SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
|
|
|
- Marybeth Ball
- 6 years ago
- Views:
Transcription
1 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion
2 The following general Surgical Technique Guide is for illustrative purposes only. As with all surgical procedures, the technique used in each case will depend on the surgeon s medical judgment as to the best treatment for each patient. Detailed preoperative clinical and diagnostic evaluation followed by carefully executed surgical technique is essential. Only those individuals with specialized training and experience in spinal surgery should attempt to use the Breckenridge Intervertebral body/vbr Fusion System. Refer to the Instructions for Use for a more complete description of indications, contraindications, warnings, cautions and other information about the system. The Breckenridge OLIF Intervertebral Body Fusion System Oblique Lumbar Interbody Fusion The Breckenridge OLIF Intervertebral Body System represents another Surgeon Driven Innovation from Lanx. The Breckenridge OLIF system is designed and engineered for ease of insertion and placement. It can be placed into the disc space through a Posterior Lumbar Interbody Fusion (PLIF) or a Transforaminal Lumbar Interbody Fusion (TLIF) approach. For a TLIF procedure, there is no need to turn the implant inside the disc space, as it is designed to be positioned obliquely across the midline. Breckenridge OLIF is manufactured from radiolucent PEEK-OPTIMA and contains tantalum makers for radiographic visualization. Features: A bullet shaped nose for self-distraction and easy insertion Curved endplate surfaces to match endplate anatomy and distribute load. Angled teeth on the endplate surfaces to allow for easy insertion and prevention of retropulsion 3 footprint options and multiple height options to match anatomic conditions A large graft space for autogenous bone graft Low profile for visualization during less invasive procedures Straight or bayoneted instruments for open or MIS procedures Compatible for use with the Descent Guided PEEK Interbody System and the Telluride Port System Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. When used as a lumbar intervertebral body fusion device, the Breckenridge OLIF implant is intended for spinal fusion procedures to be used with autogenous bone graft in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous spinal levels from L2-S1. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have had six months of non-operative treatment. These DDD patients may have had a previous non-fusion spinal surgery at the involved spinal level(s), and may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). The Breckenridge device is to be implanted via an anterior or posterior approach and is to be combined with supplemental fixation. Approved supplemental fixation systems include the Lanx Spinal Fixation Systems (Aspen, Silverton, Silverton-D and Telluride ).
3 Pre-Surgery Preparation Review and inspect all instrumentation and implants prior to sterilization. Caution: Special attention should be given to the inspection of the threads on the Draw Rod (if damaged return to Lanx and use the second Draw Rod supplied in the surgical case). Replace or add any needed components for the planned surgery. Primary surgeon must be fully experienced with the required spinal fusion techniques. Please read the Instructions for Use (IFU) for a list of warnings, cautions, contraindications, risks and product description. Surgical Exposure and Site Preparation 1. The patient is prepped, positioned and draped in the usual fashion. 2. Expose the affected levels via a standard incision and tissue dissection. 3. Perform any necessary bone and tissue removal. 4. Remove disc material and prepare endplates at the appropriate level using Lanx Disc Prep Instruments. Use a combination of curettes, rasps, osteotomes, disc shavers or box chisels to remove the disc material and cartilage from the vertebral endplates. Sequentially larger instruments are used until debridement is complete. 5. After preparing the intervertebral disc space, the bayoneted trials ( XX, -66XX, -67XX) or straight trials ( XX, -63XX, -64XX) are inserted using the t-handle ( ), starting with smallest trial height and length. (Fig. 1) Select the appropriate length of trial (23 mm, 28 mm or 32 mm) using radiographic imaging to fit the anatomic conditions, and insert progressively increasing height of the trials until the appropriate height distraction is achieved. Note: Use of trials is recommended to ensure usage of appropriate sized implant. Fig. 1 1
