Immunohistochemical expression of VEGF in normal human renal parenchyma
|
|
|
- Scott Peters
- 5 years ago
- Views:
Transcription
1 Romanian Journal of Morphology and Embryology 2006, 47(4): ORIGINAL PAPER Immunohistochemical expression of VEGF in normal human renal parenchyma FLAVIA BADERCA 1), RODICA LIGHEZAN 1), ALIS DEMA 2), AURORA ALEXA 1), M. RAICA 1) 1) Department of Histology 2) Department of Pathology, Victor Babeş University of Medicine and Pharmacy, Timişoara Abstract In the normal kidney, VEGF is constitutively expressed in podocytes and tubular epithelial cells of the renal cortex and medulla. The aim of this study was to determine distribution of VEGF in normal renal parenchyma using immunohistochemical methods. The study was retrospective, using normal kidneys samples taken from 28 patients with nephroureterectomy for different types of renal cell carcinomas. Sections were stained with routine Hematoxylin Eosin method and immunohistochemically with anti-vegf polyclonal antibody. All cases presented an intense immunoreaction in the cells lining the nephron tubular system, the higher immunoreaction intensity in the collecting and distal tubules, but weak or moderate in the proximal tubules. The Henle loop cells showed a negative immunoreaction. The immunoreaction was absent in most cells of the renal corpuscle. The cells lining the same tubule presented some variation of intensity, with large polygonal epithelial cells, bulging in the tubular lumen showing an intense cytoplasmic immunoreaction for VEGF. In the renal parenchyma adjacent to the tumor, we observed the same pattern of positive reaction distribution as in the nephron s epithelial tubular cells situated far from the tumor. Adjacent to the tumor proliferation front and in those cases with massive invasive features, we observed a partial depletion of VEGF in distal tubules, while the majority of collecting ducts remained intense positive. The VEGF immunostaining was significantly higher in the renal cortex than in the outer and respectively the inner medulla. Keywords: vascular endothelial growth factor A, kidney, distal tubules, collecting ducts, podocytes, immunohistochemistry. Introduction Vascular permeability factor (VPF) or vascular endothelial growth factor A (VEGF A) is a member of a family of dimeric glycoproteins that belong to the platelet-derived growth factor (PDGF) superfamily of growth factors. Other members of the VEGF family include VEGF B, VEGF C, VEGF D, VEGF E and placenta growth factor, PlGF [1 3]. Discovered in the late 1970s as a tumor-secreted protein that increased microvascular permeability to plasma proteins, VEGF was defined as a homodimeric heparin-binding protein, with a molecular weight of 45 kda. It was first isolated and purified from media conditioned by bovine pituitary follicular stellate cells [4]. In 1983, Senger DR et al. [5] described the partial purification of a protein able to induce vascular leakage in the skin, which was named "tumor vascular permeability factor" (VPF). The authors proposed that VPF could be a mediator of the high tumor blood vessels permeability. Because VPF was not isolated and sequenced, this factor remained molecularly unknown at that time. Senger DR et al. [6] reported the purification and amino-terminal amino acid sequencing of guinea pig VPF in In 1989, Ferrara N and Henzel WJ [4] reported the isolation of a diffusible endothelial cell-specific mitogen from medium conditioned by bovine pituitary follicular cells, which they named "vascular endothelial growth factor" to reflect the restricted target cell specificity of this molecule. This protein was distinct from the known endothelial cell mitogens such as afgf or bfgf [7]. Subsequently, in 1989, Connolly DT et al. [8] followed up on the work by Senger DR et al. [6] and independently reported the isolation and sequencing of human VPF from U937 cells. cdna cloning of VEGF and VPF, demonstrated that VEGF and VPF were the same molecule. The finding that VEGF is potent, diffusible, and specific for vascular endothelial cells led to the hypothesis that this molecule might play a role in the regulation of physiological and pathological growth of blood vessels [9, 10]. Vascular endothelial growth factor A has six isoforms that bind to high affinity receptors, predominantly located on vascular endothelium, through which it induces endothelial cell proliferation and increases vascular permeability to different macromolecules. The major isoform is the VEGF165, a basic, heparin-binding, homodimeric glycoprotein [11]. VEGF121 is a weakly acidic polypeptide that fails to bind to heparin. VEGF189 and VEGF206 are more basic and bind to heparin with greater affinity than VEGF165. Previous studies demonstrated that such differences in the isoelectric point and in affinity for
