Group 2 and Reactivity of metals. Group 2. Identification in aqueous solution.
|
|
|
- Phoebe Hart
- 5 years ago
- Views:
Transcription
1 1 2 Group 2 and H He Li Be B C N O F Ne -1.85V Na Mg Al Si P S Cl Ar V K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn ** Fr Ra Ac * Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ** Th Pa U [Ng](ns) 2 [Ng](n-1 d) 10 (ns) 2 Hg: [Xe](4f) 14 (5d) 10 (6s) 2 reactive metals - M s M aq + 2e - - less or poorly reactive hard lewis acids soft lewis acids electrostatic forces covalent O,OH -,CO 3,COO - NH 3 phenolate, RS -, ArN 21/04/2017 IC 11 Group 2 JJ 1 Reactivity of metals oxides heated give metal + O2 oxides are reduced to metals by hydrogen oxides are not reduced to metals by hydrogen Au Pt Pd Ag Hg Cu Bi As Sb Pb Sn Ni Co Cd Fe Cr Zn Mn Al Mg Na Ca Sr Ba K decreasing redox potential > form oxides only indirectly form hydrogen with cold water form hydrogen with hot water or water vapor (at high pressure) form oxides with oxygen form hydrogen with acids 21/04/2017 IC 11 Group 2 JJ 2 Group 2 Identification in aqueous solution textbook fig 12.6 Be : OH - blue solution Mg : CO 3 OH - blue precipitate Ca : CO 3 to C 2 O 4 white ppt Sr : SO 4 to CO 3 to SO 4 white ppt Ba : SO 4 to CO 3 to CrO 4 yellow ppt 21/04/2017 IC 11 Group 2 JJ 3 1
2 Some properties Be Mg Ca Sr Ba Occurence % 6x Ionisation energy / kjxmol ~ Hydration enthalpy Eo/V Ionic radius (r/å) Ionic potential (q/r) Ionic mobility Melting point/ 0 C Atomic radius /Å plm(oh) pl MCO pl MC2O pl MSO4 ~ logk MF logk MEDTA logk Mgly logk MP2O logk MATP /04/2017 IC 11 Group 2 JJ 4 Systematics? Why opposite ionic mobilities and ionic radii? Solubilities of sulfates and hydroxides? Be Mg Ca Sr Ba Ionic mobility Ionic radius /Å plm(oh) pl MSO4 ~ /04/2017 IC 11 Group 2 JJ 5 M Atom Me r (Å) E 0 (V) E 0 (n + /m + ) pkaq logkedta log en3 colour(maqn ) Be He Mg Ne Ca Ar Sr Kr Ba Xe Zn Ar 3d Cd Kr 4d Hg Xe 4f 14 5d /1: Ge Zn 4s Sn Cd 5s /2:.15 Pb Hg 6s /2: Ti Ar 3d /2: -.37 V Ar 3d /2: violet Cr Ar 3d /2: blue Mn Ar 3d /2: light red Fe Ar 3d /2: light green Co Ar 3d /2: red Ni Ar 3d green Cu Ar 3d /1: light blue Pd Kr 4d Pt Xe 4f 14 5d ? Eu Xe 4f /2: -.36 Yb Xe 4f /2: /04/2017 IC 11 Group 2 JJ 6 2
3 Calcium carbonate Calcite (low temperature) Aragonite (high temperature) 21/04/2017 IC 11 Group 2 JJ 7 Calcite Same structure MCO 3 M a/å α Ca o 55 Mg o 58 Fe o 05 Mn o 50 Zn o 27 21/04/2017 IC 11 Group 2 JJ 8 Grignard reagent Formation of C-C bonds using carbanions: R-Cl + Mg R-MgCl R R R-MgCl + C=O R-C-O - -MgCl + R R Also R-Cl + Zn R-ZnCl R 2 Zn 21/04/2017 IC 11 Group 2 JJ 9 3
4 Mg and Ca in life Hydrolytic enzymes Chlorophylls In bones Hydrolysis of polyphosphates : Lewis acid polarisation of bonds O - O P O Mg O - O P O O Calcium pectate (cell wall) In bones, teeth, shells Triggers Striated muscle contraction polyphosphates- carboxylates calmodulin 21/04/2017 IC 11 Group 2 JJ 10 Chlorophylls R =CH 3 Chl. A R =CHO Chl. B R =C 20 H 39 Green colour: 21/04/2017 IC 11 Group 2 JJ 11 End of Chapter problems 22 M + NH 3,l A A s B + C g (slow) M flame: crimson (violet through blue glass) Sr(NH 3 ) 6 Sr(NH 2 ) 2 + H 2 + 4NH 3 (12.9) XCO 3 ; X + H 2 O (0 o C) D (strong base*) D: X + 2OH - + CO 2 (qual. test) X + H 2 XH 2 drying agent (drying agents : MgH 2 CaH 2 ) X = Ba-Ca; X + 2OH - + CO 2 XCO 3 21/04/2017 IC 11 Group 2 JJ 12 4
5 End of Chapter problems 24 CaCl 2 A Polymeric in the solid state 1 BeO B Soda lime 2 Be(OH) 2 C Strong oxidising agent 3 CaO D Qualitative analysis: sulphate test 4 CaF 2 E Hygroscopic used for de-icing 5 BaCl 2 F Amphoteric 6 BeCl 2 G Quicklime 7 MgO 2 H Crystallises with Würtzite structure 8 Ca(OH) 2 /NaOH I A prototype crystal structure 9 21/04/2017 IC 11 Group 2 JJ 13 Kalk - Lime Trivial navne (names) Kalk (limestone) Kridt (chalk) marmor, (marble) CaCO 3 brændt kalk (lime, quick lime) CaO + CO 2 CaO + H 2 O Ca(OH) 2 (0.025M 0 o C, 0.01M 100 o C) Læsket kalk (slaked lime) 21/04/2017 IC 11 Group 2 JJ 14 Group 12 Zn Cd Hg r m /Å o C 1 atm HCP HCP l mp/ o C bp/ o C d/g cm resistivity* E o ΔH o ion r(m )/Å *Mg: /04/2017 IC 11 Group 2 JJ 15 5
