ReShape Integrated Dual Balloon System Procedure Manual
|
|
|
- Margaret Mitchell
- 5 years ago
- Views:
Transcription
1 ReShape Integrated Dual Balloon System Procedure Manual 1
2 TABLE OF CONTENTS Introduction... 3 Product Description... 3 Overview... 3 Component description... 4 Intragastric Balloon... 4 Placement Catheter... 4 Removal Catheter... 5 Procedure Supplies... 6 Balloon Placement Procedure... 6 Balloon Removal Procedure... 6 ReShape Dual Balloon Placement Procedure... 7 Step 1. Device preparation... 7 Step 2. Patient sedation... 8 Step 3. Diagnostic endoscopy and guidewire positioning... 8 Step 4. ReShape Dual Balloon insertion... 9 Step 5. Balloon inflation... 9 Step 6. Disengaging the Balloon from the catheter ReShape Dual Balloon Removal Step 1. Procedure preparation Step 2. Patient sedation Step 3. Balloon drainage Step 4. Capture and removal of the balloon References
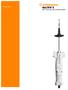
3 Introduction This procedure manual is designed to give the reader essential background information on the ReShape Integrated Dual Balloon System. The information in this manual provides a technical description of the devices and also serves as a guide on preparation, placement and removal procedures. Note: this manual does not replace the instructions for use (IFU), and as such should be used in combination with the IFUs supplied with the products. Product Description Overview The ReShape Integrated Dual Balloon is a temporary implant designed to facilitate weight loss by occupying space in the stomach and producing a sensation of satiety. The device is constructed from medical grade silicone rubber. The balloon is introduced orally via a placement catheter under endoscopic guidance and direct visualization. The balloon is designed to be placed in the stomach during an outpatient procedure using conscious sedation (MAC). It remains in the stomach for up to six months and is then removed. Fig.1 - ReShape Integrated Dual Balloon 3
4 Component description The ReShape Integrated Dual Balloon System contains (a) Intragastric Balloon / Placement Catheter Assembly, and the following accessories: (b) two Prefilled Valve Sealant Syringes, (c) Guidewire and (d) Patient ID Card. The ReShape Integrated Dual Balloon System is not made with natural rubber latex. (a) Intragastric Balloon (a) Placement Catheter (b) Prefilled Valve Sealant Syringes (c) Guidewire 4
5 (d) Patient ID Card The ReShape Removal Catheter is packaged separately 5
6 Procedure Supplies Balloon Placement Procedure Equipment/Supplies Single-use items Quantity per procedure Brand Part Number ReShape Dual Balloon 1 ReShape Medical RSM110 Guidewire 0.035in (0.89mm) diameter / 260cm length ReShape Infiltration Pump single-spike tubing Methylene blue (injectable, concentration 10mg/mL) 500cc flexible saline bags (0.9% NaCl solution) Prefilled Valve Sealant syringes (USP grade) Reusable items 1 ReShape Medical (AdvancedCath) 2 ReShape Medical ITS-10-RS Included with balloon 4 cc Clinic supply Clinic supply 2 Clinic supply Clinic supply 2 ReShape Medical Included with balloon Sterile 5cc or 6mL syringe & needle 1 Clinic supply Clinic supply Sterile graduated containers (size = min. 100 cc) Sterile adult endoscopy bite block opening size: 20mm(H) x 27mm(W) Balloon Removal Procedure Single-use items 2 Clinic supply Clinic supply 1 Olympus MAJ-168 ReShape Infiltration Pump 1 ReShape Medical KIP-II-RS Diagnostic gastroscope single-channel, 2.8mm channel mm insertion tube 1400mm total length Equipment/Supplies 1 Olympus GIF-Q160 GIF-Q180 Quantity per procedure Brand Part Number ReShape Removal Catheter 1 ReShape Medical RSM210 Large hexagonal endoscopic snare Minimum opening size: 20mm Sterile adult endoscopy bite block opening size: 20mm(H) x 27mm(W) Suction pump tubing Standard ¼ inch diameter Reusable items Suction pump (Removal Catheter independent from endoscope suction) Rat tooth graspers (wide jaw) Working Length: 165mm Opening Width: 19.5mm Minimum Channel Size: 2.8mm 1 1 Cook Medical Olympus ASH-1-S MAJ Clinic Supply Clinic supply 2 Clinic Supply Clinic Supply 1 Olympus FG-49L-1 Laryngoscope and Magill forceps 1 Clinic supply Clinic supply Diagnostic gastroscope single-channel, 2.8mm channel mm insertion tube 1400mm total length 1 Olympus GIF-Q160 GIF-Q180 6
7 ReShape Dual Balloon Placement Procedure While the patient is under conscious sedation (MAC), an endoscope is inserted through the mouth into the stomach and a guidewire is placed. The un-inflated balloons are advanced over the guidewire and placed in the stomach. Each balloon is inflated independently with a salinemethylene blue solution using an automated pump. The device is then released and remains in the stomach for up to six months. The balloons do not change the stomach s anatomy; they simply occupy space to reduce the capacity for food. In the event of a complication requiring the removal of an inflated ReShape Dual Balloon, a ReShape Removal Catheter should be available at each placement procedure. Recommended patient diet instructions to minimize the amount of food and liquid in the stomach during the procedure: - 48 hours prior to procedure: Soft foods only, no meat in any form hours prior to procedure: Clear liquids only hours prior to procedure: NPO - No food or liquids by mouth. Step 1. Device preparation Materials: - ReShape Integrated Dual Balloon - ReShape Guidewire - Lubricant - Adult-sized endoscopic bite block - ReShape Infiltration Pump and ReShape Pump Tubing - Two (2) prefilled valve sealant syringes - Two (2) 500cc saline bags - 4cc of methylene blue (10mg/mL concentration) - Syringe and needle to transfer the methylene blue into the saline bags 1. The balloon is supplied preloaded on the placement catheter. Remove the catheter from the package and inspect for any visible damage. Do not use device if damage is noted. 