Process or performance improvement is often discussed
|
|
|
- Kerry Ramsey
- 5 years ago
- Views:
Transcription
1 Process Improvement in Transfusion Medicine Ongoing efforts to decrease costs in the clinical laboratory make continuous process improvement especially important in difficult economic times. Process improvement can result in decreased workload, cost savings, and increased customer satisfaction but is an abstract concept in and of itself. To illustrate the steps of process improvement, we applied them to our blood component retrieval policy. By identifying the problems with the current system, proposing and implementing solutions, and measuring the effects before and after revamping the process, we have been able to show impressive reductions in the number of component retrievals initiated, the number acted on, wasted components, and customer complaints, all of which translate into cost savings. Once the cycle is completed, it begins anew. There must always be continuous process improvement. (Arch Pathol Lab Med. 1999;123: ) Process or performance improvement is often discussed as one means of adapting to the enormous changes, both fiscal and technological, that are currently occurring in the clinical laboratory. The alteration of a process to achieve efficiency can result in numerous benefits, including decreased workload, cost savings, and increased customer satisfaction. 1 In fact, an emphasis on process improvement is most important in difficult economic times. 2 The concept of process improvement can seem somewhat abstract; however, if applied to a real process, its benefits can become concrete. The example of process improvement that follows is our experience with streamlining the blood component retrieval (CR) process in our blood center. CR PROCESS The CR process is a system that allows blood centers to withdraw blood components that, potentially, may present risk to a transfusion recipient. The process may also be known as market withdrawal. Several facets of the CR process are regulated, 3 5 while others are common sense. The latter are carried out voluntarily by blood centers to protect the safety of the blood supply (and obviate medicolegal problems). The process can be complex, particularly as the volume of collections, and thus the number and Presented at the College of American Pathologists Conference XXXIII, Transfusion Medicine Performance Improvement, which was cosponsored with the American Association of Blood Banks, San Francisco, Calif, August 20 22, Reprints: Patricia M. Kopko, MD, Sacramento Medical Foundation Blood Center, 1625 Stockton Blvd, Sacramento, CA One Blood Center s Approach Patricia M. Kopko, MD; Paul V. Holland, MD types of CRs at a large regional blood center, increases. Retrieval of blood components that are potentially dangerous to recipients is beneficial; retrieval of those not presenting an increased risk is wasteful and costly in terms of lost components and may result in unnecessary work and confusion for hospital transfusion service (laboratory) customers. The process of retrieval of blood components is initiated when a unit of blood is identified as possibly being unfit for transfusion. There are a variety of reasons why a particular blood donation, and/or prior donations from a particular donor, may not be appropriate for transfusion (Table 1). When a blood center receives information from a donor, either at the time of the next attempted donation or after donation, that would have made the donor ineligible to donate had the information been available at the time of donation, a CR is initiated. An example of postdonation information triggering retrieval is a health care worker who remembers, subsequent to several donations, that he or she had a patient needle stick exposure several months ago that he or she had forgotten to tell prior interviewers. Since this donor would not have been eligible if this information had been known, due to the risk of potential exposure to human immunodeficiency virus and hepatitis, any blood components that are available and still unused should be withdrawn from inventory (at both the blood center and hospital transfusion services) for the safety of transfusion recipients. If a donor tests positive on a current blood donation for any of the viral markers used to screen blood donations, retrieval of in-date components from prior donations is recommended, functionally required by the Food and Drug Administration (FDA). 3 5 If an error in the manufacturing process of a unit of blood is discovered, the unit must be retrieved. Additionally, if a patient has a serious reaction to a blood component, the remaining components from that donation may have to be retrieved. For instance, if a patient has a septic transfusion reaction due to bacterial contamination of a platelet concentrate, the packed red blood cells and fresh frozen plasma from that donation should be retrieved. When a CR is initiated, the appropriate components need to be withdrawn from the market (ie, prevented from use). Any components that are still under the control of the blood center are quarantined to prevent release. Components that are potentially in a client hospital s inventory must be returned. The hospital transfusion service is notified that a particular component should not be transfused, if it has not already been used. The hospital is asked to confirm if the unit is still available or if it has been transfused. If the component is still in inventory, the Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland 569
