superficial proximal tubular length, nephron number and kidney weight
|
|
|
- Alvin Benson
- 5 years ago
- Views:
Transcription
1 J. Physiol. (1977), 272, pp With 8 teztft urea Printed in Great Britain DEVELOPMENTAL CHANGES IN NEPHRON NUMBER, PROXIMAL TUBULAR LENGTH AND SUPERFICIAL NEPHRON GLOMERULAR FILTRATION RATE OF RATS BY SIDNEY SOLOMON From the Department of Physiology, University of New Mexico, School of Medicine, Albuquerque, New Mexico 87131, U.S.A. (Received 4 January 1977) SUMMARY 1. Postnatal development of single nephron glomerular filtration rate, superficial proximal tubular length, nephron number and kidney weight have been studied in Sprague Dawley and in Wistar rats. 2. Superficial tubular length is a nonlinear function of body weight or age. There seems to be a rapid growth until animals weigh about 15 g in Wistar rats. In this strain, growth is slower thereafter. This difference is not as evident in Sprague Dawley rats. 3. Nephron numbers increase over the same period at which rapid tubular growth occurs. 4. Sprague Dawley rats have somewhat fewer, but longer, proximal tubules than do Wistar rats. 5. In all animals weighing more than 1 g, SNGFR is linearly related to ikeight. For younger, smaller Sprague Dawley rats, the same linearity holds over the age range studied older than 2 days of age. In Wistar rats, SNGFR relative to weight is less in young animals. 6. By relating SNGFR to total kidney GFR, evidence is obtained that maturation of renal function also involves a greater increase in filtration by superficial than by juxtamedullary nephrons. INTRODUCTION There are several reasons for carrying out the experiments to be described. Although the postnatal pattern of development of glomerular filtration (GFR) has been studied in quite a few mammalian species (Horster & Lewy, 197; Horster & Valtin, 1971; Potter, Jarrah, Sakai, Harrah & Holliday, 1969; Rubin, Burch & Rappaport, 1949; Solomon & Capek, 1972a, b; Spitzer & Brandis, 1974, for example), relatively few micropuncture investigations have been carried out. In the rat, a relatively restricted study on changes in superficial single nephron glomerular
2 574 S. SOLOMON filtration rate (SNGFR) has been done in this laboratory (Solomon & Capek, 1972a). Spitzer & Brandis have done a more complete micropuncture investigation of changes in SNGFR in baby guineapigs (Spitzer & Brandis, 1974). Sin,e the rat is the experimental animal used in so much micropuncture work, it was thought desirable to make a more complete study of developmental changes in SNGFR over an extended time period, not only during the immediate postnatal period, but also during the time of mature growth. A second issue to be considered in these studies is concerned with the fact that there are differences in the absolute values of SNGFR reported from different laboratories. One possibility which has been considered and shown in one study (Brenner & Daugherty, 1972) is that differences exist in different strains of rats. It was decided, therefore, to examine if the developmental patterns in Sprague Dawley and Wistar rats might be different. In addition, some attention has been paid to developmental changes in morphological parameters such as glomerular number (Bonvalet, Champion, Wanstok & Berjal, 1972) and tubular lengths and diameters (Solomon, 1974; Spitzer & Brandis, 1974; Wahl & Schnermann, 1969), but few studies exist which relate these changes to development of filtration. For example, de Rouffignac & Bonvalet found a relationship between proximal tubule length and SNGFR (de Rouffignac & Bonvalet, 197). Thus, they observed that juxtamedullary nephrons were longer and had a higher SNGFR than did superficial nephrons of the same kidney. Although the relationship between proximal tubule length and SNFGR has been studied in the guinea pig (Spitzer & Brandis, 1974), it is not known in the rat what the relationship between proximal tubule length and SNFGR is during the developmental process. It was decided to do a more complete study of changes in glomerular filtration, not only during certain aspects of immature development, but also extending into mature growth. This study reports on (a) the magnitude of superficial SNGFR in animals older than 2 days, (b) on the relationship between SNGFR and GFR, (c) on changes in the number of glomeruli during maturation, and (d) on changes in proximal tubular lengths over the same ranges of development and growth. In addition, the developmental patterns have been examined in both Sprague Dawley and Wistar rats. METHODS All of the experiments to be described were carried out on Wistar and Sprague Dawley rats obtained from Simonsen (Gilroy, California). In most studies, pregnant mothers were shipped to this laboratory. The pups were weaned 21 days after birth and were studied at varying times thereafter. Animals were not used unless there
3 RENAL DEVELOPMENT OF IMMATURE RATS 575 were at least ten and no more than thirteen siblings in a litter. Some data on Sprague Dawley rats were taken from studies carried out for other purposes. These studies were done on mature animals of known age which were obtained from Simonsen and then were acclimated to this laboratory for at least 1 week before use. No physiological studies are reported from these animals. They have been used only to provide additional data on superficial nephron proximal lengths. Because the rate of growth of these animals may have been different from that in our own laboratory, the length data are plotted as a function of body weight rather than age. Micropuncture studies and tubular measurements The preparative procedures used for measuring SNGFR and whole kidney glomerular filtration GFR have been described before in publications from this laboratory (Solomon, 1974; Solomon & Capek, 1972a; Sonnenberg & Solomon, 1969). After animals were anaesthetized, a jugular vein was cannulated for infusion of Ringer containing [3H]inulin; the ureter of the left kidney was catheterized for urine collection into preweighed plastic tubes. The left kidney was then mounted in a plastic cup, bathed with oil and illuminated for micropuncture studies. Collections were made under 'controlled suction'. Although controversy exists about the validity of measuring SNGFR by sample collection from the proximal tubule (Davis, Schnermann & Horster, 1972; Knox, Ott, Cuche, Gasser & Haas, 1974; Navar, Burke, Robinson & Clapp, 1974; Schnermann, Davis Wunderlich, Levine & Horster, 1971), it was necessary to use this site for sampling. In the youngest Wistar rats used in these studies (2 days old), there are but a few distal tubules on the surface. Additional problems arise because distal tubules