GUIDE TO FOLLOW UP TESTING FOR BLOOD OR BODY FLUID EXPOSURES AND NEEDLESTICK INJURIES
|
|
|
- Coral McCormick
- 6 years ago
- Views:
Transcription
1 GUIDE TO FOLLOW UP TESTING FOR BLOOD OR BODY FLUID EXPOSURES AND NEEDLESTICK INJURIES titis B, titis C and may be contracted through exposure to any body fluid, particularly blood. Follow up testing of exposed people is important to determine whether infection has been transmitted and to enable early treatment where this is appropriate. Any person that has positive serology on follow up testing for blood or body fluid exposure should be discussed with the on call Clinical Microbiologist/Infectious Diseases physician. Exposure from unknown source The risk of infection with titis B, titis C or following a blood or body fluid exposure from an unknown source is usually low. A risk assessment should be performed at the time of the injury by a doctor and if necessary, should be discussed with the on call Clinical Microbiologist/Infectious Diseases physician. If the risk is deemed to be low, post exposure prophylaxis will not be given. The exposed person should have serology for titis B, titis C and performed at 6 weeks, 3 months and 6 months after the exposure. They should be informed to seek medical attention if they develop jaundice or an infectious mononucleosis syndrome in the six months after their exposure. Time post exposure Six weeks Three months Six months Tests required HBsAg, and HBsAb, HCVAb, Ag/Ab, HBsAg, and HBsAb, HCVAb, Ag/Ab HBsAg, and HBsAb, HCVAb, Ag/Ab Exposure from source infected with titis C The risk of infection following a blood or body fluid exposure where the source patient is infected with titis C is around 1.8%. This risk may change depending on the circumstances of the exposure. There is no effective post-exposure prophylaxis or vaccination for titis C. It is recommended to perform follow up testing at 6 weeks, 3 months and 6 months after the exposure. The exposed person should be informed to seek medical attention if they develop nausea, abdominal pain or jaundice in the six months after their exposure. 1
2 Exposure from source infected with The risk of infection following a blood or body fluid exposure where the source patient is infected with depends on the circumstances of the exposure, but is estimated at < 1% in most situations. A risk assessment should be performed a the time of the injury and this should be discussed with the on call Clinical Microbiologist/Infectious Diseases physician. In many cases, post-exposure prophylaxis will have been offered to the exposed person and follow up should have been arranged with the Infectious Diseases service at CCDHB. If the risk of transmission was deemed very low, post-exposure prophylaxis may not be given. In either situation, it is recommended to perform follow up testing with Ag/Ab at 6 weeks, 3 months and 6 months after the exposure. The exposed person should be informed to seek medical attention if they develop fever, rash or an infectious mononucleosis syndrome in the three months following their exposure. Exposure from a source with titis B The risk of infection with titis B following a blood or body fluid exposure where the source is infected with titis B depends on the nature of the exposure, on the infectivity of the source (HBeAg positivity) and the immunity of the exposed person (vaccination status). Transmission may be as high as 30% in a non-immune recipient where the source person is HBeAg positive, or approximately 6% if the source person is HBsAg positive but HBeAg negative. A risk assessment should be performed at the time of the injury and this should be discussed with the on call Clinical Microbiologist/Infectious Diseases physician. If the exposed person has been vaccinated and is known to have HBsAb >10 IU/mL documented at any time following vaccination, there is no need for HBV PEP. No follow up testing is required. If the exposed person does not have HBsAb >10 IU/mL demonstrated at any time following vaccination, titis B Immunoglobulin and a booster dose of titis B vaccination should have been given within 72 hours of exposure. Follow up testing is outlined in the table below (HBsAb 10 IU/mL column). If the exposed person has not been vaccinated, titis B Immunoglobulin and the first dose of titis B vaccination should have been given within 72 hours of exposure. Two further doses should be given at four weekly intervals. Follow testing is outlined in the table below. 2