2. Prepare two 500cc bags of saline solution. a. Inject and mix 2cc of methylene blue (10mg/mL) into each 500cc bag of saline b. Attach the infiltration pump tubing to the saline bag c. Determine the final inflation volume for each balloon. Hold the saline bag upside down and use the pump to remove any air and excess fluid from the 500cc saline bag in order to prevent over/under-inflation of the ReShape Dual Balloon. For example, if the intended balloon size is 450cc, remove all air and 50cc of solution from the saline bag such that only 450cc of fluid remains. Repeat this step for the saline bag intended for the second balloon. i. When emptying the saline bags into the graduated cylinder, place the pump speed between 4 7 to avoid splashing Note: Maintain cleanliness of the saline and tubing. Do not allow the pump tubing to contact the floor. Do not handle the tubing with dirty gloves. 7
8 3. Refer to the ReShape Infiltration Pump Instructions for Use for setup and operating information for the ReShape Pump and its accessories. a. Set the infiltration pump flow on the back of the pump to Momentary Flow b. Increase the infiltration speed to 10 for filling the balloons c. Alternate manual inflation method: The manual inflation method requires (1) 20mL Luerlock syringe, (1) 122cm IV tubing with spike, (1) 3-way valve, and (1) pressure tubing set. Assemble the inflation instruments and connect them to the saline/methylene blue solution. Verify that the instruments are connected such that the solution is pumped from the saline bag to the distal end of the pressure tubing intended for connection to the balloon catheter. Step 2. Patient sedation Prepare and position the patient as customary for a diagnostic endoscopy (i.e. left lateral decubitis). Administer an oral anesthetic spray as necessary. Administer conscious sedation per the institution s protocol for monitored anesthesia care (MAC). Step 3. Diagnostic endoscopy and guidewire positioning 1. The placement procedure requires a single-channel gastroscope with a diameter of mm, instrument channel diameter of 2.8mm, and a maximum working length of 140cm. 2. Position the bite block in the patient s mouth. Ensure that the bite block is large enough for both the ReShape Dual Balloon and the endoscope to pass through in parallel. 3. Perform an esophagogastroduodenoscopy (EGD) to identify any significant gastrointestinal pathology that would contraindicate implantation per protocol. If excessive food or liquid is present in the stomach, the procedure should be postponed. 4. Advance the guidewire through the endoscope s instrument channel port. Place the distal portion of the guidewire into the duodenum and position the guidewire along the greater curvature of the stomach. 5. Remove the endoscope from the patient while leaving the guidewire in place. While removing the endoscope, take note of the distance from the patient s mouth to the gastro-esophageal junction. This measurement will be used when introducing the Dual Balloon. Guidewire is placed in the duodenum and positioned along the greater stomach curvature 8
9 Step 4. ReShape Dual Balloon insertion 1. Prior to insertion, apply an ample amount of lubricant to the entire surface of the balloon. 2. Apply lubricant to the proximal segment of the guidewire and pass the guidewire through the ReShape Dual Balloon s guidewire lumen. The main operating physician should maintain control of the guidewire and provide countertraction when necessary. Insert the placement catheter into the patient s mouth, down the esophagus, and into the stomach. The depth markers on the placement catheter may be used as a reference for the distance between the patient s mouth to the proximal end of the implant. a. In the event of reduction in oxygen saturation levels, anesthesia should increase the oxygen flow rate to the patient, attempt to suction the back of the throat, and hold a jaw thrust to help regain normal oxygen saturation levels. 3. Reinsert the endoscope into the stomach alongside the placement catheter. Verify that the ReShape Dual Balloon is properly positioned as described below: a. The delivery catheter detent cap of the placement catheter is distal to the GE junction. b. The balloon is lying along the greater curvature of the stomach. c. Confirm the distal balloon is at or before the angle of incisura, not extending past the pylorus. d. Reposition the ReShape Dual Balloon as necessary. 4. After verifying the balloon s placement, position the endoscope superior to the proximal balloon and withdraw the guidewire from the ReShape Dual Balloon. Step 5. Balloon inflation 1. Remove the Luer-caps from each fill tube and inflate the balloons sequentially. Note: Maintain cleanliness of the saline and tubing. Do not allow the pump tubing to contact the floor. Do not handle the tubing with dirty gloves. 2. The proximal balloon is to be inflated first. Connect the infiltration pump tubing to the proximal balloon fill tube (catheter lanyard 1) and inflate to the desired volume. Monitor inflation under endoscopic visualization. a. Confirm that the saline/methylene blue solution bag contains only the intended inflation volume for the balloon and any air and excess fluid has been removed. b. Inflate the proximal balloon. Monitor its inflation through the endoscope. 9
