Inhibition of Interneuron Firing Extends the Spread of Endocannabinoid Signaling in the Cerebellum
|
|
|
- Barnard Short
- 5 years ago
- Views:
Transcription
1 Neuron, Vol. 34, , May 30, 2002, Copyright 2002 by Cell Press Inhibition of Interneuron Firing Extends the Spread of Endocannabinoid Signaling in the Cerebellum Anatol C. Kreitzer, Adam G. Carter, and Wade G. Regehr 1 Department of Neurobiology Harvard Medical School 220 Longwood Avenue Boston, Massachusetts Summary Endocannabinoids serve as retrograde messengers in many brain regions. These diffusible lipophilic molecules are released by postsynaptic cells and regulate presynaptic neurotransmitter release. Here we describe an additional mechanism that mediates the spread of endocannabinoid signaling to distant inhibitory synapses. Depolarization of cerebellar Purkinje cells reduced the firing rate of nearby interneurons, and this reduction in firing was blocked by the cannabinoid receptor antagonist AM251. The cannabinoid receptor agonist WIN55,212-2 also reduced firing rates in interneurons, and this inhibition arose from the activation of a small potassium conductance. Thus, endocannabinoids released from the dendrites of depolarized neurons can lead to inhibition of firing in nearby cells. Because interneurons can project over several hundred micrometers, this inhibition of firing allows cells to regulate synaptic inputs at distances well beyond the limits of endocannabinoid diffusion. Introduction inhibitory synapses onto other neurons up to 75 m away (Vincent and Marty, 1993). However, the factors governing the spread of cannabinoid signaling are not known. Does the distance of spread reflect the extent of endocannabinoid diffusion, or are other mechanisms involved? Is the spatial extent of cannabinoid signaling the same for all types of synapses? Little is known about the extent of spread at excitatory synapses. Determining the mechanisms that control the spread of endocannabinoid signaling is vital to understanding their physiological role in the central nervous system. Here we examine the spread of endocannabinoid signaling in the cerebellum, where cannabinoid signaling is functionally important (DeSanty and Dar, 2001; Patel and Hillard, 2001). Purkinje cells are the only cell type in cerebellar cortex that synthesize, release, and degrade endocannabinoids (Egertova et al., 1998; Lee et al., 2000; Tsou et al., 1998b). Following Purkinje cell depolarization, both excitatory and inhibitory synapses are sup- pressed for tens of seconds (Kreitzer and Regehr, 2001b; Llano et al., 1991a). We find that at near-physio- logical temperatures, Purkinje cell depolarization suppresses inhibitory but not excitatory synapses onto neighboring cells. This differential effect arises from an additional mechanism of endocannabinoid signaling. By recording from interneuron-purkinje cell pairs, we show that endocannabinoids released from Purkinje cells can lead to a transient suppression of interneuron firing, thereby producing a widespread reduction in neuro- transmitter release at every synapse formed by these neurons. We show that these changes in excitability arise from activation of potassium channels, which are known targets of cannabinoids (Deadwyler et al., 1993; Henry and Chavkin, 1995; Mackie et al., 1995). This suppression of interneuron firing spatially extends endocannabinoid signaling and may serve to fine-tune cerebellar outputs. Endocannabinoids, such as anandamide and 2-AG, are important neuromodulators found throughout the brain (Di Marzo et al., 1998). Disruption of endocannabinoid signaling, through either genetic or pharmacological manipulations, results in altered nociception, motor coordination, and memory (Cravatt et al., 2001; Ledent et al., 1999; Sanudo-Pena et al., 1999; Sullivan, 2000; Results Zimmer et al., 1999). Most of the central effects of cannabinoids are mediated by the G protein-coupled CB1 Spread of Endocannabinoid Signaling receptor, which is widely distributed in the central ner- in the Cerebellum vous system (Pertwee, 1997). Enzymes involved in endo- We performed dual whole-cell recordings from pairs of cannabinoid synthesis and degradation are also found neighboring cerebellar Purkinje cells to examine the in many of the same brain regions as the CB1 receptor spread of endocannabinoid signaling. Excitatory gran- (Egertova et al., 1998; Lee et al., 2000; Tsou et al., 1998b). ule cell to Purkinje cell synapses and inhibitory synapses Recent studies indicate that depolarization of postsyn- from interneurons were examined. Both of these types aptic neurons can lead to the calcium-dependent re- of synapses undergo endocannabinoid-mediated suplease of endocannabinoids, which act at presynaptic pression following Purkinje cell depolarization (Diana et CB1 receptors to inhibit neurotransmitter release al., 2002; Kreitzer and Regehr, 2001a, 2001b). At room (Kreitzer and Regehr, 2001b; Ohno-Shosaku et al., 2001; temperature, Purkinje cell depolarization has been Wilson and Nicoll, 2001). shown to affect inhibitory inputs onto neighboring cells Despite recent advances in understanding the func- located up to 75 m away (Vincent and Marty, 1993). tional role of the endocannabinoid system, fundamental Spread of retrograde inhibition has not been examined issues remain unresolved regarding the spatial extent previously at parallel fiber synapses. of endocannabinoid signaling. Endocannabinoids re- Parallel fiber synapses were stimulated every 2 s, and leased during depolarization of a neuron can suppress EPSCs were simultaneously recorded from two neighboring Purkinje cells. One Purkinje cell of a pair was 1 Correspondence: wade_regehr@hms.harvard.edu depolarized from 60 mv to 0 mv for 5 s. At 24 C, a
2 Neuron 788 Figure 1. Spread of Endocannabinoid Signaling at Excitatory and Inhibitory Synapses in the Cerebellum (Aa) Dual whole-cell voltage-clamp recordings from neighboring Purkinje cells were performed at 24 C, and parallel fibers were stimulated at 2 s intervals. One Purkinje cell in a pair was depolarized from 60 to 0 mv for 5 s, while EPSCs were recorded in both the depolarized Purkinje cell (solid circles) and the neighboring cell (open circles) (n 4). The time of depolarization is marked by a thick bar in the depolarized cell and a thin bar in the neighboring cell. (Ab) Similar experiments were performed at 34 C (n 3). Note different timescale. (Ba) The effect of a 5 s Purkinje cell depolarization (marked by bar) on spontaneous IPSCs, as assayed by inhibitory synaptic charge, in the depolarized cell (solid circles) and in the neighboring cell (open circles) is shown (n 10). (Bb) Similar experiments were performed at 34 C (n 4). Error bars are SEM. decrease in the amplitude of the parallel fiber EPSC was that Purkinje cell depolarization leads to a suppression observed in both the depolarized Purkinje cell (reduced of IPSCs in both the depolarized cell and its neighbor. to 5% 1% of control, n 4) and its neighbor (58% These authors found that the spread of suppression to 10% of control, n 4). However, at 34 C, the same neighboring cells was blocked by TTX, and we have protocol resulted in a suppression of the depolarized confirmed these findings for our experimental conditions. cell (reduced to 14% 6% of control, n 3), but almost In the presence of TTX, mipscs were suppressed no suppression of the neighboring cell (91% 7% of in the depolarized cell to 64% 5% of control (n 6) control, n 3) (Figure 1A). These results suggest that and in neighboring cells, to 92% 4% of control (n at physiological temperatures, the diffusion of endocan- 6). One possible explanation for the reduced spread in nabinoids is spatially restricted. This may reflect the the presence of TTX is that a reduction in interneuron enhanced uptake of cannabinoids at physiological tem- firing contributes to the spread of endocannabinoid suppression peratures. in control conditions. This is consistent with the Similar experiments were performed while monitoring close proximity of the interneuron soma and dendrites to spontaneous inhibitory postsynaptic currents (sipscs), Purkinje cell dendrites, in contrast to granule cell so- which arise mainly from the firing of inhibitory interneu- mata, which are many hundreds of microns away from rons in the slice. At 24 C, inhibitory synaptic charge was the Purkinje cell dendrites they contact (Palay and Chan- suppressed in both the depolarized cell (reduced to Palay, 1974). 46% 4% of control, n 10) and the neighbor (77% We therefore characterized the mechanism responsi- 3% of control, n 10). At 34 C, the same protocol led ble for the spread of endocannabinoid signaling at inhibitory to a suppression of the depolarized cell (reduced to synapses. Based on the similarity of the spread at 31% 5% of control, n 4) and its neighbor (64% 24 C and 34 C for inhibitory synapses, it is likely that 6% of control, n 4) (Figure 1B), in contrast to the the mechanisms mediating spread at room temperature results at excitatory synapses. These findings indicate and at physiological temperatures are the same. Therefore, that at 34 C, the spread of endocannabinoid signaling subsequent experiments were conducted at 24 C is very different for synapses arising from interneurons in order to take advantage of improved stability relative and granule cells. to recordings at near-physiological temperatures. Together these findings indicate that the spread of endocannabinoid signaling at inhibitory synapses re- Determinants of Purkinje Cell-Interneuron Signaling sults from a mechanism that is not present at parallel We performed whole-cell recordings from Purkinje cells fiber synapses. Further support for an additional mecha- and simultaneous cell-attached recordings from interneurons nism of spread at inhibitory synapses comes from earlier to examine the mechanisms of endocannabinoid work by Vincent and Marty (1993), who demonstrated signaling at inhibitory synapses (Figures 2 4). Cerebellar
