Blood Borne Pathogen Exposure Employee
|
|
|
- Cornelia George
- 6 years ago
- Views:
Transcription
1 Blood Borne Pathogen Exposure Employee
2 INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information for Workers Compensation form 5020 Employer s Report of Occupational Injury or Illness Complete Workers Compensation form 5021 Doctor s First Report of Occupational Injury or Illness form and give to healthcare provider Complete Routes and Circumstances of Exposure Incident form Obtain Medical follow-up (see below) 2. Supervisor: see BBP Exposure packet for further instructions Obtain name and, if available, health record number of source patient 3. Provider: see BBP Exposure packet for further instructions As soon as possible, send employee to the Medical Center located at the Patient Care Center-Pomona 795 E Second Street, Suite 5 Pomona, CA (Corner of Towne Avenue and Second Street). Phone: Patient Care Center-Rancho Cucamonga 8686 Haven Avenue, Suite 200 Rancho Cucamonga, CA Phone: Draw source patient s blood. Call the Student-Employee Health Coordinator at the number below to report the exposure. The exposed employee has the choice of being seen by a WUMC healthcare provider, their own healthcare provider or they can go to the nearest emergency room for evaluation/treatment. Medical Center hours: Monday through Friday 8am to 5pm (closed weekends and holidays). NOTE: Should you have any questions, please call: Risk Manager Chique Magsino at (or extension 5452) or Student-Employee Health Coordinator, Eileen Cotter at (or extension 3870)
3 SUPERVISOR CHECKLIST 1. Provide BBP Exposure packet to the exposed/injured worker. 2. Obtain name, DOB and, if available, health record number of source patient 3. Release employee immediately to go to the PCC-Medical Center (or the employee s own healthcare provider) for medical follow up. (NOTE: If employee refuses to obtain medical care, clearly document this refusal of medical treatment on the Workers Compensation form 5020 Employer s Report of Occupational Injury or Illness and notify the WesternU Workers Compensation Coordinator in Human Resources at extension 5371.) 4. Obtain Workers Compensation form 5020 Employer s Report of Occupational Injury or Illness and assist employee in completing sections Forward form to Workers Compensation Coordinator in Human Resources. 5. Obtain Workers Compensation form 5021 Doctor s First Report of Occupational Injury or Illness form and assist employee in completing sections Instruct employee to give form to healthcare provider. 6. Access and complete the Incident Report form available on line at it will automatically be distributed to the appropriate individuals. 7. Notify the university s Workers Compensation Coordinator in Human Resources and Risk Management of BBP Exposure including the date, time of exposure, name and health record number of employee. 8. The DWC-1 and form 5020 are to be forwarded to the Worker s Compensation Coordinator in Human Resources. NOTE: Should you have any questions, please call: Risk Manager Chique Magsino at (or extension 5452) or Student-Employee Health Coordinator, Eileen Cotter at (or extension 3870)
4 HEALTHCARE PROVIDER CHECKLIST EXPOSED PERSON 1. Assess need for and provide: Review Routes and Circumstances of Exposure Incident form Tetanus/diphtheria booster following percutaneous injury, especially if not received within last 10 years (or Tdap booster if not received since age of 18 years). Verify Hepatitis B vaccination status and give HBIG if indicated. Provide HIV pre-test counseling. 2. Obtain Employee baseline tests: Human Immunodeficiency Virus antibody (HIV Ag/Ab, 4th Generation test) (blood) Hepatitis B surface antibody quantitative (HBsAb QN); Hepatitis B Surface Antigen (HBsAG), & Hepatitis B core antibody IgG/IgM (blood) Hepatitis C antibody with reflex to RIBA (HCV Ab) (blood) 3. If the source patient is already known to be HIV positive, consult with Infectious Disease physician as soon as possible. 4. If Employee has never received the Hepatitis B vaccine or has a history of HBV infection the following serum blood tests should also be ordered: HBsAg, HBcAg IgM/IgG. 5. For post-exposure prophylaxis for exposed person: a. PEP medications to order are: i. Truvada 300/200mg 1 pill by mouth daily AND ii. Raltegravir 400mg 1 tab by mouth 2 times per day b. If source person is high risk, prescribe enough PEP for 5 days i. Within 5 days, lab results will be available. ii. If the source patient is negative for HIV, PEP can be stopped. iii. If the source is positive for HIV, provide an additional 23-day supply of PEP medications. c. If source person is known to be HIV positive, prescribe enough PEP for a total of 28- days. d. If any further questions related to medications and dosages, please contact infectious disease physician at or provider may call the National PEP Hotline at e. If the exposed person is an employee, must write WU Occupational Exposure on the prescription. NOTE: Should you have any questions, please call: Risk Manager Chique Magsino at (or extension 5452) or PCC Student-Employee Health Coordinator, Eileen Cotter at (or extension 3870)
5 HEALTHCARE PROVIDER CHECKLIST SOURCE PERSON 1. Have source patient stay until the healthcare provider has had an opportunity to speak to the patient in order to determine level of risk and obtain verbal consent for testing for what is listed in item #2. 2. On known source patients, inform patient blood will be tested for HIV, Hepatitis B and C. Order the following tests: Human Immunodeficiency Virus antibody (HIV Ag/Ab, 4th Generation test) (blood) Hepatitis B surface antibody quantitative (HBsAb QN); Hepatitis B Surface Antigen (HBsAG), & Hepatitis B core antibody IgG/IgM (blood) Hepatitis C antibody with reflex to RIBA (HCV Ab) (blood) 3. When the source patient is not tested at the time of the exposure, contact the source person and obtain consent to contact their physician in order to have source patient s testing completed and results forwarded to Student-Employee Health Coordinator. 4. After explaining the need for testing to the source patient or their representative, if source patient refuses testing, please document the refusal in the health record, on the exposed employee lab requisitions, and if an employee, on the Worker s Compensation forms. 5. If test results are negative, notify source person and exposed person. 6. If test results are positive, notify source person and refer them to their personal health care provider for further follow up and/or treatment. Clearly document that the source person has been referred in their health record. 7. If test results are positive, the exposed employee should be referred to an infectious disease physician for further evaluation and follow-up. Worker s Compensation will be notified of positive results by the Student- Employee Health Coordinator. NOTE: Should you have any questions, please call: Risk Manager Chique Magsino at (or extension 5452) or Student-Employee Health Coordinator, Eileen Cotter at (or extension 3870)
