User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
|
|
|
- Marcus Bond
- 6 years ago
- Views:
Transcription
1 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting Materials Questions to detect donors at risk for HIV Group O Screening children born to mothers with or at risk for HIV infection Screening pediatric donors Other issues to consider when evaluating donors Capture Questions Documentation Flow charts Eligibility Determinations Change Control Glossary Appendices: References Flow Charts vcjd countries of risk United Kingdom vcjd countries of risk Europe HIV Group O countries of risk - Africa HPC User Instructions v1.2 July 2009
2 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Glossary terms are underlined when used in this document. Purpose: The User Instructions, Donor History Questionnaire (DHQ) and accompanying materials were designed by an interorganizational Donor History Questionnaire Cellular Therapies Task Force to screen hematopoietic progenitor cell (HPC) donors for communicable disease risk factors in accordance with requirements of the FDA, NMDP, and voluntary accrediting agencies (AABB and FACT). All of the materials are intended to aid in determining if a prospective allogeneic donor is eligible to donate HPC, Apheresis and HPC, Marrow. These materials were not designed for use with cord blood donors. Each facility must have a standard operating procedure (SOP) related to donor eligibility to be used in conjunction with the User Instructions. These materials do not replace an SOP for determining donor eligibility, but may be incorporated into the SOP. A list of references at the end of these Instructions may provide assistance in developing the SOPs. Both the User Instructions and the SOP must be available to staff performing health histories. Introduction: The DHQ is designed for use at the time of initial donor evaluation and at other times as defined by local facility SOPs. The questionnaire and accompanying materials are used as part of the process for determining donor eligibility. Other steps include a thorough medical history, review of medical records, physical examination, and laboratory testing. Many of the questions have been evaluated for comprehension in English-speaking subjects by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention. Cognitive testing, the methodology utilized by NCHS, is considered to be the state-of-the-art approach for evaluating survey questions for comprehension. Methods of Administration: The method of administration of the DHQ should be in accordance with the facility s SOP. Donor screening is an active process involving open communication between donors and interviewers, and donors should be encouraged to voice questions and concerns at any time. The Questionnaire, Instructions, and accompanying documents were designed, structured and evaluated collectively and are intended to be used together. In order to effectively complete the questionnaire, prospective donors must first read the Donor Educational Materials. They also must be provided the Medication List, and the lists of countries that are referred to in the questionnaire for determining risk factors for certain communicable diseases. HPC User Instructions v1.2 July 2009
3 DHQ Materials - Structure and Content: The DHQ questions were composed for ease of understanding by the prospective donor. They are grouped by time period beginning with a question about are you currently and ending with questions relating to have you ever. The DHQ was evaluated for comprehension; therefore, the order, content, and wording of the questions should not be changed. Facilities may choose to incorporate additional questions that are required by local SOPs. These questions should be grouped at the end of the questionnaire in the area designated for additional questions. The Donor Educational Materials must also be used without modification except for local additions, which should be placed at the end of the materials. The educational materials are intended to inform the donor of the importance of the questions they will be asked. They are also designed to educate the donor about the signs and symptoms of HIV/AIDS and what is meant by the term sexual contact. The Medication List must be used without modification except for local additions, which should be placed at the end of the materials. Medications included on this list are to be used in the assessment of donors for risk of exposure to communicable diseases. Additional questions regarding prescription or over-the-counter medications are included on the questionnaire and used to assess bacterial infections or issues that might relate to the donor s health or safety of the collection process, such as coagulation issues. Reformatting Materials: Centers may choose to format these documents to fit their own individual use. Examples of such modifications include: Formatting the questions on the page in a single column, double columns, single page, double pages, etc. Placing the numbers in front of the answer boxes, behind the answer boxes, or leaving them out entirely (though for logistical purposes, the Task Force recommends against eliminating the numbers altogether) Experimenting with different font types, sizes and colors Experimenting with shading to assist donors in staying on-line Formatting the Donor Educational Materials and/or Medication List as needed to use as a brochure, handout, poster, or whatever is appropriate to suit your own needs, as long as the order, content and wording are unchanged. Local additions should be placed at the end. Editing the flow charts to include medical criteria specific to your own facility as long as any changes or additions are more restrictive; facilities should not modify flow charts to make them less restrictive. Gender specific questions for facilities that choose to have the questionnaire administered by a historian a method should be developed whereby the historian marks the question as not appropriate when interviewing a donor of the other sex. Questions to detect donors at risk for HIV Group O: Have you ever had sexual contact with anyone who was born in or lived in Africa? and Have you ever been in Africa? are recommended by FDA to identify donors who may be at risk for HIV Group O infections. Facilities utilizing an HIV test that has been approved by FDA for donor HPC User Instructions v1.2 July 2009
