SLIT/ROBO SIGNALING IN MONOCYTE CHEMOTAXIS AND FUNCTION: A ROLE IN VASCULAR INFLAMMATION
|
|
|
- Barrie Lee
- 5 years ago
- Views:
Transcription
1 SLIT/ROBO SIGNALING IN MONOCYTE CHEMOTAXIS AND FUNCTION: A ROLE IN VASCULAR INFLAMMATION by Ilya M. Mukovozov A thesis submitted in conformity with the requirements for the degree of Master of Medical Science Institute of Medical Science University of Toronto Copyright by Ilya M. Mukovozov
2 Slit/Robo Signaling in Monocyte Chemotaxis and Function: A Role in Vascular Inflammation. ABSTRACT Ilya M. Mukovozov Master of Medical Science (MSc) Institute of Medical Science University of Toronto 2010 Vascular inflammation and associated leukocyte influx is a hallmark in the pathogenesis of atherosclerosis. In both animal models and human subjects, inhibiting monocyte recruitment is beneficial in preventing atherosclerosis and its clinical manifestations. The trafficking signals that recruit cells to areas of inflammation are provided by small secreted proteins called chemokines. Chemokines play a major role in the pathogenesis of inflammation, and redundancy among the chemokine signaling pathways means that blocking one pathway could result in another assuming its function. Therefore, we aim to block a cell s response to a range of chemokine-induced directional migration signals. Slit2 treatment inhibits monocyte migration in vitro using transwell migration assays, and in vivo, using a murine peritonitis model of inflammatory cell influx. This inhibition is shown to be dose- and time- dependent. Furthermore, Slit2 inhibits monocyte adhesion to activated endothelial cell monolayers. These data may suggest a therapeutic role for Slit2 in atherosclerosis. ii
3 ACKNOWLEDGEMENTS I am most grateful to my supervisor and mentor Dr. Lisa Robinson for her constant encouragement and guidance through these past years. Lisa s extraordinary passion for science, her positive energy and commitment to motivate young minds through the Kids Science program, have been a constant source of inspiration. Her patience and understanding have made my transition and subsequent experience in the lab very enjoyable. I would also like to thank the members of my program advisory committee, Dr. Sergio Grinstein and Dr. Thomas Waddell for their encouragement, advice, and constructive criticism. I am especially lucky to have worked with the incredible individuals that make up the Robinson lab, and am thankful for their continued support. In particular, I am grateful to Dr. Yi Wei Huang and Guang Ying Liu for their patience with me when I started working in the lab. I would also like to thank Sajeda Patel and Dr. Swasti Chaturvedi whose suggestions, feedback and advice made my experience all the more rewarding. I would also like to thank all the members of Dr. Grinstein s lab and Dr. Brumell's lab, for their technical help, constructive criticism and friendship. I would also like to acknowledge the friendly and welcoming environment on the 4th floor, especially Shahab Shahnazari and the rest of the bear pack, which brought joy and laughter to my day-to-day experiences. I would also like to thank Thomas Sabljic, Danielle Baribeau and Stephanie Byun for their support and motivation. Finally, I would like to thank my parents, my sister Irina and Buddy, for their love, dedication and support throughout my life. iii
4 DATA ATTRIBUTION The data presented here was performed in collaboration with a number of individuals. The purification of Slit2 was performed by Dr. Durocher. Dr. Huang and Sajeda Patel were responsible for performing the experiments presented in Fig In addition, Guang Ying Liu, Shirin Chahtalkhi and I performed the immunoblotting experiments presented in figure 3.4, while I performed the subsequent analysis. Min Rui-Crow performed the experiments presented in Fig Finally, the data presented in Appendix 1 represents a study that was initiated by Soumitra Tole and completed by me. We contributed equally to this work. iv
5 TABLE OF CONTENTS LIST OF FIGURES LIST OF ABBREVIATIONS vii viii CHAPTER 1: INTRODUCTION 1.1 Inflammation Leukocyte Trafficking and the Adhesion Cascade Chemoattractants The Monocyte Chemotaxis Adhesion Phagocytosis Monocytes and Vascular Inflammation Slit2: A Guidance Cue for Cell Migration Expression Slit and Robo Structure Slit2/Robo-1 Intracellular Signal Transduction Slit/Robo in Leukocyte Trafficking RHO GTPases: Rac and Cdc Structure and Regulation Role of Rho GTPases in the regulation of the actin cytoskeleton Rationale, Hypothesis & Objectives Rationale Hypothesis Objectives 35 CHAPTER 2: MATERIALS & METHODS 2.1 Reagents and Antibodies Isolation of Human Monocytes Cell Culture Slit2 Expression and Purification Immunofluorescence Transwell Migration Assay Immunoblotting Cdc42 & Rac2 Activation Assays Adhesion Murine Peritonitis Phagocytosis Statistical Analysis 46 CHAPTER 3: RESULTS 3.1 Monocytes express the Slit2 receptor, Robo Slit2 inhibits chemotaxis of human monocytic THP-1 cells Slit2 treatment inhibits activation of Rac2 and Cdc Slit2 inhibits Akt and Erk, but not p38 MAPK pathways. 51 v
6 3.5 Slit2 inhibits adhesion of monocytic THP-1 cells to activated human 52 umbilical vein endothelial cell and human arterial endothelial cell monolayers. 3.6 Slit2 inhibits monocyte recruitment in vivo Slit2 does not alter monocyte phagocytosis. 55 CHAPTER 4: DISCUSSION & CONCLUSIONS 78 REFERENCES 89 APPENDIX1: The Axonal Repellent, Slit2, Inhibits Directional Migration of Circulating Neutrophils A1.1 Abstract 123 A1.2 Introduction 124 A1.3 Materials and Methods 129 A1.4 Results 142 A1.5 Discussion 148 A1.6 Acknowledgments 155 A1.7 Authorship 156 A1.8 References 157 A1.9 Figure Legends 166 vi
7 LIST OF FIGURES Page Figure 1.1 Leukocyte Adhesion Cascade. 2 Figure 1.2 Intracellular signal transduction upon chemokine GPCR activation. 13 Figure 1.3 Rho GTPases in the polarized monocyte. 16 Figure 1.4 Primary Structure of Mammalian Slit2 and Robo-1 Proteins. 25 Figure 3.1 Robo-1 is expressed by monocytes. 52 Figure 3.2 Slit2 inhibits monocyte chemotaxis. 54 Figure 3.3 Slit2 inhibits activation of Rho GTPases (Cdc42 and Rac2). 56 Figure 3.4 Slit2 inhibits Akt and Erk but not p38 MAPKs. 59 Figure 3.5 Slit2 inhibits adhesion of monocytic THP-1 cells to human umbilical vein endothelial cells. Figure 3.6 Slit2 inhibits adhesion of monocytic THP-1 cells to human arterial endothelial cells Figure 3.7 Slit2 inhibits monocyte recruitment in vivo. 66 Figure 3.8 Slit2 dose-dependently inhibits monocyte recruitment in vivo. 68 Figure 3.9 Slit2 inhibits monocyte recruitment in vivo: time-course. 70 Figure 3.10 Slit2 does not affect RAW macrophage phagocytosis. 72 vii
8 LIST OF ABBREVIATIONS AKT Protein Kinase B CCL5 RANTES CC-chemokine Ligand 5 CNS Central Nervous System CR1 Complement Receptor 1 CXCL4 CXC Chemokine Ligand 4 CXCR4 CXC chemokine Receptor 4 DAG Diacylglycerol DAPI 4,6-diamidino-2-phenylindole DCs Dentritic Cells DH Dbl homology domain ECM Extracellular Matrix EGF Epidermal Growth Factor ERK Extracellular-signal Regulated Kinase Ena Enabled EPAC Exchange Factor Directly Activated by Cyclic AMP fmlp N-formyl-methionyl-leucyl-phenylalanine FKN Fractalkine GAP GTPase Activating Protein GDNF Glial Cell Line-derived Neurotrophic Factor GEF Guanine Nucleotide Exchange Factor GPCR G-Protein-Coupled Receptor GTPase Guanosine Triphosphatase HAEC Human Arterial Endothelial Cells HRP Horseradish Peroxidase HUVEC Human Umbilical Vein Endothelial Cells ICAM-1 Intercellular Adhesion Molecule 1 Ig Immunoglobulin IP 3 Inositol (1,4,5)-triphosphate ITAM Immunoreceptor Tyrosine Activation Motif LFA-1 Lymphocyte Function Associated Antigen 1 LRR Leucine-Rich Repeat Mac-1 Macrophage Receptor 1, α M β 2 -integrin MAPK Mitogen Activated Protein Kinase MCP-1 Monocyte Chemotactic Protein 1 Mena Mammalian Enabled MLK Myosin Light-chain Kinase OxLDL Oxidized Low Density Lipoprotein PAK1 P21-Activated Kinase PBD P21-Binding Domain PBS Phosphate Buffered Saline PDGF Platelet-Derived Growth Factor PFA Paraformaldehyde PH Pleckstrin Homology PI3K Phosphoinositide 3-kinase viii
9 PI(4,5)P 2 Phosphatidylinositol (4,5)-bisphosphate PI(3,4,5)P 3 Phosphatidylinositol (3,4,5)-trisphosphate PLC Phospholipase C PKC Protein Kinase C PKC-ζ Protein Kinase C-ζ PSGL1 P-selectin Glycoprotein Ligand 1 SDF-1 Stromal Cell-derived Factor-1 SPA1 Signal-Induced Proliferation Associated Antigen 1 VCAM-1 Vascular Cell Adhesion Molecule VEGF Vascular Endothelial Growth Factor VLA-4 Very Late Antigen 4 VVO Vesiculo-Vacuolar organelles THP-1 Human Acute Monocytic Leukemia Cell Line TNF-α Tumor Necrosis Factor α ix
10 CHAPTER 1 INTRODUCTION 1.1 INFLAMMATION Leukocyte Trafficking and the Adhesion Cascade An essential function of the inflammatory response is to selectively recruit the appropriate subsets of leukocytes to specific sites of inflammation. Leukocytes are recruited to sites of inflammation in a series of coordinated interactions with endothelial cells lining the vessel wall. The classical leukocyte adhesion cascade involves three main steps: leukocyte rolling, activation and arrest, and transmigration (Fig. 1). In the first step, circulating leukocytes are captured by selectin-mediated rolling. Rolling is mediated by L-selectin expressed on most leukocytes, as well as P-selectin and E-selectin, which are expressed by endothelial cells (Kansas, G., 1996). All of the selectins interact with P-selectin glycoprotein ligand 1 (PSGL1), although other glycoprotein ligands exist (McEver et al., 1997). The binding of leukocyte L-selectin to PSGL1 can facilitate secondary leukocyte capture by adherent leukocytes (Eriksson et al., 2001). The interactions of selectins with their ligands allows leukocytes to adhere to inflamed endothelium under flow (Alon et al., 1995). In fact, shear stress is required to support L-selectin and P- selectin adhesion, as the rolling cells detach if flow is stopped (Finger et al., 1996; Lawrence et al., 1997). This selectin-mediated slow rolling allows the leukocyte to sample the repertoire of chemokines and other activation signals presented on the luminal surface of endothelial cells. 1
11 Figure 1.1 Leukocyte Adhesion Cascade. Leukocytes are recruited to sites of inflammation in a series of coordinated interactions with endothelial cells (ECs) lining the vessel wall. The classical leukocyte adhesion cascade involves three main steps: leukocyte rolling, activation and arrest, and transmigration. ECM Extracellular Matrix. 2
12 In addition to selectins, various integrins participate in rolling. Monocytes can roll on immobilized vascular cell-adhesion molecule 1 (VCAM-1) by engaging integrin receptor very late antigen 4 (VLA-4; α 4 β 1 -integrin). Members of the β 2 - integrins can also support rolling. Resting mouse neutrophils roll on surfaces coated with E-selectin ligand and intercellular adhesion molecule 1 (ICAM-1). Ligation of E- selectin induces a conformational change in lymphocyte function-associate antigen 1 (LFA-1; α L β 2 -integrin) which allows it to bind to its ligand ICAM-1 (Salas et al., 2004). In addition, it has recently been demonstrated that the mechanochemical design of LFA-1 allows shear stress to induce and maintain a state of high ligandbinding affinity (Astrof et al., 2006). Rolling in vivo was shown to require E-selectin (Kunkel et al., 1996) and engagement of β 2 -integrins (Jung et al., 1998), LFA-1 and macrophage receptor 1 (MAC1; α M β 2 -integrin) (Dunne et al., 2002). Subsequently, leukocytes undergo integrin-dependent arrest. This is mediated by the binding of leukocyte integrins to immunoglobulin superfamily members ICAM-1 and VCAM-1 on endothelial cells and is rapidly triggered by the binding of chemokines and other chemoattractants (Campbell et al., 1996; Campbell et al., 1998). These chemokines are secreted by activated endothelial cells, although platelets can also deposit chemokines, such as CC-chemokine ligand 5 (CCL5; RANTES) and CXC-chemokine ligand 4 (CXCL4) and CXCL5, onto the inflamed endothelial lumen to trigger monocyte arrest (von Hundelshausen et al., 2001; Huo et al., 2003). Finally, following firm arrest, leukocytes transmigrate across the endothelial cell barrier, its associated basement membrane, and the pericyte sheath. Leukocytes can cross the endothelium either between adjacent 3
13 endothelial cells (paracellular route) or directly through an endothelial cell (transcellular route). Transcellular migration generally occurs in 'thin' parts of the endothelium where there is less distance for the leukocyte to migrate. In addition, caveolae containing ICAM-1 link together to form vesiculo-vacuolar organelles (VVOs). This creates a channel inside the cell through which leukocytes can migrate. Although the leukocyte adhesion cascade has been divided into several steps, these are not temporally exclusive, but instead work together to achieve the desired effect of leukocyte arrest and diapedesis. Although leukocyte diapedesis was described almost 200 years ago, its molecular mechanisms are only now beginning to be fully understood (Imhof et al., 2004). In the past decade, new insights have been gained into the signalling events that underlie integrin activation, post-adhesion strengthening of leukocyte attachment, and the molecules involved in diapedesis (Muller, W., 2003) Chemoattractants In vivo, there are several types of chemoattractant mediators that can recruit leukocytes to inflammatory foci. These include bacterial components, leukotrienes, complement factors and chemokines. C5a was the first chemoattractant to be identified, and it is a cleaved product derived from complement component C5 (Shin et al, 1968). Bacterial products such as fmlp (N-formyl-methionyl-leucylphenylalanine) and other N-formylpeptides can also act as general chemoattractants that non-specifically recruit leukocytes to inflammatory foci. 4
14 However, the main chemoattractants that specifically recruit leukocyte subsets to inflammatory foci are a family of chemoattractant cytokines called chemokines. Chemokines are a large family of small peptides that are structurally similar (Rossi et al., 2000). Most are secreted, while only two, fractalkine (FKN;CX 3 CL1) and CXCL16, are expressed on the cell surface. The physiological importance of FKN expression can be highlighted in studies of cardiac allograft rejection where FKN expression is negligible in non-rejecting cardiac isografts, but is significantly enhanced in rejecting allografts (Robinson et al., 2000). In addition, FKN -/- mice have reduced atherosclerosis compared to wild type littermates (Robinson et al., 2000). Chemokine-induced signal transduction pathways are very similar. Thus, it is the specific expression, regulation, and receptor binding patterns of each chemokine that determine functional diversity. There are four families of chemokines that are classified on the basis of the relative positions of their N- terminal cysteine residues (Luster, A.,1998). Most chemokines contain four cysteine residues and fit within the α (CXC) or the β (CC) chemokine families, although another two families exist with lone members. FKN is a lone member in its family, and its N-terminal cysteine residues are separated by three amino acids (CX 3 CL1). The fourth family is composed only of lymphotactin (XCL1), which is a lymphocyte specific chemokine (Kennedy et al., 1995). Most chemokines bind to glycosaminoglycans (GAGs) on the luminal surface of endothelial cells. This binding is required for leukocyte recruitment, since chemokines with mutations in their GAG binding domains can induce in vitro chemotaxis, but are unable to recruit leukocytes to the peritoneal cavity in vivo (Johnson et al., 2005). 5
15 The binding of chemokines to their heterotrimeric G-protein coupled receptors (GPCRs) activates leukocyte integrins instantaneously by inside-out signalling mechanisms (Shamri et al., 2005). They rapidly regulate integrin avidity by increasing both integrin affinity (by a conformational change that results in increased ligand binding energy and a decreased ligand dissociation rate), and valency (the density of integrins per area of plasma membrane involved in adhesion, determined by expression levels and lateral mobility) (Laudanna et al., 2002; Constantin et al., 2000). It is through these signaling mechanisms that chemokines act as powerful activators of integrin-mediated adhesion and leukocyte recruitment. In monocytes/macrophages, chemokines interact with specific serpentine (heptahelical) receptors on the plasma membrane, which transduce signals by coupling to heterotrimeric G proteins. Heterotrimeric G proteins are composed of an α, β, and γ subunit. The α subunit is the GDP/GTP binding element. When bound to GDP, the α subunit interacts with the β and γ subunits to form an inactive heterotrimer complex that binds to the serpentine receptor. Binding of the chemokine to the serpentine receptor induces a conformational change that causes the exchange of GDP for GTP on the α subunit. This results in the dissociation of the α subunit from the receptor and the release of the Gβγ complex. The free Gα and Gβγ subunits are then free to interact with and modulate the activity of target enzymes. Thus, chemokine binding induces the dissociation of the G protein complex into α and βγ subunits, which bind and activate target enzymes such as phosphatidylinositol 3-kinase (PI 3K), phospholipase C (PLC), or adenyl cyclase. 6
16 These enzymes play an important role in generating secondary intracellular messengers that initiate a cascade of signaling events that culminate in cytoskeletal mobilization required for the chemoattraction response. 1.2 The Monocyte Mononuclear phagocytes arise in the bone marrow from dividing monoblasts and are released into systemic circulation as nondividing monocytes (Wiktor- Jedrzejczak et al., 1996). They circulate for several days before entering tissues and replenishing resident macrophages and dendritic cells (DCs) (Akagawa et al., 1996; Chapuis et al., 1997; Randolph et al., 1998). As half of the circulating monocytes leave the bloodstream under steady-state conditions every day, monocytes constitute a large systemic reservoir of myeloid precursors. Although circulating monocytes give rise to tissue-resident macrophages, they also form specialized cells such as DCs and osteoclasts, making up the mononuclear phagocyte system (Hume et al., 2002). As precursors for microglia and osteoclasts, monocytes play a role in the physiology of the central nervous system and in bone remodeling (Servet-Delprat et al., 2002). Mononuclear phagocytes are important for both innate and adaptive immunity. Their interactions with antigen-specific T lymphocytes trigger the induction of adaptive immune responses (Geissmann et al., 2003). Monocytes, defined as blood mononuclear cells, have "bean-shaped" nuclei and express CD11b, CD11c, and CD14 in humans, and CD11b and F4/80 in mice (Muller, W., 2001). Circulating monocytes are morphologically heterogeneous and constitute approximately 5-10% of peripheral blood leukocytes (van Furth et al., 7
17 1968). In humans and mice, the two principal monocyte subpopulations are the "inflammatory" and "resident" subsets (Geissmann et al., 2003). Human inflammatory monocytes express high levels of L-selectin and several chemokine receptors, including CCR2 the CCL2 receptor, but low levels of CX 3 CR1, the FKN receptor. On the other hand, resident human macrophages express high levels of CX 3 CR1 and low to non-detectable levels of L-selectin and most chemokine receptors, such as CCR2 (Grage-Griebenow et al., 2001). Furthermore, in humans CD14 high CD16ˉ monocytes represent the inflammatory subtype, while CD14 low CD16 + monocytes represent the resident subtype (Ziegler-Heitbrock, H., 1996). In mice, inflammatory monocytes are characterized by expression of high levels of Ly- 6C, a glycosylphosphatidylinositol-linked cell surface protein with unknown function. Ly-6C high, or "inflammatory", murine monocytes are short lived under steady-state conditions, and are preferentially recruited to inflammatory foci, such as atherosclerotic lesions (Sunderktter et al., 2004; Tacke et al., 2007). Ly-6C high monocytes express higher levels of PSGL-1 than Ly-6C low, or "resident" monocytes, and thus roll at slower velocities on P-selectin and E-selectin substratum. This property allows Ly-6C high monocytes to interact preferentially with atherosclerotic endothelium, compared with Ly-6C low monocytes (An et al., 2008). Resident monocytes in mice express low to non-detectable levels of Ly-6C, persist longer and repopulate several tissues, such as the liver, lung, spleen and brain after adoptive transfer (Geissmann et al., 2003). Interestingly, Ly-6C low CX 3 CR1 high monocytes show unique patrolling behavior in mice that are deficient in natural killer cells and T lymphocytes, and are readily recruited to sites of infection (Auffray et al., 8
18 2007). Although monocytes routinely emigrate from the blood to replenish tissue macrophages, increased recruitment can be elicited by pro-inflammatory, metabolic, and immune stimuli (van Furth et al., 1985). Following recruitment, monocytes differentiate into macrophages and DCs, contributing to host defence and tissue homeostasis through the clearance of senescent cells and tissue remodelling and repair following inflammation (Gordon, S., 1986; Gordon, S., 1998). In addition to host defense, monocytes have been implicated in atherosclerosis. The mechanisms controlling monocyte functions will be outlined to better understand the role they play in tissue homeostasis and host defense Chemotaxis Chemotaxis, the directed cell migration towards external chemical gradients, is a biological phenomenon of widespread occurrence. Chemotaxis can be observed in many eukaryotic cells including: free-living organisms, leukocytes (during inflammation), endothelial cells (angiogenesis), spermatocytes (fertilization) and neurons (neurogenesis), highlighting the biological significance of this phenomenon (Singer et al., 1986). Monocytes are amoeboid cells that move by extending pseudopods. Chemotaxis is achieved by first polarizing or orienting the cell in the direction of locomotion along a chemoattractant gradient. Polarization results from preferential pseudopod extension towards areas of higher chemoattractant concentration (Zigmond, S., 1974). Efficient chemotaxis requires coordination of motile activities such as pseudopod formation at the leading edge of the cell, and uropod retraction at the trailing edge. 9
19 During chemotaxis, macrophages extend short surface protrusions called filopodia, or microspikes, which are membrane extensions of approximately µm in diameter and up to 20 µm in length. These structures act as cellular tentacles and are supported by a core bundle of actin filaments called microfilaments (Mattila et al., 2008). In macrophages, filopodia help to support thin sheets of membraneenclosed cytoplasm, called lamellipodia. Lamellipodia contain actin filaments and a meshwork of myosin II-associated microfilaments. In macrophages, as well as in other cell types, the actin network within the lamellipodia, in association with several structural and regulatory proteins, comprises the molecular motor which drives cell locomotion (Jones et al., 1998). This locomotory apparatus works against cell-tosubstratum adhesions called focal contacts or focal adhesions. These molecular structures utilize members of the integrin family of proteins to link the myosin IIcontaining bundles of cytoplasmic microfilaments, called stress fibers, to proteins in the extracellular matrix (ECM) (Critchley et al., 1999). In macrophages, integrinmediated contacts to the ECM take two forms: focal complexes and podosomes. Focal complexes are structurally similar to focal adhesions but lack stress fibers (Allen et al., 1997), while podosomes are distinct circular structures that are only observed in cells of the myeloid lineage (DeFife et al., 1999; Correia et al., 1999; Linder et al., 2003). Macrophage chemotaxis can be divided into several steps: actin-driven protrusion of filopodia and lamellipodia at the leading edge, adhesion of the leading edge to the ECM via integrin-mediated focal interactions, actomyosin-mediated cell contraction, release of contacts at the trailing edge of the cell, and recycling of 10
20 membrane receptors from the rear to the front of the cell (Allen et al., 1998; Sheetz et al., 1999; Friedl, P., 2004; Friedl et al., 2009). The cell must integrate a number of molecular events in order to efficiently move across a substratum. This coordination is required for polarization and chemotaxis, and is largely mediated by the actin cytoskeleton within the cell. Exposure of circulating monocytes to chemoattractants leads to activation and subsequent migration of monocytes across the endothelial barrier towards the inflammatory foci. Monocytes/macrophages are responsive to even minuscule gradients of extracellular signals and will undergo chemotaxis towards a variety of stimuli. These signals include chemoattractants such as fmlp, vascular endothelial growth factor (VEGF) and chemokines such as macrophage chemotactic protein 1 (MCP-1;CCL2) and stromal cell-derived factor-1α (SDF-1α;CXCL12) (Gyetko et al., 1994; Barleon et al., 1996). Binding of the chemoattractant to its cell-surface receptor activates intracellular signaling cascades, which mobilize the actin cytoskeletal machinery. Subsequently, the cell polarizes forming actin-rich filopodia and lamellipodia at the 'front' of the cell and a tail-like uropod at the cell rear. The generation and maintenance of cell polarity and actin cytoskeleton remodelling are necessary processes for efficient monocyte chemotaxis. The SDF-1α receptor (CXCR4), like other chemokine receptors, is a glycosylated seven-transmembrane domain GPCR. It has an associated heterotrimeric GDP/GTP binding protein complex made up of α, β and γ subunits. Several mechanisms for activation of GPCRs have been proposed (Gether, U., 2000; Ulloa-Aguirre et al., 1999). Small molecule agonists can bind within the 11
21 transmembrane helices and cause receptor activation, while larger molecules bind to the extracellular surface leading to a conformational change that is transmitted to an intracellular Gαβγ complex. This event is followed by exchange of GTP for GDP in the Gα protein and its subsequent dissociation from the Gβγ complex, followed by the activation of downstream signaling pathways. Both the Gα subunit and Gβγ complex interact with several downstream effectors to generate cell polarity and drive migration, although these signaling events also prime the cell for other immune functions. Furthermore, the various immune functions of the leukocyte are not temporally exclusive as they are dependent on complex interactions between intracellular signaling events. Ligation of GPCRs leads to the activation of four major signaling pathways (Fig. 2): PLC, PI3K, mitogen-activated protein kinases (MAPKs) and Rho guanosine triphosphatases (GTPases). Each of these pathways is involved in cell activation and/or the generation of cell polarity. Once the Gα subunit dissociates, the Gβγ complex activates PLC, which in turn cleaves phosphatidylinositol (4,5)- bisphosphate (PI(4,5)P 2 ) to generate inositol (1,4,5)-triphosphate (IP 3 ) and diacylglycerol (DAG). Generation of IP 3 leads to the mobilization of intracellular calcium stores from the endoplasmic reticulum and DAG activates protein kinase C (PKC) (Li et al., 2000). A convincing role for PI3Ks in GPCR signaling and chemotaxis has been established (Li et al., 2000; Sasaki et al., 2000; Hirsch et al., 2000; Servant et al., 2000; Jin et al., 2000). Although there are at least four Class I PI3K isoforms in mammalian cells (Vanhaesebroeck et al., 1999), only a single Class I B variant, 12
22 Figure 1.2 Intracellular signal transduction upon chemokine GPCR activation. Chemokine binding to GPCRs induced a conformational change that results in the dissociation of Gα subunits from the Gβγ complex. This leads to rapid outside-in signaling resulting in the activation of four major signaling pathways that influence monocyte chemotaxis, adhesion and phagocytosis: PLC, PI3K, MAPKs and Rho GTPases. Each of these pathways contributes to the generation of cell polarity and/or modulation of integrin avidity. 13
23 containing a p110γ catalytic subunit complexed with a 101 kda regulatory protein, has been shown to interact with G-proteins in leukocytes. Since chemokine responses in leukocytes, such as phagocytosis, are sensitive to pertussis toxin, it was believed that chemokine receptors are coupled to a Gα i subunit (Boulay et al., 1997). However, there is also data implicating other families of G-protein α subunits in chemokine-mediated signaling (Amatruda et al., 1993), in addition to Gβγ subunits (Clapham et al., 1993). While there is evidence that Class I B PI3K is responsive to activation by Gα subunits (Murga et al., 1998), it has also been shown that p110γ is activated via interactions with the Gβγ subunits (Neptune et al., 1997). Regardless of how PI3K is activated, the outcome leads to phosphorylation of membrane PI(4,5)P 2 by activated PI3K, resulting in the generation of PI(3,4,5)P 3 at the plasmalemma. In addition, the Gβγ complex also activates PI3Kγ which activates Src-family kinases and generates PI(3,4,5)P 3 from membrane PI(4,5)P 2 (Krugmann et al., 1999). Src-family kinases phosphorylate adapter proteins such as Shc, resulting in the recruitment of Ras GTPases and subsequent activation of MAPK pathways (Kintscher et al., 2000). Although the p38 and Erk MAPK pathways are involved in chemotaxis and adhesion, the most important biochemical events for cell polarization are the production of PIP 3 and activation of Rho GTPases at the leading edge of the cell. The PI3K dependent production of PIP 3 at the cell membrane allows for the recruitment of Rho GTPases, Rac, and Cdc42 to the cell membrane. The localization of PIP 3, Rac and Cdc42 then stimulate the polymerization of actin, a 14
24 process necessary for the formation of lamellipodia at the front of the cell (Fig 1.3). On the other hand, at the back of the cell, Rho-kinase phosphorylation results in inactivation of myosin light chain phosphatase, leading to increased myosin lightchain kinase (MLK) dependent activation of myosin (Nguyen et al., 1999). These biochemical conditions favour the formation of actomyosin bundles, contraction, deadhesion from the substratum and tail retraction (Ridley, A., 2001; Bokoch, G., 2005). Interestingly, mutual inhibition of leading edge and trailing edge proteins allows for the maintenance of cell polarity (Fenteany et al., 2004). To prevent the accumulation of PIP 3 at the trailing edge, PTEN dephosphorylates PI(3,4,5)P 3 to PI(4,5)P 2. The lack of PIP 3 in the back of the cell decreases the activation and recruitment of Rho GTPases and subsequent actin polymerization, allowing the formation of actomyosin bundles and tail retraction (Worthylake et al., 2001). Actin polymerization at the leading edge coupled to tail retraction in the back allows for directed leukocyte chemotaxis Adhesion Firm adhesion of leukocytes to activated endothelium is required for leukocyte transmigration, and is an integrin-dependent process. Integrins are large transmembrane glycoproteins that anchor the cell's cytoskeleton to other cells or to the ECM. These large protein complexes are heterodimers composed of α and β subunits. The β2 family of integrins, only expressed on leukocytes, includes LFA-1 and Mac-1. These are responsible for firm adhesion and arrest on endothelial cells, 15