6 Informed Consent for Prophylaxis After Bloodborne/Body Fluid Pathogen Exposure As an employee or student of Western University of Health Sciences, I know I have the right to be informed about the risks from a bloodborne or body fluid pathogen exposure and the recommended prophylaxis (retroviral drug) treatment. I have been informed that there are certain side effects which are associated with retroviral drugs. They can include at minimum nausea, vomiting, diarrhea, headache, sensitivity to light (called photophobia), and fatigue. I have been counseled to use precautions like use of barrier contraception, avoidance of blood or tissue donation, pregnancy and, if possible, breastfeeding for up to 12-weeks after this exposure. My healthcare provider has reviewed the recommended tests and medications that I should take because of this exposure. I have also had an opportunity to discuss the side effects of any medications recommended and what to do for follow-up care. I have read and understand the patient information booklet entitled Exposure to Blood: what healthcare personnel need to know which explains the risks of infection, prophylaxis, medications, pregnancy precautions, follow-up and special precautions. The healthcare provider has informed me of the possible risks associated with refusing these medications. The nature and purpose of the proposed prophylaxis and the risks and hazards if the treatment is withheld have been explained to me by the healthcare provider. I have had an opportunity to discuss these matters with a healthcare provider, to ask questions and receive answers about my exposure, alternatives, and the proposed treatments. I hereby consent to medication prophylaxis for exposure to blood and body fluids infection or possibly infected with HIV. I hereby decline medication prophylaxis following my exposure to bloodborne pathogens. Employee Name Date Time Employee Signature Healthcare Provider Name Date Time Witness Date Time
7 *Mandatory Vaccination Declination Waiver Form Today s Date: Center: I understand that my serum titers showed that I may be susceptible to acquiring a communicable disease. However, my signature below indicates that: I object to receiving the following required immunizations (check all that apply): Tetanus* Measles* Varicella* Diphtheria* Mumps* Hepatitis B** Pertussis* Rubella* Influenza I have read and fully understand all of the restrictions that apply, and are enforced, by this waiver. (initials) I have been provided a copy of this waiver form. (initials) The employer shall ensure that employees who decline to accept a recommended vaccination offered by the employer sign and date the following statement as required by California Code of Regulations, Title 8, subchapter 7; Group 16. Article 109; section 5199, subsection (h)(5)(e). I understand that due to my occupational exposure to aerosol transmissible disease, I may be at risk of acquiring infection with (name of disease or pathogen). I have been given the opportunity to be vaccinated against this disease or pathogen at no charge to me. However, I decline this vaccination at this time. I understand that by declining this vaccine, I continue to be at risk of acquiring, a serious disease. If in the future I continue to have occupational exposure to aerosol transmissible diseases and want to be vaccinated, I can receive the vaccination at no charge to me. (initials) ** Mandatory Vaccination Declination Statement Specific to Hepatitis B The employer shall ensure that employees who decline to accept a recommended vaccination offered by the employer sign and date the following statement as required by California Code of Regulations, Title 8, subchapter 7; Group 15. Article 109; section 5193, subsection (f)(2)(d): I understand that due to my occupational exposure to blood or other potentially infection material (OPIM) I may be at risk of acquiring hepatitis B virus (HBV) infection. I have been given the opportunity to be vaccinated with hepatitis B vaccine, at no charge to myself. However, I decline hepatitis B vaccination at this time. I understand that by declining this vaccine, I continue to be at risk of acquiring hepatitis B, a serious disease. If in the future I continue to have occupational exposure to blood or OPIM and I want to be vaccinated with hepatitis B vaccine, I can receive the vaccination series at no charge to me. (initials) Print Name Signature Date of Birth Employee ID number
8 Workers Compensation Claim Reporting Procedures (form DWC-1) Important: Employee s supervisor should be personally handed or mailed an Employee s Claim for Worker s Compensation Benefits form (DWC-1) within one (1) working day of an on the job illness or injury. Personal Delivery: Step 1: Step 2: Step 3: Step 4: Step 5: Complete lines 1-8. Enter employee s name and today s date. Supervisor completes lines 9 through12. The date on line 12 should be the same date or one working day after the date on line 11. Make a copy of the form and keep one for your files and send the original to Western University s Human Resources Department, Attention Workers Compensation Coordinator. Fill in blanks of cover letter ( sorry you re hurt letter) with employee s name and date, attach it to the DWC-1 form and give it to the employee. When employee returns completed form (sections 1-12), supervisor completes lines 13 through 18. Mail Delivery: Give a completed copy of the DWC-1 form to the employee for their records. Keep the Employer s Copy for the Workers Compensation file. Either hand deliver or fax the completed form to (909) , attention Worker s Compensation Coordinator. Steps 1 & 2 Step 3: Step 4: are the same as above, except for the following changes: On line 12 of the form, the date should reflect the day you mailed the form, which is either the same date or one working day after the date found on line 11. Complete Certified Mail Delivery form for tracking purposes. Make a copy of the mail form and keep the original form for your files. Send copies of both the Employer s Temporary Copy claim and Proof of Services to the University s Workers Compensation Coordinator. Fill in blanks of cover letter ( Sorry you re hurt letter) with employee s name and date. Attach it to the DWC-1 form, a copy of the Proof of Service and mail all forms by first class registered mail to the employee. NOTE: This must be mailed within one working day of the date of knowledge of the injury. Step 5: Same as step 5 in the Personal Delivery section above.
