Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase
|
|
|
- Bethanie Horton
- 5 years ago
- Views:
Transcription
1 Am J Physiol Endocrinol Metab 302: E1292 E1299, First published March 13, 2012; doi: /ajpendo Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase Iori Ueki, Heather B. Roman, Lawrence L. Hirschberger, Carolyn Junior, and Martha H. Stipanuk Division of Nutritional Sciences, Cornell University, Ithaca, New York Submitted 18 November 2011; accepted in final form 12 March 2012 Ueki I, Roman HB, Hirschberger LL, Junior C, Stipanuk MH. Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am J Physiol Endocrinol Metab 302: E1292 E1299, First published March 13, 2012; doi: /ajpendo Because hepatic cysteine dioxygenase (CDO) appears to play the major role in controlling cysteine catabolism in the intact rat, we characterized the effect of a lack of hepatic CDO on the regulation of cysteine and its metabolites at the whole body level. In mice with liver-specific deletion of CDO expression, hepatic and plasma cysteine levels increased. In addition, in mice with liver-specific deletion of CDO expression, the abundance of CDO and the proportion of CDO existing as the mature, more active isoform increased in extrahepatic tissues that express CDO (kidney, brown fat, and gonadal fat). CDO abundance was also increased in the pancreas, where most of the enzyme in both control and liver CDO-knockout mice was in the more active isoform. This upregulation of CDO concentration and activesite cofactor formation were not associated with an increase in CDO mrna and thus presumably were due to a decrease in CDO degradation and an increase in CDO cofactor formation in association with increased exposure of extrahepatic tissues to cysteine in mice lacking hepatic CDO. Extrahepatic tissues of liver CDO-knockout mice also had higher levels of hypotaurine, consistent with increased metabolism of cysteine by the CDO/cysteinesulfinate decarboxylase pathway. The hepatic CDO-knockout mice were able to maintain normal levels of glutathione, taurine, and sulfate. The maintenance of taurine concentrations in liver as well as in extrahepatic tissues is particularly notable, since mice were fed a taurine-free diet and liver is normally considered the major site of taurine biosynthesis. This redundant capacity for regulation of cysteine concentrations and production of hypotaurine/taurine is additional support for the body s robust mechanisms for control of body cysteine levels and indicates that extrahepatic tissues are able to compensate for a lack of hepatic capacity for cysteine catabolism. sulfur amino acids; sulfate; glutathione A MAJOR PATHWAY OF CYSTEINE METABOLISM, and the major pathway for taurine synthesis, is the oxidative pathway in which cysteine is first oxidized to cysteinesulfinate in a reaction catalyzed by cysteine dioxygenase (CDO). Cysteinesulfinic acid is further metabolized by either 1) decarboxylation to form hypotaurine, which can be further oxidized to taurine, or 2) transamination to the unstable product 3-sulfinylpyruvate, which gives rise to pyruvate and sulfite, with sulfite being further oxidized to sulfate (Fig. 1). Alternative routes of cysteine catabolism in vivo include the desulfhydration reactions catalyzed by cystathionine -lyase and cystathionine -synthase, which can give rise to sulfate, but not taurine, as an end product of cysteine metabolism. Address for reprint requests and other correspondence: M. H. Stipanuk, Div. of Nutritional Sciences, 227 Savage Hall, Cornell University, Ithaca, NY ( mhs6@cornell.edu). Hepatic CDO is highly regulated in response to changes in dietary protein or sulfur amino acid intake, undergoing up to 30-fold changes in concentration and up to 10-fold changes in catalytic efficiency (9, 10, 18, 24, 26). Studies with rodents and cultured cells (hepatocytes, adipocytes) have shown that changes in CDO concentration and activity are highly responsive to cysteine availability in the diet or culture medium (9, 11, 26). The regulation of CDO concentration has been shown to be posttranscriptional and posttranslational, and changes in CDO concentration are the consequence of changes in the rate of its ubiquitination and proteasomal degradation (9, 26). In addition to regulation at the level of enzyme concentration, the activity state of CDO is influenced by the posttranslational formation of a thioether cross-link at the active site; the protein cofactor that is produced by the cysteinyltyrosine cross-link increases the catalytic efficiency of CDO (10). Formation of this cofactor is dependent upon substrate (cysteine) turnover by CDO, making formation of the mature and more active CDO as well as an increase in CDO concentration dependent on cellular cysteine uptake. The mature form of CDO can be detected as a band that runs at a slightly lower apparent molecular mass than the immature form upon polyacrylamide gel electrophoresis. That both the response to CDO turnover and cofactor formation depend on cysteine rather than any precursor or metabolite of cysteine has been demonstrated both in vivo and in vitro (8 10). In rodents, the concentration and activity of CDO is highest in liver, with lower levels present in adipose tissue, pancreas, kidney, and lung (24, 27). Despite the expression of CDO in several tissues, studies in intact rats fed different levels of dietary protein or sulfur amino acids indicated that only hepatic CDO changed in response to the changes in sulfur amino acid intake (27). In rats fed a diet containing 400 g/kg casein, hepatic CDO activity was 34 times and CDO protein abundance was 12.5 times the levels observed in rats fed a diet containing 100 g/kg casein. These changes in CDO were associated with hepatic cysteine, glutathione, and taurine levels that were 3.5, 2.2, and 21.2 times, respectively, those in rats fed the low-protein diet. In the same study, extrahepatic tissues showed no changes in CDO abundance or activity and no changes in the tissue concentrations of cysteine, glutathione, or taurine. The lack of response of extrahepatic tissues may be a reflection of the efficiency of liver in removing sulfur amino acids from the portal circulation, converting them to glutathione, taurine, and sulfate (13). Dominy et al. (11) demonstrated that changes in CDO levels contribute to the control of intracellular cysteine concentrations. The robust regulation of hepatic CDO activity suggests that cysteine homeostasis is very important to the living organism. To gain further understanding of the physiological importance of CDO, we recently explored the ability of germ E /12 Copyright 2012 the American Physiological Society
2 E1293 MATERIALS AND METHODS Fig. 1. Simplified scheme of methionine and cysteine metabolism to taurine and inorganic sulfur. Transsulfuration of methionine transfers methionine sulfur to serine to form cysteine. Cysteine is converted to taurine and sulfate by oxidative metabolism initiated by cysteine dioxygenase. Cysteine can also be catabolized by desulfhydration reactions. Whether the oxidation of hypotaurine to taurine is enzymatic or nonenzymatic in mammalian tissues is uncertain. line CDO-knockout mice to maintain cysteine homeostasis (30). Lack of CDO resulted in extremely low plasma and tissue taurine levels (i.e., 2 7% of wild-type control levels), consistent with the major role of the CDO/cysteinesulfinate decarboxylase pathway in taurine production in mammals. Cysteine levels in CDO-knockout mice were somewhat higher than those in wild-type control mice (e.g., 2 times the wild-type level in plasma and 1.5 times the wild-type level in liver). Furthermore, evidence for excess catabolism of cysteine through desulfhydration pathways, giving rise to hydrogen sulfide, was obtained (30). Because hepatic CDO appears to play the major role in controlling cysteine catabolism in the intact rat, we pursued further characterization of the role of hepatic CDO in regulating levels of cysteine and its metabolites. We crossed conditional CDO-knockout mice with mice expressing Cre recombinase driven by the albumin promoter to generate mice with