10 i. Monitor the balloon during filling and verify that the fill tube does not disconnect. ii. Observe and make sure the orientation and location of the balloon does not change during inflation. iii. Upon completion of balloon inflation, endoscopically inspect the balloon and confirm: 1. The intended inflation volume was completely transferred from the saline bag to the balloon. 2. No leakage of fluid from balloon. c. Once inflation is complete, attach the first valve sealant syringe to the proximal balloon fill tube (catheter lanyard 1) and inject 6cc of sealant into the proximal balloon i. Leave the valve sealant syringe attached to the fill tube to serve as a confirmation that this step has been completed. 3. Then, reposition the endoscope so the distal balloon can be viewed. Connect the infiltration pump tubing to the distal balloon fill tube (catheter lanyard 2) and inflate to the desired volume. Monitor inflation under endoscopic visualization. a. Confirm the saline/methylene blue solution bag contains only the intended inflation volume for the balloon and any air and excess fluid has been removed. b. Inflate the distal balloon. Monitor its inflation through the endoscope. i. Monitor the balloon during filling and verify that the fill tube does not disconnect. ii. Upon completion of balloon inflation, endoscopically inspect the balloon and confirm: 1. The intended inflation volume was completely transferred from the saline bag to the balloon. 2. No leakage of fluid from balloon. c. Once inflation is complete, attach the second valve sealant syringe to the distal balloon fill tube (catheter lanyard 2) and inject 6cc of sealant into the distal balloon. i. Leave the valve sealant syringe attached to the fill tube to serve as a confirmation that this step has been completed. 4. DO NOT DISCONNECT THE PLACEMENT CATHETER FROM THE BALLOON UNTIL VALVE SEALANT HAS BEEN INJECTED INTO EACH BALLOON. Step 6. Disengaging the Balloon from the catheter 1. Position the endoscope proximal to the detent cap on the placement catheter. It is important to keep the endoscope proximal to the detent cap during removal of the placement catheter. If the endoscope is alongside the detent of the balloon catheter, the combined diameter of the detent cap and the endoscope may be too large to be pulled into the esophagus. 2. Hold both the Dual Balloon Catheter and the endoscope together and pull back in tandem. Take care to keep the endoscope proximal to the detent cap. Pull the inflated balloons against the GE junction; continue to pull the catheter and the endoscope with a slow and smooth constant force to release the inflated balloons from the placement catheter. 10
11 3. Once released from the Dual Balloon Catheter, continue to withdraw the placement catheter and endoscope from the patient. 4. Reinsert the endoscope to inspect the upper GI tract and the ReShape Dual Balloon. 5. Provide the patient with a Patient ID Wallet Card and instruct the patient on potential postprocedure discomfort and symptoms. 11
12 ReShape Dual Balloon Removal Attention: Appropriate precautions must be taken to ensure that the patient returns for balloon removal six months after insertion. The device is removed via endoscopy under conscious sedation (MAC). Each balloon is completely drained in a controlled manner. A snare captures the deflated dual balloon around the proximal cap and the device is removed through the mouth. Recommended patient diet instructions to minimize the amount of food and liquid in the stomach during the procedure: - 48 hours prior to procedure: Soft foods only, no meat in any form hours prior to procedure: Clear liquids only hours prior to procedure: NPO - No food or liquids by mouth. Note: If the patient does not follow these instructions, consider cancelling the removal procedure as food in the stomach during removal could increase the risk of aspiration. Step 1. Procedure preparation Equipment and Materials: - ReShape Removal Catheter - Adult-sized endoscopic bite block - Two (2) suction pumps or equivalent with ¼ inch tubing; one dedicated to the Removal Catheter. - Lubricant - Endoscopic snare - Endoscopic rat-tooth grasping forceps - Laryngoscope and Magill forceps 1. Remove the ReShape Removal Catheter from its packaging. Inspect the Removal Catheter for any damage. 2. Prepare two (2) suction canisters or equivalent with empty 1-liter canisters. 3. Have a laryngoscope and Magill forceps on hand. In case of difficulty while removing the drained balloons through the upper esophageal sphincter, a laryngoscope and Magill forceps may be used to retrieve the ReShape Dual Balloon device. Step 2. Patient sedation Prepare and position the patient as customary for a diagnostic endoscopy (i.e., left lateral). Administer conscious sedation, per the institution s protocol for monitored anesthesia care (MAC). Note: In case of emergency or complication during removal, the patient should be intubated. 12
