WNT SIGNALING IN THE PREIMPLANTATION BOVINE EMBRYO
|
|
|
- Dale McLaughlin
- 5 years ago
- Views:
Transcription
1 WNT SIGNALING IN THE PREIMPLANTATION BOVINE EMBRYO By PAULA TRIBULO A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2017
2 2017 Paula Tríbulo
3 To my husband Marcos for betting on our ambitious dreams, working hard as a team, and for making the dreams come true
4 ACKNOWLEDGMENTS I would like to express my deep esteem and thanks to my advisor, Dr. Peter J. Hansen, for his guidance and mentoring. He helped make my PhD a real milestone in my career. I truly appreciate the effort and time he has devoted to my education, and thank him for demanding more from me than what I thought I could do. I also express my admiration for Dr. Hansen s skills as a team leader by keeping the lab not only productive but making it a nice place to work in. I feel fortunate for having been educated by such an outstanding scientist and now I have the legacy of making him proud of me as one of his scientific descendants. I would also like to extend my gratitude to the members of my advisory committee, Dr. Charles Wood, Dr. Geoffrey Dahl and Dr. Paul Cooke, for their critical contributions to my research and for their positive support. I am grateful to the University of Florida, Department of Animal Sciences and the Animal Molecular and Cellular Biology Graduate Program for the opportunity to pursue my doctorate. I thank Joann Fischer (in memorium) and Renee Parks, our graduate student advisors, for their assistance and patience. I would like to state my appreciation to my lab mates for their collaboration and friendship during these years; Kyle Dobbs, Anna Denicol, Sofia Ortega, Luiz Siqueira, Jasmine Khannampuzha-Francis, Antonio Ruiz, Adriana Zolini, Liz Jannaman, Gulnur Jumatayeva, Eliab Estrada and William Ortiz. I express my deep gratitude towards Veronica Negrón-Perez for being always there for me during these years; without her company, I am sure my doctoral education would have been much more difficult. I will never forget those hours of study at night, gathering to work side by side just to support 4
5 each other and even go swimming when possible. I truly appreciate Vero s wiliness to fit everything around my son s schedule, which was not even her problem, just to help me. I also thank Jim Moss, who really understands how hard grad school is. He has always been willing to help us in those unpredicted situations so that, even when only small things were involved, he made the difference. I also thank Jim and Gail Moss for opening their house to us, and for having the fun traditional pool parties. I am very grateful to Luiz Siqueira, Lilian Oliveira, Beatriz Caetano da Silva Leão, and Khoboso C. Lehloenya for helping me with my research. Without your help I would not had been as productive! I also thank Dr. Tracy Scheffler for helping me with Western blots. Thanks also to William Rembert and Eddie Cummings, for ovary collection, and owners and employees of Central Beef Packing Co., Adena Meat Products L.P., and Florida Beef Inc. for providing ovaries. The majority of my research would not have been possible without your hard work and generosity. Thanks to the wonderful people I have met in Gainesville who have been extremely important to me: Dolo Cenoz, Alberto Gochez, Guada Vera, Juanca Giugni, Vale Zoilo, Esteban Rios, Lucas Ibarbia, Susana Braylan, Horacio Aloe, Euge Cadario, Eduardo Ribeiro, Annette Fahrenkrog, Fito Daetz, Andres Buffoni, Karen D Agostino, Pili Buteler, Alvaro Gonzalez, Jime Laporta, Pancho Peñagaricano, Maca Urrets and Lautaro Rostol. I would like to express my most sincere gratitude to my parents, Ricardo Tríbulo and Maria Manuela del Prado. My Dad taught me, with few words and lots of daily examples, that success takes passion, humility and hard work. My Mom is an exemplar 5
6 of unconditional love that allowed me to see the importance of family. Both of them are imprinted in me and have made me who I am today. I am thankful for the unconditional love and support I always had from my parents. It took me only a phone call to have my Mom come up for six month, leaving everything behind, to help me with my newborn in the last stretch of the program. I will never forget that! Thanks mami! Last but not least, thanks to my husband, Marcos, and my sons, Lautaro and Octavio, for putting up with everything that Mom s PhD implied. 6
7 TABLE OF CONTENTS page ACKNOWLEDGMENTS... 4 LIST OF TABLES LIST OF FIGURES LIST OF ABBREVIATIONS ABSTRACT CHAPTER 1 LITERATURE REVIEW Introduction Preimplantation Embryonic Development in the Cow Overview Embryonic Genome Activation Compaction First Lineage Commitment: Trophectoderm Differentiation Origin of ICM and TE Transcription factors driving ICM-TE segregation Role of Hippo signaling in regulation of TE differentiation Second Lineage Commitment: Hypoblast Differentiation Blastulation WNT Signaling Overview Canonical Wnt signaling WNT/planar cell polarity pathway Calcium signaling WNT Signaling in Embryonic Stem Cells WNT Signaling during Preimplantation Development Evidence for Association of Endometrial Expression of DKK1 and Fertility Objectives of the Present Investigations WNT REGULATION OF EMBRYONIC DEVELOPMENT LIKELY INVOLVES PATHWAYS INDEPENDENT OF NUCLEAR β-catenin Introduction Materials and Methods Embryo Production Developmental Changes in Expression of Selected Genes Involved in WNT Signaling for Embryos Produced in Vitro (Experiment 1)
8 Characteristics of the WNT Signaling System in the Morula and ICM and TE of in-vitro Produced Embryos as Revealed by RNA-Seq (Experiment 2) Localization of Total and Active β-catenin in Bovine Preimplantation Embryos as Determined by Immunofluorescence (Experiments 3 and 4) Changes in Immunoreactive Active β-catenin in Embryos Following Activation of Canonical WNT Signaling (Experiments 5-8) Localization of Active β-catenin in Mouse and Bovine Embryos as Evaluated by Confocal Microscopy (Experiment 9 and 10) Nuclear Localization of β-catenin in Bovine Embryonic Fibroblast Cells Following Activation of Canonical WNT Signaling (Experiment 11) Non-Canonical WNT Signaling Mediated by Phosphorylation of JNK (i.e., MAPK8) by WNT11 in Bovine Blastocysts (Experiment 12-14) Results Developmental Changes in Expression of Selected Genes Related to WNT Signaling for Embryos Produced In Vitro (Experiment 1) Characteristics of the WNT Signaling System in the Morula and ICM and TE of in Vitro Produced Embryos as Revealed by RNA-Seq (Experiment 2) Localization of Total and Active β-catenin in Bovine Preimplantation Embryos as Determined by Immunofluorescence (Experiments 3 and 4) Failure of Canonical WNT Activators to Induce Localization of Nuclear Active β-catenin (Experiments 5 to 8) Localization of Active β-catenin in Mouse and Bovine Embryos Evaluated by Confocal Microscopy (Experiment 9 and 10) Nuclear Localization of β-catenin in Bovine Embryonic Fibroblast Cells Following Activation of Canonical WNT Signaling (Experiment 11) Discussion CONSEQUENCES OF ENDOGENOUS AND EXOGENOUS WNT SIGNALING FOR DEVELOPMENT IN THE PREIMPLANTATION BOVINE EMBRYO Introduction Materials and Methods Embryo Production Using Non-Sex Sorted Sperm Embryo Production Using Sex-Sorted Sperm Immunolabeling of Protein in Bovine Embryos Experiment 1: Effect of Activation of Canonical WNT Signaling by Inhibition of GSK3 on Development Experiment 2: Effect of Activation of Canonical WNT Signaling by the Agonist 2-amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3- methoxyphenyl)pyrimidine (AMBMP) in the Presence or Absence of DKK1 on Development and β-catenin Labeling Experiment 3: Effects of Inhibition of Endogenous WNT Signaling with Wnt- C59 or DKK1 on Ability of Embryos to Develop to the Blastocyst Stage and Blastocyst Cell Number Experiments 4-5: Effects of DKK1 on Development and Blastocyst Cell Number
9 Experiment 6: Effects of DKK1 on Developmental Changes in YAP1 and CDX2 Localization in Morulae and Blastocysts Experiment 7: Effects of DKK1 on Activation of JNK Experiments 8-11: Embryo Responses to WNT7A Statistical Analysis Results Effect of Activation of Canonical WNT Signaling on Development (Experiments 1 and 2) Effects of Inhibition of Endogenous WNT Signaling with Wnt-C59 or DKK1 on Ability of Embryos to Develop to the Blastocyst Stage and Blastocyst Cell Number (Experiment 3) Effects of DKK1 on Development and Blastocyst Cell Number (Experiments 4-5) Effects of DKK1 on Developmental Changes in Immunoreactive YAP1 and CDX2 in Morulae and Blastocysts (Experiment 6) DKK1 Does not Activate pjnk (Experiment 7) Regulation of WNT Signaling by WNT7A (Experiments 8 and 11) Discussion CONSEQUENCES OF EXPOSURE OF EMBRYOS TO DICKKOPF-RELATED PROTEIN 1 AND COLONY STIMULATING FACTOR 2 ON BLASTOCYST YIELD, PREGNANCY RATE, AND BIRTH WEIGHT OF THE CALF Introduction Materials and Methods Animals and Experimental Design Oocyte Retrieval Oocyte Classification, Transport and Maturation Embryo Production Embryo Transfer and Pregnancy Diagnosis Birthweights of the Offspring Statistical Analyses Results In Vitro Production of Embryos Pregnancy Rate Postnatal Characteristics Discussion IDENTIFICATION OF POTENTIAL EMBRYOKINES IN THE BOVINE REPRODUCTIVE TRACT Introduction Materials and Methods Synchronization of the Estrous Cycle Collection of Oviductal and Endometrial Tissues and Uterine Flushings RNA Extraction and Gene Expression Immunofluorescence
10 Western Blotting for CSF Results Expression of Putative Embryokines Expressed in Oviduct Expression of Putative Embryokines Expressed in Endometrium Immunolocalization of Selected Embryokines within Endometrium Accumulation of CSF2 in Uterine Flushings Discussion GENERAL DISCUSSION LIST OF REFERENCES BIOGRAPHICAL SKETCH
11 LIST OF TABLES Table page 1-1 Phenotypes generated by mutation of Wnts in mouse Primer sequences used for real-time PCR Effect of stage of development and cell lineage on expression of genes involved in WNT signaling Effect of WNT11 from day 5 to day 7 after insemination on development of embryos to the blastocyst stage at day Effect of exposure of embryos to GSK3 inhibitor from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage Effect of treatment of embryos with the WNT agonist AMBMP and the endogenous regulator of WNT signaling, DKK1 from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage Effects of inhibition of endogenous WNT signaling from day 5 to day 7 of development with either Wnt-C59 or DKK1, on ability of embryos to develop to the blastocyst stage, and cell number of blastocysts Effect of exposure of embryos to DKK1 from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage and cell number of day 7 blastocysts Effect of treatment of embryos with DKK1 from day 5 to day of development on the ability of male and female embryos to develop to the blastocyst stage and cell number of day 7 blastocysts Effect of treatment of embryos with recombinant WNT7A from day 1 to day 7 of development or from day 5 to 7 of development on the ability of embryos to develop Effect of treatment of embryos with recombinant WNT7A from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage and cell number of day 7 blastocysts Effect of exposure of embryos to the CSF2, DKK1 or the combination on embryonic development and pregnancy rate of cows receiving an embryo Least-squares means for expression of 93 genes in oviduct ipsilateral to the side of ovulation during the first seven days of the estrous cycle
12 5-2 Genes whose expression in the oviduct ipsilateral to the side of ovulation was affected by day of the estrous cycle within the first 7 days after ovulation Genes whose expression in the oviduct was differentially expressed between sides ipsilateral and contralateral to the side of ovulation within the first three days after ovulation Genes differentially expressed in oviduct ipsi and contralateral to the side of ovulation that vary during the first 3 days after ovulation Least-squares means for expression of 93 genes in endometrium during the first seven days of the estrous cycle averaged from both sides of the reproductive tract Genes whose expression in the endometrium was affected by day of the estrous cycle within the first 7 days after ovulation Genes whose expression was affected by the interaction between day of the estrous cycle and side of the reproductive tract relative to ovulation
13 LIST OF FIGURES Figure page 1-1 Position-dependent Hippo signaling in preimplantation embryos Downstream cascades of WNT signaling pathway Developmental changes in expression of selected genes involved in WNT signaling for embryos produced in vitro Representative examples of localization of immunoreactive β-catenin at various stages of preimplantation development of embryos Representative examples of localization of immunoreactive non-phospho (active) β-catenin during preimplantation development Lack of localization of active β-cateninin the nucleus of in vitro produced embryos after activation of canonical WNT signaling with the GSK3 inhibitor Consequences of treatment of embryos with WNT agonists for immunolocalization of active β-catenin Localization of active β-cateninin mouse and bovine embryos by confocal microscopy Immunoreactive phospho-jnk in blastocysts produced in vitro Representative confocal microscopy images of immunoreactive active β- catenin in bovine embryonic fibroblast cells following activation of canonical WNT signaling Treatment of embryos at day 5 after insemination with DKK1 reduces amounts of immunoreactive β-catenin but does not prevent the WNT agonist (AMBMP) from increasing amounts of β-catenin Immunolocalization of the transcription factors YAP1 and CDX2 in morulae and blastocysts Effect of treatment of embryos with DKK1 from day 5 to day 7 of development on accumulation of pjnk Effect of treatment of embryos with WNT7A from day 5 to day 7 of development on accumulation of β-catenin and pjnk Effect of addition of embryokines during day 5-7 after fertilization on birth weight
14 5-1 Expression of the top 50 expressed genes in the oviduct at days 0, 3, 5 and 7 after ovulation Expression of the top 50 expressed genes in the endometrium at days 0, 3, 5 and 7 after ovulation Immunolocalization of CSF2, DKK1 and WNT5A in endometrium Immunolocalization of WNT7A in endometrium Detection of CSF2 in uterine fluid by Western blotting
15 LIST OF ABBREVIATIONS AMBMP BSA cdna COC C T DAPI DMSO DNA DPBS DPBS-PVP EGA FBS FITC ICM IgG IVF IVP mrna PVP qpcr RNA 2-Amino-4-(3,4-(methylenedioxy)benzylamino)-6- (3methoxyphenyl)pyrimidine Bovine serum albumin Complementary deoxyribonucleic acid Cumulus oocyte complex Cycle threshold 4,6-diamidino-2-phenylindole Dimethyl sulfoxide Deoxyribonucleic acid Dulbecco s phosphate buffered saline Dulbecco s phosphate buffered saline- containing 1% (w/v) polyvinylpyrrolidone Embryonic genome activation Fetal bovine serum Fluorescein isothyocyanate Inner cell mass Immunoglobulin G In vitro fertilization In vitro produced Messenger ribonucleic acid Polyvinylpyrrolidone Quantitative real time polymerase chain reaction Ribonucleic acid SOF-BE2 Synthetic oviductal fluid-bovine embryo 2 TE Trophectoderm 15
16 Note: Gene symbols are used without definition. Gene names can be retrieved from Pubmed 16
17 Abstract of Dissertation Presented to the Graduate School of the University of Florida in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy WNT SIGNALING IN THE PREIMPLANTATION BOVINE EMBRYO By Paula Tríbulo August 2017 Chair: Peter J. Hansen Major: Animal Molecular and Cellular Biology Errors during preimplantation development result in embryonic mortality and/or suboptimal phenotype after birth. Although WNT signaling regulates several developmental processes, its specific role during preimplantation development remains unclear. The aim of the research presented in this dissertation was to unravel the role of WNT signaling during preimplantation development and evaluate the participation of maternal cues on the regulation of embryonic WNT signaling pathways within the embryo. First, it was found that the typical WNT signaling mediated by nuclear localization of β-catenin is not fully functional during preimplantation development and WNT signaling relies on non-nuclear β-catenin as well as β-catenin independent pathways. Then, the consequences of endogenous and exogenous WNT signaling for development in the preimplantation embryo were evaluated. Results indicate that embryo-derived WNTs are dispensable for blastocyst formation, but participate in the regulation of inner cell mass (ICM) proliferation, likely through a mechanism independent of β-catenin. The reduction in blastocyst development upon stimulation of WNT signaling with the agonist 2-amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3- methoxyphenyl)pyrimidine (AMBMP) and the increase of embryos becoming blastocyst 17
18 when exposed to WNT7A lead to the hypothesis that maternally-derived WNTs can play a positive or negative role in regulation of preimplantation development. Subsequently, expression of WNT-related molecules was assessed in the reproductive tract during the first seven days after ovulation, the time at which preimplantation development takes place in the bovine. A large number of WNT and WNT-related proteins were detected underpinning the hypothesis that the dam regulates WNT signaling in the embryo. An additional objective of the studies presented here was to evaluate whether or not WNT signaling during preimplantation programs the embryo to have different characteristics after birth. In vitro produced embryos were treated with the WNT-regulatory molecule, DKK1, and resulted in calves with reduced birth weights. This result illustrates the ability of DKK1 to alter the pattern of development of the bovine embryo to affect postnatal phenotype. Overall, results show that WNT signaling is maternally-regulated, and although dispensable for blastocyst formation, it imposes changes in the preimplantation embryo that modify the postnatal phenotype.. 18
19 CHAPTER 1 LITERATURE REVIEW Introduction Successful development of a newly-formed embryo is a complex process. In the cow, less than 50% of zygotes are viable 7 days after fertilization (Sartori et al., 2010) and about 25% of embryos reaching the blastocyst stage are lost before calving (Hansen, 2011). Maternal errors and inherent defects in the embryo cause these embryonic losses. Knowledge on the regulatory mechanisms of embryonic development and on the optimal maternal environment to maximize pregnancy outcomes is incomplete. Although outcomes of procedures for vitro embryo production reveal that maternal signals are not essential during the preimplantation period, the importance of maternal cues as a modulator of development is revealed by the aberrant characteristics of in vitro derived blastocysts for gene expression (Corcoran et al., 2006; McHughes et al., 2009; Gad et al., 2012), metabolism (Khurana and Niemann, 2000), lipid content (Crosier et al., 2000; Sudano et al., 2012), ultrastructure (Boni et al., 1999; Rizos et al., 2002), and DNA methylation (Niemann et al., 2010). Moreover, the pregnancy rate of embryos produced in vitro is lower than that for embryos produced in vivo (Lonergan et al., 2007; Pontes et al., 2009). Another strong piece of evidence for the influence of maternal environment on embryonic development is the need of synchrony between donor and recipient animals in embryo transfer programs. (Newcomb and Rowson, 1975; Hasler et al., 1987). The environment in which the embryo undergoes preimplantation development can affect development even to the point of modifying phenotypic characteristics after birth. For instance, the offspring of pregnant rodents fed a low protein diet exclusively during the 19
20 time when the embryo undergoes preimplantation development displayed cardiovascular and metabolic diseases in adulthood (Kwong et al., 2000; Watkins et al., 2008). Bovine embryos exposed to colony stimulating factor 2 (CSF2) for two days during preimplantation development grow faster after birth than those not exposed to CSF2 (Kannampuzha-Francis et al., 2015). Similarly, offspring from in vitro derived embryos show altered postnatal phenotype compared to their in vivo counterparts such as cardiometabolic dysfunction in humans (Ceelen et al., 2008), behavioral alterations in anxiety and deficiencies in memory in mice (Fernandez-Gonzalez et al., 2004) and large offspring syndrome in cattle (Behboodi et al., 1994; Numabe et al., 2000). This dissertation focuses on the role of one potential maternal signaling system for regulation of embryonic development the WNT system. Originally described as being involved in wing formation in Drospophila (Wingless) and mammary development in mammals (Int), the WNT family of secretory ligands participates in diverse developmental processes including cell proliferation (Logan and Nusse, 2004), maintenance of pluripotency (Sato et al., 2004; Sokol, 2011), differentiation (Liu et al., 2014) and migration (Morosan-Puopolo et al., 2014). WNT signaling has been proposed as one system involved in embryo-maternal cross talk because WNT-related molecules are produced in the reproductive tract and embryonic survival in mice can be reduced through manipulation of WNT signaling pathways around the time of implantation (Mohamed et al., 2004; Hayashi et al., 2007). WNTs are also involved in regulation of pluripotency (reviewed by Nusse et al., 2008; Clevers et al., 2014) and may therefore be important for some of the early differentiation events in the preimplantation embryo. Despite the potential for WNTs to play an important role in development of the 20
21 preimplantation embryo, the specific role of these molecules during preimplantation development is rudimentary. The following literature review will begin with an overview of the key events that take place during preimplantation development. The main focus will be on the bovine embryo but information from other species is included when pertinent. Thereafter, an overview of WNT signaling will be provided and then current knowledge of the role of WNTs during preimplantation development in mammalian embryos will be summarized. Preimplantation Embryonic Development in the Cow Overview Preimplantation development refers to early developmental processes in mammalian embryos that take place before implantation and while the embryo transits through the oviduct to reach the uterus. In the species that have been most studied, the mouse and human, implantation takes place at the blastocyst stage of development so the preimplantation period encompasses the time from fertilization to blastocyst formation. In the cow, however, implantation does not formally occur (the trophoblast does not penetrate the endometrial epithelium to invade the endometrial stroma) and the first attachments of the trophoblast to the endometrium occur at about day 21 (King et al., 1981). Thus, the preimplantation period is prolonged in the bovine and involves many more differentiation events than simply formation of the blastocyst. For purposes of this dissertation, however, and to make comparisons with the mouse and human more appropriate, the preimplantation period in the cow will be considered as continuing up to formation of the blastocyst. 21
22 Development of the bovine embryo to the blastocyst stage takes approximately 7 days, with embryos entering the uterus on day 5 after fertilization (Betteridge and Fléchon, 1988). The fertilized egg, i.e. the zygote, undergoes subsequent cell divisions to originate 2-, 4-, 8-cell developmental stages and so on. These cellular divisions are often asynchronous allowing the existence of embryos with uneven number of blastomeres (Betteridge & Fléchon 1988). One characteristic of cellular divisions during the preimplantation period is that the size of individual cells is reduced as proliferation advances, as a consequence of the constant size of the entire embryo. Therefore, these divisions are referred to as cleavage divisions. Individual blastomeres are distinguishable up to the 16-cell stage but thereafter, compaction occurs, individual cell boundaries are obscured and the embryo acquires a blackberry (morus in Latin) appearance and is termed a morula. Following the appearance of a blastocoelic cavity and the first cell lineage commitment, the totipotent morula is transformed into a multipotent blastocyst. During preimplantation development there are four critical events including: 1) the transition from maternal to zygotic control of development, also termed embryonic genome activation; 2) compaction at the morula stage; 3) cell lineage commitment and 4) blastocyst formation. Embryonic Genome Activation Before the embryo is able to transcribe its own genome, cellular function and proliferation is under the control of maternal mrna accumulated and stored in the oocyte during oocyte maturation (Schier, 2007; Fair, 2010). The transition from maternally-derived mrna to embryonic-synthesized transcripts has been termed embryonic genome activation (EGA) or the maternal-zygote transition and is a key 22
23 event associated with successful early differentiation, implantation and fetal development (Niemann and Wrenzycki, 2000). The timing for initiation of EGA is species-specific, taking place at the 2 cell stage in the mouse (Wang and Dey, 2006; Li et al., 2013), at the 4-8 cell stage in humans and swine (Braude et al., 1988; Sirard, 2012), and at the 8 cell stage in the bovine (Graf et al., 2014a). Prior to EGA, developmental changes in protein synthesis depend on posttranscriptional regulation of maternally-derived mrna (Reyes and Ross, 2016). Molecules involved in this process include cis-acting regulatory sequence elements, proteins that bind to them or that modify RNA binding proteins and other proteins that participate in translation. Cytoplasmic polyadenylation element (CPE) is one of the regulatory elements present in mrna molecules that have been widely studied. Via binding to CPE binding protein (Cao and Richter, 2002), CPE labels maternal mrna for translation during oocyte maturation (Ma et al., 2013). Maternal mrna lacking CPE become deadenylated and translationally inactive (Fox and Wickens, 1990; Varnum and Michael Wormington, 1990). Deadenylated mrna are either subsequently degraded or can be readenylated after fertilization (Paynton et al., 1988). Readenylation in mammals has been proposed to be an evolutionary vestige of the mechanism used in lower vertebrates for control of protein synthesis in the embryo; in these species, readenylation of maternally derived RNA is a key event to allow development within hours from fertilization (Bachvarova et al., 1985). The processes implicated in EGA include degradation of oocyte-derived mrna, protein turnover, rearrangement of cellular organelles and cytoskeleton, uncharacterized actions of specific maternal proteins composing the subcortical 23
24 maternal complex, epigenetic changes in the chromatin to allow transcription, and acquisition of transcription machinery (reviewed by Stitzel and Seydoux, 2007). In the mouse, these changes start with resumption of meiosis in the oocyte and redistribution of mitochondria and endoplasmic reticulum (ER) (Voronina and Wessel, 2003). Fertilization is also involved in EGA because it initiates Ca + signaling inside the egg (Malcuit et al., 2006) by activating phosphatidylinositol 3-kinase signaling (Miyazaki et al., 1989). This mechanism induces the cell machinery to control subsequent events including cell cycle resumption and internalization of ER (FitzHarris et al., 2003) and is crucial for EGA (Zheng et al., 2010). Furthermore, fertilization triggers digestion of the vast majority of maternal transcripts in the oocyte (Potireddy et al., 2006), another key event for the success of EGA (Ma et al., 2013). Removal of maternal mrna involves loss of masking proteins that prevent transcripts from degradation in the oocyte, such as mouse specific Y-box protein 2 (Medvedev et al., 2008) and activation of mirna that impair translation of maternal mrna (reviewed by Lee et al., 2014). Chromatin remodeling to allow transcription involves epigenetic changes whose regulation is partially mediated by Brg1 protein. The key role of maternally-derived Brg1 in controlling EGA is reflected by the massive reduction in transcription and developmental arrest at the 2-cell stage in embryos derived from null mutant oocytes (Bultman et al., 2006). These mutant embryos show reduced methylation of histone 3, indicating that the mechanism mediating defective EGA involves epigenetic marks. Indeed, Brg1 changes the conformation of DNA around histones. The activation of embryonic transcription is a stepwise process involving two major rounds of embryonic zygotic transcription. In the first, denominated as minor 24
25 EGA, there is a small set of de novo transcripts, followed by a major round of embryonic transcription that reprograms the gene expression pattern (reviewed by Kanka, 2003). In bovine embryos, the minor activation of transcription is observed at the 2-4 cell stage (Memili and First, 2000; Kues et al., 2008), and is also thought to set the stage for the major EGA (Graf et al., 2014a, b). This idea is supported by two findings. One is that the approximately 400 genes initially transcribed (Graf et al., 2014a, b) are dispensable for embryonic development since blocking transcription at this stage does not impair blastocyst formation (Liu and Foote, 1997). The second is that these genes are involved in functions including RNA processing, protein synthesis and protein transport (Graf et al., 2014a) which are consistent with a role for preparation of the cell for the upcoming massive transcription of the genome. Gene ontology analysis of the approximately 4200 genes activated in the major EGA indicates participation of the genes in RNA splicing; mrna transcription from RNA polymerase II promoter; regulation of transcription from RNA polymerase II promoter; purine nucleotide biosynthesis; and 5S class rrna transcription from RNA polymerase III type 1 promoter. Compaction Compaction is the first morphological change in the preimplantation embryo and is characterized by a reduction in the entire volume of the embryo as well as the loss of clearly-identifiable boundaries between individual cells. The process is driven by cytoskeletal changes in the embryo and is a prerequisite for subsequent differentiation of embryos into differentiated cells of the blastocyst. Most of the current understanding of compaction comes from the mouse model. In this species, compaction starts at the 8-cell stage of development when polarization is established in blastomeres by the emergence of an apical and a basolateral domain 25
26 of the cellular membrane (Fleming and Pickering, 1985; Johnson and McConnell, 2004). At this moment, cellular contacts increase by close membrane apposition and intercellular space is minimized. Consequently, blastomeres flatten against each other becoming no longer individually distinguishable (Calarco and Brown 1969, Ducibella et al., 1977). The cells of the morula eventually show variation in appearance with outer cells remaining polar but with inner cells being not polarized. The mechanisms involved in compaction include cellular adhesion, cortical tension and filopodia. Early studies documented the key role of cell adhesion in compaction because the process could be inhibited either by blocking Ca + -dependent adhesion (Fleming et al., 2001) or targeting cell surface glycoproteins (Ducibella and Anderson, 1975). E-cadherin is the essential glycoprotein mediating compaction since the process is completely disrupted in its absence (Stephenson et al., 2010). Ligation of extracellular domains of E-cadherins from adjacent cells result in formation of adherens junctions while intracellular domains allow connection of the cellular membrane and the cortex via interaction with the cytoskeleton (Hoffman and Yap, 2015). During compaction other junctional complexes are established as well, including desmosomes, tight and gap junctions (Bell et al., 2008). The role of these other complexes in compaction is not clear. For instance, blockage of the main constitutive protein of the gap junction, connexin-43 (Cx43), results in unraveling of compaction and extrusion of blastomeres from the rest of the embryo (Becker and Davies, 1995). Connexin-43 knockout mice, however, successfully progress through compaction (De Sousa et al., 1997), suggesting the existence of compensatory mechanisms. 26
27 Even though cell adhesion is widely accepted to be critical for compaction (Kemler et al., 1977; Vestweber et al., 1985), the role of E-cadherin in this process has been questioned (Maître et al., 2012). The tension mediated by E-cadherin increases contact surfaces between cells and is referred to as adhesive tension. Cortical tension, on the other hand, is generated by a dynamic network of actin filaments connected to the cell membrane that are contracted or expanded by myosin motor proteins of the cytoskeleton (Pasternak et al., 1989) so as to reduce intercellular contacts, i.e. conferring cells with a spherical shape. The differential interfacial tension hypothesis postulates that cellular shape is dictated by the balance between cortical and adhesive tensions (Brodland, 2002). Accordingly, cells allocate in such a pattern that maximizes adhesive tension minimizing their cortical tension. Indeed, the role of cortical tension in compaction has been recently reported (Maître et al., 2015). Other players in the process of compaction are the filopodia which have been recently proposed to participate in drawing adjacent cells closer to each other (Fierro- González et al., 2013). Filopodia were first described as membrane protrusions containing E-cadherin and proteins that link it to the actin cytoskeleton i.e. α- and β- catenin observed in mouse embryos from 8-cell (at the onset of compaction) to 16-cell stage (compacted) (Fierro-González et al., 2013). Interestingly, only a fraction of blastomeres possess filopodia and they are extended to few neighboring cells simultaneously. Further characterization of these structures is needed to understand their role and mechanism of action during compaction. Regulatory pathways involved in regulation and timing of compaction are unknown. It has been proposed that the nucleus:cytoplasm ratio is responsible for 27