4 screening to include a claim for detection of Group O viruses may delete these questions from their screening questionnaire and may renumber the remainder of the questions (and related documents such as flow charts). All other facilities must continue to use these questions as formatted. See the Appendices for a list of HIV Group O countries of risk in Africa. Screening children born to mothers with or at risk for HIV infection: A child born to a mother with or at risk for HIV infection is eligible if the child is over 18 months of age and has not been breastfed within the preceding 12 months, provided that the child s HIV antibody tests, physical examination, and medical records do not indicate evidence of HIV infection. Local facility SOPs should describe further criteria to use when evaluating these donors, including the conditions under which the questions will be asked of a surrogate. Screening pediatric donors: Facilities that evaluate pediatric donors should have procedures to define the process. Screening of pediatric donors should also follow state laws. For additional information on screening infant donors one month of age or less, please refer to the FDA guidance document Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps). Other issues to consider when evaluating donors: Each facility should have written policies to further evaluate their cellular therapy donors and recipients. The evaluation should include factors such as risks of collection, and other non-communicable disease questions to assist in selecting the appropriate donor and to ensure the safety of the donor and recipient. This evaluation can occur during the history and physical examination, again on the day of collection or at other times according to facility policy. Capture Questions: The DHQ uses capture questions that may require donor historian intervention or follow-up. Capture questions are questions that cover a broad topic, and when an affirmative answer is given, follow-up questions may be asked by the donor historian to elicit additional information. Some follow-up questions are included in the flow charts, but since specific donor eligibility criteria may vary from one facility to another, an affirmative response to some questions may require consultation with the facility s SOP. Per local SOPs, facilities may implement more restrictive donor selection policies than described in the DHQ documents. Documentation: Information impacting donor eligibility determination should be meticulously documented on the DHQ. Responses should be documented with sufficient detail to determine the reason for donor eligibility or ineligibility. Each facility s SOP must define how donor responses to any follow-up questioning will be documented on the DHQ. On occasion a donor may not know the answer to a question. Facility SOPs should be in place to provide direction for determining how an I don t know response will impact the eligibility of the donor. HPC User Instructions v1.2 July 2009
5 Facility SOPs should define the action to be taken to determine if and when a product will be collected from an otherwise ineligible donor under the definition of urgent medical need. Flow charts: The User Instructions provide flow charts to guide the donor historian through the donor history questionnaire process. These flow charts are intended as a resource, and use of the flowcharts is not required. Each question is a separate flow chart, and each one contains the following information: Question: Question number and the question. Donor Eligibility: This section provides additional information to the donor historian on donor eligibility requirements for each question. Note: Optional field; additional relevant information relating to the donor question. Flow Chart: Each question is flow-charted using standard flow-charting symbols. Square / Rectangle -- Statement Diamond -- Question/decision point Oval -- Action Arrow -- Move to the next question Each question ends with an ARROW that indicates to move to the next question; however, facilities must follow their established policies concerning whether or not the donor eligibility process is terminated when it is known that the donor will not be selected. Flow charts may be revised by facilities to reflect local policy as long as eligibility decisions are not made less strict than those required by FDA or peer accrediting standards. Eligibility Determinations: Communicable Disease Questions are designed to be compliant with FDA regulations and recommendations. For some questions, a yes answer calls for a determination that the donor is ineligible per FDA regulations and or recommendations. This determination is designated in the flow chart by the Action Donor is ineligible. Refer to facility SOP for determination and documentation of urgent medical need, as appropriate. Each facility must have SOPs to describe the necessary documentation, notification, consent and labeling that is necessary when an ineligible donor will be utilized due to urgent medical need. For other questions, a yes answer may trigger a line of questioning to determine if the donor is eligible. The donor historian will need to refer to the facility s SOP to determine eligibility based on responses to the follow-up questions. This determination is designated in the flow chart by the Action Refer to facility SOP for further criteria. Change Control: Periodically the Donor History Questionnaire, the accompanying documents or the User Instructions will be updated or revised by the interorganizational Donor History Questionnaire Cellular Therapies Task Force as required for compliance with regulatory and accrediting agencies. Institutions will be notified of the changes and HPC User Instructions v1.2 July 2009
6 timeline for implementation in existing publications and on the public area of the web sites maintained by members of the Task Force. All updated documents will also be made available on the web sites. It is the responsibility of collecting facilities to make changes in their forms, procedures and processes to incorporate these revisions within the specified time. New questions that are recommended by the Task Force, or FDA, to be added to the DHQ should be placed in the area designated for additional questions until such time as the DHQ is updated to a new version date by the Task Force. HPC User Instructions v1.2 July 2009