3 Time post exposure Source HBV +, Recipient HBsAb 10 IU/mL Source HBV +, Recipient never immunised Four weeks - 2 nd dose HBV vaccine Six weeks HBsAg, HBsAb - Eight weeks - 3 rd dose HBV vaccine Three months HBsAg, HBsAb HBsAg, HBsAb Six months HBsAg, HBsAb - For persons given single booster of titis B vaccine: If HBsAb at three months still 10 IU/mL, give two further doses of titis B vaccine each four weeks apart. For persons given three doses of titis B vaccine: If HBsAb at three months still 10 IU/mL, repeat complete course (3 doses) of titis B vaccine. For further information see Ministry of Health : Immunisation Handbook 3
4 1. EXPOSED PERSON DETAILS EXPOSED PERSON NEEDLESTICK INJURY, BLOOD/BODY FLUID EXPOSURE TEST REQUEST FORM FOLLOW UP BLOOD TEST FORM 6 WEEKS POST NEEDLESTICK EXPOSURE First Name Last Name NHI No. (if known): DOB Workplace Gender (circle one) Male/Female Address Date of Exposure 2. CONTACT PERSON AND GP DETAILS Name of person managing results (e.g. GP, workplace occupational health or infection control person, manager) Position/Title Contact Daytime Phone no: After Hours Phone No: Cell phone No: If the above is not your GP, do you want a copy of your results to be sent to them?: Yes / No (circle) If Yes: Name of Dr Location of Dr s Medical Centre 3. Collect a 5ml Yellow SST tube (however alternatively EDTA, rin,sodium citrate, ACD, CPD tube may be used if SST is unable to be obtained). Label Specimen CORRECTLY. 4. INFORMED CONSENT BY EXPOSED PERSON FOR BLOOD TESTS AND PROPHYLAXIS: I understand the risk of infection following accidental blood/body fluids exposure and am aware of the treatment options. I agree to having the following blood tests performed. Tests for titis B,C and will be performed unless excluded (please cross out to exclude): titis BsAg (Diagnosis) titis BsAb (Immunity) titis C Antibody Antibody/Antigen Exposed Person Signature Date Specimen Recep on Instruc ons Test Code Tests HEG HEB HCV s B Surface Ag (diagnosis) s B Surface Ab (immunity) s C 4
5 1. EXPOSED PERSON DETAILS EXPOSED PERSON NEEDLESTICK INJURY, BLOOD/BODY FLUID EXPOSURE TEST REQUEST FORM FOLLOW UP BLOOD TEST FORM 3 MONTH POST NEEDLESTICK EXPOSURE First Name Last Name NHI No. (if known): DOB Workplace Gender (circle one) Male/Female Address Date of Exposure 2. CONTACT PERSON AND GP DETAILS Name of person managing results (e.g. GP, workplace occupational health or infection control person, manager) Position/Title Contact Daytime Phone no: After Hours Phone No: Cell phone No: If the above is not your GP, do you want a copy of your results to be sent to them?: Yes / No (circle) If Yes: Name of Dr Location of Dr s Medical Centre 3. Collect a 5ml Yellow SST tube (however alternatively EDTA, rin,sodium citrate, ACD, CPD tube may be used if SST is unable to be obtained). Label Specimen CORRECTLY. 4. INFORMED CONSENT BY EXPOSED PERSON FOR BLOOD TESTS AND PROPHYLAXIS: I understand the risk of infection following accidental blood/body fluids exposure and am aware of the treatment options. I agree to having the following blood tests performed. Tests for titis B,C and will be performed unless excluded (please cross out to exclude): titis BsAg (Diagnosis) titis BsAb (Immunity) titis C Antibody Antibody/Antigen Exposed Person Signature Date Specimen Recep on Instruc ons Test Code Tests HEG HEB HCV s B Surface Ag (diagnosis) s B Surface Ab (immunity) s C 5
6 1. EXPOSED PERSON DETAILS EXPOSED PERSON NEEDLESTICK INJURY, BLOOD/BODY FLUID EXPOSURE TEST REQUEST FORM FOLLOW UP BLOOD TEST FORM 6 MONTH POST NEEDLESTICK EXPOSURE First Name Last Name NHI No. (if known): DOB Workplace Gender (circle one) Male/Female Address Date of Exposure 2. CONTACT PERSON AND GP DETAILS Name of person managing results (e.g. GP, workplace occupational health or infection control person, manager) Position/Title Contact Daytime Phone no: After Hours Phone No: Cell phone No: If the above is not your GP, do you want a copy of your results to be sent to them?: Yes / No (circle) If Yes: Name of Dr Location of Dr s Medical Centre 3. Collect a 5ml Yellow SST tube (however alternatively EDTA, rin,sodium citrate, ACD, CPD tube may be used if SST is unable to be obtained). Label Specimen CORRECTLY. 4. INFORMED CONSENT BY EXPOSED PERSON FOR BLOOD TESTS AND PROPHYLAXIS: I understand the risk of infection following accidental blood/body fluids exposure and am aware of the treatment options. I agree to having the following blood tests performed. Tests for titis B,C and will be performed unless excluded (please cross out to exclude): titis BsAg (Diagnosis) titis BsAb (Immunity) titis C Antibody Antibody/Antigen Exposed Person Signature Date Specimen Recep on Instruc ons Test Code Tests HEG HEB HCV s B Surface Ag (diagnosis) s B Surface Ab (immunity) s C 6
