namibia UniVERSITY OF SCIEnCE AnD TECHnOLOGY
|
|
|
- Sherilyn Harrell
- 5 years ago
- Views:
Transcription
1 ' I namibia UniVERSITY OF SCIEnCE AnD TECHnOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES QUALIFICATION: QUALIFICATION CODE: LEVEL: 4 COURSE CODE: BSC410S COURSE NAME: BASIC SCIENCE SESSION: JUNE 2016 PAPER: THEORY DURATION: 3 HOURS MARKS: 100 FIRST OPPORTUNITY EXAMINATION QUESTION PAPER EXAMINER($) MR. VAINO INDOGO, MR. VICTOR UZOMA AND MRS SELMA IMENE-SCHUTI MODERATOR: PROF. HABAUKA M. KWAAMBWA INSTRUCTIONS 1. Write all your answers in the answer booklet provided, use black/blue ink pen only. 2. Read the whole question before answering 3. Begin each question on a new page PERMISSIBLE MATERIALS Scientific Calculator ATTACHMENT Periodic Table THIS QUESTION PAPER CONSISTS OF 13 PAGES (Including this front page and attachment)
2 SECTION A: BIOLOGY [35] QUESTION 1 (15) Question Type: Multiple Choice Questions. Each question equals 1 mark. 1.1 Of the six kingdoms now recognized, A. two are plants and four are animals B. four are eukaryotes and two are prokaryotes C. four are macroscopic and two are microscopic D. two are eukaryotes and four are prokaryotes 1.2 A garden pea plant forms flowers that undergo self or cross pollination to produce seeds. These seeds mature in two to three weeks. Which characteristics of living things are being described here? A. Growth and reproduction B. Cellular organization and use of energy C. Reproduction and response to environment D. Use of energy and development 1.3 Fungi that obtain their food directly from organic matter are called; A. Autotrophs B. Mutualists C. Heterotrophs D. Saprotrophs 1.4 An organism's scientific name consists of its A. Its class name and its family name B. Its kingdom name and its phylum name C. Its genus name and its species name D. Its phylum name and its species name 1.5 Dioecious plants have; A. Separate male and female flowers on the same plant B. Separate male and female flowers on different locations on the same plant C. Separate male flowers on one plant and female flowers on another plant. D. Same male and female flowers on different location on different plant. 1.6 A diet high in saturated fats can be linked to which of the following? A. kidney failure B. rickets C. anorexia D. cardiovascular or heart disease 2
3 1.7 A mineral that the body needs to work properly especially to absorb Vitamin Dis A. calcium B. iron C. magnesium D. lead 1.8 Fibre cannot actually be digested; however, it is an important part of our diet for various reasons. One of the following reasons is wrong, identify which. A. As it remains undigested it passes through the entire gut from mouth to anus and thus keeps food moving smoothly through our system. B. It causes constipation. C. The fibre absorbs poisonous waste from the digested food. D. High fibre diets are believed to reduce the risk of heart disease, bowel cancer and cholesterol in the body. 1.9 The three types of carbohydrates are A. glycerol, polysaccharide, monosaccharide B. disaccharide, monosaccharide, glycerol C. glycerol, monosaccharide, polysaccharide D. monosaccharide, polysaccharide, disaccharide 1.10 Glycogen, a polysaccharide, in your liver may be broken down to glucose by the process of A. hydrolysis B. dehydration synthesis C. condensation D. isomerization 1.11 The three macronutrients are A. monosaccharide, polysaccharide, disaccharide B. fibre, vitamins, proteins C. carbohydrates, lipids, glycerol D. lipids, carbohydrates, proteins 1.12 About 50-60% of the human body weight consists of water. One of the following is NOT a function of water in the human body: A. acts as a solvent for nutrients B. helps regulate the body temperature C. clots the blood D. participates in chemical reactions 1.13 All the following statements for the main uses of fermentation are true EXCEPT: A. It can be used for preservation B. It can used to breakdown proteins C. It can be used to change the nutritional value of food products D. It can be used to create or improve sensory characteristics of foods 3
4 1.14 A diet lacking Vitamin D and calcium can be linked to which of the following? A. kidney failure B. scurvy C. osteoporosis D. cardiovascular disease 1.15 The organic and inorganic materials in all the organisms will eventually return to the environment by the action of: (1} A. primary consumers B. producers C. decomposers D. secondary consumers QUESTION 2. [20) Question Type: Structured Questions. 2.1 Provide the 3 economic importance of Conifers Plants (6} 2.2 What is the ecological importance ofthe Decomposers in an ecosystem? (2} 2.3 Give 2 reasons why animals or plants may become endangered of extinct. (4) 2.4 Explain the food types you would recommend for an individual with the following symptoms. Justify your answer. (a) Bleeding gums (2} (b) Low hemoglobin content (2} (c) Oesteroposis 2.5 Explain why taking antibiotics for a long period oftime may lead to vitamin K deficiency? (2} SECTION B: CHEMISTRY [35] QUESTION 1 {15) Question Type: Multiple Choice Questions. Each question equals 1 mark. 1.1 The two physical quantities that define any sample of matter are; A Weight and volume B. Mass and Weight C. Mass and Volume D. Volume and Area (1} 4
