Confirmation - A Practical Approach
|
|
|
- Katherine Bridges
- 5 years ago
- Views:
Transcription
1 Data Mining i for Reference Interval Confirmation - A Practical Approach Graham Jones Chemical Pathologist St Vincent s Hospital, Sydney AACB Webinar 5 th June 2013
2 Summary A little (more) background Current status in Australian adult Common Reference Intervals (CRI) Local Validation of CRI (data mining) Bhattacharya
3 Reference Intervals Fixed proportion of a reference population Usually central 95% Usually Health population Most common interpretive tool for numerical laboratory results Changes a result (eg X mmol/l) into information (eg grossly elevated ) Customers have great faith in them Customers have great faith in them its all right I interpret the results against the laboratory s own reference interval
4 Reference Intervals Alb Cr Ratio Upper Reference Limit Number 1.0 mg/mmol mg/mmol mg/mmol (m) / 3.5 (f) mg/mmol mg/mmol Data
5 Reference Intervals Alb Cr Ratio Upper Reference Limit Number 1.0 mg/mmol 3 Highest over 3 x lowest 2.0 mg/mmol mg/mmol 5 Assay variability ~20% 2.5 (m) / 3.5 (f) mg/mmol 1 Reference interval changes the information 3.5 mg/mmol Data
6 Statement of belief Any survey of reference intervals will show a wide scatter This scatter is not related to method differences We will all select and assess the data differently This causes our laboratories to give out different information G Jones 26 th July 2012 (revised 4 th June 2013)
7 Statements of Belief (2) Laboratories should act similarly where possible Pre-analytic analytic, analytic, post-analytic This can only happen if there is: (e)meeting of people Discussion Agreement Action
8 Common Reference Intervals (CRI) Clinical Decision Points Defined by expert groups Glucose, HbA1c (diabetes Dx & Mx), Lipids (risk and treatment) Methods with comparable results Unbiased from each other CRI possible for all labs Methods with non-comparable results Biased from each other Method-specific CRI possible
9 Clinical Decision Points HbA1c HbA1c acceptable performance in EQA, standardised to international reference values, MJA 2012;197 (4) 20 th August 2012
10
11 Sample RCPA QAP Whole Blood HbA1c 2012 NGSP 11 ALP High = Median / Target = 10.3 NGSP (% %) 10 ALP Low = What we can do: - Select good method -Make method run well RCPAQAP Chemical Pathology
12 Common Clinical decision points Source same references / guidelines Interpret them in the same way Avoid minor inconsistencies Eg >1.0 mmol/l >1.00 mmol/l (1.02 not flagged / flagged depending on decimal places) Guidelines should state explicitly what should be flagged or we should decide and implement
13 Population Reference Intervals
14 Population reference intervals (Approximately) Gaussian Symmetrical Simple statistics (central 95%) Bias criteria well established Data-mining i robust Skewed / assymetrical Statistics and criteria less well established May include common pathology (eg ALT) May need clinical overlay (eg exclude overweight / obese) WARNING!!!
15 CRI - National Activities AACB Harmonisation WG National Meeting May participants 19 chemical pathologists All 6 specialist paediatric chemical pathologists
16 Process Working groups with information supplied Meta data about test Reference interval data Reference NORIP Interval to share ARQAG / SIQAG (with thanks) UK harmonised reference intervals Data extracts t from Sonic labs (with thanks) Aussie Normals (with thanks) Method comparison data QAP data,,published data BIAS study (Gus Koerbin) Small enough method bias
17 Magnesium Magnesium 1.00 Obtain ned (mmol l/l) ADVIA 2400 Architect AU2700 DxC Integra Modular Vitros Average (mmol/l)
18 Magnesium Population RI Gender differences mmol/l No No expected methodological Fasting: differences No reason to have a fasting RI Analytically there are differences Inpatients vs outpatients. No Integra and Architect possibly different Age related intervals No Serum vs Plasma Interchangeable Proposed RI: Pre-analytical Preanalytics should not have an effect on Mg
19 Creatine Kinase CK ained (U/L Obt )ADVIA Architect AU2700 DxC Integra Modular 90.0 RxL Vitros Average (U/L)
20 Reference interval Data CK MALES NORIP y NORIP - 50 y+ ARQAG SIQAG Level of LRL URL evidence (U/L) (U/L) 2a) direct ) Professional recommendation UK HARMONY 4) Consensus SONIC <60 y SONIC <90 y SONIC 90 y+ 2a) indirect AUSSIE NORMALS 2a) direct (49) (291) (exclusions to follow) (N=434) Lo et al. Hong Kong 2a) direct ejifcc March 2012 (N=378) Schumann G et al. CCA 2003;327: a) direct
21 Creatine Kinase SONIC RI data are from largely outpatients, whereas Schumann data are from hospitalised inpatients and may reflect lower muscle mass. AUSSIE NORMALS data require exclusion of individuals with increased muscle mass, e.g. athletes. Testosterone-CK correlation less change in women than men with age. Further information about the impact of haemolysis is required. Further investigation of differences between AUSSIE NORMALS and SONIC data is required, particularly for females. Caution on ethnicity investigation of the indigenous population may be required.
22 Creatine Kinase Analytically there are no differences between methods. Possible to share a common RI but need to define population p by gender & age Provisional RIs: Male: 16 -<60 y U/L Female: 60 - <90 y U/L 90 y U/L U/L
23 Analyte Male Female Calcium mmol/l Phosphate Magnesium LDH [L to mmol/l mmol/l U/L P]IFCC Sodium mmol/l Potassium mmol/l Chloride Bicarbonate mmol/l mmol/l Creatinine umol/l umol/l ALP U/L AST <40 <35 ALT <40 <30 Total Protein g/l
24 Current Paradigm Based on recommendations from the NCCLS and the IFCC Repeated in Product Information from most reagent suppliers Encoded in the NATA summary of ISO/IEC guide laboratories may perform their own detailed d reference interval studies or may validate reference intervals published elsewhere for their own methods and populations
25 Adopting (Common) Reference Intervals Laboratory must determine that reference interval is suitable for use in their lab. Two components Analytical accuracy Is my method biased compared with the method used to set the reference interval? Population accuracy Is my population the same as that included in the external reference interval?
