Breast Reconstruction Following Mastectomy or Lumpectomy
|
|
|
- Gary Dennis
- 6 years ago
- Views:
Transcription
1 Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to go to Comunicados a Proveedores, and click Cartas Circulares.] Medical Policy: MP-SU Original Effective Date: March 25, 2010 Revised: June 01, 2017 Next Revision: June, 2018 This policy applies to products subscribed by the following corporations, MCS Life Insurance Company (Commercial), and MCS Advantage, Inc. (Classicare) and, provider s contract; unless specific contract limitations, exclusions or exceptions apply. Please refer to the member s benefit certification language for benefit availability. Managed care guidelines related to referral authorization, and precertification of inpatient hospitalization, home health, home infusion and hospice services apply subject to the aforementioned exceptions. DESCRIPTION For the purposes of this medical policy, reconstructive breast surgery refers to surgery performed to individuals who underwent mastectomy or lumpectomy to correct or repair abnormal structures of the breast. Breast reconstruction is often considered after mastectomy to correct deformity or reestablish symmetry caused by previous surgery and/or the effects of therapeutic treatments. Reconstruction procedures may involve multiple techniques and stages to recreate the breast mound using prosthetic implants, tissue flaps, or autologous tissue transfers, as well as nipple/areola reconstruction or tattooing and breast reduction. These procedures can be performed immediately after a mastectomy (one stage breast reconstruction), or be delayed for weeks or years until a patient undergoes radiation, chemotherapy, or determines whether they want breast reconstruction (two-stage reconstruction). COVERAGE Benefits may vary between groups and contracts. Please refer to the appropriate member certificate and subscriber agreement contract for applicable diagnostic imaging, DME, laboratory, machine tests, benefits, and coverage. INDICATIONS Note 1 : Breast Reconstruction services of the affected and the contra-lateral unaffected breast following a mastectomy or lumpectomy are considered medically necessary for all stages of reconstruction of the breast on which the mastectomy or lumpectomy has been performed; when surgery and reconstruction 1
2 of the other breast is to produce a symmetrical appearance; and for prostheses and physical complications of all stages of mastectomy or lumpectomy, including lymphedema. For clarification purposes, in this medical policy, breast reconstruction will be consider medically necessary and cover after a lumpectomy only when the lumpectomy was performed for a diagnosis of breast cancer. I. (MCS) will consider medically necessary Breast Reconstruction services of the affected breast after a mastectomy performed for any medical reason or lumpectomy performed for a diagnosis of breast cancer. Medically necessary procedures include: a. Tissue/muscle reconstruction procedures (flaps); transverse rectus abdominus myocutaneous flap; latissimus dorsi (LD) myocutaneous flap; deep inferior epigastric perforator (DIEP) flap; superficial inferior epigastric perforator (SIEP)flap; superior or inferior gluteal free flap; and Ruben s flap b. Capsulotomy i c. Capsulectomy ii d. Implantation of tissue expander e. Implantation of U.S. FDA approved internal breast prosthesis f. Areolar and nipple reconstruction g. Areolar and nipple tattooing h. Reconstructive surgical revisions i. Removal or revision of a breast implant is considered medically necessary when it is removed for one of the following reasons: Mechanical complication of breast prosthesis; including rupture or failed implant, implant extrusion; Infection or inflammatory reaction due to a breast prosthesis; including infected breast implant, or rejection of breast implants; Other complication of internal breast implant; including siliconoma, granuloma, interference with diagnosis of breast cancer, painful capsular contracture with disfigurement. II. Medical Card system, Inc., (MCS) will consider medically necessary Breast Reconstruction services of the unaffected/contra-lateral breast, in order to produce a symmetrical appearance after a mastectomy performed for any medical reason or lumpectomy performed for a diagnosis of breast cancer. Medically necessary procedures include: a. Breast reduction by mammoplasty or mastopexy iii 2
3 b. Augmentation mammoplasty c. Augmentation with implantation of FDA approved internal breast prosthesis when the unaffected breast is smaller than the smallest available internal prosthesis d. Areolar and nipple reconstruction e. Areolar and nipple tattooing f. Reconstructive surgery revisions to produce a symmetrical appearance g. Breast implant removal and subsequent reimplantation when performed to produce a symmetrical appearance h. Capsulotomy i. Capsulectomy III., (MCS) will consider medically necessary the following products when use in association with a covered medically necessary breast reconstruction procedure: a. AlloDerm b. AlloMax IV., (MCS) considers the following products as Experimental, Investigational or Unproven, and therefore will not be covered: a. DermaMatrix Acellular Dermis b. Permacol c. Radiesse d. Strattice reconstructive tissue matrix e. Repriza Note 2 : (MCS) will consider an Experimental, Investigational or Unproven coverage for any other product not mentioned in this medical policy, until a thorough evaluation and determination is made final by the MCS Medical Advisory Committee (MAC), and/or a new statement from a Local Coverage Determination (LCD) arises. CODING INFORMATION CPT Codes (List may not be all inclusive) CPT Codes DESCRIPTION Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.0 sq cm or less Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.1 to 20.0 sq cm Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; each additional 20.0 sq cm, or part 3
