EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES
|
|
|
- Kelly Porter
- 5 years ago
- Views:
Transcription
1 ORIGINAL REPORT EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES J. Esmaeili 1, M. Mohebali 1*, G. H. Edrissian 1, S. M. Rezayat 2, M. Ghazi-Khansari 2 and S. Charehdar 1 1) Department of Medical Parasitology and Mycology, School of Public Health and Institute of Public Health Research, Medical Sciences/University of Tehran, Tehran, Iran 2) Department of Pharmacology, School of Medicine, Medical Sciences/University of Tehran, Tehran, Iran Abstract- Cutaneous leishmaniasis is endemic in 88 different countries. There are an estimated 1.5 million new cases each year, with over 90% occurring in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria (Old World) and in Brazil and Peru (New World). Miltefosine is effective in vitro and in vivo against Leishmania species and it was demonstrated efficacy in animals via the oral route. This study is the first one for evaluating the effect of miltefosine on cutaneous leishmaniasis of L. major (MRHO/IR/75/ER) by in vivo and in vitro studies in the BALB/c mouse model. As it was shown, miltefosine has a better effect on reduction of size of lesion compared to Glucantime, also it was not significant by statistical analysis. The results of this study show that miltefosine has a good activity against the proliferation of amastigotes of L. major. The results suggest that oral miltefosine might be a promising approach for developing new anti-leishmanial drugs Tehran University of Medical Sciences. All rights reserved. Acta Medica Iranica, 46(3): ; 2008 Key words: Cutaneous leishmaniasis, miltefosine, Leishmania major, treatment INTRODUCTION Cutaneous leishmaniasis is endemic in 88 different countries. There are an estimated 1.5 million new cases each year, with over 90% occurring in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria (Old World) and in Brazil and Peru (New World) (1). Both cutaneous and visceral forms of leishmaniasis are endemic in different parts of Iran. Zoonotic cutaneous leishmaniasis (CL) caused by Leishmania major is common in many rural areas of Iran (2). Antimonial compounds particularly meglumine antimoniate (Glucantime ) are the first line drugs for the treatment of all forms of leishmaniasis in Received:, Revised:, Accepted: Corresponding Author: M. Mohebali, Department of Medical Parasitology and Mycology, School of Public Health and Institute of Public Health Research, Medical Sciences/University of Tehran, Tehran, Iran Tel: Fax: mmohebali@hotmail.com Iran (3). Based on a few studies that have been carried out in recent years, about 10 to 15% of CL has not desirable response to meglumine antimoniate (Mohebali, unpublished data, 4). Recent circumstantial evidences are suggesting that an increasing number of Iranian patients with cutaneous leishmaniasis are unresponsive to meglumine antimoniate (Glucantime ) (4). Miltefosine (hexadecyl-phosphocholine, Impavido ) interacts with cell signal transduction pathways and inhibits phospholipids and sterol biosynthesis (5). It was originally developed as an anticancer agent. Miltefosine is effective in vitro and in vivo against Leishmania species (6, 7); it was demonstrated efficacy in animals via the oral route (6). The first clinical test of miltefosine used dosages that ranged from 50 mg given every other day up to 250 mg/day in Indian patients with kala azar in 2002 (8). In a later large phase II trial, treatment with mg for 28 days cured 86 (96%) of 90 viscerally infected Indian patients (9). Miltefosine is now registered to
2 Miltefosine against leshmania treat visceral leishmaniasis in Germany and India, as well as cutaneaous leishmaniasis in Colombia (10). For the first time, effectiveness and tolerability of Miltefosine were evaluated for the treatment of 63 Iranian zoonotic cutaneous leishmaniasis caused by L. major comparing to meglumine antimoniate (11). In this study, we aimed to clarify assessing the effectiveness of Miltefosine against L. major (Iranian strain) comparing with Glucantime in vitro and in vivo conditions. MATERIALS AND METHODS Materials Miltefosine was a gift from Zentaris GmbH (Zentaris, GmbH, Frankfurt, Germany) provided by Dr. M. R. Goli (Arya Daro Co.). Glucantime (Rorer Rhone-Poulenc Specia, Paris, France) received from the Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences. Parasites culture Leishmania major promastigotes, MHROM/IR/ 75/ER, were grown in Schneider s medium supplemented with 10% heat-inactivated FBS, 100 g/ml streptomycin and 100 IU/ml penicillin G at C as previously described (12). The parasites were kept in a virulent state by regular passage in susceptible BALB/c mice. Stationary phase promastigotes were harvested and centrifuged at 3,000 rpm for 10 min at 4 C. The pellet was washed 3 times in PBS (8 mm Na2HPO4, 1.75 mm KH2PO mmkcl, mm NaCl). In vivo studies Male BALB/c mice (6-8 weeks old) were obtained from the Animal Breeding Stock Facility of Razi Institute of Iran, Karaj, Iran. Male BALB/c mice were infected with subcutaneously L. major promastigotes (MHROM/IR/75/ER) at the base of tail. The weight and diameter of lesions were measured before treatment. Impression smears were prepared from lesions; methanol fixed, and stained with 10% Giemsa stain in water. The mice were randomly divided to four groups, that three (15 mice per group) and one group (5 mice) for animal Lab control. The groups included: Group 1: Control mice non-infected and nontreated Group 2: Infected but non-treated Group 3: Infected treated with miltefosine 2.5 mg/kg by daily gavage for 28 days. Group 4: Infected treated with Glucantime 60 mg/kg salt injected IP daily for 28 days. The diameters of lesions one, two and 8 weeks after the beginning the treatment were measured. Also before treatment and one, two and 8 weeks after the beginning the treatment, slides were prepared and methanol fixed, Giemsa stained and examined by light microscopy ( 1000). Drugs efficacy were determined by comparing the diameters of lesions and the number of mice which slides contained amastigotes, between treated and untreated groups. In vitro studies Mouse peritoneal macrophages The macrophages of peritoneal fluid of male BALB/c mice were collected and resuspended at /ml in RPMI 1640 supplemented with 15% FCS, as described by others (13). Cells were plated in eight-chamber LabTek tissue-culture slides, and adherent macrophages were infected with latelogarithmic promastigote parasites at a parasites-tomacrophage ratio of 5:1. After 2 h of incubation at 34 C, extracellular parasites were removed by washing, and fresh medium containing the different fixed-ratio solutions miltefosine and glucantime 1.25, 2.5, 5, 10, 20 µm or no drug was added. Each point was tested in triplicate. Each 5-ml ampoule of glucantime contained 1.5 g meglumine antimoniate corresponding to g of pentavalent antimony. The tissue-culture slides were incubated for 3 days, fresh Glucantime and miltefosine was added, and the slides were incubated for an additional 72 h. The slides were fixed and stained with Giemsa. Three slides were used for each concentration. The percentage of infected macrophages and the number of parasites per infected cell were evaluated by microscopic examination of at least 100 macrophages. The ED50 is defined in this study as 192 Acta Medica Iranica, Vol. 46, No. 3 (2008)
