Role of short-term cardiovascular regulation in heart period variability: a modeling study
|
|
|
- Giles Malone
- 6 years ago
- Views:
Transcription
1 Am J Physiol Heart Circ Physiol 284: H1479 H1493, First published December 19, 2002; /ajpheart Role of short-term cardiovascular regulation in heart period variability: a modeling study Mauro Ursino and Elisa Magosso Department of Electronics, Computer Science, and Systems, University of Bologna, I40136, Bologna, Italy Submitted 5 November 2002; accepted in final form 17 December 2002 Ursino, Mauro, and Elisa Magosso. Role of short-term cardiovascular regulation in heart period variability: a modeling study. Am J Physiol Heart Circ Physiol 284: H1479 H1493, First published December 19, 2002; /ajpheart A mathematical model of short-term cardiovascular regulation is used to investigate how heart period variability reflects the action of the autonomic regulatory mechanisms (vagal and sympathetic). The model includes the pulsating heart, the systemic (splanchnic and extrasplanchnic) and pulmonary circulation, the mechanical effect of respiration on venous return, two groups of receptors (arterial baroreceptors and lung stretch receptors), the sympathetic and vagal efferent branches, and a very low-frequency (LF) vasomotor noise. All model parameters were given on the basis of physiological data from the literature. We used data from humans whenever possible, whereas parameters for the regulation loops are derived from dog experiments. The model, with basal parameter values, produces a heart period power spectrum with two distinct peaks [a high frequency (HF) peak at the respiratory rate and a LF peak at 0.1 Hz]. Sensitivity analysis on the mechanism gains suggests that the HF peak is mainly affected by the vagal mechanism, whereas the LF peak is increased by a high sympathetic gain and reduced by a high vagal gain. Moreover, the LF peak depends significantly on the reactivity of resistance vessels and is affected by noise, amplified by the sympathetic control loop at its resonance frequency. The model may represent a new tool to study alterations in the heart period spectrum on the basis of quantitative physiological hypotheses. autonomic regulation; baroreflex; cardiovascular variability; mathematical model; power spectrum ANALYSIS OF HEART RATE VARIABILITY (HRV) has received much attention in the recent physiological literature as a method for extracting information on neural mechanisms regulating the cardiovascular system (4, 30, 31, 36). It is generally assumed that fluctuations in cardiovascular parameters originate from the interaction between the sympathetic and parasympathetic neural branches and other low-frequency (LF) sources of noise (such as those caused by humoral, thermal, or vasomotor control). In particular, two rhythms are generally observed in the heart period spectrum: a respiratory or high-frequency (HF) rhythm (located around Hz in humans), which is considered a marker of vagal activity, and a LF rhythm (around 0.1 Hz in humans), Address for reprint requests and other correspondence: M. Ursino, Dipartimento di Elettronica, Informatica e Sistemistica, viale Risorgimento 2, I40136 Bologna, Italy ( mursino@deis.unibo.it). which is thought to be a marker of sympathetic activity (28) or may reflect both vagal and sympathetic influences (3). Various maneuvers, which activate the cardiovascular control system (such as posture change or tilting) affect the heart period spectrum, leading to an increase in the LF component compared with the HF component. Because of its potential physiological and clinical impact, analysis of cardiovascular variability has been the subject of extensive research in the past decades, starting with the pioneering works by Guyton and Harris (18). Fundamental contributions in the field are the works by Akselrod et al. (4) and Koepchen (24). Recent studies include both modern signal processing techniques (7, 33) and mathematical models (11, 15, 23, 39, 43). However, the etiology of HRV in health and disease is still a matter of debate among physiologists. In particular, the role played by the various feedback regulatory loops and of sympathetic versus parasympathetic neural branches is still insufficiently clarified. Mathematical models may play an important role in clarifying the genesis of cardiovascular parameter variability and testing existing theories in accurate quantitative terms. Indeed, a few models have been presented in the literature with different purposes (11, 15, 23, 39, 43). However, all existing models are based on an oversimplified description of cardiovascular control; in particular, numerical parameter values, heart and vessel dynamics, and/or regulatory mechanism actions do not properly reflect present physiological knowledge. The aim of this study is to use a new mathematical model of short-term cardiovascular regulation to investigate the possible mechanisms leading to heart period fluctuations in reliable physiological terms. The model includes several major aspects that were not addressed in previous theoretical studies devoted to HRV analysis. In particular, the model incorporates a separate distinction of systemic and pulmonary circulation, sympathetic feedback control loops working on systemic resistance, unstressed volume and heart contractility, sympathovagal control of heart period, the mechanical effect of respiration on venous return and a very low-frequency (VLF) vasomotor term. Moreover, all these aspects are simulated on the basis of existing physiological data. The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact /03 $5.00 Copyright 2003 the American Physiological Society H1479
2 H1480 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION In the present study, as in our previous models, we assumed that heart period is the regulated quantity instead of heart rate (HR). In fact, as pointed out by Hainsworth (20), HR is not the appropriate quantity to quantify autonomic effects, because of the gross nonlinearity of the relationship linking HR to the efferent vagal and sympathetic activities [see Levy and Zieske (27)]. Conversely, these nonlinear relationships are naturally converted to linear if pulse interval is used instead of HR (see Ref. 48 for more details). Through a sensitivity analysis of model parameters, the present work aspires to clarify the conditions that may lead to clear LF oscillations in the heart period spectrum and to analyze the relationships between the LF and HF rhythms. QUALITATIVE MODEL DESCRIPTION The present model differs from a former model, described in Ref. 48, as to the following points. Simplifications Each feedback mechanism loop is described in a simpler way, without a definite distinction between the afferent and efferent neural activities. This simplification allows us to work with a more conceptual model, without any evident alteration in the overall regulatory response. Improvements The mechanical effect of respiration on venous return and cardiac output is included, and the action of lung stretch receptors on cardiovascular parameters has been taken into account. These two improvements have been introduced to obtain a physiological HF component in the heart period spectrum. In fact, both mechanisms introduce spectral components in cardiovascular quantities at the respiratory rate. In the following description only the main aspects of the model are presented in qualitative terms. Model equations are given in the APPENDIX. Heart and Vessels Fig. 1. Hydraulic analog of the cardiovascular system. P, pressure; F, flow; R, resistance; L, inertance; C, compliance; sa, systemic arteries; sp and sv, splanchnic peripheral and splanchnic venous circulation; ep and ev, extrasplanchnic peripheral and extrasplanchnic venous circulation; tv, systemic thoracic veins; ra and rv, right atrium and right ventricle; pa, pulmonary arteries; pp and pv, pulmonary peripheral and pulmonary venous circulation; la and lv, left atrium and left ventricle; P max,rv and P max,lv, right and left ventricle pressure in isometric conditions; P thor, intrathoracic pressure, i.e., the extravascular pressure for the compartments located inside the thorax (surrounded by a dashed line); P abd, abdominal pressure, which is the extravascular pressure for the splanchnic circulation (delimited by a dash-dotted line); F o,r and F o,l, blood flow from the right and left ventricle, respectively. The description of the heart and vessels is similar to that used in a previous report (48); hence, only a few details are given here. The right and left sides of the heart are modeled by means of a passive atrium (described through a linear compliance) and an active ventricle. Contractility of the ventricle is simulated by means of a time-varying elastance in series with a time-varying resistance. Shifting from the enddiastolic to the end-systolic values is governed by a pulsating activation function, which mimics the cardiac pacemaker. The vascular system comprehends a separate description of the pulmonary and the systemic circulation. The latter, in turn, contains the parallel arrangement of the splanchnic circulation and of the other, extrasplanchnic systemic vessels. Hemodynamics in each district (pulmonary, splanchnic, and systemic extrasplanchnic) is reproduced by means of resistive, capacitive, and inertial terms, as described previously (48) (see Fig. 1). In particular, blood volume stored in capacitive terms is the sum of an unstressed volume and a stressed volume, the latter being computed as the product of compliance and transmural pressure. A further compartment, not included in the previous work, represents the large systemic veins inside the thorax, carrying venous return to the right heart (see subscript tv in Fig. 1). A separate description of thoracic veins has been adopted to achieve a more accurate description of the effect of respiratory changes on venous return and cardiac output. The mechanical effect of respiration on cardiovascular quantities has been simulated by using time-varying expressions for intrathoracic pressure (P thor, which is extravascular pressure for all compartments inside the thorax; Fig. 1) and for abdominal pressure (P abd, which is extravascular pressure for the splanchnic circulation). The expressions for P thor and P abd during the respiratory cycle have been given according to the patterns reported in Ref. 35 (see APPENDIX for more details). In the present study we assumed that the subject breathes with a constant respiratory cycle 5 s long. Finally, respiratory volume (which is an input for lung stretch receptors) has been computed as a linear function of P thor. Parameters of this pressure-volume relationship were
