All abnormal results for this test will automatically have the DNA test reflexed at an additional cost.
|
|
|
- Eustace Harris
- 5 years ago
- Views:
Transcription
1 2211 Michigan Avenue Santa Monica, CA Dear Colleague: May-June 2004 REVISED At Specialty we continue to focus on working with you to reduce the problems that can result in tests not being performed. After discussions with Becton-Dickinson, we have confirmed that freezing and shipping specimens in serum separator tubes is likely to adversely affect sample integrity. This is particularly important when testing for drug levels or working with frozen samples. We have included a flyer as a reminder with this letter and will send copies with orders of serum separator tubes from our Client Supply department. We would also like to call to your attention that the wooden shaft of a swab should generally not be included with samples for TMA/GenProbe Chlamydia/Neisseria (CT/GC) tests because a substance eluted from the wood can interfere with amplification. Wooden shafts can also interfere with viral cultures. In our flyer recommending that PPT tubes be centrifuged prior to shipment, we failed to emphasize that frozen samples are preferred due to the hour specimen stability for HIV and Hepatitis C Virus RNA testing. Hexosaminidase Testing Due to changes made by the referral lab, please note the following: S50585 Hexosaminidase A,B serum is for males and non-pregnant females. S49273 Hexosaminidase, leukocytes is for pregnant, non-ashkenazic females NOTE: All abnormal results for this test will automatically have the DNA test reflexed at an additional cost. For additional information on testing or other specimen collection guidelines, please contact Client Services at Michael C. Dugan, M.D. Vice President and Co-Director of Laboratory
2 May/June 2004, page Michigan Avenue Santa Monica, CA New from Specialty Effective Tuesday, June 15, 2004 or as noted 8134 Hepatitis B Virus GenotypR Component Method Reference Range Units HBV Genotype INNO-LiPA By report Specimen/Stability 4 (2) ml Plasma EDTA; Frozen 2 Month(s) Alternate Specimens Specimen 4 (2) ml Plasma ACD Stability Frozen 2 Month(s) Collection Instructions Split specimen into 2 plastic vials and freeze immediately. Ship frozen on dry ice by overnight courier. Clinical Utility Identifies HBV Genotypes A, B, C, D, E, F, and mixture A/D. Some studies indicate that patients with HBV Genotype C experience decreased response to interferon therapy. HBV Genotype D patients may be more likely to develop recurrence than Genotype A. The predominant Genotypes are A, D, and mixtures of A/D, accounting for 89% of all genotypes in some studies. Schedule Wednesday Turnaround Time 2-8 days CPT Code 83890, 83898, 83904x6, Hepatitis B Virus Core/Pre-Core Mutant DetectR Component Method Reference Range Units Basal Core Promoter Nucleotide INNO-LiPA Wild Type Pre-Core Codon 28 INNO-LiPA Wild Type Specimen/Stability 4 (2) ml Plasma EDTA or ACD; Frozen 2 Month(s) Collection Instructions Split specimen into two plastic vials and freeze immediately. Ship frozen on dry ice by overnight courier. Clinical Utility Mutations in the basal core promoter and pre-core regions of the HBV genome may decrease or abolish HBeAg production. Schedule Wednesday Turnaround Time 2-8 days CPT Code 83890, 83892x2, 83894x2, 83898x2, Notes Identifies substitution mutations in the HBV Basal Core Promoter gene nucleotide positions 1762 and The Wild Type nucleotide combination is A1762/G1764. Identifies mutations in the HBV PreCore gene codon 28. Test Reactivation 5822 Chromosome Analysis Amniotic Fluid effective 5/17/04 Panel Component Offered as Test The following tests are generally ordered as part of clinically useful panels, but may also be ordered individually. For more information, please contact Client Services at or visit our Web site at U Amphetamines Urine 8862 Bartonella henselae DNA DetectR 5784 Blood Culture: Aerobic 8833 Brucella IgM Antibodies 2429 Ureaplasma urealyticum