28 triggering compaction, as it is important in compaction in fish and amphibians (Newport and Kirschner, 1982). Initial studies showed that artificially increasing the nuclear:cytoplasm ratio by aspirating cytoplasmic material from 1-cell mouse embryos results in early compaction at the 4-cell stage (Feng and Gordon, 1997). The experimental design of this study does not allow determination as to whether aspiration exerted its affect by altering the nuclear:cytoplasm ratio or by extraction of maternal factors regulating embryonic morphogenesis. Moreover, restoring cytoplasmic material has diverse outcomes depending upon the developmental stage of the embryo used as the cytoplasm donor (Lee et al., 2001). Specifically, cytoplasm from advanced stage donor embryos delayed embryonic morphogenesis, whereas cytoplasm from the 1-cell donor hastened it. (Lee et al., 2001). In the cow, compaction occurs at day 6 when the embryo has approximately 32 blastomeres (Betteridge & Flechón 1988). Comparison of the expression of CX43 during preimplantation development of in vivo- and in vitro-derived embryos suggests that the mechanism of cell-cell communication differ between these embryos (Wrenzycki et al., 1996). While in vivo derived embryos express CX43 throughout the preimplantation development; morulae and blastocysts derived from culture do not express CX43, but still develop to the blastocyst stage, suggesting redundant mechanisms involved in cell-cell communication. First Lineage Commitment: Trophectoderm Differentiation Although segregation of the first two distinct cell populations of the embryo, trophectoderm (TE) and inner cell mass (ICM), is not established until the blastocyst stage, events underpinning cell fate take place earlier during preimplantation development. Whether blastomere fate is established before, during or after compaction 28
29 is debated (Rossant and Tam, 2009; Zernicka-Goetz et al., 2009; Burton and Torres- Padilla, 2014) as will be discussed further in this section. Origin of ICM and TE Using the mouse model, two main models have been proposed to explain TE differentiation. The polarity model identifies polarization of blastomeres at the 8-cell stage as a key regulator of cell lineage commitment (Johnson et al., 1981). The division plane of the polar blastomeres dictates whether the two daughter cells are polarized TE cells (as a consequence of cellular division along the apical-basal plane, i.e. symmetrical division) or one TE and one ICM cell (as a consequence of cellular division along the meridional plane, i.e. asymmetrical division). The positional model, on the contrary, postulates that cell fate is dictated by the position of the cell within the embryo at the 16-cell stage (Tarkowski and Wróblewska, 1967). Thus, inner cells of the 16-cell stage embryo become ICM while outer cells become TE. In agreement with this model, changing position of blastomeres at the 16 and 32-cell stages resulted in modification of cell lineage (Hillman et al., 1972; Kelly et al., 1977; Suwinska et al., 2008). It is important to bear in mind, however, that manipulation of cell position affects both polarization and cell fate (Ziomek et al., 1982 a, b; Eckert et al., 2005). Consequently, a more recent model has been proposed to refine the polarity model by considering molecular aspects associated with polarity (Yamanaka et al., 2006). In this case the presence or absence of an apical domain (characteristic of polar cells) is proposed to drive cell fate decisions. The fact that a defined feature, i.e. apical domain, dictates the fate of the cell is less questionable than the more subjective classification of inner and outer cells, which is typically based on localization of nuclei and which can be quite variable among blastomeres (Ajduk et al., 2014). 29
30 Recently, labeling cellular membrane and recording embryo development with high resolution, followed by computationally segment of embryos allowed characterization of ICM formation in live mouse by tracking individual cell shape and position over time (Samarage et al., 2015). Behavior of most blastomeres (over 100 embryos were videotaped and studied) lead the authors to propose that ICM originates from symmetrical cell divisions of polar cells at the 12-cell stage that are subsequently internalized by apical constriction, a process driven by contraction of actomyosin networks that shrink the apical surface. Some scientists argue that cell fate commitment does not occur until the embryo undergoes compaction. Evidence includes variable distribution of inner and outer cells expressing caudal type homobox 2 (CDX2; TE marker) and Nanog homeobox (Nanog; epiblast marker) before compaction (Ditrich 2007), plasticity in cell fate when experimentally blastomeres are extracted or repositioned (Papaoiannou and Ebert 1986), and failure to identify uneven distribution of fate markers in mammalian embryos at early stages of development (Kurotaki et al., 2005; Motosugi et al., 2005). Others propose that molecular heterogeneities that predict cell fate can be observed as early as the 2 to 8-cell stage (Gardner, 2001; Plachta et al., 2011; Shi et al., 2015). Examples of predictors include sperm entrance position, which is proposed to dictate the plane of cellular division establishing heterogeneity between blastomeres (Piotrowska and Zernicka-Goetz, 2001), and timing of first cleavage (Piotrowska et al., 2001). Transcription factors driving ICM-TE segregation Whether because of position or polarity, cell fate is ultimately controlled by expression of lineage-specific transcription factors. These transcription factors are species-specific and have been substantially studied in mouse embryos. In bovine 30
31 embryos, however, little information is available and will be detailed towards the end of this section. In mouse embryos, markers for TE include Cdx2, Eomesodermin (Eomes), Gata binding protein 3 (Gata3), Kruppel-like factor 5 (Klf5), and TEA domain family member 4 (Tead4) (Beck et al., 1995; Niwa et al., 2000; Strumpf et al., 2005); while POU domain, class 5, transcription factor 1 (Pou5f1), Nanog, and SRY (sex determining region Y)-box 2 (Sox 2) are markers of cell pluripotency within the ICM (Cao, 2013). Expression of Pou5f1and Cdx2 is ubiquitous at the 8-cell embryo but Cdx2 becomes restricted to outer cells at the morula stage, while Pou5f1is confined to nuclei of the ICM at the blastocyst stage (Niwa et al., 2000; Strumpf et al., 2005). Expression of Cdx2 and Pou5f1 is mutually exclusive at the blastocyst stage, as indicated by Cdx2 -/- mouse embryos whose phenotype retains expression of Pou5f1 and Nanog in outer cells (Strumpf et al., 2005). The transcription factors driving TE differentiation regulate one another. Thus, Gata3 and Cxd2 are regulated by Tead4 (Ralston et al., 2010; Hirate et al., 2012). Moreover, Cdx2 is also regulated by Klf5 (Lin et al., 2010). Downstream of Cdx2 is Eomes (Strumpf et al., 2005). Studies using knock out embryos for the genes encoding these transcription factors show that in absence of either Klf5 or Tead4, embryos fail to form blastocyst and neither Cdx2 nor Eomes are expressed (Yagi et al., 2007; Lin et al., 2010). Subsequently, it was found that Tead4 directly activates Cdx2 enhancer (Rayon et al., 2014). When Cdx2 or Eomes are deleted, blastocysts form but abnormalities in TE result in impaired implantation (Strumpf et al., 2005). Interestingly, Klf5 and Tead4 are detected from the 2 cell stage through the blastocyst stage and they both localize to 31
32 the outer cells of the blastocyst (Yagi et al., 2007; Lin et al., 2010). Furthermore, Gata3 Cdx2 and Eomes are first expressed at the 4-cell, 8-cell and blastocyst stages of development, respectively (Strumpf et al., 2005; Home et al., 2009). Among the transcription factors characterizing ICM, Sox2 is the first to be selectively expressed in inner cells, at the 16-cell stage (Guo et al., 2010), and is confined to ICM progenitors before blastocyst formation (Wicklow et al., 2014). Expression of Sox2, however, is observed as early as the unfertilized oocyte throughout preimplantation development (Li et al., 2013). Uniform expression of Pou5f1is observed from the 8-cell stage, but in contrast to Sox2, it remains ubiquitous up to the middle-tolate blastocyst stage, when it becomes restricted to ICM (Dietrich and Hiiragi, 2007; Palmieri et al., 1994). Similarly, Nanog has a wide distribution until late blastocyst stage when it is found only in a sub population of the ICM (Strumpf et al., 2005; Dietrich and Hiiragi, 2007; Wicklow et al., 2014). In the cow, CDX2 and POU5F1 are co-expressed in all nuclei up to the morula stage, similar to the situation in mouse embryos. In contrast to the mouse, they remain co-expressed at the blastocyst stage due to lack of a mutation in the regulatory sequence of POU5F1 present in the mouse that allows CDX2 binding and repression of POU5F1 expression in TE cell (Berg et al., 2011). The key role of CDX2 on TE differentiation was initially revealed by failure of TE development after CDX2 inactivation (Berg et al., 2011). More recently, blastocyst development was observed in CDX2 depleted embryos, but embryos defects included epithelial integrity lost and upregulation of NANOG (Goissis and Cibelli, 2014; Sakurai et al., 2016). Furthermore, TE differentiation is characterized by increasing expression of CDX2 with decreasing 32
33 pluripotent capabilities that becomes irreversible by day 11 of development (Berg et al., 2011). Another difference between mouse and bovine embryos is that TEAD4 is not a master transcription factor in bovine embryos. Downregulation of TEAD4 neither impairs blastocyst formation nor affects expression of CDX2, GATA3 or POU5F1 (Sakurai et al., 2016). Master transcription factors responsible for maintenance of ICM remain unknown in bovine. In late blastocysts, Nanog and Sox2 are confined to ICM as is the case for mouse embryos (Kuijk et al., 2008). Role of Hippo signaling in regulation of TE differentiation As mentioned above, polarization, position and expression of master regulators are involved in cell lineage segregation. Recently, the Hippo signaling pathway has been implicated as a key connector of these regulators in the mouse embryo. Hippo signaling participates in the regulation of expression of the master transcription factor that mediates TE differentiation, Tead4. The core molecules of the Hippo pathway are the protein kinases Mst1/2 and Lats1/2, their transcriptional coactivators Yap1 and Taz, and the target transcription factors Tead1-4 (Figure 1-1). E- cadherin-mediated cell-cell adhesion stimulates Hippo signaling (Kim et al., 2011), which, when active, prevents nuclear translocation of Yap1, which is a transcriptional co-activator of Tead4 (Zhao et al., 2007, 2010). Consequently, activation of the pathway suppresses expression of target genes, among them, Tead4. Hippo signaling is spatially regulated, and therefore differentially activated in polar and apolar cells. The Hippo pathway components angiomotins (encoded by Amot) are proteins involved in phosphorylation-mediated inactivation of Yap1. In preimplantation embryos Amot has a highly distinctive distribution between polar and apolar cells (Hirate et al., 2013; Leung and Zernicka-Goetz, 2013). In polar (outer) cells, angiomotin (Amot) is restricted to the 33
34 apical domain where its phosphorylation is impaired so that Hippo signaling is not activated and Tead4 expression occurs (Hirate et al., 2013). In contrast, basolateral localization of Amot in apolar cells results in activation of Hippo signaling and a block to nuclear accumulation of Yap1 via phosphorylation of Lats 1/2 and Hippo-independent binding of Yap (Chan et al., 2011; Paramasivam et al., 2011; Hirate et al., 2013). Second Lineage Commitment: Hypoblast Differentiation By the late blastocyst stage, a third cell type, the hypoblast, differentiates from ICM (Kuijk et al., 2008). This cell lineage, called the primitive endoderm in mice, forms an epithelium located on the surface of the ICM lying in contact with the blastocyst cavity. When the hypoblast forms, the remaining cells of the ICM, which remain pluripotent, are denominated as epiblast. In mouse, segregation of hypoblast cells was thought to be driven by the position of the cell within ICM (Enders et al., 1978). The positional induction model proposed that cells on the surface of the ICM in contact with the blastocoel are sensitive to an inductive signal that triggers cell differentiation, and that the hypothetical signal does not rich deep in the ICM. This model was supported by the observation that cells isolated from outer ICM differentiate into hypoblast (Dziadek, 1979). One assumption of this model was that all cells within ICM have an equivalent capability to become either epiblast or hypoblast (Zernicka-Goetz et al., 2009). Subsequently, it was discovered that the ICM consists of a heterogeneous population of cells, characterized by a mix of cells expressing different levels of the transcription factors Nanog and Gata6 as markers of epiblast and hypoblast precursors. As a result, a sorting model has been proposed in which hypoblast cells first differentiate and then move to the outer part of the ICM (Rossant et al., 2003; Chazaud et al., 2006). Time-lapse dynamics of Gata6 localization 34
35 showed that hypoblast precursors move from deep to surface layers within ICM (Plusa et al., 2008). This study also revealed that some cells expressing Gata6, change their fate by switching to Nanog expression, or undergo apoptosis. The recent finding that some ICM cells contribute to both cell types (Meilhac et al., 2009) has led to a new model in which both cell movement and positional induction are proposed to participate in lineage segregation (Meilhac et al., 2009). It is now accepted that lineage allocation of cells in the ICM to hypoblast and epiblast involves a temporal sequence of 1) initial co-expression of lineage-specific transcription factors, 2) mutually-exclusive expression of transcription factors where hypoblast and epiblast precursors are distributed in the ICM in a salt-and-pepper manner, and 3) cell movement leading to the sorting and spatial segregation of the epiblast and hypoblast cell lineages (Plusa et al., 2008; Meilhac et al., 2009). The upstream mechanisms that lead to the mosaic pattern of distribution of hypoblast and epiblast precursors, and the pathways required for a fated cell to reach its final position remain unknown. One interpretation of the salt-and-pepper distribution of cells in the ICM has been that formation of cells destined for hypoblast and epiblast is random. An alternative view is the social mobility model in which it is proposed that a first wave of cells becomes epiblast and the second becomes hypoblast. Those cells in the inadequate location move, switch type, or undergo apoptosis (Zernicka-Goetz et al., 2009). Nevertheless, the exact mechanisms that connect cell polarity, cell position, and cell signaling to the cell fate in blastocysts remain to be determined. One of the first lineage-specific transcription factors expressed in hypoblast is Gata6, which has been observed around the 16 to 32-cell stage. Gata4, another 35
36 transcription factor of the Gata family, is expressed later, around the 64-cell stage, when the salt-and-pepper distribution is observed, and cells are likely to have a defined fate. Gata6 null mutant mice have defective visceral endoderm and die (Morrisey et al., 1998; Koutsourakis et al., 1999). Once hypoblast precursors are positioned underlying epiblast, Gata6 is co-expressed with Sox17, and this latter transcription factor is required for maintainance of hypoblast commitment (Artus et al., 2011). Additionally, other markers of hypoblast have been recognized including Creb312, Dab2, Fgfr2, Fn1, Grb2, Pdgfrα, Runx1, Snai1, Tcf23 (Cai et al., 2008; Plusa et al., 2008; Guo et al., 2010). Fibroblast growth factor (FGF) has been implicated in regulation of hypoblast formation since mutations in Fgf4 ligand (Feldman et al., 1995; Goldin and Papaioannou, 2003), Fgfr2 receptor (Arman et al., 1998) or Grb2, a downstream signaling molecule (Chazaud et al., 2006), result in lack of hypoblast differentiation. During preimplantation embryonic development, Fgf4 and Fgfr2 are the most highly expressed members of the FGF signaling system (Niswander and Martin, 1992; Arman et al., 1998). Although ubiquitous initially, these molecules become asymmetrically expressed by the 32-cell stage, with the ligand and receptor higher in epiblast or hypoblast precursor cells, respectively (Guo et al., 2010). By the 64-cell stage, expression of these genes becomes mutually exclusive, with Fgf4 only in epiblast and Fgfr2 in hypoblast (Messerschmidt and Kemler, 2010; Frankenberg et al., 2011). Examination of the genes regulated by Gata6 indicates that pathways upregulated in cells undergoing hypoblast differentiation include those involved in the Ras/Erk pathway, canonical WNT signaling, and a signaling axis linking G-protein 36
37 signaling to RhoA and the ERM protein moesin (Verheijen et al., 1999; Liu et al., 2002; Krawetz and Kelly, 2008). It has been suggested that the coordinate signaling by these independent pathways is required to induce cell differentiation by modulating expression of target genes and triggering cytoarchitectural changes. In cattle, the hypoblast begins to form as an epithelial layer by day 8 after insemination (Kuijk et al., 2012). The only current marker for hypoblast in cattle is GATA6. In bovine embryos GATA6 has been observed in almost all nuclei at Days 5 and 6 after insemination (Kuijk et al., 2012). Further, d 40% of ICM cells in blastocysts at day 7 and 8 after fertilization expressing GATA6 and the epiblast marker NANOG concomitantly on day 7, while only 7% of those cell retain the double expression on day 8 (Kuijk et al., 2012). Similar to the mouse, hypoblast formation in bovine embryos is also associated with the FGF/MAPK pathway. The proportion of ICM cells expressing epiblast-specific transcription factor and hypoblast expressing GATA6 is altered by modulating FGF/MAPK signaling. Thus, treatment of bovine embryos with FGF receptor or MEK inhibitors reduces GATA6 positive cells (Kuijk et al., 2012). Different from the mouse, however, GATA6 positive cells are still present when FGF receptors are inhibited, suggesting that more upstream regulators are involved in driving hypoblast differentiation (Kuijk et al., 2012). Since hypoblast cells are the only cell type of blastocyst maintaining GATA6, it is reasonable to think that GATA6 regulates expression of genes that play a central role on hypoblast function. Blastulation Concomitant with the first cell fate decision that segregates TE and ICM, the embryo cavitates to form the blastocyst. The process, known as blastulation, involves 37
38 acquisition of intercellular junctional complexes to form a sealed, epithelial like layer of cells around the embryo (Ducibella and Anderson, 1979), functional modification of cytoskeletal elements (Ducibella et al., 1977); migration of lipid vesicles and mitochondria to the cellular cortex (Wiley 1987); and concentration of Na + /K + -ATPase within the plasma membrane lining the blastocoel (Borland et al., 1977). It is proposed that the polarity of ion/solute transporters, generated as a consequence of blastomere polarization, creates an osmotic gradient across TE cells promoting diffusion of water and thereby leading to the formation of the blastocoel. Initially, the trans trophectoderm ionic gradient was thought to be energized by Na + /K + -ATPase since blastocoel formation is abolished after blockage of Na + (Betts et al., 1997). The rapid transport of water that takes place during cavitation relative to the small osmotic gradient generated by the Na + /K + -ATPase confined to the basolateral membrane domain promoted further investigation that led to the discovery of the presence of aquaporins (AQP) in TE cells (Barcroft et al., 2003). Aquaporins are transmembrane proteins functioning as molecular water channels that allow water flow in direction of osmotic gradients. Additional mechanisms exerted by other structures/molecules may exist since Aqp knockout embryos develop to the blastocyst stage (Marikawa and Alarcon, 2012). Bovine embryos express AQP at the blastocyst stage (Camargo et al., 2011 WNT Signaling Overview The gene encoding for WNT was discovered by two independent research groups. Scientists studying breast cancer in a mouse model named it Int-1(Nusse and Varmus, 1982); while the group observing a wingless phenotype in knock out 38
39 Drosophila named it wingless (Nüsslein-Volhard and Wieschaus, 1980). Subsequently, the homology between Int-1 and wingless was found (Rijsewijk et al., 1987) and the name WNT arose. WNT signaling participates in multiple developmental events during embryogenesis as well as adult tissue homeostasis. Functions of this complex signaling pathway include regulation of cell proliferation (Logan and Nusse, 2004), maintenance of pluripotency (Sato et al., 2004; Sokol, 2011), differentiation (Liu et al., 2014) and migration (Morosan-Puopolo et al., 2014). Several Wnt loss of function mutations have been generated in the mouse producing phenotypes revealing the key role of WNT on a wide range of developmental processes (Table 1-1). The role of WNT signaling during preimplantation development, however, has not been well elucidated and will be discussed in detail later in this review. The diverse functions of WNT signaling are the result of the complex nature of the signaling pathway. The signaling system is regulated by 19 WNT ligands that interact with a variety of receptors including 10 frizzled receptors (FZD), receptor tyrosine kinase like orphan receptor 2 (ROR2), protein tyrosine kinase (PTK7), knypek (KNY) and related to tyrosine kinases (RYK) and co-receptors such as LDL receptor related protein (LRP 5/6), kremen and leucine rich repeat containing G protein-coupled receptor 4 (LGR4) (reviewed by Cadigan and Nusse, 1997; Logan and Nusse, 2004). Ligands may also interact with extracellular WNT regulators such as dickkopf proteins (DKK 1-4), secreted frizzled-related proteins (SFRP 1-5), and WNT inhibitor factor (WIF1). DKK family proteins influence the signaling of WNTs by binding to the FZD coreceptors LRP5/6 (Mao et al., 2001) and kremen (Mao et al., 2002). 39
40 DKK1 has a strict inhibitory effect on FZD receptor (Kazanskaya et al., 2000) that depends on the availability of the kremen 1 coreceptor. In addition to the inhibitory effect, DKK1 can also activate alternative WNT downstream signaling mediated by small GTPases (Caneparo et al., 2007). Similarly, DKK2 and DKK4 require kremen 2 to inhibit WNT signaling, and cannot function with kremen 1 (Mao et al., 2002). The biological roles of DKK3 are still unknown since it does not interact to kremen or LRP co-receptors and does not inhibit WNT signaling (Mao et al., 2001). The SFRP prevent WNT actions by either direct interaction with WNT ligands preventing WNT-FZD binding or formation of a non-functional ligand-fzd complex (reviewed by Kawano and Kypta, 2003). Similarly, WIF1 binds to WNTs abrogating their ability to bind FZD; thus it inhibits WNT signaling (Hsieh et al., 1999). Currently, three different pathways are described to be activated upon WNTreceptor interaction (Figure 1-2): WNT/β-catenin signaling pathway (MacDonald et al., 2009) is the best described downstream pathways, and is often called the canonical WNT signaling pathway, In addition, there are the planar cell polarity pathway (PCP) (Veeman et al., 2003; Seifert and Mlodzik, 2007), and the calcium signaling pathway (Kühl et al., 2000; Kohn and Moon, 2005). The specific phenotype induced by WNT signaling depends upon the ligands and receptors in play, as well as other specific characteristics of signaling molecules in the cell; thus, specific WNT-receptor interaction can invoke different outcomes in different cell types (Amerongen et al., 2008). Canonical Wnt signaling Canonical WNT signaling is the best-described pathway downstream of WNT. The central effector of this signaling pathway is β-catenin (CTNNB1), a dual-function protein that not only modulates gene expression upon translocation to the nucleus after 40
41 WNT activation, but also serves as a constitutive protein for cellular structures involved in cell-cell adhesion (review by Perez-Moreno et al., 2003). The canonical signaling pathway is depicted in Figure 1-2 (reviews by Logan and Nusse 2004; Cadigan and Nusse 2015 ). In the absence of WNT, β-catenin is degraded in a complex reaction involving phosphorylation and targeting to the proteasome. Casein kinase I (CKI) primes the serine-threonine rich substrate within the β-catenin molecule and a subsequent dual phosphorylation reaction is performed by glycogen synthase kinase 3 (GSK3). For these phosphorylations to occur, β-catenin binds first to AXIN, a protein that presents a docking motif for GSK3 adjacent to the β-catenin binding motif. Such modification is recognized by E3 ubiquitin ligase TrCP1 and, after ubiquitination, β-catenin is degraded in proteasomes. Targeting cytosolic β-catenin for degradation takes place in a multimeric protein complex, known as the β-catenin destruction complex, and formed by oligomers of AXIN; those not only bring together GSK3 and β-catenin, but also recruit adenomatous polyposis coli (APC), a protein with 11 binding sites for β-catenin. The degradation of β-catenin is inhibited upon binding of a WNT molecule to a FZD receptor and LRP5/6 co-receptor (Figure 1-2). Subsequently, dishevelled protein (DSH) associated with the intracellular domain of FZD changes its conformation to expose a high affinity binding motif for AXIN. Consequently, AXIN oligomers are removed from the β-catenin destruction complex to halt β-catenin targeting for degradation. Cytosolic amounts of β-catenin increase, and eventually the protein translocates to the nucleus where it first displaces GROUCHO to then interact with transcription factors lymphoid enhancer-binding factor 1 (LEF1) and T-cell factor (TCF) 41
42 to regulate transcription of genes involved in cell proliferation and pluripotency such as cyclin D1 (Tetsu and McCormick, 1999) and c-myc (He et al., 1998), WNT/planar cell polarity pathway Cells and tissues experience two types of polarization: polarity along the apicalbasolateral axis, known as epithelial cell polarity; and polarity across the plane of the epithelium named, termed tissue polarity or planar cell polarity (PCP). The latter is regulated by the homonymous signaling pathway in which WNTs play a major role. Interestingly, PCP is not restricted to epithelial tissues; it regulates cell migration and cell intercalation in mesenchymal cells (Shih and Keller, 1992). Historically, knowledge of the PCP pathway comes from arthropods, and later from sophisticated genetic experiments in Drosophila (reviewed by Adler, 2002; Klein and Mlodzik, 2005). More recently, PCP has been identified as a key regulatory mechanism for developmental processes in vertebrates. In zebrafish and Xenopus it controls convergent extension during gastrulation and neurulation (reviewed by Wallingford, 2005). In mammals, PCP regulates a number of essential developmental processes including neural tube closure and left-right patterning (Gray et al., 2011). The pathway has also been implicated in malignancy since alterations of the PCP pathway can confer ability of cancer cells to migrate (reviewed by Camilli and Weeraratna, 2010). Several core components of PCP pathway are also involved in canonical WNT signaling pathway such as FZD receptors and dishevelled (Krasnow and Adler, 1994; Axelrod et al., 1998) but the downstream effects of PCP activation are independent of β- catenin (Figure 1-2). In addition to FDZ receptors, PCP can be activated through Kny (Caneparo et al., 2007), PTK7, RYK and ROR (reviewed by Cadigan and Nusse, 1997; Logan and Nusse, 2004). Receptor activation by WNTs recruits dishevelled and thereby 42
43 activates small GTPases Rac and RhoA (reviewed by Veeman et al., 2003) which culminate in cytoskeleton modifications and/or regulation of gene expression via the MAP3K-JNK pathway (Miyagi et al., 2004). Calcium signaling The least characterized downstream signaling pathway involved in WNT are those using Ca 2+ as a second messenger (Figure 1-2) (review by Kühl et al., 2000). Currently, evidence of increased intracellular Ca 2+ release as a consequence of WNT stimulation comes mainly from studies in developmental biology models, with few studies in cultured human cells. Overexpression of WNT5A (Slusarski et al., 1997) and WNT11 (Westfall et al., 2003) augment frequency of calcium fluxes in zebrafish blastulae. Similarly, in Xenopus embryos, Wn5a and Wnt11 activate Ca 2+ /calmodulindependent protein kinase II (CamKII ; Kühl et al., 2000) and protein kinase C (PKC; Sheldahl et al., 1999). Furthermore, WNT11 acts via CamKII to regulate cell movement in Xenopus embryos (Garriock and Krieg, 2007). Recombinant Wnt5a activates Ca 2+ transients in mammary epithelial cells (Dejmek et al., 2006) and thyroid carcinoma cells (Kremenevskaja et al., 2005). In addition, activation of FZD receptors in absence of ectopic WNT ligands activate CamKII and PKC, as it has been observed for FZD2 (Slusarski et al., 1997), FZD3 (Sheldahl et al., 1999) FZD4 (Robitaille et al., 2002) and FZD6 (Sheldahl et al., 1999). WNT Signaling in Embryonic Stem Cells Stem cells (SC) are undifferentiated cells that can give rise to one or more differentiated cells of specific phenotypes. Cells of the early embryo are totipotent since they can give rise to all cell lineages in the body. Once the blastocyst has formed, cells of the ICM become pluripotent while they can form all cell lineages in the fetus, the 43
44 ability to differentiate into placental tissue is lost. Stem cells in fetal and adult tissues become more restricted. For example, spermatagonial stem cells can give rise to cells of the spermatogenic lineage. One characteristic of stem cells is the capacity for selfrenewal so that cell division can be accompanied either by differentiation or maintenance of the stem cell phenotype. Embryonic stem cells are cells derived from the epiblast of blastocysts (ESC) and those from epiblast of the post-implantation embryo (EpiSC) (reviewed by Nichols and Smith, 2009). As compared to ESC, EpiSC are less pluripotent with the former being referred to as in a naïve pluripotent state and the latter having a primed pluripotent state. Alternatively ESC are referred to as pluripotent while EpiSC are multipotent. Cells derived from ESC can form cells of each of the three primary germ layers (i.e. endoderm, mesoderm, and ectoderm). Moreover, ESCs can be incorporated into the fetus when they are introduced into preimplantation embryos to make blastocyst chimeras. In contrast, EpiSCs cannot contribute to chimeras (reviewed by Rossant, 2008) nor can they give rise to ESCs. Both ESC and EpiSC express core pluripotency transcription factors Pou5f1, Nanog and Sox2 but ESCs have an unique open genome with minimal repression factors (Niwa, 2007) while EpiSCs have undergone some epigenetic changes to support differentiation towards different cell types (Tesar et al., 2007). WNT signaling is often implicated in control of self-renewal and proliferation of stem cells. In adult tissues, lineage tracing on the basis of WNT target genes revealed the critical role of WNT signaling in SC located in a wide range of organs including intestine, stomach, skin, hair follicle and mammary gland (reviewed by Clevers et al., 44
45 2014). The role of WNT signaling in maintenance of pluripotency was initially proposed based on observations that activation of WNT signaling through inhibition of GSK-3β was sufficient to maintain pluripotency in mouse ESC (mesc) and human ESC (Sato et al., 2004; Ogawa et al., 2006). In contrast, spontaneous expression of FGF4, and consequent activation of the mitogen-activated protein kinase/extra cellular signalrelated kinase (MAPK/ERK) signaling pathway triggers differentiation of mesc in culture (Kunath et al., 2007). Therefore, the standard procedure for mesc culture is the 2i strategy that consists of dual inhibition of GSK-3β and MAPK/ERK (reviewed by Ying et al., 2008). The gold standard method to assess pluripotency of ESC is by evaluation of their ability to form teratomas after being injected in mice. Teratomas are benign tumors that, because they derive from pluripotent ESC, are composed of differentiated tissues of endo-, meso-, and ectodermal origin. A number of mutations in APC in mesc result in activated β-catenin mediated WNT signaling pathway (Kielman et al., 2002). These mutant mesc had less ability to differentiate than their wild type counterparts supporting the notion that WNT/β-catenin signaling pathway maintains pluripotency. Despite the evidence mentioned above, the role of WNT/β-catenin pathway on maintenance of pluripotency in SC is controversial. One argument that call the role of WNT/β-catenin in ESC into question is that neither WNT ligand nor receptors have been shown to actively prevent differentiation (reviewed by Nusse et al., 2008). Another piece of evidence is that GSK3 participates in multiple pathways unrelated to WNT by phosphorylating a number of cellular substrates that can potentially control pluripotency in a WNT independent manner (review by Sokol et al., 2011). Further, because the 45