3 Spread of Endocannabinoid Signaling 789 Figure 2. Endocannabinoid Suppression of Individual Interneuron- Purkinje Cell Connections in the interneuron. This allowed us to distinguish between the IPSCs produced by that interneuron from those produced by the many other interneurons contacting the Purkinje cell. Aligned traces for a connected interneuron-purkinje cell pair are shown for control conditions (Figure 2A, top) and following Purkinje cell depolarization (Figure 2A, bottom). The spike-triggered averages (Figure 2B) show that Purkinje cell depolarization reduced this synaptic connection to 19% of control. Of the 46 pairs examined, 21 were connected with a mean IPSC amplitude ranging from 6 to 140 pa. Interneuron- Purkinje cell pairs with spike-triggered averages 5 pa were considered to be unconnected. For the connected interneuron-purkinje cell pairs, average synaptic currents were reduced to 45% 5% control (n 21) after Purkinje cell depolarization (Figure 2C), which is consistent with the previously described inhibition of presynaptic calcium entry by cannabinoids at this synapse (Diana et al., 2002). Depolarization suppressed every interneuron-purkinje cell connection (range 17% to 82% of control), suggesting that most, if not all, cerebellar interneurons in the molecular layer have CB1 receptors located on their presynaptic terminals. We also examined the effect of Purkinje cell depolarization on interneuron firing. For our recording conditions, interneurons fired spontaneously between 0.5 and 25 Hz, with a mean firing rate of Hz (n 46). Purkinje cell depolarization reduced interneuron firing rates significantly (p 0.01, two sample t test) in 11 of 22 interneurons within 100 m of the depolarized Purkinje cell body (as shown for one interneuron in Figure 3B). Bath application of AM251 (5 M) prevented this decrease in firing rate (Figure 3C), suggesting that it is mediated by endocannabinoids. In 4/4 cells tested in which Purkinje cell depolarization inhibited firing (61% 10% of control, Figure 3D), addition of AM251 eliminated the reduction of firing rate (105% 13% of control, Figure 3E). These results indicate that activation of CB1 receptors by endocannabinoids is required for inhibition of interneuron firing. In order to determine what factors contribute to Pur- kinje cell-interneuron signaling, we examined how con- (A) sipscs in a Purkinje cell aligned relative to spikes recorded from an interneuron (arrow) in control conditions (top) and after Purkinje cell depolarization (bottom). The depolarization from 60 to 0 mv is from t s. The spike-triggered average (B) is shown for control conditions (thin trace) and after depolarization (thick trace). The dotted line indicates the interneuron spike time. (C) Average spike-triggered IPSCs were calculated every 2 s. Mean values from nection strength and distance affected the inhibition of 21 connected interneuron-purkinje cell pairs are plotted normalized interneuron firing rate (Figure 4). In Figure 4B, the averto baseline. Error bars are SEM. age firing rates of interneurons before and after Purkinje cell depolarization are shown for interneurons within 100 m (closed circles, n 22) and for more distant interneurons in the molecular layer are classically cate- interneurons (open circles, n 24). Only interneurons gorized as either basket cells or stellate cells (Ramon y within 100 m of the Purkinje cell body were inhibited (11 Cajal, 1911). Both types of neurons provide GABAergic of 22) (Figures 4A and 4C). This distance approximately inputs to Purkinje cells, with basket cells forming an corresponds to the extent of the Purkinje cell dendritic extensive contact surrounding the initial segment of the tree, suggesting that a Purkinje cell must be in close Purkinje cell axon, and stellate cells contacting Purkinje proximity to an interneuron soma in order to inhibit its cell dendrites. However, the interneurons in this region firing rate. We also examined whether inhibition was have a continuum of properties (Sultan and Bower, correlated with connectivity. Of the 11 interneuron-pur- 1998), and the distinction between basket cells and stel- kinje cell pairs in which firing was inhibited following late cells is not clear. For this reason we refer to all of Purkinje cell depolarization, eight were connected. Howthe basket cells and stellate cells we recorded from as ever, strongly connected cells were no more likely to interneurons. We recorded from 46 interneuron-purkinje be inhibited than weakly connected cells (Figure 4D). cell pairs, whose soma-to-soma separation was be- Moreover, the firing rate was inhibited in three uncontween 15 and 190 m. nected cells, indicating that connection strength is not To determine if a spontaneously firing interneuron a primary determinant of the magnitude of firing rate made synaptic connections onto a Purkinje cell, we inhibition. Purkinje cells can therefore modulate interneuron aligned IPSC current traces in the Purkinje cell to spikes firing, even in unconnected interneurons.
4 Neuron 790 (A) Left, average connection strength is plotted against distance for 46 interneuron-purkinje cell pairs. Right, symbol legend. (B) The effect of a 5 s Purkinje cell depolarization (arrow, t 20 s) on interneuron firing frequency for all cells within 100 m of the depolar- ized Purkinje cell (n 22, solid circles) and cells more distant than 100 m (n 24, open circles). (C) Summary of the number of inhib- ited cells as a function of distance. (D) Summary of the number of inhibited cells as a function of connection strength. Error bars are SEM. Figure 3. Endocannabinoids Inhibit Spontaneous Interneuron Firing (A) Schematic of recording configuration for interneuron-purkinje cell pairs. Recordings from whole-cell voltage-clamped Purkinje cells were made simultaneously with cell-attached recordings from interneurons. The effect of Purkinje cell depolarization (t 20 s, marked by arrow) on interneuron firing in one experiment is shown for four trials in control conditions (Ba) and for four trials in the presence of 5 M AM251 (Ca). Average firing frequency for this cell is shown in control conditions (Bb) and in AM251 (Cb). Summary data for inhibition of interneuron firing are shown for control conditions (D) and after AM251 wash-in (E) (n 4). Error bars are SEM. Figure 4. The Role of Distance and Connection Strength in Endocannabinoid-Mediated Inhibition of Interneuron Firing n 3). This suggests that secondary effects involving The Mechanism of Firing Inhibition activation of metabotropic or ionotropic glutamate or by Cannabinoids GABA receptors do not contribute to the reduction in To examine the mechanism by which cannabinoids in- interneuron firing. The inhibition of firing by WIN was hibit interneuron firing, we applied the synthetic canna- reversed by AM251 (1 M), and firing typically increased binoid receptor agonist WIN55,212-2 (WIN, 5 M) above control levels (133% 12% of control in AM251, (Compton et al., 1992; D Ambra et al., 1992). In every n 5) (Figure 5A). This elevation in firing rate suggests interneuron that we recorded from, WIN reduced firing that there may be some basal inhibition of interneuron to 0% 71% of control (n 13), suggesting that the firing firing due to activity of CB1 receptors in the slice. rate of all interneurons is sensitive to cannabinoids. In Similar experiments were performed using whole-cell these experiments, bicuculline (20 M) was included in current-clamp recordings (Figure 5B). Bath application the bath to reduce firing variability. During cell-attached of WIN reduced spontaneous firing rates to 32% 11% recordings from interneurons, application of WIN re- of control (n 4). This inhibition is larger than that obduced firing frequency to 60% 7% of control (n 6) served in cell-attached recordings, and may arise from (Figure 5A). In additional cell-attached recordings per- dialysis of the cell during whole-cell recordings. Applicaformed in the presence of NBQX (10 M), CPP (5 M), tion of AM251 reversed inhibition by WIN and increased LY (100 M), and CGP55845 (2 M), WIN reduced firing rates to 157% 27% of control (n 3), similar to the firing rate to a similar extent (46% 8% of control, the cell-attached recordings.