5 1.2 Sublimation is the transformation of a state of matter from a; A. Solid to a liquid B. Liquid to gas C. Solid to gas D. Gas to liquid 1.3 An appropriate physical method that can be used to separate two liquids with different boiling points is; A. Chromatography B. Simple distillation C. Separating funnel D. Fractional distillation 1.4 Which one of the following transformations is a suitable example of a chemical change? A. Melting steel B. A plant growing C. Chopping wood D. None of the above 1.5 is an example of an intensive physical property. A. Density B. Mass C. Volume D. All of the above 1.6 Which of the following samples of matter is NOT classified as a compound? A. Blood B. Water C. Salt D. Sugar 1.7 Which of the following is NOT a characteristic of a mixture? A. No definite composition B. Cannot be separated physical and chemically C. Retain the original properties D. None of the above 1.8 The process of condensation is classified as an process and involves the in energy of the particles which make up the sample of matter. A. Endothermic and increase B. Exothermic and increase C. Endothermic and decrease D. Exothermic and decrease 5
6 1.9 In terms of the Kinetic Theory of Atoms, the intermolecular force between atoms when a solid is transformed into a liquid. A. Increase B. Decrease C. Remain the same D. Equals zero 1.10 How does the presence of an impurity affect the freezing point and boiling point of water? A. Raises the freezing point and lowers the boiling point B. Lowers the freezing point and lowers the boiling point C. Lowers the freezing point and raises the boiling point D. Raises the freezing point and raises the boiling point 1.11 When salt dissolves in water, the water is the A. solute. B. solvent. C. solubility. D. solution If the solute particles in a solution are large enough to be seen and to be filtered, the mixture is a (an) A. colloid. B. true solution. C. electrolyte. D. suspension n a mixture of salt dissolved in water, the solute is A. the salt. B. the water. C. separated by filtering. D. the precipitate All of the following processes will increase the rate of dissolving a solid in a liquid EXCEPT for A. powdering the solid. B. cooling the solution. C. heating the solution D. stirring the solution 1.15 Which ofthe following describes the carbon dioxide component of a carbonated beverage? A. Solvent B. Precipitate c. Solution D. Solute 6
7 QUESTION 2 {20) Question Type: Structured Questions. 2.1 In each of the following lists, identify the two terms which are related and provide an explanation as to either why the two terms you have chosen are related or why the third is not related. (3) a. Kelvin, Decimetre and Kilometre b. Kilogram, Cubic centimetre and litre c. Time, Distance and seconds 2.2 Perform the following mathematical operations and express each of the answers in scientific notation. a. (2.74 X 10 3 )- (4.22 X 10 5 ) + (6.35 X 10 4 ) b. [6.48X105] X (1.8 X 108) 2.4X Differentiate between the terms accuracy and precision. 2.4 Using a Kelvin thermometer, the temperature in a freezer was recorded to be 169 K. Convert the temperature to Degree Celsius loq and Fahrenheit ( F) using the appropriate formulae. 2.6 Carry out the following calculations and record the answer to the correct number of significant figures or where applicable, to the correct number of decimal places. (3) a. {0.250 I 25.00) x b c. { ) 1 { ) 2.6 Apply the rules of rounding off numbers and round off the numbers below to the number of significant figures stated. {2) a. Round off nm to two significant figures b. Round off g to three significant figures 2.7 The diagram below shows the electron arrangements of magnesium and oxygen: 7
8 a) Draw a diagram showing how a bond is made between magnesium and oxygen. {2) b) What name is given to this type of chemical bond? {1) 2.8 a) What type of chemical bond would you expect in hydrogen fluoride/ HF? {1) b) Draw a diagram to show how this bond is formed. {2) SECTION C: PHYSICS [30] QUESTION 1 (15) Question Type: Multiple Choice Questions. Each question equals 1 mark. 1.1 Which one is a renewable source of energy? A. nuclear energy B. natural gas C. hydroelectric energy D. coal 1.2 The correct order for generation of electricity from nuclear energy is {1) A. supply electricity> heat water to make steam >steam turns turbines> electrical power sent around the country B. Fusion of uranium> heat water to make steam> steam turns turbines> electrical power sent around the country C. Fission of uranium >heat water to make steam> steam turns turbines> electrical power sent around the country D. Crushing of uranium Fission of uranium heat water to make steam >steam turns turbines> electrical power sent around the country 1.3 Which of the following is true for fossil fuels? {1) A. It is obtained from remains of plant and animal materials. B. It can also be generated from biomass C. It is a tidal source of energy D. It can kill flocks of birds. 8