46 cellular context determined by the array of receptors, co-receptors and WNT regulatory molecules dictate the outcome of WNT signaling, it is likely that WNT signaling control pluripotency in some, but not all, SC. Nevertheless, the mechanism whereby WNT signaling maintains SC pluripotency may involve inhibition of differentiation through suppression of genes required for differentiation. One of the target genes of canonical WNT signaling, TCF3, regulates gene expression of ESC transcription factors. Activation of WNT signaling results in the release of TCF3-mediated repression, and consequent expression of the pluripotent markers POU5F1, NANOG and SOX2 (Cole et al., 2008). Furthermore, autocrine and paracrine actions of WNTs prevent the transition from ESC to EpiSC (Berge et al., 2014). WNT Signaling during Preimplantation Development The preimplantation mouse embryo expresses WNT related genes throughout the preimplantation development (Lloyd et al., 2003; Kemp et al., 2005). Among the genes expressed are Wnt1, Wnt3a, Wnt4, Wnt6, Wnt7a, Wnt7b, Wnt9a, Wnt10b, Sfrp1, Sfrp2, and Dkk1. The role of WNTs and WNT signaling in development of the preimplantation mouse embryo, however, is not clear. Kemler et al. (2004) generated mutant embryos whose β-catenin was resistant to ubiquitination and thus it accumulates in large amounts in the cell. Blastocyst formation was not affected by stabilization of β- catenin although gastrulation was defective. Results from that study indicate that nuclear β-catenin is dispensable for preimplantation embryonic development. In another study, Xie et al. (2008) evaluated the effect of blocking WNT/β-catenin by culturing embryos in the presence of recombinant DKK1 (which blocks WNT-FZD-LRP 46
47 inteactions) from the 2-cell to the blastocyst stage. There was no effect on blastocyst formation. To further evaluate whether or not WNT signaling during preimplantation reverberates later on development, 1-day pregnant mice were intravenously injected with an adenovirus vector carrying Dkk1 cdna, thereby creating conditional WNT inactivation (Xie et al., 2008). Blastocysts collected from these mutant mice were transferred to wild type mice at day 4, and the reciprocal transfer was also performed to distinguish between blastocyst activation and uterine receptivity defects. Results showed no differences in pregnancy when wild-type embryos were transfer to either mutant or wild-type recipients. In contrast, implantation was impaired in the group of embryos that underwent preimplantation development in a mother subjected to WNT inactivation and that were subsequently transferred to wild type females. Evaluation of these embryos developed in a mutant mother revealed that at the blastocyst stage nuclear localization of β-catenin was blocked and the target gene of β-catenin, c-myc, was downregulated. In addition, wild type blastocysts, which successfully implanted showed an overall downregulation of β-catenin independent signaling. In particular, there was low expression of the small GTPase RhoA. The authors concluded that activation of WNT/ β-catenin concomitant with inactivation of β-catenin independent WNT signaling in TE cells is required for proper function of the TE during implantation. More recently, the role of endogenous WNT signaling in embryonic and extraembryonic requirement for WNT ligand secretion was studied (Biechele et al., 2013). Two types of mutant mice were generated. One type consisted of Porcn allele deletion in zygotes and extra embryonic tissue precluding endogenous WNT secretion, 47
48 i.e. loss of function for endogenous WNTs. The other mutant type expressed a stable form of β-catenin, i.e. over activation of WNT/β-catenin signaling. Porcn mutants successfully developed to the blastocyst stage and had normal cell numbers and cell fate distribution compared with wild type embryos. Stabilization of β-catenin increased the number of ICM cells, but blastocysts had all three cell types at the blastocyst stage. Those authors concluded that Porcn-dependent WNT signaling is dispensable for blastocyst formation and does not participate in cell fate allocation. In addition, the observation that expression of WNT target genes was not affected in neither of these two phenotypes led to the conclusion that WNT/ β-catenin is not fully functional during preimplantation development. In pig and human embryos WNT signaling during preimplantation development is involved in TE differentiation, yet the mechanism underlying this function may differ between species. Pig embryos cultured in the presence of DKK1 developed into blastocysts with increased number of CDX2 + blastomeres and increased frequency of hatching (Lim et al., 2013). In the same study, stabilization of β-catenin with a GSK3 inhibitor (LiCl) reduced CDX2 expression and number of CDX2 + blastomeres. For human embryos, in contrast, stabilization of β-catenin, by either inhibition of its degradation (GSK3 inhibitor I-azakenpaullone) or WNT3 stimulation, causes upregulation of the TE marker EOMES, although there is no effect on other TE markers (CDX2 and KRT18) or on the number of CDX2 + cells (Krivega et al., 2015). In addition, loss of function of β-catenin caused by chemical induction of degradation using Cardamonin caused downregulation of CDX2 expression and reduction in the number of CDX2 + cells (Krivega et al., 2015). Neither gain nor loss of function of WNT signaling 48
49 during preimplantation development affected expression of pluripotency markers (Krivega et al., 2015). In bovine preimplantation embryos, results of the consequence of overactivation of WNT signaling have been inconsistent. Use of the agonist 2-amino-4-(3,4- methylenedioxy)benzylamino)-6-(3-methoxyphenyl)pyrimidine (AMBMP) blocks development of the embryo to the blastocyst stage in a concentration dependent manner (Denicol et al., 2013). The endogenous inhibitor of WNT/ β-catenin signaling, DKK1, negated the negative effect of AMBMP suggesting that the molecule acts through canonical WNT signaling (Denicol et al., 2013). Similarly, inhibition of β-catenin degradation using one GSK3 inhibitor (LiCl) inhibited development of embryos to the blastocyst stage (Aparicio et al., 2000). In contrast, another GSK3 inhibitor (CHIR99021) improved the ability of embryos to develop to the blastocyst stage (Aparicio et al., 2000). The observed inconsistency in results probably reflects experimental use of inhibitors or activators of canonical WNT signaling that could have effects on multiple signaling pathways, as well as generate different outcomes depending on the cellular features such as abundance of regulatory molecules and receptors. WNT signaling may also be involved in cell lineage commitment in the bovine embryo. Culture of bovine embryos in the presence of DKK1 increased the proportion of CDX2 + (TE marker) and GATA6 + (hypoblast marker) cells without affecting blastocyst development (Denicol et al., 2013, 2014). Furthermore, embryos exposed to DKK1 during the morula to blastocyst transition had better ability to establish pregnancy after transfer to recipients than control embryos (Denicol et al., 2014). 49
50 There are some indications that canonical WNT signaling is aberrant in the preimplantation embryo. In the study of mouse embryos by Kemler et al. (2004) described above, nuclear localization of β-catenin was not observed at any developmental stage in either wild type or mutant embryos. In the human, as well, there was lack of nuclear localization of β-catenin in the embryo even after stabilization of β- catenin with a mutation, and unchanged expression of a classical β-catenin target gene (TCF1) (Krivega et al., 2015). In contrast, Xie et al. (2008), also working in the mouse, found nuclear localization of β-catenin from the 1-cell to the blastocyst stage, however, by the blastocyst stage, only TE cells displayed nuclear β-catenin. Evidence for Association of Endometrial Expression of DKK1 and Fertility Two independent studies indicate that endometrial expression of the WNT inhibitor, DKK1, is reduced in sub-fertile bovine females. In one study, endometrial expression of DKK1 at day 17 of the estrous cycle was lower for lactating versus nonlactating cows (Cerri et al., 2012). Lactation is often considered as inducing subfertility in dairy cows (reviewed by Sartori et al., 2010; Hansen, 2011). A second line of evidence comes from a study where inherent fertility of heifers was determined on the basis of outcomes of four consecutive artificial inseminations (Minten et al., 2013). Pregnancy was terminated between inseminations in cases where females became pregnant. Subsequently, heifers were categorized as high fertile (pregnant 4 times) subfertile (pregnant 1-2 times), and infertile (no pregnancies). To investigate whether embryos or uteri from these heifers explain the differences in their ability to become pregnant subsequent studies were performed. No differences where observed in quantity or quality of embryos, or pregnancy rates after follicular superestimulation of the classified heifers and embryo transfer. In contrast, when these categorized heifers 50
51 were recipients of embryos, pregnancy rates were greatest for those in the high fertility group. Subsequently, reproductive tracts from cows of the three categories were obtained on day 14 of the estrous cycle to compare the transcriptome. Results show that expression of DKK1 was highest in fertile heifers, intermediate in infertile heifers and lowest in sub-fertile heifers. Results of these studies implicate WNT signaling in general and DKK1 in particular as a possible important determinant of fertility. 51
52 Table 1-1. Phenotypes generated by mutation of Wnts in mouse Gene Phenotype Reference Wnt1 Deletion portion midbrain, cerebelum (McMahon and Bradley, 1990) Wnt2 Placental defects (Monkley et al., 1996) Wnt3 Early gastrulation No formation of primitive streak (Barrow et al., 2003) (Liu et al., 1999) Wnt3a Underdevelopment of hippocampus Defect in vertebral patterning (Lee et al., 2000) (Ikeya and Takada, 2001) Wnt4 Absence of kidneys Failure in mullerian duct formation (Stark et al., 1994) (Vainio et al., 1999) Wnt5a Delayed osteoblast differentiation Impaired lung morphogenesis (Yang, 2003) (Li et al., 2002) Wnt5b Delayed osteoblast differentiation (Yang, 2003) Wnt6 Defective decidualization (Wang et al., 2013) Wnt7a malformed female reproductive tracts Lack of mullerian regression in male (Miller and Sassoon, 1998) (Parr and McMahon, 1998) Wnt7b Chorio-allantoic fusion defects (Parr et al., 2001) Wnt8a viable (Vendrell et al., 2013) Wnt8b viable * Wnt9a Skeletal abnormalities (Später et al., 2006) Wnt9b Defective urogenital development (Carroll et al., 2005) Ant10b Defective myocyte differentiation (Vertino et al., 2005) Wnt11 Defective ureters kidney hypoplasia (Majumdar et al., 2003) Wnt 16 viable * *reviewed by van Amerongen and Berns (2006) 52
53 Figure 1-1. Position-dependent Hippo signaling in preimplantation embryos. Hippo signaling is activated in inner apolar cells, and is turned off in outer polar cells. The result is inhibition of Yap phosphorylation when signaling is off, so that YAP can interact with Tead4 and activate 53
54 Figure 1-2. Downstream cascades of WNT signaling pathway. WNT/β-catenin inactive: in absence of WNT ligand, cytosolic β-catenin is targeted by a destruction complex formed by AXIN, GSK3 and APC for proteosomal degradation. Note that WNT/ -catenin can also be inactivated by DKK1 interacting with LRP5/6 and kremen1 co-receptors to impair WNT-FZD-LRP complex formation. WNT/β-catenin active: As a result of WNT-FZD-LRP binding, the β-catenin destruction complex is disassembled and β-catenin accumulates and becomes translocated to the nucleus where it regulates transcription by interacting with LEF and TCF transcription factors. WNT/PCP signaling is activated by diverse ligand-receptor interactions that recruit dishevelled (Dsh) and activates small GTPases RAC1 and RHOA. Outcomes of this signaling include cytoskeleton changes and regulation of transcription. WNT/calcium signaling can be activated by WNT-FZD interaction as well as FZD activation. It results in intracellular release of calcium via inositol 3 phosphate, calmodulin and protein kinase C. Abreviations:IP3: Inositol 3 phosphate DAG: Diacylglycerol - CamKII: calmodulin-dependent protein kinase II PKC: protein kinase C KNY: knypek - ROR2: receptor tyrosine kinase like orphan receptor 2 - DSH: - JNK: c-jun N-terminal kinase LEF: lymphoid enhancer binding factor 1 TCF: transcription factor 7 GSK3: glycogen synthase kinase 3 - LRP5/6: low density lipoprotein receptor-related protein 5/6 - APC: adenomatosis polyposis coli - DKK1: dickkopf-related protein 1 CK1:casein kinase 1-54
55 Objectives of the Present Investigations The overall objective of the research presented in this dissertation is to understand the role of WNT signaling during preimplantation development of the bovine embryo and the maternal contribution to the regulation of this signaling pathway. In cattle, exposure of embryos to the endogenous regulator of WNT signaling, DKK1, induces cell lineage commitment and confer embryos with better competence to establish and maintain pregnancy after transfer to recipient animals (Denicol et al., 2014). In contrast, overactivation of WNT signaling sometimes inhibited development (Denicol et al., 2013, Aparicio et al., 2010). Such results suggest overactivation of WNT signaling may be detrimental to embryonic development in the cow. Further evidence for this idea comes from the findings that fertility in cattle is related to endometrial expression of DKK1, which encodes for a secreted inhibitor of canonical WNT signaling (Cerri et al., 2012; Minten et al., 2013). Four series of experiments were designed to understand the role and regulation of WNT signaling during preimplantation development and consequences of that signaling for development through the prenatal period. In Chapter 2, WNT signaling was characterized during preimplantation development. It was hypothesized that 1) canonical WNT signaling (i.e. WNT signaling mediated by nuclear localization of β- catenin) is attenuated in the preimplantation embryo and 2) WNT can activate other signaling pathways in the embryo. Actions of embryo-derived and exogenous WNTs on the preimplantation embryo were evaluated in Chapter 3. The role of embryo-derived WNTs was determined by evaluating consequences of inhibition of WNT secretion by addition of a PORCN inhibitor (necessary for WNT secretion) (Takada et al., 2006) as well as the WNT inhibitor DKK1. Actions of exogenous WNT (such as might be secreted 55
56 by the endometrium) were determined by testing effects of AMBMP and WNT7A, on embryonic development. Recently, it was shown that one embryokine, CSF2, can affect embryonic development from day 5-7 of development in a way that alters characteristics of the resultant calf (Kannampuzha-Francis et al., 2015). In particular, calves born following embryo transfer of an embryo treated with CSF2 grew faster after three mo of age than calves born following transfer of a control embryo. In Chapter 4, it was tested whether actions of DKK1 during the transition from morula to the blastocyst stage would alter phenotype after birth. Because results of earlier chapters suggested a role for endometrial-derived WNT in embryonic development, an experiment described in Chapter 5 was conducted to survey oviductal and endometrial expression of genes for a number of WNT and other growth factors during the first 7 days after ovulation. 56
57 CHAPTER 2 WNT REGULATION OF EMBRYONIC DEVELOPMENT LIKELY INVOLVES PATHWAYS INDEPENDENT OF NUCLEAR β-catenin Introduction WNT signaling is a complex signaling system regulated by 19 WNT ligands that interact with a variety of receptors including FZD, ROR, PTK7 and RYK (Cadigan and Nusse, 1997; Logan & Nusse, 2004). Among the downstream signaling cascades are the canonical pathway involving binding of WNT to FZD and recruitment of the coreceptor LRP5 or LRP6 (MacDonald et al. 2009), the planar cell polarity pathway (Veeman et al., 2003; Seifert and Mlodzik, 2007), and calcium signaling pathway (Kühl et al. 2000; Kohn and Moon, 2005). The specific phenotype induced by WNT signaling depends upon the ligands and receptors in play, as well as other specific characteristics of signaling molecules in the cell; thus, a specific WNT-receptor interaction can invoke different outcomes in different cell types (Amerongen et al. 2008). Canonical WNT signaling is the most well described pathway for WNT signaling and is crucial for a number of developmental processes through regulation of cell proliferation (Logan and Nusse, 2004), maintenance of pluripotency (Sato et al. 2004; Sokol, 2011), differentiation (Liu et al. 2014) and migration (Morosan-Puopolo et al. 2014). The central effector of this signaling pathway is β-catenin, a protein that not only modulates gene expression upon translocation to the nucleus after WNT activation, but also serves as a constitutive protein for adherens junctions involved in cell-cell adhesion (Fleming et al. 2001). Nuclear accumulation of β-catenin is triggered by inhibition of its degradation by the proteasome induced by a complex consisting of CKI, GSK3 and APC. Once in the nucleus, β-catenin displaces GROUCHO to interact with the transcription factors LEF1 and TCF7 to regulate transcription of genes involved in cell 57
58 proliferation and pluripotency such as CCNDBP1 (Tetsu and McCormick, 1999) and MYC (He et al. 1998), respectively. WNTs are important regulators of mammalian development but their role during the preimplantation period has not been resolved. In the mouse, inhibition of canonical WNT signaling does not impair blastocyst development (Huelsken et al., 2000; Kemler et al., 2004; Xie et al., 2008; Lyashenko et al., 2011) or affect identity, expansion, or self-renewal of embryonic stem cells (ESC) (Lyashenko et al., 2011; Wray et al., 2011b). In other species, the role of canonical WNT signaling in the preimplantation embryo is less clear because of the experimental use of inhibitors or activators of canonical WNT signaling that could have effects on multiple signaling pathways. In the cow, for example, one inhibitor of GSK3B (which causes activation of the canonical pathway) increased competence of embryos to develop to the blastocyst stage whereas another inhibitor reduced development (Aparicio et al. 2000). A physiological antagonist of canonical WNT signaling, DKK1, enhanced the ability of porcine embryos to undergo hatching (Lim et al. 2013), increased trophectoderm (TE) differentiation in pig and cattle embryos (Lim et al. 2013; Denicol et al. 2014), and increased competence of bovine embryos to establish pregnancy after transfer to recipient females (Denicol et al. 2014). While such results suggest that activation of canonical WNT signaling may inhibit TE differentiation, DKK1 can also regulate other signaling pathways independent of canonical WNT signaling (Caneparo et al. 2007; Tahinci et al. 2007). In the human embryo, accumulation of β-catenin in the nucleus in response to inhibition of GSK3B depends upon stage of development, with accumulation being attenuated after day 3 of development and absent in blastocysts (Krivega et al. 2015). 58
59 Such a result is consistent with findings in the mouse that canonical WNT signaling is not required for development, at least after day 3, and that developmental changes in the embryo cause a dampening of canonical WNT signaling. For the current study, the bovine embryo was used as a model to test the overall hypotheses that 1) canonical WNT signaling (i.e. WNT signaling mediated by nuclear localization of β-catenin) is attenuated in the preimplantation embryo and 2) WNT can activate other signaling pathways in the embryo, as evaluated for activation of JNK. These hypotheses were evaluated in several experiments to characterize developmental changes in expression of genes involved in WNT signaling, localization of β-catenin in blastomere nuclei, and accumulation of phospho-jnk in the nucleus after WNT activation. Materials and Methods Embryo Production Bovine embryos were produced in vitro from oocytes obtained from Bos (admixture of B. taurus and B. indicus) ovaries collected at a local abattoir. Procedures for oocyte recovery and maturation were as described elsewhere (Dobbs et al. 2013). Following oocyte maturation, oocytes were fertilized for 8-10 h in groups of up to 300 oocytes with sperm pooled from three randomly selected B. taurus and B.indicus bulls using procedures described elsewhere (Denicol et al. 2014). Groups of presumptive zygotes were placed in 50 µl microdrops of SOF-BE2 (Kannampuzha- Francis et al. 2017) covered with mineral oil (Sigma-Aldrich, St. Louis, MO, USA) and cultured at 38.5 o C in a humidified atmosphere of 5% O 2 and 5% CO 2 with the balance N 2. Treatments were applied to cultured embryos by removing 5 µl of culture medium and adding the treatment in a volume of 5 µl. 59
60 For immunofluorescence experiments, embryos were produced in vitro following procedures described above with a few modifications. Oocytes were harvested using BoviPRO TM oocyte washing medium (MOFA Global, Verona, WI, USA) and fertilization of matured oocytes was performed using IVF-TL (Parrish et al. 1986) (Caisson Laboratories, Logan, UT, USA) containing PHE [80 µl of 0.5 mm penicillamine, 0.25 mm hypotaurine, and 25 µm epinephrine in 0.9% (w/v) NaCl as described by (Ortega et al. 2016). Developmental Changes in Expression of Selected Genes Involved in WNT Signaling for Embryos Produced in Vitro (Experiment 1) To prepare pools of matured oocytes, cumulus oocyte complexes (COCs) were harvested at the end of oocyte maturation (20-22 h). Cumulus cells were removed by vortexing for 5 min in HEPES-SOF medium (Denicol et al. 2014) containing 1,000 U/ ml of hyaluronidase. Denuded oocytes were washed three times in Dulbeccos s phosphate-buffered saline (DPBS) containing 1% (w/v) polyvinylpyrrolidone (DPBS- PVP), incubated in 0.1% (w/v) proteinase solution (protease from Streptomyces griseus; Sigma-Aldrich, St Louis, MO, USA) in DPBS to remove the zona pellucida, washed three times in DPBS-PVP, and suspended in groups of 30 in 100 µl extraction buffer from the PicoPure RNA isolation kit (Applied Biosystems, Foster City, CA, USA). Samples were stored at -80 o C. Embryos were produced by in vitro fertilization in 19 replicates. Embryos were harvested from culture drops at the following stages: 2-cell [28 32 h post insemination (hpi)]; 3-4 cell (44 48 hpi); 5-8 cell (50-54 hpi); 9-16 cell (72 hpi); morula (120 hpi); and blastocyst (168 hpi). Embryos were collected, processed as for denuded oocytes to remove the zona pellucida, suspended in groups of 30 in 100 µl extraction buffer from 60
61 the PicoPure RNA isolation kit (Applied Biosystems), and stored at -80 C. A separate pool of bulls was used for each replicate, resulting in a total of 19 different bulls. Transcript abundance was examined for seven genes related to WNT signaling by quantitative real time PCR (qpcr). Genes included two transcription factors (LEF1 and TCF7), two transcription factor inhibitors [AES and LOC (GROUCHO-like)], two canonical WNT co-receptors (LRP5 and LRP6), and a soluble inhibitor of canonical WNT signaling (DKK1) as well as three reference genes (GAPDH, SDHA and YWHAZ). The reference genes were chosen because expression is stable over preimplantation development (Goossens et al. 2005), and it was verified that developmental stage did not affect expression of any of these three genes. Primers are listed in Table 2-1. Primers for DKK1, GAPDH, LRP6, SDHA, and YWHAZ were previously published (Goossens et al. 2005; Denicol et al. 2013) while those for AES, LEF1, LOC505120, LRP5, and TCF7 were designed using software from Integrated DNA Technologies (Coralville, Iowa, USA). Primers were synthesized by Integrated DNA Technologies. All newly-designed primer pairs were validated using cdna from pools of day 7 bovine blastocysts by generation of a standard curve (efficiency varied from %), evaluation of melt curves and sequencing of PCR amplicons. Sequences were mapped to the B. taurus genome using the Basic Local Alignment Search Tool of the National Center for Biotechnology Information. All sequences aligned to the corresponding gene. RNA of pools of oocytes and embryos was extracted using the PicoPure RNA Isolation kit (Applied Biosystems) following the manufacturer s protocol. DNase treatment was performed using the QIAGEN DNase kit (Valencia, CA, USA) and mrna was reverse transcribed using the High Capacity cdna Reverse Transcription Kit of Applied 61
62 Biosystems. The qpcr utilized SsoFast EvaGreen Supermix reagent (Bio-Rad, Hercules, CA, USA) and was performed with a Bio-Rad CFX96-Real-Time system using conditions described previously (Dobbs et al. 2013). Two technical replicates were performed for each sample and the mean cycle threshold (C T ) calculated. Mean C T values greater than 35 were considered non-detectable and assigned a value of 35 for statistical analysis. A total of five biological replicates containing 30 oocytes or embryos each were subjected to qpcr. Data analyzed were ΔC T values, which were calculated by subtracting the geometric mean of the three reference genes from the mean C T value of the sample. For graphical purposes, the relative transcript abundance was calculated as the 2 ΔCT. Therefore, abundance of each mrna type is expressed relative to expression of reference genes. Data were analyzed by least-squares analysis of variance using the GLM procedure of SAS for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA). Assumptions of analysis of variance were tested using the Univariate procedure of SAS. Results are reported as least-squares means ± standard error of the mean. The level of significance was P < Characteristics of the WNT Signaling System in the Morula and ICM and TE of in- Vitro Produced Embryos as Revealed by RNA-Seq (Experiment 2) Data sets of the transcriptome of three pools of in-vitro produced morulae collected at day 6 after insemination, and three pools of ICM and TE purified from invitro produced blastocysts at day 8 after insemination, were examined for stage and cell type effects on expression of 80 genes involved in WNT signaling. Reads were mapped to Btau_4 ( Procedures and data for ICM and TE have been 62
63 published previously (Ozawa et al. 2012) and raw data was deposited in the DDBJ Sequence Read Archive at (Submission DRA000504). The samples of morulae (100 per pool) were produced using the same procedures and in the same replicates of in-vitro fertilization as for ICM and TE. Data were processed following the same bioinformatic methods as reported earlier (Ozawa et al. 2012). Details of the magnetic activated cell sorting procedure used to separate ICM from TE were previously published (Ozawa & Hansen, 2011). RNA-seq data was obtained using a SOLiD TM v4 sequencer (Applied Biosystems). Data on a subset of genes involved in WNT signaling were evaluated for treatment effects by least-squares analysis of variance using the GLM procedure of SAS for Windows, version 9.4. The dependent variable was number of reads of the transcript and the independent effect was cell type (morula, ICM and TE). The total transcript reads per sample was used as a covariate. Orthogonal contrasts were used to determine whether transcript abundance differed between morulae and blastocysts (morula vs. ICM+TE) or between ICM and TE. Results are reported as least-squares means ± standard error of the mean. The level of significance was P < Localization of Total and Active β-catenin in Bovine Preimplantation Embryos as Determined by Immunofluorescence (Experiments 3 and 4) Embryos produced in vitro were harvested at different stages of development using the same schedule as described earlier. Embryos were washed three times in cold DPBS- PVP, fixed in 4% (v/v) paraformaldehyde in DBPS/PVP for 15 min, and washed in DPBS/PVP three times. Immediately thereafter, embryos were incubated in permeabilization solution [DPBS containing 0.5% (v/v) Triton X-100] for 30 min at room temperature, followed by incubation for 1 h in blocking buffer [DPBS containing 5% 63
64 (w/v) bovine serum albumin (BSA)]. Embryos were then incubated overnight at 4 C with 1 µg/ ml of primary antibody in antibody buffer [DPBS containing 0.1% (v/v) Tween 20 and 1% BSA (w/v)]. For detection of total β-catenin (Experiment 3), rabbit polyclonal anti-human β-catenin antibody (Abcam, Cambridge, MA, USA) was used. For detection of active β-catenin (Experiment 4), rabbit polyclonal anti-human non-phospho (active) β- catenin antibody (Ser33/37/Thr41; Cell Signaling Technology, Beverly, MA, USA) was used, and for negative control, primary antibody was replaced with the same concentration of rabbit IgG. After three washes in washing buffer [DPBS containing 0.1% (v/v) Tween 20 and 0.1% BSA (w/v], oocytes and embryos were incubated with 1 µg/ ml goat anti-rabbit IgG conjugated with Alexa Fluor 555 (Life Technologies, Carlsbad, CA, USA) for 1 h at room temperature in the dark. Primary and secondary antibodies were diluted in antibody buffer. Nuclear labeling was achieved by incubation with 1 μg/ ml Hoechst (Sigma-Aldrich) for 15 min at room temperature. Embryos were finally rinsed in DPBS/PVP and placed on a slide containing SlowFade Gold antifade reagent (Life Technologies), covered with a coverslip, and observed with a 40x objective using a Zeiss Axioplan 2 epifluorescence microscope (Zeiss, Göttingen, Germany) and Zeiss filter sets 02 [4=,6=-diamidino-2-phenylindole (DAPI)], and 04 (rhodamine). Digital images were acquired using AxioVision software (Zeiss) and a highresolution black and white Zeiss AxioCam MRm digital camera. For Experiment 3, evaluation of total β-catenin was replicated 7 times with a total of 450 embryos. For Experiment 4, evaluation of active β-catenin was replicated 5 times with a total of 417 embryos. 64
65 Changes in Immunoreactive Active β-catenin in Embryos Following Activation of Canonical WNT Signaling (Experiments 5-8) For Experiment 5, in-vitro produced embryos were cultured in drops as described above. Culture drops were randomly assigned to stage and treatments. Developmental stages included 3-4 cell (44-48 hpi), 5-8 cell (50-54 hpi), 9-16 cell (72 hpi), and compact morula (120 hpi). At the corresponding time for each developmental stage, 5 µl of SOF- BE2 in the culture drop were replaced by either 5 µl GSK3 inhibitor [CHIR HCL; Tocris Bioscience, Bristol, UK], 5 µl of the nuclear export inhibitor leptomycin (Sigma), 5 µl leptomycin + GSK3 inhibitor, or 5 µl vehicle (SOF-BE2 containing 0.04 % (v/v) ethanol). Final concentrations were 10 µm for the GSK3 inhibitor and 22 ng/ ml for leptomycin. After 30 min of treatment while embryos were maintained in culture conditions (30 min was chosen because leptomycin B increases nuclear NFkB p65 in HeLa cells at this time; (Wolff et al. 1997), embryos were fixed and labeling for immunofluorescence was carried out as described earlier using antibody against nonphospho (active) β-catenin or the corresponding rabbit IgG. A total of 191 embryos were evaluated. For Experiment 6, canonical WNT signaling in 5-8 cell embryos and morulae was stimulated by addition of final concentrations of either 100 ng/ ml human recombinant WNT1 (Sigma-Aldrich; 99% identical amino acid sequence to bovine WNT1), 0.7 µm of the WNT agonist 2-amino-4-(3,4-methylenedioxy)benzylamino)-6-(3- methoxyphenyl)pyrimidine (AMBMP, Sigma-Aldrich) or SOF-BE2 containing 0.1% (v/v) DMSO (vehicle). Embryos were harvested 1, 6 and 24 h after treatment and analyzed by immunofluorescence for nuclear β-catenin (n=36 embryos). For Experiment 7, compact morulae were treated with 0.7 µm AMBMP or vehicle at day 5 after 65
66 insemination. Blastocysts were harvested 48 h later for assessment of nuclear active β- catenin (n=78 embryos). For experiment 8, embryos were treated with 10 µm GSK3 inhibitor (CHIR-99021) on day 6 after insemination or were used as a control group that received an equivalent amount of vehicle (SOF-BE2 containing 0.04 % (v/v) ethanol). Blastocysts were harvested 24 h after treatment for assessment of nuclear active β- catenin (n=15 embryos). Localization of Active β-catenin in Mouse and Bovine Embryos as Evaluated by Confocal Microscopy (Experiment 9 and 10) For Experiment 9, cryopreserved 5-8-cell mouse embryos (B 6 C 3 F 1 x B 6 D 2 F 1 ) were obtained from Embryotech Laboratories (Haverhill, MA, USA) and thawed following the supplier s instructions. Embryos were washed with HEPES-TALP and incubated in 50 µl oil-covered microdrops of EmbryoMax KSOM medium with amino acids (EMD Millipore, Billerica, MA, USA) for 3 h at 38.5 C in a humidified atmosphere of 5% CO 2 and 5% O 2. The 5-8 cell embryos were fixed in 10% (v/v) formalin for 30 min, permeabilized in 2.5% (v/v) Tween 20 in DPBS for 20 min, blocked with DPBS containing 5% (w/v) BSA for 1 h, and incubated overnight with 1 µg/ ml purified mouse monoclonal IgG against active β-catenin [anti-active-β-catenin (anti ABC) clone 8E7; Millipore] at 4 C. After sequential washes with DPBS containing 0.1% (w/v) BSA and 0.1 % (v/v) Tween 20, embryos were incubated in affinity-purified goat anti-mouse IgG coupled to fluorescein isothiocyanate (FITC; Abcam) for 1 h at room temperature, followed by 5 min incubation in 1 µg/ ml Hoechst Embryos were suspended in 10 µl drops of ProLong Gold anti-fade mounting medium (ThermoFisher Scientific) in chamber slides. Embryos (n=5) were observed using a spinning disk confocal scanner mounted to an Olympus DSU-IX81 inverted fluorescent microscope. Digital images 66
