DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis
|
|
|
- Sheena Randall
- 5 years ago
- Views:
Transcription
1 DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis Heiko Härtel*, Peter Dörmann, and Christoph Benning Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI Communicated by Jan A. D. Zeevaart, Michigan State University, East Lansing, MI, July 10, 2000 (received for review February 8, 2000) The galactolipids, mono- and digalactosyldiacylglycerol (DGDG), are the most common nonphosphorous lipids in the biosphere and account for 80% of the membrane lipids found in green plant tissues. These lipids are major constituents of photosynthetic membranes (thylakoids), and a large body of evidence suggests that galactolipids are associated primarily with plastid membranes in seed plants. A null-mutant of Arabidopsis (dgd1), which lacks the DGDG synthase (DGD1) resulting in a 90% reduction in the amount of DGDG under normal growth conditions, accumulated DGDG after phosphate deprivation up to 60% of the amount present in the wild type. This observation suggests the existence of a DGD1- independent pathway of galactolipid biosynthesis. The fatty acid composition of the newly formed DGDG was distinct, showing an enrichment of 16-carbon fatty acids in the C-1 position of the glycerol backbone of DGDG. Roots with their rudimentary plastids accumulated large amounts of DGDG after phosphate deprivation, suggesting that this galactolipid may be located in extraplastidic membranes. Corroborating evidence for this hypothesis was obtained directly by fractionation of subcellular membranes from leaf tissue and indirectly by lipid analysis of the phosphate-deprived fad3 mutant primarily deficient in extraplastidic fatty acid desaturation. The discovery of extraplastidic DGDG biosynthesis induced by phosphate deprivation has revealed a biochemical mechanism for plants to conserve phosphate. Apparently, plants replace phospholipids with nonphosphorous galactolipids if environmental conditions such as phosphate deprivation require this for survival. One of the most powerful environmental stimuli affecting the overall glycerolipid composition of bacterial membranes is phosphate deprivation. The replacement of phospholipids by nonphosphorous lipids was discovered first in nonphotosynthetic bacteria (1) and later in those capable of photosynthesis (2, 3). Mutants of different photosynthetic bacteria lacking the acidic nonphosphorus sulfolipid sulfoquinovosyldiacylglycerol showed impaired growth only under phosphate-limited growth conditions (2, 4). This led to the hypothesis that sulfolipid can replace the acidic thylakoid phospholipid phosphatidylglycerol under these growth conditions (5). An inverse relationship between sulfolipid and phosphatidylglycerol as a function of phosphate availability was also observed for Arabidopsis (6). Experiments with the phosphate-deficient pho1 mutant of Arabidopsis (7) suggested that not only sulfolipid but also the amounts of digalactosyldiacylglycerol are increased after phosphate deprivation (8). Thus, it seemed reasonable to ask whether the substitution of phospholipids by nonphosphorous lipids is a more general phenomenon in plants. The nonphosphorous galactolipids mono- and digalactosyldiacylgycerol (MGDG and DGDG) constitute the bulk of membrane lipids in green tissues of seed plants where they are known to be located in the photosynthetic membranes (thylakoids) of the chloroplasts (9, 10). In many plants, including Arabidopsis, two pathways exist for the biosynthesis of these lipids, one involving only enzymes located in the plastid (Fig. 1 I), the other also requiring enzymes associated with the endoplasmic reticulum (ER) (Fig. 1 II). The plastid pathway gives rise to thylakoid lipids with 16-carbon fatty acids in the carbon-2 (C-2) position of the glycerol backbone, whereas lipids derived from the ER pathway contain 18-carbon fatty acids in this position (11, 12). Therefore, fatty acid analysis of thylakoid lipids provides a convenient tool to estimate the contribution of each pathway. In addition, the availability of mutants of Arabidopsis has proven very useful in the dissection of the different lipid biosynthetic pathways in plants. For example, the act1 mutant is deficient in the plastid glycerol-3-phosphate:acyl-acp acyltransferase (Fig. 1), and as a consequence the plastidic pathway (Fig. 1 I) is impaired and 16-carbon fatty acid-containing lipids are decreased (13). The mgd1 mutant of Arabidopsis deficient in MGDG synthase activity revealed that MGD1 is responsible for the bulk of MGDG biosynthesis (14), whereas the dgd1 mutant phenotype suggested that the DGD1 gene product (Fig. 1) is responsible for over 90% of DGDG biosynthesis (15). Although the dgd1 mutant carries a stop codon in the center of the ORF and is presumably a null allele, residual DGDG is still present (16). We used this mutant as a starting point to search for a DGD1-independent pathway of galactolipid biosynthesis, which may be up-regulated after phosphate deprivation of the plant. Materials and Methods Plant Material. Surface-sterilized seeds of Arabidopsis (A. thaliana, ecotype Col-2) and the multiple-times-backcrossed act1 (13), dgd1 (15), fad3 (17), and pho1 mutants (7), were germinated on 0.8% (wt vol) agar-solidified MS medium (18) supplemented with 1% (wt vol) sucrose. The seedlings were kept on agar for 10 days before transfer to pots containing a soil mixture, as described (15). Plants were grown in growth chambers (AR- 75L, Percival Scientific, Boone, IA) under the light of a photosynthetic photon flux density ( nm) of 75 mol photons m 2 s 1. A 14-h light 10-h dark regime was applied. The day night temperature was controlled at C. All biochemical analyses were performed with leaves harvested at the rosette stage (6-week-old plants). For phosphate limitation experiments, sterile Arabidopsis medium (19) was used but at half concentration and containing 0.8% agarose, 1% sucrose, 20 mm Mes (ph 6.0), and different concentrations of KH 2 PO 4 as indicated. Plants were grown for 10 days on 0.8% (wt vol) agar-solidified MS medium supplemented with 1% (wt vol) Abbreviations: MGDG, monogalactosyldiacylgycerol; DGDG, digalactosyldiacylgycerol; ER, endoplasmic reticulum; C-2, carbon-2; PE, phosphatidylethanolamine; PC, phosphatidylcholine. *Present address: BASF Plant Science L.L.C., 26 Davis Drive, Research Triangle Park, NC Present address: Max-Planck-Institut für Molekulare Pflanzenphysiologie, Am Mühlenberg 1, D Golm, Germany. To whom reprint requests should be addressed. benning@pilot.msu.edu. The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C solely to indicate this fact. Article published online before print: Proc. Natl. Acad. Sci. USA, pnas Article and publication date are at cgi doi pnas PLANT BIOLOGY PNAS September 12, 2000 vol. 97 no
2 Subcellular Fractionation. Because dgd1 mutant thylakoids are highly unstable in vitro (23), we devised a simple and rapid protocol for the subfractionation of cell membranes and subsequent lipid analysis to preclude artifacts. Leaves (approximately 0.5 g fresh weight) were harvested directly into liquid nitrogen, stored at 70 C, and homogenized by grinding under liquid nitrogen by using a precooled mortar and pestle in cold buffer (0.35 M sorbitol 25 mm Hepes-KOH, ph mm EDTA 0.2% fat-free BSA). The homogenates were filtered through two layers of wet miracloth (Calbiochem) and centrifuged at 800 g for 1 min. The supernatant was transferred to a new tube. The pellets were gently resuspended in a small volume of cold wash buffer (10 mm Hepes-KOH, ph M sorbitol 10 mm EDTA) and centrifuged at 800 g for 1 min. The second supernatant was combined with the first in a glass tube. The pellet fraction containing mostly chloroplast membranes was immediately resuspended in 200 l chloroform methanol formic acid (10:10:1, vol vol vol), followed by the addition of 100 l 0.2MH 3 PO 4 and 1 M KCl. The combined supernatant fraction was centrifuged at 6,000 g for 6 min to remove the chloroplast membranes further. The supernatant was transferred to a new glass tube, and lipids were extracted immediately by addition of two parts of chloroform methanol formic acid (10:10:1, vol vol vol). All steps were carried out at 4 C, and the time between homogenization and lipid extraction was kept to less than 10 min. Lipids were recovered in the chloroform phase, concentrated under a stream of N 2, and lipid analysis was performed as described above. Fig. 1. Schematic representation of the three pathways of galactolipid biosynthesis discussed in the text. The glycerol backbones with the typical carbon length of fatty acids in the C-1 and C-2 positions are indicated to illustrate the main