Multi-modality Treatment Approaches to RVO-associated Macular Edema
|
|
|
- Charlene Warner
- 5 years ago
- Views:
Transcription
1 April 2014 Multi-modality Treatment Approaches to RVO-associated Macular Edema Expert insights into the importance of utilizing all of the available options Diagnosis and Monitoring of RVO and Secondary Macular Edema Reframing How We Think About Treatment for RVO Macular Laser in the RVO Treatment Paradigm Dexamethasone Intravitreal Implant Following RVO: Rescue, Adjunct or Primary Therapy?
2 For macular edema following RVO* Less here we go again. *Branch or central retinal vein occlusion. Indications and Usage Retinal Vein Occlusion: OZURDEX (dexamethasone intravitreal implant) is a corticosteroid indicated for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO). Posterior Segment Uveitis: OZURDEX is indicated for the treatment of noninfectious uveitis affecting the posterior segment of the eye. Dosage and Administration FOR OPHTHALMIC INTRAVITREAL INJECTION ONLY. The intravitreal injection procedure should be carried out under controlled aseptic conditions. Following the intravitreal injection, patients should be monitored for elevation in intraocular pressure and for endophthalmitis. Patients should be instructed to report any symptoms suggestive of endophthalmitis without delay. IMPORTANT SAFETY INFORMATION Contraindications Ocular or Periocular Infections: OZURDEX (dexamethasone intravitreal implant) is contraindicated in patients with active or suspected ocular or periocular infections including most viral diseases of the cornea and conjunctiva, including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections, and fungal diseases. Advanced Glaucoma: OZURDEX (dexamethasone intravitreal implant) is contraindicated in patients with advanced glaucoma. Aphakic Eyes with Rupture of the Posterior Lens Capsule: OZURDEX is contraindicated in patients who have aphakic eyes with rupture of the posterior lens capsule. ACIOL and Rupture of the Posterior Lens Capsule: OZURDEX is contraindicated in eyes with ACIOL (Anterior Chamber Intraocular Lens) and rupture of the posterior lens capsule. Hypersensitivity: OZURDEX is contraindicated in patients with known hypersensitivity to any components of this product. Warnings and Precautions Intravitreal Injection-related Effects: Intravitreal injections have been associated with endophthalmitis, eye inflammation, increased intraocular pressure, and retinal detachments. Patients should be monitored regularly following the injection.
3 When OCT reveals macular edema that persists or recurs in RVO, consider inflammation Inject OZURDEX (dexamethasone intravitreal implant) to help improve visual acuity 1 OCT images 2013, Dr. Szilárd Kiss. IMPORTANT SAFETY INFORMATION (continued) Warnings and Precautions (continued) Potential Steroid-related Effects: Use of corticosteroids may produce posterior subcapsular cataracts, increased intraocular pressure, glaucoma, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. Corticosteroids should be used cautiously in patients with a history of ocular herpes simplex. Risk of Implant Migration: Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber. Adverse Reactions The most common ocular adverse reactions reported by greater than 2% of patients in the first 6 months following injection of OZURDEX for retinal vein occlusion and posterior segment uveitis include: intraocular pressure increased (25%), conjunctival hemorrhage (22%), eye pain (8%), conjunctival hyperemia (7%), ocular hypertension (5%), cataract (5%), vitreous detachment (2%), and headache (4%). Increased IOP with OZURDEX peaked at approximately week 8. During the initial treatment period, 1% (3/421) of the patients who received OZURDEX required surgical procedures for management of elevated IOP. Please see Brief Summary of full Prescribing Information on next page. Keep the Opportunity in Sight 1. OZURDEX Prescribing Information Allergan, Inc., Irvine, CA marks owned by Allergan, Inc. APC30DA
4 OZURDEX (dexamethasone intravitreal implant) 0.7 mg Brief Summary Please see the OZURDEX package insert for full Prescribing Information. INDICATIONS AND USAGE Retinal Vein Occlusion: OZURDEX (dexamethasone intravitreal implant) is a corticosteroid indicated for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO). Posterior Segment Uveitis: OZURDEX is indicated for the treatment of non-infectious uveitis affecting the posterior segment of the eye. CONTRAINDICATIONS Ocular or Periocular Infections: OZURDEX (dexamethasone intravitreal implant) is contraindicated in patients with active or suspected ocular or periocular infections including most viral diseases of the cornea and conjunctiva, including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections, and fungal diseases. Advanced Glaucoma: OZURDEX is contraindicated in patients with advanced glaucoma. Aphakic Eyes with Rupture of the Posterior Lens Capsule: OZURDEX is contraindicated in patients who have aphakic eyes with rupture of the posterior lens capsule. ACIOL and Rupture of the Posterior Lens Capsule: OZURDEX is contraindicated in eyes with ACIOL (Anterior Chamber Intraocular Lens) and rupture of the posterior lens capsule. Hypersensitivity: OZURDEX is contraindicated in patients with known hypersensitivity to any components of this product. WARNINGS AND PRECAUTIONS Intravitreal Injection-related Effects: Intravitreal injections have been associated with endophthalmitis, eye inflammation, increased intraocular pressure, and retinal detachments. Patients should be monitored regularly following the injection [see Patient Counseling Information]. Potential Steroid-related Effects: Use of corticosteroids may produce posterior subcapsular cataracts, increased intraocular pressure, glaucoma, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. Corticosteroids should be used cautiously in patients with a history of ocular herpes simplex. Risk of Implant Migration: Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber. ADVERSE REACTIONS Clinical Studies Experience: Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. Adverse reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera. The following information is based on the combined clinical trial results from 3 initial, randomized, 6-month, sham-controlled studies (2 for retinal vein occlusion and 1 for posterior segment uveitis): Adverse Reactions Reported by Greater than 2% of Patients in the First Six Months MedDRA Term OZURDEX N=497 (%) Sham N=498 (%) Intraocular pressure increased 125 (25%) 10 (2%) Conjunctival hemorrhage 108 (22%) 79 (16%) Eye pain 40 (8%) 26 (5%) Conjunctival hyperemia 33 (7%) 27 (5%) Ocular hypertension 23 (5%) 3 (1%) Adverse Reactions Reported by Greater than 2% of Patients in the First Six Months (continued) MedDRA Term OZURDEX Sham N=497 (%) N=498 (%) Cataract 24 (5%) 10 (2%) Vitreous detachment 12 (2%) 8 (2%) Headache 19 (4%) 12 (2%) Increased IOP with OZURDEX (dexamethasone intravitreal implant) peaked at approximately week 8. During the initial treatment period, 1% (3/421) of the patients who received OZURDEX required surgical procedures for management of elevated IOP. Following a second injection of OZURDEX in cases where a second injection was indicated, the overall incidence of cataracts was higher after 1 year. USE IN SPECIFIC POPULATIONS Pregnancy Teratogenic Effects: Pregnancy Category C: Topical dexamethasone has been shown to be teratogenic in mice producing fetal resorptions and cleft palate. In the rabbit, dexamethasone produced fetal resorptions and multiple abnormalities involving the head, ears, limbs, palate, etc. Pregnant rhesus monkeys treated with dexamethasone sodium phosphate intramuscularly at 1 mg/kg/day every other day for 28 days or at 10 mg/kg/day once or every other day at 3 or 5 days between gestation days 23 and 49 had fetuses with minor cranial abnormalities. A 1 mg/kg/dose in pregnant rhesus monkeys would be approximately 85 times higher than an OZURDEX injection in humans (assuming 60 kg body weight). There are no adequate and well-controlled studies in pregnant women. OZURDEX (dexamethasone intravitreal implant) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Nursing Mothers: It is not known whether ocular administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when corticosteroids are administered to a nursing woman. Pediatric Use: Safety and effectiveness of OZURDEX in pediatric patients have not been established. Geriatric Use: No overall differences in safety or effectiveness have been observed between elderly and younger patients. NONCLINICAL TOXICOLOGY Carcinogenesis, Mutagenesis, Impairment of Fertility No adequate studies in animals have been conducted to determine whether OZURDEX (dexamethasone intravitreal implant) has the potential for carcinogenesis. Although no adequate studies have been conducted to determine the mutagenic potential of OZURDEX, dexamethasone has been shown to have no mutagenic effects in bacterial and mammalian cells in vitro or in the in vivo mouse micronucleus test. PATIENT COUNSELING INFORMATION In the days following intravitreal injection of OZURDEX, patients are at risk for potential complications including in particular, but not limited to, the development of endophthalmitis or elevated intraocular pressure. If the eye becomes red, sensitive to light, painful, or develops a change in vision, the patient should seek immediate care from an ophthalmologist. Patients may experience temporary visual blurring after receiving an intravitreal injection. They should not drive or use machines until this has resolved Allergan, Inc., Irvine, CA 92612, U.S.A. marks owned by Allergan, Inc. Patented. See: Made in Ireland Based on 72212US15 APC48KJ13 Rx only
5 Multi-modality Treatment Approaches to RVO-associated Macular Edema Expert insights into the importance of utilizing all of the available options Diagnosis and Monitoring of RVO and Secondary Macular Edema By Theodore Leng, MD, FACS pg 6 Reframing How We Think About Treatment for RVO By Pravin U. Dugel, MD pg 8 Macular Laser in the RVO Treatment Paradigm By Seenu M. Hariprasad, MD, and Eric W. Schneider, MD pg 11 Dexamethasone Intravitreal Implant Following RVO: Rescue, Adjunct or Primary Therapy? By Desiree Ifft, Contributing Editor with Ron Gallemore, MD, PhD, Julia Haller, MD, and Antonio Capone, Jr., MD pg 15 Editorial Staff EDITOR-IN-CHIEF, Retinal Physician: Peter K. Kaiser, MD EDITORIAL DIRECTOR, SPECIAL PROJECTS: Angela Jackson EDITOR, SPECIAL PROJECTS: Leslie Goldberg CONTRIBUTING EDITOR: Desiree Ifft Design and Production PRODUCTION DIRECTOR: Sandra Kaden Business Staff PRESIDENT: Thomas J. Wilson EXECUTIVE VICE PRESIDENT & PUBLISHER: Douglas A. Parry SALES: Molly Phillips & Scott Schmidt PROMOTIONAL EVENTS MANAGER: Michelle Kieffer Editorial and Production Offices 321 Norristown Road, Suite 150, Ambler, PA Phone: (215) Retinal Physician is published by PentaVision LLC. Copyright 2014, PentaVision LLC. All Rights Reserved. Cover image courtesy of Julia Haller, MD 5