26 Adopting Common Reference Intervals Analytical Accuracy Show method has small enough bias from method used to define reference interval Eg: Value assigned QC (manufacturer) External QA Reference Materials Certification This is all that is possible for decision points Seek multiple sources of confirmation
27 Adopting Common Reference Intervals Analytical Accuracy Show method has small enough bias from method used to define reference interval Eg: Value assigned QC (manufacturer) Eg if adopting data from Aussie External QA Normals, and using Abbott assays, Reference Materials show your results match other Abbott Certification results This is all that is possible for decision points Seek multiple sources of confirmation
28 Reference Interval Size To contain 95% of results, reference Intervals include the following: Between-person variation (CVg) Within-person variation (only one sample, CVi) Pre-analytical l variation (only one collection, CVpa) Analytical variation (only one measurement, CVa) CVri = (CVg 2 + CVi 2 + CVpa 2 + CVa 2 )
29 Common Reference Interval Size CVri = (CVg 2 + CVi 2 + CVpa 2 + CVa 2 ) CVa All samples in one run: within-run CV Same machine over time: between-run CV Different machines, same lab: CVx Different machines, different labs: CVy Different suppliers, different : CVz CVz > CVy > Cvx > CVbr > CVwr MORE MACHINES BIGGER Cva WIDER REFERENCE INTERVALS
30 Common Reference intervals When CRI applied in one lab % outside RI < 2.5% Less sensitive for abnormalities (more specific) Allows for some Wriggle room Lot to lot variation calibrator variation lab Lot-to-lot variation, calibrator variation, labto-lab variation etc
31 Adopting (Common) Reference Intervals CLSI method: run 20 samples from healthy subjects: 18 in ranges, accept. Local reference interval study Within experimental uncertainties Database mining Midpoint Number of outliers All test method and population More data = better assessment
32 Analysing the Data
33 SydPath Validation Data extracts from routine database GP samples only Ages above 18 (some upper age limits) ~9000 subjects in most groups
34 Spreadsheet Application Available from
35 Instructions Caveat: Spreadsheet put together with care. But no guarantees of being error free User assumes all responsibility. Always view data, check usual statistics and assess for reasonableness
36 Data Entry Standardised data entry List of: result, sex, age, (date) 50,000 now 65,000 max in Excel ,048,576 max in Excel 2010 Limit to outpatient data LIS, Middleware, backup server
37 More data (Excel 2010) Extend calculations (50003 x?) Copy these 2 columns down Extend Lookuo (50003 x?)
38 Additional Data Input (metadata)
39 Numerical Output
40 Application Output graph Allows visual assessment Variables: Xaxis Bin size (only affects graph)
41 Graph X- axis
42 Bin Size for Graph
43 Application Averages compared over age and sex Over time
44 Data Recording
45 Data Reporting Sydpath data analysis for Common Reference Intervals Test Sodium Potassium Chloride Bicarbonate Creatinine F Creatinine M Harmonised Limits LRL URL Current Limits LRL URL Excluded data LexL UexL Age exclusions Age-L Age-H Sex exclusions Sex(M,F,B B B B B F M Harmonised Limits Midpoint Results Average Median %low 15% 1.5% 05% 0.5% 14% 1.4% 12% 1.2% 11% 1.1% 13% 1.3% %high 1.7% 2.6% 0.2% 1.5% 2.8% 6.4% 2.5th th n start date 2/5/12 2/5/12 2/5/12 2/5/12 2/5/12 2/5/12 end date 20/9/12 22/9/12 22/9/12 22/9/12 22/9/12 22/9/12
46 Data Reporting
47 Serum Bicarbonate
48 Interpretation - Bicarbonate
49 SydPath Evaluation
50 Serum Sodium
51 Serum Creatinine - Male 6.4%
52 Serum Calcium
53 Serum ALT - Male Median= 21 (mid=21) 12.8% Hitachi IFCC no P5P
54 SydPath Evaluation
55 CRI Validations Before agreeing to the CRI there should be multiple local validations. They will only be common RI if they are very widely used. Template available Produces common analysis and format Simple to use Available now (help provided)
56 What we can do Assess the AACB CRI (whatever method) Can I use them in my lab? Feedback on decision. If enough labs say yes they become CRI!
57 What else can we do Select traceable assays JCTLM-listed if possible Reference materials, methods, laboratories Select correct version of assay: GGT (IFCC not Szasz) LDH (P L v L P) AST (P5P or P5P) Albumin (BCG or BCP)
58 Non-Standardised Tests
59 Ferritin Abbott Group Tight Group Different from others Others not the same Endocrine Program Endo-of Cycle Report (2012) RCPA QAP data used with permission.
60 Ferritin Roche ECL Group Tight group Different from others Endocrine Program Endo of Cycle Report (2012) RCPA QAP data used with permission.
61 The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again.
62 Non-Standardised Tests CRI possible within manufacturer group (not across groups) Ideally standardise assays (global manufacturer issue) Next best would be Group CRI s such that different labs give same information Again co-operative work required
63 Bhattacharya Analysis A Data mining tool to assist with establishing or verifying reference intervals using local data.
64 Bhattacharya Bhattacharya, LG. Journal of the Biometric Society. 1967;23: Example data: Frequency Distribution of the forkal length of the Porgy caught by pair-trawl fishery in the East China Sea.
