BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE. Shao Hui Chen. BS, Harbin Institute of Technology, 1994
|
|
|
- David Dorsey
- 6 years ago
- Views:
Transcription
1 BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE by Shao Hui Chen BS, Harbin Institute of Technology, 1994 MS, China Academy of Launch Vehicle Technology, 22 Submitted to the Graduate Faculty of School of Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 26
2 UNIVERSITY OF PITTSBURGH SCHOOL OF ENGINEERING This dissertation was presented by Shao Hui Chen It was defended on June 26, 26 and approved by Marwan A. Simaan, Bell of PA/Bell Atlantic Professor, Department of Electrical and Computer Engineering J. Robert Boston, Professor, Department of Electrical and Computer Engineering Luis F. Chaparro, Associate Professor, Department of Electrical and Computer Engineering Ching-Chung Li, Professor, Department of Electrical and Computer Engineering James F. Antaki, Professor, Department of Biomedical Engineering, Carnegie Mellon University Dissertation Director: Marwan A. Simaan, Bell of PA/Bell Atlantic Professor, Department of Electrical and Computer Engineering ii
3 BAROREFLEX-BASED PHYSIOLOGICAL CONTROL OF A LEFT VENTRICULAR ASSIST DEVICE Shao Hui Chen, PhD University of Pittsburgh, 26 The new generation left ventricular assist devices (LVADs) for treating end-stage heart failure are based upon turbodynamic (rotary) pumps. These devices have demonstrated several advantages over the previous pulsatile generation of LVADs, however they have also proven more difficult to control. Limited availability of observable hemodynamic variables and dynamically changing circulatory parameters impose particular difficulties for the LVAD controller to accommodate the blood flow demands of an active patient. The heart rate (HR) and systemic vascular resistance (SVR) are two important indicators of blood flow requirement of the body; but these variables have not been previously well exploited for LVAD control. In this dissertation, we will exploit these two variables and develop a control algorithm, based upon mathematical models of the cardiovascular system: both healthy and diseased, with built in autoregulatory control (baroreflex). The controller will respond to change in physiological state by adjusting the pump flow based on changes in HR and SVR as dictated by the baroreflex. Specific emphasis will be placed on hemodynamic changes during exercise in which the blood flow requirement increases dramatically to satisfy the increased oxygen consumption. As the first step in the development of the algorithm, we developed a model which will include the autoregulation of the cardiovascular system and the hydraulic power input from the pump. This model provided a more realistic simulation of the interaction between the LVAD and the cardiovascular system regulated by the baroreflex. Then the control algorithm was developed, implemented, and tested on the combined system of the LVAD and the cardiovascular system including the baroreflex. The performance of the proposed control algorithm is examined by comparing it to other control methods in response to varying levels of exercise and adding noises to the hemodynamic variables. The simulation results demonstrate that the controller is able to generate more blood flow through the pump than the constant speed and constant pump head iii
4 method, and the heart rate related pump speed method. The simulations with noise show that the controller is fairly robust to the measurement and estimate noises. iv
5 TABLE OF CONTENTS ACKNOWLEDGEMENTS... xiv 1. INTRODUCTION BACKGROUND MODELING OF CARDIOVASCULAR SYSTEM, THE BAROREFLEX AND PUMP PREVIOUS CONTROL FOR LVAD THE PROPOSED INVESTIGATIONS MODEL OF HEALTHY HEART WITH BAROREFLEX THE CARDIOVASCULAR SYSTEM MODEL THE BAROREFLEX MODEL THE COMBINED MODEL OF THE BAROREFLEX AND THE CARDIOVASCULAR SYSTEM RESPONSE TO SINGLE PARAMETER CHANGE Response to decrease in preload (blood withdrawal) Response to change in afterload (SVR) Response to change in left ventricular contractility (or Emax) Response to change in heart rate RESPONSE TO EXERCISE Single level exercise Multiple levels of exercise CONCLUSION FAILING HEART WITH BAROREFLEX HEART FAILURE AND ASSOCIATED PHYSIOLOGICAL CHANGES Changes in cardiovascular system v
6 4.1.2 Changes in baroreflex EFFECTS OF CHANGES IN HEART FOR HEART FAILURE MODEL Systolic dysfunction: decrease in Emax Diastolic dysfunction: increase in Emin Combination of systolic dysfunction and diastolic dysfunction: decrease in Emax and increase in Emin DETERMINE THE PARAMETERS OF HEART FAILURE MODEL SIMULATIONS RESULTS OF HEART FAILURE MODEL Simulation result 1 ( V = 5 ml, V = 275 ml) o Simulation result 2 ( V = 35 ml, V = 3 ml) o Simulations results of specific clinical heart failure RESPONSES OF HEART FAILURE TO EXERCISE Hypertrophic heart failure Dilated heart failure CONCLUSION THE COMBINED MODEL OF PUMP AND FAILING HEART THE PUMP MODEL THE COUPLED MODEL OF THE PUMP AND THE FAILING HEART CHANGES IN HEMODYNAMICS WITH PUMP IMPLANTED CONCLUSION PUMP CONTROL BASED ON HEART RATE AND SYSTEMIC VASCULAR RESISTANCE PUMP OPERATION PROPOSED PUMP CONTROL BASED ON HR AND SVR COMPARISON OF THE PROPOSED PUMP CONTROL WITH OTHER METHODS Constant speed method Constant pressure head method Pump speed as a linear function of heart rate PERFORMANCES OF THE PROPOSED CONTROLLER K1 and K vi T T
7 6.4.2 LVPr SVr Noise CONCLUSION CONCLUSION AND FUTURE WORK BIBLIOGRAPHY vii
8 LIST OF TABLES Table 3.1. State variables Table 3.2. Parameters and values Table 3.3. Phases in a cardiac cycle Table 3.4. State variables for baroreflex Table 3.5. Values for baroreflex parameters Table 3.6. Baseline Hemodynamics Table 3.7. Response to change in preload Table 3.8. Response to change in afterload Table 3.9. Response to change in left ventricle contractility Table 3.1. Response to change in heart rate Table Offsets in sympathetic and vagal activity Table Hemodynamic changes Table Changes in resistances Table Multiple exercise levels Table Exercise experiment data... 5 Table 4.1. Changes in parameters of the heart failure model Table 4.2. Clinical hemodynamics data for heart failure Table 4.3. Parameters for the heart failure baroreflex... 6 Table 4.4. Heart failure model combinations Table 4.5. Simulation results for heart failure (1) Table 4.6. Simulation results for heart failure (2) Table 4.7. Hypertrophic heart failure response to exercise Table 4.8. Dilated heart failure response to exercise viii
9 Table 5.1. State variables Table 5.2. Model parameters Table 5.3. Hemodynamic changes with increasing pump speed Table 5.4. Full and partial pump support Table 6.1. Multiple levels of exercise Table 6.2. Simulation results for constant speed Table 6.3. Simulation results for constant pump head Table 6.4. Simulation results for heart rate related pump speed method Table 6.5. Simulation results with K1=.5, K2= Table 6.6. Simulation results with K1=.3, K2= Table 6.7. Simulation results with LVPr = 4 mmhg Table 6.8. Simulation results with LVPr = 3 mmhg Table 6.9. Simulation results with smaller SVr Table 6.1. Simulation results with larger SVr ix
10 LIST OF FIGURES Figure 2.1. In vivo hemodynamics of calf implanted with turbodynamic LVAD... 6 Figure 2.2. Block diagram of the proposed control scheme Figure 3.1. Cardiovascular system model Figure 3.2. Typical elastance function Figure 3.3. Block diagram for the carotid baroreflex Figure 3.4. Block diagram for the afferent pathway of carotid sinus Figure 3.5. Characteristic curves for the afferent pathway Figure 3.6. Characteristic curve for the efferent sympathetic pathway Figure 3.7. Characteristic curve for the efferent vagal pathway Figure 3.8. Characteristic curve for equation (3.12)... 2 Figure 3.9. Pulsatile heart coupled with baroreflex Figure 3.1. P-V loops generated by the model and Simbiosys Figure Left ventricular pressure and left ventricular volume Figure Change in P-V loop for 2% blood withdrawal Figure Changes in hemodynamics for loss of blood Figure Change in P-V loop for -2% in SVR Figure Change in P-V loop for +2% in SVR Figure Changes in hemodynamics for changes in SVR Figure Change in P-V loop for +4% in Emax in the model Figure Changes in hemodynamics for +4 % in Emax Figure Change in P-V loop for -1% in HR Figure 3.2. Change in P-V loop for +4% in HR Figure Changes in hemodynamics for change in HR x
11 Figure Response to exercise Figure P-V loops of rest and exercise Figure Hemodynamic changes from rest to exercice Figure Changes in hemodynamics for multiple exercise levels... 5 Figure 4.1. Systolic dysfunction and diastolic dysfunction (Adopted from [56]) Figure 4.2. Changes in baroreflex Figure 4.3. Left ventricle pressure volume loop (adopted from [55]) Figure 4.4. Systolic dysfunction (adopted from [55]) Figure 4.5. Diastolic dysfunction (adopted from [55]) Figure 4.6. Combination of systolic dysfunction and diastolic dysfunction (adopted from [55]).59 Figure 4.7. P-V loops for healthy heart and systolic dysfunction heart Figure 4.8. P-V loops for healthy heart and diastolic dysfunction heart Figure 4.9. P-V loops for healthy and the combination of both failure cases Figure 4.1. Comparison of simulation results with baroreflex and without baroreflex Figure Systolic dysfunction P-V loops. ( V T = 24 ml) Figure Diastolic dysfunction P-V loops. ( V T = 25 ml) Figure P-V loops of combination of systolic and diastolic dysfunctions. ( V T = 25 ml) Figure Comparison of simulation results with baroreflex and without baroreflex Figure P-V loops of failing heart responses to changes in preload (adopted from [71]) Figure Model reproduced clinical baseline failing heart P-V loops Figure Simulation results of failing heart response to changes in preload Figure Response to exercise for hypertrophic heart failure Figure P-V loops of rest (dotted line) and exercise (solid line) Figure 4.2. Hemodynamic changes from rest to exercice Figure Changes in hemodynamics for multiple exercise levels Figure Response to exercise for dilated heart failure... 8 Figure P-V loops of rest (dotted line) and exercise (solid line)... 8 Figure Hemodynamic changes from rest to exercice Figure Changes in hemodynamics for multiple exercise levels Figure 5.1. DC motor circuit xi
12 Figure 5.2. Rotary pump characteristic curves [72] Figure 5.3. The coupled model of pump and failing heart Figure 5.4. Pump augmented failing heart with baroreflex Figure 5.5. P-V loops changes with changing pump speed Figure 5.6. Changes in hemodynamics (ratio of partial to full) Figure 6.1. Static pump characteristic curves and operating point Figure 6.2. Same SVR and different operating points Figure 6.3. Change in operating points from rest (1) to exercise (2) Figure 6.4. Block diagram for the closed-loop control based on HR and SVR... Figure 6.5. Simplified version of the combined model (aortic valve is taken out) Figure 6.6. Reduced circuit diagram in mean sense Figure 6.7. Block diagram for the controller based on the HR and SVR Figure 6.8. Controller responses to exercise level Figure 6.9. Control errors for H and Q from rest to exercise level Figure 6.1. Operating point trajectory from rest to exercise Figure LVP and LVPr Figure AOP and LVP Figure Illustration of the operating point and steady errors Figure K2 = (only H branch is applied) Figure K1 = (only CO branch is applied) Figure Steady errors for exercise level 1 and level Figure Operating point for exercise level Figure Multiple levels of exercise Figure Constant speed method response to exercise level 2. At 15s, exercise starts Figure 6.2. Constant pump head method response to exercise level 2. At 15s, exercise starts.115 Figure Heart rate related pump speed method response to exercise level Figure Response to the exercise for different control methods Figure Simulation from rest to exercise Figure K1=.7, K2=.7, LVPr=5 mmhg Figure K1=.2, K2=.2, LVPr=5 mmhg Figure K1<.4, K2>.4, noise free (rest to exercise 2) xii
13 Figure K1>.4, K2<.4. noise free (rest to exercise 2) Figure Simulation results with different K1 and K Figure LVPr <5 mmhg Figure 6.3. Simulation results with different LVP. K1=.4, K2= Figure Smaller SVr Figure Simulation results with different SVr Figure Noise features Figure Low level of noises for HR, SVR, H and CO Figure High level of noise for H only Figure High level of noise for CO only Figure High level noise for SVR only Figure High level of noise for HR only Figure High level of noise for HR, SVR, H and CO Figure 6.4. Noise for H only Figure Noise for CO only Figure Noise for SVR only Figure Noise for HR only Figure Noise for all variables r xiii
14 ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Marwan A. Simaan, for his support and guidance throughout the research. I would also like to thank Dr. James F. Antaki and Dr. J. Robert Boston for the physiology education and insightful advices in the weekly meetings. I thank Dr. Ching- Chung Li and Dr. Luis F. Chaparro for taking time and effort to serve on my committee. Special thanks to Antonio Ferreira, for the discussions and making an enjoyable environment. I would also like to thank my family, my wife Jie Li and my daughter Tianyi Chen for their love and support. This research was supported by National Science Foundation (NSF) under contract ECS- 397 and in part by National Institutes of Health / National Heart, Lung, and Blood Institute (NIH/NHLBI) under contract 1R43HL I would like to express my gratitude to them for their financial support. xiv
