UNIVERSITY OF CINCINNATI
|
|
|
- Brent Mathews
- 5 years ago
- Views:
Transcription
1 UNIVERSITY OF CINCINNATI Date: I,, hereby submit this work as part of the requirements for the degree of: in: It is entitled: This work and its defense approved by: Chair:
2 ii Intestinal lipid uptake and secretion of VLDL and chylomicron By: Andromeda Nauli August 2005 Previous degree: Bachelor of Science in Biomedical Sciences Degree to be conferred: Ph.D. Department of Pathology and Laboratory Medicine College of Medicine University of Cincinnati Committee chair: Patrick Tso, Ph.D.
3 iii ABSTRACT Despite decades of research, our understanding of intestinal lipid absorption is limited. In this Ph.D. thesis, I have dealt with two main aspects of intestinal lipid absorption, namely the uptake of lipids and the formation and secretion of triacylglycerol-rich lipoproteins (very low density lipoproteins [VLDL] and chylomicrons). In terms of uptake, CD36 is one of the plasma membrane proteins implicated in mediating lipid uptake by the intestine. In order to test this hypothesis, we utilized the CD36 knockout mouse model equipped with intraduodenal and lymph cannulas. Our studies showed that the disruption of the CD36 gene led to a significant decrease in the uptake of cholesterol but not of fatty acids. Interestingly, the role of CD36 was not limited to uptake but also appeared to affect the formation and secretion of chylomicrons, the major lipoproteins carrying the absorbed dietary fat from the gut (Chapter 2). It was first proposed by Tso et al. (202) that the small intestine secretes both VLDL and chylomicrons. Previous work by Vahouny et al. (212) suggested that female rats produced more VLDL than male rats. In addition, personal communication with Dr. Renee LeBoeuf leads us to believe that the female C57BL/6 mice may absorb lipids less efficiently than the male mice. We therefore studied the formation and secretion of lipoproteins in male and female C57BL/6 mice. Our data agree with those of Vahouny in that the female mice had a slightly higher ratio of VLDL to chylomicron secretion relative to that of the male mice. In addition, we also found that the intestinal lymphatic lipid transport of the C57BL/6
4 iv female mice segregated into two groups, a phenomenon that was absent in the male mice. In summary, our work suggests that CD36 is involved not only in intestinal cholesterol uptake, but also in regulating the formation and secretion of chylomicrons. In addition to the regulation by CD36, our studies also show that the regulation of the formation and secretion of chylomicrons are potentially different between male and female animals.
5 v
6 vi Acknowledgements I would like to express my gratitude to all of the individuals who helped me throughout this study. I am particularly thankful to Dr. Patrick Tso for all of his guidance as my mentor. I also wish to thank my committee members, Drs. Stephen Woods, Simon Newman, Ronald Jandacek, and Sean Davidson for their continuous support. I would also like to express my appreciation to University of Cincinnati Department of Pathology and Laboratory Medicine. Finally, I would like to thank my family, Susan, Surya, and mom.
7 1 Table of Contents Committee Approval Form Title page Abstract... Acknowledgements... i ii iii vi Table of Contents... 1 List of Tables and Figures. 4 List of Abbreviations.. 6 Chapter 1: Review of the Literature and Study Rationale Introduction Defining intestinal lipid absorption The importance of intestinal lipid absorption Limitation of the in vitro models Anatomy of the small intestine Structure supports function Understanding the histological layers Dietary lipids The detrimental effect of saturated fat The significance of non-dietary cholesterol Digestion of dietary lipids The early digestion processes The significance of pancreatic lipase. 22
8 Hydrolysis of cholesteryl esters Uptake of lipid digestion products by enterocytes The significance of micelles The significance of bile acids Fatty acid uptake Fatty acid transporters Mode of transport across plasma membrane Cholesterol uptake The significance of bile-acid micelles Cholesterol transporters Re-esterification of lipid digestion products inside enterocytes Lipid transport to the ER TG re-synthesis Cholesterol esterification Formation and secretion of lipoproteins Intestinal lipoproteins Assembly of lipoproteins Separate pathway for VLDL and chylomicron assembly ER to Golgi transport Regulation of lymphatic vs. portal transport Study Rationale. 39 Chapter 2: CD36 is important for intestinal cholesterol uptake and for the formation and secretion of chylomicrons
9 3 Abstract Introduction. 43 Materials and methods 45 Results 50 Discussion Chapter 3: Sex differences in intestinal lipid absorption in mice Abstract.. 77 Introduction 78 Materials and methods 81 Results. 85 Discussion. 92 Chapter 4: General Conclusions and Future Directions Insights gained on the lipid uptake Insights gained on the lipoprotein secretion 119 Literature Cited Appendix CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood Appendix Enterocyte fatty acid uptake and intestinal fatty acidbinding protein
10 4 List of Tables and Figures Table 1.1. The amount of biliary components secreted into the intestinal lumen 20 Figures 1.1. Diagram of the small intestine Analysis of fatty acid uptake by the small intestines of CD36 null and wild type mice Lymph [ 3 H]-TG transport (A), TG mass (B), and [ 14 C]-cholesterol transport (C) during continuous intraduodenal lipid infusion Total recovery of the infused TG (A) and cholesterol (B) in the stomach, colon, intestinal lumen, intestinal mucosa, and lymph at the end of the 6-h infusion period Distribution of the infused TG (A) and cholesterol (B) along 4 equal-length segments of the small intestine Mucosal distribution of [ 3 H]-fatty acids from infused triolein into the major lipid classes Mucosal distribution of [ 14 C]-cholesterol from infusate into the major lipid classes Lipoprotein particle size in the lymph of fasted mice Lipoprotein particle size of lymph from lipid infused mice Lipid composition of chylomicrons from lipid infused mice.. 73
11 Apolipoprotein secretion into the lymph by fasted or lipid infused mice The histograms of the total lymphatic triacylglycerol recovery in the male (A) and the female mice (B) The lymph flow rate during the continuous intraduodenal lipid infusion The hourly lymphatic triacylglycerol output during continuous intraduodenal lipid infusion The hourly lymphatic cholesterol output during the continuous intraduodenal lipid infusion The total radioactive triacylglycerol recovery in the lymph and the segments of gastrointestinal tract The total radioactive cholesterol recovery in the lymph and the segments of gastrointestinal tract Distribution of different classes of [ 3 H]-labeled lipids in intestinal mucosa Distribution of different classes of [ 14 C]-labeled lipids in intestinal mucosa Lipoprotein particle size of fasting lymph Lipoprotein particle size of lipid-infused lymph
12 6 List of Abbreviations ACAT = acyl coenzyme A:cholesterol acyltransferase Apo = apolipoprotein ASBT = apical sodium-dependent bile acid transporter CE = cholesterol ester CMC = critical micellar concentration COP = coat protein DG = diacylglycerol DGAT = diacylglycerol acyltransferase FA = fatty acid FATP = fatty acid transport protein G-3-P = glycerol-3-phosphate HDL = high density lipoprotein IBAT = ileal bile acid transporter I-FABP = intestinal fatty acid-binding protein ilbp = ileal binding protein KO = knock out L-FABP = liver fatty acid-binding protein LXR = liver x receptor MG = monoacylglycerol MGAT = monoacylglycerol acyltransferase MTP = microsomal triglyceride transfer protein
13 7 NaTC = sodium taurocholate NPC1L1 = Niemann-Pick C1-Like 1 PBS = phosphate-buffered saline PC = phosphatidyl choline PGC = peroxisome proliferator-activated receptors gamma coactivator PL = phospholipid SPB = sucrose polybehenate SREBP = sterol regulatory element-binding protein TG = triacylglycerol TLC = thin layer chromatography VLDL = very low density lipoprotein WT = wild type
14 8 Chapter 1 Review of the Literature and Study Rationale
15 Introduction The introductory chapter (Chapter 1) discusses our current understanding of intestinal lipid absorption with the emphasis on recent findings. This chapter complements other reviews on intestinal lipid absorption that are available in the literature (156; 174; 191; 242). Chapter 1 ends with a discussion of how our studies add to the understanding of intestinal lipid absorption, particularly on lipid uptake and the formation and secretion of intestinal triacylglycerol-rich lipoproteins. Chapters 2 and 3 discuss our studies on the role of CD36 in intestinal lipid absorption and sex differences in lipoprotein secretion, respectively Defining intestinal lipid absorption The process of intestinal lipid absorption is complex. We define intestinal absorption as the whole process that includes digestion, uptake, re-esterification of lipid digestion products, formation and secretion of lipoproteins, and transport to the circulation. Some investigators use the term intestinal absorption to refer solely to the uptake step (35), while others broaden it to the whole process as we have described (173; 190; 241). To complicate the matter further, the term uptake has been replaced with absorption by some investigators (172).
16 10 In a simplified view, intestinal lipid absorption is an important process of transferring the hydrophobic molecules of nutrients from the aqueous environment in the intestinal lumen into the aqueous environment in the circulation. An obvious problem arises: how are these hydrophobic molecules of nutrients transferred efficiently in the hydrophilic milieu? The solution to this problem is the conversion of hydrophobic molecules of nutrients through the digestive process to forms of the molecules that are less hydrophobic; and subsequently packaging them inside the intestinal epithelial cells into large lipid particles coated with phospholipids and apolipoproteins for transport in circulation. The problem of hydrophobicity is exemplified by the fact that medium chain fatty acids that are more water soluble are better absorbed than long chain fatty acids (20; 70), particularly in cases of lipase insufficiency The importance of intestinal lipid absorption The regulation of intestinal lipid absorption is not well understood. From an evolutionary viewpoint, organisms have evolved to achieve more efficient nutrient intake, storage, and utilization. A recent interest in understanding intestinal lipid absorption has arose in hopes of ultimately designing a way to reduce fat absorption. The long term goal of reducing fat absorption in Western society is obvious preventing obesity and other cardiovascular-related diseases without significantly altering the diet. For example, the drug, orlistat, is an inhibitor of pancreatic lipase that reduces the absorption of dietary fat.
17 11 The role of intestinal lipid absorption in preventing cardiovascular diseases has been considered less important than that of the liver due to the liver s role as the key organ regulating plasma lipid levels. According to this paradigm, the function of the intestine is simply to facilitate the absorption of nutrients. Due to the rapid metabolism of chylomicrons (produced by the gastrointestinal tract), the gut is not believed to be an important determinant of overall circulating plasma lipid levels. Consequently, more research has focused on the liver relative to the other organs. This paradigm is challenged by recent studies that suggest that intestine also plays a significant role in regulating plasma lipid levels. Studies from mice lacking Niemann-Pick C1-Like 1 (NPC1L1), a protein implicated in intestinal cholesterol absorption, showed that these mice were resistant to hypercholesterolemia when they ate a high-cholesterol diet (55). The liver NPC1L1 expression in these mice was negligible (5), suggesting that the intestine plays an important role in regulating lipid homeostasis. The recent studies of NPC1L1 on intestinal lipid absorption will be discussed later. An earlier study on a unique subject designated as the egg man further supports the concept that intestine is an important organ in regulating plasma lipid concentration (110). The egg man consumed about 25 eggs a day an unusually high cholesterol intake he followed for over 15 years. It is noteworthy that previous studies have confirmed elevated plasma cholesterols resulting from egg consumption (170). Contrary to expectation, however, the egg man did not
18 12 have a hypercholesterolemia. In fact, the egg man only absorbed 18% of his dietary cholesterol as compared to a normal value of about 50%, suggesting that cholesterol absorption by the intestine may be one of the important factors in determining plasma cholesterol level. In contrast to the high cholesterol diet of the egg man, the Tarahumara Indians are known for their low fat and low cholesterol diet which consists mainly of corn and beans. In addition, the Tarahumara Indians are also known for their low plasma cholesterol level. When they were put on either cholesterol-free or high cholesterol diet, their cholesterol absorption index, as determined by using the dual-isotope technique, was about 28% (141). These studies, together with the studies mentioned above, suggest that intestine can be an important organ in regulating plasma lipid levels. Consequently, altering intestinal lipid absorption should be considered one of the plausible therapeutic means in fighting cardiovascular diseases Limitation of the in vitro models Although recent findings have added significantly to our understanding of intestinal lipid absorption, many questions remained unanswered. The lack of a good cell culture model to study intestinal lipid absorption partly explains the slow progress in our understanding of the process at the cellular and molecular level. To date, the most commonly used intestinal cells to study intestinal absorption
19 13 are Caco-2 cells derived from human colon carcinoma (99; 196). Although widely used, in several aspects Caco-2 cells behave rather differently from enterocytes the epithelial cells lining the small intestine responsible for nutrient uptake. Their most important differences are in apolipoprotein B secretion and triacylglycerol (TG) synthesis. In contrast to human enterocytes that secrete only apolipoprotein B-48, Caco-2 cells secrete both apolipoprotein B-48 and B-100 (195). Furthermore, enterocytes use the monoacylglycerol (MG) pathway, while Caco-2 cells utilize primarily the glycerol-3-phosphate (G-3-P) pathway to synthesize TG (197). The significance of these two pathways will be discussed in the succeeding section. Other commonly used human colon carcinoma-derived cells are HT-29 cells. These cells, however, do not differentiate without the addition of galactose in the media (248). Readers interested in comparing different intestinal cell models should refer to studies by Chantret et al. (39). Another widely used model is the use of isolated intestinal epithelial cells (primary cell culture). However, isolation of cells from intestine has proved to cause a significant reduction in cell viability. In addition, the intestinal epithelial cells do not seem to function well when in isolation. Shakir et al. (179) reported that the viability of isolated intestinal cells dropped to about 50% after 4 hours. A recent review of the primary culture of intestinal cells also highlighted its limitation in viability (108; 153).
20 14 All of the descriptions listed above suggest that studies using in vitro models should be interpreted cautiously. The limitation of each model should be understood. As will be discussed later, many in vitro observations could not be observed in the in vivo studies Anatomy of the small intestine Structure supports function In order to facilitate a better understanding of intestinal lipid absorption, a brief overview of the anatomy of the small intestine will be presented. The gastrointestinal system consists of the gastrointestinal tract and gastrointestinal glands. The gastrointestinal tract is a continuous tube consisting of mouth, esophagus, stomach, small intestine, large intestine, rectum, and anus. The major supportive glands of gastrointestinal system are the salivary glands, pancreas, and liver. The significance of these glands in intestinal lipid absorption will be mentioned later. The whole length of an adult gastrointestinal tract is about 5 m. This extended length contributes to an enhanced area for absorption capacity. Lipid absorption mainly occurs in the small intestine, which consists of duodenum, jejunum, and ileum, with most lipids being absorbed in the duodenum and jejunum (12; 23).