molecular species derived from each pathway. The head groups are always at the glycerol C-3 position. Three pools of galactolipids are indicated, representing the plastid-derived pool (I) in the plastid, the ERderived pool in the plastid (II), and the ER-derived pool located in extraplastidic membranes (III). Pool III accumulates after phosphate deprivation. Reactions that are blocked in the act1 and dgd1 mutants are indicated. Broken lines separate plastid, cytosol, and ER. DAG, diacylglycerol; FAS, fatty acid synthase; Gal, galactose; PC, phosphatidylcholine; PCho, phosphocholine; PA, phosphatidic acid. sucrose and then transferred to agar plates containing the phosphate-limited medium on which they continued to grow for another 10 days. Phosphate Analysis. Inorganic phosphate in leaf and root tissues was determined by the method of Ames (20). Roots were washed at least two times with water and stored at 70 C before analysis. Lipid and Fatty Acid Analysis. Harvested leaves were frozen immediately in liquid nitrogen, and lipids were extracted as previously described (15). Lipid extracts were analyzed on activated ammonium sulfate-impregnated silica gel TLC plates by using a solvent system of acetone toluene water (91:30:7, vol vol vol). Lipids were visualized with iodine vapor and identified by cochromatography with lipid extracts of known composition. For quantitative analysis, individual lipids were isolated from TLC plates and used to prepare fatty acid methyl esters. The methyl esters were quantified by GLC by using myristic acid as internal standard (15). The fatty acid composition at the C-1 and C-2 positions of the glycerol backbone was determined by lipase digestion according to Siebertz and Heinz (21), with modifications as described by Miquel et al. (22). Fatty acid values were corrected for the fatty acids present in the lipase preparation (EC , Rhizopus sp., Sigma). Results Partial Suppression of the dgd1 Lipid Phenotype in a pho1 Mutant Background. To investigate the possibility of DGD1-independent galactolipid biosynthesis in response to phosphate deprivation, we crossed the DGDG-deficient dgd1 mutant and the phosphatedeprived pho1 mutant of Arabidopsis. As expected from the recessive mutations, the F 1 progeny showed wild-type lipid composition. Of 198 F 2 plants analyzed for altered lipid composition, 44 plants were similar in phenotype to dgd1 and 39 plants to pho1, but no putative double mutants were recovered. Because both mutations are located on chromosome 3 within less than 10 cm, double mutants were expected to be underrepresented. To recover the double mutant, we selected 15 homozygous dgd1 F 2 plants, a few of which we expected to be heterozygous for pho1, and screened 54 F 3 descendants each for decreased phospholipid amounts that are typical for homozygous pho1 plants. Among these 15 F 2 lines, three were found that segregated approximately 25% F 3 plants with lowered amounts of phospholipids. These were assumed to be the dgd1 pho1 homozygous plants. In addition, the amount of inorganic phosphate per fresh weight was found to be reduced to about 10% and 12% compared with wild type and dgd1, respectively, as would be expected for a homozygous pho1 mutant. These homozygous dgd1 pho1 mutants were much more stunted than either parent (Fig. 2) but were viable on soil and produced flowers and seeds. Bolting lagged behind by 5 7 days as compared with wild type. The lipid compositions of the double mutant, the single mutants, and the wild type are shown in Fig. 2. Although the homozygous dgd1 mutant contained less than 10% of wild-type amounts of DGDG, the dgd1 pho1 double mutant showed an increase in the relative amount of DGDG to 60% of wild-type levels. In the pho1 mutant, DGDG accumulated to approximately 160% of wild-type amounts. Phospholipids generally decreased in the pho1 mutant lines, whereas sulfolipid increased as observed previously (6, 8). The precursor of DGDG, MGDG, was present at similar levels in all four tested lines. Although the introduction of the pho1 mutation into the dgd1 mutant background caused an increase in DGDG content to approximately 60% of the amount found in the wild type, none of the Härtel et al.
3 photosynthetic parameters known to be altered in the dgd1 mutant (23 25) was restored (data not shown). Because partial restoration of photosynthetic defects after the ectopic expression of DGD1 in the dgd1 mutant, which resulted in intermediate amounts of DGDG, was observed (data not shown), we concluded that the DGDG formed after phosphate deprivation was either different in structure or was not as accessible to the components of the photosynthetic membrane as the DGDG derived from DGD1-mediated biosynthesis. of saturation of each fatty acid bound in galactolipids constitutes an additional level of complexity, the most crucial information with regard to the origin of galactolipids can be deduced from the ratio of 16- to 18-carbon fatty acids and their relative position on the glycerol backbone (Fig. 3). The ratio of total 16- to 18-carbon fatty acids decreased in MGDG in the two dgd1 mutant lines, indicating a larger contribution of the ER pathway to MGDG biosynthesis (Fig. 1 II). This assumption was also confirmed by positional analysis, showing an increase in 18-carbon fatty acids in the glycerol C-2 position of MGDG in the dgd1 mutants. By contrast, the ratio of total 16- to 18-carbon fatty acids in DGDG increased in pho1 and to a larger extent in the two dgd1 mutant lines (Fig. 3 Upper). Being aware of only the plastidic and ER pathways (Fig. 1 I and II) and without further positional analysis, one would conclude that the plastid pathway gives rise to DGDG in the two dgd1 mutant lines. However, positional analysis revealed that the 16-carbon fatty acids are virtually excluded from the C-2 position of the glycerol backbone of DGDG found in plants with the dgd1 mutant background. This result suggested a DGDG structure different from that of galactolipids normally derived from the plastid pathway. These are characterized by the presence of 16-carbon fatty acids in the glycerol C-2 position (Fig. 1 I). Corroborating independent evidence that DGDG biosynthesis under phosphate deprivation must be caused by a pathway other than the plastid pathway (Fig. 1 I) was provided by our analysis of lipids of the act1 mutant grown under phosphatelimited growth conditions (data not shown). The act1 mutation effectively blocks the plastid pathway of galactolipid biosynthesis (13), yet the act1 mutant showed the same increase in DGDG content under phosphate-limited growth conditions as was observed for the pho1 mutant line. DGDG Formed After Phosphate Deprivation Is Not Derived from the Plastid Pathway. A detailed fatty acid compositional analysis of DGDG Accumulates in Leaves and Roots with Decreasing Phosphate Availability. To verify independently that the changes in the lipid Fig. 2. Appearance (Top) and lipid composition (Bottom) of Arabidopsis homozygous wild type and pho1, dgd1, and dgd1兾pho1 mutant lines grown for 6 weeks on soil. Lipids were separated on activated ammonium sulfateimpregnated silica gel TLC plates and visualized with iodine vapor. The lipids were identified by cochromatography with lipid extracts of known composition. In addition, glycolipids were verified by specific staining with -naphthol reagent. The numbers at the bands indicate the relative proportion (mol%) of the respective lipids. Abbreviations not mentioned in the legend to Fig. 1. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; SQDG, sulfoquinovosyldiacylglycerol. galactolipid molecular species can provide clues regarding their biosynthetic origin, because different molecular species are predominant in galactolipids derived from the plastid or the ER pathways as summarized in Fig. 1 I and II. Although the degree Ha rtel et al. pattern in pho1 and dgd1兾pho1 were solely because of phosphate depletion and not secondary genetic defects in the pho1 mutant background, wild type and dgd1 mutant were grown on agarsolidified medium containing different concentrations of phospnas 兩 September 12, 2000 兩 vol. 97 兩 no. 19 兩 PLANT BIOLOGY Fig. 3. Fatty acid composition of MGDG and DGDG in the wild-type and pho1, dgd1, and dgd1兾pho1 mutant lines. (Upper) Ratio of total 16- to 18-carbon fatty acids in each lipid. (Lower) Relative abundance (mol%) of 16(open bars) and 18-carbon fatty acids (closed bars) at the glycerol C-2 position of MGDG and DGDG. The proportion of fatty acids (FA) in the C-2 position of the glycerol backbone was determined from the lyso derivatives after digestion of the lipids with Rhizopus sp. lipase. Values represent the means of at least three measurements. The standard error is indicated.