3 May/June 2004, page 3 Test Changes Effective Tuesday, June 15, 2004 or as noted Test Code Test Name Specific Change Also Affected 5801 Aerobic Bacterial Culture: Eye/Ear reflex to Susceptibility Name Eye/Ear Culture (reflex to 5776 Aerobic Bacterial Culture: Genital reflex to Genital Culture (reflex to Susceptibility 5775 Aerobic Bacterial Culture: Respiratory Respiratory Culture (reflex to reflex to Susceptibility 5771 Aerobic Bacterial Culture: Routine Miscellaneous reflex to Susceptibility Routine Miscellaneous Culture (reflex to 5700 Aerobic Bacterial Culture: Stool Comprehensive reflex to Susceptibility Stool Comprehensive Culture (reflex to 5701 Aerobic Bacterial Culture: Stool Routine Stool Routine Culture (reflex to reflex to Susceptibility 5772 Aerobic Bacterial Culture: Throat Throat Culture 5773 Aerobic Bacterial Culture: Urine Urine Culture (reflex to 5774 Aerobic/Anaerobic Bacterial Culture: Blood Name Blood Culture Aerobic/Anaerobic (reflex to Specimen Requirements Specimens in Isolator tube or ACD tube are not acceptable; collect specimen in blood culture bottle Collection Instructions 1. Collect a minimum of 10 ml blood in blood culture bottle prior to a predicted fever spike or before the administration of antibiotics. 2. For pediatric patients, collect 0.5 ml to 2.0 ml of blood. 3. Collecting two sets of cultures, one from each of two prepared sites at least 30 minutes intervals, is recommended Brucella abortus IgG, IgM & IgA Antibodies Name Brucella IgG, IgM & IgA Abs Clinical Utility Aids in the diagnosis of brucellosis caused by B. abortus, B. melitensis, B. suis and B. canis 7584P Epstein-Barr Virus DNA UltraQuant Plasma Alternate Specimen Plasma EDTA acceptable Alternate Specimen Stability Ambient 7 Day(s), Refrigerated 14 Day(s), Frozen 2 Month(s) 5920 F-actin IgG Autoantibodies Reference Range Negative: Less than 20.0 Units Weak Positive: Units Positive: Greater than 30.0 Units Specimen Heparinized and EDTA plasma are no longer accepted; serum is only acceptable specimen 5911 Human Anti-Mouse Antibodies (HAMA) Stability Serum Frozen 2 Month(s); Refrigerated is not acceptable 8516 Influenza Virus A & B IgG, IgM & IgA Antibodies Alternate Specimen Plasma EDTA or Heparinized; Ambient, Refrigerated or Frozen 8836 Brucella IgG Abs 5906 Hepatitis Autoimmune Plus 5908 Hepatitis Autoimmune
4 May/June 2004, page Interferon Beta (1a & 1b) Autoantibodies Specimen Temperature Ambient or Refrigerated is not acceptable. Ship serum samples frozen LDL (Low Density Lipoprotein) Sub- Fractions 9501 Legionella pneumophila Antigen Detection Urine Component Add Calculated LDL Reference Range Mean LDL Particle Size greater than 26.7 nm Calculated LDL less than 130 mg/dl Specimen Stability Frozen specimen acceptable/stability 2 Month(s) 2945 Malaria Organism Detection Specimen Whole blood (EDTA) and three thin blood smears (fixed with methanol) and three thick blood smears (unfixed) Stability Ambient 24 Hour(s) Collection Instructions 1. Prepare thin smear using the blood differential method. Air dry for 10 minutes. Fix with methanol for 5 seconds. 2. For thick smears, add 1 drop of the EDTA blood onto a clean microscopic slide. Spread the blood into a dime-sized area. Dry for 30 minutes. DO not fix with methanol. 3. Submit thin and thick slides in cardboard slide holder. These slides and the EDTA specimen should be submitted to the laboratory within 24 hours of collection Monoclonal Gammopathy Evaluation Specimen Stability Urine Ambient 5 Day(s), Refrigerated 14 Day(s), Frozen 2 Month(s) 4951 HIV MonitR - Nelfinavir Effective 5/18/04 Component Delete component Nelfinavir M8 Metabolite from panel Phosphatidylserine IgG, IgM & IgA Abs Reference Range IgG Less than 11.0 GPS U/mL IgM Less than 25.0 MPS U/mL IgA Less than 20.0 APS U/mL 1012 Rheumatoid Factor IgG, IgM & IgA Autoantibodies Method IgM change to EIA from NEPH Reference Range IgG, IgM, IgA (each) Less than 6.1 Units 5917 Soluble Liver Antigen Autoantibodies Reference Range Negative: Less than 20.1 Units Equivocal: Units Positive: Greater Than 24.9 Units 7585 Varicella-Zoster DNA DetectR Alternate Specimen 2.0(0.5) ml Amniotic Fluid Stability for Alternate Specimen Ambient 4 Day(s), Refrigerated 4 Day(s), Frozen 2 Month(s) 1080S Antiphospholipid Syndrome Eval w/o Lupus Anticoagulant 1081 Antiphospholipid Syndrome 1082 Antiphospholipid Syndrome Eval Expanded 1776 Antiphospholipid Evaluation 1013 Rheumatoid Arthritis 5906 Hepatitis Autoimmune Plus 5908 Hepatitis Autoimmune 8760 Varicella-Zoster Virus DNA UltraQuant