3 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION H1481 chosen to attain physiological values of tidal volume, minute ventilation, and end-expiration volume in humans (34, 49). Regulation Mechanisms The description of short-term regulation mechanisms includes arterial baroreceptors and lung stretch receptors. The information from these receptors modulates various cardiovascular parameters: systemic peripheral resistance (both in the splanchnic and extrasplanchnic vascular beds), venous unstressed volume (both in the splanchnic and extrasplanchnic vascular beds), heart contractility (i.e., the end-systolic elastance in the left and right ventricles), and heart period. The first three control actions are purely sympathetic in nature and are described according to the general block diagram in Fig. 2. It is worth noting the presence of two different input stimuli coming from arterial baroreceptors (sensitive to arterial pressure changes) and lung stretch receptors (sensitive to changes in respiratory volume). These input stimuli are multiplied by the respective mechanism gain and summed up. Finally, the effector response includes a sigmoidal static relationship (which accounts for the existence of upper and lower limits for the response), a pure delay, and a first-order low-pass dynamic. The last two terms reproduce the main aspects of the response time pattern. The heart period control is more complex, involving a balance between vagal and sympathetic activities. In particular, dynamics of the vagal and sympathetic mechanisms are different: the vagal control is characterized by a rapid response, which is completed within two or three cardiac beats, whereas sympathetic control requires many seconds (20, 22, 32). Accordingly, heart period regulation in the model is described through the more complex diagram in Fig. 3. Worth noting is the existence of different gains and different dynamics for the vagal and sympathetic paths, whereas the sigmoidal relationship still describes the upper and lower limits of the effector response. Of course, some of the gains in Figs. 2 and 3 can be equal to zero (see Assignment of Model Parameters) if the corresponding mechanism plays a negligible role on the specific effector response. Finally, the existence of other LF sources of noise (such as those caused by humoral and thermal control or vasomotion) has been accounted for in an empirical manner, by superimposing a LF ( 0.12 Hz) uniformly distributed noise (zero mean value and assigned variance) on the expression of peripheral extrasplanchnic systemic resistance. The power spectrum of noise used in the simulations decreases quite linearly between 0 and 0.12 Hz. Assignment of Model Parameters All parameters in the heart and vessels have been given according to the previous work (48) to simulate normal human cardiovascular dynamics. The parameters that characterize the gains and static curves of feedback regulation mechanisms in Figs. 2 and 3 have been assigned by the steps described below. Because most experiments have been carried out on dogs, all quantities were normalized to the basal level, assuming that regulation mechanisms cause the same percentage changes in humans and animals. First, a value for the gains of the lung stretch receptor mechanism on HR and resistance has been given on the basis of experimental data obtained in the dog (14, 19). These authors measured the changes in HR and in total systemic resistance during steady-state variations in pulmonary inflation pressure and/or pulmonary volume (see Fig. 4). Moreover, according to Ref. 1, we assumed that the effect of lung stretch receptors on HR is exclusively mediated by the vagus. Finally, we are not aware of any effect of lung stretch receptors on heart contractility and on venous unstressed volumes; hence, the corresponding mechanism gains were set to zero. Second, a preliminary value for the gains of all sympathetic mechanisms activated by the arterial baroreflex [i.e., parameter G a in Fig. 2] and the upper and lower limits of the sigmoidal relationships (with the exception of the relationship for heart period, which is described below) have been given to simulate the results of open-loop experiments performed in vagotomized dogs (5, 9, 12, 13, 38, 42, 44). In these experiments the carotid sinuses are isolated from the rest of the circulation and their pressure is changed in steps. However, because the vagus is cut, neither the efferent vagal activity nor the afferent activities from aortic baroreceptors and lung stretch receptors concur with the observed responses. Hence, all vagal gains and lung stretch receptor gains on peripheral resistances were set to zero during these simulations. The results are shown in Fig. 5. Third, to complete the calculation of the arterial baroreflex, we must assign the gain of the vagal control on HR. Moreover, the possible role of the extracarotid (mainly aortic) arterial baroreceptors must be assessed. In this regard, some authors have observed that these baroreceptors play a major role in the control of HR in humans and that they significantly contribute to the increase in sympathetic activity to resistance vessels (17, 40, 41). Accordingly, the vagal and sympathetic gains that characterize heart period control by the arterial baroreceptors have been reassigned to reproduce Fig. 2. Sympathetic regulation mechanisms acting on the generic effector. may represent peripheral resistance in the splanchnic or extrasplanchnic systemic vascular beds (R sp or R ep), venous unstressed volume in the splanchnic or extrasplanchnic systemic vascular bed (V usv or V uev), or end-systolic elastance in right or left ventricle (E max,rv or E max,rv). P sa and P san are instantaneous systemic arterial pressure and its normal mean value, respectively; V L indicates lung volume, and V Ln is its value at the end of expiration. P sa P san and V L V Ln are the error perceived by arterial baroreceptors and pulmonary stretch receptors, respectively; G a and G p are the maximal gains of the 2 groups of receptors on effector. The 2 pieces of information from the receptors are summed up and passed through a static sigmoidal relationship (second block). The 3rd and 4th blocks represent a pure delay, D, and a low-pass first-order dynamic with time constant.
4 H1482 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION Fig. 3. Feedback regulation of heart period, T. Control of heart period is different from those of the other effectors (see Fig. 2) because it involves a balance between vagal activity (top branch, subscript v) and sympathetic activity (bottom branch, subscript s). The meaning of the symbols is analogous to Fig. 2. the changes in heart period observed in young, healthy men (25) during pharmacological changes in arterial pressure (see Fig. 5). To account for the possible role of extracarotid baroreceptors, the gain of the sympathetic control of HR exhibits a higher value compared with the value inferred from experiments in vagotomized dogs. Similarly, the gain of the sympathetic control on systemic arterial resistances has been increased, to account for the possible effect of extracarotid baroreceptors. We assumed a ratio of extracarotid versus carotid sympathetic control on resistance and heart period as large as 2.5, which approximately agrees with data by Ferguson et al. (17). Mechanism dynamics. All dynamic parameters in the control loop have been assigned on the basis of dog experiments. The time constant and time delay of the control of contractility (elastance; E max) have been given to reproduce the frequency dependence of the open-loop transfer function from carotid sinus pressure to end-systolic elastance (26). The time constant and pure delay of the resistance control have been given according to data reported previously (16). The time constant and pure delay of the venous unstressed volume control are higher than those of the resistance control: in fact, 1 min is required before the accomplishment of complete active venoconstriction (45). The time constants and pure delays of the HR control have been assigned considering that the effect of vagus stimulation on heart period is completed within two or three beats, whereas the sympathetic control is characterized by slower dynamics (a few seconds) (20, 22, 32). A list of all model parameters (both in the vagotomized experiments and in intact conditions) can be found in Table 1. The meaning of symbols is also explained in the APPENDIX. RESULTS Figure 6A shows a segment of the heart period time pattern, simulated with the model using the basal parameter values as in Table 1. Figure 6B reports the power spectral density computed from a longer simulated signal (1,000 s) with the Welch averaged periodogram method (2). The sequence was first detrended to eliminate frequencies close to zero; then several overlapping sections (60-s duration each, windowed by means of the Hanning window) were used to compute the averaged periodogram. The results clearly show the presence of two peaks in the power spectral density. The HF peak (0.2 Hz) reflects the respiratory activity, transmitted to the cardiovascular system both via the extravascular thoracic and abdominal pressures and via action of the lung stretch receptors. The LF peak represents a resonance of the control loops, located around 0.1 Hz. Quite evident in the spectrum also is the effect of the additional noise at very low frequencies. To clarify the role of the vagal and sympathetic mechanisms in the genesis of the power spectrum, we performed a sensitivity analysis on the strength of the sympathetic and vagal branches. This is summarized in Fig. 7. Figure 7, A and B, shows the effect of increasing or decreasing the gains of all sympathetic mechanisms (working on heart period, peripheral resistance, venous unstressed volume, and heart contractility) by 20% of their basal value. Simulations show that even a moderate increase in the sympathetic gains causes a