2 316 heparin may profoundly affect the bioavailability of VEGF. VEGF121 is a freely soluble protein, but a significant fraction of VEGF165 remains bound to the cell surface and the extracellular matrix. In contrast, VEGF189 and VEGF206 are almost completely sequestered in the extracellular matrix. However, these isoforms may be released in a soluble form by heparin or heparinase, suggesting that their binding site is represented by proteoglycans containing heparin-like moieties [12]. In contrast to its transient-expression during the formation of new blood vessels, VEGF and its receptors are continuously and highly expressed in adult tissues, such as the kidney and choroid plexus. VPF/VEGF contributes to angiogenesis by both direct and indirect mechanisms. On the one hand, VEGF stimulates the endothelial cells to proliferate, to migrate, and to alter their pattern of gene expression. On the other hand, VEGF induces microvascular hyperpermeability generating a provisional plasmaderived matrix for the ingrowths of new blood vessels. The aim of this study was to determine distribution of VEGF in normal renal parenchyma using immunohistochemical methods. Material and methods Specimens Normal human kidney samples were obtained from 28 patients with different type of renal cell carcinomas, admitted in the Clinical Hospital of Timişoara. The surgical technique performed in all patients was nephroureterectomy, so that in each case specimens were taken from the tumor, kidney and ureter. Specimens were fixed in 4% buffered formalin, embedded in paraffin. Sections were stained with routine Hematoxylin Eosin method. Immunohistochemistry Immunohistochemistry was performed on formalinfixed, paraffin-embedded tissue derived from the normal pole of the nephrectomy specimens, using the three steps labeled streptavidin biotin immunoperoxidase technique (LSAB2, DAKO, Glostrup, Denmark). Five-micrometer thick sections were cut and mounted onto poly-l-lysine coated glass slides. Sections were dewaxed, rehydrated, washed in distilled water, and then rinsed in 0.01 mol/l Tris-buffered saline (PBS, ph 7.2). The antigen retrieval consisted of microwave heating in DakoCytomation Target Retrieval Solution, ph 9 at 800 watts for two cycles of 10 minutes, followed by double washing with distilled water for 5 minutes. After endogenous peroxidase inhibition with 3% hydrogen peroxide solution, the sections were washed twice with PBS and then incubated with the first step Flavia Baderca et al. antibody: polyclonal mouse anti-human vascular endothelial growth factor antibody, clone VG1, in a 1 : 50 dilution. This antibody labeled all three isoforms of VEGF: VEGF 121, VEGF 165 and VEGF 189. The slides washed twice in PBS, consecutively reacted with a labeled streptavidin biotin system (DAKO LSAB+/HRP kit). The reaction product was visualized in brown with diaminobenzidine (DAB) as a chromogen. Sections were washed twice in distilled water for 5 minutes, which stopped the reaction, then counterstained in Hematoxylin for 5 minutes, dehydrated, cleared in xylene, mounted with DPX, and glass cover slipped. Sections were examined under oil immersion with a 40 objective on a Nikon Eclipse E 400 microscope, and images were captured using a Coolpix 995 digital camera and a DN 100 digital imaging system (Nikon). Anti-vimentin (clone V9) antibody was used as a marker of the optimal fixation and embedding procedures. The negative control was a nonspecific immunoglobulin, provided by the manufacturer (DAKO, Denmark), and performed on slides from the same cases and in the same concentration as the primary antibody. The immuno-labeling of tumor cells had been used as intern positive control. All reagents for the immunohistochemical technique were supplied from DAKO, Glostrup, Denmark. Results VEGF expression The presence of VEGF was demonstrated immunohistochemically, using a polyclonal anti-vegf antibody (clone VG1). There were studied only biopsies taken from both, tumor and renal parenchyma The reaction product was visualized in brown in both, normal or tumor immunopositive cells, with cytoplasmic localization, granular pattern and a higher concentration in the perinuclear area (Figure 1). The expression of VEGF in normal renal parenchyma distant from tumor All cases presented an intense immunoreaction in the cells lining the nephron tubular system. The intensity of the immunoreaction was higher in the collecting ducts and distal tubules, but weak or moderate in the proximal tubules. The majority of Henle loop cells showed a negative immunoreaction, but some isolated cells of the Henle loop were positive, and presented a weak, focal immunoreaction. The immunoreaction was negative in the glomerulus and in most cells of the renal corpuscle. In only one case, we observed a weak positive immunoreaction for VEGF in some isolated podocytes. Isolated positive cells, localized in the outer parietal epithelium of Bowman s capsule, have been also observed on a few slides (Figure 2).
