alkalosis, which is characterized by the paradoxical
|
|
|
- Britton Ramsey
- 5 years ago
- Views:
Transcription
1 EFFECTS OF POTASSIUM ON RENAL TUBULAR REABSORPTION OF BICARBONATE"12 By KATHLEEN E. ROBERTS, HENRY T. RANDALL, HAROLD L. SANDERS, AND MARGARET HOOD WITH THE TECHNICAL ASSISTANCE OF SERGEI DENECKO, RITA LIPTON, AND FRANKIE LAWSON (From The Division of Experimental Surgery, Andre and Bella Meyer Physiology Laboratories of The Sloan-Kettering Institute, and The Departments of Surgery and Medicine, Memorial Center, New York, N. Y.) (Submitted for publication September 1, 1954; accepted December 22, 1954) It is generally recognized that potassium deprivation may result in the development of a plasma alkalosis, which is characterized by the paradoxical excretion of an acid urine (1-12). Conversely, the administration of sufficient potassium chloride results in a plasma acidosis and the simultaneous excretion of an alkaline urine (8, 13-16). Studies previously reported have shown that the dichotomous plasma and urinary findings following potassium administration result from a decrease in the renal tubular reabsorption of base-bound bicarbonate (13). The inappropriate aciduria occurring in hypokalemic alkalosis has led to the suggestion that renal reabsorption of bicarbonate is increased in circumstances where inadequate intake or excessive losses of potassium have occurred (1, 13, 17). This implies a renal failure to compensate the plasma alkalosis accompanying hypokalemia and assumes a renal contribution to the continuation of the plasma alkalosis. Although it has not been measured previously, it would appear virtually certain that hypokalemia enhances the renal tubular reabsorption of bicarbonate; the mechanism whereby the influence of potassium is mediated, however, remains more speculative (1, 13, 17). Accordingly, the studies described herein were carried out on human subjects and on dogs 1) to determine the effects of potassium depletion on the renal mechanisms implicated in stabilizing the plasma bicarbonate, and 2) to clarify the mechanisms whereby potassium influences the renal tubular reabsorption and urinary excretion of bicarbonate bound base. The studies reported below indicate that hypokalemia results in an increased renal tubular reab- 1 This work was presented, in part, at the meetings of the American Society for Clinical Investigation in Atlantic City, May, This work was supported by United States Public Health Service Grant H sorption of bicarbonate, and that the effects of potassium are independent of alterations in plasma pco2. METHODS The following experiments were carried out on 1 human subjects and on 6 dogs. Six of the patients had gastric cancer and were studied on a metabolic balance regimen: a) During a control period; and b) during an interval of potassium deprivation. The studies completed on these patients included measurements of glomerular filtration rate and urinary bicarbonate, sodium, potassium, chloride, and ph. Plasma was analyzed daily for ph, carbon dioxide content, chloride, sodium and potassium. Metabolic studies included daily measurements and analyses of intake and fecal drainage, and urinary losses of chloride, potassium and sodium. During the control period, the renal tubular reabsorption; of bicarbonate was determined previous to and following the infusion of 1 to 15 cc. of M/6 lactate or 2 per cent sodium bicarbonate to ensure frank excretion of bicarbonate and an alkaline urine. The patients were then maintained, following gastrectomy, on potassium-free, parenteral fluids for an interval of 5 to 19 days. Daily fluid replacement given the patients included glucose in water, normal saline, and B vitamins. No postoperative complications occurred in the patients during the interval of study reported here. At the termination of the hypokalemic period, the renal tubular excretion and reabsorption of bicarbonate was again measured and compared to that observed in the control period.8 Four additional patients were studied during an episode of hypokalemic alkalosis. In these patients, measurement of the renal excretion and reabsorption of bicarbonate was made during the period of alkalosis; no metabolic studies were done in these patients.4 (In two of these patients renal clearances were measured during a period of adequate potassium supplementa- Metabolic balances showed a negative potassium balance in these patients ranging between 18 and 28 meq.; no attempt was made in these studies to correlate the degree of potassium depletion with bicarbonate reabsorption. ' One of these patients had hyperadrenocorticism. Two patients were receiving cortisone with inadequate potassium supplementation and one patient had potassium depletion resulting from an inadequate dietary intake and diarrhea secondary to a severe dumping syndrome.
2 POTASSIUM AND RENAL REABSORPTION OF BICARBONATE 667 tion and at a time when plasma electrolytes were normal.) The experiments carried out on dogs were designed to determine the effects of potassium on the enhanced bicarbonate reabsorption, which has been reported to occur following elevations in plasma pco, (17-19). In these experiments, sodium bicarbonate was infused throughout to ensure adequate urinary excretion of bicarbonate; the animals were then given 1 per cent CO2 to breathe for periods of two to six hours, and the renal tubular reabsorption of bicarbonate determined before and after the infusion of potassium chloride, given at rates of.3 to.5 meq. per minute for 12 to 36 minutes. The clearance of inulin was utilized as a measure of glomerular filtration rate in the human subjects and in the dogs. The methods employed in the chemical analysis and collection of samples have been described in previous communications (13, 2). Urine was collected under oil from an indwelling catheter for ph and carbon dioxide analyses, which were done within two to five hours after collection. The remaining urine was preserved for subsequent analysis. Plasma analyses of total CO, and ph in the patients were determined on venous blood drawn without stasis; in the dogs the plasma analyses were determined from arterial blood. The reabsorption of bicarbonate was calculated as that amount reabsorbed per 1 cc. of glomerular filtrate by subtracting the excreted moiety from that filtered. RESULTS Table I shows the renal tubular reabsorption and excretion of bicarbonate in one of the patients TABLE I Reabsorption of bicarbonate bound base in the control period previous to and during the infusion of sufficient M/6 lactate to ekvate the plasma bicarbonate to lvels shown here and in the same patient fouowing an interval of potassium free fluids for six days Urine Bicarbonate Plasma Glom. Excreted Reabsorbed Time bicarbonate filt. rate meq./ meq./1 mis. meq./l. cc./mix. mi. cc. G.F. ph Lactate infusion -18 Infuse inulin in 5% g./w at 2.7 cc./min Infuse M lactate 5.5 cc./min. (Continue inulin infusion) Potassium deprivation six days -18 Infuse inulin 5% g./w at 2.46 cc./min a %i 3.2 E 