5 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION H1483 Fig. 4. Response of heart rate (HR; A) and systemic vascular resistance (B) to lung-stretch receptor activation. Continuous lines are model results. Simulations were performed in the absence of arterial baroreflex (i.e., only lung stretch receptors are operative). Hence, all the baroreflex gains were set at zero., E,, results of 3 experiments reported in Hainsworth (19); *, experimental data from Daly et al. (14). To facilitate comparison, we maintained the same input stimuli as used in in vivo experiments, i.e., inflation pressure or inflation volume. Increase in inflation volume has been expressed in % of tidal volume (V T). significant increase in the LF component, whereas the HF component exhibits just a mild decrease (Fig. 7A). By contrast, a moderate reduction in the sympathetic gains strongly attenuates the LF component (which becomes almost indistinguishable from noise) with a small increase in the HF component. Figure 7, C and D, shows the effect of a moderate change ( 20%) in the vagal gains (that is, the vagal gains on heart period from both arterial baroreceptors and lung stretch receptors). Increasing the vagal gains induces a large increase in the HF component, whereas the LF component is reduced (although the sympathetic gains were still set at their basal value). The opposite effect (significant decrease in the HF band with an increase in the LF band) occurs when the vagal gains are reduced. The previous results are summarized in Fig. 7E, where the sympathetic gains were increased by 20% and the vagal gains simultaneously decreased ( 20%). The effect is a dramatic rise in the LF component of the spectrum, with a reduction of the HF peak. Furthermore, to analyze the effect of VLF noise (such as that induced by thermal or hormonal regulation) on the spectrum, the simulation was repeated, with basal parameter values, by reducing the amplitude of the random noise by 50% of the initial value. The results, shown in Fig. 7F, attest that the presence of this VLF noise is important in the production of a clear LF component. The resonance in the control loops amplifies the existing noise at 0.1 Hz, thus resulting in a more evident peak compared with the case with almost no noise. In conclusion, sensitivity analyses in Fig. 7 point out that the HF peak mainly reflects the strength of vagal control (in fact, it is only mildly affected by the sympathetic gains), whereas the LF peak exhibits a more complex dependence on sympathetic and vagal mechanisms and on additional noise. In fact, this peak is affected both by a change in sympathetic strength and by a change in the vagal component. Moreover, the LF peak is also influenced by the level of superimposed noise. The simulation results in Fig. 7 summarize the role of the sympathetic and vagal efferent branches, and of noise, on heart period variability. However, the strength of oscillations not only depends on efferent activity but also on any other component within the control loops. In particular, any change in the sensitivity of the different effectors to efferent activity may cause a change in the oscillation strength. This point is usually ignored in the physiological literature, whereas the amplitude of the LF and HF peaks is ascribed merely to efferent activity, by neglecting the role of the other components in the loop. To analyze this aspect, we performed a sensitivity analysis on each effector response separately from the others. To this end, we selectively changed the central slope of the sigmoidal relationship for each effector (systemic peripheral resistance, venous unstressed volume, heart contractility, and heart period) by maintaining the sensitivity of all other effectors at the basal value. The slope of each effector was modified by 50% compared with the normal level, and the effect on the LF and HF peaks was evaluated. The results are shown in Fig. 8, with reference to a modification of the response of peripheral resistances (Fig. 8, A and B) and heart period (Fig. 8, C and D). The results concerning a modification of the response for venous unstressed volumes and contractility are not shown because a change in these effectors did not cause any appreciable change in the strength and position of the LF and HF peaks. The results in Fig. 8, A and B, show that the response of arterioles (which are the effectors for the resistance control) plays a pivotal role in the genesis of the LF wave. In fact, if the resistance response is depressed, the LF peak almost completely disappears, whereas a strong resistance response manifestly increases the LF peak. Moreover, as well expected, the resistance sensitivity has no effect on the HF peak. The results in Fig. 8, C and D, reveal that a strong sensitivity of the cardiac pacemaker (and hence of
6 H1484 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION Fig. 5. Summary of open-loop responses of arterial baroreflex in vagotomized conditions. A: systemic arterial pressure. B: cardiac output. C: heart period changes. D: systemic resistance. E: splanchnic unstressed volume. F: extrasplanchnic unstressed volume. Continuous thick lines are model simulation results. To simulate open-loop conditions, pressure at baroreceptors was given a constant nonpulsating value different from arterial pressure, changed in steps from 35 to 155 mmhg. To simulate vagotomy, pulmonary receptor gains (G p in Figs. 2 and 3) and baroreflex vagal gain (G atv in Fig. 3) were set at zero. Model results are compared with experimental data in the vagotomized dog in open-loop conditions. To compare model data with data in the dog, the corresponding quantities were normalized or expressed per unit weight. Experimental points are from Refs. 5, 9, 12, 13, 38, 42, and 44. Only in the case of heart period changes (C) has the simulation been repeated (dashed line) by including the baroreflex vagal gain as well as pulmonary receptor gains, to reproduce results of phenylephrine infusion in young human volunteers (ƒ; Ref. 25). Moreover, during the last simulation, baroreflex sympathetic gain on heart period (G ats in Fig. 3) and on peripheral resistances (G arep and G arsp in Fig. 2) have been enhanced to account for the role of aortic baroreceptors (17, 40, 41). The 3 experimental lines in E and F (unstressed volume control) are means SD.
7 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION H1485 Table 1. List of parameters characterizing feedback regulation mechanisms Psan 95 mmhg VLn 2.3 liters G arep (vagotom.) 0.04 mmhg 1 G prep (vagotom.) 0l 1 G arep (intact) 0.1 mmhg 1 G prep (intact) 0.33 l 1 R ep,min 0.91 mmhg s ml 1 R ep,max 1.9 mmhg s ml 1 D Rep 3s Rep 1.5 s G arsp (vagotom.) 0.04 mmhg 1 G prsp (vagotom.) 0l 1 G arsp (intact) 0.1 mmhg 1 G prsp (intact) 0.33 l 1 R sp,min 2.12 mmhg s ml 1 R sp,max 4.5 mmhg s ml 1 D Rsp 3s Rsp 1.5 s G avusv 10.8 mmhg 1 G pvusv 0l 1 V usv,min 871 ml V usv,max 1,371 ml D Vusv 5s Vusv 10 s G avuev 5.5 mmhg 1 G pvuev 0l 1 V uev,min 1,275 ml V uev,max 1,475 ml D Vuev 5s Vuev 10 s G aelv mmhg 1 G pelv 0l 1 E lv,min 2.45 mmhg/ml E lv,max 3.45 mmhg/ml D Elv 2s Elv 1.5 s G aerv mmhg 1 G perv 0l 1 D Erv 2s Erv 1.5 s E rv,min 1.25 mmhg/ml E rv,max 2.25 mmhg/ml G atv (vagotom.) 0 mmhg 1 G ptv (vagotom.) 0l 1 G atv (intact) mmhg 1 G ptv (intact) 0.25 l 1 G ats (vagotom.) mmhg 1 G pts (vagotom.) 0l 1 G ats (intact) mmhg 1 G pts (intact) 0l 1 D Tv 0.5 s Tv 0.8 s T min s T max s D Ts 3s Ts 1.8 s P san, basal systemic arterial pressure; V Ln, lung volume at end of normal expiratory act; G a, arterial receptor gain; G p pulmonary receptor gain; D, time delay;, time constant; R sp, splanchnic peripheral resistance; R ep, extrasplanchic peripheral resistance; V usv, splanchnic unstressed volume; V uev, extrasplanchnic unstressed volume; E lv, left ventricular elastance; E rv, right ventricular elastance; T s, sympathatetic control on heart period; T v, vagal control on heart period. heart period response) to efferent activity results in a higher HF peak, but this sensitivity has a modest effect on the amplitude of the LF peak. As well expected, a scarce sensitivity of the sinus node causes a depression of both LF and HF peaks. These results stress that the amplitudes of the HF and LF peaks represent not only the variations in efferent activity but also the sensitivity of the effectors (mainly arterioles and sinus node). In particular, the LF peak is especially affected by the strength of the resistance control (which depends both on variations in sympathetic efferent activity and on the reactivity of resistance vessels) whereas the HF peak is especially expressive of the vagal control on heart period and of sinus node reactivity. DISCUSSION The present work investigates the role of some shortterm regulatory mechanisms on the genesis of HRV Fig. 6. A: 200-s segment of heart period (HP) temporal pattern simulated with the model by using the basal parameter values (Table 1). B: power spectral density (PSD) computed on a longer simulated signal (1,000 s). The spectrum was obtained by breaking the signal into several sections (60-s duration each; detrended to eliminate the frequencies close to zero and then windowed by means of the Hanning window and zero-padded) and by averaging the periodograms of these sections (Welch s averaged modified periodogram method).
8 H1486 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION Fig. 7. Results of sensitivity analysis on sympathetic and vagal gains. A and B: effect on the PSD of increasing (A) and decreasing (B) the gains of all sympathetic mechanisms (working on peripheral resistances, venous unstressed volumes, heart period, and heart contractility) by 20% of their basal values. C and D: effect of increasing (C) and decreasing (D) vagal gains (i.e., the vagal gains on heart period from arterial baroreceptors and the lung stretch receptors) by 20% of their basal values. E: combined effect of increasing sympathetic gains by 20% and decreasing vagal gains by 20% (i.e., the simultaneous occurrence of the two situations depicted in A and D); F: effect of decreasing the amplitude of the very low-frequency random noise by 50%. For comparison, the basal power spectrum is reported in each panel (dashed lines).
