Received 28 December 2007/Returned for modification 9 April 2008/Accepted 27 July 2008
|
|
|
- Leonard Benson
- 6 years ago
- Views:
Transcription
1 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2008, p Vol. 46, No /08/$ doi: /jcm Copyright 2008, American Society for Microbiology. All Rights Reserved. Evaluation of the Xpert Methicillin-Resistant Staphylococcus aureus (MRSA) Assay Using the GeneXpert Real-Time PCR Platform for Rapid Detection of MRSA from Screening Specimens Angela S. Rossney, 1,2 * Celine M. Herra, 3 Gráinne I. Brennan, 4 Pamela M. Morgan, 1 and Brian O Connell 1,2,4 National MRSA Reference Laboratory, St. James s Hospital, James s St., Dublin 8, Ireland 1 ; Department of Clinical Microbiology, University of Dublin, Trinity College, St. James s Hospital, James s St., Dublin 8, Ireland 2 ; School of Biological Sciences, Dublin Institute of Technology, Kevin St., Dublin 8, Ireland 3 ; and Department of Microbiology, St. James s Hospital, James s St., Dublin 8, Ireland 4 Received 28 December 2007/Returned for modification 9 April 2008/Accepted 27 July 2008 The need for rapid methods to accurately detect methicillin-resistant Staphylococcus aureus (MRSA) is widely acknowledged, and a number of molecular assays are commercially available. This study evaluated the Xpert MRSA assay, which is run on the GeneXpert real-time PCR platform (Cepheid) for use in a clinical laboratory. The following parameters were investigated: (i) the limits of detection (LoDs) for four MRSA strains; (ii) the ability to detect isolates of MRSA from a collection representative of MRSA in Ireland since 1974 (n 114) and the ability to detect control strains with staphylococcal cassette chromosome mec types IVa (IV.1.1.1), IVb (IV.2.1.1), IVc (IV.3.1.1), IVd (IV.4.1.1), V (V.1.1.1), V T, and VI; and (iii) performance in a clinical trial with swabs from nose, throat, and groin/perineum sites from 204 patients, where results were compared with those obtained by direct and enrichment cultures. The average LoD of the four test strains was 610 CFU/ml (equivalent to 58 CFU/swab). All 114 MRSA isolates and 7 control strains tested were detected. Sensitivity, specificity, and positive and negative predictive values for clinical specimens from all sites investigated were 90%, 97%, 86%, and 98%, respectively, but throat specimens yielded poor sensitivity (75%). Sensitivity, specificity, and positive and negative predictive values for nasal specimens were 95%, 98%, 90%, and 99%, respectively. Overall, the assay was rapid and easy to perform, but performance might be enhanced by the inclusion of an equivocal interpretive category based on analysis of all available amplification data. The rapid detection of methicillin-resistant Staphylococcus aureus (MRSA) along with an early implementation of appropriate intervention has been reported to reduce the prevalence of MRSA, especially in critical care areas (1, 5, 8). In 2004, Huletsky et al. described a novel real-time PCR assay targeting DNA sequences in the region of the open reading frame orfx, where the staphylococcal cassette chromosome mec (SCCmec) integrates into the S. aureus chromosome (7). SCCmec carries the resistance determinant meca, which encodes methicillin resistance and exhibits at least six different structural types and numerous subtypes (SCCmec; T. Ito, Department of Bacteriology, School of Medicine, Juntendo University, Japan. http: // [10 November 2007, accession date]). To detect MRSA with all SCCmec types known in 2004, the assay used five forward primers designed to target sequences within SCCmec, while a sixth reverse primer and three probes were specific for orfx (7). Unlike earlier amplification techniques investigating meca, nuc, and/or fem genes, this assay could distinguish between MRSA and mixtures of methicillin-susceptible S. aureus (MSSA) and methicillin-resistant coagulase-negative staphylococci (MR-CNS) (3, 7). The assay became commercially available as the IDI-MRSA assay and is currently marketed as the BD GeneOhm MRSA assay. * Corresponding author. Mailing address: National MRSA Reference Laboratory, St. James s Hospital, James s St., Dublin 8, Ireland. Phone: Fax: arossney@stjames.ie. Published ahead of print on 6 August In 2007, a new real-time PCR MRSA assay also targeting DNA sequences in the chromosomal orfx-sccmec junction became available. This assay is the Xpert MRSA kit (Cepheid, Sunnyvale, CA), and it is used in conjunction with the GeneXpert real-time PCR platform (Cepheid). In the Xpert MRSA assay, DNA from specimens is extracted, amplified, and detected in separate chambers of single-use disposable cartridges which contain freeze-dried beads with all reagents required for the real-time process. Sample preparation time is minimal and the PCR time is 75 min. The assay is validated for use with nasal specimens taken on Copan swabs with Stuart s liquid transport medium from patients at risk for colonization with MRSA and has a quoted limit of detection (LoD) of 80 CFU per swab (Xpert MRSA assay product insert; Cepheid, Sunnyvale, CA). The present study evaluated the Xpert MRSA assay for use in a clinical laboratory. The following parameters were investigated: (i) LoD, (ii) ability to detect isolates of MRSA in a collection representative of MRSA in Ireland since 1974 and to detect isolates carrying recently described SCCmec elements, and (iii) performance in a clinical trial with swabs from nose, throat, and groin/perineum sites from 204 patients. MATERIALS AND METHODS LoD assays. LoD assays of the Xpert MRSA kit were performed using four MRSA isolates. These isolates exhibited a range of oxacillin MICs and were representative of strains recovered in Ireland, as described previously (16). LoDs were determined for 10-fold dilutions of suspensions of each strain (from 10 5 to 3285
2 3286 ROSSNEY ET AL. J. CLIN. MICROBIOL CFU/ml) adsorbed onto Copan Stuart s liquid transport medium double swabs (Copan 139C swabs; Copan Italia SPA, Brescia, Italy). Colony counts (CFU/ml) of each dilution were determined by spiral plating onto brain heart infusion agar (Oxoid CM375; Oxoid Ltd., Basingstoke, United Kingdom) by use of a Whitley automatic spiral plater (WASP; Don Whitley Scientific Ltd., Shipley, England). LoDs were determined for all four isolates in pure culture and in the presence of a mixed cocktail of bacteria consisting of 10 MSSA isolates (which were meca negative by in-house conventional PCR), five MR-CNS isolates, and single isolates of Moraxella catarrhalis, Escherichia coli, and Candida species (16). Each component of the cocktail was tested with the Xpert MRSA kit prior to use. The bacterial suspensions were also adsorbed onto Transwabs with Amies clear transport medium (MW170 Transwabs; Medical Wire and Equipment Company, Corsham, England) for subsequent culture. One Copan swab from each dilution was investigated for the presence of MRSA by use of the Xpert MRSA kit on a GeneXpert DX system, version 1.2 (Cepheid, Sunnyvale, CA), according to the manufacturer s instructions. The second Copan swab was reserved for repeat testing if required. The corresponding Transwab was cultured onto Columbia agar (LabM Lab1; International Diagnostics Group plc, Bury, United Kingdom) containing 7% (wt/vol) horse blood (BA), onto MRSA-Select chromogenic agar (CA) (Bio-Rad 63747; Bio-Rad Life Science Group, Marnes-La-Coquette, France), and into 4 ml tryptic soy broth (BD ; Becton Dickinson) containing 6.5% NaCl (salt TSB). After 18 h of incubation at 35 C, enrichment broths (10- l volumes) were subcultured onto BA and CA. All isolates from enriched and direct culture were identified as S. aureus and confirmed as MRSA as described previously (16). To express the LoD value as CFU/swab, the volume adsorbed by either Copan swabs or Transwabs was determined by measuring the difference in weights of bijoux of saline before and after adsorbance for 10 s by each of 10 Copan swabs and 10 Transwabs. The average of the 10 replicate readings was taken as the average volume adsorbed by each swab type. LoDs were recorded as the lowest concentration to give positive results by the Xpert MRSA kit or by culture. The Xpert MRSA assay defines a specimen as MRSA positive if the MRSA target has a cycle threshold (C T ) value within the valid range ( 36 cycles). The LoDs for both culture methods were calculated on the basis of growth of 1 colony on a single culture medium or 1 colony on more than one medium and were expressed as averages of the counts obtained with the four isolates. MRSA isolates and MRSA control strains. To ensure that the kit could detect MRSA strains prevalent in Ireland, 114 MRSA isolates representative of MRSA recovered in Ireland were investigated (isolate details are shown in Table 1) (18). In addition, seven control MRSA strains representing isolates carrying SCCmec IV subtypes IVa (IV.1.1.1), IVb (IV.2.1.1), IVc (IV.3.1.1), IVd (IV.4.1.1), SCCmec V (V.1.1.1), V T, and VI were tested (2, 4, 13). Isolates were prepared in saline suspensions at concentrations of 10 2 CFU/ml above the LoD of the kit (determined in the present study) and adsorbed onto Copan swabs and Transwabs. One swab of each pair of Copan swabs was tested with the Xpert MRSA assay, and the Transwab was cultured onto BA and CA. Any isolate that was negative by use of the kit was retested in pure culture and at one 10-fold-higher concentration. Clinical trial. The clinical trial was undertaken with specimens from patients attending St. James s Hospital (SJH), a large 936-bed tertiary-referral hospital catering to all specialties except maternity and pediatric services. A difficulty encountered when designing the clinical trial was that specimens processed by the Xpert MRSA assay could not be used for subsequent culture because the kit s elution reagent contains sodium hydroxide and quanidium thiocyanate. Ethical approval was obtained to take duplicate specimens from nose, throat, and groin/perineum sites from patients undergoing routine MRSA screening with Copan swabs after specimens on Transwabs had been obtained for routine diagnostic culture. Criteria for routine screening were as described previously (16). Specimens collected with Copan swabs were tested using the Xpert MRSA kit according to the manufacturer s instructions as soon as possible after collection. Specimens were tested in batches of eight by use of two four-module GeneXpert machines. If not tested immediately, specimens were stored at 4 C. Following processing, all specimens were stored at 4 C until all molecular and culture testing was complete. Specimens