4 Inserting the Breckenridge OLIF Implant 6. Once the appropriately sized trial has been chosen, select the corresponding sized Breckenridge OLIF implant from the implant caddy. Note: It is recommended to pack the central graft cavity of the implant with autogenous bone graft prior to assembly to the graft inserter. Fig Assemble the OLIF implant to the OLIF inserter, which comes in two styles for optimal visualization during implant placement: with a side handle ( ), or with a palm handle ( ) which must be ordered separately. (Fig. 2) a. Assemble the OLIF inserter draw rod ( ) through the center of the implant inserter and secure by retracting the quick connect thumb wheel of the inserter handle, fully inserting the draw rod, and then releasing the quick connect thumb wheel. (Fig. 3) b. The desired implant is loaded by threading onto the inserter assembly (turn the quick connect thumb wheel in a clockwise rotation). Fig. 3 2
5 8. Insert the OLIF implant into the disc space with the teeth oriented in the cranio-caudal direction. (Fig. 4) Caution: The implant should be inserted with the teeth in direct contact with the endplates and is not intended for rotation within the disc space. a. Slight impaction on the inserter can be used to gently advance the implant into the prepared disc space. Radiographically confirm the position and placement of the implant. b. Remove the inserter by turning the thumb wheel counter-clockwise. c. Optional fine tuning of implant position can be achieved using the straight impactor ( ) until the implant is in its final position. (Fig. 5) Additional graft material can be inserted into the disc space at this time. Fig. 4 Fig. 5 3
6 Closure 9. Compress vertebral bodies on the implant and secure with a Lanx Spinal Fixation System (Aspen, Silverton, Silverton-D or Telluride). 10. Close wound and dress in the usual fashion. POSTOPERATIVE CARE An appropriate analgesic, antibiotic, immobilization, and home care regimen must be followed. Using appropriate surgical dressings while the soft tissues are healing can help prevent injury to the related neural structures, and ultimately achieve fusion. The patient must be warned to avoid falls or sudden jolts. Removing the Breckenridge OLIF Implant 1. Attach the OLIF inserter with either the side handle ( ) or the palm handle ( ), or the OLIF removal instrument ( ) to the implant. 2. Attach the slap hammer ( ) to the instrument or use a mallet to tap the instrument until the implant is removed from disc space. PACKAGING Check packages to ensure all components are intact upon receipt. Check all sets and confirm that all instruments and implants are present. Inspect all set components for functionality to ensure there is no damage prior to use. Immediately return any damaged packages or products to Lanx without using them. HANDLING AND STORAGE Breckenridge implants and instruments must be handled, stored, and opened in such a way that they are protected from inadvertent damage or contamination. The Breckenridge device does not have a labeled shelf life. The aging process has no observed affect on the mechanical performance of the materials. Verify the proper function of the specialized surgical instruments needed for the Breckenridge procedure prior to every surgical procedure. 4
7 CLEANING AND DECONTAMINATION Breckenridge implants and instruments are not supplied sterile. Before sterilization, implants and instruments must be cleaned using the following procedures. Caution: Certain cleaning solutions such as those containing formalin, glutaraldehyde, bleach, and/or other alkaline cleaners may damage some devices, particularly instruments. Such cleaning solutions should not be used. Note: Some instruments may require disassembly prior to cleaning. Note: Special attention should be given to the draw rod. Avoid the use of pliers to disassemble the draw rod. This action will cause damage to the draw rod threads. Machine Cleaning Instructions (Recommended) 1. Prepare cleaning detergent a. Prepare an enzymatic detergent, following the manufacturer s instructions for preparation and use. b. Saline solutions should NOT be used, as saline has a corrosive effect on stainless steel. c. The detergent should have a near-neutral ph to prevent pitting and tarnishing. 2. Prepare devices for soaking a. To prevent injury, separate out sharp and pointed devices and handle with care. b. Disassemble devices with removable parts. c. Open hinged, toothed or threaded joints. d. Remove heavy or large debris using single-use, non-shedding wipes soaked in appropriate cleaning solution. 3. Clean and soak in bath a. Immerse devices in prepared bath. b. Brush all surfaces of the devices with a cleaning brush (do not use steel brushes) while they are submerged in water, ensuring that all visible soil is removed. c. Whenever applicable: i. Use a pipe cleaner and syringe to clean all cannulae, lumens, crevices, grooves and hard to reach areas. ii. Use a syringe to repeatedly apply rinsing solution under pressure to flush all cannulae, lumens, crevices, grooves and hard to reach areas. iii. Repeatedly operate/bend/articulate movable joints while cleaning. iv. Brush the inside of hollow spaces along their entire length. d. Allow devices to soak in detergent bath for the manufacturer s recommended soaking time. 4. Load devices into washer a. Place devices so they do not collide during operation. b. Place heavy items at the bottom and hollow objects in the washing machine baskets. c. Ensure no part is obstructed by large objects. d. Place articulating instruments in the fully open position and cannulated instruments horizontally. e. Place disassembled instruments in the washing machine baskets. 5