4 Table 1. Enrichment of DGDG in the extraplastidic membrane fraction Plant Pi Fraction DGDG MGDG PE D M D P WT Pellet SN Pellet SN dgd1 Pellet SN Pellet SN Lipids were isolated from different subcellular fractions of wild type and dgd1 mutant grown in medium with 1 mm or no added phosphate (Pi). The mean of three independent lipid measurements is given in mol%, and standard errors were 15% for values 10 or 30% for values 10. D M, ratio of DGDG MGDG; D P, ratio of DGDG PE; SN, supernatant; WT, wild type. Fig. 4. Changes in the total amounts of inorganic phosphate (A and B) and relative amounts of DGDG (C and D) in leaves (A and C) and roots (B and D)of wild-type and the dgd1 mutant of Arabidopsis with decreasing phosphate concentration in the nutrient medium. Open symbols denote wild-type and closed symbols dgd1 mutant values. The means of at least four measurements are shown. phate. At the same time, we were able to examine the lipid composition in roots, a nonphotosynthetic tissue with a different ratio of plastidic to extraplastidic membranes than leaves. As the content of inorganic phosphate decreased in entire plants (Fig. 4 A and B), the relative amount of DGDG increased in both leaves and roots (Fig. 4 C and D). Although in leaves of wild type and dgd1 mutant the DGDG content increased in parallel starting from different values, the same change in DGDG content with an increase of up to 10-fold was observed for roots of the wild type and the dgd1 mutant after phosphate deprivation. In roots that are well supplied with phosphate, DGDG was usually present in trace amounts. However, it accounted for 15% of all of the lipids in roots when plants were grown for 10 days in the absence of phosphate. Rudimentary plastids are typically present in roots and represent only a minor fraction of cell membranes in nongreen tissues. Thus, the discovery of a large amount of DGDG in phosphate-deprived roots immediately suggested that this newly formed galactolipid may be associated with extraplastidic membranes. Extraplastidic Membranes Are Enriched in DGDG After Phosphate Deprivation. The accumulation of large amounts of DGDG in nonphotosynthetic tissues prompted us to fractionate plastidic and extraplastidic membranes from leaves of phosphatedeprived plants to determine their lipid composition. To avoid artifacts caused by degradation of lipids in crude membrane fractions, which we previously observed to be particularly severe for dgd1 samples (23), a simple and fast procedure based on differential centrifugation was designed. Among different centrifugation regimes tested, a maximum of chloroplast membranes revealing very low contamination with extraplastidic membranes was present in the pellet fraction after a low-speed centrifugation (800 g). As a marker for extraplastidic membranes, we used phosphatidylethanolamine (PE), which is known to be excluded from plastid membranes (26). To evaluate the contamination of extraplastidic membranes with chloroplast membranes, most likely chloroplast envelopes, we also included MGDG in the analysis, which was present in constant amounts during the different phosphate treatments (Fig. 2). The chosen experimental approach and a combination of both, MGDG and PE, as markers provided a clear result with regard to DGDG localization as shown in Table 1. Very little PE is associated with the chloroplast pellet fraction, but its relative amount in the supernatant fraction is high. The reverse distribution is found for MGDG, although it is not as stringent as for PE. This may be a result of the presence of chloroplast envelope membranes containing MGDG in the supernatant. Comparing the relative amounts of DGDG to those of MGDG and PE, it is apparent that DGDG is highly enriched after phosphate deprivation in the supernatant, which represents primarily the extraplastidic membrane fraction. The relative proportion of DGDG in the supernatant fraction increased from 4.5% to 31.1% after phosphate deprivation in the wild-type and from 1.3% to 18.1% in the dgd1 mutant. This enrichment is even more drastically reflected in the ratios of DGDG-to-MGDG and DGDG-to-PE (Table 1). To obtain independent corroborating evidence for the presence of DGDG in extraplastidic membranes after phosphate deprivation, we took advantage of the fad3 mutant of Arabidopsis (17). This mutant is deficient in the 18:2 fatty acid desaturase associated with the ER resulting in increased amounts of 18:2 and decreased amounts of 18:3 fatty acids. Most importantly, the mutation primarily affects the fatty acid composition of lipids in extraplastidic membranes. Because 18:2 containing diacylglycerol moieties are thought to return from the ER to the plastid, where they are further desaturated by plastidic 18:2 fatty acid desaturases (27), chloroplast membrane lipids derived from the ER pathway are not affected by a mutation in the ER 18:2 fatty acid desaturase gene fad3. Thus we anticipated that the DGDG formed after phosphate deprivation in the fad3 mutant should show a strong increase in the 18:2- to 18:3-fatty acid ratio only if it is located in extraplastidic membranes. This effect should be particularly obvious in roots that are most enriched in DGDG after phosphate deprivation (Fig. 3) and that contain primarily extraplastidic membranes. As shown in Table 2, there is a strong effect in accordance with the fad3 desaturase defect on the fatty acid composition of DGDG (reduction of 18:3 and increase in 18:2 fatty acids) in phosphate-deprived roots of the fad3 mutant. On the other hand, there is virtually no effect of the fad3 mutation on DGDG in phosphate-sufficient roots. The overall increase in the amount of DGDG in roots was the same for wild-type and fad3 mutant. These results are in agreement with an extraplastidic location of a large fraction of DGDG synthesized in response to phosphate deprivation, but a predominant plastid association of DGDG when phosphate is plentiful in the medium. Discussion Phosphate Deficiency Induces an ACT1 DGD1-Independent Pathway for Galactolipid Biosynthesis. One of the most powerful environmental stimuli affecting the complex lipid composition of plant Härtel et al.
5 Table 2. Total amount of DGDG and degree of saturation of 18-carbon fatty acids in DGDG in roots of wild type and fad3 mutant grown in the presence ( ) and absence ( ) of 1 mm phosphate (Pi) Fatty acid Plant Pi DGDG WT fad The mean of three experiments (mol% of total fatty acids) standard error is shown. WT, wild type. membranes is a limitation in the supply of inorganic phosphate. Enticed by our previous findings of a reduction in phospholipid content and a concomitant increase in nonphosphorus lipids in Arabidopsis (6, 8), we initiated a study to understand the underlying regulatory and biochemical principles. The model plant Arabidopsis provides a rich source for mutants with defects in different aspects of metabolism, and we attempted to take full advantage of the availability of these mutants to address this problem by using a combination of genetical and biochemical approaches. To apply constant phosphate deprivation to plants, we used the pho1 mutation (7) and to deconvolute the complex pathways of galactolipid biosynthesis, we took advantage of the dgd1 mutation (15). Combining both mutations into one line led to the discovery that pho1 apparently restores the biosynthesis of DGDG in the dgd1 genetic background. This is a surprising result because the dgd1 mutation is characterized by a stop codon in the coding region of DGD1 leading to a 90% reduction in DGDG content (16). This newly formed DGDG was identified by cochromatography with authentic standards and stained identical to the DGDG commonly present in Arabidopsis. However, its fatty acid composition was different from DGDG normally found in Arabidopsis, with increased amounts of 16-carbon fatty acids in the C-1 position of the glycerol backbone (Fig. 3). The fatty acid composition is considered to be diagnostic for the origin of DGDG from either the plastid or the ER pathways (Fig. 1 I and II). Based on the exclusion of 16-carbon fatty acids from the C-2 position of glycerol, it can be inferred that this DGDG is derived from the ER pathway. This conclusion is supported further by the fact that the same molecular species of DGDG was formed in the phosphate-deprived act1 mutant (data not shown), in which the plastid pathway of galactolipid biosynthesis is disrupted (13). Therefore, phosphate deprivation induces the biosynthesis of DGDG by a DGD1 ACT1-independent pathway not previously observed, which led us to modify the pathway scheme for the biosynthesis of DGDG as shown in Fig. 1. The existence of multiple or subpools of galactolipids in addition to the commonly accepted prokaryotic (plastid-derived) and eukaryotic (ER-derived) pools (Fig. 1 I and II) has been postulated recently for different plants based on positional fatty acid analysis (28) but not in the context of phosphate-deprived plants. With regard to MGDG, we observed a strong decrease in the relative amount of 16-carbon fatty acids in the glycerol C-2 position, from 70% in the wild type to 15% in dgd1 pho1, with no detectable changes in the overall proportion of MGDG in phosphate-deprived plants (Fig. 3). This would suggest an increased contribution of the ER pathway also