Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials
|
|
|
- Mildred Snow
- 6 years ago
- Views:
Transcription
1 Received: 9 June 2016 Revised: 6 October 2016 Accepted: 17 October 2016 DOI /dom BRIEF REPORT Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials Katherine R. Tuttle MD 1 T. Dwight McKinney MD 2 Jaime A. Davidson MD 3 Greg Anglin PhD 4 Kristine D. Harper MD 5 Fady T. Botros PhD 5 1 Providence Health Care, University of Washington, Spokane, Washington 2 Eli Lilly and Company, Indianapolis, Indiana 3 Touchstone Diabetes Center, University of Texas Southwestern Medical Center, Dallas, Texas 4 Eli Lilly Canada, Toronto, Ontario, Canada 5 Eli Lilly and Company, Indianapolis, Indiana Corresponding Author: Katherine R. Tuttle MD, Providence Health Care, 104 W. 5th Avenue, Suite 350 E, Spokane, WA (katherine.tuttle@providence.org). Funding information This work was sponsored by Eli Lilly and Company. Dulaglutide is a once-weekly glucagon-like peptide-1 receptor agonist approved for the treatment of type 2 diabetes (T2D). Integrated data from 9 phase II and III trials in people with T2D (N = 05) were used to evaluate the effects of dulaglutide on estimated glomerular filtration rate (egfr [Chronic Kidney Disease Epidemiology Collaboration]), urine albumin-to-creatinine ratio (UACR) and kidney adverse events (AEs). No significant differences in egfr were observed during treatment for dulaglutide vs placebo, active comparators or insulin glargine (mean standard deviation values: dulaglutide vs placebo: vs ml/ min/1.73 m 2, P =.075; dulaglutide vs active comparators: vs ml/ min/1.73 m 2, P =.223; and dulaglutide vs insulin glargine: vs ml/ min/1.73 m 2, P =.423). Lower UACR values were observed for dulaglutide vs placebo, active comparators and insulin glargine (at 26 weeks, median [Q1-Q3] values were: dulaglutide vs placebo: 8.0 [ ] vs 8.0 [ ] mg/g, P =.023; dulaglutide vs active comparators: 8.0 [ ] vs 8.9 [ ] mg/g, P =.013; and dulaglutide vs insulin glargine: 8.9 [ ] vs 12.4 [ ] mg/g, P =.029). AEs reflecting potential acute renal failure were 3.4, 1.7 and 7.0 events/0 patient-years for dulaglutide, active comparators and placebo, respectively. In conclusion, dulaglutide treatment of clinical trial participants with T2D did not affect egfr and slightly decreased albuminuria. KEYWORDS clinical trial, diabetes complications, diabetic nephropathy, dulaglutide, GLP-1, type 2 diabetes 1 INTRODUCTION Type 2 diabetes (T2D) is the leading cause of chronic kidney disease (CKD) worldwide. 1,2 Patients with T2D and low kidney function have limited treatment options because medications that are cleared by the kidney (e.g. biguanides and some sulphonylureas) often require dosage adjustments, and many are contraindicated. 1 3 In addition, patients with T2D and CKD are at a higher risk of hypoglycaemia compared with patients with T2D because of decreased gluconeogenesis by the kidney as well as decreased clearance of insulin and of some other diabetes medications. 4 Diabetes medications not cleared by the kidney and associated with a low risk of hypoglycaemia would represent a clinically significant advance for patients with T2D and CKD. Dulaglutide is a once-weekly glucagon-like peptide-1 receptor agonist (GLP-1 RA). In phase II and phase III registration studies, dulaglutide was found to have superior glycaemic efficacy compared with placebo, exenatide, insulin glargine, metformin and sitagliptin. 5 Because of its large molecular size, dulaglutide is not cleared by the kidney, 5 but is presumably catabolized by proteolytic degradation. In a phase I study, no clinically relevant change in the pharmacokinetics of dulaglutide was observed in participants with low kidney function. 5 The primary objective of the present study was to evaluate the effects of dulaglutide on kidney function and safety in patients with T2D. This study presents integrated data from 9 clinical trials (ClinicalTrials.gov): NCT ; NCT ; NCT01104; The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd. wileyonlinelibrary.com/journal/dom Diabetes Obes Metab 2017; 19(3): This is an open access article under the terms of the Creative Commons Attribution NonCommercial NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non commercial and no modifications or adaptations are made.
2 TUTTLE ET AL. 437 NCT ; NCT ; NCT ; NCT ; NCT ; and NCT RESEARCH DESIGN AND METHODS Integrated individual-level data from 6 completed phase II and III registration studies which evaluated dulaglutide doses of 0.75 and 1.5 mg, with treatment duration of at least 26 weeks, were used to evaluate the effect of dulaglutide on kidney function (Table S1, Appendix S1). 5 In 2 studies, AWARD-1 and AWARD-5, after 26 weeks, participants receiving placebo were switched to dulaglutide or sitagliptin, respectively. 5 Data collected after the switch from placebo to active treatment were not included in the analyses of serum creatinine (scr), estimated glomerular filtration rate (egfr) and urine albumin-to-creatinine ratio (UACR). The effects of dulaglutide on scr, egfr and UACR were compared with: (1) placebo at 26 weeks (AWARD-1, AWARD-5, and a phase II ambulatory blood pressure monitoring study) 5 ; (2) all active comparators combined at 26, 52 and up to 104 weeks (AWARD 1-5: exenatide twice daily [1 study], insulin glargine [2 studies], metformin [1 study], and sitagliptin [1 study] 5 ; or (3) insulin glargine at 26 and 52 weeks (AWARD-2 and AWARD-4). 5 Dulaglutide was compared with insulin glargine alone to avoid the confounding effects of similar drug classes in the active comparator group (exenatide in AWARD-1 and sitagliptin in AWARD-5). Measurements of scr, egfr and UACR were performed by a central laboratory. egfr was calculated using scr and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. 6 UACR was based on spot urine samples and was calculated as the ratio of urinary albumin (mg) to urinary creatinine (g). Three additional placebo-controlled phase II studies, 5 which did not evaluate both 0.75 and 1.5 mg dulaglutide doses (and were of short treatment durations), were only included for the purpose of identifying potential acute renal failure adverse events (AEs) throughout the dulaglutide clinical development programme. These studies were not included in the evaluation of kidney function because of the short treatment period and the lack of both dulaglutide doses. Details of the statistical analyses are presented in Appendix S1. 3 RESULTS 3.1 Demographics and baseline characteristics A total of 05 study participants received the study drug in the 9 completed phase II and III registration studies. At baseline, across the 9 studies, 4.4% (n = 265) of participants had persistent egfr < ml/min/1.73 m 2, 3% (n = 181) had persistent macroalbuminuria (defined as UACR >300 mg/g), and 7.1% (n = 425) had egfr < ml/min/1.73 m 2 and/or macroalbuminuria. The 6 studies used to evaluate the effects of dulaglutide on kidney function included a broad range of patients with T2D whose diabetes duration ranged from 2.6 to 12.7 years (Table 1). In the 3 placebo studies, proportions of participants who used antihypertensive medications, angiotensin-converting enzyme (ACE) inhibitors and angiotension receptor blockers (ARBs) were similar in the placebo arm and the dulaglutide arm (placebo 69.9%, 43.5% and 20.6%; dulaglutide 67.5%, 41.8% and 19.5%, respectively; P =.299). Across all 6 studies, 67.6% of participants receiving dulaglutide used antihypertensive medications with 39.4% using ACE inhibitors and 20.5% using ARBs. 3.2 Effects of dulaglutide on scr, egfr and UACR In the 3 placebo-controlled studies, baseline scr, egfr and UACR values for placebo and the all-dulaglutide (0.75 mg dose and 1.5 mg dose groups combined) group were similar (Table S2, Appendix S1). No significant difference was observed in scr or egfr between placebo and the all-dulaglutide group over 26 weeks of treatment (Figure 1A,D). In response to treatment with dulaglutide or placebo up to 26 weeks, UACR decreased slightly in both groups (Figure 1G). The decrease in UACR was slightly greater in the dulaglutide group compared with placebo (median percent change 16.7% vs 