6 Group 12 Sphalerite (zinkblende) Galena Cinnober ZnS PbS (NaCl-structure) HgS MS + 3/2O 2 MO + SO 2 ZnO + C Zn + CO (Cd) Zn + CO 2 ZnO + CO HgO (heat) Hg +½O 2 21/04/2017 IC 11 Group 2 JJ 16 Aqueous chemistry H 2 O Cl - OH - NH 3 S xs Zn Zn(H 2 O) 6 ZnCl 4 Zn(OH) 4 Zn(NH 3 ) 4 ZnS ZnS Cd Cd(H 2 O) 6 CdCl 4 Cd(OH) 2 Cd(NH 3 ) 6 CdS CdS Hg Hg(H 2 O) 2 HgCl 4 HgO Hg(NH 3 ) 2 HgS HgS 2 M(NH 3 ) 4 (T d ) + 2NH 3 M(NH 3 ) 6 (O h ) L-Hg(II)-L 21/04/2017 IC 11 Group 2 JJ 17 Group 12 Identification Zn(OH) 4 ZnS white CdS Yellow HgS 2 HgS Black (cinnober) HgS + aqua regia HgCl 2 Hg + Cu Hg,Cu + Cu 21/04/2017 IC 11 Group 2 JJ 18 6
7 Hg 2 In solid (HgCl) n Hg-Hg shorter than in metal Low vibrational frequency 172 cm -1 (HgCl) n diamagnetic E-measurements not consistent with Hg + [Hg 2 ] rather than [Hg + ] 2 in equilibria 21/04/2017 IC 11 Group 2 JJ 19 M + Zn + Zn Zn 2 in fused salts Cd + Cd Cd 2 in fused salts Hg + Hg Hg 2 in aqueous solution K~160 Calomel Hg 2 Cl 2 insoluble Hg 2 (NO 3 ) 2 soluble 21/04/2017 IC 11 Group 2 JJ 20 Zn - acac - - CH 3 O=C CH 2 H + Zn(acac) 2 H 2 O O=C CH 3 [Zn(acac) 2 ] 3 21/04/2017 IC 11 Group 2 JJ 21 7
8 Some hydrolytic enzymes Enzyme Hydrolysis catalysed Metal ion Carboxypeptidase C-terminal peptide residues Zn Leucine aminopeptidase Leucine N-terminal peptide residue Zn Dipeptidase dipeptides Zn Neutral protease peptides Zn, Ca Collagenase collagen Zn Phospholipase C phospholipids Zn β-lactamase II β-lactam ring Zn Thermolysin peptides Zn, Ca Alkaline phophatase phosphate esters Zn Carbonic anhydrase carbondioxide Zn Α-amylase glucosides Zn, Ca Phopholipase A2 phospholipids Ca Inorg. pyrophosphatase pyrophosphate Mg ATPase ATP to ADP Mg 21/04/2017 IC 11 Group 2 JJ 22 Hydrolytic enzymes Carboxypeptidase Leucine aminopeptidase Dipeptidase Neutral protease Collagenase Phospholipase C β-lactamase II Thermolysin Alkaline phophatase Carbonic anhydrase α-amylase Phopholipase A 2 Inorg. Pyrophosphatase ATPase 21/04/2017 IC 11 Group 2 JJ 23 Carbonic anhydrase (H 2 O) 5 ZnOH 2 (H 2 O) 5 ZnOH + + H + pk~9.0 CAZnOH 2 CAZnOH + + H + pk~7.1 21/04/2017 IC 11 Group 2 JJ 24 8
9 Apo- and M- CA CAZn apo CA + Zn apo CA + M CAM CACd inactive in biological systems except in some Diatomers!, but active at higher ph pk 1 ~ /04/2017 IC 11 Group 2 JJ 25 9
Thin Film PV Technologies CIGS PV Technology
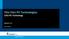 Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
GSJ Geochemical Reference Samples. Igneous Rock. Sedimentary Rock. For Instrumental analysis. Reference value for environmental analysis -1
 GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
Chelated & Essentials. For Your Pet Animals
 Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Chemical Equations Part 1
 Chemical Equations Part 1 A Directed Learning Activity for Hartnell College Chemistry 1 Funded by the Title V STEM Grant #P031S090007 through Hartnell College For information contact lyee@hartnell.edu
Chemical Equations Part 1 A Directed Learning Activity for Hartnell College Chemistry 1 Funded by the Title V STEM Grant #P031S090007 through Hartnell College For information contact lyee@hartnell.edu
NEW DEVELOPMENTS IN CATALYSIS
 You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
of human hair and nails. Part I. Analytical methodology, Sci. Tot. Environ. 2000, 250/1-3,
 Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
3a. The acid and base react to form a salt solution of ammonium propionate. CaCO s + 2 HC H O aq CO g + H O l + Ca ( aq ) + 2 C H O ( aq)
 Chapter 5 Answers Practice Examples 1a. 0.50 M Cl 1b. (a) 7.9 10-5 M F - ; (b).1 kg CaF a. (a) Al ( aq ) OH ( aq ) Al ( OH) ( s) (b) No reaction occurs. (c) Pb ( aq ) I ( aq ) PbI ( s) b. (a) Al ( aq )
Chapter 5 Answers Practice Examples 1a. 0.50 M Cl 1b. (a) 7.9 10-5 M F - ; (b).1 kg CaF a. (a) Al ( aq ) OH ( aq ) Al ( OH) ( s) (b) No reaction occurs. (c) Pb ( aq ) I ( aq ) PbI ( s) b. (a) Al ( aq )
AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES. Consumables and Supplies Reference Guide
 AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES INTRODUCTION AND OVERVIEW Capable of handling even the most difficult, high-matrix samples without dilution, the Avio 200 brings a whole new level of performance
AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES INTRODUCTION AND OVERVIEW Capable of handling even the most difficult, high-matrix samples without dilution, the Avio 200 brings a whole new level of performance
GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011
 GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011 Peter C. Webb 1 *, Michael Thompson 2, Philip J. Potts 1 and Stephen
GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011 Peter C. Webb 1 *, Michael Thompson 2, Philip J. Potts 1 and Stephen
Mike Hinds, Royal Canadian Mint
 Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
Matrix Reference Materials - SCP SCIENCE
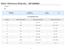 EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
WAGENINGEN EVALUATING PROGRAMS FOR ANALYTICAL LABORATORIES. Certificate of Analysis. International Soil-Analytical Exchange REFERENCE MATERIAL
 WAGENINGEN EVALUATING PROGRAMS FOR ANALYTICAL LABORATORIES Certificate of Analysis International Soil-Analytical Exchange REFERENCE MATERIAL ISE sample 946 $Dd General Information Certificate of Analysis
WAGENINGEN EVALUATING PROGRAMS FOR ANALYTICAL LABORATORIES Certificate of Analysis International Soil-Analytical Exchange REFERENCE MATERIAL ISE sample 946 $Dd General Information Certificate of Analysis
Brooks Rand Labs Detection & Reporting Limits Biota
 Biota %TS SM 2540G 0.30 1.00 % 0.020 0.100 mg/kg Al 0.14 0.80 mg/kg 0.11 0.30 mg/kg DRC 0.014 0.040 mg/kg (III) (Inorg) (Org) 0.05 0.16 mg/kg (Org) DRC 0.014 0.040 mg/kg () B 0.24 0.80 mg/kg Ba 0.03 0.16
Biota %TS SM 2540G 0.30 1.00 % 0.020 0.100 mg/kg Al 0.14 0.80 mg/kg 0.11 0.30 mg/kg DRC 0.014 0.040 mg/kg (III) (Inorg) (Org) 0.05 0.16 mg/kg (Org) DRC 0.014 0.040 mg/kg () B 0.24 0.80 mg/kg Ba 0.03 0.16
Plant Nutrients in Mineral Soils
 The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
QUALITATIVE TEST OF PROTEIN
 QUALITATIVE TEST F PRTEIN UTLINE Experment2 To detect the presence of peptide bonds or proteins in the sample Using Biuret Method Protein precipitation and denaturation Salt Strong Acid Heavy metals Heating
QUALITATIVE TEST F PRTEIN UTLINE Experment2 To detect the presence of peptide bonds or proteins in the sample Using Biuret Method Protein precipitation and denaturation Salt Strong Acid Heavy metals Heating
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS
 QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
Physicochemical Characterization of Airborne. Particulate Matter at a Mainline Underground
 Supporting information: Physicochemical Characterization of Airborne Particulate Matter at a Mainline Underground Railway Station Matthew Loxham 1,5 *, Matthew J. Cooper 2, Miriam E. Gerlofs-Nijland 3,
Supporting information: Physicochemical Characterization of Airborne Particulate Matter at a Mainline Underground Railway Station Matthew Loxham 1,5 *, Matthew J. Cooper 2, Miriam E. Gerlofs-Nijland 3,
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
Oxoacids of Phosphorus *
 OpenStax-CNX module: m35001 1 Oxoacids of Phosphorus * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Phosphorous pentoxide (P 2
OpenStax-CNX module: m35001 1 Oxoacids of Phosphorus * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Phosphorous pentoxide (P 2
People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
 Q1.Zinc forms many different salts including zinc sulfate, zinc chloride and zinc fluoride. (a) People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
Q1.Zinc forms many different salts including zinc sulfate, zinc chloride and zinc fluoride. (a) People who have a zinc deficiency can take hydrated zinc sulfate (ZnSO 4.xH 2O) as a dietary supplement.