8 5. Washing and drying cycles a. 2 minutes: Prewash with cold water; drain. b. 5 minutes: Detergent wash with hot water; drain. c. 2 minutes: Neutralize with neutral ph detergent; drain. d. 2 minutes: Rinse with hot water; drain. e. Dry with hot air at a maximum of 115 C. 6. Inspect a. Inspect the devices with the naked eye under normal lighting conditions to determine if all adherent visible soil (e.g., blood, protein substances and other debris) has been removed from surfaces, lumen, cannulae, crevices, serrations, threading, etc. b. If visible soil remains, repeat the cleaning procedure. 6 Manual Cleaning Instructions 1. Prepare cleaning detergent a. Prepare an enzymatic detergent, following the manufacturer s instructions for preparation and use. b. Saline solutions should NOT be used, as saline has a corrosive effect on stainless steel. c. The detergent should have a near-neutral ph to prevent pitting and tarnishing. 2. Prepare devices for soaking a. To prevent injury, separate out sharp and pointed devices and handle with care. b. Disassemble devices with removable parts. c. Open hinged, toothed or threaded joints. d. Remove heavy or large debris using single-use, non-shedding wipes soaked in appropriate cleaning solution. 3. Clean and soak in bath a. Immerse devices in prepared bath. b. Brush all surfaces of the devices with a cleaning brush (do not use steel brushes) while they are submerged in water, ensuring that all visible soil is removed. c. Whenever applicable: i. Use a pipe cleaner and syringe to clean all cannulae, lumens, crevices, grooves and hard to reach areas. ii. Use a syringe to repeatedly apply rinsing solution under pressure to flush all cannulae, lumens, crevices, grooves and hard to reach areas. iii. Repeatedly operate/bend/articulate movable joints while cleaning. iv. Brush the inside of hollow spaces along their entire length. d. Allow devices to soak in detergent bath for the manufacturer s recommended soaking time. 4. Rinse a. Remove the devices from the soak bath. b. Thoroughly rinse the devices under running water for a minimum of 1 minute. c. Thoroughly flush cannulae, lumens and holes.
9 5. Ultrasonic bath a. Prepare an ultrasonic bath containing a blood-dissolving detergent, following the manufacturer s instructions for preparation and use. b. Cover/seal the devices during transport from the rinse to the ultrasonic bath to prevent contamination. c. Place devices in the ultrasonic bath. d. Ensure that the devices are completely submerged and do not overlap. e. Sonicate for 15 minutes. To avoid corrosion, do not exceed 15 minutes. 6. Rinse in sterile water a. Thoroughly rinse the devices with sterile purified water (i.e., RO or DI) for a minimum of 3 minutes. 7. Dry a. Dry the devices with single-use, non-shedding absorbent wipes and/or medical compressed air (e.g., interiors of cannulae). b. Be sure to completely dry the devices immediately after rinse to inhibit corrosion. 8. Inspect a. Inspect the devices with the naked eye under normal lighting conditions to determine if all adherent visible soil (e.g., blood, protein substances and other debris) has been removed from surfaces, lumen, cannulae, crevices, serrations, threading, etc. b. If visible soil remains, repeat the cleaning procedure. STERILIZATION The Breckenridge device is provided non-sterile and is intended for the user to be cleaned and sterilized by the user. The recommended sterilization process for the implants is steam autoclave sterilization. Implants must be sterilized prior to implantation. Use of a wrap FDA-cleared wrap is recommended to maintain sterility prior to use. The following standard steam sterilization cycle must be used for the Breckenridge OLIF implants and instruments. Breckenridge OLIF Sterilization Protocol Method Cycle Temperature Exposure Time Minimum Dry Time Steam PreVacuum 132 C (270 F) 8 minutes 55 minutes Steam Gravity 132 C (270 F) 18 minutes 55 minutes Instruments are supplied non-sterile and are designed to be reused, which is typical for surgical instruments. Breckenridge Instrument Sterilization Protocol Method Cycle Temperature Exposure Time Minimum Dry Time Steam PreVacuum 132 C (270 F) 8 minutes 55 minutes Steam Gravity 132 C (270 F) 24 minutes 55 minutes The recommended sterilization cycles have been validated to assure a Sterility Assurance Level (SAL) of at least Alternative sterilization methods or cycles may be used for the instruments, but must be validated according to accepted laboratory practice. There is no validated flash sterilization method authorized. 7
10 Instruments for the The Breckenridge OLIF Intervertebral Body Fusion System Slap Hammer T-Handle Bayoneted Trials Height 10x23 11x28 13x32 7 mm mm mm mm mm mm mm mm Available in Bayoneted case straight Trials Height 10x23 11x28 13x32 7 mm mm mm mm mm mm mm mm Available in Straight case Side Handle Inserter Palm Handle Inserter* Threaded Implant Removal Instrument Inserter Draw Rod Straight Impactor Curved Impactor* *Special order Item
11 Implants for the The Breckenridge OLIF Intervertebral Body Fusion System IMPLANTS Height 10x23 11x28 13x32 7 mm mm mm mm mm mm mm mm GRAFT VOLUMES Height 10x23 11x28 13x32 7 mm 0.49 cc 0.65 cc 0.93 cc 8 mm 0.56 cc 0.74 cc 1.06 cc 9 mm 0.63 cc 0.84 cc 1.20 cc 10 mm 0.70 cc 0.93 cc 1.33 cc 11 mm 0.77 cc 1.02 cc 1.46 cc 12 mm 0.84 cc 1.12 cc 1.60 cc 13 mm 0.91 cc 1.21 cc 1.73 cc 14 mm 0.98 cc 1.30 cc 1.86 cc PRODUCT COMPLAINTS Communicate suspected deficiencies in product quality, identity, durability, reliability, safety, effectiveness and/or performance directly to Lanx ( Complaints@lanx.com Tel: ). When filing a complaint, please provide the component name(s), part number(s), lot number(s), your name and address, the nature of the complaint and patient case number. Sterilize and return all component(s) to your local Lanx representative. Notify Lanx immediately of an incident resulting in patient death or serious injury. FURTHER INFORMATION If further directions for use of this system are needed, contact Lanx Customer Service ( CustomerSupport@lanx.com, Tel: , Fax: ).
12 Implant Radiographic Marker Positions 23mm Length: 1.75mm 28mm Length: 2.0mm 32mm Length: 2.25mm 3.5mm 0.8mm Tantalum Marker Dimensions Diameter= 0.9mm Length / Height = 1.6mm With innovative solutions uniquely designed by surgeons for surgeons, Lanx specializes in devices and systems for all segments of spinal surgery. Integrating leading technology and state-of-the art engineering, our products have been created to meet the specific surgical needs of our customers and improve outcomes for their patients. Lanx, Inc. 310 Interlocken Parkway, Suite 120, Broomfield, Colorado Ph Fax Lanx, Inc. All rights reserved. Lanx, Surgeon Driven Innovation, Breckenridge, Aspen, Silverton, Silverton-D, Telluride and other associated designs and logos are registrations or trademarks of Lanx, Inc. PEEK-OPTIMA is a registration or trademark of Invibio Limited Corporation. Important: Review the instructions for use for a list of warnings, cautions, contraindications, risks and product descriptions. LIT
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Zavation Z-Link Cervical
 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
SURGICAL TECHNIQUE MANUAL. InterFuse T
 1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
1 CONTENTS InterFuse T Product Description 3 Indications for Use 3 X-Ray Marker Locations 4 Product Specifications 4 Instrument Set 5 Step 1 Preoperative Planning 8 Patient Positioning 8 Step 2 Disc Removal
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20
 VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
VTI INTERFUSE T SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. 1/20 CONTENTS InterFuse T Product Description Indications for Use X-Ray Marker Locations Product Specifications Instrument Set 3 4 5 STEP
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Silverstone Interbody System Surgical Technique
 Silverstone Interbody System Surgical Technique TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Disc Space Preparation 2. Distraction 3. Implant Selection 4. Implant Placement
Silverstone Interbody System Surgical Technique TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Disc Space Preparation 2. Distraction 3. Implant Selection 4. Implant Placement
O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion
 O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion A new implant for an evolving procedure Unique wedge nose Adaptive longer sizing Large internal graft volume Introduction Stryker Spine enters a new
O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion A new implant for an evolving procedure Unique wedge nose Adaptive longer sizing Large internal graft volume Introduction Stryker Spine enters a new
Imola Lateral IBF System Surgical Technique
 Imola Lateral IBF System Surgical Technique IMOLA CIRCUIT TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Table Mounting 2. Surgical Planning & Targeting 3. Access and Preparation
Imola Lateral IBF System Surgical Technique IMOLA CIRCUIT TABLE OF CONTENTS Design Rationale Instructions for Use Surgical Technique 1. Table Mounting 2. Surgical Planning & Targeting 3. Access and Preparation
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Surgical Technique Manual
 InterFuse S Interbody Fusion System Surgical Technique Manual VERTEBRAL TECHNOLOGIES MS 4043-02 Rev. O Product Overview Introduction The VTI InterFuse S implant is an interbody fusion device that combines
InterFuse S Interbody Fusion System Surgical Technique Manual VERTEBRAL TECHNOLOGIES MS 4043-02 Rev. O Product Overview Introduction The VTI InterFuse S implant is an interbody fusion device that combines
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK.
 VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. CONTENTS InterFuse S Product Description Indications for Use X-Ray Marker Locations and Product Specifications Instrument Set 3 4 5-7 STEP
VTI INTERFUSE S SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. CONTENTS InterFuse S Product Description Indications for Use X-Ray Marker Locations and Product Specifications Instrument Set 3 4 5-7 STEP
POSTERIOR SPINE TRUSS SYSTEM
 POSTERIOR SPINE TRUSS SYSTEM ORDERING & SHIPPING ALL FIELDS ARE REQUIRED ORDER FORM PLEASE PRINT LEGIBLY RECEIVING YOUR PSTS TRAYS & IMPLANTS All implant bins and trays have a warehouse number on them
POSTERIOR SPINE TRUSS SYSTEM ORDERING & SHIPPING ALL FIELDS ARE REQUIRED ORDER FORM PLEASE PRINT LEGIBLY RECEIVING YOUR PSTS TRAYS & IMPLANTS All implant bins and trays have a warehouse number on them
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
AVS ARIA TM Product Overview
 AVS ARIA TM Product Overview Table of Contents Indication 3 Adaptive Sizing 4-5 Wedge Nose Design 6 Graft Volumes 7 Efficient Fixation 8 Lordotic Descriptions 9 Anatomical Descriptions 10 Visualization
AVS ARIA TM Product Overview Table of Contents Indication 3 Adaptive Sizing 4-5 Wedge Nose Design 6 Graft Volumes 7 Efficient Fixation 8 Lordotic Descriptions 9 Anatomical Descriptions 10 Visualization
Solitaire Anterior Spinal System
 Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Advantage ALIF. Keith Shevlin Managing Director
 Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
MODULAR DESIGN OFFERS FREEDOM OF CHOICE. Surgical Technique
 MODULAR DESIGN OFFERS FREEDOM OF CHOICE Surgical Technique Joint Spine Sports Med MectaLIF Anterior Surgical Technique 2 INDEX 1. INTRODUCTION 4 1.1 Material & Marker 5 2. INDICATIONS 5 3. CONTRAINDICATIONS
MODULAR DESIGN OFFERS FREEDOM OF CHOICE Surgical Technique Joint Spine Sports Med MectaLIF Anterior Surgical Technique 2 INDEX 1. INTRODUCTION 4 1.1 Material & Marker 5 2. INDICATIONS 5 3. CONTRAINDICATIONS
Commander Cervical Cage - SURGICAL TECHNIQUE
 Commander Cervical Cage - SURGICAL TECHNIQUE D e s i g n e d to c l o s e l y f i t yo u! Commander Cervical Cage - SURGICAL TECHNIQUE Indications Commander cervical cages are designed primarly for restoring
Commander Cervical Cage - SURGICAL TECHNIQUE D e s i g n e d to c l o s e l y f i t yo u! Commander Cervical Cage - SURGICAL TECHNIQUE Indications Commander cervical cages are designed primarly for restoring
Thoracolumbar Solutions. Zyston Curve. Interbody Spacer System. Surgical Technique Guide
 Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Thoracolumbar Solutions Zyston Curve Interbody Spacer System Surgical Technique Guide 2 Zyston Curve Interbody Spacer System Surgical Technique Guide The Zyston Curve Interbody System is designed to optimize
Surgical Technique SUSTAIN -O. Radiolucent Spacer
 Surgical Technique SUSTAIN -O Radiolucent Spacer At Globus, we move with a sense of urgency to deliver innovations that improve the quality of life for patients with spinal disorders. We are inspired by
Surgical Technique SUSTAIN -O Radiolucent Spacer At Globus, we move with a sense of urgency to deliver innovations that improve the quality of life for patients with spinal disorders. We are inspired by
Technique Guide. T-PAL. Transforaminal posterior atraumatic lumbar spacer system.