4 thereof Replacement of tissue expander with permanent prosthesis Removal of tissue expander(s) without insertion of prosthesis Repair, complex, trunk; 1.1 cm to 2.5 cm Repair, complex, trunk; 2.6 cm to 7.5 cm Repair, complex, trunk; each additional 5 cm or less Implantation of biologic implant (e.g., acellular dermal matrix) for soft tissue reinforcement (e.g., breast, trunk) Mastopexy Reduction mammaplasty Mammaplasty, augmentation; without prosthetic implant Mammaplasty, augmentation; with prosthetic implant Removal of intact mammary implant Removal of mammary implant material Immediate insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Delayed insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction Nipple/areola reconstruction Correction of Inverted Nipples Breast reconstruction, immediate or delayed, with tissue expander, including subsequent expansion Breast reconstruction with latissimus dorsi flap, without prosthetic implant Breast reconstruction with free flap (19364 includes harvesting of the flap, microvascular transfer, closure of the donor site, and insert shaping the flap into a breast) Breast reconstruction with other technique Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; with microvascular anastomosis (supercharging) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), double pedicle, including closure of donor site Open periprosthetic capsulotomy, breast Periprosthetic capsulectomy, breast Revision of reconstructed breast Preparation of moulage for custom breast implant 4
5 20926 Tissue Grafts, other (eg, paratenon, fat, dermis) Current Procedural Terminology (CPT ) 2017 American Medical Association: Chicago, IL. HCPCS CODES (List may not be all inclusive) HCPCS Codes C1789 C9364 L8020 L8030 L8031 L8032 L8035 L8039 L8600 Q4100 Q4110 Q4116 Q4130 S2066 S2067 S2068 Prosthesis, breast (implantable) Description Porcine Implant, Permacol, per square centimeter Breast prosthesis, mastectomy form Breast prosthesis, silicone or equal, without integral adhesive Breast prosthesis, silicone or equal, with integral adhesive Nipple prosthesis, reusable, any type, each Custom breast prosthesis, post mastectomy, molded to patient model Breast prosthesis, not otherwise specified Implantable breast prosthesis, silicone or equal Skin substitute, not otherwise specified Primatrix, per square centimeter Alloderm, per square centimeter Strattice TM, per square centimeter Breast reconstruction with gluteal artery perforator (GAP) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping the flap into a breast, unilateral Breast reconstruction of a single breast with stacked deep inferior epigastric perforator (DIEP) flap(s) and/or gluteal artery perforator (GAP) flap(s),including harvesting of the flap (s), microvascular transfer, closure of donor site(s) and shaping the flap into a breast, unilateral Breast reconstruction with deep inferior epigastric perforator (DIEP) flap, or superficial inferior epigastric artery (SIEA) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping the flap into a breast, unilateral 2017 HCPCS LEVEL II Professional Edition (American Medical Association). ICD-10 Codes (List may not be all inclusive) ICD-10-Codes DESCRIPTION C Unspecified malignant neoplasm of skin of breast C Basal cell carcinoma of skin of breast C Squamous cell carcinoma of skin of breast C Other specified malignant neoplasm of skin of breast C Malignant neoplasm of nipple and areola, right female breast 5
6 C Malignant neoplasm of nipple and areola, left female breast C Malignant neoplasm of nipple and areola, right male breast C Malignant neoplasm of nipple and areola, left male breast C Malignant neoplasm of central portion of right female breast C Malignant neoplasm of central portion of left female breast C Malignant neoplasm of central portion of right male breast C Malignant neoplasm of central portion of left male breast C Malignant neoplasm of upper-inner quadrant of right female breast C Malignant neoplasm of upper-inner quadrant of left female breast C Malignant neoplasm of upper-inner quadrant of right male breast C Malignant neoplasm of upper-inner quadrant of left male breast C Malignant neoplasm of lower-inner quadrant of right female breast C Malignant neoplasm of lower-inner quadrant of left female breast C Malignant neoplasm of lower-inner quadrant of right male breast C Malignant neoplasm of lower-inner quadrant of left male breast C Malignant neoplasm of upper-outer quadrant of right female breast C Malignant neoplasm of upper-outer quadrant of left female breast C Malignant neoplasm of upper-outer quadrant of right male breast C Malignant neoplasm of lower-outer quadrant of right female breast C