Peristeen Transanal Irrigation System to Manage Bowel Dysfunction: A NICE Medical Technology Guidance
|
|
|
- Nigel Pitts
- 5 years ago
- Views:
Transcription
1 Applied Health Economics and Health Policy (2019) 17: REVIEW ARTICLE Peristeen Transanal Irrigation System to Manage Bowel Dysfunction: A NICE Medical Technology Guidance Megan Dale 1 Helen Morgan 2 Kimberly Carter 3 Judith White 1 Grace Carolan Rees 1 Published online: 14 November 2018 The Author(s) 2018 Abstract The Peristeen transanal irrigation system is intended to allow people with bowel dysfunction to flush out the lower part of the bowel as part of their bowel management strategy. Peristeen was the subject of an evaluation by the National Institute for Health and Care Excellence, through its Medical Technologies Evaluation Programme, for the management of bowel dysfunction. The company, Coloplast, submitted a case for adoption of the technology, claiming that the technology improves the severity of chronic constipation or faecal incontinence and improves quality of life for people with bowel dysfunction. Other claimed benefits included reduced frequency of UTIs, stoma surgery and hospitalisation rates, as well as reduced costs. The submission was critiqued by Cedar. The clinical evidence assessed included one randomised controlled trial, and 12 observational studies for adults and 11 studies for children. Although there are limitations in the evidence, the assessed studies show some improvement in outcomes for patients who choose to continue using Peristeen. The committee heard from patient experts that Peristeen had improved their lives and allowed them increased independence. The submitted economic evidence had numerous flaws, however following Cedar s changes to the model, and additional sensitivity analysis, the use of Peristeen was judged unlikely to be cost incurring compared with standard bowel care. The Peristeen transanal irrigation system received a positive recommendation in Medical Technologies Guidance 36. Key Points for Decision Makers * Megan Dale Megan.Dale@wales.nhs.uk Helen Morgan MorganHE1@cardiff.ac.uk Kimberly Carter Kimberley.carter@nice.org.uk Judith White Judith.White3@wales.nhs.uk Grace Carolan Rees Grace.Carolan Rees@wales.nhs.uk Peristeen is used by a wide range of people with bowel dysfunction from different causes. Not all patients find Peristeen acceptable or useful; however, for those who do, it can be a significant improvement in their quality of life and can promote dignity and independence. Peristeen can be difficult to use and people may require time, training and support to get comfortable with using it. Some people will choose to stop using Peristeen. There are considerable uncertainties in the economic evaluation; however, on average, Peristeen is unlikely to cost more than standard bowel care over a relevant time horizon Cedar, Cardiff and Vale University Health Board, Cardiff, UK Cedar, Cardiff University, Cardiff, UK National Institute for Health and Care Excellence, Manchester, UK Vol.:( )
2 26 M. Dale et al. 1 Introduction The National Institute for Health and Care Excellence (NICE) produces guidance on new or innovative medical devices or diagnostics Medical Technologies Guidance (MTG). The aim of the guidance is to support the adoption of clinically effective and cost-saving technologies in the UK National Health Service (NHS). The process for producing MTG has been previously described in detail [1]. This paper summarises Cedar s assessment report and how it was used to inform the NICE MTG on the Peristeen transanal irrigation system to manage bowel dysfunction (MTG36). Cedar is a NHS academic health care technology research collaboration based in Cardiff. This paper is part of a series that provides an insight into the development of NICE MTG [1]. 2 Background to the Condition and Technology Neurogenic bowel dysfunction can be caused by neurological conditions such as spinal cord injury, spina bifida, multiple sclerosis, Parkinson s disease and other conditions associated with impairment or loss of sphincter control and bowel mobility disorders. Bowel dysfunction can also have numerous other causes, such as injury to the rectum or bowel, or slow transit constipation. Symptoms include faecal incontinence and constipation, along with an increased rate of urinary tract infections (UTIs). Transanal irrigation can be used by people with bowel dysfunction to empty the rectum and some of the colon at a time and frequency that is suitable to them, and to avoid faecal incontinence and constipation. Peristeen is a transanal irrigation system consisting of a rectal catheter with an inflatable balloon, a manual control unit with a pump, leg straps, and a bag to hold water. The constant flow pump means that the system does not depend on gravity, and the bag does not need to be elevated. A new catheter is required for every use. The manufacturer recommends use every other day, although users will develop their own preference for how frequently to use the irrigation system. Peristeen received a CE mark in May 2003 as a class 1 medical device. A variety of systems, including Peristeen, are available that differ in design and use. These choices should be discussed by clinician and patient, and a number of systems may be tried before a preferred device for transanal irrigation is found for that particular patient. Currently, alternative treatment options include medication (oral drugs, suppositories and enemas), changes to diet, and physiotherapy. People with bowel dysfunction may also manage their symptoms using biofeedback, bowel washouts and manual removal of faeces. Some patients may need or may prefer surgery, i.e. a colostomy, ileostomy, sacral nerve stimulation or an antegrade continence enema procedure. The benefits claimed by the company to people with bowel dysfunction are that use of Peristeen improves symptoms/reduces the severity of chronic constipation; reduces the severity and frequency of faecal incontinence; improves quality of life for people with bowel dysfunction; and reduces the incidence of UTIs in people with neurogenic bowel dysfunction. The benefits claimed by the company to the healthcare system are that the use of Peristeen reduces the rate of stoma surgery in people with neurogenic bowel dysfunction; reduces the frequency, and therefore the treatment costs, of UTIs; reduces the cost of treating neurogenic bowel dysfunction in people who have experienced unsuccessful standard bowel care as first-line treatment; and reduces the rate of hospitalisation in people with neurogenic bowel dysfunction. 3 Decision Problem (Scope) In their evidence submission, the manufacturer must keep within the scope of the evaluation. The scope is defined by NICE in the form of a PICO table (population, intervention, comparator, outcomes, plus cost analysis and subgroups to be considered). 3.1 Population The population was defined as people with bowel dysfunction in any setting. Although the company claims were based on neurogenic bowel disorder, following consultation NICE broadened the scope to include all bowel dysfunction. Cedar identified that the company had inappropriately excluded most studies on children, and included such studies in their report. 3.2 Intervention The intervention was defined as the Peristeen transanal irrigation system. 3.3 Comparator The comparator was defined as conservative bowel management, which can include treatments such as diet and bowel habit advice, medication, disposable pads and anal plugs, muscle/bowel training, biofeedback and electrostimulation, and digital stimulation and manual evacuation. It was noted