3 J. Esmaeili et al. Table 1. Effect of miltefosine and Glucantime on the size of lesions (mm) in Balb/c mice infected by L. major Week after treatment Groups ± ± ± ± ± ± ± ± ± ± ± ± 7.79 Group 2: Infected but non-treated (Control group) Group 3: Infected treated with miltefosine 2.5 mg/kg by gavage daily for 28 days Group 4: Infected treated with glucantime (60 mg/kg salt injected IP) daily for 28 days the effective dose of miltefosine and glucantime that reduces the survival of Leishmania parasites by 50% ED50 values were determined by liner regression analysis. Statistical analysis Statistical significance between groups was analyzed by Student s t test using SPSS version 10. Values of P < 0.05 were considered statistically significant. RESULTS In vivo studies Effect on size of lesions The mean sizes of the lesions in three infected groups were measured before treatment and after one, two and eight weeks of treatment. The results are shown in Table 1. As shown in Table 1, both miltefosine (2.5 mg/kg by gavage for 28 days) and glucantime (60 mg/kg salt injected IP) produced a suppression and reduction effect in the size of lesions compare with control group in lesihmaniainfected mice. However, as shown in Fig 1, there is no significant difference between miltefosine and glucantime in reduction of lesion size. Parasitology The results of monitoring the slides for the present of amastigotes are shown in Table 2. The positive cases are ones which slides contained L. major amastigotes and negative cases are ones without any L. major amastigotes. The chi-square analysis of results shows a significant difference between the treated groups 3 (miltefosine) and 4 (glucantime) with control group (P < 0.05). In vitro studies In vitro EC50 for L. major amastigotes was determined after 3 days exposure to different concentration of miltefosine and Glucantime. The data represent the means ± standard deviations (SDs) of three independent experiments. The ED50 of miltefosine was 2.20 µm according to the liner regression was shown in Fig 2. More than 85% of L. major amastigotes-infected macrophages damaged when treated with 10 and 20 µm of miltefosine and was cytotoxic for both parasite and macrophage. The ED50 of Glucantime was 7.2 µm according to the liner regression was shown in Figure 2. Table 2. Parasitological results of the effect of miltefosine and Glucantime R in Balb/c mice infected by L.major Week after treatment Groups N P N P N P N P * 4 12* 3 12* * 7 8* 7 8* 7 Abbreviations: N, Negative; P, Positive Group 2: Infected but non-treated Group 3: Infected treated with miltefosine 2.5 mg/kg by gavage for 28 days Group 4: Infected treated with glucantime (60 mg/kg salt injected IP) *: P<0.001 by χ 2 test Acta Medica Iranica, Vol. 46, No. 3 (2008) 193
4 Miltefosine against leshmania Fig 1. Effect of treatment with miltefosin and Glucantim on size of lesions after one, two and eight weeks after the beginning of treatment. *: P<0.01. **: P<0.05. ***: P< DISCUSSION Fig. 2. Effect of different concentration of miltefosine on the proliferation of amastigotes of L.major. parasit count (%) Parasit count = (72.95 ± 1.097) + [( ± 1.34) * log concenteration)] Log concenteration (μm) Fig. 3. Effect of different concentration of Glucantime on the proliferation of amastigotes of L.major Leishmaniasis is a worldwide disease still treated with expensive compounds that present severe side effects, and are frequently ineffective, emphasizing the importance to search new compounds against this disease. The standard agents for leishmaniasispentavalent antimonials, pentamidine, and amphotericin B- have the disadvantages of repeated parenteral injection and of toxicity (14). Oral medications have an obvious appeal with ease of administration, domiciliary treatment, no hospital costs, no limitation by bed capacity. Thus, the quest for an effective oral antileishmanial drug has been ongoing for a long period. Drugs such as allopurinol, ketoconazole, triazoles (fluconazole), atovaquone, have been tested either alone or in combination for the treatment of VL; however, they had either no effect or only a partial effect (15). An earlier study in localized cutaneous leishmaniasis reported successful miltefosine treatment of patients with Leishmania (viannia) panamensis infections. Miltefosine has been used for up to 2 years in maintenance treatment in patients with HIV/visceral leishmaniasis, 6 but we found no reports of long-term maintenance treatment of human beings with cutaneous forms of leishmaniasis 194 Acta Medica Iranica, Vol. 46, No. 3 (2008)
5 J. Esmaeili et al. (14). Miltefosine is effective in vitro and in vivo against Leishmania species (6, 11), and Kuhlencord et al. demonstrated efficacy in animals via the oral route (6). In previous studies, it was demonstrated that mitlefosine can be applied orally without overt side effects. After a daily oral application of the mitlefosine (10 mg/kg) to rats, a steady-state level of about 100, μm was obtained in serum (16), indicating that mitlefosine is well absorbed from the gut. Furthermore, biodistribution studies of mitlefosine in mice demonstrated an accumulation of the compound in spleen and liver (17). An outstanding advantage of He-PC is its significant activity after oral administration, since few other antileishmanial drugs that are effective by oral administration are known. Ketoconazole, allopurinol, and allopurinol riboside are effective in vitro; however, clinical trials showed that cures could be achieved in only a few patients (18, 19). The results of our study shows that miltefosine has a good suppression effects on the pro suggest that meltefosine oral might be a promising approach for developing new anti-leishmanial drugs. Conflict of interests The authors declare that they have no competing interests. REFERENCES 1. Scarisbrick JJ, Chiodini PL, Watson J, Moody A, Armstrong M, Lockwood D, Bryceson A, Vega-López F. Clinical features and diagnosis of 42 travellers with cutaneous leishmaniasis. Travel Med Infect Dis Jan; 4(1): Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J Jul-Sep; 10(4-5): Momeni AZ, Aminjavaheri M. Successful treatment of non-healing cases of cutaneous leishmaniasis, using a combination of meglumine antimoniate plus allopurinol. Eur J Dermatol Jan-Feb; 13(1): Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med May; 3(5):e Soto J, Toledo J, Gutierrez P, Nicholls RS, Padilla J, Engel J, Fischer C, Voss A, Berman J. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis Oct 1;33(7):E Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother Aug; 36(8): Croft SL, Neal RA, Pendergast W, Chan JH. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol Aug 15;36(16): Sundar S, Rosenkaimer F, Makharia MK, Goyal AK, Mandal AK, Voss A, Hilgard P, Murray HW. Trial of oral miltefosine for visceral leishmaniasis. Lancet Dec 5;352(9143): Jha TK, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med Dec 9;341(24): Berman J. Miltefosine to treat leishmaniasis. Expert Opin Pharmacother Jul; 6(8): Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop Jul; 103(1): Alimohammadian MH, Darabi H, Kariminia A, Rivier D, Bovay P, Mauel J, Ajdary S, Kharazmi A. Adjuvant Effect of Leishmania major promastigotes onthe immune response of mice to ovalbumin. Iranian Biomed J. 2002; 6: Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis Aug;180(2): Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, Luz M, Gutierrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis May 1;38(9): Acta Medica Iranica, Vol. 46, No. 3 (2008) 195
6 Miltefosine against leshmania 15. Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis Apr; 24(4): Unger C, Fleer E, Damenz W, Hilgard P, Nagel G, Eibl H. Hexadecylphosphocholine: determination of serum concentrations in rats. J Lipid Mediat Jan- Feb; 3(1): Breiser A, Kim DJ, Fleer EA, Damenz W, Drube A, Berger M, Nagel GA, Eibl H, Unger C. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids Nov; 22(11): Saenz RE, Paz H, Berman JD. Efficacy of ketoconazole against Leishmania braziliensis panamensis cutaneous leishmaniasis. Am J Med Aug; 89(2): Saenz RE, Paz HM, Johnson CM, Marr JJ, Nelson DJ, Pattishall KH, Rogers MD. Treatment of American cutaneous leishmaniasis with orally administered allopurinol riboside. J Infect Dis Jul;160(1): Acta Medica Iranica, Vol. 46, No. 3 (2008)
Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study
 DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
In Vivo Efficacy of Gum Obtained Pistacia Atlantica in Experimental Treatment of Cutaneous Leishmaniasis
 Iranian J Publ Health, Vol. 39, No.1, Iranian 2010, pp.36-41 J Publ Health, Vol. 39, No.1, 2010, pp.36-41 Original Article In Vivo Efficacy of Gum Obtained Pistacia Atlantica in Experimental Treatment
Iranian J Publ Health, Vol. 39, No.1, Iranian 2010, pp.36-41 J Publ Health, Vol. 39, No.1, 2010, pp.36-41 Original Article In Vivo Efficacy of Gum Obtained Pistacia Atlantica in Experimental Treatment
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp Iranian J Parasitol. Open access Journal at ijpa.tums.ac.ir
 Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1992, p. 1630-1634 Vol. 36, No. 8 0066-4804/92/081630-05$02.00/0 Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice ARMIN KUHLENCORD,1*
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1992, p. 1630-1634 Vol. 36, No. 8 0066-4804/92/081630-05$02.00/0 Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice ARMIN KUHLENCORD,1*
Miltefosine for New World Cutaneous Leishmaniasis
 MAJOR ARTICLE for New World Cutaneous sis J. Soto, 1 B. A. Arana, 4 J. Toledo, 1 N. Rizzo, 4 J. C. Vega, 2 A. Diaz, 4 M. Luz, 1 P. Gutierrez, 1 M. Arboleda, 3 J. D. Berman, 6,a K. Junge, 5 J. Engel, 5
MAJOR ARTICLE for New World Cutaneous sis J. Soto, 1 B. A. Arana, 4 J. Toledo, 1 N. Rizzo, 4 J. C. Vega, 2 A. Diaz, 4 M. Luz, 1 P. Gutierrez, 1 M. Arboleda, 3 J. D. Berman, 6,a K. Junge, 5 J. Engel, 5
Treatment of Cutaneous Leishmaniasis with Allopurinol and Stibogluconate
 165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
Received 13 June 2010/Returned for modification 30 July 2010/Accepted 12 August 2010
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 2010, p. 4699 4704 Vol. 54, No. 11 0066-4804/10/$12.00 doi:10.1128/aac.00809-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Reductions
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 2010, p. 4699 4704 Vol. 54, No. 11 0066-4804/10/$12.00 doi:10.1128/aac.00809-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Reductions
The New England Journal of Medicine
 The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
Iranian J Parasitol: Vol. 5, No.3, 2010, pp Iranian J Parasitol. Open access Journal at ijpa.tums.ac.ir
 Tehran University of Medical Sciences Publication http:// tums.ac.ir Iranian J Parasitol Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir Original Paper
Tehran University of Medical Sciences Publication http:// tums.ac.ir Iranian J Parasitol Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir Original Paper
Pharmacokinetics of Miltefosine in Old World Cutaneous Leishmaniasis Patients
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2008, p. 2855 2860 Vol. 52, No. 8 0066-4804/08/$08.00 0 doi:10.1128/aac.00014-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2008, p. 2855 2860 Vol. 52, No. 8 0066-4804/08/$08.00 0 doi:10.1128/aac.00014-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study
 MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
Drug resistance in Indian visceral leishmaniasis
 Tropical Medicine and International Health volume6no11pp849±854november2001 Drug resistance in Indian visceral leishmaniasis Shyam Sundar Kala-azar Medical Research Centre, Banaras Hindu University, Varanasi,
Tropical Medicine and International Health volume6no11pp849±854november2001 Drug resistance in Indian visceral leishmaniasis Shyam Sundar Kala-azar Medical Research Centre, Banaras Hindu University, Varanasi,
Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial
 Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
References for Application for inclusion of paromomycin in the WHO Model List of Essential Medicines
 6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
Therapeutic Options for Visceral Leishmaniasis
 Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use
 MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
Iran J Parasitol: Vol. 12, No. 3, Jul-Sep 2017, pp Iran J Parasitol. Open access Journal at ijpa.tums.ac.ir
 Iran J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir Original Article
Iran J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir Original Article