5 Spread of Endocannabinoid Signaling 791 was activated by WIN, with a reversal potential more negative than 60 mv. This implicated a potassium conductance (E K 100 mv) since chloride (E Cl 57 mv), sodium (E Na 68 mv), and calcium (E Ca 130 mv) conductances all reverse positive to 60 mv in our recording conditions. Similar effects of WIN were observed when a high-chloride internal was used with an E Cl of 0 mv and TTX (1 M) was included in the bath saline (Figure 6B) ( I hold : pa; g: ps; n 4). This suggests that WIN does not activate a Cl conductance. When an internal solution was used in which K was replaced with Cs and TEA to block K channels, WIN had no significant effect on holding current ( pa, n 4) or membrane conductance ( ps, n 4). This indicates that activation of CB1 receptors opens a potassium conductance in these inhibitory interneurons. Previous studies have shown that CB1 receptor activation can open G protein-activated inward rectifier K channels (GIRKs) (Henry and Chavkin, 1995; Mackie et al., 1995; McAllister et al., 1999). We tested the hypothesis that the channel targeted by CB1 receptor activation in cerebellar interneurons is a GIRK. If so, WIN should not alter the conductance or the holding current in the presence of extracellular Ba 2, which blocks inward rectifier K channels (Sodickson and Bean, 1996; Standen and Stanfield, 1978). In the presence of 1 mm Ba 2,we applied WIN, followed by AM251, but neither drug had any effect on the holding current ( I hold in WIN: pa, n 5) or membrane conductance ( g in WIN: 9 4 ps, n 5) (see Experimental Procedures for analysis) (Figure 6B). This result indicates that a Ba 2 -sensitive K channel is activated by WIN, and is consistent with GIRK activation in interneurons (although Ba 2 can also block other types of K channels, see Discussion). Rapidly inactivating A type K channels can also be modulated by CB1 receptor activation (Deadwyler et al., 1993, 1995), and we tested this hypothesis in cerebellar interneurons. These channels are blocked by 4-AP, although this is a not a very selective antagonist and the A type channels are not a homogeneous population (Hille, 2001). In the presence of 1 mm 4-AP, the effect of WIN on holding current ( pa, n 7) and membrane conductance ( g: ps; n 7) was reduced, but the effect of WIN relative to AM251 was similar to control conditions ( pa; Figure 5. Cannabinoid Receptor Agonists Reduce Interneuron Firing ps; n 7). This suggested that although 4-AP may have (A) Cell-attached recording from a spontaneously firing interneuron depolarized Purkinje neurons and elevated cannabinoid in control conditions (top, left) and after application of 5 M levels in the slice, the K channel modulated by WIN is WIN55,212-2 (WIN) (top, right). Firing frequency is plotted during not blocked by 4-AP. application of WIN and 1 M AM251. (B) Current-clamp recording from an interneuron in control conditions and after application of 5 We used dynamic-clamp recordings (Sharp et al., M WIN. Firing frequency is shown during application of WIN and 1993) to determine the effect of a small potassium con- 1 M AM251. ductance on interneuron firing. A conductance of varying amplitude with a reversal potential at E K ( 100 mv) was added, while the firing rate of interneurons was To determine whether cannabinoids activate a con- monitored (Figures 6C and 6D). The addition of a 50 ps ductance that underlies the inhibition of firing, we ap- simulated potassium conductance reduced firing rates plied WIN during whole-cell voltage-clamp recordings by over 50%, while conductances larger than 100 ps from interneurons (Figure 6A). At a holding potential shut down firing almost entirely (Figures 6C and 6D). of 60 mv, bath application of WIN resulted in a positive The sensitivity of these neurons to small currents arises shift in the holding current (I hold ) (Figure 6B, left) (1.25 from their extremely high input resistance ( G, 0.28 pa, n 9) that was accompanied by an increase n 9). Therefore, the small potassium conductance in membrane conductance (Figure 6B, right) (81 26 produced by activation of CB1 receptors is of sufficient ps, n 9). These results indicate that an outward current magnitude to inhibit firing in cerebellar interneurons.
6 Neuron 792 Figure 6. Mechanism of CB1-Receptor-Mediated Firing Inhibition in Interneurons (A) Holding current (I hold ) and membrane conductance (g) were monitored in a voltage-clamped interneuron during sequential application of 5 M WIN and 1 M AM251. Dotted lines show average values used for analysis. Membrane conductance was assayed by a 50 ms 5 mv hyperpolarizing step. (B) Average effects of WIN on I hold (solid bars) and g (open bars) are shown for recordings in control conditions (n 9), with a high-chloride internal solution (n 4), with a Cs -based internal solution containing 10 mm TEA (n 4), and with 1 mm barium in the extracellular saline (n 5). (C) Interneuron firing in control conditions (top), and after adding a simulated potassium conductance of 50 ps (middle) or 100 ps (bottom) using dynamic-clamp. (D) Interneuron firing frequency is plotted against amount of simulated potassium conductance (n 4). Error bars are SEM. Discussion Mechanisms of Endocannabinoid Signaling in the Cerebellum Our findings support the involvement of multiple mechanisms of endocannabinoid signaling in the cerebellum. Cannabinoids reduce the amplitude of sipscs (Figure 2) by inhibiting calcium influx in interneuron presynaptic terminals (Diana et al., 2002). Additionally, mipsc frequency is reduced (Llano et al., 1991a), either through changes in intraterminal calcium levels or through direct actions on the release machinery, downstream of cal- cium influx. Finally, as we have shown here, cannabinoids inhibit interneuron firing frequency by activating a small potassium conductance. Spread of endocannabinoids to Purkinje cells in close proximity to the depolar- ized cell is mediated in part by changes in firing and in part by direct endocannabinoid diffusion. Spread to We describe an additional mechanism for endocannabinoid action that greatly extends the distances over which they signal. Previously, studies of endocannabinoids in synaptic transmission have focused on their direct actions at presynaptic terminals, where they inhibit IPSCs through calcium channel modulation (Kreitzer and Regehr, 2001b; Wilson et al., 2001) (Figure 7A). Here we show that endocannabinoids released by Purkinje cell dendrites also lead to a reduction in the firing rates of interneurons by activating a small potassium conductance. By inhibiting firing, endocannabinoids can reduce the rate of spontaneous IPSCs in all Purkinje cells receiving synapses from that interneuron (Figures 7B and 7C). The inhibition of stellate and basket cell firing by endocannabinoids accounts for previously described properties of depolarization-induced suppression of inhibition (DSI) in the cerebellum, which is known to be mediated by endocannabinoids. Vincent and Marty (1993) found the spread of DSI to be attenuated in the presence of TTX, when the reduction of interneuron firing rates cannot contribute to DSI. These findings are consistent with our hypothesis that firing inhibition provides the primary means for cannabinoids to exert widespread effects on inhibitory neurotransmission. In addition, these results suggest that the direct presynaptic actions of endocannabinoids are more spatially restricted than is the spread of DSI, which is consistent with a limited diffusion of these lipophilic signaling molecules.