for this lipid and, therefore, a general redirection of galactolipid synthesis from the plastid to the ER pathway in phosphate-deprived plants. Galactolipid Accumulates in Extraplastidic Membranes on Phosphate Deprivation. There are a few reports in the literature describing the presence of galactolipids in extraplastidic membranes (29, 30). These were based solely on fractionation of subcellular membranes isolated from either protoplast or intact plant tissues. Because of questions regarding the extent of unavoidable crosscontamination of different membrane fractions during preparation, these results have been largely ignored. Here, we provide four independent indirect or direct lines of evidence, which strongly suggest that a large portion of DGDG synthesized after phosphate deprivation is found in extraplastidic membranes: first, DGDG accumulated in large amounts in phosphate-deprived roots, which typically have rudimentary plastids. Because we are not aware of any evidence indicating there is a large increase in plastid membranes relative to extraplastidic membranes in roots under these conditions, this observation suggests an extraplastidic location of at least some of the newly formed DGDG. Second, with regard to different photosynthetic parameters altered in the dgd1 mutant, DGDG present in the dgd1 mutant after phosphate deprivation could not substitute for DGDG formed through the DGD1 pathway, such as DGDG in transgenic dgd1 plants expressing a wild-type DGD1 cdna (data not shown). This was because of either the altered fatty acid composition of DGDG formed after phosphate deprivation (Fig. 3) or the extraplastidic localization of most of this DGDG. Third, fractionation of plastidic and extraplastidic membranes from phosphate-sufficient and -deficient wild-type and dgd1 plants by using MGDG and PE as markers for plastidic and extraplastidic membranes showed a strong enrichment of DGDG in the extraplastidic membrane fraction. However, it should be pointed out that there was also an increase in the relative DGDG content in the chloroplast fraction of phosphatedeprived wild-type and dgd1 plants (Table 1). This finding suggests that DGDG synthesized in phosphate-deprived plants may not be associated exclusively with extraplastidic membranes. A reason for this may be that DGDG replaces phosphatidylcholine (PC), which is associated with plastid membranes as well, but which is restricted to the outer envelope of chloroplasts (31). As a matter of fact, phosphate-sufficient plants lacking the plastid pathway contain considerable amounts of DGDG with 16- carbon fatty acids in the glycerol C-1 position, the same as observed in phosphate-deprived Arabidopsis, which is presumably located in the plastid in these plants (32). Fourth, a strong change in the ratio of 18:2- to 18:3-carbon fatty acids in DGDG of the phosphate-deprived fad3 mutant (Table 2) suggests an extraplastidic location of the lipid, because the fad3 mutation is thought to affect primarily extraplastidic lipids (17). Together, these four independent experimental strategies combining biochemical, genetical, and physiological approaches provide overwhelming evidence for the location of a major portion of the DGDG synthesized in Arabidopsis deprived of phosphate in extraplastidic membranes. Enzymes Involved in the Biosynthesis of Extraplastidic DGDG. It must be emphasized that although much of the DGDG synthesized under conditions of phosphate deprivation is located in extraplastidic membranes, the enzymes involved in its biosynthesis must not necessarily be located in extraplastidic membranes (i.e., ER associated). This DGDG could also be synthesized in the outer chloroplast envelope and subsequently transferred to other membranes. Three putative MGDG synthase genes are known PLANT BIOLOGY Härtel et al. PNAS September 12, 2000 vol. 97 no
6 for Arabidopsis (33). Only one of these genes, MGD1, encodes a protein with a clearly recognizable chloroplast-targeting sequence. Similarly, DGD2, a gene encoding a protein with high similarity to DGD1, seems to lack a chloroplast transit peptide (16). Although clear evidence is not available at this time indicating that MGD2, MGD3, and DGD2 encode enzymes involved in galactolipid biosynthesis, these genes represent candidates for the biosynthesis of extraplastidic DGDG directly in the ER membranes. Because the fatty acid composition of DGDG synthesized after phosphate deprivation is very similar to that of PC (11), it seems likely that this DGDG is derived directly from PC in the ER. However, the biosynthetic mechanism may be different from that postulated for plastid galactolipid biosynthesis, which occurs by galactosylation of diacylglycerol to give rise to MGDG and by disproportioning of two MGDG molecules resulting in DGDG and diacylgycerol (34). To Conserve Phosphate, Plants Rely Less Than Animals on Phospholipids. The DGDG formed after phosphate deprivation is located in extraplastidic membranes like PC and has a similar fatty acid composition. Both lipids are neutral and bilayer forming. Thus, it is tempting to propose that DGDG synthesized in response to phosphate deprivation substitutes for PC in similar ways as sulfolipid substitutes for phosphatidylglycerol in chloroplast membranes (6, 8). Such a mechanism, the replacement of phospholipids with nonphosphorous glycolipids to adapt to phosphate-limited growth conditions, would be important for the survival of plants, which unlike animals cannot escape unfavorable environmental conditions. One-third of organic phosphate in Arabidopsis is bound normally in phospholipids (7), and conserving this phosphate may be an important survival strategy for plants. Photosynthesis requires extensive membrane systems in the chloroplast. Their function would be compromised easily under conditions of low phosphate availability if plants would rely solely on phospholipids as their main building blocks of membranes. During evolution, plants presumably avoided this potential impasse by maintaining and evolving their capability to synthesize nonphosphorous membrane lipids. It may be this evolutionary pressure that led to the fact that today nonphosphorous galactolipids represent the most abundant lipids in the biosphere. We thank John Browse for helpful discussions. Financial support was provided by the United States Department of Energy (Grant DE-FG02 98ER20305). 1. Minnikin, D. E., Abdolrahimzadeh, H. & Baddiley, J. (1974) Nature (London) 249, Benning, C., Beatty, J. T., Prince, R. C. & Somerville, C. R. (1993) Proc. Natl. Acad. Sci. USA 90, Benning, C., Huang, Z. H. & Gage, D. A. (1995) Arch. Biochem. Biophys. 317, Güler, S., Seeliger, A., Härtel, H., Renger, G. & Benning, C. (1996) J. Biol. Chem. 271, Benning, C. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, Essigmann, B., Güler, S., Narang, R. A., Linke, D. & Benning, C. (1998) Proc. Natl. Acad. Sci. USA 95, Poirier, Y., Thoma, S., Somerville, C. & Schiefelbein, J. (1991) Plant Physiol. 97, Härtel, H., Essigmann, B., Lokstein, H., Hoffmann-Benning, S., Peters-Kottig, M. & Benning, C. (1998) Biochim. Biophys. Acta 1415, Browse, J. & Somerville, C. (1991) Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, Joyard, J., Marechal, E., Miege, C., Block, M. A., Dorne, A. J. & Douce, R. (1998) in Lipids in Photosynthesis: Structure, Function and Genetics, eds. Siegenthaler, P.-A. & Murata, N. (Kluwer, Dordrecht, The Netherlands), pp Browse, J., Warwick, N., Somerville, C. R. & Slack, C. R. (1986) Biochem. J. 235, Heinz, E. & Roughan, G. (1983) Plant Physiol. 72, Kunst, L., Browse, J. & Somerville, C. (1988) Proc. Natl. Acad. Sci. USA 85, Jarvis, P., Dörmann, P., Peto, C. A., Lutes, J., Benning, C. & Chory, J. (2000) Proc. Natl. Acad. Sci. USA 97, Dörmann, P., Hoffmann-Benning, S., Balbo, I. & Benning, C. (1995) Plant Cell 7, Dörmann, P., Balbo, I. & Benning, C. (1999) Science 284, Browse, J., McConn, M., James, D., Jr. & Miquel, M. (1993) J. Biol. Chem. 268, Murashige, T. & Skoog, F. (1962) Physiol. Plant. 15, Estelle, M. A. & Somerville, C. (1987) Mol. Gen. Genet. 206, Ames, B. N. (1966) Methods Enzymol. 8, Siebertz, H. P. & Heinz, E. (1977) Z. Naturforsch. 32c, Miquel, M., Cassagne, C. & Browse, J. (1998) Plant Physiol. 117, Härtel, H., Lokstein, H., Dörmann, P., Grimm, B. & Benning, C. (1997) Plant Physiol. 115, Härtel, H., Lokstein, H., Dörmann, P., Trethewey, R. N. & Benning, C. (1998) Plant Physiol. Biochem. 36, Reifarth, F., Christen, G., Seeliger, A. G., Dörmann, P., Benning, C. & Renger, G. (1997) Biochemistry 36, Marechal, E., Block, M. A., Dorne, A.-J. & Joyard, J. (1997) Physiol. Plant. 100, Roughan, P. G. & Slack, C. R. (1982) Annu. Rev. Plant Physiol. 33, Williams, J. P. & Kahn, M. U. (1996) Plant Physiol. Biochem. 34, Kogel, K. H., Ehrlich-Rogzinski, S., Reisner, H. J. & Sharon, N. (1984) Plant Physiol. 76, Yoshida, S. & Uemura, M. (1986) Plant Physiol. 82, Dorne, A.-J., Joyard, J. & Douce, R. (1990) Proc. Natl. Acad. Sci. USA 87, Douce, R. & Joyard, J. (1980) in The Biochemistry of Plants, ed. Stumpf, P. K. (Academic, New York), Vol. 4, pp Miege, C., Marechal, E., Shimojima, M., Awai, K., Block, M. A., Ohta, H., Takamiya, K., Douce, R. & Joyard, J. (1999) Eur. J. Biochem. 265, Heemskerk, J. W. M., Schmidt, H., Hammer, U. & Heinz, E. (1991) Plant Physiol. 96, Härtel et al.
