B. PANITUMUMAB DOSE LEVEL 0 No dose reduction 1 Level -1 2 Level Other, specify in comments for this cycle
|
|
|
- Primrose Haynes
- 5 years ago
- Views:
Transcription
1 Radiation Therapy Oncology Group Phase II Study Pre-operative Chemo- Radiation + Panitumumab for Potentially Operable Lung Cancer Concurrent Summary Form AMENDED DATA YES INSTRUCTIONS: Submit all pages of this form according to time points in section 12.0 of the protocol. Record dates MM/DD/YYYY 1. ASSIGNED TREATMENT ARM(1) 2.. HEIGHT (CM)(2) 1 Arm 1 2 Arm 2 A. WAS DRUG MODIFIED/DELAYED? modification/delay 2 Reduced dose, planned (per protocol) 3 Reduced dose, not planned (not per protocol) 4 Exceeded protocol dose 5 Delayed B. PANITUMUMAB LEVEL 0 No dose reduction 1 Level -1 2 Level Other, specify in comments for this cycle C. PACLITAXEL LEVEL 0 No dose reduction 98 Other, specify in comments for this cycle D. CARBOPLATIN LEVEL 0 No dose reduction 98 Other, specify in comments for this cycle E. REASON FOR MODIFICATION modification 2 Disease progression, relapse during active treatment 3 Adverse event / side effect / complication* 4 Death on study 5 Patient withdrawal / refusal after beginning protocol therapy 6 Patient withdrawal / refusal prior to beginning protocol therapy 7 Alternative therapy, specify in comments 8 Other complicating disease, specify in comments 98 Other, specify in comments *Must code specific MedDRA Code in Q 9 Specify reason in cycle comments F. REASON TREATMENT TERMINATED 0 Not applicable, treatment not terminated 1 Treatment completed per protocol criteria 2 Disease progression, relapse during active treatment 3 Adverse event / side effect / complication* 4 Death on study 5 Patient withdrawal / refusal after beginning protocol therapy 6 Patient withdrawal / refusal prior to beginning protocol therapy 7 Alternative therapy, specify in comments 8 Patient off (protocol) treatment for other complicating disease (followup and data submission will continue) 98 Other, specify in comments *Must code specific MedDRA Code in Q 10 Specify reason in cycle comments 3. WEEK 1. WEIGHT (KG)(3). BSA(M 2 )(4) DATE AGENT START - - (6) - - (13) - - (20) DATE AGENT END - - (7) - - (14) - - (21) AGENT TOTAL (mg)(8) (mg)(15) (mg)(22) CLEARANCE ml/min(5) (USE CODE TABLE A) (9) (16) (23) MODIFIED LEVEL (USE CODE TABLE B-D) (10) (17) (24) (USE CODE TABLE E) (11) (18) (25) (USE CODE TABLE F) (12) (19) (26) COMMENTS (for this cycle) (27-28) 0839 TFa of 8
2 4. WEEK 2. WEIGHT (KG)(29). BSA(M 2 )(30) DATE AGENT START - - (32) - - (39) - - (46) DATE AGENT END - - (33) - - (40) - - (47) AGENT TOTAL (mg)(34) (mg)(41) (mg)(48) CLEARANCE ml/min(31) (USE CODE TABLE A) (35) (42) (49) MODIFIED LEVEL (USE CODE TABLE B-D) (36) (43) (50) (USE CODE TABLE E) (37) (44) (51) (USE CODE TABLE F) (38) (45) (52) COMMENTS (for this cycle) (53-54) 5. WEEK 3. WEIGHT (KG)(55). BSA(M 2 )(56 DATE AGENT START - - (58) - - (65) - - (72) DATE AGENT END - - (59) - - (66) - - (73) AGENT TOTAL (mg)(60) (mg)(67) (mg)(74) CLEARANCE ml/min(57) (USE CODE TABLE A) (61) (68) (75) MODIFIED LEVEL (USE CODE TABLE B-D) (62) (69) (76) (USE CODE TABLE E) (63) (70) (77) (USE CODE TABLE F) (64) (71) (78) COMMENTS (for this cycle) (79-80) 0839 TFa of 8
3 6. WEEK 4. WEIGHT (KG)(81). BSA(M 2 )(82) DATE AGENT START - - (84) - - (91) - - (98) DATE AGENT END - - (85) - - (92) - - (99) AGENT TOTAL (mg)(86) (mg)(93) (mg)(100) CLEARANCE ml/min(83) (USE CODE TABLE A) (87) (94) (101) MODIFIED LEVEL (USE CODE TABLE B-D) (88) (95) (102) (USE CODE TABLE E) (89) (96) (103) (USE CODE TABLE F) (90) (97) (104) COMMENTS (for this cycle) ( ) 7. WEEK 5. WEIGHT (KG)(107). BSA(M 2 )(108) DATE AGENT START - - (110) - - (117) - - (124) DATE AGENT END - - (111) - - (118) - - (125) AGENT TOTAL (mg)(112) (mg)(119) (mg)(126) CLEARANCE ml/min(109) (USE CODE TABLE A) (113) (120) (127) MODIFIED LEVEL (USE CODE TABLE B-D) (114) (121) (128) (USE CODE TABLE E) (115) (122) (129) (USE CODE TABLE F) (116) (123) (130) COMMENTS (for this cycle) ( ) 0839 TFa of 8
4 8. WEEK 6. WEIGHT (KG)(133). BSA(M 2 )(134) DATE AGENT START - - (136) - - (143) - - (150) DATE AGENT END - - (137) - - (144) - - (151) AGENT TOTAL (mg)(138) (mg)(145) (mg)(152) CLEARANCE ml/min(135) (USE CODE TABLE A) (139) (146) (153) MODIFIED LEVEL (USE CODE TABLE B-D) (140) (147) (154) (USE CODE TABLE E) (141) (148) (155) (USE CODE TABLE F) (142) (149) (156) COMMENTS (for this cycle) ( ) 0839 TFa of 8
