June 25, 2014 DURB Meeting Summary
|
|
|
- Hester Dorsey
- 5 years ago
- Views:
Transcription
1 Roll Call Present: Dr. Swee, Dr. Zanna, Dr. Gochfeld, Ms. Olson, Dr. Moore, Mr. Schafer, Dr. Gooen, Dr. Marcus, Dr. Lind (ex officio). Unable to attend: Dr. Moynihan, Dr. Barberio. Review of Minutes Pages 3-6; Tab 1 Approved Minutes from April 23, 2014 meeting was reviewed and approved. The approved meeting summary will also be posted on the DURB website at: Secretary s Report Page 7; Tab 2 Educational newsletter on Acute Pain Treatment Options has been signed by both Commissioners. It is now ready for distribution and will also be available on the DURB website. The State s Fiscal Year 2013 DURB Annual Summary was resubmitted after minor changes from the Governor s office. The Board will be informed when it is ready to be sent to the State Register. Awaiting programming changes to be made to the Asthma RetroDUR report to include Managed Care beneficiaries. The transition of HealthFirst to WellCare is anticipated during the third quarter of As requested in the last meeting, Summary of Actions of DURB is now posted on the DURB website. To address Dr. Swee s concern about utilization of atrial fibrillation (afib) drugs, a report using the Beers 2012 criteria was reviewed. Although indications for use were not available, there were 596 patients over 65 years old on these products with 2,881 claims. The Board requested further detailed report on utilization of these products. Proposed DURB meeting dates for 2015 (included in the package) are: - Wednesday, January 28 th - Wednesday, April 22 nd - Wednesday, June 24 th - Wednesday, October 21 st 1
2 Old Business HMO Response to DURB follow-up questions on protocols Pages 9-10; Tab 3 Include response in next meeting package Dr. Swee expressed his appreciation for the responses from the HMOs to the Board s previous questions. However, in reference to the ICS/LABA protocol, he requested further clarification from Plan D on what an intolerance issue is that would prompt an override. New Business A. Protocols Review Atovaquone (Mepron ): Page 11; Tab 4 Drugs for Attention Deficit/Hyperactive Disorder (ADHD): Pages 12-13; Tab 4 The Board had no comments on this protocol. The Board expressed concern about the complicated protocols for Plans A and D. They also requested: - Denial rates from those plans - Drug-drug interaction data from Plan A. - Clarification on why it is necessary to review or prior authorize all the drug claims including those drugs that have been in use for a while, and therefore have good medical profile. B. Proposed protocol for the efficient use of sofosbuvir (Sovaldi ) Informational Highlights/Reports 1. Fee-for- Service/HMO Prior Authorization Report Pages 19-25; Tab 5 Approved with minor update Pages 27-28; Tab 6 The Board reviewed a protocol for sofosbuvir, a new drug for the treatment of Chronic Hepatitis C virus. They requested that the section of the protocol that discussed alcohol/drug abuse be changed to reflect former users. This change will be made prior to sign off by the Medicaid director and the Commissioners. The protocol was a collaborative effort between DMHAS and the HMO pharmacy directors. The Board reviewed prior authorization report comparing all HMO plans including FFS for the 1 st quarter of They were concerned about the high no diagnosis denials for Horizon in the report. Dr. Gauweiler, clinical pharmacy manager with Horizon explained their process which allowed initiation of the PA process by the patient or pharmacy resulting sometimes in no diagnosis data. This is usually resolved by a phone call to the prescriber. 2
3 1. Fee-for-Service/HMO Prior Authorization Report contd. Percentage of prior authorization requests relative to total claims and denials associated with the PAs are listed below: Plan (%) PA Requests of Denial (%) claims FFS Amerigroup HealthFirst Horizon UHC WellCare Summary of DURB Recommendations April 2014: a)educational Newsletter Page 29-30; Tab 7 The Board reviewed and approved a revised educational newsletter for Acute Pain Treatment Options. b) Protocols Review and Comparison The Board reviewed HMO and FFS protocols for: 1. Ranolazine (Ranexa ) 2. Inhaled corticosteroid/laba combination (ICS/LABA) 3. Low molecular weight heparin c) October 2012 Protocol for low dose quetiapine (Seroquel ) For the ICS/LABA protocol, they expressed concern about the 60-day period to demonstrate failure and recommended that the HMO plan should be responsive to requests made prior to the 30 day trial period. The Board requested a more recent data on low-dose quetiapine utilization for further evaluation. Dr. Marcus informed the Board that trazodone, an antidepressant is being used routinely for sleep in some hospitals. He also mentioned that the Poison Control Center is getting calls reflective of increasing prescribing of quetiapine for the similar indication. 3