2 Table 1. Examples of Reasons for Component Retrieval 1. Donor calls back to: a. report illness b. report exposure to human immunodeficiency virus or hepatitis c. report travel to a malarial area d. request that donation not be used 2. Current donation tests positive for viral marker 3. Error is found in the manufacturing process 4. Patient had a septic reaction to a component hospital is asked to quarantine the component and either destroy it or return it to the blood center. When the CR is initiated and components are quarantined, it is often carried out based on preliminary information to control the product while the information is further investigated by the blood center to determine both the appropriateness of the CR and the ultimate disposition of the quarantined unit(s). The hospital transfusion service is notified of the initial reason for the market withdrawal, but often needs to be notified of the final outcome of the investigation as well, particularly if a blood component had already been transfused. An example of this is a frequent plasma donor who now tests repeatedly reactive in the screening test for the hepatitis B surface antigen. There can be up to 60 plasma donations that are still in the hospital or blood center inventory. Based on FDA recommendations, all the plasma donated in the last year is retrieved and quarantined pending the outcome of more specific confirmatory testing. 3 If the confirmatory testing results are negative, then the quarantined units can be released for transfusion. However, if the donor is confirmed positive for hepatitis B, the plasma from previous donations that has not been transfused is either destroyed or may be used for research. Additionally, hospitals that transfused plasma donated by this particular donor in the last year are notified that the donor subsequently was confirmed positive for hepatitis B so that they may notify and test transfusion recipients for evidence of hepatitis B virus infection and consider prophylaxis if they so choose. This latter notification is not required by the FDA. 3 Because the safety of the blood supply is involved in the CR process, the system needs to be as foolproof as possible but still needs to be simple, rapid, and cost-effective. However, the process in our center had focused primarily on safety without examining the CR process as a whole, which has additional ramifications, including impact on other blood center operations and hospital customers. As the number of blood donations at our blood center increased, the CR process became unwieldy and cumbersome. We decided that we needed to examine our CR system in its entirety and attempt to identify areas for potential improvement. We elected to apply process improvement to CR at our blood center as a practical exercise. PROCESS IMPROVEMENT STEP 1: IDENTIFY THE PROBLEM(S) Using our approach to revamping the CR process, we can illustrate an application of process improvement in one part of the laboratory, the blood bank. The first step in process improvement is to identify the way(s) the current system is not meeting expectations. We identified several problems, both external and internal, with the CR process of our blood center. The external complaints were primarily from customers who were unhappy with our lack of flexibility in our approach to the CR process. We received complaints because we treated every CR, no matter the cause, as immediate. We also treated all CRs with equal importance. Our procedures specified that we had to attempt to retrieve components even if they were not in-date and had been transfused up to 5 years before the CR. Additionally, our customers complained that our turnaround time, from initiation to final notification, was too long. Internally, our problems with the CR process were even more complex. The treatment of all CRs as immediate was a great burden to our hospital services department because they were expected to drop everything to retrieve components that often had long since been transfused. The work flow of the process was extremely complex and involved numerous staff in various departments (Figures 1 and 2). This complexity led to several additional problems with locating and tracking CRs, because many different staff members could handle the paperwork of the CR. In fact, CRs were often lost (usually buried on someone s desk), leading to unnecessary rework. The complexity of the process also led to long turnaround times as the CR worked its way through the system. The final internal problem identified as part of the process of retrieving blood components was unnecessary loss of product, which led to a negative financial impact on the blood center. Components were lost (unavailable for transfusion) for several reasons. Numerous unneeded CRs were initiated because there was no medical evaluation of the necessity of the CR early in the process. Medical review was only performed at the end of the process. In addition, all related components were retrieved, not just those affected by the reason for the CR. For instance, although notification of a donor s travel to a malarial area requires CR of red blood cells and platelets, but not plasma, we retrieved all components, including the plasma. We felt that this problem was due to the lack of critical medical evaluation of the reason for the CR early in the process. We were also losing product because of the long turnaround times. By the time a CR worked its way through the system and received evaluation by a blood center medical director for potential release of components, the components had often expired. PROCESS