are very narrow, thereby making puncture very difficult, and also because tubular fluid flow rates are very slow. Therefore, the use of distal tubules was precluded for measuring SNGFR. Micropuncture and clearance studies were started two hours after start of Ringer infusion. Five to nine samples were taken from each animal. SNGFR was measured by taking timed collections of fluid and transferring them in toto to vials containing a scintillation cocktail. Radioactivity was later counted using a Packard TriCarb liquid scintillation counter. Total GFR was measured as described before. At the termination of the studies, tubules were filled from a latexfilled pipette. The kidney was removed, digested in hydrochloric acid, washed with tap water and stored under tap water for one to three days. The casts of the tubules were dissected free and the length of the pars convolute only was measured using a microscope fitted with ocular micrometer. Measurements were accepted only when the tubules were attached to the glomeruli from which they originated. This segment was used since it was found possible to identify the separation between the straight and convoluted portions of the proximal tubule, but it was often difficult to see a clear separation between the end of the pars recta and the start of the thin loop of Henle. Some of the data obtained on tubule lengths of Sprague Dawley rats are taken from information obtained in a previous investigation (Sonnenberg & Solomon, 1969). Glomerular counts, kidney weigh and body weigts Other rats were used for the determination of glomerular numbers. Counting of glomeruli was started when animals were fivedays old and were continued at varying time intervals thereafter. Siblings from single litters were counted at varying times after birth in order to reduce any potential bias which could result from litter differences. Animals were removed from their cages in the late forenoon and were weighed and anaesthetized. The kidneys were removed, decapulated, weighed and macerated in 5 % hydrochloric acid at 37 'C, following in general the modification by Bonvalet,
4 576 S. SOLOMON Champion, Wanstok & Berjal (1972) of the method of Damadian, Shwayri & Bricker (1965). It was necessary to modify the procedure further, however, in this laboratory. When kidney weight (KW) was less than 5 g, effective glomerular separation could be achieved after 15 min of digestion. With kidneys weighing more than this, the central portion of the kidney could not be macerated easily with this time of digestion. Increasing the time of incubation resulted in having the outer cortex overdigested. It was decided to cut the kidneys into more than one piece when this critical weight was exceeded and to add together parts of broken glomeruli during the counting procedure. Glomerular parts were easily identified since they were more yellowbrown than tubule fragments, probably because of containing acid haemoglobin. An estimate was made of the fraction of a total glomerulus which was contained in each fragment. The fragments then were added together to the nearest glomerulus. The maximum number of glomeruli represented by fragments was always less than 2 %. This process did not result in glomerular counts different from those obtained by counting kidney sections (unpublished data of Roy Horst, Department of Anatomy, University of Vermont), and counts will be shown to be consistent with results obtained by others. After digestion, the kidney or kidney pieces were washed and transferred into 1 ml. volumetric flasks halffilled with tap water. They were kept in the refrigerator for 24 hr and then separated by gentle shaking. They were brought to volume and at least two 5 ml.. aliquots were then counted. Not all glomeruli were of the same size. One could not separate out immature and mature glomeruli by these techniques, although such a separation would have been desirable. Duplicate counts were accepted as valid when they did not differ by more than 1%. Most duplicates were actually within 5 % of each other. If, however, the differences between successive counts were excessive, counting was repeated until consistency was obtained. There are precautions which must be taken in order to get reasonable results with this method. Problems with digestion have been considered above. If, in the course of maceration, shaking is excessive, both tubules and glomeruli become highly fragmented and the apparent glomerular count is decreased. Further fragmentation can be caused by using pipettes with narrow openings, and measuring pipettes should be used with opening bores greater than 1 mm. Samples should be removed within 15 sec after termination of mixing. Differences in counts have been found between samples taken from the bottom and the top of the flask at longer times probably because of gravitational sedimentation of the glomeruli. High counts of aliquots taken from the bottom of the flask are particularly evident in samples obtained from kidneys of older, larger animals. As a final precaution, one should transfer the aliquot as rapidly as possible or else sedimentation within the pipettes can produce errors. RESULTS Anatomical considerations Fig. 1 shows the relationship between left kidney weight and body weight (BW) for both strains of rats. For both strains, no significant differences between right and left kidneys have been found, and no difference was found in the relationship between kidney weight and body weight in females. The best mathematical fit of the relationship between kidney weight and body weight has been shown to be that of a double reciprocal (Solomon & Bengele, 1974). For female Sprague Dawley rats, the equation is found to be 1/KW = /BW and for males, it is
5 RENAL DEVELOPMENT OF IMMATURE RATS 577 1/KW = X /BW. For Wistar females, it is 1/KW = / BW and for males, it is 1/KW = /BW. Using the standard errors of the regression coefficient (3.65 for male Sprague Dawley and 48 for male Wistars) it can be calculated that the kidneys of male Sprague Dawley rats are significantly, if only slightly, larger than those of male Wistars at body weights of 25 g or more (P <.5). No significant difference could be established for female rats ,11 4 g 1 _ C , r it o Body wt. 4 5 I * *.Ad*/ Ow..j#. > 6 c U o 1 1~~~~~~~~~~~~~~~~~~~~~~~~~~~~ Body wt. (g) Fig. 1. Relationship between kidney weight and body weight. Top panel shows the relationship between kidney weight and body weight for male rats. The lower panel shows the same relationship for female rats. Note change of scales between upper and lower panels. Filled circles show data obtained from Wistar strain rats, open circles show data from Sprague Dawley strain.