yielding invalid results were retested using the second Copan swab. Specimens collected with Transwabs were inoculated onto CA in the diagnostic laboratory and subsequently cultured into salt TSB. After incubation at 35 C for 18 h, 10- l volumes of salt TSB were subcultured onto BA and CA. Growth was identified as MRSA as described previously, and the presence of meca was confirmed by a conventional in-house end-point PCR assay (16). All MRSA isolates were stored at 70 C on cryoprotective beads (Protect beads; Technical TABLE 1. MRSA isolates (n 114) used to determine whether the Xpert MRSA kit could detect all strains of MRSA prevalent in Ireland MLST (no. of isolates) SCCmec type b AR type (no. of isolates) c ST5 d (10) II 07.3 d (3); 07.4 (5); 11 (2) ST5 (1) IV Unfamiliar e (1) ST8 f (11) IV or IVv 43 (8); Unfamiliar e (3 f ) ST8 (26) II or IIv 13 (7); 14 (15); New03 (3); 05 (1) ST12 (1) IV NT (1) ST22 (14) IV 06 (13); NT (1) ST30 (2) IV NT (2) ST34 (1) IV NT (1) ST36 (3) II 07.0 (2); 07.2 (1) ST45 (1) IV NT (1) ST239 (13) III or IIIv 01 (4); 09 (3); 15 (2); 44 (3); 23 (1) ST247 (3) IA 22 (2); New02 (1) ST250 (4) I or Iv 02 (4) a (6) 07 (2); New02 (1); 06 (1); SCV (2) (18) PVL-positive communityacquired MRSA (18) a, not done. b v suffixes indicate previously reported variants of SCCmec types (14, 18). c Antibiogram-resistogram (AR) types were determined from the patterns of resistance obtained when isolates were tested by disk diffusion against a panel of 23 antimicrobials as described previously (17). NT indicates AR patterns that were designated no type pending the results of DNA macrorestriction digestion analysis; PVL, Panton-Valentine leucocidin; SCV, small-colony variant. d One isolate was a double-locus variant of ST5. e Unfamiliar AR pattern. f Two isolates were single-locus variants of ST8. Service Consultants Limited, Heywood, United Kingdom). Bacterial growth from BA plates from specimens yielding positive Xpert MRSA kit results and negative MRSA culture results were also preserved at 70 C on cryoprotective beads. Discrepancies between culture and kit results were investigated by salt enrichment culture of the second Copan swab (if available). MRSA isolates recovered from specimens yielding kit-negative results were prepared in suspensions in saline at concentrations of 10 5 CFU/ml, adsorbed onto Copan swabs, and tested using the Xpert MRSA kit. Bacterial growth from BA plates from specimens which tested Xpert MRSA kit positive but from which MRSA was not recovered in culture was similarly tested to exclude the possibility that such positive results occurred as a result of MSSA or MR-CNS. Control strains. S. aureus ATCC and S. aureus ATCC were used as negative and positive controls, respectively. S. epidermidis ATCC was also used as a negative control as recommended by the manufacturer. Control isolates were prepared in saline suspensions containing 10 5 CFU/ml and adsorbed onto Copan swabs prior to testing. For determining LoDs, positive and negative controls were included with each batch of tests. When MRSA strains were tested, a negative control was included each day. During the clinical trial, a positive control was included once a week and after any batch of specimens where all specimens yielded kit-negative results. S. aureus ATCC and S. aureus ATCC were also included as negative and positive controls with each batch of CA plates. Statistics. In the clinical trial, the sensitivities, specificities, and positive and negative predictive values of the Xpert MRSA kit were calculated for all specimen types by comparison with direct culture and by comparison with enrichment culture. Because the Xpert MRSA kit detects DNA which may come from nonviable MRSA, whereas culture detects viable organisms only, Xpert MRSA kit-positive specimens that were culture negative were considered as giving possible true-positive results if the patient had been previously positive for MRSA within the last 2 years or was positive at another site. Sensitivities, specificities, and positive and negative predictive values were also calculated using these amended results for all specimen sites and for each specimen site individually. Further calculations were made when patients were grouped into those who were known to have been previously positive for MRSA and those who were not known to have been previously positive.
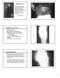
3 VOL. 46, 2008 Xpert MRSA ASSAY FOR RAPID DETECTION OF MRSA 3287 FIG. 1. Amplification data obtained with the Xpert MRSA kit for two of the four MRSA isolates used in LoD assays (A and B), two replicates of S. aureus ATCC (C and D), and two replicates of an MRSA isolate (tested at 10 6 CFU/ml) recovered from a specimen yielding a kit-negative result (E and F). All isolates demonstrated kit-negative results with appropriate amplification of the internal control. The amplification plots in panels A and B demonstrate an exponential rise in fluorescence emission that is consistent with amplification of the MRSA target, suggesting false-negative kit results. The amplification data shown in panel C are representative of results obtained with most replicates of S. aureus ATCC 25923, but on some occasions this MSSA isolate yielded a plot suggestive of MRSA target amplification (panel D). The data shown in panels E and F were obtained from an MRSA isolate tested at a bacterial concentration of 10 6 CFU/ml on two separate occasions. While both replicates yielded kit-negative results, one replicate demonstrated evidence of MRSA target amplification (panel F). RESULTS LoD assays. The average LoD value for the four MRSA test isolates in pure culture with the Xpert MRSA kit using Copan swabs was 610 CFU/ml. The average volume adsorbed by Copan swabs was 95 l/swab; hence, the average LoD/swab was 58 CFU. The amplification data obtained with two of these isolates suggested that both isolates were positive at concentrations below 58 CFU/swab, but since C T values of 36.4 and 37.1 were obtained, the kit recorded negative results (Fig. 1A and B). The average LoD values of direct and enrichment cultures using Transwabs were 750 CFU/ml and 40 CFU/ml, respectively. The average volume adsorbed by Transwabs was 228 l/swab; hence, the average LoDs of direct and enrichment cultures were 171 and 9 CFU/swab, respectively. The average LoD of the kit for the four test isolates in the presence of mixed flora was the same as the LoD obtained in pure culture (600 CFU/ml; 57 CFU/swab). During the preparation of the mixed-culture cocktail, the MSSA component yielded a kit-positive result. When the cocktail was prepared using fresh subcultures of the same 10 MSSA isolates, the cocktail tested negative and continued to test kit negative on the successive days when the LoDs of the kit were determined. However, when the cocktail was retested following completion of the LoD assays, the kit again recorded a positive result, although the accompanying amplification curve failed to demonstrate evidence of efficient amplification, suggesting that this could be a false-positive result. Conversely, although the Xpert assay always recorded negative results for
4 3288 ROSSNEY ET AL. J. CLIN. MICROBIOL. TABLE 2. Overview of results obtained with the Xpert MRSA kit and culture from 612 specimens Reference method and result No. of specimens with Xpert MRSA kit result of: Positive Negative Invalid b Total Direct culture a Positive Negative Total b 606 a Enrichment culture Positive Negative Total b 612 Amended results c Positive Negative Total b 612 c a Direct culture results were unavailable for six specimens from two patients. b Four isolates were invalid by the Xpert MRSA kit. c Amended results include numbers of specimens that were culture positive and those that were Xpert MRSA kit positive but culture negative from previously positive patients or from patients from whom a specimen from another site was positive. the MSSA control strain S. aureus ATCC (Fig. 1C), on some occasions the amplification data demonstrated evidence of amplification of MRSA target DNA with C T values just exceeding the cutoff value of 36 cycles (for example, a C T value of 37.3) and accompanying amplification curves showed an exponential rise in fluorescence emission (Fig. 1D). MRSA isolates and MRSA control strains. When this study was designed, it was intended that the MRSA isolates and MRSA control strains would be tested in the presence of the mixed cocktail of bacteria to mimic normal flora, but the inconsistent results with the MSSA component of the cocktail suggested that this would be unwise. Isolate suspensions were therefore prepared in a pure culture in saline at a concentration of 10 4 CFU/ml (i.e., 10 2 CFU/ml above the LoD determined in the present study). All MRSA isolates except two yielded positive Xpert MRSA kit results; one negative isolate was positive upon repeat testing, and the second negative isolate was positive when retested at a higher concentration (10 5 CFU/ml). All seven control MRSA strains yielded positive results. Clinical trial. Six hundred twelve specimens from 204 patients were investigated. An overview of the numbers of positive specimens obtained with the Xpert MRSA kit, with direct culture, with enrichment culture, and with amended results is shown in Table 2. By use of amended results, Xpert MRSA kit-positive specimens that were culture negative were considered to be true-positive results if the patient had been previously positive for MRSA or was positive at another site. MRSA was detected in 99 specimens (16.2%; 99/612) from 58 patients (28.4%; 58/204) by the Xpert MRSA kit. Direct culture yielded positive results from 59 specimens (9.7%; 59/606) from 37 patients (18.3%; 37/202); direct culture results were unavailable for 6 specimens from 2 patients. Specimens from 89 specimens (14.5%; 89/612) from 45 patients (22.1%; 45/204) were positive by enrichment culture. No specimen was culture positive by direct culture and negative by salt enrichment culture. When amended results were calculated, 95 specimens (15.5%; 95/612) from 47 patients (23.0%; 47/204) were deemed positive. Sixteen specimens from 14 patients yielded invalid Xpert MRSA kit results upon initial testing, but upon repeat testing only four specimen results remained invalid (throat [n 3]; groin [n 1]). MRSA isolates from all but 2 of the 19 culture-positive kit-negative specimens were kit positive when tested in pure culture at a concentration of 10 5 CFU/ml. One of the two negative isolates tested positive when retested at 10 5 CFU/ml; the other remained kit negative when