|
|
|
- Angela Cox
- 6 years ago
- Views:
Transcription
1 27027 Tourney Road Valencia, CA Test Updates November 27, 2007 Dear Colleague: Specialty Laboratories is pleased to offer two new tests to aid in the diagnosis of Primary Biliary Cirrhosis; gp210 IgG Abs (5928) and sp100 IgG Abs (5919). Another new test; F-Actin IgA Autoabs (5924) can assist in estimating the likelihood of intestinal villus atrophy in patients with Celiac Disease. The Warfarin Sensitivity DetectR (5055) replaces the discontinued Cytochrome P450 2C9 (Warfarin) GenotypR (5381). The Warfarin Sensitivity DetectR which analyzes the CYP 2C9 and VKORC1 genes is available Monday through Friday, with a 2-4 day turnaround time and an easy to read warfarin dosing recommendation chart. As a result of the recent FDA label change for the drug warfarin (Coumadin), Specialty Laboratories has developed an information packet that contains materials relevant to hospital anticoagulation policy from the FDA, Joint Commission, and others, including the handout from our recent web conference Warfarin Dosing using Genetic Information: A Model for Hospital Policy Development. Order your information packet on our website at Primary clients interested in a joint marketing opportunity to educate primary care physicians about the prevalence and wide variety of presenting symptoms for Celiac Disease can participate at no charge through this limited time offer. Specialty Laboratories will provide packages of hospital branded postcards for distribution to local physicians on the need for screening for Celiac Disease. Interested clients should contact Bobette Vikan at extension 6745 for more information. Please note: This client letter covers many changes in Specialty Laboratories CPT code recommendations. For additional information, please visit our Web site at or contact Client Relations at Thank you for choosing Specialty and we look forward to your continued support. Respectfully yours, Christopher Lockhart, M.D. Laboratory Director 1 of 4
2 New Tests: 5928 gp210 IgG Abs (Available Immediately) Component Method ReferenceRange/Units gp210 IgG Abs ELISA <20.1 Units Negative Units Equivocal > 24.9 Units Positive Specimen/Stability Serum 1.0 (0.5) ml; Refrigerated 14 days, Frozen 2 months Clinical Utility The Quanta Lite gp210 kit is an enzyme-linked immunosorbent assay (ELISA) for the semiquantitative detection of anti-gp210 antibody of the IgG class in human serum. This test is intended to aid in the diagnosis of Primary Biliary Cirrhosis (PBS). Schedule Thursday Report Same day CPT Code Note Collection Grossly hemolyzed/icteric samples are unacceptable sp100 IgG Abs (Available Immediately) Component Method ReferenceRange/Units sp100 IgG Abs ELISA <20.1 Units Negative Units Equivocal > 24.9 Units Positive Specimen/Stability Serum 1.0 (0.5) ml; Refrigerated 14 days, Frozen 2 months Clinical Utility The Quanta Lite sp100 kit is an enzyme-linked immunosorbent assay (ELISA) for the semiquantitative detection of anti-sp100 antibody of the IgG class in human serum. This test is intended to aid in the diagnosis of Primary Biliary Cirrhosis (PBS). Schedule Thursday Report Same day CPT Code Note Results are obtained using the INOVA Quanta Lite sp100 ELISA assay. sp100 values obtained with different manufacturers assay methods may not be used interchangeably. The magnitude of the reported IgG levels can not be correlated to an endpoint titer. Collection 5924 F-Actin IgA Autoabs (Available Immediately) Component Method ReferenceRange/Units F-Actin IgA Autoabs ELISA <20.0 Units Negative Units Weak positive > 30.0 Units Moderate to Strong Positive Specimen/Stability Clinical Utility Schedule Report CPT Code Note Collection Serum 1.0 (0.5) ml; Ambient 7 days, Refrigerated 14 days, Frozen 2 months The Quanta Lite Actin IgA kit is an enzyme-linked immunosorbent assay (ELISA) for the semiquantitative detection of IgA antibodies to the actin component of smooth muscle in human serum. In patients with clinical findings consistent with celiac disease, the presence of antiactin IgA antibody may assist in estimating the likelihood of intestinal villus atrophy. Saturday Same day 2 of 4