5 9. SPECIFY AE THAT CAUSED TREATMENT MODIFICATION/DELAY. USE CTC AE VERSION 4.0 MedDRA INDICATE WHICH DRUG(S) WERE MODIFIED/DELAYED USING CODE TABLE I BELOW. I. DRUG MODIFICATION/ DELAY CODE TABLE A. CTC AE ATTRIBUTION CODE ANY PROTOCOL TREATMENT (Including investigational agent) B. SAE REPORT SUBMITTED* 9 Unknown C. ATTRIBUTION CODE FOR THE INVESTIGATIONAL AGENT MODIFICATION USE CODE TABLE I MedDRA AE CODE Week # Start Date(mandatory) Pan. Pacl. Carbo. Grade A B C (848) (849) - - (850) (851) (852) (853) (854) (855) (856) (857) (858) (859) - - (860) (861) (862) (863) (864) (865) (866) (867) (868) (869) - - (870) (871) (872) (873) (874) (875) (876) (877) (878) (879) - - (880) (881) (882) (883) (884) (885) (886) (887) (888) (889) - - (890) (891) (892) (893) (894) (895) (896) (897) (898) (899) - - (900) (901) (902) (903) (904) (905) (906) (907) (908) (909) - - (910) (911) (912) (913) (914) (915) (916) (917) (918) (919) - - (920) (921) (922) (923) (924) (925) (926) (927) (928) (929) - - (930) (931) (932) (933) (934) (935) (936) (937) (938) (939) - - (940) (941) (942) (943) (944) (945) (946) (947) 10. SPECIFY AE THAT CAUSED TREATMENT. USE CTC AE VERSION 4.0 MedDRA INDICATE WHICH DRUG(S) WERE TERMINATED USING CODE TABLE II BELOW. II. DRUG CODE TABLE A. CTC AE ATTRIBUTION CODE ANY PROTOCOL TREATMENT (Including investigational agent) B. SAE REPORT SUBMITTED* 9 Unknown C. ATTRIBUTION CODE FOR THE INVESTIGATIONAL AGENT USE CODE TABLE II MedDRA AE CODE Week # Start Date(mandatory) Pan. Pacl. Carbo. GRADE A B C (1048) (1049) - - (1050) (1051) (1052) (1053) (1054) (1055) (1056) (1057) (1058) (1059) - - (1060) (1061) (1062) (1063) (1064) (1065) (1066) (1067) (1068) (1069) - - (1070) (1071) (1072) (1073) (1074) (1075) (1076) (1077) (1078) (1079) - - (1080) (1081) (1082) (1083) (1084) (1085) (1086) (1087) (1088) (1089) - - (1090) (1091) (1092) (1093) (1094) (1095) (1096) (1097) {*If SAE reported, AE grade, start date, and attributions must match on ADEERS report and CRF} 0839 TFa of 8
6 11. ANY OTHER TREATMENT RELATED ADVERSE EVENTS?(159) 9 Unknown Adverse Events: Use the CTCAE version 4 (MedDRA 12) to code all events. Score most severe grade observed during report period (grade 1-5). Adverse Events of grade 3 or higher require start date. Assign attribution to protocol treatment for each AE and indicate if an SAE was reported. Adverse events reported in Q9 or Q10 should only be reported here if the grade is worse than reported in Q9 and/or Q10. A. Attribution to Protocol Treatment Adverse Event Code B. SAE Report Submitted 9 Unknown CTCAE V4 Term (specify if "other") Grade Start Date A B (301) (302) (303) - - (304) (305) (306) (307) (308) (309) - - (310) (311) (312) (313) (314) (315) - - (316) (317) (318) (319) (320) (321) - - (322) (323) (324) (325) (326) (327) - - (328) (329) (330) (331) (332) (333) - - (334) (335) (336) (337) (338) (339) - - (340) (341) (342) (343) (344) (345) - - (346) (347) (348) (349) (350) (351) - - (352) (353) (354) (355) (356) (357) - - (358) (359) (360) (361) (362) (363) - - (364) (365) (366) (367) (368) (369) - - (370) (371) (372) (373) (374) (375) - - (376) (377) (378) (379) (380) (381) - - (382) (383) (384) (385) (386) (387) - - (388) (389) (390) (391) (392) (393) - - (394) (395) (396) (397) (398) (399) - - (400) (401) (402) (403) (404) (405) - - (406) (407) (408) (409) (410) (411) - - (412) (413) (414) Comments ( ) 0839 TFa of 8
7 UNITS(1098) 1 Conventional (use flowsheet below) 2 SI (skip to Q#17 flowsheet) 12. INSTRUCTIONS: Record laboratory values USING STANDARD U.S. UNITS and normal ranges. See protocol for lab schedules Grade lab abnormalities on page 4 and/or 5. Normal Range Pre-Rx Date (mm/dd) Year: / / / / / / / / Hgb (g/dl) Hct (%) WBC (x1000) mm 3 Platelets (x1000) mm 3 Neutrophils (%) ANC (mm 3 ) Sodium (meq/l) Potassium (meq/l) Chloride (meq/l) BUN (mg/dl) Creatinine (mg/dl) Calc. Creatinine Clear. (ml/min) Total Bilirubin (mg/dl) Alk PO4 (ImU/ml) LDH (U/L) SGOT (IU/L) SGPT (IU/L) Total Protein (g/dl) Albumin (g/dl) Uric Acid (mg/dl) Calcium (mg/dl) Glucose(mg/dl) Mg (meq/l) U/A COMMENTS ( ) The reported case report information has been reviewed and confirmed by the principal investigator. (1101) Investigator Signature - - (1102) Date 0839 TFa of 8
8 13. INSTRUCTIONS: Record laboratory values USING STANDARD INTERNATIONAL UNITS and normal ranges. See protocol for lab schedules Grade lab abnormalities on page 4 and/or 5. Normal Range Pre-Rx Date (mm/dd) Year: / / / / / / / / Hgb (mmol/l) Hct (volume fraction) WBC (x10 9 liter) Platelets (x10 9 liter) Neutrophils (fraction) ANC (mm 3 ) Sodium (mmol/l) Potassium (mmol/l) Chloride (mmol/l) BUN (mmol/l) Creatinine (umol/l) Calc. Creatinine Clear. (ml/sec/m 2 ) Total Bilirubin (umol/l) Alk PO4 (U/L) LDH (IU/L) SGOT (U/L) SGPT (U/L) Total Protein (g/l) Albumin (g/l) Uric Acid (mmol/l) Calcium (mmol/l) Glucose (mmol/l) Mg (mmol/l) U/A COMMENTS ( ) The reported case report information has been reviewed and confirmed by the principal investigator. (1105) Investigator Signature - - (1106) Date 0839 TFa of 8
ASSIGNED TREATMENT ARM
 SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
(7) VITAL SIGNS (8) LEVEL OF CONSCIOUSNESS (9) MENTAL STATUS (10) SPEECH (11) VISION (12) FUNDUS (PAPILLEDEMA)
 Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
INSTRUCTIONS: 1. Use codetable on page 1 for modifications / termination reasons
 Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
PLACE LABEL HERE. Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form
 F1 AMENDED DATA Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form YES No INSTRUCTIONS: Submit this form at the appropriate follow-up interval and at death Dates are recorded
F1 AMENDED DATA Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form YES No INSTRUCTIONS: Submit this form at the appropriate follow-up interval and at death Dates are recorded
i. Where is the participant seen?
 PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
10 Essential Blood Tests PART 1
 Presents 10 Essential Blood Tests PART 1 The Blood Chemistry Webinars With DR. DICKEN WEATHERBY Creator of the Blood Chemistry Software Essential Blood Test #1: Basic Chem Screen and CBC http://bloodchemsoftware.com
Presents 10 Essential Blood Tests PART 1 The Blood Chemistry Webinars With DR. DICKEN WEATHERBY Creator of the Blood Chemistry Software Essential Blood Test #1: Basic Chem Screen and CBC http://bloodchemsoftware.com
MEDICAL HISTORY. 23-Jan-2018 to 23-Jan VCA Miller-Robertson Animal Hospital 8807 Melrose Ave, Los Angeles, CA (310)
 8807 Melrose Ave, Los Angeles, CA 90069 (310) 657-7050 MEDICAL HISTORY 23-Jan-2018 to 23-Jan-2018 Client Linnea Engdahl (1810) C: Linnea: (310) 351-9547 Patient Abby (6487) Canine Mixed Breed 3y (22-Jan-2015)
8807 Melrose Ave, Los Angeles, CA 90069 (310) 657-7050 MEDICAL HISTORY 23-Jan-2018 to 23-Jan-2018 Client Linnea Engdahl (1810) C: Linnea: (310) 351-9547 Patient Abby (6487) Canine Mixed Breed 3y (22-Jan-2015)
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Understanding Blood Tests
 PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION
 SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
NORMAL LABORATORY VALUES FOR CHILDREN
 Pediatric Drug Lookup Normal Laboratory Values for NORMAL LABORATORY VALUES FOR CHILDREN CHEMISTRY Normal Values Albumin 0-1 y 2.0-4.0 g/dl 1 y to adult 3.5-5.5 g/dl Ammonia Newborns 90-150 mcg/dl 40-120
Pediatric Drug Lookup Normal Laboratory Values for NORMAL LABORATORY VALUES FOR CHILDREN CHEMISTRY Normal Values Albumin 0-1 y 2.0-4.0 g/dl 1 y to adult 3.5-5.5 g/dl Ammonia Newborns 90-150 mcg/dl 40-120
Clinician Blood Panel Results
 Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
EXAMPLE REPORT ONLY Contact AMS Biotechnology for current donor specific information
 EXAMPLE REPORT ONLY Contact AMS Biotechnology for current donor specific information NAME DIAGNOSIS PROTOCOL OF EVALUATION for Chronic Lymphatic Leukemia (CLL) GENERAL INFORMATION (ALL information required!!)
EXAMPLE REPORT ONLY Contact AMS Biotechnology for current donor specific information NAME DIAGNOSIS PROTOCOL OF EVALUATION for Chronic Lymphatic Leukemia (CLL) GENERAL INFORMATION (ALL information required!!)
H.6.G.2 Non-MAC Studies
 Page 63 H.6.G.2 Non-MAC Studies H.6.G.2.A Non-MAC Studies at the 600 mg dose A 600 mg dose of azithromycin was given in two non-mac studies (354/354A and 167). Please note: Study 354/354A enrolled a mixture
Page 63 H.6.G.2 Non-MAC Studies H.6.G.2.A Non-MAC Studies at the 600 mg dose A 600 mg dose of azithromycin was given in two non-mac studies (354/354A and 167). Please note: Study 354/354A enrolled a mixture
JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS. Always call results for the following test(s):
 Facilities: NoCo Laboratories JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS Always call results for the following test(s): CONSTITUENT Ethylene Glycol (Performed at CU) Time Interval (from
Facilities: NoCo Laboratories JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS Always call results for the following test(s): CONSTITUENT Ethylene Glycol (Performed at CU) Time Interval (from
SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION
 SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile.