retested at both 10 5 and 10 6 CFU/ml (Fig. 1E). Repeat testing of the latter isolate at 10 6 CFU/ml also yielded a negative kit result, but evidence of amplification (with a C T value of 36.7) was noted on this occasion (Fig. 1F). This isolate was meca gene positive by conventional in-house endpoint PCR. Non-MRSA bacterial growths from BA plates from specimens which were kit positive and MRSA culture negative were also tested by the kit, but growths from all of these non-mrsa cultures yielded negative results with the kit. Statistics. The sensitivity, specificity, and positive and negative predictive values of the Xpert MRSA kit compared with those obtained using direct culture, enrichment culture, and amended results are shown in Table 3. With nasal swabs, when the amended results were considered, the sensitivity of the kit was 95%. In comparison, its sensitivity for specimens from the throat was only 75%. When the Xpert MRSA kit results from specimens from all sites were compared with amended results, the overall sensitivity, specificity, and positive and negative predictive values were 90%, 97%, 86%, and 98%, respectively (Table 3). When this analysis was restricted to specimens from patients who were previously recorded as being MRSA positive, the sensitivity of the kit for nasal specimens was 93%, whereas with specimens from patients with no record of being previously positive (i.e., new MRSA patients), the sensitivity for nasal specimens was 100%. DISCUSSION Ireland has a serious problem with MRSA, and the proportion of MRSA among S. aureus blood culture isolates is among the highest in Europe (11). It has been reported that in areas where MRSA is endemic, the introduction of a full searchand-destroy approach, including screening of all patient contacts of all index cases and the institution of precautionary isolation for all such contacts, could reduce the prevalence of MRSA to 1% within 6 years (1). It has also been reported that rapid detection of carriage has an important role to play in such a search-and-destroy strategy (1). A study investigating the value of rapid diagnostic tests (RDTs) for MRSA when used for admission screening to a critical care area reported a reduction in the incidence of transmission of MRSA from 13.89/1,000 patient days to 4/1,000 patient days (5). While RDTs may play a major role in reducing the prevalence of MRSA, the chosen method must be sensitive and specific and have good positive and negative predictive values. Failure to recognize a particular strain of MRSA can have very serious consequences, as was recently experienced in The Netherlands, where phenotypic tests failed to detect a mecapositive strain with a low oxacillin MIC in one hospital. Over a
5 VOL. 46, 2008 Xpert MRSA ASSAY FOR RAPID DETECTION OF MRSA 3289 TABLE 3. Sensitivity, specificity, and positive and negative predictive values of the Xpert MRSA kit compared with those from direct and enrichment cultures from nose, throat, and groin/perineum specimens Reference method and analysis group Site d Value (%) of Xpert MRSA kit Sensitivity Specificity PPV e NPV f Direct culture All Enrichment culture All Nose Throat Groin/perineum Amended results a All patients All Nose Throat Groin/perineum Previously positive All patients b Nose Throat Groin/perineum Patients who were All not previously Nose positive c Throat Groin/perineum a For further explanation of amended results, see Table 2, footnote c. b Analysis restricted to specimens from patients who were previously MRSA positive. With these specimens, kit-positive culture-negative specimens were also included as true positives because the patients were known to have been previously positive for MRSA. c Analysis restricted to specimens from patients with no record of being previously MRSA positive. With these specimens, kit-positive culture-negative specimens were also included as true positives if the patient was positive for MRSA at another site. d All, nose, throat, and groin/perineum. e PPV, positive predictive value. f NPV, negative predictive value. 2-year period, this strain spread extensively not only within that hospital but in other hospitals also and it took over a year to control the resulting outbreaks (8). As more molecular RDTs for MRSA become available, the choice of an appropriate assay becomes more difficult. Evaluating new molecular assays is expensive and time-consuming, and it is hard to exclude confounding variables, even when assays are evaluated at the same time. For example, the present study required duplicate specimens to allow comparison between the Xpert MRSA kit and enrichment culture. Because sampling using the routine Transwab method had to be undertaken prior to sampling with Copan swabs to ensure that the routine diagnostic culture protocol was not compromised by the clinical trial, it is possible that less material was available on the Copan swabs for detection by the Xpert MRSA assay. In contrast, in an earlier study evaluating the IDI-MRSA kit on the SmartCycler platform, swabs were cultured by salt enrichment after they had been used with the kit (16). In that evaluation, however, the LoD of salt enrichment culture (by use of Transwabs) was 240 CFU/ml (55 CFU/swab), whereas the LoD of salt enrichment culture (by use of Transwabs) in the present study was 9 CFU/swab, indicating that the Xpert MRSA kit was subjected to a more rigorous evaluation. A possible explanation for the lower LoD of salt enrichment culture in the present study is that the volume of medium used for salt enrichment was 4 ml, whereas the protocol in the IDI-MRSA kit evaluation used 1-ml volumes and enrichment culture was performed after material on the swab was removed for testing with the IDI-MRSA kit. Interestingly, the inclusion of enrichment culture increased the number of culture-positive specimens by 23% in the earlier study and by 51% (30/59) in the present study (16). The Xpert MRSA assay demonstrated an average LoD (58 CFU/swab) threefold lower than that for direct culture (171 CFU/swab) but sixfold higher than that for enrichment culture (9 CFU/swab). In comparison, the earlier IDI-MRSA kit evaluation reported an average LoD value of 2,000 CFU/ml (equivalent to 190 CFU/swab), which was similar to the LoD of direct culture but 10-fold higher than the LoD of enrichment culture (16). The LoDs of direct culture with Transwabs were comparable in both studies: 171 CFU/swab in the present study and 800 CFU/ml (182 CFU/swab) in the earlier study (16). The Xpert MRSA assay detected all MRSA isolates investigated, including control isolates exhibiting SCCmec V, V T, and VI (2, 4, 13). Although not investigated here, both Xpert MRSA assay and IDI-MRSA are reported to lack sensitivity when detecting the pulsed-field gel electrophoresis-nontypeable MRSA strain associated with pigs in The Netherlands (15, 19). In the clinical trial, the Xpert MRSA assay exhibited sensitivities of 95% and 97% for detecting MRSA from nasal and groin/perineum sites but had difficulty detecting MRSA DNA from throat specimens (sensitivity, 75%). Interestingly although the previous evaluation of the IDI-MRSA kit reported lower sensitivities for nose and groin/perineum specimens (90% and 88%, respectively), specimens from the throat yielded a higher sensitivity (89%) (16). A major difficulty evaluating molecular assays for the detection of MRSA from clinical specimens is defining true-positive and -negative specimens. In the present study, data were analyzed by direct comparison with culture, but to overcome the problem of considering all kit-positive culture-negative specimens as false-positive kit results, kit performance was analyzed using amended results where kit-positive culture-negative results from patients who were previously positive for MRSA were regarded as true-positive results. By use of these amended results for comparison, the sensitivities and specificities of direct culture, enrichment culture, and the Xpert MRSA assay were 60.6% and 99.6%, 84.2% and 98.3%, 89.5% and 97.3%, respectively. However, this comparison is not completely valid and may overestimate the sensitivity of the assay and underestimate that of culture because culture-negative results from patients who have undergone successful treatment for MRSA should not be considered false-negative culture results. Despite the encouraging findings of the present study, the overall performance of the Xpert MRSA assay might be improved if result interpretation took account of all available assay data. The manufacturer s interpretation is based on the application of a C T cutoff value of 36 cycles, with specimens being recorded as MRSA positive if the C T is 36 and MRSA negative if the C T is 36, regardless of the presence or absence of evidence of amplification (the former being demonstrated by an amplification curve indicating an exponential rise in fluorescence emission). Data obtained in the present study suggested that this C T cutoff value might have introduced falsenegative results both in the LoD investigations and during the