67 were captured with a 60X objective and DAPI and FITC filter sets, using an attached Hamamatsu C AG monochrome CCD camera. For Experiment 10, bovine embryos were produced in vitro and harvested at the morula stage on day 5 and blastocyst stage on day 7 after insemination. Labeling of active β-catenin was performed following same procedure and antibody as for Experiment 4 except that embryos were suspended in 10 µl drops of ProLong Gold anti-fade mounting medium (ThermoFisher Scientific) in chamber slides. Embryos (n=4) were observed and images captured using the spinning disk confocal scanner and camera mentioned above, with 40 or 60X objectives and DAPI and Texas Red filter sets. Nuclear Localization of β-catenin in Bovine Embryonic Fibroblast Cells Following Activation of Canonical WNT Signaling (Experiment 11) Cells of the bovine embryonic fibroblast (BEF) cell line (Ozawa et. al 2012) were studied to verify nuclear labeling of non-phospho (active) β-catenin in non-embryonic cells. Cells were cultured with Dulbeccco s modified Eagle s Medium (Gibco, ThermoFisher Scientific) containing 10% (v/v) fetal bovine serum and 1% (v/v) antibiotic-antimycotic (10,000 units/ ml penicillin, 10 mg/ ml streptomycin and 25 µg/ ml amphotericin B; Sigma-Aldrich). Cells were initially cultured in cell culture flasks (Corning, Corning, NY, USA) at 38.5 C in a humidified atmosphere of 5% CO 2. At 90% confluency, cells were trypsinized [0.25% (w/v) trypsin, Life Technologies], mixed with an equal volume of culture medium, centrifuged for 10 min at 1750 x g, resuspended in culture medium, and counted (Automated Cell Counter, Bio-Rad, Richmond, CA, USA). Cells were seeded in 8-chamber slides (Sigma-Aldrich) at a density of 10,000 cells/well and allowed to adhere overnight in culture medium. Culture medium containing 67
68 treatments were used to replace medium. Treatments included 10 µm GSK3 inhibitor (CHIR-99021), 0.7 µm AMBMP or vehicle [0.5% (v/v) DMSO]. Concentrations were chosen based on effect of these molecules in other studies (Denicol et al. 2013; Lappas, 2014). Fixation and immunolabeling of cells was performed 24 h after treatment using 4% paraformaldehyde for 20 min, followed by permeabilization with DPBS containing 0.5% (v/v) Triton X-100 during 30 min, and blocking for 1 h with DPBS containing 5% (w/v) BSA. Incubation with primary antibody [rabbit anti-human polyclonal non-phospho (active) β-catenin (Ser33/37/Thr41); Cell Signaling Technology] or rabbit IgG proceeded overnight at 4 C. Incubation with goat anti-rabbit IgG conjugated with Alexa Fluor 555 proceeded for 1 h, followed by DNA labeling using Hoechst Cells were observed and images captured following methods described for mouse embryos; DAPI and Texas red fluorescence filters were used. Image analyses consisted of quantification of proportion of cells depicting nuclear β-catenin. A total of 41 images were analyzed. The proportion of cells showing nuclear localization of active β-catenin was analyzed by logistic regression using the LOGISTIC procedure of SAS for Windows, version 9.4 (SAS Institute Inc.) including treatment as a fixed effect. The PDIFF means separation test was used to determine which treatments differed from control cells. The level of significance was P<0.05. Non-Canonical WNT Signaling Mediated by Phosphorylation of JNK (i.e., MAPK8) by WNT11 in Bovine Blastocysts (Experiment 12-14) Experiment 12 was performed to determine whether WNTs could activate one component of the planar cell polarity pathway in bovine embryos. Embryos were produced in vitro as described. At day 7 after insemination, culture drops were randomly assigned to treatments. In each culture drop, 5 µl of SOF-BE2 were replaced by either 68
69 5 µl human recombinant WNT11 (R&D systems, Minneapolis, MN, USA; 98% amino acid sequence identity with bovine WNT11) or 5 µl vehicle [SOF-BE2 containing 0.01 % (w/v) BSA (in addition to BSA included in SOF-BE2 formulation)]. Final concentrations of WNT11 were 0.5, 1 and 2.5 µg/ml. Blastocysts were harvested 6 h after treatment and fixed in 4% (v/v) paraformaldehyde. Immunolabeling was performed as described for β-catenin except that the primary antibody was 1 µg/ ml anti-phospho- JNK [(Thr183/Tyr185,Thr221/Tyr223), Millipore] and 1 µg/ ml rabbit IgG served as a negative control. Note that the phosphorylation site is conserved among human, mouse, and bovine and that the amino acid sequence identity between these species is %. Embryos were then incubated with 1 µg/ ml goat anti-rabbit IgG conjugated with Alexa Fluor 555 (Life Technologies), and nuclear labeling was performed using Hoechst Embryos were evaluated using a Zeiss Axioplan 2 epifluorescence microscope as described earlier. Quantification of intensity of labeling with antibody was performed using ImageJ software (U.S. National Institutes of Health, Berthesda, MD, USA - version 1.70_02). Total immunoreactive phospho-jnk was measured as follows: after selection of the area encompassing the entire embryo, the mean intensity was obtained using the Measure Analysis feature and background intensity was obtained from the area surrounding the embryo. The latter was subtracted from the embryo intensity before statistical analysis. A total of 49 embryos were analyzed. Data were analyzed by least-squares analysis of variance using the PROC GLM procedure of SAS for Windows, version 9.4 (SAS Institute Inc.) including WNT11 concentration as a fixed effect. Differences between individual concentrations of WNT11 and control embryos were assessed using the PDIFF mean separation test of SAS. 69
70 Experiment 13 was performed to test whether WNT11 affected competence of embryos to develop to the blastocyst stage and allocation of blastocyst blastomeres to TE and ICM. Treatments (vehicle and 2.5 µg/ ml WNT11) were applied at day 5 after fertilization as described above. Blastocyst development was assessed on day 7 after fertilization, and blastocysts with clearly-delineated blastocoels were harvested, fixed, permeabilized, and blocked as described above. Trophectoderm cells were identified by localization of a transcription factor crucial for differentiation of TE [caudal type homeobox 2 (CDX2); (Berg et al, 2011)]. Labeling was achieved by sequential incubation with mouse anti-human polyclonal CDX2 antibody ready to use (Biogenex, Fremont, CA, USA) and 1µg/ ml goat anti-mouse IgG conjugated with FITC (Abcam).Total number of cells was determined by counting DNA-labeled nuclei by labeling with 1µg/ml Hoechst after immunolabeling for CDX2. Number of ICM was calculated as the difference between total cells and TE. The experiment was performed in 5 replicates with a total of 484 COCs and semen from 13 different bulls. Blastocyst cell number was evaluated for 74 embryos Data were analyzed for effect of treatment using Proc Glimmix of SAS for Windows, version 9.4 (SAS Institute Inc.). Each embryo was considered an observation and development (0=not developed to blastocyst; 1=developed to blastocyst) was considered a binary variable. Results are presented as least-squares means ± standard error of the mean. Results Developmental Changes in Expression of Selected Genes Related to WNT Signaling for Embryos Produced In Vitro (Experiment 1) Results on expression of seven genes related to WNT signaling in embryos produced in vitro are shown in Figure 2-1. Expression of each gene was affected by 70
71 stage of development (P= for LRP5 and P< for the other genes). There were several distinct developmental patterns in gene expression. The first was a continuous decline in transcript abundance from the oocyte to the morula stage. Only AES showed this pattern and, for this gene, there was a slight increase in transcript abundance by the blastocyst stage. A second pattern, shown for DKK1, was a continuous increase from the oocyte stage to the 9-16 cell stage followed by a decline in transcript abundance. For the other five genes, there was a slight increase in transcript abundance from the oocyte to the 2-cell or 4-cell stage followed by a decline in transcript abundance after the 2-cell, 4-cell or 5-8 cell stage. Transcripts for two genes, DKK1 and LEF1, were not detectable at the blastocyst stage. Characteristics of the WNT Signaling System in the Morula and ICM and TE of in Vitro Produced Embryos as Revealed by RNA-Seq (Experiment 2) A RNA-Seq database of the transcriptomes of in vitro produced day 6 morulae and isolated TE and ICM of day 8 blastocysts (Ozawa et al. 2012) was assessed for expression of 80 genes associated with WNT signaling. Genes were considered expressed if the average number of reads was > 5. Results are summarized in Table 2-2. Only 7 of 19 WNT genes were expressed with the remaining 12 WNT genes having < 5 reads or being not detected. Of the 7 WNT that were expressed, only one, WNT6, varied in expression between groups. Expression was higher for TE than ICM or morula. A key gene involved in post-translational modification of WNTs, PORCN, was highly expressed and transcript abundance was higher for TE than morula or ICM. A total of 6 of 10 FZD receptor genes were expressed in the morula or blastocyst. Expression of FZD1 and FZD7 was lower for ICM than for TE. In contrast, 71
72 expression of FZD6 was higher for both ICM and TE of the blastocyst than for the morula. The FZD co-receptor gene LRP6, was abundantly expressed but number of reads for LRP5 was < 5. Among the genes involved in canonical WNT signaling that were expressed were β-catenin and genes involved in the β-catenin destruction complex (APC, AXIN1, and GSK3B). DVL1, which acts to bind FZD proteins and transmit information about WNT binding, was not considered expressed (< 5 reads). Two other disheveled genes (DVL2 and DVL3) were expressed, however. The only gene involved in β-catenin metabolism that varied with stage of development was APC, which was expressed more in TE than ICM. Expression of the two WNT regulated transcription factor genes, TCF7 and LEF1 was low In addition, expression of TCF7L1 was relatively low in both morula and blastocysts. In contrast, TCF7L2 was highly expressed although expression declined from the morula to the blastocyst stage for both ICM and TE. In contrast, the transcription factor inhibitor, AES, increased in transcript abundance from the morula to blastocyst stage for both ICM and TE. Expression was also examined for several genes that can promote or antagonize canonical WNT signaling. Among such genes that were affected by developmental stage, were the WNT stimulatory molecule, RSPO3 and one of its receptors, LGR4. Expression of both genes was significantly lower for ICM and TE of the blastocyst than for morula. Two antagonists of canonical WNT signaling also declined from the morula to blastocyst stage, DKK1 and WIF1. KREMEN1, which can function as a DKK1 72
73 receptor, was also reduced in expression for ICM and TE of the blastocyst as compared to the morula. Note that the pattern of gene expression was generally consistent with the earlier experiment (Figure 2-1). In the first experiment, expression of six genes declined from the day 5 morula to the day 7 blastocyst. A similar decline occurred from the day 6 morula to day 8 blastocyst for DKK1 and LEF1. LRP6 did not change in expression, LOC and TCF7 were not detected by RNA-seq and LRP5 was barely detectable. There was one gene whose expression increased very slightly from the morula to blastocyst stage in the first experiment, AES, and a larger increase was observed when comparing the day 6 morula to day 8 blastocyst. Localization of Total and Active β-catenin in Bovine Preimplantation Embryos as Determined by Immunofluorescence (Experiments 3 and 4) Expression of genes coding for molecules involved in WNT signaling was indicative that canonical WNT signaling is partially silenced at the morula and blastocyst stages. To further explore this idea, two experiments were conducted to evaluate whether a key feature of canonical WNT signaling, nuclear localization of β-catenin, occurs during preimplantation embryonic development. For Experiment 3, an antibody recognizing both active (non-phosphorylated) and inactive (phosphorylated) β-catenin was used to determine localization of total β- catenin. Representative images are shown in Figure 2-2. Regardless of stage of development, most immunoreactive β-catenin was localized to cell membranes; immunoreactive protein in the nucleus was absent in all but the occasional cell. Since nuclear β-catenin was not observed at any developmental stage, Experiment 4 was performed using an antibody specific for active β-catenin (i.e., non- 73
74 phosphorylated β-catenin) (Figure 2-3). As for total β-catenin, most active β-catenin was localized to plasma membranes and, except for the scattered nucleus, nuclear localization was absent at all developmental stages. Failure of Canonical WNT Activators to Induce Localization of Nuclear Active β- catenin (Experiments 5 to 8) One possible reason for the lack of nuclear β-catenin in embryos is absence of stimulation by canonical WNTs. To test this hypothesis, three experiments were conducted to evaluate localization of active β-catenin after stimulation of WNT signaling. In Experiment 5, embryos were treated with a GSK3 inhibitor at the 3-4 cell, 5-8 cell, 9-16 cell and morula stages of development. Inhibition of GSK3 leads to accumulation of β-catenin and import into the nucleus (Yuan et al. 2005). Some control and GSK3 inhibitor-treated embryos were also treated with leptomycin to block nuclear exportins. This treatment was added to enhance nuclear localization of β-catenin in case nuclear β-catenin induced by GSK3 inhibition is rapidly exported from the nucleus. Representative images are shown in Figure 2-4. Active β-catenin remained localized to non-nuclear regions of the cells and to the plasma membrane in particular. With the exception of the occasional cell, there was no accumulation of β-catenin in the nucleus at any stage of development. For Experiment 6, embryos at the 5-8 cell or morulae stages of development were treated with either the WNT agonist AMBMP or human WNT1 and localization of active β-catenin determined after 1, 6, 24, and 48 h of incubation. Although both treatments increased intensity of labeling for active β-catenin in the plasma membrane, there was no accumulation of detectable β-catenin in the nucleus (Figure 2-5A). For Experiments 7 (results not shown) and 8 (Figure 2-5B), treatment of morulae with 74
75 AMBMP at day 5 or GSK3 inhibitor on day 6 after insemination also failed to increase nuclear labeling with β-catenin in resultant blastocysts at day 7. Both treatments did increase immunoreactive active β-catenin localized in the plasma membrane (Figure 2-5B). Localization of Active β-catenin in Mouse and Bovine Embryos Evaluated by Confocal Microscopy (Experiment 9 and 10) As found for bovine embryos using epifluorescence microscopy, there was no observable active β-catenin in the nucleus of mouse 5-8 cell embryos (Figure 2-6A). Similarly, no active β-catenin was observed in nuclei of bovine embryos when embryos were examined by confocal microscopy (Figure 2-6B). Nuclear Localization of β-catenin in Bovine Embryonic Fibroblast Cells Following Activation of Canonical WNT Signaling (Experiment 11) To test whether absence of nuclear localization of active β-catenin was a unique feature of preimplantation embryos, localization of the protein was also examined in BEF cells derived from bovine embryonic fibroblasts. In these cells, punctuate labeling of active β-catenin was observed in the nucleus of a fraction of cells (Figure 2-8). The proportion of cells depicting nuclear localization of active β-catenin was 310/763 (40.6%) for control cells vs. 67/148 (45.3%) for cells treated with AMBMP (P=0.29 for difference from control) and 750/839 (89.4%) for cells treated with GSK inhibitor (P<.0001 for difference from control). Actions of WNT11 on Phosphorylation of the Non-canonical Signaling Protein JNK and Development to the Blastocyst Stage (Experiment 12-13) Immunoreactive phospho-jnk was localized in nuclei (Figure 2-7A). Moreover, the pattern of nuclear labeling of phospho-jnk was punctuated. The degree of labeling varied between cells although labeling was not consistently elevated in TE or ICM. 75
76 Treatment of blastocysts with human recombinant WNT11 caused a significant increase in intensity of labeling of phospho-jnk at 2.5 µg/ ml (P<.0001) but not at 0.5 or 1 µg/ ml (Figure 2-7B). In Experiment 13, effect of WNT11 on development and blastocyst cell number was determined (Table 2-3). Treatment with WNT11 increased the proportion of oocytes that developed to the blastocyst stage (P=0.042) but had no effect on number of ICM, TE or total cells in the resulting blastocysts. Discussion The mouse embryo does not require canonical WNT signaling for either development to the blastocyst stage or ESC identity, expansion, or self-renewal (Huelsken et al. 2000; Kemler et al. 2004; Xie et al. 2008; Lyashenko et al. 2011) The situation for other species is less clear. Here it is shown that one of the characteristics of preimplantation development in the cow is a temporal decrease in expression of key genes involved in WNT signaling along with a paucity of nuclear β-catenin, even after stimulation of the embryo with molecules that activate canonical WNT signaling. These observations are consistent with the idea that, like the mouse, canonical WNT signaling is dispensable for blastocyst development in the cow. In contrast, non-canonical WNT signaling improved embryonic development because WNT11 increased the proportion of embryos becoming blastocysts while also increasing phosphorylation of JNK, a central player in the WNT/planar cell polarity (PCP) pathway (Zeke et al. (2016). Observed changes in gene expression also mean that, similar to the human (Krivega et al. 2015), characteristics of WNT signaling are likely to change during development. By the blastocyst stage, WNT signaling may play different roles in the ICM and TE because of differences in expression of several important genes in the WNT signaling system. 76
77 A key observation of the current series of experiments was, with rare exceptions, the absence of observable immunoreactive β-catenin in the nucleus of embryos at every stage examined. Failure to observe nuclear β-catenin was not because of failure of the antibodies used to recognize the molecule because immunoreactive total and active β- catenin could be localized to the plasma membrane. Lack of nuclear β-catenin was observed even after embryos were treated with molecules expected to activate canonical WNT signaling including a GSK3 inhibitor, the WNT agonist AMBMP (Liu et al. 2005) or the canonical WNT1 (Shimizu et al., 1997; Yuan et al. 2005). The lack of nuclear β-catenin was not due to rapid export from the nucleus because inhibition of nuclear exportins with leptomycin did not lead to accumulation of β-catenin in the nucleus. Failure of the molecules to induce nuclear localization was not because the molecules were inactive because all three WNT activators increased active β-catenin associated with the plasma membrane and because GSK3 inhibition increased the percent of cells with nuclear β-catenin in cells of the BEF cell line. Immunolabeling of nuclear active β-catenin in BEF cells was characterized by a punctuate pattern resembling that previously described in newly differentiated chondrocytes (Guo et al. 2004) and intrahepatic cholangiocarcinoma cells (Wang et al. 2015). An absence of β-catenin in the nucleus of the preimplantation embryo may be a widespread phenomenon in the mammal, at least for certain stages of development. In the human embryo, accumulation of β-catenin in the nucleus in response to inhibition of GSK3B depends upon stage of development, with accumulation being attenuated after day 3 of development and absent in blastocysts (Krivega et al. 2015). In the pig, immunoreactive nuclear β-catenin was faint in expanded blastocysts and absent in 77
78 hatching blastocysts (Lim et al. 2013). Moreover, accumulation in the nucleus was not induced by LiCl inhibition of GSK3 (Lim et al. 2013). Results with respect to the mouse are contradictory. No nuclear β-catenin was detected in mouse blastocysts in one study (Kemler et al. 2004) whereas active β- catenin was observed in the nucleus of embryos at the 1-cell, 2-cell, 4-cell, 8-cell, morula and blastocyst stage of development in another (Xie et al. 2008). Present results fail to replicate findings of nuclear β-catenin in mouse 5-8 cell embryos even though the antibody used in the present experiment was the same as used earlier (Xie et al. 2008). The findings that β-catenin does not translocate to the nucleus in the bovine embryo after treatment with canonical WNT activators does not mean that WNTs are not involved in regulation of embryonic development. In addition to canonical signaling, there is a variety of other signaling cascades activated by WNTs termed non-canonical pathways (Filmus et al. 2008; Chien et al. 2009; van Amerongen and Nusse, 2009; Gao, 2012). Some of these pathways use FZD as a receptor (PCP and Ca ++ mediated signaling) whereas others use other receptor molecules such as ROR and RYK. Individual WNTs preferentially stimulate canonical or non-canonical signaling depending upon ability to bind FZD and recruit LRP5/6 and other coreceptor molecules. Thus, some documented actions of WNTs on the preimplantation embryo, for example, promotion of TE development in human embryos by WNT3 (Krivega et al. 2015), could involve signaling through one or more pathways independent of accumulation of β- catenin in the nucleus. Here it was shown that WNT11, which is considered to preferentially activate non-canonical pathways (Flaherty & Dawn, 2008; Uysal-Onganer & Kypta, 2012), can activate a key component of the PCP pathway in bovine 78
79 blastocysts by phosphorylating the signaling kinase JNK in the nucleus. Activation of JNK has been implicated in actions of WNT11 in other cells (Pandur et al. 2002; Cha et al. 2008; Chen et al. 2014; Geetha-Loganathan et al. 2014) although, under certain circumstances, WNT11 can inhibit JNK signaling (Railo et al. 2008). The observation that activated JNK was localized to the nucleus suggests that the protein translocate to the nucleus after activation, as has been described for other cells (Schreck et al. 2011; Coffey, 2014). Furthermore, WNT11 participates in regulation of preimplantation developmental processes since addition of exogenous WNT11 to the culture medium resulted in higher proportion of inseminated oocytes that developed to the blastocyst stage Further investigation is needed to unravel the downstream effect of this WNT in the preimplantation bovine embryo, but the nuclear localization of phospho-jnk in response to WNT11 suggests the presence of a JNK-nuclear substrate usually associated with an effect on gene expression [reviewed in Zeke et al. (2016)]. In addition, although WNT activation did not cause accumulation of β-catenin in the nucleus in bovine embryos, it did increase β-catenin in the embryo, with the protein being localized primarily to the plasma membrane. Similar effects have been observed in human embryos (Krivega et al. 2015). Thus, certain actions of WNT on the embryo could conceivably involve signal transduction pathways utilizing membrane-bound β- catenin. In mouse embryonic stem cells, β-catenin bound to E-cadherin is required for expression of Klf4 and Nanog via STAT3 phosphorylation (Hawkins et al., 2012). It may also be possible that activation of WNT signaling does lead to some accumulation of β- catenin in the nucleus but at amounts too low to be detected by immunofluorescence. Further investigation is needed to understand the alternative WNT signaling pathways 79
80 regulating developmental processes, as well as the role of each of these pathways in preimplantation embryo development. Analysis of gene expression during development is consistent with a reduction in WNT signaling as the embryo develops. In Experiment 1, transcript abundance for all genes examined declined to a nadir at the morula or blastocyst stage of development. This was true for the WNT coreceptors, LRP5 and LRP6, the canonical WNT antagonist DKK1, two WNT-dependent transcription factors, LEF1 and TCF7, as well as two repressors of WNT-dependent transcription factors, LOC (encodes for GROUCHO-like protein) and AES. The decline in gene expression is not an artifact of in vitro fertilization or culture because similar developmental patterns of gene expression were seen for 6 of the 7 genes for embryos that developed in vivo (Supplemental Table S2-3 in Jiang et al. 2014). The only exception was for AES, which rose in transcript abundance at the blastocyst stage for in vivo embryos (Jiang et al., 2014a) but remained low for in vitro produced embryos. It is possible that the developmental decline in abundance of most transcripts examined is part of the large-scale destruction of maternally-derived mrna in the oocyte after fertilization (Tadros & Lipshitz, 2009; Graf et al., 2014) More research is required but analysis of differences in gene expression between the ICM and TE of the blastocyst are consistent with the idea that WNT signaling functions differently in the two cell types. Genes upregulated in the TE included three receptors or co-receptors (FZD1, FZD7 and LRP6) and two genes involved in inhibition of canonical WNT signaling (APC and SFRP1). Expression of WNT6 was also upregulated in the TE. This WNT, which can promote differentiation of primitive 80
81 endoderm (Krawetz & Kelly, 2008), functions as a canonical WNT when binding FZD 1/2/7 and as a non-canonical WNT when binding FZD 5/8 (Schmidt et al. 2007; Lhomond et al. 2012; Li et al. 2014). Perhaps WNT6 is secreted by TE cells to participate in differentiation of cells of the ICM to primitive endoderm. In conclusion, the accumulation of β-catenin in the nucleus in response to canonical WNT activators is blocked in the preimplantation bovine embryo. Moreover, there is a decline in expression of several genes important for canonical WNT signaling as the embryo advances in development. In contrast, at least one non-canonical signaling pathway involving JNK and the PCP pathway can be activated in the bovine preimplantation embryo. Moreover, WNT11, which causes JNK activation, improves competence of the embryo to develop to the blastocyst stage. Thus, some actions of WNTs on the preimplantation embryo are likely to involve signaling through mechanisms independent of nuclear β-catenin. Differences in gene expression between the TE and ICM mean that, by the blastocyst stage, WNT signaling may play different roles in the ICM and TE. 81
82 Table 2-1. Primer sequences used for real-time PCR. Gene Gene Reference Primers Size (bp) ID sequence AES NM_ F:5'-GACAAACACTGAGAGAGGAAGG-3' 95 R:5 -CCAGTAGGCAGCTACCATAAAT-3 DKK NM_ F: 5 -GACTGGTGGAGGCGCTCGGA R:5 -GCTGTGCCCAGAGCCGTCAT-3 GAPDH XM_ F:5 -ACCCAGAAGACTGTGGATGG R:5 -CAACAGACACGTTGGGAGTG -3 GROUCHO XM_ F:5'-AGTCGGCCAACTTTCCAGGACTTA-3' 180 R: 5 -AAGATGCAGCATTCGGTTTCAGCC-3 LEF NM_ F:5'-CTGACGCATCCTTCCAATTCT-3' 182 R: 5 -CATCCCGACCACTGTGTAATC-3 LRP XM_ F:5'-AGTATACTGC CAGCTCCGCG -3' 120 R:5 -TTCAGTCCGC CGTGGCGCTG-3 LRP XM_ F:5 -AGTGCCCTGGAACATGTGGTAGAA R:5 -ATTGGTTCCTGTGTCTGCCCAGTA-3 SDHA NM_ F:5 GCAGAACCTGATGCTTTGTG R:5 -CGTAGGAGAGCGTGTGCTT-3 TCF NM_ F:5'-GCATGGTCACAACAACCAAGCTCA-3' 121 R:5 -TGTGGGTAGAAGCTTCCCTTGGTT-3 YWHAZ XM_ F:5'-GCATCCCACAGACTATTTCC-3' 120 R:5'- GCAAAGACAATGACAGACCA-3' 82
83 Table 2-2. Effect of stage of development and cell lineage [inner cell mass (ICM) vs trophectoderm (TE)] on expression of genes involved in WNT signaling for day 6 morulae and day 8 blastocysts produced in vitro a. Number of reads P value Morula vs (ICM+TE) ICM vs TE Gene Morula ICM TE WNTs b WNT WNT2B WNT <.0001 <.0001 WNT8A WNT10A WNT WNT WNT processing PORCN Frizzled receptors and LRP co-receptors c FZD FZD FZD FZD FZD FZD LRP Proteins involved in canonical signaling d APC AXIN CNBP β-catenin DVL DVL GSK3B Transcription factors e LEF TCF7L TCF7L
84 Table 2-2. Continued Number of reads P value Morula vs (ICM+TE) ICM vs TE Genes Morula ICM TE Transcription factors inhibitors f AES TLE R-spondin signaling g LGR LOC h RSPO RSPO RSPO Other soluble WNT regulatory proteins i DKK NDP SFRP SFRP SFRP SFRP WIF Other WNT signaling proteins j DACT KREMEN RYK a Data are least-squares means of number of reads. b The following genes had < 5 reads: WNT1, WNT3, WNT3A, WNT4, WNT5A, WNT5B, WNT7A, LOC (WNT7B paralog), WNT8B, WNT9A, WNT9B,and WNT10B. c The following genes had < 5 reads: FZD2, FZD4, FZD5, FZD9, and LRP5. d The following genes had < 5 reads: AXIN2, DVL1. e The following genes had < 5 reads: TCF7 f The following genes had < 5 reads: LOC505120(GROUCHO ortholog), TLE1, TLE2, TLE4, and TLE6. g The following genes had < 5 reads: LGR5, LGR6 and RSPO5. h RSPO3 like. i The following genes had < 5 reads: DKK4 and SFRP5 j The following genes had < 5 reads: ANKRD6, DACT1, DACT3, FRAT1, KREMEN2, NKD2, VANGL1, and VANGL2 84
85 Table 2-3. Effect of 2.5 µg/ ml WNT11 from day 5 to day 7 after insemination on development of embryos to the blastocyst stage at day 7 after insemination a Blastocyst cell number Treatment Percent blastocyst Total TE ICM Vehicle 17.8 ± ± 6 89 ± 5 38 ± 3 WNT ± ± 6 93 ± 4 45 ± 3 P-value a Data are the least-squares means ± SEM of results. Data on percent blastocyst represent the percent of inseminated oocytes that developed to the blastocyst stage. The total number of oocytes was 484. Blastocyst cell number was evaluated for 74 embryos. 85
86 Figure 2-1. Developmental changes in expression of selected genes involved in WNT signaling for embryos produced in vitro (Experiment 1). Expression was assessed by qpcr. Expression of each gene was affected by stage of development (P= for LRP5 and P< for the other genes). Data are presented as least-squares means + SEM of results from 5 replicates. Blast, blastocyst (168 hpi). 86
87 Figure 2-2. Representative examples of localization of immunoreactive β-cateninat various stages of preimplantation development of embryos produced in vitro (Experiment 3). Embryos were labeled with antibody to β-catenin (red) and DNA (blue). The total number of embryos evaluated was
88 Figure 2-3. Representative examples of localization of immunoreactive non-phospho (active) β-catenin during preimplantation development of embryos produced in vitro (Experiment 4). Embryos were labeled with antibody to non-phospho (active) β-catenin (red) and DNA (blue). A total of 417 embryos was evaluated. 88
89 Figure 2-4. Lack of localization of active β-cateninin the nucleus of in vitro produced embryos after activation of canonical WNT signaling with the GSK3 inhibitor (Experiment 5). Leptomycin was added to reduce nuclear export of β-catenin. Shown are representative images of individual embryos labeled with antibody to non-phospho (active) β-catenin (red) and DNA (blue) treated at the cell stage and harvested 24 h after stimulation. Similar results were seen for embryos treated at other stages of development (total number of embryos examined = 191). 89
90 Figure 2-5. Consequences of treatment of embryos with WNT agonists for immunolocalization of active β-catenin. Shown in panel A) (Experiment 6; n=36 embryos) are representative images of individual embryos produced in vitro that were treated at the 5-8 cell stage with vehicle, the WNT agonist AMBMP or recombinant WNT1, harvested 24 h later and labeled with nonphospho (active) β-catenin (red) and DNA (blue). Shown in panel B) (Experiment 8; n=15 embryos) are representative images of individual embryos produced in vitro that were treated at day 6 after insemination with vehicle or GSK3 inhibitor, harvested 24 h later and labeled with non-phospho (active) β-catenin (red) and DNA (blue). 90
91 Figure 2-6. Localization of active β-cateninin mouse and bovine embryos by confocal microscopy. Shown in panel A) (Experiment 10) are images of 2 individual 5- cell mouse embryos labeled with non-phospho (active) β-catenin (green) and Hoechst (blue). A total of 5 embryos were examined. Shown in panel B) (Experiment 11) are representative confocal images of bovine embryos at the morula stage captured with 40X magnification (top), at the blastocyst stage captured with 40X magnification (middle), and at the blastocyst stage captured with 60X magnification (bottom). A total of 4 embryos were examined. 91
92 Figure 2-7. Immunoreactive phospho-jnk in blastocysts produced in vitro (Experiment 12). Shown in panel A) are representative images of embryos labeled with phospho-jnk (red) and Hoechst (blue) that were treated at the blastocyst stage with 0, 0.5, 1 or 2.5 µg / ml of recombinant WNT11 and harvested 6 h later. Shown in panel B) are average values of intensity of fluorescence of phospho-jnk in the whole area of the embryo (n=49 embryos). ***, P< from 0 μg/ ml. 92
93 Figure 2-8. Representative confocal microscopy images of immunoreactive active β- catenin in bovine embryonic fibroblast cells following activation of canonical WNT signaling. Cells were labeled with antibody to non-phospho (active) β- catenin (red) and Hoechst (blue) after 24 h stimulation with either vehicle, the WNT agonist AMBMP or a GSK3 inhibitor. Arrows indicate nuclei depicting nuclear β-catenin, while arrow heads indicate nuclei with no accumulation of β-catenin. 93