3., w 2.8 LI : CD I II O HYPOKALEMIC ALKALOSIS * NORMAL la** *. a PLASMA BICARBONATE (meq/l) FIG. 1. REABSORPTION OF BICARBONATE IN NoRMAL PA- TIENTS AND IN HYPOKALEMIA PATINTS The solid circles represent single determinations of bicarbonate reabsorption as calculated prior to and during the infusion of alkali. Four to five determinations of bicarbonate reabsorption were measured on each patient at varying plasma levels of bicarbonate as indicated. The open circles represent the average bicarbonate reabsorption as calculated from three clearance periods and three plasma analyses during the period of hypokalemia. (Bicarbonate reabsorption was measured in four of the patients on two separate occasions during the interval of hypokalemia.) studied: a) In the control period following the infusion of sodium lactate; and b) following an interval of six days of potassium deprivation after surgery. The infusion of lactate resulted in frank urinary excretion of bicarbonate and an alkaline urine, whereas the renal tubular reabsorption of bicarbonate remained essentially normal. The same patient during the hypokalemia interval, at a time when plasma levels of bicarbonate and ph showed an elevation comparable to that seen following lactate, showed an increase in bicarbonate reabsorption and the excretion of an acid urine containing sparse amounts of bicarbonate. The data obtained in this patient were similar to those observed in all of the patients studied, and are summarized in Figure 1. This figure illustrates the difference in renal tubular reabsorption of bicarbonate in normal patients given an alkaline load, and in patients exhibiting hypokalemic alkalosis.5 5 It has been emphasized that potassium influences bicarbonate reabsorption only with intracellular losses of considerable magnitude; no alteration in bicarbonate reabsorption occurs following an acute decrease in extracellular potassium concentration as produced by alkali in-
3 668 KATHLEEN ROBERTS, HENRY RANDALL, HAROLD SANDERS, AND MARGARET HOOD TABLE 1I Measured glomerular ration rates and clclated plasma pco2 wvues in ten paints during hypokakemia and in eight patis during a control period when adeqate potassium was being administered * Control Glom. pc2 filt. rate mm. Hg cc./min. 1) t 89 2) ) t 99 4) t 98 5) sot 1 6) ) ) 9) 1) Hypokalemia Glom. pco filt. mte Cc.1mms. mm. Hg t t t * The figures for glomerular filtration represent three separate dearance periods. The plasma pco2 represents values calculated from three separate blood samples analyzed in duplicate. t Blood drawn after morphine administration. As shown here, the reabsorption of bicarbonate was increased in the hypokalemic patients, as compared to the normal control values of 2.7 to 2.8 meq. per 1 cc. of glomerular filtrate. This fusion or glucose and insulin (13). Furthermore, it is generally recognized that plasma K levels per se are a poor reflection of total body stores of potassium as measured by metabolic balances or isotopic dilution studies (21). The potassium depleted patients presented here had sustained intracellular as well as plasma losses of potassium. was found to be true at all levels of plasma bicarbonate shown. Table II shows the calculated plasma pco2 and glomerular filtration rates in our patients during the control period and during potassium deprivation. As indicated here, no marked changes in plasma pco2 occurred in these patients despite a plasma alkalosis and an enhanced bicarbonate reabsorption. From this table it is also apparent that a decrease in glomerular filtration rate could not account for the enhanced bicarbonate reabsorption in all of the patients studied., To clarify the effects of potassium on bicarbonate reabsorption and the relationship between potassium and pco2, experiments were carried out on six dogs. In these experiments, the effects of potassium on the elevated bicarbonate reabsorption, which has been reported to occur in the presence of an elevated plasma pco2, was evaluated. A typical experiment is presented in Table III; as shown here, the elevated bicarbonate reabsorption occurring in respiratory acidosis is decreased by the simultaneous administration of potassium chloride. Furthermore, the decrease in bicarbonate reabsorption following potassium administration occurs with plasma levels of pco2 which are equivalent to those seen during the period of respiratory acidosis alone. The effects of potassium and respiratory acidosis on bicarbonate reabsorption were consistent in all the dogs studied and are summarized in Figure 2. In this figure, the bicarbonate reabsorption per 1 cc. filtrate is plotted in relation to the plasma pco2; the crosshatched area summarizes the range of normal bicarbonate reabsorption in dogs, as calculated from previously published data (13). As illustrated by the solid circles, the administration of potassium chloride decreased bicarbonate reabsorption to normal levels irrespective of elevations in plasma pco2; the open circles confirm the observations of others that elevations of plasma pco2 per se enhance bicarbonate reabsorption (17-19). 6 We have observed and there has been reported a profound decrease in glomerular filtration rate in hypokalemic alkalosis; this was, however, noted by us only in those patients who were more severely alkalotic than the patients reported here, and has been seen most often by us in situations of hypochloremic alkalosis following excessive losses of gastric contents and extracellular fluid.
4 POTASSIUM AND RENAL REABSORPTION OF BICARBONATE 669 TABLE III Efects of potassium on the enhanced bicarbonate reabsorption occurring with elevation of plasma pco2 in dogs Plasma Bicarbonate Potassium Bicarbonate Glom. Reabsorbed Time pc2 filt. rate Excreted meq./1 cc. mins. meq./l. mm. Hg cc./min. meq./mis. gkom. fil. Prime-NaHCO&-5 gm. Start infusion; NaHCOs-1 gm./l.; inulin-1 gm./l. at S cc./min Inspire 1% CO-9% Os Start KC1-.4 meq./min Urine C g 2 i V I- 8 o O RESPIRATORY ACIDOSIS * RESPIRATORY ACIDOSIS + KCI O NOMAL RANGE *26 m I. 26 'W TO7 so PLSMA pco2 Ama No.) FIG. 2. BICARBONATE REABSORPTION IN RELATION TO PLASMA PCO, IN DOGS BREATHING 1 PER CENT CO, BEFORE (OPEN CIRCLES) AND AFTER THE ADMINISTRATION OF POTASSIUM CHLORIDE (CLOSED Car ws) DISCUSSION It is apparent from the data presented that the renal tubular reabsorption of base-bound bicarbonate is increased in the situation of hypokalemia. The enhanced bicarbonate reabsorption in turn would appear to contribute to the plasma alkalosis frequently accompanying potassium depletion. Evidence has been presented that potassium per se affects those renal mechanisms implicated in the stabilization of plasma bicarbonate. Although a decrease in glomerular filtration rate is known to increase bicarbonate reabsorption (22), the changes in glomerular filtration rate reported here could not account for the enhanced bicarbonate reabsorption in all the patients studied. In addition, it has been found that potassium chloride administration will decrease bicarbonate reabsorption in dogs irrespective of alterations in glomerular filtration rate (23). The notable observations (17-19) that elevations in plasma pco2 increase the renal absorption of bicarbonate have led to the implication that elevations in plasma pco2 could enhance the reabsorption of bicarbonate in hypokalemia as well as in respiratory acidosis. The finding in our patients that the plasma pco2 was little altered does
