Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
|
|
|
- Gwendolyn Higgins
- 5 years ago
- Views:
Transcription
1 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid Exposure Management Quick Reference Sheets Blood and Body Fluid Exposure Management Whitehorse: YCDC #4Hospital Rd. Whitehorse M-F Hours: Phone: (867) Revised April 2013 WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER Yukon RNs Consult: YCDC: (867) Monday - Friday hrs *Indicate call is regarding BBF post exposure management. After Hours Contact WGH-ER Physician on call: (867) , or Community physician. MOH may be consulted after calling ER physician Work (867) or cell (867) WGH Occupational Health Practitioner ( WGH employees/inpatients) M-F Hours: Phone: (867) Yukon Physicians Consult: YCDC: (867) Monday - Friday hrs *Indicate call is regarding BBF post exposure management or MOH work (867) or cell (867) BC Centre for Excellence in HIV/AIDS (consultation service available 24 hours a day 7 days aweek) Monday Friday ( ) Tel: After hours & Weekends Tel: Management Cleanse Mucous membrane or eye: Rinse well with water and/or normal saline Skin: Wash well with soap and water. Allow injury/wound site to bleed freely, and then cover lightly. Do not promote bleeding of percutaneous injuries by cutting, scratching, squeezing, or puncturing the skin. Do not apply bleach to the injury/wound or soak it in bleach. Triage Are the four indications present for post-exposure management? See attached algorithm pg. 2 Refer to attached charts Assess risk of transmission from the source Determine status of the exposed person Counsel Access HBIG Availability of antiretroviral starter kits Arrange lab F/U for exposed person Exposed Person: Blood and body fluid exposure management algorithm; See attached pg. 2 Stratification of HIV Exposures; See attached pg. 4 Hepatitis B Post Exposure Prophylaxis; See attached pg. 5 Testing Schedule for a person exposed to HCV; See attached pg. 3 If source is known attempts should be made to have source person tested Obtain source person s consent and test for: anti-hiv, anti-hcv, HBsAg, Anti-HBs, Anti-HBc Refer to attached table: Indicators for increased risk of transmission from the source to the exposed person; See attached pg. 8 DO NOT WAIT FOR TEST RESULTS BEFORE COMMENCING TREATMENT (if indicated) Obtain consent for baseline testing which may include: anti-hiv, anti-hcv, HBsAg, Anti-HBs, Anti-HBc Refer to Counselling Guidelines: Management of Blood and Body Fluids Post Exposure pg. 36 and Probability of Transmission pg. 38 Whitehorse: Physicians request HBIG from the WGH laboratory for administration in WGH ER Afterhours: call WGH A&D to have the on-call laboratory personnel paged Outside of Whitehorse: A supply of HBIG is kept in the following communities: Beaver Creek, Ross River, Haines Junction, Dawson City, Old Crow, Mayo, Watson Lake If HBIG is not stocked in the community requiring it, arrangements will be made to have it provided from the most feasible location Whitehorse: Starter kits (5 day supply) oftherapyareavailable at the WGH ER and YCDC Outside of Whitehorse: One starter kit is supplied in each community health centre *To arrange for remainder of 23 day antiretroviral therapy call YCDC (867) Refer to attached Chart: Testing of the Exposed Person; See attached pgs. 3 & 6 Complete last page of the Blood and Body Fluid Exposure Form (BBFE) form and give to client; See attached pg. 9 Documentation Complete the Blood and Body Fluid Exposure Form (BBFE); see attached pgs. 10 & 11 Captures: exposure details, examination of injury, management of exposed, information on source, recommendations made, follow up plan of care Occupational Exposure: Worker s Compensation Board (WCB) Forms Where to send BBFE form Copy to YCDC Fax: (867) Copy to exposed person s follow up physician Provide client with the last page summary of initial management and plan for follow up; see attached pg. 9 1