7 GLOSSARY The following terms are defined in the context of their use in the Donor History Questionnaire. Capture Question A question that covers a broad topic. When an affirmative answer is given, additional follow-up questions to elicit additional information are asked by the donor historian. EXAMPLE: Have you ever been to Africa? If the donor answers yes, additional questions must be asked. Contact with Blood (1) a needlestick or other sharps injury from an instrument that has been used on any individual or patient; (2) exposure to non-intact skin (e.g., skin that is chapped, abraded, or afflicted with dermatitis); (3) a human bite that breaks the skin; (4) exposure to eye, nose, or mouth i.e., the mucous membranes. Sexual Contact The meaning of the words sexual contact with and sex are identical, and apply to any of the following activities, whether or not a condom or other protection was used: (1) Vaginal sex (contact between penis and vagina); (2) Oral sex (mouth or tongue on someone s vagina, penis, or anus); (3) Anal sex (contact between penis and anus). Close Contact with Smallpox Vaccination Site Touching the vaccination site, including the bandages covering the vaccination site; touching/handling materials that might have come into contact with an unbandaged vaccination site, including clothing, towels, and bedding. Lived With Residing in the same dwelling. EXAMPLES: house, dormitory, apartment. Eligible Screening (21 CFR ) shows that the donor is free from risk factors for, and clinical and physical evidence of, infection due to relevant communicable disease agents and diseases, and is free from communicable disease risks associated with xenotransplantation; and test results for relevant communicable disease agents are negative or nonreactive (21 CFR and ). Evaluation for risk factors in addition to those required by FDA may be required by local facility SOPs. To be deemed eligible the donor would also be eligible per local facility SOP criteria. HPC User Instructions v1.2 July 2009
8 Ineligible A donor is determined to be ineligible whenever the results of screening and testing show risk factors for, clinical and physical evidence of, or laboratory evidence of infection due to relevant communicable disease agents and diseases or communicable disease risks associated with xenotransplantation as described in 21 CFR , and Donors determined to be ineligible may be selected to donate an HCT/P if urgent medical need is documented. Requirements listed in 21 CFR must be adhered to. Urgent Medical Need No comparable HCT/P is available and the recipient is likely to suffer death or serious morbidity without the HCT/P. (21 CFR (u)). An HCT/P for allogeneic use in a first-degree or second-degree blood relative is exempt from this designation [see 21 CFR (b)(1)] HPC User Instructions v1.2 July 2009
9 The interorganizational Donor History Questionnaire Cellular Therapies Task Force includes representatives from AABB, American Association of Tissue Banks, American Society for Blood and Marrow Transplantation, American Society for Apheresis, Foundation for the Accreditation of Cellular Therapy, International Society for Cellular Therapy, National Marrow Donor Program and an ethicist. FDA provided a liaison to the Task Force. HPC User Instructions v1.2 July 2009
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Question: 1. Are you currently taking an antibiotic?
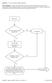 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic?
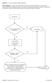 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Relevant Communicable Diseases in HCT/Ps
 Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Making Your Blood Donation Safe
 BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
NATIONAL MARROW DONOR PROGRAM. Creating Connections. Saving Lives. Fran Rabe
 Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
FormsNet Adverse Event Form
 FormsNet Adverse Event Form Registry Use Only Sequence Number: Date Received: Recipient Identification 1. CIBMTR Recipient ID (CRID): 2. NMDP Recipient ID (RID): (if applicable) - - 3. CIBMTR Center Number
FormsNet Adverse Event Form Registry Use Only Sequence Number: Date Received: Recipient Identification 1. CIBMTR Recipient ID (CRID): 2. NMDP Recipient ID (RID): (if applicable) - - 3. CIBMTR Center Number
AABB Standards and Accreditation. Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA
 AABB Standards and Accreditation Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA Objectives Review AABB standard setting process Describe accreditation program What is AABB? International association
AABB Standards and Accreditation Doug Padley, MT (ASCP) Mayo Clinic Rochester MN USA Objectives Review AABB standard setting process Describe accreditation program What is AABB? International association
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another?
 Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
NEOMED ACADEMIC POLICY
 (A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
(A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
The DRAI (Donors >12 yrs old) versus Requirements
 Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Routine Questionnaire (A1)
 Statistical Center for HIV/AIDS Research & Prevention (SCHARP) (RQ-1) RQ-1 (021) Page 1 of 8 Enrollment Date dd Staff ID: Team ID: Instructions: Use this Routine Questionnaire for all participants meeting
Statistical Center for HIV/AIDS Research & Prevention (SCHARP) (RQ-1) RQ-1 (021) Page 1 of 8 Enrollment Date dd Staff ID: Team ID: Instructions: Use this Routine Questionnaire for all participants meeting
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
The Organ and Tissue Donor Program
 The Organ and Tissue Donor Program If I needed a kidney or some other vital organ to live Would I be able to get one? Maybe. Many people who need organ transplants cannot get them because of a shortage
The Organ and Tissue Donor Program If I needed a kidney or some other vital organ to live Would I be able to get one? Maybe. Many people who need organ transplants cannot get them because of a shortage
CELLULAR THERAPY QUIZ. Correct answers and rationale
 CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
What employees should know about UNIVERSAL PRECAUTIONS. They re work practices that help prevent contact with blood and certain other body fluids.