Malignant neoplasm of lower-outer quadrant of left female breast C Malignant neoplasm of lower-outer quadrant of right male breast C Malignant neoplasm of lower-outer quadrant of left male breast C Malignant neoplasm of axillary tail of right female breast C Malignant neoplasm of axillary tail of left female breast C Malignant neoplasm of axillary tail of right male breast C Malignant neoplasm of axillary tail of left male breast C Malignant neoplasm of overlapping sites of right female breast C Malignant neoplasm of overlapping sites of left female breast C Malignant neoplasm of overlapping sites of right male breast C Malignant neoplasm of overlapping sites of left male breast C Malignant neoplasm of unspecified site of right female breast C Malignant neoplasm of unspecified site of left female breast C Malignant neoplasm of unspecified site of right male breast C Malignant neoplasm of unspecified site of left male breast C79.2 Secondary malignant neoplasm of the skin C79.81 Secondary malignant neoplasm of breast 6
7 D04.5 Carcinoma in situ of skin of trunk D05.01 Lobular carcinoma in situ of right breast D05.02 Lobular carcinoma in situ of left breast D05.11 Intraductal carcinoma in situ of right breast D05.12 Intraductal carcinoma in situ of left breast D05.81 Other specified type of carcinoma in situ of right breast D05.82 Other specified type of carcinoma in situ of left breast D05.91 Unspecified type of carcinoma in situ of right breast D05.92 Unspecified type of carcinoma in situ of left breast D24.1 Benign neoplasm of right breast D24.2 Benign neoplasm of left breast N60.01 Solitary cyst of right breast N60.02 Solitary cyst of left breast N60.11 Diffuse cystic mastopathy of right breast N60.12 Diffuse cystic mastopathy of left breast N60.21 Fibroadenosis of right breast N60.22 Fibroadenosis of left breast N60.31 Fibrosclerosis of right breast N60.32 Fibrosclerosis of left breast N60.41 Mammary duct ectasia of right breast N60.42 Mammary duct ectasia of left breast N60.81 Other benign mammary dysplasias of right breast N60.82 Other benign mammary dysplasias of left breast N60.91 Unspecified benign mammary dysplasia of right breast N60.92 Unspecified benign mammary dysplasia of left breast N64.89 Other specified disorders of breast R92.8 Other abnormal and inconclusive findings on diagnostic imaging of breast T85.41XA T85.41XD T85.41XS T85.42XA T85.42XD T85.42XS T85.43XA T85.43XD T85.43XS T85.44XA Breakdown (mechanical) of breast prosthesis and implant, initial Breakdown (mechanical) of breast prosthesis and implant, subsequent Breakdown (mechanical) of breast prosthesis and implant, sequela Displacement of breast prosthesis and implant, initial Displacement of breast prosthesis and implant, subsequent Displacement of breast prosthesis and implant, sequela Leakage of breast prosthesis and implant, initial Leakage of breast prosthesis and implant, subsequent Leakage of breast prosthesis and implant, sequela Capsular contracture of breast implant, initial 7
8 T85.44XD T85.44XS T85.49XA T85.49XD T85.49XS T85.79XA T85.79XD T85.79XS T85.818A T85.818D T85.818S T85.828A T85.828D T85.828S T85.838A T85.838D T85.838S T85.848A T85.848D T85.848S T85.858A T85.858D T85.858S T85.868A T85.868D T85.868S T85.89XA Capsular contracture of breast implant, subsequent Capsular contracture of breast implant, sequela Other mechanical complication of breast prosthesis and implant, initial Other mechanical complication of breast prosthesis and implant, subsequent Other mechanical complication of breast prosthesis and implant, sequela Infection and inflammatory reaction due to other internal prosthetic devices, implants and grafts, initial Infection and inflammatory reaction due to other internal prosthetic devices, implants and grafts, subsequent Infection and inflammatory reaction due to other internal prosthetic devices, implants and grafts, sequela Embolism due to other internal prosthetic devices, implants and grafts, initial Embolism due to other internal prosthetic devices, implants and grafts, subsequent Embolism due to other internal prosthetic devices, implants and grafts, sequela Fibrosis due to other internal prosthetic devices, implants and grafts, initial Fibrosis due to other internal prosthetic devices, implants and grafts, subsequent Fibrosis due to other internal prosthetic devices, implants and grafts, sequela Hemorrhage due to other internal prosthetic devices, implants and grafts, initial Hemorrhage due to other internal prosthetic devices, implants and grafts, subsequent Hemorrhage due to other internal prosthetic devices, implants and grafts, sequela Pain due to other internal prosthetic devices, implants and grafts, initial Pain due to other internal prosthetic devices, implants and grafts, subsequent Pain due to other internal prosthetic devices, implants and grafts, sequela Stenosis due to other internal prosthetic devices, implants and grafts, initial Stenosis due to other internal prosthetic devices, implants and grafts, subsequent Stenosis due to other internal prosthetic devices, implants and grafts, sequela Thrombosis due to other internal prosthetic devices, implants and grafts, initial Thrombosis due to other internal prosthetic devices, implants and grafts, subsequent Thrombosis due to other internal prosthetic devices, implants and grafts, sequela Other specified complication of other internal prosthetic devices, implants and grafts, initial 8
