Nova Scotia Provincial Blood Coordinating Program Provincial Dental Board of Nova Scotia. Toolkit for Allograft Usage In Dental Practice
|
|
|
- Quentin Boyd
- 5 years ago
- Views:
Transcription
1 Nova Scotia Provincial Blood Coordinating Program Provincial Dental Board of Nova Scotia Toolkit for Allograft Usage In Dental Practice September 29,
2 A. Preamble This document has been developed by the Nova Scotia Provincial Blood Coordinating Program in conjunction with the Provincial Dental Board of Nova Scotia to support compliance by dentists practicing in Nova Scotia to the Health Canada Safety of Human Cells, Tissues and Organs for Transplantation Regulations. As described within this toolkit, establishments other than source establishments that discover an unexpected adverse reaction has occurred must report the information to the source establishment or the importer (if applicable) for all suspected or known unexpected adverse reactions associated with a tissue without delay. This document is divided into sections according to responsibilities the user has: Determining whether or not a distributor or manufacturer is licensed by Health Canada; Signs and symptoms of an adverse reaction; Example cases of adverse reactions to tissues used in dental settings; Who to report adverse reactions of transplanted tissue to; and How to report adverse reactions.
3 Table of Contents A. Preamble... 2 B. Reporting Allograft Usage Data: What Dentists Need to Know... 1 C. List of Registered CTO Establishments... 2 D. Adverse Reactions to Tissues... 3 E. Example Cases... 4 F. Algorithm for Reporting Adverse Reactions to Transplanted Tissues by Dentists... 5 G. Canadian Transplantation Adverse Event (TAE) Reporting Form for Tissues... 6
4 B. Reporting Allograft Usage Data: What Dentists Need to Know Since 2016 dentists have been required to report the number of human allograft tissues transplanted in practice each year to the Provincial Dental Board of Nova Scotia. Human allograft tissues are commonly used in dental practice today. In 2009, the Health Products and Food Branch Inspectorate of Health Canada adopted the Guidance Document for Cell, Tissue, and Organ Establishments Safety of Human Cells, Tissues, and Organs for Transplantation. This document was published to assist establishments in complying with Health Canada s Safety of Human Cells, Tissues, and Organs for Transplantation Regulations, which came into force in The Canadian Dental Association published its Guidance Document for Dentists Providing Human Allogeneic Transplants in This document outlines the specific requirements that pertain to dentists using human allograft tissue products in their practice. The main requirements for dentists utilizing these products in their practice are as follows: 1. To ensure that any human allograft tissue products utilized in practice are obtained from Source Establishments that are registered with Health Canada 2. To ensure that recordkeeping practices capture all of the information required (donor identification code, description of the transplanted tissue, the Health Canada registration number of the source establishment, the notice of exceptional distribution, if applicable, information that allows for identification of the recipient, and documentation of any suspected errors, accidents, or adverse reactions and their investigation) for ten years 3. To ensure that there are procedures in place for reporting suspected errors, accidents and adverse reactions The Provincial Dental Board of Nova Scotia has a mandate to protect the public interest in matters related to the delivery of dental care. The Board does this by ensuring that only properly trained, licensed individuals provide treatment and that the treatment is of a reasonable standard. The Provincial Dental Board is responsible for the administration of the Dental Act and the Regulations pursuant to the Act. With the privilege of self-regulation comes the responsibility of responsible action. Part of the responsible action is ensuring that practitioners in Nova Scotia are aware of the regulatory requirements and are adhering to regulations and standards. As such, dentists must provide to the Board, as part of annual license renewal, the number of human allograft tissue products transplanted in practice in the previous calendar year. This is done for the safety of Nova Scotians by ensuring that transplanted tissue products are obtained from Health Canada registered source establishments, and that recipients are easily identifiable in the event of a recall. Additionally, the number and type of human allografts transplanted by dentists will be shared with the Nova Scotia Provincial Blood Coordinating Program (NSPBCP). The Program collects data on tissue usage in Nova Scotia as part of their participation in the National Cells, Tissues, and Organs Surveillance System.
