Notification of a Body in the framework of a technical harmonization directive
|
|
|
- Luke Cunningham
- 5 years ago
- Views:
Transcription
1 Notification of a Body in the framework of a technical harmonization directive From : National Organization for Medicines (EOF) Mesogeion 284, Holargos Greece To : European Commission GROWTH Directorate-General 200 Rue de la Loi, B-1049 Brussels. Other Member States Reference : Legislation : 93/42/EEC Medical devices Body name, address, telephone, fax, , website : NATIONAL EVALUATION CENTER OF QUALITY AND TECHNOLOGY IN HEALTH S.A.- EKAPTY Smyrnis GLYFADA Greece Phone : Fax : info@ekapty.gr Website : Body : NB 0653 Created : Unknown (Notifications pre-dating 2006 are not available in these lists) Last update : 12/07/2018 The body is formally accredited against : EN EN ISO/IEC EN EN ISO/IEC Name of National Accreditation Body (NAB) : ESYD The accreditation covers the product categories and conformity assessment procedures concerned by this notification : Yes 1 / 5
2 Tasks performed by the Body : Created : 29/11/2013 Last update : 11/11/2016 Product family, product /Intended use/product *MD General non-active, non-implantable medical devices - *MD Non-active devices for anaesthesia, emergency and intensive care - *MD Non-active devices for injection, infusion, transfusion and dialysis - *MD Non-active orthopaedic and rehabilitation devices - *MD Non-active medical devices with measuring function - *MD Non-active ophthalmologic devices - *MD Non-active instruments - *MD Contraceptive medical devices - *MD Non-active medical devices for disinfecting, cleaning, rinsing - *MD Non-active devices for in vitro fertilisation (IVF) and assisted reproductive technologies (ART) *MD Non-active implants - *MD Non-active orthopaedic implants - *MD Non-active soft tissue implants 2 / 5
3 *MD Devices for wound care - *MD Bandages and wound dressings - *MD Suture material and clamps - *MD Other medical devices for wound care *MD Non-active dental devices and accessories - *MD Non-active dental equipment and instruments - *MD Dental materials - *MD Dental implants *MD General active medical devices - *MD Devices for extra-corporal circulation, infusion and haemopheresis - *MD Respiratory devices, devices including hyperbaric chambers for oxygen therapy, inhalation anaesthesia - *MD Devices for stimulation or inhibition - *MD Active surgical devices - *MD Active dental devices Respiratory devices only Only for physiotherapy 3 / 5
4 - *MD Active devices for disinfection and sterilisation - *MD Active rehabilitation devices and active prostheses - *MD Software - *MD Medical gas supply systems and parts thereof *MD Devices for imaging - *MD Imaging devices utilising ionizing radiation - *MD Imaging devices utilising non-ionizing radiation *MD Monitoring devices - *MD Monitoring devices of non-vital physiological parameters - *MD Monitoring devices of vital physiological parameters *MD Devices for radiation therapy and thermo therapy - *MD Devices utilising non-ionizing radiation - *MD Devices for hyperthermia / hypothermia - *MD Devices for (extracorporal) shock-wave therapy (lithotripsy) Only for physiotherapy 4 / 5
5 Horizontal technical competence *MDS Medical devices incorporating medicinal substances, according to Directive Only for MD Codes referred above 2001/83/EC *MDS Medical devices referencing the Directive 2006/42/EC on machinery Only for MD Codes referred above *MDS Medical devices in sterile condition Including aseptic processing, ethylene oxide gas sterilisation (EOG), moist heat sterilisation, dry heat sterilisation, radiation sterilisation (gamma, x-ray, electron beam) - Only for MD Codes referred above *MDS Medical devices incorporating software /utilising software /controlled by Only for MD Codes referred above software 5 / 5
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : ANSM : Agence Nationale de Sécurité du Médicament et des produits de santé - Direction de l'inspection 143, 147, Boulevard
Notification of a Body in the framework of a technical harmonization directive From : ANSM : Agence Nationale de Sécurité du Médicament et des produits de santé - Direction de l'inspection 143, 147, Boulevard
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Ministry of Health ul. Miodowa 15 00-952 Warszawa Poland To : European Commission GROWTH Directorate-General 200 Rue