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
DEPARTMENT OF GENITO-URINARY MEDICINE HEPATITIS B
 DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
Blood and Body Fluid Exposure
 Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Hepadnaviridae family (DNA) Numerous antigenic components Humans are only known host May retain infectivity for more than 7 days at room temperature
 Hepatitis B Epidemic jaundice described by Hippocrates in 5th century BC Jaundice reported among recipients of human serum and yellow fever vaccines in 1930s and 1940s Australia antigen described in 1965
Hepatitis B Epidemic jaundice described by Hippocrates in 5th century BC Jaundice reported among recipients of human serum and yellow fever vaccines in 1930s and 1940s Australia antigen described in 1965
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC20 VACCINATION PROGRAMME FOR STAFF AND
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC20 VACCINATION PROGRAMME FOR STAFF AND
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To provide guidelines on the treatment and care of patients with Hepatitis. POLICY Hepatitis is an injury to hepatic cells and an inflammatory process in the liver. The major causes
PAGE 1 of 5 PURPOSE To provide guidelines on the treatment and care of patients with Hepatitis. POLICY Hepatitis is an injury to hepatic cells and an inflammatory process in the liver. The major causes
Address: City: State: ZIP code: Inferferon Product Requested (Include Strength):
 Please complete this entire form and fax it to: 866-940-7328. If you have questions, please call 800-310-6826. This form contains multiple pages. Please complete all pages to avoid a delay in our decision.
Please complete this entire form and fax it to: 866-940-7328. If you have questions, please call 800-310-6826. This form contains multiple pages. Please complete all pages to avoid a delay in our decision.
Management of Exposure to Needlestick Injuries & Body Fluids
 Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Bloodborne Pathogens. By Deborah Massarella RN, MSN
 Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
How to obtain vaccination records
 How to obtain vaccination records Obtaining vaccination records Follow instructions on Blackboard to navigate through to Sonia where all pre-placement information and placement associated information is
How to obtain vaccination records Obtaining vaccination records Follow instructions on Blackboard to navigate through to Sonia where all pre-placement information and placement associated information is
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
EAST LONDON INTEGRATED CARE
 CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
in pregnancy Document Review History Version Review Date Reviewed By Approved By
 GYNAECOLOGY/ ANTENATAL CARE WIRRAL WOMEN & CHILDREN S HOSPITAL Guideline No: Hepatitis B management in pregnancy VERSION 1 AMENDMENTS MADE: N/A DATE OF ISSUE: May 2012 DATE OF REVIEW: May 2015 REVIEW INTERVAL:
GYNAECOLOGY/ ANTENATAL CARE WIRRAL WOMEN & CHILDREN S HOSPITAL Guideline No: Hepatitis B management in pregnancy VERSION 1 AMENDMENTS MADE: N/A DATE OF ISSUE: May 2012 DATE OF REVIEW: May 2015 REVIEW INTERVAL:
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
RUTGERS POLICY. Errors or changes? Contact: Rutgers University Occupational Health Department
 RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Student Immunisation Record Faculty of Medicine. Section 1: Information. Notes
 Student Immunisation Record Faculty of Section 1: Information Students enrolled in programs offered by the Faculty of are REQUIRED to provide evidence of their immunisation status for the diseases listed
Student Immunisation Record Faculty of Section 1: Information Students enrolled in programs offered by the Faculty of are REQUIRED to provide evidence of their immunisation status for the diseases listed
SHARPS MANAGEMENT AND INOCULATION INJURIES
 Community Infection Prevention and Control Guidance for Health and Social Care Sharps Management and Inoculation Injuries Version 1.01 May 15 Harrogate and District NHS Foundation Trust Sharps Management
Community Infection Prevention and Control Guidance for Health and Social Care Sharps Management and Inoculation Injuries Version 1.01 May 15 Harrogate and District NHS Foundation Trust Sharps Management
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids
 Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Immunization Policy. "UIC/COD-sponsored graduate education program" is one for which UIC/COD maintains academic responsibility.
