AUSTRALIAN PATHOLOGY UNITS AND TERMINOLOGY (APUTS) Harmonised Reference Intervals Chemical Pathology. (v1.1)
|
|
|
- Steven Shepherd
- 5 years ago
- Views:
Transcription
1 AUSTRALIAN PATHOLOGY UNITS AND TERMINOLOGY (APUTS) Harmonised Reference Intervals Chemical Pathology (v1.1) ISBN: Pending 1
2 State Health Publication Number (SHPN): Pending Online copyright RCPA 2014 This work (Standards and Guidelines) is copyright. You may download, display, print and reproduce the Standards and Guidelines for your personal, noncommercial use or use within your organisation subject to the following terms and conditions: 1. The Standards and Guidelines may not be copied, reproduced, communicated or displayed, in whole or in part, for profit or commercial gain. 2. Any copy, reproduction or communication must include this RCPA copyright notice in full. 3. No changes may be made to the wording of the Standards and Guidelines including commentary, tables or diagrams. Excerpts from the Standards and Guidelines may be used. References and acknowledgments must be maintained in any reproduction or copy in full or part of the Standards and Guidelines. Apart from any use as permitted under the Copyright Act 1968 or as set out above, all other rights are reserved. Requests and inquiries concerning reproduction and rights should be addressed to RCPA, 207 Albion St, Surry Hills, NSW 2010, Australia. This material contains content from LOINC ( The LOINC table, LOINC codes and LOINC panels and forms file are copyright , Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC) Committee and available at no cost under the license athttp://loinc.org/terms-of-use. This material includes SNOMED Clinical Terms (SNOMED CT ) which is used by permission of the International Health Terminology Standards Development Organisation (IHTSDO ). All rights reserved. SNOMED CT was originally created by The College of American Pathologists. IHTSDO, SNOMED and SNOMED CT are registered trademarks of the IHTSDO First published: February, 2013, 1st Edition (version 1.0) 2
3 Australasian Reference Intervals PITUS WG4 Outcomes 1. The PITUS working group supports publication of the harmonised reference intervals developed by AACB for both adults and children as a part of the set of RCPA APUTS documents 2. Any calculation of rounding for analytes must be performed before applying the age and reference interval 3. The number of days from birth to collection must be used for age calculations ie. Day 0 is the day of birth. 4. Age intervals may use a mixture of units where appropriate in a report or in a table. This must be restricted to days, weeks and years (not months) however. 5. Because common usage for analyte reference intervals has both the low and high values included while for age limits the higher value is not included, to avoid any confusion in interpretation of boundary conditions these need to be represented in different ways in reports and tables used outside the laboratory. 6. The same method for representing age intervals must be used for adults and children 7. There is as yet no international standard for representing age intervals and the committee proposes the format 1w to <12y to show the time interval in a table or on a report. This was done to avoid confusion on reading and with the meaning of mathematical notation Example: Analyte Age Reference Interpretation of age (days) Interpretation of reference (units) Sodium 0w to <1w ( ) mmol/l 0 d x 147 mmol/l (LN-RCPA: ) 1w to <18y ( ) mmol/l 7 d x 144 mmol/l 18y to <120y ( ) mmol/l 6574 d x 145 mmol/l Where d = days from birth to collection. And x = the analyte measured in the units and rounded to the reporting unit. Assumptions used in calculations: 1 month = 4 weeks = 28 days 6 months = 26 weeks = 26w x 7 days = 182 days Each year = days (when calculating number of days always round down) Definition of age units: Days: Day 0 (the 1st day of life) is day of birth Day 1 (the 2nd day of life) is the day after the birth. Days are calendar days, starting at midnight, rather than 24 hour periods starting at the time of birth. Weeks: Week 0 (the 1st week) is 7 day period starting on day of birth. 1
4 Week 1 (the 2nd week) is the week after week 0, ie starting at the beginning of day 7 (week zero comprises days 0,1,2,3,4,5,6). Years: Year 0 (the 1st year) is the 365 day period (with leap year adjustment, LYA) starting on day of birth. Also can be described as 52 week period counting from week 0 as described previously (ie from weeks 0 51 of life). Year 1 (the 2nd year) is the year after year 0, ie starting at the beginning of day 366 (with LYA). Also can be described as starting at the start of week 52. In this setting a baby is born in day 0, (day 0 of) week 0 and (day 0 and week 0 of) year 0. 2
5 Australasian Reference Intervals - Chemical Pathology* * AACB Committee for Common Reference Intervals and AACB Paediatric Biochemistry Special Interest Group TRIM Document #: Document History: Version Reason for Change Author Date 1.0 Initial Australasian Reference Intervals. Prepared by the AACB Committee for Common Reference Intervals and AACB Paediatric Biochemistry Special Interest Group. To be published by the RCPA Donna Moore Pathology Information, Terminology and Units Standardisation (PITUS-14) Project. 06-Jun Fixed minor formatting issues. Donna Moore 3-Nov-14
