Miltefosine for New World Cutaneous Leishmaniasis
|
|
|
- Stephen Alexander
- 5 years ago
- Views:
Transcription
1 MAJOR ARTICLE for New World Cutaneous sis J. Soto, 1 B. A. Arana, 4 J. Toledo, 1 N. Rizzo, 4 J. C. Vega, 2 A. Diaz, 4 M. Luz, 1 P. Gutierrez, 1 M. Arboleda, 3 J. D. Berman, 6,a K. Junge, 5 J. Engel, 5 and H. Sindermann 5 1 Consorcio de Investigaciones Bioclínicas and 2 Fundación Fader, Bogotá, and 3 Instituto Colombiano de Medicina Tropical, Medellin, Colombia; 4 Center for Health Studies, Universidad del Valle de Guatemala, Guatemala City; and 5 Zentaris GmbH and Baxter Oncology, Frankfurt a. Main, Germany; and 6 Zentaris GmbH and Baxter Oncology, North Bethesda, Maryland The oral agent miltefosine has demonstrated a 195% cure rate in Indian visceral leishmaniasis. We performed a large, placebo-controlled study of miltefosine therapy (2.5 mg/kg per day orally for 28 days) against cutaneous leishmaniasis in Colombia and Guatemala. In regions in Colombia where vianna panamensis is common, the per-protocol cure rates for miltefosine and placebo were 91% (40 of 44 patients) and 38% (9 of 24). These values are similar to historic values for the antimony standard of care and placebo. In regions in Guatemala where L. v. and L. are common, the per-protocol cure rates were 53% (20 of 38) for miltefosine and 21% (4 of 19) for placebo. The miltefosine rate was lower than historic antimony cure rates of 190%. was well tolerated. is a useful oral agent against cutaneous leishmaniasis due to L. v. panamensis in Colombia but not against leishmaniasis due to L. v. in Guatemala. Cutaneous leishmaniasis is endemic in the New World from around the United States Mexico border, through Central America and the northern part of South America, and down to the region of Rio de Janeiro. The disease, which is characterized by papules that enlarge into ulcers, can be caused by a multitude of species: members of the vianna subgenus, such as L. v. panamensis, L. v., and L. v. guayanensis; and members of the complex, such as L. m. amazonensis and L. m.. Parasite species were named for the regions of endemicity in which the parasites are found and subsequently were differentiated by biochemical and other parameters rather than by clinical characteristics of infected patients [1 3]. Thus, the clinical course of infections Received 14 November 2003; accepted 4 January 2004; electronically published 9 April Financial support: Zentaris (to J.S. and B.A.A.). a Present affiliation: National Center for Complementary and Alternative Medicine, National Institutes of Health, Bethesda, Maryland. Reprints or correspondence: Dr. H. Sindermann, Zentaris GmbH, Weismüllerstr. 45, D Frankfurt a. Main, Germany (Herbert.Sindermann@Zentaris.De). Clinical Infectious Diseases 2004; 38: by the Infectious Diseases Society of America. All rights reserved /2004/ $15.00 due to each species the natural history of the ulcer and the response to chemotherapy has to be laboriously investigated for each combination of species and chemotherapeutic agent. It is generally thought that L. v. panamensis and L. v. infections self-cure in 112 months and that 1% 3% of patients metastasize to the mucosa of the nose and mouth (mucosal disease), whereas L. m. infection self-cures much more rapidly and does not lead to mucosal metastasis. L. v. panamensis infection and L. v. infection are treated to accelerate cure of the cutaneous lesion and to attempt to prevent mucosal metastasis, and L. m. infection may or may not be treated. Where L. m. coexists with L. v. and cannot be distinguished by presentation, all presenting lesions are treated. Standard therapy consists of parenteral administration of pentavalent antimonials daily for 20 days [4], although both shorter courses [5] and longer courses with lower amounts of the drug per day [6] have been investigated. Depending on the region of endemicity, antimony cure rates are generally 180% and are frequently 190%. Pentavalent antimonials have the disadvantages of multiple injections and mild-to-moderate clinical tox CID 2004:38 (1 May) Solo et al.
2 icity, both of which are particularly vexing for a moderate clinical problem that almost always self-cures with time. The primary chemotherapeutic need is an oral agent that has acceptable toxicity and that competes with antimonials in efficacy. A 30-year search for an effective oral agent has so far been unrewarding. For example, the promising agent allopurinol had a cure rate of 33% in Colombia [7]. (hexadecylphosphocholine) inhibits phospholipid and sterol biosynthesis [8] of trypanosomids and is effective in vivo against, including by the oral route [9, 10]. Phase 2 and then phase 3 trials against visceral leishmaniasis in India demonstrate a 97% cure rate in patients administered oral miltefosine at a dosage of 2.5 mg/kg per day for 4 weeks [11 14]. Although untreated visceral leishmaniasis results in death in contrast with cutaneous leishmaniasis, which ultimately self-cures very high efficacy against visceral disease does not guarantee similar efficacy against cutaneous disease. The antileishmanial agent paromomycin, for example, cured 73% of Indian patients with the visceral form of the disease at a dosage of 12 mg/kg per day for 3 weeks [15], but it cured 50% 60% of patients with cutaneous leishmaniasis in Colombia [16] and Central America [17], respectively, at approximately the same dose. The program to investigate miltefosine for New World cutaneous leishmaniasis was initiated with an uncontrolled, open-label, dose-ranging study in regions in Colombia where L. v. panamensis predominated (hereafter, L. v. panamensis regions ). The per-protocol cure rate for 32 patients who received 2.5 mg/kg per day for 3 4 weeks was 94% [18]. The most frequent adverse effects were a feeling of motion sickness, vomiting, and transient elevations of liver transaminase levels. To investigate the therapeutic index for oral miltefosine more generally and in a controlled manner, we performed placebocontrolled trials of miltefosine in Colombia and Guatemala. PATIENTS AND METHODS Study design. This was a randomized, placebo controlled, double-blind multicenter trial of miltefosine. There were 2 study sites. In Colombia, the patients were both civilians and soldiers who acquired infection in the provinces of provinces of Urabá and Carmen de Chucurí and who were evaluated in local hospitals for diagnosis and treatment. In Guatemala, the patients were civilians who presented, received diagnoses, and were treated at 2 clinics operated by the Universidad del Valle de Guatemala, which is located in Poptun, El Peten, Guatemala. Study population. Patients were of either sex, aged 112 years, had parasitologically confirmed cutaneous leishmaniasis, and did not have mucosal involvement. Previous treatment for the disease was permitted if therapy had stopped 4 weeks earlier and the lesions were not improving. Significant concomitant diseases were excluded by history and by the requirement for approximately normal complete blood cell counts (i.e., WBC count, hemoglobin level, and platelet count), liver transaminase levels (i.e., aspartate aminotransferase and alanine aminotransferase levels), and kidney function test results (i.e., creatinine and blood urea nitrogen level). Pregnancy and lactation were also exclusion criteria. Study treatments. (50 mg) or matching placebo capsules were administered orally for 28 days under the