 1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile. NUCLEATED CELLS 19.5 High 4.0-14.0 x 10^3/ul METAMYELOCYTES 9 % 1.8 High 0.0-0.0 x 10^3/ul BAND NEUTROPHILS 61
1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile. NUCLEATED CELLS 19.5 High 4.0-14.0 x 10^3/ul METAMYELOCYTES 9 % 1.8 High 0.0-0.0 x 10^3/ul BAND NEUTROPHILS 61
Clinician Blood Panel Results
 Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Supplementary materials
 Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Tables of Normal Values (As of February 2005)
 Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
Tables of Normal Values (As of February 2005) Note: Values and units of measurement listed in these Tables are derived from several resources. Substantial variation exists in the ranges quoted as normal
LabDriver Audit Trail Example
 LabDriver Audit Trail Example Sample details:= (SampleDId=82051) Lab no: 0902168 Centre: CR Centre (CentreId=1079) (BatchSId=1317) Status: Checked Blood date: 13/05/2009 time: 11:31:00 lab received: 13/05/2009
LabDriver Audit Trail Example Sample details:= (SampleDId=82051) Lab no: 0902168 Centre: CR Centre (CentreId=1079) (BatchSId=1317) Status: Checked Blood date: 13/05/2009 time: 11:31:00 lab received: 13/05/2009
CLL13 trial of the GCLLSG/ The GAIA Trial Page 23 of 113 IV. Baseline assessments at screening (for all patients)
 CLL13 trial of the GCLLSG/ The GAIA Trial Page 23 of 113 IV. Baseline assessments at screening (for all patients) Informed consent Inclusion-/Exclusion Criteria CIRS Score /Medical history ECOG /Disease-related
CLL13 trial of the GCLLSG/ The GAIA Trial Page 23 of 113 IV. Baseline assessments at screening (for all patients) Informed consent Inclusion-/Exclusion Criteria CIRS Score /Medical history ECOG /Disease-related
BIOCHEMICAL REPORT. Parameters Unit Finding Normal Value. Lipase U/L Amylase U/L
 Lipase U/L 88.9 10-195 Amylase U/L 1181.1 371.3-1192.6 West Delhi :- 7/148, Opp. MCD Office, Major Pankaj Batra Marg, Near Ramesh Nagar, New Delhi-15, Ph. : 011-47562566,9999830187 Liver Function Test
Lipase U/L 88.9 10-195 Amylase U/L 1181.1 371.3-1192.6 West Delhi :- 7/148, Opp. MCD Office, Major Pankaj Batra Marg, Near Ramesh Nagar, New Delhi-15, Ph. : 011-47562566,9999830187 Liver Function Test
Age: 14 Houston TX 77007
 Patient Medical History HEIGHTS HOSPITAL FOR ANIMALS Bernie Rogers Patient: JACK DOB: 08/26/1999 720 Courtlandt St. Species: FELINE Age: 14 Houston TX 77007 Breed: Domestic Shorthair Sex: MN Color: Black
Patient Medical History HEIGHTS HOSPITAL FOR ANIMALS Bernie Rogers Patient: JACK DOB: 08/26/1999 720 Courtlandt St. Species: FELINE Age: 14 Houston TX 77007 Breed: Domestic Shorthair Sex: MN Color: Black
Clinician Blood Panel Results
 Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
ENROLLMENT CONFIRMATION
 Step 1: Please review the Facility/Contact information. If any of the information is incorrect, please make the appropriate changes below: Facility/Contact Phone: (850)474-3660 Fax: (850)474-3659 6431
Step 1: Please review the Facility/Contact information. If any of the information is incorrect, please make the appropriate changes below: Facility/Contact Phone: (850)474-3660 Fax: (850)474-3659 6431
Authorised: JSWoodford, Lead of Speciality. Biochemistry Reference Intervals, October Page 1 of 5
 AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
AFP All All < 15 ug/l Albumin All 0-3M 25-40 g/l Albumin All 3-12M 32-45 g/l Albumin All 1-70Y 34-48 g/l Albumin All >70Y 32-46 g/l Alk Phos All 0-10Y 80-350 U/L Alk Phos M 10-14Y 45-400 U/L Alk Phos F
CRRT Fundamentals Pre-Test. AKI & CRRT 2017 Practice Based Learning in CRRT
 CRRT Fundamentals Pre-Test AKI & CRRT 2017 Practice Based Learning in CRRT Question 1 A 72-year-old man with HTN presents to the ED with slurred speech, headache and weakness after falling at home. He
CRRT Fundamentals Pre-Test AKI & CRRT 2017 Practice Based Learning in CRRT Question 1 A 72-year-old man with HTN presents to the ED with slurred speech, headache and weakness after falling at home. He
Serodos and Serodos plus
 Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
BC Biomedical Laboratories Adult Reference Ranges
 BC Biomedical Laboratories Adult s Name Age 25 OH VITAMIN D Blood B 0-100 nmol/l Interpretation: < 25 Deficient 25-74 Insufficient 75-199 Sufficient > 200 Toxic 5HIAA (CALC) Urine B 0-100
BC Biomedical Laboratories Adult s Name Age 25 OH VITAMIN D Blood B 0-100 nmol/l Interpretation: < 25 Deficient 25-74 Insufficient 75-199 Sufficient > 200 Toxic 5HIAA (CALC) Urine B 0-100
COG-ACNS1123: Phase 2 Trial of Response-Based Radiation Therapy for Patients with Localized Central Nervous System Germ Cell Tumors (CNS GCT)
 Page 1 of 6 COG-ACNS1123: Phase 2 Trial of Response-Based Radiation Therapy for Patients with Localized Central Nervous System Germ Cell Tumors (CNS GCT) FAST FACTS Eligibility Reviewed and Verified By
Page 1 of 6 COG-ACNS1123: Phase 2 Trial of Response-Based Radiation Therapy for Patients with Localized Central Nervous System Germ Cell Tumors (CNS GCT) FAST FACTS Eligibility Reviewed and Verified By
Get to know yourself better. Attend our health screening event.
 Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening Program. 1 SIMPLE ACTION
Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening Program. 1 SIMPLE ACTION
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Get to know yourself better. Attend our health screening event.
 Gateway Technical College Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening
Gateway Technical College Get to know yourself better. Attend our health screening event. Putting your knowledge to action is Powerful. Get the information and guidance you need with the Wellness Screening
Delta Check Calculation Guide
 Delta Check Calculation Guide National Technology 2017, All Rights Reserved By Senior Scientific Researcher, Asmaa Taher Table of Contents Definition... 2 Purpose... 2 Delta Check Research Studies... 2
Delta Check Calculation Guide National Technology 2017, All Rights Reserved By Senior Scientific Researcher, Asmaa Taher Table of Contents Definition... 2 Purpose... 2 Delta Check Research Studies... 2
1 Week Followup 5/27/2014. Nursing Home/Assisted Care Hospice Another hospital Rehabilitation Facility Unknown
 1 Week Followup t Started Please answer all questions considering all time since the previous visit and current follow-up date. Print this Form Followup Status t Started Select one of the following Inpatient
1 Week Followup t Started Please answer all questions considering all time since the previous visit and current follow-up date. Print this Form Followup Status t Started Select one of the following Inpatient
Date Time By Code Description Qty (Variance) Photo
 Adobe Animal Hospital 6331 Haven Ave., Suite 4 Rancho Cucamonga, CA 91737 909-483-3535 Patient Chart Printed: 03-16-17 at 9:44a CLIENT INFORMATION Name Ms. Amanda Barber (1394) Address 10850 Church St.