15 1. INTRODUCTION Congestive heart failure is estimated to affect five million people in the US [1], which is characterized by impaired ventricular performance, exercise intolerance, and shortened life expectancy. Although drug therapy has had significant impact on quality of life and survival for moderate heart failure, mortality remains unacceptably high. Heart transplantation is the only accepted method to treat severe cases of the disease. Unfortunately, heart transplantation is limited by the number of donor organs, less than 3 per year. The left ventricular assist device (LVAD) is therefore an alternative for many cases of end-stage heart failure [2, 3]. The first generation of LVADs was based on positive displacement (pulsatile) pumps. Recently, turbodynamic pump have received growing acceptance on account of small size and high efficiency [4-8]. The rotary part of this type of pump, which is driven by a motor, generates a pressure difference across the pump in resistance of the arterial pressure. In a typical bypass application, where the inlet and outlet of the pump connect the apex of the left ventricle and the aorta respectively, the pump helps unload the failing left ventricle by reducing its work requirement and assuming the role of providing pressure and flow to the systemic circulation. However, the control of the LVAD emerges as a challenge for the rotary pump application. Because the pump actively draws blood from the left ventricle, the flow should be adjusted according to the available blood returning to the left ventricle. For a normal heart, the cardiac output (CO) is determined by two factors: stroke volume (SV) and heart rate (HR), CO = SV HR Larger stroke volume and higher heart rate imply larger CO. Stroke volume increase is the result of a complex physiological process: increasing preload, increasing contractility and decreasing afterload. Preload is the amount of the venous blood returning to the heart. The contractility of the heart is an index of its strength of contraction. The afterload refers to the systemic vascular 1
16 resistance (SVR), the output load of the left ventricle. More generally, the physiological status of the patient may demonstrate a wide range of variation, due to exercise intensity and emotional changes. For a patient with heart failure, one or more of these functions may be damaged or attenuated therefore heart transplantation or the augment of the LVAD is needed. As learned with total artificial hearts, the inability of the device to respond to the blood flow demand of the body can dramatically impact the quality of life for these patients [9]. Thus a controller that can detect and adapt to the real time physiological changes of the body is crucial for the LVAD recipients leaving hospital, returning to normal lifestyle and improving the quality of life. Furthermore, two detrimental situations, backflow and suction, may occur for the pump operation if the pump speed is not suitably set [4, 5]. If the rotational speed (or pump flow) is too low, the blood will regurgitate from the aorta to the left ventricle through the pump (i.e. backflow). For this case, the cardiac output is not augmented but decreased. If the rotational speed is too high, the pump may attempt to draw more blood than available in the left ventricle. The latter will cause kinking at the connection of the left ventricle and cannula (suction) or the collapse of the left ventricle, which may result in damage to the heart muscle, blood, and/or vasculature. Since the LVAD is applied to unload the failing left ventricle, the basic control objective is to mimic the native heart function [4, 5]. From above we know that the native heart adjusts to the physiological cardiac output requirement by a combination of preload, contractility, afterload and heart rate. However, not all this information is readily available to the LVAD controller, especially for an ambulatory patient. Thus the main objective for the LVAD control outside the hospital settings is to incorporate varied and sometimes limited control inputs and to adapt the pump speed to the physiological state of the patient. This dissertation discusses a controller which will incorporate multiple hemodynamic variables (measured and/or estimated) and will respond to the physiological changes of the body. The specific inputs for the controller considered here are the heart rate and the systemic vascular resistance which are under the control of the baroreflex. In real life, the heart rate can be estimated from the electrical current to the drive motor of the LVAD and the systemic vascular resistance can also be estimated by using blood pressure and blood flow [1]. These will be used to estimate the physiological state changes and drive the pump speed toward the desired operating point. 2
17 This dissertation is organized as follows. First, in chapter 2, the LVAD control and the modeling of the cardiovascular system, the baroreflex and the pump are reviewed, and the proposed investigations and technical approaches are described. In chapter 3, the models of the cardiovascular system and the baroreflex are presented and coupled. The parameters for the healthy cardiovascular model are determined to simulate the normal hemodynamics. The responses to single parameter change are examined by using physiological simulation software as reference. The response to exercise is compared to the exercise experiment data in the literature. In chapter 4, model parameters are determined for the VAD patient with failing heart. Because of the progressive deterioration of the failing heart and the related cardiovascular system, there are some substantial changes in the cardiovascular system and the baroreflex. The changes in the cardiovascular system and the baroreflex are found out by surveying the literature and mapped to the heart failure model. In chapter 5, the failing heart model is coupled to the pump model and its behavior is examined. The simulation results are compared to the data available in the literature. In chapter 6, a physiological controller is developed which incorporates multiple inputs (heart rate and systemic vascular resistance). The heart rate and systemic vascular resistance are two important indicators of the physiological state of the body. Including this information will improve the pump control. The physiological controller is implemented and tested with the baroreflex + failing heart + pump model. The response to exercise of the control method is examined. The performance of this controller is compared to that of other available pump control methods such as constant pump speed, constant pump head and heart rate related pump speed method. In chapter 7, the progress to date is concluded and future work is discussed. 3
18 2. BACKGROUND Basically, there are three ways of investigating the interactions between the native circulation and the implanted pump: simulation on model, mock circulatory system, and animal experiment. The mathematic model consists of abstraction of the basic circulation elements, such as the heart and the arterial network. The mock loop is the counterpart of a certain model by using some devices instead of abstraction. The animal experiment is the preclinical feasibility test of a certain pump or pump controller. This research is focused on the model and simulation. 2.1 MODELING OF CARDIOVASCULAR SYSTEM, THE BAROREFLEX AND PUMP The cardiovascular system is usually modeled by using electrical network with the voltage representing pressure, the current representing flow and charge representing volume. The ventricular function and the arterial network are the two main foci of the modeling efforts. Generally the left ventricle is model as a time varying capacitor (or elastance) which may take form of exponential [11], sinusoidal [12] or double hill function [13]. There are two ways of modeling the arterial network: lumped and distributed system. The windkessel models are typical lumped system modeling of the arterial network. A variety of windkessel models have been developed which are basically RLC networks [14, 15]. The transmission line is a typical distributed system modeling of the arterial network which simulates the pressure wave as a function of time and location [16-18]. It is noteworthy that the parameters of the models are fixed. Besides the modeling of the heart and the arterial network, there are also efforts on modeling the baroreflex, the cardiovascular regulation system. The baroreflex is a built in 4
19 feedback system which stabilize the arterial pressure by varying the cardiac output and the systemic resistance. There are some pressure sensors located at the aortic arch and carotid sinus, which convert the pressure into nervous signal. This inbound nervous signal is then transmitted to the central nervous system and translated into outbound nervous signal. The outbound nervous signal stimulates the end organs such as the heart, the vasculature and renal system to keep the blood pressure equal or close to an intrinsically established setpoint. Models of the baroreflex can be found in [19-21]. The baroreflex in [19] is modeled as a static mapping between the arterial pressure and the heart rate (or the systemic vascular resistance). The baroreflex models in both [2] and [21] consist of the baroreceptor (pressure sensor), the afferent pathway, the efferent pathways, and end organs effectors. With the baroreflex model coupled to the cardiovascular system, the parameters of the cardiovascular system such as the heart rate and the systemic vascular resistance are not fixed any more but become pressure-dependent. The rotary pump model is effectively a current (blood flow) source connecting the ventricle to the systemic arterial system. The input of the model is the rotational speed and/or the electrical current. The variables that define the interface of the pump with the cardiovascular system are the inlet and outlet pressure, and pump flow [4, 22]. With this interface, the pump model can be coupled to the cardiovascular system model and used to simulate the interaction between the pump and the cardiovascular system and examine the performance of a control method. 2.2 PREVIOUS CONTROL FOR LVAD The principal goal of the blood pump controller is to respond to and meet the body demand for cardiac output. The inputs of the controller are available hemodynamic variables of the patient and the output of the controller is the pump speed or electrical signals such as voltage and current. Figure 2.1 shows the animal experiment data with a rotary pump implanted where the pump speed is a ramped from 7.8 to 14.5 krpm over 15 seconds. The task of the controller is to provide a speed in the range of optimal zone or safe zone while avoiding suction zone or under pumping zone. 5
20 Figure 2.1. In vivo hemodynamics of calf implanted with turbodynamic LVAD (University of Pittsburgh, unpublished data). The pump speed is a ramp from 7.8 to 14.5 krpm. From left to right, the operating zones: back flow, safe, optimal and suction. The bottom shows traces of waveforms of pump flow for low speed, optimal speed and high speed. However, the controller development is handicapped by the limited availability of physiological information of the body. Early solution for the control problem was to set a constant speed for the LVAD and recipients were supervised in the hospital [5]. While this openloop method is easy to implement, the disadvantage is that once the physiological condition of the patient changes, the patient would be at risk of adverse phenomena such as back flow and suction. Because of the limited available information for the controller, some sensor-less methods for LVAD control were developed by using the pump variables such as current, voltage and 6
21 speed [23-26]. This technique is based on the observation that pressure across, and flow through, a rotary VAD can be inferred or estimated from the electrical current and frequency of the pump s motor. Several other investigators have adopted a similar sensor-less approach to estimate pressure and flow [27-32]. However, these estimations and controllers are reliable only in a relatively narrow range of pump variables. When the pump is operating in a wide range of the patient s physiological situations, these controllers may mislead the pump to hazards for the recipients. As to control strategy, one simple idea for the controller is to maximize the flow while avoiding suction (optimal zone in Figure 2.1). Some suction indices are based on time domain characteristics and frequency domain extraction from waveform of the pump flow. The harmonic spectral index is one of them [26]. This method is based on the observation that high frequency components in pump flow or pump current increase in suction zone compared to the fundamental frequency component. Another method is using the pulsatility of the flow as control input [4]. A method reducing the uncertainty of the suction detection was also developed [33]. More generally, keeping hemodynamic variables such as the atrial pressure, the aortic pressure and cardiac output close to nominal values may lead to multiple objectives optimization [6]. Hierarchical control for LVAD, an intelligent structure based on multiple objectives optimization and expert system, is further discussed in [34, 35]. The main challenge for this class of methods is the adaptability of the suction detector if SVR changes. To further exploit the fact that the minimum pump flow achieves the extremum at the point of suction event (around s in Figure 2.1), a controller was developed which tracks the extremum even as SVR changes [36]. The disadvantage of this class of methods is that the pump speed is close to the upper bound of the optimal zone and thus is precariously close to suction. Controllers to keep the average pressure across the pump (or between the aorta and the left ventricle) constant have been developed in [31, 38-4]. This class of controllers can provide a pump speed in the safe zone or optimal zone for a certain SVR. However, the operating pump speed may move into the under pumping zone or suction zone if the SVR varies due to change in physiological state of the patient. The disadvantage for this class of methods is that these controllers require pressure sensors mounted at the inlet and outlet of the pump. If the use of pressure sensors is not practical, then the pressure difference may be estimated from the 7