6 3290 ROSSNEY ET AL. J. CLIN. MICROBIOL. clinical trial, where some tests (n 14) demonstrated clear evidence of MRSA amplification but C T values exceeded 36 cycles (Fig. 1A, B, and F). Conversely, false-positive results may have also occurred (with the MSSA component of the mixed-culture cocktail, e.g.) where C T values of 36 were obtained and reported as positive but amplification curves failed to demonstrate an exponential rise. The inclusion of an equivocal interpretative category for tests where the amplification data of the MRSA target DNA fail to support the C T cutoff result warrants investigation. Aside from the interpretative difficulties associated with the use of the C T cutoff value, findings from the present study also indicate that false-positive results may have occurred, due perhaps to a lack of specificity of the assay s proprietary target sequence for MRSA at the SCCmec-orfX junction. There are increasing numbers of reports of SCC elements that do not contain meca, for example, SCC cap1 in MSSA, which integrates into the SCCmec chromosomal attachment site attb at the end of orfx (9, 10, 12). Although the Xpert assay recorded negative results for the MSSA control, S. aureus ATCC 25923, on some occasions the accompanying amplification curves demonstrated evidence of amplification of target DNA (Fig. 1D), suggesting that like the IDI-MRSA assay, the Xpert MRSA kit may have the problem of detecting SCC in MSSA strains such as S. aureus ATCC 25923, which may be why the manufacturer recommends the use of S. epidermidis ATCC as the negative control (6). This problem requires further investigation. While the Xpert MRSA assay requires more interpretation than currently suggested by the manufacturer, it was found to perform well with nasal and groin/perineum specimens. It is rapid and easy to use and has the advantage of random access (i.e., an urgent specimen can be accommodated because specimens do not need to be tested in batches). Relative to culture, it is expensive, and results need to be confirmed by culture. A more extensive prospective study is warranted to determine its role in clinical practice in Ireland. A question that such a prospective clinical study might investigate is the duration of kit-positive results from specimens from patients who have been successfully treated for MRSA and whether there is a period during which previously culture-positive patients should not be screened by molecular methods. These data could inform how kit-positive results from patients who are known to have been previously positive for MRSA should be interpreted. ACKNOWLEDGMENTS We thank the participant patients, the infection control staff (Lisa Fetherstone and Catherine O Reilly), and the clinical staff at SJH for duplicate specimens. Thanks to Mary Kelleher, surveillance scientist, Microbiology Dept., SJH, for data on diagnostic specimens. We thank Biofact Ireland and Cepheid for providing the GeneXpert machines for the duration of the evaluation and for providing the Xpert MRSA kits at an evaluation price. We thank Teruyo Ito (Juntendo University, Tokyo, Japan), Robert Daum (University of Chicago, Chicago, IL), and Herminia de Lancastre (Instituto de Technologia Quimica e Biologica, Oeiras, Portugal) for the gift of control strains carrying SCCmec IV subtypes, SCCmec V, and SCCmec VI. There are no conflicting interests to declare. REFERENCES 1. Bootsma, M. C., O. Diekmann, and M. J. Bonten Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc. Natl. Acad. Sci. USA 103: Boyle-Vavra, S., B. Ereshefsky, C. C. Wang, and R. S. Daum Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type V T or SCCmec type IV. J. Clin. Microbiol. 43: Brown, D. F., D. I. Edwards, P. M. Hawkey, D. Morrison, G. L. Ridgway, K. J. Towner, and M. W. Wren Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J. Antimicrob. Chemother. 56: Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J. H. Song, and K. Hiramatsu Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50: Cunningham, R., P. Jenks, J. Northwood, M. Wallis, S. Ferguson, and S. Hunt Effect on MRSA transmission of rapid PCR testing of patients admitted to critical care. J. Hosp. Infect. 65: Desjardins, M., C. Guibord, B. Lalonde, B. Toye, and K. Ramotar Evaluation of the IDI-MRSA assay for detection of methicillin-resistant Staphylococcus aureus from nasal and rectal specimens pooled in a selective broth. J. Clin. Microbiol. 44: Huletsky, A., R. Giroux, V. Rossbach, M. Gagnon, M. Vaillancourt, M. Bernier, F. Gagnon, K. Truchon, M. Bastien, F. J. Picard, A. van Belkum, M. Ouellette, P. H. Roy, and M. G. Bergeron New real-time PCR assay for rapid detection of methicillin-resistant Staphylococcus aureus directly from specimens containing a mixture of staphylococci. J. Clin. Microbiol. 42: Kluytmans, J Control of meticillin-resistant Staphylococcus aureus (MRSA) and the value of rapid tests. J. Hosp. Infect. 65(Suppl. 2): Luong, T. T., S. Ouyang, K. Bush, and C. Y. Lee Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J. Bacteriol. 184: Mongkolrattanothai, K., S. Boyle, T. V. Murphy, and R. S. Daum Novel non-meca-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagf genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in Staphylococcus aureus. Antimicrob. Agents Chemother. 48: Murphy, O. M., S. Murchan, D. Whyte, H. Humphreys, A. Rossney, P. Clarke, R. Cunney, C. Keane, L. E. Fenelon, and D. O Flanagan Impact of the European Antimicrobial Resistance Surveillance System on the development of a national programme to monitor resistance in Staphylococcus aureus and Streptococcus pneumoniae in Ireland, Eur. J. Clin. Microbiol. Infect. Dis. 24: Noto, M. J., B. N. Kreiswirth, A. B. Monk, and G. L. Archer Gene acquisition at the insertion site for SCCmec, the genomic island conferring methicillin resistance in Staphylococcus aureus. J. Bacteriol. 190: Oliveira, D. C., C. Milheirico, and H. de Lencastre Redefining a structural variant of staphylococcal cassette chromosome mec, SCCmec type VI. Antimicrob. Agents Chemother. 50: Oliveira, D. C., A. Tomasz, and H. de Lencastre Secrets of success of a human pathogen: molecular evolution of pandemic clones of meticillinresistant Staphylococcus aureus. Lancet Infect. Dis. 2: Roosendaal, R., J. A. J. W. Kluytmans, J. H. C. Woudenberg, X. Huijsdens, and C. M. J. E. Vandenbroucke-Grauls Methicillin-resistant Staphylococcus aureus strains from animal origin are recognized by IDI-MRSA PCR. Clin. Microbiol. Infect. 13:S Rossney, A. S., C. M. Herra, M. M. Fitzgibbon, P. M. Morgan, M. J. Lawrence, and B. O Connell Evaluation of the IDI-MRSA assay on the SmartCycler real-time PCR platform for rapid detection of MRSA from screening specimens. Eur. J. Clin. Microbiol. Infect. Dis. 26: Rossney, A. S., M. J. Lawrence, P. M. Morgan, M. M. Fitzgibbon, A. Shore, D. C. Coleman, C. T. Keane, and B. O Connell Epidemiological typing of MRSA isolates from blood cultures taken in Irish hospitals participating in the European Antimicrobial Resistance Surveillance System ( ). Eur. J. Clin. Microbiol. Infect. Dis. 25: Shore, A., A. S. Rossney, C. T. Keane, M. C. Enright, and D. C. Coleman Seven novel variants of the staphylococcal chromosomal cassette mec in methicillin-resistant Staphylococcus aureus isolates from Ireland. Antimicrob. Agents Chemother. 49: Wassenberg, M. W. M., S. Nijssen, X. W. Huijsdens, A. M. C. Bergmans, C. M. J. E. Vandenbroucke-Grauls, M. J. M. Bonten, and J. A. J. W. Kluytmans Performance of the GeneXpert system for detection of methicillin-resistant Staphylococcus aureus including the non-typeable clone from animal origin. Clin. Microbiol. Infect. 13:S233-S234.
Evaluation of the Xpert MRSA Assay on the GeneXpert Real-Time PCR. Platform for Rapid Detection of MRSA from Screening Specimens ACCEPTED
 JCM Accepts, published online ahead of print on 6 August 2008 J. Clin. Microbiol. doi:10.1128/jcm.02487-07 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All
JCM Accepts, published online ahead of print on 6 August 2008 J. Clin. Microbiol. doi:10.1128/jcm.02487-07 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All
Received 3 November 2010/Returned for modification 26 December 2010/Accepted 1 February 2011
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1240 1244 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.02220-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Genotypic and
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1240 1244 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.02220-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Genotypic and
During High-Volume Clinical Use ACCEPTED. Healthcare, Evanston, IL, Northwestern University s Feinberg School of Medicine, Chicago,
 JCM Accepts, published online ahead of print on 11 July 2007 J. Clin. Microbiol. doi:10.1128/jcm.00670-07 Copyright 2007, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
JCM Accepts, published online ahead of print on 11 July 2007 J. Clin. Microbiol. doi:10.1128/jcm.00670-07 Copyright 2007, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Veerle Compernolle, Gerda Verschraegen, and Geert Claeys* Department of Microbiology, Ghent University Hospital, Ghent, Belgium
 JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 2007, p. 154 158 Vol. 45, No. 1 0095-1137/07/$08.00 0 doi:10.1128/jcm.01115-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Combined Use
JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 2007, p. 154 158 Vol. 45, No. 1 0095-1137/07/$08.00 0 doi:10.1128/jcm.01115-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Combined Use
Comparison of the BD MAX MRSA Assay and the BD GeneOhm MRSA. Achromopeptidase Assay for the Detection of Methicillin-Resistant Staphylococcus aureus
 JCM Accepts, published online ahead of print on 18 July 2012 J. Clin. Microbiol. doi:10.1128/jcm.01496-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 3 Comparison of the
JCM Accepts, published online ahead of print on 18 July 2012 J. Clin. Microbiol. doi:10.1128/jcm.01496-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 3 Comparison of the
Characterization of community and hospital Staphylococcus aureus isolates in Southampton, UK
 Journal of Medical Microbiology (2010), 59, 1084 1088 DOI 10.1099/jmm.0.018986-0 Characterization of community and hospital Staphylococcus aureus isolates in Southampton, UK S. M. Green, 1 P. Marsh, 1
Journal of Medical Microbiology (2010), 59, 1084 1088 DOI 10.1099/jmm.0.018986-0 Characterization of community and hospital Staphylococcus aureus isolates in Southampton, UK S. M. Green, 1 P. Marsh, 1
Michael Hombach, 1 * Gaby E. Pfyffer, 1 Malgorzata Roos, 2 and Katja Lucke 1
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2010, p. 3882 3887 Vol. 48, No. 11 0095-1137/10/$12.00 doi:10.1128/jcm.00670-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Detection
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2010, p. 3882 3887 Vol. 48, No. 11 0095-1137/10/$12.00 doi:10.1128/jcm.00670-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Detection
Received 4 September 2008/Returned for modification 24 October 2008/Accepted 25 December 2008
 JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 2009, p. 758 764 Vol. 47, No. 3 0095-1137/09/$08.00 0 doi:10.1128/jcm.01714-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Multicenter
JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 2009, p. 758 764 Vol. 47, No. 3 0095-1137/09/$08.00 0 doi:10.1128/jcm.01714-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Multicenter
Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus in Danish Hospitals
 J Antimicrob Chemother 2012; 67: 1775 1780 doi:10.1093/jac/dks125 Advance Access publication 20 April 2012 Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus in
J Antimicrob Chemother 2012; 67: 1775 1780 doi:10.1093/jac/dks125 Advance Access publication 20 April 2012 Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus in
Reduction in Workload and Reporting Time of MRSA Screening with MRSASelect. Medium versus Mannitol-Salt Medium Supplemented with Oxacillin ACCEPTED
