Studies on the role of gonadotropininhibitory. neuroendocrine regulation of reproduction in the sheep
|
|
|
- Roy Briggs
- 5 years ago
- Views:
Transcription
1 Studies on the role of gonadotropininhibitory hormone (GnIH) in the neuroendocrine regulation of reproduction in the sheep Ika Puspita Sari Department of Physiology Faculty of Medicine Monash University A thesis submitted to the Department of Physiology, Monash University in fulfilment of the requirements for the degree of Doctor of Philosophy July 2010 Supervisors: Professor Iain Clarke and Professor Alan Tilbrook
2 Copyright Notices Notice 1 Under the Copyright Act 1968, this thesis must be used only under the normal conditions of scholarly fair dealing. In particular no results or conclusions should be extracted from it, nor should it be copied or closely paraphrased in whole or in part without the written consent of the author. Proper written acknowledgement should be made for any assistance obtained from this thesis. Notice 2 I certify that I have made all reasonable efforts to secure copyright permissions for third-party content included in this thesis and have not knowingly added copyright content to my work without the owner's permission.
3 TABLE OF CONTENTS SUMMARY... V DECLARATION...VIII ACKNOWLEDGEMENTS... X LIST OF PUBLICATIONS... XII ABBREVIATIONS AND SYMBOLS... XIII Chapter 1: Review of the Literature General Introduction The Hypothalamo Pituitary Gonadal Axis GnRH cell distribution and projections within the ovine brain The Estrous Cycle of the Ewe The Luteal Phase The Follicular Phase The Preovulatory LH Surge GnRH and Regulation of GnRH secretion Factor influencing GnRH cells and GnRH secretion Sex steroid feedback mechanisms Negative feedback Seasonality and regulation of GnRH cells Positive feedback Regulation of LH and FSH Subunit Gene Expression in the Pituitary Regulation of gonadotropin subunit gene expression by GnRH Regulation of gonadotropin subunit gene expression by ovarian sex steroids and peptides 29 i
4 1.5.3 Regulation during the estrous cycle Regulation of Gonadotropin Secretion Relationship between pulsatile GnRH secretion and gonadotropin (LH and FSH) secretion Regulation by ovarian sex steroids at the level of the gonadotrope Short term negative feedback mechanism Long term negative feedback mechanism Positive feedback mechanism Gonadotropin Inhibitory Hormone (GnIH) Discovery of mammalian GnIH Characteristics of GnIH Distribution and projections of GnIH neurons Characteristics of GnIH receptors (GnIH R) Distribution of GnIH R Steroid receptors in GnIH cells Effect of GnIH on gonadotropin synthesis Effect of GnIH on gonadotropin secretion Effect of GnIH on the levels of other hormones Control of Food Intake in the Hypothalamus Effect of GnIH on food intake Gonadal Expression of GnIH and Possible Gonadal Function Overview Aims Hypotheses 48 ii
5 Chapter 2: Potent action of RFamide related peptide 3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion Declaration for Chapter Declaration by co authors Introduction Conclusion 57 Chapter 3: Effect of RF amide related peptide 3 on Luteinizing Hormone and Follicle Stimulating Hormone synthesis and secretion in ovine pituitary gonadotropes Declaration for Chapter Declaration by co authors Introduction Conclusion 96 Chapter 4: Gonadotropin inhibitory hormone (GnIH) prevents the priming effect of estradiol 17β on luteinising hormone (LH) secretion in ovine pituitary gonadotropes Declaration for Chapter Declaration by co authors Introduction Conclusion Abstract Introduction Materials and Methods Animals Pituitary collection and preparation 130 iii
6 4.6 Effect of GnIH and E2 on gonadotropin secretion Radioimmunoassay Statistical analysis Results Discussion Acknowledgements 136 Chapter 5: GnIH 3 gene expression in the follicular phase of the ewe is low and GnIH 3 blocks the LH surge Chapter 6: General Discussion General Discussion 153 Appendix I: Evidence that RF amide related peptides are inhibitors of reproduction in mammals Declaration for Appendix Declaration by co authors Introduction 160 Conclusion 160 References iv
7 SUMMARY The brain peptide GnRH provides the primary stimulus for the reproductive axis, through hypophysiotropic action on the pituitary gonadotropes. There is now strong evidence for the existence of an inhibitory factor named gonadotropin inhibitory hormone (GnIH).This thesis presents the results of a series of experiments which demonstrate that GnIH is produced in the hypothalamus of the ovine brain and acts on the pituitary gland, inhibiting gonadotropin synthesis and secretion. In addition, it is suggested that GnIH counteracts the positive feedback effect of estrogen in the pituitary gonadotropes. A demonstrated reduction in expression of GnIH in the late follicular phase of the estrous cycle may be permissive of the positive feedback effect of estrogen to cause the preovulatory LH surge. Initial studies in birds and rats suggested that GnIH 3 acts in both the brain and pituitary inhibit gonadotropin secretion. It was hypothesized that GnIH is an hypophysiotropic hormone in the sheep which acts negatively for gonadotropin synthesis and secretion. In Chapter 2, studies are reported to show that GnIH 3 producing cells are localized in the dorsomedial nucleus (DMN) and paraventricular nucleus (PVN) of the hypothalamus of the ovine brain, with GnIH terminals projecting to the neurosecretory zone of the median eminence (ME). GnIH 3 inhibited GnRHstimulated LH and FSH secretion, but did not inhibit the basal gonadotropin secretion in the pituitary gonadotropes. The intravenous infusion of GnIH 3 also reduced LH v
8 pulse amplitude in the OVX ewes. This negative effect of GnIH 3 was specific to the gonadotropes, with no effect on growth hormone (GH), cortisol and prolactin (PRL) levels. In the pituitary gonadotropes, GnIH 3 inhibited GnRH generated calcium signals, indicating at least one mechanism for reduced secretory response. The second study (Chapter 3) aimed to test the hypothesis that GnH 3 is able to inhibit gonadotropin subunit synthesis in the gonadotropes. An in vitro model of repeated stimulation of ovine pituitary cells in primary culture was established. GnIH 3 reduced both LHβ and FSHβ mrna levels in the both sexes of the sheep. Consistent with the finding in the first study, there was no effect on the expression of genes for other pituitary hormones (adrenocorticotropin, growth hormone and prolactin). GnIH 3 inhibited the GnRH induced phosphorylation of ERK 1/2, suggesting this as a possible intermediary in the action of GnIH to inhibit the synthesis of gonadotropin subunit genes. This finding provided evidence that GnIH 3 acts in the level of pituitary negatively regulates gonadotropin synthesis in the sheep. The studies conducted in the Chapter 4 aimed to examine the effect of GnIH 3 on the positive feedback of estrogen in the pituitary gonadotropes. Studies carried out with ovine pituitary cells in culture showed that estradiol 17β (E 2 ) has a priming effect on the response of gonadotropes to GnRH, as seen in vivo. The priming effect of E 2 was blocked by GnIH treatment. In Chapter 5, expression of GnIH during luteal and follicular phases of the estrous cycle were measured by in situ hybridisation. GnIH mrna expression was lower during the late follicular phase than during the luteal phase. Artificial elevation of GnIH 3 levels in vi
9 the mid follicular phase (by iv infusion) reduced mean LH levels as well as LH pulses. In a model of the E 2 benzoate (EB) induced LH surge, iv infusion of GnIH 3 had a powerful negative effect. Thus, GnIH blocked the surge in 4/6 treated animals with minor surges occurring in the other two animals. Taken together, the body of the work presented in this thesis provides strong evidence that GnIH 3 plays a role in negatively regulating reproductive function in the sheep. The data show that GnIH 3 is an hypophysiotropic hormone which acts negatively in the synthesis and secretion of gonadotropins. Reduction in expression of GnIH gene expression in the late follicular phase of the estrous cycle may be permissive of the positive feedback effect of EB to cause the preovulatory LH surge. Understanding the role of GnIH in the hypothalamo pituitary axis will lead to therapeutic uses for GnIH and analogues in the management of reproduction. vii
10 DECLARATION I hereby declare that this thesis contains no material which has been accepted for the award of any other degree or diploma in any university or other institution and affirms that to the best of my knowledge, the thesis contains no material previously published or written by another person, except where due reference is made in the text of thesis. This thesis includes 4 original papers which are either published, submitted or in preparation to be published in peer reviewed journals. The core theme of the thesis is the role of gonadotropin inhibitory hormone (GnIH) in the neuroendocrine regulation of reproduction in the sheep. The ideas, development and writing up of all the papers in the thesis were the principal responsibility of myself, the candidate, working within the Department of Physiology under the supervision of Professor Iain Clarke (Monash University). The inclusion of co authors reflects the fact that the work came from active collaboration between researchers and acknowledges input team based research. In the case of Chapters 2 5 my contribution to the work involved in the following: Thesis Publication title chapter 2 Potent action of RF amiderelated peptide 3 on pituitary gonadotropes indicative for a hypophysiotropic role in the negative regulation of gonadotropin secretion 3 Effect of RF amide related peptide 3 on Luteinizing hormone and Folliclestimulating hormone synthesis and secretion in ovine pituitary gonadotropes Publication status Published in Endocrinology Published in Endocrinology Nature and extent of candidate s contribution Cell culture conduct and LH and FSH radioimmunoassay, and preparation of manuscript. Pituitary cell culture conduct, establishing and running PCR, Western Blot and LH/FSH assays. Preparation of manuscript. viii
11 4 GnIH prevents the estradiol 17β priming effect on LH secretion in ovine pituitary gonadotropes 5 GnIH 3 gene expression in the follicular phase of the ewe is low and GnIH 3 blocks the LH surge To be submitted to Domestic Animal Endocrinology To be submitted as part of a manuscript to Nature Pituitary cell culture conduct, establishing LH/FSH assays. Preparation of manuscript. ISH and in vivo study conduct, establishing LH assay. I have renumbered and rearranged sections of submitted or published papers in order to generate a consistent presentation within the thesis. Signed: Ika Puspita Sari Date: ix
12 ACKNOWLEDGEMENTS This thesis is the result of assistance and support from many people. Therefore, I thank numerous people whose contributions have enabled the completion of my thesis. I would like to express my gratitude to my supervisor, Professor Iain Clarke, for the support over the past four and a half years. You have taught me many great things, skills, knowledge, optimism, and even management. You have also broadened my view in research, and I truly appreciate everything you have done for me. You have been very supportive, especially during the hard time with family problems, losing family members, and losing properties because of the earthquake which almost ruined my motivation for continuing my PhD. Your guidance and optimism have indeed raised me up. I would also like to thank all people in the Clarke lab, who have assisted me in the laboratory skills, data analysis, result discussions and answers to my questions Jeremy, Kath, Alix, Alda, Mandy, Jessica, Javed, Olivier, Sarah, Sofie, Qi, and Noi. My gratitude also goes to the Werribee team Bruce, Lynda, and Elaine for being great friends during the journey of my research. Jeremy, you have been of great assistance in graph and data analysis, which are a truly invaluable skill. I would never forget your Granddad s book with his Indonesian recipes, and also thanks for the Agapanthus. Alix, thanks for your assistance in an amazing lab skill (RIA, RT PCR, in situ). Alda and Jessica, thanks for the chat and the lab assistance in tissue culture. Alda, you have x
13 inspired me for being patient with the cells and immunohystochemistry. Olivier, thanks for the Western Blot tips. Mandy, thanks for your support in my study and family problems. You are the endnote lady. The Werribee team, thanks for your assistance in in vivo study, chat, coffee break with the quiz (I will adopt this in my lab). To Professor Alan Tilbrook, thank you for your statistical advice and the journals which are really useful for my University library. To Kath, thanks for your helping hands when I needed them, and thanks for being my proof reader with such invaluable advice. To Australian Development Scholarship (ADS) from Australian Government, thank you for the award. To all my friends in the Indonesian community in Victoria, thanks for your support. Anita and Adit, thanks for the invaluable supports and for being my friends since high school until Postgrad. Finally, to my family Mum, Dad, Grands, my foster parents, my hubby s family, thanks for the support. My Grandma, your love, trust and support have guided me for being a teacher and researcher. To my hubby, Mas Tunggul, thanks for being my extremely great friend and supporter, for always encouraging me in research while being a mother. xi
14 LIST OF PUBLICATIONS Publication arising from thesis: Clarke IJ., Sari IP., Qi Y., Smith JT., Parkington HC., Ubuka T., Iqbal J., Li Q., Tilbrook A., Morgan K., Pawson AJ., Tsutsui K., Millar RP., Bentley GE. (2008) Potent Action of RFamide related peptide 3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 149 (11): Sari IP., Rao A., Smith JT., Tilbrook AJ., Clarke IJ. (2009) Effect of RF amide related peptide 3 on Luteinizing Hormone and Follicle Stimulating Hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 150 (12): Sari IP., Jacobi J., Rao A., Clarke IJ. (2010) GnIH prevents the estradiol 17β priming effect on LH in ovine pituitary gonadotropes. In preparation to be submitted to Endocrinology Abstracts/Conference Proceedings : Sari IP., Tilbrook A., Clarke IJ. Effect of RFRP 3 on gonadotropin synthesis and secretion in ovine pituitary gonadotropes. Endocrine Society of Australia (ESA). Melbourne, Australia (2008) Sari IP., Smith JT., Clarke IJ. Reduced RF amide related peptide (RFRP) gene expression in the follicular phase of the ewe estrous cycle permits increased LH secretion from gonadotropes. Endocrine Society of Australia (ESA). Adelaide, Australia (2009) xii
15 ABBREVIATIONS AND SYMBOLS The abbreviation listed below applies to the entire text, excluding legends of the tables and figures where names of structures and abbreviations appear separately. Abbreviations are written in full on first appearance in each chapter. Abbreviations AMPA α amino 3 hydroxyl 5 methyl 4 isoxazole propionate ARC arcuate nucleus Arg arginine AVPV anteroventral periventricular nucleus BnST bed nucleus of the stria terminalis βend β endorphin camp cyclic adenosine mono phosphate cdna complimentary deoxyribonucleic acid DA dopamine DAG dyacylglycerol DBH dopamine β hydroxylase DIG diacylglycerol DMN dorsomedial nucleus E 2 estradiol 17 β EB estradiol benzoate ENK enkephalin ERα estrogen receptor α ERβ estrogen receptor β FSH follicle stimulating hormone GABA γ aminobutyric acid GABA A γ aminobutyric acid A GABA B γ aminobutyric acid B GAD glutamic decarboxylase GAD 65 glutamic decarboxylase isoform 65 GAL galanin GLU glutamate GnRH gonadotropin releasing hormone GnSIF gonadotropin surge inhibiting factor GnIH gonadotropin inhibitory hormone GnIH R gonadotropin inhibitory hormone receptor GnIH RP 1 gonadotropin inhibitory hormone related peptide 1 GnIH RP 2 gonadotropin inhibitory hormone related peptide 2 GnIH 3 gonadotropin inhibitory hormone 3 GnRH R gonadotropin releasing hormone receptor HPD hypothalamo pituitary disconnection HPG hypothalamo pituitary gonadal axis hpg hypogonadal ICV intracerebroventricular im intramuscular iv intravenous xiii
16 KA2 kainate receptor 2 Kiss1 kisspeptin 1 LD long day LHRIF luteinizing hormone release inhibiting factor MBH mediobasal hypothalamus MCH melanin concentrating hormone ME median eminence MPOA medial preoptic area mrna messenger ribonucleic acid MSH melanocyte stimulating hormone MTII melanotan II NA noradrenaline NMDA N methyl D aspartate NPY neuropeptide Y NPY Y1 neuropeptide Y Y1 ORX orexin OVLT organum vasculosum of the lamina terminalis OVX ovariectomised Phe phenylalanine PKC protein kinase C POA preoptic area POMC proopiomelanocortin PQRamide proline glutamine arginine PrRP prolactin releasing peptide PRL prolactin PVN paraventricular nucleus PYY 3 36 peptide YY 3 36 QRFP pyroglutamylated Rf amide peptide RFamide arginine phenylalanine amide SD short day VMN ventromedial nucleus Symbols α alpha H histidine R arginine β beta I isoleucine S serine γ gamma K lysine T threonine A alanine L leucine V valine D aspartate M methionine W tryptophan E glutamate N asparagine Y tyrosine F phenylalanine P proline / per G glycine Q glutamine % percent xiv
17 Chapter 1: Review of the Literature 1
18 Chapter 1: Review of Literature 1.1 General Introduction Reproduction is a process that involves various levels of function. In particular, the entire reproductive system depends on the production and secretion of gonadotropin releasing hormone (GnRH) by the brain. GnRH drives the synthesis and secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), by the pituitary gland. Circulating gonadotropins act on the gonads, to support gamete production and the secretion of gonadal hormones. Functions of the reproductive tract are supported by the secretions of the gonads. The GnRH cells of the hypothalamus, the gonadotropes of the pituitary gland and the gonads form a nexus termed the hypothalamo pituitary gonadal (HPG) axis. This thesis is concerned with this axis, rather than the reproductive tract. Forward drive of the axis is through synthesis and secretion of GnRH and the gonadotropins, but feedback effects of gonadal hormones regulate the system by acting on the relevant cells of the brain and the pituitary gland. In particular, the important feedback hormones are gonadal steroids, such as estradiol and progesterone as well as protein hormones such as inhibin. In the male, testosterone regulates GnRH secretion by negative feedback which occurs predominantly in the brain with minimal effects on the pituitary (Tilbrook and Clarke 2001). In the male, both testosterone and its primary metabolites (estrogen and dihydrotestosterone) regulate GnRH cells via neuronal systems which may include endogenous opiates, gamma amino butyric acid (GABA), dopamine and catecholamines amongs other neuropeptides (Tilbrook and Clarke 2001; Clarke and Tilbrook 2009). In addition, the feedback regulation of FSH secretion in the male is controlled by inhibin, 2
19 which acts at the pituitary gland (Tilbrook and Clarke 2001). In the female both negative and positive feedback effects of gonadal steroids regulate the cyclic pattern of GnRH, gonadotropins and ovarian function. The female cycle is characterized by changing GnRH and LH pulse frequency and amplitude reflecting the feedback actions of estrogen and progesterone (Clarke 1996; Tilbrook and Clarke 2001). During the luteal and follicular phases, GnRH and gonadotropin secretion are regulated negatively by gonadal steroids (Clarke 1995a; Clarke 1996). In the luteal phase, progesterone is the major negative regulator of GnRH secretion whereas estradiol is likely a dominant negative regulator in the follicular phase (Clarke 1996). Neurotransmitters such as GABA and endogenous opiates are involved in the negative feedback actions of gonadal steroids in females (reviewed in Jennes et al, 2009). Whereas negative feedback occurs in the follicular phase, the rising level of estrogen triggers a neuroendocrine switch to positive feedback when a threshold level of estrogen is reached (Clarke 1996). At this point, estrogen initiates a train of events that culminates in a surge in GnRH secretion and LH secretion. During the process of positive feedback, the rise in estrogen and an increase in the responsivity of the gonadotropes to GnRH are synchronized. GnRH pulses are important in the generation of estrogen induced LH surge and are still required once the LH surge occurs (Phillips, Cummins et al. 1990; Clarke 1995a). A detailed description of the estrous cycle of the ewe will be given in Section 1.3 and the regulation of pituitary gonadotropes will be reviewed in Section 1.5. Since GnRH neurons do not express estrogen receptor α (ERα) (Shivers, Harlan et al. 1983; Herbison and Theodosis 1992), it has been suggested that cells producing catecholamines, glutamate (GLU) and/or neuropeptide Y (NPY) are activated during 3
20 positive feedback of steroids in the female (reviewed in Jennes et al, 2009 ). More recently, the identification of kisspeptin cells in the hypothalamus has led to the realization that these cells play a major role in mediating the negative and positive feedback effects of gonadal steroids to control GnRH secretion (Goodman, Coolen et al. 2004; Estrada, Clay et al. 2006; Goodman, Lehman et al. 2007; Smith, Clay et al. 2007; Smith, Li et al. 2009). Brain systems that regulate GnRH secretion including those that mediate the feedback effects of estrogen on GnRH secretion will be reviewed in Section 1.4. For many years, the popular belief has been that GnRH is the only hypothalamic peptide that controls pituitary gonadotropin synthesis and secretion. In fact many text books have stated that this is all that is required to drive the HPG axis (Knobil and Neil 1994; Strauss and Barbieri 2009). Nevertheless, there have been suggestions from various researchers that an inhibitory peptide is also produced by the hypothalamus, known as luteinizing hormone release inhibiting factor (LHRIF) (Hwan and Freeman 1987). Another inhibitory factor, gonadotropin surge inhibiting factor (GnSIF) (Danforth and Cheng 1993), suppresses LH secretion with or without any effect on FSH. This latter is a proteinacous entity which is produced by ovarian follicles, not the brain (Littman and Hodgen 1984). In 2000, Tsutsui and colleagues (Tsutsui, Saigoh et al. 2000) identified a novel peptide from the quail brain, named gonadotropin inhibitory hormone (GnIH), because of its ability to suppress LH secretion from pituitary cells culture. The peptide has a C terminus sequence Arg Phe NH 2, identifying it as a member of the RFamide family (Satake, Hisada et al. 2001; Osugi, Ukena et al. 2004). This family includes a large number of peptides 4
21 such as PQRamide, prolactin releasing peptide (PrRP), metastin/kisspeptin, and pyroglutamylated RFamide peptide (QRFP)(Osugi, Ukena et al. 2006). Peptides that are orthologous to the avian GnIH peptide have been identified in various mammalian species (Hinuma, Shintani et al. 2000). These include, cow (Hinuma, Shintani et al. 2000; Yoshida, Habata et al. 2003), non human primates (Ubuka, Lai et al. 2009a), rats (Hinuma, Shintani et al. 2000; Ukena, Ubuka et al. 2003), mice (Hinuma, Shintani et al. 2000), hamsters (Kriegsfeld, Mei et al. 2006) and humans (Ubuka, Ukena et al. 2006; Ubuka, Morgan et al. 2009b) (Table 1). The Tsutsui group characterized cdna for GnIH from quail brain. The quail GnIH gene encodes one GnIH and two putative gene related peptide sequences (GnIH RP 1 and GnIH RP 2. These peptides all share a common C terminal LPXRF NH 2 motif (where X represents L in both GnIH and GnIH RP 1, and Q in GnIH RP 2) [reviewed in (Fukusumi, Fujii et al. 2006), reviewed in (Ukena and Tsutsui 2005)] (Table 1). Since GnIH or orthologous peptides inhibit gonadotropin release from the pituitary in various animals [reviewed in (Fukusumi, Fujii et al. 2006);(Ubuka, Lai et al. 2009a);(Smith and Clarke 2010)], the function of the peptide has been substantiated (Tsutsui, Saigoh et al. 2000; Kriegsfeld, Mei et al. 2006; Johnson, Tsutsui et al. 2007; Murakami, Matsuzaki et al. 2008). In some reports, however, lack of effect has been shown (Anderson, Relf et al. 2009; Rizwan, Porteous et al. 2009). Such reports have raised skepticism as to whether GnIH plays a significant role in mammalian gonadotropin secretion [reviewed in (Smith and Clarke 2010)]. 5
22 Table 1. GnIH peptides that have been identified in mammals (bird and fish peptide as a comparison) Species Peptide Sequence Reference Quail Human Rat Mouse Bovine Goldfish Hamster White Crowned Sparrow Starling Monkey GnIH GnIH RP 1 GnIH RP 2 RFRP 1 RFRP 2 RFRP 3 RFRP 1 RFRP 3 RFRP 1 RFRP 3 RFRP 1 RFRP 3 gflpxrfa 1 gflpxrfa 2 gflpxrfa 3 RFRP 1 RFRP 3 GnIH GnIH RP 1 GnIH RP 2 GnIH GnIH RP 1 GnIH RP 2 RFRP 3 SIKPSAYLPLRF SLNFEEMKDWGSKNFMKVNTPTVNKVPNSVANLPLRF SSIQSLLNLPQRF MPHSFANLPLRF SAGATANLPLRS VPNLPQRF VPHSAANLPLRF ANMEAGTMSHFPSLPQRF VPHSAANLPLRF NMEAGTMSHFPSLPQRF SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRF AMAHLPLRLGKNREDSLSRWVPNLPQRF SPAPANKVPHSAANLPLRF ILSRVPSLPQRF SIKPFSNLPLRF SLNFEEMEDWGSKDIIKMNPFTASKMPNSVANLPLRF SPLVKGSSQSLLNLPQRF SIKPFSNLPLRF SLNSEDMEDWGSKDIIKMNPFTASKMPNSVANLPLRF SPLLKGSSQSLLNLPQRF SLTFEEVKDWAPKIKMNTPAVNKMPPSAANLPLRF VPNLPQRF SGRNMEVSLVRQVLNLPQRF SGTGLSATLPQRF (Tsutsui, Saigoh et al. 2000) (Satake, Hisada et al. 2001) (Satake, Hisada et al. 2001) (Hinuma, Shintani et al. 2000; Ubuka, Morgan et al. 2009b) (Hinuma, Shintani et al. 2000) (Hinuma, Shintani et al. 2000; Ubuka, Morgan et al. 2009b) (Hinuma, Shintani et al. 2000) (Hinuma, Shintani et al. 2000) (Ukena and Tsutsui 2001) (Hinuma, Shintani et al. 2000) (Hinuma, Shintani et al. 2000; Fukusumi, Habata et al. 2001) (Yoshida, Habata et al. 2003) (Sawada, Ukena et al. 2002) (Sawada, Ukena et al. 2002) (Sawada, Ukena et al. 2002) (Kriegsfeld, Mei et al. 2006) (Kriegsfeld, Mei et al. 2006) (Osugi, Ukena et al. 2004) (Osugi, Ukena et al. 2004) (Osugi, Ukena et al. 2004) (Ubuka, Kim et al. 2008) (Ubuka, Kim et al. 2008) (Ubuka, Kim et al. 2008) (Ubuka, Lai et al. 2009a) Modified from (Clarke, Qi et al. 2009). When the work in this thesis was begun, there was still some considerable doubt as to whether GnIH is an important regulatory peptide in mammals. It was hypothesized that GnIH is a negative regulator of GnRH cells and/or gonadotropin synthesis and/or secretion by the pituitary. A better understanding of how such inhibition might occur would enhance our knowledge of the regulation of reproductive function in mammals. It was also considered possible that GnIH may regulate other systems, such as those that control food intake, based on some earlier studies in rats (Johnson, Tsutsui et al. 2007). This thesis examines the role of GnIH in the neuroendocrine regulation of reproduction the ewe. Accordingly, the following review focuses on the reproductive neuroendocrine 6
23 system in the ewe and a consideration of the state of knowledge regarding the action of GnIH. 1.2 The Hypothalamo Pituitary Gonadal Axis GnRH is a decapeptide which was originally isolated from porcine brain by Schally and coworkers (Schally, Arimura et al. 1971; Matsuo, Baba et al. 1976). GnRH is released from GnRH nerve terminals in the external zone of the ME into the hypophysial portal vessels, to act on the gonadotropes of the anterior pituitary gland (Fink, Chiappa et al. 1976). At the level of the gonadotrope, GnRH acts to stimulate synthesis as well as secretion of gonadotropin, luteinizing hormone (LH) and follicle stimulating hormone (FSH). The gonadotropins then circulate in the peripheral blood system to act on the gonads to control the synthesis and secretion of the ovarian steroid and ovarian follicle peptide as well as inhibin (Clarke 1996) GnRH cell distribution and projections within the ovine brain In the ovine brain, GnRH neurons are found in the medial preoptic area (MPOA) at the level of the organum vasculosum of the lamina terminalis (OVLT), with a few cells in the arcuate nucleus (ARC) and the ventromedial nucleus of hypothalamus (VMN). Occasional cells are also seen in the bed nucleus of the stria terminalis (BnST), the perifornical area, the supraoptic nucleus and paraventricular nucleus (PVN) (Lehman, Robinson et al. 1986). GnRH neurons from the preoptic area (POA) project to the median eminence (ME) and OVLT (Samson, Snyder et al. 1980; Lehman, Robinson et al. 1986). The ME of mammalian species contains the greatest amount of GnRH, reflecting the fact that GnRH is stored in 7
24 neuronal terminals prior to release into hypophysial portal blood (Polkowska, Dubois et al. 1980). The mutation in the GnRH genes causes hypogonadism which has been demonstrated in the hypogonadal mouse model (Cattanach, Iddon et al. 1977). The deletion of exons III and IV of the GnRH gene leads to the failure of gonadal development in the hypogonadal mouse (Mason, Hayflick et al. 1986). This demonstrates the principle that GnRH is the primary factor responsible for sexual development and the function of the reproductive axis (Pfaff and Keiner 1973). The necessity of GnRH secretion for successful reproductive function has also been demonstrated in studies where hypothalamo pituitary disconnection (HPD) of ewes leads to the loss of LH and FSH synthesis and secretion (Clarke, Cummins et al. 1983; Hamernik, Crowder et al. 1986). HPD is a surgical technique which effectively isolates the pituitary gland from the hypothalamus without disturbance of blood supply to the pars distalis of pituitary gland, providing a model of an isolated pituitary gland for in vivo study (Clarke, Cummins et al. 1983; Mercer, Clements et al. 1989). Similarly, ARC lesions in monkey reduce LH and FSH concentration. In both primate and the sheep models of isolation of the pituitary gland, gonadotropin secretion can be restored by the pulsatile administration of GnRH (Plant, Nakai et al. 1978; Clarke, Cummins et al. 1983). Furthermore, the essential requirement for GnRH in the reproductive process is seen in animals immunized against GnRH; this lowers LH and FSH levels and prevents estrous cycles and ovulation (Clarke, Fraser et al. 1978). 8
25 1.3 The Estrous Cycle of the Ewe The female estrous cycle is characterised by four distinct phases, being the luteal, the follicular and pre ovulatory phases and the metestrous period [(Clarke 1996) reviewed in (Clarke and Pompolo 2005)]. Estrus refers to the period of time before and during ovulation when females display sexual behaviour and are receptive to males, and differs among species. In the ewe, the estrous cycle is days (Marshall 1904) and each phase is characterised by a particular pattern of GnRH, LH, FSH, estrogen and progesterone secretion [reviewed in (Clarke and Pompolo 2005)]; this is detailed in Figure 1. 9
26 Figure 1. Schematic representation of the ovine estrous cycle. During the luteal phase progesterone is the main negative regulator of GnRH pulse frequency. During the luteal phase, estrogen acts in concert with progesterone to exert a negative feedback effect. In the follicular phase, the absence of progesterone allows an increase in GnRH pulse frequency and estrogen; the latter reduces GnRH pulse amplitude. The increase in GnRH pulse frequency causes the reduction in LH pulse amplitude due to an inverse relationship between the two (Wildt, Hausler et al. 1981; Clarke, Cummins et al. 1984). In the midfollicular (preovulatory) phase, at a critical level of estrogen, there is a switch from negative feedback action of estrogen to a positive feedback effect. This switch is a unique phenomenon that drives the preovulatory GnRH/LH surge (Clarke and Pompolo 2005) The Luteal Phase The luteal phase of the estrous cycle is characterized by high progesterone secretion from the corpus luteum, with basal levels of estradiol which is secreted by ovarian follicles. During this period, high amplitude, low frequency GnRH pulses occur (Clarke 1987a), as a result of the elevated progesterone levels (Karsch 1987). In concordance with the general 10
27 principle (GnRH: LH), LH pulses also occur with high amplitude and low frequency at this time (Clarke 1987a; Moenter, Caraty et al. 1991; Clarke 1995a; Clarke 1995b). This pattern of GnRH/LH secretion is also seen in the luteal phase of the menstrual cycle in the nonhuman primate (Goodman and Karsch 1980; Ellinwood, Norman et al. 1984) and women (Yen, Lasley et al. 1975). FSH levels are relatively constant throughout the luteal phase (Salamonsen, Jonas et al. 1973). At the level of the pituitary gonadotrope, progesterone does not directly affect LH release (Clarke and Cummins 1984; Girmus and Wise 1991) but progesterone is thought to enhance negative feedback of estrogen on the pituitary (Clarke and Cummins 1984; Clarke, Cummins et al. 1989). On the other hand, others (Batra and Miller 1985a; Batra and Miller 1985b) have provided evidence that progesterone alone reduces GnRH stimulated LH and FSH secretion by direct action on gonadotropes. It has been suggested that progesterone elicits this effect by decreasing GnRH receptor number (Batra and Miller 1985a). Whether this is a major means of feedback by progesterone, directed at the gonadotrope, remains somewhat open to question and the predominant effect of progesterone is thought to be the regulation of pulsatile GnRH secretion (Clarke 1996) The Follicular Phase The follicular phase begins when progesterone levels fall due to the demise of the corpus luteum. Thus, during the follicular phase, plasma progesterone levels are low and estrogen secretion from the ovary increases as a result of growing ovarian follicles, under the positive influence of increased GnRH/LH pulse frequency (Baird and Scaramuzzi 1976; Clarke, Cummins et al. 1987). The fall in progesterone removes one component of negative feedback on GnRH neurons, resulting in an increase in GnRH pulse frequency 11
28 and a subsequent increase in LH secretion (Karsch, Foster et al. 1983), without affecting LH pulse amplitude (Clarke 1995a). During this phase, estrogen exhibits negative feedback on both the hypothalamus and pituitary (Clarke 1996). Thomas et al 1988 suggested that the progressive decline in LH amplitude during the follicular phase of the ewe is likely due to a combination of negative feedback actions of estrogen and the increase of LH pulse frequency. The direct effect of estrogen on the pituitary and the possible progesterone involvement in the action of estrogen has been studied in ovariectomised HPD ewes in which the GnRH input was kept constant (Clarke and Cummins 1984; Clarke and Cummins 1985). The LH response to estrogen is greater in OVX/HPD animals primed with progesterone than in those given estrogen alone (Clarke and Cummins 1984). During the follicular phase, FSH levels are at a nadir as a result of both rising estrogen levels and the increase in plasma inhibin levels (inhibin is produced by ovarian follicles) (Baird, Swanston et al. 1981). Mid way through the follicular phase estrogen levels reach a threshold and, being unconstrained by progesterone, this results in a switch from negative to positive feedback (Clarke 1995a). The positive feedback event is due to a rise in GnRH secretion to surge levels and an increase in the responsiveness of the pituitary gonadotrophs to GnRH (Clarke 1995a; Clarke 1995b). The mechanism of positive feedback will be discussed in Section
29 Since LH secretion is initially clamped during the follicular phase and held at tonic levels, it has been suggested that there is some co ordinated time delay event between the hypothalamus and the pituitary gland in response to the rising levels of estrogen (Clarke 1995a). The time delayed effect of estrogen is seen in OVX ewes which are given a single injection of estrogen, whereby these animals produce a surge within hours following estrogen treatment (Clarke 1993). Others have shown a similar time delay in the positive feedback event in progesterone primed OVX ewes. In this case a model of the estrous cycle has been generated by Karsch et al (Goodman and Karsch 1981; Goodman, Legan et al. 1981; Karsch, Foster et al. 1983; Moenter, Caraty et al. 1991; Moenter, Brand et al. 1992; Moenter, Caraty et al. 1993; Evans, Dahl et al. 1994; Evans, Dahl et al. 1997; Harris, Dye et al. 1999). Early studies (Scaramuzzi, Tillson et al. 1971) showed that maintenance of tonic high progesterone levels blocked the estrogen induced LH surge in OVX ewes and this was later shown to be due to a blockade of the GnRH surge (Kasa Vubu, Dahl et al. 1992). Another useful model in the sheep is that of anestrus, because these animals are ovaryintact but do not experience estrous cycles (Goding, Catt et al. 1969; Clarke 1988). In such animals, a single intramuscular injection of estrogen causes a GnRH/LH surge within hours (Clarke 1988). Interestingly, the GnRH surges that are obtained in anestrous ewes in response to an injection of estrogen are greater magnitude than those in estrogentreated OVX ewes (Clarke 1988; Clarke 1993). 13
30 1.3.3 The Preovulatory LH Surge. As indicated above, the preovulatory surge phase is characterized by an increase in the secretion of GnRH, an increase in the pituitary gonadotrope responsiveness to GnRH and the resultant outpouring of LH from the anterior pituitary (Clarke 1987a; Clarke 1995b). GnRH secretion increases by fold at the time of LH surge (Moenter, Caraty et al. 1991) and GnRH secretion continues for some hours after the termination of the LH surge reflecting the exhaustion of LH stores within the pituitary; over 90% of the pituitary LH stores are secreted during this time (Roche, Foster et al. 1970). The preovulatory LH surge is the result of a unique positive feedback event that is caused by estrogen (Baird, Swanston et al. 1981; Clarke and Cummins 1985; Caraty, Locatelli et al. 1989). It has been suggested that the full expression of the positive feedback of estrogen is initiated by the increased frequency and/or amplitude of GnRH pulses (Clarke, Cummins et al. 1989; Clarke 1993), although others have argued that the pulsatile mode of secretion of GnRH is lost at this time (Karsch and Evans 1996). Arguing for the former, it has been demonstrated that the increase in the GnRH pulse frequency from an hourly to 2pulses/hour increases the positive feedback effect of estrogen on LH surge (Clarke, Cummins et al. 1989) and pulses of GnRH are more effective than continuous treatment in the priming the pituitary gland for the positive feedback event (Phillips, Cummins et al. 1990). In the ewe, it is thought that estrogen acts within mediobasal hypothalamus (MBH) on the neurons that contain ERα which then relay positive and negative feedback signals to GnRH cells within the POA (Clarke, Pompolo et al. 2001; Pompolo, Rawson et al. 2001; Pompolo, Pereira et al. 2003a). As indicated in the introduction, neither ERα nor 14
31 progesterone receptors are expressed by GnRH neurons (Shivers, Harlan et al. 1983; Watson, Skolnik et al. 1992). ERβ is expressed by GnRH neurons, as demonstrated in the rat (Herbison and Pape 2001; Hrabovszky, Steinhauser et al. 2001), however it is appears that ERβ plays a minor role in the feedback of sex steroids on GnRH neurons (Krege, Hodgin et al. 1998). Thus, it is understood that an intermediary pathway must be involved in the steroid feedback mechanism. Such interneuronal pathways may form a complex network of serial and converging systems arising from different regions of the brain. These include neurons that produce noradrenaline (NA), NPY, kisspeptin, GABA, dopamine, enkephalin (ENK), GLU, melanocyte stimulating hormone (MSH), β endorphin (βend), galanin (GAL), orexin (ORX), melanin concentrating hormone (MCH) and POMC (Estrada, Pompolo et al. 2003; Clarke and Pompolo 2005; Smith, Cunningham et al. 2005; Estrada, Clay et al. 2006; Clarke and Tilbrook 2009). 1.4 GnRH and Regulation of GnRH secretion Factor influencing GnRH cells and GnRH secretion Prior to relevant technology, it was considered that GnRH secretion into the hypophysial portal vessels was pulsatile (Sarkar, Chiappa et al. 1976) but final evidence for this was not forthcoming until a model was developed in sheep (Clarke and Cummins 1982). GnRH pulses vary in terms of frequency and amplitude as a result of synchronous release from multiple GnRH neurons (Clarke and Cummins 1982; Clarke 1996). It has been hypothesised that there is the pulse generator system which is responsible for the 15