9 Information to be Provided to the Evaluating Healthcare Provider (Routes and Circumstances of Exposure Incident) Please Print Employee's Name Date Date of Birth SS# Telephone (Business) (Home/Cell) Department/Job Title Date of Exposure, 20 Time of Exposure AM PM Hepatitis B Vaccination Status Completed series Never received series Unknown Location of Incident Describe what job duties you were performing when the exposure incident occurred. Describe the circumstances under which the exposure incident occurred (what happened that resulted in the incident) What body fluid(s) were you exposed to? What was the route of exposure (e.g., mucosal contact, contact with nonintact skin, percutaneous)? Describe any personal protective equipment (PPE) in use at time of exposure incident: gloves cover gown face shield face mask Other Did PPE fail? If yes, how? Identification of source individual(s) (names) Other pertinent information
10 To the Evaluating Healthcare Provider: Post-Bloodborne Pathogen Exposure WRITTEN OPINION The evaluation of this Western University of Health Sciences employee has been completed. The exposed person has been: Informed of the results of this evaluation. Advised about any medical conditions resulting from exposure to blood or other potentially infectious materials which require further evaluation and treatment. NOTE: No other findings should be included on this report. Please give one copy of this completed and signed form to the exposed person and send one copy to: Western University of Health Sciences Attention: Student-Employee Health Coordinator 479 E Second Street, Room 110 Secure fax line: (909) Healthcare Provider's signature Healthcare Provider's name (printed) Date
11 Bloodborne/Body Fluid Post Exposure Protocol In the event that an occupational exposure to blood or body fluids (that is not the employee s) occurs, and the employee chooses to be seen in one of the Western University of Health Sciences Medical Center (WUMC), the following steps should be performed: I. MANAGE THE EXPOSURE A. Employee should cleanse/wash any exposed skin immediately with soap and water. B. Exposure to eyes, nose, and/or mouth should be thoroughly flushed with a lot of lukewarm to warm water. II. NOTIFY SUPERVISOR/FACULTY IMMEDIATELY A. Supervisor/faculty shall release the employee from their duties immediately to seek postexposure care. a. Employees have the choice of going to: i. The WUMC located in the PCC building in Pomona or in Rancho Cucamonga ii. US Healthworks iii. Their own healthcare provider iv. Nearest hospital s emergency room B. Supervisor will assist the employee in contacting the Student-Employee Health Coordinator at extension 3870 ( ) about the exposure C. If instructed by the health care provider, supervisors will assist in obtaining the source patient s consent for required lab work. III. REQUIRED REPORTS/DOCUMENTATION A. Following an exposure incident, the following forms must be completed: 1) DWC-1 known as a Workers Compensation Claim form 2) DWC form 5021 known as Doctor s First Report of Occupational Injury or Illness 3) DWC form 5020 known as Employer s Report of Occupational Injury or Illness 4) Western U s Human Resources form Accident Investigation and Prevention Report B. These forms can be found online or at the Medical Center located in the Patient Care Center in Pomona and Rancho Cucamonga. C. The Employee, along with their Supervisor should: 1) Complete the DWC-1 entirely. 2) Complete the Employer and Employee section on form 5020.
12 i. These two forms should then be given to the Workers Compensation Coordinator in Human Resources. 3) Form 5021 items 1 through 17 only and then give the form to the healthcare provider. i. Healthcare provider is responsible for forwarding on this form to the Workers Compensation Coordinator. IV. MEDICAL EVALUATION/COUNSELING A. Employee should be aware that time is of the essence when a blood/body fluid exposure has occurred. Immediate medical assessment and intervention should be sought after the exposure. B. The first available healthcare provider is to see the individual(s). The first available Medical Assistant is to assist in drawing/processing the blood. C. Employee and source patient (if known) will have to be counseled and advised that their blood will be tested for HIV, Hepatitis B and Hepatitis C. A cheek swab may also be obtained at the initial visit. The counseling is to be done by the licensed healthcare provider. Post-exposure counseling must be consistent with the current US Public Health Services Guidelines. 1) Please note that when the source patient is already known to be infected with HBV, HCV and/or HIV, testing the source s blood is not necessary.. D. Neither the employee nor the source patient is to be charged for any of these tests or if needed, medications. This is per state law. E. The route of exposure and circumstances under which the incident occurred should be clearly documented. F. All information obtained at this evaluation is to be kept confidential and only the minimum necessary information can be released to the Workers Compensation Coordinator. G. If the Medical Center is closed (after normal business hours or holidays), the employee should be referred to US Health Works, their own healthcare provider, or the nearest emergency room for immediate medical evaluation and follow up. H. If an exposure should occur after normal business hours, on weekends or holidays, please contact the Student-Employee Health Coordinator as and leave brief message. 1) NOTE: If any BBP exposure occurs in or on the Oregon WesternU campus, please contact the Director of Operations at if the exposure is with an employee or a student. Be sure to also call the Student-Employee Health Coordinator at I. Employees/students with off campus exposure shall notify Student-Employee Health Coordinator as soon as possible even when evaluation and/or treatment are initiated elsewhere. The Student-Employee Health Coordinator will notify Risk Management and the Workers Compensation Coordinator in Human Resources. V. PROCEDURE FOR SOURCE TESTING NOTE: California law requires that at no time is the exposed person to approach the source patient and request verbal consent to test the source s blood. The verbal consent must be obtained from a neutral party, e.g., healthcare provider or employee s supervisor.