25 Figure 1.3 Rho GTPases in the polarized monocyte. The PI3K dependent production of PIP 3 at the cell membrane allows for the recruitment of Rho GTPases, Rac, and Cdc42 to the cell membrane. The localization of PIP 3, Rac and Cdc42 stimulates the polymerization of actin, a process necessary for the formation of lamellipodia at the front of the cell. At the back of the cell, Rho-kinase phosphorylation results in inactivation of myosin light chain phosphatase, leading to increased myosin light-chain kinase (MLK) dependent activation of myosin. 16
26 and later attachment with ECM components. LFA-1 and Mac-1 can both bind VCAM-2 and ICAM-1 (Ley et al., 2007). Chemokines are powerful activators of integrin-mediated firm adhesion. GPCR stimulation results in rapid inside-out signaling cascades that result in a net increase in the average integrin affinity and valency (Chan et al., 2003; Carman et al., 2003). Although the signaling cascades downstream of GPCRs are incompletely understood, several pathways have been at least partially elucidated. For example, PLC is known to be recruited after GPCR signaling. Recruitment and activation of PLC results in the production of IP 3 and DAG followed by an increase in intracellular calcium. This calcium flux and the production of DAG activates guanine nucleotide exchange factors (GEFs), such as Vav1 and CALDAG1, and results in the recruitment and activation of Rho GTPases (Constantin et al., 2000; Vielkind et al., 2005; Crittenden et al., 2004). Thus, Rho GTPases are also involved in the signaling cascades linking GPCR activation and changes in integrin affinity. For example, chemokine-mediated conformational changes in LFA-1 are induced by GTPases RhoA (Giagulli et al., 2004) and Rap-1 (Shimonaka et al., 2003). Once recruited and activated, Rho GTPases associate with actin binding proteins, including talin-1 and α-actinin, to modulate integrin affinity (Sampath et al., 1998; Jones et al., 1998; Brakebusch et al., 2003). Thus, chemokine-mediated changes in integrin affinity are dependent on interactions with the actin cytoskeleton. In addition to GPCR-dependent inside-out signaling, binding of ligands to integrins also induces outside-in signaling cascades (Ley et al., 2007). Paxillin is a scaffold protein for signaling molecules that can bind integrins. Ligand induced 17
27 integrin clustering activates Src-family kinases. These phosphorylate paxillin allowing the recruitment of downstream effectors, such as ADP-ribosylating factor GTPase activating protein (ArkGAP) and PAK interacting exchange factor (PIX). ArfGAP inactivates Rac, while PIX activates Cdc42 (DeMali et al., 2003). Src-family kinases also activate Vav1, a Cdc42 and Rac GEF (DeMali et al., 2003). Thus, Rho GTPases are required for the mobilization of the actin cytoskeleton to form and maintain adhesive contacts. Both GPCR-induced inside-out signaling and ligandinduced outside-in signaling modulate the activity of Rho GTPases to mobilize the actin cytoskeleton for adhesion Phagocytosis Phagocytosis is a cellular process in which solid particles are engulfed by the cell membrane to form internal phagosomes. Although this process can be used for the acquisition of nutrients and to clear dead cells and debris, in leukocytes, this is a major mechanism used to clear invading microorganisms. Although phagocytosis of uncoated particles can occur, the efficiency of phagocytosis is greatly enhanced by the binding of opsonins, such as complement factors or Igs, to the surface of a particle. Opsonins can be recognized by receptors on the leukocyte surface, including Fc (Ravetch et al., 1991) and complement receptors (Brown, E., 1991), which mobilize the actin cytoskeleton and aid in the subsequent internalization. Fc receptors facilitate the engulfment of Ig coated particles. These receptors are members of the multichain immune recognition receptor family. Their signaling parallels signaling via T and B cell antigen receptors, which signal via homologous 18
28 cytoplasmic sequences, called immunoreceptor tyrosine activation motif (ITAM) (Strzelecka et al., 1997; Imboden et al., 1985). Fc receptor activation results in recruitment of PI3K, which converts PI(4,5)P 2 to PIP(3,4,5)P 3 and DAG. DAG is also produced by PLC and activates PKC (Botelho et al., 2000). Phagocytosis requires normal signaling through phosphoinositide kinases and PLC (Greenberg et al., 2003). Ultimately, Fc receptor ligation leads to actin mobilization and membrane remodeling, which is mediated by Rho GTPases. GEFs, such as Vav-1, contain a pleckstrin homology (PH) domain with a high affinity for PI(3,4,5)P 3, promoting their recruitment to membrane PI(3,4,5)P 3 at sites of Fc receptor ligation (Bustelo, X., 2002). Recruitment of GEFs results in localized activation of Rho GTPases Rac and Cdc42, which mobilize the actin machinery to extend pseudopods to engulf the particle (Greenberg et al., 2002). For example, Fcγ phagocytosis results in activation of the Rho GTPases Cdc42 and Rac (Caron et al., 1998). Complement-derived opsonins, such as C3b, can be generated by both the classical and the alternative pathways. Complement receptor 1 (CR1) on the surface of leukocytes recognizes and binds C3b. Unlike the active phagocytosis seen in Fc receptor-mediated engulfment, complement-mediated phagocytosis is slow and gentle (Greenberg et al., 2002). The phagocytic ability of monocytes is important for host defence. In addition, monocyte phagocytosis of oxidized low density lipoprotein (OxLDL) is implicated in the formation of foam cells in atherosclerotic lesions Monocytes and Vascular Inflammation 19
29 Atherosclerosis is an inflammatory disease that can be characterized by intense immunological activity in the vessel wall. It is the major cause of coronary heart disease (CHD) the leading cause of death in North America (Castelli et al., 1984; Pasternak et al., 2004). The process of atherogenesis is also involved in cerebrovascular atherosclerotic disease, and in the aortic artery, renal artery and peripheral vasculature. Thus, the pathogenesis of atherosclerosis is wide ranging and threatens human health worldwide (Murray et al., 1997). Atherosclerosis involves the formation of vessel lesions, called plaques, that are characterized by lipid accumulation, inflammation, cell death and fibrosis. Over time, these plaques can mature and grow in size. The main complications of atherosclerosis result from plaques that occlude the vessel, causing stenoses by limiting blood flow and starving the downstream tissues of oxygen and nutrients. In some instances, the plaque may rupture, sending an embolus down the vessel and exposing the prothrombotic material in the plaque to the blood. This may also result in an abrupt formation of a thrombotic clot, which can occlude the vessel at the site of plaque rupture (Libby et al., 2002). In the coronary vessels, this can lead to a myocardial infarction. Atherosclerosis in the carotid vessels can result in ischaemic stroke and transient ischaemic attacks. Atherosclerotic plaques are composed of a mixture of immune cells (mainly macrophages and T lymphocytes), extracellular matrix, lipids and lipid-rich debris. This accumulation of immune cells and lipids in the intima is located between the vascular endothelial cells and the smooth muscle cells of the vascular media. Although the cellular compositions of plaques changes with time, lipid-laden 20
30 macrophages, or foam cells, tend to outnumber other cell types. The immune cells in the plaque become activated and produce proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interferon-γ (IFNγ). This proinflammatory environment induces the vascular endothelial cells to express increased leukocyte adhesion molecules, allowing monocyte and other immune cells to transmigrate into the plaque. In addition, vascular endothelial cells increase adhesion molecule expression in response to cholesterol accumulation in the intima (Cybulsky et al., 1991). Macrophage colony-stimulating factor (M-CSF) produced by endothelial cells and smooth muscle cells (Rajavashisth et al., 1990) allows monocytes to differentiate into macrophages in the plaque (Smith et al., 1995). In addition, atherosclerotic plaques produce the chemokine FKN, which can be shed by proteolysis. Shed FKN can engage its receptor CX 3 CR1 on blood-borne monocytes and macrophages, stimulating their recruitment to the atherosclerotic vessel wall (Combadire et al., 2003; Lesnik et al., 2003). Thus, the recruitment of immune cells, especially monocytes, initiates the formation of atherosclerotic plaques. Since the recruitment of monocytes to the vessel wall is pivotal to atherogenesis, targeting the recruitment of monocytes to the subintima may produce clinical benefits and slow the atherogenetic process. 1.3 SLIT2: A GUIDANCE CUE FOR CELL MIGRATION During the development of the central nervous system (CNS), neurons must migrate and project axons over long distances. Most axons emanating from the CNS must cross the midline and then project longitudinally towards their synaptic 21
31 targets. The molecular mechanisms that guide this pathfinding include: contact attraction, chemoattraction, contact repulsion and chemorepulsion. These mechanisms act simultaneously in a coordinated manner to direct axonal pathfinding (Tessier-Lavigne et al., 1996). Generally, guidance cues can either promote or repress migration of neurons and axonal projections. For example, netrins are diffusible chemotropic factors that attract commissural axons to the midline (Kennedy et al., 1994). The Slit family of secreted proteins, together with their cell-surface receptor Roundabout (Robo), act to repel neurons during CNS development. Once commissural axons have crossed the midline, midline glial cells express Slit to prevent the axons from re-crossing the midline. Drosophila Slit mutants exhibit midline defects, such as collapse of the regular scaffold of commissural and longitudinal axon tracts in the embryonic CNS (Rothberg et al., 1988; Rothberg et al., 1990). A similar defect is observed in Robo mutants, where projecting axon tracts cross the midline repeatedly (Kidd et al., 1998). Recent studies demonstrate a role for Slit and Robo as guidance cues outside of the CNS. For example, in Drosophila mesoderm migration, myocyte precursors migrate away from the midline towards peripheral target sites where they fuse to form muscle fibers. In Slit and Robo mutants, these cells do not migrate away from the midline and instead fuse across the midline (Rothberg et al., 1990). Interestingly, this defect can be reversed by expressing Slit protein in midline cells (Kramer et al., 2001). Slit and Robo signaling also plays a role in nephrogenesis. The proper localization of the kidney is dependent on the formation of a structure called the ureteric bud. This process requires secretion of glial cell line-derived 22
32 neurotrophic factor (GDNF) by nearby mesenchymal cells. Slit and Robo knockout mice display abnormal patterns of GDNF secretion and develop multiple ureteric buds and multiple urinary collecting systems, implicating Slit and Robo in nephrogenesis (Ray, L., 2004). Furthermore, variations in the human Robo gene have been associated with familial vesicoureteral reflux (Bertoli-Avella et al., 2008), a condition with improper insertion of ureters into the bladder resulting in retrograde flow of urine from the bladder to the kidney. Therefore, Slit and Robo appear to play a role in normal human urinary tract formation. In addition to its role in embryogenesis, Slit also plays a role in the mature organism. A recent study demonstrated Slit2-mediated inhibition of aortic smooth muscle cell migration toward a gradient of platelet-derived growth factor (PDGF) (Liu et al., 2006). This inhibition was shown to be mediated by suppressing the activation of small GTPase Rac1. Furthermore, Slit2 can prevent breast cancer cell metastasis. Both Robo and the chemokine receptor CXCR4 are expressed by some human breast cancer cells, allowing them to migrate towards gradients of SDF-1α. The lungs may produce high levels of SDF-1α, promoting breast cancer metastasis to this tissue. Slit2 inhibited the chemotaxis, adhesion and chemoinvasion of breast cancer cells (Prasad et al., 2004). Several other studies have demonstrated a role for Slit2 as a tumor suppressor. Slit2 was shown to inhibit colony formation in lung, colorectal and breast cancer cell lines (Dallol et al., 2002). Furthermore, Slit2 was often epigenetically silenced in more aggressive forms of these and other cancers (Dallol et al., 2003; Dallol et al., 2003; Dickinson et al., 2004). Therefore, these studies imply a role for Slit and Robo in the adult organism and in cancer biology. 23
33 1.3.1 Expression The expression of the Slit genes has been demonstrated in many organisms, including Drosophila (Battye et al., 1999), Caenorhabditis elegans (Hao et al., 2001), Xenopus (Chen et al., 2000), Gallus gallus domesticus (Holmes et al., 2001; Vargesson et al., 2001), mice (Holmes et al., 1998; Piper et al., 2000), rats (Marillat et al., 2002) and humans (Itoh et al., 1998). In mammals, there are three members of the Slit family. Although Slit1 is predominantly expressed in the developing CNS (Yuan et al., 1999), Slit2 and Slit3 are expressed outside the CNS, in the lung, kidney and heart tissues (Wu et al., 2001). Importantly, Slit expression persists in the adult organisms, suggesting a role for the Slit family beyond embryogenesis. Expression of Robo has been demonstrated in Drosophila (Kidd et al., 1998), mice (Yuan et al., 1999) and humans (Kidd et al., 1998). There are four isoforms of Robo in mammals. Robo-1 has been shown to be most highly expressed in tissues outside the CNS, including human leukocytes (Wu et al., 2001). Interestingly, the tissue expression of Slit and Robo is relatively complementary, suggesting a functional relationship in the adult organism (Yuan et al., 1999) Slit and Robo Structure The Slit family of proteins contains an N-terminal signal peptide, four leucinerich repeats (LRRs), nine epidermal growth factor (EGF) repeats and a C-terminal cysteine knot (Fig 1.4) (Rothberg et al., 1988; Rothberg et al., 1990; Rothberg et al., 1992). The EGF repeats and LRR allow the Slit proteins to interact with ECM 24
34 Figure 1.4 Primary Structure of Mammalian Slit2 and Robo-1 Proteins.. Mammalian Slit2 contains four leucine rich repeats (LRRs), nine epidermal growth factor (EGF) repeats, a laminin G (G) domain, and a cysteine rich C terminus. The Robo-1 receptor contains five immunoglobulin (Ig) repeats, three fibronectin (FN) type III, a transmembrane Domain (TM) and four conserved cytoplasmic (CC) signaling motifs. 25
35 components, such as glypican-1 (Ronca et al., 2001), enabling them to act as localized, non-diffusible, signaling molecules. Furthermore, Slit2 can be cleaved after the fifth EGF repeat by proteases to form N-terminal (Slit-N) and C-terminal (Slit-C) fragments (Brose et al., 1999; Wang et al., 1999). The N-terminal fragment includes the first 1118 amino acids and contains the four LRRs and the first five EGF repeats, while the C-terminal fragment contains the remaining residues (Brose et al., 1999). Importantly, it is the four LRRs that are necessary and sufficient for interaction with the Robo receptor and downstream signaling (Battye et al., 1999). Therefore, both the full length protein and the N-terminal Slit2 fragment can bind Robo receptors to repel migrating cells and projecting axons (Nguyen Ba-Charvet et al., 2001). Although the cleavage of Slit2 does not eliminate its activity, it may play a role in its diffusion, since N-Slit appears to be more tightly associated with the cell membrane. In rat neural tissue both the N-terminal and C-terminal fragments of Slit were shown to bind heparan sulfate proteoglycan glypican-1 (Liang et al., 1999), although the C-terminal fragment bound with higher affinity, suggesting a possible regulatory mechanism for its diffusion. Robo is a single-pass type-1 receptor and signaling molecule for the Slit family of proteins. The extracellular region of Robo-1 contains five immunoglobulin (Ig) repeats and three fibronectin type III domains. The cytoplasmic region of Robo- 1 contains four conserved cytoplasmic signaling motifs, CC0, CC1, CC2 and CC3 (Kidd et al., 1998; Zallen et al., 1998). Only the Ig domains of Robo are required to bind to the LRRs in full length and N-terminal Slit2 (Battye et al., 2001; Chen et al., 26
36 2001; Nguyen Ba-Charvet et al., 2001). The cytoplasmic CC motifs of Robo are required for its response to Slit (Bashaw et al., 2000) Slit2/Robo-1 intracellular signal transduction Studies of neuronal tissue have demonstrated that Robo-1 can signal through two pathways that lead to mobilization and remodeling of the cytoskeleton: Enabled (Ena) protein and Rho GTPases. Both of these pathways require the CC motifs in the cytoplasmic domain of Robo to signal. Ena and its mammalian homologue (Mena) are members of a family of proteins that are believed to link signal transduction to localized remodeling of the actin cytoskeleton by binding to profilin, an actin binding protein which regulates actin polymerization (Lanier et al, 1999; Wills et al., 1999). The bacteria Listeria monocytogenes utilizes the Ena proteins for actin-based motility (Laurent et al, 1999). Ena was demonstrated to be a substrate for the Abelson kinase (Gertler et al., 1989). Ena and Abelson can both bind to Robo. Ena binds to the CC1 motifs while Abelson binds to the CC3 motif (Bashaw et al., 2000). Impairing Ena binding reduced Robo function while mutations in Abelson results in Robo hyperactivity (Bashaw et al., 2000). A second pathway through which Slit/Robo mediates cell repulsion is through modulation of Rho GTPase activity. A family of GTPase activating proteins, Slit Robo GTPase activating proteins (srgaps), were shown to bind Robo (Wong et al., 2001). The SH3 domain of these proteins is required to bind to the CC3 motif of Robo, while the GAP domain has activity for the Rho GTPases Rac, Cdc42 and 27
37 Rho (Wong et al., 2001). This suggests a model where Slit ligation of Robo induces the recruitment of srgap followed by inactivation of Rho GTPases and inhibition of actin remodeling and cell motility. The literature supporting the role of Rho GTPases in cell motility is consistent with this model Slit and Robo in leukocyte trafficking Both neuronal and leukocyte cell migration require the recognition of guidance cues, polarization of the cell and mobilization of the actin cytoskeleton. Thus, a conservation of cell migration guidance mechanisms was proposed when Slit2 was found to inhibit leukocyte migration, in addition to its well established role in neuronal guidance (Guan et al., 2003; Kanellis et al., 2004; Prasad et al., 2007). The first study to demonstrate Slit-mediated inhibition of leukocyte migration was published in Nature by Wu et al., This study utilized transwell migration assays to demonstrate Slit-mediated inhibition of chemotaxis of rat lymph node cells to gradients of MCP-1 and neutrophil-like HL-60 cells to fmlp gradients (Wu et al., 2003). Subsequently, Kanellis et al. demonstrated Slit2-mediated inhibition of chemotaxis towards MCP-1 and fmlp in rat-derived macrophages (Kanellis et al., 2004). Another study showed that Slit2 inhibited migration of dendritic cells (DCs) (Guan et al., 2003). Although these early studies implicated Slit2 in modulation of leukocyte chemotaxis, clear evidence for this role was lacking in primary human cells. However, in 2007, Prasad et al. were able to demonstrate that Slit2 can inhibit chemotaxis and transendothelial migration of primary CD4+ T lymphocytes and the human Jurkat T cell line (Prasad et al., 2007). 28
38 These results are indicative of a role for Slit2 in human leukocyte chemotaxis, as Slit2 has been shown to inhibit migration of human DCs and lymphocytes. Furthermore, we have shown that Slit2 inhibits the in vitro chemotaxis of primary human neutrophils, and the in vivo recruitment of mouse neutrophils (Tole et al., 2009). Importantly, we have demonstrated that Slit2 inhibits neutrophil chemotaxis towards a range of chemokines, both in vivo and in vitro, including fmlp, IL-8, C5a and FKN (Tole et al., 2009). Therefore, Slit2 may have a therapeutic role as a universal inhibitor of leukocyte migration. 1.4 RHO GTPases: Rac and Cdc42 Small GTPases of the Rho family are a part of the Ras superfamily of small GTP-binding proteins. They are pivotal regulators of many signaling networks that are activated by a diverse variety of receptor types. To date, over 20 mammalian Rho GTPases have been characterized, and these can be grouped into 6 different classes: Rac (Rac1, Rac2, Rac3, RhoG), Rho (RhoA, RhoB, RhoC), Cdc42 (Cdc42Hs, G25K, TC10), Rnd (Rnd3/RhoE, Rnd1/Rho6, Rnd2/Rho7), RhoD, and TFF (Aspenström, P., 1999; Kjoller et al., 1999). When activated, Rho GTPases regulate many important processes in all eukaryotic cells, including actin cytoskeleton dynamics, transcriptional regulation, cell cycle progression, and membrane trafficking. The activation, and hence the activity of Rho GTPases is regulated by outside-in signals from a variety of receptor types, including GPCRs, tyrosine kinase receptors, cytokine receptors and adhesion receptors. Rho GTPases play an critical role in leukocytes as regulators of signaling networks that 29
39 allow these cells to perform specialized functions, such as chemotaxis, adhesion and phagocytosis Structure and Regulation All Rho GTPases contain two main structural domains, the C-terminal 'CAAX' motif and a catalytic GTP domain. The 'CAAX' motif undergoes post-translational processing, involving carboxy-terminal proteolysis of the AAX residues followed by carboxyl-methylation. The modified C-terminal domain can then attach to membrane lipids and facilitates membrane association and subcellular localization of Rho GTPases (Gutierrez et al., 1989; Casey et al., 1989; Fujiyama et al., 1990). The catalytic domain contains two regions, switch I and switch II. These domains correspond to different structural conformations in the GTP-bound and GDP-bound forms. Rho GTPases function as molecular switches by cycling between GDPbound and GTP-bound forms. When bound to GDP, Rho GTPases are inactive. Upstream signaling events leading to the exchange of GDP for GTP switches the protein to an active state. The active form of the protein can transduce signals via interactions with downstream targets or effector molecules to produce a cellular response. The intrinsic GTPase activity of Rho GTPases completes the cycle, by hydrolyzing GTP, returning the GTPase to its inactive GDP-bound state. There are three classes of molecules that interact with Rho GTPases and are capable of regulating their activation state: guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs catalyze the exchange of GDP for GTP, leading to the 30
40 activation of Rho GTPases. GEFs stimulate the release of GDP allowing GTP, which is present at higher concentrations in cells than GDP, to bind and activate GTPases. To date, over 69 mammalian GEFs for Rho GTPases have been identified (Rossman et al., 2005). They are characterized by the presence of a Dbl homology domain (DH), which is capable of interacting with both the switch I and switch II regions and catalyses the exchange of GDP for GTP. In addition, many of these DH-domain containing proteins, such as Vav, contain a PH domain. The PH domain allows GEFs to bind phosphoinositides, such as PIP 3. This allows GEFs to be localized to the plasma membrane where they can interact with other Rho GTPase interacting proteins. Thus, GEFs promote the activation of Rho GTPases and also facilitate their interaction with downstream effector molecules. On the other hand, GAPs enhance the intrinsic GTPase activity of Rho GTPases, resulting in the suppression of their activity. Although GTPases posses intrinsic GTPase activity, the actual rate of GTP hydrolysis is relatively slow. Therefore, the interaction with a GAP is required for efficient GTP hydrolysis, as this accelerates the cleavage step by several orders of magnitude (Vetter et al., 2001). To date, more than 70 eukaryotic RhoGAPs have been discovered, 35 of these can be found in humans (Tcherkezian et al., 2007). There exists a large diversity in the primary sequences of the various GAPs. However, each one contains a Rho GAP domain with a conserved tertiary structure composed of α helices and a catalytically critical 'arginine finger' which stabilizes the formation of the transition state during GTP hydrolysis (Nassar et al., 1998). In addition, the Rho GAP domain can interact with both the switch I and switch II regions on the GTPase domain (Gamblin et al., 31
41 1998). This interaction allows GAPs to facilitate the intrinsic hydrolysis of GTP, resulting in the inactivation of Rho GTPases. Finally, GDIs associate with Rho GTPases in their inactive GDP-bound state and inhibit their activation by GEFs. In addition, GDIs have also been shown to bind to GTP-bound GTPases, such as Rho GTPase, and suppress GTPase activity (Oloffson, B., 1999). Finally, there is evidence that GDIs can bind to isoprenyl moieties on the C-terminus of GTPases in order to sequester them in the cytosol (Keep et al., 1997). The role of GDIs in partitioning GTPases between the membrane and cytosol may be physiologically more important than the inhibition of their activation. It is possible that the GDImediated partitioning of GTPases may provide a storage pool of Rho GTPases that may be readily utilized upon cell activation. Ultimately, the function of GDIs is to prevent the activation of Rho GTPases, prevent their interaction with membranes, and inhibit their downstream signaling networks Role of Rho GTPases in the regulation of the actin cytoskeleton Rho GTPases are pivotal regulators of signaling networks that are activated by chemokine and cytokine receptors, along with other receptor types, and result in the mobilization of the cytoskeleton (Machesky et al., 1997). Actin polymerization is a common response of motile cells to chemoattractants, and occurs following the activation of Rho GTPases (Carson et al., 1986; Hall et al., 1989; Howard et al., 1984). The movement of eukaryotic cells relies on coordinated extension of actinrich lamellipodia in the leading edge and retraction of the uropod at the rear of the cell. The extension of lamellae in the leading edge involves rapid turnover of actin 32
42 filaments (Symons et al., 1991; Wang, Y., 1985). More stable actin-myosin cables can be found in more established protrusions and in the middle and rear of the cell (DeBiasio et al., 1988). Thus, cell motility requires the coordinated polymerization of actin in protrusions at the leading edge and contraction of actin-myosin cables at the middle and rear of the cell. In addition, other factors such as recycling of the plasma membrane and integrin-mediated adhesion are important for cell motility (Bretscher, M., 1996; Martenson et al., 1993; Yamada et al., 1995; Mitra et al., 2005). Furthermore, coordinated actin assembly is important for integrin-mediated adhesion and phagosome formation (Defacque et al., 2000; Calderwood et al., 2000). All of these processes are dependent on coordinated mobilization of the actin cytoskeleton, and are regulated by deployment of actin-binding proteins by activated Rho GTPases. Rho GTPases are in an ideal position to control cell motility and morphological changes in response to extracellular stimuli, such as chemokine gradients. For example activation of Rho in fibroblasts results in the assembly of stress fibers and focal adhesions (Ridley et al., 1992). The activation of Rac causes extension of lamellipodia and assembly of small focal complexes (Nobes et al., 1995; Ridley et al., 1992). Finally, activation of the Cdc42 Rho GTPase leads to the formation of filopodial extensions (Nobes et al., 1995). 1.5 Rationale, Hypothesis & Objectives Rationale 33
43 Monocyte recruitment and proliferation in the subintima is a hallmark of atherosclerosis and vascular inflammation. The trafficking signals that recruit monocytes to sites of inflammation are provided by chemoattractants. Although we can target certain individual chemoattractants and their receptors, redundancy exists in the chemokine signaling pathways that allow other pathways to compensate for the loss of one or more. Therefore, it would be more efficient to knock-out chemoattractant-mediated cell recruitment with a universal inhibitor of chemokine GPCR signaling pathways. The Slit family of proteins have long been known to act as inhibitors of cell migration and axon projection in the CNS. More recent studies have implicated a role for Slit2/Robo-1 signaling in diverse cell types, including leukocytes, both in vitro and in vivo (Dallol et al., 2002; Guan et al., 2003; Kanellis et al. 2004; Liu et al., 2006; Prasad et al., 2004; Prasad et al., 2007). Furthermore, we have demonstrated Slit2-mediated inhibition of circulating human and mouse neutrophils to several chemoattractant gradients (Tole et al., 2009). These data suggest that Slit2 may inhibit cellular migration outside of the CNS, implying that the guidance mechanisms controlling cell migration may be conserved across cell types. Indeed, Slit2 was also shown to inhibit the chemotaxis of circulating human neutrophils induced by several classes of chemoattractants, including: fmlp, IL-8 and C5a (Tole et al., 2009). In addition, Slit2 was shown to dramatically decrease neutrophil recruitment in an in vivo model of murine peritonitis induced by sodium periodate or other chemoattractants (C5a, mouse inflammatory protein 2). However, limited data is available on the effect of Slit2 on monocyte migration and function. Studies in 34
44 neuronal cells have implicated the Rho GTPases in the Slit2-mediated inhibition of migration. In human neutrophils, Slit2 was shown to inhibit the activation of Rho GTPases, Cdc42 and Rac2, after fmlp stimulation (Tole et al., 2009). Since Rho GTPases are important regulators of the cytoskeleton, the effects of Slit2 may go beyond affecting the migration of a cell to include modulation of other functions such as adhesion or phagocytosis, as these functions all involve actin cytoskeleton remodelling Hypothesis We hypothesize that Slit2/Robo-1 signaling can inhibit monocyte/macrophage chemotaxis and modulate immune functions such as adhesion to endothelial cells and phagocytosis of Ig-opsonized beads. We hypothesize that Slit2 exerts its effects by suppressing the activity of Rho GTPases Rac and Cdc42. However, we hypothesize that Slit2/Robo-1 signaling will have no effect on the activation of MAPKs, as was observed in primary human neutrophils (Tole et al., 2009). In addition, we hypothesize that Slit2, administered to mice intraperitoneally or intravenously will inhibit monocyte/macrophage recruitment to the peritoneal cavity, in vivo, in a murine model of sodium periodate-induced inflammation Objectives The first objective of this study is to determine if monocytes/macrophages express the Slit2 receptor Robo-1. Monocytes/macrophages must express the 35