6 Diagnosis and Monitoring of RVO and Secondary Macular Edema Advances in technology, such as ultra-widefield angiography and spectral domain OCT, aid in patient management. By Theodore Leng, MD, FACS Dr. Leng is Director of Ophthalmic Diagnostics and a Clinical Assistant Professor of Ophthalmology at the Byers Eye Institute at Stanford, Stanford University School of Medicine, in Palo Alto, Calif. We don t have to think too far into the past to remember a time when we diagnosed and planned treatment for our retinal vein occlusion (RVO) patients without several of the advanced technologies we have today. Clinical exam, fluorescein angiography (FA) and OCT continue to be our best tools for evaluating RVO and associated macular edema, but our imaging capabilities have improved significantly. Clinical Exam. RVO patients commonly report decreased vision and blurring in one eye, but it s typically painless with sudden onset. They may also report distortion of images, and their visual disturbances may be limited to one part of the visual field. A dilated fundus exam often reveals the classic features of branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO), which include intraretinal hemorrhages and tortuosity and dilation of the retinal blood vessels. Macular edema, exudates and cotton wool spots may be present. If so, their location should be noted in the chart so any changes that occur over time or with treatment can be documented. In addition to a measurement of visual acuity, which may or may not be affected depending on the location of the occlusion, a confrontation visual field test should be performed. Depression of the field in a certain quadrant could indicate macular edema or areas of ischemia. Ischemic RVO has a much poorer prognosis than nonischemic. 1 The pupillary light reflex should also be checked as the presence of an afferent pupillary defect may be a sign of ischemia. Questions about cardiovascular disease, stroke, high cholesterol and diabetes are particularly relevant when eliciting the medical history of RVO patients. All of those conditions are associated with RVO, with hypertension being the number one associated risk factor. Many patients aren t aware of the connection between these systemic conditions and retinal vein occlusion. As such, it s important that we educate them about the link between these conditions and RVO and thus, the importance of getting certain risk factors under control. We should also encourage them to follow up with their primary care physician on a regular basis. Fluorescein Angiography. When symptoms and clinical examination suggest RVO, FA is the next step. Ultra-widefield angiography (Optos) is preferred because it enables efficient imaging of the retinal periphery out to approximately 200 degrees. This reveals whether areas of the peripheral retina are ischemic, which is useful for determining a prognosis, and planning your treatment approach, follow-up steps and schedule. Peripheral ischemia should be monitored closely with suspicion. Ischemia induces production of vascular endothelial growth factor (VEGF) that in turn can lead to increased vascular permeability and macular edema, as well as neovascularization in the retina and iris. 2,3 6
7 Figure 1 Figure 2 Figures 1 and 2. In this eye imaged with ultra-widefield angiography and OCT, peripheral ischemia is present without associated macular edema. The ischemia should be closely monitored, however, given its propensity to cause either neovascularization or persistent/recurrent macular edema. Ultra-widefield angiography has shown us that even if the macula is not ischemic, ischemia in the periphery can be extensive. Eyes with peripheral ischemia are likely to require repeat anti-vegf injection therapy, whereas eyes without peripheral ischemia may not require anti-vegf treatment at all, provided the patient s vision isn t being affected by the macular edema. Peripheral ischemia often exists without associated macular edema (Figures 1 and 2). However, when both are present, persistent ischemia can perpetuate a cycle of posttreatment resolution and recurrence of the edema. Most photographers will capture some color fundus photos as part of performing FA. In general, their diagnostic value doesn t extend beyond serving as documentation of what was observed during the clinical exam. OCT. OCT provides us with a more efficient, less invasive and less costly way than repeat FA to evaluate and monitor RVO and associated macular edema over time. With the progression from time-domain to spectral-domain technology, we have gained the ability to capture volumetric scans of the retina, rather than only single-line scans. This allows generation of thickness maps that more precisely identify focal areas of retinal thickening. In our clinic, for all patients in whom macular disease is suspected, we obtain a high-resolution, highdensity scan through the center of the fovea. We also capture a cube scan that covers a 6-by-6-mm area of the macula. For RVO patients in particular, it s important to look at the entire cube to determine whether focal areas of edema are present outside the fovea. It may be faster to look only at one scan through the fovea, but doing so can lead to the assumption that the entire macula is free of edema when it may not be. Making use of the thickness maps derived from the cube is also a good way to pick up all edematous areas. Edema outside the fovea can be observed rather than treated, but it should be monitored for changes over time. After initial examination and baseline OCT and FA, most physicians choose to follow RVO patients with monthly OCT scans. Repeat FA is reserved for when it is unclear why a patient isn t responding to treatment as expected or experiences worsening vision or if neovascularization is suspected. Once macular edema becomes quiescent or doesn t require monthly treatment, and depending on the extent of ischemia, the interval between follow-up visits can be extended to every 3, 6 or 12 months. Even when the eye is quiet, it is prudent to examine any patient with a history of RVO once or twice each year. Advances in Imaging on the Horizon Further improvements that will enhance our ability to manage RVO patients are being researched and developed. Among them is the use of OCT for performing angiography for imaging retinal capillaries. Having a noncontact method for viewing the retinal vasculature would be a welcome addition. Swept-source OCT also promises to provide wider views of the peripheral retina. We can also look forward to the development of new visualization tools based on volumetric data. References 1. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118(1): Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4): Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113(12):
8 Reframing How We Think About Treatment for RVO Changing therapy options require a change in our approach. By Pravin U. Dugel, MD Dr. Dugel is Managing Partner with Retinal Consultants of Arizona and a founding member of Spectra Eye Institute in Sun City. He is also a Clinical Associate Professor at the USC Department of Ophthalmology, Keck School of Medicine, University of Southern California. The number of options retina specialists have for managing macular edema associated with retinal vein occlusion (RVO) has increased since observation was the prevailing approach and the only proven treatment, solely for branch occlusion, was laser photocoagulation. The increase in options has led to a focus on debating the question: Which treatment is best for RVO? I venture to say there is a better way to frame the issue. First, we should understand that RVO, like any other chronic, complex disease, has a life cycle consisting of various stages. Oncologists, for example, have approached cancer this way for many years. Rather than simply diagnosing lung cancer or colon cancer, they delve deeper to categorize it at a specific stage, stage 1, stage 2, and so on, and use that insight to implement the appropriate treatment plan. Ideally, we would do the same with RVO. Clinically, we see the repercussions of RVO disease stages that follow the initial occlusive event capillary permeability and leakage, edema, inflammation, vessel remodeling and recanalization, neovascularization if ischemia is present, and fibrosis. However, not all patients go through all the stages. Furthermore, although we know a multitude of chemical factors are involved, e.g., angiogenic and inflammatory cytokines, 1-3 we re still working to clearly define the stages at the cellular level and discern the relationships between them. Until we re armed with that information, we aren t able to predict, for instance, that a specific patient s RVO is at the leakage stage and therefore an anti- VEGF agent alone will resolve his macular edema. In the meantime, what we can do is work with the life cycle of RVO in a roundabout way, by titrating our treatment options and perhaps combining* them until we arrive at what works for the individual patient. By doing this, we not only provide the best possible current care but also adopt the mindset that will be most helpful to us as our knowledge of RVO and associated macular edema expands. Here, I summarize other key aspects of my approach to macular edema secondary to retinal vein occlusion. Treatment Criteria The treatment criteria used in the early studies of laser for RVO and in subsequent anti-vegf studies are outdated. Rather than observe patients for 3 months to see if macular edema spontaneously resolves and visual acuity improves to 20/40 or better, I treat all patients who have edema and a vision complaint, even if their Snellen visual acuity is 20/20. One reason is that the longer we wait to treat, the less vision we can expect the patient to recover. Another reason is that Snellen acuity is not a true assessment of visual function. It doesn t take into account the real-world aspects of what constitutes clear, comfortable vision for patients, such as contrast sensitivity and color perception. I often see RVO patients with 20/20 Snellen acuity who say they re having trouble seeing. 8 * In my practice, more than 50 percent of patients have combination therapy.