13 A. Obtain the source and exposed person s verbal consent to test their blood for HIV, Hepatitis B and Hepatitis C. Once consent has been obtained, complete the required documents for the reference lab. Obtain and label the blood specimens. Be sure each set of specimens are clearly marked as Source or Exposed and that the correct requisition is attached to each specimen. B. If the source person refuses to have their blood drawn this should be documented on the exposed person s health record and on the Lab Requisition for the employee s exposure. C. It is important to notify WesternU Risk Management Department (at extension 5452 [ ]) of the exposure and if the source person grants or refuses consent for their blood to be drawn and tested for HIV, Hepatitis B and Hepatitis C. D. Document exposure in source patient s health record and document that the following labs were drawn for source of occupational exposure. ii. On source patients, order the following tests: Human Immunodeficiency Virus (HIV Ag/Ab, 4th Generation test) (blood) Hepatitis B surface antibody quantitative (HBsAb QN); Hepatitis B Surface Antigen (HBsAG), & Hepatitis B core antibody IgG/IgM (blood) Hepatitis C antibody with reflex to RIBA (HCV Ab) [serum blood test] E. Laboratory results for the exposed employee and source person will be sent to the healthcare provider for review and to determine if medical follow-up is needed. F. The Source s laboratory results will be reviewed by the healthcare provider to determine necessary follow-up. Results will then be scanned in the source person s health record and, if requested by the source patient, notified of their test results. VI. POST EXPOSURE CARE: TESTING/TREATMENT A. The Medical Center, located at the Patient Care Center in Pomona or in Rancho Cucamonga, is open Monday through Friday 8AM to 5PM. B. Holidays, weekends, or after hours Employees are to be immediately referred to the nearest hospital Emergency room or personal healthcare provider. 1) Determine if exposure occurred and to what extent in order to ensure that the initiation of prophylaxis is begun within hours of exposure. 2) Inform employee that the blood will be tested for HIV, Hepatitis B and Hepatitis C. 3) Submit Employee specimens using the lab order form from LabCorp (they are already premarked for the required tests for the employee and source patient). a. Order labs for exposed individual: HIV Ag/Ab, 4th Generation test (blood), Hepatitis B surface antibody quantitative (HBsAb QN); Hepatitis B Surface, Antigen (HBsAg), & Hepatitis B core antibody IgG/IgM (blood); Hepatitis C antibody with reflex to RIBA (HCV Ab) (blood) b. If the healthcare provider determines that the employee should be started on antiretroviral prophylaxis, the following labs should also be ordered: CBC, ALT, AST, total bilirubin,
14 Creatinine and BUN at baseline and 2-weeks post-exposure. Further testing may be indicated if abnormalities are detected. c. Females of childbearing age must have a urine or serum pregnancy test performed prior to starting medications. d. Send blood specimens to reference lab per protocol. 4) Post-exposure Treatment and Follow up a. Offer tetanus/diphtheria booster following percutaneous injury, especially if not received within last 10 years (or Tdap booster if not received since age of 18 years). b. If employee has not been vaccinated against Hepatitis B: i. Offer Hepatitis B vaccine if source is high risk for hepatitis B. ii. Offer Hepatitis B Immune Globulin 0.06 ml/kg IM along with HBV vaccine if source is known to be positive for Hepatitis B. c. Employees testing positive for Hepatitis B and/or C initially or at 6 months, will be referred immediately to an appropriate healthcare provider for further evaluation. If tests are positive for HIV initially, at 6 weeks or at 6 months, referral to an infectious disease physician will be recommended. d. Any follow up labs are to be arranged by the employee s primary care provider or US Healthworks for the exposed employee. e. Recommend HIV prophylaxis following percutaneous injury or contamination of mucous membranes or nonintact skin with blood, body fluids visibly contaminated with blood, unfixed tissue, semen, vaginal secretions, and cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids (goal is to begin within hours of exposure). Post-exposure prophylaxis for exposed person, prescription for the following should be given: a. If source person is high risk or known to be HIV positive, prescribe enough for 5 days i. Truvada 300/200mg 1 pill by mouth daily AND ii. Raltegravir 400mg 1 tab by mouth 2 times per day b. Within 5 days, lab results will be available. i. If the source patient is negative for HIV, PEP can be stopped. ii. If the source is positive for HIV, provide an additional 23-day supply of PEP medications. c. If any further questions related to medications and dosages, please contact infectious disease physician at or provider may call the National PEP Hotline at d. Prophylaxis medications write WU Occupational Exposure on prescription if it is for an employee of WesternU. i. Provide with 5 day supply of medication. ii. Assure Consent for Post-Exposure Prophylaxis is completed and a signed copy of the consent is given to patient to give to pharmacist.