 I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
Student Health Requirements Master of Arts, Biomedical Sciences Program
 Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官
 C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
EMPLOYEE HEALTH TABLE OF CONTENTS
 EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
keyword: hepatitis Hepatitis
 www.bpac.org.nz keyword: hepatitis Hepatitis Key reviewers: Dr Susan Taylor, Microbiologist, Diagnostic Medlab, Auckland Dr Tim Blackmore, Infectious Diseases Physician and Microbiologist, Wellington Hospital,
www.bpac.org.nz keyword: hepatitis Hepatitis Key reviewers: Dr Susan Taylor, Microbiologist, Diagnostic Medlab, Auckland Dr Tim Blackmore, Infectious Diseases Physician and Microbiologist, Wellington Hospital,
Emergency Department Blood/Body Fluid Exposure Instructions
 Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
End Stage Liver Disease & Disease Specific Indications for Liver Transplant. Susan Kang, RN, MSN, ANP-BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
HNE Health Immunisation Requirements for Health Care Students
 FREQUENTLY ASKED QUESTIONS (FAQ) When is my information to be provided to NSW Health? Your immunisation compliance must be completed within six months of commencement of the course and well in advance
FREQUENTLY ASKED QUESTIONS (FAQ) When is my information to be provided to NSW Health? Your immunisation compliance must be completed within six months of commencement of the course and well in advance
HEPATITIS HEPATITIS A. The Hepatitis Alphabet HOW DOES ONE GET HEPATITIS A? THE SYMPTOMS of HEPATITIS A
 HEPATITIS Hepatitis is an inflammation of the liver that can be caused by viruses, chemicals or drugs. The two most common types of viral hepatitis are Hepatitis A (also called infectious hepatitis ) and
HEPATITIS Hepatitis is an inflammation of the liver that can be caused by viruses, chemicals or drugs. The two most common types of viral hepatitis are Hepatitis A (also called infectious hepatitis ) and
Nursing and Midwifery students only. Section 1: Information
 Nursing and Midwifery students only. Section 1: Information Students enrolled in programs offered by our School are REQUIRED to provide evidence of their immunisation status for the diseases listed in
Nursing and Midwifery students only. Section 1: Information Students enrolled in programs offered by our School are REQUIRED to provide evidence of their immunisation status for the diseases listed in
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE
 CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
Hepatitis B Positive: Care of Mother Policy
 Document ID: MATY031 Version: 1.0 Facilitated by: Jo McMullan, CMM Issue Date: July 2012 Approved by: Maternity Quality Committee Review date: October 2016 Hepatitis B Positive: Care of Mother Policy Purpose
Document ID: MATY031 Version: 1.0 Facilitated by: Jo McMullan, CMM Issue Date: July 2012 Approved by: Maternity Quality Committee Review date: October 2016 Hepatitis B Positive: Care of Mother Policy Purpose
HEPATITIS B NON-IMMEDIATE NOTIFICATION EPIDEMIOLOGY PROGRAM
 HEPATITIS B NON-IMMEDIATE NOTIFICATION EPIDEMIOLOGY PROGRAM Event Name: Event Time Period: ACUTE Clinical Presentation (CDC 2012): CDC Event (2012): HEPB Lifelong immunity An acute illness with a discrete
HEPATITIS B NON-IMMEDIATE NOTIFICATION EPIDEMIOLOGY PROGRAM Event Name: Event Time Period: ACUTE Clinical Presentation (CDC 2012): CDC Event (2012): HEPB Lifelong immunity An acute illness with a discrete
Sunny Smiles Clinical Guidelines
 Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
HEPATITIS B INFECTION and Pregnancy. Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011
 HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
Media centre. WHO Hepatitis B. Key facts. 1 of :12 AM.
