The Role of Teneurin C-Terminal Associated Peptide (TCAP)-1 in the Regulation of Stress-Related Behaviours
|
|
|
- Betty Stevenson
- 5 years ago
- Views:
Transcription
1 The Role of Teneurin C-Terminal Associated Peptide (TCAP)-1 in the Regulation of Stress-Related Behaviours by Laura Alexandra Tan A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Cell and Systems Biology University of Toronto Copyright by Laura Alexandra Tan 2011
2 The Role of Teneurin C-Terminal Associated Peptide (TCAP)-1 in the Regulation of Stress-Related Behaviours Abstract Laura Alexandra Tan Doctor of Philosophy Cell and Systems Biology University of Toronto 2011 The teneurin C-terminal associated peptides (TCAPs) are a newly-elucidated family of four bioactive peptides that were found during a screen for novel corticotropin-releasing factor (CRF)-like peptide families. The predicted peptide sequences have the characteristics of a bioactive peptide and are 40 or 41 amino acid residues long. One of the peptides in the family, TCAP-1, has numerous in vitro effects, where it modulates camp accumulation, neuronal proliferation, neurite outgrowth, brain-derived neurotrophic factor levels, and possesses neuroprotective effects under alkalotic or hypoxic conditions. However, little is known about TCAP-1 s in vivo effects. Given the structural similarity of TCAP-1 to the CRF family, it is expected that these peptide systems may interact in vivo. The aims of this research were to 1) investigate the role of TCAP-1 on CRF- and stress-induced behaviours in rats, and determine if intracerebral TCAP-1 could modulate stress-induced anxiety-like behaviours; 2) determine the areas of the brain where TCAP-1 is taken up and is active; and 3) investigate the role of TCAP- 1 s cytoskeletal modulation on stress-sensitive areas of the brain so as to determine a mechanism for long-term behavioural changes in the brain. I have established that TCAP-1 modulates anxiety-like behaviour in exploratory tests of anxiety, and that TCAP-1 is particularly active in the limbic system, including the hippocampus, amygdala, septum, and medial prefrontal cortex, ii
3 and that TCAP-1 increases the dendritic spine density in the hippocampus, a brain area important for anxiety, learning, and memory. These studies have confirmed that TCAP-1 indeed plays a role in stress-like behaviours and modulates stress-related processes in the brain. iii
4 Acknowledgments A doctorate is no doubt a long and arduous process and an adventure that cannot be completed alone. I have been blessed by the best network of supervisors, advisors, coworkers, friends, and family who have supported me throughout my studies at the University of Toronto and have made my time here memorable beyond all imagining. I have been extremely fortunate to have been supported by a number of sources, including the Natural Sciences and Engineering Research Council of Canada (NSERC), the University of Toronto, and the Department of Cell and Systems Biology. I would not have been able to complete these studies without this support. As graduate students consider themselves lucky if they have a good working relationship with their supervisor, I consider myself doubly blessed to have been under the wings of Drs. Susan Rotzinger and David Lovejoy. My heartfelt thanks to Susan for her patience and guidance, especially in the early years of my Ph.D. when projects were unspeakably long and data was fleeting. Susan, you have been a stellar role model and I have thoroughly enjoyed our long discussions on science, family, and life. To David, thank you for the stellar projects that have opened up so many opportunities for me. You have put unwavering faith in my abilities over the years and have allowed me to blossom under your supervision. Our conversations, whether about the intricacies of behavioural data or the subtleties of a recently reviewed red wine, have always been stimulating and appreciated. To Susan and David, being your graduate student has been, without a doubt, one of the greatest honours I could have received. I would also like to thank my wonderful supporting cast of advisors, both official and unofficial. To my supervisory committee members, Drs. Franco Vaccarino and Les Buck, thank you for the many helpful suggestions over the years that have shaped my studies and given me perspectives from two very different points of view. I would also like to thank Dr. Jackson Bittencourt, whose advice through several visits has both challenged and encouraged me in my studies. Special thanks to Ian Buglass, who has helped me navigate the administrative ins and outs of the department. During my long tenure at U of T, I have been surrounded by a wonderfully talented laboratory family. Thanks to Arij Al Chawaf, who was a nurturing mentor when I was both an iv
5 undergraduate and an early graduate student; to Gina Trubiani, whose drive has always been an inspiration; Dr. Cláudio Casatti, whose Brazilian hospitality knew no bounds; to Tiffany Ng, for being an incredible friend in the lab, the best of travel buddies, and a lifelong adopted sister; to Dhan Chand, for the many discussions, crazy times on various conference trips, and for pushing the TCAP project along at an dizzying pace; to Tanya Nock, for her infectious smile and everneeded optimism; to Reuben DeAlmeida and Mei Xu, for their enthusiasm and random teatimes; to Lifang Song for her intense professionalism; and to Rasha Ahmed and Yvonne Kwok, two undergraduate students whose presence in the lab made summers a blast. Lastly, I would like to thank Karen Xu, who worked side-by-side with me through various behavioural projects and who never turned down a request for her exceptional technical wizardry. Outside of the lab, I have been eternally grateful to be involved in some amazing endeavours. Thanks to my various choirs, especially the Newman Centre Choir, with whom I have been able to perform magnificent works that I never thought I d have the chance to perform. A shout out to my various sports teams, especially my Tri-Campus Volleyball and SGS intramural teams, who have given me an outlet to get rid of some of my lab frustrations! And of course, to my teammates of the Varsity Blues women s lacrosse team, I am extremely blessed and honoured to have been one of you for the past two years. I have also had a network of friends whose support has been unwavering. Thanks to Heather Spares, Mel Ballou, and Maree Martinez-Day for their long-distance love, Adriane and Victor Castellino, and Matthew Otto for the lovely musical times, and Angela Tran, Deanna Langer and Kevin Brown for their constant Ph.D. encouragement, providing a push when I needed it the most. I know I have missed names, as my list of supporting cast is long, but to all of you, know that you have all played a part in this milestone. Finally, I am greatly indebted to my wonderful parents, Rose and Michael, who have always encouraged me to follow the career that made me happy. Thank you, Mom and Dad; because of you, I have had the freedom to build upon your steadfast foundation, lifelong curiosity, and immense courage. To my brother, Christopher, thank you for your long-distance but unconditional support and your constant influx of levity. The three of you have always challenged me to be my best, whether in science, sport, music, or art; and I am grateful to have shared my adventures with you. v
6 In closing, to all who have helped me arrive at this point, I will quote a certain friend. When I asked her how to write the conclusions to the thesis, she provided me with a succinct suggestion which I will amend: To everyone: You are awesome, thank you, and goodnight. vi
7 Table of Contents Acknowledgments... iv Table of Contents... vii List of Tables... xi List of Figures... xii List of Abbreviations... xv 1 Chapter One: Introduction: The stress response and the search for novel neuropeptides Abstract Homeostasis, allostasis, and the stress response Stress and the HPA axis Neuroanatomy of the HPA axis Excitatory input to the HPA axis Inhibitory inputs into the HPA axis Neuroanatomy of the behavioural stress response The role of corticotropin-releasing factor (CRF) family: mediators of stress CRF receptors The role of the urocortins in the stress response The HPA axis and behavioural stress responses: two sides of the same coin The search for novel CRF-like peptides: the teneurin C-terminal associated peptides Objectives and Hypothesis References Chapter Two: TCAP-1 and its role in anxiety-like behaviours Abstract Introduction Materials and Methods vii
8 2.3.1 Animals Surgery Peptides Injection procedure Cannula placement verification Elevated Plus Maze (EPM) Open Field Test (OF) Ethovision parameters Experiment 1: Acute effects of TCAP-1 treatment Experiment 2: Effects of 5-day repeated TCAP-1 pre-treatment Experiment 3: Effects of 10-day repeated TCAP-1 treatment Data analysis Results Experiment 1: Acute effects of TCAP-1 treatment Experiment 2: Effects of 5-day repeated TCAP-1 pre-treatment Experiment 3: Effects of 10-day repeated TCAP-1 treatment Discussion References Chapter Three: Elucidating TCAP-1 responsive areas of the brain Abstract Introduction Materials and Methods Animals Surgery Peptides Experiment 1: Acute effects of TCAP-1 on c-fos immunoreactivity viii
9 3.3.5 Experiment 2: Repeated TCAP-1 pre-treatment on c-fos immunoreactivity Statistical Analysis Results Experiment 1: Acute TCAP-1 attenuates CRF-induced c-fos in the limbic system Experiment 2: Repeated TCAP-1 pre-treatment does not affect CRF-induced c-fos accumulation TCAP-1 treatment increases variability in CRF-treated populations Discussion References Chapter Four: TCAP-1 and the regulation of neural morphology Abstract Introduction Materials and Methods Animals Surgery TCAP-1 and vehicle administration Restraint protocol Tissue fixation and histology Dendritic spine analysis Statistical analysis Results Effects of TCAP-1 on spine density under basal conditions Effects of TCAP-1 on spine density under stressed conditions Linear regression analysis Discussion References ix
10 5 Chapter Five: Uptake of intravenous TCAP-1 into body tissues and the brain Abstract Introduction Materials and Methods Animals Peptides Distribution of [ 125 I]-TCAP-1 in brain and tissues Results Uptake of intravenous TCAP-1 into body tissues Uptake of intravenous TCAP-1 into the brain Discussion References Chapter Six: Significance of findings and conclusions Abstract The neuroanatomy of TCAP TCAP-1: An enigmatic peptide TCAP-1 and CRF: Preparing the brain for stress TCAP-1 in stress behaviours: My spider sense is tingling Stress as a risk factor for disease: Too much of a good thing Future directions How do the effects of endogenous TCAP-1 differ from synthetic TCAP-1? Does TCAP-1 affect HPA axis regulation? Does TCAP-1 have a role in learning and memory? Conclusions References Copyright Acknowledgements... Error! Bookmark not defined. x
11 List of Tables Table 2.1: Experiment 1: Acute injections of CRF are anxiogenic on the EPM and OF Table 2.2: Experiment 1: Mean percent of control for exploratory behaviours in the EPM and OF Table 2.3: Experiment 2: Acute injections of CRF in the EPM and OF Table 2.4: Experiment 2: Mean percent of control for exploratory behaviours in the EPM and OF Table 2.5: Experiment 2: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the OF for the full 60 minute trial as a mean percent of control Table 2.6: Experiment 3: Restraint as a stressor in the EPM Table 2.7: Experiment 3: Mean percent of control for exploratory behaviours in the EPM Table 3.1: Cumulative c-fos immunoreactivity counts in other areas of the brain in the repeated TCAP-1 administration study Table 4.1: Raw behaviour of rats in the EPM subjected to 10 days of TCAP-1 injections and restraint Table 5.1: Partitioning of [ 125 I]-TCAP-1 in tissues following i.v. administration to male rats xi
12 List of Figures Figure 1.1: The hypothalamic-pituitary-adrenal axis Figure 1.2: Overview of excitatory inputs into the HPA axis Figure 1.3: Overview of inhibitory inputs into the HPA axis Figure 1.4: Inputs into the BnST from the amygdala Figure 1.5: Distribution of CRF receptors and CRF-like peptides Figure 1.6: The CRF peptide family and its binding partners Figure 1.7: The CRF and TCAP families Figure 2.1: Schematics of the elevated plus maze and open field tests Figure 2.2: Schematic of Acute TCAP-1 experiment Figure 2.3: Schematic of 5-Day Pre-Treatment Administration experiment Figure 2.4: Schematic of 10-Day Repeated Administration experiment Figure 2.5: Acute TCAP-1 on CRF-induced behaviour in the EPM Figure 2.6: Acute TCAP-1 on CRF-induced behaviour in the OF Figure 2.7: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the EPM Figure 2.8: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the OF Figure 2.9: Repeated TCAP-1 on restraint-induced behaviour in the EPM Figure 3.1: Representative images of 25 µm coronal rat brain sections Figure 3.2: Representative images of 25 µm coronal rat brain sections Figure 3.3: Cumulative c-fos immunoreactivity counts in the subfields of the hippocampus in the acute TCAP-1 administration study xii
13 Figure 3.4: Cumulative c-fos immunoreactivity counts in the subnuclei of the amygdala in the acute TCAP-1 administration study Figure 3.5: Cumulative c-fos immunoreactivity counts in other areas of the brain in the acute TCAP-1 administration study Figure 3.6: Cumulative c-fos immunoreactivity counts in areas of the brain where TCAP-1 had no effect on c-fos induction in the acute TCAP-1 administration study Figure 3.7: Cumulative c-fos immunoreactivity counts in the subfields of the hippocampus in the repeated TCAP-1 administration study Figure 3.8: Cumulative c-fos immunoreactivity counts in the subnuclei of the amygdala in the repeated TCAP-1 administration study Figure 3.9: Mean coefficient of variation (c υ ) in the (A) acute TCAP-1 administration study and the (B) repeated TCAP-1 administration study Figure 3.10: Percent difference from mean for 5 rats in the TCAP+CRF group in the (A) acute TCAP-1 administration study and (B) repeated TCAP-1 administration study Figure 4.1: Pathways of the hippocampal formation Figure 4.2: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA1 region of the hippocampus Figure 4.3: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA3 region of the hippocampus Figure 4.4: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA1 region of the hippocampus under repeated restraint conditions Figure 4.5: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA3 region of the hippocampus under repeated restraint conditions Figure 4.6: Effects of repeated injections of TCAP-1 on dendritic spine density in the BLA under non-restraint and repeated restraint conditions xiii
14 Figure 4.7: Correlations between spine density in the stratum radiatum (SR) of the CA3 and EPM behavior Figure 5.1: The distribution of injected [ 125 I]-TCAP-1 in the rat brain Figure 6.1: Neuroanatomy of TCAP-1 within stress circuitry Figure 6.2: Model of TCAP-1 s intracellular effects. Exogenous TCAP-1 binds to a membranebound receptor and is taken up in a punctate-like manner Figure 6.3: Photomicrographs of TCAP-1 immunoreactivity in hippocampal neurons from rat and capuchin monkey (Cebus apella) xiv
15 List of Abbreviations α-msh ACTH AMPA ANOVA AP AP-1 AVP BBB BDNF BLA BnST BSA CA(1-3) camp cdna CeA cpm CRE CREB CRF CRF-BP c υ DAB ddh 2 O DG DRE DREAM DV EF-1 EPM EW FGF-1 FITC GABA GR GRE HPA HPG HPLC IC i.c.v. ID IgG i.p. i.v. alpha-melanocyte-stimulating hormone adrenocorticotrophic hormone α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid analysis of variance anterior-posterior activator protein-1 arginine vasopressin blood-brain barrier brain-derived neurotrophic factor basolateral nucleus of the amygdala bed nucleus of the stria terminalis bovine serum albumin cornu Ammonis cyclic adenosine monophosphate complementary deoxyribonucleic acid central nucleus of the amygdala counts per minute camp response element camp response element binding protein corticotropin-releasing factor corticotropin-releasing factor binding protein coefficient of variation diaminobenzidine double distilled water dentate gyrus downstream response element downstream response element antagonist modulator dorsal-ventral elongation factor-1 elevated plus-maze Edinger-Westphal fibroblast growth factor-1 fluorescein isothiocyanate γ-aminobutyric acid glucocorticoid receptor glucocorticoid response element hypothalamic-pituitary-adrenal hypothalamic-pituitary-gonadal high-performance liquid chromatography inferior colliculus intracerebroventricular injected dose immunoglobulin G intraperitoneal intravenous xv
16 LC MAP MCH MeA MGN ML MN mpfc mpvn mrna MR NGF NMDA NPY NTS OCT OF OVLT PAG PFA POMC ppvn PVN PVT SAL SCN SEM SFO SOD SRE TBS TBS-T TCAP TRH Ucn VTA locus coeruleus microtubule-associated protein melanin concentrating hormone medial nucleus of the amygdala medial geniculate nucleus medial-lateral mammillary nucleus medial prefrontal cortex magnocellular PVN messenger ribonucleic acid mineralocorticoid receptor nerve growth factor N-methyl-D-aspartic acid neuropeptide Y nucleus of the solitary tract optimal cutting temperature open field organum vasculosum of the lamina terminalis periaqueductal grey paraformaldehyde proopiomelanocortin parvocellular PVN paraventricular nucleus of the hypothalamus paraventricular nucleus of the thalamus saline suprachiasmatic nucleus standard error of the mean subfornical organ superoxide dismutase serum response element tris-buffered saline tris-buffered saline with tween-20 teneurin C-terminal associated peptide thyrotropin-releasing hormone urocortin ventral tegmental area xvi
17 1 1 Chapter One: Introduction: The stress response and the search for novel neuropeptides 1.1 Abstract The study of stress has spanned the greater part of the last century, and a particular milestone in the field was the publishing of Walter Cannon s early work on homeostasis and Hans Selye s work on the general adaptation syndrome in the 1930s. The work popularized the idea of the stress response, which involved autonomic, neuroendocrine, and behavioural responses to stimuli that could upset the homeostatic balance. involved in the hypothalamic-pituitary-adrenal (HPA) axis. Later work would identify the releasing factors Corticotropin-releasing factor (CRF), the hypothalamic activating peptide of the HPA axis, was sequenced in 1981, which started an explosion of stress-related research that identified the neuroanatomy of stress-related circuits, identified three more CRF-related peptides (the urocortins), and explored CRF s role as a mediator of the stress response. Quite recently, a new distantly related but CRF-like peptide family was found, called the teneurin C-terminal associated peptides (TCAPs). The TCAP sequence possesses the characteristics of a bioactive peptide, and synthetic versions of the predicted TCAP-1 peptide possesses both in vitro and in vivo effects, including reorganization of the cytoskeleton, neuroprotection under alkalotic conditions, and modulation of acoustic startle behaviour. However, much of TCAP-1 s in vivo characteristics were unknown, especially TCAP-1 s role in stress-like behaviours, an attribute suggested by TCAP-1 s sequence similarity to CRF and TCAP s mrna distribution in the brain. Therefore, the objective of this thesis is to determine if TCAP-1 can regulate CRF-mediated processes in the brain, including neuronal activation of brain areas, alteration of neuronal morphology, and/or modulation of stress-like behaviours. 1.2 Homeostasis, allostasis, and the stress response In his seminal 1936 letter to Nature, Hans Selye (1936) described a non-specific syndrome that exists in rats after exposure to noxious stimuli such as cold, injury, or immune challenge, which he coined the general adaptation syndrome. This occurred in three parts: 1) the alarm reaction, in which there are a myriad of physiological and behavioural changes, such as: an increase in the immune response, disappearance of fat tissue, a decrease in body temperature, enlargement of
18 2 the adrenals, and sexual inhibition; 2) resistance, where small but repeated exposure to noxious stimuli will elicit physiological and behavioural changes that will allow the organs and tissues to return to a semblance of normality; and 3) exhaustion, in which chronic noxious stimuli cause the rats to be unable to cope with ongoing insults such that the rat succumbs to the effects of the stimuli, exhibiting symptoms similar to the alarm reaction (Selye, 1936). Selye had developed his theories during his time at McGill University, and was heavily influenced by researchers such as Walter Cannon, who termed the fight or flight response and developed theories on homeostasis, and Claude Bernard, who coined the term milieu interieur. During the 1930s, the term stress had been used in passing in physiological circles (Viner, 1999), as well as by Walter Cannon, who used the term in 1935 to describe physiological responses as a result of homeostatic perturbation (Cannon, 1935). However, it was not until Selye that the general adaptation syndrome and stress became linked, and the theory was highly controversial throughout the 1940s and 1950s, such that even Cannon was a vocal opponent (Viner, 1999). Selye categorized stress into two extremes: eustress responses were beneficial or behaviourally activating responses to a stressor, whereas distress responses were damaging responses to a protracted stressor. Over the next few decades, Selye s model would be altered and thoroughly transmuted by the time it became accepted as the norm. By the 1970s the word stress, by constant usage, became part of the normal lexicon (Viner, 1999), although by his own admission later in life, Selye admitted that the term strain would have been the better term, as in the field of physics, the applied force, or stress, on a material produces the strain. Modern stress theories now include the idea of allostasis, the changing of homeostatic setpoints in response to stimuli from the environment (McEwen and Wingfield, 2003). Allostasis was first formulated by Sterling and Eyer (1988) as a reimagining of Cannon s original theories on homeostasis (Day, 2005), and was later championed by Bruce McEwen (McEwen and Stellar, 1993). McEwen suggested that allostatic mechanisms are processes in the body that return the organism to a state of homeostasis and anticipate the demand for resources. This produces the allostatic state, in which the body maintains an altered level of allostatic mediators, such as heightened inflammatory cytokines or blood pressure (McEwen and Wingfield, 2003). Over time, strains on the systems create allostatic load, the wear-and-tear on a body from multiple noxious insults that could produce illness or other detrimental effects ( allostatic overload ) (McEwen and Stellar, 1993). McEwen hypothesized that allostatic
19 3 overload occurs in two forms: Type 1 occurs when energy demands exceed energy stores, and Type 2 occurs when energy intake is greater than energy demand. Type 1 allostatic overload elicits escape responses and other coping strategies, whereas Type 2 allostatic overload does not elicit an escape response and social conflict drives the detrimental effects. However, the theory of allostasis is somewhat controversial (Dallman, 2003; Day, 2005) in that the allostasis theory suggests that any threat to homeostasis, however minor or beneficial, should be categorized as a stressor. As the whole brain is involved in maintaining homeostasis, then the whole brain should be seen as part of the stress circuit which is of no use to the stress neurocircuitry field (Day, 2005). Instead, Day suggests that stimuli that perturb homeostasis can elicit either selective responses (a small subset of tissues acting upon a minor challenge) or nonselective responses (a co-ordinated and simultaneous activation of tissues acting upon a real or perceived major challenge) to meet a challenge, and that Selye s non-specific label on stress refers to responses that are common to all stressors, such as activation of the hypothalamicpituitary-adrenal axis. Stress, therefore, can be redefined as an organism s multi-system response to a challenge or stimuli that cannot be contained by the body s homeostatic mechanisms (Day, 2005). Therefore, the stress response allows for appropriate reaction to novel and potentially harmful stimuli and allows the organism to interact with its environment. Stressors are a part of everyday life. Organisms exist as a network of tissues and organs that must survive within a narrow range of physiological conditions. On a daily basis, organisms are subjected to a variety of noxious stimuli that could potentially be damaging to an organism, whether as a result of stressors such as predation, immune challenge, temperature, ph, exercise, lack of nutrition, or inter-species competition. In the human condition, stress has become a leading risk factor in a number of economically significant diseases, such as heart disease, mental illness, substance abuse, neurodegenerative disorders, and gastrointestinal disorders. However, stressors are not altogether detrimental; a small amount of stress is necessary for arousal and motivated behaviour. The stress response is a multimodal system to protect the organism from perturbation. Harmful or stressful stimuli activate the sympathetic branch of the autonomic nervous system, inducing the secretion of catecholamines such as epinephrine (adrenaline) from the adrenal medulla and norepinephrine (noradrenaline) from the sympathetic ganglia neurons. Activation of the
20 4 sympathetic nervous system via brainstem efferents and the secretion of catecholamines are associated with the cardiovascular and visceral changes that prepare the body for the fight or flight response, which occurs rapidly after the onset of the stressor. Stressors will elicit the release of monoamines in the brain, such as norepinephrine, serotonin, and dopamine, which enhance the processing and integration of environmental or somatosensory stimuli. Perception of a stressor also results in activation of the hypothalamic-adrenal-pituitary (HPA) axis, the result of which is a slightly delayed secretion of glucocorticoids from the adrenal cortex. Tissues respond to glucocorticoids by increasing energy metabolism and availability so that the organism has sufficient energy to survive the stressful episode. 1.3 Stress and the HPA axis The HPA axis is a seemingly simple feedback loop in its construction: the paraventricular nucleus of the hypothalamus (PVN) receives afferents from the sensory system and brainstem, secreting corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) during periods of stress, which projects to the median eminence and the hypophyseal portal system. CRF then reaches the anterior pituitary corticotropes, which are stimulated to secrete adrenocorticotropic hormone (ACTH) which enters the systemic circulation. ACTH then stimulates the adrenal cortex to secrete glucocorticoids (such as cortisol in humans and corticosterone in rodents), which will cause a host of different effects, including the increase of gluconeogenesis, mobilization of amino acids, increased lipolysis, and suppression of the immune system. Glucocorticoids will then feedback on the hypothalamus and pituitary to inhibit its own synthesis (Figure 1.1). Basal glucocorticoid secretion follows a circadian rhythm, with concentrations peaking in the perceived morning. The rhythm is dependent on the suprachiasmatic nucleus (SCN), the brain s internal clock (Herman et al., 2005), as lesions of the SCN abolish glucocorticoid rhythmicity (Moore and Eichler, 1972). Glucocorticoids exert a multitude of effects that could be potentially detrimental over long durations, and therefore its secretion is highly regulated. Glucocorticoids will feedback to acutely inhibit the release of CRF and chronically downregulate CRF and AVP expression in the PVN, and hence glucocorticoid secretion (Keller-Wood and Dallman, 1984). The fast feedback depends on the rate of glucocorticoid secretion, and is non-genomic in nature. This effect occurs within seconds but the feedback pathways are still not fully elucidated
21 5 (Dallman, 2005), although this feedback is thought to involve inhibition of membrane-bound receptors that have rapid glutamatergic signalling to the PVN (Di et al., 2003). The more familiar delayed feedback system utilizes genomic changes that depend on the concentration of glucocorticoids (Herman et al., 2005) and works via inhibition of CRF and AVP synthesis and release in the PVN and ACTH and proopiomelanocortin (POMC) synthesis in the pituitary. Glucocorticoid negative feedback also occurs via limbic structures that have inputs to the HPA axis (Herman et al., 2003; 2005). Glucocorticoid actions occur by binding to two distinct cytosolic receptors. The first is the glucocorticoid receptor (GR), which is a type-ii low-affinity receptor. GR is abundant in the brain and is only bound during periods of high glucocorticoid secretion, such as during stress. The mineralocorticoid receptor (MR), is a type-i high-affinity receptor with a much more limited distribution. The MR is bound even at basal levels of glucocorticoids, suggesting that the MR controls basal HPA axis tone (de Kloet et al., 1999). Inhibition of the HPA axis also occurs without glucocorticoid input. The bed nucleus of the stria terminalis (BnST), medial preoptic nucleus, dorsomedial nucleus, and lateral hypothalamic area send γ-aminobutyric acid (GABA)-ergic projections to the PVN, which decrease CRF release, a process which is independent of glucocorticoids (Boudaba et al., 1996; Dong et al., 2001). The HPA axis is not a static system; being essential for survival, its sensitivity changes in response to previous experience. Chronic stressors that are the same, and thus, predictable (for example, repeated exposure to restraint or white noise, known as a homotypic stressor), will decrease the responsiveness of the HPA axis to that stressor (Bhatnagar et al., 2002; Chowdhury et al., 2000; Girotti et al., 2006; Vallès et al., 2006). This attenuation, known as habituation, can be seen as a decrease in both glucocorticoid and ACTH levels (Culman et al., 1991; File, 1982; Helmreich et al., 1997) as well as a decrease in immunoreactivity of the immediate-early gene protein, c-fos, in certain areas of the brain (Girotti et al., 2006). Habituation can be blocked by peripheral injection of MR antagonists (Cole et al., 2000), and GR expression in the hippocampus is correlated with the degree of habituation to a repeated stressor (Helmreich et al., 1997). The effects of habituation are reversible, although effects have been noted to last for at least 4-6 weeks, depending on the intensity of the stressor (Vallès et al., 2006). The HPA axis also exhibits facilitation when exposed to a stressor and then presented with a different (also known as heterotypic) type of stressor. Heterotypic stressors will elicit increased
22 6 glucocorticoid and ACTH release, an effect that lasts for weeks (Akana et al., 1992; Helmreich et al., 1997; Kant et al., 1985; van Dijken et al., 1993). Both the GR and MR are involved in this potentiation, as GR and MR antagonists abolish this effect (Calvo and Volosin, 2001). Facilitation also increases GR and MR binding capacity in the hippocampus (van Dijken et al., 1993). Figure 1.1: The hypothalamic-pituitary-adrenal axis. Noxious stimuli will stimulate the parvocellular cells of the paraventricular nucleus of the hypothalamus (PVN) to release both corticotropin-releasing factor (CRF) and arginine vasopressin (AVP), which enter the hypophyseal portal system. CRF and AVP stimulate the corticotropes of neuroendocrine tissues of the anterior pituitary to release adrenocorticotropic hormone (ACTH). ACTH is then released into the systemic blood flow, where it stimulates the adrenal cortex to release glucocorticoids, such as cortisol (corticosterone in rodents). Glucocorticoids mobilize glucose in target tissues but also feedback on the brain to inhibit release of both CRF and ACTH, thus limiting its own release. Glucocorticoids will also feedback on limbic and other brain areas with input into the PVN to limit HPA axis output.
23 7 1.4 Neuroanatomy of the HPA axis Activation of the HPA axis is accomplished through three pathways: local hypothalamic pathways, ascending input from the brainstem, and input from the circumventricular organs (Figure 1.2) (Ziegler and Herman, 2002). Meanwhile, the regulation of the HPA axis is highly influenced by a set of brain structures from the limbic system (such as the hippocampus, amygdala, and septum), thalamus, and cortex. These areas possess a multitude of interconnections with the HPA axis and each other and work synergistically to maintain HPA axis responsiveness to homeostatic challenges Excitatory input to the HPA axis The hypothalamus consists of a network of distinct nuclei that are intimately interconnected, acting to secrete a variety of hormones into the hypophyseal portal system or through neurosecretory cells. For the stress response, the most important of these nuclei is the PVN, which is subdivided into distinct populations of neurons: the two largest are the magnocellular part (mpvn), which secretes oxytocin and vasopressin, and projects to the posterior pituitary; and the parvocellular part (ppvn), which secretes CRF, AVP, and thyrotropin-releasing hormone (TRH) into the hypophyseal portal system. Conventional wisdom would assume that the ppvn, as the main source of CRF in the HPA axis, would receive direct input from the stress-regulatory areas of the limbic system, but this is not the case (Herman et al., 2003; 2005). The ppvn is mostly confined to its own borders, with only a few connections directly from limbic structures. Instead, many stress-regulatory areas, such as the hippocampus, amygdala, and medial prefrontal cortex (mpfc), innervate the area surrounding the PVN, known as the peri-pvn, which sends inhibitory GABAergic projections to the ppvn (Herman et al., 2003). The PVN also receives direct connections from relays such as the preoptic area, dorsomedial nucleus of the hypothalamus, BnST, and the nucleus of the solitary tract (NTS) (Boudaba et al., 1996; Cunningham and Sawchenko, 1988). Circumventricular organ input to the PVN is achieved by the subfornical organ (SFO) and the organum vasulosum of the lamina terminalis (OVLT) (Figure 1.2). These two areas lack a blood-brain barrier and provide a mechanism for peripheral signals to reach the PVN. The SFO sends angiotensinergic input to the PVN and is sensitive to osmotic stressors (Larsen and
24 8 Mikkelsen, 1995; Plotsky et al., 1988), and activation of this pathway increases CRF and ACTH release (Plotsky et al., 1988). These areas are most likely more sensitive to systemic stressors. Figure 1.2: Overview of excitatory inputs into the HPA axis. Excitatory inputs stimulate the parvocellular PVN to release CRF. Purple nuclei represent direct inputs to the PVN, green nuclei represent polysynaptic inputs to the PVN. Inputs from the nucleus of the solitary tract (NTS, A2) and the ventrolateral medulla (VLM, A1/C1) catecholaminergic efferents (NE) directly project to the PVN. The dorsal raphe nucleus (DRN) also sends sparse serotonergic (5- HT) connections directly to the PVN, but along with the locus coeruleus (LC), it also sends polysynaptic connections through other PVN-regulating areas. The amygdala (Amy) and lateral septum (LS) send excitatory input via disinhibition of GABAergic projections in the peri-pvn and bed nucleus of the stria terminals (BnST) area. Two circumventricular organs, the OVLT and the SFO directly innervate the PVN. Adapted from Ziegler and Herman et al., 2002.
25 9 The brainstem is a source of excitatory input in the HPA axis (Figure 1.2). Both the noradrenergic and serotonergic systems play roles in arousal and attention and it is therefore no surprise that they exert activating effects on the HPA axis. The brainstem sends both ascending noradrenergic input from the NTS and the locus coeruleus (LC) and serotonergic input from the dorsal raphe nucleus into the forebrain. The NTS is associated with the baroreflex response (Herman et al., 2003). It receives input from the mpfc, central nucleus (CeA) and medial nucleus (MeA) of the amygdala, although the CeA/MeA delivers opposing input (Dayas and Day, 2002). In addition, the NTS receives rich innervation from the area postrema, a circumventricular organ. The PVN receives direct input from the A2 noradrenergic cells around the NTS region (Cunningham and Sawchenko, 1988), and the NTS sends projections to the LC, CeA, and MeA (van Bockstaele et al., 1999). The NTS is sensitive to both psychological and physical stressors, although the area has been implicated as vitally important for the HPA axis response to physical or visceral challenges (Buller, 2003; Dayas et al., 2001; Herman and Cullinan, 1997; Pacak, 2000) and expresses GR and MR (Ahima and Harlan, 1990; Ahima et al., 1991). Another important source of excitatory input into the PVN is the LC, which is involved in arousal (Ziegler and Herman, 2002) and is one of the most stressor-sensitive areas in the brain (Herman and Cullinan, 1997). The LC receives input from the medial preoptic area, dorsal raphe, periaqueductal grey (PAG) (Luppi et al., 1995), and projects to forebrain regions such as the amygdala, mpfc, and hippocampus, hypothalamus, cerebellum, and cortex via the A6 noradrenergic cell group (Cunningham and Sawchenko, 1988; Grant and Redmond, 1981; Lehnert et al., 1998) which contains the highest concentration of noradrenergic cells in the brain (Foote et al., 1983). However, it does not send direct connections to the PVN (Cunningham and Sawchenko, 1988) and instead may activate stress-related pathways via activation of other areas connected to the PVN (Ziegler and Herman, 2002). The LC has a role in HPA axis modulation, as lesions reduce ACTH and corticosterone secretion in response to restraint (Ziegler et al., 1999). The dorsal raphe nucleus is a source of serotonergic input to the PVN. This nucleus receives input from the mpfc, hypothalamus, CeA, and medulla (Lee et al., 2003; Peyron et al., 1998) and sends projections to the lateral septum, striatum, PAG, hippocampus, thalamus, and BnST (Vertes, 1991). The dorsal raphe nucleus sends both direct and indirect projections to the PVN (Sawchenko and Swanson, 1983), and lesions of the raphe nuclei attenuate restraintinduced plasma ACTH (Jørgensen et al., 1998). The raphe nuclei contain dense bodies that
26 10 supply the brain with serotonin, which suggests that the raphe may indirectly influence the HPA axis via stress-regulating centres. Unlike other components of the limbic system, such as the hippocampus or mpfc, the amygdala provides mostly excitatory input into the HPA axis through disinhibition of GABAergic relays to the PVN (Forray and Gysling, 2004). The amygdala is not a homogeneous structure, and distinct populations of nuclei are interconnected and respond to different stressors. Three nuclei will be discussed here: the MeA, CeA and BLA. The MeA receives innervation from the olfactory system, infralimbic cortex of the mpfc, and ventral subiculum of the hippocampus, and sends connections to the periaqueductal grey (PAG), ventral tegmental area (VTA), median raphe, medial preoptic area, and BnST (Canteras et al., 1995; Cullinan et al., 1993; Dong et al., 2001). The MeA expresses GR receptors, and to a lesser degree, expresses MR receptors (Ahima and Harlan, 1990; Ahima et al., 1991), and GR mrna is upregulated in the MeA in times of chronic pain (Ulrich-Lai et al., 2006). The MeA has been implicated in the habituation process, as lesions block the attenuation to repeated restraint (Carter et al., 2004), and stimulation of the MeA increases plasma corticosterone levels (Dunn and Whitener, 1986). The CeA receives innervation from the PAG, somatosensory cortex, LC, and the BLA, (Dong et al., 2001; Prewitt and Herman, 1998) and sends projections to the BnST, lateral hypothalamic area, medial preoptic area, and NTS (Petrov et al., 1993; Prewitt and Herman, 1998). The CeA expresses both types of glucocorticoid receptors with high concentrations of GR (Ahima an Harlan, 1990), levels which are upregulated by chronic stressors (Ulrich-Lai et al., 2006). Lesions of the CeA cause a blunting of the ACTH response to immobilization stressors (Beaulieu et al., 1986). Interestingly, the MeA and CeA appear to be sensitive to different types of stimuli, as the MeA is sensitive to stimuli that are noxious but do not present a direct threat to the organism, such as restraint, forced swimming, noise, or open space (Dayas et al., 1999). These stimuli are known as psychological or emotional stressors. The CeA is sensitive to stimuli that are physical in nature, such as haemorrhage or immune challenge (Dayas et al., 2001). Finally, the BLA receives input from the thalamus and ventral subiculum (Canteras et al., 1995), but its efferent connections are not as extensive as the MeA or CeA. Instead, the BLA sends sparse projections to the anterior BnST and mpfc, but most of its connections are between amygdalar nuclei, including the MeA and CeA (Dong et al., 2001). Like the MeA, the BLA is activated by emotional or anticipatory stressors (Cullinan et al., 1995; Sawchenko et al., 2000) and is
27 11 particularly active in response to heterotypic stressors (Bhatnagar and Dallman, 1998). The BLA expresses both GR and MR (Ahima and Harlan, 1990; Ahima et al., 1991), and appears to have a role in the integration of stimuli. The septum is thought to have a modulatory role on the HPA axis. The lateral septum is interconnected with the limbic system and hypothalamus, and sends projections to the peri-pvn area, medial preoptic area, and lateral hypothalamic area (Canteras and Swanson, 1992; Risold and Swanson, 1997). These areas are both GABAergic and glutamatergic (Risold and Swanson, 1996), indicating that the region may modulate HPA axis input by differential input into these areas. The region expresses both GR and MR (Calfa et al., 2006), and lesions of the lateral septum increased ACTH and corticosterone release after forced swimming (Singewald et al., 2011) Inhibitory inputs into the HPA axis The hippocampus is one of the most widely studied limbic structures and is associated with spatial reasoning, learning, and the consolidation of memory (Buwalda et al., 2005). The structure consists of Ammon s Horn (CA1-CA3), dentate gyrus, fimbria, and the subiculum, and is divided into the dorsal (anterior) and ventral (posterior) parts. The hippocampus is innervated by the NTS, LC, amygdala, cortex, and thalamus, amongst other areas (Castle et al., 2005; Moga et al., 1995). Information generally enters through the entorhinal cortex and the dentate gyrus and is processed through the CA3 to CA1 before exiting through the subiculum. The hippocampus also receives input to the CA2 region from the supramammillary nucleus of the hypothalamus (Mercer et al., 2007). The hippocampus is mostly inhibitory on the HPA axis, sending excitatory connections to GABAergic neurons in the BnST and peri-pvn (Figure 1.3) (Cullinan et al., 1993). The hippocampus contains some of the highest concentrations of both GR and MR receptors in the brain (Ahima and Harlan, 1990; Ahima et al., 1991), suggesting that the hippocampus is a main feedback site for the HPA axis. For example, electrical stimulation of the hippocampus causes a decrease in glucocorticoid secretion (Dunn and Orr, 1984), lesions increase the sensitivity to mild stressors (Herman et al., 1995), and chronic stressors increase GR levels in the hippocampus (Mizoguchi et al., 2003). The mpfc is often associated with the integration of cognitive and emotional information, and the consolidation of past and present stimuli. Like the hippocampus, the mpfc is a source of
28 12 inhibitory input to the HPA axis (Figure 1.3). It is involved in higher-order cognitive processes, such as goal-directed behaviour, decision making, and reward seeking (Walton et al., 2002). The mpfc is located in the anterior forebrain and although it has several subdivisions (anterior cingulate, prelimbic, and infralimbic cortices), they are poorly delineated. The mpfc is innervated by the BLA, receives both noradrenergic input from the LC and dopaminergic input from the ventral tegmental area (Bacon et al., 1996; Herman et al., 2003), and sends projections to the amygdala, BnST, paraventricular nucleus of the thalamus (PVT), and dorsal raphe (McDonald et al., 1996; Radley et al., 2009; Vertes, 2002). The mpfc expresses GR and MR and their expression is altered by chronic stressors (Diorio et al., 1993; Mizoguchi et al., 2003). Lesions of the mpfc cause elevations of both ACTH and corticosterone in response to a restraint stressor, but not ether (Diorio et al., 1993) suggesting that this area may discriminate between stressor modalities. The thalamus is situated above the hypothalamus and is well positioned in the brain to exert effects on the stress response. Particularly important is the paraventricular nucleus of the thalamus (PVT), which is vital to the habituation response. The PVT receives connections from many stress-regulating nuclei, such as the ventral subiculum, infralimbic cortex, prelimbic cortex, BnST, NTS, LC, and raphe nuclei (Otake et al., 2002). In turn, the PVT sends projections to the BnST, SCN, MeA, CeA, BLA, and ventral subiculum (Moga et al., 1995). Lesions of the PVT block the habituation of the HPA axis to homotypic stressors without affecting the baseline stress response (Bhatnagar et al., 2002) and may block facilitation to heterotypic stressors (Bhatnagar and Dallman, 1998). The PVT expresses both glucocorticoid receptors (Ahima and Harlan, 1990; Ahima et al., 1991), and MR antagonists, but not GR antagonists, abolish habituation to repeated restraint (Cole et al., 2000).