5 What products are included in the project? Below are some examples to help you identify the type of products that fall within this scope: 1. Demineralized bone (Raptos by Citagenix; PentOs Ol Fill by Citagenix, Straumann AlloGraft) 2. Mineralized bone (Mineralized cancellous powder by Synthes) 3. Demineralized bone strips (PentOs Ol Fec by Citagenix) 4. Demineralized bone putty ( PentOs Ol Putty by Citagenix, DBM Putty by Synthes, AlloOss Putty with demineralized bone chips by ACE Surgical) 5. Injectable bone morphogenetic protein (INFUSE Bone Graft by Medtronic) 6. Particulate allograft (Puros by Zimmer) 7. Extracellular dermal matrix (DynaMatrix by Citagenix) 8. Acellular dermal matrix (NeoDerm by Citagenix, AlloDerm Regenerative Tissue Matrix by LifeCell; DermanMatrix Acellular Dermis by Synthes) 9. Placental allograft membrane (BioXclude by Citagenix) 10.Any other product that contains human cells These are some examples of the products that are in scope. If you have other products and are unsure whether they should be counted, you can contact the Nova Scotia Provincial Blood Coordinating Program at (902) C. List of Registered CTO Establishments In accordance with the Safety of Human Cells, Tissues and Organs for Transplantation Regulations (CTO Regulations), all source establishments and establishments that distribute or import for further distribution, cells, tissues and organs (CTO), must register with Health Canada. The List of Registered CTO Establishments should be used as a reference tool to identify those establishments that have registered with Health Canada prior to requesting tissues from suppliers. The List of Registered Cells, Tissues and Organs Establishments can be found at: Commonly used registered establishments in Nova Scotia include: BioHorizons
6 Citagenix Zimmer BioMet Nobel BioCare Straumann Musculoskeletal Transplant Foundation Medical devices establishment licences held with Health Canada can be determined by entering the appropriate information in this website: Commonly used licensed medical device establishments include: Dentsply Depuy D. Adverse Reactions to Tissues Establishments, other than source establishments, that discover an unexpected adverse reaction has occurred must report to the source establishment and the importer (if applicable) all suspected or known unexpected adverse reactions associated with a tissue without delay as well as the NSPBCP. Upon receipt of the notice above, an importer of tissue is required to notify the source establishment of the adverse reaction. An unexpected adverse reaction following the transplantation of a tissue includes the unintended and unforeseen transmission of any bacterial, viral or fungal infection (infection disease or disease agents), as well as malignancies or any other disease/disorders (e.g. genetic disorder, immunological disorder, etc.) that is suspected to originate from the donor. Signs and Symptoms include but are not limited to: Chills Fever Localized tenderness Abscess formation Osteonecrosis Rash
7 Pain Swelling Dry socket Bleeding If the infectious agent was a virus such as HBV or HIV etc., the presentation would of course differ depending on the disease and may be a positive screening test in an asymptomatic patient. E. Example Cases Tissue Mineralized cortical powder Cortical bone demineralized and dental pericardium AlloDerm Mineralized allograft cortical/cancellous bone granules AlloDerm (acellular human skin graft) Adverse Reaction(s) Jaw ache/pain, dry socket and premature loss of sutures Rash Peri-implantitis, infection of the AlloDerm membrane Failed osteointegration of a bone allograft possibly due to a post-operative infection of the graft site Swelling and bleeding
8 F. Algorithm for Reporting Adverse Reactions to Transplanted Tissues by Dentists
9 G. Canadian Transplantation Adverse Event (TAE) Reporting Form for Tissues The TAE Reporting Form for Tissues can be obtained by calling The NSPBCP at or on the NSPBCP website: Instructions for Completing the Canadian Transplantation Adverse Event (TAE) Reporting Form for Tissues are as follows: 1) Complete the Date of birth and sex fields. 2) Complete the information in regards to your facility. This includes: Name of facility Address Name and contact information of the transplanting dentist Name and contact information of the individual completing the form if different from the transplanting dentist
10 3) Complete the information in regards to the Source Establishment. This includes: Name Address Contact Person and Health Canada registration number, you must also indicate if they were notified
11 4) Complete the information in regards to the transplantation adverse event. This includes: Date and location of the event Tissue type Product code Supplier name Donor ID code Expiry date Quantity Dates of Transplant, Pre-Transplant and Post-Transplant.
12 5) Complete the clinical history section of the form with as much information as possible. 6) Complete the Clinical Signs section providing as much information as possible.
13 7) Complete the diagnosis of TAE section. This includes details on: the specific event severity imputability outcome status of the investigation
14 8) Complete the conclusion section. The transplanting dentist would sign off on all information documented verifying its accuracy as well as providing a contact number and date/time the report was completed.
15 Once complete, the TAE Reporting Form should be forwarded to the source establishment or importer (if applicable) as well as the NSPBCP by fax at or to The source establishment or importer and the NSPBCP may contact you for further information not captured on the form if the situation requires it. Documentation of submitting the form to the source establishment or the importer as well as the NSPBCP should be kept in the patients file to prove compliance to the regulations.