5 May/June 2004, page 5 Discontinued Tests Effective Tuesday, June 8, 2004 or as noted The following test(s) are no longer routinely available from Specialty. Whenever possible, alternate tests are recommended. Please note that if a test is designated as a replacement, contractual pricing will be copied from discontinued test to replacement test. Contractual pricing does not apply to alternate tests or sendout tests. Please contact Client Services or your Sales Representative if you have any questions. Test Test Name Reason Alternate or Replacement Tests Code 5802 Aerobic Bacterial Culture: Environ l Low-volume No recommendations S41181 Allergen Douglas Fir IgE In-house test available [alternate] RT207 Allergen Douglas Fir IgE S41522 Allergen Scallops IgE In-house test available [alternate] RF338 Allergen-Scallops IgE S41747 Allergen Watermelon IgE In-house test available [alternate] RF329 Allergen Watermelon IgE S42056 Allergen Olive Tree - European IgE In-house test available [alternate] T9 Allergen Allergen-Olive IgE S42961 Allergen Cranberry IgE In-house test available [alternate] RF341 Allergen Cranberry IgE S43073 Allergen Cypress Arizona IgE In-house test available [alternate] RT222 Allergen Cypress, Arizona IgE S43862 Allergen Bean White/Navy IgE In-house test available [alternate] F15 Allergen White bean IgE S46006 Allergen Oak, Virginia Live IgE In-house test available [alternate] RT218 Allergen Oak, Virginia Live IgE S46007 Allergen Sorrel, Sheep IgE In-house test available [alternate] W18 Allergen Sheep sorrel IgE S46798 Allergen Dog Hair/Dander IgE In-house test available [alternate] E4 Allergen Dog Dander IgE S46972 Allergen Sage (Food) IgE In-house test available [alternate] RF344 Allergen Sage(Food) IgE S47430 Allergen Macadamia Nut In-house test available [alternate] RF345 Allergen Macadamia Nut S48200 Allergen Allspice IgE In-house test available [alternate] RF339 Allergen Allspice IgE S48730 Allergen Pine White IgE In-house test available [alternate] T16 Allergen White Pine IgE S48985 Allergen Rape Seed/Canola [364] In-house test available [alternate] RF316 Allergen Rape Seed IgE S49083 Allergen Carmine Dye Extract IgE In-house test available [alternate] RF340 Allergen Carmine Dye Extract IgE S49522 Allergen Gelatine Bovine [468] In-house test available [alternate] C74 Allergen Gelatine Bovine IgE S49531 Allergen Protamine IgE [1041] In-house test available [alternate] RC207 Allergen Protamine IgE S50335 Allergen German Cockroach IgE In-house test available [alternate] I6 Allergen Cockroach IgE 5642 Escherichia coli Enteroinvasive Toxin Reagents not available 5640 E. coli 0157:H7 Culture [alternate] 5639 E. coli Enterohemorrhagic (0157:H7) Stool Culture [alternate] 2504 Legionella Culture: Environmental No recommendations For additional information please call Client Services at or visit our Web site at
Please note that our serum opiates EIA screen assay is not appropriate for therapeutic drug monitoring of Oxycodone.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
5701 Aerobic Stool Culture, Routine Component Method Reference Range. Organism/Pathogen CULTURE Not detected. Echinococcus IgG EIA Not detected
 2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
New Test Announcements
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
Test Catalog Updates August 3, 2016 Updates noted in Red