29 13 Figure 1.3: Overview of inhibitory inputs into the HPA axis. Inhibitory inputs into the PVN prevent the release of CRF to the pituitary. Purple nuclei represent direct connections to the PVN, green nuclei represent polysynaptic input to the PVN. Inhibitory input from the hippocampus (Hi) exits through the ventral subiculum (vsub), which sends glutamatergic connections (+) to the GABA-rich (-) peri-pvn and bed nucleus of the stria terminalis (BnST) area, which inhibit the PVN. The medial prefrontal cortex (mpfc) also sends similar glutamatergic projections to the BnST and peri-pvn area. Adapted from Ziegler and Herman, The HPA axis receives direct input from the BnST, which serves as a relay between the PVN and stress-regulating brain structures. The BnST is thoroughly subdivided and each brain area that innervates the BnST does so in a specific pattern, although there is considerable overlap (for an example, see Figure 1.4). The BnST has connections with the CeA, NTS, PAG, VTA, and the hypothalamus (Forray and Gysling, 2004). Notably, the BnST also receives noradrenergic input from the A1 and A2 noradrenergic cell groups in the brainstem (Forray and Gysling, 2004). The BnST sends mostly inhibitory inputs into the PVN (Boudaba et al., 1996; Cullinan et al., 1993), although electrical stimulation of the anterodorsal and fusiform subnuclei in the anterior BnST increase plasma corticosterone (Dunn, 1987).
30 14 Figure 1.4: Inputs into the BnST from the amygdala. Projections from the CeA or MeA to the BnST produce overlapping, but unique patterns. CeA projections are represented by red dots, and MeA projections are represented by blue dots. Adapted from Dong et al., 2001, BnST diagram adapted from Paxinos and Watson, Together, excitatory inputs from the brainstem, circumventricular organs, septum, and amygdala, and inhibitory inputs from the hippocampus and mpfc (via the BnST and peri-pvn area), shape the HPA axis response to a variety of psychological and physical stressors. Physical stressors, such as ether inhalation, haemorrhage, or injection of inflammatory cytokines, activate catecholaminergic brainstem neurons which activate the PVN directly (Herman and Cullinan, 1997). On the other hand, psychological stressors, such as restraint and noise, are first processed by the limbic circuitry and the cortex before being relayed to the HPA axis, as interpretation of the stimulus requires integration of multiple sensory modalities before execution of the response. Activation of the HPA axis in this case requires inhibition of GABAergic connections to the PVN. Therefore, these areas serve to categorize and modulate the output of the HPA axis to form the appropriate physiological response.
31 Neuroanatomy of the behavioural stress response Noxious stimuli have multi-pronged effects in that they activate neuroendocrine, autonomic, and behavioural responses. Perception of a stressor can result in anticipatory states modulated by the sympathomedullary system and HPA axis, such as the behavioural dimension known as anxiety. This state is the anticipatory behavioural response to a future stressor, whether real or imagined, and is exacerbated by continued presence of the stressor. It has also been linked to fear, the behavioural response to an identifiable potential threat that subsides shortly after its onset (Davis et al., 1997). Anxiety-like behaviour, which can include freezing, hypervigilance, risk assessment, avoidance of open spaces, xenophobia, and defensive behaviours, are measured by a variety of rodent tests (for a review, see Rotzinger et al., 2010) that produce stressful environments in which behaviours such as startle, movement, and approach-avoidance can be quantified. Tests of anxiety have been validated using anxiolytic and anxiogenic (anxietyreducing and anxiety-inducing, respectively) drugs that have efficacy in humans, suggesting that the behaviour exhibited in the test is related to anxious states. The amygdala is important in fear behaviours and emotional memory (Rodrigues et al., 2009), and it receives input from multiple sensory modalities and projects to limbic, hypothalamic, and brainstem nuclei. The amygdala is intimately connected to the central autonomic system components, such as the BnST, LC, NTS, parabrachial nucleus, and ventrolateral medulla, but also has connections to the limbic system and the HPA axis. The BLA serves as the input to the amygdaloid complex, whereas the CeA serves as a major output. Lesions of the BLA or CeA eliminate fear-potentiated acoustic startle (Davis et al., 1997; Oakes and Coover, 1997) but have no effect on unconditioned startle (Melia et al., 1992). Amygdala lesions also impair approachavoidance behaviour in the elevated T-maze (Strauss et al., 2003), which can be interpreted as reduced anxiety-like behaviour; however another group found no effect in the elevated plus maze (EPM), a similar model (Treit et al., 1993). The same group also noted that amygdala lesion induced altered behaviours toward painful stimuli in the shock-probe burying test (Treit et al., 1993). As mentioned previously, the amygdala is also sensitive to glucocorticoids, as implants of corticosterone increased anxiety-like behaviour, an effect that was reversed by MR or GR antagonists (Myers and Greenwood-Van Meerveld, 2007). It has also been suggested that the amygdala is important for passive coping strategies (Roozendaal et al., 1997), and the amygdala
32 16 is well positioned to work in concert with the limbic system and brainstem efferents to modulate stress behaviours. The septo-hippocampal pathway, consisting of the septum, fimbria-fornix, and the hippocampus, has also been implicated in regulating anxiety-like behaviour. The hippocampus is also bidirectionally linked to the BLA, and the septum is connected with the MeA and CeA, suggesting that the two areas may work in concert to regulate stress behaviours. Lesions in the ventral but not the dorsal hippocampus generally reduce anxiety-like behaviour in the EPM, behaviour that was not a result of deficits in spatial reasoning (Degroot and Treit, 2004; Kjelstrup et al., 2002). Tetrodotoxin lesions of the fimbria-fornix, an area generally important in learning, had anxiolytic effects in the EPM and Vogel conflict tests (Degroot and Treit, 2004). Lesions of the septum, a structure closely associated with the hippocampus, decrease anxiety-like behaviour in the EPM (Degroot and Treit, 2004; Treit et al., 1993) and shock-probe burying test (Degroot and Treit, 2004), although it potentiated unconditioned acoustic startle (Melia et al., 1992). MR antagonists have fast-acting effects in the hippocampus, reducing anxiety-like behaviour (Bitran et al., 1998; Smythe et al., 1997). Another area that is closely linked with both the HPA axis and the limbic system is the mpfc, which is involved in decision making and cognitive functions. Lesions of the mpfc decrease anxiety-like behaviour in the EPM and social interaction tests (Gonzalez et al., 2000), although increased anxiety-like behaviour in the social interaction test was seen 5 weeks after lesion (Rangel et al., 2003). Inactivation of the mpfc produces similar anxiety-reducing effects in the Vogel conflict test (Resstel et al., 2008). As reviewed previously, these areas integrate the response to psychological stress and express glucocorticoid receptors. Although glucocorticoids have been implicated in mediating stress-like behaviours such as freezing, memory consolidation, and fear (de Kloet, 2004), other peptide systems are involved in stress-like behaviours that are independent of HPA axis activation. 1.6 The role of corticotropin-releasing factor (CRF) family: mediators of stress The existence of a factor that could be released by the median eminence to trigger pituitary hormone release was first hypothesized in the late 1930s (Harris, 1937). In the mid-1950s, two
33 17 groups identified that hypothalamic extracts contained a factor that could induce ACTH release from the pituitary (Guillemin and Rosenberg, 1955; Saffran and Schally, 1955). However, it was not until 1981 that Wylie Vale and colleagues determined the structure and sequence of ovine CRF (Spiess et al., 1981; Vale et al., 1981), and the sequence was subsequently determined in rats, pigs, man, and other species (for a review, see Rivier and Plotsky, 1986). The newly synthesized peptide could elicit ACTH release and behavioural activation in rats (Britton et al., 1982; Sutton et al., 1982; Vale et al., 1981). Stressor exposure increased CRF mrna levels in the PVN (Imaki et al., 1991; Lightman and Young, 1988; 1989), and thus CRF was soon heralded as an endogenous mediator of stress-like responses. CRF co-localized with oxytocin, AVP, and neurotensin (Sawchenko et al., 1984) and CRF-immunoreactivity was found in the PVN but was also found centrally (i.e. outside of the hypothalamus) in areas such as the CeA, BnST, septum, NTS, LC, neocortex, and hippocampus (Figure 1.5) (Sakanaka et al., 1987; Swanson et al., 1983). Radioiodination of CRF with iodine-125 located high-affinity uptake sites in the anterior pituitary (De Souza et al., 1984; Wynn et al., 1984) as well as a pattern in the brain similar to CRF-immunoreactivity, suggesting that CRF had a neurotransmitter role (De Souza et al., 1985; Wynn et al., 1984). This was strengthened by experiments from the Nemeroff group showing that amygdala, striatum, and midbrain tissue could Ca 2+ -dependently release CRF (Smith et al., 1986). Chronic stressors such as cold, or unpredictable stressors increased CRF-immunoreactivity in the LC and anterior hypothalamic area, and elicited CRF release from median eminence, arcuate nucleus, medial preoptic nucleus (Chappell et al., 1986), CeA (Merali et al., 1998), and hippocampus (Chen et al., 2004; 2006). These results suggest that whereas PVN CRF is vital for the HPA axis response (Beyer et al., 1988), initiation of stress-like behaviours may be regulated by central CRF actions that are independent of HPA axis activation (Britton et al., 1986a; 1986b).
34 Figure 1.5: Distribution of CRF receptors and CRF-like peptides. (Top) Distribution of the CRF 1 and CRF 2 receptors in the brain. (Bottom) Distribution of the CRF, Ucn 1, Ucn 2, Ucn 3, and TCAP in the brain. 7, facial nucleus; 10, vagus nucleus; 12, hypoglossal nucleus; AO, accessory olfactory nucleus; ARC, arcuate nucleus; BG, basal ganglia; BLA, basolateral nucleus of amygdala; BnST, bed nucleus of the stria terminalis; CB, cerebellum; CeA, central nucleus of amygdala; CgCx, cingulate cortex; EW, Edinger-Westphal nucleus; FrCx, frontal cortex; Hi, hippocampus; IC, inferior colliculus; LC, locus coeruleus; LDTg, lateral tegmental nucleus; LS, lateral septum; LSO, lateral superior olive; MeA, medial nucleus of amygdala; MnPO, medial preoptic nucleus; MS, medial septum; NTS, nucleus of the solitary tract; OB, olfactory bulb; OccCx, occipital cortex; OTu, olfactory tubercle; PAG, periaqueductal grey; ParCx, parietal cortex; PB, parabrachial nucleus; PF, perifornical area; PG, pontine grey; PPTg, pedunculopontine tegmental nucleus; PVN, paraventricular nucleus of the hypothalamus; R, red nucleus; RN, raphe nucleus; SON, supraoptic nucleus; SC, superior colliculus; SN, substantia 18
35 19 nigra; Sp5n, spinal trigeminal nucleus; SPO, superior paraolivary nucleus; Thal, thalamus; VLM, ventrolateral medulla; VMH, ventromedial hypothalamic nucleus. Adapted from Reul and Holsboer, CRF receptors Two G protein-coupled receptors which activate adenylate cyclase were identified. The type 1 receptor (CRF 1 ) is a 415-amino acid residue protein that binds CRF with high affinity (Chang et al., 1993; Chen et al., 1993; Perrin et al., 1993) and belongs to the family B of G protein-coupled receptors, like those that bind calcitonin and growth hormone-releasing hormone (Chen et al., 1993). Eight splice variants have been identified (α, β, c-h) (Zmijewski and Slominski, 2010), although the effects of the CRF 1α isoform, which is involved in classical CRF signalling, will be reviewed here. A second distinct but structurally-related type 2 receptor (CRF 2 ) was later identified with 70% identity to the CRF 1 receptor sequence (Lovenberg et al., 1995b; Perrin et al., 1995), which is a 431-amino acid residue protein, but differs in the N-terminus from the CRF 1 receptor (Perrin et al., 1995). Although CRF is able to bind to the CRF 2 receptor, it preferentially binds the CRF 1 receptor. Two splice variants of the CRF 2 receptor were identified in rats and are differentially expressed as CRF 2(a) in the brain and CRF 2(b) in the periphery (Lovenberg et al., 1995a). A third isoform, the CRF 2(c) receptor, was later identified in humans, although not in any other species to date (Hauger et al., 2003; Kostich et al., 1998). The CRF 2(a) receptor will be discussed further. A binding protein, CRF-BP, was also identified in both the brain and periphery (Potter et al., 1991; 1992). The CRF-BP inhibits ACTH release and binds to CRF, modulating its effects (Behan et al., 1995). The distribution of the CRF 1 receptor in the brain is widespread, for the most part overlapping with areas of CRF immunoreactivity (Figure 1.5). CRF 1 receptor mrna is found throughout brain, including the anterior pituitary, layer IV of the cortex, parts of the olfactory pathway, hippocampus, BLA, MeA, medial septum, supramammillary nucleus, lateral hypothalamic area, parts of the brainstem, and cerebellum (Bittencourt and Sawchenko, 2000; Chalmers et al., 1995; van Pett et al., 2000). Stressors cause a biphasic change in pituitary CRF 1 receptor expression, decreasing mrna 2 hours after and increasing mrna 4 hours after stressor exposure (Aguilera et al., 2004). Under basal conditions, CRF 1 is not expressed in the PVN, although different stressors increase CRF 1 receptor mrna in the PVN, such as lipopolysaccharide injection, immobilization (Rivest et al., 1995), haemorrhage, or footshock (van Pett et al., 2000).
36 20 Immobilization also causes a decrease in CRF 1 receptor mrna in the cortex and BLA (Rivest et al., 1995). Repeated i.c.v. CRF administration increases CRF 1 receptor expression in the mpfc and hippocampus and downregulates CRF 1 receptors in the amygdala (Hauger et al., 2006). In contrast to the CRF 1 receptor, the CRF 2 has a more limited distribution. CRF 2 receptor mrna is found in the lateral septum, ventromedial hypothalamic nucleus, MeA, dorsal and median raphe nuclei, parts of the BnST, hippocampus, olfactory bulb, NTS, arcuate nucleus, magnocellular PVN, and PAG (Bittencourt et al., 2000; Chalmers et al., 1995; van Pett et al., 2000). Injections of CRF revealed that activation of the CRF receptors has a role in the stress response. As expected, intravenous (i.v.) CRF induced ACTH release by acting upon the anterior pituitary through activation of the CRF 1 receptor (Orth et al., 1983; Vale et al., 1981). Pharmacological studies showed that i.v. CRF 1 receptor antagonists that do not cross the blood-brain barrier, such as Astressin B, blocked the stressor-induced release of ACTH (Rivier et al., 2003). However, intracerebroventricular (i.c.v.) injection of CRF, a route of administration that reaches the brain parenchyma (Bittencourt and Sawchenko, 2000), also had central effects, as it elevated plasma catecholamines (Brown et al., 1982a; 1982b), increased mean arterial pressure and heart rate (Fisher et al., 1982), decreased growth hormone, luteinizing hormone (Ono et al., 1984), and gonadotropin-releasing hormone (Petraglia et al., 1987), and increased pituitary ACTH release (Brown et al., 1982b). Moreover, i.c.v. injection of CRF (for a review, see Dunn and Berridge, 1990) or intra-amygdalar injections of CRF (Daniels et al., 2004; Liang and Lee, 1988; Sajdyk et al., 1999) have well-documented effects increasing anxiety-like behaviours in rats. In addition, hypophysectomized rats, which lack the pituitary gland, are still behaviourally responsive to i.c.v. CRF (Berridge and Dunn, 1989; Eaves et al., 1985). Most of these effects were shown to be mediated by the CRF 1 receptor, as CRF 1 antagonists abolished behavioural responses to stressors (for a review, see Rotzinger et al., 2010), and CRF 1 receptor-deficient mice displayed reduced stressor-induced release of ACTH and corticosterone (Smith et al., 1998; Timpl et al., 1998), and decreased anxiety-like behaviour (Chotiwat et al., 2010; Contarino et al., 1999; Smith et al., 1998; Timpl et al., 1998). However, this effect could likely be a result of corticosterone deficiency in development because of impaired HPA axis signalling. However, a particularly elegant study supports that central CRF 1 is vital for the behavioural response: a mouse line with a conditional knockout of cortical and limbic CRF 1 receptor but intact pituitary CRF 1 receptor showed decreased anxiety-like behaviour but enhanced restraint-induced ACTH and
37 21 corticosterone release that persisted longer than controls (Müller et al., 2003). This indicates that pituitary CRF 1 receptor is vital for HPA axis activation, central CRF 1 receptor is important for HPA axis feedback, and reduced anxiety-like behaviour in conditional knockouts is HPA axisindependent. In addition, lentiviral knockdown of the CRF 1 receptor in the BLA (Sztainberg et al., 2010) or oligonucleotide injection into the CeA (Liebsch et al., 1995) reduced anxiety-like behaviour. These data support theories that the behavioural response to stress is mediated by extrahypothalamic CRF circuits and independent of pituitary CRF 1 receptors. In contrast to the CRF 1 receptor, the role of central CRF 2 receptors is not as clear. I.c.v. injections of anti-svg-30, a CRF 2 receptor antagonist, attenuated stress-like behaviour (Takahashi et al., 2001) and CRF-induced stress-like behaviour (Risbrough et al., 2003). Deletions of the CRF 2 receptor resulted in normal basal ACTH and corticosterone levels with enhanced ACTH and corticosterone responses to restraint (Bale et al., 2000; Coste et al., 2000; Preil et al., 2001) and prolonged elevated corticosterone after restraint (Coste et al., 2000). Deletion of the CRF 2 receptor in mice produced varying effects on stress-like behaviour, showing no effect (Coste et al., 2000) or an increase in stress-like behaviour (Bale et al., 2000; Chotiwat et al., 2010; Kishimoto et al., 2000). CRF 1 and CRF 2 receptor double knockout in mice abolished restraint- and CRF-induced ACTH and corticosterone (Bale et al., 2002; Preil et al., 2001), and the reduced HPA axis responsiveness attributed to CRF 1 deficiency could not be compensated for by CRF 2 receptor deletion. It does not appear that the CRF 1 -CRF 2 receptor relationship is purely antagonistic. HPA CRF 1 receptor activation may be responsible for ACTH and corticosterone output, whereas central CRF 1 receptor activation may be responsible for behavioural effects and the termination of HPA axis output. Meanwhile, the CRF 2 receptor may have a dual role depending on the phase of the stress response, as it increases the sensitivity to stressors in the short term and decreases the sensitivity to stress and inhibits the HPA axis in the long term after the stressor has abated (Gysling et al., 2004; Reul and Holsboer, 2002). In addition, CRF 2 receptor activation may induce stress-coping behaviours, such as grooming, exploration, and hypophagia (Coste et al., 2000).
38 The role of the urocortins in the stress response Additional CRF-like peptides were also identified, which began with fish urotensin 1 and frog sauvagine (Lederis et al., 1982; Montecucchi et al., 1980). However, more closely related peptides soon emerged. First was urocortin 1 (Ucn 1), a 40 amino acid peptide with 45% identity to CRF which binds to the CRF 1 and CRF 2 receptors with high affinity (Vaughan et al., 1995) and binds to the CRF-BP (Behan et al., 1996). Ucn 1 is richly expressed in the nonpreganglionic Edinger-Westphal (EW) nucleus, as well as the lateral olivary nucleus, and PAG (Figure 1.5) (Vaughan et al., 1995). In addition, Ucn 1-immunoreactivity was found in the PVN and supraoptic nucleus. However, Ucn 1-immunoreactive terminals were found in only a few nuclei bearing CRF 2 receptors, such as the lateral septum (Bittencourt et al., 1999), and i.c.v. injection of Ucn 1 induced activation of CRF 2 -positive areas (Bittencourt and Sawchenko, 2000; Vaughan et al., 1995). However, Ucn 1 expression does not overlap with CRF 2 receptor expression, suggesting that other endogenous ligands must exist that bind to the CRF 2. Urocortin 2 (Ucn 2) and urocortin 3 (Ucn 3), both of which are 38 amino acid residues long were then identified (Hsu and Hsueh, 2001; Lewis et al., 2001; Reyes et al., 2001). Unlike Ucn 1, these peptides bind the CRF 2 receptor with high affinity, with little affinity for CRF 1 receptor. Ucn 2 is expressed in the PVN, arcuate nucleus, and LC, and i.c.v. injection induces activation in the BnST, PVN, CeA, and the NTS (Reyes et al., 2001). Ucn 3 is expressed in the BnST, MeA, medial preoptic area, and perifornical area (Figure 1.5) (Lewis et al., 2001). The urocortins added to the family of peptides that have the potential to influence central CRF receptors (Figure 1.6), and, by their expression in stress-regulating areas, the stress response. The roles of the urocortins are also not fully elucidated. Ucn 1 injection induces the release of ACTH (Asaba et al., 1998; Vaughan et al., 1995) and corticosterone (Asaba et al., 1998), and increases anxiety-like behaviour (Jones et al., 1998; Moreau et al., 1997; Spiga et al., 2006). These actions are presumably through activation of the CRF 1 receptor, although these effects may be a result of exogenous Ucn 1 acting upon sites not normally associated with Ucn 1 activity. Although injection of Ucn 1 can activate the HPA axis, it is not likely an endogenous HPA axis activator; adrenalectomy, which normally elevates CRF output from the hypothalamus, had no effect on Ucn 1 levels, and Ucn 1-antiserum did not affect ACTH output (Oki and Sasano, 2004). Instead, endogenous Ucn 1 appears to be involved in modulating stress behaviour. Cells from the EW send Ucn 1-rich terminals to the lateral septum, and injections of
39 23 CRF 2 receptor antagonist into the area abolished immobilization or CRF-induced stress behaviours (Radulovic et al., 1999). Restraint, footshock, or acute pain increased Ucn 1 mrna in the EW (Cespedes et al., 2010; Harris et al., 2006; Kozicz et al., 2001; Okere et al., 2010 Weninger et al., 2000), and EW Ucn 1-immunoreactivty remains elevated 18 h after stressor (Kozicz et al., 2001). Mice deficient for CRF but with intact CRF receptors showed normal anxiety-like behaviour to stressors, but this effect was blocked by CRF 1 receptor antagonists (Weninger et al., 1999), suggesting that endogenous Ucn 1 may be mediating this behaviour. Mice deficient in Ucn 1 showed normal stress-induced endocrine responses (Vetter et al., 2002; Wang et al., 2002) and normal (Wang et al., 2002) or increased anxiety-like behaviour (Vetter et al., 2002). In particular, Vetter et al. suggest that the increase in anxiety behaviour may be a result of decreased CRF 2 activation in the lateral septum. Figure 1.6: The CRF peptide family and its binding partners. CRF binds with high affinity to the CRF 1 receptor and CRF binding protein (CRF-BP), and with low affinity to the CRF 2 receptor. Ucn 1 binds to the CRF 1, CRF 2, and CRF-BP with high affinity. Ucn 2 and Ucn 3 are selective agonists for the CRF 2 receptor.
40 24 Injections of the CRF 2 receptor-specific agonists, Ucn 2 and Ucn 3, had no effect on the HPA axis (Pelleymounter et al., 2004), although other reports indicated that Ucn 2 (Maruyama et al., 2007) and Ucn 3 (Jamieson et al., 2006) injection induced ACTH release. Activation of the CRF 2 receptor has produced mixed results in behavioural tests. Injections of Ucns 2 or 3 increased stress-like behaviour (Pelleymounter et al., 2002, 2004; Risbrough et al., 2003; Skórzewska et al., 2011) or elicited delayed but reduced stress-like behaviour (Reul and Holsboer, 2002; Valdez et al., 2002, 2003; Venihaki et al., 2004; Zhao et al., 2007). However, aside from the NTS, i.c.v. Ucn 2 activated cell groups that do not strongly express CRF 2 receptors, such as the CeA, PVN, and BnST, and did not activate areas of CRF 2 receptor expression, such as the lateral septum or raphe nuclei (Reyes et al., 2001). Immobilization increased Ucn 2 expression in the PVN but not the LC (Tanaka et al., 2003), and restraint increased Ucn 3 mrna in the MeA and perifornical area (Harris et al., 2006). Ucn 2 or Ucn 3 knockout in mice did not alter HPA axis output or anxiety-like behaviour, although Ucn 3 knockout blocked the acquisition of social memories (Deussing et al., 2010). A triple knockout of all three urocortins resulted in normal basal and post-stress anxiety-like behaviour, but increased anxiety-like behaviour when tested 24 hours later, indicating that Ucn knockout mice do not recover from stressor exposure, although there was no difference in post-stress corticosterone levels (Neufeld-Cohen et al., 2010). This supports the theory that the urocortins, via activation of the CRF 2 receptor, are important endogenous mediators of the behavioural stress response. The urocortins represent peptides that preferentially bind to the CRF 2 receptor and have been associated with coping behaviour and the termination of stress behaviours during the recovery phase. Ucn 1, in particular, may have a role in stress adaptation through its CRF 1 and CRF 2 receptor activity, but it has been hypothesized that the Ucn 1 pathway from the EW may constitute a separate, but complementary system in concert with the CRF-PVN pathway (Kozicz, 2007). Endogenous Ucn 2 and Ucn 3 appear to have a role in recovery after stress, although it may not initiate the HPA axis or stress behaviours. Although, it should be noted that whereas injections of Ucn 2 have modulatory effects on behaviour, it does not activate CRF 2 receptor-rich areas, except the NTS, which suggests that the Ucn 2 (or Ucn 3) form predicted by cdna may not be the endogenous form.
41 The HPA axis and behavioural stress responses: two sides of the same coin Clearly, both the HPA axis response and the behavioural response allow the individual to cope and survive homeostatic threats. However, the circuitries responsible for HPA output and behaviour, although they overlap, are not necessarily the same. Adrenalectomy itself does not produce anxiolytic effects on the EPM (Bitran et al., 1998), although it blocks anxiety-like responses after a stressor (Calvo and Volosin, 2001). Loss of forebrain MR (including the mpfc, hippocampus, and amygdala) also does not induce anxiety-like behaviour (Berger et al., 2006), and overexpression of forebrain MR reduces anxiety-like behaviour (Rozenboom et al., 2007). Forebrain knockout of the GR indicates that HPA axis reactivity is still intact after chronic stressor (Furay et al., 2008), whereas GR is required for HPA axis negative feedback, and GR knockout mice exhibit less anxiety-like behaviour (Boyle et al., 2006). These results suggest that glucocorticoids are required for anxiety-like behaviours. However, the study by Müller et al. (2003) clearly demonstrated in CRF 1 receptor forebrain knockout mice that whereas HPA axis output was intact, anxiety-like behaviour was impaired, and that the central, but not pituitary CRF 1 receptors are required for anxiety-like behaviours. Together, these data suggest that although glucocorticoids produce anxiety-like states in the brain, they cannot do so without central CRF input, and therefore the central CRF system is vital for stress behaviours. 1.8 The search for novel CRF-like peptides: the teneurin C- terminal associated peptides The CRF peptide family is well-conserved and phylogenetically old, consisting of four members with an ancient common ancestor. CRF and Ucns 1-3 appear to have evolved after two genome duplications, one of which may have occured before the formation of the vertebrate lineage (for a review, see Lovejoy and Jahan, 2006). Duplications have allowed for the evolution of new peptides to create families with similar, but not necessarily overlapping functions. Peptides are some of the oldest signalling molecules, being present in the simplest of organisms. Therefore, a gene duplication before the vertebrate divergence, providing CRF with a sister peptide lineage in both vertebrates and invertebrates, could be the basis for a phylogenetically old but previously undiscovered CRF-related family.
42 26 In an attempt to identify CRF-like paralogues, a Ucn 1 probe was used as a low-stringency screen on a rainbow trout cdna library. Of the 600,000 clones that were screened, a fragment was identified belonging to the C-terminus of the teneurin-3 transmembrane protein (Qian et al., 2004). The screen identified a sequence encoding a 40-amino acid residue peptide, which was deemed teneurin C-terminal associated peptide-3 (TCAP-3) for its position on the terminal end of the teneurin protein. Vertebrates possess four teneurin proteins (teneurins 1-4) and each have a TCAP sequence on the C-terminal end (TCAPs 1-4), consisting of peptides 40 or 41 amino acids long that are encoded by the terminal 31 st exon of the teneurin gene (Figure 1.7). Sequence analysis revealed that the TCAP sequences have about 20% amino acid identity with CRF (Wang et al., 2005) and possess cleavage and amidation motifs befitting a bioactive peptide (Qian et al., 2004). The TCAPs are highly conserved, but are also phylogenetically old, suggesting that they are important in the development and survivability of a variety of species (Lovejoy et al., 2006). Figure 1.7: The CRF and TCAP families. Alignment of the CRF family of peptides with the teneurin C-terminal associated peptides. Blue boxes represent amino acid identity to CRF family peptides. Green boxes represent amino acid similarity. Dashed line represents a possible relation from a common ancestor with the CRF peptide family. Adapted from Wang et al., 2005.
43 27 As their name suggests, TCAPs are closely associated with the teneurin proteins. Teneurins are large type II transmembrane proteins with their N-terminus on the cytosolic face and their C- terminus on the extracellular face. Teneurins were originally discovered in Drosophila as the ten-m/odz genes by two separate groups (Baumgartner et al., 1994; Levine et al., 1994), and were subsequently found in C. elegans, chicken, mouse, and humans. Teneurins appear to have a role in development, as mutations in Drosophila result in embryonic lethality (Baumgartner et al., 1994; Levine et al., 1994). Teneurins are expressed in the developing nervous system (Kenzelmann et al., 2008), visual system (Kenzelmann et al., 2008; Kenzelmann-Broz et al., 2010; Rubin et al., 2002) and developing limbs (Kenzelmann-Broz et al., 2010; Tucker et al., 2001), and appear to promote neurite growth, cell adhesion, and axon guidance (Young and Leamey, 2009). TCAPs are active in vitro, producing effects in neuronal culture models. Synthetic TCAP-3 increased camp accumulation at low levels, modulated cell proliferation depending on concentration, decreased teneurin-1 gene expression at low levels and increased teneurin-1 gene expression at high levels in immortalized Gn11 neurons (Qian et al., 2004). Further study continued with synthetic TCAP-1. Like TCAP-3, TCAP-1 increased camp accumulation at low levels, but decreased camp accumulation at high levels, suggesting a modulatory effect (Wang et al., 2005). The increase of camp was not blocked by a Ucn 1 antagonist, suggesting that this effect was not occurring via CRF 1 or CRF 2 receptors. TCAP-1 could also dose-dependently inhibit forskolin-induced camp, and like TCAP-3, TCAP-1 modulated cell proliferation (Wang et al., 2005). TCAP-1 was neuroprotective in alkalotic environments in vitro, allowing TCAP-1- treated N38 immortalized neurons to survive at a ph of 8 or 8.4. TCAP-1 treatment prevented cell death via inhibition of necrotic, but not apoptotic cell pathways, increasing superoxide dismutase (SOD)-1 and increasing SOD copper chaperone and catalase (Trubiani et al., 2007). TCAP-1 could also affect neuron morphology. TCAP-1 treatment in N38 cells modulated the outgrowth of neurites, which are projections from the soma of the neuron in culture, such that long neurites got longer whereas short neurites got shorter (Al Chawaf et al., 2007a). In primary hippocampal culture, TCAP-1 administration induced an increase in neurite outgrowth and the fasciculation of neurites, showing that effects could occur in a non-immortalized culture (Al
44 28 Chawaf et al., 2007a). Modulation and reorganization of the neuron likely requires reorganization of the cytoskeleton, and TCAP-1 increased both β-tubulin and β-actin levels in N38 cells, and caused a reorganization of β-tubulin in neurons (Al Chawaf et al., 2007a). These data suggest that TCAP-1 is involved in axonal growth and morphology. TCAP-1 also had intriguing in vivo effects. In situ hybridization studies in the rat revealed that TCAP-1 mrna could be found throughout the brain, such as in the cortex, hippocampus, amygdala, ventromedial nucleus of the hypothalamus, subthalamic nucleus, olfactory bulb, brainstem, and cerebellum (Figure 1.5) (Wang et al., 2005). These areas are associated with the regulation and integration of the stress response, suggesting that TCAP-1 has a role in regulating the HPA axis or stress behaviours. To determine if TCAP-1 could affect behaviour, TCAP-1 was tested in the acoustic startle test, in which high startle responses are associated with increased anxiety (Yeomans and Frankland, 1995). Rats were separated out according to basal startle behaviour prior to injections, producing a low-startle group and a high-startle group. Injections of TCAP-1 into the BLA modulated acoustic startle behaviour, increasing startle in the low-startle group and decreasing startle in the high-startle group (Wang et al., 2005). Furthermore, i.c.v. injections for 5 days produced a decrease in startle behaviour 21 days after the last injection, showing that TCAP-1 has long-lasting behavioural effects (Wang et al., 2005). These behavioural effects, along with the distribution of mrna in key limbic and regulatory brain areas, indicate that TCAP-1 has an important role in stress-related and anxiety-like behaviours, and it may do so via HPA axis modulation or interaction with the CRF system. 1.9 Objectives and Hypothesis The TCAP family presents a newly elucidated but evolutionarily old lineage, as TCAPs have been found in every vertebrate genome that has been studied thus far. TCAP-1 has many documented effects in intracellular signalling, neuroprotection, and cytoskeleton regulation, but very little work had been done on its effects in vivo. I hypothesize that TCAP-1 is an intrinsic component of the stress response system and therefore, interacts with the CRF system. By virtue of TCAP-1 s mrna distribution in stress-regulating brain areas, its effects on acoustic startle, and its structural similarity to CRF, it was necessary to determine if TCAP-1 could regulate the effects of CRF, and ultimately the behavioural stress response in vivo. To do so, it was first important to determine TCAP-1 s effects on anxiety-like behaviours, given the ability
45 29 of extrahypothalamic CRF to mediate the behavioural response. Secondly, it was essential to determine both uptake sites and neuronal activation sites in the brain, to determine if TCAP-1 mrna expression and activity overlapped. Due to TCAP-1 s effects on the acoustic startle, it was important to focus on limbic circuits, such as the hippocampus and amygdala, as well as higher-order structures that process information, such as the mpfc. Finally, it was important to determine a mechanism for such behavioural modifications, as TCAP-1 s acoustic startle effects were still present long after TCAP-1 was eliminated from the system. TCAP-1 s role in remodelling the neural cytoskeleton could promote long-term modifications in brain connectivity. To realize these goals, the following objectives were accomplished: 1) Explored the effects of TCAP-1 on stress-induced anxiety-like behaviours in the rat 2) Determined the activation and uptake areas in the brain where TCAP-1 exerts its effects 3) Examined the mechanism underlying TCAP-1 s long-term changes in stress-sensitive structures in the brain 1.10 References Aguilera G, Nikodemova M, Wynn PC, Catt KJ. (2004) Corticotropin releasing hormone receptors: two decades later. Peptides 25(3): Ahima RS, Harlan RE. (1990) Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39(3): Ahima R, Krozowski Z, Harlan R. (1991) Type 1 corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol 313(3): Akana SF, Dallman MF, Bradbury MF, Scribner KA, Strack AM, Walker CD. (1992) Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology 131(1): Al Chawaf A, St Amant K, Belsham D, Lovejoy DA. (2007a) Regulation of neurite growth in immortalized mouse hypothalamic neurons and rat hippocampal primary cultures by teneurin C-terminal associated peptide-1. Neuroscience 144(4): Al Chawaf A, Xu K, Tan L, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2007) Corticotropinreleasing factor (CRF)-induced behaviors are modulated by intravenous administration of teneurin C-terminal associated peptide-1 (TCAP-1). Peptides 28(7):
46 30 Asaba K, Makino S, Hashimoto K. (1998) Effect of urocortin on ACTH secretion from rat anterior pituitary in vitro and in vivo: comparison with corticotropin-releasing hormone. Brain Res 806(1): Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. (1996) Amygdala input to the medial prefrontal cortex (mpfc) in the rat: a light and electron microscope study. Brain Res 720(1-2): Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. (2000) Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat Genet 224(4): Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. (2002) Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci 22(1): Baumgartner S, Martin D, Hagios C, Chiquet-Ehrismann R. (1994) Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J 13(16): Beaulieu S, Di Paolo T, Barden N. (1986) Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotonergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 44(2): Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. (1995) Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol 16(4): Behan DP, Khonsaly O, Ling N, De Souza EB. (1996) Urocortin interaction with corticotropinreleasing factor (CRF) binding protein (CRF-BP): a novel mechanism for elevating free CRF levels in human brain. Brain Res 725(2): Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schütz G. (2006) Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A 103(1): Berridge CW, Dunn AJ. (1989) CRF and restraint-stress decrease exploratory behavior in hypophysectomized rats. Pharmacol Biochem Behav 34(3): Beyer HS, Matta SG, Sharp BM. (1988) Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology 123(4): Bhatnagar S, Dallman M. (1998) Neuroanatomical basis for facilitation of hypothalamicpituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84(4):
47 31 Bhatnagar S, Huber R, Nowak N, Trotter P. (2002) Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14(5): Bitran D, Shiekh M, Dowd JA, Dugan MM, Renda P. (1998) Corticosterone is permissive to the anxiolytic effect that results from the blockade of hippocampal mineralocorticoid receptors. Pharmacol Biochem Behav 60(4): Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. (1999) Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 415(3): Bittencourt JC, Sawchenko PE. (2000) Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci 20(3): Boudaba C, Szabó K, Tasker JG. (1996) Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci 16(22): Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. (2006) Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 26(7): Britton DR, Koob GF, Rivier J, Vale W. (1982) Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci 31(4): Britton KT, Lee G, Dana R, Risch SC, Koob GF. (1986a) Activating and anxiogenic effects of corticotropin releasing factor are not inhibited by blockade of the pituitary-adrenal system with dexamethasone. Life Sci 39(14): Britton KT, Lee G, Vale W, Rivier J, Koob GF. (1986b) Corticotropin releasing factor (CRF) receptor antagonist blocks activating and anxiogenic actions of CRF in the rat. Brain Res 369(1-2): Brown MR, Fisher LA, Rivier J, Spiess J, Rivier C, Vale W. (1982a) Corticotropin-releasing factor: effects on the sympathetic nervous system and oxygen consumption. Life Sci 30(2): Brown MR, Fisher LA, Spiess J, Rivier J, Rivier C, Vale W. (1982b) Comparison of the biologic actions of corticotropin-releasing factor and sauvagine. Regul Pept 4(2): Buller KM. (2003) Neuroimmune stress responses: reciprocal connections between the hypothalamus and the brainstem. Stress 6(1): Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. (2005) Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29(1):
48 32 Calfa G, Volosin M, Molina VA. (2006) Glucocorticoid receptors in lateral septum are involved in the modulation of the emotional sequelae induced by social defeat. Behav Brain Res 172(2): Calvo N, Volosin M. (2001) Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology 73(4): Cannon WB. (1935) Stresses and strains of homeostasis. Am J Med Sci 189(1): Canteras NS, Swanson LW. (1992) Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol 324(2): Canteras NS, Simerly RB, Swanson LW. (1995) Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 360(2): Carter RN, Pinnock SB, Herbert J. (2004) Does the amygdala modulate adaptation to repeated stress? Neuroscience 126(1): Castle M, Comoli E, Loewy AD. (2005) Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience 134(2): Cespedes IC, de Oliveira AR, da Silva J, da Silva AV, Sita LV, Bittencourt JC. (2010) mrna expression of corticotropin-releasing factor and urocortin 1 after restraint and foot shock together with alprazolam administration. Peptides 31(12): Chalmers DT, Lovenberg TW, De Souza EB. (1995) Localization of novel corticotropinreleasing factor (CRF 2 ) mrna expression to specific subcortical nuclei in rat brain: comparison with CRF 1 receptor mrna expression. J Neurosci 15(10): Chang CP, Pearse RV 2 nd, O Connell S, Rosenfeld MG. (1993) Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 11(6): Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. (1986) Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 6(10): Chen R, Lewis KA, Perrin MH, Vale WW. (1993) Expression cloning of a human corticotropinreleasing-factor receptor. Proc Natl Acad Sci U S A 90(19): Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. (2004) Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience 126(3): Chen Y, Fenoglio KA, Dubé CM, Grigoriadis DE, Baram TZ. (2006) Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry 11(11):
49 33 Chotiwat C, Kelso EW, Harris RB. (2010) The effects of repeated restraint stress on energy balance and behavior of mice with selective deletion of CRF receptors. Stress 13(3): Chowdhury GH, Fujioka T, Nakamura S. (2000) Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull 52(3): Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MF, Spencer RL. (2000) Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol 12(10): Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. (1999) Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res 835(1): 1-9. Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. (2000) Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24(4): Cullinan WE, Herman JP, Watson SJ. (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332(1): Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64(2): Culman J, Kopin IJ, Saavedra JM. (1991) Regulation of corticotropin-releasing hormone and pituitary-adrenocortical response during acute and repeated stress in the rat. Endocr Regul 25(3): Cunningham ET Jr, Sawchenko PE. (1988) Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274(1): Dallman, MF. (2003) Stress by any other name..? Horm Behav 43(1): 18-20; discussion Dallman, MF. (2005) Fast glucocorticoid actions on brain: back to the future. Neuroendocrinol 26(3-4): Front Daniels WM, Richter L, Stein DJ. (2004) The effects of repeated intra-amygdala CRF injections on rat behavior and HPA axis function after stress. Metab Brain Dis 19(1-2): Davis M, Walker DL, Lee Y. (1997) Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci 352(1362):