CTO Establishment Inspections. an Atlantic update - Tissues
 Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Bone Grafting for Socket Preservation
 Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Report on the Transplantation Transmission Sentinel Network (TTSN)
 Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs
 Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
Dental Bone Graft Market Research Report - Forecast to 2027
 Report Information More information from: https://www.marketresearchfuture.com/reports/867 Dental Bone Graft Market Research Report - Forecast to 2027 Report / Search Code: MRFR/DM/0365-HCRR Publish Date:
Report Information More information from: https://www.marketresearchfuture.com/reports/867 Dental Bone Graft Market Research Report - Forecast to 2027 Report / Search Code: MRFR/DM/0365-HCRR Publish Date:
Understanding the New Access to Cannabis for Medical Purposes Regulations
 Understanding the New Access to Cannabis for Medical Purposes Regulations Health Canada August 2016 Table of Contents 1. Introduction 2. Health Canada's role 3. What it means for health care practitioners
Understanding the New Access to Cannabis for Medical Purposes Regulations Health Canada August 2016 Table of Contents 1. Introduction 2. Health Canada's role 3. What it means for health care practitioners
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
Please find enclosed Dosimetry Service Licence No which replaces Dosimetry Service Licence No
 Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
User Guide for Transition to the Drug Information System
 User Guide for Transition to the Drug Information System Document Revision History Date Description Version Updated By August 12, 2013 New/revised content 1.0 Lori Emery Version 1.0 Page 2 of 13 Table
User Guide for Transition to the Drug Information System Document Revision History Date Description Version Updated By August 12, 2013 New/revised content 1.0 Lori Emery Version 1.0 Page 2 of 13 Table
Coding and Labelling of
 Coding and Labelling of Tissues in Europe Deirdre Fehily Why are Tissue Coding and Labelling Important? Anonimity Traceability global uniqueness Standardised product information Potential for electronic
Coding and Labelling of Tissues in Europe Deirdre Fehily Why are Tissue Coding and Labelling Important? Anonimity Traceability global uniqueness Standardised product information Potential for electronic
Canadian Grain Commission
 Canadian Grain Commission Licensing feed mills Discussion paper February 9, 2015 Ce document est aussi disponible en français. 1 About the Canadian Grain Commission The Canadian Grain Commission is a federal
Canadian Grain Commission Licensing feed mills Discussion paper February 9, 2015 Ce document est aussi disponible en français. 1 About the Canadian Grain Commission The Canadian Grain Commission is a federal
closed tray technique using the indirect transfer coping
 closed tray technique using the indirect transfer coping impression techniques closed tray technique using the indirect transfer coping Use this technique to make a single or multiple-unit, implant-level
closed tray technique using the indirect transfer coping impression techniques closed tray technique using the indirect transfer coping Use this technique to make a single or multiple-unit, implant-level
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
TABLE 4 U.S. TEETH CLEANING VISITS BY SELECTED CHARACTERISTICS (%) TABLE 4 (CONTINUED) FIGURE 2 U.S. POPULATION WITH TEETH CLEANING
 CHAPTER ONE: INTRODUCTION... 1 STUDY GOALS AND OBJECTIVES... 1 REASONS FOR DOING THE STUDY... 1 SCOPE OF REPORT... 1 METHODOLOGY... 2 ANALYST... 3 RELATED BCC STUDIES... 3 BCC ONLINE SERVICES... 3 DISCLAIMER...
CHAPTER ONE: INTRODUCTION... 1 STUDY GOALS AND OBJECTIVES... 1 REASONS FOR DOING THE STUDY... 1 SCOPE OF REPORT... 1 METHODOLOGY... 2 ANALYST... 3 RELATED BCC STUDIES... 3 BCC ONLINE SERVICES... 3 DISCLAIMER...