IMPROVEMENT STEP 2: PROPOSE AND IMPLEMENT SOLUTIONS The first part of the next step in process improvement is to propose solutions to address the problems identified; the second part of this step is to implement the chosen solution(s). All departments affected by the process should have input into possible solutions and the changes proposed and then attempted. In this part of the process, means should also be established to measure the efficacy of the corrective actions as well as identify any new problems or issues with the improvements. Several actions were proposed and then certain ones taken to correct the problems with the CR process. We stopped treating all CRs as needing immediate and urgent attention. We separated the CRs into two classes: a group that needed to be dealt with urgently and another group that needed CRs to be performed as soon as possible (ie, quarantine and initial customer notification occurring within 72 hours). 570 Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland
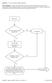
3 Figure 1. Work flow of original component retrieval (CR) process. Triangles indicate decision points; rectangles, actions. Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland 571
4 Figure 2. Work flow of original component retrieval (CR) process continued. Triangles indicate decision points; rectangles, actions; and FDA, Food and Drug Administration. Information on CRs that requires immediate attention includes infection with, or possible exposure to, human immunodeficiency virus; exposure to, or diagnosis of, hepatitis; fever, chills, vomiting, or diarrhea with 24 hours of donation; and donor hotline calls when the donor indicates that he or she does not want his or her blood used, but does not specify a reason for this postdonation call. When a CR that fits into the immediate category is initiated, the hospital services department immediately retrieves the appropriate blood components based on defined medical criteria (Table 2). Any CR information received that is not clearly urgent, but is of concern, is referred to the medical director on call for a decision on whether immediate action needs to be taken. Instead of retrieving all components donated in the last year, or 5 years, we decided that it would be more appro- 572 Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland
5 Table 2. Medical Directive for Immediate Component Retrievals Component Retrieval Information Symptoms of fever, chills, vomiting, or diarrhea within 24 h of donation Call from the donor stating, Don t use my blood Donor exposure to hepatitis or human immunodeficiency virus Not defined but may represent a recipient safety issue Retrieve All components of the unit in question (most recent donation) Platelets back 5 d, red blood cells back 42 d, cryoprecipitate and plasma back 1 y Platelets back 5 d, red blood cells back 42 d, and cryoprecipitate and plasma back 1 y or retrieve all components back to initial exposure (whichever time frame is shorter) Refer to on-call physician for direction priate to follow FDA recommendations and retrieve only those components that were still in-date and potentially available for transfusion. This allowed both our facility and our hospital transfusion services to stop wasting time and effort determining the disposition of long-expired components. In-date was defined as 5 days for platelets, 42 days for red blood cells, and 1 year for fresh frozen plasma and cryoprecipitate. If the FDA recommendation defined a shorter period for CR (eg, 3 months for human immunodeficiency virus 1 antigen), this period was used. 5 We greatly simplified the work flow of the CR process, in part by assigning a CR coordinator who is responsible for guiding the CRs through the system (Figure 3). This individual directly hands-off CRs to the next employee responsible for an action needed in the process. The desired action is performed and the CR paperwork is returned to the CR coordinator. In addition, we decreased the number of staff responsible for performing actions in the CR process. This combination of changes has greatly decreased the difficulties of tracking and locating CRs and has virtually abolished the problem of lost CRs. Further, the CR turnaround was decreased so that in-date components could be released from quarantine, if appropriate to do so. For nonimmediate CRs, the decision of the appropriate components to retrieve, if any, is accomplished in one of two ways. For commonly occurring reasons for CRs, a medical directive that details which components to retrieve has defined an algorithm for the CR coordinator to follow. Commonly occurring CRs include, for example, repeat reactive viral marker tests and postdonation information about a diagnosis of cancer, exposure to hepatitis or human immunodeficiency virus, and travel to a malarial area. Those CRs falling outside this specified algorithm are reviewed by a medical director before retrieval. Our final alteration of the CR process was moving the timing of the medical review of the CRs. Instead of a nurse or a medical director reviewing a CR for appropriateness at the end of the process, after numerous components had been retrieved unnecessarily, a physician reviews all CRs up front that do not clearly fit into the defined medical directive for CRs. This change was made possible by the fact that all CRs are no longer treated as immediate. There is now time in the process for the CR coordinator to deliver an entire day s worth of nonimmediate CRs to a medical director for review, before any retrieval of