9 T85.898D Other specified complication of other internal prosthetic devices, implants and grafts, subsequent T85.898S Other specified complication of other internal prosthetic devices, implants and grafts, sequela Z15.01 Genetic susceptibility to malignant neoplasm of breast Z40.01 Encounter for prophylactic removal of breast Z42.1 Encounter for breast reconstruction following mastectomy Z42.8 Encounter for other plastic and reconstructive surgery following medical procedure or healed injury Z44.31 Encounter for fitting and adjustment of external right breast prosthesis Z44.32 Encounter for fitting and adjustment of external left breast prosthesis Z Encounter for adjustment or removal of right breast implant Z Encounter for adjustment or removal of left breast implant Z80.3 Family history of malignant neoplasm of breast Z85.3 Personal history of malignant neoplasm of breast Z90.10 Acquired absence of unspecified breast and nipple Z90.11 Acquired absence of right breast and nipple Z90.12 Acquired absence of left breast and nipple Z90.13 Acquired absence of bilateral breasts and nipples REFERENCES 1. Thomson Reuters - FindLaw. (2017) 1998 Federal Breast Reconstruction Law - Women s Health and Cancer Rights Act: Overview. Generated by the American Society of Plastic Surgeons (ASPS). Signed into Law on October Accessed March 25, Available at URL address: 2. Centers for Medicare and Medicaid Services. Local Coverage Determination (LCD): Cosmetic and Reconstructive Surgery (L34698). Contractor Name: Wisconsin Physicians Service Insurance Corporation. Contractor Number: Original Determination Effective Date: For services performed on or after 10/01/2015. Revision Effective Date: For services performed on or after 01/01/2017. Accessed March 25, Available at URL address: ive%7cretired&s=all&bc=aggaaaiaiaaaaa%3d%3d& 3. Centers for Medicare and Medicaid Services. National Coverage Determination (NCD) for Breast Reconstruction Following Mastectomy. Publication Number: Manual Section Number: 9
10 Version Number: 1. Effective date: 1/1/1997. Accessed March 25, Available at URL address: 4. MayoClinic. (2016, November 30). Tests and Procedures: Breast lift. Accessed on June 1, Available at URL Address: 5. Puerto Rico: Ley 186 Cirugía Reconstructiva (P. de la C. 186); Texto de Aprobación final por la Cámara, 19 de enero de Accessed March 25, Available at URL address: 6. Puerto Rico: Ley 275 del año 2012; Carta de Derechos de los Pacientes y Sobrevivientes de Cáncer. Accessed March 25, Available at URL address: 7. RTI Surgical, Inc. (2017, April 8). RTI Surgical Expands Line of Matrices in International Markets. Accessed May 30, Available at URL Address: 8. Uptodate. Maurice Nahabedian, MD. Complications of reconstructive and aesthetic breast surgery. Literature review current through: April This topic last updated: July 25, Accessed May 26, Available at URL Address: #H Uptodate. Maurice Nahabedian, MD. Options for flap based breast reconstruction. Literature review current through: April This topic last updated: Aug 15, Accessed May 25, Available at URL Address: t+reconstruction+procedures&selectedtitle=6~150&view=print&displayedview=full# 10. Uptodate. Maurice Nahabedian, MD. Overview of Breast reconstruction.literature review current through: April This topic last updated: May 23, Accessed May 25, Available at URL Address: =1~ Uptodate. Maurice Nahabedian, MD. Implant Based Breast reconstruction and augmentation.literature review current through: April This topic last updated: April 24, 10
11 2017. Accessed May 25, Available at URL Address: WebMD.Gardner, Stephanie S. MD. Breast Cancer Health Center: Breast Reconstruction. Date of revision: August 11, 2015.Accessed April 25, Available at URL Address: Verywell. (2017, March 13). What Is a Capsulotomy? Fixing the Most Common Complication of Breast Augmentation. Accessed on June 1, Available at URL Address: American Society of plastic Surgeons. Practice Parameters: Breast Reconstruction with Expanders and Implants.. Published: Accessed April 25, Available at URL: or American Society of plastic Surgeons. Practice Parameters: Breast Reconstruction Following Diagnosis and Treatment for Breast Cancer. Dated: March Accessed May 26, Available at URL Address: POLICY HISTORY DATE ACTION COMMENT March 11, 2010 Origination of Policy March 24, 2011 Yearly Review 1. HCPCS Codes added to the policy L8020-L CPT Codes added to the policy March 9, 2012 Yearly Review March 19, 2013 Revised References updated. Added new References, numbers 1, 2, & 7. To the Indications Section: Revised previous indication: Breast implant removal and subsequent reimplantation; and substituted with: Removal or revision of a breast implant is considered medically necessary when it is removed for one of the following reasons: Mechanical complication of breast prosthesis; including rupture or failed implant, implant extrusion; Infection or inflammatory reaction due to a breast prosthesis; including infected breast implant, or rejection of breast implants; Other complication of internal breast implant; including siliconoma, granuloma, interference with diagnosis of breast cancer, painful capsular contracture with disfigurement. 11