9 1.4 Peter uses energy of 800 J to carry a suitcase upstairs, a distance of 4 m. Assuming the acceleration due to gravity is 9.81 m/s/s, Find the mass of the suitcase. A. 20g B kg c g D kg 1.5 A 2500 gram Ball is thrown into the air with an initial velocity of 30 em/sec. Which statement is true about the ball when it reaches the top of its ascent? A. Kinetic energy is at its highest B. The kinetic energy is equal to gravitational energy. C. The ball does not have energy at this position. D. Total energy is equal to potential energy of a ball. 1.6 During the ascent phase of a rep of the bench press, the lifter exerts an average vertical force of 200 N against a barbell while the barbell moves 0.8 m upward. How much work did the lifter do to the barbell? BEilCH-PRESS A. 0.8J B. 200 J c. 800 J D. 160 J 9
10 1.7 This graph shows a ball rolling from A to G. The ball starts at point A and rolls through to point G. Which letter shows when the ball has the highest potential energy? {1) A. A only B. D and G C. C and E D. D and A 1.8 A cart at the top of a 0.3 Km hill has a mass of 500 g? Assume that acceleration due to gravity is 10m/s/s. t 0.3km 1 What is the cart's gravitational potential energy? {1) A. 150 J B J c J D Nm (Use information from question 1.8) Assuming that energy is conserved and there is no friction, what is the speed at the bottom of the hill. {1) A.137 m/s B. 173 m/s C. 173 m/s/s D m/s 10
11 1.10 Law of Conservation of Energy states that; A. Energy can be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. B. Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. C. A. Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy changes. D. Energy can be created or destroyed; it may be transformed from one form into another, but the total amount of energy changes A disturbance that transfers energy through a medium from one location to the other is known as A. Gas B. Sound C. Potential energy D. Wave 1.12 Waves that are produced by means of charged particle accelerations are: A. Mechanical waves B. Ocean waves C. Electromagnetic waves B. S-Type earthquake waves 1.13 Which type of wave vibrates forming 90 to the direction of motion of the wave? A. Transverse wave B. Longitudinal wave C. Both transverse and longitudinal wave D. None of the above A distance between any two repeating points on a wave A. Amplitude B. Wavelength C. Velocity D. Frequency What is the wavelength of a sound wave with a frequency of 0.05 KHz? (Speed of sound is 900 km/h). A. 230m B. 50 Hz C. 250 m/s D. 5 m 11
12 QUESTION 2 (15) Question Type: Structured questions 1. An experiment carried out in the physics laboratory to determine the rate of change of velocity has the following results presented in graphical form below. Using the diagram answer the following question: a. State the two important pieces of information missing from the graph? Information 1 is (i) Information 2 is (ii) b. From the question above, identify the independent variable and the dependent variable. Independent variable is (i) Dependent variable is (ii) c. The equation of a straight line is given by; Y = MX + C (i) From the equation above, what does the letters "M" and "C" mean? M meaning: C meaning: (ii) What quantity of measurement does M represent? State the 51 units for this quantity of measurement. Quantity is (i) 51 units is (ii) (iii) From the graph above, what is the value of C? Value of Cis (iv) At what time is the velocity= 2? 12
13 2. An applied force (Fapp) of 75 N is used to accelerate an OBJECT A to the right across a frictional surface. The object encounters 10 N of friction (Ffrict)... Fnorm - Ffrict = 1 ON.. OBJECT ' A.. r Fapp = 75 N "' I" Fgrav Use the diagram above to determine the: (a) Normal force, Fnorm 80 N (b) Net force, Fnet 3. Define the term vector. END 13
14 M セ H Periodic Table H He Li Be B c N 0 F Ne Na Mg AI Si p 5 Cl Ar K Ca Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe (98) Cs Ba La Hf Ta w Re Os lr Pt Au Hg Tl Pb Bi Po At Rn (209) (210) (222) Fr Ra Ac Rf Db Sg Bh Hs Mt (289) (223) (226) (227) (261) (262) (263) (262) (265) (266) (269) (272) (277) (287) (289)_ L_(25)3) Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (145) Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No Lr (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (262)
Name: Mode of Study: Student Number: Qualification: Instruction: Answer all the questions on the questions paper.