 What are Universal Precautions? What employees should know about UNIVERSAL PRECAUTIONS They re work practices that help prevent contact with blood and certain other body fluids. Universal precautions are:
What are Universal Precautions? What employees should know about UNIVERSAL PRECAUTIONS They re work practices that help prevent contact with blood and certain other body fluids. Universal precautions are:
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
HPV HUMAN PAPILLOMA VIRUS
 HPV HUMAN PAPILLOMA VIRUS WHAT IS HPV? HPV is the most common sexually transmitted infection (STI). There are more than 40 HPV types that can infect the genital areas of males and females. Four of these
HPV HUMAN PAPILLOMA VIRUS WHAT IS HPV? HPV is the most common sexually transmitted infection (STI). There are more than 40 HPV types that can infect the genital areas of males and females. Four of these
SPECIALIZED FAMILY CARE Provider Training
 SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
UDHQ Project Overview and Update 7 May 2012
 Introduction Organ, tissue, and eye (OTE) donation and transplantation professionals have long understood the value of collecting medical and behavioral history information on potential donors to assess
Introduction Organ, tissue, and eye (OTE) donation and transplantation professionals have long understood the value of collecting medical and behavioral history information on potential donors to assess
What do I need to know about HIV and sex? What are my responsibilities and choices?
 Patient and Family Education HIV: Teens and Sex This handout has information about sex and HIV. This handout answers common questions you might ask about sex. It is important for you to talk to your parents
Patient and Family Education HIV: Teens and Sex This handout has information about sex and HIV. This handout answers common questions you might ask about sex. It is important for you to talk to your parents
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
IMPORTANT HEALTH INFORMATION
 IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
Question 1. Blood Borne Viruses: Some important basic facts 6. What does BBV stand for? A. Blood Based Virus. B. Blood Borne Viruses
 Question 1 What does BBV stand for? A. Blood Based Virus B. Blood Borne Viruses C. Basic Blood Viruses Blood Borne Viruses: Some important basic facts 6 Question 1 What does BBV stand for? A. Blood Based
Question 1 What does BBV stand for? A. Blood Based Virus B. Blood Borne Viruses C. Basic Blood Viruses Blood Borne Viruses: Some important basic facts 6 Question 1 What does BBV stand for? A. Blood Based
DETERMINING WHETHER AN ACTIVITY IS HUMAN SUBJECTS RESEARCH AS DEFINED BY FEDERAL REGULATIONS
 Page 1 of 6 Human Research Protection Page: 1 of 6 DETERMINING WHETHER AN ACTIVITY IS HUMAN SUBJECTS RESEARCH AS DEFINED BY FEDERAL REGULATIONS DESCRIPTION Any proposed activity involving contact with
Page 1 of 6 Human Research Protection Page: 1 of 6 DETERMINING WHETHER AN ACTIVITY IS HUMAN SUBJECTS RESEARCH AS DEFINED BY FEDERAL REGULATIONS DESCRIPTION Any proposed activity involving contact with
CHAPTER Committee Substitute for Committee Substitute for House Bill No. 321
 CHAPTER 2015-110 Committee Substitute for Committee Substitute for House Bill No. 321 An act relating to HIV testing; amending s. 381.004, F.S.; revising and providing definitions; specifying the notification
CHAPTER 2015-110 Committee Substitute for Committee Substitute for House Bill No. 321 An act relating to HIV testing; amending s. 381.004, F.S.; revising and providing definitions; specifying the notification
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
EXPOSING DANGERS OF HUMAN PAPILLOMAVIRUS IN BOTH MEN AND WOMEN
 EXPOSING DANGERS OF HUMAN PAPILLOMAVIRUS IN BOTH MEN AND WOMEN Aishatu Abdullahi Adamu 3rd Year Student, Department of Medical Laboratory Technology, NIMS University Jaipur (India) ABSTRACT The human papillomavirus
EXPOSING DANGERS OF HUMAN PAPILLOMAVIRUS IN BOTH MEN AND WOMEN Aishatu Abdullahi Adamu 3rd Year Student, Department of Medical Laboratory Technology, NIMS University Jaipur (India) ABSTRACT The human papillomavirus
For People Who Have Been Sexually Assaulted... What You Need To Know about STDs and Emergency Contraception
 For People Who Have Been Sexually Assaulted... What You Need To Know about STDs and Emergency Contraception FOR PEOPLE WHO HAVE BEEN SEXUALLY ASSAULTED What You Need to Know about STDs and Emergency Contraception
For People Who Have Been Sexually Assaulted... What You Need To Know about STDs and Emergency Contraception FOR PEOPLE WHO HAVE BEEN SEXUALLY ASSAULTED What You Need to Know about STDs and Emergency Contraception
Teen Sexual Health Survey
 Instructions Teen Sexual Health Survey Thank you for taking part in our survey. DO NOT write your name on this survey. The answers you give will be kept private. No one will know what you write. Answer
Instructions Teen Sexual Health Survey Thank you for taking part in our survey. DO NOT write your name on this survey. The answers you give will be kept private. No one will know what you write. Answer
Global Perspective on Donor and Recipient Safety: Biovigilance in Hematopoietic Cell Transplantation
 Global Perspective on Donor and Recipient Safety: Biovigilance in Hematopoietic Cell Transplantation John P. Miller, MDPhD Vice President and Senior Medical Director National Marrow Donor Program / Be
Global Perspective on Donor and Recipient Safety: Biovigilance in Hematopoietic Cell Transplantation John P. Miller, MDPhD Vice President and Senior Medical Director National Marrow Donor Program / Be