2 Blood and Body Fluid Exposure Management Algorithm: Exposed Person: Guidelines pg. 8 Are the four indications present for post-exposure management? percutaneous, mucosal or non-intact skin exposure to blood or infectious body fluids potentially infectious source susceptible exposed person Yes No Assess risk of the source for HIV, HBV and HCV Refer to Appendix B: Risk Factors for Possible Transmission No post exposure management required Provide education, counseling or advice as appropriate HIV HBV HCV Obtain baseline anti HIV Assess eligibility for PEP Refer totable 3, pg 16: Stratification of HIV Exposure Significant risk: Start PEP: Refer to Table 1, pg 13:Testing of the Exposed Person Complete baseline serology Determine HBV susceptibility Refer to Table 4, pg 21: Hepatitis B Post- Exposure prophylaxis Negligible Risk: No PEP Complete: 3, 6 week and 3, 6 month serology Complete baseline Anti- HCV serology Note: There is no initial PEP for HCV Source Unknown Complete 3 and 6 month, serology (Anti HCV) Negative: Complete 3 and 6 month serology (Anti HCV) Source HCV -ve Complete 3 and 6 month Serology (Anti HCV) Source Known Source HCV +ve or High Risk Complete 3 week HCV RT-PCR Positive: Repeat PCR test to confirm result on a separate specimen *Antiretrovirals are not recommended for needlesticks from an abandoned needle in a community setting when there is no history of the origin of the needle or the time of its abandonment. If positive, active infection is confirmed - consult with YCDC about monitoring and treatment options 2
3 Testing of the Exposed Person Guidelines pg. 13 TIME SINCE EXPOSURE Anti- HIV Anti-HCV HCV PCR HBsAg Anti- Anti- HBs HBc RATIONALE FOR TESTING OF THE EXPOSED PERSON ASAP To check baseline status of the exposed person. Negative or non-reactive test results suggest no prior infection. 3 weeks after exposure If source is HCV+ or in a high risk group, test exposed person for HCV infection by RT-PCR ) If HCV RT-PCR +, early treatment may be beneficial. If the exposed person is confirmed PCR+, active infection is present and there is no need to test for anti-hcv. 6 weeks after exposure 3 months after exposure 6 months after exposure To check whether seroconversion has occurred. A change from the initial negative (or non-reactive) test result to a positive (or reactive) result indicates that seroconversion has occurred. Seroconversion following a blood or body fluid exposure does not definitively establish that the exposure was the source of the virus if the exposed person has other risk factors. If the source person tests negative for HBV, HCV, and HIV and is not in a high-risk group, only baseline testing of the exposed person is indicated. See Table 4, pg. 21: Hepatitis B Post-Exposure Prophylaxis If PCR+, a second sample needs to be tested to confirm the result. Note: If the exposed person is a pregnant woman, request HBV testing as close to delivery as possible. 3
4 4 Hospital Rd., Whitehorse, YT. Y1A 3H8 Stratification of HIV Exposures: Guidelines pg. 16 EXPOSURE RISK TYPE OF EXPOSURE RECOMMENDATION SIGNIFICANT RISK: Any percutaneous exposure to infectious Antiretroviral starter kit (5 body fluids day kit) Infectious body fluid and an HIV positive source or a known high-risk source. (See Appendix B in Guideline) Mucous membrane or non-intact skin exposure (3 or more drops for 3 or more minutes) In the event of a large prolonged exposure of blood on intact skin, assess the integrity of the skin. If appropriate, treat as a significant risk exposure. Consult: YCDC Weekdays hrs. Tel: (867) Medical Officer of Health: Dr. Brendan Hanley Tel: (867) Cell: (867) BC Centre for Excellence in HIV/AIDS Monday Friday ( ) Tel: (604) Afterhours and weekends Tel: (604) NEGLIGIBLE RISK: Source known or presumed to be HIV negative OR Injury not known to transmit HIV OR Body fluid not known to transmit HIV Percutaneous, mucous membrane or skin exposure to non-infectious body fluid source HIV positive or negative. Bites unless there has clearly been transmission of infected blood. A superficial scratch that does not bleed. Injuries received in fights would rarely be appropriate indications for prophylaxis unless it is clear that transfer of infected blood has occurred. * Community Nurses When using BC-CfE consultation service, please be advised that should HIV post exposure prophylaxis be recommended, this should be discussed and prescribed by a licensed Yukon Physician. No antiretrovirals recommended. Offer counselling clarifying the negligible risk of HIV infection and advise re: risk prevention (i.e. preventing recurrences of exposure incidents). Antiretrovirals (ARTs) are not provided free to persons exposed to HIV as part of their personal lives (i.e. consensual adult sex, or sharing drug injection equipment). However, the assessing physician may elect to prescribe ARTs for these situations and should consult with YCDC or the BC Centre for Excellence in HIV/AIDS regarding which ARTs to prescribe. Note: Prophylaxis is not recommended for needlesticks from abandoned needles when they are outside the healthcare setting or when there is no history of the needle or the time of abandonment.. 4
5 Hepatitis B Post-Exposure Prophylaxis: Guidelines pg. 21 Vaccination history of exposed person Test exposed person for: HBsAg, anti- HBc & anti- HBs. If source is known HBsAg positive or high risk or tests positive within 48 hours of exposure If source is unknown or not tested or low risk or tests HBsAG negative within 48 hours of exposure (eg. Abandoned needle) Post-exposure re-testing Documented anti-hbs level ( 10 IU/L) on prior testing Test for all three markers for medical-legal purposes Unvaccinated Test for all 3 markers Known non- responder to one Hep B series Received 1 dose of Hep B vaccine, anti- HBs status unknown Received 2 doses of a 3 dose Hep B series, anti-hbs status unknown Complete Hep B vaccination (2 or 3 dose series) and anti- HBs status unknown or anti- HBs < 10 when tested > 6 months postseries Known non- responder after two courses of Hep B vaccine Test for all 3 markers Test for all 3 markers Test for all 3 markers. If anti-hbs is 10 IU/L, then Test for all 3 markers. If anti- HBs is 10 U/L, then Test for all 3 markers. If anti-hbs is 10 IU/L, then Test for HBsAg & anti-hbc. Do not test for anti- HBs. No action required. Give Hepatitis B Immune Globulin (HBIg) and Hepatitis B vaccineseries Give HBIg & complete Hep B vaccine series. Give HBIg & 3rd dose of Hep B vaccine. Repeat 3 rd dose if given too early in series. Do not give HBIg. Complete Hep B vaccine series. Give HBIg and 1 dose of vaccine. Give HBIg only & give another dose of HBIg in 1 mo. No action required. Give Hep B vaccine series Give 2 nd Hep B vaccine series Complete Hep B vaccine series. Give 1 dose of Hep B vaccine & retest for anti- HBs in 4 wks; if 10 IU/L repeat series. Do not give HBIg. Complete Hep B vaccine series. 1 dose Hep B vaccine & retest for anti-hbs in 4wks; if 10 IU/L complete second series. No action required. No action required. Re-test for HBsAg at 3 months & for all 3 markers at 6 months Re-test for HBsAg at 3 months & for all 3 markers at 6 months Re-test for HBsAg at 3 months & for all 3 markers at 6 months No re-testing required. Re-test for HBsAg at 3 months & for all 3 markers at 6 months Re-test for HBsAg at 3 months & for HBsAg & anti-hbc at 6 months. A non-responder to a series of Hepatitis B vaccine is someone who demonstrates an anti-hbs level of 10 UI/L, when measured 1 to 6 months post-vaccination. Consensual adult sex with known Sex Trade Worker or IDU is not an indication for HBIg, nor is a community acquired needlestick injury: the risk of transmission is low and the number needed to treat to prevent infection is extremely high. HBIg is indicated in the case of sexual assault or if one of the individuals is known to have acute or chronic Hepatitis B infection. HBIg dose for all clients 8.3kg is 0.06ml/kg. Give HBIg as soon as possible, preferably within 48 hours of the exposure. For a percutaneous exposure, HBIg may be given up to 7days following the exposure. If the client presents 7 days following a percutaneous exposure, give Hepatitis B vaccine only. For permucosal or sexual exposures, HBIg may be given up to 14 days following the last exposure. If the client presents 14 days following a permucosal or sexual exposure, give Hepatitis B vaccine only. Hepatitis B vaccine schedule is 0, 1 and 6 months for post-exposure prophylaxis. A second series of Hepatitis B vaccine should be offered to non-responders Note: This table does not apply to post-exposure management of immunocompromised persons. This group requires consultation with a physician specializing in infectious diseases. 5
6 HCV Exposure: Guidelines pg. 23 While HCV is transmitted more efficiently by the parenteral route than HIV, it is transmitted by sexual contact much less efficiently than either HBV or HIV. Transmission probabilities for HCV are summarized in Appendix N, page 38 in Guideline. At the present time, no immediate post-exposure treatment is recommended for HCV. However, the anti-hcv status of the exposed person should be determined to assess whether the person has been infected with HCV in the past. Testing schedule for a person exposed to HCV: If suspected exposure to HCV has occurred complete an anti-hcv base line test 1 st Test If anti-hcv reactive * If anti-hcv non reactive 2 nd Test weeks after the initial initial anti-hcv anti-hcv testing, testing, if source s if source s Complete an immediate follow-up test for HCV RNA HCV status status is positive is positive or unknown, or source test is for in HCV a high RNA risk group, perform testing for HCV RNA to look for PCR to look for active infection should be performed to look for active infection. active infection HCV RNA PCR performed If HCV RNA PCR positive If HCV RNA PCR negative Follow up Testing Repeat PCR testing to confirm result Repeat anti-hcv anti HCV at: at: 33 months 12 6months If HCV RNA positive; If HCV RNA negative; active infection active infection not confirmed, consult with confirmed If If all anti HCV both anti-hcv tests a health care provider are tests negative, are negative, about monitoring and transmission has has treatment options not not occurred occurred and and monitoring may may cease cease If either anti-hcv test is positive, active infection confirmed. Consult with a health care provider about monitoring and treatment options *Anti-HCV takes about 5-10 weeks after infection to develop. If your baseline anti-hcv test is reactive, then you may have been infected in the past and a follow-up HCV RNA test can be perform to determine if you are actively infected (i.e., have the virus in your blood at the present time). 6
7 4 Hospital Rd., Whitehorse, YT. Y1A 3H8 Fluids and Tissues Capable of Transmitting Bloodborne Pathogens: Guidelines pg. 24 FLUID HIV HBV HCV Blood and fluids visibly contaminated with blood Yes Yes Yes Semen Yes Yes Yes * Vaginal secretions Yes Yes Yes * Pleural, amniotic, pericardial, peritoneal, synovial and cerebrospinal fluids and inflammatory exudates Saliva Transplanted tissue or organs Yes Yes Yes No, unless contaminated with blood Yes No, unless contaminated with blood Yes Yes Yes Breast milk Yes Plausible, particularly if nipples are cracked or bleeding or if the mother is HBeAg positive Plausible, particularly if nipples are cracked or bleeding Faeces Nasal secretions Sputum Sweat Tears Urine Vomitus No, unless they contain visible blood. *While HCV is transmitted more efficiently by the parenteral route than HIV, it is transmitted by sexual contact much less efficiently than either HBV or HIV. Transmission probabilities for HCV are summarized in the Guideline, Appendix N, page 38. 7
8 Risk Factors for Possible Transmission from the Source to the Exposed Person: Guidelines pg. 25 HIV HBV HCV The source is a person who has ever had: The source is a person who has ever had: injection drug use injection drug use and/or inhalational drug use high-risk sexual behaviour (i.e., multiple sex partners, anal sex) high-risk sexual behaviour (i.e., multiple sex partners, anal sex) The source is a person who has ever had: injection drug use and/or inhalational drug use high-risk sexual behaviour (i.e. multiple sex partners, anal sex) a sexual partner who is an injection drug user (IDU), or who is HIV+ a sexual partner who is an IDU, or who has acute or chronic HBV a sexual partner who is an IDU, or who is HCV+ blood contact with a known case of HIV infection blood contact with a known case of HBV infection for which there was no provision of postexposure prophylaxis blood contact with a known case of HCV infection emigration from a country where HIV is endemic a history of multiple transfusions of blood or blood products prior to Nov OR a history of receipt of blood-derived coagulation products before July 1988 emigration from a country where HBV is endemic a history of multiple transfusions of blood or blood products prior to Jan OR a history of receipt of bloodderived coagulation products before January 1972 a history of multiple transfusions of blood or blood products prior to May 1992 OR a history of receipt of blood-derived coagulation products before July 1988 or a history of receipt of IV immunoglobulin products prior to 1997 a diagnosis of sexually transmitted disease(s) a diagnosis of sexually transmitted disease(s) tattoo, body piercing, electrolysis, acupuncture tattoo, body piercing, electrolysis, acupuncture tattoo, body piercing, electrolysis, acupuncture a history of dialysis a history of dialysis a history of dialysis In Canada, testing of donated blood for anti-hiv began in November 1985; for HBsAg in January 1972; and for anti-hcv first generation in June 1990 and anti-hcv second generation in May All factor concentrates distributed in Canada were heat treated after July IV immunoglobulin products were either PCR tested for HCV or had solvent detergent virucidal treatment after 1997 High risk examples include snorting and sniffing of cocaine and smoking crack pipes. 8
9 Yukon Communicable D is e a s e Control Exposed Person s Follow up Plan for Post Exposure to Blood & Body Fluids: Guidelines pg. 35 was seen at Name Date o f Birth location of Health Care Facility on Date following an exposure to blood or body fluids. Initial assessment was done by Name of initial Health Care Provider The date of exposure was You received the following post-exposure treatment: Wound cleaning Started on antiretroviral starter kit (5 days) Tetanus Immunization Hepatitis B Vaccine List medications name, dose, & instructions Hepatitis B immune globulin (HBIG) Initial counseling f or blood and body f luid exposure Other You have had baseline Blood tests for: Hepatitis B Hepatitis C HIV Your follow up Health Care Provide r is: Location of Health Care Facility Phone Number It is recommended that you have the following follow up: (Check all that may apply). Follow up is recommended for: Location for follow -up Date Further doses of Hepatitis B vaccine Hepatitis B Immune Globulin (HBIG) You have been started on HIV antiretroviral medications. You must see your f ollow up Health Care Provider within 3 days to determine if you should continue taking the medication f or a remaining 23 days Results of baseline blood tests. Follow up blood work Other (specify) For information related to exposure of blood and body f luids and disease specific information please visit If you have any questions or concerns please contact your follow up Health Care Provider or YG (5673Q) F1 02/2010 YCDC at #4 Hospital Road Whitehorse, YT Y1A 3H8 Tel: (867)
10 10
11 11
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
Hepatitis B Control. Table of Contents
 Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
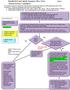 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
TABLE OF CONTENTS 1.0 INTRODUCTION Goals... 2
 (Adapted with permission from: BCCDC Communicable Disease Control Hepatitis B Guideline September 2009) TABLE OF CONTENTS 1.0 INTRODUCTION... 2 1.1 Goals... 2 2.0 CLINICAL DESCRIPTION... 2 3.0 EPIDEMIOLOGY...
(Adapted with permission from: BCCDC Communicable Disease Control Hepatitis B Guideline September 2009) TABLE OF CONTENTS 1.0 INTRODUCTION... 2 1.1 Goals... 2 2.0 CLINICAL DESCRIPTION... 2 3.0 EPIDEMIOLOGY...
HEPATITIS B INFECTION and Pregnancy. Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011
 HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Blood Borne Pathogens. Becky Walch, R.N. Micheel Valdez, L.V.N.
 Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Greater Glasgow and Clyde. Blood Borne Viruses: Some important basic facts
 Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B. Acute HBV Questionnaire
 BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Hepatitis B and C Overview, Outbreaks, and Recommendations. Viral Hepatitis Language. Types of Viral Hepatitis 7/1/2013
 Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses
 Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
BLOODBORNE DISEASES. Prevention of transmission for school staff. for staff not directly responsible for providing care or cleaning up blood
 BLOODBORNE DISEASES Prevention of transmission for school staff for staff not directly responsible for providing care or cleaning up blood MASSACHUSETTS DIVISION OF OCCUPATIONAL SAFETY Robert Prezioso,
BLOODBORNE DISEASES Prevention of transmission for school staff for staff not directly responsible for providing care or cleaning up blood MASSACHUSETTS DIVISION OF OCCUPATIONAL SAFETY Robert Prezioso,
Bloodborne Pathogens Training For School Personnel
 Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
Bloodborne Pathogens: Recognition and Treatment 2011
 Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
CMC Annual Review of BLOODBORNE DISEASES. Prevention of Transmission for School Staff
 CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
CMC Annual Review of BLOODBORNE DISEASES Prevention of Transmission for School Staff Standard on Bloodborne Pathogens OSHA sets the standard of care We must have standards to follow in schools for everyone
Hepatitis Case Investigation
 * indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
* indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
Chapter 2 Hepatitis B Overview
 Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Pre and Post Exposure Prophylaxis. Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018
 Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Management of Exposure to Needlestick Injuries & Body Fluids
 Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Infection/Disease Control HEPATITIS CONTROL PROGRAM
 OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Module 1: HIV epidemiology, transmission and prevention
 Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Uses and Misuses of Viral Hepatitis Testing. Origins of Liver Science
 Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
DEPARTMENT OF GENITO-URINARY MEDICINE HEPATITIS B
 DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
Transmission/Prevention
 Transmission/Prevention Section Three Transmission/Prevention Hepatitis C is transmitted by blood-to-blood contact. Any break in the skin may allow HCV to enter the body, even if no blood is visible. The
Transmission/Prevention Section Three Transmission/Prevention Hepatitis C is transmitted by blood-to-blood contact. Any break in the skin may allow HCV to enter the body, even if no blood is visible. The
Blood Borne Pathogens
 Bloomer School District Blood Borne Pathogens Developed by: Tammy Kornesczuk, RN Act Rather Than Re-act School Staff tend to be nurturing and care-taking people Don t rush to help without putting on gloves
Bloomer School District Blood Borne Pathogens Developed by: Tammy Kornesczuk, RN Act Rather Than Re-act School Staff tend to be nurturing and care-taking people Don t rush to help without putting on gloves
PEDIATRIC EMERGENCY DEPARTMENT CLINICAL GUIDELINE: NON- OCCUPATIONAL EXPOSURE TO BLOOD-BORNE PATHOGENS (HIV, Hepatitis B, AND Hepatitis C)
 General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
Blood and Body Fluid Exposure
 Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
EMPLOYEE HEALTH TABLE OF CONTENTS
 EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
Greenwood School District 50 OSHA UPDATE BLOODBORNE PATHOGENS
 Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
2017 BLOODBORNE PATHOGENS
 2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
Post Exposure Prophylaxis (PEP)
 Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Post Blood & Body Fluid Exposure Checklist
 Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Bloodborne Pathogens. By Deborah Massarella RN, MSN
 Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES
 NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Non-Occupational Post-Exposure HIV Prophylaxis npep
 Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
Obstetric Complications in HIV-Infected Women. Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School
 Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
I. PROCEEDINGS AFTER EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (OPIM) WHICH MIGHT TRANSFER AN INFECTION WITH HAEMATOGENOUS VIRUSES
 Annex no. 1 to the enactment No. 13/XIV R/2010 of the Rector of the Medical University in Wrocław dated April 12, 2010 Proceedings after occupational exposure to HIV, HBV and HCV 1 LEGAL GROUNDS: 1. The