45 receptor in order to be responsive to the effects of Slit2. Next, the effect of Slit2 on monocyte/macrophage chemotaxis will be characterized in vitro using transwell chemotaxis assays and treatment with the monocyte/macrophage chemoattractant SDF-1α. In addition, the intracellular signaling cascades that mediate Slit2/Robo-1 signaling in monocytes/macrophages will be investigated by observing the role of Slit2 on Rho GTPases Cdc42 and Rac1, and on the Akt, Erk, and p38 MAPKs. Pulldown assays for activated, or GTP-bound, Rho GTPases will be performed, following incubation with Slit2 and activation with SDF-1α. To determine the effect of Slit2 on MAPKs, western blots for phosphorylated and total MAPKs will be performed, following incubation of monocytes/macrophages with Slit2 and treatment with SDF-1α. The effect of Slit2 on monocyte/macrophage adhesion to activated endothelial cells will also be investigated. Confluent endothelial cell monolayers will be activated with a proinflammatory cytokine (TNF-α), and the adhesion of monocytes/macrophages following incubation with Slit2 will be tested. Furthermore, the effect of Slit2 on monocyte/macrophage recruitment in vivo using a murine model of sodium periodate induced peritonitis will be conducted. Slit2 will be administered intraperitoneally or intravenously an hour prior to the induction of peritonitis, and peritoneal lavages will be performed to determine the number of recruited monocytes/macrophages. In addition, the dose of Slit2 required to optimally inhibit monocyte/macrophage recruitment in vivo will be determined by performing a dose-response experiment using the murine peritonitis model and intravenously administered Slit2. Furthermore, a time-course experiment will be performed by administering Slit2 at 1 day, 4 days, and 10 days prior to inducing 36
46 peritonitis, to determine the biological half-life of intravenously administered Slit2. Finally, the effect of Slit2 on other monocyte/macrophage functions involving Rho GTPases, such as phagocytosis, will be investigated. To do this, phagocytosis assays will be conducted with Ig-opsonized latex beads, and the monocyte/macrophage phagocytosis following incubation with Slit2 will be quantified. 37
47 CHAPTER 2 MATERIALS & METHODS 2.1 Reagents and antibodies Unless otherwise stated, reagents were purchased from Sigma-Aldrich. Monocyte isolation kit was purchased from StemCell Technologies. The following primary antibodies were used: anti human Robo-1 (ab7279, Abcam, Cambridge, MA), anti-myc 9E10 (Covance, QC, Canada), anti-human Cdc42 (Cell Signaling, Danvers, MA), anti-human Rac1 (Upstate Biotechnology, Lake Placid, NY). The following secondary antibodies were used: Cy-3 conjugated anti rabbit IgG, Cy-2 conjugated anti-human IgG, and horseradish peroxidase-conjugated anti rabbit IgG (Jackson Immunoresearch Laboratories, Bar Harbor, ME). MAPK Antibodies were purchased from Invitrogen Canada (Burlington, Ontario, Canada). 2.2 Isolation of human monocytes Blood from healthy volunteers was obtained on each day of experimentation. The monocytes were isolated using a Polymorphprep gradient separation solution (Axis-Shield, Norway) and an EasySep Negative Selection kit (StemCell Technologies). A volume of blood was gently layered over an equal volume of Polymorphprep solution, and centrifuged at 460 g for 35 minutes at an acceleration of 2 units and deceleration of 0 units in order to prevent cell activation. The lower layer containing peripheral blood mononuclear cells (PBMCs) was collected, washed in cold PBS with 2% fetal bovine serum (FBS) and 1mM EDTA and centrifuged at 260 g, room temperature for 5 min. When redness could still be seen 38
48 in the pellet, indicating the presence of red blood cells, an additional wash in cold PBS with 2% FBS and 1 mm EDTA was performed. The EasySep Negative Selection procedure was performed according to the manufacturer s protocols. Briefly, PBMCs (5x10 7 cells/ml) are labelled with EasySep Human Monocyte Enrichment Cocktail (50µg/mL cells)(stemcell Technologies) for 10 minutes at 4 C. The Negative Selection Enrichment Cocktail contains a combination of monoclonal antibodies that were purified from hybridoma culture supernatant by affinity chromatography using Protein A or Protein G Sepharose. These antibodies are bound in bispecific Tetrameric Antibody Complexes which are directed against cell surface antigens on human leukocytes (CD2, CD3, CD16, CD19, CD20, CD56, CD66b, CD123, glycophorin A) and dextran. These mouse monoclonal antibodies are of the IgG 1 subclass. In addition, this cocktail also contains an FcR blocker to prevent non-specific binding of monocytes. The antibody subclass of the FcR blocker is IgG 2b. The cells were then labelled with EasySep Magnetic Microparticles (50 µg/ml cells)(stemcell Technologies) for 5 minutes at 4 C. The Magnetic Microparticles contain a suspension of magnetic dextran iron particles in TRIS buffer. Then, the EasySep magnet was used to remove the magnetically labelled cells, while the pure monocytes are poured off. The purified monocytes were then resuspended in ice cold PBS with 2% FBS and 1 mm EDTA for subsequent experiments. Experiments were performed within 1-2 hours of cell isolation. Cell viability was determined to be >98% by Trypan blue staining 2.3 Cell culture 39
49 Human acute monocytic leukemia (THP-1) cells were cultured in RPMI-1640 (Sigma Chemical, St Louis, MO) supplemented with 5% FBS. Primary human umbilical vein endothelial cells (HUVECs) and human arterial endothelial cells (HAECs) were grown in endothelial basal medium 2 (EBM-2) supplemented with Clonetics EGM-2 SingleQuots (Lonza, Walkersville, MD) These include: 10mL FBS, 2mL of recombinant human fibroblast growth factor-b, 0.5mL of ascorbic acid, 0.5mL of recombinant human vascular endothelial growth factor, 0.5mL of recombinant human epidermal growth factor, 0.5mL heparin, 0.2mL hydrocortisone, 0.5mL recombinant insulin-like growth factor-1, and 0.5mL gentamicin sulfate amphotericin-b for 500mL of EBM-2. Only low passage cells (up to passage 11) were used for adhesion experiments. Once cellular confluency was reached, cells were passaged and/or seeded into 96-well clear bottom tissue culture plates for adhesion experiments. 2.4 Slit2 expression and purification Stable human embryonic kidney 293 cell line expressing full-length or N- terminal human Slit2 with a His tag at its carboxyl terminus was used for Slit2 purification. Recombinant Slit2 was purified by Sylvie Perret and Dr. Yves Durocher at the National Research Council of Canada. The presence of purified Slit2 was confirmed by immunoblotting with poly anti-his antibody (Sigma A-7058). Following purification, Slit2 was aliquotted, snap frozen and stored at -80 C for future use. Aliquots were never re-frozen or used after storage at 4 C. In the experiments described below, Slit2 was generally used at a concentration of 4.6 µg/ml diluted in 40
50 ice cold PBS. Endotoxin concentrations in our Slit2 preparation ranged from ng/ml, yielding final experimental concentrations of pg/ml which are well below those thought to activate leukocytes (Moore et al., 2000). To verify this, we added similar concentrations of endotoxin in neutrophil Transwell assays, and found that such levels of endotoxin had no effect on neutrophil migration (Tole et al., 2009). 2.5 Immunofluorescence Primary human monocytes, murine RAW macrophages and THP-1 cells were allowed to settle onto fibronectin-coated coverslips and allowed to adhere for 3 minutes at room temperature (RT). The cells were then fixed with 4% paraformaldehyde (PFA) for 10 minutes at RT. The cells were stained with rabbit anti-robo-1 (1µg/ml) for 2 hours at RT, washed with phosphate buffered saline (PBS) and then incubated with Cy3-conjugated anti-rabbit secondary antibody for 1 hour at RT. A Leica DMIRE2 spinning disc confocal microscope (Leica Microsystems, Toronto, Ontario, Canada) equipped with a Hamamatsu backthinned EM-CCD camera and Volocity software (Improvision Inc., Lexington, MA) was used to capture images. 2.6 Transwell migration assay Human THP-1 cells (1x10 6 cells/ml, 100 µl/condition) were incubated with PBS vehicle or Slit2 at concentrations ranging from 46 ng/ml to 4.6 µg/ml for 10 minutes at 37 C and 5% CO 2. The cells were then loaded into the top chamber of a 41
51 5 µm Transwell insert (Corning Life Sciences, Corning, NY) designed for a 24-well plate. A glass coverslip was placed in the bottom well. The bottom chamber was filled with 600 µl of serum-free RPMI-1640 alone or with SDF-1α (100ng/mL) in the presence or absence of Slit2. Monocytes were allowed to migrate into the bottom chamber for 3.5 hours at 37 C and 5% CO 2. Following incubation, the monocytes were rapidly spun down onto the coverslips (by centrifugation of the entire plate at 100 g, 1 min), fixed with 4% PFA, washed with PBS and labeled with DAPI dye for visualization of cell nuclei. A Leica DMIRE microscope was used to take representative high-power (40X) images and total number of cells was counted in at least 10 random fields. The data represent the mean values ± SEM from at least 4 independent experiments. 2.7 Immunoblotting THP-1 cells were serum starved overnight, resuspended in serum-free RPMI-1640 (1x10 6 cells/ml) and incubated with either PBS vehicle or Slit2 (4.6 µg/ml) for 10 min at 37 C, 5% CO 2. The cells were subsequently activated with SDF-1α (100ng/mL) for 0, 2 and 5 minutes. The cells were then washed with 1 ml of ice cold PBS. Next, the cells were lysed using ice-cold lysis buffer (50 mm Tris, ph 7.5, 10% glycerol, 100 mm NaCl, 1% NP-40, 5 mm MgCl2, 1 mm DTT, 1 mm PMSF, 1/100 protease inhibitor cocktail and 1 mm NaVO3). Protein samples were added to 6x SDS gel loading buffer (1% ß-mercaptoethanol, 1% SDS, 30% glycerol, % bromophenol blue, Tris HCl 0.28 M, ph 6.8). Samples were centrifuged briefly at 10,000 rpm for 1 min. Protein gels were electrotransferred to poly- 42
52 vinyldene fluoride (PVDF) membranes (Millipore) in transfer buffer (25 mm Tris base, 190 mm Glycine, 0.05% SDS, and 20% methanol) for 1.5 hr at 350 A at 4 C. Membranes were probed for i) phosphorylated and total Akt, ii) phophorylated and total Erk, and iii) phophorylated and total p38 MAP kinase. The membranes were always probed with antibody detecting the phophorylated protein first, stripped, and then reprobed with the antibody detecting total protein as a loading control. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Biosciences, UK Ltd, Buckinghamshire, UK) and the signal captured onto Kodak-Biomax film (Rochester, NY, USA). Image J software (NIH, Bethesda, MA, USA) was used for densitometry analysis, and subsequent statistical analysis was performed using Microsoft Excel. 2.8 Cdc42 and Rac2 activation assays The pull-down assay for the Rho GTPases Cdc42 and Rac1 was performed as previously described (Benard et al., 1999; Tole et al., 2009) with slight modifications. The phosphate binding domain (PBD; aa) of PAK1 in pgex- 4T3 vector was expressed as a GST fusion protein in BL21 (DE3) E. coli cells. The GST-PBD fusion protein was affinity purified using glutathione sepharose 4B beads (GE Healthcare). THP-1 cells (1x10 7 cells/sample) diluted in 500 µl 37 C warmed HEPES-HBSS were incubated with PBS vehicle or with 4.6 µg/ml Slit2 at 37 C and 5% CO 2 for 10 minutes. Cells were then stimulated with SDF-1α (100ng/mL) for 0, 2, or 5 minutes at 37 C and the reactions were stopped by adding 500 µl ice-cold lysis buffer. Samples were centrifuged at maximal speed in a bench-top centrifuge 43
53 for 5 min at 4 C and an aliquot of supernatant was used as a loading control. The remaining supernatants were added to GST-PBD glutathione beads (20 mg beads/sample). Samples were rotated at 4 C for 1 hour, washed 3 times with cold wash buffer (50 mm Tris, ph 7.5, 40 mm NaCl, 0.5% NP-40, 30 mm MgCl2, 1 mm DTT, 1 mm PMSF, 0.1 mm NaVO3 ) and added with 20 µlof 2 x Laemmli loading buffer. The samples were then run on SDS-PAGE and transferred onto a 0.2 mm PVDF membrane (Millipore). Cdc42 and Rac2 were detected using rabbit antihuman Cdc42 (Cell Signaling, Danvers, MA) and rabbit anti-human Rac2 (Upstate Biotechnology, Lake Placid, NY) primary antibodies and goat anti-rabbit HRPconjugated secondary antibodies. Densitometry analysis was performed on the blots using Image J software (NIH, Bethesda, MA, USA). The data represent the mean values ± SEM from 3 independent experiments. 2.9 Adhesion Primary human endothelial cells (HUVECs and HAECs) were seeded (~1x10 4 cells/well) in 96-well tissue culture plates and grown to confluence. Once confluence was confirmed using a light microscope, the wells were aspirated and replenished with endothelial basal medium 2 alone or with TNF-α (20 ng/ml). The plates are then incubated at 37 C and 5% CO 2 for 4 hours. THP-1 cells were simultaneously labeled with Calcein AM at 37 C and 5% CO 2 for 30 mins. After labeling, monocytic THP-1 cells were washed once in 45 ml of PBS, pelleted (1500 rpm, 5 min) and resuspended in serum-free RPMI-1640 at 1x10 6 cells/ml. THP-1 cells were then incubated with PBS in the presence or absence of Slit2 (4.6 ug/ml) 44
54 at 37 C and 5% CO 2 for 10 mins. The THP-1 cells were allowed to settle onto the endothelial cell monolayers (1x10 5 cells/well) and incubated at 37 C and 5% CO 2 for 30 mins. The plates were then centrifuged (100g, 1 min) upside down to remove non-adherent cells. A fluorescent plate reader was used to measure the fluorescence intensity of each well ( nm for Calcein AM). Fluorescence intensities are normalized to the unstimulated condition. The data represent the mean values ± SEM from at least 4 independent experiments Murine peritonitis Experimental murine peritonitis was carried out as previously described with slight modification (Lotero et al., 2001; Jiang et al., 2005; Viriyakosol et al., 2005). All procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals and were approved by The Hospital for Sick Children Research Institute Animal Care Committee. For the experiments in Fig. 3.7, Slit2 (1.8 µg/mouse) was administered intraperitoneally to BALB/c mice (Chares River Canada) an hour prior to sodium periodate induced peritonitis. For the experiments in Fig. 3.8 and 3.9, CD1 mice were utilized. For the dose-titration experiments presented in Fig. 3.8, Slit2 (4.6 µg, 460 ng or 46 ng) was administered intravenously via tail-vein injection. For the time-course experiments presented in Fig 3.9, we administered Slit2 intravenously (1.8 µg/mouse) at 1 day, 4 days, and 10 days prior to inducing peritonitis with sodium periodate (1mg/mouse) injected intraperitoneally. For the experiments in Fig 3.7 and Fig. 3.8, peritonitis was induced an hour after Slit2 or PBS treatment, with an intraperitoneal injection of sodium 45
55 periodate (1mg/mouse). Peritoneal lavages were performed after 24 hours with 5 ml of cold PBS containing 2% FBS. The cells were washed, red blood cells lysed and hemocytometer counts performed. The data represent the mean values ± SEM from at least 4 independent experiments Phagocytosis Monocyte phagocytosis was performed as previously described (Yan et al., 2007) with slight modifications. Human IgG (1 mg/ml) was coated onto 3.8 µm latex beads for 2 hours at room temperature. RAW macrophages were incubated with Slit2 (600 ng/ml) or control medium (equal volume) for 10 minutes, exposed to the latex beads, centrifuged (1000 rpm for 30s) to initiate phagocytosis, and plated onto fibronectin-coated (20 µg/ml) coverslips. Phagocytosis was terminated after 30 min and external beads were labeled on ice using anti human Cy-2 conjugated secondary antibody. Slit2 or control medium were present throughout the course of phagocytosis. Images of at least 10 random fields were acquired using a Leica deconvolution microscope. To determine the number of ingested particles, total beads were counted using DIC and the number of external, fluorescently-labeled beads were subtracted. The phagocytic index (number of ingested beads/ number of cells) was used as an outcome measure Statistical analysis Analysis of variance (ANOVA) followed by Bonferonni post-hoc tests were performed using SPSS statistical software to analyze the data from adhesion 46
56 experiments. In all other cases, the Student s t-test was used. Significant difference was considered for p<0.05. Graphic representation show mean ± SEM as variance bars. 47
57 CHAPTER 3 RESULTS 3.1 Monocytes express the Slit2 receptor, Robo-1. Immunoblotting confirmed Robo-1 protein expression in primary human monocytes, mouse RAW macrophages and human THP-1 monocytic cells. Furthermore, immunofluorescence staining confirmed the presence of Robo-1 on the surface of primary human monocytes and human monocytic THP-1 cells, colocalizing with a membrane marker (Fig. 3.1). Collectively, these data demonstrate that primary human monocytes, mouse RAW macrophages and human monocytic THP-1 cells express the Slit2 receptor, Robo Slit2 inhibits chemotaxis of human monocytic THP-1 cells. The neuronal literature has clearly demonstrated the role of Slit2 as a repellent of neuronal cells and projecting axons. More recent studies have shown that Slit2 may act as a general chemorepellent, since it was shown to inhibit the chemotaxis of diverse cell types, including: smooth muscle cells (Liu, et al., 2006), DCs (Guan, et al. 2003), T lymphocytes (Prasad, et al., 2007), RAW macrophages (Kanellis, et al., 2004) and primary human neutrophils (Tole et al., 2009). Since human monocytes also expressed Robo-1, we hypothesized that Slit2 also inhibits monocyte chemotaxis. Transwell migration assays were performed to determine the effect of Slit2 on monocyte chemotaxis. Human monocytic THP-1 cells were utilized for these experiments. THP-1 cells failed to migrate in the absence of the chemokine SDF-1α 48
58 (Fig. 3.2A&C). When SDF-1α (100 ng/ml) was added to the bottom chamber, monocytic THP-1 cell migration to the lower chamber was significantly increased (Fig. 3.2B), p<0.01. To test the effect of Slit2 on monocytic THP-1 cell chemotaxis, we incubated the cells with Slit2 (4.6 µg/ml) for 10 minutes and tested their ability to migrate when Slit2 (4.6 µg/ml) alone (Fig. 3.2C) or Slit2 with SDF-1α (100 ng/ml) (Fig. 3.2D) was added to the bottom chamber. In the absence of a chemokine gradient, monocytic THP-1 cells pre-treated with Slit2 failed to migrate to the bottom chamber (Fig. 3.2C). However, Slit2 treated cells exhibited decreased chemotaxis in the presence of a chemokine gradient (Fig. 3.2D), p<0.01. In addition, we tested the effect of N-Slit2, a cleaved N-terminal fragment containing all four LRR required for signaling, on the chemotaxis of monocytic THP-1 cells. Monocytic THP-1 cells treated with N-Slit2 also exhibited decreased chemotaxis in the presence of a chemokine gradient (Fig. 3.2D), p<0.01. These data demonstrate that both the full length Slit2 and N-Slit2 can inhibit SDF-1α-mediated chemotaxis of monocytic THP- 1 cells, but no effect on monocytic THP-1 cell chemotaxis is observed in the absence of a chemokine gradient Slit2 treatment inhibits activation of Rac2 and Cdc42 Slit2 has been shown to inhibit migration of neuronal cells via recruitment to the intracellular domain of Robo of a novel family of Slit Robo Rho GTPase activating proteins (srgaps). srgaps convert the active GTP-bound forms of Rho GTPases, Cdc42 and Rac1, to their inactive GDP-bound forms. (Wong et al., 2001). In a study of vascular smooth muscle cell migration, Slit2 was shown to suppress 49
59 the activation of Rac1 (Liu et al., 2006). Rac1 and Cdc42 have been demonstrated to play critical roles in leukocyte polarization and chemotaxis. HL-60 cells transfected with dominant-negative construct of Cdc42 show impaired migration (Srinivasan et al., 2003). Therefore, we hypothesized that the observed decrease in monocyte chemotaxis may be due to Slit2-mediated inactivation of Rac1 and/or Cdc42. We utilized the p21-binding domain (PBD) of PAK1, which only binds to active GTP-bound forms of Rac1 and Cdc42 (Benard et al., 1999), conjugated to GST beads (GST-PBD) in order to pull down activated forms of Rac1 and Cdc42. Human monocytic THP-1 cells were incubated with PBS in the presence or absence of Slit2 (4.6 µg/ml) for 10 minutes and then stimulated with SDF-1α (100 ng/ml) for 2 minutes. Activated Rho GTPases were pulled down using GST-PBD beads. Subsequently, immunoblotting was performed for Cdc42 and Rac1. Unstimulated monocytic THP-1 cells had low basal levels of activated Rac1 and Cdc42. Stimulation with SDF-1α led to a 6.4 fold increase (, p<0.05) in GTP-bound Cdc42 and a 21.6 fold (, p<0.05) increase in GTP-bound Rac1, compared with unstimulated cells (Fig. 3.3). Slit2 treatment alone had no effect on baseline activation of Rac1 and Cdc42 (Fig. 3.3). However, Slit2 treatment significantly reduced SDF-1α mediated activation of Cdc42 and Rac1 (Fig. 3.3). Monocytic THP- 1 cells incubated with Slit2 had a 3.8 fold (, p<0.05) increase in GTP-bound Cdc42 and a 12.2 fold (, p<0.05) increase in GTP-bound Rac1, compared to unstimulated cells. These data suggest that a disruption in SDF-1α-mediated Rho GTPase activation is involved in Slit2-mediated inhibition of monocytic THP-1 cell 50
60 chemotaxis towards SDF-1α. The data represent the mean values ± SEM from 4 independent experiments Akt and Erk, but not p38 MAPK pathways are affected by Slit2 treatment. The signal transduction pathways leading from chemoattractant receptor activation to chemotaxis are not fully understood. Generally, chemoattractant receptor stimulation activates several MAPK pathways including: PI3K/Akt, Erk and p38. In neutrophils, PI3K-dependent production of PIP 3 and subsequent recruitment and activation of Akt MAPK at the leading edge is important for migration (Heit et al., 2002). Another study in human monocytes demonstrated that an inhibitor of MEK inhibited MAPK activation and MCP-1-mediated chemotaxis (Yen et al., 1997). In fact, MCP-1-mediated chemotaxis of monocytic THP-1 cells was shown to be Erk MAPK dependent (Kintscher et al., 2000). Thus, MAPK inhibitors can arrest chemotaxis. Therefore, we tested the effect of Slit2 on chemoattractant-induced activation of Akt, Erk and p38 MAPK pathways in human monocytic THP-1 cells. Stimulation with SDF-1α induced robust activation of the Akt and Erk MAPK pathways (Fig. 3.4A). However, no activation of the p38 MAPK was observed (Fig 3.4A & D). Stimulation with SDF-1α for 5 minutes led to a 2.9 fold increase (, p<0.05) in phosphorylated Akt and a 3.2 fold (, p<0.05) increase in phosphorylated Erk, compared with unstimulated cells (Fig. 3.4A-C). Incubation with Slit2 alone had no effect on baseline activation of Akt, Erk and p38 MAPKs (Fig. 3.3). However, incubation with Slit2 significantly reduced SDF-1α-mediated activation of Akt and Erk MAPKs at 5 minutes, although no effect was observed for p38 MARK (Fig. 3.3). 51
61 Monocytic THP-1 cells incubated with Slit2 had a 1.7 fold (, p<0.05) increase in phosphorylated Akt and a 1.9 fold (, p<0.05) increase in phosphorylated Erk, compared to unstimulated cells. Therefore, incubation with Slit2 decreased the activation of Akt and Erk MAPKs (Fig. 3.4A-C) at 5 minutes after SDF-1α stimulation. However, incubation with Slit2 had no effect on the p38 MAPK pathway (Fig. 3.4A & D). These results suggest that Slit2 treated monocytes might have a defect in the synthesis of PIP 3 and subsequent recruitment and activation of Akt MAPK Slit2 inhibits adhesion of monocytic THP-1 cells to activated human umbilical vein endothelial cell and human arterial endothelial cell monolayers. After their initial recruitment, monocytes must firmly arrest on the endothelium and undergo diapedesis to reach inflammatory foci. Integrins on the surface of leukocytes bind to Ig superfamily members such as ICAM-1 and VCAM-1 on the surface of endothelial cells (Ley et al., 2007). Rho GTPases participate in many cellular processes that transmit signals from the cell surface to influence the activity of the actin cytoskeleton (Sechi et al., 2000). Leukocyte adhesion to endothelial cells requires outside-in signaling which can be initiated by integrin ligation and clustering, which is partially dependent on Rho GTPases (Ley et al., 2007). Since Slit2 inhibits the activation of Cdc42 and Rac2 Rho GTPases, we tested the effect of Slit2 on adhesion of monocytic THP-1 cells to endothelial cells. Endothelial cells were activated with TNF-α for 4 hours in order to simulate inflammation and increase endothelial expression of adhesion molecules. Monocytic 52
62 THP-1 cells were allowed to adhere for 30 minutes and the plates centrifuged upside down at 100xg for 1 min to remove non-adherent cells. For HUVECs (Fig. 3.5), Slit2 treatment alone had no effect on cell adhesion, whereas TNF-α stimulation of endothelial monolayers increased baseline adhesion to almost 400%, p<0.005 (Fig. 3.5). When monocytic THP-1 cells were incubated with Slit2, adhesion was abolished to near baseline levels, P<0.05 (Fig. 3.5). Since adhesion characteristics differ for different types of endothelial cells, we wanted to use cell types similar to those affected in human cardiovascular disease. Therefore, we utilized primary HAECs to confirm our findings from HUVECs. The same trend was observed for HAECs (Fig. 3.6), Slit-treatment alone had no effect of cell adhesion, while TNF-α stimulation of endothelial monolayers increased baseline adhesion by over 200%, p<0.01 (Fig. 3.6). When monocytic THP-1 cells were incubated with Slit2, adhesion was abolished to near baseline levels, P<0.005 (Fig. 3.6). These data suggest that Slit2 may play a role in monocyte adhesion to vascular and arterial endothelium under inflammatory conditions. 3.6 Slit2 inhibits monocyte recruitment in vivo. We showed that Slit2 inhibits monocyte chemotaxis and adhesion to activated endothelial cells. Thus, we wanted to investigate the functional relevance of these observations. To study the effects of Slit2 on monocyte recruitment in vivo, we used a previously described mouse model of chemical irritant peritonitis (Jiang et al., 2005; Viriyakosol et al., 2005). Sodium periodate injection alone induced peritonitis, with robust monocyte recruitment compared to control mice (Fig.3.7, 53
63 p<0.001). Intraperitoneal administration of Slit2 diminished monocyte recruitment nearly four-fold (Fig. 3.7; p<0.001). Next, we wanted to test whether the effect of Slit2 is dose-dependent. Again, injection of sodium periodate alone induced vigorous peritonitis when compared to control (Fig. 3.8, p<0.05). Slit2 significantly inhibited monocyte recruitment at doses of 4.6 and 0.46 µg (Fig. 3.8, p<0.05). Although administration of 46 ng of Slit2 decreased monocyte recruitment by half, this effect was not statistically significant (Fig. 3.8). Finally, we performed a timecourse experiment to determine the duration of Slit2 biological activity following intravenous administration. Again, female CD1 mice were utilized for these experiments. Slit2 (1.8 µg/mouse) was administered intravenously at 10 days, 4 days and 1 day prior to induction of experimental peritonitis. Sodium periodate alone induced vigorous peritonitis when compared to control (Fig 3.9, p<0.001). Intravenous administration of Slit2 one day before peritonitis completely abolished cell recruitment to baseline (Fig 3.9, p<0.001). When Slit2 was administered intravenously 4 days prior to the induction of peritonitis, monocyte recruitment was again significantly inhibited (Fig 3.9, p<0.01). However, pre-treatment with Slit2 at 10 days prior to induction of peritonitis had no effect on monocyte recruitment. Thus, these data indicate that Slit2 has very potent effects on in vivo monocyte recruitment, with persistent biological activity even when administered 4 days prior to an inflammatory insult. This suggests that local or systemic administration of Slit2 may be used to alleviate monocyte recruitment in inflammation and atherosclerosis. Because Slit2 is heavily glycosylated, it is relatively 'sticky' and may achieve high 54
64 local concentrations by interacting with ECM components such as glypican-1 (Ronca et al., 2001). 3.7 Slit2 does not alter monocyte phagocytosis. Monocytes/macrophages are professional antigen presenting cells and can therefore internalize and destroy pathogens and cellular debris. They can also internalize opsonized or non-opsonized targets. This is mediated by Fc receptors for Igs and the integrin Mac-1 for complement components (Aderem et al., 1999). Rho GTPases were shown to be required for calcium signaling and phagocytosis by Fcγ receptors in macrophages (Hackam et al., 1997; Caron et al., 1998). Since Slit2 inhibits the activation of Rho GTPases, we tested the effect of Slit2 on monocyte phagocytosis. RAW macrophages were centrifuged together with IgG-opsonized latex beads to initiate phagocytosis for 10 minutes. External beads were subsequently fluorescently labeled and images of at least 10 random fields were captured. Slit2 treatment had no effect on the phagocytic index (number of ingested particles/ number of cells) of RAW macrophages (Fig 3.10A&B; Min Rui-Crow performed these experiments). 55
65 56
66 Figure 3.1 Slit2 is expressed by monocytes. Robo-1 expression has been confirmed in primary human monocytes and human monocytic THP-1 cells. A, western blotting for Robo1 protein in murine RAW macrophages, human monocytic THP-1 cells and primary human monocytes. B, Surface immunofluorescence staining showing the co-localization of cell surface Robo-1 and a membrane marker. These results confirm the presence of the Robo-1 receptor on the surface of monocytic THP-1 cells, RAW macrophages and primary human monocytes. 57
67 58
68 Figure 3.2 Slit2 inhibits monocyte chemotaxis. Monocyte chemotaxis was studied in vitro using a Transwell membrane inserts. A, Human monocytic THP-1 cells failed to migrate in the absence of SDF-1α (Fig. 3.2A). When SDF-1α (100 ng/ml) was added to the bottom chamber, THP-1 cells exhibited increased migration to the lower chamber (Fig. 3.2A) (, p<0.001). To test the effect of Slit2 on THP-1 chemotaxis, we incubated the cells with Slit2 (4.6 µg/ml) for 10 minutes and tested their ability to migrate when Slit2 (4.6 µg/ml) alone (Fig. 3.2A) or Slit2 with SDF-1α (Fig. 3.2A) was added to the bottom chamber. In the absence of a chemokine gradient, THP-1 cells incubated with Slit2 failed to migrate to the bottom chamber (Fig. 3.2A). Slit2 treatment decreased chemotaxis towards an SDF-1α gradient (Fig. 3.2A) (, p<0.001). B, THP-1 cells were incubated with full-length Slit2, N-terminal Slit2 or PBS vehicle for 10 minutes prior to migration. Slit2 and the chemokine SDF-1α were added to the bottom well only. After 3.5 hours, the number of migrated cells was quantified microscopically. Both the full length protein and the N-terminal fragment of Slit2 were able to inhibit THP-1 cell chemotaxis towards an SDF-1α gradient (, p<0.001). 59
69 60
70 Figure 3.3 Slit2 inhibits activation of Rho GTPases (Cdc42 and Rac1). Human monocytic THP-1 cells were incubated with PBS alone or containing Slit2 (4.6 μg/ml) for 10 minutes and stimulated with SDF-1α (100ng/mL) for 2 minutes and lysates collected. GTP-bound or activated Cdc42 and Rac1 were pulled down using GST-PBD beads. A, Western blots showing the activation of Rho GTPases Cdc42 and Rac1 with SDF-1α stimulation. B&C, The graphs depicts the band intensities normalized to the loading controls. Unstimulated monocytic THP-1 cells had low basal levels of activated Rac1 and Cdc42. Stimulation with SDF-1α led to a 6 fold increase (, p<0.05) in GTP-bound Cdc42 and a 21 fold (, p<0.05) increase in GTP-bound Rac1, compared with unstimulated cells. Slit2 treatment alone had no effect on baseline activation of Rac1 and Cdc42. However, Slit2 treatment significantly reduced SDF-1α mediated activation of Cdc42 and Rac1. Monocytic THP-1 cells incubated with Slit2 had a 4 fold (, p<0.05) increase in GTP-bound Cdc42 and a 12 fold (, p<0.05) increase in GTP-bound Rac1, compared to unstimulated cells. The data represent the mean values ±SEM from 4 independent experiments. 61