Nova Scotia Drug Information System
 Nova Scotia Drug Information System Functions Presentation Details: Slides: 19 Duration: 00:32:04 Filename: DIS Module4.Functions.ppt Presenter Details: Slide 1 Nova Scotia Drug Information System Duration:
Nova Scotia Drug Information System Functions Presentation Details: Slides: 19 Duration: 00:32:04 Filename: DIS Module4.Functions.ppt Presenter Details: Slide 1 Nova Scotia Drug Information System Duration:
Alberta - US Comparator: Standard-Making and Enforcement Functions
 Alberta - US Comparator: Standard-Making and Enforcement Functions Current Reliability Standards Below is a link to current reliability standards in Alberta: http://www.aeso.ca/rulesprocedures/17006.html
Alberta - US Comparator: Standard-Making and Enforcement Functions Current Reliability Standards Below is a link to current reliability standards in Alberta: http://www.aeso.ca/rulesprocedures/17006.html
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Tell Us About Your Child. Who is Accompanying Your Child Today? Parent Information. Primary Dental Insurance
 1 Today s Date: 2 (225) 664-2646 (225) 664-2640 (fax) 245 VETERANS BLVD. DENHAM SPRINGS, LA 70726 Who is Accompanying Your Child Today? Name: Relation: Do you have legal custody of this child? Yes No Tell
1 Today s Date: 2 (225) 664-2646 (225) 664-2640 (fax) 245 VETERANS BLVD. DENHAM SPRINGS, LA 70726 Who is Accompanying Your Child Today? Name: Relation: Do you have legal custody of this child? Yes No Tell
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
closed tray pick-up technique using the snap coping
 closed tray pick-up technique using the snap coping impression techniques closed tray pick-up technique using the snap coping Use this technique to make a single or multiple-unit, implant-level impression
closed tray pick-up technique using the snap coping impression techniques closed tray pick-up technique using the snap coping Use this technique to make a single or multiple-unit, implant-level impression
open tray technique using the direct pick-up coping
 open tray technique using the direct pick-up coping impression techniques open tray technique using the direct pick-up coping Use this technique to make a single or multiple-unit, implant-level impression
open tray technique using the direct pick-up coping impression techniques open tray technique using the direct pick-up coping Use this technique to make a single or multiple-unit, implant-level impression
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Guidelines on the Sourcing of Medicinal Products for Sale or Supply by a Retail Pharmacy Business
 Guidelines on the Sourcing of Medicinal Products for Sale or Supply by a Retail Pharmacy Business to Facilitate Compliance with Regulations 5(1)(g), 6, 8 and 11 of the Regulation of Retail Pharmacy Businesses
Guidelines on the Sourcing of Medicinal Products for Sale or Supply by a Retail Pharmacy Business to Facilitate Compliance with Regulations 5(1)(g), 6, 8 and 11 of the Regulation of Retail Pharmacy Businesses
Bone & Membrane. Catalogue Biologic Regenerative Products ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
international biologics product catalog
 international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
Demand for Ocular Tissue in Canada - Final Report
 Demand for Ocular Tissue in Canada - Final Report January 2010 Table of Contents Executive Summary... 3 Background... 4 Purpose... 4 Overview... 4 Limitations... 4 Waiting Lists for Cornea Transplants...
Demand for Ocular Tissue in Canada - Final Report January 2010 Table of Contents Executive Summary... 3 Background... 4 Purpose... 4 Overview... 4 Limitations... 4 Waiting Lists for Cornea Transplants...
PHYSIOTHERAPY ACT AUTHORIZATION REGULATIONS
 c t PHYSIOTHERAPY ACT AUTHORIZATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to July 11, 2009. It is intended
c t PHYSIOTHERAPY ACT AUTHORIZATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to July 11, 2009. It is intended
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
Continuing Education Requirements
 Continuing Education Requirements Updated May 2015 Contents 2 Introduction Program Principles 3 Program Objective 4 Quality Assurance Requirements, including: Reporting Credits Records of Continuing Education
Continuing Education Requirements Updated May 2015 Contents 2 Introduction Program Principles 3 Program Objective 4 Quality Assurance Requirements, including: Reporting Credits Records of Continuing Education
abutment selection guide
 abutment selection guide Use the abutment selection guide to determine what abutments are best suited for the desired final prosthesis. Simply determine if the case is a single-tooth, multiple-unit or
abutment selection guide Use the abutment selection guide to determine what abutments are best suited for the desired final prosthesis. Simply determine if the case is a single-tooth, multiple-unit or
screw-retained crown using a PEEK temporary abutment
 screw-retained crown using a PEEK temporary abutment temporary restorations screw-retained crown using a PEEK temporary abutment Use this technique for the fabrication of short term (30 days), screw-retained
screw-retained crown using a PEEK temporary abutment temporary restorations screw-retained crown using a PEEK temporary abutment Use this technique for the fabrication of short term (30 days), screw-retained
Prescribing and Dispensing Drugs
 STANDARDS & GUIDELINES Prescribing and Dispensing Drugs TABLE OF CONTENTS Standards and guidelines inform practitioners and the public of CDSBC s expectations for registrants. This document primarily contains
STANDARDS & GUIDELINES Prescribing and Dispensing Drugs TABLE OF CONTENTS Standards and guidelines inform practitioners and the public of CDSBC s expectations for registrants. This document primarily contains
Product Information. Straumann CARES Scan & Shape Turning into digital solutions.
