ARTIFICIAL HEART VALVES
|
|
|
- Sherilyn Copeland
- 6 years ago
- Views:
Transcription
1 ARTIFICIAL HEART VALVES 1. INTRODUCTION DAN T. SIMIONESCU Clemson University Clemson, South Carolina The cardiovascular system is a closed circulatory system that ensures blood flow through the human body. Blood circulates through two systems; the first is responsible for pushing blood from the heart to the lungs to capture oxygen, and the second is responsible for distributing oxygenated blood to body organs. This system is composed of a double pump with branched tubes that leads blood from the heart to the organs (arteries) and returns it to the heart (veins). The heart is essentially a muscular pump with four rooms, two for receiving incoming blood (atria) and two for pushing blood toward the organs (ventricles). In order to maintain unidirectional flow of blood, 3-D open-close gateways (valves) are optimally located between atria and ventricles, and between the ventricles and the emerging arteries. The workload of heart valves is nothing short of extraordinary. The valves open and close once per second totaling more than 3 billion times in a lifetime. These movements take place under constant pressure and in flowing blood, a very viscous fluid rich in minerals, proteins, lipids, and cells. Valves may progressively become defective and critically influence the performance of the heart. These defects are collectively named valve diseases. As the properties of normal and diseased valves are not sufficiently understood, no drugs are available to treat and cure valve diseases. The only curative solution is to perform open-heart surgery to replace diseased valves with artificially engineered devices. These valve substitutes are nonliving materials fashioned in the form of valves that reestablish initial mechanical functions and restore the functionality of the heart. However, these devices may eventually fail because of imperfections in design, composition, or biocompatibility. Once a device shows signs of failure, the patient requires a second open-heart surgery for replacement of the defective device. Reoperations are generally risky and pose additional problems for children who naturally outgrow their implants. Thus, the long-term goal of biomedical engineering is to find better methods for treating valvular diseases and thus impact lives of millions of patients worldwide. This daunting effort brings together a multidisciplinary team of specialists in medicine, biology, engineering, and mechanics. This chapter describes the structure, function, biology, and pathology of heart valves; information on current replacement devices; and the necessary prerequisites for constructing an ideal replacement valve. Ongoing research is aimed at improving existing devices by enhancing biocompatibility, as well as pioneering work on novel tissue-engineering approaches, which would facilitate complete regeneration of valve tissues. 2. NATURAL HEART VALVES ARE SPECIALIZED CARDIOVASCULAR TISSUES The mechanical action of the human heart is similar to that of the two-cylinder engine. Four natural heart valves maintain the direction of blood flow within the circulatory system (Fig. 1). The tricuspid and mitral valves (collectively called the atrioventricular valves) open to allow blood to fill the ventricular cavities. These two admission (inflow) valves are connected to the subjacent heart muscle via fibrous extensions (chordae tendinae). When the inflow valves are closed, blood is forced to flow through the aortic and pulmonary valves (collectively called the semilunar valves). These two ejection (outflow) valves open in response to blood flow and immediately close after ejection. Although not initially apparent, a structural and functional correlation between the heart muscle and the valves exists (Fig. 1). A 3-D network of extra cellular matrix (mainly collagen fibers), formally known as the cardiac skeleton, maintains the spatial structure and function of the heart muscle. This cardiac skeleton includes large structures (macro-skeleton), such as the Mi P Ao Tri Tri P Ao Mi Macroskeleton Fibrous ring Matrix Tri Tricuspid P Pulmonary Ao Aortic Mi Mitral Figure 1. Functional anatomy of the heart valves. Longitudinal section diagram depicting venous blood (dark blue arrows) collecting in the right ventricle via the tricuspid valve (Tri) and sent through the pulmonary valve (P) to the lungs. Oxygenated blood (red arrows) returns to the left ventricle via the mitral valve (Mi) and is directed to the organs via the aortic valve (Ao). Insert at left shows a cross section through the heart at the level of the four heart valves. Microskeleton 1 Wiley Encyclopedia of Biomedical Engineering, Copyright & 2006 John Wiley & Sons, Inc.
2 2 ARTIFICIAL HEART VALVES heart valves, the chordae tendinae, and the septum, and a fibrous micro-skeleton of connective tissue, which comprises the entire collagenous network that encloses heart muscle fibers. Besides providing structural support for the cells, the cardiac skeleton allows a mechanical coupling function [i.e., the concomitant contraction of all muscle fibers during ventricular contraction (systole)] (1). The heart valves are structurally composed of thin sheets of tissue (cusps or leaflets), which represent anatomical extensions of the cardiac endocardium (Fig. 2). These cusps are strengthened with a hydrophobic, collagenous, load-bearing tissue layer (fibrosa) that maintains continuity with the fibrous ring into which it is inserted (2). Elastic fibers that provide resilience and recoil are predominantly present in the inflow layers of the heart (e.g., the ventricularis of the semilunar valves) (3). Between these two layers is a loose connective tissue comprised predominantly of hydrophilic, shock-absorbing glycosaminoglycans (spongiosa) (4). This gel-like matrix acts as a space-filler and a lubricant to permit continuous bending, rearranging, straightening, and rotating of structural fibers with minimal friction (5). In addition to these explicit biochemical and 3D constructs, specific cells exhibiting metabolic and contractile Atrial endocardium Ann Fibr Aortic media Ann Fibr Auricularis Chordae Ventricular endocardium Aortic intima Arterialis Ventricularis Ventricular endocardium Spongiosa Fibrosa Fibrosa Ventricularis Spongiosa Atrium Ventricle Aorta Ventricle Figure 2. General structure of the heart valves. Representative schematic depicting a simplified cross section through an atrioventricular valve (top) and a semilunar valve (bottom); direction of blood flow is shown by thick arrow. Note the central supporting structure (fibrosa) inserted in the fibrous ring (Ann. Fibr.) and the presence of shock-absorbing layers (spongiosa), which are structural extensions of the cardiac endocardium. properties are also found in each heart valve, which include matrix-producing cells known as fibroblasts, active valvular interstitial cells known as myofibroblasts, and endothelium and resident macrophages (Fig. 3). Valvular cells exhibit high metabolic activities related to matrix homeostasis (synthesis and degradation of collagen and proteoglycans) as well as reactivity toward vasoactive compounds such as epinephrine and angiotensins. Therefore, this multivariate cell design clearly indicates that specific cellular activities characterize the form and function of each valve, which permits continuous adaptation to subtle hemodynamic modifications (6). In conclusion, heart valves are unique, specialized components of the cardiac connective tissue network that rely on delicately balanced homeostatic activities for their mechanical and biological functions. 3. HEART VALVE DISEASES ARE CURED BY SURGICAL REPLACEMENT Valvular pathology is an important aspect of cardiovascular diseases. Mechanically speaking, valve dysfunction can be expressed as either imperfect closure (insufficiency) or incomplete opening (stenosis). In the majority of pathological cases, valvular tissue appears either excessively deformed (floppy) or very thick and rigid, characterized by leaflet calcification (Fig. 4). The importance of the structural/functional connection of the heart muscle and the valves is clearly demonstrated by known pathologic situations. For example, in aortic stenosis, the heart muscle attempts to compensate for deficiencies in blood flow by growing in mass (hypertrophy). Consequently, regression of myocardial mass after valve replacement is one of the major clinical indicators of effective treatment of valvular pathology (7). Valvular diseases associate with cell activation and alterations in metabolism of collagen and proteoglycans. These structural changes induce functional modifications that eventually lead to malfunction. The causes of valvular diseases are typically associated with congenital defects, atherosclerosis, infections, or postrheumatic episodes (6). However, the mechanisms of onset and progression of valvular diseases are largely unknown, specifically because of the lack of adequate experimental models. Heart valve diseases progress rapidly and may become fatal. As the majority of heart valve disease is irreversible, its progression cannot be prevented by pharmacological treatments. Moreover, damaged heart valves lack the ability to spontaneously regenerate (8). The only treatment option presently available is open-heart surgery, in which the diseased valve is removed and replaced with an artificial device. Although effective, this procedure is quite traumatic for the patient. For a fortunate few, however, small areas of the valve can be reconstructed or repaired using biomaterials, obviating the need for major surgery to install new artificial heart valves. Pathological heart valves exhibit significantly altered mechanical and biological properties that eventually require surgical replacement with artificial devices. For an excellent update on
3 ARTIFICIAL HEART VALVES 3 Atrium Ventricle Aorta Ventricle Legend Myocytes Smooth muscle cells Endothelium Valvular int. cells Fibroblasts Macrophages Figure 3. Cell types and distribution in heart valves. Representative schematic depicting a simplified cross section through an atrioventricular valve (top) and a semilunar valve (bottom); direction of blood flow is shown by arrow. Myocytes and smooth muscle cells populate the base of the valve indicating a transition area from a muscular structure to a specialized fibrous connective tissue. Fibroblasts and interstitial cells mainly populate the load-bearing valve structures; valves are fully covered by a continuous layer of endothelial cells. (a) (b) Figure 4. Pathology of human mitral valves (viewed from the atrial side). Photo depicting (a) an apparently normal valve, with some signs of leaflet thickening (o); (b) stenotic valve with signs of thickening (o); and extensive calcium deposits (m) in both (c) and (d) with signs of chordae thickening. (c) (d) heart valve pathology, please refer to the work of F. J. Schoen (6). 4. ARTIFICIAL HEART VALVES ARE EXCELLENT MECHANO- MIMETIC DEVICES Heart valve replacement, pioneered in the early 1970s, is now a routine surgical procedure that employs devices made of nonliving, nonresorbable biomaterials for substitution of the valvular mechanical functions (9). Replacement of heart valves offers an excellent improvement in the quality of life for thousands of patients and can be considered one of the major accomplishments of biomedical engineering. It is estimated that more than 275,000 replacement heart valves are implanted annually worldwide; thus the social and economic impact of heart valves research and development is considerable (10).