71 62
72 Figure 3.4 Slit2 inhibits Akt and Erk but not p38 MAPKs. Human monocytic THP-1 cells were incubated with PBS vehicle or containing Slit2 (4.6 μg/ml) for 10 minutes and stimulated with SDF-1α (100ng/mL) for 5 minutes and lysates collected. A, Western blots showing the activation of Erk, Akt and p38 MAPKs over time with SDF-1α stimulation. Blotting with phospho antibodies was performed first, then the membranes were stripped and reprobed with antibodies for the total protein to use for loading controls. B&C&D, Graphs depict the band intensities normalized to the loading controls. Stimulation with SDF-1α induced robust activation of the Akt and Erk MAPK pathways (Fig. 3.4A&B&C). However, no activation of the p38 MAPK was observed (Fig 3.4A&D). Stimulation with SDF-1α for 5 minutes led to a 2.9 fold increase (, p<0.05) in phosphorylated Akt and a 3.2 fold (, p<0.05) increase in phosphorylated Erk, compared with unstimulated cells (Fig. 3.4A&B&C). Incubation with Slit2 alone had no effect on baseline activation of Akt, Erk and p38 MAPKs (Fig. 3.4). However, incubation with Slit2 significantly reduced SDF-1α-mediated activation of Akt and Erk MAPKs at 5 minutes, although no effect was observed for p38 MARK (Fig. 3.4). Monocytic THP-1 cells incubated with Slit2 had only a 1.7 fold (, p<0.05) increase in phosphorylated Akt and a 1.9 fold (, p<0.05) increase in phosphorylated Erk. Therefore, incubation with Slit2 decreased the activation of Akt and Erk MAPKs (Fig. 3.4A&B&C) at 5 minutes after SDF-1α stimulation. However, incubation with Slit2 had no effect on the p38 MAPK pathway (Fig. 3.4A&D). The data represent the mean values ±SEM from 8 independent experiments. 63
73 64
74 Figure 3.5 Slit2 inhibits adhesion of monocytic THP-1 cells to human umbilical vein endothelial cells. HUVEC monolayers were stimulated with TNF-α for 4 hours and human monocytic THP-1 cells were incubated with PBS vehicle alone or containing Slit2 (4.6µg/mL) for 10 minutes. Slit-treatment alone had no effect on cell adhesion, while adhesion to activated endothelial monolayers increased from baseline to almost 400% (, p<0.005). When THP-1 cells were incubated with Slit2, adhesion was abolished to near baseline levels (, P<0.05). 65
75 66
76 Figure 3.6 Slit2 inhibits adhesion of monocytic THP-1 cells to human arterial endothelial cells. HAEC monolayers were activated with TNF-α for 4 hours and human monocytic THP-1 cells were incubated with PBS vehicle alone or containing Slit2 (4.6µg/mL). Slit2 treatment alone had no effect of cell adhesion, whereas TNF-α stimulation of the endothelial monolayers increased baseline adhesion by over 200% (, p<0.01). When monocytic THP-1 cells were incubated with Slit2, adhesion was abolished to near baseline levels (, p<0.005). 67
77 68
78 Figure 3.7 Slit2 inhibits monocyte recruitment in vivo. Monocyte recruitment was determined in vivo using a model of murine peritonitis. PBS alone or containing Slit2 (1.8µg/mouse) was administered intraperitoneally one hour prior to sodium periodate induced peritonitis. Sodium periodate (1mg/mouse) was injected intraperitoneally and peritoneal lavages were performed after 24 hours. Sodium periodate alone induced vigorous peritonitis, reflected in the robust monocyte recruitment (, p<0.001). Intraperitoneal pretreatment with 1.8µg of Slit2 significantly inhibited monocyte recruitment (, p<0.001) (n=5). 69
79 70
80 Figure 3.8 Slit2 dose-dependently inhibits monocyte recruitment in vivo. Monocyte recruitment was determine in vivo using a model of murine peritonitis. Slit2 was administered intravenously via tail-vein injections at 4.6 µg/mouse, 460 ng/mouse and 46 ng/mouse prior to sodium periodate induced peritonitis. Sodium periodate (1 mg/mouse) was injected intraperitoneally and peritoneal lavages were performed after 24 hours. Sodium periodate alone induced vigorous peritonitis, reflected in the robust monocyte recruitment (, p<0.05). However, pre-treatment with 4600 ng or 460 ng of Slit2 significantly diminished monocyte recruitment (, p<0.05). 71
81 72
82 Figure 3.9 Slit2 inhibits monocyte/macrophage recruitment in vivo: time-course. Monocyte/macrophage recruitment was studied in an in vivo model of murine peritonitis. Slit2 (1.8 µg/mouse) was administered intravenously via tail-vein injections at 1, 4 and 10 days prior to sodium periodate induced peritonitis. Sodium periodate (1 mg/mouse) was injected intraperitoneally and peritoneal lavages were performed after 24 hours. Sodium periodate alone induced vigorous peritonitis, reflected in the high number of recruited monocytes/macrophages (, p<0.001). Intravenous pre-treatment with Slit2 at 1 day and 4 days prior to induction of peritonitis significantly diminished monocyte/macrophage recruitment (,p<0.001) at 1 day and (,p<0.01) at 4 days. 73
83 74
84 Figure 3.10 Slit2 does not affect RAW macrophage phagocytosis. Murine RAW macrophages were centrifuged together with IgGopsonized latex beads to initiate phagocytosis for 10 minutes. External beads were then fluorescently labeled and images of at least 10 random fields were captured. A, Representative images of control and Slit2 treated RAW macrophages performing IgG-mediated phagocytosis. B, Slit2 treatment had no effect on the phagocytic index (number of ingested particles/ number of cells) of RAW macrophages. 75
85 CHAPTER 4 DISCUSSION & CONCLUSIONS The aim of this project was to determine the effect of Slit2 on monocyte chemotaxis, adhesion, and phagocytosis in vitro. Furthermore, we wanted to determine whether Slit2 can inhibit monocyte recruitment in vivo. We have shown that primary human monocytes and human monocytic THP-1 cells express the Slit2 receptor, Robo-1, and that Slit2 blocks monocyte migration in response to a SDF-1α gradient. This finding is consistent with observations in the literature on the effect of Slit2 on Robo-1 expressing cells. In fact, Slit2 has been shown to inhibit the chemotaxis of a number of human hematopoetic cell types, including T-cells and DCs (Guan et al., 2003; Kanellis et al., 2004; Prasad et al., 2007). In addition, we have previously demonstrated that Slit2 inhibited the chemotaxis of circulating human neutrophils (Tole et al., 2009). Although Slit2 has been demonstrated to inhibit the chemotaxis of diverse cell types, the mechanisms underlying its actions are not understood completely. Chemotaxis is a complex process in which the cell polarizes to form a wide lamella at the leading edge and a tail-like uropod in the trailing edge. Forward propulsion is dependent on rapid turnover and polymerization of actin filaments. We have previously shown that treatment of circulating human neutrophils with Slit2 reduced chemokine-mediated generation of free barbed ends required for actin polymerization at the leading edge (Tole et al., 2009; Glogauer et al., 2000). This observation is consistent with previous findings in neuronal cells linking Slit2/Robo-1 signaling with proteins that are involved in the mobilization of the actin cytoskeleton, 76
86 such as Ena and srgap (Bashaw et al., 2000; Wong et al., 2001). srgap activates Rho GTPases Rac and Cdc42, which are important for actin turnover in migrating cells. Cdc42 is responsible for maintaining directionality, by driving the formation of filopodia to sample extracellular cues, while Rac drives actin assembly in lamellipodia required for forward propulsion during chemotaxis (Srinivasan et al., 2003). We have found that Slit2 inhibits chemokine-mediated activation of Cdc42 in human monocytic THP-1 cells. Our finding is consistent with studies in neuronal cells and in our previous studies in primary human neutrophils, where Slit2 was shown to inhibit activation of Cdc42, preventing these cells from undergoing directional migration up a chemotactic gradient (Wong et al., 2001; Tole et al., 2009). Furthermore, we have shown that Slit2 decreased chemokine-mediated activation of Rac1 in monocytic THP-1 cells. Our observation is consistent with Slit2-mediated suppression of Rac activation in studies of human vascular smooth muscle cells and human T lymphocytes (Liu et al., 2006; Kanellis et al., 2004;Prasad et al., 2007). Indeed, we have previously shown that Slit2 suppressed the activation of Rac in circulating human neutrophils (Tole et al., 2009). GPCR-mediated signaling in monocytes, as in other leukocytes, leads to rapid phospholipid metabolism and the activation of MAPK pathways, including Akt, Erk, and p38. Disruption of these pathways, using chemical inhibitors, has been shown to inhibit chemotaxis (Heit et al., 2001). We have shown that Slit2 inhibited chemokine-induced activation of Akt MAPK. This suggests that Slit2 may affect phospholipid metabolism, specifically the generation of PIP 3 at the plasma membrane, which is required for the recruitment and activation of Akt MAPK. Our 77
87 observation is consistent with a previous report which found that in human breast cancer cells, Slit2 inhibited chemokine-mediated activation of PI3K, and subsequent activation of Akt MAPK (Prasad et al., 2004). Moreover, Slit2 was also shown to inhibit SDF-1α-induced activation of Akt in Jurkat T cells (Prasad et al., 2007). However, this trend in Slit2-mediated inhibition of SDF-1α-induced Akt MAPK activation is inconsistent with our study in circulating human neutrophils, where Slit2 was shown to have no effect on the fmlp-induced activation of Akt, Erk and p38 MAPKs (Tole et al., 2009). We have also found that Slit2 inhibited SDF-1α-induced activation of Erk MAPK. This finding is consistent with a study in human breast cancer cells, where Slit2 inhibited SDF-1α-induced activation of Erk MAPK (Prasad et al., 2004). However, our observation is inconsistent with studies in granulocytic cells (Wu et al., 2001) and in our previous report in circulating human neutrophils, where no Slit2-mediated inhibition in Erk MAPK activation was observed (Tole et al., 2009). Finally, we have shown that Slit2 did not affect SDF-1α-induced p38 MAPK activation, consistent with findings on Jurkat T lymphocytes and in our previous report in circulating human neutrophils (Prasad et al, 2007; Tole et al., 2009). These differential effects of Slit2 on MAPK activity may be attributable to differences in cell types used or in the chemoattractants used for stimulation. For example, our previous study on circulating human neutrophils utilized the bacterial product fmlp to stimulate the MAPK pathways, while this study utilized the chemokine SDF-1α. This is further supported by the consistency of our findings with those in human breast cancer cells, where SDF-1α was also utilized for MAPK activation. 78
88 In order to be recruited from circulation and extravasate, monocytes must undergo a series of coordinated interactions with vascular endothelial cells. During acute inflammation, or in chronic inflammatory conditions such as atherosclerosis, the local cytokine microenvironment activates vascular endothelial cells to express increased levels of adhesion molecules. These activated endothelial cells are able to efficiently capture circulating leukocytes, including monocytes, facilitating their arrest and diapedesis across the vessel wall. To determine if Slit2 affects monocyte adhesion, we performed adhesion assays using endothelial monolayers activated with the proinflammatory cytokine TNF-α. We have shown that Slit2 inhibited adhesion of monocytic THP-1 cells to activated endothelial monolayers, specifically HUVECs and HAECs. Our observations are consistent with a study of human breast cancer cells which found that Slit2 inhibited CXCL12-mediated adhesion to ligands such as fibronectin and collagen (Prasad et al., 2004). Furthermore, Slit2 has previously been shown to block Jurkat T cell adhesion to activated HUVEC monolayers. Consistent with this line of evidence is a recent report showing that the Slit/Robo pathway functions to antagonize E-cadherin-mediated cell adhesion of Drosophila cardioblasts during development (Santiago-Martnez et al., 2008). Since Rho GTPases are also involved in the actin mobilization required for cell adhesion, it is likely that the signaling events downstream of Slit2/Robo-1 influence the ability of the cell to form adhesive contacts. Further studies should explore the effect of Slit2 on the adhesion of monocytes to Ig superfamily ligands, ICAM-1 and VCAM-1, which are important in physiological cell adhesion. In addition, GPCR-mediated activation of monocytes during rolling induces outside-in and inside-out signaling 79
89 pathways which lead to changes in integrin avidity on monocytes. Thus, future studies should also explore the effect of Slit2 on transient upregulation of monocyte integrin affinity induced by chemokines and other chemoattractants, using the methods of Chan et al. (2003). Signals elicited by chemokines and other chemoattractants activate leukocyte β 1 - and β 2 -integrins, resulting in tight adhesion to the vascular endothelium and induction of cytoskeleton-driven leukocyte migration. Many chemokines can bind to transmembrane heparan sulphate proteoglycans on the luminal surface of the endothelium in order to be presented to leukocytes (Spillmann et al., 1998; Halden et al., 2004). When chemokines bind to these proteoglycans, the chemokine receptor binding site remains exposed (Proudfoot et al., 2000). This allows the chemokine to interact with its chemokine receptor expressed on leukocytes in order to elicit a rapid integrin activation signal. Complex signaling networks regulate the affinity of integrins, via spatial separation and unfolding of the two integrin chains, and the avidity of integrins, by increasing lateral mobility and clustering (Kim et al., 2003). RAP1 and RAP2 are small GTPases of the RAS family that play an important role in chemokine-mediated inside-out signaling, which activates the integrins LFA-1 and VLA-4 (Katagiri et al., 2000; McLeod et al., 2004). RAP1 is expressed by most haematopoietic cells, cycling between an inactive GDP-bound form and an active GTP-bound form. Like other GTPases, its activity is regulated by the GEF exchange factor directly activated by cyclic AMP (EPAC) and the GAPs signal-induced proliferation associated antigen 1 (SPA1) and RAPGAPII (Bos, L., 2003). Recent 80
90 reports have shown that CCL21 and SDF-1α rapidly activate RAP1 to its active, GTP-bound, form (Bos, L., 2003, Shimonaka et al., 2003). The chemokine-mediated activation of RAP1 induces LFA-1- and VLA-4-dependent adhesion and migration (McLeod et al., 2004; Shimonaka et al., 2003). This is controlled by leukocyte adhesion to ICAM-1 and VCAM-1 expressed by activated endothelial cells. The importance of RAP1 is highlighted in studies of leukocytes transfected with constitutively active RAP1, which are able to adhere and migrate independently of a chemokine signal (Shimonaka et al., 2003; Tohyama et al., 2003). Furthermore, transfection with RAP1 GAPs, SPA1 or RAPGAPII, blocks integrin-mediated cell adhesion and migration. RAP1 modulates integrin affinity by binding to RAPL. This complex then activates integrins by binding to a conserved GLY-PHE-PHE-LYS- ARG motif on the integrin α-chain. Interestingly, overexpression of RAPL has been shown to activate integrin-mediated cell adhesion, while overexpression of a RAPL mutant that is incapable of binding RAP1 inhibits adhesion (Katagiri et al., 2003). Therefore, the chemokine-induced activation of RAP1 is important for the signaling networks that activate leukocyte integrins. In order to gain a better mechanistic understanding of the Slit2-mediated inhibition of monocyte adhesion to activated endothelium, future studies should explore the effect of Slit2/Robo-1 signaling on the activation of RAP1 and on the activity and localization of its GEFs and GAPs. In addition to RAP1 and RAPL, there are other signaling networks that contribute to the rapid chemokine-induced integrin activation. Talin is a cytoskeletal protein consisting of a globular head and a rod-like domain. The head domain can bind to the ASN-PRO-XAA-TYR/PHE motif on the β-chain of integrins. This binding 81
91 activates the integrin by keeping the cytoplasmic domains of the α- and β-chains separated, allowing the unfolding of the extracellular domain and exposure of the ligand-binding pocket (Kim et al., 2003; Tadokoro et al., 2003). Although the mechanism by which talin regulates chemokine-mediated integrin activation is incompletely understood, it is believed to be required for integrin activation downstream of several signaling pathways (Tadokoro et al., 2003). Furthermore, the protease calpain can cleave talin between the head and rod domains. Once cleaved, the head domain has a six fold higher affinity for the integrin β-chain than does the intact molecule, allowing for more efficient integrin activation (Calderwood, A., 2004). This cleavage may allow for further regulation of talin-mediated integrin activation. In addition, the binding of phosphoinositol phosphate kinase type Iγ to talin regulates talin-integrin interactions by enhancing the binding affinity of talin for the integrin β-chain (Di Paulo et al., 2002; Ling et al., 2002; Martel et al., 2001). Finally, phosphorylation of a tyrosine residue on the integrin talin-binding motif by SRC-family kinases prevents talin-mediated integrin activation (Datta et al., 2002; Sakai et al., 2001). Thus, talin may play a regulatory role in the chemokine-induced integrin-mediated leukocyte adhesion. Future experiments to elucidate the mechanism by which Slit2/Robo-1 signaling inhibits monocyte adhesion to activated endothelial cells should include an investigation of talin. Specifically, the effect of Slit2/Robo-1 signaling on talin phosphorylation and cleavage should be addressed. Another important regulatory signaling pathway leading to integrin activation involves RhoA. RhoA is a member of the RAS superfamily of GTPases, and is involves in integrin activation, membrane ruffling, stress fiber formation and cell 82
92 migration (Alblas et al., 2001; Ridley et al., 1994; Laudanna et al., 2002). Several studies have demonstrated that blocking RhoA or its downstream targets increases monocyte adhesion to ICAM-1 ligand (Worthylake et al., 2003; Smith et al., 2003). The effect of RhoA is complex, as it is activated by both chemokines and ligandbound integrins on adherent cells. Several RhoA-interacting adaptors are required for β 2 -integrin-dependent adhesion to ICAM-1, and these provide another mechanism for the tuning of integrin-mediated adhesion induced by chemokines. Thus, RhoA regulates integrin-mediated adhesion via the activation of integrins, the regulation of lateral integrin mobility in the plasma membrane and the effect on the actin cytoskeleton. Future experiments to shed light on the mechanism by which Slit2/Robo-1 signaling inhibits monocyte adhesion to activated endothelial cells should include an investigation of RhoA activity. In addition, the effect of Slit2/Robo- 1 signaling on RhoA GAPs and GEFs should be addressed. Another signaling network regulating integrin-mediated leukocyte adhesion via the modulation of cell polarity involves atypical protein kinase C-δ (PKC-δ) (Wang et al., 2003; Etienne-Manneville et al., 2003). Chemokines induce the kinase activity of PKC-δ, via its interaction with PI3K and RhoA, resulting in its targeting to the plasma membrane where it leads to increased integrin mobility (Giagulli et al., 2004). This is required for the clustering of activated integrins, leading to high integrin avidity, allowing leukocytes to rapidly induce firm adhesion during rolling. Furthermore, it has been shown that further activation of adherent cells by chemokines results in PKC-δ localization to the lamellipodium (Wang et al., 2003). Therefore, PKC-δ plays a role in leukocyte polarity and in the reinforcement of 83
93 integrin-mediated cell adhesion. Future experiments into the mechanism by which Slit2/Robo-1 signaling inhibits monocyte adhesion should include an investigation of PKC-δ. Specifically, the effect of Slit2/Robo-1 signaling on PKC-δ activation and membrane targetting should be addressed. Since we found that Slit2 can inhibit monocyte chemotaxis and adhesion in vitro, we next sought to investigate if it will work in vivo. To test the in vivo recruitment of monocytes to inflammatory foci, we used a sodium periodate-induced model of experimental murine peritonitis. When Slit2 is administered intraperitoneally, it gets absorbed systemically (Kanellis et al., 2004). We have shown that when Slit2 was administered intraperitoneally an hour prior to inducing peritonitis, monocyte recruitment to the peritoneal cavity was significantly abolished. This supports our previous observation which demonstrated that Slit2 administered intraperitoneally was able to effectively diminish neutrophil recruitment in the same murine model of experimental peritonitis induced by sodium periodate (Tole et al., 2009). Thus, Slit2 may inhibit the recruitment of different subsets of leukocytes in vivo. Moreover, we have previously shown that Slit2 can inhibit the recruitment of neutrophils in vivo to diverse chemoattractants administered intraperitoneally, including MIP-1 and C5a (Tole et al., 2009). To determine the dose of Slit2 required to exert an optimal biological effect, we administered Slit2 intravenously at decreasing doses (46 ng µg). We have found that Slit2 was able to exert a significant effect on monocyte recruitment in vivo even when administered at 460 ng/mouse, although the effect wore off with further dilution. Furthermore, due to the potent effect of Slit2 on leukocyte recruitment in vivo, we sought to determine the 84
94 timing of Slit2 administration required to induce an optimal biological effect. We found that Slit2 significantly abolished monocyte recruitment when administered 1 day or 4 days prior to induction of peritonitis, although the effect of Slit2 was slightly diminished at 4 days, compared to the effect at 1 day. Literature on the exogenous application of Slit2 is scarce. These findings suggests that Slit2 can persist in the circulation in order to exert a biological effect for up to 4 days. Due to its extensive glycosylation and hence 'stickiness', Slit2 may associated with GAGs on the endothelial lumen. This is consistent with findings that human full-length Slit2 and N-terminal Slit2 are tightly associated with the cell membrane (Brose et al., 1999). This property allows Slit2 to be concentrated on the endothelial lumen, where it can signal and exert an effect on leukocytes as they interact with the vessel wall. In addition, the extensive glycosylation on Slit2 may confer protection from degradation by proteases, which may further increase its biological half life. Phagocytosis is a vital monocyte/macrophage function required for innate and adaptive immunity. Once monocytes are recruited to inflammatory foci, they must engulf pathogens for immune clearance or antigen presentation. Since Rho GTPases are involved in the actin mobilization required to form pseudopods and engulf particles, we speculated that Slit2 might have an effect on monocyte phagocytosis. To determine the effect of Slit2 on monocyte phagocytosis, we performed phagocytosis assays with Ig-opsonized latex beads. We have found that Slit2 had no effect on monocyte phagocytosis. Although this finding was surprising, since Rho GTPases Rac and Cdc42 are involved in phagocytosis, this finding is consistent with a recent report demonstrating that Slit2 treatment had no effect on 85
95 neutrophil phagocytosis of Ig-opsonized latex beads (unpublished observations). This observations may be due to the fact that Slit2 only acts on polarized or polarizing cells. Thus, further studies are needed to elucidate the precise effect of Slit2 on leukocyte phagocytosis. In our current study, we have shown that Slit2 can inhibit the chemotaxis of monocytic THP-1 cells to gradients of SDF-1α. It is likely that this inhibition is mediated by the ability of Slit2 to inhibit Rho GTPases Cdc42 and Rac1, and therefore, the polymerization and turnover of actin, and mobilization of the actin cytoskeleton. Consistent with this hypothesis is our observation that Slit2 inhibited the adhesion of monocytic THP-1 cells to activated endothelial cells, since adhesion is also dependent on Rho GTPase-mediated actin mobilization. However, it is surprising that Slit2 had no effect on monocyte phagocytosis, as this process is also dependent on Rho GTPases-mediated dynamic actin regulation. Although further studies into the mechanism of Slit2 action is necessary, our data strongly support the use of Slit2 as a novel anti-inflammatory agent. Currently, many antiinflammatory agents act via general suppression of immune activation and function, and thus have serious side effects. Although Slit2 may also have immunosuppressive effects, targeted local delivery of Slit2 could be utilized to prevent localized inflammatory cell recruitment and the associated tissue damage, while preserving the overall function of the immune system in the host. Our data demonstrate that Slit2 can selectively inhibit monocyte recruitment and adhesion to the vessel wall, while preserving vital immune functions such as phagocytosis. 86
96 REFERENCES Aderem, A., & Underhill, D. M. (1999). Mechanisms of phagocytosis in macrophages. Annual Review of Immunology, 17, Akagawa, K. S., Takasuka, N., Nozaki, Y., Komuro, I., Azuma, M., Ueda, M., et al. (1996). Generation of CD1 䗩 dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood, 88(10), Alblas, J., Ulfman, L., Hordijk, P., & Koenderman, L. (2001). Activation of rhoa and ROCK are essential for detachment of migrating leukocytes. Molecular Biology of the Cell, 12(7), Allen, W. E., Jones, G. E., Pollard, J. W., & Ridley, A. J. (1997). Rho, rac and Cdc42 regulate actin organization and cell adhesion in macrophages. Journal of Cell Science, 110(6), Allen, W. E., Zicha, D., Ridley, A. J., & Jones, G. E. (1998). A role for Cdc42 in macrophage chemotaxis. The Journal of Cell Biology, 141(5), Alon, R., Hammer, D. A., & Springer, T. A. (1995). Lifetime of the P-selectincarbohydrate bond and its response to tensile force in hydrodynamic flow. Nature, 374(6522),
97 Amatruda, T. T., Gerard, N. P., Gerard, C., & Simon, M. I. (1993). Specific interactions of chemoattractant factor receptors with G-proteins. The Journal of Biological Chemistry, 268(14), An, G., Wang, H., Tang, R., Yago, T., McDaniel, J. M., McGee, S., et al. (2008). P- selectin glycoprotein ligand-1 is highly expressed on ly-6chi monocytes and a major determinant for ly-6chi monocyte recruitment to sites of atherosclerosis in mice. Circulation, 117(25), Aspenström, P. (1999). Effectors for the rho GTPases. Current Opinion in Cell Biology, 11(1), 95. Astrof, N., Salas, A., Shimaoka, M., Chen, J., & Springer, T. (2006). Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry, 45(50), Auffray, C., Fogg, D., Garfa, M., Elain, G., Join-Lambert, O., Kayal, S., et al. (2007). Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science, 317(5838), Barleon, B., Sozzani, S., Zhou, D., Weich, H. A., Mantovani, A., & Marm, D. (1996). Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood, 87(8),
98 Bashaw, G. J., Kidd, T., Murray, D., Pawson, T., & Goodman, C. S. (2000). Repulsive axon guidance: Abelson and enabled play opposing roles downstream of the roundabout receptor. Cell, 101(7), Battye, R., Stevens, A., & Jacobs, J. R. (1999). Axon repulsion from the midline of the drosophila CNS requires slit function. Development, 126(11), Battye, R., Stevens, A., Perry, R. L., & Jacobs, J. R. (2001). Repellent signaling by slit requires the leucine-rich repeats. The Journal of Neuroscience, 21(12), Benard, V., Bohl, B. P., & Bokoch, G. M. (1999). Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. The Journal of Biological Chemistry, 274(19), Bertoli-Avella, A., Conte, M., Punzo, F., de Graaf, B., Lama, G., La Manna, A., et al. (2008). ROBO2 gene variants are associated with familial vesicoureteral reflux. Journal of the American Society of Nephrology, 19(4), Bokoch, G. M. (2005). Regulation of innate immunity by rho GTPases. Trends in Cell Biology, 15(3), 163. Bos, J. L. (2003). Epac: A new camp target and new avenues in camp research. Nature Reviews.Molecular Cell Biology, 4(9),
99 Botelho, R. J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J. D., et al. (2000). Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. The Journal of Cell Biology, 151(7), Boulay, F., Naik, N., Giannini, E., Tardif, M., & Brouchon, L. (1997). Phagocyte chemoattractant receptors. Annals of the New York Academy of Sciences, 832, Brakebusch, C., & Fssler, R. (2003). The integrin-actin connection, an eternal love affair. EMBO Journal, 22(10), Bretscher, M. S. (1996). Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell, 87(4), 601. Brose, K., Bland, K. S., Wang, K. H., Arnott, D., Henzel, W., Goodman, C. S., et al. (1999). Slit proteins bind robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell, 96(6), Brown, E. J. (1991). Complement receptors and phagocytosis. Current Opinion in Immunology, 3(1), 76. Bustelo, X. (2002). Regulation of vav proteins by intramolecular events. Frontiers in Bioscience, 7, d24-d30. Calderwood, D. A. (2004). Integrin activation. Journal of Cell Science, 117(5),
100 Calderwood, D. A., Shattil, S. J., & Ginsberg, M. H. (2000). Integrins and actin filaments: Reciprocal regulation of cell adhesion and signaling. The Journal of Biological Chemistry, 275(30), Campbell, J. J., Hedrick, J., Zlotnik, A., Siani, M. A., Thompson, D. A., & Butcher, E. C. (1998). Chemokines and the arrest of lymphocytes rolling under flow conditions. Science, 279(5349), 381. Campbell, J. J., Qin, S., Bacon, K. B., Mackay, C. R., & Butcher, E. C. (1996). Biology of chemokine and classical chemoattractant receptors: Differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. The Journal of Cell Biology, 134(1), 255. Carman, C., & Springer, T. (2003). Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Current Opinion in Cell Biology, 15(5), Caron, E., & Hall, A. (1998). Identification of two distinct mechanisms of phagocytosis controlled by different rho GTPases. Science, 282(5394), Carson, M., Weber, A., & Zigmond, S. H. (1986). An actin-nucleating activity in polymorphonuclear leukocytes is modulated by chemotactic peptides. The Journal of Cell Biology, 103(6),
101 Casey, P. J., Solski, P. A., Der, C. J., & Buss, J. E. (1989). p21ras is modified by a farnesyl isoprenoid. Proceedings of the National Academy of Sciences of the United States of America, 86(21), Castelli, W. P. (1984). Epidemiology of coronary heart disease: The framingham study. The American Journal of Medicine, 76(2), 4. Chan, J., Hyduk, S., & Cybulsky, M. (2003). Detecting rapid and transient upregulation of leukocyte integrin affinity induced by chemokines and chemoattractants. Journal of Immunological Methods, 273(1-2), Chapuis, F., Rosenzwajg, M., Yagello, M., Ekman, M., Biberfeld, P., & Gluckman, J. C. (1997). Differentiation of human dendritic cells from monocytes in vitro. European Journal of Immunology, 27(2), Chen, J. H., Wen, L., Dupuis, S., Wu, J. Y., & Rao, Y. (2001). The N-terminal leucine-rich regions in slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. The Journal of Neuroscience, 21(5), Chen, J., Wu, W., Li, H., Fagaly, T., Zhou, L., Wu, J., et al. (2000). Embryonic expression and extracellular secretion of xenopus slit. Neuroscience, 96(1), 231. Clapham, D. E., & Neer, E. J. (1993). New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature, 365(6445),