liver-specific knockout of CDO. Because other cells that express CDO respond to changes in cysteine concentration in vitro (28), we hypothesized that plasma cysteine levels would increase in mice without hepatic CDO and that this would then result in an increase in CDO abundance and activity in other tissues. Generation of floxed CDO targeting construct and CDO / mice. C57BL/6 mice were generated using a Flox/Cre protocol. The floxed mice contained a targeted construct with loxp sites on either side of exon 3 of the CDO1 gene (Fig. 2). Recombination of these loxp sites in liver of mice expressing Cre recombinase driven by the albumin promoter yields hepatocyte-specific knockout of CDO. Conditional CDO-knockout (CDO F/F ) mice generated in our laboratory (30) were crossed with C57BL/6-Tg(Alb-cre)21Mgn/J mice (Jackson Laboratories) that express a transgene for Cre recombinase driven by the albumin gene promoter (AlbCre). Offspring with the CDO F/ AlbCre genotype were further crossed to obtain CDO F/F AlbCre mice. Finally, CDO F/F AlbCre and CDO F/F mice were interbred to obtain the CDO F/F AlbCre and CDO F/F littermates used in this study. Also included in this study as a control for the insertion of the AlbCre gene into the genome were CDO / AlbCre mice. Because albumin expression begins around the time of birth in mice, the disruption of the floxed CDO gene is expected to occur postnatally. Design of targeting vectors and genotyping were done as described previously (30). In this article, the experimental mice are referred to as follows: LKO (liver knockout; CDO F/F AlbCre ), FC (floxed control; CDO F/F ), and CC (Cre control; CDO / AlbCre ). Mice were housed in a pathogen-free barrier facility maintained at 23 C and 45 50% humidity, with a 14-h dark period (lights off from 0600 to 2000). All animals in the breeding colony had access to an irradiated semipurified rodent diet (7012 Teklad LM-485; Harlan Laboratories), and access to water was ad libitum. All experimental procedures involving live animals were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee. Dietary treament. Mice were weaned at 28 days and placed on a semipurified diet that provided 8.0 g/kg diet of methionine equivalents [1 g of cyst(e)ine 1.24 g of methionine equivalents]; this level of sulfur amino acids is similar to that supplied by the semipurified rodent diet. Pups had unrestricted access to this diet up until postnatal day 49. At the beginning of week 8, mice were switched to a diet that provided a slightly higher level of sulfur amino acids (i.e., 12.3 g/kg diet of methionine equivalents). Mice consumed this diet for 1 wk before the experiment was terminated at postnatal day 56, when tissue samples were collected. The composition of the experimental diets is shown in Table 1; diets were prepared in pelleted form by Dyets (Bethlehem, PA). Fig. 2. Generation and analysis of the cysteine dioxygenase (CDO)-null mutation in hepatocytes. Schematic representation of the wild-type (WT) allele ( ), the floxed CDO gene (F) showing the targeted region containing exon 3 flanked by loxp sites (and with an Frt site remaining from removal of the Neo cassette), and the null CDO allele ( ) after Cre-mediated deletion of the targeted region. Genotyping of tail DNA was performed using primer sets indicated by the arrows; the sequences for these primers have been reported (30).
3 E1294 Table 1. Composition of mouse diets 20% Soy Protein Isolate 0.31% L-Methionine (Basal) 20% Casein 0.42% L-Methionine 0.15% L-Cystine (High SAA) Soy protein isolate Vitamin-free casein L-Methionine L-Cystine Cornstarch Dextrinized cornstarch Sucrose Fiber (Solka-Floc) Soybean oil AIN-93G-MX mineral mix AIN-93G-VX vitamin mix Choline bitartrate tert-butylhydroquinone Total methionine equivalents Values are in g/kg diet; 1gofcyst(e)ine 1.24-g methionine equivalents. SAA, sulfur amino acids. Sample collection. At the end of the 7-day period on the sulfur amino acid-enriched diet (i.e., between 1000 and 1400 on day 8), mice were euthanized with CO 2, and blood and tissues were collected. Blood was drawn via cardiac puncture into EDTA-coated tubes. Then the liver, kidneys, pancreas, brown fat (interscapular) pads, and gonadal fat (epididymal or periuterine plus periovarian) pads were removed and immediately frozen in liquid nitrogen. Blood was centrifuged at 18,000 g at 4 C, and plasma was removed and frozen in liquid nitrogen. Tissues and plasma were stored at 80 C until analysis; the stability of CDO protein, aminothiols, and other measured metabolites in samples stored for 6 mo under these conditions was verified in preliminary studies. Measurement of CDO relative abundance. Frozen tissues samples were homogenized in nine volumes of ice-cold lysis buffer (50 mm Tris, ph 7.5, 150 mm NaCl, 1 mm EDTA, 0.5% NP-40) containing 1 Complete Protease Inhibitor Cocktail (Roche; except 4 protease inhibitor for pancreas homogenates) to prepare 10% (wt/vol) tissue homogenates of liver, kidney, brown fat, gonadal fat, and pancreas. Homogenates were centrifuged at 20,000 g for 20 min at 4 C, and the supernatants were stored at 80 C until Western blotting of the soluble proteins was performed. Protein concentration of the supernatant was determined using the BCA Protein Assay kit (Thermo Scientific Pierce Protein Research Products). Proteins were separated by SDS-PAGE, transferred to a membrane, and immunoblotted for CDO, as described previously (25, 30). Immunoreactive protein bands were visualized and quantified using a Li-COR Odyssey infrared imaging system and software scanner (Li-COR Biosciences). CDO values were normalized using actin (liver, kidney, gonadal fat) or GAPDH (brown fat) as a control for loading and transfer. Measurement of CDO mrna relative abundance. RNA was isolated using the RNeasy mini kit or the RNeasy Lipid tissue mini kit (for fat) according to the manufacturer s directions (Qiagen). Complementary DNA was reverse transcribed using Applied Biosystems High Capacity cdna kit (Applied Biosystems) and quantified using Power Sybr Green (Applied Biosystems) in conjunction with a Roche 480 Lightcycler (Roche Diagnostics). Primers for CDO were forward (5=-3=) GATTCTGTGCTGGGGTGAA and reverse (5=-3=) CAGT- GGGAGTCCGTGTGAT. Values for CDO mrna were normalized to values for actin mrna. Measurement of total cystine, total homocysteine, and total glutathione. Aminothiols (thiols plus disulfides, after reduction) were measured by an HPLC method based on components of the methods described by Nolin et al. (19), Frick et al. (12), and Kuhn et al. (16). For liver and fat, an aliquot of the ice-cold homogenate prepared for CDO Western blotting, as described above, was immediately mixed with an equal volume of 10% (wt/vol) trichloroacetic acid (TCA) solution to denature and precipitate protein. To prevent hydrolysis of glutathione by -glutamyltranspeptidase, kidney and pancreas were homogenized directly in 5% (wt/vol) TCA. The acid supernatant was obtained by centrifuging for 20 min at 20,000 g at 4 C. The supernatant was then frozen at 80 C until the HPLC analysis was performed. Standards ranged from 0.1 to 10 M homocysteine, 2 to 200 M cysteine, and 20 to 2,000 M glutathione. Standards were diluted 1:1 in 10% (wt/vol) TCA. Recoveries for internal standards added to tissue samples were complete for all tissue samples as prepared and analyzed. On the day the tissue samples were to be analyzed, supernatants were thawed on ice. To reduce any disulfides present, samples were treated with tris(2-carboxyethyl)phosphine (TCEP). To a 50- l aliquot of tissue supernatant or standard, 125 l of 125 mm borate buffer (ph 9.0) with 1 mm EDTA was added, followed by 4 l of 200 mm TCEP in 125 mm borate buffer (final ph 7 8) and 11 l of 1.55 M NaOH. After the diluted sample was incubated at room temperature for 15 min, thiols were derivatized by adding 50 l of a solution of 1.5 mg/ml borate buffer of ammonium 7-fluorobenzo-2-oxa-I,3-diazole- 4-sulphonate, ph 9.0, and incubating the sample mixture at 60 C for 1 h. Then 20 l of the final sample solution was injected onto a C18 reversed-phase column, and aminothiol