MEDICAL POLICY MEDICARE ADVANTAGE ONLY (See Product Variations)
|
|
|
- Theodora Newman
- 5 years ago
- Views:
Transcription
1 Original Issue Date (Created): 11/1/2010 Most Recent Review Date (Revised): 1/18/2018 Effective Date: 9/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY I. POLICY Denosumab (Prolia ) Denosumab (Prolia ) may be considered medically necessary for the treatment of osteoporosis in postmenopausal women and men when the patient is receiving supplemental calcium and vitamin D and any ONE of the following indications are met: History of a hip or vertebral (clinical or morphometric) fracture, fragility or low-impact fracture, or multiple risks of fracture (see policy guidelines); or Bone mineral density (BMD) T score of [-2.5] or less at the femoral neck or spine after appropriate evaluation to exclude secondary causes; or The patient has contraindications or is unresponsive to oral osteoporosis agents. Denosumab (Prolia ) may be considered medically necessary for the following cancerrelated indications: To increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer; or To increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer. Denosumab (Prolia ) for all other indications than those listed above is considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this drug. Page 1
2 Denosumab (Xgeva ) Denosumab (Xgeva ) may be considered medically necessary for the following: Prevention of skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors. Treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity. Treatment of hypercalcemia of malignancy refractory to bisphosphonate therapy Denosumab (Xgeva ) for all other indications than those listed is considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this drug. Policy Guidelines Patients receiving Prolia should not receive Xgeva - same active ingredient. All postmenopausal women and men age 50 and older should be evaluated for osteoporosis risk in order to determine the need for BMD testing and/or vertebral imaging. In general, the more risk factors that are present, the greater the risk of fracture. The FRAX tool has been developed by WHO to evaluate fracture risk of patients. It is based on individual patient models that integrate the risks associated with clinical risk factors as well as bone mineral density (BMD) at the femoral neck. Use of WHO Fracture Risk Algorithm (FRAX ) in the U.S. FRAX was developed to calculate the 10-year probability of a hip fracture and the 10- year probability of a major osteoporotic fracture (defined as clinical vertebral, hip, forearm or proximal humerus fracture) taking into account femoral neck BMD and the clinical risk factors shown in the table below. The FRAX algorithm is available at and at It is also available on newer DXA machines or with software upgrades that provide the FRAX scores on the bone density report. Page 2
3 FRAX is most useful in patients with low femoral neck BMD. Utilizing FRAX in patients with low BMD at the lumbar spine but a relatively normal BMD at the femoral neck underestimates fracture risk in these individuals. Specifically, the WHO algorithm has not been validated for the use of lumbar spine BMD. NOF recommends treatment of individuals with osteoporosis of the lumbar spine as well as the hip. Application of U.S.-FRAX in the U.S FRAX is intended for postmenopausal women and men age 50 and older; it is not intended for use in younger adults or children, however the FRAX tool has been validated for use in men and women from age 40 to 90. The FRAX tool has not been validated in patients currently or previously treated with pharmacotherapy for osteoporosis. In such patients, clinical judgment must be exercised in interpreting FRAX scores. The following examples of untreated patients are offered21: a) No ET/HT or estrogen agonist/antagonist (SERM) for the past one year b) No calcitonin for the past one year c) No PTH for the past one year d) No denosumab for the past one year e) No bisphosphonate for the past two years (unless it is an oral taken for <2 months) Note: Calcium and vitamin D do NOT constitute treatment in this context. FRAX can be calculated with either femoral neck BMD or total hip BMD but when available, femoral neck BMD is preferred. The use of BMD from non-hip sites is not recommended. The WHO determined that for many causes of secondary osteoporosis, fracture risk was mediated primarily through impact on BMD. For this reason, when femoral neck BMD is inserted into FRAX, the secondary osteoporosis button is automatically inactivated Conditions, Diseases and Medications That Cause or Contribute to Osteoporosis and Fractures Lifestyle factors Alcohol Abuse High salt intake Falling Low calcium intake Inadequate physical activity Excessive thinness Vitamin D insufficiency Immobilization Excess Smoking (active or passive) Page 3