32 control of pulsatile GnRH and gonadotropin secretion (O'Byrne and Knobil 1993), but this has never been defined at the cellular level. It has been suggested that GnRH neurons may have intrinsic phasic properties that result in pulsatile secretion (Cardenas, Ordog et al. 1993). In addition, Lehman and co workers (1986, 1988) (Lehman, Robinson et al. 1986; Lehman, Karsch et al. 1988) suggest that the phasic activity of GnRH neurons may be caused by the connections existing between GnRH cell bodies. Others suggest that the pulse generator resides within the hypothalamus (Blake and Sawyer 1974; Krey, Butler et al. 1975), but other extrahypothalamic pulse generator systems may exist (Clifton and Sawyer 1979). It has been further assumed that GnRH neurons exhibit inherent pulsatility after GnRH neurons from the olfactory placode of rhesus monkey and embryonic rat cultures displayed a pulsatile nature (Terasawa, Keen et al. 1999; Funabashi, Daikoku et al. 2000). GnRH neurons are influenced by various neuronal systems in various brain areas that either contain or do not contain ERα. For example many GnRH regulating cells located in the ARC, the VMN, the BnST, the POA and brain stem have been found to express express ERα, whereas the cells of the lateral hypothalamus area do not express ERα but may influence GnRH secretion through multi synaptic pathways [reviewed in (Clarke and Tilbrook 2009)]. The cells of these areas may also modulate GnRH secretion via a range of neurotransmitters. The BnST and POA regions of the sheep contain GABA and GLU cells (Pompolo, Scott et al. 2002; Pompolo, Pereira et al. 2003a), the ARC/VMN region contains cells that produce dopamine (DA), NPY, MSH, βend, ENK, GLU, GABA, and GAL and the cells of the lateral hypothalamus produce ORX and MCH [reviewed in (Clarke and Tilbrook 2009)]. In the brain stem cells in various groupings produce NA (Rawson, Scott et al. 2001; 16
33 Clarke and Tilbrook 2009; Pereira, Rawson et al. 2010) and recently, it has been demonstrated that A1 and A2 regions in the brain stem which contain NA neurons project to the BnST in the ewe brain (Pereira, Rawson et al. 2010). These neurons express ERα and are stimulated by estrogen. Input to GnRH cells may be indirect via the BnST (Pereira, Rawson et al. 2010). NA neurons may further provide direct input to GnRH cells as 20% of GnRH cells in the POA receive contact from terminals that contain dopamine βhydroxylase (DBH), a marker for NA neurons (Pompolo, Pereira et al. 2003b) Sex steroid feedback mechanisms The function of GnRH cells is influenced by indirect sex steroid feedback within the brain, via cells expressing the relevant sex steroid receptors (Herbison 1998). There are three different types of steroidal feedback namely short term negative, long term negative and positive feedback (Clarke, Thomas et al. 1987) Negative feedback The short term negative feedback actions of estrogen can be demonstrated by the single injection of estrogen to an OVX female sheep (Clarke and Cummins 1984). Such a feedback mechanism may be operative in the early phases of the female estrous cycle (Thomas, Martin et al. 1988), but is not a generally overt phenomenon at other stages of the estrous cycle nor in males. Long term or chronic negative feedback pertains throughout most of the female estrous cycle, in males and during the seasonal non breeding season (see below) in both sexes. The negative feedback effect of testosterone in males is due to effects on the brain via 17
34 regulation of GnRH secretion and is not by direct action on the pituitary gland (Tilbrook and Clarke 2001). Thus, testosterone inhibits GnRH pulse frequency in the castrated rams (Tilbrook, de Kretser et al. 1991). During the luteal and follicular phases of the estrous cycle of the ewe, progesterone and estrogen act in combination to inhibit GnRH pulse frequency. During the follicular phase, chronic estrogen negative feedback increases the frequency and reduces the amplitude of GnRH/LH pulses (Karsch 1987; Karsch, Cummins et al. 1987; Thomas, Martin et al. 1988). The neural elements that mediate these feedback effects of sex steroids have been detailed for the ovine brain and are reviewed in (Goodman 1996) One major neurotransmitter that is involved in the negative feedback effect is the GABA and GABAergic cells which possess the relevant sex steroid receptors. For example, in the ovine brain a high percentage of cells that are immunopositive for Glutamic decarboxylase (GAD) possess ERα (Herbison, Robinson et al. 1993). GABA input to GnRH cell bodies appear to inhibit GnRH neurons in the ewe (Pompolo, Scott et al. 2002), and a reduction in GABA activity may permit the positive feedback effect of estrogen (Robinson, Kendrick et al. 1991; Scott and Clarke 1993a). Accordingly, Glutamic decarboxylase isoform 65 (GAD 65 ) mrna levels are higher in both BnST and POA during the luteal phase and fall prior to the LH surge (Robinson, Kendrick et al. 1991; Pompolo, Scott et al. 2002). In OVX and estrogen treated ewes, both GABA A agonist and GABA B antagonist inhibited LH secretion, but a GABA B agonist had no effect on LH release (Scott and Clarke 1993a; Scott and Clarke 1993b). This transmitter is clearly involved in feedback effects of estrogen but the exact role that it plays remains unclear. 18
35 The so called negative feedback of estrogen may also be due to removal of a positive effect on GnRH neurons. Indeed, it has been observed that ovariectomy of ewe causes a significant up regulation of Kiss1 mrna and kisspeptin in the ARC and replacement with estrogen reverses this effect (Smith, Clay et al. 2007; Smith, Rao et al. 2008; Clarke, Smith et al. 2009; Smith, Li et al. 2009). The role of kisspeptin is discussed below. The negative feedback of progesterone in the brain may be mediated by opioid neurons (Goodman 1996). Treatment with naloxone, an opioid antagonist, in the early and midluteal phases of the ewe estrous cycle (but not the follicular phase) increases GnRH/LH frequency (Brooks, Lamming et al. 1986). Furthermore, naloxone treatment into the medial preoptic area (MPOA) or the mediobasal hypothalamus (MBH) of the ewe increases plasma LH concentrations during the luteal phase (Weesner and Malven 1990). Treatment with the opioid antagonist WIN , specific for the kappa opioid receptor subtype, increases LH pulse frequency during the luteal phase of the ewe estrous cycle (Wood 1983; Whisnant and Goodman 1988) and in the progesterone treated OVX ewe (Whisnant and Goodman 1988; Yang, Haynes et al. 1988). Implantation of WIN into POA and MBH of the ewe also stimulates LH pulse frequency (Whisnant, Havern et al. 1991). The involvement of the opiate βend is less clear as intracerebroventricular (ICV) administration of this endogenous peptide during the luteal phase of the ewe did not affect LH release (Horton, Francis et al. 1989), questioning the importance of this particular endogenous opioid in the feedback regulation of GnRH. In addition, POMC mrna levels remain unchanged in the ARC of the ewe across the estrous cycle (Walsh, Rao et al. 1998). Since POMC encodes βend (Wilkinson 2006), the participation of this system in feedback regulation is uncertain. Other endogenous opiates which have 19
36 inhibitory effects on LH frequency are enkephalin and dynorphin [reviewed in (LJennes, Ulloa Aguire et al. 2009)]. Treatment with methionine enkephalin analogue FK during the follicular phase of the ewe decreases episodic LH secretion and replacement with naloxone in combination with FK reverses this effect (Brooks, Lamming et al. 1986). Furthermore, (Walsh and Clarke 1998) showed that enkephalin levels vary during the estrous cycle of the ewe, strongly suggesting a role in GnRH feedback regulation as enkephalin neurons in rodents express ERα (Olster and Blaustein 1990; Akesson and Micevych 1991; Yuri and Kawata 1994; Zhu and Pfaff 1995; Simerly, Young et al. 1996; Walsh, Rao et al. 2001). Other studies by (Goodman, Coolen et al. 2004) suggest a role for dynorphin in the negative feedback actions of progesterone in the brain as dynorphin neurons express the progesterone receptor and contact GnRH perikarya (Foradori, Coolen et al. 2002; Goodman, Coolen et al. 2004). Almost all dynorphin neurons in the ARC of the ewe also contain kisspeptin, suggesting that these are master neurons involved in the negative feedback action of progesterone as well as estrogen (Goodman, Lehman et al. 2007). The melanocortin system may have a role in the control of GnRH secretion, as the treatment with melanocortin agonist melanotan II (MTII) increases mean plasma LH levels and LH pulse frequency in the ewe during the luteal phase (Backholer, Smith et al. 2009). Furthermore, MTII treatment increases the expression of orexin mrna in the dorsomedial hypothalamus (DMH) and Kiss1 mrna in the POA but decreases Kiss1 mrna in the ARC (Backholer, Smith et al. 2009). The melanocortin system may thus regulate GnRH cells via the activation of kisspeptin cells in the POA and/or orexin cells in the DMH. 20
37 Seasonality and regulation of GnRH cells Sheep are seasonal breeders, with breeding activity activated by reducing day length (Lincoln and Short 1980; Karsch, Bittman et al. 1984). The non breeding season or anestrus is a special case with respect to estrogen feedback. Classic studies conducted by (Legan, Karsch et al. 1977) showed that the sensitivity of the hypothalamo pituitary axis to estrogen in the female sheep is increased in the non breeding season. During the nonbreeding season when the response to the negative feedback of estrogen is high, estrogen is unable to cause an increase LH secretion even though there is no difference in binding of estrogen to plasma protein between anestrus and breeding season (Legan, Karsch et al. 1977). Estrogen treatment eliminates GnRH pulses in the ovary intact ewe during the anestrous season (Karsch, Cummins et al. 1987; Karsch, Dahl et al. 1993). There are strong indications for the involvement of the A15 dopaminergic system as a regulatory centre that directs seasonal breeding in sheep (Lehman, Durham et al. 1996). Dopaminergic antagonist (pimozide) treatment increases LH pulse frequency in OVX estrogen treated anestrous ewes suggesting a role for DA neurons in the negative feedback of anestrous ewes (Meyer and Goodman 1985). Furthermore, injection of the neurotoxin 6 hydroxy dopamine into the region of the A15 consistently decreases the ability of estrogen to inhibit LH pulse frequency in anestrous ewes (Thiery, Martin et al. 1989). Thus, dopaminergic D2 receptors are involved in the estrogen dependent inhibition of GnRH secretion during anestrus in the ewe (Thiery, Gayrard et al. 1995). The possible role of GLU input to A15 neurons has been demonstrated in ovary intact anestrous ewes as local administration into the A15 of either a N methyl D aspartate (NMDA) receptor or kainite/α amino 3 hydroxyl 5 methyl 4 isoxazole propionate (AMPA) receptor antagonist stimulates LH pulse frequency and mean LH levels (Eyigor and Jennes 21
38 2000). Immunohistocemistry data from estrogen treated OVX ewes shows that GLU input to A15 neurons is higher in anestrous than during the breeding season, suggesting that in anestrus, estrogen suppresses GnRH pulse frequency by stimulating release of GLU from synapses onto inhibitory A15 DA neurons afferent to GnRH cells (Singh, Hileman et al. 2009). More recently it has become apparent that the function of kisspeptin cells is involved in the seasonal change in breeding activity in the ewe. Kisspeptin input to GnRH neurons in MBH, and Kiss1 mrna in the ARC are higher in the breeding season compared to the nonbreeding season in OVX estrogen treated ewes (Smith, Coolen et al. 2008). Kiss1 mrna in the POA of the OVX ewe is similar in anestrous and the breeding seasons (Clarke, Smith et al. 2009), however following estrogen treatment to OVX ewes, expression of Kiss1 mrna in the POA of ewe is seen to be higher in the non breeding season (Smith, Coolen et al. 2008). Since season does not have any effect on kisspeptin expression in the POA, it is suggested that kisspeptin neurons in the POA are not fundamentally involved in the transition from breeding to non breeding seasons and vice versa (Smith, Coolen et al. 2008). Kisspeptin treatment peripherally increases LH levels in ewes during both the nonbreeding and the breeding season with the highest LH response to kisspeptin during the non breeding season (Smith, Saleh et al. 2009). This higher response may be because of the lower LH pulse frequency during the anestrous season (Clarke 1988), allowing for a build up of releasable stores of LH in gonadotropes. Studies on kisspeptin involvement in seasonality of breeding in the Syrian and Siberian hamster present a more complex picture, because kisspeptin levels in the ARC, 22
39 determined by immunohistochemistry, were seen to be low in Syrian hamster but high in Siberian hamster during short day (SD) photoperiod when the animals are sexually inactive (Revel, Saboureau et al. 2006; Simonneaux, Ansel et al. 2009). Further studies demonstrate down regulation of Kiss1 mrna expressions in the ARC and anteroventral periventricular nucleus (AVPV) of male and female Syrian hamster during SD exposure (Ansel, Bolborea et al. 2010). Since this species is a long day breeder, the results are somewhat perplexing. Treatment with melatonin in long day (LD) adapted Siberian hamsters and pinealectomy before SD exposure suggest that melatonin drives the change in Kiss1 mrna expression in response to photoperiod (Ansel, Bolborea et al. 2010) Positive feedback The positive feedback of estrogen occurs during the estrous/menstrual period, due to rising levels of estrogen in the follicular phase. This specific effect of an acute and transient rise in estrogen to cause an LH surge has been demonstrated by estrogen treatment of seasonally anestrous ewes (Goding, Catt et al. 1969), OVX ewes (Radford, Wheatley et al. 1969), estrogen primed OVX rats (Caligaris, Astrada et al. 1967), OVX monkeys (Yamaji, Dierschke et al. 1972) and eugonadal women (Swerdloff and Odell 1968). It has been suggested that this action requires a reduction in the negative tone of the opioid system because treatment with the opioid agonist FK delays the LH surge in ewes (Brooks, Lamming et al. 1986). Moreover, central treatment with FK to the ewe reduces the magnitude of LH surge (Knight, Stansfield et al. 1990). However, icv injection of βend in the early follicular phase of the ewe does not affect the onset of LH 23
40 surge (Curlewis, Naylor et al. 1991). The involvement of opioid systems in the positive feedback mechanism (reduction in tone), was further questioned by another study which showed that neither morphine nor naloxone treatment altered the estrogen induced LH surge response in anestrous ewes (Horton and Clarke 1988). The role of NPY in the positive feedback of estrogen has been demonstrated after passive immunization of ewes with NPY antibodies delayed the onset of LH surge (Porter, Naylor et al. 1993). Injection of specific anti NPY antibody or antisense oligonucleotides blocks the estrogen induced LH surge in rats (Wehrenberg, Corder et al. 1989; Kalra, Bonavera et al. 1995). NPY cells provide synaptic input to GnRH cell bodies in the sheep and NPY Y1 receptor protein is present in many GnRH fibers and terminals in the OVLT and ME of rats (Porter, Naylor et al. 1993; Li, Chen et al. 1999). The NPY input to GnRH cells may, however, arise from the ARC population of NPY cells or the noradrenergic cells of the brain stem that co produce NPY (Pompolo, Pereira et al. 2003a). Rat studies suggest that the activation of the NPY Y1 receptor subtype is required for the occurrence of a steroidinduced LH surge (Kalra and Crowley 1992; Leupen, Besecke et al. 1997). NPY Y1 receptor expression in the hypothalamus is increased at the time of proestrous in rats (Xu, Urban et al. 2000). In sheep the effect of NPY on GnRH cells is most likely mediated through Y2 receptors as treatment with the Y2 receptor agonist (human PYY 3 36 ) delays the timing of LH surge in the ewes (Clarke, Backholer et al. 2005). Thus NPY effects on GnRH cells appear to differ between species. Initial studies showed that intravenous injection of phenoxybenzamine (an α receptor antagonist) in the ewe did not block the estrogen induced LH surge (Jackson 1977). 24
41 However, in the rat, estrogen increases the firing rate of A1 cells of the ventrolateral medulla of the brain stem and increases NA secretion in these cells on the afternoon of proestrus, indicating the possible involvement of NA neurons in the positive feedback of sex steroids (Kaba, Saito et al. 1983; Mohankumar, Thyagarajan et al. 1994). The activation of A1 neurons is observed in OVX ewes within 1 hour injection of estrogen (Rawson, Scott et al. 2001). Similar results are obtained in OVX, estrogen primed progesterone treated rats (Temel, Lin et al. 2002). Additionally, NA release is increased in the ME of the rat and sheep at the time of the preovulatory LH surge (Sheaves, Warburton et al. 1984; Domanski, Chomicka et al. 1991). Robinson et al further confirmed this concept in the ewe by demonstrating an increase in the frequency of NA pulses, measured by brain dialysis, at the time of preovulatory LH surge. Treatment with α 1 adrenergic receptor antagonist blocks the LH surge in rats and reduces the magnitude of LH surge in the ewes (Drouva, Laplante et al. 1982; Le, Berghorn et al. 1997; Clarke, Scott et al. 2006). Moreover, during the follicular phase of the ewe estrous cycle, the number of varicosities immunostained for dopamine β hydroxylase (DBH) in the dorsal and lateral BnST is decreased, indicating an increase in NA release in these areas. Collectively, these data indicate that the noradrenergic system is involved in generation of the GnRH/LH surge via projections to the BnST and relay to GnRH cells (Clarke, Scott et al. 2006). Based on data from ewes it has been proposed that NA is a permissive factor for LH surge (Clarke, Scott et al. 2006). The MBH is the major site of estrogen positive feedback action in the ewe (Blache, Fabre Nys et al. 1991; Caraty, Fabre Nys et al. 1998) and, as indicated in the introduction, kisspeptin plays a major role in the positive feedback regulation of GnRH. Kiss1 mrna and 25
42 kisspeptin protein expression in the ARC (Estrada, Clay et al. 2006; Smith, Li et al. 2009) and the lateral POA (Smith, Li et al. 2009) of the ewe increase just prior to and during the LH surge. Further studies have revealed that kisspeptin cells in the ARC of ewe coexpress neurokinin B (NKB), a positive mediator of GnRH secretion (Goodman, Lehman et al. 2007). Kisspeptin neurons located in the caudal extent of the ARC are involved in generating the preovulatory GnRH/LH surge in the ewe (Estrada, Clay et al. 2006; Smith, Li et al. 2009). Continuous infusion of kisspeptin synchronizes the LH surge in progesterone primed cyclic ewes and causes ovulation in seasonally acyclic ewes (Caraty, Smith et al. 2007). In the late follicular phase kisspeptin treatment effectively increases LH secretion (Smith, Saleh et al. 2009). Further studies have demonstrated that the kisspeptin antagonist, peptide 234, blocks kisspeptin induced firing of GnRH neurons in transgenic female mice, inhibits kisspeptin stimulated LH secretion in male rats and mice, and reduces LH pulses in OVX ewes (Roseweir, Kauffman et al. 2009). Collectively, these data indicate that kisspeptin neurons which also express steroid receptors (Smith, Cunningham et al. 2005; Franceschini, Lomet et al. 2006; Smith, Popa et al. 2006; Smith, Clay et al. 2007) play a major role in the generation of the positive feedback effect of estrogen to generate the preovulatory GnRH/LH surge. The GLU system within the hypothalamus may also be involved in the positive feedback of estrogen. GLU is released in the POA just prior to and during the steroid induced LH surge in rats (Demling, Fuchs et al. 1985; Jarry, Hirsch et al. 1992; Ping, Mahesh et al. 1994; Jarry, Leonhardt et al. 1995). GLU neurons in the ARC of the ewe are stimulated after 26