65 Porgy Forkal Length
66 Graphical Technique
67
68 Bhattacharaya Find a Gaussian Distribution in the midst of other data Outputs are midpoint and SD of Gaussian Distribution Needs The majority of results to be unaffected by disease Underlying Gaussian distribution ib ti Appropriate comparator (outpatients) Note: not affected by outliers
69 Bhattacharya Spreadsheet
70 Instructions Caveat: Spreadsheet put together with care. But no guarantees of being error free User assumes all responsibility. Always view data, check usual statistics and assess for reasonableness
71 Bhattacharya Spreadsheet Data Entry 3 columns Value, Sex, Age 65,000 (as is) More in Excel 2010
72 Bhattacharya Spreadsheet Analysis Inputs
73 Bhattacharya Spreadsheet Outputs (1) Basic Statistics
74 Bhatacharya Spreadsheet Bin Size (smaller more noise; larger fewer bins) - Operator dependent - Good data resistant to changes - More data is better - Aim for very straight line >3 data points
75 Error example Non-Gaussian Data Upward curve on Bhattacharya line
76 WARNING Log Transformation Can often make a good fit May be including disease state Log transform is only one of many transforms (eg Cox-Box) (has no effect on narrow distributions)
77 Assessment Criteria CLSI- number of outliers Data Mining Bias of midpoint (+/- 1/10th of RI) Number of outliers Raw data Data distribution Raw data Bhattacharya analysis Analytical Bias, QAP ALE (total error)
78 CRI The next Steps Adult Common RI Local validations Will I use them in my lab? Each lab (spreadsheet available) Agreement gee e t(some e/a all?) Together Implementation Each lab Other developments Bias 2 Study Gus Koerbin Liquid serum chemistry from RCPA QAP Method alignment (traceable assays) Next analytes?
79 Conclusions To provide the same information from different labs we need the same results and the same reference intervals (or RI that match the method) The current stated occurred through many decisions in many labs A change requires many more (good) decisions, based on good data Data mining (done well) provides a useful tool
The Whats and Hows of Reference Intervals. Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney
 The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
Quality Initiative In Pathology. Harmonisation of Laboratory Testing
 Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
Evidence Based Commutability: Bias 2 Study. Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA
 Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
Reference Intervals. Graham Jones / Gus Koerbin
 Reference Intervals Graham Jones / Gus Koerbin Adult CRI - Harmonisation Harmonisation 1 2012: (13 tests + 1 calculation) Harmonisation 2 2013: Confirm 2012 recommendations. Discussed: albumin, globulin,
Reference Intervals Graham Jones / Gus Koerbin Adult CRI - Harmonisation Harmonisation 1 2012: (13 tests + 1 calculation) Harmonisation 2 2013: Confirm 2012 recommendations. Discussed: albumin, globulin,
Setting of quality standards
 Setting of quality standards Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney AACB ASM Adelaide October 2014 Setting of Quality Standards - 2013 The 2013 QC workshop revealed
Setting of quality standards Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney AACB ASM Adelaide October 2014 Setting of Quality Standards - 2013 The 2013 QC workshop revealed
Impact of Proposed HRI s on Laboratory Report Flagging Rates
 Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Why Traceability Matters to Patients?
 Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
Harmonisation of Reference Ranges
 Harmonisation of Reference Ranges Ken Sikaris BSc(Hons), MBBS, FRCPA, FAACB, FFSc Vice President, AACB (Education) Chemical Pathologist, Melbourne Pathology Director of Clinical Support Systems, Sonic
Harmonisation of Reference Ranges Ken Sikaris BSc(Hons), MBBS, FRCPA, FAACB, FFSc Vice President, AACB (Education) Chemical Pathologist, Melbourne Pathology Director of Clinical Support Systems, Sonic
Assessment of latest LFT guidelines from American College of Gastroenterology. Gus Koerbin
 Assessment of latest LFT guidelines from American College of Gastroenterology Gus Koerbin Focus on ALT Review AACB current recommendations RI (examples) Bias Clinical Significance BMI AGC Guidelines Response
Assessment of latest LFT guidelines from American College of Gastroenterology Gus Koerbin Focus on ALT Review AACB current recommendations RI (examples) Bias Clinical Significance BMI AGC Guidelines Response
AUSTRALIAN PATHOLOGY UNITS AND TERMINOLOGY (APUTS) Harmonised Reference Intervals Chemical Pathology. (v1.1)
 AUSTRALIAN PATHOLOGY UNITS AND TERMINOLOGY (APUTS) Harmonised Reference Intervals Chemical Pathology (v1.1) ISBN: Pending 1 State Health Publication Number (SHPN): Pending Online copyright RCPA 2014 This
AUSTRALIAN PATHOLOGY UNITS AND TERMINOLOGY (APUTS) Harmonised Reference Intervals Chemical Pathology (v1.1) ISBN: Pending 1 State Health Publication Number (SHPN): Pending Online copyright RCPA 2014 This
Global Report #01 27/11/2013. Patient percentile monitoring compared to internal quality control (IQC) monitoring
 Patient Percentile Monitoring Global Report #01 CONTENT Project status Patient percentile monitoring compared to internal quality control (IQC) monitoring Comparison of outpatient/all patient monitoring
Patient Percentile Monitoring Global Report #01 CONTENT Project status Patient percentile monitoring compared to internal quality control (IQC) monitoring Comparison of outpatient/all patient monitoring
Breakout Session C: Harmonisation of the Alert Table.
 Breakout Session C: Harmonisation of the Alert Table. RCPA-AACB High Risk Results Working Party Andrew Georgiou Craig Campbell Grahame Caldwell Hans Schneider Penelope Coates Que Lam Rita Horvath Robert
Breakout Session C: Harmonisation of the Alert Table. RCPA-AACB High Risk Results Working Party Andrew Georgiou Craig Campbell Grahame Caldwell Hans Schneider Penelope Coates Que Lam Rita Horvath Robert
Coping with Analytical Interferences
 Coping with Analytical Interferences (Handling Icteric, Hemolytic and Lipaemic Samples) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements
Coping with Analytical Interferences (Handling Icteric, Hemolytic and Lipaemic Samples) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) 2009 GH2-B (fresh pooled samples) * = NGSP certified at the time of the survey
 College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK.
 Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK. Bill.Bartlett@nhs.net www.biologicalvariation.com Biological variation affects the clinical utility of reference
Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK. Bill.Bartlett@nhs.net www.biologicalvariation.com Biological variation affects the clinical utility of reference
General Chemistry Scheme Guide
 General Chemistry Scheme Guide Copyright WEQAS. All rights reserved. No part of this document may be reproduced or utilised in any form without permission from WEQAS Contents. Scheme details and repertoire.....