 s Updates noted in Red BLOD1424 Allergen Food Basil No page Specimen Requirement 0.5 ml (0.3 ml minimum) serum. Send Stability: RMT - NA REFT - 14 days Frozen - 14 days BLOD0248 Allergen Food Green Bean
s Updates noted in Red BLOD1424 Allergen Food Basil No page Specimen Requirement 0.5 ml (0.3 ml minimum) serum. Send Stability: RMT - NA REFT - 14 days Frozen - 14 days BLOD0248 Allergen Food Green Bean
TEST REQUEST INFORMATION- VIROLOGY
 Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
March Immunoglobulins, Total (IgA, IgE, IgG, IgM) Interleukin-6 (IL6) Protein Electrophoresis, CSF. Thyrotropin Receptor Antibody
 University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Alkaline Phosphatase, Bone Specific
 Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
Alkaline Phosphatase, Bone Specific Order Name: ALK P BONE Test Number: 3656500 REV DATE:11/5/2010 Alkaline Phosphatase, Bone Specific IMMENZ Preferred 1 ml (0.3) Serum Clot Activator SST (Red/Gray or
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2010 Dear Colleague: Our May communication to you brings very exciting news of a new test offering available
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2010 Dear Colleague: Our May communication to you brings very exciting news of a new test offering available
Diagnostic Methods of HBV and HDV infections
 Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Anti-FceR (CU Index) Antibody
 Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
MICROBIOLOGY SPECIMEN COLLECTION MANUAL
 Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
December Metanephrines, Fractionated, Free, Plasma Plasminogen Activator Inhibitor Type 1 Stool Cultures and Sensitivities
 University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
Rapid-VIDITEST Swine Flu
 Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Business Unit: Quest Diagnostics Nichols Institute, Valencia. June 2011 Update Dear Colleague,
 Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
12/10/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
The pages that follow contain information critical to protecting the health of your patients and the citizens of Colorado.
 Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Follow us on Twitter @TCHDHealth and @TCHDEmergency John
Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Follow us on Twitter @TCHDHealth and @TCHDEmergency John
DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations
 Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
November Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Collection (Specimen Source Required on all tests) Sputum: >5 ml required. First morning specimen preferred.
 Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
Type Acid Fast (Mycobacteria) Sputum: >5 ml required. First morning specimen preferred. For blood, sodium heparin tube preferred. Lithium heparin acceptable. Do not centrifuge.. delay. Swabs are not appropriate
October Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
ANNOUNCEMENTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Page # Reactivated
Memo No: Date: 25-Nov NEW Serology and Virology Testing
 Memo No: 2014-61 Date: 25-Nov-2014 www.hicl.on.ca Re: NEW Serology and Virology Testing In Common Laboratories has been working hard to expand our test menu. We are pleased to announce the availability
Memo No: 2014-61 Date: 25-Nov-2014 www.hicl.on.ca Re: NEW Serology and Virology Testing In Common Laboratories has been working hard to expand our test menu. We are pleased to announce the availability
HML Update. Summer 2009 Volume 15, No. 3. Keeping Our Clients Informed Questions or Comments: Call
 HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Laboratory diagnosis of congenital infections
 Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
TELEFAX. Toxoplasma Serology Laboratory (TSL) DATE: TO: FAX: FROM:
 TELEFAX DATE: TO: FAX: FROM: Toxoplasma Serology Laboratory RE: SPECIMEN REQUIREMENTS AND TESTING INFORMATION (effective 1/15/16) THIS TRANSMISSION CONSISTS OF 16 PAGES INCLUDING THIS COVER PAGE. IF THERE
