Semaglutide the new kid on the block in the field of glucagonlike peptide-1 receptor agonists?
|
|
|
- Barnaby Perry
- 5 years ago
- Views:
Transcription
1 Editorial Page 1 of 5 Semaglutide the new kid on the block in the field of glucagonlike peptide-1 receptor agonists? Cristian Guja 1,2, Rucsandra Dănciulescu Miulescu 1,2 1 National Institute of Diabetes, Nutrition and Metabolic Diseases Prof. N.C. Paulescu, Bucharest, Romania; 2 Carol Davila University of Medicine and Pharmacy, Bucharest, Romania Correspondence to: Cristian Guja. Carol Davila University of Medicine and Pharmacy Bucharest, Romania; National Institute of Diabetes, Nutrition and Metabolic Diseases Prof. N.C. Paulescu, 5-7 Ion Movila Street, Bucharest, Romania. cristian.guja@b.astral.ro. Provenance: This is an invited Editorial commissioned by Section Editor Dr. Kaiping Zhang, PhD (AME College, AME Group, Hangzhou, China). Comment on: Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017;5: Submitted Sep 28, Accepted for publication Oct 12, doi: /atm View this article at: Introduction It is a known fact that, due to the progressive nature of type 2 diabetes (T2D) and decline of beta cell function, diabetes patients require over time treatment intensification to maintain good glycemic control (1,2). In addition, international treatment guidelines recommend achieving glycemic targets while minimizing the risk of hypoglycemia and weight gain, two of the most frequent adverse effects of classical diabetes medications (1,2). The last decade witnessed the advent of the glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs), one of the most promising classes of modern diabetes medications (3). GLP-1RAs decrease glycaemia by stimulating insulin secretion from the pancreatic beta cells in a glucose dependent manner and inhibiting glucagon secretion from the pancreatic alpha cells, in the same time promoting satiety (direct effect on cerebral GLP-1 receptors and prolongation of gastric emptying), and, consequently, weight loss (4). According to their mode of action, GLP-1RAs can be classified into short-acting preparations with once (lixisenatide) or twice (eventide) daily administration and long-acting with once daily (liraglutide) or once weekly (QW) administration (eventide QW, dulaglutide, albiglutide and semaglutide) (5). While long-acting GLP-1RAs have a stronger effect on fasting plasma glucose and overall glycemic control, short-acting preparations decrease more post-prandial blood-glucose (3,5). In addition, once-weekly GLP-1RAs may improve patient compliance and quality of life compared to the short-action preparations (6). Headto-head clinical trials as well as systematic reviews/metaanalyses showed that long-acting GLP1RAs are superior to short-acting GLP-1RAs in reducing HbA1c (7). Moreover, the compounds with long duration of action proved to be superior to basal insulin in reducing HbA1c as shown by recent meta-analyses (5,8). Semaglutide development Semaglutide is a GLP-1RA in the final phases of authorization for the treatment of T2D in humans. Semaglutide has a high degree of homology (~94%) with the human GLP-1 molecule, rendering it a human GLP-1 analogue (9). There are three key changes in the molecule of semaglutide compared to human GLP-1: alanine in position 8 is replaced by α-aminoisobutyric acid (which increases resistance to DPP-4 action); a C-18 fatty acid is conjugated to Lys in position 26 via a glutamate spacer, providing specific binding to albumin; and Lys in position 34 is substituted by Arg (preventing the C-18 fatty acid to bind at a wrong site) (10). After s.c. injection, the halflife of semaglutide is approximately one week and an early phase 2 randomized study showed that doses to be used in clinical trials should be 0.5 and 1.0 mg (11). A formulation of semaglutide with oral administration is currently in the phase 3 clinical trials period (12).
2 Page 2 of 5 Guja and Dănciulescu Miulescu. Semaglutide in T2D Table 1 Effect of long-acting GLP-1RAs in treatment naïve T2D patients (13-17) SUSTAIN-1 (13) DURATION-4 (14) LEAD-3 (15) AWARD-3 (16) HARMONY-2 (17) GLP1-RA Semaglutide Exe QW Liraglutide Dulaglutide Albiglutide Comparator Placebo Metformin, pioglitazone, sitagliptin Glimepiride Metformin Placebo Duration (weeks) Dose (mg) Baseline HbA1c 8.09% 8.12% % 8.3% 8.3% 7.6% 7.6% 8% 8.2% δhba1c 1.45% 1.55% 1.53% 0.84% 1.14% 0.71% 0.78% 0.7% 0.89% δhba1c vs. comparator 1.43% 1.53% 0.05% Met; +0.1% Pio; 0.38% Sita 0.33% 0.62% 0.15% 0.22% 0.84% 1.04% % HbA1c <7% 74% 72% 63% 42.8% 50.9% 63% 62% 40.2% 49% δweight (kg) δweight vs. comparator (kg) Met; 3.5 Pio; 1.2 Sita δsbp (mmhg) Hypoglycemia 0% 0% 2% 12% 8% 11.1% 12.3% 5.9% 6.1% GI side effects 38% 38% 49% 51% 31.7% 30.3% Nausea 20% 24% 11.3% 27% 29% 10.7% 19% 9.9% 9.1% Diarrhea 13% 11% 10.9% 16% 19% 5.2% 10% 9.9% 13.1%, only severe or blood-glucose confirmed episodes reported;, only individual GI side effects but not overall percentage of GI side effects reported; δ, change. Exe QW, eventide once weekly; GLP-1RAs, glucagon-like peptide-1 receptor agonists; T2D, type 2 diabetes; Met, Metformin; Pio, Pioglitazone; Sita, Sitagliptin. Semaglutide efficacy and safety in clinical trials Now, in The Lancet Diabetes Endocrinology, Sorli and colleagues report the results of SUSTAIN 1 (NCT ), a 30-week, phase 3, randomized controlled trial investigating the efficacy and safety of semaglutide monotherapy versus placebo in treatment naïve T2D patients uncontrolled by lifestyle changes alone (Table 1) (13). Overall 388 patients were randomized 2:2:1:1 to once-weekly semaglutide (0.5 or 1.0 mg) or injected placebo. Mean baseline age was 53.7 years, mean diabetes duration 4.18 years and mean HbA1c 8.05%. Patients were obese (mean BMI kg/m 2 ) with a good sex and race balance. After 30 weeks of treatment, HbA1c decreased significantly with both semaglutide doses, the placebo subtracted difference being 1.43% (0.5 mg dose), respectively 1.53% (1.0 mg semaglutide). Overall 74% of patients treated with 0.5 mg semaglutide and 72% of those with 1.0 mg semaglutide reached a target HbA1c of <7%, while 59%, respectively 60% attained a HbA1c 6.5%. Most importantly, this target was achieved without severe or blood-glucose-confirmed hypoglycemia. In the same time, treatment with semaglutide was associated with a significant weight loss: 3.73 kg for the 0.5 mg semaglutide treated patients and 4.53 kg for the 1.0 mg semaglutide group, in both cases significantly better compared to placebo treated patients. In SUSTAIN-1, treatment with the dose of 1.0 mg of semaglutide was also associated with a significant decrease (compared to placebo) in total and LDL-cholesterol as well as in the levels of free fatty acids. In addition to the decrease with the 2 3 mmhg of the systolic blood pressure (SBP), this completes the favorable CV safety profile of semaglutide which was already proven in patients with already established cardiovascular disease (CVD) or very high cardiovascular (CV) risk in the SUSTAIN-6 trial (18). Otherwise, treatment with semaglutide was rather well tolerated, with a profile of adverse events comparable with that already reported for GLP-1RAs (7), with