10.0%; P =.043 [Table S2, Appendix S1]). In the 5 phase III studies that evaluated dulaglutide vs active comparator, baseline scr and egfr levels were comparable between the all-dulaglutide and active comparator groups (Table S2, Appendix S1). No significant difference was observed in scr or egfr between the 2 groups with treatment up to 104 weeks (Figure 1B,E). Baseline UACR was slightly higher in the active comparator group (Table S2, Appendix S1 and Figure 1H). In response to treatment with dulaglutide or active comparator up to 104 weeks, UACR tended to decrease in both groups (Figure 1H). UACR levels were slightly lower in the dulaglutide group compared with the active comparator group (Figure 1H). At 26 weeks, median percent changes were 20.0% vs 12.5%, respectively (Table S2, Appendix S1). In the 2 phase III studies that evaluated dulaglutide vs insulin glargine, baseline scr and egfr levels were similar in the all-dulaglutide and insulin glargine groups (Table S2, Appendix S1). No significant difference was observed in scr or egfr between the 2 groups with treatment up to 52 weeks (Figure 1C,F). Baseline UACR was slightly higher in the insulin glargine group (Table S2, Appendix S1 and Figure 1I). UACR levels tended to be lower in the dulaglutide group compared with the insulin glargine group (Figure 1I and Table S2, Appendix S1). At 26 weeks, median percent changes in UACR from baseline were 20.0% for dulaglutide vs 9.4% for insulin glargine. At 52 weeks, the median percent decrease in UACR was 16.7% for dulaglutide and 3.7% for insulin glargine (Table S2, Appendix S1). 3.3 Proportions of participants with 30% or 40% decline in egfr The number of participants who experienced a 30% or 40% decline in egfr over time was not significantly different for dulaglutidetreated participants compared with placebo and all active comparators combined (Table S3, Appendix S1); however, in comparison with insulin glargine, there were significantly fewer dulaglutide-treated participants who experienced a 40% decline in egfr at any point
3 438 TUTTLE ET AL. TABLE 1 Summary of baseline demographics and renal characteristics in clinical trials of duration 26 weeks AWARD-1 AWARD-2 AWARD-3 AWARD-4 AWARD-5 ABPM study Variable N = 976 N = 7 N = 7 N = 884 N = 1202 N = 755 Sex: female, n (%) 406 (41.6) 393 (48.7) 454 (56.3) 411 (46.5) 643 (53.5) 363 (48.1) Mean (s.d.) age, years 55.7 (9.8) 56.7 (9.5) 55.6 (10.4) 59.4 (9.2) 54.1 (9.9) 56.5 (10.3) Age <65 years, n (%) 820 (84.0) 646 (.0) 664 (82.3) 641 (72.5) 1048 (87.2) 8 (.5) Ethnicity, n (%) Not Hispanic or Latino 644 (66.0) 516 (63.9) 535 (66.3) 581 (65.7) 950 (79.0) 468 (62.0) Hispanic or Latino 331 (33.9) 291 (36.1) 272 (33.7) 303 (34.3) 251 (20.9) 287 (38.0) Unknown 1 (.1) 0 (0) 0 (0) 0 (0) 1 (<.1) 0 (0) Race, n (%) White 726 (74.4) 570 (70.6) 0 (74.3) 697 (78.8) 613 (51.0) 8 (.5) Asian 24 (2.5) 137 (17.0) 61 (7.6) 35 (4.0) 285 (23.7) 69 (9.1) American-Indian or Alaska Native 135 (13.8) 89 (11.0) 85 (10.5) 46 (5.2) 1 (<.1) 2 (.3) African-American 76 (7.8) 4 (.5) 53 (6.6) 85 (9.6) 50 (4.2) 66 (8.7) Multiple or unknown 1 12 (1.2) 7 (.9) 7 (.9) 20 (2.3) 252 (21.0) 9 (1.2) Native Hawaiian or Other Pacific Islander 3 (.3) 0 (0) 1 (.1) 1 (.1) 1 (<.1) 1 (.1) Mean (s.d.) BMI, kg/m (5.4) 31.6 (5.5) 33.3 (5.5) 32.5 (5.2) 31.3 (4.4) 33.0 (6.0) Mean (s.d.) duration of diabetes, years 8.8 (5.6) 9.1 (6.0) 2.6 (1.8) 12.7 (7.0) 7.1 (5.1) 8.3 (5.9) Mean (s.d.) HbA1c, % 8.1 (1.3) 8.1 (1.0) 7.6 (0.9) 8.5 (1.0) 8.1 (1.1) 7.9 (0.8) UACR > 300 mg/g and/or egfr < ml/min/1.73 m 2,n 50 (5.1) 37 (4.6) 50 (6.2) 132 (14.9) 53 (4.4) 69 (9.1) (%) 2,3 Macroalbuminuria (UACR > 300 mg/g), n (%) 4 23 (2.4) 28 (3.5) 20 (2.5) 52 (5.9) 30 (2.5) 24 (3.2) egfr < ml/min/1.73 m 2, n (%) 2,5 27 (2.8) 9 (1.1) 30 (3.7) 93 (10.5) 25 (2.1) 51 (6.8) Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; s.d., standard deviation. 1 Multiple or Unknown includes patients self-declared as Hispanic race in AWARD-5. 2 egfr was calculated based on the CKD-EPI equation 6 and serum creatinine value, using the highest measured value of egfr (CKD-EPI). 3 Patients were included if they met criteria for either the UACR > 300 mg/g or egfr (CKD-EPI) < ml/min/1.73 m 2 group as shown in Footnotes 4 and 5 below. 4 Patients were included if UACR >300 mg/g at all measured timepoints during baseline. 5 Patients were included if egfr (CKD-EPI) < ml/min/1.73 m 2 at all measured timepoints during baseline. during a 1-year treatment period (0.26% vs 1.25%; P =.012 [Table S3, Appendix S1]). 3.4 AEs reflecting potential acute renal failure In the 9 completed phase II and III studies, 4006 received dulaglutide (3531 patient-years), 703 received placebo (284 patient-years) and 1541 received active comparator (1722 patient-years). Some study participants received placebo followed by dulaglutide (n = 121) or sitagliptin (n = 124) and are included in the totals for each drug. 5 AEs reflecting potential acute renal failure (Table S4, Appendix S1) were reported at rates of 3.4 (n = 12), 1.7 (n = 3) and 7.0 (n = 2) events per 0 patient-years of exposure for dulaglutide, active comparators and placebo, respectively. Details regarding the 12 dulaglutide participants who reported an AE reflecting potential acute renal failure are included in Appendix S1. 4 DISCUSSION Dulaglutide treatment in clinical trials for T2D did not affect egfr, was associated with a slight decrease in albuminuria, and was not associated with an increase in AEs reflecting potential acute renal failure. Several studies have also shown that treatment of patients with T2D with other GLP-1 RAs did not alter scr or egfr 7 9 ; however, post-marketing cases of pre-renal acute decreases in kidney function have been reported with some GLP-1 RAs This has been attributed to volume depletion that may occur in patients who experience excessive nausea, diarrhoea and vomiting In patients with low kidney function, treatment with GLP-1 RAs that are cleared by the kidney may increase gastrointestinal AEs 13 because of increased exposure to the medication. Consequently, the renally cleared GLP-1 RAs are not recommended to be used in patients with severely reduced kidney function Elevated rates of gastrointestinal AEs in patients with low kidney function have also been reported with other GLP-1 RAs that are not cleared by the kidney; 7,17 therefore, it is recommended to use these medications with caution when initiating or escalating doses in patients with renal impairment. 17,18 Dulaglutide exposure is not altered in patients with various degrees of reduced kidney function. 5 Although long-term treatment with GLP-1 RAs did not alter egfr in patients with T2D, 7 9 controversy exists regarding acute effects of GLP-1 RAs on renal haemodynamics The present data show that dulaglutide
4 TUTTLE ET AL. 439 (A) Placebo (B) Active Comparators (C) All Dulaglutide All Dulaglutide Insulin Glargine All Dulaglutide scr (µmol/l) p=0.096 p=0.133 p=0.561 p=0.542 p=0.413 p=0.428 Baseline 26 Weeks Baseline 26 Weeks 52 Weeks Weeks Baseline 26 Weeks 52 Weeks PL= AC= IG= DU= DU= DU= (D) (E) (F) egfr (ml/min/1.73 m 2 ) Baseline PL= 568 DU= 1669 p= Weeks Baseline AC= 1417 DU= 2836 p= Weeks p= Weeks p= Weeks (G) (H) (I) p=0.587 p=0.423 Baseline 26 Weeks 52 Weeks IG= DU= UACR (mg/g) p= p=0.013 p=0.035 p= p=0.029 p= Baseline PL= 557 DU= Weeks Baseline 26 Weeks 52 Weeks Weeks Baseline 26 Weeks AC= IG= DU= DU= Weeks P<0.05 indicates a significant difference between dulaglutide compared with placebo, active comparators or insulin glargine at the corresponding time point. FIGURE 1 scr, egfr and UACR values for dulaglutide compared with placebo, all active comparators combined and insulin glargine. Plotted values are mean (standard deviation) for scr and egfr and median and interquartile range (Q1-Q3) for UACR. All dulaglutide = 0.75 and 1.5 mg groups combined; active comparators = sitagliptin, exenatide (twice daily), insulin glargine and metformin groups combined. P values for difference in least squares means at specified time points from a repeated measures model of logarithm of the response variable, with terms adjusting for study, treatment, study by treatment, time, time by treatment