7 Spread of Endocannabinoid Signaling 793 from single Purkinje cells. However, synaptic stimulation, such as synchronized parallel fiber activation, which is known to evoke endocannabinoid release from Purkinje cells (Maejima et al., 2001), is likely to activate large numbers of Purkinje cells. Even though synaptic stimulation probably generates less endocannabinoid release from individual Purkinje cells, the total amount of endocannabinoids released could be much greater. This would lead to inhibition of many more interneurons and significant widespread effects. Furthermore, at 34 C, Purkinje cell depolarization resulted in a reduction of IPSCs in the neighboring cell that was even larger than that observed at 24 C (Figure 1), indicating that at physiological temperatures, cannabinoid uptake and degradation does not prevent the spread of endocannabinoids to nearby interneurons. These results suggest that inhibition of interneuron firing by endocannabinoids is likely to occur under physiological conditions. Direct versus Indirect Effects of Cannabinoids Another important question is whether endocannabinoids released from Purkinje cells inhibit interneuron firing directly, or whether they act as an intermediate messenger leading to the generation of another factor, which could reduce interneuron firing. In the cerebellar cortex, Purkinje cells are the sole source of endocannabinoids. They are the only cell type in this region that contain either PLD (Lee et al., 2000), which is involved in the synthesis of anandamide, or FAAH (Egertova et al., 1998; Tsou et al., 1998b), which is involved in the breakdown of endocannabinoids and is thought to be a marker of cells that generate endocannabinoids. Cerebellar interneurons in the molecular layer contain CB1 receptors (Diana et al., 2002; Matsuda et al., 1993; Tsou et al., 1998a) and are located in close proximity to Pur- kinje cell dendrites, making them well suited to detect endocannabinoids released from Purkinje cells. Moreover, we find that cannabinoids inhibit firing, even in the presence of antagonists of GABA A, AMPA, NMDA, GABA B, and metabotropic glutamate receptors, which would eliminate any secondary effects of cannabinoids mediated by these receptors. Furthermore, in the presence of these blockers and TTX, a CB1 receptor agonist activated a potassium conductance sufficient to ac- count for the inhibition in firing. Together, these findings indicate that the most likely explanation for our observa- tions is that cannabinoids released from Purkinje cell dendrites bind directly to CB1 receptors on interneurons, leading to the activation of a K conductance and an inhibition of firing. Figure 7. Schematic Depicting Various Mechanisms of Endocannabinoid Signaling in the Cerebellum Purkinje cell depolarization leads to calcium influx and release of endocannabinoids (light gray), which spread locally from the depo- larized cell. In (A), endocannabinoids released from a Purkinje cell directly inhibit a presynaptic terminal (presynaptic inhibition, black bouton) from a distant interneuron. Other synapses formed by that interneuron are unaffected. In (B), the spontaneous firing of an interneuron near the depolarized Purkinje cell is reduced, and consequently all synapses formed by that interneuron are inhibited (inhibi- tion of firing, dark gray boutons), even though no synapses exist between the interneuron and the depolarized Purkinje cell. In (C), locally released endocannabinoids act both directly to inhibit the presynaptic terminals of the interneuron and indirectly to reduce firing frequency at that interneuron. Mechanism of Cannabinoid Inhibition of Interneuron Firing Rate The firing of cerebellar interneurons is sensitive to small conductance changes. Our results suggest that endo- cannabinoids inhibit interneuron firing by activating a conductance of less than 100 ps that reverses at potentials more negative than 60 mv. Assuming a single channel conductance of 10 ps, this corresponds to fewer than ten open channels. Based on the reversal potential, and the sensitivity to intracellular K channel blockers, the conductance change produced by cannabinoid receptor agonists likely reflects the opening of more distant cells is mediated primarily by changes in firing rate. Although firing was affected in only 50% of nearby cells following Purkinje cell depolarization, we have found that all interneurons are sensitive to the CB1 agonist WIN55, It is likely that the specific location of individual interneurons relative to Purkinje cell dendrites contributes to this variability in firing inhibition. An important issue that we did not directly examine is whether inhibition of firing by endocannabinoids occurs under physiological conditions. Our experiments were focused on mechanisms of endocannabinoid signaling and were conducted using voltage clamp, primarily at 24 C, using long postsynaptic depolarizations that were designed to evoke maximal endocannabinoid release
8 Neuron 794 potassium channels. Although the location of these the cerebellar cortex. Because Purkinje cells are the channels is not known, the magnitude of firing inhibition output cells of the cerebellar cortex, it is interesting to depends upon the proximity of interneuron cell bodies to consider the effect that they may have on their targets Purkinje cells and does not depend upon the connection in the deep cerebellar nuclei. Such a lateral excitation strength, favoring a location in the soma and/or den- may be transformed into lateral inhibition in these nuclei, drites. since Purkinje cells are themselves inhibitory. In this Although the small size of the CB1 receptor-activated way, endocannabinoid modulation of interneuron firing potassium current in cerebellar interneurons has limited may serve to fine-tune cerebellar outputs. our ability to identify the channel that mediates the modulation, there are several candidate channel types. Can- Experimental Procedures nabinoids can shift the inactivation of A type potassium currents to more positive values (Deadwyler et al., 1993), Sagittal slices (250 m thick) were cut from the cerebellar vermis of 14- to 21-day-old Sprague Dawley rats. Slices were superfused which results in increased potassium conductance at with an external saline solution containing (in mm): 125 NaCl, 2.5 normal resting potentials. CB1 receptors can also acti- KCl, 2 CaCl 2, 1 MgCl 2, 26 NaHCO 3, 1.25 NaH 2 PO 4, and 25 glucose, vate GIRKs (Henry and Chavkin, 1995; Mackie et al., bubbled with 95% O 2 /5% CO 2. NBQX (10 M) was added to the 1995; McAllister et al., 1999). Our results indicate that external solution to suppress synaptic currents mediated by AMPA the channels activated by CB1 receptors are blocked receptors. Bicuculline (20 M), CGP55845 (2 M), and CPP (5 M) by 1 mm barium, but not by 1 mm 4-AP. These findings were also included in all current-clamp, dynamic-clamp, and in- terneuron voltage-clamp experiments. LY341495, CGP55845, CPP, suggest that A type potassium channels are not inand AM251 were purchased from Tocris Cookson (Ballwin, MO). volved, whereas a Ba 2 -sensitive conductance, such as Bicuculline, WIN55,212-2, and TTX were purchased from Sigma (St. a GIRK, may mediate the inhibition of interneuron firing Louis, MO). by endocannabinoids. However, in addition to blocking Whole-cell recordings of Purkinje cells were obtained as de- inward rectifier K channels, Ba 2 is also known to block scribed previously (Llano et al., 1991b). Glass electrodes (2-4 M ) B were filled with one of two internal solutions. Solution 1 contained: K channels and some types of delayed rectifier K 120 CsGlu, 15 CsCl, 8 NaCl, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 channels (Hille, 2001), and therefore the exact identity of Na-GTP, adjusted to ph 7.3 with CsOH. Solution 2 contained: 150 the channel mediating the inhibition of interneuron firing CsCl, 1 EGTA, 10 HEPES, 0.1 CaCl 2, 4.6 MgCl 2, 2 Mg-ATP, 0.3 Naby cannabinoids remains unknown. Electrophysiologi- GTP, adjusted to ph 7.3 with CsOH. Internal solutions also contained cal studies of cerebellar interneurons in the molecular 5 mm QX-314 to block voltage-dependent sodium channels. Solu- layer suggest that they contain a variety of voltagemore tion 1 gave smaller inward inhibitory currents but provided much sensitive potassium channels (Midtgaard, 1992). Immukinje stability and was therefore used for paired interneuron-pur- cell recordings. Solution 2 provided large inward inhibitory nohistochemical studies (Liao et al., 1996) and in situ synaptic currents, but cells were less stable for long recordings. hybridization (Karschin et al., 1996) suggest that GIRKs Inhibitory synaptic currents were monitored at a holding potential 1, 2, and 3 are present in the cerebellar molecular layer, of 60 mv. The 80 s trials used to assay DSI were separated by 60 although it remains unclear from these studies whether s. Access resistance and leak currents were monitored and expericerebellar interneurons express GIRKs. ments were rejected if these parameters changed significantly dur- ing recording. Whole-cell voltage-clamp and current-clamp recordings from cer- Comparison with the Hippocampus ebellar interneurons were obtained using 3 5 M electrodes con- In the hippocampus, it is unclear whether changes in taining (in mm): 130 KMeSO 3, 10 NaCl, 2 MgCl 2, 0.16 CaCl 2, 0.5 EGTA, the excitability of inhibitory cells contribute to the spread 10 HEPES, 4 Na-ATP, and 0.4 Na-GTP, 14 Tris-creatine phosphate, of endocannabinoid signaling. The spread of endocanliquid adjusted to ph 7.3. The holding potential was 60 mv, and the nabinoid signaling appears more restricted ( 20 m) junction potential ( 8 mv) was not corrected. For the high- than in the cerebellum (Wilson and Nicoll, 2001). One chloride internal solution, KCl was substituted for KMeSO 3. For the Cs -TEA internal, 120 CsMeSO 3 and 10 TEA-Cl were substituted for possibility is that interneuron firing in the hippocampus KMeSO 3. Cell-attached recordings from interneurons were obtained is insensitive to cannabinoids. A more restricted spread using 3 5 M electrodes containing (in mm): 165 NaCl, 2.5 KCl, 3 of endocannabinoid signaling may also arise due to the CaCl 2, 2 MgCl 2, 10 HEPES, 1.25 NaH 2 PO 4, and 25 glucose, adjusted different morphology and connectivity of CA1 pyramidal to ph 7.3. cells and inhibitory interneurons in the hippocampus. During voltage-clamp experiments in cerebellar interneurons (Fig- ure 6), a baseline was obtained in control conditions and then 5 M As a result, it may be possible that depolarization of a WIN was bath applied, followed by, in some cases, 1 M AM251. single pyramidal cell is insufficient to significantly alter In all conditions except 1 mm 4-AP, no significant difference existed interneuron firing, but that elevated activity in multiple between control levels and the levels after AM251 application, and pyramidal cells can affect firing and enhance the spread in these cases, the effect of WIN was calculated by subtracting the of endocannabinoid signaling. values in WIN from the average of the values in control conditions and AM251. Dynamic-clamp recordings were made at 50 khz using an ITC- Role of Endocannabinoid Signaling 18 computer interface controlled by an Igor Pro XOP (Instrutech, in the Cerebellum Port Washington, NY). For these recordings, E K was set at 100 By reducing interneuron firing, endocannabinoids re- mv, and DC conductance values between 0 and 150 ps (in 25 ps leased from a depolarized Purkinje cell can disinhibit increments) were randomly applied to cells for 1 s intervals. During surrounding Purkinje cells. The spatial extent of this recordings, cell capacitance was maximally compensated, and ac- disinhibition is dictated by the axonal arborization of cess resistance ranged between 10 and 15 M. DSI was quantified by calculating synaptic charge as described basket and stellate cells, which project in the parasagitpreviously (Kreitzer and Regehr, 2001a). Analysis was performed tal plane over several hundred micrometers (Palay and using custom routines written in Igor Pro (Wavemetrics, Lake Os- Chan-Palay, 1974). This will increase activity in neigh- wego, OR). Current-clamp and dynamic-clamp recordings were performed boring Purkinje cells and produce lateral excitation in with an AxoClamp 2B amplifier, and outputs were digitized
9 Spread of Endocannabinoid Signaling 795 at 50 khz with an 18-bit D/A converter (Instrutech, Port Washington, presynaptic calcium influx by endogenous cannabinoids at excitatory NY) and filtered at 10 khz. All other recordings were performed synapses onto Purkinje cells. Neuron 29, with Axon Instruments 200A and 200B amplifiers, and outputs were Ledent, C., Valverde, O., Cossu, G., Petitet, F., Aubert, J., Beslot, digitized at 2 5 khz with a 16-bit D/A converter (Instrutech, Port F., Bohme, G.A., Imperato, A., Pedrazzini, T., Roques, B.P., et al. Washington, NY), using Pulse Control software (Herrington and (1999). Unresponsiveness to cannabinoids and reduced addictive Bookman, 1995), and filtered at 1 khz with a 4-pole Bessel filter. effects of opiates in CB1 receptor knockout mice. Science 283, Acknowledgments Lee, M., Jo, Y., Chun, M., Chung, J., Kim, M., and Min, D. (2000). Immunohistochemical localization of phospholipase D1 in rat central We thank Dawn Blitz, Kelly Foster, Solange Brown, Matthew Xunervous system. Brain Res. 864, Friedman, Stephan Brenowitz, and Patrick Safo for comments on the manuscript. This work was supported by NIH R01-NS Liao, Y.J., Jan, Y.N., and Jan, L.Y. (1996). Heteromultimerization of G-protein-gated inwardly rectifying K channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 16, Received: December 19, Revised: March 29, 2002 Llano, I., Leresche, N., and Marty, A. (1991a). Calcium entry increases References the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6, Compton, D.R., Gold, L.H., Ward, S.J., Balster, R.L., and Martin, Llano, I., Marty, A., Armstrong, C.M., and Konnerth, A. (1991b). Syn- B.R. (1992). Aminoalkylindole analogs: cannabimimetic activity of a aptic- and agonist-induced excitatory currents of Purkinje cells in class of compounds structurally distinct from delta 9-tetrahydrocan- rat cerebellar slices. J. Physiol. 434, nabinol. J. Pharmacol. Exp. Ther. 263, Mackie, K., Lai, Y., Westenbroek, R., and Mitchell, R. (1995). Canna- Cravatt, B.F., Demarest, K., Patricelli, M.P., Bracey, M.H., Giang, binoids activate an inwardly rectifying potassium conductance and D.K., Martin, B.R., and Lichtman, A.H. (2001). Supersensitivity to inhibit Q-type calcium currents in AtT20 cells transfected with rat anandamide and enhanced endogenous cannabinoid signaling in brain cannabinoid receptor. J. Neurosci. 15, mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA Maejima, T., Hashimoto, K., Yoshida, T., Aiba, A., and Kano, M. 98, (2001). Presynaptic inhibition caused by retrograde signal from met- D Ambra, T.E., Estep, K.G., Bell, M.R., Eissenstat, M.A., Josef, K.A., abotropic glutamate to cannabinoid receptors. Neuron 31, Ward, S.J., Haycock, D.A., Baizman, E.R., Casiano, F.M., and Beglin, Matsuda, L.A., Bonner, T.I., and Lolait, S.J. (1993). Localization of N.C. (1992). Conformationally restrained analogues of pravadoline: cannabinoid receptor mrna in rat brain. J. Comp. Neurol. 327, nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 35, McAllister, S.D., Griffin, G., Satin, L.S., and Abood, M.E. (1999). Deadwyler, S.A., Hampson, R.E., Bennett, B.A., Edwards, T.A., Mu, Cannabinoid receptors can activate and inhibit G protein-coupled J., Pacheco, M.A., Ward, S.J., and Childers, S. (1993). Cannabinoids inwardly rectifying potassium channels in a xenopus oocyte expresmodulate potassium current in cultured hippocampal neurons. Resion system. J. Pharmacol. Exp. Ther. 291, ceptors Channels 1, Midtgaard, J. (1992). Membrane properties and synaptic responses Deadwyler, S.A., Hampson, R.E., Mu, J., Whyte, A., and Childers, of Golgi cells and stellate cells in the turtle cerebellum in vitro. J. S. (1995). Cannabinoids modulate voltage sensitive potassium Physiol. 457, A-current in hippocampal neurons via a camp-dependent process. Ohno-Shosaku, T., Maejima, T., and Kano, M. (2001). Endogenous J. Pharmacol. Exp. Ther. 273, cannabinoids mediate retrograde signals from depolarized postsyn- DeSanty, K.P., and Dar, M.S. (2001). Cannabinoid-induced motor aptic neurons to presynaptic terminals. Neuron 29, incoordination through the cerebellar CB(1) receptor in mice. Phar- Palay, S.L., and Chan-Palay, V. (1974). Cerebellar Cortex (New York: macol. Biochem. Behav. 69, Springer-Verlag). Diana, M., Levenes, C., Mackie, K., and Marty, A. (2002). Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje Patel, S., and Hillard, C.J. (2001). Cannabinoid CB(1) receptor ago- cells is mediated by endogenous cannabinoids. J. Neurosci. 22, nists produce cerebellar dysfunction in mice. J. Pharmacol. Exp Ther. 297, Di Marzo, V., Melck, D., Bisogno, T., and De Petrocellis, L. (1998). Pertwee, R.G. (1997). Pharmacology of cannabinoid CB1 and CB2 Endocannabinoids: endogenous cannabinoid receptor ligands with receptors. Pharmacol. Ther. 74, neuroupmodulatory action. Trends Neurosci. 21, Ramon y Cajal, S. (1911). Histologie du systeme nerveux de l homme Egertova, M., Giang, D.K., Cravatt, B.F., and Elphick, M.R. (1998). et des vertebres., Tome II edition (Paris: Maloine). A new perspective on cannabinoid signalling: complementary localof Sanudo-Pena, M.C., Tsou, K., and Walker, J.M. (1999). Motor actions ization of fatty acid amide hydrolase and the CB1 receptor in rat cannabinoids in the basal ganglia output nuclei. Life Sci. 65, brain. Proc. R. Soc. Lond. B Biol. Sci. 265, Henry, D.J., and Chavkin, C. (1995). Activation of inwardly rectifying Sodickson, D.L., and Bean, B.P. (1996). GABAB receptor-activated potassium channels (GIRK1) by co-expressed rat brain cannabinoid inwardly rectifying potassium current in dissociated hippocampal receptors in Xenopus oocytes. Neurosci. Lett. 186, CA3 neurons. J. Neurosci. 16, Herrington, J., and Bookman, R.J. (1995). Pulse Control V4.5: IGOR Sharp, A.A., O Neil, M.B., Abbott, L.F., and Marder, E. (1993). Dy- XOPs for Patch Clamp Data Acquisition (Miami, FL: University of namic clamp: computer-generated conductances in real neurons. Miami). J. Neurophysiol. 69, Hille, B. (2001). Ion Channels of Excitable Membranes, Third edition Standen, N.B., and Stanfield, P.R. (1978). A potential- and time- (Sunderland, MA: Sinauer Associates). dependent blockade of inward rectification in frog skeletal muscle Karschin, C., Dissmann, E., Stuehmer, W., and Karschin, A. (1996). fibres by barium and strontium ions. J. Physiol. 280, IRK(1 3) and GIRK(1 4) inwardly rectifying K channel mrnas are Sullivan, J.M. (2000). Cellular and molecular mechanisms underlying differentially expressed in the adult rat brain. J. Neurosci. 16, 3559 learning and memory impairments produced by cannabinoids Learn. Mem. 7, Kreitzer, A.C., and Regehr, W.G. (2001a). Cerebellar depolarizationcerebellar Sultan, F., and Bower, J.M. (1998). Quantitative Golgi study of the rat induced suppression of inhibition is mediated by endogenous cananalysis. molecular layer interneurons using principal component nabinoids. J. Neurosci. 21, RC174, 1 5. J. Comp. Neurol. 393, Kreitzer, A.C., and Regehr, W.G. (2001b). Retrograde inhibition of Tsou, K., Brown, S., Sanudo-Pena, M.C., Mackie, K., and Walker,
10 Neuron 796 J.M. (1998a). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, Tsou, K., Nogueron, M.I., Muthian, S., Sanudo-Pena, M.C., Hillard, C.J., Deutsch, D.G., and Walker, J.M. (1998b). Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci. Lett. 254, Vincent, P., and Marty, A. (1993). Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron 11, Wilson, R.I., and Nicoll, R.A. (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, Wilson, R.I., Kunos, G., and Nicoll, R.A. (2001). Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31, Zimmer, A., Zimmer, A.M., Hohmann, A.G., Herkenham, M., and Bonner, T.I. (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 96,
Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids
 The Journal of Neuroscience, 2001, Vol. 21 RC174 1of5 Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids Anatol C. Kreitzer and Wade G. Regehr Department
The Journal of Neuroscience, 2001, Vol. 21 RC174 1of5 Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids Anatol C. Kreitzer and Wade G. Regehr Department
Supplementary Information
 Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids
 Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids Solange P Brown 1 3,Stephan D Brenowitz 1,3 & Wade G Regehr 1 Many types of neurons can release
Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids Solange P Brown 1 3,Stephan D Brenowitz 1,3 & Wade G Regehr 1 Many types of neurons can release
BIPN140 Lecture 8: Synaptic Transmission II
 BIPN140 Lecture 8: Synaptic Transmission II 1. Postsynaptic Receptors: Metabotropic & Ionotropic 2. Postsynaptic Responses (Postsynaptic Potentials, PSPs) 3. Neurotransmitters Su (FA16) Chemical Synapse:
BIPN140 Lecture 8: Synaptic Transmission II 1. Postsynaptic Receptors: Metabotropic & Ionotropic 2. Postsynaptic Responses (Postsynaptic Potentials, PSPs) 3. Neurotransmitters Su (FA16) Chemical Synapse:
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Synaptic Integration
 Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Calcium Dependence of Retrograde Inhibition by Endocannabinoids at Synapses onto Purkinje Cells
 The Journal of Neuroscience, July 16, 2003 23(15):6373 6384 6373 Cellular/Molecular Calcium Dependence of Retrograde Inhibition by Endocannabinoids at Synapses onto Purkinje Cells Stephan D. Brenowitz
The Journal of Neuroscience, July 16, 2003 23(15):6373 6384 6373 Cellular/Molecular Calcium Dependence of Retrograde Inhibition by Endocannabinoids at Synapses onto Purkinje Cells Stephan D. Brenowitz
Endocannabinoids Inhibit Transmission at Granule Cell to Purkinje Cell Synapses by Modulating Three Types of Presynaptic Calcium Channels
 The Journal of Neuroscience, June 16, 2004 24(24):5623 5631 5623 Cellular/Molecular Endocannabinoids Inhibit Transmission at Granule Cell to Purkinje Cell Synapses by Modulating Three Types of Presynaptic
The Journal of Neuroscience, June 16, 2004 24(24):5623 5631 5623 Cellular/Molecular Endocannabinoids Inhibit Transmission at Granule Cell to Purkinje Cell Synapses by Modulating Three Types of Presynaptic
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Sample Lab Report 1 from 1. Measuring and Manipulating Passive Membrane Properties
 Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
The control of spiking by synaptic input in striatal and pallidal neurons
 The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
Neurons of the Bed Nucleus of the Stria Terminalis (BNST)
 Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Chang Chun City, Jilin Province , P.R of China. School of Medicine, Yamagata University Faculy of Medicine, Yamagata, Japan
 Yamagata Med J 2005 23 (1) 1-10 Xinyi Gu*, ***, Satoshi Fujii**, Kunio Kato*** *Department of Neurology,The Norman Bethun University of Medical Science, Chang Chun City, Jilin Province 140031, P.R of China
Yamagata Med J 2005 23 (1) 1-10 Xinyi Gu*, ***, Satoshi Fujii**, Kunio Kato*** *Department of Neurology,The Norman Bethun University of Medical Science, Chang Chun City, Jilin Province 140031, P.R of China
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Presynaptic Cannabinoid Sensitivity Is a Major Determinant of Depolarization-Induced Retrograde Suppression at Hippocampal Synapses
 The Journal of Neuroscience, May 15, 2002, 22(10):3864 3872 Presynaptic Cannabinoid Sensitivity Is a Major Determinant of Depolarization-Induced Retrograde Suppression at Hippocampal Synapses Takako Ohno-Shosaku,