TELEFAX DATE: TO: FAX: FROM: Toxoplasma Serology Laboratory RE: SPECIMEN REQUIREMENTS AND TESTING INFORMATION (effective 1/15/16) THIS TRANSMISSION CONSISTS OF 16 PAGES INCLUDING THIS COVER PAGE. IF THERE
Diagnostic Methods of HBV infection. Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO)
 Diagnostic Methods of HBV infection Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO) Hepatitis B-laboratory diagnosis Detection of HBV infection involves
Diagnostic Methods of HBV infection Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO) Hepatitis B-laboratory diagnosis Detection of HBV infection involves
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT
 SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
READ HIGHLIGHTED CHANGES
 BIO-FLASH anti-hbs 3000-8581 100 tests The BIO-FLASH anti-hbs is a fully automated chemiluminescent simultaneous immunoassay for quantitative measurement of antibodies to Hepatitis B surface antigen (anti-hbs)
BIO-FLASH anti-hbs 3000-8581 100 tests The BIO-FLASH anti-hbs is a fully automated chemiluminescent simultaneous immunoassay for quantitative measurement of antibodies to Hepatitis B surface antigen (anti-hbs)
Test Menu. Infectious Diseases Laboratory. Division of Infectious Diseases Department of Medicine
 Test Menu Infectious Diseases Laboratory Division of Infectious Diseases Department of Medicine July 2017 1 Atypical Pneumonia PCR Panel (APP)* The APP is an in-house developed, real-time (RT) PCR assay,
Test Menu Infectious Diseases Laboratory Division of Infectious Diseases Department of Medicine July 2017 1 Atypical Pneumonia PCR Panel (APP)* The APP is an in-house developed, real-time (RT) PCR assay,
Please contact Customer Service with any questions. Ph: option 5
 Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Sarasota Memorial Laboratory Services is pleased to announce that we are now performing the following testing in house: Immature Platelet Fraction (IPF) The Immature Platelet Fraction (IPF) is used to
Review the following alphabetical listing of our lab test catalog for each of our offered tests. Each page has at least the following elements:
 Site: Fremont Rideout Health Group Laboratory Services Policy and Procedure Creation Date: 08/17/2009 Subject/Title: FRHG Laboratories' Catalog of Tests Q-S Document Owner: Corson, Karen Pagination: Page
Site: Fremont Rideout Health Group Laboratory Services Policy and Procedure Creation Date: 08/17/2009 Subject/Title: FRHG Laboratories' Catalog of Tests Q-S Document Owner: Corson, Karen Pagination: Page
October Courier Service on Holidays There will be no courier service Thursday, Dec. 24th, 2011.
 University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS
 MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS OVERVIEW (1) Background (2) Sample collection vials useful for different types of specimens (3) Stability of nucleic
MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS OVERVIEW (1) Background (2) Sample collection vials useful for different types of specimens (3) Stability of nucleic
Aerobic Wound Culture and Stain
 Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Recommendations for Celiac Disease Testing. IgA & ttg IgA
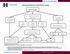 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
 27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Ward Manual. PHC Diagnostic Virology & Reference Laboratory
 PHC Diagnostic Virology & Reference Laboratory I. General Information The SPH Virology Laboratory is located at St. Paul s Hospital 1081 Burrard Street, Room 625, Burrard Building, Vancouver, B.C. V6Z
PHC Diagnostic Virology & Reference Laboratory I. General Information The SPH Virology Laboratory is located at St. Paul s Hospital 1081 Burrard Street, Room 625, Burrard Building, Vancouver, B.C. V6Z
February Billing and Compliance. Chemistry/Immunology. Help Us Help You. Microbiology. Referral Testing. Supplies. Website