 1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
Bloodborne Pathogens. Penn State University Environmental Health & Safety
 Bloodborne Pathogens Penn State University Environmental Health & Safety Diseases of Concern Hepatitis B (Serum Hepatitis) Hepatitis C (non-a non-b Hepatitis) HIV (Human Immunodeficiency Virus aka the
Bloodborne Pathogens Penn State University Environmental Health & Safety Diseases of Concern Hepatitis B (Serum Hepatitis) Hepatitis C (non-a non-b Hepatitis) HIV (Human Immunodeficiency Virus aka the
Hepatitis B and C Overview, Outbreaks, and Recommendations. Viral Hepatitis Language. Types of Viral Hepatitis 7/1/2013
 Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B Positive: Care of Mother Policy
 Document ID: MATY031 Version: 1.0 Facilitated by: Jo McMullan, CMM Last reviewed: October 2014 Approved by: Maternity Quality Committee Review date: October 2016 Hepatitis B Positive: Care of Mother Policy
Document ID: MATY031 Version: 1.0 Facilitated by: Jo McMullan, CMM Last reviewed: October 2014 Approved by: Maternity Quality Committee Review date: October 2016 Hepatitis B Positive: Care of Mother Policy
BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees
 BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees Buncombe County Public Schools require employees to receive annual training for Bloodborne Pathogens. This online training
BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees Buncombe County Public Schools require employees to receive annual training for Bloodborne Pathogens. This online training
SUMMARY OF PRODUCT CHARACTERISTICS. Human proteins g/l g/l of which human immunoglobulin at least to. 180 IU/ml 180 IU/vial
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT UMAN BIG 180 IU/ml Solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. UMAN BIG 180 IU/1
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT UMAN BIG 180 IU/ml Solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. UMAN BIG 180 IU/1
Immunisation Requirements and Mandatory Health Screenings
 Immunisation Requirements and Mandatory Health Screenings The purpose of pre-employment screening is to ensure that you are fit for the position you have applied for and that you don t have any condition
Immunisation Requirements and Mandatory Health Screenings The purpose of pre-employment screening is to ensure that you are fit for the position you have applied for and that you don t have any condition
Viral hepatitis Blood Born hepatitis. Dr. MONA BADR Assistant Professor College of Medicine & KKUH
 Viral hepatitis Blood Born hepatitis Dr. MONA BADR Assistant Professor College of Medicine & KKUH Outline Introduction to hepatitis Characteristics of viral hepatitis Mode of transmission Markers of hepatitis
Viral hepatitis Blood Born hepatitis Dr. MONA BADR Assistant Professor College of Medicine & KKUH Outline Introduction to hepatitis Characteristics of viral hepatitis Mode of transmission Markers of hepatitis
MA PERINATAL HEPATITIS B PREVENTION PROGRAM
 MA PERINATAL HEPATITIS B PREVENTION PROGRAM Massachusetts Department of Public Health Immunization Program MIAP 2016 1 1 Presenter Disclosure Information I, Theodora Wohler, have been asked to disclose
MA PERINATAL HEPATITIS B PREVENTION PROGRAM Massachusetts Department of Public Health Immunization Program MIAP 2016 1 1 Presenter Disclosure Information I, Theodora Wohler, have been asked to disclose
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
HNE Health Immunisation Requirements for Health Care Students
 FREQUENTLY ASKED QUESTIONS (FAQ) When is my information to be provided to NSW Health? Your immunisation compliance must be completed within six months of commencement of the course and well in advance
FREQUENTLY ASKED QUESTIONS (FAQ) When is my information to be provided to NSW Health? Your immunisation compliance must be completed within six months of commencement of the course and well in advance
Questions & Answers 1 November 2005 Vaccine Schedule
 Questions & Answers 1 November 2005 Vaccine Schedule General Questions Q. When can clinics order the new vaccine? A. From early October 2005. Orders will be placed on back order & stock will be sent out
Questions & Answers 1 November 2005 Vaccine Schedule General Questions Q. When can clinics order the new vaccine? A. From early October 2005. Orders will be placed on back order & stock will be sent out
Staff Immunisation Policy
 Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE
 Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses
 Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Measles Elimination Dr. Suzanne Cotter 4 th National Immunisation Conference 2007
 Measles Elimination 2010 Dr. Suzanne Cotter 4 th National Immunisation Conference 2007 Measles Viral illness Highly infectious Human reservoir only Respiratory transmission Responsible for ~500,000 deaths
