|
|
|
- Victoria Jennings
- 5 years ago
- Views:
Transcription
1 27027 Tourney Road Valencia, CA Test Updates May 27, 2010 Dear Colleague: Our May communication to you brings very exciting news of a new test offering available in July FcGammaRIIa & FcGammaRIIIa Mutation Analysis (5388). Combined FcγRIIa/FcγRIIIa polymorphisms are shown to be prognostic factors for disease progression in metastatic colorectal cancer in patients treated with cetuximab plus irinotecan. These polymorphisms have also been found to predict rituximab response in patients with follicular lymphoma. Human Fcγ receptors on hematopoietic cells are involved in several important biological responses, including phagocytosis, release of inflammatory mediators, antibody-dependent cellular toxicity, enhancement of antigen presentation, and platelet activation. The efficacy of Fcγ receptor function displays inter-individual heterogeneity due to genetic polymorphisms of FcγR subclasses including FcγRIIa (CD32a), FcγRIIIa (CD16a). These polymorphisms have been associated with immune related disorders and furthermore serve as markers for therapeutic efficacy and side-effects of treatment with monoclonal antibodies. We are also pleased to inform you of Specialty s recent New York approval for Fentanyl & Norfentanyl Urine w/reflex Confirmation (4175U). This also applies to panel Expanded Drugs Of Abuse Screen Ur + Fentanyl W/Reflex Conf (4472U). This will enable our New York clients and those serving New York residents to have these testing provided within our facility. In addition, please note that EBV Nuclear Ag (EBNA) IgM Abs (2271) and F-Actin IgA Autoabs (5924) are now FDA approved tests. Please also see this important reminder from our Billing Department: You are likely aware of Medicare s requirement for physician and non-physician practitioners to be enrolled in the Medicare Provider Enrollment, Chain and Ownership System (PECOS). We are currently receiving warning messages that certain ordering/referring providers are not enrolled in PECOS. Under the current rule, claims will be denied and payments will stop on January 1, If that occurs, your facility could potentially be responsible for payment. Please work with your physician and clients to ensure that they are enrolled. We thank you for choosing Specialty and look forward to your continued support. For additional information, please visit our Web site at or contact Client Relations at Respectfully Yours, Christopher Lockhart, M.D. Laboratory Director
2 New Tests (Specialty): 3750 Respiratory Allergy Profile Region XI (Available ) Dermatophagoides pteronyssinus IgE ImmunoCAP <0.35 ku/l Dermatophagoides farinae IgE ImmunoCAP <0.35 ku/l Cat epithelium/dander IgE ImmunoCAP <0.35 ku/l Dog dander IgE ImmunoCAP <0.35 ku/l Bermuda grass IgE ImmunoCAP <0.35 ku/l Timothy grass IgE ImmunoCAP <0.35 ku/l Cockroach, German IgE ImmunoCAP <0.35 ku/l Penicillium chrysogenum IgE ImmunoCAP <0.35 ku/l Cladosporium herbarum IgE ImmunoCAP <0.35 ku/l Aspergillus fumigatus IgE ImmunoCAP <0.35 ku/l Alternaria alternata/tenuis IgE ImmunoCAP <0.35 ku/l Grey alder IgE ImmunoCAP <0.35 ku/l Mountain juniper/cedar IgE ImmunoCAP <0.35 ku/l Oak IgE ImmunoCAP <0.35 ku/l Elm IgE ImmunoCAP <0.35 ku/l Olive IgE ImmunoCAP <0.35 ku/l Cottonwood IgE ImmunoCAP <0.35 ku/l Box elder IgE ImmunoCAP <0.35 ku/l Mulberry IgE ImmunoCAP <0.35 ku/l Short (common) ragweed IgE ImmunoCAP <0.35 ku/l Mugwort IgE ImmunoCAP <0.35 ku/l Saltwort, Russian thistle IgE ImmunoCAP <0.35 ku/l Pigweed IgE ImmunoCAP <0.35 ku/l Sheep sorrel IgE ImmunoCAP <0.35 ku/l IgE Total ImmunoCAP 0 6 weeks < 5.2 IU/mL 7 weeks 3 months < 9.2 IU/mL 4 6 months < 16.4 IU/mL 7 9 months < 22.6 IU/mL months < 29.2 IU/mL 13 months 2 years < 51.7 IU/mL 3 years < 72.0 IU/mL 4 years < 90.0 IU/mL 5 years < IU/mL 6 years < IU/mL 7 years < IU/mL 8 years < IU/mL 9 years < IU/mL 10 years < IU/mL >10 years < IU/mL Specimen/Stability Serum 6.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Sunday- Saturday Same day CPT Code 86003x24, Regulatory Status FDA Approved Always Statement REFERENCE RANGES for Allergen IgE tests: IgE (ku/l) Interpretation < 0.35 Class 0 - Below Detection Class 1 - Low Class 2 - Moderate Class 3 - High Class 4 - Very High Class 5 - Very High >=100 Class 6 - Very High Note: Omalizumab (Xolair, Genentech; humanized IgG1 antihuman IgE Fc) treatment does not significantly interfere with the accuracy of total IgE on the ImmunoCAP (Phadia) platform. J Allergy Clin Immunol 2006;117:759-66). 2 of 18
3 New Tests (Specialty): (cont d) 3751 Respiratory Allergy Profile Region XV (Available ) Dermatophagoides pteronyssinus IgE ImmunoCAP <0.35 ku/l Dermatophagoides farinae IgE ImmunoCAP <0.35 ku/l Cat epithelium/dander IgE ImmunoCAP <0.35 ku/l Dog dander IgE ImmunoCAP <0.35 ku/l Bermuda grass IgE ImmunoCAP <0.35 ku/l Timothy grass IgE ImmunoCAP <0.35 ku/l Cockroach, German IgE ImmunoCAP <0.35 ku/l Penicillium chrysogenum IgE ImmunoCAP <0.35 ku/l Cladosporium herbarum IgE ImmunoCAP <0.35 ku/l Aspergillus fumigatus IgE ImmunoCAP <0.35 ku/l Alternaria alternata/tenuis IgE ImmunoCAP <0.35 ku/l Mountain juniper/cedar IgE ImmunoCAP <0.35 ku/l Oak IgE ImmunoCAP <0.35 ku/l Elm IgE ImmunoCAP <0.35 ku/l Olive IgE ImmunoCAP <0.35 ku/l Cottonwood IgE ImmunoCAP <0.35 ku/l Box elder IgE ImmunoCAP <0.35 ku/l Mulberry IgE ImmunoCAP <0.35 ku/l Short (common) ragweed IgE ImmunoCAP <0.35 ku/l Mugwort IgE ImmunoCAP <0.35 ku/l Saltwort, Russian thistle IgE ImmunoCAP <0.35 ku/l Pigweed IgE ImmunoCAP <0.35 ku/l IgE Total ImmunoCAP 0 6 weeks < 5.2 IU/mL 7 weeks 3 months < 9.2 IU/mL 4 6 months < 16.4 IU/mL 7 9 months < 22.6 IU/mL months < 29.2 IU/mL 13 months 2 years < 51.7 IU/mL 3 years < 72.0 IU/mL 4 years < 90.0 IU/mL 5 years < IU/mL 6 years < IU/mL 7 years < IU/mL 8 years < IU/mL 9 years < IU/mL 10 years < IU/mL >10 years < IU/mL Specimen/Stability Serum 6.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Sunday- Saturday Same day CPT Code 86003x22, Regulatory Status FDA Approved Always Statement REFERENCE RANGES for Allergen IgE tests: IgE (ku/l) Interpretation < 0.35 Class 0 - Below Detection Class 1 - Low Class 2 - Moderate Class 3 - High Class 4 - Very High Class 5 - Very High >=100 Class 6 - Very High Note: Omalizumab (Xolair, Genentech; humanized IgG1 antihuman IgE Fc) treatment does not significantly interfere with the accuracy of total IgE on the ImmunoCAP (Phadia) platform. J Allergy Clin Immunol 2006;117:759-66). 3 of 18