derivatives were separated using 100 mm potassium phosphate buffer, ph 2.1, with 5% (vol/vol) acetonitrile as the mobile phase and a flow rate of 1.5 ml/min. Fluorescence of derivatives in the eluate was detected using an excitation wavelength of 385 nm and an emission wavelength of 515 nm. Following chromatography of each sample, the HPLC column was washed with 70% acetonitrile and then reequilibrated with mobile phase. Plasma samples were similarly analyzed for total aminothiol levels except for their initial preparation. For plasma analysis, plasma was thawed on ice on the day of analysis. A 30- l aliquot of the plasma was mixed with 3 l of a 75-mM solution of TCEP in 125 mm borate buffer (final ph 7 8), and the mixture was incubated for 30 min at 4 C. Then 30 l of 10% (wt/vol) TCA-1 mm EDTA was added, and the mixture was centrifuged to obtain the acid supernatant. To 20 l of the acid supernatant, 4 l of 1.55 M NaOH, 50 l of 125 mm borate buffer (ph 9.5) containing 5 mm EDTA, and 20 l of ammonium 7-fluorobenzo-2-oxa-I,3-diazole-4-sulphonate solution (1.5 mg/ml in borate buffer, ph 9.5) were added. This mixture was allowed to incubate for 1 h at 60 C. HPLC analysis was then carried out as described for tissue samples. Measurement of hypotaurine and taurine. For the measurement of taurine and hypotaurine, aliquots of plasma and of the tissue homogenates prepared as described for CDO Western blotting were treated by addition of three volumes of 5% (wt/vol) sulfosalicylic acid and mixing. Acidified samples were centrifuged at 20,000 g for 20 min at 4 C. The supernatants were then frozen at 80 C for later analysis. On the day of analysis, the acid supernatants or standards (1 1,000 M) were diluted 1:50 in 200 mm borate buffer, ph 10.4, and then analyzed by an HPLC method using precolumn derivatization with o-phthaldialdehyde (OPA). Chromatography of derivatized samples was conducted on a mm Nova-Pak C18 column equipped with a C18 guard cartridge. Under conditions of no flow, 75 l of OPA-2-mercaptoethanol-derivatizing reagent and then a 50- l volume of the diluted sample were injected into the precolumn tubing by an automatic sample injector (WISP Model 712; Waters). The derivatizing reagent was prepared fresh daily in a small brown glass container by mixing 7 mg of OPA with 300 l of 95% ethanol, 10 ml of 100 mm borate buffer (ph 10.4), and 20 l of 2-mercaptoethanol. A 1-min delay was programmed prior to flow of mobile phase being initiated to allow reaction of amines with OPA. Amino acids were separated by gradient elution using two buffers. Buffer A was 50 mm potassium phosphate buffer (ph 7.0) 3.5% (vol/vol) tetrahydrofuran, and buffer B was 50 mm potassium phosphate buffer (ph 7.0) 3.5%
4 Table 2. Body weight of LKO and control mice E1295 Body Weight at 7 wk (When Placed on SAA-Enriched Diet) Body Weight at 8 wk (When Experiment was Terminated) Change in Weight While on SAA-Enriched Diet Male Female Male Female Male Female LKO FC CC Values are means SE for 4 8 mice and in g. LKO, liver knockout; FC, floxed control; CC, Cre control. Significant effect of sex difference on body weight, P Change in body weight over the week on SAA-enriched diet was not significantly different from zero for any of the groups. (vol/vol) tetrahydrofuran-40% (vol/vol) acetonitrile. Buffers were filtered through m filters before use. Flow rate was 1.0 ml/min, and column temperature was maintained at room temperature. Mobile phase was started at 80% A-20% B and then changed to 30% A-70% B over 20 min. In between samples, the column was washed with 100% B and then reequilibrated to 80% A-20% B. Detection of OPA-derivatized compounds was performed using excitation and emission peaks at 360 and 455 nm, respectively. Measurement of plasma sulfate. Plasma sulfate levels were measured by HPLC separation and conductivity detection, as described by Hoffman et al. (14). Plasma was centrifuged using a 3,000-MW centrifugal filter (Amicon Ultracel; Millipore) to remove proteins prior to HPLC on a Hamilton PRP X-100 anion exchange column (Hamilton, Reno, NV), using a 2.5-mM phthalate buffer as the mobile phase. A conductivity detector was used to measure levels of sulfate in the eluate. Statistical analysis. Results were analyzed by ANOVA for effects of genotype and sex. Tukey s test was used for comparison of individual means. Differences were considered significant at P RESULTS As shown in Table 2, disruption of the CDO gene in liver tissue had no effect on the body weight of the LKO mice, which was the same as that of the FC and CC mice. Additionally, the body weights of both LKO and control mice were stable during the experimental feeding of the sulfur amino acid-enriched diet. Male mice weighed significantly more (P 0.01) than female mice, as expected. No clinical phenotype was observed in the LKO mice; they appeared to be identical to the FC and CC control mice. Relative CDO abundance was measured in the livers of LKO, FC, and CC mice, as shown in Fig. 3. The absent or negligible CDO in the liver of the LKO mice demonstrates that expression of albumin-cre recombinase had disrupted the CDO1 gene in hepatocytes before the mice were 8 wk of age. As expected, liver of the FC and CC mice expressed CDO, which migrated as two bands. CDO abundance was measured by western blotting in kidney, pancreas, brown fat, and gonadal fat, as shown in Fig. 4. The relative abundance of CDO increased significantly (P 0.05) in the pancreas of both female and male LKO mice and in kidney and brown fat of male mice relative to either FC or CC control mice. CDO abundance in kidney and brown fat of female mice and in the epididymal fat of male LKO mice tended to be greater than that in control mice (P 0.10). CDO abundance in gonadal fat of female mice did not differ from that of control mice (P 0.10). Analysis of tissue CDO mrna by real-time PCR confirmed that the mechanism for upregulation of extrahepatic CDO concentration was not transcriptional, as reported previously for liver CDO. The abundance of CDO mrna in the extrahepatic tissues was the same in LKO, FC, and CC mice for male mice and also for female mice. In addition to the increase in total CDO abundance, the proportion of CDO existing as the mature, more active isoform also increased in the kidney, brown fat, and gonadal fat of LKO mice. As shown in Fig. 5, the fraction of total CDO present as the mature isoform was 18 58% higher in extrahepatic tissues of LKO mice than in control mice. CDO in pancreas (not shown in Fig. 5) ran almost entirely as the lower band, or mature isoform, in both LKO and control mice. The presence of most CDO as the mature isoform in pancreas suggests either that CDO degradation occurs more slowly or that the catalytic turnover of cysteine by CDO is more rapid in the pancreas than in other tissues. To assess whether knockout of hepatic CDO influenced the plasma or tissue levels of cysteine and its metabolites or precursors, cysteine, glutathionine, homocysteine, hypotaurine, and taurine were measured in liver, kidney, pancreas, and plasma, and sulfate was measured in plasma. As shown in Table 3, 8-wk-old LKO mice had sufficient CDO to allow them to maintain relatively normal levels of cysteine and its metabolites. Plasma (P 0.001) and liver (P 0.01) cysteine levels Fig. 3. Western blots of liver of liver knockout (LKO), floxed control (FC), and Cre control (CC) mice for CDO expression. Each lane was loaded with 50 g of total soluble liver protein. Two bands are typically observed when samples containing CDO are separated by SDS-PAGE. These 2 bands correspond to an immature less active isoform (upper band) and a mature more active isoform (lower band). The upper and lower bands migrate with apparent molecular masses of 23 and 22.5 kda, respectively. Actin is also shown as a loading control. Because we used CDO antibody raised in rabbits, we used -actin antibody raised in mice; the -actin antibody raised in mice reacts with another protein in liver samples that is seen here as a lighter band above the -actin band. This 2nd band is not observed if we use -actin antibody raised in rabbits.