components. This has resulted in numerous CRs being canceled at the medical review step and has greatly decreased the number of components retrieved unnecessarily. It also allows for early release of components that were quarantined inappropriately or unnecessarily. This is particularly important for platelet products, because they expire 5 days after donation. PROCESS IMPROVEMENT STEP 3: MEASURE THE IMPACT OF THE SOLUTION(S) The third step in process improvement is to measure the effect(s) of changing the process. Quantifying the effects of the changes and determining both the benefits and improvements and fallout and unanticipated impacts are essential. Measurements to determine effectiveness need to be made before and after implementation of changes in the process. The changes made to the CR process have had a dramatic impact on the number of CRs and the cost of the CR process. In the fourth quarter of 1996, before implementation of any of these changes, 441 CRs were initiated. Because all these were treated as immediate, they were all acted on. To measure the effects of the changes in the CR process and to quantify the process improvement, we evaluated the same parameters during a similar period after changes were implemented. In the fourth quarter of 1997, there were only 281 CRs initiated, with approximately the same total number of units drawn. Now, only 156 of these actually needed any retrieval action. This represents a 36% reduction in the number of CRs initiated and a 65% reduction in the number of CRs acted on. Not only did the total number of CRs acted on decrease, so did the number of components retrieved. Table 3 shows the actual components retrieved in the fourth quarter of 1997 compared with the components that would have been retrieved before this process improvement. Overall, there was a 67% reduction in the number of components retrieved. The most dramatic differences are seen with platelet and red blood cell components. This is primarily due to the fact that we are no longer attempting to retrieve red blood cells and platelets beyond their expiration date and the relatively short shelf-lives of these components compared with plasma products. We estimated that, on average, each CR needed 3 employee hours to complete under the old CR system. After the process improvement implementation, we found that it now takes about 1 hour per CR. For the 1 year period before the changes in the CR system (10/96 9/97), there were 1039 CRs initiated. Assuming a 36% reduction in the number of CRs initiated, a 2-hour reduction in employee hours worked per CR and an average hourly wage (including benefits) of $22.30, the CR process has saved our blood center approximately $ in salary expenses (Table 4). We also found that there was less product wastage due to blood components expiring before their release because the CRs are being turned around more rapidly, although the extent of this has been difficult to quantify. Finally, customer satisfaction has increased. As mentioned previously, before beginning this process improvement program, several of our customers had complained that we were attempting to retrieve expired components, that we treated all CRs as urgent when most were not, and that our turnaround times were too long. To date, since implementing the changes described, we have not Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland 573
6 Figure 3. Work flow on new component retrieval (CR) process. Triangles indicate decision points; rectangles, actions; and FDA, Food and Drug Administration. Table 3. Retrieved (actual), No. Not retrieved, No. Total, No. Reduction, % Fourth Quarter 1997 Component Retrievals Actual Plus Those Not Required to Be Retrieved Red Blood Cells Cryoprecipitate Fresh Frozen Plasma Jumbo Fresh Frozen Plasma Recovered Plasma Platelet Concentrates Platelet Apheresis Total received a single complaint about our CR process. Instead, we have received both praise and a few inquiries about our new approach. Once the cycle of process improvement has been completed, it begins anew. Identification of residual problems, or new ones created, starts over. Solutions are proposed, agreed on, and implemented. Then, remeasurement of efficacy, or lack of efficacy, is carried out to assess the degree of process improvement (eg, lack of rework, cost savings, and decreased complaints). By closely examining the CR system in our blood center and looking for ways to improve the process, we have simplified a complex system and saved money in terms of both wages and lost product, decreased turnaround times, 574 Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland
7 Table 4. Estimated Cost Savings of Process Improvement to Component Retrieval System Component Retrievals (New System) [Component Retrievals (old system)] (1 % reduction) (1039 Component Retrievals) (1 0.36) 665 Component Retrievals * 374 fewer component retrievals are initiated Total Savings (Component Retrievals Not Initiated) (3 h/component Retrieval) (Cost of Labor) (Component Retrievals) (2 h/component Retrieval) (Cost of Labor) (374 Component Retrievals) (3 h/component Retrieval) ($22.30/h) (665 Component Retrievals) (2 h/component Retrieval) ($22.30/h) $ and essentially eliminated customer complaints. We continue to evaluate this process improvement to determine if our goals are being accomplished. If not, the process will be modified further, with input from those affected; there must be ongoing evaluation for continuous process improvement. For example, at this point, we are considering our ability to capture information electronically by putting the entire CR system on our internal, linked personal computer system. This would allow all employees with CR responsibilities to access the status of CR information and potentially perform their CR tasks via their personal computers. References 1. West NR. Observations on outcomes. Health Care Division Newsletter, American Society for Quality, Health Care Division. 