3 Peristeen NICE MTG that the type of treatment is dependent on personal preference, ability and carer support. 3.4 Outcomes The following outcomes were included in the scope: severity and frequency of incontinence, severity of constipation, quality of life, length and frequency of irrigation, devicerelated adverse events, frequency of UTI, incidence of stoma surgery and hospitalisations, staff time (including primary care and community care visits), and individual length of use/user satisfaction. 4 Cedar s Review of Evidence The company provided an evidence submission to NICE that should be in line with the scope. This submission presented the available clinical and cost evidence, alongside a de novo cost model produced by the company. Cedar s assessment report aimed to provide the Medical Technologies Advisory Committee (MTAC) with a balanced, fair, and independent appraisal of the evidence surrounding the use of Peristeen for bowel dysfunction [2]. Broadly, this involved (1) identifying any additional information not included in the manufacturer s submission; (2) appraising the published clinical and cost evidence and, where necessary, presenting additional data; (3) critiquing the de novo cost model by checking inputs, assumptions, and structural integrity; (4) highlighting any key issues; and (5) presenting any new analyses carried out. 4.1 Review of Clinical Effectiveness Evidence The company submitted 10 studies consisting of 1 randomised controlled trial (RCT) of adults [3], 7 observational studies of adults [4 10], and 1 observational study of children [11], plus 1 prepublication study of adults. Cedar did not agree with the company s justification for excluding all but one paediatric study (because they did not use validated scoring systems) and included all studies with relevant outcomes. Cedar undertook a comprehensive search and selection process (Fig. 1) and included a further 4 observational studies of adults[12 15] and a further 10 observational studies of children [16 25]. The main outcomes in the included studies were patient-reported outcomes, which, due to the nature of the device, was appropriate. Included studies are listed in Table Adult Studies One reasonable-quality RCT that included 87 adults with spinal cord injuries and neurogenic bowel dysfunction was identified [3]. This unblinded study compared transanal irrigation using Peristeen (n = 42) with standard bowel care (n = 45) for 10 weeks and reported significant improvements in validated patient-reported outcomes for bowel function. Twelve patients in the Peristeen arm and two standard care patients withdrew from the study. There was a significant improvement in a series of patientreported outcome measures (PROMs) Cleveland Clinic Constipation Scoring System (CCCS), St Mark s Fecal Incontinence Grading System (FIGS), and Neurogenic Bowel Dysfunction Score (NBDS) for the Peristeen group compared with standard care (Table 2). In the observational studies of adults, which reported comparative data (before and after), there was an improvement in patient-reported outcomes for bowel function when using Peristeen. Most of these studies used appropriate standardised PROMs. Outcome measures such as incidence of UTIs and faecal incontinence also improved when using Peristeen, while outcomes related to general quality-of-life measures were less widely reported and changes were either not significant or were significantly improved in only some domains. There were considerable numbers of people who stopped using Peristeen, particularly in the first few months Paediatric Studies The paediatric studies were non-comparative, observational, case series. Six of these observational studies were prospective and five were retrospective in design. The studies were of variable quality and used less-consistent outcome measures. General findings indicated the use of Peristeen resulted in improvements in bowel management, however the evidence is weaker than for adults, partly due to the difficulty in obtaining valid PROM data for children. For both adults and children, there were variable numbers of people who stopped using Peristeen, particularly in the first few months. This was frequently because they disliked using the device or found it painful or ineffective Safety Outcomes Bowel perforation is a rare but potentially serious adverse event that can occur with Peristeen use. A global audit [26] based on voluntary reporting to the company found 49 incidents of bowel perforation, 35 of which were certainly caused by Peristeen. Less-serious adverse events include symptoms such as abdominal pain or nausea, which may lead individual patients affected to seek an alternative management strategy. 27
4 28 M. Dale et al. Records iden fied through database searching duplicates removed (n =227) Addi onal records iden fied through other sources (n =60) plus 62 MAUDE reports; 1 MHRA report; 5 clinical trials First screen: tle/abstract (n = 287) +68 (63 safety reports and 5 clinical trials) Records excluded (n = 247) + 3 clinical trials Full-text ar cles excluded (n = 14) Second screen: assessed for eligibility at full-text (n = 40) Publica ons included in clinical evalua on (n = 24 full text) Publica ons included in economic evalua on (n = 2) Included: safety reports (n=63) & ongoing studies (n=2) Fig. 1 Cedar s study selection process. MHRA Medicines and Healthcare products Regulatory Agency, MAUDE manufacturer and user facility device experience 4.2 Review of Economic Evidence The company identified two published economic studies [27, 28]. The Christensen et al. [27]. model is from a societal perspective, however it did not match the scope and was therefore excluded by both Cedar and the company. The work of Emmanuel et al. [28]. was the basis for the de novo cost model which is required from the company as part of the evidence submission, and is critiqued in the following sections. The company did not update the costs in the submitted model, but did comment on the impact the updated costs would have Model Structure The submitted model was based on the work of Emmanuel et al. [28], a Markov model with a 6-month cycle, comparing the use of the Peristeen transanal irrigation system with standard bowel care for a 30-year-old subject with spinal cord injury. The model time horizon was 37 years, with a discount rate of 3.5% and an NHS and social services perspective. The available states in the model are management using Peristeen, standard bowel care, surgery, or stoma. Stoma is an end state in this model and patients cannot move from this, as shown in Fig. 2. Key assumptions in the model are as follows. All patients enter the model at age 30 and survive for 37 years. There is no death state in the model. The probability of ceasing to use Peristeen is assumed to be constant. Adverse events are included as hospital admissions, and are assumed to be split equally between gastrointestinal infections, pressure ulcers, falls or trauma, abdominal pain and UTI. Bowel perforation is not explicitly included. The model is stated as being for a patient with spinal cord injuries, and patients are assumed to be homogeneous.
5 Peristeen NICE MTG 29 Table 1 Studies included by both the company and Cedar References Country Study type Population n Company Cedar Adults Chan et al. [12] UK OBS Mixed 91 Christensen et al. [4] Europe including UK OBS a SCI 62 Christensen et al. [3] Europe, including the UK RCT SCI 87 Del Popolo et al. [5] Italy OBS SCI 36 Pre-publication study provided as academic in confidence Hamonet-Torny et al. [6] France OBS NBD 16 Kim et al. [13] South Korea OBS SCI 52 Loftus et al. [7] Ireland OBS NBD 11 Nafees et al. [14] UK DC Mixed 129 Passananti et al. [8] UK OBS MS 49 Preziosi et al. [9] UK OBS MS 30 Rosen et al. [10] Austria OBS ARS 14 Switzerland Whitehouse et al. [15] UK OBS FBD 113 Children Alenezi et al. [16] Saudi Arabia OBS NBD 18 Ausili et al. [17] Italy OBS SB 60 Choi et al. [32] South Korea OBS SB 44 Corbett et al. [18] UK OBS Mixed 24 Kelly et al. [19] USA OBS NBD 24 King et al. [20] Australia OBS SB 20 Koppen et al. [21] Netherlands OBS IC 67 Lopez Pereira et al. [22] Spain OBS SB 40 Marzheuser et al. [23] Germany OBS ARM 40 Midrio et al. [11] Italy OBS Mixed 83 Nasher et al. [24] UK OBS IC 13 Pacilli et al. [25] UK OBS Mixed 23 a RCT and OBS paper by Christensen et al. used the same patients ARM Anorectal malformation, FBD Functional bowel disorder, MS multiple sclerosis, SB spina bifida, SCI spinal cord injury, NBD neurogenic bowel dysfunction, IC idiopathic constipation, ARS anterior resection syndrome, RCT randomised controlled trial, OBS observational, single-arm study, DC discrete choice experiment, indicates included, indicates explicitly excluded Transition probabilities and variables are constant over time for all patients. Many variables are likely to change with age for all patients, and will also change over time for patients with progressive diseases such as multiple sclerosis. Transition probabilities for patients who start using Peristeen and then revert to standard bowel care are stated in the submission as being the same as probabilities in the standard bowel care arm Data Sources for Outcomes and Resources The model uses audit data for 227 patients from three UK hospitals as the main source of clinical and resource data. Data for standard bowel care are taken from the baseline questionnaires for patients, except for the number of patients progressing to stoma, which is taken from the retrospective data of 157 patients prior to the introduction of Peristeen. Limited information on the audit is published [8, 28], but an extract of some information was shared with Cedar by the company. The audit appears to have an appropriate NHS setting, with suitable patient pathways, and an appropriate, if heterogeneous, population. However, Cedar were unable to reconcile differences in the data from the available sources, and had insufficient information available to critique the audit data used in the model. The statistical techniques used for calculating transition probabilities from patients enrolled at different time points, with different lengths of follow-up, were not stated Data Sources for Adverse Events Adverse events are modelled as UTIs that respond to treatment, and events requiring hospitalisation. The