50 34 Day TA. (2005) Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry 29(8): Dayas CV, Buller KM, Day TA. (1999) Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11(7): Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. (2001) Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14(7): Dayas CV, Day TA. (2002) Opposing roles for medial and central amygdala in the initiation of noradrenergic cell responses to a psychological stressor. Eur J Neurosci 15(10): De Kloet ER, Oitzl MS, Joëls M. (1999) Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22(10): De Kloet ER. (2004) Hormones and the stressed brain. Ann N Y Acad Sci 1018: De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. (1984) Corticotropinreleasing factor receptors in rat forebrain: autoradiographic identification. Science 224(4656): De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. (1985) Corticotropinreleasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci 5(12): Degroot A, Treit D. (2004) Anxiety is functionally segregated within the septo-hippocampal system. Brain Res 1001(1-2): Deussing JM, Breu J, Kühne C, Kallnik M, Bunck M, Glasl L, Yen YC, Schmidt MV, Zurmühlen R, Vogl AM, Gailus-Durner V, Fuchs H, Hölter SM, Wotjak CT, Landgraf R, de Angelis MH, Holsboer F, Wurst W. (2010) Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J Neurosci 30(27): Di S, Malcher-Lopes R, Halmos KC, Tasker JG. (2003) Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23(12): Diorio D, Viau V, Meaney MJ. (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13(9): Dong HW, Petrovich GD, Watts AG, Swanson LW. (2001) Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436(4):
51 35 Dunn JD, Orr SE. (1984) Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res 54(1): 1-6. Dunn JD, Whitener J. (1986) Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 42(3): Dunn JD. (1987) Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res 407(2): Dunn JD, Berridge CW. (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15(2): Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF. (1985) Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides 6(5): File SE. (1982) The rat corticosterone response: habituation and modification by chlordiazepoxide. Physiol Behav 29(1): Fisher LA, Rivier J, Rivier C, Spiess J, Vale W, Brown MR. (1982) Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology 110(6): Foote SL, Bloom FE, Aston-Jones G. (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63(3): Forray MI, Gysling K. (2004) Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev 47(1-3): Furay AR, Bruestle AE, Herman JP. (2008) The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149(11): Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. (2006) Habituation to repeated restraint is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 138(4): Gonzales LE, Rujano M, Tucci S, Paredes D, Silva E, Alba G, Hernandez L. (2000) Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res 887(1): Grant SJ, Redmond DE Jr. (1981) The neuroanatomy and pharmacology of the nucleus locus coeruleus. Prog Clin Biol Res 71:5-27. Guillemin R, Rosenberg B. (1955) Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology 57(5): Gysling K, Forray MI, Haeger P, Daza C, Rojas R. (2004) Corticotropin-releasing hormone and urocortin: redundant or distinctive functions? Brain Res Brain Res Rev 47(1-3):
52 36 Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. (2006) Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav 49(5): Harris GW. (1937) The induction of ovulation in the rabbit by electrical stimulation of the hypothalamo-hypophysial mechanism. Proc Roy Soc Lond B 122: Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. (2003) International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55(1): Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. (2006) Corticotropin-releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets 5(4): Helmreich DL, Morano MI, Akil H, Watson SJ. (1997) Correlation between changes in stressinduced corticosterone secretion and GR mrna levels. Stress 2(2): Herman JP, Cullinan WE, Morano Mi, Akil H, Watson SJ. (1995) Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol 7(6): Herman JP, Cullinan WE. (1997) Neurocircuitry of stress: central control of the hypothalamopituitary-adrenocortical axis. Trends Neurosci 20(2): Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24(3): Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29(8): Hsu SY, Hsueh AJ. (2001) Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med 7(5): Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. (1991) Differential regulation of corticotropin-releasing factor mrna in rat brain regions by glucocorticoids and stress. J Neurosci 11(3): Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. (2006) Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology 147(10): Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. (1998) The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology (Berl) 138(2):
53 37 Jørgensen H, Knigge U, Kjaer A, Vadsholt T, Warberg J. (1998) Serotonergic involvement in stress-induced ACTH release. Brain Res 811(1-2): Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. (1985) Habituatin to repeated stress is stressor specific. Pharmacol Biochem Behav 22(4): Keller-Wood ME, Dallman MF. (1984) Corticosteroid inhibition of ACTH secretion. Endocr Rev 5(1): Kenzelmann D, Chiquet-Ehrismann R, Leachman NT, Tucker RP. (2008) Teneurin-1 is expressed in interconnected regions of the developing brain and is processed in vivo. BMC Dev Biol 8:30. Kenzelmann-Broz D, Tucker RP, Leachman NT, Chiquet-Ehrismann R. (2010) The expression of teneurin-4 in the avian embryo: potential roles in patterning of the limb and nervous system. Int J Dev Biol 54(10): Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. (2000) Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet 24(4): Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. (2002) Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 99(16): Kostich WA, Chen A, Sperle K, Largent BL. (1998) Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF 2γ receptor. Mol Endocrinol 12(8): Kozicz T, Min L, Arimura A. (2001) The activation of urocortin immunoreactive neurons in the Edinger-Westphal nucleus following acute pain stress in rats. Stress 4(2): Kozicz T. (2007) CRF and CRF-related peptides in stress adaptation: from invertebrates to man. Gen Comp Endocrinol 153(1-3): Larsen PJ, Mikkelsen JD. (1995) Functional identification of central afferent projections conveying information of acute stress to the hypothalamic paraventricular nucleus. J Neurosci 15(4): Lederis K, Letter A, McMaster D, Moore G, Schlesinger D. (1982) Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science 218(4568): Lee HS, Kim MA, Valentino RJ, Waterhouse BD. (2003) Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res 963(1-2): Lehnert H, Schulz C, Dieterich K. (1998) Physiological and neurochemical aspects of corticotropin-releasing factor actions in the brain: the role of the locus coeruleus. Neurochem Res 23(8):
54 38 Levine A, Bashan-Ahrend A, Budai-Hadrian O, Gartenberg D, Menasherow S, Wides R. (1994) odd Oz: a novel Drosophila pair rule gene. Cell 77(4): Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. (2001) Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF 2 receptor. Proc Natl Acad Sci U S A 98(13): Liang KC, Lee EH. (1988) Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology (Berl) 96(2): Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. (1995) Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept 59(2): Lightman SL, Young WS 3 rd. (1988) Corticotropin-releasing factor, vasopressin and proopiomelanocortin mrna responses to stress and opiates in the rat. J Physiol 403: Lovejoy DA, Al Chawaf A, Cadinouche MZ. (2006) Teneurin C-terminal associated peptides: an enigmatic family of neuropeptides with structural similarity to the corticotropin-releasing factor and calcitonin families of peptides. Gen Comp Endocrinol 148(3): Lovejoy DA, Jahan S. (2006) Phylogeny of the corticotropin-releasing factor family of peptides in the metazoa. Gen Comp Endocrinol 146(1): 1-8. Lovenberg TW, Chalmers DT, Liu C, De Souza EB. (1995a) CRF2 alpha and CRF2 beta receptor mrnas are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136(9): Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Olstersdorf T. (1995b) Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A 92(3): Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. (1995) Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience 65(1): Maruyama H, Makino S, Noguchi T, Nishioka T, Hashimoto K. (2007) Central type 2 corticotropin-releasing hormone receptor mediates hypothalamic-pituitary-adrenocortical axis activation in the rat. Neuroendocrinology 86(1): McDonald AJ, Mascagni F, Guo L. (1996) Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71(1):
55 39 McEwen BS, Stellar E. (1993) Stress and the individual. Mechanisms leading to disease. Arch Interl Med 153(18): McEwen BS, Wingfield JC. (2003) The concept of allostasis in biology and medicine. Horm Behav 43(1): Melia KR, Sananes CB, Davis M. (1992) Lesions of the central nucleus of the amygdala block the excitatory effects of septal ablation on the acoustic startle relflex. Physiol Behav 51(1): Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. (1998) Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 18(12): Mercer A, Trigg HL, Thomson AM. (2007) Characterization of neurons in the CA2 subfield of the adult rat hippocampus. J Neurosci 27(27): Mizoguchi K, Ishige A, Aburada M, Tabira T. (2003) Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 119(3): Moga MM, Weis RP, Moore RY. (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359(2): Montecucchi PC, Anastasi A, de Castiglione R, Erspamer V. (1980) Isolation and amino acid composition of sauvagine. An active polypeptide from methanol extracts of the skin of the South American frog Phyllomedusa sauvagei. Int J Pept Protein Res 16(3): Moore RY, Eichler VB. (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42(1): Moreau JL, Kilpatrick G, Jenck F. (1997) Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport 8(7): Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kühn R, Reul JM, Holsboer F, Wurst W. (2003) Limbic corticotropinreleasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6(10): Myers B, Greenwood-Van Meerveld B. (2007) Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol 292(6):G Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. (2010) A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci U S A 107(44): Oakes ME, Coover GD. (1997) Effects of small amygdala lesions on fear, but not aggression, in the rat. Physiol Behav 61(1):
56 40 Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. (2010) Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res 1317: Oki Y, Sasano H. (2004) Localization and physiological roles of urocortin. Peptides 25(10): Ono N, Lumpkin MD, Samson WK, McDonald JK, McCann SM. (1984) Intrahypothalamic action of corticotropin-releasing factor (CRF) to inhibit growth hormone and LH release in the rat. Life Sci 35(10): Orth DN, Jackson RV, DeCherney GS, DeBold CR, Alexander AN, Island DP, Rivier J, Rivier C, Spiess J, Vale W. (1983) J Clin Invest 71(3): Otake K, Kin K, Nakamura Y. (2002) Fos expressin in afferents to the rat midline thalamus following immobilization stress. Neurosci Res 43(3): Qian X, Barsyte-Lovejoy D, Wang L, Chewpoy B, Gautam N, Al Chawaf A, Lovejoy DA. (2004) Cloning and characterization of teneurin C-terminal associated peptide (TCAP)-3 from the hypothalamus of an adult rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 137(2): Pacák K. (2000) Stressor-specific activation of the hypothalamic-pituitary-adrenocortical axis. Physiol Res 49 Suppl 1: S11-7. Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4 th Ed., Academic Press, Sydney. Pelleymounter MA, Joppa M, Ling N, Foster AC. (2002) Pharmacological evidence supporting a role for central corticotropin-releasing factor 2 receptors in behavioral, but not endocrine, response to environmental stress. J Phamacol Exp Ther 302(1): Pelleymounter MA, Joppa M, Ling N, Foster AC. (2004) Behavioral and neuroendocrine effects of the selective CRF 2 receptor agonists urocortin II and urocortin III. Peptides 25(4): Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. (1993) Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 133(6): Perrin MH, Donaldson CJ, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko PE, Vale W. (1995) Identification of a second corticotropin-releasing factor receptor gene and characterization of a cdna expressed in heart. Proc Natl Acad Sci U S A 92(7): Petraglia F, Sutton S, Vale W, Plotsky P. (1987) Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology 120(3):
57 41 Petrov T, Krukoff TL, Jhamandas JH. (1993) Branching projections of catecholaminergic brainstem neurons to the paraventricular nucleus and the central nucleus of the amygdala in the rat. Brain Res 609(1-2): Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82(2): Plotsky PM, Sutton SW, Bruhn TO, Ferguson AV. (1988) Analysis of the role of angiotensin II in mediation of adrenocorticotropin secretion. Endocrinology 122(2): Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. (1991) Cloning and characterization of the cdnas for human and rat corticotropin-releasing factor-binding proteins. Nature 349(6308): Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. (1992) The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A 89(9): Preil J, Müller MB, Gesing A, Reul JM, Sillaber I, van Gaalen MM, Landgrebe J, Holsboer F, Stenzel-Poore M, Wurst W. (2001) Regulation of the hypothalamic-pituitaryadrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology 142(11): Prewitt CM, Herman JP. (1998) Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat 15(3): Radley JJ, Gosselink KL, Sawchenko PE. (2009) A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29(22): Radulovic J, Rühmann A, Liepold T, Spiess J. (1999) Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci 19(12): Rangel A, Gonzalez LE, Villarroel V, Hernandez L. (2003) Anxiolysis followed by anxiogenesis relates to coping and corticosterone after medial prefrontal cortical damage in rats. Brain Res 992(1): Resstel LB, Souza RF, Guimarães FS. (2008) Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav 93(1-2): Reul JM, Holsboer F. (2002) Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2(1): Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. (2001) Urocortin II: a member of the corticotropin-
58 42 releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A 98(5): Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. (2003) Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 170(2): Risold PY, Swanson LW. (1996) Structural evidence for functional domains in the rat hippocampus. Science 272(5267): Risold PY, Swanson LW. (1997) Connections of the rat lateral septal complex. Brain Res Brain Res Rev 24(2-3): Rivest S, Laflamme N, Nappi RE. (1995) Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci 15(4): Rivier CL, Plotsky PM. (1986) Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Ann Rev Physiol 48: Rivier CL, Grigoriadis DE, Rivier JE. (2003) Role of corticotropin-releasing factors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 144(6): Rodrigues SM, LeDoux JE, Sapolsky RM. (2009) The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32: Roozendaal B, Koolhaas JM, Bohus B. (1997) The role of the central amygdala in stress and adaptation. Acta Physiol Scand Suppl 640: Rotzinger S, Lovejoy DA, Tan LA. (2010) Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31(4): Rozeboom AM, Akil H, Seasholtz AF. (2007) Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters stress responsiveness in mice. Proc Natl Acad Sci 104(11): Rubin BP, Tucker RP, Brown-Luedi M, Martin D, Chiquet-Ehrismann R. (2002) Teneurin 2 is expressed by the neurons of the thalamofugal visual system in situ and promotes homophilic cell-cell adhesion in vitro. Development 129(20): Saffran M, Schally AV. (1955) The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol 33(3): Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. (1999) Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res 100(1-2):
59 43 Sakanaka M, Shibasaki Y, Lederis K. (1987) Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidasediaminobenzidine method. J Comp Neurol 260(2): Sawchenko PE, Swanson LW. (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218: Sawchenko PE, Swanson LW, Vale W. (1984) Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasoppresin-, and neurotensin-immunoreactive neurons in the hypothalamus of the rat. J Neurosci 4(4): Sawchenko PE, Li HY, Ericsson A. (2000) Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res 122: Selye H. (1936) A syndrome produced by diverse nocuous agents. Nature 138: 32. Singewald GM, Rjabokon A, Singewald N, Ebner K. (2011) The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36(4): Skórzewska A, Bidziński A, Lehner M, Turzyńska D, Sobolewska A, Wisłowska-Stanek A, Maciejak P, Szyndler J, Płaźnik A. (2010) The localization of brain sites of anxiogeniclike effects of urocortin 2. Neuropeptides 45(1): Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. (1998) Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20(6): Smith MA, Bissette G, Slotkin TA, Knight DL, Nemeroff CB. (1986) Release of corticotropinreleasing factor from rat brain regions in vitro. Endocrinology 118(5): Smythe JW, Murphy D, Timothy C, Costall B. (1997) Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol Biochem Behav 56(3): Spiess J, Rivier J, Rivier C, Vale W. (1981) Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A 78(10): Spiga F, Lightman SL, Shekhar A, Lowry CA. (2006) Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-fos expression in brainstem serotonergic neurons. Neuroscience 138(4): Sterling P, Eyer J. (1988) Allostasis: a new paradigm to explain arousal pathology. In: Handbook of Life Stress, Cognition, and Health (Fisher S, Reason J, eds). New York, NY: J. Wiley & Sons
60 44 Strauss CV, Maisonnette SS, Coimbra NC, Zangrossi H Jr. (2003) Effects of N-methyl-Daspartate-induced amygdala lesion in rats submitted to the elevated T-maze test of anxiety. Physiol Behav 78(1): Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. (1982) Corticotropin releasing factor produces behavioural activation in rats. Nature 297(584): Swanson LW, Sawchenko PE, Rivier J, Vale WW. (1983) Organization of ovine corticotropinreleasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36(3): Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. (2010) The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry 15(9): Tanaka Y, Makino S, Noguchi T, Tamura K, Kaneda T, Hashimoto K. (2003) Effect of stress and adrenalectomy on urocortin II mrna expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology 78(1): Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. (2001) Antagonism of CRF 2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res 902(2): Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. (1998) Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19(2): Treit D, Pesold C, Rotzinger S. (1993) Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci 107(5): Trubiani G, Al Chawaf A, Belsham DD, Barsyte-Lovejoy D, Lovejoy DA. (2007) Teneurin carboxy (C)-terminal associated peptide-1 inhibits alkalosis-associated necrotic neuronal death by stimulating superoxide dismutase and catalase activity in immortalized mouse hypothalamic cells. Brain Res 116: Tucker RP, Chiquet-Ehrismann R, Chevron MP, Martin D, Hall RJ, Rubin BP. (2001) Teneurin- 2 is expressed in tissues that regulate limb and somite pattern formation and is induced in vitro and in situ by FGF8. Dev Dyn 220(1): Ulrich-Lai YM, Xie W, Meij JTA, Dolgas CM, Yu L, Herman JP. (2006) Limbic and HPA axis function in an animal model of chronic pain neuropathic pain. Physiol Behav 88(1-2): Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. (2002) Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropinreleasing factor related peptide. Brain Res 943(1):
61 45 Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. (2003) Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selectie type 2 corticotropin-releasing factor agonist. Brain Res 980(2): Vale W, Spiess J, Rivier C, Rivier J. (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotrophin and β-endorphin. Science 213(4514): Vallès A, Martí O, Amario A. (2006) Long-term effects of a single exposure to immobilization: a c-fos mrna study of the response to the homotypic stress in the rat brain. J Neurobiol 66(6): van Bockstaele EJ, Peoples J, Telegan P. (1999) Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol 412(3): van Dijken HH, de Goeij DC, Sutanto W, Mos J, de Kloet ER, Tilders FJ. (1993) Short inescapable stress produces long-lasting changes in the brain-pituitary-adrenal axis of adult male rats. Neuroendocrinology 58(1): Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. (2000) Distribution of mrnas encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428(2): Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. (1995) Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378(6554): Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. (2004) Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol 16(5): Vertes RP. (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313: Vertes RP. (2002) Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 442(2): Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. (2002) Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet 31(4): Viner R. (1999) Putting stress in life: Hans Selye and the making of stress theory. Social Studies of Science 29(3): Walton ME, Bannerman DM, Rushworth MF. (2002) The role of the rat medial frontal cortex in effort-based decision making. J Neurosci 22(24):
62 46 Wang L, Rotzinger S, Al Chawaf A, Elias CF, Barsyte-Lovejoy D, Qian X, Wang NC, De Cristofaro A, Belsham D, Bittencourt JC, Vaccarino F, Lovejoy DA. (2005) Teneurin proteins possess a carboxy terminal sequence with neuromodulatory activity. Brain Res Mol Brain Res 133(2): Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De Biasi M, Paylor R, Bradley A. (2002) Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol 22(18): Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. (1999) Stress-induced behaviors require the cortiotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A 96(14): Weninger SC, Peters LL, Majzoub JA. (2000) Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology 141(1): Wynn PC, Hauger RL, Holmes MC, Millan MA, Catt KJ, Aguilera G. (1984) Brain and pituitary receptors for corticotropin releasing factor: localization and differential regulation after adrenalectomy. Peptides 5(6): Yeomans JS, Frankland PW. (1995) The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 21(3): Young TR, Leamey CA. (2009) Teneurins: important regulators of neural circuitry. Int J Biochem Cell Biol 41(5): Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. (2007) Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behaviour in rats. J Pharmacol Exp Ther 323(3): Ziegler DR, Cass WA, Herman JP. (1999) Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol 11(5): Ziegler DR, Herman JP. (2002) Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integ Comp Biol 42: Zmijewski MA, Slominski AT. (2010) Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol 57(1): 1-13.
63 47 2 Chapter Two: TCAP-1 and its role in anxiety-like behaviours Parts of this chapter have been published in modified forms in: Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2008) Repeated intracerebral teneurin C-terminal associated peptide (TCAP)-1 injections produce enduring changes in behavioral responses to corticotropin-releasing factor (CRF) in rat models of anxiety. Behav Brain Res 188(1): (Authorization for reproduction obtained from Elsevier) Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009) Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201(1): (Authorization for reproduction obtained from Elsevier) Tan LA, Al Chawaf A, Vaccarino FJ, Boutros PC, Lovejoy DA. (2011) Teneurin C-terminal associated peptide (TCAP)-1 modulates dendritic morphology in hippocampal neurons and decreases anxiety-like behaviors in rats. Physiol Behav In Press. (Authorization for reproduction obtained from Elsevier) 2.1 Abstract Teneurin C-terminal associated peptide (TCAP)-1 mrna has been localized in areas of the brain associated with the stress response, such as the hippocampus, amygdala, and hypothalamus, suggesting that TCAP-1 is ideally positioned to play a role in stress behaviours. Indeed, previous studies showed that injections of TCAP-1 into the basolateral nucleus of the amygdala modulated the acoustic startle response in rats, effects which were long-lasting. Previous in vitro experiments showing TCAP-1 s neuroprotective effects had indicated that TCAP-1 has its most pronounced effects in the presence of a stressor, and that TCAP-1 could have different acute and chronic effects. In the current studies, Wistar rats were injected with i.c.v. TCAP-1 either acutely (1 day) or repeatedly (5 days or 10 days) and then challenged with either corticotropinreleasing factor or restraint. Rats were then tested in the elevated plus maze and open field tests, which are standard rodent exploratory tests of anxiety. In the absence of a stressor, TCAP-1 increased exploratory behaviours and decreased risk assessment, enhancing the effect of mild stress, whereas in the presence of a stressor, TCAP-1 appeared to increase the anxiety state, enhancing the effect of severe stress.
64 Introduction The novel neuropeptide family of the teneurin C-terminal associated peptides (TCAPs) have about 20% amino acid identity with corticotropin-releasing factor (CRF), the principal activating peptide of the hypothalamic-pituitary-adrenal stress axis (Qian et al., 2004). The TCAP family has four members (TCAPs 1-4) that are 40 or 41 amino acids long. Of these peptides, TCAP-1 mrna is expressed in key forebrain and limbic structures in the brain, including the hippocampus, amygdala, hypothalamus, and cortex (Wang et al., 2005). These areas have been implicated in the regulation of stress behaviours, indicating that TCAP-1 expression is wellpositioned to regulate or modify stress-related behaviour. Synthetic TCAP-1 induces a number of bioactive effects, both in vitro and in vivo. In cell culture, TCAP-1 modulates camp accumulation and cell proliferation (Wang et al., 2005), influences neurite outgrowth in mouse immortalized hypothalamic culture (Al Chawaf et al., 2007a), and protects against alkalotic cell death by upregulation of superoxide dismutase (Trubiani et al., 2007). Behaviourally, TCAP-1 elicits significant and long-lasting effects in a test of anxiety known as the acoustic startle test. This is an unconditioned test that does not require learning or motivated behaviour. When injected into the basolateral nucleus of the amygdala, an area of the brain associated with fear, TCAP-1 modulated the response to the acoustic startle. TCAP-1-treated rats that initially had a low baseline startle had a relatively increased startle response; whereas TCAP-1-treated rats that initially had a high baseline startle had a relatively decreased startle response (Wang et al., 2005). Injections of TCAP-1 into the lateral ventricles produced long-lasting decreases in acoustic startle relative to saline-treated animals, an effect that lasted 21 days after the last TCAP-1 injection (Wang et al., 2005). Finally, TCAP-1 administration for 5 days can decrease CRF-induced anxiety-like behaviour in the acoustic startle response 21 days after the final TCAP-1 injection (Tan et al., 2008). Together, these results indicated that TCAP-1 may not have effects under basal conditions, and that conditions that induce stress, such as alkaline conditions in culture or noxious auditory stimuli, may be required to see TCAP-1 s stress-modulating effects. The elevated plus maze (EPM) and the open field test (OF) are two widely-used rodent tests of anxiety. These tests have been used to investigate the role of peptides (for example, CRF, cholecystokinin, urocortin, substance P, arginine vasopressin, and oxytocin) in anxiety behaviour
65 49 (for a review, see Rotzinger et al., 2010). In particular, CRF produces anxiogenic effects in tests such as the acoustic startle, social interaction, and EPM tests (for a review, see Dunn and Berridge, 1990). Repeated restraint in a Plexiglas tube has also been used to induce anxiety-like behaviour in social interaction (Albonetti and Farabollini, 1993; Doremus-Fitzwater et al., 2009), although this treatment induces habituation of the stress response in the EPM (Jaferi and Bhatnagar, 2007; Thorsell et al., 1999) and light-dark box (Cancela et al., 1995), producing no effect or anxiolytic effects. A stressed state may be required to potentiate the effects of TCAP-1 on behaviour, and therefore, injections of CRF or repeated restraint in a Plexiglas tube were used. We have subsequently shown that TCAP-1 may have a role in CRF-induced stress behaviours, as pretreatment with intravenous (i.v.) TCAP-1 for 5 days resulted in modulation of CRF-induced behaviour, depending on the route of CRF administration. In the OF, i.v.-administered TCAP-1 attenuated the effects of i.c.v.-administered CRF, whereas it potentiated the effects of i.v.- administered CRF (Al Chawaf et al., 2007b). The results of the acoustic startle test suggested that TCAP-1 has a role in stress-related behaviours. Few behavioural experiments had been attempted previously; therefore, I investigated the role of both acute and repeated injections of TCAP-1 on stress (via CRF or restraint)-induced behaviours in the EPM and OF. The effects of TCAP-1 on these behavioural experiments are critical to formulate the questions postulated in the rest of the thesis. 2.3 Materials and Methods Animals Male Wistar rats weighing g were obtained from Charles River Laboratories (Montreal, QC). Rats were singly housed in Plexiglas shoebox cages under standard laboratory conditions (12:12 h light:dark cycle, lights on at 0700 h, temperature C) with food and water available ad libitum. Rats were given one week to acclimatize to laboratory conditions before surgery. All procedures were approved by the University of Toronto Animal Care Committee in accordance with the Canadian Council on Animal Care.
66 Surgery Male Wistar rats were anesthetized with isoflurane (3% induction, 2-3% maintenance in 100% O 2 ) and fit into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Analgesics (0.5 mg/kg buprenorphine) were administered subcutaneously at the beginning of surgery. A midline incision was made along the top of the head, exposing bregma. A 22-gauge guide cannula (Plastics One, Roanoke, VA, USA) was implanted into the right lateral ventricle using the following flat-skull coordinates: AP 1.0 and ML 1.4 from bregma, DV 2.7 from dura (Paxinos and Watson, 1998). The guide cannula was secured to the skull using four jewellers screws and dental cement. The opening of the cannula was covered by a removable cannula dummy. The wound was treated with Hibitane (Pfizer Animal Health, Kirkland, QC) to prevent infection. Animals were kept under warm lamps until regaining consciousness and provided wet chow with Nutri-Cal (Vetoquinol USA, Fort Worth, TX) in a clean cage for 12 h. Rats were then transferred to a clean cage and were given one week to recover from surgery Peptides Synthetic mouse TCAP-1 was synthesized at 95% purity using f-moc-based solid phase synthesis (American Peptide Company, Sunnyvale, CA). For the acute TCAP-1 administration, TCAP-1 stocks were prepared to a concentration of M (equivalent to 1 μg/μl TCAP-1) by treating lyophilized TCAP-1 with ammonium hydroxide vapour, then dissolving in sterile saline. For the acute TCAP-1 treatment, the dose was based on the molar equivalent of 3 μg of CRF (630 pmol/rat). For the repeated TCAP-1 treatments, TCAP-1 stocks were prepared to a concentration of 10-4 M, and the dose was 300 pmol/rat. CRF (Sigma Aldrich, Oakville, ON) was dissolved in sterile saline at a concentration of 1 μg/μl Injection procedure Injections were given between 0800 and 1200 h. Lateral ventricle (i.c.v) injections were delivered at a rate of 2 µl/min by a Hamilton syringe with a pump (Razel Scientific Instrument Inc., Stamford, CT) connected via PE-50 tubing to a 28-gauge stainless steel injector that extended 1 mm below the cannula guide tip. After injection, the injector was left in place for 60 s following infusion to prevent backflow up the cannula.
67 Cannula placement verification Cannula placement was confirmed upon sacrifice of the animal. The acute administration and 5- day treatment rats were sacrificed under CO 2 gas. Rats were then decapitated and their brains were i.c.v. injected with 1 µl of 1% methylene blue before their brains were retrieved. The brains were then obtained and fixed in 4% formaldehyde. 24 h later, brains were sliced coronally with a razor blade to expose the lateral ventricles. Rats were considered to have a proper cannula placement if methylene blue was found in the lateral ventricles. Rats with improper cannula placement were removed from the sample. The 10-day treatment rats were deeply anesthetized under 3% isoflurane and quickly decapitated. The brain was obtained and placed in Golgi impregnation solution (see Chapter 4). Brain sections from the Golgi stain procedure were viewed under a microscope and cannula placement was verified by identifying a transection of the cortex and corpus callosum by the cannula tract into the right lateral ventricle. Rats with improper cannula placement were removed from the sample Elevated Plus Maze (EPM) The elevated plus maze test (EPM) was conducted using a standard plus maze apparatus elevated 65 cm above the floor (Figure 2.1A). It consisted of two arms (50 cm x 10 cm) enclosed by black Plexiglas walls 40 cm high (i.e. the closed arms ), and two arms (50 cm x 10 cm) which were open and not enclosed (i.e. the open arms ). Each pair of arms was separated by 180 and connected by a centre platform (10 cm x 10 cm). The entire apparatus was painted with matte black paint and the maze was illuminated by two dim red lights (25 W bulbs) suspended above the two open arms. The floor around the maze was covered in dull black material to avoid shine that would cause background noise for the tracking software.
68 52 Figure 2.1: Schematics of the elevated plus maze (EPM, Fig. 2.1A) and open field (OF, Fig. 2.1B) tests. In the EPM, the maze is arranged in a cross with two closed arms with unscalable walls (thick lines) and two open arms (thin lines) that are elevated 65 cm from the ground. Rats are individually introduced to the middle of the maze facing an open arm and are allowed to freely explore for 5 min. Rats will generally avoid the open arms of the maze, and anxiolytic treatments will increase both open arm time and entries. In the OF, rats are allowed to freely explore in a novel box for 10 or 60 min. Rats will generally avoid the centre of the maze and remain in the perimeter. Anxiolytic treatments will increase the amount of time and entries in the centre. For each test, rats were placed on the centre platform facing an open arm. Rats were allowed to freely explore the maze for 5 min and movements were recorded from above using a digital camera suspended above the centre of the maze. Rat movements were tracked, quantified, and compiled using an Ethovision video tracking system (Noldus Information Technology). Only data from rats that completed the full 5 min without falling off the maze were retained for the results. Supplementary behaviours, such as rearing (the rat assumes a vertical posture standing on its back two legs), head-dipping (the rat places four feet in the open arm and directs its head below the open arm looking at the floor below), or stretched-attend (the rat s two hind legs remain stationary and the rat makes a forward locomotory motion with its front legs, stretching its body, and retreats back to a more compact position with its hind legs never changing position) were manually counted by an observer blind to the study treatments. The maze was cleaned with mild soap and ethanol between tests.
69 Open Field Test (OF) The open field test (OF) apparatus consisted of a 50 cm x 50 cm arena with 40 cm high walls made of black particle board (Figure 2.1B). A 30 cm x 30 cm square in the centre of the open field was defined as the centre zone for data analysis. The apparatus was illuminated by either dim red lights [25 W bulbs] or white lights [100 W bulbs]). Rat movements were tracked, quantified, and compiled using Ethovision. For each test, rats were placed in the centre of the arena. Rats were allowed to freely explore the maze for 10 min or 60 min. Supplementary behaviours (rearing) were manually counted by an observer blind to the study treatments. The maze was cleaned with mild soap and ethanol between tests Ethovision parameters To maintain the same parameters for the EPM test, an initial background picture was taken of the maze before each round of tests to calibrate the maze. A small white cardboard cross (four 2.5 cm arms extending from a 10 cm x 10 cm centre) was placed in the middle of the maze to ensure that when the rat had extended all four paws across the threshold of either the open or closed arm, the Ethovision program would construe this as a true arm entry. The arena was determined by outlining the background picture with extra room allowed for the open arms, so that the rat signal would not be lost if the rat head-dipped over the open arm. To calibrate the distance moved, a line was drawn from tip to tip of the white cardboard cross (i.e. 15 cm). For the OF, a 30 cm x 30 cm square was introduced to the initial background before each round of tests to calibrate the maze and ensure the same centre area could be acquired regardless of changes to the camera position. To calibrate the distance moved in the OF arena, a line was drawn diagonally from one corner of the maze to the other (i.e cm). Both cardboard backgrounds were removed prior to testing in the maze, so that the entire arena had a uniform black surface. During quantification of both the EPM and the OF, rat detection (white on black) was set to gray scaling, and the image was filtered, first with erosion (2 pixels) and then dilation (2 pixels) to eliminate background noise. 2 cm of minimum movement was required to be scored as genuine locomotion.
70 Experiment 1: Acute effects of TCAP-1 treatment A timeline schematic of Experiment 1 can be found in Figure 2.2. Male Wistar rats (n = 158) were cannulated into the right lateral ventricle. After surgery, rats were handled for 5 min per day for five consecutive days and introduced to mock injections. On test day, rats were first given an i.c.v. injection of either saline or 630 pmol (in 3 µl) of TCAP-1. After waiting 60 s to prevent backflow up the cannula, rats were given a second i.c.v. injection of saline (0 µg CRF), 1 µg CRF, or 3 µg CRF. Rats were returned to their home cages in the colony for 2 h. Rats were then tested on the EPM, and returned to the colony upon completion of the test. Figure 2.2: Schematic of Acute TCAP-1 experiment. On Day 1, rats were given an i.c.v. injection of 630 pmol (3 µg) of TCAP-1, followed directly by an i.c.v. challenge of 0, 1 or 3 µg CRF and were returned to the colony. Two hours later, rats were tested on the EPM. On Day 4, the same rats were again treated with i.c.v. TCAP-1 (630 pmol, 3 µg), followed directly by a challenge of 0, 1, or 3 µg CRF (i.c.v.) and returned to the colony. Two hours later, rats were tested on the OF.
71 55 Three days after the EPM test, rats were again injected with either saline or 630 pmol of TCAP- 1, followed by either saline, 1 µg CRF or 3 µg CRF. Rats were returned to the colony for 2 h. Rats were then tested in the OF and returned to the colony upon completion of the test. There were 6 treatment groups; 3 of these were controls: saline injection with saline injection ( SAL+CRF (0 µg) ), saline injection with 1 µg CRF injection ( SAL+CRF (1 µg) ), and saline injection with 3 µg CRF injection ( SAL+CRF (3 µg) ); 3 of these were experimental groups: TCAP-1 injection with saline injection ( TCAP+CRF (0 µg) ), TCAP-1 injection with 1 µg CRF ( TCAP+CRF (1 µg) ), and TCAP-1 injection with 3 µg ( TCAP+CRF (3 µg) ) Experiment 2: Effects of 5-day repeated TCAP-1 pre-treatment A timeline schematic of Experiment 2 can be found in Figure 2.3. Male Wistar rats (n = 79) were cannulated into the right lateral ventricle. After surgery, rats were allowed to recover for 1 week. Rats were then i.c.v.-injected for 5 consecutive days (Days 1-5) with either saline or 300 pmol (in 3 µl) of TCAP-1. Rats were then allowed to rest for 1 week before behavioural tests. On Day 12, rats were injected with an acute challenge of saline (0 µg of CRF), 1 µg, or 3 µg of CRF (i.c.v.). Rats were then returned to the colony for 30 min. The rats were then individually tested in the EPM and returned to the colony. On Day 15, three days after the EPM, rats were again administered i.c.v. saline (0 µg of CRF), 1 µg, or 3 µg of CRF. Rats were returned to the colony for 30 min. The rats were then tested in the OF and returned to the colony. There were 6 treatment groups; 3 of these were controls: saline pre-treatment with acute saline injection ( SAL+CRF (0 µg) ), saline pre-treatment with acute 1 µg CRF injection ( SAL+CRF (1 µg) ), and saline pre-treatment with acute 3 µg CRF injection ( SAL+CRF (3 µg) ); 3 of these were experimental groups: TCAP-1 pre-treatment with acute saline injection ( TCAP+CRF (0 µg) ), TCAP-1 pre-treatment with acute 1 µg CRF injection ( TCAP+CRF (1 µg) ), and TCAP-1 pre-treatment with acute 3 µg CRF injection ( TCAP+CRF (3 µg) ).
72 56 Figure 2.3: Schematic of 5-Day Pre-Treatment Administration experiment. Rats were treated daily with i.c.v. TCAP-1 (300 pmol) on Days 1-5. On Day 12, rats were injected (i.c.v.) with a challenge of 0, 1, or 3 µg CRF. Rats were returned to the colony for 30 min and then tested on the EPM. On Day 15, rats were injected (i.c.v.) with a challenge of 0, 1, or 3 µg CRF. Rats were returned to the colony for 30 min and then tested in the OF Experiment 3: Effects of 10-day repeated TCAP-1 treatment A timeline schematic of Experiment 3 can be found in Figure 2.4. Male Wistar rats (n = 30) were cannulated into the right lateral ventricle. After surgery, rats were allowed to recover for 1 week. Rats were then injected (i.c.v.) with either saline or 300 pmol TCAP-1 for 10 consecutive days. In the afternoon of each of these 10 consecutive treatment days ( h) rats were either handled lightly and allowed to run through the restraint tube (for rats to experience the novelty of the tube) as a non-stressed control, or were subjected to 2 h per day of restraint stress in a clear Plexiglas tube (length = 15.8 cm, interior diameter = 7 cm) in their home cages. 24 h after the last injection/restraint day, rats were tested on the EPM. Rats were then sacrificed and the brains were subjected to a Golgi impregnation study (see Chapter 4). There were 4 treatment groups: saline treatment with no restraint ( SAL ), TCAP-1 treatment with no restraint ( TCAP ), saline treatment with restraint ( SAL+Restraint ), and TCAP-1 treatment with restraint ( TCAP+Restraint ).
73 57 Figure 2.4: Schematic of 10-Day Repeated Administration experiment. Rats were treated each morning with i.c.v. TCAP-1 (300 pmol) and subjected to restraint stress in a Plexiglas tube for 2 h in the afternoon for 10 consecutive days. Rats were tested on the EPM on Day Data analysis Behaviour in the EPM and OF was quantified using Ethovision software. Raw data was transferred to Microsoft Excel for sorting. Statistical analysis of the data was carried out with GraphPad Prism 4.0. For the EPM, percent open arm time was calculated as [open arm time/(open arm time + closed arm time) x 100], and percent open arm entries was calculated as [open arm entries/(open arm entries + closed arm entries) x 100]. Data was converted to a percent of control of the corresponding saline group at each dose of CRF or restraint. Values were analyzed by a one-sample t-test against a hypothetical mean of 100%. 2.4 Results Experiment 1: Acute effects of TCAP-1 treatment As expected, i.c.v. injections of either 1 µg or 3 µg CRF produced significant dose-dependent increases in anxiety-like behaviour in the EPM and OF tests (Table 2.1) as measured by one-way ANOVA. CRF significantly decreased percent open arm entries (F 2,63 = 3.507, p = 0.036), percent open arm time (F 2,63 = 4.474, p = ), rearing (F 2,63 = 7.43, p = ), and headdipping (F 2,63 = 5.239, p = ) in the EPM. CRF also significantly decreased centre entries (F 2,75 = 8.28, p = ), centre time (F 2,75 = 8.928, p = ), centre distance travelled (F 2,75 = 9.689, p = ), total distance travelled (F 2,75 = 5.595, p = ), and rearing (F 2,75 =
74 , p < ) in the OF. These data indicate that CRF could serve as suitable positive controls for TCAP-1-treated groups. Table 2.1: Experiment 1: Acute injections of CRF are anxiogenic on the EPM and OF SAL+CRF (0 µg) SAL+CRF (1 µg) SAL+CRF (3 µg) EPM % Open Arm Entries (%) * % Open Arm Time (%) * Rearing ** Head-Dipping ** Stretched-Attend OF Centre Entries *** Centre Time (s) *** Centre Distance (cm) *** Total Distance (cm) ** Rearing *** (*p < 0.05, **p < 0.01, ***p < 0.001, One-way ANOVA). Figure 2.5: Acute TCAP-1 on CRF-induced behaviour in the EPM. Percent of control data for (A) percent open arm entries and (B) percent open arm time. TCAP-1-treated groups are presented as a percentage of saline-treated controls injected with a challenge of 0, 1, or 3 µg CRF. Acute TCAP-1 had no effect on open arm measures in the EPM at any CRF dose. Bars represent mean percent of control + SEM.
