Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study
|
|
|
- Blaze Simpson
- 6 years ago
- Views:
Transcription
1 Diabetes Research and Clinical Practice 48 (2000) Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study Nakayasu Wake a, Akinori Hisashige b, Takafumi Katayama b, Hideki Kishikawa a, *, Yasuo Ohkubo a, Masakazu Sakai a, Eiichi Araki a, Motoaki Shichiri a a Department of Metabolic Medicine, Kumamoto Uni ersity School of Medicine, Honjo, Kumamoto , Japan b Department of Pre enti e Medicine, School of Medicine, The Uni ersity of Tokushima, Tokushima, Japan Received 15 November 1999; received in revised form 5 January 2000; accepted 5 January 2000 Abstract To evaluate the cost and effectiveness of intensive insulin therapy for type 2 diabetes on the prevention of diabetes complications in Japan, we performed economic evaluation based on a randomized controlled trial. A total of 110 patients with type 2 diabetes were randomly assigned into two groups, a multiple insulin injection therapy (MIT) group or a conventional insulin injection therapy (CIT) group, and were followed-up for 10 years. Economic evaluation (cost consequences analysis) was applied to evaluate both health and economic outcomes. As outcome measures for effectiveness of intensive insulin therapy, the frequency of complications, such as retinopathy, nephropathy, neuropathy, macrovascular event, and diabetes-related death, was used. For estimating costs, a viewpoint of the payer (the National Health Insurance) was adopted. Direct medical costs associated with diabetes care during 10 years were calculated and evaluated. In a base case analysis, all costs were discounted to the present value at an annual rate of 3%. Sensitivity analyses were carried out to assess the robustness of the results to changes in the values of important variables. MIT reduced the relative risk in the progression of retinopathy by 67%, photocoagulation by 77%, progression of nephropathy by 66%, albuminuria by 100% and clinical neuropathy by 64%, relative to CIT. Moreover, MIT prolonged the period in which patients were free of complications, including 2.0 years for progression of retinopathy (P ), 0.3 years for photocoagulation (P 0.05), 1.5 years for progression of nephropathy (P 0.01) and 2.2 years for clinical neuropathy (P ). The total cost (discounted at 3%) per patient during the 10-year period for each group was $ and , respectively. The reduction of total costs in MIT over CIT was mainly due to reduced costs for management of diabetic complications. Our results show that MIT is more beneficial than CIT in both cost and effectiveness. Therefore, MIT is recommended for the treatment of type 2 diabetic patients who require insulin therapy as early as possible from the perspective of both patients and health policy Elsevier Science Ireland Ltd. All rights reserved. Keywords: Type 2 diabetes; Microvascular complications; Intensive glycemic control; Multiple insulin injection treatment; Cost-effectiveness * Corresponding author. Tel.: ; fax: /00/$ - see front matter 2000 Elsevier Science Ireland Ltd. All rights reserved. PII: S (00)
2 202 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) Introduction Diabetes mellitus is a major disease with high morbidity and mortality consuming high health care expenditure in most developed countries [1]. Clinical care of diabetic patients requires a relatively high use of health care resources compared with non-diabetic patients. In particular, the longterm complications associated with diabetes are expensive in terms of health care and quality of life. In the United States, where the prevalence rate of diabetes is approximately 7% in adults, it has been estimated that the combined direct and indirect costs of diabetes in 1997 were $98 billion [2]. In Japan, the number of patients with diabetes has markedly increased in recent decades. Approximately 6.9 million individuals or 6% of the Japanese population suffered from diabetes in 1997 [3]. Moreover, the health care expenditure for diabetes was $8 billion, which constituted 4% of total health care expenditure [4]. Although diabetes implies a relatively poor prognosis, there is a distinct prospect of significant improvement in prognosis with the implementation of effective preventive strategies [1]. Recently, a remarkable medical innovation has emerged in the prevention of diabetic complications, known as intensive insulin therapy, which is designed to achieve blood glucose level as close to the non-diabetic range as possible with three or more daily insulin injections [5 7]. Several small randomized controlled trials (RCTs) have compared intensive with conventional treatment in patients with type 1 diabetes [8 10]. These results and their systematic review showed a reduction in the development and progression of microvascular complications. A subsequent long-term and large RCT (the Diabetes Control and Complication Trial (DCCT)) has extended these findings [11]. Moreover, in type 2 diabetes, which constitutes about 80 90% of all cases of diabetes in developed countries, the Kumamoto Study demonstrated almost the same results as DCCT [12,13]. The United Kingdom Prospective Diabetes Study (UKPDS) also confirmed these findings [14]. However, it was suggested that intensive therapy consumed more resources and was substantially more expensive than conventional treatment [15]. Under considerable pressure on health care expenditure, additional resources for this therapy may need to be diverted from other health care practices or non-health care activities. Therefore, it is essential to analyze and consider the cost-effectiveness of intensive insulin therapy. In type 1 diabetes, economic evaluation of lifetime benefits and costs of intensive insulin therapy based on DCCT suggested that this therapy was within the range of cost-effectiveness [15]. In contrast, in type 2 diabetes, although a simulation model for economic evaluation of intensive therapy was developed and examined [16], the actual cost-effectiveness of intensive therapy based on RCT has not yet been reported. In the present study, we evaluate the cost-effectiveness of intensive insulin therapy for type 2 diabetes based on RCT, the Kumamoto Study, compared with conventional insulin therapy [12,13]. 2. Research design and methods 2.1. E aluation of clinical outcome For economic evaluation, cost consequences analysis based on RCT (the Kumamoto Study) was performed in this study [17,18]. The costs and consequences of intensive insulin therapy for type 2 diabetes, compared with conventional insulin therapy, were computed separately and listed. This analysis makes few assumptions in economic evaluation [17,18]. In evaluating consequences or health outcomes of each treatment modality, RCT was performed. During , a total of 110 patients with insulin-requiring type 2 diabetes were randomly assigned to a multiple insulin injection therapy (MIT) group or to a conventional insulin injection therapy (CIT) group [12,13]. Each group was further divided into two cohorts: the primary-prevention cohort (no retinopathy, and urinary albumin excretion 30 mg/24 h; n=55), and the secondary-intervention cohort (simple retinopathy, and urinary albumin excretion 300 mg/24 h; n=55). Follow-up examinations specific for diabetic complications were performed at the third month and every 6 months
3 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) after the commencement of the study. The endpoints were defined as progression of retinopathy, preproliferative or proliferative retinopathy, photocoagulation, progression of nephropathy, clinical neuropathy, macrovascular complications and diabetes-related death. Progression of retinopathy was defined as changes upward of at least two or more stages in modified Early Treatment Diabetic Retinopathy Study classification in the Kumamoto Study [19 21]. Patients with nephropathy were divided into three stages according to urinary albumin excretion (normoalbuminuria, 30 mg/day; microalbuminuria, mg/day; or albuminuria, 300 mg/day), and the change from any one stage to a higher stage up was defined as progression of nephropathy [12,13,22,23]. Clinical neuropathy was defined as an abnormal neurologic examination that was consistent with the presence of either abnormal somatic-nerve testing (nerve conduction velocities and vibration thresholds) or abnormal autonomic-nerve testing (orthostatic hypotension and the coefficients of variation of R R interval on the electrocardiogram). Macrovascular complications were defined as the appearance of cerebrovascular, cardiovascular, or peripheral vascular disease. Diabetes-related death was defined as death due to myocardial infarction, stroke, peripheral vascular disease, renal disease, hyperglycemia, hypoglycemia or sudden death. The clinical goal of the CIT group was to avoid the development of symptoms related to hyperglycemia or hypoglycemia, and maintain fasting blood glucose concentration (FBG) below 140 mg/dl. The clinical goal of the MIT group was to maintain the FBG below 140 mg/dl, 2-h postprandial blood glucose concentration less than 200 Table 1 Characteristics of patients at baseline a MIT group CIT group Number (M/F) 55 (28/27) 55 (26/29) Age (years) Duration of diabetes (years) Body mass index (kg/m 2 ) a Values presented as mean S.D. mg/dl, hemoglobin A1c below 7.0% and mean amplitude of glycemic excursions below 100 mg/ dl. There were no significant differences at entry between CIT and MIT groups, with regard to age, gender, duration of diabetes, and body mass index, as reported previously (Table 1). After 10 years, 97 patients remained in the study, nine patients died (three in the MIT group and six in the CIT group) and four patients moved to other cities (two in each of the MIT and CIT groups). The reduction in risk for the development and progression of diabetic microvascular and macrovascular complications, and diabetesrelated death in the combined cohort (primaryprevention and secondary-intervention groups) was calculated, and the number of years free from endpoints in the CIT and MIT groups were also calculated based on the 10-year results of the Kumamoto Study E aluation of costs From a viewpoint of the payer (the National Health Insurance), all medical services and costs for MIT and CIT were identified and estimated. This viewpoint considers all direct medical costs relative to diabetes such as those associated with inpatient care, outpatient care, medications, medical equipment, and laboratory tests [17,18]. Capital costs were not evaluated due to the lack of data related to such costs. Direct non-medical costs born by patients and their families, such as the costs of transportation, lodging and family care, were not included. Furthermore, indirect costs such as production losses associated with lost or impaired ability to work or to engage in leisure activities were not included. In this study, the costs of MIT and CIT were based on actual medical services used in Kumamoto Study, excluding research costs. All medical and insurance records of the patients during were collected and analyzed. All costs of medical services were determined based on the uniform reimbursement fee schedule of the Japanese National Heath Insurance in Table 2 summarizes the main sources of information of unit costs. These unit costs were combined with the resource volumes to obtain a net cost per
4 204 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) Table 2 List of unit costs a Outpatient service $ Doctor s fee 8.4/visit Tests Blood Fee for interpretation of 9.2/month hematological examination Fee for interpretation of blood 10.0/month chemistry test Hemoglobin A1c 7.1/month Plasma glucose 1.5/test Serum creatinin 1.5/test Clinical chemistry test 16.7/test Lipid profile 2.3/test Urine Fee for interpretation of urinalysis 2.0/test Urinalysis 2.3/month Urine albumin 18.8/test Urine C-peptide 20.4/test Other tests Funduscopic analysis 9.3/test Fluorescein angiography 33.3/test Fee for interpretation of 9.2/month neurophysiological tests Electromyogram 20.8/test Procedure Venipuncture 1.0/procedure Electrocardiogram 12.5/recording Insulin R (rapid-acting) 5 ample 40.3 N (intermediate-acting) 5 ample 40.3 Administration cost of self-injection 76.7/month Insulin syringe 25.0/month Self-monitoring Once per day 41.7/month Twice per day Three times per day 58.3/month 66.7/month Inpatient service Nursing Nursing assistance ( 30 hospital days) Nursing assistance ( 30 hospital days) Room charge General room (1 14 days) General room (14 days 1 month) General room (1 2 months) 73.0/day 72.0/day 47.8/day 39.5/day 28.0/day Table 2 (Continued) Inpatient service $ Extra charge for specific functional Hp. 8.8/month Diet Cost of basic diet 17.7/test Additional cost for therapeutic diet 2.9/day Tests Basal test ( 21days) 12.1/test Basal test ( 21days) 10.0/test Fee of management and interpretation Fee for test 16.7/month Fee for interpretation of basal test 41.7/month Fee for management of drugs 40.0/month Fee for management of discharge 25.0/patient a This table shows the list of several unit costs in Japan. Inpatient service represents costs specific for inpatients or different from the cost for outpatients. Other costs for inpatients are the same as costs for outpatients. The basal test in inpatients represents the upper limit of the cost for blood chemistry. patient over the entire period in this trial. Costs were calculated in Japanese yen in 1998 and converted to US dollars using the exchange rate of $1=Y 120. In comparing costs between MIT and CIT, first, mean total costs per patient by category of cost were compared. All costs were classified into two categories: treatment and complication costs. Treatment costs included cost of outpatient clinic visits, cost of laboratory tests (blood, urine and other special tests), insulin-related cost (insulin, syringes and management cost for self-injection) and cost of self-monitoring of blood glucose level. On the other hand, complication costs include cost of hospitalization, cost of drugs (drugs for macrovascular and microvascular complications), and cost of specific treatment of ophthalmic complications such as cost of photocoagulation. Second, annual and cumulative costs over time were compared between MIT and CIT. Adjustments for differential timing of costs were made because of the existence of time preference [17,18]. Individuals and a society place a higher value on resources used today than at some point in the future. Therefore, costs were presented both undiscounted, and discounted at a rate of 3% as recommended by the US Panel on Cost-Effectiveness [18], or discounted at a rate of 5% as consistent with current practice [17].
5 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) Fig. 1. Risk reduction rates of microvascular and macrovascular complications, and diabetes-related death by intensive insulin therapy, over 10 years of the Kumamoto Study. The percentages of risk reduction by intensive glycemic control using multiple insulin injection therapy were calculated by subtracting the relative risk of multiple insulin injection therapy compared with conventional insulin injection therapy from one and multiplying by 100. Data represent the mean 95% CI Statistical analysis All analyses and comparisons were performed on the basis of intention to treat. The crude relative risk method and Kaplan Meier method were undertaken to calculate the risk reduction rates of endpoints and the average years free from endpoints, respectively. A t-test was used for the comparison of the annual costs and their unit costs, and the cumulative cost between CIT and MIT groups. In addition, sensitivity analyses were performed to assess the robustness of the results to changes in the values of variables incorporated into the analysis. The sensitivity to changes of complication costs with discounting varied between 0 and 5% were examined. P 0.05 was defined as statistically significant. Values were expressed as mean S.D. and mean differences in costs were expressed with 95% confidence intervals (CI). All analyses and comparisons were performed with the consent of participating subjects, and the study protocol was approved by the Human Ethics Review Committee of our institution. 3. Results 3.1. Clinical outcomes Fig. 1 shows the effect of treatment on the development and progression of diabetic microvascular and macrovascular complications, and diabetes-related death over 10 years. Compared with CIT, MIT reduced the relative risks of progression of retinopathy, preproliferative or proliferative retinopathy, photocoagulation, progression of nephropathy, albuminuria, clinical neuropathy, macrovascular complications and diabetes-related death by 67% (95% CI, 49 79%), 69% (34 86%), 77% (47 90%), 66% (42 80%), 100%, 64% (45 76%), 54% (2 78%) and 81% (28 95%), respectively. Since none of the patients suffered from end-stage renal disease, vision loss or lower extremity amputation during the period of the study, the reduction in these risks could not be evaluated. The years free from endpoints in the CIT and MIT groups, and their differences are shown in Table 3. Intensive insulin therapy prolonged the free period by 2.0 years for progression of retinopathy, 0.2 years for preproliferative or proliferative retinopathy, 0.3 years for photocoagulation, 1.5 years for progression of nephropathy, 2.2 years for clinical neuropathy, 0.3 years for macrovascular complications and 0.2 years for diabetes-related death, compared with CIT. The numbers of years free from preproliferative or proliferative retinopathy, macrovascular complications, and diabetes-related death were not statistically significant between MIT and CIT groups (P=0.06, 0.17 and 0.097, respectively). Discounted at 3 and 5% rates, intensive insulin therapy prolonged free period by 1.4 and 1.1 years for progression of retinopathy, 0.2 and 0.1 years for photocoagulation, 1.1 and 0.8 years for
6 206 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) progression of nephropathy, and 1.5 and 1.1 years for clinical neuropathy, respectively, compared with CIT Costs Treatment and complication costs Table 4 shows the total costs per patient by category of cost over the duration of the study. Treatment costs, such as costs of clinic visits, laboratory tests, and self-monitoring were significantly higher for MIT (by $475, 631 and 3834, respectively) compared with those of CIT. Furthermore, the total treatment costs per patient for MIT was significantly higher (P 0.001) by $6080 (95% CI, $ ) compared with the costs for CIT. In contrast, for costs of complications, CIT was associated with increased costs of hospitalization, drugs, and specific treatment of ophthalmic disorders by $3239, 3419 and 1315, respectively, compared with those under MIT. Total complication costs in the CIT group was $7974 higher than that in MIT (95% CI, $ ; P 0.001). The total cost per patient, representing the combined cost of treatment and complications costs, in MIT was lower than that in CIT ($ versus $36 685). The difference in total cost between CIT and MIT was $1893 (95% CI, $6220 to 2433). Discounted at 3 and 5% rates, the total costs in MIT were still lower than those in CIT, albeit statistically insignificant Costs o er time Fig. 2 shows the annual trend of total costs and costs by category over time. In the first year, the annual total cost per patient was higher in MIT than in CIT ($3260 vs. $2655; P 0.001). However, these costs crossed each other in the fifth year. In the final year of observation, annual total costs ($5760) in CIT were higher ($4462) than in MIT. In CIT, while the treatment costs increased gradually from $2272 to 2555 over time with some fluctuation, complication costs increased more steeply from $383 to On the other hand, in MIT, treatment costs increased slightly from $2807 to 3148 over time, and complication costs increased progressively from $453 to Therefore, the difference in treatment costs between MIT and CIT remained at almost the same level throughout the study period, while that in complication costs widened during the same period. Consequently, as shown in Fig. 3, the cumulative total cost per patient in CIT exceeded that in MIT from the eighth year of the study. Even if the total cost per patient was discounted at the 5% rate, the cumulative total cost per patient in CIT exceeded that in MIT from the ninth year of the study Sensiti ity analysis The presented data suggest that total cost in the CIT and MIT groups was influenced by changes Table 3 Average years free from endpoint complications a MIT CIT P value Differences b Undiscounted 3% Discounted 5% Discounted Progression of retinopathy Preproliferative or proliferative retinopathy Photocoagulation Progression of nephropathy Albuminuria NA NA Clinical neuropathy Macrovascular complications Diabetes-related death a NA, not applicable. The P value indicates the difference of the years free from endpoint complication between MIT and CIT (calculated by log-rank test). b Differences were calculated by subtracting CIT from MIT (mean difference over median 10 years). The discount was not applicable in albuminuria, since there were no patients suffering from albuminuria in MIT during 10 years.
7 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) Table 4 Total costs per patient by category of cost Cost in MIT a Cost in CIT a Cost difference between MIT and CIT b ($/patient) ($/patient) ($/patient) Treatment costs Clinic visits cc 324 to 625 Tests c 165 to 1096 Insulin-related to 2675 Self-monitoring cc 3519 to 4148 Others * 101 to 36 Total cc 3891 to 8268 Complication costs Hospitalization *,cc 4673 to 1805 Drugs *,c 5730 to 1109 Specific treatment for ophthalmic *,cc 1983 to 647 complications Total *,cc to 4694 Total costs of treatment and complications Undiscounted * 6220 to % Discounted * 4832 to % Discounted * 4081 to 2376 a Data presented as mean S.D. b Presented as 95% CI. * Negative cost differences indicate that cost in CIT exceeded that in MIT c P 0.05 of significant difference between MIT and CIT, respectively. cc P of significant difference between MIT and CIT, respectively. in complication costs. Therefore, sensitivity analysis was performed by changing the risk reduction rate of each incidence of major complications (retinopathy, nephropathy and neuropathy) by MIT within the 95% CI. When the relative risk reduction for retinopathy, nephropathy, and neuropathy under MIT relative to CIT changed within the 95% CI, the total costs in MIT (undiscounted) varied in the range $ , and , respectively, in contrast to the total cost in CIT ($36 685). Discounted at the 3% rate, these costs become in the range $ , and , respectively, while total cost in CIT was $ at a rate of 3% discount. Furthermore, discounted at the 5% rate, total costs became $ , and , respectively, while total costs in CIT was $ at 5% discount rate. 4. Discussion The results of this study confirmed and extended the major findings of 6- and 8-year follow-up of the Kumamoto Study that intensive insulin therapy could significantly reduce the risk of diabetic microvascular complications in Japanese patients with type 2 diabetes [12,13]. We also showed that intensive insulin therapy could prolong the period in which the patient is free from endpoint complications such as retinopathy, nephropathy and neuropathy. These findings were in principle consistent with the results of large scale RCTs, DCCT with type 1 diabetes [11] and UKPDS with type 2 diabetes [14]. In our study, cost-effectiveness analysis was performed to evaluate the economic effects of intensive insulin therapy for insulin-requiring type 2 diabetic patients. The results showed that intensive insulin therapy
8 208 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) was not as expensive as we had expected. The total cost (discounted at 3%) per patient throughout a period of 10 years under MIT and CIT was $ and , respectively (Table 4). In MIT, increased treatment costs by 30% was offset by a reduction in complication costs of 50%. It is possible to assume that costs were equal among the two treatment modalities, since statistical significance in the reduction of total costs was not observed. If costs are identical, the largest outcome alternative should be selected, considering the decision matrix for incremental cost and effectiveness of the two programs. It could be called effectiveness-maximization analysis. Intensive insulin therapy is just this example. Therefore, from the viewpoint of the National Health Insurance, intensive insulin therapy is efficient (or value for money) health care technology. We also applied the sensitivity analysis to confirm the robustness of the results. In our study, the risk reduction rate of retinopathy was most potent factor in robustness of total cost for type 2 diabetic patients. For example, decreasing the relative risk reduction of MIT for retinopathy to the lower limit of the 95% CI increased the total costs of MIT from $ to In type 1 diabetes, the DCCT research group estimated the lifetime benefits and costs of intensive insulin therapy for approximately persons in the US who met DCCT eligibility criteria, using a simulation model. They reported that intensive insulin therapy costs $ per year of life gained and $ per quality-adjusted life-year (QALY) gained [15]. Therefore, intensive insulin therapy for type 1 diabetic patients is in the category of technology that has strong evidence for adoption and appropriate utilization [24]. In this model, the DCCT group adopted annual treatment costs for intensive insulin therapy and conventional insulin therapy of $4014 and 1666 per year per patient, respectively [25]. Therefore, the annual treatment cost for intensive insulin therapy in DCCT was more expensive compared with ours, while that of conventional insulin therapy was less expensive than ours. The annual treatment costs in our study were $ in MIT and $ in CIT over 10 years. The differences in annual treatment Fig. 2. Comparison of annual trend of total, treatment and complication costs between patients under intensive insulin therapy and conventional insulin therapy in the Kumamoto Study., Annual total costs in the MIT group;, annual total costs in the CIT group;, annual treatment costs in the MIT group;, annual treatment costs in the CIT group;, annual complication costs in the MIT group;, annual complication costs in the CIT group; broken lines, costs of MIT; solid lines, costs of CIT. Discount was not performed for the values in this figure.
9 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) Fig. 3. Comparison of cumulative costs over a period of 10 years between MIT and CIT groups. (A) Undiscounted, (B) discount at 5%.,, cumulative medical costs of MIT and CIT groups, respectively. cost between DCCT and our study seemed to be mainly due to differences in costs of outpatient services and case management services. In DCCT, the annual cost of outpatient services and case management services was almost three times higher in MIT than in CIT group, while those costs were almost identical in our study [25]. Further analysis showed that differences in the frequency and costs of clinical visits and laboratory tests for glycemic control were also responsible for the difference in annual treatment costs between DCCT and our study. In type 2 diabetes, the economical effects of intensive insulin treatment have not been sufficiently evaluated. Recently, a simulation model based on the DCCT model has been developed and applied to evaluate the cost-effectiveness of intensive insulin therapy in type 2 diabetes [16]. In that simulation study, the treatment cost in MIT increased almost twofold, which was partially offset by a reduction in the complication costs. The incremental cost per QALY was $16 002, which was comparable with the costs reported in type 1 diabetes. In this model, $1099 per year per patient was used for the treatment of patients with standard insulin therapy and $3324 per year per patient for patients with multiple daily insulin injection therapy. In contrast, in our study, the difference of annual treatment costs in CIT and MIT ($2112 and 2720 per year per patient, respectively) was small but statistically significant (calculated from the total treatment costs of 10 years; shown in Table 4). Therefore, the difference in annual treatment costs between conventional insulin therapy and intensive insulin therapy in simulation model might induce incremental cost per QALY gained. The smaller difference between costs of MIT and CIT in Japan than that in the US could be mainly due to the difference in costs of outpatient service including doctor s fee and tests ($730 in US versus $110 in Japan) [25]. It could also be due to the lower costs for treatment of side effects of therapy, since there were no patients who experienced severe hypoglycemia or weight gain greater than 20% in the Kumamoto Study. Although the UKPDS research group reported the cost-effectiveness of tight blood pressure control compared with a less tight control, they could not demonstrate the cost-effectiveness of intensive insulin therapy in type 2 diabetes [26]. Economic analysis of glycemic control study in UKPDS would strengthen our result of the economic effects of intensive therapy using the actual costs in type 2 diabetic patients. In conclusion, these results indicate that intensive insulin therapy in patients with type 2 diabetes could prolong the period in which patients are free from diabetes-related endpoints and improve quality of life, thereby saving money through its beneficial effects on diabetic complications. Therefore, we recommend application of intensive therapy in insulin-requiring type 2 diabetic patients as early as possible.
10 210 N. Wake et al. / Diabetes Research and Clinical Practice 48 (2000) References [1] WHO Study Group on Prevention of Diabetes Mellitus, Prevention of diabetes mellitus, WHO Technical report series, 844, WHO, Geneva, [2] American Diabetes Association, Economic consequences of diabetes mellitus in the U.S. in 1997, Diabetes Care 21 (1998) [3] Ministry of Health and Welfare in Japan, Preliminary Report of Diabetes Mellitus Fact-Finding Survey, Japan Association for Diabetes Care and Education, Tokyo, Japan, [4] Report of Expenditure of Diabetes Mellitus, Ministry of Health and Welfare in Japan, Tokyo, Japan, [5] H. Kishikawa, Y. Hashimoto, Y. Hashiguchi, et al., Insulin treatment regimes for Type II (non-insulin dependent) diabetes mellitus as judged by residual B-cell function, Diabetic Nutr. Metab. 4 (1991) [6] P.J. O Connor, S.J. Spann, S.H. Woolf, Care of adults with type 2 diabetes mellitus, a review of evidence, J. Fam. Pract. 47 (1998) S13 S22. [7] W.H. Herman, Glycaemic control in diabetes, Br. Med. J. 319 (1999) [8] B. Feldt-Rasmussen, E.R. Mathiesen, T. Jensen, T. Lauritzen, T. Deckert, Effect of improved metabolic control on loss of kidney function in Type I (insulin-dependent) diabetic patients, an update of the Steno studies, Diabetologia 34 (1991) [9] The Kroc Collaborative Study Group, Diabetic retinopathy after two years of intensified insulin treatment, J. Am. Med. Assoc. 260 (1988) [10] O. Brinchmann-Hansen, K. Dahl-Jørgensen, L. Sandvik, K.F. Hanssen, Blood glucose concentrations and progression of diabetic retinopathy, the seven year results of the Oslo study, Br. Med. J. 304 (1992) [11] The Diabetes Control and Complications Trial Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus, N. Engl. J. Med. 329 (1993) [12] Y. Ohkubo, H. Kishikawa, E. Araki, et al., Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with noninsulin-dependent diabetes mellitus a randomized prospective 6-year study, Diabetes Res. Clin. Pract. 28 (1995) [13] M. Shichiri, H. Kishikawa, Y. Ohkubo, N. Wake, Longterm results of the Kumamoto Study on optimal diabetes control in Type 2 patients, Diabetes Care (2000) (in press). [14] UKPDS, Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33), Lancet 352 (1998) [15] The Diabetes Control and Complication Trial Research Group, Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complication trial, J. Am. Med. Assoc. 276 (1996) [16] R.C. Eastman, J.C. Javitt, W.H. Herman, et al., Model of complications of NIDDM, II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia, Diabetes Care 20 (1997) [17] M.F. Drummond, B.J. O Brien, G.L. Stoddart, G.W. Torrance, Methods for the Economic Evaluation of Health Care Programmes, 2nd ed., Oxford University Press, Oxford, [18] M.R. Gold, J.E. Siegel, L.B. Russell, M.C. Weinstein, Cost-effectiveness in Health and Medicine, Oxford University Press, New York, [19] Early Treatment Diabetic Retinopathy Study Research Group, Early Treatment Diabetic Retinopathy Study, Manual of Operations, Bethesda, MD, Public Health Service, [20] Early Treatment Diabetic Retinopathy Study Research Group, ETDRS report no. 10: grading diabetic retinopathy from stereoscopic color fundus photographs an extension of the modified Airlie House classification, Ophthalmology 98 (1991) [21] Early Treatment Diabetic Retinopathy Study Research Group, ETDRS report no. 12: fundus photographic risk factors for progression of diabetic retinopathy, Ophthalmology 98 (1991) [22] B. Feldt-Rasmussen, E.R. Mathiesen, T. Jensen, T. Lauritzen, T. Deckert, Effect of improved metabolic control on loss of kidney function in Type I (insulin-dependent) diabetic patients, an update of the Steno studies, Diabetologia 34 (1991) [23] C.E. Mogensen, Management of diabetic renal involvement and disease, Lancet 1 (1988) [24] A. Laupacis, D. Feeny, A.S. Detsky, P.X. Tugwell, How attractive dose a new technology have to be to warrant adoption and utilization?, Chin. Med. Assoc. J. 146 (1992) [25] The Diabetes Control and Complications Trial Research Group, Resource utilization and costs of care in the diabetes control and complications trial, Diabetes Care 18 (1995) [26] UK Prospective Diabetes Study Group, Cost effectiveness analysis of improved blood pressure control if hypertensive patients with type 2 diabetes, UKPDS 40, Br. Med. J. 317 (1998)
Managing Diabetes for Improved Health and Economic Outcomes
 Managing Diabetes for Improved Health and Economic Outcomes Based on a presentation by David McCulloch, MD Presentation Summary The contribution of postprandial glucose to diabetes progression and diabetes-related
Managing Diabetes for Improved Health and Economic Outcomes Based on a presentation by David McCulloch, MD Presentation Summary The contribution of postprandial glucose to diabetes progression and diabetes-related
Improvement of glycemic control and quality-of-life by insulin lispro therapy: Assessing benefits by ITR-QOL questionnaires
 diabetes research and clinical practice 81 (2008) 169 178 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/diabres Improvement of glycemic control and quality-of-life by insulin
diabetes research and clinical practice 81 (2008) 169 178 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/diabres Improvement of glycemic control and quality-of-life by insulin
Source of effectiveness data The effectiveness data were derived from a review or synthesis of completed studies.
 Cost effectiveness of ACE inhibitor treatment for patients with Type 1 diabetes mellitus Dong F B, Sorensen S W, Manninen D L, Thompson T J, Narayan V, Orians C E, Gregg E W, Eastman R C, Dasbach E J,
Cost effectiveness of ACE inhibitor treatment for patients with Type 1 diabetes mellitus Dong F B, Sorensen S W, Manninen D L, Thompson T J, Narayan V, Orians C E, Gregg E W, Eastman R C, Dasbach E J,
A computer simulation model for cost effectiveness analysis of mass screening for Type 2 diabetes mellitus
 Diabetes Research and Clinical Practice 54 Suppl. 1 (2001) S37 S42 www.elsevier.com/locate/diabres A computer simulation model for cost effectiveness analysis of mass screening for Type 2 diabetes mellitus
Diabetes Research and Clinical Practice 54 Suppl. 1 (2001) S37 S42 www.elsevier.com/locate/diabres A computer simulation model for cost effectiveness analysis of mass screening for Type 2 diabetes mellitus
Diabetes Mellitus Type 2 Evidence-Based Drivers
 This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
A factorial randomized trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Hypotheses: Among individuals with type 2 diabetes, the risks of major microvascular
Setting The setting appears to have been secondary care. The economic study was conducted in the USA.
 Cost-effectiveness of use of the GlucoWatch Biographer in children and adolescents with type 1 diabetes: a preliminary analysis based on a randomized controlled trial Eastman R C, Leptien A D, Chase H
Cost-effectiveness of use of the GlucoWatch Biographer in children and adolescents with type 1 diabetes: a preliminary analysis based on a randomized controlled trial Eastman R C, Leptien A D, Chase H
Type of intervention Secondary prevention and treatment; Other (medication coverage policy design).
 Cost-effectiveness of full Medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes Rosen A B, Hamel M B, Weinstein M C, Cutler D M, Fendrick A, Vijan S Record Status
Cost-effectiveness of full Medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes Rosen A B, Hamel M B, Weinstein M C, Cutler D M, Fendrick A, Vijan S Record Status
Microvascular Complications in Diabetes:
 Microvascular Complications in Diabetes: Perspectives on Glycemic Control to Prevent Microvascular Complications Discussion Outline: Glycemia and Microvascular Compliations Clinical Trials - A Brief History
Microvascular Complications in Diabetes: Perspectives on Glycemic Control to Prevent Microvascular Complications Discussion Outline: Glycemia and Microvascular Compliations Clinical Trials - A Brief History
Diabetic Nephropathy. Objectives:
 There are, in truth, no specialties in medicine, since to know fully many of the most important diseases a man must be familiar with their manifestations in many organs. William Osler 1894. Objectives:
There are, in truth, no specialties in medicine, since to know fully many of the most important diseases a man must be familiar with their manifestations in many organs. William Osler 1894. Objectives:
Microvascular Disease in Type 1 Diabetes
 Microvascular Disease in Type 1 Diabetes Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine The Course
Microvascular Disease in Type 1 Diabetes Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine The Course
Ο ρόλος των τριγλυκεριδίων στην παθογένεια των μικροαγγειοπαθητικών επιπλοκών του σακχαρώδη διαβήτη
 Ο ρόλος των τριγλυκεριδίων στην παθογένεια των μικροαγγειοπαθητικών επιπλοκών του σακχαρώδη διαβήτη Κωνσταντίνος Τζιόμαλος Επίκουρος Καθηγητής Παθολογίας Α Προπαιδευτική Παθολογική Κλινική, Νοσοκομείο
Ο ρόλος των τριγλυκεριδίων στην παθογένεια των μικροαγγειοπαθητικών επιπλοκών του σακχαρώδη διαβήτη Κωνσταντίνος Τζιόμαλος Επίκουρος Καθηγητής Παθολογίας Α Προπαιδευτική Παθολογική Κλινική, Νοσοκομείο
Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus
 British Journal of Nutrition (2000), 84, Suppl. 2, S177±S181 S177 Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus Takeshi Kuzuya* JA Shioya General Hospital, Tomita
British Journal of Nutrition (2000), 84, Suppl. 2, S177±S181 S177 Early diagnosis, early treatment and the new diagnostic criteria of diabetes mellitus Takeshi Kuzuya* JA Shioya General Hospital, Tomita
Diabetes is a metabolic disorder primarily
 P O S I T I O N S T A T E M E N T Implications of the United Kingdom Prospective Diabetes Study AMERICAN DIABETES ASSOCIATION Diabetes is a metabolic disorder primarily characterized by elevated blood
P O S I T I O N S T A T E M E N T Implications of the United Kingdom Prospective Diabetes Study AMERICAN DIABETES ASSOCIATION Diabetes is a metabolic disorder primarily characterized by elevated blood
DIABETES MEASURES GROUP OVERVIEW
 2014 PQRS OPTIONS F MEASURES GROUPS: DIABETES MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN DIABETES MEASURES GROUP: #1. Diabetes: Hemoglobin A1c Poor Control #2. Diabetes: Low Density Lipoprotein (LDL-C)
2014 PQRS OPTIONS F MEASURES GROUPS: DIABETES MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN DIABETES MEASURES GROUP: #1. Diabetes: Hemoglobin A1c Poor Control #2. Diabetes: Low Density Lipoprotein (LDL-C)
Diabetes Care 24: , 2001
 Pathophysiology/Complications O R I G I N A L A R T I C L E Prevalence and Significance of Retinopathy in Subjects With Type 1 Diabetes of Less Than 5 Years Duration Screened for the Diabetes Control and
Pathophysiology/Complications O R I G I N A L A R T I C L E Prevalence and Significance of Retinopathy in Subjects With Type 1 Diabetes of Less Than 5 Years Duration Screened for the Diabetes Control and
This article identifies key challenges in incorporating costs
 ARENAS OF APPLICATION Using Costs in Cost-Effectiveness Models for Chronic Diseases Lessons From Diabetes Thomas J. Hoerger, PhD From the RTI-UNC Center of Excellence in Health Promotion Economics, RTI
ARENAS OF APPLICATION Using Costs in Cost-Effectiveness Models for Chronic Diseases Lessons From Diabetes Thomas J. Hoerger, PhD From the RTI-UNC Center of Excellence in Health Promotion Economics, RTI
What s the Goal? Individualizing Glycemic Targets. Matthew Freeby M.D. December 3 rd, 2016
 What s the Goal? Individualizing Glycemic Targets Matthew Freeby M.D. December 3 rd, 2016 Diabetes Mellitus: Complications and Co-Morbid Conditions Retinopathy Between 2005-2008, 28.5% of patients with
What s the Goal? Individualizing Glycemic Targets Matthew Freeby M.D. December 3 rd, 2016 Diabetes Mellitus: Complications and Co-Morbid Conditions Retinopathy Between 2005-2008, 28.5% of patients with
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes
 Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
Obesity, Insulin Resistance, Metabolic Syndrome, and the Natural History of Type 2 Diabetes Genetics, environment, and lifestyle (obesity, inactivity, poor diet) Impaired fasting glucose Decreased β-cell
American Academy of Insurance Medicine
 American Academy of Insurance Medicine October 2012 Dr. Alison Moy Liberty Mutual Dr. John Kirkpatrick Thrivent Financial for Lutherans 1 59 year old male, diagnosed with T2DM six months ago Nonsmoker
American Academy of Insurance Medicine October 2012 Dr. Alison Moy Liberty Mutual Dr. John Kirkpatrick Thrivent Financial for Lutherans 1 59 year old male, diagnosed with T2DM six months ago Nonsmoker
Health technology The use of an angiotensin-converting enzyme (ACE) inhibitor, enalapril, at a dose of 10 mg/day.
 Cost-effectiveness of using angiotensin-converting enzyme inhibitors to slow nephropathy in normotensive patients with diabetes type II and microalbuminuria Sakthong P, Tangphao O, Eiam-Ong S, Kamolratanakul
Cost-effectiveness of using angiotensin-converting enzyme inhibitors to slow nephropathy in normotensive patients with diabetes type II and microalbuminuria Sakthong P, Tangphao O, Eiam-Ong S, Kamolratanakul
The retinal renin-angiotensin system: implications for therapy in diabetic retinopathy
 (2002) 16, S42 S46 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh : implications for therapy in diabetic retinopathy AK Sjølie 1 and N Chaturvedi 2 1 Department
(2002) 16, S42 S46 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh : implications for therapy in diabetic retinopathy AK Sjølie 1 and N Chaturvedi 2 1 Department
Metformin should be considered in all patients with type 2 diabetes unless contra-indicated
 November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
Why is Earlier and More Aggressive Treatment of T2 Diabetes Better?
 Blood glucose (mmol/l) Why is Earlier and More Aggressive Treatment of T2 Diabetes Better? Disclosures Dr Kennedy has provided CME, been on advisory boards or received travel or conference support from:
Blood glucose (mmol/l) Why is Earlier and More Aggressive Treatment of T2 Diabetes Better? Disclosures Dr Kennedy has provided CME, been on advisory boards or received travel or conference support from:
Setting The setting was not explicitly stated. The economic study was carried out in the UK.
 Cost-effectiveness of rosiglitazone combination therapy for the treatment of type 2 diabetes mellitus in the UK Beale S, Bagust A, Shearer A T, Martin A, Hulme L Record Status This is a critical abstract
Cost-effectiveness of rosiglitazone combination therapy for the treatment of type 2 diabetes mellitus in the UK Beale S, Bagust A, Shearer A T, Martin A, Hulme L Record Status This is a critical abstract
How to Reduce CVD Complications in Diabetes?
 How to Reduce CVD Complications in Diabetes? Chaicharn Deerochanawong M.D. Diabetes and Endocrinology Unit Department of Medicine Rajavithi Hospital, Ministry of Public Health Framingham Heart Study 30-Year
How to Reduce CVD Complications in Diabetes? Chaicharn Deerochanawong M.D. Diabetes and Endocrinology Unit Department of Medicine Rajavithi Hospital, Ministry of Public Health Framingham Heart Study 30-Year
Diabetes: What is the scope of the problem?
 Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Study population The study population comprised type 1 and 2 diabetic patients without renal complications.
 Diabetes nephropathy in the Netherlands: a cost effectiveness analysis of national clinical guidelines van Os N, Niessen L W, Bilo H J, Casparie A F, van Hout B A Record Status This is a critical abstract
Diabetes nephropathy in the Netherlands: a cost effectiveness analysis of national clinical guidelines van Os N, Niessen L W, Bilo H J, Casparie A F, van Hout B A Record Status This is a critical abstract
Page # 1. Objectives. History of Clinical Trials. Developing New Therapies Clinical Studies and Trials. FDA Classification of Clinical Studies/Trials
 Developing New Therapies Clinical Studies and Trials Richard C. Eastman M.D. Objectives Discuss how new treatments are studied in humans. Explain how the rights of human volunteers are safeguarded. Discuss
Developing New Therapies Clinical Studies and Trials Richard C. Eastman M.D. Objectives Discuss how new treatments are studied in humans. Explain how the rights of human volunteers are safeguarded. Discuss
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus. Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre
 Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
Chapter 37: Exercise Prescription in Patients with Diabetes
 Chapter 37: Exercise Prescription in Patients with Diabetes American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Chapter 37: Exercise Prescription in Patients with Diabetes American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Update on Diabetes. Ketan Dhatariya. Why it s Not Just About Glucose Lowering Any More. Consultant in Diabetes NNUH
 Update on Diabetes Why it s Not Just About Glucose Lowering Any More Ketan Dhatariya Consultant in Diabetes NNUH The Story So Far.. DCCT Retinopathy Neuropathy Nephropathy Intensive glucose control in
Update on Diabetes Why it s Not Just About Glucose Lowering Any More Ketan Dhatariya Consultant in Diabetes NNUH The Story So Far.. DCCT Retinopathy Neuropathy Nephropathy Intensive glucose control in
Prevention And Treatment of Diabetic Nephropathy. MOH Clinical Practice Guidelines 3/2006 Dr Stephen Chew Tec Huan
 Prevention And Treatment of Diabetic Nephropathy MOH Clinical Practice Guidelines 3/2006 Dr Stephen Chew Tec Huan Prevention Tight glucose control reduces the development of diabetic nephropathy Progression
Prevention And Treatment of Diabetic Nephropathy MOH Clinical Practice Guidelines 3/2006 Dr Stephen Chew Tec Huan Prevention Tight glucose control reduces the development of diabetic nephropathy Progression
Cost-effectiveness ratios are commonly used to
 ... HEALTH ECONOMICS... Application of Cost-Effectiveness Analysis to Multiple Products: A Practical Guide Mohan V. Bala, PhD; and Gary A. Zarkin, PhD The appropriate interpretation of cost-effectiveness
... HEALTH ECONOMICS... Application of Cost-Effectiveness Analysis to Multiple Products: A Practical Guide Mohan V. Bala, PhD; and Gary A. Zarkin, PhD The appropriate interpretation of cost-effectiveness
The Burden of the Diabetic Heart
 The Burden of the Diabetic Heart Dr. Ghaida Kaddaha (MBBS, MRCP-UK, FRCP-london) Diabetes Unit Rashid Hospital Dubai U.A.E Risk of CVD in Diabetes Morbidity and mortality from CVD is 2-4 fold higher than
The Burden of the Diabetic Heart Dr. Ghaida Kaddaha (MBBS, MRCP-UK, FRCP-london) Diabetes Unit Rashid Hospital Dubai U.A.E Risk of CVD in Diabetes Morbidity and mortality from CVD is 2-4 fold higher than
Setting The setting was a hospital. The economic study was conducted in the USA.
 Incremental cost-effectiveness of laser therapy for visual loss secondary to branch retinal vein occlusion Brown G C, Brown M M, Sharma S, Busbee B, Brown H Record Status This is a critical abstract of
Incremental cost-effectiveness of laser therapy for visual loss secondary to branch retinal vein occlusion Brown G C, Brown M M, Sharma S, Busbee B, Brown H Record Status This is a critical abstract of
Clinical Research and Methods. Vol. 37, No. 2
 Clinical Research and Methods Vol. 37, No. 2 125 Glycemic Control and the Risk of Multiple Microvascular Diabetic Complications Kenneth G. Schellhase, MD, MPH; Thomas D. Koepsell, MD, MPH; Noel S. Weiss,
Clinical Research and Methods Vol. 37, No. 2 125 Glycemic Control and the Risk of Multiple Microvascular Diabetic Complications Kenneth G. Schellhase, MD, MPH; Thomas D. Koepsell, MD, MPH; Noel S. Weiss,
Clinical impact and health economic consequences of post-transplant type 2 diabetes mellitus Chilcott J B, Whitby S M, Moore R
 Clinical impact and health economic consequences of post-transplant type 2 diabetes mellitus Chilcott J B, Whitby S M, Moore R Record Status This is a critical abstract of an economic evaluation that meets
Clinical impact and health economic consequences of post-transplant type 2 diabetes mellitus Chilcott J B, Whitby S M, Moore R Record Status This is a critical abstract of an economic evaluation that meets
Type of intervention Primary prevention; secondary prevention. Economic study type Cost-effectiveness analysis and cost utility analysis.
 A predictive model of the health benefits and cost effectiveness of celiprolol and atenolol in primary prevention of cardiovascular disease in hypertensive patients Milne R J, Hoorn S V, Jackson R T Record
A predictive model of the health benefits and cost effectiveness of celiprolol and atenolol in primary prevention of cardiovascular disease in hypertensive patients Milne R J, Hoorn S V, Jackson R T Record
A Primer on Health Economics & Integrating Findings from Clinical Trials into Health Technology Assessments and Decision Making
 Presenter: Chris Cameron CANNeCTIN November 8, 2013 A Primer on Health Economics & Integrating Findings from Clinical Trials into Health Technology Assessments and Decision Making Acknowledgements Vanier
Presenter: Chris Cameron CANNeCTIN November 8, 2013 A Primer on Health Economics & Integrating Findings from Clinical Trials into Health Technology Assessments and Decision Making Acknowledgements Vanier
Session 6. Evaluating the Cost of Pharmaceuticals
 Drug and Therapeutics Committee Training Course Session 6. Evaluating the Cost of Pharmaceuticals Trainer s Guide Drug and Therapeutics Committee Training Course Trainer s Guide This document was made
Drug and Therapeutics Committee Training Course Session 6. Evaluating the Cost of Pharmaceuticals Trainer s Guide Drug and Therapeutics Committee Training Course Trainer s Guide This document was made
Health technology The use of the antihypertensive drug losartan for the prevention of stroke.
 Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial Jonsson B, Carides G W, Burke T A, Dasbach E J, Lindholm L H, Dahlof B Record Status
Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial Jonsson B, Carides G W, Burke T A, Dasbach E J, Lindholm L H, Dahlof B Record Status
Course Instructors: Mark S. Roberts, MD, MPP Ken Smith, MD, MS Stanley Kuo, MS
 CLRES 2120/HSADM 2220 Cost Effectiveness Analysis 9/3 10/01 2009 (M, Th 3:00 5:00) PKVL 305A Phone contact: 692 4826 Course Instructors: Mark S. Roberts, MD, MPP Ken Smith, MD, MS Stanley Kuo, MS Email
CLRES 2120/HSADM 2220 Cost Effectiveness Analysis 9/3 10/01 2009 (M, Th 3:00 5:00) PKVL 305A Phone contact: 692 4826 Course Instructors: Mark S. Roberts, MD, MPP Ken Smith, MD, MS Stanley Kuo, MS Email
Renal Protection Staying on Target
 Update Staying on Target James Barton, MD, FRCPC As presented at the University of Saskatchewan's Management of Diabetes & Its Complications (May 2004) Gwen s case Gwen, 49, asks you to take on her primary
Update Staying on Target James Barton, MD, FRCPC As presented at the University of Saskatchewan's Management of Diabetes & Its Complications (May 2004) Gwen s case Gwen, 49, asks you to take on her primary
An Evaluation of Cost Sharing to Finance a Diet and Physical Activity Intervention to Prevent Diabetes
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E An Evaluation of Cost Sharing to Finance a Diet and Physical Activity Intervention to Prevent Diabetes RONALD T. ACKERMANN,
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E An Evaluation of Cost Sharing to Finance a Diet and Physical Activity Intervention to Prevent Diabetes RONALD T. ACKERMANN,
Update on CVD and Microvascular Complications in T2D
 Update on CVD and Microvascular Complications in T2D Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine
Update on CVD and Microvascular Complications in T2D Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of Miami Miller School of Medicine
Diabetes Day for Primary Care Clinicians Advances in Diabetes Care
 Diabetes Day for Primary Care Clinicians Advances in Diabetes Care Elliot Sternthal, MD, FACP, FACE Chair New England AACE Diabetes Day Planning Committee Welcome and Introduction This presentation will:
Diabetes Day for Primary Care Clinicians Advances in Diabetes Care Elliot Sternthal, MD, FACP, FACE Chair New England AACE Diabetes Day Planning Committee Welcome and Introduction This presentation will:
ESC GUIDELINES ON DIABETES AND CARDIOVASCULAR DISEASES
 ESC GUIDELINES ON DIABETES AND CARDIOVASCULAR DISEASES Pr. Michel KOMAJDA Institute of Cardiology - IHU ICAN Pitie Salpetriere Hospital - University Pierre and Marie Curie, Paris (France) DEFINITION A
ESC GUIDELINES ON DIABETES AND CARDIOVASCULAR DISEASES Pr. Michel KOMAJDA Institute of Cardiology - IHU ICAN Pitie Salpetriere Hospital - University Pierre and Marie Curie, Paris (France) DEFINITION A
Kidney and heart: dangerous liaisons. Luis M. RUILOPE (Madrid, Spain)
 Kidney and heart: dangerous liaisons Luis M. RUILOPE (Madrid, Spain) Type 2 diabetes and renal disease: impact on cardiovascular outcomes The "heavyweights" of modifiable CVD risk factors Hypertension
Kidney and heart: dangerous liaisons Luis M. RUILOPE (Madrid, Spain) Type 2 diabetes and renal disease: impact on cardiovascular outcomes The "heavyweights" of modifiable CVD risk factors Hypertension
The Diabetes Link to Heart Disease
 The Diabetes Link to Heart Disease Anthony Abe DeSantis, MD September 18, 2015 University of WA Division of Metabolism, Endocrinology and Nutrition Oswald Toosweet Case #1 68 yo M with T2DM Diagnosed DM
The Diabetes Link to Heart Disease Anthony Abe DeSantis, MD September 18, 2015 University of WA Division of Metabolism, Endocrinology and Nutrition Oswald Toosweet Case #1 68 yo M with T2DM Diagnosed DM
Analysis for the Improvement of Inadequate Glycemic Control in Patients with Type 2 Diabetes Mellitus in Nagano, Japan
 Shinshu Med J, 66⑸:319~324, 2018 Analysis for the Improvement of Inadequate Glycemic Control in Patients with Type 2 Diabetes Mellitus in Nagano, Japan Ai Sato 1 ), Yoshihiko Sato 1)*, Yuki Kobayashi 1)
Shinshu Med J, 66⑸:319~324, 2018 Analysis for the Improvement of Inadequate Glycemic Control in Patients with Type 2 Diabetes Mellitus in Nagano, Japan Ai Sato 1 ), Yoshihiko Sato 1)*, Yuki Kobayashi 1)
Long-Term Visual Outcome in Proliferative Diabetic Retinopathy Patients After Panretinal Photocoagulation
 Long-Term Visual Outcome in Proliferative Diabetic Retinopathy Patients After Panretinal Photocoagulation Murat Dogru, Makoto Nakamura, Masanori Inoue and Misao Yamamoto Department of Ophthalmology, Kobe
Long-Term Visual Outcome in Proliferative Diabetic Retinopathy Patients After Panretinal Photocoagulation Murat Dogru, Makoto Nakamura, Masanori Inoue and Misao Yamamoto Department of Ophthalmology, Kobe
Welcome and Introduction
 Welcome and Introduction This presentation will: Define obesity, prediabetes, and diabetes Discuss the diagnoses and management of obesity, prediabetes, and diabetes Explain the early risk factors for
Welcome and Introduction This presentation will: Define obesity, prediabetes, and diabetes Discuss the diagnoses and management of obesity, prediabetes, and diabetes Explain the early risk factors for
Diabetes. Health Care Disparities: Medical Evidence. A Constellation of Complications. Every 24 hours.
 Health Care Disparities: Medical Evidence Diabetes Effects 2.8 Million People in US 7% of the US Population Sixth Leading Cause of Death Kenneth J. Steier, DO, MBA, MPH, MHA, MGH Dean of Clinical Education
Health Care Disparities: Medical Evidence Diabetes Effects 2.8 Million People in US 7% of the US Population Sixth Leading Cause of Death Kenneth J. Steier, DO, MBA, MPH, MHA, MGH Dean of Clinical Education
Glucose and CV disease
 Glucose and CV disease Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic,
Glucose and CV disease Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic,
Evaluation of a mass influenza vaccination campaign Takahashi H, Tanaka Y, Ohyama T, Sunagawa T, Nakashima K, Schmid G P, Okabe N
 Evaluation of a mass influenza vaccination campaign Takahashi H, Tanaka Y, Ohyama T, Sunagawa T, Nakashima K, Schmid G P, Okabe N Record Status This is a critical abstract of an economic evaluation that
Evaluation of a mass influenza vaccination campaign Takahashi H, Tanaka Y, Ohyama T, Sunagawa T, Nakashima K, Schmid G P, Okabe N Record Status This is a critical abstract of an economic evaluation that
Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care
 Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
Quality ID #119 (NQF 0062): Diabetes: Medical Attention for Nephropathy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process
2/20/2012. New Technology #1. The Horizon of New Health Technologies. Introduction to Economic Evaluation
 Introduction to Economic Evaluation Sean D. Sullivan, PhD Professor and Director Pharmaceutical Outcomes Research and Policy Program University of Washington The Horizon of New Health Technologies Diagnostics:
Introduction to Economic Evaluation Sean D. Sullivan, PhD Professor and Director Pharmaceutical Outcomes Research and Policy Program University of Washington The Horizon of New Health Technologies Diagnostics:
A pilot Study of 25-Hydroxy Vitamin D in Egyptian Diabetic Patients with Diabetic Retinopathy
 A pilot Study of 25-Hydroxy Vitamin D in Egyptian Diabetic Patients with Diabetic Retinopathy El-Orabi HA 1, Halawa MR 1, Abd El-Salam MM 1, Eliewa TF 2 and Sherif NSE 1 Internal Medicine and Endocrinology
A pilot Study of 25-Hydroxy Vitamin D in Egyptian Diabetic Patients with Diabetic Retinopathy El-Orabi HA 1, Halawa MR 1, Abd El-Salam MM 1, Eliewa TF 2 and Sherif NSE 1 Internal Medicine and Endocrinology
Meaghan St Charles, PhD, 1 Peter Lynch, MPH, 2 Claudia Graham, PhD, 3 Michael E. Minshall, MPH 1. Introduction. Methods
 Volume 12 Number 5 2009 VALUE IN HEALTH A Cost-Effectiveness Analysis of Continuous Subcutaneous Insulin Injection versus Multiple Daily Injections in Type 1 Diabetes Patients: A Third-Party US Payer Perspectivevhe_478
Volume 12 Number 5 2009 VALUE IN HEALTH A Cost-Effectiveness Analysis of Continuous Subcutaneous Insulin Injection versus Multiple Daily Injections in Type 1 Diabetes Patients: A Third-Party US Payer Perspectivevhe_478
The DiSC assay: a cost-effective guide to treatment for chronic lymphocytic leukemia? Mason J M, Drummond M F, Bosanquet A G, Sheldon T A
 The DiSC assay: a cost-effective guide to treatment for chronic lymphocytic leukemia? Mason J M, Drummond M F, Bosanquet A G, Sheldon T A Record Status This is a critical abstract of an economic evaluation
The DiSC assay: a cost-effective guide to treatment for chronic lymphocytic leukemia? Mason J M, Drummond M F, Bosanquet A G, Sheldon T A Record Status This is a critical abstract of an economic evaluation
Impact of Glycemic Control on Survival of Diabetic Patients on Chronic Regular Hemodialysis
 Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Impact of Glycemic Control on Survival of Diabetic Patients on Chronic Regular Hemodialysis A 7-year observational study TAKESHI OOMICHI,
Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Impact of Glycemic Control on Survival of Diabetic Patients on Chronic Regular Hemodialysis A 7-year observational study TAKESHI OOMICHI,
Diabetes Guidelines in View of Recent Clinical Trials Are They Still Applicable?
 Diabetes Guidelines in View of Recent Clinical Trials Are They Still Applicable? Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of
Diabetes Guidelines in View of Recent Clinical Trials Are They Still Applicable? Jay S. Skyler, MD, MACP Division of Endocrinology, Diabetes, and Metabolism and Diabetes Research Institute University of
Blood Pressure Treatment Goals
 Blood Pressure Treatment Goals Kenneth Izuora, MD, MBA, FACE Associate Professor UNLV School of Medicine November 18, 2017 Learning Objectives Discuss the recent studies on treating hypertension Review
Blood Pressure Treatment Goals Kenneth Izuora, MD, MBA, FACE Associate Professor UNLV School of Medicine November 18, 2017 Learning Objectives Discuss the recent studies on treating hypertension Review
DR as a Biomarker for Systemic Vascular Complications
 DR as a Biomarker for Systemic Vascular Complications Lihteh Wu MD Asociados de Mácula, Vítreo y Retina de Costa Rica San José, Costa Rica LW65@cornell.edu Disclosures Dr Wu has received lecture fees from
DR as a Biomarker for Systemic Vascular Complications Lihteh Wu MD Asociados de Mácula, Vítreo y Retina de Costa Rica San José, Costa Rica LW65@cornell.edu Disclosures Dr Wu has received lecture fees from
Diabetes Overview. How Food is Digested
 Diabetes Overview You are The Teacher, The Coach and the Fan Pathophysiology of Diabetes Complications Know the Numbers Treatment Can Good Control Make a Difference? Can Tight Control Be too Tight? How
Diabetes Overview You are The Teacher, The Coach and the Fan Pathophysiology of Diabetes Complications Know the Numbers Treatment Can Good Control Make a Difference? Can Tight Control Be too Tight? How
CTAF Overview. Agenda. Evidence Review. Insulin Degludec (Tresiba, Novo Nordisk) for the Treatment of Diabetes
 CTAF Overview Insulin Degludec (Tresiba, Novo Nordisk) for the Treatment of Diabetes February 12, 2016 Core program of the Institute for Clinical and Economic Review (ICER) Goal: Help patients, clinicians,
CTAF Overview Insulin Degludec (Tresiba, Novo Nordisk) for the Treatment of Diabetes February 12, 2016 Core program of the Institute for Clinical and Economic Review (ICER) Goal: Help patients, clinicians,
Link between effectiveness and cost data The effectiveness and cost data came from the same sample of patients and were prospectively evaluated.
 Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS) Raikou M, McGuire A, Colhoun
Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS) Raikou M, McGuire A, Colhoun
Modified version focused on CCNC Quality Measures and Feedback Processes
 Executive Summary: Standards of Medical Care in Diabetes 2010 Modified version focused on CCNC Quality Measures and Feedback Processes See http://care.diabetesjournals.org/content/33/supplement_1/s11.full
Executive Summary: Standards of Medical Care in Diabetes 2010 Modified version focused on CCNC Quality Measures and Feedback Processes See http://care.diabetesjournals.org/content/33/supplement_1/s11.full
Cost-effectiveness of pravastatin for primary prevention of coronary artery disease in Japan Nagata-Kobayashi S, Shimbo T, Matsui K, Fukui T
 Cost-effectiveness of pravastatin for primary prevention of coronary artery disease in Japan Nagata-Kobayashi S, Shimbo T, Matsui K, Fukui T Record Status This is a critical abstract of an economic evaluation
Cost-effectiveness of pravastatin for primary prevention of coronary artery disease in Japan Nagata-Kobayashi S, Shimbo T, Matsui K, Fukui T Record Status This is a critical abstract of an economic evaluation
Eugene Barrett M.D., Ph.D. University of Virginia 6/18/2007. Diagnosis and what is it Glucose Tolerance Categories FPG
 Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
TYPE 2 DIABETES IS A MAJOR
 ORIGINAL CONTRIBUTION Cost-effectiveness of Intensive Glycemic Control, Intensified Hypertension Control, and Serum Cholesterol Level Reduction for Type 2 Diabetes The CDC Diabetes Cost-effectiveness Group
ORIGINAL CONTRIBUTION Cost-effectiveness of Intensive Glycemic Control, Intensified Hypertension Control, and Serum Cholesterol Level Reduction for Type 2 Diabetes The CDC Diabetes Cost-effectiveness Group
DCCT Diabetes Control & Complications Trial
 DCCT Diabetes Control & Complications Trial A. Vinik MD, PhD, FCP, MACP, FACE, Καθηγητής και Αντιπρόεδρος για την Έρευνα, Ιατρική Σχολή Ανατολικής Βιρτζίνια, Κέντρο Ενδοκρινολογικών και Μεταβολικών Νοσημάτων,
DCCT Diabetes Control & Complications Trial A. Vinik MD, PhD, FCP, MACP, FACE, Καθηγητής και Αντιπρόεδρος για την Έρευνα, Ιατρική Σχολή Ανατολικής Βιρτζίνια, Κέντρο Ενδοκρινολογικών και Μεταβολικών Νοσημάτων,
Disclosures. Diabetes and Cardiovascular Risk Management. Learning Objectives. Atherosclerotic Cardiovascular Disease
 Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Moving to an A1C-Based Screening & Diagnosis of Diabetes. By Prof.M.Assy Diabetes&Endocrinology unit
 Moving to an A1C-Based Screening & Diagnosis of Diabetes By Prof.M.Assy Diabetes&Endocrinology unit is the nonenzymatic glycated product of the hemoglobin beta-chain at the valine terminal residue. Clin
Moving to an A1C-Based Screening & Diagnosis of Diabetes By Prof.M.Assy Diabetes&Endocrinology unit is the nonenzymatic glycated product of the hemoglobin beta-chain at the valine terminal residue. Clin
ADVANCE post trial ObservatioNal Study
 Hot Topics in Diabetes 50 th EASD, Vienna 2014 ADVANCE post trial ObservatioNal Study Sophia Zoungas The George Institute The University of Sydney Rationale and Study Design Sophia Zoungas The George Institute
Hot Topics in Diabetes 50 th EASD, Vienna 2014 ADVANCE post trial ObservatioNal Study Sophia Zoungas The George Institute The University of Sydney Rationale and Study Design Sophia Zoungas The George Institute
Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes
 PRESS RELEASE Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes Dublin, Ireland (15 June 2012) Sanofi presented results
PRESS RELEASE Sanofi Announces Results of ORIGIN, the World s Longest and Largest Randomised Clinical Trial in Insulin in Pre- and Early Diabetes Dublin, Ireland (15 June 2012) Sanofi presented results
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness
 Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
LIRAGLUTIDE VS EXENATIDE IN COMBINATION WITH METFORMIN AND/OR SULFONYLUREA THERAPY IN TYPE 2 DIABETES MELLITUS THERAPY IN BULGARIA. A MODELLING STUDY.
 LIRAGLUTIDE VS EXENATIDE IN COMBINATION WITH METFORMIN AND/OR SULFONYLUREA THERAPY IN TYPE 2 DIABETES MELLITUS THERAPY IN BULGARIA. A MODELLING STUDY. Petrova Guenka, Anna Ivanova, Marcin Czech, Vasil
LIRAGLUTIDE VS EXENATIDE IN COMBINATION WITH METFORMIN AND/OR SULFONYLUREA THERAPY IN TYPE 2 DIABETES MELLITUS THERAPY IN BULGARIA. A MODELLING STUDY. Petrova Guenka, Anna Ivanova, Marcin Czech, Vasil
Why Do We Care About Prediabetes?
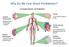 Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Cost Effectiveness of canagliflozin (Invokana )
 Cost Effectiveness of canagliflozin (Invokana ) for adults with type 2 diabetes mellitus to improve glycaemic control as monotherapy or add-on therapy with other anti-hyperglycaemic agents including insulin,
Cost Effectiveness of canagliflozin (Invokana ) for adults with type 2 diabetes mellitus to improve glycaemic control as monotherapy or add-on therapy with other anti-hyperglycaemic agents including insulin,
Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone
 Index Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication GAD glutamic acid decarboxylase GLP-1 glucagon-like peptide 1 NPH neutral
Index Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication GAD glutamic acid decarboxylase GLP-1 glucagon-like peptide 1 NPH neutral
Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism Perlroth D J, Sanders G D, Gould M K
 Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism Perlroth D J, Sanders G D, Gould M K Record Status This is a critical abstract of an economic evaluation that meets
Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism Perlroth D J, Sanders G D, Gould M K Record Status This is a critical abstract of an economic evaluation that meets
Setting The setting was primary and secondary care. The economic study was carried out in Canada.
 Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective Tarride J E, Gordon A, Vera-Llonch
Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective Tarride J E, Gordon A, Vera-Llonch
Complications in IDDM are caused by elevated blood glucose level: The Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up
 Diabetologia (1996) 39: 1483 1488 Springer-Verlag 1996 Complications in IDDM are caused by elevated blood glucose level: The Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up P. Reichard
Diabetologia (1996) 39: 1483 1488 Springer-Verlag 1996 Complications in IDDM are caused by elevated blood glucose level: The Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up P. Reichard
ICD-10CM, HCC and Risk Adjustment Factor
 ICD-10CM, HCC and Risk Adjustment Factor Not everyone is aware of what CMs calls the risk adjustment model. It was developed under the Patient Protection and Affordable Care Act (also known as the PACA)
ICD-10CM, HCC and Risk Adjustment Factor Not everyone is aware of what CMs calls the risk adjustment model. It was developed under the Patient Protection and Affordable Care Act (also known as the PACA)
The cost-effectiveness of expanded testing for primary HIV infection Coco A
 The cost-effectiveness of expanded testing for primary HIV infection Coco A Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
The cost-effectiveness of expanded testing for primary HIV infection Coco A Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
Clinical Practice Guideline Key Points
 Clinical Practice Guideline Key Points Clinical Practice Guideline 2008 Key Points Diabetes Mellitus Provided by: Highmark Endocrinology Clinical Quality Improvement Committee In accordance with Highmark
Clinical Practice Guideline Key Points Clinical Practice Guideline 2008 Key Points Diabetes Mellitus Provided by: Highmark Endocrinology Clinical Quality Improvement Committee In accordance with Highmark
Diabetologia. Coping with Type 11 diabetes: the patient's perspective. Abstract
 Diabetologia (2002) 45:S 18-S22 DOI 1O.1007/s00125-002-0861-2 Diabetologia Coping with Type 11 diabetes: the patient's perspective M. Koopmanschap* Institute for Medical Technology Assessment, Erasmus
Diabetologia (2002) 45:S 18-S22 DOI 1O.1007/s00125-002-0861-2 Diabetologia Coping with Type 11 diabetes: the patient's perspective M. Koopmanschap* Institute for Medical Technology Assessment, Erasmus
Ischemic Heart and Cerebrovascular Disease. Harold E. Lebovitz, MD, FACE Kathmandu November 2010
 Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Type 2 diabetes affects 18% to 20% of people more
 Reduction in Use of Healthcare Services With Combination Sulfonylurea and Rosiglitazone: Findings From the Rosiglitazone Early vs SULfonylurea Titration (RESULT) Study William H. Herman, MD, MPH; Riad
Reduction in Use of Healthcare Services With Combination Sulfonylurea and Rosiglitazone: Findings From the Rosiglitazone Early vs SULfonylurea Titration (RESULT) Study William H. Herman, MD, MPH; Riad
Setting The setting was primary care. The economic study was carried out in the UK and the USA.
 Cost-effectiveness of losartan-based therapy in patients with hypertension and left ventricular hypertrophy: a UK-based economic evaluation of the Losartan Intervention For Endpoint Reduction in Hypertension
Cost-effectiveness of losartan-based therapy in patients with hypertension and left ventricular hypertrophy: a UK-based economic evaluation of the Losartan Intervention For Endpoint Reduction in Hypertension
The Open Hypertension Journal, 2015, 7, 1-6 1
 Send Orders for Reprints to reprints@benthamscience.ae The Open Hypertension Journal, 2015, 7, 1-6 1 Open Access Home Blood Pressure Measurement in the Morning Related to Cancer in Patients with Type 2
Send Orders for Reprints to reprints@benthamscience.ae The Open Hypertension Journal, 2015, 7, 1-6 1 Open Access Home Blood Pressure Measurement in the Morning Related to Cancer in Patients with Type 2
Complications of Diabetes: Screening and Prevention
 Complications of Diabetes: Screening and Prevention Dr Steve Cleland Consultant Physician GGH and QEUH Diabetes Staff Education Course June 17 Diabetic Complications Microvascular: Retinopathy Nephropathy
Complications of Diabetes: Screening and Prevention Dr Steve Cleland Consultant Physician GGH and QEUH Diabetes Staff Education Course June 17 Diabetic Complications Microvascular: Retinopathy Nephropathy
Diabetes Mellitus: Evaluation and Care Management
 Diabetes Mellitus: Evaluation and Care Management Michael King, MD Assistant Professor Residency Program Director University of Kentucky Dept. of Family & Community Medicine Learning Objectives 1. Review
Diabetes Mellitus: Evaluation and Care Management Michael King, MD Assistant Professor Residency Program Director University of Kentucky Dept. of Family & Community Medicine Learning Objectives 1. Review
Diabetes Control and Complications in Public Hospitals in Malaysia
 ORIGINAL ARTICLE Diabetes Control and Complications in Public Hospitals in Malaysia Mafauzy M. FRCP For the Diabcare-Malaysia Study Group, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian,
ORIGINAL ARTICLE Diabetes Control and Complications in Public Hospitals in Malaysia Mafauzy M. FRCP For the Diabcare-Malaysia Study Group, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian,
Study of Impact of Serum Uric Acid in Type 2 Diabetic Patients And Its Relationship with Development of Complications
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 3 Ver. V (March. 2017), PP 58-62 www.iosrjournals.org Study of Impact of Serum Uric Acid in
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 3 Ver. V (March. 2017), PP 58-62 www.iosrjournals.org Study of Impact of Serum Uric Acid in
Summary 1. Comparative effectiveness
 Cost-effectiveness of Sacubitril/Valsartan (Entresto) for the treatment of symptomatic chronic heart failure in adult patients with reduced ejection fraction. The NCPE has issued a recommendation regarding
Cost-effectiveness of Sacubitril/Valsartan (Entresto) for the treatment of symptomatic chronic heart failure in adult patients with reduced ejection fraction. The NCPE has issued a recommendation regarding
Diabete: terapia nei pazienti a rischio cardiovascolare
 Diabete: terapia nei pazienti a rischio cardiovascolare Giorgio Sesti Università Magna Graecia di Catanzaro Cardiovascular mortality in relation to diabetes mellitus and a prior MI: A Danish Population
Diabete: terapia nei pazienti a rischio cardiovascolare Giorgio Sesti Università Magna Graecia di Catanzaro Cardiovascular mortality in relation to diabetes mellitus and a prior MI: A Danish Population