1998: Laszlo GP. Cost reduction through quality improvement. Quality Manage Forum. February 1998: Recommendations for the quarantine and disposition of units from prior collections from donors with repeatedly reactive screening tests for hepatitis B virus (HBV), hepatitis C virus (HCV) and human T-lymphotropic virus type I (HTLV-I). Rockville, Md: Dept of Health and Human Services, Food and Drug Administration; 1996: Department of Health and Human Services, Food and Drug Administration. Current good manufacturing practices for blood and blood components: notification of consignees receiving blood and blood components at increased risk for transmitting HIV infection. 61 Federal Register (1996). 5. Recommendations for donor screening with licensed test for HIV-1 antigen. Rockville, Md: Dept of Health and Human Services, Food and Drug Administration; 1995:2 15. Arch Pathol Lab Med Vol 123, July 1999 Blood Center Process Improvement Kopko & Holland 575
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Strategies to Prevent Pharmaceutical Waste: Modifying Co-Pay Structures
 Modifying Co-Pay Structures Extended producer responsibility (EPR) is a policy approach in which the producer s responsibility for their product extends to the post-consumer management of that product
Modifying Co-Pay Structures Extended producer responsibility (EPR) is a policy approach in which the producer s responsibility for their product extends to the post-consumer management of that product
Department of Health and Human Services
 Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB
 Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB Plasma Fractionation Evolved from a medical service to a global manufacturing industry Involves sophisticated industrial process High
Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB Plasma Fractionation Evolved from a medical service to a global manufacturing industry Involves sophisticated industrial process High
[Submitted Electronically]
![[Submitted Electronically] [Submitted Electronically]](/thumbs/82/85246007.jpg) [Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
[Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
HOW RARE IS YOUR BLOOD? DONATE ( ) CANADIAN BLOOD SERVICES RARE BLOOD PROGRAM
 HOW RARE IS YOUR BLOOD? CANADIAN BLOOD SERVICES RARE BLOOD PROGRAM If you have a rare blood type, become a donor and help save lives across Canada and around the world. WHAT IS RARE BLOOD? Did you know
HOW RARE IS YOUR BLOOD? CANADIAN BLOOD SERVICES RARE BLOOD PROGRAM If you have a rare blood type, become a donor and help save lives across Canada and around the world. WHAT IS RARE BLOOD? Did you know
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
Armed Services Blood Program
 Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
Ministry of Children and Youth Services. Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW
 Chapter 4 Section 4.01 Ministry of Children and Youth Services Autism Services and Supports for Children Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of
Chapter 4 Section 4.01 Ministry of Children and Youth Services Autism Services and Supports for Children Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
HIV/AIDS. Kuna High School Mr. Stanley
 HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
Consent to Shipment of Frozen Embryos to and Short Term Storage of Frozen Embryos at the Family Fertility Center
 1. Background On or about and requested clinic and treating physician) at (date), name(s) of parties underwent IVF treatment) (name of (address of clinic) to perform in vitro fertilization using: a. Oocyte
1. Background On or about and requested clinic and treating physician) at (date), name(s) of parties underwent IVF treatment) (name of (address of clinic) to perform in vitro fertilization using: a. Oocyte
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
Blood Supply and Wastage
 Blood Supply and Wastage Inga Willett Lab Matters, Oake Manor, 21 st June 2017 Blood supply UK supplied by 4 blood services: SNBTS NIBTS NHSBT WBS http://commons.wikimedia.org/wiki/file:uk_map_home_nations.png
Blood Supply and Wastage Inga Willett Lab Matters, Oake Manor, 21 st June 2017 Blood supply UK supplied by 4 blood services: SNBTS NIBTS NHSBT WBS http://commons.wikimedia.org/wiki/file:uk_map_home_nations.png
Answers. To Your. Questions
 Blood Transfusions Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
Blood Transfusions Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
BLOOD COMPONENTS are regulated as
 Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
Making Your Blood Donation Safe
 BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
AIDS Prevention and Control Act
 AIDS Prevention and Control Act The Regulations became legally effective after public notification was made by the President on Dec. 17, 1990 An amendment of the Regulations comprising the addition of
AIDS Prevention and Control Act The Regulations became legally effective after public notification was made by the President on Dec. 17, 1990 An amendment of the Regulations comprising the addition of
SOP 17 BLOOD BANK. 3. Overall Responsibility: Blood Bank In-Change/Pathologist.