CERTIFICATE TB SAMPLE PREPARATION ANALYTICAL PROCEDURES. Signature: Colin Ramshaw, Vancouver Laboratory Manager
 Page: 1 This copy reported on 6-FEB-2015 CERTIFICATE TB13103717 This report is for 31 Drill Core samples submitted to our lab in Thunder Bay, ON, Canada on 12-JUN-2013. The following have access to data
Page: 1 This copy reported on 6-FEB-2015 CERTIFICATE TB13103717 This report is for 31 Drill Core samples submitted to our lab in Thunder Bay, ON, Canada on 12-JUN-2013. The following have access to data
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
Qualitative test of protein-lab2
 1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
Soil Composition. Air
 Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
Soil Composition Air Soil Included Air Approximately 40 to 60% of the volume of a soil is actually empty space between the solid particles (voids). These voids are filled with air and/or water. The air
Corrosion Inhibitors. Background & Mechanisms. Applications & Formulating Strategies
 Corrosion Inhibitors for Waterborne Alkyd Technology 28 th Biennial Western Coatings Societies Symposium & Show Las Vegas, Nevada October 2007 Outline: Corrosion Inhibitors Background & Mechanisms Applications
Corrosion Inhibitors for Waterborne Alkyd Technology 28 th Biennial Western Coatings Societies Symposium & Show Las Vegas, Nevada October 2007 Outline: Corrosion Inhibitors Background & Mechanisms Applications
TREE ESSENTIAL ELEMENT. ZINC (Zn)
 Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Pub. No. 17 April 2016 TREE ESSENTIAL ELEMENT ZINC (Zn) By Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Zinc (Zn) is a
Classes at: - Topic: Redox & Volumetric Titration
 PHYSICAL CHEMISTRY by: SHAILENDRA KR. Classes at: - SCIENCE TUTRIALS; pp. Khuda Baksh Library, Ashok Rajpath, Patna PIN PINT STUDY CIRCLE; House No. 5A/65, pp. Mahual Kothi, Alpana Market, Patna Topic:
PHYSICAL CHEMISTRY by: SHAILENDRA KR. Classes at: - SCIENCE TUTRIALS; pp. Khuda Baksh Library, Ashok Rajpath, Patna PIN PINT STUDY CIRCLE; House No. 5A/65, pp. Mahual Kothi, Alpana Market, Patna Topic:
C 6 H 12 O 6 + 6O 2 6CO 2 + 6H 2 O
 Sample Questions for the Individual Round Exam 1. Glucose is the most basic sugar involved in human metabolism. Its structure is provided below: a. The overall reaction of glucose metabolism is given below.
Sample Questions for the Individual Round Exam 1. Glucose is the most basic sugar involved in human metabolism. Its structure is provided below: a. The overall reaction of glucose metabolism is given below.