Epidemic Outbreak of Cutaneous Leishmaniasis due to Leishmania major in Ghanavat Rural District, Qom Province, Central Iran
 Epidemic Outbreak of Cutaneous Leishmaniasis due to Leishmania major in Ghanavat Rural District, Qom Province, Central Iran *AA Akhavan 1, MR Yaghoobi-Ershadi 1, D Mehdipour 2, H Abdoli 3, B Farzinnia
Epidemic Outbreak of Cutaneous Leishmaniasis due to Leishmania major in Ghanavat Rural District, Qom Province, Central Iran *AA Akhavan 1, MR Yaghoobi-Ershadi 1, D Mehdipour 2, H Abdoli 3, B Farzinnia
Efficacies of Vesicular and Free Sodium Stibogluconate Formulations against Clinical Isolates of Leishmania donovani
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 2001, p. 3555 3559 Vol. 45, No. 12 0066-4804/01/$04.00 0 DOI: 10.1128/AAC.45.12.3555 3559.2001 Copyright 2001, American Society for Microbiology. All Rights
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 2001, p. 3555 3559 Vol. 45, No. 12 0066-4804/01/$04.00 0 DOI: 10.1128/AAC.45.12.3555 3559.2001 Copyright 2001, American Society for Microbiology. All Rights
A Comparison of Miltefosine and Sodium. Ritmeijer, K; Dejenie, A; Assefa, Y; Hundie, T B; Mesure, J; Boots, G; den Boer, M; Davidson, R N
 MSF Field Research A Comparison of and Sodium Stibogluconate for Treatment of Visceral Leishmaniasis in an Ethiopian Population with High Prevalence of HIV Infection. Authors Citation Ritmeijer, K; Dejenie,
MSF Field Research A Comparison of and Sodium Stibogluconate for Treatment of Visceral Leishmaniasis in an Ethiopian Population with High Prevalence of HIV Infection. Authors Citation Ritmeijer, K; Dejenie,
Epidemiological Study of Cutaneous Leishmaniasis in Tuz
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 1 (2017) pp. 477-483 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2017.601.056
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 1 (2017) pp. 477-483 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2017.601.056
Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis. Shyam Sundar Banaras Hindu University Varanasi, India
 Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis Shyam Sundar Banaras Hindu University Varanasi, India History of the Current Epidemic in India & Antimony Resistance 6 Official
Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis Shyam Sundar Banaras Hindu University Varanasi, India History of the Current Epidemic in India & Antimony Resistance 6 Official
Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment
 MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
Leishmaniasis. CDR R.L. Gutierrez Oct 2014
 Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Cutaneous Leishmaniasis with Unusual Clinical and Histological Presentation: Report of Four Cases
 CASE REPORT Cutaneous Leishmaniasis with Unusual Clinical and Histological Presentation: Report of Four Cases Hamideh Moravvej 1, Mohammadreza Barzegar 1, Soheila Nasiri 1, Ehsan Abolhasani 1, and Mehdi
CASE REPORT Cutaneous Leishmaniasis with Unusual Clinical and Histological Presentation: Report of Four Cases Hamideh Moravvej 1, Mohammadreza Barzegar 1, Soheila Nasiri 1, Ehsan Abolhasani 1, and Mehdi
Miltefosine for Old World cutaneous leishmaniasis: An experimental study on Leishmania major infected mice
 Alexandria Journal of Medicine (2012) 48, 261 271 Alexandria University Faculty of Medicine Alexandria Journal of Medicine www.sciencedirect.com ORIGINAL ARTICLE Miltefosine for Old World cutaneous leishmaniasis:
Alexandria Journal of Medicine (2012) 48, 261 271 Alexandria University Faculty of Medicine Alexandria Journal of Medicine www.sciencedirect.com ORIGINAL ARTICLE Miltefosine for Old World cutaneous leishmaniasis:
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan
 Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
Leishmaniasis is a cosmopolitan disease
 Pakistan J. Zool., vol. 36(1), pp. 45-52, 24. Anti-Leishmanial Drug Delivery: Acetylated LDL as A Site-Specific Delivery Ligand AKRAM SHAH * AND DAVID HART Life Sciences Department, School of Life, Basic
Pakistan J. Zool., vol. 36(1), pp. 45-52, 24. Anti-Leishmanial Drug Delivery: Acetylated LDL as A Site-Specific Delivery Ligand AKRAM SHAH * AND DAVID HART Life Sciences Department, School of Life, Basic
Laboratory diagnosis of Blood and tissue flagellates
 Laboratory diagnosis of Blood and tissue flagellates (Leishmania and trypanosma) Sarah Alharbi Clinical Laboratory department Collage of Applied Medical Sciences King Saud University Leishmania and trypanosma:
Laboratory diagnosis of Blood and tissue flagellates (Leishmania and trypanosma) Sarah Alharbi Clinical Laboratory department Collage of Applied Medical Sciences King Saud University Leishmania and trypanosma:
Leishmania major Infection
 Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
The Most Common Parasitic Infections In Yemen. Medical Parasitology
 The Most Common Parasitic Infections In Yemen Medical Parasitology ﻓﺎﯾز اﻟﺧوﻻﻧﻲ / د 2 : is a vector-borne disease that transmitted by sandflies and caused by obligate intracellular protozoa of the genus
The Most Common Parasitic Infections In Yemen Medical Parasitology ﻓﺎﯾز اﻟﺧوﻻﻧﻲ / د 2 : is a vector-borne disease that transmitted by sandflies and caused by obligate intracellular protozoa of the genus
HAEMOFLAGELLATES. Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine
 HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
Topical effectiveness of different concentrations of nanosilver solution on Leishmania major lesions in Balb/c mice
 J Vector Borne Dis 49, Dec 2012, pp. 249 253 Topical effectiveness of different concentrations of nanosilver solution on Leishmania major lesions in Balb/c mice Mohammad Ali Nilforoushzadeh 1, Leila Shirani-Bidabadi
J Vector Borne Dis 49, Dec 2012, pp. 249 253 Topical effectiveness of different concentrations of nanosilver solution on Leishmania major lesions in Balb/c mice Mohammad Ali Nilforoushzadeh 1, Leila Shirani-Bidabadi
Efficacies of KY62 against Leishmania amazonensis and Leishmania donovani in Experimental Murine Cutaneous Leishmaniasis and Visceral Leishmaniasis
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 1998, p. 2542 2548 Vol. 42, No. 10 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Efficacies of KY62 against Leishmania
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 1998, p. 2542 2548 Vol. 42, No. 10 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Efficacies of KY62 against Leishmania
Downloaded from irje.tums.ac.ir at 8:16 IRST on Friday March 8th 2019
 .8-34 :1 1 1395 +98-34-3357541 : 4 3 1 1 HSR 3 4 HSR :. 86 : : iraj.sharifi@yahoo.com :. :.... P 0.05 94/10/5 : 94/6/1 :. 3 138-86 :. 1.. :... : 65....(1 ). 30 10 30 138.(3-5) 9/. :() : 48-1 6 - ( ). (PHC).