22 rotational speed and/or the motor current by using sensor-less methods [31]. This would be limited by the applicable physiological range. An investigation using oxygen saturation of the blood for control purpose has also been reported [41]. In an animal experiment a proportional control law was implemented that increased the flow of a total artificial heart in proportion to changes in the mixed venous oxygen saturation (MVO2) which is acquired by an indwelling sensor. The addition of a MVO2 sensor would benefit the overall robustness of the controller; however changes in MVO2 are relatively slow, as compared to the rapid changes in vascular resistance, for example. A controller based on MVO2 alone would not be able to respond to the rapid physiological changes and to avoid suction. Other control approaches such as using the heart rate as a controller input have been reported [42]. As one part of the circulation regulatory system, the heart rate is an indicator of blood flow demand of the body. In the animal experiment, the controller adjusted the pump speed in response to increasing or decreasing heart rate in a linear relationship. The HR in this study was calculated from the pump current. In-vivo results demonstrated a positive response of this control scheme to treadmill exercises. However, this method does not take the change in SVR into account. From rest to exercise, there is a dramatic decrease in SVR accompanying the increase in heart rate. For the case of heart failure where the heart is not pumping effectively, change in SVR is a major mechanism to generate the desired cardiac output. The controller based on the HR alone may fail to provide the appropriate cardiac output for the physiologically changing body. This dissertation will discuss an improved controller that incorporates the heart rate and the systemic vascular resistance and respond to the physiological changes of the body instantaneously based on the baroreflex, the built in cardiovascular regulation system. The heart rate can be inferred from pump current and the systemic vascular resistance can be estimated from blood flow and blood pressure. By incorporating the information of the heart rate and the systemic vascular resistance, the controller can vary the pump speed in response to the change in physiological state of the body, even for the challenging case of exercise. 8
23 2.3 THE PROPOSED INVESTIGATIONS The main goal of this work is to improve the LVAD support for patients with heart failure. A physiological control algorithm will be developed, based on the models of implanted LVAD, the heart, circulation and regulatory system. The underlying principle of demand based control is the baroreflex, the autoregulatory system of the circulation, which manages the blood pressure and cardiac output. For a healthy person, the baroreflex regulates the blood pressure and cardiac output according to different physiological states of the body by changing heart rate, heart contractility, systemic vascular resistance and total blood volume. For a patient with heart failure, the baroreflex is preserved fairly well even though some end organs functions are attenuated or damaged. The proposed controller will use the estimated heart rate and estimated systemic vascular resistance as control inputs to generate the optimal pump speed for a specific physiological state. The investigations will be based on a combination of existing theory and new models. The specific aims and associated technical approaches are: (1) To improve the combined model of the cardiovascular system and the LVAD. The coupled model of a LVAD and a cardiovascular system with a built in baroreflex will be established for simulating the interaction between the LVAD and the cardiovascular system and testing a physiological controller. This model will include the autoregulation of the cardiovascular system and the hydraulic power input from the pump. This model will be more realistic to simulate the interactions between the LVAD and the circulatory system regulated by the baroreflex. (2) To develop a physiological control algorithm for the LVAD that can incorporate various sensors inputs and/or estimations. A physiological controller for the LVAD will be developed which incorporates the information of heart rate and systemic resistance. This controller will behave like a part of the autoregulation of the cardiovascular system and thus will be responsive to changes in hemodynamic parameters and variables for different physiological states. (3) To implement and validate this control algorithm on the combined model of the LVAD and the cardiovascular system, and examine the performances of the proposed control algorithm by comparing to constant pump speed, constant pump head, and heart rate 9
24 related pump speed method. The resistance of the controller to noise will also be examined. These are illustrated in Figure 2.2. IMPLANTED PUMP NATIVE HEART PUMP CONTROLLER CIRCULATION INTRINSIC AUTOREGULATION END ORGANS Figure 2.2. Block diagram of the proposed control scheme. The following chapters will present the up-to-date progress of the investigation, including the healthy and failing cardiovascular models with built in baroreflex, the combined model of the pump and the cardiovascular system with baroreflex, and the proposed pump controller using the heart rate and the systemic vascular resistance as inputs. 1
25 3. MODEL OF HEALTHY HEART WITH BAROREFLEX In this chapter, the pulsatile heart model is introduced in section 3.1 first and the baroreflex model in 3.2. Then the two models are coupled to simulate the interaction between them. The parameters of the coupled model are tuned in section 3.3 by using physiological simulation software Simbiosys as a reference. Then the response of the coupled model to single parameter change in preload, afterload, left ventricular contractility and heart rate is compared to that of Simbiosys in section 3.3. The response of the model to exercise is also examined in section THE CARDIOVASCULAR SYSTEM MODEL The cardiovascular system model employed here is from [43-46] which is represented by the lumped parameter circuit shown in Figure 3.1. Table 3.1 lists the state variables, and Table 3.2 lists the system parameters and their associated values. R 1 R 2 D 1 R 3 D 2 R 4 L C 2 x c2 C 1 (t) x c1 x c4 C 3 x c3 Figure 3.1. Cardiovascular system model. 11
26 Table 3.1. State variables Variables Physiological meaning (units) x c1 Left ventricular volume (ml) x c2 Left atrial pressure (mmhg) x c3 Arterial pressure (mmhg) x Aortic Flow (ml/s) c4 Table 3.2. Parameters and values Parameters Physiological Value Units Meaning Resistances R 1 Systemic Resistance R 2 Mitral valve.5 mmhg/ml/s R 3 Aortic valve.1 R 4 Characteristic.398 resistance Compliances C 1 (t) Left ventricular compliance Timevarying C 2 Left atrial compliance 4.4 ml/mmhg C 3 Systemic compliance 1.33 Inertances L Valves D 1 D 2 Inertance of blood in Aorta Mitral valve Aortic valve.5 mmhg.s 2 /ml In this lumped parameter circuit, the left ventricle is described as a time-varying capacitor. One way to model its behavior is by means of the elastance function, which is the reciprocal of the compliance. It determines the change in pressure for a given change in volume within a chamber and was defined in [47] as following: 12
27 LVP() t Et () = 1/ C1() t LVV () t V = (3.1) Where E(t) is the time varying elastance (mmhg/ml), LVP(t)= x1(t) is the left ventricular pressure (mmhg), LVV(t) is the left ventricular volume (ml) and V is a reference volume (V = 5 ml for a normal heart), the theoretical volume in the ventricle at zero pressure. The elastance function Et () = ( E E ) E( t) + E max min n n min (3.2) Where constants Emax and Emin are related to the end systolic pressure volume relationship (ESPVR) and the end diastolic pressure volume relationship (EDPVR), respectively. En(tn) is the normalized time-varying elastance, so called double hill function from [13], tn = t/tmax, Tmax =.2+.15tc and tc is the cardiac cycle. 1.9 t n.7 1 En( tn) = tn tn (3.3) Notice that E(t) is a re-scaled version of En(tn). Figure 3.2 shows the elastance function for Emax = 2., Emin =.6, and heart rate 6 bpm. Elastance (mmhg/ml) Emax Cardiac cycle Emin Figure 3.2. Typical elastance function E max = 2., E min =.6, and normalized cardiac cycle. 13
28 Since this model includes two diodes, the following phases will occur, over four different time intervals in a normal cardiac cycle, as illustrated in Table 3.3. Table 3.3. Phases in a cardiac cycle Modes Valves Phases D 1 D 2 1 Closed Closed Isovolumic contraction 2 Closed Open Ejection 1 Closed Closed Isovolumic relaxation 3 Open Closed Filling - Open Open Not feasible For each phase of the cardiac cycle, the state equation can be written into the form of dx A() tx dt = (3.4) T c1 c2 c3 c4 with different matrix A(t) for each phase, x [ x x x x ] =. 1) Isovolumic phase: In this phase of the cardiac cycle, the aortic and mitral valves are closed. Since the aortic valve is closed, the aortic flow is zero, i.e., x c4 =. In this phase, we have: 1 1 RC 1 2 RC 1 2 At () = 1 1 (3.5) RC 1 3 RC 1 3 2) Ejection phase: In this phase, the aortic valve is open, and the mitral valve is closed. In this phase the left ventricle is pumping blood into the circulatory system, where 14
29 1 1 1 RC 1 2 RC 1 2 At () = RC 1 3 RC 1 3 C3 Et () 1 ( R3 + R4) L L L (3.6) 3) Filling phase: When the heart is filling, blood from the left atrium goes into the left ventricle. The mitral valve is open, and the aortic valve is closed which again implies phase, x c4 =. For this At () Et () Et () R2 R 2 Et () ( R1+ R2) 1 RC RRC RC = RC 1 3 RC 1 3 (3.7) The case with both valves open does not occur for a normal heart and thus is not included in this model. For a sequence of these phases in a normal cardiac cycle, for example, fillingcontraction-ejection-relaxation, the end states of the last phase are initial conditions for the next phase. 3.2 THE BAROREFLEX MODEL The baroreflex model employed here is from [48-5] with some parameters tuned to simulate the human dynamics. In this model, the baroreflex consists of the baroreceptor, the afferent pathway, the efferent pathways and the regulation effectors. The baroreceptor is a pressure sensor located in the carotid sinus or aorta which converts pressure into afferent firing frequency. Then the afferent firing frequency is translated into efferent signals by the nervous system: sympathetic 15
30 firing frequency and vagal firing frequency. These efferent signals are the inputs of the regulation effectors. The regulation effects include changes in vessels resistances and heart rate (or cardiac cycle). For example, if the pressure is lower than the set point, the systemic resistance and heart rate will increase to reduce the error between the current pressure and the set point pressure. The closed loop baroreflex of the block diagram in Figure 3.3 is applied on the cardiovascular model in Figure 3.1. The different parts of the baroreflex are described as follows. Baroreceptor Afferent Pathway Pressure Nervous system Regulation Effectors Efferent Pathways Figure 3.3. Block diagram for the carotid baroreflex. The baroreflex consists of baroreceptor, afferent pathway, efferent pathway and regulation effectors. Afferent pathway. In [48] the afferent baroreflex pathway is described as the series arrangement of a linear derivative first-order dynamic block and a sigmoidal function as shown in Figure 3.4. Pressure Linear Derivative Static Sigmoid Firing frequency Figure 3.4. Block diagram for the afferent pathway of carotid sinus. The linear derivative block in Figure 3.4 dp τ dt dp dt in p = pin + τz p (3.8) 16
31 Where τ p and τ z are the time constants for the real pole and the real zero in the linear dynamic block (usually with τ z / τ p > 1), pin is the carotid sinus pressure, p is the output variable of the linear derivative dynamic block (with dimension of pressure). The static sigmoidal function in Figure 3.4 is f as ( P ) = f + f e min 1 + e P ( Po ) Ka max P ( Po ) Ka where f as is the frequency of spikes in the afferent fibers, fmax (3.9) and fmin are the upper and lower saturation of the frequency discharge, P o is the value of the intrasinus pressure at the central point of the sigmoidal functional, K a is a parameter with the dimension of pressure, related to the slope of the static sigmoidal function at the central point. The characteristic curves for the linear derivative and static sigmoid functions are shown in Figure 3.5. Efferent sympathetic pathway. The monotonically decreasing function that relates the activity in the afferent and efferent neural pathways is described by an exponential shaped function [48]. Kes fas f ( f ) = f + ( f f ) e (3.1) es as es es es Where fes is the frequency of spikes in the efferent sympathetic nerves, es, K es f and fes are constants (with f > fes ), fas is the output in (3.9). The characteristic curve is shown in Figure 3.6. es a. Characteristic curve (frequency domain) for afferent pathway (linear derivative). 17
32 τ z =6.37 s; τ p =2.76 s; P o = 8 mmhg; b. Characteristic curve for afferent pathway (static sigmoid). K a = mmhg; f min = 2.52 spikes/s; f max = spikes/s. Figure 3.5. Characteristic curves for the afferent pathway. Figure 3.6. Characteristic curve for the efferent sympathetic pathway. fes = 2.1 spikes/s; f es = spikes/s; K es =.675 s/spikes. 18
33 Efferent vagal pathway. The efferent vagal activity is a monotonically increasing function of the activity in the sinus nerve with an upper saturation. The sigmoidal equation similar to (3.11) is used [48] f ev ( f as ) = f + f e ev 1 + e f ( as faso ) Kev ev f f ( as aso ) Kev (3.11) Where fev is the frequency of spikes in the efferent vagal fibers, ev, f K ev and fev are constant parameters (with fev > fev ), faso is the central value in the characteristic curve in (3.9) and fas is the output in (3.9). The characteristic curve is shown in Figure 3.7. Figure 3.7. Characteristic curve for the efferent vagal pathway. f ev = 3.2 spikes/s; fev = 6.3 spikes/s; f aso = 25 spikes/s; K ev = 7.6 spikes/s. Regulation effectors A. Sympathetic effectors on resistances To simulate the blood flow distribution among the different parts of the body, the systemic vascular resistance is divided into three parts: R 1, R 2, R 3. R 1 is the splanchnic resistance; R 2 is the resistance other than active muscle and splanchnic resistance; R 3 is the active muscle resistance. 19
34 The response of the resistances to the sympathetic drive includes a delay, a logarithmic static function, and a low-pass first-order dynamics [48]. e () t = R i G ln[ f f + 1] f f R es ( t Di) i esmin es esmin f es < f esmin (3.12) dδri () t 1 = ( Δ R dt i ( t )) + er i () t (3.13) τ Ri R () t = Δ R() t + R (3.14) i i i Where R i is the resistances with i = 1, 2, 3, er i is the output of the static logarithmic characteristic function, is the value of f ( t Di ) es es f evaluated at ( t Di), R i τ and Di are the time constants and delay of the mechanism, f esmin is the minimum sympathetic stimulation, and ΔR () t is the resistance change with respect to caused by sympathetic stimulation and G is a i constant gain factor. R i R i y x Figure 3.8. Characteristic curve for equation (3.12). y ln[ x fesmin + 1] fes f = f < f es f esmin = 2.66 esmin esmin 2