4 New Tests (Specialty): (cont d) 3752 Respiratory Allergy Profile Region XVI (Available ) Dermatophagoides pteronyssinus IgE ImmunoCAP <0.35 ku/l Dermatophagoides farinae IgE ImmunoCAP <0.35 ku/l Cat epithelium/dander IgE ImmunoCAP <0.35 ku/l Dog dander IgE ImmunoCAP <0.35 ku/l Timothy grass IgE ImmunoCAP <0.35 ku/l Cockroach, German IgE ImmunoCAP <0.35 ku/l Penicillium chrysogenum IgE ImmunoCAP <0.35 ku/l Cladosporium herbarum IgE ImmunoCAP <0.35 ku/l Aspergillus fumigatus IgE ImmunoCAP <0.35 ku/l Alternaria alternata/tenuis IgE ImmunoCAP <0.35 ku/l Grey alder IgE ImmunoCAP <0.35 ku/l Mountain juniper/cedar IgE ImmunoCAP <0.35 ku/l Oak IgE ImmunoCAP <0.35 ku/l Elm IgE ImmunoCAP <0.35 ku/l Common silver birch IgE ImmunoCAP <0.35 ku/l Cottonwood IgE ImmunoCAP <0.35 ku/l Box elder IgE ImmunoCAP <0.35 ku/l Mugwort IgE ImmunoCAP <0.35 ku/l Saltwort, Russian thistle IgE ImmunoCAP <0.35 ku/l Pigweed IgE ImmunoCAP <0.35 ku/l Sheep sorrel IgE ImmunoCAP <0.35 ku/l IgE Total ImmunoCAP 0 6 weeks < 5.2 IU/mL 7 weeks 3 months < 9.2 IU/mL 4 6 months < 16.4 IU/mL 7 9 months < 22.6 IU/mL months < 29.2 IU/mL 13 months 2 years < 51.7 IU/mL 3 years < 72.0 IU/mL 4 years < 90.0 IU/mL 5 years < IU/mL 6 years < IU/mL 7 years < IU/mL 8 years < IU/mL 9 years < IU/mL 10 years < IU/mL >10 years < IU/mL Specimen/Stability Serum 6.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Sunday- Saturday Same day CPT Code 86003x21, Regulatory Status FDA Approved Always Statement REFERENCE RANGES for Allergen IgE tests: IgE (ku/l) Interpretation < 0.35 Class 0 - Below Detection Class 1 - Low Class 2 - Moderate Class 3 - High Class 4 - Very High Class 5 - Very High >=100 Class 6 - Very High Note: Omalizumab (Xolair, Genentech; humanized IgG1 antihuman IgE Fc) treatment does not significantly interfere with the accuracy of total IgE on the ImmunoCAP (Phadia) platform. J Allergy Clin Immunol 2006;117:759-66). 4 of 18
5 New Tests (Specialty): (cont d) 3753 Respiratory Allergy Profile Region XVII (Available ) Dermatophagoides pteronyssinus IgE ImmunoCAP <0.35 ku/l Dermatophagoides farinae IgE ImmunoCAP <0.35 ku/l Cat epithelium/dander IgE ImmunoCAP <0.35 ku/l Dog dander IgE ImmunoCAP <0.35 ku/l Timothy grass IgE ImmunoCAP <0.35 ku/l Cockroach, German IgE ImmunoCAP <0.35 ku/l Penicillium chrysogenum IgE ImmunoCAP <0.35 ku/l Cladosporium herbarum IgE ImmunoCAP <0.35 ku/l Aspergillus fumigatus IgE ImmunoCAP <0.35 ku/l Alternaria alternata/tenuis IgE ImmunoCAP <0.35 ku/l Mountain juniper/cedar IgE ImmunoCAP <0.35 ku/l Oak IgE ImmunoCAP <0.35 ku/l Elm IgE ImmunoCAP <0.35 ku/l Grey alder IgE ImmunoCAP <0.35 ku/l Cottonwood IgE ImmunoCAP <0.35 ku/l Common silver birch IgE ImmunoCAP <0.35 ku/l Box elder IgE ImmunoCAP <0.35 ku/l White ash IgE ImmunoCAP <0.35 ku/l Walnut tree pollen IgE ImmunoCAP <0.35 ku/l Short (common) ragweed IgE ImmunoCAP <0.35 ku/l Pigweed IgE ImmunoCAP <0.35 ku/l Sheep sorrel IgE ImmunoCAP <0.35 ku/l Nettle IgE ImmunoCAP <0.35 ku/l IgE Total ImmunoCAP 0 6 weeks < 5.2 IU/mL 7 weeks 3 months < 9.2 IU/mL 4 6 months < 16.4 IU/mL 7 9 months < 22.6 IU/mL months < 29.2 IU/mL 13 months 2 years < 51.7 IU/mL 3 years < 72.0 IU/mL 4 years < 90.0 IU/mL 5 years < IU/mL 6 years < IU/mL 7 years < IU/mL 8 years < IU/mL 9 years < IU/mL 10 years < IU/mL >10 years < IU/mL Specimen/Stability Serum 6.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Sunday- Saturday Same day CPT Code 86003x23, Regulatory Status FDA Approved Always Statement REFERENCE RANGES for Allergen IgE tests: IgE (ku/l) Interpretation < 0.35 Class 0 - Below Detection Class 1 - Low Class 2 - Moderate Class 3 - High Class 4 - Very High Class 5 - Very High >=100 Class 6 - Very High Note: Omalizumab (Xolair, Genentech; humanized IgG1 antihuman IgE Fc) treatment does not significantly interfere with the accuracy of total IgE on the ImmunoCAP (Phadia) platform. J Allergy Clin Immunol 2006;117:759-66). 5 of 18
6 New Tests (Specialty): (cont d) 3606 Sucrase (Available June 1) Sucrase S um/min/gram protein Specimen/Stability Tissue 5.0 (2.0) mg: Frozen 31 days Collection Instructions Place 5 mg (2 mg) small bowel biopsy in a polypropylene screw cap collection tube and freeze within 2 hours of collection. Keep frozen on dry ice. Store at -50 to -90 degrees C. Tuesday - Saturday Next day CPT Code Regulatory Status Laboratory Developed Test Always Statement Units are reported as um/min/gram protein Lactase, maltase, palatinase and sucrase are mucosal disaccharidases that are present in normal small intestine and fetal colon. A decrease in the enzyme activity of these disaccharidases has been noted in colonic adenomas, adenocarcinomas and malabsorption. Decreased disaccharidase activity is a good indicator of mucosal injury with the exception of lactase Palatinase (Available June 1) Palatinase S um/min/gram protein Specimen/Stability Tissue 5.0 (2.0) mg: Frozen 31 days Collection Instructions Place 5 mg (2 mg) small bowel biopsy in a polypropylene screw cap collection tube and freeze within 2 hours of collection. Keep frozen on dry ice. Store at -50 to -90 degrees C. Tuesday - Saturday Next day CPT Code Regulatory Status Laboratory Developed Test Always Statement Units are reported as um/min/gram protein Lactase, maltase, palatinase and sucrase are mucosal disaccharidases that are present in normal small intestine and fetal colon. A decrease in the enzyme activity of these disaccharidases has been noted in colonic adenomas, adenocarcinomas and malabsorption. Decreased disaccharidase activity is a good indicator of mucosal injury with the exception of lactase Maltase (Available June 1) Maltase S um/min/gram protein Specimen/Stability Tissue 5.0 (2.0) mg: Frozen 31 days Collection Instructions Place 5 mg (2 mg) small bowel biopsy in a polypropylene screw cap collection tube and freeze within 2 hours of collection. Keep frozen on dry ice. Store at -50 to -90 degrees C. Tuesday - Saturday Next day CPT Code Regulatory Status Laboratory Developed Test Always Statement Units are reported as um/min/gram protein Lactase, maltase, palatinase and sucrase are mucosal disaccharidases that are present in normal small intestine and fetal colon. A decrease in the enzyme activity of these disaccharidases has been noted in colonic adenomas, adenocarcinomas and malabsorption. Decreased disaccharidase activity is a good indicator of mucosal injury with the exception of lactase. 6 of 18
7 New Tests (Specialty): (cont d) 3614 Lactase (Available June 1) Lactase S um/min/gram protein Specimen/Stability Tissue 5.0 (2.0) mg: Frozen 31 days Collection Instructions Place 5 mg (2 mg) small bowel biopsy in a polypropylene screw cap collection tube and freeze within 2 hours of collection. Keep frozen on dry ice. Store at -50 to -90 degrees C. Tuesday - Saturday Next day CPT Code Regulatory Status Laboratory Developed Test Always Statement Units are reported as um/min/gram protein Lactase, maltase, palatinase and sucrase are mucosal disaccharidases that are present in normal small intestine and fetal colon. A decrease in the enzyme activity of these disaccharidases has been noted in colonic adenomas, adenocarcinomas and malabsorption. Decreased disaccharidase activity is a good indicator of mucosal injury with the exception of lactase Histoplasma Total Antibody (Available June 14) Histoplasma Total Ab EIA <0.90 Index Specimen/Stability Serum 1.0 (0.3) ml: Ambient 48 hours, Refrigerated 2 weeks, Frozen 2 months Thursday Same day CPT Code Regulatory Status FDA Approved Always Statement Reference Ranges for Histoplasma Total Ab: Negative: < 0.90 Index Equivocal: Index Positive: > 1.10 Index This test is indicated for testing persons having symptoms of respiratory disease, as an aid in the presumptive laboratory diagnosis of histoplasma infection. The test is not approved for testing blood or plasma donors Beta-2-Glycoprotein (Beta-2-GPI) IgG Autoabs (Available July 6) Beta-2-GPI IgG Autoabs EIA <21 Units Specimen/Stability Serum 1.0 (0.6) ml: Refrigerated 48 hours, Frozen 2 months Sunday - Saturday Same day CPT Code Regulatory Status FDA Approved Beta-2-GPI autoantibodies are found in patients with antiphospholipid syndrome (APS) and are associated with increased risk of venous and arterial thrombosis and thrombocytopenia. Beta-2-GPI autoantibodies are found only in patients with autoimmune diseases, while cardiolipin autoantibodies can be transiently found in infectious diseases. 7 of 18
8 New Tests (Specialty): (cont d) 1183 Beta-2-Glycoprotein (Beta-2-GPI) IgM Autoabs (Available July 6) Beta-2-GPI IgM Autoabs EIA <21 Units Specimen/Stability Serum 1.0 (0.6) ml: Refrigerated 48 hours, Frozen 2 months Sunday - Saturday Same day CPT Code Regulatory Status FDA Approved Beta-2-GPI autoantibodies are found in patients with antiphospholipid syndrome (APS) and are associated with increased risk of venous and arterial thrombosis and thrombocytopenia. Beta-2-GPI autoantibodies are found only in patients with autoimmune diseases, while cardiolipin autoantibodies can be transiently found in infectious diseases Beta-2-Glycoprotein (Beta-2-GPI) IgA Autoabs (Available July 6) Beta-2-GPI IgA Autoabs EIA <21 Units Specimen/Stability Serum 1.0 (0.6) ml: Refrigerated 48 hours, Frozen 2 months Sunday - Saturday Same day CPT Code Regulatory Status FDA Approved Beta-2-GPI autoantibodies are found in patients with antiphospholipid syndrome (APS) and are associated with increased risk of venous and arterial thrombosis and thrombocytopenia. Beta-2-GPI autoantibodies are found only in patients with autoimmune diseases, while cardiolipin autoantibodies can be transiently found in infectious diseases FcGammaRIIa & FcGammaRIIIa Mutation Analysis (Available July 12) This test is not approved for the testing of patient samples from New York State. FcGammaRIIa & RIIIa PCR Mutation not detected Specimen/Stability Whole Blood EDTA 5.0 (3.0) ml: Ambient 7 days, Refrigerated 7 days Alt Specimen Whole Blood ACD (A or B) 5.0 (3.0) ml: Ambient 7 days, Refrigerated 7 days Collection Instructions EDTA is the preferred anticoagulant, but ACD (A or B) is also acceptable. Heparin is not acceptable. Ship ambient. Frozen specimens are not acceptable. Wednesday Within 3 days CPT Code 83891, 83898, 83896x2, Regulatory Status Laboratory Developed Test Always Statement This test(s) was developed and its performance characteristics have been determined by Specialty Laboratories. Performance characteristics refer to the analytical performance of the test. Note Test detects the FcGammaRIIa and FcGammaRIIIa V158F genomic variants. This test is not approved for the testing of patient samples from New York State. FcGammaRIIa H131R and FcGammaRIIIa V158F variants have been associated with immune related disorders and serve as markers for therapeutic efficacy and side-effects of treatment with monoclonal antibodies. These variants have been found to predict rituximab response in patients with follicular lymphoma. Combined FcGammaRIIa/FcGammaRIIIa variants are also shown to be prognostic factors for disease progression in metastatic colorectal cancer, in patients treated with cetuximab plus irinotecan. 8 of 18
9 New Tests (Specialty): (cont d) 3515W Vitamin B1 (Thiamine) Whole Blood (Available July 12) Vitamin B1 WB HPLC nmol/l Specimen/Stability Whole Blood EDTA 1.0 (0.5) ml: Refrigerated 24 hours, Frozen 30 days Collection Instructions Protect from light; use foil wrapping or amber tubes. Ship frozen. Monday, Wednesday, Friday Next day CPT Code Regulatory Status Laboratory Developed Test Vitamin B1 helps to maintain the health of mucous membranes such as those lining the intestines. Thiamine diphosphate (also referred to as "thiamine pyrophosphate") is the physiologically active form of Vitamin B1 thus the monitoring of this diphosphate should be preferred to the analysis of total thiamine. 9 of 18
10 Test Changes: 2271 Epstein-Barr Virus Nuclear Ag (EBNA) IgM Abs Regulatory Status FDA Approved (NEW) 2364 Rapid Plasma Reagin (RPR) Always Statement Reactive results for this nontreponemal test are not confirmed. All reactive nontreponemal tests should be confirmed using a standard treponemal test unless the patient has had a known/documented prior syphilis infection. Also Affected DOS Codes 2366, 2366C 3114 Angiotensin Converting Enzyme (ACE) Collection Instructions Grossly hemolyzed samples will be rejected. (NEW) 3131 Adenosine Deaminase Specimen/Stability Serum 1.0 (0.5) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Alt Specimen CSF 1.0 (0.5) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Pleural Fluid 1.0 (0.5) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Note: Ambient and frozen specimens now accepted; increased refrigerated stability. 4094U Propoxyphene Confirmation Urine Specimen/Stability Urine 4.0 (2.0) ml: Ambient 14 days, Refrigerated 14 days, Frozen 30 days Note: Increased ambient, refrigerated and frozen stability. 4101U Phencyclidine (PCP) Screen Urine w/reflex Confirmation Specimen/Stability Urine 5.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Note: Increased ambient, refrigerated and frozen stability. 10 of 18
11 Test Changes: (cont d) 4109U Methadone Screen Urine w/reflex Confirmation Specimen/Stability Urine 5.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Note: Increased ambient and frozen stability. 4127U Propoxyphene Screen Urine w/reflex Confirmation Specimen/Stability Urine 5.0 (4.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Note: Increased ambient, refrigerated and frozen stability. 4183U Phencyclidine (PCP) Confirmation Urine Specimen/Stability Urine 4.0 (2.0) ml: Ambient 14 days, Refrigerated 14 days, Frozen 30 days Note: Increased ambient, refrigerated and frozen stability. 4185UR Opiates Confirmation Urine Specimen/Stability Urine 10.0 (5.0) ml: Ambient 14 days, Refrigerated 14 days, Frozen 30 days Note: Increased ambient and decreased frozen stability. Also Affected DOS Codes 4179U, 4186UR, 4187UR, 4490UR, 4492UR 4192U Methadone Confirmation Urine Specimen/Stability Urine 4.0 (2.0) ml: Ambient 14 days, Refrigerated 14 days, Frozen 30 days Note: Increased ambient and frozen stability. 4250U Opiates Screen Urine w/reflex Confirmation Specimen/Stability Urine 10.0 (5.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 30 days Note: Increased ambient and decreased frozen stability F-Actin IgA Autoabs Regulatory Status FDA Approved (NEW) 11 of 18
12 Test Changes: (cont d) 9640 Streptococcus Group B DNA DetectR Note This test is not approved by the State of New York for the testing of ThinPrep and SurePath samples Vitamin D, 25-Hydroxy Total [LC-MS-MS] June 7 Reference Range Vitamin D, 25-OD Total: ng/ml (NEW) Also Affected DOS Code U Oxycodone & Metabolite Urine June 15 Always Statement Limit of quantitation Oxycodone 50 ng/ml (NEW) Oxymorphone 50 ng/ml (NEW) Also Affected Reflex of DOS Codes 4422U, 4470U, 4472U 1519 Transferrin June 29 Reference Range mg/dl (NEW) 1600 Complement Functional Activity CH50 June 29 Specimen/Stability Serum 1.0 (0.8) ml: Frozen 1 month Note: Reduced frozen stability Follicle-Stimulating Hormone June 29 Specimen/Stability Serum 2.0 (1.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 2 months Note: Ambient now accepted; increased refrigerated stability. Reference Range Female see below Male: miu/ml (NEW) Always Statement Female >= 18 Years: Follicular miu/ml (same) Mid Cycle Peak miu/ml (NEW) Luteal miu/ml (same) Postmenopausal miu/ml (same) Female < 18 Years: FSH reference ranges established on post-pubertal patient population. Reference range not established for pre-pubertal patients using this assay. Also Affected DOS Codes 2016, 2020 (reference range only), 2023 (reference range only) 12 of 18
13 Test Changes: (cont d) 3198 Luteinizing Hormone June 29 Specimen/Stability Serum 2.0 (1.0) ml: Ambient 7 days, Refrigerated 7 days, Frozen 2 months Note: Ambient now accepted; increased refrigerated stability. Reference Range Female see below Male < 70 Years: miu/ml (NEW) >=70 Years: miu/ml (NEW) Always Statement Female >= 18 Years: Follicular miu/ml (same) Mid Cycle Peak miu/ml (same) Luteal miu/ml (same) Postmenopausal miu/ml (same) Female < 18 Years: LH reference ranges established on post-pubertal patient population. Reference range not established for pre-pubertal patients using this assay. Also Affected DOS Codes 2016, 2020 (reference range only), 2023 (reference range only) 3228 Thyroxine (T4), Free June 29 Specimen/Stability Serum 1.0 (0.5) ml: Ambient 7 days, Refrigerated 7 days, Frozen 2 months Note: Ambient now accepted; increased refrigerated stability. Reference Range < 1 Year Not established 1 9 Years ng/dl (NEW) Years ng/dl (NEW) > 18 Years ng/dl (NEW) Also Affected DOS Codes 3072 (reference range only), 3074 (reference range only) 3318U Vanillylmandelic Acid 24Hr Urine June 29 Specimen/Stability Urine 24 hour 5.0 (2.5) ml: Ambient 7 days, Refrigerated 14 days, Frozen 14 days Note: Decreased frozen stability. 3318UR Vanillylmandelic Acid Urine Random June 29 Specimen/Stability Urine 5.0 (2.5) ml: Ambient 7 days, Refrigerated 14 days, Frozen 14 days Note: Decreased frozen stability. 13 of 18
14 Test Changes: (cont d) 3522 Folate June 29 Specimen/Stability Serum 2.0 (1.0) ml: Ambient 1 day, Refrigerated 7 days, Frozen 2 months Note: Increased refrigerated stability. Reference Range < 5 Years: Not established 5 9 Years: > 7.1 ng/ml (NEW) Years: > 8.0 ng/ml (NEW) > 17 Years: > 5.4 ng/ml (NEW) Always Statement Reference Range Interpretation (> 17 Years): Low <3.4 ng/ml (NEW) Borderline ng/ml (NEW) Normal > 5.4 ng/ml (NEW) Also Affected DOS Code 3020 (reference range only) 4129U Drugs of Abuse Screen Urine w/reflex Confirmation June 29 Specimen/Stability Urine 10.0 (5.0) ml: Refrigerated 7 days, Frozen 14 days Note: Decreased frozen stability. 4175U Fentanyl Screen Urine w/reflex Confirmation June 29 Methodology ELISA (NEW) Always Statement ing Limit: 0.3 ng/ml Synonym: Sublimaze Approximately 6% of dose is excreted in the urine as unchanged drug in 3-4 days. Analysis by Enzyme-Linked Immunosorbent Assay (ELISA) 4933 Olanzapine June 29 Specimen/Stability Serum 3.0 (2.0) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Note: Ambient and refrigerated specimens now accepted; decreased frozen stability. Collection Instructions Serum separator tubes are not acceptable. Ship samples refrigerated Clozapine & Norclozapine June 29 Specimen/Stability Plasma Heparinzed 2.0 (1.0) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Alt Specimen Plasma EDTA 2.0 (1.0) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Serum 2.0 (1.0) ml: Ambient 30 days, Refrigerated 30 days, Frozen 30 days Note: Ambient specimens now accepted; increased refrigerated and decreased frozen stability. 14 of 18
15 Test Changes: (cont d) 5382 Cytochrome P450 2C19 GenotypR June 29 Name Cytochrome P450 2C19 (Clopidogrel) GenotypR 1083 Beta-2-Glycoprotein I (Beta-2-GPI) IgG, IgM & IgA Autoabs July 6 Reference Range Beta-2-GPI IgG < 21 Units (NEW) Beta-2-GPI IgM < 21 Units (NEW) Beta-2-GPI IgA < 21 Units (NEW) Also Affected DOS Code Glomerular Basement Membrane IgG Autoabs July 6 Reference Range < 21 Units (NEW) Always Statement Negative < 21 Units Weak Positive Units Moderate to Strong Positive > 30 Units Also Affected DOS Code 1726 The CPT Codes provided are based on AMA Guidelines and are for informational purposes only. CPT Coding is the sole responsibility of the billing party. Please direct any questions regarding CPT Coding to the payer being billed. 15 of 18
16 New Referral Tests: The following tests are now available from Quest Diagnostics and may be referred through Specialty Laboratories. S52159 CellSearch Circulating Tumor Cells, Prostate [16812] Test performed at Quest Diagnostics, San Juan Capistrano S52160 CellSearch Circulating Tumor Cells, Colon [16811] Test performed at Quest Diagnostics, San Juan Capistrano Please call client relations at or visit our website at for ordering information. 16 of 18
17 Discontinued Tests: : S52043 HTLV-I/II Antibody, WB (CSF) [61105] No replacement S52040 Cartilage Oligomeric Matrix Protein (COMP) [157] No replacement S51728 KRAS Mutation Analysis [16510X] Recommended replacement: 5032 KRAS Mutation Analysis Test performed at Specialty Laboratories S51883 NRAS Mutation Analysis [16818] Recommended replacement: 5030 NRAS Mutation Analysis Test performed at Specialty Laboratories S51674 RAS Mutation Analysis, Cell-Based [16128X] Recommended replacement: 5034 RAS Mutation Analysis, Cell-Based Test performed at Specialty Laboratories S41700NY FENTANYL RANDOM URINE (9176U) Recommended replacement: 4175U-Fentanyl screen urine w/reflex confirmation Test performed at Specialty Laboratories June 15: 8322 Histoplasma IgG Abs [EIA] Recommended replacement: 8328 Histoplasma Total Antibody [ELISA] Test performed at Specialty Laboratories 8323 Histoplasma IgM Abs [EIA] Recommended replacement: 8328 Histoplasma Total Antibody [ELISA] Test performed at Specialty Laboratories 8324 Histoplasma IgG & IgM Abs [EIA] Recommended replacement: 8328 Histoplasma Total Antibody [ELISA] Test performed at Specialty Laboratories 17 of 18
18 Discontinued Tests: (cont d) July 1: S50826 DRUG SCREEN MECONIUM COMPREHENSIVE [941510] Recommended replacement: S52037-Drug Screen Panel 9, Meconium (30427X) Test performed at Quest Diagnostics, Chantilly S50870 PHENOBARBITAL [90194] Recommended replacement: S52035-Phenobarbital (708X) Test performed at Quest Diagnostics, Chantilly S50902 S49582 S42830 S50710 CALPROTECTIN Recommended replacement: S52034-Calprotectin (16796) Test performed at Quest Diagnostics, San Juan Capistrano INTERLEUKIN 1 BETA Recommended replacement: S52033-Interleukin-1 Beta (1757Z) Test performed at Quest Diagnostics, San Juan Capistrano PNH CD59 EXPRESSION Recommended replacement: S CD55 AND CD59 EXPRESSION, RED CELLS & GRANULOCYTES [19835X] Test performed at Quest Diagnostics, San Juan Capistrano Anti RNA Polymerase III Recommended replacement: S52032-RNA Polymerase III Antibody (19899X) Test performed by Quest Diagnostics, San Juan Capistrano S50878 QUETIAPINE (SEROQUEL) [90069] Recommended replacement: S QUETIAPINE [4051SP] Test performed at National Medical Services July 6: 5394 JAK2 V617F Mutation, Qual PCR, Plasma w/reflex Exons 12, 13 Recommended replacement: 5396 JAK2 V617F Mutation, Ql, w/rfx Exons 12, 13 & MPL W515, S505 Test performed at Specialty Laboratories 18 of 18
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
May Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
New Test Announcements
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
All abnormal results for this test will automatically have the DNA test reflexed at an additional cost.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
Allergy IgE Allergy Test Sensitivity and Specificity 6/23/17
 Allergy IgE Allergy Test Sensitivity and Specificity 6/23/17 1 Sensitivity and Specificity Benchmarking Goal Test pooled human sera with known positive and negative reactivity to determine Allergy sensitivity
Allergy IgE Allergy Test Sensitivity and Specificity 6/23/17 1 Sensitivity and Specificity Benchmarking Goal Test pooled human sera with known positive and negative reactivity to determine Allergy sensitivity
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
Test Catalog Updates August 3, 2016 Updates noted in Red
 s Updates noted in Red BLOD1424 Allergen Food Basil No page Specimen Requirement 0.5 ml (0.3 ml minimum) serum. Send Stability: RMT - NA REFT - 14 days Frozen - 14 days BLOD0248 Allergen Food Green Bean
s Updates noted in Red BLOD1424 Allergen Food Basil No page Specimen Requirement 0.5 ml (0.3 ml minimum) serum. Send Stability: RMT - NA REFT - 14 days Frozen - 14 days BLOD0248 Allergen Food Green Bean
Please note that our serum opiates EIA screen assay is not appropriate for therapeutic drug monitoring of Oxycodone.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
ImmunoCAP. Specific IgE blood test
 Allergy- Specific IgE blood test provides clinicians with an accurate and convenient method of helping to rule in or rule out allergy in patients with allergy-like symptoms. Allergy- Positive About Allergy-
Allergy- Specific IgE blood test provides clinicians with an accurate and convenient method of helping to rule in or rule out allergy in patients with allergy-like symptoms. Allergy- Positive About Allergy-
Business Unit: Quest Diagnostics Nichols Institute, Valencia. June 2011 Update Dear Colleague,
 Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 25, 2010 Dear Colleague: This client letter contains many changes to our reference ranges in the area of chemistry
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 25, 2010 Dear Colleague: This client letter contains many changes to our reference ranges in the area of chemistry
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 27, 2007 Dear Colleague: Specialty Laboratories is pleased to offer two new tests to aid in the diagnosis
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 27, 2007 Dear Colleague: Specialty Laboratories is pleased to offer two new tests to aid in the diagnosis
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
November Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
CMS will not implement the new tier codes for Medicare/Medicaid claims for calendar year 2012.
 January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
Human Allergen Specific IgE ELISA Kits
 Intended Use Human Allergen Specific IgE ELISA Kits These kits are in vitro assays for the qualitative or quantitative detection of allergen-specific IgE antibodies in human serum. They are intended for
Intended Use Human Allergen Specific IgE ELISA Kits These kits are in vitro assays for the qualitative or quantitative detection of allergen-specific IgE antibodies in human serum. They are intended for
Documentation, Codebook, and Frequencies
 Documentation, Codebook, and Frequencies Laboratory Component: Allergen Specific IgE(s) and Total IgE in Serum Survey Years: 2005 to 2006 SAS Export File: AL_IGE_D.XPT First Published: June 2008 Last Revised:
Documentation, Codebook, and Frequencies Laboratory Component: Allergen Specific IgE(s) and Total IgE in Serum Survey Years: 2005 to 2006 SAS Export File: AL_IGE_D.XPT First Published: June 2008 Last Revised:
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
 27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates June 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the improvement of our turnaround times
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates June 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the improvement of our turnaround times
Effective Mitigation of Allergen-Induced Asthma
 Effective Mitigation of Allergen-Induced Asthma John C. Carlson, MD, PhD Tulane University, School of Medicine John.Carlson@Tulane.edu Disclosures Federal research funding, including salary support: HUD:
Effective Mitigation of Allergen-Induced Asthma John C. Carlson, MD, PhD Tulane University, School of Medicine John.Carlson@Tulane.edu Disclosures Federal research funding, including salary support: HUD:
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
INTERPATH LABORATORY, INC. TEST UPDATES
 EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
Please contact Customer Service with any questions. Ph: option 5
 Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
IMMEDIATE HOT LINE: Effective January 6, 2014
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Milan Analytica AG Baslerstrasse Rheinfelden
 Milan Analytica AG Baslerstrasse 15 4310 Rheinfelden www.milananalytica.ch Product list valid from 01.09.2012 Please ask for evaluation samples under Product Article number Circulating immune complexes
Milan Analytica AG Baslerstrasse 15 4310 Rheinfelden www.milananalytica.ch Product list valid from 01.09.2012 Please ask for evaluation samples under Product Article number Circulating immune complexes
Allergy Immunotherapy: A New Role for the Family Physician
 Allergy Immunotherapy: A New Role for the Family Physician Louis Kuritzky MD Clinical Assistant Professor Emeritus Department of Community Health and Family Medicine College of Medicine University of Florida,
Allergy Immunotherapy: A New Role for the Family Physician Louis Kuritzky MD Clinical Assistant Professor Emeritus Department of Community Health and Family Medicine College of Medicine University of Florida,
12/10/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Allergology product list 2018
 Milan Analytica AG Baslerstrasse 15 4310 Rheinfelden Switzerland www.milananalytica.ch Allergology product list 2018 Please ask for more information under Product Article number Circulating immune complexes
Milan Analytica AG Baslerstrasse 15 4310 Rheinfelden Switzerland www.milananalytica.ch Allergology product list 2018 Please ask for more information under Product Article number Circulating immune complexes
March Immunoglobulins, Total (IgA, IgE, IgG, IgM) Interleukin-6 (IL6) Protein Electrophoresis, CSF. Thyrotropin Receptor Antibody
 University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
October Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
5701 Aerobic Stool Culture, Routine Component Method Reference Range. Organism/Pathogen CULTURE Not detected. Echinococcus IgG EIA Not detected
 2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
Alkaline Phosphatase, Bone Specific
 Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
DNA & Allergen Compatibility Report
 DNA & Allergen Compatibility Report REPORT FOR: JOHN W. DOE 11.21.2017 John W. Doe Personal Profile CURRENT MEDICATIONS: FOOD: Advil 07.11.2017 Clopidogrel 07.23.2017 Doxepin 09.04.2017 Fluoxetine 09.17.2017
DNA & Allergen Compatibility Report REPORT FOR: JOHN W. DOE 11.21.2017 John W. Doe Personal Profile CURRENT MEDICATIONS: FOOD: Advil 07.11.2017 Clopidogrel 07.23.2017 Doxepin 09.04.2017 Fluoxetine 09.17.2017
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
January Billing and compliance. Chemistry. Help us help you. Referral testing. Additional CPT code changes announced for 2015
 January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
10/7/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Quality Controls in Allergy Diagnosis
 Quality Controls in Allergy Diagnosis Alistair Crockard Royal Hospitals Belfast Northern Ireland Quality Controls in Allergy What do we want? Diagnosis What can be controlled? What can be achieved? What
Quality Controls in Allergy Diagnosis Alistair Crockard Royal Hospitals Belfast Northern Ireland Quality Controls in Allergy What do we want? Diagnosis What can be controlled? What can be achieved? What
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Allergy Testing and Allergy Immunotherapy
 All Panels and individual tests below are billed by CPT Code 86003 or contain a component that bills 86003 Test Name Allergic Bronchopulmonary Aspergillosis Panel II Allergy Panel 1 Allergy Panel 2 Allergy
All Panels and individual tests below are billed by CPT Code 86003 or contain a component that bills 86003 Test Name Allergic Bronchopulmonary Aspergillosis Panel II Allergy Panel 1 Allergy Panel 2 Allergy
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT
 SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
In-Common Laboratories
 Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Coverage Criteria: Express Scripts, Inc. monograph dated 03/03/2010
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Xolair (omalizumab) Commercial HMO/PPO/CDHP HMO/PPO/CDHP: Rx
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Xolair (omalizumab) Commercial HMO/PPO/CDHP HMO/PPO/CDHP: Rx
This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma tests/panels.
 February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
The information contained here may be very important to your practice. Please take a moment to review this document.
 September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
New Test ANNOUNCEMENT
 March 2003 W New Test ANNOUNCEMENT A Mayo Reference Services Publication Pediatric Allergy Screen
March 2003 W New Test ANNOUNCEMENT A Mayo Reference Services Publication Pediatric Allergy Screen
PCL ALVERNO INSTALLING NEW ANALYZERS AND AUTOMATION
 October 2014 Dear Healthcare Provider, The information contained in the packet may be very important to your practice. Below is a quick summary of the items that are included in this mailing. Please take
October 2014 Dear Healthcare Provider, The information contained in the packet may be very important to your practice. Below is a quick summary of the items that are included in this mailing. Please take
Chemiluminescent Assay Versus Immunoblotting for Detection of Positive Reaction to Allergens
 Chemiluminescent Assay Versus Immunoblotting for Detection of Positive Reaction to Allergens Yoo Kyoung Ihm, MD, So-Young Kang, MD, PhD, Myeong Hee Kim, MD, Woo In Lee, MD (Department of Laboratory Medicine,
Chemiluminescent Assay Versus Immunoblotting for Detection of Positive Reaction to Allergens Yoo Kyoung Ihm, MD, So-Young Kang, MD, PhD, Myeong Hee Kim, MD, Woo In Lee, MD (Department of Laboratory Medicine,
Test Bulletin. Effective November March 1, 2014
 Test Bulletin Effective November March 1, 2014 BD Vacutainer Tube Conversion Red, Gold, and Green Tubes On Wednesday, December 3, 2014, the final conversion from Greiner Vacuette to BD Vacutainer collection
Test Bulletin Effective November March 1, 2014 BD Vacutainer Tube Conversion Red, Gold, and Green Tubes On Wednesday, December 3, 2014, the final conversion from Greiner Vacuette to BD Vacutainer collection
Patient Information Specimen Information Client Information. Specimen: EN255254W. Requisition:
 Patient Information Specimen Information Client Information MAIL000 Requisition: 0014895 DIRECT TO PATIENT- WEST HILLS Lab Ref #: PRO76199 8401 FALLBROOK AVE WEST HILLS, CA 91304-3226 Phone: 530.347.6380
Patient Information Specimen Information Client Information MAIL000 Requisition: 0014895 DIRECT TO PATIENT- WEST HILLS Lab Ref #: PRO76199 8401 FALLBROOK AVE WEST HILLS, CA 91304-3226 Phone: 530.347.6380
Tests that have had changes to the method/ CPT code, units of measurement, scope of analysis, reference comments, or specimen requirements.
 In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below are the
In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below are the
THE STAR
 THE STAR 210-521-6886 Fall Allergies The beginning of fall means the return of cooler weather. From our favorite pumpkin-spiced latte, to the changing color of the leaves there are tons of things to love
THE STAR 210-521-6886 Fall Allergies The beginning of fall means the return of cooler weather. From our favorite pumpkin-spiced latte, to the changing color of the leaves there are tons of things to love
Cleveland Clinic Laboratories
 Cleveland Clinic Laboratories Technical Update September 2012 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That's why we are happy to provide
Cleveland Clinic Laboratories Technical Update September 2012 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That's why we are happy to provide
Prevalence of Fungal Allergy in Patients with Allergic Rhinosinusitis
 Prevalence of Fungal Allergy in Patients with Allergic Rhinosinusitis Kyle Kennedy, M.D. Karen Calhoun,, M.D. June 5, 1999 Hypothesis Subset of patients with signs and symptoms of allergic rhinosinusitis
Prevalence of Fungal Allergy in Patients with Allergic Rhinosinusitis Kyle Kennedy, M.D. Karen Calhoun,, M.D. June 5, 1999 Hypothesis Subset of patients with signs and symptoms of allergic rhinosinusitis
Patient Information Specimen Information Client Information. Specimen: EN255254W. Requisition: TSENG, JUSTINA J DOB: 10/26/1955 AGE: 59
 Report Status: Final Patient Information Specimen Information Client Information Specimen: EN255254W Client #: 44123510 MAIL001 Requisition: 0014895 Lab Ref #: PRO76199 GREENVILLE RANCHERIA 1425 MONTGOMERY
Report Status: Final Patient Information Specimen Information Client Information Specimen: EN255254W Client #: 44123510 MAIL001 Requisition: 0014895 Lab Ref #: PRO76199 GREENVILLE RANCHERIA 1425 MONTGOMERY
Testing Profiles Available -
 The Clontarf Clinic Allergy Centre & Laboratory (Co. Reg No. 383229) The Clontarf Clinic 63 Clontarf Road Clontarf Dublin 3 Berkeley Allergy Clinic (Wednesday) 12 Berkeley Road Dublin 7 Tel: 01 8338207
The Clontarf Clinic Allergy Centre & Laboratory (Co. Reg No. 383229) The Clontarf Clinic 63 Clontarf Road Clontarf Dublin 3 Berkeley Allergy Clinic (Wednesday) 12 Berkeley Road Dublin 7 Tel: 01 8338207
February Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
April Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 Revision Message! Please note: 4/20/15 communication revision for test code 17306- Vitamin D, 25-Hydroxy, Total, Immunoassay, New York patient message NEW TESTS Please Note: Not all test codes assigned
Revision Message! Please note: 4/20/15 communication revision for test code 17306- Vitamin D, 25-Hydroxy, Total, Immunoassay, New York patient message NEW TESTS Please Note: Not all test codes assigned
Annual Notice to Providers (2014)
 8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
Tests recently added to the NMS Labs test menu. New Tests are effective immediately.
 Immediate Action In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed
Immediate Action In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed
December Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
The pages that follow contain information critical to protecting the health of your patients and the citizens of Colorado.
 Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Follow us on Twitter @TCHDHealth and @TCHDEmergency John
Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Follow us on Twitter @TCHDHealth and @TCHDEmergency John
I look forward to talking with many of you in the future and welcome your calls, whether they be technical, consultative or quality oriented.
 Volume 4 Aug/2011 Business Unit: Quest Diagnostics Nichols Institute, Valencia September 2011 Update Dear Colleague, This letter represents the first under my signature as the Medical Director of the Quest
Volume 4 Aug/2011 Business Unit: Quest Diagnostics Nichols Institute, Valencia September 2011 Update Dear Colleague, This letter represents the first under my signature as the Medical Director of the Quest
University of Pretoria
 University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
Characteristics of allergy in autoimmune thyroid diseases. Ildikó Molnár MD, PhD, EndoMed, Hungary
 Characteristics of allergy in autoimmune thyroid diseases Ildikó Molnár MD, PhD, EndoMed, Hungary Relationship between allergic responses and thyroid autoimmunity IgE levels IgE deposits are present in
Characteristics of allergy in autoimmune thyroid diseases Ildikó Molnár MD, PhD, EndoMed, Hungary Relationship between allergic responses and thyroid autoimmunity IgE levels IgE deposits are present in
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 5 Immunology MLE-M2 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 5 Immunology MLE-M2 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
HOSPITALS IN-COMMON LABORATORY INC.
 Immunoglobulin E Ab., Single Allergen: Allergens are now searchable and viewable in the web test manual database but are not listed individually in the printable pdf-format documents. Trace Metals: Trace
Immunoglobulin E Ab., Single Allergen: Allergens are now searchable and viewable in the web test manual database but are not listed individually in the printable pdf-format documents. Trace Metals: Trace
Cleveland Clinic Laboratories
 Cleveland Clinic Laboratories Technical Update April 2015 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That s why we are happy to provide
Cleveland Clinic Laboratories Technical Update April 2015 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That s why we are happy to provide
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
Recommendations for Celiac Disease Testing. IgA & ttg IgA
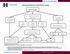 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M1 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M1 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
Case History Number One. Case History Two. Selection and Preparation of Allergen Vaccines (Extracts) Single Allergen Associated With Allergic Disease
 Selection and Preparation of Allergen Vaccines (Extracts) Richard F. Lockey, M.D. Division of Allergy and Immunology Department of Internal Medicine University of South Florida College of Medicine and
Selection and Preparation of Allergen Vaccines (Extracts) Richard F. Lockey, M.D. Division of Allergy and Immunology Department of Internal Medicine University of South Florida College of Medicine and
Environmental and occupational disorders. Analytic precision and accuracy of commercial immunoassays for specific IgE: Establishing a standard
 Environmental and occupational disorders Analytic precision and accuracy of commercial immunoassays for specific IgE: Establishing a standard P. Brock Williams, PhD, a James H. Barnes, PhD, b Sheryl L.
Environmental and occupational disorders Analytic precision and accuracy of commercial immunoassays for specific IgE: Establishing a standard P. Brock Williams, PhD, a James H. Barnes, PhD, b Sheryl L.
ALLERGEN TEST REQUEST FORM Department of Laboratories St. Louis, Missouri 63110
 Date} NAME ADDRESS ACCOUNT INFORMATION PHONE ORDERING PHYSICIAN BILL TO: 9 ACCOUNT 9 PATIENT/INSURANCE 9 ALTERNATE SEND ADDITIONAL COPY OF REPORT TO: CLIENT NUMBER/PHYSICIAN NAME PHONE/FAX NUM. PHYSICIAN
Date} NAME ADDRESS ACCOUNT INFORMATION PHONE ORDERING PHYSICIAN BILL TO: 9 ACCOUNT 9 PATIENT/INSURANCE 9 ALTERNATE SEND ADDITIONAL COPY OF REPORT TO: CLIENT NUMBER/PHYSICIAN NAME PHONE/FAX NUM. PHYSICIAN
PLS UPDATE J A N U A R Y
 www.physlab.com PLS UPDATE J A N U A R Y 2 0 1 5 Changes to HPV CPT Codes: Effective 01/01/2015, the AMA created new CPT Codes for HPV testing. These codes are as follows: 87623 Infectious agent detection
www.physlab.com PLS UPDATE J A N U A R Y 2 0 1 5 Changes to HPV CPT Codes: Effective 01/01/2015, the AMA created new CPT Codes for HPV testing. These codes are as follows: 87623 Infectious agent detection
MEDICAL PRACTICE. In theory it should be possible to predict the IgE-mediated
 BRITISH MEDICAL JOURNAL VOLUME 285 14 AUGUST 1982 483 Computers in Medicine Allergy screening using a microcomputer C F A PANTIN, Abstract T G MERRETT Data are presented to show that a microcomputer can
BRITISH MEDICAL JOURNAL VOLUME 285 14 AUGUST 1982 483 Computers in Medicine Allergy screening using a microcomputer C F A PANTIN, Abstract T G MERRETT Data are presented to show that a microcomputer can
Selection of Antigen Therapy. Hector P. Rodriguez MD Department of Otolaryngology Columbia University
 Selection of Antigen Therapy Hector P. Rodriguez MD Department of Otolaryngology Columbia University Selection of Antigen for Therapy The number of antigens available from supply houses for treatment of
Selection of Antigen Therapy Hector P. Rodriguez MD Department of Otolaryngology Columbia University Selection of Antigen for Therapy The number of antigens available from supply houses for treatment of
Cleveland Clinic Laboratories
 Cleveland Clinic Laboratories Technical Update February 2016 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That s why we are happy to provide
Cleveland Clinic Laboratories Technical Update February 2016 Cleveland Clinic Laboratories is dedicated to keeping you updated and informed about recent testing changes. That s why we are happy to provide
July Billing and Compliance. Chemistry. Help Us Help You. Referral Testing
 July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
APPENDIX Allergy (ImmunoCap ) Testing Allergen IgE Antibodies
 Hypersensitivity to the allergens included in this catalog are detected by (ImmunoCAP ). Testing is done by FEIA measuring circulating allergen specific immunoglobulin E. Results are reported as class
Hypersensitivity to the allergens included in this catalog are detected by (ImmunoCAP ). Testing is done by FEIA measuring circulating allergen specific immunoglobulin E. Results are reported as class
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 6 Immunology 2016 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 6 Immunology 2016 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
TEST NAME RESULT-FLAG TEST NAME RESULT-FLAG
 Abnormal (s) Summary Summary may not contain all abnormal results, especially those of Tests with an interpretation. Please review the entire report. TEST NAME RESULT-FLAG TEST NAME RESULT-FLAG GLUCOSE
Abnormal (s) Summary Summary may not contain all abnormal results, especially those of Tests with an interpretation. Please review the entire report. TEST NAME RESULT-FLAG TEST NAME RESULT-FLAG GLUCOSE
Analyte Specimen Demographic Reference Range Units
 Acetone Negative titer Alanine aminotransferase (ALT/SGPT) 10-49 U/L Albumin 3.2-4.8 g/dl Alcohol < 10 Alpha-fetoprotein (AFP) < 1.3-8.1 ng/ml Alkaline phosphatase 0 7 days 7 30 days 1 3 3 6 6 12 1 3 3
Acetone Negative titer Alanine aminotransferase (ALT/SGPT) 10-49 U/L Albumin 3.2-4.8 g/dl Alcohol < 10 Alpha-fetoprotein (AFP) < 1.3-8.1 ng/ml Alkaline phosphatase 0 7 days 7 30 days 1 3 3 6 6 12 1 3 3
December Metanephrines, Fractionated, Free, Plasma Plasminogen Activator Inhibitor Type 1 Stool Cultures and Sensitivities
 University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
e. Elm Correct Question 2 Which preservative/adjuvant has the greatest potential to breakdown immunotherapy because of protease activity? a.
 Allergen Immunotherapy Practical Quiz Question 1 Which of the following pollens shows cross-reactivity with birch pollen? a. Alder b. Olive c. Ash d. Black walnut e. Elm Question 2 Which preservative/adjuvant
Allergen Immunotherapy Practical Quiz Question 1 Which of the following pollens shows cross-reactivity with birch pollen? a. Alder b. Olive c. Ash d. Black walnut e. Elm Question 2 Which preservative/adjuvant
THE COMPLETE ALLERGY SERVICE ALLERGIES & YOUR PET IMPROVE YOUR PET S QUALITY OF LIFE INNOVATION IN ANIMAL HEALTHCARE
 THE COMPLETE ALLERGY SERVICE ALLERGIES & YOUR PET IMPROVE YOUR PET S QUALITY OF LIFE INNOVATION IN ANIMAL HEALTHCARE WHAT IS AN ALLERGY? An allergy is a reaction of the immune system to a common substance
THE COMPLETE ALLERGY SERVICE ALLERGIES & YOUR PET IMPROVE YOUR PET S QUALITY OF LIFE INNOVATION IN ANIMAL HEALTHCARE WHAT IS AN ALLERGY? An allergy is a reaction of the immune system to a common substance
Please contact the Client Services Team if you require further information.
 Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
M E D I C A L L A B O R A T O R Y
 M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
M E D I C A L L A B O R A T O R Y E V A L U A T I O N PARTICIPANT SUMMARY 2 0 1 7 Immunology 2017 MLE-M3 Total Commitment to Education and Service Provided by ACP, Inc. Table of Contents Evaluation Criteria...