13 Step 3. Balloon drainage 1. The removal procedure requires a single-channel gastroscope with a diameter of mm, instrument channel diameter of 2.8mm, and a maximum working length of 140cm. 2. Position an adult-size endoscopic bite block into the patient s mouth. 3. Perform an endoscopic inspection of the esophagus and stomach. If excessive food or liquid is present, the procedure should be postponed. 4. With the endoscope in the stomach, insert the Removal Catheter through the endoscope s instrument channel. 5. The order in which the balloons are drained is at the discretion of the physician. Position the endoscope adjacent to the first balloon to be drained and orient the Removal Catheter perpendicular to the surface of the balloon. 6. Advance the Removal Catheter up to the balloon surface, apply light pressure and turn the crank handle clockwise until it stops to advance the needle and puncture the balloon. a. Insert the Removal Catheter into the balloon up to the second depth marking, and then fully retract the needle by turning the crank handle counter clockwise. It is important to ensure that the needle has been retracted before inserting the catheter beyond the second marking. b. Insert the catheter deeper into the balloon up to the third depth marking. c. Open the Removal Catheter s valve and apply suction to drain the balloon. d. Hold the endoscope steady and ensure that the catheter remains positioned within the balloon until the balloon is completely drained. 13
14 e. After draining the balloon, withdraw the catheter from the balloon. Note: If necessary, temporarily leave the Removal Catheter s valve open and disconnect the suction pump tube. This will relieve the vacuum within the deflated balloon and allow the catheter to be pulled out of the balloon. 7. Close the Removal Catheter s valve and reconnect the suction pump tube. Reposition the Removal Catheter perpendicular to the surface of the next balloon and repeat the same steps as was done to drain the first balloon. 8. Completely withdraw the Removal Catheter from the endoscope when the second balloon has been drained. 9. Insert endoscopic rat-toothed grasping forceps to tear a hole in each of the balloons. This allows trapped fluid and air to completely drain from the balloon and reduce resistance during removal. 10. Before removing the graspers, search for the proximal cap of the balloon device. If necessary, use the graspers to reposition the balloon device such that the proximal cap is easily seen. Note: If the proximal cap is unreachable or sitting too far into the fundus, grasp the central shaft and push the entire balloon device distally. This will allow snaring of the proximal cap in an antegrade position. Alternatively, flip the balloon to place the proximal cap in the distal portion of the stomach where the distal cap can be snared by retroflexing the endoscope. 11. Remove the grasper and any remaining fluid from the stomach using endoscopic suction. Step 4. Capture and removal of the balloon 1. Insert an endoscopic snare and prepare to capture the drained ReShape Dual Balloon. a. Position the endoscope as necessary in order to access the proximal cap. b. Capture the proximal cap with the endoscopic snare. 14
15 Note: Snaring the proximal cap provides a secure hold of the drained ReShape Dual Balloon. Do not capture the device by the balloon skin or along the shaft. 2. With the ReShape Dual Balloon proximal cap firmly grasped within the snare, remove the endoscope and the captured balloon from the patient. Make sure to hold both the snare and endoscope when pulling the balloon up the esophagus. The snared balloon should remain under direct endoscopic visualization during the entire removal. a. In case of difficulty during removal, use the following technique: i. If the balloon releases from the snare in the lower esophagus: 1. Push the balloon with the tip of the endoscope until the balloon has been pushed from the esophagus back down into the stomach. Note: this should be done with light and steady pressure. Too much force could cause the scope to bend instead of pushing the balloon. 2. Reposition the balloon device as necesary and snare the proximal cap, again. ii. If the balloon releases from the snare in the upper esophagus, use a laryngoscope and Magill forceps to retrieve the ReShape Dual Balloon device. 3. Reinsert the endoscope to inspect the patient s esophagus and stomach. References Instructions for Use ReShape Integrated Dual Balloon System Instructions for Use ReShape Gastroenterology Guidewire Instructions for Use ReShape Removal Catheter Instructions for Use ReShape Infiltration Pump 15
16 ReShape Medical, Inc. 100 Calle Iglesia San Clemente, CA YES-RESHAPE ReShape Medical, Inc. All rights reserved. RESHAPE and the RESHAPE MEDICAL Logo are registered trademarks of ReShape Medical, Inc Rev. C 16
ReShape Integrated Dual Balloon System Instructions for Use
 ReShape Integrated Dual Balloon System Instructions for Use CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION The ReShape Integrated Dual Balloon
ReShape Integrated Dual Balloon System Instructions for Use CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION The ReShape Integrated Dual Balloon