9 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION H1487 Downloaded from Fig. 8. Results of sensitivity analysis on effector responses. A and B: effect on the PSD of increasing (A) and decreasing (B) the slope of the sigmoidal curve of the peripheral resistances (both splanchnic and extrasplanchnic) by 50% of its basal value. C and D: effect on the PSD of increasing (C) and decreasing (D) the slope of the sigmoidal curve of the heart period by 50% of its basal value. Changes in the slope of the sigmoidal curve of the other 2 effectors (heart contractility and venous unstressed volume) do not cause any appreciable alterations in the power spectrum; hence the corresponding data are omitted. with the use of mathematical modeling and computer simulation techniques. Although various models have been presented in recent years for the study of HRV, the present model introduces several new aspects and overcomes previous limitations. The idea that the arterial pressure control loop can cause oscillations in cardiovascular quantities has been portrayed in the physiological literature for many decades [see Guyton and Harris (18); see also Koepchen (24) for an ample review]. Kitney (23) first proposed a nonlinear model that involves a negative feedback loop, a pure time delay, and a switching element to theoretically analyze the oscillatory behavior of the blood pressure control system. A popular model of HRV was proposed by deboer et al. (15) in the late 1980s. It consists of a set of difference equations describing the baroreflex control, the input impedance of the systemic arterial tree, the contractile properties of the myocardium, and the mechanical effect of respiration. With that model, the authors were able to explain the presence of a LF peak in the HR spectrum, ascribing it to a resonance of the baroreflex control loop, mainly due to the sympathetic time delay. However, these models are based on a very simplistic description of the cardiovascular system and of the heart, and they include just a few features of the pressure control loop. Hence, their value lies more in the analysis of the mathematical properties of the equations than in physiological soundness. A simple model of HRV, which may exhibit chaotic behavior, was recently presented by Cavalcanti and Belardinelli (11). In this case, too, however, the emphasis is more on the mathematical route leading to chaos than on physiological reliability. Reduced models, oriented to the analysis of a simple feedback loop, have also been presented (10, 39). by on November 26, 2017
10 H1488 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION Seydnejad and Kitney (43) recently presented a more comprehensive model of cardiovascular regulation, finalized to the study of blood pressure and HRV. The model consists of a set of differential equations and includes several aspects of cardiovascular regulation, such as the afferent baroreflex, the vagal and sympathetic modulation of HR, the sympathetic control of the vasculature, the mechanical effect of respiration, a centrogenic oscillator, and a VLF vasorhythm. However, the nonlinear dependence of blood pressure on HR, on respiration, and on sympathetic excitation (i.e., the overall circulatory response) was identified empirically, through the analysis of Volterra series expansion, without the use of a cardiovascular model. Empirical mathematical models, based on the identification of autoregressive equations, were extensively used by Baselli et al. (see Ref. 7 for a review). Although these last models represent helpful empirical tools to quantitatively analyze spectra and extract specific features from data, they do not provide a physiological underpinning for the mechanisms leading to cardiovascular variability. The previous compendium underlines the need for a comprehensive mathematical model that carefully embodies physiological knowledge and can elucidate the possible origin of cardiovascular variability without the need for empirical learning procedures. The present model incorporates several aspects that were previously neglected, such as the control of venous unstressed volume and contractility, the lung stretch receptor reflex, heart pulsatility, the effects of intrathoracic and abdominal pressure changes on pulmonary circulation and on venous return, and a VLF vasomotor noise. Moreover, each feedback loop is separately described with its own parameter values. We are not aware of any previous model that summarizes all these aspects into a single theoretical structure. Furthermore, parameters in the model were given on the basis of physiological experiments in the literature (generally performed in open-loop conditions) and not on the basis of the final desired behavior. The pattern of HRV arises ultimately as an emergent property, when all model components are combined, and the model works in its natural closed-loop condition. This aspect strongly differentiates physiological models (like the present) from empirical models. The primary result of the present study is that a spectrum of heart period variability, similar to that observed in human subjects, emerges spontaneously from model simulations using basal parameter values. Subsequently, the sensitivity analysis of the effect of parameter changes furnished interesting clues on the origin and physiological significance of the HF and LF spectral components. HF Component The HF or respiratory component of the spectrum is determined by two concurrent mechanisms. The first is the effect of systemic arterial pressure (SAP) changes mediated by the baroreflex. SAP exhibits respiratory fluctuations caused by the intrathoracic and abdominal pressure changes (mechanical effect) and by the lung stretch receptor reflex working on resistance (neurogenic effect). The fluctuations systematically stimulate the baroreflex at the respiratory period (0.2 Hz in the present study). At this frequency, however, the baroreflex works entirely through its strong and fast vagal component, whereas the sympathetic component is almost completely damped out because of its low-pass filtering dynamic. The second mechanism, showing the direct effect of the lung stretch receptor reflex on heart period, is also mediated by the vagus. A common viewpoint in the literature is that the HF peak can be considered as an index of vagal activity. The previous description, and the sensitivity analysis shown in Fig. 7, confirm that the HF peak is significantly affected by the vagal gains and it is quite independent of the sympathetic gains. Hence, changes in the HF peak can be considered to be almost entirely caused by vagal control actions. However, care must be taken in considering the HF peak as an index of the strength in vagal control. In fact, the HF peak is modulated by all factors affecting the input to baroreflex and the lung stretch reflex [such as the depth and frequency of breathing (21), venous compliances in the thoracic and abdominal cavity, posture changes (47), etc.]. Moreover, as shown in Fig. 8, C and D, the HF peak depends strongly on the sensitivity of the cardiac pacemaker to efferent activity. Hence, as suggested by Akselrod (3) and Malpas (31), in different subjects and/or under different breathing conditions, the HF spectral component may be largely different even in the presence of an equivalent vagal gain. LF Peak As suggested by several authors (3, 28, 30, 31), model simulations confirm that the LF component of the power spectrum is strongly affected by the sympathetic system. A change in the sympathetic gains, in fact, causes a dramatic alteration in this component of the spectrum, leaving the HF component almost unchanged. In particular, to achieve values of LF oscillations in agreement with those observed in humans, the model requires values of sympathetic gains working on heart period and resistance much higher than those deducible from open-loop experiments in vagotomized dogs (see Fig. 5 and Table 1). These high values are justified by the presence of aortic baroreceptors (not operating in vagotomized conditions) and by the strong role of sympathetic control in humans (17, 40, 41). Actually, the present model results underscore the existence of a resonance induced by the slow sympathetic control loops around 0.1 Hz: this is congruent with the physiological considerations included in the model by Kitney (23) and deboer et al. (15). However, the sensitivity analysis in Fig. 7 shows that other factors may modulate the LF peak considerably. First, a reduction in the vagal gains induces an appreciable increase in the LF peak, and vice versa. The model suggests that if the vagal mechanisms are
11 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION H1489 strong they can damp the alterations in heart period induced by the sympathetic loop. By contrast, if the vagal gains are reduced, oscillations in heart period induced by the sympathetic resonance can be entirely manifested. In addition, the LF peak is dramatically affected by the presence of vasomotor noise in the LF and VLF range. This noise is amplified by the resonance in the baroreflex loop, placed around 0.1 Hz: the higher the noise, the higher is its amplification and so the higher the peak in the power spectrum. The latter result underlines the difficulty in considering the LF peak merely as a marker of short-term baroreflex regulation, without regard for other factors (such as vasomotion, thermal regulation, or hormonal regulation) that may represent noise terms for the autonomic control loops. This conclusion agrees with the observation that calcium blockade, which attenuates vasomotor fluctuations, also reduces the LF peak (50). The effect of changes in vagal gains on the LF peak (Fig. 7, C and D) deserves a comment. The result in Fig. 7 seems to contradict the observation by some authors (47), according to whom the LF peak is reduced by atropine, which is a vagal blocking agent. Conversely, in our model a reduction in vagal gain causes an increase in the LF peak. This contradiction, however, is only apparent and can be explained by the difference between average activity and fluctuations. The peaks in power spectrum reflect the fluctuations in neural activities at that given frequency, not the mean value of the neural activity. The fluctuations are conceptually different from the mean activity, and the relationship between these two aspects is far from being fully understood (3, 31). In the model, fluctuations are especially determined by the mechanism gains but are also affected by the working point along the sigmoidal characteristic (see Fig. 2). All of the sensitivity analyses illustrated in Fig. 7 explore the effect of variations in the mechanism gains only, which cause large changes in fluctuations without significantly modifying the working point along the sigmoidal relationship. Indeed, throughout this study, the working point was always located in the central linear part of the sigmoidal curve. In contrast, maneuvers that modify the average neural activity (such as atropine infusion, tilting, or exercise) presumably cause a shift in the working point along the sigmoidal relationship. If the working point moves from the central part of the sigmoidal relationship toward saturation, fluctuations are reduced. By contrast, moving the working point from a saturation region toward the central region causes an increase in fluctuations. By way of example, during exercise or heart failure, average sympathetic activity increases but the LF power may be reduced (6, 46). Similarly, if vagal activity is partially abolished by atropine administration, the working point of the heart period shifts toward the lower saturation region (i.e., we have cardioacceleration), which dampens heart period variability. This effect should not be confused with a reduction in the vagal gain. Furthermore, the sensitivity analysis in Fig. 8 reveals that the amplitude of the LF peak not only depends on efferent neural activity (sympathetic vs. vagal) but is also markedly affected by the individual sensitivity of different effectors. In particular, the present results suggest that the sensitivity of resistance vessels (arterioles) to sympathetic efferent activity plays a major role in the origin of the LF peak, whereas the other effectors (such as venous unstressed volume or contractility) are less important. The reason for this finding is that LF fluctuations originate from oscillations in blood pressure, which are detected by the baroreflex system and transmitted to heart period fluctuations. Pressure oscillations, in turn, are directly correlated with oscillations in resistance. Hence, if the ability of resistance vessels to respond