 JCM Accepts, published online ahead of print on 30 January 2008 J. Clin. Microbiol. doi:10.1128/jcm.01253-07 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All
JCM Accepts, published online ahead of print on 30 January 2008 J. Clin. Microbiol. doi:10.1128/jcm.01253-07 Copyright 2008, American Society for Microbiology and/or the Listed Authors/Institutions. All
Received 21 October 2005/Returned for modification 23 December 2005/Accepted 21 May 2006
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2006, p. 2904 2908 Vol. 44, No. 8 0095-1137/06/$08.00 0 doi:10.1128/jcm.02211-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Concurrent
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2006, p. 2904 2908 Vol. 44, No. 8 0095-1137/06/$08.00 0 doi:10.1128/jcm.02211-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Concurrent
Ueli von Ah, Dieter Wirz, and A. U. Daniels*
 JOURNAL OF CLINICAL MICROBIOLOGY, June 2008, p. 2083 2087 Vol. 46, No. 6 0095-1137/08/$08.00 0 doi:10.1128/jcm.00611-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Rapid Differentiation
JOURNAL OF CLINICAL MICROBIOLOGY, June 2008, p. 2083 2087 Vol. 46, No. 6 0095-1137/08/$08.00 0 doi:10.1128/jcm.00611-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Rapid Differentiation
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Whole genome sequencing & new strain typing methods in IPC. Lyn Gilbert ACIPC conference Hobart, November 2015
 Whole genome sequencing & new strain typing methods in IPC Lyn Gilbert ACIPC conference Hobart, November 2015 Why do strain typing? Evolution, population genetics, geographic distribution 2 Why strain
Whole genome sequencing & new strain typing methods in IPC Lyn Gilbert ACIPC conference Hobart, November 2015 Why do strain typing? Evolution, population genetics, geographic distribution 2 Why strain
Retrospective and Prospective Verification of the Cepheid Xpert Flu Assay
 JCM Accepts, published online ahead of print on 20 July 2011 J. Clin. Microbiol. doi:10.1128/jcm.01162-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
JCM Accepts, published online ahead of print on 20 July 2011 J. Clin. Microbiol. doi:10.1128/jcm.01162-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil
 224 BJID 2003; 7 (June) Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil Larissa Lutz, Adão Machado, Nadia Kuplich and Afonso Luís
224 BJID 2003; 7 (June) Clinical Failure of Vancomycin Treatment of Staphylococcus aureus Infection in a Tertiary Care Hospital in Southern Brazil Larissa Lutz, Adão Machado, Nadia Kuplich and Afonso Luís
Sensitivity of Surveillance Testing for Multidrug-Resistant Gram-Negative Bacteria in the
 JCM Accepts, published online ahead of print on 20 August 2014 J. Clin. Microbiol. doi:10.1128/jcm.02369-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 Sensitivity of Surveillance
JCM Accepts, published online ahead of print on 20 August 2014 J. Clin. Microbiol. doi:10.1128/jcm.02369-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 Sensitivity of Surveillance
Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory
 Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory Dr Danièle Meunier, AMRHAI BSAC - Antimicrobial Susceptibility Testing User Days Oxazolidinone Linezolid
Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory Dr Danièle Meunier, AMRHAI BSAC - Antimicrobial Susceptibility Testing User Days Oxazolidinone Linezolid
Received 31 January 2007/Returned for modification 29 March 2007/Accepted 12 June 2007
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2007, p. 2554 2563 Vol. 45, No. 8 0095-1137/07/$08.00 0 doi:10.1128/jcm.00245-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. The Emergence
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2007, p. 2554 2563 Vol. 45, No. 8 0095-1137/07/$08.00 0 doi:10.1128/jcm.00245-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. The Emergence
Multi-clonal origin of macrolide-resistant Mycoplasma pneumoniae isolates. determined by multiple-locus variable-number tandem-repeat analysis
 JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
Genetic diversity of community-associated methicillin-resistant Staphylococcus aureus in southern Stockholm,
 ORIGINAL ARTICLE 10.1111/j.1469-0691.2007.01941.x Genetic diversity of community-associated methicillin-resistant Staphylococcus aureus in southern Stockholm, 2000 2005 H. Fang, G. Hedin, G. Li and C.
ORIGINAL ARTICLE 10.1111/j.1469-0691.2007.01941.x Genetic diversity of community-associated methicillin-resistant Staphylococcus aureus in southern Stockholm, 2000 2005 H. Fang, G. Hedin, G. Li and C.
Evaluation of Antibacterial Effect of Odor Eliminating Compounds
 Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
Received 2 October 2002/Returned for modification 29 November 2002/Accepted 7 January 2003
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2003, p. 1687 1693 Vol. 41, No. 4 0095-1137/03/$08.00 0 DOI: 10.1128/JCM.41.4.1687 1693.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2003, p. 1687 1693 Vol. 41, No. 4 0095-1137/03/$08.00 0 DOI: 10.1128/JCM.41.4.1687 1693.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
(multidrug-resistant Pseudomonas aeruginosa; MDRP)
 220 2009 (multidrug-resistant Pseudomonas aeruginosa; MDRP) 21 4 1 21 10 4 amikacin (AMK), imipenem/cilastatin (IPM), ciprofloxacin (CPFX) multidrug-resistant Pseudomonas aeruginosa (MDRP) CHROMagar TM
220 2009 (multidrug-resistant Pseudomonas aeruginosa; MDRP) 21 4 1 21 10 4 amikacin (AMK), imipenem/cilastatin (IPM), ciprofloxacin (CPFX) multidrug-resistant Pseudomonas aeruginosa (MDRP) CHROMagar TM
Evaluation of methicillin-resistant Staphylococcus aureus (MRSA) colonization in pigs and people that work with pigs in Ontario Veterinary College
 Evaluation of methicillin-resistant Staphylococcus aureus (MRSA) colonization in pigs and people that work with pigs in Ontario Veterinary College Final Report September 2007 This research has been possible
Evaluation of methicillin-resistant Staphylococcus aureus (MRSA) colonization in pigs and people that work with pigs in Ontario Veterinary College Final Report September 2007 This research has been possible
A new selective blood agar medium for Streptococcus pyogenes and other haemolytic streptococci
 J. clin. Path. (1964), 17, 231 A new selective blood agar medium for Streptococcus pyogenes and other haemolytic streptococci E. J. L. LOWBURY, A. KIDSON, AND H. A. LILLY From the Medical Research Council
J. clin. Path. (1964), 17, 231 A new selective blood agar medium for Streptococcus pyogenes and other haemolytic streptococci E. J. L. LOWBURY, A. KIDSON, AND H. A. LILLY From the Medical Research Council
An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
 Asian Biomedicine Vol. 4 No. 1 February 2010; 141-145 Brief communication (Original) An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
Asian Biomedicine Vol. 4 No. 1 February 2010; 141-145 Brief communication (Original) An evaluation of 2.0 McFarland Etest method for detection of heterogeneous vancomycin-intermediate Staphylococcus aureus
ANNUAL REPORT National Meticillin-Resistant Staphylococcus aureus Reference Laboratory
 ANNUAL REPORT 2015 National Meticillin-Resistant Staphylococcus aureus Reference Laboratory INTRODUCTION... 2 ROLE OF THE LABORATORY... 3 SERVICES... 3 ISOLATES... 3 ROUTINE LABORATORY WORK... 6 REFERENCE
ANNUAL REPORT 2015 National Meticillin-Resistant Staphylococcus aureus Reference Laboratory INTRODUCTION... 2 ROLE OF THE LABORATORY... 3 SERVICES... 3 ISOLATES... 3 ROUTINE LABORATORY WORK... 6 REFERENCE
Received 30 March 2005; returned 16 June 2005; revised 8 September 2005; accepted 12 September 2005
 Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Western Australian Methicillin-Resistant Staphylococcus aureus (MRSA) and Vancomycin Resistant Enterococcus (VRE) Epidemiology and Typing Report
 Australian Collaborating Centre for Enterococcus and Staphylococcus Species (ACCESS) Typing and Research Microbiology and Infectious Diseases Department PathWest Laboratory Medicine-WA, Royal Perth Hospital
Australian Collaborating Centre for Enterococcus and Staphylococcus Species (ACCESS) Typing and Research Microbiology and Infectious Diseases Department PathWest Laboratory Medicine-WA, Royal Perth Hospital
OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae
 OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae I. Importance of prenatal screening strategies II. Past approaches Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory
OUTLINE Laboratory Detection and Reporting of Streptococcus agalactiae I. Importance of prenatal screening strategies II. Past approaches Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory
Infectious Disease Diagnostics in the Geisinger Health System
 10X Essentials Infectious Disease Diagnostics in the Geisinger Health System NEW: Rapid GeneXpert PCR NEW Test Codes: MRSAP, MRSA Screen PCR CDIFP, C. difficile/ Epi PCR EVP, Enterovirus PCR Specimens
10X Essentials Infectious Disease Diagnostics in the Geisinger Health System NEW: Rapid GeneXpert PCR NEW Test Codes: MRSAP, MRSA Screen PCR CDIFP, C. difficile/ Epi PCR EVP, Enterovirus PCR Specimens
Exploring the evolution of MRSA with Whole Genome Sequencing
 Exploring the evolution of MRSA with Whole Genome Sequencing PhD student: Zheng WANG Supervisor: Professor Margaret IP Department of Microbiology, CUHK Joint Graduate Seminar Department of Microbiology,
Exploring the evolution of MRSA with Whole Genome Sequencing PhD student: Zheng WANG Supervisor: Professor Margaret IP Department of Microbiology, CUHK Joint Graduate Seminar Department of Microbiology,
Laboratory Detection and Reporting of Streptococcus agalactiae
 Laboratory Detection and Reporting of Streptococcus agalactiae Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory Milwaukee, Wisconsin The presenter states no conflict of interest and has
Laboratory Detection and Reporting of Streptococcus agalactiae Erik Munson Clinical Microbiology Wheaton Franciscan Laboratory Milwaukee, Wisconsin The presenter states no conflict of interest and has
GBS Screening, diagnosis and clinically relevant resistance
 GBS Screening, diagnosis and clinically relevant resistance Pierrette Melin National Reference Centre for GBS Medical Microbiology, University Hospital of Liege 1 Background Evolution of culture methods
GBS Screening, diagnosis and clinically relevant resistance Pierrette Melin National Reference Centre for GBS Medical Microbiology, University Hospital of Liege 1 Background Evolution of culture methods
Bacterial Survival In Synovial Fluid: Is S. aureus in the Knee Joint Persisting Despite Antibiotic Treatment?