5 -67 KATHLEEN ROBERTS, HENRY RANDALL, HAROLD SANDERS, AND MARGARET HOOD not furnish credence to this assumption. The consistent finding of an elevated total CO2 and blood ph with little change in plasma pco2 indicates that hypokalemic alkalosis as studied in our patients was uncompensated. Similar elevations of the blood ph with a normal pco2 have been observed by us in hypochloremic alkalosis and following alkali infusion in both dog and man. Only with superimposed pulmonary disease, anesthesia or with morphine administration have we consistently observed "respiratory compensations" to occur in metabolic alkalosis (24). The finding in dogs that potassium decreased the renal tubular reabsorption of bicarbonate to normal levels, irrespective of the level of plasma pco2, indicates that potassium per se influences bicarbonate reabsorption even with elevations of plasma pco2. From these experiments and those reported elsewhere, it would appear that potassium and pco2 affect the renal tubular bicarbonate reabsorption independently and in the opposite direction. This apparent antagonism and the mechanisms whereby pco2 and potassium affect bicarbonate reabsorption may be reconciled, if Hypadmlic AUuclosis I I~h Pd_wbmI A_boni lcvvv consideration is given to implicated alterations of the ph within the renal tubular cell, as summarized in Figure 3 (1, 17, 25-27). With excessive potassium losses or deprivation, there occurs a depletion of the major intracellular cation. In this situation, a relative intracellular acidosis occurs (1, 2, 12, 27, 28), and the measm ALOS INTRACELLULAR, el p2 I Psm _ phi RofWEAOIPTION I f FIG. 13,r *t FIG. 3. RELAIONSHIP BETWEEN ESTIMATED INTrAcELLULAR PH IN THE RENAL TUBULES AND MEASURE BICABONATE REBSORPON f ured bicarbonate reabsorption is increased. The opposite occurs following potassium administration; the increase in intracellular cation concentration which occurs could result in an elevation of the intracellular ph (16). In this situation, there is a decrease in the measured bicarbonate reabsorption. Conceivably, a change in extracellular pco2 results in an equivalent change in intracellular pco2, since carbon dioxide is believed to permeate all cells, and rapid equilibration is achieved between the extra- and intracellular compartments. Thus, changes in the extracellular ph resulting from alterations in carbonic acid could effect a similar alteration in the intracellular compartment (29). In respiratory acidosis, elevation of the plasma pco2 and thus, intracellular carbonic acid, conceivably decreases the intracellular ph. Under these circumstances, the measured bicarbonate reabsorption is increased (17-19). If the simultaneous administration of potassium minimizes the alterations in intracellular ph by increasing the intracellular cation concentration coincident with the increase of intracellular carbonic acid, the enhanced bicarbonate reabsorption occasioned by the elevated pco2 should decrease. The experiments summarized in Figure 2 indicate that this does occur, and also provide indirect evidence that alterations in intracellular ph, secondary to alterations in intracellular pco2 and potassium, influence the renal tubular reabsorption of bicarbonate. Presumably the decrease in bicarbonate reabsorption following carbonic anhydrase inhibition could also be mediated through changes in intracellular ph (1). Inhibition of the formation of carbonic acid within the renal tubular cell could result in an increased ph and thus, a measured decrease in bicarbonate reabsorption. Similarly, the increased bicarbonate excretion, which has been observed following respiratory alkalosis (19, 3, 31) with a decrease in plasma pco2, could be accounted for by a decrease in intracellular carbonic acid and elevation of the ph; under these circumstances, the measured renal reabsorption of bicarbonate is decreased. Although it is impossible by methods currently available to state with certainty that alterations in intracellular potassium do not occur following administration of carbonic anhydrase inhibitors or with alterations in plasma pco2, evidence pre-
6 POTASSIUM AND RENAL REABSORPTION OF BICARBONATE sented elsewhere indicates that the potassium concentration within the renal tubular cell is unchanged acutely in respiratory acidosis or following the administration of carbonic anhydrase inhibitors (32). From these studies in which measurements of the renal extraction of potassium from arterial blood was compared to the urinary excretion, it was concluded that all potassium removed from the arterial blood during a given interval was excreted in the urine during the same interval. Only with potassium chloride administration was there evidence that the potassium extracted from the arterial blood was not excreted in toto and presumably entered the renal tubular cell. This indirect evidence precludes the implication that alterations in the intracellular potassium per se are responsible for the changes in bicarbonate reabsorption observed in respiratory acidosis or following a carbonic anhydrase inhibitor. The importance of intracellular ph alterations in conditioning the reabsorption of bicarbonate has obvious importance in maintaining the bicarbonate concentration of extracellular fluid. SUMMARY AND CONCLUSIONS 1. The renal tubular reabsorption of bicarbonate is increased in hypokalemic patients. 2. The increased reabsorption of bicarbonate could not be accounted for by elevations of plasma pco2. 3. In dogs, the elevated bicarbonate reabsorption, which occurs following elevation of the plasma pco2, is returned to normal by the administration of potassium chloride without a change in pco2. 4. Indirect evidence has been presented that a relative change in ph within the renal tubular cells may be the common denominator by which alterations in potassium and pco2 affect bicarbonate reabsorption. REFERENCES 1. Berliner, R. W., Kennedy, T. J., Jr., and Orloff, J., Relationship between acidification of urine and potassium metabolism: Effect of carbonic anhydrase inhibition on potassium excretion. Am. J. Med., 1951, 11, Black, D. A. K, and Milne, M. D., Experimental potassium depletion in man. Clin. Sc., 1952, 11, Willson, D. M., Power, M. H., and Kepler, E. J., Alkalosis and low plasma potassium in a case of Cushing's Syndrome: A metabolic study. J. Clin. Invest., 194, 19, Van Slyke, K K., and Evans, E. I., The paradox of aciduria in the presence of alkalosis caused by hypochloremia. Ann. Surg., 1947, 126, Randall, H. T., Habif, D. V., Lockwood, J. S., and Werner, S. C., Potassium deficiency in surgical patients. Surgery, 1949, 26, Peters, J. P., and Van Slyke, D. D., Quantitative Clinical Chemistry, Vol. I. Baltimore, Williams & Wilkins, Gamble, J. L., Fahey, K. R, Appleton, J., and Mac- Lachlan, E., Congenital alkalosis with diarrhea. J. Pediat., 1945, 26, Broch,. J., Low potassium alkalosis with acid urine in ulcerative colitis. Scandinav. J. Gin. & Lab. Invest., 195, 2, Eliel, L. P., Pearson,. H., and Rawson, R. W., Postoperative potassium deficit and metabolic alkalosis. New England J. Med., 195, 243, Howard, J. E., and Carey, R. A., The use of potassium in therapy.. Clin. Endocrinol., 1949, 9, Darrow, D. C., Congenital alkalosis with diarrhea. J. Pediat., 1945, 26, Darrow, D. C., Body-fluid physiology: The role of potassium in clinical disturbances of body water and electrolyte. New England J. Med., 195, 242, 978, and 114 (concluded). 