone, ether, alcohol, benzine), - Oxidizing agents (for example: peroxide) - Halogens (chlorine, jodine, bromine) - Aromated, halogenated hydrocarbons Please do not clean any instruments, sterilization trays, and sterilization containers by use of metal brushes or steel wool. Please do not expose any instruments, sterilization trays, and sterilization containers to temperatures higher than 137 C (279 F). 10. Reusability Implants are single use products - once they had contact to patients or they had been contaminated, it is not allowed to reuse them. The Instruments can be reused in case of adequate care and if they are undamaged. The user is responsible for each further use as well as for the use of damaged and dirty instruments (no liability in case of disregard). 11. Handling returning products Medical products are considered as returns independent from their use or disuse when they are sent back to the supplier. Possible reasons for returns are: repairs, returns of borrowed instrument sets or product quality claims. All persons who have contact to these products during the returning process face a possible or real risk to get in contact to contaminated products and therefore a higher risk of infections. Swemac will only process returned products that has been cleaned and disinfected in a validated cleaning process. A completed Decontamination report which states that the product is disinfected shall accompany the returned product. Any product returned to Swemac without or with and uncompleted Decontamination report will be considered as contaminated and will not be processed. Inability to present a completed Decontamination report up on the return of borrowed intruments or/and implants will result in a decontamination fee for cleaning and disinfection of the products. The Decontamination report can be requested from Swemac free of charge. 12. Warnings and precaution actions - The correct choice of the implants is of initial importance. The therapeutic success is depending on the choice of the right implant. bone size and tissue situation limit the sizes and thickness of the implants. Please request the latest developments and improvements to your supplier. - Implants are not regarded as full substitutes for the damaged bone functions. The operating doctor has to consider the nature and comprehensions of the medical capacity after the operation - For exact instruction how to place the implant please read the concerned op-instruction. - Please use for clavicula fractures only our special clavicula plates. - Please be carefully when anatomically preforming reco plates: all pressure on the plates and in different directions can maybe later cause a break of the plate as the material was stressed. - Please avoid drilling on metal, this may cause a damage to the drill that cannot be repaired. Implants which already had contact to patients are not allowed to be reused. Please dispose used implants correctly. Even when it seems undamaged, small defects or internal disturbances traces may occur, which could cause technical fatigues of the material.
INSTRUCTIONS FOR CLEANING, DISINFECTION AND STERILIZATION OF DFS INSTRUMENTS
 Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
Instructions for use 0483 Permanent and temporary YASARGIL Aneurysm and Vessel Clips
 1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
Hygienic Reprocessing
 Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
UNICLIP DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM FOR DENTAL USE ONLY. FISDR / F EN / 11 / updated 06/2016 1/7
 UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
ProUltra Ultrasonic Non Surgical Endo Tips
 ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
 EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
INFORMATION TO THE PRODUCT RANGES. Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS
 INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
1) INDICATIONS FOR USE These products have to be used only in hospital environments, clinics or dental offices by qualified dental personnel.
 ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
Cleaning and sterilization instructions for Astra Tech Implant System EV
 Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
ASTM Workshop on Medical Device Cleanliness
 ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Reprocessing of Implants: What are the Issues?
 Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
Aesculap Spine Instructions for use
 Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
CURVES. Anatomically Adaptable IPR strips. Brochure Instructions for use Processing instructions
 CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset
 SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
THE. Curve THE CURVE STRIPPING TOOL
 THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care
 a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
ATHLET VBR. The real winner. VBR in PEEK-OPTIMA PRODUCT INFORMATION
 ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Doxpal. Self-retaining Retractor
 Doxpal Self-retaining Retractor Doxpal Easy to use and reposition Doxpal retractors are easy to use and easy to reposition due to the elastic properties of the plastic. There are no locking devices to
Doxpal Self-retaining Retractor Doxpal Easy to use and reposition Doxpal retractors are easy to use and easy to reposition due to the elastic properties of the plastic. There are no locking devices to
Surgical Technique Guide
 Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Complex Medical Devices and Steam Sterilization. By Andrew Gay Director - Sterilizer Validation Australia
 Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
TABLE OF CONTENTS. PAL-650 Instrument System Instruction Manual. Applicable Part Numbers Introduction General Warnings...