observation of study staff. To administer 2.5 mg/kg per day, patients 45 kg received 3 capsules per day (1 capsule in the morning, 1 capsule at lunch, and 1 capsule in the evening, after meals), and patients!45 kg received 2 capsules per day (1 capsule in the morning and 1 capsule in the evening, after meals). Toxicity evaluation. Patients were interviewed for subjective adverse events daily during treatment. Blood samples were obtained for repeated blood cell counts, liver function tests, and kidney function tests weekly during therapy, at the end of therapy, and at 2 and 6 months after the end of therapy. Subjective and laboratory adverse events were graded according to the Common Toxicity Criteria (CTC) of the National Cancer Institute ( Efficacy evaluation. For each lesion, the sizes of its ulcer and induration were measured at 2 weeks, 2 months, and 6 months after the end of therapy. Lesions for which there was incomplete reepithelialization of the ulcer or incomplete elimination of induration underwent repeated parasitological investigation. A lesion was defined as a treatment failure if it enlarged by 50% or was positive for parasites 2 weeks to 6 months after the end of therapy, relapsed (enlarged) after previously diminishing in size, or did not completely reepithelialize by 6 months after the end of therapy. Appearance of a new lesion from which could be demonstrated was also a criterion for failure. Cure was defined as complete healing of all lesions by 6 months after the end of therapy. Thus, for a patient to be cured, no lesion could enlarge by 50%, be parasite positive, relapse, or heal incompletely, and no new -positive lesion could appear. Parasitology. Lesion samples were obtained by 1 ofthe following methods: slit skin smear, aspirate, and biopsy. Proof of infection before treatment or of continued infection after treatment consisted of microscopic identification of amastigotes in the direct Giemsa stain of the smear or the demonstration of motile promastigotes cultured from the aspirate and/or biopsy of a lesion. In Colombia, cultures of baseline lesion aspirates were identified to the species level via monoclonal antibody binding [2]. In Guatemala, cultures or for Cutaneous sis CID 2004:38 (1 May) 1267
3 Table 1. Presenting characteristics of patients with cutaneous leishmaniasis in Colombia and Guatemala who received therapy with miltefosine. Characteristic (n p 49) Colombian site (n p 24) (n p 40) Guatemalan site (n p 20) Age, mean years SD Male sex, % Weight, mean kg SD Median no. of lesions (range) 1 (1 8) 1 (1 5) 1 (1 10) 1 (1 3) Ulcer size, median mm 2 (range) 171 ( ) 238 (6 2110) 165 (6 1650) 154 (6 3300) No. (%) of patients with previous therapy failure 3 (6) 2 (8) 10 (25) 8 (40) clinical samples preserved in absolute alcohol were identified to the species levels by PCR [19, 20]. Statistical analysis. The primary end point of the trial was the rate of cured patients. A 2-sided Cochran-Mantel- Haenszel (CHM) test stratified by site was performed to compare the cures after miltefosine and placebo. The comparison of cure rates within each site was performed by 2-sided x 2 tests. Ethics. The study protocol and amendments were approved by the responsible authority at the Colombian study site (Comite de Etica en Investigacion, Hospital Militar Central, Bogota, Colombia) and at the Guatemalan study site (Universidad del Valle Ethics Committee). The first visit of the first patient was in June The last visit of the last patient was in December Table 2. Efficacy of miltefosine used to treat cutaneous leishmaniasis in Colombian and Guatemalan patients. Variable Colombian site (n p 49) RESULTS Patient characteristics. The presenting characteristics of the miltefosine and placebo at the Colombian and Guatemalan sites are listed in table 1. On average, patients were in the third decade of life, weighed 60 kg, and had 1 lesion. The ulcer size was 200 mm 2. Of the 133 patients, 119 (89%) were men. Compliance with therapy. Six of the 133 treated patients did not receive the full 28 days of medication. Two were miltefosine in Colombia: one patient missed his last day of therapy and then was lost to follow-up; the other patient stopped therapy on day 27 because of intolerability. Two were miltefosine in Guatemala; both did not appear for (n p 24) Guatemalan site (n p 40) (n p 20) No. of patients cured No. of patients with treatment failure All Parasite-positive lesions Size of lesion doubled 2 5 a 4 b 2 Relapse No. of unassessable patients All Lost after therapy Lost after 2 weeks Lost after 3 months Cure rate, n/n (%) Intent-to-treat 40/49 (82) 9/24 (38) 20/40 (50) 4/20 (20) Per-protocol 40/44 (91) 9/24 (38) 20/38 (53) 4/19 (21) a All 5 of these patients also had parasite-positive lesions. b Two of these 4 patients also had parasite-positive lesions CID 2004:38 (1 May) Solo et al.
4 Table 3. species infecting Guatemalan patients with cutaneous leishmaniasis. Response vianna Unknown vianna recipient Unknown Cure Treatment failure Unassessable NOTE. Data are no. of patients. P values for intent-to-treat cure rates for patients for whom species was identified: L. v., P p.18, by Fisher s exact test; and L. m., P p.54, by Fisher s exact test. treatment on day 21 and then were lost to follow-up. Two were placebo patients in Guatemala: one did not appear on day 21 and then was lost to follow up, and the other had his last week of treatment stolen and did appear for follow-up. All 6 partially treated patients are included with the 127 fully treated patients in the efficacy and tolerance evaluations below. Efficacy. In Colombia, 40 miltefosine were cured, 4 had illness that failed to respond to therapy, and 5 were lost to follow-up (table 2). The intent-to-treat cure rate was 82%. The per-protocol cure rate, in which the 5 patients who were lost to follow-up are not included, was 91%. There is no reason to hypothesize that the illness of these 5 patients would have failed to respond to therapy. For the 3 patients who were seen after treatment but not at 6 months, 1 patient had completely healed by 2 weeks after therapy, 1 patient had 1 completely healed lesion and one 85% healed lesion 2 weeks after therapy, and 1 patient had completely healed by 3 months after therapy. There were 9 placebo who were cured and 15 who had illness that failed to respond to therapy. The intent-to-treat and per-protocol cure rates were 38%. The difference between the intent-to-treat and per-protocol miltefosine cure rates and the respective placebo cure rates were statistically significant ( P!.001, by the x 2 test) In Guatemala, 20 miltefosine were cured, 18 had illness that failed to respond to therapy, and 2 were unassessable (table 2). Four placebo were cured, and 15 had illness that failed to respond to the placebo. The intent-to-treat cure rates were 50% (miltefosine) versus 20% (placebo), and the per-protocol cure rates were close to these values, because few patients were lost to follow-up. The difference between the intent-to-treat and per-protocol miltefosine cure rates and the respective placebo cure rates was statistically significant (P p.025 for intent-to-treat cure rate and P p.023 for per-protocol cure rate, by x 2 test). The overall treatment comparison of intent-to-treat and per-protocol cure rates stratified by site revealed significant differences ( P!.001, by CMH test). Parasitology. In Colombia, cultures of 7 baseline lesion aspirates were