35 B. Heart rate effectors The response of the cardiac cycle is a result of both the vagal and sympathetic activities. The cardiac cycle changes induced by sympathetic stimulation are achieved through equations similar to (3.12) and (3.13) [48]. G ln[ f ( t D ) f + 1] f f ets() t = fes < f Ts es Ts esmin es esmin esmin (3.15) dδts() t 1 = ( Δ Ts dt ( t )) + ets () t (3.16) τ Ts The cardiac cycle change induced by vagal activity differs from the sympathetic case because cardiac cycle increases linearly with the efferent vagal excitation [48]. e () t = G fev( t D (3.17) Tv Tv Tv ) dδtv() t 1 = ( Δ Tv dt ( t )) + etv () t (3.18) τ Where the meanings of the symbols are similar to that of (3.12) and (3.13). The cardiac cycle is calculated by assuming a linear interaction between the sympathetic and vagal caused changes [48]. Tv Tt () Tst () Tvt () T = Δ +Δ + (3.19) Where Tt () is the overall altered cardiac cycle due to sympathetic and vagal stimulation, Δ Ts() t is the change due to sympathetic stimulation, Δ Tv() t is the change due to vagal stimulation, is the constant cardiac cycle without any nervous excitation. T 3.3 THE COMBINED MODEL OF THE BAROREFLEX AND THE CARDIOVASCULAR SYSTEM Based on the cardiovascular model in section 3.1, the baroreflex model is coupled to it. In the combined model of Figure 3.9, R1 in the cardiovascular circuit model is the SVR which is divided into 3 parallel parts to simulate the blood flow distribution among different parts of the 21
36 body. The left ventricle contractility (Emax) and total blood volume (VT, summation of the charge in capacitors and inductors) are results of sympathetic excitation in the model. The arterial pressure is the input for the baroreflex. The SVR, HR, Emax and VT are under the control of the baroreflex. Specifically, the SVR, Emax and VT vary instantaneously; the HR (6/ cardiac cycle) varies cycle by cycle, in other words, the HR remains constant in a cardiac cycle. The hemodynimic variables generated by Simbiosys (Critical Concepts, Inc) [51] are used as reference for tuning the parameters for this coupled model. Simbiosys is a physiology simulation software which uses mathematical models to simulate the function of the heart and the autonomic control of a human. Arterial Pressure Baroreflex SVR, HR, Emax, VT Cardiovascular System baroreflex. Figure 3.9. Pulsatile heart coupled with baroreflex. The arterial pressure is the input of the baroreflex, SVR, HR, Emax, VT are under the control of the Table 3.4 shows the state variables for the baroreflex block and Table 3.5 parameters (most from [48-51]) and values for tuning the baroreflex to generate normal hemodynamics. The resulting steady total blood volume is about 25 ml. The SVR (R1 in the circuit) in this model is still divided into three parallel parts: R 11, R 12, R 13. x b1 Table 3.4. State variables for baroreflex the change in splanchnic resistance due to sympathetic stimulation x the change in the resistance other than active muscle and splanchnic b2 resistance due to sympathetic stimulation 22
37 x b3 Table 3.4. (continued) the change in active muscle resistance due to sympathetic stimulation x the change in cardiac cycle due to sympathetic stimulation b4 x the change in cardiac cycle due to vagal stimulation b5 x the change in heart contractility due to sympathetic stimulation b6 x the change in total blood volume due to sympathetic stimulation b7 State equations: dx dt bi = 1 x u ( x ) for i = 1,2,3,4,5,6,7 (3.2) τ + i bi i c3 Where x c3 is the arterial pressure in section 3.1. Wher i es t d ] i es fes t d f i es ui xc = g ln[ f ( ) f min + 1 ( ) min 1, 2, 3, 4, 6, 7 ( 3) fes t d < f ( i ) esmin i = (3.21) u5( xc3) = g5 fev ( t d 5 (3.22) ) fes t d is f i es evaluated att di, fev ( t d5 ) is f ev evaluated at t d 5. e ( ) xc3 Po Kes [ fmin + fmax exp( K )] a = + offset _ es + ( es ) exp (3.23) xc3 P o [1 + exp( K )] a f f f f f es es es ev = xc3 Po { K } a xc3 Po { K f } a aso Kev f + f exp [exp( ) f ]/ offset _ ev + 1+ exp [exp( ) ]/ ev ev aso ev K (3.24) R11 = R1 + x b 1 (3.25) R12 = R2 + x b 2 (3.26) R13 = R3 + x b 3 (3.27) T = T (3.28) + xb4 + xb5 Emax = Emax + xb (3.29) 6 V = V + x T T b7 (3.3) 23
38 Where τ i, gi, di, f, esmin fes, es f, K es, offset _ es, f ev, fev, K ev, offset _ ev, f aso, f min, f, Ka, Po, SV, R 1, R 2, R 3, T, max f es min, max, V are constants, E T fes is the sympathetic activity, f ev is the vagal activity. Table 3.5. Values for baroreflex parameters Parameter Value Physiological meaning τ 1 s Time constant for resistance 1 τ 1 s Time constant for resistance 2 τ 1 s Time constant for resistance 3 τ 4 s Time constant for sympathetic 4 stimulated cardiac cycle change τ 1.5 s Time constant for vagal stimulated 5 cardiac cycle change τ 1 s Time constant for sympathetic 6 stimulated heart contractility change τ 2 s Time constant for sympathetic 7 stimulated total blood volume change g.695 Gain for splanchnic resistance change 1 g.53 Gain for other resistance change 2 g 2.81 Gain for muscle resistance 3 g -.6 Gain for sympathetic stimulated 4 cardiac cycle change g.1 Gain for vagal stimulated cardiac 5 cycle change g.475 Gain for sympathetic stimulated heart 6 contractility change g 2 Gain for sympathetic stimulated total 7 blood volume change 24
39 Table 3.5. (continued) d 1 2 s Time delay for sympathetic stimulated resistance change d 2 s Time delay for sympathetic 2 stimulated resistance change d 2 s Time delay for sympathetic 3 stimulated resistance change d 2 s Time constant for sympathetic 4 stimulated cardiac cycle change d.2 s Time constant for vagal stimulated 5 cardiac cycle change d 2 s Time delay for sympathetic 6 stimulated heart contractility change d 5 s Time delay for sympathetic 7 τ 6.37 s Constant z τ 2.76 s Constant p stimulated total blood volume change f esmin 2.66 spikes/s Threshold value for sympathetic excitation fes 2.1 spikes/s Constant f spikes/s Constant es K.675 s/spikes Constant es offset _ es spikes/s Offset in sympathetic activity f 3.2 spikes/s Constant ev fev 6.3 spikes/s Constant K 7.6 spikes/s Constant ev offset _ ev spikes/s Offset in vagal activity f 25 spikes/s Constant aso 25
40 Table 3.5. (continued) f 2.52 spikes/s Constant min f max spikes/s Constant K mmhg Constant a P 92 mmhg Constant o R 2.49 mmhg/ml/s Constant 1 R.96 mmhg/ml/s Constant 2 R 4.13 mmhg/ml/s Constant 3 T.2 s Constant E max 2.2 mmhg/ml Constant V 25 ml Constant T The baseline P-V loops for Simbiosys and the model are shown in Figure 3.1 and the waveforms of left ventricular pressure and left ventricular volume are shown in Figure Table 3.6 lists the baseline hemodynamics for both the model and Simbiosys. It can be seen that the model reproduces fairly well the baseline hemodynamics generated by Simbiosys. a. Baseline P-V loop from Simbiosys. 26
41 b. P-V loop generated by the model. Figure 3.1. P-V loops generated by the model and Simbiosys. a. Left ventricular pressure and left ventricular volume from Simbiosys. 27
42 b. Left ventricular pressure and left ventricular volume generated by the model. Figure Left ventricular pressure and left ventricular volume. Table 3.6. Baseline Hemodynamics Simbiosys Model LVEDP (mmhg) 7 LVEDP (mmhg) 7 LVESP (mmhg) 9 LVESP (mmhg) 89 EDV (ml) 118 EDV (ml) 121 ESV (ml) 4 ESV (ml) 44 MAP (mmhg) 88 MAP (mmhg) 89 SV (ml) 78 SV (ml) 77 HR (bpm) 68 HR (bpm) 69 CO (l/min) 5.3 CO (l/min) 5.3 LV contractility 1.3 Emax (mmhg/ml) 2.7 Arterial contractility 1.16 SVR (mmhg/ml/s).91 Sympathetic tone.137 Sympathetic activity 2.78 Parasympathetic tone.36 Parasympathetic activity 6.11 LVEDP: left ventricular end diastolic pressure; LVESP: left ventricular end systolic pressure; EDV: end diastolic volume; ESV: end systolic volume; MAP: mean arterial pressure; SV: stroke volume; HR: heart rate; CO: cardiac 28
43 output; Emax: peak left ventricular contractility; SVR: systemic arterial resistance. Sympathetic activity and parasympathetic activity are in mean value (spikes/s). LVESP for Simbiosys is read directly from the panel; LVESP for the model is hard to read thus assumed the same as MAP. The values for LV contractility, arterial contractility are dimensionless relative parameters (needed to be multiplied by a constant contractility); sympathetic tone and parasympathetic tone are dimensionless parameters range from (no tone) to 1 (maximum tone). 3.4 RESPONSE TO SINGLE PARAMETER CHANGE The behaviors of the model and Simbiosys are compared by examining the response of the both to single parameter change in preload, afterload, left ventricular contractility and heart rate Response to decrease in preload (blood withdrawal) This subsection will examine the response of the model to forced change in preload by using blood withdrawal. The percentage of blood loss is set the same for Simbiosys and the model. For example, for the normal value of total blood volume 5 ml, -5 ml implies 1 % loss of blood in Simbiosys. Similarly, for the model with total blood volume of 25 ml, -25 ml implies 1% loss of blood. The maximum available withdrawal rate ml/hr (or 2.78 ml/s) in Simbiosys is used to avoid fluid compensation from the renal system. For the model, the rate of bleeding is the same by decreasing the left ventricular volume. The steady state values are recorded in Table 3.7 after the desired loss of blood is finished. P-V loops are shown in Figure 3.12 for 2% loss of blood. Figure 3.13 shows the changes in hemodynamic variables compared with corresponding baseline values. Table 3.7. Response to change in preload Hemodynamics Baseline 1% loss 2% loss of Tendency of blood blood LVEDP (mmhg) 7 4 Down LVESP (mmhg) Down 29
44 Table 3.7. (continued) EDV (ml) Down ESV (ml) Down MAP (mmhg) Down SV (ml) Down HR (bpm) Up Simbiosys CO (l/min) Down LV contractility Up Arterial contractility Up Sympathetic tone Up Parasympathetic tone Down LVEDP (mmhg) Down LVESP (mmhg) Down EDV (ml) Down ESV (ml) Down MAP (mmhg) Down Model SV (ml) Down HR (bpm) Up CO (l/min) Down Emax (mmhg/ml) Up SVR (mmhg/ml/s) Up Sympathetic activity Up Parasympath activity Down 3
45 a. 2% Blood withdrawal for Simbiosys. b. 2% Blood withdrawal for the model. Figure Change in P-V loop for 2% blood withdrawal. Simbiosys Model % MAP HR CO Emax SVR 31
46 a. 1 % loss of blood. Simbiosys Model % MAP HR CO Emax SVR b. 2 % loss of blood. Figure Changes in hemodynamics for loss of blood. With loss of blood, CO and MAP decrease; HR, SVR, and Emax increase. For both Simbiosys and the model, when preload decreases, (1) P-V loops shrink towards the left bottom corner of the coordinate; (2) stoke volume decreases and heart rate increases but cardiac output decreases; (3) mean arterial pressure decreases even though systemic vascular resistance increases; (4) left ventricular contractility and sympathetic activity increases, parasympathetic activity decreases Response to change in afterload (SVR) This subsection will examine the response of the model to forced change in afterload by using forced change in SVR. The change in SVR for Simbiosys is induced by forced change in arterial contractility. For the model, it is induced by forced change in SVR directly. The steady state values are recorded in Table 3.8 after the changes. P-V loops in Figure 3.14 and Figure 3.15 are shown respectively for -2% and +2% change in SVR for Simbiosys and the model. Figure 3.16 shows the changes in hemodynamic variables compared with corresponding baseline values. 32
47 a. -2% in SVR for Simbiosys Left ventricle pressure (mmhg) Left ventricle volume (ml) b. -2% in SVR for the model. Figure Change in P-V loop for -2% in SVR. 33
48 a. +2% in SVR for Simbiosys. b. +2% in SVR for the model. Figure Change in P-V loop for +2% in SVR. Sim biosys Table 3.8. Response to change in afterload Hemo- -2% -1% Base +1% +2% Tendency dynamics in SVR in SVR line in SVR in SVR LVEDP (mmhg) Up LVESP (mmhg) Up EDV (ml) Up ESV (ml) Up MAP (mmhg) Up SV (ml) Down HR (bpm) Down CO (l/min) Down LV contractility Down Arterial Up contractility Sympathetic tone Down Parasympathetic same 34
49 Model Table 3.8. (continued) LVEDP (mmhg) same LVESP (mmhg) EDV (ml) Down ESV (ml) Up MAP (mmhg) SV (ml) Down HR (bpm) Down CO (l/min) Down Emax Down (mmhg/ml) SVR Up (mmhg/ml/s) Sympathetic activity Parasympathetic Up activity Simbiosys Model 15 % 5 MAP HR SV CO Emax a. -2 % in SVR. HR, CO and Emax increase with decrease in SVR. 35
50 Simbiosys Model 15 % 5 MAP HR SV CO Emax b. +2 % in SVR. HR, CO and Emax decrease with increase in SVR. Figure Changes in hemodynamics for changes in SVR. For both Simbiosys and the model, when afterload increases, (1) P-V loops do not change greatly; (2) stroke volume, heart rate and cardiac output decrease; (3) mean arterial pressures do not increase greatly; (4) left ventricular contractility (or Emax) and sympathetic activity decrease. The difference is that: when afterload increases, the parasympathetic tone does not change in Simiosys, but it increases in the model Response to change in left ventricular contractility (or Emax) This subsection will examine the response of the model to forced change in left ventricular contractility by using forced change in Emax. The change in Emax for Simbiosys is induced by forced change in left ventricular contractility. For the model, it is induced by forced change in Emax directly. The steady state values are recorded in Table 3.9 after the changes. P-V loops in Figure 3.17 are shown +4% for change in left ventricular contractility for Simbiosys and +4% changes in Emax for the model. Figure 3.18 shows the changes in hemodynamic variables compared with corresponding baseline values. 36
51 a. +4% change in left ventricular contractility for Simbiosys. b. +4% change in Emax in the model. Figure Change in P-V loop for +4% in Emax in the model. Table 3.9. Response to change in left ventricle contractility Hemodynamics Base +2% +4% Tendency line in Emax in Emax LVEDP (mmhg) Down LVESP (mmhg) Up EDV (ml) Down ESV (ml) Down MAP (mmhg) Up SV (ml) Up 37
52 Simbio sys Table 3.9. (continued) HR (bpm) Down CO (l/min) LV contractility Up Arterial contractility Down Sympathetic tone Down Parasympathetic tone Same LVEDP (mmhg) Same LVESP (mmhg) Up EDV (ml) Same ESV (ml) Down Model MAP (mmhg) Up SV (ml) Up HR (bpm) Down CO (l/min) Up Emax (mmhg/ml) Up SVR (mmhg/ml/s) Down Sympathetic activity Down Parasympath activity Up Simbiosys Model 15 % 5 MAP HR SV CO SVR Figure Changes in hemodynamics for +4 % in Emax. MAP and CO increase; HR and SVR decrease with increase in Emax. 38