.8-34 :1 1 1395 +98-34-3357541 : 4 3 1 1 HSR 3 4 HSR :. 86 : : iraj.sharifi@yahoo.com :. :.... P 0.05 94/10/5 : 94/6/1 :. 3 138-86 :. 1.. :... : 65....(1 ). 30 10 30 138.(3-5) 9/. :() : 48-1 6 - ( ). (PHC).
Leishmaniasis: A forgotten disease among neglected people
 ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
Kala-Azar- Treatment Update
 JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
Int.J.Curr.Res.Aca.Rev.2017; 5(4):
 International Journal of Current Research and Academic Review ISSN: 2347-3215 (Online) Volume 5 Number 4 (April-2017) Journal homepage: http://www.ijcrar.com doi: https://doi.org/10.20546/ijcrar.2017.504.014
International Journal of Current Research and Academic Review ISSN: 2347-3215 (Online) Volume 5 Number 4 (April-2017) Journal homepage: http://www.ijcrar.com doi: https://doi.org/10.20546/ijcrar.2017.504.014
Leishmaniasis chemotherapy challenges and opportunities
 REVIEW 10.1111/j.1469-0691.2011.03630.x Leishmaniasis chemotherapy challenges and opportunities S. L. Croft 1 and P. Olliaro 2,3 1) Faculty of Infectious and Tropical Diseases, London School of Hygiene
REVIEW 10.1111/j.1469-0691.2011.03630.x Leishmaniasis chemotherapy challenges and opportunities S. L. Croft 1 and P. Olliaro 2,3 1) Faculty of Infectious and Tropical Diseases, London School of Hygiene
An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran
 An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran ORIGINAL ARTICLE ABSTRACT Cutaneous leishmaniasis (CL) is a widespread tropical infection which has a high incidence
An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran ORIGINAL ARTICLE ABSTRACT Cutaneous leishmaniasis (CL) is a widespread tropical infection which has a high incidence
Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India
 Indian J Med Res 120, September 2004, pp 166-172 Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India C.P. Thakur, S. Narayan* & A. Ranjan* Balaji
Indian J Med Res 120, September 2004, pp 166-172 Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India C.P. Thakur, S. Narayan* & A. Ranjan* Balaji
The status of Visceral Leishmaniasis in India
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(8):195-201 Review Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 The status of Visceral Leishmaniasis in India Naveen
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(8):195-201 Review Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 The status of Visceral Leishmaniasis in India Naveen
Studying the Chemical Composition in Vitro Activity of Cinnamomum zeylanicum and Eugenia caryophyllata Essential Oils on Leishmania major
 Studying the Chemical Composition in Vitro Activity of Cinnamomum zeylanicum and Eugenia caryophyllata Essential Oils on Leishmania major Oghlniaz. Jorjani 1, Mojtaba Raeisi 1*, Hajar Ziaei Hezarjaribi
Studying the Chemical Composition in Vitro Activity of Cinnamomum zeylanicum and Eugenia caryophyllata Essential Oils on Leishmania major Oghlniaz. Jorjani 1, Mojtaba Raeisi 1*, Hajar Ziaei Hezarjaribi
Visceral leishmaniasis - current therapeutic modalities
 Review Article Indian J Med Res 123, March 2006, pp 345-352 Visceral leishmaniasis - current therapeutic modalities Shyam Sundar & Mitali Chatterjee* Kala-azar Medical Research Center, Department of Medicine,
Review Article Indian J Med Res 123, March 2006, pp 345-352 Visceral leishmaniasis - current therapeutic modalities Shyam Sundar & Mitali Chatterjee* Kala-azar Medical Research Center, Department of Medicine,
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course
 Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Leishmaniasis, Kala Azar(The Black Fever)
 Leishmaniasis, Kala Azar(The Black Fever) By Lawrence Hall Etiologic agent Protist obligate intracellular parasite, Transmission Vectors Phylum: Euglenozoa (genus Leishmania) Over 21 species that infect
Leishmaniasis, Kala Azar(The Black Fever) By Lawrence Hall Etiologic agent Protist obligate intracellular parasite, Transmission Vectors Phylum: Euglenozoa (genus Leishmania) Over 21 species that infect
Leishmaniasis. MAJ Kris Paolino September 2014
 Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Nanomedicine & Drug Delivery Systems Group Research Institute for Medicines and Pharmaceutical Sciences (imed.ul)
 Faculty of Pharmacy of the University of Lisbon Nanomedicine & Drug Delivery Systems Group Research Institute for Medicines and Pharmaceutical Sciences (imed.ul) Leishmania in southern Europe. (Duiardin,
Faculty of Pharmacy of the University of Lisbon Nanomedicine & Drug Delivery Systems Group Research Institute for Medicines and Pharmaceutical Sciences (imed.ul) Leishmania in southern Europe. (Duiardin,
New insights on leishmaniasis in immunosuppressive conditions
 New insights on leishmaniasis in immunosuppressive conditions Javier Moreno Immunoparasitology Unit WHO Collaborative Center for Leishmaniasis Centro Nacional de Microbiología INSTITUTO DE SALUD CARLOS
New insights on leishmaniasis in immunosuppressive conditions Javier Moreno Immunoparasitology Unit WHO Collaborative Center for Leishmaniasis Centro Nacional de Microbiología INSTITUTO DE SALUD CARLOS
Transactions of the Royal Society of Tropical Medicine and Hygiene
 Transactions of the Royal Society of Tropical Medicine and Hygiene 104 (2010) 225 229 Contents lists available at ScienceDirect Transactions of the Royal Society of Tropical Medicine and Hygiene journal
Transactions of the Royal Society of Tropical Medicine and Hygiene 104 (2010) 225 229 Contents lists available at ScienceDirect Transactions of the Royal Society of Tropical Medicine and Hygiene journal
Leishmaniasis impact and treatment access
 REVIEW 10.1111/j.1469-0691.2011.03635.x Leishmaniasis impact and treatment access M. den Boer, D. Argaw, J. Jannin and J. Alvar Leishmaniasis Control Programme, WHO/IDM. Av. Appia, Geneva, Switzerland
REVIEW 10.1111/j.1469-0691.2011.03635.x Leishmaniasis impact and treatment access M. den Boer, D. Argaw, J. Jannin and J. Alvar Leishmaniasis Control Programme, WHO/IDM. Av. Appia, Geneva, Switzerland
Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes
 Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes Undergraduate Honors Thesis The Ohio State University, College of Pharmacy Division of Medicinal Chemistry and Pharmacognosy
Mechanistic Studies of Pentamidine Analogs on Leishmania donovani Promastigotes Undergraduate Honors Thesis The Ohio State University, College of Pharmacy Division of Medicinal Chemistry and Pharmacognosy
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar
 Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
Effects of Miltefosine and Other Alkylphosphocholines on Human Intestinal Parasite Entamoeba histolytica
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2001, p. 1505 1510 Vol. 45, No. 5 0066-4804/01/$04.00 0 DOI: 10.1128/AAC.45.5.1505 1510.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2001, p. 1505 1510 Vol. 45, No. 5 0066-4804/01/$04.00 0 DOI: 10.1128/AAC.45.5.1505 1510.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved.