 Technique Guide T-PAL. Transforaminal posterior atraumatic lumbar spacer system. Table of Contents Introduction T-PAL 2 AO Principles 4 Indications and Contraindications 5 Surgical Technique Preparation
Technique Guide T-PAL. Transforaminal posterior atraumatic lumbar spacer system. Table of Contents Introduction T-PAL 2 AO Principles 4 Indications and Contraindications 5 Surgical Technique Preparation
Ardis. Surgical Technique. Interbody System. Solutions by the people of Zimmer Spine. zimmerspine.com
 Ardis Interbody System Surgical Technique Solutions by the people of Zimmer Spine. zimmerspine.com A more advanced interbody solution. From the people of Zimmer Spine. The easily inserted Ardis Interbody
Ardis Interbody System Surgical Technique Solutions by the people of Zimmer Spine. zimmerspine.com A more advanced interbody solution. From the people of Zimmer Spine. The easily inserted Ardis Interbody
Interbody fusion cage for the transforaminal approach. Travios. Surgical Technique
 Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
ATHLET VBR. The real winner. VBR in PEEK-OPTIMA PRODUCT INFORMATION
 ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
Cervical Solutions. Alta. ACDF System. Surgical Technique Guide
 Cervical Solutions Alta ACDF System Surgical Technique Guide 2 Alta ACDF System Surgical Technique Guide A comprehensive system to address a continuum of fixation requirements and anatomic demands. Alta
Cervical Solutions Alta ACDF System Surgical Technique Guide 2 Alta ACDF System Surgical Technique Guide A comprehensive system to address a continuum of fixation requirements and anatomic demands. Alta
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
SURGICAL TECHNIQUE GUIDE
 The following general surgical technique is for illustrative purposes only. As with all surgical procedures, the technique used in each case will depend on the surgeon s medical judgment as to the best
The following general surgical technique is for illustrative purposes only. As with all surgical procedures, the technique used in each case will depend on the surgeon s medical judgment as to the best
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
The Implant. (Klappen außen sind nur 192 mm breit!)
 SEMIAL product information The Implant SEMIAL implants are designed to restore interbody vertebral disc height and maintain solid bony fusion when used in conjunction with bone graft material and supportive
SEMIAL product information The Implant SEMIAL implants are designed to restore interbody vertebral disc height and maintain solid bony fusion when used in conjunction with bone graft material and supportive
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
INTERVERTEBRAL BODY FUSION DEVICE. Surgical Technique
 INTERVERTEBRAL BODY FUSION DEVICE Surgical Technique Joint Spine Sports Med MectaLIF Oblique & Posterior Surgical Technique 2 INDEX 1. INTRODUCTION 4 1.1 Materials & Markers 5 2. INDICATIONS 6 3. CONTRAINDICATIONS
INTERVERTEBRAL BODY FUSION DEVICE Surgical Technique Joint Spine Sports Med MectaLIF Oblique & Posterior Surgical Technique 2 INDEX 1. INTRODUCTION 4 1.1 Materials & Markers 5 2. INDICATIONS 6 3. CONTRAINDICATIONS
SURGICAL TECHNIQUE. Minimally Invasive Lateral Approach. Spinal fusion...simplified HA-COATED MINUTEMAN G3-R
 Minimally Invasive Lateral Approach SURGICAL TECHNIQUE Spinal fusion...simplified HA-COATED MINUTEMAN G3-R THE MINUTEMAN G3-R DESIGN RATIONALE The Minuteman G3-R is a minimally invasive, interspinous-interlaminar
Minimally Invasive Lateral Approach SURGICAL TECHNIQUE Spinal fusion...simplified HA-COATED MINUTEMAN G3-R THE MINUTEMAN G3-R DESIGN RATIONALE The Minuteman G3-R is a minimally invasive, interspinous-interlaminar
BAK/C Cervical Anterior Interbody Fusion System
 Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