and log-scale baseline values of renal analytes and HbA1c. Numbers presented below the graphs are the number of patients included in the analysis at each time point. AC, active comparators; DU, dulaglutide; IG, insulin glargine; PL, placebo. Grey circles = dulaglutide, black diamonds = placebo, active comparators, or insulin glargine. A, scr for dulaglutide compared with placebo. B, scr for dulaglutide compared with active comparators. C, scr for dulaglutide compared with insulin glargine. D, egfr for dulaglutide compared with placebo. E, egfr for dulaglutide compared with active comparators. F, egfr for dulaglutide compared with insulin glargine. G, UACR values for dulaglutide compared with placebo. H, UACR values for dulaglutide compared with active comparators. I, UACR values for dulaglutide compared with insulin glargine. P <.05 indicates a significant difference between dulaglutide compared with placebo, active comparators or insulin glargine at the corresponding time point. treatment of study participants with T2D did not alter egfr compared with placebo, all active comparators combined, or insulin glargine. A small decline in egfr of 1 to 3 ml/min/1.73 m 2 was observed in all treatment groups (treatment duration weeks), an expected finding based on normal ageing. 22 egfr typically declines even more, ~2 to 4 ml/min/y, in patients with T2D and diabetic kidney disease In addition, proportions of patients experiencing a decline in egfr by 30% or 40% were generally similar for dulaglutide and placebo, active comparators or insulin glargine, except that proportion of patients experiencing a decline in egfr by 40% was significantly lower with dulaglutide compared with insulin glargine. In the dulaglutide studies, there were very few patient reports of AEs potentially reflecting acute renal failure and the rate of patients in the dulaglutide groups who experienced these events was similar to the placebo and active comparator groups, taking into account the duration of treatment exposure. Only 2 events indicated typical acute renal failure; 1 attributable to sepsis and 1 to dehydration (Appendix S1). These AE data, together with the egfr data, indicate that dulaglutide is not associated with an increased risk of acute renal failure. A small decrease in UACR was observed in the dulaglutide group compared with placebo, all active comparators combined and insulin glargine, but the observed decrease was not of immediate clinical significance. Nevertheless, the dulaglutide albuminuria-lowering effect, similar to that of other GLP-1 RAs, 25,26 suggests that treatment with dulaglutide could exert long-term renoprotective effects. Preclinical and early clinical data show that GLP-1 receptors are expressed in the kidney and provide rationale that GLP-1 RAs may exert
5 440 TUTTLE ET AL. renoprotective effects by decreasing albuminuria through GLP-1- mediated anti-inflammatory effects, amelioration of oxidative stress and vascular endothelium protection in the kidney In addition, GLP-1 is also involved in sodium and water homeostasis via stimulation of natriuresis and diuresis, attributed to inhibition of sodium-hydrogen exchange in the proximal tubule. 36,37 Recent cardiovascular outcomes studies showed a lower risk of new-onset persistent macroalbuminuria with GLP-1 RAs liraglutide and semaglutide. 38,39 To determine the clinical significance of this albuminuria-lowering signal, the effects of dulaglutide should be further evaluated in patients with elevated UACR at baseline. Limitations of the present study include the inclusion of a small number of participants with low egfr or high levels of albuminuria. In addition, the studies included in the present analyses were not prospectively designed to evaluate the effects of dulaglutide on kidney function. The study s strengths include the availability of individuallevel data at common timepoints from a large number of participants with T2D, relatively long treatment periods, and the availability of both placebo and active comparator data. In conclusion, dulaglutide treatment of clinical trial participants with T2D did not affect egfr and slightly decreased albuminuria; however, because of the limited clinical experience in patients with severely reduced kidney function or end-stage renal disease, the US label indicates that kidney function should be monitored in dulaglutide-treated patients with renal impairment who experience severe gastrointestinal side effects, 40 and the European Union label indicates that dulaglutide is not recommended in patients with severely reduced kidney function. 41 Effects of dulaglutide treatment in patients with T2D and moderate or severe CKD are being prospectively evaluated in the currently ongoing phase III AWARD-7 study (clinicaltrials.gov NCT ). ACKNOWLEDGMENTS The authors wish to thank Marie Geissler from INC Research for editorial assistance. Prior Presentation: portions of these data were presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, June 5-9, 2015 and at the 51st Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, September 14-18, Conflict of interest K. D. H., G. A. and F. T. B. are employees of Eli Lilly and Company. J. A. D. is a consultant for Amgen, Aspire Bariatrics, Astra Zeneca, Eli Lilly, Glaxo Smith Kline, Janssen, Merck Sharp and Dohme, Novo Nordisk, REMD Biotherapeutics and Sanofi and is a participant on a Speaker s Bureau for Astra Zeneca, Janssen, Novo Nordisk and Takeda. K. R. T. is a consultant for Eli Lilly, Amgen, Noxxon. T. D. M. is a retired distinguished medical fellow from Eli Lilly and Company. No other potential conflicts of interest relevant to this article are reported. Author contributions K. R. T, T. D. M. and J. A. D. were involved in the interpretation of the data and critically revised the manuscript for important intellectual content and scientific significance. K. R. T also led the development of the revised and final versions of the manuscript. G. A. was involved in the statistical analysis and interpretation of the data and critically revised the manuscript for important intellectual content. K. D. H. was involved in the interpretation of the data and critically revised the manuscript for important intellectual content. F. T. B. was involved in the analysis and interpretation of the data, critically revised the manuscript for important intellectual content and scientific significance, and assisted with drafting and supervising the development of the manuscript. F. T. B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. REFERENCES 1. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD. Am J Kidney Dis. 2007;49(2)(suppl 2):S12-S National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;(5): Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4): Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6): Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev Apr 21;32(8): doi: 10.2/dmrr Review. PMID: [Epub ahead of print]. 6. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9): Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract. 2011;17(3): Tuttle KR, Heilmann C, Hoogwerf BJ, Brown C, Anderson PW. Effects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetes. Am J Kidney Dis. 2013;62(2): Pawaskar M, Tuttle KR, Li Q, Best JH, Anderson PW. Observational study of kidney function and albuminuria in patients with type 2 diabetes treated with exenatide BID versus insulin glargine. Ann Pharmacother. 2014;48(5): López-Ruiz A, del Peso-Gilsanz C, Meoro-Avilés A, et al. Acute renal failure when exenatide is co-administered with diuretics and angiotensin II blockers. Pharm World Sci. 2010;32(5): Weise WJ, Sivanandy MS, Block CA, Comi RJ. Exenatide-associated ischemic renal failure. Diabetes Care. 2009;32(2):e22-e Kaakeh Y, Kanjee S, Boone K, Sutton J. Liraglutide-induced acute kidney injury. Pharmacotherapy. 2012;32(1):e7-e Linnebjerg H, Kothare PA, Park S, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64(3): Byetta [prescribing information]. Wilmington, DE: AstraZeneca; Bydureon [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; Lyxumia [summary of product characteristics]. Paris, France: Sanofi- Aventis Group; 2013.
6 TUTTLE ET AL Tanzeum [prescribing information]. Wilmington, DE: GlaxoSmithKline LLC; Victoza [prescribing information]. Princeton, NJ: Novo Nordisk Inc.; Von Scholten BJ, Hansen TW, Goetze JP, Persson F, Rossing P. Glucagon-like peptide 1 receptor agonist (GLP-1 RA): long-term effect on kidney function in patients with type 2 diabetes. J Diabetes Complications. 2015;29(5): Muskiet MH, Tonneijck L, Smits MM, et al. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab. 2016;18(2): Tonneijck L, Smits MM, Muskiet MH, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59(7): Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59(2): Hoefield RA, Kalra PA, Baker PG, et al. The use of egfr and ACR to predict decline in renal function in people with diabetes. Nephrol Dial Transplant. 2011;26(3): Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD). Nephrol Dial Transplant. 2012;27(5): Hirai A, Imamura S, Hirai K. A novel therapeutic use of glucagon-like peptide1 receptor agonist for the treatment of overt diabetic nephropathy in patients with type 2 diabetes. (Abstract). Diabetes. 2012;61- (suppl 1):A240, A 943-P. 26. Mohan V, Kannan A, Jebarani S. A retrospective analysis of the effect of liraglutide in Asian Indian patients with type 2 diabetes with proteinuria. (Abstract). Diabetes. 2012;61(suppl 1):A569, A-2249-PO. 27. Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85(3): Imamura S, Hirai K, Hirai A. The glucagon-like peptide-1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patients. Tohoku J Exp Med. 2013;231(1): Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagonlike peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18(4): Tanaka T, Higashijima Y, Wada T, Nangaku M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014;86(4): Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol. 2002;434(3): Gutzwiller JP, Hruz P, Huber AR, et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion. 2006;73: Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89(6): Skov J, Dejgaard A, Frøkiær J, et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab. 2013;98(4):E664-E Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38(1): Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept. 2007;141(1-3): Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;297(6):F1647-F Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375: Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med Sep 15. PMID: [Epub ahead of print]. 40. Trulicity [prescribing information]. Indianapolis, IN: Lilly USA, LLC; Trulicity [summary of product characteristics]. Houten, The Netherlands: Eli Lilly and Company; SUPPORTING INFORMATION Additional Supporting Information may be found online in the supporting information tab for this article. How to cite this article: Tuttle KR, McKinney TD, Davidson JA, Anglin G, Harper KD and Botros FT. Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials, Diabetes Obes Metab, 2017;19(3):
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol
 Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Glucagon-like peptide-1 (GLP-1) Agonists Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.18 Line of Business: Health Insurance Marketplace See Important
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.18 Line of Business: Health Insurance Marketplace See Important
The first stop for professional medicines advice
 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1 receptor analogues The first stop for professional medicines advice 1 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1
London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1 receptor analogues The first stop for professional medicines advice 1 London Medicines Evaluation Network Overview: Glucagon-Like Peptide-1
Safety profile of Liraglutide: Recent Updates. Mohammadreza Rostamzadeh,M.D.
 Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO
 The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO DEPARTMENT OF EMERGENCY AND ORGAN TRANSPLANTATION SECTION OF INTERNAL MEDICINE, ENDOCRINOLOGY, ANDROLOGY AND METABOLIC DISEASES Disclaimer
The Highlights of the AWARD Clinical Program FRANCESCO GIORGINO DEPARTMENT OF EMERGENCY AND ORGAN TRANSPLANTATION SECTION OF INTERNAL MEDICINE, ENDOCRINOLOGY, ANDROLOGY AND METABOLIC DISEASES Disclaimer
Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications
 Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications Nathan Woolever, Pharm.D., Resident Pharmacist Pharmacy Grand Rounds November 6 th, 2018 Franciscan Healthcare La Crosse, WI 2017
Cardiovascular Benefits of Two Classes of Antihyperglycemic Medications Nathan Woolever, Pharm.D., Resident Pharmacist Pharmacy Grand Rounds November 6 th, 2018 Franciscan Healthcare La Crosse, WI 2017
Hot Topics in Diabetic Kidney Disease a primary care perspective
 Hot Topics in Diabetic Kidney Disease a primary care perspective DR SARAH DAVIES GP PARTNER WITH SPECIAL INTEREST IN DIABETES, CARDIFF DUK CLINICAL CHAMPION NB MEDICAL HOT TOPICS PRESENTER AND DIABETES
Hot Topics in Diabetic Kidney Disease a primary care perspective DR SARAH DAVIES GP PARTNER WITH SPECIAL INTEREST IN DIABETES, CARDIFF DUK CLINICAL CHAMPION NB MEDICAL HOT TOPICS PRESENTER AND DIABETES
SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection
 SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection Hiddo Lambers Heerspink Department of Clinical Pharmacy and Pharmacology University Medical Center
SGLT2 inhibition in diabetes: extending from glycaemic control to renal and cardiovascular protection Hiddo Lambers Heerspink Department of Clinical Pharmacy and Pharmacology University Medical Center
STEP THERAPY CRITERIA
 CATEGORY DRUG CLASS BRAND NAME (generic) STEP THERAPY CRITERIA AMYLIN ANALOG: SYMLIN/SYMLINPEN (pramlintide acetate) ANTIDIABETIC AGENTS GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST (GLP-1): ADLYXIN (lixisenatide)
CATEGORY DRUG CLASS BRAND NAME (generic) STEP THERAPY CRITERIA AMYLIN ANALOG: SYMLIN/SYMLINPEN (pramlintide acetate) ANTIDIABETIC AGENTS GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST (GLP-1): ADLYXIN (lixisenatide)
Achieving and maintaining good glycemic control is an
 Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists Yehuda Handelsman, MD, FACP, FNLA, FASPC, MACE; Kathleen Wyne, MD, PhD, FACE, FNLA; Anthony Cannon,
Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists Yehuda Handelsman, MD, FACP, FNLA, FASPC, MACE; Kathleen Wyne, MD, PhD, FACE, FNLA; Anthony Cannon,
Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors. Bryce Fukunaga PharmD April 25, 2018
 Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors Bryce Fukunaga PharmD April 25, 2018 Objectives For each drug class: Identify the overall place in therapy Explain the mechanism of action
Side Effects of: GLP-1 agonists DPP-4 inhibitors SGLT-2 inhibitors Bryce Fukunaga PharmD April 25, 2018 Objectives For each drug class: Identify the overall place in therapy Explain the mechanism of action
Early treatment for patients with Type 2 Diabetes
 Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Lilly Diabetes: Pipeline Update
 Lilly Diabetes: Pipeline Update June 16, 2014 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's current expectations, but actual results may differ
Lilly Diabetes: Pipeline Update June 16, 2014 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's current expectations, but actual results may differ
This program applies to Commercial, GenPlus and Health Insurance Marketplace formularies.
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists
 Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: LA.PST.14 Effective Date: 03.18 Last Review Date: 3.18 Line of Business: Medicaid Revision Log 1Revision Log 1Revision
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: LA.PST.14 Effective Date: 03.18 Last Review Date: 3.18 Line of Business: Medicaid Revision Log 1Revision Log 1Revision
Let s not sugarcoat it! Update on Pharmacologic Management of Type II DM
 Let s not sugarcoat it! Update on Pharmacologic Management of Type II DM Gregory Castelli, PharmD, BCPS, BC-ADM Clinical Pharmacist UPMC St. Margaret Objectives By the end of this presentation, participants
Let s not sugarcoat it! Update on Pharmacologic Management of Type II DM Gregory Castelli, PharmD, BCPS, BC-ADM Clinical Pharmacist UPMC St. Margaret Objectives By the end of this presentation, participants
GLP-1 (glucagon-like peptide-1) Agonists (Byetta, Bydureon, Tanzeum, Trulicity, Victoza ) Step Therapy and Quantity Limit Criteria Program Summary
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My!
 DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My! Kevin M. Pantalone, DO, ECNU, CCD Associate Staff Director of Clinical Research Department of Endocrinology Endocrinology and
DM-2 Therapy Update: GLP-1, SGLT-2 Inhibitors, and Inhaled Insulin, Oh My! Kevin M. Pantalone, DO, ECNU, CCD Associate Staff Director of Clinical Research Department of Endocrinology Endocrinology and
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: Last Review Date: 02.
 Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: HIM Revision Log See Important Reminder
Clinical Policy: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Reference Number: HIM.PA.53 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: HIM Revision Log See Important Reminder
Variability in drug response: towards more personalized diabetes care Petrykiv, Sergei
 University of Groningen Variability in drug response: towards more personalized diabetes care Petrykiv, Sergei IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
University of Groningen Variability in drug response: towards more personalized diabetes care Petrykiv, Sergei IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you
Very Practical Tips for Managing Type 2 Diabetes
 Very Practical Tips for Managing Type 2 Diabetes Jean-François Yale, MD, FRCPC McGill University Health Centre, Montreal, Canada Jean-francois.yale@mcgill.ca www.dryale.ca OBJECTIVES DISCLOSURES The participant
Very Practical Tips for Managing Type 2 Diabetes Jean-François Yale, MD, FRCPC McGill University Health Centre, Montreal, Canada Jean-francois.yale@mcgill.ca www.dryale.ca OBJECTIVES DISCLOSURES The participant
Update on Therapies for Type 2 Diabetes: Angela D. Mazza, DO July 31, 2015
 Update on Therapies for Type 2 Diabetes: 2015 Angela D. Mazza, DO July 31, 2015 Objectives To present the newer available therapies for the management of T2D To discuss the advantages and disadvantages
Update on Therapies for Type 2 Diabetes: 2015 Angela D. Mazza, DO July 31, 2015 Objectives To present the newer available therapies for the management of T2D To discuss the advantages and disadvantages
Incredible Incretins Abby Frye, PharmD, BCACP
 Incredible Incretins Abby Frye, PharmD, BCACP Objectives & Disclosures Review the pathophysiology of T2DM and the impact of the incretin system Describe the defining characteristics of the available glucagonlike
Incredible Incretins Abby Frye, PharmD, BCACP Objectives & Disclosures Review the pathophysiology of T2DM and the impact of the incretin system Describe the defining characteristics of the available glucagonlike
Soo LIM, MD, PHD Internal Medicine Seoul National University Bundang Hospital
 Soo LIM, MD, PHD Internal Medicine Seoul National University Bundang Hospital Agenda Association between Cardiovascular Disease and Type 2 Diabetes Importance of HbA1c Management esp. High risk patients
Soo LIM, MD, PHD Internal Medicine Seoul National University Bundang Hospital Agenda Association between Cardiovascular Disease and Type 2 Diabetes Importance of HbA1c Management esp. High risk patients
Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists
 Texas Vendor Drug Program Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists Publication History Developed February 2006. Revised September 2018; September 2016; June 2015; October 2013; December
Texas Vendor Drug Program Drug Use Criteria: Glucagon-Like Peptide 1 Receptor Agonists Publication History Developed February 2006. Revised September 2018; September 2016; June 2015; October 2013; December
HEART FAILURE AND DIABETES MELLITUS: DANGEROUS LIASONS MICHEL KOMAJDA, MD
 HEART FAILURE AND DIABETES MELLITUS: DANGEROUS LIASONS MICHEL KOMAJDA, MD Author affiliations: Department of Cardiology, Hôpital Saint Joseph, Paris, France Address for correspondence: Michel Komajda,
HEART FAILURE AND DIABETES MELLITUS: DANGEROUS LIASONS MICHEL KOMAJDA, MD Author affiliations: Department of Cardiology, Hôpital Saint Joseph, Paris, France Address for correspondence: Michel Komajda,
PROTEZIONE DAL DANNO RENALE NEL DIABETE TIPO 2: RUOLO DEI NUOVI FARMACI. Massimo Boemi UOC Malattie Metaboliche e Diabetologia IRCCS INRCA Ancona
 PROTEZIONE DAL DANNO RENALE NEL DIABETE TIPO 2: RUOLO DEI NUOVI FARMACI Massimo Boemi UOC Malattie Metaboliche e Diabetologia IRCCS INRCA Ancona Disclosure Dr Massimo Boemi has been granted as speaker
PROTEZIONE DAL DANNO RENALE NEL DIABETE TIPO 2: RUOLO DEI NUOVI FARMACI Massimo Boemi UOC Malattie Metaboliche e Diabetologia IRCCS INRCA Ancona Disclosure Dr Massimo Boemi has been granted as speaker
Renal safety of newer medications
 Amar Puttanna MBChB, MRCP, Specialist Registrar, Department of Diabetes and Endocrinology, City Hospital, Birmingham, UK Lakshminarayanan Varadhan MBBS, MRCP (UK), MRCP Diabetes and Endocrinology, DipMedEd
Amar Puttanna MBChB, MRCP, Specialist Registrar, Department of Diabetes and Endocrinology, City Hospital, Birmingham, UK Lakshminarayanan Varadhan MBBS, MRCP (UK), MRCP Diabetes and Endocrinology, DipMedEd
Dulaglutide: designed with patients in mind
 31 st Panhellenic Annual Congress of the Hellenic Association for the Study & Education of Diabetes Mellitus Thessaloniki 11 th, November 2017 Dulaglutide: designed with patients in mind Imre Pavo MD PhD
31 st Panhellenic Annual Congress of the Hellenic Association for the Study & Education of Diabetes Mellitus Thessaloniki 11 th, November 2017 Dulaglutide: designed with patients in mind Imre Pavo MD PhD
Primary Care Approach to Management of CKD
 Primary Care Approach to Management of CKD This PowerPoint was developed through a collaboration between the National Kidney Foundation and ASCP. Copyright 2018 National Kidney Foundation and ASCP Low
Primary Care Approach to Management of CKD This PowerPoint was developed through a collaboration between the National Kidney Foundation and ASCP. Copyright 2018 National Kidney Foundation and ASCP Low
New Drug Evaluation: Dulaglutide
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes
 Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Exploring Non-Insulin Therapies in Type 1 Diabetes
 Exploring Non-Insulin Therapies in Type 1 Diabetes Susan Cornell, BS, PharmD, CDE, FAPhA, FAADE Associate Professor Midwestern University - Chicago College of Pharmacy Disclosures Dr. Cornell: Advanced
Exploring Non-Insulin Therapies in Type 1 Diabetes Susan Cornell, BS, PharmD, CDE, FAPhA, FAADE Associate Professor Midwestern University - Chicago College of Pharmacy Disclosures Dr. Cornell: Advanced
LVHN Scholarly Works. Lehigh Valley Health Network. Nelson Kopyt DO, FASN, FACP Lehigh Valley Health Network,
 Lehigh Valley Health Network LVHN Scholarly Works Department of Medicine Efficacy and Safety of Oral Ferric Maltol (FM) in Treating Iron-Deficiency Anemia (IDA) in Patients with Chronic Kidney Disease
Lehigh Valley Health Network LVHN Scholarly Works Department of Medicine Efficacy and Safety of Oral Ferric Maltol (FM) in Treating Iron-Deficiency Anemia (IDA) in Patients with Chronic Kidney Disease
Help the Heart. An Update on GLP-1 Agonists and SGLT2 Inhibitors. Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire
 Help the Heart An Update on GLP-1 Agonists and SGLT2 Inhibitors Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire Mayo Clinic Grand Rounds May 16, 2017 2017 MFMER slide-1
Help the Heart An Update on GLP-1 Agonists and SGLT2 Inhibitors Tara Hawley, PharmD PGY1 Pharmacy Resident Mayo Clinic Health System Eau Claire Mayo Clinic Grand Rounds May 16, 2017 2017 MFMER slide-1
Have you seen a patient like Elaine *?
 (linagliptin) 5mg tablets Have you seen a patient like Elaine *? *Hypothetical patient profile Elaine * : 60 years old Housewife *Hypothetical patient profile ELAINE*: T2D Patient with early signs of kidney
(linagliptin) 5mg tablets Have you seen a patient like Elaine *? *Hypothetical patient profile Elaine * : 60 years old Housewife *Hypothetical patient profile ELAINE*: T2D Patient with early signs of kidney
Horizon Scanning Technology Summary. Liraglutide for type 2 diabetes. National Horizon Scanning Centre. April 2007
 Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Managing patients with renal disease
 Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Insulin Initiation and Intensification. Disclosure. Objectives
 Insulin Initiation and Intensification Neil Skolnik, M.D. Associate Director Family Medicine Residency Program Abington Memorial Hospital Professor of Family and Community Medicine Temple University School
Insulin Initiation and Intensification Neil Skolnik, M.D. Associate Director Family Medicine Residency Program Abington Memorial Hospital Professor of Family and Community Medicine Temple University School
dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd.
 dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd. 04 December 2015 The Scottish Medicines Consortium (SMC) has completed its
dulaglutide 0.75mg and 1.5mg solution for injection in pre-filled pen (Trulicity ) SMC No. (1110/15) Eli Lilly and Company Ltd. 04 December 2015 The Scottish Medicines Consortium (SMC) has completed its
Higher levels of Urinary Albumin Excretion within the Normal Range Predict Faster Decline in Glomerular Filtration Rate in Diabetic Patients
 Diabetes Care Publish Ahead of Print, published online May 12, 2009 Albuminuria and GFR Decline in Diabetes Higher levels of Urinary Albumin Excretion within the Normal Range Predict Faster Decline in
Diabetes Care Publish Ahead of Print, published online May 12, 2009 Albuminuria and GFR Decline in Diabetes Higher levels of Urinary Albumin Excretion within the Normal Range Predict Faster Decline in
exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited
 exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
exenatide 2mg powder and solvent for prolonged-release suspension for injection (Bydureon ) SMC No. (748/11) Eli Lilly and Company Limited 09 December 2011 The Scottish Medicines Consortium (SMC) has completed
Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists
 Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists Robert R. Henry, MD Professor of Medicine University of California, San Diego Relevant Conflict
Can We Reduce Heart Failure by Treating Diabetes? CVOT Data on SGLT2 Inhibitors and GLP-1Receptor Agonists Robert R. Henry, MD Professor of Medicine University of California, San Diego Relevant Conflict
GLP-1 Agonists for Diabetes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
FARXIGA (dapagliflozin) Jardiance (empagliflozin) tablets. Synjardy (empagliflozin and metformin hydrochloride) tablets. GLUCOPHAGE* (metformin)
 Type 2 Medications Drug Class How It Works Brand and Generic Names Manufacturers Usual Starting Dose The kidneys filter sugar and either absorb it back into your body for energy or remove it through your
Type 2 Medications Drug Class How It Works Brand and Generic Names Manufacturers Usual Starting Dose The kidneys filter sugar and either absorb it back into your body for energy or remove it through your
Beyond A1C. Non-glycemic Effects of GLP-1 Receptor Agonists. Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows
 Beyond A1C Non-glycemic Effects of GLP-1 Receptor Agonists Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows Disclosures No conflicts of interest. Learning Objectives 1. Understand the physiological
Beyond A1C Non-glycemic Effects of GLP-1 Receptor Agonists Olga Astapova MD, PhD Luis Chavez MD URMC Endocrinology Fellows Disclosures No conflicts of interest. Learning Objectives 1. Understand the physiological
Exploring Non-Insulin Therapies in Type 1 Diabetes. Objectives. Pre-Assessment Question #1. Disclosures
 Exploring Non-Insulin Therapies in Type 1 Diabetes Disclosures Dr. Cornell: Advanced Practitioner Advisory Board and Speakers Bureau: Novo Nordisk Susan Cornell, BS, PharmD, CDE, FAPhA, FAADE Associate
Exploring Non-Insulin Therapies in Type 1 Diabetes Disclosures Dr. Cornell: Advanced Practitioner Advisory Board and Speakers Bureau: Novo Nordisk Susan Cornell, BS, PharmD, CDE, FAPhA, FAADE Associate
Chief of Endocrinology East Orange General Hospital
 Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
GLP-1 agonists. Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK
 GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
GLP-1 agonists Ian Gallen Consultant Community Diabetologist Royal Berkshire Hospital Reading UK What do GLP-1 agonists do? Physiology of postprandial glucose regulation Meal ❶ ❷ Insulin Rising plasma
GLP-1. GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4.
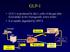 GLP-1 GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4 Food intake éinsulin Gut églucose uptake Pancreas Beta cells Alpha cells
GLP-1 GLP-1 is produced by the L-cells of the gut after food intake in two biologically active forms It is rapidly degraded by DPP-4 Food intake éinsulin Gut églucose uptake Pancreas Beta cells Alpha cells
GLP-1 Receptor Agonists and SGLT-2 Inhibitors. Debbie Hicks
 GLP-1 Receptor Agonists and SGLT-2 Inhibitors Debbie Hicks Prescribing and Adverse Event reporting information is available at this meeting from the AstraZeneca representative The views expressed by the
GLP-1 Receptor Agonists and SGLT-2 Inhibitors Debbie Hicks Prescribing and Adverse Event reporting information is available at this meeting from the AstraZeneca representative The views expressed by the
Diabetes and Hypertension
 Diabetes and Hypertension M.Nakhjvani,M.D Tehran University of Medical Sciences 20-8-96 Hypertension Common DM comorbidity Prevalence depends on diabetes type, age, BMI, ethnicity Major risk factor for
Diabetes and Hypertension M.Nakhjvani,M.D Tehran University of Medical Sciences 20-8-96 Hypertension Common DM comorbidity Prevalence depends on diabetes type, age, BMI, ethnicity Major risk factor for
GLP-1 RECEPTOR AGONIST SHOULD I TRY IT? VERONICA BRADY, PHD, BC-ADM, CDE PROJECT ECHO JUNE 21, 2018
 GLP-1 RECEPTOR AGONIST SHOULD I TRY IT? VERONICA BRADY, PHD, BC-ADM, CDE PROJECT ECHO JUNE 21, 2018 SOMETHING TO CONSIDER IF YOU COULD PRESCRIBE A MEDICATION FOR YOUR PATIENT WITH DIABETES THAT: DECREASED
GLP-1 RECEPTOR AGONIST SHOULD I TRY IT? VERONICA BRADY, PHD, BC-ADM, CDE PROJECT ECHO JUNE 21, 2018 SOMETHING TO CONSIDER IF YOU COULD PRESCRIBE A MEDICATION FOR YOUR PATIENT WITH DIABETES THAT: DECREASED
Scottish Medicines Consortium
 Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Terapia con agonisti GLP1 e outcome cardiovascolare. Edoardo Mannucci
 Terapia con agonisti GLP e outcome cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca,
Terapia con agonisti GLP e outcome cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca,
Clinical Relevance of Blood Pressure Lowering Effect of Modern Antidiabetic Drugs
 Clinical Relevance of Blood Pressure Lowering Effect of Modern Antidiabetic Drugs Professor Guntram Schernthaner Medical University of Vienna, Austria guntram.schernthaner@meduniwien.ac.at Agenda Glucose
Clinical Relevance of Blood Pressure Lowering Effect of Modern Antidiabetic Drugs Professor Guntram Schernthaner Medical University of Vienna, Austria guntram.schernthaner@meduniwien.ac.at Agenda Glucose
Diabetic Nephropathy
 Diabetic Nephropathy Larry Lehrner, Ph.D.,M.D. llehrner@ksosn.com Commercial Support Acknowledgement: There is no outside support for this activity Financial Disclosure: stocks > 50,000 Bayer, J&J, Norvartis,Novo
Diabetic Nephropathy Larry Lehrner, Ph.D.,M.D. llehrner@ksosn.com Commercial Support Acknowledgement: There is no outside support for this activity Financial Disclosure: stocks > 50,000 Bayer, J&J, Norvartis,Novo
Objectives. Kidney Complications With Diabetes. Case 10/21/2015
 Objectives Kidney Complications With Diabetes Brian Boerner, MD Diabetes, Endocrinology, and Metabolism University of Nebraska Medical Center Review screening for, and management of, albuminuria Review
Objectives Kidney Complications With Diabetes Brian Boerner, MD Diabetes, Endocrinology, and Metabolism University of Nebraska Medical Center Review screening for, and management of, albuminuria Review
Diabetic Nephropathy Larry Lehrner, Ph.D.,M.D.
 Diabetic Nephropathy Larry Lehrner, Ph.D.,M.D. llehrner@ksosn.com Commercial Support Acknowledgement: There is no outside support for this activity Financial Disclosure: stocks > 50,000 Bayer, J&J, Norvartis,Novo
Diabetic Nephropathy Larry Lehrner, Ph.D.,M.D. llehrner@ksosn.com Commercial Support Acknowledgement: There is no outside support for this activity Financial Disclosure: stocks > 50,000 Bayer, J&J, Norvartis,Novo
University of Groningen. Evaluation of renal end points in nephrology trials Weldegiorgis, Misghina Tekeste
 University of Groningen Evaluation of renal end points in nephrology trials Weldegiorgis, Misghina Tekeste IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
University of Groningen Evaluation of renal end points in nephrology trials Weldegiorgis, Misghina Tekeste IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
VICTOSA and Renal impairment DR.R.S.SAJAD
 VICTOSA and Renal impairment DR.R.S.SAJAD February 2019 Main effect of GLP-1 is : Stimulating glucose dependent insulin release from the pancreatic islets. Slow gastric emptying Inhibit inappropriate
VICTOSA and Renal impairment DR.R.S.SAJAD February 2019 Main effect of GLP-1 is : Stimulating glucose dependent insulin release from the pancreatic islets. Slow gastric emptying Inhibit inappropriate
Diabetic Kidney Disease in the Primary Care Clinic
 Diabetic Kidney Disease in the Primary Care Clinic Jess Wheeler, DO Nephrology 2015 Outline: 1. CKD/DKD is a growing problem 2. Diagnosis of Chronic Kidney Disease (CKD) 3. Diagnosis of Diabetic Kidney
Diabetic Kidney Disease in the Primary Care Clinic Jess Wheeler, DO Nephrology 2015 Outline: 1. CKD/DKD is a growing problem 2. Diagnosis of Chronic Kidney Disease (CKD) 3. Diagnosis of Diabetic Kidney
Multiple Factors Should Be Considered When Setting a Glycemic Goal
 Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
Multiple Facts Should Be Considered When Setting a Glycemic Goal Patient attitude and expected treatment effts Risks potentially associated with hypoglycemia, other adverse events Disease duration Me stringent
New and Emerging Therapies for Type 2 DM
 Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
Dale Clayton MHSc, MD, FRCPC Dalhousie University/Capital Health April 28, 2011 New and Emerging Therapies for Type 2 DM The science of today, is the technology of tomorrow. Edward Teller American Physicist
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES
 INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
INJECTABLE THERAPY FOR THE TREATMENT OF DIABETES ARSHNA SANGHRAJKA DIABETES SPECIALIST PRESCRIBING PHARMACIST OBJECTIVES EXPLORE THE TYPES OF INSULIN AND INJECTABLE DIABETES TREATMENTS AND DEVICES AVAILABLE
Dr Brandon Orr-Walker
 Dr Brandon Orr-Walker Endocrinologist Clinical Head of Endocrinology and Diabetes Middlemore Auckland 17:45-18:10 What Can New Agents Offer Us? Diabetes Management What do the new agents offer us? Brandon
Dr Brandon Orr-Walker Endocrinologist Clinical Head of Endocrinology and Diabetes Middlemore Auckland 17:45-18:10 What Can New Agents Offer Us? Diabetes Management What do the new agents offer us? Brandon
Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American
 Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American Association of Diabetes Educators (AADE) for nurses, dietitians,
Update on GLP-1 Agonists in Type 2 Diabetes is supported by an educational grant from Novo Nordisk Inc. It has been accredited by the American Association of Diabetes Educators (AADE) for nurses, dietitians,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care
 Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
Translating GLP-1 trial outcomes to cardiology practice
 Translating GLP-1 trial outcomes to cardiology practice Which patients will benefit? Lars Rydén Senior Professor Department of Medicine K2 Karolinska Institutet Stockholm, Sweden Munich August 27, 2018
Translating GLP-1 trial outcomes to cardiology practice Which patients will benefit? Lars Rydén Senior Professor Department of Medicine K2 Karolinska Institutet Stockholm, Sweden Munich August 27, 2018
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes
 Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
 Study Summary DAILY ORAL SODIUM BICARBONATE PRESERVES GLOMERULAR FILTRATION RATE BY SLOWING ITS DECLINE IN EARLY HYPERTENSIVE NEPHROPATHY This was a 5-year, single-center, prospective, randomized, placebo-controlled,
Study Summary DAILY ORAL SODIUM BICARBONATE PRESERVES GLOMERULAR FILTRATION RATE BY SLOWING ITS DECLINE IN EARLY HYPERTENSIVE NEPHROPATHY This was a 5-year, single-center, prospective, randomized, placebo-controlled,
Effect of Saxagliptin on Renal Outcomes in the SAVOR TIMI- 53 study- Appendixes:
 Effect of Saxagliptin on Renal Outcomes in the SAVOR TIMI- 53 study- Appendixes: Appendix Table 1: Number and % of patients at the saxagliptin and placebo arms, according to egfr and on treatment ACR groups
Effect of Saxagliptin on Renal Outcomes in the SAVOR TIMI- 53 study- Appendixes: Appendix Table 1: Number and % of patients at the saxagliptin and placebo arms, according to egfr and on treatment ACR groups
Stages of Chronic Kidney Disease (CKD)
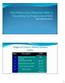 Early Treatment is the Key Stages of Chronic Kidney Disease (CKD) Stage Description GFR (ml/min/1.73 m 2 ) >90 1 Kidney damage with normal or GFR 2 Mild decrease in GFR 60-89 3 Moderate decrease in GFR
Early Treatment is the Key Stages of Chronic Kidney Disease (CKD) Stage Description GFR (ml/min/1.73 m 2 ) >90 1 Kidney damage with normal or GFR 2 Mild decrease in GFR 60-89 3 Moderate decrease in GFR
Diabetes and the Elderly: Medication Considerations When Determining Benefits and Risks
 Diabetes and the Elderly: Medication Considerations When Determining Benefits and Risks Gretchen M. Ray, PharmD, PhC, BCACP, CDE Associate Professor UNM College of Pharmacy September 7 th, 2018 DISCLOSURES
Diabetes and the Elderly: Medication Considerations When Determining Benefits and Risks Gretchen M. Ray, PharmD, PhC, BCACP, CDE Associate Professor UNM College of Pharmacy September 7 th, 2018 DISCLOSURES
Non-insulin treatment in Type 1 DM Sang Yong Kim
 Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
in patients with diabetes, nephropathy and/or chronic kidney disease Summary of recommendations July 2017
 Association of British Clinical Diabetologists (ABCD) and Renal Association clinical guidelines: Hypertension management and reninangiotensin-aldosterone system blockade in patients with diabetes, nephropathy
Association of British Clinical Diabetologists (ABCD) and Renal Association clinical guidelines: Hypertension management and reninangiotensin-aldosterone system blockade in patients with diabetes, nephropathy
TREATMENTS FOR TYPE 2 DIABETES. Susan Henry Diabetes Specialist Nurse
 TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
Can Treating Diabetes with SGLT2 inhibitors Prevent Heart Failure?
 UCSD Hawaii 2017 Symposium Can Treating Diabetes with SGLT2 inhibitors Prevent Heart Failure? Gregg C. Fonarow, MD, FACC, FAHA Elliot Corday Professor of Cardiovascular Medicine UCLA Division of Cardiology
UCSD Hawaii 2017 Symposium Can Treating Diabetes with SGLT2 inhibitors Prevent Heart Failure? Gregg C. Fonarow, MD, FACC, FAHA Elliot Corday Professor of Cardiovascular Medicine UCLA Division of Cardiology
Summary. Introduction REVIEW ARTICLE. Johan Jendle 1 * George Grunberger 2 Thomas Blevins 3 Francesco Giorgino 4 Ryan T. Hietpas 5 Fady T.
 REVIEW ARTICLE DIABETES/METABOLISM RESEARCH AND REVIEWS Diabetes Metab Res Rev 2016; 32: 776 790. Published online 15 May 2016 in Wiley Online Library (wileyonlinelibrary.com).2810 Efficacy and safety
REVIEW ARTICLE DIABETES/METABOLISM RESEARCH AND REVIEWS Diabetes Metab Res Rev 2016; 32: 776 790. Published online 15 May 2016 in Wiley Online Library (wileyonlinelibrary.com).2810 Efficacy and safety
SUPPLEMENTARY DATA. Supplementary Table 1. Baseline Patient Characteristics
 Supplementary Table 1. Baseline Patient Characteristics Normally distributed data are presented as mean (±SD), data that were not of a normal distribution are presented as median (ICR). The baseline characteristics
Supplementary Table 1. Baseline Patient Characteristics Normally distributed data are presented as mean (±SD), data that were not of a normal distribution are presented as median (ICR). The baseline characteristics
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Management of Type 2 Diabetes Cardiovascular Outcomes Trials Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas
 Management of Type 2 Diabetes Cardiovascular Outcomes Trials 2018 Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas Speaker Disclosure Dr. Blevins has disclosed that he has received grant support
Management of Type 2 Diabetes Cardiovascular Outcomes Trials 2018 Tom Blevins MD Texas Diabetes and Endocrinology Austin, Texas Speaker Disclosure Dr. Blevins has disclosed that he has received grant support
Semaglutide the new kid on the block in the field of glucagonlike peptide-1 receptor agonists?
 Editorial Page 1 of 5 Semaglutide the new kid on the block in the field of glucagonlike peptide-1 receptor agonists? Cristian Guja 1,2, Rucsandra Dănciulescu Miulescu 1,2 1 National Institute of Diabetes,
Editorial Page 1 of 5 Semaglutide the new kid on the block in the field of glucagonlike peptide-1 receptor agonists? Cristian Guja 1,2, Rucsandra Dănciulescu Miulescu 1,2 1 National Institute of Diabetes,
8/12/2016. Diabetes Management Across the Spectrum of Kidney Function. Andrew Bzowyckyj. Learning Objectives. Ashley Crowl
 Diabetes Management Across the Spectrum of Kidney Function Andrew Bzowyckyj PharmD, BCPS, CDE Clinical Assistant Professor School of Pharmacy University of Missouri-Kansas City Kansas City, MO Ashley Crowl
Diabetes Management Across the Spectrum of Kidney Function Andrew Bzowyckyj PharmD, BCPS, CDE Clinical Assistant Professor School of Pharmacy University of Missouri-Kansas City Kansas City, MO Ashley Crowl
SYSTEMATIC REVIEW. Introduction. X. Xue, 1 Z. Ren, 1 A. Zhang, 2 Q. Yang, 3 W. Zhang, 4 F. Liu 1,4
 SYSTEMATIC REVIEW Efficacy and safety of once-weekly glucagon-like peptide-1 receptor agonists compared with exenatide and liraglutide in type 2 diabetes: a systemic review of randomised controlled trials
SYSTEMATIC REVIEW Efficacy and safety of once-weekly glucagon-like peptide-1 receptor agonists compared with exenatide and liraglutide in type 2 diabetes: a systemic review of randomised controlled trials
Glucagon-Like Peptide-1 (GLP-1) Agonists
 Glucagon-Like Peptide-1 (GLP-1) Agonists Policy Number: 5.01.565 Last Review: 07/2018 Origination: 06/2014 Next Review: 07/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Glucagon-Like Peptide-1 (GLP-1) Agonists Policy Number: 5.01.565 Last Review: 07/2018 Origination: 06/2014 Next Review: 07/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
QUICK REFERENCE FOR HEALTHCARE PROVIDERS
 KEY MESSAGES 1 SCREENING CRITERIA Screen: Patients with DM and/or hypertension at least yearly. Consider screening patients with: Age >65 years old Family history of stage 5 CKD or hereditary kidney disease
KEY MESSAGES 1 SCREENING CRITERIA Screen: Patients with DM and/or hypertension at least yearly. Consider screening patients with: Age >65 years old Family history of stage 5 CKD or hereditary kidney disease
Diabetic Nephropathy 2009
 Diabetic Nephropathy 2009 Michael T McDermott MD Director, Endocrinology and Diabetes Practice University of Colorado Hospital Michael.mcdermott@ucdenver.edu Diabetic Nephropathy Clinical Stages Hyperfunction
Diabetic Nephropathy 2009 Michael T McDermott MD Director, Endocrinology and Diabetes Practice University of Colorado Hospital Michael.mcdermott@ucdenver.edu Diabetic Nephropathy Clinical Stages Hyperfunction
For Personal Use Only. Any commercial use is strictly prohibited. Role of glucagon-like peptide 1 receptor agonists in management of obesity
 Role of glucagon-like peptide 1 receptor agonists in management of obesity Diana Isaacs, Pharm.D., BCPS, BC-ADM, CDE, Chicago State University, Chicago, IL, and Oak Lawn VA Clinic of Edward Hines Jr. VA
Role of glucagon-like peptide 1 receptor agonists in management of obesity Diana Isaacs, Pharm.D., BCPS, BC-ADM, CDE, Chicago State University, Chicago, IL, and Oak Lawn VA Clinic of Edward Hines Jr. VA
Chronic kidney disease (CKD) has received
 Participant Follow-up in the Kidney Early Evaluation Program (KEEP) After Initial Detection Allan J. Collins, MD, FACP, 1,2 Suying Li, PhD, 1 Shu-Cheng Chen, MS, 1 and Joseph A. Vassalotti, MD 3,4 Background:
Participant Follow-up in the Kidney Early Evaluation Program (KEEP) After Initial Detection Allan J. Collins, MD, FACP, 1,2 Suying Li, PhD, 1 Shu-Cheng Chen, MS, 1 and Joseph A. Vassalotti, MD 3,4 Background:
Have you seen a patient like Carol *?
 (linagliptin) 5mg tablets Have you seen a patient like Carol *? *Hypothetical patient profile Carol * : 70 years old Retired schoolteacher *Hypothetical patient profile CAROL*: T2D patient with moderate
(linagliptin) 5mg tablets Have you seen a patient like Carol *? *Hypothetical patient profile Carol * : 70 years old Retired schoolteacher *Hypothetical patient profile CAROL*: T2D patient with moderate
The CARI Guidelines Caring for Australians with Renal Impairment. Specific effects of calcium channel blockers in diabetic nephropathy GUIDELINES
 Specific effects of calcium channel blockers in diabetic nephropathy Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES a. Non-dihydropyridine calcium channel
Specific effects of calcium channel blockers in diabetic nephropathy Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES a. Non-dihydropyridine calcium channel
Diabetes in Renal Patients. Contents. Understanding Diabetic Nephropathy
 Diabetes in Renal Patients Contents Understanding Diabetic Nephropathy What effect does CKD have on a patient s diabetic control? Diabetic Drugs in CKD and Dialysis Patients Hyper and Hypoglycaemia in
Diabetes in Renal Patients Contents Understanding Diabetic Nephropathy What effect does CKD have on a patient s diabetic control? Diabetic Drugs in CKD and Dialysis Patients Hyper and Hypoglycaemia in
Patients characteristics associated with better glycemic response to teneligliptin and metformin therapy in type 2 diabetes: a retrospective study
 International Journal of Advances in Medicine Gadge PV et al. Int J Adv Med. 2018 Apr;5(2):424-428 http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20181082
International Journal of Advances in Medicine Gadge PV et al. Int J Adv Med. 2018 Apr;5(2):424-428 http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20181082
Disclosure. Learning Objectives. Case. Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare
 Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
The CARI Guidelines Caring for Australasians with Renal Impairment. ACE Inhibitor and Angiotensin II Antagonist Combination Treatment GUIDELINES
 ACE Inhibitor and Angiotensin II Antagonist Combination Treatment Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES No recommendations possible based on Level
ACE Inhibitor and Angiotensin II Antagonist Combination Treatment Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES No recommendations possible based on Level
La lezione dei trials di safety cardiovascolare. Edoardo Mannucci
 La lezione dei trials di safety cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer
La lezione dei trials di safety cardiovascolare Edoardo Mannucci Conflitti di interessi Negli ultimi due anni, E. Mannucci ha ricevuto compensi per relazioni e/o consulenze da: Abbott, AstraZeneca, Boehringer

 www.usrds.org www.usrds.org 1 1,749 + (2,032) 1,563 to
www.usrds.org www.usrds.org 1 1,749 + (2,032) 1,563 to