Measles Elimination 2010 Dr. Suzanne Cotter 4 th National Immunisation Conference 2007 Measles Viral illness Highly infectious Human reservoir only Respiratory transmission Responsible for ~500,000 deaths
Appendix 11 Roles and Responsibilities October, 2013 Page 1 of 5
 October, 2013 Page 1 of 5 Exposed Person To present to a health care facility as soon as possible following the exposure (ideally within 2 hours). To answer assessment questions. (Refer to Appendix 15
October, 2013 Page 1 of 5 Exposed Person To present to a health care facility as soon as possible following the exposure (ideally within 2 hours). To answer assessment questions. (Refer to Appendix 15
MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES
 NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
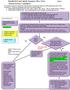 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needle Stick. Mr. Fadi J. Zaben RN MSN IMET 2000, Ramallah IMET 2000
 Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
SAMPLE OF PRE-COURSE OCCUPATIONAL HEALTH QUESTIONNAIRE 2017
 SAMPLE OF PRE-COURSE OCCUPATIONAL HEALTH QUESTIONNAIRE 2017 PLEASE NOTE THIS IS FOR GUIDANCE ONLY AND IS SUBJECT TO CHANGE PART A Applicant Personal Information PART B Applicant General Health Information
SAMPLE OF PRE-COURSE OCCUPATIONAL HEALTH QUESTIONNAIRE 2017 PLEASE NOTE THIS IS FOR GUIDANCE ONLY AND IS SUBJECT TO CHANGE PART A Applicant Personal Information PART B Applicant General Health Information
Primary Care Services for blood borne viral hepatitis prevention, treatment and care
 Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
CARE OF HEPATITIS B POSITIVE MOTHER
 CARE OF HEPATITIS B POSITIVE MOTHER SCOPE All medical and midwifery staff employed by Hutt Valley DHB. All Hutt Valley DHB Maternity access holders. All Special Care Baby Unit staff PURPOSE The purpose
CARE OF HEPATITIS B POSITIVE MOTHER SCOPE All medical and midwifery staff employed by Hutt Valley DHB. All Hutt Valley DHB Maternity access holders. All Special Care Baby Unit staff PURPOSE The purpose
Department of Health. Year 8. vaccination program. Important information for parents and students
 Department of Health Year 8 vaccination program Important information for parents and students Contents Why immunise? 1 Vaccination program 1 Schedule of vaccinations 2 Vaccination records 2 Vaccine safety
Department of Health Year 8 vaccination program Important information for parents and students Contents Why immunise? 1 Vaccination program 1 Schedule of vaccinations 2 Vaccination records 2 Vaccine safety
Primary Sample Manual Infectious Serology Issue No Effective Date: 20/09/17 Page 1 of 15 EUROFINS BIOMNIS
 Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
Uses and Misuses of Viral Hepatitis Testing. Origins of Liver Science
 Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Blood Borne Pathogen Exposure Employee
 Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
Health Unit Coordinator
 Health Unit Coordinator Health Requirements Checklist All MATC Health Science students are required to complete and upload health requirements prior to petitioning for courses which contain a clinical
Health Unit Coordinator Health Requirements Checklist All MATC Health Science students are required to complete and upload health requirements prior to petitioning for courses which contain a clinical
Management of Hepatitis B - Information for primary care providers
 Management of Hepatitis B - Information for primary care providers July 2018 Chronic hepatitis B (CHB) is often a lifelong condition. Not everyone infected needs anti-viral therapy. This document outlines
Management of Hepatitis B - Information for primary care providers July 2018 Chronic hepatitis B (CHB) is often a lifelong condition. Not everyone infected needs anti-viral therapy. This document outlines
Chapter 2 Hepatitis B Overview
 Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT
 GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: For active immunisation of individuals
GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: For active immunisation of individuals
OCCUPATIONAL HEALTH PROTOCOL
 Faculty of Medicine and Surgery OCCUPATIONAL HEALTH PROTOCOL (Applicable to applicants for the Doctor of Medicine and Surgery M.D. - Degree Course) Applicability for Courses commencing in October 2015
Faculty of Medicine and Surgery OCCUPATIONAL HEALTH PROTOCOL (Applicable to applicants for the Doctor of Medicine and Surgery M.D. - Degree Course) Applicability for Courses commencing in October 2015
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B. Acute HBV Questionnaire
 BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