75 59 Figure 2.6: Acute TCAP-1 on CRF-induced behaviour in the OF. Percent of control for (A) centre entries, (B) centre time, (C) centre distance travelled, and (D) total distance travelled. Acute TCAP-1 had no effect on centre measures, although TCAP-1 in the absence of a stressor (TCAP+CRF (0 µg)) increased total distance travelled in the OF. Bars represent mean percent of control + SEM (***p < 0.001). In the EPM, data from the SAL+CRF (0 µg), SAL+CRF (1 µg), SAL+CRF (3 µg) control groups (n = 29, 18, 19, respectively) and from the TCAP+CRF (0 µg), TCAP+CRF (1 µg), TCAP+CRF (3 µg) experimental groups (n = 31, 22, 21, respectively) were obtained. TCAP-1 group data was converted to a percent of control compared to the mean of the corresponding SAL+CRF group at each dose of CRF (0, 1, or 3 µg). Several measures were investigated: percent open arm entries, percent arm time, stretched-attend postures, head-dipping, and rearing. In percent open arm entries (Figure 2.5A), TCAP-1 at any concentration of CRF (0, 1, or 3 µg) had no effect relative to controls treated with CRF (0, 1, or 3 µg) (t = 1.365, p = 0.182; t = 1.212,
76 60 p = 0.239; t = , p = 0.932, respectively). Similarly, in percent open arm time (Figure 2.5B), TCAP-1 with CRF treatment (0, 1, or 3 µg) was not significantly different from its respective control (t = 1.136, p = 0.265; t = 0.082, p = 0.935; t = 0.246, p = 0.808, respectively). In exploratory behaviours, such as rearing, TCAP-1 had no effect relative to controls at 1 or 3 µg treatments of CRF (Table 2.2; t = 0.741, p = and t = 0.958, p = 0.35), however, TCAP-1 had a significant effect of increasing rearing behaviours by 11.8% relative to control in the absence of a stressor (i.e. TCAP+CRF (0 µg)) (t = 2.436, p = 0.021). TCAP-1 had no effect on stretched-attend postures (Table 2.2; TCAP+CRF (0 µg): t = 1.922, p = ; TCAP+CRF (1 µg): t = 1.239, p = 0.229; TCAP+CRF (3 µg): t = 0.398, p = 0.695) or head-dipping behaviours (Table 2.2; TCAP+CRF (0 µg): t = 0.226, p = 0.823; TCAP+CRF (1 µg): t = 0.820, p = 0.422; TCAP+CRF (3 µg): t = 0.530, p = 0.602). Table 2.2: Experiment 1: Mean percent of control for exploratory behaviours in the EPM and OF (*p < 0.05) TCAP+CRF (0 µg) TCAP+CRF (1 µg) TCAP+CRF (3 µg) EPM Rearing 111.8%* % % Head-Dipping 97.60% 86.30% 89.60% Stretched-Attend % % % OF Rearing % % %
77 61 In the OF, data from the SAL+CRF (0 µg), SAL+CRF (1 µg), SAL+CRF (3 µg) control groups (n = 36, 22, 20, respectively) and from the TCAP+CRF (0 µg), TCAP+CRF (1 µg), TCAP+CRF (3 µg) experimental groups (n = 34, 23, 23, respectively) were obtained. TCAP-1 group data was converted to a percent of control compared to the mean of the corresponding SAL+CRF group at each dose of CRF (0, 1, or 3 µg). Several measures were investigated: centre entries, centre time, centre distance, total distance, and rearing. TCAP-1 did not affect centre entries at any concentration of CRF (0, 1, or 3 µg) relative to controls (Figure 2.6A; t = 1.246, p = 0.222; t = 0.354, p = 0.727; t = 1.474, p = 0.155, respectively). TCAP-1 also did not affect centre time (Figure 2.6B; TCAP+CRF (0 µg): t = 0.806, p = 0.426; TCAP+CRF (1 µg): t = , p = 0.941; TCAP+CRF (3 µg): t = 1.635, p = 0.116) or centre distance (Figure 2.6C; TCAP+CRF (0 µg): t = 1.091, p = 0.283; TCAP+CRF (1 µg): t = 1.048, p = 0.306; TCAP+CRF (3 µg): t = 1.738, p = ). However, TCAP-1 produced a significant increase in total distance travelled by 25.3% relative to control in the absence of CRF (Figure 2.6D; TCAP+CRF (0 µg): t = 4.004, p = ), although it had no effect with 1 µg CRF (t = 1.419, p = 0.170) or 3 µg CRF (t = 0.558, p = 0.582). Rearing, a vertical exploratory behaviour, was also measured, although TCAP-1 had no effect on these behaviours relative to controls (Table 2.2; TCAP+CRF (0 µg): t = 1.941, p = ; TCAP+CRF (1 µg): t = 0.151, p = 0.882; TCAP+CRF (3 µg): t = 0.149, p = 0.883) Experiment 2: Effects of 5-day repeated TCAP-1 pre-treatment Like Experiment 1, CRF was used as a stressor to distinguish TCAP-1 s effects on anxiety as determined by one-way ANOVA (Table 2.3). CRF significantly reduced percent open arm time in the EPM (F 2,27 = 4.321, p = ) whereas CRF tended to reduce percent open arm entries, although this trend did not reach significance (F 2,27 = 2.367, p = 0.113). In the OF, CRF had non-significant effects on centre entries, time, distance travelled, and total distance travelled (p > 0.05).
78 62 Table 2.3: Experiment 2: Acute injections of CRF in the EPM and OF. SAL+CRF (0 µg) SAL+CRF (1 µg) SAL+CRF (3 µg) EPM % Open Arm Entries (%) % Open Arm Time (%) * Rearing a Head-Dipping a Stretched-Attend a OF Centre Entries Centre Time (s) Centre Distance (cm) Total Distance (cm) a Exploratory behaviours could only be obtained from a portion of the group; SAL+CRF (0 µg): n = 5, SAL+CRF (1 µg): n = 6, SAL+CRF(3 µg): n = 7, (*p < 0.05). Figure 2.7: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the EPM. Percent of control data for (A) percent open arm entries and (B) percent open arm time. Rats were pre-treated with 5 days of TCAP-1 and then given an i.c.v. challenge of 0, 1, or 3 µg CRF on test day. TCAP-1-treated groups are presented as a percentage of their respective saline-pretreated controls. Percent open arm entries and percent open arm time were significantly reduced in the TCAP+CRF (1 µg) group relative to the SAL+CRF (1 µg) group, indicating that TCAP-1 potentiated the effects of 1 µg CRF. Bars represent mean percent of control + SEM (*p < 0.05).
79 63 EPM data from the SAL+CRF (0 µg), SAL+CRF (1 µg), SAL+CRF (3 µg) groups (n = 10, 13, 7, respectively) and TCAP+CRF (0 µg), TCAP+CRF (1 µg), TCAP+CRF (3 µg) groups (n = 11, 8, 8, respectively) were obtained. TCAP-1 group data was converted to a percent of control for the corresponding saline group at each dose of CRF (0, 1, 3 µg). Percent open arm entries, percent open arm time, stretched-attend postures, head-dipping, and rearing were measured. TCAP-1 had no effect on percent open arm entries without CRF (Figure 2.7A; TCAP+CRF (0 µg): t = 1.006; p = 0.338) or with 3 µg CRF (TCAP+CRF (3 µg): t = 0.218, p = 0.834), however, TCAP-1 pre-treatment with a 1 µg CRF challenge had a significant effect reducing percent open arm entries by 39.4% relative to its saline-treated treated control (t = 4.986, p = ). Similarly, in percent open arm time, TCAP-1 pre-treatment had no effect without CRF or with a 3 µg injection of CRF on test day (Figure 2.7B; TCAP+CRF (0 µg): t = 1.059, p = 0.315; TCAP+CRF (3 µg): t = 0.674, p = 0.522), although TCAP-1 reduced percent open arm time by 37.6% under a 1 µg CRF challenge on test day (t = 2.718, p = ). In exploratory behaviours, TCAP-1 pre-treatment decreased rearing by 51.4% relative to its control when challenged with 3 µg CRF on test day (Table 2.4; t = 3.384, p = ) but had no effect at 0 µg or 1 µg doses of CRF (TCAP+CRF (0 µg): t = 0.916, p = ; TCAP+CRF (1 µg): t = 0.618, p = 0.599). TCAP-1 pre-treatment increased head-dipping by 63.0% in the absence of CRF relative to control (t = 4.493, p = ) but decreased head-dipping by 37.9% relative to controls when challenged with 1 µg CRF (Table 2.4; t = 6.351, p = ), but this effect was not seen in the TCAP-1 pre-treatment group that was challenged with 3 µg CRF (t = 0.938, p = 0.380). Finally, TCAP-1 pre-treatment had no effect on stretched-attend behaviours (Table 2.4) when challenged with either no CRF (t = 1.079, p = 0.330) or with 3 µg CRF (t = 1.210, p = 0.265), although TCAP-1 with 1 µg virtually eliminated stretched-attend postures in this experiment, relative to control.
80 64 Figure 2.8: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the OF. Percent of control data for (A) centre entries, (B) centre time, (C) centre distance travelled, and (D) total distance travelled. Rats treated with TCAP-1 and challenged with CRF (1, 3 µg) had significantly lower centre entries, time, distance, and total distance than saline pre-treated controls. Bars represent mean percent of control + SEM (*p < 0.05, **p < 0.01).
81 65 Table 2.4: Experiment 2: Mean percent of control for exploratory behaviours in the EPM and OF. TCAP+CRF (0 µg) TCAP+CRF (1 µg) TCAP+CRF (3 µg) EPM Rearing 118.6% 79.5% 48.6%* Head-Dipping 163.0%** 62.1%* 72.9% Stretched-Attend 55.6% 0.0% 145.8% OF Rearing 108.2% 94.6% 44.5%** Exploratory behaviours could only be obtained from a portion of the EPM group; SAL+CRF (0 µg): n = 5, SAL+CRF (1 µg): n = 6, SAL+CRF(3 µg): n = 7; TCAP+CRF (0 µg): n = 6, TCAP+CRF (1 µg): n = 3, TCAP+CRF(3 µg): n = 8, (*p < 0.05, **p < 0.01). OF data from the SAL+CRF (0 µg), SAL+CRF (1 µg), SAL+CRF (3 µg) groups (n = 7, 6, 7, respectively) and the TCAP+CRF (0 µg), TCAP+CRF (1 µg), TCAP+CRF (3 µg) groups (n = 7, 7, 6, respectively) were obtained. Data from the first 10 min of the 60 min trial were analyzed, in order to facilitate comparisons with Experiment 1. TCAP-1 group data was converted to a percent of control for the corresponding saline group at each dose of CRF (0, 1, 3 µg), and the following measures were analyzed: centre entries, centre time, centre distance travelled, and total distance travelled. TCAP-1 had no effect on centre entries relative to saline-treated controls for CRF doses of 0 or 1 µg (Figure 2.8A; t = , p = ; t = 1.550, p = 0.172, respectively), although pre-treatment of TCAP-1 with an acute challenge of 3 µg CRF reduced centre entries by 37.6% relative to its saline control (t = 3.419, p = ). TCAP-1 had no effect on centre time under unchallenged conditions or 3 µg CRF (Figure 2.8B; t = 0.656, p = 0.537; t = 2.036, p = , respectively) although TCAP-1 as a pre-treatment had significant effects in rats challenged with 1 µg CRF (t = 2.853, p = ), decreasing the time spent in the centre of the OF by 43.3%. Similarly, in centre distance travelled, TCAP-1 had no effects in the absence of a challenge (Figure 2.8C; t = 1.300, p = 0.241), although it significantly reduced centre distance travelled in rats pre-treated with TCAP-1 and acutely challenged with 1 µg CRF (t = 2.550, p = ) and 3 µg CRF (t = 2.737, p = ) by 35.3% and 43.0%, respectively. TCAP-1 had no effects on total distance travelled at the 0 µg and 1 µg dose of CRF (Figure 2.8D; t = 0.586, p = 0.580; t = 0.345, p = 0.742, respectively) but TCAP-1 treatment reduced total distance in the 3 µg CRF group by 25.2% relative to its control (t = 4.637, p = ). Finally, TCAP-1 had no
82 66 effect on rearing on its own in the first 10 min of the trial, although it significantly reduced rearing in rats pre-treated with TCAP-1 and challenged with 3 µg of CRF (Table 2.4; t = 4.506, p = ). Table 2.5: Experiment 2: Repeated TCAP-1 pre-treatment on CRF-induced behaviour in the OF for the full 60 minute trial as a mean percent of control TCAP+CRF (0 µg) TCAP+CRF (1 µg) TCAP+CRF (3 µg) Centre Entries % % %** Centre Time (s) % %* %* Centre Distance (cm) % %* %* Total Distance (cm) % % % (*p < 0.05, **p < 0.01) OF data were also analyzed for the full 60 min trial, producing results that were similar to the 10 min test (Table 2.5). TCAP-1 pre-treatment with an acute 3 µg CRF dose produced the most consistent effects, reducing centre entries by 49.5%, centre time by 51.0%, and distance travelled by 44.8%, and relative to controls (p = , p = , p = , respectively). TCAP-1 pre-treatment with an acute dose of 1 µg CRF decreased centre time by 52.4% and decreased centre distance by 38.9% relative to saline-treated controls (p = , p = , respectively) Experiment 3: Effects of 10-day repeated TCAP-1 treatment In contrast to Experiment 1 and 2, restraint was used as a long-term stressor in this experiment (Table 2.6). Restraint produced a tendency to increase percent open arm entries and time, but this was not significant using a one-way ANOVA (p > 0.05). Table 2.6: Experiment 3: Restraint as a stressor in the EPM. Saline Restraint % Open Arm Entries (%) % Open Arm Time (%) Rearing Head-Dipping Stretched-Attend
83 67 Figure 2.9: Repeated TCAP-1 on restraint-induced behaviour in the EPM. Percent of control data for (A) percent open arm entries and (B) percent open arm time. The TCAP-1 treatment group had a significantly higher mean percent open arm time than its saline-treated group, whereas the TCAP-1 treatment group subjected to daily restraint had a significantly lower mean percent open arm time relative to its saline-treated group. Bars represent mean percent of control + SEM (*p < 0.05). For the EPM, data were obtained from SAL (n = 4), TCAP (n = 5), SAL+Restraint (n = 5), and TCAP+Restraint (n = 4) groups. TCAP-1 group data were converted to a percent of control compared to the mean of the corresponding saline-treated group without or with restraint. Several measures were investigated: percent open arm entries, percent arm time, stretched-attend postures, head-dipping, and rearing. In percent open arm entries (Figure 2.9A), TCAP-1 with restraint had no significant effect relative to its saline-treated controls (t = 1.971, p = 0.143), and TCAP-1 in the absence of restraint may have increased percent open arm entries, but this effect was just below statistical significance (t = 2.624, p = ). TCAP-1 had effects on the percent open arm time in the absence of restraint, increasing the percent open arm time by 24.9% relative to its control (Figure 2.9B; t = 4.191, p = ), and decreasing the percent open arm time in restraint groups by 11.5% relative to its control (t = 3.373, p = ). TCAP-1 had no effects on rearing, without or with restraint (Table 2.7; t = 0.923, p = 0.409; t = 0.951, p = 0.412, respectively), nor did TCAP-1 have effects on head-dipping (Table 2.7; TCAP: t = 1.432, p = 0.225; TCAP+Restraint: t = 1.725, p = 0.183). However, TCAP-1 did have effects on stretchedattend postures, a type of risk-assessment behaviour. TCAP-1 treatment, in the absence of restraint, decreased stretched-attend postures by 25.7% relative to control (Table 2.7; t = 3.087, p
84 68 = ), but this effect was not seen in the TCAP+Restraint group relative to its restraint control (t = 1.984, p = 0.142). There were no differences in any of the groups in total distance travelled in the maze (data not shown). Table 2.7: Experiment 3: Mean percent of control for exploratory behaviours in the EPM (*p < 0.05) TCAP+No Restraint TCAP+Restraint Rearing 75.30% % Head-Dipping 80.00% 66.86% Stretched-Attend 74.29%* 77.40% 2.5 Discussion The experiments described in this chapter were designed to explore the effects of acute or repeated i.c.v. TCAP-1 on stress-related behaviours in two unconditioned tests of anxiety, the EPM and OF tests. TCAP-1 administered acutely did not have robust effects on behaviour with or without CRF co-treatment. In contrast, 5 days of TCAP-1 pre-treatment significantly potentiated CRF-induced behaviour in both the EPM and OF tests, but did not have robust effects in the absence of a stressor. Finally, 10 days of TCAP-1 treatment affected behaviours in the EPM both in the presence and absence of a restraint stressor. These sometimes contrasting results indicate that TCAP-1 may be acting as a behavioural modulator, which is supported by previous work (Wang et al., 2005), which showed that acute injections of TCAP-1 into the basolateral nucleus of the amygdala of rats had differential effects on the acoustic startle response depending upon the rat s baseline reactivity. Thus, these studies confirmed that TCAP- 1 administration is capable of modulating stress-associated behaviour and may act in part by regulating the CRF system. The EPM test has been used extensively as a test of anxiety (Carobrez and Bertoglio, 2005; Pellow et al., 1985; Pellow and File, 1986; Rodgers and Dalvi, 1997). The maze has been validated using known anxiolytics that have efficacy in humans, such as chlordiazepoxide and diazepam (Pellow et al., 1985), which increase the percentage of entries into, and time spent on the open arms of the maze. Drugs that increase anxiety in humans, such as amphetamine or caffeine, reduce these parameters (Pellow et al., 1985). However, the EPM may not predict
85 69 anxiety-modulating measures in all substances, as it does not predict the anxiolytic-like properties of antidepressants, such as selective serotonin reuptake inhibitors, which are used in the treatment of some anxiety disorders (Borsini et al., 2002). Exposure to the open arms of the maze increases serum corticosterone levels (Pellow et al., 1985), as the aversion to open spaces, but not the height or novelty of the maze, produces a stressful environment (Treit et al., 1993). Supplementary exploratory behaviours in the EPM, such as rearing, head-dipping, and stretchedattend postures, have been used as additional measures that have ethological validity but may be more sensitive to anxiety-modulating drugs than traditional open arm measures (Carobrez and Bertoglio, 2005). Rearing is a type of vertical exploratory activity (Ohl, 2003), head-dipping is classified as directed exploration beneath the maze, and the stretched-attend posture is a defensive posture and risk assessment behaviour (Weiss et al., 1998). In particular, risk assessment behaviours may be separate from anxiety, and may persist even in animals that readily venture into unprotected spaces (Ohl, 2003). Manipulations that increase anxiety tend to decrease rearing (Rodgers and Dalvi, 1997) and increase head-dipping and stretched-attend postures (Kaesermann, 1986; Mikics et al., 2005; Rodgers and Cole, 1993). The OF test is similar to the EPM test in that it measures the time, entries, and distance travelled in the open space of a novel arena (i.e. the centre), away from tactile stimuli such as inescapable walls (Lister, 1990). Rats will generally avoid the centre portion of the chamber, and anxiolytic manipulations will increase the time spent in the centre of the arena (Crawley, 1985; Prut and Belzung, 2003). Rearing, a type of exploratory behaviour, was also measured; anxiety-inducing treatments, such as i.c.v. injection of CRF, decreases rearing behaviour (Britton et al., 1984; Sherman and Kalin 1986; Sutton et al., 1982). Although not as widely used as the EPM, the OF can be used to elucidate effects on locomotion to ensure that anxiolytic effects seen in either test are not confounded simply by an alteration in locomotion. Two different types of stimuli, acute CRF and repeated restraint, were used in these studies to induce stress-like conditions. Acute CRF produced anxiogenic effects in Experiments 1 and 2, similar to previous studies (Adamec et al., 1991; Adamec and McKay, 1993; File et al., 1988; Sutton et al., 1982). In contrast, repeated restraint produced a tendency, although not significant, to increase percent open arm entries and time. It may seem paradoxical that repeated stressors may decrease anxiety; however, as displayed in Experiment 3, organisms can habituate to the same (i.e. homotypic) repeated stressor, as repeated restraint had little, if any, effect on the EPM.
86 70 For example, 7 days of restraint (2 h per day) produced anxiolytic effects in the light-dark box (Cancela et al., 1995) although a single restraint session produced anxiogenic effects. Similarly, 9-10 days of restraint (1 h per day) had no effect on EPM behaviour although a single restraint session increased anxiety in the EPM test (Thorsell et al., 1999), and 8 days of restraint (30 min per day) reduced anxiety in the EPM compared to acute restraint (Jaferi and Bhatnagar, 2007). Thus, a small amount of predictable stressors may be beneficial to the organism. Habituation of the stress response occurs at an endocrine level, as a single restraint session significantly increases corticosterone and ACTH levels, but multiple restraint exposures decrease corticosterone and ACTH (Culman et al., 1991; Dhabhar et al., 1997; File, 1982). However, although the effects of repeated restraint on endocrine and behavioural measures may appear attenuated, multiple exposures to restraint still have lasting effects in the brain, as evidenced by atrophy of the dendrites of hippocampal cells and hypertrophy of the dendrites in amygdala cells (see Chapter 4). Acutely, TCAP-1 did not have any robust behavioural effects with or without a co-injection of CRF. TCAP-1 increased rearing exploratory behaviours in the EPM and increased total distance travelled in the OF in the absence of CRF relative to controls. Exploratory behaviours may be used as another stress-like behavioural dimension but does not replace the traditional open arm measures; therefore, although rearing behaviours are decreased under stressful conditions, we cannot conclude that this treatment of TCAP-1 decreased anxiety per se. Similarly, an increase in distance moved in TCAP-1-treated populations, although not necessarily an indication of anxiety, may indicate an increase in exploratory behaviour in a novel environment. This is in contrast to the effects of CRF in this experiment, which decreased total locomotion. The doses of CRF used in this experiment are relatively high (1 µg CRF is equivalent to 0.21 nmol, whereas 3 µg CRF is equivalent to 0.63 nmol), and high doses (0.15 nmol) of i.c.v. CRF decrease locomotion and rearing in a novel open field (Sutton et al., 1982). However, the effects of CRF exhibit an inverse-u shaped curve: low doses ( nmol) increase locomotion and rearing, and evoke a behaviourally activating or hypervigilance response similar to behaviour elicited after non-traumatic mild stress such as white noise or bright light (Dunn and Berridge, 1990; Roth and Katz, 1979). TCAP-1, therefore, may be inducing an acutely behaviourally active state which may manifest itself by an increase of activity and exploratory
87 71 behaviour in order to actively obtain information about a potential threat (Ohl, 2003), but this behaviour is eliminated in the presence of more stressful stimuli. The results of acute injection of TCAP-1 were not as pronounced as expected, given that a sisterstudy that investigated c-fos protein induction (see Chapter 3) provided intriguing and robust results. Previously, acute injections of TCAP-1 into the amygdala had modulatory effects in the absence of an additional stressor in the acoustic startle test (Wang et al., 2005). However, it is possible that the time course used in this study, which is identical to the time course used in the c-fos induction experiment, was not appropriate to see behavioural modifications to CRFinduced exploratory behaviour. The anxiogenic effects of CRF are still present 2 h after i.c.v. administration (Spina et al., 2002), but other peptides may have shorter windows with which to see effects. For example, low doses of neuropeptide Y (NPY) in mice have anxiogenic effects on the EPM 10 min after injection but these effects are not detected 30 min post-injection (Nakajima et al., 1998), which may be a result of a biphasic disappearance curve with relatively short half-lives (4.1 and 20 min) (Pernow et al., 1987). Similarly, TCAP-1 has a relatively short half-life of roughly min in blood when given intravenously (Song, Barsyte, and Lovejoy, manuscript in preparation), suggesting that although 2 h was sufficient to see the effects of TCAP-1 on CRF-induced c-fos induction, the behavioural effects of reduced c-fos in specific areas of the brain may have already subsided prior to behavioural testing. Repeated injections of TCAP-1 had differential effects, depending on the treatment regimen. The first group of experiments consisted of 5 days of daily TCAP-1 injections (Experiment 2) followed by a 1-week rest period before CRF challenge and behavioural testing, which I have referred to as pre-treatment. This treatment paradigm was used previously (Wang et al., 2005) to demonstrate long-term effects of TCAP-1 in the acoustic startle test. This regimen is similar to the sensitization protocol seen in stress literature demonstrating that multiple exposures to a single type of stressor, for example, CRF, swim stress, or drugs of abuse, potentiates the effects of a challenge, such as amphetamine, cocaine, or a heterotypic stressor, after a stress-free period (Cador et al., 1993; Cole et al., 1990; Dronjak et al., 2004; Piazza et al., 1990; Schmidt et al., 1999; Stewart and Badiani, 1993). Therefore, the sensitization paradigm was utilized to potentially enhance the effect of repeated injections of TCAP-1 with a challenge of i.c.v. CRF.
88 72 In the EPM, pre-treatment with TCAP-1 without an added stress challenge did not affect open arm measures, although it significantly increased head-dipping, a type of exploratory behaviour. This increase in directed exploration is reminiscent of the results seen in the acute studies, although in the TCAP-1 pre-treatment experiment, testing was performed one week after the last TCAP-1 treatment; long-term changes would have to occur that were facilitated by multiple exposures to TCAP-1. Exposure to the EPM produces a significant rise in plasma corticosterone (Rodgers et al., 1999) and is generally regarded as a mild stressor. Therefore, like the acute study, TCAP-1 could be potentiating the effect of a mild stress: in this case, facilitating behavioural activation (Sutton et al., 1982) and increasing directed exploration. In the two stress conditions, pre-treatment with TCAP-1 potentiated the effect of the CRF challenge. Rats treated with 5 days of TCAP-1 showed decreased percent open arm time and percent open arm entries in response to a 1 µg CRF challenge relative to rats treated with saline and CRF. This treatment also decreased head-dipping and eliminated stretched-attend postures relative to controls. TCAP-1 pre-treatment with a higher dose of CRF (3 µg) did not increase these stresspotentiating effects in the EPM, although it did decrease rearing relative to controls. The absence of an effect may be due to an unexpected blunted effect of the higher dose of CRF, as 3 µg did not appear to be as severe a stressor as the 1 µg dose, although I have shown that 3 µg of CRF indeed does have anxiogenic effects (Experiment 1). However, TCAP-1 did decrease rearing when challenged with 3 µg CRF compared to controls, which suggests that TCAP-1 potentiated the effects of CRF at this dose. In the OF test, rats were tested for 60 min and analyzed to record data for both the full 60 min session as well as the first 10 min of the session in order to compare data with Experiment 1. The 60 min test investigated whether TCAP-1 altered behaviour after the novelty of the OF subsided, but in general the results were similar to the first 10 min of the trial. Pre-treatment of rats with 5 days of i.c.v. TCAP-1 had no effect without an added stressor challenge, as TCAP-1 pre-treated rats were virtually indistinguishable from their saline pre-treated controls. Interestingly, TCAP-1 treatment did not increase total locomotion, either in the first 10 min or for the whole 60 min trial, unlike the results from Experiment 1. In the first 10 min, pretreatment of TCAP-1 potentiated the effects of both 1 µg and 3 µg CRF, as TCAP-1 pretreatment decreased centre time and centre distance in rats challenged with 1 µg CRF relative to controls, whereas TCAP-1 pre-treatment decreased centre entries, centre distance, total distance
89 73 travelled and rearing in rats challenged with 3 µg CRF relative to controls. Data from the whole 60 min were similar to the results from the first 10 min, with the exception that after 60 min, TCAP-1 did not decrease total distance moved in rats treated with 3 µg CRF relative to control. The results from the OF test suggest that TCAP-1 pre-treatment, although it does not itself alter behaviour in the test, it did potentiate the effects of both 1 and 3 µg of CRF. Taken together, the results of the EPM and OF tests in TCAP-1 pre-treated populations indicate that TCAP-1 by itself does not affect traditional measures in either test, but may increase exploratory behaviour such as head-dipping. However, under an acute challenge such as an i.c.v. injection of CRF, TCAP-1 pre-treatment increases the effect of the stressor and appears to be increasing anxiety-like behaviour. The second repeated treatment paradigm (Experiment 3) consisted of 10 days of i.c.v. TCAP-1 injections with EPM testing performed 24 h after the last injection. During the 10-day injection period, rats were subjected to 2 h per day of restraint in a clear Plexigas tube in their home cages. Restraint was used as a psychological stressor that utilizes limbic system integration before activation of the HPA axis and behavioural responses. Rats from these behavioural tests were used to investigate dendritic spines in the hippocampus and amygdala (see Chapter 4), and thus the treatment paradigm with the concurrent restraint sessions followed a timeline similar to the Vyas et al. (2002) study that produced dendritic remodelling. The premise of this experiment was that rats will respond to the repeated stressor by altering levels of catecholamines, ACTH, and hormones such as prolactin (Culman et al., 1991; Kant et al., 1983; McCarty et al., 1988), increasing basal corticosterone levels (Dronjak et al., 2004), blunting the corticosterone response to the homotypic stressor (Kim and Han, 2006; Ma et al., 1999), potentiating the corticosterone response to a heterotypic stressor (Dronjak et al., 2004; Ma et al., 1999), and inducing anxietylike behaviour (Chotiwat and Harris, 2006; Suvrathan et al., 2010; Vyas et al., 2002). However, if TCAP-1 affects behaviour primarily in the presence of a stressor, we hypothesized that daily TCAP-1 injections a few hours prior to daily restraint may alter the effect of the stressor on the rat, modulating both neuronal morphology (see Chapter 4) and behaviour. In the EPM, 10 days of TCAP-1 increased percent open arm time in the absence of a stressor, which is in contrast to other experiments that have used the EPM (Al Chawaf et al., 2007b; Tan et al., 2008, 2009) where TCAP-1 treatment under basal conditions did not affect open arm
90 74 measures. This may be a result of the number of doses given under this treatment regimen, as long-term exposure to TCAP-1 may be required to elicit effects in the absence of a challenge. Ten days of TCAP-1 administration also decreased stretched-attend postures. Taken together these two measures suggest that long-term i.c.v. administration of TCAP produces anxiolytic effects. In restrained animals, TCAP-1 treatment decreased percent open arm time relative to saline-treated controls. However, it should be noted that the restraint stress did not induce anxiety-like behaviour in saline-treated rats, and therefore we cannot conclude that TCAP-1 potentiated the effect of restraint as it did in Experiment 2. As previously discussed above, repeated restraint induced a trend towards decreased anxiety, which is in disagreement with numerous previous reports (Gameiro et al., 2006; Sevgi et al., 2006; Suvrathan et al., 2010; Vyas et al., 2002), but in agreement with others (Cancela et al., 1995; Jaferi and Bhatnagar, 2007). This discrepancy can possibly be explained by the severity of the stressor used in the experiment. For example, the Chattarji group (Suvrathan et al., 2010; Vyas et al., 2002) utilized complete immobilization in a Decapicone, which is a more severe form of restraint than the Plexiglas restraint tube, which still allows a small, but noticeable, amount of movement. The restraint in which we exposed our rats may be described as both predictable and mild, as our rats continually entered restraint tubes each day without a struggle. The predictability and low severity of the stressor may be beneficial, as chronic predictable restraint stress decreased anxiety in the EPM and novel object recognition test (Parihar et al., 2011). The authors postulate that predictable chronic stress produces a lower level of corticosterone output from the HPA axis, which has procognitive and anxiety-reducing effects. Therefore, as TCAP-1 had decreased percent open arm time relative to saline controls, TCAP-1 may indeed be eliciting increased anxiety in the presence of a mild stressor without potentiating the effect of the stressor. Although these behavioural studies indicate that TCAP-1 has the capability to regulate CRFdependent behaviours, the mechanism is not clear. A previous study indicated that TCAP-1 does not interact with either CRF receptor directly (Wang et al., 2005), although this does not discount the possibility that TCAP-1 may be involved in downstream signal transduction systems in the cell. For example, TCAP-1 modulates camp accumulation in Gn11 immortalized hypothalamic neurons independently of CRF receptor binding (Wang et al., 2005), and in high concentrations, TCAP-1 may decrease camp accumulation without interacting directly with the CRF receptors, attenuating the cell s response to CRF. TCAP-1 may also interfere with regulatory proteins,
91 75 such as transcription factors that are involved in genomic responses to CRF. For example, immediate-early gene proteins such as Fos and Jun are highly upregulated after exposure to stressors, such as restraint or CRF injection. Fos, for example, is upregulated via increases in camp which causes a phosphorylation of camp response element binding protein (CREB). Immediate-early gene proteins then dimerize and bind to activator protein-1 (AP-1) sites which then produce a host of effects, such as increased transcription of brain-derived neurotrophic factor (BDNF), which increases synaptic plasticity, and increased trafficking of CRF receptors, which could increase sensitivity to additional stress insults. Together, these studies indicate that TCAP-1 has differential effects based on the severity of the stressor. Under low-stress conditions (CRF (0 µg) and no restraint), TCAP-1 increased exploratory behaviours and decreased risk assessment, and appeared to decrease the anxiety state. In contrast, under high-stress conditions (CRF (1 µg), CRF (3 µg), or restraint), TCAP-1 appeared to increase the anxiety state. It is possible that TCAP-1 may affect stress along CRF s inverse-u shaped curve; TCAP-1 could be enhancing the effect of the low-stress experienced in novel environments such as the EPM and OF by increasing behavioural activation (Sutton et al., 1982) and enhancing the effect of high-stress elicited by exogenous stressors, thereby increasing the anxiety state. In summary, I have shown that i.c.v. TCAP-1 does exert behavioural effects in exploratory tests of anxiety, and that TCAP-1 enhances the effects of low and high levels of stress. 2.6 References Adamec RE, Sayin U, Brown A. (1991) The effects of corticotrophin releasing factor (CRF) and handling stress on behavior in the elevated plus-maze test of anxiety. J Psychopharmacology 5(3): Adamec RE, McKay D. (1993) Amygdala kindling, anxiety, and corticotrophin releasing factor (CRF). Physiol Behav 54(3): Al Chawaf A, St Amant K, Belsham D, Lovejoy DA. (2007a) Regulation of neurite growth in immortalized mouse hypothalamic neurons and rat hippocampal primary cultures by teneurin C-terminal associated peptide-1. Neuroscience 144(4): Al Chawaf A, Xu K, Tan L, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2007b) Corticotropinreleasing factor (CRF)-induced behaviors are modulated by intravenous administration of teneurin C-terminal associated peptide-1 (TCAP-1). Peptides 28(7):
92 76 Albonetti ME, Farabollini F. (1993) Effects of single and repeated restraint on the social behavior of male rats. Physiol Behav53(5): Bittencourt JC, Sawchenko PE. (2000) Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci 20(3): Borsini F, Podhorna J, Marazziti D. (2002) Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 163(2): Britton DR, Hoffman DK, Lederis K, Rivier J. (1984) A comparison of the behavioral effects of CRF, sauvagine and urotensin I. Brain Res 304(2): Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. (1993) Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res 606(2): Cancela LM, Bregonzio C, Molina VA. (1995) Anxiolytic-like effect induced by chronic stress is reversed by naloxone treatment. Brain Res Bull 36(3): Carobrez AP, Bertoglio LJ. (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus maze model 20 years on. Neurosci Biobehav Rev 29(8): Chotiwat C, Harris RB. (2006) Increase anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav 50(3): Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M. (1990) Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res 512(2): Crawley JN. (1985) Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev 9(1): Culman J, Kopin IJ, Saavedra JM. (1991) Regulation of corticotropin-releasing hormone and pituitary-adrenocortical response during acute and repeated stress in the rat. Endocr Regul 25(3): Dhabhar FS, McEwen BS, Spencer RL. (1997) Adaptation to prolonged or repeated stress comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 65(5): Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. (2009) Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav 97(3-4): Dronjak S, Jezova D, Kvetnansky R. (2004) Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci 1018:
93 77 Dunn JD, Berridge CW. (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15(2): File SE. (1982) The rat corticosterone response: habituation and modification by chlordiazepoxide. Physiol Behav 29(1): File SE, Mabbutt PS, Walker JH. (1988) Comparison of adaptive responses in familiar and novel environments: modulatory factors. Ann N Y Acad Sci 525: Gameiro GH, Gameiro PH, Andrade Ada S, Pereira LF, Arthuri MT, Marcondes FK, Veiga MC. (2006) Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav 87(4): Jaferi A, Bhatnagar S. (2007) Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 1186: Kaesermann HP. (1986) Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action. Psychopharmacology (Berl) 89(1): Kant GJ, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. (1983) Effects of repeated stress on pituitary cyclic AMP, and plasma prolactin, corticosterone and growth hormone in male rats. Pharmacol Biochem Behav 18(6): Kim KS, Han PL. (2006) Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res 83: Lister RG. (1990) Ethologically-based animal models of anxiety disorders. Pharmacol Ther 46(3): Ma XM, Lightman SL, Aguilera G. (1999) Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology 140(8): McCarty R, Horwatt K, Konarska M. (1988) Chronic stress and sympathetic-adrenal medullary responsiveness. Soc Sci Med 26(3): Mikics E, Barsy B, Barsvári B, Haller J. (2005) Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Horm Behav 48(2): Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, Baba S, Kasuga M. (1998) Neuropeptide Y produces anxiety via Y2-type receptors. Peptides 19(2): Ohl F. (2003) Testing for anxiety. Clin Neurosci Res 3:233-8.
94 78 Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. (2010) Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry 16(2): Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4 th Ed., Academic Press, Sydney. Pellow S, Chopin P, File SE, Briley M. (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3): Pellow S, File SE. (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24(3): Pernow J, Lundberg JM, Kaijser L. (1987) Vasoconstrictor effects in vivo and plasma disappearance rate of neuropeptide Y in man. Life Sci 40(1): Piazza PV, Deminiere JM, le Moal M, Simon H. (1990) Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine selfadministration. Brain Res 514(1): Prut L, Belzung C. (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1-3): Qian X, Barsyte-Lovejoy D, Wang L, Chewpoy B, Gautam N, Al Chawaf A, Lovejoy DA. (2004) Cloning and characterization of teneurin C-terminal associated peptide (TCAP)-3 from the hypothalamus of an adult rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 137(2): Rodgers RJ, Cole JC. (1993) Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol Behav 53(2): Rodgers RJ, Dalvi A. (1997) Anxiety, defense and the elevated plus-maze. Neurosci Biobehav Rev 21(6): Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. (1999) Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav 688(1-2): Roth KA, Katz RJ. (1979) Stress, behavioral arousal, and open field activity a reexamination of emotionality in the rat. Neurosci Biobehav Rev 3(4): Rotzinger S, Lovejoy DA, Tan LA. (2010) Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31(4): Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. (1999) Stressor- or druginduced sensitization of the corticosterone response is not critically involved in the longterm expression of behavioural sensitization to amphetamine. Neuroscience 92(1):
95 79 Sevgi S, Ozek M, Eroglu L. (2006) L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find Exp Clin Pharmacol 28(2): Sherman JE, Kalin NH. (1986) ICV-CRH potently affects behavior without altering antinociceptive responding. Life Sci 39(5): Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, Britton KT, Rivier J, Vale WW, Koob GF. (2002) Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl) 160(2): Stewart J, Badiani A. (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4(4): Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. (1982) Corticotropin releasing factor produces behavioural activation in rats. Nature 297(584): Suvrathan A, Tomar A, Chattarji S. (2010) Effects of chronic and acute stress on rat behaviour in the rat forced-swim test. Stress 13(6): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2008) Repeated intracerebral teneurin C-terminal associated peptide (TCAP)-1 injections produce enduring changes in behavioral responses to corticotropin-releasing factor (CRF) in rat models of anxiety. Behav Brain Res 188(1): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009) Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201(1): Thorsell A, Carlsson K, Ekman R, Heilig M. (1999) Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport 10(14): Treit D, Menard J, Royan C. (1993) Anxiogenic stimuli in the elevated plus maze. Pharmacol Biochem Behav 44(2): Trubiani G, Al Chawaf A, Belsham DD, Barsyte-Lovejoy D, Lovejoy DA. (2007) Teneurin carboxy (C)-terminal associated peptide-1 inhibits alkalosis-associated necrotic neuronal death by stimulating superoxide dismutase and catalase activity in immortalized mouse hypothalamic cells. Brain Res 116: Wang L, Rotzinger S, Al Chawaf A, Elias CF, Barsyte-Lovejoy D, Qian X, Wang NC, De Cristofaro A, Belsham D, Bittencourt JC, Vaccarino F, Lovejoy DA. (2005) Teneurin proteins possess a carboxy terminal sequence with neuromodulatory activity. Brain Res Mol Brain Res 133(2):
96 80 Weiss SM, Wadsworth G, Fletcher A, Dourish CT. (1998) Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci Biobehav Rev 23(2): Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22(15):
97 81 3 Chapter Three: Elucidating TCAP-1 responsive areas of the brain Part of this chapter has been published in a modified form in: Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009) Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201(1): (Authorization for reproduction obtained from Elsevier) 3.1 Abstract The teneurin C-terminal associated peptides (TCAPs) are a novel family of four endogenous peptides that have shown bioactive properties both in vitro and in vivo. Previously, I and others have shown that both acute and repeated intracerebral injections of synthetic TCAP-1 modulate anxiety-like behaviours in male rats in tests of anxiety, although the neural substrates responsible for these effects were unknown. In the current study, I examined the induction of the immediateearly gene, c-fos, after acute or repeated intracerebral injections of TCAP-1 into male rats, alone or in concert with CRF. The results indicate that whereas an acute injection of TCAP-1 did not induce c-fos on its own, an acute TCAP-1 injection attenuated the CRF-induced increase in c- Fos expression in the limbic system and many of the areas associated with the behavioural responses to stress, including the hippocampus, central and basolateral nuclei of the amygdala, medial prefrontal cortex, and dorsal raphe nucleus. Other areas, such as the paraventricular nucleus of the hypothalamus, bed nucleus of the stria terminalis, medial nucleus of the amygdala, and locus coeruleus, displayed CRF-induced c-fos levels that were unaffected by TCAP-1. However, when TCAP-1 was injected daily for five days as a pre-treatment regimen, TCAP-1 did not affect basal levels of c-fos, nor did it attenuate the effects of a CRF challenge in any of the areas measured, including the areas that had been attenuated in the acute study. In both the acute and repeated TCAP-1 treatment experiments, there was no difference in the coefficient of variation between TCAP-1 and the saline treated groups, however, the coefficient of variation in the TCAP-1 + CRF treated groups was significantly greater than groups treated with CRF in both experiments. This indicates that whereas the acute TCAP-1 can attenuate CRF in certain areas of the brain, but repeated TCAP-1 does not, either treatment regimen of TCAP-1 can increase the
98 82 variability of c-fos responses under stress; this suggests that like previous behavioural experiments, individual differences may play an important role in responses to TCAP Introduction TCAP-1 is a novel biologically active peptide that in vitro can modulate camp accumulation and cell proliferation (Qian et al., 2004) and, when injected into the basolateral nucleus of the amygdala, can modulate acoustic startle behaviour (Wang et al., 2005). Previous work had indicated that TCAP-1 mrna is found in areas of the brain associated with the regulation of behavioural adaptations to stress, such as the hippocampus, amygdala, and hypothalamus (Wang et al., 2005). Furthermore, TCAP-1 has behavioural effects in animal approach-avoidance conflict tests, such as the EPM and open field tests (see Chapter 2; Tan et al., 2008). Therefore, it was imperative to elucidate the areas of the brain sensitive to TCAP-1 under both acute treatment and repeated pre-treatment regimens, as these treatment regimens were successful in eliciting behavioural effects (Tan et al., 2008; Wang et al., 2005). Although the expression of TCAP-1 was located in the limbic system and throughout the brain, peptide localization does not necessarily translate to peptide action. For example, the lateral central nucleus of the amygdala is strongly immunoreactive for CRF but is relatively devoid of CRF receptor expression (Bittencourt and Sawchenko, 2000; Justice et al., 2008). Therefore, I used the immediate-early gene protein, c-fos, which is a transcription factor of the activator protein-1 (AP-1) family, as a measure of general neuronal activation in the brain. AP-1 proteins can form heterodimers between the Jun proteins (c-jun, Jun B, and Jun D) and the Fos proteins (c-fos, Fos B, Fra 1, Fra 2) to form the AP-1 complex, which binds to the AP-1 response element to activate the transcription of genes that regulate neuronal proliferation, apoptosis, and differentiation (Herdegen and Waetzig, 2001). The c-fos protein has a low basal expression but is highly inducible, as c-fos is increased in neurons in response to a variety of stimuli such as stress, learning, and nociception (Herrera and Robertson, 1996). C-Fos mrna is increased within a few minutes of the stimuli (Imaki et al., 1995), and the protein shows its maximal signal 2 hours after induction, with c-fos returning to basal levels after 4 hours (Bittencourt and Sawchenko, 2000; Imaki et al., 1993), which makes c-fos an ideal marker for neuronal activation.