4 Genetic factors Cystic fibrosis Homocystinuria Osteogenesis imperfect Ehlers-Danlos Hypophosphatasia Parental history of hip fracture Gaucher s disease Idiopathic hypercalciuria Porphyria Glycogen storage diseases Marfan syndrome Riley-Day syndrome Hemochromatosis Hypogonadal states Menkes steely hair syndrome Androgen insensitivity Hyperprolactinemia Premature ovarian failure Anorexia nervosa and bulimia Premature menopause Athletic amenorrhea Turner s & Klinefelter s syndromes Panhypopituitarism Endocrine disorders Adrenal insufficiency Cushing s syndrome Central Adiposity Diabetes mellitus (Types 1 & 2) Hyperparathyroidism Thyrotoxicosis Gastrointestinal disorders Celiac disease Inflammatory bowel disease Primary biliary cirrhosis Gastric bypass GI surgery Hematologic disorders Malabsorption Pancreatic disease Multiple myeloma Monoclonal gammopathies Sickle cell disease Hemophilia Leukemia and lymphomas Systemic mastocytosis Thalassemia Rheumatologic and autoimmune diseases Ankylosing spondylitis Lupus Rheumatoid arthritis Other rheumatic and autoimmune diseases Central nervous system disorders Epilepsy Parkinson s disease Stroke Multiple sclerosis Spinal cord injury Miscellaneous conditions and diseases AIDS/HIV Congestive heart failure Muscular dystrophy Page 4
5 Alcoholism Depression Post-transplant bone disease Amyloidosis End stage renal disease Sarcoidosis Chronic metabolic acidosis Hypercalciuria Weight loss Chronic obstructive lung disease Idiopathic scoliosis Medications Aluminum (in antacids) Cyclosporine A and tacrolimus Proton pump inhibitors Anticoagulants (heparin) Depo-medroxyprogesterone Selective serotonin reuptake (premenopausal inhibitors contraception) Anticonvulsants Aromatase inhibitors Barbiturates Glucocorticoids ( 5 mg/d prednisone or equivalent for 3 months) GnRH (Gonadotropin releasing hormone) antagonists and agonists Lithium Cancer chemotherapeutic Methotrexate drugs From: The Surgeon General s Report, with modification Cross-references: Tamoxifen (premenopausal use) Thiazolidinediones (such as Actos and Avandia ) Thyroid hormones (in excess) Parenteral nutrition MP Off-Label Use of Medications MP Bone Turnover Markers For Diagnosis and Management of Osteoporosis and Diseases Associated with High Bone Turnover MP Zoledronic Acid (Reclast, Zometa ) MP Whole Body Dual X-ray Absorptiometry to Determine Body Composition II. PRODUCT VARIATIONS TOP This policy is only applicable to Medicare Advantage products administered by Capital BlueCross. For all other products, please refer to Prime Therapeutics policy for any preauthorization and medical necessity requirements related to drugs covered under a member s medical benefit. Page 5
6 Notes: 1. FDA approved drugs used for indications other than what is indicated on the FDA approved product label may be covered under Medicare if it is determined that the use is medically accepted, taking into consideration the Medicare recognized national drug compendia, authoritative medical literature and/or accepted standards of medical practice. Refer to Medicare Benefit Policy Manual (100-2, Chapter 15, Section Unlabeled Use of Drug) In accordance with CMS letter issued on September 17, 2012, entitled Prohibition on Imposing Mandatory Step Therapy for Access to Part B Drugs and Services. Step therapy that is not part of the FDA label does not apply to Medicare Advantage. III. DESCRIPTION/BACKGROUND Denosumab (Prolia ) TOP Osteoporosis is a condition characterized by a loss of bone mass that occurs throughout the skeleton, which predisposes a patient to fractures. The Dual energy X-ray absorptiopmetry (DEXA) measures bone mineral density with results reported as a T- score. T-scores are reported according to standard deviations (SD) of World Health Criteria. The BMD diagnosis of normal, low bone mass, osteoporosis and severe or established osteoporosis is based on the WHO diagnostic classification: Normal: - T-score within 1 SD of a young normal adult Osteopenia-T-score of -1 to 2.5 SD below that of a young normal adult Osteoporosis- T-score of -2.5 or less SD below that of a young normal adult Severe osteoporosis- T-score of -2.5 or less SD below normal with fragility fractures Denosumab (Prolia, Amgen) is the first monoclonal antibody approved in the U.S. for the treatment of postmenopausal osteoporosis.. The drug was approved by the U.S. Food and Drug Administration (FDA) on June 1, 2010 It inhibits osteoclast-mediated bone resorption; thus, it is an antiresorptive agent like the bisphosphonates but has a different mechanism of action. Denosumab is a fully human monoclonal antibody which specifically targets the receptor activity of nuclear factor kappa B ligand (RANKL). RANKL is a protein which is essential for the formation, survival and function of osteoclasts, which are the cells responsible for bone resorption. On September 19, 2011, the U.S. Food and Drug Administration (FDA) approved two new indications for Prolia (denosumab) as a treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer and Page 6