Adobe Animal Hospital 6331 Haven Ave., Suite 4 Rancho Cucamonga, CA 91737 909-483-3535 Patient Chart Printed: 03-16-17 at 9:44a CLIENT INFORMATION Name Ms. Amanda Barber (1394) Address 10850 Church St.
SydPath Reference Intervals for Clinical Trials (Contract Pathology Unit) Unauthorised Copy
 HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
REFERENCE INTERVALS. Units Canine Feline Bovine Equine Porcine Ovine
 REFERENCE INTERVALS Biochemistry Units Canine Feline Bovine Equine Porcine Ovine Sodium mmol/l 144-151 149-156 135-151 135-148 140-150 143-151 Potassium mmol/l 3.9-5.3 3.3-5.2 3.9-5.9 3.0-5.0 4.7-7.1 4.6-7.0
REFERENCE INTERVALS Biochemistry Units Canine Feline Bovine Equine Porcine Ovine Sodium mmol/l 144-151 149-156 135-151 135-148 140-150 143-151 Potassium mmol/l 3.9-5.3 3.3-5.2 3.9-5.9 3.0-5.0 4.7-7.1 4.6-7.0
ROTUNDA HOSPITAL DEPARTMENT OF LABORATORY MEDICINE
 This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
REGISTRATION FORM 1 / 2
 IDENTIFICATION AND BASELINE DATA REGISTRATION FORM 1 / 2 M Patient s initials: Sequential case N : SEX : F Date of birth: Place of birth I I Centre I I Address I I Telephone number I I Actual profession
IDENTIFICATION AND BASELINE DATA REGISTRATION FORM 1 / 2 M Patient s initials: Sequential case N : SEX : F Date of birth: Place of birth I I Centre I I Address I I Telephone number I I Actual profession
What is PlaqueOff (PO)? A new study in Beagle dogs. Oral effects of
 Oral effects of What is? PO is a dry food supplement. Sprinkle it onto your pet s food daily. PO is an algae that has been harvested in the Atlantic ocean in northern Norway and contains nothing else such
Oral effects of What is? PO is a dry food supplement. Sprinkle it onto your pet s food daily. PO is an algae that has been harvested in the Atlantic ocean in northern Norway and contains nothing else such
Kathryn Jones 8/11/2015
 1 of 8 8/11/2015 2:25 PM This informa on is copyrighted 2014 by Balancing Body Chemistry with Nutri on Seminars. No part may be copied or reproduced without wri en approval of Balancing Body Chemistry
1 of 8 8/11/2015 2:25 PM This informa on is copyrighted 2014 by Balancing Body Chemistry with Nutri on Seminars. No part may be copied or reproduced without wri en approval of Balancing Body Chemistry
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017
 M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
Cancer Research Group Version Date: June 22, 2016 NCI Update Date: November 13, Schema
 ev. 5/14 Cancer esearch Group Schema NDUCTON 1, 3 rm Step 0 P E - E G S T T O Step 1 N D O M Z T O Stratification: ntent to stem cell transplant at progression: Yes or No Bortezomib 1.3 mg/m2 SQ or V days
ev. 5/14 Cancer esearch Group Schema NDUCTON 1, 3 rm Step 0 P E - E G S T T O Step 1 N D O M Z T O Stratification: ntent to stem cell transplant at progression: Yes or No Bortezomib 1.3 mg/m2 SQ or V days
*** To get the most out of this report and the consultation, send us blood test results that cover as many of the following markers as possible:
 Rick Gold, Certified FDN Practitioner Gold Functional Wellness, Inc. Web: http://goldfunctionalwellness.com/ Phone: (561)270-6364 Email: Rick@goldfunctionalwellness.com Schedule a consultation: https://snapappointments.com/listing/38c
Rick Gold, Certified FDN Practitioner Gold Functional Wellness, Inc. Web: http://goldfunctionalwellness.com/ Phone: (561)270-6364 Email: Rick@goldfunctionalwellness.com Schedule a consultation: https://snapappointments.com/listing/38c
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck
 Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Breast Pathway Group Gemcitabine & Paclitaxel in Advanced Breast Cancer
 Breast Pathway Group Gemcitabine & Paclitaxel in Advanced Breast Cancer Indication: Alternative palliative treatment for advanced breast cancer in patients where docetaxel monotherapy or docetaxel/capecitabine
Breast Pathway Group Gemcitabine & Paclitaxel in Advanced Breast Cancer Indication: Alternative palliative treatment for advanced breast cancer in patients where docetaxel monotherapy or docetaxel/capecitabine
Patient: Becky Smith DOB: 01/26/XXXX Age: 5 y/o Attending: Dr. D. Miles Allergies: NKA MR#: 203. Patient Chart #203 Becky Smith
 Patient Chart #203 Becky Smith 1 Property of CSCLV CSCLV Rev: 06/04/2018 Chief Complaint: Abdominal pain. Informant: Parents. HISTORY & PHYSICAL HPI: Ill looking patient, healthy until 2 days ago when
Patient Chart #203 Becky Smith 1 Property of CSCLV CSCLV Rev: 06/04/2018 Chief Complaint: Abdominal pain. Informant: Parents. HISTORY & PHYSICAL HPI: Ill looking patient, healthy until 2 days ago when
Session 1: Circuit, Anticoagulation and Monitoring. Ashita Tolwani, MD, MSc Noel Oabel, BSN, RN, CNN 2019
 Session 1: Circuit, Anticoagulation and Monitoring Ashita Tolwani, MD, MSc Noel Oabel, BSN, RN, CNN 2019 Goals n Learn how to set up citrate anticoagulation for CVVH, CVVHD, CVVHDF using Prismaflex n Determine
Session 1: Circuit, Anticoagulation and Monitoring Ashita Tolwani, MD, MSc Noel Oabel, BSN, RN, CNN 2019 Goals n Learn how to set up citrate anticoagulation for CVVH, CVVHD, CVVHDF using Prismaflex n Determine
Test Result Reference Range Flag
 Date of Last Result Test Result Reference Range Flag Dec 07, 2016 25-Hydroxy Vitamin D Total 53 ng/ml 30-100 ng/ml Activated Partial Thromboplast Time Alanine Aminotransferase (ALT/SGPT) 25 sec 24-35 sec
Date of Last Result Test Result Reference Range Flag Dec 07, 2016 25-Hydroxy Vitamin D Total 53 ng/ml 30-100 ng/ml Activated Partial Thromboplast Time Alanine Aminotransferase (ALT/SGPT) 25 sec 24-35 sec