Leishmaniasis. By Joseph Knight, PA-C. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India.
 Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
Therapy of Visceral Leishmaniasis Due to Leishmania infantum: Experimental Assessment of Efficacy of AmBisome
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 1996, p. 1214 1218 Vol. 40, No. 5 0066-4804/96/$04.00 0 Copyright 1996, American Society for Microbiology Therapy of Visceral Leishmaniasis Due to Leishmania
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 1996, p. 1214 1218 Vol. 40, No. 5 0066-4804/96/$04.00 0 Copyright 1996, American Society for Microbiology Therapy of Visceral Leishmaniasis Due to Leishmania
Therapy of Visceral Leishmaniasis Due to Leishmania infantum: Experimental Assessment of Efficacy of AmBisome
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 1996, p. 1214 1218 Vol. 40, No. 5 0066-4804/96/$04.00 0 Copyright 1996, American Society for Microbiology Therapy of Visceral Leishmaniasis Due to Leishmania
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 1996, p. 1214 1218 Vol. 40, No. 5 0066-4804/96/$04.00 0 Copyright 1996, American Society for Microbiology Therapy of Visceral Leishmaniasis Due to Leishmania
Acta Dermatovenerol Croat 2008;16(2):60-64 CLINICAL ARTICLE
 2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
Antileishmanial Activity of Lavandula angustifolia and Rosmarinus Officinalis Essential Oils and Nano-emulsions on Leishmania major (MRHO/IR/75/ER)
 Iran J Parasitol: Vol. 12, No. 4, Oct-Dec 2017, pp.622-631 Iran J Parasitol Tehran University of Medical Sciences Publication http://tums.ac.ir Open access Journal at http://ijpa.tums.ac.ir Iranian Society
Iran J Parasitol: Vol. 12, No. 4, Oct-Dec 2017, pp.622-631 Iran J Parasitol Tehran University of Medical Sciences Publication http://tums.ac.ir Open access Journal at http://ijpa.tums.ac.ir Iranian Society
Control of leishmaniasis
 SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
World Health Organization Department of Communicable Disease Surveillance and Response
 WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the
WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the
A Novel Noninvasive Method for Diagnosis of Visceral Leishmaniasis by. rk39 Test in Sputum Samples
 JCM Accepts, published online ahead of print on 0 June 00 J. Clin. Microbiol. doi:0./jcm.00-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
JCM Accepts, published online ahead of print on 0 June 00 J. Clin. Microbiol. doi:0./jcm.00-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
Research Article Some Epidemiological Aspects of Cutaneous Leishmaniasis in a New Focus, Central Iran
 Dermatology Research and Practice Volume 2015, Article ID 286408, 5 pages http://dx.doi.org/10.1155/2015/286408 Research Article Some Epidemiological Aspects of Cutaneous Leishmaniasis in a New Focus,
Dermatology Research and Practice Volume 2015, Article ID 286408, 5 pages http://dx.doi.org/10.1155/2015/286408 Research Article Some Epidemiological Aspects of Cutaneous Leishmaniasis in a New Focus,
Hamid M. Niknam, Ph.D.
 Hamid M. Niknam, Ph.D. PERSONAL IDENTIFICATIONS: Name: Hamid Family name: Mahmoudzadeh Niknam Date of birth: 1959 Place of birth: Tehran Address: Department of Immunology, Pasteur Institute of Iran, No
Hamid M. Niknam, Ph.D. PERSONAL IDENTIFICATIONS: Name: Hamid Family name: Mahmoudzadeh Niknam Date of birth: 1959 Place of birth: Tehran Address: Department of Immunology, Pasteur Institute of Iran, No
Anthroponotic Cutaneous Leishmaniasis in Nonendemic Quarters of a Centeral City in Iran
 Iranian J Publ Health, Vol. 6, No., 7, pp.7- Iranian J Publ Health, Vol. 6, No., 7, pp.7- Original Articles Anthroponotic Cutaneous Leishmaniasis in Nonendemic Quarters of a Centeral City in Iran *AR Zahraei-Ramazani,
Iranian J Publ Health, Vol. 6, No., 7, pp.7- Iranian J Publ Health, Vol. 6, No., 7, pp.7- Original Articles Anthroponotic Cutaneous Leishmaniasis in Nonendemic Quarters of a Centeral City in Iran *AR Zahraei-Ramazani,
Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India
 The new england journal of medicine original article Single-Dose Liposomal for Visceral Leishmaniasis in India Shyam Sundar, M.D., Jaya Chakravarty, M.D., Dipti Agarwal, M.D., Madhukar Rai, M.D., and Henry
The new england journal of medicine original article Single-Dose Liposomal for Visceral Leishmaniasis in India Shyam Sundar, M.D., Jaya Chakravarty, M.D., Dipti Agarwal, M.D., Madhukar Rai, M.D., and Henry
1. Introduction. Correspondence should be addressed to Homa Hajjaran; and Mehdi Mohebali;
 BioMed Research International Volume 2013, Article ID 789326, 7 pages http://dx.doi.org/10.1155/2013/789326 Research Article Molecular Identification and Polymorphism Determination of Cutaneous and Visceral
BioMed Research International Volume 2013, Article ID 789326, 7 pages http://dx.doi.org/10.1155/2013/789326 Research Article Molecular Identification and Polymorphism Determination of Cutaneous and Visceral
THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
 Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran Tel: ( )
 Name: Mahin Farahmand Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran 1316943551 Tel: (+ 98-21) 66968855 E-mail: sana@pasteur.ac.ir Mahin362008@yahoo.com Nationality: Iranian QUALIFICATION:
Name: Mahin Farahmand Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran 1316943551 Tel: (+ 98-21) 66968855 E-mail: sana@pasteur.ac.ir Mahin362008@yahoo.com Nationality: Iranian QUALIFICATION:
Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines:
 Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines: Comments re Feb 2011 reviews of the v 2010 application February 23, 2011 1. The application and its review In v 2010,
Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines: Comments re Feb 2011 reviews of the v 2010 application February 23, 2011 1. The application and its review In v 2010,
SMe. Me NITROIMIDAZOLES FOR VISCERAL LEISHMANIASIS FEXINIDAZOLE AND VL-2098 SHYAM SUNDAR
 SMe O 2 CH 2 O Me ITROIMIDAZOLES FOR VISCERAL LEISHMAIASIS FEXIIDAZOLE AD VL-2098 SHYAM SUDAR Target Product Profile for a CE Optimal Target Profile Target Label VL and PKDL VL Spp All species L. donavani