53 For both Simbiosys and the model, when left ventricular contractility (or Emax) increases, (1) P-V loops expand to the left; (2) stoke volume increases and heart rate decreases; (3) systemic vascular resistance decreases; (4) sympathetic activity decreases Response to change in heart rate This section will examine the response of the model to forced change in heart rate by using forced change in heart rate (to simulate drug intervention). The forced change in heart rate for Simbiosys is induced by forced change in sinus rate. For the model, it is induced by forced change in heart rate directly. The steady values are recorded in Table 3.1 after the changes. P-V loops in Figure 3.19 are shown for -1% changes in heart rate for Simbiosys and the model. P-V loops in Figure 3.2 are shown for +4% changes in heart rate for Simbiosys and the model. Figure 3.21 shows the changes in hemodynamic variables compared with corresponding baseline values. a. -1% change in heart rate for Simbiosys. 39
54 b. -1% change in heart rate for the model. Figure Change in P-V loop for -1% in HR. a. +4% change in heart rate for Simbiosys. 4
55 b. +4% change in heart rate for the model. Figure 3.2. Change in P-V loop for +4% in HR. Simbiosys Table 3.1. Response to change in heart rate Hemodynamics -1% in HR Base line +1% in HR +2% in HR +4% in HR Tendency LVEDP Down (mmhg) LVESP Down (mmhg) EDV (ml) Down ESV (ml) Down MAP Up (mmhg) SV (ml) Down HR (bpm) Up CO (l/min) Up LV Down contractility 41
56 Model Table 3.1. (continued) Arterial Down contractility Sympathetic Down tone Parasympathe Same tic tone LVEDP Down (mmhg) LVESP (mmhg) EDV (ml) Down ESV (ml) Same MAP Up (mmhg) SV (ml) Down HR (bpm) Up CO (l/min) Up Emax Down (mmhg/ml) SVR Down (mmhg/ml/s) Sympathetic activity Down Parasympath Up activity 42
57 Simbiosys Model % MAP SV CO Emax SVR Figure 5.15a -1 % in HR. CO decreases; SVR and Emax increase with decrease in heart rate. Simbiosys Model % MAP SV CO Emax SVR Figure 5.15b +4 % in HR. CO increases; SVR and Emax decrease with increase in heart rate. Figure Changes in hemodynamics for change in HR. For both Simbiosys and the model, when heart rate increases, (1) P-V loops shrink to the left; (2) left ventricular contractility decreases; (3) stoke volume decreases but cardiac output increases; (4) systemic vascular resistance decreases; (5) mean arterial pressure does not increase greatly; (6) sympathetic activity decreases. The difference is that: when heart rate increases, the parasympathetic tone does not change in Simbiosys, but parasympathetic activity increases in the model. 43
58 3.5 RESPONSE TO EXERCISE This section will examine the response of the model to exercise. The exercise level is determined by the combination of nervous offsets (central command) and forced change in active muscle resistance. The exercise experiment hemodynamic data for healthy people (9 subjects) from [52] are used as reference for tuning the combinations. The simulation results are compared to the data from [52] Single level exercise In the simulation of a certain level of exercise, the set point change is induced by adding offsets to efferent pathways progressively, by changing the sympathetic offset offset _ es in (3.23) and the vagal offset offset _ ev in (3.24) linearly in 5 seconds. In Figure 3.22, the offsets start changing progressively from 1s with values in Table At 15 s, the exercise begins, R 13 starts decreasing due to local mechanism in active muscle (forced change from 7.1 mmhg/ml/s to.8 mmhg/ml/s linearly in 1 seconds), but heart rate and left ventricle contractility increase continually until they achieve new steady values. The complex of changes is shown in Figure The new set point consists of higher pressure, higher heart rate and lower SVR which is consistent with exercise physiology [53]. Table Offsets in sympathetic and vagal activity Rest (steady) Exercise (steady) offset _ es.24 offset _ ev.2 As shown in Figure 3.23, the changes in P-V loops from rest to exercise include increases in end diastolic volume and end systolic pressure. The hemodynamic changes are listed in Table Since the blood flow is inverse proportional to resistance, the changes in resistances imply the redistribution of the blood flow. The redistribution of blood flow is shown in Table
59 15 HR AF AOP Emax SVR Time (seconds) Figure Response to exercise. MAP in mmhg, AF (aortic flow) in L/min, HR in bpm, SVR in mmhg/ml/s, (MAP, CO, SVR) changes from (89, 5.3,.91) to (16, 1.8,.51) during exercise Rest Exercise Left ventricle pressure (mmhg) Left ventricle volume (ml) decreases. Figure P-V loops of rest and exercise. The loop for exercise expands to the right. The end diastolic volume increases and end systolic volume 45
60 Table Hemodynamic changes Experiment data from [52] Rest Exercise Tendency MAP (mmhg) 86 ± 3 96 ± 3 Up HR (bpm) 68 ± ± 4 Up SV 93 ± ± 8 Up CO (l/min) 6.2 ± ± 1.2 Up SVR* (mmhg/ml/s) Down LVR** (mmhg/ml/s).77 SVR/LVR (%) 58.6 Simulation results Rest Exercise Tendency LVEDP (mmhg) 7 6 Down LVESP (mmhg) Up EDV (ml) Up ESV (ml) Down MAP (mmhg) Up SV (ml) Up HR (bpm) Up CO (l/min) Up Emax (mmhg/ml) Up SVR (mmhg/ml/s) Down Sympathetic activity Up Parasympath activity Down *: calculated from systemic vascular conductance. **: leg vascular resistance, also calculated from systemic vascular conductance. 46
61 Table Changes in resistances Resistance Rest Exercise R R R SVR Multiple levels of exercise Multiple exercise levels are simulated by using the experimental data from [52] as reference to tweak the combinations of nervous offsets and forced change in R 13 (muscle resistance) to make the hemodynamic variables in the simulation close to the real data. The simulation results are shown in Table The hemodynamic changes in percentage from rest to different levels of exercise (ratio of exercise to rest) are illustrated in Figure 3.24, compared to that of experiment data from [52]. Table Multiple exercise levels Exercise level Data from [52] (9 subjects) Rest 71 w 97 w 125 w MAP (mmhg) 86 ± 3 96 ± 3 98 ± 3 17 ± 2 HR (bpm) 68 ± ± ± ± 3 SV (ml) 93 ± ± ± ± 6 CO (L/min) 6.2 ± ± ± ±.8 SVR* (mmhg/ml/s) LVR** (mmhg/ml/s) SVR/LVR (%)
62 Table (continued) Simulation result Offsets (,) (.24,.2) (.3,.24) (.33,.24) (O1,O2)*** MAP (mmhg) HR (bpm) SV (ml) CO (L/min) SVR (mmhg/ml/s) R (mmhg/ml/s) SVR/R13 (%) *: calculated from systemic vascular conductance. **: leg vascular resistance, also calculated from systemic vascular conductance. ***: outbound nervous signals offsets: O1 (sympathetic offset), O2 (parasympathetic offset). Experiment Simulation % MAP HR SV CO SVR Exercise level 1 48
63 Experiment Simulation % MAP HR SV CO SVR Exercise level 2 Experiment Simulation % MAP HR SV CO SVR Exercise level 3 Figure Hemodynamic changes from rest to exercice. MAP, CO, and HR increase SVR decreases. According to exercise physiology [52-54], for the human exercise experiment, there is an increase in stroke volume (SV) accompanying the increase in blood pressure at low level excercise, and stroke volume reaches a plateau at a submaximal exercise level. Other hemodanymic variables (MAP, HR, CO) increase with exercise levels. To demonstrate the trends in hemodynamics with exercise intensity, comparable exercise experimental data from [54] are listed in Table The experiment data in Table 3.14 and Table 3.15 from [52, 54] and simulation results for multiple exercise levels are illustrated in Figure
64 Table Exercise experiment data Exercise level MAP (mmhg) HR (bpm) SV (ml) CO (L/min) SVR(mmHg/ml/s) Data read from figures in [54]. MAP (mmhg) SV (ml) HR (bpm) Exercise level CO (L/min) Exercise level Figure Changes in hemodynamics for multiple exercise levels. Diamond: experiment data from [52]; Square: experiment data from [54]; Triangle: simulation results. 5
65 3.6 CONCLUSION The cardiovascular system model is described and the baroreflex model is coupled to it. Using physiological simulation software Simbiosys and exercise experiment data in the literature as reference, the coupled model of the cardiovascular system and the baroreflex reproduced the hemodynamic response fairly well to single parameter change in preload, afterload, left ventricular contractility and heart rate. The responses to multiple levels of exercise are simulated and the results are consistent with exercise experiment data. Thus this coupled model can be considered as a model for a healthy person. The next chapter is to determine the parameters for the people with heart failure based on this healthy heart model. 51
66 4. FAILING HEART WITH BAROREFLEX In this chapter, the qualitative and quantitative changes in the cardiovascular system and the baroreflex will be found out by surveying the literature for patients with heart failure, and these changes will be mapped into the model. The parameters are tuned by using heart failure hemodynamic data in the literature as reference. The responses of the model to multiple levels of exercise are examined and compared to that in the literature. 4.1 HEART FAILURE AND ASSOCIATED PHYSIOLOGICAL CHANGES Heart failure is the inability of the heart to supply adequate blood flow and therefore oxygen delivery to peripheral tissues and organs. Heart failure is the final result of a variety of primary cardiovascular diseases [1]. The common cause of heart failure is coronary artery disease (CAD). CAD reduces coronary blood flow and oxygen delivery to the myocardium and thus causes impaired function. Another common cause of heart failure is myocardial infarction which needs to be compensated by non-infarcted regions for the loss of function. The other factors like valvular disease and congenital defects place increased demands upon the ailing heart and precipitate failure. External factors for heart failure include increased afterload and increased body demands. There are a series of changes associated with heart failure which include the changes in the cardiovascular system and the changes in the baroreflex [1, 55]. 52
67 4.1.1 Changes in cardiovascular system 1) Cardiac Changes The changes in cardiac function associated with heart failure result in a decrease in stroke volume as well as cardiac output. The decline in stroke volume is due to systolic dysfunction, diastolic dysfunction, or a combination of the two [1, 55]. Simply stated, systolic dysfunction is the result of decreased left heart contractility. Diastolic dysfunction means that the ventricle becomes less compliant and impairs ventricular filling. As illustrated in Figure 4.1, the systolic dysfunction is usually caused by the dilated myocardium, which is characterized by increased end diastolic volume and decreased ejection fraction. The diastolic dysfunction is usually caused by the hypertrophic myocardium, which is characterized by decreased end diastolic volume. 2) Neurohumoral Changes Neurohumoral responses include increased sympathetic nervous activities and increased release of antidiuretic hormone. The net effect of these neurohumoral responses is to help maintain arterial pressure and increase heart rate and blood volume. Otherwise, the arterial pressure will drop out of acceptable range due to decreased stroke volume and cardiac output. 3) Systemic Vascular Resistance changes To compensate for reduced cardiac output associated with heart failure, some feedback mechanisms within the body will try to maintain normal arterial pressure by constricting arterial resistance vessels thus increase the systemic vascular resistance. The baroreflex is an important component of this feedback system. 4) Blood Volume changes In heart failure, the compensatory increase in blood volume can increase ventricular preload and stroke volume. Blood volume is augmented by decreased urine output and retention of fluid. There is also an increase in circulating anti-diuretic hormone that contributes to renal retention of water. The resulting increase in blood volume helps to maintain cardiac output. On the other 53
68 hand, the increased volume can be deleterious because it increases venous pressures and leads to pulmonary and systemic edema. Figure 4.1. Systolic dysfunction and diastolic dysfunction (Adopted from [56]) Changes in baroreflex Initially, a reduction in cardiac output associated with heart failure leads to a decrease in the arterial pressure applied to the baroreceptors which, in turn, cause increased heart rate and 54
69 systemic vascular resistance through sympathetic and vagal systems. The sympathetic excitation is in effect for the duration of the failure [57]. In neck chamber experiment for examining carotid baroreceptor-cardiac reflex mechanisms in patients with congestive heart failure, the shape of the sigmoid baroreceptor stimulus-cardiac response relation is qualitatively normal in heart failure patients and the time delay of the baroreflex is not changed, but the baroreflex sensitivity is depressed [58]. It was reported that there was a diminished sensitivity of the afferent limb while the gain of the central portion of the reflex was normal in rats with cardiac failure [59]. Patients with heart failure have increased sympathetic nerve activity. In addition, the increase in sympathetic activity is well related to severity of the heart failure and the sympathetic nerve activity progressively increases from mild to severe heart failure [6]. It is suggested that the depressed end-organ response of the baroreflex and the blunted response at the receptor level account for the decrease in baroreflex gain [61, 62]. It has been reported that the vasodilatory response is impaired in patients with congestive heart failure [63]. Reduced baroreflex sensitivity for heart failure is well known where baroreflex sensitivity is defined as the ratio of change in cardiac cycle to change in the arterial pressure [64-66]. In summary, there are some changes in the cardiovascular system and the baroreflex with heart failure. These changes can be mapped into the changes in parameters of the heart failure model. The tendencies of parameters changes for heart failure model are listed in Table 4.1. The parts with parameter change in the baroreflex loop are illustrated in Figure 4.2. Table 4.1. Changes in parameters of the heart failure model Parameters Tendency Emax Down Cardiovascular Emin Up System SVR Up Heart rate Up Baroreceptor sensitivity Down Baroreflex Sympathetic heart rate gain Down Paraympathetic heart rate gain Down Resistances gains Down 55
70 Sensitivity decreases Baroreceptor Afferent Pathway Pressure Nervous system Gains decrease Regulation Effectors Efferent Pathway Figure 4.2. Changes in baroreflex. The baroreceptor gain, and regulation effectors gains (sympathetic heart rate and parasympathetic gain, resitances gains) decrease for patients with heart failure. 4.2 EFFECTS OF CHANGES IN HEART FOR HEART FAILURE MODEL The change in heart contractility and/or compliance is the primary change of heart failure. As shown in Figure 4.3, the left ventricular contractility (Emax) refers to the slope of end systolic pressure volume relationship (ESPVR) and the left ventricular compliance (1/Emin) refers to the reciprocal of the slope of end diastolic pressure volume relationship (ESPVR). There are basically two types of physical changes with heart failure: decrease in the left ventricular contractility and decrease in the left ventricular compliance. The change in ejection fraction (defined as the ratio of stroke volume to the end diastolic volume) is usually a result of the systolic dysfunction. In the following examples, it is assumed that change in heart contractility and/or compliance is the only change and other parameters (heart rate, systemic vascular resistance and total blood volume) are fixed. 56
71 Figure 4.3. Left ventricle pressure volume loop (adopted from [55]). Four phases in a cardiac cycle: a. filling, b. isovolumetric contraction, c. ejection, d. isovolumetric relaxation. EDV: end diastolic volume. ESV: end systolic volume. SV: stroke volume, the difference between EDV and ESV Systolic dysfunction: decrease in Emax As shown in Figure 4.4, the slope of the end systolic pressure volume relationship (ESPVR) decreases with loss of left ventricular contractility (Emax). This causes an increased end systolic Figure 4.4. Systolic dysfunction (adopted from [55]). Emax decreased (loss of contractility), Emin and heart rate unchanged. 57
72 volume and an increased end diastolic volume; however the increase in end diastolic volume is not as great as the increase in end systolic volume. Thus the resulting stroke volume decreases. Since stroke volume decreases and end diastolic volume increases, there is a decrease in ejection fraction (EF) Diastolic dysfunction: increase in Emin As shown in Figure 4.5, a decrease in ventricular compliance (increase in Emin) accompanies with diastolic dysfunction, as occurs in ventricular hypertrophy. This will result in a decreased end diastolic volume and a greater end diastolic pressure as shown by changes in the ventricular pressure-volume loop. As a result of these changes, stroke volume decreases. Dependant on the relative change in stroke volume and end diastolic volume, there may or may not be a small change in ejection fraction. Figure 4.5. Diastolic dysfunction (adopted from [55]). Emin increased, Emax and heart rate unchanged Combination of systolic dysfunction and diastolic dysfunction: decrease in Emax and increase in Emin As shown in Figure 4.6, the slope of the ESPVR is decreased (Emax decreased) and the slope of the passive filling curve is increased (Emin increased). There is a significant decrease in stroke 58
73 volume because of decreased EDV and increased ESV. As a result, the ejection fraction decreased. Figure 4.6. Combination of systolic dysfunction and diastolic dysfunction (adopted from [55]). Emax decreased, Emin increased and heart rate unchanged. 4.3 DETERMINE THE PARAMETERS OF HEART FAILURE MODEL The parameters for heart failure model are tuned by using heart failure hemodynamic data in the literature as reference. These changes are made according to section 4.1 including both the cardiovascular model and the baroreflex model. 1) Hemodynamic data for heart failure in the literature The hemodynamics data for patients with heart failure in [67-69] are listed in Table 4.2 as reference for tuning the parameters of heart failure model. These data of heart failure was collected before the implants of LVADs. Table 4.2. Clinical hemodynamics data for heart failure Hemodynamic Data from [67] Data from [68] Data from [69] (23 patients ) (1 patients) (2 patients) Systolic pressure (mmhg).6 ± 12.4 No 97 ± 11 Diastolic pressure (mmhg) 56.8 ± 1.4 No 59 ± 12 59
Physiological Control of Left Ventricular Assist Devices Based on Gradient of Flow (1)
 2005 American Control Conference June 8-10, 2005. Portland, OR, USA FrA13.4 Physiological Control of Left Ventricular Assist Devices Based on Gradient of Flow (1) Shaohui Chen (2), James F. Antaki (3),
2005 American Control Conference June 8-10, 2005. Portland, OR, USA FrA13.4 Physiological Control of Left Ventricular Assist Devices Based on Gradient of Flow (1) Shaohui Chen (2), James F. Antaki (3),
Suction Detection And Feedback Control For The Rotary Left Ventricular Assist Device
 University of Central Florida Electronic Theses and Dissertations Doctoral Dissertation (Open Access) Suction Detection And Feedback Control For The Rotary Left Ventricular Assist Device 2013 Yu Wang University
University of Central Florida Electronic Theses and Dissertations Doctoral Dissertation (Open Access) Suction Detection And Feedback Control For The Rotary Left Ventricular Assist Device 2013 Yu Wang University
CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE
 CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE VASILE MANOLIU Electrical Engineering Faculty, POLITEHNICA University of Bucharest, Splaiul
CONSIDERATIONS ABOUT THE LUMPED PARAMETER WINDKESSEL MODEL APPLICATIVITY IN THE CARDIOVASCULAR SYSTEM STRUCTURE VASILE MANOLIU Electrical Engineering Faculty, POLITEHNICA University of Bucharest, Splaiul
ENT 318/3 Artificial Organs
 ENT 318/3 Artificial Organs Modeling of cardiovascular system and VAD Lecturer Ahmad Nasrul bin Norali 1 What is modeling and why we need it? In designing product, sometimes we have to make sure that the
ENT 318/3 Artificial Organs Modeling of cardiovascular system and VAD Lecturer Ahmad Nasrul bin Norali 1 What is modeling and why we need it? In designing product, sometimes we have to make sure that the
Chapter 9, Part 2. Cardiocirculatory Adjustments to Exercise
 Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
An Investigation of the Pump Operating Characteristics as a Novel Control Index for LVAD Control
 International Seongjin Journal of Choi, Control, J. Robert Automation, Boston, and and Systems, James F. vol. Antaki 3, no. 1, pp. -8, March 5 An Investigation of the Pump Operating Characteristics as
International Seongjin Journal of Choi, Control, J. Robert Automation, Boston, and and Systems, James F. vol. Antaki 3, no. 1, pp. -8, March 5 An Investigation of the Pump Operating Characteristics as
Principles of Biomedical Systems & Devices. Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont
 Principles of Biomedical Systems & Devices Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont Review of Cardiac Anatomy Four chambers Two atria-receive blood from the vena cave and pulmonary veins Two
Principles of Biomedical Systems & Devices Lecture 8: Cardiovascular Dynamics Dr. Maria Tahamont Review of Cardiac Anatomy Four chambers Two atria-receive blood from the vena cave and pulmonary veins Two
Cardiovascular Physiology. Heart Physiology. Introduction. The heart. Electrophysiology of the heart
 Cardiovascular Physiology Heart Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through
Cardiovascular Physiology Heart Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through
A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS
 A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS R. Mukkamala, R. G. Mark, R. J. Cohen Haard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA Abstract We
A NONINVASIVE METHOD FOR CHARACTERIZING VENTRICULAR DIASTOLIC FILLING DYNAMICS R. Mukkamala, R. G. Mark, R. J. Cohen Haard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA Abstract We
HEART transplantation has now been recognized as the
 IEEE TRANSACTIONS ON CONTROL SYSTEMS TECHNOLOGY, VOL. 17, NO. 1, JANUARY 2009 15 A Dynamical State Space Representation and Performance Analysis of a Feedback-Controlled Rotary Left Ventricular Assist
IEEE TRANSACTIONS ON CONTROL SYSTEMS TECHNOLOGY, VOL. 17, NO. 1, JANUARY 2009 15 A Dynamical State Space Representation and Performance Analysis of a Feedback-Controlled Rotary Left Ventricular Assist
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Mechanics of Cath Lab Support Devices
 Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Professor of Medicine Mayo Clinic College of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida DISCLOSURE Presenter:
Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Professor of Medicine Mayo Clinic College of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida DISCLOSURE Presenter:
SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006
 SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006 The goal of this exercise to explore the behavior of the heart as a mechanical pump. For
SymBioSys Exercise 2 Cardiac Function Revised and reformatted by C. S. Tritt, Ph.D. Last updated March 20, 2006 The goal of this exercise to explore the behavior of the heart as a mechanical pump. For
AS Level OCR Cardiovascular System
 AS Level OCR Cardiovascular System Learning Objectives The link between the Cardiac Cycle and the Conduction system of the heart. The relationship between Stroke volume, Heart rate and Cardiac Output.
AS Level OCR Cardiovascular System Learning Objectives The link between the Cardiac Cycle and the Conduction system of the heart. The relationship between Stroke volume, Heart rate and Cardiac Output.
Mechanics of Cath Lab Support Devices
 Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Chief Medical Officer First Coast Cardiovascular Institute, Jacksonville, FL Professor of Medicine, UCF, Orlando, FL None DISCLOSURE Percutaneous
Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Chief Medical Officer First Coast Cardiovascular Institute, Jacksonville, FL Professor of Medicine, UCF, Orlando, FL None DISCLOSURE Percutaneous
Interaction between carotid baroregulation and the pulsating heart: a mathematical model
 Interaction between carotid baroregulation and the pulsating heart: a mathematical model MAURO URSINO Department of Electronics, Computer Science and Systems, University of Bologna, I40136 Bologna, Italy
Interaction between carotid baroregulation and the pulsating heart: a mathematical model MAURO URSINO Department of Electronics, Computer Science and Systems, University of Bologna, I40136 Bologna, Italy
Ventricular Assisting Devices in the Cathlab. Unrestricted
 Ventricular Assisting Devices in the Cathlab Unrestricted What is a VAD? A single system device that is surgically attached to the left ventricle of the heart and to the aorta for left ventricular support
Ventricular Assisting Devices in the Cathlab Unrestricted What is a VAD? A single system device that is surgically attached to the left ventricle of the heart and to the aorta for left ventricular support
Eindhoven University of Technology. Exam Modeling Cardiac Function (8W160)
 Eindhoven University of Technology department of Biomedical Engineering group Cardiovascular Biomechanics Exam Modeling Cardiac Function (8W160) January 21, 2011, 14.00 17.00 h This exam consists of 6
Eindhoven University of Technology department of Biomedical Engineering group Cardiovascular Biomechanics Exam Modeling Cardiac Function (8W160) January 21, 2011, 14.00 17.00 h This exam consists of 6
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Heart Pump and Cardiac Cycle. Faisal I. Mohammed, MD, PhD
 Heart Pump and Cardiac Cycle Faisal I. Mohammed, MD, PhD 1 Objectives To understand the volume, mechanical, pressure and electrical changes during the cardiac cycle To understand the inter-relationship
Heart Pump and Cardiac Cycle Faisal I. Mohammed, MD, PhD 1 Objectives To understand the volume, mechanical, pressure and electrical changes during the cardiac cycle To understand the inter-relationship
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1
 BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
Modeling, Estimation and Control of Human Circulatory System with A Left Ventricular Assist Device
 Modeling, Estimation and Control of Human Circulatory System with A Left Ventricular Assist Device Yi Wu, Paul Allaire 2, Gang Tao 3 and Don Olsen 4 Corresponding Author, Department of Mechanical Engineering,
Modeling, Estimation and Control of Human Circulatory System with A Left Ventricular Assist Device Yi Wu, Paul Allaire 2, Gang Tao 3 and Don Olsen 4 Corresponding Author, Department of Mechanical Engineering,
Cardiac Output MCQ. Professor of Cardiovascular Physiology. Cairo University 2007
 Cardiac Output MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 90- Guided by Ohm's law when : a- Cardiac output = 5.6 L/min. b- Systolic and diastolic BP
Cardiac Output MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 90- Guided by Ohm's law when : a- Cardiac output = 5.6 L/min. b- Systolic and diastolic BP
2.6 Cardiovascular Computer Simulation
 2.6 Cardiovascular Computer Simulation ROOM 23G22 Contents 1. INTRODUCTION... 4 1.1. GENERAL REMARKS... 4 1.2. LEARNING GOALS... 4 1.3. PHYSIOLOGICAL PARAMETERS... 5 1.4. GLOSSARY... 5 2. USING THE COMPUTER
2.6 Cardiovascular Computer Simulation ROOM 23G22 Contents 1. INTRODUCTION... 4 1.1. GENERAL REMARKS... 4 1.2. LEARNING GOALS... 4 1.3. PHYSIOLOGICAL PARAMETERS... 5 1.4. GLOSSARY... 5 2. USING THE COMPUTER
Blood Pressure and its Regulation
 Blood Pressure and its Regulation Blood pressure in your blood vessels is closely monitored by baroreceptors; they send messages to the cardio regulatory center of your medulla oblongata to regulate your
Blood Pressure and its Regulation Blood pressure in your blood vessels is closely monitored by baroreceptors; they send messages to the cardio regulatory center of your medulla oblongata to regulate your
Impedance Cardiography (ICG) Method, Technology and Validity
 Method, Technology and Validity Hemodynamic Basics Cardiovascular System Cardiac Output (CO) Mean arterial pressure (MAP) Variable resistance (SVR) Aortic valve Left ventricle Elastic arteries / Aorta
Method, Technology and Validity Hemodynamic Basics Cardiovascular System Cardiac Output (CO) Mean arterial pressure (MAP) Variable resistance (SVR) Aortic valve Left ventricle Elastic arteries / Aorta
THESIS NEW FRANK-STARLING BASED CONTRACTILITY AND VENTRICULAR STIFFNESS INDICES: CLINICALLY APPLICABLE ALTERNATIVE TO EMAX. Submitted by.