General Chemistry Scheme Guide Copyright WEQAS. All rights reserved. No part of this document may be reproduced or utilised in any form without permission from WEQAS Contents. Scheme details and repertoire.....
Narelle Hadlow, Peter Ward, Ken Sikaris
 Narelle Hadlow, Peter Ward, Ken Sikaris Analytes for consideration Vascular, renal and Na and water changes Consider physiology and review data Na, K, Cl, Urea, Cr, Osm Acid base status Consider physiology
Narelle Hadlow, Peter Ward, Ken Sikaris Analytes for consideration Vascular, renal and Na and water changes Consider physiology and review data Na, K, Cl, Urea, Cr, Osm Acid base status Consider physiology
Traceability in External Quality Assessment: How Weqas ensures traceability in EQA and stresses its importance to users. David Ducroq.
 Traceability in External Quality Assessment: How Weqas ensures traceability in EQA and stresses its importance to users David Ducroq Weqas Unit 6, Parc Tŷ Glas Llanishen Cardiff UK www.weqas.com Programme
Traceability in External Quality Assessment: How Weqas ensures traceability in EQA and stresses its importance to users David Ducroq Weqas Unit 6, Parc Tŷ Glas Llanishen Cardiff UK www.weqas.com Programme
External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA -
 External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA - Anja Kessler Bonn, Germany 1 RELA Surveys www.dgkl-rfb.de 2 RELA Annual Process Each participant receives two different
External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA - Anja Kessler Bonn, Germany 1 RELA Surveys www.dgkl-rfb.de 2 RELA Annual Process Each participant receives two different
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
Use of Target Values in EQA
 Use of Target Values in EQA Dr. Anja Kessler Bonn, Germany 1 External Quality Assessment Example: Progesterone 580 participants Measurement principles: Luminescence detection Radioactivity detection Fluorescence
Use of Target Values in EQA Dr. Anja Kessler Bonn, Germany 1 External Quality Assessment Example: Progesterone 580 participants Measurement principles: Luminescence detection Radioactivity detection Fluorescence
Serodos and Serodos plus
 Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
TDM A biochemists approach (Vancomycin)
 TDM A biochemists approach (Vancomycin) Graham Jones Chemical Pathologist SydPath, St Vincent s Hospital, Sydney ANZ TDM Workshop Sydney May 2015 Declaration I am a chemical Pathologist (not a clinical
TDM A biochemists approach (Vancomycin) Graham Jones Chemical Pathologist SydPath, St Vincent s Hospital, Sydney ANZ TDM Workshop Sydney May 2015 Declaration I am a chemical Pathologist (not a clinical
ROTUNDA HOSPITAL DEPARTMENT OF LABORATORY MEDICINE
 This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
Individual Lab Report Ci-Trol Nov,2016. Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC Your Lab
 Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Biochemistry Adult Reference Ranges
 Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes
 Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Harmonizing Reference Intervals (hris) ± Result Uncertainty (RU)
 Harmonizing Reference Intervals (hris) ± Result Uncertainty (RU) What do you think the main concerns will be for harmonized reference intervals in your lab? How can we do a better job of conveying what
Harmonizing Reference Intervals (hris) ± Result Uncertainty (RU) What do you think the main concerns will be for harmonized reference intervals in your lab? How can we do a better job of conveying what
What tests should be on the Alert List?
 What tests should be on the Alert List? Dr Que Lam On behalf RCPA-AACB High Risk Results Working Party: Alan McNeil, Grahame Caldwell, Craig Campbell, Penelope Coates, Robert Flatman, Andrew Georgiou,
What tests should be on the Alert List? Dr Que Lam On behalf RCPA-AACB High Risk Results Working Party: Alan McNeil, Grahame Caldwell, Craig Campbell, Penelope Coates, Robert Flatman, Andrew Georgiou,
State of the Art of HbA1c Measurement
 State of the Art of HbA1c Measurement XXI Congreso Latinoamericano de Patologia Clinica Y XLII Congreso Mexicano de Patologia Clinica, Cancun October 2012 Randie R. Little, Ph.D. NGSP Network Laboratory
State of the Art of HbA1c Measurement XXI Congreso Latinoamericano de Patologia Clinica Y XLII Congreso Mexicano de Patologia Clinica, Cancun October 2012 Randie R. Little, Ph.D. NGSP Network Laboratory
The Integrated Cardiovascular Clinical Network CHSA
 The Integrated Cardiovascular Clinical Network CHSA Roche cobas b 101 HbA1c and Lipids Evaluation July 2013 Introduction Cardiovascular disease (CVD) is the leading cause of mortality in Australia, accounting
The Integrated Cardiovascular Clinical Network CHSA Roche cobas b 101 HbA1c and Lipids Evaluation July 2013 Introduction Cardiovascular disease (CVD) is the leading cause of mortality in Australia, accounting
The analytical phase
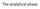 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
Method Comparison Report Semi-Annual 1/5/2018
 Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
Cortisol Assays. The Good, The Bad and The Indifferent. David Ducroq. Cardiff and Vale ulhb WEQAS. Unit 6, Parc Tŷ Glas. Llanishen.
 Cortisol Assays The Good, The Bad and The Indifferent David Ducroq Cardiff and Vale ulhb WEQAS Unit 6, Parc Tŷ Glas Llanishen Cardiff www.weqas.com Summary Brief overview of current methods and challenges
Cortisol Assays The Good, The Bad and The Indifferent David Ducroq Cardiff and Vale ulhb WEQAS Unit 6, Parc Tŷ Glas Llanishen Cardiff www.weqas.com Summary Brief overview of current methods and challenges
1. PROTOCOL. Comparison Study Summary. Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR July 30, 2014
 Comparison Study Summary Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR 97123 July 30, 2014 1. PROTOCOL This study was conducted on July 24 th, 2014 at Tuality Healthcare, Hillsboro, OR. The
Comparison Study Summary Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR 97123 July 30, 2014 1. PROTOCOL This study was conducted on July 24 th, 2014 at Tuality Healthcare, Hillsboro, OR. The
The Use of Tests in Clinical Biochemistry Interpreting Blood Results. Rowland Reece Principal Clinical Biochemist St. Vincent s University Hospital
 The Use of Tests in Clinical Biochemistry Interpreting Blood Results Rowland Reece Principal Clinical Biochemist St. Vincent s University Hospital Topic Headlines What is Clinical Biochemistry? How does
The Use of Tests in Clinical Biochemistry Interpreting Blood Results Rowland Reece Principal Clinical Biochemist St. Vincent s University Hospital Topic Headlines What is Clinical Biochemistry? How does
Authorised: JSWoodford, Lead of Speciality. Biochemistry Reference Intervals, October Page 1 of 5
 AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing. Vincent Delatour, PhD
 Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Body Fluids EQA. Rizzi de Leon. Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia
 Body Fluids EQA Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia 22-24 February 2019 What is a Body Fluid? https://en.wikipedia.org/wiki/ascites
Body Fluids EQA Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia 22-24 February 2019 What is a Body Fluid? https://en.wikipedia.org/wiki/ascites
Venous Blood Gas Reference Intervals
 Venous Blood Gas Reference Intervals The NSW Health Pathology approach Andrea Rita Horvath Department of Clinical Chemistry & Endocrinology NSW Health Pathology, Prince of Wales Hospital, Sydney andrea.horvath@health.nsw.gov.au
Venous Blood Gas Reference Intervals The NSW Health Pathology approach Andrea Rita Horvath Department of Clinical Chemistry & Endocrinology NSW Health Pathology, Prince of Wales Hospital, Sydney andrea.horvath@health.nsw.gov.au
Evaluation Report of the Pneumatic Tube Transport System (PEVCO) connecting Dialysis Hospital to. Mubarak Hospital. Dr.
 5 Evaluation Report of the Transport System (PEVCO) connecting Dialysis Hospital to Mubarak Hospital Dr. Anwar AlAnjeri Senior Registrar Clinical Biochemistry Laboratory Mubarak Hospital Introduction:
5 Evaluation Report of the Transport System (PEVCO) connecting Dialysis Hospital to Mubarak Hospital Dr. Anwar AlAnjeri Senior Registrar Clinical Biochemistry Laboratory Mubarak Hospital Introduction:
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube
 Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
Analytical performance specifications two years after Milan conference. Prof Mauro Panteghini CIRME Scientific Coordinator
 Analytical performance specifications two years after Milan conference Prof Mauro Panteghini CIRME Scientific Coordinator Definition Analytical performance specifications: Criteria that specify (in numerical
Analytical performance specifications two years after Milan conference Prof Mauro Panteghini CIRME Scientific Coordinator Definition Analytical performance specifications: Criteria that specify (in numerical
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions
 Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Patient: 55 y female (ambulatory)
 Disclosures Speaking Honoraria Radiometer (Canada) Nova Biomedical, Draeger Roche Diagnostics (Canada) Research Support (Reagents, Instrumentation, Travel) Nova Biomedical Abbott Laboratories (Canada)
Disclosures Speaking Honoraria Radiometer (Canada) Nova Biomedical, Draeger Roche Diagnostics (Canada) Research Support (Reagents, Instrumentation, Travel) Nova Biomedical Abbott Laboratories (Canada)
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Evaluation of new MiniCollect Z Serum (Separator) Tubes
 Evaluation of new MiniCollect Z Serum (Separator) Tubes Background: Greiner Bio-One has developed a newly designed MiniCollect tube offering an integrated collection scoop. The advantage of the new tube
Evaluation of new MiniCollect Z Serum (Separator) Tubes Background: Greiner Bio-One has developed a newly designed MiniCollect tube offering an integrated collection scoop. The advantage of the new tube
Dubrovnik 2014 Sverre Sandberg, Norwegian Quality Improvement of Primary Care Laboratories Noklus, Bergen, Norway
 POC testing instruments for diagnosing and monitoring diabetes in clinical settings Dubrovnik 2014 Sverre Sandberg, Norwegian Quality Improvement of Primary Care Laboratories Noklus, Bergen, Norway Does
POC testing instruments for diagnosing and monitoring diabetes in clinical settings Dubrovnik 2014 Sverre Sandberg, Norwegian Quality Improvement of Primary Care Laboratories Noklus, Bergen, Norway Does
CALIBRATION SERUM LEVEL 3 (CAL 3)
 CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 907UE SIZE: 20 x 5ml EXPIRY: 2019-07-28 GTIN: 05055273200966 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration
CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 907UE SIZE: 20 x 5ml EXPIRY: 2019-07-28 GTIN: 05055273200966 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration
CALIBRATION SERUM - LEVEL 2 (CAL 2)
 CAT. NO. CAL 2350 LOT NO. 1242UN SIZE: 20 x 5ml EXPIRY: 2020-02-28 GTIN: 05055273200959 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
CAT. NO. CAL 2350 LOT NO. 1242UN SIZE: 20 x 5ml EXPIRY: 2020-02-28 GTIN: 05055273200959 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
CALIBRATION SERUM LEVEL 3 (CAL 3)
 CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 709UE SIZE: 20 x 5ml EXP: 2016-12 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 709UE SIZE: 20 x 5ml EXP: 2016-12 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
HIV Viral Load Quality Assessment Program Summary for Panel HIVVL 2018Oct26
 1 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Viral Load Quality Assessment Program Summary
1 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Viral Load Quality Assessment Program Summary
What information on measurement uncertainty should be communicated to clinicians, and how? Mario Plebani
 What information on measurement uncertainty should be communicated to clinicians, and how? Mario Plebani OUTLINE OF TALK Uncertainty in medicine and shared decision making Measurement uncertainty in laboratory
What information on measurement uncertainty should be communicated to clinicians, and how? Mario Plebani OUTLINE OF TALK Uncertainty in medicine and shared decision making Measurement uncertainty in laboratory
Laboratory Medicine Standardization Activity in Japan
 JCTLM 15/11/2005 Sevres, Paris oratory Medicine Standardization Activity in Japan National Institute Metrology of Japan (NMIJ) Koichi Chiba Fujirebio Inc. Katsuhiko Yamamoto University of Tsukuba Katsuhiko
JCTLM 15/11/2005 Sevres, Paris oratory Medicine Standardization Activity in Japan National Institute Metrology of Japan (NMIJ) Koichi Chiba Fujirebio Inc. Katsuhiko Yamamoto University of Tsukuba Katsuhiko
1 PROTOCOL. Comparison Study Summary. Springs Memorial Hospital. Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC (803)
 Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Should HbA1C measured by POC instruments be used for diagnosis of diabetes? Sverre Sandberg, Norwegian Quality Improvement of Primary Care
 Should HbA1C measured by POC instruments be used for diagnosis of diabetes? Sverre Sandberg, Norwegian Quality Improvement of Primary Care Laboratories Noklus, Bergen, Norway NOKLUS a POC organisation
Should HbA1C measured by POC instruments be used for diagnosis of diabetes? Sverre Sandberg, Norwegian Quality Improvement of Primary Care Laboratories Noklus, Bergen, Norway NOKLUS a POC organisation
METHOD VALIDATION: WHY, HOW AND WHEN?
 METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
EQAS. Hemoglobin Program (BC80) Cycle 12: December 2014 December 2015 Sample No: 1 Sample Date: 17 Dec 14. Exceptions. Customer Information
 (BC80) : December 2014 December 20 Exceptions None at this time. Legend: No Warnings Missing Result Late Results < < > * Amended Result (per participant s request) News Customer Information Please refer
(BC80) : December 2014 December 20 Exceptions None at this time. Legend: No Warnings Missing Result Late Results < < > * Amended Result (per participant s request) News Customer Information Please refer
HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2)
 0843 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 899UN SIZE: 20 x 5ml / 5 x 5ml EXPIRY: 2018-02 INTENDED USE This product is intended for in vitro diagnostic
0843 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 899UN SIZE: 20 x 5ml / 5 x 5ml EXPIRY: 2018-02 INTENDED USE This product is intended for in vitro diagnostic
Questionnaire. Traceability in EQA. Traceability
 Questionnaire in EQA QUESTIONNAIRE ON TRACEABILITY QUESTIONNAIRE ON TRACEABILITY GENERAL INFORMATION Name EQA organisation Country Specify the total number of measurands in the schemes of your EQA organisation
Questionnaire in EQA QUESTIONNAIRE ON TRACEABILITY QUESTIONNAIRE ON TRACEABILITY GENERAL INFORMATION Name EQA organisation Country Specify the total number of measurands in the schemes of your EQA organisation
Understanding Quality Control for Infectious Disease Testing. Wayne Dimech ASLM Quality Control Workshop Abuja, Nigeria 9 th December 2018
 Understanding Quality Control for Infectious Disease Testing Wayne Dimech ASLM Quality Control Workshop Abuja, Nigeria 9 th December 2018 Disclosure Attendance at ASLM part-sponsored by CDC No personal
Understanding Quality Control for Infectious Disease Testing Wayne Dimech ASLM Quality Control Workshop Abuja, Nigeria 9 th December 2018 Disclosure Attendance at ASLM part-sponsored by CDC No personal
REFERENCE INTERVALS OF COMMON CLINICAL CHEMISTRY ANALYTES FOR ADULTS IN HONG KONG
 The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine CHEMISTRY ANALYTES FOR ADULTS IN HONG KONG YC Lo 1, David A. Armbruster 2 1 Pamela Youde Nethersole Eastern Hospital,
The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine CHEMISTRY ANALYTES FOR ADULTS IN HONG KONG YC Lo 1, David A. Armbruster 2 1 Pamela Youde Nethersole Eastern Hospital,
Total Cost of Ownership (TCO): An evidence-based approach to compare laboratory equipment
 Total Cost of Ownership (TCO): An evidence-based approach to compare laboratory equipment P.C.G. Gontard 1, L.I. Stankevich 1, B.G. Gorodetsky 1 SUMMARY Clinical laboratories across the globe operate in
Total Cost of Ownership (TCO): An evidence-based approach to compare laboratory equipment P.C.G. Gontard 1, L.I. Stankevich 1, B.G. Gorodetsky 1 SUMMARY Clinical laboratories across the globe operate in
1. Calibra - H - Store at 2-8 ºC.
 CALIBRA H Insert Ref.:80 Lot Expiration Calibrator Attention. It is suggested to verify carefully if the lot number printed in this insert corresponds to the lot on the bottle label. Intended use. is a
CALIBRA H Insert Ref.:80 Lot Expiration Calibrator Attention. It is suggested to verify carefully if the lot number printed in this insert corresponds to the lot on the bottle label. Intended use. is a
ENROLLMENT CONFIRMATION
 Step 1: Please review the Facility/Contact information. If any of the information is incorrect, please make the appropriate changes below: Facility/Contact Phone: (850)474-3660 Fax: (850)474-3659 6431
Step 1: Please review the Facility/Contact information. If any of the information is incorrect, please make the appropriate changes below: Facility/Contact Phone: (850)474-3660 Fax: (850)474-3659 6431
Paediatric reference intervals an update. Tina Yen Harmonisation Workshop Sydney 18 th May 2017
 Paediatric reference intervals an update Tina Yen Harmonisation Workshop Sydney 18 th May 2017 Meeting of the AACB Paediatric SIG Tuesday 16 th May 2017 Prof Frank Bowling (NSW/ TAS) Prof Rita Horvath
Paediatric reference intervals an update Tina Yen Harmonisation Workshop Sydney 18 th May 2017 Meeting of the AACB Paediatric SIG Tuesday 16 th May 2017 Prof Frank Bowling (NSW/ TAS) Prof Rita Horvath
Agenda. 15 Dec
 Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
cobas c 501 analyzer and cobas c 311 analyzer Within Run Imprecision Guidelines
 cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
Evidence Based HbA1c Accuracy. Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology
 Evidence Based HbA1c Accuracy Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology Fresh lycohaemoglobin This program is designed to assist laboratories to assess the accuracy of their hihba1c
Evidence Based HbA1c Accuracy Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology Fresh lycohaemoglobin This program is designed to assist laboratories to assess the accuracy of their hihba1c
Tables of Normal Values (As of February 2005)
 Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
SydPath Reference Intervals for Clinical Trials (Contract Pathology Unit) Unauthorised Copy
 HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
Guide to Fulfillment of Measurement Uncertainty
 DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
INTERNAL QUALITY CONTROL ( Q I C) QC)
 EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
Biochemistry Department Laboratory Handbook
 Biochemistry Department Laboratory Handbook Version : 3.3 Page 1 of 12 Table of contents Biochemistry Department... 1 Laboratory Handbook... 1 Introduction... 3 The Biochemistry Department... 3 High risk
Biochemistry Department Laboratory Handbook Version : 3.3 Page 1 of 12 Table of contents Biochemistry Department... 1 Laboratory Handbook... 1 Introduction... 3 The Biochemistry Department... 3 High risk
Can We Now Recommend a Common Calculation for Osmolar Gap?
 Can We Now Recommend a Common Calculation for Osmolar Gap? Oral Presentation AACB 4 th Harmonisation Workshop, Sydney, May 2015 Dr James Doery INTRODUCTION What is the osmolal gap? Why measure? Clinical
Can We Now Recommend a Common Calculation for Osmolar Gap? Oral Presentation AACB 4 th Harmonisation Workshop, Sydney, May 2015 Dr James Doery INTRODUCTION What is the osmolal gap? Why measure? Clinical
Correcting laboratory results for the effects of interferences: an approach incorporating uncertainty of measurement
 Original Article Annals of Clinical Biochemistry 2015, Vol. 52(2) 226 231! The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalspermissions.nav DOI: 10.1177/0004563214533516 acb.sagepub.com
Original Article Annals of Clinical Biochemistry 2015, Vol. 52(2) 226 231! The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalspermissions.nav DOI: 10.1177/0004563214533516 acb.sagepub.com
WSLH. Calibration Verification/ Linearity Products. roficiency. esting. Products provided in partnership with:
 WSLH PT roficiency esting Calibration Verification/ Linearity Products Products provided in partnership with: www.wslhpt.org 800-462-5261 PTService@slh.wisc.edu General Chemistry Ammonia/Ethanol - 5 x
WSLH PT roficiency esting Calibration Verification/ Linearity Products Products provided in partnership with: www.wslhpt.org 800-462-5261 PTService@slh.wisc.edu General Chemistry Ammonia/Ethanol - 5 x
New York State Soluble Tumor Markers Proficiency Test
 ADREW M. CUOMO Governor October 5, 2016 HOWARD A. ZUCKER, M.D., J.D. Commissioner SALLY DRESLI, M.S., R.. Executive Deputy Commissioner ew York State Soluble Tumor Markers Proficiency Test 9-2016 1 Dear
ADREW M. CUOMO Governor October 5, 2016 HOWARD A. ZUCKER, M.D., J.D. Commissioner SALLY DRESLI, M.S., R.. Executive Deputy Commissioner ew York State Soluble Tumor Markers Proficiency Test 9-2016 1 Dear
Excellence and confidence
 Thermo Scientific System Reagents Fully supported and guaranteed, CE marked system solutions Streamlined supply and support Maximum stability and reduced waste Standardised performance in External Quality
Thermo Scientific System Reagents Fully supported and guaranteed, CE marked system solutions Streamlined supply and support Maximum stability and reduced waste Standardised performance in External Quality
NQLM Nordic Committee for External Quality Assurance Programmes in Laboratory Medicine
 1(4) NQLM 2001-11-23 Nordic Committee for External Quality Assurance Programmes in Laboratory Medicine NORDIC INTERFERENCE STUDY, March 2001 Effects of bilirubin on some common serum analysis Enclosed
1(4) NQLM 2001-11-23 Nordic Committee for External Quality Assurance Programmes in Laboratory Medicine NORDIC INTERFERENCE STUDY, March 2001 Effects of bilirubin on some common serum analysis Enclosed
Controls & Calibrators Clinical Chemistry
 Controls & Calibrators Clinical Chemistry Clinical Chemistry Controls & Lipids Clinical Chemistry and lipid quality controls have been manufactured from true human serum to ensure they perform the same
Controls & Calibrators Clinical Chemistry Clinical Chemistry Controls & Lipids Clinical Chemistry and lipid quality controls have been manufactured from true human serum to ensure they perform the same
Seeing is not Believing (13-Nov-2004)
 In: 55th Annual Meeting of the American College of Veterinary Pathologists (ACVP) & 39th Annual Meeting of the American Society of Clinical Pathology (ASVCP), ACVP and ASVCP (Eds.) Publisher: American
In: 55th Annual Meeting of the American College of Veterinary Pathologists (ACVP) & 39th Annual Meeting of the American Society of Clinical Pathology (ASVCP), ACVP and ASVCP (Eds.) Publisher: American
TRACEABILITY and UNCERTAINTY
 ACP Acid phosphatase total 1-naphthyl phosphate NPP Acid phosphatase, non-prostatic 1-naphthyl phosphate (Inhib.:tartrate) ACP-P Acid phosphatase, prostatic 1-naphthyl phosphate (Inhib.:tartrate) ALB Albumin
ACP Acid phosphatase total 1-naphthyl phosphate NPP Acid phosphatase, non-prostatic 1-naphthyl phosphate (Inhib.:tartrate) ACP-P Acid phosphatase, prostatic 1-naphthyl phosphate (Inhib.:tartrate) ALB Albumin
AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST. RCPA-AACB Working Party for High Risk result Management
 AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST RCPA-AACB Working Party for High Risk result Management RCPA-AACB WORKING PARTY FOR HIGH RISKS RESULTS Craig Campbell Grahame Caldwell
AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST RCPA-AACB Working Party for High Risk result Management RCPA-AACB WORKING PARTY FOR HIGH RISKS RESULTS Craig Campbell Grahame Caldwell
Manufacturer Report for Siemens Unassayed Chemistry Lot Exp 30 Jun 2018
 Acetaminophen Enzymatic, colorimetric µg/ml.09 0..0.09 0..0 0. 0. 0. 0. 9.. 9.0 0.9.0..9.. Albumin Bromcresol Purple (BCP) g/dl.0 0.0..0 0.00.. 0.0.. 0.09..9 0.0..9 0.0..0 0.0..0 0.0. Alkaline Phosphatase