3 Test Changes: 1580U Protein Electrophoresis Urine Effective Stability Also Affected Immediately Ambient 14 days, Refrigerated 14 days, Frozen 1 month 1595U, 1584U, 1584UR 1580C Protein Electrophoresis CSF Effective Stability Also Affected Immediately Refrigerated 7 days, Frozen 1 month 1584C 8315UR Histoplasma Antigen Urine Effective December 4 Ref Range < 0.7 Negative Borderline > 2.9 Moderate to Strong positive 4873W Mercury Whole Blood Effective Alt. Specimen Stability Immediately 2 (0.5) ml Whole Blood EDTA now accepted Ambient 7 days, Refrigerated 1 month, Frozen 2 months 1859 IHC Stain & Interpretation: Pathologist Chooses 1-6 Stains Effective Panel Name Immediately IHC Stain & Interpretation: Pathologist Chooses 1-12 Stains 1833 ER, PR, Ki67, p53, HER-2/neu w/reflex FISH, Breast Cancer Effective December 18 Component ER Breast Interpretation ADD PR Breast Interpretation ADD HER-2/neu Interpretation ADD Reference ranges: 0, 1+ = No overexpression 2+, 3+ = Overexpression Ki67 (MIB-1) Interpretation ADD Reference ranges: <10% = Low 10-20% = Intermediate > 20% = High p53 Interpretation ADD 3125U Monoclonal Gammopathies Urine Effective December 18 Note Detected results will automatically reflex to Kappa & Lambda Light Chain and IgG, IgM & IgA quantitation by Nephelometry, for an additional charge 3 of 4
4 Discontinued: Effective December 4, 2007: 3912 Interleukin-12 Replaced by: S51504 Interleukin 12, S,P (Non-NY approved lab) for 3912 and 3912PL 3912F Interleukin-12 Fluid Replaced by: S51505 Interleukin 12, Fluid (Non-NY approved lab) for 3912F 3912PL Interleukin-12 Plasma Replaced by: S51504 Interleukin 12, S,P (Non-NY approved lab) for 3912 and 3912PL 4135 Methotrexate Replaced by: S51506 Methotrexate for Quinidine Replaced by: S51507 Quinidine for Cytochrome P450 2C9 (Warfarin) GenotypR Replaced by: 5055 Warfarin Sensitivity DetectR (VKORC1 & CYP2C9) 1857 IHC Stain & Interpretation: Pathologist Chooses 1-3 Stains Replaced by: 1859 IHC Stain & Interpretation: Pathologist Chooses 1-12 Stains 4 of 4
5 CPT Coding Change Recommendations The following CPT code changes are part of an ongoing CPT coding standardization. The CPT codes provided are based upon AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. If you have any questions, please refer to the Current Procedural Terminology (CPT) Manual published by the American Medical Association. To verify reimbursement, or to ask questions regarding usage of a CPT code, please contact your local Medicare carrier. Please contact Specialty Client Services at if you have questions regarding CPT coding changes to custom panels. *Effective: December 1, Tissue Total Autoabs Screen (TABS) 83516, 86255x Myasthenia Gravis Evaluation 83519, Myasthenia Gravis Evaluation PLUS 83519x3, Transglutaminase IgG Autoabs [EIA] Transglutaminase IgA Autoabs [EIA] Transglutaminase IgG & IgA Autoabs 83516x Celiac Disease EvaluatR w/iga 82784, 83516x3, 86255x Celiac Disease Autoabs Evaluation 83516x2, 86255x Celiac Disease EvaluatR 83516x3, 86255x Antiphospholipid Syndrome EvaluatR, Expanded 86147x3, 86148x3, 83516x9, Myocardial Total Autoabs Parietal Cell Total Autoabs Reticulin Total Autoabs Smooth Muscle Total Autoabs Striational Total Autoabs Centromere Autoabs Skin Total Autoabs, Inter-Epithelial & Dermal-Epidermal 86255x Adrenal Total Autoantibodies Chromatin (Histone-DNA Complex) IgG Autoantibodies Reticulin IgA Autoabs Endomysial IgA Autoabs DNA Autoantibodies, Double-Stranded [Crithidia] SRP Autoantibodies Proliferating Cell Nuclear Ag Autoabs Gliadin IgG Abs Gliadin IgG & IgA Abs 83516x Gliadin IgA Abs HLA-A, B, C DetectR HLA-A, B, C, DR DetectR 86813, HLA-A DetectR HLA-B DetectR HLA-C DetectR HLA-DR DetectR Plasminogen, Quantitative Tourney Rd., Valencia, CA Phone Fax
6 2007 CPT Code Changes, page Alpha-1-Antitrypsin Deficiency Fetal Study w/reflex MCC 83891, 83900, 83909, 83912, 83914x Lymphocyte Enumeration, T Cell 86359, Lymphocyte Enumeration, Helper/Inducer Lymphocyte Enumeration, Helper/Suppressor Lymphocyte Enumeration, T & B Cell 86355, Lymphocyte Enumeration, Helper/Suppressor - Limited Lymphocyte Enumeration, Basic & NK Cells 86355, 86357, 86359, Lymphocyte Enumeration, Basic 86355, 86359, Rapidly Progressive Glomerulonephritis Evaluation 83520, 86060, 86021x3, 86038, 86160x Phosphatidylcholine IgG, IgM & IgA Autoabs x Phosphatidic Acid IgG, IgM & IgA Autoabs x Phosphatidylinositol IgG, IgM & IgA Autoabs x Antiphospholipid Evaluation 86147x3, 83516x9, 83520x x3, 86148x Phosphatidylethanolamine IgG, IgM & IgA Autoabs 83516x Natural Killer Cell Quantitation Complement C4 Binding Protein Candida albicans Evaluation 86628x3, Ova & Parasite: Comprehensive Exam w/coccidia Evaluation 87177, 87207x2, Ova & Parasite: Coccidia Evaluation 87015, 87207x Antistreptolysin O Antibodies (ASO) Herpes Simplex Virus Typing from Tissue Culture (FA) Ureaplasma urealyticum/mycoplasma hominis Culture Bordetella pertussis/parapertussis Culture Bordetella pertussis/parapertussis Evaluation 87081, 87265x Fecal Leukocytes Estrogens, Fractionated Serum Growth Hormone Deficiency MonitR 82397, Myositis AssessR 83516x5, 86235x Mi-2 Autoantibodies Myositis AssessR Plus Jo-1 Autoantibodies 83516x5, 86235x Epinephrine Plasma Valproic Acid, Free S-100B AccuQuant Serum Amylase Isoenzymes 82150x2, Chromogranin A, End-Point Titer Chromogranin A Insulin-Like Growth Factor Binding Protein (IGFBP-3) Alkaline Phosphatase, Bone Specific Benzodiazepines Serum Carbamazepine & Metabolites (10,11 Epoxide) Risperidone Amiodarone & Metabolites Tricyclic Antidepressants (TCA) Confirm Serum Extended Tricyclic Antidepressants (TCA) Confirmation Serum Tricyclics Antidepressants (TCA) Screen Serum Cocaine & Metabolites Confirmation Serum Oxycodone & Metabolite Serum 83925