13. Roberts, K. E., Magida, M. G., and Pitts, R. F., Relationship between potassium and bicarbonate in blood and urine. Am. J. Physiol., 1953, 172, Winkler, A. W., and Smith, P. K, Renal excretion of potassium salts. Am. J. Physiol., 1942, 138, Berliner, R. W., Kennedy, T. J., Jr., and Hilton, J. G., Renal mechanisms for excretion of potassium. Am. J. Physiol., 195, 162, Bourdillon, J., Distribution in body fluids and excretion of ingested ammonium chloride, potassium chloride, and sodium chloride. Am. J. Physiol., 1937, 12, Relman, A. S., Etsten, B., and Schwartz, W. B., The regulation of renal bicarbonate reabsorption by plasma carbon dioxide tension. 3. Gin. Invest., 1953, 32, Dorman, P. J., Sullivan, W. J., and Pitts, R. F., The renal response to acute respiratory acidosis. J. Gin. Invest., 1954, 33, Brazeau, P., and Gilman, A., Effect of plasma CO, tension on renal tubular reabsorption of bicarbonate. Am. J. Physiol., 1953, 175, Roberts, K. E., Randall, H. T., Farr, H. W., Kidwell, A. P., McNeer, G. P., and Pack, G. T., Cardiovascular and blood volume alterations resulting from intrajejunal administration of hypertonic solutions to gastrectomized patients: The
7 672 KATHLEEN ROBERTS, HENRY RANDALL, HAROLD SANDERS, AND MARGARET HOOD relationship of these changes to the dumping syndrome. Ann. Surg., 1954, 14, Moore, F. D., Edelman, I. S., Olney, J. M., James, A. H., Brooks, L., and Wilson, G. M., Body sodium and potassium: III. Interrelated trends in alimentary, renal and cardiovascular disease; lack of correlation between body stores and plasma concentration. Metabolism, 1954, 3, Thompson, D. D., and Barrett, W. J., Effects of variations in glomerular filtration rate on bicarbonate absorption. Federation Proc., 1953, 12, Roberts, K E., Unpublished observations. 24. Roberts, K. E., Poppell, J. W., Beals, R., Randall, H. T., and Vanamee, P., Failure of respiratory compensations in metabolic alkalosis. In preparation. 25. Berliner, R. W., Renal secretion of potassium and hydrogen ions. Federation Proc., 1952, 11, Orloff, J., Kennedy, T. J., Jr., and Berliner, R. W., The effect of potassium in nephrectomized rats with hypokalemic alkalosis. J. Clin. Invest., 1953, 32, Gardner, L. I., MacLachlan, E. A., and Berman, H., Effect of potassium deficiency on carbon dioxide, cation, and phosphate content of muscle, with a note on the carbon dioxide content of human muscle. J. Gen. Physiol., 1952, 36, Cooke, R. E., Segar, W. E., Cheek, D. B., Coville, F. E., and Darrow, D. C., The extrarenal correction of alkalosis associated with potassium deficiency. J. Clin. Invest., 1952, 31, Wallace, W. M., and Hastings, A. B., The distribution of the bicarbonate ion in mammalian muscle. J. Biol. Chem., 1942, 144, Stanbury, S. W., and Thomson, A. E., The renal response to respiratory alkalosis. Clin. Sc., 1952, 11, Singer, R. B., Clark, J. K., Barker, E. S., and Elkinton, J. R., The effects of acute respiratory alkalosis on electrolyte excretion and renal hemodynamics in man. J. Clin. Invest., 1952, 31, Milch, R. A., Randall, H. T., and Roberts, K. E., Unpublished observations. SPECIAL NOTICE TO SUBSCRIBERS Post Offices will no longer forward the Journal when you move. Please notify The Journal of Clinical Investigation, Business Office, 622 West 168th Street, New York 32, N. Y. at once when you have a change of address, and do not omit the zone number if there is one.
2.5 per cent solution). A Magill endotracheal tube fitted. with an inflatable balloon was introduced into the trachea
 THE REGULATION OF RENAL BICARBONATE REABSORPTION BY PLASMA CARBON DIOXIDE TENSION ' By ARNOLD S. RELMAN, BENJAMIN ETSTEN, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals
THE REGULATION OF RENAL BICARBONATE REABSORPTION BY PLASMA CARBON DIOXIDE TENSION ' By ARNOLD S. RELMAN, BENJAMIN ETSTEN, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals
only. Analysis of human muscle obtained by biopsy suggests that changes
 A PROPOSED MECHANISM OF EXTRACELLLAR REGLATION OF MSCLE COMPOSITION* ROBERT E. COOKE** AND WILLIAM E. SEGAR The changes in muscle composition which occur in disturbances of extracellular electrolyte composition
A PROPOSED MECHANISM OF EXTRACELLLAR REGLATION OF MSCLE COMPOSITION* ROBERT E. COOKE** AND WILLIAM E. SEGAR The changes in muscle composition which occur in disturbances of extracellular electrolyte composition
rapidity. It has also been reported that addition
 THE ACIDIFYING EFFECT OF RUBIDIUM IN NORMAL AND POTASSIUM-DEFICIENT ALKALOTIC RATS 1 By ARNOLD S. RELMAN, ARLENE M. ROY, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals,
THE ACIDIFYING EFFECT OF RUBIDIUM IN NORMAL AND POTASSIUM-DEFICIENT ALKALOTIC RATS 1 By ARNOLD S. RELMAN, ARLENE M. ROY, AND WILLIAM B. SCHWARTZ (From the Evans Memorial, Massachusetts Memorial Hospitals,
The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency *
 lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
lournal of Clinical Investigation Vol. 45, No. 4, 1966 The Induction of Metabolic Alkalosis by Correction of Potassium Deficiency * HOWARD L. BLEICH,t RICHARD L. TANNEN,t AND WILLIAM B. SCHWARTZ t (From
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
Singer and Hastings (7) have indicated that the. data of Shock and Hastings (8) gives an apparent
 INTRACELLULAR CATION EXCHANGES IN ACIDOSIS DUE TO RENAL INSUFFICIENCY. EFFECTS OF ALKALI THERAPY1 By J. R. ELKINTON,2 R. D. SQUIRES,3 AND R. B. SINGER (From the Chemical Section of the Department of Medicine,
INTRACELLULAR CATION EXCHANGES IN ACIDOSIS DUE TO RENAL INSUFFICIENCY. EFFECTS OF ALKALI THERAPY1 By J. R. ELKINTON,2 R. D. SQUIRES,3 AND R. B. SINGER (From the Chemical Section of the Department of Medicine,
Acid-Base Imbalance-2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD
 AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
AcidBase Imbalance2 Lecture 9 (12/4/2015) Yanal A. Shafagoj MD. PhD Introduction Disturbance in acidbase balance are common clinical problem that range in severity from mild to life threatening, the acute
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE*
 ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
ELECTROLYTE DISTURBANCES IN CONGESTIVE HEART FAILURE* DAVID P. B.AUMANN, M.D. Department of Medicine, University of Arkansas School of Medicine, Little Rock, Arkansas The retention of salt and water secondary
Laboratory of Kidney and Electrolyte. Metabolism, National Heart Institute, Bethesda, Maryland THE REGULATION OF
 JACK ORLOFF* Laboratory of Kidney and Electrolyte Metabolism, National Heart Institute, Bethesda, Maryland THE R6LE OF THE KIDNEY IN ACID-BASE BALANCE THE REGULATION OF It would be presumptuous to ascribe
JACK ORLOFF* Laboratory of Kidney and Electrolyte Metabolism, National Heart Institute, Bethesda, Maryland THE R6LE OF THE KIDNEY IN ACID-BASE BALANCE THE REGULATION OF It would be presumptuous to ascribe
ACIDOSIS * to enter during respiratory acidosis is the red cell. 10). However, in the rat, Epstein, Branscome
 TISSUE AND RENAL RESPONSE TO CHRONIC RESPIRATORY ACIDOSIS * By NORMAN W. CARTER,t DONALD W. SELDIN AND H. C. TENG (From the Department of Internal Medicine, The University of Texas Southwestern Medical
TISSUE AND RENAL RESPONSE TO CHRONIC RESPIRATORY ACIDOSIS * By NORMAN W. CARTER,t DONALD W. SELDIN AND H. C. TENG (From the Department of Internal Medicine, The University of Texas Southwestern Medical
UNIT VI: ACID BASE IMBALANCE
 UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
MIGUEL CHIAPPORI 4. Renal function. Twelve healthy Peruvian males between the ages of 20 and 28 years were studied. None
 ORAL SODIUM LOADING IN NORMAL INDIVIDUALS By KEHL MARKLEY,1 MANUEL BOCANEGRA,2 GUILLERMO MORALES,3 AND MIGUEL CHIAPPORI 4 (From the U. S. Public Health Service, U. S. Department of Health, Education, and
ORAL SODIUM LOADING IN NORMAL INDIVIDUALS By KEHL MARKLEY,1 MANUEL BOCANEGRA,2 GUILLERMO MORALES,3 AND MIGUEL CHIAPPORI 4 (From the U. S. Public Health Service, U. S. Department of Health, Education, and
The kidney. (Pseudo) Practical questions. The kidneys are all about keeping the body s homeostasis. for questions Ella
 The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
The kidney (Pseudo) Practical questions for questions Ella (striemit@gmail.com) The kidneys are all about keeping the body s homeostasis Ingestion Product of metabolism H 2 O Ca ++ Cl - K + Na + H 2 O
HUMAN SUBJECT 1. Syracuse, N. Y.) the urine of increasing quantities of these buffers, it has been found in man as in the dog that (1)
 THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. II. FACTORS AFFECTING THE EXCRETION OF TITRATABLE ACID BY THE NORMAL HUMAN SUBJECT 1 By W. A. SCHIESS, J. L. AYER, W. D. LOTSPEICH AND R. F. PITTS WITH
THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. II. FACTORS AFFECTING THE EXCRETION OF TITRATABLE ACID BY THE NORMAL HUMAN SUBJECT 1 By W. A. SCHIESS, J. L. AYER, W. D. LOTSPEICH AND R. F. PITTS WITH
chloride and ammonia excretion were measured for a period of 80 consecutive days in five normal young adult
 POTENTIATION OF DIURETIC ACTION OF MERCUHYDRIN1 BY AMMONIUM CHLORIDE 2 By JAMES G. HILTON 8 WITH THE TECHNICAL ASSISTANCE OF HELEN KALINSKY (From the Research Service, First Division, Goldwater Memorial
POTENTIATION OF DIURETIC ACTION OF MERCUHYDRIN1 BY AMMONIUM CHLORIDE 2 By JAMES G. HILTON 8 WITH THE TECHNICAL ASSISTANCE OF HELEN KALINSKY (From the Research Service, First Division, Goldwater Memorial
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
meq of sodium, less than 1 meq of chloride, and 0.1 meq
 Journal of Clinical Investigation Vol. 4, No., 96 ON THE MECHANISM OF NITRATE-INDUCED ALKALOSIS. THE POSSIBLE ROLE OF SELECTIVE CHLORIDE DEPLETION IN ACID-BASE REGULATION * By PAUL F. GULYASSY,t CHARLES
Journal of Clinical Investigation Vol. 4, No., 96 ON THE MECHANISM OF NITRATE-INDUCED ALKALOSIS. THE POSSIBLE ROLE OF SELECTIVE CHLORIDE DEPLETION IN ACID-BASE REGULATION * By PAUL F. GULYASSY,t CHARLES
blood (1-5). This syndrome of carbon dioxide
 THE RENL RESPONSE TO CUTE RESPIRTORY CIDOSIS 1 BY PHILIP J. DORMN,2 W. JMES SULLIVN,2 ND ROBERT F. PITTS WITH THE TECHNICL SSISTNCE OF SIBYL F. LNGE ND MRTH B. MCLEOD (From the Department of Physiology,
THE RENL RESPONSE TO CUTE RESPIRTORY CIDOSIS 1 BY PHILIP J. DORMN,2 W. JMES SULLIVN,2 ND ROBERT F. PITTS WITH THE TECHNICL SSISTNCE OF SIBYL F. LNGE ND MRTH B. MCLEOD (From the Department of Physiology,
CHAPTER 27 LECTURE OUTLINE
 CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CASE 27. What is the response of the kidney to metabolic acidosis? What is the response of the kidney to a respiratory alkalosis?
 CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
ACID-BASE BALANCE URINE BLOOD AIR
 ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
(5), the presumed site of potassium secretion. RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM
 RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM DEPLETED DOGS' By HELEN M. ANDERSON AND JOHN H. LARAGH WITH THE TECHNICAL ASSISTANCE OF CLARA W. HALL, SALLY MOORE, AND JOSEPH HARTOG (From the Department
RENAL EXCRETION OF POTASSIUM IN NORMAL AND SODIUM DEPLETED DOGS' By HELEN M. ANDERSON AND JOHN H. LARAGH WITH THE TECHNICAL ASSISTANCE OF CLARA W. HALL, SALLY MOORE, AND JOSEPH HARTOG (From the Department
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Metabolic Alkalosis: Vomiting
 RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-
 Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
to assess the renal reabsorptive capacity after restoration of normal carbon dioxide tension in
 Journal of Clinical nvestigation Vol. 41, No. 12, 1962 EFFECTS OF CHRONC HYPERCAPNA ON ELECTROLYTE AND ACD-BASE EQULBRUM.. CHARACTERSTCS OF THE ADAPTVE AND RECOVERY PROCESS AS EVALUATED BY PROVSON OF ALKAL
Journal of Clinical nvestigation Vol. 41, No. 12, 1962 EFFECTS OF CHRONC HYPERCAPNA ON ELECTROLYTE AND ACD-BASE EQULBRUM.. CHARACTERSTCS OF THE ADAPTVE AND RECOVERY PROCESS AS EVALUATED BY PROVSON OF ALKAL
There are number of parameters which are measured: ph Oxygen (O 2 ) Carbon Dioxide (CO 2 ) Bicarbonate (HCO 3 -) AaDO 2 O 2 Content O 2 Saturation
 Arterial Blood Gases (ABG) A blood gas is exactly that...it measures the dissolved gases in your bloodstream. This provides one of the best measurements of what is known as the acid-base balance. The body
Arterial Blood Gases (ABG) A blood gas is exactly that...it measures the dissolved gases in your bloodstream. This provides one of the best measurements of what is known as the acid-base balance. The body
The Role of Chloride in Hypokalemic Alkalosis in the Rat *
 Journal of Clinical Investigation Vol. 44, No. 2, 1965 The Role of Chloride in Hypokalemic Alkalosis in the Rat * (From the Renal Unit, Department of Clinical Endocrinology and Metabolic Diseases, University
Journal of Clinical Investigation Vol. 44, No. 2, 1965 The Role of Chloride in Hypokalemic Alkalosis in the Rat * (From the Renal Unit, Department of Clinical Endocrinology and Metabolic Diseases, University
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM
 CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM B. I. Heller,, J. F. Hammarsten, F. L. Stutzman J Clin Invest. 1953;32(9):858-861. https://doi.org/10.1172/jci102803.