4 4 ARTIFICIAL HEART VALVES Engineered devices, or mechanical valves, are used to replace diseased human heart valves in approximately 50% of the cases. Valves made of processed biological tissues are used in an additional 45% of cases. Pulmonary autograft valves (whereby the patient s own pulmonary valve is transplanted into the aortic position) and human cryopreserved allograft valves represent the remainder of implanted valves. Autografts and allografts exhibit excellent durability after implantation, but are not readily available for all patients (11). Mechanical heart valves are constructed from rigid supporting materials and mobile components whose designs range from that of a caged ball device to designs encompassing free-moving tilting discs or flaps with restricted movement (Fig. 5). The components ensuring proper opening and closing of valvular orifices are made of inert, biocompatible materials such as pyrolitic carbon, polyester (Dacron)-covered polymers, or metals (12). Blood pressure differences within the chambers of the heart enable these mechanical valves to properly function. For example, for a caged ball valve inserted in the aortic position, contraction of the left ventricle will create sufficient blood pressure to thrust the ball toward the opposite end of the cage, thus allowing blood to flow around the ball. Immediately after systole (ventricular contraction), the ventricular pressure decreases and the ball is pushed toward the circular valve base, closing the orifice completely. However, imperfect blood flow through some of these devices can create nonphysiological patterns that may induce the formation of blood clots. Furthermore, the surfaces exposed directly to blood flow are not entirely antithrombogenic. For these reasons, in order to avoid complications caused by thrombus formation, patients with mechanical heart valves are required to take anticoagulation medications for the rest of their lives (13). As such, because patients may have possible episodic internal bleeding, mechanical valves are of limited use for pregnant women or women considering pregnancy and patients with coagulation diseases. Biological heart valves (bioprostheses) are made of porcine aortic valves or bovine pericardial sheets that are mounted on adequate supports (stents) to mimic the (c) (a) (b) (d) (e) (f) Figure 5. Artificial heart valves. Photo showing mechanical valves including devices that employ a caged ball (a) or two hinged leaflets (b) for functioning. Tissue valves are either made from bovine pericardium (c), porcine heart valves mounted on stents (d), or left unmounted (e and f). Valves in (a), (c) (e) are shown in closed position, and valve (b) in a partially open position. Images (a), (c), and (f) were provided courtesy of Edwards Lifesciences, Inc. Models depicted are: Starr-Edwardss Ball Valves (a), Carpentier-Edwardss PERIMOUNT Magnas Pericardial Bioprosthesis (c), and Edwards Prima Pluss Stentless Bioprosthesis (f). Copyright Edwards Lifesciences, Inc All rights reserved. Images (b), (d), and (e) were provided courtesy of St. Jude Medical, Inc. Models depicted are: St Jude Medicals Regent Valve (b), Biocor Aortic (d), and Toronto SPV (e). Copyright St. Jude Medical, Inc All rights reserved.
5 ARTIFICIAL HEART VALVES 5 valvular architecture, or they are left unmounted (stentless) (Fig. 5) (14). To understand how these devices are created, a typical procedure for creating a pericardiumderived heart valve is shown in Fig. 6. Bovine pericardium (the external fibrous sac that encloses the heart) is collected at the slaughterhouse, chemically stabilized, cut into three leaflets, and mounted on a three-legged (stented) ring using surgical sutures. The final product is adapted with a circular ring at the base that allows the surgeon to secure the device to the human fibrous ring. Similar to natural valves, the function of bioprostheses is pressure-driven. When the blood pressure below the valve is greater than the pressure above, the leaflets are forced toward the outside, thus reversing curvature and causing the valve to open. When the blood pressure above the valve is greater than the pressure below, the valve closes by pushing the leaflets toward the center. As the total surface of the leaflets exceeds that of the orifice, the leaflets overlap in the center (coaptation) and allow for complete closure of the orifice, without backflow (regurgitation). Cross-species implantation of animal tissues is clearly prone to severe immune rejection and rapid tissue degeneration. For this reason, bovine or porcine tissues are treated with glutaraldehyde, a water-soluble cross-linker, which almost completely reduces tissue antigenicity (15). In addition, glutaraldehyde devitalizes tissues and kills all resident cells, prevents degradation by host enzymes, and sterilizes the tissue for implantation. Interestingly, the use of glutaraldehyde was inspired from its use in microscopy (as a fixative) and as a hospital sterilant (in the form of Cidex, a commonly used bactericidal liquid sterilization medium for heat-sensitive materials) (16). Tissuederived heart valves are less thrombogenic than their mechanical counterparts and do not require long-term anticoagulation. For an excellent overview of different models and properties of heart valves, please refer to the work of J. Butany et al. (14). Overall, mechanical and tissue-derived heart valves perform exceptionally well as devices intended to open and close valvular orifices, and thus could be considered as admirable mechano-mimetic replacements. 5. ARTIFICIAL HEART VALVES HAVE A LIMITED BIOLOGICAL DURABILITY Artificial heart valves function quite effectively for many years after implantation, but their long-term durability is quite limited. Clinical follow-ups indicate that more than 50% of patients with artificial valve implants develop complications within 10 years (6). This disturbing trend suggests that the majority of implanted valves would have to be explanted after 20 years. From the perspective of the valvular patients, a second open-heart surgery to retrieve and replace the defective device is prone to high clinical risks, and therefore undesirable. The lack of long-term (a) (b) (c) (d) (e) (f) Figure 6. Artificial heart valve from bovine pericardium. Diagram showing the steps involved in creating a heart valve device. Bovine pericardium is collected (a) then crosslinked in glutaraldehyde (b), fashioned into three leaflets (c), mounted on a stent (d), and fitted with a sewing ring. While opening, blood flow (arrow) pushes leaflets toward the outside (e) and creates an almost circular orifice (insert). Upon closure, leaflets are pushed toward the center of the valve (f) and complete closure is ensured by central coaptation of leaflets (insert).
6 6 ARTIFICIAL HEART VALVES Figure 7. Pathology of tissue heart valves. Diagram showing typical aspects observed in explanted devices, including (1) tissue ruptures, (2) abrasions, (3) thickening of the tissue, (4) calcification, and (5) host tissue overgrowth. 5 valve durability is especially important in the case of pediatric patients, where supplemental surgical procedures are required to accommodate the natural growth of the patients. The choice between bioprosthetic and mechanical valves depends on specific patient characteristics. Mechanical valves are more durable but require life-long anticoagulant therapy, whereas bioprostheses tend to deteriorate more rapidly because of degenerative processes but do not require anticoagulation therapy. In general, tissue valves are implanted in elderly patients (60 65 years or older), which have a lower tendency to calcify tissue implants and a mean life expectancy of 10 to 15 years (17). Conversely, more mechanical valves are implanted in younger patients and children. Analysis of explanted valves revealed that the principal causes of device failure are thrombosis, degeneration, and lack of full integration into host tissue, perivalvular leaks, host tissue overgrowth, and susceptibility to infections. Complications related to thrombogenicity are the major problems of mechanical valves, whereas tissue-derived valves are mainly affected by the degeneration and calcification of the tissue components (18). For example, explanted pericardial valves reveal tissue abrasion and erosion, tearing and perforations, deformations and loss of bending stiffness, along with deposition of calcium minerals (Fig. 7). Tissue degeneration in artificial heart valves occurs as a result of the interplay between host-related factors and implant-related factors. As a response to the implant, the host selects one or more defense mechanisms that correspond to the degree of activation induced by the implant. Implantation of tissue heart valves induces a chronic foreign-body response, a low degree of immune reaction, activation of the coagulation cascade, and inflammatory response. These reactions are translated into deposition of fibrin immunoglobulins and complement infiltration of activated macrophages and lymphocytes and growth of host fibrous tissue over nonmoving parts of the implant (18). The main pathways of bioprosthetic heart valve failure are structural damage and calcification of the tissue component. Analysis of explanted (failed) bioprosthetic heart valves typically reveals the coexistence of structural damage and calcification. Structural damage may be pure (noncalcific), stress-induced disruption of fiber architecture (19), or mediated by enzymatic degradation (20). Mechanical deformation was shown to accelerate calcification and, conversely, calcific deposits significantly alter mechanical properties (21). These processes may be synergistic, but convincing evidence also exists that each may occur independently. Many improvements in design and geometry of the leaflets have reduced, but not fully eliminated, the incidence of mechanical damage. For an excellent overview of heart valve design issues, please refer to the work of I. Vesely (22). Calcium deposition in tissue-derived artificial heart valves is one of the major causes of their clinical failure. For this reason, noteworthy efforts have been made to elucidate the mechanisms of valve calcification and to implement treatments that would prevent this unwarranted side effect (23). Calcification typically appears as granular, fibrillar, or lamellar deposits, which greatly reduce tissue mechanical properties and can effectively contribute to erosion, abrasions, tearing, and perforations. The major factors involved in calcification are young age, hypercholesterolemia, and flexural stress, implant composition, and chemical pretreatments of the biological tissues. Calcification may occur through passive mechanisms (direct deposition of calcium and phosphate) but host cells, via remodeling mechanisms involving proteasemediated degeneration of matrix components and deposition of bone proteins, can mediate it (24). Mechanisms underlying calcification of tissue-derived artificial heart valves in patients are still not fully understood, although tissue composition and the use of glutaraldehyde appear to be two essential factors. In most