 1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml
 EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
PROTAPER GOLD TM Treatment
 PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
care NCDC-Cleanser
 Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
Medical White Oil in an oil pen 24.950.12 Optimum cleaning with care As an instrument manufacturer the company Helmut Zepf developed a product line for the optimum processing of dental instruments, in
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
Sample Enhanced Visual Inspection of medical devices. SUBJECT: Enhanced Visual Inspection of medical devices
 Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
S5 Rotary Files. only. Manufacturer. Packing unit. Batch code. For professional use only (0)
 EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS
 CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS w w w. c e r a r o o t. c o m Contents 3 Precise instruments 6 Be AWARE 7 Your responsability 8 Preoperative, intraoperative, and postoperative
CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS w w w. c e r a r o o t. c o m Contents 3 Precise instruments 6 Be AWARE 7 Your responsability 8 Preoperative, intraoperative, and postoperative
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Contents. Helpline No. UK/ North Ireland Rep. Ireland Web Support Model Number: 12005
 Contents 1 02 03 04 06 07 11 12 13 15 17 18 19 19 Contents Welcome Safety Instructions Box Contents Notes on Measurements Getting Started Measuring Weight Saving Personal Data Measuring Body Fat, Water
Contents 1 02 03 04 06 07 11 12 13 15 17 18 19 19 Contents Welcome Safety Instructions Box Contents Notes on Measurements Getting Started Measuring Weight Saving Personal Data Measuring Body Fat, Water
Healthcare Sterilisation: Challenging Practices Volume 2
 Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Chapter 5: Checking and Maintaining Ultrasound Equipment
 Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Workshop on the reprocessing of medical devices. 5. December Brussels. Nikou Ghassemieh Managing Director
 Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT. Instruction Manual
 HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Reprocessing of Implants What are the Issues? Dr. Michelle Alfa, Diagnostic Services of Manitoba A Webber Training Teleclass
 Reprocessing of Implants What are the Issues? Reprocessing of Implants: What are the Issues? When You Open Pandora s Box. Hear no evil. See no evil. Speak no evil. Dr. Michelle J. Alfa, Ph.D., FCCM Medical
Reprocessing of Implants What are the Issues? Reprocessing of Implants: What are the Issues? When You Open Pandora s Box. Hear no evil. See no evil. Speak no evil. Dr. Michelle J. Alfa, Ph.D., FCCM Medical
TONTARRA Medizintechnik GmbH. Surgical Instruments in Demand. Laminectomy Punches and Rongeurs
 TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
Implant Locator. User Manual
 Implant Locator User Manual Table of Contents Introduction..... 2 1. Indications for use..... 3 2. Contraindications... 3 3. Warnings........ 3 4. Precautions........ 3 5. Adverse Reactions.... 3 6. Step-by-Step
Implant Locator User Manual Table of Contents Introduction..... 2 1. Indications for use..... 3 2. Contraindications... 3 3. Warnings........ 3 4. Precautions........ 3 5. Adverse Reactions.... 3 6. Step-by-Step
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
TTA Wedge System INSTRUCTIONS FOR USE
 TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Surgical Technique and Product Specifications
 StelKast Acetabular Surgical Technique and Product Specifications Including Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Technique Introduction StelKast offers a variety
StelKast Acetabular Surgical Technique and Product Specifications Including Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Technique Introduction StelKast offers a variety
Aesculap Long and Short Ventriculoscope Systems Instructions for Use
 Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
AS Medizintechnik GmbH Sattlerstraße Tuttlingen Germany
 Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
Before using the intramedullary. please ensure that it is functioning properly and free of any defects. In addition, please follow these instructions, including the information regarding repair as wellas
Zavation Z-Link Cervical
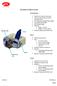 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING. An effective degreaser for use on all surfaces
 Version: 3 RE EC/453/2010 - ISO 11014-1 1. IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING 1. 1. Product identifier: 1. 2. Relevant identified uses of the substance or mixture
Version: 3 RE EC/453/2010 - ISO 11014-1 1. IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING 1. 1. Product identifier: 1. 2. Relevant identified uses of the substance or mixture
Massage to support. Postnatal 11/11/2014. Infection Control 2013 in your Massage Practice. Monica Pasinato-Forchielli
 Massage to support World Pregnancy, Massage Conference Birth Presents: and Postnatal The Down for mother & Dirty and baby on World Sanitation Massage and Conference June Infection Control 2013 in your
Massage to support World Pregnancy, Massage Conference Birth Presents: and Postnatal The Down for mother & Dirty and baby on World Sanitation Massage and Conference June Infection Control 2013 in your
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES
 Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