6 Australasian Reference Intervals - Chemical Pathology Analyte Age Reference Interpretation of age (days) Interpretation of reference (units) Sodium 0d to <1w ( ) mmol/l 0 d x 147 mmol/l (LN-RCPA: ) 1w to <18y ( ) mmol/l 7 d x 144 mmol/l 18y to <120y ( ) mmol/l 6574 d x 145 mmol/l Potassium 0d to <1w ( ) mmol/l 0 d x 6.5 mmol/l (LN-RCPA: ) 1w to <26w ( ) mmol/l 7 d x 6.7 mmol/l See note 1 26w to <2y ( ) mmol/l 182 d x 5.6 mmol/l 2y to <18y ( ) mmol/l 730 d x 5.3 mmol/l 18y to <120y ( ) mmol/l 6574 d x 5.2 mmol/l Chloride 0d to <1w (98 115) mmol/l 0 d 6 98 x 115 mmol/l (LN-RCPA: ) 1w to <18y (97 110) mmol/l 7 d x 110 mmol/l 18y to <120y (95 110) mmol/l 6574 d x 110 mmol/l Bicarbonate 0d to <1w (15 28) mmol/l 0 d 6 15 x 28 mmol/l (LN-RCPA: ) 1w to <2y (16 29) mmol/l 7 d x 29 mmol/l 2y to <10y (17 30) mmol/l 730 d x 30 mmol/l 10y to <18y (20 32) mmol/l 3652 d x 32 mmol/l 18y to <120y (22 32) mmol/l 6574 d x 32 mmol/l Creatinine 0d to <1w (22 93) umol/l 0 d 6 22 x 93 umol/l (LN-RCPA: ) 1w to <4w (17 50) umol/l 7 d x 50 umol/l See note 2 and 3 4w to <2y (11 36) umol/l 28 d x 36 umol/l 2y to <6y (20 44) umol/l 730 d x 44 umol/l 6y to <12y (27 58) umol/l 2191 d x 58 umol/l Male 12y to <15y (35 83) umol/l 4383 d x 83 umol/l 15y to <19y (50 100) umol/l 5478 d x 100 umol/l 19y to <60y (60 110) umol/l 6939 d x 110 umol/l Female 12y to <15y (35 74) umol/l 4383 d x 74 umol/l 15y to <19y (38 82) umol/l 5478 d x 82 umol/l 19y to <60y (45 90) umol/l 6939 d x 90 umol/l Calcium 0d to <1w ( ) mmol/l 0 d x 2.80 mmol/l (LN-RCPA: ) 1w to <26w ( ) mmol/l 7 d x 2.80 mmol/l 26w to <2y ( ) mmol/l 182 d x 2.70 mmol/l 2y to <18y ( ) mmol/l 730 d x 2.65 mmol/l 18y to <120y ( ) mmol/l 6574 d x 2.60 mmol/l Calcium corrected for albumin (LN-RCPA: ) 18y to <120y ( ) mmol/l 6574 d x 2.60 mmol/l Phosphate 0d to <1w ( ) mmol/l 0 d x 2.85 mmol/l (LN-RCPA: ) 1w to <4w ( ) mmol/l 7 d x 2.75 mmol/l 4w to <26w ( ) mmol/l 28 d x 2.50 mmol/l 26w to <1y ( ) mmol/l 182 d x 2.30 mmol/l 1y to <4y ( ) mmol/l 365 d x 2.20 mmol/l 4y to <15y ( ) mmol/l 1461 d x 2.00 mmol/l 15y to <18y ( ) mmol/l 5478 d x 1.85 mmol/l 18y to <20y ( ) mmol/l 6574 d x 1.65 mmol/l 20y to <120y ( ) mmol/l 7305 d x 1.50 mmol/l Magnesium 0d to <1w ( ) mmol/l 0 d x 1.00 mmol/l (LN-RCPA: ) 1w to <18y ( ) mmol/l 7 d x 1.10 mmol/l 18y to <120y ( ) mmol/l 6574 d x 1.10 mmol/l Lactate dehydrogenase (LN-RCPA: ) See note 4 Alkaline phosphatase 18y to <120y 0d to <1w ( ) U/L (80 380) U/L 6574 d d x 250 U/L 80 x 380 U/L (LN-RCPA: ) 1w to <4w ( ) U/L 7 d x 550 U/L 4w to <26w ( ) U/L 28 d x 650 U/L 26w to <2y ( ) U/L 182 d x 450 U/L 2y to <6y ( ) U/L 730 d x 370 U/L 6y to <10y ( ) U/L 2191 d x 440 U/L Male Total Protein (LN-RCPA: ) 10y to <14y ( ) U/L 3652 d x 530 U/L 14y to <15y ( ) U/L 5113 d x 480 U/L 15y to <17y (80-380) U/L 5478 d x 380 U/L 17y to <19y (50 220) U/L 6209 d x 220 U/L 19y to <22y (45 150) U/L 6939 d x 150 U/L 22y to <120y (30 110) U/L 8035 d x 110 U/L Female 10y to <13y ( ) U/L 3652 d x 460 U/L 13y to <14y (70 330) U/L 4748 d x 330 U/L 14y to <15y (50 280) U/L 5113 d x 280 U/L 15y to <16y (45 170) U/L 5478 d x 170 U/L 16y to <22y (35 140) U/L 5844 d x 140 U/L 22y to <120y (30 110) U/L 8035 d x 110 U/L 18y to <120y (60 80) g/l 6574 d x 80 g/l Unless otherwise specified, the intervals are for serum or plasma for adults (18 years of age and older). The intervals are for use by laboratories using methods which are traceable to JCTLM-listed reference materials, methods and services (except bicarbonate where no references are listed). LN-RCPA is the LOINC code from the RCPA dataset to be used for each analyte. Note:
7 1. For reference intervals between 0w to <18y, the Potassium Reference Intervals listed in the table are for serum specimens only. Below are the Potassium Reference Intervals for when a plasma specimen is collected. Potassium (Plasma) 0d to <1w ( ) mmol/l 0 d x 6.2 mmol/l 1w to <26w ( ) mmol/l 7 d x 6.4 mmol/l 26w to <2y ( ) mmol/l 182 d x 5.4 mmol/l 2y to <18y ( ) mmol/l 730 d x 4.9 mmol/l For reference intervals from 18y to <120y, the Potassium Reference Intervals listed are for use for both serum and plasma. Laboratories testing only heparin plasma may choose to use a lower interval. 2. Creatinine RIs are by Vitros enzymatic assay 3. Creatinine has harmonised reference intervals for adults up to the age of 60 years. For older ages laboratories may elect to maintain these. 4. Lactate dehydrogenase [L to P] (IFCC), lactate to pyruvate method (IFCC method).
8 Australasian Adult Reference Intervals - Chemical Pathology Analyte Age Reference Sodium (LN-RCPA: ) Potassium (LN-RCPA: ) note 1 Chloride (LN-RCPA: ) Bicarbonate (LN-RCPA: ) See Interpretation of age (days) Interpretation of reference (units) Creatinine Male (LN-RCPA: ) 19y to <60y (60 110) umol/l 6574 d x 110 umol/l See note 2 Calcium (LN-RCPA: ) 18y to <120y ( ) mmol/l 6574 d y to <120y Female ( ) mmol/l 6574 d y to <120y (95 110) mmol/l 6574 d x 145 mmol/l 3.5 x 5.2 mmol/l 95 x 110 mmol/l 18y to <120y (22 32) mmol/l 6574 d x 32 mmol/l 19y to <60y (45 90) umol/l 6574 d x 90 umol/l 18y to <120y ( ) mmol/l 6574 d x 2.60 mmol/l Calcium corrected for albumin (LN-RCPA: ) Phosphate (LN-RCPA: ) Magnesium (LN-RCPA: ) Lactate dehydrogenase (LN-RCPA: ) See note 3 Alkaline phosphatase (LN-RCPA: ) Total Protein (LN-RCPA: ) 18y to <120y ( ) mmol/l 6574 d x 2.60 mmol/l 20y to <120y ( ) mmol/l 6574 d x 1.50 mmol/l 18y to <120y ( ) mmol/l 6574 d x 1.10 mmol/l 18y to <120y ( ) U/L 6574 d x 250 U/L 22y to <120y (30 110) U/L 6574 d x 110 U/L 18y to <120y (60 80) g/l 6574 d x 80 g/l Unless otherwise specified, the intervals are for serum or plasma for adults (18 years of age and older). The intervals are for use by laboratories using methods which are traceable to JCTLM-listed reference materials, methods and services (except bicarbonate where no references are listed). LN-RCPA is the LOINC code from the RCPA dataset to be used for each analyte. Note: 1. This range is proposed for use for both serum and plasma. Laboratories testing only heparin plasma may choose to use a lower interval. 2. Creatinine has harmonised reference intervals for adults up to the age of 60 years. For older ages laboratories may elect to maintain these. 3. Lactate dehydrogenase [L to P] (IFCC), lactate to pyruvate method (IFCC method).