 THESIS NEW FRANK-STARLING BASED CONTRACTILITY AND VENTRICULAR STIFFNESS INDICES: CLINICALLY APPLICABLE ALTERNATIVE TO EMAX Submitted by Haiming Tan Graduate Degree Program in Bioengineering In partial
THESIS NEW FRANK-STARLING BASED CONTRACTILITY AND VENTRICULAR STIFFNESS INDICES: CLINICALLY APPLICABLE ALTERNATIVE TO EMAX Submitted by Haiming Tan Graduate Degree Program in Bioengineering In partial
Blood pressure. Formation of the blood pressure: Blood pressure. Formation of the blood pressure 5/1/12
 Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
Blood pressure Blood pressure Dr Badri Paudel www.badripaudel.com Ø Blood pressure means the force exerted by the blood against the vessel wall Ø ( or the force exerted by the blood against any unit area
QUIZ 1. Tuesday, March 2, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Evaluation of Native Left Ventricular Function During Mechanical Circulatory Support.: Theoretical Basis and Clinical Limitations
 Review Evaluation of Native Left Ventricular Function During Mechanical Circulatory Support.: Theoretical Basis and Clinical Limitations Tohru Sakamoto, MD, PhD Left ventricular function on patients with
Review Evaluation of Native Left Ventricular Function During Mechanical Circulatory Support.: Theoretical Basis and Clinical Limitations Tohru Sakamoto, MD, PhD Left ventricular function on patients with
Analysis of Human Cardiovascular System using Equivalent Electronic System
 Analysis of Human Cardiovascular System using Equivalent Electronic System N. Vinoth 1, S. Nagarjuna Chary 2 Dept of Electronics and Instrumentation Engineering, Annamalai University, Annamalai nagar,
Analysis of Human Cardiovascular System using Equivalent Electronic System N. Vinoth 1, S. Nagarjuna Chary 2 Dept of Electronics and Instrumentation Engineering, Annamalai University, Annamalai nagar,
Veins. VENOUS RETURN = PRELOAD = End Diastolic Volume= Blood returning to heart per cardiac cycle (EDV) or per minute (Venous Return)
 Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Veins Venous system transports blood back to heart (VENOUS RETURN) Capillaries drain into venules Venules converge to form small veins that exit organs Smaller veins merge to form larger vessels Veins
Chapter 20: Cardiovascular System: The Heart
 Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
BIOL 219 Spring Chapters 14&15 Cardiovascular System
 1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
A New Development Of Feedback Controller For Left Ventricular Assist Device
 University of entral Florida Electronic Theses and Dissertations Masters Thesis (Open Access) A New Development Of Feedback ontroller For Left Ventricular Assist Device 2 Yu Wang University of entral Florida
University of entral Florida Electronic Theses and Dissertations Masters Thesis (Open Access) A New Development Of Feedback ontroller For Left Ventricular Assist Device 2 Yu Wang University of entral Florida
Practice Exercises for the Cardiovascular System
 Practice Exercises for the Cardiovascular System On the diagram below, color the oxygen-rich blood red and the oxygen-poor blood blue. Label the parts: Continued on the next page... Label the parts on
Practice Exercises for the Cardiovascular System On the diagram below, color the oxygen-rich blood red and the oxygen-poor blood blue. Label the parts: Continued on the next page... Label the parts on
Design of an automated peripheral resistance
 Design of an automated peripheral resistance Renske Hoeben BMTE 08.44 October 2008 Supervisor: dr.ir. M.C.M. Rutten 1. Abstract To evaluate the function and the assist properties of ventricular assist
Design of an automated peripheral resistance Renske Hoeben BMTE 08.44 October 2008 Supervisor: dr.ir. M.C.M. Rutten 1. Abstract To evaluate the function and the assist properties of ventricular assist
THE CARDIOVASCULAR SYSTEM. Heart 2
 THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
Forward Looking Statement
 Forward Looking Statement This presentation contains forward-looking statements. All forward looking statements are management s (Dave Rosa) present expectations of future events and are subject to a number
Forward Looking Statement This presentation contains forward-looking statements. All forward looking statements are management s (Dave Rosa) present expectations of future events and are subject to a number
BME 5742 Bio-Systems Modeling and Control. Lecture 41 Heart & Blood Circulation Heart Function Basics
 BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
Application of a PExSim for modeling a POLVAD artificial heart and the human circulatory system with left ventricle assistance
 Pol J Med Phys Eng 2010;16(2):107-124. PL ISSN 1425-4689 doi: 10.2478/v10013-010-0010-z website: http://www.pjmpe.waw.pl Alicja Siewnicka, Bartlomiej Fajdek, Krzysztof Janiszowski Application of a PExSim
Pol J Med Phys Eng 2010;16(2):107-124. PL ISSN 1425-4689 doi: 10.2478/v10013-010-0010-z website: http://www.pjmpe.waw.pl Alicja Siewnicka, Bartlomiej Fajdek, Krzysztof Janiszowski Application of a PExSim
GIGA - In Silico Medicine, University of Liege, Belgium, 2
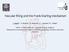 S. Kosta 1,*, A. Pironet 1, J.A. Negroni 2, E.C. Lascano 2, P.C. Dauby 1 1 GIGA - In Silico Medicine, University of Liege, Belgium, 2 Department of Comparative Cellular and Molecular Biology, Favaloro
S. Kosta 1,*, A. Pironet 1, J.A. Negroni 2, E.C. Lascano 2, P.C. Dauby 1 1 GIGA - In Silico Medicine, University of Liege, Belgium, 2 Department of Comparative Cellular and Molecular Biology, Favaloro
Achieving Physiologic Perfusion with Ventricular Assist Devices: Comparison of Control Strategies
 25 American Control Conference June 8-1, 25. Portland, OR, USA FrA13.3 Achieving Physiologic Perfusion with Ventricular Assist Devices: Comparison of Control Strategies Guruprasad Giridharan, George Pantalos,
25 American Control Conference June 8-1, 25. Portland, OR, USA FrA13.3 Achieving Physiologic Perfusion with Ventricular Assist Devices: Comparison of Control Strategies Guruprasad Giridharan, George Pantalos,
From PV loop to Starling curve. S Magder Division of Critical Care, McGill University Health Centre
 From PV loop to Starling curve S Magder Division of Critical Care, McGill University Health Centre Otto Frank 1890 s Frank-Starling Relationship ( The Law of the Heart ) The greater the initial stretch
From PV loop to Starling curve S Magder Division of Critical Care, McGill University Health Centre Otto Frank 1890 s Frank-Starling Relationship ( The Law of the Heart ) The greater the initial stretch
Cardiovascular Physiology
 Cardiovascular Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through two vascular systems
Cardiovascular Physiology Introduction The cardiovascular system consists of the heart and two vascular systems, the systemic and pulmonary circulations. The heart pumps blood through two vascular systems
VENTRICULAR assist devices (VAD) are mechanical
 IEEE TRANSACTIONS ON AUTOMATIC CONTROL, VOL. 43, NO. 6, JUNE 1998 765 Estimation of Systemic Vascular Bed Parameters for Artificial Heart Control Yih-Choung Yu, Student Member, IEEE, J. Robert Boston,
IEEE TRANSACTIONS ON AUTOMATIC CONTROL, VOL. 43, NO. 6, JUNE 1998 765 Estimation of Systemic Vascular Bed Parameters for Artificial Heart Control Yih-Choung Yu, Student Member, IEEE, J. Robert Boston,
The effect of captopril on the performance of the control strategies of BJUT II VAD
 DOI 10.1186/s12938-016-0247-1 BioMedical Engineering OnLine RESEARCH Open Access The effect of captopril on the performance of the control strategies of BJUT II VAD Kaiyun Gu, Bin Gao, Yu Chang * and Yi
DOI 10.1186/s12938-016-0247-1 BioMedical Engineering OnLine RESEARCH Open Access The effect of captopril on the performance of the control strategies of BJUT II VAD Kaiyun Gu, Bin Gao, Yu Chang * and Yi
The circulatory system
 Introduction to Physiology (Course # 72336) 1 הלב עקרונות בסיסיים (הכנה למעבדת לב) Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
Introduction to Physiology (Course # 72336) 1 הלב עקרונות בסיסיים (הכנה למעבדת לב) Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp , , , I. Major Functions of the Circulatory System
 P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
Introduction to Physiology (Course # 72336) 1. Adi Mizrahi Textbook Chapter 12
 Introduction to Physiology (Course # 72336) 1 עקרונות בסיסיים (הכנה למעבדת לב) הלב Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
Introduction to Physiology (Course # 72336) 1 עקרונות בסיסיים (הכנה למעבדת לב) הלב Adi Mizrahi mizrahia@cc.huji.ac.il Textbook Chapter 12 2 The circulatory system To the heart Away from the heart 3 L 2.5
PHYSIOLOGY MeQ'S (Morgan) All the following statements related to blood volume are correct except for: 5 A. Blood volume is about 5 litres. B.
 PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
-12. -Ensherah Mokheemer - ABDULLAH ZREQAT. -Faisal Mohammad. 1 P a g e
 -12 -Ensherah Mokheemer - ABDULLAH ZREQAT -Faisal Mohammad 1 P a g e In the previous lecture we talked about: - cardiac index: we use the cardiac index to compare the cardiac output between different individuals,
-12 -Ensherah Mokheemer - ABDULLAH ZREQAT -Faisal Mohammad 1 P a g e In the previous lecture we talked about: - cardiac index: we use the cardiac index to compare the cardiac output between different individuals,
254 IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 57, NO. 2, FEBRUARY 2010
 254 IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 57, NO. 2, FEBRUARY 2010 Parameter-Optimized Model of Cardiovascular Rotary Blood Pump Interactions Einly Lim, Socrates Dokos, Shaun L. Cloherty, Member,
254 IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 57, NO. 2, FEBRUARY 2010 Parameter-Optimized Model of Cardiovascular Rotary Blood Pump Interactions Einly Lim, Socrates Dokos, Shaun L. Cloherty, Member,
The relation between stroke work and end-diastolic volume in the ventricles
 Modelling in Medicine and Biology VI 123 The relation between stroke work and end-diastolic volume in the ventricles R. M. Shoucri Department of Mathematics and Computer Science, Royal Military College
Modelling in Medicine and Biology VI 123 The relation between stroke work and end-diastolic volume in the ventricles R. M. Shoucri Department of Mathematics and Computer Science, Royal Military College
Cardiovascular Physiology
 Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
The Cardiovascular System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 11 The Cardiovascular System Slides 11.1 11.19 Lecture Slides in PowerPoint by Jerry L. Cook The Cardiovascular System
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 11 The Cardiovascular System Slides 11.1 11.19 Lecture Slides in PowerPoint by Jerry L. Cook The Cardiovascular System
10. Thick deposits of lipids on the walls of blood vessels, called, can lead to serious circulatory issues. A. aneurysm B. atherosclerosis C.
 Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Chapter 20 (2) The Heart
 Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
*Generating blood pressure *Routing blood: separates. *Ensuring one-way blood. *Regulating blood supply *Changes in contraction
 *Generating blood pressure *Routing blood: separates pulmonary and systemic circulations *Ensuring one-way blood flow: valves *Regulating blood supply *Changes in contraction rate and force match blood
*Generating blood pressure *Routing blood: separates pulmonary and systemic circulations *Ensuring one-way blood flow: valves *Regulating blood supply *Changes in contraction rate and force match blood
Modeling and Simulation of Arterial Pressure Control
 Modeling and Simulation of Arterial Pressure Control EUGEN IANCU*, IONELA IANCU** * Department of Automation and Mechatronics ** Department of Physiology, Medical Informatics and Biostatistics University
Modeling and Simulation of Arterial Pressure Control EUGEN IANCU*, IONELA IANCU** * Department of Automation and Mechatronics ** Department of Physiology, Medical Informatics and Biostatistics University
Electrical Conduction
 Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
Sinoatrial (SA) node Electrical Conduction Sets the pace of the heartbeat at 70 bpm AV node (50 bpm) and Purkinje fibers (25 40 bpm) can act as pacemakers under some conditions Internodal pathway from
The Cardiovascular System
 The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
The Heart. Size, Form, and Location of the Heart. 1. Blunt, rounded point; most inferior part of the heart.
 12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
How does the heart pump? From sarcomere to ejection volume
 How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
PROBLEM SET 2. Assigned: February 10, 2004 Due: February 19, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
IP: Regulation of Cardiac Output
 ANP 1105D Winter 2013 Assignment 9: The Heart, part 2: Chap... Assignment 9: The Heart, part 2: Chapter 18 Signed in as Alex Sokolowski Help Close Resources Due: 11:59pm on Monday, March 25, 2013 Note:
ANP 1105D Winter 2013 Assignment 9: The Heart, part 2: Chap... Assignment 9: The Heart, part 2: Chapter 18 Signed in as Alex Sokolowski Help Close Resources Due: 11:59pm on Monday, March 25, 2013 Note:
Cardiac Output (C.O.) Regulation of Cardiac Output
 Cardiac Output (C.O.) Is the volume of the blood pumped by each ventricle per minute (5 Litre) Stroke volume: Is the volume of the blood pumped by each ventricle per beat. Stroke volume = End diastolic
Cardiac Output (C.O.) Is the volume of the blood pumped by each ventricle per minute (5 Litre) Stroke volume: Is the volume of the blood pumped by each ventricle per beat. Stroke volume = End diastolic
CHAPTER 4 Basic Physiological Principles
 4-1 CHAPTER 4 Basic Physiological Principles Now that we have a working anatomical knowledge of the heart and circulatory system, we will next develop a functional and quantitative knowledge of the cardiovascular
4-1 CHAPTER 4 Basic Physiological Principles Now that we have a working anatomical knowledge of the heart and circulatory system, we will next develop a functional and quantitative knowledge of the cardiovascular
MECHANICAL PROPERTIES OF THE HEART I & II
 MECHANICAL PROPERTIES OF THE HEART I & II Recommended Reading: Guyton, A. Textbook of Medical Physiology, 1 th Edition. Chapters 9, 14, 2. Berne & Levy. Principles of Physiology. 4 th Edition. Chapter
MECHANICAL PROPERTIES OF THE HEART I & II Recommended Reading: Guyton, A. Textbook of Medical Physiology, 1 th Edition. Chapters 9, 14, 2. Berne & Levy. Principles of Physiology. 4 th Edition. Chapter
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 20 The Cardiovascular System: The Heart Introduction The purpose of the chapter is to: 1. Learn about the components of the cardiovascular system
Principles of Anatomy and Physiology 14 th Edition CHAPTER 20 The Cardiovascular System: The Heart Introduction The purpose of the chapter is to: 1. Learn about the components of the cardiovascular system
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
Blood Pressure Regulation. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Καθετηριασμός δεξιάς κοιλίας. Σ. Χατζημιλτιάδης Καθηγητής Καρδιολογίας ΑΠΘ
 Καθετηριασμός δεξιάς κοιλίας Σ. Χατζημιλτιάδης Καθηγητής Καρδιολογίας ΑΠΘ The increasing interest in pulmonary arterial hypertension (PAH), the increasing interest in implantation of LVADs, and the evolution
Καθετηριασμός δεξιάς κοιλίας Σ. Χατζημιλτιάδης Καθηγητής Καρδιολογίας ΑΠΘ The increasing interest in pulmonary arterial hypertension (PAH), the increasing interest in implantation of LVADs, and the evolution
Physiology lecture 15 Hemodynamic
 Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
Physiology lecture 15 Hemodynamic Dispensability (D) : proportional change in volume per unit change in pressure D = V/ P*V It is proportional (divided by the original volume). Compliance (C) : total change
A PARADIGM FOR QUANTIFYING VENTRICULAR CONTRACTION USA
 CELLULAR & MOLECULAR BIOLOGY LETTERS 331 A PARADIGM FOR QUANTIFYING VENTRICULAR CONTRACTION JOSEPH L. PALLADINO 1 and ABRAHAM NOORDERGRAAF 2 1 Department of Engineering, Trinity College, Hartford, CT,
CELLULAR & MOLECULAR BIOLOGY LETTERS 331 A PARADIGM FOR QUANTIFYING VENTRICULAR CONTRACTION JOSEPH L. PALLADINO 1 and ABRAHAM NOORDERGRAAF 2 1 Department of Engineering, Trinity College, Hartford, CT,
FUNDAMENTALS OF HEMODYNAMICS, VASOACTIVE DRUGS AND IABP IN THE FAILING HEART
 FUNDAMENTALS OF HEMODYNAMICS, VASOACTIVE DRUGS AND IABP IN THE FAILING HEART CINDY BITHER, MSN, ANP, ANP, AACC, CHFN CHIEF NP, ADV HF PROGRAM MEDSTAR WASHINGTON HOSPITAL CENTER CONFLICTS OF INTEREST NONE
FUNDAMENTALS OF HEMODYNAMICS, VASOACTIVE DRUGS AND IABP IN THE FAILING HEART CINDY BITHER, MSN, ANP, ANP, AACC, CHFN CHIEF NP, ADV HF PROGRAM MEDSTAR WASHINGTON HOSPITAL CENTER CONFLICTS OF INTEREST NONE
Modeling, Estimation and Control of Cardiovascular Systems with A Left Ventricular Assist Device
 25 merican Control Conference June 8-, 25. Portland, OR, US Fr3.6 Modeling, Estimation and Control of Cardiovascular Systems with eft entricular ssist Device Yi Wu, Paul llaire 2, Gang Tao 3 and Don Olsen
25 merican Control Conference June 8-, 25. Portland, OR, US Fr3.6 Modeling, Estimation and Control of Cardiovascular Systems with eft entricular ssist Device Yi Wu, Paul llaire 2, Gang Tao 3 and Don Olsen
Cardiovascular System
 Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Properties of Pressure
 OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
OBJECTIVES Overview Relationship between pressure and flow Understand the differences between series and parallel circuits Cardiac output and its distribution Cardiac function Control of blood pressure
UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS
 UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS CARDIAC MECHANICAL SUPPORT PROGRAM GUIDELINES CARDIAC MECHANICAL SUPPORT: LVAD BASICS FREQUENT SCENARIOS AND TROUBLESHOOTING Review Date: July 2011
UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS CARDIAC MECHANICAL SUPPORT PROGRAM GUIDELINES CARDIAC MECHANICAL SUPPORT: LVAD BASICS FREQUENT SCENARIOS AND TROUBLESHOOTING Review Date: July 2011
CIRCULATORY SYSTEM BLOOD VESSELS
 Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
The elastance model implemented in a left ventricle finite element model. Pim van Ooij TU Eindhoven BMTE Juni 2006
 The elastance model implemented in a left ventricle finite element model Pim van Ooij TU Eindhoven BMTE 06.29 Juni 2006 1 Summary Since heart diseases are the second largest cause of death in the western
The elastance model implemented in a left ventricle finite element model Pim van Ooij TU Eindhoven BMTE 06.29 Juni 2006 1 Summary Since heart diseases are the second largest cause of death in the western
The Arterial and Venous Systems Roland Pittman, Ph.D.
 The Arterial and Venous Systems Roland Pittman, Ph.D. OBJECTIVES: 1. State the primary characteristics of the arterial and venous systems. 2. Describe the elastic properties of arteries in terms of pressure,
The Arterial and Venous Systems Roland Pittman, Ph.D. OBJECTIVES: 1. State the primary characteristics of the arterial and venous systems. 2. Describe the elastic properties of arteries in terms of pressure,
The Cardiovascular System
 11 PART A The Cardiovascular System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Cardiovascular
11 PART A The Cardiovascular System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Cardiovascular
Georgios C. Bompotis Cardiologist, Director of Cardiological Department, Papageorgiou Hospital,
 Georgios C. Bompotis Cardiologist, Director of Cardiological Department, Papageorgiou Hospital, Disclosure Statement of Financial Interest I, Georgios Bompotis DO NOT have a financial interest/arrangement
Georgios C. Bompotis Cardiologist, Director of Cardiological Department, Papageorgiou Hospital, Disclosure Statement of Financial Interest I, Georgios Bompotis DO NOT have a financial interest/arrangement
Anatomy Review: The Heart Graphics are used with permission of A.D.A.M. Software, Inc. and Benjamin/Cummings Publishing Co.
 Anatomy Review: The Heart Graphics are used with permission of A.D.A.M. Software, Inc. and Benjamin/Cummings Publishing Co. Anatomy Views Label the diagrams of the heart below: Interactive Physiology Study
Anatomy Review: The Heart Graphics are used with permission of A.D.A.M. Software, Inc. and Benjamin/Cummings Publishing Co. Anatomy Views Label the diagrams of the heart below: Interactive Physiology Study
ThinkIR: The University of Louisville's Institutional Repository
 University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 7-2011 RPM and flow modulation for a continuous flow left ventricular assist
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 7-2011 RPM and flow modulation for a continuous flow left ventricular assist
Contents 1 Computational Haemodynamics An Introduction 2 The Human Cardiovascular System
 Contents 1 Computational Haemodynamics An Introduction... 1 1.1 What is Computational Haemodynamics (CHD)... 1 1.2 Advantages of CHD... 3 1.3 Applications in the Cardiovascular System... 4 1.3.1 CHD as
Contents 1 Computational Haemodynamics An Introduction... 1 1.1 What is Computational Haemodynamics (CHD)... 1 1.2 Advantages of CHD... 3 1.3 Applications in the Cardiovascular System... 4 1.3.1 CHD as
Cardiovascular System: The Heart
 Cardiovascular System: The Heart I. Anatomy of the Heart (See lab handout for terms list) A. Describe the size, shape and location of the heart B. Describe the structure and function of the pericardium
Cardiovascular System: The Heart I. Anatomy of the Heart (See lab handout for terms list) A. Describe the size, shape and location of the heart B. Describe the structure and function of the pericardium
Outline. Electrical Activity of the Human Heart. What is the Heart? The Heart as a Pump. Anatomy of the Heart. The Hard Work
 Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Detection of Cardiovascular Anomalies: An Observer-Based Approach. Thesis by Fernando Díaz Ledezma, B.S. in Mechatronics Engineering
 Detection of Cardiovascular Anomalies: An Observer-Based Approach Thesis by Fernando Díaz Ledezma, B.S. in Mechatronics Engineering Submitted in Partial Fulfillment of the Requirements for the degree of
Detection of Cardiovascular Anomalies: An Observer-Based Approach Thesis by Fernando Díaz Ledezma, B.S. in Mechatronics Engineering Submitted in Partial Fulfillment of the Requirements for the degree of
Circulation: Chapter 25. Cardiac Output. The Mammalian Heart Fig Right side of the heart
 Circulation: Chapter 25 1. Limits of Diffusion A. Small organisms use diffusion B. rapid over small distances 2. Most animals have circulatory systems A. Blood B. Pump (Heart) or propulsive structures
Circulation: Chapter 25 1. Limits of Diffusion A. Small organisms use diffusion B. rapid over small distances 2. Most animals have circulatory systems A. Blood B. Pump (Heart) or propulsive structures
Impedance Cardiography (ICG) Application of ICG for Hypertension Management
 Application of ICG for Hypertension Management 1mA @ 100 khz Impedance Cardiography (ICG) Non-invasive Beat-to-beat Hemodynamic Monitoring Diastole Systole Aortic valve is closed No blood flow in the aorta
Application of ICG for Hypertension Management 1mA @ 100 khz Impedance Cardiography (ICG) Non-invasive Beat-to-beat Hemodynamic Monitoring Diastole Systole Aortic valve is closed No blood flow in the aorta
BioMedical Engineering OnLine 2013, 12:8
 BioMedical Engineering OnLine This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A multi-scale
BioMedical Engineering OnLine This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A multi-scale
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS
 HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS TABLE OF CONTENTS Welcome... 3 HVAD Waveforms 1. Characteristics... 4 2. Theory of Operation... 5 3. Ao & LV Pressure... 6 4. HQ Curve... 7 5. PV Loops... 8 Home
HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS TABLE OF CONTENTS Welcome... 3 HVAD Waveforms 1. Characteristics... 4 2. Theory of Operation... 5 3. Ao & LV Pressure... 6 4. HQ Curve... 7 5. PV Loops... 8 Home
Cardiovascular Structure & Function
 Cardiovascular Structure & Function Cardiovascular system: The heart Arteries Veins Capillaries Lymphatic vessels Weighting of the heart ceremony: Ancient Egyptians William Harvey and Blood Flow April
Cardiovascular Structure & Function Cardiovascular system: The heart Arteries Veins Capillaries Lymphatic vessels Weighting of the heart ceremony: Ancient Egyptians William Harvey and Blood Flow April