 Bacterial Survival In Synovial Fluid: Is S. aureus in the Knee Joint Persisting Despite Antibiotic Treatment? Sana Dastgheyb 1, Sommer Hammoud 2, Constantinos Ketonis, MD 3, James Purtill, MD 3, Michael
Bacterial Survival In Synovial Fluid: Is S. aureus in the Knee Joint Persisting Despite Antibiotic Treatment? Sana Dastgheyb 1, Sommer Hammoud 2, Constantinos Ketonis, MD 3, James Purtill, MD 3, Michael
RAPID REDUCTION OF STAPHYLOCOCCUS AUREUS POPULATIONS ON STAINLESS STEEL SURFACES BY ZEOLITE CERAMIC COATINGS CONTAINING SILVER AND ZINC IONS
 RAPID REDUCTION OF STAPHYLOCOCCUS AUREUS POPULATIONS ON STAINLESS STEEL SURFACES BY ZEOLITE CERAMIC COATINGS CONTAINING SILVER AND ZINC IONS INE 2 (CAR HEADLINE 2) By: K. R. Bright C. P. Gerba P. A. Rusin
RAPID REDUCTION OF STAPHYLOCOCCUS AUREUS POPULATIONS ON STAINLESS STEEL SURFACES BY ZEOLITE CERAMIC COATINGS CONTAINING SILVER AND ZINC IONS INE 2 (CAR HEADLINE 2) By: K. R. Bright C. P. Gerba P. A. Rusin
β CARBA Test Rapid detection of carbapenemase-producing Enterobacteriaceae strains Contents 1. INTENDED USE
 β CARBA Test 25 68260 Rapid detection of carbapenemase-producing Enterobacteriaceae strains 881159 2015/05 Contents 1. INTENDED USE 2. SUMMARY AND EXPLANATION OF THE TEST 3. PRINCIPLE OF THE PROCEDURE
β CARBA Test 25 68260 Rapid detection of carbapenemase-producing Enterobacteriaceae strains 881159 2015/05 Contents 1. INTENDED USE 2. SUMMARY AND EXPLANATION OF THE TEST 3. PRINCIPLE OF THE PROCEDURE
Laboratory Evaluation of the BD MAX TM MRSA Assay
 JCM Accepts, published online ahead of print on 14 May 2014 J. Clin. Microbiol. doi:10.1128/jcm.00237-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 Laboratory Evaluation
JCM Accepts, published online ahead of print on 14 May 2014 J. Clin. Microbiol. doi:10.1128/jcm.00237-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 Laboratory Evaluation
Practical Aspects of Standardisation for a Global Controls Manufacturer
 Practical Aspects of Standardisation for a Global Controls Manufacturer MRSA SoGAT Clinical Diagnostic Meeting NIBSC, UK June 25, 2008 Frank Opdam PhD, AcroMetrix Standardisation A fundamental goal of
Practical Aspects of Standardisation for a Global Controls Manufacturer MRSA SoGAT Clinical Diagnostic Meeting NIBSC, UK June 25, 2008 Frank Opdam PhD, AcroMetrix Standardisation A fundamental goal of
Xpert CT/NG. Detect it in here. Stop it out there. Three targets, 90 minutes. No repeat testing. Xpert CT/NG. Xpert. A better way.
 / Detect it in here. Stop it out there. / Three targets, 90 minutes. No repeat testing. In Vitro Diagnostic Medical Device A better way. For the first time, we are able to offer highly accurate results
/ Detect it in here. Stop it out there. / Three targets, 90 minutes. No repeat testing. In Vitro Diagnostic Medical Device A better way. For the first time, we are able to offer highly accurate results
Typing of ônh ôstaphylococcus epidermidis ôns ô Colonizing in Human Nares by Pulsed-Field Gel Electrophoresis
 Microbiol. Immunol., 39(5), 315-319, 1995 Typing of ônh ôstaphylococcus epidermidis ôns ô Colonizing in Human Nares by Pulsed-Field Gel Electrophoresis Lan Hu*, Akiko Umeda, and Kazunobu Amako Department
Microbiol. Immunol., 39(5), 315-319, 1995 Typing of ônh ôstaphylococcus epidermidis ôns ô Colonizing in Human Nares by Pulsed-Field Gel Electrophoresis Lan Hu*, Akiko Umeda, and Kazunobu Amako Department
Comparison of flocked and rayon swabs for detection of nasal carriage of Staphylococcus aureus amongst pathology staff
 JCM Accepts, published online ahead of print on 26 May 2010 J. Clin. Microbiol. doi:10.1128/jcm.01617-09 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
JCM Accepts, published online ahead of print on 26 May 2010 J. Clin. Microbiol. doi:10.1128/jcm.01617-09 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Extreme Genetic Diversity of Methicillin-Resistant Staphylococcus epidermidis Strains Disseminated among Healthy Japanese Children
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2008, p. 3778 3783 Vol. 46, No. 11 0095-1137/08/$08.00 0 doi:10.1128/jcm.02262-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Extreme
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2008, p. 3778 3783 Vol. 46, No. 11 0095-1137/08/$08.00 0 doi:10.1128/jcm.02262-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Extreme
Pathogens in the twilight zone: Update on emerging disease issues with implications for the pork industry
 Pathogens in the twilight zone: Update on emerging disease issues with implications for the pork industry Peter Davies BVSc, PhD University of Minnesota Introduction For an industry under increasing public
Pathogens in the twilight zone: Update on emerging disease issues with implications for the pork industry Peter Davies BVSc, PhD University of Minnesota Introduction For an industry under increasing public
CONSIDERATIONS IN UTI DETECTION AND POTENTIAL IMPACT ON ANTIBIOTIC STEWARDSHIP
 CONSIDERATIONS IN UTI DETECTION AND POTENTIAL IMPACT ON ANTIBIOTIC STEWARDSHIP ERIN H. GRAF, PHD, D(ABMM) Director, Infectious Disease Diagnostics Laboratory Assistant Professor, Clinical Pathology and
CONSIDERATIONS IN UTI DETECTION AND POTENTIAL IMPACT ON ANTIBIOTIC STEWARDSHIP ERIN H. GRAF, PHD, D(ABMM) Director, Infectious Disease Diagnostics Laboratory Assistant Professor, Clinical Pathology and
In vitro assessment of dual drug combinations to inhibit growth of Neisseria gonorrhoeae
 AAC Accepted Manuscript Posted Online 26 January 2015 Antimicrob. Agents Chemother. doi:10.1128/aac.04127-14 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 In vitro assessment
AAC Accepted Manuscript Posted Online 26 January 2015 Antimicrob. Agents Chemother. doi:10.1128/aac.04127-14 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 In vitro assessment
PAMET Continuing Education 2016
 PAMET Continuing Education 2016 Agent of gastroenteritis Medium/method] used for routine screening/detection in stool samples Salmonella, Shigella, MacConkey, Hektoen, Bismuth sulfite,etc. Plesiomonas
PAMET Continuing Education 2016 Agent of gastroenteritis Medium/method] used for routine screening/detection in stool samples Salmonella, Shigella, MacConkey, Hektoen, Bismuth sulfite,etc. Plesiomonas
The importance of considering BACTERIAL LOAD in OTITIS MEDIA research
 The importance of considering BACTERIAL LOAD in OTITIS MEDIA research Heidi Smith-Vaughan, Robyn Marsh, Michael Binks, Mirjam Kaestli, Peter S Morris, Amanda J Leach OM in Indigenous children OM is endemic
The importance of considering BACTERIAL LOAD in OTITIS MEDIA research Heidi Smith-Vaughan, Robyn Marsh, Michael Binks, Mirjam Kaestli, Peter S Morris, Amanda J Leach OM in Indigenous children OM is endemic
Title: Rapid PCR Tests for MRSA in Hospitalized Patients: Diagnostic Accuracy, Clinical and Cost-Effectiveness
 Title: Rapid PCR Tests for MRSA in Hospitalized Patients: Diagnostic Accuracy, Clinical and Cost-Effectiveness Date: 23 Oct 2007 Context and Policy Issues: Staphylococcus aureus (SA), a gram positive bacterium,
Title: Rapid PCR Tests for MRSA in Hospitalized Patients: Diagnostic Accuracy, Clinical and Cost-Effectiveness Date: 23 Oct 2007 Context and Policy Issues: Staphylococcus aureus (SA), a gram positive bacterium,
Respiratory Pathogen Panel TEM-PCR Test Code:
 Respiratory Pathogen Panel TEM-PCR Test Code: 220000 Tests in this Panel Enterovirus group Human bocavirus Human coronavirus (4 types) Human metapneumovirus Influenza A - Human influenza Influenza A -
Respiratory Pathogen Panel TEM-PCR Test Code: 220000 Tests in this Panel Enterovirus group Human bocavirus Human coronavirus (4 types) Human metapneumovirus Influenza A - Human influenza Influenza A -
Bacterial Screening of Platelet Concentrates
 Bacterial Screening of Platelet Concentrates Enda Cadden Platelet Donations Very Short shelf-life
Bacterial Screening of Platelet Concentrates Enda Cadden Platelet Donations Very Short shelf-life
Staphylococci. What s to be Covered. Clinical Scenario #1
 Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Xpert MTB/RIF Ultra: Understanding this new diagnostic and who will have access to it
 Xpert MTB/RIF Ultra: Understanding this new diagnostic and who will have access to it Angela Starks, PhD Chief, Laboratory Branch Division of TB Elimination Matt Bankowski, PhD, MS, D(ABMM), HCLD/CC(ABB)
Xpert MTB/RIF Ultra: Understanding this new diagnostic and who will have access to it Angela Starks, PhD Chief, Laboratory Branch Division of TB Elimination Matt Bankowski, PhD, MS, D(ABMM), HCLD/CC(ABB)
Ken Jost, BA, has the following disclosures to make:
 Diagnosis of TB Disease: Laboratory Ken Jost, BA May 10, 2017 TB Intensive May 9-12, 2017 San Antonio, TX EXCELLENCE EXPERTISE INNOVATION Ken Jost, BA, has the following disclosures to make: No conflict