speciated by monoclonal antibody binding. All 7 parasites were L. v. panamensis. In Guatemala, 46 of the 60 infecting parasites were speciated by PCR (table 3). A total of 63% of speciated parasites were L. v., and 37% of speciated parasites were L. m.. The distribution of L. v. and L. m. with cure and failure in response to miltefosine and placebo is shown in table 3. The rate of cure of L. v. was low (33%), compared with the rate of cure of L. m. (60%). Table 4. Tolerance to miltefosine therapy in patients with cutaneous leishmaniasis in Colombia and Guatemala. Variable No. (%) of patients (n p 89) (n p 44) Treatment-emergent adverse events Nausea 32 (36) 4 (9) a Motion sickness 26 (29) 10 (23) Headache 24 (27) 9 (20) Vomiting 1 28 (31) 2 (5) b (25) 1 (2) (3) 1 (2) 14 3 (3) 0 (0) Diarrhea 1 5 (6) 1 (2) (4) 1 (2) 12 1 (1) 0 (0) Laboratory parameters Creatinine level Increased 29 (33) 4 (9) c CTC grade 1 d 28 (31) 4 (9) CTC grade 2 e 1(1) 0(0) Elevated aspartate aminotransferase level 7 (8) 8 (18) Elevated alanine aminotransferase level 9 (10) 5 (11) NOTE. CTC, Common Toxicity Criteria of the National Cancer Institute. a P!.001, byx 2 test. b P!.001, byx 2 test. c P p.003, byx 2 test. d Less than 1.5 times the upper limit of normal. e Between 1.5 and 3.0 times the upper limit of normal. for Cutaneous sis CID 2004:38 (1 May) 1269
5 Table 5. Efficacy of standard antimonial therapy to treat cutaneous leishmaniasis in Colombia and Guatemala. No. of subjects Cure rate, % Study, therapy group Colombian studies All Cured Had treatment failure Lost to follow-up ITT PP Species Glucantime % vianna panamensis Reference(s) Allopurinol Glucantime ND [21] Untreated Glucantime ND [22] Guatemalan studies Glucantime vianna [23] Glucantime L. v. [24] Glucantime At least 50% L. v. [5] [25] NOTE. ITT, intent to treat; ND, not determined; PP, per protocol. Tolerance. Symptomatic and laboratory adverse events for all miltefosine and placebo are summarized in table 4, which lists treatment-emergent subjective events that occurred in at least 10% of patients. The frequency of nausea and vomiting was significantly higher in miltefosine. The large majority of patients who vomited did so on 1 2 occasions. For 3 patients, vomiting occurred 5, 6, or 7 times. No patient discontinued therapy prematurely for these reasons. The single premature discontinuation was due to persistent motion sickness and headache. The creatinine level increased to more than the normal range in 32% of miltefosine, compared with 4% of placebo. In all cases but one, the increase was to CTC grade 1. There was no difference between miltefosine and placebo in the percentage of patients who experienced increases in the liver function tests. All increases were CTC grade 1 (i.e.,!2.5 times the upper limit of normal). DISCUSSION This trial of miltefosine in Colombia and Guatemala is the largest placebo-controlled trial of chemotherapy for cutaneous leishmaniasis yet reported from the New World. Because New World cutaneous leishmaniasis almost always self-cures, and because the rate of self-cure varies with species and region of endemicity, data from uncontrolled trials are difficult to interpret. The present trial was planned after an initial open-label dose-ranging trial of miltefosine against Colombian cutaneous disease generated attractive results. For miltefosine in Colombia, the 6-month intent-to-treat cure rate was 82%, and the 6-month per-protocol cure rate, considering that 5 patients were not assessable, was 91%. In contrast, for placebo, the 6-month intent-to-treat and perprotocol cure rates were 38%. The miltefosine efficacy data in this study well parallels the data from the preceding open-label trial in the same regions of Colombia, for which the 6-month cure rates were 81% (30 of 37 cases) on an intent-to-treat basis and 94% (30 of 32 cases) on a per-protocol basis, with 5 patients being lost to follow-up [18]. A summary of cure rates due to standard therapy with antimony and with placebo is provided in table 5. In Colombia, the per-protocol glucantime cure rates in L. panamensis regions historically have been 84% 93%, and the placebo cure rates have been 36% 37%. The 91% and 38% miltefosine and placebo per-protocol cure rates, respectively, for the present study are very similar to those values. This comparison indicates that the efficacy of miltefosine is equivalent to historic values of standard therapy with glucantime with respect to L. v. panamensis disease in Colombia. For miltefosine in Guatemala, the 6-month intent-to-treat cure rate for miltefosine was 50%, and for placebo, it was 20%. [7] 1270 CID 2004:38 (1 May) Solo et al.
6 Historic values of cure rates due to antimony and placebo for L. v. and L. m. are also summarized in table 5. In Guatemala, L. v. is highly susceptible to antimonial therapy, with a cure rate of generally 190%. Because 63% of speciated parasites were L. v. in the present study, and because 67% were identified as L. v. in previous work in this region [23], the species of did not differ between the present and past work. Thus, the miltefosine cure rate of 50% in the present study can be compared unfavorably to the historic antimony cure rates of 190% for Guatemalan patients infected with comparable species of. Patients with cutaneous leishmaniasis experience only a skin ulcer and are systemically healthy. Because this was the first blinded trial of miltefosine in an essentially normal population, our trial permits determination of the inherent clinical tolerance of this drug. Motion sickness and nausea were each reported by 30% of patients, with nausea but not motion sickness being specifically attributable to the drug. Vomiting but not diarrhea was also specifically attributable to miltefosine and was experienced by 32% of patients. Nevertheless, approximately three-quarters of these patients had only 1 2 episodes of vomiting during the 28-day course of therapy, and no patient stopped therapy for this reason. The creatinine level was more frequently elevated in the miltefosine group than in the placebo group, but almost all creatinine level elevations were mild (CTC grade 1). Aspartate aminotransferase and alanine aminotransferase levels were not more frequently elevated in the miltefosine group than in control subjects. The mild changes in laboratory parameters suggest that in the cutaneous leishmaniasis population, in contrast to visceral leishmaniasis patients who have systemic disease, routine recording of laboratory parameters need not be performed. Chemotherapeutic agents are evaluated on the basis of efficacy, tolerance, and convenience and cost of administration. The standard agents for leishmaniases pentavalent antimonials, pentamidine, and amphotericin B all are effective for New World cutaneous leishmaniasis [26, 27], but they all have the disadvantages of repeated parenteral injection and of toxicity. is an oral agent that this trial has revealed to have acceptable tolerance. The Colombian data from this trial show that miltefosine has demonstrable efficacy, and to a degree similar to historic values of antimony, against apparent L. v. panamensis disease in Colombia. This is the first firm demonstration that any oral agent is either an improvement over placebo or has efficacy comparable to historic values of standard therapy for a form of New World cutaneous leishmaniasis. For Old World cutaneous disease, the only placebo-controlled demonstration of efficacy of an oral agent is that of fluconazole for L. major disease in Saudi Arabia [28]. The efficacy of miltefosine against disease in Guatemala, although higher than that of placebo, was lower than historic values of antimony. The general value of miltefosine for New World cutaneous disease remains to be demonstrated by further studies against L. v. and other endemic species. Acknowledgments We are grateful for the assistance of the personnel of the Seccional de Salud de Santander, the Laboratorio Departamental de Santander, and the Centro de Salud Carmen de Chucurí, Colombia; and of Dr. J Argueta and Lic E. Flores, Ministry of Health, Guatemala. References 1. Kreutzer RD, Semko ME, Hendricks LD, et al. Identification of spp by multiple isozyme analysis. Am J Trop Med Hyg 1983; 32: Chico ME, Guderian RH, Cooper PJ, Armijos R, Grogl M. Evaluation of a direct immunofluorescent antibody (DIFMA) test using genus specific monoclonal antibody in the routine diagnosis of cutaneous leishmaniasis. Rev Soc Bras Med Trop 1995; 28: Gangneux J-P, Menotti J, Lorenzo F, et al. Prospective value of PCR amplification and sequencing for diagnosis and typing of Old World infections in an area of nonendemicity. J Clin Microbiol 2003; 41: Herwaldt BL, Berman JD. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg 1992; 46: Arana BA, Navin TR, Arana FE, et al. Efficacy of a short course (10 days) of high-dose meglumine antimonate with or without interferongamma in treating cutaneous leishmaniasis in Guatemala. Clin Infect Dis 1994; 18: Oliveira-Neto MP, Schubach A, Mattos M, Goncalves-Costa SC, Pirmez C. A low-dose antimony treatment in 159 patients with American cutaneous leishmaniasis: extensive follow-up studies (up to 10 years). Am J Trop Med Hyg 1997; 57: Velez I, Agudelo S, Hendrickx E, et al. Inefficacy of Allopurinol for Colombian cutaneous leishmaniasis: a randomized, controlled trial. Ann Intern Med 1997; 126: Urbina JA. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 1997; 114(Suppl):S Croft SL, Neal RA, Pendergast W, et al. The activity of alkylphosphorylcholines and related derivatives against donovani. Biochem Pharmacol 1987; 36: Kuhlencord A, Maniera T, Eibl H, et al. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother 1992; 36: Sundar S, Rosenkaimer K, Makharia MK, et al. Trial of oral miltefosine for visceral leishmaniasis. Lancet 1998; 352: Jha TK, Sundar S, Thakur CP, et al., an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med 1999; 341: Sundar S, Jha TK, Thakur CP, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 2002; 347: Bhattacharya SK, Jha TK, Sundar S, et al. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin Infect Dis 2004; 38: Jha TK, Olliaro P, Thakur CP, et al. Randomised controlled trial of aminosidine (paromomycin) v sodium stibogluconate for treating visceral leishmaniasis in North Bihar, India. BMJ 1998; 316: for Cutaneous sis CID 2004:38 (1 May) 1271
7 16. Soto J, Grogl M, Berman J, Olliaro P. Limited efficacy of injectable aminosidine as single-agent therapy for Colombian cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 1994; 88: Hepburn NC, Tidman MJ, Hunter JA. Aminosidine (paromomycin) versus sodium stibogluconate for the treatment of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 1994; 88: Soto J, Toledo J, Gutierrez P, et al. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis 2001; 33: E Noyes HA, Belli AA, Miangon R. Appraisal of various random amplified polymorphic DNA polymerase chain reaction primers for identification. Am J Trop Med Hyg 1996; 55: Noyes HA, Reyburn H, Bailey JW, Smith D. A nested-pcr based schizodeme method for identifying kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of tropica in Pakistan. J Clin Microbiol 1998; 36: Soto-Mancipe J, Grogl M, Berman JD. Evaluation of pentamidine for the treatment of cutaneous leishmaniasis in Colombia. Clin Infect Dis 1993; 16: Soto J, Fuya P, Herrera R, Berman J. Topical paromomycin/methylbenzethonium chloride plus parenteral meglumine antimonate as treatment for American cutaneous leishmaniasis: controlled study. Clin Infect Dis 1998; 26: Navin TR, Arana BA, Arana FE, Berman JD, Chajon JF. -controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis 1992; 165: Navin TR, Arana BA, Arana FE, de Merida AM, Castillo AL, Pozuelos JL. -controlled clinical trial of meglumine antimonate (glucantime) vs localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. Am J Trop Med Hyg 1990; 42: Herwaldt BL, Arana BA, Navin TR. The natural history of cutaneous leishmaniasis in Guatemala. J Infect Dis 1992; 165: Soto J, Buffet P, Grogl M, Berman J. Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine. Am J Trop Med Hyg 1994; 50: Sampaio SAP, Castro RM, Dillon NL, Martins JEC. Treatment of mucocutaneous (American) leishmaniasis with amphotericin B: report of 70 cases. Int J Dermatol 1971; 10: Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by major. N Engl J Med 2002; 346: CID 2004:38 (1 May) Solo et al.
Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial
 Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
The New England Journal of Medicine
 The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment
 MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use
 MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES
 ORIGINAL REPORT EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES J. Esmaeili 1, M. Mohebali 1*, G. H. Edrissian 1, S. M. Rezayat 2, M. Ghazi-Khansari 2 and
ORIGINAL REPORT EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES J. Esmaeili 1, M. Mohebali 1*, G. H. Edrissian 1, S. M. Rezayat 2, M. Ghazi-Khansari 2 and
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Treatment of Cutaneous Leishmaniasis with Allopurinol and Stibogluconate
 165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
data indicating a 31% efficacy in northwest Colombia. 6
 Am. J. Trop. Med. Hyg., 64(3, 4), 2001, pp. 187 193 Copyright 2001 by The American Society of Tropical Medicine and Hygiene TREATMENT FAILURE IN CHILDREN IN A RANDOMIZED CLINICAL TRIAL WITH 10 AND 20 DAYS
Am. J. Trop. Med. Hyg., 64(3, 4), 2001, pp. 187 193 Copyright 2001 by The American Society of Tropical Medicine and Hygiene TREATMENT FAILURE IN CHILDREN IN A RANDOMIZED CLINICAL TRIAL WITH 10 AND 20 DAYS
Therapeutic Options for Visceral Leishmaniasis
 Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study
 MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
References for Application for inclusion of paromomycin in the WHO Model List of Essential Medicines
 6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian referral centre
 512 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 111(8): 512-516, August 2016 Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian
512 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 111(8): 512-516, August 2016 Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian
Visceral Leishmaniasis combination therapies
 Best Science for the Most Neglected From patient needs to implementation of new treatments Visceral Leishmaniasis combination therapies Shyam Sundar Institute of Medical Sciences Banaras Hindu University
Best Science for the Most Neglected From patient needs to implementation of new treatments Visceral Leishmaniasis combination therapies Shyam Sundar Institute of Medical Sciences Banaras Hindu University
COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
 Am. J. Trop. Med. Hyg., 72(2), 2005, pp. 133 137 Copyright 2005 by The American Society of Tropical Medicine and Hygiene COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
Am. J. Trop. Med. Hyg., 72(2), 2005, pp. 133 137 Copyright 2005 by The American Society of Tropical Medicine and Hygiene COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis. Shyam Sundar Banaras Hindu University Varanasi, India
 Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis Shyam Sundar Banaras Hindu University Varanasi, India History of the Current Epidemic in India & Antimony Resistance 6 Official
Antimonial Resistance & Combination therapy in Indian Visceral Leishmaniasis Shyam Sundar Banaras Hindu University Varanasi, India History of the Current Epidemic in India & Antimony Resistance 6 Official
Acta Dermatovenerol Croat 2008;16(2):60-64 CLINICAL ARTICLE
 2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
Intralesional Infiltration with Meglumine Antimoniate for the Treatment of Leishmaniasis Recidiva Cutis in Ecuador
 In order to provide our readers with timely access to new content, papers accepted by the American Journal of Tropical Medicine and Hygiene are posted online ahead of print publication. Papers that have
In order to provide our readers with timely access to new content, papers accepted by the American Journal of Tropical Medicine and Hygiene are posted online ahead of print publication. Papers that have
Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India
 The new england journal of medicine original article Single-Dose Liposomal for Visceral Leishmaniasis in India Shyam Sundar, M.D., Jaya Chakravarty, M.D., Dipti Agarwal, M.D., Madhukar Rai, M.D., and Henry
The new england journal of medicine original article Single-Dose Liposomal for Visceral Leishmaniasis in India Shyam Sundar, M.D., Jaya Chakravarty, M.D., Dipti Agarwal, M.D., Madhukar Rai, M.D., and Henry
Drug resistance in Indian visceral leishmaniasis
 Tropical Medicine and International Health volume6no11pp849±854november2001 Drug resistance in Indian visceral leishmaniasis Shyam Sundar Kala-azar Medical Research Centre, Banaras Hindu University, Varanasi,
Tropical Medicine and International Health volume6no11pp849±854november2001 Drug resistance in Indian visceral leishmaniasis Shyam Sundar Kala-azar Medical Research Centre, Banaras Hindu University, Varanasi,
Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil
 Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
A Comparison of Miltefosine and Sodium. Ritmeijer, K; Dejenie, A; Assefa, Y; Hundie, T B; Mesure, J; Boots, G; den Boer, M; Davidson, R N
 MSF Field Research A Comparison of and Sodium Stibogluconate for Treatment of Visceral Leishmaniasis in an Ethiopian Population with High Prevalence of HIV Infection. Authors Citation Ritmeijer, K; Dejenie,
MSF Field Research A Comparison of and Sodium Stibogluconate for Treatment of Visceral Leishmaniasis in an Ethiopian Population with High Prevalence of HIV Infection. Authors Citation Ritmeijer, K; Dejenie,
Randomized, Double-Blinded, Phase 2 Trial of WR 279,396 (Paromomycin and Gentamicin) for Cutaneous Leishmaniasis in Panama
 Am. J. Trop. Med. Hyg., 89(3), 2013, pp. 557 563 doi:10.4269/ajtmh.12-0736 Copyright 2013 by The American Society of Tropical Medicine and Hygiene Randomized, Double-Blinded, Phase 2 Trial of WR 279,396
Am. J. Trop. Med. Hyg., 89(3), 2013, pp. 557 563 doi:10.4269/ajtmh.12-0736 Copyright 2013 by The American Society of Tropical Medicine and Hygiene Randomized, Double-Blinded, Phase 2 Trial of WR 279,396
Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis
 ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
Treatment of cutaneous leishmaniasis among travellers
 Journal of Antimicrobial Chemotherapy (2004) 53, 158 166 DOI: 10.1093/jac/dkh058 Advance Access publication 16 January 2004 Treatment of cutaneous leishmaniasis among travellers J. Blum 1 *, P. Desjeux
Journal of Antimicrobial Chemotherapy (2004) 53, 158 166 DOI: 10.1093/jac/dkh058 Advance Access publication 16 January 2004 Treatment of cutaneous leishmaniasis among travellers J. Blum 1 *, P. Desjeux
Leishmaniasis chemotherapy challenges and opportunities
 REVIEW 10.1111/j.1469-0691.2011.03630.x Leishmaniasis chemotherapy challenges and opportunities S. L. Croft 1 and P. Olliaro 2,3 1) Faculty of Infectious and Tropical Diseases, London School of Hygiene
REVIEW 10.1111/j.1469-0691.2011.03630.x Leishmaniasis chemotherapy challenges and opportunities S. L. Croft 1 and P. Olliaro 2,3 1) Faculty of Infectious and Tropical Diseases, London School of Hygiene
Kala-Azar- Treatment Update
 JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp Iranian J Parasitol. Open access Journal at ijpa.tums.ac.ir
 Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
HAEMOFLAGELLATES. Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine
 HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
Original Article Chloroquine in cutaneous leishmaniasis
 Original Article Chloroquine in cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin, Iqbal Sidiqui Department of Dermatology, Pakistan Institute of Medical Sciences, Islamabad Abstract Background Chloroquine
Original Article Chloroquine in cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin, Iqbal Sidiqui Department of Dermatology, Pakistan Institute of Medical Sciences, Islamabad Abstract Background Chloroquine
Transactions of the Royal Society of Tropical Medicine and Hygiene
 Transactions of the Royal Society of Tropical Medicine and Hygiene 104 (2010) 225 229 Contents lists available at ScienceDirect Transactions of the Royal Society of Tropical Medicine and Hygiene journal
Transactions of the Royal Society of Tropical Medicine and Hygiene 104 (2010) 225 229 Contents lists available at ScienceDirect Transactions of the Royal Society of Tropical Medicine and Hygiene journal
POST KALA-AZAR DERMAL LEISHMANIASIS WITH ULCERATION ON FOOT: AN ATYPICAL CASE PRESENTATION SUCCESSFULLY TREATED WITH MILTEFOSINE
 American Journal of Infectious Diseases 10 (2): 50-55, 2014 ISSN: 1553-6203 2014 Science Publication doi:10.3844/ajidsp.2014.50.55 Published Online 10 (2) 2014 (http://www.thescipub.com/ajid.toc) POST
American Journal of Infectious Diseases 10 (2): 50-55, 2014 ISSN: 1553-6203 2014 Science Publication doi:10.3844/ajidsp.2014.50.55 Published Online 10 (2) 2014 (http://www.thescipub.com/ajid.toc) POST
Received 13 June 2010/Returned for modification 30 July 2010/Accepted 12 August 2010
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 2010, p. 4699 4704 Vol. 54, No. 11 0066-4804/10/$12.00 doi:10.1128/aac.00809-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Reductions
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 2010, p. 4699 4704 Vol. 54, No. 11 0066-4804/10/$12.00 doi:10.1128/aac.00809-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Reductions
WMLN Case Study. Necrotizing Palate Biopsy Specimen. Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP)
 WMLN Case Study Necrotizing Palate Biopsy Specimen Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP) 44 year old male from Honduras Immigrated to US 12 years ago to WI 4 years ago Seen
WMLN Case Study Necrotizing Palate Biopsy Specimen Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP) 44 year old male from Honduras Immigrated to US 12 years ago to WI 4 years ago Seen
Leishmaniasis. By Joseph Knight, PA-C. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India.
 Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
I nfectious diseases steal the headlines on a
 649 REVIEW Leishmaniasis Tonio V Piscopo, Charles Mallia Azzopardi... Epidemiology, disease patterns, immunology, diagnosis, treatment and control measures of are described. Various issues relating to
649 REVIEW Leishmaniasis Tonio V Piscopo, Charles Mallia Azzopardi... Epidemiology, disease patterns, immunology, diagnosis, treatment and control measures of are described. Various issues relating to
Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1992, p. 1630-1634 Vol. 36, No. 8 0066-4804/92/081630-05$02.00/0 Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice ARMIN KUHLENCORD,1*
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1992, p. 1630-1634 Vol. 36, No. 8 0066-4804/92/081630-05$02.00/0 Hexadecylphosphocholine: Oral Treatment of Visceral Leishmaniasis in Mice ARMIN KUHLENCORD,1*
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Pharmacokinetics of Miltefosine in Old World Cutaneous Leishmaniasis Patients
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2008, p. 2855 2860 Vol. 52, No. 8 0066-4804/08/$08.00 0 doi:10.1128/aac.00014-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2008, p. 2855 2860 Vol. 52, No. 8 0066-4804/08/$08.00 0 doi:10.1128/aac.00014-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India
 Indian J Med Res 120, September 2004, pp 166-172 Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India C.P. Thakur, S. Narayan* & A. Ranjan* Balaji
Indian J Med Res 120, September 2004, pp 166-172 Epidemiological, clinical & pharmacological study of antimonyresistant visceral leishmaniasis in Bihar, India C.P. Thakur, S. Narayan* & A. Ranjan* Balaji
Leishmania major Infection
 Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
Leishmaniasis. CDR R.L. Gutierrez Oct 2014
 Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course
 Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Randomised controlled trial of aminosidine (paromomycin) v sodium stibogluconate for treating visceral leishmaniasis in North Bihar, India
 belly-achers 13 do not grow up to be big belly achers but do grow up to suffer from anxiety or depression. We thank Warren Hilder and Erol Yusef for their assistance in data handling. Contributors: MH
belly-achers 13 do not grow up to be big belly achers but do grow up to suffer from anxiety or depression. We thank Warren Hilder and Erol Yusef for their assistance in data handling. Contributors: MH
Visceral leishmaniasis - current therapeutic modalities
 Review Article Indian J Med Res 123, March 2006, pp 345-352 Visceral leishmaniasis - current therapeutic modalities Shyam Sundar & Mitali Chatterjee* Kala-azar Medical Research Center, Department of Medicine,
Review Article Indian J Med Res 123, March 2006, pp 345-352 Visceral leishmaniasis - current therapeutic modalities Shyam Sundar & Mitali Chatterjee* Kala-azar Medical Research Center, Department of Medicine,
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan
 Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014
 I S T M 116 REVIEW LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014 Johannes Blum, MD, Pierre Buffet, MD, Leo Visser, MD, # Gundel Harms, MD, Mark S. Bailey,
I S T M 116 REVIEW LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014 Johannes Blum, MD, Pierre Buffet, MD, Leo Visser, MD, # Gundel Harms, MD, Mark S. Bailey,
18 : 1. Shyam Sundar, Anup Singh, Arun Shah, Varanasi EPIDEMIOLOGY: DIAGNOSIS OF VL:
 18 : 1 Visceral leishmaniasis-current scenario Visceral leishmaniasis (VL) is a systemic disease that is fatal if left untreated and is caused by an obligate intracellular protozoan parasite of genus leishmania.
18 : 1 Visceral leishmaniasis-current scenario Visceral leishmaniasis (VL) is a systemic disease that is fatal if left untreated and is caused by an obligate intracellular protozoan parasite of genus leishmania.
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar
 Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
 Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole
 CSE REPORT Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole udi, MD 1, ilza ellamy R. Limcangco, MD, FPDS 2, Johannes F, Dayrit, MD, FPDS 2,3 Introduction:
CSE REPORT Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole udi, MD 1, ilza ellamy R. Limcangco, MD, FPDS 2, Johannes F, Dayrit, MD, FPDS 2,3 Introduction:
Visceral childhood leishmaniasis in southern Turkey: experience of twenty years
 The Turkish Journal of Pediatrics 2009; 51: 1-5 Original Visceral childhood leishmaniasis in southern Turkey: experience of twenty years Oğuz Dursun 1, Seyhan Erişir 2, Akif Yeşilipek 3 Divisions of 1
The Turkish Journal of Pediatrics 2009; 51: 1-5 Original Visceral childhood leishmaniasis in southern Turkey: experience of twenty years Oğuz Dursun 1, Seyhan Erişir 2, Akif Yeşilipek 3 Divisions of 1
A review of clinical trials of treatments for visceral leishmaniasis in the Indian subcontinent (India, Bangladesh and Nepal)
 Review A review of clinical trials of treatments for visceral leishmaniasis in the Indian subcontinent (India, Bangladesh and Nepal) Messiah M Cheeran 1, Shyam Sundar 2, Sumar Rijal 3, Abul Faiz 4, Pascal
Review A review of clinical trials of treatments for visceral leishmaniasis in the Indian subcontinent (India, Bangladesh and Nepal) Messiah M Cheeran 1, Shyam Sundar 2, Sumar Rijal 3, Abul Faiz 4, Pascal
Review Article. Management of visceral leishmaniasis: Indian perspective
 Review Article www.jpgmonline.com Management of visceral leishmaniasis: Indian perspective S. Agrawal, M. Rai, S. Sundar Department of Medicine, Institute of Medical Sciences, Banaras Hindu University,
Review Article www.jpgmonline.com Management of visceral leishmaniasis: Indian perspective S. Agrawal, M. Rai, S. Sundar Department of Medicine, Institute of Medical Sciences, Banaras Hindu University,
Tropical medicine rounds
 Oxford, IJD International 0011-9059 Blackwell 41 UK Science, Journal Ltd 2002 of Dermatology Tropical medicine rounds Cutaneous André Báfica, Leishmaniasis Fabiano Oliveira, Luiz A. R. Freitas, Eliane
Oxford, IJD International 0011-9059 Blackwell 41 UK Science, Journal Ltd 2002 of Dermatology Tropical medicine rounds Cutaneous André Báfica, Leishmaniasis Fabiano Oliveira, Luiz A. R. Freitas, Eliane
Blood Smears Only 6 October Sample Preparation and Quality Control 15B-K
 NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 6 October 5 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 6 October 5 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
Mucosal leishmaniasis: the experience of a Brazilian referral center
 Rev Soc Bras Med Trop 5(3):38-33, May-June, 08 doi: 0.590/0037-88-0478-07 Major Article Mucosal leishmaniasis: the experience of a Brazilian referral center Mariana Junqueira Pedras [],[], Janaína de Pina
Rev Soc Bras Med Trop 5(3):38-33, May-June, 08 doi: 0.590/0037-88-0478-07 Major Article Mucosal leishmaniasis: the experience of a Brazilian referral center Mariana Junqueira Pedras [],[], Janaína de Pina
Departments of Dermatology, Venereology and Leprosy and *Microbiology, Indira Gandhi Medical College, Shimla, India.
 Net Study Comparative efþ cacy of intralesional sodium stibogluconate (SSG) alone and its combination with intramuscular SSG to treat localized cutaneous leishmaniasis: Results of a pilot study Ajeet K.
Net Study Comparative efþ cacy of intralesional sodium stibogluconate (SSG) alone and its combination with intramuscular SSG to treat localized cutaneous leishmaniasis: Results of a pilot study Ajeet K.
Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics
 Clinical dermatology Review article CED Clinical and Experimental Dermatology Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics M. Ameen Royal Free Hospital, Royal
Clinical dermatology Review article CED Clinical and Experimental Dermatology Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics M. Ameen Royal Free Hospital, Royal
Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial
 Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
Leishmaniasis: A forgotten disease among neglected people
 ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças Red Room 17: 00h
 CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000 deaths (6.7%) 310 million at
CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000 deaths (6.7%) 310 million at
A Novel Noninvasive Method for Diagnosis of Visceral Leishmaniasis by. rk39 Test in Sputum Samples
 JCM Accepts, published online ahead of print on 0 June 00 J. Clin. Microbiol. doi:0./jcm.00-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
JCM Accepts, published online ahead of print on 0 June 00 J. Clin. Microbiol. doi:0./jcm.00-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
 Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Micafungin, a new Echinocandin: Pediatric Development
 Micafungin, a new Echinocandin: Pediatric Development Andreas H. Groll, M.D. Infectious Disease Research Program Center for Bone Marrow Transplantation and Department of Pediatric Hematology/Oncology University
Micafungin, a new Echinocandin: Pediatric Development Andreas H. Groll, M.D. Infectious Disease Research Program Center for Bone Marrow Transplantation and Department of Pediatric Hematology/Oncology University
DOI /j x
 THERAPEUTICS DOI 10.1111/j.1365-2133.2007.07872.x Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses O. Zerpa, M. Ulrich, B. Blanco, M. Polegre, A. Avila, N. Matos,* I. Mendoza,
THERAPEUTICS DOI 10.1111/j.1365-2133.2007.07872.x Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses O. Zerpa, M. Ulrich, B. Blanco, M. Polegre, A. Avila, N. Matos,* I. Mendoza,
Sponsor / Company: Sanofi Drug substance(s): SSR103371
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
SUMMARY. Cutaneous leishmaniasis with only skin involvement: single to multiple skin ulcers, satellite lesions and nodular lymphangitis.
 SUMMARY Leishmaniasis is a disease affecting predominantly people in the developing countries; 350 million people worldwide are at risk and yearly more than 2 million new cases occur. Leishmaniasis is
SUMMARY Leishmaniasis is a disease affecting predominantly people in the developing countries; 350 million people worldwide are at risk and yearly more than 2 million new cases occur. Leishmaniasis is
Paromomycin for the Treatment of Visceral Leishmaniasis in Sudan: A Randomized, Open-Label, Dose-Finding Study
 Paromomycin for the Treatment of Visceral Leishmaniasis in Sudan: A Randomized, Open-Label, Dose-Finding Study Ahmed M. Musa 1 *, Brima Younis 1, Ahmed Fadlalla 2, Catherine Royce 3, Manica Balasegaram
Paromomycin for the Treatment of Visceral Leishmaniasis in Sudan: A Randomized, Open-Label, Dose-Finding Study Ahmed M. Musa 1 *, Brima Younis 1, Ahmed Fadlalla 2, Catherine Royce 3, Manica Balasegaram
Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review
 COLLECTION REVIEW Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review Nayara Castelano Brito, Ana Rabello, Gláucia Fernandes Cota* Pesquisas
COLLECTION REVIEW Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review Nayara Castelano Brito, Ana Rabello, Gláucia Fernandes Cota* Pesquisas
Dr Monique Wasunna MBChB, PhD, DTM&H DNDi Africa Regional office. WorldLeish6 Congress, Toledo Spain
 Phase II Randomized Clinical Trial of the Efficacy and Safety of AmBisome in Combination with SSG or Miltefosine versus Miltefosine Monotherapy for African VL Dr Monique Wasunna MBChB, PhD, DTM&H DNDi
Phase II Randomized Clinical Trial of the Efficacy and Safety of AmBisome in Combination with SSG or Miltefosine versus Miltefosine Monotherapy for African VL Dr Monique Wasunna MBChB, PhD, DTM&H DNDi
SMe. Me NITROIMIDAZOLES FOR VISCERAL LEISHMANIASIS FEXINIDAZOLE AND VL-2098 SHYAM SUNDAR
 SMe O 2 CH 2 O Me ITROIMIDAZOLES FOR VISCERAL LEISHMAIASIS FEXIIDAZOLE AD VL-2098 SHYAM SUDAR Target Product Profile for a CE Optimal Target Profile Target Label VL and PKDL VL Spp All species L. donavani
SMe O 2 CH 2 O Me ITROIMIDAZOLES FOR VISCERAL LEISHMAIASIS FEXIIDAZOLE AD VL-2098 SHYAM SUDAR Target Product Profile for a CE Optimal Target Profile Target Label VL and PKDL VL Spp All species L. donavani
Leishmaniasis impact and treatment access
 REVIEW 10.1111/j.1469-0691.2011.03635.x Leishmaniasis impact and treatment access M. den Boer, D. Argaw, J. Jannin and J. Alvar Leishmaniasis Control Programme, WHO/IDM. Av. Appia, Geneva, Switzerland
REVIEW 10.1111/j.1469-0691.2011.03635.x Leishmaniasis impact and treatment access M. den Boer, D. Argaw, J. Jannin and J. Alvar Leishmaniasis Control Programme, WHO/IDM. Av. Appia, Geneva, Switzerland
Efficacies of KY62 against Leishmania amazonensis and Leishmania donovani in Experimental Murine Cutaneous Leishmaniasis and Visceral Leishmaniasis
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 1998, p. 2542 2548 Vol. 42, No. 10 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Efficacies of KY62 against Leishmania
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 1998, p. 2542 2548 Vol. 42, No. 10 0066-4804/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Efficacies of KY62 against Leishmania
on November 20, 2018 by guest
 AAC Accepts, published online ahead of print on 13 September 2010 Antimicrob. Agents Chemother. doi:10.1128/aac.00985-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions.
AAC Accepts, published online ahead of print on 13 September 2010 Antimicrob. Agents Chemother. doi:10.1128/aac.00985-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions.