ReShape Integrated Dual Balloon System Instructions for Use
 ReShape Integrated Dual Balloon System Instructions for Use CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION The ReShape Integrated Dual Balloon
ReShape Integrated Dual Balloon System Instructions for Use CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION The ReShape Integrated Dual Balloon
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
How to perform: HALO 360 Radiofrequency Ablation of Barrett s s Esophagus
 How to perform: HALO 360 Radiofrequency Ablation of Barrett s s Esophagus Used abbreviations BE: EID: ER: RFA: Barrett s esophagus Esophageal inner diameter Endoscopic resection Radiofrequency ablation
How to perform: HALO 360 Radiofrequency Ablation of Barrett s s Esophagus Used abbreviations BE: EID: ER: RFA: Barrett s esophagus Esophageal inner diameter Endoscopic resection Radiofrequency ablation
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
PRODUCTS FOR THE DIFFICULT AIRWAY. Courtesy of Cook Critical Care
 PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
Division 1 Introduction to Advanced Prehospital Care
 Division 1 Introduction to Advanced Prehospital Care Chapter 7 Intravenous Access and Medication Administration Part 1 Principles and Routes of Medication Administration Topics Aseptic Technique Medication
Division 1 Introduction to Advanced Prehospital Care Chapter 7 Intravenous Access and Medication Administration Part 1 Principles and Routes of Medication Administration Topics Aseptic Technique Medication
Policies & Procedures. RNSP - RN Procedure. I.D. Number: 1097
 Policies & Procedures Title: ESOPHAGEAL TAMPONADE TUBE (MINNESOTA Tube) ASSISTING WITH INSERTION, CARE OF A PATIENT, ASSISTING WITH REMOVAL RNSP - RN Procedure I.D. Number: 1097 Authorization [x] Nursing
Policies & Procedures Title: ESOPHAGEAL TAMPONADE TUBE (MINNESOTA Tube) ASSISTING WITH INSERTION, CARE OF A PATIENT, ASSISTING WITH REMOVAL RNSP - RN Procedure I.D. Number: 1097 Authorization [x] Nursing
Information Technology Solutions
 2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
AXIOS Stent and Electrocautery Enhanced Delivery System. Quick Reference Guide
 AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
Danis Procedure Pack - BASIC
 Danis Procedure Pack - BASIC Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: volenec@ellacs.eu Danis
Danis Procedure Pack - BASIC Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: volenec@ellacs.eu Danis
ThruPort systems ProPlege peripheral retrograde cardioplegia device
 ThruPort systems ProPlege peripheral retrograde cardioplegia device Training Module Lessons Lesson 1: ProPlege device Lesson 2: Preparing for the case Lesson 3: Utilizing the device Lesson 4: Troubleshooting
ThruPort systems ProPlege peripheral retrograde cardioplegia device Training Module Lessons Lesson 1: ProPlege device Lesson 2: Preparing for the case Lesson 3: Utilizing the device Lesson 4: Troubleshooting
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to
 1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only. In
1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only. In
Chapter 40 Advanced Airway Management
 1 2 3 4 5 Chapter 40 Advanced Airway Management Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only.
1 2 3 4 5 Chapter 40 Advanced Airway Management Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only.
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Placing PEG and Jejunostomy Tubes in Dogs and Cats
 Placing PEG and Jejunostomy Tubes in Dogs and Cats I. Gastrostomy tube A. Percutaneous Endoscopic Gastrostomy (PEG) tube placement Supplies for PEG tube placement: Supplies and equipment for general anesthesia
Placing PEG and Jejunostomy Tubes in Dogs and Cats I. Gastrostomy tube A. Percutaneous Endoscopic Gastrostomy (PEG) tube placement Supplies for PEG tube placement: Supplies and equipment for general anesthesia
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
A-Tube Instructions for Use
 a s s i s t A-Tube Instructions for Use Caution: US Federal law restricts this device to sale on or by the order of a physician. IFU-029 Rev. B (06/2016) Rx Only Aspire Bariatrics, Inc. 2016 TABLE OF CONTENTS
a s s i s t A-Tube Instructions for Use Caution: US Federal law restricts this device to sale on or by the order of a physician. IFU-029 Rev. B (06/2016) Rx Only Aspire Bariatrics, Inc. 2016 TABLE OF CONTENTS
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
TJF-160F/VF Cleaning and Disinfection Checklist
 TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES
 EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION
 MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
Part 1 Principles and Routes of Medication Administration
 1 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration 2 Caution: Administering medications is business Always take appropriate Standard measures to reduce your
1 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration 2 Caution: Administering medications is business Always take appropriate Standard measures to reduce your
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CCTC Minnesota Procedure: Minnesota Tube, Assisting with Insertion and Care of Patient
 CCTC Minnesota Procedure: Minnesota Tube, Assisting with Insertion and Care of Patient Purpose: To control bleeding from esophageal or gastric varices that have not responded to medical therapy (ie. Sclerotherapy,