 SOP 17 BLOOD BANK 1. Purpose: To ensure the availability of safe blood unit with facility for compatibility testing, storage and issue of blood in an aseptic environment on 24*7 basis trough trained professionals.
SOP 17 BLOOD BANK 1. Purpose: To ensure the availability of safe blood unit with facility for compatibility testing, storage and issue of blood in an aseptic environment on 24*7 basis trough trained professionals.
HIV SCREENING WORKSHOP Exercise
 HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
BLOOD TRANSFUSIONS. Answers. To Your. Questions
 BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
New Advances in Transfusion EM I LY CO BERLY, M D
 New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
CERTIFIED MAIL RETURN RECEIPT REQUESTED. Esther Hernandez President United States Blood Bank, Inc NW 95th Avenue Miami, Florida
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
CTYOMEGALOVIRUS (CMV) - BACKGROUND
 CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
Transfusion 2004: Current Practice Standards. Kay Elliott, MT (ASCP) SBB SWMC Transfusion Service
 Transfusion 2004: Current Practice Standards Kay Elliott, MT (ASCP) SBB SWMC Transfusion Service Massive Transfusion Protocol (MTP) When should it be activated? Massive bleeding i.e. loss of one blood
Transfusion 2004: Current Practice Standards Kay Elliott, MT (ASCP) SBB SWMC Transfusion Service Massive Transfusion Protocol (MTP) When should it be activated? Massive bleeding i.e. loss of one blood
Understanding Blood Transfusion and The Zimmer OrthoPAT * System
 Understanding Blood Transfusion and The Zimmer OrthoPAT * System Know Your Options A global leader in the provision of high-quality, hands-on education and training for orthopaedic surgeons. *Trademark
Understanding Blood Transfusion and The Zimmer OrthoPAT * System Know Your Options A global leader in the provision of high-quality, hands-on education and training for orthopaedic surgeons. *Trademark
Blood Transfusion. What is blood transfusion? What are blood banks? When is a blood transfusion needed? Who can donate blood?
 What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
 Fact Sheet Blood Collection and Use Hong Kong Red Cross Blood Transfusion Service The Hong Kong Red Cross began its voluntary, nonremunerated blood donation programme to supply blood to all hospitals in
Fact Sheet Blood Collection and Use Hong Kong Red Cross Blood Transfusion Service The Hong Kong Red Cross began its voluntary, nonremunerated blood donation programme to supply blood to all hospitals in
AdvaMedDx Value Assessment Framework in Practice
 AdvaMedDx Value Assessment Framework in Practice Application of the Comprehensive Assessment of the Value of Diagnostic Technologies Framework to Abbott s 4th Generation ARCHITECT HIV Antigen/Antibody
AdvaMedDx Value Assessment Framework in Practice Application of the Comprehensive Assessment of the Value of Diagnostic Technologies Framework to Abbott s 4th Generation ARCHITECT HIV Antigen/Antibody
Boot Camp Transfusion Reactions
 Boot Camp Transfusion Reactions Dr. Kristine Roland Regional Medical Lead for Transfusion Medicine, VCH Objectives By the end of this session, you should be able to: Describe in common language the potential
Boot Camp Transfusion Reactions Dr. Kristine Roland Regional Medical Lead for Transfusion Medicine, VCH Objectives By the end of this session, you should be able to: Describe in common language the potential
Blood Transfusion. There are three types of blood cells: Red blood cells. White blood cells. Platelets.
 Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Note: Staff who work in case management programs should attend the AIDS Institute training, "Addressing Prevention in HIV Case Management.
 Addressing Prevention with HIV Positive Clients This one-day training will prepare participants to help people living with HIV to avoid sexual and substance use behaviors that can result in transmitting
Addressing Prevention with HIV Positive Clients This one-day training will prepare participants to help people living with HIV to avoid sexual and substance use behaviors that can result in transmitting
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
East Carolina University Student Health Service
 Page 1 of 5 Policy: BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIAL EXPOSURE POLICY FOR STUDENTS, WITH CLINICAL EXPOSURES s (SHS) will adapt and modify the policies and procedures of ECU Prospective Health
Page 1 of 5 Policy: BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIAL EXPOSURE POLICY FOR STUDENTS, WITH CLINICAL EXPOSURES s (SHS) will adapt and modify the policies and procedures of ECU Prospective Health
2/2/2011. Blood Components and Transfusions. Why Blood Transfusion?