3 Immunohistochemical expression of VEGF in normal human renal parenchyma 317 Small clusters of epithelial cells, localized in the outer parietal layer of Bowman s capsule, at the urinary pole, showed an intense positive immunoreaction. A similar staining pattern was observed in the epithelial cells of the proximal convoluted tubules (Figure 3). The reaction intensity was higher in the distal tubules epithelial cells, situated at the vascular pole of glomerulus. The intensity of VEGF expression varied along the different segments of the tubular system. It was higher in collecting ducts and distal tubules epithelial cells than in those of nephron s proximal tubules (Figures 4 and 5). Even if the intensity of reaction was almost uniform in the tubules, in transversal section, cells lining the same tubule presented some variation of intensity, due to the presence of some large polygonal epithelial cells, bulging in the tubular lumen that expressed an intense but diffuse cytoplasmic immunoreaction for VEGF (Figure 6). Such an intense positive reaction, cited also by other authors, could be related to one of the most important functions of VEGF, the increasing of vascular permeability. It is known that VEGF was initially named vascular permeability factor. The strong positive immunoreaction for VEGF in the collecting ducts and distal tubules epithelial cells suggest a relationship between VEGF expression and adjacent straight vessels (vasa recta), with important roles in gradient concentration. The high levels of VEGF can be correlated with the particular evolution of renal tumors, because the pre-existing VEGF in renal parenchyma represents a local source of growth factor. VEGF expression in renal parenchyma adjacent to the tumor Renal parenchyma showed a consistent VEGF staining in the nephron s tubular system, with no evidence of positive immunoreaction in the renal corpuscle or renal interstitium (Figure 7). Regarding to the intensity of VEGF immunostaining, we observed, in the renal parenchyma adjacent to the tumor, the same pattern of positive reaction distribution as in the nephron s epithelial tubular cells situated far by the tumor. The positive reaction was more intense in the epithelial cells of distal tubules and collecting ducts than in those of the proximal tubules. The collecting ducts epithelial cells labeled for VEGF with some variation of positive reaction, while the Henle loop cells kept a negative or very weak positive reaction for VEGF (Figure 8). The VEGF immunostaining was significantly higher in the renal cortex than in the outer and respectively the inner medulla. In the renal parenchyma, adjacent to the tumor proliferation front, and in those cases with massive invasive features, we observed a partial depletion of VEGF in distal tubules epithelial cells, while the majority of collecting ducts were still intense positive (Figure 9), characteristics not mentioned by other authors. The depletion of the reaction product in tubular epithelial cell cytoplasm was associated with changes in tubular architecture and with the presence of a partially tubules atrophy. These changes were constant in those cases associated with glomerular sclerosis, where the tubular cells of sclerotic glomeruli were negative for VEGF. In one case of clear cell renal carcinoma, we observed the presence of isolated tubules surrounded by the tumor proliferation (Figure 10). The tubules were identified according to their apparent normal morphology. The positive immunoreaction for VEGF was significantly higher in the epithelial tubular cells within the tumor than in tumor cells (Figure 11). This pattern of VEGF expression was similar for normal tubules within tumor area and for small clusters of epithelial tubular cells, localized between tumor cells, staining negative or weak positive for VEGF (Figure 12). Discussions The human VEGF-A gene is located on chromosome 6 and contains eight exons separated by seven introns. There are six different isoforms named after the amino acids number of the peptide chain, VEGF 121, VEGF 145, VEGF 165, VEGF 183, VEGF 189 and VEGF 206 [12, 13]. VEGF 165 is the most abundantly expressed isoform [14] and VEGF 206 the rarest [13, 15]. VEGF mediates its biological effects by binding to more than two receptors belonging to the family of tyrosine kinases [10]. The VEGF A receptors initially described were VEGFR 1 (Flt 1) and VEGFR 2 (KDR, flk 1) localized on the microvascular endothelium of the normal kidneys and tumors, healing wounds and inflammatory sites [16 18]. The third receptor for VEGF A has been later demonstrated to be neuropilin 1 [13, 19]. The expression of neuropilin 1 by normal human podocytes was shown on studies in vitro and in vivo [17, 20, 21]. VEGF has been shown to reduce apoptosis in a large number of cells and cell lines, many of which express VEGF R1. The complex, formed by the tumor secreted VEGF and its receptors, may become a potential target for antiangiogenic therapy [22, 23]. VEGF has been demonstrated to have an important role in kidney organogenesis, especially glomerulogenesis, acting as a paracrine messenger molecule between capillary endothelial cells and Bowman's capsule, in order to maintain the glomerular integrity [24]. In human embryonic and adult kidney, VEGF was localized in the glomerular epithelial cells, while its receptors were present in endothelial cells of both glomerular and peritubular capillaries [24]. Some authors studied the VEGF expression during embryonic development and discovered that is first