99 83 CRF acts as a neurotransmitter/neuromodulator outside of the HPA axis, and the wide distribution of CRF 1 receptors in areas of processing and integration of sensory information (van Pett et al., 2000) allows for modulation of adaptive behaviours in response to stress. As highlighted in Chapter 2, i.c.v. CRF can be used to elicit many of the behavioural and physiological manifestations that are likewise elicited by stressors such as restraint, forced swim, and immune challenge (Koob et al., 1993; Korte and De Boer, 2003). I.c.v. CRF can dosedependently increase c-fos in the brain (Bittencourt and Sawchenko, 2000) in patterns similar to stressors such as restraint, footshock, forced swim, and immune challenge (Crane et al., 2005; Cullinan et al., 1995; Imaki et al., 1993; Rivest and Laflamme, 1995). C-Fos induction has also been used to gauge long-term changes in responses to stress, such as habituation, where multiple administrations of a homotypic stressor results in an attenuated stress-induced c-fos response (Girotti et al., 2006; Melia et al., 1994; Watanabe et al., 1994) and sensitization, where a heterotypic stressor results in a potentiated stress-induced c-fos response (Armario et al., 2004; Melia et al., 1994;). Therefore, i.c.v. CRF has been used as a predictable and dose-dependent stressor, as protocols for administering stressors such as restraint, swim stress, and footshock can vary between laboratories. The mechanism by which TCAP-1 regulates CRF-mediated behaviour is not known. Therefore, these experiments determined the areas of the brain that are sensitive to TCAP-1, and examined potential interactions between TCAP-1 and CRF in the brain. I.c.v.-administered CRF was used in the current study as a positive control, and also to assess the effects of i.c.v.-administered TCAP-1 on CRF-induced c-fos immunoreactivity. Therefore, I treated rats with TCAP-1 and CRF to determine if TCAP-1 could modulate the expected CRF-induced c-fos accumulation. 3.3 Materials and Methods Animals Male Wistar rats (n = 80; acute study, n = 40, repeated study, n= 40) weighing g were obtained from Charles River Laboratories (Montreal, QC). Rats were singly housed in Plexiglas shoebox cages under standard laboratory conditions (12:12 h light:dark cycle, lights on at 0700 h, temperature C) with food and water available ad libitum. Rats were given one week to acclimatize to laboratory conditions before surgery. All procedures were approved by the
100 84 University of Toronto Animal Care Committee in accordance with the Canadian Council on Animal Care Surgery Male Wistar rats were anesthetized with isoflurane (3% induction, 2 3% maintenance in 100% O 2 ) and fit into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Analgesics (0.5 mg/kg buprenorphine for acute administration animals; 2.5 mg/kg ketaprofen for repeated administration animals) were administered subcutaneously at the beginning of surgery. A midline incision was made along the top of the head, exposing bregma. A 22-gauge guide cannula (Plastics One, Roanoke, VA, USA) was implanted into the right lateral ventricle using the following flat-skull coordinates: AP 1.0 and ML 1.4 from bregma, DV 2.7 from dura (Paxinos and Watson, 1998). The guide cannula was secured to the skull using four jewellers screws and dental cement. The opening of the cannula was covered by a removable cannula dummy. The wound was treated with Hibitane (Pfizer Animal Health, Kirkland, QC) to prevent infection. Animals were kept under warm lamps until regaining consciousness and provided with wet chow fortified with Nutri-Cal (Vetoquinol USA, Fort Worth, TX) in a clean cage for 12 h. Rats were then transferred to a clean cage and were given one week to recover from surgery Peptides Synthetic mouse TCAP-1 was synthesized at 95% purity using f-moc-based solid phase synthesis (American Peptide Company, Sunnyvale, CA). For the acute TCAP-1 administration, TCAP-1 stocks were prepared to a concentration of M (equivalent to 1 μg/μl TCAP-1) by treating lyophilized TCAP-1 with ammonium hydroxide vapour, then dissolving in sterile saline. The TCAP-1 dose was based on the molar equivalent of 3 μg of CRF. For the repeated TCAP-1 administration, TCAP-1 stocks were prepared to a concentration of 10-4 M. CRF (Sigma Aldrich, Oakville, ON) was dissolved in sterile saline at a concentration of 1 μg/μl Experiment 1: Acute effects of TCAP-1 on c-fos immunoreactivity Injections Injections were given between 0800 h and 1000 h. I.c.v. injections were delivered at a rate of 2 μl/min by a Hamilton syringe connected to a pump (Razel Scientific Instrument Inc., Stamford, CT) and connected via PE-50 tubing to a 28-gauge stainless steel injector that extended 1 mm
101 85 below the cannula guide tip. Animals were injected first with either 3 μl of sterile saline (vehicle) or 3 μg of TCAP-1. The injector was left in place for 60 s following infusion to prevent backflow up the cannula. Afterwards, the injector was removed, and a second injector was inserted into the cannula. An infusion of either 1 μl of saline or 1 μl of 1 μg/μl CRF was injected. There were four treatment groups, each with a total volume of 4 μl: saline + saline ( SAL ), saline + CRF ( CRF ), TCAP-1 + saline ( TCAP ), and TCAP-1 + CRF ( TCAP + CRF ) Tissue processing After injections, rats were returned to their home cages and the colony for 2 h. After that period, rats were removed from the colony and overdosed with isoflurane and decapitated. The brains were removed within 90 s, rinsed in PBS, and snap-frozen in 70 C isopentane. The brains were then stored at 80 C until they could be processed. Brains were mounted to the cryostat pedestal with Tissue-Tek OCT media (Sakura Finetek, Torrance, CA) and brains were sectioned on the coronal plane in a 1-in-6 series on a cryostat at a thickness of 25 μm. Sections were thawmounted onto Superfrost Plus slides (Fisher Scientific, Canada) and allowed to dry overnight before being stored at 80 C until processing Immunohistochemistry Immunohistochemical protocols were adapted from Sundquist and Nisenbaum (2005). All wash steps were performed with Tris-buffered saline with Tween-20 (TBS-T; 50 mm Tris HCl, 150 mm NaCl, 0.05% Tween-20, ph 7.6). Antibodies were diluted in TBS-T with 1% weight by volume IgG-free bovine serum albumin fraction V (BSA; Jackson ImmunoResearch, Burlington, ON). Slides were immersed in 4% phosphate-buffered paraformaldehyde (PFA) for 10 min. Endogenous peroxidases were quenched with a 1% hydrogen peroxide/methanol solution for 15 min. Endogenous proteins were blocked with 10% normal goat serum (Vector Laboratories, Burlington, ON). The sections were incubated with rabbit anti-c-fos IgG (1:2000; sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h in a standard humid chamber, then incubated in biotinylated goat anti-rabbit IgG (1:200; BA-1000, Vector Laboratories) for 30 min. The sections were reacted with an avidin biotin peroxidase complex (Vectastain ABC Elite Kit, Vector Laboratories) for 30 min according to kit instructions. Subsequently, the sections were visualized using diaminobenzidine chromagen (DAB Substrate Kit, Vector Laboratories). The sections were dehydrated using graded ethanols, cleared in xylene, and coverslipped with
102 86 Permount and coverglass (Fisher Scientific, Ottawa, ON). An adjoining series of sections were Nissl-stained by dehydrating the sections in graded ethanols, defatting in xylene, rehydration in graded ethanols and staining in 0.1% cresyl violet. Slides were then dehydrated in ethanol, cleared in xylene, and coverslipped with Permount and coverglass Analysis Areas of the brain that are associated with the stress response and demonstrate high c-fos activity in response to CRF were investigated. This included components of the limbic system (amygdala and hippocampus) and other areas linked to both the limbic system and the regulation of stress, including the medial prefrontal cortex (infralimbic and prelimbic cortices), paraventricular nucleus of the hypothalamus, septum (medial and lateral), bed nucleus of the stria terminalis, locus coeruleus, and the dorsal raphe nucleus. Sections were scanned for c-fos activity using a Nikon Optiphot microscope (Nikon Canada, Mississauga, ON). C-Fos-positive cells were manually counted for each area using NIS Elements Software Basic v2.10 (Nikon Canada, Mississauga, ON). Cumulative counts through the entire series for each region of interest were obtained for each brain. The observer was blind to the study Experiment 2: Repeated TCAP-1 pre-treatment on c-fos immunoreactivity Injections Injections were given between 0800 h and 1000 h. I.c.v. injections were delivered as indicated above. The rats were injected once daily for five days with either 3 µl sterile saline (vehicle) or 3 µl of 10-4 M TCAP-1 and then returned to their cages. Rats were then allowed to rest for 7 days. On test day, rats were injected (i.c.v.) with 1 µl of saline (vehicle) or 1 µg/µl CRF and then returned to the colony. There were four treatment groups: saline pre-treatment with acute saline challenge ( SAL+SAL ), saline pre-treatment with acute CRF challenge ( SAL+CRF ), TCAP-1 pre-treatment with acute saline challenge ( TCAP+SAL ), and TCAP-1 pre-treatment with acute CRF challenge ( TCAP+CRF ). Rats were sacrificed 2 h after CRF injection and their brains were excised and snap-frozen in cold isopentane. Brains were mounted to the cryostat pedestal with Tissue-Tek OCT media (Sakura Finetek, Torrance, CA) and brains were
103 87 sectioned on the coronal plane in a 1-in-6 series on a cryostat at a thickness of 25 µm and thawmounted on SuperFrost Plus slides and stored at -80 C until immunohistochemical preparation Immunohistochemistry Owing to antibody lots from Santa Cruz Biotechnologies with vastly different efficacy, a modified immunohistochemistry protocol was adapted from Experiment 1. Immunohistochemical protocols were adapted from Sundquist and Nisenbaum (2005). All wash steps were performed with TBS-T. Antibodies were diluted in TBS-T with 1% weight by volume IgG-free BSA. Sections were immersed in 4% phosphate-buffered PFA for 10 minutes. Exogenous peroxidases were quenched with a 1% hydrogen peroxide/methanol solution for 15 min. Sections were then blocked with an avidin-biotin block (Vector Laboratories) at half-strength for 15 min. Endogenous proteins were blocked with 10% normal goat serum and then incubated with rabbit anti-c-fos IgG (1:1000, Santa Cruz Biotechnology) for 24 h at room temperature in a standard humid chamber. Sections were then incubated in biotinylated goat anti-rabbit IgG (1:200) for 30 mins, reacted with the ABC complex for 30 min, and then visualized with DAB. The sections were dehydrated with alcohol, cleared in xylene, and coverslipped with Permount and coverglass. An adjoining series of sections was stained using cresyl violet for reference purposes Analysis Areas of the brain that were quantified in Experiment 1 were also quantified in Experiment 2, with the inclusion of olfactory areas on the ventral side of the brain. Sections were scanned for c-fos-positive cells as detailed in Experiment 1. The observer was blind to the treatment groups Statistical Analysis Statistical analysis was performed using GraphPad Prism 4.0. Data was analyzed using a twoway ANOVA with TCAP-1 and CRF as factors, followed by Bonferonni's post hoc test. To compare populations where the means differ greatly from each other, the coefficient of variation was used, which is a unitless and normalized measure of dispersion in a sample, with the equation: c υ = σ/µ, where c υ is the coefficient of variation, σ is the standard deviation, and µ is the mean. The mean coefficient of variation across treatments was analyzed using a two-tailed Student s t-test. P < 0.05 was considered significant.
104 Results Experiment 1: Acute TCAP-1 attenuates CRF-induced c-fos in the limbic system Data from the saline (n = 9), CRF (n = 9), TCAP (n = 9) and TCAP+CRF (n = 7) groups were analyzed using two-way ANOVA with Bonferonni s post hoc test, with TCAP and CRF as factors. Figure 3.1: Representative images of 25 µm coronal rat brain sections. Photomicrographs show c-fos immunoreactivity in the subfields of the hippocampus from the acute TCAP-1 administration study: the CA1 (A-D), CA2 (E-H), CA3 (I-L) and the dentate gyrus (DG; M-P). Rats were cannulated into the lateral ventricle and treated with either saline only (SAL; A, E, I, and M), TCAP-1 and saline (TCAP; B, F, J, and N), saline and CRF (CRF; C, G, K, and O), or TCAP-1 and CRF (TCAP+CRF; D, H, L, and P). Scale bar = 100 µm.
105 89 Saline, as expected, did not induce c-fos protein, which served as a negative control. CRF elicited high c-fos immunoreactivity in areas associated with the stress response, including the hippocampus (Figure 3.1), amygdala, medial prefrontal cortex (Figure 3.2), dorsal raphe nuclei, and the PVN (not shown). Figure 3.2: Representative images of 25 µm coronal rat brain sections. Photomicrographs show the central nucleus of the amygdala (CeA; A-D), the basolateral nucleus of the amygdala (BLA; E-H), and the medial prefrontal cortex (mpfc; I-L) from the acute TCAP-1 administration study. Rats were cannulated into the lateral ventricle and treated with either saline only (SAL; A, E, and I), TCAP-1 and saline (TCAP; B, F, and J), saline and CRF (CRF; C, G, and K), or TCAP-1 and CRF (TCAP+CRF; D, H, and L). Scale bar = 100 µm. In the hippocampus, the two-way ANOVA revealed that there was a significant main effect of CRF on c-fos expression in the CA1 (Figure 3.3A; F 1,30 = 17.03, p = ), CA2 (Figure 3.3B; F 1,30 = 19.82, p = ), and CA3 (Figure 3.3C; F 1,30 = 25.00, p < ) subfields, as well as in the dentate gyrus (Figure 3.3D; F 1,29 = 33.52, p < ). There was a significant main effect of TCAP-1 on c-fos expression in the CA1 (F 1,30 = 4.655, p = ) and CA3 (F 1,30 = 9.943, p = ) subfields as well as in the dentate gyrus (F 1,29 = 5.671, p = 0.024). As well, there was a significant interaction between CRF and TCAP-1 in the CA1 (F 1,30 = 5.834, p = 0.022), CA2
106 90 (F 1,30 = 7.276, p = ), and CA3 (F 1,30 = 13.77, p = ) subfields of the hippocampus, as well as in the dentate gyrus (F 1,29 = 8.32, p = ). Bonferroni's post hoc test revealed that CRF significantly increased c-fos expression relative to saline in the CA1, CA2, and CA3 subfields of the hippocampus and the dentate gyrus (p < for all). Treatment with TCAP-1 in CRF-treated animals significantly reduced c-fos expression relative to CRF-only treated animals in the CA1 (p < 0.05), CA2 (p < 0.05), and CA3 (p < 0.001) subfields, and the dentate gyrus (p < 0.01). Figure 3.3: Cumulative c-fos immunoreactivity counts in the subfields of the hippocampus in the acute TCAP-1 administration study: (A) the CA1, (B) CA2, (C) CA3, and (D) dentate gyrus (DG). CRF significantly increased c-fos and TCAP-1 significantly blocked the effect of CRF on c-fos induction across all four subfields of the hippocampus. Values represent means + SEM (*p < 0.05, **p < 0.01, ***p < 0.001).
107 91 Figure 3.4: Cumulative c-fos immunoreactivity counts in the subnuclei of the amygdala in the acute TCAP-1 administration study: (A) the central nucleus (CeA), (B) the basolateral nucleus (BLA), and the (C) medial nucleus (MeA). There were significant main effects of CRF in all three nuclei, but TCAP-1 attenuated CRF-induced c-fos in the CeA and BLA. Values represent means + SEM (*p < 0.05, **p < 0.01, ***p < 0.001). In the amygdala, two-way ANOVA revealed that CRF had a significant main effect of increasing c-fos expression in the central nucleus (CeA, Figure 3.4A; F 1,30 = 13.89, p = ) and basolateral nucleus (BLA, Figure 3.4B; F 1,30 = 17.16, p = ). Bonferroni's post hoc test revealed that treatment of CRF significantly increased c-fos expression relative to saline in the CeA (p < 0.05) and BLA (p < 0.001). In these two nuclei, TCAP-1 treatment in CRF-treated animals decreased c-fos expression such that it was no longer significantly different from saline. CRF had a significant main effect on the medial nucleus of the amygdala (MeA, Figure 3.4C; F 1,30 = 7.663, p = ), but TCAP-1 did not attenuate CRF-induced c-fos, nor was there any interaction between CRF and TCAP-1. CRF had a significant main effect of increasing c-fos immunoreactivity in the medial prefrontal cortex (mpfc, Figure 3.5A; F 1,30 = 4.989, p = ). There was also a main interaction effect between TCAP-1 and CRF (F 1,30 = 5.121, p = 0.031). Bonferroni's post hoc test revealed that CRF significantly increased c-fos expression relative to saline (p < 0.05), and TCAP-1 administration to CRF-treated animals attenuated c-fos expression so that it did not significantly differ from saline.
108 92 Figure 3.5: Cumulative c-fos immunoreactivity counts in other areas of the brain in the acute TCAP-1 administration study: (A) the medial prefrontal cortex (mpfc), (B) lateral septum, (C) medial septum, and (D) dorsal raphe. There were significant main effects of CRF in all four areas, and TCAP-1 attenuated CRF-induced c-fos in the mpfc, and septum. TCAP-1 significantly blocked CRF-induced c-fos in the dorsal raphe. Values represent means + SEM (*p < 0.05, **p < 0.01, ***p < 0.001). Likewise, CRF had a significant main effect of increasing c-fos expression in the lateral septum (Figure 3.5B; F 1,30 = 16.52, p = ) and medial septum (Figure 3.5C; F 1,30 = 16.44, p = ). Bonferroni's post hoc test indicated that CRF significantly increased c-fos expression relative to saline in the medial and lateral septum (p < 0.01 for both). This increase was attenuated by TCAP-1 administration, as TCAP-1 and CRF co-treated animals did not significantly differ from saline controls.
109 93 In the dorsal raphe nucleus, CRF had a significant main effect of increasing c-fos immunoreactivity (Figure 3.5D; F 1,30 = 20.45, p < ), and there was a main interaction effect between CRF and TCAP-1 (F 1,30 = 8.32, p = ). Bonferonni's post hoc test revealed that CRF significantly increased c-fos expression relative to saline (p < 0.001), whereas TCAP-1 treatment to CRF-treated animals significantly decreased c-fos levels relative to CRF-only treated animals (p < 0.05). Figure 3.6: Cumulative c-fos immunoreactivity counts in areas of the brain where TCAP-1 had no effect on c-fos induction in the acute TCAP-1 administration study: (A) the bed nucleus of the stria terminalis (BnST), (B) parvocellular paraventricular nucleus of the hypothalamus (parvocellular PVN), (C) magnocellular PVN, and (D) locus coeruleus. There were significant main effects of CRF in all four areas; however, TCAP-1 had no effect on c-fos induction when TCAP-1 and CRF were co-injected together. Values represent means + SEM (*p < 0.05, **p < 0.01).
110 94 CRF significantly increased c-fos expression in areas associated with the stress response, but not all these areas were sensitive to TCAP-1. CRF had significant main effects in the bed nucleus of the stria terminalis (BnST) (Figure 3.6A; F 1,30 = 10.28, p = ), the parvocellular part of the paraventricular nucleus of the hypothalamus (ppvn, Figure 3.6B; F 1,30 = 15.47, p = ), magnocellular part of the PVN (mpvn, Figure 3.6C; F 1,30 = 6.258, p = ), and the locus coeruleus (Figure 3.6D; F 1,30 = 9.628, p = ). Bonferroni post hoc analysis revealed that CRF increased c-fos expression relative to saline in the PVN (p < 0.05). However, TCAP-1 did not attenuate CRF-induced c-fos, nor were there any interactions between CRF and TCAP-1 in any of these areas Experiment 2: Repeated TCAP-1 pre-treatment does not affect CRF-induced c-fos accumulation Data from the SAL+SAL (n = 9), SAL+CRF (n = 7), TCAP+SAL (n = 8) and TCAP+CRF (n = 8) groups were analyzed using two-way ANOVA with Bonferonni s post hoc test, with TCAP and CRF as factors. Similar to Experiment 1, saline did not strongly induce c-fos in any of the areas measured, and served as the negative control. CRF induced c-fos throughout the brain and served as the positive control. In the hippocampus, there was a significant main effect of CRF in the CA1 (Figure 3.7A; F 1,29 = 18.18, p = ), CA2 (Figure 3.7B; F 1,29 = 8.631, p = ), CA3 (Figure 3.7C; F 1,29 = 22.49, p < ), and the dentate gyrus (Figure 3.7D; F 1,29 = 13.42, p = 0.001). However, TCAP-1 had no significant main effects in the CA1 (p = 0.485), CA2 (p = 0.513), CA3 (p = 0.713), or dentate gyrus (p = 0.545), and there were no interactions between TCAP-1 pretreatment and CRF. Bonferonni s post hoc test revealed that in the CA1, CA2, and dentate gyrus, there were significant increases in c-fos immunoreactivity between TCAP+SAL and TCAP+CRF (p < 0.01 for all). In the CA3, there were significant increases in c-fos immunoreactivity between saline and CRF (p < 0.01) and TCAP+SAL and TCAP+CRF (p < 0.01).
111 Figure 3.7: Cumulative c-fos immunoreactivity counts in the subfields of the hippocampus in the repeated TCAP-1 administration study: (A) the CA1, (B) CA2, (C) CA3, and (D) dentate gyrus (DG). There were significant main effects of CRF in all four areas, but TCAP-1 had no effect on CRF-induced c-fos immunoreactivity. Values represent means + SEM (*p < 0.05, **p < 0.01). 95
112 96 Figure 3.8: Cumulative c-fos immunoreactivity counts in the subnuclei of the amygdala in the repeated TCAP-1 administration study: (A) the central nucleus (CeA), (B) the basolateral nucleus (BLA), and the (C) medial nucleus (MeA). There were significant main effects of CRF in all three areas, but TCAP-1 had no effect on CRF-induced c-fos immunoreactivity. Values represent means + SEM (**p < 0.01). As expected, CRF increased c-fos levels in the amygdala. There were significant main effects of CRF in the CeA (Figure 3.8A; F 1,29 = 14.78, p = ), BLA (Figure 3.8B; F 1,29 = 7.083, p = 0.126), and MeA (Figure 3.8C; F 1,29 = 5.587, p = 0.025). There were no main effects of TCAP-1 on the CeA (p = 0.537), BLA (p = 0.902) or MeA (p = 0.396), nor were there any interactions between TCAP pre-treatment and CRF. Bonferonni s post hoc test indicated that c-fos induction in the CeA under TCAP+CRF treatment was significantly greater than TCAP-1 (p < 0.01). In the mpfc, CRF had a significant main effect on c-fos induction (Table 3.1; F 1,29 =25.27, p < ) but there was no effect of TCAP (p = 0.640), nor was there any interaction of TCAP-1 and CRF. Post hoc tests reveal that the CRF group had significantly higher c-fos levels than saline (p < 0.01), and the TCAP+CRF group had significantly higher c-fos levels than the TCAP group (p < 0.01). CRF had significant main effect in the lateral septum (Table 3.1; F 1,29 = 16.01, p = ) and the medial septum (Table 3.1; F 1,29 = 15.39, p = ). There was no effect of TCAP in either the lateral (p = 0.789) or medial (p = 0.852), nor were there any interactions between TCAP-1 treatment and CRF. Post hoc analysis revealed that CRF groups had significantly higher c-fos than saline groups in both the lateral and medial septum (p < 0.05 for both) and TCAP+CRF groups had significantly higher c-fos than TCAP-1 groups in both the lateral and medial septum (p < 0.05 for both).
113 97 Table 3.1: Cumulative c-fos immunoreactivity counts in other areas of the brain in the repeated TCAP-1 administration study. Saline+Saline TCAP+Saline Saline+CRF TCAP+CRF mpfc PVN Parvo PVN Magno Dorsal Raphe Locus Coeruleus Lateral Septum Medial Septum BnST Piriform Cortex Olfactory Tubercle CxA BnST, bed nucleus of the stria terminalis; CxA, cortical-amygdala transition area; mpfc, medial prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus. Likewise, CRF had main effects in the dorsal raphe nucleus (Table 3.1; F 1,29 = 19.90, p = ), locus coeruleus (Table 3.1; F 1,29 = 8.415, p = 0.007), and BnST (Table 3.1; F 1,29 = 10.28, p = 0.003), but there were no main effects of TCAP-1 in the dorsal raphe (p = 0.573), locus coeruleus (p = 0.583), or BnST (p = 0.816) or any interactions between TCAP-1 and CRF. Post hoc analysis indicated that there c-fos was significantly higher in CRF groups relative to saline (p < 0.01) and in TCAP+CRF groups relative to TCAP (p < 0.01). In the olfactory areas, CRF had significant main effects in the piriform cortex (Table 3.1; F 1,29 = 14.91, p = ), olfactory tubercle (Table 3.1; F 1,29 = 7.750, p = 0.009), and the cortexamygdalar transition zone (CxA, Table 3.1; F 1,29 = 13.64, p = ), although there were no significant main effects of TCAP in any of these areas (p = 0.368, p = 0.855, and p = 0.573, respectively). Post hoc analysis revealed that there was significantly higher c-fos in CRF groups over saline groups in the piriform cortex and olfactory tubercle (p < 0.05 for both) and significantly higher c-fos in TCAP+CRF groups over TCAP-1 groups in the piriform cortex and CxA (p < 0.05 for both). Finally, in the PVN, CRF had significant main effects in the ppvn (Table 3.1; F 1,29 = 11.90, p = ) and the mpvn (Table 3.1; F 1,29 = 10.00, p = ), but there was no main effect of TCAP (p = and p = 0.638, respectively) or interaction between TCAP-1 and CRF. There was significantly more c-fos induction in TCAP+CRF groups relative to TCAP-1 groups in both the ppvn and mpvn (p < 0.05 for both).
114 TCAP-1 treatment increases variability in CRF-treated populations The coefficient of variation (c υ ) was calculated in groups from Experiment 1 and Experiment 2 to elucidate whether TCAP treatment increases variability in basal and CRF-challenged populations. For each brain area, the c υ was calculated from the group mean and the group standard deviation. The c υ from all brain areas were then averaged for a treatment score to determine the average variation elicited by the treatment. In experiment 1, there was no difference in the average c υ between TCAP and saline. However, the average c υ was significantly higher in the TCAP+CRF group over the CRF group (Figure 3.9A; p < 0.05). Similarly, in experiment 2, there was no difference in the average c υ between TCAP-1 and saline, although the average c υ was significantly higher in the TCAP+CRF group over the CRF group (Figure 3.9B; p < 0.01). As TCAP-1 effects could be dependent on individual differences, the % difference from mean for each area was calculated and plotted for each rat in the TCAP+CRF groups (Figure 3.10A- B). The graphs show that the rats that had high c-fos levels in one area tended to have high c- Fos throughout their brain, whereas rats that exhibited below-average c-fos levels in one area tended to have low c-fos throughout their brain.
115 99 Figure 3.9: Mean coefficient of variation (c υ ) in the (A) acute TCAP-1 administration study and the (B) repeated TCAP-1 administration study. TCAP-1 increased the coefficient of variation in the CRF-challenged group in both the acute and repeated TCAP-1 administration studies. Bars represent means + SEM (**p < 0.01, ***p < 0.001). Figure 3.10: Percent difference from mean for 5 rats in the TCAP+CRF group in the (A) acute TCAP-1 administration study and (B) repeated TCAP-1 administration study. Both graphs indicate that in general, rats that had high c-fos immunoreactivity in one area tended to have high c-fos immunoreactivity in other areas, whereas rats that had low c-fos immunoreactivity in one area tended to have low c-fos immunoreactivity in other areas. Brain areas: (1) CA1; (2) CA2; (3) CA3; (4) DG; (5) MeA; (6) BLA; (7) CeA; (8) mpfc; (9) ppvn; (10) mpvn; (11) lateral septum; (12) medial septum; (13) dorsal raphe; (14) LC; (15) lateral BnST; (16) medial BnST; (17) oval nucleus (BnST).
116 Discussion The aim of the studies in this chapter was to delineate the areas of the brain that are responsive to i.c.v. TCAP-1 administered either acutely or repeatedly, as well as to determine if TCAP-1 can alter CRF-induced activation in the brain. The major finding of these studies is that acute i.c.v. TCAP-1 administration can attenuate CRF-induced c-fos expression in the hippocampus, central (CeA) and basolateral (BLA) nuclei of the amygdala, medial prefrontal cortex (mpfc), lateral and medial septum, and dorsal raphe nucleus in the male rat brain. Conversely, repeated pretreatment of TCAP-1 did not attenuate CRF-induced c-fos in any areas of the brain found in the acute study; however, like the acute study, TCAP-1 treatment increased the coefficient of variation in CRF-treated groups. These studies were the first to map the neuroanatomical activity pattern of TCAP-1, both under basal and stressed conditions, revealing important sites in the limbic system. These findings are consistent with previous in situ hybridization data, which indicated that the limbic system and cortex are structures that strongly express TCAP mrna (Wang et al., 2005). Thus, these studies provide a strong set of evidence indicating the regions of the brain where TCAP-1 interacts with CRF-associated functions. The data obtained from the acute TCAP administration study strongly indicated that the hippocampus is a major area in which TCAP-1 exerts its effects. The hippocampus also has a major role in memory and spatial reasoning (Eichenbaum, 2000; Nadel, 1991; Squire and Cave, 1991), and is important in encoding contextual information associated with stressful or fearful environments. The hippocampus is sensitive to stress, and plays a mostly inhibitory role on the HPA axis, although this role is only a small subset of the structure s functions (Herman et al., 2003). The hippocampus appears to be the most sensitive area to acute TCAP-1 administration in all the brain structures investigated; acute TCAP-1 significantly blocked CRF-induced c-fos as well as had a significant main effect on c-fos induction, whereas repeated pre-treatment of TCAP-1 had no effect on CRF-induced c-fos. Subsequent work by Dhan Chand in our laboratory using a TCAP-1-specific antibody has shown that TCAP-1 is strongly expressed in the CA1, CA2, and CA3 regions of the hippocampus, with weaker staining found in the dentate gyrus (Chand et al., manuscript in preparation). Other subsequent studies have shown that immortalized E14 hippocampal and primary hippocampal culture neurons can take up a fluorescein isothiocyanate (FITC)-[K 8 ]-TCAP-1 analogue in a punctate-like manner, and in some cases it can be translocated to the nucleus where it may associate with chromatin (Chand et al.,
117 101 manuscript in preparation). Additionally, as will be discussed in Chapter 4, TCAP-1 can influence cytoskeletal proteins in vitro and modulate hippocampal morphology in vivo. The mpfc is associated with the integration and consolidation of stimuli as well as higher-order processes such as decision making and reward. The mpfc is highly interconnected with the limbic system and like the hippocampus, the mpfc selectively inhibits the HPA axis (Herman et al., 2003). The mpfc is differentially activated depending on the type of stress, as mpfc lesions increased PVN c-fos mrna in response to restraint stress but not ether (Figueiredo et al., 2003). In particular, it has been postulated that this brain area is involved in psychological or anticipatory stressors: stressors that do not pose a direct threat to the organism, such as footshock (Imaki et al., 1993) or restraint (Cullinan et al., 1995), as opposed to stressors that are physical or reactive stressors such as lipopolysaccharide injection (Yokoyama and Sasaki, 1999). I.c.v. CRF induces c-fos in the mpfc (Bittencourt and Sawchenko, 2000), similar to results in this study. Acute TCAP-1 attenuated CRF-induced c-fos protein, such that it was no longer significantly different from saline, whereas repeated pre-treatment of TCAP-1 had no effect on CRF-induced c-fos. Recent work has shown that the mpfc is immunoreactive for TCAP-1 (Chand et al., manuscript in preparation), and therefore, the attenuation of stressinduced processes in the mpfc may impact HPA axis responsiveness. TCAP-1 administration attenuated CRF-induced c-fos in the lateral and medial septum. The area appears to respond to psychological stressors, such as restraint or noise (Arnold et al., 1992), but less so to physiological stressors, such as immune challenge (Emmert and Herman, 1999), and contributes inhibitory input to the HPA axis (Herman et al., 2003). I.c.v. CRF induces c-fos in the medial and lateral septum (Arnold et al., 1992; Bittencourt and Sawchenko, 2000), although the medial septum preferentially expresses CRF 1 receptors whereas the lateral septum expresses CRF 2 receptors (Bittencourt and Sawchenko, 2000). However, infusion of α- helical-crf, a CRF 1 and CRF 2 receptor antagonist, did not block restraint-induced c-fos in the lateral septum, indicating that activation in this area may be due to other inputs into the septal region (Arnold et al., 1992). In these studies, acute TCAP-1 attenuated CRF-induced c-fos in both the medial and lateral septum, indicating that TCAP-1 may have role in regulating the effects of CRF 1 and CRF 2 receptor ligands.
118 102 The amygdala plays a vital role in the integration of fear and anxiety (Davis, 1992), and unlike the hippocampus and mpfc, the amygdala plays a mostly excitatory role on the HPA axis (Herman et al., 2003). Three of the main nuclei, the CeA, BLA, and MeA, appear to have differing functions in stress regulation; the CeA appears to be activated in response to physical stressors, whereas the MeA appears to be activated in response to psychological stressors (Dayas et al., 1999, 2001). The BLA has intrinsic connections with other nuclei in the amygdala, and may be important activating the CeA, which is relatively devoid of CRF 1 receptors (Koo et al., 2004). Our experiments confirm that acute CRF induces c-fos in the CeA, BLA, and MeA, which is similar to previous reports (Bittencourt and Sawchenko, 2000; Imaki et al., 1993). In addition, acute, but not repeated i.c.v. TCAP-1 attenuated CRF-induced c-fos in the BLA and CeA, but not the MeA, which may indicate that TCAP-1 has a stressor-specific role in stress regulation. We have previously shown that the infusions of TCAP-1 into the BLA can modulate behaviour in the acoustic startle test (Wang et al., 2005), indicating that the amygdala is a sensitive substrate for TCAP-1. The sensitivity of the dorsal raphe nucleus to acute TCAP-1 suggests that the serotonergic system may play a role in TCAP-1 signalling. This nucleus has serotonergic neurons that express high c-fos activity during stress (Rioja et al., 2006) and has connections to the mpfc, hippocampus, and amygdala (Lowry, 2002; Vertes, 1991) that are mostly excitatory. However, the dorsal raphe also receives input from the mpfc and CeA (Lee et al., 2003; Peyron et al., 1998) and CRFergic inputs to the dorsal raphe nucleus terminate on GABAergic rather than serotonergic cell dendrites (Waselus et al., 2005). Acute TCAP-1 significantly reduced c-fos elicited by CRF challenge, and the significant decrease in c-fos could be a result of direct effects on raphe neurons or reduced input from forebrain afferents. Likewise, reduced c-fos in forebrain areas such as the limbic system may be a result of reduced serotonergic or GABAergic input. Several regions of the brain that play a significant role in CRF signalling were not affected by TCAP-1 administration. TCAP-1 did not attenuate CRF-induced c-fos in either the parvocellular or magnocellular region of the paraventricular nucleus of the hypothalamus (ppvn and mpvn). In particular, the ppvn is the source of CRF to the HPA axis, which indicates that TCAP-1 may act independently of the canonical HPA pathway. These results agree with a previous experiment in that intravenously-administered TCAP-1 did not affect CRF-induced
119 103 increases corticosterone secretion (Al Chawaf et al., 2007b). TCAP-1 did not affect the bed nucleus of the stria terminalis (BnST), which is a source of many limbic relays to the PVN (Herman et al., 2005). Likewise, the locus coeruleus, a source of ascending and behaviourally activating noradrenergic input into the forebrain, did not show any TCAP-1 effects. Although experiments have shown that the olfactory areas are immunoreactive for TCAP-1 (Chand et al., manuscript in preparation), there were no effects of TCAP-1 seen in any of these areas. As a whole, acute TCAP-1 had CRF-attenuating effects in many, but not all forebrain areas measured, indicating a specificity of action instead of a global decrease in activity. TCAP-1 on its own did not significantly induce c-fos when given acutely nor did it affect basal c-fos when given as a repeated pre-treatment. This may represent a primed state: one where TCAP-1 treatment does not itself induce a response, but instead selectively inhibits the activation of behaviourally relevant brain areas. For example, CRF receptor antagonists, such as CP-154,526 and CRA1000/CRA1001 are inactive in the light/dark box in the absence of stress; however, pretreatment with a stressor (swim stress) was required to see behavioural effects (Okuyama et al., 1999). At the completion of these studies, the mechanism by which TCAP-1 attenuates heightened c- Fos remained unknown. Subsequent work by Tanya Nock (Nock, 2009) used reporter assays to dissect apart the promoter sequences of the c-fos gene to determine which promoter elements were being affected by TCAP-1. The c-fos gene contains multiple response elements, such as the serum response element (SRE), the activator protein-1 (AP-1) element, and the camp response element (CRE). TCAP-1 was previously shown to modulate camp accumulation in vitro, such that high TCAP-1 decreased camp and low TCAP-1 increased camp (Wang et al., 2005). In the signal transduction pathway, camp is upstream of the camp response element binding protein (CREB) which binds with the AP-1 and CRE. Immortalized N3 hypothalamic cells were transfected with luciferase plasmids containing the promoter sequences, and then treated with TCAP-1. TCAP-1 tended to decrease AP-1 activity and increased basal SRE activity (Nock, 2009). However, there was no effect of TCAP-1 on the basal activity of the c- Fos promoter that contains the CRE, AP-1 and SRE elements. This indicated that there may be additional elements required for c-fos regulation. The downstream response element (DRE) is downstream of the TATA box and is activated by calcium, which inactivates the DRE-antagonist modulator (DREAM) protein to allow transcription (Carrión et al., 1999). DRE was significantly
120 104 decreased following TCAP-1 treatment in transfected N38 cells (Nock, 2009), indicating that the modulation of response elements in concert may be required for a reduction in basal c-fos activity. In the current study, the best results were seen after acute administration of TCAP-1 under CRF challenge. Although many of the brain areas highlighted in the c-fos study are rich in CRF 1 expression, Nock (2009) showed that TCAP-1 does not attenuate c-fos via the CRF receptor. In an elegant study, she transfected 293T cells, which do not bind TCAP-1, with either the CRF 1 or CRF 2 receptor. The transfected 293T cells were treated with CRF and/or TCAP-1 to determine the reporter activity of the CRE, a response element on the c-fos gene. Whereas CRF significantly activated the CRE, indicating CRF receptor binding and c-fos activation, TCAP-1 administration did not activate the CRE nor did it attenuate CRF-induced CRE activation (Nock, 2009). Furthermore, TCAP-1 did not affect the glucocorticoid response element (GRE) or attenuate dexamethasone induction of the GRE on the crf promoter. There is also a possibility that the acute mechanisms involved in attenuating CRF-induced c-fos may differ from long-term mechanisms. Pharmacokinetic data obtained in our laboratory has shown that TCAP-1 has a short half-life in serum when given i.v. (between min) and that TCAP-1 is quickly cleared from the bloodstream, returning to undetectable levels a few hours after bolus injection (Song, Barsyte, and Lovejoy, manuscript in preparation). This suggests that i.c.v. TCAP-1 is cleared in a similarly expedient fashion, such that an acute injection can affect c-fos when measured two hours later, but repeated TCAP-1 administration is cleared from the brain within hours and changes to c-fos expression could be absent when measured one week later. However, as TCAP-1 does have long-term behavioural effects (Chapter 2), TCAP-1 may be modifying expression of downstream elements of the CRF response, for example, modulating expression of the CRF receptors to increase the response to stress. Acute or repeated TCAP-1 treatment in CRF-challenged groups resulted in increased variability relative to CRF-challenged groups, indicating that individual differences may be an important factor. In fact, analysis of the individuals in both acute and repeated TCAP-1 administration groups indicate that the effects of TCAP-1 on CRF-induced c-fos were consistent across brain areas: some rats had consistently high levels of c-fos over all their brain areas, whereas some
121 105 rats had consistently low levels of c-fos over all their brain areas. The effectiveness of TCAP-1 in behavioural tests may also rely on individual differences; TCAP-1 administration decreased anxiety-like behaviour in high emotionality rats and increased anxiety-like behaviour in low emotionality rats (Wang et al., 2005), indicating that the individual differences in responsiveness may help determine the activational/behavioural responses to the peptide. The current study also provides an indicator of how TCAP-1 may be affecting CRF-induced behaviour (Tan et al., 2008), as TCAP-1 pre-treatment potentiated CRF-induced behaviour in the EPM and open field, whereas it attenuated CRF-induced behaviour in the acoustic startle test. The results may be explained by the role of brain areas required for each task. The amygdala is important in fear and anxiety behaviours, which would be manifested in approach-avoidance conflict tests such as the EPM and open field, as well as in reflexive fear tests such as the acoustic startle test. However, the hippocampus exerts inhibitory control on behavioural responses and may play a larger role in exploration tasks in novel environments such as the EPM and the open field. According the theories by O Keefe and Nadel (as reviewed by Crusio, 2001), the hippocampus is intimately involved with initiating exploratory behaviour in novel environments to build a cognitive map. Therefore, decreased activation in the hippocampus could result in increased anxiety-like behaviour. Together, the data in this Chapter indicate that acute, but not repeated injections of TCAP-1 can attenuate CRF-induced c-fos in specific areas of the brain associated with the extrahypothalamic stress circuitry, particularly in the limbic system and the dorsal raphe nucleus. TCAP-1 did not alter CRF-induced c-fos in all areas, such as the PVN, BnST, or locus coeruleus, which indicates that TCAP-1 may be acting independently of the HPA axis. Analysis of the coefficient of variation of both acute and repeated treatments indicate that individual differences may play a role in the response to TCAP-1 under stress, as there was no difference in the coefficient of variation between the control groups treated with either saline or TCAP-1; however, TCAP-1 significantly increased the coefficient of variation in groups treated with CRF, such that pretreatment of TCAP-1, whether acute or repeated, increased the coefficient of variation within the population. Thus, one possibility is that TCAP-1, acting on a receptor system distinct from the CRF receptors, modulates either signal transduction or transcriptional elements downstream of CRF receptor activation.