7 as a treatment to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for non-metastatic prostate cancer. On September. 20, 2012 the U.S. Food and Drug Administration (FDA) approved Prolia (denosumab) as a treatment to increase bone mass in men with osteoporosis at high risk for fracture. Prolia, the first FDA-approved RANK Ligand inhibitor, is a subcutaneous injection administered by a health care professional every six months. While osteoporosis and osteoporosis-related fractures are more commonly associated with postmenopausal women, osteoporosis in men is a significant issue that is increasing in prevalence as life expectancies rise, According to the National Osteoporosis Foundation, two million men in the U.S. have osteoporosis and another 12 million are at risk. Osteoporosis and osteoporotic fractures in men remain under diagnosed and under treated. Denosumab (Xgeva ) Xgeva is a monoclonal antibody that targets a protein involved in cancer-related bone destruction called human RANKL. Other FDA-approved drugs for similar conditions include Zometa (zoledronic acid) and Aredia (pamidronate disodium). Xgeva is not indicated for the prevention of skeletal-related events in patients with multiple myeloma. Administer calcium and vitamin D as necessary to treat or prevent hypocalcemia. Xgeva is available as a 120mg/1.7mL single-use vial. Skeletal-related events (SRE s) On November 19, 2010, the U.S. Food and Drug Administration (FDA) approved Amgen s (Xgeva ) (denosumab) for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors. Skeletal-related events include bone fractures from cancer and bone pain requiring radiation. For patients with bone metastasis from solid tumors, XGEVA is administered as a subcutaneous injection (120 mg) every four weeks in the upper arm, upper thigh, or abdomen. On January 5, 2018, the U.S. Food and Drug Administration (FDA) approved the supplemental Biologics License Application (sbla) for XGEVA (denosumab) to expand the currently approved indication for the prevention of skeletal-related events in patients with bone metastases from solid tumors to include patients with multiple myeloma. The approval is based on data from the pivotal Phase 3 '482 study, the largest international multiple myeloma clinical trial ever conducted, which enrolled 1,718 patients. Page 7
8 Hypercalcemia of malignancy On Dec. 8, 2014 the U.S. Food and Drug Administration (FDA) approved Xgeva (denosumab) for the treatment of hypercalcemia of malignancy (HCM) refractory to bisphosphonate therapy. Xgeva was approved and granted Orphan Drug Designation by the FDA, which is reserved for drugs that are intended for the treatment of rare diseases affecting fewer than 200,000 people in the U.S. HCM is a serious complication in patients with advanced cancer, including those with hematologic malignancies, and indicates poor prognosis. 1, 2 The condition results from cancer-driven increases in bone resorption, and if untreated, can lead to renal failure, progressive mental impairment, coma and death. For patients with hypercalcemia of malignancy, XGEVA is administered as a subcutaneous injection (120 mg) every four weeks with additional 120 mg doses on days eight and 15 of the first month of therapy Giant cell tumor of bone On June 13, 2013 the U.S. Food and Drug Administration (FDA) approved XGEVA (denosumab) for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone (GCTB) that is unresectable or where surgical resection is likely to result in severe morbidity. XGEVA was approved following a priority review by the FDA, a designation reserved for drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. GCTB typically affects individuals between the ages of 20 to 40. The disease is characterized by a bone destructive tumor that often results in fractures. When untreated, it often results in complete destruction of the affected bone, leading to bone fracture, joint dysfunction, deformity or amputation. For patients with GCTB, XGEVA is administered as a subcutaneous injection (120 mg) every four weeks with additional 120 mg doses on days eight and 15 of the first month of therapy. IV. RATIONALE TOP For information on clinical studies for denosumab (Prolia and Xgeva), refer to the Prescribing Information. Page 8
9 V. DEFINITIONS TOP BONE RESORPTION is bone loss due to osteoclastic activity. FRACTURE is a traumatic injury to a bone in which the continuity of the bone tissue is broken. MENOPAUSE cessation of menses as a result of failure of follicular maturation in the presence of elevated gonadotropin. OSTEOCLASTIC refers to osteoclasts, especially with reference to their activity in the absorption and removal of osseous (bone) tissue. OSTEOCLASTS are large multinucleated cells formed from differentiated macrophages that are responsible for the breakdown of bone. VI. BENEFIT VARIATIONS TOP The existence of this medical policy does not mean that this service is a covered benefit under the member's contract. Benefit determinations should be based in all cases on the applicable contract language. Medical policies do not constitute a description of benefits. A member s individual or group customer benefits govern which services are covered, which are excluded, and which are subject to benefit limits and which require preauthorization. Members and providers should consult the member s benefit information or contact Capital BlueCross for benefit information. VII. DISCLAIMER TOP Capital BlueCross s medical policies are developed to assist in administering a member s benefits, do not constitute medical advice and are subject to change. Treating providers are solely responsible for medical advice and treatment of members. Members should discuss any medical policy related to their coverage or condition with their provider and consult their benefit information to determine if the service is covered. If there is a discrepancy between this medical policy and a member s benefit information, the benefit information will govern. Capital BlueCross considers the information contained in this medical policy to be proprietary and it may only be disseminated as permitted by law. VIII. CODING INFORMATION TOP Note: This list of codes may not be all-inclusive, and codes are subject to change at any time. The identification of a code in this section does not denote coverage as Page 9