7 2007 CPT Code Changes, page Opiates Confirmation Serum Nicotine & Cotinine Serum Methadone Confirmation Serum Lamotrigine Fentanyl & Norfentanyl Serum Flunitrazepam & Metabolites Confirmation Serum Hydromorphone Serum Dihydrocodeine Serum Hydrocodone Serum AmpliChip CYP450 2D6 & 2C19 GenotypR 83891, 83892x2, 83900, 83901x4, Mycophenolic Acid Amitriptyline & Nortriptyline Flurazepam (Dalmane) Doxepin & Nordoxepin Imipramine & Desipramine Fluoxetine & Norfluoxetine Clomipramine & Desmethylclomipramine Clozapine & Norclozapine Sickle Cell MonitR Fungus Culture & Stain - Skin, Hair or Nail 87101, M. avium (MAC) Complex Culture ID [DNA Probe] M. tuberculosis Complex Culture ID [DNA Probe] AFB Suscept: MAI Complex (MAC) by Agar Proportion Method 87190x Gram Negative Susceptibility Panel: Urine/Non-Urine Gram Positive Susceptibility Panel: Urine/Non-Urine Anaerobic Susceptibility Panel 87076, 87181x Fungus Culture: Yeast Screen-Skin, Hair, or Nail 87101, Hepatitis Autoimmune EvaluatR PLUS 86021, 86038, 86255, 86376x2, 87522, 83516x Hepatitis Autoimmune EvaluatR 86038, 86376x2, 83516x F-Actin IgG Autoantibodies Platelet Glycoprotein (Direct & Indirect) 86022x3, 86023x Platelet Associated Gycoprotein (Direct) 86023x Hypersensitivity Pneumonitis Evaluation 86001x8, 86331x Hypersensitivity Evaluation II 86331x Cryptococcus Ag Candida Ag Detection HIV p24 Antigen, Qualitative HIV-1 RNA UltraQuant [bdna] & CD4 Cell Count 86361, SR Lymphocyte Enumeration, Helper/Inducer with Serial Reporting C Alpha-Fetoprotein (AFP) Tumor Marker CSF R Cholinesterase RBC U Epinephrine 24hr Urine UR Epinephrine Urine Random C S-100B AccuQuant CSF U Benzodiazepines Confirmation Urine U Barbiturates Confirmation Urine U Propoxyphene Confirmation Urine 83925
8 2007 CPT Code Changes, page U Alcohol, Ethyl Urine U Lysergic Acid Diethylamide (LSD) Urine U Cannabinoids Confirmation Urine U Cocaine & Metabolites Confirmation Urine U Oxycodone & Metabolite Urine UR Opiates Confirmation Urine UR Opiates Confirmation w/6-mam Urine UR Opiates Confirmation (Morphine & Codeine) Urine U Amphetamines Confirmation Urine U Nicotine & Metabolite Urine U Methadone Confirmation Urine U Meperidine & Normeperidine Uurine U Fentanyl & Norfentanyl Urine U Flunitrazepam & Metabolites Confirmation Urine UR Hydrocodone Urine AccuQuant (including Hydromorphone) UR Hydromorphone Urine AccuQuant U Clonazepam & 7-Amino Clonazepam Urine NY HIV-1 Phenoscript New York 87903, 87904x4 7440SW Chlamydia trachomatis/n. gonorrhoeae rrna PLUS [TMA] Swab w/ rfx Confirm C Cryptococcus Antigen CSF SR HIV-1 RNA UltraQuant [bdna] & CD4 Cell Count w/serial Report 86361, 87536
9 2008 CPT Coding Change Recommendations The following CPT code changes are based on information in the 2008 CPT and AMA instructions. The CPT codes provided are based upon AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. If you have any questions, please refer to the Current Procedural Terminology (CPT) Manual published by the American Medical Association. To verify reimbursement or to ask questions regarding usage of a CPT code, please contact your local Medicare carrier. Please contact Specialty Client Services at if you have questions regarding CPT coding changes to custom panels. *Effective: January 1, Cystatin C Cellular Immune Dysfunction Evaluation 86355, 86359, 86360, 86356x Chronic Fatigue & Immune Dysfunction Syndrome Evaluation 86360, 86356x3, 86663, 86359, 86665x2, 86664, 86790x CD4 Surface Marker CD8 Surface Marker CD16/56 Surface Marker S42830 PNH CD59 Expression S50647 Fetal Hemoglobin Distribution S50902 Calprotectin Tourney Rd., Valencia, CA Phone Fax
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates February 27, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the introduction of an advanced kidney
New Test Announcements
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com New Test Announcements Dear Colleague: We are pleased to announce many new test offerings this month. January 25, 2007 In oncology,
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 26, 2008 Dear Colleague: We are pleased to announce the availability of two new assays for an expanded evaluation
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 21, 2007 Dear Colleague: As part of our continuing efforts to improve testing turnaround times, quality, and service,
Dynacare Laboratories
 Dynacare Laboratories Affiliated with Froedtert & the Medical College of Wisconsin January 2015 2015 CPT Code Updates Dear Client: The American Medical Association (AMA) publishes the Current Procedural
Dynacare Laboratories Affiliated with Froedtert & the Medical College of Wisconsin January 2015 2015 CPT Code Updates Dear Client: The American Medical Association (AMA) publishes the Current Procedural
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates October 28, 2009 Dear Colleague: Specialty Laboratories is pleased to announce the January availability of two trace
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
5701 Aerobic Stool Culture, Routine Component Method Reference Range. Organism/Pathogen CULTURE Not detected. Echinococcus IgG EIA Not detected
 2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
2211 Michigan Avenue Santa Monica, CA 90404 800-421-4449 Coming SOON Coronavirus (SARS) RNA by PCR Specialty has a TaqMan assay for the detection of the SARS coronavirus directly from patient respiratory
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
INTERPATH LABORATORY, INC. TEST UPDATES
 EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
EFFECTIVE DATE: February 16, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to
All abnormal results for this test will automatically have the DNA test reflexed at an additional cost.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: May-June 2004 REVISED 6-15-04 At Specialty we continue to focus on working with you to reduce the problems that can result in tests
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2010 Dear Colleague: Our May communication to you brings very exciting news of a new test offering available
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates May 27, 2010 Dear Colleague: Our May communication to you brings very exciting news of a new test offering available
1/27/ New Release, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
CMS will not implement the new tier codes for Medicare/Medicaid claims for calendar year 2012.
 January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Hammersmith Medicines Research Limited Cumberland Avenue Park Royal London NW10 7EW Contact: Juan Naveda Tel: +44 (0) 20 8961 4130 Fax: +44
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Hammersmith Medicines Research Limited Cumberland Avenue Park Royal London NW10 7EW Contact: Juan Naveda Tel: +44 (0) 20 8961 4130 Fax: +44
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma tests/panels.
 February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
December Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
September HCMC Toxicology Transition: Additional information and Frequently Asked Questions
 September 2016 HCMC Toxicology Transition: Additional information and Frequently Asked Questions Many clinicians have asked for more information about the Urine Drug Compliance Analysis (LAB8742) switch
September 2016 HCMC Toxicology Transition: Additional information and Frequently Asked Questions Many clinicians have asked for more information about the Urine Drug Compliance Analysis (LAB8742) switch
Reflex Test Protocols
 University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
Please note that our serum opiates EIA screen assay is not appropriate for therapeutic drug monitoring of Oxycodone.
 2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
2211 Michigan Avenue Santa Monica, CA 90404 800 421 4449 Dear Colleague: October, 2004 Last month, in anticipation of cold and flu season, we introduced the Respiratory Virus DetectR (Hexaplex ) with Influenza
Aerobic Wound Culture and Stain
 Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
Aerobic Wound Culture and Stain Order Name: C WOUN RTS Test Number: 6000153 Revision Date: 03/27/2014 Aerobic Wound Culture and Stain Culture Preferred 1 ml Tissue Sterile Screwtop Container Room Temperature
2015 Annual Physician Notice
 0 Annual Physician Notice The Office of Inspector General (OIG) recommends clinical laboratories send notices to physicians and other providers who use their services, at least once a year, to inform the
0 Annual Physician Notice The Office of Inspector General (OIG) recommends clinical laboratories send notices to physicians and other providers who use their services, at least once a year, to inform the
IMMEDIATE HOT LINE: Effective January 6, 2014
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
Overseas Referred Tests 2014
 OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
3703 Camino del Rio South 100-A San Diego, CA, Phone Fax CLIA# 05D Director: David J.
 Drug Adherence Assessment Report Prescribed Medications: NO MEDICATION LIST PROVIDED CONSISTENT RESULTS - REPORTED MEDICATION DETECTED (PARENT DRUG AND/OR METABOLITE) REPORTED PRESCRIPTION FLAG ANTICIPATED
Drug Adherence Assessment Report Prescribed Medications: NO MEDICATION LIST PROVIDED CONSISTENT RESULTS - REPORTED MEDICATION DETECTED (PARENT DRUG AND/OR METABOLITE) REPORTED PRESCRIPTION FLAG ANTICIPATED
Test Definition: PCDSO Pain Clinic Drug Screen, Urine
 Reporting Title: Pain Clinic Drug Screen, U Performing Location: Rochester Specimen Requirements: Collection Container/Tube: Plastic urine container Submission Container/Tube: Plastic, 60-mL urine bottle
Reporting Title: Pain Clinic Drug Screen, U Performing Location: Rochester Specimen Requirements: Collection Container/Tube: Plastic urine container Submission Container/Tube: Plastic, 60-mL urine bottle
Test Definition: PDSOX Pain Clinic Drug Screen, Chain of Custody, Urine
 Reporting Title: Pain Clinic Drug Screen, CoC, U Performing Location: Rochester Specimen Requirements: Container/Tube: Chain-of-Custody Kit (Supply T282) containing the specimen containers, seals, and
Reporting Title: Pain Clinic Drug Screen, CoC, U Performing Location: Rochester Specimen Requirements: Container/Tube: Chain-of-Custody Kit (Supply T282) containing the specimen containers, seals, and
Alcohol. Ethanol Highlands Parkway, Suite 100 Smyrna, GA 30082
 Alcohol The alcohol of interest is ethanol. Ethanol has a sedative effect in the brain. Ethanol intoxication symptoms include blurred vision, slurred speech, poor coordination and difficulty thinking depending
Alcohol The alcohol of interest is ethanol. Ethanol has a sedative effect in the brain. Ethanol intoxication symptoms include blurred vision, slurred speech, poor coordination and difficulty thinking depending
Your Results are Our Priority.
 Your Results are Our Priority. Enzyme Linked Immunosorbent Assay (ELISA) One year shelf life Ready to use, color coded reagents and bottles Single dilution factor Adaptable for all forensic matrices Forensic
Your Results are Our Priority. Enzyme Linked Immunosorbent Assay (ELISA) One year shelf life Ready to use, color coded reagents and bottles Single dilution factor Adaptable for all forensic matrices Forensic
INTERPATH LABORATORY, INC. TEST UPDATES
 EFFECTIVE DATE: January 25, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to contact
EFFECTIVE DATE: January 25, 2016 INTERPATH LABORATORY, INC. TEST UPDATES Please take note of the following test updates and make the appropriate changes in your Interpath Service Manual. Feel free to contact
12/10/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens
 Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens This document describes the validation of a Neogen Fentanyl kit for the semi-quantitative analysis of
Validation Report for the Neogen Fentanyl Kit for ELISA Screening of Whole Blood and Urine Specimens This document describes the validation of a Neogen Fentanyl kit for the semi-quantitative analysis of
Annual Notice to Providers (2014)
 8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
8901 West Lincoln Avenue, West Allis, WI 53227 5400 Pearl, Rosemont, IL 60018 Annual Notice to Providers (2014) May 2014 Dear Physician/Client: The Medicare Program encourages clinical laboratories to
December Metanephrines, Fractionated, Free, Plasma Plasminogen Activator Inhibitor Type 1 Stool Cultures and Sensitivities
 University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
University of Washington December 2012 TEST change 1,25 Dihydroxy Vitamin D Frequency AFB Blood Cultures Alcohol Screen Anti Deaminated Gliadin, IgG Anti Gliadin Package Anti Mullerian Hormone Package
10/7/ Immediate Action, Quest Diagnostics Nichols Institute, Valencia
 REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
REDIRECTS Please Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date Page
Recommendations for Celiac Disease Testing. IgA & ttg IgA
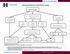 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
MEMORANDUM. Carol Ghere, MT (ASCP) SI, Compliance Officer CPT4 Code Changes from American Medical Association (AMA)
 MEMORANDUM TO: FROM: RML Clients Carol Ghere, MT (ASCP) SI, Compliance Officer DATE: January 2, 2015 SUBJECT: 2015 CPT4 Code Changes from American Medical Association (AMA) 2015 CPT4 Code Changes from
MEMORANDUM TO: FROM: RML Clients Carol Ghere, MT (ASCP) SI, Compliance Officer DATE: January 2, 2015 SUBJECT: 2015 CPT4 Code Changes from American Medical Association (AMA) 2015 CPT4 Code Changes from
DRUGS OF ABUSE
 DRUGS OF ABUSE www.btnx.com 1-888-339-9964 Rapid Response Drugs of Abuse and Alcohol Screening Devices BTNX Inc. prides itself in offering quality drug detection tests for accurate, safe and rapid testing.
DRUGS OF ABUSE www.btnx.com 1-888-339-9964 Rapid Response Drugs of Abuse and Alcohol Screening Devices BTNX Inc. prides itself in offering quality drug detection tests for accurate, safe and rapid testing.