SMe O 2 CH 2 O Me ITROIMIDAZOLES FOR VISCERAL LEISHMAIASIS FEXIIDAZOLE AD VL-2098 SHYAM SUDAR Target Product Profile for a CE Optimal Target Profile Target Label VL and PKDL VL Spp All species L. donavani
Medical Science. Jyoti Joshi, Sukhbir Kaur RESEARCH
 Medical Science The International Weekly journal ISSN 2321 7359 EISSN 2321 7367 RESEARCH To investigate the protective efficacy and immunomodulatory potential of single dose combination of SSG along with
Medical Science The International Weekly journal ISSN 2321 7359 EISSN 2321 7367 RESEARCH To investigate the protective efficacy and immunomodulatory potential of single dose combination of SSG along with
Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial
 Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
WRAIR- GEIS 'OPERATIONAL CLINICAL INFECTIOUS DISEASE' COURSE
 Leishmaniasis WRAIR- GEIS 'OPERATIONAL CLINICAL INFECTIOUS DISEASE' COURSE The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as
Leishmaniasis WRAIR- GEIS 'OPERATIONAL CLINICAL INFECTIOUS DISEASE' COURSE The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as
Phlebotomus argentipes. Amphotericin B has long been recognized as a powerful anti-kala-azar drug
 A STUDY OF EFFICACY AND ADVERSE DRUG REACTIONS OF CONVENTIONAL AMPHOTERICIN B IN THE TREATMENT OF KALA-AZAR Rajesh Kumar Pandey 1, S. N. Singh 2, Bharat Kumar 3, Kashif Shahnawaz 4 HOW TO CITE THIS ARTICLE:
A STUDY OF EFFICACY AND ADVERSE DRUG REACTIONS OF CONVENTIONAL AMPHOTERICIN B IN THE TREATMENT OF KALA-AZAR Rajesh Kumar Pandey 1, S. N. Singh 2, Bharat Kumar 3, Kashif Shahnawaz 4 HOW TO CITE THIS ARTICLE:
Some Ecological Aspects of Phlebotomine Sand Flies in an Endemic Focus of Cutaneous Leishmaniasis in Iran
 Original Article Some Ecological Aspects of Phlebotomine Sand Flies in an Endemic Focus of Cutaneous Leishmaniasis in Iran H Abdoli 1, SH Hejazi 2, *AA Akhavan 3, AR Zahraei-Ramazani 3, MR Yaghoobi-Ershadi
Original Article Some Ecological Aspects of Phlebotomine Sand Flies in an Endemic Focus of Cutaneous Leishmaniasis in Iran H Abdoli 1, SH Hejazi 2, *AA Akhavan 3, AR Zahraei-Ramazani 3, MR Yaghoobi-Ershadi
18 : 1. Shyam Sundar, Anup Singh, Arun Shah, Varanasi EPIDEMIOLOGY: DIAGNOSIS OF VL:
 18 : 1 Visceral leishmaniasis-current scenario Visceral leishmaniasis (VL) is a systemic disease that is fatal if left untreated and is caused by an obligate intracellular protozoan parasite of genus leishmania.
18 : 1 Visceral leishmaniasis-current scenario Visceral leishmaniasis (VL) is a systemic disease that is fatal if left untreated and is caused by an obligate intracellular protozoan parasite of genus leishmania.
Cutaneous Leishmania
 DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY Cutaneous Leishmania Ibrahim Khalifeh, MD I do not have any relevant relationships with industry. Cutaneous Leishmania: Is it a Legend? Ibrahim Khalifeh,
DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY Cutaneous Leishmania Ibrahim Khalifeh, MD I do not have any relevant relationships with industry. Cutaneous Leishmania: Is it a Legend? Ibrahim Khalifeh,
Cutaneous Leishmaniasis in an Immigrant Saudi Worker: A Case Report
 J HEALTH POPUL NUTR 2014 Jun;32(2):372-376 ISSN 1606-0997 $ 5.00+0.20 INTERNATIONAL CENTRE FOR DIARRHOEAL DISEASE RESEARCH, BANGLADESH CASE STUDY Cutaneous Leishmaniasis in an Immigrant Saudi Worker: A
J HEALTH POPUL NUTR 2014 Jun;32(2):372-376 ISSN 1606-0997 $ 5.00+0.20 INTERNATIONAL CENTRE FOR DIARRHOEAL DISEASE RESEARCH, BANGLADESH CASE STUDY Cutaneous Leishmaniasis in an Immigrant Saudi Worker: A
ISPUB.COM. Cutaneous Leishmaniasis in Iran. C Yazdi, M Narmani, B Sadri INTRODUCTION CASE 1 CASE 2
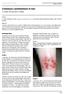 ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
Elimination of VL in the Indian subcontinent is it achievable?
 Elimination of VL in the Indian subcontinent is it achievable? P Das Rajendra Memorial Research Institute, Patna Jorge Alvar DNDi, Geneva Bhawna Sharma DNDi, India Leishmaniasis 350 million at risk worldwide
Elimination of VL in the Indian subcontinent is it achievable? P Das Rajendra Memorial Research Institute, Patna Jorge Alvar DNDi, Geneva Bhawna Sharma DNDi, India Leishmaniasis 350 million at risk worldwide
Visceral Leishmaniasis combination therapies
 Best Science for the Most Neglected From patient needs to implementation of new treatments Visceral Leishmaniasis combination therapies Shyam Sundar Institute of Medical Sciences Banaras Hindu University
Best Science for the Most Neglected From patient needs to implementation of new treatments Visceral Leishmaniasis combination therapies Shyam Sundar Institute of Medical Sciences Banaras Hindu University
Title: Treatment failure and miltefosine susceptibility in dermal leishmaniasis
 AAC Accepts, published online ahead of print on 21 October 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.01023-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Title:
AAC Accepts, published online ahead of print on 21 October 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.01023-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Title:
Serological Survey and Associated Risk Factors of Visceral Leishmaniasis in Qom Province, Central Iran
 Iranian J Publ Health, Vol. 43, No. 1, Jan 2014, pp.50-55 Original Article Serological Survey and Associated Risk Factors of Visceral Leishmaniasis in Qom Province, Central Iran Arash RAKHSHANPOUR 1, Mehdi
Iranian J Publ Health, Vol. 43, No. 1, Jan 2014, pp.50-55 Original Article Serological Survey and Associated Risk Factors of Visceral Leishmaniasis in Qom Province, Central Iran Arash RAKHSHANPOUR 1, Mehdi
Control of leishmaniasis
 EXECUTIVE BOARD EB118/4 118th Session 11 May 2006 Provisional agenda item 5.1 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
EXECUTIVE BOARD EB118/4 118th Session 11 May 2006 Provisional agenda item 5.1 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis
 ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
on November 20, 2018 by guest
 AAC Accepts, published online ahead of print on 13 September 2010 Antimicrob. Agents Chemother. doi:10.1128/aac.00985-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions.