4 318 expressed in the trophoblast, within the first days after implantation, suggesting a role for this factor in the angiogenesis in the decidua and placenta. In the human fetus (16 22 weeks), VEGF mrna expression was present in many tissues and was highly expressed in the lungs, kidneys, and spleen [12, 25]. Previous studies have shown that VEGF is continuously expressed in organs with fenestrated endothelia such as choroid plexus and kidney glomeruli [26]. In some studies has been emitted the hypothesis that the continuous expression of VEGF is some particular tissues might be involved in the induction or maintenance of endothelial fenestrations [7, 27, 28]. In most adult tissues, the VEGF positive reaction present during embryonic vasculogenesis and angiogenesis is down-regulated parallel with the reduction of endothelial proliferation. Using immunohistochemical methods, the VEGF presence has been identified in some epithelial cells, kidney glomeruli and myocytes, but not in vascular endothelial cells [26]. On human kidney, a positive VEGF reaction product was demonstrated in visceral glomerular epithelial cells, also known as podocytes [29, 30]. Normal human podocytes are also known to express VEGF receptors such as neuropilin 1, but are VEGF R2 negative. The main VEGF isoform expressed by podocytes is VEGF165. Other VEGF isoforms such as 121, 165, and 189 has been also demonstrated in activated mesangial cells, during mesangioproliferative nephritis. Other authors demonstrated that in kidney glomeruli the major site of VEGF expression was the podocyte [31] but its functional role and the factors responsible for its regulation are poorly understood and controversial [27, 32, 33]. Vascular endothelial growth factor seems to be anti-cytotoxic in podocytes. Moreover, it has been suggested that nephrin, a cell adhesion molecule of the podocyte slit diaphragm, can contribute to antiapoptotic mechanisms in these cells [21]. In our study, opposite with data shown by other authors, the majority of renal corpuscle cells showed no positive reaction for anti-vegf antibody. The lack of positive immunoreaction in glomerular cells was similar for the renal corpuscle adjacent to the tumor or situated distant from tumor. We identified only a small number of visceral glomerular epithelia cells VEGF immunopositive, but the immunoreaction intensity was weak and restricted. In comparaison, Dvorak HF et al. [34] identified a strong VEGF expression in glomerular podocytes and tubular epithelium of normal kidney [29, 34]. In 2003, Eremina V [35] demonstrated the VEGF expression in the podocytes of normal human kidney, the epithelial cells of the distal tubules and collecting ducts. Using immunohistochemical and in situ hybridization methods, the VEGF mrna and protein have been co-localized in glomerular epithelia and collecting duct cells. Flavia Baderca et al. Some studies revealed that mature podocytes express significant levels of distally spliced isoforms of VEGF that have been shown to be inhibitory and antiangiogenic [36]. The polyclonal antibody used in our study binds both isoforms of VEGF: 165 and 165b, but even so we did not find a positive reaction in glomerular cells. Regarding the expression of VEGF in the cells of Bowman s capsule, we identified a negative expression in the majority of Bowman s capsule outer epithelial cells, with only few isolated positive parietal cells. The immunoreaction was moderate at urinary pole, present in small clusters of intense positive parietal epithelial cells, localized adjacent to the proximal tubule. In conformity with the hypothesis emitted in 1997 by Kitamoto Y [24], Bowman's capsule secretes VEGF in order to stimulate capillary endothelial cells to develop a glomerulus. In the nephron s tubules, the upregulation of VEGF is initiated also by the hypoxic signal resulting from poor peritubular vascularity. In distal tubules, VEGF expression may be necessary to maintain the fenestration of capillary endothelial cells. Some authors have demonstrated other VEGF positive cells, such as: mesangial cells [37], glomerular endothelial cells [31], activated macrophages [37], renal glomerular visceral epithelia [29]. These observations suggested that VEGF might play an important regulatory role for glomerular endothelial cells [31, 38]. Opposite to these studies, we did not identify any VEGF positive mesangial cells by immunohistochemistry. The distal tubules and collecting ducts showed a strong positive reaction, these findings being similar with data of Brown LF et al. [29] that identified the VEGF protein in the epithelia of collecting ducts. In 1997, Kitamoto Y [24] highlighted the evidence of VEGF message in proximal and distal convoluted tubules of the adult rat. Previous immunohistochemical studies have provided solid evidence of VEGF presence in normal human kidney, confined to distal tubule and collecting ducts [18, 34]. The expression of VEGF in collecting ducts and of VEGF receptors in medullar capillaries may play a role in maintaining the medullar osmolarity. VEGF may promote renal tubular epithelial cell survival in vivo in situations associated with cellular stress, for example acute ischemia or toxic injury of the kidney. These data suggest an expanded role for VEGF in pathological conditions in the kidney [39]. Our study revealed that even if the positive reaction for VEGF had a constant presence in normal renal parenchyma, its intensity varied along the different segments of the tubular system. It was higher in epithelial cells of the collecting and distal tubules, with minimal reaction in proximal tubules of the nephron. The Henle loop epithelial cells showed no VEGF staining.