cases analyzed thus far, the mineral phase is composed of bone-like hydroxyapatite associated with collagen, elastin, and chemically devitalized cells. Ongoing research focuses on prevention of calcification in tissue-derived artificial heart valves by attempts to remove or extract cells, by structural modification of collagen and elastin, and by stabilization or addition of natural calcification inhibitors (25,26). Glutaraldehyde induces adequate preservation of collagenous structures, but also triggers severe cell alterations, which result in the formation of cell debris that resemble matrix vesicles formed by bone cells. For this reason, treatments are under study to extract all cellular components before implantation (23). Residual loosely bound or unreacted aldehyde groups may also be involved in tissue calcification. Neutralization of free aldehyde groups with compounds that possess reactive amines such as glutamine, glycine, homocysteic acid, and lysine has been shown to reduce calcification in animal models. In addition to simple aldehyde neutralization, amino-oleic acid, a treatment recently implemented in clinical use, may also hinder calcification by mechanisms related to reduction of calcium diffusion through tissues (27). Additionally, amino-biphosphonates (28) may also act as crystal growth inhibitors to hinder calcification. A different approach involving the use of stabilization chemistries, which do not employ glutaraldehyde, is also under investigation. These chemistries include carbodiimides, dyemediated photo fixation, and epoxy-based crosslinkers (29). Tissues cross-linked with nonglutaraldehyde agents do not calcify in experimental models to the same extent as glutaraldehyde-fixed tissues, thus directly linking the
7 ARTIFICIAL HEART VALVES 7 presence of glutaraldehyde as one direct cause of tissue calcification. Extensive research on alternative cross-linking methods is currently being pursued, but none have yet reached clinical use. These results indicate that mechanical and biological factors contribute significantly to failure of artificial heart valves. Although they perform well mechanically, artificial heart valves do not possess sufficient biological assets to fulfill the requirements of true biomimetic devices. Their lack of biologic properties may account for their limited durability after implantation. As artificial heart valves lack live cells, they are deficient in their ability to maintain and adapt the valvular matrix composition or to maintain an adequate calcium homeostasis. In the absence of live cells, degenerative processes induced by mechanical fatigue, proteolytic enzymes, and calcium deposition slowly erode the structural components and lead to progressive valve deterioration. In addition, the lack of an intact layer of endothelial cells may allow free influx of blood components and could also contribute to device-related thrombogenicity. Finally, the majority of current artificial heart valves, except newer stentless valves, do not maintain their cardiac skeleton-valve continuity and thus may progressively impair heart function. In conclusion, pathological artificial valves exhibit altered mechanical properties, similar to human pathological valves. However, exciting research is currently ongoing to improve these devices and increase their biological durability. It is expected that efforts targeted toward understanding the pathology of artificial heart valves will facilitate greater insights into human valvular pathology. 6. THE IDEAL ARTIFICIAL HEART VALVE One of the greatest biomedical engineering challenges today is to develop an implantable device that resists the natural conditions to which heart valves are subjected, without eliciting host reactions that would impair their function. This research field is very exciting and poses abundant questions whose answers will positively impact the lives millions of patients. Specifically, these natural conditions affecting the lifespan of an artificial heart valve include their durability to 40 million beats per year for about 80 years (summing up to about 3.2 billion cycles in a lifetime) under a cyclical pressure of about 200 g/cm 2 in a very aggressive, corrosive fluid of high viscosity. Other research endeavors include testing valves within elevated concentrations of calcium and phosphate, proteins, lipids, enzymes, and cells, in the presence of potentially destructive defense mechanisms such as the immune system, coagulation cascade, inflammation, encapsulation, and calcification (30). This ongoing research has initially concluded that the ideal artificial heart valve should fulfill the following specific clinical, mechanical, and biological prerequisites. These prerequisites include, but are not limited to: 1. Full valve closure and release, and an internal orifice area close to the natural valve; 2. Limited resistance to blood flow while moving, and laminar flow through valve; 3. Mechanically durable, resistant to wear, maintain properties throughout lifetime; 4. Perfect integration into host tissue with minimal healing responses; 5. Chemically stable, nonleachable, noncytotoxic components; 6. Nonhemolytic, nonthrombogenic, substrate not supportive of infections; 7. Nonimmunogenic, noninflammatory, noncalcifying; 8. Long-term shelf life without changes in properties and sterility; 9. Continuity with the cardiac skeleton; 10. Ease of implantation, preferably using minimally invasive surgery; 11. Available in all sizes and reasonably priced. To assess these properties, artificial heart valves are rigorously tested in vitro and in vivo, before being approved for human use. In vitro testing includes analysis of flow patterns by computer modeling and evaluation of hydrodynamic functions using ventricle simulators (31). In addition, valve components are routinely tested for mechanical properties such as elasticity, tensile strength, and wear resistance. For long-term durability evaluation, valves are subjected to fatigue testing at accelerated rates (up to 20 times faster that normal heart rate) (32). In vivo tests include subdermal implantation of tissue valve components for calcification studies (33) and valve replacement in sheep (34). Currently, no artificial heart valve device, either mechanical or tissue-derived, fulfills all those previously described prerequisites. No material produced by the human imagination or through cutting-edge technology available can fully reproduce the complexity of the natural heart valve. However, the possibilities for creating such a naturally complex construct are endless if biomaterials or approaches can be developed that, instead of waging war with biology, will promote perfect integration within local and systemic physiologic systems. One such promising avenue is the regenerative medicine/tissue engineering approach. 7. REGENERATIVE MEDICINE APPROACHES TO HEART VALVE REPLACEMENT The field of regenerative medicine is based on the innovative and visionary principle of using the patient s own cells and extracellular matrix components to restore or replace tissues and organs that have failed. This regenerative approach is a derivative of reconstructive surgery, where surgeons use the patient s tissues to rebuild injured or aging body parts. Modern approaches to heart valve regenerative medicine include several research methodologies, collectively known as tissue engineering. The most intensely researched approaches are (1) the use of decellularized tissues as scaffolds for in situ regeneration, (2)
8 8 ARTIFICIAL HEART VALVES construction of tissue equivalents in the laboratory before implantation, and (3) use of scaffolds preseeded with stem cells. A widespread approach is to use native (uncrosslinked) decellularized valves obtained from processed human or animal tissues. Once implanted, decellularized tissues are expected to provide proper environment and sufficient stimuli for host cells to infiltrate, remodel, and eventually regenerate the valvular tissue. As antigenic determinants are mainly concentrated on the surface of cells, removal of the original cells (decellularization) is necessary for avoiding complications related to immune rejection, which can be satisfactorily attained using combinations of detergents and enzymes, leaving behind 3-D scaffolds comprising apparently intact matrix molecules. Experimental data obtained with decellularized porcine valves yielded spectacular results (35). In vitro hydrodynamic performance was excellent, and valves performed well after implantation in sheep for 5 months as replacement of pulmonary valves. Explanted valves showed good repopulation of porcine scaffolds with fibroblast-like cells as well as an almost complete re-endothelialization of valve surfaces. These encouraging studies prompted several smallscale studies in humans using a commercially available decellularized porcine heart valve. However, despite the initial enthusiasm, the results of a small study in children were catastrophic. In a breakthrough and intrepid study, a group of cardiac surgeons from Austria reported that three out of four children implanted with decellularized porcine heart valves had died within 1 year after implantation because of tissue rupture, degeneration, and calcification of the implant (36). Analysis of explanted decellularized porcine heart valves revealed lack of cell repopulation or endothelialization. The collagen matrix induced a severe inflammatory response and encapsulation of the graft and also served as a substrate for development of numerous calcification sites. The same group of researchers also recently identified the presence of the porcine cell-specific disaccharide galactose-alpha-1-3-galactose (alpha-gal epitope) in decellularized porcine heart valves as the possible source of immunogenicity (37). Similarly, decellularized blood vessels also elicited inflammatory responses (38) and failed to maintain patency (39). Evidently, more basic studies are required for further development of these products as well as to reevaluate the relevance of animal models. Several academic groups, as well as a group of medical device companies, continue to pursue research and development of decellularized cardiovascular tissues (40 42). A second approach involves construction of tissue equivalents in the laboratory prior to implantation, with the expectation that assembling the tissue-engineered valvular constructs from appropriate cells, and synthetic or natural matrix components, would create mechanically competent, nonthrombogenic, living tissues capable of adaptation and growth. Compared with decellularized tissues, this approach provides better control of the device properties before implantation. However, because of the enormity of this task, and because of possible early clinical failures of decellularized tissues, researchers in the field of heart valve tissue regeneration have taken a cautious, stepwise approach. The in vitro assembly of heart valve tissue from its individual components was pioneered by I. Vesely et al. (43,44). In this ingenious approach, a chemically crosslinked nonbiodegradable glycosaminoglycan gel was seeded