9 Australasian Paediatric Reference Intervals - Chemical Pathology Analyte Age Reference Interpretation of age (days) Interpretation of reference (units) Sodium 0d to <1w ( ) mmol/l 0 d x 147 mmol/l (LN-RCPA: ) 1w to <18y ( ) mmol/l 7 d x 144 mmol/l 18y to <120y ( ) mmol/l 6574 d x 145 mmol/l Potassium 0d to <1w ( ) mmol/l 0 d x 6.5 mmol/l (LN-RCPA: ) 1w to <26w ( ) mmol/l 7 d x 6.7 mmol/l See note 1 26w to <2y ( ) mmol/l 182 d x 5.6 mmol/l 2y to <18y ( ) mmol/l 730 d x 5.3 mmol/l 18y to <120y ( ) mmol/l 6574 d x 5.2 mmol/l Chloride 0d to <1w (98 115) mmol/l 0 d 6 98 x 115 mmol/l (LN-RCPA: ) 1w to <18y (97 110) mmol/l 7 d x 110 mmol/l 18y to <120y (95 110) mmol/l 6574 d x 110 mmol/l Bicarbonate 0d to <1w (15 28) mmol/l 0 d 6 15 x 28 mmol/l (LN-RCPA: ) 1w to <2y (16 29) mmol/l 7 d x 29 mmol/l 2y to <10y (17 30) mmol/l 730 d x 30 mmol/l 10y to <18y (20 32) mmol/l 3652 d x 32 mmol/l 18y to <120y (22 32) mmol/l 6574 d x 32 mmol/l Creatinine 0d to <1w (22 93) umol/l 0 d 6 22 x 93 umol/l (LN-RCPA: ) 1w to <4w (17 50) umol/l 7 d x 50 umol/l See note 2 and 3 4w to <2y (11 36) umol/l 28 d x 36 umol/l 2y to <6y (20 44) umol/l 730 d x 44 umol/l 6y to <12y (27 58) umol/l 2191 d x 58 umol/l Male 12y to <15y (35 83) umol/l 4383 d x 83 umol/l 15y to <19y (50 100) umol/l 5478 d x 100 umol/l 19y to <60y (60 110) umol/l 6939 d x 110 umol/l Female 12y to <15y (35 74) umol/l 4383 d x 74 umol/l 15y to <19y (38 82) umol/l 5478 d x 82 umol/l 19y to <60y (45 90) umol/l 6939 d x 90 umol/l Calcium 0d to <1w ( ) mmol/l 0 d x 2.80 mmol/l (LN-RCPA: ) 1w to <26w ( ) mmol/l 7 d x 2.80 mmol/l 26w to <2y ( ) mmol/l 182 d x 2.70 mmol/l 2y to <18y ( ) mmol/l 730 d x 2.65 mmol/l 18y to <120y ( ) mmol/l 6574 d x 2.60 mmol/l Phosphate 0d to <1w ( ) mmol/l 0 d x 2.85 mmol/l (LN-RCPA: ) 1w to <4w ( ) mmol/l 7 d x 2.75 mmol/l 4w to <26w ( ) mmol/l 28 d x 2.50 mmol/l 26w to <1y ( ) mmol/l 182 d x 2.30 mmol/l 1y to <4y ( ) mmol/l 365 d x 2.20 mmol/l 4y to <15y ( ) mmol/l 1461 d x 2.00 mmol/l 15y to <18y ( ) mmol/l 5478 d x 1.85 mmol/l 18y to <20y ( ) mmol/l 6574 d x 1.65 mmol/l 20y to <120y ( ) mmol/l 7305 d x 1.50 mmol/l Magnesium 0d to <1w ( ) mmol/l 0 d x 1.00 mmol/l (LN-RCPA: ) 1w to <18y ( ) mmol/l 7 d x 1.10 mmol/l 18y to <120y ( ) mmol/l 6574 d x 1.10 mmol/l Alkaline phosphatase 0d to <1w (80 380) U/L 0 d 6 80 x 380 U/L (LN-RCPA: ) 1w to <4w ( ) U/L 7 d x 550 U/L 4w to <26w ( ) U/L 28 d x 650 U/L 26w to <2y ( ) U/L 182 d x 450 U/L 2y to <6y ( ) U/L 730 d x 370 U/L 6y to <10y ( ) U/L 2191 d x 440 U/L Male 10y to <14y ( ) U/L 3652 d x 530 U/L 14y to <15y ( ) U/L 5113 d x 480 U/L 15y to <17y (80-380) U/L 5478 d x 380 U/L 17y to <19y (50 220) U/L 6209 d x 220 U/L 19y to <22y (45 150) U/L 6939 d x 150 U/L 22y to <120y (30 110) U/L 8035 d x 110 U/L Female 10y to <13y ( ) U/L 3652 d x 460 U/L 13y to <14y (70 330) U/L 4748 d x 330 U/L 14y to <15y (50 280) U/L 5113 d x 280 U/L 15y to <16y (45 170) U/L 5478 d x 170 U/L 16y to <22y (35 140) U/L 5844 d x 140 U/L 22y to <120y (30 110) U/L 8035 d x 110 U/L LN-RCPA is the LOINC code from the RCPA dataset to be used for each analyte. Note: 1. Potassium Reference Intervals are for serum specimens. Below are the Potassium Reference Intervals for when a plasma specimen is collected.
10 Potassium (Plasma) 0d to <1w ( ) mmol/l 0 d x 6.2 mmol/l See note 1 1w to <26w ( ) mmol/l 7 d x 6.4 mmol/l 26w to <2y ( ) mmol/l 182 d x 5.4 mmol/l 2y to <18y ( ) mmol/l 731 d x 4.9 mmol/l 2. Reference intervals for patients <19y are specific for labs who use the Ortho Vitros Enzymatic creatinine assay. 3. Creatinine has harmonised reference intervals for patients up to the age 60 years. The reference intervals above this age are currently under review.