20. HIV and AIDS. Objectives. How is HIV transmitted?
 20. HIV and AIDS Objectives By the end of this session, group members will be able to: Explain what HIV and AIDS are. Describe how HIV is transmitted. Explain the difference between HIV and AIDS. List
20. HIV and AIDS Objectives By the end of this session, group members will be able to: Explain what HIV and AIDS are. Describe how HIV is transmitted. Explain the difference between HIV and AIDS. List
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Strategy,, policy and commissioning on hepatitis B and C testing NICE Pathways bring together everything NICE says on a topic in an interactive flowchart. NICE Pathways are interactive and designed to
Strategy,, policy and commissioning on hepatitis B and C testing NICE Pathways bring together everything NICE says on a topic in an interactive flowchart. NICE Pathways are interactive and designed to
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
STIs and BBVs. The facts
 EASY ENGLISH STIs and BBVs Some people say sex germs The facts New words There may be words in this factsheet that are new to you and you may not know what they mean or you may be unsure what they mean.
EASY ENGLISH STIs and BBVs Some people say sex germs The facts New words There may be words in this factsheet that are new to you and you may not know what they mean or you may be unsure what they mean.
SEXUALLY TRANSMITTED INFECTIONS (STIS)
 SEXUALLY TRANSMITTED INFECTIONS (STIS) CONTENTS Causes Prevention Methods of Transmission Decisions to Have Sexual Intercourse Signs and symptoms Screening Pop Quiz Diagnosis Management: Public Health
SEXUALLY TRANSMITTED INFECTIONS (STIS) CONTENTS Causes Prevention Methods of Transmission Decisions to Have Sexual Intercourse Signs and symptoms Screening Pop Quiz Diagnosis Management: Public Health
Uniform DRAI - Donor >12 yrs old Requirements Crosswalk
 Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
CMC Annual Review of BLOODBORNE DISEASES. Prevention of Transmission for School Staff
 CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
Zika 101 for Occupational Safety and Health Professionals
 CDC Responds to ZIKA Zika 101 for Occupational Safety and Health Professionals CDR Jill M. Shugart, MSPH, REHS CDC/National Institute for Occupational Safety and Health Emergency Preparedness and Response
CDC Responds to ZIKA Zika 101 for Occupational Safety and Health Professionals CDR Jill M. Shugart, MSPH, REHS CDC/National Institute for Occupational Safety and Health Emergency Preparedness and Response
HIV/AIDS. Saskatchewan. Saskatchewan Health Population Health Branch
 HIV/AIDS In Saskatchewan 26 Saskatchewan Health Population Health Branch HIV/AIDS in Saskatchewan to December 31, 26 This epidemiological report profiles HIV and AIDS in Saskatchewan from the commencement
HIV/AIDS In Saskatchewan 26 Saskatchewan Health Population Health Branch HIV/AIDS in Saskatchewan to December 31, 26 This epidemiological report profiles HIV and AIDS in Saskatchewan from the commencement
Sexually Transmitted Infections. Kim Dawson October 2010
 Sexually Transmitted Infections Kim Dawson October 2010 Objectives: You will learn about: Sexually Transmitted Infections (STI s). How they are transferred. High risk behavior. The most common STI s. How
Sexually Transmitted Infections Kim Dawson October 2010 Objectives: You will learn about: Sexually Transmitted Infections (STI s). How they are transferred. High risk behavior. The most common STI s. How
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Implementation of the Stem Cell Therapeutic and Research Act of 2005
 Implementation of the Stem Cell Therapeutic and Research Act of 2005 The 6 th Annual Somatic Cell Therapy Symposium Bethesda, MD September 26, 2006 James F. Burdick, M.D. U.S. Department of Health and
Implementation of the Stem Cell Therapeutic and Research Act of 2005 The 6 th Annual Somatic Cell Therapy Symposium Bethesda, MD September 26, 2006 James F. Burdick, M.D. U.S. Department of Health and
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
IRB GRAND ROUNDS SOCIAL AND BEHAVIORAL RESEARCH: NEED TO KNOW
 IRB GRAND ROUNDS SOCIAL AND BEHAVIORAL RESEARCH: NEED TO KNOW Vivienne Carrasco, MPH,CIP Senior IRB Regulatory Analyst, Social and Behavioral Sciences Human Subject Research Office University of Miami
IRB GRAND ROUNDS SOCIAL AND BEHAVIORAL RESEARCH: NEED TO KNOW Vivienne Carrasco, MPH,CIP Senior IRB Regulatory Analyst, Social and Behavioral Sciences Human Subject Research Office University of Miami