CCTC Minnesota Procedure: Minnesota Tube, Assisting with Insertion and Care of Patient Purpose: To control bleeding from esophageal or gastric varices that have not responded to medical therapy (ie. Sclerotherapy,
Patient Information Publications Warren Grant Magnuson Clinical Center National Institutes of Health
 Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Integra. Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE
 Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Managing Foreign Objects in the GI Tract Tips & Tricks
 Managing Foreign Objects in the GI Tract Tips & Tricks STEPHEN LANDRENEAU, MD Take home o Perform emergent endoscopy in cases of: Esophageal obstruction due to food bolus impaction or foreign object. Disk
Managing Foreign Objects in the GI Tract Tips & Tricks STEPHEN LANDRENEAU, MD Take home o Perform emergent endoscopy in cases of: Esophageal obstruction due to food bolus impaction or foreign object. Disk
Distal Radius Surgical Technique Guide
 Distal Radius Surgical Technique Guide About IlluminOss IlluminOss minimally invasive procedure incorporates a PET or Dacron balloon catheter that is delivered through a small pathway into the intramedullary
Distal Radius Surgical Technique Guide About IlluminOss IlluminOss minimally invasive procedure incorporates a PET or Dacron balloon catheter that is delivered through a small pathway into the intramedullary
RX Biliary System. Start
 Start We re in! When you control the wire, efficiency comes along for the ride. The of Physician-Controlled Wireguided Cannulation Physician-controlled wireguided cannulation (WGC) facilitates deep biliary
Start We re in! When you control the wire, efficiency comes along for the ride. The of Physician-Controlled Wireguided Cannulation Physician-controlled wireguided cannulation (WGC) facilitates deep biliary
FAST1 Intraosseous Infusion System. Training Session
 FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Instruction for use of Foley Catheter
 Instruction for use of Foley Catheter General Description: Foley Catheter for single use consists of a thin, sterile tube made of Natural latex 100% coated with medical solid Silicone, ended with soft
Instruction for use of Foley Catheter General Description: Foley Catheter for single use consists of a thin, sterile tube made of Natural latex 100% coated with medical solid Silicone, ended with soft
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Instruction For Use for All Silicon Foley Catheter
 General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
Tools of the Gastroenterologist: Introduction to GI Endoscopy
 Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always
 1 3 4 5 6 7 8 9 10 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always take appropriate Standard measures
1 3 4 5 6 7 8 9 10 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always take appropriate Standard measures
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
limbsandthings.com Advanced Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL
 Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
TRACHEOBRONCHIAL FOREIGN BODY REMOVAL ADVICE IN DOGS AND CATS
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk TRACHEOBRONCHIAL FOREIGN BODY REMOVAL ADVICE IN DOGS AND CATS Author : MIKE STAFFORD-JOHNSON, MIKE MARTIN Categories : Vets
Vet Times The website for the veterinary profession https://www.vettimes.co.uk TRACHEOBRONCHIAL FOREIGN BODY REMOVAL ADVICE IN DOGS AND CATS Author : MIKE STAFFORD-JOHNSON, MIKE MARTIN Categories : Vets
Itemized Billing and Procedure Description for the AspireAssist
 Itemized Billing and Procedure Description for the AspireAssist The following describes the recommended therapy course for the first year for a patient undergoing AspireAssist therapy. Although providers
Itemized Billing and Procedure Description for the AspireAssist The following describes the recommended therapy course for the first year for a patient undergoing AspireAssist therapy. Although providers
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 127 B A C D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. Bard 12 French, 5 cc balloon,
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 127 B A C D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. Bard 12 French, 5 cc balloon,
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
Procedure for removal and reinsertion of a supra pubic catheter
 Procedure for removal and reinsertion of a supra pubic catheter Equipment required collect prior to procedure Perform this procedure as an aseptic technique to minimise the risk of introducing Clean the
Procedure for removal and reinsertion of a supra pubic catheter Equipment required collect prior to procedure Perform this procedure as an aseptic technique to minimise the risk of introducing Clean the
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
MULTIFIX S Knotless Implants
 Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
Technique Guide MULTIFIX S Knotless Implants The MULTIFIX S system offers 5.5mm and 6.5mm knotless, all-peek, poundin implants. The system provides multiple fixation options, a streamlined technique, and
Technique Guide. *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System
 Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
Endoscopic Retrograde Cholangiopancreatography (ERCP)
 Endoscopic Retrograde Cholangiopancreatography (ERCP) Medical Imaging and Treatment of the Bile and Pancreatic Ducts CIE-02718 Understanding ERCP Brochure Update_F.indd 1 7/11/18 9:51 A Minimally Invasive
Endoscopic Retrograde Cholangiopancreatography (ERCP) Medical Imaging and Treatment of the Bile and Pancreatic Ducts CIE-02718 Understanding ERCP Brochure Update_F.indd 1 7/11/18 9:51 A Minimally Invasive
SARASOTA MEMORIAL HOSPITAL. NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) 12/18 12/18 1 of 7 RESPONSIBILITY:
 SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB
 The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB Akerman, Paul A. 1 ; Cantero, Daniel 2 ; Avila, Jose 2 ; Pangtay, Jesus 3 ; Agrawal, Deepak 1 Introduction:
The Spiral Enteroscopy Experience in 101 Consecutive Patients: Safety and Efficacy Using the Discovery SB Akerman, Paul A. 1 ; Cantero, Daniel 2 ; Avila, Jose 2 ; Pangtay, Jesus 3 ; Agrawal, Deepak 1 Introduction:
Adult Intubation Skill Sheet
 Adult Intubation 2. Opens the airway manually and inserts an oral airway *** 3. Ventilates the patient with BVM attached to oxygen at 15 lpm *** 4. Directs assistant to oxygenate the patient 5. Selects
Adult Intubation 2. Opens the airway manually and inserts an oral airway *** 3. Ventilates the patient with BVM attached to oxygen at 15 lpm *** 4. Directs assistant to oxygenate the patient 5. Selects
Part 1: General requirements
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Waitin In The Wings. Esophageal/Tracheal Double Lumen Airway (Combitube ) Indications and Use for the Pre-Hospital Provider
 Waitin In The Wings Esophageal/Tracheal Double Lumen Airway (Combitube ) Indications and Use for the Pre-Hospital Provider 1 CombiTube Kit General Description The CombiTube is A double-lumen tube with
Waitin In The Wings Esophageal/Tracheal Double Lumen Airway (Combitube ) Indications and Use for the Pre-Hospital Provider 1 CombiTube Kit General Description The CombiTube is A double-lumen tube with
BULKAMID STANDARD OPERATING PROCEDURE
 BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
Procedure. P/N Rev B
 Patient Information Guide for the ReShape Procedure Before your procedure, please review this important information P/N 03 0312 Rev B Table of Contents Why is it Important to Lose Weight?... 3 Why Do Doctors
Patient Information Guide for the ReShape Procedure Before your procedure, please review this important information P/N 03 0312 Rev B Table of Contents Why is it Important to Lose Weight?... 3 Why Do Doctors
SOP: Urinary Catheter in Dogs and Cats
 SOP: Urinary Catheter in Dogs and Cats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Urinary Catheter in Dogs and Cats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
CrackCast Episode 30 GI Bleeding
 CrackCast Episode 30 GI Bleeding Episode overview: 1) List 5 causes of UGIB in adults and pediatrics 2) List 5 causes of LGIB in adults and pediatrics 3) Describe your management approach for severe UGIB
CrackCast Episode 30 GI Bleeding Episode overview: 1) List 5 causes of UGIB in adults and pediatrics 2) List 5 causes of LGIB in adults and pediatrics 3) Describe your management approach for severe UGIB
Overview. The Team Concept. Chapter 7. Assisting the ALS Provider 9/11/2012. The Team Concept ALS Procedures and Equipment
 Chapter 7 Assisting the ALS Provider Slide 1 Overview The Team Concept ALS Procedures and Equipment Electrocardiogram (ECG) Monitoring Slide 2 The Team Concept Prehospital care involves many individuals
Chapter 7 Assisting the ALS Provider Slide 1 Overview The Team Concept ALS Procedures and Equipment Electrocardiogram (ECG) Monitoring Slide 2 The Team Concept Prehospital care involves many individuals
Pulmonary. Pulmonary Endoscopy. Alair Bronchial Thermoplasty System. Transbronchial Aspiration Needles. Cytology Brushes.
 Pulmonary Endoscopy Alair Bronchial Thermoplasty System Alair Bronchial Thermoplasty System... 79 Airway Stents Dynamic (Y) Stent... 79 Polyflex Self-Expanding Silicone Airway Stent... 82 Ultraflex Partially
Pulmonary Endoscopy Alair Bronchial Thermoplasty System Alair Bronchial Thermoplasty System... 79 Airway Stents Dynamic (Y) Stent... 79 Polyflex Self-Expanding Silicone Airway Stent... 82 Ultraflex Partially
Educational Session: Evaluation and Management of the Difficult Airway
 Educational Session: Evaluation and Management of the Difficult Airway Diane M. Birnbaumer, MD, FACEP 3/24/2010 7:30 AM - 8:30 AM The Difficult Airway What s Up YOUR Sleeve? Diane M. Birnbaumer, M.D.,
Educational Session: Evaluation and Management of the Difficult Airway Diane M. Birnbaumer, MD, FACEP 3/24/2010 7:30 AM - 8:30 AM The Difficult Airway What s Up YOUR Sleeve? Diane M. Birnbaumer, M.D.,
AIRWAY MANAGEMENT PRODUCTS
 QUICK REFERENCE GUIDE AIRWAY MANAGEMENT PRODUCTS Shiley Endotracheal Tubes SHILEY ENDOTRACHEAL TUBE CATALOG NUMBER CHARTS DESCRIPTION QTY PER BOX SIZE (MM) 1.0 1.5 2.0 2.5 3.0 3.5 4.0 VENTILATOR-ASSOCIATED
QUICK REFERENCE GUIDE AIRWAY MANAGEMENT PRODUCTS Shiley Endotracheal Tubes SHILEY ENDOTRACHEAL TUBE CATALOG NUMBER CHARTS DESCRIPTION QTY PER BOX SIZE (MM) 1.0 1.5 2.0 2.5 3.0 3.5 4.0 VENTILATOR-ASSOCIATED
Rhinology Products. Rhinology Products. Superior solutions for superior patient care.