3 Annals of Translational Medicine, Vol 5, No 23 December 2017 Page 3 of 5 gastrointestinal (GI) side effects (nausea and diarrhea especially) being most often reported. Overall prevalence of GI side effects was 38% for both doses of semaglutide compared with 15% in placebo treated subjects. There were no episodes of pancreatitis reported during the follow-up treatment. Three other major randomized controlled studies with semaglutide already published their full results. These are SUSTAIN-2 in which efficacy and safety of semaglutide compared with sitagliptin was tested in T2D patients treated with metformin, a thiazolidinedione (TZD) or both (19), SUSTAIN-4 in which the efficacy and safety of semaglutide was compared with that of basal insulin glargine in T2D patients treated with metformin plus minus a sulphonylurea (SU) (20) and, finally, SUSTAIN-6 which tested de CV safety of semaglutide in patients with high CVD risk (18). Interestingly, the hypoglycemic effects of semaglutide were quite consistent across these studies, despite the fact they included rather different patient populations in various stages of T2D evolution. Thus, mean HbA1c decrease varied between 1.1% and 1.3% for the 0.5 mg dose and between 1.4% and 1.64% for the 1.0 mg dose (18-20), comparable with the 1.45%, respectively 1.55% recorded in SUSTAIN-1. The same is true for the percentage of subjects reaching a HbA1c <7%, 72 74% in the SUSTAIN-1 trial, between 60% (0.5 mg dose) and 78% (1.0 mg dose) in the other three semaglutide studies. Regarding the weight loss, this again was consistently reproducible, ranging between 3.5 and 4.3 kg for the 0.5 mg dose and between 5 and 6.1 kg for the 1.0 mg dose (18-20). Interestingly, in a post-hoc analysis of the DURATION-6 data (21) it was shown that weight loss is a direct effect of semaglutide and not related with the GI side effects (nausea and vomiting) associated with GLP1- RA treatment. Concerning adverse events, the most frequently reported in all SUSTAIN trials were GI side effects, the prevalence ranging from 40% to 50%, partially dependent with the duration of exposure to semaglutide. However, usually these were mild in intensity and very rarely (3 5%) led to discontinuation of study medication (18-20). While differences in the study populations included in clinical trials and trial design per se make it difficult to compare the findings of different randomized controlled trials (RCTs), we are giving in Table 1 the main results of the studies that used other long acting GLP-1RAs in T2D patients that were treatment naïve at the time of randomization. As shown, semaglutide led to similar HbA1c decreases compared with once weekly eventide (however higher baseline HbA1c for Exe QW) and higher than once daily liraglutide and once weekly albiglutide (both with similar HbA1c at baseline). Once weekly dulaglutide was also associated with a lower drop in HbA1c compared to semaglutide but lower HbA1c at baseline makes difficult to interpret this difference. Overall, semaglutide was associated with the highest decrease in weight but also with a higher incidence of nausea compared with the other once weekly GLP-1RAs (24% for 0.5 mg semaglutide, 11.3% for Exe QW, 19% for 1.5 mg dulaglutide and 9.1% for 50 mg albiglutide). Obviously more relevant are the results of head to head clinical trials comparing these molecules. Thus, the SUSTAIN-3 trial (22) compared the efficacy and safety of once weekly (QW) semaglutide 1.0 mg with that of Exe QW on 813 T2D subjects not controlled on oral drugs (HbA1c at baseline 8.3%). After 56 weeks of treatment, semaglutide reduced HbA1c significantly greater than Exe QW: 1.5% compared with 0.9%, with 67% of semaglutide treated patients reaching the HbA1c target of <7% compared with 40%. In the same time, patients treated with semaglutide lost significantly more weight compared with those treated with Exe QW: mean body weight reduction 5.6 vs. 1.9 kg. However, semaglutide was associated with a higher percentage of GI adverse events (41.8% vs. 33.3%) while Exe QW had more injection site reactions (1.2% for semaglutide vs. 22% for Exe QW) (22). More recently, a press release from Novo Nordisk (23) communicated some of the results of the SUSTAIN-7 trial which compared the efficacy and safety of semaglutide QW 0.5 and 1.0 mg with that of dulaglutide QW 0.75 and 1.5 mg. The trial included 1201 T2D subjects uncontrolled on metformin alone, with a mean HbA1c at baseline of 8.2% and a mean body weight of 95 kg. After 40-week of treatment, 0.5 mg of semaglutide QW reduced HbA1c by 1.5% compared to 1.1% for 0.75 mg of dulaglutide QW, while 1.0 mg of semaglutide QW reduced HbA1c by 1.8% vs. 1.4% for dulaglutide QW 1.5 mg. The HbA1c target of <7% was achieved by 79% of patients treated with 1.0 mg of semaglutide compared with 68% of the patients treated with 1.5 mg dulaglutide. Moreover, semaglutide was associated with significantly higher reduction of body weight: 4.6 vs. 2.3 kg for 0.5 mg semaglutide and 0.75 mg of dulaglutide, 6.5 vs. 3 kg for
4 Page 4 of 5 Guja and Dănciulescu Miulescu. Semaglutide in T2D 1.0 mg semaglutide, respectively 1.5 mg dulaglutide (23). Conclusions Overall, the results of the SUSTAIN trial program, including SUSTAIN-1, indicate a robust efficacy of once weekly semaglutide in improving metabolic control and decreasing body weight, with a safety profile characteristic for a GLP-1RA, in two trials semaglutide QW being superior to other two GLP-1RAs with once weekly administration: exenatide and dulaglutide. This combines with the results of the SUSTAIN-6 trial indicating the CV benefits of semaglutide: significant 26% reduction (P<0.001) of the composite of CV death, non-fatal myocardial infarction and non-fatal stroke for T2D patients treated with semaglutide versus placebo (18). So, currently, five GLP-1RAs are already approved for the treatment of T2D while semaglutide is in the final phases of approval. Some significant differences exist between the existing GLP-1RAs and semaglutide seems to have some advantages over currently existing drugs. However, finally, the choice of a specific GLP-1RA will be based on several factors, including patient preferences, potential adverse effects, and cost. Acknowledgements None. Footnote Conflicts of Interest: C Guja has received consulting fees from Alfa Wasserman, AstraZeneca, Bayer AG, Berlin- Chemie Mennarini, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. R Dănciulescu Miulescu has no conflicts of interest to declare. References 