Qualitative chemical reaction of functional group in protein
 Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
Copyright 2016 Dan Dill 1
 carbonate These solutions are mixed and a precipitate forms. After the precipitation, the solution 1. will be positively charged 2. will be electrically neutral 3. will be negatively charged 4. More information
carbonate These solutions are mixed and a precipitate forms. After the precipitation, the solution 1. will be positively charged 2. will be electrically neutral 3. will be negatively charged 4. More information
(Writing model for laboratory note book)
 Paper: Lab 50 Syllabus *************************************************************************** Experiment: Organic Qualitative analysis 1) Detection of elements (Nitrogen, Sulphur and halogens). 2)
Paper: Lab 50 Syllabus *************************************************************************** Experiment: Organic Qualitative analysis 1) Detection of elements (Nitrogen, Sulphur and halogens). 2)
CERTIFICATE OF ANALYSIS
 Quality Analysis... Innovative Technologies Dan Whaley Box 771 Atikoken Ontario POT 1C0 Germany ATTN: Dan Whaley Date Submitted: Invoice No.: Invoice Date: Your Reference: CERTIFICATE OF ANALYSIS 18-Feb-15
Quality Analysis... Innovative Technologies Dan Whaley Box 771 Atikoken Ontario POT 1C0 Germany ATTN: Dan Whaley Date Submitted: Invoice No.: Invoice Date: Your Reference: CERTIFICATE OF ANALYSIS 18-Feb-15
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A. Ac-ag...Au-Zr. Bearbeitet von B Predel
 Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Receptor Modeling and Wet Deposition Measurements
 Receptor Modeling and Wet Deposition Measurements NADP Annual Meeting and Scientific Symposium October 15, 2008 Presented by Robert K. Stevens Florida DEP Some Elements of Research Presented were conducted
Receptor Modeling and Wet Deposition Measurements NADP Annual Meeting and Scientific Symposium October 15, 2008 Presented by Robert K. Stevens Florida DEP Some Elements of Research Presented were conducted
Chapter 17 Acids and Bases (same as Exam 2 Worksheet Answers)
 xam 3 Wrksheet Answers xam 3 Wrksheet Answers Chemistry 4 Chapter 17 Acids and Bases (same as xam Wrksheet Answers) 1. Fr the weak acids, what is the pk a and rank the acids accrding t strength? 4.74 6.38;.3
xam 3 Wrksheet Answers xam 3 Wrksheet Answers Chemistry 4 Chapter 17 Acids and Bases (same as xam Wrksheet Answers) 1. Fr the weak acids, what is the pk a and rank the acids accrding t strength? 4.74 6.38;.3
Figure 1. Location of 43 benchmark sites across Alberta.
 1.0 INTRODUCTION This report describes the micronutrient and trace element status of the AESA (Alberta Environmentally Sustainable Agriculture) Soil Quality Benchmark Sites. Previous reports completed
1.0 INTRODUCTION This report describes the micronutrient and trace element status of the AESA (Alberta Environmentally Sustainable Agriculture) Soil Quality Benchmark Sites. Previous reports completed
Course Content
 Biology Induction Course Content AS Biology A-Level Biology AS Practical Work Career options Degree options Research Based IS Task Due date: 1 st lesson back after the summer holidays 1. Compare and contrast
Biology Induction Course Content AS Biology A-Level Biology AS Practical Work Career options Degree options Research Based IS Task Due date: 1 st lesson back after the summer holidays 1. Compare and contrast
0620 CHEMISTRY. 0620/23 Paper 2 (Core Theory), maximum raw mark 80
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/23 Paper 2 (Core Theory), maximum raw mark 80
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/23 Paper 2 (Core Theory), maximum raw mark 80
Biochemistry. Chapter 6
 Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biological Molecules B Lipids, Proteins and Enzymes. Triglycerides. Glycerol
 Glycerol www.biologymicro.wordpress.com Biological Molecules B Lipids, Proteins and Enzymes Lipids - Lipids are fats/oils and are present in all cells- they have different properties for different functions
Glycerol www.biologymicro.wordpress.com Biological Molecules B Lipids, Proteins and Enzymes Lipids - Lipids are fats/oils and are present in all cells- they have different properties for different functions
Chemistry 1120 Exam 1 Study Guide
 Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Micronutrient Compatibility with Pesticides and NPK Fertilizers. Brian Haschemeyer Director of Discovery and Innovation
 Micronutrient Compatibility with Pesticides and NPK Fertilizers Brian Haschemeyer Director of Discovery and Innovation When you have a tank mix question or issue what do you do? Google it Jar Test If its
Micronutrient Compatibility with Pesticides and NPK Fertilizers Brian Haschemeyer Director of Discovery and Innovation When you have a tank mix question or issue what do you do? Google it Jar Test If its
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
CHAPTER BIOMOLECULES. Chapterwise Previous year Qs. (c) hydrolysis of ATP (d) defence mechanism Ans: (b)
 259 CHAPTER BIOMOLECULES 1. On hydrolysis of starch, we finally get [1991] (a) Glucose (b) Fructose (c) Both (d) and 2. The couplings between base units of DNA is through: [1992] (a) Hydrogen bonding (b)
259 CHAPTER BIOMOLECULES 1. On hydrolysis of starch, we finally get [1991] (a) Glucose (b) Fructose (c) Both (d) and 2. The couplings between base units of DNA is through: [1992] (a) Hydrogen bonding (b)
Molecules of Life. Chapter 22. Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1
 Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Alcohol aldehydes cetones and carboxylic acids
 Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
Aim: To study the effect of ph on the action of salivary amylase. NCERT
 Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins
 BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins The word protein is derived from the Greek word proteios, which means of primary importance. In fact, proteins plays an important role in all
BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins The word protein is derived from the Greek word proteios, which means of primary importance. In fact, proteins plays an important role in all
In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent.
 Acids and Bases Acids and Bases In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent. i.e. Salt water is an example of Na + and Cl - in a solution
Acids and Bases Acids and Bases In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent. i.e. Salt water is an example of Na + and Cl - in a solution
TNPSC Chemistry Study Material Fertilizers
 TNPSC Chemistry Study Material A fertilizer is any material of natural or synthetic origin (other than liming materials) that is applied to soils or to plant tissues to supply one or more plant nutrients
TNPSC Chemistry Study Material A fertilizer is any material of natural or synthetic origin (other than liming materials) that is applied to soils or to plant tissues to supply one or more plant nutrients
Actual Excipient Test Data on Metal Impurities Submitted to IPEC-Americas from Industry
 Actual Excipient Test Data on Metal Impurities Submitted to IPEC-Americas from Industry Priscilla Zawislak Chair Compendial Review Committee IPEC Americas Global Regulatory Affairs Manager Ashland Inc.
Actual Excipient Test Data on Metal Impurities Submitted to IPEC-Americas from Industry Priscilla Zawislak Chair Compendial Review Committee IPEC Americas Global Regulatory Affairs Manager Ashland Inc.
Zeolite Clinoptilolite Powder
 Catalog 2018 Zeolite Clinoptilolite Powder Formula: (Ca,K 2,Na 2,Mg) 4 Al 8 Si 40 O 96.24H 2 O Clinoptilolite content 90-92% TBA - tribomechanical pulverized
Catalog 2018 Zeolite Clinoptilolite Powder Formula: (Ca,K 2,Na 2,Mg) 4 Al 8 Si 40 O 96.24H 2 O Clinoptilolite content 90-92% TBA - tribomechanical pulverized
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Enzyme Mimics. Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites
 Enzyme Mimics Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites 1 Enzyme Mimics Biochemical systems: Binding is a trigger to events: Binding induces a conformational change in the receptor
Enzyme Mimics Principles Cyclodextrins as Mimics Corands as Mimics Metallobiosites 1 Enzyme Mimics Biochemical systems: Binding is a trigger to events: Binding induces a conformational change in the receptor
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
There are two groups of minerals: Major salt components: K, Na, Ca, Mg, Cl -, sulfate, phosphate, and HCO
 MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
MINERALS INTRODUCTION 90 elements in the earth s s crust, 25 are known to be essential to life, they are present in living cells, including in food. Food contains additional, non-essential elements. Some
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
2.1. thebiotutor. Unit F212: Molecules, Biodiversity, Food and Health. 1.1 Biological molecules. Answers
 thebiotutor Unit F212: Molecules, Biodiversity, Food and Health 1.1 Biological molecules Answers 1 1. δ + H hydrogen bond δ + H O δ - O δ - H H δ + δ+ 1 hydrogen bond represented as, horizontal / vertical,
thebiotutor Unit F212: Molecules, Biodiversity, Food and Health 1.1 Biological molecules Answers 1 1. δ + H hydrogen bond δ + H O δ - O δ - H H δ + δ+ 1 hydrogen bond represented as, horizontal / vertical,
Advanced Placement. Chemistry. Lab Based Questions
 Advanced Placement Chemistry Lab Based Questions 2014 47.90 91.22 178.49 (261) 50.94 92.91 180.95 (262) 52.00 93.94 183.85 (263) 54.938 (98) 186.21 (262) 55.85 101.1 190.2 (265) 58.93 102.91 192.2 (266)
Advanced Placement Chemistry Lab Based Questions 2014 47.90 91.22 178.49 (261) 50.94 92.91 180.95 (262) 52.00 93.94 183.85 (263) 54.938 (98) 186.21 (262) 55.85 101.1 190.2 (265) 58.93 102.91 192.2 (266)
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
CERTIFICATE OF ANALYSIS
 Quality Analysis... Innovative Technologies North Star Gold Corp 17 Wellington Street North P.O. Box 2529 New Liskard ON P0J 1P0 Canada Date Submitted: 09-Jul-14 Invoice No.: A14-04604 Invoice Date: 25-Jul-14
Quality Analysis... Innovative Technologies North Star Gold Corp 17 Wellington Street North P.O. Box 2529 New Liskard ON P0J 1P0 Canada Date Submitted: 09-Jul-14 Invoice No.: A14-04604 Invoice Date: 25-Jul-14
2.1.1 Biological Molecules
 2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
Chapter 2 pt 2. Atoms, Molecules, and Life. Gregory Ahearn. John Crocker. Including the lecture Materials of
 Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
General Certificate of Secondary Education Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] TUESDAY 25 FEBRUARY 2014, MORNING
![General Certificate of Secondary Education Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] TUESDAY 25 FEBRUARY 2014, MORNING General Certificate of Secondary Education Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] TUESDAY 25 FEBRUARY 2014, MORNING](/thumbs/73/69571061.jpg) General Certificate of Secondary Education 2013 2014 Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] TUESDAY 25 FEBRUARY 2014, MORNING MARK SCHEME 9018.01 F General Marking Instructions Introduction
General Certificate of Secondary Education 2013 2014 Double Award Science: Chemistry Unit C1 Higher Tier [GSD22] TUESDAY 25 FEBRUARY 2014, MORNING MARK SCHEME 9018.01 F General Marking Instructions Introduction
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS
 SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
CHAPTER4 ANSWERS. Multiple Choice Questions. Short Answer Questions. 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b)
 CHAPTER4 ANSWERS Multiple Choice Questions 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b) 9. (a) 10. (d) 11. (a) 12. (d) 13. (b) 14. (a) 15. (c) 16. (c) 17. (c) 18. (d) 19. (c) 20. (a) 21. (b)
CHAPTER4 ANSWERS Multiple Choice Questions 1. (b) 2. (d) 3. (a) 4. (c) 5. (c) 6. (b) 7. (a) 8. (b) 9. (a) 10. (d) 11. (a) 12. (d) 13. (b) 14. (a) 15. (c) 16. (c) 17. (c) 18. (d) 19. (c) 20. (a) 21. (b)
Serial No. 366,637. Filing Date 30 December 1994 NOTICE
 Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
R 3 NH + + H 2 O R 3 N + H 3 O +
 KEY BAS PARMAEUTAL SEE FR TE PRATG PARMAST MED 527, Fall 2008 Exam #1, Dr. Desai, 200 points; ctober 7, 2008 STUDET AME (in APTAL (Signature) Letters LY) R Pledge V V V V 2 F 3 Si P S 4 Ge As Se Br 5 1.
KEY BAS PARMAEUTAL SEE FR TE PRATG PARMAST MED 527, Fall 2008 Exam #1, Dr. Desai, 200 points; ctober 7, 2008 STUDET AME (in APTAL (Signature) Letters LY) R Pledge V V V V 2 F 3 Si P S 4 Ge As Se Br 5 1.
PRECIPITATON TITRATION
 PRECIPITATON TITRATION Precipitation titration is a special type of titrimetric procedure, which involves the formation of precipitates during the course of titration. The titrant react with the analyte
PRECIPITATON TITRATION Precipitation titration is a special type of titrimetric procedure, which involves the formation of precipitates during the course of titration. The titrant react with the analyte
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
5124 SCIENCE (PHYSICS AND CHEMISTRY)
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level www.xtremepapers.com MARK SCHEME for the October/November 2010 question paper for the guidance of teachers 5124 SCIENCE (PHYSICS AND
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level www.xtremepapers.com MARK SCHEME for the October/November 2010 question paper for the guidance of teachers 5124 SCIENCE (PHYSICS AND
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Fertilization Programming
 Fertilization Plant Composition Water composes 90% of plant weight (fresh weight) Dry weight is composed of 17 essential elements: Non-fertilizer elements: Carbon (C) -- 41% of dry weight (DW) Hydrogen
Fertilization Plant Composition Water composes 90% of plant weight (fresh weight) Dry weight is composed of 17 essential elements: Non-fertilizer elements: Carbon (C) -- 41% of dry weight (DW) Hydrogen
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
These biogenic elements are divided into: six major biogenic elements (elements found in almost all of Earth's living systems, often in relatively
 BIOGENIC ELEMENTS All matter in the Universe occurs in the form of atoms of a small number of elements. There are 92 naturally occurring chemical elements in the Universe. Almost every one of the chemical
BIOGENIC ELEMENTS All matter in the Universe occurs in the form of atoms of a small number of elements. There are 92 naturally occurring chemical elements in the Universe. Almost every one of the chemical
3150:112 SAMPLE TEST 2. Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck!
 SAMPLE TEST 2 3150:112 Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck! QUESTIONS 1-3 REFER TO TE FOLLOWING: A. C 2 O O B. C 2 O O O C 2 O C. O C 2 O 1.
SAMPLE TEST 2 3150:112 Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck! QUESTIONS 1-3 REFER TO TE FOLLOWING: A. C 2 O O B. C 2 O O O C 2 O C. O C 2 O 1.