122 106 In light of previous studies indicating that TCAP-1 can also modulate elements of the cytoskeleton (Al Chawaf et al., 2007), the effects that TCAP-1 have on the cell may be more global and could also play a role in neuronal plasticity. If this is so, changes in synaptic interaction or plasticity among cells in CRF-responsive nuclei may modulate the actions of various efferent systems that also modulate the action of CRF. 3.6 References Al Chawaf A, St Amant K, Belsham D, Lovejoy DA. (2007) Regulation of neurite growth in immortalized mouse hypothalamic neurons and rat hippocampal primary cultures by teneurin C-terminal associated peptide-1. Neuroscience 144(4): Armario A, Vallès A, Dal-Zotto S, Márquez C, Belda X. (2004) A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress 7(3): Arnold FJ, De Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. (1992) Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience 51(2): Bittencourt JC, Sawchenko PE. (2000) Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci 20(3): Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR. (1999) DREAM is a Ca 2+ -regulated transcriptional repressor. Nature 398(6722): Crane JW, French KR, Buller KM. (2005) Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress 8(3): Crusio WE. (2001) Genetic dissection of mouse exploratory behaviour. Behav Brain Res 125(1-2): Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64(2): Davis M. (1992) The role of the amygdala in fear and anxiety. Ann Rev Neurosci 15: Dayas CV, Buller KM, Day TA. (1999) Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11(7):
123 107 Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. (2001) Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14(7): Eichenbaum H. (2000) Hippocampus: mapping or memory? Curr Biol 10(21): R Emmert MH, Herman JP. (1999) Differential forebrain c-fos mrna induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res 845(1): Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. (2003) The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 18(8): Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. (2006) Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 138(4): Herdegen T, Waetzig V. (2001) AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene 20(19): Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24(3): Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29(8): Herrera DG, Robertson HA. (1996) Activation of c-fos in the brain. Prog Neurobiol 50(2-3): Imaki T, Shibasaki T, Hotta M, Demura H. (1993) Intracerebroventricular administration of corticotropin-releasing factor induced c-fos mrna expression in brain regions related to stress responses: comparison with pattern of c-fos mnra induction after stress. Brain Res 616(1-2): Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. (1995) Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest 96(1): Justice NJ, Yuan ZF, Sawchenko PE, Vale W. (2008) Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligandreceptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511(4):
124 108 Koo JW, Han JS, Kim JJ. (2004) Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci 24(35): Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. (1993) The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp 172: Korte SM, De Boer SF. (2003) A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus maze. Eur J Pharmacol 463(1-3): Lee HS, Kim MA, Valentino RJ, Waterhouse BD. (2003) Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res 963(1-2): Lowry CA. (2002) Functional subsets of serotonergic neurones: implications for control of the hypothamic-pituitary-adrenal axis. J Neuroendocrinol 14(11): Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. (1994) Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci 14(10): Nadel L. (1991) The hippocampus and space revisited. Hippocampus 1(3): Nock TG. (2009) Development of an enzyme immunoassay and cellular function assays to probe the function of teneurin C-terminal associated peptide (TCAP). M.Sc. Thesis. University of Toronto. Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Nakazato A, Kumagai T, Okubo T, Tomisawa K. (1999) Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther 289(2): Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4 th Ed., Academic Press, Sydney. Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82(2): Qian X, Barsyte-Lovejoy D, Wang L, Chewpoy B, Gautam N, Al Chawaf A, Lovejoy DA. (2004) Cloning and characterization of teneurin C-terminal associated peptide (TCAP)-3 from the hypothalamus of an adult rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 137(2): Rioja J, Santín LJ, Doña A, de Pablos L, Minano FJ, Gonzalez-Baron S, Aguirre JA. (2006) 5- HT 1A receptor activation counteracts c-fos immunoreactivity induced in serotonin neurons of the raphe nuclei after immobilization stress in the male rat. Neurosci Lett 397(3):
125 109 Rivest S, Laflamme N. (1995) Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol 7(7): Squire LR, Cave CB. (1991) The hippocampus, memory, and space. Hippocampus 1(3): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2008) Repeated intracerebral teneurin C-terminal associated peptide (TCAP)-1 injections produce enduring changes in behavioral responses to corticotropin-releasing factor (CRF) in rat models of anxiety. Behav Brain Res 188(1): Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. (2000) Distribution of mrnas encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428(2): Vertes RP. (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313: Wang L, Rotzinger S, Al Chawaf A, Elias CF, Barsyte-Lovejoy D, Qian X, Wang NC, De Cristofaro A, Belsham D, Bittencourt JC, Vaccarino F, Lovejoy DA. (2005) Teneurin proteins possess a carboxy terminal sequence with neuromodulatory activity. Brain Res Mol Brain Res 133(2): Waselus M, Valentino RJ, Van Bockstaele EJ. (2005) Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol 482(2): Watanabe Y, Stone E, McEwen BS. (1994) Induction and habituation of c-fos and zif/268 by acute and repeated stressors. Neuroreport 5(11): Yokoyama C, Sasaki K. (1999) Regional expressions of Fos-like immunoreactivity in rat cerebral cortex after stress; restraint and intraperitoneal lipopolysaccharide. Brain Res 816(2):
126 110 4 Chapter Four: TCAP-1 and the regulation of neural morphology Part of this chapter has been published in: Tan LA, Al Chawaf A, Vaccarino FJ, Boutros PC, Lovejoy DA. (2011) Teneurin C-terminal associated peptide (TCAP)-1 modulates dendritic morphology in hippocampal neurons and decreases anxiety-like behaviors in rats. Physiol Behav In Press. (Authorization for reproduction obtained from Elsevier) 4.1 Abstract Teneurin C-terminal associated peptide (TCAP)-1 is highly expressed in the hippocampus and amygdala, two areas that are important in regulating behavioural responses to stress. In vitro, TCAP-1 upregulates cytoskeletal proteins in immortalized neurons and increases neurite outgrowth in primary hippocampal culture. In vivo, TCAP-1 blocks stress-induced c-fos in the hippocampus and amygdala, and decreases anxiety-like behaviour in the elevated plus maze. This suggests that TCAP-1 plays a role in the remodelling of limbic system networks to modulate stress-induced behaviours. Different chronic stressors, such as social defeat or restraint, cause a remodelling of the pyramidal neurons in the hippocampus and amygdala. Both areas are neuroplastic, and dendritic spines are sensitive to stress and receive excitatory synaptic connections. In this study, repeated daily injection of TCAP-1 (300 pmol) for 10 days increased spine density in the CA1 and CA3 regions of the hippocampus, without affecting spine density in the amygdala. Interestingly, 10 days of restraint increased spine density in CA1 and CA3 neurons. Repeated TCAP-1 had no effect on spine density under restraint conditions; however, linear regressions indicate that there may be a correlation between spine density and EPM behaviour, a relationship that changes when treated with TCAP Introduction Teneurin C-terminal associated peptide (TCAP)-1 is a novel neuropeptide found in the metazoan central nervous system. In situ hybridization experiments indicate that TCAP-1 is expressed in the limbic system, especially in the pyramidal layer of the hippocampus and in neurons of the basolateral nucleus of the amygdala (BLA) (Wang et al., 2005). TCAP-1 is neuroprotective against alkalotic stress (Trubiani et al., 2007), and influences brain-derived neurotrophic factor (BDNF) expression and translation (Ng, 2010) in immortalized hypothalamic neurons.
127 111 Moreover, acutely-administered TCAP-1 attenuates CRF-induced c-fos accumulation in the limbic system, notably in the hippocampus and amygdala (Chapter 3; Tan et al., 2009) by attenuation of the AP-1 and DRE response elements on the c-fos gene (Nock, 2009). Five days of repeated injections of TCAP-1 in a pre-treatment regimen modulated CRF-induced behaviour whereas ten days of TCAP-1 reduced anxiety-like behaviour under basal conditions (Chapter 2; Tan et al., 2008). Furthermore, acute administration of TCAP-1 into the BLA of rats modulated acoustic startle, and i.c.v. injections of TCAP-1 produced behavioural effects that lasted at least 3 weeks after treatment (Wang et al., 2005), indicating that TCAP-1 has long-lasting effects. Although these studies indicate that TCAP-1 may have effects on CRF regulation, and hence, elements of the stress response, the mechanism by which this occurs is not known. The hippocampus and amygdala are structures that are associated with the regulation of the behavioural stress response (Chapter 3; Herman et al., 2003, 2005; Jacobson and Sapolsky, 1991) and recent data indicate that these areas are important substrates for TCAP-1 action. Dendrites on the pyramidal neurons of these two regions are morphologically plastic, and dendritic branches can reversibly change in complexity in response to a variety of stimuli, such as stress (Lambert et al., 1998; Magariños et al., 1996; Watanabe et al., 1992) and learning (Mahajan and Desiraju, 1988). The dendrites of these two areas contain numerous spines that form some of the incoming excitatory synapses. The formation and pruning of spines is also highly dynamic and are more sensitive to stimuli than the remodelling of dendritic trees (Dalla et al., 2009; Morales-Medina et al., 2009; Shors et al., 2001; Silva- Gómez et al., 2003). In particular, chronic restraint or immobilization drastically alters the number of dendritic spines in the CA1 and CA3 regions of the hippocampus (McLaughlin et al., 2005; Sunanda et al., 1995) and the BLA (Mitra et al., 2005; Vyas et al., 2006), which involves remodelling of cytoskeletal proteins and other elements (Gu et al., 2008; Tada and Sheng, 2006). In vitro, TCAP-1 increases cytoskeletal proteins, including β-actin and β-tubulin, and modulates neurite outgrowth in immortalized hypothalamic neurons, and increases neurite outgrowth in primary hippocampal neurons (Al Chawaf et al., 2007a). Inputs into the dendrites of the hippocampus are laminar (Figure 4.1), such that they receive input from sources unique to each stratum (Amaral and Lavenex, 2007; Knowles, 1992). Cortical input arrives from the entorhinal cortex, which sends projections to the dentate gyrus via the perforant path, as well as directly to the distal apical dendrites of CA1 and CA3 neurons, a
128 112 layer known as the stratum lacunosum-moleculare. Dentate gyrus mossy fibre input extends to the thorny excrescences of the stratum lucidum, a layer proximal to the soma, of CA3 neurons. CA3 axons project to the CA1 via the Schaffer collaterals to the stratum radiatum, a layer in between the stratum lacunosum-moleculare and lucidum, and to the stratum oriens, which exists on the basilar tree. The CA1 then projects to the subiculum, one of the main outputs of the hippocampus. Intrinsically, axons of the CA3 project their axons both contralaterally and ipsilaterally to other CA3 neurons in addition to CA1 neurons, as well as to interneurons within the layer (Amaral and Lavenex, 2007). These projections terminate in the strata radiatum and oriens of CA3 neurons. Figure 4.1: Pathways of the hippocampal formation. (A) In the hippocampus, information is unidirectional and arrives from the entorhinal cortex (ECx) via the perforant path (PP), arriving at the dentate gyrus (DG) mossy fibres. These fibres project to the thorny excrescences of CA3 pyramidal neurons. Direct input from the ECx via the PP projects to the distal dendrites of CA3 neurons. The CA3 sends associational and commissural collaterals to other CA3 neurons both contralaterally and ipsilaterally. The CA3 also sends axons to CA1 neurons via the Schaffer Collateral pathway. CA1 neurons then project to the subiculum (Sb) which projects to the ECx. Inputs to the pyramidal neuron dendrites are laminar (red box, CA3). (B) For example, in CA3 neurons, in the apical tree, the distal most part of the dendrites (stratum lacunosum-moleculare) receives information from the PP. The middle part of the apical tree (stratum radiatum) receives commissural and associational collaterals from other CA3 neurons. The proximal part of the apical tree (stratum lucidum) receives axons from the dentate gyrus mossy fibres. In the basilar tree, the stratum oriens receives commissural intrinsic connections. Scale bar = 100 µm. Adapted from
129 113 Previous work has shown that 10 days of TCAP-1 can modulate dendritic morphology in CA3 hippocampal neurons under unstressed and restraint conditions (Al Chawaf, 2008). In unstressed conditions, TCAP-1 treatment decreased dendritic branching in the basilar tree µm away from the soma, an area that corresponds to the stratum oriens. Under restraint conditions, TCAP- 1 treatment increased dendritic branching in the apical tree µm away from the soma, which corresponds to the stratum radiatum. This indicated that TCAP-1 may have a layerspecific role in modulating pyramidal neuron morphology, and dendritic spines were investigated as a more sensitive measure of stress-induced changes in morphological plasticity than dendritic arbour, as some stress-induced spine changes can be seen after a single stress episode (Mitra et al., 2005; Shors et al., 2001). A recent study indicated that a 5 h multimodal bout of stressors resulted in loss of dendritic spines in the CA3 stratum radiatum (Chen et al., 2010), indicating that stimuli can differentially alter strata in the hippocampus. Furthermore, this effect could be blocked by i.c.v. infusion of a CRF 1 receptor blocker (NBI 30775), which prevented spine loss and restored memory function. I hypothesized that as 10 days of TCAP-1 treatment significantly affected dendrite branching in CA3 neurons, TCAP-1 should have a role in remodelling dendritic spines in the CA1 and CA3 of the hippocampus as well. In addition, the BLA was analyzed, as it highly expresses TCAP-1 mrna and injections to the BLA modulate anxiety-like behaviour. I propose that changes in dendritic spines underlie modifications of anxiety-like behaviour, and that these spine changes may be a mechanism for long-term behavioural effects seen in previous studies. 4.3 Materials and Methods Animals All experiments were performed using methods approved by the University of Toronto Animal Care Committee and the Canadian Council on Animal Care. Male Wistar rats (n=30, g, Charles River Laboratories, Montreal, QC) were individually housed in shoebox cages on a 12 h light/dark cycle (lights on at 0700 h) at a constant temperature of 21 C, and were provided with standard rat chow and tap water ad libitum.
130 Surgery Upon delivery, rats were allowed one week to acclimatize to housing conditions. Rats were anaesthetized with 2-3% isoflurane (mixed with oxygen) and fit into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Prior to surgery, rats were administered subcutaneous analgesics (0.5 mg/kg buprenorphine). Rats were then surgically implanted with a 22-gauge guide cannula (Plastics One, Roanoke, VA) into the right lateral ventricle (AP: -1.0 and ML: -1.4 from bregma, DV: -3.7 from dura, co-ordinates according to Paxinos and Watson, 1998). The guide cannula was secured to the skull using four jeweller s screws and dental acrylic, and the opening was covered with a cannula dummy to ensure cannula patency. The wound was treated with Hibitane (Pfizer Animal Health, Kirkland, QC) to prevent infection. Rats were allowed to recover from anaesthesia in clean cages under warm heating lamps. Rats were then transferred to a clean cage and provided with wet chow and Nutri-Cal (Vetoquinal USA, Fort Worth, TX) for 12 h. Rats were then transferred to a clean cage and rats were allowed one week to recover from surgery TCAP-1 and vehicle administration Synthetic TCAP-1 was synthesized as previously described (Wang et al., 2005). The lyophilized peptide was solubilized by exposure to ammonium hydroxide vapour for 2 min before dilution in phosphate buffered saline (10 mm PBS, ph 7.4) at a concentration of 10-4 M. Injections were delivered between 1000 h and 1200 h in a randomized order. Intracerebroventricular (i.c.v.) injections were delivered at a rate of 2 µl/min by a syringe pump (Razel Scientific Instrument Inc., Stamford, CT) connected by PE-50 tubing to a 28-gauge stainless steel injector that extended 1 mm below the cannula guide opening. Animals were injected once daily for 10 consecutive days with either 3 µl of saline (control) or 3 µl of 10-4 M TCAP-1. The injector needle was left in place for 60 s following injection to allow for the peptide to diffuse into the CSF and to prevent backflow up the cannula Restraint protocol Rats were either handled lightly and run through the restraint tube (for rats to experience the novelty of the tube) as a non-stressed control, or were subjected to 2 h per day for 10 consecutive days of restraint in a clear Plexiglas tube (length = 15.8 cm; interior diameter = 7 cm) in their
131 115 home cages. Restraint took place between 1300 h and 1500 h each day after completion of daily i.c.v. injections Tissue fixation and histology Twenty-four hours after the last injection/restraint treatment, rats were deeply anaesthetized with 3% isoflurane in oxygen and decapitated. The brain was removed within 2 min and sectioned to remove the olfactory lobe and 2-3 mm of the anterior part of the frontal lobe and the brainstem and cerebellum to yield a tissue block between AP 1.0 mm and -7.0 mm from bregma (coordinates according to Paxinos and Watson, 1998). Brain blocks were trimmed to allow Golgi stain to fully impregnate the tissue block. Fresh brain blocks were rinsed with PBS and placed into a Golgi impregnation solution containing potassium dichromate, mercuric chloride, and potassium chromate (Solutions A and B, Rapid GolgiStain kit, FD Neurotechnologies Inc., Ellicott City, MD), and were stored in the dark in separate scintillation tubes for 14 days. Brains were then rinsed in PBS and transferred to a cryoprotectant solution (Solution C, Rapid GolgiStain kit) for 48 h, rapidly frozen in -70 C isopentane and stored at -80 C. Brain blocks were mounted on the cryostat pedestal using Tissue-Tek OCT media (Sakura Finetek, Torrance, CA). Brains were sectioned on a cryostat (Leica CM3050, Richmond Hill, ON) at -30 C to obtain 100 µm brain slices on the coronal plane through the amygdala and the dorsal hippocampus. Brain sections were thaw-mounted with a drop of water on 3% gelatinized slides, air-dried in the dark, and stored in slide boxes at room temperature prior to staining. The brain sections were then rehydrated in ddh 2 O and incubated in proprietary solution (Solutions D and E) which visualizes neurons previously impregnated with Golgi stain. The sections were then dehydrated in graded ethanols, cleared in xylene, and cover-slipped with Permount and coverglass (Fisher Scientific, Ottawa, ON). Slides were subsequently stored in the dark until analysis. All brains were checked using a light microscope for proper cannula placement into the lateral ventricle, and those with improper placement were removed from the experiment Dendritic spine analysis Pyramidal cells from the CA1 and CA3 regions of the hippocampus (between bregma: to 4.80 mm, Paxinos and Watson, 1998) and from the BLA (between bregma: to mm) were selected for quantitative analysis. For each brain, 5 CA1, 5 CA3, and 5 BLA neurons were analyzed. To be included in the analysis, Golgi-impregnated pyramidal neurons had to be
132 116 uniformly stained throughout the basilar and apical trees and discernible from neighbouring cells. Because inputs to the CA1 and CA3 neuron dendrites are laminar, the major strata of each tree were analyzed separately. For the CA1 and CA3 regions, spines on primary and secondary branches in the stratum oriens in the basilar tree, and spines on secondary and tertiary dendritic branches in the stratum radiatum and stratum lacunosum-moleculare of the apical tree were investigated. In the BLA, spines on primary and secondary branches were quantified. For each area, 5 segments of 10 µm each were photographed using an inverted light microscope at 1000x magnification with oil immersion (Leica DMI3000, Richmond Hill, ON). In some cases, the segments were from the same branch. Dendritic spines from photomicrographs were manually counted using NIS Elements Software Basic v2.30 (Nikon, Mississauga, ON) by an observer blind to the rat treatments. Spines were required to be distinct from the dendritic branch to be counted Statistical analysis For purposes of the analysis, rats were divided into non-restraint groups and restraint groups. Spine density counts from each neuron were averaged for a cell mean, and five neurons from each animal were averaged for an animal mean for each of the strata analyzed. To determine the effect of TCAP-1 on spine density under basal (unstressed) conditions, in the CA1 and CA3 areas of the hippocampus, strata were analyzed by two-way ANOVA between the saline + no restraint and the TCAP-1 + no restraint groups, with TCAP-1 treatment and strata as independent factors. For the BLA, individual student s t-tests were performed. To determine the effects of restraint on spine density, two-way ANOVAs were performed between saline + no restraint and saline + restraint groups, with restraint and strata as independent factors. For the BLA, individual t-tests between the saline + no restraint and saline + restraint groups were performed. Finally, to determine the effect of TCAP-1 on spine density under restraint (stressed) conditions, two-way ANOVAs were performed in the restraint groups with TCAP-1 treatment and strata as independent factors. For the BLA, individual student s t-tests were performed in the restraint groups. Data obtained from the elevated plus maze that was an adjunct to this study (see Chapter 2) were analyzed in conjunction with spine density; rats that had completed the EPM and had brain areas analyzed for spine density measures were included in a linear regression correlation analysis. Data was analyzed using GraphPad Prism 4.0. P < 0.05 was considered significant.
133 Results In each of the treatment groups, Golgi-stained neurons in the CA1 and CA3 areas of the hippocampus and pyramidal-like neurons of the BLA were easily identifiable. Representative dendritic segments are illustrated in Figure 4.2A and Figure 4.4A (CA1 hippocampus), Figure 4.3A and Figure 4.5A (CA3 hippocampus) and Figure 4.6A (BLA) Effects of TCAP-1 on spine density under basal conditions Animal means were analyzed by two-way ANOVA with TCAP-1 and stratum as independent factors. In the CA1 region of the hippocampus (Figure 4.2B), TCAP-1 treatment had a significant main effect on spine density. (n = 5; F 1,24 = 8.162, p = ). There was also a significant main effect of stratum (F 2, 24 = 6.151, p = 0.007), indicating that there is a difference in spine density across the different strata. There was no interaction between spine density and stratum (F 2,24 = , p = 0.63). Bonferonni s post hoc tests indicate that the stratum radiatum possessed a significantly greater number of spines than the strata lacunosum-moleculare or oriens (p < 0.05).
134 118 Figure 4.2: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA1 region of the hippocampus. (A) Representative photomicrographs of Golgi-stained dendrites of CA1 pyramidal neurons show three laminar regions (stratum lacunosum-moleculare [SLM] and stratum radiatum [SR] of the apical tree and stratum oriens [SO] of the basilar tree) treated with saline + no restraint or TCAP-1 + no restraint. (B) Spine density counts from secondary and tertiary branches of dendrites in the SLM and SR regions and counts from primary and secondary branches of the SO region. TCAP-1 had significant main effects on spine density (F 1,24 = 8.162, p = ). Bars are mean + SEM with n = 5 rats per group (5 x 10 μm segments measured for 5 neurons per animal). Scale bar = 10 μm. In the CA3 region of the hippocampus, (Figure 4.3B), TCAP-1 treatment had a significant main effect on spine density (n = 5; F 1, 24 = 18.26, p = ), however, the stratum did not have any significant main effects (F 2,24 = , p = ), nor were there any interactions between TCAP-1 and stratum (F 2,24 = , p = ). TCAP-1 did not affect spine density in the BLA region (n = 5; Figure 4.6B; p = , t-test).
135 119 Figure 4.3: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA3 region of the hippocampus. (A) Representative photomicrographs of Golgi-stained dendrites of CA3 pyramidal neurons show three laminar regions (SLM, SR, and SO) treated with saline + no restraint or TCAP-1 + no restraint. (B) Spine density counts from secondary and tertiary branches of dendrites in the SLM and SR regions and counts from primary and secondary branches of the SO region. TCAP-1 had significant main effects on spine density (F 1,24 = 18.26, p = ). Bars are mean + SEM with n = 5 rats per group (5 x 10 μm segments measured for 5 neurons per animal). Scale bar = 10 μm Effects of TCAP-1 on spine density under stressed conditions Analyses were performed between saline-treated animals in non-restrained and restrained groups. Animal means were analyzed by two-way ANOVA with restraint and stratum as independent factors. In the saline treated groups, restraint had significant main effects in the CA1 (n = 5;F 1,24 = 5.071, p = ) and the CA3 (F 1,24 = 8.570, p = ), indicating that restraint, indeed, increased spine density in the hippocampus, and could be a control to investigate how TCAP-1 affects spine density in stressed conditions. However, restraint did not significantly alter spine density in the BLA (p = , t-test).
136 120 Figure 4.4: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA1 region of the hippocampus under repeated restraint conditions. (A) Representative photomicrographs of Golgi-stained dendrites of CA1 pyramidal neurons show three laminar regions (SLM, SR, and SO) treated with saline + restraint or TCAP-1 + restraint. (B) Spine density counts from secondary and tertiary branches of dendrites in the SLM and SR regions and counts from primary and secondary branches of the SO region. TCAP-1 had no significant effects on spine density. Bars are mean + SEM with n = 5 rats per group (5 x 10 μm segments measured for 5 neurons per animal). Scale bar = 10 μm. Figure 4.5: Effects of repeated injections of TCAP-1 on dendritic spine density in the CA3 region of the hippocampus under repeated restraint conditions. (A) Representative photomicrographs of Golgi-stained dendrites of CA3 pyramidal neurons show three laminar regions (SLM, SR, and SO) treated with saline + restraint or TCAP-1 + restraint. (B) Spine density counts from secondary and tertiary branches of dendrites in the SLM and SR regions and counts from primary and secondary branches of the SO region. TCAP-1 had no significant effects on spine density. Bars are mean + SEM with n = 5 rats per group (5 x 10 μm segments measured for 5 neurons per animal). Scale bar = 10 μm.
137 121 Data from restrained animals (animal means) were analyzed by two-way ANOVA with TCAP-1 and stratum as independent factors. In the CA1 region (Figure 4.4B), TCAP-1 did not have any significant main effects (n = 5; F 1,24 = 1.365, p = ), although there was a significant main effect of stratum (F 2,24 = 4.711, p = ) but no interaction between TCAP-1 and stratum (F 2,24 = , p = ). In the CA3 region (Figure 4.5B), there were no main effects of TCAP-1 (F 1,24 = , p = ), stratum (F 2,24 = , p = ), or interaction of TCAP-1 and stratum (F 2,24 = , p = ). There was no effect of TCAP-1 in the BLA (n = 5; Figure 4.6C; p = , t-test). Figure 4.6: Effects of repeated injections of TCAP-1 on dendritic spine density in the BLA under non-restraint and repeated restraint conditions. (A) Representative photomicrographs of Golgi-stained dendrites of BLA pyramidal-like neurons treated with saline + no restraint, TCAP-1 + no restraint, saline + restraint, and TCAP-1 + restraint conditions. (B) Spine density counts from primary and secondary branches of dendrites under non-restraint conditions and (C) restraint conditions. TCAP-1 had no significant effects on spine density in the BLA, and restraint did not cause a change in spine density. Bars are mean + SEM with n = 5 rats per group (5 x 10 μm segments measured for 5 neurons per animal). Scale bar = 10 μm Linear regression analysis Linear regression analysis was performed on all rats that had their brains analyzed for spine density, and had completed the EPM test (Table 4.1, Figure 4.7). In the CA1 or BLA, no
138 122 consistent correlations could be drawn between spine density and behavioural measures on the EPM. However, in the stratum radiatum of the CA3, there was a strong positive correlation between spine density and open arm entries in the maze in rats treated with saline + restraint (n = 4; r 2 = , p < ), and a negative correlation in the TCAP + restraint group (n = 3; r 2 = , p < ). Similar results were seen in correlations of the same measures in the stratum lacunosum-moleculare and oriens (data not shown). Negative correlations in the stratum radiatum of the CA3 between spine density and open arm time were also found in TCAP + no restraint (n = 4; r 2 = , p = ) and TCAP + restraint groups (n = 3; r 2 = , p = ). Finally, there was a positive correlation between spine density and closed arm entries in the TCAP + no restraint (n = 3; r 2 = 0.63, p < ) and a negative correlation between spine density and closed arm entries in the TCAP + restraint group (n = 4; r 2 = ; p = ). Table 4.1: Raw behaviour of rats in the EPM subjected to 10 days of TCAP-1 injections and restraint. Saline+No Restraint TCAP+No Restraint Saline+Restraint TCAP+Restraint Open Arm Entries Open Arm Time (s) Closed Arm Entries Values are means + SEM. Figure 4.7: Correlations between spine density in the stratum radiatum (SR) of the CA3 and EPM behavior. Linear regression analyses indicated correlations between spine density of the CA3 SR and (A) open arm entries, (B) open arm time, and (C) closed arm entries.
139 Discussion TCAP is a novel neuropeptide that is strongly expressed in the hippocampus, amygdala, and other nuclei in the limbic system (Wang et al., 2005). The present studies show that TCAP-1, when injected i.c.v. over a period of 10 days, increases spine density in CA1 and CA3 hippocampal neurons under basal conditions but does not affect BLA neurons. TCAP-1 did not affect spine density in restrained rats in the hippocampus or BLA. Furthermore, performance on the elevated plus maze (EPM, Chapter 2) correlated with spine density changes in CA3 neurons. Thus, these studies indicate that TCAP-1 has the potential to modulate neuronal plasticity and could therefore facilitate the actions of afferent fibres regulating stress-sensitive regions of the brain. Previous work had demonstrated that 10 days of TCAP-1 administration can cause a remodelling of the dendrites of CA3 hippocampal neurons in areas corresponding to the stratum radiatum and stratum oriens of the dendritic trees (Al Chawaf, 2008). These results indicated that TCAP-1 may be selectively affecting different parts of the dendritic trees. As inputs to the CA3 are laminar, and these two areas receive intrinsic connections both contralaterally and ipsilaterally from within the CA3 (Amaral and Lavenex, 2007), I hypothesized that TCAP-1 may be modulating synaptic connectivity within these layers. Under non-restrained conditions, TCAP-1 increases spine density in the CA1 and CA3 regions across all the strata of the dendritic trees. There was a significant effect of strata in the CA1 region, as the stratum radiatum had more spines than the strata lacunosum-moleculare or oriens. In the CA3, there was no effect of stratum, nor were there any interactions between spine density and stratum, indicating that whereas TCAP-1 increases spine density in the hippocampus, it does not differentially affect the spines on the different strata of the dendritic trees. TCAP-1 did not affect BLA neurons under basal conditions, although the BLA is a site of TCAP-1 mrna expression (Wang et al., 2005), immunoreactivity (Chand et al., manuscript in preparation), CRF attenuation (Tan et al., 2009), and behavioural activity (Wang et al., 2005). The observed changes in spine formation provide a mechanism that explains, in part, the changes in the EPM behaviour (Chapter 2). Ten days of injections of TCAP-1 significantly increased open arm time and decreased closed arm entries on the EPM.