6 578 S. SOLOMON Since in Wistar rats, it has been shown that kidney weight to body weight ratio is inversely relate& to growth rate for up to at least 4 days postnatally (Solomon & Bengele, 1973; Solomon & Capek, 1972b), the possibility that the male Sprague Dawley rats grow slower than the Wistars was examined. No such difference in growth rate was detected (Fig. 2). 5 K 41 3F cm 3: co 2F 8 * T a 11 U 4. ). so i o Age (days) 8 1 Fig. 2. Relationship between body weight and age of neonatal rats of the Wistar strain (filled circles) and of Sprague Dawley strain (open circles). Two obvious possibilities exist as to differences in the developmental processes which underlie the difference in kidney weight. There could be different numbers of nephrons in the two strains, or else the mass of each
7 RENAL DEVELOPMENT OF IMMATURE RATS 579 nephron could be different. In Fig. 3, the number of glomeruli as a function of age is shown for both species. No sex difference exists in the number of glomeruli for either strain of rat. In both strains of rats, the developmental patterns are similar. Thus, there is an increase in the number of glomeruli 35 r 3k 25p. * O To * * O a Go. 8 ) 2 F Cu ' 15 F C,, 1. E C X 35 o3 I.. I 25 o I o. I~ no 8 2k o 15k o ic Age (days) Fig. 3. Dependence of glomerular number of age on rats. Upperpanel represents data from Wistar strain and lower panel from Sprague Dawley. Data from males are filled circles and open circles are from females. until animals are 346 days old. If one puts the data in terms of kidney weight, the full complement of glomeruli is attained when the kidney has reached a weight between 7 and 8 g for Wistar rats. Referring the number of glomeruli to kidney weight may be more appropriate since, in unpublished studies, we have found that fast growing Wistar rats reach 24 PHY 272 I
8 58 S. SOLOMON their full complement at the same kidney weight but at an earlier age than normally growing rats. Furthermore, one cannot ascribe the difference in kidney weight between male rats of the two strains to differences in the number of nephrons. On the contrary, a small but significant difference in nephron number in the opposite direction to that which one might expect on the basis of kidney 7 6 E 8 o. 4 _ *O o o S o. CD 2 I~~~C Body wt. (g) Fig. 4. Changes in proximal tubular length with body weight of superficial nephrons of left kidney of Wistar (filled circles) and Sprague Dawley (openl circles) strains of rats. weight, is found in these studies. Sprague Dawley rats have a lesser number of glomeruli than do Wistars despite the fact that they have bigger kidneys. If one fits the developmental curves with couple reciprocal curves, the number of nephrons of rats over 15 g in weight is 31, for Wistar, and 28, for Sprague Dawley. Although this is but a difference of 27 nephrons, it is a highly significant difference (P <.1). The right kidney has more nephrons than the left by a small number. Thus, in Sprague Dawley rats, glomerular counts of the right kidney are greater by 132±+49 (P <.2). In Wistar rats, the difference is (P <.1). One would expect that the differences between the two strains of rats could be explained by differences in nephron mass. Fig. 4 shows the developmental pattern of superficial proximal tubular length. Because some of the
9 RENAL DEVELOPMENT OF IMMATURE RATS 581 data is taken from animals used for other studies (Sonnenberg & Solomon, 1969), ages and kidney weights are not always available and, as a result, the lengths are expressed as a function of body weight. By referring, however, to Figs. 1 and 2, it is possible to approximate the corresponding ages from the body weights. As has been shown previously (Solomon, 1974), there are two apparent phases of growth of the tubules in Wistar rats, a rapid phase until animals are between 125 to 15 g weight, followed by a 4 3 U 2 z 1 _ * o. I, I,, I Body wt. (g) Fig. 5. Relationship between SNGFR and body weight of Wistar (filled circles) and Sprague Dawley (open circles) strains of rats. phase of slower growth. This weight would correspond to an age of 35 4 days. Statistical analysis of the regression between body weight and tubular length shows that in Sprague Dawley rats, tubules are longer than in Wistar rats for animals over 15 g body weight. Sprague Dawley rats have the relationship, length = ( ± 3.7) body weight. The number in parenthesis indicates the standard error of the regression. For Wistar rats, the relationship is tubular length = (± 2.649) body weight. Over the range of mature animal weights studied, tubules from Sprague Dawley rats are significantly longer than those from Wistars. The absence of a significant slope between body weight and tubular length would show that in this strain there is also a change in growth rate in mature animals as compared to growth rates in young pups. 242
10 582 S. SOLOMON Development of glomerular filtration rate When total kidney GFR of animals weighing over 15 g is expressed in terms of body weight, no significant differences are evident. In Sprague Dawley rats, filtration rate is 3 39 ml./min. kg ( ±.143), while in Wistars, it is 377 (+ 251) ml./min. kg. Nor are there any differences when expressed in terms of kidney weight ( ml./min. g for Sprague o1 r n 1 4 C 9 F 8 1 CU E,. CU. Q 7 F , a co I Age (days) Fig. 6. Apparent number of nephrons (obtained by dividing GFR by SNGFR) as a function of age. Filled circles show data from Wistar strain. Open circles show data from Sprague Dawley strain. Circled points indicate data from Wistar strain rats weighing less than 5 g. o * I I 7 Dawley versus ml./min. g for Wistar rats). Measurements in GFR of animals younger than this show the previously described age dependent increase (Horster & Lewy, 197; Horster & Valtin, 1971; Solomon & Capek, 1972b).