5 Immunohistochemical expression of VEGF in normal human renal parenchyma 319 Figure 1 Cytoplasmic positive immunoreaction for VEGF with a higher perinuclear concentration and a granular pattern in normal renal tubules. Immunoreaction for VEGF ( 400) Figure 2 Immunoreaction for VEGF in the renal corpuscle. Weak positive reaction in some isolated or small clusters of podocytes ( 400) Figure 3 Urinary pole of renal corpuscle Proximal convoluted tubule epithelial cells showed an intense positive reaction. Immunoreaction for VEGF ( 400) Figure 4 Intense immunostaining in distal and collecting tubules of the outer medulla. Immunoreaction for VEGF ( 400) Figure 5 Proximal tubules of the outer medulla with weak positive reaction. Immunoreaction for VEGF ( 400) Figure 6 Heterogenic positive immunoreaction in tubular epithelial cells ( 400)
6 320 Flavia Baderca et al. Figure 7 Normal kidney with diffuse immunohistochemical staining for VEGF in all tubules of the renal cortex, and no staining of renal corpuscle or renal interstitium ( 100) Figure 8 Collecting ducts with intense positive cells, and Henle loop epithelial cells with negative immunoreaction for VEGF ( 400) Figure 9 The partial depletion of VEGF in distal tubules and the Henle loop. The majority of collecting ducts were still intense positive ( 100) Figure 10 Renal tubule with apparent normal morphology within the tumor. Immunoreaction for VEGF ( 400) Figure 11 The intensity of positive reaction is higher in tubular cells within the tumor than in tumor cells). Small cluster of tubular cells, intense positive for VEGF and tumor cells weak positive. Immunoreaction for VEGF ( 400) Figure 12 Small cluster of tubular cells, intense positive for VEGF and tumor cells weak positive. Immunoreaction for VEGF ( 400)
7 Immunohistochemical expression of VEGF in normal human renal parenchyma 321 There are no other studies reporting about the VEGF presence in the renal parenchyma adjacent to the tumor. In our study, we identified a partial depletion of VEGF in distal tubules epithelial cells, while the majority of collecting ducts remained intense positive. In addition, the presence of apparent normal tubules within the tumor area could reflect the high rate of tumor proliferation, but these aspects need to be further investigated. One researcher group that have investigated the expression of VEGF in human renal tissues, have found an increased VEGF staining only in the tumor area and in surrounding vascular tissue, but not in the area of normal renal tissue. Although these studies included a relatively small number of patients, the decreased expression of VEGF, support the hypothesis that in patients with minimal change disease there was a specific down-regulation of VEGF gene expression [7]. We consider that the differences noticed between our results and those published by other researcher groups can be explained by the type of antibody (polyclonal versus monoclonal) and by the used method for the VEGF determination (immunohistochemistry versus polymerase chain reaction or in situ hybridization). Conclusions Our study confirms the presence of VEGF in the nephron s tubular system situated adjacent or distant from tumor. The VEGF immunoreaction was intense in the epithelial cells of the distal tubules and collecting ducts and moderate in the proximal tubules epithelial cells. The Henle loop epithelial cells were VEGF negative. The cells of renal corpuscle did not react with the polyclonal anti VEGF antibody. Although recent studies have identified an intense positive reaction for VEGF in podocytes, our study revealed only a few podocytes with a weak positive reaction and a minimal positive reaction in some parietal epithelial cells of the Bowman s capsule. This positive reaction, in some podocytes and parietal epithelial cells, was observed in a scarce number of cases. On one slide, along the tumor proliferation front, there was observed the presence of an apparent normal tubule with positive VEGF cells surrounded by weakly stained tumor cells. The same positive immunoreaction was observed even in small clusters of normal tubular epithelial cells within the tumor. In the peritumoral area, the proximal and distal tubules epithelial cells showed a less intense positive immunoreaction for VEGF than those localized in renal parenchyma situated distant from tumor. The immunoreaction intensity for VEGF decreased from kidney cortex to inner medulla. The glomerular sclerosis has been associated with a negative reaction in the epithelial tubular cells. References [1] MATSUMOTO T., CLAESSON-WELSH L., VEGF receptor signal transduction, Sci STKE, 2001, 112:re21. [2] CARMELIET P., MOONS L., LUTTUN A. et al., Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions, Nat Med, 2001, 7: [3] DVORAK H. F., Vascular Permeability Factor/Vascular Endothelial Growth Factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy, J Clin Oncology, 2002, 20: [4] FERRARA N., HENZEL W. J., Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cell, Biochem Biophys Res Commun, 1989, 161: [5] SENGER D. R., GALLI S. J., DVORAK A. M., PERRUZZI C. A. et al., Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid, Sci, 1983, 219: [6] SENGER D. R., CONNOLLY D. T., VAN DE WATER L., FEDER J. et al., Purification and NH 2 -terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor, Cancer Res, 1990, 50: [7] KANG D. H., JOHNSON R. J., Vascular endothelial growth factor: a new player in the pathogenesis of renal fibrosis, Curr Opin Nephrol Hypertens, 2003, 12(1): [8] CONNOLLY D. T., OLANDER J. V., HEUVELMAN D. et al., Human vascular permeability factor. Isolation from U937 cells, J Biol Chem, 1989, 264: [9] FERRARA N., HOUCK K. A., JAKEMAN L. B. et al., The vascular endothelial growth factor family of polypeptides, J Cell Biochem, 1991, 47: [10] FERRARA N., Vascular Endothelial Growth Factor: basic science and clinical progress, Endocrine Reviews, 2004, 25(4): [11] SATCHELL S. C., ANDERSON K. L., MATHIESON P. W., Angiopoietin 1 and Vascular Endothelial Growth Factor modulate human glomerular endothelial cell barrier properties, J Am Soc Nephrol, 2004, 15: [12] FERRARA N., KEYT B., Vascular endothelial growth factor. Basic biology and clinical implications. In: ROSEN E., GOLDBERG I. D. (eds), Regulation of Angiogenesis, Springer Verlag, New York, 1997, [13] ROBINSON C. J., STRINGER S. E., The splice variants of vascular endothelial growth factor (VEGF) and their receptors, J Cell Sci, 2001, 114(5): [14] KEYT B. A., NGUYEN H. V., BERLEAU L. T. et al., Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptorselective VEGF variants by site-directed mutagenesis, J Biol Chem, 1996, 8; 271(10): [15] INOUE K., SAKURADA Y., MURAKAMI M. et al., Detection of gene expression of vascular endothelial growth factor and flk-1 in the renal glomeruli of the normal rat kidney using the laser microdissection system, Virchows Archiv, 2003, 442(2): [16] FENG D., NAGY J. A., BREKKEN R. A., PETTERSSON A. et al., Ultrastructural localization of the Vascular Permeability Factor/Vascular Endothelial Growth Factor (VPF/VEGF) Receptor-2 (FLK-1, KDR) in normal mouse kidney and in the hyperpermeable vessels induced by VPF/VEGFexpressing tumors and adenoviral vectors, J Histochem Cytochem, 2000, 48: [17] HARPER S. J., XING C. Y., WHITTLE C. et al., Expression of neuropilin by human glomerular epithelial cells in vitro and in vivo, Clin Sci (Colch), 2001, 101: [18] OSTENDORF T., KUNTER U., EITNER F. et al., VEGF 165 mediates glomerular endothelial repair, J Clin Invest, 1999, 104(7): [19] ROBERT B., ZHAO X., ABRAHAMSON D. R., Coexpression of neuropilin-1, Flk1, and VEGF 164 in developing and mature mouse kidney glomeruli, Am J Physiol Renal Physiol, 2000, 279: F275 F282.
8 322 [20] KANELLIS J., MUDGE S. J., FRASER S. et al., Redistribution of cytoplasmic VEGF to the basolateral aspect of renal tubular cells in ischemia-reperfusion injury, Kidney Int, 2000, 57(6): [21] FOSTER R. R., SALEEM M. A., MATHIESON P. W. et al., Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes, Am J Physiol Renal Physiol, 2005, 288(1):F [22] DVORAK H. F., NAGY J. A., DVORAK A. M., Structure of solid tumors and their vasculature: Implications for therapy with monoclonal antibodies, Cancer Cells, 1991, 3: [23] BREKKEN R. A., HUANG X., KING S. W. et al., Vascular endothelial growth factor as a marker of tumor endothelium, Cancer Res, 1998, 58: [24] KITAMOTO Y., TOKUNAGA H., TOMITA K., Vascular Endothelial Growth Factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis, J Clin Invest, 1997, 99(10): [25] JAKEMAN L. B., WINER J., BENNETT G. L. et al., Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues, J Clin Invest, 1992, 89: [26] SÖRENSSON J., FIERLBECK W., HEIDER T. et al., Glomerular endothelial fenestrae in vivo are not formed from caveolae, J Am Soc Nephrol, 2002, 13: [27] SATCHELL S. C., HARPER S. J., TOOKE J. E. et al., Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor, J Am Soc Nephrol, 2002, 13(2): [28] ESSER S., WOLBURG K., WOLBURG H. et al., Vascular Endothelial Growth Factor induces endothelial fenestrations in vitro, Cell Biol, 1998, 140(4): [29] BROWN L. F., BERSE B., TOGNAZZI K. et al., Vascular permeability factor mrna and protein expression in human kidney, Kidney Int, 1992, 42: [30] CHEN J., BRAET F., BRODSKY S. et al., VEGF-induced mobilization of caveolae and increase in permeability of endothelial cells, Am J Physiol Cell Physiol, 2002, 282(5):C1053 C1063. Flavia Baderca et al. [31] UCHIDA K., UCHIDA S., NITTA K. et al., Glomerular endothelial cells in culture express and secrete vascular endothelial growth factor, Am J Physiol, 1994, 266(1 Pt 2): F [32] BATES D. O., CUI T. G., DOUGHTY