5 E1296 Fig. 4. Relative abundance of CDO in nonhepatic tissues of LKO, FC, and CC mice. Representative blots are shown, with each lane loaded with 50 g of total soluble protein. Boxes surrounded by solid lines were from different gels. Dotted lines indicate where a lane was deleted so that the order of blots would be the same as in the bar graph. Values in bar graph are means SE for 4 7 mice, with the mean for the FC mice shown as 1.0. *Value for LKO mice is greater than those for control mice, P Value for LKO mice tends to be greater than those for control mice, P F, female; M, male. were 15 and 45% higher, respectively, in LKO mice than in control mice, whereas kidney and pancreas cysteine levels were not different between LKO and control mice. The levels of cysteine metabolites (glutathione and taurine) were not different from control levels, even in liver of the LKO mice that were no longer expressing hepatic CDO. In contrast, hypotaurine levels were significantly (P 0.01) greater in plasma, kidney, and pancreas of LKO mice than in CC or FC control mice, whereas liver hypotaurine levels were not different for LKO and control mice. The higher levels of hypotaurine in pancreas and kidney are consistent with the upregulation of CDO in these tissues. Although taurine and hypotaurine may be taken up from the plasma, most of the body hypotaurine/taurine pool is in the form of taurine, and the highest amounts of hypotaurine are found in tissues that actively synthesize it by the CDO/cysteinesulfinate decarboxylase pathway. It is notable that, for mice of all genotypes, hypotaurine levels in liver, kidney, and plasma were 3% of the taurine level in the same tissue. In contrast, hypotaurine levels in the pancreas were two to three orders of magnitude higher than those in other tissues, ranging from 24 to 137% of the pancreatic taurine level. It was surprising to us that hypotaurine was so high in pancreas, but the identity of hypotaurine was confirmed by the addition of hydrogen peroxide to samples to oxidize the hypotaurine to taurine, with the hypotaurine peak disappearing and the taurine peak increasing by an equivalent molar amount, as measured with the HPLC method used for this study. Further support for accuracy of these pancreatic hypotaurine values is the absence of hypotaurine in these same tissues from CDO / mice (30). An overall effect of sex on cysteine and some cysteine metabolite levels was observed; plasma cysteine, liver cysteine, plasma homocysteine, and liver homocysteine were higher in female mice, whereas kidney cysteine, liver glutathione, kidney glutathione, pancreas glutathione, and kidney homocysteine were lower in female mice than in male mice. The metabolic basis of these differences was not explored. In previous work, we noted that hepatic CDO levels are significantly higher in female than in male mice, whereas hepatic cysteinesulfinate decarboxylase levels are significantly lower in female than in male mice (30). DISCUSSION Fig. 5. Fraction of total CDO present as the mature isoform (i.e., lower band) in nonhepatic tissues of LKO, FC, and CC mice. Values are means SE for 4 7 mice. *Value for LKO mice is greater than those for both control groups, P Value for LKO mice is greater than that for F/F control mice but not different from that for the CC control group, P In the intact mammal, the liver is normally the most active site for metabolism of dietary sulfur amino acids. Arteriovenous difference studies show that the liver removes methionine and cyst(e)ine from the portal blood and releases taurine, glutathione, and sulfate into the circulation (13). The dominant role of the liver in dietary sulfur amino acid metabolism is further illustrated by the robust upregulation of CDO in the liver, but not extrahepatic tissues, in response to an increased intake of dietary sulfur amino acids or protein (2, 27). Thus, hepatic CDO appears to play a somewhat unique role in
6 Table 3. Cysteine, glutathione, homocysteine, taurine, and sulfate levels in plasma and tissues of CDO LKO and control mice Females Males Statistics (P Value) E1297 LKO FC CC LKO FC CC G S G S Plasma cysteine, mol/l Liver cysteine, mol/g tissue Kidney cysteine, mol/g tissue Pancreas cysteine, mol/g tissue Plasma glutathione, mol/l Liver glutathione, mol/g tissue Kidney glutathione, mol/g tissue Pancreas glutathione, mol/g tissue Plasma homocysteine, mol/l Liver homocysteine, nmol/g tissue Kidney homocysteine, nmol/g tissue Pancreas homocysteine, nmol/g tissue Plasma sulfate, mol/l 1, , , , , Plasma taurine, mol/l Liver taurine, mol/g tissue Kidney taurine, mol/g tissue Pancreas taurine, mol/g tissue Plasma hypotaurine, mol/l Liver hypotaurine, mol/g tissue Kidney hypotaurine, mol/g tissue Pancreas hypotaurine, mol/g tissue Values are means SE for 4 8 mice; values for tissues are expressed as amount per gram wet weight of tissue. CDO, cysteine dioxygenase. Significant effects of genotype (G) or sex difference (S) and their interaction (G S) were determined by ANOVA. Differences were accepted at P Levels of significance ( 0.05) are reported in the last 3 columns. regulating body cysteine homeostasis. Because CDO is also expressed in other tissues, including the adipose tissue, kidney, and pancreas, we questioned whether these tissues could also play an important role in cysteine metabolism, particularly in situations in which a first pass of amino acids through the liver does not occur (i.e., parenteral feeding) or in liver disease, in which hepatic sulfur amino acid metabolism is impaired. Our previous observations that CDO abundance and activity in adipocytes respond to changes in cysteine concentration in vitro suggested that CDO in extrahepatic tissues might respond in a similar manner should these tissues be exposed to an elevated cysteine concentration (28). Thus, we hypothesized that plasma cysteine levels would increase in mice with a liver-specific disruption of the CDO gene and that this would then result in an increase in CDO abundance and activity in extrahepatic tissues. In this study, expression of CDO in hepatocytes was essentially eliminated by the time the mice were 7 wk old and used for this study. Previous studies have shown that the expression of genes driven by the albumin promoter occurs as a result of the process of hepatocyte differentiation, which begins before birth and is complete by about postnatal day 42 in mice (20, 32). In these LKO mice, the relative abundance of CDO in brown and white adipose tissues, kidney, and pancreas was significantly higher compared with FC and CC control mice, demonstrating that extrahepatic tissues of intact animals are able to upregulate CDO abundance in the absence of hepatic CDO or presumably in the face of an increase in circulating cyst(e)ine levels. Furthermore, greater increases were observed for the relative abundance of the more catalytically active isoform of CDO compared with the less active isoform, demonstrating that CDO activity in these tissues was increased by both an increase in CDO concentration and an increased formation of the mature isoform of CDO, just as is observed in livers of wild-type mice fed sulfur amino acid- or proteinenriched diets (9, 30). In our previous work, we have shown that both the increase in CDO abundance, which is due mainly to decreased ubiquitination and degradation of CDO when cysteine levels are high, and the increase in catalytic efficiency, which is due to formation of the mature isoform of CDO, are due specifically to an increase in cellular cysteine supply or concentration (8, 9, 17, 21, 26). A greater abundance of total CDO and a higher proportion of CDO present as the mature isoform were observed in extrahepatic tissues of LKO mice. Thus, it is most likely that CDO in extrahepatic tissues in LKO mice was upregulated in response to higher circulating concentrations of cysteine, at least in the postprandial period. Indeed, plasma cysteine (P 0.01) and hepatic cysteine (P 0.001) levels were significantly higher in LKO mice than in control mice, consistent with the liver having an impaired ability to dispose of excess cysteine, resulting in higher circulating cysteine levels. Liver cysteine was 17 85% higher in LKO mice, with an average of 47% higher, than in control mice, which compares favorably with the 50% higher hepatic cysteine concentrations observed in the CDO / mouse and underscores the physiologically important role of liver in cysteine disposal (30). Plasma cysteine changes in the LKO mice were relatively small, ranging from 7% above control to 20% above control values (average of 15%). Nevertheless, a consistent and significant (P 0.001) increase in plasma cysteine was observed in the LKO mice. Even in our earlier studies with the CDO / mouse (i.e., germ line CDO-knockout), plasma cysteine was only double that of wild-type mice (30). Kidney and pancreas cysteine levels were similar in LKO and control mice. Based on the current understanding of regulation of CDO in response to changes in cysteine, the observation of a sustained increase in extrahepatic CDO abundance and mature isoform abundance indicates that extrahepatic tissues were exposed to higher cysteine levels at some points during each 24-h period, perhaps mostly during the feeding periods, and this is consistent with the higher hepatic and plasma cysteine levels in LKO mice.