 Product Information Turning into digital solutions. I have been using the Scan & Shape service since its introduction by Straumann. I have found it fast, reliable and cost-effective. The ability to preview
Product Information Turning into digital solutions. I have been using the Scan & Shape service since its introduction by Straumann. I have found it fast, reliable and cost-effective. The ability to preview
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE The following languages are included in this packet:
 BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Multi-unit abutment open tray technique using the direct pick-up coping
 Multi-unit abutment open tray technique using the direct pick-up coping impression techniques Multi-unit abutment open tray technique using the direct pick-up coping Use this technique to make an impression
Multi-unit abutment open tray technique using the direct pick-up coping impression techniques Multi-unit abutment open tray technique using the direct pick-up coping Use this technique to make an impression
surgical and prosthetic options & impression technique overview
 surgical and prosthetic options & impression technique overview surgical options Multiple surgical protocols are used to achieve the prosthetic outcome of choice. The technique selected may be dependent
surgical and prosthetic options & impression technique overview surgical options Multiple surgical protocols are used to achieve the prosthetic outcome of choice. The technique selected may be dependent
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
iphis Case ID #: Reported Date: Reporting Source: Diagnosing Health Unit: Branch Office: Outbreak Number:
 Case Investigation Form: Hepatitis C Legend iphis system mandatory Required Case Information (add dates as YYYY-MM-DD) Case s Last Name: Case s First Name: Middle Name: Birth Date: Male Gender: Female
Case Investigation Form: Hepatitis C Legend iphis system mandatory Required Case Information (add dates as YYYY-MM-DD) Case s Last Name: Case s First Name: Middle Name: Birth Date: Male Gender: Female
ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS
 c t ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to December
c t ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to December
Procedure. Identify all possible radiation hazards.
 Guidance Notes on Radiation Risk Assessment Prior to commencing any new work practice involving a source of ionising radiation it is important that a realistic assessment of the radiation risks is carried
Guidance Notes on Radiation Risk Assessment Prior to commencing any new work practice involving a source of ionising radiation it is important that a realistic assessment of the radiation risks is carried
fabricating a custom impression coping using the closed tray technique
 fabricating a custom impression coping using the closed tray technique impression techniques fabricating a custom impression coping using the closed tray technique Use this technique to fabricate a customized,
fabricating a custom impression coping using the closed tray technique impression techniques fabricating a custom impression coping using the closed tray technique Use this technique to fabricate a customized,
Minnesota. Prescribing and Dispensing Profile. Research current through November 2015.
 Prescribing and Dispensing Profile Minnesota Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
Prescribing and Dispensing Profile Minnesota Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
screw-retained crown using the Laser-Lok Easy Ti temp abutment
 screw-retained crown using the Laser-Lok Easy Ti temp abutment temporary restorations screw-retained crown using the Laser-Lok Easy Ti temp abutment Use this technique for the fabrication of long-term
screw-retained crown using the Laser-Lok Easy Ti temp abutment temporary restorations screw-retained crown using the Laser-Lok Easy Ti temp abutment Use this technique for the fabrication of long-term
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances
 QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
Multi-unit abutment closed tray technique using the indirect transfer coping
 Multi-unit abutment closed tray technique using the indirect transfer coping impression techniques Multi-unit abutment closed tray technique using the indirect transfer coping Use this technique to make
Multi-unit abutment closed tray technique using the indirect transfer coping impression techniques Multi-unit abutment closed tray technique using the indirect transfer coping Use this technique to make
Commercial Feed Mill Verification Task Procedures
 Introduction The Canadian Food Inspection Agency (CFIA) is dedicated to safeguarding food, animals and plants, which enhances the health and well-being of Canada s people, environment and economy. As part
Introduction The Canadian Food Inspection Agency (CFIA) is dedicated to safeguarding food, animals and plants, which enhances the health and well-being of Canada s people, environment and economy. As part
Background Paper for the Tissue Expert Committee:
 Background Paper for the Tissue Expert Committee: What will the role of Canadian allograft processing be in the future? Contents 1. Introduction... 2 A. Background... 2 2. Scope... 3 3. Tissue Processing
Background Paper for the Tissue Expert Committee: What will the role of Canadian allograft processing be in the future? Contents 1. Introduction... 2 A. Background... 2 2. Scope... 3 3. Tissue Processing
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
FREQUENTLY ASKED QUESTIONS Naturopathic Profession Regulation Proposed Key Elements
 FREQUENTLY ASKED QUESTIONS Naturopathic Profession Regulation Proposed Key Elements July 2016 PROPOSED KEY ELEMENTS Q. What are key elements? A. Key elements cover the most important parts of the proposed
FREQUENTLY ASKED QUESTIONS Naturopathic Profession Regulation Proposed Key Elements July 2016 PROPOSED KEY ELEMENTS Q. What are key elements? A. Key elements cover the most important parts of the proposed
15. Procuring, processing and transporting gametes and