Notification of a Body in the framework of a technical harmonization directive From : Ministry of Health ul. Miodowa 15 00-952 Warszawa Poland To : European Commission GROWTH Directorate-General 200 Rue
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Ministero dello Sviluppo Economico - Direzione Generale per il Mercato, la Concorrenza, il Consumatore, la Vigilanza
Notification of a Body in the framework of a technical harmonization directive From : Ministero dello Sviluppo Economico - Direzione Generale per il Mercato, la Concorrenza, il Consumatore, la Vigilanza
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK ISO/IEC 17021-1:2015 to provide quality management systems Davy Avenue Knowhill Milton Keynes MK5 8NL Contact: Dr Eugene Kotlirov Tel: +44
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK ISO/IEC 17021-1:2015 to provide quality management systems Davy Avenue Knowhill Milton Keynes MK5 8NL Contact: Dr Eugene Kotlirov Tel: +44
Office No. E E, Amber Gem Tower, SHeikh Khalifa Bin Zayed Road 2, Ajman, U.A.E
 IAS Accreditation Number MSCB-119 Company Name AQC MIDDLE EAST F.Z.E Address Office No. E1-1401 E, Amber Gem Tower, SHeikh Khalifa Bin Zayed Road 2, Ajman, U.A.E Contact Name Nadeem Pasha Telephone +971
IAS Accreditation Number MSCB-119 Company Name AQC MIDDLE EAST F.Z.E Address Office No. E1-1401 E, Amber Gem Tower, SHeikh Khalifa Bin Zayed Road 2, Ajman, U.A.E Contact Name Nadeem Pasha Telephone +971
EUROPEAN COMMISSION DIRECTORATE-GENERAL III INDUSTRY Industrial affairs III: Consumer goods industries Pharmaceuticals and Cosmetics
 EUROPEAN COMMISSION DIRECTORATE-GENERAL III INDUSTRY Industrial affairs III: Consumer goods industries Pharmaceuticals and Cosmetics Community Basic Format for Manufacturing Authorisation Explanatory Notes
EUROPEAN COMMISSION DIRECTORATE-GENERAL III INDUSTRY Industrial affairs III: Consumer goods industries Pharmaceuticals and Cosmetics Community Basic Format for Manufacturing Authorisation Explanatory Notes
Notice Information: - Advisory 01 November 2010
 Notice Information: - Advisory 01 November 2010 Part 1. Product Information a) Title: Single Use & Single Patient Use Medical Devices b) Product Name/Type: Single Use & Single Patient Use Medical Devices
Notice Information: - Advisory 01 November 2010 Part 1. Product Information a) Title: Single Use & Single Patient Use Medical Devices b) Product Name/Type: Single Use & Single Patient Use Medical Devices
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
inspection body type A
 inspection body type A legal entity HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen web www.hygcen.at ident no. 0196 site HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen initial date of
inspection body type A legal entity HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen web www.hygcen.at ident no. 0196 site HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen initial date of
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 22794 First edition 2007-07-15 Corrected version 2009-01-15 Dentistry Implantable materials for bone filling and augmentation in oral and maxillofacial surgery Contents of a
INTERNATIONAL STANDARD ISO 22794 First edition 2007-07-15 Corrected version 2009-01-15 Dentistry Implantable materials for bone filling and augmentation in oral and maxillofacial surgery Contents of a
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
Healthcare Sterilisation: Challenging Practices Volume 2
 Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
BS EN ISO 14971: Risk Analysis and Risk Management Report
 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Gates and
BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Gates and
EC Declaration of Conformity
 EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
BS EN ISO 14971: Risk Analysis and Risk Management Report
 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Maxflex Pop
BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Maxflex Pop
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Derby Manor Derby Road Bournemouth Dorset BH1 3QB Contact: Mr D Riggs Tel: +44 (0)1202 552 153 Fax: +44 (0)1202 290 716 E-Mail: info@urs-certifcation.co.uk
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Derby Manor Derby Road Bournemouth Dorset BH1 3QB Contact: Mr D Riggs Tel: +44 (0)1202 552 153 Fax: +44 (0)1202 290 716 E-Mail: info@urs-certifcation.co.uk