10 coverage is determined by the terms of member benefit information. In addition, not all covered services are eligible for separate reimbursement. Covered when medically necessary: HCPCS Code Description J0897 Injection, denosumab, 1 mg (i.e., Prolia, Xgeva ) The following diagnoses are considered medically necessary for Prolia. All other indications are considered investigational, and therefore will not be covered. ICD-10-CM Diagnosis Description Codes C Malignant neoplasm of nipple and areola, right female breast C Malignant neoplasm of nipple and areola, left female breast C Malignant neoplasm of nipple and areola, unspecified female breast C Malignant neoplasm of central portion of right female breast C Malignant neoplasm of central portion of left female breast C Malignant neoplasm of central portion of unspecified female breast C Malignant neoplasm of upper-inner quadrant of right female breast C Malignant neoplasm of upper-inner quadrant of left female breast C Malignant neoplasm of upper-inner quadrant of unspecified female breast C Malignant neoplasm of lower-inner quadrant of right female breast C Malignant neoplasm of lower-inner quadrant of left female breast C Malignant neoplasm of lower-inner quadrant of unspecified female breast C Malignant neoplasm of upper-outer quadrant of right female breast C Malignant neoplasm of upper-outer quadrant of left female breast C Malignant neoplasm of upper-outer quadrant of unspecified female breast C Malignant neoplasm of lower-outer quadrant of right female breast C Malignant neoplasm of lower-outer quadrant of left female breast Page 10
11 ICD-10-CM Diagnosis Codes Description C Malignant neoplasm of lower-outer quadrant of unspecified female breast C Malignant neoplasm of axillary tail of right female breast C Malignant neoplasm of axillary tail of left female breast C Malignant neoplasm of axillary tail of unspecified female breast C Malignant neoplasm of overlapping sites of right female breast C Malignant neoplasm of overlapping sites of left female breast C Malignant neoplasm of overlapping sites of unspecified female breast C Malignant neoplasm of unspecified site of right female breast C Malignant neoplasm of unspecified site of left female breast C Malignant neoplasm of unspecified site of unspecified female breast C61 Malignant neoplasm of prostate C79.81 Secondary malignant neoplasm of breast D29.1 Benign neoplasm of prostate D49.3 Neoplasm of unspecified behavior of breast M80.00XA M80.00XD M80.00XG M80.00XK M80.00XP M80.00XS Age-related osteoporosis with current pathological fracture, unspecified site, initial encounter for fracture Age-related osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with routine healing Age-related osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with delayed healing Age-related osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with nonunion Age-related osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with malunion Age-related osteoporosis with current pathological fracture, unspecified site, sequela Page 11
12 ICD-10-CM Diagnosis Codes M80.08XA M80.08XD M80.08XG M80.08XK M80.08XP M80.08XS M80.80XA M80.80XD M80.80XG M80.80XK M80.80XP M80.80XS M80.88XA M80.88XD M80.88XG Description Age-related osteoporosis with current pathological fracture, vertebra(e), initial encounter for fracture Age-related osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with routine healing Age-related osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with delayed healing Age-related osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with nonunion Age-related osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with malunion Age-related osteoporosis with current pathological fracture, vertebra(e), sequela Other osteoporosis with current pathological fracture, unspecified site, initial encounter for fracture Other osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with routine healing Other osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with delayed healing Other osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with nonunion Other osteoporosis with current pathological fracture, unspecified site, subsequent encounter for fracture with malunion Other osteoporosis with current pathological fracture, unspecified site, sequela Other osteoporosis with current pathological fracture, vertebra(e), initial encounter for fracture Other osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with routine healing Other osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with delayed healing Page 12