11 RENAL DEVELOPMENT OF IMMATURE RATS 583 As far as SNGFR is concerned, there is an increase as the animals develop. As shown in Fig. 5, no obvious difference exists between the two different strains for older animals. In order to compare these data in terms of body weight to those of other workers they will be discussed further. The calculation of SNGFR per 1 g body weight for animals heavier than 15 g is 1'3 + 8 nl./min for Sprague Dawley rats and nl./min for Wistar rats. For Sprague Dawley rats weighing more than 5 g, there is no significant difference in the relationship. In Wistar rats, SNGFR/I g body weight is less in neonates than in mature animals. In animals weighing 575 g, it is 67 nl./min; for animals weighing 75 1 g, it is 1*9 nl./min. Although the last figure is not statistically different from SNGFR of animals weighing over 15 g, at lower weights the difference is probably valid (P <.5). Since it has been shown that there is a redistribution of filtration to superficial nephrons during early development (Solomon & Capek, 1972b; Spitzer & Brandis, 1974), it was decided to determine what the developmental pattern of this redistribution is in rats. To get at this problem, an apparent number of nephrons was calculated by dividing total kidney GFR by SNGFR. Fig. 6 shows the apparent number of nephrons as a function of age. No difference between strains is seen. A marked decrease occurs in the apparent number of nephrons from the time animals reach an age of about 4 days. Older rats do not seem to show any decrease in apparent number of nephrons. Fig. 6 also includes nephron numbers on a few Wistar pups weighing less than 5 g and aged 222 days old. Despite the large scatter of the data, the apparent nephron number is less in some animals when they weigh less than 455 g than in the age range of 254 days. DISCUSSION Following birth, the GFR per gram kidney weight shows an increase until some postnatal time at which the value is constant. Changes in GFR have generally been attributed to two factors: (a) an increase in the number of filtering nephrons, and (b) an increase in the amount of filtrate per nephlon. The results obtained in this study generally support both of these views. Thus, we find an increase in the number of nephrons over about the first 5 days postnatally and secondly, we find an increase in absolute values of SNGFR throughout the age span and range of animal weights used in this study. These events occur in both strains. The results of this study also support a second conclusion which can be made regarding development of overall renal function. The fact that the apparent number of nephrons decrease in animals weighing over 5 g until they reach about 4 days of age indicates that superficial nephron GFR increased relatively
12 584 S. SOLOMON more than did juxtamedullary nephron glomerular filtration rate (JNGFR) over this period of development. (The increase in apparent number of nephrons in the smaller animals probably reflects the developmental increase in the number of filtering nephrons.) After that, filtration rates of both populations of nephrons increase proportionally. Total GFR/g kidney wt. (or GFR expressed as a function of body weight) reaches stable values before the changes in distribution of nephron filtration rate are completed (Horster & Valtin, 1971; Sonnenberg & Solomon, 1969). As a result, it should be recognized that just measuring total GFR per se need not indicate maturation. These studies show that development of changes in distribution of GFR are also a part of the maturation process. CD _ 16 C. C E 1 C c8 *. 6 <4 2_ 1~~~~ Body wt. (g) Fig. 7. Apparent number of nephrons (calculated by dividing GFR by SNGFR) for guineapigs of different weights. Points calculated from data of Spitzer & Brandis (1974). A similar conclusion is implicit in the work of Spitzer & Brandis on the guineapig (Spitzer & Brandis, 1974). They calculated that JNGFR (juxtamedullary nephrons) increased for the first 15 days postnatally, and then remained constant while SNGFR increased continuously over the 3 postnatal days of their study. If one calculates an apparent number of nephrons as a function of animal weight, there is an increase over the time range of that study (Fig. 7) (in contrast with the findings in rats, see Fig.6). In a comparable micropuncture investigation by Horster & Valtin on the dog, an apparent number of nephrons calculated from their data do not
13 RENAL DEVELOPMENT OF IMMATURE RATS 585 show any age dependent changes (Horster & Valtin, 1971). The values are so scattered, however, that only a marked trend would be evident. Studies on different species therefore show that there are at least quantitative differences in the pattern of development. If, in fact, the ratio of filtration by superficial and juxtamedullary nephrons remains constant during development of the dog, there may even be qualitative differences between species. Both strains of rats do not develop in quantitatively identical patterns. In the youngest and smallest Wistar rats which were punctured, the SNGFR when normalized in terms of body weight was less than that of the adult. (As will be discussed later, an adult is defined by me as an animal being more than 4 days old or weighing more than 15 g.) On the TABLE 1. SNGFR of Wistar and Sprague Dawley rats as found in various laboratories Wistars Weight range SNGFR (g) (nl./min. 1 g) Reference 254.4* 93 ± 3t Coelho, J. B. (1973) t Horster, M. & Lewy, J. E. (197) Solomon, S. (1974) Damadian, R. V., Shwayri, E. & Bricker, N. S. (1965) 