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines:
 Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines: Comments re Feb 2011 reviews of the v 2010 application February 23, 2011 1. The application and its review In v 2010,
Application for Inclusion of MILTEFOSINE on WHO Model List of Essential Medicines: Comments re Feb 2011 reviews of the v 2010 application February 23, 2011 1. The application and its review In v 2010,
Control of leishmaniasis
 SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
The Most Common Parasitic Infections In Yemen. Medical Parasitology
 The Most Common Parasitic Infections In Yemen Medical Parasitology ﻓﺎﯾز اﻟﺧوﻻﻧﻲ / د 2 : is a vector-borne disease that transmitted by sandflies and caused by obligate intracellular protozoa of the genus
The Most Common Parasitic Infections In Yemen Medical Parasitology ﻓﺎﯾز اﻟﺧوﻻﻧﻲ / د 2 : is a vector-borne disease that transmitted by sandflies and caused by obligate intracellular protozoa of the genus
A bs tr ac t. n engl j med 356;25 june 21,
 The new england journal of medicine established in 1812 june 21, 2007 vol. 356 no. 25 Injectable Paromomycin for Visceral Leishmaniasis in India Shyam Sundar, M.D., T.K. Jha, M.D., Chandreshwar P. Thakur,
The new england journal of medicine established in 1812 june 21, 2007 vol. 356 no. 25 Injectable Paromomycin for Visceral Leishmaniasis in India Shyam Sundar, M.D., T.K. Jha, M.D., Chandreshwar P. Thakur,
PHYTOTHERAPY: AN INNOVATIVE PERSPECTIVE IN LEISHMANIASIS TREATMENT
 PHYTOTHERAPY: AN INNOVATIVE PERSPECTIVE IN LEISHMANIASIS TREATMENT LUIZ FILIPE GONÇALVES DE OLIVEIRA Fundação Oswaldo Cruz Instituto Oswaldo Cruz Laboratório de Biologia Molecular e Doenças Endêmicas Leishmaniases
PHYTOTHERAPY: AN INNOVATIVE PERSPECTIVE IN LEISHMANIASIS TREATMENT LUIZ FILIPE GONÇALVES DE OLIVEIRA Fundação Oswaldo Cruz Instituto Oswaldo Cruz Laboratório de Biologia Molecular e Doenças Endêmicas Leishmaniases
Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis
 62 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(1): 62-66, February 2009 Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis Zélia Maria
62 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(1): 62-66, February 2009 Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis Zélia Maria
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study
 DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B
 MAJOR ARTICLE Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B Paulo R. L. Machado, 1,2 Maria Elisa A. Rosa, 1 Luís H. Guimarães, 1,2 Fernanda V. O. Prates, 1 Adriano Queiroz, 1,2
MAJOR ARTICLE Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B Paulo R. L. Machado, 1,2 Maria Elisa A. Rosa, 1 Luís H. Guimarães, 1,2 Fernanda V. O. Prates, 1 Adriano Queiroz, 1,2
Leishmaniasis. MAJ Kris Paolino September 2014
 Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin
 Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin Department of Dermatology, Pakistan Institute of Medical Sciences,
Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin Department of Dermatology, Pakistan Institute of Medical Sciences,
Downloaded from:
 Wise, ES; Armstrong, MS; Watson, J; Lockwood, DN (2012) Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travellers with New World cutaneous leishmaniasis
Wise, ES; Armstrong, MS; Watson, J; Lockwood, DN (2012) Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travellers with New World cutaneous leishmaniasis
Leishmaniasis, Kala Azar(The Black Fever)
 Leishmaniasis, Kala Azar(The Black Fever) By Lawrence Hall Etiologic agent Protist obligate intracellular parasite, Transmission Vectors Phylum: Euglenozoa (genus Leishmania) Over 21 species that infect
Leishmaniasis, Kala Azar(The Black Fever) By Lawrence Hall Etiologic agent Protist obligate intracellular parasite, Transmission Vectors Phylum: Euglenozoa (genus Leishmania) Over 21 species that infect
Eastern Mediterranean Health Journal, Vol. 9, No. 4,
 Eastern Mediterranean Health Journal, Vol. 9, No. 4, 2003 837 Antimony-resistant Leishmania donovani in eastern Sudan: incidence and in vitro correlation M.G. Abdo, 1 W.M. Elamin, 1 E.A.G. Khalil 1 and
Eastern Mediterranean Health Journal, Vol. 9, No. 4, 2003 837 Antimony-resistant Leishmania donovani in eastern Sudan: incidence and in vitro correlation M.G. Abdo, 1 W.M. Elamin, 1 E.A.G. Khalil 1 and
ISPUB.COM. Cutaneous Leishmaniasis in Iran. C Yazdi, M Narmani, B Sadri INTRODUCTION CASE 1 CASE 2
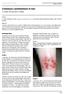 ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
Int.J.Curr.Res.Aca.Rev.2017; 5(4):
 International Journal of Current Research and Academic Review ISSN: 2347-3215 (Online) Volume 5 Number 4 (April-2017) Journal homepage: http://www.ijcrar.com doi: https://doi.org/10.20546/ijcrar.2017.504.014
International Journal of Current Research and Academic Review ISSN: 2347-3215 (Online) Volume 5 Number 4 (April-2017) Journal homepage: http://www.ijcrar.com doi: https://doi.org/10.20546/ijcrar.2017.504.014
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Authors Chappuis, François; Alirol, Emilie; Worku, Dagemlidet T; Mueller, Yolanda; Ritmeijer, Koert
 MSF Field Research High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B Item type Article
MSF Field Research High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B Item type Article
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List
 Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
Topical Par o mo my cin with or without Gentamicin for Cutaneous Leish ma ni a sis
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Topical Par o mo my cin with or without Gentamicin for Cutaneous Leish ma ni a sis Afif Ben Salah, M.D., Nathalie Ben Messaoud, M.D.,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Topical Par o mo my cin with or without Gentamicin for Cutaneous Leish ma ni a sis Afif Ben Salah, M.D., Nathalie Ben Messaoud, M.D.,
VISERAL LEISHMANIASI S (KALA-AZAR)
 VISERAL LEISHMANIASI S (KALA-AZAR) :OUTLINES DEFINITION. EPIDEMIOLOGY. PARASITE & VECTOR. PATHOLOGY CLINICAL & LIFE CYCLE. PICTURE. COMPLICATIONS. DIAGNOSIS. INVESTIGATIONS. MANAGEMENT TREATMENT S CONTROL.
VISERAL LEISHMANIASI S (KALA-AZAR) :OUTLINES DEFINITION. EPIDEMIOLOGY. PARASITE & VECTOR. PATHOLOGY CLINICAL & LIFE CYCLE. PICTURE. COMPLICATIONS. DIAGNOSIS. INVESTIGATIONS. MANAGEMENT TREATMENT S CONTROL.
New insights on leishmaniasis in immunosuppressive conditions
 New insights on leishmaniasis in immunosuppressive conditions Javier Moreno Immunoparasitology Unit WHO Collaborative Center for Leishmaniasis Centro Nacional de Microbiología INSTITUTO DE SALUD CARLOS
New insights on leishmaniasis in immunosuppressive conditions Javier Moreno Immunoparasitology Unit WHO Collaborative Center for Leishmaniasis Centro Nacional de Microbiología INSTITUTO DE SALUD CARLOS
It has been estimated that 90% of individuals
 Famciclovir for Cutaneous Herpesvirus Infections: An Update and Review of New Single-Day Dosing Indications Manju Chacko, MD; Jeffrey M. Weinberg, MD Infections with herpes simplex virus (HSV) types 1
Famciclovir for Cutaneous Herpesvirus Infections: An Update and Review of New Single-Day Dosing Indications Manju Chacko, MD; Jeffrey M. Weinberg, MD Infections with herpes simplex virus (HSV) types 1
14/05/2013,, 50,,, 16 /4/2012, 2,. 38,5,.,.., (30py).,. ( t:36,7%, Hb:11,8g/dl), (PLT: ) (WBC:2400, lymph:700),, 2 /2012,. 1
 ,, 50,,, 16 /4/2012, 2,. 38,5,.,.., (30py).,. ( t:36,7%, Hb:11,8g/dl), (PLT:114.000) (WBC:2400, lymph:700),, 2 /2012,. 1 Ht 36.7 Hb 11.8 2.400 67/29 114 MCV 88 33 CRP 0.54 PCT 0.24 Fe 58 790 ng/ml INR
,, 50,,, 16 /4/2012, 2,. 38,5,.,.., (30py).,. ( t:36,7%, Hb:11,8g/dl), (PLT:114.000) (WBC:2400, lymph:700),, 2 /2012,. 1 Ht 36.7 Hb 11.8 2.400 67/29 114 MCV 88 33 CRP 0.54 PCT 0.24 Fe 58 790 ng/ml INR