21 15 There is little structural difference among the duodenum, jejunum, and ileum, only that the jejunum has more foldings (plicae circulares), which further increase the surface area for absorption. The presence of villi and microvilli of the small intestine contributes additional 10- and 20-fold increases in surface area, respectively. Altogether, these structures serve to enhance the efficiency of nutrient absorption Understanding the histological layers The gastrointestinal tract is made out of 4 histological layers, known as the mucosa, submucosa, muscularis, and serosa. As depicted in Figure 1.1., the small intestine has many projections (villi) that are lined by enterocytes (intestinal epithelial cells, also known as absorptive cells) facing toward the lumen. This outer (luminal) layer is called the mucosal layer, which is made out of epithelial lining, lamina propria, and muscularis mucosae (layers of muscle cells). The enterocytes lining the villi (epithelial lining) are the cells responsible for taking up the nutrients from the lumen. These nutrients will then be transported to the circulation, either through blood (Figure 1, left) or lymphatic (Figure 1, center) systems. The blood capillaries and lymphatic lacteals are located in the lamina propria and extend to the outer most layer, serosa, composed of loose connective tissues. In between the epithelial lining and the lamina propria lies the basement membrane. The second layer, the submucosa, consists of connective tissues infiltrated by nerve cells, immune cells, and capillaries. The
22 16 third layer, the muscularis, consists of inner and outer mucularis, both of which are also infiltrated by nerve cells and capillaries. The function of the muscularis is for intestinal motility, allowing for a proper mechanical mixture and transit of the ingested nutrients in the lumen. Figure 1.1. Diagram of the small intestine showing blood circulation (left), lymphatic circulation (center) and smooth muscle cells (right). Adapted from Basic Histology, 9 th ed. (120)
23 Dietary lipids A recent survey showed that about 30-40% of total energy of a typical Western diet was from fat (246). These studies also highlighted a significantly higher amount of fat consumed in the Western relative to the Eastern countries. About 95% of dietary fat is long-chain TG. The other 5% are phospholipids, fatty acids, and cholesterol. Most dietary cholesterol is free cholesterol. Dietary cholesteryl esters need to be hydrolyzed prior to uptake by the enterocytes (228). In this chapter, we will only discuss intestinal absorption of TG and cholesterol The detrimental effect of saturated fat It is known that saturated fat is hyperlipidemic, and polyunsaturated fat is not. Recent studies from Spiegelman s laboratory (114) suggest that saturated fat, but not polyunsaturated fat, stimulates the expression of liver coactivator PGC-1β, which in turn mediates the transcription of two subsets of genes. These two subsets of genes are genes responsible for lipid synthesis in the liver (regulated by SREBP) and genes responsible for lipid transport from the liver to the circulation (regulated by LXRα). As a result of the increased synthesis and the concomitant mobilization of lipid from the liver to the circulation, plasma lipid levels increase (hyperlipidemia).
24 18 While we know that saturated fats gain entry to the body through the gastrointestinal tract, we do not know the extent of the gastrointestinal tract contribution to the hyperlipidemic effect of saturated fat. Studies comparing the absorption of saturated and unsaturated fat showed that the unsaturated fat was more efficiently absorbed (69; 152). The reason may be that the unsaturated fat is more soluble in micelles than the saturated fat. The importance of micelle solubilization will be discussed shortly The significance of non-dietary cholesterol The source of cholesterol in the intestinal lumen is often thought to be derived mainly from diet. This view is clearly not accurate. Average cholesterol consumption is mg/day (247). In contrast, biliary cholesterol contributes to about mg/day (210) (See also Table 1.1. on page 20). In addition to diet and bile, enterocyte turnover also provides a significant amount of cholesterol into the intestinal lumen. Although its value is hard to determine, we estimated from the fecal sterol studies (143) that enterocyte turnover contributes about mg/day. The high amount of luminal cholesterol derived from enterocyte turnover is not suprising, considering that the intestine is one of the organs that produce the highest amount of cholesterol (11; 183) and that enterocyte turnover rate is about 3-4 days. An interesting recent study showed that mice infected with parasites could increase their enterocyte turnover rate as a mechanism to expel the parasites from the gut (49). It remains to be
25 19 determined whether intestinal parasites could indirectly affect intestinal cholesterol absorption. Since biliary cholesterol represents about 2/3 of total luminal cholesterol, the importance of biliary cholesterol absorption becomes obvious. The difference between biliary and dietary cholesterol absorption has often been discussed (177; 240). Several studies have concluded that biliary cholesterol is more efficiently absorbed partly due to its better solubilization in micelles (176). However, other investigators believe that dietary cholesterol will also be eventually solubilized in micelles to the same extent as biliary cholesterol; therefore their net percent absorption should be comparable (239). The absorption of biliary cholesterol is often ignored partly due to the erroneous assumption that the source of luminal cholesterol is mainly from diet. In fact, Breslow et al. believe that the mechanism underlying the regulation of dietary cholesterol absorption depends strongly on how much biliary cholesterol is secreted into the small intestine (178). A high cholesterol diet will supply more cholesterol to the liver, which in turn will secrete more biliary cholesterol. The more biliary cholesterol is secreted, the less dietary cholesterol is absorbed. They proposed that both biliary and dietary cholesterol absorption compete for incorporation into micelles, and that this is the reason for reduced dietary cholesterol absorption in individuals with high biliary cholesterol secretion. Biliary cholesterol secretion may be one of the factors regulating cholesterol absorption.
26 20 However, other non-biliary factors may also be critical for its regulation (119), as will be discussed later. Bile components Bile flow Bile salt Total bilirubin Cholesterol Phospholipids Values 7.1 ± 0.3 µl/100 g b. wt./min 383 ± 18 nmol/100 g b. wt./min 0.29 ± 0.01 nmol/100 g b. wt./min 5.2 ± 0.6 nmol/100 g b. wt./min 47.7 ± 3.2 nmol/100 g b. wt./min Table 1.1. The amount of biliary components secreted into the intestinal lumen. Data represent means ± SEM with n=6 (222) American Association for the Study of Liver Diseases. Reproduced with permission of John Wiley & Sons, Inc. (
27 Digestion of dietary lipids The early digestion processes Mastication (chewing) and antral peristalsis of the stomach provide grinding and mechanical mixing of food. In addition to grinding and mixing, the stomach also plays an important role in emptying the food into the small intestine a complex and important process that has been reviewed recently (166). The early digestion of TG occurs in the stomach (1) by the action of two major enzymes, namely lingual and gastric lipase. These two enzymes share many similarities; both enzymes function at low ph of about 4-6 (66; 94) and are more efficient in hydrolyzing shorter chain than longer chain TGs (113). The major products of both enzymes are diacylglycerols (DGs) and fatty acids (FAs) (65; 91; 93; 184). The fact that these enzymes hydrolyze TGs into DGs and FAs suggests that these enzymes are also critical in emulsifying the dietary lipids prior to entry into the small intestine. It has been estimated that this emulsification is responsible for reducing the size of fat droplets from 2-5 µm into about 0.5 µm (171). The significance of these enzymes has been thought to be more pronounced in neonates since these enzymes work better on medium chain TGs, and that the major dietary fat of neonates is milk, which contains some medium chain TGs
28 22 (92). It is also believed that pancreatic lipase as well as bile acid secretion have not been fully developed in neonates (88). As will be discussed later, pancreatic lipase is the main enzyme that hydrolyzes TG. However, a study on a 5-year-old child with congenital defect reducing the activity of both pancreatic lipase and colipase showed that this child could absorb about 50% of the dietary fat (78), suggesting that other lipases, eg., gastric lipase, can compensate for TG digestion up to a certain level The significance of pancreatic lipase Pancreatic lipase is secreted abundantly by the pancreas. However, its activity is significantly enhanced by the presence of a colipase, a coenzyme that is originally secreted in its inactive procolipase form and later activated by trypsin. The colipase interacts with the oil-water interface allowing the lipase to bind to the interface to hydrolyze TGs. A model of how TG can get in the active site of the pancreatic lipase has been proposed. This model suggests that the pancreatic lipase has a displaceable lid that can expose its catalytic site and allow its nonpolar surface to stabilize its TG substrate (21; 26). The hydrolysis of TGs by the pancreatic lipase yields 2-monoacylglycerols (2- MGs) and FAs (133; 135; 154). However, isomerization of 2-MGs can occasionally result in a complete hydrolysis of TGs into glycerols and FAs. The digestion of TG by the pancreatic lipase is critical for its micellar solubilization.
29 23 TGs are poorly soluble in aqueous environment. In contrast, MGs and FAs are readily soluble in micellar phase Hydrolysis of cholesteryl esters Biliary cholesterol and most dietary cholesterol are not esterified. Esterified cholesterol is not taken up by the enterocytes (227). As reported in previous studies, cholesterol esters were less efficiently absorbed than cholesterol (187). Therefore, it can be concluded that the hydrolysis of cholesteryl esters is required for them to be taken up by the enterocytes. The main, but not the only, enzyme that mediates cholesteryl ester hydrolysis is likely to be cholesterol esterase (cholesterol ester lipase/sterol ester hydrolase). Mice lacking cholesterol ester lipase displayed a significant decrease in cholesteryl ester absorption but had a normal absorption of free cholesterol (226) Uptake of lipid digestion products by enterocytes The significance of micelles As mentioned earlier, the uptake of FAs, MGs, and cholesterol depends critically on the micelle formation. Micelles are lipid aggregates consisting of bile acids, FAs, MGs, cholesterol, and phospholipids (122). The presence of a µm thick unstirred water layer in the intestine warrants the need for the micelles (234;
30 24 238) since they greatly increase the aqueous solubility of the lipid digestion products (97; 235). Although the size of the micelles is larger than the monomeric FAs and would, therefore, reduce the diffusion rate across the unstirred water layer, the enhanced solubility by the micelles (up to about 1,000 times) overcomes this diffusion barrier. It is noteworthy that micelles are not taken up by enterocytes as a whole (96; 112). In fact, studies showed that MGs and FAs were taken up at different rate (95; 144). Bile acids, the principal components of intestinal micelles, are subsequently taken up in the ileum. The uptake off bile acids is mediated by ileal bile acid transporter (IBAT), also known as apical sodium-dependent bile acid transporter (ASBT) The significance of bile acids The concentration of bile acids required for micelle formation is called critical micellar concentration (CMC), which is about mm depending on the bile acid species and luminal ph (14). Due to their significant role in micelle formation, bile acids are indispensable for lipid uptake. Several studies have reported that steatorrhea (fat malabsorption) could occur when bile acid concentration was below the CMC (13; 175).
31 25 In the absence of the micelle formation, lipid uptake may depend primarily on the ability of the monomeric FAs to pass through the unstirred water layer. This diffusion process is certainly not efficient, especially for the long chain FAs. It has been proposed that lipid digestion products can also be carried in vesicles (35). However, previous studies showed that the FAs from the micelles were more efficiently taken up by the Caco-2 cells, as compared to the FAs from the vesicles (148). Nevertheless, the role of the vesicles in facilitating FA uptake may be critical in certain clinical presentations, such as in patients with cholestasis (obstruction of bile flow to the intestine) Fatty acid uptake Fatty acid transporters The plasma membrane molecules responsible for the uptake of FAs are still largely unknown. Several of the candidate molecules have been proposed with little or no in vivo evidence supporting them. The scavenger receptor CD36 has been implicated in the uptake of FAs in many tissues, such as muscles and adipose. Chapter 2 and Appendix 1 discuss our studies on the role of CD36 in the FA uptake by the intestine. Using both a continuous lipid infusion method and a fecal fat balance method (103), we could not find any differences in the uptake of FA by the intestine between the CD36 null and wild type (WT) mice.
32 26 Consequently, our studies suggest that CD36 may not be a FA transporter in the intestine. One of the earliest suggestions for a fatty acid transporter came from a study by Stremmel et al. (186) which showed that a fatty acid binding protein located at the rat jejunal microvillous membrane was involved in the uptake of FAs by the small intestinal epithelial cells. It is noteworthy that this protein is different from both the intracellular fatty acid binding protein and the liver fatty acid binding protein. The authors showed that they could inhibit the binding of FAs to the brush border membrane vesicles using the antibodies that they raised against their isolated protein. Consequently, their studies generated a considerable amount of interest since inhibition of this protein may potentially inhibit the absorption of fat by the small intestine. Unfortunately, it was later reported by the same group that their plasma membrane fatty acid binding protein is closely related to the mitochondrial glutamic-oxaloacetic transaminase, therefore raising the question of whether or not the protein that they isolated and characterized was an artifact from the subcellular fractionation (19). Another FA transporter that has been proposed is the fatty acid transport protein 4 (FATP4). FATP4 is the only FATP expressed in the small intestine. It is localized in the apical brush border of the enterocytes. Previous studies showed that the heterozygous FATP null enterocytes had a 40% reduction in FA uptake as compared to that of FATP WT enterocytes (82). However, in vivo studies did
33 27 not show that the heterozygous FATP null mice had an increase in percent of fecal fat relative to that of the WT mice. (Homozygous FATP4 KO mice were embryonic lethal.) The authors suggest that although FATP4 may play a role in the uptake of FAs, its absence may not be enough to cause any physiological differences in lipid absorption Mode of transport across plasma membrane Although the search for FA transporters in the intestine has remained vigorous, the concept of passive transport (non carrier-mediated diffusion) has not been considered adequately. Recent studies (90; 109) briefly reviewed the significance of a flip flop mechanism on FA uptake. The flip flop process is thought to consist of adsorption, translocation, and desorption. It is believed that desorption (the process of transferring from plasma membrane to cytosol) is the rate-limiting step in the flip flop mechanism. It is likely that both the passive diffusion mechanism and the carrier-mediated mechanism coexist in the uptake of lipids by the intestine, especially when considering that the lipid concentration in the intestinal lumen under normal absorption is relatively high. At a low FA concentration, the carrier-mediated mechanism may be critical. And at a high FA concentration, typical of that seen after a fatty meal, the passive diffusion mechanism may be more significant. This concept, though accepted and often discussed by a number of investigators in
34 28 the field (47; 81; 146; 209), is often missed by the other investigators who are searching for the fatty acid transporters. For further discussion on this topic, please refer to Appendix Cholesterol uptake The significance of bile-acid micelles Cholesterol uptake by the enterocytes also depends heavily on micelles. Earlier studies showed that bile-diverted rats did not transport dietary cholesterol to the lymph (181), implying that bile is obligatory for cholesterol absorption. Recent outpatient studies (243) showed that subjects given a cholic acid supplement had a higher cholesterol absorption than control subjects. Subjects with cholic acid supplements also showed increased intraluminal bile acid concentrations with a concomitant increase in micellar cholesterol. Although this study did not prove a causal effect, it is highly suggestive that bile acids can significantly increase cholesterol uptake by the intestine through the formation of micelles. These studies are a good illustration of the importance of translational research. Other investigators have also showed a similar increase in cholesterol absorption when sodium taurocholate was supplemented in rats (188).
35 Cholesterol transporters Several studies have attempted to isolate cholesterol transporters from the small intestinal brush-border membrane by using the method of subcellular fractionation. One of the molecules isolated was shown to have a cholesterol transfer activity, and was subsequently proposed to be a cholesterol transporter (nsl-tp, non-specific lipid transfer protein) (192). However, later studies showed that the protein was mainly an intracellular molecule, ruling out its possibility as a cholesterol transporter (193; 244). These studies show us that the isolation and identification of cholesterol transporters by the subcellular fractionation should be done with caution. Recent studies have argued that NPC1L1 is one of the cholesterol transporters in the intestine (7; 54; 77). NPC1L1 is highly expressed in the enterocytes of mice (6). In contrast, the highest NPC1L1 expression in human is in the liver with relatively low expression in the intestine (51), suggesting that NPC1L1 may have a different physiological significance among species. There have also been some conflicting results concerning the localization of NPC1L1 in the enterocytes. Some investigators argued that the protein was localized in the plasma membrane (8); others showed that it was mainly localized in the intracellular compartment, and suggested that the function of NPC1L1 is to direct cholesterol to the Golgi aparatus (50).
36 30 As mentioned earlier, recent studies reported that NPC1L1 KO mice had a significant reduction in cholesterol absorption (9) and were resistant to hypercholesterolemia (53). These mice also showed a significant reduction in biliary cholesterol (52), arguing against the concept that biliary cholesterol level is the sole determining factor in regulating dietary cholesterol absorption. The exact role of intestinal NPC1L1 is unclear, and more studies are needed before NPC1L1 can be considered as an intestinal cholesterol transporter. Another plasma membrane protein that may mediate cholesterol uptake by the intestine is CD36. Studies using isolated human intestinal brush border membranes showed that antibodies against CD36 could block the uptake of cholesterol but not cholesterol esters (230). Chapter 2 further discusses our studies on the role of CD36 in intestinal cholesterol uptake. Our studies showed that mice disrupted in the CD36 gene had a significant accumulation of infused cholesterol in the intestinal lumen, suggesting that CD36 may play a role in mediating cholesterol uptake in the intestine. Further studies are needed to determine whether or not CD36 is a true intestinal cholesterol transporter Re-esterification of digestion products inside enterocytes Once lipids cross the plasma membrane of the enterocyte, they will be transported to the endoplasmic reticulum (ER) for re-esterification. The process of how lipids are transported to the ER is unclear. A brief discussion on how they
37 31 may be transported to the ER has been presented elsewhere (136). Much of our recent understanding of the re-esterification of lipid digestion products in the intestine comes from the work of Farese et al Lipid transport to the ER I-FABP is believed to be a protein mediating intracellular lipid transport in the intestine (15; 130; 137). It is localized in the cytosol of the small intestine. Studies comparing a naturally occurring polymorphism of I-FABP showed that some Pima Indians of Arizona had a substitution of alanine (Ala 54 ) to threonine (Thr 54 ) in their I-FABP (17). The Pima Indians with the less prevalent threonine (Thr 54 ) I-FABP were associated with insulin resistance. Interestingly, Thr 54 I- FABP had a higher affinity for long chain FAs than Ala 54 I-FABP. Further studies showed that when incubated with long chain FAs Caco-2 cells expressing Thr 54 I- FABP were able to secrete more TGs than those expressing Ala 54 I-FABP (16). The authors suggested that I-FABP played an important role in the intracellular lipid transport in the intestine. However, in vivo studies showed that the I-FABP KO mice had a higher body weight than the WT mice (221). Interestingly, disruption of I-FABP gene did not lead to the upregulation of the other FABPs, namely liver FABP (L-FABP) and ileal lipid binding protein (ilbp). These data suggest that FABPs may not play an important role in the intestinal lipid absorption, or that there exists such a redundancy in the system that makes it hard to be perturbed.
38 TG re-synthesis There exist two pathways for synthesizing TG in the enterocyte, namely the monoacylglycerol (MG) pathway and the glycerol-3-phosphate (G-3-P) pathway. The MG pathway consists of: 1) Acylation of 2-MG into diacylglycerol (DG) by monoacylglycerol acyltransferase (MGAT). 2) Acylation of DG into TG by diacylglycerol acyltransferase (DGAT (36)). In contrast, the G-3-P pathway, which uses a different starting substrate, consists of: 1) Acylations of glycerol-3-phosphate into phosphatidic acid by glycerophosphate acyltransferase. 2) Hydrolysis of phosphatidic acid into DG by phosphatidate phosphohydrolase. 3) Acylation of DG into TG by DGAT. The relative significance of these two pathways depends on the substrate abundance. Since there are more 2-MGs present during lipid-fed stage, the MG pathway becomes the predominant pathway under these conditions. A previous study further supports this notion by showing that the presence of 2-MG could inhibit the G-3-P pathway (165).
39 33 Since MG pathway is more critical in intestinal lipid absorption, the subsequent discussion will focus on the enzymes involved in the MG pathway. So far, there are three MGATs that have been identified, namely MGAT1, MGAT2, and MGAT3. MGAT2 but not MGAT1 is expressed in the intestine of both mice and humans (245). The importance of MGAT2 in intestinal lipid absorption has been suggested based on its high expression in the intestine and its regulation by dietary fat (34). As depicted above, DGAT enzyme is required for both pathways. Therefore, the absence of DGAT activity would suggest a reduction in lipid absorption or perhaps even lethality. However, studies using DGAT KO mice showed that although TG synthesis was reduced in many tissues examined, these mice appeared relatively healthy (182). This data strongly suggest that there are more than one DGAT enzymes. In fact, a second DGAT enzyme, DGAT2, has been identified (38). DGAT2 KO mice were lethal shortly after birth, suggesting that its function cannot be compensated and that it is a critical enzyme (185). However, the significance of DGAT2 in intestinal lipid absorption in humans is questionable since its expression was relatively low in the human intestine (37). Although DGAT1 may be important in intestinal lipid absorption, its absence did not cause a significant reduction in intestinal TG absorption, as determined by the fecal analysis method (30).
40 34 Interestingly, in rats the re-synthesis of the lipid digestion products into TG by the intestine turns out to be very rapid less than 2 minutes (129). However, the TG re-synthesis does not seem to be rapid in mice since there were only about 50% of the labeled FAs incorporated into TGs by the end of the 6 h infusion (see Chapter 2 and 3). Interestingly, the lipid transport into the lymph is also more efficient in rats than mice (unpublished observation). It remains to be determined whether this difference in lymphatic lipid transport between rats and mice is due to differences in their esterification Cholesterol esterification About 70-80% of the total cholesterol transported to the lymph is cholesteryl esters. As discussed earlier, enterocytes take up cholesterol but not cholesteryl esters (225), implying that cholesterol must be esterified prior to its transport to the lymph. The main enzymes that are thought to be responsible for esterifying cholesterol in the enterocytes are the cholesterol esterase and acyl coenzyme A:cholesterol acyltransferases (ACATs). It is believed that the origin of the cholesterol esterase is the pancreas, and that the enzyme is taken up by the enterocytes (71). It was also argued that cholesterol esterase was more important than ACAT in esterifying cholesterol in the intestine (72; 76). As mentioned earlier, mice lacking cholesterol esterase absorbed cholesterol normally, but had a reduced cholesterol ester absorption (224). Later studies
41 35 suggested that ACAT may, indeed, be more critical for cholesterol absorption (27). There are 2 ACATs identified so far: ACAT1 and ACAT2. ACAT1 KO mice did not display any abnormal cholesterol absorption by the intestine, as determined by dual isotope fecal analysis (142). On the other hand, ACAT2 KO mice displayed a significant reduction in intestinal cholesterol absorption only when put on a high fat high cholesterol diet, as determined by using the similar method (28; 167). These findings suggest that ACAT2 may be an important cholesterol esterifying enzyme in the intestine (29) Formation and secretion of lipoproteins Intestinal lipoproteins Lipoproteins are lipid particles with hydrophobic core and hydrophilic coats. By packaging the lipids with hydrophilic coats (phospholipids and apolipoproteins), lipoproteins can greatly enhance the transport of lipids to the circulation. The major lipoproteins secreted by the intestine are chylomicrons and very low density lipoproteins (VLDLs). The distinction between the two is empirical. Chylomicrons are defined as lipoproteins with Svedberg flotation (S f ) rate of more than 400, and VLDL are defined as lipoproteins with S f rate of Intestine secretes mostly VLDL during fasting state. In contrast, chylomicrons are the
42 36 major lipoproteins secreted during lipid-fed state. In addition to these two lipoproteins, the intestine has also been reported to secrete high density lipoproteins (HDLs) (18) Assembly of lipoproteins Most of our understanding of how lipoproteins are assembled in the intestine is derived from studies of the liver. Accordingly, nascent Apo B that is partially translated is brought into the ER. The nascent Apo B then moves into the lumen of ER. The addition of lipids occurs mainly in the lumen of the smooth ER. However, if there are not enough lipids available, Apo B will be degraded. This initial process of addition of lipids is regulated by microsomal triglyceride transfer protein (MTP), suggesting that MTP may play a more important role during the initial formation of (primordial) lipoproteins (83). As the translation of Apo B is completed, it will be released to the lumen of ER, forming a lipid-poor primordial lipoprotein. More lipids will be added to this lipid-poor lipoprotein, and the mature lipoprotein will be assembled and secreted from the Golgi. Additional discussions on the intracellular assembly of lipoproteins are available in the literature (220; 237).
43 Separate pathways for VLDL and chylomicron assembly Tso et al. proposed that VLDL and chylomicron are assembled using different pathways (199; 203). The supporting evidences for this thesis come from the studies that showed that the hydrophobic surfactant, Pluronic L-81, could inhibit chylomicron but not VLDL secretion (200; 201). The previous data showing that the uptake of certain FAs by the intestine resulted in a unique preference towards being transported as either VLDL or chylomicron also supports the proposal (151). This concept is worthy of further investigation because chylomicrons are thought to be more efficient in transporting lipids than VLDL. In addition, chylomicron clearance is rapid, suggesting that the intestine may potentially regulate the metabolism of absorbed lipids via its regulation of VLDL and chylomicron secretion ER to Golgi transport The importance of ER to Golgi transport has been highlighted recently (121). However, the mechanism of how lipids are transported from the ER to Golgi in the intestine is unclear at the moment. It seems that the transport of nascent lipoproteins (PCTV, pre-chylomicron transport vesicles) from the ER to Golgi uses the common COPII machinery (180). The COPII machinery, a common machinery used for export of nascent proteins out from the ER, involves the assembly of several COPII proteins in a stepwise fashion. In humans, mutations
44 38 in Sar1 GTPase, a protein critical for the initial assembly of COPII proteins, have been associated with lipid absorption disorders (107), suggesting that COPII machinery is critical in the process of intestinal lipid absorption. It has also been proposed that the rate limiting step in the intestinal lipid absorption is at the ER to Golgi transport (127). Subsequently, the lipoproteins from Golgi are targeted to the basolateral membrane for exocytosis Regulation of lymphatic vs. portal transport Once the lipoproteins are secreted by the enterocytes, they have to pass the basement membrane to get to the lamina propria. Earlier studies suggested that the integrity of basement membrane was disrupted during lipid absorption, allowing for the lipoproteins to get to the lamina propria (198). The lamina propria is filled with lymph and blood capillaries. It is commonly accepted that lipoproteins and hydrophobic molecules enter the lymphatic capillaries but small and polar molecules enter the blood capillaries. However, several studies have provided evidence that lipids could also be transported via the portal route (31; 124; 126; 140). If such regulation exists, then it may be possible that the regulation of lymphatic vs. blood (portal) transport is determined early during the packaging of the ingested materials in the enterocytes (223). This proposal is worth pursuing, especially when considering that materials transported via lymphatic and portal route may have a different metabolic fate, as will be
45 39 discussed in Chapter 3. Although materials transported via lymphatic system will be delivered eventually into the blood circulation, they first enter the mesenteric lymph, then the thoracic duct, and finally the blood circulation via the subclavian vein. In contrast, materials transported portally enter directly to the liver, the key metabolic organ. So although both transport systems eventually enter the blood circulation, the metabolic fate may be significantly different Study Rationale The review presented above suggests that there are several concepts regarding the intestinal lipid absorption which remain unclear. First, it is not clear as to what extent uptake of lipids by the intestine is mediated by the plasma membrane proteins and the passive process. We tested a well-known candidate molecule, CD36, for its involvement in lipid uptake by using a KO mouse model. Chapter 2 presents the results and discussions of this study. Second, it is not clear how lipoprotein formation and secretion are regulated in the intestine. In order to gain a better understanding of this regulation, we utilized two models to study: the CD36 KO mouse model and the C57BL/6 male and female mouse model. In the CD36 KO mouse model, we sought to test the hypothesis that CD36 may also be involved in chylomicron formation and secretion (Chapter 2). The use of C57BL/6 mouse strain for studying the mechanism of chylomicron formation and secretion was suggested to us by Dr. Renee LeBoeuf (personal
46 40 communication) who found that the female C57BL/6 mice absorbed lipids less efficiently than their male counterpart. The sex differences in lipoprotein secretion has also been suggested by the previous studies in rats (211). Hence, we decided to study the lipoprotein formation and secretion in male and female C57BL/6 mice. These studies are presented in Chapter 3. Chapter 4 discusses and summarizes the overall implications of our findings and some possible future experiments.
47 41 Chapter 2 CD36 is important for intestinal cholesterol uptake and for the formation and secretion of chylomicrons (Submitted to Gastroenterology for consideration for publication)
48 42 Abstract Background & Aims: The goal of our studies is to determine the role of the scavenger receptor CD36 in intestinal lipid absorption. Methods: A knock out (KO) mouse model equipped with lymph and duodenal cannulas was used. Results: The CD36 KO, as compared to wild type (WT) mice, exhibited significant accumulation of infused cholesterol in the intestinal lumen and significantly reduced cholesterol transport into the lymph. Triacylglycerol (TG) in the lumen of KO mice trended higher but the effect was not significant. However, there was marked TG accumulation in the intestinal mucosa and a significant reduction in their lymphatic transport. The ratio of TG to fatty acids in the mucosa of the KO mice was slightly higher than that of the WT, arguing against impaired lipid esterification but rather for a deficiency in the formation and secretion of chylomicrons that is responsible as the cause for the reduced lymphatic TG transport. This is further supported by a marked reduction of lymphatic apolipoprotein B-48, A-IV, and A-I and by the formation of smaller lipoprotein particles in the lymph of CD36 KO mice. Conclusions: Our data suggest that CD36 plays an important role in: 1) mediating intestinal cholesterol uptake; and 2) the formation and secretion of chylomicrons. We propose that inactivation of intestinal CD36 may lead to reduced cholesterol absorption with potential benefits in the treatment of hypercholesterolemia and the development of atherosclerosis.
49 43 Introduction The transmembrane protein CD36 is expressed in many cell types, including platelets (158), monocytes (189), capillary endothelial cells (111), erythroblasts (60), adipocytes (3), and intestinal (41; 117; 164), mammary, and retinal epithelial cells (89). In the rat intestinal epithelial cells, CD36 is localized in the apical brush border membranes of mainly the duodenum and jejunum (42; 163). Using human intestinal tissues, Lobo et al. (115) also localized the CD36 expression along the proximal brush border membranes of the intestinal epithelium. CD36 binds to a wide range of ligands, such as native and modified lipoproteins (33; 61), anionic phospholipids (169), cholesterol (229), and fatty acids (2). Evidence from studies conducted both in vitro (100; 159) and in vivo (63) supports an important role for CD36 in facilitating fatty acid uptake by adipose and muscle tissues. Its role in intestinal lipid uptake is less clear. In support for such a role are the high expression levels of CD36 and its expression pattern along the brush border membrane of the proximal intestine, which is typical of proteins implicated in lipid uptake would support such a role (43; 162). A recent study by Drover et al. (58) showed that CD36 KO mice did not exhibit reduced intestinal fatty acid uptake but had impairments in lymph TG secretion and in clearance of blood chylomicrons. Whether or not CD36 plays a
50 44 significant role in intestinal cholesterol uptake and their transport in TG-rich lipoproteins in vivo remains unknown. A role in uptake has been suggested by the report that the CD36 protein bound cholesterol and that CD36 antibodies inhibited cholesterol uptake by brush border membranes (233). The goal of this study is to further our understanding of the role of CD36 in intestinal uptake and transport of cholesterol and fatty acids using the well established conscious lymph fistula model. We explored further the effect of CD36 deficiency on formation and secretion of chylomicrons. Finally, we reexamined using a Sucrose Polybehenate (SPB) method (102) if disruption of the CD36 gene would lead to an overall reduction in intestinal fat absorption.
51 45 Materials and Methods Materials. Triolein, cholesterol, egg phosphatidylcholine (PC), and sodium taurocholate were purchased from Sigma (St. Louis, MO). The radioactive [9, 10-3 H (N)] triolein and [4-14 C] cholesterol were purchased from New England Nuclear (Boston, MA). Silica gel 60 plates were purchased from Fisher Scientific (Pittsburgh, PA). Animals. CD36 WT and KO mice were generated as described previously (57). Animals were maintained on regular chow diet under a 12 h light / 12 h dark cycle at University of Cincinnati Laboratory Animal Medical Services. We used 4-12 month-old male animals were used in our studies. Sucrose Polybehenate (SPB) method. The SPB method was developed and validated by Jandacek et al. (104). It is a non-invasive method for studying overall lipid absorption. The method relies on analyzing intestinal fat absorption by determining the ratio of fecal fat vs. a fecal non-absorbable fat marker. Lymph and duodenal cannulation. Intestinal lymph ducts of anesthesized (ketamine, 80 mg/kg and xylazine, 20 mg/kg) mice were cannulated with PVC tubing (I.D., 0.20 mm; O.D., 0.50 mm) as described by Bollman, et al. (22) with the following modifications. Suture of the lymph cannula was replaced by application of cyanoacrylate glue (Krazy Glue, Itasca, IL); in addition, a PVC tube (I.D., 0.5 mm; O.D., 0.8 mm) was inserted into the duodenum through a fundal
52 46 incision of the stomach and secured by a purse-string. Following the surgery, mice were infused with 5% glucose in saline (145 mm NaCl, 4 mm KCl and 0.28 M glucose) at a rate of 0.3 ml/h. The glucose/saline solution was replaced with the prepared lipid infusate the next morning. Lipid infusate preparation. Triolein, [ 3 H] triolein, cholesterol, [ 14 C] cholesterol and PC were combined dissolved in chloroform. The chloroform content was evaporated by using nitrogen gas. The chloroform-free lipid mixture was then suspended with 19 mm NaTC and sonicated. Lipid infusate was checked for homogeneity by sampling the bottom, the middle, and the top part of the emulsion. The counts usually agreed with one another within 2 %. Aliquots of the infusate were also taken at the beginning and at the end of the infusion period to check for stability of the infusate. The counts usually agreed with one another within 5%. The lipid emulsion was infused for 6 hours. The hourly infusate contained 4 µmol triolein labeled with [ 3 H] triolein, 0.78 µmol cholesterol labeled with [ 14 C] cholesterol, 0.78 µmol egg phosphatidylcholine (PC), and 5.7 µmol sodium taurocholate (NaTC) in phosphate-buffered saline (PBS) (0.958 g Na 2 HPO 4, g NaH 2 PO 4, 6.8 g NaCl, and g KCl per 1 L H 2 O) at ph=6.4. Collection of lymph, luminal, and mucosal samples. Lymph samples were collected hourly. At the end of the 6-h infusion period, the animals were
53 47 anesthetized with the ketamine-xylazine mixture, and stomach, small intestine (cut into 4 equal segments), colon were tied off separately, and the luminal content were collected. In some experiments the lipid was administered by gavage, ([ 3 H] oleate and olive oil, 100 µl/mouse, to overnight fasted mice and the mice were sacrificed 45 min later). Tissue samples were homogenized using a Polytron homogenizer, and radioactivity of each sample was measured. For later analyses of tissue lipid content, small intestinal segments were immediately extracted using the Folch method (68). Lymphatic triacylglycerol mass was determined using a triacylglycerol kit from Randox as previously described (149). Thin-layer chromatography (TLC) analysis of mucosal lipids. Lipids extracted from the small intestinal segments were run on silica gel 60 plates using a solvent system of petroleum ether/ethyl ether/glacial acetic acid, 25:5:1 volume ratio. After visualizing the samples and the co-migrating reference standards with iodine vapor, samples were scraped and added with 1 ml of absolute alcohol before adding the scintillation liquid (Opti Fluor for aqueous samples) for counting of radioactivity. Lipoprotein particle size analysis. Carbon-coated formvar film on a 400 mesh copper grid (Electron Microscopy Sciences) was floated on top of a drop of the lymph sample. The grid was dried with filter paper and briefly added to 2% phosphotungstic acid (ph 6.0). For lipid-infused lymph samples, 5h and 6h lymph samples were pooled and diluted with sterile water 1:4, vol:vol. Fasting
54 48 lymph samples were not diluted and were added on grids as described above. Standard beads (200 nm) were used for calibration (Duke Scientific Corp). Electron microscopic images were taken immediately by using JEOL JEM The size of lipoprotein particles was measured by using Adobe Photoshop and software from Reindeer Graphics. An average of 800 particles was sized per lymph sample. A previous pilot study showed that the manual and the digital counting methods agreed closely (data not shown). Chylomicron composition. To determine the lipid composition of chylomicron in the lymph, equal aliquots of lymph samples were layered under 0.15 M NaCl and subjected to centrifugation for 30 min at 50,000 rpm in MLA- 130 rotor in a table top ultracentrifuge (Beckman instruments). Chylomicrons were removed and lipid composition was determined as described using kits from Wako chemicals. Western blot analysis of apolipoproteins. One minute lymph output at fasting and after 4-h infusion from CD36 WT and KO mice were run on 4-20% gradient gel. Proteins were transferred to polyvinylidene difluoride membranes, reacted with primary antibodies against Apo B (1:7500 dilution), Apo A-IV (1:5000), and Apo A-I (1:5000), and appropriate secondary antibodies. The immunocomplexes were detected using the ECL system (Amersham Biosciences) according to the manufacturer s instructions. Data were quantified using Scion Image software.
55 49 Statistical analysis. The data shown are means ± SE (standard errors). To compare groups throughout the 6-h infusion, two-way repeated measures ANOVA with Tukey as a post-test analysis was used. A t-test was used for the rest of the data analyses. Statistical analyses were performed using Sigmastat (SPSS Inc.), and were considered significant if P < 0.05.
56 50 Results Fat absorption in CD36 null mice The SPB method allows the analysis of fat absorption in ad libitum fed mice by determining in fecal samples the content of excreted fatty acids relative to the content of a non-absorbable marker. Both CD36 WT (n=4) and KO (n=4) mice showed a 91% overall fatty acid absorption (Figure 2.1.), suggesting that ablation of CD36 is not sufficient to reduce fatty acid uptake. However, this method could not determine the rate and the site at which the fat is ingested and absorbed by the gut. To acquire that information, we used the conscious lymph fistula model to study intestinal fatty acid and cholesterol absorption. Analysis of lipid transport by the small intestine To further determine whether CD36 plays a role in lipid transport by the small intestine, we analyzed the lymphatic lipid output of both CD36 WT and KO mice. CD36 KO mice had a marked reduction in lymphatic output of both TG (Figure 2.2. A) and cholesterol (Figure 2.2. C). The reduction in TG output (P < 0.001) was evident as early as the first hour of infusion (Figure 2.2. A), reaching only 25% of the hourly infused TG as compared to 50% in the WT mice. The total TG mass (Figure 2.2. B) in the lymph of the CD36 KO mice was also lower (P = 0.003) than that of CD36 WT mice throughout the entire 6 h study. About 80%
57 51 of the TG mass was calculated to be derived from the infused TG in both the CD36 WT and KO mice. CD36 KO mice also showed a reduction (P = 0.002) in cholesterol transport, reaching only 10% of the hourly infused as compared to the 30% for the WT mice. The decrease was evident starting at the third hour of infusion (Figure 2.2. C). Analysis of the luminal and the mucosal lipid of the small intestine Most of the absorption of TG and cholesterol occurred in the small intestine since only a small amount of the infused radioactive TG and cholesterol was recovered from the colon (Figures 2.3. A and B). There was also little if any reflux of the radioactive TG and cholesterol back to the stomach (Figure 2.3., stomach). There was a trend for luminal TG to be higher in CD36 KO than in WT mice but the difference was not statistically significant (Figure 2.3. A, lumen). This observation would support the data obtained from the SPB studies (Figure 2.1.) that showed no major difference in fatty acid absorption between the CD36 WT and KO mice. Under the same conditions, lymphatic TG transport of CD36 KO mice was significantly decreased (P = , Figure 2.3. A, lymph) and CD36 KO mice retained more of the infused TGs in their intestinal mucosa (Figure 2.3. A, mucosa). In a separate study, we observed a similar distribution of radioactivity when the lipid was administered by gavage instead of duodenal infusion (data not shown). Thus the fate of the infused TG was similar between the CD36 KO and the wild type animals, irrespective of whether the lipid was
58 52 infused as an emulsion at a constant rate (in this study) or as a gavage (data not presented). Significantly higher luminal cholesterol counts (P = ) were obtained from the null mice (Figure 2.3. B, lumen). However, under the same conditions where CD36 KO mice exhibited defective cholesterol output into the lymph (P = ) (Figure 2.3. B, lymph), there was no evidence for more cholesterol counts in the CD36-deficient mucosa (Figure 2.3. B, mucosa). Distribution of the infused lipid along the segments of the small intestine The infused lipid was retained more in the proximal than in the distal segments of the intestinal mucosa (M1 being the most proximal and M4 being the most distal) (Figure 2.4, A and B). However, while more TG was retained in all segments of the small intestine of CD36 null mice (Figure 2.4. A), slightly more cholesterol was retained only in M2 and M3 relative to the WT mice (Figure 2.4. B). The low recovery of both [ 3 H] and [ 14 C] in the M4 segments further imply that the lipid infused was not excreted. Lipid distribution of the labeled fatty acids in intestinal segments Figure 2.5. shows the distribution of the infused triolein in various enterocyte lipid fractions; cholesteryl esters (CE), triacylglycerols (TG), fatty acids (FA),
59 53 diacylglycerols (DG), and monoacylglycerols+phospholipids (MG+PL). In the proximal half of the mucosa of the CD36 KO mice, most of the [ 3 H]-labeled lipids were in the form of TG (Figure 2.5. A and B) as compared to MG+PL in the WT mucosa; these differences were significant only in M2 (P = for CE and P = for MG+PL). This trend was partially reversed in distal segments (M3 and M4) where CD36-deficient mucosa exhibited less accumulation of TG and more in CE. Figure 2.6. shows the distribution of [ 14 C]-labeled cholesterol. About 80-90% of the [ 14 C]-label was recovered as free cholesterol in both CD36 WT and KO mice. No significant differences in distribution were observed between the two genotypes, although CE trended higher in distal segments of CD36 KO mice. Lipoprotein particle size analysis Figure 2.7.A shows the size distribution of the lymph lipoprotein particles from the fasted CD36 WT and KO mice. Similar to CD36 WT animals, CD36 KO mice had most of the lipoproteins in the VLDL size range during fasting (Figure 2.7. B). Figures 2.7. C and D show the fasting lipoprotein particles of CD36 WT and KO mice as analyzed by negative staining, respectively. Figure 2.8.A shows the size distribution of the lipoprotein particles during the lipid-fed stage of both the CD36 WT and KO mice. During the lipid-fed stage, the CD36 KO mice secreted appreciably less chylomicron relative to VLDL as compared to that of the CD36
60 54 WT mice (Figure 2.8. B). The lipid-fed lipoprotein particles of the CD36 WT and KO mice are shown in Figures 2.8. C and D, respectively. The average size of the lipoprotein particles of the CD36 KO mice (841.5 ± 76.1 Å) was significantly lower (P = ) than that of the CD36 WT mice ( ± Å) during the lipid-fed stage. Chylomicron composition Although less chylomicrons were secreted in the lymph of CD36 KO mice, their lipid composition was similar to that of chylomicrons from WT mice (Figure 2.9.). The chylomicrons secreted contain mostly TG (about 88%) with small amounts of phospholipids (about 8%) and cholesterol (about 4%). Apolipoprotein secretion by the small intestine The CD36 KO mice secreted slightly less apolipoprotein B-48 (Figure A), A- IV (Figure B), and A-I (Figure C) than the CD36 WT mice during the fasting stage. During the lipid-fed stage, apolipoprotein B-48 (Figure D) and A-I (Figure F) were also secreted slightly less by the CD36 KO relative to those of the CD36 WT. The apolipoprotein A-IV secretion was comparable between both WT and KO in the lipid-fed stage (Figure E).
61 55 Discussion The scavenger receptor CD36 is expressed in the brush border membranes of the proximal small intestine of both rodents (40; 161) and humans (118). CD36 binds to a wide variety of ligands, including lipoproteins (32; 62), anionic phospholipids (168), and long-chain fatty acids (4). Its high expression levels and defined localization in the small intestine together with its high-affinity for lipids would suggest a role for CD36 in lipid absorption. In this study, using the CD36 deficient mouse model equipped with lymph and intraduodenal cannulas, we directly examined the role of CD36 in uptake and lymphatic transport of both TGs and cholesterol. Previous work by Goudriaan et al. (86) and by Drover et al. (56) suggested that CD36 does not function in the uptake of dietary fatty acids. We reexamined this using the SPB method, which measures the percentage of the un-absorbed (fecal) fatty acids to the non-absorbable lipid marker. No deficiency in absorption could be detected in CD36 deficient mice. This is also supported by the similar recovery of [ 3 H]-labeled fatty acids from the intestinal lumen at the end of the 6-h infusion. Despite the fact that CD36 has been well characterized as mediating the uptake of free fatty acids in adipose tissue and muscle, in the intestine its deletion does not appear to cause an overall reduction in steady state fatty acid uptake. Our findings are consistent with those by Drover et al. (59). As a result of the high
62 56 fatty acid concentrations that occur in the intestine during a lipid meal, it is possible that passive transport of fatty acids may mediate most of the uptake in this tissue(208). Although the uptake of fatty acids by the small intestine is not dependent on CD36, the formation and secretion of chylomicrons appear to be, as supported by two lines of evidence. First, lymphatic TG transport was significantly reduced in the CD36 KO mice for both the dietary TG (infused) and the endogenous TG (originating from the fatty acids derived from biliary phospholipids). Second, the recovery of [ 3 H] TG counts in the intestinal mucosa was considerably higher in the CD36 KO than in the WT mice, thus indicating that the fatty acids were not incorporated into chylomicrons efficiently. The function of CD36 in chylomicron formation will be examined in future studies using subcellular fractionation and pulse chase experiments. The CD36 KO mice as compared to the WT mice had higher percentages of [ 3 H]- labeled lipids in the mucosal of the proximal intestine (segments M1 and M2). This is in line with normal CD36 expression being high in proximal segments and with the concept that proximal as opposed to distal segments play the major role in fatty acid absorption and secretion (24). This interpretation is further supported by the relatively lower [ 3 H] counts recovered in the distal segments (M3 and M4). Noteworthy, fatty acid esterification into TG does not appear to be impaired in CD36 deficiency. In addition to the higher percentage of [ 3 H]-labeled
63 57 TG, there were also higher [ 3 H] counts. These data suggest that the reduced lymphatic TG transport reflected a defect at a step downstream of fatty acid esterification, such as packaging of the synthesized TG into chylomicrons and secretion into the lymph. Analysis of several major apolipoproteins coating the chylomicrons, namely Apo B-48, A-IV, and A-I also shows that CD36 KO mice secreted appreciably less chylomicron apolipoproteins than WT mice. This together with the fact that chemical composition of the secreted chylomicrons was similar for both genotypes would support the interpretation of a reduction in production rate as opposed to a defect in a specific step of chylomicron pathway. Although chylomicrons are empirically defined as TG-rich lipoprotein particles of size 800 nm in diameter or larger in contrast to less than 800 nm in diameter for VLDL, Tso et al. (204) proposed that secretion of both particles involved two different pathways. The data in CD36 KO mice would support this concept. However, there was a discrepancy between our studies and those of Goudriaan et al. (85). Using an inhibitor of intravascular lipolysis, Triton WR1339, they showed that CD36 KO and WT mice were comparable in the plasma recovery of infused TG and fatty acids. We cannot explain the discrepancy in the findings of their study with ours, but it should be noted that their plasma TG recovery was only about 15% of the total infused by the end of 4 hours as compared to our lymphatic recoveries of about 44% of the total infused for WT and 18% for the
64 58 KO animals. The lymph fistula model provides us with a direct measurement of the secretion of chylomicrons and VLDL by the small intestine; and this measurement is not complicated by factors such as stomach emptying. In contrast, in the WR1339 study, stomach emptying could be potentially a complicating factor. In addition, we do not know if WR1339 affects the formation and secretion of intestinal chylomicrons and VLDL, which could potentially explain the low plasma recovery in the study reported by Goudriaan et al. (84). Lastly, the dose they infused was significantly high. Mice eat about 4 grams of chow a day. Assuming the chow is 5% fat, mice consumed about 200 mg or about 200 µl of oil in a day. In their studies, however, mice were challenged with a single bolus of 200 µl of oil. In contrast to the uptake of fatty acids, the uptake of cholesterol was markedly reduced in the CD36 knockout animals since there was a significant accumulation of the infused cholesterol in the lumen. In addition, there was a significant reduction in lymphatic cholesterol transport which was not correlated with the accumulation of mucosal [ 14 C] cholesterol counts. Finally, the ratio of [ 14 C] cholesterol to [ 14 C] cholesterol esters was similar for the KO and WT mice, implying that there was no defect in the esterification of infused cholesterol. These data would support the interpretation that uptake of cholesterol from the lumen into the enterocyte is impaired in CD36 KO mice. However, it should be noted that infused fatty acids were less readily esterified with cholesterol in these mice. Thus the possibility that the defect in cholesterol uptake may be
65 59 consequent to a change in FA utilization should be considered. Another possibility is that in the absence of CD36 cholesterol and fatty acid may not be efficiently targeted to the same intracellular compartment. Our findings that CD36 mediates cholesterol uptake were in agreement with Werder s studies showing that CD36 mediates the uptake of free cholesterol in the isolated human intestinal brush border membranes (231). Another plasma membrane protein that may mediate cholesterol uptake by the gut is the Niemann-Pick C1 Like 1 (NPC1L1) protein (10). Genetic ablation of NPC1L1 gene showed that about 9% of the infused cholesterol remained in the lumen, a percentage comparable to that observed in our studies (about 11%). The exact contribution of each of these molecules in cholesterol uptake remains unclear. Furthermore, it is not known if disruption of both genes would result in an even more marked reduction in cholesterol uptake by the gut.
66 60 Acknowledgements This work was supported by the National Institute of Health (grants DK-56910, and DK to P Tso and DK60022 and DK33301 to N Abumrad), the predoctoral fellowship award from the American Heart Association, Ohio Valley Affiliate and the National Health Research Institute scholarship for cardiovascular diseases (A. Nauli). We thank the University of Cincinnati Mouse Metabolic Phenotype Center DK for providing many of the phenotypic tests relevant to the study.
67 61 Figure Legends Figure 2.1. Analysis of fatty acid uptake by the small intestines of CD36 null and wild type mice. A minimum of 9 fecal samples from each group were analyzed by using the SPB method that determines the ratio of fecal fat vs. a fecal non-absorbable fat marker. Values are means ± SE. Figure 2.2. Lymph [ 3 H]-TG transport (A), TG mass (B), and [ 14 C]-cholesterol transport (C) during continuous intraduodenal lipid infusion. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. Values are means ± SE. Figure 2.3. Total recovery of the infused TG (A) and cholesterol (B) in the stomach, colon, intestinal lumen, intestinal mucosa, and lymph at the end of the 6-h infusion period. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. The recovery of labeled lipids in intestinal mucosa, lymph, stomach, lumen, and colon were determined at the end of the 6 h by scintillation counter. Values are means ± SE. Figure 2.4. Distribution of the infused TG (A) and cholesterol (B) along 4 equal-length segments of the small intestine. At the end of the 6hr infusion period, the intestines were harvested and divided into 4 equal length segments.
68 62 The recoveries of labeled lipids in these segments were determined by scintillation counter. From proximal to distal: M1, M2, M3, M4 (n=5). Values are means ± SE. Figure 2.5. Mucosal distribution of [ 3 H]-fatty acids from infused triolein into the major lipid classes. Mucosa was divided into 4 equal segments, from proximal to distal: M1 (A), M2 (B), M3 (C), and M4 (D). Mucosal lipids were extracted and separated by TLC into CE, TG, FA, DG, and MG+PL. CE, cholesteryl esters; TG, TGs; FA, fatty acids; DG, diacylglycerols; MG+PL, monoacylglycerols and phospholipids. Values are means ± SE. Figure 2.6. Mucosal distribution of [ 14 C]-cholesterol from infusate into the major lipid classes. Mucosa was divided into 4 equal segments, from proximal to distal: M1 (A), M2 (B), M3 (C), and M4 (D). Mucosal lipids were extracted and separated by TLC into cholesterol and cholesterol esters. Values are means ± SE. Figure 2.7. Lipoprotein particle size in the lymph of fasted mice. Distribution of particle size (A), relative VLDL/chylomicron ratio (B), and representative pictures of the fasting lipoprotein particles from CD36 WT (C) and KO (D) mice are shown. Particles < 800 Å are considered VLDL, and 800 Å or more are considered chylomicrons. Standard bars represent 5000 Å (500 nm). Values are means ± SE.
69 63 Figure 2.8. Lipoprotein particle size of lymph from lipid infused mice. Distribution of particle size (A), relative VLDL/chylomicron ratio (B), and representative pictures of the lipoprotein particles of CD36 WT (C) and KO (D) mice during the lipid-fed stage are shown. Particles < 800 Å are VLDL, and 800 Å or are chylomicrons. Standard bars are 5000 Å (500 nm). Values are means ± SE. Figure 2.9. Lipid composition of chylomicrons from lipid infused mice. Each chylomicron lipid, TG (white), total cholesterol (grey) and phospholipids (black) is expressed as percentage of the total content. Figure Apolipoprotein secretion into the lymph by fasted or lipid infused mice. Apolipoprotein B-48 (A), A-IV (B), and A-I (C) secretions during fasting stage, and apolipoprotein B-48 (D), A-IV (E), and A-I (F) secretions during lipid-fed stages were quantified by using Scion Image software. Equal amount of samples relative to their lymph output were loaded. OD = optical density. Values are means ± SE.
70 64 Figure % fatty acid uptake CD36 WT CD36 KO
71 65 Figure 2.2. A) Lymph [ 3 H]-TG transport % of hourly infused 75 CD36 WT (n=7) CD36 KO (n=7) * * * * * * Time (h) B) Lymph TG (mass) transport Lymph triacylglycerol (mg/h) * * CD36WT (n=4) CD36KO (n=7) * * Time (h) * * *
72 66 C) Lymph [ 14 C]-cholesterol transport % of hourly infused CD36 WT (n=7) CD36 KO (n=7) * * * * Time (h)
73 67 Figure 2.3. A) 60 The recovery of [ 3 H]-labeled FA % of total infused * CD36 WT (n=5) CD36 KO (n=5) 0 Mucosa Lymph Stomach Lumen Colon B) The recovery of [ 14 C]-labeled cholesterol % of total infused * CD36 WT (n=5) CD36 KO (n=5) Mucosa Lymph Stomach Lumen Colon *
74 68 Figure 2.4. A) % [ 3 H] Count CD36 WT CD36 KO 0 M1 M2 M3 M4 Mucosal Segment B) % [ 14 C] Count CD36 WT CD36 KO 0 M1 M2 M3 M4 Mucosal Segment
75 69 Figure 2.5. A) M1 % [ 3 H] Count CD36 WT (n=3) CD36 KO (n=3) 0 CE TG FA DG MG+PL B) M2 50 CD36 WT (n=3) CD36 KO (n=3) % [ 3 H] Count * 10 0 * CE TG FA DG MG+PL C) M3 % [ 3 H] Count CD36 WT (n=3) CD36 KO (n=3) 0 CE TG FA DG MG+PL D) M4 % [ 3 H] Count CD36 WT (n=3) CD36 KO (n=3) 0 CE TG FA DG MG+PL
76 70 Figure 2.6. A) M1 % [ 14 C] Count 100 CD36 WT (n=3) CD36 KO (n=3) Cholesterol Cholesterol Ester B) M2 % [ 14 C] Count 100 CD36 WT (n=3) CD36 KO (n=3) Cholesterol Cholesterol Ester C) M3 % [ 14 C] Count CD36 WT (n=3) CD36 KO (n=3) 0 Cholesterol Cholesterol Ester D) M4 % [ 14 C] Count CD36 WT (n=3) CD36 KO (n=3) 0 Cholesterol Cholesterol Ester
77 71 Figure 2.7. A) 50 % Particles 40 CD36 WT (n=3) CD36 KO (n=4) Diameter (Å) B) % Particles 100 CD36WT CD36KO VLDL C) D) Chylomicron
78 72 Figure 2.8. A) % Particles CD36 WT (n=2) CD36 KO (n=4) Diameter (Å) B) CD36WT CD36KO % Particles VLDL C) D) Chylomicron
79 Figure
80 74 Figure A) B-48 (fast) 100 OD (Mean ± SE) WT (n=4) KO (n=4) B) A-IV (fast) 50 OD (Mean ± SE) WT (n=4) KO (n=4) C) A-I (fast) 50 OD (Mean ± SE) WT (n=4) KO (n=4)
81 75 D) B-48 (lipid-fed) 75 OD (Mean ± SE) WT (n=4) KO (n=4) E) A-IV (lipid-fed) 50 OD (Mean ± SE) WT (n=4) KO (n=4) F) A-I (lipid-fed) 40 OD (Mean ± SE) WT (n=4) KO (n=4)
82 76 Chapter 3 Sex differences in intestinal lipid absorption in mice
83 77 Abstract: Intestinal lipid absorption is an essential process that governs how dietary lipid is digested and absorbed by the intestine and transported to the circulation. Although the basic process of intestinal lipid absorption is understood, many critical aspects remain unclear. One question in particular is whether or not intestinal lipid absorption differs between sexes. In order to address potential sex differences in intestinal lipid absorption, we used the lymph fistula model to monitor uptake, re-esterification, and transport of lipids by the small intestine before they are metabolized by the peripheral tissues. Using this model, we showed that female mice, unlike male mice, segregated into either the high or the low lymphatic transport groups. The high group had similar lymphatic triacylglycerol transport to the males, and the low group had significantly less. These differences are not due to the differences in lipid uptake or re-esterification by enterocytes, but rather may be due to the regulation of lipid transport toward lymphatic vs. portal circulation. We then tested if estrous stages regulate this lipid transport, and found that it was not. We also found that the female mice both the high and the low transport groups secreted more VLDL, and the male mice secreted more chylomicrons. Collectively, our data suggest the existence of sex differences in intestinal lipoprotein secretion and transport that may result in different metabolism of lipids.
84 78 Introduction Lipids are an essential part of our diet. The main dietary lipids consist of triacylglycerols, cholesterols, and phospholipids. The absorption of dietary lipids is important for the acquisition of energy and lipid-soluble vitamins. Dietary lipid absorption is undoubtedly a complex physiological process. It is a dynamic process that consists of digestion, uptake, re-esterification, packaging, and transport (155). The main enzyme responsible for the digestion of triacylglycerols is pancreatic lipase. Pancreatic lipase hydrolyzes triacylglycerol into 2-monoacylglycerol and two molecules of fatty acids (131; 132; 134). In the presence of bile, these lipid digestion products form mixed micelles that allow them to pass through the unstirred water layer of the small intestine (236). These micelles consist of bile salts, cholesterol, phospholipids, and lipid digestion products. As micelles pass through the small intestine, their lipid digestion products are taken up by enterocytes. Whether the uptake step is an active or passive process remains controversial. However, both processes may take place depending on the physiological stage (207). During fasting or a substantially low fat diet, the active process may predominate. In contrast, during normal to high lipid load, the passive process may instead be more predominant.
85 79 The lipid digestion products that are taken up by enterocytes are re-esterified into triacylglycerols by an ATP-dependent process. They are then packaged mainly as chylomicrons and transported via the lymphatic route. Although the lymphatic route is the major avenue for lipid transport, portal route has also been implicated in the transport of absorbed lipid (123; 125; 139). Like triacylglycerol absorption, dietary cholesterol absorption also involves several steps. Dietary cholesterol enters the intestinal lumen mainly as free cholesterol. It is also taken up by enterocytes and undergoes esterification. The two enzymes responsible for cholesterol esterification are acyl-coa cholesterol acyltransferase (ACAT) and cholesterol esterase (25; 48; 75). Once esterified, the cholesteryl esters will be packaged together with triacylglycerols into chylomicrons and transported into the lymphatic circulation. There is evidence that cholesterol esterification regulates cholesterol absorption (74). Most of our understanding of lipid absorption has been derived from studies using male animal models. In fact, our understanding of lipid absorption in females is very limited. There have only been a few studies that directly compare the sex differences in lipid absorption. Vahouny et al. (216; 217) reported that female rats had a higher intestinal very low density lipoprotein (VLDL) protein than male rats, and suggested that VLDL played a significant role in cholesterol transport in female rats.
86 80 Our present studies showed that the female mice could be segregated into two groups based on their lymphatic lipid absorption; one group had lipid absorption comparable to that of the males, and the other group had significantly less. Furthermore, we showed that this segregation was not due to lipid uptake or esterification by enterocytes, but potentially lipid transport regulation. Finally, we tested whether this segregation observed in the female mice was due to the stage of their estrous cycle or not. Our data did not support that estrous cycle regulates this segregation of lipid transport. We also found that irrespective of whether the female mice were in the high or the low output group, they had a tendency to secrete more VLDL than the male mice.
87 81 Materials and Methods Materials. Triolein, cholesterol, egg phosphatidylcholine (PC), and sodium taurocholate were purchased from Sigma (St. Louis, MO). The radioactive [9, 10-3 H (N)] triolein and [4-14 C] cholesterol were obtained from New England Nuclear (Boston, MA). Silica gel 60 plates were purchased from Fisher Scientific (Pittsburgh, PA). Animals. Male and female C57BL/6 mice aged 8-10 weeks were maintained at 12 h light / 12 h dark cycle with regular chow diet at University of Cincinnati Laboratory Animal Medical Services. To initiate the cannulation procedure, an anesthetic mixture of ketamine (80 mg/kg) and xylazine (20 mg/kg) was injected intraperitoneally into the mice. Intestinal lymph ducts of mice were cannulated with PVC tubing (I.D., 0.20 mm; O.D., 0.50 mm) according to techniques described by Bollman, et al. (22) Some modifications to the methods included using cyanoacrylate glue (Krazy Glue, Itasca, IL) instead of suture to secure the lymph cannula; additional insertion of a PVC tube (I.D., 0.5 mm; O.D., 0.8 mm) into the duodenum through a fundal incision of the stomach was secured by a purse-string. After surgery, mice were infused overnight with 5% glucose in saline (145 mm NaCl, 4 mm KCl and 0.28 M glucose) at a rate of 0.3 ml/h. The next morning the glucose/saline solution was replaced with the prepared lipid infusate. A total of 15 male and 32 female animals were studied. We had to use more female animals for the purpose of verifying their bimodal
88 82 distribution (See Result). For estrous cycle determination, vaginal smear was collected at the end of the 6h infusion (see below) and determined under the light microscope. All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC). Lipid infusate preparation. Four µmol triolein with a trace amount of [ 3 H] triolein, 0.78 µmol cholesterol with a trace amount of [ 14 C] cholesterol, 0.78 µmol egg phosphatidylcholine (PC), and 5.7 µmol sodium taurocholate (NaTC) in phosphate-buffered saline (PBS) (0.958 g Na 2 HPO 4, g NaH 2 PO 4, 6.8 g NaCl, and g KCl per 1 L H 2 O) at ph=6.4 were the amounts infused every hour. To prepare the emulsion, appropriate amounts of triolein, cholesterol (dissolved in chloroform), PC (dissolved in chloroform), [ 3 H] triolein, and [ 14 C] cholesterol were pooled and evaporated under a gentle stream of nitrogen gas. The mixture was then suspended with the corresponding volume of 19 mm NaTC and sonicated to form a homogenous lipid emulsion. Collection of lymph, luminal, and mucosal samples. The fasting lymph was collected for one hour prior to the lipid infusion. During the 6-h lipid infusion, hourly lymph was collected. At the end of the 6-h infusion, the animals were anesthetized with ketamine-xylazine mixture. Stomach, small intestine, and colon were removed. The lumen of the small intestine was washed with 0.5 % taurodeoxycholic acid. The pre-washed small intestine was divided into four equal lengths. The four segments of the small intestine, the stomach, and the
89 83 colon were homogenized individually in taurodeoxycholic acid by using Polytron homogenizer. An aliquot of each sample was added to Opti-Fluor (Packard Bioscience, Meriden, CT) and counted by liquid scintillation spectrometer (Model TR1900 tri-carb; Packard). The total count for each sample was calculated from the total volume of the sample and the aliquot count obtained from the scintillation counter. The disintegration per minute (dpm) was converted to percent recovery by multiplying the ratio of the dpm of the sample to the dpm of the infused lipid by 100. The remaining small intestinal segments were extracted immediately according to the method described by Folch et al. (67) Thin-layer chromatography (TLC) analysis of mucosal lipids. Lipids extracted from the segments of the small intestine were analyzed by TLC. In brief, TLC was carried out by running the samples that were dissolved in chloroform on silica gel 60 plates using a solvent system of petroleum ether/ethyl ether/glacial acetic acid with 25:5:1 volume ratio. After visualizing the plates with iodine vapor, each separated sample was scraped using co-migrating standards as reference. The scraped lipids were added to 1 ml of absolute alcohol, and radioactivity was counted as described above. Analysis of lipoprotein particle size by electron microscopy. For fasting lymph samples, carbon-coated formvar film on a 400 mesh copper grid (Electron Microscopy Sciences) was added with 20 µl of fasting lymph samples. After 1 min, the grid was dried gently with filter paper, and added with 2%
90 84 phosphotungstic acid (ph 6.0) was added. The phosphotungstic acid was also dried gently after 1 min of incubation time. For lipid-infused lymph samples, 5h and 6h lymph samples were pooled and then diluted with sterile water to 1:4. Twenty µl of the diluted pooled samples were used as described above. Additionally, 200 nm standard beads were used for calibration (Duke Scientific Corp). Pictures were taken immediately by using transmission electron microscopy (JEOL JEM-1230). The lipoprotein particle size was analyzed by using Adobe Photoshop version 6.0 with an added plugin (IPTK) purchased from Reindeer Graphics, Inc. A minimum of about 300 particles were counted per sample. Our pilot study has shown that the manual and the digital counting methods agreed closely (data not shown). Statistical analysis. All values are expressed as means ± SE. When comparing all groups throughout the 6-h infusion (hourly data), two-way repeated measures ANOVA with Tukey as a post-test analysis was used. For comparison of total recovery of three groups, One-way ANOVA with Tukey was performed as a post-test analysis. A t-test was used for comparing two groups. The statistical analyses were performed by using Sigmastats version 2.03 (SPSS Inc.), and were considered significant if the P values were <0.05.
91 85 RESULTS Histogram of the total lymphatic triacylglycerol output in the male and the female mice The histograms of the lipid absorption of the male (Figure 3.1. A) and the female mice (Figure 1B) were derived from the distribution of their total lymphatic [ 3 H]- triacylglycerol outputs. The total lymphatic [ 3 H]-triacylglycerol outputs were obtained by summing the total [ 3 H] counts recovered in the lymph during the six hour of infusion. Figure 3.1. A shows that the male mice had a range of total lymphatic [ 3 H]-triacylglycerol output of 21-70% with a bell shape distribution. However, the female mice had a distinct bimodal distribution with 12 mice falling in the range of 0-30%, and 20 mice falling in the range of 31-70%. Due to this distribution, the female mice were grouped into either high output group (High, 31-70% of the total lymphatic triacylglycerol recovery) or low output group (Low, 0-30% of the total lymphatic triacylglycerol recovery) in our subsequent figures and analysis. Lymph flow during continuous intraduodenal lipid infusion Figure 3.2. shows that the male group had steady lymph flow rate of 0.25 ml/h. The high-output female group had a lymph flow rate that did not differ from that of the males. The low-output female group, however, showed a decrease in the
92 86 lymph flow rate at the second hour of the infusion (from 0.24 ml/h to 0.14 ml/h) and remained at ~0.14 ml/h throughout the end of the infusion period. The lymph flow rate of the low-output female group was significantly different (P < 0.001) from both the male (starting at 2h) and the high-output female groups (starting at 1h). Hourly lymphatic triacylglycerol output Figure 3.3.A displays hourly lymphatic [ 3 H]-triacylglycerol recovery in the male, the low-, and the high-output female groups. As shown, the hourly lymphatic triacylglycerol output of the high-output group superimposed that of the male group, reaching a maximum of about 55% recovery of hourly infused [ 3 H] at the third hour of the infusion. As expected, the low-output female group had a lower hourly lymphatic triacylglycerol recovery, reaching a maximum of only 23% recovery at the third hour. The differences in hourly lymphatic triacylglycerol output were significant (P < 0.001) when comparing the low-output female group with both the high-output female and the male groups (from the first hour onwards). Figure 3.3.B shows hourly lymphatic [ 3 H]-triacylglycerol recovery in proestrous, estrous, and diestrous mice. There was no statistical significant among them. The steady state recovery was about 35% in each group, suggesting that the low- and the the high-output females were distributed quite equally into proestrous, estrous, and diestrous.
93 87 Hourly lymphatic cholesterol output As shown in Figure 3.4.A, the difference in hourly lymphatic [ 14 C]-cholesterol output between the high and the low-output female groups was more pronounced than the difference in the hourly lymphatic [ 3 H]-triacylglycerol output (Figure 3.3.A). The output curve of the males was between those of the high- and the low-output female groups. All three groups were significantly different from one another (P < 0.001) starting at 2h infusion. Figure 3.4.B shows that hourly lymphatic [ 14 C]-cholesterol output was not significantly different among the proestrous, estrous, and diestrous groups, and that they were all comparable to that of the males. Total triacylglycerol recovery Figure 3.5.A shows the percent recovery of the total [ 3 H]-triacylglycerol in the stomach, lumen, colon, and mucosa (small intestine) at the end of the 6-h infusion period. It also includes the percent recovery of the [ 3 H]-triacylglycerols in the lymph and those that could not be recovered at the end of the experiment ( Others ). The percent recoveries of the [ 3 H]-triacylglycerols in the stomach, lumen, colon, and mucosa were ~1.1%, 2.0%, 0.4%, and 13.0%, respectively, and do not show any significant difference among the three groups studied. However, the total [ 3 H]-lymphatic triacylglycerol recovery in the low-output female group (19.1%) was significantly lower than those in the male (46.4%, P < 0.001)
94 88 and the high-output female groups (51.2%, P < 0.001). The percent of the[ 3 H]- triacylglycerols that could not be recovered in the low-output female group (63.3%) was higher than those in the male (39.8%, P < 0.001) and the highoutput female groups (31.8%, P < 0.001). In contrast, figure 3.5.B shows that the proestrous, estrous, and diestrous mice were comparable in their recoveries in lumen, stomach, colon, mucosa, and lymph. The total lymphatic [ 3 H]- triacylglycerol transport was about 35% for proestrous, estrous, and diestrous mice. Total cholesterol recovery Figure 3.6.A shows the percent of recovery of [ 14 C]-cholesterol in the stomach, the lumen, colon, mucosa (small intestine), and lymph at the end of the 6h infusion period. The percent of total [ 14 C]-cholesterol recovery in the stomach, lumen, and colon was ~1.3%, 6.0%, and 1.2%, respectively. There was no significant difference among the three groups studied. When the hourly lymphatic cholesterol output was expressed as a cumulative recovery of the whole 6-h infusion, a similar trend as depicted in Figure 3.4. was evident. All three groups were significantly different (P < ) from one another in their total lymphatic [ 14 C]-cholesterol recovery (P < 0.01 when comparing the male group [19.7%] to the high-output female group [28.9%]; P < when comparing the low-output female group [10.0%] with the other two groups). The total [ 14 C]-cholesterol recoveries in the mucosa of both female groups were
95 89 significantly lower (P = 0.001) than those in the male group (P < 0.05 when comparing the male [43.5%] to the high-output female groups [31.5%]; P < when comparing the male to the low-output female groups [26.3%]). The percent of the [ 14 C]-cholesterol that could not be recovered (unaccounted cholesterols) was not significantly different between the male and the high-output female groups ( Others ). The unaccounted cholesterol in low-output female group was 54.1% of the dose, significantly higher than those of the male [28.7%] (P < 0.001) and the high-output female group [28.9%] (P < 0.001). Figure 3.6.B did not show any significant different among proestrous, estrous, diestrous mice in their [ 14 C] recoveries in lumen, stomach, colon, mucosa, and lymph. Their total lymphatic [ 14 C]-cholesterol transport was about 20%, comparable to that of the males. Thin-layer chromatography analysis of mucosal lipids Lipids extracted from intestinal mucosa were separated according to their classes by TLC. Figure 3.7. shows the distribution of [ 3 H]-labeled lipids into cholesteryl esters, triacylglycerols, fatty acids, diacylglycerols, and monoacylglycerols+phospholipids. As shown, about 50% of [ 3 H]-labeled lipids in the proximal half of the intestinal mucosa was in the form of triacylglycerols (Figure 3.7. A and 7B). The [ 3 H]-labeled mucosal lipids in the distal half, however, distributed more equally into triacylglycerols, fatty acids, diacylglycerols,
96 90 and monoacylglycerols+phospholipids (Figure 3.7. C and D). It is important to point out that the proximal half of the intestinal mucosa plays a more critical role in lipid absorption since it represented more than 80% of the total radiolabeled lipids in the mucosa (data not shown). That is, the proximal region of the small intestine, at least in our model, was the region where most triacylglycerol and cholesterol absorption occurred. There is no difference among the three groups in either one of their mucosal segments. Figure 3.8. shows the distribution of [ 14 C]-labeled lipids into cholesterol and cholesterol esters. As portrayed, about 75% of the [ 14 C]-labeled lipids were in the form of cholesterol. Statistical analysis of the three groups also did not show any significant difference. Lipoprotein particle size analysis. As shown in Figure 3.9. A, the lipoprotein particles of fasting lymph of the male mice (Figure 9C) were slightly larger than those of the high-output female mice (Figure 3.9. D), and the low-output female mice (Figure 3.9. E). When the lipoprotein particles were expressed as a ratio of VLDL (particles smaller than 800 Å) to chylomicron (particles of 800 Å or larger), the male mice were shown to have a slightly lower ratio of VLDL to chylomicron compared to those of the other two female groups (Figure 3.9. B). This slight difference in particle size was also
97 91 evident in the lipid-fed stage. During the lipid infusion, the male mice (Figure C) also made slightly larger particles than both the high-output (Figure D) and the low output (Figure E) female mice. The male mice showed an increase in relative chylomicron percentage from about 31% (fasting stage) to about 62% (lipid-fed stage). The female mice only reached 40% during the lipidfed stage. Although the difference in the ratio of VLDL to chylomicron in the lipid-fed stage was not significant between the male and the other two female groups, the male mice had a tendency to produce relatively more chylomicrons while the female mice produced predominantly VLDLs during the lipid-fed stage.
98 92 DISCUSSION Lipid absorption is a dynamic process that involves many complex steps. These steps include digestion, uptake, re-esterification, packaging, and transport (157). Our lipid absorption studies used the lymph fistula model to allow us not only to determine any lipid uptake difference between males and females, but also to monitor the quantity of lipids transported to the lymph prior to their metabolism by the periphery. The lymphatic triacylglycerol transport of the female mice segregated into high and low output groups. Unlike the female mice, the lymphatic transport by the male mice did not show any segregation, but rather a normal unimodal distribution. Analysis of lymphatic cholesterol transport revealed that the female mice that had low triacylglycerol transport also had low cholesterol transport to the lymph; and similarly, the female mice that had high triacylglycerol transport had high cholesterol transport. Although lymph flow in the low-output female group was significantly lower, lymph flow may not the factor responsible for these differences. The male and the high-output female groups did not show any differences in lymph flow, yet the cholesterol transport to lymph between these two groups was significantly different. To determine the reason for the differences in lymphatic lipid transport, we investigated lipid uptake into the enterocytes and re-esterification by the small
99 93 intestine in these mice. Analysis of lipid uptake did not show any significant difference among the three groups studied. Although the luminal cholesterol in the male group was slightly higher than those in the two female groups, this small difference could not account for the large difference in their lymphatic transport. The possibility that the infused lipids were excreted cannot explain the difference as shown by the low recovery in the colons and negligible radioactivity in fecal samples (data not shown). The possibility of reverse transit into the stomach was neglected by the low counts in the stomach in all three groups. Collectively these data imply that the male and the female mice were comparable in lipid uptake by the enterocytes. To determine if there was any difference in lipid re-esterification by the enterocytes, we performed TLC analysis on the mucosal lipids. The three groups were not significantly different in the distribution of the classes of lipid from the proximal throughout the distal intestinal mucosa. Cholesterol esterification by the enterocytes has been shown to affect lipid absorption (73), however, our data did not support any difference in lipid esterification by the enterocytes. As mentioned in the introduction, absorbed lipid can be transported via both the lymphatic and the portal routes. Cannulating both the lymph duct and the portal vein simultaneously is not a feasible approach since it will cause severe distress to the mice that could alter normal absorption processes. In the case of the lymph cannulation model, the infused lipid that did not enter the lymph and was
100 94 not excreted should have entered the portal blood. Cholesterol is not metabolized in the intestine, but 5-7% of the infused triacylglycerol may be metabolized by the gut (194). However, possible metabolism would represent only a small fraction of the unaccounted lipid. A study using both thoracic duct and portal cannulation in rats suggested that about 50% of the infused lipid can enter the portal circulation (138). By carefully measuring flow rate and the amount of lipid in the portal circulation, Mansbach et al. (10) also showed that there was about 39% of absorbed lipid entered the portal vein. Therefore, our derived portal transport data are in agreement with Mansbach s report. The low-output group had higher portal triacylglycerol transport than that of the high-output group, and vice versa. Similarly, the low-output group had higher portal cholesterol transport than that of the high-output group, and vice versa. The differences between the two female groups in lymphatic lipid transport were not due to lipid uptake or re-esterification by the enterocytes, but were likely due to the regulation of lipid transport: lymphatic vs. portal route. The detailed mechanism that governs this process remains elusive. Body weight was not associated with the difference in lipid transport in the female mice. The weights of the high- and the low-output groups were not different (average body weights ± SE were ± 0.52 g and ± 0.44 g, respectively). One factor that could be linked to these observations is the variation in the hormonal status in the females. When we tested if the estrous cycle was associated with the high
101 95 and the low lymphatic lipid output in the female mice, we found that it was not. We cannot completely rule out the hormonal factor at the moment since in a particular estrous cycle, there exists a complex hormonal interaction. It remains to be determined if a particular hormone, such as estrogen, could regulate the lipid transport. The idea of hormonal regulation of intestinal lipid absorption was indicated by Vahouny et al. (215; 219). Their studies showed that female rats had higher VLDL protein production compared to that of the male rats. To investigate if female mice produced more VLDLs to chylomicron than male mice, we performed an analysis on the lipoprotein particle size of lymph samples using a negative staining method. Our analysis showed that the female mice had a slightly higher VLDL to chylomicron ratio compared to that of the male mice both in fasting and lipid-fed stages. It is interesting to note that the amount of infused cholesterol in the mucosa was significantly higher in the male than in the two female groups. It remains to be determined if VLDL formation and transport is more efficient than chylomicron in transporting cholesterol, thereby clearing the cholesterol that was taken up by the enterocytes more quickly. This is possible in view that VLDL may play a more significant role in transporting dietary cholesterol than dietary triacylglycerols in females as suggested by Vahouny et al. (214; 218). However, the ratio of VLDL to chylomicron could not explain the differences in the lymphatic lipid transport between the two female groups since the two groups were quite comparable in their VLDL to chylomicron ratios.
102 96 Our studies raise critical questions. How are the lipids transported in portal circulation (what are they bound to)? Is the proposed regulation of lipid transport mediated by a sex hormone(s)? Despite these unanswered questions, we have contributed to the understanding that lipid absorption is not merely a process that dictates how much lipid is absorbed, but also how lipids are transported once absorbed. The amount of lipids absorbed is clearly important, however the major route for lipid absorption is also important. Lipids entering portal route will drain into the key metabolic organ, the liver. On the other hand, lipids entering the lymphatic route may have a significant time lapse before they enter the liver. Hence, it is plausible that lipid transport may affect metabolic fate. In fact, several studies have reported such differences in dietary lipid uptake and retention by peripheral tissues between men and women. Dietary lipid uptake and retention by leg muscle tissues were reported to be greater in women than in men (98). The splanchnic uptake of dietary lipids, however, was greater in men (150). These observations suggest that the metabolic fate of absorbed lipids can differ between male and female (105). Whether or not regulation of lipid transport plays a significant role in this process remains to be determined.