CONCERNING THE EFFECTS OF MAGNESIUM SULFATE ON RENAL FUNCTION, ELECTROLYTE EXCRETION, AND CLEARANCE OF MAGNESIUM B. I. Heller,, J. F. Hammarsten, F. L. Stutzman J Clin Invest. 1953;32(9):858-861. https://doi.org/10.1172/jci102803.
Arterial Blood Gases Interpretation Definition Values respiratory metabolic
 Arterial Blood Gases Interpretation Definition A blood gas test measures the amount of oxygen and carbon dioxide in the blood. It is also useful in determining the ph level of the blood. The test is commonly
Arterial Blood Gases Interpretation Definition A blood gas test measures the amount of oxygen and carbon dioxide in the blood. It is also useful in determining the ph level of the blood. The test is commonly
Boston, Mass.) per L. and the bicarbonate 35 meq. per L. The bath was maintained at approximately ph 7.4 by equilibration
 CHANGES IN ACID-BASE BALANCE OF UREMIC PATIENTS DURING HEMODIALYSIS1 By JOHN M. WELLER,2 ROY C. SWAN,3 AND JOHN P. MERRILL 4 (From the Department of Medicine, Peter Bent Brigham Hospital and Harvard Medical
CHANGES IN ACID-BASE BALANCE OF UREMIC PATIENTS DURING HEMODIALYSIS1 By JOHN M. WELLER,2 ROY C. SWAN,3 AND JOHN P. MERRILL 4 (From the Department of Medicine, Peter Bent Brigham Hospital and Harvard Medical
man of the effects of diabetes and of insulin on the maximum ability of the tubules to reabsorb glucose.
 EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
Chapter 20 8/23/2016. Fluids and Electrolytes. Fluid (Water) Fluid (Water) (Cont.) Functions
 Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
Fluid and Electrolytes P A R T 4
 Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
simultaneously excreted. They also brought forward some evidence to
 THE EXCRETION OF CHLORIDES AND BICARBON- ATES BY THE HUMAN KIDNEY. BY H. W. DAVIES, M.B., B.S., J. B. S. HALDANE, M.A. AND G. L. PESKETT, B.A. (From the Laboratory, Cherwell, Oxford.) AM BARD and PAPI
THE EXCRETION OF CHLORIDES AND BICARBON- ATES BY THE HUMAN KIDNEY. BY H. W. DAVIES, M.B., B.S., J. B. S. HALDANE, M.A. AND G. L. PESKETT, B.A. (From the Laboratory, Cherwell, Oxford.) AM BARD and PAPI
Slide 1. Slide 2. Slide 3. Learning Outcomes. Acid base terminology ARTERIAL BLOOD GAS INTERPRETATION
 Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Acid/Base Disorders 2015
 Objectives - 2 1. Identify acid/base disorders 2. Discuss etiologies for 1 0 acid/base disorders (will not include mixed disorders) 3. Interpret acid/base disorders by interpreting arterial blood gas &
Objectives - 2 1. Identify acid/base disorders 2. Discuss etiologies for 1 0 acid/base disorders (will not include mixed disorders) 3. Interpret acid/base disorders by interpreting arterial blood gas &
Effect of Potassium Deficiency on the Reabsorption of Bicarbonate in the Proximal Tubule of the Rat Kidney *
 Journal of Clinical Investigation Vol. 43, No. 10, 1964 Effect of Potassium Deficiency on the Reabsorption of Bicarbonate in the Proximal Tubule of the Rat Kidney * FLOYD C. RECTOR, JR., H. ALLAN BLOOMER,t
Journal of Clinical Investigation Vol. 43, No. 10, 1964 Effect of Potassium Deficiency on the Reabsorption of Bicarbonate in the Proximal Tubule of the Rat Kidney * FLOYD C. RECTOR, JR., H. ALLAN BLOOMER,t
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
The Effect of Hyperventilation on Distal Nephron Hydrogen Ion Secretion
 The Effect of Hyperventilation on Distal Nephron Hydrogen Ion Secretion R. A. GIAMMARCo, M. B. GOLDSTEIN, M. L. HALPERIN, and B. J. STINEAUGH From the Department of Medicine, University of Toronto, Toronto
The Effect of Hyperventilation on Distal Nephron Hydrogen Ion Secretion R. A. GIAMMARCo, M. B. GOLDSTEIN, M. L. HALPERIN, and B. J. STINEAUGH From the Department of Medicine, University of Toronto, Toronto
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE
 Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Hyperaldosteronism: Conn's Syndrome
 RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
Acids, Bases, and Salts
 Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
Disclaimer. Chapter 3 Disorder of Water, Electrolyte and Acid-base Professor A. S. Alhomida. Disorder of Water and Electrolyte
 Disclaimer King Saud University College of Science Department of Biochemistry The texts, tables, figures and images contained in this course presentation (BCH 376) are not my own, they can be found on:
Disclaimer King Saud University College of Science Department of Biochemistry The texts, tables, figures and images contained in this course presentation (BCH 376) are not my own, they can be found on:
IT HAS BECOME apparent, in clinical
 Electrocardiographic Alterations Produced by a Decrease in Plasma ph, Bicarbonate and Sodium as Compared with those Produced by an Increase in Potassium By ATHLEEN E. ROBERTS, M.D., AND MELVILLE G. MAGIDA,
Electrocardiographic Alterations Produced by a Decrease in Plasma ph, Bicarbonate and Sodium as Compared with those Produced by an Increase in Potassium By ATHLEEN E. ROBERTS, M.D., AND MELVILLE G. MAGIDA,
Renal physiology V. Regulation of acid-base balance. Dr Alida Koorts BMS
 Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
Renal physiology V Regulation of acidbase balance Dr Alida Koorts BMS 712 012 319 2921 akoorts@medic.up.ac.za Hydrogen ions (H + ): Concentration and origin Concentration in arterial blood, resting: [H
SALLY RENSHAW. with that to inorganic mercury under different. whereas an organic mercurial would be relatively
 THE EFFECTS OF ACID-BASE BALANCE ON THE DIURESIS PRODUCED BY ORGANIC AND INORGANIC MERCURIALS 1 By ROBERT I. LEVY,2 I. M. WEINER,3 AND GILBERT H. MUDGE WITH THE TECHNICAL ASSISTANCE OF FLORENCE RISSER,
THE EFFECTS OF ACID-BASE BALANCE ON THE DIURESIS PRODUCED BY ORGANIC AND INORGANIC MERCURIALS 1 By ROBERT I. LEVY,2 I. M. WEINER,3 AND GILBERT H. MUDGE WITH THE TECHNICAL ASSISTANCE OF FLORENCE RISSER,
clamped. At 30- or 60-minute intervals urine specimens were collected and the bladder washed out with saline
 Downloaded from http://www.jci.org on January 11, 218. https://doi.org/1.1172/jci11171 THE MECHANISM OF THE EXCRETION OF VITAMIN C BY THE HUMAN KIDNEY AT LOW AND NORMAL PLASMA LEVELS OF ASCORBIC ACID 1
Downloaded from http://www.jci.org on January 11, 218. https://doi.org/1.1172/jci11171 THE MECHANISM OF THE EXCRETION OF VITAMIN C BY THE HUMAN KIDNEY AT LOW AND NORMAL PLASMA LEVELS OF ASCORBIC ACID 1
Water, Electrolytes, and Acid-Base Balance
 Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Medicine, Cambridge, England, and Wuppertal, B.A.O.R.