to sympathetic influences is depressed, the power of the LF spectral component will similarly decline. The previous considerations stress that the LF peak not only reflects efferent neural activity but is also sensibly affected by vessel reactivity. As remarked by Malpas (31), the latter aspect is often ignored in the clinical/physiological literature, where the ratio LF/HF is just considered a marker of efferent neural activity, thus neglecting all other components participating to the pressure control loop. Of course, the present model implies some simplifications and omissions, which may be the target of future improvements and extensions. The main limitations are critically discussed below. First, in the present study we focused attention on the autonomic pressure control as the main factor affecting the LF waves, whereas other possible mechanisms have been neglected. This choice was adopted because the aim of this work was just to investigate the role of autonomic control system in the genesis of HRV. However, as discussed by Koepchen in his review (24), several other factors, and their complex nonlinear interactions, may concur with the formation of LF waves besides autonomic regulation. These include central, humoral, and vasomotor factors. Moreover, not only the strength of these factors but also their spectral distribution may be important in the etiology of LF fluctuations. A possible effect of some of these mechanisms has been included in the present study only in an empirical way, in the form of VLF noise. Second, the present description of the autonomic loops is necessarily simplified and omits some mechanisms that may have a role. In particular, experimental evidence suggests that cardiovascular afferent sympathetic fibers (located in the cardiac structures and in large thoracic vessels) are capable of mediating excitatory reflex actions, with positive feedback characteristics (29). This reflex seems to normally participate in the neural regulation of cardiovascular system, interacting with the negative feedback mechanisms (originating in the arterial baroreceptive and vagal afferents). Sympathosympathetic circuits can play a role in the genesis of HRV and in particular in the genesis of the LF oscillations, because these mechanisms are sympathetic in origin. The description of factors involved in HF waves is also simplified and neglects some mechanisms de-
12 H1490 CARDIOVASCULAR VARIABILITY AND SHORT-TERM REGULATION scribed in the literature. In particular, the baroreflex system includes not only arterial (or high pressure) baroreceptors but also cardiopulmonary (low pressure) baroreceptors (located in the atria, ventricles, and pulmonary veins). Activity in these receptors may be significantly affected by changes in venous pressure induced by respiration; hence, these receptors may parallel the effect of lung stretch receptors. Moreover, several authors have hypothesized that an important constituent of HF waves may be irradiation of impulses from the respiratory centers to cardiac vagal motor neurons (central mechanism). Furthermore, in the present simulations only metronomic breathing was simulated, with a respiratory period as great as 5 s. As a consequence, the HF peak and the changes in vagal activity are more evident than during normal respiration, because of a synchronism between all respiratory components (28). This consideration justifies why, with basal parameter values, the model predicts a HF spectral component much greater than the LF component. Finally, the mechanical effect of respiration on cardiac output is imputable not only to venous compression (i.e., the respiratory pump included in the present model) but also to ventricular interdependence. Ventricles share a common septal wall and are situated in a space limited by pericardial constraints. Filling and emptying of one ventricle are thus directly influenced by changes in the volume or pressure in the other ventricle. As a consequence of this phenomenon, called ventricular interdependence, the inspiratory increase in right ventricle filling (due to the fall in intrathoracic pressure) results in a larger decrease in left ventricle filling from the pulmonary circulation (8, 37). Thereby, ventricular interdependence mechanically contributes to the respiratory fluctuations in systemic arterial pressure. The mechanical coupling between ventricles is increased in pericardial diseases (such as cardiac tamponade and constrictive pericarditis), producing an exacerbation of respiratory-induced hemodynamic events (pulsus paradoxus). In the present study, the model has been validated by demonstrating that the autonomic pressure reflex may induce oscillations in heart period with physiological characteristics and by studying the size of the LF and HF components at different values of the parameters for the autonomic control. Of course, a broader validation is necessary in future works. This should include the following major items: 1) analysis of arterial pressure fluctuations and comparison of the corresponding spectra at different locations along the vascular system, 2) cross-spectral analysis among different quantities in the model, with special emphasis on phase differences among fluctuations, 3) characterization of model behavior with classic methods for nonlinear analysis (such as a study of bifurcations, computation of embedded dimensions and entrainments among oscillators). All these topics may be the target of future works. An important aspect that deserves discussion is the choice of species used to assign the numerical parameter values. Of course, the results obtained in this work depend on this choice, and scaling to other species requires caution. In the present study, parameters of the cardiovascular system (resistances, compliances, volumes, cardiac output, etc.) have been assigned to simulate hemodynamics of a normal man (see Ref. 48 for a thorough description). By contrast, as discussed in QUALITATIVE MODEL DESCRIPTION, the parameters of the autonomic regulation have been assigned mainly on the basis of experiments performed in dogs. The latter choice has two main implications. First, the use of a parameter setting based on dog experiments may lead to spectra more similar to those observed in dogs than in humans. Second, as discussed by Malpas (31), the dynamic characteristics of the autonomic loops (time delays, time constants) are significantly different between animal species. As is well known from the automatic control theory, time delays are particularly important in the genesis of instability phenomena. Small animals (such as rabbits and rats) exhibit time delays shorter than those measured in the dog s baroreflex arc: these differences determine a higher resonance frequency for the control loop. For instance, the LF band is at 0.1 Hz in the human, 0.14 Hz in the dog, 0.3 Hz in the rabbit, and 0.4 Hz in the rat (see Ref. 31 for extensive references). Hence, we emphasize that new ad hoc values should be assigned for the baroreflex loop parameters (especially time delays) if one intends to use the present model to simulate experiments in smaller animals. In conclusion, the present study demonstrates that LF and HF peaks in the heart period spectrum spontaneously emerge from a model of short-term cardiovascular regulation based on actual physiological knowledge. The model suggests that the LF peak reflects a resonance of the pressure control loop, especially connected with sympathetic regulation and with the reactivity of resistance vessels, but it is also intensified by other LF sources of noise and is attenuated by a strong vagal control. The HF peak is dominated by the vagal control of heart period. The model can be used to achieve deeper understanding of the mechanisms causing HRV, and of the significance of spectral changes, on the basis of rigorous quantitative hypotheses and physiological considerations. APPENDIX: QUANTITATIVE MODEL DESCRIPTION Cardiovascular System Equations for the heart and hemodynamics are formally similar to those used in the previous article (48); hence, they are not repeated for the sake of brevity. However, compared with the previous work, the present paper accounts for an extravascular pressure different from atmospheric pressure (i.e., not null) in the thoracic and abdominal cavity. As a consequence, vessel transmural pressure inside the thoracic cavity is computed as the difference between the intravascular and intrathoracic pressure (P thor), whereas transmural pressure at splanchnic vessels is intravascular pressure minus abdominal pressure (P abd).
Interaction between carotid baroregulation and the pulsating heart: a mathematical model
 Interaction between carotid baroregulation and the pulsating heart: a mathematical model MAURO URSINO Department of Electronics, Computer Science and Systems, University of Bologna, I40136 Bologna, Italy
Interaction between carotid baroregulation and the pulsating heart: a mathematical model MAURO URSINO Department of Electronics, Computer Science and Systems, University of Bologna, I40136 Bologna, Italy
Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model
 Am J Physiol Heart Circ Physiol 279: H149 H165, 2000. Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model MAURO URSINO AND ELISA MAGOSSO Department of Electronics, Computer Science,
Am J Physiol Heart Circ Physiol 279: H149 H165, 2000. Acute cardiovascular response to isocapnic hypoxia. I. A mathematical model MAURO URSINO AND ELISA MAGOSSO Department of Electronics, Computer Science,
CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE
 CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE VASILE MANOLIU Electrical Engineering Faculty, POLITEHNICA University of Bucharest, Splaiul
CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE VASILE MANOLIU Electrical Engineering Faculty, POLITEHNICA University of Bucharest, Splaiul
Modeling cardiovascular autoregulation of the preterm infant
 Modeling cardiovascular autoregulation of the preterm infant Marco Dat BMTE 1.18 November 8th 21 ID nr: 547385 Faculty of medical engineering Department of cardiovascular biomechanics Supervisors: ir.
Modeling cardiovascular autoregulation of the preterm infant Marco Dat BMTE 1.18 November 8th 21 ID nr: 547385 Faculty of medical engineering Department of cardiovascular biomechanics Supervisors: ir.
Chapter 9, Part 2. Cardiocirculatory Adjustments to Exercise
 Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1
 BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS
 A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS R. Mukkamala, R. G. Mark, R. J. Cohen Haard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA Abstract We
A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS R. Mukkamala, R. G. Mark, R. J. Cohen Haard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA Abstract We
Figure removed due to copyright restrictions.
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 An Example of a Fetal Heart Rate Tracing Figure removed
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 An Example of a Fetal Heart Rate Tracing Figure removed
The Arterial and Venous Systems Roland Pittman, Ph.D.
 The Arterial and Venous Systems Roland Pittman, Ph.D. OBJECTIVES: 1. State the primary characteristics of the arterial and venous systems. 2. Describe the elastic properties of arteries in terms of pressure,
The Arterial and Venous Systems Roland Pittman, Ph.D. OBJECTIVES: 1. State the primary characteristics of the arterial and venous systems. 2. Describe the elastic properties of arteries in terms of pressure,
Blood Pressure Regulation. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Blood pressure. Formation of the blood pressure: Blood pressure. Formation of the blood pressure 5/1/12
 Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Cardiovascular Physiology. Heart Physiology. Introduction. The heart. Electrophysiology of the heart
 Cardiovascular Physiology Heart Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through
Cardiovascular Physiology Heart Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through
Review Article. Interactive Physiology in Critical Illness : Pulmonary and Cardiovascular Systems. Introduction
 310 Indian Deepak J Physiol Shrivastava Pharmacol 2016; 60(4) : 310 314 Indian J Physiol Pharmacol 2016; 60(4) Review Article Interactive Physiology in Critical Illness : Pulmonary and Cardiovascular Systems
310 Indian Deepak J Physiol Shrivastava Pharmacol 2016; 60(4) : 310 314 Indian J Physiol Pharmacol 2016; 60(4) Review Article Interactive Physiology in Critical Illness : Pulmonary and Cardiovascular Systems
The Cardiac Cycle Clive M. Baumgarten, Ph.D.