13 ICD-10-CM Diagnosis Codes M80.88XK M80.88XP M80.88XS Description Other osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with nonunion Other osteoporosis with current pathological fracture, vertebra(e), subsequent encounter for fracture with nonunion Other osteoporosis with current pathological fracture, vertebra(e), sequela M81.0 Age-related osteoporosis without current pathological fracture M81.6 Localized osteoporosis [Lequesne] M81.8 Other osteoporosis without current pathological fracture M85.80 Other specified disorders of bone density and structure, unspecified site M85.88 Other specified disorders of bone density and structure, other site M85.89 Other specified disorders of bone density and structure, multiple sites M85.9 "Disorder of bone density and structure, unspecified Z85.3 Personal history of malignant neoplasm of breast Z Personal history of (healed) osteoporosis fracture Z Personal history of (healed) other pathological fracture Z Personal history of (healed) stress fracture Z Long term (current) use of aromatase inhibitors Z Long term (current) use of other agents affecting estrogen receptors and estrogen levels The following diagnoses are considered medically necessary for Xgeva. All other indications are considered investigational, and therefore will not be covered. ICD-10-CM Diagnosis Codes Description C79.51 Secondary malignant neoplasm of bone C90.00 Multiple myeloma not having achieved remission Page 13
14 ICD-10-CM Diagnosis Description Codes C90.01 Multiple myeloma in remission C90.02 Multiple myeloma in relapse D48.0 Neoplasm of uncertain behavior of bone and articular cartilage E83.52 Hypercalcemia IX. REFERENCES TOP Centers for Medicare and Medicaid Services (CMS) Medicare Benefit Policy Manual. Publication Chapter 15. Section Unlabeled Use of Drug. Effective 10/01/03. [Website]: Accessed January 18, Centers for Medicare and Medicaid Services (CMS) Medicare Benefit Policy Manual. Publication Chapter 15. Section Off-Label Use of Anti- Cancer Drugs and Biologicals. [Website]: Accessed January 18, Centers for Medicare and Medicaid Services (CMS) Medicare Benefit Policy Manual Publication Chapter 15 Sections 50, , Drugs and Biologicals Effective 10/01/03. [Website]: Accessed January 18, Taber s Cyclopedic Medical Dictionary 20th edition Denosumab (Prolia ) BCBSA Specialty Pharmacy Combined Capacity (SPCC) Report # Denosumab (Prolia ) December Cummings SR, San Martin J, et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N Engle J Med 361; 8 August 20, FRAX WHO Fracture Risk Assessment Tool [Website]: Accessed January 18, Lerwicki, M. Ostéoporotic Fracture Risk Assessment In: UpToDate Online Journal [serial online]. Waltham, MA: UpToDate; updated January 4, [Website]: Accessed January 18, Page 14
15 National Institute for Health and Clinical Excellence (NICE). Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Clinical Guidance TA204. October [Website]: Accessed January 18, Prolia (denosumab) prescribing information. Revised 01/17.Prolia [Website]: Accessed January 18, WHO Fracture risk assessment tool. [Website]: Accessed January 18, Denosumab (Xgeva ) BCBSA Specialty Pharmacy Combined Capacity (SPCC) Report #8/15 Denosumab (Prolia.Xgeva ) December Smith MR, Egerdie B, et al. Denosumab in Men Receiving Androgen-Deprivation Therapy for Prostate Cancer N Engl J Med 361; 8 August 20, 2009 The National Osteoporosis Foundation [Website]: Accessed January 18, PR Newswire. January 5, FDA Approves XGEVA (denosumab) For The Prevention Of Skeletal-Related Events In Patients With Multiple Myeloma. [Website]: Accessed January 18, Xgeva (denosumab) prescribing information. Revised 01/18..Xgeva [Website]: Accessed January 18, X. POLICY HISTORY TOP CAC 9/27/10 New Policy. CAC 10/25/11 Consensus review. CAC 11/22/11 Minor revision to add recent (9/19/2011 ) FDA approved cancer-related indications for treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer and as a treatment to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for non-metastatic prostate cancer. These indications will not require preauthorization. CAC 10/30/12 Consensus review. References updated but no changes to the policy statements. Medicare variation revised to state step therapy Page 15
16 does not apply. Codes reviewed 10/25/12 CAC 7/30/13 Minor. Denosumab (Prolia ) and Denosumab (Xgeva ) combined into one policy. MP Denosumab (Xgeva ) retired. Indications for treatment of men with osteoporosis added as a medically necessary indication for Prolia. Treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity added as an indication for Xgeva. Admin code review. CAC 3/25/14 Consensus review. References updated. No changes to the policy statements. Rationale added. Medicare variation revised. Admin code review. Added Admin change 7/1/14 deleted notation regarding preauthorization requirement. All Users should refer to officially posted preauthorization resources for requirements. CAC 3/24/15 Minor. Added hypercalcemia of malignancy as a medically necessary indication for Xgeva. Other policy statements unchanged. References and rationale updated. CAC 1/26/16 Coding review. No changes to the policy statements. References updated. FEP variation revised to refer to the FEP medical policy manual. Coding updated. Admin update 1/1/17: Product variation section reformatted CAC 3/28/17 Consensus review. No changes to the policy statements. References updated. Coding reviewed. Coding updated 4/20/17 Admin update 9/1/17: Administrative wording corrections to policy statement. Admin update 6/1/18: Effective 6/1/18 for Medicare Advantage only. See Prime Therapeutics for all other products. 