102 Combadire, C., Potteaux, S., Gao, J., Esposito, B., Casanova, S., Lee, E., et al. (2003). Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation, 107(7), Constantin, G., Majeed, M., Giagulli, C., Piccio, L., Kim, J. Y., Butcher, E. C., et al. (2000). Chemokines trigger immediate beta2 integrin affinity and mobility changes: Differential regulation and roles in lymphocyte arrest under flow. Immunity, 13(6), 759. Correia, I., Chu, D., Chou, Y., Goldman, R., & Matsudaira, P. (1999). Integrating the actin and vimentin cytoskeletons: Adhesion-dependent formation of fimbrinvimentin complexes in macrophages. The Journal of Cell Biology, 146(4), 831. Critchley, D. R., Holt, M. R., Barry, S. T., Priddle, H., Hemmings, L., & Norman, J. (1999). Integrin-mediated cell adhesion: The cytoskeletal connection. Biochemical Society Symposia, 65, Crittenden, J., Bergmeier, W., Zhang, Y., Piffath, C., Liang, Y., Wagner, D., et al. (2004). CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nature Medicine, 10(9), Cybulsky, M. I., & Gimbrone, M. A. (1991). Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science, 251(4995),
103 Dallol, A., Da Silva, N., Viacava, P., Minna, J., Bieche, I., Maher, E., et al. (2002). SLIT2, a human homologue of the drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Research, 62(20), Dallol, A., Krex, D., Hesson, L., Eng, C., Maher, E., & Latif, F. (2003). Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene, 22(29), Dallol, A., Morton, D., Maher, E., & Latif, F. (2003). SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Research, 63(5), Datta, A., Huber, F., & Boettiger, D. (2002). Phosphorylation of beta3 integrin controls ligand binding strength. The Journal of Biological Chemistry, 277(6), DeBiasio, R. L., Wang, L. L., Fisher, G. W., & Taylor, D. L. (1988). The dynamic distribution of fluorescent analogues of actin and myosin in protrusions at the leading edge of migrating swiss 3T3 fibroblasts. The Journal of Cell Biology, 107(6), Defacque, H., Egeberg, M., Habermann, A., Diakonova, M., Roy, C., Mangeat, P., et al. (2000). Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO Journal, 19(2),
104 DeFife, K. M., Jenney, C. R., Colton, E., & Anderson, J. M. (1999). Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. The Journal of Histochemistry and Cytochemistry, 47(1), DeMali, K., Wennerberg, K., & Burridge, K. (2003). Integrin signaling to the actin cytoskeleton. Current Opinion in Cell Biology, 15(5), Di Paolo, G., Pellegrini, L., Letinic, K., Cestra, G., Zoncu, R., Voronov, S., et al. (2002). Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature, 420(6911), Dickinson, R., Dallol, A., Bieche, I., Krex, D., Morton, D., Maher, E., et al. (2004). Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers. The British Journal of Cancer, 91(12), Dunne, J., Ballantyne, C., Beaudet, A., & Ley, K. (2002). Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and mac-1. Blood, 99(1), 336. Eriksson, E. E., Xie, X., Werr, J., Thoren, P., & Lindbom, L. (2001). Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. The Journal of Experimental Medicine, 194(2), 205. Etienne-Manneville, S., & Hall, A. (2003). Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature, 421(6924),
105 Fenteany, G., & Glogauer, M. (2004). Cytoskeletal remodeling in leukocyte function. Current Opinion in Hematology, 11(1), Finger, E. B., Puri, K. D., Alon, R., Lawrence, M. B., von Andrian, U. H., & Springer, T. A. (1996). Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature, 379(6562), 266. Friedl, P. (2004). Prespecification and plasticity: Shifting mechanisms of cell migration. Current Opinion in Cell Biology, 16(1), 14. Friedl, P., & Wolf, K. (2009). Proteolytic interstitial cell migration: A five-step process. Cancer and Metastasis Reviews, 28(1-2), Fujiyama, A., & Tamanoi, F. (1990). RAS2 protein of saccharomyces cerevisiae undergoes removal of methionine at N terminus and removal of three amino acids at C terminus. The Journal of Biological Chemistry, 265(6), Gamblin, S. J., & Smerdon, S. J. (1998). GTPase-activating proteins and their complexes. Current Opinion in Structural Biology, 8(2), Geissmann, F., Jung, S., & Littman, D. (2003). Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity, 19(1), Gertler, F. B., Bennett, R. L., Clark, M. J., & Hoffmann, F. M. (1989). Drosophila abl tyrosine kinase in embryonic CNS axons: A role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell, 58(1),
106 Gether, U. (2000). Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocrine Reviews, 21(1), 90. Giagulli, C., Scarpini, E., Ottoboni, L., Narumiya, S., Butcher, E., Constantin, G., et al. (2004). RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: Critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity, 20(1), Glogauer, M., Hartwig, J., & Stossel, T. (2000). Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. The Journal of Cell Biology, 150(4), Glogauer, M., Marchal, C., Zhu, F., Worku, A., Clausen, B., Foerster, I., et al. (2003). Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. The Journal of Immunology, 170(11), Gordon, S. (1986). Biology of the macrophage. Journal of Cell Science.Supplement, 4, Gordon, S. (1998). The role of the macrophage in immune regulation. Research in Immunology, 149(7-8), 685. Grage-Griebenow, E., Flad, H. D., & Ernst, M. (2001). Heterogeneity of human peripheral blood monocyte subsets. Journal of Leukocyte Biology, 69(1), Greenberg, S., & Grinstein, S. (2002). Phagocytosis and innate immunity. Current Opinion in Immunology, 14(1),
107 Guan, H., Zu, G., Xie, Y., Tang, H., Johnson, M., Xu, X., et al. (2003). Neuronal repellent Slit2 inhibits dendritic cell migration and the development of immune responses. The Journal of Immunology, 171(12), Gutierrez, L., Magee, A. I., Marshall, C. J., & Hancock, J. F. (1989). Posttranslational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO Journal, 8(4), Gyetko, M. R., Todd, R. F., Wilkinson, C. C., & Sitrin, R. G. (1994). The urokinase receptor is required for human monocyte chemotaxis in vitro. Journal of Clinical Investigation, 93(4), Hackam, D., Rotstein, O., Schreiber, A., Zhang, W., & Grinstein, S. (1997). Rho is required for the initiation of calcium signaling and phagocytosis by fcgamma receptors in macrophages. The Journal of Experimental Medicine, 186(6), 955. Halden, Y., Rek, A., Atzenhofer, W., Szilak, L., Wabnig, A., & Kungl, A. (2004). Interleukin-8 binds to syndecan-2 on human endothelial cells. The Biochemical Journal, 377(Pt 2), 533. Hall, A. L., Warren, V., Dharmawardhane, S., & Condeelis, J. (1989). Identification of actin nucleation activity and polymerization inhibitor in ameboid cells: Their regulation by chemotactic stimulation. The Journal of Cell Biology, 109(5),
108 Hao, J. C., Yu, T. W., Fujisawa, K., Culotti, J. G., Gengyo-Ando, K., Mitani, S., et al. (2001). C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron, 32(1), Heit, B., Tavener, S., Raharjo, E., & Kubes, P. (2002). An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. Science Signaling, 159(1), 91. Hirsch, E., Katanaev, V. L., Garlanda, C., Azzolino, O., Pirola, L., Silengo, L., et al. (2000). Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science, 287(5455), Holmes, G. P., Negus, K., Burridge, L., Raman, S., Algar, E., Yamada, T., et al. (1998). Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mechanisms of Development, 79(1-2), Holmes, G., & Niswander, L. (2001). Expression of slit-2 and slit-3 during chick development. Developmental Dynamics, 222(2), Howard, T. H., & Meyer, W. H. (1984). Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. The Journal of Cell Biology, 98(4),
109 Hume, D., Ross, I., Himes, S. R., Sasmono, R. T., Wells, C., & Ravasi, T. (2002). The mononuclear phagocyte system revisited. Journal of Leukocyte Biology, 72(4), Huo, Y., Schober, A., Forlow, S. B., Smith, D., Hyman, M., Jung, S., et al. (2003). Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nature Medicine, 9(1), 61. Imboden, J. B., & Stobo, J. D. (1985). Transmembrane signalling by the T cell antigen receptor. perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. The Journal of Experimental Medicine, 161(3), Imhof, B., & Aurrand-Lions, M. (2004). Adhesion mechanisms regulating the migration of monocytes. Nature Reviews.Immunology, 4(6), 432. Itoh, A., Miyabayashi, T., Ohno, M., & Sakano, S. (1998). Cloning and expressions of three mammalian homologues of drosophila slit suggest possible roles for slit in the formation and maintenance of the nervous system. Molecular Brain Research, 62(2), Jiang, N., & Pisetsky, D. (2005). The effect of inflammation on the generation of plasma DNA from dead and dying cells in the peritoneum. Journal of Leukocyte Biology, 77(3),
110 Jin, T., Zhang, N., Long, Y., Parent, C. A., & Devreotes, P. N. (2000). Localization of the G protein betagamma complex in living cells during chemotaxis. Science, 287(5455), Johnson, Z., Proudfoot, A. E., & Handel, T. M. (2005). Interaction of chemokines and glycosaminoglycans: A new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Reviews, 16(6), 625. Jones, G. E., Allen, W. E., & Ridley, A. J. (1998). The rho GTPases in macrophage motility and chemotaxis. Cell Adhesion Communication, 6(2-3), Jones, S. L., Wang, J., Turck, C. W., & Brown, E. J. (1998). A role for the actinbundling protein L-plastin in the regulation of leukocyte integrin function. Proceedings of the National Academy of Sciences of the United States of America, 95(16), Jung, U., Norman, K. E., Scharffetter-Kochanek, K., Beaudet, A. L., & Ley, K. (1998). Transit time of leukocytes rolling through venules controls cytokineinduced inflammatory cell recruitment in vivo. Journal of Clinical Investigation, 102(8), Kanellis, J., Garcia, G., Ping, L., Parra, G., Wilson, C., Rao, Y., et al. (2004). Modulation of inflammation by slit protein in vivo in experimental crescentic glomerulonephritis. The American Journal of Pathology, 165(1),
111 Kansas, G. S. (1996). Selectins and their ligands: Current concepts and controversies. Blood, 88(9), Katagiri, K., Hattori, M., Minato, N., Irie, S. k., Takatsu, K., & Kinashi, T. (2000). Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-oh kinase. Molecular and Cellular Biology, 20(6), Katagiri, K., Maeda, A., Shimonaka, M., & Kinashi, T. (2003). RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nature Immunology, 4(8), Keep, N. H., Barnes, M., Barsukov, I., Badii, R., Lian, L. Y., Segal, A. W., et al. (1997). A modulator of rho family G proteins, rhogdi, binds these G proteins via an immunoglobulin-like domain and a flexible N-terminal arm. Structure, 5(5), Kennedy, J., Kelner, G. S., Kleyensteuber, S., Schall, T. J., Weiss, M. C., Yssel, H., et al. (1995). Molecular cloning and functional characterization of human lymphotactin. The Journal of Immunology, 155(1), 203. Kennedy, T. E., Serafini, T., de la Torre, J. R., & Tessier-Lavigne, M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell, 78(3),
112 Kidd, T., Brose, K., Mitchell, K. J., Fetter, R. D., Tessier-Lavigne, M., Goodman, C. S., et al. (1998). Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell, 92(2), Kim, M., Carman, C., & Springer, T. (2003). Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science, 301(5640), Kintscher, U., Goetze, S., Wakino, S., Kim, S., Nagpal, S., Chandraratna, R. A., et al. (2000). Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. European Journal of Pharmacology, 401(3), Kintscher, U., Goetze, S., Wakino, S., Kim, S., Nagpal, S., Chandraratna, R. A., et al. (2000). Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. European Journal of Pharmacology, 401(3), Kjoller, L., & Hall, A. (1999). Signaling to rho GTPases. Experimental Cell Research, 253(1), Kramer, S. G., Kidd, T., Simpson, J. H., & Goodman, C. S. (2001). Switching repulsion to attraction: Changing responses to slit during transition in mesoderm migration. Science, 292(5517),
113 Krugmann, S., Hawkins, P. T., Pryer, N., & Braselmann, S. (1999). Characterizing the interactions between the two subunits of the p101/p110gamma phosphoinositide 3-kinase and their role in the activation of this enzyme by G beta gamma subunits. The Journal of Biological Chemistry, 274(24), Kunkel, E. J., & Ley, K. (1996). Distinct phenotype of E-selectin-deficient mice. E- selectin is required for slow leukocyte rolling in vivo. Circulation Research, 79(6), Lanier, L. M., Gates, M. A., Witke, W., Menzies, A. S., Wehman, A. M., Macklis, J. D., et al. (1999). Mena is required for neurulation and commissure formation. Neuron, 22(2), Laudanna, C., Kim, J., Constantin, G., & Butcher, E. (2002). Rapid leukocyte integrin activation by chemokines. Immunological Reviews, 186, 37. Laurent, V., Loisel, T. P., Harbeck, B., Wehman, A., Grbe, L., Jockusch, B. M., et al. (1999). Role of proteins of the Ena/VASP family in actin-based motility of listeria monocytogenes. The Journal of Cell Biology, 144(6), Lawrence, M. B., Kansas, G. S., Kunkel, E. J., & Ley, K. (1997). Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E). The Journal of Cell Biology, 136(3),
114 Lesnik, P., Haskell, C., & Charo, I. (2003). Decreased atherosclerosis in CX 3 CR1 / mice reveals a role for fractalkine in atherogenesis. Journal of Clinical Investigation, 111(3), 333. Ley, K., Laudanna, C., Cybulsky, M., & Nourshargh, S. (2007). Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nature Reviews.Immunology, 7(9), Li, Z., Jiang, H., Xie, W., Zhang, W., Smrcka, A., & Wu, D. (2000). Roles of PLC-2 and-3 and PI3K in chemoattractant-mediated signal transduction. Science's STKE, 287(5455), Liang, Y., Annan, R. S., Carr, S. A., Popp, S., Mevissen, M., Margolis, R. K., et al. (1999). Mammalian homologues of the drosophila slit protein are ligands of the heparan sulfate proteoglycan glypican-1 in brain. The Journal of Biological Chemistry, 274(25), Libby, P., & Aikawa, M. (2002). Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nature Medicine, 8(11), Linder, S., & Aepfelbacher, M. (2003). Podosomes: Adhesion hot-spots of invasive cells. Trends in Cell Biology, 13(7), Ling, K., Doughman, R., Firestone, A., Bunce, M., & Anderson, R. (2002). Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature, 420(6911),
115 Liu, D., Hou, J., Hu, X., Wang, X., Xiao, Y., Mou, Y., et al. (2006). Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circulation Research, 98(4), Lotero, L. A., Jordn, J. A., Olmos, G., Alvarez, F. J., Tejedor, M. C., & Diez, J. C. (2001). Differential in vitro and in vivo behavior of mouse ascorbate/fe3 and diamide oxidized erythrocytes. Bioscience Reports, 21(6), Luster, A. D. (1998). Chemokines--chemotactic cytokines that mediate inflammation. New England Journal of Medicine, the, 338(7), 436. Machesky, L. M., & Hall, A. (1997). Role of actin polymerization and adhesion to extracellular matrix in rac- and rho-induced cytoskeletal reorganization. The Journal of Cell Biology, 138(4), Marillat, V., Cases, O., Nguyen-Ba-Charvet, K. T., Tessier-Lavigne, M., Sotelo, C., & Chdotal, A. (2002). Spatiotemporal expression patterns of slit and robo genes in the rat brain. Journal of Comparative Neurology, 442(2), Martel, V., Racaud-Sultan, C., Dupe, S., Marie, C., Paulhe, F., Galmiche, A., et al. (2001). Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. The Journal of Biological Chemistry, 276(24),
116 Martenson, C., Stone, K., Reedy, M., & Sheetz, M. (1993). Fast axonal transport is required for growth cone advance. Nature, 366(6450), Mattila, P., & Lappalainen, P. (2008). Filopodia: Molecular architecture and cellular functions. Nature Reviews.Molecular Cell Biology, 9(6), McEver, R. P., & Cummings, R. D. (1997). Perspectives series: Cell adhesion in vascular biology. role of PSGL-1 binding to selectins in leukocyte recruitment. Journal of Clinical Investigation, 100(3), 485. McLeod, S., Shum, A., Lee, R., Takei, F., & Gold, M. (2004). The rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. The Journal of Biological Chemistry, 279(13), Mitra, S., Hanson, D., & Schlaepfer, D. (2005). Focal adhesion kinase: In command and control of cell motility. Nature Reviews.Molecular Cell Biology, 6(1), Moore, K. J., Andersson, L. P., Ingalls, R. R., Monks, B. G., Li, R., Arnaout, M. A., et al. (2000). Divergent response to LPS and bacteria in CD14-deficient murine macrophages. The Journal of Immunology, 165(8), Muller, W. A. (2001). New mechanisms and pathways for monocyte recruitment. The Journal of Experimental Medicine, 194(9),
117 Muller, W. A. (2003). Leukocyte endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology, 24(6), Murga, C., Laguinge, L., Wetzker, R., Cuadrado, A., & Gutkind, J. S. (1998). Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-oh kinasegamma. The Journal of Biological Chemistry, 273(30), Murray, C. J. L., & Lopez, D. (1997). Global mortality, disability, and the contribution of risk factors: Global burden of disease study. The Lancet, 349, Nassar, N., Hoffman, G. R., Manor, D., Clardy, J. C., & Cerione, R. A. (1998). Structures of Cdc42 bound to the active and catalytically compromised forms of Cdc42GAP. Nature Structural Biology, 5(12), Neptune, E. R., & Bourne, H. R. (1997). Receptors induce chemotaxis by releasing the betagamma subunit of gi, not by activating gq or gs. Proceedings of the National Academy of Sciences of the United States of America, 94(26), Nguyen Ba-Charvet, K. T. N., Brose, K., Ma, L., Wang KH., Marillat, V., Sotelo, C., et al. (2001). Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. The Journal of Neuroscience, 21(12),
118 Nguyen, D. H., Catling, A. D., Webb, D. J., Sankovic, M., Walker, L. A., Somlyo, A. V., et al. (1999). Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. The Journal of Cell Biology, 146(1), Nobes, C. D., & Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81(1), Olofsson, B. (1999). Rho guanine dissociation inhibitors:: Pivotal molecules in cellular signalling. Cellular Signalling, 11(8), 545. Pasternak, R., Criqui, M., Benjamin, E., Fowkes, F. G. R., Isselbacher, E., McCullough, P., et al. (2004). Atherosclerotic vascular disease conference: Writing group I: Epidemiology. Circulation, 109(21), Piper, M., Georgas, K., Yamada, T., & Little, M. (2000). Expression of the vertebrate slit gene family and their putative receptors, the robo genes, in the developing murine kidney. Mechanisms of Development, 94(1-2), Prasad, A., Fernandis, A., Rao, Y., & Ganju, R. (2004). Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. The Journal of Biological Chemistry, 279(10),
119 Prasad, A., Qamri, Z., Wu, J., & Ganju, R. (2007). Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. Journal of Leukocyte Biology, 82(3), Proudfoot, A. E., Power, C. A., & Wells, T. N. (2000). The strategy of blocking the chemokine system to combat disease. Immunological Reviews, 177, Rajavashisth, T. B., Andalibi, A., Territo, M. C., Berliner, J. A., Navab, M., Fogelman, A. M., et al. (1990). Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature, 344(6263), Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M., & Muller, W. A. (1998). Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science, 282(5388), Ravetch, J. V., & Kinet, J. P. (1991). Fc receptors. Annual Review of Immunology, 9, Ray, L. B. (2004). STKE: Slit and robo in kidney formation. Science, 304(5675), 1215c. Ridley, A. J. (2001). Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Letters, 498(2-3),
120 Ridley, A. J., & Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70(3), Ridley, A. J., & Hall, A. (1994). Signal transduction pathways regulating rhomediated stress fibre formation: Requirement for a tyrosine kinase. EMBO Journal, 13(11), Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D., & Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell, 70(3), Robinson, L. A., Nataraj, C., Thomas, D. W., Howell, D. N., Griffiths, R., Bautch, V., et al. (2000). A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. The Journal of Immunology, 165(11), Ronca, F., Andersen, J. S., Paech, V., & Margolis, R. U. (2001). Characterization of slit protein interactions with glypican-1. The Journal of Biological Chemistry, 276(31), Rossi, D., & Zlotnik, A. (2000). The biology of chemokines and their receptors. Annual Review of Immunology, 18, Rossman, K., Der, C., & Sondek, J. (2005). GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Reviews.Molecular Cell Biology, 6(2),
121 Rothberg, J. M., & Artavanis-Tsakonas, S. (1992). Modularity of the slit protein. characterization of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. Journal of Molecular Biology, 227(2), Rothberg, J. M., Hartley, D. A., Walther, Z., & Artavanis-Tsakonas, S. (1988). Slit: An EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell, 55(6), Rothberg, J. M., Jacobs, J. R., Goodman, C. S., & Artavanis-Tsakonas, S. (1990). Slit: An extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Development, 4(12A), Sakai, T., Jove, R., Fssler, R., & Mosher, D. F. (2001). Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proceedings of the National Academy of Sciences of the United States of America, 98(7), Salas, A., Shimaoka, M., Kogan, A., Harwood, C., von Andrian, U., & Springer, T. (2004). Rolling adhesion through an extended conformation of integrin alphalbeta2 and relation to alpha I and beta I-like domain interaction. Immunity, 20(4), 393. Sampath, R., Gallagher, P. J., & Pavalko, F. M. (1998). Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. activation-dependent 112
122 regulation of associations with talin and alpha-actinin. The Journal of Biological Chemistry, 273(50), Santiago-Martnez, E., Soplop, N., Patel, R., & Kramer, S. (2008). Repulsion by slit and roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during drosophila heart tube lumen formation. The Journal of Cell Biology, 182(2), Sasaki, T., Irie-Sasaki, J., Jones, R. G., Oliveira-dos-Santos, A. J., Stanford, W. L., Bolon, B., et al. (2000). Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science, 287(5455), Sechi, A. S., & Wehland, J. (2000). The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. Journal of Cell Science, 113 Pt 21, Servant, G., Weiner, O. D., Herzmark, P., Balla, T., Sedat, J. W., & Bourne, H. R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science, 287(5455), Servet-Delprat, C., Arnaud, S., Jurdic, P., Nataf, S., Grasset, M., Soulas, C., et al. (2002). Flt3 macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunology, 3, Shamri, R., Grabovsky, V., Gauguet, J., Feigelson, S., Manevich, E., Kolanus, W., et al. (2005). Lymphocyte arrest requires instantaneous induction of an 113
123 extended LFA-1 conformation mediated by endothelium-bound chemokines. Nature Immunology, 6(5), 497. Sheetz, M. P., Felsenfeld, D., Galbraith, C. G., & Choquet, D. (1999). Cell migration as a five-step cycle. Biochemical Society Symposia, 65, Shimonaka, M., Katagiri, K., Nakayama, T., Fujita, N., Tsuruo, T., Yoshie, O., et al. (2003). Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. The Journal of Cell Biology, 161(2), Shin, H. S., Snyderman, R., Friedman, E., Mellors, A., & Mayer, M. M. (1968). Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science, 162(851), 361. Singer, S. J., & Kupfer, A. (1986). The directed migration of eukaryotic cells. Annual Review of Cell Biology, 2, Smith, A., Bracke, M., Leitinger, B., Porter, J., & Hogg, N. (2003). LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. Journal of Cell Science, 116(15), Smith, J. D., Trogan, E., Ginsberg, M., Grigaux, C., Tian, J., & Miyata, M. (1995). Decreased atherosclerosis in mice deficient in both macrophage colonystimulating factor (op) and apolipoprotein E. Proceedings of the National Academy of Sciences of the United States of America, 92(18),
124 Spillmann, D., Witt, D., & Lindahl, U. (1998). Defining the interleukin-8-binding domain of heparan sulfate. The Journal of Biological Chemistry, 273(25), Srinivasan, S., Wang, F., Glavas, S., Ott, A., Hofmann, F., Aktories, K., et al. (2003). Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. The Journal of Cell Biology, 160(3), Strzelecka, A., Kwiatkowska, K., & Sobota, A. (1997). Tyrosine phosphorylation and fcgamma receptor-mediated phagocytosis. FEBS Letters, 400(1), Sunderktter, C., Nikolic, T., Dillon, M., Van Rooijen, N., Stehling, M., Drevets, D., et al. (2004). Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. The Journal of Immunology, 172(7), Symons, M. H., & Mitchison, T. J. (1991). Control of actin polymerization in live and permeabilized fibroblasts. The Journal of Cell Biology, 114(3), Tacke, F., Alvarez, D., Kaplan, T., Jakubzick, C., Spanbroek, R., Llodra, J., et al. (2007). Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. Journal of Clinical Investigation, 117(1), Tadokoro, S., Shattil, S., Eto, K., Tai, V., Liddington, R., de Pereda, J., et al. (2003). Talin binding to integrin beta tails: A final common step in integrin activation. Science, 302(5642),
125 Tcherkezian, J., & Lamarche-Vane, N. (2007). Current knowledge of the large RhoGAP family of proteins. Biology of the Cell, 99(2), Tcherkezian, J., & Lamarche-Vane, N. (2007). Current knowledge of the large RhoGAP family of proteins. Biology of the Cell, 99(2), Tessier-Lavigne, M., & Goodman, C. S. (1996). The molecular biology of axon guidance. Science, 274(5290), Tohyama, Y., Katagiri, K., Pardi, R., Lu, C., Springer, T., & Kinashi, T. (2003). The critical cytoplasmic regions of the alphal/beta2 integrin in Rap1-induced adhesion and migration. Molecular Biology of the Cell, 14(6), Tole, S., Mukovozov, I., Huang, Y., Magalhaes, M. A. O., Yan, M., Crow, M., et al. (2009). The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. Journal of Leukocyte Biology, 86(6), Ulloa-Aguirre, A., Stanislaus, D., Janovick, J. A., & Conn, P. M. (1999). Structureactivity relationships of G protein-coupled receptors. Archives of Medical Research, 30(6), Van Furth, R., & Cohn, Z. (1968). The origin and kinetics of mononuclear phagocytes. The Journal of Experimental Medicine, 128(3), 415. van Furth, R., Nibbering, P. H., van Dissel, J. T., & Diesselhoff-den Dulk, M. M. (1985). The characterization, origin, and kinetics of skin macrophages during inflammation. The Journal of Investigative Dermatology, 85(5),
126 Vanhaesebroeck, B., & Waterfield, M. D. (1999). Signaling by distinct classes of phosphoinositide 3-kinases. Experimental Cell Research, 253(1), Vargesson, N., Luria, V., Messina, I., Erskine, L., & Laufer, E. (2001). Expression patterns of slit and robo family members during vertebrate limb development. Mechanisms of Development, 106(1-2), Vetter, I. R., & Wittinghofer, A. (2001). The guanine nucleotide-binding switch in three dimensions. Science, 294(5545), Vielkind, S., Gallagher-Gambarelli, M., Gomez, M., Hinton, H., & Cantrell, D. (2005). Integrin regulation by RhoA in thymocytes. The Journal of Immunology, 175(1), Viriyakosol, S., Fierer, J., Brown, G., & Kirkland, T. (2005). Innate immunity to the pathogenic fungus coccidioides posadasii is dependent on toll-like receptor 2 and dectin-1. Infection and Immunity, 73(3), von Hundelshausen, P., Weber, K. S., Huo, Y., Proudfoot, A. E., Nelson, P. J., Ley, K., et al. (2001). RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation, 103(13), Wang, H., Zhang, Y., Ozdamar, B., Ogunjimi, A., Alexandrova, E., Thomsen, G., et al. (2003). Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science, 302(5651),
127 Wang, K. H., Brose, K., Arnott, D., Kidd, T., Goodman, C. S., Henzel, W., et al. (1999). Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell, 96(6), Wang, Y. L. (1985). Exchange of actin subunits at the leading edge of living fibroblasts: Possible role of treadmilling. The Journal of Cell Biology, 101(2), 597. Wiktor-Jedrzejczak, W., & Gordon, S. (1996). Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1- deficient op/op mouse. Physiological Reviews, 76(4), Wills, Z., Marr, L., Zinn, K., Goodman, C. S., & Van Vactor, D. (1999). Profilin and the abl tyrosine kinase are required for motor axon outgrowth in the drosophila embryo. Neuron, 22(2), Wong, K., Ren, X. R., Huang, Y. Z., Xie, Y., Liu, G., Saito, H., et al. (2001). Signal transduction in neuronal migration: Roles of GTPase activating proteins and the small GTPase Cdc42 in the slit-robo pathway. Cell, 107(2), Worthylake, R. A., Lemoine, S., Watson, J. M., & Burridge, K. (2001). RhoA is required for monocyte tail retraction during transendothelial migration. The Journal of Cell Biology, 154(1),
128 Worthylake, R., & Burridge, K. (2003). RhoA and ROCK promote migration by limiting membrane protrusions. The Journal of Biological Chemistry, 278(15), Wu, J. Y., Feng, L., Park, H. T., Havlioglu, N., Wen, L., Tang, H., et al. (2001). The neuronal repellent slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature, 410(6831), Yamada, K. M., & Miyamoto, S. (1995). Integrin transmembrane signaling and cytoskeletal control. Current Opinion in Cell Biology, 7, 681. Yan, M., Di Ciano-Oliveira, C., Grinstein, S., & Trimble, W. (2007). Coronin function is required for chemotaxis and phagocytosis in human neutrophils. The Journal of Immunology, 178(9), Yen, H., Zhang, Y., Penfold, S., & Rollins, B. J. (1997). MCP-1-mediated chemotaxis requires activation of non-overlapping signal transduction pathways. Journal of Leukocyte Biology, 61(4), Yuan, W., Zhou, L., Chen, J. H., Wu, J. Y., Rao, Y., & Ornitz, D. M. (1999). The mouse SLIT family: Secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Developmental Biology, 212(2),
129 Zallen, J. A., Yi, B. A., & Bargmann, C. I. (1998). The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell, 92(2), Ziegler-Heitbrock, H. W. L. (1996). Heterogeneity of human blood monocytes: The CD14 CD16 subpopulation. Immunology Today, 17(9), 424. Zigmond, S. H. (1974). Mechanisms of sensing chemical gradients by polymorphonuclear leukocytes. Nature, 249,
130 APPENDIX 1 The Axonal Repellent, Slit2, Inhibits Directional Migration of Circulating Neutrophils A1.1 Abstract In inflammatory diseases circulating neutrophils are recruited to sites of injury. Attractant signals are provided by many different chemotactic molecules, such that blockade of one may not effectively prevent neutrophil recruitment. The Slit family of secreted proteins, and their transmembrane receptor, Roundabout (Robo), repel axonal migration during central nervous system development. Emerging evidence shows that by inhibiting the activation of Rho-family GTPases, Slit2/Robo also inhibit migration of other cell types towards a variety of chemotactic factors, in vitro and in vivo. The role of Slit2 in inflammation, however, has been largely unexplored. We isolated primary neutrophils from human peripheral blood and mouse bone marrow, and detected Robo-1 expression. Using video-microscopic live cell tracking, we found that Slit2 selectively impaired directional migration, but not random movement, of neutrophils towards formyl-methionyl-leucyl-phenylalanine (fmlp). Slit2 also inhibited neutrophil migration towards other chemoattractants, namely C5a and interleukin (IL)-8. Slit2 inhibited neutrophil chemotaxis by preventing chemoattractant-induced actin barbed end formation and cell polarization. Slit2 mediated these effects by suppressing inducible activation of Cdc42 and Rac2, but did not impair activation of other major kinase pathways involved in neutrophil migration. We further tested the effects of Slit2 in vivo using mouse models of peritoneal inflammation induced by sodium periodate, C5a, and 121