 15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
Regulatory Framework for Medical Devices in South Africa. 23 November 2018 Andrea Keyter Deputy Director: Medical Devices
 Regulatory Framework for Medical Devices in South Africa 23 November 2018 Andrea Keyter Deputy Director: Medical Devices Medicines and Related Substances Act, 1965 (Act 101 of 1965) Act 72 of 2008 and
Regulatory Framework for Medical Devices in South Africa 23 November 2018 Andrea Keyter Deputy Director: Medical Devices Medicines and Related Substances Act, 1965 (Act 101 of 1965) Act 72 of 2008 and
ARKANSAS STATE UNIVERSITY GOVERNING PRINCIPLES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH
 ARKANSAS STATE UNIVERSITY GOVERNING PRINCIPLES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH 1.0 INTRODUCTION Arkansas State University (ASU) is committed to enhancing the growth of research and other
ARKANSAS STATE UNIVERSITY GOVERNING PRINCIPLES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH 1.0 INTRODUCTION Arkansas State University (ASU) is committed to enhancing the growth of research and other
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Protocol for Healthcare Worker Influenza Immunization Surveillance in Acute Care Hospitals in Nova Scotia
 Protocol for Healthcare Worker Influenza Immunization Surveillance in Acute Care Hospitals in Nova Scotia Patient Safety Act Final March 2016 The following protocol is an appendix to the Patient Safety
Protocol for Healthcare Worker Influenza Immunization Surveillance in Acute Care Hospitals in Nova Scotia Patient Safety Act Final March 2016 The following protocol is an appendix to the Patient Safety
Bassett Medical Center Transfusion Services Tissue Reference Log for LIS Entry and Assignment. Room Temperature C
 Bassett Medical Center Transfusion Services Tissue Reference Log for LIS Entry and Assignment EPIC OR Order Groups Group 1: Bone Putty, Matrix, BNP, Trinity Elite Group 2: Bone Filler, Wrap, Strips, Dural
Bassett Medical Center Transfusion Services Tissue Reference Log for LIS Entry and Assignment EPIC OR Order Groups Group 1: Bone Putty, Matrix, BNP, Trinity Elite Group 2: Bone Filler, Wrap, Strips, Dural
Hepatitis C January 26, 2018
 Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Executive Summary for Executive Licensing Panel 20 May Dr Kamal Ahuja. Follow-up inspection 14 April 2010
 Summary for Licensing Panel 20 May 2010 Centre number 0011 Centre name The London Sperm Bank Person Responsible Dr Kamal Ahuja Background Follow-up inspection 14 April 2010 1. The London Sperm Bank underwent
Summary for Licensing Panel 20 May 2010 Centre number 0011 Centre name The London Sperm Bank Person Responsible Dr Kamal Ahuja Background Follow-up inspection 14 April 2010 1. The London Sperm Bank underwent
Changing practice to support service delivery
 Nurse and Midwife Medicinal Product Prescribing Toolkit Authorised Medicinal Products, Off-label Prescription and Exempt Medicinal Products Toolkit Changing practice to support service delivery Introduction
Nurse and Midwife Medicinal Product Prescribing Toolkit Authorised Medicinal Products, Off-label Prescription and Exempt Medicinal Products Toolkit Changing practice to support service delivery Introduction
Product Information. Straumann CARES Scan & Shape Turn into digital.
 Product Information Turn into digital. I have been using the Scan & Shape service since its introduction by Straumann. I have found it fast, reliable and cost-effective. The ability to preview the case
Product Information Turn into digital. I have been using the Scan & Shape service since its introduction by Straumann. I have found it fast, reliable and cost-effective. The ability to preview the case
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
OD Secure chairside pick-up using existing denture
 OD Secure chairside pick-up using existing denture full-arch restorations OD Secure chairside pick-up using existing denture Use this technique for chairside pick-up of the OD Secure housing into an existing
OD Secure chairside pick-up using existing denture full-arch restorations OD Secure chairside pick-up using existing denture Use this technique for chairside pick-up of the OD Secure housing into an existing
HOSPITAL INNOVATIONS
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
REGENERATION BY DESIGN
 REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
2. STRAUMANN PRODUCTS COVERED BY THE STRAUMANN GUARANTEE. Abutment attached to an implant* Replacement with equivalent ceramic abutment**
 Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
UNIVERSITY POLICY. Revised: Contact:
 UNIVERSITY POLICY Policy Name: Policy for Controlled Substances Section #: 90.2.3 Section Title: Compliance Approval Authority: Responsible Executive: Responsible Office: Formerly Book: President Adopted:
UNIVERSITY POLICY Policy Name: Policy for Controlled Substances Section #: 90.2.3 Section Title: Compliance Approval Authority: Responsible Executive: Responsible Office: Formerly Book: President Adopted:
This Chapter Contains:
 This Chapter Contains: State and Federal Laws Relating to Controlled Substances and Other Medications Drugs Used in Animal Shelters Acquiring Controlled Substances Record Keeping of Controlled Substances
This Chapter Contains: State and Federal Laws Relating to Controlled Substances and Other Medications Drugs Used in Animal Shelters Acquiring Controlled Substances Record Keeping of Controlled Substances
WORLD HEALTH ORGANIZATION. Human organ and tissue transplantation
 WORLD HEALTH ORGANIZATION EXECUTIVE BOARD EB113/14 113th Session 27 November 2003 Provisional agenda item 3.17 Human organ and tissue transplantation Report by the Secretariat 1. At its 112th session in
WORLD HEALTH ORGANIZATION EXECUTIVE BOARD EB113/14 113th Session 27 November 2003 Provisional agenda item 3.17 Human organ and tissue transplantation Report by the Secretariat 1. At its 112th session in
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative
 Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
Oklahoma. Prescribing and Dispensing Profile. Research current through November 2015.
 Prescribing and Dispensing Profile Oklahoma Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
Prescribing and Dispensing Profile Oklahoma Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
CHAPTER Committee Substitute for Committee Substitute for Senate Bill No. 2760
 CHAPTER 2008-64 Committee Substitute for Committee Substitute for Senate Bill No. 2760 An act relating to dentistry; amending s. 466.003, F.S.; providing a definition; amending s. 466.006, F.S.; revising
CHAPTER 2008-64 Committee Substitute for Committee Substitute for Senate Bill No. 2760 An act relating to dentistry; amending s. 466.003, F.S.; providing a definition; amending s. 466.006, F.S.; revising
verification jig fabrication
 verification jig fabrication full-arch restorations verification jig fabrication Use this technique to verify and achieve passive fitting metal framework for a bridge, hybrid prosthesis or for an overdenture
verification jig fabrication full-arch restorations verification jig fabrication Use this technique to verify and achieve passive fitting metal framework for a bridge, hybrid prosthesis or for an overdenture
DRIVING THE FUTURE. About HOSPITAL INNOVATIONS. Strong commitment to healthcare innovation
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
Operatory Units TABLE 20 SELECT MANUFACTURERS AND DISTRIBUTORS OF DENTAL FURNITURE TABLE 20 (CONTINUED) Cavity Preparation Systems
 CHAPTER ONE: INTRODUCTION... 1 STUDY GOALS AND OBJECTIVES... 1 REASONS FOR DOING THE STUDY... 1 SCOPE AND FORMAT... 1 METHODOLOGY... 1 RELATED BCC STUDIES... 2 ANALYST CREDENTIALS... 2 BCC ON-LINE SERVICES...
CHAPTER ONE: INTRODUCTION... 1 STUDY GOALS AND OBJECTIVES... 1 REASONS FOR DOING THE STUDY... 1 SCOPE AND FORMAT... 1 METHODOLOGY... 1 RELATED BCC STUDIES... 2 ANALYST CREDENTIALS... 2 BCC ON-LINE SERVICES...
Prior Authorization for Level 4 Deep Sedation and General Anesthesia Provided in Conjunction with Therapeutic Dental Treatment
 16.1.25.2 Prior Authorization for Level 4 Deep Sedation and General Anesthesia Provided in Conjunction with Therapeutic Dental Treatment Notice: MM/DD/YYYY Effective: July 1, 2017 Impacted Programs Health
16.1.25.2 Prior Authorization for Level 4 Deep Sedation and General Anesthesia Provided in Conjunction with Therapeutic Dental Treatment Notice: MM/DD/YYYY Effective: July 1, 2017 Impacted Programs Health
Proposal to Modify ABO Determination, Reporting, and Verifications Requirements. Operations and Safety Committee June 2015
 Proposal to Modify ABO Determination, Reporting, and Verifications Requirements Operations and Safety Committee June 2015 1 The Problem Rules are misunderstood resulting in compliance issues Rules vary
Proposal to Modify ABO Determination, Reporting, and Verifications Requirements Operations and Safety Committee June 2015 1 The Problem Rules are misunderstood resulting in compliance issues Rules vary
Within the Scope of Practice/Role of _X APRN RN LPN CNA ADVISORY OPINION PAIN MANAGEMENT GUIDELIINES
 Wyoming State Board of Nursing 130 Hobbs Avenue, Suite B Cheyenne, WY 82002 Phone (307) 777-7601 Fax (307) 777-3519 E-Mail: wsbn-info-licensing@wyo.gov Home Page: https://nursing-online.state.wy.us/ OPINION:
Wyoming State Board of Nursing 130 Hobbs Avenue, Suite B Cheyenne, WY 82002 Phone (307) 777-7601 Fax (307) 777-3519 E-Mail: wsbn-info-licensing@wyo.gov Home Page: https://nursing-online.state.wy.us/ OPINION:
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR VACCINE PART III: PATIENT MEDICATION INFORMATION
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR VACCINE PART III: PATIENT MEDICATION INFORMATION SHINGRIX Herpes Zoster vaccine (non-live recombinant, AS01B adjuvanted) Suspension for Injection Read this
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR VACCINE PART III: PATIENT MEDICATION INFORMATION SHINGRIX Herpes Zoster vaccine (non-live recombinant, AS01B adjuvanted) Suspension for Injection Read this
Automated external defibrillators
 MEDICAL DEVICES Automated external defibrillators Our advice www.hpra.ie Automated external defibrillators MEDICAL DEVICES Automated external defibrillators An automated external defibrillator (AED) is