14±6 Coelho, J. B., Chien, K.C.H. & Bradley, S. E. (1972) Sprague Dawleys Weight range SNGFR (g) (nl./min. 1 g) Reference Bartoli, E. & Earley, L. E. (1973) 318.1* Coelho, J. B. (1973) * Only average body weights given. t Values obtained on MunichWistar strain. Weights of animals not given in the data. other hand, SNGFR per 1 g body weight of Sprague Dawley rats was the same over all ranges of weight and age used in these studies. These studies indicate that during development of Wistar rats, the filtration rate of individual nephrons first show a disproportionately large increase after the onset of filtration and later show slower changes which may reflect general aspects of growth; i.e. the continuous increase in body size and organ weights which accompany aging. Whether comparable changes also occur in Sprague Dawley rats cannot be concluded from this study since
14 586 S. SOLOMON such a developmental pattern could have taken place at a postnatal time earlier than that of the animals used in this study. Another point of interest is that the mature stable SNGFR, when expressed in terms of body weight, is greater in Sprague Dawley rats than in Wistars. Brenner obtained comparable results using the MunichWistar strain (Brenner &. Daugherty, 1972). Table 1 shows a summary of data (as originally presented or recalculated) which have appeared in the literature and wherein animal weights were published so that it has been possible to normalize SNGFR in terms of body weight. It is clear that Wistar rats, except for the Munich strain, do have higher SNGFR than do Sprague Dawley in other laboratories. Also, this way of normalizing the data results in a marked reduction in quantitative differences between laboratories (Wright & Giebisch, 1972). In contrast to the above, total kidney GFR is not significantly different between strains although Wistar rats do have a higher mean GFR. If one considers that GFR is about the same per kidney in both strains, while nephron number and SNGFR are lower in mature Sprague Dawley rats, one would predict that Sprague Dawley rats would have a greater JNGFR. Another possibility is that the number of juxtamedullary nephrons is greater in Sprague Dawley rats and that they have a higher nephron GFR. This point has not been established. The fact that nephron numbers are lower in Sprague Dawley rats while the kidney weight is larger in older male rats and the same as Wistars in females, can be explained by the observation that the superficial proximal convolutions are longer. If the remainder of the nephron has comparable proportions in both strains, the Sprague Dawley nephrons would be longer overall. It is of interest that the early increases in length do not seem to be much different in the two strains of rats, but that it is during mature stages of growth that the tubule lengths become more disparate. Accordingly, it is only when male animals are over 25 g in body weight that the kidney weight of male Sprague Dawleys is greater than that of Wistars. When SNGFR is related to proximal convoluted tubular length, one does not find a constant relationship in the rat. Whereas SNGFR is a linear function of body weight in older animals (Fig. 5), proximal tubular length is a nonlinear function of body weight as shown in Fig. 4. Statistical analysis of these data in this Figure shows that they are better fitted by a hyperbolic function. (An Ftest using a comparison of mean square deviations from each regression was used for computing this difference between fits.) As shown in Fig. 8 for those animals where enough data were obtained to calculate SNGFR per unit length of tubule, it would appear that a constant relationship is found at the early stages of development. Mature growth is characterized by a disproportionate increase in SNGFR. This
15 RENAL DEVELOPMENT OF IMMATURE RATS 587 finding is in contrast with the developmental pattern of guineapig (Spitzer & Brandis, 1974). Since this species was studied only for the first 3 days postnatally, it is possible that dissociation of SNGFIR and tubular length may have occurred at a later stage of growth. The observation that SNGFR is proportional to tubular length in a single kidney (de Rouffignac & Bonvalet, 197) does not always apply in comparisons between animals of the same species at different ages. 1 _ 8 E~~~~~~~~~~~~~~~~~.~ E 6 CL O L_ CD 4 Z D.~~~ 2 ~~~~~~~ Body wt. (g) Fig. 8. Relationship between SNGFR per mm proximal tubular length and body weight of rats. Filled circles show data from Wistar strain and open circles data from Sprague Dawley strain. As a final point in this discussion. I should like to consider the definition of maturity of kidney function in the rat. As can be seen in this study, there are transitions in developmental patterns with respect to distribution of SNGFR, growth rate of tubules, number of nephrons. Although some kidney functions show mature characteristics at relatively early ages (see Bengele & Solomon, 1974, for some references on this matter), others show changes at a postnatal age of about 354 days or when animals are about g in weight. In our laboratory, we have found that other functions which show maturity at this time are the response to blood volume expansion (Bengele & Solomon, 1974) and basal fractional excretion of sodium (Solomon, Bengele & Smith, 1974). It would therefore appear that renal maturity does not occur until animals are at least 4 days of age or 15 g in weight.
16 588 S. SOLOMON This work was supported in part by grants from the National Institutes of Health, AM 16171, and the National Science Foundation, BMS I should like to thank Mrs Lucy Moore and Mrs Arlene Dieterle for technical assistance. REFERENCES BARToII, E. & EARL Y, L. E. (1973). Measurements of nephron filtration rate in the rat with and without occlusion of the proximal tubule. Kidney int. 3, BENGELE, H. H. & SOLOMON, S. (1974). Development of renal response to blood volume expansion in the rat. Am. J. Physiol. 227, BONVALET, J. P., CI[APIoN, M., WANSTOK, F. & BERJAL, G. (1972). Compensatory renal hypertrophy in young rats: Increase in the number of nephrons. Kidney int. 1, BRENmiR, B. M. & DAUGHERTY, T. M. (1972). The measurement of glomerular filtration rate in single nephrons of the rat kidney. Yale J. Biol. Med. 45, 221. COELHO, J. B. (1973). Effect of dietary sodium intake on the intrarenal distribution of nephron glomerular filtration rates in the rat. Circulation Ree. 23, CoEImo, J. B., CHIEN, K. C. H. & BRADLEY, S. E. (1972). Measurement of single nephron glomerular filtration rate without micropuncture. Am. J. Phyeiol. 223, DmADniA, R. V., SHWAYRI, E. & BRIcKER, N. S. (1965). On the existence of nonurine forming nephrons in the diseased kidney of the dog. J. Lab. din. Med. 65, DAVIS, J. M., ScHNERMANN, J. & HoRsTER, M. (1972). Micropuncture method for the determination of nephron filtration rate a recollection study. Pfluger8 Arch. gee. Phyeiol. 333, DE ROUFFIGNAC, C. & BONVALET, J. P. (197). Etude chez le rat des variations du d6bit individual de filtration glomerulaire des n6phrons superficiels et profonds de I'apport sod6. Pfluiger8 Arch. gee. Phyeiol. 317, HORSTER, M. & LEWY, J. E. (197). Filtration fraction and extraction of PAH during neonatal period in the rat. Am. J. Phyeiol. 219, HORSTER, M. & VALTIN, H. (1971). Postnatal development of renal function: Micropuncture and clearance studies in the dog. J. dlin. Inveet. 5, KNOX, F. G., OTr, C., CuCHE, J. L., GAssER, J. & HAAS, J. (1974). Autoregulation of single nephron filtration rate in the presence and the absence of flow to the macula densa. Circulation Ree. 34, NAvAR, L. G., BURE, T. J., ROBINSON, R. R. & CLAPP, J. R. (1974). Distal tubular feedback in the autoregulation of single nephron glomerular filtration rate. J. din. Inve8t. 53, POTrER, D., JOmSiH, A., SAKAI, T., HARRuAH, J. & HOLLIDAY, M. A. (1969). Character of function and size in kidney during normal growth of rats. Pediat. Ree. 3, RUBIN, M. E., BUnCH, E. & RAPPAPORT, M. (1949). Maturation of renal function in childhood: Clearance studies. J. dlin. Invest. 28, SCHNERMANN, J., DAVIS, J. M., WUNDERLICH, P., LEVINE, D. Z. & HORSTER, M. (1971). Technical problems in the micropuncture determination of nephron filtration rate and their functional implications. Pflugere Arch. gee. Phyeiol. 329, SOLOMON, S. (1974). Absolute rates of sodium and potassium reabsorption by proximal tubule of immature rats. Biologia neonat. 25, SOLOMON, S. & BENGELE, H. H. (1973). Growth rates and organ weights of rats. Biologia neonat. 22,
17 RENAL DEVELOPMENT OF IMMATURE RATS 589 SOLOMON, S. & BENGELE, H. H. (1974). Effect of deoxycorticosterone on organ weights of rats during maturation. Proc. Soc. exp. Biol. Med. 147, SOLOMON, S., BENGELE, H. H. & SMITH, W. M. (1974). Size and composition of body fluid compartments as related to regulation of renal function in postnatal rats. XXVI Internal. Cong. Physiol. Sci. New Delhi, India. SOLOMON, S. & CAPEK, K. (1972a). Regulation of superficial single nephron glomerular filtration rates in infant rats. Proc. Soc. exp. Biol. Med. 139, SOLOMON, S. & CAPEK, K. (1972 b). Increased food availability and renal development of neonatal rats. Biologia neonat. 21, 915. SONNENBERG, H. & SOLOMON, S. (1969). Mechanism of natriuresis following intravascular and extracellular volume expansion. Can. J. Physiol. Pharmacol. 47, SPITZER, A. & BRa"DIs, M. (1974). Function and morphological maturation of the superficial nephrons. J. Olin. Invest. 53, WAJHL, M. & ScHNEP, J. (1969). Microdissection study of the length of different tubular segments of rat superficial nephrons. Z. Anat. Entw. GeSch. 129, WWIGHr, F. S. & GIEBISCH, G. (1972). Glomerular filtration in single nephrons. Kidney int. 1, 2129.
nephrons, this was not accompanied by concomitant disproportionate
 J. Phy8iol. (1976), 262, pp. 119-129 119 With 4 text-figures Printed in Great Britain FUNCTIONAL AND MORPHOLOGIC MATURATION OF SUPERFICIAL AND JUXTAMEDULLARY NEPHRONS IN THE RAT BY C. DE ROUFFIGNAC AND
J. Phy8iol. (1976), 262, pp. 119-129 119 With 4 text-figures Printed in Great Britain FUNCTIONAL AND MORPHOLOGIC MATURATION OF SUPERFICIAL AND JUXTAMEDULLARY NEPHRONS IN THE RAT BY C. DE ROUFFIGNAC AND
(0*24+0*01, P < 0.05). development. chloride osmotic diuresis does not exist during maturation in rats.
 J. Phyeiol. (1976), 258, pp. 83-98 83 With 4 text-figuree Printed in Great Britain MATURATION OF THE RENAL RESPONSE TO HYPERTONIC SODIUM CHLORIDE LOADING IN RATS: MICROPUNCTURE AND CLEARANCE STUDIES BY
J. Phyeiol. (1976), 258, pp. 83-98 83 With 4 text-figuree Printed in Great Britain MATURATION OF THE RENAL RESPONSE TO HYPERTONIC SODIUM CHLORIDE LOADING IN RATS: MICROPUNCTURE AND CLEARANCE STUDIES BY
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa
 Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Changes in renal function during pregnancy in humans have been well documented
 J. Physiol. (1982), 329, pp. 389-409 389 With 7 text-figures Printed in Great Britain MICROPUNCTURE STUDY OF CHANGES IN GLOMERULAR FILTRATION AND ION AND WATER HANDLING BY THE RAT KIDNEY DURING PREGNANCY
J. Physiol. (1982), 329, pp. 389-409 389 With 7 text-figures Printed in Great Britain MICROPUNCTURE STUDY OF CHANGES IN GLOMERULAR FILTRATION AND ION AND WATER HANDLING BY THE RAT KIDNEY DURING PREGNANCY
Effect of Variatidn in Dietary NaCl Intake
 Effect of Variatidn in ary NaCl Intake on the Intrarenal Distribution of Plasma Flow in the Rat ROLAND C. BLANTZ, JoH DONALD W. SELDIN D. WALLIN, FLOYD C. RECTOR, JR., and From the Department of Internal
Effect of Variatidn in ary NaCl Intake on the Intrarenal Distribution of Plasma Flow in the Rat ROLAND C. BLANTZ, JoH DONALD W. SELDIN D. WALLIN, FLOYD C. RECTOR, JR., and From the Department of Internal
Compensatory renal hypertrophy in young rats: Increase in the number of nephrons
 Kidney International, Vol 1 (1972), p 391 396 Compensatory renal hypertrophy in young rats: Increase in the number of nephrons JAN-PIRR BONVALT, MONIQU CHAMPION, FRIDA WANTOK, and Gu BRJAL Unite de recherches
Kidney International, Vol 1 (1972), p 391 396 Compensatory renal hypertrophy in young rats: Increase in the number of nephrons JAN-PIRR BONVALT, MONIQU CHAMPION, FRIDA WANTOK, and Gu BRJAL Unite de recherches
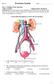
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Micropuncture study of renal tubular reabsorption of calcium in normal rodentd
 Micropuncture study of renal tubular reabsorption of calcium in normal rodentd WILLIAM E. LASSITER,3 CARL W. GOTTSCHALK,4 AND MARGARET MYLLE Department of Medicine, University of North Carolina, School
Micropuncture study of renal tubular reabsorption of calcium in normal rodentd WILLIAM E. LASSITER,3 CARL W. GOTTSCHALK,4 AND MARGARET MYLLE Department of Medicine, University of North Carolina, School
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog
 Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
~~I li~jllwl t... ~i d.. ud...!j.i..:.jj..u_,. ~I.:,.o \\- oo ~~1.:,-o L::...l_,
 Qatar Univ. Sci. J. (1993), 13(2): 264-270 PRENATAL DEVELOPMENT OF THE GUINEA PIG KIDNEY QUANTITATIVE AND QUALITATIVE MORPHOLOGY By ZEINAB M. A. EL GOHARY Department of Zoology, Faculty of Science, University
Qatar Univ. Sci. J. (1993), 13(2): 264-270 PRENATAL DEVELOPMENT OF THE GUINEA PIG KIDNEY QUANTITATIVE AND QUALITATIVE MORPHOLOGY By ZEINAB M. A. EL GOHARY Department of Zoology, Faculty of Science, University
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
fluid, *7 in peritubular capillary plasma and in
 J. Phy8iol. (1977), 273, pp. 765-773 765 With 2 text-ftgurem Printed in Great Britain COMPARISON OF CHLORIDE CONCENTRATION AND OSMOLALITY IN PROXIMAL TUBULAR FLUID, PERITUBULAR CAPILLARY PLASMA AND SYSTEMIC
J. Phy8iol. (1977), 273, pp. 765-773 765 With 2 text-ftgurem Printed in Great Britain COMPARISON OF CHLORIDE CONCENTRATION AND OSMOLALITY IN PROXIMAL TUBULAR FLUID, PERITUBULAR CAPILLARY PLASMA AND SYSTEMIC
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Functions of the Urinary System
 The Urinary System Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Regulate aspects of homeostasis Water balance Electrolytes Acid-base balance in the blood
The Urinary System Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Regulate aspects of homeostasis Water balance Electrolytes Acid-base balance in the blood
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Glomerular filtration in single nephrons
 Kidney International, Vol. 1 (1972), p. 201 209 EDITORIAL REVIEW Glomerular filtration in single nephrons In recent years as many investigators have turned their attention to the study of tubular transport
Kidney International, Vol. 1 (1972), p. 201 209 EDITORIAL REVIEW Glomerular filtration in single nephrons In recent years as many investigators have turned their attention to the study of tubular transport
The Urinary System PART A
 15 The Urinary System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Functions of the Urinary
15 The Urinary System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Functions of the Urinary
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Actualités néphrologiques Jean Hamburger 23 Avril Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris
 Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
1. Anatomy / Vascularisation. 2. Urine concentration. 3. Axial heterogeneity of some segments
 Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Changes in glomerular filtration rate (G.F.R.) in human pregnancy are well. (Received 23 January 1981) significantly enhanced.