YANG Wen, FENG Fu-Ying, HOU Hai-Tong, JIANG Gui-Zhen, XU Yi-Nong *, KUANG Ting-Yun
 Acta Botanica Sinica 2004, 46 (2): 211 215 http://www.chineseplantscience.com Alternation in Lipid Composition of Wheat Leaves Induced by Phosphate Deficiency Is Related to Both Lipid Biosynthesis and
Acta Botanica Sinica 2004, 46 (2): 211 215 http://www.chineseplantscience.com Alternation in Lipid Composition of Wheat Leaves Induced by Phosphate Deficiency Is Related to Both Lipid Biosynthesis and
acyltransferase activity
 Proc. Natl. Acad. Sci. USA Vol. 85, pp. 4143-4147, June 1988 Biochemistry Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase
Proc. Natl. Acad. Sci. USA Vol. 85, pp. 4143-4147, June 1988 Biochemistry Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase
A mutant in Arabidopsis Lacking a Chloroplast Specific Lipid. Lewis Kurschner and Karen Thulasi Masters in Botany
 A mutant in Arabidopsis Lacking a Chloroplast Specific Lipid Lewis Kurschner and Karen Thulasi Masters in Botany Fatty acid nomenclature Fatty acyl composition Chain length Degree of unsaturation and position
A mutant in Arabidopsis Lacking a Chloroplast Specific Lipid Lewis Kurschner and Karen Thulasi Masters in Botany Fatty acid nomenclature Fatty acyl composition Chain length Degree of unsaturation and position
Fatty Acid Desaturation
 Fatty Acid Desaturation Objectives: 1. Isolation of desaturase mutants 2. Substrates for fatty acid desaturation 3. ellular localization of desaturases References: Buchanan et al. 2000. Biochemistry and
Fatty Acid Desaturation Objectives: 1. Isolation of desaturase mutants 2. Substrates for fatty acid desaturation 3. ellular localization of desaturases References: Buchanan et al. 2000. Biochemistry and
Probing Arabidopsis Chloroplast Diacylglycerol Pools by Selectively Targeting Bacterial Diacylglycerol Kinase to Suborganellar Membranes
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biochemistry -- Faculty Publications Biochemistry, Department of 2013 Probing Arabidopsis Chloroplast Diacylglycerol Pools
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biochemistry -- Faculty Publications Biochemistry, Department of 2013 Probing Arabidopsis Chloroplast Diacylglycerol Pools
The photosynthetic membranes of seed plants are rich in
 Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth Bin Yu, Changcheng Xu, and Christoph Benning* Department of Biochemistry and Molecular Biology, Michigan
Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth Bin Yu, Changcheng Xu, and Christoph Benning* Department of Biochemistry and Molecular Biology, Michigan
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Disruption of the FATB Gene in Arabidopsis Demonstrates an Essential Role of Saturated Fatty Acids in Plant Growth
 The Plant Cell, Vol. 15, 1020 1033, April 2003, www.plantcell.org 2003 American Society of Plant Biologists Disruption of the FATB Gene in Arabidopsis Demonstrates an Essential Role of Saturated Fatty
The Plant Cell, Vol. 15, 1020 1033, April 2003, www.plantcell.org 2003 American Society of Plant Biologists Disruption of the FATB Gene in Arabidopsis Demonstrates an Essential Role of Saturated Fatty
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
BERND ESSIGMANN*, SINAN GÜLER, RAM AVTAR NARANG, DIRK LINKE, AND CHRISTOPH BENNING* MATERIALS AND METHODS
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 1950 1955, February 1998 Plant Biology Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 1950 1955, February 1998 Plant Biology Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid
Lipid Trafficking between the Endoplasmic Reticulum and the Plastid in Arabidopsis Requires the Extraplastidic TGD4 Protein W
 This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
Oleate accumulation, induced by silencing of microsomal omega-6 desaturase, declines with leaf expansion in transgenic tobacco
 Journal of Plant Physiology 164 (2007) 23 30 www.elsevier.de/jplph Oleate accumulation, induced by silencing of microsomal omega-6 desaturase, declines with leaf expansion in transgenic tobacco Mingfeng
Journal of Plant Physiology 164 (2007) 23 30 www.elsevier.de/jplph Oleate accumulation, induced by silencing of microsomal omega-6 desaturase, declines with leaf expansion in transgenic tobacco Mingfeng
Glycerolipidome responses to freezingand chilling-induced injuries: examples in Arabidopsis and rice
 Zheng et al. BMC Plant Biology (2016) 16:70 DOI 10.1186/s12870-016-0758-8 RESEARCH ARTICLE Open Access Glycerolipidome responses to freezingand chilling-induced injuries: examples in Arabidopsis and rice
Zheng et al. BMC Plant Biology (2016) 16:70 DOI 10.1186/s12870-016-0758-8 RESEARCH ARTICLE Open Access Glycerolipidome responses to freezingand chilling-induced injuries: examples in Arabidopsis and rice
Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana
 Biochemistry: Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana 38 CAMERON E. NIENABER ʻ04 Abstract Long recognized as essential plant nutrients and metabolites, inorganic and organic
Biochemistry: Signaling in the Nitrogen Assimilation Pathway of Arabidopsis Thaliana 38 CAMERON E. NIENABER ʻ04 Abstract Long recognized as essential plant nutrients and metabolites, inorganic and organic
ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd
 ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd I. Classes of membrane lipids A. Glycerolipids (quantitatively the most important of the three membrane lipids) B. Shingolipids
ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd I. Classes of membrane lipids A. Glycerolipids (quantitatively the most important of the three membrane lipids) B. Shingolipids
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Plant Physiology Preview. Published on April 20, 2018, as DOI: /pp
 Plant Physiology Preview. Published on April 20, 2018, as DOI:10.1104/pp.17.01805 1 2 3 4 5 6 7 8 9 10 11 12 Running head: Development of HFA-accumulating Arabidopsis Corresponding author: John Browse,
Plant Physiology Preview. Published on April 20, 2018, as DOI:10.1104/pp.17.01805 1 2 3 4 5 6 7 8 9 10 11 12 Running head: Development of HFA-accumulating Arabidopsis Corresponding author: John Browse,
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Enzymes: Helper Protein molecules
 Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enrichment of Phospholipids from Biological Matrices with Zirconium Oxide-Modified Silica Sorbents
 Enrichment of Phospholipids from Biological Matrices with Zirconium Oxide-Modified Silica Sorbents Xiaoning Lu, Jennifer E. Claus, and David S. Bell Supelco, Div. of Sigma-Aldrich Bellefonte, PA 16823
Enrichment of Phospholipids from Biological Matrices with Zirconium Oxide-Modified Silica Sorbents Xiaoning Lu, Jennifer E. Claus, and David S. Bell Supelco, Div. of Sigma-Aldrich Bellefonte, PA 16823
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Total Phosphatidic Acid Assay Kit
 Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Chloroplast lipid transfer processes in Chlamydomonas reinhardtii involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) orthologue
 The Plant Journal (2015) 84, 1005 1020 doi: 10.1111/tpj.13060 Chloroplast lipid transfer processes in Chlamydomonas reinhardtii involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) orthologue Jaruswan Warakanont
The Plant Journal (2015) 84, 1005 1020 doi: 10.1111/tpj.13060 Chloroplast lipid transfer processes in Chlamydomonas reinhardtii involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) orthologue Jaruswan Warakanont
Sequential Extraction of Plant Metabolites
 ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
Molecular, Cellular, and Developmental Biology Program, Kansas State University, Manhattan, Kansas c
 This article is published in Online, Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs, but before the final, complete
This article is published in Online, Preview Section, which publishes manuscripts accepted for publication after they have been edited and the authors have corrected proofs, but before the final, complete
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
Chloroplast lipid transfer processes in Chlamydomonas reinhardtii involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) ortholog