TECHNICAL BROCHURE. Capture Facet Fixation System
 TECHNICAL BROCHURE Capture Facet Fixation System Table of Contents Product Overview...2 Instruments...4 Capture Facet Screw Surgical Technique Patient Preparation and Positioning...6 Guide Pin Placement...7
TECHNICAL BROCHURE Capture Facet Fixation System Table of Contents Product Overview...2 Instruments...4 Capture Facet Screw Surgical Technique Patient Preparation and Positioning...6 Guide Pin Placement...7
OPERATIVE TECHNIQUE PTC PEEK FORZA. spacer system
 OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
OPERATIVE TECHNIQUE PTC PEEK FORZA spacer system TABLE OF CONTENTS Introduction 1 Operative Technique 2 Instruments 12 FORZA PEEK Part Numbers 20 FORZA PTC Part Numbers 22 Modular Implant Inserter 24 Disassembly
A U X I L I A R Y C O N N E C T O R S Surgical Technique
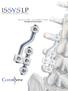 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Transforaminal Lumbar Interbody Fusion Cage (TLIF)
 Transforaminal Lumbar Interbody Fusion age (TLIF) 990100010 DOULE ENGINE MEDIL MTERIL O., LTD. No. 218 Houxiang Road, Haicang District, Xiamen 361022, P.R.hina Tel: +86 592 6087101 Fax: +86 592 6587078
Transforaminal Lumbar Interbody Fusion age (TLIF) 990100010 DOULE ENGINE MEDIL MTERIL O., LTD. No. 218 Houxiang Road, Haicang District, Xiamen 361022, P.R.hina Tel: +86 592 6087101 Fax: +86 592 6587078
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
XYcor Expandable Spinal Spacer System
 XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
SYNEX The vertebral body replacement with ratchet mechanism
 SYNEX The vertebral body replacement with ratchet mechanism Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image
SYNEX The vertebral body replacement with ratchet mechanism Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Surgical Technique. TraXis. Precision. Transforaminal Lumbar Interbody Fusion Spacer. The Art & Science of Spine Surgery
 Surgical Technique Precision TraXis Transforaminal Lumbar Interbody Fusion Spacer The Art & Science of Spine Surgery Table of Contents Introduction 3 Indications 4 Key Instruments 5 Surgical Technique
Surgical Technique Precision TraXis Transforaminal Lumbar Interbody Fusion Spacer The Art & Science of Spine Surgery Table of Contents Introduction 3 Indications 4 Key Instruments 5 Surgical Technique
Aesculap Spine T-Space PEEK. Transforaminal Lumbar Interbody Fusion System
 Aesculap Spine T-Space PEEK Transforaminal Lumbar Interbody Fusion System Forword Growing socio-economic pressure in conjunction with the high incidence of spinal disorders and consequent conditions calls
Aesculap Spine T-Space PEEK Transforaminal Lumbar Interbody Fusion System Forword Growing socio-economic pressure in conjunction with the high incidence of spinal disorders and consequent conditions calls
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Fusion Device. Surgical Technique. Cervical Interbody Fusion with Trabecular Metal Technology
 TM-S Fusion Device Surgical Technique Cervical Interbody Fusion with Trabecular Metal Technology 2 TM-S Fusion Device Surgical Technique Disclaimer This surgical technique is not intended for use in the
TM-S Fusion Device Surgical Technique Cervical Interbody Fusion with Trabecular Metal Technology 2 TM-S Fusion Device Surgical Technique Disclaimer This surgical technique is not intended for use in the
Apache Interbody Surgical Technique. LC004 Rev F
 LC004 Rev F Apache Interbody Surgical Technique Apache Anterior Lumbar Interbody Fusion Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion
LC004 Rev F Apache Interbody Surgical Technique Apache Anterior Lumbar Interbody Fusion Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion
Veyron -C Anterior Cervical System Surgical Technique
 Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