12 To the Coding Information: added the new CPT Code 19355, and the new ICD-9 Codes , 175.9, 217, 232.5, , , , & V51.8. March 27, 2014 Revised Annual review, no changes to coverage. References updated. To the Indication Section: New Medical products were added in the Subsection III (b) and Subsection IV (e). Medical product NeoForm Dermis was retired as suggested by The Medical Advisory Committee MAC. Note#2 was added to the Medical policy to clarify the Word Lumpectomy" that was including in the Title as suggested in the Medical Advisory Committee MAC at October 14, Autologous Fat Transplant was retired from this medical policy as suggested by the MAC for be considered experimental at this time. Note#3 was added to the medical policy to facilitate the process to determine when a new product will be approved by the MCS medical team. To the Coding section: New HCPCS codes were added to the policy: C9358, Q4116, Q4100, Q4110, C9364, Q4130, C9358, and C9360. A new ICD-10 Codes (Preview Draft) section was added to the policy. October 14, 2014 To the References section: New References (#7, 9, 10, and 11) were added to the Policy. This medical policy was present for MAC approval on October 14, To the Indications Section: Section I was restructured to read as: Medical Card System, Inc. (MCS) will consider medically necessary Breast Reconstruction services of the affected breast after a mastectomy performed for any medical reason or lumpectomy performed for a diagnosis of breast cancer. Medically necessary procedures include. Section II was restructured to read as: Medical Card system, Inc., (MCS) will consider medically necessary Breast Reconstruction services of the unaffected/contra-lateral breast, in order to produce a symmetrical appearance after a mastectomy performed for any medical reason or lumpectomy performed for a diagnosis of breast cancer. Medically necessary procedures include: A new Statement was added to the Medical Policy as suggested for the Medical Advisory Committee MAC on October 14, 2014: For clarification purposes, in this medical policy, breast reconstruction will be consider medically necessary and cover after a lumpectomy only when the lumpectomy was performed for a diagnosis of breast cancer. 12
13 In section III Medical Product NeoForm Dermis was retired from this medical policy after MAC determination on October 14, In section IV Autologous Fat Graft was removed from this medical policy after MAC determination on October 14, The new products suggested as part of the revision of March 2014, will not be included as per MAC determination on October 14, 2014, except for Repriza, that has a clear statement of not coverage from Medicare. A new note was added to the Medical Policy as suggested for the Medical Advisory Committee MAC on October 14, 2014: Note2: (MCS) will consider an Experimental, Investigational or Unproven coverage for any other product not mentioned in this medical policy, until a thorough evaluation and determination is made final by the MCS Medical Advisory Committee (MAC), and/or a new statement from a Local Coverage Determination (LCD) arises. November 23, 2015 Revised To the coding section: Eliminate ICD-9 codes since they are no longer valid for diagnosis classification. Add new section of ICD-10 codes which are the valid diagnosis classification since October 1, March 31, 2016 Revised References were updated. To the Coding Section: CPT Codes Section: New CPT Codes were added to the Policy ( and 20926) ICD-10 Codes Section: The following ICD-10 Codes were deleted from This Policy: C44.500, C44.510, C44.520, C50.021, C50.022, C50.029, C50.121, C50.122, C50.129, C50.221, C50.222, C50.229, C50.321, C50.322, C50.329, C50.421, C50.422, C50.429, C50.521, C50.522, C50.529, C50.621, C50.622, C50.629, C50.821, C50.822, C50.829, C50.921, C50.922, C50.929, T85.72XA, T86.842, T86.848, and T ICD-10 Codes Section: The following ICD-10 Codes were added to the Policy: C44.591, C79.2, N64.89, R92.8, Z15.01, and Z80.3. To the References Section: The following References were deleted from this Policy: #4 and 5. The following References were added to this Medical Policy: #9, 10, 11 and 12. June 1, 2017 Revised To the Coding Section: To the ICD-10 Codes Section: The following ICD-10 Codes were added to the Policy: C50.021, C50.022, C50.121, C50.122, C50.221, C50.222, C50.321, 13
14 C50.322, C50.421, C50.521, C50.522, C50.621, C50.622, C50.821, C50.822, C50.921, C50.922, T85.41XD, T85.41XS, T85.42XD, T85.42XS, T85.43XD, T85.43XS, T85.44XD, T85.44XS, T85.49XD, T85.49XS, T85.79XD, T85.79XS, T85.818A, T85.818D, T85.818S, T85.828A, T85.828D, T85.828S, T85.838A, T85.838D, T85.838S, T85.848A, T85.848D, T85.848S, T85.858A, T85.858D, T85.858S, T85.868A, T85.868D, T85.868S, T85.868A, T85.868D, T85.868S, and Z42.8. The following ICD-10 Codes were deleted from This Policy: C44.509, C44.519, C44.529, C50.019, C50.119, C50.219, C50.319, C50.419, C50.519, C50.619, C50.819, C50.919, D05.00, D05.10, D05.80, D05.90, D24.9, N60.09, N60.19, N60.29, N60.39, N60.49, N60.89, N60.99, T85.81XA, T85.82XA, T85.83XA, T85.84XA, T85.85XA, T85.86XA, T85.89XA, Z44.30, and Z To the References Section: The following References were added to this Medical Policy: #4, 7, 9, 14, and 16. The following References were deleted from this Policy: #8, and 15. This document is for informational