4 3. DHS and DHSS Programs Top Drugs Report Pages 31-42; Tab 8 The Board reviewed April 2014 report of the top drugs, by dollar amount, claims count, and service units. They also looked at the same report sorted by unit count by Dr. Marcus. This report indicated high utilization of oxycodone, ranking it at number one in this category as well as claim count. (A similar report will be available in the next meeting package). Dr. Marcus suggested that the State should work with the NJ Prescription Monitoring Program (PMP) to see why utilization of this product is so high. The Board also requested a report that examines the indications for these prescriptions in order to rule out diversion and/or abuse. 5. Medication Information Pages 43-48; Tab 9 The following medical information were also included and discussed: (a) FDA Advisors Reject Combination Pain Pill Moxduo (b) Lawyers for Zohydro maker urge judge to strike down Mass. restrictions Dr. Swee had requested follow-up information on the activities (e.g. restrictions) around this product. (c) Certain Sedatives Tied to Breathing Problems in Older COPD Patients (d) WHO Guidelines May Help With Price Reductions For Hepatitis C Drugs 4
5 Follow up items: (a) 2013 DURB annual report to the NJ Register (a) Report will be sent to the NJ Register after review by the Governor s office (b) Confirm the definition of intolerance with Plan D. (c) ADHD protocol- Plans A and D to report on denial rates and DDIs (d)update for HCV protocol former drug/etoh abuser vs. six months (e) Oxycodone utilization report (f) Updated low dose quetiapine (Seroquel ) report (b) ICS-LABA (inhaled corticosteroid/long-acting beta agonist) combo inhaler will be provided if the patient did not exhibit an adequate response to treatment with an ICS; experienced intolerance/adverse reaction to previous therapy with an ICS or, has a documented contraindication to treatment with an ICS. (c) HMO responses included in meeting package (d) Done (e ) Included in meeting package (f ) Included in meeting package 5
February 4, 2015 DURB Meeting Summary
 Roll Call Present: Dr. Swee, Dr. Zanna, Dr. Gochfeld, Dr. Moynihan, Mr. Schafer, Dr. Gooen, Dr. Marcus, Dr. Lind (ex officio). Unable to attend: Dr. Barberio, Dr. Moore, Ms. Olson Review of Minutes Pages
Roll Call Present: Dr. Swee, Dr. Zanna, Dr. Gochfeld, Dr. Moynihan, Mr. Schafer, Dr. Gooen, Dr. Marcus, Dr. Lind (ex officio). Unable to attend: Dr. Barberio, Dr. Moore, Ms. Olson Review of Minutes Pages
January 11, 2017 DURB Meeting Summary
 Roll Call Present: Dr. Swee, Dr. Zanna (ex officio), Dr. Gooen, Dr. Marcus, Dr. Gochfeld, Dr. Barberio, Dr. Lind (ex officio). Unable to attend: Mr. Schafer Dr. Moynihan, Dr. Moore, Ms. Olson, Public Notice
Roll Call Present: Dr. Swee, Dr. Zanna (ex officio), Dr. Gooen, Dr. Marcus, Dr. Gochfeld, Dr. Barberio, Dr. Lind (ex officio). Unable to attend: Mr. Schafer Dr. Moynihan, Dr. Moore, Ms. Olson, Public Notice
August 10, 2017 DURB Meeting Summary
 Roll Call Present: Dr. Swee, Dr. Zanna (ex officio), Dr. Gooen, Dr. Marcus, Dr. Gochfeld, Dr. Moynihan, Dr. Moore, Ms. Olson, Dr. Lind (ex officio) Unable to attend: Mr. Schafer, Dr. Barberio Public Notice
Roll Call Present: Dr. Swee, Dr. Zanna (ex officio), Dr. Gooen, Dr. Marcus, Dr. Gochfeld, Dr. Moynihan, Dr. Moore, Ms. Olson, Dr. Lind (ex officio) Unable to attend: Mr. Schafer, Dr. Barberio Public Notice
April 21, 2010 DURB Meeting Summary
 Roll Call Present: Dr. Swee, Ms. Olson, Ms. Martinez- Rodriguez, Dr. Barberio, Dr. Gooen, Dr. Gochfeld, Dr. Lichtbroun Absent: Dr. Moore, Dr. Marcus, Mr. Schafer, Dr. Moynihan, Review of Minutes Pages
Roll Call Present: Dr. Swee, Ms. Olson, Ms. Martinez- Rodriguez, Dr. Barberio, Dr. Gooen, Dr. Gochfeld, Dr. Lichtbroun Absent: Dr. Moore, Dr. Marcus, Mr. Schafer, Dr. Moynihan, Review of Minutes Pages
June 23, 2010 DURB Meeting Summary
 Roll Call Present: Dr. Swee, Ms. Olson, Ms. Martinez- Rodriguez, Dr. Barberio, Dr. Gooen, Dr. Gochfeld, Dr. Lichtbroun, Dr. Marcus Absent: Dr. Moore, Mr. Schafer, Dr. Moynihan, Review of Minutes Pages
Roll Call Present: Dr. Swee, Ms. Olson, Ms. Martinez- Rodriguez, Dr. Barberio, Dr. Gooen, Dr. Gochfeld, Dr. Lichtbroun, Dr. Marcus Absent: Dr. Moore, Mr. Schafer, Dr. Moynihan, Review of Minutes Pages
October 2015 news bulletin
 October 2015 news bulletin Claims tip of the month billing medical injectables For single dose vials, providers should bill Amerigroup Washington, Inc. for the total amount of the drug contained in the
October 2015 news bulletin Claims tip of the month billing medical injectables For single dose vials, providers should bill Amerigroup Washington, Inc. for the total amount of the drug contained in the
Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy. Date Prescription will Start Denying at the
 Maryland Medicaid Pharmacy Program & News ViewsNovember 2013 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy The Antipsychotic Peer Review Program is Expanding!
Maryland Medicaid Pharmacy Program & News ViewsNovember 2013 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy The Antipsychotic Peer Review Program is Expanding!
Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year,
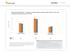 ASTHMA CHART 1 Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year, 215-216 7 6 5 4 3 2 1 1 Controller medication 1 Rescue medication
ASTHMA CHART 1 Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year, 215-216 7 6 5 4 3 2 1 1 Controller medication 1 Rescue medication
Policy Evaluation: Step Therapy Prior Authorization of Combination Inhaled Corticosteroid / Long-Acting Beta-Agonists
 Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Policy Evaluation: Step Therapy Prior
Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Policy Evaluation: Step Therapy Prior
Idaho DUR Board Meeting Minutes
 Idaho DUR Board Meeting Minutes Date: January 21, 2016 Time: 9am-1:30pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Members
Idaho DUR Board Meeting Minutes Date: January 21, 2016 Time: 9am-1:30pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Members
Idaho DUR Board Meeting Minutes
 Idaho DUR Board Meeting Minutes Date: July 20, 2017 Time: 9am-12:30pm Location: Holiday Inn Boise Airport 2970 West Elder Street, Boise, Idaho, 83705 Moderator: David Agler, M.D. Committee Member Present:
Idaho DUR Board Meeting Minutes Date: July 20, 2017 Time: 9am-12:30pm Location: Holiday Inn Boise Airport 2970 West Elder Street, Boise, Idaho, 83705 Moderator: David Agler, M.D. Committee Member Present:
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 September 2018 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke, R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Ram Illindala, MD, Dennis Chapron, Pharm.D. Ex Officio
September 2018 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke, R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Ram Illindala, MD, Dennis Chapron, Pharm.D. Ex Officio
Idaho DUR Board Meeting Minutes
 Date: July 16, 2015 Time: 9am-1:30pm Idaho DUR Board Meeting Minutes Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Member Present:
Date: July 16, 2015 Time: 9am-1:30pm Idaho DUR Board Meeting Minutes Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Member Present:
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE July 5, 2016 SUBJECT EFFECTIVE DATE July 11, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants - Pharmacy Services Leesa M. Allen, Deputy Secretary
ISSUE DATE July 5, 2016 SUBJECT EFFECTIVE DATE July 11, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants - Pharmacy Services Leesa M. Allen, Deputy Secretary
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 September 2008 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair); Dennis Chapron, M.S.; Richard Gannon, Pharm.D.; Keith Lyke R.Ph., Mike Moore, R.Ph., MPH; Bhupesh Mangla, M.D., Ram
September 2008 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair); Dennis Chapron, M.S.; Richard Gannon, Pharm.D.; Keith Lyke R.Ph., Mike Moore, R.Ph., MPH; Bhupesh Mangla, M.D., Ram
Patient Review and Coordination Program
 Patient Review and Coordination Program For Medical Assistance Clients Who Need Assistance In Appropriate Use of Phyllis Coolen, RN, MN Division of Healthcare Health and Recovery Administration January
Patient Review and Coordination Program For Medical Assistance Clients Who Need Assistance In Appropriate Use of Phyllis Coolen, RN, MN Division of Healthcare Health and Recovery Administration January
Drug Use Evaluation: Low Dose Quetiapine
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Public Policy HCA Public Policy No
 Public Policy HCA Public Policy No.11-2016 TO: FROM: RE: HCA HOSPICE PROVIDER MEMBERS PATRICK CONOLE, VICE PRESIDENT, FINANCE & MANAGEMENT UPDATES FROM NGS HOSPICE ADVISORY MEETING DATE: JUNE 10, 2016
Public Policy HCA Public Policy No.11-2016 TO: FROM: RE: HCA HOSPICE PROVIDER MEMBERS PATRICK CONOLE, VICE PRESIDENT, FINANCE & MANAGEMENT UPDATES FROM NGS HOSPICE ADVISORY MEETING DATE: JUNE 10, 2016
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 June 2008 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair); Dennis Chapron, M.S.; Richard Gannon, Pharm.D.; Keith Lyke R.Ph., Mike Moore, R.Ph., MPH; Bhupesh Mangla, M.D., Ram Illindala,
June 2008 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair); Dennis Chapron, M.S.; Richard Gannon, Pharm.D.; Keith Lyke R.Ph., Mike Moore, R.Ph., MPH; Bhupesh Mangla, M.D., Ram Illindala,
Daklinza Sovaldi. Daklinza (daclatasvir) and Sovaldi (sofosbuvir) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Daklinza Sovaldi Page: 1 of 4 Last Review Date: September 18, 2015 Daklinza Sovaldi Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Daklinza Sovaldi Page: 1 of 4 Last Review Date: September 18, 2015 Daklinza Sovaldi Description
2017 United Healthcare Services, Inc.
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2055-10 Program Prior Authorization/Medical Necessity Medication Olysio (simeprevir) P&T Approval Date 4/2015, 11/2015, 8/2016,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2055-10 Program Prior Authorization/Medical Necessity Medication Olysio (simeprevir) P&T Approval Date 4/2015, 11/2015, 8/2016,
Curbing Prescription Drug Abuse in Medicaid
 Curbing Prescription Drug Abuse in Medicaid Joint Legislative Health Care Oversight Committee October 12, 2010 Dr. Lisa Weeks, BSPharm, PharmD Pharmacy and Ancillary Services Division of Medical Assistance
Curbing Prescription Drug Abuse in Medicaid Joint Legislative Health Care Oversight Committee October 12, 2010 Dr. Lisa Weeks, BSPharm, PharmD Pharmacy and Ancillary Services Division of Medical Assistance
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE January 6, 2016 SUBJECT EFFECTIVE DATE January 20, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of COPD Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE January 6, 2016 SUBJECT EFFECTIVE DATE January 20, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of COPD Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Changing patterns of sedative use over time in older adults in Ontario
 Changing patterns of sedative use over time in older adults in Ontario Andrea Iaboni, MD, DPhil, FRCPC Toronto Rehabilitation Institute University Health Network Faculty/Presenter Disclosure Faculty: Andrea
Changing patterns of sedative use over time in older adults in Ontario Andrea Iaboni, MD, DPhil, FRCPC Toronto Rehabilitation Institute University Health Network Faculty/Presenter Disclosure Faculty: Andrea
Rexulti (brexpiprazole)
 Market DC Rexulti (brexpiprazole) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Indiana see State Specific Mandates below *Maryland see State Specific Mandates below *Virginia
Market DC Rexulti (brexpiprazole) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Indiana see State Specific Mandates below *Maryland see State Specific Mandates below *Virginia
PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL
 PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Hepatitis C Virus (HCV) in Prevalence + + Approximately 80,000 individuals in are living with hepatitis C virus (HCV).
PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Hepatitis C Virus (HCV) in Prevalence + + Approximately 80,000 individuals in are living with hepatitis C virus (HCV).
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 March 2015 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Charles Caley Pharm.D. BCPP, Ram Illindala, MD, Carol
March 2015 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Charles Caley Pharm.D. BCPP, Ram Illindala, MD, Carol
Eliminating Hepatitis C in the United States Treatment Access for All! Ryan Clary Executive Director December 7, 2016
 Eliminating Hepatitis C in the United States Treatment Access for All! Ryan Clary Executive Director December 7, 2016 History The cure arrives! Sovaldi (Dec. 2013) List price: $84,000 Immediate payer outrage/restrictions
Eliminating Hepatitis C in the United States Treatment Access for All! Ryan Clary Executive Director December 7, 2016 History The cure arrives! Sovaldi (Dec. 2013) List price: $84,000 Immediate payer outrage/restrictions
PCC EHR Meaningful Use Measures. Maria Horn July 18, :15 pm. Including CQM Reports
 PCC EHR Meaningful Use Measures Maria Horn July 18, 2014 2:15 pm Including CQM Reports Meaningful Use and PCC EHR This presentation reviews the measures that are housed in PCC EHR which is 2011 CEHRT (Certified
PCC EHR Meaningful Use Measures Maria Horn July 18, 2014 2:15 pm Including CQM Reports Meaningful Use and PCC EHR This presentation reviews the measures that are housed in PCC EHR which is 2011 CEHRT (Certified
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 December 2012 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Dennis Chapron, M.S., Bhupesh Mangla, M.D., Charles Caley, Pharm.D., Ram Illindala, M.D., Richard
December 2012 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Dennis Chapron, M.S., Bhupesh Mangla, M.D., Charles Caley, Pharm.D., Ram Illindala, M.D., Richard
Daklinza Sovaldi. Daklinza (daclatasvir) and Sovaldi (sofosbuvir) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Daklinza Sovaldi Page: 1 of 7 Last Review Date: June 24, 2016 Daklinza Sovaldi Description Daklinza
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Daklinza Sovaldi Page: 1 of 7 Last Review Date: June 24, 2016 Daklinza Sovaldi Description Daklinza
requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group
 requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group Canterbury and Coastal Clinical Consortium Group Medicine Management plans 2013/14
requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group Canterbury and Coastal Clinical Consortium Group Medicine Management plans 2013/14
Idaho DUR Board Meeting Minutes
 Idaho DUR Board Meeting Minutes Date: October 16, 2014 Time: 9am-2pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Member Present:
Idaho DUR Board Meeting Minutes Date: October 16, 2014 Time: 9am-2pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Member Present:
PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S.
 Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
E-Prescribing, EPCS & PDMP: An Update
 E-Prescribing, EPCS & PDMP: An Update Melissa A. Kotrys, MPH Chief Executive Officer July 27, 2018 Arizona Health-e Connection is now Health Current Arizona s primary resource for health information technology
E-Prescribing, EPCS & PDMP: An Update Melissa A. Kotrys, MPH Chief Executive Officer July 27, 2018 Arizona Health-e Connection is now Health Current Arizona s primary resource for health information technology
Molina Healthcare of Texas Hepatitis C Drugs (Medicaid)
 Texas Standard Prior Authorization Form Addendum Molina Healthcare of Texas Hepatitis C Drugs (Medicaid) This fax machine is located in a secure location as required by HIPAA Regulations. Complete / Review
Texas Standard Prior Authorization Form Addendum Molina Healthcare of Texas Hepatitis C Drugs (Medicaid) This fax machine is located in a secure location as required by HIPAA Regulations. Complete / Review
Antipsychotic Medications Age and Step Therapy
 Market DC *- Florida Healthy Kids Antipsychotic Medications Age and Step Therapy Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Virginia Medicaid See State Specific Mandates *Indiana
Market DC *- Florida Healthy Kids Antipsychotic Medications Age and Step Therapy Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Virginia Medicaid See State Specific Mandates *Indiana
News & Views. Antipsychotics on Maryland Medicaid PDL and Coverage of a 30-day Emergency Supply of Atypical Antipsychotics
 Maryland Medicaid Pharmacy Program News & Views February 2011 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy Antipsychotics on Maryland Medicaid PDL and Coverage
Maryland Medicaid Pharmacy Program News & Views February 2011 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy Antipsychotics on Maryland Medicaid PDL and Coverage
CME/CE POSTTEST CME/CE QUESTIONS
 CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
Texas Vendor Drug Program Specialty Drug List Process. February 2019
 Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
The Louis de la Parte Florida Mental Health Institute
 Data Brief December 2003 Mary Rose Murrin, M.A. Kelley Dhont, M.S. David Thornton, M.A. The Louis de la Parte Florida Mental Health Institute Children s Psychotropic Medication Use by Age and Diagnostic
Data Brief December 2003 Mary Rose Murrin, M.A. Kelley Dhont, M.S. David Thornton, M.A. The Louis de la Parte Florida Mental Health Institute Children s Psychotropic Medication Use by Age and Diagnostic
Mental Illness and Addiction:
 Mental Illness and Addiction: Impact on the Workforce Presented by Terri White, MSW Commissioner Oklahoma Department of Mental Health and Substance Abuse Services Mental Illness and Addiction: Diseases
Mental Illness and Addiction: Impact on the Workforce Presented by Terri White, MSW Commissioner Oklahoma Department of Mental Health and Substance Abuse Services Mental Illness and Addiction: Diseases
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 March 2018 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke, R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Ram Illindala, MD, Dennis Chapron, PharmD., Carol Drufva,
March 2018 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke, R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Ram Illindala, MD, Dennis Chapron, PharmD., Carol Drufva,
2. Does the patient have chronic urticaria? Y N
 Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Xolair (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Xolair (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmaceutical Management Medicaid 2019
 Pharmaceutical Management Medicaid 2019 Toll-free Contact Number: (888) 327-0671 Pharmacy Administration: (810) 244-1660 Introduction Pharmaceutical Management promotes the use of the most clinically appropriate,
Pharmaceutical Management Medicaid 2019 Toll-free Contact Number: (888) 327-0671 Pharmacy Administration: (810) 244-1660 Introduction Pharmaceutical Management promotes the use of the most clinically appropriate,
New Generics: Specialty Network: Retail Pharmacies Dispensing Specialty Products
 SUPPORTING OUR PROVIDER PARTNERS THROUGH COMMUNICATION AND COLLABORATION. DATE JANUARY 2016 ISSUE 1 HELPFUL NUMBERS FOR PROVIDERS Magellan: 1-800-846-7971 Bin: 016523 Processor control: 747 HELPFUL NUMBERS
SUPPORTING OUR PROVIDER PARTNERS THROUGH COMMUNICATION AND COLLABORATION. DATE JANUARY 2016 ISSUE 1 HELPFUL NUMBERS FOR PROVIDERS Magellan: 1-800-846-7971 Bin: 016523 Processor control: 747 HELPFUL NUMBERS
Comprehensive Research Plan: Inhaled corticosteroids + long-acting beta agonists (ICS+LABA) for the treatment of asthma
 Comprehensive Research Plan: Inhaled corticosteroids + long-acting beta agonists (ICS+LABA) for the treatment of asthma Pharmacoepidemiology Unit July 10, 2014 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca
Comprehensive Research Plan: Inhaled corticosteroids + long-acting beta agonists (ICS+LABA) for the treatment of asthma Pharmacoepidemiology Unit July 10, 2014 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca
Alabama Medicaid Pharmacy Override
 Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
I. UNIFORM FORMULARY REVIEW PROCESS
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID
 CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID BACKGROUND Cytokine and cell-adhesion molecule (CAM) antagonists have a major role in the treatment of chronic inflammatory diseases such
CYTOKINE AND CAM ANTAGONIST UTILIZATION IN MISSISSIPPI MEDICAID BACKGROUND Cytokine and cell-adhesion molecule (CAM) antagonists have a major role in the treatment of chronic inflammatory diseases such
Clinical Policy: Roflumilast (Daliresp) Reference Number: CP.PMN.46. Line of Business: Medicaid
 Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Do not open the test booklet prior to being told to do so.
 Last Name: Pharmacy 4054 Pharmacy Law Exam II Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
Last Name: Pharmacy 4054 Pharmacy Law Exam II Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
Proposal on subsidy changes for some respiratory inhalation products and access restrictions to combination inhalers.
 30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
April 10 th, Bond Street, Toronto ON, M5B 1W8
 Comprehensive Research Plan: Inhaled long-acting muscarinic antagonists (LAMAs; long-acting anticholinergics) for the treatment of chronic obstructive pulmonary disease (COPD) April 10 th, 2014 30 Bond
Comprehensive Research Plan: Inhaled long-acting muscarinic antagonists (LAMAs; long-acting anticholinergics) for the treatment of chronic obstructive pulmonary disease (COPD) April 10 th, 2014 30 Bond
Questions and answers about HCA s opioid clinical policy for Apple Health (Medicaid)
 Questions and answers about HCA s opioid clinical policy for Apple Health (Medicaid) This Q&A covers questions the Health Care Authority (HCA) received during webinars about the opioid clinical policy
Questions and answers about HCA s opioid clinical policy for Apple Health (Medicaid) This Q&A covers questions the Health Care Authority (HCA) received during webinars about the opioid clinical policy
Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx
 Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx Nick Reuter Division of Pharmacologic Therapy Substance Abuse and Mental Health Services
Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx Nick Reuter Division of Pharmacologic Therapy Substance Abuse and Mental Health Services
Daklinza Sovaldi. Daklinza (daclatasvir) and Sovaldi (sofosbuvir) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.36 Subject: Daklinza Page: 1 of 8 Last Review Date: March 18, 2016 Daklinza Description Daklinza (daclatasvir)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.36 Subject: Daklinza Page: 1 of 8 Last Review Date: March 18, 2016 Daklinza Description Daklinza (daclatasvir)
Arkansas Department of Health
 Arkansas Department of Health 4815 West Markham Street Little Rock, Arkansas 72205-3867 Telephone (501) 661-2000 Governor Mike Beebe Nathaniel Smith, MD, MPH, Interim Director and State Health Officer