MEDICAL DEVICES Automated external defibrillators Our advice www.hpra.ie Automated external defibrillators MEDICAL DEVICES Automated external defibrillators An automated external defibrillator (AED) is
NEW PROVIDER ENROLLMENT FOR ADULT SITE
 New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
ball abutment overdenture: chairside pick-up using existing denture
 ball abutment overdenture: chairside pick-up using existing denture full-arch restorations ball abutment overdenture: chairside pick-up using existing denture Use this technique for chairside pick-up of
ball abutment overdenture: chairside pick-up using existing denture full-arch restorations ball abutment overdenture: chairside pick-up using existing denture Use this technique for chairside pick-up of
Communicable Diseases
 Policy 1016 Elk Grove Police Department 1016.1 PURPOSE AND SCOPE This policy provides general guidelines to assist in minimizing the risk of department members contracting and/or spreading communicable
Policy 1016 Elk Grove Police Department 1016.1 PURPOSE AND SCOPE This policy provides general guidelines to assist in minimizing the risk of department members contracting and/or spreading communicable
Template Standard Operating Procedure For: Handling of Midazolam and other controlled drugs in Dental Practices
 Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
THINKING OUTSIDE THE PALATE
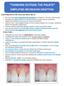 THINKING OUTSIDE THE PALATE SIMPLIFIED RECESSION GRAFTING Course Objectives-In this course you will be able to: See how much less complicated this technique is compared to Pin-Hole and tunneling. Recognize
THINKING OUTSIDE THE PALATE SIMPLIFIED RECESSION GRAFTING Course Objectives-In this course you will be able to: See how much less complicated this technique is compared to Pin-Hole and tunneling. Recognize
OUR VISION OUR MISSION
 Health Canada Santé Canada Your health and safety... our priority. Votre santé et votre sécurité... notre priorité. INSPECTORATE PROGRAM ANNUAL INSPECTION SUMMARY REPORT 2012 2013 22 OUR VISION To be a
Health Canada Santé Canada Your health and safety... our priority. Votre santé et votre sécurité... notre priorité. INSPECTORATE PROGRAM ANNUAL INSPECTION SUMMARY REPORT 2012 2013 22 OUR VISION To be a
Louisiana. Prescribing and Dispensing Profile. Research current through November 2015.
 Prescribing and Dispensing Profile Louisiana Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
Prescribing and Dispensing Profile Louisiana Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS
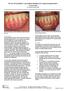 The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
Pennsylvania s Medical Marijuana Program
 Pennsylvania s Medical Marijuana Program John Collins Director, Office of Medical Marijuana October 26, 2018 Welcome to Pennsylvania s Medical Marijuana Program The Pennsylvania Department of Health's
Pennsylvania s Medical Marijuana Program John Collins Director, Office of Medical Marijuana October 26, 2018 Welcome to Pennsylvania s Medical Marijuana Program The Pennsylvania Department of Health's
Increased Risk Donors for Organ Transplantation
 A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
3. Guarantee conditions
 Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Standards for Professional Conduct In The Practice of Dentistry
 Standards for Professional Conduct In The Practice of Dentistry Preamble The Standards for Professional Conduct for licensees of the Virginia Board of Dentistry establishes a set of principles to govern
Standards for Professional Conduct In The Practice of Dentistry Preamble The Standards for Professional Conduct for licensees of the Virginia Board of Dentistry establishes a set of principles to govern
Department of Dental Hygiene Policy on Bloodborne Pathogens
 Department of Dental Hygiene Policy on Bloodborne Pathogens The Bridgemont Department of Dental Hygiene Policy on Bloodborne Pathogens is modeled after the policy statement published by the American Dental
Department of Dental Hygiene Policy on Bloodborne Pathogens The Bridgemont Department of Dental Hygiene Policy on Bloodborne Pathogens is modeled after the policy statement published by the American Dental