 The Cardiac Cycle Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe periods comprising cardiac cycle and events within each period 2. Describe the temporal relationships between pressure, blood flow,
The Cardiac Cycle Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe periods comprising cardiac cycle and events within each period 2. Describe the temporal relationships between pressure, blood flow,
Figure 1 Uncontrolled state where ZPFV is increased by 500 ml.
 Question 1 Part I Description The initial venous zero pressure filling volume was 2500 ml. The heart rate was fixed at 72 beats/min. The systemic arterial pressure (SAP) was 114/75 mmhg (average = 94 mmhg).
Question 1 Part I Description The initial venous zero pressure filling volume was 2500 ml. The heart rate was fixed at 72 beats/min. The systemic arterial pressure (SAP) was 114/75 mmhg (average = 94 mmhg).
Heart Pump and Cardiac Cycle. Faisal I. Mohammed, MD, PhD
 Heart Pump and Cardiac Cycle Faisal I. Mohammed, MD, PhD 1 Objectives To understand the volume, mechanical, pressure and electrical changes during the cardiac cycle To understand the inter-relationship
Heart Pump and Cardiac Cycle Faisal I. Mohammed, MD, PhD 1 Objectives To understand the volume, mechanical, pressure and electrical changes during the cardiac cycle To understand the inter-relationship
Computational modeling of cardiovascular response to orthostatic stress
 J Appl Physiol 92: 1239 1254, 2002; 10.1152/japplphysiol.00241.2001. Computational modeling of cardiovascular response to orthostatic stress THOMAS HELDT, 1 EUNB.SHIM, 2 ROGER D. KAMM, 3 AND ROGER G. MARK
J Appl Physiol 92: 1239 1254, 2002; 10.1152/japplphysiol.00241.2001. Computational modeling of cardiovascular response to orthostatic stress THOMAS HELDT, 1 EUNB.SHIM, 2 ROGER D. KAMM, 3 AND ROGER G. MARK
BRAIN IN THE HEART. Eternity In Our Hearts
 BRAIN IN THE HEART Rediscovering the Biblical Significance of the Heart Eternity In Our Hearts He has made everything beautiful (appropriate) in its time. He has also set eternity in their heart, yet so
BRAIN IN THE HEART Rediscovering the Biblical Significance of the Heart Eternity In Our Hearts He has made everything beautiful (appropriate) in its time. He has also set eternity in their heart, yet so
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006
 SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006 The goal of this exercise to explore the behavior of the heart as a mechanical pump. For
SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006 The goal of this exercise to explore the behavior of the heart as a mechanical pump. For
Jan M. Headley, R.N. BS
 Fluid First: Using PLR & SVV to Optimize Volume Jan M. Headley, R.N. BS Disclosure Director, Clinical Marketing & Professional Education Edwards Lifesciences Does this Patient NEED Fluid?? WE Have a Problem
Fluid First: Using PLR & SVV to Optimize Volume Jan M. Headley, R.N. BS Disclosure Director, Clinical Marketing & Professional Education Edwards Lifesciences Does this Patient NEED Fluid?? WE Have a Problem
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Supplemental methods Pericardium In several studies, it has been shown that the pericardium significantly modulates ventricular interaction. 1-4 Since ventricular interaction has
SUPPLEMENTAL MATERIAL Supplemental methods Pericardium In several studies, it has been shown that the pericardium significantly modulates ventricular interaction. 1-4 Since ventricular interaction has
Note: At the end of the instructions, you will find a table which must be filled in to complete the exercise.
 Autonomic Nervous System Theoretical foundations and instructions for conducting practical exercises carried out during the course List of practical exercises 1. Deep (controlled) breath test 2. Cold pressor
Autonomic Nervous System Theoretical foundations and instructions for conducting practical exercises carried out during the course List of practical exercises 1. Deep (controlled) breath test 2. Cold pressor
ENT 318/3 Artificial Organs
 ENT 318/3 Artificial Organs Modeling of cardiovascular system and VAD Lecturer Ahmad Nasrul bin Norali 1 What is modeling and why we need it? In designing product, sometimes we have to make sure that the
ENT 318/3 Artificial Organs Modeling of cardiovascular system and VAD Lecturer Ahmad Nasrul bin Norali 1 What is modeling and why we need it? In designing product, sometimes we have to make sure that the
Approaches to the Autonomic Nervous System in Female Athletes
 Health Topics for Tokyoites Juntendo Medical Journal 2017. 63(2), 83-87 Approaches to the Autonomic Nervous System in Female Athletes NOBUHIRO SUETAKE *,HIROYUKI KOBAYASHI * *Department of Hospital Administration,
Health Topics for Tokyoites Juntendo Medical Journal 2017. 63(2), 83-87 Approaches to the Autonomic Nervous System in Female Athletes NOBUHIRO SUETAKE *,HIROYUKI KOBAYASHI * *Department of Hospital Administration,
Eindhoven University of Technology. Exam Modeling Cardiac Function (8W160)
 Eindhoven University of Technology department of Biomedical Engineering group Cardiovascular Biomechanics Exam Modeling Cardiac Function (8W160) January 21, 2011, 14.00 17.00 h This exam consists of 6
Eindhoven University of Technology department of Biomedical Engineering group Cardiovascular Biomechanics Exam Modeling Cardiac Function (8W160) January 21, 2011, 14.00 17.00 h This exam consists of 6
Determination of Cardiac Output By Equating Venous Return Curves With Cardiac Response Curves1
 Determination of Cardiac Output By Equating Venous Return Curves With Cardiac Response Curves1 ARTHUR C. GUYTQN From the Department of Physiology and Biophysics, School of Medicine, University of Mississippi,
Determination of Cardiac Output By Equating Venous Return Curves With Cardiac Response Curves1 ARTHUR C. GUYTQN From the Department of Physiology and Biophysics, School of Medicine, University of Mississippi,
*Generating blood pressure *Routing blood: separates. *Ensuring one-way blood. *Regulating blood supply *Changes in contraction
 *Generating blood pressure *Routing blood: separates pulmonary and systemic circulations *Ensuring one-way blood flow: valves *Regulating blood supply *Changes in contraction rate and force match blood
*Generating blood pressure *Routing blood: separates pulmonary and systemic circulations *Ensuring one-way blood flow: valves *Regulating blood supply *Changes in contraction rate and force match blood
The Journal of Physiology
 J Physiol 590.23 (2012) pp 5975 5992 5975 A new conceptual paradigm for the haemodynamics of salt-sensitive hypertension: a mathematical modelling approach Viktoria A. Averina 1, Hans G. Othmer 1, Gregory
J Physiol 590.23 (2012) pp 5975 5992 5975 A new conceptual paradigm for the haemodynamics of salt-sensitive hypertension: a mathematical modelling approach Viktoria A. Averina 1, Hans G. Othmer 1, Gregory
10. Thick deposits of lipids on the walls of blood vessels, called, can lead to serious circulatory issues. A. aneurysm B. atherosclerosis C.
 Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart-rate Variability Christoph Guger,
 Heart-rate Variability Christoph Guger, 10.02.2004 Heart-rate Variability (HRV) 1965 Hon & Lee Fetal distress alterations in interbeat intervals before heart rate (HR) changed 1980 HRV is strong and independent
Heart-rate Variability Christoph Guger, 10.02.2004 Heart-rate Variability (HRV) 1965 Hon & Lee Fetal distress alterations in interbeat intervals before heart rate (HR) changed 1980 HRV is strong and independent
Veins. VENOUS RETURN = PRELOAD = End Diastolic Volume= Blood returning to heart per cardiac cycle (EDV) or per minute (Venous Return)
 Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Principles of Biomedical Systems & Devices. Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont
 Principles of Biomedical Systems & Devices Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont Review of Cardiac Anatomy Four chambers Two atria-receive blood from the vena cave and pulmonary veins Two
Principles of Biomedical Systems & Devices Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont Review of Cardiac Anatomy Four chambers Two atria-receive blood from the vena cave and pulmonary veins Two
Physiology lecture 15 Hemodynamic
 Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
Effects of Light Stimulus Frequency on Phase Characteristics of Brain Waves
 SICE Annual Conference 27 Sept. 17-2, 27, Kagawa University, Japan Effects of Light Stimulus Frequency on Phase Characteristics of Brain Waves Seiji Nishifuji 1, Kentaro Fujisaki 1 and Shogo Tanaka 1 1
SICE Annual Conference 27 Sept. 17-2, 27, Kagawa University, Japan Effects of Light Stimulus Frequency on Phase Characteristics of Brain Waves Seiji Nishifuji 1, Kentaro Fujisaki 1 and Shogo Tanaka 1 1
CASE 13. What neural and humoral pathways regulate arterial pressure? What are two effects of angiotensin II?
 CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
BME 5742 Bio-Systems Modeling and Control. Lecture 41 Heart & Blood Circulation Heart Function Basics
 BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
Chapters 9 & 10. Cardiorespiratory System. Cardiovascular Adjustments to Exercise. Cardiovascular Adjustments to Exercise. Nervous System Components
 Cardiorespiratory System Chapters 9 & 10 Cardiorespiratory Control Pulmonary ventilation Gas exchange Left heart Arterial system Tissues Right heart Lungs Pulmonary ventilation Cardiovascular Regulation-
Cardiorespiratory System Chapters 9 & 10 Cardiorespiratory Control Pulmonary ventilation Gas exchange Left heart Arterial system Tissues Right heart Lungs Pulmonary ventilation Cardiovascular Regulation-
The Cardiovascular System
 The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
PHYSIOLOGY MeQ'S (Morgan) All the following statements related to blood volume are correct except for: 5 A. Blood volume is about 5 litres. B.
 PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
Cardiovascular Physiology
 Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Cardiovascular Responses to Exercise
 CARDIOVASCULAR PHYSIOLOGY 69 Case 13 Cardiovascular Responses to Exercise Cassandra Farias is a 34-year-old dietician at an academic medical center. She believes in the importance of a healthy lifestyle
CARDIOVASCULAR PHYSIOLOGY 69 Case 13 Cardiovascular Responses to Exercise Cassandra Farias is a 34-year-old dietician at an academic medical center. She believes in the importance of a healthy lifestyle
Mutual interactions of respiratory sinus arrhythmia and the carotid baroreceptor-heart rate reflex
 Clinical Science (1992) 82, 19-145 (Printed in Great Britain) Mutual interactions of respiratory sinus arrhythmia and the carotid baroreceptor-heart rate reflex I9 S. J. CROSS*, M. R. COWlEt and J. M.
Clinical Science (1992) 82, 19-145 (Printed in Great Britain) Mutual interactions of respiratory sinus arrhythmia and the carotid baroreceptor-heart rate reflex I9 S. J. CROSS*, M. R. COWlEt and J. M.
Modeling and Simulation of Arterial Pressure Control
 Modeling and Simulation of Arterial Pressure Control EUGEN IANCU*, IONELA IANCU** * Department of Automation and Mechatronics ** Department of Physiology, Medical Informatics and Biostatistics University
Modeling and Simulation of Arterial Pressure Control EUGEN IANCU*, IONELA IANCU** * Department of Automation and Mechatronics ** Department of Physiology, Medical Informatics and Biostatistics University
Blood Pressure Regulation. Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure
 Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
Cardiovascular Physiology
 Cardiovascular Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through two vascular systems
Cardiovascular Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through two vascular systems
Estimating Mental Stress Using a Wearable Cardio-Respiratory Sensor
 Estimating Mental Stress Using a Wearable Cardio-Respiratory Sensor Jongyoon Choi and Ricardo Gutierrez-Osuna Department of Computer Science and Engineering Texas A&M University, College Station, TX {goonyong,
Estimating Mental Stress Using a Wearable Cardio-Respiratory Sensor Jongyoon Choi and Ricardo Gutierrez-Osuna Department of Computer Science and Engineering Texas A&M University, College Station, TX {goonyong,
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM
 Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Blood Pressure and its Regulation
 Blood Pressure and its Regulation Blood pressure in your blood vessels is closely monitored by baroreceptors; they send messages to the cardio regulatory center of your medulla oblongata to regulate your
Blood Pressure and its Regulation Blood pressure in your blood vessels is closely monitored by baroreceptors; they send messages to the cardio regulatory center of your medulla oblongata to regulate your
Regulation of respiration
 Regulation of respiration Breathing is controlled by the central neuronal network to meet the metabolic demands of the body Neural regulation Chemical regulation Respiratory center Definition: A collection
Regulation of respiration Breathing is controlled by the central neuronal network to meet the metabolic demands of the body Neural regulation Chemical regulation Respiratory center Definition: A collection
The Influence of Altered Pulmonarv
 The Influence of Altered Pulmonarv J Mechanics on the Adequacy of Controlled Ventilation Peter Hutchin, M.D., and Richard M. Peters, M.D. W ' hereas during spontaneous respiration the individual determines
The Influence of Altered Pulmonarv J Mechanics on the Adequacy of Controlled Ventilation Peter Hutchin, M.D., and Richard M. Peters, M.D. W ' hereas during spontaneous respiration the individual determines
REGULATION OF CARDIOVASCULAR SYSTEM
 REGULATION OF CARDIOVASCULAR SYSTEM Jonas Addae Medical Sciences, UWI REGULATION OF CARDIOVASCULAR SYSTEM Intrinsic Coupling of cardiac and vascular functions - Autoregulation of vessel diameter Extrinsic
REGULATION OF CARDIOVASCULAR SYSTEM Jonas Addae Medical Sciences, UWI REGULATION OF CARDIOVASCULAR SYSTEM Intrinsic Coupling of cardiac and vascular functions - Autoregulation of vessel diameter Extrinsic
The Vigileo monitor by Edwards Lifesciences supports both the FloTrac Sensor for continuous cardiac output and the Edwards PreSep oximetry catheter
 1 2 The Vigileo monitor by Edwards Lifesciences supports both the FloTrac Sensor for continuous cardiac output and the Edwards PreSep oximetry catheter for continuous central venous oximetry (ScvO2) 3
1 2 The Vigileo monitor by Edwards Lifesciences supports both the FloTrac Sensor for continuous cardiac output and the Edwards PreSep oximetry catheter for continuous central venous oximetry (ScvO2) 3
HRV in Diabetes and Other Disorders
 HRV in Diabetes and Other Disorders Roy Freeman, MD Center for Autonomic and Peripheral Nerve Disorders Beth Israel Deaconess Medical Center Harvard Medical School Control Propranolol Atropine Wheeler
HRV in Diabetes and Other Disorders Roy Freeman, MD Center for Autonomic and Peripheral Nerve Disorders Beth Israel Deaconess Medical Center Harvard Medical School Control Propranolol Atropine Wheeler
11/10/2014. Muscular pump Two atria Two ventricles. In mediastinum of thoracic cavity 2/3 of heart's mass lies left of midline of sternum
 It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
HRV ventricular response during atrial fibrillation. Valentina Corino
 HRV ventricular response during atrial fibrillation Outline AF clinical background Methods: 1. Time domain parameters 2. Spectral analysis Applications: 1. Evaluation of Exercise and Flecainide Effects
HRV ventricular response during atrial fibrillation Outline AF clinical background Methods: 1. Time domain parameters 2. Spectral analysis Applications: 1. Evaluation of Exercise and Flecainide Effects
Cardiac Output (C.O.) Regulation of Cardiac Output
 Cardiac Output (C.O.) Is the volume of the blood pumped by each ventricle per minute (5 Litre) Stroke volume: Is the volume of the blood pumped by each ventricle per beat. Stroke volume = End diastolic
Cardiac Output (C.O.) Is the volume of the blood pumped by each ventricle per minute (5 Litre) Stroke volume: Is the volume of the blood pumped by each ventricle per beat. Stroke volume = End diastolic
EKG Abnormalities. Adapted from:
 EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
Cardiovascular System
 Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Chapter 20 (2) The Heart
 Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Project 1: Circulation
 Project 1: Circulation This project refers to the matlab files located at: http://www.math.nyu.edu/faculty/peskin/modsimprograms/ch1/. Model of the systemic arteries. The first thing to do is adjust the
Project 1: Circulation This project refers to the matlab files located at: http://www.math.nyu.edu/faculty/peskin/modsimprograms/ch1/. Model of the systemic arteries. The first thing to do is adjust the
Heart. Large lymphatic vessels Lymph node. Lymphatic. system Arteriovenous anastomosis. (exchange vessels)
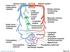 Venous system Large veins (capacitance vessels) Small veins (capacitance vessels) Postcapillary venule Thoroughfare channel Heart Large lymphatic vessels Lymph node Lymphatic system Arteriovenous anastomosis
Venous system Large veins (capacitance vessels) Small veins (capacitance vessels) Postcapillary venule Thoroughfare channel Heart Large lymphatic vessels Lymph node Lymphatic system Arteriovenous anastomosis
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Inner Balance Assessment Using Methods of the Heart Rate Variability Analysis
 Inner Balance Assessment Using Methods of the Heart Rate Variability Analysis Practical Use Manual 2000 2009 Biocom Technologies. All Rights reserved. Biocom Technologies makes no claims that its products
Inner Balance Assessment Using Methods of the Heart Rate Variability Analysis Practical Use Manual 2000 2009 Biocom Technologies. All Rights reserved. Biocom Technologies makes no claims that its products
Outline. Echocardiographic Assessment of Pericardial Effusion/Tamponade: The Essentials
 Echocardiographic Assessment of Pericardial Effusion/Tamponade: The Essentials John R Schairer DO FACC Henry Ford Heart and Vascular Institute No Disclosures Outline Normal Anatomy and Physiology Pathophysiology
Echocardiographic Assessment of Pericardial Effusion/Tamponade: The Essentials John R Schairer DO FACC Henry Ford Heart and Vascular Institute No Disclosures Outline Normal Anatomy and Physiology Pathophysiology
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Biology 236 Spring 2002 Campos/Wurdak/Fahey Laboratory 4. Cardiovascular and Respiratory Adjustments to Stationary Bicycle Exercise.
 BACKGROUND: Cardiovascular and Respiratory Adjustments to Stationary Bicycle Exercise. The integration of cardiovascular and respiratory adjustments occurring in response to varying levels of metabolic
BACKGROUND: Cardiovascular and Respiratory Adjustments to Stationary Bicycle Exercise. The integration of cardiovascular and respiratory adjustments occurring in response to varying levels of metabolic
0. Where do you think blood tends to pool when a witch flies on her broomstick? (1 point)
 1 Vertebrate Physiology 437 EXAM II 31 October 2002 NAME 0. Where do you think blood tends to pool when a witch flies on her broomstick? (1 point) True or False (write true or false ; 6 points total; 1
1 Vertebrate Physiology 437 EXAM II 31 October 2002 NAME 0. Where do you think blood tends to pool when a witch flies on her broomstick? (1 point) True or False (write true or false ; 6 points total; 1
Anaesthesia. Update in. An Introduction to Cardiovascular Physiology. James Rogers Correspondence
 Update in Anaesthesia Originally published in Update in Anaesthesia, edition 10 (1999) An Introduction to Cardiovascular Physiology Correspondence Email: James.Rogers@nbt.nhs.uk INTRODUCTION The cardiovascular
Update in Anaesthesia Originally published in Update in Anaesthesia, edition 10 (1999) An Introduction to Cardiovascular Physiology Correspondence Email: James.Rogers@nbt.nhs.uk INTRODUCTION The cardiovascular
University of Groningen. Short-term cardiovascular effects of mental tasks Roon, Arie Matthijs van
 University of Groningen Short-term cardiovascular effects of mental tasks Roon, Arie Matthijs van IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
University of Groningen Short-term cardiovascular effects of mental tasks Roon, Arie Matthijs van IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
AS Level OCR Cardiovascular System
 AS Level OCR Cardiovascular System Learning Objectives The link between the Cardiac Cycle and the Conduction system of the heart. The relationship between Stroke volume, Heart rate and Cardiac Output.
AS Level OCR Cardiovascular System Learning Objectives The link between the Cardiac Cycle and the Conduction system of the heart. The relationship between Stroke volume, Heart rate and Cardiac Output.
Spirometry and Flow Volume Measurements
 Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Analysis of Human Cardiovascular System using Equivalent Electronic System
 Analysis of Human Cardiovascular System using Equivalent Electronic System N. Vinoth 1, S. Nagarjuna Chary 2 Dept of Electronics and Instrumentation Engineering, Annamalai University, Annamalai nagar,
Analysis of Human Cardiovascular System using Equivalent Electronic System N. Vinoth 1, S. Nagarjuna Chary 2 Dept of Electronics and Instrumentation Engineering, Annamalai University, Annamalai nagar,
d) Cardiovascular System Higher Human Biology
 d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
Received November 19, 2012; revised December 20, 2012; accepted December 27, 2012
 Journal of Behavioral and Brain Science, 2013, 3, 26-48 doi:10.4236/jbbs.2013.31004 Published Online February 2013 (http://www.scirp.org/journal/jbbs) Heart Rate Variability, Standard of Measurement, Physiological
Journal of Behavioral and Brain Science, 2013, 3, 26-48 doi:10.4236/jbbs.2013.31004 Published Online February 2013 (http://www.scirp.org/journal/jbbs) Heart Rate Variability, Standard of Measurement, Physiological
BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE. Shao Hui Chen. BS, Harbin Institute of Technology, 1994
 BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE by Shao Hui Chen BS, Harbin Institute of Technology, 1994 MS, China Academy of Launch Vehicle Technology, 22 Submitted to the
BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE by Shao Hui Chen BS, Harbin Institute of Technology, 1994 MS, China Academy of Launch Vehicle Technology, 22 Submitted to the
4. The two inferior chambers of the heart are known as the atria. the superior and inferior vena cava, which empty into the left atrium.
 Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Chapter 18 - Heart. I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity)
 Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Normal Pericardial Physiology
 Normal Pericardial Physiology Normal pericardium contains 20-30 ml of lymphoid fluid lubricating function that facilitates normal myocardial rotation and translation during each cardiac cycle in that the
Normal Pericardial Physiology Normal pericardium contains 20-30 ml of lymphoid fluid lubricating function that facilitates normal myocardial rotation and translation during each cardiac cycle in that the
Properties of Pressure
 OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
On the Track of Syncope induced by Orthostatic Stress - Feedback Mechanisms Regulating the Cardiovascular System
 Proceedings of the 7th IFAC Symposium on Modelling and Control in Biomedical Systems On the Track of Syncope induced by Orthostatic Stress - Feedback Mechanisms Regulating the Cardiovascular System Ottesen
Proceedings of the 7th IFAC Symposium on Modelling and Control in Biomedical Systems On the Track of Syncope induced by Orthostatic Stress - Feedback Mechanisms Regulating the Cardiovascular System Ottesen
10/23/2017. Muscular pump Two atria Two ventricles. In mediastinum of thoracic cavity 2/3 of heart's mass lies left of midline of sternum
 It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
The circulatory system
 Introduction to Physiology (Course # 72336) 1 הלב עקרונות בסיסיים (הכנה למעבדת לב) Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
Introduction to Physiology (Course # 72336) 1 הלב עקרונות בסיסיים (הכנה למעבדת לב) Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits:
 1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
Complexity of cardiovascular control in amyotrophic lateral sclerosis patients is related to disease duration
 Complexity of cardiovascular control in amyotrophic lateral sclerosis patients is related to disease duration 1,2, Laura Dalla Vecchia 1, Kalliopi Marinou 1, Gabriele Mora 1, Alberto Porta 3,4 ¹ IRCCS
Complexity of cardiovascular control in amyotrophic lateral sclerosis patients is related to disease duration 1,2, Laura Dalla Vecchia 1, Kalliopi Marinou 1, Gabriele Mora 1, Alberto Porta 3,4 ¹ IRCCS
Adel Hasanin Ahmed 1
 Adel Hasanin Ahmed 1 PERICARDIAL DISEASE The pericardial effusion ends anteriorly to the descending aorta and is best visualised in the PLAX. PSAX is actually very useful sometimes for looking at posterior
Adel Hasanin Ahmed 1 PERICARDIAL DISEASE The pericardial effusion ends anteriorly to the descending aorta and is best visualised in the PLAX. PSAX is actually very useful sometimes for looking at posterior
THE CARDIOVASCULAR SYSTEM. Heart 2
 THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
Introduction to Physiology (Course # 72336) 1. Adi Mizrahi Textbook Chapter 12
 Introduction to Physiology (Course # 72336) 1 עקרונות בסיסיים (הכנה למעבדת לב) הלב Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
Introduction to Physiology (Course # 72336) 1 עקרונות בסיסיים (הכנה למעבדת לב) הלב Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
2.6 Cardiovascular Computer Simulation
 2.6 Cardiovascular Computer Simulation ROOM 23G22 Contents 1. INTRODUCTION... 4 1.1. GENERAL REMARKS... 4 1.2. LEARNING GOALS... 4 1.3. PHYSIOLOGICAL PARAMETERS... 5 1.4. GLOSSARY... 5 2. USING THE COMPUTER
2.6 Cardiovascular Computer Simulation ROOM 23G22 Contents 1. INTRODUCTION... 4 1.1. GENERAL REMARKS... 4 1.2. LEARNING GOALS... 4 1.3. PHYSIOLOGICAL PARAMETERS... 5 1.4. GLOSSARY... 5 2. USING THE COMPUTER
PROBLEM SET 2. Assigned: February 10, 2004 Due: February 19, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
CRITICAL THINKING QUESTIONS AND ANSWERS AND CYCLE 2 LAB EXAM TEMPLATE. There are two main mechanisms that work in conjunction to return the blood
 CRITICAL THINKING QUESTIONS AND ANSWERS AND CYCLE 2 LAB EXAM TEMPLATE There are two main mechanisms that work in conjunction to return the blood THE CARDIAC PUMP 1) The forward pull(vis a fronte) This
CRITICAL THINKING QUESTIONS AND ANSWERS AND CYCLE 2 LAB EXAM TEMPLATE There are two main mechanisms that work in conjunction to return the blood THE CARDIAC PUMP 1) The forward pull(vis a fronte) This
Heart Rate Variability in Athletes
 Heart rate variability in athletes. AE Aubert, B Seps and F Beckers. Sports Medicine 33(12):889-919, 2003 1 Heart Rate Variability in Athletes A.E. Aubert, B. Seps and F. Beckers Laboratory of Experimental
Heart rate variability in athletes. AE Aubert, B Seps and F Beckers. Sports Medicine 33(12):889-919, 2003 1 Heart Rate Variability in Athletes A.E. Aubert, B. Seps and F. Beckers Laboratory of Experimental
1. Distinguish among the types of blood vessels on the basis of their structure and function.
 Blood Vessels and Circulation Objectives This chapter describes the structure and functions of the blood vessels Additional subjects contained in Chapter 13 include cardiovascular physiology, regulation,
Blood Vessels and Circulation Objectives This chapter describes the structure and functions of the blood vessels Additional subjects contained in Chapter 13 include cardiovascular physiology, regulation,