94 CHAPTER 3 CONSEQUENCES OF ENDOGENOUS AND EXOGENOUS WNT SIGNALING FOR DEVELOPMENT IN THE PREIMPLANTATION BOVINE EMBRYO Introduction The WNT are a family of 19 extracellular growth factors whose secretion depends upon palmitoylation mediated by acytransferases (Willert et al., 2003; Ho and Keller, 2015). WNT signaling participates in a number of developmental processes including cellular proliferation (Logan and Nusse, 2004), maintenance of pluripotency (Sato et al., 2004; Sokol, 2011), cellular differentiation (Liu et al., 2014), asymmetrical cell division (Sawa, 2012), the epithelial-mesenchymal transition (Kleber and Sommer, 2004), and axis elongation (Silhankova and Korswagen, 2007; Zinovyeva et al., 2008). The outcome of WNT signaling depends upon the cellular context established by the availability of receptors, co-receptors and molecules involved in different signaling pathways. WNTs may interact with any of ten frizzled (FZD) receptors as well as alternative receptors including ROR, PTK7 and RYK to activate different downstream signaling cascades (Cadigan and Nusse, 1997; Logan and Nusse, 2004). The most well-described signaling pathway, the so-called canonical pathway, is mediated by nuclear β-catenin. This protein has a dual function because it provides an indirect link between cadherin and the contractile cortical actin cytoskeleton (Abe et al., 2013) and can also localize into the nucleus where it interacts with transcription factors to regulate gene expression (He et al., 1998; Tetsu and McCormick, 1999). Other downstream signaling pathways activated by WNTs include the planar cell polarity pathway (PCP) (Veeman et al., 2003; Seifert and Mlodzik, 2007), and Ca + mediated cascade (Kühl et al., 2000; Kohn and Moon, 2005). More recently, non-nuclear β-catenin has also been associated with downstream signaling (Hawkins et al., 2012). 94
95 The role of endogenous and maternally-derived WNTs in preimplantation development is unclear. Blastocyst-stage embryos express only a subset of WNT genes. Of the 19 WNT ligands, only 10, 8 and 6 are expressed in mouse, human and bovine blastocysts respectively (Lloyd et al., 2003; Kemp et al., 2005; Ozawa et al., 2012; Denicol et al., 2013a; Yan et al., 2013). There also is limited or no detectable nuclear accumulation of β-catenin in these three species even in the presence of WNT agonists (Kemler et al., 2004; Krivega et al., 2015). In mouse embryos, inhibition of secretion of endogenous WNT does not affect blastocyst formation (Biechele et al., 2013) and neither does depletion of β-catenin (Huelsken et al., 2000). Furthermore, inhibition of β-catenin mediated WNT signaling with DKK1 does not impair blastocyst formation either in mouse, cow, or pig embryos (Li et al., 2008; Xie et al., 2008b; Denicol et al., 2013a; Lim et al., 2013). Taken together, evidence suggests that endogenous WNT signaling mediated through nuclear accumulation of β-catenin is dispensable for blastocyst formation. A role for embryo-derived WNT acting through other pathways is possible however. Moreover, maternally-derived WNTs could also contribute to regulation of embryonic development although the consequences of maternal WNT for the embryo are unclear. There may also be a physiological role for the WNT antagonist DKK1 in preimplantation development. A product of the uterine endometrium (Kao et al., 2002; Tulac et al., 2003; Peng et al., 2008; Cerri et al., 2012), DKK1 can both block intracellular accumulation of β-catenin by interfering with the formation of a WNT-FZD- LRP5/6 complex (Logan and Nusse, 2004; MacDonald et al., 2009) and can itself signal through activation of JNK and the PCP pathway (Caneparo et al., 2007; Killick et al., 95
96 2014). Exposure of pig embryos to DKK1 increased the number of TE cells in blastocyst, without changing the number of ICM, thereby increasing the proportion of cells in the blastocyst that were TE (Lim et al., 2013). Similarly, exposure of bovine embryos to DKK1 increased the proportion of blastocyst blastomeres classified as TE and improved competence of embryos to establish pregnancy after transfer (Denicol et al., 2014). In the pig, DKK1 increased the proportion of blastocysts that hatched in vitro (Lim et al., 2013). The purpose of the series of experiments documented in this chapter was to determine consequences of activation and inhibition of β-catenin dependent and - independent WNT signaling on development of bovine preimplantation embryos and allocation of cells in the blastocyst into ICM and TE lineages. Results indicate a limited role of embryo-derived WNTs in blastocyst development and suggest that, depending on the type of ligand, maternally-derived WNT can potentially either promote or inhibit competence of the embryo to become a blastocyst. Materials and Methods Embryo Production Using Non-Sex Sorted Sperm Formulation of media used for production of bovine embryos in vitro are described elsewhere (Ortega et al., 2016). Cumulus-oocyte complexes (COC) were obtained from cattle ovaries (including Bos taurus and cattle that are an admixture of B. taurus and B. indicus) collected at a local abattoir by bisecting follicles 3 to 8 mm in diameter with a scalpel. Procedures for oocyte recovery and maturation, fertilization and embryo culture were performed following procedures described elsewhere (Dobbs et al., 2013) with a few modifications. Oocytes were harvested using BoviPRO TM oocyte washing medium (MOFA Global, Verona, WI, USA) and matured for h in groups 96
97 of 10 in oocyte maturation medium (OMM). Groups of up to 300 matured oocytes were then fertilized for 8-10 h in 300 µl IVF-TL (Caisson Laboratories, Logan, UT, USA) to which sperm (final concentration, 1 x 10 6 cells/ml) and 80 µl of a solution of PHE (0.5 mm penicillamine, 0.25 mm hypotaurine, and 25 µm epinephrine) were added. Sperm used for each fertilization procedure consisted of a pool from three B. taurus or Brangus bulls that were randomly selected from available bulls. A different assortment of bulls was used for each procedure. Sperm from frozen-thawed straws were purified before fertilization using an Isolate gradient [(Irvine Scientific, Santa Ana, CA; 50% (v/v) and 90% (v/v) isolate] and diluted in IVF-TALP (Caisson Laboratories). After removal of cumulus cells, groups of presumptive zygotes were placed in 50 µl microdrops of SOF-BE2 covered with mineral oil (Sigma-Aldrich, St. Louis, MO, USA) and cultured at 38.5 o C in a humidified atmosphere of 5% O 2 and 5% CO 2 with the balance N 2. Unless stated otherwise, treatments were administered on day 5 of development [120 hours post insemination (hpi)]. The procedure consisted of removing 5 µl of culture medium and replacing it with 5 µl of culture medium containing ten times the desired concentration of the treatment in the drop. Embryo Production Using Sex-Sorted Sperm Procedures were as described above except for semen preparation and fertilization. Commercially-available X and Y-sorted sperm from Angus sires were obtained from ABS Global (De Forest, WI, USA) and Genex Cooperative, Inc. (Shawano, WI, USA). Separated pools of X and Y-sorted sperm from the same two bulls, randomly-selected from those available, were used in each fertilization procedure. The total number of bulls used was six. Sperm were purified before fertilization using Puresperm 40/80 gradient column (Nidacon International AB, Mölndal, Sweden). 97
98 Sperm was first centrifuged (2,600 x g for 5 min) in 2.0 ml microcentifuge tubes containing 250 µl sperm over two layers of 200 µl of Puresperm (top layer of Puresperm 40 and bottom layer of Puresperm 80). The pellet representing the bottom 100 µl was transferred to a new microcentrifuge tube, washed in 1000 µl of IVF-TL that had been pre-equilibrated at 38.5 C under 5% CO 2, and centrifuged at 600 x g for 3 min. Fertilization of groups of 30 matured cumulus-oocyte complexes was performed in 60 μl oil-covered microdrops of IVF-TL medium containing 3.5 µl of PHE. Final concentration of sperm in the fertilization drop was 2 x 10 6 cells/ml. Fertilization was carried out for h at 38.5 C and a humidified atmosphere of 5% (v/v) CO 2. Treatments were administered as described for embryos produced with non-sex sorted semen. Immunolabeling of Protein in Bovine Embryos Procedures for labeling embryos against β-catenin and pjnk were as follows. Embryos were fixed in 4% (v/v) paraformaldehyde, and permeabilized in Dulbeccos s phosphate-buffered saline (DPBS) containing 0.5% (v/v) Triton X-100. Blocking was performed using DPBS containing 5% (w/v) bovine serum albumin (BSA), and incubation with primary antibody diluted in antibody buffer [DPBS containing 0.1% (v/v) Tween 20 and 1% BSA (w/v)] was performed overnight at 4 C. Total immunoreactive β- catenin was detected using 1µg/ml rabbit polyclonal anti-human β-catenin (Abcam). Immunoreactive pjnk was detected using 1 µg/ml rabbit polyclonal anti-phospho-jnk [(Thr183/Tyr185,Thr221/Tyr223) (Millipore, Billerica, MA, USA)]. Note that the phosphorylation site is conserved among human and bovine JNK and that the amino acid sequence identity between these species is 99%. Incubation with labeled second antibody (goat anti-rabbit IgG conjugated with Alexa Fluor 555 (Life Technologies; 98
99 1µg/ml diluted in antibody buffer) proceeded for 1 h at room temperature. Nuclear labeling was performed with 1µg/ml Hoechst (Sigma-Aldrich) in antibody buffer. Slides were mounted using SlowFade Gold antifade reagent (Life Technologies, Carlsbad, CA, USA), and observed with a 40x objective using a Zeiss Axioplan 2 epifluorescence microscope (Zeiss, Göttingen, Germany) and Zeiss filter sets 02 [4=,6=-diamidino-2-phenylindole (DAPI)], 03 (FITC filter), and 04 (rhodamine). Digital images of individual blastocysts were acquired using AxioVision software (Zeiss) and a high-resolution black and white Zeiss AxioCam MRm digital camera. For negative control, IgG of the same species was used to replace primary antibody using the same concentration. For dual immunolocalization of YAP1 and CDX2, embryos were processed as described above with few modifications. After overnight incubation with rabbit monoclonal anti-human YAP1 (Cell Signaling Technology, Beverly, MA, USA) at a concentration of 0.01µg/ml. embryos were washed 3 times with washing buffer [DPBS containing 0.1% (v/v) Tween 20 and 0.1% BSA (w/v] and incubated with secondary antibody [goat anti-rabbit IgG conjugated with Alexa Fluor 555 (Life Technologies; 1µg/ml] for 1 h at room temperature. Embryos were washed again 3 times and incubated for 1 h with primary antibody against CDX2 (mouse anti-human polyclonal CDX2 antibody, ready to use; Biogenex, Fremont, CA, USA) and 1 h with 1µg/ml goat anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC; Abcam, Cambridge, MA, USA). Nuclear labeling, slide mounting, and image acquisition were performed as describe above. 99
100 Quantification of intensity of labeling in either the entire embryo or in the nuclei was performed using ImageJ software (U.S. National Institutes of Health, Berthesda, MD, USA). For labeling of the entire embryo, the area encompassing the entire embryo was selected and the mean intensity obtained using the Measure Analysis feature of ImageJ. Background intensity was obtained from the area surrounding the embryo using the same technique and the value subtracted from embryo intensity. Labeling in the nucleus was determined using a similar technique except that the software was used to isolate nuclear regions within each embryo based on labeling with Hoechst Number of ICM and TE cells was determined for embryos labeled with anti-cdx2 as described above. Those cells with nuclei labeled with CDX2 were considered TE and the number of ICM cells was determined by subtracting number of TE cells from the total number of cells determined by counting number of nuclei labeled with Hoechst Experiment 1: Effect of Activation of Canonical WNT Signaling by Inhibition of GSK3 on Development Embryos were cultured in 50 µl microdrops of SOF-BE2. Treatments were either 10 µm CHIR99021 (Tocris Bioscience, Avonmouth, Bristol, UK; final concentrations in the drop) or vehicle (SOF-BE2). The concentration of inhibitor was chosen because it was effective at blocking lipopolysaccharide-induced inflammation in adipose tissue (Lappas, 2014). Blastocyst development was evaluated on day 7 of development (168 hpi). The experiment was performed in 5 replicates using a total of 803 COC and 10 different bulls. 100
101 Experiment 2: Effect of Activation of Canonical WNT Signaling by the Agonist 2- amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl)pyrimidine (AMBMP) in the Presence or Absence of DKK1 on Development and β-catenin Labeling The source of AMBMP was Calbiochem (San Diego, CA, USA). Human recombinant DKK1 was purchased from R&D Systems (Minneapolis, MN, USA). Both reagents were reconstituted as previously described (Denicol et al., 2013a). Embryos were cultured in 50 µl microdrops of SOF-BE2. Treatments were either vehicle [SOF- BE2 containing 0.1% (v/v) DMSO], 0.7 µm AMBMP, 100 ng/ml DKK1, or 0.7 µm AMBMP plus 100 ng/ml DKK1 (final concentrations in the drop). Blastocyst development was evaluated on day 7 of culture (168 hpi), and a fraction of blastocysts with a clearly delineable blastocoel were randomly selected for immunolabeling of β- catenin. The experiment was performed in 7 replicates using a total of 1,006 COC and 10 different bulls. A total of 165 blastocysts were analyzed for immunolabeling of β- catenin. Experiment 3: Effects of Inhibition of Endogenous WNT Signaling with Wnt-C59 or DKK1 on Ability of Embryos to Develop to the Blastocyst Stage and Blastocyst Cell Number Wnt-C59 [2-(4-(2-methylpyridin-4-yl)phenyl)-N-(4-(pyridine-3- yl)phenyl)acetamide] was purchased as a 10 mm stock in dimethyl sulfoximine (DMSO) from Cellagen Technology (San Diego, CA, USA). The Wnt-C59 was serially diluted to 100 nm in SOF-BE2 containing 0.001% (v/v) DMSO so that the final concentration of Wnt-C59 in the culture drop was 10 nm. The concentration of inhibitor was chosen because it blocked WNT activation in HeLa cells (Proffitt et al, 2013). Treatments were vehicle [SOF-BE2 containing 0.01% (w/v) BSA (in addition to the BSA included in SOF- BE2 formulation) and % (v/v) DMSO]; 10 nm Wnt-C59; or 100 ng/ml DKK1 (final 101
102 concentrations in the drop). Blastocyst development was assessed on day 7 of development (168 hpi). A fraction of blastocysts with a clearly delineable blastocoel was randomly selected and subjected to immunolabeling to determine the numbers of TE and ICM cells. The experiment was performed in 5 replicates with a total of 905 COC and 13 different bulls. Treatment effect was evaluated for 91 blastocysts. Experiments 4-5: Effects of DKK1 on Development and Blastocyst Cell Number In each experiment, treatments were 100 ng/ml DKK1 and vehicle [SOF-BE2 containing 0.01% (w/v) BSA (in addition to the BSA included in SOF-BE2 formulation)]. The concentration of DKK1 was chosen because it was effective at blocking actions of WNT signaling agonist on development of bovine embryos to the blastocyst stage (Denicol et al., 2013a). Both experiments 4 and 5 were designed to test whether DKK1 alters competence of embryos to become blastocysts and the number of TE and ICM cells in the blastocyst. An additional goal of experiment 5 was to determine whether effects of DKK1 varied with embryo sex. Experiment 4 was performed in 10 replicates with a total of 1,545 COC and conventional semen from 7 bulls. Experiment 5 was replicated 5 times with a total of 1,348 COC and X- and Y-sorted semen from 6 bulls. Blastocyst development was evaluated on day 7 of development (168 hpi) and a fraction of blastocysts with a clearly delineated blastocoel (n=89 for Experiment 4 and 204 for Experiment 5) were randomly selected for labeling with anti-cdx2 and Hoechst to determine number of TE and ICM. 102
103 Experiment 6: Effects of DKK1 on Developmental Changes in YAP1 and CDX2 Localization in Morulae and Blastocysts The transcription factor YAP1 plays an important role in TE formation in the mouse by interacting with TEAD4 to induce transcription of CDX2 (Nishioka et al., 2007; Yagi et al., 2007). For Experiment 6, it was determined whether DKK1 modifies the developmental pattern of immunoreactive YAP1 and CDX2 from day 5 to day 7 of development (when the embryo transitions from the morula to the blastocyst stage). Embryos were treated with either 100 ng/ml DKK1 or vehicle [SOF-BE2 containing 0.01% (w/v) BSA (in addition to the BSA included in SOF-BE2 formulation)]. Morulae on day 5 (6 hours after treatment), morulae on day 6 (24 hours after treatment) and blastocysts with a clearly delineated blastocoel on day 7 (48 hours after treatment) were harvested and fixed for immunolocalization of YAP1 and CDX2. Number of cells was counted based on DNA staining; number of YAP1 + and CDX2 + were also quantified. Intensity of the nuclear YAP1 and nuclear CDX2 was obtained. Data are presented as absolute and relative number of CDX2 + and YAP1 + nuclei. This experiment was performed in 5 replicates with a total of 1,510 COC and semen from 7 bulls. A total of 232 individual embryos were assessed for immunofluorescence. Experiment 7: Effects of DKK1 on Activation of JNK Experiment 7 tested the hypothesis that DKK1 could activate JNK signaling in bovine embryos. Activation was assessed as the accumulation of pjnk. Treatments, which were added at day 5 of development, included vehicle [SOF-BE2 containing 0.01% (w/v) BSA (in addition to the BSA included in SOF-BE2 formulation)], and 100 ng/ml DKK1 (final concentration in the drop). Morulae were harvested 6 hours after adding the treatment and immunolabeled for pjnk. The experiment was performed in 4 103
104 replicates with a total of 587 COC and semen from 11 different bulls. Treatment effect on JNK accumulation was evaluated in 91 embryos. Experiments 8-11: Embryo Responses to WNT7A For these experiments, treatments were 66 ng/ml human recombinant WNT7A (ebioscience Inc., San Diego, CA, USA) or vehicle [SOF-BE2 containing 0.01 mm NaPO 4, 0.5 mm NaCl and % (w/v) CHAPS in water]. The concentration of WNT7A was chosen because it is the upper limit of the range suggested for biological activity of the product by the manufacturer, as determined by inhibition of Wnt-3ainduced alkaline phosphatase production in MC3T3-E1 cells, and 50 ng/ml was effective activating Akt/mTOR anabolic growth pathway in skeletal muscle (von Maltzahn et al., 2012). Blastocyst rate was assessed on day 7 of development (180 hpi) and a fraction of blastocysts with clearly delineated blastocoel was randomly selected for further analyses. For experiment 8, embryos were produced in vitro as described above with few modifications. Oocytes were matured in BO-IVM (IVF Bioscience, Falmouth, Cornwall, UK) for 24 h, and fertilization proceeded for h. Culture drops were randomly assigned to one of four treatments in a 2 x 2 arrangement with two treatments (recombinant WNT7A or vehicle) and treatment on either day 1 (15 hpi) or day 5 of development (115 hpi). Blastocysts were harvested for gene expression analysis. Pools of 10 blastocysts were washed three times in DPBS-PVP, incubated in 0.1% (w/v) proteinase solution (protease from Streptomyces griseus; Sigma-Aldrich) in DPBS to remove the zona pellucida, washed three times in DPBS-PVP, and snap frozen in 5 µl DPBS-PVP. Samples were stored at -80 o C until processed for gene expression analysis 104
105 by Fludigm qpcr. This experiment was performed in 6 replicates with a total of 1,566 COC and semen from 8 bulls. For experiments 9 and 10, embryos were produced in vitro. Treatments were added on day 5 of development (120 hpi). Blastocysts were harvested to determine number of ICM and TE cells (experiment 9) and intensity of total β-catenin (experiments 10) by immunolabeling as described above. Experiment 9 was performed in 7 replicates with a total of 1,413 COC and semen from 7 bulls. A total of 54 individual blastocysts were assessed by immunofluorescence. Experiment 10 was performed in 10 replicates with a total of 1,471 COC and semen from 11 bulls. A total of 239 individual blastocysts were assessed by immunofluorescence to quantify total β-catenin. For experiment 11, embryos were produced following procedures for experiment 8. Treatments were added on day 5 of development (120 hpi), and blastocysts were harvested on day 7 of culture for immunolabeling of pjnk as described above. A total of 42 individual blastocysts were assessed by immunofluorescence to quantify total pjnk. Statistical Analysis Effects of treatment on the percent of oocytes or cleaved embryos developing to the blastocyst stage were evaluated using Proc Glimmix of SAS for Windows, version 9.4 (SAS Institute Inc, Cary, NC, USA). Each embryo was considered an observation (0=not developed to blastocyst; 1=developed to blastocyst). The analysis was performed with the dependent variable considered as a binomial distribution, and treatments as fixed effects. Results are presented as least-squares means ± standard error of the mean. Data on intensity of immunolabeling were analyzed by least-squares analysis of variance using the PROC MIXED procedure of SAS. Treatments were fixed effects and 105
106 replicate was considered a random effect. Results are presented as least-squares means ± standard error of the mean. Results Effect of Activation of Canonical WNT Signaling on Development (Experiments 1 and 2) Two experiments were conducted to determine whether activation of canonical WNT signaling would affect development of embryos to the blastocyst stage. In Experiment 1, addition of the GSK3 inhibitor CHIR to cultured embryos from day 5 to day 7 of development decreased the proportion of oocytes and cleaved embryos becoming blastocysts (P=0.03 and P=0.05, respectively; Table 3-1). In Experiment 2, canonical WNT signaling was activated by addition of the WNT agonist AMBMP. To test whether AMBMP acts by increasing β-catenin accumulation, embryos were treated with DKK1 which blocks coactivation of WNT receptors. Immunoreactive β-catenin was localized primarily to the plasma membrane and was never found in the nucleus even after addition of AMBMP (Figure 3-1A). Intensity of immunoreactive β-catenin was increased by AMBMP (P<0.0001) and decreased by DKK1 (P=0.0001). There was no interaction between AMBMP and DKK1 because AMBMP increased immunoreactive β- catenin even in the presence of DKK1. Note, however, that the amount of β-catenin in embryos treated with AMBMP combined with DKK1 was similar to the amount of β- catenin in embryos treated with vehicle alone. Thus, while DKK1 did not prevent actions of AMBMP to increase β-catenin, the total amount of β-catenin was not elevated as compared to controls (Figure 3-1B). AMBMP reduced development to the blastocyst stage and, while DKK1 alone did not alter development, the effect of AMBMP was 106
107 blocked by DKK1 (DKK1 by AMBMP interaction: P=0.04 for blastocysts/oocyte and P=0.03 for blastocysts/cleaved; Table 3-2). Effects of Inhibition of Endogenous WNT Signaling with Wnt-C59 or DKK1 on Ability of Embryos to Develop to the Blastocyst Stage and Blastocyst Cell Number (Experiment 3) Neither Wnt-C59, which blocks acylation and secretion of WNTs, nor DKK1, which interferes with activation of the WNT-FZD-LRP5/6 receptor complex, altered the proportion of oocytes or cleaved embryos developing to the blastocyst stage (Table 3-3). However, blastocysts formed in the presence of Wnt-C59 had increased number of ICM cells (P=0.02) and tended to have reduced TE:ICM ratio (P=0.06) relative to embryos treated with vehicle. There was no effect of DKK1 on numbers of ICM or TE cells in the blastocyst (Table 3-3). Effects of DKK1 on Development and Blastocyst Cell Number (Experiments 4-5) There was no effect of addition of DKK1 from day 5 to day 7 of development on the proportion of oocytes or cleaved embryos that became blastocysts or on the numbers of ICM or TE cells in the resulting blastocysts. This was true whether embryos were produced using conventional semen (Table 3-4) and for male and female embryos tested separately after production using sexed semen (Table 3-5). The only exception was a tendency for female embryos to have smaller TE:ICM ratio (P=0.10) Effects of DKK1 on Developmental Changes in Immunoreactive YAP1 and CDX2 in Morulae and Blastocysts (Experiment 6) The transcription factor YAP1 plays an important role in TE formation in the mouse by interacting with TEAD4 to induce transcription of CDX2. For Experiment 6, it was tested whether DKK1 would modify developmental changes in the number of cells positive for YAP1 and CDX2 from day 5 to 7 of development. Representative examples 107
108 of labeling are shown in Figure 3-2A. Both YAP1 and CDX2 were localized exclusively to nuclei. For day 5 morulae, nuclei positive for YAP1 and CDX2 were not frequent and labeling was faint. By day 6, however, there were abundant numbers of YAP1 + and CDX2 + cells. By day 7, both type of cells were confined to the TE. Quantification of nuclei that were positive for YAP1 and CDX2 indicated that the number of cells positive for both markers increased during development (Figure 3-2B, 3.2F). However, the proportion of total cells that were YAP1 + declined after day 5 (Figure 3-2C) while the proportion of total cells that were CDX2 + increased from day 5 to 7 (Figure 3-2G). Similarly, the percent of CDX2+ cells that were also YAP1+ declined over time (Figure 3-2H) while the percent of YAP1+ cells that were also CDX2+ increased (Figure 3-2G). Thus, as the embryo developed, an increasing number of CDX2+ cells lost expression of YAP1. Intensity of labeling for YAP1 and CDX2 in nuclei positive for the marker increased during development (Figure 3-2E and 3-2I). There was no effect of DKK1 on total number of blastomeres or on the number or proportion of blastomeres that were YAP1 + or CDX2 +. Similarly, DKK1 did not affect intensity of CDX2 + cells.in contrast, the intensity of YAP1 labeling in YAP1 + cells was reduced by DKK1 (P=0.04; Figure 3-2E). DKK1 Does not Activate pjnk (Experiment 7) Addition of DKK1 to embryos at day 5 of development did not increase accumulation of immunoreactive pjnk in blastocysts at day 7 of development (Figure 3-3). Regulation of WNT Signaling by WNT7A (Experiments 8 and 11) Results of Experiment 8 are summarized in Table 3-6. Treatment of embryos with WNT7A beginning at either day 1 (20 hpi) or day 5 (115 hpi) increased the 108
109 proportion of oocytes (P=0.0005) and cleaved embryos (P=0.02) that became a blastocyst at day 7 of development. There was no effect of day of treatment or interaction of day with treatment. Subsequent experiments with WNT7A involved treatment on day 5 of development. In Experiment 9, WNT7A again increased the proportion of oocytes (P=0.02) and cleaved embryos (P=0.04) that became a blastocyst at day 7 of development. There was, however, no effect of WNT7A on blastocyst cell number (Table 3-7). Effects of WNT7A on accumulation of β-catenin and pjnk were evaluated in Experiments 10 and 11. There was no effect of WNT7A on total immunoreactive β- catenin on day 7 (Figure 3-4B), Moreover, there was no nuclear localization of β-catenin regardless of treatment (Figure 3-4A-B). Treatment with WNT7A actually reduced JNK signaling as indicated by a reduction in immunoreactive pjnk in either the total area of the embryo (P<.0001) or in nuclei (P<.0001; Figure 3-4C-E). Discussion Collectively, data suggest that embryo-derived WNTs are dispensable for blastocyst formation in bovine embryos, but participate in regulation of ICM proliferation, likely through a mechanism independent of β-catenin. In contrast, exogenous WNTs, can regulate competence of the embryo to develop to the blastocyst stage, with WNT agonists that increase intracellular β-catenin inhibiting development and WNTs like WNT7A that do not regulate intracellular β-catenin improving competence of the embryo to develop to the blastocyst stage. There are two lines of evidence that endogenous WNT are not required for development to the blastocyst stage. Competence of embryos to develop to the 109
110 blastocyst stage was not reduced by inhibition of secretion of endogenous WNTs through a Wnt-C59-mediated block of PORCN. Moreover, inhibition of β-catenin mediated WNT signaling with DKK1 did not alter the proportion of oocytes or cleaved embryos becoming a blastocyst. In earlier studies as well, there was no effect of DKK1 on development (Xie et al., 2008b; Denicol et al., 2013a). The lack of role for endogenous WNTs in development to the blastocyst is also true for mouse embryos. Blastocyst formation is not impaired in either β-catenin deficient mice (Huelsken et al., 2000) or embryos in which Wnt signaling is blocked (Xie et al., 2008b) or in which Porcn-dependent Wnt signaling is inhibited (Biechele et al., 2013). There was also no effect of Dkk1 on development of the mouse embryo (Xie et al., 2008b). Although the cow parallels the mouse with respect to the dispensability of WNT signaling for development to the blastocyst stage, there may be divergence between species in role of endogenous WNT in formation of the ICM. As shown here, inhibition of PORCN increased the number of cells in the ICM of the bovine embryo, indicating that endogenous WNTs limit the number of these cells. In contrast, numbers of ICM and TE cells were unperturbed in Porcn-mutant mouse blastocysts (Biechele et al., 2013). The number of cells in the ICM could be determined by the proportion of blastomeres in the morula that remain pluripotent after differentiation of the TE or by the degree of proliferation and apoptosis of cells in the ICM. Specific WNT can promote differentiation (Krivega et al., 2015), and apoptosis (Famili et al., 2015) and decrease proliferation (Qin et al., 2016). 110
111 Treatment of embryos with DKK1, which was shown to reduce β-catenin in the embryo, did not affect the number of ICM or TE cells. Thus, it is likely that the endogenous WNT regulating number of ICM cells acts through a mechanism independent of β-catenin. There was also no effect of DKK1 on TE cell number in the blastocyst. Additional evidence against a role for DKK1 in differentiation of the bovine blastocyst was the finding that DKK1 reduced accumulation of YAP1, a transcription factor important for TE formation in mouse (Nishioka et al., 2009) and did not affect the amount of the transcription factor CDX2 that is responsible for TE differentiation (Sakurai et al., 2016). These findings stand in contrast to earlier studies in cattle (Denicol et al., 2014) and pigs (Lim et al., 2013) that DKK1 increases the number of TE cells in the blastocyst. In addition, DKK1 increased expression of AMOT in bovine morulae (Denicol et al., 2015); this gene participates in TE formation in mice (Hirate et al., 2012). The reason for the discrepancy between current findings and earlier ones with respect to actions of DKK1 on TE numbers is not known. Although sex can affect response of the bovine embryo to embryokines (Siqueira and Hansen, 2016), there was no effect of DKK1 on TE numbers in either male or female embryos. Although embryo-derived WNT have little effect on the competence of an embryo to become a blastocyst, activation of WNT signaling, either by treatment with AMBMP or WNT7A, can modify the proportion of embryos developing to the blastocyst stage. The uterine endometrium expresses a wide number of WNT ligands including WNT1, WNT5A, WNT6, WNT7A, WNT8A, WNT9A and WNT9B (Mamo et al., 2012; Tribulo et al., 2015) and it is likely that maternally-derived WNT participate in embryonic development. Consequences of maternal WNT signaling are likely to depend on a 111
112 complex array of factors including the abundance of specific WNT ligand, receptor and co-receptor availability, and presence of WNT regulatory molecules such as DKK1 and soluble frizzled receptors, which are also expressed in the endometrium (Tribulo et al., 2015). Results of the present study indicate that WNTs that increase β-catenin decrease developmental competence because two treatments that increase cellular β-catenin, GSK3 inhibitor and the WNT mimetic AMBMP, decreased the proportion of embryos that developed to the blastocyst stage. In an earlier study, as well, AMBMP decreased development of bovine embryos (Denicol et al., 2013a). Effects of AMBMP were decreased by DKK1, which also decreased the amount of β-catenin in the embryo. This result is an indication that actions of AMBMP involve accumulation of β-catenin and that maternally-derived molecules like DKK1 can modify responses of the embryo to WNTs that increase cellular β-catenin. As reported earlier (Tribulo et al., 2017), β-catenin was not localized in the nucleus of the embryo after treatment with GSK inhibitor or AMBMP and thus it is likely that β-catenin acts independent of a nuclear site of action. Not all maternally-derived WNT are likely to inhibit development. Present results indicate that WNT7A, which does not affect amounts of β-catenin in the embryo but does decreases phosphorylation of JNK, increased the proportion of embryos that developed to the blastocyst stage. WNT7A is not expressed in the bovine preimplantation embryo (Denicol et al., 2013a) but is highly expressed in bovine endometrium (Tribulo et al., 2015). Although it has been described that WNT signaling mediated by JNK is required for cavity formation in mouse embryos (Lu et al., 2008; Xie 112