Arkansas Department of Health 4815 West Markham Street Little Rock, Arkansas 72205-3867 Telephone (501) 661-2000 Governor Mike Beebe Nathaniel Smith, MD, MPH, Interim Director and State Health Officer
Embeda. Embeda (morphine sulfate and naltrexone hydrochloride) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.39 Subject: Embeda Page: 1 of 6 Last Review Date: March 18, 2016 Embeda Description Embeda (morphine
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.39 Subject: Embeda Page: 1 of 6 Last Review Date: March 18, 2016 Embeda Description Embeda (morphine
Louisiana Medicaid. Provider Update. Volume 27, Issue 3 May/June UNISYS Health Information Management Acquired. by Molina Healthcare
 Louisiana Medicaid Provider Update Volume 27, Issue 3 May/June 2010 UNISYS Health Information Management Acquired by Molina Healthcare Effective May 1, 2010, Molina Healthcare purchased the Health Information
Louisiana Medicaid Provider Update Volume 27, Issue 3 May/June 2010 UNISYS Health Information Management Acquired by Molina Healthcare Effective May 1, 2010, Molina Healthcare purchased the Health Information
USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT
 BACKGROUND: USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT Approximately 10% of patients who are prescribed opioids and seek care from multiple doctors, are prescribed
BACKGROUND: USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT Approximately 10% of patients who are prescribed opioids and seek care from multiple doctors, are prescribed
Statement Of. The National Association of Chain Drug Stores. For. U.S. Senate Committee on Finance. Hearing on:
 Statement Of The National Association of Chain Drug Stores For U.S. Senate Committee on Finance Hearing on: 10:00 a.m. National Association of Chain Drug Stores (NACDS) 1776 Wilson Blvd., Suite 200 Arlington,
Statement Of The National Association of Chain Drug Stores For U.S. Senate Committee on Finance Hearing on: 10:00 a.m. National Association of Chain Drug Stores (NACDS) 1776 Wilson Blvd., Suite 200 Arlington,
Zepatier. Zepatier (elbasvir, grazoprevir) and Ribavirin. Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Zepatier Page: 1 of 6 Last Review Date: March 18, 2016 Zepatier Description Zepatier (elbasvir,
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Zepatier Page: 1 of 6 Last Review Date: March 18, 2016 Zepatier Description Zepatier (elbasvir,
Sovaldi Ribavirin. Sovaldi (sofosbuvir) with Ribavirin (Copegus, Moderiba, Rebetol, RibaPak, Ribasphere, RibaTab, ribavirin) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Sovaldi Ribavirin Page: 1 of 7 Last Review Date: December 3, 2015 Sovaldi Ribavirin Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Sovaldi Ribavirin Page: 1 of 7 Last Review Date: December 3, 2015 Sovaldi Ribavirin Description
BEFORE THE ALASKA OFFICE OF ADMINISTRATIVE HEARINGS ON REFERRAL BY THE COMMISSIONER OF HEALTH AND SOCIAL SERVICES DECISION
 BEFORE THE ALASKA OFFICE OF ADMINISTRATIVE HEARINGS ON REFERRAL BY THE COMMISSIONER OF HEALTH AND SOCIAL SERVICES In the Matter of: ) ) B N ) OAH No. 15-1051-MDX ) Agency No. I. Introduction DECISION The
BEFORE THE ALASKA OFFICE OF ADMINISTRATIVE HEARINGS ON REFERRAL BY THE COMMISSIONER OF HEALTH AND SOCIAL SERVICES In the Matter of: ) ) B N ) OAH No. 15-1051-MDX ) Agency No. I. Introduction DECISION The
Medicare Advantage Outreach and Education Bulletin
 Medicare Advantage Outreach and Education Bulletin Blue Cross and Blue Shield of Georgia Prescription Drug Monitoring Programs Prescription drug abuse and diversion are acute problems in the area of pain
Medicare Advantage Outreach and Education Bulletin Blue Cross and Blue Shield of Georgia Prescription Drug Monitoring Programs Prescription drug abuse and diversion are acute problems in the area of pain
mehealth for ADHD Parent Manual
 mehealth for ADHD adhd.mehealthom.com mehealth for ADHD Parent Manual al Version 1.0 Revised 11/05/2008 mehealth for ADHD is a team-oriented approach where parents and teachers assist healthcare providers
mehealth for ADHD adhd.mehealthom.com mehealth for ADHD Parent Manual al Version 1.0 Revised 11/05/2008 mehealth for ADHD is a team-oriented approach where parents and teachers assist healthcare providers
STEP THERAPY IN MEDICARE PART D
 STEP THERAPY IN MEDICARE PART D Sarkis Kavarian, PharmD Candidate 15 Preceptor Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. May 1 st, 2015 Objectives Why is this important? Medicare Part
STEP THERAPY IN MEDICARE PART D Sarkis Kavarian, PharmD Candidate 15 Preceptor Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. May 1 st, 2015 Objectives Why is this important? Medicare Part
2017 UnitedHealthcare Services, Inc.
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2052-10 Program Prior Authorization/Medical Necessity Medication Harvoni (ledipasvir/sofosbuvir) P&T Approval Date 4/2015, 8/2015,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2052-10 Program Prior Authorization/Medical Necessity Medication Harvoni (ledipasvir/sofosbuvir) P&T Approval Date 4/2015, 8/2015,
ATTENDANCE PRESENT ABSENT EXCUSED Robert Weiss, M.D., Cardiologist, Chair X Amy Enos, Pharm. D. Waltz LTC Pharmacy
 TO: Maine Drug Utilization Review Board DATE: April 10, 2014 RE: Maine DUR Board Meeting minutes from April 8, 2014 ATTENDANCE PRESENT ABSENT ECUSED Robert Weiss, M.D., Cardiologist, Chair Amy Enos, Pharm.
TO: Maine Drug Utilization Review Board DATE: April 10, 2014 RE: Maine DUR Board Meeting minutes from April 8, 2014 ATTENDANCE PRESENT ABSENT ECUSED Robert Weiss, M.D., Cardiologist, Chair Amy Enos, Pharm.