TruGuard Custom Tongue and Jaw Positioner Instructions for Use
 TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
Healthcare Sterilisation: Introduction and Standard Practices, Volume 1
 Contents 1. Overview of Sterilisation and Background... 1 1.1 Magic Wand... 1 1.1.1 Definition of Sterilisation... 2 1.1.2 Detectives or Forensic Scientists... 3 1.1.3 Idealised Process... 6 1.1.4 Lesser
Contents 1. Overview of Sterilisation and Background... 1 1.1 Magic Wand... 1 1.1.1 Definition of Sterilisation... 2 1.1.2 Detectives or Forensic Scientists... 3 1.1.3 Idealised Process... 6 1.1.4 Lesser
Sterilization of health care products Radiation. Part 3: Guidance on dosimetric aspects of development, validation and routine control
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-3 Second edition 2017-06 Sterilization of health care products Radiation Part 3: Guidance on dosimetric aspects of development, validation
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-3 Second edition 2017-06 Sterilization of health care products Radiation Part 3: Guidance on dosimetric aspects of development, validation
Workshop on the reprocessing of medical devices. 5. December Brussels. Nikou Ghassemieh Managing Director
 Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
BS EN ISO 14971: Risk Analysis and Risk Management Report
 BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Tungsten Carbide
BLACKHORSE ROAD, LETCHWORTH, HERTS, SG6 1HB, ENGLAND Tel: + 44 (0) 1462 686221 www.stoddard.co.uk Fax: + 44 (0) 1462 480711 BS EN ISO 14971: Risk Analysis and Risk Management Report Product: Tungsten Carbide
A high-level overview of the requirements of medical packaging standards
 Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
Electrodes (for TransAeris System)
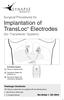 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
not Registered with the Ministry of Justice of the Russian Federation dated July 9, 2012, No MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER
 not Registered with the Ministry of Justice of the Russian Federation dated July 9, 2012, No. 24852 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER dated June 6, 2012, No. 4n ON APPROVAL OF THE MEDICAL
not Registered with the Ministry of Justice of the Russian Federation dated July 9, 2012, No. 24852 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER dated June 6, 2012, No. 4n ON APPROVAL OF THE MEDICAL
Pulpdent Corporation Revision Date: May 7, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation 1.2 1.2.2 Application / Use SIC 1.2.3 Use Category 1.3 Manufacturer Pulpdent Corporation 80 Oakland Street, P.O. Box 780
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation 1.2 1.2.2 Application / Use SIC 1.2.3 Use Category 1.3 Manufacturer Pulpdent Corporation 80 Oakland Street, P.O. Box 780
5101: of 5 APPENDIX B. Revenue Center Codes Requiring CPT or HCPCS Coding
 ACTION: Final ENACTED Appendix 5101:3-2-21 DATE: 03/17/2011 1:51 PM 5101:3-2-21 1 of 5 IV Therapy 0260 General Classification 0261 Infusion Pump 0269 Other IV Therapy Oncology 0280 General Classification
ACTION: Final ENACTED Appendix 5101:3-2-21 DATE: 03/17/2011 1:51 PM 5101:3-2-21 1 of 5 IV Therapy 0260 General Classification 0261 Infusion Pump 0269 Other IV Therapy Oncology 0280 General Classification
Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations Brussels,
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals : regulatory framework and market authorisations Brussels,
O(K) SUMMARY. Mega'Gen Co., Ltd , Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: , Fax:
 EZ PLUS IMPLANT SYSTEM 13. 510O(K) SUMMARY Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
EZ PLUS IMPLANT SYSTEM 13. 510O(K) SUMMARY Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
ต วอย าง AF เคร องม อทางการแพทย ท จะศ กษาท ม ความเส ยงน อยและความเส ยงมาก
 EXAMPLES: ต วอย าง เคร องม อทางการแพทย ท จะศ กษาท ม ความเส ยงน อยและความเส ยงมาก ต วอย างเคร องม อทางการแพทย ท จะศ กษาท ม ความเส ยงน อย NON-SIGNIFICANT RISK DEVICE STUDIES Bio-stimulation Lasers for treatment
EXAMPLES: ต วอย าง เคร องม อทางการแพทย ท จะศ กษาท ม ความเส ยงน อยและความเส ยงมาก ต วอย างเคร องม อทางการแพทย ท จะศ กษาท ม ความเส ยงน อย NON-SIGNIFICANT RISK DEVICE STUDIES Bio-stimulation Lasers for treatment
Facility Information for Initial Assessment
 DIAGNOSTIC ACCREDITATION PROGRAM 300 669 Howe Street Telephone: 604-733-7758 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca Fax: 604-733-3503 Facility Information for Initial Assessment
DIAGNOSTIC ACCREDITATION PROGRAM 300 669 Howe Street Telephone: 604-733-7758 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca Fax: 604-733-3503 Facility Information for Initial Assessment
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
GUIDELINE ON THE CATEGORISATION OF EXTENSION APPLICATIONS (EA) versus VARIATIONS APPLICATIONS (V) OCTOBER 2003
 EUROPN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, implementation and legislation for consumer goods Pharmaceuticals : regulary framework and market authorisations Brussels, F2/AW D(2002)
EUROPN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, implementation and legislation for consumer goods Pharmaceuticals : regulary framework and market authorisations Brussels, F2/AW D(2002)
Compressor Nebulizer. Model UN-014
 Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
REVENUE CODE LIST REQUIRING CPT/HCPCS CODES FOR OUTPATIENT FACILITY CLAIMS
 REVENUE CODE LIST REQUIRING CPT/HCPCS CODES FOR OUTPATIENT FACILITY CLAIMS For Providers Effective July 15, 2018 Revenue Code Description 240 All inclusive ancillary, general 250 Pharmacy 251 Drugs, generic
REVENUE CODE LIST REQUIRING CPT/HCPCS CODES FOR OUTPATIENT FACILITY CLAIMS For Providers Effective July 15, 2018 Revenue Code Description 240 All inclusive ancillary, general 250 Pharmacy 251 Drugs, generic
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon
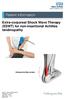 Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon Source: Trauma & Orthopaedics Reference No: 6459-1 Issue date: 1/2/19 Review date: 1/2/22 Page
Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon Source: Trauma & Orthopaedics Reference No: 6459-1 Issue date: 1/2/19 Review date: 1/2/22 Page
ko~ Tk (K) SUMMARY
 ko~3367-3 13. 510(K) SUMMARY 7-Tk 1 92006 Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun, Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
ko~3367-3 13. 510(K) SUMMARY 7-Tk 1 92006 Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun, Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
Schedule of Benefits PPO MASSACHUSETTS
 Schedule of s PPO MASSACHUSETTS ID: MD0000017711_A5 X This Schedule of s states any Limits and the amounts you must pay for Covered s. However, it is only a summary of your benefits. Please see your Handbook
Schedule of s PPO MASSACHUSETTS ID: MD0000017711_A5 X This Schedule of s states any Limits and the amounts you must pay for Covered s. However, it is only a summary of your benefits. Please see your Handbook
COMPARATIVE MEDICINE LABORATORY ANIMAL FACILITIES STANDARD OPERATING PROCEDURE FOR RODENT SURGERY
 2.A.3 COMPARATIVE MEDICINE LABORATORY ANIMAL FACILITIES STANDARD OPERATING PROCEDURE FOR RODENT SURGERY 1.0 Purpose: Post-operative infections in rodents can and do occur. Such infections, which may not
2.A.3 COMPARATIVE MEDICINE LABORATORY ANIMAL FACILITIES STANDARD OPERATING PROCEDURE FOR RODENT SURGERY 1.0 Purpose: Post-operative infections in rodents can and do occur. Such infections, which may not
Pulpdent Corporation Revision Date: May 1, 2017
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
Pulpdent Corporation Revision Date: May 1, 2017
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Ortho-Coat 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Ortho-Coat 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
Pulpdent Corporation Revision Date: May 1, 2017 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Etch-All 10% Phosphoric Acid Etching Gel 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer Pulpdent
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Etch-All 10% Phosphoric Acid Etching Gel 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer Pulpdent
Knee Arthroscopy. Anatomy
 Knee Arthroscopy Knee arthroscopy is a surgical procedure that allows doctors to view the knee joint without making a large incision (cut) through the skin and other soft tissues. Arthroscopy is used to
Knee Arthroscopy Knee arthroscopy is a surgical procedure that allows doctors to view the knee joint without making a large incision (cut) through the skin and other soft tissues. Arthroscopy is used to
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers
 INTERNATIONAL STANDARD ISO 14708-2 Second edition 2012-08-15 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs médicaux implantables actifs
INTERNATIONAL STANDARD ISO 14708-2 Second edition 2012-08-15 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs médicaux implantables actifs
powered by technology easy laser guide
 powered by technology easy laser guide Rev. 1.0 / Oct. 2009 2 Easy Laser Guide The elexxion Easy Laser Guide offers both general information about dental lasers as well as specific information about elexxion
powered by technology easy laser guide Rev. 1.0 / Oct. 2009 2 Easy Laser Guide The elexxion Easy Laser Guide offers both general information about dental lasers as well as specific information about elexxion
DENTAL INFECTION PREVENTION AND CONTROL MANUAL
 DENTAL INFECTION PREVENTION AND CONTROL MANUAL www.infectioncontroldental.com.au Infection Prevention and Control Manual Copyright 2016: Infection Control Dental Pty Ltd All Rights Reserved. Without limiting
DENTAL INFECTION PREVENTION AND CONTROL MANUAL www.infectioncontroldental.com.au Infection Prevention and Control Manual Copyright 2016: Infection Control Dental Pty Ltd All Rights Reserved. Without limiting
The National Board for Certification of Orthopaedic Technologists. Standards of Practice For the Orthopaedic Technologist Certified (OTC )
 The National Board for Certification of Orthopaedic Technologists Standards of Practice For the Orthopaedic Technologist Certified (OTC ) Disclaimer: The intent of these Standards of Practice is to provide
The National Board for Certification of Orthopaedic Technologists Standards of Practice For the Orthopaedic Technologist Certified (OTC ) Disclaimer: The intent of these Standards of Practice is to provide
Radiography. 1. Introduction. 2. Documentation of Compliance. 3. Didactic Competency Requirements. 4. Clinical Competency Requirements
 PRIMARY CERTIFICATION AND REGISTRATION Radiography 1. Introduction Candidates for certification and registration are required to meet the Professional Education Requirements specified in the ARRT Rules
PRIMARY CERTIFICATION AND REGISTRATION Radiography 1. Introduction Candidates for certification and registration are required to meet the Professional Education Requirements specified in the ARRT Rules
BSD-500 Hyperthermia System
 BSD-500 Hyperthermia System What are you doing for your recurrent cancer patients? BSD-500 No shielding room needed Portable FDA Approved for pallative treatment of certain tumors Delivers both superficial
BSD-500 Hyperthermia System What are you doing for your recurrent cancer patients? BSD-500 No shielding room needed Portable FDA Approved for pallative treatment of certain tumors Delivers both superficial
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 17065:2012 to provide product conformity certification Unit 1 Pendle Place Skelmersdale West Lancashire WN8 9PN Contact: Mr Simon Barrowcliff Tel: +44 (0)1695 556666 Fax: +44 (0)1695 557077 E-Mail: Simon.Barrowcliff@element.com
17065:2012 to provide product conformity certification Unit 1 Pendle Place Skelmersdale West Lancashire WN8 9PN Contact: Mr Simon Barrowcliff Tel: +44 (0)1695 556666 Fax: +44 (0)1695 557077 E-Mail: Simon.Barrowcliff@element.com
Guidance for Industry
 MEDICAL DEVICES SECTOR Guidance for Industry Format and Content of Proposed Bundling/Grouping Criteria for Medical Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes
MEDICAL DEVICES SECTOR Guidance for Industry Format and Content of Proposed Bundling/Grouping Criteria for Medical Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes
Table 1 Model of STRIDESMOOTH (Stride) and STRIDESMOOTH+ Microcatheter
 AMM-Q269 Ver.7 Page 2 of 5 Table 1 Model of STRIDESMOOTH (Stride) and STRIDESMOOTH+ Microcatheter Product name STRIDESMOOTH (Stride) Model No. STD105-22S STD125-22S STD125-22A STD130-22S STD150-22S STD105-26S
AMM-Q269 Ver.7 Page 2 of 5 Table 1 Model of STRIDESMOOTH (Stride) and STRIDESMOOTH+ Microcatheter Product name STRIDESMOOTH (Stride) Model No. STD105-22S STD125-22S STD125-22A STD130-22S STD150-22S STD105-26S
Emergency Care Progress Log
 Emergency Care Progress Log For further details on the National Occupational Competencies for EMRs, please visit www.paramedic.ca. Check off each skill once successfully demonstrated the Instructor. All
Emergency Care Progress Log For further details on the National Occupational Competencies for EMRs, please visit www.paramedic.ca. Check off each skill once successfully demonstrated the Instructor. All
Trust Policy 218 Ionising Radiation Safety Policy
 Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
ARIZONA CTE CAREER PREPARATION STANDARDS & MEASUREMENT CRITERIA DENTAL ASSISTING
 DENTAL ASSISTING - 51.0600 1.0 DEMONSTRATE DENTAL OFFICE BUSINESS PROCEDURES 1.1 Explain the importance of patient scheduling depending on treatment time requirement 1.2 Describe the function of a recall
DENTAL ASSISTING - 51.0600 1.0 DEMONSTRATE DENTAL OFFICE BUSINESS PROCEDURES 1.1 Explain the importance of patient scheduling depending on treatment time requirement 1.2 Describe the function of a recall
Active Ingredient in Disinfectants & antiseptics (others)
 Public Health Lab Ministry of Health Active Ingredient in Disinfectants & antiseptics (others) Chemist. Saleh A. El-Taweel Head of Drug Analysis Dept. Introduction During patient treatment, surfaces in
Public Health Lab Ministry of Health Active Ingredient in Disinfectants & antiseptics (others) Chemist. Saleh A. El-Taweel Head of Drug Analysis Dept. Introduction During patient treatment, surfaces in
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
Pulpdent Corporation Revision Date: May 6, 2014 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL VOLUME 2C. Guidelines. Medicinal products for human use
 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels, Draft Revision 2 NOTICE TO APPLICANTS VOLUME 2C Guidelines Medicinal products for human use Safety, environment and information
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels, Draft Revision 2 NOTICE TO APPLICANTS VOLUME 2C Guidelines Medicinal products for human use Safety, environment and information
2.2. Label elements There are no statutory labelling requirements under Directive 1999/45/EC, regulation 1272/2008 and regulation 453/2012.
 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product Identifier 1.2. Relevant identified uses of the substance or mixture and uses advised against Relevant uses:
SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product Identifier 1.2. Relevant identified uses of the substance or mixture and uses advised against Relevant uses:
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
INDEPENDANT CONSULTING FOR QUALITY AND REGULATORY AFFAIRS EXPERTS FOR MEDICAL DEVICES REFERENCES. DATE POSITION ACTIVITIES MEDICAL DEVICES 2009 to NOW
 INDEPENDANT CONSULTING FOR QUALITY AND EXPERTS FOR MEDICAL DEVICES REFERENCES OUTSOURCED SERVICES DATE POSITION ACTIVITIES MEDICAL DEVICES 2009 to NOW QUALITY AND MANAGER company which designs and manufactures
INDEPENDANT CONSULTING FOR QUALITY AND EXPERTS FOR MEDICAL DEVICES REFERENCES OUTSOURCED SERVICES DATE POSITION ACTIVITIES MEDICAL DEVICES 2009 to NOW QUALITY AND MANAGER company which designs and manufactures
Advanced Training Program Infection Prevention and Control By Dr. Ahmad Farouk EBFM, MRCGP, CIC
 Advanced Training Program Infection Prevention and Control By Dr. Ahmad Farouk EBFM, MRCGP, CIC Tel: +973 172 80 8 50 Mobile: +973 343 58 323 Fax: +973 a 11446 Address: BMMI Tower, Office 1423, 14 th Floor,
Advanced Training Program Infection Prevention and Control By Dr. Ahmad Farouk EBFM, MRCGP, CIC Tel: +973 172 80 8 50 Mobile: +973 343 58 323 Fax: +973 a 11446 Address: BMMI Tower, Office 1423, 14 th Floor,
Instructions for use 0483 Permanent and temporary YASARGIL Aneurysm and Vessel Clips
 1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
Complex Medical Devices and Steam Sterilization. By Andrew Gay Director - Sterilizer Validation Australia
 Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
: GLYCEROL FORMAL CRS
 in accordance with Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 14/12/2011 Version: 1.0 SECTION 1: Identification of the substance/mixture
in accordance with Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 14/12/2011 Version: 1.0 SECTION 1: Identification of the substance/mixture
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL
 EN EN EN EUROPEAN COMMISSION Brussels, 27.8.2010 COM(2010) 443 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Report on the issue of the reprocessing of medical devices in
EN EN EN EUROPEAN COMMISSION Brussels, 27.8.2010 COM(2010) 443 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Report on the issue of the reprocessing of medical devices in
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606
 PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
QUESTIONS AND AGREED ANSWERS
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Chemicals, metals, mechanical, electrical and construction industries; Raw materials Chemicals - Classification & Labelling, Specific Products,
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Chemicals, metals, mechanical, electrical and construction industries; Raw materials Chemicals - Classification & Labelling, Specific Products,
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset
 SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
See the benefits table below. None. $2,000 per Member per Calendar Year $4,000 per family per Calendar Year
 Schedule of s Harvard Pilgrim Health Care, Inc. THE HARVARD PILGRIM HMO MAINE ID: MD0000017741_A4 X This Schedule of s states any Limits and Member Cost Sharing amounts you must pay for Covered s. However,
Schedule of s Harvard Pilgrim Health Care, Inc. THE HARVARD PILGRIM HMO MAINE ID: MD0000017741_A4 X This Schedule of s states any Limits and Member Cost Sharing amounts you must pay for Covered s. However,
: HUMAN ALBUMIN FOR ELECTROPHORESIS BRP
 Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 22/08/2008 Version: 6.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product
Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 22/08/2008 Version: 6.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product
SAFETY DATA SHEET. according to 1907/2006/EC, Article 31. Reinforced ZOE Cement Liquid
 SAFETY DATA SHEET according to 907/2006/EC, Article 3 Page /5 date SECTION : Identification of the substance/mixture and of the company/undertaking.. Product identifier Product name.2. Relevant identified
SAFETY DATA SHEET according to 907/2006/EC, Article 3 Page /5 date SECTION : Identification of the substance/mixture and of the company/undertaking.. Product identifier Product name.2. Relevant identified
Extra-corporeal Shock Wave Therapy (ESWT) for plantar fasciitis / heel pain
 Extra-corporeal Shock Wave Therapy (ESWT) for plantar fasciitis / heel pain Source: Trauma & Orthopaedics Reference No: 6460-1 Issue date: 1/2/19 Review date: 1/2/22 Page 1 of 6 Plantar fasciitis / heel
Extra-corporeal Shock Wave Therapy (ESWT) for plantar fasciitis / heel pain Source: Trauma & Orthopaedics Reference No: 6460-1 Issue date: 1/2/19 Review date: 1/2/22 Page 1 of 6 Plantar fasciitis / heel
Sterilization of health care products Radiation. Part 2: Establishing the sterilization dose
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-2 Third edition 2013-06-01 Sterilization of health care products Radiation Part 2: Establishing the sterilization dose Stérilisation des
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11137-2 Third edition 2013-06-01 Sterilization of health care products Radiation Part 2: Establishing the sterilization dose Stérilisation des
Scope of Accreditation of Testing Laboratory (EN ISO/IEC 17025:2005) HygCen Austria GmbH / (ID No.: 0196)
 HygCen Austria GmbH / (ID : 0196) issue title remarks PvO 1 02-030 2008-05 Testing on the cleaning efficacy of chemo-thermal laundry disinfection procedures in laundries 2 11-001 2016-04 Hygienic examination
HygCen Austria GmbH / (ID : 0196) issue title remarks PvO 1 02-030 2008-05 Testing on the cleaning efficacy of chemo-thermal laundry disinfection procedures in laundries 2 11-001 2016-04 Hygienic examination
TONTARRA Medizintechnik GmbH. Surgical Instruments in Demand. Laminectomy Punches and Rongeurs
 TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
S5 Rotary Files. only. Manufacturer. Packing unit. Batch code. For professional use only (0)
 EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
Kidner procedure. For the treatment of accessory navicular. Information for patients Department of Podiatric Surgery
 Kidner procedure For the treatment of accessory navicular Information for patients Department of Podiatric Surgery What is an accessory? An accessory is an additional /bony segment which is not usually
Kidner procedure For the treatment of accessory navicular Information for patients Department of Podiatric Surgery What is an accessory? An accessory is an additional /bony segment which is not usually
Safety Data Sheet ULTRAPLAN RENOVATION
 Safety Data Sheet dated 13/5/2010, version 1 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Trade name: Product type and use: Cement based levelling mortar. Supplier: MAPEI
Safety Data Sheet dated 13/5/2010, version 1 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Trade name: Product type and use: Cement based levelling mortar. Supplier: MAPEI
: ASCORBIC ACID IMPURITY C CRS
 in accordance to Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 04/02/2010 Version: 3.0 SECTION 1: Identification of the substance/mixture and
in accordance to Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 04/02/2010 Version: 3.0 SECTION 1: Identification of the substance/mixture and
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 17510 First edition 2015-08-01 Medical devices Sleep apnoea breathing therapy Masks and application accessories Dispositifs médicaux Thérapie respiratoire de l apnée du sommeil
INTERNATIONAL STANDARD ISO 17510 First edition 2015-08-01 Medical devices Sleep apnoea breathing therapy Masks and application accessories Dispositifs médicaux Thérapie respiratoire de l apnée du sommeil
: FOLIC ACID IMPURITY A CRS
 Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 07/12/2009 Version: 2.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product
Date of issue: 27/06/2013 Revision date: 27/06/2013 Supersedes: 07/12/2009 Version: 2.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product
STANDARD OPERATING PROCEDURE #203 LARGE ANIMAL SURGERY
 STANDARD OPERATING PROCEDURE #203 LARGE ANIMAL SURGERY 1. PURPOSE This Standard Operating Procedure (SOP) describes procedures for general surgery of large animal species such as swine, dogs, rabbits,
STANDARD OPERATING PROCEDURE #203 LARGE ANIMAL SURGERY 1. PURPOSE This Standard Operating Procedure (SOP) describes procedures for general surgery of large animal species such as swine, dogs, rabbits,
Martin Bullemer Key Account Manager Medical 2008 April 16th
 Medical Device e-manufacturing Targets Martin Bullemer Key Account Manager Medical 2008 April 16th The Medical Market for Laser Sintering Every human is different, which results in a wide variety of different
Medical Device e-manufacturing Targets Martin Bullemer Key Account Manager Medical 2008 April 16th The Medical Market for Laser Sintering Every human is different, which results in a wide variety of different
See the benefits table below. $250 per Member per Calendar Year $500 per family per Calendar Year
 Schedule of s HMO MASSACHUSETTS ID: MD0000017703_A9 X This Schedule of s states any Limits and the Member Cost Sharing amounts you must pay for Covered s. However, it is only a summary of your benefits.
Schedule of s HMO MASSACHUSETTS ID: MD0000017703_A9 X This Schedule of s states any Limits and the Member Cost Sharing amounts you must pay for Covered s. However, it is only a summary of your benefits.
: Human Immunoglobulin (Fc Function and Molecular size) BRP
 Human Immunoglobulin (Fc Function and Molecular size) BRP in accordance with Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 : Version: 1.0 SECTION 1: Identification
Human Immunoglobulin (Fc Function and Molecular size) BRP in accordance with Regulation (EC) No. 1907/2006, as amended. Date of issue: 27/06/2013 Revision date: 27/06/2013 : Version: 1.0 SECTION 1: Identification
Controlling Natural Occurring Radioactive Material (NORM) Exposure
 Controlling Natural Occurring Radioactive Material (NORM) Exposure Agenda What is NORM? What is Radiation? Radiation Types Health Effects NORM Oil & Gas Where is NORM Located? How Could I Be Exposed? NORM
Controlling Natural Occurring Radioactive Material (NORM) Exposure Agenda What is NORM? What is Radiation? Radiation Types Health Effects NORM Oil & Gas Where is NORM Located? How Could I Be Exposed? NORM
SEKUROKA Eye first-aid station (Wall box)
 18.12.2013 Kit Components Product code Description CK44 SEKUROKA Eye first-aid station (Wall box) Components: A: (Y828) 1x500ml Disposable eye wash bottle ( saline-solution) B: (AA81) 1x200ml Disposable
18.12.2013 Kit Components Product code Description CK44 SEKUROKA Eye first-aid station (Wall box) Components: A: (Y828) 1x500ml Disposable eye wash bottle ( saline-solution) B: (AA81) 1x200ml Disposable
ASEPT -System ASEPT Pleural/Peritoneal. ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories. Quality and Experience
 Quality and Experience ASEPT -System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Quality and Experience ASEPT -System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