113 et al., 2008b), the role of WNT signaling mediated by pjnk remains unknown in the bovine. One of the objectives of the series of experiments documented here was to identify downstream pathways affected by DKK1. This molecule, which can block canonical WNT signaling by interfering with recruitment of the LRP5/6 co-receptor to the WNT-FZD ligand receptor (Bafico et al., 2001; Nusse, 2001) maybe an important determinant of fertility in the cow. Bovine embryos treated with DKK1 were more likely to establish and maintain pregnancy after transfer to recipient cows than embryos not treated with DKK1(Denicol et al., 2014). Also, expression of DKK1 in endometrium was lower for heifers diagnosed as infertile compared to heifers considered fertile (Minten et al., 2013) and was lower for endometrium of lactating cows than non-lactating cows (Cerri et al., 2012). Actions of DKK1 are complex because, in addition to interfering with WNT-FZD signaling, DKK1 can act as a WNT agonist to activate non-canonical signaling pathways such as WNT/PCP pathway (Caneparo et al., 2007). In the present work the ability of DKK1 to reduce accumulation of β-catenin was documented, showing that it can reduce β-catenin and block actions of molecules like AMBMP. Treatment with DKK1 did not prevent AMBMP from increasing β-catenin. Thus, AMBMP acts to increase β-catenin in the cell by acting downstream from formation of the WNT-receptor-co-receptor complex. However, DKK1 did block actions of AMBMP on development, probably because the total β-catenin accumulated in embryos treated with AMBMP and DKK1 in combination was the same as for embryos treated with vehicle alone. In contrast to regulation of β- catenin in the embryo, DKK1 had no effect on JNK phosphorylation even though DKK1 113
114 can activate JNK signaling in other cellular systems (Killick et al., 2014; Krause et al., 2014). The lack of effect of DKK1 on activation of JNK in the bovine embryo may reflect insufficient amounts of one or more molecules involved in the pathway by which DKK1 activates JNK signaling. To our knowledge, data presented here are first description of localization of YAP1 in the bovine embryo. This transcription factor plays an important role in TE formation in the mouse by interacting with TEAD4 to induce transcription of CDX2. The pattern of expression of YAP1 from the morula stage at day 5 to the blastocyst stage at day 7 indicates that YAP1 accumulation in the nucleus precedes that of CDX2, as revealed by higher number of YAP1 + than CDX2 + nuclei at day 5 morulae, and presence of nuclear YAP1 in every nucleus that was CDX2 + at this developmental stage. As embryos developed, however, the proportion of blastomeres that were YAP1 + declined and fewer nuclei were YAP1 + than were CDX2 +. By the blastocyst stage, both YAP1 and CDX2 were localized in the TE. This observation, as well as the observation that over 90% of YAP1 + nuclei in the blastocyst were also CDX2 +, is consistent with a role of YAP1 in CDX2 expression and TE differentiation. Functional studies are needed to determine whether or not YAP1 is required for CDX2 expression in bovine embryos. Taken together, data suggest that embryo-derived WNTs are dispensable for blastocyst formation in bovine embryos but do participate in formation of the ICM. In contrast, exogenous WNTs can affect embryonic development in a positive or negative manner depending upon nature of the WNT ligand and the downstream outcome. This latter result implies that maternally-derived WNT could play important roles in development of the preimplantation embryo. 114
115 Table 3-1. Effect of exposure of embryos to GSK3 inhibitor from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage a Treatment Vehicle GSK3 inhibitor P-value Blastocysts/oocyte (%) 28.5 ± ± Blastocysts/cleaved embryo (%) 34.5 ± ± a Data are the least-squares means ± SEM of results from 5 replicates. 115
116 Table 3-2. Effect of treatment of embryos with the WNT agonist AMBMP and the endogenous regulator of WNT signaling, DKK1 from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage a Treatments Blastocysts/cleaved embryo AMBMP DKK1 Blastocysts/oocyte (%) (%) ± ± ± ± ± ± ± ± 4.8 Statistical significance (P) AMBMP DKK Interaction a Data are the least-squares means ± SEM of results from 7 replicates. 116
117 Table 3-3. Effects of inhibition of endogenous WNT signaling from day 5 to day 7 of development with either Wnt-C59 or DKK1, on ability of embryos to develop to the blastocyst stage, and cell number of blastocysts at day 7 of development. a Treatment Blastocysts/ oocyte (%) Development Vehicle 16.6 ± ± 3.9 Wnt-C ± ± 3.7 Blastocyst cell number Blastocysts/ cleaved embryo (%) Total TE ICM ± DKK ± ± 3.3 a Data are the least-squares means ± SEM of results from 5 replicates. b Differs from control (P=0.02). c Differs from control (P=0.06). TE:ICM ratio ± ± ± ± ± ± 4.1 b 1.7 ± 0.1 c ± ± ± ±
118 Table 3-4. Effect of exposure of embryos to DKK1 from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage and cell number of day 7 blastocysts a. Treatment Blastocysts/ oocyte (%) Development Blastocyst cell number Blastocysts/ cleaved embryo (%) Total TE ICM ± TE:ICM ratio Vehicle 25.1 ± ± ± ± ± ± DKK ± ± ± ± ± 0.1 a Data are the least-squares means ± SEM of results from 10 replicates. There were no effects of treatment (P>0.10). 118
119 Table 3-5. Effect of treatment of embryos with DKK1 from day 5 to day of development on the ability of male and female embryos to develop to the blastocyst stage and cell number of day 7 blastocysts a. Development Blastocyst cell number Treatment Sex Blastocysts/ oocyte (%) Blastocysts/ cleaved embryo (%) Total TE ICM TE:ICM ratio Female 20.6 ± ± ± ± ± b ±0.1 Vehicle Male 14.3 ± ± ± ± ± c ± 0.1 Female 17.1 ± ± ± ± ± b ±0.1 DKK1 Male 15.9 ± ± ± ± ± c ±0.1 a Data are the least-squares means ± SEM of results from 5 replicates. Treatment consisted of 100 ng/ml DKK1 (dickkopf-related protein 1). bc indicate tendency of sex effect on TE:ICM ratio P=
120 Table 3-6. Effect of treatment of embryos with recombinant WNT7A from day 1 to day 7 of development or from day 5 to 7 of development (i.e., during the morula to blastocyst transition) on the ability of embryos to develop to the blastocyst stage a. Development Blastocysts/cleaved Blastocysts/oocyte (%) embryo (%) Timing of treatment Treatment Vehicle 24.4 ± ± 3.6 day 1-7 WNT7A 31.0 ± ± 3.6 Vehicle 20.1 ± ± 3.6 day 5 to 7 WNT7A 29.8 ± ± 3.6 Statistical significance (P) WNT7A day Interaction a Data are the least-squares means ± SEM of results from 6 replicates. 120
121 Table 3-7. Effect of treatment of embryos with recombinant WNT7A from day 5 to day 7 of development on the ability of embryos to develop to the blastocyst stage and cell number of day 7 blastocysts a. Treatment Blastocysts/ oocyte (%) Development Blastocysts/ cleaved embryo (%) Blastocyst cell number Total TE ICM TE:ICM ratio Vehicle 24.5 ± ± ± ± ± ± 0.2 WNT7A 30.7 ± ± ± ± ± ± 0.2 P-value a Data are the least-squares means ± SEM of results from 4 replicates. 121
122 Figure 3-1. Treatment of embryos at day 5 after insemination with 100 ng/ml DKK1 reduces amounts of immunoreactive β-catenin but does not prevent the WNT agonist (AMBMP) from increasing amounts of β-catenin. A) Representative images of embryos immunolabeled for β-catenin (red) and DNA (blue). B) Quantification of intensity of β-catenin. Immunoreactive β-catenin was affected by AMBMP (***P<0.0001) and DKK1 (****P=0.0001) but not by the AMBMP by DKK1 interaction (P=0.9). Data are least-squares means ± SEM of results from 7 replicates, with a total of 165 labeled blastocyst 122
123 Figure 3-2. Immunolocalization of the transcription factors YAP1 and CDX2 in morulae and blastocysts. A) Shown are representative images of individual embryos immunolabeled for YAP1 (red), CDX2 (green), and DNA (blue) at Days 5 (morulae), 6 (morulae) and 7 (blastocyst) of development. B to I) Effect of treatment of embryos with DKK1 at day 5 of development on immunoreactive YAP1 and CDX2 at Days 5 (morulae), 6 (morulae) and 7 (blastocysts) of development. Data represent the absolute B) and relative C) (percent of total cells) number of cells positive for YAP1, absolute F) and relative G) number of cells positive for nuclear CDX2. Panels D and H show quantification of dual labeling for YAP1 and CDX2 expressed relative to YAP1 + D) and CDX2 + nuclei H). Panels E and I show intensity of labeling of nuclei for YAP1 E) and CDX2 I). Data are least-squares means ± SEM of results from 5 replicates. Embryos treated with vehicle are represented by closed circles and solid lines while embryos treated with DKK1 are represented by broken lines and open circles. H). Data are least-squares means ± SEM of results from 5 replicates, with a total of 232 labeled embryos. Asterisks indicate effect of DKK1 on intensity of YAP1 immunofluorescence (P=0.04). 123
124 Figure 3-3. Effect of treatment of embryos with DKK1 from day 5 to day 7 of development on accumulation of pjnk. A) Representative images of individual blastocysts immunolabeled for pjnk (red) and DNA (blue). B) Quantification of intensity of pjnk in whole embryonic area.(left pannel) Quantification of intensity of pjnk in nuclear area (right pannel). Data are least-squares means ± SEM of results from four replicates with a total of 91 labeled blastocysts. 124
125 Figure 3-4. Effect of treatment of embryos with WNT7A from day 5 to day 7 of development on accumulation of β-catenin and pjnk. A) Representative images of individual blastocysts immunolabeled for β-catenin (red) and DNA (blue). B) Quantification of intensity of β-catenin in whole embryonic area. Data are least-squares means ± SEM of results from ten replicates with 239 labeled embryos. C) Representative images of individual blastocysts immunolabeled for pjnk (red) and DNA (blue). D) Quantification of intensity of pjnk in whole embryonic area. E) Quantification of intensity of pjnk in nuclear area. Data are least-squares means ± SEM 125
126 CHAPTER 4 CONSEQUENCES OF EXPOSURE OF EMBRYOS TO DICKKOPF-RELATED PROTEIN 1 AND COLONY STIMULATING FACTOR 2 ON BLASTOCYST YIELD, PREGNANCY RATE, AND BIRTH WEIGHT OF THE CALF Introduction Embryonic development is under regulation of maternally-derived molecules called embryokines. These molecules can affect competence of the embryo to develop to the blastocyst stage, establish pregnancy after transfer to females and even change postnatal phenotype. In the cow, both insulin-like growth factor-1 (IGF1) (Block et al., 2008, 2011) and the cytokine colony stimulating factor 2 (CSF2) can increase the percent of embryos becoming blastocyst in culture and increase the proportion of embryos that establish pregnancy after transfer to females (de Moraes and Hansen., 1997; Loureiro et al., 2011a, b; Denicol et al., 2014) s. In addition, while not affecting competence to form a blastocyst, the WNT regulatory protein DKK1 can increase the percent of embryos establishing pregnancy after transfer (Denicol et al., 2014). Moreover, exposure of embryos to CSF2 from day 5 to 7 of culture improves postnatal growth of the resultant calves (Kannampuzha-Francis et al., 2015). The existence of embryokines leads to the possibility that addition of appropriate embryokines into culture systems for in vitro produced embryos may increase the outcomes of this increasingly-important procedure. Embryos produced in vitro have several abnormal features compared to embryos produced in vivo including altered gene expression (Corcoran et al., 2006; McHughes et al., 2009; Gad et al., 2012), metabolism (Khurana and Niemann, 2000), lipid content (Crosier et al., 2000; Sudano et al., 2012), ultrastructure (Boni et al., 1999; Rizos et al., 2002), and DNA methylation (Niemann et al., 2010). Moreover, the pregnancy rate of embryos produced in vitro is 126
127 lower than for embryos produced in vivo (Lonergan et al., 2007; Pontes et al., 2009). However, most embryos produced in vitro under commercial conditions are cultured in medium containing fetal bovine serum (FBS) and it is possible that the existence of an array of growth factors and other regulatory molecules in serum preclude additional actions of exogenously added embryokines. Indeed, the beneficial effects of CSF2 on blastocyst yield were abrogated when embryos were cultured in serum containing medium (de Moraes and Hansen, 1997). Here the effect of CSF2 and DKK1 alone and in combination on in vitro embryo production in beef cattle was tested. The working hypothesis was that co-exposure of embryos to CSF2 and DKK1 will improve development of preimplantation embryos to the blastocyst stage and will confer greater ability to successfully establish and maintain pregnancy. It was also hypothesized that exposure of preimplantation embryos to CSF2 in combination with DKK1 will improve birthweight of the offspring. The experiment was performed using culture medium containing FBS to evaluate the value of adding these embryokines under conditions commonly used in commercial in vitro fertilization (IVF) laboratories. Materials and Methods Animals and Experimental Design The experiment was conducted using 70 cows selected for oocyte collection while housed on pasture on four commercial beef farms located near Córdoba, Argentina ( S, W). Donor cows were included in the study from August 2015 to August Donors were both heifers and multiparous cows of various breeds including Angus (n=7), Red Angus (n=3), Bonsmara (n=14), Brahman (n=2), 127
128 Brangus (n=19), Red Brangus (n=8), and Braford (n=17). Body condition score at the time of oocyte retrieval ranged between 3 and 4 (1-5 scale; Ferguson et al., 1994). Each donor female was subjected to 1 to 4 rounds of oocyte retrieval using transvaginal, ultrasound-guided ovum pickup procedures, referred to as ovum pick-up (OPU). Oocytes were recovered by OPU at each farm, transported in cryotubes (Corning, NY, USA) with maturation medium (Bioklone, Jaboticabal, SP, Brazil) and conditioned atmosphere (5% (v/v) CO 2 and 7% (v/v) O 2 ) in an oocyte transport incubator (Cryologic Pty Ltd., Blackburn, Victoria, Australia) and subjected to in vitro maturation and fertilization at the IVF laboratory of the Instituto de Reproducción Animal Córdoba (IRAC), Córdoba, Argentina. For each IVF session, spermatozoa from a single bull were used to fertilize all viable cumulus-oocyte complexes from a single donor. Sire (n=27 total) was selected by the cow owners. Different sires were used for individual donors. Moreover, the same sire was not always used for an individual oocyte donor for each OPU/IVF session. Blastocysts were then transferred to recipient cows located at one of the four commercial farms. The study was conducted as a randomized block design. Within each farm, donors were randomly allocated to one of the four treatment groups (vehicle, CSF2, DKK1, CSF2 + DKK1). Embryos from a given donor were always allocated to the same treatment for each round of OPU. Oocyte Retrieval The complete procedure for OPU was performed while donor cows were under epidural anesthesia (5-7 ml 2% (w/v) lidocaine, (Lidocaina VT, Laboratorio Vetue, Venado Tuerto, Argentina). All visible follicles were aspirated using a transvaginal ultrasound-guided 5 MHz microconvex probe (Mindray DP-30, Mindray Medical 128
129 International Ltd, Shenzhen, China) mounted to a transvaginal handle (WTA, Cravinhos, Brazil) equipped with a disposable 18 ga x 3.81 cm vacutainer needle attached to a 50 ml Falcon tube (Fisher Scientific, Altham, MA, USA) and a vacuum pump (SV-003, WTA) via a clean silicon tubing. The handle-mounted-probe was positioned dorsally to the vaginal fornix and one ovary held against the probe through rectal manipulation. Ovarian follicles were targeted with the needle across the vaginal wall, and aspiration was performed with a vacuum of mm Hg, and ml/min flow. Cumulus oocyte complexes (COC) were collected in a phosphate-buffered saline based collection medium (PICTOR-GEN ; Biogen Argentina SA, Córdoba, Argentina) supplemented with heparin (10 IU/ml, Sobrius, Fada Pharma, Ciudad Autónoma de Buenos Aires, Argentina). Immediately after OPU of both ovaries, the aspiration fluids were filtered (50 µm, Millipore, Alphaville, SP, Brazil). The COC were washed with collection medium and COC retrieved by searching in a petri dish (Falcon) under a stereomicroscope. Oocyte Classification, Transport and Maturation Upon recovery, COC were classified based on appearance of the oocyte cytoplasm and number of cumulus cell layers as described previously (Hasler et al., 1995). Grade 1, 2 and 3 COC were considered viable, while grade 4 COC (which ordinarily include denuded and expanded COC, as well as those with pycnotic cytoplasm) were subdivided into two categories based on cytoplasmic appearance. Thus, COC with pycnotic cytoplasm were discarded while COC that were either denuded or expanded with homogenous cytoplasm were manipulated with their viable counterparts. Viable and non-pycnotic grade 4 COC were transported in cryotubes (Corning), containing 400 µl maturation medium (Bioklone) covered with 120 µl mineral 129
130 oil (Sigma, USA) with an atmosphere of 5% (v/v) CO 2 and 7% (v/v) O 2. Cryotubes were prepared at the laboratory and kept at 4 C. Immediately before OPU, cryotubes were equilibrated in a 37 C water bath. After transferring oocytes into the cryotubes, the atmosphere was re-conditioned to 5% CO 2 and 7% O 2 and tubes were placed into an oocyte transport incubator (Cryologic Pty Ltd.) set at 38 C. Upon arrival to the IVF laboratory, groups of up to 27 COC from the same donor were transferred to equilibrated 100 µl drops of maturation medium. When the number of COC from a donor was larger than 27, COC were split into groups of the same size. Maturation was conducted for a total of 22 to 26 h (including transportation time) in an incubator at 38.5 C with an atmosphere of 5% CO 2 in humidified air. Embryo Production Matured COC were washed in TL sperm medium (Bioklone) and placed in 50 µl drops of IVF medium (Bioklone) covered by mineral oil, to which sperm (final concentration, 2 x 10 6 cells/ml) were added. The bull used to provide sperm for each fertilization procedure was chosen by the client. Sperm from frozen-thawed straws were purified using Percoll before insemination. Co-culture of gametes proceeded for 24 h at 38.5 C in a humidified atmosphere of 5% (v/v) CO 2. After removal of cumulus cells by pipetting, presumptive zygotes were placed in 100 µl oil-covered drops of culture medium [synthetic oviduct fluid (SOF) containing FBS, Bioklone]. At 3 and 5 days after insemination, culture medium (50 µl/drop) was replaced by the same volume of fresh SOF medium. At day 5, the fresh medium contained either vehicle [Dulbecco s phosphate-buffered saline with 0.1% (w/v) bovine serum albumin (DPBS-BSA), final concentration in the drop =0.002% (v/v)], CSF2, DKK1 or CSF2 and DKK1 at twice the final concentration to achieve final concentrations of 10 ng/ml CSF2 and 100 ng/ml 130
131 DKK1 in a culture medium consisting of 0.001% (v/v) DPBS-BSA in culture medium (Bioklone). The source of lyophilized human recombinant DKK1 was R&D Systems (Minneapolis, MN, USA). Protein was reconstituted using DPBS-BSA and stored at 10 µg/ml in aliquots at -20 C until use. Lyophilized recombinant bovine CSF2 was from Kingfisher Biotech, Inc. (Saint Paul, MN) and was described by the supplier as GM- CSF. It was reconstituted in DPBS-BSA to 1 µg/ml and stored at -20 C in aliquots until use. The proportion of cleaved zygotes was assessed 3 days after insemination. Embryonic development was assessed 7 days after insemination using developmental stage classifications described by the International Embryo Transfer Society (Stringfellow and Givens, 2010). Transferable embryos had a quality score of grade 1 or 2 and one of the following stages: early blastocyst (Stage 5), blastocyst (stage 6), expanded blastocyst (stage 7) and hatching blastocyst (stage 8). Embryos were washed in Hepes-SOF medium (Bioklone), and individually packed in straws using Hepes-SOF medium. Embryos were transported in an embryo transport incubator (WTA) at 38.5 C to the farm, which was located 2 to 15 hours from the laboratory. Embryo Transfer and Pregnancy Diagnosis Recipient animals were synchronized to be receptive to a day 7 blastocyst using different hormonal treatments according to the characteristics of the herd. A total of 452 embryos were transferred including Bos taurus [Angus (n=31), Red Angus (n=34), and Bonsmara (n=103)], Bos indicus [Brahman (n=11)], and Brahman-influenced [Brangus (n=88), Red Brangus (n=46), Braford (n=139)] embryos. Pregnancy diagnosis was performed by transrectal ultrasonography using a 5 MHz linear-array transducer 131
132 (Chison D 600 Vet; Chison Medical Imaging co., China) between day 30 and 40 of gestation. Birthweights of the Offspring Birthweight data were available on a subset of 31 male and female calves from five breeds (Angus, Red Angus, Brangus Negro, Red Brangus and Bonsmara). Weights were measured within 72 h after birth. Statistical Analyses Binary responses were analyzed by multivariable logistic regression using the GLIMMIX procedure of SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) with binomial distribution. Three response variables were investigated including proportion of either putative or cleaved zygotes that developed to the blastocyst stage at day 7 after insemination, and proportion of transferred blastocysts that established pregnancy. The final statistical model for each variable was built using a backward selection method in which variables were continuously removed when P > Class variables tested for effect on embryonic development and pregnancy included farm and breed of the donor. Covariates tested for effect on embryonic development included number of retrieved COC, number of viable COC, average quality score of COC, percent of viable COC that cleaved. Covariates tested for effect on pregnancy also included number of blastocysts at day 7 after insemination, percent of viable COC becoming a blastocyst on day 7, and the percent of cleaved zygotes becoming a blastocyst on day 7. Data are presented as least-squares means ± SEM. Treatment effects on birthweight were determined by ANOVA using the MIXED procedure of SAS including the fixed effects of DKK1, CSF2, interaction of DKK1 by CSF2, sex and breed. 132
133 Results In Vitro Production of Embryos Overall, the average number of recovered COC per donor per OPU procedure was 23.3, of those, 80.4 % were classified as viable (including grade 4 with homogenous oocyte nucleus) and subjected to in vitro maturation. The overall proportion of putative zygotes (i.e., those matured COC that were exposed to sperm) that cleaved by day 3 after insemination was 59.4%. Moreover, 32% of putative zygotes developed to the blastocyst stage by day 7 after insemination. Neither treatment nor any covariate affected (P>0.05) any characteristic of in vitro production (Table 4-1). Pregnancy Rate A total of 452 embryos were transferred to recipients. Overall, the proportion of transferred embryos that established pregnancy was 42.2%. There was no effect of treatment on pregnancy rate (Table 4-1) and no covariate was found to affect the effect of treatment related to pregnancy rate. Postnatal Characteristics In a dataset of 31 calves (n=15 males and 16 females), birthweight was affected by the main effect of DKK1 (P=0.04), but not by CSF2 or the interaction between DKK1 and CSF2 (Figure 4-1). In particular, birthweight was lower for calves derived from embryos treated with DKK1 from day 5 to 7 after insemination regardless of whether CSF2 was also in the culture medium. Discussion Both CSF2 and DKK1 have been shown to modify embryonic development in cattle when embryos are treated from day 5 to 7 of development (i.e., during the period when the embryo transitions from the morula to the blastocyst stage). Treatment with 133
134 CSF2 increased the percent of embryos developing to the blastocyst stage, particularly when the level of development in control embryos was low (de Moraes and Hansen, 1997; Loureiro et al., 2009). Moreover, embryos treated with CSF2 were more likely to establish pregnancy rate after transfer to females (Loureiro et al., 2009; Denicol et al., 2014). Treatment of embryos with DKK1 has been reported to increase subsequent pregnancy rates following embryo transfer (Denicol et al., 2014). Despite the effects of these two embryokines, there was no beneficial effect of either molecule on embryonic development or post-transfer survival in the present experiment. Such a result is interpreted to indicate that the characteristics of the embryo culture system can modify effectiveness of specific embryo regulatory molecules in a commercial embryo transfer production facility. Nonetheless, there were effects of one of the molecules, DKK1, on subsequent development. In particular, calves born from embryos cultured with DKK1 were lighter at birth than embryos cultured in absence of exogenous DKK1. Thus, actions of specific molecules on development of the preimplantation embryo can cause long-term changes affecting postnatal phenotype. The most likely explanation for failure to repeat effects of CSF2 and DKK1 on embryonic development and competence for establishment of pregnancy is the presence of FBS in the medium used to produce embryos. Addition of serum to embryo culture medium can increase the percent of embryos advancing to a transferrable stage of development (Van Langendonckt et al., 1997; Rizos, 2002) and is commonly added to commercial embryo culture systems for that purpose. It is likely that CSF2 and DKK1 were ineffective at stimulating development to the blastocyst stage and increasing pregnancy rate because other growth factors in serum were exerting similar actions on 134
135 the embryo. Indeed, for CSF2 beneficial effects on blastocyst yield occurred in the absence of serum but not in the presence of 5% (v/v) bovine steer serum (de Moraes and Hansen, 1997). The use of FBS can be associated with detrimental effects on embryonic quality as reflected by accumulation of lipid droplets (Abe et al., 2002), alterations in the embryonic transcriptome (Cagnone and Sirard, 2014) and exacerbation of fetal growth leading to the large offspring syndrome reviewed by Young et al. (1998). Perhaps discovery of additional molecules that can serve as embryokines can lead to a replacement of serum in embryo culture medium with specific bioactive molecules. There are other some differences between earlier studies and the present experiment that could be responsible for differences in results. The studies in which effects of CSF2 and DKK1 on pregnancy rate after transfer were observed involved dairy animals (vs. beef cattle in the present experiment), use of sexed semen to produce predominately-female embryos (vs. conventional semen), and a source of CSF2 different than the one for the present experiment. For CSF2, embryonic responses vary between female and male embryos (Siqueira and Hansen, 2016) but effects of DKK1 on morula gene expression were largely similar for female and male embryos (Denicol et al., 2015). In an earlier experiment, heifer calves produced from embryos exposed to CSF2 in vitro had similar birth weights as control embryos but, beginning at 4 mo of age, grew faster than control calves (Kannampuzha-Francis et al., 2015). Similarly, there was no effect of CSF2 on birthweight in the present experiment. Although CSF2 treatment during the period of the morula and blastocyst stage did not have a significant effect on 135
136 postnatal phenotype, there was a developmental programming action of DKK1. Calves derived from embryos treated with DKK1 were smaller at birth than calves from embryos not exposed to DKK1. This result has several implications including the need to understand how changes in molecular processes in the morula- and blastocyst-stage embryo can modify development in a way that causes phenotype to be altered many months later in postnatal life. One possibility is that DKK1 changes the embryonic transcriptome or epigenome in ways that affect downstream cell differentiation. Treatment of embryos with DKK1 causes small changes in the transcriptome at the morula state (Denicol et al, 2015) as well as in trophectoderm and hypoblast formation in one study (Denicol et al, 2014) although not in results presented above (Chapter 3). From a practical perspective, these data provide additional evidence that postnatal function of livestock species can potentially be modified by the environment affecting the embryo during the preimplantation period. Such an idea is well supported from the rodent literature (Fleming et al., 2015a, b). Indeed, bovine embryos actively respond to the environment as early as 8-16 cell stage of development by modifying their transcriptome (Cagnone and Sirard, 2014). Perhaps, modification of postnatal phenotype for optimal livestock production should consider not only genetic selection and provision of an optimal environment after birth but should also consider how to alter the microenvironment of the preimplantation embryo as well. 136
137 Table 4-1. Effect of exposure of embryos to the CSF2, DKK1 or the combination on embryonic development and pregnancy rate of cows receiving an embryo a Treatment Embryonic development at day 7 Pregnancy rate Blastocysts/viable Blastocysts/cleaved (%) CSF2 DKK1 COC (%) zygote (%) ± ± ± ± ± ± ± ± ± ± ± ± 4.9 Statistical significance (P) CSF CSF2 DKK DKK1 CSF2 x DKK CSF2 x DKK1 a Data are least-squares means ± SEM from a total of 70 donors subjected to OPU on 1 to 4 occasions to yield 2339 cumulus-oocyte complexes (COC). Vehicle N=85; CSF2 N=104; DKK1 N=120; CSF2+DKK1 N=
138 Figure 4-1. Effect of addition of embryokines during day 5-7 after fertilization on birth weight of the resultant calves. Data are least-squares means ± SEM from 31 calves after adjusting for calf sex and breed. Birth weight was affected by exposure to dickkopf-related protein 1 (DKK1) from day 5 to day 7 of culture (P=0.04) but not by colony-stimulating factor 2 (CSF2) or the CSF2 x DKK1 interaction. 138
139 CHAPTER 5 IDENTIFICATION OF POTENTIAL EMBRYOKINES IN THE BOVINE REPRODUCTIVE TRACT Introduction The environment established by the mother for the preimplantation embryo plays a key role in ensuring that development proceeds in a manner that optimizes pregnancy success and postnatal development. Disruption of maternal physiology during the preimplantation period can compromise embryonic survival. Examples include the effect of establishing an abnormal ratio of estradiol to progesterone in mice (Yoshinaga and Adams, 1966) and humans (Simon et al., 1995) and exposure of female embryo transfer recipients to bisphenol A in mice (Xiao et al., 2011). Other conditions can enhance maternal capacity for supporting development, as shown in cattle for treatment with somatotropin (Moreira et al., 2002). The maternal environment during the preimplantation period can also alter the developmental program of the embryo in a manner that alters postnatal phenotype (review by Hansen et al., 2016). For example, feeding a diet low in protein during the preimplantation period modified postnatal growth and accumulation of body fat in rodents (Fleming et al., 2015). The importance of the maternal environment for embryonic development is illustrated by the consequences of embryo production in vitro. In cattle, for example, embryos produced in vitro experience altered gene expression (Corcoran et al., 2006; McHughes et al., 2009; Gad et al., 2012), metabolism (Khurana and Niemann, 2000), lipid content (Crosier et al., 2000; Sudano et al., 2012), ultrastructure (Boni et al., 1999; Rizos et al., 2002), DNA methylation (Niemann et al., 2010), competence to establish pregnancy (Lonergan et al., 2007; Pontes et al., 2009) and properties of the resultant offspring (Fernandez-Gonzalez et al., 2004; Farin et al., 2006; Ceelen et al., 2008). 139
140 Transfer of embryos produced in vitro to the oviduct can mitigate at least some of these abnormalities (Enright et al., 2000; Lazzari et al., 2010; Gad et al., 2012), indicating the importance of the absence of maternal signals as a cause of aberrant development. An important mechanism by which the maternal oviduct and endometrium directs embryonic development is through secretion of regulatory molecules called embryokines. A number of growth factors can affect embryonic development in various species. Among the most studied embryokines are CSF2 (Sjoblom et al., 1999; Loureiro et al., 2009; Denicol et al., 2014), IGF1 (Lin et al., 2003; Jousan and Hansen, 2007; Bonilla et al., 2011) and LIF (Kauma and Matt, 1995; Mohamed et al., 2004; Neira et al., 2010). In many cases, it is unknown whether molecules that affect embryonic development in vitro are present in the reproductive tract at times coincident with development of the preimplantation embryos. In addition, there are likely other regulatory molecules produced by the reproductive tract that can act on the preimplantation embryo. Indeed, the embryo is poised to respond to a plethora of maternal regulatory molecules because of the wide range of growth factor and hormone receptor genes that it expresses (Graf et al., 2014; Zuo et al., 2016). The objective of the present study was to identify potential embryokines during the first 7 days after ovulation using the cow as a model. It is during this period that the bovine embryo develops from the zygote to the blastocyst stage, where it spends the first 4-5 days in the oviduct and then moves into the uterine lumen (Betteridge and Fléchon, 1988). The approach was to collect oviductal and endometrial tissue and determine the relative amounts of expression of genes for 93 hormones, growth factors, 140