purposes only. It is not an authorization, certification, explanation of benefits, or contract. Receipt of benefits is subject to satisfaction of all terms and conditions of coverage. Eligibility and benefit coverage are determined in accordance with the terms of the member s plan in effect as of the date services are rendered., (MCS) medical policies are developed with the assistance of medical professionals and are based upon a review of published and unpublished information including, but not limited to, current medical literature, guidelines published by public health and health research agencies, and community medical practices in the treatment and diagnosis of disease. Because medical practice, information, and technology are constantly changing, Medical Card System, Inc., (MCS) reserves the right to review and update its medical policies at its discretion., (MCS) medical policies are intended to serve as a resource to the plan. They are not intended to limit the plan s ability to interpret plan language as deemed appropriate. Physicians and other providers are solely responsible for all aspects of medical care and treatment, including the type, quality, and levels of care and treatment they choose to provide. i Capsulotomy is a procedure in which part of the "capsule" of scar tissue surrounding a breast implant is removed or the tissue altered or released in some way. ii Capsulectomy is a procedure in which the entire "capsule" of scar tissue surrounding a breast implant is surgically removed. iii Mastopexy or breast lift surgery refers to a group of elective surgical operations designed to lift or change the shape of a person's breasts. 14
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery after Mastectomy or Lumpectomy
 Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
CMS Limitations Guide Mammography & Bone Mass Measurement
 Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations guide provides you with the latest changes.
Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations guide provides you with the latest changes.
Medical Policy Original Effective Date: Revised Date: 09/26/2018 Page 1 of 26
 Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Breast Surgery Corporate Medical Policy
 File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC
 Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Breast Reconstruction Options
 Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Reconstructive Breast Surgery and Management of Breast Implants
 Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Clinical Medical Policy Department Clinical Affairs Division DESCRIPTION
 Prothrombin Time/International Normalized Ratio (PT/INR) Monitor for Home Anticoagulation Management [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr.
Prothrombin Time/International Normalized Ratio (PT/INR) Monitor for Home Anticoagulation Management [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr.
National Medical Policy
 National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
Breast Reconstruction: Current Strategies and Future Opportunities
 Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Direct Current Therapy for Treatment of Hemorrhoids
 Direct Current Therapy for Treatment of Hemorrhoids [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Direct Current Therapy for Treatment of Hemorrhoids [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Current Strategies in Breast Reconstruction
 Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Breast Restoration Surgery After a mastectomy
 UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
Breast Reconstruction
 Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C. Your Guide To BREAST RECONSTRUCTION
 Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Endovenous Radiofrequency and Laser Ablation
 Endovenous Radiofrequency and Laser Ablation [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.]
Endovenous Radiofrequency and Laser Ablation [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.]
Breast Reconstruction. Westmead Breast Cancer Institute
 Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
2017 PHYSICIAN CODING GUIDE
 2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Oncoplastic and Reconstructive Surgery
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Prophylactic Mastectomy & Reconstructive Implications
 Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Breast Cancer Reconstruction
 Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
In a second stage or a second operation that tissue expander is removed through the same incision and the implant is placed within the chest pocket.
 Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
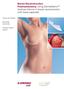 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Selected Operative Procedure Categories for KNHSS SSI Surveillance
 Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Clinical Medical Policy Department Clinical Affairs Division DESCRIPTION
 Ambulatory Blood Pressure Monitoring (ABPM) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr. go to Comunicados a Proveedores, and click Cartas Circulares.]
Ambulatory Blood Pressure Monitoring (ABPM) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr. go to Comunicados a Proveedores, and click Cartas Circulares.]