The Journal of Neuroscience, May 15, 2002, 22(10):3864 3872 Presynaptic Cannabinoid Sensitivity Is a Major Determinant of Depolarization-Induced Retrograde Suppression at Hippocampal Synapses Takako Ohno-Shosaku,
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Endocannabinoid Signaling in the Brain
 REVIEW: NEUROSCIENCE Endocannabinoid Signaling in the Brain Rachel I. Wilson 1 and Roger A. Nicoll 2 * The primary psychoactive ingredient in cannabis, 9 -tetrahydrocannabinol ( 9 -THC), affects the brain
REVIEW: NEUROSCIENCE Endocannabinoid Signaling in the Brain Rachel I. Wilson 1 and Roger A. Nicoll 2 * The primary psychoactive ingredient in cannabis, 9 -tetrahydrocannabinol ( 9 -THC), affects the brain
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus
 Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
Cholinergic Activation of M2 Receptors Leads to Context- Dependent Modulation of Feedforward Inhibition in the Visual Thalamus Miklos Antal., Claudio Acuna-Goycolea., R. Todd Pressler, Dawn M. Blitz, Wade
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1
 BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
Synaptic Communication. Steven McLoon Department of Neuroscience University of Minnesota
 Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche
 Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche Sean J. Markwardt, Cristina V. Dieni, Jacques I. Wadiche & Linda Overstreet-Wadiche Supplementary Methods. Animals We used hemizygous
Ivy/Neurogliaform Interneurons Coordinate Activity in the Neurogenic Niche Sean J. Markwardt, Cristina V. Dieni, Jacques I. Wadiche & Linda Overstreet-Wadiche Supplementary Methods. Animals We used hemizygous
Chapter 5 subtitles GABAergic synaptic transmission
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Resonant synchronization of heterogeneous inhibitory networks
 Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
Analysis of the Effects of Cannabinoids on Synaptic Transmission Between Basket and Purkinje Cells in the Cerebellar Cortex of the Rat
 JPET This Fast article Forward. has not been Published copyedited and on formatted. May 3, The 2004 final as version DOI:10.1124/jpet.104.066670 may differ from this version. JPET # 66670 1 Analysis of
JPET This Fast article Forward. has not been Published copyedited and on formatted. May 3, The 2004 final as version DOI:10.1124/jpet.104.066670 may differ from this version. JPET # 66670 1 Analysis of
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
Structure of a Neuron:
 Structure of a Neuron: At the dendrite the incoming signals arrive (incoming currents) At the soma current are finally integrated. At the axon hillock action potential are generated if the potential crosses
Structure of a Neuron: At the dendrite the incoming signals arrive (incoming currents) At the soma current are finally integrated. At the axon hillock action potential are generated if the potential crosses
What effect would an AChE inhibitor have at the neuromuscular junction?
 CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
Chapter 6 subtitles postsynaptic integration
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 6 subtitles postsynaptic integration INTRODUCTION (1:56) This sixth and final chapter deals with the summation of presynaptic currents. Glutamate and
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
CYTOARCHITECTURE OF CEREBRAL CORTEX
 BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
Short-Term Retrograde Inhibition of GABAergic Synaptic Currents in Rat Purkinje Cells Is Mediated by Endogenous Cannabinoids
 The Journal of Neuroscience, January 1, 2002, 22(1):200 208 Short-Term Retrograde Inhibition of GABAergic Synaptic Currents in Rat Purkinje Cells Is Mediated by Endogenous Cannabinoids Marco A. Diana,
The Journal of Neuroscience, January 1, 2002, 22(1):200 208 Short-Term Retrograde Inhibition of GABAergic Synaptic Currents in Rat Purkinje Cells Is Mediated by Endogenous Cannabinoids Marco A. Diana,
Chapter 4 Neuronal Physiology
 Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
5-Nervous system II: Physiology of Neurons
 5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse
 Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
CHAPTER 44: Neurons and Nervous Systems
 CHAPTER 44: Neurons and Nervous Systems 1. What are the three different types of neurons and what are their functions? a. b. c. 2. Label and list the function of each part of the neuron. 3. How does the
CHAPTER 44: Neurons and Nervous Systems 1. What are the three different types of neurons and what are their functions? a. b. c. 2. Label and list the function of each part of the neuron. 3. How does the
Dendritic Mechanisms of Phase Precession in Hippocampal CA1 Pyramidal Neurons
 RAPID COMMUNICATION Dendritic Mechanisms of Phase Precession in Hippocampal CA1 Pyramidal Neurons JEFFREY C. MAGEE Neuroscience Center, Louisiana State University Medical Center, New Orleans, Louisiana
RAPID COMMUNICATION Dendritic Mechanisms of Phase Precession in Hippocampal CA1 Pyramidal Neurons JEFFREY C. MAGEE Neuroscience Center, Louisiana State University Medical Center, New Orleans, Louisiana
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
Cell communication. Gated ion channels. Allow specific ions to pass only when gates are open
 increase decrease Cell communication Gated ion channels Allow specific ions to pass only when gates are open Triggered by: potential change, chemical binding, temperature change, stretching 1 Voltage-Gated
increase decrease Cell communication Gated ion channels Allow specific ions to pass only when gates are open Triggered by: potential change, chemical binding, temperature change, stretching 1 Voltage-Gated
Cell communication. Gated ion channels. Voltage-Gated Na + Channel. Allow specific ions to pass only when gates are open
 increase decrease Cell communication Gated ion channels Allow specific ions to pass only when gates are open Voltage-Gated Na + Channel Activation gate ECF Triggered by: change, chemical binding, temperature
increase decrease Cell communication Gated ion channels Allow specific ions to pass only when gates are open Voltage-Gated Na + Channel Activation gate ECF Triggered by: change, chemical binding, temperature
Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex
 Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex Christoph Kapfer 1,2, Lindsey L Glickfeld 1,3, Bassam V Atallah 1,3 & Massimo Scanziani 1 The balance between
Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex Christoph Kapfer 1,2, Lindsey L Glickfeld 1,3, Bassam V Atallah 1,3 & Massimo Scanziani 1 The balance between
Astrocyte signaling controls spike timing-dependent depression at neocortical synapses
 Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Excitatory synaptic transmission in the central nervous system. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus
 Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus M. Frerking*, C. C. H. Petersen*, and R. A. Nicoll* Departments of *Cellular and Molecular Pharmacology and Physiology,
Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus M. Frerking*, C. C. H. Petersen*, and R. A. Nicoll* Departments of *Cellular and Molecular Pharmacology and Physiology,
CURRENT REVIEW. Endocannabinoids and Their Implications for Epilepsy. Neurophysiological Properties of the Endocannabinoids Systems
 CURRENT REVIEW Endocannabinoids and Their Implications for Epilepsy Bradley E. Alger, Ph.D. University of Maryland School of Medicine, Baltimore, Maryland This review covers the main features of a newly
CURRENT REVIEW Endocannabinoids and Their Implications for Epilepsy Bradley E. Alger, Ph.D. University of Maryland School of Medicine, Baltimore, Maryland This review covers the main features of a newly
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Na + K + pump. The beauty of the Na + K + pump. Cotransport. The setup Cotransport the result. Found along the plasma membrane of all cells.