In the interests of disclosure
 2 In the interests of disclosure 26-May-2017 Contents 3 December, 2018 (Ancient) History of PI in Australia 1969 Medicheck,,Shepherd & Preventicare Transmission of first pathology reports to GP with IBM
2 In the interests of disclosure 26-May-2017 Contents 3 December, 2018 (Ancient) History of PI in Australia 1969 Medicheck,,Shepherd & Preventicare Transmission of first pathology reports to GP with IBM
Quality Initiative In Pathology. Harmonisation of Laboratory Testing
 Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
Reference Intervals. Graham Jones / Gus Koerbin
 Reference Intervals Graham Jones / Gus Koerbin Adult CRI - Harmonisation Harmonisation 1 2012: (13 tests + 1 calculation) Harmonisation 2 2013: Confirm 2012 recommendations. Discussed: albumin, globulin,
Reference Intervals Graham Jones / Gus Koerbin Adult CRI - Harmonisation Harmonisation 1 2012: (13 tests + 1 calculation) Harmonisation 2 2013: Confirm 2012 recommendations. Discussed: albumin, globulin,
The Whats and Hows of Reference Intervals. Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney
 The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
Standards for Pathology Informatics in Australia (SPIA)
 Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Cytopathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards and Guidelines
Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Cytopathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards and Guidelines
Impact of Proposed HRI s on Laboratory Report Flagging Rates
 Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Breakout Session C: Harmonisation of the Alert Table.
 Breakout Session C: Harmonisation of the Alert Table. RCPA-AACB High Risk Results Working Party Andrew Georgiou Craig Campbell Grahame Caldwell Hans Schneider Penelope Coates Que Lam Rita Horvath Robert
Breakout Session C: Harmonisation of the Alert Table. RCPA-AACB High Risk Results Working Party Andrew Georgiou Craig Campbell Grahame Caldwell Hans Schneider Penelope Coates Que Lam Rita Horvath Robert
Setting of quality standards
 Setting of quality standards Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney AACB ASM Adelaide October 2014 Setting of Quality Standards - 2013 The 2013 QC workshop revealed
Setting of quality standards Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney AACB ASM Adelaide October 2014 Setting of Quality Standards - 2013 The 2013 QC workshop revealed
Confirmation - A Practical Approach
 Data Mining i for Reference Interval Confirmation - A Practical Approach Graham Jones Chemical Pathologist St Vincent s Hospital, Sydney AACB Webinar 5 th June 2013 Summary A little (more) background Current
Data Mining i for Reference Interval Confirmation - A Practical Approach Graham Jones Chemical Pathologist St Vincent s Hospital, Sydney AACB Webinar 5 th June 2013 Summary A little (more) background Current
HealtheNet Pathology - overview
 HealtheNet Pathology - overview Joshua Bunting HealtheNet Integration Manager - ehealth NSW Alex Eigenstetter Director of Performance NSW Health Pathology August 2017 1 A de-identified patient case study
HealtheNet Pathology - overview Joshua Bunting HealtheNet Integration Manager - ehealth NSW Alex Eigenstetter Director of Performance NSW Health Pathology August 2017 1 A de-identified patient case study
Evidence Based Commutability: Bias 2 Study. Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA
 Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
Standards for Pathology Informatics in Australia (SPIA)
 Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Chemical Pathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards
Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Chemical Pathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards
ROTUNDA HOSPITAL DEPARTMENT OF LABORATORY MEDICINE
 This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
Why Traceability Matters to Patients?
 Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
2018 CMS Web Interface
 CMS Web Interface PREV-7 (NQF 0041): Preventive Care and Screening: Influenza Immunization Measure Steward: PCPI CMS Web Interface V2.0 Page 1 of 19 11/13/2017 Contents INTRODUCTION... 3 CMS WEB INTERFACE
CMS Web Interface PREV-7 (NQF 0041): Preventive Care and Screening: Influenza Immunization Measure Steward: PCPI CMS Web Interface V2.0 Page 1 of 19 11/13/2017 Contents INTRODUCTION... 3 CMS WEB INTERFACE
2018 CMS Web Interface
 CMS Web Interface PREV-7 (NQF 0041): Preventive Care and Screening: Influenza Immunization Measure Steward: PCPI CMS Web Interface V2.1 Page 1 of 19 06/25/ Contents INTRODUCTION... 3 CMS WEB INTERFACE
CMS Web Interface PREV-7 (NQF 0041): Preventive Care and Screening: Influenza Immunization Measure Steward: PCPI CMS Web Interface V2.1 Page 1 of 19 06/25/ Contents INTRODUCTION... 3 CMS WEB INTERFACE
Sweat Electrolytes Quality Assurance Program th February 2008 Summary report of the Sweat Electrolyte Questionnaire 2007
 RCPA QUALITY ASSURANCE PROGRAMS PTY LIMITED ABN 32 3 52 2 RCPA Chemical Pathology Programs In association with the Australasian Association of Clinical Biochemists Sweat Electrolytes Quality Assurance
RCPA QUALITY ASSURANCE PROGRAMS PTY LIMITED ABN 32 3 52 2 RCPA Chemical Pathology Programs In association with the Australasian Association of Clinical Biochemists Sweat Electrolytes Quality Assurance
General Chemistry Scheme Guide
 General Chemistry Scheme Guide Copyright WEQAS. All rights reserved. No part of this document may be reproduced or utilised in any form without permission from WEQAS Contents. Scheme details and repertoire.....
General Chemistry Scheme Guide Copyright WEQAS. All rights reserved. No part of this document may be reproduced or utilised in any form without permission from WEQAS Contents. Scheme details and repertoire.....
Harmonisation of Reference Ranges
 Harmonisation of Reference Ranges Ken Sikaris BSc(Hons), MBBS, FRCPA, FAACB, FFSc Vice President, AACB (Education) Chemical Pathologist, Melbourne Pathology Director of Clinical Support Systems, Sonic
Harmonisation of Reference Ranges Ken Sikaris BSc(Hons), MBBS, FRCPA, FAACB, FFSc Vice President, AACB (Education) Chemical Pathologist, Melbourne Pathology Director of Clinical Support Systems, Sonic
Serodos and Serodos plus
 Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
December 12, Dear Colleague:
 1100 13th Street NW, Third Floor Washington, DC 20005 phone 202.955.3500 fax 202.955.3599 www.ncqa.org December 12, 2018 Dear Colleague: NCQA released a stop-work notification on November 28 for all six
1100 13th Street NW, Third Floor Washington, DC 20005 phone 202.955.3500 fax 202.955.3599 www.ncqa.org December 12, 2018 Dear Colleague: NCQA released a stop-work notification on November 28 for all six
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
NEW RCPCH REFERENCE RANGES-
 s vary between populations and age groups and it is important to always check the reference Haematology: Haemoglobin Male 130 175 g/l 0 6 days 145-220 g/l Female 115 165 g/l 7 days 140-186 g/l 8 days 3
s vary between populations and age groups and it is important to always check the reference Haematology: Haemoglobin Male 130 175 g/l 0 6 days 145-220 g/l Female 115 165 g/l 7 days 140-186 g/l 8 days 3
2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY. MEASURE TYPE: Process
 Measure #226 (NQF 0028): Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention National Quality Strategy Domain: Community / Population Health 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Measure #226 (NQF 0028): Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention National Quality Strategy Domain: Community / Population Health 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube
 Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
EXAM COVER SHEET. Course Code: CLS 432. Course Description: Clinical Biochemistry. Final Exam. Duration: 2 hour. 1st semester 1432/1433.