7 E1298 In the LKO mouse, the greater ability of extrahepatic tissues to catabolize cysteine probably accounts for the negligible effect of liver-specific CDO-knockout on plasma and extrahepatic tissue cysteine levels. Indeed, a rough estimate of the change in the capacity of pancreas for cysteine dioxygenation suggests that the observed changes in extrahepatic tissue CDO could compensate for the absent hepatic CDO. Based on relative measurements of CDO in various tissues (24), pancreas normally has about 25% as much CDO as the liver per gram of tissue, and this increases in the LKO mouse to about 75% as much CDO as in the wild-type liver. However, the mouse pancreas weighs about 17% as much as the liver, yielding an amount of CDO in the LKO mouse pancreas that is only about 13% of that in the liver of a wild-type mouse. However, essentially all of the pancreatic CDO is the the mature form compared with only about 25% of hepatic CDO in the wild-type mouse being in the mature form. Because the mature form has more than 10 times the catalytic efficiency of immature CDO, the pancreas of the LKO mouse would have about 100% of the capacity of the wild-type liver. In addition to this, the two- to fourfold higher cysteine concentration in pancreas than in liver should also increase flux through the cysteine dioxygenase reaction because the K m of CDO for cysteine is substantially higher ( 4 mm) than tissue cysteine concentrations ( 0.1 mol/g for liver and 0.25 mol/g for pancreas) (21). In the wild-type animal, hepatic metabolism of cysteine is responsible for the majority of taurine synthesis (3, 4, 13). In the CDO / mouse, tissue and plasma taurine concentrations were reduced to less than 10% of wild-type levels (30). It is notable that the plasma and tissue levels of taurine were not different between the LKO and control mice in this study despite the animals being fed a diet that contained no preformed taurine. Clearly, the extrahepatic tissues that express CDO are capable of taurine synthesis in vivo, and the liver is able to actively take up taurine under these circumstances. This observation is consistent with the reported expression of CDO and cysteinesulfinate decarboxylase in white and brown adipose tissue, kidney, and pancreas and also with the ability of adipocytes to produce taurine (15, 24, 28, 29). This indicates that in vivo taurine synthesis by tissues that express CDO is determined largely by cysteine availability, which serves as both the substrate and the molecule that triggers an increase in CDO concentration and activity. Normally liver plays the major role, but if cysteine is administered intravenously or if hepatic metabolism of sulfur amino acids is impaired, other tissues should be able to supply sufficient taurine. In contrast to taurine, greater levels of hypotaurine were present in plasma, kidney, and pancreas of the LKO mice compared with those in control mice. This observation is consistent with the increased CDO activity in extrahepatic tissues of LKO mice and with a role of these tissues in supplying hypotaurine and taurine to the circulation for uptake by other tissues, including liver in the LKO mice. Thus, although most of the body s hypotaurine/taurine pool is in the form of taurine, hypotaurine is a better indicator of active taurine biosynthesis. Previous work has shown that CDO activity plays the major role in determining the flux of cysteine to hypotaurine/taurine (1, 2, 5). Cysteinesulfinate decarboxyase, which catalyzes the decarboxylation of cysteinesulfinate to hypotaurine, is highly expressed in pancreas and kidney as well as in liver (24). Compared with other tissues, pancreas maintains a large proportion of its hypotaurine/taurine pool in the form of hypotaurine. It is possible that this reflects both active synthesis and high turnover of the hypotaurine/taurine pool in the pancreas. Tissue and plasma glutathione, tissue and plasma homocysteine, and plasma sulfate levels were also sustained in the LKO mice, not being different than levels in control mice. Cysteine was clearly not limiting for glutathione synthesis, and liverspecific disruption of the CDO gene would not be expected to affect the ability of liver to synthesize glutathione. Similarly, the metabolism of methionine should be relatively normal in mice lacking hepatic CDO, and cysteine levels were minimally affected, consistent with our observation of similar homocysteine levels in LKO and control mice. Although disruption of the liver CDO gene would block sulfate production via the cysteinesulfinate transamination pathway, the observation that the plasma sulfate level was not different between wild-type and CDO / mice indicates that sulfate was produced by other pathways. This most likely was due largely to the ability of desulfhydration enzymes (i.e., cystathionine -lyase and cystathionine -synthase) to release the reduced sulfur of cysteine, followed by the rapid conversion of sulfide to sulfate (6, 7, 22, 23, 24). Indeed, a block in hepatic taurine synthesis could actively promote inorganic sulfur production from cysteine because all catabolism of cysteine by desulfhydration enzymes eventually results in sulfate production, whereas cysteinesulfinate formed from cysteine by CDO is partitioned between conversion to taurine and catabolism to sulfate and pyruvate. The LKO mice had a normal clinical phenotype, consistent with their ability to upregulate CDO in extrahepatic tissues to maintain cysteine catabolism by the oxidative pathway and hence, to maintain tissue taurine levels and minimize flux of cysteine through desulfhydration pathways. This is in contrast to the phenotype of germ line CDO-knockout mice (CDO / ), which consists of a severe postnatal growth deficit, a high rate of postnatal mortality, joint hypermobility, wry nose, and microscopically visible abnormalities in the elastin fibers in the large arteries and in the parenchyma and small blood vessels of the lungs (30). CDO / mice have elevated levels of cysteine and glutathione, very low levels of taurine, and essentially no hypotaurine in plasma and tissues. Additionally, CDO / mice exhibit excess catabolism of cysteine by desulfhydration pathways as assessed by elevated acid-labile sulfur in tissues, elevated thiosulfate excretion in the urine, loss of cytochrome c oxidase activity, and slightly elevated plasma sulfate levels. Much of the pathology observed in CDO / mice is likely due to elevated cysteine levels and the consequential excess production of H 2 S because taurine supplementation had little effect on the phenotype of CDO / mice (30) and also because the pathologies observed in CDO / mice generally appear earlier and are different from those reported for mice lacking expression of the taurine transporter (31). This study demonstrates that CDO in extrahepatic tissues is able to undergo upregulation of enzyme concentration and activity state in response to an increase in cysteine concentrations in the plasma and/or tissue. Generally, this is not necessary because the liver removes much of the cysteine during the first pass of the portal blood through the liver. The ability of extrahepatic CDO-expressing tissues to assume the roles of cysteine disposal and taurine biosynthesis when cysteine levels are elevated, perhaps because of a lack of liver function, can compensate largely for the loss of hepatic CDO activity.