6 30 M. Dale et al. Table 2 Summary of RCTs Included studies Design and intervention(s) Participants and setting Outcomes Results Withdrawals Cedar comments Christensen et al. [3] RCT comparing transanal irrigation using Peristeen with SBC for 10 weeks Company-funded 87 randomised (42 Peristeen vs. 45 SBC) 62 men, 25 women, average age 49.1 years 73 completed 5 European spinal cord injury centres (Sweden, Italy, Germany, UK, Denmark) All patients 18 years or older, at least 3 months after spinal cord injury Primary outcomes CCCS and FIGS Secondary outcomes NBDS, modified ASCRS, numeric box score on bowel function, influence on daily activities and general satisfaction Outcomes collected at weeks 0 and 10, plus weekly telephone interview CCCS, FIGS and NBDS were significantly improved for Peristeen vs. SBC Subgroup analysis found no significant difference for patients who could walk, but significant improvement for those who used wheelchairs or were confined to bed ASCRS scores were significantly improved for Peristeen vs. SBC in domains of coping/ behaviour, but no significant difference was noted for the lifestyle and depression/selfperception domains The numeric box scores were significantly improved for bowel function, general satisfaction and improvement in quality of life, but not for influence on daily activities 14 w/d (12 Peristeen, 2 SBC) 73 completed, 5 lost to follow-up Blinding was not possible A large number of patients stopped using Peristeen before the end of the study, and were subsequently included in ITT analysis using baseline data in place of missing data Baseline imbalance between groups for those using a wheelchair or confined to bed Subgroup analysis not stated as planned Study supported by the company ASCRS American Society of Colon and Rectal Surgeons, CCCS Cleveland Clinic Constipation Score, FIGS St Mark s Faecal Incontinence Grading Score, NBDS Neurogenic Bowel Dysfunction Score, SBC standard bowel care, RCTs randomised controlled trials, ITT intention to treat, w/d withdrawals
7 Peristeen NICE MTG 31 Fig. 2 Markov model structure Peristeen Standard bowel care Standard bowel care Surgery Surgery Stoma Stoma hospitalisations are split equally between abdominal pain, gastrointestinal infection, pressure ulcers, falls and trauma, and UTIs. The assumption of an equal split is not justified and is likely to overestimate treatment for pressure ulcers, which can be very costly Errors Cedar identified several errors in the model, the most important of which were all related to people in the Peristeen arm who returned to using standard care. The main errors for these people returning to standard care were: the cost of a health care professional s time was not included; the cost of standard bowel care-related adverse events was not included; there was a lower use of consumables assumed, compared with people in the model who had never used Peristeen; a reduced probability of moving to surgery or stoma for people in the model receiving standard bowel care who had previously received Peristeen. Cedar considered all these points to be modelling errors as they did not reflect the intended model function as described in the company submission. No evidence was submitted to suggest that people who used Peristeen and then changed back to standard care would have any different resource requirement or outcomes compared with those who had never used Peristeen. Once these errors were corrected, the cost saving due to the use of Peristeen was reduced from 21,768 to Changes Made by Cedar The change made by Cedar that had the largest impact was to change the cost of a pressure ulcer from 24,214 (Touche Ross [29], inflated) to 15,134 (Dealy [30], inflated). In addition, Cedar changed the cost of a UTI from per episode to (Berningham [31], inflated) in line with the costs listed in the original study referenced. In order to reflect the high number of people ceasing to use Peristeen at an early stage, Cedar introduced a variable transition probability. Due to the limited data available, this was set as three different fixed values to empirically recreate the reported dropout rate. Background mortality was also added due to the long time horizon for this model. The combined impact of these changes further reduced the cost saving to 3176, as shown in Table One Way Sensitivity Analysis Cedar added frequency of use to the one-way sensitivity analysis and carried out additional sensitivity analysis. The model is very sensitive to the frequency of use and also sensitive to pressure ulcer treatment and faecal incontinence, and there is uncertainty about each of these variables. There is limited clinical evidence around the frequency of use and for an improvement in faecal incontinence. Furthermore, there is no direct clinical evidence
8 32 M. Dale et al. Table 3 Impact on the incremental cost of additional work by Cedar Model version Incremental cost (over 37 years) Base-case submitted by the company 21,768 Changes 1 8: correction of errors 7829 Patients in the Peristeen arm returning to SBC have full costs, including appropriate medication, HCP time and adverse events Carer time in both arms is corrected Transition probability for all patients receiving SBC is standardised Changes 9 10: Cedar-suggested refinements Reduced number of Peristeen users in the first year, using variable transition probability 10. Background mortality added 11. Pressure ulcer cost changed to 15, UTI cost changed to Final Cedar base-case with all corrections and refinements 3175 SBC standard bowel care, HCP healthcare professional, UTI urinary tract infection linking the use of Peristeen to a reduction in pressure ulcers Key Drivers A key driver in the model is the frequency of use, due to the need for a new catheter at each use. The average frequency is likely to be correctly modelled every other day, however the actual use for each patient may vary from daily to weekly depending on their preferences and circumstances. The cost saving identified in Cedar s corrected model in the Peristeen arm is largely due to reduced time for health care professional visits and carer time; reduced incidence of faecal incontinence (requiring fewer incontinence pads); and reduced incidence of UTIs and fewer hospitalisations. 5 National NICE Guidance 5.1 Preliminary Guidance The evidence submitted by the company and Cedar s report were presented to the MTAC, who produced the following draft recommendations: The case for adopting Peristeen for managing neurogenic bowel dysfunction in adults is supported by the evidence. Peristeen can reduce the severity of constipation and incontinence, improve bowel-related quality of life, and promote dignity and independence. Peristeen may not be suitable for all people with neurogenic bowel dysfunction and can be difficult to use. It may take several weeks before a person is comfortable using it themselves, and some people may choose to stop using it. Peristeen is therefore most effective when it is offered with specialist training for users, carers and NHS staff, along with structured patient support. Cost modelling for Peristeen is associated with significant uncertainties, but it is likely that, overall, Peristeen provides additional clinical benefits without costing more than standard bowel care. 5.2 Consultation Response During the consultation period, NICE received 43 consultation comments from 18 consultees (9 NHS professionals, 1 private sector healthcare professional, 1 professional society, 1 patient group and 6 manufacturers). The comments related to Peristeen use in children, functional bowel disorders, user experience, other transanal irrigation devices, frequency of use, cost and adverse events. New information was submitted from centres using Peristeen in children and in people of all ages with functional bowel disease. This information was reviewed by Cedar and presented to the committee. Because of this, the committee broadened the recommendations to include people with bowel dysfunction of any age. 6 Key Challenges and Learning Points The Peristeen transanal irrigation system can be used successfully by a wide range of people whose bowel dysfunction may have different causes and symptoms. This leads to a very broad scope, and the studies that inform the clinical evidence have diverse populations and outcomes. This breadth and diversity mean that combining the clinical evidence presents particular challenges. In addition, the outcomes