 February 2014 Billing and Compliance ICD 10 update It s coming! Chemistry/Immunology Hepatitis B Antibody reporting change Help Us Help You Completion of Allina Health Laboratory manual request forms Unscheduled
February 2014 Billing and Compliance ICD 10 update It s coming! Chemistry/Immunology Hepatitis B Antibody reporting change Help Us Help You Completion of Allina Health Laboratory manual request forms Unscheduled
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
TISSUE COLLECTION. SCPA 603- Histopathological Techniques for Routine and Research
 TISSUE COLLECTION SCPA 603- Histopathological Techniques for Routine and Research Somphong Narkpinit, MD. Department of Pathobiology Faculty of Science, Mahidol University somphong.nar@mahidol.ac.th Learning
TISSUE COLLECTION SCPA 603- Histopathological Techniques for Routine and Research Somphong Narkpinit, MD. Department of Pathobiology Faculty of Science, Mahidol University somphong.nar@mahidol.ac.th Learning
Rapid-VIDITEST. Influenza A
 Rapid-VIDITEST Influenza A (One step Influenza A Card test for the detection of Influenza type A antigen from human nasopharyngeal specimens (swab, nasopharyngeal wash and aspirate). Instruction manual
Rapid-VIDITEST Influenza A (One step Influenza A Card test for the detection of Influenza type A antigen from human nasopharyngeal specimens (swab, nasopharyngeal wash and aspirate). Instruction manual
In-Common Laboratories
 Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Luton and Dunstable University Hospital NHS Foundation Trust Lewsey Road Luton Bedfordshire LU4 0DZ Contact: Pauline Philip Tel: +44 (0)1582
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Luton and Dunstable University Hospital NHS Foundation Trust Lewsey Road Luton Bedfordshire LU4 0DZ Contact: Pauline Philip Tel: +44 (0)1582
Microbiology Collection
 Microbiology Collection Notify the Microbiology Laboratory at 920-738-6317 if any of the following etiological agents are suspected to be present in the specimens sent to the laboratory. Bacillius anthracis
Microbiology Collection Notify the Microbiology Laboratory at 920-738-6317 if any of the following etiological agents are suspected to be present in the specimens sent to the laboratory. Bacillius anthracis
AbBaltis. Product Catalogue. Contact us now for free samples!
 AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 27, 2007 Dear Colleague: Specialty Laboratories is pleased to offer two new tests to aid in the diagnosis
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 27, 2007 Dear Colleague: Specialty Laboratories is pleased to offer two new tests to aid in the diagnosis
Test Definition: FMCPS Meningoencephalitis Comprehensive Panel (Serum)
 Reporting Title: Meningoencephalitis Comp Panel, S Performing Location: Focus Diagnostics, Specimen Requirements: Draw blood in a plain, red-top tube(s). (Serum gel tube is acceptable.) Spin down and send
Reporting Title: Meningoencephalitis Comp Panel, S Performing Location: Focus Diagnostics, Specimen Requirements: Draw blood in a plain, red-top tube(s). (Serum gel tube is acceptable.) Spin down and send
Technical Bulletin No. 162
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 162 cobas 6800 HCV Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 162 cobas 6800 HCV Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma tests/panels.
 February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
IMMUNOFLUORESCENT STAINING FOR RESPIRATORY VIRUS DETECTION: INFLUENZA A, INFLUENZA B AND RESPIRATORY SYNCYTIAL VIRUS
 IMMUNOFLUORESCENT STAINING FOR RESPIRATORY VIRUS DETECTION: INFLUENZA A, INFLUENZA B AND RESPIRATORY SYNCYTIAL VIRUS A. Introduction Influenza A, Influenza B and RSV are responsible for serious respiratory
IMMUNOFLUORESCENT STAINING FOR RESPIRATORY VIRUS DETECTION: INFLUENZA A, INFLUENZA B AND RESPIRATORY SYNCYTIAL VIRUS A. Introduction Influenza A, Influenza B and RSV are responsible for serious respiratory