15 f. For pregnant women, prophylaxis should be reserved for those with HIGH RISK exposures and in consultation with her obstetrician. i. Provided with 5 day supply of medication until test results are available. ii. Assure Consent for Post-Exposure Prophylaxis is completed. VII. POST EXPOSURE FOLLOW-UP ON TEST RESULTS A. A. Healthcare provider is to review lab results and complete the Post-Bloodborne Pathogen Exposure Written Opinion form. Once completed, scan into employee s medical record. B. The employee is to be given a written copy of the treating healthcare provider s opinion within 15-days after the medical evaluation. All other findings or diagnoses excluding the following should remain confidential and not be included in the written report: 1) The written opinion in relation to the Hepatitis B vaccine should be limited to and include whether the vaccine is indicated or the employee has received the vaccine previously. 2) The written opinion in relation to the post-exposure evaluation and follow-up should be limited to and include that the employee was informed of the evaluation results and that the employee was informed of any medical condition resulting from the exposure to blood/body fluids which will require further medical evaluation or treatment. VIII. EXPOSURE/INJURY BILLING A. All employee bloodborne pathogen exposures or injuries are to be reported to the Workers Compensation Coordinator (WCC) in Human Resources. B. All Workers Compensation forms are to be forward to the WCC so that the appropriate documents can be completed and processed for payment and filing. C. WCC will also be responsible for contacting our Workers Compensation Carrier to report the injury/exposure. IX. MEDICAL RECORDKEEPING A. Medical recordkeeping shall include: 1. Name and social security number of the employee 2. Copy of the employee s HBV vaccination status, including dates of the all HBV vaccines and any medical records relative to the employee s ability to receive the vaccines 3. Results of all exams, medical testing and follow-up procedures, including counseling 4. Copies of all required/pertinent forms as mentioned above 5. Post-exposure prophylaxis 6. A copy of the healthcare provider s written opinion
16 BILLING GUIDELINES EXPOSURE/INJURY A. All exposures or injuries are to be reported to the Workers Compensation Coordinator (WCC) in Human Resources. B. All Workers Compensation forms and bills, including the source person s bills for the lab/office visit are to be forwarded to the WCC in the HR office so that the appropriate documents can be completed and processed for payment. C. WCC will also be responsible for contacting our Workers Compensation Carrier to report the injury/exposure. D. If employee was seen by healthcare provider and/or an infectious disease physician, an office visit must be charged. MEDICAL ASSISTANT S INSTRUCTIONS FOR BLOOD OR BODY FLUID EXPOSURE The exposed employee presents to the front desk of the medical center and will be checked in following the usual check in process. The exposed employee will be immediately roomed and the first available healthcare provider will examine the employee. The healthcare provider will inform the employee and source person that their blood will be tested for HIV, Hepatitis B and Hepatitis C. PROCEDURE FOR TESTING EXPOSED PERSON A. The medical assistant completes lab requisition for exposed individual HIV 1/HIV 2 antibody (see ii below) - HbsAb Quantitative, HCV Antibody with reflex to RIBA. i. HBsAg, HBcAg IgM/IgG should be ordered for persons who have never received the Hepatitis B vaccine or have previous history of Hepatitis B infection. ii. For HIV 1/HIV 2 antibody, please use rapid HIV swab test kit if available otherwise order blood test. B. If the healthcare provider determines that the employee should be started on antiretroviral prophylaxis, the following labs should also be ordered: CBC, ALT, AST, total bilirubin, Creatinine and BUN at baseline and 2-weeks post-exposure. Further testing may be indicated if abnormalities are detected. C. Females of childbearing age must have a urine or serum pregnancy test prior to starting on the antiretroviral medications. D. Send specimens to reference lab per protocol. E. Laboratory results for the exposed person will be sent to the provider that examined the exposed patient for review and necessary follow-up. Results will then be documented in the source s medical record. Submit all specimens using the appropriate lab order form.
17 PROCEDURE FOR SOURCE TESTING NOTE: California law requires that at no time is the exposed person to approach the source patient and request verbal consent to test the source s blood for HIV, Hepatitis B or C. The notification must be obtained from a neutral party, e.g., healthcare provider or employee s supervisor. A. Once the source and exposed persons are notified to have their blood drawn, the medical assistant completes the required documents for the reference lab, performs the blood draw and labels the blood specimens. The Medical Assistant must ensure each set of specimens are clearly marked as Source or Exposed and that the correct requisition is attached to the correct specimen. B. The medical assistant should perform the Rapid HIV swab test for the source patient if available and provide the results to healthcare provider. C. If the source person refuses to have their blood drawn this must be documented on the exposed person s health record and on the Lab Requisition. D. The medical assistant will notify the Student-Employee Health Coordinator [SEHC] (ext [ ]) who will notify WesternU Risk Management Department (at extension 5452 [ ]) of the exposure and if the source patient grants or refuses to have their blood drawn to be drawn and tested for HIV-1/HIV-2, Hepatitis B and Hepatitis C. E. The medical assistant will document exposure in source patient s health record and that the labs were drawn for source of occupational exposure (ICD- 9 code V15.95) for an employee and for student exposure if for a student. F. Laboratory results for the exposed person will be sent to the healthcare provider that examined the exposed patient for review and necessary follow-up. Results will then be documented in the source person s health record. If the source person consents to receive the results of the blood testing, the healthcare provider will inform the source person of the results. G. If the source patient consents to receive the results of the blood testing, the provider will inform the source patient of the results. EXPOSURE/INJURY BILLING GUIDELINES A. All exposures or injuries are to be reported to the Workers Compensation Coordinator (WCC) in Human Resources. B. All Workers Compensation forms and bills, including the source person s bills for the lab/office visit are to be forward to the WCC in the HR office so that the appropriate documents can be completed and processed for payment and filing by the SEHC. C. WCC will also be responsible for contacting our Workers Compensation Carrier to report the injury/exposure. D. If employee was seen by a healthcare provider, an office visit must be charged.