Phlebotomy Health Requirements Checklist
 Phlebotomy Health Requirements Checklist The applicant must: 1). Upload the original completed form to your CertifiedBackground profile. 2). Retain a copy for your records. www.certifiedbackground.com
Phlebotomy Health Requirements Checklist The applicant must: 1). Upload the original completed form to your CertifiedBackground profile. 2). Retain a copy for your records. www.certifiedbackground.com
Student Health Services 881 Commonwealth Ave, West / Student Information (To be completed by the student) Student Name Last First Middle
 Medical Clearance The following information must be completed on the medical history form, if any information is missing the form will be considered incomplete and will not be processed. If you have questions,
Medical Clearance The following information must be completed on the medical history form, if any information is missing the form will be considered incomplete and will not be processed. If you have questions,
OCCUPATIONAL HEALTH PROTOCOL
 Faculty of Dental Surgery OCCUPATIONAL HEALTH PROTOCOL Applicable to applicants for the: Master of Dental Surgery Course Bachelor of Science (Honours) in Dental Hygiene Course Bachelor of Science (Honours)
Faculty of Dental Surgery OCCUPATIONAL HEALTH PROTOCOL Applicable to applicants for the: Master of Dental Surgery Course Bachelor of Science (Honours) in Dental Hygiene Course Bachelor of Science (Honours)
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Chapter 5 Serology Testing
 Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
P L E A S E N O T E - There will be a charge for each person, even if your travel has been discussed previously with your own GP.
 I M P O R T A N T - It is preferable that your Travel Consultation appointment is made one month prior to your departure. Please return this record this form promptly. You will be advised of an appointment
I M P O R T A N T - It is preferable that your Travel Consultation appointment is made one month prior to your departure. Please return this record this form promptly. You will be advised of an appointment
LeadingAge Florida February 24, 2016
 BLOODBORNE PATHOGENS EMPLOYEE HANDBOOK 1 POLICY EMPLOYEE ORIENTATION AND IN-SERVICE TO BLOODBORNE PATHOGENS AWARENESS It is the policy of Healthcare Services Group, Inc., to ensure that all employees with
BLOODBORNE PATHOGENS EMPLOYEE HANDBOOK 1 POLICY EMPLOYEE ORIENTATION AND IN-SERVICE TO BLOODBORNE PATHOGENS AWARENESS It is the policy of Healthcare Services Group, Inc., to ensure that all employees with
Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September 20, 2001
 Policy # MI/SER/04/v01 Page 1 of 9 Section: Subject Title: Hepatitis Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September
Policy # MI/SER/04/v01 Page 1 of 9 Section: Subject Title: Hepatitis Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September
PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS
 PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS IMMUNISATION AND HEALTH RECORDS Q: When should I commence my immunisations? A: All immunisation requirements must be completed prior to placement. It is
PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS IMMUNISATION AND HEALTH RECORDS Q: When should I commence my immunisations? A: All immunisation requirements must be completed prior to placement. It is
Policy # MI/SER/02/v03 Page 1 of 9 Policy & Procedure Manual
 Policy # MI/SER/02/v03 Page 1 of 9 Section: Subject Title: AxSYM System Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: October 28, 2003 AXSYM
Policy # MI/SER/02/v03 Page 1 of 9 Section: Subject Title: AxSYM System Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: October 28, 2003 AXSYM
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019
 Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis
 Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
CUSOM Student Health Immunization Requirements
 CUSOM Student Health Immunization Requirements Regulatory and legislative authorities require that students demonstrate immunization, immunity and/or protection from multiple contagious diseases before
CUSOM Student Health Immunization Requirements Regulatory and legislative authorities require that students demonstrate immunization, immunity and/or protection from multiple contagious diseases before
Viral Hepatitis - Historical Perspective
 Viral Hepatitis - Historical Perspective Infectious A E Enterically transmitted Viral hepatitis NANB Serum B D F, G,? other C Parenterally transmitted Before the discovery of hepatitis A virus (HAV) and
Viral Hepatitis - Historical Perspective Infectious A E Enterically transmitted Viral hepatitis NANB Serum B D F, G,? other C Parenterally transmitted Before the discovery of hepatitis A virus (HAV) and