131 macrophage inflammatory protein-2 (MIP-2). In all instances, Slit2 effectively reduced neutrophil recruitment (p < 0.01). Collectively, these data demonstrate that Slit2 potently inhibits chemotaxis, but not random motion, of circulating neutrophils, and point to Slit2 as a potential new therapeutic for preventing localized inflammation. 122
132 A1.2 Introduction Neutrophils are a critical component of the innate immune system and provide the first line of defense against bacterial and fungal pathogens. During an inflammatory response, neutrophils are recruited to sites of inflammation in a series of coordinated interactions with vascular endothelial cells. Traffic signals are provided by diverse chemoattractant molecules, including chemokines such as IL-8, and bacterial products such as formylated peptides. These chemoattractants recruit circulating neutrophils to sites of inflammation, and activate recruited neutrophils to adhere firmly to the endothelium. While their potent anti-microbial arsenal makes neutrophils efficient at fighting microorganisms, it is also capable of causing injury to the surrounding tissue. Indeed, neutrophils inflict significant tissue damage in inflammatory conditions including ischemia-reperfusion injury of solid organs, acute respiratory distress syndrome, and rheumatoid arthritis [1-4]. Once recruited to sites of injury, infiltrating neutrophils release reactive oxygen species and degradative enzymes, fuelling local tissue destruction. Anti-inflammatory drugs such as aspirin and glucocorticoids are widely used, and yet, have shown modest success in reducing neutrophil-mediated injury. These drugs attenuate activation of transcription factors such as NF-κB, thereby reducing expression of cytokines [5]. An alternative approach to prevent neutrophil-mediated tissue damage would be blockade of chemotactic pathways that recruit neutrophils to sites of inflammation. Indeed, some chemokine receptor antagonists or blocking antibodies have shown success in animal models and are undergoing clinical trials [6]. However, given the number of chemoattractant signals that recruit neutrophils, it 123
133 is unlikely that targeting a single chemokine/chemokine receptor pathway would achieve widespread clinical success. Thus, localized general blockade of inflammatory chemoattractants could represent a clinically useful strategy to reduce neutrophil-mediated tissue damage. Clues as to how generalized blockade of neutrophil chemoattractant signals might be realized are provided in the neurodevelopmental literature. The Slit family of secreted proteins, together with their transmembrane receptor Roundabout (Robo), repel migration of axons and neurons during development of the central nervous system. Slit is expressed along the midline of the developing central nervous system and its interaction with Robo prevents axons from repeatedly and randomly crossing the midline [7, 8]. While the importance of Slit/Robo interactions in development has been demonstrated, the intracellular signaling pathways that lead to Slit-mediated inhibition of migration remain unclear. Data from Drosophila suggests that Abelson kinase (Abl) and Enabled (Ena) proteins associate with the intracellular domains of Robo-1 and may be involved in the repulsive response to Slit2 [9]. Addition of extracellular Slit2 to neuronal cells results in the recruitment of soluble Slit Robo guanosine triphosphatase (GTPase) activating protein 1 (srgap1) to the cytoplasmic tail of Robo-1 [10]. In addition to neuronal cells, Slit2 and Robo-1 also inhibit migration of other cell types, including vascular smooth muscle cells, breast cancer cells, and brain tumor cells [11-13]. Several studies have demonstrated that Slit2 inhibits migration of haematopoietic cells, including murine macrophages, cultured cells of granulocytic lineage, dendritic cells, and primary human T-lymphocytes, towards 124
134 chemoattractant signals [14-17]. Importantly, Slit2 not only inhibits cell migration towards one type of chemoattractant signal, but towards many diverse signals, including platelet-derived growth factor (PDGF) and the chemokines, CXCL12 and CCL2 [12,13, 16, 17]. In vivo, Slit2 inhibits neoangiogenesis by impairing pathologic migration of endothelial cells to vascular endothelial growth factor [18]. Existing data point to a role for Slit2 as a generalized anti-migration signal, which universally inhibits cell migration. However, the potential use of Slit2 to prevent inflammation has been largely unexplored. In particular, there is a paucity of data addressing the effects of Slit2 on migration of human leukocytes, especially neutrophils. Moreover, the mechanisms by which Slit2 mediates its anti-migratory effects are incompletely understood. The aim of this study was to assess, in real-time, the effect of Slit2 on recruitment of primary neutrophils. We observed that primary human and murine neutrophils express the Slit2 receptor, Robo-1, and that Slit2 inhibits directional migration, but not random migration, of neutrophils towards a chemotactic stimulus. Our studies demonstrate that Slit2 mediates these effects by preventing chemoattractant-induced cell polarization and generation of actin free barbed ends, a pre-requisite for directional migration of neutrophils. Our data further suggest that Slit2 prevents chemoattractant-induced free barbed end formation by suppressing inducible activation of the small GTPases, Cdc42 and Rac2, but does not affect activation of other major kinase pathways involved in neutrophil migration. To investigate whether Slit2 prevents neutrophil chemotaxis in vivo, we used mouse models of peritoneal inflammation, and observed a significant reduction in the 125
135 number of neutrophils recruited to the peritoneum in response to diverse inflammatory stimuli, in the presence of Slit2 [19]. Taken together, these data indicate a novel role for the axonal repellent, Slit2, as an anti-inflammatory agent which specifically prevents chemotactic trafficking of circulating neutrophils. 126
136 A1.3 Materials and Methods Reagents and antibodies. Unless otherwise stated, reagents were purchased from Sigma-Aldrich (St. Louis, MO). Polymorphprep neutrophil separation medium was purchased from Axis- Shield, Norway. The following primary antibodies were used: anti-robo-1 (Abcam, Cambridge, MA, and Santa Cruz Biotechnology, Santa Cruz, CA), anti-myc 9E10 (Covance, QC, Canada), anti-human Cdc42 (Cell Signaling, Danvers, MA), anti-human Rac2 (Upstate Biotechnology, Lake Placid, NY), antimouse CD3 (BD Biosciences, Mississauga, Ontario, Canada), anti-b220 (BD Biosciences), anti-nk1.1 (BD Biosciences), anti-f4/80 (Serotec, Raleigh, NC), anti- Erk, anti-phospho-erk, anti-p38 MAPK, anti-phospho-p38 MAPK, anti-akt, and antiphospho-akt. Rhodamine-conjugated phalloidin was from Invitrogen Canada (Burlington, Ontario, Canada). The following secondary antibodies were used: Cy3- conjugated anti-rabbit IgG, Cy2- conjugated anti-human IgG, phycoerythrin (PE)- conjugated anti-rat IgG and anti-mouse IgG (Jackson Immunoresearch Laboratories, Bar Harbor, ME), and horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG (Jackson Immunoresearch Laboratories). C5a was purchased from Biovision, Inc. (Mountain View, CA), interleukin-8 (IL-8) from Invitrogen, and macrophage inflammatory protein-2 (MIP-2) from R&D Systems (Minneapolis, MN). Isolation of primary human and murine neutrophils. Human blood was obtained from healthy volunteers and neutrophils were isolated using two methods. For experiments testing the activation of Rac and Cdc42, neutrophils were isolated by 127
137 dextran sedimentation as described with slight modifications [20]. Briefly, two volumes of blood were mixed with one volume of 6% dextran T-500 in 0.9% NaCl and set at room temperature until clear separation of layers was seen (about 30 min). The leukocyte-rich upper layer was collected and centrifuged at 260g at room temperature for 5 min. The cell pellet was re-suspended in a volume of 0.9% NaCl equal to the starting volume of blood, laid onto 10 ml of Ficoll-hypaque solution, and centrifuged at 460g for 30 min. Red blood cells were lysed by adding 20 ml of ice-cold 0.2% NaCl for 30 s, resuspended in 20 ml of ice-cold 1.6% NaCl and centrifuged at 250g at 4 C for 5 min. Neutrophils were re-suspended in ice-cold PBS with 0.5% BSA. Cells were kept on ice for subsequent experimental use. The purity of neutrophils isolated in this manner was assessed by modified Wright- Giemsa stain (Hema-Tek Stain Pack; Bayer, Elkhart, IN) using an automated stainer (Hema-Tek 2000; Bayer), and was consistently greater than 95%. For all other experiments, the Polymorphprep gradient separation procedure was performed according to the manufacturer s recommendations. Purified neutrophils were suspended in PBS without calcium and kept at room temperature. Prior to use, the neutrophils were re-suspended in HBSS with 1mM CaCl2 and 1mM MgCl2. Experiments were performed within 1-2 h of isolation of neutrophils. Cell purity was consistently >85-90%. Cell viability was >98% by Trypan blue exclusion. For RTPCR experiments, a QIAmp RNA Blood Mini Kit (QIAGEN, Ontario, Canada) was used to isolate total RNA from human leukocytes isolated from whole blood, according to the manufacturer s specifications. Primary murine neutrophils were isolated as previously described [19, 21]. Briefly, adult CD1 mice were killed by CO2 128
138 inhalation. Femurs and tibias were removed and bone marrow was extracted. Bone marrow cells were layered onto discontinuous Percoll gradients of 81%/65%/55%. Mature neutrophils were isolated from the 81%/65% interface. More than 85% of cells were neutrophils as assessed by Wright-Giemsa staining. Slit2 expression and purification. Stable human embryonic kidney (HEK) 293 cell line expressing full-length human Slit2 with a c-myc-tag at its carboxyl terminus was a kind gift from Drs. Rolando del Maestro (McGill University, Montreal, Canada) and Yi Rao (Washington University, St. Louis, MO) and grown as described [22]. Recombinant Slit2 was purified from the conditioned medium using two methods. Conditioned medium was concentrated and Slit2 purified by affinity chromatography using anti-c-myc Ab 9E10 (Covance, QC, Canada) and Size Primary Immunoprecipitation kit (Thermo Scientific, Rockford, IL) following the manufacturer's instructions. Slit2 was also obtained by Superdex-200 size exclusion chromatography. Briefly, conditioned medium was concentrated using Centricon Plus-20 (Millipore, Billerica, MA) and loaded onto the column [16]. The column was then washed with PBS and fractions containing Slit2 were pooled, concentrated, aliquoted and stored in 80 C before use. The presence of Slit2 was verifed using silver staining and immunoblotting with anti-myc Ab (Supplementary Figure 1A & B). The above protocol was repeated with conditioned medium from control HEK293 cells to obtain control medium. This preparation of Slit2 was titrated and used at a concentration of 0.6 µg/ml. In parallel assays, control medium was used in lieu of Slit2. 129
139 Large scale preparation of Slit2 was performed by transfection of HEK293-EBNA1 cells. Briefly, human Slit2 cdna (MGC: ; aa of NP_004778) was amplified using forward (CTATCTAGACCTCAGGCGTGCCCGGCGCAGTGC) and reverse (CTAGGATCCGGACACACACCTCGTACAGC) primers containing XbaI and BamHI restriction sites. The amplified cdna was cloned into the ptt28 vector digested with NheI and BamHI. The ptt28 vector is a derivative of the ptt5 vector [23, 24] and contains a synthetic and codon-optimized signal peptide (MGELLLLLLLGLRLQLSLG) and a C-terminal (His)8G tag separated by NheI and BamHI restriction sites. HEK293-EBNA1 cells (clone 6E) were transfected with 1 µg/ml cdna as previously described [25]. Culture medium was harvested 120 h post-transfection, clarified by centrifugation (4,000 x g for 15 min), and filtered through a 0.45 µm membrane. Slit2 secreted into the medium was purified by immobilized metal-affinity chromatography using a Fractogel-cobalt column equilibrated in PBS. Following washing steps with 5 column volumes (CV) of Wash1 Buffer (50 mm sodium phosphate ph 7.0 and 300 mm NaCl) followed by 5 CV of Wash2 Buffer (50 mm sodium phosphate ph 7.0, 300 mm NaCl and 25 mm imidazole), bound Slit2 was eluted with Elution Buffer (50 mm sodium phosphate ph 7.0, 300 mm NaCl and 25 mm imidazole). The pooled eluted material was immediately desalted on Econo-Pac 10 columns (Bio-Rad Laboratories, Mississauga, ON) previously equilibrated with PBS according to the manufacturer s specifications. Protein concentration was determined by absorbance at 280 nm using a calculated Slit2 molar extinction coefficient of ( For Western blots, proteins were 130
140 resolved on reducing SDS-PAGE (4 12% Nu-PAGE Bis-Tris gradient gel, Invitrogen) followed by transfer to a 0.2 mm Protran nitrocellulose membrane (Schleicher & Schuell, Keene, NH) in Tris-glycine buffer for 1 h at 300 ma. Purity was verified by Ponceau staining and immunoblotting (Supplementary Figure 1C & D). The membrane was incubated in blocking reagent (Roche Diagnostics, Laval, Canada), and then probed with anti-polyhis-hrp Ab (Sigma-Aldrich) for 1 h (Supplementary Figure 1D). Detection was performed using BM Chemiluminescence Blotting Substrate (Roche Diagnostics) with a Kodak Digital Science Image Station 440cf equipped with Kodak Digital Science 1D image analysis software version 3.0 (Eastman Kodak, New York, NY). We measured endotoxin levels in purified Slit2 stock preparations using ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript Corp., Piscataway, NJ). Endotoxin concentrations ranged from ng/ml, yielding final experimental concentrations of pg/ml which are well below those thought to activate leukocytes [26]. To verify this point, we added similar concentrations of endotoxin in neutrophil Transwell assays, and found that such levels of endotoxin had no effect on neutrophil migration (Supplementary Figure 2). RT-PCR. RNA isolation and RT-PCR were performed using the QIAamp RNA blood mini kit and the QIAGEN one-step RT-PCR kit (QIAGEN, Missisauga, ON) as described [13]. As previously described, the following primers specific for Robo-1 were used: GGCCCCACTCCCCCTGTTCG (forward primer) and TCCTCTTCTGGCGCATCCGTATCC (reverse primer) [13]. Amplified products were 131
141 analyzed by electrophoresis on 2% agarose gels containing ethidium bromide to confirm primer specificity and PCR product size (278 bp). Immunofluorescent labeling. Primary human and mouse neutrophils were allowed to settle onto fibronectin-coated coverslips and to adhere for 3 minutes at room temperature. The cells were fixed with 4% paraformaldehyde for 10 min at 4 C. Neutrophils were stained with rabbit anti-robo-1 Ab (1 µg/ml) for 2 h, washed and then incubated with anti-rabbit-cy3 secondary Ab for 1 h. In some experiments, human or mouse neutrophils were incubated with fmlp (1 µm) for 3 min, following incubation with purified Slit2 (4.5 µg/ml). Cells were fixed, permeabilized, and incubated with rhodamine-conjugated phalloidin (1:500) for 30 min to visualize actin. A Leica DMIRE2 spinning disc confocal microscope (Leica Microsystems, Toronto, Ontario, Canada) equipped with a Hamamatsu back-thinned EM-CCD camera and Volocity software (Improvision Inc., Lexington, MA) was used to capture images. Flow cytometry. Cell surface expression of Robo-1 was verified by incubating human and mouse neutrophils with anti-robo-1 Ab, followed by PE-conjugated secondary Ab. Analysis was performed using a FACScalibur flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Inc., Ashland, OR), as previously described [27, 28]. Immunoblotting. Freshly isolated human or mouse neutrophils were pre-treated with either control medium or Slit2 for 10 min and then activated with fmlp (1 µm). Cells 132
142 were lysed using ice-cold 2x lysis buffer (1 x = 50 mm Tris, ph 7.5, 10% glycerol, 100 mm NaCl, 1% NP- 40, 5 mm MgCl2, 1 mm DTT, 1 mm PMSF, 1/100 protease inhibitor cocktail and 1 mm NaVO3). Samples were run on SDS-PAGE, transferred to 0.2 mm PVDF (Millipore) membrane and probed for Robo-1 or for both phosphorylated and total Akt, Erk and p38 MAP kinase. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Biosciences, UK Ltd, Buckinghamshire, UK) recorded on x-ray film. Prior to performing experiments, a time-course study was performed to determine the optimal point at which to measure phosphorylation of Akt, Erk, and p38 MAPK following exposure to fmlp. Of samples harvested at s, the maximum signal was observed at 30 s, and therefore, a 30 s timepoint was used for all subsequent experiments. Migration assay. Freshly isolated neutrophils (10 6 cells/ 100µl) were incubated with medium alone, Slit2 (0.6 µg/ml), or control medium at 37 C for 10 minutes. Cells were loaded into the top chamber of a 3 µm Transwell insert (Corning Life Sciences, Corning, NY) in a 24-well plate. A coverslip was added to the bottom chamber which was filled with 600 µl of HBSS alone, fmlp (1 µm), C5a (2 µg/ml), or IL-8 (0.1 µg/ml) [29-32]. Into the bottom chamber Slit2, control medium, or HBSS was dispensed. Transwell plates were incubated for 1 h at 37 C. To determine the number of neutrophils which had migrated from the top to the bottom chamber, the filter was removed and neutrophils in the lower chamber were rapidly spun down onto the coverslips, fixed with 4% paraformaldehyde, washed, and labeled with DAPI. A Leica DMIRE microscope was used to take representative 40x and 63x 133
143 high-power images. A Nikon light microscope was used to count at least 10 random fields from each coverslip. The data represent the mean value ± SEM from at least 4 independent experiments for each treatment condition. Micropipette chemotaxis assays. To measure neutrophil migration, round glass coverslips (25-mm diameter; Thomas Scientific, Swedesboro, NJ) coated with fibronectin were mounted in Leiden chambers, overlaid with 0.5 ml of the indicated solution, and placed on the heated stage of a Leica DM IRB microscope (Leica Microsystem, Richmond Hill, Ontario, Canada). Next, a 100 µl aliquot of the neutrophil suspension containing 10 6 cells was added, and cells allowed to settle for 10 min. To induce chemotaxis, a point-source of chemoattractant was delivered using a glass micropipette [33-36]. Micropipettes were prepared from borosilicate capillaries with an outer diameter of 1.0 mm and an inner diameter of 0.78 mm (Sutter, Novato, CA) using a model P-97 micropipette puller (Sutter). The tips of the micropipettes were 1.0 µm in diameter. Precise positioning of the micropipette in the visual field was accomplished using a model 5171 micromanipulator (Eppendorf, Hamburg, Germany). Although the distance between the pipette and the individual cells adherent to the coverslip varied, the initial average distance of the cells under observation (i.e., those in the microscopic field under observation) ranged between 40 and 50 µm. The pipette remained stationary, and diffusion of the chemoattractant generated a standing gradient [33-36]. Images were acquired every 10 s until completion of the experiment. Only cells which started and remained in the field of view over the entire course of videocapture were 134
144 analyzed. Using Volocity TM software (Improvision, Waltham, MA), the distance traveled was measured by tracking the centroid of each cell over time. Four different measures of chemotactic activity were assessed: total migration (distance), net migration (displacement), speed (distance/time) and directionality (displacement/distance). Total migration was defined as the sum of the absolute distances traveled in all the individual time intervals. The net migration was calculated as the difference between the initial distance of the cell with respect to the pipette and that at the end of the experiment. Migration speed was calculated by dividing the total distance travelled over the elapsed time. Directionality was measured by obtaining a ratio of displacement over distance. Actin free barbed end assay. To assess the effects of Slit2 on fmlp-induced actin polymerization, actin nucleation activity was measured as enhancement of pyrene actin fluorescence as previously described [21, 37, 38]. Briefly, human neutrophils (5x10 6 /ml) were permeabilized for 10 s using 0.1 vol of OG buffer (PHEM buffer containing 4% octyl glucoside, 10 µm phallacidin, 42 nm leupeptin, 10 mm benzamidine, and mm aprotinin) or NP-40 (final concentration of 1%). Permeabilization was stopped by diluting the detergent with 3 vol of buffer B (1 mm Tris, 1 mm EGTA, 2 mm MgCl2, 10 mm KCl, 5 mm β- mercaptoethanol, 5 mm ATP; ph 7.4). We then assayed for nuclei by adding pyrene-labeled rabbit skeletal muscle actin to a final concentration of 1 µm, and followed the fluorescence increase with a microplate reader (FLUOstaroptima, BMG 135
145 Labtech, Nepean, Ontario, Canada) at excitation and emission wavelengths of 366 and 386 nm, respectively [21, 37]. Cdc42 and Rac2 activation assays. Prior to performing these experiments, a timecourse study was performed to determine the optimal point at which to measure activation of Rac2 and Cdc42 following exposure to fmlp. Of samples harvested at s, the maximum signal was observed at 30 s, and therefore, a 30 s timepoint was used for subsequent experiments. To assess the effects of Slit2 on fmlpinduced activation of Cdc42 and Rac2, pull-down assays were performed as previously described with slight modifications [39]. The p21-binding domain (PBD; aa ) of PAK1 in pgex-4t3 vector was expressed as a GST fusion protein in BL21 (DE3) E. coli cells. The GST-PBD fusion protein was affinity purified using glutathione sepharose 4B beads (GE Healthcare Bio-Sciences, Piscataway, NJ). Protein bound beads were aliquoted and stored at 80 C for later use. Human neutrophils purified by dextran sedimentation (~1 x 10 7 /sample) were diluted in 0.5 ml 37 C warmed HEPES-HBSS and incubated with purified Slit2 (0.6 µg/ml) at 37 C for 10 min. Cells were stimulated with fmlp (1 µm) for 30 s at 37 C and the reaction was stopped by adding 0.5 ml ice-cold 2x lysis buffer (1x = 50 mm Tris, ph 7.5, 10% glycerol, 100 mm NaCl, 1% NP-40, 5 mm MgCl2, 1 mm DTT, 1mM PMSF, 1/100 protease inhibitor cocktail, and 1 mm NaVO3). Samples were centrifuged at maximal speed in a bench-top centrifuge for 5 min at 4 C and an aliquot of supernatant was used as loading control. The remaining supernatants were added to GST-PBD glutathione beads (20 µg GST-PBD/sample). Samples were rotated at 136
146 4 C for 1 h, washed 3 times with cold wash buffer (50 mm Tris, ph 7.5, 40 mm NaCl, 0.5% NP-40, 30 mm MgCl2, 1 mm DTT, 1 mm PMSF, 0.1 mm NaVO3) and 20 µl of 2x Laemmli loading buffer added. Samples were run on SDS-PAGE and transferred onto a 0.2 mm PVDF (Millipore) membrane. Cdc42 and Rac2 were detected using anti-human Cdc42 and anti-human Rac2 primary Ab and HRPconjugated secondary Ab. Densitometry analysis was performed on the blots using Image J software. To examine the effects of Slit2 on spatial distribution of activated Rac and Cdc42, assays were performed as previously described [33]. Briefly, mouse bone marrow-derived neutrophils were isolated and 1x 10 6 cells were suspended in Nucleofector solution supplemented with 6 µg cdna expression plasmids encoding each of yellow fluorescent protein-tagged p21-binding domain of PAK (PAK-PBD-YFP), which selectively detects activated Rac and Cdc42, together with red fluorescent protein-tagged H-Ras (H-Ras-RFP) to label the plasma membrane [21, 33]. Cells were transfected using a Cell Line V Nucleofector TM kit (Amaxa Biosystems, Amaxa, Inc.) and the Nucleofector TM program Y-001 [21, 33]. Transfected cells were carefully recovered and transferred to Iscove s Modified Dulbecco s Medium pre-warmed to 37 C and allowed to recover for 2 h. Neutrophils were placed on coverslips coated with 1% BSA mounted in an Attafluor cell chamber (Invitrogen) and exposed to a point source of fmlp (1 µm) dispensed through a glass micropipette [21, 33]. In some experiments, neutrophils were pre-incubated with purified Slit2 (4.5 µg/ml) for 10 min. Cells were maintained on a microscope stage heated to 37 C, and digital images were acquired every 3-5 s using a Leica DMIRE2 inverted fluorescence microscope 137
147 equipped with a Hamamatsu backthinned EM-CCD camera and spinning disc confocal scan head [21, 33]. Images were acquired and analyzed using Volocity TM software. Following chemotactic stimulation with fmlp, the ratio of the fluorescence intensity of PAKPBD-YFP: H-Ras-RFP was compared at the leading edge of the cell and the trailing edge of the cell [21, 33]. The normalized mean fluorescence intensity was calculated for 19 cells from three independent experiments [33]. Mouse peritonitis experiments. To determine the effects of Slit2 on neutrophil chemotaxis in vivo, we used a mouse model of sodium periodate-induced peritonitis as previously described [19]. All procedures were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals and were approved by the Hospital for Sick Children Research Institute Animal Care Committee. Adult CD1 mice were injected intraperitoneally with Slit2 (100 ng) or control medium, then 1 h later with 1 ml of 5 mm sodium periodate in PBS [15]. After 3 h, mice were euthanized and the peritoneal exudate collected by lavage with chilled PBS (5 ml/mouse). Infiltrating neutrophils were counted using an electronic cell counter (Becton Dickinson) and neutrophil influx was confirmed by analyzing cytospun slides. To determine whether Slit2 administered systemically prevents neutrophil recruitment, purified Slit2 (1.8 µg in 0.2 ml normal saline) was administered by intravenous tail-vein injection. One hour later, 1 ml PBS containing sodium periodate (5 mm), C5a (10 µg), or MIP-2 (2.5 µg) was injected intraperitoneally [40, 41]. After 3 h, mice were euthanized, peritoneal exudate collected, and infiltrating neutrophils counted as described above. The number of infiltrating 138
148 monocytes/macrophages, T lymphocytes, B lymphocytes, and natural killer cells was determined by labeling cells with Ab directed to F4/80 (10 µg/ml), CD3 (5 µg/ml), B220 (2 µg/ml), or NK1.1 (2 µg/ml), respectively, and performing flow cytometry as previously described [27, 28]. Statistical analysis. Analysis of variance (ANOVA) followed by Bonferonni post-hoc testing was performed using SPSS statistical software to analyze the data from Transwell experiments. In all other cases, the Student s t-test was used. p < 0.05 was considered significant. 139
149 A1.4 Results 1) Primary human and mouse neutrophils express the Slit2 receptor, Robo-1. Robo- 1 mrna and protein expression were detected in both human and mouse neutrophils (Figure 1A & B) [19, 21]. Since Robo-1 expression has previously been demonstrated in primary human lymphocytes, as a positive control, we verified Robo-1 expression in human leukocytes isolated from whole blood (Figure 1A) [16]. We detected two distinct bands for Robo-1 protein in mouse neutrophils, consistent with the splice variants previously reported (Figure 1B) [42]. Using immunofluorescence microscopy and flow cytometry, we detected Robo-1 expression on the surface of human and murine neutrophils (Figure 1C-E). 2) Slit2 inhibits migration of human neutrophils towards fmlp. We studied the effects of Slit2 on Transwell migration of human neutrophils. As expected, basal migration was minimal (Figure 2A & E), but increased in the presence of an fmlp chemotactic gradient (Figure 2B & E; p < 0.001). When no chemotactic gradient was present, purified Slit2 did not stimulate neutrophil transmigration (Figure 2D). However, Slit2 prevented neutrophil migration towards fmlp in the lower chamber, in a dose-dependent fashion (compare Figure 2B & C; Figure 2E, p < for the two highest Slit2 concentrations tested). When fplc-enriched Slit2 from conditioned medium of Slit2-expressing HEK-293T cells was tested, very similar results were obtained (Supplementary Figure 3). In this instance, control medium from mock-transfected cells had no effect on neutrophil migration, verifying that the Slit2 preparation did not contain any factors that could inadvertently affect neutrophil 140
150 migration (Supplementary Figure 3; p < 0.05 vs no fmlp). Together, these data demonstrate that Slit2 inhibits fmlp-induced migration of primary human neutrophils in a dose-dependent fashion. 3) Slit2 inhibits migration of human neutrophils towards other chemoattractants. To determine whether Slit2 inhibits neutrophil migration towards different chemoattractant signals, we performed Transwell assays in which C5a or IL-8 were placed in the lower chamber. Slit2 resulted in a four-fold and six-fold decrease in neutrophil migration towards IL-8 and C5a, respectively (Figure 2F; IL-8: 93 ± 23 cells/field; IL-8 + Slit2: 23 ± 5 cells/field; C5a: 51 ± 10 cells/field; C5a + Slit2: 8 ± 3 cells/field; p < for C5a and IL-8). These data demonstrate that Slit2 is a potent inhibitor of neutrophil migration towards diverse types of chemotactic cue. 4) Slit2 inhibits directional but not random migration of human neutrophils. We next determined whether the observed effects of Slit2 on neutrophil migration were due to inhibition of cell chemotaxis or chemokinesis. Chemokinesis is defined as random movement in response to a stimulant. Unlike chemokinesis, chemotaxis includes a vectoral assessment of migration and is defined as directional migration in response to a chemotactic gradient. Therefore, defects in chemokinesis result in the failure of a cell to move while defects in chemotaxis result in the failure of a cell to move in the right direction. In the absence of Slit2, neutrophils migrated efficiently towards a point-source of fmlp (Supplementary Video 1 and Supplementary Figure 4A-C). In the presence of Slit2, neutrophils moved randomly but failed to move towards the 141
151 micropipette (Supplementary Video 2 and Supplementary Figure 4D-F). These data suggest that Slit2 does not inhibit generalized movement of neutrophils but rather, their directionality. To refine the analysis, we tracked the centroid of each neutrophil over time. Figure 3A depicts the migratory tracks of neutrophils exposed to an fmlp gradient while Figure 3B represents the migratory tracks of neutrophils exposed to fmlp in the presence of Slit2. The displacement, speed, and directionality were determined for each cell. A neutrophil migrating efficiently (directly) up a chemotactic gradient would have very similar displacement and distance measurements. As such, its directionality value would be close to 1. Conversely, a neutrophil moving randomly would have a smaller net displacement despite traveling the same distance, thereby having a directionality value closer to 0. Neutrophils incubated with fmlp alone had an average speed of 6.8 ± 0.6 µm/min, no different from those incubated with fmlp together with Slit2 (6.5 ± 0.6 µm/min; Figure 3C). In the presence of Slit2, the directionality ratio was significantly reduced (Figure 3D; fmlp 0.61 ± 0.04; fmlp + Slit ± 0.04; p < 0.002). Taken together, these data demonstrate that Slit2 does not inhibit the random movement and speed of neutrophil migration but, rather, prevents directional migration towards a chemotactic gradient. 5) Slit2 inhibits chemoattractant-stimulated actin free barbed end formation in human neutrophils. We directly assayed the effects of Slit2 on actin free barbed end formation, an event critical for formation of protruding lamellipodia and neutrophil migration [21, 37, 43-46]. In pyrene-actin polymerization curves generated, the 142