with vascular cells and cultured in vitro, which resulted in the formation of a thin sheet of elastin at the interface between cells and the glycosaminoglycan gel. The collagen structural component was created by fibroblast-mediated compaction of soluble collagen, with the expectation that in vitro assembly of these building blocks would, at some point, create a valve-like structure. For more details, please refer to the chapter on Tissue Engineering of Heart Valves in this Encyclopedia. In an exemplary collaborative effort, clinicians, basic scientists, polymer chemists, and biomedical engineers focused on creating functional tissue-engineered heart valves in vitro using biodegradable scaffolds (45). These efforts included scaffold design and characterization, optimization of cell sources, finding adequate mechanisms for cell delivery, and optimizing in vitro culture of the seeded scaffolds, culminating with the surgical replacement of heart valves in sheep. A wide variety of scaffolding models created from biodegradable polymers have been tested for heart valve tissue engineering, including polyglycolic acid, polylactic acid, polycaprolactone, and biodegradable elastomers, which are manufactured into nonwoven textiles in shapes and conformations that mimic the natural architecture of the heart valve. These scaffolds are then seeded with cells that can be obtained from (1) fully differentiated cells such as myofibroblasts and endothelial cells derived from systemic arteries, or (2) pluripotent stem cells derived from adipose tissue, bone marrow, or peripheral blood (46). As mature cells have a limited lifespan, an attractive cell source was stem cells for heart valve tissue engineering. Recently, in a landmark experiment, mesenchymal stem cells obtained from ovine bone marrow were seeded onto biodegradable scaffolds and the constructs were cultured in vitro before implantation as a valve in the pulmonary position of sheep (47) for up to 8 months. Tissue-engineered valves performed well hemodynamically, with signs of slow degradation of the scaffolds, and concomitant deposition of new extracellular matrix. Moreover, cell types resembled those present in natural heart valves. Overall, these exciting results hold great promise for truly effective regenerative approaches to treatment of heart valve disease. 8. FUTURE AND PERSPECTIVES By virtue of its clinical applications, biomedical engineering has matured into an interdisciplinary medical specialty, which necessitates intertwining expertise from numerous fields, including surgery, pathology, cell and molecular biology, matrix biochemistry, engineering, and mechanics. In anticipation of major scientific breakthroughs in this field, surgeons will continue to treat these diseases by first reestablishing the mechanical functions of the heart using artificial valves. The yellow brick
9 ARTIFICIAL HEART VALVES 9 road leading to effective treatments of valvular disease continues to present multiple challenges. Three extremely exciting lines of investigation are: A. Finding causes and developing nonsurgical therapy approaches for valvular disease. To achieve this goal, more detailed molecular, biochemical, and cellular studies are needed on normal and diseased human valves as well as development of more clinically relevant animal models for valve diseases. B. Improvement of current artificial devices. Regarding mechanical valves, it would be a milestone of success to drastically reduce the incidence of thrombogenicity caused by valve replacement with mechanical devices. For tissue valves, more effective stabilization and anticalcification treatments are required to reduce the incidence of tissue degeneration and extend device durability. More studies on implant-host tissue interactions are needed to develop innovative materials that elicit minimal healing responses that are caused by surgically invasive and inherently traumatic valve replacement techniques. Finally, a need to develop minimally invasive implantation approaches that would reduce the need for open-heart surgery for valve replacement exists. C. Regenerative medicine approaches. Heart valve tissue engineering is only in the nascent stage of research. Therefore, to succeed, it is imperative that we fully understand the structural-functional properties of normal heart valves and to determine how the valvular cells remodel the tissue. As stem cell research offers a marvelous potential for effective regeneration of heart valve tissue by differentiation into the proper cells, insights into embryological development are also essential (8). 9. CONCLUSIONS Heart valves are connective tissues that maintain their remarkable mechanical and biological functions under austere biomechanical and biochemical conditions within the human body. As heart valve injuries lead to diseases that cause progressive valve degeneration, open-heart surgery to replace diseased tissue with an engineered device is, at present, the only curative method for correcting these altered mechanical and biological properties. Mechanical and tissue-derived artificial heart valves successfully reestablish mechanical functions but do not replenish the biological requisites for long-term durability and eventually fail. Remarkable research efforts are underway to develop better devices as well as to open new and exciting avenues for tissue engineering approaches, which could potentially facilitate a complete regeneration of valve tissues. BIBLIOGRAPHY 1. J. M. Icardo and E. Colvee, Atrioventricular valves of the mouse: III. Collagenous skeleton and myotendinous junction. Anat. Rec. 1995; 243(3): I. Vesely and R. Noseworthy, Micromechanics of the fibrosa and the ventricularis in aortic valve leaflets. J. Biomech. 1992; 25(1): I. Vesely, The role of elastin in aortic valve mechanics. J. Biomech. 1998; 31(2): J. Lovekamp and N. Vyavahare, Periodate-mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J. Biomed. Mater. Res. 2001; 56(4): J. J. Lovekamp, D. T. Simionescu, J. J. Mercuri, B. Zubiate, M. S. Sacks, and N. R. Vyavahare, Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials, in press. 6. F. J. Schoen, Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc. Pathol. 2005; 14(4): X. Y. Jin and J. R. Pepper, Do stentless valves make a difference? Eur. J. Cardiothorac. Surg. 2002; 22(1): E. Rabkin-Aikawa, J. E. Mayer, Jr., and F. J. Schoen, Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 2005; 94: M. I. Ionescu, B. C. Pakrashi, D. A. Mary, I. T. Bartek, and G. H. Wooler, Replacement of heart valves with frame-mounted tissue grafts. Thorax 1974; 29(1): Y. S. Morsi, I. E. Birchall, and F. L. Rosenfeldt, Artificial aortic valves: an overview. Int. J. Artif. Organs 2004; 27(6): F. J. Schoen and R. J. Levy, Founder s Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28 May 2, Tissue heart valves: current challenges and future research perspectives. J. Biomed. Mater. Res. 1999; 47(4): J. Butany, M. S. Ahluwalia, C. Munroe, C. Fayet, C. Ahn, P. Blit, C. Kepron, R. J. Cusimano, and R. L. Leask, Mechanical heart valve prostheses: identification and evaluation. Cardiovasc. Pathol. 2003; 12(6): D. Horstkotte, Prosthetic valves or tissue valves a vote for mechanical prostheses. Z. Kardiol. 1985; 74 (Suppl 6): J. Butany, C. Fayet, M. S. Ahluwalia, P. Blit, C. Ahn, C. Munroe, N. Israel, R. J. Cusimano, and R. L. Leask, Biological replacement heart valves. Identification and evaluation. Cardiovasc. Pathol. 2003; 12(3): M. Dahm, M. Husmann, M. Eckhard, D. Prufer, E. Groh, and H. Oelert, Relevance of immunologic reactions for tissue failure of bioprosthetic heart valves. Ann. Thorac. Surg. 1995; 60(2 Suppl):S348 S D. T. Cheung, N. Perelman, E. C. Ko, and M. E. Nimni, Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connect. Tissue Res. 1985; 13(2): A. M. Borkon, L. M. Soule, K. L. Baughman, W. A. Baumgartner, T. J. Gardner, L. Watkins, V. L. Gott, K. A. Hall, and B. A. Reitz, Aortic valve selection in the elderly patient. Ann. Thorac. Surg. 1988; 46(3): F. J. Schoen and C. E. Hobson, Anatomic analysis of removed prosthetic heart valves: causes of failure of 33 mechanical valves and 58 bioprostheses, 1980 to Hum. Pathol. 1985; 16(6):
10 10 ARTIFICIAL HEART VALVES 19. M. S. Sacks and F. J. Schoen, Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J. Biomed. Mater. Res. 2002; 62(3): D. T. Simionescu, J. J. Lovekamp, and N. R. Vyavahare, Extracellular matrix degrading enzymes are active in porcine stentless aortic bioprosthetic heart valves. J. Biomed. Mater. Res. A 2003; 66(4): I. Vesely, J. E. Barber, and N. B. Ratliff, Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J. Heart Valve Dis. 2001; 10(4): I. Vesely, The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovasc. Pathol. 2003; 12(5): D. T. Simionescu, Prevention of calcification in bioprosthetic heart valves: challenges and perspectives. Expert Opin. Biol. Ther. 2004; 4(12): A. Simionescu, D. Simionescu, and R. Deac, Biochemical pathways of tissue degeneration in bioprosthetic cardiac valves. The role of matrix metalloproteinases. Asaio. J. 1996; 42(5):M561 M N. Vyavahare, M. Ogle, F. J. Schoen, and R. J. Levy, Elastin calcification and its prevention with aluminum chloride pretreatment. Am. J. Pathol. 1999; 155(3): C. M. Giachelli, Ectopic calcification: gathering hard facts about soft tissue mineralization. Am. J. Pathol. 1999; 154(3): J. P. Gott, C. Pan, L. M. Dorsey, J. L. Jay, G. K. Jett, F. J. Schoen, J. M. Girardot, and R. A. Guyton, Calcification of porcine valves: a successful new method of antimineralization. Ann. Thorac. Surg. 1992; 53(2): ; discussion R. J. Levy, G. Golomb, J. Wolfrum, S. A. Lund, F. J. Schoen, and R. Langer, Local controlled-release of diphosphonates from ethylenevinylacetate matrices prevents bioprosthetic heart valve calcification. Trans. Am. Soc. Artif. Intern. Organs 1985; 31: M. A. Moore, PhotoFix: unraveling the mystery. J. Long Term Eff. Med. Implants 2001; 11(3 4): T. Gudbjartsson, S. Aranki, and L. H. Cohn, Mechanical/bioprosthetic mitral valve replacement. In: L. H. Cohn and L. H. Edmunds, Jr., eds., Cardiac Surgery in the Adult. New York: McGraw-Hill, 2003, pp A. P. Yoganathan, Z. He, and S. Casey Jones, Fluid mechanics of heart valves. Annu. Rev. Biomed. Eng. 2004; 6: K. Iwasaki, M. Umezu, K. Iijima, and K. Imachi, Implications for the establishment of accelerated fatigue test protocols for prosthetic heart valves. Artif. Organs 2002; 26(5): F. J. Schoen, G. Golomb, and R. J. Levy, Calcification of bioprosthetic heart valves: a perspective on models. J. Heart Valve Dis. 1992; 1(1): M. F. Ogle, S. J. Kelly, R. W. Bianco, and R. J. Levy, Calcification resistance with aluminum-ethanol treated porcine aortic valve bioprostheses in juvenile sheep. Ann. Thorac. Surg. 2003; 75(4): S. Goldstein, D. R. Clarke, S. P. Walsh, K. S. Black, and M. F. O Brien, Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann. Thorac. Surg. 2000; 70(6): P. Simon, M. T. Kasimir, G. Seebacher, G. Weigel, R. Ullrich, U. Salzer-Muhar, E. Rieder, and E. Wolner, Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur. J. Cardiothorac. Surg. 2003; 23(6): ; discussion M. T. Kasimir, E. Rieder, G. Seebacher, E. Wolner, G. Weigel, and P. Simon, Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng. 2005; 11(7 8): F. Sayk, I. Bos, U. Schubert, T. Wedel, and H. H. Sievers, Histopathologic findings in a novel decellularized pulmonary homograft: an autopsy study. Ann. Thorac. Surg. 2005; 79(5): M. A. Sharp, D. Phillips, I. Roberts, and L. Hands, A cautionary case: the SynerGraft vascular prosthesis. Eur. J. Vasc. Endovasc. Surg. 2004; 27(1): H. E. Wilcox, S. A. Korossis, C. Booth, K. G. Watterson, J. N. Kearney, J. Fisher, and E. Ingham, Biocompatibility and recellularization potential of an acellular porcine heart valve matrix. J. Heart Valve Dis. 2005; 14(2): ; discussion E. Rieder, G. Seebacher, M. T. Kasimir, E. Eichmair, B. Winter, B. Dekan, E. Wolner, P. Simon, and G. Weigel, Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation 2005; 111(21): D. T. Simionescu, Q. Lu, Y. Song, J. Lee, T. N. Rosenbalm, C. Kelley, and N. R. Vyavahare, Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials 2006; 27(5): A. Ramamurthi and I. Vesely, Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials 2005; 26(9): Y. Shi, A. Ramamurthi, and I. Vesely, Towards tissue engineering of a composite aortic valve. Biomed. Sci. Instrum. 