AAC Accepts, published online ahead of print on 13 September 2010 Antimicrob. Agents Chemother. doi:10.1128/aac.00985-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions.
Design and Antileishmanial Activity of Amphotericin B-Loaded Stable Ionic Amphiphile Biovector Formulations
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2002, p. 1597 1601 Vol. 46, No. 5 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.5.1597 1601.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2002, p. 1597 1601 Vol. 46, No. 5 0066-4804/02/$04.00 0 DOI: 10.1128/AAC.46.5.1597 1601.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
Leishmaniasis: Challenges for Vaccine Development
 Leishmaniasis: Challenges for Vaccine Development Steven G. Reed Infectious Disease Research Institute Seattle IDRI Goals: Leishmaniasis To improve existing vaccines for use in therapy and prevention.
Leishmaniasis: Challenges for Vaccine Development Steven G. Reed Infectious Disease Research Institute Seattle IDRI Goals: Leishmaniasis To improve existing vaccines for use in therapy and prevention.
Review Article. Management of visceral leishmaniasis: Indian perspective
 Review Article www.jpgmonline.com Management of visceral leishmaniasis: Indian perspective S. Agrawal, M. Rai, S. Sundar Department of Medicine, Institute of Medical Sciences, Banaras Hindu University,
Review Article www.jpgmonline.com Management of visceral leishmaniasis: Indian perspective S. Agrawal, M. Rai, S. Sundar Department of Medicine, Institute of Medical Sciences, Banaras Hindu University,
In Vitro Antiparasitic and Apoptotic Effects of Antimony Sulfide Nanoparticles on Leishmaniainfantum
 Journal of Parasitology Research Volume 2012, Article ID 756568, 7 pages doi:10.1155/2012/756568 Research Article In Vitro Antiparasitic and Apoptotic Effects of Antimony Sulfide Nanoparticles on Leishmaniainfantum
Journal of Parasitology Research Volume 2012, Article ID 756568, 7 pages doi:10.1155/2012/756568 Research Article In Vitro Antiparasitic and Apoptotic Effects of Antimony Sulfide Nanoparticles on Leishmaniainfantum
Utility of Western Blot Analysis for the Diagnosis of Cutaneous Leishmaniasis
 Iran J Parasitol: Vol. 1, No. 4, Oct -Dec 215, pp.599-64 Iran J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian Society
Iran J Parasitol: Vol. 1, No. 4, Oct -Dec 215, pp.599-64 Iran J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian Society
Incidence Rate of Cutaneous Leishmaniasis in Chabahar within
 Journal of Community Health Research. 2016;5(1): 29-35. Original Article Incidence Rate of Cutaneous Leishmaniasis in Chabahar within 2008-2010 Mojtaba Moghateli 1, faiz Mohammad Atesh Bahar 2, Nooshin
Journal of Community Health Research. 2016;5(1): 29-35. Original Article Incidence Rate of Cutaneous Leishmaniasis in Chabahar within 2008-2010 Mojtaba Moghateli 1, faiz Mohammad Atesh Bahar 2, Nooshin
EPIDEMIOLOGICAL, CLINICAL AND THERAPEUTIC FEATURES OF PEDIATRIC KALA-AZAR
 EPIDEMIOLOGICAL, CLINICAL AND THERAPEUTIC FEATURES OF PEDIATRIC KALA-AZAR Kadivar Mohammad Rahim 1 and Moslehi Mohammad Ashkan 1 1 Department of Pediatrics, Nemazee Hospital, Shiraz University of Medical
EPIDEMIOLOGICAL, CLINICAL AND THERAPEUTIC FEATURES OF PEDIATRIC KALA-AZAR Kadivar Mohammad Rahim 1 and Moslehi Mohammad Ashkan 1 1 Department of Pediatrics, Nemazee Hospital, Shiraz University of Medical
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
Wild Poliovirus*, 03 Aug 2004 to 02 Aug 2005
 Wild Poliovirus*, 03 Aug 2004 to 02 Aug 2005 Wild virus type 1 Wild virus type 3 Wild virus type 1 & 3 Endemic countries Re-established transmission countries Case or outbreak following importation *Excludes
Wild Poliovirus*, 03 Aug 2004 to 02 Aug 2005 Wild virus type 1 Wild virus type 3 Wild virus type 1 & 3 Endemic countries Re-established transmission countries Case or outbreak following importation *Excludes
Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil
 Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
More than 98 countries affected by Leishmaniasis
 More than 98 countries affected by Leishmaniasis ACL In many urban & sub urban areas (8 provinces) Tehran, Mashhad, Neishabur, Shiraz, Kerman, Bam (Sharifi et al. 2011) ZCL In 2012, totally ( ZCL & ACL)
More than 98 countries affected by Leishmaniasis ACL In many urban & sub urban areas (8 provinces) Tehran, Mashhad, Neishabur, Shiraz, Kerman, Bam (Sharifi et al. 2011) ZCL In 2012, totally ( ZCL & ACL)
Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran M.S. P.H.: Medical parasitology (Tehran University of Medical Science)
 Name: Mahin Farahmand Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran 1316943551 Tel : (+ 98-21) 64112811 E-mail: sana@pasteur.ac.ir Mahin362008@yahoo.com Nationality: Iranian QUALIFICATION:
Name: Mahin Farahmand Address: Pasteur Institute of Iran (IPI), Pasteur Ave. Tehran, Iran 1316943551 Tel : (+ 98-21) 64112811 E-mail: sana@pasteur.ac.ir Mahin362008@yahoo.com Nationality: Iranian QUALIFICATION:
