Disclaimer. Chapter 3 Disorder of Water, Electrolyte and Acid-base Professor A. S. Alhomida. Disorder of Water and Electrolyte
|
|
|
- Christian Jones
- 6 years ago
- Views:
Transcription
1 Disclaimer King Saud University College of Science Department of Biochemistry The texts, tables, figures and images contained in this course presentation (BCH 376) are not my own, they can be found on: References supplied Atlases or The web Chapter 3 Disorder of Water, Electrolyte and Acid-base Professor A. S. Alhomida 1 Disorder of Water and Electrolyte 1. Dehydration It is a disturbance of water or electrolytes or both It is disturbance of water balance in which the output exceeds the intakes, causing a reduction of body water below the normal level 2. Water Intoxication It is caused by excess of water retention in the body 2 1
2 Types of Dehydration 1. Primary Depletion (Pure water depletion) 2. Secondary Depletion (Pure salts depletion, Sodium depletion) 3. Mixed Depletion; in which both occur 3 Primary Depletion Definition It occurs when intake is stopped or water intake is inadequate and there is no parallel loss of salt in the secretions from the body 4 2
3 Primary Depletion, Cont d Causes 1. When person is too weak or too ill to satisfy his/her water needs 2. In mental person who refuses to drink 3. In cases of coma, dysphagia (difficultly in swallowing) 4. In individual lost in desert or shipwrecked 5 Pathophysiology 1. Water depletion occurs almost always because of lack of intake, rather than because of losses from the body 2. When a person stops his intake of water, body water stores become depleted, because of continuing obligatory losses and later on, supplemented by the continued excretion of minimal volume of urine required for excretion of metabolic loads 6 3
4 Pathophysiology, Cont d 3. Only source of water supply for the body in the complete absence of intake of water becomes the water obtained from oxidation of food staffs (metabolic water) 4. As obligatory water loss continues, the concentration of the electrolytes rises in the ECF, which becomes hypertonic (hyperosmolar) 5. Water moves from ICF to ECF to correct this imbalance and to maintain uniform osmotic pressure throughout the body 7 Pathophysiology, Cont d 5. Thus the volume of ECF is maintained almost to normal at the expense of ICF which is grossly reduced in volume causing intracellular dehydration 8 4
5 ICF ECF C Water B A A = Plasma; B = Tissue Fluid; A + B = Extracellular Fluid (ECF); C = Intracellular Fluid (ICF) 1. Cellular dehydration 2. ECF volume is almost maintained Figure Showing distribution of body water in three compartments in primary dehydration 9 Clinical and Biochemical Significance 1. Thirst is the earliest symptom due to intracellular dehydration 2. Dehydration is shown by a dry tongue and pinched fancies 3. Oliguria: hyperosmolarity stimulates the releases of ADH which causes reassertion of water from the kidney tubules, causing a gradual diminution of urine volume 10 5
6 Clinical and Biochemical Significance, Cont d 4. A normal or slightly increased blood urea, reduced plasma volume may occur 5. There is usually no circulatory collapse or fall in blood pressure as the plasma volume is maintained 6. Urinary chloride will contain NaCl rather than it is to higher side 11 Death Occurs when has loss amounts to approximately 15% of body weight (about % of total body water) which happens on about the 7 th to 10 th day of complete water deprivation and if NOT treated 12 6
7 Secondary Depletion Definition It occurs when fluids of high sodium or chloride content are lost from the body and are replaced by salt-deficient fluids such as water by mouth or glucose solution IV It is called now sodium depletion rather than salt depletion to lay stress on the fact that Na is the significant ion concerted with the maintenance of ECF volume 13 Causes 1. Loss of Na can occur by excessive sweating when only water is taken in as replacement 2. Loss of gastrointestial fluids as in vomiting, diarrhea or biliary fistulae's, cholera and continue aspirations through intubations 14 7
8 Causes, Cont d 3. Urinary loses of Na are NOT as common as Addison s syndrome, but can occur as in diabetic acidosis, cerebral salt-washing syndrome and certain instances of chronic renal diseases 4. Vigorous uses of diuretics and low Na or salt-free diets in the management of congestive heart failure may induce Na depletion 15 Pathophysiology 1. ECF becomes hypotonic 2. The lowered osmotic pressure inhibits the release of ADH and the kidneys excrete water in an attempt to maintain normal EC Na levels 16 8
9 Pathophysiology, Cont d 3. Because of the above, plasma and interstitial fluid volume are decreased 4. Extracellular hypotonic allows water to flow into the cells where the level is greater, thus further reducing the volume of ECF and it leads to the cellular hydration is contrast to cellular dehydration noted in primary depletion 17 ICF ECF C B Water A A = Plasma; B = Tissue Fluid; A + B = Extracellular Fluid (ECF); C = Intracellular Fluid (ICF) 1. Cellular hydration 2. Reduction in tissue fluid volume more than plasma volume Figure Showing distribution of body water in three compartments in secondary dehydration 18 9
10 Clinical and Biochemical Significance 1. Because of hypotonicity, thirst is NOT a striking feature and absence of thirst is an important negative finding 2. Person appears apathetic and listless, mental changes are common and hallucinations and confusion are common and sometimes delirious 3. Anorexia and nausea and vomiting often aggravates the vicious circle of Na depletion: 19 Vicious Circle of Na Depletion Anorexia Na Depletion Loss of NaCl Salt depletion 20 10
11 Clinical and Biochemical Significance, Cont d 4. Cramps are common and may occur in high, duodecimal and respiratory muscles 5. Loss of interstitial fluid is manifested clinically by sunken eyes and inelastic skin 6. Reduced plasma volume leads to hemo concentration. As result of lowered blood volume there are decreased cardiac output, lowering of blood pressure and a tendency to orthostatic fainting 21 Clinical and Biochemical Significance, Cont d 7. Decreased glomerular filtration leads to nitrogen retention with increase urea concentration 8. Urinary analysis: person drinking freely maintain a normal or slightly increased urinary volume, but there is no salt present in the urine (except in Addison s disease) Death Due to oligemic shock 22 11
12 Mixed Water and Sodium Depletion Definition It is more common than depletion of either alone It occurs when there is loss of fluids containing high concentration of Na and Cl without of a free intake of water Pathophysiology Initially the ECF is hypotonic. Later water loss outstrips the salt loss and ECF becomes hypertonic 23 Clinical and Biochemical Significance 1. The clinical picture is a mixture of primary and secondary depletion 2. The volume of fluid in both ECF and ICF is reduced 3. The person appears dehydrated and complains of thirst 24 12
13 Clinical and Biochemical Significance, Cont d 4. The blood pressure may be lowered, blood urea and hemo concentration are raised 5. Urinary output is diminished and excretion of salt is reduced 25 Types of Fluids to Administer For Primary Dehydration Water by mouth or per rectum or 5% glucose by IV, SC or IP routes depending on the cases Note: never give isotonic saline which increase hypertonicity 26 13
14 Types of Fluids to Administer, Cont d For Secondary Dehydration Isotonic saline solution For Mixed Type Dehydroation A mixture of saline and 5% glucose solution usually: 1: 1 (0.5 normal saline solution) 1: 2 (0.33 normal saline solution) 27 Regulation of Dehydration and Rehydratoin 28 14
15 Action of ADH 29 Regulation of Water Intake: Thirst Mechanism 30 15
16 Mechanisms and Consequences of ADH Release 31 Water Intoxication Definition It is caused by excess of water retention in the body Causes 1. Renal failure 2. Excessive of administration of fluids 3. Hyper secretion of ADH following administration of an anesthetic for surgery 4. Excess of aldosterone 32 16
17 Clinical and Biochemical Significance 1. Headache, nausea, in coordination of movements, muscular weakness and delirium 2. PCV, Hb concentration, and plasma proteins concentrations are reduced 3. Plasma electrolytes are lowered 4. Urinary volume is usually increased and its specific gravity is low Treatment Withholding fluids by mouth and administering 3-5% hypertonic saline IV 33 Acid-base Disorders Basic Terminology 1. Acidemia is present when blood ph < Alkalemia is present when blood ph > Acidosis is a process that will result in acidemia if left unopposed 4. Alkalosis is a process that will result in alkalemia if left unopposed 5. Metabolic refers to a disorder that results from a primary alteration in [H + ] or [HCO - 3 ] 6. Respiratory refers to a disorder that results from a primary alteration in PCO 2 due to altered CO 2 elimination 34 17
18 Acid-base Disorders 1. Simple (Primary) Acid-base Disorder: have one primary abnormality Respiratory acidosis Respiratory alkalosis Metabolic acidosis Metabolic alkalosis 2. Mixed Acid-base Disorder: have more than one primary abnormality Two to three primary disorders can be combined together to result in a mixed disorder 35 Acid-base Disorder, Cont d 36 18
19 Acidosis ph of body fluids below Respiratory acidosis Caused by inadequate ventilation 2. Metabolic acidosis Results from all conditions other than respiratory that decrease ph 37 Alkalosis ph of body fluids above Respiratory alkalosis Caused by hyperventilation 2. Metabolic alkalosis Results from all conditions other than respiratory that increase ph 38 19
20 Respiratory Ventilation 1. Normal, Unassisted Breathing An increase in arterial PCO 2 acts through the respiratory center to increase the rate of pulmonary ventilation A decrease in arterial PCO 2 reduces the rate of ventilation 2. Assisted Breathing A respirator is used to assist breathing by expelling CO 2, thus reducing PCO 2 in blood 39 Respiratory Acidosis 40 20
21 Respiratory Acidosis Causes 1. Damage of CNS 2. Respiratory problem Hypoventilation Asthma Pneumonia 41 Respiratory Acidosis Causes, Cont d 3. Pain Chest Tightness Cough Wheeze 42 21
22 Respiratory Alkalosis 43 Respiratory Alkalosis Causes 1. Stimulation of respiratory center CNS diseases Salicylate poisoning Hyperpyrexia Fevercoma 44 22
23 Respiratory Alkalosis Causes, Cont d 2. Hysteria 3. Apprehensive blood donor 4. High altitude ascending 5. Hepatic 45 Metabolic Acidosis 46 23
24 Metabolic Acidosis, Cont d Acidosis will cause more potassium ions to be moved extracellularly in exchange for hydrogen ions Hyperkalemia may result 47 Metabolic Acidosis Causes 1. Diabetic acidosis 2. Lactic acidosis 3. Starvation 4. High fever 5. Violent exercise 48 24
25 Metabolic Acidosis Causes, Cont d 6. Hemorrhage 7. Anoxia 8. Renal failure 9. Heart failure 10. Diarrhoea 49 Metabolic Alkalosis 50 25
26 Metabolic Alkalosis, Cont d Alkalosis will cause more hydrogen ions to be moved extracellularly in exchange for potassium ions Hypokalemia may result 51 Metabolic Alkalosis Causes 1. Vomiting 2. Alkali administration 3. Potassium deficiency 4. Radiation therapy 52 26
27 Acid-base Changes in Acidosis and Alkalosis Acid-base Disturbance Respiratory acidosis Respiratory alkalosis Metabolic acidosis Metabolic alkalosis ph HCO - 3 PCO 2 Thicker arrows indicate primary disorder 53 Acid-base Changes in Acidosis and Alkalosis, Cont d Memorize Acid-base Disturbance ph HCO 3 - PCO 2 Respiratory acidosis Respiratory alkalosis ph, PCO 2 in opposite directions; HCO 3- will follow PCO 2 Metabolic acidosis Metabolic alkalosis Thicker arrows indicate primary disorder ph, HCO 3 - in same direction; PCO 2 will follow HCO
28 Acidosis and Alkalosis 55 THE END Any questions? 56 28
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
UNIT VI: ACID BASE IMBALANCE
 UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
Water, Electrolytes, and Acid-Base Balance
 Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Fluid and Electrolytes P A R T 4
 Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Fluid, Electrolyte, and Acid Base Balance
 25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
Acid-Base Tutorial 2/10/2014. Overview. Physiology (2) Physiology (1)
 Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE
 Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES
 Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Fluid and Electrolytes P A R T 2
 Fluid and Electrolytes P A R T 2 Fluid Shifts Extracellular fluid distribution is dynamic Interstitial fluid formation is continuous Venous system Large veins (capacitance vessels) Small veins (capacitance
Fluid and Electrolytes P A R T 2 Fluid Shifts Extracellular fluid distribution is dynamic Interstitial fluid formation is continuous Venous system Large veins (capacitance vessels) Small veins (capacitance
Fluids and electrolytes
 Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
BIOL 221 Chapter 26 Fluids & Electrolytes. 35 slides
 BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender
 BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
CHAPTER 27 LECTURE OUTLINE
 CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
Acid Base Balance. Chapter 26 Balance. ph Imbalances. Acid Base Balance. CO 2 and ph. Carbonic Acid. Part 2. Acid/Base Balance
 Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline
 BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline Fluid divisions 1. Extracellular fluid (ECF) 2. Intracellular fluid (ICF) Stabilization 1. Fluid balance 2. Electrolyte balance
BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline Fluid divisions 1. Extracellular fluid (ECF) 2. Intracellular fluid (ICF) Stabilization 1. Fluid balance 2. Electrolyte balance
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Technical University of Mombasa Faculty of Applied and Health Sciences
 Technical University of Mombasa Faculty of Applied and Health Sciences DEPARTMENT OF MEDICAL SCIENCES UNIVERSITY EXAMINATION FOR THE DEGREE OF BACHELOR OF MEDICAL LABORATORY SCIENCES BMLS 12S -Regular
Technical University of Mombasa Faculty of Applied and Health Sciences DEPARTMENT OF MEDICAL SCIENCES UNIVERSITY EXAMINATION FOR THE DEGREE OF BACHELOR OF MEDICAL LABORATORY SCIENCES BMLS 12S -Regular
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
ACID BASE BALANCE & BODY FLUID. Ani Retno Prijanti Renal and Body Fluids Module Juni 2008
 ACID BASE BALANCE & BODY FLUID Ani Retno Prijanti Renal and Body Fluids Module Juni 2008 1 Continuous Mixing of Body Fluids 2 Water Balance and ECF Osmolality To remain properly hydrated, water intake
ACID BASE BALANCE & BODY FLUID Ani Retno Prijanti Renal and Body Fluids Module Juni 2008 1 Continuous Mixing of Body Fluids 2 Water Balance and ECF Osmolality To remain properly hydrated, water intake
Fluid, electrolyte, and acid-base balance
 Fluid, electrolyte, and acid-base balance Chapter 50 Ra'eda Almashaqba 1 Fluid, electrolyte, and acid-base balance About 46% to 60%of the average adult's weight is water, which is vital to health and normal
Fluid, electrolyte, and acid-base balance Chapter 50 Ra'eda Almashaqba 1 Fluid, electrolyte, and acid-base balance About 46% to 60%of the average adult's weight is water, which is vital to health and normal
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
1. 09/07/16 Ch 1: Intro to Human A & P 1
 Table of Contents # Date Title Page # 1. 09/07/16 Ch 1: Intro to Human A & P 1 2. 09/19/16 Ch 18: Water, Electrolyte, and Acid-Base Balance 5 i 1 09/19/16 Chapter 18: Water, Electrolyte, and Acid-Base
Table of Contents # Date Title Page # 1. 09/07/16 Ch 1: Intro to Human A & P 1 2. 09/19/16 Ch 18: Water, Electrolyte, and Acid-Base Balance 5 i 1 09/19/16 Chapter 18: Water, Electrolyte, and Acid-Base
Acid-Base Imbalance-2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD
 AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
Acids, Bases, and Salts
 Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
5/18/2017. Specific Electrolytes. Sodium. Sodium. Sodium. Sodium. Sodium
 Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Chapter 24 Water, Electrolyte and Acid-Base Balance
 Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
Principles of Fluid Balance
 Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender
 BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides!1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides!1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
Body water content. Fluid compartments. Regulation of water output. Water balance and ECF osmolallty. Regulation of water intake
 Body water content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; females 50% This difference reflects
Body water content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; females 50% This difference reflects
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Nursing Process Focus: Patients Receiving Dextran 40 (Gentran 40)
 Assess for presence/history of hypovolemia, shock, venous thrombosis. Assess vital signs: Hypovolemic shock secondary to surgery, burns, hemorrhage, other serious condition PT and PTT abnormalities Venous
Assess for presence/history of hypovolemia, shock, venous thrombosis. Assess vital signs: Hypovolemic shock secondary to surgery, burns, hemorrhage, other serious condition PT and PTT abnormalities Venous
Biochemistry of acid-base disorders. Alice Skoumalová
 Biochemistry of acid-base disorders Alice Skoumalová Main topics of the lecture: Measurement of acid-base dysbalance Classification of the acid-base disorders 4 basic acid-base disorders and their compensaiton
Biochemistry of acid-base disorders Alice Skoumalová Main topics of the lecture: Measurement of acid-base dysbalance Classification of the acid-base disorders 4 basic acid-base disorders and their compensaiton
IV Fluids. I.V. Fluid Osmolarity Composition 0.9% NaCL (Normal Saline Solution, NSS) Uses/Clinical Considerations
 IV Fluids When administering IV fluids, the type and amount of fluid may influence patient outcomes. Make sure to understand the differences between fluid products and their effects. Crystalloids Crystalloid
IV Fluids When administering IV fluids, the type and amount of fluid may influence patient outcomes. Make sure to understand the differences between fluid products and their effects. Crystalloids Crystalloid
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE*
 ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
Acid-Base Physiology. Dr. Tamás Bense Dr. Alexandra Turi
 Acid-Base Physiology Dr. Tamás Bense Dr. Alexandra Turi What is a blood gas assessment? We get it from an arterial sample (a.radialis, a. brachialis, a. femoralis) Invasive technique If the patient is
Acid-Base Physiology Dr. Tamás Bense Dr. Alexandra Turi What is a blood gas assessment? We get it from an arterial sample (a.radialis, a. brachialis, a. femoralis) Invasive technique If the patient is
Chapter 21. Diuretic Agents. Mosby items and derived items 2008, 2002 by Mosby, Inc., an affiliate of Elsevier Inc.
 Chapter 21 Diuretic Agents Renal Structure and Function Kidneys at level of umbilicus Each weighs 160 to 175 g and is 10 to 12 cm long Most blood flow per gram of weight in body 22% of cardiac output (CO)
Chapter 21 Diuretic Agents Renal Structure and Function Kidneys at level of umbilicus Each weighs 160 to 175 g and is 10 to 12 cm long Most blood flow per gram of weight in body 22% of cardiac output (CO)
Fluid, Electrolyte, and Acid Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid Base Balance Bi 233 Body Water Content Largest component of the body Infants have low body fat, low bone mass, and are 73% or more water Healthy males are about
Chapter 26 Fluid, Electrolyte, and Acid Base Balance Bi 233 Body Water Content Largest component of the body Infants have low body fat, low bone mass, and are 73% or more water Healthy males are about
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Disorders of Acid-Base
 Disorders of Acid-Base Balance Bởi: OpenStaxCollege Normal arterial blood ph is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood ph below 7.35 is considered to be in acidosis
Disorders of Acid-Base Balance Bởi: OpenStaxCollege Normal arterial blood ph is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood ph below 7.35 is considered to be in acidosis
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
The Urinary System 15PART B. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART B Ureters Slender tubes attaching the kidney to the bladder Continuous with
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART B Ureters Slender tubes attaching the kidney to the bladder Continuous with
Acid Base Balance by: Susan Mberenga RN, BSN, MSN
 Acid Base Balance by: Susan Mberenga RN, BSN, MSN Acid Base Balance Refers to hydrogen ions as measured by ph Normal range: 7.35-7.45 Acidosis/acidemia: ph is less than 7.35 Alkalosis/alkalemia: ph is
Acid Base Balance by: Susan Mberenga RN, BSN, MSN Acid Base Balance Refers to hydrogen ions as measured by ph Normal range: 7.35-7.45 Acidosis/acidemia: ph is less than 7.35 Alkalosis/alkalemia: ph is
BUFFERING OF HYDROGEN LOAD
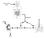 BUFFERING OF HYDROGEN LOAD 1. Extracellular space minutes 2. Intracellular space minutes to hours 3. Respiratory compensation 6 to 12 hours 4. Renal compensation hours, up to 2-3 days RENAL HYDROGEN SECRETION
BUFFERING OF HYDROGEN LOAD 1. Extracellular space minutes 2. Intracellular space minutes to hours 3. Respiratory compensation 6 to 12 hours 4. Renal compensation hours, up to 2-3 days RENAL HYDROGEN SECRETION
Acid-Base Balance * OpenStax
 OpenStax-CNX module: m46409 1 Acid-Base Balance * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be
OpenStax-CNX module: m46409 1 Acid-Base Balance * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be
PARAMEDIC RESOURCE MANUAL
 ONTARIO BASE HOSPITAL GROUP PARAMEDIC RESOURCE MANUAL ACID-BASE BALANCE SECTION SIX Version 1.1 2010 Update PARAMEDIC RESOURCE MANUAL OBJECTIVES: ACID-BASE BALANCE The objectives indicate what you should
ONTARIO BASE HOSPITAL GROUP PARAMEDIC RESOURCE MANUAL ACID-BASE BALANCE SECTION SIX Version 1.1 2010 Update PARAMEDIC RESOURCE MANUAL OBJECTIVES: ACID-BASE BALANCE The objectives indicate what you should
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Chapter 2. Fluid, Electrolyte, and Acid-Base Imbalances
 Chapter 2 Fluid, Electrolyte, and Acid-Base Imbalances Review of Concepts and Processes The major component of the body is water. Water is located in these compartments: Intracellular fluid (ICF) compartment
Chapter 2 Fluid, Electrolyte, and Acid-Base Imbalances Review of Concepts and Processes The major component of the body is water. Water is located in these compartments: Intracellular fluid (ICF) compartment
3/17/2017. Acid-Base Disturbances. Goal. Eric Magaña, M.D. Presbyterian Medical Center Department of Pulmonary and Critical Care Medicine
 Acid-Base Disturbances Eric Magaña, M.D. Presbyterian Medical Center Department of Pulmonary and Critical Care Medicine Goal Provide an approach to determine complex acid-base disorders Discuss the approach
Acid-Base Disturbances Eric Magaña, M.D. Presbyterian Medical Center Department of Pulmonary and Critical Care Medicine Goal Provide an approach to determine complex acid-base disorders Discuss the approach
9/14/2017. Acid-Base Disturbances. Goal. Provide an approach to determine complex acid-base disorders
 Acid-Base Disturbances NCNP October 10, 2017 Eric Magaña, M.D. Presbyterian Medical Center Department of Pulmonary and Critical Care Medicine Goal Provide an approach to determine complex acid-base disorders
Acid-Base Disturbances NCNP October 10, 2017 Eric Magaña, M.D. Presbyterian Medical Center Department of Pulmonary and Critical Care Medicine Goal Provide an approach to determine complex acid-base disorders
Body fluids. Lecture 13:
 Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Acid/Base Disorders 2015
 Objectives - 2 1. Identify acid/base disorders 2. Discuss etiologies for 1 0 acid/base disorders (will not include mixed disorders) 3. Interpret acid/base disorders by interpreting arterial blood gas &
Objectives - 2 1. Identify acid/base disorders 2. Discuss etiologies for 1 0 acid/base disorders (will not include mixed disorders) 3. Interpret acid/base disorders by interpreting arterial blood gas &
Part 1 The Cell and the Cellular Environment
 1 Chapter 3 Anatomy and Physiology Part 1 The Cell and the Cellular Environment 2 The Human Cell The is the fundamental unit of the human body. Cells contain all the necessary for life functions. 3 Cell
1 Chapter 3 Anatomy and Physiology Part 1 The Cell and the Cellular Environment 2 The Human Cell The is the fundamental unit of the human body. Cells contain all the necessary for life functions. 3 Cell
Metabolic Alkalosis: Vomiting
 RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
Overview. Fluid & Electrolyte Disorders. Water distribution. Introduction 5/10/2014
 Overview Fluid & Electrolyte Disorders Dr Nicola Barlow Clinical Biochemistry Department, City Hospital Introduction Fluid and electrolyte homeostasis Electrolyte disturbances Analytical parameters Methods
Overview Fluid & Electrolyte Disorders Dr Nicola Barlow Clinical Biochemistry Department, City Hospital Introduction Fluid and electrolyte homeostasis Electrolyte disturbances Analytical parameters Methods
Inorganic pharmaceutical chemistry. Replacement Therapy Lec 2
 Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
Acid/Base Balance. the concentrations of these two ions affect the acidity or alkalinity of body fluids
 Acid/Base Balance some of most critical ions in body fluids are H + (hydrogen) and OH - (hydroxyl) ions the concentrations of these two ions affect the acidity or alkalinity of body fluids acidity/alkalinity
Acid/Base Balance some of most critical ions in body fluids are H + (hydrogen) and OH - (hydroxyl) ions the concentrations of these two ions affect the acidity or alkalinity of body fluids acidity/alkalinity
1. What is the acid-base disturbance in this patient?
 /ABG QUIZ QUIZ 1. What is the acid-base disturbance in this patient? Presenting complaint: pneumonia 1 point Uncompensated metabolic alkalosis Partially compensated respiratory alkalosis Mixed alkalosis
/ABG QUIZ QUIZ 1. What is the acid-base disturbance in this patient? Presenting complaint: pneumonia 1 point Uncompensated metabolic alkalosis Partially compensated respiratory alkalosis Mixed alkalosis
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter
 Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
Instrumental determination of electrolytes in urine. Amal Alamri
 Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017
 ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017 WHAT INFORMATION DOES AN ABG GIVE US? ph = measure of hydrogen ion concentration (acidity or alkalinity) PaCO2 = partial pressure
ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017 WHAT INFORMATION DOES AN ABG GIVE US? ph = measure of hydrogen ion concentration (acidity or alkalinity) PaCO2 = partial pressure
Interpretation of ABG. Chandra Shekhar Bala, FCPS( Medicine) Junior Consultant NINS and Hospital, Dhaka
 Interpretation of ABG Chandra Shekhar Bala, FCPS( Medicine) Junior Consultant NINS and Hospital, Dhaka ABG analysis of Ms Rubi Ms. Rubi, 20 year-old lady PH 7.29 presented with breathlessness. She had
Interpretation of ABG Chandra Shekhar Bala, FCPS( Medicine) Junior Consultant NINS and Hospital, Dhaka ABG analysis of Ms Rubi Ms. Rubi, 20 year-old lady PH 7.29 presented with breathlessness. She had
There are number of parameters which are measured: ph Oxygen (O 2 ) Carbon Dioxide (CO 2 ) Bicarbonate (HCO 3 -) AaDO 2 O 2 Content O 2 Saturation
 Arterial Blood Gases (ABG) A blood gas is exactly that...it measures the dissolved gases in your bloodstream. This provides one of the best measurements of what is known as the acid-base balance. The body
Arterial Blood Gases (ABG) A blood gas is exactly that...it measures the dissolved gases in your bloodstream. This provides one of the best measurements of what is known as the acid-base balance. The body
Disorders of Acid-Base Balance
 OpenStax-CNX module: m46413 1 Disorders of Acid-Base Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m46413 1 Disorders of Acid-Base Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
Physiological Causes of Abnormal ABG s
 Physiological Causes of Abnormal ABG s Major Student Performance Objective 1 1. The student will be able to discuss causes for various types of blood gas results. 2. They will also be required to discuss
Physiological Causes of Abnormal ABG s Major Student Performance Objective 1 1. The student will be able to discuss causes for various types of blood gas results. 2. They will also be required to discuss
CASE 27. What is the response of the kidney to metabolic acidosis? What is the response of the kidney to a respiratory alkalosis?
 CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
Hypoglycemia, Electrolyte disturbances and acid-base imbalances
 Hypoglycemia, Electrolyte disturbances and acid-base imbalances Pediatric emergency PICU division Pediatric department Medical faculty, University of Sumatera Utara H. Adam Malik Hospital 1 Hypoglycemia
Hypoglycemia, Electrolyte disturbances and acid-base imbalances Pediatric emergency PICU division Pediatric department Medical faculty, University of Sumatera Utara H. Adam Malik Hospital 1 Hypoglycemia
Water (Dysnatremia) & Sodium (Dysvolemia) Disorders Ahmad Raed Tarakji, MD, MSPH, PGCertMedEd, FRCPC, FACP, FASN, FNKF, FISQua
 Water (Dysnatremia) & Sodium (Dysvolemia) Disorders Ahmad Raed Tarakji, MD, MSPH, PGCertMedEd, FRCPC, FACP, FASN, FNKF, FISQua Assistant Professor Nephrology Unit, Department of Medicine College of Medicine,
Water (Dysnatremia) & Sodium (Dysvolemia) Disorders Ahmad Raed Tarakji, MD, MSPH, PGCertMedEd, FRCPC, FACP, FASN, FNKF, FISQua Assistant Professor Nephrology Unit, Department of Medicine College of Medicine,
Mannitol-induced Metabolic Alkalosis
 Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
Electrolyte & Blood Pressure :, 00 ) Mannitolinduced Metabolic Alkalosis Kyung Pyo Kang, M.D., Sik Lee, M.D., Kyung Hoon Lee, M.D., and Sung Kyew Kang, M.D. Department of Internal Medicine, Research Institute
Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D.
 Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D. Normal Blood PH 7.35 to 7.45 Crucial importance to maintain homeostatic function of Body. Any Significant
Inter Inter Pretation of Acid Base Disturbance in Critically ill Patients. By :-: Dr. Vinay Bhomia M.D. Normal Blood PH 7.35 to 7.45 Crucial importance to maintain homeostatic function of Body. Any Significant
SODIUM BALANCE Overview
 SODIUM BALANCE Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS III Seminar VJ Temple 1 How are solute and solvent related to solution?
SODIUM BALANCE Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS III Seminar VJ Temple 1 How are solute and solvent related to solution?
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
Cardiorenal and Renocardiac Syndrome
 And Renocardiac Syndrome A Vicious Cycle Cardiorenal and Renocardiac Syndrome Type 1 (acute) Acute HF results in acute kidney injury Type 2 Chronic cardiac dysfunction (eg, chronic HF) causes progressive
And Renocardiac Syndrome A Vicious Cycle Cardiorenal and Renocardiac Syndrome Type 1 (acute) Acute HF results in acute kidney injury Type 2 Chronic cardiac dysfunction (eg, chronic HF) causes progressive
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
Water compartments inside and outside cells maintain a balanced distribution of total body water.
 Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
Physiology questions review
 Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
Physiology questions review 1- The consumption of O2 by the kidney: a- decrease as blood flow increases b- regulated by erythropoiten c- remains constant as blood flow increase d- direct reflects the level
營養學. 營養學 Nutrition ~ Water (H 2 O) ~
 營養學 營養學 Nutrition ~ Water (H 2 O) ~ 謝明哲 M.J.Shieh 臺北醫學大學公共衛生暨營養學院 保健營養學系 研究所 clark@tmu.edu.tw 1 Outline Function Homeostasis Requirement Deficiency syndrome Water Toxicity 2 Water (H 2 O) ~ An essential
營養學 營養學 Nutrition ~ Water (H 2 O) ~ 謝明哲 M.J.Shieh 臺北醫學大學公共衛生暨營養學院 保健營養學系 研究所 clark@tmu.edu.tw 1 Outline Function Homeostasis Requirement Deficiency syndrome Water Toxicity 2 Water (H 2 O) ~ An essential
Basic Fluid and Electrolytes
 Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Chapter 20 8/23/2016. Fluids and Electrolytes. Fluid (Water) Fluid (Water) (Cont.) Functions
 Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of:
 [Acid-Base Balance] [Dr. Bashir Khasawneh] [5 th February 2012] Acid-Base Basic Concepts: The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of: Which is modified from Henderson-Hasselbach
[Acid-Base Balance] [Dr. Bashir Khasawneh] [5 th February 2012] Acid-Base Basic Concepts: The relationship between H+,PaCO₂ and HCO₃ are expressed in the equation of: Which is modified from Henderson-Hasselbach
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Are you ready to have fun?
 Arterial Blood Gas INTERPRETATION By Nena Bonuel, MSN, RN, CCRN, CNS, ACNS-BC Nurse Specialist, Center for Professional Excellence Are you ready to have fun? 1. Yes! 2. I rather go shopping 3. I still
Arterial Blood Gas INTERPRETATION By Nena Bonuel, MSN, RN, CCRN, CNS, ACNS-BC Nurse Specialist, Center for Professional Excellence Are you ready to have fun? 1. Yes! 2. I rather go shopping 3. I still
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013
 Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Slide 1. Slide 2. Slide 3. Learning Outcomes. Acid base terminology ARTERIAL BLOOD GAS INTERPRETATION
 Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Salty Solutions or Salty Problems? Outline. Outline 29/04/2013
 Salty Solutions or Salty Problems? 18 th October 2012 Richard Seigne Anaesthetist 1 - Non fluid 40% T o t a l b o d y f l u i d 60% NaCl NaCl Intra-cellular fluid 2/3 KCl Interstitial fluid 3/4 of ECF
Salty Solutions or Salty Problems? 18 th October 2012 Richard Seigne Anaesthetist 1 - Non fluid 40% T o t a l b o d y f l u i d 60% NaCl NaCl Intra-cellular fluid 2/3 KCl Interstitial fluid 3/4 of ECF
Objectives Body Fluids Electrolytes The Kidney and formation of urine
 Objectives Body Fluids Outline the functions of water in the body. State how water content varies with age and sex. Differentiate between intracellular and extra-cellular fluid. Explain how water moves
Objectives Body Fluids Outline the functions of water in the body. State how water content varies with age and sex. Differentiate between intracellular and extra-cellular fluid. Explain how water moves
Physiology week 16 Renal 2 (volume/buffers)
 Physiology week 16 Renal 2 (volume/buffers) Defense of Tonicity and Volume Defense of tonicity Tonicity = osmolality of a solution relative to plasma Osmolality measures [ ] all particles in solution,
Physiology week 16 Renal 2 (volume/buffers) Defense of Tonicity and Volume Defense of tonicity Tonicity = osmolality of a solution relative to plasma Osmolality measures [ ] all particles in solution,
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D.
 Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines
 Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines Author: Richard Pugh June 2015 Guideline for management of hyponatraemia in intensive care Background
Wales Critical Care & Trauma Network (North) Management of Hyponatraemia in Intensive Care Guidelines Author: Richard Pugh June 2015 Guideline for management of hyponatraemia in intensive care Background
Renal physiology V. Regulation of acid-base balance. Dr Alida Koorts BMS
 Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
UNIT 9 INVESTIGATION OF ACID-BASE DISTURBANCES
 UNIT 9 INVESTIGATION OF ACIDBASE DISTURBANCES LEARNING OBJECTIVES At the end of this chapter, students must be able to: 1. Describe the main parametres that define the acidbase equilibrium 2. Identify
UNIT 9 INVESTIGATION OF ACIDBASE DISTURBANCES LEARNING OBJECTIVES At the end of this chapter, students must be able to: 1. Describe the main parametres that define the acidbase equilibrium 2. Identify
FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS
 Chapter 27 1 BIOLOGY 2402 Anatomy and Physiology Lecture Chapter 27 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS Chapter 27 2 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTAIS Body fluid refers to the body
Chapter 27 1 BIOLOGY 2402 Anatomy and Physiology Lecture Chapter 27 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS Chapter 27 2 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTAIS Body fluid refers to the body