140 124 The increase in CA3 spine density in the stratum oriens but decrease in basilar branching indicates that overall spine availability may remain relatively unchanged in the basilar tree, as spine density as a whole changes as a function of dendritic length (Radley and Morrison, 2005). However, in the apical tree, there was no change in the branching but an increase in spine density, suggesting an increase in available spines. Together, this indicates that there is an increase in the apical, but not necessarily basal dendritic spine availability of CA3 neurons, although one should note that an increase of spines does not necessarily equate with an increase in synapses, as a proportion of synapses are silent and lack AMPA receptors (Bourne and Harris, 2008). Further studies that investigate the role of TCAP-1 on the type of spines and their receptor disposition will be required to determine if TCAP-1 has a role in increasing excitatory synapse input into the CA3 region. These studies provide new insight into the role of TCAP-1 in the hippocampus and support previous studies indicating a significant role in this region of the brain. The pyramidal neurons of the hippocampal CA3 region are strongly immunoreactive for TCAP-1, whereas the CA1 region is TCAP-1-immunoreactive, albeit less than the CA3 (Chand et al., manuscript in preparation). Recent in vitro experiments have demonstrated that primary hippocampal neurons readily take up fluorescein isothiocyanate (FITC)-labelled TCAP-1, after which it is trafficked to the cytosol, and in some cases, to the nucleus (Chand et al., manuscript in preparation). My observations that TCAP-1 induces changes in spine density indicate that TCAP-1 must have a role in cytoskeletal element regulation. Dendritic spine dynamics depend on the actin filaments in the cytoskeleton, which mediate the formation, elimination, and stability of spines. In addition, the cytoskeleton is also dependent on the regulation of microtubules, where new spines anchor their actin filaments (Hotulainen and Hoogenraad, 2010). Within the neuron, TCAP-1 increases the cytoskeletal proteins, β-actin and β-tubulin, and modulates neurite outgrowth in immortalized hypothalamic culture (Al Chawaf et al., 2007a). Furthermore, in vitro knockdown of all four TCAPs using sirna caused a significant decrease in neurite number in N38 cells (Trubiani, 2008), indicating that TCAPs may be important for neurite stabilization. Recent data has shown that 100 nm TCAP-1 administration on immortalized hippocampal E14 cells causes a remodelling of the cytoskeleton and reorganization of α-tubulin, β-tubulin, and β-actin to the periphery and neurites of the cell (Dhan Chand, personal communications). Our laboratory has indicated that TCAP-1 binds to several kda proteins, one of which is a 35 kda binding
141 125 protein, identified by mass spectrometry as elongation factor-1 (EF-1; Dhan Chand, personal communications). One isoform of the protein, EF-1α, is expressed in pyramidal cell bodies and dendrites of the hippocampus, and has been localized in the strata radiatum and oriens, which receive associational and commissural afferents (Huang et al., 2005). Interestingly, EF-1α binds actin and may anchor β-actin mrna to the cytoskeleton in dendrites (Liu et al., 2002). EF-1α is also increased after perforant path activation although this may represent a reorganization of the actin cytoskeleton to redistribute EF-1α to dendritic areas. EF-1 co-localizes with endogenous TCAP-1 in the cytosol of E14 hippocampal cells (Dhan Chand, personal communications); however, FITC-labelled TCAP-1 does not co-localize with EF-1, as exogenous TCAP-1 appears to be sequestered in vesicles. However, it is possible that TCAP-1 treatment can increase expression of endogenous TCAP-1, which would bind to EF-1α to exert cytoskeletal changes. TCAP-1 also binds to the 15 kda protein, stathmin (Dhan Chand, personal communications), which is involved in the decoupling of α,β-tubulin heterodimers (Cassimeris, 2002). Stathmin mrna is increased in the hippocampus following a single but not chronic methamphetamine dose, a treatment which increases dendritic spines (Ujike et al., 2002). This indicates that stathmin may only be required for spine sprouting rather than upkeep. However, it remains to be seen whether TCAP-1 can affect other components of the actin cytoskeleton, such as microtubule-associated protein-2 (MAP2) and synaptopodin, which have been implicated in anxiety disorders in human patients (Soetanto et al., 2010), and MAP1 and synaptophysin, which are increased and decreased respectively in the hippocampus after restraint (Xu et al., 2004). Neurotrophins like brain-derived neurotrophic factor (BDNF), may also play a role in the upkeep of hippocampal complexity, as haploinsufficient BDNF +/- mice have simplified hippocampal branching and are resistant to stress-induced atrophy (Magariños et al., 2010). TCAP-1 has been shown to reduce BDNF in vitro (Ng, 2010), indicating that a decrease of neurotrophic support may be responsible for a lack of effect in restraint populations. Restraint treatment increased spine density in saline-treated controls relative to unstressed controls, similar to some studies (McLaughlin et al., 2005; Sunanda et al., 1995). Some stressors, such as acute tail shock, increased CA1 spines (Shors et al., 2001). However, other stressors, such as prenatal stress (Martínez-Téllez et al., 2009), postweaning social isolation
142 126 (Silva- Gómez et al., 2003), corticosterone treatment (Morales-Medina et al., 2009), or oxidative stressor (Avila-Costa et al., 1999) reduce spine density in the hippocampus. Stress-induced spine loss has been attributed to high corticosterone working synergistically with excitatory amino acids (for a review, see Fuchs and Flügge, 1998). However, the increase in spines seen in this study may be a result of habituation to the restraint conditions, as repeated restraint results in decreased ACTH secretion (for a review, see Martí and Armario, 1998) and corticosterone (Girotti et al., 2006; Magariños and McEwen, 1995) over time. Restraint is regarded as a mild emotional stressor and is less severe than immobilization (Briski and Gillen, 2001), a stressor treatment that was used to induce dendritic spine changes over 10 days of stress (Mitra et al., 2005). In this study, mild restraint, instead of inducing a reduction in spines associated with other stressors, could have produced an arousing environment which increased possible synaptic connections, and the predictability of the stressor could have resulted in an increase in spines. For example, Parihar et al. (2011) have shown that predictable chronic mild stress, in contrast to unpredictable mild stress, results in increased open arm time in the elevated plus maze, increased cognitive ability in the Morris water maze, and an increase in neurogenesis in the hippocampus. Alternatively, Sunanda et al. (1995) suggest that the increase in spines could be due to abnormal sprouting of new spines, such as that induced by a brief seizure, enough to induce long-term potentiation (Ben-Ari and Represa, 1990). Unfortunately, blood samples to measure corticosterone levels were not obtained so we could not determine if repeated restraint treatment resulted in levels of corticosterone lower than other stressors (i.e. immobilization, oxidative stressor). Unlike other experiments where a stressor was required to see effects (Al Chawaf et al., 2007b; Tan et al., 2008, 2009), 10 days of TCAP-1 administration did not appear to have effects on dendritic spines under restraint conditions relative to saline-treated restraint controls in the hippocampus or amygdala. However, an increase in spines may compensate for a loss in surface area due to decreased branching (Radley and Morrison, 2005). Similar to other studies in the literature (Magariños and McEwen, 1995; Vyas et al., 2002), 10 days of restraint reduced branching in the apical shaft of CA3 neurons (both the strata radiatum and lacunosummoleculare) without affecting the basilar tree (Al Chawaf, 2008 Thesis). TCAP-1 treatment in restrained rats also increased apical branching of CA3 neurons, whereas the basilar tree was unchanged (Al Chawaf, 2008). Therefore, restraint decreased branching but increased spines in
143 127 the apical tree of CA3 neurons in saline-treated rats, indicating that overall there may be no change in spine availability. However, TCAP-1 treatment both increased dendritic branching and increased spine density in the apical tree of CA3 neurons, suggesting and increase in spines µm away from the soma, an area corresponding to the stratum radiatum. This suggests that TCAP-1 may indeed increase available spines particularly in the layer of the hippocampus that receives intrinsic input from other ipsilateral and contralateral CA3 neurons. TCAP-1 and restraint did not alter dendritic branching in the distal parts of the apical tree (Al Chawaf, 2008), although it increased spine density, increasing the number of spines relative to the saline-treated control. These results indicate that TCAP-1 did have effects on spine density in the apical tree, and the stratum radiatum was particularly affected. However, it is unknown whether these results translate to the CA1 region, as the effect of TCAP-1 on dendritic branching in the region is unknown. However, the stratum radiatum of the CA1 had significantly more spines than either the strata oriens or lacunosum-moleculare, and perhaps TCAP-1 had differential effects on this area as well. In the amygdala, immobilization induces hypertrophy of dendritic arbour in BLA neurons (Mitra et al., 2005; Vyas et al., 2004, 2006), and an increase in dendritic spines (Vyas et al., 2006), however our findings indicate that daily restraint did not affect BLA spine density, and TCAP-1 had no effect in the BLA. It is possible that the daily restraint regimen used in these studies was not severe enough to cause morphological changes in amygdalar neurons although it significantly affected hippocampal neurons. This is possibly due to an insufficient induction of corticosterone (as discussed above), as only a single dose of physiologically high levels of corticosterone is sufficient to induce BLA hypertrophy and heightened anxiety (Mitra and Sapolsky, 2008). Recent studies have indicated that individual differences to TCAP-1 may be important (Wang et al., 2005; Chapter 3), and therefore pooled results may confound significant data. The unstressed and restrained groups were analyzed together, and regression analyses indicated that there is a correlation between spine density in the CA3 and behaviours in the EPM (from Chapter 2). In restrained saline-treated animals, there was a significant positive correlation between spine density and open arm entries. This suggests that in saline-treated animals, higher spine density in the CA3 region is associated with greater entries into the open arm (and thus, decreased anxiety-like behaviour). As many stress manipulations, such as corticosterone treatment, result
144 128 in decreased spine density in the hippocampus (Morales-Medina et al., 2009) and increased anxiety-like behaviour in the EPM (Mitra and Sapolsky, 2008), one would surmise that increased spines result in a decrease in anxiety-like behaviour. However, the correlations with TCAP-1- treated animals indicate that the correlation is a negative relationship in restrained rats treated with TCAP-1, as higher spine density is associated with fewer entries into the open arm (and therefore, increased anxiety-like behaviour). Although one should expect that the relationship between spine number and anxiety-like behaviour should be independent of treatment, it is possible that with TCAP-1 treatment, TCAP-1 could have had an effect on silent spines (Bourne and Harris, 2008), or could be producing aberrant spines (Sunanda et al., 1995) which could result in increased anxiety-like behaviour in the EPM. To our knowledge, no studies have directly correlated spine density changes with anxiety-like behaviour in rats; although, a recent study in female rats reported a negative correlation between escape tendencies in a learned helplessness paradigm and hippocampal spine synapses (Hajszan et al., 2010). However, despite the high significance of the correlation in the current study, sample sizes are small and more anxiety tests must be used to properly delineate the role of individual differences on responses to TCAP-1. This Chapter has shown that 10 days of TCAP-1 injection results in an increase in spine density in the hippocampus, but not the amygdala. While it appeared that these effects are lost under restraint conditions, as spine density in restrained TCAP-1-treated rats did not differ from restrained saline-treated rats, companion data describing the dendritic branching suggested that indeed, TCAP-1 may have increased total spine availability in the strata radiatum and lacunosum-moleculare in the CA3 region. Finally, there were strong correlations between spine density and EPM behaviour (from Chapter 2), indicating that TCAP-1 might be altering anxietylike behaviour, in part, via modification of dendritic spines. 4.6 References Al Chawaf A, St Amant K, Belsham D, Lovejoy DA. (2007a) Regulation of neurite growth in immortalized mouse hypothalamic neurons and rat hippocampal primary cultures by teneurin C-terminal associated peptide-1. Neuroscience 144(4): Al Chawaf A, Xu K, Tan L, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2007b) Corticotropinreleasing factor (CRF)-induced behaviors are modulated by intravenous administration of teneurin C-terminal associated peptide-1 (TCAP-1). Peptides 28(7):
145 129 Al Chawaf A. (2008) Teneurin C-terminal associated peptide (TCAP): Molecular and cellular characterization of in vivo and in vitro neuromodulatory effects of TCAP-1. Ph.D. Thesis. University of Toronto. Amaral D, Lavenex P. (2007) Hippocampal neuroanatomy. In: The Hippocampus Book. (Andersen P, Morris R, Amaral D, Bliss T, O Keefe J, eds). Oxford, UK: Oxford University Press. pp Avila-Costa MR, Colín-Barenque L, Fortoul TI, Machado-Salas P, Espinosa-Villanueva J, Rugerio-Vargas C, Rivas-Arancibia S. (1999) Memory deterioration in an oxidative stress model and its correlation with cytological changes on rat hippocampus CA1. Neursci Lett 270(2): Ben-Ari Y, Represa A. (1990) Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci 13(8): Bourne JN, Harris KM. (2008) Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31: Briski K, Gillen E. (2001) Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull 55(3): Cassimeris L. (2002) The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol 14(1): Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. (2010) Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropinreleasing hormone signaling. Proc Natl Acad Sci U S A 107(29): Dalla C, Whetstone AS, Hodes GE, Shors TJ. (2009) Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculine females. Neurosci Lett 449(1): Fuchs E, Flügge G. (1998) Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev 23(2): Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. (2006) Habituation to repeated stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 138(4): Gu J, Firestein BL, Zheng JQ. (2008) Microtubles in dendritic spine development. J Neurosci 28(46): Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. (2010) Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 65(5):
146 130 Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24(3): Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29(8): Hotulainen P, Hoogenraad CC. (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189(4): Huang F, Chotiner JK, Steward O. (2005) The mrna for elongation factor 1α is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci 25(31): Jacobson L, Sapolsky R. (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12(2): Knowles WD. (1992) Normal anatomy and neurophysiology of the hippocampal formation. J Clin Neurophysiol 9(2): Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. (1998) Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav 65(1): Liu G, Grant WM, Persky D, Latham VM Jr, Singer RH, Condeelis J. (2002) Interactions of elongation factor 1α with F-actin and β-actin mrna: implications for anchoring mrna in cell protrusions. Mol Biol Cell 13(2): Magariños AM, McEwen BS. (1995) Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 69(1): Magariños AM, McEwen BS, Flügge G, Fuchs E. (1996) Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16(10): Magariños AM, Li CJ, Toth JG, Bath KG, Jing D, Lee FS, McEwen BS. (2010) Effect of brainderived neurotropic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus In Press. Mahajan DS, Desiraju T. (1988) Alterations of dendritic branching and spine densities of hippocampal CA3 pyramidal neurons induced by operant conditioning in the phase of brain growth spurt. Exp Neurol 100(1): Martí O, Armario A. (1998) Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci 16(3-4):
147 131 Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G. (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse 63(9): McLaughlin KJ, Baran SE, Wright RL, Conrad CD. (2005) Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience 135(4): Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. (2005) Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A 102(26): Mitra R, Sapolsky RM. (2008) Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A 105(14): Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. (2009) Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens, and amygdala in the rat. J Chem Neuroanat 38(4): Ng, TSJ. (2010) The effect of teneurin C-terminal associated peptide (TCAP)-1: Protection against hypoxic-stress and regulation of brain-derived neurotrophic factor (BDNF) in immortalised hypothalamic N38 cells. M.Sc. Thesis. University of Toronto. Nock TG. (2009) Development of an enzyme immunoassay and cellular function assays to probe the function of teneurin C-terminal associated peptide (TCAP). M.Sc. Thesis. University of Toronto. Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. (2011) Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry 16(2): Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4 th Ed., Academic Press, Sydney. Radley JJ, Morrison JH. (2005) Repeated stress and structural plasticity in the brain. Ageing Res Rev 4(2): Shors TJ, Chua C, Falduto J. (2001) Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21(16): Silva-Gómez AB, Rojas D, Juárez I, Flores G. (2003) Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983(1-2): Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, Kelly J, Leurgans S, Bennett DA, Arnold SE. (2010) Association of anxiety and depression with microtubuleassociated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry 67(5):
148 132 Sunanda, Rao MS, Raju TR. (1995) Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons a quantitative study. Brain Res 694(1-2): Tada T, Sheng M. (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 16(1): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2008) Repeated intracerebral teneurin C-terminal associated peptide (TCAP)-1 injections produce enduring changes in behavioral responses to corticotropin-releasing factor (CRF) in rat models of anxiety. Behav Brain Res 188(1): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009) Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201(1): Trubiani G, Al Chawaf A, Belsham DD, Barsyte-Lovejoy D, Lovejoy DA. (2007) Teneurin carboxy (C)-terminal associated peptide-1 inhibits alkalosis-associated necrotic neuronal death by stimulating superoxide dismutase and catalase activity in immortalized mouse hypothalamic cells. Brain Res 116: Trubiani G. (2008) Elucidating the neuroprotective role of teneurin C-terminal associated peptide (TCAP)-1. Ph.D. Thesis. University of Toronto. Ujike H, Takaki M, Kodama M, Kuroda S. (2002) Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci 965: Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22(15): Vyas A, Pillai AG, Chattarji S. (2004) Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128(4): Vyas A, Jadhav S, Chattarji S. (2006) Prolonged behavioural stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143(2): Wang L, Rotzinger S, Al Chawaf A, Elias CF, Barsyte-Lovejoy D, Qian X, Wang NC, De Cristofaro A, Belsham D, Bittencourt JC, Vaccarino F, Lovejoy DA. (2005) Teneurin proteins possess a carboxy terminal sequence with neuromodulatory activity. Brain Res Mol Brain Res 133(2): Watanabe Y, Gould E, McEwen BS. (1992) Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588(2):
149 Xu H, He J, Richardson JS, Li XM. (2004) The response of synaptophysin and microtubuleassociated protein 1 to restraint stress in rat hippocampus and its modulation by venlafaxine. J Neurochem 91(6):
150 134 5 Chapter Five: Uptake of intravenous TCAP-1 into body tissues and the brain This chapter is in preparation for submission to Behavioural Brain Research: Tan LA, Song L, Gifford A, Barsyte D, Lovejoy DA. Activity of subcutaneously-administered teneurin C-terminal associated peptide (TCAP)-1 in the blood and brain. 5.1 Abstract Previous studies indicate that TCAP-1 is active in vivo at modulating anxiety-like behaviours under stress, and that TCAP-1 has promising neuroprotective effects under alkalotic (Trubiani, et al., 2007) or hypoxic stress (Ng, 2010). These results suggest that TCAP-1 could have therapeutic potential; however, in vivo TCAP-1 treatments have been mainly administered through the i.c.v. route, which is an invasive procedure. In contrast, one recent study showed that peripherally-administered TCAP-1 through the intravenous route modulated CRF-induced behaviour in the EPM and OF, and that a fluorescently-tagged TCAP-1 analogue could cross the blood-brain barrier (Al Chawaf et al., 2007). Previous results had indicated that TCAP-1 can be found in the limbic system, cortex, cerebellum, and hypothalamus of the rat brain, but it was unknown if these areas possessed binding sites for the peptide. Therefore, TCAP-1 was labelled with radioactive 125 I and injected intravenously into rats. Organs and tissues were obtained and counted in a gamma counter, and the brain was sectioned to elucidate uptake sites in the brain. The results indicate that [ 125 I]-TCAP-1 is rapidly cleared from the blood via the gastrointestinal and renal tracts, and that only a small fraction of [ 125 I]-TCAP-1 enters the brain. Within the brain, [ 125 I]-TCAP-1 signal was found in areas of known TCAP-1 activity, such as the cortex, cerebellum, hippocampus, amygdala, and hypothalamus, but also in areas not known to have significant TCAP-1 expression such as the nucleus accumbens, substantia nigra, caudate putamen, superior and inferior colliculi, medial geniculate nucleus, and mammillary nucleus. The functions of these areas suggest that TCAP-1 may have additional roles in addiction, movement, memory, and the processing of auditory stimuli. 5.2 Introduction The TCAP family consists of four novel neuropeptides that are found throughout the brain but also peripherally in the body (Chand et al., manuscript in preparation). Our laboratory has
151 135 demonstrated that in vitro, TCAP-1 is neuroprotective in immortalized neurons under alkaline (Trubiani et al., 2007) or hypoxic conditions (Ng, 2010). Previous chapters have also shown that TCAP-1 indeed has many in vivo effects that can affect behaviour, neuronal activation, and neuron morphology. However, to garner these in vivo effects, TCAP-1 was delivered in a large bolus directly into the lateral ventricles of rats, which is an undoubtedly invasive procedure. However, a previous study (Al Chawaf et al., 2007) indicated that TCAP-1 could modulate the effects of CRF when given peripherally. In addition, the study showed that a [K 8 ]-TCAP-1 analogue with a fluorescein isothiocyanate (FITC) tag could cross the blood-brain barrier (BBB) and could be found in blood vessels and fibres in the brain. Recent studies have indicated that TCAP-1 is rapidly cleared from the bloodstream after i.v. administration of the peptide and is undetectable a few hours after i.v. injection (Song, Barsyte and Lovejoy, manuscript in preparation). However, the mechanism by which TCAP-1 is eliminated is unknown. Radioiodination is a method of labelling a peptide with a radionuclide such as 125 I or 131 I, which are both γ-emitters. 125 I is the most common for radioiodination (Bailey, 1994) and 125 I can be attached to tyrosine residues in a peptide, making it amenable to localization in tissues by radioactive counts or localization in brain sections by visualization on radiographic film. Studies utilizing this technique allow researchers to detect low concentrations of peptide in brain sections. Previous studies (Wang et al., 2005; Chand et al., manuscript in preparation) had determined that TCAP-1 is expressed and immunoreactive throughout the brain, especially in areas such as the hippocampus, hypothalamus, and cerebellum, and I have previously shown that TCAP-1 is active in reducing CRF-induced c-fos (Chapter 3; Tan et al., 2009). However, the putative TCAP-1 receptor has not been found, and immunoreactive peptide labelling does not necessarily equate with peptide binding. Therefore, in collaboration with Dr. A. Gifford at InvivoPharm (Calverton, NY), TCAP-1 was iodinated and injected i.v. to determine uptake sites in the brain after [ 125 I]-TCAP-1 has crossed the BBB, as well as to determine the organs in the body that readily take up [ 125 I]-TCAP-1.
152 Materials and Methods Animals Male Sprague-Dawley rats (n = 5) (160 g, Charles River, Wilmington MA) were housed in standard laboratory conditions. All procedures were in accordance with the InvivoPharm company policies Peptides Lyophilized TCAP-1 powder was provided to colleagues at InvivoPharm (Calverton, NY), where TCAP-1 radioiodination was completed by Dr. A. Gifford. 5 µl iodogen was added to 50 µl dichloromethane and allowed to dry. 30 µl of ammonium bicarbonate buffer was added, followed by 15 µg of TCAP-1. The solution was incubated with 1.5 mci Na 125 I in 40 µl phosphate buffer (ph 7.8) for 15 min at RT. The reaction was stopped with 2 µl trifluoroacetic acid (TFA) and purified by high-performance liquid chromatography (HPLC). A radioactive peak eluting between min was collected and 870 µci of radiolabelled peptide was obtained. [ 125 I]-TCAP-1 was stored in the mobile phase with 1% bovine serum albumen and 0.1% mercaptoethanol. Prior to injection, [ 125 I]-TCAP-1 was dried under nitrogen, re-dissolved in 0.1% TFA and loaded onto a C-18 sep pack cartridge. Following washing, the peptide was eluted with 50% acetonitrile with 0.1% TFA, evaporated to dryness and re-dissolved in 0.1 ml of ammonium bicarbonate and diluted in 0.5 ml ddh 2 O Distribution of [ 125 I]-TCAP-1 in brain and tissues To determine the distribution of [ 125 I]-TCAP-1 in rat tissues, Sprague-Dawley rats (n = 2) were first injected with 0.1 ml of intraperitoneal (i.p.) 0.9% sodium iodide prior to injection of radioiodinated TCAP-1 (10-12 µci) via the tail vein. Rats were sacrificed 30 min after injection and tissues and organs were collected, weighed, and the radioactivity was counted in a gamma counter (CRC-5, Capintec Inc., Ramsey, NJ). Counts for the stomach, intestine, cecum, and colon included organ contents. Counts for the pancreas, prostate, skin, muscle, and bone were tissue samples rather than whole organs. To determine the distribution of [ 125 I]-TCAP-1 in the regions of the brain, Sprague-Dawley rats (n = 3) were weighed and injected with 0.2 ml of 0.9% sodium iodide (i.p.) prior to tail vein injection with [ 125 I]-TCAP-1. The dose ( µci) was proportional to the rat weight. Rats were sacrificed 30 min after [ 125 I]-TCAP-1 injection.
153 137 Following sacrifice, the brains were dissected out and weighed. Brains were cooled in ice-cold saline and sectioned in 300-µm slices on a vibratome. Sections were dried on a slide warmer and exposed to a phosphor screen for 7-15 days and scanned. Areas of [ 125 I]-TCAP-1 uptake in the brain were analyzed according to the atlas of Paxinos and Watson (1998). 5.4 Results Uptake of intravenous TCAP-1 into body tissues After i.v. injection, [ 125 I]-TCAP-1 could be found in the tissues throughout the rat body (Table 5.1) but tended to be associated with highly vascular tissues. The highest levels of 125 I- TCAP-1 in terms of counts per minute (cpm) per gram of tissue included bladder, kidney, plasma stomach and liver. Lower concentrations were found in the adrenal gland, lungs, intestine and thyroid gland. The lowest concentrations were found in brain, cecum, muscle, testes and thymus. The bladder, when full, accounted for 2.7% of the total injected dose (ID), but when empty accounted for only 0.09% of the total ID. The kidneys took up 9.98% of the ID. A measurement of 5.39% of the ID of 125 I-mTCAP-1 could also be found in the stomach and 6.41% could be found in the liver. The small intestine accounted for 4.59% of the ID. Lower activity was detected in the lungs, pancreas, spleen, cecum, adrenals, and skin. Only 0.13% of the total ID entered the brain Uptake of intravenous TCAP-1 into the brain I.v. injection of [ 125 I]-TCAP-1 into the tail vein produced a distribution of radioactivity in the rat brain (Figure 5.1). Positive signals could be detected in the caudate putamen, nucleus accumbens (shell), thalamus, basolateral nucleus of the amygdala, hippocampus, substantia nigra, medial geniculate nucleus, mammillary nucleus, superior and inferior colliculus, and cerebellum. Positive but weaker signals could be detected in the preoptic area, hypothalamus, posteromedial cortical nucleus of the amygdala, and lateral entorhinal cortex. No signal could be found in the bed nucleus of the stria terminalis, septum, paraventricular nucleus of the hypothalamus, and locus coeruleus.
154 138 Table 5.1: Partitioning of [ 125 I]-TCAP-1 in tissues following i.v. administration to male rats. Tissue Mean (cpm/g) Range (cpm/g) %ID/organ % ID/g Adrenals % 0.75% Bladder (full) # 2.70% 12.29% Bladder (empty) # 0.09% 1.21% Bone * 0.45% Brain % 0.07% Cecum % 0.14% Colon % 0.28% Heart % 0.42% Kidneys % 6.05% Liver % 1.10% Lungs % 0.85% Muscle * 0.27% Pancreas * 0.64% Plasma * 1.85% Prostate * 0.43% Skin * 0.53% Small Intestine % 0.65% Spleen % 0.67% Stomach % 1.77% Tail (injection site) % 1.28% Testis % 0.24% Thymus % 0.27% cpm/g = counts per minute per gram of tissue. %ID = percent radioactivity of injected dose. N = 2 for all tissues except for the bladder measurements where # n = 1. * = full organs could not be obtained.
155 Figure 5.1: The distribution of intravenously injected [ 125 I]-TCAP-1 in the rat brain. Autoradiograms of 300 µm sections (left) with brain atlas reference (right; Paxinos and Watson, 2008) indicate areas of TCAP-1 binding. AcbSh, nucleus accumbens, shell; Amyg, amygdala; CB, cerebellum; CPu, caudate putamen; Hi, hippocampus; IC, inferior colliculus; mgn, medial geniculate nucleus; MN, mammillary nucleus; PAG, periaqueductal grey; SC, superior colliculus; SN, substantia nigra. 139
156 Discussion TCAP-1 is a novel neuropeptide that is expressed and immunoreactive throughout the brain but is also found peripherally in areas such as the ovaries and testes (Chand et al., manuscript in preparation; Wang et al., 2005). However, areas of mrna and peptide expression do not necessarily equate with peptide binding, and a membrane-bound receptor has yet to be conclusively identified. The work detailed in this chapter investigated the uptake sites of radioiodinated [ 125 I]-TCAP-1 both in the brain and in the body. I.v. injection of 125 I-TCAP-1 resulted in uptake by tissues and organs of the body 30 min after administration. These studies indicate that i.v. TCAP-1 was preferentially taken up by regions in the limbic system, areas of high dopaminergic activity, and acoustic processing. Because the uptake was found in these particular regions and not necessarily those associated with high vascularity, this suggests a specific uptake mechanism for TCAP-1. In the body, a large proportion of the labelled [ 125 I]-TCAP-1 was found in the kidneys and the full but not the empty bladder, indicating that exogenously-administered TCAP-1 is rapidly cleared via renal elimination. Large amounts of activity was also found in the liver, stomach, small intestine and its contents, indicating that TCAP-1 may be excreted through the gastrointestinal system. Although the gonads have been identified as an area of high TCAP-1 immunoreactivity (Chand et al., manuscript in preparation), the testes and prostate did not take up a notable amount of [ 125 I]-TCAP-1. However, low levels in the testes may be due to the protective effect of the blood-testis barrier (BTB), which acts like the BBB to prevent toxins from entering the seminiferous tubules (Mruk and Cheng, 2010). Interestingly, although [ 125 I]- TCAP-1 appears to cross the BBB, only a fraction of the dose at the 30 min time-point entered the brain. As low doses of i.v.-administered TCAP-1 have been shown to modulate anxiety-like behaviour (Al Chawaf et al., 2007), this supports previous results (Wang et al., 2005) that TCAP- 1 is behaviourally active at very low doses. The idea that a peptide could cross the BBB was once controversial; researchers had once believed the BBB to be almost impenetrable to larger molecules like peptides. However, the BBB is now seen as a regulatory system which allows some, but not all, peptides into the brain through transmembrane diffusion (TRH and α-msh, for example) and carrier-mediated transport or saturable transport (AVP, leptin, and insulin, for example) (Banks et al., 1992). However,
157 141 peptides like melanin concentrating hormone (MCH), NPY, CART, orexin A, and orexin B cannot cross the BBB (Banks, 2006). BBB-crossing mechanisms involve proteins that allow transport into the brain; however, peptides that have similar or even the same receptors do not necessarily cross the BBB with equal efficiency. For example, the peptides MIF-1 and interleukin-1 cross the BBB but Tyr-MIF-1 and interleukin-2 do not (Kastin et al., 1999). CRF, which has a structure and molecular weight similar to TCAP-1, has an energy-dependent saturable transport out of the brain and a slow saturable influx into the brain (Kastin and Pan, 2003), but transport of peptides out of the brain may make the passage of peptides across the BBB to appear low. At the 30 min-timepoint, only 0.13% of TCAP-1 entered the brain. However, although peptide transport systems may exist to cross the BBB, this does not necessarily ensure that large amounts of peptide will enter the brain. The pancreatic peptides, amylin and insulin, require BBB transport mechanisms to access hypothalamic and cortical receptors, and the small amounts that cross the BBB are sufficient to reduce food intake and body weight. Only 0.12% and 0.046% of the injected dose of radioiodinated amylin and insulin, respectively, could be found in the brain (Banks and Kastin, 1998). While this may appear to be a very small proportion, in comparison, only 0.02% of a therapeutically active peripheral dose of morphine crosses the BBB (Kastin and Pan, 2003), and therefore, low concentrations in the brain can still have high efficacy. Other peripheral peptides, such as leptin, require BBB-crossing mechanisms to exert their effects. For example, leptin transport across the BBB is decreased in obese animals, and leptin administered to the brain, but not peripherally, caused the animals to lose weight (Banks, 2006). Thus, saturable influx mechanisms allow peripherally secreted peptides to enter the brain to exert its effects. In situ hybridization, TCAP-1 immunohistochemistry, and c-fos immunohistochemistry have indicated that the amygdala, medial prefrontal cortex, hypothalamus, cerebellum, and dorsal raphe nucleus are areas where TCAP-1 is either expressed or is active (Chand et al., manuscript in preparation; Tan et al., 2009; Wang et al., 2005). Using a FITC-[K 8 ]-TCAP-1 analog, we previously showed that peripherally-administered TCAP-1 can cross the BBB. FITC-[K 8 ]- TCAP-1 was found in several regions along large blood vessels, fibres, and highly vascularized areas such as the caudate putamen and choroid plexus, but not at any of the circumventricular organs (Al Chawaf et al., 2007). In vitro neuron cultures will readily take up FITC-[K 8 ]-TCAP-1 in a punctate-like manner (Chand et al., manuscript in preparation), but the Al Chawaf et al.
158 142 (2007) study did not confirm uptake in many of the areas that previously showed TCAP-1 expression, and questions arose as to whether FITC-[K 8 ]-TCAP-1 was reaching the brain parenchyma. Radioiodinated [ 125 I]-TCAP-1 was utilized as a different method to identify TCAP-1 binding sites in the brain. [ 125 I]-TCAP-1 was found, as expected, in areas previously known to have TCAP-1 expression, such as the hippocampus, amygdala, cortex, and cerebellum, but also in areas previously unknown to have significant TCAP-1 expression in the rat, such as the caudate putamen, nucleus accumbens, substantia nigra, mammillary nucleus, superior and inferior colliculi and medial geniculate nucleus. Interestingly, there was a strong signal from the nucleus accumbens, substantia nigra, and caudate putamen, three areas of high dopaminergic activity. Although the resolution was not sufficient to detect a signal in the ventral tegmental area, TCAP-1 uptake in the nucleus accumbens suggests a role in reward systems (Leshner and Koob 1999; Self, 1998; Wise, 1987). Interestingly, recent data has indicated that TCAP-1 blocks CRF-induced cocaine reinstatement and behavioural sensitization (Kupferschmidt et al., 2011), although TCAP-1 had no effect on footshock- or cocaine-induced reinstatement or sensitization. The substantia nigra in the midbrain is the source of ascending dopaminergic connections to the dorsal striatum via the nigrastriatal pathway to control movement (Amalric and Koob, 1993). Uptake was also found in the caudate putamen in the rat, which suggests that TCAP-1 may have a role in elements of both the mesolimbic and nigrastriatal dopaminergic pathways and thus may have a role in motivation and addictions. The mammillary nucleus (MN) is considered a part of the dorsal hypothalamus that has connections to the hippocampus and thalamic nuclei. The MN has been implicated for its role in memory (Vann and Aggleton, 2004) and the head-direction signal which helps encode the animal s direction in the context of the environment (Taube, 2007). The nucleus is divided into the lateral and medial parts, which have different connections. The medial nucleus receives information from the septum, supramammillary nuclei, ventral tegmental area, entorhinal cortex, and the hippocampus, whereas the lateral nucleus receives input from the septum, supramammillary nuclei, dorsal tegmental nucleus, and the hippocampus, but it is assumed that the two nuclei are synergistic (Vann and Aggleton, 2004). Unfortunately, the resolution of the
159 143 autoradiogram did not allow for dissection of this nucleus, although in the capuchin monkey, TCAP-1 peptide has been found in the lateral but not medial MN (Casatti and Torres, personal communications). Lesions of the MN increase open arm entries in the EPM (Beracochea and Krazem, 1991), increase activity in the open field (Field et al., 1978), and impair memory in the radial-arm maze and the Morris water maze (Vann and Aggleton, 2004). These results are similar to those seen after hippocampal lesion (Bannerman et al., 2004) in which ventral lesions decrease anxiety-like behaviour and dorsal lesions impair spatial learning. Behaviourally, injections of TCAP-1 results in modulation of the stress response (see Chapter 2), and this suggests that TCAP-1 could have a role in spatial reasoning via uptake in this region. The inferior colliculus (IC) is one of the largest midbrain structures that receives multimodal auditory information from the cochlear nuclei in the brainstem and sends ipsilateral and contralateral connections to the medial geniculate nucleus (mgn). Electrical stimulation of the IC elicits arousal, freezing, and escape behaviours in a dose-dependent manner in rats (Brandão et al., 2003), suggesting that the IC is intimately involved in the responses to a spectrum of stress severity. Moreover, injections of excitatory amino acids into the IC, enough to stimulate freezing behaviour, increase the amplitude of neuron potentials in the IC, similar to a response seen after the presentation of a conditioned stimuli (light) or ultrasonic signals (Brandão et al., 2001). It has been suggested that the IC, in addition to the dorsal periaqueductal grey, is responsible for the brain aversion system which controls defensive behaviours, such as vigilance, freezing, risk-assessment, and escape (Brandão et al., 2003). The mgn is a thalamic relay between the amygdala, thalamus, IC, and auditory cortex. The mgn also has indirect and direct connections to the amygdala, which are required for the conditioned fear response (LeDoux et al., 1990; LeDoux, 1993). Lesions of the mgn abolish HPA activation to noxious auditory stimuli, but not to restraint or ether (Campeau et al., 1997). Injections of TCAP-1 directly into the BLA modulated anxiety-like behaviour in the acoustic startle test (Wang et al., 2005) whereas i.c.v. injections of TCAP-1 decrease startle both in the absence (Wang et al., 2005) or presence of a stressor (Tan et al., 2008). The BLA is downstream of mgn input to modulate fear, suggesting that the decrease in the startle response may be a result of i.c.v. TCAP- 1 reaching the mgn in addition to BLA modulation. Indeed, the BLA is required for synaptic plasticity in the mgn in response to auditory fear conditioning (Maren et al., 2001). The cells of the mgn are also sensitive to stressors, as chronic stressors have been shown to induce dendritic
160 144 atrophy in the mgn without altering auditory fear conditioning (Dagnino-Subiabre et al., 2009). That TCAP-1 appears to be taken up in both the IC and mgn suggests that TCAP-1 may be modulating both acoustic startle and defensive behaviours (see Chapter 2) via modulation of both these auditory inputs. [ 125 I]-labelling of TCAP-1 is a less disruptive method of peptide labelling because of the comparatively small size of the iodine addition. This would be expected to have less of an effect on transport and binding in cells compared to a larger label such as FITC. This method also uses a native form of TCAP-1, and does not require substitution of lysine residues to attach the FITC tag. However, [ 125 I] is an indirect method and we cannot be sure how much of the radioactive signal is associated with the nuclide per se or digested fragments of the peptide. On the other hand, the FITC label may provide more direct evidence of TCAP-1 localization because FITC by itself does not significantly label regions in the brain (Al Chawaf et al., 2007) and in vitro the FITC-labelled regions in cells show co-localization with immunoreactive peptide indicating that the peptide is intact (Chand et al., manuscript in preparation). FITC labels are also superior to radioiodination in that higher resolution images can be obtained from brain sections and no special radioactive protocols need be in place to perform the experiments. Additional studies using a comparable dose of i.c.v. FITC-[K 8 ]-TCAP-1 will need to be completed, as the current study indicates that TCAP-1 does reach brain parenchyma. Previous work has demonstrated that TCAP-1 has numerous effects in the brain; however, if a mechanism exists to transport peripheral TCAP-1 to the brain, this suggests that there must be some interaction between TCAP-1 in the periphery and TCAP-1 in the brain. At this time, it is unknown whether there is transport of TCAP-1 out of the brain into the blood, like the saturable system that exists for CRF. In addition to its numerous effects in the brain, CRF has important stress-related effects in the periphery. CRF receptors are richly expressed in the gastrointestinal tract (Larauche et al., 2009) and mediate gut motility during stress. In fact, the CRF 1 receptor has been implicated in gastrointestinal diseases such as irritable bowel syndrome. Therefore, TCAP-1 could also have peripheral effects in body tissues, perhaps separate from its effects in the brain; however, it is unknown whether the gastrointestinal tract expresses TCAP-1. There is high TCAP-1 immunoreactivity in the testes and ovaries, particularly in cell types that have high proliferative rates, such as the spermatagonia and spermatocytes in the testes, and the granulosa cells and oocytes of the ovaries (Chand et al., manuscript in preparation). Stress mediators, such
161 145 as CRF and glucocorticoids, interfere with tissues secreting sex steroids (Rivier and Rivest, 1991), inhibiting reproduction during periods of stress. TCAP-1 may therefore be interacting with the hypothalamic-pituitary-gonadal (HPG) axis, modulating the effects of these stress mediators at the level of the gonads but also feeding back and affecting centres in the brain governing reproduction. Alternatively, TCAP-1 release from the gonads to the brain may mediate stress sensitivity during receptive periods to affect reproductive behaviours. In summary, the data detailed in this chapter indicates that peripherally-administered [ 125 I]- TCAP-1 enters the bloodstream and is most likely rapidly eliminated via the renal and gastrointestinal systems. Like other bioactive peptides that pass through the BBB, only a small fraction of the peptide entered the brain. There, [ 125 I]-TCAP-1 was taken up by structures in the limbic system as well as elements of the dopaminergic system and auditory processing relays. 5.6 References Al Chawaf A, Xu K, Tan L, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2007) Corticotropinreleasing factor (CRF)-induced behaviors are modulated by intravenous administration of teneurin C-terminal associated peptide-1 (TCAP-1). Peptides 28(7): Amalric M, Koob GF. (1993) Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res 99: Bailey GS. (1994) Labeling of peptides and proteins by radioiodination. Methods Mol Biol 32: Banks WA, Audus KL, Davis TP. (1992) Permeability of the blood-brain barrier to peptides: an approach to the development of therapeutically useful analogs. Peptides 13(6): Banks WA, Kastin AJ. (1998) Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19(5): Banks WA. (2006) The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav 89(4): Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. (2004) Regional dissociations within the hippocampus memory and anxiety. Neurosci Biobehav Rev 28(3): Beracochea DJ, Krazem A. (1991) Effects of mammillary body and mediodorsal thalamic lesions on elevated plus maze exploration. Neuroreport 2(12): Brandão ML, Coimbra NC, Osaki MY. (2001) Changes in the auditory-evoked potenitals induced by fear-evoking stimulations. Physiol Behav 72(3):
162 146 Brandão ML, Troncoso AC, de Souza Silva MA, Huston JP. (2003) The relevance of neuronal substractes of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur J Pharmacol 463(1-3): Campeau S, Akil H, Watson SJ. (1997) Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mrna in the forebrain associated with audiogenic stress in rats. J Neurosci 17(15): Dagnino-Subiabre A, Muñoz-Llancao P, Terreros G, Wyneken U, Díaz-Véliz G, Porter B, Kilgard MP, Atzori M, Aboitiz F. (2009) Chronic stress induces dendritic atrophy in the rat medial geniculate nucleus: effects on auditory conditioning. Behav Brain Res 203(1): Field TD, Rosenstock J, King EC, Greene E. (1978) Behavioral role of the mammillary efferent system. Brain Res Bull 3(5): Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusal observations. Brain Res 848(1-2): Kastin AJ, Pan W. (2003) Peptide transport across the blood-brain barrier. Prog Drug Res 61: Kupferschmidt DA, Lovejoy DA, Rotzinger S, Erb S. (2011) Teneurin C-terminal associated peptide-1 blocks the effects of corticotropin-releasing factor on reinstatement of cocaine seeking and on cocaine-induced behavioural sensitization. Br J Pharmacol 162(3): Larauche M, Kiank C, Taché Y. (2009) Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 60 Suppl 7: LeDoux JE, Farb C, Ruggiero DA. (1990) Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 10(4): LeDoux JE. (1993) Emotional memory: in search of systems and synapses. Ann N Y Acad Sci 702: Leshner AI, Koob GF. (1999) Drugs of abuse and the brain. Proc Assoc Am Physicians 111(2): Maren S, Yap SA, Goosens KA. (2001) The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci 21(6): RC135. Mruk DD, Cheng CY. (2010) Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci 365(1546):
163 147 Ng, TSJ. (2010) The effect of teneurin C-terminal associated peptide (TCAP)-1: Protection against hypoxic-stress and regulation of brain-derived neurotrophic factor (BDNF) in immortalised hypothalamic N38 cells. M.Sc. Thesis. University of Toronto. Rivier C, Rivest S. (1991) Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 45(4): Self DW. (1998) Neural substractes of drug craving and relapse in drug addiction. Ann Med 30(4): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2008) Repeated intracerebral teneurin C-terminal associated peptide (TCAP)-1 injections produce enduring changes in behavioral responses to corticotropin-releasing factor (CRF) in rat models of anxiety. Behav Brain Res 188(1): Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009) Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201(1): Taube JS. (2007) The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci 30: Trubiani G, Al Chawaf A, Belsham DD, Barsyte-Lovejoy D, Lovejoy DA. (2007) Teneurin carboxy (C)-terminal associated peptide-1 inhibits alkalosis-associated necrotic neuronal death by stimulating superoxide dismutase and catalase activity in immortalized mouse hypothalamic cells. Brain Res 116: Vann SD, Aggleton JP. (2004) The mammillary bodies: two memory systems in one? Nat Rev Neurosci 5(1): Wang L, Rotzinger S, Al Chawaf A, Elias CF, Barsyte-Lovejoy D, Qian X, Wang NC, De Cristofaro A, Belsham D, Bittencourt JC, Vaccarino F, Lovejoy DA. (2005) Teneurin proteins possess a carboxy terminal sequence with neuromodulatory activity. Brain Res Mol Brain Res 133(2): Wise, RA. (1987) The role of reward pathways in the development of drug dependence. Pharmacol Ther 35(1-2):
164 148 6 Chapter Six: Significance of findings and conclusions 6.1 Abstract The studies completed in this thesis have advanced the knowledge of TCAP-1 in vivo. I determined that intracerebral TCAP-1 can modulate anxiety-like behaviours in rodent exploratory tests of anxiety, depending on the level of stress perceived by the rat. Next, I elucidated the areas in the brain where TCAP-1 binds and is active in blocking CRF-induced neuronal activation, such as within the limbic system and brainstem nuclei. Finally, I determined that administration of TCAP-1 can remodel dendritic spines in the hippocampus, an important stress-sensitive and regulatory centre of the HPA axis, without affecting the amygdala. Although TCAP-1 has structural similarity to the CRF family of peptides, TCAP-1 itself may not induce a stress response, instead lowering the threshold of the stimulus required to initiate stress behaviours, resulting in what may seem like paradoxical behaviour. Furthermore, the results of this thesis provide a set of clear questions to further investigate the role of TCAP-1 in stress, memory, and behavioural adaptation. Possible experimental approaches have been proposed that could tackle these new questions. 6.2 The neuroanatomy of TCAP-1 The neuroanatomy involved in the stress response is understandably a very complex network of interconnected nuclei that activate, inhibit, and disinhibit both the endocrine and autonomic outputs of the brain to integrate sensory information and mount the correct response to harmful stimuli. CRF, glucocorticoids, and monoamines all participate in this integration, but a new player, namely TCAP-1, provides another level of endogenous control on specific stressregulating nodes in the brain.
165 Figure 6.1: Neuroanatomy of TCAP-1 within stress circuitry. TCAP-1 is acutely active in areas of the limbic system and the brainstem (yellow arrows) where it decreases the activation of stress-sensitive nuclei that have direct or indirect connections to the PVN. GABAergic (red lines) and glutamatergic (green lines) input into PVN-projecting areas, such as the BnST and peri- PVN, which themselves are GABAergic, modulate input from the limbic areas. Monoaminergic input from the brainstem (grey arrows) project to both PVN-projecting areas as well as the mpfc, but its CRF-induced activation from the PVN may be blunted by TCAP-1. Adapted from Tan and Lovejoy,
166 150 Based on the studies completed in this research, along with emerging new evidence on the mechanism of TCAP-1, I propose a mechanism for the actions of TCAP-1. Once peripheral TCAP-1 has crossed the BBB, or has been released in the brain, TCAP-1 is taken up by the hippocampus, amygdala, cortex, and dopaminergic and serotonergic nuclei. Under normal conditions, TCAP-1 increases spine density in the hippocampus but not the amygdala, although it does not activate c-fos-mediated processes. However, during times of stress, such as during the release of CRF in the brain, which mimics the onset of a stress-like episode, TCAP-1 blocks the effect of CRF in the mpfc, hippocampus, amygdala, and dorsal raphe (Figure 6.1). This may result in a decrease of the glutamatergic input from the mpfc and hippocampus to areas such as the BnST and peri-pvn, which send GABAergic connections to the PVN. Additionally, TCAP-1 may decrease GABAergic input from the amygdala to the BnST and peri-pvn area and serotonergic input from the dorsal raphe to the peri-pvn area, perhaps through activation of a different set of neurons in these relay areas than those innervated by inhibitory inputs. As TCAP-1 administration did not decrease activation of the BnST as a whole, it is possible that decreased glutamatergic input to the BnST from the mpfc and hippocampus compensated for a decrease in GABAergic input from the amygdala, resulting in unabated PVN activation. But because each of these brain areas is involved in different aspects of the behavioural stress response, TCAP-1 may increase the sensitivity to stimuli which would alter behavioural, rather than HPA axis output. Although the neuroanatomy of TCAP-1 suggests a role in glutamate and GABA modulation, it was not determined directly as a part of my work. Previous studies indicate that TCAP-1 can modulate the actions of glutamate on immortalized hypothalamic cells (Trubiani, 2008). However, further studies will need to be performed to confirm such a mechanism. 6.3 TCAP-1: An enigmatic peptide During the time in which my thesis research was performed, a set of recent data has become available that helps conceptualize the conditions under which TCAP-1 can be released. TCAP-1 can be expressed as a separate transcript from the teneurin transcript, as cdna probes directed towards the TCAP-1 sequence revealed two separate bands in a Northern blot, representing a full-length teneurin transcript and a shorter TCAP transcript roughly the size of the terminal exon (Chand et al., manuscript in preparation). This suggests that although the TCAPs have been
167 151 associated with the teneurins, TCAP-1 could be independently transcribed from the TCAP sequence that is associated with the teneurins. Recent immunohistochemistry data support this theory. E14 immortalized hippocampal cells were labelled with antibodies generated against epitopes within the TCAP-1 sequence, and compared with labelling from antibodies for teneurin just upstream of the TCAP-1 sequence; whereas the TCAP-1 antiserum predominantly labelled the cytosol, the teneurin antiserum labelled the plasma membrane. Although there was some overlap, there were areas of distinct TCAP-1-only signals (Chand et al., manuscript in preparation). Endogenous TCAP-1 does not appear to be sequestered in vesicles, and it is possible that TCAP-1 is transcribed by free ribosomes, which is common in peptides that are soluble in the cytosol, such as fibroblast growth factor (FGF)-1 (Jackson et al., 1992). Thus, a reservoir of cytosolic TCAP-1 exists in these cells expressing endogenous TCAP-1. Addition of fluorescently-labelled FITC-[K 8 ]-TCAP-1 to either E14 or primary hippocampal cells shows that FITC-[K 8 ]-TCAP-1 is readily taken up by neurons in a punctate-like manner, and in some cases, the FITC signal can be found in the nucleus (Chand et al., manuscript in preparation). The mechanism for this uptake has not been elucidated as yet, although it is not clathrin-dependent (Chand and Song, unpublished findings). Before the evolution of multicellular organisms and circulatory systems, one of the simplest forms of communication between cells was the paracrine release of molecules into the media in which the cells were living, which would reach the target cells via diffusion. These chemical signals could be released under a variety of conditions, including upon necrotic cell death, when the cellular membrane would degenerate and the cytosolic contents would be released into the surrounding area. Recent data has indicated that TCAP-1 has neuroprotective effects under both alkalotic and hypoxic conditions. Under conditions of high ph, TCAP-1 upregulated superoxide dismutase-1 as well as the copper chaperone, inhibiting necrosis in the N38 neuronal cell line (Trubiani et al., 2007). Additionally, TCAP-1 increased cell proliferation of N38 cells under 1% oxygen relative to vehicle-treated controls (Ng, 2010). Thus, TCAP-1 could be acting upon neighbouring cells as a paracrine signal that cells around it were perishing so as to upregulate survival pathways in order to survive (Figure 6.2).