J. M. et al., VEGF 165 b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma, Cancer Research, 2002, 62: [33] FOSTER R. R., HOLE R., ANDERSON K. et al., Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes, Am J Physiol Renal Physiol, 2003, 284:F1263 F1273. [34] DVORAK H. F., NAGY J. A., FENG D. et al., Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis, Curr Top Microbiol Immunol, 1999, 237: [35] EREMINA V., SOOD M., HAIGH J. et al., Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases, Clin Invest, 2003, 111: [36] CUI G. T., FOSTER R. R., SALEEM M., MATHIESON P. W. et al., Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF 165 b) mrna and protein, Am J Physiol Renal Physiol, 2004, 286:F767 F773. [37] IIJIMA K., YOSHIKAWA N., CONNOLLY D. T., NAKAMURA H., Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor, Kidney Int, 1993, 44: [38] CHEONG H. I., LEE J. H., HAHN H. et al., Circulating VEGF and TGF-β1 in children with idiopathic nephrotic syndrome, J Nephrol, 2001, 14: [39] KANELLIS J., MUDGE S. J., FRASER S., KATERELOS M. et al., Redistribution of cytoplasmic VEGF to the basolateral aspect of renal tubular cells in ischemia-reperfusion injury, Kidney Int, 2000, 57(6): Corresponding author Flavia Baderca, Assistant Professor, MD, PhD, Department of Histology, Victor Babeş University of Medicine and Pharmacy, 2 Eftimie Murgu Square, Timişoara, Romania; Phone , flaviabaderca@yahoo.com Received: February 15 th, 2007 Accepted: March 10 th, 2007
Angiogenesis in urothelial tumors of the upper urinary tract
 Romanian Journal of Morphology and Embryology 2005, 46(4):263 268 Angiogenesis in urothelial tumors of the upper urinary tract FLAVIA BADERCA 1), RODICA LIGHEZAN 1), ALIS DEMA 2), AURORA ALEXA 1), M. RAICA
Romanian Journal of Morphology and Embryology 2005, 46(4):263 268 Angiogenesis in urothelial tumors of the upper urinary tract FLAVIA BADERCA 1), RODICA LIGHEZAN 1), ALIS DEMA 2), AURORA ALEXA 1), M. RAICA
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
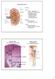 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
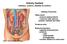
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M
 SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Chapter 25: Urinary System
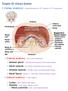 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
RENAL HISTOPATHOLOGY
 RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Histopathology: Glomerulonephritis and other renal pathology
 Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Renal Pathology 1: Glomerulus. With many thanks to Elizabeth Angus PhD for EM photographs
 Renal Pathology 1: Glomerulus With many thanks to Elizabeth Angus PhD for EM photographs Anatomy of the Kidney http://www.yalemedicalgroup.org/stw/page.asp?pageid=stw028980 The Nephron http://www.beltina.org/health-dictionary/nephron-function-kidney-definition.html
Renal Pathology 1: Glomerulus With many thanks to Elizabeth Angus PhD for EM photographs Anatomy of the Kidney http://www.yalemedicalgroup.org/stw/page.asp?pageid=stw028980 The Nephron http://www.beltina.org/health-dictionary/nephron-function-kidney-definition.html
LABORATORY EXERCISES FOR THE URINARY SYSTEM
 LABORATORY EXERCISES FOR THE URINARY SYSTEM cortex Medulla DEMO SLIDE BOX 172 (450-E001-H-76). Kidney, horse. the inner medulla medullary rays, Uriniferous tubules expand both the cortex and medulla corticomedullary
LABORATORY EXERCISES FOR THE URINARY SYSTEM cortex Medulla DEMO SLIDE BOX 172 (450-E001-H-76). Kidney, horse. the inner medulla medullary rays, Uriniferous tubules expand both the cortex and medulla corticomedullary
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE
 THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
HISTOLOGY OF THE URINARY SYSTEM
 HISTOLOGY OF THE URINARY SYSTEM The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the
HISTOLOGY OF THE URINARY SYSTEM The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Surgical Pathology Report
 Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
URINARY SYSTEM. These organs lie posterior or inferior to the. (membrane).
 URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
CD15 and CEA expression in thymic epithelial neoplasms
 Turkish Journal of Cancer Volume 8, No., 8 CD and CEA expression in thymic epithelial neoplasms AYTEKİN AKYOL, AYŞEGÜL ÜNER Hacettepe University, Department of Pathology, Ankara-Turkey ABSTRACT The aim
Turkish Journal of Cancer Volume 8, No., 8 CD and CEA expression in thymic epithelial neoplasms AYTEKİN AKYOL, AYŞEGÜL ÜNER Hacettepe University, Department of Pathology, Ankara-Turkey ABSTRACT The aim
Introduction. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats
 Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
What is excretion? Excretion is the removal of metabolic waste from the body.
 Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
Excretion What is excretion? Excretion is the removal of metabolic waste from the body. Excretion in Plants Plants produce very little waste products. Plants lose oxygen and water vapour through the stomata.
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19](/thumbs/77/74887026.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Urinary Anatomy. Lab 40. Kidneys. Nephrons. Renal Corpuscle
 Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
THE RENAL / URINARY SYSTEM
 1 THE RENAL / URINARY SYSTEM Definition/Description: The Renal/Urinary system is the body system, which plays a vital role in the maintenance of homeostasis by the following processes: 1. Production of
1 THE RENAL / URINARY SYSTEM Definition/Description: The Renal/Urinary system is the body system, which plays a vital role in the maintenance of homeostasis by the following processes: 1. Production of
Human Anatomy Unit 3 URINARY SYSTEM
 Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
The topic of normal vascular and glomerular anatomy is introduced
 Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Expression of Vascular Permeability Factor (VPF/VEGF) Is Altered in Many Glomerular Diseases1
 Expression of Vascular Permeability Factor (VPF/VEGF) Is Altered in Many Glomerular Diseases1 Kenneth Shulman, Seymour Rosen, Kathi Tognazzi, Eleanor J. Manseau, and Lawrence F. Brown2 K. Shulman. S. Rosen,
Expression of Vascular Permeability Factor (VPF/VEGF) Is Altered in Many Glomerular Diseases1 Kenneth Shulman, Seymour Rosen, Kathi Tognazzi, Eleanor J. Manseau, and Lawrence F. Brown2 K. Shulman. S. Rosen,
Unit #4 Waste and Excretion. The Kidneys
 Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Basic Urinary Tract Anatomy and Histology
 Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
Frontal section of human kidney. (Scheme).
 15 Urinary system 15-001 Kidney Cortex Medulla Papilla renalis Medial Lateral Medullary ray Labyrinth Columna renalis Pelvis renalis Calix renalis Ureter Capsula fibrosa Lobus renalis Columna renalis 15-01.
15 Urinary system 15-001 Kidney Cortex Medulla Papilla renalis Medial Lateral Medullary ray Labyrinth Columna renalis Pelvis renalis Calix renalis Ureter Capsula fibrosa Lobus renalis Columna renalis 15-01.
1. The Fibrous Capsule covers the outside of the kidney. It is made of fat and fibers.
 Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
Outline Urinary System
 Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
5.Which part of the nephron removes water, ions and nutrients from the blood?
 Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
Chapter 24: The Urinary System
 Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
R J M E Romanian Journal of Morphology & Embryology
 Rom J Morphol Embryol 2012, 53(3 Suppl):749 753 ORIGINAL PAPER R J M E Romanian Journal of Morphology & Embryology http://www.rjme.ro/ The characterization of PDGFR-alpha and PDGFR-beta expression in malignant
Rom J Morphol Embryol 2012, 53(3 Suppl):749 753 ORIGINAL PAPER R J M E Romanian Journal of Morphology & Embryology http://www.rjme.ro/ The characterization of PDGFR-alpha and PDGFR-beta expression in malignant
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer
 Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma
 Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Fifth Year Biology. Excretion. Miss Rochford
 Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
General Anatomy of Urinary System
 General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Chapter 44: Osmoregulation and Excretion
 Name Period The steady-state physiological condition that organisms must maintain is termed homeostasis. Osmoregulation and excretion are frequently cited examples of homeostasis and are the central ideas
Name Period The steady-state physiological condition that organisms must maintain is termed homeostasis. Osmoregulation and excretion are frequently cited examples of homeostasis and are the central ideas
Overview of glomerular diseases
 Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Question 1: Solution 1: Question 2: Question 3: Question 4: Class X The Excretory System Biology
 A. MULTIPLE CHOICE TYPE: (select the most appropriate option in each case) Book Name: Selina Concise Question 1: Excretion primarily involves (a) removal of all byproducts during catabolism (b) removal
A. MULTIPLE CHOICE TYPE: (select the most appropriate option in each case) Book Name: Selina Concise Question 1: Excretion primarily involves (a) removal of all byproducts during catabolism (b) removal
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
URINARY SYSTEM. Urinary System
 URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
Urinary System (Anatomy & Physiology)
 Urinary System (Anatomy & Physiology) IACLD CME, Monday, February 20, 2012 Mohammad Reza Bakhtiari, DCLS, PhD Iranian Research Organization for Science & Technology (IROST) Tehran, Iran The Urinary System
Urinary System (Anatomy & Physiology) IACLD CME, Monday, February 20, 2012 Mohammad Reza Bakhtiari, DCLS, PhD Iranian Research Organization for Science & Technology (IROST) Tehran, Iran The Urinary System
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 25.1 Using Figure 25.1, match the following: 1) Glomerulus. 2) Afferent arteriole. 3)
Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 25.1 Using Figure 25.1, match the following: 1) Glomerulus. 2) Afferent arteriole. 3)