Print version. Lecture #31 Coordination Chemistry: Case Studies: EDTA, detergents. (Stumm & Morgan, Chapt.6: pg ) Benjamin; Chapter
 Updated: 11 April 2018 Print version Lecture #31 Coordination Chemistry: Case Studies: EDTA, detergents (Stumm & Morgan, Chapt.6: pg.317-319) Benjamin; Chapter 8.1-8.6 David Reckhow CEE 680 #31 1 EDTA
Updated: 11 April 2018 Print version Lecture #31 Coordination Chemistry: Case Studies: EDTA, detergents (Stumm & Morgan, Chapt.6: pg.317-319) Benjamin; Chapter 8.1-8.6 David Reckhow CEE 680 #31 1 EDTA
Bio 12 Chapter 2 Test Review
 Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
ENDF/B-VII.1 and its covariances
 ENDF/B-VII.1 and its covariances Mike Herman National Nuclear Data Center Brookhaven National Laboratory mwherman@bnl.gov Brookhaven Science Associates ENDF/B-VII.0 ENDF/B-VII.1 Release December 2011 5
ENDF/B-VII.1 and its covariances Mike Herman National Nuclear Data Center Brookhaven National Laboratory mwherman@bnl.gov Brookhaven Science Associates ENDF/B-VII.0 ENDF/B-VII.1 Release December 2011 5
Micro-review. Element reducing oxidizing Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) S HS - (high) SO4 2- (high) Mo
 Micro-review Element reducing oxidizing environment environment Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) (low) S HS - (high) S4 2- (high) Mo [MonS4-n] 2- MoS2 (low) V V 3+, V 4+ sulfides
Micro-review Element reducing oxidizing environment environment Fe Fe 2+ (high) Fe 3+ (low) Cu Cu sulfides Cu 2+ (moderate) (low) S HS - (high) S4 2- (high) Mo [MonS4-n] 2- MoS2 (low) V V 3+, V 4+ sulfides
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Unit 1, Section C.1. In which you will learn about: Solutions Electrolytes Saturation Solubility curves
 Unit 1, Section C.1 In which you will learn about: Solutions Electrolytes Saturation Solubility curves Some Definitions A solution is a homogeneous mixture of 2 or more substances in a single phase. One
Unit 1, Section C.1 In which you will learn about: Solutions Electrolytes Saturation Solubility curves Some Definitions A solution is a homogeneous mixture of 2 or more substances in a single phase. One
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
ELEMENTS WITH MORE TOXIC EFFECTS
 BIOL 695 ELEMENTS WITH MORE TOXIC EFFECTS Chapter 20 MENGEL et al, 5th Ed IODINE Not shown to be essential, stimulating effects reported for some crops at 0.1 µg g -1 Toxic effects at 0.5-1.0 µg g -1 in
BIOL 695 ELEMENTS WITH MORE TOXIC EFFECTS Chapter 20 MENGEL et al, 5th Ed IODINE Not shown to be essential, stimulating effects reported for some crops at 0.1 µg g -1 Toxic effects at 0.5-1.0 µg g -1 in
OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK. Tyrone R.L. John, Chartered Biologist
 NAME: OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK Tyrone R.L. John, Chartered Biologist 1 Tyrone R.L. John, Chartered Biologist 2 Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular
NAME: OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK Tyrone R.L. John, Chartered Biologist 1 Tyrone R.L. John, Chartered Biologist 2 Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular
Adenosine triphosphate (ATP)
 Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
Supplying Nutrients to Crops
 Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
Supplying Nutrients to Crops What is Plant Nutrition? Plants need nutrients for healthy growth and development. Plant nutrition involves the absorption of nutrients for plant growth and is dependent on
Bioapatites. BeátaKerémi DMD, PhD Department of Oral Biology
 Bioapatites BeátaKerémi DMD, PhD Department of Oral Biology Mineralized ( hard ) tissues: Bones Teeth Enamel Cementum Dentin Hydroxy(l)-apatite Data correspond to 100 g dry weight. Composition of hard
Bioapatites BeátaKerémi DMD, PhD Department of Oral Biology Mineralized ( hard ) tissues: Bones Teeth Enamel Cementum Dentin Hydroxy(l)-apatite Data correspond to 100 g dry weight. Composition of hard
Chemical Pharmaceutical Quality Control. Prof.Dr.Joumaa Al- Zehouri Damascus university Faculty of Pharmacy
 Chemical Pharmaceutical Quality Control Prof.Dr.Joumaa Al- Zehouri Damascus university Faculty of Pharmacy COMPLEXOMETRIC REACTIONS AND TITRATIONS Prof.J.Al-Zehouri 1 ml of 0.1Msodium edetate is equivalent
Chemical Pharmaceutical Quality Control Prof.Dr.Joumaa Al- Zehouri Damascus university Faculty of Pharmacy COMPLEXOMETRIC REACTIONS AND TITRATIONS Prof.J.Al-Zehouri 1 ml of 0.1Msodium edetate is equivalent
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK. Tyrone R.L. John, Chartered Biologist
 NAME: OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK Tyrone R.L. John, Chartered Biologist 1 Tyrone R.L. John, Chartered Biologist 2 Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular
NAME: OPTION GROUP: BIOLOGICAL MOLECULES 3 PROTEINS WORKBOOK Tyrone R.L. John, Chartered Biologist 1 Tyrone R.L. John, Chartered Biologist 2 Instructions REVISION CHECKLIST AND ASSESSMENT OBJECTIVES Regular
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