 Name: Mode of Study: Student Number: Qualification: Instruction: Answer all the questions on the questions paper. SECTION A: BIOLOGY QUESTION 1 [20 MARKS] Question Type: Multiple Choices. Each answer equals
Name: Mode of Study: Student Number: Qualification: Instruction: Answer all the questions on the questions paper. SECTION A: BIOLOGY QUESTION 1 [20 MARKS] Question Type: Multiple Choices. Each answer equals
FEEDBACK TUTORIAL LETTER TEST 2 SECOND SEMESTER 2018 BASIC SCIENCE [BSC410S]
![FEEDBACK TUTORIAL LETTER TEST 2 SECOND SEMESTER 2018 BASIC SCIENCE [BSC410S] FEEDBACK TUTORIAL LETTER TEST 2 SECOND SEMESTER 2018 BASIC SCIENCE [BSC410S]](/thumbs/87/96785039.jpg) FEEDBACK TUTORIAL LETTER TEST 2 SECOND SEMESTER 2018 BASIC SCIENCE [BSC410S] 1 FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BSC PROGRAMME NAME ST. #:. QUALIFICATION(S):
FEEDBACK TUTORIAL LETTER TEST 2 SECOND SEMESTER 2018 BASIC SCIENCE [BSC410S] 1 FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BSC PROGRAMME NAME ST. #:. QUALIFICATION(S):
Thin Film PV Technologies CIGS PV Technology
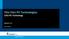 Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
CHEMISTRY OF LIFE 30 JANUARY 2013
 CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
Chelated & Essentials. For Your Pet Animals
 Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Full file at
 General, Organic, and Biological Chemistry, 3e (Timberlake) Chapter 2 Energy and Matter 2.1 Multiple-Choice Questions 1) An example of kinetic energy is A) a coiled spring. B) running water. C) a tree.
General, Organic, and Biological Chemistry, 3e (Timberlake) Chapter 2 Energy and Matter 2.1 Multiple-Choice Questions 1) An example of kinetic energy is A) a coiled spring. B) running water. C) a tree.
GSJ Geochemical Reference Samples. Igneous Rock. Sedimentary Rock. For Instrumental analysis. Reference value for environmental analysis -1
 GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
FOOD. Why do we need food? What's in our food? There are 3 trace elements, Iron (Fe), Copper (Cu) and Zinc (Zn).
 Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
5. Groups A and B in the table below contain molecular formulas of compounds.
 1. Which group consists entirely of organic molecules? A) protein, oxygen, fat B) protein, starch, fat C) water, carbon dioxide, oxygen D) water, starch, protein 2. Which statement describes starches,
1. Which group consists entirely of organic molecules? A) protein, oxygen, fat B) protein, starch, fat C) water, carbon dioxide, oxygen D) water, starch, protein 2. Which statement describes starches,
Maharashtra State Board Class IX Science and Technology Sample Paper 3
 Maharashtra State Board Class IX Science and Technology Sample Paper 3 Time: 3 hrs Max. Marks: 80 Note: 1. Use the same answer-sheet for Section A and Section B. 2. Draw well-labelled diagrams wherever
Maharashtra State Board Class IX Science and Technology Sample Paper 3 Time: 3 hrs Max. Marks: 80 Note: 1. Use the same answer-sheet for Section A and Section B. 2. Draw well-labelled diagrams wherever
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Year 8 Assessment. Autumn Term Remember!
 Remember! Each section of questions gets harder as you go through (higher level questions at the end of each section). Try all questions. Write down an idea even if you re not sure you might get a mark!
Remember! Each section of questions gets harder as you go through (higher level questions at the end of each section). Try all questions. Write down an idea even if you re not sure you might get a mark!
CHEMISTRY OF LIFE 13 MARCH 2013
 CHEMISTRY OF LIFE 13 MARCH 2013 Lesson Description In this lesson, we revise: How molecules are classified The importance of water How to test samples for glucose and starch Key Concepts Terminology A
CHEMISTRY OF LIFE 13 MARCH 2013 Lesson Description In this lesson, we revise: How molecules are classified The importance of water How to test samples for glucose and starch Key Concepts Terminology A
CHEMISTRY OF LIFE 05 FEBRUARY 2014
 CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
What are the most common elements in living organisms? What is the difference between monomers, dimers and polymers?
 What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Topic 3.1 Nutrients. - Lipids are an essential part of the and are a part of cell in the body.
 Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
Choosing What You Eat and Why. Chapter 1 BIOL1400 Dr. Mohamad H. Termos
 Choosing What You Eat and Why Chapter 1 BIOL1400 Dr. Mohamad H. Termos Objectives Following this lecture, you should be able to describe: - Nutrition definition - Sources of nutrients - Energy sources
Choosing What You Eat and Why Chapter 1 BIOL1400 Dr. Mohamad H. Termos Objectives Following this lecture, you should be able to describe: - Nutrition definition - Sources of nutrients - Energy sources
Ch 2 Molecules of life
 Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ms. Golub & Ms. Sahar Date: Unit 2- Test #1
 Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Environmental Literacy Project Michigan State University. Lesson 2.3: Materials Plants Are Made Of
 Environmental Literacy Project Michigan State University Lesson 2.3: Materials Plants Are Made Of Benchmark Scale Power of Ten Large Scale: Farm field Decimal Style Large scale Larger 10 5 10 4 10 3 Larger
Environmental Literacy Project Michigan State University Lesson 2.3: Materials Plants Are Made Of Benchmark Scale Power of Ten Large Scale: Farm field Decimal Style Large scale Larger 10 5 10 4 10 3 Larger
Digestive and Excretory Systems
 Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Importance of Nutrition
 The EAT WELL Plate Canada s food guide Food pyramid Importance of Nutrition Energy for body metabolism (nerve impulses, contraction of muscles, repair and replacement of cells Raw materials for building
The EAT WELL Plate Canada s food guide Food pyramid Importance of Nutrition Energy for body metabolism (nerve impulses, contraction of muscles, repair and replacement of cells Raw materials for building
Chapter 6, Part Read Activity 6A - Choosing a Meal and orally attempt the procedure and discussion on page 99.
 Science 9 Unit 1 Worksheet Chapter 6, Part 1. 1. Read Activity 6A - Choosing a Meal and orally attempt the procedure and discussion on page 99. 2. Your body is made up of,,,, and many other materials.
Science 9 Unit 1 Worksheet Chapter 6, Part 1. 1. Read Activity 6A - Choosing a Meal and orally attempt the procedure and discussion on page 99. 2. Your body is made up of,,,, and many other materials.
NEW DEVELOPMENTS IN CATALYSIS
 You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
SCIENCE (REVISED SYLLABUS) HIGHER LEVEL
 J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2010 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL TIME : 2 HOURS INSTRUCTIONS 1. Write your name, school name and teacher s name in the boxes provided on this page.
J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2010 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL TIME : 2 HOURS INSTRUCTIONS 1. Write your name, school name and teacher s name in the boxes provided on this page.
Class- 1X SCIENCE Paper- 4 Time allowed: 3 hours maximum Marks: 90
 Class- 1X SCIENCE Paper- 4 Time allowed: 3 hours maximum Marks: 90 General Instructions: 1. The question paper comprises of two sections, A and B you are to attempt both the sections. 2. All questions
Class- 1X SCIENCE Paper- 4 Time allowed: 3 hours maximum Marks: 90 General Instructions: 1. The question paper comprises of two sections, A and B you are to attempt both the sections. 2. All questions
Standard B-3: The student will demonstrate an understanding of the flow of energy within and between living systems.
 B-3.1 Summarize the overall process by which photosynthesis converts solar energy into chemical energy and interpret the chemical equation for the process. Taxonomy Level: 2.4-B and 2.1-B Understand Conceptual
B-3.1 Summarize the overall process by which photosynthesis converts solar energy into chemical energy and interpret the chemical equation for the process. Taxonomy Level: 2.4-B and 2.1-B Understand Conceptual
Biochemical Concepts. Section 4.6 The Chemistry of Water. Pre-View 4.6. A Covalent Polar Molecule
 Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Lesson 3 Understanding Nutrients and Their Importance
 Unit B Understanding Animal Body Systems Lesson 3 Understanding Nutrients and Their Importance 1 Terms Balanced ration Carbohydrates Complex carbohydrates Disaccharides Essential nutrients Ether Fat Fat-soluble
Unit B Understanding Animal Body Systems Lesson 3 Understanding Nutrients and Their Importance 1 Terms Balanced ration Carbohydrates Complex carbohydrates Disaccharides Essential nutrients Ether Fat Fat-soluble
INTERMEDIATE 1 1 Food and Diet. These elements are present in compounds - not as free elements.
 INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
INTERMEDIATE 1 1 Food and Diet FOOD AND DIET The main elements present in the human body are: Hydrogen Oxygen Nitrogen Carbon These elements are present in compounds - not as free elements. Unlike plants,
UNIT 2 DIABETES REVIEW
 UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
The building blocks for this molecule are A) amino acids B) simple sugars C) fats D) molecular bases
 1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
Chapter 2 pt 2. Atoms, Molecules, and Life. Gregory Ahearn. John Crocker. Including the lecture Materials of
 Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Chapter 11 Nutrition: Food for Thought
 Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins
 I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins Inorganic compounds = compounds that DO NOT contain carbon Ex: Vitamins, minerals,
I. ROLE OF CARBON IN ORGANISMS: Organic compounds = compounds that contain carbon Ex: Carbohydrates, lipids, proteins Inorganic compounds = compounds that DO NOT contain carbon Ex: Vitamins, minerals,
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Lipids.
 Lipids Mark Scheme Level Subject Exam Board Topic Sub-Topic Booklet International A Level Biology Edexcel Molecules, Transport and Health Lipids Mark Scheme Time Allowed: 63 minutes Score: /52 Percentage:
Lipids Mark Scheme Level Subject Exam Board Topic Sub-Topic Booklet International A Level Biology Edexcel Molecules, Transport and Health Lipids Mark Scheme Time Allowed: 63 minutes Score: /52 Percentage:
I, 2013 SUMMATIVE ASSESSMENT I, 2013 / SCIENCE. IX / Class IX. Time Allowed : 3 hours Maximum Marks : 90
 EWFGDSK I, 0 SUMMATIVE ASSESSMENT I, 0 / SCIENCE IX / Class IX 90 Time Allowed : hours Maximum Marks : 90 General Instructions : The question paper comprises of two Sections, A and B. You are to attempt
EWFGDSK I, 0 SUMMATIVE ASSESSMENT I, 0 / SCIENCE IX / Class IX 90 Time Allowed : hours Maximum Marks : 90 General Instructions : The question paper comprises of two Sections, A and B. You are to attempt
Organic Molecules. 1. The structural formulas shown represent certain organic compounds found in living cells.
 Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
Digestion and Human Health
 Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
I. ROLE OF CARBON IN ORGANISMS:
 Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Molecules of Life. Carbohydrates Lipids Proteins Nucleic Acids
 Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
Half Yearly Exam 2016
 GOZO COLLEGE Secondary School KULLEĠĠ TA GĦAWDEX Skola Sekondarja Half Yearly Exam 2016 Year 8 Levels: 5-8 SCIENCE Time: 1:30min Name: Class: Year 8 Instructions to Candidates Answer all twenty questions.
GOZO COLLEGE Secondary School KULLEĠĠ TA GĦAWDEX Skola Sekondarja Half Yearly Exam 2016 Year 8 Levels: 5-8 SCIENCE Time: 1:30min Name: Class: Year 8 Instructions to Candidates Answer all twenty questions.
Stored Mechanical Gravitational Electrical. measured as. Forms of Energy
 Thermodynamics is the branch of science that studies the transformation of energy from one form to another. Thermochemistry specifically studies heat changes that accompany chemical reactions and phase
Thermodynamics is the branch of science that studies the transformation of energy from one form to another. Thermochemistry specifically studies heat changes that accompany chemical reactions and phase
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic
 Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Summary Consumer Products
 Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
Chapter 11 Nutrition: Food for Thought
 Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
1.3.1 Function of Food. Why do we need food?
 1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
Science. Area of Experience: Science. At Junior Certificate level the student can:
 Science Area of Experience: Science At Junior Certificate level the student can: 1 The Non-Living Environment Describe the characteristics and structures of different materials and explain how they change
Science Area of Experience: Science At Junior Certificate level the student can: 1 The Non-Living Environment Describe the characteristics and structures of different materials and explain how they change
Nutrition, Nutrition, Nutrition! Because food is life! Oh, I m hungry!
 Nutrition, Nutrition, Nutrition! Because food is life! Oh, I m hungry! Topics of Study 1. What is metabolism? 2. Energy and chemical changes 3. Nutrients needed for a healthy lifestyle 4. Calories and
Nutrition, Nutrition, Nutrition! Because food is life! Oh, I m hungry! Topics of Study 1. What is metabolism? 2. Energy and chemical changes 3. Nutrients needed for a healthy lifestyle 4. Calories and
Proteins. Biomolecules. Nucleic Acids. The Building Blocks of Life
 Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
Serial No. 366,637. Filing Date 30 December 1994 NOTICE
 Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
NUTRITION. Professor Andrea Garrison Biology 11 Illustrations 2010 Pearson Education, Inc.
 NUTRITION Professor Andrea Garrison Biology 11 Illustrations 2010 Pearson Education, Inc. Proper Diet Carbohydrates Proteins Lipids (fats) Water Vitamins Fiber Inorganic salts Nutrition 2 Carbohydrates
NUTRITION Professor Andrea Garrison Biology 11 Illustrations 2010 Pearson Education, Inc. Proper Diet Carbohydrates Proteins Lipids (fats) Water Vitamins Fiber Inorganic salts Nutrition 2 Carbohydrates
of human hair and nails. Part I. Analytical methodology, Sci. Tot. Environ. 2000, 250/1-3,
 Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS
 SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
The six elements that make up 99.9% of all living things include
 The six elements that make up 99.9% of all living things include 1. C, K, O, N, Ca and S 2. C, P, S, H, O and N 3. C, P, K, I, O and N 4. N, O, P, H, S and T 75% 15% 1 C, K, O, N, Ca and S C, P, S, H,
The six elements that make up 99.9% of all living things include 1. C, K, O, N, Ca and S 2. C, P, S, H, O and N 3. C, P, K, I, O and N 4. N, O, P, H, S and T 75% 15% 1 C, K, O, N, Ca and S C, P, S, H,
Biology Chapter 2 Review
 Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
Properties of Water. 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin.
 Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
2.1.1 Biological Molecules
 2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
Proteins. Biomolecules. Nucleic Acids. The Building Blocks of Life
 Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are 1. Organic molecules that are (at least 1 Carbon molecule and often chains of Carbon) They all contain.
Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are 1. Organic molecules that are (at least 1 Carbon molecule and often chains of Carbon) They all contain.
Matrix Reference Materials - SCP SCIENCE
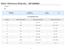 EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
CP Biology Chapter 2: Molecules of Life Name Amatuzzi #1: Carbohydrates pp Period Homework
 Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells
 Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells Objectives Summarize the characteristics of organic compounds. Compare the structures and function of different types of biomolecules.
Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells Objectives Summarize the characteristics of organic compounds. Compare the structures and function of different types of biomolecules.
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014
 REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Chapter 1. What You Eat and Why
 Chapter 1 What You Eat and Why What is nutrition? Nutrition is the science of food, the nutrients and the substances therein, their action, interaction, and balance in relation to health and disease,
Chapter 1 What You Eat and Why What is nutrition? Nutrition is the science of food, the nutrients and the substances therein, their action, interaction, and balance in relation to health and disease,
Chemical Formulas. Chemical Formula CH 3 COCHCHOCHClCHNH Lewis Dot Structure
 Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
DE LA SALLE SCHOOL LEARNING PROGRAMME YEAR 7. Half Term 1a
 Half Term 1a Laboratory equipment and the hazards associated with them. How hazardous chemicals can affect the environment around us. Hazards Irritant Corrosive Toxic Sodium hydroxide Hydrochloric acid
Half Term 1a Laboratory equipment and the hazards associated with them. How hazardous chemicals can affect the environment around us. Hazards Irritant Corrosive Toxic Sodium hydroxide Hydrochloric acid