Acetaminophen Enzymatic, colorimetric µg/ml.09 0..0.09 0..0 0. 0. 0. 0. 9.. 9.0 0.9.0..9.. Albumin Bromcresol Purple (BCP) g/dl.0 0.0..0 0.00.. 0.0.. 0.09..9 0.0..9 0.0..0 0.0..0 0.0. Alkaline Phosphatase
HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2)
 0120 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 995UN SIZE: 20 x 5ml / 5 x 5ml EXP: 2018-12 INTENDED USE This product is intended for in vitro diagnostic use,
0120 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 995UN SIZE: 20 x 5ml / 5 x 5ml EXP: 2018-12 INTENDED USE This product is intended for in vitro diagnostic use,
HIV Viral Load Quality Assessment Program Summary for Panel HIVVL 2017Oct27
 1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
HUMAN ASSAYED MULTI-SERA - LEVEL 3 (HUM ASY CONTROL 3)
 0086 HUMAN ASSAYED MULTI-SERA - LEVEL 3 (HUM ASY CONTROL 3) CAT. NO. HE1532 / HS2611 LOT NO. 789UE SIZE: 20 x 5ml / 5 x 5ml EXP: 2019-05 INTENDED USE This product is intended for in vitro diagnostic use
0086 HUMAN ASSAYED MULTI-SERA - LEVEL 3 (HUM ASY CONTROL 3) CAT. NO. HE1532 / HS2611 LOT NO. 789UE SIZE: 20 x 5ml / 5 x 5ml EXP: 2019-05 INTENDED USE This product is intended for in vitro diagnostic use
GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS
 GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS Ferruccio Ceriotti Servizio di Medicina di Laboratorio, Ospedale San Raffaele, Milano F. Ceriotti, Milano, 27-11-2015 2 Preamble Most of the
GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS Ferruccio Ceriotti Servizio di Medicina di Laboratorio, Ospedale San Raffaele, Milano F. Ceriotti, Milano, 27-11-2015 2 Preamble Most of the
(a) y = 1.0x + 0.0; r = ; N = 60 (b) y = 1.0x + 0.0; r = ; N = Lot 1, Li-heparin whole blood, HbA1c (%)
 cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
It is a requirement of ISO that laboratories shall determine the uncertainty of measurement of results, where relevant and possible. (5.6.
 Guideline Subject: Uncertainty of Measurement Approval Date: November 2004, November 2009 Review Date: November 2013 Review By: PPAC Number: 2/2004 Introduction This Guideline has been developed to provide
Guideline Subject: Uncertainty of Measurement Approval Date: November 2004, November 2009 Review Date: November 2013 Review By: PPAC Number: 2/2004 Introduction This Guideline has been developed to provide
DIAZYME PRODUCT LIST LIST INNOVATIONS IN CLINICAL DIAGNOSTICS
 DIAZYME PRODUCT LIST LIST PRODUCT 2 13 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
DIAZYME PRODUCT LIST LIST PRODUCT 2 13 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
Define performance goals in standardization: Is fitness for medical purpose the key?
 Define performance goals in standardization: Is fitness for medical purpose the key? 7th CIRME International Scientific Meeting Metrological traceability and assay standardization Stresa, May 24th, 2013
Define performance goals in standardization: Is fitness for medical purpose the key? 7th CIRME International Scientific Meeting Metrological traceability and assay standardization Stresa, May 24th, 2013
TRENDS IN TIME. EXTERNAL EVALUATION PROGRAM FOR Immmunoassays - Chemistry
 Commissie voor Klinische Biologie Commission de Biologie Clinique EXTERNAL EVALUATION PROGRAM FOR Immmunoassays - Chemistry TRENDS IN TIME CM Van Campenhout N Hamers JC Libeer WIV/ISP/IPH Clinical Biology
Commissie voor Klinische Biologie Commission de Biologie Clinique EXTERNAL EVALUATION PROGRAM FOR Immmunoassays - Chemistry TRENDS IN TIME CM Van Campenhout N Hamers JC Libeer WIV/ISP/IPH Clinical Biology
1 PROTOCOL. Comparison Study Summary
 Comparison Study Summary OnSite Health 210 S. Michigan Street, Suite 110 South Bend, IN 46601 574-243-5108 ext. 203 July 27, 2015 1 PROTOCOL This internal evaluation was conducted on July 17 & 20, 2015
Comparison Study Summary OnSite Health 210 S. Michigan Street, Suite 110 South Bend, IN 46601 574-243-5108 ext. 203 July 27, 2015 1 PROTOCOL This internal evaluation was conducted on July 17 & 20, 2015
Part No. J04498 Cat No Performance Verifiers: Training Module
 Cat No. 680 0325 : Training Module Export authorized under general license GTDA (General Technical Data Available) IMPORTANT The information contained herein is based on the experience and knowledge relating
Cat No. 680 0325 : Training Module Export authorized under general license GTDA (General Technical Data Available) IMPORTANT The information contained herein is based on the experience and knowledge relating
Update: Body Fluid Testing. Fernando San Gil
 Update: Body Fluid Testing Fernando San Gil Background Notes - 1 Year Significant moments in IVD 2013 NATA and the TGA publish new validation and registration requirements for in-house IVDs and for in-house
Update: Body Fluid Testing Fernando San Gil Background Notes - 1 Year Significant moments in IVD 2013 NATA and the TGA publish new validation and registration requirements for in-house IVDs and for in-house