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Policy for the use of Cytomegalovirus (CMV) negative blood products
 Policy for the use of Cytomegalovirus (CMV) negative blood products SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory Cancer and Specialist Care Sub Area Haematology and Blood
Policy for the use of Cytomegalovirus (CMV) negative blood products SharePoint Location Clinical Policies and Guidelines SharePoint Index Directory Cancer and Specialist Care Sub Area Haematology and Blood
A Sexual Health Study with Africans in Frankfurt am Main
 A Sexual Health Study with Africans in Frankfurt am Main Working together for Health Promotion INFORMATION FOR STUDY PARTICIPANTS WHO ARE WE? We are a group of Africans, researchers and persons doing
A Sexual Health Study with Africans in Frankfurt am Main Working together for Health Promotion INFORMATION FOR STUDY PARTICIPANTS WHO ARE WE? We are a group of Africans, researchers and persons doing
CDC Responds to ZIKA Zika 101
 CDC Responds to ZIKA Zika 101 Updated April 29, 2016 What is Zika virus disease (Zika)? Disease spread primarily through the bite of an Aedes species mosquito infected with Zika virus. Many people infected
CDC Responds to ZIKA Zika 101 Updated April 29, 2016 What is Zika virus disease (Zika)? Disease spread primarily through the bite of an Aedes species mosquito infected with Zika virus. Many people infected
BLOOD TRANSFUSION MEDICINE TECHNICAL MANUAL SARAN
 page 1 / 5 page 2 / 5 blood transfusion medicine technical pdf In 2010, the World Health Assembly deliberated on challenges related to the availability, safety and quality of blood products and defined
page 1 / 5 page 2 / 5 blood transfusion medicine technical pdf In 2010, the World Health Assembly deliberated on challenges related to the availability, safety and quality of blood products and defined
Hematopoiesis i starts from. HPC differentiates t to sequentially more mature. HPC circulate in very low
 Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
STI Feud Instructions
 STI Feud Instructions This game works best with groups of 4 20. What you will need: The STI question cards, 2 easy buttons from staples, chart paper, markers (if no black or white board) 1. Break the group
STI Feud Instructions This game works best with groups of 4 20. What you will need: The STI question cards, 2 easy buttons from staples, chart paper, markers (if no black or white board) 1. Break the group
Sexually Transmitted. Diseases
 Sexually Transmitted Diseases How can I get an STD? Many STDs are carried and transmitted through semen and vaginal fluids. Some STDs can be spread through skin to skin contact Mother to child STDs: Signs
Sexually Transmitted Diseases How can I get an STD? Many STDs are carried and transmitted through semen and vaginal fluids. Some STDs can be spread through skin to skin contact Mother to child STDs: Signs
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Virginia Beach Department of Emergency Medical Services. CAAS # Index # Administration VACCINATION POLICY
 Virginia Beach Department of Emergency Medical Services CAAS # 106.01.01 Index # Administration VACCINATION POLICY PURPOSE: This policy is designed to provide guidance in operating a program for the administration
Virginia Beach Department of Emergency Medical Services CAAS # 106.01.01 Index # Administration VACCINATION POLICY PURPOSE: This policy is designed to provide guidance in operating a program for the administration
Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation
 Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation Presented by Ngaire Elwood, PhD Vice President, Foundation for the Accreditation of Cellular Therapy (FACT); Director of
Introduction to FACT Immune Effector Cellular Therapy Standards and Accreditation Presented by Ngaire Elwood, PhD Vice President, Foundation for the Accreditation of Cellular Therapy (FACT); Director of
Greater Glasgow and Clyde. Blood Borne Viruses: Some important basic facts
 Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Malaria prevention and control
 COMMUNITY TOOLS Tool 1 Malaria prevention and control Point to first picture: What do you see in this picture? 1. A small child who looks very sick. The child is sweating and shaking. There are mosquitoes
COMMUNITY TOOLS Tool 1 Malaria prevention and control Point to first picture: What do you see in this picture? 1. A small child who looks very sick. The child is sweating and shaking. There are mosquitoes