168 152 Figure 6.2: Model of TCAP-1 s intracellular effects. Exogenous TCAP-1 binds to a membrane-bound receptor and is taken up in a punctate-like manner. Exogenous TCAP-1 upregulates neuroprotective mechanisms, such as superoxide dismutase (SOD), SOD copper chaperone (SDCC), and catalase, and the modulation of BDNF expression and release. TCAP-1 upregulates α-actinin and β-tubulin, which increases cytoskeletal proteins. Endogenous TCAP-1 in the cytosol binds to elongation factor-1 (EF-1), which helps anchor actin mrna to the cytoskeleton to produce new dendritic spines. Adapted from Tan and Lovejoy, 2009.
169 153 These signals could also have major effects on the cytoskeleton, stimulating the neurons in a network to prepare for a decrease in number, thus preparing new connections to compensate for a loss of connectivity. TCAP-1 affected neurite outgrowth in N38 cultures, modulating neurite number under normal (Al Chawaf et al., 2007a) and hypoxic conditions (Ng, 2010). Furthermore, TCAP-1 modulated neurite length, increasing the length of long neurites and decreasing the length of short neurites (Al Chawaf et al., 2007a). It appears that TCAP-1 is vital for this reorganization of neurites in the neuron. Knockdown of all four TCAPs using sirna resulted in a decrease in neurite number (Trubiani, 2008). These effects could only be accomplished via remodelling of the neuronal cytoskeleton. TCAP-1 increased β-actin and β- tubulin cytoskeletal proteins and increased α-actinin-4 and β-tubulin transcription in vitro (Al Chawaf et al., 2007a). Interestingly, administration of TCAP-1 on E14 hippocampal neurons caused a reorganization of β-tubulin-immunoreactive fibres from a cytosolic scaffold to the periphery of the cell (Chand and Lovejoy, personal communications). This indicates that TCAP- 1, in addition to protecting the cell from a potential insult, could be promoting active mechanisms to maintain network integrity. 6.4 TCAP-1 and CRF: Preparing the brain for stress In the brain, the perception of a stressor, whether real or imagined, produces a cascade of signals throughout autonomic and neuroendocrine pathways to prepare the body for the insult. Increasing the concentration of a neuroprotective peptide could therefore modify the activity of these systems to modulate the effect of the incoming stimuli. CRF is released both in the PVN to the HPA axis as well as within central brain circuits like the limbic system and brainstem. Acute TCAP-1 on its own, although it had no effects on c-fos protein, blocked CRF-induced c-fos immunoreactivity in several regulatory areas that project to the PVN and hence the HPA axis (Chapter 3). However, TCAP-1 did not affect recruitment of the PVN neurons, suggesting that TCAP-1 could act independently of the HPA axis. Previously, i.v. TCAP-1 did not affect i.v. CRF-induced corticosterone release (Al Chawaf et al., 2007b), indicating that TCAP-1 does not interfere with CRF 1 receptors in the pituitary. On the other hand, central CRF 1 receptors may have different responsibilities that are independent of HPA axis, as conditional knockout of forebrain CRF 1 receptors decreased anxiety-like behaviour independently of the HPA axis
170 154 (Müller et al., 2003). Therefore, TCAP-1 may be blocking CRF 1 receptor activity without affecting HPA axis recruitment. In contrast to acute administration, repeated administration of TCAP-1 in a pre-treatment protocol did not affect CRF-induced c-fos immunoreactivity (Chapter 3). C-Fos protein has low basal expression, increases rapidly after stimuli, and returns to basal levels 4 hours after an insult (Bittencourt and Sawchenko, 2000). Therefore, TCAP-1 s effects on c-fos may only extend to this short window, and not one week after TCAP-1 administration, instead producing long-term effects of TCAP-1 administration downstream of the c-fos pathway. Acute and repeated administration of TCAP-1 could have different effects on CRF-induced c-fos. In both the acute and repeated treatments, TCAP-1 increased the variability in the group treated with both TCAP-1 and CRF. This suggests that there may be individual differences in the responses of rats to TCAP-1 under stress; some rats had consistently high c-fos levels, whereas others had consistently low c-fos levels. TCAP-1 could be modulating stress responses in the brain, polarizing the responses to either potentiate or attenuate a response, depending on the rat. Over time, repeated exposure to mediators of stress produces profound effects on the brain. Chronic exposure to corticosterone remodels neurons in the hippocampus (Morales-Medina et al., 2009) but exposure to mild stressors, such as our model of repeated restraint, may induce compensatory measures to maintain connectivity. Prolonged exposure to a peptide released during cell death could prepare the brain for additional insults by increasing the connections in a network. Repeated administration of TCAP-1 increased spine density in the hippocampus without affecting the amygdala (Chapter 4). Prolonged exposure to stressors also affected spine density, as repeated restraint appeared to increase spine density with or without exogenous TCAP-1. In these rats, restraint treatment decreased dendritic branching relative to nonrestrained rats in the apical tree, whereas TCAP-1 increased branching in the apical tree in restrained rats relative to saline-treated restrained rats (Al Chawaf, 2008). Total spine density changes as a function of dendritic length and an increase of spines may compensate for decreased dendritic branching (Radley and Morrison, 2005). Therefore, in saline-treated and restrained rats, spine density in the apical tree would effectively remain the same, whereas in TCAP-1-treated and restrained rats, spine density in the apical tree would be increased. The mechanism for increased spine density remains elusive (Figure 6.2). As mentioned previously, TCAP-1 remodels the cytoskeleton, and in addition, recent evidence has suggested that TCAP-1
171 155 can bind to elongation factor-1 (Chand and Song, personal communications), which helps anchor β-actin mrna to the cytoskeleton (Liu et al., 2002) and is found in the apical dendrites of the hippocampus (Huang et al., 2005). CRF is released from the interneurons of the hippocampus (Chen et al., 2001; 2004a), where it is well positioned to be released after stressful stimuli, and knockout of the CRF 1 receptor results in increased dendritic arborisation (Chen et al., 2004b). Therefore, blockade of CRF by TCAP-1 administration (Chapter 3), at least acutely, could account for increased branching in the hippocampus. 6.5 TCAP-1 in stress behaviours: My spider sense is tingling The ability to actively perceive and cope with potentially harmful situations is no doubt beneficial for survival. In a new environment, animals are constantly faced with approachavoidance conflict: whether to explore the new environment and gain valuable information or remain protected but ignorant to its surroundings. The stress state of the animal may play a factor. For example, in a new environment, low levels of CRF will be behaviourally activating, increasing exploration; whereas under high levels, CRF will inhibit exploration (Dunn and Berridge, 1990). A peptide that prepares the brain for stressors could therefore promote different coping mechanisms by increasing the exploratory drive in a novel environment (active coping) and increasing protective and defensive behaviours (passive coping) in a particularly harmful environment. In the absence of a stressor, TCAP-1 appeared to have mild anxiolytic effects in the elevated plus maze (EPM) and open field (OF) (Chapter 2). TCAP-1 appeared to increase directed exploratory behaviour and increased open arm exploration when TCAP-1 was given repeatedly. As the open spaces in the EPM and OF induce a stress response, TCAP-1 could have lowered the stimulus threshold necessary to initiate active coping behaviours, enhancing the effects of a low-stress environment that culminated in increased exploratory behaviour. Although acute TCAP-1 on its own did not affect c-fos expression (Chapter 3), it is possible that TCAP-1 may have blocked the effects of CRF released in response to the behavioural test, as the open spaces can produce an HPA axis response (Pellow et al., 1985). In the presence of a stressor, TCAP-1 appeared to have anxiogenic effects in the EPM and OF tests. Acutely, TCAP-1 had no effect on CRF-induced behaviours, although TCAP-1 attenuated CRF-activation of the limbic system (Chapter 3). However, repeated exposure to TCAP-1
172 156 promoted stress-like behaviours. Five days of TCAP-1 pre-treatment or 10 days of repeated TCAP-1 treatment produced behaviours where rats avoided open spaces and decreased exploratory behaviour. As TCAP-1 release in the brain may signal brain distress or injury, the effects of repeated TCAP-1 may be compounded. This increase in anxiety-like behaviour may be adaptive over time, as continued exposure to a peptide that elicits a prepared state, including increased hippocampal connectivity (Chapter 4), but not necessarily a stressed state, could make an organism more aware of injurious cues. Instead, TCAP-1 may lower the threshold to initiate passive coping behaviours in the presence of a stressor, such as decreased exploration and xenophobia. Previous studies have shown that TCAP-1 can have modulatory effects on stress behaviour. I.v. administration of TCAP-1 modulated CRF-induced behaviours in the EPM and OF, depending on the route of administration of CRF. TCAP-1 potentiated the effects of i.v. CRF but attenuated the effects of i.c.v. CRF. I.v. CRF does not appreciably cross the blood-brain barrier (BBB; Chrousos et al., 1985), although a saturable transport system across the BBB exists (Kastin and Pan, 2003). On the other hand, TCAP-1 does cross the BBB (Chapter 5; Al Chawaf et al., 2007b), but in very small quantities. I.v. TCAP-1 did not alter HPA axis induction (Al Chawaf et al., 2007b), and i.v. CRF mainly affects the pituitary CRF 1 receptors, suggesting that behavioural effects may have been the result of the central effects of TCAP-1. However, the dose of TCAP-1 that reached the brain is considerably lower than concentrations that were delivered i.c.v. in the above experiments. As TCAP-1 appears to exert its pharmacological effects via a dose-dependent inverse-u shaped curve (Wang et al., 2005), low central concentrations of TCAP-1 may have different effects than the higher doses seen in the i.c.v. TCAP-1 experiments. In the acoustic startle test, two studies demonstrated that i.c.v. TCAP-1 has anxiety-reducing effects. On its own, pre-treatment of TCAP-1 for 5 days decreased average startle both in the absence (Wang et al., 2005) and presence of CRF (Tan et al., 2008). In response to noxious stimuli such as loud noise, like the results from the EPM and OF, TCAP-1 could have promoted passive coping styles, such as freezing and decreased reactivity to the environment (Bandler et al., 2000). This passive coping behaviour, which can occur in response to stressors that are inescapable, may culminate in decreased startle. Thus, TCAP-1 may be enhancing the switch between coping styles from active to passive depending on the level of stress, hence driving the
173 157 organism towards what may seem like paradoxical behaviour in these behavioural tests. This effect may be mediated by the trafficking of CRF receptors, as TCAP-1 may have an effect on the proportion of CRF 1 and CRF 2 receptors at the cell membrane. For example, in the serotonergic neurons of the dorsal raphe nucleus, exposure to stress induces an internalization of CRF 1 receptors from the membrane to the cytosol and the trafficking of CRF 2 receptors from the cytosol to the membrane (Waselus et al., 2009). As the CRF 1 receptor activation generally promotes active coping behaviours, and CRF 2 receptor activation generally promotes passive coping behaviours, altered trafficking of the CRF receptors may account for enhancements of both active and passive coping styles. 6.6 Stress as a risk factor for disease: Too much of a good thing The stress response is essential for the survivability of an organism, and hence its genes, and is understandably a complex and deeply regulated system. The ability of an organism to cope with perturbations to homeostasis put it at a considerable advantage over those that cannot and therefore endogenous systems that mediate allostatic mechanisms, or stability through change (McEwen, 2008), are retained and conserved across species. However, continued exposure to even sub-threshold but damaging stimuli can overload a taxed system ( allostatic overload ). We see the consequences of this in the human population: the inability to deal with chronic stressors is a risk factor for mental, neurodegenerative, cardiovascular, and gastrointestinal diseases (McEwen, 2008). A prolonged or exaggerated response to stress causes damage to the brain, which may be a factor in the development of brain diseases. For example, chronic stress can induce hippocampal atrophy and a decrease in neurogenesis (Bremner, 2006; McEwen and Magariños, 1997), whereas small hippocampal sizes have been correlated with major depressive disorder and posttraumatic stress disorder (Bonne et al., 2008; Campbell et al., 2004). Furthermore, chronic activation of the CRF 1 receptor increases phosphorylation of tau proteins, a prerequisite for the formation of β-amyloid plaques that are prevalent in Alzheimer s disease pathology (Rissman et al., 2007). Therefore, understanding the mechanisms that change the stress response from adaptive to maladaptive is vital to discovering new therapies, and can be accomplished by investigating the component parts of the stress response. The TCAP family provides a new thread in the network of endogenous stress mediators. Although many questions remain as to how endogenous TCAP behaves, it is becoming clear that
174 158 TCAP s role in the brain is an important one. Its influence may extend beyond the stress response to development, metabolism, and reproduction, and can therefore be a novel therapeutic target for numerous economically significant diseases. 6.7 Future directions Throughout this thesis I have highlighted many of the findings that have advanced the knowledge of TCAP-1 in vivo. However, many questions remain as to how TCAP-1 may seemingly polarize the behavioural response to stress, and as to what other effects TCAP-1 has on stress behaviours, such as learning and memory How do the effects of endogenous TCAP-1 differ from synthetic TCAP-1? Much of the early work on TCAP-1 has centred on the use of the synthetic peptide in both in vitro and in vivo experiments. However, as can be seen in experiments with i.c.v. Ucn 1, central injection can affect the whole brain, not only places where the endogenous peptide is released (Jones et al., 1998; Spiga et al., 2006). Therefore, i.c.v. TCAP-1 may be acting on sites where TCAP-1 is not normally found or released. Although the administration of TCAP-1 will no doubt be an important strategy in determining its role of in regulating CRF- and stress-related behaviours, deciphering the role of endogenous TCAP-1 in the brain will also be required. TCAP-1 antisera and 35 S-TCAP-1 antisense probes have been successful in locating TCAP-1 distribution in the brain; however, these techniques can also be used to detect changes in TCAP- 1 immunoreactivity or TCAP-1 mrna expression in response to psychological or physical stressors. Additionally, these experiments may reveal the conditions upon which endogenous TCAP-1 is released in the brain. The advent of new TCAP-1 over-expressing immortalized cells (Barsyte-Lovejoy and Lovejoy, personal communications) as well as TCAP-1 sirna (Trubiani, 2008) will enable us to discern the role of endogenous TCAP-1 in mediating intracellular processes, whereas TCAP-1 sirna could be microinfused into the hippocampus, amygdala, septum, or BnST to determine if endogenous TCAP-1 is mediating stress behaviour via the limbic system.
175 Does TCAP-1 affect HPA axis regulation? Systemic TCAP-1 does not appear to affect HPA axis activation (Al Chawaf et al., 2007b), although this does not discount the possibility that higher concentrations of central TCAP-1 may affect HPA axis output by affecting PVN-regulating areas such as the mpfc, hippocampus, or amygdala. Therefore, it will be important to determine whether acute or repeated i.c.v. injections of TCAP-1 can alter HPA axis output (ACTH or corticosterone), and whether central TCAP-1 can block HPA axis recruitment in response to psychological stimuli, such as restraint or social defeat, that utilize forebrain processing before its relay to the PVN. TCAP-1 appears to be most effective under multiple administrations of the peptide. Additional strategies such as longer dose regimens may enhance the effects seen in my experiments. Another possibility is the use of osmotic minipumps, usually used to continually administer a drug over time. TCAP-1 has a short half-life in serum, and daily i.c.v. injections expose the organism to daily peaks of high TCAP-1 concentration. Administration by minipump would facilitate a steady concentration of TCAP-1 for the duration of the regimen. This strategy may simulate an increased basal level of release rather than intermittent release modelled by daily injection. Multiple administrations of TCAP-1 have produced the strongest behavioural effects. These effects last long after the peptide has been cleared from the bloodstream, indicating that longterm modifications have taken place. Whereas this could have occurred via remodelling of hippocampal dendritic spines, it is possible that TCAP-1 administration could have affected expression of a variety of receptors, including CRF 1, CRF 2, GR, or MR. Although TCAP-1 does not bind to CRF receptors (Nock, 2009; Wang et al., 2005), TCAP-1 could alter the expression of these receptors; for example, the expression patterns of CRF 1 receptors and immunoreactivity patterns of TCAP-1 overlap in areas such as the cortex, cerebellum, hippocampus, BLA, and pontine grey. TCAP-1 could upregulate CRF 1 receptors in these areas under conditions of TCAP-1 release (for example, an ischemic insult in which there is neural trauma), modulating a stress response, depending on the area in which CRF 1 receptor is upregulated. The GR:MR ratio has been implicated in regulating stress responsiveness and adaptation (Oitzl et al., 1997). Whereas the GR is important in long-term memory processes, the MR appears to be implicated in short-term processes and novel behaviour (Oitzl et al., 1997). A higher GR:MR
176 160 ratio could result in increased responsiveness to stressors, whereas a lower GR:MR ratio could result in increased resilience and neuroprotection (Joëls and de Kloet, 1994). As the hippocampus highly expresses both GR and MR, it is the ideal place to investigate whether endogenous TCAP-1 and these receptors co-localize, and whether the GR:MR ratio in hippocampal cells changes in response to TCAP-1 administration Does TCAP-1 have a role in learning and memory? Recent immunohistochemical work in both rat and monkey has supported c-fos data indicating that the hippocampus is an important site for TCAP-1 activity. In the rat, TCAP-1 immunoreactivity is widespread in the hippocampus, with strong signals in the CA3 and CA2, a moderate signal in the CA1 and a weaker signal in the DG (Chand et al., manuscript in preparation). Interestingly, TCAP-1 antisera labelled dendritic branches in the apical tree of the CA1-CA3 subfields of the hippocampus (Casatti and Torres, personal communications) where the effects of TCAP-1 on increasing spine density were their greatest, with little immunoreactivity in the basilar tree (Figure 6.3). In the capuchin monkey (Cebus apella), TCAP-1 immunoreactivity is also found in the apical fibres of the hippocampal pyramidal neurons (Casatti and Torres, personal communications), with no immunoreactivity in the basilar tree. The hippocampus has an established role in inhibiting the HPA axis and a role in stress behaviours, but perhaps its strongest influence is in spatial reasoning, learning, and memory. TCAP-1 s strong presence in the hippocampus, especially in the dorsal hippocampus, indicates that TCAP-1 could have a role in the processing and retention of salient information, perhaps promoting behavioural adaptation after continued exposure to a stimulus. Future work could utilize a variety of hippocampal-dependent rodent memory tasks, such as the 12-arm radial maze or the Morris water maze (Sharma et al., 2010) in which intrahippocampal administration of synthetic TCAP-1 or TCAP-1 sirna could be used to investigate the roles of high TCAP-1 and low TCAP-1 on spatial learning. Other types of learning, such as fear conditioning (Pitts et al., 2009) are dependent upon the CeA for memory consolidation but not for the expression of fear. The CeA contains CRFimmunoreactive cell bodies and fibres but does not express CRF receptors. As TCAP-1 attenuated the activation of the CeA, future studies could investigate whether microinjections of
177 161 TCAP-1 could interfere with consolidation of fear memories, uncoupling the relationship between the conditioned and unconditioned stimuli. Figure 6.3: Photomicrographs of TCAP-1 immunoreactivity in hippocampal neurons from rat and capuchin monkey (Cebus apella). Left: Rat brain labelled with TNR408 antisera (1:1700) with nickel-enhanced DAB (magnification = 400x). Note that TCAP is found in the pyramidal layer (Py), stratum lucidum (SL), stratum radiatum (SR) and stratum lacunosummoleculare (SLM) but not the stratum oriens (SO). Right: Monkey brain labelled with TNR408 antisera (1:1700) with nickel-enhanced DAB (magnification = 160x). Note the absence of pyramidal soma labelling in the Py layer, but positive labelling in the dendritic fibres of the SR and SLM. Photomicrographs are courtesy of Prof. Claudio Casatti and Kelly Torres, Universidade Estadual Paulista (UNESP), Brazil. 6.8 Conclusions In closing, these investigations have shown that TCAP-1, a newly-elucidated, endogenous, and highly-conserved peptide, has numerous in vivo effects that build upon recent in vitro data. My studies have confirmed a role for TCAP-1 in stress behaviours and brain plasticity, but the results suggest further roles in learning and memory. TCAP-1 could indeed be an allostatic mediator, limiting the allostatic overload that results in damage to the brain, and increasing protective behavioural strategies to ensure survivability. Thus, the data presented here lay a foundation of work that have both furthered our understanding of this enigmatic peptide and posed questions as to the nature of its irreplaceable functions in the body.
Stress and the aging brain
 Stress and the aging brain Stress and the aging brain: What are the issues? Aging makes us less able to adjust to change Reactions of elderly to change generate stress Stress response involves acute reactions
Stress and the aging brain Stress and the aging brain: What are the issues? Aging makes us less able to adjust to change Reactions of elderly to change generate stress Stress response involves acute reactions
Neurobiology of Addiction
 Neurobiology of Addiction Domenic A. Ciraulo, MD Director of Alcohol Pharmacotherapy Research Center for Addiction Medicine Department of Psychiatry Massachusetts General Hospital Disclosure Neither I
Neurobiology of Addiction Domenic A. Ciraulo, MD Director of Alcohol Pharmacotherapy Research Center for Addiction Medicine Department of Psychiatry Massachusetts General Hospital Disclosure Neither I
Stress and Emotion. Stressors are things that challenge homeostasis -- these challenges may be real or merely anticipated
 Stress and Emotion 1 Stressors are things that challenge homeostasis -- these challenges may be real or merely anticipated Stress responses are what the body does about it 2 1 Two broad stressor categories
Stress and Emotion 1 Stressors are things that challenge homeostasis -- these challenges may be real or merely anticipated Stress responses are what the body does about it 2 1 Two broad stressor categories
The Role of Oxytocin in the Stress and Anxiety Response
 The Role of Oxytocin in the Stress and Anxiety Response by Rose C. Mantella BS, Allegheny College, 1998 Submitted to the Graduate Faculty of the School of Pharmacy in partial fulfillment of the requirements
The Role of Oxytocin in the Stress and Anxiety Response by Rose C. Mantella BS, Allegheny College, 1998 Submitted to the Graduate Faculty of the School of Pharmacy in partial fulfillment of the requirements
Chemical Control of Behavior and Brain 1 of 9
 Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Investigation of the role of nesfatin-1/nucb2 in the central nervous system. Ph.D. thesis Katalin Könczöl
 Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Central Neurocircuitry Functioning during the Wake-Sleep Cycle
 Chapter 1 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Central Neurocircuitry Functioning during the Wake-Sleep Cycle The
Chapter 1 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Central Neurocircuitry Functioning during the Wake-Sleep Cycle The
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Stress and Disease. Chapter 8. Elsevier items and derived items 2008 by Mosby, Inc., an affiliate of Elsevier Inc.
 Stress and Disease Chapter 8 Stress A person experiences stress when a demand exceeds a person s coping abilities, resulting in reactions such as disturbances of cognition, emotion, and behavior that can
Stress and Disease Chapter 8 Stress A person experiences stress when a demand exceeds a person s coping abilities, resulting in reactions such as disturbances of cognition, emotion, and behavior that can
Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003
 Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003 Name: Student #: BEFORE YOU BEGIN!!! 1) Count the number of pages in your exam. The exam is 8 pages long; if you do not
Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003 Name: Student #: BEFORE YOU BEGIN!!! 1) Count the number of pages in your exam. The exam is 8 pages long; if you do not
ZNZ Advanced Course in Neuroscience Mon Limbic System II. David P. Wolfer MD
 ZNZ Advanced Course in Neuroscience Mon 05.05.2014 Limbic System II David P. Wolfer MD Institute of Anatomy, University of Zurich Institute for Human Movement Sciences and Sport, ETH Zurich http://www.dpwolfer.ch
ZNZ Advanced Course in Neuroscience Mon 05.05.2014 Limbic System II David P. Wolfer MD Institute of Anatomy, University of Zurich Institute for Human Movement Sciences and Sport, ETH Zurich http://www.dpwolfer.ch
processes in the central nervous system (CNS), affecting many of the during the course of ethanol treatment. Ethanol stimulates the release of
 INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Page 1 L 58. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems /2013 RETICULAR FORMATION
 Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Hompes Method. Practitioner Training Level II. Lesson Thirty-one The Adrenals
 Hompes Method Practitioner Training Level II Lesson Thirty-one The Adrenals Health for the People Ltd not for reuse without expressed permission Hompes Method is a trading name of Health For The People
Hompes Method Practitioner Training Level II Lesson Thirty-one The Adrenals Health for the People Ltd not for reuse without expressed permission Hompes Method is a trading name of Health For The People
NEUROBIOLOGY ALCOHOLISM
 NEUROBIOLOGY ALCOHOLISM THERE HAS BEEN A MAJOR THEORETICAL SHIFT IN MEDICATION DEVELOPMENT IN ALCOHOLISM Driven by animal models of intermittent ethanol administration followed by termination, then access
NEUROBIOLOGY ALCOHOLISM THERE HAS BEEN A MAJOR THEORETICAL SHIFT IN MEDICATION DEVELOPMENT IN ALCOHOLISM Driven by animal models of intermittent ethanol administration followed by termination, then access
Hypothalamus. Marco Celio Mai 2010
 Hypothalamus Marco Celio Mai 2010 Historical Plates from the seventh book of the first edition (1543) of the Fabrica by Andreas Vesalius, showing what is believed to be the oldest anatomical images in
Hypothalamus Marco Celio Mai 2010 Historical Plates from the seventh book of the first edition (1543) of the Fabrica by Andreas Vesalius, showing what is believed to be the oldest anatomical images in
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
Hypothalamus. lies below the hypothalamic sulcus. includes the following ventral surface structures:
 Hypothalamus I. Overview The Hypothalamus is a division of the diencephalon. lies within the floor and ventral part of the walls of the third ventricle, functions primarily in the maintenance of homeostasis.
Hypothalamus I. Overview The Hypothalamus is a division of the diencephalon. lies within the floor and ventral part of the walls of the third ventricle, functions primarily in the maintenance of homeostasis.
Central catecholamine pathways in stress reactions
 Central catecholamine pathways in stress reactions Palkovits Miklós Semmelweis University, Budapest 2016 Selye János (1907-1982) In 1936, when this definition was formulated, we knew of only three objective
Central catecholamine pathways in stress reactions Palkovits Miklós Semmelweis University, Budapest 2016 Selye János (1907-1982) In 1936, when this definition was formulated, we knew of only three objective
Autonomic Nervous System and Hypothalamus
 Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Lu Chen LSA room 201 Phone: (510) 643-8163 Email: luchen@berkeley.edu Office hours: M,W,F, 10-11 am 1 Autonomic Nervous System and Hypothalamus Lu Chen, Ph.D. MCB, UC Berkeley 2 Hypothalamus Brain stem
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Brain Mechanisms of Emotion 1 of 6
 Brain Mechanisms of Emotion 1 of 6 I. WHAT IS AN EMOTION? A. Three components (Oately & Jenkins, 1996) 1. caused by conscious or unconscious evaluation of an event as relevant to a goal that is important
Brain Mechanisms of Emotion 1 of 6 I. WHAT IS AN EMOTION? A. Three components (Oately & Jenkins, 1996) 1. caused by conscious or unconscious evaluation of an event as relevant to a goal that is important
Copyright 2017 The Guilford Press
 This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
PHYSIOLOGY AND MAINTENANCE Vol. III - Glucocorticoids and Brain - Natalia E. Ordyan and Vera G. Shalyapina
 GLUCOCORTICOIDS AND BRAIN Natalia E. Ordyan and Vera G. Shalyapina Pavlov Institute of Physiology, Russian Academy of Sciences, St.-Petersburg, Russia. Keywords: Adaptation, hypothalamic-pituitary-adrenal
GLUCOCORTICOIDS AND BRAIN Natalia E. Ordyan and Vera G. Shalyapina Pavlov Institute of Physiology, Russian Academy of Sciences, St.-Petersburg, Russia. Keywords: Adaptation, hypothalamic-pituitary-adrenal
PSYCH 260 Exam 2. March 2, Answer the questions using the Scantron form. Name:
 PSYCH 260 Exam 2 March 2, 2017 Answer the questions using the Scantron form. Name: 1 1 Main Please put in their proper order the steps that lead to synaptic communication between neurons. Begin with the
PSYCH 260 Exam 2 March 2, 2017 Answer the questions using the Scantron form. Name: 1 1 Main Please put in their proper order the steps that lead to synaptic communication between neurons. Begin with the
Orexin and Sleep. Team: A Little Bit of Leptin
 Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
CNS Control of Food Intake. Adena Zadourian & Andrea Shelton
 CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
Introduction to Systems Neuroscience. Nov. 28, The limbic system. Daniel C. Kiper
 Introduction to Systems Neuroscience Nov. 28, 2017 The limbic system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html LIMBIC SYSTEM The term limbic system mean
Introduction to Systems Neuroscience Nov. 28, 2017 The limbic system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html LIMBIC SYSTEM The term limbic system mean
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
9.14 Class 32 Review. Limbic system
 9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Stress and autonomic dysfunction
 Stress and autonomic dysfunction Autonomic dysfunction during critical illness Djillali ANNANE, Hôpital Raymond Poincaré (AP-HP) Université de Versailles SQY, Garches Host response to stress Cannon (1930)
Stress and autonomic dysfunction Autonomic dysfunction during critical illness Djillali ANNANE, Hôpital Raymond Poincaré (AP-HP) Université de Versailles SQY, Garches Host response to stress Cannon (1930)
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
Stress, memory and the amygdala
 Stress, memory and the amygdala Benno Roozendaal, Bruce S. McEwen and Sumantra Chattarji Abstract Emotionally significant experiences tend to be well remembered, and the amygdala has a pivotal role in
Stress, memory and the amygdala Benno Roozendaal, Bruce S. McEwen and Sumantra Chattarji Abstract Emotionally significant experiences tend to be well remembered, and the amygdala has a pivotal role in
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior
 Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
Chapter 26. Stress and Associated Problems
 Chapter 26 Stress and Associated Problems Stress Over 40% of adults experience adverse effects from stress. 2 The History of Stress 1935 Hans Selye conducted studies to see if he could find a new sex hormone
Chapter 26 Stress and Associated Problems Stress Over 40% of adults experience adverse effects from stress. 2 The History of Stress 1935 Hans Selye conducted studies to see if he could find a new sex hormone
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
4/23/2018. Endocrine System: Overview. Endocrine System: Overview
 Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
Art labeling Activity: Figure 16.1
 ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
HANS SELYE DESERVES much of the credit for introducing
 0163-769X/01/$03.00/0 Endocrine Reviews 22(4):502 548 Printed in U.S.A. Copyright 2001 by The Endocrine Society Stressor Specificity of Central Neuroendocrine Responses: Implications for Stress-Related
0163-769X/01/$03.00/0 Endocrine Reviews 22(4):502 548 Printed in U.S.A. Copyright 2001 by The Endocrine Society Stressor Specificity of Central Neuroendocrine Responses: Implications for Stress-Related
The Endocrine System. I. Overview of the Endocrine System. II. Three Families of Hormones. III. Hormone Receptors. IV. Classes of Hormone Receptor
 The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy
 Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Course Booklet. We have felt the pain that Neuroscience is giving you.
 Exams Stressing You Out? Take Action! Course Booklet NEUR 1202 Carleton University* *TranscendFinals is not affiliated with the university We have felt the pain that Neuroscience is giving you. Our mission
Exams Stressing You Out? Take Action! Course Booklet NEUR 1202 Carleton University* *TranscendFinals is not affiliated with the university We have felt the pain that Neuroscience is giving you. Our mission
The Emotional Nervous System
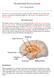 The Emotional Nervous System Dr. C. George Boeree Emotion involves the entire nervous system, of course. But there are two parts of the nervous system that are especially significant: The limbic system
The Emotional Nervous System Dr. C. George Boeree Emotion involves the entire nervous system, of course. But there are two parts of the nervous system that are especially significant: The limbic system
Neurology and Trauma: Impact and Treatment Implications Damien Dowd, M.A. & Jocelyn Proulx, Ph.D.
 Neurology and Trauma: Impact and Treatment Implications Damien Dowd, M.A. & Jocelyn Proulx, Ph.D. Neurological Response to a Stressor Information from the senses goes to the thalamus which sends the information
Neurology and Trauma: Impact and Treatment Implications Damien Dowd, M.A. & Jocelyn Proulx, Ph.D. Neurological Response to a Stressor Information from the senses goes to the thalamus which sends the information
Class 16 Emotions (10/19/17) Chapter 10
 Class 16 Emotions (10/19/17) Chapter 10 Notes By: Rashea Psych 302 10/19/17 Emotions The issues o Innate or learned? o Voluntary or involuntary? (conscious/unconscious) o Adaptive behavior or communication?
Class 16 Emotions (10/19/17) Chapter 10 Notes By: Rashea Psych 302 10/19/17 Emotions The issues o Innate or learned? o Voluntary or involuntary? (conscious/unconscious) o Adaptive behavior or communication?
Chapter 20. Endocrine System Chemical signals coordinate body functions Chemical signals coordinate body functions. !
 26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki
 TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
The Endocrine response to Stress. Dr. Sajeda Al-Chalabi Assistant Professor Head of Dept. Of Physiology
 The Endocrine response to Stress Dr. Sajeda Al-Chalabi Assistant Professor Head of Dept. Of Physiology The Physiology of Stress A series of neural and chemical reactions meant for physical survival
The Endocrine response to Stress Dr. Sajeda Al-Chalabi Assistant Professor Head of Dept. Of Physiology The Physiology of Stress A series of neural and chemical reactions meant for physical survival
Hypothalamic Control of Posterior Pituitary
 Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Internal Regulation II Energy
 Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
MOLECULAR BIOLOGY OF DRUG ADDICTION. Sylvane Desrivières, SGDP Centre
 1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
Motivation 1 of 6. during the prandial state when the blood is filled
 Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
74. Hormone synthesis in the adrenal cortex. The glucocorticoids: biosynthesis, regulation, effects. Adrenal cortex is vital for life!
 74. Hormone synthesis in the adrenal cortex. The glucocorticoids: biosynthesis, regulation, effects. Adrenal cortex is vital for life! 5 g each Zona glomerulosa : Mineralocorticoids ALDOSTERON Zona fasciculata:
74. Hormone synthesis in the adrenal cortex. The glucocorticoids: biosynthesis, regulation, effects. Adrenal cortex is vital for life! 5 g each Zona glomerulosa : Mineralocorticoids ALDOSTERON Zona fasciculata:
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Nsci 2100: Human Neuroanatomy 2017 Examination 3
 Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Chapter 2: Studies of Human Learning and Memory. From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D.
 Chapter 2: Studies of Human Learning and Memory From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Medium Spiny Neuron A Current Conception of the major memory systems in the brain Figure
Chapter 2: Studies of Human Learning and Memory From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Medium Spiny Neuron A Current Conception of the major memory systems in the brain Figure
Neurobiology of Aggression and Violence: Systems, Intervention, and Impact
 Neurobiology of Aggression and Violence: Systems, Intervention, and Impact Neal G. Simon, Ph. D. Professor Dept. of Biological Sciences Lehigh University Outline: Goals 1. Overview 2. Regulatory Systems
Neurobiology of Aggression and Violence: Systems, Intervention, and Impact Neal G. Simon, Ph. D. Professor Dept. of Biological Sciences Lehigh University Outline: Goals 1. Overview 2. Regulatory Systems
Monday, 7 th of July 2008 ( ) University of Buea MED30. (GENERAL ENDOCRINOLOGY) Exam ( )
 .. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
.. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
Chapter 18: Endocrine Glands
 Chapter 18: Endocrine Glands I. Functions of the Endocrine System A. List and describe the eight major functions of the endocrine system: 1. 2. 3. 4. 5. 6. 7. 8. Page 1 of 19 C II. Pituitary Gland and
Chapter 18: Endocrine Glands I. Functions of the Endocrine System A. List and describe the eight major functions of the endocrine system: 1. 2. 3. 4. 5. 6. 7. 8. Page 1 of 19 C II. Pituitary Gland and
Classes of Neurotransmitters. Neurotransmitters
 1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
Chronic Stress, Neurotransmitter Plasticity, and Body Weight
 Chronic Stress, Neurotransmitter Plasticity, and Body Weight A dissertation submitted to the Division of Research and Advanced Sciences of the University of Cincinnati In partial fulfillment of the requirement
Chronic Stress, Neurotransmitter Plasticity, and Body Weight A dissertation submitted to the Division of Research and Advanced Sciences of the University of Cincinnati In partial fulfillment of the requirement
8/26/13. Announcements
 Announcements THM questions will start for points on Wednesday. Make sure you are registered correctly! Problems registering for BioPortal? Make sure you are using the link from the syllabus or FAQ. 30
Announcements THM questions will start for points on Wednesday. Make sure you are registered correctly! Problems registering for BioPortal? Make sure you are using the link from the syllabus or FAQ. 30
Unit 3: The Biological Bases of Behaviour
 Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Cortisol, stress and robustness in cattle. Pierre MORMEDE Génétique, Physiologie et Systèmes d Elevage GenPhySE UMR1388 Castanet-Tolosan
 Cortisol, stress and robustness in cattle Pierre MORMEDE Génétique, Physiologie et Systèmes d Elevage GenPhySE UMR1388 Castanet-Tolosan CORTISOL IS NOT STRESS CORTISOL AND STRESS CORTISOL AND ROBUSTNESS
Cortisol, stress and robustness in cattle Pierre MORMEDE Génétique, Physiologie et Systèmes d Elevage GenPhySE UMR1388 Castanet-Tolosan CORTISOL IS NOT STRESS CORTISOL AND STRESS CORTISOL AND ROBUSTNESS
Chapter 11 - Endocrine System
 Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Strategies to Gain Acceptance for Wellness/ Mind-Body Programs by Skeptical Residents and Clinical Faculty: Stress Physiology
 Strategies to Gain Acceptance for Wellness/ Mind-Body Programs by Skeptical Residents and Clinical Faculty: Stress Physiology Michael D. Lumpkin, PhD Professor of Integrative Physiology and Biochemistry
Strategies to Gain Acceptance for Wellness/ Mind-Body Programs by Skeptical Residents and Clinical Faculty: Stress Physiology Michael D. Lumpkin, PhD Professor of Integrative Physiology and Biochemistry
Exam 2 PSYC Fall (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
 Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07)
 NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
BAPTIST HEALTH SCHOOL OF NURSING NSG 3036A: PSYCHIATRIC-MENTAL HEALTH HEALTH PROMOTION: STRESS ADAPTATION AND CONCEPTS OF PSYCHOBIOLOGY
 BAPTIST HEALTH SCHOOL OF NURSING NSG 3036A: PSYCHIATRIC-MENTAL HEALTH HEALTH PROMOTION: STRESS ADAPTATION AND CONCEPTS OF PSYCHOBIOLOGY LECTURE OBJECTIVES: 1. Define adaptation and maladaptation. 2. Describe
BAPTIST HEALTH SCHOOL OF NURSING NSG 3036A: PSYCHIATRIC-MENTAL HEALTH HEALTH PROMOTION: STRESS ADAPTATION AND CONCEPTS OF PSYCHOBIOLOGY LECTURE OBJECTIVES: 1. Define adaptation and maladaptation. 2. Describe
Stress: The Good, Bad, and the Ugly Part One. Catherine Nelson, Ph.D. University of Utah
 Stress: The Good, Bad, and the Ugly Part One Catherine Nelson, Ph.D. University of Utah Cathy.nelson@utah.edu Course Overview: Stress Session One Definitions Physiology Toxic Stress Risk factors for experiencing
Stress: The Good, Bad, and the Ugly Part One Catherine Nelson, Ph.D. University of Utah Cathy.nelson@utah.edu Course Overview: Stress Session One Definitions Physiology Toxic Stress Risk factors for experiencing
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone
 Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Acetylcholine (ACh) Action potential. Agonists. Drugs that enhance the actions of neurotransmitters.
 Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Autonomic nervous system
 Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
An Introduction to Neural Systems
 C H A P T E R 1 An Introduction to Neural Systems 1.1 INTRODUCTION The purpose of this book is to provide a comprehensive understanding of the neurobiology of social behavior in mammals, including humans.
C H A P T E R 1 An Introduction to Neural Systems 1.1 INTRODUCTION The purpose of this book is to provide a comprehensive understanding of the neurobiology of social behavior in mammals, including humans.
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Biology 218 Human Anatomy
 Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Endocrine system. Coordination & regulation Glands Hormones
 Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
Endocrine system Coordination & regulation Glands Hormones Endocrine system structures Anatomy - Dispersed system of glands that communicate with each other & all body cells via hormones. Endocrine glands:
Hormonal regulation of. Physiology Department Medical School, University of Sumatera Utara
 Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
The Central Nervous System I. Chapter 12
 The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
Stress, Health, & Coping. Radwan Banimustafa MD
 Stress, Health, & Coping Radwan Banimustafa MD Introduction: What Is Stress? Stress a negative emotional state occurring in response to events that are perceived as taxing or exceeding a person s resources
Stress, Health, & Coping Radwan Banimustafa MD Introduction: What Is Stress? Stress a negative emotional state occurring in response to events that are perceived as taxing or exceeding a person s resources
Stress in fishes. Stress in fishes
 Stress in fishes Definitions Over the years a definition of stress has proved difficult to form A shift in normal, homeostatic, physiological processes resulting from the action of any biotic or abiotic
Stress in fishes Definitions Over the years a definition of stress has proved difficult to form A shift in normal, homeostatic, physiological processes resulting from the action of any biotic or abiotic
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis
 Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis
 Progress in Neuro-Psychopharmacology & Biological Psychiatry 29 (2005) 1201 1213 www.elsevier.com/locate/pnpbp Review article Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical
Progress in Neuro-Psychopharmacology & Biological Psychiatry 29 (2005) 1201 1213 www.elsevier.com/locate/pnpbp Review article Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical