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patientcentered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38: American Diabetes Association. Pharmacologic approaches to glycemic treatment. sec. 8. in standards of medical care in Diabetes Diabetes Care 2017;40:S64-S Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6: ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015;58: Abd El Aziz MS, Kahle M, Meier JJ, et al. A meta-analysis comparing clinical effects of short- or long-acting GLP- 1 receptor agonists versus insulin treatment from headto-head studies in type 2 diabetic patients. Diabetes Obes Metab 2017;19: Blundell J, Finlayson G, Axelsen M, et al. Effects of onceweekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab 2017;19: Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19: Singh S, Wright EE Jr, Kwan AY, et al. Glucagonlike peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2017;19: Lau J, Bloch P, Schäffer L, et al. Discovery of the onceweekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 2015;58: Lorenz M, Evers A, Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett 2013;23: Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care 2016;39: Tran KL, Park YI, Pandya S, et al. Overview of glucagonlike peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits 2017;10: Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallelgroup, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017;5:
5 Annals of Translational Medicine, Vol 5, No 23 December 2017 Page 5 of Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drugnaive patients with type 2 diabetes (DURATION-4): a 26- week double-blind study. Diabetes Care 2012;35: Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373: Umpierrez G, Tofé Povedano S, Pérez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37: Nauck MA, Stewart MW, Perkins C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016;59: Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375: Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017;5: Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5: Consoli A, Bain SC, Davies M, et al. Semaglutide provides sustained reductions in body weight over 2 years in subjects with type 2 diabetes (SUSTAIN 6). Diabetologia 2017;60:S Ahmann A, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide vs. exenatide ER in subjects with type 2 diabetes (SUSTAIN 3). Diabetes 2016;65:A Novo Nordisk A/S. Semaglutide superior to dulaglutide on glucose control and weight loss in people with type 2 diabetes in SUSTAIN 7. Novo Nordisk A/S Available online: Cite this article as: Guja C, Dănciulescu Miulescu R. Semaglutide the new kid on the block in the field of glucagon-like peptide-1 receptor agonists? Ann Transl Med 2017;5(23):475. doi: /atm
Early treatment for patients with Type 2 Diabetes
 Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
SUSTAINable management of type 2 diabetes: feasibility of use and safety of semaglutide
 Editorial Page 1 of 5 SUSTAINable management of type 2 diabetes: feasibility of use and safety of semaglutide Jun Shirakawa, Yasuo Terauchi Department of Endocrinology and Metabolism, Graduate School of
Editorial Page 1 of 5 SUSTAINable management of type 2 diabetes: feasibility of use and safety of semaglutide Jun Shirakawa, Yasuo Terauchi Department of Endocrinology and Metabolism, Graduate School of
Achieving and maintaining good glycemic control is an
 Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists Yehuda Handelsman, MD, FACP, FNLA, FASPC, MACE; Kathleen Wyne, MD, PhD, FACE, FNLA; Anthony Cannon,
Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists Yehuda Handelsman, MD, FACP, FNLA, FASPC, MACE; Kathleen Wyne, MD, PhD, FACE, FNLA; Anthony Cannon,
OTE. Semaglutide (Ozempic ): A New GLP-1 Agonist for Type 2 Diabetes Mellitus. Vol. 33, Issue 12 September Established 1985
 HAR Vol. 33, Issue 12 September 2018 M A N OTE Established 1985 semaglutide in the treatment of T2DM. Semaglutide (Ozempic ): A New GL-1 Agonist for Type 2 Diabetes Mellitus Graciela Meshkalla, harmd Candidate
HAR Vol. 33, Issue 12 September 2018 M A N OTE Established 1985 semaglutide in the treatment of T2DM. Semaglutide (Ozempic ): A New GL-1 Agonist for Type 2 Diabetes Mellitus Graciela Meshkalla, harmd Candidate
The first stop for professional medicines advice
 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1 receptor analogues The first stop for professional medicines advice 1 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1
London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1 receptor analogues The first stop for professional medicines advice 1 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1
Albiglutide, a Once-Weekly GLP-1RA, for the Treatment of Type 2 Diabetes
 St. Onge et al. Medical Research Archives, vol. 5, issue 11, November 2017 issue Page 1 of 10 REVIEW ARTICLE Albiglutide, a Once-Weekly GLP-1RA, for the Treatment of Type 2 Diabetes Erin St. Onge 1*, Shannon
St. Onge et al. Medical Research Archives, vol. 5, issue 11, November 2017 issue Page 1 of 10 REVIEW ARTICLE Albiglutide, a Once-Weekly GLP-1RA, for the Treatment of Type 2 Diabetes Erin St. Onge 1*, Shannon
MOA: Long acting glucagon-like peptide 1 receptor agonist
 Alexandria Rydz MOA: Long acting glucagon-like peptide 1 receptor agonist Increases glucose dependent insulin secretion Decreases inappropriate glucagon secretion Increases β- cell growth and replication
Alexandria Rydz MOA: Long acting glucagon-like peptide 1 receptor agonist Increases glucose dependent insulin secretion Decreases inappropriate glucagon secretion Increases β- cell growth and replication
Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors
 Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors Timothy Bailey, MD, FACE, CPI Director, AMCR Institute,
Incretin-based Therapies for Type 2 Diabetes Comparisons Between Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors Timothy Bailey, MD, FACE, CPI Director, AMCR Institute,
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE. CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010
 Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
Practical Strategies for the Clinical Use of Incretin Mimetics CME/CE Robert R. Henry, MD Authors and Disclosures CME/CE Released: 09/15/2009; Valid for credit through 09/15/2010 Introduction Type 2 diabetes
GLP 1 agonists Winning the Losing Battle. Dr Bernard SAMIA. KCS Congress: Impact through collaboration
 GLP 1 agonists Winning the Losing Battle Dr Bernard SAMIA KCS Congress: Impact through collaboration CONTACT: Tel. +254 735 833 803 Email: kcardiacs@gmail.com Web: www.kenyacardiacs.org Disclosures I have
GLP 1 agonists Winning the Losing Battle Dr Bernard SAMIA KCS Congress: Impact through collaboration CONTACT: Tel. +254 735 833 803 Email: kcardiacs@gmail.com Web: www.kenyacardiacs.org Disclosures I have
GLP-1RA and insulin: friends or foes?
 Tresiba Expert Panel Meeting 28/06/2014 GLP-1RA and insulin: friends or foes? Matteo Monami Careggi Teaching Hospital. Florence. Italy Dr Monami has received consultancy and/or speaking fees from: Merck
Tresiba Expert Panel Meeting 28/06/2014 GLP-1RA and insulin: friends or foes? Matteo Monami Careggi Teaching Hospital. Florence. Italy Dr Monami has received consultancy and/or speaking fees from: Merck
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol
 Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
GLP-1 agonists. Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK
 GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
GLP-1 (glucagon-like peptide-1) Agonists (Byetta, Bydureon, Tanzeum, Trulicity, Victoza ) Step Therapy and Quantity Limit Criteria Program Summary
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
The Many Faces of T2DM in Long-term Care Facilities
 The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
Francesca Porcellati
 XX Congresso Nazionale AMD Razionali e Benefici dell Aggiunta del GLP-1 RA Short-Acting all Insulina Basale Francesca Porcellati Dipartimento di Medicina Interna, Sezione di Medicina Interna, Endocrinologia
XX Congresso Nazionale AMD Razionali e Benefici dell Aggiunta del GLP-1 RA Short-Acting all Insulina Basale Francesca Porcellati Dipartimento di Medicina Interna, Sezione di Medicina Interna, Endocrinologia
OBESITY IN TYPE 2 DIABETES
 OBESITY IN TYPE 2 DIABETES Ashley Crowl, PharmD, BCACP Assistant Professor University of Kansas Objectives Review how to manage obesity in patients with type-2 diabetes mellitus Compare antiobesity agents
OBESITY IN TYPE 2 DIABETES Ashley Crowl, PharmD, BCACP Assistant Professor University of Kansas Objectives Review how to manage obesity in patients with type-2 diabetes mellitus Compare antiobesity agents
Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American
 Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American Association of Diabetes Educators (AADE) for nurses, dietitians,
Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American Association of Diabetes Educators (AADE) for nurses, dietitians,
Chief of Endocrinology East Orange General Hospital
 Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Non-insulin treatment in Type 1 DM Sang Yong Kim
 Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Scottish Medicines Consortium
 Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Du gusts is megl che one. Edoardo Mannucci
 Du gusts is megl che one Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer Ingelheim, Eli
Du gusts is megl che one Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer Ingelheim, Eli
exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited
 exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
Beyond A1C. Non-glycemic Effects of GLP-1 Receptor Agonists. Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows
 Beyond A1C Non-glycemic Effects of GLP-1 Receptor Agonists Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows Disclosures No conflicts of interest. Learning Objectives 1. Understand the physiological
Beyond A1C Non-glycemic Effects of GLP-1 Receptor Agonists Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows Disclosures No conflicts of interest. Learning Objectives 1. Understand the physiological
Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications
 Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications Nathan Woolever, Pharm.D., Resident Pharmacist Pharmacy Grand Rounds November 6 th, 2018 Franciscan Healthcare La Crosse, WI 2017
Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications Nathan Woolever, Pharm.D., Resident Pharmacist Pharmacy Grand Rounds November 6 th, 2018 Franciscan Healthcare La Crosse, WI 2017
DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My!
 DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My! Kevin M. Pantalone, DO, ECNU, CCD Associate Staff Director of Clinical Research Department of Endocrinology Endocrinology and
DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My! Kevin M. Pantalone, DO, ECNU, CCD Associate Staff Director of Clinical Research Department of Endocrinology Endocrinology and
The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO
 The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO DEPARTMENT OF EMERGENCY AND ORGAN TRANSPLANTATION SECTION OF INTERNAL MEDICINE, ENDOCRINOLOGY, ANDROLOGY AND METABOLIC DISEASES Disclaimer
The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO DEPARTMENT OF EMERGENCY AND ORGAN TRANSPLANTATION SECTION OF INTERNAL MEDICINE, ENDOCRINOLOGY, ANDROLOGY AND METABOLIC DISEASES Disclaimer
3/8/2011. Julie M. Sease, Pharm D, BCPS, CDE Associate Professor of Pharmacy Practice Presbyterian College School of Pharmacy
 Summarize revisions to the 2011 American Diabetes Association clinical practice guidelines. Evaluate bromocriptine as a therapeutic option in the management of type 2 diabetes. Compare and contrast the
Summarize revisions to the 2011 American Diabetes Association clinical practice guidelines. Evaluate bromocriptine as a therapeutic option in the management of type 2 diabetes. Compare and contrast the
Multiple Factors Should Be Considered When Setting a Glycemic Goal
 Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
Management of Type 2 Diabetes
 Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
New Drug Evaluation: Dulaglutide
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
For Personal Use Only. Any commercial use is strictly prohibited. Role of glucagon-like peptide 1 receptor agonists in management of obesity
 Role of glucagon-like peptide 1 receptor agonists in management of obesity Diana Isaacs, Pharm.D., BCPS, BC-ADM, CDE, Chicago State University, Chicago, IL, and Oak Lawn VA Clinic of Edward Hines Jr. VA
Role of glucagon-like peptide 1 receptor agonists in management of obesity Diana Isaacs, Pharm.D., BCPS, BC-ADM, CDE, Chicago State University, Chicago, IL, and Oak Lawn VA Clinic of Edward Hines Jr. VA
Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists
 Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists Robert R. Henry, MD Professor of Medicine University of California, San Diego Relevant Conflict
Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists Robert R. Henry, MD Professor of Medicine University of California, San Diego Relevant Conflict
semaglutide 0.25mg, 0.5mg and 1mg solution for injection in pre-filled pen (Ozempic ) Novo Nordisk Ltd.
 1 www.scottishmedicines.org.uk semaglutide 0.25mg, 0.5mg and 1mg solution for injection in pre-filled pen (Ozempic ) Novo Nordisk Ltd. SMC2092 9 November 2018 (Issued 7 December 2018) The Scottish Medicines
1 www.scottishmedicines.org.uk semaglutide 0.25mg, 0.5mg and 1mg solution for injection in pre-filled pen (Ozempic ) Novo Nordisk Ltd. SMC2092 9 November 2018 (Issued 7 December 2018) The Scottish Medicines
Lilly Diabetes: Pipeline Update
 Lilly Diabetes: Pipeline Update June 16, 2014 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's current expectations, but actual results may differ
Lilly Diabetes: Pipeline Update June 16, 2014 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's current expectations, but actual results may differ
Terapia con agonisti GLP1 e outcome cardiovascolare. Edoardo Mannucci
 Terapia con agonisti GLP e outcome cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca,
Terapia con agonisti GLP e outcome cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca,
NEW DIABETES CARE MEDICATIONS
 NEW DIABETES CARE MEDICATIONS James Bonucchi DO, ECNU, FACE Adult Medicine and Endocrinology Specialists Disclosures Speakers bureau Sanofi AZ BI Diabetes Diabetes cost ADA 2017 data Ever increasing disorder.
NEW DIABETES CARE MEDICATIONS James Bonucchi DO, ECNU, FACE Adult Medicine and Endocrinology Specialists Disclosures Speakers bureau Sanofi AZ BI Diabetes Diabetes cost ADA 2017 data Ever increasing disorder.
ADA Analyst Presentation Saturday 9 th June
 ADA Analyst Presentation Saturday 9 th June Carlo Russo Senior Vice-President & Albiglutide Team Leader, GSK Property of GlaxoSmithKline Agenda Welcome & introduction to the Harmony Clinical Programme
ADA Analyst Presentation Saturday 9 th June Carlo Russo Senior Vice-President & Albiglutide Team Leader, GSK Property of GlaxoSmithKline Agenda Welcome & introduction to the Harmony Clinical Programme
Incredible Incretins Abby Frye, PharmD, BCACP
 Incredible Incretins Abby Frye, PharmD, BCACP Objectives & Disclosures Review the pathophysiology of T2DM and the impact of the incretin system Describe the defining characteristics of the available glucagonlike
Incredible Incretins Abby Frye, PharmD, BCACP Objectives & Disclosures Review the pathophysiology of T2DM and the impact of the incretin system Describe the defining characteristics of the available glucagonlike
GLUCAGON LIKE PEPTIDE (GLP) 1 AGONISTS FOR THE TREATMENT OF TYPE 2 DIABETES, WEIGHT CONTROL AND CARDIOVASCULAR PROTECTION.
 GLUCAGON LIKE PEPTIDE (GLP) 1 AGONISTS FOR THE TREATMENT OF TYPE 2 DIABETES, WEIGHT CONTROL AND CARDIOVASCULAR PROTECTION. Patricia Garnica MS, ANP-BC, CDE, CDTC Inpatient Diabetes Nurse Practitioner North
GLUCAGON LIKE PEPTIDE (GLP) 1 AGONISTS FOR THE TREATMENT OF TYPE 2 DIABETES, WEIGHT CONTROL AND CARDIOVASCULAR PROTECTION. Patricia Garnica MS, ANP-BC, CDE, CDTC Inpatient Diabetes Nurse Practitioner North
This program applies to Commercial, GenPlus and Health Insurance Marketplace formularies.
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
Advances in Outpatient Diabetes Care: Algorithms for Care and the Role of Injectable Therapies. Module D
 Advances in Outpatient Diabetes Care: Algorithms for Care and the Role of Injectable Therapies Module D 1 Learning Objectives Apply the principles of the comprehensive diabetes algorithms to patients with
Advances in Outpatient Diabetes Care: Algorithms for Care and the Role of Injectable Therapies Module D 1 Learning Objectives Apply the principles of the comprehensive diabetes algorithms to patients with
SYSTEMATIC REVIEW. Introduction. X. Xue, 1 Z. Ren, 1 A. Zhang, 2 Q. Yang, 3 W. Zhang, 4 F. Liu 1,4
 SYSTEMATIC REVIEW Efficacy and safety of once-weekly glucagon-like peptide-1 receptor agonists compared with exenatide and liraglutide in type 2 diabetes: a systemic review of randomised controlled trials
SYSTEMATIC REVIEW Efficacy and safety of once-weekly glucagon-like peptide-1 receptor agonists compared with exenatide and liraglutide in type 2 diabetes: a systemic review of randomised controlled trials
Basal & GLP-1 Fixed Combination Use
 Basal & GLP-1 Fixed Combination Use Michelle M. Mangual, MD Diplomate of the American board of Internal Medicine and Endocrinology, Diabetes and Metabolism San Juan City hospital Learning Objectives o
Basal & GLP-1 Fixed Combination Use Michelle M. Mangual, MD Diplomate of the American board of Internal Medicine and Endocrinology, Diabetes and Metabolism San Juan City hospital Learning Objectives o
Intensifying Treatment Beyond Monotherapy in T2DM: Where Do Newer Therapies Fit?
 Intensifying Treatment Beyond Monotherapy in T2DM: Where Do Newer Therapies Fit? Vanita R. Aroda, MD Scientific Director & Physician Investigator MedStar Community Clinical Research Center MedStar Health
Intensifying Treatment Beyond Monotherapy in T2DM: Where Do Newer Therapies Fit? Vanita R. Aroda, MD Scientific Director & Physician Investigator MedStar Community Clinical Research Center MedStar Health
New Medicine Assessment Semaglutide (Ozempic ) Treatment of Adults with Insufficiently Controlled Type 2 Diabetes
 December 2018 New Medicine Assessment Semaglutide (Ozempic ) Treatment of Adults with Insufficiently Controlled Type 2 Diabetes Recommendation: GREEN Semaglutide is an appropriate treatment option for
December 2018 New Medicine Assessment Semaglutide (Ozempic ) Treatment of Adults with Insufficiently Controlled Type 2 Diabetes Recommendation: GREEN Semaglutide is an appropriate treatment option for
Liraglutide: First Once-Daily Human GLP-1 Analogue
 DRUG PROFILE KERALA MEDICAL JOURNAL Liraglutide: First Once-Daily Human GLP-1 Analogue Sreejith N Kumar Research Cell, IMA Kerala State, K-5, Kochar Road, Sasthamangalam Thiruvananthapuram* ABSTRACT Published
DRUG PROFILE KERALA MEDICAL JOURNAL Liraglutide: First Once-Daily Human GLP-1 Analogue Sreejith N Kumar Research Cell, IMA Kerala State, K-5, Kochar Road, Sasthamangalam Thiruvananthapuram* ABSTRACT Published
GLP-1 receptor agonists for type 2 diabetes currently available in the U.S.
 GLP-1 receptor agonists for type 2 diabetes currently available in the U.S. GLP-1 agonists are a class of antidiabetic agents that mimic the actions of the glucagon-like peptide. GLP-1 is one of several
GLP-1 receptor agonists for type 2 diabetes currently available in the U.S. GLP-1 agonists are a class of antidiabetic agents that mimic the actions of the glucagon-like peptide. GLP-1 is one of several
Insulin Initiation and Intensification. Disclosure. Objectives
 Insulin Initiation and Intensification Neil Skolnik, M.D. Associate Director Family Medicine Residency Program Abington Memorial Hospital Professor of Family and Community Medicine Temple University School
Insulin Initiation and Intensification Neil Skolnik, M.D. Associate Director Family Medicine Residency Program Abington Memorial Hospital Professor of Family and Community Medicine Temple University School
La lezione dei trials di safety cardiovascolare. Edoardo Mannucci
 La lezione dei trials di safety cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer
La lezione dei trials di safety cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer
Soliqua (insulin glargine and lixisenatide), Xultophy (insulin degludec and liraglutide)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.48 Subject: Insulin GLP-1 Combinations Page: 1 of 5 Last Review Date: September 15, 2017 Insulin GLP-1
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.48 Subject: Insulin GLP-1 Combinations Page: 1 of 5 Last Review Date: September 15, 2017 Insulin GLP-1
Horizon Scanning Technology Summary. Liraglutide for type 2 diabetes. National Horizon Scanning Centre. April 2007
 Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Faculty. Timothy S. Reid, MD (Co-Chair, Presenter) Medical Director Mercy Diabetes Center Janesville, WI
 Activity Overview In this case-based webcast, meet Jackie, a 62-year-old woman with type 2 diabetes. Her glycated hemoglobin (HbA1C) is 9.2%, and she is taking 2 oral agents and basal insulin; however,
Activity Overview In this case-based webcast, meet Jackie, a 62-year-old woman with type 2 diabetes. Her glycated hemoglobin (HbA1C) is 9.2%, and she is taking 2 oral agents and basal insulin; however,
Selecting GLP-1 RA Treatment
 Selecting GLP-1 RA Treatment Dr Felicity Kaplan March 2017 Objectives Review the progressive nature of type 2 diabetes Understand the need for timely treatment intensification Examine the place of GLP-1
Selecting GLP-1 RA Treatment Dr Felicity Kaplan March 2017 Objectives Review the progressive nature of type 2 diabetes Understand the need for timely treatment intensification Examine the place of GLP-1
New Drug Evaluation: lixisenatide injection, subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
GLP-1-based therapies in the management of type 2 diabetes
 GLP-1-based therapies in the management of type 2 diabetes Makbul Aman Mansyur Division Endocrine & Metabolism Department of Internal Medicine Faculty of Medicine Hasanuddin University/ RSUP Dr. Wahidin
GLP-1-based therapies in the management of type 2 diabetes Makbul Aman Mansyur Division Endocrine & Metabolism Department of Internal Medicine Faculty of Medicine Hasanuddin University/ RSUP Dr. Wahidin
Disclosures of Interest. Publications Diabetologia Key points to emphasize
 Disclosures of Interest No conflicts or disclosures How to Use the American Diabetes Association s Type 2 Diabetes Treatment Algorithm Rashida Downing, MD, FAAFP Primary Care Physician JenCare Medical
Disclosures of Interest No conflicts or disclosures How to Use the American Diabetes Association s Type 2 Diabetes Treatment Algorithm Rashida Downing, MD, FAAFP Primary Care Physician JenCare Medical
sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd
 sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the
sitagliptin, 25mg, 50mg and 100mg film-coated tablets (Januvia ) SMC No. (1083/15) Merck Sharp and Dohme UK Ltd 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Le incretine: un passo avanti. Francesco Dotta
 Le incretine: un passo avanti Francesco Dotta U.O.C. Diabetologia, Policlinico Le Scotte Università di Siena Fondazione Umberto Di Mario ONLUS Toscana Life Science Park Incretins: multiple targets multiple
Le incretine: un passo avanti Francesco Dotta U.O.C. Diabetologia, Policlinico Le Scotte Università di Siena Fondazione Umberto Di Mario ONLUS Toscana Life Science Park Incretins: multiple targets multiple
No Increased Cardiovascular Risk for Lixisenatide in ELIXA
 ON ISSUES IN THE MANAGEMENT OF TYPE 2 DIABETES JUNE 2015 Coverage of data from ADA 2015, June 5 9 in Boston, Massachusetts No Increased Cardiovascular Risk for Lixisenatide in ELIXA First Cardiovascular
ON ISSUES IN THE MANAGEMENT OF TYPE 2 DIABETES JUNE 2015 Coverage of data from ADA 2015, June 5 9 in Boston, Massachusetts No Increased Cardiovascular Risk for Lixisenatide in ELIXA First Cardiovascular
Case Report Off-Label Use of Liraglutide in the Management of a Pediatric Patient with Type 2 Diabetes Mellitus
 Case Reports in Pediatrics Volume 2013, Article ID 703925, 4 pages http://dx.doi.org/10.1155/2013/703925 Case Report Off-Label Use of Liraglutide in the Management of a Pediatric Patient with Type 2 Diabetes
Case Reports in Pediatrics Volume 2013, Article ID 703925, 4 pages http://dx.doi.org/10.1155/2013/703925 Case Report Off-Label Use of Liraglutide in the Management of a Pediatric Patient with Type 2 Diabetes
Type 2 Diabetes: Where Do We Start with Treatment? DIABETES EDUCATION. Diabetes Mellitus: Complications and Co-Morbid Conditions
 Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
Help the Heart. An Update on GLP-1 Agonists and SGLT2 Inhibitors. Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire
 Help the Heart An Update on GLP-1 Agonists and SGLT2 Inhibitors Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire Mayo Clinic Grand Rounds May 16, 2017 2017 MFMER slide-1
Help the Heart An Update on GLP-1 Agonists and SGLT2 Inhibitors Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire Mayo Clinic Grand Rounds May 16, 2017 2017 MFMER slide-1
Diabetes: Three Core Deficits
 Diabetes: Three Core Deficits Fat Cell Dysfunction Impaired Incretin Function Impaired Appetite Suppression Obesity and Insulin Resistance in Muscle and Liver Hyperglycemia Impaired Insulin Secretion Islet
Diabetes: Three Core Deficits Fat Cell Dysfunction Impaired Incretin Function Impaired Appetite Suppression Obesity and Insulin Resistance in Muscle and Liver Hyperglycemia Impaired Insulin Secretion Islet
Management of Type 2 Diabetes Cardiovascular Outcomes Trials Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas
 Management of Type 2 Diabetes Cardiovascular Outcomes Trials 2018 Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas Speaker Disclosure Dr. Blevins has disclosed that he has received grant support
Management of Type 2 Diabetes Cardiovascular Outcomes Trials 2018 Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas Speaker Disclosure Dr. Blevins has disclosed that he has received grant support
Update on Insulin-based Agents for T2D
 Update on Insulin-based Agents for T2D Injectable Therapies for Type 2 Diabetes Mellitus (T2DM) and Obesity This presentation will: Describe established and newly available insulin therapies for treatment
Update on Insulin-based Agents for T2D Injectable Therapies for Type 2 Diabetes Mellitus (T2DM) and Obesity This presentation will: Describe established and newly available insulin therapies for treatment
Initiating Injectable Therapy in Type 2 Diabetes
 Initiating Injectable Therapy in Type 2 Diabetes David Doriguzzi, PA C Learning Objectives To understand current Diabetes treatment guidelines To understand how injectable medications fit into current
Initiating Injectable Therapy in Type 2 Diabetes David Doriguzzi, PA C Learning Objectives To understand current Diabetes treatment guidelines To understand how injectable medications fit into current
GLP-1 Agonists for Diabetes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes
 Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs
 Butler University Digital Commons @ Butler University Undergraduate Honors Thesis Collection Undergraduate Scholarship 5-2017 A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs Shelby
Butler University Digital Commons @ Butler University Undergraduate Honors Thesis Collection Undergraduate Scholarship 5-2017 A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs Shelby
Glucagon-Like Peptide-1 (GLP-1) Agonists
 Glucagon-Like Peptide-1 (GLP-1) Agonists Policy Number: 5.01.565 Last Review: 07/2018 Origination: 06/2014 Next Review: 07/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Glucagon-Like Peptide-1 (GLP-1) Agonists Policy Number: 5.01.565 Last Review: 07/2018 Origination: 06/2014 Next Review: 07/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Novel anti-diabetic therapies
 Prof. Manfredi Rizzo, MD, PhD ASSOCIATE PROFESSOR OF INTERNAL MEDICINE School of Medicine University of Palermo, Italy & ASSOCIATE PROFESSOR OF INTERNAL MEDICINE School of Medicine University of South
Prof. Manfredi Rizzo, MD, PhD ASSOCIATE PROFESSOR OF INTERNAL MEDICINE School of Medicine University of Palermo, Italy & ASSOCIATE PROFESSOR OF INTERNAL MEDICINE School of Medicine University of South
Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance
 580257TAE0010.1177/2042018815580257Therapeutic Advances in Endocrinology and MetabolismSH Tella and MS Rendell research-article2015 Therapeutic Advances in Endocrinology and Metabolism Review Glucagon-like
580257TAE0010.1177/2042018815580257Therapeutic Advances in Endocrinology and MetabolismSH Tella and MS Rendell research-article2015 Therapeutic Advances in Endocrinology and Metabolism Review Glucagon-like
Role of incretins in the treatment of type 2 diabetes
 Role of incretins in the treatment of type 2 diabetes Jens Juul Holst Department of Medical Physiology Panum Institute University of Copenhagen Denmark Diabetes & Obesity Spanish Society of Internal Medicine
Role of incretins in the treatment of type 2 diabetes Jens Juul Holst Department of Medical Physiology Panum Institute University of Copenhagen Denmark Diabetes & Obesity Spanish Society of Internal Medicine
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP
 Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Agenda. Indications Different insulin preparations Insulin initiation Insulin intensification
 Insulin Therapy F. Hosseinpanah Obesity Research Center Research Institute for Endocrine sciences Shahid Beheshti University of Medical Sciences November 11, 2017 Agenda Indications Different insulin preparations
Insulin Therapy F. Hosseinpanah Obesity Research Center Research Institute for Endocrine sciences Shahid Beheshti University of Medical Sciences November 11, 2017 Agenda Indications Different insulin preparations
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy
 Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
PROCEEDINGS CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES * Vivian A. Fonseca, MD, FRCP ABSTRACT
 CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES Vivian A. Fonseca, MD, FRCP ABSTRACT Despite proven lifestyle recommendations and the availability of a range of oral antidiabetic agents,
CLINICAL RESEARCH AND EXPERIENCE WITH INCRETIN-BASED THERAPIES Vivian A. Fonseca, MD, FRCP ABSTRACT Despite proven lifestyle recommendations and the availability of a range of oral antidiabetic agents,
dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd.
 dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd. 04 December 2015 The Scottish Medicines Consortium (SMC) has completed its
dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd. 04 December 2015 The Scottish Medicines Consortium (SMC) has completed its
Dulaglutide (LY ) for the treatment of type 2 diabetes
 Expert Review of Clinical Pharmacology ISSN: 1751-2433 (Print) 1751-2441 (Online) Journal homepage: http://www.tandfonline.com/loi/ierj20 (LY-2189265) for the treatment of type 2 diabetes André J. Scheen
Expert Review of Clinical Pharmacology ISSN: 1751-2433 (Print) 1751-2441 (Online) Journal homepage: http://www.tandfonline.com/loi/ierj20 (LY-2189265) for the treatment of type 2 diabetes André J. Scheen
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS
 IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
Professor Rudy Bilous James Cook University Hospital
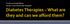 Professor Rudy Bilous James Cook University Hospital Rate per 100 patient years Rate per 100 patient years 16 Risk of retinopathy progression 16 Risk of developing microalbuminuria 12 12 8 8 4 0 0 5 6
Professor Rudy Bilous James Cook University Hospital Rate per 100 patient years Rate per 100 patient years 16 Risk of retinopathy progression 16 Risk of developing microalbuminuria 12 12 8 8 4 0 0 5 6
Update on Diabetes Cardiovascular Outcome Trials
 Update on Diabetes Cardiovascular Outcome Trials Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine
Update on Diabetes Cardiovascular Outcome Trials Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine
Dulaglutide: designed with patients in mind
 31 st Panhellenic Annual Congress of the Hellenic Association for the Study & Education of Diabetes Mellitus Thessaloniki 11 th, November 2017 Dulaglutide: designed with patients in mind Imre Pavo MD PhD
31 st Panhellenic Annual Congress of the Hellenic Association for the Study & Education of Diabetes Mellitus Thessaloniki 11 th, November 2017 Dulaglutide: designed with patients in mind Imre Pavo MD PhD
DR. SUBHASH K. WANGNOO
 Photograph DR. SUBHASH K. WANGNOO M.D, D.M, FRCP (London) Senior Consultant, Endocrinologist & Diabetologist Apollo Centre for Obesity, Diabetes and Endocrinology Indraprastha Apollo Hospital, Sarita Vihar,
Photograph DR. SUBHASH K. WANGNOO M.D, D.M, FRCP (London) Senior Consultant, Endocrinologist & Diabetologist Apollo Centre for Obesity, Diabetes and Endocrinology Indraprastha Apollo Hospital, Sarita Vihar,
NCT Number: NCT
 Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec 100 U/mL in insulin-naïve adults with type 2 diabetes mellitus: Design and baseline characteristics of the BRIGHT study Alice Cheng
Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec 100 U/mL in insulin-naïve adults with type 2 diabetes mellitus: Design and baseline characteristics of the BRIGHT study Alice Cheng
Bedtime-to-Morning Glucose Difference and iglarlixi in Type 2 Diabetes: Post Hoc Analysis of LixiLan-L
 Diabetes Ther (2018) 9:2155 2162 https://doi.org/10.1007/s13300-018-0507-0 BRIEF REPORT Bedtime-to-Morning Glucose Difference and iglarlixi in Type 2 Diabetes: Post Hoc Analysis of LixiLan-L Ariel Zisman.
Diabetes Ther (2018) 9:2155 2162 https://doi.org/10.1007/s13300-018-0507-0 BRIEF REPORT Bedtime-to-Morning Glucose Difference and iglarlixi in Type 2 Diabetes: Post Hoc Analysis of LixiLan-L Ariel Zisman.
Disclosure. Learning Objectives. Case. Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare
 Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
A Review of the Long-Term Efficacy, Tolerability, and Safety of Exenatide Once Weekly for Type 2 Diabetes
 Adv Ther (2017) 34:1791 1814 DOI 10.1007/s12325-017-0499-6 REVIEW A Review of the Long-Term Efficacy, Tolerability, and Safety of Exenatide Once Weekly for Type 2 Diabetes Stefano Genovese. Edoardo Mannucci.
Adv Ther (2017) 34:1791 1814 DOI 10.1007/s12325-017-0499-6 REVIEW A Review of the Long-Term Efficacy, Tolerability, and Safety of Exenatide Once Weekly for Type 2 Diabetes Stefano Genovese. Edoardo Mannucci.
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES
 INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists
 Texas Vendor Drug Program Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists Publication History Developed February 2006. Revised September 2018; September 2016; June 2015; October 2013; December
Texas Vendor Drug Program Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists Publication History Developed February 2006. Revised September 2018; September 2016; June 2015; October 2013; December
Safety profile of Liraglutide: Recent Updates. Mohammadreza Rostamzadeh,M.D.
 Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
Presented By: Creative Educational Concepts, Inc. Lexington, KY
 Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of April 2015. The content and views presented in this educational activity are those of the
Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of April 2015. The content and views presented in this educational activity are those of the
Case Studies in Type 2 Diabetes Mellitus: Focus on Cardiovascular Outcomes Trials
 Case Studies in Type 2 Diabetes Mellitus: Focus on Cardiovascular Outcomes Trials Louis Kuritzky MD Clinical Assistant Professor Emeritus Department of Community Health and Family Medicine College of Medicine
Case Studies in Type 2 Diabetes Mellitus: Focus on Cardiovascular Outcomes Trials Louis Kuritzky MD Clinical Assistant Professor Emeritus Department of Community Health and Family Medicine College of Medicine
Dr Matthew Coghlan Novo Nordisk
 Dr Matthew Coghlan Novo Nordisk Preventing diabetes and its complications Matthew Coghlan PhD Senior Director Search & Evaluation Global Drug Discovery Novo Nordisk A/S Obesity rates worldwide are increasing
Dr Matthew Coghlan Novo Nordisk Preventing diabetes and its complications Matthew Coghlan PhD Senior Director Search & Evaluation Global Drug Discovery Novo Nordisk A/S Obesity rates worldwide are increasing
Current Status of Incretin Based Therapies in Type 2 Diabetes
 Current Status of Incretin Based Therapies in Type 2 Diabetes DR.M.Mukhyaprana Prabhu Professor of Internal Medicine Kasturba Medical College, Manipal, Manipal University, India 2 nd International Endocrine
Current Status of Incretin Based Therapies in Type 2 Diabetes DR.M.Mukhyaprana Prabhu Professor of Internal Medicine Kasturba Medical College, Manipal, Manipal University, India 2 nd International Endocrine
Adlyxin. (lixisenatide) New Product Slideshow
 Adlyxin (lixisenatide) New Product Slideshow Introduction Brand name: Adlyxin Generic name: Lixisenatide Pharmacological class: Glucagon-like peptide-1 (GLP-1) receptor agonist Strength and Formulation:
Adlyxin (lixisenatide) New Product Slideshow Introduction Brand name: Adlyxin Generic name: Lixisenatide Pharmacological class: Glucagon-like peptide-1 (GLP-1) receptor agonist Strength and Formulation:
STEP THERAPY CRITERIA
 CATEGORY DRUG CLASS BRAND NAME (generic) STEP THERAPY CRITERIA AMYLIN ANALOG: SYMLIN/SYMLINPEN (pramlintide acetate) ANTIDIABETIC AGENTS GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST (GLP-1): ADLYXIN (lixisenatide)
CATEGORY DRUG CLASS BRAND NAME (generic) STEP THERAPY CRITERIA AMYLIN ANALOG: SYMLIN/SYMLINPEN (pramlintide acetate) ANTIDIABETIC AGENTS GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST (GLP-1): ADLYXIN (lixisenatide)
Brigham and Women s Hospital Type 2 Diabetes Management Program Physician Pharmacist Collaborative Drug Therapy Management Protocol
 Brigham and Women s Hospital Type 2 Diabetes Management Program Physician Pharmacist Collaborative Drug Therapy Management Protocol *Please note that this guideline may not be appropriate for all patients
Brigham and Women s Hospital Type 2 Diabetes Management Program Physician Pharmacist Collaborative Drug Therapy Management Protocol *Please note that this guideline may not be appropriate for all patients
GLP-1 receptor agonists for type 2 diabetes A rationale drug. development. Bo Ahrén
 PROF. BO AHREN (Orcid ID : 0000-0002-9804-5340) Article type : Mini Review GLP-1 receptor agonists for type 2 diabetes A rationale drug development Bo Ahrén Department of Clinical Sciences Lund, Lund University,
PROF. BO AHREN (Orcid ID : 0000-0002-9804-5340) Article type : Mini Review GLP-1 receptor agonists for type 2 diabetes A rationale drug development Bo Ahrén Department of Clinical Sciences Lund, Lund University,