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
Blood-borne Pathogens Exposure Policy
 Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
EMPLOYEE HEALTH TABLE OF CONTENTS
 EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
Bloodborne Pathogens. Post-Exposure Incident Packet. An Informational Guide
 Bloodborne Pathogens Post-Exposure Incident Packet An Informational Guide Faribault Public Schools Bloodborne Pathogens Post-Exposure Incident Packet This packet has been developed as an informational
Bloodborne Pathogens Post-Exposure Incident Packet An Informational Guide Faribault Public Schools Bloodborne Pathogens Post-Exposure Incident Packet This packet has been developed as an informational
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
Bloodborne Pathogens Training (OHS_BIO500) Course Material
 Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Post Blood & Body Fluid Exposure Checklist
 Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
ADMINISTRATIVE SERVICES MANUAL
 1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
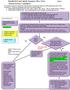 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
HOFSTRA UNIVERSITY DEPARTMENT OF PHYSICIAN ASSISTANT STUDIES
 HOFSTRA UNIVERSITY DEPARTMENT OF PHYSICIAN ASSISTANT STUDIES Date: March 15, 2017 To: Class of 2019 Re: Health Clearance Forms for Didactic Year Please visit your primary health care provider to complete
HOFSTRA UNIVERSITY DEPARTMENT OF PHYSICIAN ASSISTANT STUDIES Date: March 15, 2017 To: Class of 2019 Re: Health Clearance Forms for Didactic Year Please visit your primary health care provider to complete
Bloodborne Pathogens. Montclair Kimberley Academy 1
 Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
Safety Committee Prototypical Safety Program Manual
 1 Bloodborne Pathogens Exposure Control Plan Policy The Department Bloodborne Pathogens Exposure Control Plan is designed to comply with the requirements of the OSHA Bloodborne Pathogens Standard, 29 CFR
1 Bloodborne Pathogens Exposure Control Plan Policy The Department Bloodborne Pathogens Exposure Control Plan is designed to comply with the requirements of the OSHA Bloodborne Pathogens Standard, 29 CFR
Bloodborne Pathogens Presentation. Itasca County Public Health
 Bloodborne Pathogens Presentation Itasca County Public Health 1 Could You Contract a Disease at Work? Administering first aid? Cleaning the restrooms? Using an item covered with dried blood? A co-worker
Bloodborne Pathogens Presentation Itasca County Public Health 1 Could You Contract a Disease at Work? Administering first aid? Cleaning the restrooms? Using an item covered with dried blood? A co-worker
Health Clearance FAQ s
 Immunizations and Tuberculosis Clearance Q Why do I need to submit my immunization records and serum titers? A Many clinical rotation sites that our student s rotate through require copies of both your
Immunizations and Tuberculosis Clearance Q Why do I need to submit my immunization records and serum titers? A Many clinical rotation sites that our student s rotate through require copies of both your
Drew University Bloodborne Pathogens Exposure Control Plan and Procedures
 PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Bloodborne Pathogen Module. Chelmsford Public Schools September, 2016
 Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Healthcare Professionals
 EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]
![BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens] BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]](/thumbs/73/68341667.jpg) BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP)
 Harvey Ingham 30 2804 Forest Ave Des Moines, IA 50311 515-271-3804 ehs@drake.edu www.drake.edu/ehs May 2016 BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP) PURPOSE This document serves as the written
Harvey Ingham 30 2804 Forest Ave Des Moines, IA 50311 515-271-3804 ehs@drake.edu www.drake.edu/ehs May 2016 BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP) PURPOSE This document serves as the written
Bloodborne Pathogens Training. IEA, Inc.
 Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
A. Background for Trainer: B. What OSHA Requires: Bloodborne Pathogens. Lesson Plan 6080a
 Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Bloodborne Pathogens
 Bloodborne Pathogens Disclaimer This training material presents very important information. Your organization must do an evaluation of all exposures, applicable codes and regulations, and establish proper
Bloodborne Pathogens Disclaimer This training material presents very important information. Your organization must do an evaluation of all exposures, applicable codes and regulations, and establish proper
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
RUTGERS POLICY. Errors or changes? Contact: Rutgers University Occupational Health Department
 RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS)
 Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS) PURPOSE To reduce the risk of exposure of Washtenaw County Community Mental Health (CMH)
Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS) PURPOSE To reduce the risk of exposure of Washtenaw County Community Mental Health (CMH)
Blood borne Pathogen Exposure Policy for Students
 Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
SCHOOL OF MEDICINE IMMUNIZATION COMPLIANCE FORM
 SCHOOL OF MEDICINE IMMUNIZATION COMPLIANCE FORM Louisiana R.S. 17:170 Schools of Higher Learning Tulane University Campus Health, Health Center Downtown 504-988-6929, Uptown 504-865-5255 Upload this form
SCHOOL OF MEDICINE IMMUNIZATION COMPLIANCE FORM Louisiana R.S. 17:170 Schools of Higher Learning Tulane University Campus Health, Health Center Downtown 504-988-6929, Uptown 504-865-5255 Upload this form
East Carolina University Student Health Service
 Page 1 of 5 Policy: BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIAL EXPOSURE POLICY FOR STUDENTS, WITH CLINICAL EXPOSURES s (SHS) will adapt and modify the policies and procedures of ECU Prospective Health
Page 1 of 5 Policy: BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIAL EXPOSURE POLICY FOR STUDENTS, WITH CLINICAL EXPOSURES s (SHS) will adapt and modify the policies and procedures of ECU Prospective Health
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS
 TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
Bloodborne Pathogens 29 CFR
 Bloodborne Pathogens 29 CFR 1910.1030 Revised OSHA Bloodborne Pathogens Compliance Directive (CPL2-2.44D) Could You Contract a Disease at Work? Administering first aid? Cleaning the restrooms? Using a
Bloodborne Pathogens 29 CFR 1910.1030 Revised OSHA Bloodborne Pathogens Compliance Directive (CPL2-2.44D) Could You Contract a Disease at Work? Administering first aid? Cleaning the restrooms? Using a
Goldenrod Hills Community Action. Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR
 Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Bloodborne Pathogens Training
 Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Immunization Policy. "UIC/COD-sponsored graduate education program" is one for which UIC/COD maintains academic responsibility.