Annex no. 1 to the enactment No. 13/XIV R/2010 of the Rector of the Medical University in Wrocław dated April 12, 2010 Proceedings after occupational exposure to HIV, HBV and HCV 1 LEGAL GROUNDS: 1. The
Information for Health Care Workers
 Information for Health Care Workers This Bulletin highlights precautions to be taken by health care workers who may be exposed to blood and body fluids. In general, workers should minimize direct contact
Information for Health Care Workers This Bulletin highlights precautions to be taken by health care workers who may be exposed to blood and body fluids. In general, workers should minimize direct contact
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids
 Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Bloodborne Pathogens. Kathleen Stefek, RN, MSN
 Bloodborne Pathogens Kathleen Stefek, RN, MSN What are Bloodborne Pathogens? Infectious agents carried in the blood and other body fluids that are capable of infecting a host (people like you and me) with
Bloodborne Pathogens Kathleen Stefek, RN, MSN What are Bloodborne Pathogens? Infectious agents carried in the blood and other body fluids that are capable of infecting a host (people like you and me) with
Drew University Bloodborne Pathogens Exposure Control Plan and Procedures
 PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
Occupational Exposures to Bloodborne Pathogen (BBP) Training
 Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
WVU Occupational Medicine Program for evaluation and treatment of persons exposed to Lentivirus Vectors
 Hazard Description: Lentivirus vector systems are derived from HIV and similar viruses because of being very effective at inserting genetic material into the genome of target cells. While most researchers
Hazard Description: Lentivirus vector systems are derived from HIV and similar viruses because of being very effective at inserting genetic material into the genome of target cells. While most researchers
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
Hepatitis B at a Glance
 Return completed form, preferably within 30 days of U.S. date of arrival, to address on reverse side of this form. Review overseas medical exam if available and document immunization dates. Indicate if
Return completed form, preferably within 30 days of U.S. date of arrival, to address on reverse side of this form. Review overseas medical exam if available and document immunization dates. Indicate if
Blood-borne Pathogens Exposure Policy
 Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
OB Provider Guide to Alaska s Perinatal Hepatitis B Prevention Program
 OB Provider Guide to Alaska s Perinatal Hepatitis B Prevention Program Dear Colleague, This letter is to introduce myself and explain the role I play with the Alaska Perinatal Hepatitis B Program. Alaska
OB Provider Guide to Alaska s Perinatal Hepatitis B Prevention Program Dear Colleague, This letter is to introduce myself and explain the role I play with the Alaska Perinatal Hepatitis B Program. Alaska
Sandi Mitchell Nurse Educator Clinical Prevention Services BCCDC
 Sandi Mitchell Nurse Educator Clinical Prevention Services BCCDC sandi.mitchell@bccdc.ca Hepatitis A, B and C Testing Transmission Treatment What Is Hepatitis? Hepatitis means inflammation of the liver
Sandi Mitchell Nurse Educator Clinical Prevention Services BCCDC sandi.mitchell@bccdc.ca Hepatitis A, B and C Testing Transmission Treatment What Is Hepatitis? Hepatitis means inflammation of the liver
HIV Occupational Transmission and Exposure. Marsh Gelbart 2010
 HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
Environmental Health and Safety Offices BLOODBORNE PATHOGENS
 Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Appendix 11 Roles and Responsibilities October, 2013 Page 1 of 5
 October, 2013 Page 1 of 5 Exposed Person To present to a health care facility as soon as possible following the exposure (ideally within 2 hours). To answer assessment questions. (Refer to Appendix 15
October, 2013 Page 1 of 5 Exposed Person To present to a health care facility as soon as possible following the exposure (ideally within 2 hours). To answer assessment questions. (Refer to Appendix 15
Lifetime risk of infection >60% Early childhood infections common
 Hepatitis Community Medicine HBV Public health sig HBV is 100 times more infectious than HIV. >350 million chronically infected worldwide. >1 million people die annually of HBV- related chronic liver disease.
Hepatitis Community Medicine HBV Public health sig HBV is 100 times more infectious than HIV. >350 million chronically infected worldwide. >1 million people die annually of HBV- related chronic liver disease.
How does HBV affect the liver?
 Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification
 SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