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Test Definition: LCMS Leukemia/Lymphoma Immunophenotyping by Flow Cytometry
 Reporting Title: Leukemia/Lymphoma, Phenotype Performing Location: Rochester Advisory Information: This test is appropriate for hematopoietic specimens only. If your specimen is a solid tissue, order LLPT
Reporting Title: Leukemia/Lymphoma, Phenotype Performing Location: Rochester Advisory Information: This test is appropriate for hematopoietic specimens only. If your specimen is a solid tissue, order LLPT
Influenza-Associated Pediatric Mortality rev Jan 2018
 rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
About HPV. Human papillomavirus (HPV) is a group of viruses that are extremely common worldwide. Theree are more than 100 types of HPV, of which at
 Cervical Cancer & HPV What is HPV Human papillomavirus (HPV) is a group of viruses that are extremely common worldwide. About HPV Theree are more than 100 types of HPV, of which at least 13 are cancer-causing
Cervical Cancer & HPV What is HPV Human papillomavirus (HPV) is a group of viruses that are extremely common worldwide. About HPV Theree are more than 100 types of HPV, of which at least 13 are cancer-causing
Collection Container Category Source Tests Order Codes
 Nasal Nares culture for MRSA screen ONLY (Outpatient) NSL Room BD BBL Dual Culture Swab (Red Cap) Ear Ear culture EARC Room Eye Eye Culture EYEC Room Cultures Vaginal Genital Culture GEN or GC Room Superficial
Nasal Nares culture for MRSA screen ONLY (Outpatient) NSL Room BD BBL Dual Culture Swab (Red Cap) Ear Ear culture EARC Room Eye Eye Culture EYEC Room Cultures Vaginal Genital Culture GEN or GC Room Superficial
Schedule of Accreditation
 Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS
 AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS Take Control, Molecular Control. 02 04 05 06 06 07 08 08 AMPLIRUN TOTAL RESPIRATORY INFECTIONS TUBERCULOSIS INFECTIONS GASTROINTESTINAL
AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS Take Control, Molecular Control. 02 04 05 06 06 07 08 08 AMPLIRUN TOTAL RESPIRATORY INFECTIONS TUBERCULOSIS INFECTIONS GASTROINTESTINAL
BIO-FLASH. BIOKIT, S.A. - Can Malé s/n Lliçà d Amunt - Barcelona - SPAIN
 READ HIGHLIGHTED CHANGES BIO-FLASH anti-hbc 3000-8578 100 tests The BIO-FLASH anti-hbc is a fully automated chemiluminescent two-step immunoassay for qualitative measurement of total antibodies to hepatitis
READ HIGHLIGHTED CHANGES BIO-FLASH anti-hbc 3000-8578 100 tests The BIO-FLASH anti-hbc is a fully automated chemiluminescent two-step immunoassay for qualitative measurement of total antibodies to hepatitis
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates June 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the improvement of our turnaround times
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates June 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the improvement of our turnaround times
IMMEDIATE HOT LINE: Effective January 6, 2014
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
Trends in molecular diagnostics
 Trends in molecular diagnostics Detection of target genes of interest Quantification Infectious diseases HIV Hepatitis C & B TB / MAC Cytomegalovirus Herpes simplex Varicella zoster CT/GC HPV Profiling
Trends in molecular diagnostics Detection of target genes of interest Quantification Infectious diseases HIV Hepatitis C & B TB / MAC Cytomegalovirus Herpes simplex Varicella zoster CT/GC HPV Profiling
4. RED AND BLUE TOP COLLECTION SWABS WILL NO LONGER BE SUPPLIED FOR BACTERIAL CULTURE
 Laboratory Testing Update Specimen Transport Changes dated 12-24-14 SEH Laboratory is making significant changes to specimen transports! The Laboratory is introducing new testing platforms along with working
Laboratory Testing Update Specimen Transport Changes dated 12-24-14 SEH Laboratory is making significant changes to specimen transports! The Laboratory is introducing new testing platforms along with working
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document.
 February 2018 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN CHLAMYDIA/GONORRHEA SPECIMEN COLLECTION
February 2018 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN CHLAMYDIA/GONORRHEA SPECIMEN COLLECTION
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
Accredited entity according to ČSN EN ISO 15189:2013 Laboratoře Mikrochem a.s. Mikrochem Laboratories Nezvalova 984/2, Hodolany, Olomouc
 Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
December Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Test Requested Specimen Ordering Recommendations
 Microbiology Essentials Culture and Sensitivity (C&S) Urine C&S Catheter Surgical (excluding kidney aspirates) Voided Requisition requirements o Specific method of collection MUST be indicated o Indicate
Microbiology Essentials Culture and Sensitivity (C&S) Urine C&S Catheter Surgical (excluding kidney aspirates) Voided Requisition requirements o Specific method of collection MUST be indicated o Indicate
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Microbiology Department Crawley Hospital West Green Drive Crawley RH11 7DH United Kingdom Contact: Clare Reynolds Tel: +44 (0) 1293 600379
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Microbiology Department Crawley Hospital West Green Drive Crawley RH11 7DH United Kingdom Contact: Clare Reynolds Tel: +44 (0) 1293 600379
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World
 MIT OpenCourseWare http://ocw.mit.edu SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World Spring 2009 For information about citing these materials or our
MIT OpenCourseWare http://ocw.mit.edu SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World Spring 2009 For information about citing these materials or our
METABOLITE TEST REQUISITION FORM SPECIMEN INFORMATION. Specimen Date: Time: ADDITIONAL INFORMATION
 METABOLITE TEST REQUISITION FORM Baylor Institute of Metabolic Disease Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION - OR Place Patient Sticker
METABOLITE TEST REQUISITION FORM Baylor Institute of Metabolic Disease Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION - OR Place Patient Sticker
Rapid-VIDITEST. Influenza A+B
 Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
(DNA) Real-time PCR. Exicycler 96 Rotor-Gene Q/6000 PCR
 Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets (TaqMan Real-time ' FAM ' BHQ1
Real-Time (DNA) Real-time Exicycler 96 Rotor-Gene Q/6000 IU Mix1 Mix2 IU/μl IU/μl IU/μl IU/μl IU/μl IPC NTC C 1 Lot# 2 Freeze & thawing 1 MSDS: Material Safety Data Sheets (TaqMan Real-time ' FAM ' BHQ1
Notes from the Sentinel Liaison
 ADPH Unplugged Notes from the Sentinel Liaison Thank you for participating! Survey ListServ Notes Trainings Sentinel Training August 9-10, 2017 Packaging and Shipping September 14,2017 You can be a Sentinel
ADPH Unplugged Notes from the Sentinel Liaison Thank you for participating! Survey ListServ Notes Trainings Sentinel Training August 9-10, 2017 Packaging and Shipping September 14,2017 You can be a Sentinel
INTERPATH LABORATORY, INC. TEST UPDATES
 EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
HSV-1 IgM ELISA. Catalog No (96 Tests) For Research Use Only. Not for use in Diagnostic Procedures.
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. HSV-1 IgM ELISA Kit is intended for the detection of IgM antibody to HSV-1 in human serum or plasma. SUMMARY AND
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. HSV-1 IgM ELISA Kit is intended for the detection of IgM antibody to HSV-1 in human serum or plasma. SUMMARY AND
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
Current September 2017
 1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
Respiratory Pathogen Panel TEM-PCR Test Code:
 Respiratory Pathogen Panel TEM-PCR Test Code: 220000 Tests in this Panel Enterovirus group Human bocavirus Human coronavirus (4 types) Human metapneumovirus Influenza A - Human influenza Influenza A -
Respiratory Pathogen Panel TEM-PCR Test Code: 220000 Tests in this Panel Enterovirus group Human bocavirus Human coronavirus (4 types) Human metapneumovirus Influenza A - Human influenza Influenza A -
For Research Use Only Ver
 INSTRUCTION MANUAL Quick-RNA Viral Kit Catalog Nos. R1034 & R1035 Highlights Quick, spin-column purification of viral RNA from plasma, serum, CSF, cell culture media, cellular suspensions, urine, blood,
INSTRUCTION MANUAL Quick-RNA Viral Kit Catalog Nos. R1034 & R1035 Highlights Quick, spin-column purification of viral RNA from plasma, serum, CSF, cell culture media, cellular suspensions, urine, blood,
METABOLITE TEST REQUISITION FORM
 METABOLITE TEST REQUISITION FORM Baylor Scott and White Research Institute Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION OR Place Patient Sticker
METABOLITE TEST REQUISITION FORM Baylor Scott and White Research Institute Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION OR Place Patient Sticker