43 estrogen treatment (Blache, Fabre Nys et al. 1991; Caraty, Fabre Nys et al. 1998; Pompolo, Pereira et al. 2003a). In addition, several studies indicate GLU receptors such as the kainate receptor (KA2) and AMPA receptors (GluR1, GluR3 and GluR5), expressed on GnRH neurons, are activated during the steroid induced LH surge (Eyigor and Jennes 2000; Bailey, Centers et al. 2006). 1.5 Regulation of LH and FSH Subunit Gene Expression in the Pituitary Gonadotropes are the pituitary cells that synthesise and secrete gonadotropins under the control of GnRH. The gonadotropins, LH and FSH, are members of the pituitary glycoprotein hormone family (Canfield, Birken et al. 1978) and comprise of two peptide chains, namely alpha (α) and beta (β). The α subunit is the same for all the glycoproteins within a species and is encoded by a single gene (Boothby, Ruddon et al. 1981). In contrast, the β subunit is different for each pituitary hormone (Jutisz and Tertrin Clary 1974). The α subunit contains two asparagine linked carbohydrate chains, while the β subunit chain contains one or two of these chains (Sairam and Li 1975). Some studies have been conducted to elucidate the regions which are involved in receptor interaction and antibody binding (Ward and Moore 1979; Keutmann, Charlesworth et al. 1987; Keutmann, Charlesworth et al. 1988; Ryan, Charlesworth et al. 1988; Charlesworth, Bergert et al. 1991) and have defined the region of the LHβ subunit that is important for specificity binding (Ward and Moore 1979); other regions are important for receptor activation and secondary structure (Ryan, Charlesworth et al. 1988; Keutmann 1992). Additionally, two dominant regions of the α subunit are involved in antibody binding (Charlesworth, Bergert et al. 1991). The amino acid sequences of the α and β subunits shows homology across species, for example, the alpha subunit of the rat and the mouse 27
44 have 96% homology, whereas ovine and human homology is 74%. For the FSHβ subunit homology between ovine and bovine is 93% and for the LHβ subunit of the rat and dog, homology is 87% (Chin 1985). The genes for the gonadotropin subunits have been cloned and sequenced in most important laboratory and domesticated species. The α and β subunits are encoded by separate genes (Gharib, Wierman et al. 1990). The α subunit gene consists of four exons and three introns while both the LHβ and FSHβ genes have three exons and two introns (Gharib, Roy et al. 1989). The expression of the α subunit gene has been determined in some species such as human (Fiddes and Goodman 1979), mouse (Chin, Kronenberg et al. 1981), rat (Godine, Chin et al. 1982), bovine (Nilson, Nejedlik et al. 1983), primate and equine (Fenstermaker, Farmerie et al. 1990). The expression of the FSHβ subunit has been determined in the cow (Esch, Mason et al. 1986), the rat (Maurer 1987), the human (Watkins, Eddy et al. 1987), and the pig (Kato 1988), while LHβ gene has been characterized in the rat (Chin, Godine et al. 1983), human (Talmadge, Vamvakopoulos et al. 1984), cow (Maurer 1985) and dog (Wolf, Appleby et al. 1987) Regulation of gonadotropin subunit gene expression by GnRH During embryogenesis, basal GnRH gene expression is activated and maintained throughout development, but may be independent of GnRH stimulation. As GnRH secretion increases, basal gonadotropin gene expression is up regulated reaching its maximum at puberty (Brown and McNeilly 1999). GnRH stimulation of the gonadotrope is an absolute requirement for the maintenance of gonadotropin synthesis as demonstrated in OVX/HPD sheep. In this model, pituitary gland expression of gonadotropin subunits 28
45 ceases in the absence of GnRH replacement, (Hamernik, Crowder et al. 1986; Mercer, Clements et al. 1989). GnRH pulsatility is necessary to stimulate the synthesis of gonadotropin subunits in the sheep and rat pituitary (Leung, Kaynard et al. 1987; Hamernik and Nett 1988; Mercer, Clements et al. 1988; Mercer and Clarke 1989; Mercer, Clements et al. 1989; Haisenleder, Dalkin et al. 1991). Interestingly, either low or high levels of GnRH input can maintain LHβ mrna levels (Mercer and Clarke 1989), even without discernable secretion. Shupnik et al. (1990) demonstrated that pulsatile GnRH is able to stimulate α and LHβ subunits in the rats. Other studies showed that pulsatile GnRH stimulates mrna levels for all three gonadotropin subunits in rats (Haisenleder, Yasin et al. 1993), whereas a constant infusion of GnRH increases α subunit only (Jakubowiak, Tong et al. 1991). Using GnRHdeficient male rats, fast physiologic GnRH pulses increase expression of all three gonadotropin subunits (Haisenleder, Katt et al. 1988; Dalkin, Haisenleder et al. 1989). It appears that the synthesis of LHβ is much more closely regulated by GnRH pulsatility than the synthesis of FSHβ and α gene. In summary, the synthesis of different gonadotropin subunits requires a particular type of GnRH signal Regulation of gonadotropin subunit gene expression by ovarian sex steroids and peptides The gonadal steroids regulate gonadotropin gene expression both negatively and positively. Since gonadectomy leads to an increase in mrna levels for all three gonadotropins in some species (Gharib, Wierman et al. 1987; Mercer, Clements et al. 1989; Attardi, Marshall et al. 1992), these animals have been used to determine the effects of steroids on gonadotropin synthesis. Chronic administration of estrogen to OVX 29
46 animals decreases gonadotropin subunit mrna to levels seen in the intact rat and ewe (Gharib, Bowers et al. 1986; Mercer, Clements et al. 1989). Using OVX/HPD ewes with GnRH replacement, (Mercer and Clarke 1989) demonstrated that oestradiol had no effect on LHβ subunit but reduced FSHβ and stimulated α subunit (Mercer, Clements et al. 1989). Short term estrogen treatment (8 hours) of hypothalamo pituitary intact OVX ewes decreased the levels of mrna for all three gonadotropin subunits whereas in OVX/HPD ewes this treatment reduced both α and FSHβ subunits (Mercer, Phillips et al. 1993). LHβ mrna expression in the pregnant ewe (with high progesterone levels) was shown to be low (Wise, Nilson et al. 1985), but other experiments with OVX ewes showed that progesterone treatment alone does not affect gonadotropin synthesis (Hamernik, Kim et al. 1987). Similar results were obtained in rat studies (Counis, Corbani et al. 1983; Simard, Labrie et al. 1988). A direct pituitary effect of testosterone to down regulate α and LHβ mrna was demonstrated in cultured pituitary cells which were treated with testosterone (Gharib, Wierman et al. 1990). The peptides, inhibin, activin and follistatin, are produced by granulosa cells of the ovarian follicles, placenta, and pituitary (Roberts, Meunier et al. 1989; Petraglia, Sacerdote et al. 1991; Findlay 1993; Fowler, Evans et al. 1998; Peng, Ohno et al. 1999; Debieve, Pampfer et al. 2000; Manuelpillai, Schneider Kolsky et al. 2001). Inhibin reduces FSHβ mrna expression in OVX/HPD, GnRH pulsed sheep and in rat pituitary cell cultures, with no effect on both α and LHβ subunits (Mercer, Clements et al. 1987; Carroll, Corrigan et al. 1989). Follistatin also decreases FSHβ mrna levels in cultured pituitary cells. On the other hand, activin stimulates FSHβ mrna in pituitary cell culture (Carroll, Corrigan et al. 1989). 30
47 1.5.3 Regulation during the estrous cycle Studies of gonadotropin subunit mrna levels throughout the estrous cycle of rats and sheep have demonstrated variation in expression of the genes for both the α and β subunits of LH (Landefeld, Kaynard et al. 1985; Landefeld, Maurer et al. 1985; Zmeili, Papavasiliou et al. 1986). Other data confirmed that LHβ mrna levels in cycling rats increases during proestrus whereas α subunit mrna levels increases during diestrus (Zmeili, Papavasiliou et al. 1986). Using intact normally cycling ewes, it was shown that both α subunit and LHβ mrna levels increase at the onset of estrus (Landefeld, Kaynard et al. 1985; Landefeld, Maurer et al. 1985). However, in the estradiol induced preovulatory like LH surge in OVX ewes, the amount of LHβ mrna is low while levels of α subunit mrna remain unchanged (Hamernik and Nett 1988). 1.6 Regulation of Gonadotropin Secretion Relationship between pulsatile GnRH secretion and gonadotropin (LH and FSH) secretion It is now well accepted that gonadotropin secretion from the anterior pituitary is released in a pulsatile fashion. The concept of pulsatility was revealed in early studies by Dierschke et al with frequent blood sampling of OVX rhesus monkies. LH secretion is characterized by regular bursts of release which occur at intervals of 20 60min in OVX rhesus monkies, sheep, rats and humans (Dierschke, Bhattacharya et al. 1970; Midgley and Jaffe 1971; Butler, Malven et al. 1972; Gay and Sheth 1972; Yen, Tsai et al. 1972). Malven 1975 electrically stimulated the ME of OVX ewes, causing an increase in LH release, substantiating the notion that LH pulses are due to GnRH pulses. This was also supported by GnRH immunization in sheep (Clarke, Fraser et al. 1978). There is an 31
48 absence of LH pulses in GnRH immunisized sheep but the pulses can be restored by injection of GnRH analogues (Fraser, Clarke et al. 1981). The simultaneous measurement of both GnRH pulses in the hypophysial portal blood and LH pulses in the jugular plasma of ewes provided the final proof that GnRH pulses cause LH pulses (Clarke and Cummins 1982) (Fig.2). This model was used to characterise GnRH secretion in a wide range of physiological conditions (Clarke, Thomas et al. 1987), providing extensive data to demonstrate the close association between GnRH and LH pulsatility. 32
49 Figure 2. Relationship between pulsatile GnRH secretion from the hypophysial portal blood and pulsatile LH secretion from jugular blood. Arrows indicate GnRH/LH pulses (Clarke and Cummins 1982). 33
50 The pattern of GnRH pulses (amplitude and frequency) is important in determining the quantity and quality of gonadotropin secretion. Using arcuate lesioned OVX rhesus monkey, Knobil 1981 determined the effect of various frequencies of GnRH treatment on LH and FSH secretion. Pulsatile administration of GnRH at a frequency of 1 pulse/hour maintained LH and FSH secretion. An increase to 5 pulses/hour of GnRH treatment decreased LH and FSH secretion dramatically. Wildt et al demonstrated the effect of varying the amplitude of GnRH. At a constant frequency, decreasing the amplitude of GnRH leads to low plasma levels of LH, with FSH levels becoming undetectable. Increasing GnRH amplitude did not alter LH levels, but lowered FSH levels. These results suggested that changes in the frequency of GnRH have a greater effect on LH secretion than varying the amplitude. The effect of amplitude and frequency of GnRH pulses has also been clearly demonstrated in OVX/HPD sheep (Clarke and Cummins 1984). Increasing GnRH pulse frequency from 1/hour to 2/hour led to an increase in baseline plasma LH and a decline in LH pulse amplitude. The data from primates and sheep established this inverse relationship between GnRH pulse frequency and LH pulse amplitude which partly explains the pattern of LH secretion during the ovine estrous cycle (Clarke 1996). The LH pulse amplitude relates to the amount of releaseable LH available in the gonadotrope (Clarke and Cummins 1985). The secretion of FSH is different to that of LH secretion. Even though (Padmanabhan, McFadden et al. 1997) obtained the synchrony between GnRH and FSH pulses in sheep, more other studies show that FSH secretion is not strictly a reflection of the pulsatile secretion of GnRH (Clarke 2002b). In OVX/HPD ewes, the cessation of pulsatile GnRH input terminates LH secretion whereas FSH secretion continues, but diminishes with time 34
51 (Clarke, Burman et al. 1986). In addition, central treatment with a GnRH antagonist to rams blocks pulsatile LH secretion with no suppression on FSH secretion (Lincoln and Fraser 1987). Secretion of FSH without pulsatile input of GnRH indicates a passive secretory mode for FSH, reflecting available stores in the gonadotropes (Clarke 2002) Regulation by ovarian sex steroids at the level of the gonadotrope Short-term negative feedback mechanism Testosterone negatively regulates GnRH pulse frequency in the castrated sheep (Tilbrook, de Kretser et al. 1991). Using HPD wethers, central testosterone, dihydrotestosterone or estrogen treatment does not affect plasma LH pulse amplitude or plasma FSH levels there by providing evidence that the site of negative feedback of gonadal hormones in ram is in the hypothalamus (Tilbrook, de Kretser et al. 1991). At the level of the pituitary, estrogen decreases LH pulse amplitude during follicular phase of the estrous cycle (Thomas, Martin et al. 1988). The short term negative feedback effect of estrogen on the pituitary, to reduce both LH and FSH secretion, has been further demonstrated in OVX/HPD sheep (Clarke and Cummins 1984). A decrease in FSH secretion is also seen in ovine pituitary cells in culture treated with estrogen and progesterone treatment for 24h (Alexander and Miller 1982; Phillips, Lin et al. 1988). A similar effect of estrogen is also seen in a variety of pituitary types and cell lines (Arreguin Arevalo and Nett 2005; Bulayeva, Wozniak et al. 2005). Rapid acute suppression of LH was observed when OVX sheep pituitary cell cultures were treated by estrogen. This occurs in a dose dependent manner with maximum suppression occurring between min (Arreguin Arevalo and Nett 2005). It has been proposed that this is due to a non genomic cellular mechanism (Kelly and Wagner 1999; Kelly and Levin 2001; Kelly, Qiu et al. 2005). In OVX and OVX/HPD ewes 35
52 estradiol reduces plasma LH secretion within 60 and 90min (Iqbal, Latchoumanin et al. 2007). Furthermore, estradiol treatment reduces GnRH stimulated LH levels within 15 60min in pituitary cell culture and inhibits GnRH stimulated Ca 2+, suggesting evidence of rapid nongenomic event (Iqbal, Latchoumanin et al. 2009) Long-term negative feedback mechanism Chronic treatment with a combination of estrogen and progesterone clearly reduces LH synthesis and secretion (Nett, Flores et al. 1990). However, progesterone treatment for 3 weeks reduced LH secretion but not FSH secretion (Hamernik, Kim et al. 1987). Thus, at least one component of the long term negative feedback effect of estrogen and progesterone is due to action on the gonadotropes, as well as GnRH secretion (see above) Positive feedback mechanism The positive feedback effect of estrogen on gonadotropin secretion is due to a surge release of GnRH as well as an increase in the responsiveness of gonadotropes to GnRH. The latter involves a change in the second messenger signalling systems within the gonadotrope (Clarke 1995b). It has been shown that the sensitivity of the cells to GnRH increases at the time of positive feedback which may be only partly due to an increase in GnRH receptor (GnRH R) levels (Clarke 2002b). This increase in GnRH R expression prior to the cyclic LH surge has been demonstrated in a number of studies in different species (Savoy Moore, Schwartz et al. 1980; Adams and 36
53 Spies 1981; Marian, Cooper et al. 1981; Crowder and Nett 1984; Brooks, Taylor et al. 1993; Turzillo, Juengel et al. 1995; Cowley, Rao et al. 1998). In OVX, estrogen treated ewes there is an increase in GnRH R number prior to the positive feedback event (Nett, Crowder et al. 1984; Schoenemann, Brown et al. 1985; Schoenemann, Humphrey et al. 1985; Adams, Sakurai et al. 1996). In OVX/HPD ewes receiving pulsatile GnRH input, a single injection of estrogen during the positive feedback timeframe also increases GnRH R number and GnRH R mrna levels (Clarke, Cummins et al. 1988; Cowley, Rao et al. 1998), demonstrating this is a due to a direct effect of estrogen on the gonadotropes to prime them to respond to GnRH at the time of the surge. Estrogen increases the activity of protein kinase C (PKC) and increases the calcium current at the time of the LH surge (Drouva, Gorenne et al. 1990; Heyward and Clarke 1995). Estrogen also increases the mobilization of LH secretory granules in the gonadotropes (Lewis, Morris et al. 1985; Currie and McNeilly 1995; Thomas and Clarke 1997) demonstrating the preparation for the massive secretory event that occurs during the surge. 1.7 Gonadotropin Inhibitory Hormone (GnIH) Discovery of mammalian GnIH The identification of RFamide peptides were first described by Price and Greenberg (1977), who reported the isolation of a tetrapeptide Phe Met Arg Phe NH 2 (FMRFamide) from Macrocallista nimbosa. This peptide showed a cardioexcitatory effect (Price and Greenberg 1977). The first RFamide isolated from a vertebrate was chicken pentapeptide FMFRamide, which increases rat arterial blood pressure (Dockray, Reeve et al. 1983). 37
54 Almost two decades later, Perry and co workers extracted mammalian RFamide peptide from bovine brain namely neuropeptide FF (NPFF) and AF (NPAF) which showed various biological activities including an anti opioid effect in mammals (Panula, Aarnisalo et al. 1996). A further RFamide peptide was then extracted from bovine brain as a ligand for an orphan G protein coupled receptor (GPCR) and was named prolactin releasing peptide (PrRP) because of its prolactin releasing activity in rat anterior pituitary cells (Hinuma, Habata et al. 1998). Other RFamide which was isolated in the ganglia of the venus clam on the basis of its cardiovascular properties in 1977 was kisspeptin (Price and Greenberg 1977). The anti metastic properties were then established in 1996 (Lee, Miele et al. 1996) and kisspeptin was discovered as the natural ligand for the previously orphaned G coupled protein receptor GPR54 in 2001 (Kotani, Detheux et al. 2001). Ohtaki et al was the first to identify kisspeptin in the human. Avian GnIH was isolated from the brain of the Japanese quail using High Performance Liquid Chromatography (HPLC) and a competitive enzyme linked immunosorbent assay (ELISA) (Tsutsui, Saigoh et al. 2000). The isolated peptide is a 12 amino acid sequence Ser Ile Lys Pro Ser Ala Tyr Leu Pro Leu Arg Phe NH 2 (SIKPSAYLPLRFamide), and was named GnIH based on its ablility to inhibit gonadotropin secretion in birds (Tsutsui, Saigoh et al. 2000). It was claimed that this neuropeptide had not been previously identified in vertebrates, even though its C terminal LPLRFamide was identical to chicken LPLRFamide peptide identified earlier (Dockray, Reeve et al. 1983). 38
55 1.7.2 Characteristics of GnIH The GnIH precursor polypeptide in the quail brain consists of 173 amino acid residues, encoding one GnIH and two GnIH related peptide (GnIH RP) sequences. These peptides all share a common C terminal Leu Pro X Arg Phe NH2 (LPXRF NH 2 ) motif (where X represents L in both GnIH and GnIH RP 1, and Q in GnIH RP 2) (Satake, Hisada et al. 2001; Ukena and Tsutsui 2001; Fukusumi, Fujii et al. 2006) (Table 1). Using gene database, other RFamide peptides in mammalian brains, namely RFamide related peptides 1, 2 and 3 (RFRP 1, 2 and 3), were identified (Hinuma, Shintani et al. 2000). These may also be named GnIH 1, 2 and 3, even though GnIH 2 does not activate the same receptor as GnIH 1 and 3 (Hinuma, Shintani et al. 2000). The mammalian GnIH has a C terminal sequence of Leu Pro X Arg Phe NH2 (LPXRF NH 2 ) where X is either Gln or Leu [reviewed in (Clarke, Qi et al. 2009)] Distribution and projections of GnIH neurons Using ELISA, immunoreactive GnIH cells are detected in the highest concentration in the diencephalon of the quail brain (Tsutsui, Saigoh et al. 2000). Subsequently, the precise localization of GnIH cells in avian brain was demonstrated by immunohistochemistry (IHC) and in situ hybridization (ISH) to be located in the paraventricular nucleus (PVN) in the hypothalamus (Bentley, Perfito et al. 2003; Ukena, Ubuka et al. 2003; Osugi, Ukena et al. 2004; Ukena and Tsutsui 2005). The most prominent fibre beds of these neurons are seen in the median eminence (ME) of the hypothalamus (Bentley, Perfito et al. 2003; Ukena, Ubuka et al. 2003). In the mouse brain, GnIH cells are located in the hypothalamus, pons and medulla oblongata as well as spinal cord (Ukena and Tsutsui 2001). In the hypothalamus, GnIH 39