1/18/18 Minor revision. Policy revised to add the new FDA-approved indication for the prevention of skeletal-related events in patients with multiple myeloma. Previously, this indication had been considered investigational. Background and references updated. Rationale section revised to refer to the prescribing information for the clinical studies. Coding reviewed. Effective 9/1/18. Top Health care benefit programs issued or administered by Capital BlueCross and/or its subsidiaries, Capital Advantage Insurance Company, Capital Advantage Assurance Company and Keystone Health Plan Central. Independent licensees of the BlueCross BlueShield Association. Communications issued by Capital BlueCross in its capacity as administrator of programs and provider relations for all companies. Page 16
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
IEHP UM Subcommittee Approved Authorization Guidelines DEXA Scan
 Policy: IEHP UM Subcommittee Approved Authorization Guidelines IEHP considers bone mineral density testing using DEXA medically necessary for members who meet any of the following criteria: Women aged
Policy: IEHP UM Subcommittee Approved Authorization Guidelines IEHP considers bone mineral density testing using DEXA medically necessary for members who meet any of the following criteria: Women aged
denosumab (Prolia ) Policy # Original Effective Date: 07/21/2011 Current Effective Date: 04/19/2017
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Based on review of available data, the Company may consider the use of denosumab (Prolia) for the
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy: Denosumab (Prolia, Xgeva) Reference Number: CP.PHAR.58
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Case Finding and Risk Assessment for Osteoporosis
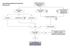 Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Prior Authorization Required: Yes as shown below
 PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 : assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
: assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Xgeva. Xgeva (denosumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Practical Management Of Osteoporosis
 Practical Management Of Osteoporosis CONFERENCE 2012 Education Centre, Bournemouth.19 November The following companies have given funding towards the cost of this meeting but have no input into the agenda
Practical Management Of Osteoporosis CONFERENCE 2012 Education Centre, Bournemouth.19 November The following companies have given funding towards the cost of this meeting but have no input into the agenda
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Xgeva. Xgeva (denosumab) Description. Section: Prescription Drugs Effective Date: January 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
BMD: A Continuum of Risk WHO Bone Density Criteria
 Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Bone Mineral Density Studies in Adult Populations
 Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 1/1/2012 Most Recent Review Date (Revised): 1/18/2018 Effective Date: 8/1/2018 RETIRED POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 1/1/2012 Most Recent Review Date (Revised): 1/18/2018 Effective Date: 8/1/2018 RETIRED POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Medication Policy Manual. Topic: Prolia, denosumab Date of Origin: August 11, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
Bone Mass Measurement BONE MASS MEASUREMENT HS-042. Policy Number: HS-042. Original Effective Date: 8/25/2008
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
BREAST CANCER AND BONE HEALTH
 BREAST CANCER AND BONE HEALTH Rowena Ridout, MD, FRCPC Toronto Western Hospital Osteoporosis Program University Health Network / Mount Sinai Hospital rowena.ridout@uhn.ca None to declare Conflicts of Interest
BREAST CANCER AND BONE HEALTH Rowena Ridout, MD, FRCPC Toronto Western Hospital Osteoporosis Program University Health Network / Mount Sinai Hospital rowena.ridout@uhn.ca None to declare Conflicts of Interest
Osteoporosis. When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of.
 Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Bone (Mineral) Density Studies
 Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section: Radiology Place(s)
Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section: Radiology Place(s)
Disclosure and Conflicts of Interest Steven T Harris MD Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis
 Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Contractor Number 03201
 Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.