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
Chapter 2: Biochemistry
 Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
10. The diagram below shows two different kinds of substances, A and B, entering a cell.
 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
30.1 Organization of the Human Body
 30.1 Organization of the Human Body Organization of the Body The levels of organization in the body include cells, tissues, organs, and organ systems. At each level of organization, these parts of the
30.1 Organization of the Human Body Organization of the Body The levels of organization in the body include cells, tissues, organs, and organ systems. At each level of organization, these parts of the
UGRC 145: FOOD AND NUTRITION IN EVERYDAY LIFE
 UGRC 145: FOOD AND NUTRITION IN EVERYDAY LIFE Session 2 MACRONUTRIENTS Lecturer: PROF. MATILDA STEINER-ASIEDU, SBS, CBAS; University of Ghana, Email: tillysteiner@gmail.com College of Education School
UGRC 145: FOOD AND NUTRITION IN EVERYDAY LIFE Session 2 MACRONUTRIENTS Lecturer: PROF. MATILDA STEINER-ASIEDU, SBS, CBAS; University of Ghana, Email: tillysteiner@gmail.com College of Education School
Human Biology *P41558A0128* P41558A. Unit: 4HB0 Paper: 01. Edexcel International GCSE. Tuesday 7 May 2013 Morning Time: 2 hours.
 Write your name here Surname Other names Edexcel International GCSE Centre Number Human Biology Unit: 4HB0 Paper: 01 Candidate Number Tuesday 7 May 2013 Morning Time: 2 hours You must have: Ruler Candidates
Write your name here Surname Other names Edexcel International GCSE Centre Number Human Biology Unit: 4HB0 Paper: 01 Candidate Number Tuesday 7 May 2013 Morning Time: 2 hours You must have: Ruler Candidates
By: Mochamad Nurcholis Food Science Department Brawijaya University 2013
 PHYSIOLOGY & METABOLISMS of Microorganisms By: Mochamad Nurcholis Food Science Department Brawijaya University 2013 What is metabolisms? Can you explain it? Overall biochemical reaction within cells of
PHYSIOLOGY & METABOLISMS of Microorganisms By: Mochamad Nurcholis Food Science Department Brawijaya University 2013 What is metabolisms? Can you explain it? Overall biochemical reaction within cells of
Chapter 3: Biochemistry Adapted from PPT by S. Edwards. By PresenterMedia.com
 Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
3. Describe the study in mimicry, using king snakes and coral snakes. Identify the control in the experiment.
 Biology Semester 1 Exam Review Guide Chapter 1 Biology in the 21 st Century 1. Distinguish between the following key terms: Biology Name : Pd: Hypothesis Variable Controlled experiment Theory Model Technology
Biology Semester 1 Exam Review Guide Chapter 1 Biology in the 21 st Century 1. Distinguish between the following key terms: Biology Name : Pd: Hypothesis Variable Controlled experiment Theory Model Technology
Biochemistry. Chapter 6
 Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
SPECIFICATION CONTINUED Glucose has two isomers, α-glucose and β-glucose, with structures:
 alevelbiology.co.uk SPECIFICATION Monosaccharides are the monomers from which larger carbohydrates are made. Glucose, galactose and fructose are common monosaccharides. A condensation reaction between
alevelbiology.co.uk SPECIFICATION Monosaccharides are the monomers from which larger carbohydrates are made. Glucose, galactose and fructose are common monosaccharides. A condensation reaction between
2014 National Curriculum Science Band 1
 2014 National Curriculum Science Band 1 ask simple questions and recognise that they can be answered in different ways (Year 1 variety of common animals including fish, amphibians, reptiles, birds and
2014 National Curriculum Science Band 1 ask simple questions and recognise that they can be answered in different ways (Year 1 variety of common animals including fish, amphibians, reptiles, birds and
Chapter 15 Food and Digestion
 Chapter 15 Food and Digestion Activity: Use Qualitative Observations (5 senses) to describe: What happens when you see candy? How does it smell? How do you chomp it into smaller pieces or swallow candy
Chapter 15 Food and Digestion Activity: Use Qualitative Observations (5 senses) to describe: What happens when you see candy? How does it smell? How do you chomp it into smaller pieces or swallow candy
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
Biochemistry: Macromolecules
 1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
National 5 Unit Two : Nature s Chemistry
 National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
Unit 2 - Characteristics of Living Things
 Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Mike Hinds, Royal Canadian Mint
 Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
30.1 Organization of the Human Body
 30.1 Organization of the Human Body Lesson Objectives Describe how the human body is organized. Explain homeostasis. Lesson Summary Organization of the Body The levels of organization in a multicellular
30.1 Organization of the Human Body Lesson Objectives Describe how the human body is organized. Explain homeostasis. Lesson Summary Organization of the Body The levels of organization in a multicellular
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
I. ROLE OF CARBON IN ORGANISMS:
 Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