 The beauty of the Na + K + pump Na + K + pump Found along the plasma membrane of all cells. Establishes gradients, controls osmotic effects, allows for cotransport Nerve cells have a Na + K + pump and
The beauty of the Na + K + pump Na + K + pump Found along the plasma membrane of all cells. Establishes gradients, controls osmotic effects, allows for cotransport Nerve cells have a Na + K + pump and
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Axon initial segment position changes CA1 pyramidal neuron excitability
 Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Axon initial segment position changes CA1 pyramidal neuron excitability Cristina Nigro and Jason Pipkin UCSD Neurosciences Graduate Program Abstract The axon initial segment (AIS) is the portion of the
Prolonged Synaptic Integration in Perirhinal Cortical Neurons
 RAPID COMMUNICATION Prolonged Synaptic Integration in Perirhinal Cortical Neurons JOHN M. BEGGS, 1 JAMES R. MOYER, JR., 1 JOHN P. MCGANN, 2 AND THOMAS H. BROWN 1 3 1 Department of Psychology, 2 Interdepartmental
RAPID COMMUNICATION Prolonged Synaptic Integration in Perirhinal Cortical Neurons JOHN M. BEGGS, 1 JAMES R. MOYER, JR., 1 JOHN P. MCGANN, 2 AND THOMAS H. BROWN 1 3 1 Department of Psychology, 2 Interdepartmental
Dendritic Signal Integration
 Dendritic Signal Integration 445 Dendritic Signal Integration N Spruston, Northwestern University, Evanston, IL, USA ã 2009 Elsevier Ltd. All rights reserved. Overview: Questions Most neurons have elaborately
Dendritic Signal Integration 445 Dendritic Signal Integration N Spruston, Northwestern University, Evanston, IL, USA ã 2009 Elsevier Ltd. All rights reserved. Overview: Questions Most neurons have elaborately
Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons
 11488 Journal of Physiology (2001), 532.3, pp.731 748 731 Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons Chiung-Chun Huang, Shiow-Win
11488 Journal of Physiology (2001), 532.3, pp.731 748 731 Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons Chiung-Chun Huang, Shiow-Win
Neurobiology. Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2010 1 The nervous system Central nervous system (CNS) Peripheral nervous system (PNS) 2 Enteric nervous system (digestive tract, gall bladder and
Neurobiology Cells of the nervous system Anthony Heape 2010 1 The nervous system Central nervous system (CNS) Peripheral nervous system (PNS) 2 Enteric nervous system (digestive tract, gall bladder and
Problem Set 3 - Answers. -70mV TBOA
 Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey HST 131/ Neuro 200 18 September 05 Explanation in text below graphs. Problem
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey HST 131/ Neuro 200 18 September 05 Explanation in text below graphs. Problem
Long-term depression and recognition of parallel "bre patterns in a multi-compartmental model of a cerebellar Purkinje cell
 Neurocomputing 38}40 (2001) 383}388 Long-term depression and recognition of parallel "bre patterns in a multi-compartmental model of a cerebellar Purkinje cell Volker Steuber*, Erik De Schutter Laboratory
Neurocomputing 38}40 (2001) 383}388 Long-term depression and recognition of parallel "bre patterns in a multi-compartmental model of a cerebellar Purkinje cell Volker Steuber*, Erik De Schutter Laboratory
GABA B Receptor-Mediated Presynaptic Inhibition Has History-Dependent Effects on Synaptic Transmission during Physiologically Relevant Spike Trains
 The Journal of Neuroscience, June 15, 2003 23(12):4809 4814 4809 Brief Communication GABA B Receptor-Mediated Presynaptic Inhibition Has History-Dependent Effects on Synaptic Transmission during Physiologically
The Journal of Neuroscience, June 15, 2003 23(12):4809 4814 4809 Brief Communication GABA B Receptor-Mediated Presynaptic Inhibition Has History-Dependent Effects on Synaptic Transmission during Physiologically
Neurons, Synapses, and Signaling
 Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Action Potentials and Synaptic Transmission. BIO 219 Napa Valley College Dr. Adam Ross
 Action Potentials and Synaptic Transmission BIO 219 Napa Valley College Dr. Adam Ross Review of action potentials Nodes of Ranvier Nucleus Dendrites Cell body In saltatory conduction, the nerve impulses
Action Potentials and Synaptic Transmission BIO 219 Napa Valley College Dr. Adam Ross Review of action potentials Nodes of Ranvier Nucleus Dendrites Cell body In saltatory conduction, the nerve impulses
Communication within a Neuron
 Neuronal Communication, Ph.D. Communication within a Neuron Measuring Electrical Potentials of Axons The Membrane Potential The Action Potential Conduction of the Action Potential 1 The withdrawal reflex
Neuronal Communication, Ph.D. Communication within a Neuron Measuring Electrical Potentials of Axons The Membrane Potential The Action Potential Conduction of the Action Potential 1 The withdrawal reflex
Changes in Extracellular Ionic Composition q
 Changes in Extracellular Ionic Composition q JL Stringer, Baylor College of Medicine, Houston, TX, United States Ó 2017 Elsevier Inc. All rights reserved. Introduction 1 Background 1 Methods 2 Recent Results
Changes in Extracellular Ionic Composition q JL Stringer, Baylor College of Medicine, Houston, TX, United States Ó 2017 Elsevier Inc. All rights reserved. Introduction 1 Background 1 Methods 2 Recent Results
The action potential travels down both branches because each branch is a typical axon with voltage dependent Na + and K+ channels.
 BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
Miniature Synaptic Events Elicited by Presynaptic Ca 2 Rise Are Selectively Suppressed by Cannabinoid Receptor Activation in Cerebellar Purkinje Cells
 86 The Journal of Neuroscience, January 4, 2006 26(1):86 95 Cellular/Molecular Miniature Synaptic Events Elicited by Presynaptic Ca 2 Rise Are Selectively Suppressed by Cannabinoid Receptor Activation
86 The Journal of Neuroscience, January 4, 2006 26(1):86 95 Cellular/Molecular Miniature Synaptic Events Elicited by Presynaptic Ca 2 Rise Are Selectively Suppressed by Cannabinoid Receptor Activation
Local Interneurons Regulate Synaptic Strength by Retrograde Release of Endocannabinoids
 The Journal of Neuroscience, September 27, 2006 26(39):9935 9943 9935 Cellular/Molecular Local Interneurons Regulate Synaptic Strength by Retrograde Release of Endocannabinoids Michael Beierlein and Wade
The Journal of Neuroscience, September 27, 2006 26(39):9935 9943 9935 Cellular/Molecular Local Interneurons Regulate Synaptic Strength by Retrograde Release of Endocannabinoids Michael Beierlein and Wade
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Cannabinoids 101. Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary
 Cannabinoids 101 Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary Disclosures Have received honoraria for Scientific Consultation: Pfizer International GW Pharmaceuticals Receive operating
Cannabinoids 101 Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary Disclosures Have received honoraria for Scientific Consultation: Pfizer International GW Pharmaceuticals Receive operating
3) Most of the organelles in a neuron are located in the A) dendritic region. B) axon hillock. C) axon. D) cell body. E) axon terminals.
 Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
The Influence of Multivesicular Release and Postsynaptic Receptor Saturation on Transmission at Granule Cell to Purkinje Cell Synapses
 The Journal of Neuroscience, December 14, 2005 25(50):11655 11665 11655 Cellular/Molecular The Influence of Multivesicular Release and Postsynaptic Receptor Saturation on Transmission at Granule Cell to
The Journal of Neuroscience, December 14, 2005 25(50):11655 11665 11655 Cellular/Molecular The Influence of Multivesicular Release and Postsynaptic Receptor Saturation on Transmission at Granule Cell to
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]](/thumbs/86/94924826.jpg) QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ YOURSELF COLOSSAL NEURON ACTIVITY
 QUIZ YOURSELF What are the factors that produce the resting potential? How is an action potential initiated and what is the subsequent flow of ions during the action potential? 1 COLOSSAL NEURON ACTIVITY
QUIZ YOURSELF What are the factors that produce the resting potential? How is an action potential initiated and what is the subsequent flow of ions during the action potential? 1 COLOSSAL NEURON ACTIVITY
Synapses and synaptic plasticity. Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember
 Synapses and synaptic plasticity Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember 1 Brain is comprised of networks of neurons connected and communicating via synapses ~10
Synapses and synaptic plasticity Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember 1 Brain is comprised of networks of neurons connected and communicating via synapses ~10
3.E.2 Continued. This is the essential knowledge statement from the curriculum framework. Detect---process--- response
 Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
MCB MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY
 MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
Neurotransmitter Systems II Receptors. Reading: BCP Chapter 6
 Neurotransmitter Systems II Receptors Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important chemical
Neurotransmitter Systems II Receptors Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important chemical
Notes are online at The Neuron
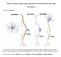 Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
vesicle diameter 0.5 nm 5 nm 50 nm 500 nm Lipid bilayer thickness.05 nm.5 nm 5 nm 50 nm
 Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Name (write your name on every sheet) HST 131/Neuro 200 Exam I, Sept 29, 2004
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Name (write your name on every sheet) HST 131/Neuro 200 Exam I, Sept 29, 2004
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