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biochemistry -- Faculty Publications Biochemistry, Department of 2015 Chloroplast lipid transfer processes in Chlamydomonas
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biochemistry -- Faculty Publications Biochemistry, Department of 2015 Chloroplast lipid transfer processes in Chlamydomonas
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Quantification of Free Sterols, Sterol Esters, Sterol Glucosides and Acylated Sterol Glucosides in Plants by
 Quantification of Free Sterols, Sterol Esters, Sterol Glucosides and Acylated Sterol Glucosides in Plants by Q-TOF Mass Spectrometry Vera Wewer and Peter Dörmann Seville, Spain, July 2012 Institute of
Quantification of Free Sterols, Sterol Esters, Sterol Glucosides and Acylated Sterol Glucosides in Plants by Q-TOF Mass Spectrometry Vera Wewer and Peter Dörmann Seville, Spain, July 2012 Institute of
EXPERIMENT 13: Isolation and Characterization of Erythrocyte
 EXPERIMENT 13: Isolation and Characterization of Erythrocyte Day 1: Isolation of Erythrocyte Steps 1 through 6 of the Switzer & Garrity protocol (pages 220-221) have been performed by the TA. We will be
EXPERIMENT 13: Isolation and Characterization of Erythrocyte Day 1: Isolation of Erythrocyte Steps 1 through 6 of the Switzer & Garrity protocol (pages 220-221) have been performed by the TA. We will be
Tocopherols Modulate Extraplastidic Polyunsaturated Fatty Acid Metabolism in Arabidopsis at Low Temperature W
 The Plant Cell, Vol. 20: 452 470, February 2008, www.plantcell.org ª 2008 American Society of Plant Biologists Tocopherols Modulate Extraplastidic Polyunsaturated Fatty Acid Metabolism in Arabidopsis at
The Plant Cell, Vol. 20: 452 470, February 2008, www.plantcell.org ª 2008 American Society of Plant Biologists Tocopherols Modulate Extraplastidic Polyunsaturated Fatty Acid Metabolism in Arabidopsis at
Lipids. Lipids. Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague
 Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
MONO- and DIGALACTOSYLDIACYLGLYCEROLS and RELATED LIPIDS from PLANTS and MICROORGANISMS
 MN- and DIGALACTSYLDIACYLGLYCERLS and RELATED LIPIDS from PLANTS and MICRRGANISMS STRUCTURE, CCURRENCE, BISYNTHESIS AND ANALYSIS 1. Mono- and Digalactosyldiacylglycerols from Plants Monogalactosyldiacylglycerols
MN- and DIGALACTSYLDIACYLGLYCERLS and RELATED LIPIDS from PLANTS and MICRRGANISMS STRUCTURE, CCURRENCE, BISYNTHESIS AND ANALYSIS 1. Mono- and Digalactosyldiacylglycerols from Plants Monogalactosyldiacylglycerols
Lipid Characterization in Vegetative Tissues of High Saturated Fatty Acid Sunflower Mutants
 78 J. Agric. Food Chem. 1999, 47, 78 82 Lipid Characterization in Vegetative Tissues of High Saturated Fatty Acid Sunflower Mutants Sara Cantisán, Enrique Martínez-Force, Rosario AÄ lvarez-ortega, and
78 J. Agric. Food Chem. 1999, 47, 78 82 Lipid Characterization in Vegetative Tissues of High Saturated Fatty Acid Sunflower Mutants Sara Cantisán, Enrique Martínez-Force, Rosario AÄ lvarez-ortega, and
A simple technique for the analysis of positional distribution of fatty acids on di- and triacylglycerols using lipase and phospholipase A2
 paper on methodology A simple technique for the analysis of positional distribution of fatty acids on di- and triacylglycerols using lipase and phospholipase A2 John P. Williams,' Mobashsher U. Khan, and
paper on methodology A simple technique for the analysis of positional distribution of fatty acids on di- and triacylglycerols using lipase and phospholipase A2 John P. Williams,' Mobashsher U. Khan, and
SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE
 California Avocado Society 1968 Yearbook 52: 102-108 SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE Yoshio Kikuta Present address: Department of Botany, Faculty of Agriculture,
California Avocado Society 1968 Yearbook 52: 102-108 SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE Yoshio Kikuta Present address: Department of Botany, Faculty of Agriculture,
Review. Molecular aspects of plastid envelope biochemistry
 Eur. J. Biochem. 199,489-59 (1991) FEBS 1991 14295691467F Review Molecular aspects of plastid envelope biochemistry Jacques JOYARD, Maryse A. BLOCK and Roland DOUCE Laboratoire de Physiologie Cellulaire
Eur. J. Biochem. 199,489-59 (1991) FEBS 1991 14295691467F Review Molecular aspects of plastid envelope biochemistry Jacques JOYARD, Maryse A. BLOCK and Roland DOUCE Laboratoire de Physiologie Cellulaire
Chloroplast lipid synthesis and lipid trafficking through ER plastid membrane contact sites
 Cellular Traffic of Lipids and Calcium at Membrane Contact Sites 457 Chloroplast lipid synthesis and lipid trafficking through ER plastid membrane contact sites Zhen Wang and Christoph Benning 1 Department
Cellular Traffic of Lipids and Calcium at Membrane Contact Sites 457 Chloroplast lipid synthesis and lipid trafficking through ER plastid membrane contact sites Zhen Wang and Christoph Benning 1 Department
Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis
 Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis Aardra Kachroo*, Srivathsa C. Venugopal*, Ludmila Lapchyk*, Deane Falcone, David Hildebrand, and Pradeep
Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis Aardra Kachroo*, Srivathsa C. Venugopal*, Ludmila Lapchyk*, Deane Falcone, David Hildebrand, and Pradeep
Lipids and Fatty Acids
 Lipids and Fatty Acids Objectives: 1. What are Lipids? properties glycerolipids vs. isoprenoids glycerolipid structure glycerolipid nomenclature 2. Fatty acid biosynthesis ellular localization Substrate
Lipids and Fatty Acids Objectives: 1. What are Lipids? properties glycerolipids vs. isoprenoids glycerolipid structure glycerolipid nomenclature 2. Fatty acid biosynthesis ellular localization Substrate
Fatty Acid Methylation Kits
 Methyl esterification kit for fatty acids analysis Fatty Acid Methylation Kits Below are two methods for efficiently preparing fatty acid samples for GC analysis. Neither method requires high temperatures,
Methyl esterification kit for fatty acids analysis Fatty Acid Methylation Kits Below are two methods for efficiently preparing fatty acid samples for GC analysis. Neither method requires high temperatures,
MPS Advanced Plant Biochemistry Course
 MPS 587 - Advanced Plant Biochemistry Course Lipids section guest lecture: Philip Bates Post-doc Browse lab Office: 453 Clark Hall Email: phil_bates@wsu.edu Lecture 9 Lipids I 1. What is a lipid? 2. Fatty
MPS 587 - Advanced Plant Biochemistry Course Lipids section guest lecture: Philip Bates Post-doc Browse lab Office: 453 Clark Hall Email: phil_bates@wsu.edu Lecture 9 Lipids I 1. What is a lipid? 2. Fatty
FIRST MIDTERM EXAMINATION
 FIRST MIDTERM EXAMINATION 1. True or false: because enzymes are produced by living organisms and because they allow chemical reactions to occur that would not otherwise occur, enzymes represent an exception
FIRST MIDTERM EXAMINATION 1. True or false: because enzymes are produced by living organisms and because they allow chemical reactions to occur that would not otherwise occur, enzymes represent an exception
The Plant Cell, Vol. 15, , December 2003, American Society of Plant Biologists
 , Vol. 15, 2952 2965, December 2003, www.plantcell.org 2003 American Society of Plant Biologists Plastidial Fatty Acid Signaling Modulates Salicylic Acid and Jasmonic Acid Mediated Defense Pathways in
, Vol. 15, 2952 2965, December 2003, www.plantcell.org 2003 American Society of Plant Biologists Plastidial Fatty Acid Signaling Modulates Salicylic Acid and Jasmonic Acid Mediated Defense Pathways in
Mass Spectrometry based metabolomics
 Mass Spectrometry based metabolomics Metabolomics- A realm of small molecules (
Mass Spectrometry based metabolomics Metabolomics- A realm of small molecules (
Cell wall components:
 Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. The Cell Wall The primary cell wall is capable of rapid expansion during
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. The Cell Wall The primary cell wall is capable of rapid expansion during
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure.
 Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. Animal cells have a lysosome (related to vacuole) and centrioles (function
Main differences between plant and animal cells: Plant cells have: cell walls, a large central vacuole, plastids and turgor pressure. Animal cells have a lysosome (related to vacuole) and centrioles (function
Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag
 Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag Jonathan A. Brain Galina Gulis, Ph.D. 1 Kevin E. Redding, Ph.D. 2 Associate Professor of Chemistry Adjunct
Affinity Purification of Photosystem I from Chlamydomonas reinhardtii using a Polyhistidine Tag Jonathan A. Brain Galina Gulis, Ph.D. 1 Kevin E. Redding, Ph.D. 2 Associate Professor of Chemistry Adjunct
A Tour of the Cell Lecture 2, Part 1 Fall 2008
 Cell Theory 1 A Tour of the Cell Lecture 2, Part 1 Fall 2008 Cells are the basic unit of structure and function The lowest level of structure that can perform all activities required for life Reproduction
Cell Theory 1 A Tour of the Cell Lecture 2, Part 1 Fall 2008 Cells are the basic unit of structure and function The lowest level of structure that can perform all activities required for life Reproduction
BIOCHEMICAL CHARACTERIZATION OF TRIACYLGLYCEROL METABOLISM IN MICROALGAE. Bensheng Liu A DISSERTATION
 BIOCHEMICAL CHARACTERIZATION OF TRIACYLGLYCEROL METABOLISM IN MICROALGAE By Bensheng Liu A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree
BIOCHEMICAL CHARACTERIZATION OF TRIACYLGLYCEROL METABOLISM IN MICROALGAE By Bensheng Liu A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree
Chapters 2 and 3. Pages and Pages Prayer Attendance Homework
 Chapters 2 and 3 Pages 44-45 and Pages 59-62 Prayer Attendance Homework The Cell The cell is the basic unit of life on Earth, separated from its environment by a membrane and sometimes an outer wall. Prokaryotic
Chapters 2 and 3 Pages 44-45 and Pages 59-62 Prayer Attendance Homework The Cell The cell is the basic unit of life on Earth, separated from its environment by a membrane and sometimes an outer wall. Prokaryotic
DAG (Diacylglycerol) Assay Kit
 Product Manual DAG (Diacylglycerol) Assay Kit Catalog Number MET-5028 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Diacylglycerols (DAG) are key intermediates in the
Product Manual DAG (Diacylglycerol) Assay Kit Catalog Number MET-5028 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Diacylglycerols (DAG) are key intermediates in the
IDENTIFICATION AND QUANTIFICATION OF LIPID METABOLITES IN COTTON FIBERS: RECONCILIATION WITH METABOLIC PATHWAY PREDICTIONS FROM DNA DATABASES.
 IDENTIFICATION AND QUANTIFICATION OF LIPID METABOLITES IN COTTON FIBERS: RECONCILIATION WITH METABOLIC PATHWAY PREDICTIONS FROM DNA DATABASES. Sylvia W. Wanjie, B.S. Thesis Prepared for the Degree of MASTER
IDENTIFICATION AND QUANTIFICATION OF LIPID METABOLITES IN COTTON FIBERS: RECONCILIATION WITH METABOLIC PATHWAY PREDICTIONS FROM DNA DATABASES. Sylvia W. Wanjie, B.S. Thesis Prepared for the Degree of MASTER
PHOSPHOLIPIDS METABOLISM. BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
Explain the reason for this difference in resolving power.
 1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry
 [Frontiers in Bioscience 12, 2494-2506, January 1, 2007] Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry Ruth Welti 1, Jyoti Shah 1, Weiqi Li
[Frontiers in Bioscience 12, 2494-2506, January 1, 2007] Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry Ruth Welti 1, Jyoti Shah 1, Weiqi Li
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
On the Metabolic Relationships between Monogalactosyldiacylglycerol and Digalactosyldiacylglycerol Molecular Species in Dunaliella salina"
 ~~ THE JOURNAL OF BIOLOGICAL CHEMISTRY Val. 262, No. 16, Issue of June 5, pp. 75867593,1987 0 1987 by The Amencan Society of Btologicel Chemists, Inc. Prinled in U.S.A. On the Metabolic Relationships between
~~ THE JOURNAL OF BIOLOGICAL CHEMISTRY Val. 262, No. 16, Issue of June 5, pp. 75867593,1987 0 1987 by The Amencan Society of Btologicel Chemists, Inc. Prinled in U.S.A. On the Metabolic Relationships between
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Title: A Central Role for Triacylglycerol in Membrane Lipid Breakdown, Fatty Acid ß- Oxidation and Plant Survival under Extended Darkness
 Plant Physiology Preview. Published on June 1, 2017, as DOI:10.1104/pp.17.00653 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Short title: Role of Triacylglycerol in Stress Tolerance Address for correspondence:
Plant Physiology Preview. Published on June 1, 2017, as DOI:10.1104/pp.17.00653 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Short title: Role of Triacylglycerol in Stress Tolerance Address for correspondence:
MPS Advanced Plant Biochemistry Course. Fall Semester Lecture 11. Lipids III
 MPS 587 - Advanced Plant Biochemistry Course Fall Semester 2011 Lecture 11 Lipids III 9. Triacylglycerol synthesis 10. Engineering triacylglycerol fatty acid composition Today s topics on the Arabidopsis
MPS 587 - Advanced Plant Biochemistry Course Fall Semester 2011 Lecture 11 Lipids III 9. Triacylglycerol synthesis 10. Engineering triacylglycerol fatty acid composition Today s topics on the Arabidopsis
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Structural Characterization of Sulfoquinovosyl, Monogalactosyl and Digalactosyl Diacylglycerols by FAB-CID-MS/MS
 JOURNAL OF MASS SPECTROMETRY, VOL. 32, 968È977 (1997) Structural Characterization of Sulfoquinovosyl, Monogalactosyl and Digalactosyl Diacylglycerols by FAB-CID-MS/MS Young Hwan Kim,1,2* Jong Shin Yoo1
JOURNAL OF MASS SPECTROMETRY, VOL. 32, 968È977 (1997) Structural Characterization of Sulfoquinovosyl, Monogalactosyl and Digalactosyl Diacylglycerols by FAB-CID-MS/MS Young Hwan Kim,1,2* Jong Shin Yoo1
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Chloroplast Biogenesis. Regulation of Lipid Transport to the Thylakoid in Chloroplasts Isolated from Expanding and Fully Expanded Leaves of Pea 1
 Chloroplast Biogenesis. Regulation of Lipid Transport to the Thylakoid in Chloroplasts Isolated from Expanding and Fully Expanded Leaves of Pea 1 Mats X. Andersson, J. Magnus Kjellberg, and Anna Stina
Chloroplast Biogenesis. Regulation of Lipid Transport to the Thylakoid in Chloroplasts Isolated from Expanding and Fully Expanded Leaves of Pea 1 Mats X. Andersson, J. Magnus Kjellberg, and Anna Stina
Division of Biology and Molecular, Cellular, and Developmental Biology Program, Kansas State University, Manhattan, Kansas
 The Plant Cell, Vol. 16, 465 477, February 2004, www.plantcell.org ª 2004 American Society of Plant Biologists The Arabidopsis thaliana Dihydroxyacetone Phosphate Reductase Gene SUPPRESSOR OF FATTY ACID
The Plant Cell, Vol. 16, 465 477, February 2004, www.plantcell.org ª 2004 American Society of Plant Biologists The Arabidopsis thaliana Dihydroxyacetone Phosphate Reductase Gene SUPPRESSOR OF FATTY ACID
GLOSSY MUTANTS OF MAIZE
 Heredity (1979), 42 (3), 391-395 GLOSSY MUTATS OF MAIZE IX. CHEMISTRY OF GLOSSY 4, GLOSSY 8, GLOSSY 15 AD GLOSSY 18 SURFACE WAXES* G. BtACHI, P. AVATO and F. SALAMII Istituto di Ch,mica organica, Viale
Heredity (1979), 42 (3), 391-395 GLOSSY MUTATS OF MAIZE IX. CHEMISTRY OF GLOSSY 4, GLOSSY 8, GLOSSY 15 AD GLOSSY 18 SURFACE WAXES* G. BtACHI, P. AVATO and F. SALAMII Istituto di Ch,mica organica, Viale
Accumulation of D6-unsaturated fatty acids in transgenic tobacco plants expressing a D6-desaturase from Borago officinalis
 Journal of Experimental Botany, Vol. 0, No. 340, pp. 1647 162, November 1999 Accumulation of D6-unsaturated fatty acids in transgenic tobacco plants expressing a D6-desaturase from Borago officinalis Olga
Journal of Experimental Botany, Vol. 0, No. 340, pp. 1647 162, November 1999 Accumulation of D6-unsaturated fatty acids in transgenic tobacco plants expressing a D6-desaturase from Borago officinalis Olga
Lipids in Photosynthesis
 Lipids in Photosynthesis Essential and Regulatory Functions Edited by Hajime Wada Department of Life Sciences Graduate School of Arts and Science University of Tokyo Japan and Norio Murata National Institute
Lipids in Photosynthesis Essential and Regulatory Functions Edited by Hajime Wada Department of Life Sciences Graduate School of Arts and Science University of Tokyo Japan and Norio Murata National Institute
Annotation of Genes Involved in Glycerolipid Biosynthesis in Chlamydomonas reinhardtii: Discovery of the Betaine Lipid Synthase BTA1 Cr
 EUKARYOTIC CELL, Feb. 2005, p. 242 252 Vol. 4, No. 2 1535-9778/05/$08.00 0 doi:10.1128/ec.4.2.242 252.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Annotation of Genes Involved
EUKARYOTIC CELL, Feb. 2005, p. 242 252 Vol. 4, No. 2 1535-9778/05/$08.00 0 doi:10.1128/ec.4.2.242 252.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Annotation of Genes Involved
Effects of a sub-lethal copper exposure on non-green Acer pseudoplatanus cell lipids
 Plant Physiology and Biochemistry 41 (2003) 471 477 www.elsevier.com/locate/plaphy Original article Effects of a sub-lethal copper exposure on non-green Acer pseudoplatanus cell lipids Mário Pádua a,b,
Plant Physiology and Biochemistry 41 (2003) 471 477 www.elsevier.com/locate/plaphy Original article Effects of a sub-lethal copper exposure on non-green Acer pseudoplatanus cell lipids Mário Pádua a,b,
Chapter 7: How Cells Harvest Energy AP
 Chapter 7: How Cells Harvest Energy AP Essential Knowledge 1.B.1 distributed among organisms today. (7.1) 1.D.2 Organisms share many conserved core processes and features that evolved and are widely Scientific
Chapter 7: How Cells Harvest Energy AP Essential Knowledge 1.B.1 distributed among organisms today. (7.1) 1.D.2 Organisms share many conserved core processes and features that evolved and are widely Scientific
Supplemental Data. Beck et al. (2010). Plant Cell /tpc
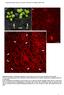 Supplemental Figure 1. Phenotypic comparison of the rosette leaves of four-week-old mpk4 and Col-0 plants. A mpk4 vs Col-0 plants grown in soil. Note the extreme dwarfism of the mpk4 plants (white arrows)
Supplemental Figure 1. Phenotypic comparison of the rosette leaves of four-week-old mpk4 and Col-0 plants. A mpk4 vs Col-0 plants grown in soil. Note the extreme dwarfism of the mpk4 plants (white arrows)
CHAPTER 4 - CELLS. All living things are made up of one or more cells. A cell is the smallest unit that can carry on all of the processes of life.
 CHAPTER 4 - CELLS Objectives Name the scientists who first observed living and nonliving cells. Summarize the research that led to the development of the cell theory. State the three principles of the
CHAPTER 4 - CELLS Objectives Name the scientists who first observed living and nonliving cells. Summarize the research that led to the development of the cell theory. State the three principles of the
Phospholipids Metabolism
 Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Quantity of phosphorus spared via phospholipid substitutions by picocyanobacteria. (1 6 P atoms cell -1 )* (% of P in DNA) Synechococcus WH812.7 ±.1 15 ± 2 Synechococcus WH783 2.7
Supplementary Table 1. Quantity of phosphorus spared via phospholipid substitutions by picocyanobacteria. (1 6 P atoms cell -1 )* (% of P in DNA) Synechococcus WH812.7 ±.1 15 ± 2 Synechococcus WH783 2.7
Cloning and Expression of a Bacterial CGTase and Impacts on Phytoremediation. Sarah J. MacDonald Assistant Professor Missouri Valley College
 Cloning and Expression of a Bacterial CGTase and Impacts on Phytoremediation Sarah J. MacDonald Assistant Professor Missouri Valley College Phytoremediation of Organic Compounds Phytodegradation: Plants
Cloning and Expression of a Bacterial CGTase and Impacts on Phytoremediation Sarah J. MacDonald Assistant Professor Missouri Valley College Phytoremediation of Organic Compounds Phytodegradation: Plants
Cell Structure. Present in animal cell. Present in plant cell. Organelle. Function. strength, resist pressure created when water enters
 Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
The phosphatidylcholine to phosphatidylethanolamine ratio of Saccharomyces cerevisiae varies with the growth phase
 Chapter 3 The phosphatidylcholine to phosphatidylethanolamine ratio of Saccharomyces cerevisiae varies with the growth phase M.J.F.W. Janssen, M.C. Koorengevel, B. de Kruijff, A.I.P.M. de Kroon Yeast 16
Chapter 3 The phosphatidylcholine to phosphatidylethanolamine ratio of Saccharomyces cerevisiae varies with the growth phase M.J.F.W. Janssen, M.C. Koorengevel, B. de Kruijff, A.I.P.M. de Kroon Yeast 16
The Role of Lipids in Flowering Development of Arabidopsis Enhanced pah1pah2 Plants. Toshiro Ito 1 & Lee Lishi 2
 The Role of Lipids in Flowering Development of Arabidopsis Enhanced pah1pah2 Plants Toshiro Ito 1 & Lee Lishi 2 Department of Biological Sciences, Faculty of Science, National University of Singapore,
The Role of Lipids in Flowering Development of Arabidopsis Enhanced pah1pah2 Plants Toshiro Ito 1 & Lee Lishi 2 Department of Biological Sciences, Faculty of Science, National University of Singapore,
Studies on arabiopsides using two Arabidopsis thaliana ecotypes
 Studies on arabiopsides using two Arabidopsis thaliana ecotypes Nilay Peker Practical work, July - August 2010, 15 hp Department of Plant and Environmental Sciences University of Gothenburg Abstract In
Studies on arabiopsides using two Arabidopsis thaliana ecotypes Nilay Peker Practical work, July - August 2010, 15 hp Department of Plant and Environmental Sciences University of Gothenburg Abstract In
Name # Class Regents Review: Characteristics of Life and Biochemistry
 Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Midi Plant Genomic DNA Purification Kit
 Midi Plant Genomic DNA Purification Kit Cat #:DP022MD/ DP022MD-50 Size:10/50 reactions Store at RT For research use only 1 Description: The Midi Plant Genomic DNA Purification Kit provides a rapid, simple
Midi Plant Genomic DNA Purification Kit Cat #:DP022MD/ DP022MD-50 Size:10/50 reactions Store at RT For research use only 1 Description: The Midi Plant Genomic DNA Purification Kit provides a rapid, simple
Supporting Information
 Supporting Information Bansal-Mutalik and Nikaido 10.1073/pnas.1112572108 Relative lipid release (% ) Relative lipid release (%) 15 30 60 120 240 1440 Extraction time (minutes) Step 1 Step 2 Step 3 Number
Supporting Information Bansal-Mutalik and Nikaido 10.1073/pnas.1112572108 Relative lipid release (% ) Relative lipid release (%) 15 30 60 120 240 1440 Extraction time (minutes) Step 1 Step 2 Step 3 Number
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
4/12/17. Cells. Cell Structure. Ch. 2 Cell Structure and Func.on. Range of Cell Sizes BIOL 100
 Ch. 2 Cell Structure and Func.on BIOL 100 Cells Fundamental units of life Cell theory All living things are composed of one or more cells. The cell is the most basic unit of life. All cells come from pre-existing
Ch. 2 Cell Structure and Func.on BIOL 100 Cells Fundamental units of life Cell theory All living things are composed of one or more cells. The cell is the most basic unit of life. All cells come from pre-existing
Zackary Johnson Department of Oceanography
 Zackary Johnson Department of Oceanography http://www.soest.hawaii.edu/oceanography/zij/education/ocn621 Application of Bioenergetics to Biological Oceanography Biochemical parameters indicative of stock
Zackary Johnson Department of Oceanography http://www.soest.hawaii.edu/oceanography/zij/education/ocn621 Application of Bioenergetics to Biological Oceanography Biochemical parameters indicative of stock
Cells & Cell Organelles. Doing Life s Work
 Cells & Cell Organelles Doing Life s Work AP Biology 2009-2010 Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles Doing Life s Work AP Biology 2009-2010 Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
A. Major parts 1. Nucleus 2. Cytoplasm a. Contain organelles (see below) 3. Plasma membrane (To be discussed in Cellular Transport Lecture)
 Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Don t Freak Out. Test on cell organelle on Friday!
 Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
The use of mass spectrometry in lipidomics. Outlines
 The use of mass spectrometry in lipidomics Jeevan Prasain jprasain@uab.edu 6-2612 utlines Brief introduction to lipidomics Analytical methodology: MS/MS structure elucidation of phospholipids Phospholipid
The use of mass spectrometry in lipidomics Jeevan Prasain jprasain@uab.edu 6-2612 utlines Brief introduction to lipidomics Analytical methodology: MS/MS structure elucidation of phospholipids Phospholipid
Chapter 1-2 Review Assignment
 Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Study Guide for Biology Chapter 5
 Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
Synthesis and elongation of fatty acids
 Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
Synthesis and elongation of fatty acids A molecular caliper mechanism for determining very long-chain fatty acid length Vladimir Denic and Jonathan S. Weissman (2007) Cell 130, 663-677 February 28, 2008
20X Buffer (Tube1) 96-well microplate (12 strips) 1
 PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