 182 J. Physiol. (I948) I07, i82-i86 6I2.46I.62 PHOSPHATE CLEARANCES IN INFANTS AND ADULTS BY R. F. A. DEAN AND R. A. McCANCE From the Medical Research Council, Department. of Experimental Medicine, Cambridge,
182 J. Physiol. (I948) I07, i82-i86 6I2.46I.62 PHOSPHATE CLEARANCES IN INFANTS AND ADULTS BY R. F. A. DEAN AND R. A. McCANCE From the Medical Research Council, Department. of Experimental Medicine, Cambridge,
THE RENAL EXCRETION OF CITRATE
 THE RENAL EXCRETION OF CITRATE Arthur P. Grollman,, Helen C. Harrison, Harold E. Harrison J Clin Invest. 1961;4(7):129-1296. https://doi.org/1.1172/jci14358. Research Article Find the latest version: http://jci.me/14358-pdf
THE RENAL EXCRETION OF CITRATE Arthur P. Grollman,, Helen C. Harrison, Harold E. Harrison J Clin Invest. 1961;4(7):129-1296. https://doi.org/1.1172/jci14358. Research Article Find the latest version: http://jci.me/14358-pdf
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis.
 Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
BUFFERING OF HYDROGEN LOAD
 BUFFERING OF HYDROGEN LOAD 1. Extracellular space minutes 2. Intracellular space minutes to hours 3. Respiratory compensation 6 to 12 hours 4. Renal compensation hours, up to 2-3 days RENAL HYDROGEN SECRETION
BUFFERING OF HYDROGEN LOAD 1. Extracellular space minutes 2. Intracellular space minutes to hours 3. Respiratory compensation 6 to 12 hours 4. Renal compensation hours, up to 2-3 days RENAL HYDROGEN SECRETION
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
Carbon Dioxide Transport. Carbon Dioxide. Carbon Dioxide Transport. Carbon Dioxide Transport - Plasma. Hydrolysis of Water
 Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Are you ready to have fun?
 Arterial Blood Gas INTERPRETATION By Nena Bonuel, MSN, RN, CCRN, CNS, ACNS-BC Nurse Specialist, Center for Professional Excellence Are you ready to have fun? 1. Yes! 2. I rather go shopping 3. I still
Arterial Blood Gas INTERPRETATION By Nena Bonuel, MSN, RN, CCRN, CNS, ACNS-BC Nurse Specialist, Center for Professional Excellence Are you ready to have fun? 1. Yes! 2. I rather go shopping 3. I still
D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor
![D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor](/thumbs/75/72756397.jpg) Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
[Chapter 25] The Liver and the Gut. John A Kellum
![[Chapter 25] The Liver and the Gut. John A Kellum [Chapter 25] The Liver and the Gut. John A Kellum](/thumbs/85/92127350.jpg) [Chapter 25] The Liver and the Gut John A Kellum 25.1 Introduction Throughout this book we have seen that the Stewart formulation can be used to help understand the mechanisms of acid-base equilibrium
[Chapter 25] The Liver and the Gut John A Kellum 25.1 Introduction Throughout this book we have seen that the Stewart formulation can be used to help understand the mechanisms of acid-base equilibrium
during the 8 days prior to the first intraperitoneal
 EXPERIMENTAL HYPERTONICITY: ALTERATIONS IN THE DISTRIBUTION OF BODY WATER, AND THE CAUSE OF DEATH 1 By ALEXANDER W. WINKLER, J. RUSSELL ELKINTON,2 JAMES HOPPER, JR.,8 AND HEBBEL E. HOFF (From the Department
EXPERIMENTAL HYPERTONICITY: ALTERATIONS IN THE DISTRIBUTION OF BODY WATER, AND THE CAUSE OF DEATH 1 By ALEXANDER W. WINKLER, J. RUSSELL ELKINTON,2 JAMES HOPPER, JR.,8 AND HEBBEL E. HOFF (From the Department
Instrumental determination of electrolytes in urine. Amal Alamri
 Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
Acid Base Balance. Chapter 26 Balance. ph Imbalances. Acid Base Balance. CO 2 and ph. Carbonic Acid. Part 2. Acid/Base Balance
 Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
dynamic action of ingested amino acids effected
 THE.EFFECT OF GLYCINE ON THE PRODUCTION AND EXCRETION OF URIC ACID1 BY MEYER FRIEDMAN (Fromn the Harold Brunn Institute for Cardiovascular Research, San Francisco, California) Mt. Zion Hospital, (Received
THE.EFFECT OF GLYCINE ON THE PRODUCTION AND EXCRETION OF URIC ACID1 BY MEYER FRIEDMAN (Fromn the Harold Brunn Institute for Cardiovascular Research, San Francisco, California) Mt. Zion Hospital, (Received
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Fluid, Electrolyte, and Acid Base Balance
 25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
Acid-Base Alterations and Renal Gluconeogenesis: Effect
 Journal of Clinical Investigation Vol. 46, No. 7, 1967 Acid-Base Alterations and Renal Gluconeogenesis: Effect of ph, Bicarbonate Concentration, and Pco2 * DONALD E. KAMM,t ROBERT E. Fuisz,4 A. DAVID GOODMAN,
Journal of Clinical Investigation Vol. 46, No. 7, 1967 Acid-Base Alterations and Renal Gluconeogenesis: Effect of ph, Bicarbonate Concentration, and Pco2 * DONALD E. KAMM,t ROBERT E. Fuisz,4 A. DAVID GOODMAN,
Acid-Base Imbalance. Shu-Yi (Emily) Wang, PhD, RN, CNS Denver School of Nursing
 Acid-Base Imbalance Shu-Yi (Emily) Wang, PhD, RN, CNS gpwsy@hotmail.com Denver School of Nursing ph Ranges Compatible With Life In blood, the ph represents the relationship between the respiratory and
Acid-Base Imbalance Shu-Yi (Emily) Wang, PhD, RN, CNS gpwsy@hotmail.com Denver School of Nursing ph Ranges Compatible With Life In blood, the ph represents the relationship between the respiratory and
Inorganic pharmaceutical chemistry. Replacement Therapy Lec 2
 Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
Inorganic pharmaceutical chemistry Replacement Therapy Lec 2 Replacement Therapy The basic objective of replacement therapy is to restore the volume and composition of the body fluids to normal one. Volume
ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017
 ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017 WHAT INFORMATION DOES AN ABG GIVE US? ph = measure of hydrogen ion concentration (acidity or alkalinity) PaCO2 = partial pressure