141 cytokines, chemokines, and WNT-related molecules that could potentially function as embryokines. Materials and Methods Synchronization of the Estrous Cycle The reproductive status of non-lactating Holstein cows was assessed by transrectal ultrasonography and 20 cows with a detectable corpus luteum were subjected to a hormonal protocol to synchronize ovulation. On day -18 (day of expected ovulation = day 0), cows were injected, i.m., with 25 mg prostaglandin F 2 (PGF, Lutalyse, Zoetis, Florham Park, NJ, USA) followed by 100 µg gonadorelin (GnRH; Cystorelin, Merial Inc., Duluth, GA, USA) on day -16. A second, identical injection of GnRH was injected on day -9 and a progesterone-containing controlled internal drug release device (CIDR, Zoetis) was inserted intravaginally. At day -4, each cow was administered 25 mg PGF, i.m., and the intravaginal device was removed. Another 25 mg PGF was injected at day -3 and 100 µg GnRH was injected i.m. at day -2, i.e. 24 h after PGF. Transrectal ultrasonography of ovaries was performed on day -4, -1 and 0 to confirm ovulation. A total of 15 cows were successfully synchronized and slaughtered at either day 0 (n=4), 3 (n=4), 5 (n=3) or 7 (n=4) relative to the expected day of ovulation. Slaughter was by captive-bolt stunning and exsanguination. Collection of Oviductal and Endometrial Tissues and Uterine Flushings Reproductive tracts were obtained immediately after slaughter and placed on ice. Processing of all organs was completed within a maximum of 4 h from slaughter of the first cow. Side of the reproductive tract was identified as being ipsilateral or contralateral to the side of ovulation. Ovulation of cows slaughtered at day 0 was confirmed by 141
142 absence of a preovulatory follicle in 3 of 4 cows, and the presence of an ovulatory fossa. For the remaining cow, ovaries were lost during processing and ovulation could not be confirmed. After dissecting the oviduct free from the mesosalpinx, the lower third of the oviduct, corresponding to the isthmus, was used to cut transversal 1-mm sections while the oviduct was gently stretched. Samples were snap frozen in liquid N 2 for evaluation of gene expression. Samples were stored in liquid N 2 until transport to the laboratory and storage at -80 C. Uterine flushings and samples of endometrium were collected separately from both uterine horns. The mesometrium was removed and each uterine horn was clamped near the uterine body. The end near the uterotubal junction was opened with a 0.5 cm incision and 30 ml of Dulbeccos s phosphate-buffered saline (DPBS) at room temperature were flushed into the uterine horn from the opposite end using an 18 ga needle. The fluid was propelled by massage along the uterine horn through the incision. Recovered fluid was kept on ice; after centrifugation at 3000 x g for 15 min at 4 C, the supernatant fraction was obtained and stored at -20 C. After flushing, each uterine horn was opened with a longitudinal incision along the curvature. Intercaruncular regions of endometrial tissue were harvested from the middle section of uterine horns using a scalpel and tweezers. Some samples were snap frozen, while others were frozen in optimal cutting temperature medium (O.C.T., Sakura Finetek USA Inc., Torrance, CA, USA) on dry ice covered with 2 methylbutyrate for immunofluorescence analyses. All samples were stored in liquid N 2 until transport to the laboratory where they were stored at -80 C. 142
143 RNA Extraction and Gene Expression Snap-frozen samples of endometrium and oviduct were thawed and homogenized in lysis buffer from Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA) using a tissue homogenizer (Tissue Master 125, OMNI International, Kennesaw, GA, USA) for 10 sec at speed 4. Homogenized tissue in lysis buffer was transferred to the silica columns of the Qiagen RNeasy Mini kit and RNA was extracted by following manufacturer instructions including DNase treatment. Abundance of specific mrna molecules for 93 genes potentially involved in control of embryonic development was determined using the NanoString ncounter analysis system (NanoString Technologies, Seattle, WA, USA) (Geiss et al., 2008). This analytical procedure consists of gene-specific 100-mer probe pairs (i.e. one probe that captures the target gene, and one probe that serves as reporter) that are hybridized to the sample in solution. The reporter probe carries a fluorescent signal, and only probe pairs that hybridize to transcripts in the sample are immobilized and captured for data collection. Probes were designed to quantify mrna for 28 growth factors, 12 chemokines, 22 cytokines, 3 hormones, 19 WNT ligands and 9 WNT regulatory molecules. In addition to the 93 regulatory molecule genes, expression of an aminopeptidase (ANPEP) was also measured as an internal control because it has been shown to be highly expressed in bovine endometrium at days 5 and 7 after ovulation (Forde et al., 2009). Expression of a total of 6 housekeeping genes (ACTB, RPL19, ERK1, GAPDH, SLC30A6, SUZ12) was also assessed. The 100-probe set was designed and synthesized by NanoString Technologies by identifying an optimal sequence within the target transcript that met the criteria of 143
144 uniqueness in the genome and mutual independence from the other probes in the set. In addition to the customized arrays of probes, internal controls were included in the hybridization reaction. The level of expression of these mrna was assessed in six multiplexed hybridization reactions that occurred in solution, post-hybridization steps handled on a custom liquid-handling robot. Subsequently, purified reactions were subjected to the digital analyzer that automatically acquired images and collected data by counting the number of times the reporter probe for each gene was detected. Data were first normalized to external RNA spike-in controls and then normalized to housekeeping genes (ACTB, ERK1, GAPDH, RPL19, SLC30A6, SUZ12) using two normalization factors (i.e. one to adjust for spike-in controls and one for housekeeping genes). Each normalization factor was calculated by for each sample dividing average geometric mean of spike-in controls or housekeeping genes for all samples by the geometric mean for an individual sample. Genes were considered expressed if the number of reads was greater than two standard deviations above the mean of negative controls (i.e., 10.4). For cases in which transcript was detectable for some samples but not others, the value used for statistical analysis for non-detectable samples was the minimum detection level (10.4). Data were analyzed by least-squares analysis of variance using the GLM procedure of SAS for Windows, version 9.4 (SAS Institute Inc, Cary, NC, USA). The statistical model for expression in endometrium included the fixed effects of day, side, and the day by side interaction, and the random effect of cow nested within day. Orthogonal contrasts were performed to identify the pattern of variation over days, including linear, quadratic and cubic effects of day. Two statistical models were used for 144
145 analysis of variance of gene expression in oviduct. Gene expression data for tissue ipsilateral to the side of ovulation were analyzed with day as a fixed effect and the random effect of cow nested within day. Day effects were separated into individual degree-of-freedom comparisons using the same contrasts described for endometrial tissue. In a separate analysis, side was included in the statistical model as a fixed effect along with day and day by side interaction. In this case, only changes between day 0 and day 3 of the estrous cycle were evaluated because this is period that coincides with embryonic development in the oviduct. Data are presented as least-squares means ± standard error of the mean. Immunofluorescence Frozen tissue was processed on a Microm HM550 cryostatic microtome (ThermoFisher Scientific Inc., Waltham, MA, USA) to obtain 4 µm sections that were mounted on Superfrost plus slides (Fisher Scientific, Suwanee, GA, USA) and kept at - 80 C. Slides were fixed in ice- cold acetone for 10 min and allowed to dry for 1 h at room temperature. Re-hydration was performed using Tris-buffered saline (TBS; 20 mm Tris-HCl, ph 7.5 containing mm NaCl) for 20 min, followed by 1 h blocking with the same buffer containing either 10% (v/v) goat serum (Millipore, Billerica, MA, USA) for WNT5A, WNT7A and CSF2 or 10% (v/v) horse serum (Atlanta Biologicals, Flowery Branch, GA, USA) for DKK1. Primary antibodies included rabbit polyclonal antibodies against human WNT5A and human WNT7A (Abcam, Cambridge, MA, USA; 98.4 and 98.6% predicted amino acid sequence identity with bovine WNT5A and WNT7A, respectively), a mouse IgG1 monoclonal antibody against bovine CSF2 (GM-CSF 17.2 IgG1, Washington State University Monoclonal Antibody Center, Pullman, WA, USA); and goat polyclonal anti- 145
146 human DKK1 antibody (R&D Systems Minneapolis, MN, USA; 90.5% predicted amino acid sequence identity with bovine DKK1). All antibodies were diluted with TBS containing 1% (v/v) goat serum, except for anti-human DKK1, which was diluted in TBS. Concentrations were 5 µg/ml, 10 µg/ml, 10 µg/ml and 0.2 µg/ml for anti WNT5A, WNT7A, CSF2, and DKK1, respectively. As a negative control, primary antibody was substituted with IgG of the species corresponding to the primary antibody. Incubation with primary antibodies proceeded overnight at 4 C. Sections were then washed three times for 5 min using TBS containing 1% (v/v) goat serum, except for anti-human DKK1, which was washed with TBS. Secondary antibodies were diluted to a concentration of 6.66 µg/ml and incubated with tissue at room temperature for 1 h. A goat anti-rabbit antibody conjugated to Alexa 555 was used for WNT5A, a goat anti-rabbit antibody conjugated to Alexa 488 was used for WNT7A, a goat anti-mouse antibody conjugated to Alexa 488 was used for CSF2, and a rabbit anti goat antibody conjugated to Alexa 488 was used for DKK1. All secondary antibodies were from Life Technologies (Carlsbad, CA, USA). Following labeling with second antibody and washing of sections with TBS, sections were incubated with 1 µg/ml Hoechst for 5 min to label nuclei. Slides were mounted using SlowFade Gold antifade reagent (Life Technologies), covered with a coverslip, and observed with a 40x objective using a Zeiss Axioplan 2 epifluorescence microscope (Zeiss, Göttingen, Germany) and Zeiss filter sets 02 (4,6- diamidino-2-phenylindole), 03 (fluorescein isothiocyanate filter), and 04 (rhodamine). Digital images were acquired using AxioVision software (Zeiss) and a high-resolution black and white Zeiss AxioCam MRm digital camera. 146
147 Western Blotting for CSF2 All reagents were purchased from Fisher Scientific unless otherwise stated. Uterine flushings were thawed and 1 ml aliquots were precipitated with ice-cold acetone (1:4 dilution, v:v) for 60 min at -20 C. After centrifugation at 13,000 x g for 10 min, pellets were allowed to dry and then reconstituted with double distilled water. Samples were mixed with Laemmli buffer, heated for 5 min at 95 C, and stored at -80 C until analysis. Equal volumes were loaded into precast polyacrylamide gels (Mini- PROTEAN TGX 4-15% polyacrylamide; Bio-Rad, Hercules, CA, USA) and separated by sodium dodecyl sulfate, polyacrylamide gel electrophoresis at 110 V for 60 min. Proteins were then transferred to a nitrocellulose membrane (Hybond ECL, GE Healthcare Life Sciences, Pittsburgh, PA, USA) in a wet system using Tris-glycine transfer buffer [0.06 % (w/v) Tris base, 2.88 % (w/v) glycine, 0.01 % (w/v) sodium dodecyl sulfate, 20 % (v/v) methanol]. Protein transfer proceeded for 60 min at 65 V at 4 C. After 1 h incubation with blocking buffer (StartingBlock, Thermo Scientific), blots were probed with 1 µg/ml mouse IgG1 monoclonal antibody against bovine CSF2 (GM- CSF 17.2 IgG1, VMRD) diluted in blocking buffer containing 0.2% (v/v) Tween 20 and rocked overnight at 4 C. As a negative control, other blots were probed with an equivalent concentration of mouse IgG1. After washing, membranes were incubated with 0.1 µg/ml goat anti-mouse IRDye 800CW conjugated anti-igg antibody (LI-COR Biosciences, Lincoln, NE, USA) for 1 h in the dark at 4 C. Bands were visualized using Odyssey Infrared Imaging System (LI-COR Biosciences). 147
148 Results Expression of Putative Embryokines Expressed in Oviduct Levels of expression of the 93 evaluated genes at each day of the estrous cycle are shown in Table 5-1 and the 50-highest expressed on each day are presented in Figure 5-1. Overall, there was wide variation in the magnitude of expression among genes. Average reads varied from 13,625 to 16 for the 50 most-expressed genes. Of the 93 genes evaluated, all were expressed in the oviduct ipsilateral to the side of ovulation on day 0 and day 3 of the estrous cycle. By day 5, however, only 71 genes were expressed. Those genes whose transcripts were not detectable were AMH, BMP15, CCL4, DKK4, IFNB1, IL2, IL3, IL4, IL5, IL13, IL17A, IL21, SFRP5, TSHB, WNT1, WNT3, WNT3A, WNT7B, WNT8A, WNT8B, WNT9B, and WNT10B. Transcripts for these genes were also non-detectable at day 7. Moreover, 10 other genes were not expressed at day 7 (FGF11, FGF1, GDF9, IL10, IL1B, INHBA, PGF, WNT7A, WNT10A, WNT11). The 10 most highly-expressed genes in descending magnitude were CXCL12, CTGF, IGF2, SFRP2, WNT5A, IK, CXCL10, CXCL3, HDGFRP2 and HDGF at day 0; CXCL12, CTGF, IGF2, IK, IGF1, HDGF, WNT5A, CXCL3, CXCL10 and CCL14 at day 3; CTGF, GRO1, CXCL3, WNT5A, SFRP1 and IL8,. DKK3, CXCL12, IK, and HDGF at day 5; and IK, CTGF, HDGF, CXCL12, HDGFRP2, CXCL3, GRO1, VEGFA, IGF2, and VEGFB at day 7 after ovulation. Of these, CTGF, CXCL3, and IK were among the 10 most abundant transcripts at each day. A total of 21 genes were significantly affected by day of the estrous cycle (Table 5-2). Of these genes, 11 genes were most highly expressed at estrus (CCL21, CTGF, CXCL10, CXCL16, DKK3, FGF10, IL18, IL33, IL34, PGF, and SFRP2), one at day 3 148
149 (WNT4), 8 at day 5 (BMP7, HGF, IL6, SFRP1, TGFB1, WIF1, WNT2, and WNT5A), and 1 at day 7 (IK). Further analysis was conducted to determine if gene expression was affected by side of the oviduct relative to ovulation during the first three days of the estrous cycle when the embryo is resident within the oviduct. There was an effect of side of the oviduct for only 5 genes (DKK2, IL10, IL13, TGFB3, and WNT4). In each case, expression was higher for the oviduct ipsilateral to the side of ovulation than for the oviduct contralateral to the side of ovulation (Table 5-3). For 9 genes (BMP4, CXCL12, CXCL16, FGF10, GRO1, HDGFRP2, IL34, SFRP2, and VEGFB), the effect of side of ovulation depended on the day of the estrous cycle (Table 5-4). All but 3 of these genes (BMP4, GRO1 and SFRP2) were more abundant in the oviduct ipsilateral to the side of ovulation at day 0 whereas side had no effect at day 3. Expression of BMP4, GRO1 and SFRP2 was higher for the ipsilateral side at day 0 but lower for the ipsilateral side at day 3. Expression of Putative Embryokines Expressed in Endometrium Data for all 93 evaluated genes are summarized in Table 5-5 and the transcript abundance for the top 50 expressed genes at each day of the estrous cycle are displayed in Figure 5-2. Overall, there was wide variation in the magnitude of gene expression. For example, average reads varied from to 442 for the top 50 most expressed genes across days. All genes were expressed at day 0, 3 and 7 of the estrous cycle, but only 69 of the 93 genes were expressed on day 5. Genes whose transcripts were not detected at day 5 were AMH, BMP15, CCL21, CCL26, DKK4, FGF14, GDF9, IL2, IL3, IL4, IL5, 149
150 IL10, IL13, IL21, SFRP5, TSHB, WNT1, WNT2B, WNT3, WNT7B, WNT8A, WNT8B, WNT9B,and WNT10B. The 10 most highly expressed genes in descending magnitude were CTGF, CXCL12, WNT5A, WNT6, IGF2, CXCL3, WIF1, HDGF, IK, and VEGFA at day 0; WNT5A, CTGF, CXCL10, HDGF, CXCL3, IK, IGF2, CXCL12, VEGFA, and SFRP1 at day 3; WNT5A, TDGF1, CXCL3, CTGF, SFRP1, GRO1, IK, HDGF, IGF1, and VEGFA at day 5; and TDGF, WNT5A, CTGF, VEGFB, IK, HDGF, SFRP1, VEGFA, HDGFRP2 and WNT7A at day 7 after ovulation. A total of 5 genes (CTGF, HDGF, IK, VEGFA and WNT5A) were among the ten highest expressed genes at each day of the estrous cycle. day of the estrous cycle affected expression of 34 genes (Table 5-6). Of these, 10 were most highly expressed at day 0 (BMP7, CCL14, CCL21, CCL26, CTGF, CXCL12, IGF2, IL33, SFRP2, and WIF1), 2 at day 3 (HDGF, IL15), 16 at day 5 (CSF2, CX3CL1, CXCL3, FGF1, FGF2, GRO1, HGF, IGF1, IL1B, IL6, IL8, SFRP1, SFRP4, TDGF1, WNT16, and WNT5A) and six at day 7 (CXCL16, FGF13, HDGFRP2, VEGFB, WNT7A and WNT11). Only one gene (IK) was differentially expressed between uterine horns, being higher for endometrium ipsilateral to the side of ovulation than for endometrium contralateral to the side of ovulation (900 ± 42 vs 788 ± 43). There were also significant interactions between day and side for 6 genes (Table 5-7). Expression of each of these 6 genes was higher for endometrium contralateral to the side of ovulation at day 0 but expression was either higher for the contralateral side or not different between sides at other days. Immunolocalization of Selected Embryokines within Endometrium Protein localization in the endometrium was evaluated for two known embryokines (CSF2 and DKK1) and two putative embryokines that were highly 150
151 expressed in endometrium (WNT5A and WNT7A). Immunofluorescence was conducted using tissue from the day of the estrous cycle at which gene expression was highest, i.e, day 5 for CSF2, 7 for DKK1, 5 WNT5A, and 7 for WNT7A. Both CSF2 (Figure 5-3) and WNT5A (Figure 5-3) were localized to the luminal epithelium, glandular epithelium and stroma. DKK1 was localized to endometrial stromal cells but was not detectable in glandular or luminal epithelium (Figure 5-3). In contrast to the other proteins, immunoreactive WNT7A was localized to the nucleus in all three major compartments of the endometrium. Nuclear immunolabeling was greater for luminal and glandular epithelium than for stroma. In addition to nuclear localization, immunoreactive WNT7A was also strongly localized to the apical domain of the luminal epithelium only (Figure 5-4). Accumulation of CSF2 in Uterine Flushings Immunoreactive CSF2 was detected in uterine flushings at day 3, 5 and 7 of the estrous cycle, and, faintly, on day 0 (Figure 5-5). On all days, there was a band of immunoreactive protein of molecular weight (24,600). Additional lower and higher molecular weight bands were identified at day 7. The major immunoreactive bands of CSF2 in uterine flushings were of higher molecular weight than either of the two immunoreactive bands identified in a preparation of recombinant CSF2 produced in yeast (22,700 and 19,600). Discussion These results reveal that a large number of genes encoding for cell signaling proteins are expressed by the oviduct and endometrium of the reproductive tract of the cow during the first 7 days after ovulation. It is during this time when the bovine embryo 151
152 develops to the blastocyst stage, first in the oviduct and, after day 4-5, in the uterus (Betteridge and Fléchon, 1988). Many of the proteins encoded by these genes are likely to play important roles in development of the preimplantation embryo. Indeed, several proteins whose genes were expressed by the oviduct and endometrium in this study have been shown to modify embryonic development in the cow. These include activin A (Trigal et al., 2011; Kannampuzha-Francis et al., 2016), BMP4 (La Rosa et al., 2011), CSF2 (Loureiro et al., 2009; Denicol et al., 2014), CTGF (Kannampuzha-Francis et al., 2016), DKK1 (Denicol et al., 2014), EGF (Sakagami et al., 2012), FGF2 (Fields et al., 2011), HDGF (Gómez et al., 2014), IGF1 (Jousan and Hansen, 2007; Bonilla et al., 2011), ILB1 (Paula-Lopes et al., 1998), LIF (Mohamed et al., 2004; Neira et al., 2010; Mo et al., 2014), TGFβ (Neira et al., 2010), and WNT7A (Chapter 3). Other genes expressed in the oviduct and endometrium that have been implicated in regulation of preimplantation embryonic development in other species include NGF (Menino et al., 1989), TGFA (Paria and Dey, 1990; Dardik et al., 1993) and WNT3A (Krivega et al., 2015). Further studies on the effects of the proteins encoded by each of the 93 genes expressed in the oviduct and endometrium can be useful to identify additional cellsignaling molecules that act as embryokines. Many of the cell signaling molecules encoded by the transcripts under investigation may function to regulate physiology of the reproductive tract itself. The angiogenic factor VEGFA was among the most 20 highly-expressed genes in the oviduct and among the 10 highest expressed genes in the endometrium at each day of the estrous cycle. Differential expression of genes involved in angiogenesis have been related to capacity of heifers to become pregnant after embryo transfer (Ponsuksili et 152
153 al., 2014). In addition, various chemokine genes highly expressed in both oviduct (including CXCL3, CXCL10, CXCL12, CCL14 and GRO1) and endometrium (CXCL3 and CXCL10). Chemokines participate in limiting bacterial infection in the reproductive tract (Sheldon, 2015). Another highly-expressed gene in endometrium, WNT7A, is involved in uterine gland morphogenesis (Dunlap et al., 2011). Accumulation of transcripts does not necessarily mean that the protein is synthesized and placed in a location where it can act on the embryo. This is particularly a concern for genes expressed in oviduct because tissue sections analyzed included all layers of the oviduct. Nonetheless, it is likely that accumulation of mrna is indicative of protein synthesis in many cases. Indeed, all the proteins examined immunohistochemically - CSF2, DKK1, WNT5A, and WNT7A - were present in the endometrium. Moreover, CSF2 was secreted into the uterine lumen as indicated by the presence of immunoreactive protein in uterine flushings. The main form of CSF2 detected in uterine flushes was of larger molecular weight (24,600) than the two immunoreactive bands in recombinant bovine CSF2 (22,700 and 19,600). Although the recombinant CSF2 used was produced in yeast, and is subject to posttranslational modifications, it is likely that CSF2 is more glycosylated. Multiple forms of CSF2 have previously been reported in bovine uterine fluid (de Moraes et al., 1999) and by lymphocytes (Cebon et al., 1990). Interestingly, WNT7A was localized to the nucleus of cells of the three endometrial compartments as well as to the apical domain of the luminal epithelium. It is likely that WNT7A has a dual function within the reproductive tract since it can increase the competence of bovine embryos to develop to the blastocyst stage (Chapter 3), and 153
154 regulate proliferation of endometrial epithelium in mouse (Dunlap et al., 2011). To the best of our knowledge, nuclear localization of WNT has not been documented before. Secretion of WNT requires palmitoylation (Willert et al., 2002) and it is possible that the resultant increase in hydrophobicity could lead to localization to the nucleus. There was a great deal of variation in transcript abundance among genes. It would be overly simplistic, however, to assume that genes that are most highly expressed are more important for embryonic development. For cell-signaling molecules, active concentrations required for receptor binding are usually low, in the nanomolar range (for example, Kannampuzha-Francis et al., 2016). Endometrial expression of two well-characterized embryokines, CSF2 and DKK1, was low compared to many other genes. Nonetheless, transcript abundance was sufficient to allow production of detectable amounts of immunoreactive protein in the endometrium. Examination of cyclic changes in gene expression in both oviduct and endometrium is consistent for a role of ovarian steroids in regulation of some of the genes examined. In oviduct, all 93 genes were expressed at day 0 and day 3 of the estrous cycle but only 71 were expressed by day 5 and 61 by day 7. Of the genes significantly affected by day of the estrous cycle, 11 were most highly expressed at day 0, when concentrations of estradiol were high and those of progesterone low; only one was most highly expressed at day 7. For the endometrium, some genes were upregulated at day 0 whereas another set was upregulated at day 7, when estradiol concentrations would be low and progesterone concentrations elevated. While all genes were expressed at day 0, 3 and 7 of the estrous cycle, only 69 of the 93 genes were expressed on day 5. Of genes significantly affected by day of the estrous cycle, 10 were 154
155 most highly expressed at day 0 and another 22 at either day 5 of 7. In other studies on changes in oviductal and endometrial gene expression during the estrous cycle of the cow, there was a fraction of genes differentially expressed between estrus and diestrus (Bauersachs et al., 2004, 2005; Mitko et al., 2008). Steroids play a key role on the regulation of the reproductive tract. Exogenous supplementation of estradiol and progesterone during early stages of the estrous cycle alters the transcriptome of oviduct and endometrium (Groothuis et al., 2007; Forde et al., 2010). Early studies indicated that side of the reproductive tract relative to ovulation had an effect on secretion of proteins by oviduct and endometrium (Malayer et al., 1988; Williams et al., 1992). Such a result is consistent with local regulation of the reproductive tract by the preovulatory follicle or incipient corpus luteum. In contrast, expression of few of the genes in the present experiment was affected by side of the reproductive tract. Only 14 genes were differentially expressed between oviducts ipsilateral and contralateral to ovulation, with the majority being upregulated in the ipsilateral oviduct. Moreover, expression of only one gene in the endometrium differed between uterine horns. Consistent with our findings was the general lack of effect of side of the reproductive tract on the endometrial transcriptome (Bauersachs et al., 2005). Earlier studies (Malayer et al., 1988; Williams et al., 1992) were based on examination of protein secretion and an effect of side of the reproductive tract on posttranscriptional mechanisms cannot be discounted. Results indicate that the oviduct and endometrium express a wide range of cellsignaling genes that have the potential to participate in regulation of development of the preimplantation embryo. Expression of many of these genes varies with stage of the 155
156 estrous cycle, suggesting importance of both estradiol and progesterone in regulation of gene expression. 156
157 Table 5-1. Least-squares means for expression of 93 genes in oviduct ipsilateral to the side of ovulation during the first seven days of the estrous cycle Normalized number of transcripts Gene day 0 day 3 day 5 day 7 AMH ANPEP BMP BMP BMP BMP CCL CCL CCL CCL CSF CTGF CX3CL CXCL CXCL CXCL CXCL DKK DKK DKK DKK EGF FGF FGF FGF FGF FGF FGF FGF FIGF GDF GRO HDGF HDGFRP HDGFRP HGF IFNB IGF IGF IK
158 Table 5-1. Continued Normalized number of transcripts Gene day 0 day 3 day 5 day 7 IL IL12A IL12B IL IL IL IL17A IL IL1A IL1B IL IL IL IL IL IL IL IL IL IL INHBA NGF PGF SFRP SFRP SFRP SFRP TDGF TGFB TGFB TSHB VEGFA VEGFB WIF WNT WNT10A WNT10B WNT WNT WNT WNT2B WNT WNT3A
159 Table 5-1. Continued Normalized number of transcripts Gene day 0 day 0 day 0 day 0 WNT WNT5A WNT5B WNT WNT7A WNT7B WNT8A WNT8B WNT9A WNT9B
160 Table 5-2. Genes whose expression in the oviduct ipsilateral to the side of ovulation was affected by day of the estrous cycle within the first 7 days after ovulation a Normalized number of transcripts P-value Gene day 0 day 3 day 5 day 7 linear quadratic cubic BMP7 148 ± ± ± ± CCL ± ± ± ± CTGF 3123 ± ± ± ± CXCL ± ± ± ± CXCL ± ± ± ± DKK3 244 ± ± ± ± FGF ± ± ± ± HGF 160 ± ± ± ± IK 845 ± ± ± ± IL ± ± ± ± IL ± ± ± ± IL ± ± ± ± IL6 32 ± ± ± ± PGF 61 ± ± ± ± SFRP1 528 ± ± ± ± SFRP2 991 ± ± ± ± TGFB1 132 ± ± ± ± WIF1 370 ± ± ± ± WNT2 28 ± ± ± ± WNT4 61 ± ± ± ± WNT5A 916 ± ± ± ± a Data are least-squares means ± SEM. -) indicates P >
161 Table 5-3. Genes whose expression in the oviduct was differentially expressed between sides ipsilateral and contralateral to the side of ovulation within the first three days after ovulation a Normalized number of transcripts Gene Ipsilateral Contralateral P-value DKK2 96 ± ± IL10 46 ± ± IL13 46 ± ± TGFB3 137 ± ± WNT4 69 ± ± a Data are least-squares means ± SEM. 161
162 Table 5-4. Genes differentially expressed in oviduct ipsi and contralateral to the side of ovulation that vary during the first 3 days after ovulation a Normalized number of transcripts day 0 day 3 Gene Ipsilateral Contralateral Ipsilateral Contralateral P-value BMP4 191 ± ± ± ± CXCL ± ± ± ± CXCL ± ± ± ± FGF ± ± ± ± GRO1 202 ± ± ± ± HDGFRP2 773 ± ± ± ± IL ± ± ± ± SFRP2 991 ± ± ± ± VEGFB 473 ± ± ± ± a Data are least-squares means ± SEM for effect of day by side interaction. 162
163 Table 5-5. Least-squares means for expression of 93 genes in endometrium during the first seven days of the estrous cycle averaged from both sides of the reproductive tract. Gene Normalized number of transcripts day 0 day 3 day 5 day 7 AMH ANPEP BMP BMP BMP BMP CCL CCL CCL CCL CSF CTGF CX3CL CXCL CXCL CXCL CXCL DKK DKK DKK DKK EGF FGF FGF FGF FGF FGF FGF FGF FIGF GDF GRO HDGF HDGFRP HDGFRP HGF IFNB IGF IGF IK IL
164 Table 5-5. Continued Gene Normalized number of transcripts day 0 day 3 day 5 day 7 IL12A IL12B IL IL IL IL17A IL IL1A IL1B IL IL IL IL IL IL IL IL IL IL INHBA NGF PGF SFRP SFRP SFRP SFRP TDGF TGFB TGFB TSHB VEGFA VEGFB WIF WNT WNT10A WNT10B WNT WNT WNT WNT2B WNT WNT3A WNT
165 Table 5-5. Continued Gene Normalized number of transcripts day 0 day 3 day 5 day 7 WNT5A WNT5B WNT WNT7A WNT7B WNT8A WNT8B WNT9A WNT9B
166 Table 5-6. Genes whose expression in the endometrium was affected by day of the estrous cycle within the first 7 days after ovulation a Normalized number of transcripts P-values Gene day 0 day 3 day 5 day 7 linear quadratic cubic BMP7 184 ± ± ± ± CCL ± ± ± ± CCL21 78 ± ± ± ± CCL26 40 ± ± ± ± CSF2 43 ± ± ± ± CTGF 3310± ± ± ± CX3CL1 98 ± ± ± ± CXCL3 951 ± ± ± ± CXCL ± ± ± ± CXCL ± ± ± ± 28 < FGF1 33 ± ± ± ± FGF2 186 ± ± ± ± FGF13 77 ± 8 41 ± 8 30 ± ± < GRO1 474 ± ± ± ± HDGF 825 ± ± ± ± HDGFRP2 452 ± ± ± ± HGF 137 ± ± ± ± IGF1 391 ± ± ± ± IGF2 1015± ± ± ± IL1B 33 ± ± ± ± IL8 47 ± ± ± ± IL15 53 ± ± ± ± IL16 60 ± 8 48 ± 8 57 ± 8 35 ± IL ± ± ± ± SFRP1 513 ± ± ± ± SFRP2 195 ± ± ± ± SFRP4 47 ± ± ± ± TDGF1 159 ± ± ± ±980 <.0001 < VEGFB 442 ± ± ± ± 71 <.0001 < WIF1 832 ± ± ± ± WNT5A 1625± ± ± ± WNT7A 36 ± ± ± ± 22 < WNT11 81 ± ± ± ± WNT ± ± ± ± a Data are least squares means ± SEM. -) indicate P >
167 Table 5-7. Genes whose expression was affected by the interaction between day of the estrous cycle and side of the reproductive tract relative to ovulation a Gene Normalized number of transcripts day 0 day 3 day 5 day 7 P-value Ipsilateral Contralateral Ipsilateral Contralateral Ipsilateral Contralateral Ipsilateral Contralateral 222 ± 291 ± 814 ± 202 ± DKK ± ± ± ± IL16 33 ± ± ± ± ± ± ± ± IL34 18 ± ± ± ± ± ± ± ± NGF 16 ± ± ± ± ± ± ± ± WNT2 51 ± ± ± ± ± ± ± ± WNT5B 13 ± ± ± ± ± ± ± ± a Data are least-squares means ± SEM 167
168 Figure 5-1. Expression of the top 50 expressed genes in the oviduct at days 0, 3, 5 and 7 after ovulation. Data are least-squares means. 168
169 Figure 5-2. Expression of the top 50 expressed genes in the endometrium at days 0, 3, 5 and 7 after ovulation. Data are least-squares means. 169
170 Figure 5-3. Immunolocalization of CSF2, DKK1 and WNT5A in endometrium. DNA was labeled with Hoescht (blue). A) Representative images showing CSF2 (green) localized to luminal epithelium (LE), glandular epithelium (GE) and stroma. B) Representative images showing DKK1 (green) localized to stroma. C) Representative images showing WNT5A (red) localized to LE, GE and stroma. 170
171 Figure 5-4. Immunolocalization of WNT7A in endometrium. Representative images showing WNT7A (green) localized to luminal epithelium (LE), glandular epithelium (GE) and stroma. DNA was labeled with Hoescht (blue). Scale bar 100 µm. 171
172 Figure 5-5. Detection of CSF2 in uterine fluid by Western blotting. Lanes samples of uterine flushings from individual cows at day 0 (n=4), day 3 (n=4), day 5 (n=3) and day 7 (n=4) after ovulation. The location of molecular weight standards at 25 and 20 kda is shown to the left of the blot while the migration distance of two bands in recombinant bovine CSF2 are shown by the arrows to the right of the blot. 172
Biology 4361 Developmental Biology Exam 1 ID#: October 11, 2005
 Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Totipotency and lineage segregation in the human embryo
 Molecular Human Reproduction, Vol.20, No.7 pp. 599 618, 2014 Advanced Access publication on April 3, 2014 doi:10.1093/molehr/gau027 REVIEW Totipotency and lineage segregation in the human embryo C. De
Molecular Human Reproduction, Vol.20, No.7 pp. 599 618, 2014 Advanced Access publication on April 3, 2014 doi:10.1093/molehr/gau027 REVIEW Totipotency and lineage segregation in the human embryo C. De
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
DEVELOPMENTAL PROGRAMMING OF THE PREIMPLANTATION BOVINE EMBRYO BY COLONY STIMULATING FACTOR 2
 DEVELOPMENTAL PROGRAMMING OF THE PREIMPLANTATION BOVINE EMBRYO BY COLONY STIMULATING FACTOR 2 By KYLE BRADLEY DOBBS A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL
DEVELOPMENTAL PROGRAMMING OF THE PREIMPLANTATION BOVINE EMBRYO BY COLONY STIMULATING FACTOR 2 By KYLE BRADLEY DOBBS A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL
Research programs involving human early embryos
 Research programs involving human early embryos 1. Understanding normal mammalian embryo development 2. Understanding errors in genetic and epigenetic programs 3. Providing research and therapeutic tools
Research programs involving human early embryos 1. Understanding normal mammalian embryo development 2. Understanding errors in genetic and epigenetic programs 3. Providing research and therapeutic tools
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Preimplantation genetic diagnosis: polar body and embryo biopsy
 Human Reproduction, Vol. 15, (Suppl. 4), pp. 69-75, 2000 Preimplantation genetic diagnosis: polar body and embryo biopsy Luca Gianaroli SISMER, Via Mazzini 12, 40138 Bologna, Italy Scientific Director
Human Reproduction, Vol. 15, (Suppl. 4), pp. 69-75, 2000 Preimplantation genetic diagnosis: polar body and embryo biopsy Luca Gianaroli SISMER, Via Mazzini 12, 40138 Bologna, Italy Scientific Director
Strategic delivery: Setting standards Increasing and. Details: Output: Demonstrating efficiency. informing choice.
 Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Single-cell RNA-Seq profiling of human pre-implantation embryos and embryonic stem cells
 Single-cell RNA-Seq profiling of human pre-implantation embryos and embryonic stem cells Liying Yan,2,5, Mingyu Yang,5, Hongshan Guo, Lu Yang, Jun Wu, Rong Li,2, Ping Liu, Ying Lian, Xiaoying Zheng, Jie
Single-cell RNA-Seq profiling of human pre-implantation embryos and embryonic stem cells Liying Yan,2,5, Mingyu Yang,5, Hongshan Guo, Lu Yang, Jun Wu, Rong Li,2, Ping Liu, Ying Lian, Xiaoying Zheng, Jie
Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Chapter 18 Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development and is responsible for differences
Chapter 18 Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development and is responsible for differences
C. elegans Embryonic Development
 Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin 8 Oocyte and cu
 Models for study of oocyte competence: George W. Smith (Smithge7@msu.edu) Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin
Models for study of oocyte competence: George W. Smith (Smithge7@msu.edu) Foundational questions Oocyte-derived functional mediators of early embryonic development (EST and candidate gene) JY-1 Nobox Importin
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
REPRODUCTIVE BIOTECHNOLOGY IN SWINE
 REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade)
 HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
The Biology and Genetics of Cells and Organisms The Biology of Cancer
 The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
Nucleic acids. Nucleic acids are information-rich polymers of nucleotides
 Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
The Influence of Culture Medium on Embryonic Viability. Klaus E. Wiemer PhD Director of Embryology and Reproductive Sciences
 The Influence of Culture Medium on Embryonic Viability Klaus E. Wiemer PhD Director of Embryology and Reproductive Sciences Developmental Difference In Vivo vs. In Vitro Developmental Environment Attempts
The Influence of Culture Medium on Embryonic Viability Klaus E. Wiemer PhD Director of Embryology and Reproductive Sciences Developmental Difference In Vivo vs. In Vitro Developmental Environment Attempts
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
5/12/2015. Cell Size. Relative Rate of Reaction
 Cell Makeup Chapter 4 The Cell: The Fundamental Unit of Life We previously talked about the cell membrane The cytoplasm is everything inside the membrane, except the nucleus Includes Cytosol = liquid portion
Cell Makeup Chapter 4 The Cell: The Fundamental Unit of Life We previously talked about the cell membrane The cytoplasm is everything inside the membrane, except the nucleus Includes Cytosol = liquid portion
Fertilization depends on mechanisms that help sperm meet eggs of the same species.
 Fertilization depends on mechanisms that help sperm meet eggs of the same species. www.uchsc.edu/ltc/fertilization.html Fertilization union of sperm and egg Is a chain of events. Interruption of any step
Fertilization depends on mechanisms that help sperm meet eggs of the same species. www.uchsc.edu/ltc/fertilization.html Fertilization union of sperm and egg Is a chain of events. Interruption of any step
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Gene Regulation Part 2
 Michael Cummings Chapter 9 Gene Regulation Part 2 David Reisman University of South Carolina Other topics in Chp 9 Part 2 Protein folding diseases Most diseases are caused by mutations in the DNA that
Michael Cummings Chapter 9 Gene Regulation Part 2 David Reisman University of South Carolina Other topics in Chp 9 Part 2 Protein folding diseases Most diseases are caused by mutations in the DNA that
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
(a) TEM of a plasma. Fimbriae. Nucleoid. Ribosomes. Plasma membrane. Cell wall Capsule. Bacterial chromosome
 0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
basic unit structure and function
 Chapter 3 Cells Introduction The cell is the basic unit of structure and function in living things. Cells vary in their shape, size, and arrangements, but all cells have similar components with a particular
Chapter 3 Cells Introduction The cell is the basic unit of structure and function in living things. Cells vary in their shape, size, and arrangements, but all cells have similar components with a particular
Allelic reprogramming of the histone modification H3K4me3 in early mammalian development
 Allelic reprogramming of the histone modification H3K4me3 in early mammalian development 张戈 Method and material STAR ChIP seq (small-scale TELP-assisted rapid ChIP seq) 200 mouse embryonic stem cells PWK/PhJ
Allelic reprogramming of the histone modification H3K4me3 in early mammalian development 张戈 Method and material STAR ChIP seq (small-scale TELP-assisted rapid ChIP seq) 200 mouse embryonic stem cells PWK/PhJ
The Animal Cell. English. Introduction
 R04 (1000523) English The Animal Cell Introduction Cells in animal multicellular organisms principally only occur in groups of similar cells or together with other differentiated cells, or embedded in
R04 (1000523) English The Animal Cell Introduction Cells in animal multicellular organisms principally only occur in groups of similar cells or together with other differentiated cells, or embedded in
Effect of addition of exogenous growth factor on in vitro development of preimplantation stage buffalo embryos
 Effect of addition of exogenous growth factor on in vitro development of preimplantation stage buffalo embryos CONTENTS 5. EFFECT OF ADDITION OF EXOGENOUS GROWTH FACTOR ON IN VITRO DEVELOPMENT OF PREIMPLANTATION
Effect of addition of exogenous growth factor on in vitro development of preimplantation stage buffalo embryos CONTENTS 5. EFFECT OF ADDITION OF EXOGENOUS GROWTH FACTOR ON IN VITRO DEVELOPMENT OF PREIMPLANTATION
Human height. Length of some nerve and muscle cells. Chicken egg. Frog egg. Most plant and animal cells Nucleus Most bacteria Mitochondrion
 10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
Name: Xueming Zhao. Professional Title: Professor. Animal embryo biotechnology, mainly including in vitro maturation (IVM), in vitro fertilization
 Name: Xueming Zhao Professional Title: Professor Telephone:86-010-62815892 Fax:86-010-62895971 E-mail: zhaoxueming@caas.cn Website: http://www.iascaas.net.cn/yjspy/dsjj/sssds/dwyzyzypz1/62040.htm Research
Name: Xueming Zhao Professional Title: Professor Telephone:86-010-62815892 Fax:86-010-62895971 E-mail: zhaoxueming@caas.cn Website: http://www.iascaas.net.cn/yjspy/dsjj/sssds/dwyzyzypz1/62040.htm Research
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Fragile X Syndrome. Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype
 Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Src-INACTIVE / Src-INACTIVE
 Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
7.012 Problem Set 6 Solutions
 Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
CELL PARTS TYPICAL ANIMAL CELL
 AP BIOLOGY CText Reference, Campbell v.8, Chapter 6 ACTIVITY1.12 NAME DATE HOUR CELL PARTS TYPICAL ANIMAL CELL ENDOMEMBRANE SYSTEM TYPICAL PLANT CELL QUESTIONS: 1. Write the name of the cell part in the
AP BIOLOGY CText Reference, Campbell v.8, Chapter 6 ACTIVITY1.12 NAME DATE HOUR CELL PARTS TYPICAL ANIMAL CELL ENDOMEMBRANE SYSTEM TYPICAL PLANT CELL QUESTIONS: 1. Write the name of the cell part in the
Chapter 3 Part 2! Pages (10 th and 11 th eds.)! The Cellular Level of Organization! Cellular Organelles and Protein Synthesis!
 Chapter 3 Part 2! Pages 65 89 (10 th and 11 th eds.)! The Cellular Level of Organization! Cellular Organelles and Protein Synthesis! The Cell Theory! Living organisms are composed of one or more cells.!
Chapter 3 Part 2! Pages 65 89 (10 th and 11 th eds.)! The Cellular Level of Organization! Cellular Organelles and Protein Synthesis! The Cell Theory! Living organisms are composed of one or more cells.!
Cell cycle and apoptosis
 Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Chapter 11 How Genes Are Controlled
 Chapter 11 How Genes Are Controlled PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Mary
Chapter 11 How Genes Are Controlled PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Mary
Understanding eggs, sperm and embryos. Marta Jansa Perez Wolfson Fertility Centre
 Understanding eggs, sperm and embryos Marta Jansa Perez Wolfson Fertility Centre What does embryology involve? Aims of the embryology laboratory Creation of a large number of embryos and supporting their
Understanding eggs, sperm and embryos Marta Jansa Perez Wolfson Fertility Centre What does embryology involve? Aims of the embryology laboratory Creation of a large number of embryos and supporting their
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
A. Major parts 1. Nucleus 2. Cytoplasm a. Contain organelles (see below) 3. Plasma membrane (To be discussed in Cellular Transport Lecture)
 Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
The intra-follicular molecular biology mandating advancement of egg retrieval in some women
 The intra-follicular molecular biology mandating advancement of egg retrieval in some women David H. Barad, USA Director of Assisted Reproductive Technology, The Center for Human Reproduction New York
The intra-follicular molecular biology mandating advancement of egg retrieval in some women David H. Barad, USA Director of Assisted Reproductive Technology, The Center for Human Reproduction New York
Don t Freak Out. Test on cell organelle on Friday!
 Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
Cell Structure 1 Don t Freak Out Test on cell organelle on Friday! This test should be a buffer test and help raise your overall test score. All information will come from this week! 2 Cells Provide Compartments
I) Development: tissue differentiation and timing II) Whole Chromosome Regulation
 Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
Dissecting gene regulation network in human early embryos. at single-cell and single-base resolution
 Dissecting gene regulation network in human early embryos at single-cell and single-base resolution Fuchou Tang BIOPIC, College of Life Sciences Peking University 07/10/2015 Cockburn and Rossant, 2010
Dissecting gene regulation network in human early embryos at single-cell and single-base resolution Fuchou Tang BIOPIC, College of Life Sciences Peking University 07/10/2015 Cockburn and Rossant, 2010
Bi-potent Gonads. Sex Determination
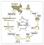 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
The Cell Cycle M G2 G1 G0 S 1
 The Cell Cycle M G2 G1 G0 S 1 Cell Cycle G1 (Gap 1) 3 to 12 hours in length Respond to cues from the environment External cues Growth factors that signal the cell to stay in G1 or continue to through the
The Cell Cycle M G2 G1 G0 S 1 Cell Cycle G1 (Gap 1) 3 to 12 hours in length Respond to cues from the environment External cues Growth factors that signal the cell to stay in G1 or continue to through the
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
ORGANELLES OF THE ENDOMEMBRANE SYSTEM
 Membranes compartmentalize the interior of the cell and facilitate a variety of metabolic activities. Chloroplasts and a rigid cell wall are what distinguish a plant cell from an animal cell. A typical
Membranes compartmentalize the interior of the cell and facilitate a variety of metabolic activities. Chloroplasts and a rigid cell wall are what distinguish a plant cell from an animal cell. A typical
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
REGULATION OF GROWTH AND THERMOPROTECTION OF THE BOVINE PREIMPLANTATION EMBRYO BY INSULIN-LIKE GROWTH FACTOR-1
 REGULATION OF GROWTH AND THERMOPROTECTION OF THE BOVINE PREIMPLANTATION EMBRYO BY INSULIN-LIKE GROWTH FACTOR-1 By ALINE QUADROS SANTOS BONILLA A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY
REGULATION OF GROWTH AND THERMOPROTECTION OF THE BOVINE PREIMPLANTATION EMBRYO BY INSULIN-LIKE GROWTH FACTOR-1 By ALINE QUADROS SANTOS BONILLA A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY
Cellular compartments
 Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
BIO 5099: Molecular Biology for Computer Scientists (et al)
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
Animal Science 434! Tonic and Preovulatory Surge of GnRH! Tonic and Preovulatory Surge of GnRH! Lecture 11: The Follicular Phase of the Estrous Cycle!
 Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
Derived copy of Fertilization *
 OpenStax-CNX module: m56433 1 Derived copy of Fertilization * Stephanie Fretham Based on Fertilization by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m56433 1 Derived copy of Fertilization * Stephanie Fretham Based on Fertilization by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
M.Sc. (Reproductive Biology & Clinical Embryology)
 M.Sc. (Reproductive Biology & Clinical Embryology) ACADEMIC SCHEDULE Theory (Didactic) Lectures + Seminars + Journal Clubs Semester I & II (2 hours / day) Practicals (Hands-on + Demo) Semester I & II (first
M.Sc. (Reproductive Biology & Clinical Embryology) ACADEMIC SCHEDULE Theory (Didactic) Lectures + Seminars + Journal Clubs Semester I & II (2 hours / day) Practicals (Hands-on + Demo) Semester I & II (first
AP Biology Cells: Chapters 4 & 5
 AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System 26.1 Multiple-Choice Questions 1) Hormones are chemicals produced by the endocrine system that
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System 26.1 Multiple-Choice Questions 1) Hormones are chemicals produced by the endocrine system that
Early scientists who observed cells made detailed sketches of what they saw.
 Early scientists who observed cells made detailed sketches of what they saw. Early scientists who observed cells made detailed sketches of what they saw. CORK Early scientists who observed cells made detailed
Early scientists who observed cells made detailed sketches of what they saw. Early scientists who observed cells made detailed sketches of what they saw. CORK Early scientists who observed cells made detailed
Chapter 3: Cells. I. Overview
 Chapter 3: Cells I. Overview A. Characteristics 1. Basic structural/functional unit 2. Diameter is too small to see by the naked eye 3. Can be over 3 feet long 4. Trillions of cells in over 200 basic types
Chapter 3: Cells I. Overview A. Characteristics 1. Basic structural/functional unit 2. Diameter is too small to see by the naked eye 3. Can be over 3 feet long 4. Trillions of cells in over 200 basic types
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature19360 Supplementary Tables Supplementary Table 1. Number of monoclonal reads in each sample Sample Number of cells Total reads Aligned reads Monoclonal reads
SUPPLEMENTARY INFORMATION doi:10.1038/nature19360 Supplementary Tables Supplementary Table 1. Number of monoclonal reads in each sample Sample Number of cells Total reads Aligned reads Monoclonal reads
New Assisted Reproductive Techniques for Horses. Dirk K. Vanderwall, DVM, PhD, Dipl. ACT
 New Assisted Reproductive Techniques for Horses Dirk K. Vanderwall, DVM, PhD, Dipl. ACT Northwest Equine Reproduction Laboratory Department of Animal and Veterinary Science Center for Reproductive Biology
New Assisted Reproductive Techniques for Horses Dirk K. Vanderwall, DVM, PhD, Dipl. ACT Northwest Equine Reproduction Laboratory Department of Animal and Veterinary Science Center for Reproductive Biology
DEVELOPMENTS in reproduction continue
 Important Reproductive Technologies DEVELOPMENTS in reproduction continue to advance. Like humans, animals struggle with reproduction from time to time. Challenges facing the animal industry include the
Important Reproductive Technologies DEVELOPMENTS in reproduction continue to advance. Like humans, animals struggle with reproduction from time to time. Challenges facing the animal industry include the
Hold On To Your Dreams
 Hold On To Your Dreams Dr. Michael Kettel Dr. Sandy Chuan 1. THE BASICS OF IVF & EMBRYO DEVELOPMENT 2. IVF ADD-ONS - MYTH VS. SCIENCE IN VITRO FERTILIZATION 1. Ovarian Stimulation 2. Egg Retrieval 3. Create
Hold On To Your Dreams Dr. Michael Kettel Dr. Sandy Chuan 1. THE BASICS OF IVF & EMBRYO DEVELOPMENT 2. IVF ADD-ONS - MYTH VS. SCIENCE IN VITRO FERTILIZATION 1. Ovarian Stimulation 2. Egg Retrieval 3. Create
Chapter 36 Active Reading Guide Reproduction and Development
 Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
Regulators of Cell Cycle Progression
 Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
General Biology 1004 Chapter 11 Lecture Handout, Summer 2005 Dr. Frisby
 Slide 1 CHAPTER 11 Gene Regulation PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Neil Campbell, Jane Reece,
Slide 1 CHAPTER 11 Gene Regulation PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Neil Campbell, Jane Reece,
STEM CELL RESEARCH: MEDICAL PROGRESS WITH RESPONSIBILITY
 STEM CELL RESEARCH: MEDICAL PROGRESS WITH RESPONSIBILITY A REPORT FROM THE CHIEF MEDICAL OFFICER S EXPERT GROUP REVIEWING THE POTENTIAL OF DEVELOPMENTS IN STEM CELL RESEARCH AND CELL NUCLEAR REPLACEMENT
STEM CELL RESEARCH: MEDICAL PROGRESS WITH RESPONSIBILITY A REPORT FROM THE CHIEF MEDICAL OFFICER S EXPERT GROUP REVIEWING THE POTENTIAL OF DEVELOPMENTS IN STEM CELL RESEARCH AND CELL NUCLEAR REPLACEMENT
6 functions of membrane proteins integral & peripheral proteins Membrane Junctions
 Cells Cells are the structural units of all living organisms ranging from unicellular to multicellular organisms. Biochemical activities of cells are dictated by cell shape and specific subcellular structures.
Cells Cells are the structural units of all living organisms ranging from unicellular to multicellular organisms. Biochemical activities of cells are dictated by cell shape and specific subcellular structures.
Gene Regulation. Bacteria. Chapter 18: Regulation of Gene Expression
 Chapter 18: Regulation of Gene Expression A Biology 2013 1 Gene Regulation rokaryotes and eukaryotes alter their gene expression in response to their changing environment In multicellular eukaryotes, gene
Chapter 18: Regulation of Gene Expression A Biology 2013 1 Gene Regulation rokaryotes and eukaryotes alter their gene expression in response to their changing environment In multicellular eukaryotes, gene
Distributions of Mitochondria and the Cytoskeleton in Hamster Embryos Developed In Vivo and In Vitro
 J. Mamm. Ova Res. Vol. 23, 128 134, 2006 128 Original Distributions of Mitochondria and the Cytoskeleton in Hamster Embryos Developed In Vivo and In Vitro Hiroyuki Suzuki 1 *, Manabu Satoh 1 ** and Katsuya
J. Mamm. Ova Res. Vol. 23, 128 134, 2006 128 Original Distributions of Mitochondria and the Cytoskeleton in Hamster Embryos Developed In Vivo and In Vitro Hiroyuki Suzuki 1 *, Manabu Satoh 1 ** and Katsuya
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Cell Cycle Notes chromatin, somatic cells gametes mitosis sister chromatids, centromere cytokinesis binary fission,
 Cell Cycle Notes 1. Importance of Cell Division a. For single celled organisms, cell division increases the number of individuals. b. In a multicellular organism, cell division functions to repair and
Cell Cycle Notes 1. Importance of Cell Division a. For single celled organisms, cell division increases the number of individuals. b. In a multicellular organism, cell division functions to repair and
Keywords: Daughter Cells Asexual Reproduction Sexual Reproduction Chromosomes Chromatin Homologous Chromosomes Diploid
 Name: CP Biology Unit 6: Cell Growth and Development Students will be able to: 6.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
Name: CP Biology Unit 6: Cell Growth and Development Students will be able to: 6.1 Understand and explain the different aspects of the eukaryotic cell cycle. Explain how cell size is related to cell division
'''''''''''''''''Fundamental'Biology' BI'1101' ' an'interdisciplinary'approach'to'introductory'biology' Five'Levels'of'Organiza-on' Molecular'
 '''''''''''''''''Fundamental'Biology' BI'1101' ' an'interdisciplinary'approach'to'introductory'biology' Anggraini'Barlian,' Iriawa-' Tjandra'Anggraeni' SITH4ITB' Five'Levels'of'Organiza-on' Molecular'
'''''''''''''''''Fundamental'Biology' BI'1101' ' an'interdisciplinary'approach'to'introductory'biology' Anggraini'Barlian,' Iriawa-' Tjandra'Anggraeni' SITH4ITB' Five'Levels'of'Organiza-on' Molecular'
Ovarian stimulation and inflammation, not always friends! Marc-André Sirard DVM PhD, Canadian Research Chair in Reproduction Genomics
 Ovarian stimulation and inflammation, not always friends! Marc-André Sirard DVM PhD, Canadian Research Chair in Reproduction Genomics Conflict of interest Controlled Ovarian Stimulation Timing Test (COST2)GFI
Ovarian stimulation and inflammation, not always friends! Marc-André Sirard DVM PhD, Canadian Research Chair in Reproduction Genomics Conflict of interest Controlled Ovarian Stimulation Timing Test (COST2)GFI
Cancer. October is National Breast Cancer Awareness Month
 Cancer October is National Breast Cancer Awareness Month Objectives 1: Gene regulation Explain how cells in all the different parts of your body develop such different characteristics and functions. Contrast
Cancer October is National Breast Cancer Awareness Month Objectives 1: Gene regulation Explain how cells in all the different parts of your body develop such different characteristics and functions. Contrast
AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is
 AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is continuous v Small cell size is becoming more necessary as
AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is continuous v Small cell size is becoming more necessary as
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Structures in Cells. Cytoplasm. Lecture 5, EH1008: Biology for Public Health, Biomolecules
 Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
Propagation of the Signal
 OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
Realizing dreams booklet.indd 1 5/20/ :26:52 AM
 Realizing dreams. 18891booklet.indd 1 5/20/2010 11:26:52 AM The Journey To Parenthood The first Gator Baby was born in 1988 through the in vitro fertilization program at the University of Florida. Since
Realizing dreams. 18891booklet.indd 1 5/20/2010 11:26:52 AM The Journey To Parenthood The first Gator Baby was born in 1988 through the in vitro fertilization program at the University of Florida. Since
MicroRNA and Male Infertility: A Potential for Diagnosis
 Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
5.1. KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. 68 Reinforcement Unit 2 Resource Book
 5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
5.1 THE CELL CYCLE KEY CONCEPT Cells have distinct phases of growth, reproduction, and normal functions. Cells have a regular pattern of growth, DNA duplication, and division that is called the cell cycle.
RNA Processing in Eukaryotes *
 OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
Cell Structure and Function. Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages and 68-69
 Cell Structure and Function Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages 45 59 and 68-69 Assignments for this Unit Pick up the notes/worksheet for this unit and the project There
Cell Structure and Function Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages 45 59 and 68-69 Assignments for this Unit Pick up the notes/worksheet for this unit and the project There