 EXAM COVER SHEET Course Code: CLS 432 Course Description: Clinical Biochemistry Final Exam Duration: 2 hour 1st semester 1432/1433 Student Name: Student Uni No: Part 1 Multiple choice questions Answer
EXAM COVER SHEET Course Code: CLS 432 Course Description: Clinical Biochemistry Final Exam Duration: 2 hour 1st semester 1432/1433 Student Name: Student Uni No: Part 1 Multiple choice questions Answer
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Measure #143 (NQF 0384): Oncology: Medical and Radiation Pain Intensity Quantified National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Measure #143 (NQF 0384): Oncology: Medical and Radiation Pain Intensity Quantified National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Biochemistry Adult Reference Ranges
 Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Manufacturer Report for Siemens Unassayed Chemistry Lot Exp 30 Jun 2018
 Acetaminophen Enzymatic, colorimetric µg/ml.09 0..0.09 0..0 0. 0. 0. 0. 9.. 9.0 0.9.0..9.. Albumin Bromcresol Purple (BCP) g/dl.0 0.0..0 0.00.. 0.0.. 0.09..9 0.0..9 0.0..0 0.0..0 0.0. Alkaline Phosphatase
Acetaminophen Enzymatic, colorimetric µg/ml.09 0..0.09 0..0 0. 0. 0. 0. 9.. 9.0 0.9.0..9.. Albumin Bromcresol Purple (BCP) g/dl.0 0.0..0 0.00.. 0.0.. 0.09..9 0.0..9 0.0..0 0.0..0 0.0. Alkaline Phosphatase
1. Calibra - H - Store at 2-8 ºC.
 CALIBRA H Insert Ref.:80 Lot Expiration Calibrator Attention. It is suggested to verify carefully if the lot number printed in this insert corresponds to the lot on the bottle label. Intended use. is a
CALIBRA H Insert Ref.:80 Lot Expiration Calibrator Attention. It is suggested to verify carefully if the lot number printed in this insert corresponds to the lot on the bottle label. Intended use. is a
Measure #69 (NQF 0380): Hematology: Multiple Myeloma: Treatment with Bisphosphonates National Quality Strategy Domain: Effective Clinical Care
 Measure #69 (NQF 0380): Hematology: Multiple Myeloma: Treatment with Bisphosphonates National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Measure #69 (NQF 0380): Hematology: Multiple Myeloma: Treatment with Bisphosphonates National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions
 Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
KEY FACTS IN ANAESTHESIA AND INTENSIVE CARE
 KEY FACTS IN ANAESTHESIA AND INTENSIVE CARE Alcira Serrano Gomez MD Fellow John Farman Intensive Care Unit Addenbrooke s NHS Trust Cambridge, UK Gilbert R Park MD DMed Sci FRCA Director of Intensive Care
KEY FACTS IN ANAESTHESIA AND INTENSIVE CARE Alcira Serrano Gomez MD Fellow John Farman Intensive Care Unit Addenbrooke s NHS Trust Cambridge, UK Gilbert R Park MD DMed Sci FRCA Director of Intensive Care
SRI NATHELLA SAMPATHU CHETTY CLINICAL LABORATORY (UNIT OF MEDICAL RESEARCH FOUNDATION) Test Master List
 S. No Lab/ Ref/ code Name of the Test CLINICAL BIOCHEMISTRY TESTS MASTER LIST Storage of Specimen Anti Reportable examined Required Coagulants interval specimen / (vacutainer) (vacutainer) (TAT) Temp.
S. No Lab/ Ref/ code Name of the Test CLINICAL BIOCHEMISTRY TESTS MASTER LIST Storage of Specimen Anti Reportable examined Required Coagulants interval specimen / (vacutainer) (vacutainer) (TAT) Temp.
What tests should be on the Alert List?
 What tests should be on the Alert List? Dr Que Lam On behalf RCPA-AACB High Risk Results Working Party: Alan McNeil, Grahame Caldwell, Craig Campbell, Penelope Coates, Robert Flatman, Andrew Georgiou,
What tests should be on the Alert List? Dr Que Lam On behalf RCPA-AACB High Risk Results Working Party: Alan McNeil, Grahame Caldwell, Craig Campbell, Penelope Coates, Robert Flatman, Andrew Georgiou,
Measure #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health
 Measure #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Measure #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Preventive Care and Screening: Influenza Immunization
 e Title e Identifier ( Authoring Tool) Preventive Care and Screening: Influenza Immunization 147 e Version number 6.1.000 NQF Number 0041 GUID a244aa29-7d11-4616-888a-86e376bfcc6f ment Period Steward Developer
e Title e Identifier ( Authoring Tool) Preventive Care and Screening: Influenza Immunization 147 e Version number 6.1.000 NQF Number 0041 GUID a244aa29-7d11-4616-888a-86e376bfcc6f ment Period Steward Developer
2018 CMS Web Interface
 CMS Web Interface CARE-2 (NQF 0101): Falls: Screening for Future Fall Risk Measure Steward: NCQA CMS Web Interface V2.1 Page 1 of 18 12/11/2017 Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface CARE-2 (NQF 0101): Falls: Screening for Future Fall Risk Measure Steward: NCQA CMS Web Interface V2.1 Page 1 of 18 12/11/2017 Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION...
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes
 Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Delta Check Calculation Guide
 Delta Check Calculation Guide National Technology 2017, All Rights Reserved By Senior Scientific Researcher, Asmaa Taher Table of Contents Definition... 2 Purpose... 2 Delta Check Research Studies... 2
Delta Check Calculation Guide National Technology 2017, All Rights Reserved By Senior Scientific Researcher, Asmaa Taher Table of Contents Definition... 2 Purpose... 2 Delta Check Research Studies... 2
Royal College of Pathologists of Australasia Allowable Limits of Performance
 The RCPA (Royal College of Pathologists of Australasia) have developed a thorough set of specifications for allowable error. For those not bound by the CLIA regulations, these can serve as a valuable resource.