 Blood Components and Transfusions Describe blood components Identify nursing responsibilities r/t blood transfusion Discuss factors r/t blood transfusion including blood typing, Rh factor, and cross matching
Blood Components and Transfusions Describe blood components Identify nursing responsibilities r/t blood transfusion Discuss factors r/t blood transfusion including blood typing, Rh factor, and cross matching
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
2017 BLOODBORNE PATHOGENS
 2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
RISK OF DISEASE TRANSMISSION FROM ORGAN DONORS PATIENT INFORMATION GUIDE
 Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Specialty Drugs in Workers Compensation
 Specialty Drugs in Workers Compensation A GUIDE FOR CLAIMS HANDLERS Specialty Drugs Specialty drugs have been developed in response to demand for specialized medications for complex medical conditions.
Specialty Drugs in Workers Compensation A GUIDE FOR CLAIMS HANDLERS Specialty Drugs Specialty drugs have been developed in response to demand for specialized medications for complex medical conditions.
Organ Procurement and Transplantation Network
 OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
Donor Registration and Consent for HLA Typing
 Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
Question: 1. Are you currently taking an antibiotic?
 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies
 2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
IRB USE ONLY Approval Date: September 10, 2013 Expiration Date: September 10, 2014
 Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: The goal of Assisted Reproductive Technology (ART) is to help infertile couples to become pregnant. During treatment, some cellular
Approval : September 10, 2013 Expiration : September 10, 2014 DESCRIPTION: The goal of Assisted Reproductive Technology (ART) is to help infertile couples to become pregnant. During treatment, some cellular
A Patient s Guide to Blood Components and Products
 2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
2006 HIV Diagnostics Survey
 Public Health Laboratory Issues in Brief: 2006 HIV Diagnostics Survey Association of Public Health Laboratories November 2007 Background Nearly 20 years ago, the Association of Public Health Laboratories
Public Health Laboratory Issues in Brief: 2006 HIV Diagnostics Survey Association of Public Health Laboratories November 2007 Background Nearly 20 years ago, the Association of Public Health Laboratories
Hepatitis is an epidemic disease that can be caused by different viruses including hepatitis viruses A, B, C, D or E.
 What is Hepatitis? Hepatitis is an epidemic disease that can be caused by different viruses including hepatitis viruses A, B, C, D or E. Hepatitis A and E are not chronic and are mostly present in areas
What is Hepatitis? Hepatitis is an epidemic disease that can be caused by different viruses including hepatitis viruses A, B, C, D or E. Hepatitis A and E are not chronic and are mostly present in areas
Greenwood School District 50 OSHA UPDATE BLOODBORNE PATHOGENS
 Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
As a result of this training, participants will be able to:
 Addressing Sexual Risk with Drug Users and their Partners 1 Day Training This one-day training will build participant knowledge and skills in offering sexual harm reduction options to substance users.
Addressing Sexual Risk with Drug Users and their Partners 1 Day Training This one-day training will build participant knowledge and skills in offering sexual harm reduction options to substance users.