2002; 38: S. P. Hoerstrup, R. Sodian, S. Daebritz, J. Wang, E. A. Bacha, D. P. Martin, A. M. Moran, K. J. Guleserian, J. S. Sperling, S. Kaushal, J. P. Vacanti, F. J. Schoen, and J. E. Mayer, Jr., Functional living trileaflet heart valves grown in vitro. Circulation 2000; 102(19 Suppl 3):III44 III F. W. Sutherland and J. E. Mayer, Jr., Tissue engineering for cardiac surgery. In: L. H. Cohn and L. H. Edmunds, Jr., eds., Cardiac Surgery in the Adult. New York: McGraw-Hill, 2003, pp F. W. Sutherland, T. E. Perry, Y. Yu, M. C. Sherwood, E. Rabkin, Y. Masuda, G. A. Garcia, D. L. McLellan, G. C. Engelmayr, Jr., M. S. Sacks, F. J. Schoen, and J. E. Mayer, Jr., From stem cells to viable autologous semilunar heart valve. Circulation 2005; 111(21):
Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets
 Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets Introduction Chi Zheng, M1 - University of Pittsburgh; BSE - University of Michigan In association
Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets Introduction Chi Zheng, M1 - University of Pittsburgh; BSE - University of Michigan In association
Iatrogenic pathology of the heart:
 Iatrogenic pathology of the heart: Complications of mitral valve plasty and replacement Patrick Bruneval D pt of Pathology Hôpital Européen Georges Pompidou Enterprise Interest None Mitral valve surgery
Iatrogenic pathology of the heart: Complications of mitral valve plasty and replacement Patrick Bruneval D pt of Pathology Hôpital Européen Georges Pompidou Enterprise Interest None Mitral valve surgery
TSDA Boot Camp September 13-16, Introduction to Aortic Valve Surgery. George L. Hicks, Jr., MD
 TSDA Boot Camp September 13-16, 2018 Introduction to Aortic Valve Surgery George L. Hicks, Jr., MD Aortic Valve Pathology and Treatment Valvular Aortic Stenosis in Adults Average Course (Post mortem data)
TSDA Boot Camp September 13-16, 2018 Introduction to Aortic Valve Surgery George L. Hicks, Jr., MD Aortic Valve Pathology and Treatment Valvular Aortic Stenosis in Adults Average Course (Post mortem data)
The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to:
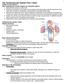 The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to: 1. Describe the functions of the heart 2. Describe the location of the heart,
The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to: 1. Describe the functions of the heart 2. Describe the location of the heart,
CorMatrix ECM Bioscaffold
 CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
CIRCULATORY SYSTEM BLOOD VESSELS
 Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
Late failure of transcatheter heart valves: An open question
 Late failure of transcatheter heart valves: An open question A comparison with surgically implanted bioprosthetic heart valves. A. Rashid The Cardiothoracic Centre Liverpool, UK. Conflict of Interest Statement
Late failure of transcatheter heart valves: An open question A comparison with surgically implanted bioprosthetic heart valves. A. Rashid The Cardiothoracic Centre Liverpool, UK. Conflict of Interest Statement
Chapter 20 (1) The Heart
 Chapter 20 (1) The Heart Learning Objectives Describe the location and structure of the heart Describe the path of a drop of blood from the superior vena cava or inferior vena cava through the heart out
Chapter 20 (1) The Heart Learning Objectives Describe the location and structure of the heart Describe the path of a drop of blood from the superior vena cava or inferior vena cava through the heart out
Two semilunar valves. Two atrioventricular valves. Valves of the heart. Left atrioventricular or bicuspid valve Mitral valve
 The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
THE HEART OBJECTIVES: LOCATION OF THE HEART IN THE THORACIC CAVITY CARDIOVASCULAR SYSTEM
 BIOLOGY II CARDIOVASCULAR SYSTEM ACTIVITY #3 NAME DATE HOUR THE HEART OBJECTIVES: Describe the anatomy of the heart and identify and give the functions of all parts. (pp. 356 363) Trace the flow of blood
BIOLOGY II CARDIOVASCULAR SYSTEM ACTIVITY #3 NAME DATE HOUR THE HEART OBJECTIVES: Describe the anatomy of the heart and identify and give the functions of all parts. (pp. 356 363) Trace the flow of blood
UNDERSTANDING YOUR HEART VALVE. Mosaic Tissue Valve
 UNDERSTANDING YOUR HEART VALVE Mosaic Tissue Valve A Message to You from the Employees at Medtronic, Inc. We understand that having heart valve replacement surgery is an important change in your life.
UNDERSTANDING YOUR HEART VALVE Mosaic Tissue Valve A Message to You from the Employees at Medtronic, Inc. We understand that having heart valve replacement surgery is an important change in your life.
Adult Cardiac Surgery
 Adult Cardiac Surgery Mahmoud ABU-ABEELEH Associate Professor Department of Surgery Division of Cardiothoracic Surgery School of Medicine University Of Jordan Adult Cardiac Surgery: Ischemic Heart Disease
Adult Cardiac Surgery Mahmoud ABU-ABEELEH Associate Professor Department of Surgery Division of Cardiothoracic Surgery School of Medicine University Of Jordan Adult Cardiac Surgery: Ischemic Heart Disease
Cardiovascular Anatomy Dr. Gary Mumaugh
 Cardiovascular Anatomy Dr. Gary Mumaugh Location of Heart Approximately the size of your fist Location o Superior surface of diaphragm o Left of the midline in mediastinum o Anterior to the vertebral column,
Cardiovascular Anatomy Dr. Gary Mumaugh Location of Heart Approximately the size of your fist Location o Superior surface of diaphragm o Left of the midline in mediastinum o Anterior to the vertebral column,
The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle.
 The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle. From this camber, it passes through the pulmonary semilunar valve
The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle. From this camber, it passes through the pulmonary semilunar valve
Cardiovascular System. I. Structures of the heart A. : Pericardium sack that surrounds the heart
 Cardiovascular System I. Structures of the heart A. : Pericardium sack that surrounds the heart 1. : Pericardial Cavity serous fluid filled space between the heart and the pericardium B. Heart Wall 1.
Cardiovascular System I. Structures of the heart A. : Pericardium sack that surrounds the heart 1. : Pericardial Cavity serous fluid filled space between the heart and the pericardium B. Heart Wall 1.
Section 5.1 The heart and heart disease
 Section 5.1 The heart and heart disease Mammals are too large to rely on diffusion. They need a circulatory system to move substances around the body. Blood moves down pressure gradients, from high to
Section 5.1 The heart and heart disease Mammals are too large to rely on diffusion. They need a circulatory system to move substances around the body. Blood moves down pressure gradients, from high to
The Heart. Size, Form, and Location of the Heart. 1. Blunt, rounded point; most inferior part of the heart.
 12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
Ch 19: Cardiovascular System - The Heart -
 Ch 19: Cardiovascular System - The Heart - Give a detailed description of the superficial and internal anatomy of the heart, including the pericardium, the myocardium, and the cardiac muscle. Trace the
Ch 19: Cardiovascular System - The Heart - Give a detailed description of the superficial and internal anatomy of the heart, including the pericardium, the myocardium, and the cardiac muscle. Trace the
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.2 The Blood System D.4 The Heart Essential Questions: 6.2 The blood system continuously transports substances to cells and simultaneously collects waste products. D.3 The chemical
Topic 6: Human Physiology 6.2 The Blood System D.4 The Heart Essential Questions: 6.2 The blood system continuously transports substances to cells and simultaneously collects waste products. D.3 The chemical
LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART
 LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART Because mammals are warm-blooded and generally very active animals, they require high metabolic rates. One major requirement of a high metabolism is
LAB 12-1 HEART DISSECTION GROSS ANATOMY OF THE HEART Because mammals are warm-blooded and generally very active animals, they require high metabolic rates. One major requirement of a high metabolism is
Heart Anatomy. 7/5/02 Stephen G Davenport 1
 Heart Anatomy Copyright 1999, Stephen G. Davenport, No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form without prior written permission. 7/5/02 Stephen
Heart Anatomy Copyright 1999, Stephen G. Davenport, No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form without prior written permission. 7/5/02 Stephen
The Cardiovascular System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Cardiovascular System 11 PART A The Cardiovascular System A closed system of the heart and blood
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Cardiovascular System 11 PART A The Cardiovascular System A closed system of the heart and blood
Hemodynamics Benefit of Supra-Annular Design in Failed Bio-Prosthetic Valves
 Hemodynamics Benefit of Supra-Annular Design in Failed Bio-Prosthetic Valves Speaker's name: I have the following potential conflicts of interest to report: Proctorship for Medtronic Agenda Failure modes
Hemodynamics Benefit of Supra-Annular Design in Failed Bio-Prosthetic Valves Speaker's name: I have the following potential conflicts of interest to report: Proctorship for Medtronic Agenda Failure modes
Blood and Heart. Student Learning Objectives:
 Blood and Heart Student Learning Objectives: Identify the major components of the blood. Identify the primary structures associated with the heart Follow the blood through the path of the circulation.
Blood and Heart Student Learning Objectives: Identify the major components of the blood. Identify the primary structures associated with the heart Follow the blood through the path of the circulation.