Diagnosis of TB Disease: Laboratory Ken Jost, BA May 10, 2017 TB Intensive May 9-12, 2017 San Antonio, TX EXCELLENCE EXPERTISE INNOVATION Ken Jost, BA, has the following disclosures to make: No conflict
Strep-a-Test Twister Test
 Strep-a-Test Twister Test Code: 24524 A rapid test for the qualitative detection of Strep A antigen in throat swab specimens. For professional in vitro diagnostic use only. INTENDED USE The Strep A Twist
Strep-a-Test Twister Test Code: 24524 A rapid test for the qualitative detection of Strep A antigen in throat swab specimens. For professional in vitro diagnostic use only. INTENDED USE The Strep A Twist
The Bacteriology of Bronchiectasis in Australian Indigenous children
 The Bacteriology of Bronchiectasis in Australian Indigenous children Kim Hare, Amanda Leach, Peter Morris, Heidi Smith-Vaughan, Anne Chang Presentation outline What is bronchiectasis? Our research at Menzies
The Bacteriology of Bronchiectasis in Australian Indigenous children Kim Hare, Amanda Leach, Peter Morris, Heidi Smith-Vaughan, Anne Chang Presentation outline What is bronchiectasis? Our research at Menzies
Chapter 2.1. Meticillin-resistant Staphylococcus aureus epidemiology and transmission in a Dutch hospital
 Meticillin-resistant Staphylococcus aureus epidemiology and transmission in a Dutch hospital MML van Rijen 1, T Bosch 2, MEOC Heck 2, JAJW Kluytmans 1,3 1 Laboratory for Microbiology and Infection Control,
Meticillin-resistant Staphylococcus aureus epidemiology and transmission in a Dutch hospital MML van Rijen 1, T Bosch 2, MEOC Heck 2, JAJW Kluytmans 1,3 1 Laboratory for Microbiology and Infection Control,
DIABETIC FOOT ULCERS AND Staphylococcus aureus : NEW INSIGHTS
 DIABETIC FOOT ULCERS AND Staphylococcus aureus : NEW INSIGHTS Lyon 9 décembre 2010 Some important points Prevalence of diabetes mellitus in France: 3 millions (4.5% of population, 11,2% > 65 ans) In 2000,
DIABETIC FOOT ULCERS AND Staphylococcus aureus : NEW INSIGHTS Lyon 9 décembre 2010 Some important points Prevalence of diabetes mellitus in France: 3 millions (4.5% of population, 11,2% > 65 ans) In 2000,
Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese
 Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese July 2013 By: Dr. Francisco Diez Gonzalez University of Minnesota Dr. Mastura Akhtar Partners: Midwest Dairy Association
Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese July 2013 By: Dr. Francisco Diez Gonzalez University of Minnesota Dr. Mastura Akhtar Partners: Midwest Dairy Association
Affinity of Doripenem and Comparators to Penicillin-Binding Proteins in Escherichia coli and ACCEPTED
 AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
AAC Accepts, published online ahead of print on February 00 Antimicrob. Agents Chemother. doi:./aac.01-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
The first Staphylococcus aureus isolates with reduced susceptibility to vancomycin in Poland
 Journal of Antimicrobial Chemotherapy (2002) 50, 1065 1069 DOI: 10.1093/jac/dkf252 The first Staphylococcus aureus isolates with reduced susceptibility to vancomycin in Poland Jolanta Krzysztoá-Russjan
Journal of Antimicrobial Chemotherapy (2002) 50, 1065 1069 DOI: 10.1093/jac/dkf252 The first Staphylococcus aureus isolates with reduced susceptibility to vancomycin in Poland Jolanta Krzysztoá-Russjan
Educational Workshops 2016
 Educational Workshops 2016 Keynote CPE Screening We are grateful to Dr Andrew Dodgson, Consultant Microbiologist, Public Health England and Central Manchester Hospitals NHS Foundation Trust Terminology
Educational Workshops 2016 Keynote CPE Screening We are grateful to Dr Andrew Dodgson, Consultant Microbiologist, Public Health England and Central Manchester Hospitals NHS Foundation Trust Terminology
EUROTRAVNET SCIENCE WATCH NOVEMBER-DECEMBER 2009
 www.eurotravnet.eu E u r o p e a n T r a v e l a n d T r o p i c a l M e d i c i n e N e t w o r k o f t h e I n t e r n a t i o n a l S o c i e t y o f T r a v e l M e d i c i n e E u r o p e a n C e
www.eurotravnet.eu E u r o p e a n T r a v e l a n d T r o p i c a l M e d i c i n e N e t w o r k o f t h e I n t e r n a t i o n a l S o c i e t y o f T r a v e l M e d i c i n e E u r o p e a n C e
Guidance on screening and confirmation of carbapenem resistant Enterobacteriacae (CRE) December 12, 2011
 Guidance on screening and confirmation of carbapenem resistant Enterobacteriacae (CRE) December 12, 2011 Objectives: To discuss the guidelines for detection of CRE in the laboratory setting. To review
Guidance on screening and confirmation of carbapenem resistant Enterobacteriacae (CRE) December 12, 2011 Objectives: To discuss the guidelines for detection of CRE in the laboratory setting. To review
Pseudomonas aeruginosa
 JOURNAL OF CLINICAL MICROBIOLOGY, July 1983, p. 16-164 95-1137/83/716-5$2./ Copyright C) 1983, American Society for Microbiology Vol. 18, No. 1 A Three-Year Study of Nosocomial Infections Associated with
JOURNAL OF CLINICAL MICROBIOLOGY, July 1983, p. 16-164 95-1137/83/716-5$2./ Copyright C) 1983, American Society for Microbiology Vol. 18, No. 1 A Three-Year Study of Nosocomial Infections Associated with
Steven D. Brown* and Maria M. Traczewski. The Clinical Microbiology Institute, 9725 SW Commerce Circle, Wilsonville, Oregon 97070
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
PNEUMOCOCCUS VALENT LATEX KIT
 PNEUMOCOCCUS 7-10-13-VALENT LATEX KIT Latex particles coated with pneumococcal antiserum raised in rabbits for in vitro diagnostic use Application The Pneumococcus 7-10-13-valent Latex Kit is a ready to
PNEUMOCOCCUS 7-10-13-VALENT LATEX KIT Latex particles coated with pneumococcal antiserum raised in rabbits for in vitro diagnostic use Application The Pneumococcus 7-10-13-valent Latex Kit is a ready to
Rifampin Resistance. Charlottesville, Virginia i0w organisms in Trypticase soy broth (BBL Microbiology
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
S. M. Clark*, A. Loeffler and R. Bond
 J Antimicrob Chemother 2015; 70: 2048 2052 doi:10.1093/jac/dkv056 Advance Access publication 5 March 2015 Susceptibility in vitro of canine methicillin-resistant and -susceptible staphylococcal isolates
J Antimicrob Chemother 2015; 70: 2048 2052 doi:10.1093/jac/dkv056 Advance Access publication 5 March 2015 Susceptibility in vitro of canine methicillin-resistant and -susceptible staphylococcal isolates
Chlamydia Rapid Screen Test (RAP-2858) RUO in the USA. Revised 28 Jul 2006
 INDICATION For the rapid detection of Chlamydia Trachomatis antigens in swab specimens. For in vitro diagnostic use only, except in the United States where it is intended for Research Use Only. SUMMARY
INDICATION For the rapid detection of Chlamydia Trachomatis antigens in swab specimens. For in vitro diagnostic use only, except in the United States where it is intended for Research Use Only. SUMMARY
What s to be Covered. Microbiology of staphylococci Epidemiology of S. aureus infections Pathogenesis of S. aureus infections
 Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
Staphylococci Micrococcus, which, when limited in its extent and activity, causes acute suppurative inflammation (phlegmon), produces, when more extensive and intense in its action on the human system,
New genomic typing method MLST
 New genomic typing method MLST Bon KIMURA fingerprinting PFGE DNA multilocus sequence typingmlst alleles PFGE MLST 1990 PCR 1 PCR DNA PFGE 1 PFGE RAPDrandomly amplified polymorphic DNA 3 AFLPAmplified
New genomic typing method MLST Bon KIMURA fingerprinting PFGE DNA multilocus sequence typingmlst alleles PFGE MLST 1990 PCR 1 PCR DNA PFGE 1 PFGE RAPDrandomly amplified polymorphic DNA 3 AFLPAmplified
ANNUAL REPORT National Meticillin-Resistant Staphylococcus aureus Reference Laboratory
 ANNUAL REPORT 2016 National Meticillin-Resistant Staphylococcus aureus Reference Laboratory CONTACT DETAILS National MRSA Reference Laboratory St. James s Hospital, James s Street, Dublin 8 Ireland Tel:
ANNUAL REPORT 2016 National Meticillin-Resistant Staphylococcus aureus Reference Laboratory CONTACT DETAILS National MRSA Reference Laboratory St. James s Hospital, James s Street, Dublin 8 Ireland Tel:
ANTIBACTERIAL TOOTHPASTE: DO NOT SWALLOW
 ANTIBACTERIAL TOOTHPASTE: DO NOT SWALLOW Sarah McCuaig BACKGROUND, PURPOSE, HYPOTHESES Market statistics indicate a significant increase in the use of antibacterial products in North American households.
ANTIBACTERIAL TOOTHPASTE: DO NOT SWALLOW Sarah McCuaig BACKGROUND, PURPOSE, HYPOTHESES Market statistics indicate a significant increase in the use of antibacterial products in North American households.