ARTERIAL BLOOD GASES PART 1 BACK TO BASICS SSR OLIVIA ELSWORTH SEPT 2017 WHAT INFORMATION DOES AN ABG GIVE US? ph = measure of hydrogen ion concentration (acidity or alkalinity) PaCO2 = partial pressure
The Effects of Selective Depletion of Hydrochloric Acid on Acid-Base and Electrolyte Equilibrium *
 Journal of Clinical Investigation Vol. 43, No. 9, 1964 The Effects of Selective Depletion of Hydrochloric Acid on Acid-Base and Electrolyte Equilibrium * MARK A. NEEDLE, GEORGE J. KALOYANIDES, AND WILLIAM
Journal of Clinical Investigation Vol. 43, No. 9, 1964 The Effects of Selective Depletion of Hydrochloric Acid on Acid-Base and Electrolyte Equilibrium * MARK A. NEEDLE, GEORGE J. KALOYANIDES, AND WILLIAM
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Objectives. Blood Buffers. Definitions. Strong/Weak Acids. Fixed (Non-Volatile) Acids. Module H Malley pages
 Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
Blood Buffers Module H Malley pages 120-126 Objectives Define a buffer system and differentiate between the buffering systems present in the body. Given an arterial blood-gas result, determine the degree
Chapter 24 Water, Electrolyte and Acid-Base Balance
 Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
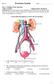 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013
 Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Basic Fluid and Electrolytes
 Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
hold for the human kidney.2 Shannon and Smith (4) have rightfully stressed
 THE RENAL EXCRETION OF INULIN AT LOW PLASMA CONCEN- TRATIONS OF THIS COMPOUND, AND ITS RELATIONSHIP TO THE GLOMERULAR FILTRATION RATE IN NORMAL, NEPHRITIC AND HYPERTENSIVE INDIVIDUALS' By BENJAMIN F. MILLER,
THE RENAL EXCRETION OF INULIN AT LOW PLASMA CONCEN- TRATIONS OF THIS COMPOUND, AND ITS RELATIONSHIP TO THE GLOMERULAR FILTRATION RATE IN NORMAL, NEPHRITIC AND HYPERTENSIVE INDIVIDUALS' By BENJAMIN F. MILLER,
ance of the sugar, until at plasma levels of 140 mgm. per cent the creatinine/sugar clearance ratio
 THE RENAL EXCRETION OF CREATININE IN MAN BY JAMES A. SHANNON 1 (From The Department of Physiology, New York University College of Medicine, New York City) In a previous paper the evidence on the excretion
THE RENAL EXCRETION OF CREATININE IN MAN BY JAMES A. SHANNON 1 (From The Department of Physiology, New York University College of Medicine, New York City) In a previous paper the evidence on the excretion
STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD. Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO
 STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO Department of Physiology, Kyoto Prefectural University of Medicine, Kyoto
STUDY ON MECHANISM OF ACID AND AMMONIA EXCRETION BY KIDNEY AFTER ACID LOAD Hisato YOSHIMURA, Mamoru FUJIMOTO AND Junichi SUGIMOTO Department of Physiology, Kyoto Prefectural University of Medicine, Kyoto
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
The Physiologic Limits of the Defense of ph *
 lournal of Clinical Investigation Vol. 44, No. 2, 1965 The Response of Extracellular Hydrogen Ion Concentration to Graded Degrees of Chronic Hypercapnia: The Physiologic Limits of the Defense of ph * WILLIAM
lournal of Clinical Investigation Vol. 44, No. 2, 1965 The Response of Extracellular Hydrogen Ion Concentration to Graded Degrees of Chronic Hypercapnia: The Physiologic Limits of the Defense of ph * WILLIAM
Pressure Diuresis 9 Sample Student Essays
 Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Managing Acid Base and Electrolyte Disturbances with RRT
 Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
already been published [O'Connor, 1958 b]. emphasized that the most prominent action of adrenaline on the kidney is to
![already been published [O'Connor, 1958 b]. emphasized that the most prominent action of adrenaline on the kidney is to already been published [O'Connor, 1958 b]. emphasized that the most prominent action of adrenaline on the kidney is to](/thumbs/88/116101114.jpg) THE EFFECT ON THE VOLUME AND COMPOSITION OF THE URINE OF THE INFUSION OF ADRENALINE AND NORADRENALINE. By W. J. O'CoNNoR. From the Department of Physiology, School of Medicine, University of Leeds. (Received
THE EFFECT ON THE VOLUME AND COMPOSITION OF THE URINE OF THE INFUSION OF ADRENALINE AND NORADRENALINE. By W. J. O'CoNNoR. From the Department of Physiology, School of Medicine, University of Leeds. (Received
Sodium Bicarbonate Injection, USP
 Sodium Bicarbonate Injection, USP FOR THE CORRECTION OF METABOLIC ACIDOSIS AND OTHER CONDITIONS REQUIRING SYSTEMIC ALKALINIZATION. Plastic Ansyr II Syringe R x only DESCRIPTION Sodium Bicarbonate Injection,
Sodium Bicarbonate Injection, USP FOR THE CORRECTION OF METABOLIC ACIDOSIS AND OTHER CONDITIONS REQUIRING SYSTEMIC ALKALINIZATION. Plastic Ansyr II Syringe R x only DESCRIPTION Sodium Bicarbonate Injection,
DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE. Jules B. Puschett, M.D.
 DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE Jules B. Puschett, M.D. Diuretic Resistance A clinical circumstance in which patients do not respond to a combination of salt restriction and even large
DIAGNOSIS AND MANAGEMENT OF DIURETIC RESISTANCE Jules B. Puschett, M.D. Diuretic Resistance A clinical circumstance in which patients do not respond to a combination of salt restriction and even large
DIURETICS-3 Dr. Shariq Syed
 DIURETICS-3 Dr. Shariq Syed AIKTC - Knowledge Resources & Relay Center 1 Pop Quiz!! Diuretics primarily prevent the reabsorption of K Na Cl I Don t know, Too busy with periodic exams! AIKTC - Knowledge
DIURETICS-3 Dr. Shariq Syed AIKTC - Knowledge Resources & Relay Center 1 Pop Quiz!! Diuretics primarily prevent the reabsorption of K Na Cl I Don t know, Too busy with periodic exams! AIKTC - Knowledge
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