Heart Valve Replacement
 Heart Valve Replacement Introduction Sometimes people have serious problems with the valves in their hearts. A heart valve repair or replacement surgery restores or replaces a defective heart valve. If
Heart Valve Replacement Introduction Sometimes people have serious problems with the valves in their hearts. A heart valve repair or replacement surgery restores or replaces a defective heart valve. If
The Cardiovascular and Lymphatic Systems Cardiovascular System Blood Vessels Blood Vessels Arteries Arteries Arteries
 CH 12 The Cardiovascular and s The Cardiovascular and s OUTLINE: Cardiovascular System Blood Vessels Blood Pressure Cardiovascular System The cardiovascular system is composed of Blood vessels This system
CH 12 The Cardiovascular and s The Cardiovascular and s OUTLINE: Cardiovascular System Blood Vessels Blood Pressure Cardiovascular System The cardiovascular system is composed of Blood vessels This system
Chapter 20: Cardiovascular System: The Heart
 Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
The Heart and Cardiovascular System
 The Heart and Cardiovascular System What you will learn The location of the heart 3 layers and covering of the heart Explain the function of the heart as 2 separate pumps Identify the 4 chambers of the
The Heart and Cardiovascular System What you will learn The location of the heart 3 layers and covering of the heart Explain the function of the heart as 2 separate pumps Identify the 4 chambers of the
Echocardiographic Evaluation of Mitral Valve Prostheses
 Echocardiographic Evaluation of Mitral Valve Prostheses Dennis A. Tighe, M.D., FACC, FACP, FASE Cardiovascular Medicine University of Massachusetts Medical School Worcester, MA www.asecho.org 1 Nishimura
Echocardiographic Evaluation of Mitral Valve Prostheses Dennis A. Tighe, M.D., FACC, FACP, FASE Cardiovascular Medicine University of Massachusetts Medical School Worcester, MA www.asecho.org 1 Nishimura
Cardiovascular System. Heart Anatomy
 Cardiovascular System Heart Anatomy 1 The Heart Location & general description: Atria vs. ventricles Pulmonary vs. systemic circulation Coverings Walls The heart is found in the mediastinum, the medial
Cardiovascular System Heart Anatomy 1 The Heart Location & general description: Atria vs. ventricles Pulmonary vs. systemic circulation Coverings Walls The heart is found in the mediastinum, the medial
PATIENT BOOKLET MEDTRONIC SURGICAL VALVE REPLACEMENT. Tissue Valve for Aortic and Mitral Valve Replacement
 PATIENT BOOKLET MEDTRONIC SURGICAL VALVE REPLACEMENT Tissue Valve for Aortic and Mitral Valve Replacement ARE MEDTRONIC SURGICAL TISSUE HEART VALVES RIGHT FOR YOU? Medtronic surgical heart valves are for
PATIENT BOOKLET MEDTRONIC SURGICAL VALVE REPLACEMENT Tissue Valve for Aortic and Mitral Valve Replacement ARE MEDTRONIC SURGICAL TISSUE HEART VALVES RIGHT FOR YOU? Medtronic surgical heart valves are for
Heart Dissection. 5. Locate the tip of the heart or the apex. Only the left ventricle extends all the way to the apex.
 Heart Dissection Page 1 of 6 Background: The heart is a four-chambered, hollow organ composed primarily of cardiac muscle tissue. It is located in the center of the chest in between the lungs. It is the
Heart Dissection Page 1 of 6 Background: The heart is a four-chambered, hollow organ composed primarily of cardiac muscle tissue. It is located in the center of the chest in between the lungs. It is the
The Cardiovascular System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Slides 11.1 11.19 Seventh Edition Chapter 11 The Cardiovascular System Functions of the Cardiovascular system Function of the heart: to pump blood
Essentials of Human Anatomy & Physiology Elaine N. Marieb Slides 11.1 11.19 Seventh Edition Chapter 11 The Cardiovascular System Functions of the Cardiovascular system Function of the heart: to pump blood
The Cardiovascular and Lymphatic Systems
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 12 The Cardiovascular and Lymphatic Systems Lecture Presentation Anne Gasc Hawaii Pacific University and
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 12 The Cardiovascular and Lymphatic Systems Lecture Presentation Anne Gasc Hawaii Pacific University and
By the end of this session, the student should be able to:
 Valvular Heart disease HVD By Dr. Ashraf Abdelfatah Deyab VHD- Objectives By the end of this session, the student should be able to: Define and classify valvular heart disease. Enlist the causes of acquired
Valvular Heart disease HVD By Dr. Ashraf Abdelfatah Deyab VHD- Objectives By the end of this session, the student should be able to: Define and classify valvular heart disease. Enlist the causes of acquired
CIE Biology GCSE. 9: Transport in animals. Notes.
 CIE Biology GCSE 9: Transport in animals Notes The circulatory system acts as the main transport system in animals. It is made up of blood vessels such as arteries, veins and capillaries, in which blood
CIE Biology GCSE 9: Transport in animals Notes The circulatory system acts as the main transport system in animals. It is made up of blood vessels such as arteries, veins and capillaries, in which blood
The HEART. What is it???? Pericardium. Heart Facts. This muscle never stops working It works when you are asleep
 This muscle never stops working It works when you are asleep The HEART It works when you eat It really works when you exercise. What is it???? Located between the lungs in the mid thoracic region Apex
This muscle never stops working It works when you are asleep The HEART It works when you eat It really works when you exercise. What is it???? Located between the lungs in the mid thoracic region Apex
Lower Secondary Science Blood Circulatory System Notes / Advanced Notes
 Lower Secondary Science Blood Circulatory System Notes / Advanced Notes Double Circulation in Mammals In mammals, there is a double circulation (i.e. blood passes through the heart twice in one complete
Lower Secondary Science Blood Circulatory System Notes / Advanced Notes Double Circulation in Mammals In mammals, there is a double circulation (i.e. blood passes through the heart twice in one complete
Chapter 18 - Heart. I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity)
 Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Chapter 18 - Heart I. Heart Anatomy: size of your fist; located in mediastinum (medial cavity) A. Coverings: heart enclosed in double walled sac called the pericardium 1. Fibrous pericardium: dense connective
Approximately the size of your fist Location Superior surface of diaphragm Left of the midline in mediastinum Anterior to the vertebral column,
 Dr. Gary Mumaugh Approximately the size of your fist Location Superior surface of diaphragm Left of the midline in mediastinum Anterior to the vertebral column, posterior to the sternum Posteriorly the
Dr. Gary Mumaugh Approximately the size of your fist Location Superior surface of diaphragm Left of the midline in mediastinum Anterior to the vertebral column, posterior to the sternum Posteriorly the
CV Anatomy Quiz. Dr Ella Kim Dr Pip Green
 CV Anatomy Quiz Dr Ella Kim Dr Pip Green Q1 The location of the heart is correctly described as A) lateral to the lungs. B) medial to the sternum. C) superior to the diaphragm. D) posterior to the spinal
CV Anatomy Quiz Dr Ella Kim Dr Pip Green Q1 The location of the heart is correctly described as A) lateral to the lungs. B) medial to the sternum. C) superior to the diaphragm. D) posterior to the spinal
CARDIOVASCULAR SYSTEM
 CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
Mosaic tissue-engineered porcine pulmonary artery valved conduit: long-term follow-up after implantation in an ovine model RETRACTED
 Interactive CardioVascular and Thoracic Surgery (2014) 1 5 doi:10.1093/icvts/ivu350 CONGENITAL a b c d Mosaic tissue-engineered porcine pulmonary artery valved conduit: long-term follow-up after implantation
Interactive CardioVascular and Thoracic Surgery (2014) 1 5 doi:10.1093/icvts/ivu350 CONGENITAL a b c d Mosaic tissue-engineered porcine pulmonary artery valved conduit: long-term follow-up after implantation
LECTURE 5. Anatomy of the heart
 LECTURE 5. Anatomy of the heart Main components of the CVS: Heart Blood circulatory system arterial compartment haemomicrocirculatory (=microvascular) compartment venous compartment Lymphatic circulatory
LECTURE 5. Anatomy of the heart Main components of the CVS: Heart Blood circulatory system arterial compartment haemomicrocirculatory (=microvascular) compartment venous compartment Lymphatic circulatory
Prevention of calcification in bioprosthetic heart valves: challenges and perspectives
 Review 1. Introduction 2. Current status and presentation of bioprosthetic heart valves 3. Macroscopic alterations in explanted bioprosthetic heart valves 4. Factors that influence bioprosthetic heart
Review 1. Introduction 2. Current status and presentation of bioprosthetic heart valves 3. Macroscopic alterations in explanted bioprosthetic heart valves 4. Factors that influence bioprosthetic heart
Circulatory System Function Move circulatory fluid (blood) around body Gas Transport Nutrient Transport Excretory Product Transport
 Lecture 37 Introduction to Circulation BY DR QAZI IMTIAZ RASOOL OBJECTIVES Functions of the Heart Generating blood pressure Routing blood: separates pulmonary and systemic circulations Ensuring one-way
Lecture 37 Introduction to Circulation BY DR QAZI IMTIAZ RASOOL OBJECTIVES Functions of the Heart Generating blood pressure Routing blood: separates pulmonary and systemic circulations Ensuring one-way
Tissue-Engineered Blood Vessels in Pediatric Cardiac Surgery
 YALE JOURNAL OF BIOLOGY AND MEDICINE 81 (008), pp.1-6. Copyright 008. REVIEW Tissue-Engineered Blood Vessels in Pediatric Cardiac Surgery Toshiharu Shinoka, MD,* Christopher Breuer, MD Department of Surgery,
YALE JOURNAL OF BIOLOGY AND MEDICINE 81 (008), pp.1-6. Copyright 008. REVIEW Tissue-Engineered Blood Vessels in Pediatric Cardiac Surgery Toshiharu Shinoka, MD,* Christopher Breuer, MD Department of Surgery,
The Circulatory System. The Heart, Blood Vessels, Blood Types
 The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
I will not discuss off label use or investigational use in my presentation.
 I will not discuss off label use or investigational use in my presentation. Surgical valves Design and Durability Testing Potential Concerns Real Practice 1952-1962 1963-1966 1967-1969 1969-1977 1977-1984
I will not discuss off label use or investigational use in my presentation. Surgical valves Design and Durability Testing Potential Concerns Real Practice 1952-1962 1963-1966 1967-1969 1969-1977 1977-1984
Carpentier-Edwards ThermaFix Process: A Method for Extracting Calcium Binding Sites from Pericardial Tissue
 Carpentier-Edwards ThermaFix Process: A Method for Extracting Calcium Binding Sites from Pericardial Tissue Jeffrey S. Dove, Principal Engineer Myron Howanec, Principal Engineer Mano J. Thubrikar, PhD,
Carpentier-Edwards ThermaFix Process: A Method for Extracting Calcium Binding Sites from Pericardial Tissue Jeffrey S. Dove, Principal Engineer Myron Howanec, Principal Engineer Mano J. Thubrikar, PhD,
BEGO BIOMATERIALS When the result counts
 BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
Chapter 14. The Cardiovascular System
 Chapter 14 The Cardiovascular System Introduction Cardiovascular system - heart, blood and blood vessels Cardiac muscle makes up bulk of heart provides force to pump blood Function - transports blood 2
Chapter 14 The Cardiovascular System Introduction Cardiovascular system - heart, blood and blood vessels Cardiac muscle makes up bulk of heart provides force to pump blood Function - transports blood 2
THE HEART. A. The Pericardium - a double sac of serous membrane surrounding the heart
 THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Anatomy of the Heart. Figure 20 2c
 Anatomy of the Heart Figure 20 2c Pericardium & Myocardium Remember, the heart sits in it s own cavity, known as the mediastinum. The heart is surrounded by the Pericardium, a double lining of the pericardial
Anatomy of the Heart Figure 20 2c Pericardium & Myocardium Remember, the heart sits in it s own cavity, known as the mediastinum. The heart is surrounded by the Pericardium, a double lining of the pericardial
Heart. Heart 2-Tunica media: middle layer (media ='middle') muscle fibers (smooth or cardiac).
 t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
THE CIRCULATORY SYSTEM
 Biology 30S THE CIRCULATORY SYSTEM Name: This module adapted from bblearn.merlin.mb.ca 1 Introduction to Circulation The first organ to form, and the last organ to die. The heart is the pump of life. The
Biology 30S THE CIRCULATORY SYSTEM Name: This module adapted from bblearn.merlin.mb.ca 1 Introduction to Circulation The first organ to form, and the last organ to die. The heart is the pump of life. The
The problem of the missing organ
 The problem of the missing organ 1. Irreversible injury (acute and chronic) destroys organ function. 2. Five basic therapies for the missing organ. 3. Examples of widespread clinical problems that have
The problem of the missing organ 1. Irreversible injury (acute and chronic) destroys organ function. 2. Five basic therapies for the missing organ. 3. Examples of widespread clinical problems that have
Cardiac Surgery A Resource of Experimental Design
 Cardiac Surgery A Resource of Experimental Design Complete Transposition: a. Atrial switch the chronically systemic right ventricle b. Arterial switch the suddenly systemic left ventricle Fontan operation
Cardiac Surgery A Resource of Experimental Design Complete Transposition: a. Atrial switch the chronically systemic right ventricle b. Arterial switch the suddenly systemic left ventricle Fontan operation
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 20 The Cardiovascular System: The Heart Introduction The purpose of the chapter is to: 1. Learn about the components of the cardiovascular system
Principles of Anatomy and Physiology 14 th Edition CHAPTER 20 The Cardiovascular System: The Heart Introduction The purpose of the chapter is to: 1. Learn about the components of the cardiovascular system
A FUTURE WITHOUT RE-OPERATIONS?
 A FUTURE WITHOUT RE-OPERATIONS? New horizons in pulmonary heart valve therapy January 29, 2018 Prof. Dr. Gerardus Bennink Chief and head of pediatric cardio-thoracic congenital surgery Heart Center of
A FUTURE WITHOUT RE-OPERATIONS? New horizons in pulmonary heart valve therapy January 29, 2018 Prof. Dr. Gerardus Bennink Chief and head of pediatric cardio-thoracic congenital surgery Heart Center of
Scrub In: Red blood cells are called: Which component of blood is necessary for the initiation of the blood clotting process:
 Scrub In: Red blood cells are called: a. erythrocytes b. leukocytes c. melanocytes d. thrombocytes Which component of blood is necessary for the initiation of the blood clotting process: a. erythrocytes
Scrub In: Red blood cells are called: a. erythrocytes b. leukocytes c. melanocytes d. thrombocytes Which component of blood is necessary for the initiation of the blood clotting process: a. erythrocytes
the Cardiovascular System I
 the Cardiovascular System I By: Dr. Nabil A Khouri MD, MsC, Ph.D MEDIASTINUM 1. Superior Mediastinum 2. inferior Mediastinum Anterior mediastinum. Middle mediastinum. Posterior mediastinum Anatomy of
the Cardiovascular System I By: Dr. Nabil A Khouri MD, MsC, Ph.D MEDIASTINUM 1. Superior Mediastinum 2. inferior Mediastinum Anterior mediastinum. Middle mediastinum. Posterior mediastinum Anatomy of
Circulatory System 10.1
 1 Circulatory System 10.1 2 ARTERIES Arteries-blood vessels that carry blood away from the heart Thick walls Inner & Outer layers: connective tissue Middle layers are muscle and elastic connective tissue
1 Circulatory System 10.1 2 ARTERIES Arteries-blood vessels that carry blood away from the heart Thick walls Inner & Outer layers: connective tissue Middle layers are muscle and elastic connective tissue
12.1 The Function of Circulation
 12.1 The Function of Circulation The Circulatory System Magnetic Resonance Angiography (MRA) Heart pump beats 100 000 times a day Deliver oxygen and nutrients Function of Circulation Multicellular organisms
12.1 The Function of Circulation The Circulatory System Magnetic Resonance Angiography (MRA) Heart pump beats 100 000 times a day Deliver oxygen and nutrients Function of Circulation Multicellular organisms
The Cardiovascular System home study course
 The Cardiovascular System home study course harmony house holistic therapy treatment centre and training academy www.harmony-house.org 1 Copyright 2010 by Mark and Katy Rogers All rights reserved. No part
The Cardiovascular System home study course harmony house holistic therapy treatment centre and training academy www.harmony-house.org 1 Copyright 2010 by Mark and Katy Rogers All rights reserved. No part
The Circulatory System
 The Circulatory System Dr. Sami Zaqout The circulatory system Circulatory system Blood vascular systems Lymphatic vascular systems Blood vascular systems Blood vascular systems The circulatory system Circulatory
The Circulatory System Dr. Sami Zaqout The circulatory system Circulatory system Blood vascular systems Lymphatic vascular systems Blood vascular systems Blood vascular systems The circulatory system Circulatory
SCPA602 Cardiovascular System
 SCPA602 Cardiovascular System Associate Professor Dr. Wannee Jiraungkoorskul Department of Pathobiology, Faculty of Science, Mahidol University Tel: 02-201-5563, E-mail: wannee.jir@mahidol.ac.th 1 Objectives
SCPA602 Cardiovascular System Associate Professor Dr. Wannee Jiraungkoorskul Department of Pathobiology, Faculty of Science, Mahidol University Tel: 02-201-5563, E-mail: wannee.jir@mahidol.ac.th 1 Objectives
PATIENT BOOKLET MEDTRONIC MITRAL AND TRICUSPID HEART VALVE REPAIR
 PATIENT BOOKLET MEDTRONIC MITRAL AND TRICUSPID HEART VALVE REPAIR ARE MEDTRONIC HEART VALVE REPAIR THERAPIES RIGHT FOR YOU? Prosthetic (artificial) heart valve repair products are used by physicians to
PATIENT BOOKLET MEDTRONIC MITRAL AND TRICUSPID HEART VALVE REPAIR ARE MEDTRONIC HEART VALVE REPAIR THERAPIES RIGHT FOR YOU? Prosthetic (artificial) heart valve repair products are used by physicians to
Reconstruction of the intervalvular fibrous body during aortic and
 Aortic and mitral valve replacement with reconstruction of the intervalvular fibrous body: An analysis of clinical outcomes Nilto C. De Oliveira, MD Tirone E. David, MD Susan Armstrong, MSc Joan Ivanov,
Aortic and mitral valve replacement with reconstruction of the intervalvular fibrous body: An analysis of clinical outcomes Nilto C. De Oliveira, MD Tirone E. David, MD Susan Armstrong, MSc Joan Ivanov,
Major Function of the Cardiovascular System. Transportation. Structures of the Cardiovascular System. Heart - muscular pump
 Structures of the Cardiovascular System Heart - muscular pump Blood vessels - network of tubes Blood - liquid transport vehicle brachiocephalic trunk superior vena cava right pulmonary arteries right pulmonary
Structures of the Cardiovascular System Heart - muscular pump Blood vessels - network of tubes Blood - liquid transport vehicle brachiocephalic trunk superior vena cava right pulmonary arteries right pulmonary
Unit 1: Human Systems. The Circulatory System
 Unit 1: Human Systems The Circulatory System nourish all cells with oxygen, glucose, amino acids and other nutrients and carry away carbon dioxide, urea and other wastes Purposes Transport chemical messengers
Unit 1: Human Systems The Circulatory System nourish all cells with oxygen, glucose, amino acids and other nutrients and carry away carbon dioxide, urea and other wastes Purposes Transport chemical messengers
STRUCTURES OF THE CARDIOVASCULAR SYSTEM
 STRUCTURES OF THE CARDIOVASCULAR SYSTEM CARDIOVASCULAR SYSTEM Also called the circulatory system Consists of the heart, arteries, veins, and capillaries Main function is to pump/circulate oxygenated blood