152 slope is proportional to the free barbed end numbers [21, 37]. As expected, unstimulated neutrophils demonstrated low basal levels of free barbed end generation, but fmlp promoted a rapid, six-fold increase (Figure 4A & B; p < 0.04). Similarly, when neutrophils were treated with control medium prior to stimulation with fmlp, we observed a five-fold increase in the rate of actin polymerization as compared to unstimulated cells (Figure 4A & B; p < 0.01). In the presence of Slit2, fmlp-induced actin polymerization was considerably more modest, resulting in less than a three-fold increase compared to unstimulated cells (Figure 4A & B; p < 0.04). Slit2 significantly reduced fmlp-stimulated generation of actin filaments (Figure 4A & B; p < 0.05 vs control medium). Accordingly, Slit2 inhibited accumulation of actin at the leading edge of neutrophils following exposure to fmlp (Figure 4C). Collectively, these data suggest that Slit2 inhibits directional migration of neutrophils by disrupting generation of high-affinity free barbed ends that drive actin filament elongation. This in turn inhibits actin assembly at the leading edge of migrating cells, thus preventing efficient chemotaxis. 6) Slit2 inhibits chemoattractant-induced polarization and activation of Rac2 and Cdc42 in primary human neutrophils. Following chemotactic stimulation, activation of the Rho GTPases, Rac and Cdc42, plays a key role in the re-organization of actin filaments [19, 21, 34]. Since the predominant isoform of Rac in human neutrophils is Rac2, not Rac1, we specifically studied activation of Rac2 [47, 48]. We used GST beads conjugated to the p21-binding domain of p21-activated kinase-1 (PAK-PBD) to detect the activated, GTP-bound species of Rac and Cdc42 [39]. Unstimulated 143
153 neutrophils had low basal levels of activated Rac2 and Cdc42 (Figure 5A & B). Exposure to fmlp increased levels of activated Cdc42 by five-fold, and of activated Rac2 by three-fold (Figure 5A and B; p < 0.01 vs unstimulated for both Cdc42 and Rac2). Slit2 did not affect basal levels of activated Rac2 and Cdc42, but significantly inhibited fmlp-induced activation of these GTPases (Figure 5A & B; p < 0.05). Upon stimulation with fmlp, levels of activated Cdc42 and Rac2 in the presence of Slit2 were less than half those observed when Slit2 was not present (Figure 5B; p < 0.05). Moreover, Slit2 prevented spatial accumulation of activated Rac and Cdc42 at the leading edge of fmlp-stimulated neutrophils (Figure 5C & D; p < 0.001). These data demonstrate that Slit2 inhibits neutrophil chemotaxis and actin polymerization by preventing cell polarization and disrupting generation and recruitment to the lamellipodium of activated Rac2 and Cdc42. 7) Slit2 does not inhibit chemoattractant-induced activation of other major kinase pathways. We examined the effects of Slit2 on activation of a number of other kinase pathways associated with neutrophil chemotaxis, namely, phosphoinositide 3-kinase (PI3K), Akt, Extracellular signal related kinase (Erk), and p38 mitogenactivated protein kinase (MAPK) [49-52]. As expected, stimulation of neutrophils with fmlp led to rapid phosphorylation of Akt, Erk and p38-mapk (Figure 6A-D; p < for Akt; p < 0.05 for Erk; p < 0.05 for p-38 MAPK). Slit2 treatment had no effect on the basal level of kinase activation (Figure 6A-D). Upon stimulation with fmlp, resulting levels of activated Akt were comparable in the presence or absence of Slit2, suggesting that Slit2 does not impair the ability of neutrophils to generate 144
154 PI(3,4,5)P3 (Figure 6A & B). Similarly, Slit2 treatment had no effect on fmlp-induced phosphorylation of Erk and p38 MAP kinase (Figure 6A, C, and D). Collectively, these data suggest that Slit2 inhibits neutrophil chemotaxis by specifically preventing activation of Cdc42 and Rac2, but not activation of Akt, Erk, or p38 MAPKs. 8) Slit2 inhibits leukocyte recruitment in peritoneal inflammation. To study the effects of Slit2 on neutrophil recruitment in vivo, we used a well-described mouse model of chemical irritant peritonitis [43]. In the presence of control medium, sodium periodate administration resulted in influx of 1.90 x10 6 ± 0.50 x10 6 neutrophils (Figure 7A). When Slit2 was pre-administered by intraperitoneal injection, neutrophil recruitment to the peritoneal cavity decreased six-fold (Figure 7A; 0.30 x10 6 ± 0.11x10 6 ; p < 0.05). When purified Slit2 was pre-administered intravenously by tail vein injection, neutrophil influx fell from 0.86 x10 6 ± 0.10 x10 6 to 0.05 x10 6 ± 0.02 x10 6 (Figure 7B; p < 0.001). Although the number of other leukocyte subsets recruited to the peritoneal cavity was small, Slit2 also inhibited infiltration of several of them, especially monocytes/macrophages (Supplementary Table 1; p < 0.01). Slit2 prevented neutrophil recruitment to the peritoneum in response to other chemoattractant factors, namely C5a and MIP-2 (Figure 7B; C5a: 1.50 x10 6 ± 0.60x10 6 ; C5a + Slit2: 0.30 x10 6 ± 0.08 x10 6 ; p < 0.001; MIP-2: 1.12 x x10 6 ± 0.24x10 6 ; MIP-2 + Slit2: 0.65 x 10 6 ± 0.19 x10 6, p < 0.01). These data demonstrate that Slit2 acts as a potent inhibitor of chemotaxis for circulating neutrophils, as well as for other leukocytes, towards diverse inflammatory stimuli. 145
155 A1.5 Discussion The aim of this study was to assess the effect of Slit2 on the migration of circulating neutrophils. We demonstrated that primary human neutrophils express Robo-1 and that exogenous application of Slit2 blocks migration of neutrophils in response to a chemotactic gradient. This observation is consistent with the effect of Slit2 on other cells expressing Robo-1 on their surface. Indeed, Slit2/Robo-1 have recently been shown to inhibit the migration of a number of different cell types, including cells of hematopoetic lineage such as dendritic cells and T lymphocytes [14-16]. A major finding of our study is that Slit2 did not inhibit all movement but specifically the directed migration of neutrophils. This is a particularly important distinction because neutrophil chemotaxis to sites of injury is an important component of inflammatory tissue injury. Indeed, neutrophil-mediated tissue damage is associated with a number of inflammatory conditions, including rheumatoid arthritis and ischemia-reperfusion injury [2, 53]. The ability of Slit2 to specifically disrupt neutrophil chemotaxis points to the potential use of this agent as a novel therapeutic for inflammatory tissue injury. While Slit2 has been shown to inhibit chemotactic migration of several cell types, the mechanisms that mediate these effects remain poorly understood. Neutrophil migration involves a complex series of events in which the cell, upon sensing a chemotactic gradient, develops a polarized morphology with a wide lamella at the front and a narrow tail-like uropod at the back. Critical to the maintenance of this asymmetry and to forward propulsion is the rapid turnover of actin filaments at the lamella. In this study, we demonstrated that treatment of 146
156 neutrophils with Slit2 led to a significant reduction in fmlp-stimulated generation of free barbed ends which are required for rapid actin polymerization at the leading edge [37]. This observation is consistent with data from neuronal cells linking Robo- 1 to proteins associated with the actin cytoskeleton, including Enabled kinase (Ena) and slit-robo GTPase activating protein-1 (srgap1) [9, 10]. However, to the best of our knowledge, this study provides the first evidence directly linking Slit2 treatment to a reduction in chemoattractant-stimulated high affinity actin filament ends. In neutrophils undergoing chemotaxis, the family of small GTPases mediate turnover of actin. Indeed, treatment of cells with Clostridium difficile toxin, which inhibits GTPases by monoglucosylation, results in severe defects in actin turnover and migration [54]. Seminal work describing the effects of introducing dominantnegative cdna constructs into HL-60 granolucytic cells identified Rac as the key determinant of actin assembly, and Cdc42 as being responsible for maintaining the direction of migration [34]. We observed that exogenous application of Slit2 prevented chemoattractant-induced activation and recruitment of both Cdc42 and Rac2. These data are consistent with data from neuronal cells where Slit2 treatment has been shown to recruit the novel GTPase activating protein srgap1, and to subsequently inactivate Cdc42 and inhibit axonal migration [10]. In HL-60 neutrophil-like cells, inhibition of Cdc42 using a dominant negative allele prevents cells from efficiently moving up a chemotactic gradient, and results in extension of random lamellae in all directions [34]. We found that Slit2 also prevented chemoattractant-induced activation of Rac2. Similarly, Slit2 has been shown to suppress Rac activation in human vascular 147
157 smooth muscle cells, human T lymphocytes, and murine RAW macrophages [13, 15, 16]. In murine neutrophils, Rac1 and Rac2 are expressed at similar levels, and each isoform has distinct functions. Neutrophils deficient in Rac1 display normal migratory velocity but reduced directionality towards chemotactic gradients [19]. In contrast, Rac2-deficient neutrophils demonstrate reduced migration speed, but normal chemotactic migration [19]. Rac1-deficient neutrophils show a partial reduction in chemoattractant-induced actin polymerization, and the kinetics of actin assembly are delayed, preferentially inhibiting early rather than later events [43]. Overall, the effects of Slit2 we observed on neutrophil migratory characteristics are highly reminiscent of Rac1 deficiency. In our experiments, rather than evaluate overall actin assembly, we focused on a key regulatory feature of this process, namely, generation of free high-affinity actin filament ends. Measurement of free barbed end formation specifically measures the initial burst of actin activity following chemotactic stimulation. Indeed, free barbed end generation of actin is required for efficient cell chemotaxis. We found that Slit2 inhibited chemoattractant-induced generation of free barbed ends by over 50%. This falls in between values observed in Rac1- and Rac2-deficient neutrophils, in which a 30% defect and a 70% defect in free barbed end generation has been reported, respectively [21]. It is interesting to note that following chemotactic stimulation of both Slit2-treated human neutrophils and Rac1-deficient murine neutrophils, random migration of cells remains intact despite a partial defect in generation of actin high-affinity free barbed ends. Emerging data supports the concept that it is not the total amount of actin polymerization that governs cell motility, but rather, the spatiotemporal 148
158 dynamics of actin assembly within the migrating cell. In support of this notion is the recent discovery that hematopoietic protein 1 (Hem-1) constitutes part of an organizational complex that localizes to propagating waves of actin nucleation within migrating neutrophils [55, 56]. These waves interact reciprocally with actin to define and organize the leading edge of neutrophils [56]. In this way, net cell movement results from the collective actions of multiple self-organizing actin-based waves. At the molecular and cellular level, Slit2 s effects on neutrophil migration share features akin to those seen in both Rac1- and Rac2-null mice. This may be explained by the differences in expression of Rac isoforms between murine and human neutrophils. In murine neutrophils, Rac1 and Rac2 are expressed at equivalent concentrations. In human neutrophils, Rac2 expression is 4 to 40 times greater than that of Rac1 [47, 48, 57]. Thus, in human neutrophils it is likely that Rac2 mediates functions assumed by Rac1 in murine neutrophils. In human neutrophils it has proven very difficult to delineate the individual functions of Rac1 and Rac2. The two GTPases are 92% homologous and the guanine nucleotide exchange factors that regulate them are the same, rendering expression of mutant proteins in neutrophil-like cell lines an ineffective means of dissecting the individual roles played by Rac1 and Rac2 in chemotaxis. Moreover, human neutrophils are small, terminally differentiated cells which are difficult to transfect, further complicating the ability to experimentally manipulate them. Together, our data suggest a mechanism of action whereby Slit2 binding to Robo-1 in human neutrophils prevents chemoattractant-induced activation of Rac2 and Cdc42, with 149
159 consequent disruption of actin free barbed end formation, and ultimately, inhibition of directional neutrophil migration. Stimulation of neutrophils by fmlp also leads to rapid phospholipid metabolism and activation of major kinase pathways, including Akt, Erk, and p38- MAPK, responsible for transcriptional changes. Studies using specific inhibitors demonstrate that disrupting each of these pathways significantly disrupts neutrophil chemotaxis. However, exogenous treatment with Slit2 had no effect on the chemoattractant-induced activation of any of the above pathways. We observed normal activation of the Akt pathway in response to chemotactic stimulation, suggesting that Slit2 does not inhibit phospholipid metabolism and specifically, generation of PI(3,4,5)P 3. These results were somewhat surprising, given the important role played by PI(3,4,5)P 3 in chemotactic migration of neutrophils. In one study, neutrophils from PI3Kγ-deficient mice displayed reduced directional migration towards chemotactic gradients [50]. Our data is, however, consistent with observations in human HL-60 granulocytic cells expressing a dominant negative allele of Cdc42. In these studies, suppression of Cdc42 still led to normal PI(3,4,5)P 3 production and Akt activation [34]. In yet another study, Slit2 prevented chemokine-induced activation of PI3K in human breast cancer cells [12]. We further found that in human neutrophils, Slit2 did not inhibit chemoattractant-induced activation of Erk, nor p38-mapk. These data are in concordance with those of others, demonstrating that neither activation of p38-mapk in Jurkat T lymphocytes nor activation of Erk in human granulocytic cells was affected by Slit2 [16, 17]. In another study, Slit2 prevented chemotaxis and chemoinvasion of breast cancer 150
160 cells towards the chemokine, CXCL12, and inhibited CXCL12-induced activation of Erk [12]. These differential effects of Slit2 on inducible kinase activity may be attributable to the different cell types used and to the different chemotactic agents used to stimulate them. To determine whether Slit2 can prevent neutrophil recruitment in vivo, we used mouse models of peritoneal inflammation induced by local instillation of sodium periodate, C5a, or MIP-2. We found that administration of Slit2, either intraperitoneally or intravenously, significantly reduced neutrophil recruitment. This is the first direct demonstration of Slit2 s potent anti-chemotactic actions on neutrophils in vivo. These data confirm a universal antimigratory role for Slit2, and are in keeping with recent work showing that Slit2 prevents pathologic neovascularization within the eye by inhibiting chemotaxis of endothelial cells towards vascular endothelial growth factor [18]. In another study, Slit2 ameliorated glomerulonephritis-associated kidney injury by inhibiting chemotactic infiltration of macrophages [15]. Our results would suggest that localized or systemic delivery of Slit2 may reduce neutrophil recruitment and subsequent tissue damage associated with inflammation. Soluble Slit2 is relatively sticky and could potentially be locally maintained at high concentration by adhering to extracelleular matrix proteins such as glypican-1 [58]. Thus, after regional administration, Slit2 could be retained at sites of inflammation, such as joints and transplanted organs, thereby alleviating neutrophil-inflicted tissue injury associated with rheumatoid arthritis and ischemia reperfusion injury. Because Slit2 blocks migration of several types of inflammatory cells, including neutrophils, T lymphocytes, macrophages, and dendritic cells, 151
161 towards diverse chemotactic stimuli, it could act as a highly effective antiinflammatory agent [14-17]. Further studies are needed to explore the clinical use of Slit2, or a Slit-like agent, for prevention and treatment of localized inflammation. 152
162 A1.6 Acknowledgments The authors wish to thank Dr. Mohabir Ramjeesingh for technical assistance, and Drs. Gilles St-Laurent, and Sylvie Perret for reagents. We are grateful to Dr. Sergio Grinstein for reagents and for helpful advice. This work was supported by the Canadian Institute of Health Research (L.A.R.), the Kidney Foundation of Canada (L.A.R.), and an Early Researcher Award from the Ministry of Research and Innovation, Government of Ontario (L.A.R.). L.A.R. holds a Canada Research Chair, Tier
163 A1.7 Authorship S.T. designed and performed experiments, analyzed results, and helped with manuscript preparation. I.M.M., Y-W.H., M.A.O.M., and M.Y. designed and performed experiments and analyzed results. M.R.C., G-Y.L., and C.X.S. designed and performed experiments. Y.D. generated critical reagents and helped with manuscript preparation. M.G. designed experiments and helped with manuscript preparation. L.A.R. designed experiments, interpreted results, and prepared the manuscript. 154
164 A1.8 References 1. Reutershan, J., Ley, K. (2004) Bench-to-bedside review: Acute respiratory distress syndrome- how neutrophils migrate into the lumg. Crit Care 8, Weissmann, G., Korchak, H. (1984) Rheumatoid arthritis: the role of neutrophil activation. Inflammation 8 Suppl, S3-S Rossen, R.D., Swain, J.L., Michael, L.H., Weakley, S., Giannini, E., Entman, M.L. (1985) Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res 57, Lucchesi, B.R., Mullane, K.M. (1986) Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol 26, Panes, J., Perry, M., Granger, D.N. (1999) Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol 126, Charo, I.F., Ransohoff, R.M. (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354, Nguyen Ba-Charvet, K.T., Brose, K., Ma, L., Wang, K.H., Marillat, V., Sotelo, C., Tessier-Lavigne, M., Chedotal, A. (2001) Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci 21, Brose, K., Bland, K.S., Wang, K.H., Arnott, D., Henzel, W., Goodman, C.S., Tessier-Lavigne, M., Kidd, T. (1999) Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96,
165 9. Bashaw, G.J., Kidd, T., Murray, D., Pawson, T., Goodman, C.S. (2000) Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the Roundabout receptor. Cell 101, Wong, K., Ren, X.-R., Huang, Y.-Z., Xie, Y., Liu, G., Saito, H., Tang, H., Wen, L., Brady-Kalnay, S.M., Mei, L., Wu, J.Y., Xiong, W.-C., Rao, Y. (2001) Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 107, Werbowetski-Ogilvie, T.E., Seyed Sadr, M., Jabado, N., Angers-Lostau, A., Agar, N.Y.R., Wu, J., Bjerkvig, R., Antel, J.P., Faury, D., Rao, Y., Del Maestro, R.F. (2006) Inhibition of medulloblastoma cell invasion by Slit. Oncogene 25, Prasad, A., Fernandis, A.Z., Rao, Y., Ganju, R.K. (2004) Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem 279, Liu, D., Hou, J., Hu, X., Wang, X., Xiao, Y., Mou, Y., De Leon, H. (2006) Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circ Res 98, Guan, H., Zu, G., Tang, H., Johnson, M., Xu, X., Kevil, C., Xiong, W.-C., Elmets, C., Rao, Y., Wu, J.Y., Xu, H. (2003) Neuronal repellent Slit2 inhibits dendritic cell migration and the development of immune responses. J Immunol 171, Kanellis, J., Garcia, G.E., Li, P., Parra, G., Wilson, C.B., Han, S., Smith, C.W., Johnson, R.J., Wu, J.Y., Feng, L. (2004) Modulation of inflammation by Slit protein in vivo in experimental crescentic glomerulonephritis. Am J Pathol 165, Prasad, A., Qamri, Z., Wu, J., Ganju, R.K. (2007) Slit-2/Robo-1 modulates the 156
166 CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol 82, Wu, J.Y., Feng, L., Park, H.-T., Havlioglu, N., Wen, L., Tang, H., Bacon, K.B., Jiang, Z.-h., Zhang, X.-c., Rao, Y. (2001) The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, Jones, C.A., Londin, N.R., Chen, H., Park, K.W., Sauvaget, D., Stockton, R.A., Wythe, J.D., Suh, W., Larrieu-Lahargue, F., Mukoutama, Y.-s., Lindblom, P., Seth, P., Frias, A., Nishiya, N., Ginsberg, M.H., Gerhardt, H., Zhang, K., Li, D.Y. (2008) Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 14, Sun, C.X., Downey, G.P., Zhu, F., Koh, A.L.Y., Thang, H., Glogauer, M. (2004) Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104, Clark, R.A., Volpp, B.D., Leidal, K.G., Nauseef, W.M. (1990) Two cytosolic components of the human neutrophil respiratory burst oxidase traslocate to the plasma membrane during cell activation. J Clin Invest 85, Sun, C.X., Magalhaes, M.A.O., Glogauer, M. (2007) Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fmlp receptor. J Cell Biol 179, Li, H.S., Chen, J.H., Wu, W., Fagaly, T., Zhou, L., Yuan, W., Dupuis, S., Jiang, Z.H., Nash, W., Gick, C., Ornitz, D.M., Wu, J.Y., Rao, Y. (1999) Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96,
167 23. Zhang, J., Liu, X., Bell, A., To, R., Nath Berall, T., Azizi, A., Li, J., Cass, B., Durocher, Y. (2009) Transient expression and purification of chimeric heavy chain antibodies. Protein Expr Purif 65, Shi, C., Shin, Y.-O., Hanson, J., Cass, B., Loewen, M.C., Durocher, Y. (2005) Purification and characterization of a recombinant G-protein-coupled receptor, Saccharomyces cerevisiae Ste2p, transiently expressed in HEK203 EBNA1 cells. Biochemistry 44, Durocher, Y., Perret, S., Kamen, A. (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30, E Moore, K.J., Andersson, L.P., Ingalls, R.R., Monks, B.G., Li, R., Arnaout, M.A., Golenbock, D.G., Freeman, M.W. (2000) Divergent responses to LPS and bacteria in CD14-deficient murine macrophages. J Immunol 165, Robinson, L.A., Nataraj, C., Thomas, D.W., Cosby, J.M., Griffiths, R., Bautch, V.L., Patel, D.D., Coffman, T.M. (2003) The chemokine CX3CL1 regulates NK cell activity in vivo. Cellular Immunol 225, Robinson, L.A., Nataraj, C., Thomas, D.W., Howell, D.N., Griffiths, R., Bautch, V., Patel, D.D., Feng, L., Coffman, T.M. (2000) A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J Immunol 165, Yan, M., Di Ciano-Oliveira, C., Grinstein, S., Trimble, W.S. (2007) Coronin function is required for chemotaxis and phagocytosis in human neutrophils. J Immunol 178,
168 30. Veldkamp, K.E., Heezius, H.C.J.M., Verhoef, J., van Strijp, J.A.G., Van Kessel, K.P.M. (2000) Modulation of neutrophil chemokine receptors by Staphylococcus aureus supernate. Infect Immun 68, Crawford, M.A., Aylott, C.V., Bourdeau, R.W., Bokoch, G.M. (2006) Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol 176, Fickl, H., Theron, A.J., Anderson, R., Mitchell, T.J., Feldman, C. (2007) Palladium attenuates the pro-inflammatory interactions of C5a, interleukin-8 and pneumolysin with human neutrophils. J Immunotoxicol 4, Magalhaes, M.A.O., Zhu, F., Sarantis, H., Gray-Owen, S.D., Ellen, R.P., Glogauer, M. (2007) Expression and translocation of fluorescent-tagged p21- activated kinase-binding domain and PH domain of protein kinase B during murine neutrophil chemotaxis. J Leukoc Biol 82, Srinivasan, S., Wang, F., Glavas, S., Ott, A., Hofmann, F., Aktories, K., Kalman, D., Bourne, H.R. (2003) Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160, Hayashi, H., Aharonovitz, O., Alexander, R.T., Touret, N., Furuya, W., Orlowski, J., Grinstein, S. (2008) Na+/H+ exchange and ph regulation in the control of neutrophil chemokinesis and chemotaxis. Am J Physiol Cell Physiol 294, C526- C Gardiner, E.M., Pestonjamasp, K.N., Bohl, B.P., Chamberlain, C., Hahn, K.M., Bokoch, G.M. (2002) Spatial and temporal analysis of Rac activation during live cell chemotaxis. Curr Biol 12,
169 37. Glogauer, M., Hartwig, J.H., Stossel, T.P. (2000) Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. J Cell Biol 150, Barkalow, K., Witke, W., Kwiakowski, D.J., Hartwig, J.H. (1996) Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J Cell Biol 134, Benard, V., Bohl, B.P., Bokoch, G.M. (1999) Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem 274, de Haas, C.J.C., Veldkamp, K.E., Peschel, A., Weerkamp, F., Van Wamel, W.J.B., Heezius, E.C.J.M., Poppelier, M.J.J.G., Van Kessel, K.P.M., van Strijp, J.A.G. (2004) Chemotaxis inhibitory protein of Staphylcoccus aureus, a bacterial antiinflammatory agent. J Exp Med 199, Bajt, M.L., Farhood, A., Jaeschke, H. (2001) Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol 281, G1188-G Clark, K., Hammond, E., Rabbitts, P. (2002) Temporal and spatial expression of two isoforms of the Dutt1/Robo1 gene in mouse development. FEBS Letters 523, Glogauer, M., Marchal, C.C., Zhu, F., Worku, A., Clausen, B.E., Foerster, I., Marks, P., Downey, G.P., Dinauer, M., Kwiakowski, D.J. (2003) Rac1 deletion in mouse neutrophils has selective effects on neutrophil function. J Immunol 170,
170 44. Condeelis, J. (2001) How is actin polymerization nucleated in vivo? Trends Cell Biol 11, Ichetovkin, I., Grant, W., Condeelis, J. (2002) Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp/3 complex. Curr Biol 12, Huang, T.Y., DerMardirossian, C., Bokoch, G.M. (2006) Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 18, Li, S., Yamauchi, A., Marchal, C.C., Molitoris, J.K., Quilliam, L.A., Dinauer, M.C. (2002) Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac1 and Rac2 gene dosage effect on neutrophil functions. J Immunol 169, Quinn, M.T., Evans, T., Loetterle, L.R., Jesaitis, A.J., Bokoch, G.M. (1993) Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem 268, Coxon, P.Y., Rane, M.J., Uriarte, S., Powell, D.W., Singh, S., Butt, W., Chen, Q., McLeish, K.R. (2003) MAPK-activated protein kinase-2 participates in p38 MAPKdependent and ERK-dependent functions in human neutrophils. Cell Signal 15, Hannigan, M., Zhan, L., Li, Z., Ai, Y., Wu, D., Huang, C.-K. (2002) Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N- formyl-met-leu- Phe-induced chemotaxis. Proc Natl Acad Sci USA 99,
171 51. Heit, B., Tavenere, S., Raharjo, E., Kubes, P. (2002) An intracellular signaling hierarchy determines direction of migration in opposing gradients. J Cell Biol 159, Zu, Y.-L., Qi, J., Gilchrist, A., Fernandez, G.A., Vazquez-Abad, D., Kreutzer, D.L., Huang, C.-K., Sha'afi, R.I. (1998) p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or fmlp stimulation. J Immunol 160, Kaminski, K.A., Bonda, T.A., Korecki, J., Musial, W.J. (2002) Oxidative stress and neutrophil activation- the two keystones of ischemia/reperfusion injury. Int J Cardiol 86, Sehr, P., Joseph, G., Genth, H., Just, I., Pick, E., Aktories, K. (1998) Glucosylation and ADP ribosylation of rho proteins: effects of nucleotide binding, GTPase activity, and effector coupling. Biochemistry 37, Weiner, O.D., Rentel, M.C., Ott, A., Brown, G.E., Jedrychowski, M., Yaffe, M.B., Gygi, S.P., Cantley, L.C., Bourne, H.R., Kirschner, M.W. (2006) Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol 4, e Weiner, O.D., Marganski, W.A., Wu, L.F., Altschuler, S.J., Kirschner, M.W. (2007) An actin-based wave generator organizes cell motility. PLoS Biol 5, e Gu, Y., Filippi, M.-D., Cancelas, J.A., Siefring, J.E., Williams, E.P., Jasti, A.C., Harris, C.E., Lee, A.W., Prabhakar, R., Atkinson, S.J., Kwiakowski, D.J., Williams, D.A. (2003) Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302,
172 58. Ronca, F., Andersen, J.S., Paech, V., Margolis, R.U. (2001) Characterization of Slit protein interactions with glypican-1. J Biol Chem 276,
173 A1.9 Figure Legends Figure 1. Primary human and murine neutrophils express Robo-1. A, Primary human neutrophils were isolated from venous blood of healthy volunteers, RNA was extracted, and RTPCR was performed using specific primers for Robo-1. For comparison, total RNA was isolated from human leukocytes from whole blood, and RT-PCR similarly performed. B, Cell lysates from primary human neutrophils and bone marrow-derived murine neutrophils were harvested and immunoblotting was performed using anti-robo-1 primary Ab and HRP-conjugated secondary Ab. C, Human neutrophils were plated on fibronectin-coated coverslips and labeled with anti-robo-1 Ab followed by Cy3-conjugated secondary Ab. Cells were examined using a Leica DMIRE2 spinning disc confocal microscope at 100x magnification. Scale bar is 10 µm. Representative image from one of three separate experiments. D, To detect cell surface expression of Robo-1, primary human neutrophils were fixed, incubated with anti-robo-1 Ab followed by PE-conjugated secondary Ab or with secondary Ab alone, and analyzed using a FACScalibur flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Inc., Ashland, OR). Representative image from one of three similar independent experiments. Value indicates % of cells with positive labeling. E, Mouse bone marrow-derived neutrophils were isolated and cell surface Robo-1 labeled as described in D. Representative image from one of three similar independent experiments. Value indicates % of cells with positive labeling. 164
174 Figure 2. Slit2 inhibits migration of human neutrophils towards diverse chemoattractants. A-D, Primary human neutrophils were incubated with purified Slit2 (4.5 µg/ml) for 10 min at 37 C, then migration assays performed across 3 µm Transwell inserts. The lower chamber contained HBSS or Slit2-containing HBSS in the presence or absence of fmlp (1 µm). Neutrophils were placed in the upper chamber and Transwell plates incubated for 1 h at 37 C. The insert was removed, and cells which had migrated from the upper to the lower chamber were gently centrifuged onto coverslips and cell nuclei labeled with DAPI to facilitate visualization. Representative high-power (63x) images of migrated cells from four independent experiments were taken using a Leica deconvolution microscope: A, HBSS. B, HBSS with fmlp. C, Slit2 with fmlp. D, Slit2. E, Transwell assays were performed as described above, in the presence of the indicated concentrations of Slit2. Random fields were counted using a Nikon light microscope. Mean number of cells counted per 63x field ± SEM. *, p < 0.001; n=10. F, Transwell migration assays were performed as described above. In the lower chamber was placed either C5a (2 µg/ml) or IL-8 (0.13 µg/ml), in the presence or absence of purified Slit2 (4.5 µg/ml). *, p < 0.001; n = 4. Figure 3: Slit2 inhibits neutrophil chemotaxis. Primary human neutrophils were allowed to settle onto fibronectin-coated coverslips. A micropipette containing fmlp (1 µm) was used to dispense a point-source and gradient of chemoattractant, and neutrophil migration was monitored using time-lapse video microscopy. The cells were maintained on the 37 C-heated stage of a Leica DMIRE2 inverted microscope 165