![[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4. [If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.](/thumbs/86/94943423.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
3. Has bone specific alkaline phosphatase level increased OR does the member have symptoms related to active Paget s?
 Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Osteoporosis and Bone Health. Heather Schickedanz, MD Geriatric Knowledge Network, 08/10/16
 Osteoporosis and Bone Health Heather Schickedanz, MD Geriatric Knowledge Network, 08/10/16 1 Learning Objectives Recognize the risk factors for osteoporosis Diagnose and treat osteoporosis Reduce the risk
Osteoporosis and Bone Health Heather Schickedanz, MD Geriatric Knowledge Network, 08/10/16 1 Learning Objectives Recognize the risk factors for osteoporosis Diagnose and treat osteoporosis Reduce the risk
Pharmacy Management Drug Policy
 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
FYI ONLY Generic Name. Generics available. zoledronic acid N/A
 Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Sarena Ravi MD, MPH Endocrinologist. Franciscan Physicians Network Division of Endocrinology Chicago, IL
 Sarena Ravi MD, MPH Endocrinologist Franciscan Physicians Network Division of Endocrinology Chicago, IL Definition & Diagnosis of Osteoporosis Management of Osteoporosis in all Populations Long term Management
Sarena Ravi MD, MPH Endocrinologist Franciscan Physicians Network Division of Endocrinology Chicago, IL Definition & Diagnosis of Osteoporosis Management of Osteoporosis in all Populations Long term Management
Bone (Mineral) Density Studies
 Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2014 Section: Radiology Place(s) of Service:
Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2014 Section: Radiology Place(s) of Service:
Clinical Practice. Presented by: Internist, Endocrinologist
 Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents
 AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
New Developments in Osteoporosis: Screening, Prevention and Treatment
 Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Bone Densitometry Pathway
 Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Cross-reference: MP Whole Body Dual X-Ray Absorptiometry (DEXA) to Determine Body Composition MP Bone Mineral Density
 Original Issue Date (Created): April 26, 2011 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Screening for vertebral fractures using dual x-ray absorptiometry
Original Issue Date (Created): April 26, 2011 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Screening for vertebral fractures using dual x-ray absorptiometry
Subject: Denosumab (Prolia ; Xgeva ) Injection
 09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Osteoporosis. Open Access. John A. Kanis. Diseases, University of Sheffield, UK
 Journal of Medical Sciences (2010); 3(3): 00-00 Review Article Osteoporosis Open Access John A. Kanis WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK incorporated into
Journal of Medical Sciences (2010); 3(3): 00-00 Review Article Osteoporosis Open Access John A. Kanis WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK incorporated into
Metabolic Bone Disease and the Gastroenterologist
 VOLUME 8, ISSUE 3, YEAR 2009 Metabolic Bone Disease and the Gastroenterologist Peter R. McNally, DO, FACP, FACG University Colorado at Denver, School of Medicine, Center for Human Simulation Series Introduction:
VOLUME 8, ISSUE 3, YEAR 2009 Metabolic Bone Disease and the Gastroenterologist Peter R. McNally, DO, FACP, FACG University Colorado at Denver, School of Medicine, Center for Human Simulation Series Introduction:
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Clinical Appropriateness Guidelines: Advanced Imaging
 Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Use of DXA / Bone Density in the Care of Your Patients. Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist
 Use of DXA / Bone Density in the Care of Your Patients Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist Important Websites Resources for Clinicians and Patients www.nof.org www.iofbonehealth.org
Use of DXA / Bone Density in the Care of Your Patients Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist Important Websites Resources for Clinicians and Patients www.nof.org www.iofbonehealth.org
WCHQ Ambulatory Measure Specification Screening For Osteoporosis Measurement Period 07/01/16-06/30/17 Submission Period: 09/05/17-10/20/17
 MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Beyond the Break. After Breast Cancer: Osteoporosis in Survivorship. Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO
 Beyond the Break After Breast Cancer: Osteoporosis in Survivorship Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO Disclosures No disclosures Osteoporosis in Breast Cancer Survivorship
Beyond the Break After Breast Cancer: Osteoporosis in Survivorship Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO Disclosures No disclosures Osteoporosis in Breast Cancer Survivorship
Prolia /Xgeva (denosumab) Document Number: IC-0098
 / (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
/ (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
COURSE OUTLINE - Module I
 Module I MEDICAL DISCLAIMER The information in this program is for educational purposes only. It is meant to as a guide towards health and does not replace the evaluation by and advice of a qualified licensed
Module I MEDICAL DISCLAIMER The information in this program is for educational purposes only. It is meant to as a guide towards health and does not replace the evaluation by and advice of a qualified licensed
Pediatric Use: Safety and effectiveness of Ustekinumab (STELARA ) in pediatric patients have not been evaluated.
 Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
MEDICAL POLICY I. POLICY POLICY TITLE POLICY NUMBER CANAKINUMAB (ILARIS ) MP-2.147
 Original Issue Date (Created): May 1, 2010 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Preauthorization is required for injectable Canakinumab (Ilaris ): Note:
Original Issue Date (Created): May 1, 2010 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Preauthorization is required for injectable Canakinumab (Ilaris ): Note:
Major Recommendations Recommendations apply to postmenopausal women and men age 50 and older.
 Clinical Practice Guideline Effective August 2007 (Revised 10/09) This guideline is based upon the guideline developed by an expert committee of the National Osteoporosis Foundation (NOF) in collaboration
Clinical Practice Guideline Effective August 2007 (Revised 10/09) This guideline is based upon the guideline developed by an expert committee of the National Osteoporosis Foundation (NOF) in collaboration
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Approval of a drug under this criteria document does not ensure full coverage of the drug.
 Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Current and Emerging Strategies for Osteoporosis
 Current and Emerging Strategies for Osteoporosis I have nothing to disclose. Anne Schafer, MD Assistant Professor of Medicine Division of Endocrinology & Metabolism December 12, 2014 Outline Osteoporosis
Current and Emerging Strategies for Osteoporosis I have nothing to disclose. Anne Schafer, MD Assistant Professor of Medicine Division of Endocrinology & Metabolism December 12, 2014 Outline Osteoporosis
Interpreting DEXA Scan and. the New Fracture Risk. Assessment. Algorithm
 Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
Management of Osteoporosis Clinical Practice Guideline September 2013
 Management of Osteoporosis Clinical Practice Guideline September 2013 MedStar Health and MedStar Family Choice accept and endorse the clinical guidelines set forth by the National Osteoporosis Foundation
Management of Osteoporosis Clinical Practice Guideline September 2013 MedStar Health and MedStar Family Choice accept and endorse the clinical guidelines set forth by the National Osteoporosis Foundation
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Prevalence of Osteoporosis 5/3/2017. Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist
 Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist Prevalence of Osteoporosis 1.5 million fractures annually in the U.S. Overall lifetime risk for an osteoporotic fracture is about
Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist Prevalence of Osteoporosis 1.5 million fractures annually in the U.S. Overall lifetime risk for an osteoporotic fracture is about
Osteoporosis: fragility fracture risk. Costing report. Implementing NICE guidance
 Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options
 ISPUB.COM The Internet Journal of Academic Physician Assistants Volume 1 Number 1 Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options K Ihrke Citation K Ihrke.. The Internet Journal of
ISPUB.COM The Internet Journal of Academic Physician Assistants Volume 1 Number 1 Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options K Ihrke Citation K Ihrke.. The Internet Journal of
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Skeletal Manifestations
 Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb ligand
 Page 2 of 1765 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Denosumab (AMG 162) Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb
Page 2 of 1765 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Denosumab (AMG 162) Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT
 NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
the health outcomes or benefits associated with this procedure.
 Original Issue Date (Created): October 4, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Cognitive rehabilitation may be considered medically necessary for
Original Issue Date (Created): October 4, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Cognitive rehabilitation may be considered medically necessary for
Aromatase Inhibitors & Osteoporosis
 Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Quality ID #39 (NQF 0046): Screening for Osteoporosis for Women Aged Years of Age National Quality Strategy Domain: Effective Clinical Care
 Quality ID #39 (NQF 0046): Screening for Osteoporosis for Women Aged 65-85 Years of Age National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #39 (NQF 0046): Screening for Osteoporosis for Women Aged 65-85 Years of Age National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bone_mineral_density_studies 12/1996 9/2017 9/2018 9/2017 Description of Procedure or Service Bone density
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bone_mineral_density_studies 12/1996 9/2017 9/2018 9/2017 Description of Procedure or Service Bone density
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR XGEVA. Notes
 For prevention of skeletal-related events XGEVA is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors. XGEVA is not indicated for the prevention
For prevention of skeletal-related events XGEVA is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors. XGEVA is not indicated for the prevention
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment. William D. Leslie, MD MSc FRCPC
 Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Clinician s Guide to Prevention and Treatment of Osteoporosis
 Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Learning Objectives. Controversies in Osteoporosis Prevention and Management. Etiology. Presenter Disclosure Information. Epidemiology.
 12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now?
 Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now? Steven M. Petak, MD, JD, FACE, FCLM Texas Institute for Reproductive Medicine And Endocrinology, Houston, Texas
Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now? Steven M. Petak, MD, JD, FACE, FCLM Texas Institute for Reproductive Medicine And Endocrinology, Houston, Texas
Breast Cancer and Bone Loss. One in seven women will develop breast cancer during a lifetime
 Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