Detailed instructions for each learning activity may be found below. Here is an overview of learning activities for the instructor to choose from:
 Learning Activities Detailed instructions for each learning activity may be found below. Here is an overview of learning activities for the instructor to choose from: Number Name Methods Time 3.9.1 Knowing
Learning Activities Detailed instructions for each learning activity may be found below. Here is an overview of learning activities for the instructor to choose from: Number Name Methods Time 3.9.1 Knowing
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
HIV AND AIDS FACT SHEETS
 The Human Immunodeficiency Virus (HIV) has been one of the most devastating new diseases to emerge over the course of the past century. Even though HIV may not always be in the headlines now, it is still
The Human Immunodeficiency Virus (HIV) has been one of the most devastating new diseases to emerge over the course of the past century. Even though HIV may not always be in the headlines now, it is still
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
STD Notes. Myths about STDs
 STD Notes Sexually transmitted diseases (STD's) or sexually transmitted infections (STI's) are infectious diseases that spread from person to person through intimate contact. STD's can affect males and
STD Notes Sexually transmitted diseases (STD's) or sexually transmitted infections (STI's) are infectious diseases that spread from person to person through intimate contact. STD's can affect males and
Training Objectives. Provide a basic understanding of:
 Training Objectives Provide a basic understanding of: 1. Bloodborne pathogens (BBP) 2. Common modes of transmission of BBP 3. Methods to prevent transmission of BBP 4. Information to help school staff
Training Objectives Provide a basic understanding of: 1. Bloodborne pathogens (BBP) 2. Common modes of transmission of BBP 3. Methods to prevent transmission of BBP 4. Information to help school staff
May Safety Subject. Bloodborne Pathogens
 May Safety Subject Bloodborne Pathogens Everyone is at risk to contact bloodborne pathogens. Some more than others. Universal precautions means treating all objects as potentially contaminated Personal
May Safety Subject Bloodborne Pathogens Everyone is at risk to contact bloodborne pathogens. Some more than others. Universal precautions means treating all objects as potentially contaminated Personal
Key Concepts Guide. Rev. March 2015 Page 1 of 13
 Key Concepts Guide Key concepts are main ideas. They convey big-picture ideas. Birth control is good at preventing pregnancy and Everyone has the right to say who touches their body and how are both key
Key Concepts Guide Key concepts are main ideas. They convey big-picture ideas. Birth control is good at preventing pregnancy and Everyone has the right to say who touches their body and how are both key
Strategic Peer-Enhanced Care and Treatment Retention Model (SPECTRuM) Initiative. Intervention Protocol #2
 MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH BUREAU OF INFECTIOUS DISEASE AND LABORATORY SCIENCES HIV/AIDS AND STD SURVEILLANCE PROGRAM AND OFFICE OF HIV/AIDS Strategic Peer-Enhanced Care and Treatment Retention
MASSACHUSETTS DEPARTMENT OF PUBLIC HEALTH BUREAU OF INFECTIOUS DISEASE AND LABORATORY SCIENCES HIV/AIDS AND STD SURVEILLANCE PROGRAM AND OFFICE OF HIV/AIDS Strategic Peer-Enhanced Care and Treatment Retention
HIV/AIDS MODULE. Rationale
 HIV/AIDS MODULE Rationale According to WHO HIV/AIDS remains one of the world's most significant public health challenges, particularly in low- and middle-income countries. As a result of recent advances
HIV/AIDS MODULE Rationale According to WHO HIV/AIDS remains one of the world's most significant public health challenges, particularly in low- and middle-income countries. As a result of recent advances
Name of Recipient: Recipient s DOB (if known) Relationship to Recipient: (Example: mother, father, sister, brother, friend, etc)
 The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
Diane Lockman, Public Health Nurse. Marilyn Mitchell, Registered Nurse. Sue Mulhall, Public Health Nurse
 Diane Lockman, Public Health Nurse Marilyn Mitchell, Registered Nurse Sue Mulhall, Public Health Nurse Agenda Pre-evaluation Minimizing Your Risk Emergency Service Worker Exposures Scenarios Questions