Corporate Medical Policy
 Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Post-mastectomy breast reconstruction
 Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Breast Reconstruction: Patient Information Document
 breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
Breast Reconstruction Following Mastectomy or Lumpectomy
 Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 1/15/2019 Coverage Policy Number... 0178 Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Related Coverage
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni
 SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni Icro Meattini, MD Radiation Oncology Department - University of Florence Azienda Ospedaliero Universitaria Careggi Firenze Breast
SIMPOSIO Ricostruzione mammaria ed implicazioni radioterapiche Indicazioni Icro Meattini, MD Radiation Oncology Department - University of Florence Azienda Ospedaliero Universitaria Careggi Firenze Breast
Advances in Breast Surgery. Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015
 Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Plastic surgery of the breast includes; augmentation, reduction, Plastic Surgery of the Breast. Abstract. Continuing Education Column
 Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST RECONSTRUCTIONS
 PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy. Zhang Jin, Ph.D MD
 How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
Author's response to reviews
 Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION
 Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review
 Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
Research Article http://www.alliedacademies.org/advanced-surgical-research/ Reconstructive Breast Surgery following Mastectomy for Breast Cancer: A Review Gurnam Virdi* Department of surgery, Queen Elizabeth
BCCCP Approved ICD-9 Code List Fiscal Year 2010
 BCCCP Approved ICD-9 List Fiscal Year 2010 Diagnosis Description 174.0 Malignant neoplasm of female breast; Nipple and areola 174.1 Malignant neoplasm of female breast; Central portion 174.2 Malignant
BCCCP Approved ICD-9 List Fiscal Year 2010 Diagnosis Description 174.0 Malignant neoplasm of female breast; Nipple and areola 174.1 Malignant neoplasm of female breast; Central portion 174.2 Malignant
Blepharoptosis repair is covered as functional/reconstructive surgery to correct: Visual impairment due to droop or displacement of the upper lid.
 Premier Health Insuring Corporation POLICY AND PROCEDURE MANUAL MP.074.PC - Blepharoplasty This policy applies to the following line(s) of business: Premier Health Insuring Corporation MA DSNP Premier
Premier Health Insuring Corporation POLICY AND PROCEDURE MANUAL MP.074.PC - Blepharoplasty This policy applies to the following line(s) of business: Premier Health Insuring Corporation MA DSNP Premier
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
National Mastectomy & Breast Reconstruction Audit Datasheet - Mastectomy +/- Immediate Reconstruction
 Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
MEDICAL POLICY No R10 BREAST RELATED PROCEDURES*
 BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
(https://www.aetna.com/) Number: Policy *Please see amendment for Pennsylvania Medicaid at the end
 (https://www.aetna.com/) Number: 0185 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers reconstructive breast surgery medically necessary after a medically
(https://www.aetna.com/) Number: 0185 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers reconstructive breast surgery medically necessary after a medically
Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry?
 ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
Protocol. Reconstructive Breast Surgery/Management of Breast Implants
 Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
A Combined Practice. Why Its Worked. Barriers to Breast Reconstruction. As a breast oncologist the patient gets seemless care
 A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
Women s Imaging ICD-10-CM
 Women s Imaging ICD-10-CM Clinical Documentation Guides Brought to you by www.codingstrategies.com The Resource for Physician and Outpatient Coding, Compliance & ICD-10-CM OTHER CLINICAL DOCUMENTATION
Women s Imaging ICD-10-CM Clinical Documentation Guides Brought to you by www.codingstrategies.com The Resource for Physician and Outpatient Coding, Compliance & ICD-10-CM OTHER CLINICAL DOCUMENTATION
Supplementary Online Content
 Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Clinical Policy Title: Cosmetic, plastic, and scar revision surgery
 Clinical Policy Title: Cosmetic, plastic, and scar revision surgery Clinical Policy Number: CCP.1184 Effective Date: October 1, 2015 Initial Review Date: August 19, 2015 Most Recent Review Date: September
Clinical Policy Title: Cosmetic, plastic, and scar revision surgery Clinical Policy Number: CCP.1184 Effective Date: October 1, 2015 Initial Review Date: August 19, 2015 Most Recent Review Date: September
Sponsored by: INOVA August 19, Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT
 Sponsored by: INOVA August 19, 2015 Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT WWW.KARENZUPKO.COM 2 1 FOCUS Documentation! Diagnosis codes that get paid. Diagnosis codes that accurately
Sponsored by: INOVA August 19, 2015 Presented by: Teri Romano, RN, MBA, CPC, CMDP CONNECT WITH US AT WWW.KARENZUPKO.COM 2 1 FOCUS Documentation! Diagnosis codes that get paid. Diagnosis codes that accurately
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
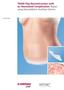 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
The decision to repair a partial mastectomy CME. State of the Art and Science in Postmastectomy Breast Reconstruction.
 CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
CME State of the Art and Science in Postmastectomy Breast Reconstruction Steven J. Kronowitz, M.D. Houston, Texas Learning Objectives: After reading this article, the participant should be able to: 1.