 Rhinology Products Superior solutions for superior patient care. The Doyle Open-Lumen Splint addresses the problem of potential closure of the airway lumen by a hypertrophied turbinate. We also offer the
Rhinology Products Superior solutions for superior patient care. The Doyle Open-Lumen Splint addresses the problem of potential closure of the airway lumen by a hypertrophied turbinate. We also offer the
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER
 INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF ENBREL POWDER Introduction The following instructions explain how to prepare and inject Enbrel powder for injection. Please read the instructions carefully
Introducing the Fastrach-LMA. Prepared by Jim Medeiros, NREMT-P Regional Field Coordinator Lord Fairfax EMS Council
 Introducing the Fastrach-LMA Prepared by Jim Medeiros, NREMT-P Regional Field Coordinator Lord Fairfax EMS Council Objectives Review Anatomy of the Upper Airway Review LFEMSC LMA Protocol Discuss Indications
Introducing the Fastrach-LMA Prepared by Jim Medeiros, NREMT-P Regional Field Coordinator Lord Fairfax EMS Council Objectives Review Anatomy of the Upper Airway Review LFEMSC LMA Protocol Discuss Indications
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0
 fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
Gastric Balloon for Weight Loss. Karol A Gutowski, MD, FACS Hot Topics
 Gastric Balloon for Weight Loss Karol A Gutowski, MD, FACS Hot Topics Disclosures None related to this topic Will use brand names due to lack of distinguishing generic names Presentation Level of Evidence
Gastric Balloon for Weight Loss Karol A Gutowski, MD, FACS Hot Topics Disclosures None related to this topic Will use brand names due to lack of distinguishing generic names Presentation Level of Evidence
Phoenix Atherectomy System Product Overview /LB
 Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
SHOULDER PATIENTS. Diagnostic Shoulder Arthroscopy Technique Guide
 SHOULDER PATIENTS Diagnostic Shoulder Arthroscopy Technique Guide mi-eye 2 Indications for Use The mi-eye 2 system is indicated for use in diagnostic and operative arthroscopic and endoscopic procedures
SHOULDER PATIENTS Diagnostic Shoulder Arthroscopy Technique Guide mi-eye 2 Indications for Use The mi-eye 2 system is indicated for use in diagnostic and operative arthroscopic and endoscopic procedures
Instruction Guide to Sterile Self-Intermittent Catheterization For Men Using the Cure Catheter Closed System
 Cure Medical donates 10% of net income to medical research in pursuit of a cure for spinal cord injuries and central nervous system disorders. For information on scientific advancements, visit www.curemedical.com.
Cure Medical donates 10% of net income to medical research in pursuit of a cure for spinal cord injuries and central nervous system disorders. For information on scientific advancements, visit www.curemedical.com.
How to Change a. Foley catheter. Patient Education Rehabilitation Nursing. Step-by-step instructions for the caregiver
 Patient Education How to Change a Foley Catheter Step-by-step instructions for the caregiver This handout gives step-bystep instructions for changing a Foley catheter, which is a tube in your bladder to
Patient Education How to Change a Foley Catheter Step-by-step instructions for the caregiver This handout gives step-bystep instructions for changing a Foley catheter, which is a tube in your bladder to
Conventus CAGE PH Surgical Techniques
 Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES
 Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
SYNFLATE SYSTEM SURGICAL TECHNIQUE
 SYNFLATE SYSTEM Vertebral augmentation system for the reduction of fractures and/or creation of a void in cancellous bone in the spine SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SynFlate 2 AO Principles
SYNFLATE SYSTEM Vertebral augmentation system for the reduction of fractures and/or creation of a void in cancellous bone in the spine SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SynFlate 2 AO Principles
IO considerations. Daniel Dunham
 IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
LESSON ASSIGNMENT. Urinary System Diseases/Disorders. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
Technique Guide. *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant
 Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
PREPARING FOR REFLUX TESTING. Bravo Reflux Testing System. A simple way to evaluate your gastroesophageal reflux symptoms
 PREPARING FOR REFLUX TESTING Bravo Reflux Testing System A simple way to evaluate your gastroesophageal reflux symptoms HOW IT WORKS The test involves a miniature ph capsule, which is approximately the
PREPARING FOR REFLUX TESTING Bravo Reflux Testing System A simple way to evaluate your gastroesophageal reflux symptoms HOW IT WORKS The test involves a miniature ph capsule, which is approximately the
Helping to protect you from exposure to hazardous drugs
 Helping to protect you from exposure to hazardous drugs Closed system solution: Texium closed male luer and SmartSite needle-free valve products What you can t see can hurt you The risks are well documented
Helping to protect you from exposure to hazardous drugs Closed system solution: Texium closed male luer and SmartSite needle-free valve products What you can t see can hurt you The risks are well documented
mild Devices Kit - Instructions for Use
 INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