 J. Physiol. (1981), 319, pp. 153-164 153 With 6 text-figures Printed in Great Britain THE EFFECT OF PREGNANCY ON GLOMERULAR FILTRATION RATE AND SALT AND WATER REABSORPTION IN THE RAT BY J. C. ATHERTON
J. Physiol. (1981), 319, pp. 153-164 153 With 6 text-figures Printed in Great Britain THE EFFECT OF PREGNANCY ON GLOMERULAR FILTRATION RATE AND SALT AND WATER REABSORPTION IN THE RAT BY J. C. ATHERTON
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
The Urinary System. Copyright 2003 Pearson Education, Inc. publishing as Benjamin Cummings
 The Urinary System Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Functions of the Urinary System Regulate aspects of homeostasis Water balance Electrolytes
The Urinary System Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Functions of the Urinary System Regulate aspects of homeostasis Water balance Electrolytes
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Compensatory Renal Hypertrophy in Dogs: Single Nephron Glomerular Filtration Rate
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 51 (1978), 307-313 Compensatory Renal Hypertrophy in Dogs: Single Nephron Glomerular Filtration Rate SERGE CARRIiRE Montreal, P.Q., Canada Kidney weight, length
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 51 (1978), 307-313 Compensatory Renal Hypertrophy in Dogs: Single Nephron Glomerular Filtration Rate SERGE CARRIiRE Montreal, P.Q., Canada Kidney weight, length
H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M
 SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Urinary System BIO 250. Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat. Routes of Waste Elimination
 Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Functional and Morphologic Maturation
 Functional and Morphologic Maturation of the Superficial Nephrons RELATIONSHIP TO TOTAL KIDNEY FUNCTION ADRIAN SprrzmiR and MATTHAS BRANDis From the Department of Pediatrics and The Rose F. Kennedy Center,
Functional and Morphologic Maturation of the Superficial Nephrons RELATIONSHIP TO TOTAL KIDNEY FUNCTION ADRIAN SprrzmiR and MATTHAS BRANDis From the Department of Pediatrics and The Rose F. Kennedy Center,
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Class XI Chapter 19 Excretory Products and their Elimination Biology
 Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Anatomy/Physiology Study Guide: Unit 9 Excretory System
 Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Urinary system. Lab-7
 Urinary system Lab-7 Excretion: processes that remove wastes and excess materials from the body Urinary system (kidneys): excretes nitrogenous wastes, excess solutes, and water The Kidneys Regulate Water
Urinary system Lab-7 Excretion: processes that remove wastes and excess materials from the body Urinary system (kidneys): excretes nitrogenous wastes, excess solutes, and water The Kidneys Regulate Water
Dietary Protein Suppresses Feedback Control of Glomerular Filtration in Rats
 Dietary Protein Suppresses Feedback Control of Glomerular Filtration in Rats Frank D. Seney, Jr., and Fred S. Wright Departments ofmedicine and Physiology, Yale University, New Haven, Connecticut 651;
Dietary Protein Suppresses Feedback Control of Glomerular Filtration in Rats Frank D. Seney, Jr., and Fred S. Wright Departments ofmedicine and Physiology, Yale University, New Haven, Connecticut 651;
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
The Urinary System 15PART A. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART A Functions of the Urinary System Elimination of waste products Nitrogenous
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART A Functions of the Urinary System Elimination of waste products Nitrogenous
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Nephrology - the study of the kidney. Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system
 Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Lund, 1948), the effect of which was to produce glomerular lesions without. relationship between increased protein loads and the tubular reabsorption
 544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
AP2, Lab 7 - THE URINARY SYSTEM
 AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
Fractional excretion of uric acid in infancy and childhood
 Archives of Disease in Childhood, 1974, 49, 878. Fractional excretion of uric acid in infancy and childhood Index of tubular maturation J. H. PASSWELL, M. MODAN, M. BRISH, S. ORDA, and H. BOICHIS From
Archives of Disease in Childhood, 1974, 49, 878. Fractional excretion of uric acid in infancy and childhood Index of tubular maturation J. H. PASSWELL, M. MODAN, M. BRISH, S. ORDA, and H. BOICHIS From
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Renal Tubular Transport of 3 H-Digoxin in Saline Diuresis in Rats
 85 Renal Tubular Transport of 3 H-Digoxin in Saline Diuresis in Rats Evaluation by Micropuncture RICHARD J. ROMAN AND MICHAEL L. KAUKER, PH.D. SUMMARY We evaluated urinary excretion and tubular transport
85 Renal Tubular Transport of 3 H-Digoxin in Saline Diuresis in Rats Evaluation by Micropuncture RICHARD J. ROMAN AND MICHAEL L. KAUKER, PH.D. SUMMARY We evaluated urinary excretion and tubular transport
Basic Urinary Tract Anatomy and Histology
 Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
The excretory system
 Key Words The excretory system haemoglobin Convoluted tubule Loop of Henle nephron ureter/-s sweat glands Bowman s capsule Glomerulus cholesterol Bladder Excretion is the removal of the waste products
Key Words The excretory system haemoglobin Convoluted tubule Loop of Henle nephron ureter/-s sweat glands Bowman s capsule Glomerulus cholesterol Bladder Excretion is the removal of the waste products
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Excretion and Water Balance
 Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
during Saline Loading in the Dog
 Superficial and Juxtamedullary Nephron Function during Saline Loading in the Dog FRANK J. BRUNS, EDWARD A. ALEXANDER, ARHu NORMAN G. LEVINsY L. RILEY, and From the Departments of Medicine, Boston University
Superficial and Juxtamedullary Nephron Function during Saline Loading in the Dog FRANK J. BRUNS, EDWARD A. ALEXANDER, ARHu NORMAN G. LEVINsY L. RILEY, and From the Departments of Medicine, Boston University
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Preparation of Animals for Live Animal Imaging
 Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Chapter 25: Urinary System
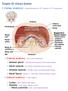 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
28/04/2013 LEARNING OUTCOME C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS STUDENT ACHIEVEMENT INDICATORS URINARY SYSTEM & EXCRETION
 LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Sciences, Madison, WI 53792, U.S.A.
 J. Phy8iol. (1984), 356, pp. 919 9 With 4 textfigures Printed in Great Britain SEGMENTAL ANALYSIS OF RENAL GLUCOSE TRANSPORT IN YOUNG FEMALE RATS BY NONA R. McSHERRY AND SUNGFENG WENt From the Department
J. Phy8iol. (1984), 356, pp. 919 9 With 4 textfigures Printed in Great Britain SEGMENTAL ANALYSIS OF RENAL GLUCOSE TRANSPORT IN YOUNG FEMALE RATS BY NONA R. McSHERRY AND SUNGFENG WENt From the Department