Breast debridement and closure cpt
 Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
Clinical Policy Title: Breast reconstruction following breast cancer surgery
 Clinical Policy Title: Breast reconstruction following breast cancer surgery Clinical Policy Number: 13.03.04 Effective Date: April 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date:
Clinical Policy Title: Breast reconstruction following breast cancer surgery Clinical Policy Number: 13.03.04 Effective Date: April 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date:
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION
 MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
MICHAEL R. ZENN, M.D. INFORMATION ABOUT BREAST RECONSTRUCTION The purpose of breast reconstruction is to restore body image and to enable you to wear all types of clothes without restriction. Most women
COPE Library Sample
 Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
Breast Anatomy LOBULE LOBE ACINI (MILK PRODUCING UNITS) NIPPLE AREOLA COMPLEX ENLARGEMENT OF DUCT AND LOBE LOBULE SUPRACLAVICULAR NODES INFRACLAVICULAR NODES DUCT DUCT ACINI (MILK PRODUCING UNITS) 8420
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Figure 1. Anatomy of the breast
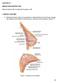 CHAPTER 12 BREAST RECONSTRUCTION Mihaela Rapolti, MD and Michelle Roughton, MD I. BREAST ANATOMY A. Mastering breast anatomy is essential for understanding how the breast changes with aging and principles
CHAPTER 12 BREAST RECONSTRUCTION Mihaela Rapolti, MD and Michelle Roughton, MD I. BREAST ANATOMY A. Mastering breast anatomy is essential for understanding how the breast changes with aging and principles
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Faslodex (fulvestrant) Policy Number: MP-044-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Breast cancer has become so
 The three stages of breast reconstruction BY FORTUNE C IWUAGWU Breast cancer has become so common that most people reading this article will know someone (either professionally or personally) who has been
The three stages of breast reconstruction BY FORTUNE C IWUAGWU Breast cancer has become so common that most people reading this article will know someone (either professionally or personally) who has been
Reconstructive and Cosmetic Services
 Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Classification System
 Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
AHM Breast Reconstruction
 AHM Breast Reconstruction Clinical Indications Breast Reconstruction o TIP: State and Federal regulations concerning breast reconstruction after a medically necessary mastectomy impact plans variously,
AHM Breast Reconstruction Clinical Indications Breast Reconstruction o TIP: State and Federal regulations concerning breast reconstruction after a medically necessary mastectomy impact plans variously,
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Allograft Based Breast Reconstruction: Opportunity for a Second Look
 Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Breast Reconstruction in Women Under 30: A 10-Year Experience
 ORIGINAL ARTICLE Breast Reconstruction in Women Under 30: A 10-Year Experience Warren A. Ellsworth, MD,* Barbara L. Bass, MD, FACS, Roman J. Skoracki, MD, à and Lior Heller, MD* *Division of Plastic Surgery,
ORIGINAL ARTICLE Breast Reconstruction in Women Under 30: A 10-Year Experience Warren A. Ellsworth, MD,* Barbara L. Bass, MD, FACS, Roman J. Skoracki, MD, à and Lior Heller, MD* *Division of Plastic Surgery,
TOTAL Head and Neck Congenital Defects 50
 Operative Minimums Effective July 1, 2014 Review Committee for Plastic Surgery NOTE: The index procedure number for Laser is tracked by Total Laser and not by the subcategories of Aesthetic Laser and Reconstructive
Operative Minimums Effective July 1, 2014 Review Committee for Plastic Surgery NOTE: The index procedure number for Laser is tracked by Total Laser and not by the subcategories of Aesthetic Laser and Reconstructive
Clinical Policy Title: Breast reconstruction following breast cancer surgery
 Clinical Policy Title: Breast reconstruction following breast cancer surgery Clinical Policy Number: 13.03.04 Effective Date: April 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date:
Clinical Policy Title: Breast reconstruction following breast cancer surgery Clinical Policy Number: 13.03.04 Effective Date: April 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date:
Extending breast conservation and other new oncoplastic techniques
 Extending breast conservation and other new oncoplastic techniques Dick Rainsbury BSBR 11-12 November 2013 Liverpool What s the maximum volume of the breast which can be resected during lumpectomy without
Extending breast conservation and other new oncoplastic techniques Dick Rainsbury BSBR 11-12 November 2013 Liverpool What s the maximum volume of the breast which can be resected during lumpectomy without
2018 Clinical Revisions
 InterQual Guidelines for Surgery and Procedures Performed in the Inpatient Setting 2018 Clinical Revisions Review and Incorporation of Recent Medical Literature Change Healthcare is committed to keeping
InterQual Guidelines for Surgery and Procedures Performed in the Inpatient Setting 2018 Clinical Revisions Review and Incorporation of Recent Medical Literature Change Healthcare is committed to keeping
Breast Reconstruction. Breast Care
 Breast Reconstruction Breast Care We put our patients first by working as one team; leading and listening, and striving for the best. Together, we make the difference. Patient information Musgrove Park
Breast Reconstruction Breast Care We put our patients first by working as one team; leading and listening, and striving for the best. Together, we make the difference. Patient information Musgrove Park
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- AIDED DETECTION (CAD) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
can see several late effects. Asymmetry is probably the most common and the thing that patients notice the most. We can also see implant wrinkling or
 Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
rupture, you may notice silicone in their lymph nodes on radiographs. This may be seen and help us detect that there is a rupture.
 Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Exercise & Breast Cancer Recovery
 Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