Diane Lockman, Public Health Nurse Marilyn Mitchell, Registered Nurse Sue Mulhall, Public Health Nurse Agenda Pre-evaluation Minimizing Your Risk Emergency Service Worker Exposures Scenarios Questions
H1N1 and Flu Shots During Pregnancy
 H1N1 and Flu Shots During Pregnancy HEALTH EDUCATION HEALTH EDUCATION The H1N1 flu and seasonal vaccines are safe and recommended for pregnant women. Be sure to: Get the H1N1 flu vaccine for yourself and
H1N1 and Flu Shots During Pregnancy HEALTH EDUCATION HEALTH EDUCATION The H1N1 flu and seasonal vaccines are safe and recommended for pregnant women. Be sure to: Get the H1N1 flu vaccine for yourself and
ELDER ABUSE SCREENING TOOL (EAST)
 ELDER ABUSE SCREENING TOOL (EAST) NOVEMBER 2011 CONTENTS PAGE 1. BACKGROUND... 1 2. HOW TO USE THE ELDER ABUSE SCREENING TOOL (EAST)... 2 APPENDICES Annexure A: Annexure B: Annexure C: Elder Abuse Screening
ELDER ABUSE SCREENING TOOL (EAST) NOVEMBER 2011 CONTENTS PAGE 1. BACKGROUND... 1 2. HOW TO USE THE ELDER ABUSE SCREENING TOOL (EAST)... 2 APPENDICES Annexure A: Annexure B: Annexure C: Elder Abuse Screening
Emergency, Community and Health Outreach
 ECHO Q&A Emergency, Community and Health Outreach Draft 4 FINAL DRAFT June 16, 2009 ECHO Questions and Answers 10 Minute Conversation STD Prevention and Treatment GUEST: Please modify question #2 for your
ECHO Q&A Emergency, Community and Health Outreach Draft 4 FINAL DRAFT June 16, 2009 ECHO Questions and Answers 10 Minute Conversation STD Prevention and Treatment GUEST: Please modify question #2 for your
HUMAN PAPILLOMAVIRUS
 HUMAN PAPILLOMAVIRUS HUMAN PAPILLOMAVIRUS The Human Papillomavirus (HPV) is responsible for 60% of cancers of the throat including base of the tongue and tonsils. AN OVERVIEW TO HUMAN PAPILLOMAVIRUS Human
HUMAN PAPILLOMAVIRUS HUMAN PAPILLOMAVIRUS The Human Papillomavirus (HPV) is responsible for 60% of cancers of the throat including base of the tongue and tonsils. AN OVERVIEW TO HUMAN PAPILLOMAVIRUS Human
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
Hepatitis Trivia Game
 Hepatitis Trivia Game Materials: 30 cards with a multiple-choice, fill-in-the-blank, or true/false question written on them. Facilitator s answer sheet Description Trivia Game gives participants the opportunity
Hepatitis Trivia Game Materials: 30 cards with a multiple-choice, fill-in-the-blank, or true/false question written on them. Facilitator s answer sheet Description Trivia Game gives participants the opportunity
INFORMATION SHEET FOR THE DEPARTMENT OF PAIN AND PALLIATIVE CARE
 INFORMATION SHEET FOR THE DEPARTMENT OF PAIN AND PALLIATIVE CARE Please review the following instructions as it contains important information regarding the management of your pain. Once reviewed, our
INFORMATION SHEET FOR THE DEPARTMENT OF PAIN AND PALLIATIVE CARE Please review the following instructions as it contains important information regarding the management of your pain. Once reviewed, our
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet
 JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
Guidance for Industry
 Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Supervisors, Department Heads and Principals will:
 The Greater Victoria School District is committed to each student s success in learning within a responsive and safe environment. REGULATION 4213 UNIVERSAL PRECAUTIONS Preamble Universal Precautions are
The Greater Victoria School District is committed to each student s success in learning within a responsive and safe environment. REGULATION 4213 UNIVERSAL PRECAUTIONS Preamble Universal Precautions are
Take out CST test corrections What do you know about STDs?
 Assignment #5 STDs LO: To understand sexually transmitted diseases. EQ: What are all of the ways to contract STDs? (4-5 sentences underlining key words) AGENDA 5/12-5/13 1. Group work 2. Notes Homework
Assignment #5 STDs LO: To understand sexually transmitted diseases. EQ: What are all of the ways to contract STDs? (4-5 sentences underlining key words) AGENDA 5/12-5/13 1. Group work 2. Notes Homework
Manitoba Health Statistical Update on HIV/AIDS
 Manitoba Health Statistical Update on HIV/AIDS 1985 - Dec 2001 Communicable Disease Control Unit Public Health Branch MANITOBA HEALTH STATISTICAL UPDATE ON HIV/AIDS 1985 TO DECEMBER 2001 HIV January 1,
Manitoba Health Statistical Update on HIV/AIDS 1985 - Dec 2001 Communicable Disease Control Unit Public Health Branch MANITOBA HEALTH STATISTICAL UPDATE ON HIV/AIDS 1985 TO DECEMBER 2001 HIV January 1,