Informed Consent for Liver Transplant Patients
 Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID
 UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
ECHO-Antibiotic Stewardship Program
 ECHO-Antibiotic Stewardship Program More Interesting Recent Literature Updates April 20, 2017 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Antibiotic
ECHO-Antibiotic Stewardship Program More Interesting Recent Literature Updates April 20, 2017 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Antibiotic
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
BLOOD PROGRAM. The American Legion
 BLOOD PROGRAM The American Legion BLOOD AND COMMUNITY HEALTH The use of the blood of one human being to save the life of another is one of the world s greatest medical achievements. Thousands of people
BLOOD PROGRAM The American Legion BLOOD AND COMMUNITY HEALTH The use of the blood of one human being to save the life of another is one of the world s greatest medical achievements. Thousands of people
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Transfusion Management of IgA deficiency. Emily Coberly, MD Medical Director, Transfusion Services University of Missouri Columbia
 Transfusion Management of IgA deficiency Emily Coberly, MD Medical Director, Transfusion Services University of Missouri Columbia Our patient 63 year-old female with a duodenal stricture who was admitted
Transfusion Management of IgA deficiency Emily Coberly, MD Medical Director, Transfusion Services University of Missouri Columbia Our patient 63 year-old female with a duodenal stricture who was admitted
Autologous plasma eye drops. Patient information
 Autologous plasma eye drops Patient information Autologous plasma eye drops are made from the patient s own blood and are used to help treat their corneal surface defects and diseases. This leaflet gives
Autologous plasma eye drops Patient information Autologous plasma eye drops are made from the patient s own blood and are used to help treat their corneal surface defects and diseases. This leaflet gives
Blood Transfusion Orientation & Information 2010
 Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
An Uncured Disease. By: Katie Farley. A disease so prominent, so recently discovered and so deadly has
 Katie Farley (505) 522-4594 kmfarley@hotmail.com Las Cruces, New Mexico 1500 words An Uncured Disease By: Katie Farley A disease so prominent, so recently discovered and so deadly has infected an estimated
Katie Farley (505) 522-4594 kmfarley@hotmail.com Las Cruces, New Mexico 1500 words An Uncured Disease By: Katie Farley A disease so prominent, so recently discovered and so deadly has infected an estimated
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019
![[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019 [Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019](/thumbs/95/123651537.jpg) SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
Database on Blood Safety 2009 in ECO Member States
 Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
As a result of this training, participants will be able to:
 Addressing Prevention with HIV Positive Clients 1 Day Training This one-day training will prepare participants to help people living with HIV to avoid sexual and substance use behaviors that can result
Addressing Prevention with HIV Positive Clients 1 Day Training This one-day training will prepare participants to help people living with HIV to avoid sexual and substance use behaviors that can result
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
IQPP Qualified Donor Standard
 IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
19/08/2014. What is Injection Safety?
 Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
IMPORTANT HEALTH INFORMATION
 IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
Arts Administrators and Healthcare Providers
 Arts Administrators and Healthcare Providers Table of Contents Part One: Creating Music and Programs 2 Preparing to Start a Program 2 Finding Funding 2 Selecting Partner Arts Organizations or Healthcare
Arts Administrators and Healthcare Providers Table of Contents Part One: Creating Music and Programs 2 Preparing to Start a Program 2 Finding Funding 2 Selecting Partner Arts Organizations or Healthcare
New York State Vaccine Program Vaccine Restitution Policy
 Purpose New York State (NYS) provides vaccine worth approximately $79 million to Vaccines for Children (VFC) providers in New York State (outside of New York City) each year at no cost to providers. As
Purpose New York State (NYS) provides vaccine worth approximately $79 million to Vaccines for Children (VFC) providers in New York State (outside of New York City) each year at no cost to providers. As
School Nursing and Health. Standard Precautions. (aka Universal Precautions)
 School Nursing and Health Standard Precautions (aka Universal Precautions) August 2016 1 Standard Precautions Occupational Safety and Health Administration Federal Law 29 CFR bloodborne Pathogens 1910.1030(g)(2)(i)
School Nursing and Health Standard Precautions (aka Universal Precautions) August 2016 1 Standard Precautions Occupational Safety and Health Administration Federal Law 29 CFR bloodborne Pathogens 1910.1030(g)(2)(i)
FDA Reentry Guidance
 FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
Pathology s Role in Liver Transplantation Surgery: Offering Patients a New Lease on Life
 Pathology s Role in Liver Transplantation Surgery: Offering Patients a New Lease on Life Montefiore recently became the first health system in New York City to perform a liver transplant using a donor
Pathology s Role in Liver Transplantation Surgery: Offering Patients a New Lease on Life Montefiore recently became the first health system in New York City to perform a liver transplant using a donor
Self-Instructional Packet (SIP)
 Self-Instructional Packet (SIP) Advanced Infection Prevention and Control Training Module 1 Intro to Infection Prevention Control February 11, 2013 Page 1 Learning Objectives Module One Introduction to
Self-Instructional Packet (SIP) Advanced Infection Prevention and Control Training Module 1 Intro to Infection Prevention Control February 11, 2013 Page 1 Learning Objectives Module One Introduction to
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories
 Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
BLOOD TRANSFUSIONS. Answers. To Your. Questions
 BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