Asthma Policy. September 2015
 Asthma Policy September 2015 Contents Policy Statement... 2 Guidance... 2 Management of Asthma in Schools... 2 Implementing the Policy... 3 Record Keeping... 3 Exercise and Activity... 3 Visits, Outings
Asthma Policy September 2015 Contents Policy Statement... 2 Guidance... 2 Management of Asthma in Schools... 2 Implementing the Policy... 3 Record Keeping... 3 Exercise and Activity... 3 Visits, Outings
Prior Authorization Guideline
 Guideline GL-35952 Opioid Quantity Limit Overrides Formulary OptumRx Formulary Note: Approval Date 7/10/2017 Revision Date 7/10/2017 Technician Note: P&T Approval Date: 2/16/2010; P&T Revision Date: 7/12/2011
Guideline GL-35952 Opioid Quantity Limit Overrides Formulary OptumRx Formulary Note: Approval Date 7/10/2017 Revision Date 7/10/2017 Technician Note: P&T Approval Date: 2/16/2010; P&T Revision Date: 7/12/2011
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax:
 REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Anthem Blue Cross Cal MediConnect Plan 1-844-493-9213 Medicare Prior Authorization
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Anthem Blue Cross Cal MediConnect Plan 1-844-493-9213 Medicare Prior Authorization
Technivie. Technivie (ombitasvir, paritaprevir, ritonavir) and Ribavirin. Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Technivie Page: 1 of 6 Last Review Date: March 18, 2016 Technivie Description Technivie (ombitasvir,
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Technivie Page: 1 of 6 Last Review Date: March 18, 2016 Technivie Description Technivie (ombitasvir,
Medicare Part D Opioid Policies for 2019 Information for Patients
 CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Opioid Policies for 2019 Information for Patients Introduction Prescription opioid pain medications like oxycodone (OxyContin ), hydrocodone (Vicodin
CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Opioid Policies for 2019 Information for Patients Introduction Prescription opioid pain medications like oxycodone (OxyContin ), hydrocodone (Vicodin
Pharmacy Medical Necessity Guidelines: Medications for the Treatment of Hepatitis C
 Pharmacy Medical Necessity Guidelines: Medications for the Treatment of Hepatitis C Effective: July 1, 2018 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical
Pharmacy Medical Necessity Guidelines: Medications for the Treatment of Hepatitis C Effective: July 1, 2018 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical
Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs. June 5, 2015
 Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs 1 June 5, 2015 Introductions Mark Steck Pharm.D Independent Consultant, MAXIMUS John J.P. Crouse Vice President, MAXIMUS Market Lead
Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs 1 June 5, 2015 Introductions Mark Steck Pharm.D Independent Consultant, MAXIMUS John J.P. Crouse Vice President, MAXIMUS Market Lead
Psychotropic Medication
 FOM 802-1 1 of 10 OVERVIEW The use of psychotropic medication as part of a child s comprehensive mental health treatment plan may be beneficial and should include consideration of all alternative interventions.
FOM 802-1 1 of 10 OVERVIEW The use of psychotropic medication as part of a child s comprehensive mental health treatment plan may be beneficial and should include consideration of all alternative interventions.
WEBINAR. Difficult-to-treat and severe asthma: changing the paradigm
 WEBINAR Difficult-to-treat and severe asthma: changing the paradigm A multidisciplinary discussion on new therapies, and how to identify and manage difficult-to-treat and severe asthma DIFFICULT-TO-TREAT
WEBINAR Difficult-to-treat and severe asthma: changing the paradigm A multidisciplinary discussion on new therapies, and how to identify and manage difficult-to-treat and severe asthma DIFFICULT-TO-TREAT
Algorithm for the use of inhaled therapies in COPD Version 2 May 2017
 Algorithm for the use of inhaled therapies in COPD This document has been revised by the Berkshire West Respiratory Network to support clinicians in selecting the most appropriate, cost effective treatments
Algorithm for the use of inhaled therapies in COPD This document has been revised by the Berkshire West Respiratory Network to support clinicians in selecting the most appropriate, cost effective treatments
HEDIS 2014 (CY 2013) GOAL MET OR EXCEEDED
 Buckeye Health Plan 2016 Medicaid Ratings: The Healthcare Effectiveness Data and Information Set () is a tool that helps health plans measure the quality of care their members receive. Buckeye uses data
Buckeye Health Plan 2016 Medicaid Ratings: The Healthcare Effectiveness Data and Information Set () is a tool that helps health plans measure the quality of care their members receive. Buckeye uses data
MEMBER GRIEVANCES AND APPEALS PROCEDURES
 MEMBER GRIEVANCES AND APPEALS PROCEDURES We value our members. We want you to let us know right away if you are not happy with our health plan. This includes if you have any questions, complaints or problems
MEMBER GRIEVANCES AND APPEALS PROCEDURES We value our members. We want you to let us know right away if you are not happy with our health plan. This includes if you have any questions, complaints or problems
RE: CMS-4130-P (Medicare Program; Policy and Technical Changes to the Medicare Prescription Drug Benefit)
 Herb Kuhn, Acting Deputy Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Department of Health and Human Services Washington, D.C. 20201
Herb Kuhn, Acting Deputy Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Department of Health and Human Services Washington, D.C. 20201
Design - Multicentre prospective cohort study. Setting UK Community Pharmacies within one CCG area within the UK
 Enabling Patient Health Improvements through COPD (EPIC) Medicines Optimisation within Community Pharmacy: a prospective cohort study Abstract Objectives To improve patients ability to manage their own
Enabling Patient Health Improvements through COPD (EPIC) Medicines Optimisation within Community Pharmacy: a prospective cohort study Abstract Objectives To improve patients ability to manage their own