 I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
Blood Borne Pathogens (BBP)
 Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES
 City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
Laboratory Exposure Control Plan WAC
 A. Introduction Because laboratories and classrooms working directly with blood or other potentially infectious materials have different bloodborne pathogen exposure concerns than the general university,
A. Introduction Because laboratories and classrooms working directly with blood or other potentially infectious materials have different bloodborne pathogen exposure concerns than the general university,
Occupational Exposures to Bloodborne Pathogen (BBP) Training
 Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training
 Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids
 Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools
 Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
Infection Prevention and Control - General Orientation
 Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
COMMONWEALTH SCAFFOLD Quality Scaffolding Across New England
 Prepared by Allied Insurance Brokers, Inc. Commonwealth Scaffold, LLC Bloodborne Pathogens Program and Training Materials Effective Date: 12/14/2012 Revision #: Table of Contents Bloodborne Pathogens Program
Prepared by Allied Insurance Brokers, Inc. Commonwealth Scaffold, LLC Bloodborne Pathogens Program and Training Materials Effective Date: 12/14/2012 Revision #: Table of Contents Bloodborne Pathogens Program
Bloodborne Pathogens
 Bloodborne Pathogens Introduction How to Use this Presentation This presentation contains base material for use in an instructor-led training setting. You may modify this presentation to satisfy the specific
Bloodborne Pathogens Introduction How to Use this Presentation This presentation contains base material for use in an instructor-led training setting. You may modify this presentation to satisfy the specific
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Exposure Control Plan
 San Bernardino County Superintendent of Schools Bloodborne Pathogens Exposure Control Plan BIOHAZARD 2018/2019 Title 8 California Code of Regulations Section 5193 Table of Contents PURPOSE OF THE PLAN...3
San Bernardino County Superintendent of Schools Bloodborne Pathogens Exposure Control Plan BIOHAZARD 2018/2019 Title 8 California Code of Regulations Section 5193 Table of Contents PURPOSE OF THE PLAN...3
Doc No: BLOOD Midland Engineering Co., Inc. Initial Issue Date 12/04/15 Safety Management System
 Revision Preparation: Safety Mgr Authority: President Issuing Dept: Safety Page: Page 1 of 15 INTRODUCTION The Occupational Safety and Health Administration (OSHA) has a variety of regulations that all
Revision Preparation: Safety Mgr Authority: President Issuing Dept: Safety Page: Page 1 of 15 INTRODUCTION The Occupational Safety and Health Administration (OSHA) has a variety of regulations that all
SUMMER HEALTH PROFESSIONS EDUCATION PROGRAM FOR ACCEPTED STUDENTS
 SUMMER HEALTH PROFESSIONS EDUCATION PROGRAM FOR ACCEPTED STUDENTS Immunization Information To manage issues related to infection control, The University of Texas Health Science Center at Houston (UTHealth)
SUMMER HEALTH PROFESSIONS EDUCATION PROGRAM FOR ACCEPTED STUDENTS Immunization Information To manage issues related to infection control, The University of Texas Health Science Center at Houston (UTHealth)
Bloodborne Pathogen Exposure Control Plan
 Bloodborne Pathogen Exposure Control Plan Table of Contents I. Purpose II. Exposure Determination III. Methods of Compliance A. Universal Precautions B. Engineering Controls C. Work Practice Controls D.
Bloodborne Pathogen Exposure Control Plan Table of Contents I. Purpose II. Exposure Determination III. Methods of Compliance A. Universal Precautions B. Engineering Controls C. Work Practice Controls D.
Use the steps below to complete the CertifiedBackground (CB) electronic health record tracking process.
 Renal Dialysis Health Requirements Checklist All MATC Health Science students are required to complete and upload health requirements prior to petitioning for courses which contain a clinical component.
Renal Dialysis Health Requirements Checklist All MATC Health Science students are required to complete and upload health requirements prior to petitioning for courses which contain a clinical component.