The RCPA (Royal College of Pathologists of Australasia) have developed a thorough set of specifications for allowable error. For those not bound by the CLIA regulations, these can serve as a valuable resource.
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #440: Basal Cell Carcinoma (BCC)/Squamous Cell Carcinoma (SCC): Biopsy Reporting Time Pathologist to Clinician National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Quality ID #440: Basal Cell Carcinoma (BCC)/Squamous Cell Carcinoma (SCC): Biopsy Reporting Time Pathologist to Clinician National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Biochemistry Department Laboratory Handbook
 Biochemistry Department Laboratory Handbook Version : 3.3 Page 1 of 12 Table of contents Biochemistry Department... 1 Laboratory Handbook... 1 Introduction... 3 The Biochemistry Department... 3 High risk
Biochemistry Department Laboratory Handbook Version : 3.3 Page 1 of 12 Table of contents Biochemistry Department... 1 Laboratory Handbook... 1 Introduction... 3 The Biochemistry Department... 3 High risk
Hamilton Regional Laboratory Medicine Program
 Created: April 2002 of Review: February 2004 of Review: June 2006 of Review: July 2007, St. Joseph s Healthcare went live with Meditech as of June18, 2007. of Review: August 2009 of Review: December 2011;
Created: April 2002 of Review: February 2004 of Review: June 2006 of Review: July 2007, St. Joseph s Healthcare went live with Meditech as of June18, 2007. of Review: August 2009 of Review: December 2011;
Hamilton Regional Laboratory Medicine Program
 Created: April 2002 of Review: February 2004 of Review: June 2006 of Review: July 2007, St. Joseph s Healthcare went live with Meditech as of June18, 2007. of Review: August 2009 of Review: December 2011;
Created: April 2002 of Review: February 2004 of Review: June 2006 of Review: July 2007, St. Joseph s Healthcare went live with Meditech as of June18, 2007. of Review: August 2009 of Review: December 2011;
Quality ID #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health
 Quality ID #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #110 (NQF 0041): Preventive Care and Screening: Influenza Immunization National Quality Strategy Domain: Community/Population Health 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Measure #109: Osteoarthritis (OA): Function and Pain Assessment National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #109: Osteoarthritis (OA): Function and Pain Assessment National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK.
 Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK. Bill.Bartlett@nhs.net www.biologicalvariation.com Biological variation affects the clinical utility of reference
Dr Bill Bartlett Blood Sciences, Ninewells Hospital & Medical School, NHS Tayside, Scotland, UK. Bill.Bartlett@nhs.net www.biologicalvariation.com Biological variation affects the clinical utility of reference
AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST. RCPA-AACB Working Party for High Risk result Management
 AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST RCPA-AACB Working Party for High Risk result Management RCPA-AACB WORKING PARTY FOR HIGH RISKS RESULTS Craig Campbell Grahame Caldwell
AN EVIDENCE AND RISK-BASED APPROACH TO A HARMONISED LABORATORY ALERT LIST RCPA-AACB Working Party for High Risk result Management RCPA-AACB WORKING PARTY FOR HIGH RISKS RESULTS Craig Campbell Grahame Caldwell
Quality ID #225 (NQF 0509): Radiology: Reminder System for Screening Mammograms National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #225 (NQF 0509): Radiology: Reminder System for Screening Mammograms National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #225 (NQF 0509): Radiology: Reminder System for Screening Mammograms National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
The order of draw - myth or science? Dr Mike Cornes: Principal Clinical Scientist Royal Wolverhampton NHS Trust
 The order of draw - myth or science? Dr Mike Cornes: Principal Clinical Scientist Royal Wolverhampton NHS Trust Where is Wolverhampton? Samples Errors Where is Order of draw Important? Problems Associated
The order of draw - myth or science? Dr Mike Cornes: Principal Clinical Scientist Royal Wolverhampton NHS Trust Where is Wolverhampton? Samples Errors Where is Order of draw Important? Problems Associated
Test Profiles Time for a Change. Tony Badrick (AACB Harmonisation Committee)
 Test Profiles Time for a Change Tony Badrick (AACB Harmonisation Committee) 1. Why Profiles 2. Profiles A. LFT B. Electrolyte C. Renal D. Bone 3. Summary Same clothes!! Harmonisation! Same car!! Harmonisation!
Test Profiles Time for a Change Tony Badrick (AACB Harmonisation Committee) 1. Why Profiles 2. Profiles A. LFT B. Electrolyte C. Renal D. Bone 3. Summary Same clothes!! Harmonisation! Same car!! Harmonisation!
VITROS MicroSlide Assay Summary
 ACET Acetaminophen ALB Albumin EDTA 10 9 TDM PV Specialty 5.5 4 PV Isotonic saline or 10 200 μg/ml 66 1323 μmol/l (μmol/l = μg/ml x 6.616) 1.00 6.00 g/dl 10.0-60.0 g/l (g/l = g/dl x 10) Therapeutic: 670
ACET Acetaminophen ALB Albumin EDTA 10 9 TDM PV Specialty 5.5 4 PV Isotonic saline or 10 200 μg/ml 66 1323 μmol/l (μmol/l = μg/ml x 6.616) 1.00 6.00 g/dl 10.0-60.0 g/l (g/l = g/dl x 10) Therapeutic: 670
Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination
 Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage of new patients whose
Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage of new patients whose
Australian and New Zealand College of Veterinary Scientists. Membership Examination. Small Animal Medicine Paper 1
 Australian and New Zealand College of Veterinary Scientists Membership Examination June 2017 Small Animal Medicine Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours after perusal Answer
Australian and New Zealand College of Veterinary Scientists Membership Examination June 2017 Small Animal Medicine Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours after perusal Answer
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #102 (NQF 0389): Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low Risk Prostate Cancer Patients National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS
Quality ID #102 (NQF 0389): Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low Risk Prostate Cancer Patients National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #7 (NQF 0070): Coronary Artery Disease (CAD): Beta-Blocker Therapy Prior Myocardial Infarction (MI) or Left Ventricular Systolic Dysfunction (LVEF < 40%) National Quality Strategy Domain: Effective
Quality ID #7 (NQF 0070): Coronary Artery Disease (CAD): Beta-Blocker Therapy Prior Myocardial Infarction (MI) or Left Ventricular Systolic Dysfunction (LVEF < 40%) National Quality Strategy Domain: Effective
Definition: Active injection drug users - Those who have injected any drug(s) within the 12 month reporting period
 Quality ID #387 (NQF 3060): Annual Hepatitis C Virus (HCV) Screening for Patients who are Active Injection Drug Users National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
Quality ID #387 (NQF 3060): Annual Hepatitis C Virus (HCV) Screening for Patients who are Active Injection Drug Users National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
Quality ID #76: Prevention of Central Venous Catheter (CVC) - Related Bloodstream Infections National Quality Strategy Domain: Patient Safety
 Quality ID #76: Prevention of Central Venous Catheter (CVC) - Related Bloodstream Infections National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #76: Prevention of Central Venous Catheter (CVC) - Related Bloodstream Infections National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Body Fluids EQA. Rizzi de Leon. Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia
 Body Fluids EQA Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia 22-24 February 2019 What is a Body Fluid? https://en.wikipedia.org/wiki/ascites
Body Fluids EQA Royal College of Pathologists Australasia Quality Assurance Programs (RCPAQAP) Pathology Update Melbourne, Australia 22-24 February 2019 What is a Body Fluid? https://en.wikipedia.org/wiki/ascites
Specific Accreditation Guidance OECD GLP
 Specific Accreditation Guidance OECD GLP Information for GLP Study Sponsors January 2018 Copyright National Association of Testing Authorities, Australia 2013 This publication is protected by copyright
Specific Accreditation Guidance OECD GLP Information for GLP Study Sponsors January 2018 Copyright National Association of Testing Authorities, Australia 2013 This publication is protected by copyright
Supplementary materials
 Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination
 Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage of
Measure #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage of
Authorised: JSWoodford, Lead of Speciality. Biochemistry Reference Intervals, October Page 1 of 5
 AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
i. Where is the participant seen?
 PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
Measure #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care
 Measure #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
Measure #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Process High Priority
 Quality ID #440: Basal Cell Carcinoma (BCC)/Squamous Cell Carcinoma (SCC): Biopsy Reporting Time Pathologist to Clinician National Quality Strategy Domain: Communication and Care Coordination Meaningful
Quality ID #440: Basal Cell Carcinoma (BCC)/Squamous Cell Carcinoma (SCC): Biopsy Reporting Time Pathologist to Clinician National Quality Strategy Domain: Communication and Care Coordination Meaningful
Update: Body Fluid Testing. Fernando San Gil
 Update: Body Fluid Testing Fernando San Gil Background Notes - 1 Year Significant moments in IVD 2013 NATA and the TGA publish new validation and registration requirements for in-house IVDs and for in-house
Update: Body Fluid Testing Fernando San Gil Background Notes - 1 Year Significant moments in IVD 2013 NATA and the TGA publish new validation and registration requirements for in-house IVDs and for in-house
Solution for cardiac perfusion in viaflex plastic container
 CARDIOPLEGIA SOLUTION A Solution for cardiac perfusion in viaflex plastic container DESCRIPTION Cardioplegia Solution A is a sterile, non-pyrogenic solution in a Viaflex bag. It is used to induce cardiac
CARDIOPLEGIA SOLUTION A Solution for cardiac perfusion in viaflex plastic container DESCRIPTION Cardioplegia Solution A is a sterile, non-pyrogenic solution in a Viaflex bag. It is used to induce cardiac
B. PANITUMUMAB DOSE LEVEL 0 No dose reduction 1 Level -1 2 Level Other, specify in comments for this cycle
 Radiation Therapy Oncology Group Phase II Study Pre-operative Chemo- Radiation + Panitumumab for Potentially Operable Lung Cancer Concurrent Summary Form AMENDED DATA YES INSTRUCTIONS: Submit all pages
Radiation Therapy Oncology Group Phase II Study Pre-operative Chemo- Radiation + Panitumumab for Potentially Operable Lung Cancer Concurrent Summary Form AMENDED DATA YES INSTRUCTIONS: Submit all pages
Standard Operating Procedure
 Subject Alkaline Phosphatase C111 Index Number Lab-1504 Section Laboratory Subsection Regional Clinic/Affiliate Hospital Laboratories Category Departmental Contact Kamprud, Elizabeth Last Revised 4/12/2017
Subject Alkaline Phosphatase C111 Index Number Lab-1504 Section Laboratory Subsection Regional Clinic/Affiliate Hospital Laboratories Category Departmental Contact Kamprud, Elizabeth Last Revised 4/12/2017
SydPath Reference Intervals for Clinical Trials (Contract Pathology Unit) Unauthorised Copy
 HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
Glossary of terms used in College examinations. The Royal College of Emergency Medicine
 Glossary of terms used in College examinations The Royal College of Emergency Medicine The CEM uses several terms in examinations that may cause confusion. The following definitions are intended as a guide
Glossary of terms used in College examinations The Royal College of Emergency Medicine The CEM uses several terms in examinations that may cause confusion. The following definitions are intended as a guide
QUALITROL 2H Insert. 01 Inglês - Ref.: 72. Ref.:72. Precautions and warnings
 QUALITROL 2H Insert Ref.:72 Lot 6002 Expiration 2019/08/23 Precautions and warnings Qualitrol 2H is for in vitro diagnostic use only. Attention. It is suggested to verify carefully if the lot number pressed
QUALITROL 2H Insert Ref.:72 Lot 6002 Expiration 2019/08/23 Precautions and warnings Qualitrol 2H is for in vitro diagnostic use only. Attention. It is suggested to verify carefully if the lot number pressed
Tables of Normal Values (As of February 2005)
 Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
The positive side of overdiagnosis
 The positive side of overdiagnosis Does an immediate comprehensive panel of laboratory tests in outpatient care improve patient outcomes? Bram Vrijsen, Maarten ten Berg, Christiana Naaktgeboren, Jolande
The positive side of overdiagnosis Does an immediate comprehensive panel of laboratory tests in outpatient care improve patient outcomes? Bram Vrijsen, Maarten ten Berg, Christiana Naaktgeboren, Jolande
Please contact the Client Services Team if you require further information.
 Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
DENOMINATOR: All patients aged 18 years and older seen for at least two visits or at least one preventive visit during the measurement period
 Quality ID #431 (NQF 2152): Preventive Care and Screening: Unhealthy Alcohol Use: Screening & Brief Counseling - National Quality Strategy Domain: Community / Population Health 2018 OPTIONS F INDIVIDUAL
Quality ID #431 (NQF 2152): Preventive Care and Screening: Unhealthy Alcohol Use: Screening & Brief Counseling - National Quality Strategy Domain: Community / Population Health 2018 OPTIONS F INDIVIDUAL
Denominator Criteria (Eligible Cases): Patient encounter during the performance period (CPT): 78300, 78305, 78306, 78315, 78320
 Quality ID #147: Nuclear Medicine: Correlation with Existing Imaging Studies for All Patients Undergoing Bone Scintigraphy National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Quality ID #147: Nuclear Medicine: Correlation with Existing Imaging Studies for All Patients Undergoing Bone Scintigraphy National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Supplementary Online Content
 Supplementary Online Content Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559. eequation. Applying the
Supplementary Online Content Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559. eequation. Applying the
HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2)
 0843 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 899UN SIZE: 20 x 5ml / 5 x 5ml EXPIRY: 2018-02 INTENDED USE This product is intended for in vitro diagnostic
0843 HUMAN ASSAYED MULTI-SERA - LEVEL 2 (HUM ASY CONTROL 2) CAT. NO. HN1530 / HS2611 LOT NO. 899UN SIZE: 20 x 5ml / 5 x 5ml EXPIRY: 2018-02 INTENDED USE This product is intended for in vitro diagnostic
NUMERATOR: Patients who had baseline cytogenetic testing performed on bone marrow
 Quality ID #67 (NQF 0377): Hematology: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow National Quality Strategy Domain: Effective Clinical Care
Quality ID #67 (NQF 0377): Hematology: Myelodysplastic Syndrome (MDS) and Acute Leukemias: Baseline Cytogenetic Testing Performed on Bone Marrow National Quality Strategy Domain: Effective Clinical Care
Quality ID #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care
 Quality ID #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
Quality ID #178: Rheumatoid Arthritis (RA): Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
CALIBRATION SERUM LEVEL 3 (CAL 3)
 CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 709UE SIZE: 20 x 5ml EXP: 2016-12 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
CALIBRATION SERUM LEVEL 3 (CAL 3) CAT. NO. CAL 2351 LOT NO. 709UE SIZE: 20 x 5ml EXP: 2016-12 INTENDED USE For use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised
SAT24 Supersaturation Profile, 24 Hour, Urine
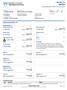 1-800-533-1710 SAT24 Supersaturation Profile, 24 Hour, Urine Patient ID Patient Name SAMPLEREPORT, SAT24 NORMAL Birth Date 1976-05-13 Gender M Age 40 Order Number Client Order Number Ordering Physician
1-800-533-1710 SAT24 Supersaturation Profile, 24 Hour, Urine Patient ID Patient Name SAMPLEREPORT, SAT24 NORMAL Birth Date 1976-05-13 Gender M Age 40 Order Number Client Order Number Ordering Physician
cobas c 501 analyzer and cobas c 311 analyzer Within Run Imprecision Guidelines
 cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
cobas c 501 analyzer and cobas c 311 analyzer General Information How to use these guidelines Unless otherwise indicated, the data presented is the same for both the cobas c 501 analyzer and the cobas
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Quality ID #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL
Quality ID #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL
Evaluation of VACUETTE SECONDARY Tubes
 Evaluation of VACUETTE SECODARY Tubes Background VACUETTE SECODARY Tubes are used as a secondary container for aliquoting, storing and transporting blood, blood components and urine from the primary tube
Evaluation of VACUETTE SECODARY Tubes Background VACUETTE SECODARY Tubes are used as a secondary container for aliquoting, storing and transporting blood, blood components and urine from the primary tube
CASE-BASED SMALL GROUP DISCUSSION MHD II
 MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
BC Biomedical Laboratories Adult Reference Ranges
 BC Biomedical Laboratories Adult s Name Age 25 OH VITAMIN D Blood B 0-100 nmol/l Interpretation: < 25 Deficient 25-74 Insufficient 75-199 Sufficient > 200 Toxic 5HIAA (CALC) Urine B 0-100
BC Biomedical Laboratories Adult s Name Age 25 OH VITAMIN D Blood B 0-100 nmol/l Interpretation: < 25 Deficient 25-74 Insufficient 75-199 Sufficient > 200 Toxic 5HIAA (CALC) Urine B 0-100
Quality ID #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
Quality ID #265: Biopsy Follow-Up National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
2) Patients who are 18 years and older with a diagnosis of CAD or history of cardiac surgery who have a prior myocardial infarction
 Measure #7 (NQF 0070): Coronary Artery Disease (CAD): Beta-Blocker Therapy Prior Myocardial Infarction (MI) or Left Ventricular Systolic Dysfunction (LVEF < 40%) National Quality Strategy Domain: Effective
Measure #7 (NQF 0070): Coronary Artery Disease (CAD): Beta-Blocker Therapy Prior Myocardial Infarction (MI) or Left Ventricular Systolic Dysfunction (LVEF < 40%) National Quality Strategy Domain: Effective
Measuring Sodium in dialysis patients. UCL Center for Nephrology
 Measuring Sodium in dialysis patients UCL Center for Nephrology sodium Na-heparin sulphate Na-dermatan sulphate Na-chondroitin sulphate endothelium Na-heparin sulphate Na-Sulfate plasma protein Na-Citrate
Measuring Sodium in dialysis patients UCL Center for Nephrology sodium Na-heparin sulphate Na-dermatan sulphate Na-chondroitin sulphate endothelium Na-heparin sulphate Na-Sulfate plasma protein Na-Citrate
Narelle Hadlow, Peter Ward, Ken Sikaris
 Narelle Hadlow, Peter Ward, Ken Sikaris Analytes for consideration Vascular, renal and Na and water changes Consider physiology and review data Na, K, Cl, Urea, Cr, Osm Acid base status Consider physiology
Narelle Hadlow, Peter Ward, Ken Sikaris Analytes for consideration Vascular, renal and Na and water changes Consider physiology and review data Na, K, Cl, Urea, Cr, Osm Acid base status Consider physiology
Get to know yourself better. Attend our health screening event.
 Gateway Technical College Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening
Gateway Technical College Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening
NOTICES DEPARTMENT OF HEALTH
 NOTICES DEPARTMENT OF HEALTH Approved Drugs for ALS Ambulance Services [42 Pa.B. 4229] [Saturday, July 7, 2012] Under 28 Pa. Code 1005.11(b) (relating to drug use, control and security), the following
NOTICES DEPARTMENT OF HEALTH Approved Drugs for ALS Ambulance Services [42 Pa.B. 4229] [Saturday, July 7, 2012] Under 28 Pa. Code 1005.11(b) (relating to drug use, control and security), the following
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #400 (NQF 3059): One-Time Screening for Hepatitis C Virus (HCV) for Patients at Risk National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #400 (NQF 3059): One-Time Screening for Hepatitis C Virus (HCV) for Patients at Risk National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY
DENOMINATOR: All female patients aged 65 years and older with a visit during the measurement period
 Measure #48: Urinary Incontinence: Assessment of Presence or Absence of Urinary Incontinence in Women Aged 65 Years and Older National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR
Measure #48: Urinary Incontinence: Assessment of Presence or Absence of Urinary Incontinence in Women Aged 65 Years and Older National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR