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Analgesics, Narcotic Long Acting and Analgesics, Narcotic Short
ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Analgesics, Narcotic Long Acting and Analgesics, Narcotic Short
Zepatier is contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh B or C) due to potential toxicity (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Zepatier Page: 1 of 6 Last Review Date: June 24, 2016 Zepatier Description Zepatier (elbasvir,
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Zepatier Page: 1 of 6 Last Review Date: June 24, 2016 Zepatier Description Zepatier (elbasvir,
Background. Background. Background 3/14/2014. Conflict of Interest Statement:
 Platform Presentations Comparison of zolpidem to other drugs associated with falls in hospitalized patients Ed Rainville, MSPharm. Conflict of Interest Statement: The speaker has no conflict of interest
Platform Presentations Comparison of zolpidem to other drugs associated with falls in hospitalized patients Ed Rainville, MSPharm. Conflict of Interest Statement: The speaker has no conflict of interest
Hepatitis C Virus Management
 Hepatitis C Virus Management FDA-Approved Medications Hepatitis C is caused by a virus and results in liver inflammation, which can lead to advanced liver disease and/or liver cancer. An estimated 3 to
Hepatitis C Virus Management FDA-Approved Medications Hepatitis C is caused by a virus and results in liver inflammation, which can lead to advanced liver disease and/or liver cancer. An estimated 3 to
Prescription Drug Abuse: Developing Evidence-Based State Level Interventions
 Prescription Drug Abuse: Developing Evidence-Based State Level Interventions Signe Shackelford, MPH Policy Analyst APHA Annual Meeting November 5, 2013 Presenter Disclosure The following personal financial
Prescription Drug Abuse: Developing Evidence-Based State Level Interventions Signe Shackelford, MPH Policy Analyst APHA Annual Meeting November 5, 2013 Presenter Disclosure The following personal financial
INFLUENZA & PNEUMOCCOCAL VACCINATIONS
 INFLUENZA & PNEUMOCCOCAL VACCINATIONS ONE HEALTH PLAN S PERSPECTIVE Paige Reichert, MD Senior Medical Director of Quality May 2015 THE CIGNA-HEALTHSPRING FOOTPRINT o Cigna-HealthSpring serves the senior
INFLUENZA & PNEUMOCCOCAL VACCINATIONS ONE HEALTH PLAN S PERSPECTIVE Paige Reichert, MD Senior Medical Director of Quality May 2015 THE CIGNA-HEALTHSPRING FOOTPRINT o Cigna-HealthSpring serves the senior
Drug Class Prior Authorization Criteria Hepatitis C
 Drug Class Prior Authorization Criteria Hepatitis C Line of Business: Medicaid P & T Approval Date: November 14, 2018 Effective Date: January 1, 2019 This drug class prior authorization criteria have been
Drug Class Prior Authorization Criteria Hepatitis C Line of Business: Medicaid P & T Approval Date: November 14, 2018 Effective Date: January 1, 2019 This drug class prior authorization criteria have been
New Jersey Department of Human Services Quarterly Newsletter Division of Mental Health Services June 2006
 Dear Mental Health Community, On February 10, 2006, the Division of Mental Health Services released a Wellness and Recovery Transformation Statement guiding the direction for future activities of New Jersey
Dear Mental Health Community, On February 10, 2006, the Division of Mental Health Services released a Wellness and Recovery Transformation Statement guiding the direction for future activities of New Jersey
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 2018 (2018Q3)
 NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 218 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 218 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
September 1, The Honorable Tom Price, MD Secretary Department of Health and Human Services 200 Independence Avenue SW Washington, DC 20201
 September 1, 2017 The Honorable Tom Price, MD Secretary Department of Health and Human Services 200 Independence Avenue SW Washington, DC 20201 Dear Secretary Price: The National Association of County
September 1, 2017 The Honorable Tom Price, MD Secretary Department of Health and Human Services 200 Independence Avenue SW Washington, DC 20201 Dear Secretary Price: The National Association of County
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS. Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails
 STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
Medicare Advantage Outreach and Education Bulletin
 Medicare Advantage Outreach and Education Bulletin Anthem Blue Cross and Blue Shield Prescription Drug Monitoring Programs Prescription drug abuse and diversion are acute problems in the area of pain management.
Medicare Advantage Outreach and Education Bulletin Anthem Blue Cross and Blue Shield Prescription Drug Monitoring Programs Prescription drug abuse and diversion are acute problems in the area of pain management.
Utah. Prescribing and Dispensing Profile. Research current through November 2015.
 Prescribing and Dispensing Profile Utah Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view
Prescribing and Dispensing Profile Utah Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view
Dental Providers - For Action Health Maintenance Organizations - For Information Only. Updates and Information for Dental Services
 State of New Jersey Department of Human Services Division of Medical Assistance & Health Services Volume 18 No. 08 July 2008 TO: SUBJECT: Dental Providers - For Action Health Maintenance Organizations
State of New Jersey Department of Human Services Division of Medical Assistance & Health Services Volume 18 No. 08 July 2008 TO: SUBJECT: Dental Providers - For Action Health Maintenance Organizations
J codes for drugs alphabetical list
 J codes for drugs alphabetical list The Borg System is 100 % J codes for drugs alphabetical list GO J codes for drugs alphabetical list j codes You are here: Home > Medicare > HCPCS Release & Code Sets
J codes for drugs alphabetical list The Borg System is 100 % J codes for drugs alphabetical list GO J codes for drugs alphabetical list j codes You are here: Home > Medicare > HCPCS Release & Code Sets