9 Gauging Response to Treatment My most frequent first-line therapy for RVO-associated macular edema is an anti-vegf agent, as it is for most retina specialists. I gauge whether it is adequate for a given patient based on a post hoc analysis from the BRAVO and CRUISE studies of ranibizumab 4 (Lucentis, Genentech). The analysis showed that in patients with central retinal vein occlusion, 90% of the response to treatment occurred within the first three injections. Therefore, if I see no response after three injections, I m confident that monotherapy will not be adequate, and I move to the next option. If I see robust improvement in macular thickness and visual acuity by the third injection, I m inclined to continue with the injections until the response plateaus. I use another subanalysis from BRAVO and CRUISE, the RETAIN study, 5 as guidance. This analysis showed that despite a good visual response, approximately 50% of patients continue to need anti-vegf injections for edema as much as 4 years later. This indicates that at least half of patients are likely better off if we use additional treatment to keep the retina dry, thus reducing the treatment burden. Updated Approaches to Laser Treatment The role of traditional laser treatment is becoming less and less relevant. It does produce benefits, but, as we know, it is also photodestructive, producing scarring and scotoma. Scars can expand slowly over time, which is especially troubling for young patients. On the other hand, some recent developments in laser technology are encouraging. For example, Endpoint Management (Topcon Medical), for use with the PASCAL and Streamline lasers, is an innovative way to precisely control laser output. Through photostimulation, the retinal pigment epithelium cells can be selectively targeted with subthreshold treatment to prompt the necessary biologic response for decreasing edema, without collateral tissue damage. It will be interesting to see what further studies reveal about the safety and efficacy of these types of technology. In other words, although traditional laser photocoagulation may be less relevant in the treatment of RVO, selective laser photostimulation may have a role. Steroid Pharmacokinetics All steroids and steroid delivery devices are not the same. The pharmacokinetics (PK) of each is an important consideration in the treatment of macular edema. Injecting a bolus of steroid, such as intravitreal triamcinolone, results in a large increase of drug in the eye followed by a sudden decrease, which is not a favorable PK profile. Beneficial effects are minimized, and complications are maximized. In comparison, the dexamethasone intravitreal implant provides an initial burst of drug elution followed by a gradual decrease over time, a much more favorable profile. Complications are more predictable, and therefore more easily managed. Thinking in terms of the life cycle of RVOassociated edema, we can see how using an anti- VEGF agent is a sensible option at the relatively early stage of capillary permeability. When inflammation becomes more of a factor, based on how the eye responds to anti-vegf, the steroid implant makes sense. Studies have confirmed the safety of using it multiple times as well. Should the fluocinolone intravitreal insert (Alimera Sciences) receive FDA approval for RVO treatment, it could be a useful next step, in particular for eyes with very diffuse edema that could benefit from the device s 3-year steadystate release of steroid. I m more likely to use the dexamethasone implant rather than anti-vegf therapy in a vitrectomized eye, because its sustained presence in the eye has been show to be effective in this scenario. Pravin U. Dugel, MD Attention to Potential Complications All of the available treatments for macular edema have side effects, which we must understand and manage. Anti-VEGF agents are certainly effective, yet they may increase the risk for systemic arteriothromboembolic events (ATEs). Even though there s a lack of definitive data, we must be mindful of the trends. We do have to use caution in our patients who are at highest risk for ATEs, particularly patients 85 and older. Whether different anti-vegf agents have different systemic safety profiles is not known. One theory that has been put forth suggests that the IgG1 protein could have an impact by allowing for greater systemic exposure. We are also learning that chronic anti-vegf treatment may additionally have local side effects. This has been seen in neovascular AMD as geographic atrophy and fibrosis. The systemic and local side effects of chronic anti-vegf treatment for RVO requires more study. Our main concerns with steroid treatments are cataract formation and elevated IOP. As mentioned previously, using steroids with known favorable PK profiles improves our ability to predict and manage these side effects. 9
10 Whether we re using monotherapy or combination therapies, we must use them in the context of the risk/ benefit ratio for the individual patient. For example, in an 85-year-old patient with a history of heart attack or stroke, I prefer to use an anti-vegf agent without the Fc fragment for a short period of time, and I m more likely to switch to the dexamethasone implant quickly. Our main concerns with steroid treatments are cataract formation and elevated IOP. As mentioned previously, using steroids with known favorable PK profiles improves our ability to predict and manage these side effects. Pravin U. Dugel, MD I m less likely to use the implant in steroid-responsive patients or patients with uncontrolled glaucoma, although several studies indicate the side effects are quite manageable in glaucoma patients with good IOP control. 6 I do monitor such patients more closely than patients without these added risks. When deciding whether to use the dexamethasone implant in phakic patients, the risk/benefit equation is also an important consideration. For patients in danger of losing vision to fibrosis, for instance, the need for future cataract surgery becomes less of a concern. The clinical trials for both the dexamethasone and fluocinolone implants showed that eyes with these devices do very well with cataract surgery. As a final example, I m more likely to use the dexamethasone implant rather than anti-vegf therapy in a vitrectomized eye, because its sustained presence in the eye has been shown to be effective in this scenario. 7 Further Progress to Come As we learn more about the pathophysiology of RVO and its sequelae, we ll be better able to select targeted treatment plans. We ll also continue to benefit from the knowledge that most diseases of the retina share a final common pathway, leading to vision loss. Therefore, lessons learned from new therapies for age-related macular degeneration and diabetic macular edema will be applicable to vein occlusion. Anti-platelet-derived growth factor is an example of this potential cross-pollination of seemingly disparate diseases. Its potent anti-fibrotic effect is now being studied in AMD and may be applicable in RVO in the future. In conclusion, we ve made meaningful progress in RVO treatment in the past several years, and I believe additional treatment options are on the horizon. However, a better understanding of this and other chronic retinal diseases begins with an understanding of the life cycle of the disease. References 1. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22): Feng J, Zhao T, Zhang Y, Ma Y, Jiang Y. Differences in aqueous concentrations of cytokines in macular edema secondary to branch and central retinal vein occlusion. PLoS ONE. 2013;8(7):e Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 2012;96(11): Bhisitkul RB, Campochiaro PA, Shapiro H, Rubio RG. Predictive value in retinal vein occlusions of early versus late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology. 2013;120(5): Campochiaro PA, Sophie R, Pearlman J, et al. Longterm outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN Study. Ophthalmology. 2014;121(1): Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014;34(2): Boyer DS, Faber D, Gupta S, et al.; Ozurdex CHAMPLAIN Study Group. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):
11 Macular Laser in the RVO Treatment Paradigm This treatment option remains useful in many situations and is being examined anew as part of combination therapy. By Seenu M. Hariprasad, MD, & Eric W. Schneider, MD Dr. Hariprasad is an Associate Professor, Chief of the Vitreoretinal Service and Director of Clinical Research in the Department of Ophthalmology and Visual Science at the University of Chicago Medical Center. He is the editor of the 2014 book Management of Retinal Vein Occlusion: Current Concepts. Dr. Schneider is a Senior Vitreoretinal Fellow at the Duke Eye Center in Durham, N.C. He is a contributing author for Management of Retinal Vein Occlusion: Current Concepts. Anti-vascular endothelial growth factor (VEGF) therapy has certainly raised our expectations regarding treatment of macular edema secondary to retinal vein occlusion (RVO). However, there remains a distinct role for macular grid laser (MGL) treatment, either alone or as part of combination therapy. We recently completed a textbook 1 on current concepts in RVO management, which includes a discussion of the utility of MGL for treating macular edema, the most common cause of vision loss after RVO. This article is a summary of the body of literature and expert experience on which the book is based. Macular Grid Laser as Monotherapy As prospective, randomized, controlled clinical trials, the Branch Vein Occlusion Study (BVOS) and Central Vein Occlusion Study (CVOS) were the first to provide us with Level I evidence on the use of laser photocoagulation in the treatment of RVO and its sequelae. From the BVOS, we learned that argon MGL is preferable to observation for patients with macular edema secondary to branch retinal vein occlusion (BRVO) and vision of 20/40 or worse. Patients in the BVOS were divided into four groups. One group included 139 eyes with vision loss from macular edema secondary to BRVO. Key inclusion criteria were onset of BRVO from 3 to 18 months prior to the study, presence of macular edema involving the fovea, and best-corrected visual acuity of 20/40 or worse. Eyes with foveal hemorrhage or foveal capillary nonperfusion were excluded, as were those with any other ocular disease that could compromise visual acuity. Patients were randomized to either observation or treatment with argon MGL (n=71). The treatment group received MGL treatment in the area of capillary leakage as identified by fluorescein angiography (FA) within the vascular arcades but not closer to the fovea than the outer edge of the avascular zone. Additional MGL was performed at 4 months if foveal edema remained with attendant decreased visual acuity. The majority of treated eyes required only a single session of laser. At 3 years, 65% of the eyes that underwent MGL gained at least 2 lines of vision compared with 37% in the untreated group. Mean visual acuity at the 3-year follow-up visit was 20/40 to 20/50 in the treated group and 20/70 in the untreated group. BVOS thus established that MGL applied to discrete areas of leakage can be useful as monotherapy in eyes with macular edema secondary to BRVO. We have further confirmation of this finding from the Standard Care versus Corticosteroid for Retinal Vein Occlusion (SCORE) study. SCORE compared MGL with intravitreal corticosteroid injections for BRVO-related macular edema. In this large, prospective comparative clinical trial, MGL was shown to result in similar visual acuity gains when compared with steroid monotherapy, but with fewer complications. 11
12 Case Report Macular Grid Laser Following Incomplete Response to Anti-VEGF Injections Anti-VEGF therapy does not lead to complete resolution of RVO-associated macular edema in every patient. Adding macular grid laser treatment to the therapy regimen may dry the retina and allow cessation of anti-vegf injections, as this case demonstrates. The patient is a 48-year-old female with a history of hypertension and waxing/waning blurry vision in the left eye for 10 months for which she had not sought treatment. Her initial dilated fundus exam revealed macular edema in the superior foveal region OS secondary to non-ischemic BRVO with exudates extending through the fovea (Figure 1). Fluorescein angiography was consistent with a diagnosis of chronic BRVO with macular edema (Figure 2). OCT imaging was performed (Figure 3). Visual acuity was 20/20 OD and 20/20-2 OS. Given the visual acuity at this visit, the decision was made to observe the patient. The patient returned for follow-up 3 months later. Visual acuity OS had declined to 20/25 and macular edema was still present. The patient was treated at this visit with intravitreal bevacizumab (Avastin, Genentech). One month later, visual acuity OS was reduced to 20/30, persistent macular edema was noted, and the patient was given a second bevacizumab injection. After another month, the edema OS improved slightly but vision did not, and the patient was given a third anti-vegf injection. Approximately 6 weeks after the third injection, edema in the affected eye had stabilized, but vision declined further to 20/40 (Figure 4). At this visit, the patient received a macular grid laser treatment OS. By 3 months post laser, macular edema in the treated eye had significantly improved and visual acuity increased to 20/30 (Figure 5) Since that time, no further treatment has been needed. Macular edema OS completely resolved, and VA improved to 20/20-1 (Figure 6). Figure 1 Figure 2 Figure 1. Prior to treatment, BRVO-associated macular edema was observed in the superior foveal region of the patient s left eye. Exudates extended through the fovea. Figure 2. The initial fluorescein angiogram demonstrated microvascular abnormalities in the distribution of a macular branch vein with associated leakage consistent with a diagnosis of chronic BRVO with macular edema. According to the results of the CVOS, macular grid photocoagulation is not recommended for treating macular edema due to central retinal vein occlusion (CRVO). Out of the 150 patients enrolled in the macular edema portion of this study, 77 were randomized to receive argon MGL while the remainder were observed. Patients were included if they had confirmed CRVO for at least 3 months, foveal-involving macular edema verified by FA, visual acuity between 20/50 and 5/200 and intraocular pressure less than 30 mmhg. Exclusion criteria included foveal nonperfusion documented on FA as well as a history of retinal laser photocoagulation, diabetic retinopathy, retinal neovascularization or lens abnormalities (significant opacity, aphakia/pseudophakia). In the treatment group, eyes received argon MGL applied to areas of capillary leakage not extending into the foveal avascular zone. Collateral vessels and/or hemorrhages were avoided. Patients received additional laser treatment after their first treatment if they gained nine or fewer letters of vision and had persistent macular edema at a follow-up visit. At the end of 1 year, all of the eyes in the observation group had some degree of edema. In contrast, 31% of patients in the treatment group experienced complete resolution of angiographically evident edema 1 year after MGL. Despite the disparity in anatomic outcomes, however, no significant difference in post-treatment visual acuity was observed between the two groups. 12
13 Figure 3 Figure 4 Figure 5 Figure 6 Figures 3-6. OCT thickness maps and representative B-scans of the patient s left eye from presentation to last follow-up visit. After initial treatment with 3 anti- VEGF injections, macular edema was still present, and the patient underwent one macular grid laser treatment. By 3 months post laser, the edema in the treated eye had significantly improved. Since that time, no further treatment has been needed. The macular edema is completely resolved, and visual acuity is 20/20-1. Macular Grid Laser in Combination Therapy It is notable that while MGL did not improve patients vision in the CVOS, it did reduce macular leakage. Since that study was conducted, intravitreal pharmacologic therapies (i.e., steroid and anti-vegf agents) have proven to be effective, albeit short-term, treatments for macular edema associated with RVO. In light of these new therapeutic options, it makes sense to re-evaluate the potential utility of MGL applied in combination with intravitreal agents for CRVO. In BRVO, in which laser monotherapy has been proven beneficial, combination therapy may have the added advantage of limiting toxicity and/or lessening the treatment burden as smaller doses or less frequent treatments may achieve similar results. Combination therapy may also be used to increase efficacy in patients who are not adequately responsive to monotherapy. In either situation, the benefit of combination treatments is due to the synergistic effects achieved. Each agent has a different pharmacokinetic profile and acts at a different point in the pathway leading to macular edema. Unfortunately, only a small number of studies exploring RVO combination therapies have been published to date. Most are short-term, retrospective and/or involve small numbers of patients. Nonetheless, 13
14 as retinal specialists are increasingly using MGL in combination with other treatments, it remains a treatment paradigm very much worth exploring. MGL + Anti-VEGF Laser in addition to intravitreal anti-vegf therapy is an often-used combination in clinical practice. Given the previously documented high levels of VEGF in the aqueous and vitreous of eyes with RVO, it is not surprising that anti-vegf monotherapy is effective in combating the associated macular edema. Although the exact mechanism whereby MGL reduces macular edema is unclear, one possible explanation relates to a reduction in VEGF levels secondary to a decrease in retinal hypoxia induced by laser. When laser is combined with anti-vegf agents, which bind to and directly inhibit VEGF, a synergistic anti-vegf effect is produced. Furthermore, anti-vegf agents mitigate some of the limitations of MGL, including the inability to treat in the presence of dense macular hemorrhage and the need for increased laser power in the setting of severe macular edema with an associated greater risk of collateral tissue damage. In contrast, because anti-vegf injections work quickly and can be given regardless of the severity of macular hemorrhage or edema, they may quiet the eye to allow us to utilize MGL earlier with greater efficacy and safety. The studies 1 of anti-vegf/mgl combination therapy published to date have shown mixed results. In some, the combination clearly outperformed standalone anti-vegf treatment in achieving better anatomic and visual outcomes. In others, impressive anatomical improvements were not matched by gains in vision. In published studies in which injection frequency was tracked, however, combination laser/anti-vegf treatment resulted in a reduced need for anti-vegf injections. MGL + Corticosteroid Combining MGL with corticosteroid may also increase the efficacy of our treatment for RVO-related macular edema compared to either treatment alone. The literature 1 confirms that in addition to VEGF, inflammatory factors play a role in RVO-related edema. A combination of steroid therapy with MGL may result in synergistic effects as it targets both the inflammatory component (steroids) while also addressing VEGF-related drivers of macular edema at multiple different points along the pathway (reduced VEGF production due to lessened retinal hypoxia from laser; reduced activity of effectors downstream of the VEGF receptor from steroids). Only two prospective studies 1 of an intravitreal triamcinolone/mgl combination for BRVO-related macular edema have been published. In one, MGL applied after an average of 1.13 steroid injections led to impressive structural and functional improvements. In a separate comparative study, both a combination therapy group (consisting of patients receiving steroid therapy after failed MGL) and a steroid monotherapy group demonstrated significant anatomic and functional improvements at early timepoints. However, these improvements were maintained at later timepoints only in the steroid monotherapy group. The inclusion of only patients refractory to MGL in the combination group may be a significant confounder in this study. Of note, in both studies, 1 the rate of IOP elevation was similar to the rate with intravitreal triamcinolone monotherapy. Two abstracts 1 presented in 2012 described non-comparative studies in which the dexamethasone implant (Ozurdex, Allergan) was utilized in conjunction with MGL. Significant decreases in retinal thickness were observed in both studies, but corresponding visual gains were noted in only one study. The authors of a prospective study 1 comparing subthreshold micropulse grid laser (Iridex) alone to micropulse laser plus intravitreal triamcinolone for macular edema in BRVO reported better visual results in the combination group. While these initial results are promising, future studies will be required to further evaluate safety and efficacy of these MGL combination therapies as well as to establish the ideal timing, frequency and optimal settings for adjunctive laser therapy. Role of MGL May Be Expanding Although retina specialists have been utilizing MGL as a treatment for RVO-related macular edema for decades, recent technological developments in laser delivery may lead to even greater efficacy and safety. For example, image-guided laser platforms, such as Navilas (OD-OS), have the potential to drastically improve treatment accuracy. Moreover, improvements in diagnostic imaging capabilities and laser lenses offer further hope for improved MGL application. Indeed, in light of these technological advances and the potential for synergistic effects when used in combination with intravitreal therapy, it appears now may be an ideal time to revisit the role of MGL in the treatment of macular edema secondary to RVO. Reference 1. Hariprasad SM. Management of Retinal Vein Occlusion: Current Concepts. 1st ed. Thorofare, NJ: Slack Incorporated;
15 CVSi21 CVS-PC Snellen Chart Patient Education Children s Charts ( ) Disparity ETDRS Video Or Your Local Lombart Representative. Corporate Office Robin Hood Road, Norfolk, VA FAX ATLANTA BALTIMORE/ WASHINGTON D. C. BOSTON BOYNTON BEACH/ MIAMI BRADENTON CHARLOT TE CHICAGO CINCINNATI DALLAS DENVER DETROIT GREENSBORO HOUSTON K ANSAS CIT Y KNOXVILLE LOS ANGELES MILWAUKEE MINNEAPOLIS NEW JERSEY/ NEW YORK NORFOLK PORTLAND SACRAMENTO SAN DIEGO SAN FRANCISCO
16 Dexamethasone Intravitreal Implant Following RVO: Rescue, Adjunct or Primary Therapy? Ozurdex can be a safe, effective treatment option for macular edema in all three scenarios. By Desiree Ifft, Contributing Editor Since the Phase III GENEVA clinical trials demonstrated the safety and effectiveness of the dexamethasone intravitreal implant (Ozurdex, Allergan) for treatment of macular edema following retinal vein occlusion (RVO), how best to utilize the implant in clinical practice has been further studied. In addition, doctors have gained several years of experience using it, and VEGF therapies have been FDA-approved for the same indication. In light of what has been learned, where the implant fits into retina specialists approach to treatment of this group of patients has evolved. Studies, Experience Lead to Expanded Use of Ozurdex We re more comfortable using Ozurdex overall, says Ron Gallemore, MD, PhD, Founder and Director of the Retina Macula Institute and Research Center in Los Angeles and Assistant Professor at the Jules Stein Eye Institute, UCLA School of Medicine. The subset of patients that we consider good candidates for the implant is larger as well. We don t hesitate to switch to it quickly when macular edema is resistant to intravitreal anti-vegf therapy and a patient s vision isn t bouncing back after a few injections. In cases where the patient has a partial response to anti-vegf therapy, but a hint of edema remains, we can often achieve a line or two more vision when we add Ozurdex. As many as 30% of our RVO patients are at least partially resistant to anti-vegf treatment. Also, in our experience, using anti-vegf injections and Ozurdex together can extend the interval between treatments compared with either of the two drugs alone. Dr. Gallemore and colleagues conducted a retrospective chart review involving RVO patients who received an Ozurdex implant to treat macular edema that had persisted after multiple bevacizumab (Avastin, Genentech) injections. On average, the patients receiving Ozurdex had a decrease in central foveal thickness of ± μm and improvement in visual acuity. 1 The retrospective study was subsequently repeated as a prospective trial. In that study, in which eyes with RVO recalcitrant to anti-vegf therapy were randomized to receive Ozurdex either every 16 weeks or prn, the implant significantly reduced retinal thickness and improved visual acuity. 2 A higher incidence of posterior subcapsular cataract was seen with repeat administration of Ozurdex, Dr. Gallemore explains, but, interestingly, multifocal electroretinogram showed statistically significant improvement in macular function after treatment with consecutive dexamethasone implants. The macular function improvement was seen in all of the patients who received Ozurdex, whether they received implants every 16 weeks or prn during the 48-week study. In the original GENEVA clinical trials, the implant was administered every 6 months, but the effect waned during that interval. 3 However, the extension portions of GENEVA showed that further reduction of edema and recovery of vision can occur with repeat administration. 4 In the GENEVA extension trials, visual recovery was better for patients who were treated at the outset with Ozurdex, suggesting that earlier use of the implant may achieve better visual 16
17 results, Dr. Gallemore notes. That said, it s also important to know that patients who were initially randomized to the sham group also had a reduction in edema and recovery of vision when they were switched to treatment with Ozurdex, indicating that even if treatment with Ozurdex is delayed, it can still be beneficial. Today, most doctors using Ozurdex for patients with RVO-associated macular edema administer the implant every 2-3 months, especially in eyes that had not responded to, or had an inadequate response to, anti-vegf agents. Dr. Gallemore may choose Ozurdex as primary therapy in specific situations, including RVO patients who refuse to have frequent intravitreal injections and patients with a recent history of heart attack or stroke. Anti-VEGF treatment is relatively contraindicated in patients who have recently suffered MI or a stroke, he says. He may also choose the implant as initial therapy for pregnant women for safety reasons and for maximizing visual results in patients who ve had a vitrectomy because he expects the sustained-release dexamethasone to remain in the eye longer than other intravitreally injected steroids or anti-vegf agents. 5 Julia Haller, MD, Ophthalmologist-in-Chief of the Wills Eye Institute and Professor and Chair of the Department of Ophthalmology at Jefferson Medical College of Thomas Jefferson University in Philadelphia, agrees. We know that anti-vegf drugs have a short half-life in vitrectomized eyes, so for those patients, I m more likely to use the dexamethasone implant, which is our only current FDA-approved therapeutic option that provides extended drug delivery, she says. Given their effectiveness, 6-9 most of us start with anti-vegf injections for treating RVO-associated macular edema, but it s important to keep in mind that steroids have anti-vegf properties as well. Therefore, there is room for both treatments, either both upfront or sequentially. In some cases, before I combine treatments, I may try a different anti-vegf agent if the initial choice is not effective. In other cases, such as in eyes that are already pseudophakic, I tend to get Ozurdex on board much sooner than I may have in the past. The same applies to eyes with a severe occlusion and very poor visual acuity, which I know from experience will likely have hard-to-control, recurring macular edema. (See Case Report: Dexamethasone Implant Plus Anti-VEGF Agent as Initial Treatment, on the adjacent page.) Intravitreal triamcinolone is a reasonable option when the decision is made to use a steroid, Dr. Haller points out, but she prefers the dexamethasone implant. Triamcinolone can be effective for a few weeks or a month or two, but the dexamethasone implant is more potent, yet with a more favorable safety profile in terms of cataract progression, IOP elevation and potential toxicity Also, not all formulations of triamcinolone are FDA-approved for ocular use, and problems with compounding pharmacies have raised concerns. Unless a patient has issues with insurance coverage and can t afford it cost-wise, I would much rather use the implant than triamcinolone injections. Based on her own experience and the cumulative experience of other retina specialists, Dr. Haller says she s more aggressive than in the past using Ozurdex for patients who are already using a topical IOP-lowering medication. Once I started using Ozurdex in this group of patients, I realized how well it works and that the complications are very manageable. That put the implant on my Triamcinolone can be effective for a few weeks or a month or two, but the dexamethasone implant is more potent, yet with a more favorable safety profile in terms of cataract progression, IOP elevation and potential toxicity. Julia Haller, MD radar screen more often as a solid option sooner rather than later. It s very important to balance any potential implant-related complications with the need to eliminate the macular edema to preserve vision, she says. She cites the recently published Shasta study as an indication that Ozurdex is being used safely and effectively in patients with controlled glaucoma. A Real-world Evaluation of Ozurdex Use Shasta is a 26-site, retrospective chart review study of patients with branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO) who received two or more dexamethasone implants. 13 Patients who had previously received an Ozurdex implant as part of a clinical study were excluded. The 289 study subjects received a mean 3.2 implants (range of 2 to 9) during the study time period, either as monotherapy (for 29.1% of the patients) or in conjunction with other treatments. At baseline (i.e., at the time of their first implant), 31.5% of the patients had glaucoma or ocular hypertension, and 15.6% had a history of IOP elevation in response to steroid treatment documented in their charts. According to Shasta data analysis, the percentage of patients who experienced a 2- to 3-line improvement in best-corrected visual acuity compared with baseline was similar after each implant. Overall, 62.9% of patients demonstrated at least a 2-line improvement in BCVA, and 48.1% of patients demonstrated at least a 3-line improvement at some point after treatment with Ozurdex. With the reminder that Shasta is a retrospective study, lead author Antonio Capone, Jr., MD, considers its safety 17
18 Case Report Dexamethasone Implant Plus Anti-VEGF Agent as Initial Treatment Figure 1 Figure 2 In eyes with severe retinal vein occlusion and very poor visual acuity on presentation, macular edema is often recurrent despite treatment. In some of these cases, a reasonable course of action may be combining therapies at the outset, as in this case. The patient is a 45-year-old otherwise healthy male who presented with sudden loss of vision in his left eye. Visual acuity OS was counting fingers inferiorly at 6 feet. Clinical exam and color fundus photography (Figure 1) revealed a hemiretinal vein occlusion, which fluorescein angiography confirmed (Figure 2). Central retinal thickness was 624 µm as measured by Spectralis SD-OCT (Heidelberg Engineering) (Figure 3). Given the severity of the occlusion and macular edema and the patient s lack of other health issues, the decision was made to immediately administer an anti-vegf injection and a dexamethasone intravitreal implant. The patient indicated he understood the risks and benefits. For example, he was willing to accept the risk of steroid-induced cataract in favor of receiving the two treatments in an effort to restore as much of his vision as possible. The patient returned for monthly follow-up visits, receiving four additional anti-vegf injections. By the first follow-up visit, central retinal thickness had decreased to 153 µm. Five months after the initial visit, extreme ischemia, which is known to upregulate VEGF, was still present, so the patient received peripheral laser treatment, which was his last treatment of any type to date. As of 1 month post laser (6 months after presentation), the macular edema remained completely resolved and visual acuity had improved to 20/50. Julia Haller, MD Figure 3 Figure 1. Color fundus image at presentation. Figure 2. Fluorescein angiography (4:37:16) at presentation. Figure 4 Figure 3. At the patient s initial visit, central retinal thickness was 624 µm as measured by Spectralis SD-OCT. Figure 4. Six months after initiation of therapy with a dexamethasone implant and an anti-vegf injection, 1 month after a peripheral laser treatment, macular edema remained completely resolved. 18
19 findings the most notable from the perspective of clinicians. Very few patients had meaningful pressure rises, he explains. 32.6% had an increase in IOP from baseline of at least 10 mmhg, and a similar proportion required pressure-lowering medications, but the vast majority were effectively managed with drops. A very small percentage, 1.7%, required incisional surgery. Also, the steroid response was very predictable, it peaked at 6 weeks and no stairstep increase in pressure was seen with subsequent implants. All of this runs counter to what I think many doctors would anticipate with a steroid treatment. Dr. Capone, who practices with Associated Retinal Consultants in Michigan and is a Clinical Professor of Biomedical Sciences at Oakland University s William Beaumont School of Medicine, comments further on Shasta, pointing out that it involves somewhat of a selection bias. These were patients who had been treated in clinical practices, so most had received anti-vegf, laser and/or other steroid therapy before receiving Ozurdex implants. Only 39 Shasta patients were treatment-naïve when they began receiving Ozurdex. As such, this cohort is much more laden with treatment-refractory patients than most prospective clinical trials, which typically enroll treatment-naïve patients. Therefore, more of them have blunted vision outcomes. Along the same lines, Dr. Capone continues, Other studies have shown us that patients with protracted References 1. Sharareh B, Gallemore R, Taban M, Onishi S, Wallsh J. Recalcitrant macular edema after intravitreal bevacizumab is responsive to an intravitreal dexamethasone implant in retinal vein occlusion. Retina. 2013;33(6): Gallemore RP, Wallsh J, Sharareh B, Liu S. Dexamethasone implant improves retinal function in retinal vein occlusion recalcitrant to anti-vegf therapy. Scientific Poster PO244; presented during the Annual Meeting of the American Academy of Ophthalmology, Nov 17, 2013, New Orleans, USA. 3. Haller JA, Bandello F, Belfort R Jr, et al., for the OZURDEX GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6): Haller JA, Bandello F, Belfort R Jr, et al., for the Ozurdex GENEVA Study Group. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12): Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52(7): Campochiaro PA, Heier JS, Feiner L, et al., BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6): Brown DM, Campochiaro PA, Singh RP, et al., CRUISE Investigators. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6): RVO-related macular edema don t do as well as those whose edema is resolved sooner, 14,15 and in patients who have a significant response to anti-vegf monotherapy, the response is most dramatic with the first injection. There may be additional positive impact with subsequent injections, but we re learning that if macular edema isn t at least 50% resolved after one injection, it is appropriate to consider adjunctive or alternative therapy with Ozurdex promptly. Again, the longer the edema persists, the less vision we re able to preserve. Individualized Treatment is the Best Course of Action An advantage of the Shasta study relative to prospective studies is that it reflects results obtained with Ozurdex in the real world, Dr. Capone says. More guidance on how best to treat RVO-associated macular edema will likely come from several studies currently under way (such as NCT ) that directly compare the safety and effectiveness of anti-vegf agents with the dexamethasone implant. In the meantime, according to Dr. Haller, Taking care of patients with this condition to the best of our ability means determining which approach is best for each individual in terms of safety, efficacy, potential complications and burden of care. 8. Boyer D, Heier J, Brown DM, Clark WL, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5): Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97(3): Chu YK, Chung EJ, Kwon OW, Lee JH, Koh HJ. Objective evaluation of cataract progression associated with a high dose intravitreal triamcinolone injection. Eye. 2008;22(7): Lang Y, Zemel E, Miller B, Perlman I. Retinal toxicity of intravitreal kenalog in albino rabbits. Retina. 2007;27(6): Roth DB, Verma V, Realini T, et al. Long-term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology. 2009;116: Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (shasta study). Retina. 2014;34(2): Yeh WS, Haller JA, Lanzetta P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology 2012;119(6): Daien V, Navarre S, Fesler P, et al. Visual acuity outcome and predictive factors after bevacizumab for central retinal vein occlusion. Eur J Ophthalmol 2012;22:
20
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Vascular Disease Ocular Manifestations of Systemic Hypertension
 Vascular Disease Ocular Manifestations of Systemic Hypertension Maynard L. Pohl, OD, FAAO Pacific Cataract & Laser Institute 10500 NE 8 th Street, Suite 1650 Bellevue, WA 98004 USA 425-462-7664 Cerebrovascular
Vascular Disease Ocular Manifestations of Systemic Hypertension Maynard L. Pohl, OD, FAAO Pacific Cataract & Laser Institute 10500 NE 8 th Street, Suite 1650 Bellevue, WA 98004 USA 425-462-7664 Cerebrovascular
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England. Retinal Vein Occlusion (RVO) with Macular oedema (MO)
 Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Macular edema (ME) is the most common
 MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
What you can expect with OZURDEX
 Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
OZURDEX. (dexamethasone intravitreal implant) 0.7 mg
 OZURDEX (dexamethasone intravitreal implant).7 mg HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing
OZURDEX (dexamethasone intravitreal implant).7 mg HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing
The Era of anti- - - VEGF Kirk L. Halvorson, OD
 The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA
 OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
Facts About Diabetic Eye Disease
 Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
An Update on Branch Retinal Vein Occlusion Treatment Studies. Amiee Ho, O.D. Pacific University College of Optometry
 An Update on Branch Retinal Vein Occlusion Treatment Studies Amiee Ho, O.D. Pacific University College of Optometry Course Description This course focuses on current treatment options available for macular
An Update on Branch Retinal Vein Occlusion Treatment Studies Amiee Ho, O.D. Pacific University College of Optometry Course Description This course focuses on current treatment options available for macular
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
A Patient s Guide to Diabetic Retinopathy
 Diabetic Retinopathy A Patient s Guide to Diabetic Retinopathy 840 Walnut Street, Philadelphia PA 19107 www.willseye.org Diabetic Retinopathy 1. Definition Diabetic retinopathy is a complication of diabetes
Diabetic Retinopathy A Patient s Guide to Diabetic Retinopathy 840 Walnut Street, Philadelphia PA 19107 www.willseye.org Diabetic Retinopathy 1. Definition Diabetic retinopathy is a complication of diabetes
Diabetic Retinopathy A Presentation for the Public
 Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
A Guide to Administering
 A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
Anti VEGF Agents in Retinal Disorders Current Scenario
 Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
FA Conference. Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences
 FA Conference Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences Patient Presentation CC: (sent by optometrist) Blurry/foggy vision HPI: 62 yo
FA Conference Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences Patient Presentation CC: (sent by optometrist) Blurry/foggy vision HPI: 62 yo
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
What you can expect with OZURDEX
 Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Treatment of Retinal Vein Occlusion (RVO)
 Manchester Royal Eye Hospital Medical Retina Services Information for Patients Treatment of Retinal Vein Occlusion (RVO) What is a Retinal Vein Occlusion (RVO)? The retina is the light sensitive layer
Manchester Royal Eye Hospital Medical Retina Services Information for Patients Treatment of Retinal Vein Occlusion (RVO) What is a Retinal Vein Occlusion (RVO)? The retina is the light sensitive layer
Retinal Vein Occlusion
 Retinal Update 2018 Retinal Vein Occlusion Case Presentations to Myself Branch Vein Occlusion What medical evaluation do you recommend for this 72 year old patient? Is there anything you ask of your medical
Retinal Update 2018 Retinal Vein Occlusion Case Presentations to Myself Branch Vein Occlusion What medical evaluation do you recommend for this 72 year old patient? Is there anything you ask of your medical
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION
 THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington
 Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg
 An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
The Human Eye. Cornea Iris. Pupil. Lens. Retina
 The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
Diabetic maculopathy 11/ An update on. Miss Vasuki Sivagnanavel
 Miss Vasuki Sivagnanavel Consultant Ophthalmologist An update on Diabetic maculopathy Despite advances in the management of diabetes, diabetic retinopathy is already the commonest cause of blindness among
Miss Vasuki Sivagnanavel Consultant Ophthalmologist An update on Diabetic maculopathy Despite advances in the management of diabetes, diabetic retinopathy is already the commonest cause of blindness among
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
SCHEDULING STATUS Schedule 4. PROPRIETARY NAME AND DOSAGE FORM OZURDEX intravitreal implant
 Page 1 of 10 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM intravitreal implant COMPOSITION One intravitreal implant contains: Dexamethasone 700 μg Excipients: Ester terminated 50:50 poly
Page 1 of 10 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM intravitreal implant COMPOSITION One intravitreal implant contains: Dexamethasone 700 μg Excipients: Ester terminated 50:50 poly
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
 Research Article http://www.alliedacademies.org/clinical-ophthalmology-and-vision-science/ A 2-year retrospective study of the treatment of retinal vein occlusion with dexamethasone 0.7 mg intravitreal
Research Article http://www.alliedacademies.org/clinical-ophthalmology-and-vision-science/ A 2-year retrospective study of the treatment of retinal vein occlusion with dexamethasone 0.7 mg intravitreal
CONTRAINDICATIONS Active ocular infections (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
Yoshiro Minami 1*, Taiji Nagaoka 2, Akihiro Ishibazawa 1,2 and Akitoshi Yoshida 2
 Minami et al. BMC Ophthalmology (2017) 17:90 DOI 10.1186/s12886-017-0485-4 RESEARCH ARTICLE Open Access Correlation between short- and long-term effects of intravitreal ranibizumab therapy on macular edema
Minami et al. BMC Ophthalmology (2017) 17:90 DOI 10.1186/s12886-017-0485-4 RESEARCH ARTICLE Open Access Correlation between short- and long-term effects of intravitreal ranibizumab therapy on macular edema
Recalcitrant Diabetic Macular Oedema: Therapeutic Options
 December 2007 A. Giridhar et al. - Recalcitrant DME 451 CONSULTATION S E C T I O N Recalcitrant Diabetic Macular Oedema: Therapeutic Options Dr. Cyrus M Shroff 1, Dr. N S Muralidhar 2, Dr. R Narayanan
December 2007 A. Giridhar et al. - Recalcitrant DME 451 CONSULTATION S E C T I O N Recalcitrant Diabetic Macular Oedema: Therapeutic Options Dr. Cyrus M Shroff 1, Dr. N S Muralidhar 2, Dr. R Narayanan
Clinical Case Presentation. Branch Retinal Vein Occlusion. Sarita M. Registered Nurse Whangarei Base Hospital
 Clinical Case Presentation on Branch Retinal Vein Occlusion Sarita M. Registered Nurse Whangarei Base Hospital Introduction Case Study Pathogenesis Clinical Features Investigations Treatment Follow-up
Clinical Case Presentation on Branch Retinal Vein Occlusion Sarita M. Registered Nurse Whangarei Base Hospital Introduction Case Study Pathogenesis Clinical Features Investigations Treatment Follow-up
Retinal vein occlusion (RVO) is a vascular disease
 INTRAVITREAL RANIBIZUMAB FOR RETINAL VEIN OCCLUSION THROUGH 1 YEAR IN CLINICAL PRACTICE TROELS BRYNSKOV, MD,* HENRIK KEMP, MD,* TORBEN L. SØRENSEN, MD, DMSC* Purpose: To evaluate the efficacy and safety
INTRAVITREAL RANIBIZUMAB FOR RETINAL VEIN OCCLUSION THROUGH 1 YEAR IN CLINICAL PRACTICE TROELS BRYNSKOV, MD,* HENRIK KEMP, MD,* TORBEN L. SØRENSEN, MD, DMSC* Purpose: To evaluate the efficacy and safety
Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012
 Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012 Proliferative Diabetic Retinopathy Laser Treatments Medical Treatment Surgical Treatment Diabetic Macular Edema Laser
Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012 Proliferative Diabetic Retinopathy Laser Treatments Medical Treatment Surgical Treatment Diabetic Macular Edema Laser
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS
 ORIGINAL ARTICLE ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS Aggarwal Somesh VP 1, Shah Sonali N 2, Bharwada Rekha M 3,
ORIGINAL ARTICLE ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS Aggarwal Somesh VP 1, Shah Sonali N 2, Bharwada Rekha M 3,
OCT Angiography in Primary Eye Care
 OCT Angiography in Primary Eye Care An Image Interpretation Primer Julie Rodman, OD, MS, FAAO and Nadia Waheed, MD, MPH Table of Contents Diabetic Retinopathy 3-6 Choroidal Neovascularization 7-9 Central
OCT Angiography in Primary Eye Care An Image Interpretation Primer Julie Rodman, OD, MS, FAAO and Nadia Waheed, MD, MPH Table of Contents Diabetic Retinopathy 3-6 Choroidal Neovascularization 7-9 Central
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
PART 1: GENERAL RETINAL ANATOMY
 PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
Diabetic Retinopatathy
 Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Research Article Differentiation between Good and Low-Responders to Intravitreal Ranibizumab for Macular Edema Secondary to Retinal Vein Occlusion
 Hindawi Publishing Corporation Journal of Ophthalmology Volume 2016, Article ID 9875741, 6 pages http://dx.doi.org/10.1155/2016/9875741 Research Article Differentiation between Good and Low-Responders
Hindawi Publishing Corporation Journal of Ophthalmology Volume 2016, Article ID 9875741, 6 pages http://dx.doi.org/10.1155/2016/9875741 Research Article Differentiation between Good and Low-Responders
Venous Occlusive Diseases
 Venous Occlusive Diseases Bruce R. Saran, MD Adjunct Assistant Clinical Professor of Medicine Scheie Eye Institute University of Pennsylvania School of Medicine Philadelphia, PA -a division of: RVO Demographics
Venous Occlusive Diseases Bruce R. Saran, MD Adjunct Assistant Clinical Professor of Medicine Scheie Eye Institute University of Pennsylvania School of Medicine Philadelphia, PA -a division of: RVO Demographics
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
MAXIMIZING PATIENT OUTCOMES IN DME:
 Insert to July/August 2015 MAXIMIZING PATIENT OUTCOMES IN DME: A DISCUSSION OF EVOLVING, EVIDENCE-BASED OPTIONS FOR CLINICAL PRACTICE An expert panel explores evolving treatment options. Supported via
Insert to July/August 2015 MAXIMIZING PATIENT OUTCOMES IN DME: A DISCUSSION OF EVOLVING, EVIDENCE-BASED OPTIONS FOR CLINICAL PRACTICE An expert panel explores evolving treatment options. Supported via
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014
 From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
ZEISS AngioPlex OCT Angiography. Clinical Case Reports
 Clinical Case Reports Proliferative Diabetic Retinopathy (PDR) Case Report 969 PROLIFERATIVE DIABETIC RETINOPATHY 1 1-year-old diabetic female presents for follow-up of proliferative diabetic retinopathy
Clinical Case Reports Proliferative Diabetic Retinopathy (PDR) Case Report 969 PROLIFERATIVE DIABETIC RETINOPATHY 1 1-year-old diabetic female presents for follow-up of proliferative diabetic retinopathy
The US Food and Drug Administration
 Insert to July/August 2011 Injection Techniques for the Dexamethasone Intravitreal Implant Covered topics include: Profiles of the Experienced Injector Preparing the Patient for the Injection Injection
Insert to July/August 2011 Injection Techniques for the Dexamethasone Intravitreal Implant Covered topics include: Profiles of the Experienced Injector Preparing the Patient for the Injection Injection
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
Applying New Data to Improve the Standard of Care in Retinal Disease. With articles by Gaurav K. Shah, MD Carl D. Regillo, MD.
 Supplement to July/August 2012 CME Activity Applying New Data to Improve the Standard of Care in Retinal Disease With articles by Gaurav K. Shah, MD Carl D. Regillo, MD Release date: August 2012. Expiration
Supplement to July/August 2012 CME Activity Applying New Data to Improve the Standard of Care in Retinal Disease With articles by Gaurav K. Shah, MD Carl D. Regillo, MD Release date: August 2012. Expiration
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Dexamethasone Intravitreal Implant Rescue Treatment for Bevacizumab Refractory Macular Edema Secondary to Branch Retinal Vein Occlusion
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2017;31(2):108-114 https://doi.org/10.3341/kjo.2017.31.2.108 Original Article Dexamethasone Intravitreal Implant Rescue Treatment for Bevacizumab Refractory
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2017;31(2):108-114 https://doi.org/10.3341/kjo.2017.31.2.108 Original Article Dexamethasone Intravitreal Implant Rescue Treatment for Bevacizumab Refractory
The Foundation WHAT IS THE RETINA? continued next page. RETINA HEALTH SERIES Facts from the ASRS
 The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Intravitreal Injections An intravitreal (pronounced in tra VIT re al)
The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Intravitreal Injections An intravitreal (pronounced in tra VIT re al)
Original Effective Date: 10/24/2016. Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant)
 Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
New Developments in the treatment of Diabetic Retinopathy
 New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
Brampton Hurontario Street Brampton, ON L6Y 0P6
 Diabetic Retinopathy What is Diabetic Retinopathy Diabetic retinopathy is one of the leading causes of blindness world-wide. Diabetes damages blood vessels in many organs of the body including the eyes.
Diabetic Retinopathy What is Diabetic Retinopathy Diabetic retinopathy is one of the leading causes of blindness world-wide. Diabetes damages blood vessels in many organs of the body including the eyes.
SUMMARY. Heather Casparis, MD,* and Neil M. Bressler, MD MARINA AND ANCHOR
 The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
Diabetic Retinopathy: Managing the Extremes. J. Michael Jumper, MD West Coast Retina
 Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
PRODUCT MONOGRAPH. (dexamethasone) Intravitreal Implant 0.7 mg. Corticosteroid. Allergan Inc. Markham, ON L6G 0B5. Date of Revision: April 14, 2015
 PRODUCT MONOGRAPH Pr OZURDEX (dexamethasone) Intravitreal Implant 0.7 mg Corticosteroid Allergan Inc. Markham, ON L6G 0B5 Date of Revision: April 14, 2015 Submission Control No: 169671 Page 1 of 36 Table
PRODUCT MONOGRAPH Pr OZURDEX (dexamethasone) Intravitreal Implant 0.7 mg Corticosteroid Allergan Inc. Markham, ON L6G 0B5 Date of Revision: April 14, 2015 Submission Control No: 169671 Page 1 of 36 Table
Posterior Segment Macular Edema
 Posterior Segment Macular Edema Treatment of Macular Edema following Branch Retinal Vein Occlusion Raafay Sophie, MD 1 and Peter A Campochiaro, MD 2 1. Post-doctoral Fellow; 2. Eccles Professor of Ophthalmology
Posterior Segment Macular Edema Treatment of Macular Edema following Branch Retinal Vein Occlusion Raafay Sophie, MD 1 and Peter A Campochiaro, MD 2 1. Post-doctoral Fellow; 2. Eccles Professor of Ophthalmology
RVO RETINAL VEIN OCCLUSION
 RVO RETINAL VEIN OCCLUSION A guide to understanding RVO Take some time to learn about RVO - it may help you hold on to your vision Retinal vein occlusion is a common disorder of the retina and a leading
RVO RETINAL VEIN OCCLUSION A guide to understanding RVO Take some time to learn about RVO - it may help you hold on to your vision Retinal vein occlusion is a common disorder of the retina and a leading
Intravitreal Injection
 for patients Eye Clinic Ipswich Hospital Tel: 01473 703230 Intravitreal Injection What is an intravitreal injection? An intravitreal injection is the injection of a drug into the vitreous body (the jelly
for patients Eye Clinic Ipswich Hospital Tel: 01473 703230 Intravitreal Injection What is an intravitreal injection? An intravitreal injection is the injection of a drug into the vitreous body (the jelly
The Foundation WHAT IS THE RETINA?
 The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Branch Retinal Vein Occlusion Retinal vein occlusions occur when there
The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Branch Retinal Vein Occlusion Retinal vein occlusions occur when there
Applying New Data to Improve the Standard of Care in Retinal Diseases Managing Macular Edema Associated With Retinal Venous Occlusions
 Supplement to November/December 2013 CME Activity Applying New Data to Improve the Standard of Care in Retinal Diseases Managing Macular Edema Associated With Retinal Venous Occlusions By Michael Singer,
Supplement to November/December 2013 CME Activity Applying New Data to Improve the Standard of Care in Retinal Diseases Managing Macular Edema Associated With Retinal Venous Occlusions By Michael Singer,
Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283
 Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283 NICE 2018. All rights
Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion Technology appraisal guidance Published: 22 May 2013 nice.org.uk/guidance/ta283 NICE 2018. All rights
COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY
 Original Article COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY Aggarwal Somesh V 1, Shah Sonali N 2, Bharwada Rekha
Original Article COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY Aggarwal Somesh V 1, Shah Sonali N 2, Bharwada Rekha
The Foundation. continued next page. RETINA HEALTH SERIES Facts from the ASRS
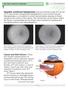 The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Idiopathic Juxtafoveal Telangiectasis (pronounced tell an gee ACT te
The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Idiopathic Juxtafoveal Telangiectasis (pronounced tell an gee ACT te
Package leaflet: information for the user. OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone
 Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Supplement to March Ranibizumab: Expanding Horizons in Retinal Vein Occlusion Management. Sponsored by Novartis Pharma AG
 Supplement to March 2015 Ranibizumab: Expanding Horizons in Retinal Vein Occlusion Management Sponsored by Novartis Pharma AG Ranibizumab: Expanding Horizons in Retinal Vein Occlusion Management This supplement
Supplement to March 2015 Ranibizumab: Expanding Horizons in Retinal Vein Occlusion Management Sponsored by Novartis Pharma AG Ranibizumab: Expanding Horizons in Retinal Vein Occlusion Management This supplement
FROM OUTDATED TO UPDATED Eminence-Based Medicine
 FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer
 aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
Ophthalmology Macular Pathways
 Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Age-Related Macular Degeneration (AMD)
 Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Neuropathy (NAION) and Avastin. Clinical Assembly of the AOCOO-HNS Foundation May 9, 2013
 Non Arteritic Ischemic Optic Neuropathy (NAION) and Avastin Shalom Kelman, MD Clinical Assembly of the AOCOO-HNS Foundation May 9, 2013 Anterior Ischemic Optic Neuropathy Acute, painless, visual loss,
Non Arteritic Ischemic Optic Neuropathy (NAION) and Avastin Shalom Kelman, MD Clinical Assembly of the AOCOO-HNS Foundation May 9, 2013 Anterior Ischemic Optic Neuropathy Acute, painless, visual loss,
IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
Instudies of vascular endothelial growth factor
 MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY
 EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY Diwakar chaudhary *1, 2, Hu shuqiong, Long Yuan and Xiong kun 1 Yangtze University, 1 Nanhuan Road
EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY Diwakar chaudhary *1, 2, Hu shuqiong, Long Yuan and Xiong kun 1 Yangtze University, 1 Nanhuan Road
DIABETIC RETINOPATHY
 THE UK GUIDE DIABETIC RETINOPATHY Everything you need to know about diabetic retinopathy Jaheed Khan BSc (Hons) MBBS MD FRCOphth Fellow of the Royal College of Ophthalmologists Association for Research
THE UK GUIDE DIABETIC RETINOPATHY Everything you need to know about diabetic retinopathy Jaheed Khan BSc (Hons) MBBS MD FRCOphth Fellow of the Royal College of Ophthalmologists Association for Research
CORNEAL CONDITIONS CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
AUSTRALIAN PRODUCT INFORMATION OZURDEX (DEXAMETHASONE) INTRAVITREAL IMPLANT
 AUSTRALIAN PRODUCT INFORMATION OZURDEX (DEXAMETHASONE) INTRAVITREAL IMPLANT 1 NAME OF THE MEDICINE Dexamethasone 2 QUALITATIVE AND QUANTITATIVE COMPOSITION OZURDEX is a biodegradable intravitreal implant
AUSTRALIAN PRODUCT INFORMATION OZURDEX (DEXAMETHASONE) INTRAVITREAL IMPLANT 1 NAME OF THE MEDICINE Dexamethasone 2 QUALITATIVE AND QUANTITATIVE COMPOSITION OZURDEX is a biodegradable intravitreal implant
REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - Executive Summary Table below presents the key metrics of Eylea for macular edema (ME) and age-related
REFERENCE CODE GDHC482DFR PUBLICAT ION DATE OCTOBER 2014 EYLEA (MACULAR EDEMA AND MACULAR DEGENERATION) - Executive Summary Table below presents the key metrics of Eylea for macular edema (ME) and age-related
Past ocular history. DME Case 1. Patient presents blurred VA. Hemoglobin A1c 11.5% -- patient states sugars have not been in good control
 Past ocular history Patient presents blurred VA DME Case 1 Hemoglobin A1c 11.5% -- patient states sugars have not been in good control PDR with macular edema OU Rishi Singh MD Cleveland Clinic OD OS 1
Past ocular history Patient presents blurred VA DME Case 1 Hemoglobin A1c 11.5% -- patient states sugars have not been in good control PDR with macular edema OU Rishi Singh MD Cleveland Clinic OD OS 1
Comparison of BRVO and CRVO management
 Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection
 Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
Information for Patients Manchester Royal Eye Hospital Medical Retina Services Treatment of Age Related Macular Degeneration (AMD) by Intravitreal Injection What is age related macular degeneration (AMD)?
Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant
 Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Retinal Complications of Obstructive Sleep Apnea A Growing Concern!
 Retinal Complications of Obstructive Sleep Apnea A Growing Concern! Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl
Retinal Complications of Obstructive Sleep Apnea A Growing Concern! Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl
Clinical Trials in Diabetic Retinopathy. Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D.
 1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? WHAT CAUSES DIABETIC RETINOPATHY? WHAT ARE THE STAGES OF DIABETIC RETINOPATHY?
 Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? Diabetic retinopathy affects 8 million Americans with diabetes. A leading cause of blindness in American adults, it is caused by damage to the small blood
Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? Diabetic retinopathy affects 8 million Americans with diabetes. A leading cause of blindness in American adults, it is caused by damage to the small blood