56 immunoreactive cells are concentrated in the dorsomedial nucleus (DMN) in mouse (Ukena and Tsutsui 2001; Ukena and Tsutsui 2005; Smith and Clarke 2010). GnIH cells are identified in the dorsomedial nucleus of the hypothalamus (DMH) in mice, hamsters and rats using ISH (Kriegsfeld, Mei et al. 2006). Other studies in rats have detected GnIH cells in the area between the ventromedial nucleus (VMN) and the DMN (Hinuma, Shintani et al. 2000; Fukusumi, Habata et al. 2001; Yano, Iijima et al. 2003; Ukena and Tsutsui 2005; Johnson, Tsutsui et al. 2007; Rizwan, Porteous et al. 2009; Smith and Clarke 2010) with cells extend rostrally to the perifornical area (Legagneux, Bernard Franchi et al. 2009; Smith and Clarke 2010). ISH studies in the ovine brain reveals a population of GnIH cells in the dorsal PVN and the DMN (Clarke, Sari et al. 2008; Dardente, Birnie et al. 2008), with additional mrna signal in ependyma (Dardente, Birnie et al. 2008; Clarke, Qi et al. 2009). GnIH expressing cells are identified in the non human primate and human hypothalamus (Ubuka, Lai et al. 2009a; Ubuka, Morgan et al. 2009b). Using ISH and immunocytochemistry (ICC), GnIH cell bodies were found in the periventricular region of the hypothalamus of monkey with the highest level in the intermediate periventricular nucleus (IPe) (Ubuka, Lai et al. 2009a). In the human brain, GnIH cell bodies are seen in the dorsomedial region of the hypothalamus (Ubuka, Morgan et al. 2009b). GnIH containing neurons project to many areas in the mammalian brain. Fibers have been located in the nucleus of the stria terminalis and habenular nucleus, and in the PVN of thalamus of monkey. In the hypothalamus, fibers have been identified in the POA, PVN, IPe, ARC, and dorsal hypothalamic area of monkey (Ubuka, Lai et al. 2009a). (Johnson, 40
57 Tsutsui et al. 2007) identified projections from GnIH neurons in the terminal beds in the amygdala and dienchephalon, in the region of the BnST and PVN in the thalamus of the rat. GnIH ir terminals are found in the neurosecretory zone of the ME in hamster, sheep and primate (Kriegsfeld, Mei et al. 2006; Dardente, Birnie et al. 2008; Clarke, Qi et al. 2009; Ubuka, Lai et al. 2009a). However, similar projections are not identified in the rat (Johnson, Tsutsui et al. 2007; Rizwan, Porteous et al. 2009). GnIH terminal beds exist in the same region as where GnRH cells are found in the sheep (Lehman, Robinson et al. 1986). The interaction of GnIH immunoreactive (GnIH ir) fibers with GnRH neurons is 40 80% in rat, hamster, mouse, sheep and primate (Wu, Irby et al. 1987; Kriegsfeld, Mei et al. 2006; Johnson, Tsutsui et al. 2007; Smith, Coolen et al. 2008; Qi, Oldfield et al. 2009), consistent with results in birds (Bentley, Perfito et al. 2003; Ubuka, Kim et al. 2008). In addition, chronic infusion of GnIH reduces the activation of GnRH neurons and decreases neuronal activation in the anteroventral periventricular region in rats (Anderson, Kieser et al. 2008). Further studies in male and female mice reveal that GnIH treatment inhibits the firing rate of GnRH neurons (Ducret, Anderson et al. 2009). These data suggested that GnIH regulates GnRH cells directly [reviewed in (Smith and Clarke 2010)] Characteristics of GnIH receptors (GnIH-R) Hinuma and co workers identified a specific receptor for GnIH, namely OT7T022, using the same strategy for identifying endogenous ligands for orphan seven transmembranedomain receptors (7TMRs) through monitoring extracellular acidification rate from Chinese hamster ovary cell lines expressing various 7TMRs (CHO) (Hinuma, Habata et al. 1998; Hinuma, Shintani et al. 2000). The GnIH receptor (GnIH R) that was identified by 41
58 (Hinuma, Shintani et al. 2000) is the same as that identified by Bonini et al (Bonini, Jones et al. 2000). (Bonini, Jones et al. 2000) identified two G protein couple receptors (GPCR) for neuropeptide FF (NPFF), namely NPFF1 and NPFF2. The sequence of NPFF, FLFQPQRF NH2, is closely related to that of GnIH, and the four amino acids on the C terminal of NPFF and GnIH are identical (Ukena and Tsutsui 2005). In addition, GnIH (also referred to as RFRP) displays a higher affinity for NPFF1 whereas NPFF has potent activity for NPFF2, suggesting that NPFF1 is the receptor for GnIH (Liu, Guan et al. 2001; Yoshida, Habata et al. 2003). (Dockray 2004) designed five genes that encode GnIH GPCR (RFamide GPCR in that paper) (rfr 1 to rfr 5). Consistent with earlier findings, rfr 2 (NPFF 1, OT7T022, GPCR 147, GPR 147) is proposed as the cognate receptor for GnIH (Dockray 2004). This receptor has been cloned for a range of species including rat, sheep and human (Hinuma, Shintani et al. 2000; Ikemoto and Park 2005; Yin, Ukena et al. 2005; Dardente, Birnie et al. 2008; Ubuka, Morgan et al. 2009b). Additionally, NPFF receptors have been cloned from mouse and bovine (Yang, Tao et al. 2008) Distribution of GnIH-R In quail, the GnIH R was identified in the hypothalamus, the cerebrum, mesencephalon, spinal cord and pituitary (Yin, Ukena et al. 2005). Additional localization of GnIH receptor has been described in gonads and reproductive tract of birds and rats (Hinuma, Shintani et al. 2000; Bentley, Ubuka et al. 2008). GnIH R has been also demonstrated to be expressed by the GnRH cells in the brain of birds (Bentley, Ubuka et al. 2008; Ubuka, McGuire et al. 2008). 42
59 In the sheep, the GnIH receptor (rfr 2) is seen to have widespread distribution in the hypothalamus, including the supraoptic nucleus (SON), suprachiasmatic nucleus (SCN) and the periventricular nucleus (PeVN). There is also strong expression in the pars tuberalis (PT) of the pituitary gland (Dardente, Birnie et al. 2008). Using RT PCR and DNA sequencing methods, Ubuka and co workers (2009) identify the expression of GnIH cognate receptor (GPR 147 in that paper) in human hypothalamus and pituitary. (Bonini, Jones et al. 2000) identified NPFF1 mrna in the same area of the human and rat brain, with a high level of expression in the hypothalamus. Expression was also seen in the spinal cord, amygdala, hippocampus and substantia nigra with additional expression in the caudate putamen and thalamus of human brain. NPFF1 mrna has also been found to be expressed in the testes and ovary of rat (Bonini, Jones et al. 2000) Steroid receptors in GnIH cells In the hamster brain at least 40% of GnIH neurons were seen to express ERα (Kriegsfeld, Mei et al. 2006). It has further been demonstrated in the Syrian hamster that there is a change of GnIH gene expression during the estrous cycle, being low during the preovulatory LH surge and highest during the proestrus phase (Gibson, Humber et al. 2008). In contrast, others report no change in GnIH expression in both castrated and castrated testosterone treated male Syrian hamster (Revel, Saboureau et al. 2008) Effect of GnIH on gonadotropin synthesis Initial studies on the role of GnIH in the synthesis of gonadotropin showed that a dose of 10 7 M GnIH reduces the expression of α and FSHβ subunit in the chicken pituitary. However, at doses of M GnIH did not inhibit LHβ subunit gene expression (Ciccone, Dunn et al. 2004). Different results were obtained from an in vivo study in the 43
60 male quail, where intraperitoneal injection of GnIH reduced the expression of α subunit and LHβ subunit with no reduction on FSHβ subunit expression (Ubuka, Ukena et al. 2006) Effect of GnIH on gonadotropin secretion Intitial studies showed that ICV injection of GnIH reduced plasma LH levels in OVX hamsters and gonad intact rats (Kriegsfeld, Mei et al. 2006; Johnson, Tsutsui et al. 2007; Johnson and Fraley 2008). Other studies, showed no inhibitory effect of ICV injection of GnIH in either OVX rats or OVX estrogen treated rats (Murakami, Matsuzaki et al. 2008; Anderson, Relf et al. 2009). Further support for a central action of GnIH was demonstrated in pre pubertal rats, after ICV injection of GnIH antisense oligonucleotide led to an increase in plasma LH concentrations (Johnson and Fraley 2008). Peripheral administration of GnIH in OVX rats reduces plasma LH levels (Murakami, Matsuzaki et al. 2008) and GnRH stimulated LH secretion (Rizwan, Porteous et al. 2009). Furthermore, GnIH inhibits GnRH stimulated LH release from pituitary cell cultures of rat, and bovine (Murakami, Matsuzaki et al. 2008; Kadokawa, Shibata et al. 2009). Inconsistent results have been obtained using human fetal pituitary cells (Ubuka, Morgan et al. 2009b). Studies on the avian species demonstrated the inhibitory action of GnIH on gonadotropin secretion both in vivo and in vitro (Tsutsui, Saigoh et al. 2000; Osugi, Ukena et al. 2004) Effect of GnIH on the levels of other hormones GnIH affects pituitary hormones other than gonadotropins such as prolactin (PRL) and growth hormone (GH). GnIH stimulates both PRL and GH (Hinuma, Shintani et al. 2000) but does not affect corticosteroid secretion in the rat (Johnson, Tsutsui et al. 2007). Some 44
61 GnIH cells express the glucocorticoid receptor and this proportion of cells expressing the relevant receptor increase in response to stress in male rats (Kirby, Geraghty et al. 2009) 1.8 Control of Food Intake in the Hypothalamus The central regulation of appetite involves many neuropeptides which are termed orexigenic (peptides that stimulate food intake) and anorexigenic (peptides that inhibit food intake) [reviewed in Schwartz et al, 2000 (Schwartz 2000)]. Many appetite regulating peptides also regulate aspects of the reproductive system. NPY, POMC (including the melanocortins), orexin, and kisspeptin are all examples of peptides with such dual functions (Barker Gibb, Scott et al. 1995; Watanobe, Suda et al. 1999; Clarke, Backholer et al. 2005; Backholer, Smith et al. 2009). The DMN is considered an important feeding hub where many appetite regulating peptides are synthesised and released. A role for DMN in food intake control was demonstrated in lesion studies; lesions of this nucleus cause hypophagia [reviewed in Bernardis and Bellinger, 1998 (Bernardis and Bellinger 1998)]. In rats, the DMN contains NPY cells and fibers (Chronwall, DiMaggio et al. 1985), which may be involved in the control of feeding. The ARC also contains a high concentration of NPY cell bodies which project to DMN and PVN (Chronwall, DiMaggio et al. 1985). The nutritional status (starvation refeeding) is reflected in NPY levels of both DMN and PVN in Zucker (fa/fa) and corpulent (cp/cp JCR: LA) rats [reviewed in Bernardis and Bellinger, 1998 (Bernardis and Bellinger 1998)]; (McKibbin, Cotton et al. 1991; Williams, Shellard et al. 1992). There are also several animal models in which NPY mrna expression in the DMN is elevated, such as the lethal yellow A y, MC4 receptor knockout mice (Kesterson, Huszar et al. 1997), 45
62 as well as diet induced obese (DIO) mice (Guan, Yu et al. 1998), tubby mice (Guan, Yu et al. 1998), and brown adipose tissue (BAT) deficient obese mice (Tritos, Elmquist et al. 1998). Intense exercise and food restriction increase in NPY expression in the DMN of the rat brain (Lewis, Shellard et al. 1993). Moreover, the long form leptin receptor (OB Rb) mrna is observed in DMN in rats (Elmquist 2001), supporting the notion that this nucleus is involved in the regulation of food intake. In the sheep, NPY expression is high in the ARC, but is also produced in a few cells of the POA, (Antonopoulos, Karamanlidis et al. 1989; Norgren and Lehman 1989; Tillet, Caldani et al. 1989; Barker Gibb, Scott et al. 1995; Skinner and Herbison 1997) Effect of GnIH on food intake GnIH stimulates food intake in birds and male rats (Tachibana, Sato et al. 2005; Johnson, Tsutsui et al. 2007; Murakami, Matsuzaki et al. 2008), consistent with the role of the GnIH peptide rich area of the DMN in regulating appetite. In sheep, GnIH cells project to cells that produce appetite regulating peptides such as NPY and POMC in the ARC, those that produce orexin and MCH in the lateral hypothalamus, and those that produce orexin in the DMN (Qi, Oldfield et al. 2009). In addition, other peptides from the RFamide family also stimulate food intake. PrRP stimulates feeding in chicks (Tachibana, Sato et al. 2005), 26 and 43RFa QRFP increases food intake in mice (Chartrel, Dujardin et al. 2003; do Rego, Leprince et al. 2006; Moriya, Sano et al. 2006; Takayasu, Sakurai et al. 2006). The dual function of GnIH in relation to food intake and reproduction is similar to that of other peptides such as NPY and melanocortins as mentioned above [reviewed in Smith and Clarke, 2009 (Smith and Clarke 2010)]. 46
63 1.9 Gonadal Expression of GnIH and Possible Gonadal Function As mentioned above, expression of the GnIH receptor has been shown in the gonads and reproductive tract of birds and rats (Hinuma, Shintani et al. 2000; Bentley, Ubuka et al. 2008) as well as NPFF1 mrna in the testes and ovary of the rat (Bonini, Jones et al. 2000). Thus, it is possible that GnIH affects gonadal function as demonstrated in male quail. GnIH administration for 2 weeks in mature birds induces testicular apoptosis and decreases the diameter of seminiferous tubules. Moreover, injection of GnIH to immature birds inhibits testicular growth and spermatogenic activity (Ubuka, Ukena et al. 2006) Overview The discovery of GnIH counters the popular belief that GnRH is the only hypothalamic peptide that controls pituitary gonadotropin synthesis and secretion. GnIH cells are localized in the brain and there is evidence of input to GnRH neurons. GnIH neurons also project to the median eminence in some species, providing a strong precedent for GnIH to act as an hypophysiotropic peptide, at the level of the pituitary. GnIH inhibits GnRH neurons and reduces both synthesis and secretion of gonadotropin in the pituitary. Because the GnIH receptor is expressed in the brain, pituitary and gonads, it is possible that GnIH acts at multiple levels. Dual function is exhibited by GnIH, in that it reduces reproductive function and increases food intake Aims The general aim of this thesis was to seek further evidence that GnIH plays an important role in the reproductive process in the sheep. In particular, these studies aimed to provide significant new information for an inhibitory system that counters the effect of GnRH. At 47
64 the time that the work in this thesis began, this challenged dogma, but there has been accumulating data supporting a role for GnIH in the past few years. This body of work in this thesis aimed to prove that GnIH is an hypophysiotropic hormone which acts specifically at the level of the gonadotrope to inhibit gonadotropin synthesis and secretion. My work also aimed to determine how GnIH and estrogen act in concert at the level of the pituitary gonadotrope. It is hoped that my work will lead to therapeutic uses for GnIH and analogues in the management of reproduction Hypotheses The unifying hypothesis tested in this thesis was that GnIH is a potent inhibitor of gonadotropin synthesis and secretion. The specific hypotheses tested were: 1. GnIH is an hypophysiotropic hormone specifically inhibiting gonadotropin secretion. 2. GnIH inhibits gonadotropin subunit gene synthesis in the pituitary. 3. GnIH acts in concert with estrogen at the level of the pituitary gonadotrope. The results chapters of this thesis are formed by a series of papers. In some cases, only part of the work in the presented paper represents the work of the candidate and this is indicated at the start of each chapter. 48
65 Chapter 2: Potent action of RFamide related peptide 3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion 49
66 Declaration for Chapter 2 50
67 Declaration by co authors 51
68 52
69 53
70 54
71 55
72 2.1 Introduction Foregoing work in other species showed that GnIH cells are located in the PVN in avian brain (Bentley, Perfito et al. 2003; Ukena, Ubuka et al. 2003; Osugi, Ukena et al. 2004); reviewed in (Ukena and Tsutsui 2005) and DMH in rodents (Ukena and Tsutsui 2001); reviewed in (Ukena and Tsutsui 2005); and (Smith and Clarke 2010). GnIH has also been shown to inhibit gonadotropin secretion in several species including hamster, rat, bird (Osugi, Ukena et al. 2004; Kriegsfeld, Mei et al. 2006; Johnson, Tsutsui et al. 2007; Murakami, Matsuzaki et al. 2008; Rizwan, Porteous et al. 2009). GnIH reduces gonadotropin secretion in vivo in the rat and bovine species, and in vitro in the avian species (Tsutsui, Saigoh et al. 2000; Murakami, Matsuzaki et al. 2008; Kadokawa, Shibata et al. 2009). The present study was undertaken to clone the gene for GnIH in the sheep and to determine the location of GnIH cells by in situ hybridisation and immunohistochemistry. Tracing studies were undertaken to determine the projections of GnRH to the median eminence in the sheep. The work in this paper that was undertaken by the candidate was to establish pituitary cell cultures and to test the effects of GnIH 3 (RFRP 3) on gonadotropin secretion, with and without GnRH treatment. Cell cultures were treated with amidated and non amidated forms of hamster GnIH to elucidate the importance of amidation of the peptide. In addition, the candidate tested the effect of GnIH 3 on gonadotropin secretion in vivo. The candidate assayed LH and FSH levels by radioimmunoassay (RIA). Because GnIH 3 was shown to reduce GnRH stimulated gonadotropin secretion, further studies were undertaken to determine whether this was due to a reduction in GnRH stimulated mobilisation of intracellular calcium. 56
73 2.2 Conclusion The ovine GnIH 3 (RFRP 3) amino acid sequence was seen to differ from the original avian GnIH sequence (Hinuma, Shintani et al. 2000; Tsutsui, Saigoh et al. 2000) but was identical to the human sequence (Clarke and Tilbrook 2009). Consistent with observations in birds (Bentley, Perfito et al. 2003; Ukena, Ubuka et al. 2003; Osugi, Ukena et al. 2004); reviewed in (Ukena and Tsutsui 2005) and rodents (Ukena and Tsutsui 2001); reviewed in (Ukena and Tsutsui 2005; Smith and Clarke 2010), GnIH 3 neurons were found to be localized to the DMH and PVN. Furthermore, the tracing studies showed that the cells project to the neurosecretory zone of the median eminence. The projection to this area of the sheep may indicate secretion of the peptide into the hypophysial portal blood with possible action of the peptide in the pituitary. Ovine pituitary cells respond to GnRH in vitro, showing a robust increase in the secretion of LH and FSH within 2h. GnIH 3 did not reduce the basal secretion of LH and FSH from the pituitary gonadotropes. On the other hand, GnIH 3 inhibited GnRH stimulated LH and FSH secretion in vitro and in vivo. Gonadotropin secretion is maintained by the presence of GnRH stimulation, so it is important to test the inhibitor under the condition of GnRH stimulation. GnIH 3 inhibited GnRH stimulated LH and FSH secretion in a dose dependent manner. The importance of amidation of the peptide was indicated by the fact that nonamidated peptide was inactive in terms of blocking GnRH stimulated LH and FSH secretion. Thus, non amidated hamster GnIH did not inhibit GnRH stimulated gonadotropin secretion, whereas amidated hamster GnIH reduced gonadotropin secretion to the same extent as GnIH 3. 57
74 In this study we also tested the specificity action of GnIH on gonadotropin secretion and/or whether it also affects secretion of other pituitary hormones. GnIH did not inhibit PRL secretion from the pituitary gonadotropes of the rats (Tsutsui, Saigoh et al. 2000), but GnIH 3 reduced PRL release in rat anterior primary pituitary cell cultures (Anderson, Kieser et al. 2008). However, the in vivo study on the rat indicated that GnIH stimulates PRL release (Hinuma, Shintani et al. 2000) and GH secretion (Johnson, Tsutsui et al. 2007; Johnson and Fraley 2008) as well as growth hormone releasing hormone mrna (Johnson and Fraley 2008). Our in vivo study in sheep showed that GnIH 3 acts specifically to inhibit gonadotropin secretion with no effect on the PRL or GH or cortisol levels (an indicator of the activity of the hypothalamo pituitary adrenal axis). This specific effect of GnIH 3 was demonstrated in vivo by infusion into OVX ewes. Importantly, the infusion was intravenous, so that action was likely to be directly at the level of the pituitary gonadotropes. The ability of this peptide to cross the blood brain barrier is not known, so effects of this treatment on GnRH secretion are not known. The trophic stimulus of GnRH to secretion of gonadotropins has been shown to be dependent upon an increase in free cytoplasmic calcium levels (Smith, Wakefield et al. 1987; Leong 1991; Stojilkovic and Catt 1992). In rats, GnRH induced calcium signals in gonadotropes are influenced by gonadal steroids (Heyward and Clarke 1995; Tobin and Canny 1996; Tobin, Millar et al. 1997; Tobin and Canny 1998). In the pituitary gonadotropes of ewes, GnIH 3 suppressed GnRH generated calcium signal Our study indicated the direct action of GnIH on the gonadotropes and this is most likely mediated via GnIH R, which have been demonstrated in the pituitary glands of rats and 58
75 Siberian hamster (Hinuma, Shintani et al. 2000; Gibson, Humber et al. 2008; Quennell, Rizwan et al. 2010). The body of work presented in this chapter is not without limitations. This study was conducted in OVX ewes in the absence of steroid hormones; this particular issue is addressed in Chapter 4. The inhibitory effect of GnIH 3 in gonad intact animals may differ to that seen in OVX animals. Thus, during the estrous cycle of the ewes, dominance of either progesterone (in the luteal phase) or estrogen (in the follicular phase) may affect the response to GnIH. Furthermore, there may be different responses in the breeding and non breeding seasons. Further studies of the responses in these different reproductive states would be informative. It would also be informative to measure levels of GnRH R in ovine pituitary gonadotropes. Overall, the data from this study provided strong evidence that GnIH 3 is a hypophysiotropic hormone in the sheep, inhibiting GnRH stimulated gonadotropin secretion at the level of the pituitary gonadotropes. 59
76 Potent action of RFRP 3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion Published in Endocrinology (2008)
77 Abstract We identified a gene in the ovine hypothalamus encoding for RFRP 3 and tested the hypothesis that this system produces an hypophysiotropic hormone which inhibits the function of pituitary gonadotropes. The RFRP 3 gene encodes for a peptide that appears identical to human RFRP 3 homologue. Using an antiserum raised against RFRP 3, cells were localised to the dorsomedial hypothalamic nucleus/paraventricular nucleus of the ovine brain and shown to project to the neurosecretory zone of the ovine median eminence, predicating a role for this peptide in the regulation of anterior pituitary gland function. Ovine RFRP 3 peptide was tested for biological activity in vitro and in vivo and was shown to reduce LH and FSH secretion in a specific manner. RFRP 3 potently inhibited GnRH stimulated mobilisation of intracellular calcium in gonadotropes. These data indicate that RFRP 3 is a specific and potent mammalian gonadotropin inhibiting hormone and that it acts upon pituitary gonadotropes to reduce GnRH stimulated gonadotropin secretion. 61
78 Introduction Gonadotropin releasing hormone (GnRH) is the primary stimulator of gonadotropin secretion (Clarke 1996), but there is mounting evidence of a functional inhibitory hormone in the hypothalamus (Bentley, Kriegsfeld et al. 2006; Tsutsui, Ubuka et al. 2006). Such an entity has been sought for many years (Clarke 1989), with definitive evidence of the existence of gonadotropin inhibitory hormone (GnIH) first obtained in the avian brain (Tsutsui, Saigoh et al. 2000). Data have since accumulated to indicate that GnIH negatively regulates reproductive function in hamsters and rats (Kriegsfeld, Mei et al. 2006; Johnson, Tsutsui et al. 2007). Most focus in mammalian studies has been on central actions of GnIH to inhibit GnRH function, but there is also an indication that GnIH acts on pituitary gonadotropes in birds (Tsutsui, Saigoh et al. 2000; Ciccone, Dunn et al. 2004; Osugi, Ukena et al. 2004; Yin, Ukena et al. 2005; Bentley, Jensen et al. 2006). The GnIH receptor is also expressed in the avian pituitary and brain (Yin, Ukena et al. 2005; Ubuka, Kim et al. 2008), predicating a role for the hormone as an hypophysiotropic factor. There are various homologues of GnIH (for review, see (Tsutsui and Ukena 2006)), which belong to a large family of peptides with a common Arg Phe NH2 C terminus (RFamide)(for review, see (Ukena and Tsutsui 2005)). We now report the existence of an ovine gene encoding for RFRP 3 (GnIH). We localised RFRP 3 cells in the ovine hypothalamus and determined that the cells project to the neurosecretory zone of the median eminence. Physiological studies on pituitary gonadotropes then revealed specific and potent effects to reduce gonadotropin secretion, by counteracting the effect of GnRH to increase intracellular free calcium. 62
79 Material and Methods All animal procedures were conducted with prior institutional ethical approval under the requirements of the Australian Prevention of Cruelty to Animals Act 1986 and the National Health and Medical Research Council/Commonwealth Scientific and Industrial Research Organization/Australian Animal Commission Code of Practice for the Care and Use of Animals for Scientific Purposes. Animals The animals used in this study were female Corriedale ewes of 3 5 years, that were ovariectomised to remove the influence of gonadal steroids. When required, the animals were euthanased with an i.v. injection of 20 ml of Lethobarb (pentobarbitone sodium 325mg/ml, Virbac, Peakhurst, NSW, Australia). For the purpose of blood sampling, animals were contained in single pens, within an animal facility subject to natural light and temperature. When confined, the animals received chaffed lucerne hay and water ad libitum Otherwise, the animals were maintained on pasture with supplemental feeding of meadow hay. Gene cloning A partial sequence of the ovine GnIH precursor gene was generated by rtpcr cloning using ovine hypothalamic cdna extract and primers based on the known bovine RFRP sequence (GenBank accession no. NM_174168) (Hinuma, Shintani et al. 2000), as shown in Figure 1. 63
80 In situ hybridization and immunohistochemistry The regional localization of GnIH cells in the ovine hypothalamus was mapped by in situ hybridization and further definition of localization was obtained by labeling the same sections with antisera that define locality to the PVN/DMN. For the former, we used a monoclonal neurophysin antiserum that identifies oxytocin (OT) and vasopressin (AVP) cells (PS45) (gift from Dr. H Gainer) (Ben Barak, Russell et al. 1985). For the latter we used a rabbit polyclonal antiserum against orexin (ORX) (Sakurai, Amemiya et al. 1998) that we have used previously for ovine brain (Iqbal, Pompolo et al. 2001). This neuropeptide is produced in the dorsomedial nucleus (Iqbal, Pompolo et al. 2001). This combined in situ hybridization and immunohistochemistry procedure was conducted on free floating paraformaldehyde fixed sections (40µm) of ovine hypothalamus, prehybridised for 2h at 58 C in a hybridization buffer (50% formamide, 5XSSC, ph 7.0, 250 µg/ml herring sperm DNA, 100 µg/ml yeast trna, 100 µg/ml heparin, 5% dextran sulfate, 1 x Denhardt s solution and 0.1% Tween 20). The probe was then added to a final concentration of ng/ml and sections were incubated at 58 C overnight. After hybridization, the sections were washed 2 x 30 min in 2 x SSC, 0.1% Tween 20 at 58 C, followed by 2 x 30 min in 0.1 SSC, 0.1% Tween 20 at 58 C. The sections were rinsed in maleic acid buffer 2 x 15 min (0.1M maleic acid, 0.15 M NaCl, 0.1% Tween 20) at room temperature and incubated for 2h in maleic acid buffer containing 2% Boehringer Blocking Reagent (BBR, Roche), 10% goat serum and 2 mm levamisole. A mixture of 3 primary antibodies, including alkaline phosphatase conjugated sheep anti digoxigenin antibody (1:1000, Roche), PS45 (neurophysin) (1:5000) and rabbit anti ORX (dilution 1:2000) was then applied for h at 4 C. The sections were washed in 10 mm Tris HCl 64
81 buffer with 0.9% NaCl (ph 7.4) and the PS45 and ORX labelling was visualised with goat anti mouse Alexa 488 and goat anti rabbit Alexa 546 (1:500, Molecular Probes Inc, Eugene, OR, USA) respectively. To visualize the digoxigenin labelling, the sections were washed 3 x 1 h in maleic acid buffer with 2 mm levamisole. This was followed by 2 x 15 min equilibration steps in alkaline buffer (0.1 M NaCl, 0.1 M Tris HCl, ph 9.5, 0.1 M MgCl 2, 0.1% Tween 20, 2 mm levamisole) with nitroblue tetrazolium (NBR). Then 5 bromo 4 chloro 3 indolyl phosphate (BCIP) salts (Roche) were applied to reveal the digoxigeninlabelled neurons. The sections were then mounted on gelatine chrome coated slides and coverslipped with anti fade fluorescent medium (Dako Corp., Botany, NSW, Australia). A negative control (exclusion of primary antibody) was included for each immunohistochemical run. For in situ hybridisation with 35S label, the 460 base cdna sequence shown in Figure 1 was inserted into a pgemt easy plasmid. Under a standard transcription protocol, antisense and sense ovine GnIH riboprobes were transcribed with T7 and SP6 polymerase respectively (Promega Corp, Madison, WI, USA) with 35S UTP (GE Healthcare Life Sciences, Boston, MA). The riboprobe was separated from unincorporated nucleotides on a Sephadex G 25 column. No signal was observed after the application of sense probe (data not shown). This was applied to sections using a protocol previously described (Estrada, Clay et al. 2006). Anterograde tracing from the PVN to the median eminence A polyclonal antiserum was raised in guinea pig using RFRP 3 conjugated to keyhole limpet hemocyanin as an immunogen (#1354, Antibodies Australia, Melbourne, 65
82 Australia). The titre (1:4000) and specificity of this antiserum was tested by immunohistochemistry on sections of formalin perfused tissues prepared as described previously (Qi, Iqbal et al. 2008). Coronal sections (40µm) of the hypothalamus were cut on a cryostat and prepared for immunohistochemistry as described (Beckman, Shi et al. 2009). Sections that included the region of the paraventricular nucleus (PVN) and the dorsomedial hypothalamic nucleus (DMN) were incubated with the antiserum at a dilution of 1:4000, with or without 0.1mg/ml RFRP 3, quail GnIH, hampster GnIH, kisspeptin, NFF, PrRP, chemerin, GnRH and QRFP43. RFRP 3, quail and hamster GnIH abolished staining, whereas the other peptides did not reduce the intensity of immunostaining (data not shown). In order to determine whether GnIH cells of the PVN/DMN region project to the external zone of the median eminence, we injected 50 70µl biotinylated dextran amine (BDA) into 3 sheep, using methods previously described (Beckman, Shi et al. 2009). The animals were euthanazed after 3 weeks and hypothalamic blocks were processed for immunohistochemistry (Qi, Iqbal et al. 2008). One animal with an injection that was clearly localised to the central region of the PVN was selected for detailed study. BDA was detected by avidin FITC (1:500) and GnIH immunostaining was visualised with Alexa 546 (1:500). Images of the median eminence were merged to determine whether anterograde tracer and GnIH were present in neuronal terminals in the external neurosecretory zone. In vitro analysis of effect of RFRP 3 Pituitaries were obtained from sheep immediately following euthanasia and placed in sterile DMEM solution. The tissues were minced and the cells dissociated by incubation 66
83 (25 min at 37ºC) in 10 ml of DMEM + 0.5% BSA/PBS. This solution contained (per 20 ml) collagenase (0.03 g), DNase (200 μl), hyaluronidase (200 μl), trypsin inhibitor (200 μl) and pancreatin (500 μl). The cells were triturated and the process was repeated twice at 5 min intervals. After passing through mesh and centrifugation, the cells were resuspended in 50 ml 0.1% BSA/PBS. The percentages of live cells (>95%) were estimated by trypan blue exclusion. Finally the cells were pelleted and resuspended in DMEM containing FCS (10%) including antibiotic (1% antimycotic penicillin) and plated out at a density of 0.5 x 106/well in 1 ml DMEM containing 10% FCS and antibiotic for 72h before experimentation. On the day of experimentation, the medium was removed and replaced with serum free DMEM containing 0.1% BSA and allowed to equilibrate for 2h. This medium was then replaced with medium containing either GnRH (10 9 M) or GnRH plus RFRP 3 (Val Pro Asn Leu Pro Gln Arg Phe NH 2 ) that was synthesized by conventional solid phase methodology and purified on preparative HPLC. The peptide was 95% pure on analytical HPLC. The molecular mass was confirmed by mass spectrometry. RFRP 3 was used at doses of 10 8 to M with 6 wells/ treatment. The supernatant was collected 2 h later and assayed for LH and FSH by radioimmunoassay. These experiments were replicated 3 times with similar results, although data are presented for only one of the replicates. In order to determine the specificity of effect on LH and FSH secretion, we treated cells with a non amidated form of GnIH, using the same protocol as above. In this case, a non amidated hamster GnIH was available so the experiments were performed with amidated and non amidated hamster peptide (Ukena, Iwakoshi et al. 2002). 67
84 Effect of RFRP 3 on GnRH stimulated generation of intracellular free calcium. Cells obtained from ovariectomised ewes as described above were allowed to settle on glass coverslips (9 mm diameter) at 35 C, in an atmosphere containing 5% CO and at 2 85% humidity. The cells were used h after plating. The cells were washed with physiological saline solution (PSS) containing (mm): NaCl 117, KCl 5, MgCl 2 2, KH 2 PO 4 0.5, NaHCO 3 5, HEPES 10, glucose 10, and BSA 0.1%, at ph 7.35 and were then loaded with Fluo 4 AM ( M) in PSS containing 0.01% pluronic for 30 min at room temperature (23 C) in the dark. Coverslips with loaded cells attached were transferred to a Warner tissue bath mounted on an Olympus IX71 inverted microscope and continuously superfused with PSS at 33 C for 20 min to allow cleavage of Fluo 4 AM by cytoplasmic esterases. The cells were viewed under confocal conditions during which they were illuminated by a Kr/Ar laser at 488 nm, and the light passed through a Yokogawa CSU22 Nipkow spinning disc to a high sensitivity electron multiplying Andor ixon CCD camera. Full frames were collected at 2 s intervals. Cells that responded to GnRH with an increase in cytoplasmic calcium were accepted as gonadotropes. These cells had low, stable cytoplasmic levels in the absence of stimulation and responded to GnRH with a large spike in cytoplasmic calcium that was sustained for approximately 30 s. Gonadotropes thus defined constituted approximately 10% of pituitary cells. Cytoplasmic calcium fluctuated spontaneously in an additional 10% of cells, but neither the amplitude nor the frequency of these oscillations was changed by GnRH or by RFRP 3. RFRP 3 did not alter cytoplasmic calcium in cells that were unresponsive to GnRH. Following 5 min of control recording, the cells were exposed to M GnRH for 1 min (exposure 1, to identify gonadotropes) followed by a 30 min period of superfusion without GnRH. The cells were 68
85 then challenged with GnRH for a second time at 35 min (exposure 2). Either vehicle or RFRP 3 ( M) was applied for 20 min prior to and during exposure 2, and the laser was turned off in this period to prevent photobleaching of the cells. This was followed by a 1 min application of PSS containing 30 mm KCl (isosmotic substitution of NaCl) at 40 min to confirm integrity of the cells. The experiment included replicates from 8 sheep for vehicle treatment and 3 4 sheep for RFRP 3 treatment. Effect of RFRP 3 on pituitary hormone release in vivo Adult ewes were used at least 1 month after ovariectomy. The animals received bilateral jugular venous cannulae for sampling and infusion of human RFRP 3 (n = 3) or saline vehicle (n = 3). Blood samples were collected at 10 min intervals over 5 h. At 120 min, the ewes received an initial priming dose of 50 μg human RFRP 3 followed by a continuous infusion of 200 μg over 1 h. Blood samples were rapidly transferred to heparinised tubes on ice and then centrifuged at 4 C. Plasma was stored at 20 C until assayed by RIA to measure levels of LH and FSH. In order to determine the specificity of the response, plasma levels of GH, prolactin and cortisol were also measured. Radioimmunoassays Established methods were used for the measurement of LH, (Lee, Cumming et al. 1976), FSH (Bremner, Findlay et al. 1980), GH (Thomas, Mercer et al. 1990), prolactin (Iqbal, Kurose et al. 2006), and cortisol (Bocking, McMillen et al. 1986). 69
86 Data Analysis Treatment means for in vitro data were analysed by analysis of variance. For analysis of in vivo hormone data the mean area under the curve (AUC) was calculated prior to and during infusion of RFRP 3 and LH pulse amplitude was also calculated (Clarke 1993). These data were then analysed by analysis of variance. We used Scheffe s test for post hoc comparisons. For the measurement of cytoplasmic free calcium, responses for single cells to GnRH were expressed as (change in fluorescence exposure 2) x 100/ (change in fluorescence exposure 1). The mean values from cells for each sheep were used for statistical analysis. The least squares method was used to construct a sigmoid concentration response curve, and to determine the maximum amplitude of exposure 2 (E max ) in response to GnRH and the pa 2 value ( log IC 50 ) for the RFRP 3 effect (GraphPad Software, USA). 70
87 Results Cloning of ovine RFRP 3 cdna The partial sequence obtained by cloning of ovine hypothalamic extract is shown in Fig 1. Ovine RFRP 1 could be 34 amino acids or 12 amino acids, depending on where it is cleaved. Ovine RFRP 3 appears identical to human RFRP 3 (Ukena and Tsutsui 2005; Ubuka, Binns et al. 2006)(GenBank EU177779), but isolation of mature ovine peptide is required for confirmation. Identification of RFRP 3 neurons in the ovine brain with projections to the neurosecretory zone of the median eminence RFRP 3 expressing neuronal cell bodies were found in the DMH and the PVN as well as the region between these two nuclei (Fig 2). Further indication of the density of these cells is shown by radiolabelling in Figure 3. Labelling of RFRP 3 expressing cells was not seen in any other region of the ovine brain (data not shown). Anterograde tracing from the PVN showed that cells of this region projected to the neurosecretory zone of the median eminence, since RFRP ir fibres were double labelled with BDA (Fig 4A) and were found in the vicinity of hypophysial portal blood vessels (Fig 4B). Effect of RFRP 3 on LH and FSH release in vitro Dose related inhibition of GnRH stimulated LH and FSH secretion was observed when RFRP 3 was applied to cell cultures (Fig 5A and B), with no effect of RFRP 3 alone (Fig 5C and D). The effect on LH secretion was greater than the effect on FSH secretion. The specificity of effect was confirmed by the lack of effect of de amidated hamster GnIH. Whereas hamster GnIH had an effect on LH and FSH secretion (Fig 6A & B) that was 71
88 similar to that seen with RFRP 3, the non amidated hamster peptide had no effect on GnRH stimulated LH or FSH secretion (Fig 6A & B). Effect of RFRP 3 on LH and FSH release in vivo Administration (iv) of RFRP 3 to ovariectomised ewes suppressed LH pulse amplitude (Control 2.13 ± 0.38 ng/ml, RFRP ± 0.29 ng/ml, P < 0.05) and LH AUC (Fig. 7A), but had no effect on FSH secretion (Fig. 7B) Specificity of action on the gonadotropes was indicated by the absence of effects on either prolactin (Fig. 7C), GH (Fig. 7D) or cortisol (Fig. 7E). Effect of RFRP 3 on GnRH stimulated mobilisation of intracellular calcium A standard dose of GnRH increased cytoplasmic calcium in isolated Fluo 4 labelled gonadotropes and this response was reduced by RFRP3 (Fig 8A). RFRP 3 reduced this response to GnRH in a dose dependent manner, with an IC 50 of M (Fig. 8B). Discussion The results of this study provide strong evidence that RFRP 3 is a hypophysiotropic hormone, that provides an inhibitory signal from the hypothalamus which acts on the pituitary gonadotropes. Thus, RFRP 3 appears to be a bona fide GnIH. This conculusion was reached in the following ways: We identified a cdna in the ovine hypothalamus, which encodes a homolog of RFRP 3. This homolog is identical to that found in human brain. 72
89 RFRP 3 neurons were localised to the PVN and DMH in the ovine brain and anterograde tracing from the PVN indicated projections of RFRP 3 neurons to the neurosecretory zone of the median eminence. RFRP 3 potently inhibited pituitary gonadotropin secretion from the pituitary gland in vitro and when administered i.v. to ovariectomised ewes in vivo. RFRP 3 blocked the calcium signal generated by GnRH in gonadotropes in vitro. The form of RFRP 3 that is found in the ovine brain encodes for a peptide that appears identical to sequence found in the human brain (Hinuma, Shintani et al. 2000; Yoshida, Habata et al. 2003), so it was used in all but one of the experiments in the present study. Ovine/human RFRP 3 differs from the original avian GnIH (SIKPSAYLPLRFamide) that was isolated from quail brain (Tsutsui, Saigoh et al. 2000), as well as rat (Ukena, Iwakoshi et al. 2002) and hamster (Kriegsfeld, Mei et al. 2006) forms of GnIH. These peptides belong to a large family of RF amide peptides that exert various neuroendocrine effects. For example, prolactin releasing peptide (PrRP) is a potent releaser of prolactin in rodents (Hinuma, Habata et al. 1998). Another member of this family that is important in the function of the reproductive axis is kisspeptin, which has a positive influence on GnRH secretion (Smith and Clarke 2007). Clearly the potential exists for the involvement of other RF amide peptides in neuroendocrine function, making this an emerging field of interest. It is possible that these molecules form a tier of modulators of the well recognised hypophysiotropic factors, and GnIH seems to specifically oppose the actions of GnRH. The location of RFRP 3 expressing cells in the ovine brain is within the ventral region of the PVN and throughout the DMH, with cells found between these two nuclei. Whereas 73
90 the GnIH cells are confined to the PVN in birds (Tsutsui, Saigoh et al. 2000; Ukena, Ubuka et al. 2003; Bentley, Kriegsfeld et al. 2006), the location of the neurons is in the DMH of rat, mouse and hamster brain (Ukena and Tsutsui 2001; Kriegsfeld, Mei et al. 2006). Our anterograde tracing data indicate that the GnIH (RFRP 3) cells of this region of the ovine brain project to the external zone of the median eminence, which is consistent with the notion that this peptide acts as a hypophysiotropic factor. It now remains to be determined whether RFRP 3 is actually secreted into hypophysial portal blood and this will require the development of an appropriate assay; such work is in progress. Clearly, the sheep is an ideal species in which to resolve this important issue, since hypophysial portal blood sampling is possible (Clarke and Cummins 1982). Our in vitro data indicate that RFRP 3 is a potent inhibitor of GnRH stimulated gonadotropin release from gonadotropes, with an effect obtained at picomolar concentrations. Whereas this may appear anomalous with many other physiological systems where peptides generally act in the nanomolar range, it should be noted that another RF amide, kisspeptin, is similarly potent in its ability to influence the reproductive axis (Gottsch, Cunningham et al. 2004). Our present data obtained with sheep cells add to earlier work in hamsters showing that either intracerebroventricular or intraperitoneal injection of GnIH reduced plasma LH levels (Kriegsfeld, Mei et al. 2006). This earlier work did not determine whether GnIH had a central or pituitary site of action. Certainly, there is good evidence in birds, hamsters, rats and mice that GnIH neurons project to GnRH cells (Bentley, Perfito et al. 2003; Kriegsfeld, Mei et al. 2006; Ubuka, Kim et al. 2008) and the peptide may therefore act as a neuromodulator, regulating GnRH secretion (Bentley, Kriegsfeld et al. 2006). In addition, avian pituitary expresses GnIH receptor (Yin, Ukena et 74
91 al. 2005; Ubuka, Kim et al. 2008) and there is some evidence in birds for a role for GnIH in the regulation of gonadotropin secretion by direct action on the pituitary gland, but definitive proof of a specific effect in mammals has not been forthcoming until now. We present data to show that RFRP 3 potently inhibits gonadotropin secretion in the sheep and the effect is specific to the gonadotrope. Firstly, a non amidated preparation of hamster GnIH had no effect on pituitary cells, whereas amidated hamster GnIH inhibited gonadotropin secretion to the same extent as RFRP 3. Secondly, with in vivo administration, an effect was seen only on LH secretion and not on plasma levels of prolactin, GH or cortisol. This demonstration of specificity is important, since there are reports of an effect of kisspeptin and RFRP 3 to stimulate the GH axis in cattle (Johnson, Tsutsui et al. 2007; Kadokawa, Matsui et al. 2008), so specificity of RF amide peptides action becomes an issue. Although we did not examine an effect on the thyroid axis, it appears that the effect of GnIH is specific to the pituitary gonadotropes. It will be informative to determine the location of the GPR147, the putative GnIH receptor (Ikemoto and Park 2005). Our in vitro experiments showed an effect of RFRP 3 treatment on GnRH stimulated FSH secretion, but there was no effect of a 1 h infusion on FSH levels in ovariectomised ewes in vivo. Notably, the in vitro effect on FSH secretion was less than that on LH secretion and this may be due to the difference in the mechanism of control of secretion of the two gonadotropins. In addition, an in vivo effect of GnIH upon LH levels was shown in quail, with reduction in alpha subunit and LHβ mrna in the pituitary gland, but not on FSHβ subunit or plasma FSH levels (Ubuka, Ukena et al. 2006). That study did not determine a direct pituitary effect, although another study using avian pituitary cultures showed that 75
92 GnIH reduced FSH and LH secretion with an effect on alpha subunit and FSHβ expression and not LHβ (Ciccone, Dunn et al. 2004). Whereas pulsatile LH secretion is wholly dependent upon secretagogue actions of GnRH, this is not the case for FSH secretion (Clarke, Burman et al. 1986), and apparent pulsatile secretion of FSH is not tightly coupled to GnRH input (Clarke, Burman et al. 1986; Clarke, Moore et al. 2002). Accordingly, one would expect that the relatively lower FSH secretory response to GnRH that is seen in vitro would be less influenced by a suppressive factor than would the LH response. It remains possible that more prolonged treatment with RFRP 3 negatively regulates FSH secretion. We found that GnIH alone had no effect on the secretion of gonadotropins from ovine cells in culture, demonstrating that there is no singular action of the peptide on these cells. This is in accord with the well known fact that gonadotropes require the tropic stimulus of GnRH to function. In the absence of pulsatile GnRH input, gonadotropes neither produce nor secrete gonadotropins (Clarke, Cummins et al. 1983; Mercer, Clements et al. 1989). Our data unequivocally show that RFRP 3 potently blocks the generation of intracellular free calcium elicited by GnRH. Similar to the finding on gonadotropin secretion, RFRP 3 alone was without effect on basal cytoplasmic calcium levels (data not shown). An increase in cytoplasmic calcium levels is essential for the secretion of LH by GnRH in these cells (Smith, Wakefield et al. 1987). The generation of inositol trisphosphate is responsible for the initial large increase in cytoplasmic calcium (Izumi, Stojilkovic et al. 1989) and LH release in response to GnRH (Leong 1991; Stojilkovic and Catt 1992) and it is this 76
93 component of the response that is reduced by RFRP 3. Studies are in progress to probe the site(s) of RFRP 3 action within this second messenger system. Further studies are also in progress to determine whether RFRP 3 acts on other intracellular pathways and the extent to which it may affect the expression of gonadotropin subunits. In summary, we present data that indicates a role for GnIH as a hypophysiotropic hormone. A gene encoding RFRP 3 is identified in the ovine hypothalamus and the cells are localised to the PVN/DMH. These cells project to the neurosecretory zone of the median eminence and RFRP 3 exerts potent and specific effects on pituitary gonadotropes. Accordingly, the secretory response of these cells is profoundly suppressed by RFRP 3, through a negative influence on the generation of intracellular free calcium. Acknowledgements We thank Alix Rao, Alda Pereira, Bruce Doughton and Lynda Morrish for technical support and NIH for assay reagents. The work was supported by grants to GEB from NSF (IOB ) and University California, Berkeley and a Grant in Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan ( and ) to KT. JTS is supported by a Monash University Strategic Grant ECD
94 Figure 1. Ovine GnIH precursor cdna sequence and its deduced amino acid sequence. Ovine RFRP 1 could be 34 amino acids or 12 amino acids, depending on where it is cleaved (first highlighted sequence). Sheep RFRP 3 is also highlighted (VPNLPQRF) and is identical to human and bovine RFRP 3 (Hinuma, Shintani et al. 2000; Yoshida, Habata et al. 2003). This ovine cdna has 93% common identity with bovine RFamide related precursor polypeptide (Accession # AB040291) The C terminal amino acid sequences for ovine GnIH peptides [ LPXRF amide (X = L or Q)] including Gly as an amidation signal and Arg as an endoproteolytic basic amino acid are shown in bold. 78
95 Figure 2. Combined in situ hybridisation localisation of RFRP 3 cells and immunohistochemical localisation of orexin (ORX) and OT and AVP cells (OT + AVP) cells in the ovine brain. A series of sections in the region of the paraventricular nucleus (PVN) and the dorsomedial nucleus (DMN) were used to localise RFRP 3 by in situ hybridisation with digoxigenin label (left column). The same sections were then immunostained for ORX (middle column, red Alexa 546 visualisation) and neurophysin to localise OT and AVP cells (right column, green Alexa 488 visualisation). ORX defines the region of the DMN and 79
96 OT/AVP defines the region of the PVN. Arrows indicate examples of labelled cells. 3V = third ventricle. 80
97 Figure 3. In situ hybridisation with 35S labelled riboprobe, indicating the location of RFRP 3 expressing cells in the paraventricular nucleus (PVN) and the dorsomedial nucleus (DMN). Panel A shows cells mapped to this region (F = fornix; 3V = third ventricle; ME = median eminence). Panel B shows low power expression (scale bar 200 µm) of cells visualised by in situ hybridisation and Panel C shows a higher power (scale bar 200 µm) view of cellular labelling. 81
KISSPEPTIN AND GNIH CONTROL OF GNRH IN FEMALE MAMMALS
 KISSPEPTIN AND GNIH CONTROL OF GNRH IN FEMALE MAMMALS M.J. Zamiri Department of Animal Science, College of Agriculture, Shiraz University, Shiraz, Iran mjzamiri@gmail.com Introduction Since the discovery
KISSPEPTIN AND GNIH CONTROL OF GNRH IN FEMALE MAMMALS M.J. Zamiri Department of Animal Science, College of Agriculture, Shiraz University, Shiraz, Iran mjzamiri@gmail.com Introduction Since the discovery
Menstrual Cycle. Last example of how a circle works. Course Outline. Topic #! Topic lecture! Silverthorn! Membranes (pre-requisite material)!!
 The goal of these lectures is to discuss how control system is formed and operates. For this, basic physiology associated with the control the menstrual cycle will be used. The sections for this lecture
The goal of these lectures is to discuss how control system is formed and operates. For this, basic physiology associated with the control the menstrual cycle will be used. The sections for this lecture
Reproduction. Introduction
 Reproduction The goal of these lectures is to discuss basic physiology associated with the control of reproduction (from sexual diferentiation to adult reproductive function). 26 The sections for this
Reproduction The goal of these lectures is to discuss basic physiology associated with the control of reproduction (from sexual diferentiation to adult reproductive function). 26 The sections for this
Reproductive cyclicity 19. Introduction. Page 1. repro and its story lines. Male repro: a simpler way of control
 Reproductive cyclicity 19 Male repro: a simpler way of control Menstrual cycles: ovary / uterine anatomy and cell types, follicular phase, ovulation, luteal phase, cyclicity Race events: removal of P4
Reproductive cyclicity 19 Male repro: a simpler way of control Menstrual cycles: ovary / uterine anatomy and cell types, follicular phase, ovulation, luteal phase, cyclicity Race events: removal of P4
Endocrine Glands. Endocrine glands
 ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
Development Team. Department of Zoology, University of Delhi. Department of Zoology, University of Delhi
 Paper Module : 06 : 17 Development Team Principal Investigator : Prof. Neeta Sehgal Department of Zoology, University of Delhi Co-Principal Investigator : Prof. D.K. Singh Department of Zoology, University
Paper Module : 06 : 17 Development Team Principal Investigator : Prof. Neeta Sehgal Department of Zoology, University of Delhi Co-Principal Investigator : Prof. D.K. Singh Department of Zoology, University
Animal and Veterinary Science Department University of Idaho. REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5
 Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
Animal and Veterinary Science Department University of Idaho REGULATION OF REPRODUCTION AVS 222 (Instructor: Dr. Amin Ahmadzadeh) Chapter 5 I. DEFINITIONS A. Endocrine Gland B. Hormone Chemical messenger
The Role of Kisspeptin and KNDy Cells in the Reproductive Neuroendocrine System
 Western University Scholarship@Western Electronic Thesis and Dissertation Repository August 2013 The Role of Kisspeptin and KNDy Cells in the Reproductive Neuroendocrine System Christina M. Merkley The
Western University Scholarship@Western Electronic Thesis and Dissertation Repository August 2013 The Role of Kisspeptin and KNDy Cells in the Reproductive Neuroendocrine System Christina M. Merkley The
Hypothalamic Expression of KISS1 and Gonadotropin Inhibitory Hormone Genes During the Menstrual Cycle of a Non-Human Primate 1
 BIOLOGY OF REPRODUCTION 83, 568 577 (2010) Published online before print 23 June 2010. DOI 10.1095/biolreprod.110.085407 Hypothalamic Expression of KISS1 and Gonadotropin Inhibitory Hormone Genes During
BIOLOGY OF REPRODUCTION 83, 568 577 (2010) Published online before print 23 June 2010. DOI 10.1095/biolreprod.110.085407 Hypothalamic Expression of KISS1 and Gonadotropin Inhibitory Hormone Genes During
REPRODUCTION & GENETICS. Hormones
 REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
REPRODUCTION & GENETICS Hormones http://www.youtube.com/watch?v=np0wfu_mgzo Objectives 2 Define what hormones are; Compare and contrast the male and female hormones; Explain what each hormone in the mail
REPRODUCTIVE ENDOCRINOLOGY OF THE MALE
 Reproductive Biotechnologies Andrology I REPRODUCTIVE ENDOCRINOLOGY OF THE MALE Prof. Alberto Contri REPRODUCTIVE ENDOCRINOLOGY OF THE MALE SPERMATOGENESIS AND REPRODUCTIVE BEHAVIOR RELATED TO THE ACTIVITY
Reproductive Biotechnologies Andrology I REPRODUCTIVE ENDOCRINOLOGY OF THE MALE Prof. Alberto Contri REPRODUCTIVE ENDOCRINOLOGY OF THE MALE SPERMATOGENESIS AND REPRODUCTIVE BEHAVIOR RELATED TO THE ACTIVITY
Evaluation of the Hypothalamic Kisspeptin System Throughout the Estrous Cycle and During the Attainment of Puberty in Gilts
 South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Theses and Dissertations 2016 Evaluation of the Hypothalamic Kisspeptin System
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Theses and Dissertations 2016 Evaluation of the Hypothalamic Kisspeptin System
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
Direct Steroidal Regulation and Inhibitory Mode of Action of Gonadotropin-Inhibitory Hormone (GnIH or RFRP-3) in Immortalized Hypothalamic Cell Models
 Direct Steroidal Regulation and Inhibitory Mode of Action of Gonadotropin-Inhibitory Hormone (GnIH or RFRP-3) in Immortalized Hypothalamic Cell Models by Nicole Gojska A thesis submitted in conformity
Direct Steroidal Regulation and Inhibitory Mode of Action of Gonadotropin-Inhibitory Hormone (GnIH or RFRP-3) in Immortalized Hypothalamic Cell Models by Nicole Gojska A thesis submitted in conformity
Regulation of Kisspeptin-expressing neurons and stimulatory mode of action of Kisspeptin in Immortalized Hypothalamic Cell Models
 Regulation of Kisspeptin-expressing neurons and stimulatory mode of action of Kisspeptin in Immortalized Hypothalamic Cell Models Zoey Friedman A thesis submitted in conformity with the requirements for
Regulation of Kisspeptin-expressing neurons and stimulatory mode of action of Kisspeptin in Immortalized Hypothalamic Cell Models Zoey Friedman A thesis submitted in conformity with the requirements for
The Endocrine System. I. Overview of the Endocrine System. II. Three Families of Hormones. III. Hormone Receptors. IV. Classes of Hormone Receptor
 The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
DEVELOPMENTAL CHANGES IN GONADOTROPltf RELEASING HORMONE NEURONS IN THE BRAIN OF THE FEMALE RABBIT (oryctolagus cuniculus)
 DEVELOPMENTAL CHANGES IN GONADOTROPltf RELEASING HORMONE NEURONS IN THE BRAIN OF THE FEMALE RABBIT (oryctolagus cuniculus) By WARREN G. FOSTER, B.Sc., M.Sc. A Thesis Submitted to the School of Graduate
DEVELOPMENTAL CHANGES IN GONADOTROPltf RELEASING HORMONE NEURONS IN THE BRAIN OF THE FEMALE RABBIT (oryctolagus cuniculus) By WARREN G. FOSTER, B.Sc., M.Sc. A Thesis Submitted to the School of Graduate
The reproductive system
 The reproductive system THE OVARIAN CYCLE HORMONAL REGULATION OF OOGENSIS AND OVULATION hypothalamic-pituitary-ovary axis Overview of the structures of the endocrine system Principal functions of the
The reproductive system THE OVARIAN CYCLE HORMONAL REGULATION OF OOGENSIS AND OVULATION hypothalamic-pituitary-ovary axis Overview of the structures of the endocrine system Principal functions of the
/97/$03.00/0 Vol. 138, No. 12
 0013-7227/97/$03.00/0 Vol. 138, No. 12 Endocrinology Printed in U.S.A. Copyright 1997 by The Endocrine Society Estradiol Requirements for Induction and Maintenance of the Gonadotropin-Releasing Hormone
0013-7227/97/$03.00/0 Vol. 138, No. 12 Endocrinology Printed in U.S.A. Copyright 1997 by The Endocrine Society Estradiol Requirements for Induction and Maintenance of the Gonadotropin-Releasing Hormone
Endocrine System. Chapter 18. Introduction. How Hormones Work. How Hormones Work. The Hypothalamus & Endocrine Regulation
 Introduction Endocrine System Chapter 18 The endocrine system consists of cells, tissues, & organs that secrete into the blood Hormone an organic substance secreted by a cell that has an effect on the
Introduction Endocrine System Chapter 18 The endocrine system consists of cells, tissues, & organs that secrete into the blood Hormone an organic substance secreted by a cell that has an effect on the
Lecture 11, 27 Sept 2005 Chapter 14 & 15. Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005
 Lecture 11, 27 Sept 2005 Chapter 14 & 15 Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 instr: Kevin Bonine t.a.: Kristen Potter 1 Vertebrate Physiology 437 Chapter
Lecture 11, 27 Sept 2005 Chapter 14 & 15 Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 instr: Kevin Bonine t.a.: Kristen Potter 1 Vertebrate Physiology 437 Chapter
CASE 41. What is the pathophysiologic cause of her amenorrhea? Which cells in the ovary secrete estrogen?
 CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
CASE 41 A 19-year-old woman presents to her gynecologist with complaints of not having had a period for 6 months. She reports having normal periods since menarche at age 12. She denies sexual activity,
Iranian Journal of Basic Medical Sciences
 Iranian Journal of Basic Medical Sciences ijbms.mums.ac.ir Is precocious puberty linked to hypothalamic expression of arginine-phenylalanine-amide-related peptide? Yuanyuan He 1, Wen Sun 1, Jian Yu 1 *
Iranian Journal of Basic Medical Sciences ijbms.mums.ac.ir Is precocious puberty linked to hypothalamic expression of arginine-phenylalanine-amide-related peptide? Yuanyuan He 1, Wen Sun 1, Jian Yu 1 *
The Effects of the Novel Reproductive Peptide Phoenixin-20 Amide on GnRH and Kisspeptin Hypothalamic Cell Models
 The Effects of the Novel Reproductive Peptide Phoenixin-20 Amide on GnRH and Kisspeptin Hypothalamic Cell Models by Alice K. Treen A thesis submitted in conformity with the requirements for the degree
The Effects of the Novel Reproductive Peptide Phoenixin-20 Amide on GnRH and Kisspeptin Hypothalamic Cell Models by Alice K. Treen A thesis submitted in conformity with the requirements for the degree
Model Answer. M.Sc. Zoology (First Semester) Examination Paper LZT 103 (Endocrinology)
 Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Kisspeptin and other neuropeptides. New opportunities for reproductive endocrinology Nobel Laureates. Richard A Anderson
 Kisspeptin and other neuropeptides Higher centres Timing of puberty Stress New opportunities for reproductive endocrinology E2 +ve E2 -ve Richard A Anderson Obstetrics and Gynaecology University of Edinburgh
Kisspeptin and other neuropeptides Higher centres Timing of puberty Stress New opportunities for reproductive endocrinology E2 +ve E2 -ve Richard A Anderson Obstetrics and Gynaecology University of Edinburgh
CHARACTERIZATION OF HYPOTHALAMIC NEUROPEPTIDES IN MAMMALIAN REPRODUCTION
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2015 CHARACTERIZATION OF HYPOTHALAMIC NEUROPEPTIDES IN MAMMALIAN REPRODUCTION
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2015 CHARACTERIZATION OF HYPOTHALAMIC NEUROPEPTIDES IN MAMMALIAN REPRODUCTION
Effects of Prenatal Testosterone on the Reproductive and Metabolic Neurons of the Sheep Hypothalamus
 Western University Scholarship@Western Electronic Thesis and Dissertation Repository August 2013 Effects of Prenatal Testosterone on the Reproductive and Metabolic Neurons of the Sheep Hypothalamus Maria
Western University Scholarship@Western Electronic Thesis and Dissertation Repository August 2013 Effects of Prenatal Testosterone on the Reproductive and Metabolic Neurons of the Sheep Hypothalamus Maria
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Female Reproductive System. Lesson 10
 Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
Female Reproductive System Lesson 10 Learning Goals 1. What are the five hormones involved in the female reproductive system? 2. Understand the four phases of the menstrual cycle. Human Reproductive System
Evidence that estrogen mediates the positive feedback effect on GnRH
 Advances in Environmental Biology, 3(3): 244-248, 2009 ISSN 1995-0756 2009, American-Eurasian Network for Scientific Information 244 This is a refereed journal and all articles are professionally screened
Advances in Environmental Biology, 3(3): 244-248, 2009 ISSN 1995-0756 2009, American-Eurasian Network for Scientific Information 244 This is a refereed journal and all articles are professionally screened
Chapter 27 The Reproductive System. MDufilho
 Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
Chapter 27 The Reproductive System 1 Figure 27.19 Events of oogenesis. Before birth Meiotic events 2n Oogonium (stem cell) Mitosis Follicle development in ovary Follicle cells Oocyte 2n Primary oocyte
Endocrine Glands: Hormone-secreting organs are called endocrine glands
 University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
Reproductive FSH. Analyte Information
 Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
Monday, 7 th of July 2008 ( ) University of Buea MED30. (GENERAL ENDOCRINOLOGY) Exam ( )
 .. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
.. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH.
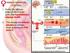 1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
1. During the follicular phase of the ovarian cycle, the hypothalamus releases GnRH. 2. This causes the anterior pituitary to secrete small quantities of FSH and LH. 3. At this time, the follicles in the
Molecular and Cellular Endocrinology
 Molecular and Cellular Endocrinology 324 (2010) 102 109 Contents lists available at ScienceDirect Molecular and Cellular Endocrinology journal homepage: www.elsevier.com/locate/mce Review Seasonal breeding
Molecular and Cellular Endocrinology 324 (2010) 102 109 Contents lists available at ScienceDirect Molecular and Cellular Endocrinology journal homepage: www.elsevier.com/locate/mce Review Seasonal breeding
Ch 11: Endocrine System
 Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
Two important cells in female are the theca cells and the granulose cells. Granulosa cells are affected by the two gonadotropin hormones; FSH and LH.
 1 UGS physiology sheet #13 lecture 3 Dr.Saleem Khresha. Now we will start discussing the female reproductive system Ovarian Steroids Two important cells in female are the theca cells and the granulose
1 UGS physiology sheet #13 lecture 3 Dr.Saleem Khresha. Now we will start discussing the female reproductive system Ovarian Steroids Two important cells in female are the theca cells and the granulose
Hormonal Control of Human Reproduction
 Hormonal Control of Human Reproduction Bởi: OpenStaxCollege The human male and female reproductive cycles are controlled by the interaction of hormones from the hypothalamus and anterior pituitary with
Hormonal Control of Human Reproduction Bởi: OpenStaxCollege The human male and female reproductive cycles are controlled by the interaction of hormones from the hypothalamus and anterior pituitary with
Course: Animal Production. Instructor: Ms. Hutchinson. Objectives: After completing this unit of instruction, students will be able to:
 Course: Animal Production Unit Title: Hormones TEKS: 130.3 (C)(6)(A) Instructor: Ms. Hutchinson Objectives: After completing this unit of instruction, students will be able to: A. Define what hormones
Course: Animal Production Unit Title: Hormones TEKS: 130.3 (C)(6)(A) Instructor: Ms. Hutchinson Objectives: After completing this unit of instruction, students will be able to: A. Define what hormones
Neuroendocrinology an integrative approach
 Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 04 Course website: rci.rutgers.edu/~advis Material to be covered:
Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 04 Course website: rci.rutgers.edu/~advis Material to be covered:
Chapter 14 Reproduction Review Assignment
 Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
The reproductive lifespan
 The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
The reproductive lifespan Reproductive potential Ovarian cycles Pregnancy Lactation Male Female Puberty Menopause Age Menstruation is an external indicator of ovarian events controlled by the hypothalamicpituitary
Hypothalamus & Pituitary Gland
 Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
Ying Wan Master of Neuroscience
 The refinement of the pulsatile Luteinizing Hormone (LH) profile following pubertal maturation: a potential mechanism for enhanced recruitment of ovarian follicles for improved fertility Ying Wan Master
The refinement of the pulsatile Luteinizing Hormone (LH) profile following pubertal maturation: a potential mechanism for enhanced recruitment of ovarian follicles for improved fertility Ying Wan Master
10.7 The Reproductive Hormones
 10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
10.7 The Reproductive Hormones December 10, 2013. Website survey?? QUESTION: Who is more complicated: men or women? The Female Reproductive System ovaries: produce gametes (eggs) produce estrogen (steroid
The decapeptide GnRH is the primary factor responsible
 NEUROENDOCRINOLOGY Identification, Expression, and Physiological Functions of Siberian Hamster Gonadotropin-Inhibitory Hormone Takayoshi Ubuka,* Kazuhiko Inoue,* Yujiro Fukuda, Takanobu Mizuno, Kazuyoshi
NEUROENDOCRINOLOGY Identification, Expression, and Physiological Functions of Siberian Hamster Gonadotropin-Inhibitory Hormone Takayoshi Ubuka,* Kazuhiko Inoue,* Yujiro Fukuda, Takanobu Mizuno, Kazuyoshi
The Endocrine System. PowerPoint Lecture Presentations prepared by Jason LaPres. Lone Star College North Harris
 18 The Endocrine System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris NOTE: Presentations extensively modified for use in MCB 244 & 246 at the University of Illinois
18 The Endocrine System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris NOTE: Presentations extensively modified for use in MCB 244 & 246 at the University of Illinois
Animal Reproduction. Reproductive Cyclicity. # lectures for cumulative test # 02 book 12. Reproductive cyclicity: terminology and basic concepts
 Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 15 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 15 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Emerging ideas about kisspeptin GPR54 signaling in the neuroendocrine regulation of reproduction
 Review TRENDS in Neurosciences Vol.30 No.10 Emerging ideas about kisspeptin GPR54 signaling in the neuroendocrine regulation of reproduction Alexander S. Kauffman 1, Donald K Clifton 2 and Robert A. Steiner
Review TRENDS in Neurosciences Vol.30 No.10 Emerging ideas about kisspeptin GPR54 signaling in the neuroendocrine regulation of reproduction Alexander S. Kauffman 1, Donald K Clifton 2 and Robert A. Steiner
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Endocrine System Hormones (Ch. 45)
 Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
Art labeling Activity: Figure 16.1
 ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
Hypothalamic Control of Posterior Pituitary
 Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior
 Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
Reproductive physiology
 Reproductive physiology Sex hormones: Androgens Estrogens Gestagens Learning objectives 86 (also 90) Sex Genetic sex Gonadal sex Phenotypic sex XY - XX chromosomes testes - ovaries external features Tha
Reproductive physiology Sex hormones: Androgens Estrogens Gestagens Learning objectives 86 (also 90) Sex Genetic sex Gonadal sex Phenotypic sex XY - XX chromosomes testes - ovaries external features Tha
Chapter 20. Endocrine System Chemical signals coordinate body functions Chemical signals coordinate body functions. !
 26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
Endocrine system. Coordination & regulation Glands Hormones
 Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1
 GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
INTERACTIVE ROLES OF GONADOTROPIN-RELEASING HORMONE AND RF-AMIDE RELATED PEPTIDE 3 IN ADENOHYPOPHYSEAL PHYSIOLOGY AND REPRODUCTION IN THE MARE
 INTERACTIVE ROLES OF GONADOTROPIN-RELEASING HORMONE AND RF-AMIDE RELATED PEPTIDE 3 IN ADENOHYPOPHYSEAL PHYSIOLOGY AND REPRODUCTION IN THE MARE A Dissertation by JENNIFER FRANCES THORSON Submitted to the
INTERACTIVE ROLES OF GONADOTROPIN-RELEASING HORMONE AND RF-AMIDE RELATED PEPTIDE 3 IN ADENOHYPOPHYSEAL PHYSIOLOGY AND REPRODUCTION IN THE MARE A Dissertation by JENNIFER FRANCES THORSON Submitted to the
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Female Reproductive System. Justin D. Vidal
 Female Reproductive System Justin D. Vidal If you cannot identify the tissue, then it is probably part of the female reproductive system! Introduction The female reproductive system is constantly changing,
Female Reproductive System Justin D. Vidal If you cannot identify the tissue, then it is probably part of the female reproductive system! Introduction The female reproductive system is constantly changing,
The Endocrine System PART A
 9 The Endocrine System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Endocrine System
9 The Endocrine System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Endocrine System
Endocrine System Hormones. AP Biology
 Endocrine System Hormones 2007-2008 Regulation Why are hormones needed? u chemical messages from one body part to another u communication needed to coordinate whole body u daily homeostasis & regulation
Endocrine System Hormones 2007-2008 Regulation Why are hormones needed? u chemical messages from one body part to another u communication needed to coordinate whole body u daily homeostasis & regulation
Unit Eleven - The Endocrine System
 Unit Eleven - The Endocrine System I. Introduction A. Overview: the endocrine system and nervous system work to control homeostasis within the body. The endocrine system operates at a much pace but the
Unit Eleven - The Endocrine System I. Introduction A. Overview: the endocrine system and nervous system work to control homeostasis within the body. The endocrine system operates at a much pace but the
Reproductive hormones and epilepsy
 3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Teaching Course 7 Treatment of women with epilepsy - Level 1-2 Reproductive hormones and epilepsy Gerhard
3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Teaching Course 7 Treatment of women with epilepsy - Level 1-2 Reproductive hormones and epilepsy Gerhard
Peripubertal, leptin-deficient ob/ob female mice were used in an investigation of
 ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle?
 Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
Investigation: The Human Menstrual Cycle Research Question: How do hormones control the menstrual cycle? Introduction: The menstrual cycle (changes within the uterus) is an approximately 28-day cycle that
Neuroendocrine Regulation of the Estrous Cycle and Seasonal Breeding in the Ewe
 BIOLOGY OF REPRODUCTION 20, 74-85 (1979) Neuroendocrine Regulation of the Estrous Cycle and Seasonal Breeding in the Ewe SANDRA J. LEGAN and FRED J. KARSCH INTRODUCTION One of the most intriguing aspects
BIOLOGY OF REPRODUCTION 20, 74-85 (1979) Neuroendocrine Regulation of the Estrous Cycle and Seasonal Breeding in the Ewe SANDRA J. LEGAN and FRED J. KARSCH INTRODUCTION One of the most intriguing aspects
Ch45: Endocrine System
 Ch45: Endocrine System Endocrine System Homeostasis is the tendency to maintain a stable internal environment. Function = coordinate and control the body with hormones to maintain homeostasis Works with
Ch45: Endocrine System Endocrine System Homeostasis is the tendency to maintain a stable internal environment. Function = coordinate and control the body with hormones to maintain homeostasis Works with
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Reproductive Health and Pituitary Disease
 Reproductive Health and Pituitary Disease Janet F. McLaren, MD Assistant Professor Division of Reproductive Endocrinology and Infertility Department of Obstetrics and Gynecology jmclaren@uabmc.edu Objectives
Reproductive Health and Pituitary Disease Janet F. McLaren, MD Assistant Professor Division of Reproductive Endocrinology and Infertility Department of Obstetrics and Gynecology jmclaren@uabmc.edu Objectives
NOTES 11.5: ENDOCRINE SYSTEM. Pages
 NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Human Biochemistry. Hormones
 Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Interactions of Gonadotropin-Releasing Hormone (GnRH) and Gonadotropin-Inhibitory Hormone (GnIH) in Birds and Mammals
 JOURNAL OF EXPERIMENTAL ZOOLOGY 305A:807 814 (2006) Interactions of Gonadotropin-Releasing Hormone (GnRH) and Gonadotropin-Inhibitory Hormone (GnIH) in Birds and Mammals GEORGE E. BENTLEY 1, LANCE J. KRIEGSFELD
JOURNAL OF EXPERIMENTAL ZOOLOGY 305A:807 814 (2006) Interactions of Gonadotropin-Releasing Hormone (GnRH) and Gonadotropin-Inhibitory Hormone (GnIH) in Birds and Mammals GEORGE E. BENTLEY 1, LANCE J. KRIEGSFELD
8/26/13. Announcements
 Announcements THM questions will start for points on Wednesday. Make sure you are registered correctly! Problems registering for BioPortal? Make sure you are using the link from the syllabus or FAQ. 30
Announcements THM questions will start for points on Wednesday. Make sure you are registered correctly! Problems registering for BioPortal? Make sure you are using the link from the syllabus or FAQ. 30
ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES:
 ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES: -In a living organism there must be coordination of number of physiological activities taking place simultaneously such as: movement, respiration,
ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES: -In a living organism there must be coordination of number of physiological activities taking place simultaneously such as: movement, respiration,
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara
 Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara 1 Endocrine Control: Three Levels of Integration Hormones of the hypothalamic-anterior pituitary
Reproductive System (Hormone Function) Physiology Department Medical School, University of Sumatera Utara 1 Endocrine Control: Three Levels of Integration Hormones of the hypothalamic-anterior pituitary
Summary
 Summary 118 This thesis is focused on the background of elevated levels of FSH in the early follicular phase of women with regular menstrual cycles. In the introduction (chapter 1) we describe the characteristics
Summary 118 This thesis is focused on the background of elevated levels of FSH in the early follicular phase of women with regular menstrual cycles. In the introduction (chapter 1) we describe the characteristics
Principles of Endocrinology
 Principles of Endocrinology 凌雁 Yan Ling Department of Endocrinology and Metabolism Zhongshan Hospital Fudan University Scope of endocrinology Endocrinology is a branch of biology and medicine dealing with
Principles of Endocrinology 凌雁 Yan Ling Department of Endocrinology and Metabolism Zhongshan Hospital Fudan University Scope of endocrinology Endocrinology is a branch of biology and medicine dealing with
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
BIOL 2458 A&P II CHAPTER 18 SI Both the system and the endocrine system affect all body cells.
 BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
9.4 Regulating the Reproductive System
 9.4 Regulating the Reproductive System The Reproductive System to unite a single reproductive cell from a female with a single reproductive cell from a male Both male and female reproductive systems include
9.4 Regulating the Reproductive System The Reproductive System to unite a single reproductive cell from a female with a single reproductive cell from a male Both male and female reproductive systems include
GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH)
 GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH) Naturally occurring hormone, produced by the hypothalamus and transferred to the anterior pituitary gland in the hypophyseal
GONADOTROPHIN (LUTEINISING)- RELEASING HORMONE AND ANALOGUES (GnRH OR LHRH) Naturally occurring hormone, produced by the hypothalamus and transferred to the anterior pituitary gland in the hypophyseal
Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges in Female Rats
 Southern Illinois University Carbondale OpenSIUC Honors Theses University Honors Program 5-10-2014 Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges
Southern Illinois University Carbondale OpenSIUC Honors Theses University Honors Program 5-10-2014 Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges
Endocrine System Notes
 Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine system. Coordination & regulation Glands Hormones
 Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
Reproductive Endocrinology
 Reproductive Endocrinology Reproductive Endocrinology Hypothalamic hormones Gonadotropin releasing hormone (GnRH) - stimulate release of FSH = follicle stimulating hormone LH = luteinizing hormone from
Reproductive Endocrinology Reproductive Endocrinology Hypothalamic hormones Gonadotropin releasing hormone (GnRH) - stimulate release of FSH = follicle stimulating hormone LH = luteinizing hormone from
Homeostasis. Endocrine System Nervous System
 Homeostasis Endocrine System Nervous System 2004-2005 Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body homeostasis & regulation
Homeostasis Endocrine System Nervous System 2004-2005 Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body homeostasis & regulation
Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats
 105 Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats Masahiro Murakami, Toshiya Matsuzaki, Takeshi Iwasa, Toshiyuki Yasui, Minoru Irahara, Tomohiro Osugi
105 Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats Masahiro Murakami, Toshiya Matsuzaki, Takeshi Iwasa, Toshiyuki Yasui, Minoru Irahara, Tomohiro Osugi
Lecture 3. Reproductive Endocrinology. (Sep 30, 2008)
 Lecture 3 Reproductive Endocrinology (Sep 30, 2008) Major achievements that speed up the development: 1. The anterior pituitary controls the function of the gonads. 2. Gonads produce steroid hormones that
Lecture 3 Reproductive Endocrinology (Sep 30, 2008) Major achievements that speed up the development: 1. The anterior pituitary controls the function of the gonads. 2. Gonads produce steroid hormones that
Chapter 26. Hormones and the Endocrine System. Lecture by Edward J. Zalisko
 Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
The Endocrine System PART A
 9 The Endocrine System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Endocrine System
9 The Endocrine System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Endocrine System
Endocrine system. General principle of endocrinology. Mode of hormone delivery to target. Mode of hormone delivery to target
 Endocrine system General principle of endocrinology Co-ordinating system to regulate and integrate function of different cells - Nervous system -Endocrine system Neuro-endocrine system Hormone Molecules
Endocrine system General principle of endocrinology Co-ordinating system to regulate and integrate function of different cells - Nervous system -Endocrine system Neuro-endocrine system Hormone Molecules
I. Endocrine System & Hormones Figure 1: Human Endocrine System
 I. Endocrine System & Hormones Figure 1: Human Endocrine System Endocrine System: a) Endocrine glands are ductless since they lack specific vessels for the transport of hormones throughout the body. Instead,
I. Endocrine System & Hormones Figure 1: Human Endocrine System Endocrine System: a) Endocrine glands are ductless since they lack specific vessels for the transport of hormones throughout the body. Instead,