103 97 In conclusion, our studies showed that the female mice, unlike the male mice, had two distinct populations based on lymphatic triacylglycerol transport. Their transport segregated into either high or low-output groups, with the high-output group having similar lymphatic triacylglycerol transport to the males and the lowoutput group having significantly less. These differences in lymphatic lipid transport were not due to the difference in net lipid uptake or re-esterification by enterocytes, but most likely were due to the regulation of lipid transport. Male mice did not seem to regulate lipid transport in this manner. This observation raises the question of whether or not the proposed regulation of lipid transport was mediated by estrous cycle. Our studies, however, did not support that the estrous cycle regulates lipid transport. The mechanism underlying the regulation of lipid transport remains to be determined.
104 98 Acknowledgements: This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases Grants DK-56910, DK-54504, DK-56863, and by a pre-doctoral fellowship award from the American Heart Association, Ohio Valley Affiliate. The authors would like to thank Ronald Jandacek and Cali Smith for critical reading of the manuscript.
105 99 Figure Legends: Figure 3.1. The histograms of the total lymphatic triacylglycerol recovery in the male (A) and the female mice (B). Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. The total lymphatic lipid recoveries were plotted in a histogram. Figure 3.2. The lymph flow rate during the continuous intraduodenal lipid infusion. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. 0- h lymph represents fasting lymph. The female mice were divided into two groups, the high-output group (High) and the low-output group (Low), as discussed in Results. Groups not having common letters are significantly different (p < 0.05). Values are means ± SE. Figure 3.3. The hourly lymphatic triacylglycerol output during continuous intraduodenal lipid infusion. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. 0-h lymph represents fasting lymph. The female mice were either divided into two groups, high-output group (High) and low-output
106 100 group (Low), as discussed in Results (A), or according to their estrous cycle (B). Groups not having common letters are significantly different (p<0.05). Values are means ± SE. Figure 3.4. The hourly lymphatic cholesterol output during the continuous intraduodenal lipid infusion. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. 0-h lymph represents fasting lymph. The female mice were either divided into two groups, high-output group (High) and low-output group (Low), as discussed in Results (A), or according to their estrous cycle (B). Groups not having common letters are significantly different (p < 0.05). Values are means ± SE. Figure 3.5. The total radioactive triacylglycerol recovery in the lymph and the segments of gastrointestinal tract. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. The female mice were either divided into two groups, high-output group (High) and low-output group (Low), as discussed in Results (A), or according to their estrous cycle (B). Lymph represents the cumulative amount of radioactive triacylglycerol recovered in the lymph over the 6-h infusion. Stomach, lumen, colon, and mucosa (small intestine) were analyzed as explained in Methods. Others indicates portal
107 101 transport. Groups not having common letters are significantly different (p < 0.05). Values are means ± SE. Figure 3.6. The total radioactive cholesterol recovery in the lymph and the segments of gastrointestinal tract. Mice were equipped with lymph and duodenal cannulae, and were intraduodenally infused with a lipid emulsion containing labeled triolein and cholesterol for a period of 6 hours. The female mice were either divided into two groups, high-output group (High) and low-output group (Low), as discussed in Results (A), or according to their estrous cycle (B). Lymph represents the cumulative amount of radioactive cholesterol recovered in the lymph over the 6-h infusion. Stomach, lumen, colon, and mucosa (small intestine) were analyzed as explained in Methods. Others represents portal transport. Groups not having common letters are significantly different (p < 0.05). Values are means ± SE. Figure 3.7. Distribution of different classes of [ 3 H]-labeled lipids in intestinal mucosa. Mucosa was divided into 4 equal segments, from proximal to distal: M1 (A), M2 (B), M3 (C), and M4 (D). Mucosal lipids were extracted and separated by TLC into CE, TG, FA, DG, and MG+PL. CE, cholesteryl esters; TG, TGs; FA, fatty acids; DG, diacylglycerols; MG+PL, monoacylglycerols and phospholipids. Values are means ± SE. Figure 3.8. Distribution of different classes of [ 14 C]-labeled lipids in intestinal mucosa. Mucosa was divided into 4 equal segments, from proximal to distal: M1
108 102 (A), M2 (B), M3 (C), and M4 (D). Mucosal lipids were extracted and separated by TLC into cholesterol and cholesterol esters. Values are means ± SE. Figure 3.9. Lipoprotein particle size of fasting lymph. Distribution of particle size (A), relative VLDL/chylomicron ratio (B), and representative pictures of the fasting lymph of the male (C), the high- (D), and the low-output female (E) are shown. Particles < 800 Å are considered VLDL, and 800 Å or more are considered chylomicrons. Standard bars represent 5000 Å (500 nm). Values are means ± SE. Figure Lipoprotein particle size of lipid-infused lymph. Distribution of particle size (A), relative VLDL/chylomicron ratio (B), and representative pictures of the fasting lymph of the male (C), the high- (D), and the low-output female (E) are shown. Particles < 800 Å are considered VLDL, and 800 Å or more are considered chylomicrons. Standard bars represent 5000 Å (500 nm). Values are means ± SE.
109 103 Figure 3.1. A) Male 10 Number of Animals Total lymph TG recovered (%) B) Female 10 Number of Animals Total lymph TG recovered (%)
110 104 Figure 3.2. Lymph flow: male, high- and low-output groups Lymph Flow (ml/h) Male (n=15) Female, High (n=12) Female, Low (n=20) a b Time (h)
111 105 Figure 3.3. Lymphatic triacylglycerol output: male, high- and low-output groups A) % of Hourly Infused (Mean ± SE) Male (n=15) Female, High (n=12) Female, Low (n=20) a b Time (h) Lymphatic triacylglycerol output: proestrous, estrous, and diestrous B) % of hourly infused (Mean ± SE) Proestrous (n=6) Diestrous (n=11) Estrous (n=6) Time (h)
112 106 Figure 3.4. Lymphatic cholesterol output: male, high- and low-output groups A) % of Hourly Infused (Mean ± SE) Male (n=15) Female, High (n=12) Female, Low (n=20) a b c Time (h) Lymphatic cholesterol output: proestrous, estrous, and diestrous B) 50 Proestrous (n=6) % of hourly infused (Mean ± SE) Diestrous (n=11) Estrous (n=6) Time (h)
113 107 Figure 3.5. A) Total triacylglycerol recovery: male, high- and low-output groups % of Total Infused (Mean ± SE) Male (n=15) Female, High (n=12) Female, Low (n=20) a a b a a b 0 Stomach Lumen Colon Mucosa Lymph Others B) Total triacylglycerol recovery: proestrous, estrous, and diestrous % of total infused (Mean ± SE) Proestrous (n=6) Diestrous (n=11) Estrous (n=6) 0 Stomach Lumen Colon Mucosa Lymph Others
114 108 Figure 3.6. Total cholesterol recovery: male, high- and low-output groups A) % of Total Infused (Mean ± SE) 75 Male (n=15) Female, High (n=12) Female, Low (n=20) 50 a 25 b b a b c a a b 0 Stomach Lumen Colon Mucosa Lymph Others Total cholesterol recovery: proestrous, estrous, and diestrous B) % of total infused (Mean ± SE) Proestrous (n=6) Diestrous (n=11) Estrous (n=6) 0 Stomach Lumen Colon Mucosa Lymph Others
115 109 Figure 3.7. A) % [ 3 H] Count Male (n=3) Female (High, n= 5) Female (Low, n= 3) B) 75 0 CE TG FA DG MG+PL % [ 3 H] Count CE TG FA DG MG+PL C) 75 % [ 3 H] Count CE TG FA DG MG+PL D) 40 % [ 3 H] Count CE TG FA DG MG+PL
116 110 Figure 3.8. A) % [ 14 C] Count Male (n=3) Female (High, n= 5) Female (Low, n= 3) 0 Cholesterol Cholesterol Ester B) 100 % [ 14 C] Count C) Cholesterol Cholesterol Ester % [ 14 C] Count Cholesterol Cholesterol Ester D) 100 % [ 14 C] Count Cholesterol Cholesteryl Ester
117 111 Figure 3.9. A) % Particles Male (n=4) Female (High, n=4) Female (Low, n=5) Diameter (Å) B) % Particles Male (n=4) Female (High, n=4) Female (Low, n=5) 0 VLDL Chylomicron
118 112 C) D) E)
119 113 Figure A) % Particles Male (n=3) Female (High, n=4) Female (Low, n=3) Diameter (Å) B) % Particles Male (n=3) Female (High, n=4) Female (Low, n=3) 0 VLDL Chylomicron
120 114 C) D) E)
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
CHM333 LECTURE 34: 11/30 12/2/09 FALL 2009 Professor Christine Hrycyna
 Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
Lipid Metabolism β-oxidation FA Acetyl-CoA Triacylglycerols (TAGs) and glycogen are the two major forms of stored energy in vertebrates Glycogen can supply ATP for muscle contraction for less than an hour
Digestion and transport of TAG by plasma lipoproteins
 Digestion and transport of TAG by plasma lipoproteins Lipoproteins are multimolecular complexes of lipids and proteins, they are not macromolecules They transport lipids in the plasma because lipids are
Digestion and transport of TAG by plasma lipoproteins Lipoproteins are multimolecular complexes of lipids and proteins, they are not macromolecules They transport lipids in the plasma because lipids are
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
BIOL2171 ANU TCA CYCLE
 TCA CYCLE IMPORTANCE: Oxidation of 2C Acetyl Co-A 2CO 2 + 3NADH + FADH 2 (8e-s donated to O 2 in the ETC) + GTP (energy) + Heat OVERVIEW: Occurs In the mitochondrion matrix. 1. the acetyl portion of acetyl-coa
TCA CYCLE IMPORTANCE: Oxidation of 2C Acetyl Co-A 2CO 2 + 3NADH + FADH 2 (8e-s donated to O 2 in the ETC) + GTP (energy) + Heat OVERVIEW: Occurs In the mitochondrion matrix. 1. the acetyl portion of acetyl-coa
Digestive System. Part 3
 Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
- Most nutrients are absorbed before reaching the ileum. - Colon is responsible for final removal of electrolytes and water.
 University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
Digestion and Absorption
 Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
Gastrointestinal Anatomy and Physiology. Bio 219 Napa Valley College Dr. Adam Ross
 Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Moh Tarek + Suhayb. Tamara Al-Azzeh + Asmaa Aljeelani ... Faisal
 28 Moh Tarek + Suhayb Tamara Al-Azzeh + Asmaa Aljeelani... Faisal Digestion of dietary lipids Lipid digestion and absorption are complex processes. They involve soluble enzymes, substrates with different
28 Moh Tarek + Suhayb Tamara Al-Azzeh + Asmaa Aljeelani... Faisal Digestion of dietary lipids Lipid digestion and absorption are complex processes. They involve soluble enzymes, substrates with different
Abdulrahman Alhanbali. Lojayn Salah. Mohammad Khatatbeh. 1 P a g e
 7 Abdulrahman Alhanbali Lojayn Salah Mohammad Khatatbeh 1 P a g e In this lecture we will talk about digestion and absorption of food in the alimentary tract. But first of all we have some important points
7 Abdulrahman Alhanbali Lojayn Salah Mohammad Khatatbeh 1 P a g e In this lecture we will talk about digestion and absorption of food in the alimentary tract. But first of all we have some important points
Physiology Unit 4 DIGESTIVE PHYSIOLOGY
 Physiology Unit 4 DIGESTIVE PHYSIOLOGY In Physiology Today Functions Motility Ingestion Mastication Deglutition Peristalsis Secretion 7 liters/day! Exocrine/endocrine Digestion Absorption Digestion of
Physiology Unit 4 DIGESTIVE PHYSIOLOGY In Physiology Today Functions Motility Ingestion Mastication Deglutition Peristalsis Secretion 7 liters/day! Exocrine/endocrine Digestion Absorption Digestion of
Chapter 14: The Digestive System
 Chapter 14: The Digestive System Digestive system consists of Muscular tube (digestive tract) alimentary canal Accessory organs teeth, tongue, glandular organs 6 essential activities 1. 2. 3. 4. 5. 6.
Chapter 14: The Digestive System Digestive system consists of Muscular tube (digestive tract) alimentary canal Accessory organs teeth, tongue, glandular organs 6 essential activities 1. 2. 3. 4. 5. 6.
Lipoprotein Formation, Structure and Metabolism: Cholesterol Balance and the Regulation of Plasma Lipid Levels
 Lipoprotein Formation, Structure and Metabolism: Balance and the Regulation of Plasma Lipid Levels David E. Cohen, MD, PhD Director of Hepatology, Gastroenterology Division, Brigham and Women s Hospital
Lipoprotein Formation, Structure and Metabolism: Balance and the Regulation of Plasma Lipid Levels David E. Cohen, MD, PhD Director of Hepatology, Gastroenterology Division, Brigham and Women s Hospital
L1, 2 : Biochemical Aspects of Digestion of Lipids, Proteins, and Carbohydrates
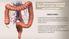 L1, 2 : Biochemical Aspects of Digestion of Lipids, Proteins, and Carbohydrates OBJECTIVES: Understand the process of digestion of dietary lipids, protein and carbohydrates including, the organs involved,
L1, 2 : Biochemical Aspects of Digestion of Lipids, Proteins, and Carbohydrates OBJECTIVES: Understand the process of digestion of dietary lipids, protein and carbohydrates including, the organs involved,
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
Cellular control of cholesterol. Peter Takizawa Department of Cell Biology
 Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
4. ABSORPTION. Transport mechanisms. Absorption ABSORPTION MECHANISMS. Active transport. Active transport uses metabolic energy
 4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
Lipid Diges.on 11/4/ CLASSIFICATION OF LIPID LIPID GLYCEROL BASED NON- GLYCEROL BASED SIMPLE COMPOUND GLYCOLIPID PHOSPHOGLYCERIDES
 Lipid Diges.on 3.1 CLASSIFICATION OF LIPID LIPID GLYCEROL BASED NON- GLYCEROL BASED SIMPLE COMPOUND GLYCOLIPID PHOSPHOGLYCERIDES FATS GLUCOLIPIDS GALACTOLIPIDS LECITHINS CEPHALINS SPHINGOMYELINS CEREBROSIDES
Lipid Diges.on 3.1 CLASSIFICATION OF LIPID LIPID GLYCEROL BASED NON- GLYCEROL BASED SIMPLE COMPOUND GLYCOLIPID PHOSPHOGLYCERIDES FATS GLUCOLIPIDS GALACTOLIPIDS LECITHINS CEPHALINS SPHINGOMYELINS CEREBROSIDES
Lipoproteins Metabolism
 Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
Lipoproteins Metabolism LEARNING OBJECTIVES By the end of this Lecture, the student should be able to describe: What are Lipoproteins? Describe Lipoprotein Particles. Composition of Lipoproteins. The chemical
BPK 312 Nutrition for Fitness & Sport. Lecture 2. Digestion & Absorption of Food Nutrients
 BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Digestion and Absorption
 Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Includes mouth, pharynx, esophagus, stomach, small intestine, large intestine, rectum, anus. Salivary glands, liver, gallbladder, pancreas
 Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
A. Incorrect! The esophagus connects the pharynx and the stomach.
 Human Physiology - Problem Drill 19: Digestive Physiology and Nutrition Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. This organ
Human Physiology - Problem Drill 19: Digestive Physiology and Nutrition Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. This organ
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Chemical Digestion and Absorption: A Closer Look
 Chemical Digestion and Absorption: A Closer Look Bởi: OpenStaxCollege As you have learned, the process of mechanical digestion is relatively simple. It involves the physical breakdown of food but does
Chemical Digestion and Absorption: A Closer Look Bởi: OpenStaxCollege As you have learned, the process of mechanical digestion is relatively simple. It involves the physical breakdown of food but does
Chapter 15 Gastrointestinal System
 Chapter 15 Gastrointestinal System Dr. LL Wang E-mail: wanglinlin@zju.edu.cn Rm 608, Block B, Research Building, School of Medicine, Zijingang Campus Pancreatic Secretion The exocrine cells in the pancreas
Chapter 15 Gastrointestinal System Dr. LL Wang E-mail: wanglinlin@zju.edu.cn Rm 608, Block B, Research Building, School of Medicine, Zijingang Campus Pancreatic Secretion The exocrine cells in the pancreas
Lipid Digestion. An Introduction to Lipid Transport and Digestion with consideration of High Density and Low Density Lipoproteins.
 Digestion An Introduction to Transport and Digestion with consideration of High Density and Low Density Lipoproteins By Noel Ways Suspension and Nutralization of Chyme ph Boli containing lipids enters
Digestion An Introduction to Transport and Digestion with consideration of High Density and Low Density Lipoproteins By Noel Ways Suspension and Nutralization of Chyme ph Boli containing lipids enters
10/23/2013 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
Digestive System Processes *
 OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
The Digestive System. What is the advantage of a one-way gut? If you swallow something, is it really inside you?
 The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
Anatomy & Histology of The Small intestine
 Anatomy & Histology of The Small intestine Prof. Abdulameer Al-Nuaimi E-mail: a.al-nuaimi@sheffield.ac.uk E. mail: abdulameerh@yahoo.com Jejunum Ileum Histology: Duodenum, jejunum, and ileum
Anatomy & Histology of The Small intestine Prof. Abdulameer Al-Nuaimi E-mail: a.al-nuaimi@sheffield.ac.uk E. mail: abdulameerh@yahoo.com Jejunum Ileum Histology: Duodenum, jejunum, and ileum
Figure Nutrition: omnivore, herbivore, carnivore
 Figure 41.1 Nutrition: omnivore, herbivore, carnivore Essential Nutrients: Amino acids Fatty acids Vitamins Minerals Figure 41.2 Complete vs incomplete Omnivore vs herbivore (vegetarian) Table 41.1 Table
Figure 41.1 Nutrition: omnivore, herbivore, carnivore Essential Nutrients: Amino acids Fatty acids Vitamins Minerals Figure 41.2 Complete vs incomplete Omnivore vs herbivore (vegetarian) Table 41.1 Table
General Structure of Digestive Tract
 Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
AN ANIMAL S DIET MUST SUPPLY CHEMICAL ENERGY, ORGANIC MOLECULES, AND ESSENTIAL NUTRIENTS
 1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
Ch 7 Nutrition in humans
 Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Corrected by. numb. Done. Doctor. Asma Karameh. Faisal Al Khateeb. 1 P age
 numb 27 Done Asma Karameh Corrected by ا لاء العجرمي Doctor Faisal Al Khateeb 1 P age DIGESTION AND TRANSPORT OF TRIACYL-GLYCEROL BY PLASMA LIPOPROTEIN General Lipids refer to a collection ofheterogeneous
numb 27 Done Asma Karameh Corrected by ا لاء العجرمي Doctor Faisal Al Khateeb 1 P age DIGESTION AND TRANSPORT OF TRIACYL-GLYCEROL BY PLASMA LIPOPROTEIN General Lipids refer to a collection ofheterogeneous
Nutrition. Autotrophs. plants, some protists & bacteria producers
 Nutrition Autotrophs plants, some protists & bacteria producers Nutrition Heterotrophs animals, fungi, some protists & bacteria consumers Animal Nutrition Most obtain food by ingestion take in their food
Nutrition Autotrophs plants, some protists & bacteria producers Nutrition Heterotrophs animals, fungi, some protists & bacteria consumers Animal Nutrition Most obtain food by ingestion take in their food
History. Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added. Fat deficiency severely affected
 Chapter 5 LIPIDS History 1918 Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added Fat deficiency severely affected Bone growth Reproduction Called Vitamin
Chapter 5 LIPIDS History 1918 Aron first proposed that fat may be essential for normal growth Tested on animals-vitamins A,D,E added Fat deficiency severely affected Bone growth Reproduction Called Vitamin
23.1 Lipid Metabolism in Animals. Chapter 23. Micelles Lipid Metabolism in. Animals. Overview of Digestion Lipid Metabolism in
 Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
Tissues and organs PART 1
 Tissues and organs PART 1 Animals and plants are multicellular (made of many cells). Cells become specialised according to their function Tissues: Many cells that perform one or several functions; they
Tissues and organs PART 1 Animals and plants are multicellular (made of many cells). Cells become specialised according to their function Tissues: Many cells that perform one or several functions; they
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Digestive Lecture Test Questions Set 4
 Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Ingestion Digestion- Absorption- Elimination
 DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
Lipid Digestion. and Human Nutrition. An Introduction to Lipid Transport and Digestion with consideration of High Density and Low Density Lipoproteins
 Digestion and Human Nutrition An Introduction to Transport and Digestion with consideration of High Density and Low Density Lipoproteins By Noel Ways Emulsification of s and release of Pancreatic Lipase
Digestion and Human Nutrition An Introduction to Transport and Digestion with consideration of High Density and Low Density Lipoproteins By Noel Ways Emulsification of s and release of Pancreatic Lipase
Regulating Hepatic Cellular Cholesterol
 Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
LAB 3: Biomolecules and Digestion
 Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
PHYSIOLOGY OF THE DIGESTIVE SYSTEM
 Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material.
 Chapter 3 Fats Chapter 3 Lesson 3.1 Key Concepts Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material. Foods from animal and plant sources supply distinct
Chapter 3 Fats Chapter 3 Lesson 3.1 Key Concepts Dietary fat supplies essential body tissue needs, both as an energy fuel and a structural material. Foods from animal and plant sources supply distinct
Development of in vitro Chylomicron Assay Using Caco-2 Cells
 East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations 12-2013 Development of in vitro Chylomicron Assay Using Caco-2 Cells Yuxi Sun East
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations 12-2013 Development of in vitro Chylomicron Assay Using Caco-2 Cells Yuxi Sun East
Questions on Digestion
 Name: Questions on Digestion Directions: The following questions are taken from previous IB Final Papers on Topic 6.1 (Digestion). Answer all questions. This will serve as a study guide for the next quiz.
Name: Questions on Digestion Directions: The following questions are taken from previous IB Final Papers on Topic 6.1 (Digestion). Answer all questions. This will serve as a study guide for the next quiz.
10/18/2017 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY. DIGESTIVE SYSTEM Dr.B.Jyothi
 KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
Class XI Chapter 16 Digestion and Absorption Biology
 Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Learning Targets. The Gastrointestinal (GI) Tract. Also known as the alimentary canal. Hollow series of organs that food passes through
 Digestion the multistep process of breaking down food into molecules the body can use Learning Targets Describe the path food takes through the digestive system. Identify the major organs of the digestive
Digestion the multistep process of breaking down food into molecules the body can use Learning Targets Describe the path food takes through the digestive system. Identify the major organs of the digestive
An introduction to Liposomal Encapsulation Technology
 An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS. Mar 16 10:34 PM
 DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
 Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
The Digestive System. Basic process of digestion. Mouth and Teeth 10/30/2016
 The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
Energy, Chemical Reactions and Enzymes
 Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
How to maximize fat energy? Swine. Poultry. Shrimp. Technical brochure about the molecular structure and mode of action of lysolecithins
 Introduction «Lecithin and lysolecithin «Normal fat digestion «Mode of action lysolecithins «Conclusions «Swine Poultry Fish How to maximize fat energy? Shrimp Technical brochure about the molecular structure
Introduction «Lecithin and lysolecithin «Normal fat digestion «Mode of action lysolecithins «Conclusions «Swine Poultry Fish How to maximize fat energy? Shrimp Technical brochure about the molecular structure
Small intestine. Small intestine
 General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
CONTENTS. Digestion of carbohydrates. Absorption of carbohydrates. Clinical significance
 CONTENTS Digestion of carbohydrates Absorption of carbohydrates Clinical significance Carbohydrates present in the diet Polysaccharides Disaccharides Monosaccharides Starch Glycogen Lactose Maltose Sucrose
CONTENTS Digestion of carbohydrates Absorption of carbohydrates Clinical significance Carbohydrates present in the diet Polysaccharides Disaccharides Monosaccharides Starch Glycogen Lactose Maltose Sucrose
B4 NUTRITION 4.3 Animal Nutrition
 B4 NUTRITION 4.3 Animal Nutrition 1. State the term balanced diet & describe how balanced diet is related to age, sex & activity of an individual. Balanced diet: A diet that contains all the main nutrients
B4 NUTRITION 4.3 Animal Nutrition 1. State the term balanced diet & describe how balanced diet is related to age, sex & activity of an individual. Balanced diet: A diet that contains all the main nutrients
The Digestive System. Prepares food for use by all body cells.
 The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
An overview of the digestive system. mouth pharynx esophagus stomach small intestine large intestine rectum anus
 An overview of the digestive system mouth pharynx esophagus stomach small intestine large intestine rectum anus Why GIT? What are the main steps in the digestive process? Ingestion intake of food via the
An overview of the digestive system mouth pharynx esophagus stomach small intestine large intestine rectum anus Why GIT? What are the main steps in the digestive process? Ingestion intake of food via the
Cholesterol and its transport. Alice Skoumalová
 Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Summary and general discussion
 Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
a. parotid b. sublingual c. submandibular
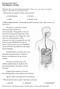 Bozeman Science/ Nature The Digestive System Watch the videos, and answer the questions below. Please write your answers in complete sentences, and explain all concepts thoroughly. 1. What are the four
Bozeman Science/ Nature The Digestive System Watch the videos, and answer the questions below. Please write your answers in complete sentences, and explain all concepts thoroughly. 1. What are the four
Sphincters heartburn diaphragm The Stomach gastric glands pepsin, chyme The Small Intestine 1-Digestion Is Completed in the Small Intestine duodenum
 Sphincters are muscles that encircle tubes and act as valves. The tubes close when the sphincters contract and they open when the sphincters relax. When food or saliva is swallowed, the sphincter relaxes
Sphincters are muscles that encircle tubes and act as valves. The tubes close when the sphincters contract and they open when the sphincters relax. When food or saliva is swallowed, the sphincter relaxes
Digestive System. In one end and out the other.
 Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
The Digestive System. Chapter 25
 The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Small Intestine. The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine.
 The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions.
 Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions. 1. What is digestion? What is the difference between mechanical and enzymatic digestion?
Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions. 1. What is digestion? What is the difference between mechanical and enzymatic digestion?
- Digestion occurs during periods of low activity - Produces more energy than it uses. - Mucosa
 Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
The new guidelines issued in PRESENTATIONS... Future Outlook: Changing Perspectives on Best Practice
 ... PRESENTATIONS... Future Outlook: Changing Perspectives on Best Practice Based on a presentation by Daniel J. Rader, MD Presentation Summary The guidelines recently released by the National Cholesterol
... PRESENTATIONS... Future Outlook: Changing Perspectives on Best Practice Based on a presentation by Daniel J. Rader, MD Presentation Summary The guidelines recently released by the National Cholesterol
Topic 11. Coronary Artery Disease
 Topic 11 Coronary Artery Disease Lipid metabolism http://news.bbc.co.uk/2/hi/health/7372495.stm Sterol Metabolism and Coronary Artery Disease Big Picture: Exogenous Cholesterol and Fat Metabolism Fats-Triglycerides
Topic 11 Coronary Artery Disease Lipid metabolism http://news.bbc.co.uk/2/hi/health/7372495.stm Sterol Metabolism and Coronary Artery Disease Big Picture: Exogenous Cholesterol and Fat Metabolism Fats-Triglycerides
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System
 The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
Summary of chemical breakdown of food by hydrolytic enzymes (Protein enzymes).
 Biology 12 Digestive System Digestion Overview: The digestive process can be divided into 4 phases: 1. ingestion - includes swallowing and peristalsis 2. digestion - the physical (by teeth) and chemical
Biology 12 Digestive System Digestion Overview: The digestive process can be divided into 4 phases: 1. ingestion - includes swallowing and peristalsis 2. digestion - the physical (by teeth) and chemical
Digestion and Nutrition. Chapter 40
 Digestion and Nutrition Chapter 40 Impacts, Issues Hormones and Hunger Fat cells secrete leptin, which reduces appetite; an empty stomach secretes ghrelin, which makes you hungry the goal is healthy nutrition
Digestion and Nutrition Chapter 40 Impacts, Issues Hormones and Hunger Fat cells secrete leptin, which reduces appetite; an empty stomach secretes ghrelin, which makes you hungry the goal is healthy nutrition
Digestive Tract. Also called alimentary canal or gastrointestinal tract. stomach small intestine large intestine - anus
 Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, ,
 IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
MCAT Biology Problem Drill 20: The Digestive System
 MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
Title: Dec 12 8:42 AM (1 of 37) Chapter 11: Digestion and Excretion
 Title: Dec 12 8:42 AM (1 of 37) Chapter 11: Digestion and Excretion Introduction to Digestion Read pages 352 358 Make summary notes on this section Creat a Concept Map on the Essential Nutrients, including:
Title: Dec 12 8:42 AM (1 of 37) Chapter 11: Digestion and Excretion Introduction to Digestion Read pages 352 358 Make summary notes on this section Creat a Concept Map on the Essential Nutrients, including:
Chapter 26 The Digestive System
 Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Peptic Ulcer Disease: Zollinger-Ellison Syndrome
 GASTROINTESTINAL PHYSIOLOGY 235 Case 41 Peptic Ulcer Disease: Zollinger-Ellison Syndrome Abe Rosenfeld, who is 47 years old, owns a house painting business with his brothers. The brothers pride themselves
GASTROINTESTINAL PHYSIOLOGY 235 Case 41 Peptic Ulcer Disease: Zollinger-Ellison Syndrome Abe Rosenfeld, who is 47 years old, owns a house painting business with his brothers. The brothers pride themselves
Ch 18. Physiology of the Digestive System
 Ch 18 Physiology of the Digestive System SLOs List the functions of the digestive system Distinguish and describe the different patterns of motility observed in the GI tract. Name and explain the various
Ch 18 Physiology of the Digestive System SLOs List the functions of the digestive system Distinguish and describe the different patterns of motility observed in the GI tract. Name and explain the various
THE DIGESTIVE SYSTEM
 THE DIGESTIVE SYSTEM Composed of two parts: 1. 2. There are 4 main parts of digestion: 1. Ingestion: 2. Digestion: a. Mechanical Digestion: Example: b. Chemical Digestion: Example: 3. Absorption: 4. Egestion:
THE DIGESTIVE SYSTEM Composed of two parts: 1. 2. There are 4 main parts of digestion: 1. Ingestion: 2. Digestion: a. Mechanical Digestion: Example: b. Chemical Digestion: Example: 3. Absorption: 4. Egestion:
Human Nutrition (IGCSE Biology Syllabus )
 Human Nutrition (IGCSE Biology Syllabus 2016-2018) o Balanced diet: getting all the right nutrients in correct proportions o Diet related to: - Age - Gender - Activity - Pregnant women o Malnutrition:
Human Nutrition (IGCSE Biology Syllabus 2016-2018) o Balanced diet: getting all the right nutrients in correct proportions o Diet related to: - Age - Gender - Activity - Pregnant women o Malnutrition:
Digestion, Absorption, Transport, and Excretion of Nutrients
 Digestion, Absorption, Transport, and Excretion of Nutrients (Session 6) Mohsen Karamati Department of Nutrition Sciences, Varastegan Institute for Medical Sciences, Mashhad, Iran E-mail: karamatim@varastegan.ac.ir
Digestion, Absorption, Transport, and Excretion of Nutrients (Session 6) Mohsen Karamati Department of Nutrition Sciences, Varastegan Institute for Medical Sciences, Mashhad, Iran E-mail: karamatim@varastegan.ac.ir
Biology 12 - Digestion Notes
 Biology 12 - Digestion Notes Anatomy Physiology Functions of the Digestive System -------------------------------------------------------------------------------------- food (enzymes, bile, HCl) to assist
Biology 12 - Digestion Notes Anatomy Physiology Functions of the Digestive System -------------------------------------------------------------------------------------- food (enzymes, bile, HCl) to assist
Nutrition and Digestion
 Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
Two main groups Alimentary canal continuous coiled hollow tube Accessory digestive organs
 Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Chapter 8: Digestion. Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes
 Chapter 8: Digestion Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes What organisms need Digestion? Heterotrophs - rely on ingestion of organic molecules for production of
Chapter 8: Digestion Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes What organisms need Digestion? Heterotrophs - rely on ingestion of organic molecules for production of