Bloodborne Pathogens Training Lakeville Area Public Schools
 Bloodborne Pathogens Training Lakeville Area Public Schools Questions? Contact the District s Health & Safety Consultant Dan Fitch at IEA, Inc. 763-315-7900 dan.fitch@ieasafety.com OR District s Exposure
Bloodborne Pathogens Training Lakeville Area Public Schools Questions? Contact the District s Health & Safety Consultant Dan Fitch at IEA, Inc. 763-315-7900 dan.fitch@ieasafety.com OR District s Exposure
MiraCosta Community College District Bloodborne Pathogens Exposure Control Plan
 1 MiraCosta Community College District General Information & Instructions This form is provided to facilitate compliance with federal and state Bloodborne Pathogens Standards, OSHA 29 CFR 1910.1030 and
1 MiraCosta Community College District General Information & Instructions This form is provided to facilitate compliance with federal and state Bloodborne Pathogens Standards, OSHA 29 CFR 1910.1030 and
SUBJECT: Management of Human Body Fluids/Waste (Bloodborne Pathogens)
 Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Bloodborne Pathogens Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan RIM of the World Unified School District 27315 North Bay Road Blue Jay, CA 92352 (909) 336-4100 July 2016 Safety and Risk Management Department RIM of the World
Bloodborne Pathogens Exposure Control Plan RIM of the World Unified School District 27315 North Bay Road Blue Jay, CA 92352 (909) 336-4100 July 2016 Safety and Risk Management Department RIM of the World
Oley Valley School District Bloodborne Pathogen Policy. Exposure Control Plan
 Oley Valley School District Bloodborne Pathogen Policy Exposure Control Plan Commonwealth of Pennsylvania Resources: A copy of the PA State Guidelines on Bloodborne Pathogens for the Public Sector can
Oley Valley School District Bloodborne Pathogen Policy Exposure Control Plan Commonwealth of Pennsylvania Resources: A copy of the PA State Guidelines on Bloodborne Pathogens for the Public Sector can
Needle Stick/Blood Borne Pathogen Exposure Guidelines
 Needle Stick/Blood Borne Pathogen Exposure Guidelines Purpose: To outline the expected behavior to be followed by all student pharmacists who have received an accidental exposure incident while in an educational
Needle Stick/Blood Borne Pathogen Exposure Guidelines Purpose: To outline the expected behavior to be followed by all student pharmacists who have received an accidental exposure incident while in an educational
How are Blood borne pathogens harmful? How do you come into contact with Blood-borne pathogens?
 What are Blood borne pathogens? How are Blood borne pathogens harmful? How do you come into contact with Blood-borne pathogens? How do I protect myself? A blood-borne disease is one that can be spread
What are Blood borne pathogens? How are Blood borne pathogens harmful? How do you come into contact with Blood-borne pathogens? How do I protect myself? A blood-borne disease is one that can be spread
FULL-TIME ADULT STUDENT Acceptance Package Phase II
 Revised 6/2013 FULL-TIME ADULT STUDENT Acceptance Package Phase II THE FOLLOWING FORMS ARE NOT TO BE COMPLETED AND RETURNED UNLESS YOU ARE ACCEPTED INTO A PROGRAM Connecticut Technical High School System
Revised 6/2013 FULL-TIME ADULT STUDENT Acceptance Package Phase II THE FOLLOWING FORMS ARE NOT TO BE COMPLETED AND RETURNED UNLESS YOU ARE ACCEPTED INTO A PROGRAM Connecticut Technical High School System
Bloodborne Pathogens
 Bloodborne Pathogens Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Risk of exposure Protecting yourself from exposure through prevention Responding appropriately
Bloodborne Pathogens Session Objectives Identify bloodborne pathogens (BBPs) Understand how diseases are transmitted Risk of exposure Protecting yourself from exposure through prevention Responding appropriately
2014 OSHA Blood-borne Pathogens (BBP) Update JHS Annual Mandatory Education
 2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
Infection Control Program (ICP) ICP Components 1. Exposure Determination 2. Control Methods A. Universal Precautions
 Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN
 The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN Table of Contents SCOPE... 3 DEFINITIONS... 3 EXPOSURE DETERMINATION... 3 METHODS OF COMPLIANCE... 4 HEPATITIS B VACCINATION
The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN Table of Contents SCOPE... 3 DEFINITIONS... 3 EXPOSURE DETERMINATION... 3 METHODS OF COMPLIANCE... 4 HEPATITIS B VACCINATION
Dear Parent or Guardian,
 Dear Parent or Guardian, This summer may be a period of transition for you and your child. For a lot of our students it may even be the first time they are taking the lead in their personal care, including
Dear Parent or Guardian, This summer may be a period of transition for you and your child. For a lot of our students it may even be the first time they are taking the lead in their personal care, including
CHAPTER Committee Substitute for Committee Substitute for House Bill No. 321
 CHAPTER 2015-110 Committee Substitute for Committee Substitute for House Bill No. 321 An act relating to HIV testing; amending s. 381.004, F.S.; revising and providing definitions; specifying the notification
CHAPTER 2015-110 Committee Substitute for Committee Substitute for House Bill No. 321 An act relating to HIV testing; amending s. 381.004, F.S.; revising and providing definitions; specifying the notification
Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN April 2018 Table of Contents Exposure Determination... 3 Hepatitis B Vaccine... 4 Universal
Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN April 2018 Table of Contents Exposure Determination... 3 Hepatitis B Vaccine... 4 Universal
Dare County Schools. Bloodborne Pathogens Exposure Control Plan
 Dare County Schools Bloodborne Pathogens Exposure Control Plan 2017 Dare County Schools Bloodborne Pathogens Exposure Control Page 1 of 12 Dare County Schools Bloodborne Pathogen Program Purpose An infection
Dare County Schools Bloodborne Pathogens Exposure Control Plan 2017 Dare County Schools Bloodborne Pathogens Exposure Control Page 1 of 12 Dare County Schools Bloodborne Pathogen Program Purpose An infection
Bloodborne Pathogens LVHN s Annual Safety Course
 Slide 1 Bloodborne Pathogens LVHN s Annual Safety Course Nucleus Medical Media (2010). Blood Carries Nutrients. Smart Imagebase. Lehigh Valley Health Network cares about your safety especially about protecting
Slide 1 Bloodborne Pathogens LVHN s Annual Safety Course Nucleus Medical Media (2010). Blood Carries Nutrients. Smart Imagebase. Lehigh Valley Health Network cares about your safety especially about protecting
Environmental Health and Safety Offices BLOODBORNE PATHOGENS
 Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