8 Except for increases in hypotaurine levels in plasma and CDO-expressing extrahepatic tissues, sulfur amino acid metabolites were maintained largely at normal levels in LKO mice at the expense of small increases in hepatic and plasma cysteine concentrations. This is yet another example of the body s robust mechanisms (and backup mechanisms) for control of body cysteine levels. When hepatic metabolism fails to control cysteine levels, CDO is induced in extrahepatic tissues, ensuring sustained production of hypotaurine and taurine and ensuring that cysteine levels in tissues do not rise markedly, which would give rise to the unregulated and excess production of H 2 S and other possibly other reduced sulfur compounds. GRANTS This project was supported by Grant DK from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DISCLOSURES No conflicts of interest, financial or otherwise, are reported by the authors. AUTHOR CONTRIBUTIONS I.U. and M.H.S. did the conception and design of the research; I.U., H.B.R., L.L.H., and C.C.J. performed the experiments; I.U., C.C.J., and M.H.S. analyzed the data; I.U., C.C.J., and M.H.S. interpreted the results of the experiments; I.U. and M.H.S. prepared the figures; I.U., H.B.R., L.L.H., C.C.J., and M.H.S. edited and revised the manuscript; I.U., H.B.R., L.L.H., C.C.J., and M.H.S. approved the final version of the manuscript; C.C.J. and M.H.S. drafted the manuscript. REFERENCES 1. Bagley PJ, Stipanuk MH. The activities of rat hepatic cysteine dioxygenase and cysteinesulfinate decarboxylase are regulated in a reciprocal manner in response to dietary casein level. J Nutr 124: , Bagley PJ, Stipanuk MH. Rats fed a low protein diet supplemented with sulfur amino acids have increased cysteine dioxygenase activity and increased taurine production in hepatocytes. J Nutr 125: , Bella DL, Stipanuk MH. Effects of protein, methionine, or chloride on acid-base balance and on cysteine catabolism. Am J Physiol Endocrinol Metab 269: E910 E917, Bella, Hahn C, Stipanuk H. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol Endocrinol Metab 277: E144 E153, Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol Endocrinol Metab 276: E326 E335, Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H 2S by cystathionine -synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: , Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H 2S biogenesis by human cystathionine -lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: , Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr 133: , Dominy JE, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem J 394: , Dominy JE, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem 283: , E Dominy JE, Hwang J, Stipanuk MH. Overexpression of cysteine dioxygenase reduces intracellular cysteine and glutathione pools in HepG2/C3A cells. Am J Physiol Endocrinol Metab 293: E62 E69, Frick B, Schröcksnadel K, Neurauter G, Wirleitner B, Artner-Dworzak E, Fuchs D. Rapid measurement of total plasma homocysteine by HPLC. Clin Chim Acta 331: 19 23, Garcia RA, Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122: , Hoffman DA, Wallace SM, Verbeeck RK. Simple method for the determination of inorganic sulfate in human serum and urine using single-column ion chromatography. J Chromatogr B 565: , Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mrna expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51: , Kuhn KS, Krasselt AI, Fürst P. Glutathione and glutathione metabolites in small tissue samples and mucosal biopsies. Clin Chem 46: , Kwon YH, Stipanuk MH. Cysteine regulates expression of cysteine dioxygenase and -glutamylcysteine synthetase in cultured rat hepatocytes. Am J Physiol Endocrinol Metab 280: E804 E815, Lee JI, Londono M, Hirschberger LL, Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J Nutr Biochem 15: , Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci 852: , Postic C, Magnuson MA. DNA excision in liver by an albumin-cre transgene occurs progressively with age. Genesis 26: , Siakkou E, Rutledge MT, Wilbanks SM, Jameson GN. Capturing crosslink formation with enzymatic activity in cysteine dioxygenase. Biochim Biophys Acta 1814: , Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine -synthase and -cystathionase to H 2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: , Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: , Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis 34: 17 32, Stipanuk MH, Dominy JE, Ueki I, Hirschberger LL. Measurement of Cysteine Dioxygenase Activity and Protein Abundance. Curr Protoc Toxicol 38: , Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab 286: E439 E448, Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr 132: , Ueki I, Stipanuk MH. 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr 139: , Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 137: , Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab 301: E668 E684, Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Häussinger D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol 428: , Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. Cre activity in fetal albcre mouse hepatocytes: Utility for developmental studies. Genesis 47: , 2009.
The oxidation of the sulfur atom of the sulfur amino
 ANTIOXIDANTS & REDOX SIGNALING Volume 19, Number 12, 2013 ª Mary Ann Liebert, Inc. DOI: 10.1089/ars.2012.5010 ORIGINAL RESEARCH COMMUNICATION The Cysteine Dioxgenase Knockout Mouse: Altered Cysteine Metabolism
ANTIOXIDANTS & REDOX SIGNALING Volume 19, Number 12, 2013 ª Mary Ann Liebert, Inc. DOI: 10.1089/ars.2012.5010 ORIGINAL RESEARCH COMMUNICATION The Cysteine Dioxgenase Knockout Mouse: Altered Cysteine Metabolism
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Supporting Information
 Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
Cysteine Supplementation in Parenteral-fed Critically ill Neonates:
 Cysteine Supplementation in Parenteral-fed Critically ill Neonates: A Randomized Trial Investigating Glutathione Synthesis Stephen B. Shew, M.D. UCLA Division of Pediatric Surgery Nov 29, 2006 Background
Cysteine Supplementation in Parenteral-fed Critically ill Neonates: A Randomized Trial Investigating Glutathione Synthesis Stephen B. Shew, M.D. UCLA Division of Pediatric Surgery Nov 29, 2006 Background
Influence of Dietary Protein Levels on Hepatic Cysteine Dioxygenase Activity in Rainbow Trout
 Fisheries Science 60(2), 229-233 (1994) Influence of Dietary Protein Levels on Hepatic Cysteine Dioxygenase Activity in Rainbow Trout Masahito Yokoyama, Miho Udagawa, and Jun-ichi Nakazoe National Research
Fisheries Science 60(2), 229-233 (1994) Influence of Dietary Protein Levels on Hepatic Cysteine Dioxygenase Activity in Rainbow Trout Masahito Yokoyama, Miho Udagawa, and Jun-ichi Nakazoe National Research
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
Preparation of SG3249 antibody-drug conjugates
 Preparation of SG3249 antibody-drug conjugates Conjugate A: Herceptin-SG3249 (ConjA) Antibody (15 mg, 100 nanomoles) was diluted into 13.5 ml of a reduction buffer containing 10 mm sodium borate ph 8.4,
Preparation of SG3249 antibody-drug conjugates Conjugate A: Herceptin-SG3249 (ConjA) Antibody (15 mg, 100 nanomoles) was diluted into 13.5 ml of a reduction buffer containing 10 mm sodium borate ph 8.4,
Amino acids. Ing. Petrová Jaroslava. Workshop on Official Controls of Feed AGR 46230, , Ankara. Turkey ÚKZÚZ - NRL RO Praha 1
 Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Student Number: To form the polar phase when adsorption chromatography was used.
 Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
Choline Assay Kit (Fluorometric)
 Product Manual Choline Assay Kit (Fluorometric) Catalog Number MET- 5042 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Choline is a water soluble amine that is an essential
Product Manual Choline Assay Kit (Fluorometric) Catalog Number MET- 5042 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Choline is a water soluble amine that is an essential
For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin
 Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
 Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were
 Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were separated via ultracentrifugation and lysed to analyze
Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were separated via ultracentrifugation and lysed to analyze
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Curriculum Vitae. Martha Harney Stipanuk
 Curriculum Vitae Martha Harney Stipanuk Educational Background: BS: University of Kentucky, Lexington, KY, Home Economics/Education, 1970 MS: Cornell University, Ithaca, NY, Human Nutrition and Biochemistry,
Curriculum Vitae Martha Harney Stipanuk Educational Background: BS: University of Kentucky, Lexington, KY, Home Economics/Education, 1970 MS: Cornell University, Ithaca, NY, Human Nutrition and Biochemistry,
Nature Protocols: doi: /nprot Supplementary Figure 1. Fluorescent titration of probe CPDSA.
 Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2*
 Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit User Manual (v5)
 Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
BIOCHEMISTRY & MEDICINE:
 BIOCHEMISTRY & MEDICINE: INTRODUCTION Biochemistry can be defined as the science of the chemical basis of life (Gk bios "life"). The cell is the structural unit of living systems. Thus, biochemistry can
BIOCHEMISTRY & MEDICINE: INTRODUCTION Biochemistry can be defined as the science of the chemical basis of life (Gk bios "life"). The cell is the structural unit of living systems. Thus, biochemistry can
UV Tracer TM Maleimide NHS ester
 UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
Free Fatty Acid Assay Kit (Fluorometric)
 Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo
 Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo BERNHARD H. LAUTERBURG, GEORGE B. CORCORAN, and JERRY R. MITCHELL, Baylor College of
VaTx1 VaTx2 VaTx3. VaTx min Retention Time (min) Retention Time (min)
 a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
a Absorbance (mau) 5 2 5 3 4 5 6 7 8 9 6 2 3 4 5 6 VaTx2 High Ca 2+ Low Ca 2+ b 38.2 min Absorbance (mau) 3 2 3 4 5 3 2 VaTx2 39.3 min 3 4 5 3 2 4. min 3 4 5 Supplementary Figure. Toxin Purification For
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
DAG (Diacylglycerol) Assay Kit
 Product Manual DAG (Diacylglycerol) Assay Kit Catalog Number MET-5028 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Diacylglycerols (DAG) are key intermediates in the
Product Manual DAG (Diacylglycerol) Assay Kit Catalog Number MET-5028 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Diacylglycerols (DAG) are key intermediates in the
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Module C CHEMISTRY & CELL BIOLOGY REVIEW
 Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Total Phosphatidic Acid Assay Kit
 Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
20S Proteasome Activity Assay Kit
 20S Proteasome Activity Assay Kit For 100 Assays Cat. No. APT280 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23
20S Proteasome Activity Assay Kit For 100 Assays Cat. No. APT280 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard. Product Number: AD0013
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Mammalian Membrane Protein Extraction Kit
 Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Prerequisites Protein purification techniques and protein analytical methods. Basic enzyme kinetics.
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
Effect of Excess of Individual Essential Amino Acids in Diets on Chicks
 135 Effect of Excess of Individual Essential Amino Acids in Diets on Chicks Jun-ichi OKUMURA and Kiyoto YAMAGUCHI Laboratory of Animal Nutrition, Faculty of Agriculture, Nagoya University, Nagoya-shi 464
135 Effect of Excess of Individual Essential Amino Acids in Diets on Chicks Jun-ichi OKUMURA and Kiyoto YAMAGUCHI Laboratory of Animal Nutrition, Faculty of Agriculture, Nagoya University, Nagoya-shi 464
LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade
 AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h]
![1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h] 1.5 ASK1KO fed. fasted 16 hrs w/o water. Fed. 4th. 4th WT ASK1KO N=29, 11(WT), ,5(ASK1KO) ASK1KO ASK1KO **** Time [h]](/thumbs/87/97258189.jpg) 7: 13: 19: 1: 7: 151117 a 151117 4th 4th b c RQ.95 KO.9.85.8.75.7 light dark light dark.65 7: 19: 7: 19: 7: Means ± SEM, N=6 RQ 1..9.8.7.6.6 KO CL (-) CL (+) ibat weight ratio (/body weight) [%].5.4.3.2.1
7: 13: 19: 1: 7: 151117 a 151117 4th 4th b c RQ.95 KO.9.85.8.75.7 light dark light dark.65 7: 19: 7: 19: 7: Means ± SEM, N=6 RQ 1..9.8.7.6.6 KO CL (-) CL (+) ibat weight ratio (/body weight) [%].5.4.3.2.1
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry. Supporting Information
 Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
Characterization of Disulfide Linkages in Proteins by 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry M. Montana Quick, Christopher M. Crittenden, Jake A. Rosenberg, and Jennifer S. Brodbelt
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin
 SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
Application Note. Introduction. Analysis of Total Aflatoxins in Food by HPLC and UHPLC
 Introduction Aflatoxins are a group of mycotoxins produced by microorganisms such as Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius living in tropical or subtropical regions and have
Introduction Aflatoxins are a group of mycotoxins produced by microorganisms such as Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius living in tropical or subtropical regions and have
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
DELFIA Tb-DTPA ITC Chelate & Terbium Standard
 AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana
 Biochemistry: Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana 38 CAMERON E. NIENABER ʻ04 Abstract Long recognized as essential plant nutrients and metabolites, inorganic and organic
Biochemistry: Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana 38 CAMERON E. NIENABER ʻ04 Abstract Long recognized as essential plant nutrients and metabolites, inorganic and organic
MagCapture Exosome Isolation Kit PS Q&A
 MagCapture Exosome Isolation Kit PS Q&A Specifications and performance P.1 Comparison of the conventional method P.2 Operation methods and composition P.4 Amount of starting sample P.5 Analysis after exosomes
MagCapture Exosome Isolation Kit PS Q&A Specifications and performance P.1 Comparison of the conventional method P.2 Operation methods and composition P.4 Amount of starting sample P.5 Analysis after exosomes
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
PAF Acetylhydrolase Assay Kit
 PAF Acetylhydrolase Assay Kit Catalog Number KA1354 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
PAF Acetylhydrolase Assay Kit Catalog Number KA1354 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
Item Catalog Number Manufacturer 1,4-Dithioerythritol (1 g) D9680 Sigma-Aldrich
 SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Information
 Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Supplementary Information Akt regulates hepatic metabolism by suppressing a Foxo1 dependent global inhibition of adaptation to nutrient intake Mingjian Lu 1, Min Wan 1, Karla F. Leavens 1, Qingwei Chu
Effects of Addition of Sulfur-containing Amino Acids and Their Catabolites to a Low Protein Diet on Liver Fat Content in Rats
 Agr. Biol. Chem., 40 (3), 593 `597, 1976 Effects of Addition of Sulfur-containing Amino Acids and Their Catabolites to a Low Protein Diet on Liver Fat Content in Rats Toshizo KIMURA and Akira YOSHIDA Laboratory
Agr. Biol. Chem., 40 (3), 593 `597, 1976 Effects of Addition of Sulfur-containing Amino Acids and Their Catabolites to a Low Protein Diet on Liver Fat Content in Rats Toshizo KIMURA and Akira YOSHIDA Laboratory
Glutathione Assay Kit
 Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Supplementary Information. Deciphering the Venomic Transcriptome of Killer- Wasp Vespa velutina
 Supplementary Information Deciphering the Venomic Transcriptome of Killer- Wasp Vespa velutina Zhirui Liu, Shuanggang Chen, You Zhou, Cuihong Xie, Bifeng Zhu, Huming Zhu, Shupeng Liu, Wei Wang, Hongzhuan
Supplementary Information Deciphering the Venomic Transcriptome of Killer- Wasp Vespa velutina Zhirui Liu, Shuanggang Chen, You Zhou, Cuihong Xie, Bifeng Zhu, Huming Zhu, Shupeng Liu, Wei Wang, Hongzhuan
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Note: During 30 minute incubation; proceed thru appropriate sections below (e.g. sections II, III and V).
 LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography
 Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Lecture Notes 2: Protiens
 Lecture Notes 2: Protiens BY/ARSHED ABD ALI SHIHAD Proteins and Amino Acids What Are Proteins? Large molecules Made up of chains of amino acids Are found in every cell in the body Are involved in most
Lecture Notes 2: Protiens BY/ARSHED ABD ALI SHIHAD Proteins and Amino Acids What Are Proteins? Large molecules Made up of chains of amino acids Are found in every cell in the body Are involved in most
NITROGEN METABOLISM An Overview
 1 University of Papua New Guinea School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PBL Seminar & Health Sciences NITROGEN METABOLISM
1 University of Papua New Guinea School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PBL Seminar & Health Sciences NITROGEN METABOLISM
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
E.Z.N.A. SQ Blood DNA Kit II. Table of Contents
 E.Z.N.A. SQ Blood DNA Kit II Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Blood Storage and DNA Yield...4 Preparing Reagents...5 100-500 μl Whole Blood Protocol...6
E.Z.N.A. SQ Blood DNA Kit II Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Blood Storage and DNA Yield...4 Preparing Reagents...5 100-500 μl Whole Blood Protocol...6
Supporting Information File S2
 Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Mouse Hydrogen Peroxide (H2O2) Fluorescent Detection Kit
 Mouse Hydrogen Peroxide (H2O2) Fluorescent Detection Kit CATALOG NO: IRAAKT2552 LOT NO: SAMPLE INTENDED USE The Hydrogen Peroxide Fluorescent Detection Kit is designed to quantitatively measure H2O2 in
Mouse Hydrogen Peroxide (H2O2) Fluorescent Detection Kit CATALOG NO: IRAAKT2552 LOT NO: SAMPLE INTENDED USE The Hydrogen Peroxide Fluorescent Detection Kit is designed to quantitatively measure H2O2 in
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Improve Protein Analysis with the New, Mass Spectrometry- Compatible ProteasMAX Surfactant
 Improve Protein Analysis with the New, Mass Spectrometry- Compatible Surfactant ABSTRACT Incomplete solubilization and digestion and poor peptide recovery are frequent limitations in protein sample preparation
Improve Protein Analysis with the New, Mass Spectrometry- Compatible Surfactant ABSTRACT Incomplete solubilization and digestion and poor peptide recovery are frequent limitations in protein sample preparation
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow
 Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow Purpose Described herein is a workflow that combines the isobaric tagging reagents, itraq Reagents, with the separation power
Multiplex Protein Quantitation using itraq Reagents in a Gel-Based Workflow Purpose Described herein is a workflow that combines the isobaric tagging reagents, itraq Reagents, with the separation power
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
SUPPLEMENTARY DATA Supplementary Figure 1. Body weight and fat mass of AdicerKO mice.
 SUPPLEMENTARY DATA Supplementary Figure 1. Body weight and fat mass of AdicerKO mice. Twelve week old mice were subjected to ad libitum (AL) or dietary restriction (DR) regimens for three months. (A) Body
SUPPLEMENTARY DATA Supplementary Figure 1. Body weight and fat mass of AdicerKO mice. Twelve week old mice were subjected to ad libitum (AL) or dietary restriction (DR) regimens for three months. (A) Body
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
III. TOXICOKINETICS. Studies relevant to the toxicokinetics of inorganic chloramines are severely
 III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis
 Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis Product # LS-UR2-2.1x100 Ludger Document # LS-uR2-DMB-Guide-v2.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United Kingdom
Product Guide for LudgerSep TM ur2 UHPLC Column for DMB Sialic Acid Analysis Product # LS-UR2-2.1x100 Ludger Document # LS-uR2-DMB-Guide-v2.1 Ludger Ltd Culham Science Centre Oxford OX14 3EB United Kingdom
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
Human Hydrogen Peroxide Fluorescent Detection Kit
 Human Hydrogen Peroxide Fluorescent Detection Kit CATALOG NO: IRAAKT2525 LOT NO: SAMPLE INTENDED USE The Hydrogen Peroxide Fluorescent Detection Kit is designed to quantitatively measure H₂O₂ in a variety
Human Hydrogen Peroxide Fluorescent Detection Kit CATALOG NO: IRAAKT2525 LOT NO: SAMPLE INTENDED USE The Hydrogen Peroxide Fluorescent Detection Kit is designed to quantitatively measure H₂O₂ in a variety
Human Leptin ELISA Kit
 Product Manual Human Leptin ELISA Kit Catalog Numbers MET-5057 MET-5057-5 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Leptin is a polypeptide hormone
Product Manual Human Leptin ELISA Kit Catalog Numbers MET-5057 MET-5057-5 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Leptin is a polypeptide hormone
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):
 Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
The following protocol describes the isolation of nuclei from tissue. Item. Catalog No Manufacturer
 SOP: Nuclei isolation from tissue and DNaseI treatment Date modified: 090923 Modified by: P. Sabo. (UW) The following protocol describes the isolation of nuclei from tissue. Ordering Information Item.
SOP: Nuclei isolation from tissue and DNaseI treatment Date modified: 090923 Modified by: P. Sabo. (UW) The following protocol describes the isolation of nuclei from tissue. Ordering Information Item.
BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice
 SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
Supplementary Information
 Supplementary Information Overexpression of Fto leads to increased food intake and results in obesity Chris Church, Lee Moir, Fiona McMurray, Christophe Girard, Gareth T Banks, Lydia Teboul, Sara Wells,
Supplementary Information Overexpression of Fto leads to increased food intake and results in obesity Chris Church, Lee Moir, Fiona McMurray, Christophe Girard, Gareth T Banks, Lydia Teboul, Sara Wells,
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Supplemental Figures
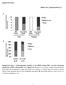 Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
Supplemental Figures Supplemental Figure 1. Fasting-dependent regulation of the SREBP ortholog SBP-1 and lipid homeostasis mediated by the SIRT1 ortholog SIR-2.1 in C. elegans. (A) Wild-type or sir-2.1(lof)
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