Dissemination of New Methicillin-Resistant Staphylococcus aureus Clones in the Community
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2002, p. 4289 4294 Vol. 40, No. 11 0095-1137/02/$04.00 0 DOI: 10.1128/JCM.40.11.4289 4294.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2002, p. 4289 4294 Vol. 40, No. 11 0095-1137/02/$04.00 0 DOI: 10.1128/JCM.40.11.4289 4294.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
Clinical Importance of Purulence in Methicillin- Resistant Staphylococcus aureus Skin and Soft Tissue Infections
 Clinical Importance of Purulence in Methicillin- Resistant Staphylococcus aureus Skin and Soft Tissue Infections Susan E. Crawford, MD, Michael Z. David, MD, PhD, Daniel Glikman, MD, Kimberly J. King,
Clinical Importance of Purulence in Methicillin- Resistant Staphylococcus aureus Skin and Soft Tissue Infections Susan E. Crawford, MD, Michael Z. David, MD, PhD, Daniel Glikman, MD, Kimberly J. King,
ALERT. Clinical microbiology considerations related to the emergence of. New Delhi metallo beta lactamases (NDM 1) and Klebsiella
 ALERT Clinical microbiology considerations related to the emergence of New Delhi metallo beta lactamases (NDM 1) and Klebsiella pneumoniae carbapenemases (KPC) amongst hospitalized patients in South Africa
ALERT Clinical microbiology considerations related to the emergence of New Delhi metallo beta lactamases (NDM 1) and Klebsiella pneumoniae carbapenemases (KPC) amongst hospitalized patients in South Africa
Case Studies, or Verification Vignettes
 Case Studies, or Verification Vignettes 1 Vignette #1 Change from One Automated AST to Another Your lab is changing from one FDA-cleared automated AST to another Is a verification study required? 2 Yes
Case Studies, or Verification Vignettes 1 Vignette #1 Change from One Automated AST to Another Your lab is changing from one FDA-cleared automated AST to another Is a verification study required? 2 Yes
LESSON 2.6 WORKBOOK Diagnosing infections, and, what s up your nose?
 Staphylococcus aureus Morphology: The physical form or structure of a microbe.. LESSON 2.6 WORKBOOK Diagnosing infections, and, what s up your nose? Now we have discussed the different requirements that
Staphylococcus aureus Morphology: The physical form or structure of a microbe.. LESSON 2.6 WORKBOOK Diagnosing infections, and, what s up your nose? Now we have discussed the different requirements that
Performance of Various Testing Methodologies for Detection of Heteroresistant Vancomycin-Intermediate Staphylococcus aureus in Bloodstream Isolates
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1489 1494 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.02302-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Performance
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2011, p. 1489 1494 Vol. 49, No. 4 0095-1137/11/$12.00 doi:10.1128/jcm.02302-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Performance
Frequency of Nasal Carriage of Staphylococcus Aureus and Its Antimicrobial Resistance Pattern in Patients on Hemodialysis
 Dialysis Frequency of Nasal Carriage of Staphylococcus Aureus and Its Antimicrobial Resistance Pattern in Patients on Hemodialysis Roya Ghasemian, 1 Narges Najafi, 1 Atieh Makhlough, 2 Mohammad Khademloo
Dialysis Frequency of Nasal Carriage of Staphylococcus Aureus and Its Antimicrobial Resistance Pattern in Patients on Hemodialysis Roya Ghasemian, 1 Narges Najafi, 1 Atieh Makhlough, 2 Mohammad Khademloo
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com FORMULATION AND EVALUATION OF HERBAL WASH FOR NASAL HEALTH
Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com FORMULATION AND EVALUATION OF HERBAL WASH FOR NASAL HEALTH
Comparison of Different Phenotypic Approaches to Screen and Detect mecc-harboring Methicillin-Resistant Staphylococcus aureus
 Edinburgh Research Explorer Comparison of Different Phenotypic Approaches to Screen and Detect mecc-harboring Methicillin-Resistant Staphylococcus aureus Citation for published version: Kriegeskorte, A,
Edinburgh Research Explorer Comparison of Different Phenotypic Approaches to Screen and Detect mecc-harboring Methicillin-Resistant Staphylococcus aureus Citation for published version: Kriegeskorte, A,
466 Biomed Environ Sci, 2014; 27(6):
 466 Biomed Environ Sci, 2014; 27(6): 466-470 Letter to the Editor Modification and Evaluation of Brucella Broth Based Campylobacter jejuni Transport Medium * BAI Yao 1,2,$, CUI Sheng Hui 3,$, XU Xiao 3,
466 Biomed Environ Sci, 2014; 27(6): 466-470 Letter to the Editor Modification and Evaluation of Brucella Broth Based Campylobacter jejuni Transport Medium * BAI Yao 1,2,$, CUI Sheng Hui 3,$, XU Xiao 3,
International Clones of Methicillin-Resistant Staphylococcus aureus in Two Hospitals in Miami, Florida
 JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 2004, p. 542 547 Vol. 42, No. 2 0095-1137/04/$08.00 0 DOI: 10.1128/JCM.42.2.542 547.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. International
JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 2004, p. 542 547 Vol. 42, No. 2 0095-1137/04/$08.00 0 DOI: 10.1128/JCM.42.2.542 547.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. International
Rapid-VIDITEST. Strep A Blister. One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture.
 Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
Healthcare-associated infections acquired in intensive care units
 SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Healthcare-associated infections acquired in intensive care units Key facts In 2015, 11 788 (8.3%) of patients staying in an intensive care unit
Adenosine Triphosphate Bioluminescence Test of the Nasal Spray Nozzles Attached to an Ear-nose-throat Treatment Unit
 Original J.TWMU Advance Publication Adenosine Triphosphate Bioluminescence Test of the Nasal Spray Nozzles Attached to an Ear-nose-throat Treatment Unit Yukie YAMAMURA, Tomoko EGAWA, Yukako SEO, and Manabu
Original J.TWMU Advance Publication Adenosine Triphosphate Bioluminescence Test of the Nasal Spray Nozzles Attached to an Ear-nose-throat Treatment Unit Yukie YAMAMURA, Tomoko EGAWA, Yukako SEO, and Manabu
Enrichment culture of CSF is of limited value in the diagnosis of neonatal meningitis
 Enrichment culture of CSF is of limited value in the diagnosis of neonatal S. H. Chaudhry, D. Wagstaff, Anupam Gupta, I. C. Bowler, D. P. Webster To cite this version: S. H. Chaudhry, D. Wagstaff, Anupam
Enrichment culture of CSF is of limited value in the diagnosis of neonatal S. H. Chaudhry, D. Wagstaff, Anupam Gupta, I. C. Bowler, D. P. Webster To cite this version: S. H. Chaudhry, D. Wagstaff, Anupam
Costs and effects of MRSA control in Dutch hospitals
 Costs and effects of MRSA control in Dutch hospitals Costs and effects of MRSA control in Dutch hospitals M.W.M. Wassenberg, de Bilt, the Netherlands Thesis, University of Utrecht, the Netherlands, including
Costs and effects of MRSA control in Dutch hospitals Costs and effects of MRSA control in Dutch hospitals M.W.M. Wassenberg, de Bilt, the Netherlands Thesis, University of Utrecht, the Netherlands, including
Report of Typing & Antimicrobial Susceptibilities of Isolates Causing Invasive Pneumococcal Disease in Ireland,
 Report of Typing & Antimicrobial Susceptibilities of Isolates Causing Invasive Pneumococcal Disease in Ireland, 2011-2013 1. Background Streptococcus pneumoniae is a major cause of life-threatening infections
Report of Typing & Antimicrobial Susceptibilities of Isolates Causing Invasive Pneumococcal Disease in Ireland, 2011-2013 1. Background Streptococcus pneumoniae is a major cause of life-threatening infections
A Norazah, S M Liew, A G M Kamel, Y T Koh, V K E Lim. O r i g i n a l A r t i c l e
 Singapore Med J 2001 Vol 42(1) : 015-019 O r i g i n a l A r t i c l e DNA Fingerprinting of Methicillin-Resistant Staphylococcus Aureus by Pulsed-Field Gel Electrophoresis (PFGE): Comparison of Strains
Singapore Med J 2001 Vol 42(1) : 015-019 O r i g i n a l A r t i c l e DNA Fingerprinting of Methicillin-Resistant Staphylococcus Aureus by Pulsed-Field Gel Electrophoresis (PFGE): Comparison of Strains
Validation of Vitek version 7.01 software for testing staphylococci against vancomycin
 Diagnostic Microbiology and Infectious Disease 43 (2002) 135 140 www.elsevier.com/locate/diagmicrobio Validation of Vitek version 7.01 software for testing staphylococci against vancomycin P.M. Raney a,
Diagnostic Microbiology and Infectious Disease 43 (2002) 135 140 www.elsevier.com/locate/diagmicrobio Validation of Vitek version 7.01 software for testing staphylococci against vancomycin P.M. Raney a,
Staphylococcus aureus Colonization and Infection in New York State Prisons
 MAJOR ARTICLE Staphylococcus aureus Colonization and Infection in New York State Prisons Franklin D. Lowy, 1,2 Allison E. Aiello, 5 Meera Bhat, 1 Vicki D. Johnson-Lawrence, 5 Mei-Ho Lee, 1 Earl Burrell,
MAJOR ARTICLE Staphylococcus aureus Colonization and Infection in New York State Prisons Franklin D. Lowy, 1,2 Allison E. Aiello, 5 Meera Bhat, 1 Vicki D. Johnson-Lawrence, 5 Mei-Ho Lee, 1 Earl Burrell,
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Rapid-VIDITEST. Strep A Card. One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture.
 Rapid-VIDITEST Strep A Card One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec,
Rapid-VIDITEST Strep A Card One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec,
GeneXpert System. On-Demand Molecular Testing for the Physician Office Laboratory
 On-Demand Molecular Testing for the Physician Office Laboratory GeneXpert System How can on-site testing positively impact patient care and clinic operations? A better way. On-site molecular diagnostics
On-Demand Molecular Testing for the Physician Office Laboratory GeneXpert System How can on-site testing positively impact patient care and clinic operations? A better way. On-site molecular diagnostics
Discussion points CLSI M100 S19 Update. #1 format of tables has changed. #2 non susceptible category
 Discussion points 2009 CLSI M100 S19 Update Nebraska Public Health Laboratory Changes most important to routine antimicrobial susceptibility testing. Documents available Janet Hindler discussion slide
Discussion points 2009 CLSI M100 S19 Update Nebraska Public Health Laboratory Changes most important to routine antimicrobial susceptibility testing. Documents available Janet Hindler discussion slide