175 equipped with a Hamamatsu back-thinned EM-CCD camera and spinning disc confocal scan head. Digital pictures were acquired every 3 s. In some experiments, neutrophils were also exposed to anti-myc Ab affinity-purified Slit2 (0.6 µg/ml). Volocity TM (Improvision) software was used to track the centroid of migrating neutrophils and thus calculate the total distance, net distance and speed of migration. Directionality (displacement/distance) was used as a measure of chemotaxis. A minimum of 8-10 cells for each condition were examined from each of three separate experiments. Two to 3 cells from each quadrant were randomly selected prior to initiating tracking. Only cells which started and remained in the field of view over the entire course of video capture were analyzed. A, Migratory tracks from one experiment where neutrophils were exposed to fmlp. X marks the position of the micropipette. B, Migratory tracks from one experiment where neutrophils were exposed to fmlp together with Slit2. X marks the position of the micropipette. Panel inset depicts an enlarged view of the tracks made by a single neutrophil. C, Graph depicting the mean migratory speed of neutrophils exposed to fmlp alone or to fmlp in conjunction with Slit2. Mean values ± SEM for 3 separate experiments. D, Graph depicting the mean directionality of neutrophils exposed to fmlp alone or to fmlp together with Slit2. Mean values ± SEM for 3 separate experiments. *, p < Figure 4: Slit2 inhibits chemoattractant-stimulated formation of actin free barbed ends in human neutrophils. A, Time series analysis of the fluorescence increase associated with actin polymerization. Briefly, 1x10 6 freshly isolated human 166
176 neutrophils were permeabilized for 10 s with 0.2% OG buffer, and the permeabilization process was stopped by diluting the detergent with 3 vol of buffer B, as described in Materials and Methods. Cells were stimulated with fmlp (1 µm) for 120 s in the presence of fplc-enriched Slit2 (0.6 µg/ml) from conditioned medium or control medium. Free barbed end generation was assayed by adding pyrene-labeled rabbit skeletal muscle actin to a final concentration of 1 µm and following the fluorescence increase using a microplate reader (FLUOstaroptima) with fluorescence excitation and emission wavelengths of 355 and 405 nm, respectively. Representative results of four separate experiments are shown. B, Pyrene-actin incorporation was monitored as in (A) for 150s and the change in slope of the curve was used as a measure of the rate of actin polymerization. Mean rate of actin polymerization normalized to the unstimulated control ± SEM. *, p < 0.05; **, p < 0.04; ***, p < C, Freshly isolated human and mouse neutrophils were incubated with Slit2 and plated on fibronectin-coated coverslips in a 6 well tissue culture plate. Cells were incubated with fmlp (1 µm) for 3 min, then fixed, permeabilized with 0.1% Triton, and incubated with rhodamine-conjugated phalloidin for 30 min to visualize actin. Cells were examined using a Leica DMIRE2 spinning disc confocal microscope at 100x magnification. Figure 5: Slit2 prevents chemoattractant-induced activation and redistribution of Rac2 and Cdc42. A, Neutrophils were activated with PBS or fmlp (1 µm for 30 s) in the presence or absence of anti-myc Ab affinity-purified Slit2 (0.6 µg/ml), and cell lysates collected. GST beads conjugated to the p21-binding domain of PAK1 167
177 were used to pull down activated Cdc42 and Rac and immunoblotting was performed using specific Ab directed against Cdc42 or Rac2. Blots shown are representative of five independent experiments. B, Mean values ± SEM of normalized band intensities from five independent experiments (*p < 0.01; ** p < 0.05). C, Neutrophils were isolated from murine bone marrow as described in Materials and Methods. One million cells were suspended in 100 µl NucleofectorTM solution (Amaxa, Inc.) supplemented with 6 µg cdna for PAK-PBD-YFP and H-Ras- RFP. Cells were transfected using a Cell Line V Nucleofector TM kit and the Nucleofector TM program Y-001. Transfected cells were carefully recovered with 500 µl Iscove s Modified Dulbecco s Medium (IMDM) pre-warmed to 37 C, and transferred to 1.5 ml pre-warmed IMDM supplemented with 10% FBS in six-well plates for 2 h. After the recovery period, cells were incubated with purified Slit2 (4.5 µg/ml) for 10 min. Cells were mounted on a 1% BSA-coated coverslip in an Attafluor cell chamber mounted on the 37 C heated stage of a Leica DMIRE2 inverted fluorescence microscope quipped with a Hamamatsu back-thinned EM-CCD camera and spinning disc confocal scan head. Cells were exposed to a pointsource of chemoattractant using a glass micropipette containing fmlp (1 µm). Digital pictures were taken every 3 s for 5 min, and images were acquired and analyzed using Volocity software (Improvision Ltd). Images showing the distribution of PAK-PBD-YFP, H-Ras-RFP, and the resulting GFP-RFP ratio at the leading edge compared to the trailing edge of cells exposed to fmlp alone or fmlp in the presence of Slit2. Arrow indicates the direction of the chemotactic gradient. Images 168
178 are representative of at least 19 cells analyzed from 3 separate experiments. D, Experiments were performed as described in (C). Mean values ± SEM for the normalized mean fluorescence intensity (MFI), calculated as the GFP:RFP ratio at the leading edge compared to the trailing edge of the cell. A minimum of 19 cells were analyzed from 3 separate experiments. *, p < Figure 6: Slit2 does not inhibit chemoattractant-induced activation of Akt, Erk, or p38-mapk. A, Neutrophils were incubated with fmlp and/or Slit2, as described for Figure 5A. Cell lysates were collected, and immunoblotting was performed using specific Ab detecting phospho-akt, phospho-erk, and phospho-p38 MAPK. Blots were stripped and re-probed using Ab detecting total Akt, total Erk, and total p38 MAPK, respectively. Blots are representative of 3 independent experiments. B, Band intensities for phospho-akt (p-akt) normalized to total Akt. Mean values ± SEM for 3 independent experiments (*, p < ; **, p < 0.05). C, Band intensities for phospho-erk (p-erk) normalized to total Erk. Mean values ± SEM for 3 independent experiments (**, p < 0.05). D, Band intensities for phospho-p38-mapk (p-p38) normalized to total p38-mapk. Mean values ± SEM for 3 independent experiments. (**, p < 0.05; ***, p < 0.005). Figure 7: Slit2 inhibits neutrophil chemotaxis in vivo towards diverse attractant stimuli. A, Adult CD1 mice were injected intraperitoneally with Slit2 (0.1 µg/mouse) or control medium, and 1 h later, with 1 ml of 5 mm sodium periodate (NaIO 4 ). After 3 h, mice were euthanized and the peritoneal exudates collected by 169
179 lavage with chilled PBS (5 ml/mouse). Infiltrating leukocytes were counted using an electronic cell counter and the number of neutrophils quantified using Wright- Giemsa stain. Mean values ± SEM from 5 separate experiments. *, p < 0.05; **, p < B, Adult CD1 mice received an intravenous dose of purified Slit2 (1.8 µg in 0.2 ml normal saline) by tail-vein injection. One hour later, mice were given 1 ml of NaIO 4 (5 mm), C5a (10 µg), or MIP-2 (2.5 µg) by intraperitoneal injection. After 3 h, mice were euthanized and the peritoneal exudates collected by lavage with chilled PBS (5 ml/mouse). Infiltrating leukocytes were counted using an electronic cell counter and the number of neutrophils quantified using Wright-Giemsa stain. Mean values ± SEM from 4 to 6 separate experiments per treatment condition. *, p < 0.001; **, p < Supplementary Figure 1: Recombinant hslit2 purified by size-exclusion chromatography and cobalt-affinity chromatography. A-B, Conditioned medium was harvested from HEK293- hslit2-myc cells and control HEK-293 cells as described in Materials and Methods. Using size-exclusion chromatography, fractionated samples were collected and were run in 8% SDSPAGE. A, Representative gel for a sample from pooled fractions was silver stained. B, Representative gel, transferred to a PVDF membrane and immunoblotting performed using monoclonal anti-myc Ab. C-D, For larger-scale preparation of Slit2, conditioned medium was harvested from HEK293-EBNA1 cells transfected with ptt28-slit2 expression plasmid, as described in Materials and Methods. Slit2 secreted into the medium was purified by immobilized metal-affinity chromatography 170
180 using Fractogel-cobalt columns. Samples were desalted and immunoblotting performed. Proteins were resolved on reducing NuPAGE 4-12% Bis-Tris gradient gels, and transferred to nitrocellulose membranes. C, Representative membrane, stained with Ponceau red solution. D, Representative membrane, probed with antipolyhis- HRP Ab. For C and D, lanes are marked as follows: 1) harvested medium 5 days posttransfection; 2) IMAC flow-through; 3) Wash1; 4) Wash 2; 5) pooled eluted fractions from Fractogel-cobalt column. Supplementary Figure 2: Measurement and verification of endotoxin levels and activity present in Slit2 preparations. From each separate Slit2 preparation, endotoxin levels were measured using ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript Corp., Piscataway, NJ), according to the manufacturer s specifications. Endotoxin concentrations ranged from EU/ml, corresponding to ng/ml endotoxin, and yielding final experimental concentrations of pg/ml, which are well below those thought to activate leukocytes. To verify this, endotoxin (40 pg/ml) was added to Transwell assays, and effects on neutrophil transmigration examined as described in Materials and Methods and in Figure 2. n=2. Supplementary Figure 3: Slit2 inhibits migration of primary human neutrophils. Primary human neutrophils were incubated with FPLC-enriched Slit2 from conditioned medium (0.6 µg/ml) or with similar fractions from control medium for 10 min at 37 C, then migration assays performed across 3 µm Transwell 171
181 inserts. The lower chamber contained HBSS, control medium or Slit2 in the presence or absence of fmlp (1 µm). Neutrophils were placed in the upper chamber and Transwell plates incubated for 1 h at 37 C. The insert was removed, and cells which had migrated from the upper to the lower chamber were gently centrifuged onto coverslips, fixed and random fields were counted using a Nikon light microscope. Representative high-power (63x) images of migrated cells from four independent experiments were taken using a Leica deconvolution microscope. Mean number of cells counted per 63x field ± SEM for 4 independent experiments. (*, p < 0.05). Supplementary Figure 4: Slit2 inhibits directional migration of human neutrophils. Primary human neutrophils were allowed to settle onto fibronectincoated coverslips. A micropipette containing fmlp (1 µm) was used to dispense a point-source and gradient of chemoattractant, and neutrophil migration was monitored using time-lapse video microscopy at 37 C. A-C, Migration of neutrophils exposed to a gradient of fmlp over the course of 5 minutes. D-F, Migration of neutrophils exposed to a gradient of fmlp together with Slit2 (0.6 µg/ml) over 5 minutes. Representative images from one of five separate experiments. For 4 independent experiments. (*, p < 0.05). Supplementary Video 1. Human neutrophils migrate effectively towards a point source of fmlp. Glass coverslips were coated with fibronectin, mounted in a Leiden chamber, and placed on the heated stage of a microscope. A suspension of 172
182 human neutrophils containing 10 6 cells/ 100 µl was added and allowed to settle for 10 min. To induce chemotaxis, a point-source of fmlp (1 µm) was delivered using a borosilicate capillary micropipette. The pipette was held stationary and diffusion of fmlp generated a standing gradient. Images were acquired using MetaMorph software (Universal Imaging, West Chester, PA) running on a Dell Optiplex DGX 590 computer interfaced with a Photometrics camera via a 12-bit GPIB/IIA board (National Instruments, Foster City, CA). Image acquisition was started upon the pipette entering the field and images were obtained every 10 s until completion of the experiment. Representative video from one of five separate experiments. Supplementary Video 2. Slit2 inhibits directional migration of human neutrophils towards a point source of fmlp. Experiments were performed as described in Supplementary Video 1. Neutrophils were also exposed to anti-myc Ab affinity-purified Slit2 (0.6 µg/ml) and cell migration was monitored by time-lapse videomicroscopy. Representative video from one of five separate experiments. Supplementary Table 1. Leukocyte subsets recovered from peritoneal lavage fluid following sodium periodate-induced peritonitis. Adult CD1 mice received an intravenous dose of purified Slit2 (1.8 µg in 0.2 ml normal saline) by tail-vein injection. One hour later, mice were given 1 ml of NaIO 4 (5 mm) by intraperitoneal injection. After 3 h, mice were euthanized and the peritoneal exudates collected by lavage with chilled PBS (5 ml/mouse). The total number of cells was counted, and the numbers of monocytes/macrophages, T lymphocytes, B lymphocytes, and 173
183 natural killer cells determined by labeling with Ab directed to F4/80, CD3, B220, and NK1.1, respectively, followed by PE-conjugated secondary Ab. Flow cytometry was performed using a FACScalibur flow cytometer and FlowJo software. Mean values ± SEM from 4 separate experiments. 174
184 175
185 176
186 177
187 178
188 179
The recruitment of leukocytes and plasma proteins from the blood to sites of infection and tissue injury is called inflammation
 The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
Acute Kidney Injury: Basic Research Interactive Workshop
 Acute Kidney Injury: Basic Research Interactive Workshop Lisa Robinson, MD, FRCP(C) Division of Nephrology, The Hospital for Sick Children Renal Transplant Program, SickKids Transplant Centre Program in
Acute Kidney Injury: Basic Research Interactive Workshop Lisa Robinson, MD, FRCP(C) Division of Nephrology, The Hospital for Sick Children Renal Transplant Program, SickKids Transplant Centre Program in
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Chapter 3, Part A (Pages 37-45): Leukocyte Migration into Tissues
 Allergy and Immunology Review Corner: Chapter 3, Part A (pages 37-45) of Cellular and Molecular Immunology (Seventh Edition), by Abul K. Abbas, Andrew H. Lichtman and Shiv Pillai. Chapter 3, Part A (Pages
Allergy and Immunology Review Corner: Chapter 3, Part A (pages 37-45) of Cellular and Molecular Immunology (Seventh Edition), by Abul K. Abbas, Andrew H. Lichtman and Shiv Pillai. Chapter 3, Part A (Pages
Lecture: CHAPTER 13 Signal Transduction Pathways
 Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction Pathways. Part 2
 Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
Biosignals, Chapter 8, rearranged, Part I
 Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Chapter 15: Signal transduction
 Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Propagation of the Signal
 OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
Chapter 20. Cell - Cell Signaling: Hormones and Receptors. Three general types of extracellular signaling. endocrine signaling. paracrine signaling
 Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Signal Transduction: G-Protein Coupled Receptors
 Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
BIOLOGY. Cell Communication CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson. Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 11 Cell Communication Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Cellular Messaging Cells can signal to
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 11 Cell Communication Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Cellular Messaging Cells can signal to
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Lecture 9: Cell Communication I
 02.05.10 Lecture 9: Cell Communication I Multicellular organisms need to coordinate cellular functions in different tissues Cell-to-cell communication is also used by single celled organisms to signal
02.05.10 Lecture 9: Cell Communication I Multicellular organisms need to coordinate cellular functions in different tissues Cell-to-cell communication is also used by single celled organisms to signal
Chem Lecture 10 Signal Transduction
 Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Membrane associated receptor transfers the information. Second messengers relay information
 Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
Sarah Jaar Marah Al-Darawsheh
 22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
Basis of Immunology and
 Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Cell Signaling (part 1)
 15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
Effects of Second Messengers
 Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Intercellular indirect communication
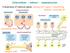 Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS
 LECTURE: 07 Title: IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS LEARNING OBJECTIVES: The student should be able to: The chemical nature of the cellular surface receptors. Define the location of the
LECTURE: 07 Title: IMMUNE CELL SURFACE RECEPTORS AND THEIR FUNCTIONS LEARNING OBJECTIVES: The student should be able to: The chemical nature of the cellular surface receptors. Define the location of the
2013 W. H. Freeman and Company. 12 Signal Transduction
 2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
MCB*4010 Midterm Exam / Winter 2008
 MCB*4010 Midterm Exam / Winter 2008 Name: ID: Instructions: Answer all 4 questions. The number of marks for each question indicates how many points you need to provide. Write your answers in point form,
MCB*4010 Midterm Exam / Winter 2008 Name: ID: Instructions: Answer all 4 questions. The number of marks for each question indicates how many points you need to provide. Write your answers in point form,
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Subject Index. Bcl-2, apoptosis regulation Bone marrow, polymorphonuclear neutrophil release 24, 26
 Subject Index A1, apoptosis regulation 217, 218 Adaptive immunity, polymorphonuclear neutrophil role 31 33 Angiogenesis cancer 178 endometrium remodeling 172 HIV Tat induction mechanism 176 inflammatory
Subject Index A1, apoptosis regulation 217, 218 Adaptive immunity, polymorphonuclear neutrophil role 31 33 Angiogenesis cancer 178 endometrium remodeling 172 HIV Tat induction mechanism 176 inflammatory
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
The dynamic regulation of blood vessel caliber
 INVITED BASIC SCIENCE REVIEW The dynamic regulation of blood vessel caliber Colleen M. Brophy, MD, Augusta, Ga BACKGROUND The flow of blood to organs is regulated by changes in the diameter of the blood
INVITED BASIC SCIENCE REVIEW The dynamic regulation of blood vessel caliber Colleen M. Brophy, MD, Augusta, Ga BACKGROUND The flow of blood to organs is regulated by changes in the diameter of the blood
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
Regulation of cell function by intracellular signaling
 Regulation of cell function by intracellular signaling Objectives: Regulation principle Allosteric and covalent mechanisms, Popular second messengers, Protein kinases, Kinase cascade and interaction. regulation
Regulation of cell function by intracellular signaling Objectives: Regulation principle Allosteric and covalent mechanisms, Popular second messengers, Protein kinases, Kinase cascade and interaction. regulation
Lymphoid architecture & Leukocyte recirculation. Thursday Jan 26th, 2017
 Lymphoid architecture & Leukocyte recirculation Thursday Jan 26th, 2017 Topics The life of immune cells Where are they born? Where are they educated? Where do they function? How do they get there? The
Lymphoid architecture & Leukocyte recirculation Thursday Jan 26th, 2017 Topics The life of immune cells Where are they born? Where are they educated? Where do they function? How do they get there? The
BCOR 011 Lecture 19 Oct 12, 2005 I. Cell Communication Signal Transduction Chapter 11
 BCOR 011 Lecture 19 Oct 12, 2005 I. Cell Communication Signal Transduction Chapter 11 External signal is received and converted to another form to elicit a response 1 Lecture Outline 1. Types of intercellular
BCOR 011 Lecture 19 Oct 12, 2005 I. Cell Communication Signal Transduction Chapter 11 External signal is received and converted to another form to elicit a response 1 Lecture Outline 1. Types of intercellular
Chapter 2 (pages 22 33): Cells and Tissues of the Immune System. Prepared by Kristen Dazy, MD, Scripps Clinic Medical Group
 Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS; Andrew H. H. Lichtman, MD, PhD; and Shiv Pillai, MBBS, PhD. Chapter 2 (pages 22 33): Cells and
Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS; Andrew H. H. Lichtman, MD, PhD; and Shiv Pillai, MBBS, PhD. Chapter 2 (pages 22 33): Cells and
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Receptors Families. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
The elements of G protein-coupled receptor systems
 The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression
 Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Lecture Outline. Hormones & Chemical Signaling. Communication Basics: Overview. Communication Basics: Methods. Four methods of cell communication
 Lecture Outline Hormones & Chemical Signaling Communication Basics Communication Overview Communication Methods Signal pathways Regulation (modulation) of signal pathways Homeostasis... again Endocrine
Lecture Outline Hormones & Chemical Signaling Communication Basics Communication Overview Communication Methods Signal pathways Regulation (modulation) of signal pathways Homeostasis... again Endocrine
Signaling Through Immune System Receptors (Ch. 7)
 Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Model Answer. M.Sc. Zoology (First Semester) Examination Paper LZT 103 (Endocrinology)
 Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Signal Transduction: Information Metabolism. Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire
 Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Cell signaling. How do cells receive and respond to signals from their surroundings?
 Cell signaling How do cells receive and respond to signals from their surroundings? Prokaryotes and unicellular eukaryotes are largely independent and autonomous. In multicellular organisms there is a
Cell signaling How do cells receive and respond to signals from their surroundings? Prokaryotes and unicellular eukaryotes are largely independent and autonomous. In multicellular organisms there is a
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cell-Derived Inflammatory Mediators
 Cell-Derived Inflammatory Mediators Introduction about chemical mediators in inflammation Mediators may be Cellular mediators cell-produced or cell-secreted derived from circulating inactive precursors,
Cell-Derived Inflammatory Mediators Introduction about chemical mediators in inflammation Mediators may be Cellular mediators cell-produced or cell-secreted derived from circulating inactive precursors,
T Cell Effector Mechanisms I: B cell Help & DTH
 T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
Chapter 11. Cell Communication
 Chapter 11 Cell Communication Overview: The Cellular Internet Cell-to-cell communication Is absolutely essential for multicellular organisms Concept 11.1: External signals are converted into responses
Chapter 11 Cell Communication Overview: The Cellular Internet Cell-to-cell communication Is absolutely essential for multicellular organisms Concept 11.1: External signals are converted into responses
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Communication. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas
 Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Computational Systems Biology: Biology X
 Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#10:(November-22-2010) Cancer and Signals 1 1 Micro-Environment Story How does the rest of our body influences the cancer cell?
Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#10:(November-22-2010) Cancer and Signals 1 1 Micro-Environment Story How does the rest of our body influences the cancer cell?
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Newly Recognized Components of the Innate Immune System
 Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Cell Motility. Junfeng Ji ( 纪俊峰 ), PhD
 Cell Motility Junfeng Ji ( 纪俊峰 ), PhD Professor Laboratory of Somatic Reprogramming and Stem Cell Aging Center for Stem Cell and Tissue Engineering School of Medicine Zhejiang University Email: jijunfeng@zju.edu.cnedu
Cell Motility Junfeng Ji ( 纪俊峰 ), PhD Professor Laboratory of Somatic Reprogramming and Stem Cell Aging Center for Stem Cell and Tissue Engineering School of Medicine Zhejiang University Email: jijunfeng@zju.edu.cnedu
Cell Communication. Chapter 11. PowerPoint Lectures for Biology, Seventh Edition. Lectures by Chris Romero. Neil Campbell and Jane Reece
 Chapter 11 Cell Communication PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: The Cellular Internet Cell-to-cell communication Is absolutely
Chapter 11 Cell Communication PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: The Cellular Internet Cell-to-cell communication Is absolutely
Principles of cell signaling Lecture 4
 Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Introduction! Introduction! Introduction! Chem Lecture 10 Signal Transduction & Sensory Systems Part 2
 Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
10. Which of the following immune cell is unable to phagocytose (a) neutrophils (b) eosinophils (c) macrophages (d) T-cells (e) monocytes
 Chapter 2. Acute and chronic inflammation(6): 1. In acute inflammation, which events occur in the correct chronological order? (Remembered from 2000, 2004 exam.) p50 (a) transient vasoconstriction, stasis
Chapter 2. Acute and chronic inflammation(6): 1. In acute inflammation, which events occur in the correct chronological order? (Remembered from 2000, 2004 exam.) p50 (a) transient vasoconstriction, stasis
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
A novel role for reactive oxygen species in the regulation of RhoA; implications for endothelial permeability and leukocyte transmigration.
 A novel role for reactive oxygen species in the regulation of RhoA; implications for endothelial permeability and leukocyte transmigration. Amir Aghajanian A dissertation submitted to the faculty of the
A novel role for reactive oxygen species in the regulation of RhoA; implications for endothelial permeability and leukocyte transmigration. Amir Aghajanian A dissertation submitted to the faculty of the
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
The Tissue Engineer s Toolkit
 The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
Disease causing organisms Resistance Immunity
 Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Cell Communication. Chapter 11. Key Concepts in Chapter 11. Cellular Messaging. Cell-to-cell communication is essential for multicellular organisms
 Chapter 11 Cell Communication Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 11 1. External signals are converted to responses within the cell. 2. Reception: A signaling
Chapter 11 Cell Communication Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 11 1. External signals are converted to responses within the cell. 2. Reception: A signaling
Receptors and Drug Action. Dr. Subasini Pharmacology Department Ishik University, Erbil
 Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Cellular Messengers. Intracellular Communication
 Cellular Messengers Intracellular Communication Most common cellular communication is done through extracellular chemical messengers: Ligands Specific in function 1. Paracrines Local messengers (neighboring
Cellular Messengers Intracellular Communication Most common cellular communication is done through extracellular chemical messengers: Ligands Specific in function 1. Paracrines Local messengers (neighboring
CMB621: Cytoskeleton. Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved
 CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Lecture 36: Review of membrane function
 Chem*3560 Lecture 36: Review of membrane function Membrane: Lipid bilayer with embedded or associated proteins. Bilayers: 40-70% neutral phospholipid 10-20% negative phospholipid 10-30% cholesterol 10-30%
Chem*3560 Lecture 36: Review of membrane function Membrane: Lipid bilayer with embedded or associated proteins. Bilayers: 40-70% neutral phospholipid 10-20% negative phospholipid 10-30% cholesterol 10-30%
Generation of the Immune Response
 Generation of the Immune Response Sheet 18 immunity I only added extra notes that were explained in the lecture, refer back to the slides. SLIDE 3: In the generation of Immune response whether by B or
Generation of the Immune Response Sheet 18 immunity I only added extra notes that were explained in the lecture, refer back to the slides. SLIDE 3: In the generation of Immune response whether by B or
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Signal-Transduction Cascades - 2. The Phosphoinositide Cascade
 Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
Cell Biology (BIOL 4374 and BCHS 4313) Third Exam 4/24/01
 Cell Biology (BIOL 4374 and BCHS 4313) Third Exam 4/24/01 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. For multiple choice questions,
Cell Biology (BIOL 4374 and BCHS 4313) Third Exam 4/24/01 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. For multiple choice questions,
ANATOMY & PHYSIOLOGY - CLUTCH CH. 6 - CELL COMMUNICATION.
 !! www.clutchprep.com CONCEPT: CELL-TO-CELL CONNECTIONS AND SIGNALING Gap and Tight Junctions: Adjacent cells communicate and hold on to each other via junctions. Two important kinds: Gap Junctions are
!! www.clutchprep.com CONCEPT: CELL-TO-CELL CONNECTIONS AND SIGNALING Gap and Tight Junctions: Adjacent cells communicate and hold on to each other via junctions. Two important kinds: Gap Junctions are
MACROPHAGE "MONOCYTES" SURFACE RECEPTORS
 LECTURE: 13 Title: MACROPHAGE "MONOCYTES" SURFACE RECEPTORS LEARNING OBJECTIVES: The student should be able to: Describe the blood monocytes (size, and shape of nucleus). Enumerate some of the monocytes
LECTURE: 13 Title: MACROPHAGE "MONOCYTES" SURFACE RECEPTORS LEARNING OBJECTIVES: The student should be able to: Describe the blood monocytes (size, and shape of nucleus). Enumerate some of the monocytes
Revision. camp pathway
 االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease
 Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
Molecular Cell Biology 5068 In Class Exam 1 September 29, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
General Principles of Endocrine Physiology
 General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
Cellular Physiology (PHSI3009) Contents:
 Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
1. Activated receptor tyrosine kinases (RTKs) phosphorylates themselves
 Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Biochemie 4. Cell communication - GPCR
 Biochemie 4 Cell communication - GPCR 1 Lecture outline General principles - local and long-distance signaling - classes of receptors - molecular switches and second messengers Receptor tyrosine kinases
Biochemie 4 Cell communication - GPCR 1 Lecture outline General principles - local and long-distance signaling - classes of receptors - molecular switches and second messengers Receptor tyrosine kinases
Mechanisms of Hormone Action
 Mechanisms of Hormone Action General principles: 1. Signals act over different ranges. 2. Signals have different chemical natures. 3. The same signal can induce a different response in different cells.
Mechanisms of Hormone Action General principles: 1. Signals act over different ranges. 2. Signals have different chemical natures. 3. The same signal can induce a different response in different cells.
LECTURE OUTLINE: CTP (Connective Tissues Proper) (Ordinary Connective Tissues)
 LECTURE OUTLINE: CTP (Connective Tissues Proper) (Ordinary Connective Tissues) General Definition: Tissues composed of cells embedded in an extracellular (intercellular) matrix, consisting of ground substance
LECTURE OUTLINE: CTP (Connective Tissues Proper) (Ordinary Connective Tissues) General Definition: Tissues composed of cells embedded in an extracellular (intercellular) matrix, consisting of ground substance