Doxorubicin and Ifosfamide Sarcoma
 Systemic Anti Cancer Treatment Protocol Doxorubicin and Ifosfamide Sarcoma PROTOCOL REF: MPHADOXIFO (Version No:.0) Approved for use in: Soft tissue sarcoma Dosage: Drug Dosage Route Frequency Doxorubicin
Systemic Anti Cancer Treatment Protocol Doxorubicin and Ifosfamide Sarcoma PROTOCOL REF: MPHADOXIFO (Version No:.0) Approved for use in: Soft tissue sarcoma Dosage: Drug Dosage Route Frequency Doxorubicin
Blood Test Results Report
 Blood Test Results Report The Blood Test Results Report lists the results of the patient s Chemistry Screen and CBC and shows you whether or not an individual element is outside of the optimal range and/or
Blood Test Results Report The Blood Test Results Report lists the results of the patient s Chemistry Screen and CBC and shows you whether or not an individual element is outside of the optimal range and/or
FBC interpretation. Dr. Gergely Varga
 FBC interpretation Dr. Gergely Varga #1 71 Y/O female, c/o weakness Test Undertaken : FBC (FBC) Sample Type: Whole Blood [ - 26.09.11 14:59] Hb 7.3 g/dl* 12.0-15.5 RBC 3.5 10^12/l * 3.80-5.60 Hct 0.24
FBC interpretation Dr. Gergely Varga #1 71 Y/O female, c/o weakness Test Undertaken : FBC (FBC) Sample Type: Whole Blood [ - 26.09.11 14:59] Hb 7.3 g/dl* 12.0-15.5 RBC 3.5 10^12/l * 3.80-5.60 Hct 0.24
Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma
 Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Plattenepithelkarzinom des Ösophagus, 1 st -line
 Plattenepithelkarzinom des Ösophagus, 1 st -line AIO-STO-0309 An open-label, randomized phase III trial of cisplatin and 5-fluorouracil with or without panitumumab for patients with nonresectable, advanced
Plattenepithelkarzinom des Ösophagus, 1 st -line AIO-STO-0309 An open-label, randomized phase III trial of cisplatin and 5-fluorouracil with or without panitumumab for patients with nonresectable, advanced
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE:
 Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
MHD I SESSION X. Renal Disease
 MHD I, Session X, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION X Renal Disease Monday, November 11, 2013 MHD I, Session X, Student Copy Page 2 Case #1 Cc: I have had weeks of diarrhea
MHD I, Session X, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION X Renal Disease Monday, November 11, 2013 MHD I, Session X, Student Copy Page 2 Case #1 Cc: I have had weeks of diarrhea
ROUTINE LAB STUDIES. Routine Clinic Lab Studies
 ROUTINE LAB STUDIES Routine Clinic Lab Studies With all lab studies, a tacrolimus or cyclosporine level will be obtained. These drug levels are routinely assessed to ensure that there is enough or not
ROUTINE LAB STUDIES Routine Clinic Lab Studies With all lab studies, a tacrolimus or cyclosporine level will be obtained. These drug levels are routinely assessed to ensure that there is enough or not
PENN STATE MILTON S. HERSHEY MEDICAL CENTER TABLE OF CRITICAL LAB VALUES CURRENT AS OF: 1/2015
 PENN STATE MILTON S. HERSHEY MEDICAL CENTER TABLE OF CRITICAL LAB VALUES CURRENT AS OF: 1/2015 Section: Chemistry For inpatients, once 3 consecutive critical values for the same analyte (test result) have
PENN STATE MILTON S. HERSHEY MEDICAL CENTER TABLE OF CRITICAL LAB VALUES CURRENT AS OF: 1/2015 Section: Chemistry For inpatients, once 3 consecutive critical values for the same analyte (test result) have
MHD I Session VIII Renal Disease November 6, 2013 STUDENT COPY
 MHD I, Session VIII, Student Copy Page 1 MHD I Session VIII Renal Disease November 6, 2013 STUDENT COPY MHD I, Session VIII, Student Copy Page 2 Case #1 Chief Complaint: I have been feeling just lousy
MHD I, Session VIII, Student Copy Page 1 MHD I Session VIII Renal Disease November 6, 2013 STUDENT COPY MHD I, Session VIII, Student Copy Page 2 Case #1 Chief Complaint: I have been feeling just lousy
ELIGIBILITY: small cell or neuroendocrine cancer (pure or mixed) administration of this protocol is restricted to BCCA Cancer Centres
 BCCA Protocol Summary for Treatment of Small Cell or Neuroendocrine Carcinoma of Gynecologic System Origin using PACLitaxel, CISplatin, Etoposide and CARBOplatin with Radiation (GO 95 02) Protocol Code:
BCCA Protocol Summary for Treatment of Small Cell or Neuroendocrine Carcinoma of Gynecologic System Origin using PACLitaxel, CISplatin, Etoposide and CARBOplatin with Radiation (GO 95 02) Protocol Code:
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
MidMichigan Health LABORATORY POLICY Title: Effecti ve te: Key Words: places: Category: Applicability: reference laboratory
 Page 1 of 5 PURPOSE This policy describes the responsibilities for determining which tests performed and or provided by the MidMichigan Health Laboratories fulfill the criteria for critical values / critical
Page 1 of 5 PURPOSE This policy describes the responsibilities for determining which tests performed and or provided by the MidMichigan Health Laboratories fulfill the criteria for critical values / critical
Impact of Proposed HRI s on Laboratory Report Flagging Rates
 Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Impact of Proposed HRI s on Laboratory Report Flagging Rates A/Prof. Ken Sikaris Melbourne Pathology BSc(Hons), MBBS, FRCPA, FAACB, FFSc CBN 2011 WORKSHOP 2012 WORKSHOP 2013 WORKSHOP 2014 GENERAL CONCEPTS
Provided by MedicalStudentExams.com NORMAL LABORATORY VALUES
 NORMAL LABORATORY VALUES 1. BLOOD, PLASMA, SERUM 2. CEREBROSPINAL FLUID 3. HEMATOLOGIC 4. SWEAT 5. URINE 6. SYNOVIAL FLUID 7. TOXIC LEVELS 8. Tumour Markers 9. Differential of Cerebral Spinal Fluid 10.
NORMAL LABORATORY VALUES 1. BLOOD, PLASMA, SERUM 2. CEREBROSPINAL FLUID 3. HEMATOLOGIC 4. SWEAT 5. URINE 6. SYNOVIAL FLUID 7. TOXIC LEVELS 8. Tumour Markers 9. Differential of Cerebral Spinal Fluid 10.
COMPANY OR UNIVERSITY
 CONTRIBUTOR NAME Daniel Heinrich, DVM CONTRIBUTOR EMAIL dheinric@umn.edu COAUTHORS Jed Overmann, DVM, DACVP; Davis Seelig DVM, PhD, DACVP & Matthew Sturos, DVM COMPANY OR UNIVERSITY University of Minnesota
CONTRIBUTOR NAME Daniel Heinrich, DVM CONTRIBUTOR EMAIL dheinric@umn.edu COAUTHORS Jed Overmann, DVM, DACVP; Davis Seelig DVM, PhD, DACVP & Matthew Sturos, DVM COMPANY OR UNIVERSITY University of Minnesota
ELIGIBILITY: Newly diagnosed acute promyelocytic leukemia (APL) with high risk (WBC more than 10 x 10 9 /L)
 BC Cancer Protocol Summary for First-Line Induction and Consolidation Therapy of Acute Promyelocytic Leukemia Using Arsenic Trioxide, Tretinoin (All-Trans Retinoic Acid) and DAUNOrubicin Protocol Code
BC Cancer Protocol Summary for First-Line Induction and Consolidation Therapy of Acute Promyelocytic Leukemia Using Arsenic Trioxide, Tretinoin (All-Trans Retinoic Acid) and DAUNOrubicin Protocol Code
Comments / Frequency (if not each time)
 s CHEMISTRY Acetaminophen High: > 250 ug/ml Bicarbonate Low: < 10 meq/l High: >40 meq/l Bilirubin (< 5 years old) High: > 15 mg/dl Calcium, total (serum) Low: < 6.0 mg/dl High: > 13.0 mg/dl > 15 Absurd
s CHEMISTRY Acetaminophen High: > 250 ug/ml Bicarbonate Low: < 10 meq/l High: >40 meq/l Bilirubin (< 5 years old) High: > 15 mg/dl Calcium, total (serum) Low: < 6.0 mg/dl High: > 13.0 mg/dl > 15 Absurd
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes
 Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Ana Luisa Stuckett, PhD, MS
 Ana Luisa Stuckett, PhD, MS alstuckett@mdanderson.org Clinical Studies Coordinator Investigational Cancer Therapeutics (Phase I) MD Anderson Cancer Center Houston, Texas Education Ph.D., Microbiology,
Ana Luisa Stuckett, PhD, MS alstuckett@mdanderson.org Clinical Studies Coordinator Investigational Cancer Therapeutics (Phase I) MD Anderson Cancer Center Houston, Texas Education Ph.D., Microbiology,
Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of
 Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of Gastroenterology & Hepatology www.livermd.org HCV in advanced disease In principle
Hepatitis C: How sick can we treat? Robert S. Brown, Jr., MD, MPH Vice Chair, Transitions of Care Interim Chief, Division of Gastroenterology & Hepatology www.livermd.org HCV in advanced disease In principle
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour
 TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
Postanalytical phase
 Postanalytical phase Test request POSTANALYTICAL Result interpretation PHASE Result Sampling Black box: the lab And the RESULT is created The technician approves the result; it is transferred to the lab
Postanalytical phase Test request POSTANALYTICAL Result interpretation PHASE Result Sampling Black box: the lab And the RESULT is created The technician approves the result; it is transferred to the lab
1 Week Followup - Intermacs
 version date: 9/27/2017 1 Week Followup - Intermacs Followup Status (1 Week Followup (+/- 3 days)) Select one of the following Inpatient Outpatient Other Facility Unable to obtain follow-up information
version date: 9/27/2017 1 Week Followup - Intermacs Followup Status (1 Week Followup (+/- 3 days)) Select one of the following Inpatient Outpatient Other Facility Unable to obtain follow-up information
Form 8: Post Transplant Annual Followup
 Page 1 of 8 Patient Details Hidden Show Show/Hide Annotations Stickies: Toggle All Toggle Open Toggle Resolved Form 8: Post Transplant Annual Followup Toggle Question Year/Info Print this Form t Started
Page 1 of 8 Patient Details Hidden Show Show/Hide Annotations Stickies: Toggle All Toggle Open Toggle Resolved Form 8: Post Transplant Annual Followup Toggle Question Year/Info Print this Form t Started
CORE CLINICAL DATASET
 The following pages are the CORE CLINICAL DATASET for Ebolavirus disease. The forms are featured in order of priority. Therefore, when resources are limited, data collection can be reduced as required
The following pages are the CORE CLINICAL DATASET for Ebolavirus disease. The forms are featured in order of priority. Therefore, when resources are limited, data collection can be reduced as required
Gemcitabine + Cisplatin Regimen
 Gemcitabine + Cisplatin Regimen Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication
Gemcitabine + Cisplatin Regimen Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication
Patient Encounters in the Primary Care Setting
 Patient Encounters in the Primary Care Setting Carmine D Amico, D.O. Clinical Cases Overview Learning objectives Clinical case presentations Questions for audience participation 1 Clinical Cases Learning
Patient Encounters in the Primary Care Setting Carmine D Amico, D.O. Clinical Cases Overview Learning objectives Clinical case presentations Questions for audience participation 1 Clinical Cases Learning
R-GDP: Rituximab, Gemcitabine, Dexamethasone &Cisplatin
 : Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
: Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
CRRT Fundamentals Pre- and Post- Test Answers. AKI & CRRT 2017 Practice Based Learning in CRRT
 CRRT Fundamentals Pre- and Post- Test Answers AKI & CRRT 2017 Practice Based Learning in CRRT Question 1 A 72-year-old man with HTN presents to the ED with slurred speech, headache and weakness after falling
CRRT Fundamentals Pre- and Post- Test Answers AKI & CRRT 2017 Practice Based Learning in CRRT Question 1 A 72-year-old man with HTN presents to the ED with slurred speech, headache and weakness after falling
CASE-BASED SMALL GROUP DISCUSSION MHD II
 MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
Rapid Laboratories In House Tests
 Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions
 Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Complete Medical History
 Lab Results for Ben Greenfield Last Test Date: Your medical history is not complete. Complete Medical History Complete Medical History What's Next Blood Draw Blood draw scheduled Complete your medical
Lab Results for Ben Greenfield Last Test Date: Your medical history is not complete. Complete Medical History Complete Medical History What's Next Blood Draw Blood draw scheduled Complete your medical
Final Case Study: Renal Disease Due 3/19/14 60 points
 NUT 116BL Name: CHRISTINE WOO Winter 2014 Section: 1 Final Case Study: Renal Disease Due 3/19/14 60 points Part I: Initial Presentation Present Illness: Jenny is a 19 yo F student referred to the renal
NUT 116BL Name: CHRISTINE WOO Winter 2014 Section: 1 Final Case Study: Renal Disease Due 3/19/14 60 points Part I: Initial Presentation Present Illness: Jenny is a 19 yo F student referred to the renal
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary
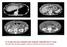 A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
SUMMARY OF CHANGES Amendment 6, Version Date: March 29, 2010 [Broadcast: April 8, 2010]
![SUMMARY OF CHANGES Amendment 6, Version Date: March 29, 2010 [Broadcast: April 8, 2010] SUMMARY OF CHANGES Amendment 6, Version Date: March 29, 2010 [Broadcast: April 8, 2010]](/thumbs/78/77615238.jpg) Amendment 6, Version Date: March 29, 2010 [Broadcast: April 8, 2010] RTOG 0233, "A Phase II Randomized Trial for Patients With Muscle-Invading Bladder Cancer Evaluating Transurethral Surgery and BID Irradiation
Amendment 6, Version Date: March 29, 2010 [Broadcast: April 8, 2010] RTOG 0233, "A Phase II Randomized Trial for Patients With Muscle-Invading Bladder Cancer Evaluating Transurethral Surgery and BID Irradiation
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine
 BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
SMITH, JOHN D.C. Functional Health Report Practitioner Copy JANE DOE. Lab Test on Mar 11, 2017 Conventional US Units
 SMITH, JOHN D.C. Functional Health Report Practitioner Copy JANE DOE Conventional US Units Table of Contents Health Improvement Plan 3 This report shows customized recommendations based on the blood test
SMITH, JOHN D.C. Functional Health Report Practitioner Copy JANE DOE Conventional US Units Table of Contents Health Improvement Plan 3 This report shows customized recommendations based on the blood test
Ascend Clinical Reference Ranges (November 2018) Chemistry
 24 Hour Urine Creatinine mg/24 hr 800-2000 (Male) 600-1800 (Female) Kinetic Alkaline Picrate (Jaffe Reaction) for Creatinine 24 hour Urine Creatinine Clearence (Creatinine ml/min/1.73m^2 85-125 (Male)
24 Hour Urine Creatinine mg/24 hr 800-2000 (Male) 600-1800 (Female) Kinetic Alkaline Picrate (Jaffe Reaction) for Creatinine 24 hour Urine Creatinine Clearence (Creatinine ml/min/1.73m^2 85-125 (Male)
Ascend Clinical Reference Ranges (July 2018) Chemistry
 24 Hour Urine Creatinine mg/24 hr 800-2000 (Male) Kinetic Alkaline Picrate (Jaffe Reaction) for 24 hour Urine Creatinine Clearence (Creatinine ml/min/1.73m^2 Clearance) 600-1800 (Female) 85-125 (Male)
24 Hour Urine Creatinine mg/24 hr 800-2000 (Male) Kinetic Alkaline Picrate (Jaffe Reaction) for 24 hour Urine Creatinine Clearence (Creatinine ml/min/1.73m^2 Clearance) 600-1800 (Female) 85-125 (Male)
Cisplatin / Paclitaxel Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Docetaxel + Nintedanib
 Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
Module 7 Your Blood Work
 Module 7 Your Blood Work Every month you will need to collect a sample of your blood just before you start dialysis, and depending on your doctor s recommendation, at the end of your dialysis treatment.
Module 7 Your Blood Work Every month you will need to collect a sample of your blood just before you start dialysis, and depending on your doctor s recommendation, at the end of your dialysis treatment.
Cisplatin Doxorubicin Sarcoma
 Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
Gemcitabine, Dexamethasone and Cisplatin GDP Regimen
 Gemcitabine, Dexamethasone and Cisplatin GDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A Derby outreach
Gemcitabine, Dexamethasone and Cisplatin GDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A Derby outreach