9 Peristeen NICE MTG most important to the people who use Peristeen may not be the outcomes that are most easily captured and reported in clinical studies. The economic evaluation aspect presented significant challenges due to the lack of transparency in the data used for clinical and resource inputs, and the significant structural errors contained in the model. The focus of the sensitivity analysis on costs rather than resource use, and the omission of key factors, such as frequency of use, was potentially misleading. 7 Conclusions The Peristeen transanal irrigation system received a positive recommendation from NICE and should be considered as an option for people requiring additional treatment strategies to manage bowel dysfunction. Published evidence showed that the Peristeen transanal irrigation system improved patient-reported outcomes in those patients who continued to use it. The evidence is more robust for the adult population, partially due to the availability of appropriate validated outcome measures. Consultation responses reported the successful use of Peristeen in children in the UK. The submitted economic evidence was based on audit data that were not available for scrutiny, and the model contained several errors. Despite the uncertainty that remains in the model, it is likely that use of the Peristeen transanal irrigation system will not cost any more than standard care. Acknowledgements Shaun Harris (Swansea Centre for Health Economics, Swansea University) provided advice on the health economic analysis; Joelle Williams and Robert Palmer (Cedar, Cardiff and Vale University Health Board) assisted in inputs and checking for economic modelling; and Andrew Cleves and Ruth Poole (Cedar, Cardiff and Vale University Health Board) assisted in the preparation of the assessment report. The authors thank Miss Karen Nugent (Consultant, Pelvic Floor and Colorectal Surgery, University Hospital Southampton), Professor Anton Emmanuel (University College London), Mr. Oliver Jones (Consultant, Colorectal Surgery, Oxford University Hospital) and Dr Ian Beales (Consultant, Gastroenterology, Norfolk and Norwich University Hospital) for providing expert clinical advice. Author Contributions MD, JW, HM, KC and GCR contributed to the preparation of this article. GCR reviewed the full assessment report, as well as this article, and acts as a guarantor for the overall content. Compliance with Ethical Standards Cedar were funded for their work by the NICE Medical Technologies Evaluation Programme. MD, JW and GCR are NHS employees, and the NHS has a financial interest in the guidance on which this project is based. HM is a Cardiff University employee and has no conflicts of interest to declare. KC is a NICE employee and had no role in the production of the assessment report, but contributed to the preparation of this article. This summary of the Medical Technology Guidance was produced following publication of the final guidance report. The article has not been externally peer reviewed by Applied Health Economics and Health Policy. Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. References 1. Campbell B, Campbell M. NICE medical technologies guidance: a novel and rigorous methodology to address a new health technology assessment challenge. Appl Health Econ Health Policy. 2012;10(5): Dale M, Ray A, Morgan H, Poole R, Carolan-Rees G. External assessment centre report: peristeen anal irrigation system to manage bowel dysfunction. NICE commissioned report https :// nce/gid-mt511 /docum ents/asses sment -repor t-2. Accessed 8 Oct Christensen P, Bazzocchi G, Coggrave M, Abel R, Hultling C, Krogh K, et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology. 2006;131(3): Christensen P, Bazzocchi G, Coggrave M, et al. Outcome of transanal irrigation for bowel dysfunction in patients with spinal cord injury. J Spinal Cord Med. 2008;31(5): Del Popolo G, Mosiello G, Pilati C, Lamartina M, Battaglino F, Buffa P, et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study. Spinal Cord. 2008;46(7): Hamonet-Torny J, Bordes J, Daviet JC, Dalmay F, Joslin F, Salle JY. Long-term transanal irrigation s continuation at home. Preliminary study. Ann Phys Rehabil Med. 2013;56(2): Loftus C, Wallace E, McCaughey M, Smith E. Transanal irrigation in the management of neurogenic bowel dysfunction. Ir Med J. 2012;105(7): Passananti V, Wilton A, Preziosi G, Storrie JB, Emmanuel A. Long-term efficacy and safety of transanal irrigation in multiple sclerosis. Neurogastroenterol Motil. 2016;28(9): Preziosi G, Gosling J, Raeburn A, et al. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis Colon Rectum. 2012;55(10): Rosen H, Robert-Yap J, Tentschert G, Lechner M, Roche B. Transanal irrigation improves quality of life in patients with low anterior resection syndrome. Colorectal Dis. 2011;13(10):e Midrio P, Mosiello G, Ausili E, Gamba P, Marte A, Lombardi L, et al. Peristeen transanal irrigation in paediatric patients with anorectal malformations and spinal cord lesions: a multicentre Italian study. Colorectal Dis. 2016;18(1): Chan DS, Saklani A, Shah PR, Lewis M, Haray PN. Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders. Colorectal Dis. 2012;14(6): Kim HR, Lee BS, Lee JE, Shin HI. Application of transanal irrigation for patients with spinal cord injury in South Korea: a 6-month follow-up study. Spinal Cord. 2013;51(5): Nafees B, Lloyd AJ, Ballinger RS, Emmanuel A. Managing neurogenic bowel dysfunction: what do patients prefer? A discrete choice experiment of patient preferences for transanal irrigation and standard bowel management. Patient Pref Adherence. 2016;10:
10 34 M. Dale et al. 15. Whitehouse PA, McWilliams D, Katt C, et al. Peristeen rectal irrigation for functional bowel disorders: which patients benefit? Gastrointest Nurs. 2010;8(2): Alenezi H, Alhazmi H, Trbay M, et al. Peristeen anal irrigation as a substitute for the MACE procedure in children who are in need of reconstructive bladder surgery. J Can Urol Assoc. 2013;8(1 2):E Ausili E, Focarelli B, Tabacco F, et al. Transanal irrigation in myelomeningocele children: an alternative, safe and valid approach for neurogenic constipation. Spinal Cord. 2010;48(7): Corbett P, Denny A, Dick K, et al. Peristeen integrated transanal irrigation system successfully treats faecal incontinence in children. J Pediatr Urol. 2014;10(2): Kelly M, Dorgalli C, McLorie G, Khoury A. Prospective evaluation of Peristeen transanal irrigation system with the validated neurogenic bowel dysfunction score sheet in the pediatric population. Neurourol Urodyn. 2017;36(3): King SK, Stathopoulos L, Pinnuck L, Wells J, Hutson J, Heloury Y. Retrograde continence enema in children with spina bifida: not as effective as first thought. J Paediatr Child Health. 2017;53(4): Koppen IJN, Kuizenga-Wessel S, Voogt HW, et al. transanal irrigation in the treatment of children with intractable functional constipation. J Pediatr Gastroenterol Nutr. 2017;64(2): Lopez Pereira P, Salvador OP, Arcas JA, et al. Transanal irrigation for the treatment of neuropathic bowel dysfunction. J Pediatr Urol. 2010;6(2): Marzheuser S, Karsten K, Rothe K. Improvements in incontinence with self-management in patients with anorectal malformations. Eur J Pediatr Surg. 2016;26(2): Nasher O, Hill R, Peeraully R, et al. Peristeen transanal irrigation system for paediatric faecal incontinence: a single centre experience. Int J Pediatr. 2014;2014: Pacilli M, Pallot D, Andrews A, et al. Use of Peristeen transanal colonic irrigation for bowel management in children: a singlecenter experience. J Pediatr Surg. 2014;49(2): Christensen P, Andreasen J, Ehlers L. Global audit on bowel perforations related to transanal irrigation. Techn Coloproctol. 2016;20(2): Christensen P, Andreasen J, Ehlers L. Cost-effectiveness of transanal irrigation versus conservative bowel management for spinal cord injury patients. Spinal Cord. 2009;47(2): Emmanuel A, Kumar G, Christensen P, et al. Long-term costeffectiveness of transanal irrigation in patients with neurogenic bowel dysfunction. PLoS One. 2016;11(8):e Touche Ross and Co. The cost of pressure sores. London: Touche Ross and Co.; Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care. 2012;21(6): Bermingham SL, Hodgkinson S, Wright S, Hayter E, Spinks J, Pellowe C. Intermittent self catheterisation with hydrophilic, gel reservoir, and non-coated catheters: a systematic review and cost effectiveness analysis. BMJ. 2013;346:e Choi EK, Han SW, Shin SH, Ji Y, Chon J, Im Y. Long-term outcome of transanal irrigation for children with spina bifida. Spinal Cord. 2015;53:
External Assessment Centre report
 External Assessment Centre report Title: Peristeen anal irrigation system to manage bowel dysfunction Produced by: Authors: Cedar Megan Dale (Cedar, Cardiff & Vale University Health Board) Grace Carolan-Rees
External Assessment Centre report Title: Peristeen anal irrigation system to manage bowel dysfunction Produced by: Authors: Cedar Megan Dale (Cedar, Cardiff & Vale University Health Board) Grace Carolan-Rees
Transanal irrigation systems for neurogenic bowel dysfunction, chronic constipation, and chronic faecal incontinence
 Transanal irrigation systems for neurogenic bowel dysfunction, chronic constipation, and chronic faecal incontinence Lead author: Hayley Johnson Regional Drug & Therapeutics Centre (Newcastle) March 2016
Transanal irrigation systems for neurogenic bowel dysfunction, chronic constipation, and chronic faecal incontinence Lead author: Hayley Johnson Regional Drug & Therapeutics Centre (Newcastle) March 2016
Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings
 Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings Tiffany Gordon, MSN, RN, CRRN, CPN David Vandersteen, MD John Belew RN, PhD Gillette
Efficacy of Peristeen transanal irrigation system for neurogenic bowel in the pediatric population: Preliminary findings Tiffany Gordon, MSN, RN, CRRN, CPN David Vandersteen, MD John Belew RN, PhD Gillette
Transanal irrigation for the management of neurogenic bowel dysfunction: Evidence summary
 Transanal irrigation for the management of neurogenic bowel dysfunction: Evidence summary Transanal irrigation for the management of neurogenic bowel dysfunction: evidence summary A randomized, controlled
Transanal irrigation for the management of neurogenic bowel dysfunction: Evidence summary Transanal irrigation for the management of neurogenic bowel dysfunction: evidence summary A randomized, controlled
Transanal irrigation for the management of neurogenic bowel dysfunction: evidence summary
 Transanal irrigation for the management of neurogenic bowel dysfunction: evidence summary A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured
Transanal irrigation for the management of neurogenic bowel dysfunction: evidence summary A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida
 Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Bowel Dysfunction in Neurological Disease Best Practice in an Evolving Disorder
 Bowel Dysfunction in Neurological Disease Best Practice in an Evolving Disorder Anton Emmanuel October 2016 National Hospital for Neurology & Neurosurgery Regulation of colonic function Brain gut axis
Bowel Dysfunction in Neurological Disease Best Practice in an Evolving Disorder Anton Emmanuel October 2016 National Hospital for Neurology & Neurosurgery Regulation of colonic function Brain gut axis
Rectal irrigation (DROP-List)
 B171 February 2017 2.0 Community Interest Company Rectal irrigation (DROP-List) This is one of a number of bulletins providing further information on medicines and medical devices contained in the PrescQIPP
B171 February 2017 2.0 Community Interest Company Rectal irrigation (DROP-List) This is one of a number of bulletins providing further information on medicines and medical devices contained in the PrescQIPP
Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders
 Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
When the bowel works, life works
 When the bowel works, life works Feeling better by taking control It can be difficult to establish a regular bowel routine for people living with chronic constipation, incontinence or time-consuming bowel
When the bowel works, life works Feeling better by taking control It can be difficult to establish a regular bowel routine for people living with chronic constipation, incontinence or time-consuming bowel
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen
 Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
1. What evidence exists that prevention of constipation in the first year of life improves outcome of bowel management in later childhood?
 BOWEL FUNCTION AND CARE Overall Outcomes Primary Outcomes o Maintenance of social continence as appropriate for age level Secondary Outcomes o Maximization of independence with managing bowel program o
BOWEL FUNCTION AND CARE Overall Outcomes Primary Outcomes o Maintenance of social continence as appropriate for age level Secondary Outcomes o Maximization of independence with managing bowel program o
Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study
 (), & International Society All rights reserved -/ $. www.nature.com/sc ORIGINAL ARTICLE Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study G Del Popolo,
(), & International Society All rights reserved -/ $. www.nature.com/sc ORIGINAL ARTICLE Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study G Del Popolo,
Management of Neurogenic Bowel Dysfunction. Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders
 Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Transanal irrigation. Toolbox for neurogenic bowel management. Information for patients Spinal Injuries
 Transanal irrigation Toolbox for neurogenic bowel management Information for patients Spinal Injuries Neurogenic bowel dysfunction A number of people with a spinal injury experience faecal incontinence
Transanal irrigation Toolbox for neurogenic bowel management Information for patients Spinal Injuries Neurogenic bowel dysfunction A number of people with a spinal injury experience faecal incontinence
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Bowel Function and Care. Pat Beierwaltes, Chair Paige Church Lusine Ambartsumyan Sharon Braille Julie Dicker Tiffany Gordon Sue Liebold
 Bowel Function and Care Pat Beierwaltes, Chair Paige Church Lusine Ambartsumyan Sharon Braille Julie Dicker Tiffany Gordon Sue Liebold Outcomes Primary Outcomes Maintenance of social continence as appropriate
Bowel Function and Care Pat Beierwaltes, Chair Paige Church Lusine Ambartsumyan Sharon Braille Julie Dicker Tiffany Gordon Sue Liebold Outcomes Primary Outcomes Maintenance of social continence as appropriate
A70.4 Insertion of neurostimulator electrodes into peripheral nerve Z12.2 Posterior tibial nerve R15.X Faecal incontinence
 The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
Transanal colonic irrigation has recently become an
 ORIGINAL CONTRIBUTION Long-Term Outcome and Safety of Transanal Irrigation for Constipation and Fecal Incontinence Peter Christensen, Ph.D. 1,2 & Klaus Krogh, D.M.Sci. 2 & Steen Buntzen, D.M.Sci. 1 Fariborz
ORIGINAL CONTRIBUTION Long-Term Outcome and Safety of Transanal Irrigation for Constipation and Fecal Incontinence Peter Christensen, Ph.D. 1,2 & Klaus Krogh, D.M.Sci. 2 & Steen Buntzen, D.M.Sci. 1 Fariborz
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE The management of faecal incontinence in adults 1.1 Short title Faecal incontinence 2 Background (a) (b) (c) The National Institute
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE The management of faecal incontinence in adults 1.1 Short title Faecal incontinence 2 Background (a) (b) (c) The National Institute
Latest developments in transanal irrigation therapy CHRONIC CONSTIPATION
 CONTINENCE Latest developments in transanal irrigation therapy Sharon Holroyd Transanal irrigation has been acknowledged as a minimally invasive in patients with neurogenic bowel disorders. The severity
CONTINENCE Latest developments in transanal irrigation therapy Sharon Holroyd Transanal irrigation has been acknowledged as a minimally invasive in patients with neurogenic bowel disorders. The severity
Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211
 Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
National Institute for Health and Clinical Excellence. Health Technology Appraisal. Prucalopride for the treatment of chronic constipation in women
 Health Technology Appraisal Summary form Prucalopride for the treatment of chronic constipation in women Comment 1: the draft remit Appropriateness Movetis Movetis entirely welcomes the opportunity to
Health Technology Appraisal Summary form Prucalopride for the treatment of chronic constipation in women Comment 1: the draft remit Appropriateness Movetis Movetis entirely welcomes the opportunity to
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
SACRAL NERVE STIMULATION FOR EXPERIENCE IN CHILDREN
 SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
Guidelines for the Manual Evacuation of Faeces
 Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
HELPING YOU HELP OTHERS RECTAL IRRIGATION WITH AQUAFLUSH CONE BASED RECTAL IRRIGATION SYSTEMS FOR HEALTHCARE PROFESSIONALS
 HELPING YOU HELP OTHERS RECTAL IRRIGATION WITH AQUAFLUSH CONE BASED RECTAL IRRIGATION SYSTEMS FOR HEALTHCARE PROFESSIONALS Effective bowel emptying is the norm for most people. However, for your patients,
HELPING YOU HELP OTHERS RECTAL IRRIGATION WITH AQUAFLUSH CONE BASED RECTAL IRRIGATION SYSTEMS FOR HEALTHCARE PROFESSIONALS Effective bowel emptying is the norm for most people. However, for your patients,
Incontinence in neurological disease
 nice bulletin Incontinence in neurological disease NICE provided the content for this booklet which is independent of any company or product advertised NICE Bulletin - Incontinence in neurological disease.indd
nice bulletin Incontinence in neurological disease NICE provided the content for this booklet which is independent of any company or product advertised NICE Bulletin - Incontinence in neurological disease.indd
encathopedia Volume 8 NEUROGENIC BOWEL DYSFUNCTION AND TAI
 encathopedia Volume 8 NEUROGENIC BOWEL DYSFUNCTION AND TAI Neurogenic Bowel Dysfunction Neurogenic Bowel Dysfunction (NBD) is when the bowel does not work properly due to loss of normal sensory or motor
encathopedia Volume 8 NEUROGENIC BOWEL DYSFUNCTION AND TAI Neurogenic Bowel Dysfunction Neurogenic Bowel Dysfunction (NBD) is when the bowel does not work properly due to loss of normal sensory or motor
Bowel Function and Care
 Bowel Function and Care Workgroup Members: Patricia Beierwaltes, DNP, CPNP (Chair); Lusine Ambartsumyan, MD; Sharon Baillie, RN, CNC, MN; Paige Church, MD; Julie Dicker, RN; Tiffany Gordon, MSN, RN, CPN;
Bowel Function and Care Workgroup Members: Patricia Beierwaltes, DNP, CPNP (Chair); Lusine Ambartsumyan, MD; Sharon Baillie, RN, CNC, MN; Paige Church, MD; Julie Dicker, RN; Tiffany Gordon, MSN, RN, CPN;
Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39
 ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Premeeting briefing Prucalopride for the treatment of chronic constipation in women
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Premeeting briefing Prucalopride for the treatment of chronic constipation in women This briefing presents the key issues arising from the manufacturer
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Premeeting briefing Prucalopride for the treatment of chronic constipation in women This briefing presents the key issues arising from the manufacturer
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical Technologies Advisory Committee (MTAC) 71 st MTAC Meeting Friday 21 st July 2017 Park Suite, 4th floor, Mercure Manchester Piccadilly Hotel, Portland
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical Technologies Advisory Committee (MTAC) 71 st MTAC Meeting Friday 21 st July 2017 Park Suite, 4th floor, Mercure Manchester Piccadilly Hotel, Portland
Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41
 Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
In keeping with the Scottish Diabetes Group criteria, use should be restricted to those who:
 Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Technology appraisal guidance Published: 26 September 2012 nice.org.uk/guidance/ta264
 Alteplase for treating acute ischaemic stroke Technology appraisal guidance Published: 26 September 2012 nice.org.uk/guidance/ta264 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Alteplase for treating acute ischaemic stroke Technology appraisal guidance Published: 26 September 2012 nice.org.uk/guidance/ta264 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: management of idiopathic constipation in children in primary and secondary care 1.1 Short title Constipation
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: management of idiopathic constipation in children in primary and secondary care 1.1 Short title Constipation
Medical technologies guidance Published: 1 February 2018 nice.org.uk/guidance/mtg35
 Memokath-051 stent for ureteric obstruction Medical technologies guidance Published: 1 February 2018 nice.org.uk/guidance/mtg35 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Memokath-051 stent for ureteric obstruction Medical technologies guidance Published: 1 February 2018 nice.org.uk/guidance/mtg35 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tertiary, regional and local pelvic floor service providers: the future. model? Andrew Williams
 Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Stapled transanal rectal resection for obstructed defaecation syndrome
 Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471
 Eluxadoline for treating irritable bowel syndrome with diarrhoea Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471 NICE 2017. All rights reserved. Subject to Notice of
Eluxadoline for treating irritable bowel syndrome with diarrhoea Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta471 NICE 2017. All rights reserved. Subject to Notice of
Childhood constipation, a real problem..? Marc Benninga, Emma Children s Hospital, AMC, Amsterdam, the Netherlands
 Childhood constipation, a real problem..? Marc Benninga, Emma Children s Hospital, AMC, Amsterdam, the Netherlands Constipation 0-10% >10-20% >20-30% >30-40% Mugie SM, et al. Best Pract & Res Clin Gastroenterol
Childhood constipation, a real problem..? Marc Benninga, Emma Children s Hospital, AMC, Amsterdam, the Netherlands Constipation 0-10% >10-20% >20-30% >30-40% Mugie SM, et al. Best Pract & Res Clin Gastroenterol
Patient Information Leaflet
 Patient Information Leaflet Background Faecal incontinence is a common health problem, affecting over 1% of community-dwelling adults (1;2). Chronic constipation may affect 3-5% of the population depending
Patient Information Leaflet Background Faecal incontinence is a common health problem, affecting over 1% of community-dwelling adults (1;2). Chronic constipation may affect 3-5% of the population depending
Integrated Continence Service Policy. January SafeCare Council January Carol Giffin, Continence Advisor
 Policy No: OP51 Version: 1.0 Name of Policy: Integrated Continence Service Policy Effective From: January 2008 Approved by: SafeCare Council January 2008 Next Review Date: January 2010 Reviewed by: Carol
Policy No: OP51 Version: 1.0 Name of Policy: Integrated Continence Service Policy Effective From: January 2008 Approved by: SafeCare Council January 2008 Next Review Date: January 2010 Reviewed by: Carol
Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442
 Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta318
 Lubiprostone for treating chronic idiopathic constipation Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta318 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Lubiprostone for treating chronic idiopathic constipation Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta318 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Continence Promotion in Children with Additional Needs
 Continence Promotion in Children with Additional Needs Understanding bladder and bowel comorbidities the importance of assessment: Information for professionals Children and young people with physical
Continence Promotion in Children with Additional Needs Understanding bladder and bowel comorbidities the importance of assessment: Information for professionals Children and young people with physical
CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE
 CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE The minimum standards required to initiate specialised conservative treatment
CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE The minimum standards required to initiate specialised conservative treatment
Fecal Incontinence. What is fecal incontinence?
 Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
PROCEDURE FOR THE USE OF PERISTEEN ANAL IRRIGATION SYSTEM FOR ADULTS (FOR THE PURPOSE OF RECTAL IRRIGATION)
 PROCEDURE FOR THE USE OF PERISTEEN ANAL IRRIGATION SYSTEM FOR ADULTS (FOR THE PURPOSE OF RECTAL IRRIGATION) Issue History Issue Version Purpose of Issue/Description of Change Planned Review Date One To
PROCEDURE FOR THE USE OF PERISTEEN ANAL IRRIGATION SYSTEM FOR ADULTS (FOR THE PURPOSE OF RECTAL IRRIGATION) Issue History Issue Version Purpose of Issue/Description of Change Planned Review Date One To
Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493
 Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
Incidence of Colorectal Cancers- Australia. Anterior Resection 5/23/2018. What spurs us to investigate?
 Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Module 5 Management Of Urinary Incontinence
 Management Of Urinary Incontinence V3: Last Reviewed September 2017 Learning Outcomes Outline conservative management options Discover the options available to manage the different types of incontinence
Management Of Urinary Incontinence V3: Last Reviewed September 2017 Learning Outcomes Outline conservative management options Discover the options available to manage the different types of incontinence
Faecal incontinence. Faecal incontinence: the management of faecal incontinence in adults
 Issue date: June 2007 Faecal incontinence Faecal incontinence: the management of faecal incontinence in adults NICE clinical guideline 49 Developed by the National Collaborating Centre for Acute Care NICE
Issue date: June 2007 Faecal incontinence Faecal incontinence: the management of faecal incontinence in adults NICE clinical guideline 49 Developed by the National Collaborating Centre for Acute Care NICE
Transanal irrigation for bowel dysfunction in chronic stage of spinal cord-injured patients
 9 Japanese Journal of Comprehensive Rehabilitation Science (2019) Original Article Transanal irrigation for bowel dysfunction in chronic stage of spinal cord-injured patients Megumi Ozeki, MD, DMSc, 1
9 Japanese Journal of Comprehensive Rehabilitation Science (2019) Original Article Transanal irrigation for bowel dysfunction in chronic stage of spinal cord-injured patients Megumi Ozeki, MD, DMSc, 1
Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245
 Apixaban for the prevention ention of venous thromboembolism after total hip or knee replacement in adults Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245 NICE 2018.
Apixaban for the prevention ention of venous thromboembolism after total hip or knee replacement in adults Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245 NICE 2018.
Care Trigger Continence Management Publication code: HCR
 Care Trigger Continence Management Publication code: HCR-0312-045 Care Trigger Check list of Evidence National Care Does the care service have policies in place for: Promotion of continence that separates
Care Trigger Continence Management Publication code: HCR-0312-045 Care Trigger Check list of Evidence National Care Does the care service have policies in place for: Promotion of continence that separates
Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459
 Collagenase clostridium histolyticum for treating Dupuytren's contracture Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459 NICE 2017. All rights reserved. Subject to Notice
Collagenase clostridium histolyticum for treating Dupuytren's contracture Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459 NICE 2017. All rights reserved. Subject to Notice
Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325
 Nalmefene for reducing alcohol consumption in people with alcohol dependence Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325 NICE 2018. All rights reserved. Subject
Nalmefene for reducing alcohol consumption in people with alcohol dependence Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325 NICE 2018. All rights reserved. Subject
Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta472
 Obinutuzumab with bendamustine for treating follicular lymphoma refractory to rituximab Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta472 NICE 2018. All rights reserved.
Obinutuzumab with bendamustine for treating follicular lymphoma refractory to rituximab Technology appraisal guidance Published: 30 August 2017 nice.org.uk/guidance/ta472 NICE 2018. All rights reserved.
Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340
 Ustekinumab for treating active psoriatic arthritis Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ustekinumab for treating active psoriatic arthritis Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Biofeedback Therapy A nurse led management service for functional bowel disorders
 Biofeedback Therapy A nurse led management service for functional bowel disorders Brigitte Collins Lead Nurse BSc, MSc GI Nursing, Dip/Hypnotherapy St Marks Hospital Is biofeedback necessary? Conservative
Biofeedback Therapy A nurse led management service for functional bowel disorders Brigitte Collins Lead Nurse BSc, MSc GI Nursing, Dip/Hypnotherapy St Marks Hospital Is biofeedback necessary? Conservative
Developing spinal cord compression care guidelines at WPH
 Developing spinal cord compression care guidelines at WPH Spinal cord compression team: Sue Banks, Jean Buchanan, Bernie Foran, Suzanne Hodson, Jane Mason, Rebecca Mills, Jan Siddall, Rebecca Walsh, Clare
Developing spinal cord compression care guidelines at WPH Spinal cord compression team: Sue Banks, Jean Buchanan, Bernie Foran, Suzanne Hodson, Jane Mason, Rebecca Mills, Jan Siddall, Rebecca Walsh, Clare
GI Physiology - Investigating and treating patients with pelvic floor dysfunction. Lynne Smith Department of GI Physiology NGH Sheffield
 GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence
 Research for Patient Benefit Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence Thin NN 1, Taylor SJC 2, Bremner SA 2, Hounsome N 2, Alam A
Research for Patient Benefit Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence Thin NN 1, Taylor SJC 2, Bremner SA 2, Hounsome N 2, Alam A
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: the diagnosis and management of idiopathic childhood constipation in primary and secondary care 1.1 Short title
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Constipation: the diagnosis and management of idiopathic childhood constipation in primary and secondary care 1.1 Short title
Alcohol interventions in secondary and further education
 National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
Chronic Care. Coloplast Capital Market Day Jan Rolin Frederiksen, President Coloplast US. Coloplast Capital Market Day 2009
 Coloplast Capital Market Day 2009 Jan Rolin Frederiksen, President Coloplast US Page 1 Agenda Product Portfolio Ostomy Care Continence Care Wound and Skin Care Page 2 Portfolio covers four product-business
Coloplast Capital Market Day 2009 Jan Rolin Frederiksen, President Coloplast US Page 1 Agenda Product Portfolio Ostomy Care Continence Care Wound and Skin Care Page 2 Portfolio covers four product-business
Scottish Health Technologies Group
 Scottish Health Technologies Group Advice Statement Portable bladder ultrasound scanners 8 March 2011 Advice Statement 002/11 Question: Can the use of a portable 3D ultrasound bladder scanner to measure
Scottish Health Technologies Group Advice Statement Portable bladder ultrasound scanners 8 March 2011 Advice Statement 002/11 Question: Can the use of a portable 3D ultrasound bladder scanner to measure
Global Continence Care Market with Focus on Intermittent Catheter Market ( ) December 2016
 Global Continence Care Market with Focus on Intermittent Catheter Market (2016-2020) December 2016 Global Continence Care Market Report Scope of the Report The report titled Global Continence Care Market
Global Continence Care Market with Focus on Intermittent Catheter Market (2016-2020) December 2016 Global Continence Care Market Report Scope of the Report The report titled Global Continence Care Market
PREPARING FOR ANORECTOAL MANOMETRY. ManoScan Anorectal Manometry System
 PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
Measuring outcomes to improve the management of continence care. Dr Adrienne Rivlin KPMG Global Strategy Group
 Measuring outcomes to improve the management of continence care Dr Adrienne Rivlin KPMG Global Strategy Group Background of the study Many treatment options, few find a cure High quality daily continence
Measuring outcomes to improve the management of continence care Dr Adrienne Rivlin KPMG Global Strategy Group Background of the study Many treatment options, few find a cure High quality daily continence
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transabdominal artificial bowel sphincter implantation for faecal incontinence
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transabdominal artificial bowel sphincter implantation for faecal incontinence
Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416
 Osimertinib for treating locally advanced or metastatic EGFR T790M mutation- positive non-small-cell lung cancer Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416 NICE
Osimertinib for treating locally advanced or metastatic EGFR T790M mutation- positive non-small-cell lung cancer Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416 NICE
GUIDELINES FOR THE MANAGEMENT OF URINARY INCONTINENCE IN THE PALLIATIVE CARE SETTING
 GUIDELINES FOR THE MANAGEMENT OF URINARY INCONTINENCE IN THE PALLIATIVE CARE SETTING 43.1 GENERAL PRINCIPLES Urinary continence can be defined as the ability to store urine in the bladder and to excrete
GUIDELINES FOR THE MANAGEMENT OF URINARY INCONTINENCE IN THE PALLIATIVE CARE SETTING 43.1 GENERAL PRINCIPLES Urinary continence can be defined as the ability to store urine in the bladder and to excrete
Technology appraisal guidance Published: 14 December 2011 nice.org.uk/guidance/ta239
 Fulvestrant for the treatment of locally advanced or metastatic breast cancer Technology appraisal guidance Published: 14 December 2011 nice.org.uk/guidance/ta239 NICE 2018. All rights reserved. Subject
Fulvestrant for the treatment of locally advanced or metastatic breast cancer Technology appraisal guidance Published: 14 December 2011 nice.org.uk/guidance/ta239 NICE 2018. All rights reserved. Subject
Bowel Function After Spinal Cord Injury
 Bowel Function After Spinal Cord Injury A resource for individuals with SCI and their supporters This presentation is based on SCI Model Systems research and was developed with support from the National
Bowel Function After Spinal Cord Injury A resource for individuals with SCI and their supporters This presentation is based on SCI Model Systems research and was developed with support from the National
Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180)
 Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
A joint venture between the Department of Uro-Neurology, Queen Square, University College London and the Neuro-Urology Promotion Committee of the ICS
 Neurogenic bladder 2-day course A joint venture between the Uro-Neurology,, University College London and the Neuro-Urology Promotion Committee of the ICS November 27 and 28, 2014 Venue: Clinical Neuroscience
Neurogenic bladder 2-day course A joint venture between the Uro-Neurology,, University College London and the Neuro-Urology Promotion Committee of the ICS November 27 and 28, 2014 Venue: Clinical Neuroscience
Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546
 Padeliporfin for untreated localised prostate cancer Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546 NICE 2019. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Padeliporfin for untreated localised prostate cancer Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546 NICE 2019. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345
 Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Faecal Incontinence Information Leaflet THE DIGESTIVE SYSTEM
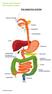 THE DIGESTIVE SYSTEM This factsheet is about faecal incontinence Faecal (or anal) incontinence is the loss of stool, liquid or gas from the bowel at an undesirable time. Males and females of any age may
THE DIGESTIVE SYSTEM This factsheet is about faecal incontinence Faecal (or anal) incontinence is the loss of stool, liquid or gas from the bowel at an undesirable time. Males and females of any age may
Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566
 Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Access to clinical trial information and the stockpiling of Tamiflu. Department of Health
 MEMORANDUM FOR THE COMMITTEE OF PUBLIC ACCOUNTS HC 125 SESSION 2013-14 21 MAY 2013 Department of Health Access to clinical trial information and the stockpiling of Tamiflu 4 Summary Access to clinical
MEMORANDUM FOR THE COMMITTEE OF PUBLIC ACCOUNTS HC 125 SESSION 2013-14 21 MAY 2013 Department of Health Access to clinical trial information and the stockpiling of Tamiflu 4 Summary Access to clinical
Faecal incontinence. The management of faecal incontinence in adults. Issued: June NICE clinical guideline 49. guidance.nice.org.
 Faecal incontinence The management of faecal incontinence in adults Issued: June 2007 NICE clinical guideline 49 guidance.nice.org.uk/cg49 NICE has accredited the process used by the Centre for Clinical
Faecal incontinence The management of faecal incontinence in adults Issued: June 2007 NICE clinical guideline 49 guidance.nice.org.uk/cg49 NICE has accredited the process used by the Centre for Clinical
Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547
 Tofacitinib for moderately to severelyerely active ulcerative colitis Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547 NICE 2019. All rights reserved. Subject to Notice
Tofacitinib for moderately to severelyerely active ulcerative colitis Technology appraisal guidance Published: 28 November 2018 nice.org.uk/guidance/ta547 NICE 2019. All rights reserved. Subject to Notice
Botulinum toxin type A for the prevention of headaches in adults with chronic migraine
 Botulinum toxin type A for the prevention of headaches in adults with chronic migraine Issued: June 2012 guidance.nice.org.uk/ta260 NICE has accredited the process used by the Centre for Health Technology
Botulinum toxin type A for the prevention of headaches in adults with chronic migraine Issued: June 2012 guidance.nice.org.uk/ta260 NICE has accredited the process used by the Centre for Health Technology
Duc M. Vo, MD, FACS Northwest Surgical Specialists
 Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
15. Prevention of UTI and lifestyle modifications
 15. Prevention of UTI and lifestyle modifications Key questions: Does improving poor voiding habits help prevent UTI recurrence? Does improving constipation help prevent UTI recurrence? Does increasing
15. Prevention of UTI and lifestyle modifications Key questions: Does improving poor voiding habits help prevent UTI recurrence? Does improving constipation help prevent UTI recurrence? Does increasing
OHTAC Recommendation
 OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
Addendum to clinical guideline 131, Colorectal cancer
 : National Institute for Health Care Excellence Final Addendum to clinical guideline 131, Colorectal cancer Clinical guideline addendum 131.1 Methods, evidence recommendations December 2014 Final version
: National Institute for Health Care Excellence Final Addendum to clinical guideline 131, Colorectal cancer Clinical guideline addendum 131.1 Methods, evidence recommendations December 2014 Final version
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Clinical and economic consequences of volume- or time-dependent intermittent catheterization in patients with spinal cord lesions and neuropathic bladder Polliack T, Bluvshtein V, Philo O, Ronen J, Gelernter
Clinical and economic consequences of volume- or time-dependent intermittent catheterization in patients with spinal cord lesions and neuropathic bladder Polliack T, Bluvshtein V, Philo O, Ronen J, Gelernter
Motility Disorders. Pelvic Floor. Colorectal Center for Functional Bowel Disorders (N = 701) January 2010 November 2011
 Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Policy #: 049 Latest Review Date: April 2009 Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
 Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