STRUCTURES OF THE CARDIOVASCULAR SYSTEM CARDIOVASCULAR SYSTEM Also called the circulatory system Consists of the heart, arteries, veins, and capillaries Main function is to pump/circulate oxygenated blood
Pulmonary Valve Replacement
 Pulmonary Valve Replacement with Fascia Lata J. C. R. Lincoln, F.R.C.S., M. Geens, M.D., M. Schottenfeld, M.D., and D. N. Ross, F.R.C.S. ABSTRACT The purpose of this paper is to describe a technique of
Pulmonary Valve Replacement with Fascia Lata J. C. R. Lincoln, F.R.C.S., M. Geens, M.D., M. Schottenfeld, M.D., and D. N. Ross, F.R.C.S. ABSTRACT The purpose of this paper is to describe a technique of
16 YEAR RESULTS Carpentier-Edwards PERIMOUNT Mitral Pericardial Bioprosthesis, Model 6900
 CLINICAL COMMUNIQUé 6 YEAR RESULTS Carpentier-Edwards PERIMOUNT Mitral Pericardial Bioprosthesis, Model 69 The Carpentier-Edwards PERIMOUNT Mitral Pericardial Valve, Model 69, was introduced into clinical
CLINICAL COMMUNIQUé 6 YEAR RESULTS Carpentier-Edwards PERIMOUNT Mitral Pericardial Bioprosthesis, Model 69 The Carpentier-Edwards PERIMOUNT Mitral Pericardial Valve, Model 69, was introduced into clinical
Unit 10 Cardiovascular System
 Unit 10 Cardiovascular System I. Functions Deliver nutrients to cells > O 2, sugars, amino acids, lipids, ions, H 2 O... Remove waste from cells > CO 2, pathogens, toxins, lactic acid... Fight off infection
Unit 10 Cardiovascular System I. Functions Deliver nutrients to cells > O 2, sugars, amino acids, lipids, ions, H 2 O... Remove waste from cells > CO 2, pathogens, toxins, lactic acid... Fight off infection
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
Tissue engineering of cartilage
 Tissue engineering of cartilage Cartilage responds to mechanical forces and is able to remodel in response to the prevailing stress Cartilage, like bone, may respond to mechanical stimulation by increasing
Tissue engineering of cartilage Cartilage responds to mechanical forces and is able to remodel in response to the prevailing stress Cartilage, like bone, may respond to mechanical stimulation by increasing
Decellularization of Aortic Homografts: South American and European Current Experience
 Department of Cardiac Surgery Instituto de Neurologia e Cardiologia de Curitiba (INC-Cardio) Decellularization of Aortic Homografts: South American and European Current Experience Francisco Diniz Affonso
Department of Cardiac Surgery Instituto de Neurologia e Cardiologia de Curitiba (INC-Cardio) Decellularization of Aortic Homografts: South American and European Current Experience Francisco Diniz Affonso
THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH
 A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
Read Chapters 21 & 22, McKinley et al
 ACTIVITY 9: BLOOD AND HEART OBJECTIVES: 1) How to get ready: Read Chapters 21 & 22, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. Read dissection instructions BEFORE YOU
ACTIVITY 9: BLOOD AND HEART OBJECTIVES: 1) How to get ready: Read Chapters 21 & 22, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. Read dissection instructions BEFORE YOU
PROSTHETIC VALVE BOARD REVIEW
 PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
STABILIZATION OF EXTRACELLULAR MATRIX COMPONENTS IN BIOPROSTHETIC HEART VALVES USING NEOMYCIN AND PENTAGALLOYL GLUCOSE ENHANCED CROSSLINKING
 Clemson University TigerPrints All Theses Theses 8-2012 STABILIZATION OF EXTRACELLULAR MATRIX COMPONENTS IN BIOPROSTHETIC HEART VALVES USING NEOMYCIN AND PENTAGALLOYL GLUCOSE ENHANCED CROSSLINKING Daniel
Clemson University TigerPrints All Theses Theses 8-2012 STABILIZATION OF EXTRACELLULAR MATRIX COMPONENTS IN BIOPROSTHETIC HEART VALVES USING NEOMYCIN AND PENTAGALLOYL GLUCOSE ENHANCED CROSSLINKING Daniel
CoreValve in a Degenerative Surgical Valve
 CoreValve in a Degenerative Surgical Valve Ran Kornowski, MD, FESC, FACC Chairman Department of Cardiology Rabin Medical Center, Petach Tikva, Israel Disclosure Statement of Financial Interest I, Ran Kornowski,
CoreValve in a Degenerative Surgical Valve Ran Kornowski, MD, FESC, FACC Chairman Department of Cardiology Rabin Medical Center, Petach Tikva, Israel Disclosure Statement of Financial Interest I, Ran Kornowski,
Anatomy of the Heart
 Biology 212: Anatomy and Physiology II Anatomy of the Heart References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning this lab: Chapter
Biology 212: Anatomy and Physiology II Anatomy of the Heart References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning this lab: Chapter
10. Thick deposits of lipids on the walls of blood vessels, called, can lead to serious circulatory issues. A. aneurysm B. atherosclerosis C.
 Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
BIOMATERIALS. What is a Biomaterial? Attention! Info on Term Projects. Anything in contact with human body?
 Week Topic Instructor 1 Biomedical Engineering World Dr. S. Takaç 2 Biomed. Instrumentation and Signals Dr. Ö. Birgül YOU 3 Medical Imaging ARE Dr. Ö. Birgül 4 Biomechanics HERE Dr. C. Evrensel Attention!
Week Topic Instructor 1 Biomedical Engineering World Dr. S. Takaç 2 Biomed. Instrumentation and Signals Dr. Ö. Birgül YOU 3 Medical Imaging ARE Dr. Ö. Birgül 4 Biomechanics HERE Dr. C. Evrensel Attention!
7.L.1.4 Circulatory System Guided Study Notes. Circulation
 1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
Chapter 27 The Heart and Blood Vessels
 Chapter 27 The Heart and Blood Vessels Most animals have a closed blood system. The blood flows continuously in vessels back to the heart. In an open system the blood is pumped into open ended tubes and
Chapter 27 The Heart and Blood Vessels Most animals have a closed blood system. The blood flows continuously in vessels back to the heart. In an open system the blood is pumped into open ended tubes and
The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits:
 1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES
 EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES JAMES M. ANDERSON, MD, PhD DISTINGUISHED UNIVERSITY PROFESSOR DEPARTMENTS OF PATHOLOGY, MACROMOLECULAR SCIENCE, AND BIOMEDICAL ENGINEERING CASE WESTERN
EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES JAMES M. ANDERSON, MD, PhD DISTINGUISHED UNIVERSITY PROFESSOR DEPARTMENTS OF PATHOLOGY, MACROMOLECULAR SCIENCE, AND BIOMEDICAL ENGINEERING CASE WESTERN
Indications and Late Results of Aortic Valve Repair
 Indications and Late Results of Aortic Valve Repair Prof. Gebrine El Khoury Department of Cardiovascular and Thoracic Surgery Cliniques St. Luc Brussels, Belgium Aortic Valve Repair Question # 1 Can the
Indications and Late Results of Aortic Valve Repair Prof. Gebrine El Khoury Department of Cardiovascular and Thoracic Surgery Cliniques St. Luc Brussels, Belgium Aortic Valve Repair Question # 1 Can the
Blood Functions. Blood and the Cardiovascular System. Blood. Plasma. Erythrocytes (RBCs) Erythrocytes (RBCs) 4/7/2017
 Blood Functions Blood and the Cardiovascular System Distribution Delivery of oxygen and nutrients to all body cells; Transport of wastes to lungs and excretory organs; Transport of hormones Regulation
Blood Functions Blood and the Cardiovascular System Distribution Delivery of oxygen and nutrients to all body cells; Transport of wastes to lungs and excretory organs; Transport of hormones Regulation
Pathology of Coronary Artery Disease
 Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
As a courtesy to your fellow classmates please refrain from talking, beating, or snoring. And Now Our Feature Presentation.
 As a courtesy to your fellow classmates please refrain from talking, beating, or snoring. And Now Our Feature Presentation. Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood
As a courtesy to your fellow classmates please refrain from talking, beating, or snoring. And Now Our Feature Presentation. Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood
Circulation And Blood. Circulation And Blood. Circulation And Blood. Circulation And Blood. Blood 10/22/2012
 Cells in our body build their own membranes and organelles Make their own ATP Assemble their own enzymes and other proteins And may manufacture substances used elsewhere in the body To do these things,
Cells in our body build their own membranes and organelles Make their own ATP Assemble their own enzymes and other proteins And may manufacture substances used elsewhere in the body To do these things,
THE HEART. Unit 3: Transportation and Respiration
 THE HEART Unit 3: Transportation and Respiration The Circulatory System Also called the Cardiovascular System Circulates blood in the body Transports nutrients, oxygen, carbon dioxide, hormones, and blood
THE HEART Unit 3: Transportation and Respiration The Circulatory System Also called the Cardiovascular System Circulates blood in the body Transports nutrients, oxygen, carbon dioxide, hormones, and blood
Basic principles of Rheumatic mitral valve Repair
 Basic principles of Rheumatic mitral valve Repair Prof. Gebrine El Khoury, MD DEPARTMENT OF CARDIOVASCULAR AND THORACIC SURGERY ST. LUC HOSPITAL - BRUSSELS, BELGIUM 1 Rheumatic MV disease MV repair confers
Basic principles of Rheumatic mitral valve Repair Prof. Gebrine El Khoury, MD DEPARTMENT OF CARDIOVASCULAR AND THORACIC SURGERY ST. LUC HOSPITAL - BRUSSELS, BELGIUM 1 Rheumatic MV disease MV repair confers
Ch.15 Cardiovascular System Pgs {15-12} {15-13}
 Ch.15 Cardiovascular System Pgs {15-12} {15-13} E. Skeleton of the Heart 1. The skeleton of the heart is composed of rings of dense connective tissue and other masses of connective tissue in the interventricular
Ch.15 Cardiovascular System Pgs {15-12} {15-13} E. Skeleton of the Heart 1. The skeleton of the heart is composed of rings of dense connective tissue and other masses of connective tissue in the interventricular