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
September 04, 2008
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 04, 2008 Dear Valued Client: As you may be aware, in recent years there has been a tremendous challenge in
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 04, 2008 Dear Valued Client: As you may be aware, in recent years there has been a tremendous challenge in
ORAL FLUID AS A CHEMICAL TEST FOR THE DRE PROGRAM : HISTORY, THE FUTURE, AND PRACTICAL CONSIDERATIONS
 ORAL FLUID AS A CHEMICAL TEST FOR THE DRE PROGRAM : HISTORY, THE FUTURE, AND PRACTICAL CONSIDERATIONS Barry K Logan PhD, DABFT National Director of Forensic Services, NMS Labs Willow Grove PA Disclaimer
ORAL FLUID AS A CHEMICAL TEST FOR THE DRE PROGRAM : HISTORY, THE FUTURE, AND PRACTICAL CONSIDERATIONS Barry K Logan PhD, DABFT National Director of Forensic Services, NMS Labs Willow Grove PA Disclaimer
MEDICAL POLICY Drug Testing
 POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 11/13/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 11/13/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
Specific Panels. Celiac disease panel. Pancreas Panel:
 Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
November Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
Test Bulletin. Specimen Type. Preferred Volume. PCA3 PCA3TM Urine 2.5 ml KIT Frozen. URCTFR Urine 2.5 ml Urine Refrigerated
 Bulletin New Referral ing s Effective March January 1, 2017 2014 Description Current New Specimen Type Preferred Volume Tube Type Temperature to Transport Notes/Comments Prostate Cancer Biomarker PCA3
Bulletin New Referral ing s Effective March January 1, 2017 2014 Description Current New Specimen Type Preferred Volume Tube Type Temperature to Transport Notes/Comments Prostate Cancer Biomarker PCA3
PROFICIENCY TESTING. Clinical Laboratory Improvement Amendments (CLIA) DOs and DON Ts. Brochure # 8
 Clinical Laboratory Improvement Amendments (CLIA) Brochure # 8 PROFICIENCY TESTING DOs and DON Ts NOTE: Congress passed the Clinical Laboratory Improvement Amendments (CLIA) in 1988 establishing quality
Clinical Laboratory Improvement Amendments (CLIA) Brochure # 8 PROFICIENCY TESTING DOs and DON Ts NOTE: Congress passed the Clinical Laboratory Improvement Amendments (CLIA) in 1988 establishing quality
Drug & Alcohol Testing in. Canada. Point of Care Testing
 Drug & Alcohol Testing in Canada Point of Care Testing Introduction We are the leading Canadian innovator of diagnostic testing products and services Verify Diagnostics has assisted governments, corporations,
Drug & Alcohol Testing in Canada Point of Care Testing Introduction We are the leading Canadian innovator of diagnostic testing products and services Verify Diagnostics has assisted governments, corporations,
 27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
27027 Tour ney Road Valencia, CA 91355 8 0 0 4 2 1 7 1 1 0 www.specialtylabs.com Test Updates December 25, 2010 Dear Colleague: Please review the following specific test listings for important information
Business Unit: Quest Diagnostics Nichols Institute, Valencia. June 2011 Update Dear Colleague,
 Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Forensic Toxicology Scope of Testing and Detection Limits
 Forensic Toxicology Scope of Testing and Detection Limits Table of Contents QUALITATIVE ANALYSES... 2 Volatile Screen by GC/FID... 2 Carbon Monoxide by Microdiffusion... 2 Ethylene Glycol by GC/MS... 2
Forensic Toxicology Scope of Testing and Detection Limits Table of Contents QUALITATIVE ANALYSES... 2 Volatile Screen by GC/FID... 2 Carbon Monoxide by Microdiffusion... 2 Ethylene Glycol by GC/MS... 2
ALPS Screen Order Set B-Cell Panel Order Set (% and absolute #) (% and Absolute #) ALPS (CD3+/CD4-/CD8-/TCR αβ+)
 All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
LCMS-8050 Drugs of Abuse: 113 Analytes with Polarity Switching
 Liquid Chromatography Mass Spectrometry SSI-LCMS-8 LCMS-8 Drugs of Abuse: Analytes with Polarity Switching LCMS-8 Summary Seventy six analytes and their internal standards are described below. Multiple
Liquid Chromatography Mass Spectrometry SSI-LCMS-8 LCMS-8 Drugs of Abuse: Analytes with Polarity Switching LCMS-8 Summary Seventy six analytes and their internal standards are described below. Multiple
March Immunoglobulins, Total (IgA, IgE, IgG, IgM) Interleukin-6 (IL6) Protein Electrophoresis, CSF. Thyrotropin Receptor Antibody
 University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
MICROBIOLOGY SPECIMEN COLLECTION MANUAL
 Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
Lee Memorial Health System Lee County, FL CLINICAL LABORATORY MICROBIOLOGY SPECIMEN COLLECTION MANUAL ACID FAST CULTURE Specimen Type see Specimen Chart ACID FAST STAIN see Specimen Chart Acid Fast stain
2017 Drug Screen Tests
 2017 Drug Screen Tests The ABCs of Applying the CMS 2017 HCPCS Codes Written by: Karen D. Chappell, EJD, MBA, CIRCC, CPMA, CPC-I, CHC Terminology Immunoassay Qualitative Therapeutic Quantitative GC/MS
2017 Drug Screen Tests The ABCs of Applying the CMS 2017 HCPCS Codes Written by: Karen D. Chappell, EJD, MBA, CIRCC, CPMA, CPC-I, CHC Terminology Immunoassay Qualitative Therapeutic Quantitative GC/MS
Clinical Laboratory. 14:41:00 Complement Component 3 50 mg/dl Oct-18
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
cobas c 501 analyzer and cobas c 311 analyzer Within Run Imprecision Guidelines
 cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
Clinical Laboratory. [None
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
PCL ALVERNO INSTALLING NEW ANALYZERS AND AUTOMATION
 October 2014 Dear Healthcare Provider, The information contained in the packet may be very important to your practice. Below is a quick summary of the items that are included in this mailing. Please take
October 2014 Dear Healthcare Provider, The information contained in the packet may be very important to your practice. Below is a quick summary of the items that are included in this mailing. Please take
Urine Drug Testing Methods 3-5
 Urine Drug Testing Methods 3-5 Type of Test Logistics Pearls Initial Screening Test: Immunoassay Confirmatory Test: Gas chromatography-mass spectrometry (GCMS) + or Liquid chromatography-mass spectrometry
Urine Drug Testing Methods 3-5 Type of Test Logistics Pearls Initial Screening Test: Immunoassay Confirmatory Test: Gas chromatography-mass spectrometry (GCMS) + or Liquid chromatography-mass spectrometry
What Your Drug Test Really Means. Krista Beiermann, RN, OHS Occupational Health Services, Columbus Hospital
 What Your Drug Test Really Means Krista Beiermann, RN, OHS Occupational Health Services, Columbus Hospital Disclosure There are no relevant financial relationships with commercial interests associated.
What Your Drug Test Really Means Krista Beiermann, RN, OHS Occupational Health Services, Columbus Hospital Disclosure There are no relevant financial relationships with commercial interests associated.
PROFILES MENU. Page no. 9 Page no. 8 Page no. 7 Page no. 6 Page no. 5. Page no. 2. Page no. 4 Page no. 3
 Health Profiles PROFILES MENU Page no. 1 Page no. 2 Page no. 4 Page no. 3 1 Basic Metabolic Panel (BMP) 2 Liver Function Tests (LFT) 3 Comprehensive Metabolic Panel (CMP) 4 Lipid Profile 5 Chem 7 Panel
Health Profiles PROFILES MENU Page no. 1 Page no. 2 Page no. 4 Page no. 3 1 Basic Metabolic Panel (BMP) 2 Liver Function Tests (LFT) 3 Comprehensive Metabolic Panel (CMP) 4 Lipid Profile 5 Chem 7 Panel
3703 Camino del Rio South 100-A San Diego, CA, Phone Fax CLIA# 05D years
 Drug Adherence Assessment Report CleanAssure TM (DRIED BLOOD SPOT): Detection Range see NOTES. Prescribed Medications: HYDROMORPHONE (DILAUDID, EXALGO), CYCLOBENZAPRINE (FLEXERIL), METHADONE (METHADOSE),
Drug Adherence Assessment Report CleanAssure TM (DRIED BLOOD SPOT): Detection Range see NOTES. Prescribed Medications: HYDROMORPHONE (DILAUDID, EXALGO), CYCLOBENZAPRINE (FLEXERIL), METHADONE (METHADOSE),
MEDICAL POLICY Drug Testing
 POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 04/10/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 04/10/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
March Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
Therapeutic Drugs Monitoring TDM 2016 Therapeutic Drugs Monitoring Scheme Application Form
 complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: TM Purchase order no.: (compulsory) TDM Distribution Schedule Samples for the
complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: TM Purchase order no.: (compulsory) TDM Distribution Schedule Samples for the
Anti-FceR (CU Index) Antibody
 Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Powering Efficient Behavioral Health Care Services
 Powering Efficient Behavioral Health Care Services Streamlining behavioral health care decisions through comprehensive and efficient drug monitoring Quest Diagnostics has you and your patients covered
Powering Efficient Behavioral Health Care Services Streamlining behavioral health care decisions through comprehensive and efficient drug monitoring Quest Diagnostics has you and your patients covered
HOSPITALS IN-COMMON LABORATORY INC.
 Immunoglobulin E Ab., Single Allergen: Allergens are now searchable and viewable in the web test manual database but are not listed individually in the printable pdf-format documents. Trace Metals: Trace
Immunoglobulin E Ab., Single Allergen: Allergens are now searchable and viewable in the web test manual database but are not listed individually in the printable pdf-format documents. Trace Metals: Trace
BID ANNUAL CONTRACT FOR LAB TESTING
 1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
Therapeutic Drugs Monitoring TDM 2018 Therapeutic Drugs Monitoring Scheme Application Form
 complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: TM Purchase order no.: (compulsory) TDM Distribution Schedule Samples for the
complete all sections below and return to LGC Standards Proficiency Testing by email, fax or post. Returning customer Lab ID: TM Purchase order no.: (compulsory) TDM Distribution Schedule Samples for the
CLIA APPROVED PROFICIENCY TESTING PROGRAMS ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts (800)
 ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts 01886 (800) 665-2575 MICROBIOLOGY Bacteriology Aerobic Culture and Identification Antibiotic Susceptibility Testing Direct Antigen Detection Gram Stain
ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts 01886 (800) 665-2575 MICROBIOLOGY Bacteriology Aerobic Culture and Identification Antibiotic Susceptibility Testing Direct Antigen Detection Gram Stain
Refugee Health Funding Models: A Review of PA Models and A Vision for the Future
 Refugee Health Funding Models: A Review of PA Models and A Vision for the Future Gretchen Shanfeld, MPH Director of Health and Wellness, Nationalities Service Center Coordinator, Philadelphia Refugee Health
Refugee Health Funding Models: A Review of PA Models and A Vision for the Future Gretchen Shanfeld, MPH Director of Health and Wellness, Nationalities Service Center Coordinator, Philadelphia Refugee Health
GENERAL INFORMATION CLINICAL LABORATORY PHONE DIRECTORY
 GENERAL INFORMATION CLINICAL LABORATORY PHONE DIRECTORY SECTION PHONE NUMBER Clinical Pathologist 431-5888 Laboratory Main Laboratory Administrative Director Janis Nall Accessioning/Client Services Section
GENERAL INFORMATION CLINICAL LABORATORY PHONE DIRECTORY SECTION PHONE NUMBER Clinical Pathologist 431-5888 Laboratory Main Laboratory Administrative Director Janis Nall Accessioning/Client Services Section
DrugConfirm Advanced Urine Dip Cards.
 DrugConfirm Advanced Urine Dip Cards The information in this presentation is a general overview on using the Confirm BioSciences DrugConfirm Advanced drug test cup but can be applied to many similarly
DrugConfirm Advanced Urine Dip Cards The information in this presentation is a general overview on using the Confirm BioSciences DrugConfirm Advanced drug test cup but can be applied to many similarly
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Schedule of ccreditation United Kingdom ccreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK ccredited to Laboratory locations: Gavenny Court Brecon Road bergavenny Monmouthshire
Schedule of ccreditation United Kingdom ccreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK ccredited to Laboratory locations: Gavenny Court Brecon Road bergavenny Monmouthshire
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
VASCULITIS PRODUCT HIGHLIGHTS
 VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
The Drug Testing Process. Employer or Practice
 Disclosures Clinical Professor, Jefferson Medical College BOD MROCC [Medical Review Officer Certification Council] BOD National Sleep Foundation BOD POEMS [Pennsylvania Occupational & Environmental Medicine
Disclosures Clinical Professor, Jefferson Medical College BOD MROCC [Medical Review Officer Certification Council] BOD National Sleep Foundation BOD POEMS [Pennsylvania Occupational & Environmental Medicine
Saskatchewan ND Price List
 Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
Jonathan Rochon, Pier-Luc Plante, Serge Auger, Réal Paquin, Jacques Corbeil, Pierre Picard
 Dried Blood Spots in Tips Targeted Drug Screening in 9 Seconds per Sample Using Laser Diode Thermal Desorption Mass Spectrometry (LDTD-MS/MS) Jonathan Rochon, Pier-Luc Plante, Serge Auger, Réal Paquin,
Dried Blood Spots in Tips Targeted Drug Screening in 9 Seconds per Sample Using Laser Diode Thermal Desorption Mass Spectrometry (LDTD-MS/MS) Jonathan Rochon, Pier-Luc Plante, Serge Auger, Réal Paquin,
Acetylcholine Receptor Modulating Antibody
 Acetylcholine Receptor Modulating Antibody Order Name: ACETY MOD Test Number: 5516500 TEST COMPONENTS REV DATE: 09/25/2012 Acetylcholine Receptor Modulating Antibody SEMI-FC Preferred 1 ml (0.5) Serum
Acetylcholine Receptor Modulating Antibody Order Name: ACETY MOD Test Number: 5516500 TEST COMPONENTS REV DATE: 09/25/2012 Acetylcholine Receptor Modulating Antibody SEMI-FC Preferred 1 ml (0.5) Serum
February Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
October Courier Service on Holidays There will be no courier service Thursday, Dec. 24th, 2011.
 University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
THROMBOSIS PRODUCT HIGHLIGHTS
 PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
A Simple and Accurate Method for the Rapid Quantitation of Drugs of Abuse in Urine Using Liquid Chromatography
 Application Note LCMS-109 A Simple and Accurate Method for the Rapid Quantitation of Drugs of Abuse in Urine Using Liquid Chromatography Time of Flight (LC-TOF) Mass Spectrometry Introduction Many clinical
Application Note LCMS-109 A Simple and Accurate Method for the Rapid Quantitation of Drugs of Abuse in Urine Using Liquid Chromatography Time of Flight (LC-TOF) Mass Spectrometry Introduction Many clinical
DrugConfirm Advanced Instant Urine Drug Test Cups Training Guide.
 DrugConfirm Advanced Instant Urine Drug Test Cups Training Guide The information in this presentation is a general overview on using Confirm BioSciences DrugConfirm Advanced urine drug test cup; however,
DrugConfirm Advanced Instant Urine Drug Test Cups Training Guide The information in this presentation is a general overview on using Confirm BioSciences DrugConfirm Advanced urine drug test cup; however,
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
