Advanced Placement. Chemistry. Lab Based Questions
|
|
|
- Ezra Lloyd
- 5 years ago
- Views:
Transcription
1 Advanced Placement Chemistry Lab Based Questions 2014
2 (261) (262) (263) (98) (262) (265) (266) H Li Na K Rb Cs Fr He Ne Ar Kr Xe Rn Ce Th Pr Pa Nd U Pm Np Sm Pu Eu Am Gd Cm Tb Bk Dy Cf Ho Es Er Fm Tm Md Yb No Lu Lr Be Mg Ca Sr Ba Ra B Al Ga In Tl C Si Ge Sn Pb N P As Sb Bi O S Se Te Po F Cl Br I At Sc Y La Ac Ti Zr Hf Rf V Nb Ta Db Cr Mo W Sg Mn Tc Re Bh Fe Ru Os Hs Co Rh Ir Mt Ni Pd Pt Cu Ag Au Zn Cd Hg (223) (222) (145) (244) (243) (247) (247) (251) (252) (257) (258) (259) (260) (209) (210) (269) (272) (277) *Lanthanide Series: Actinide Series: * Periodic Table of the Elements Not yet named
3 Throughout the test the following symbols have the definitions specified unless otherwise noted. L, ml liter(s), milliliter(s) mm Hg millimeters of mercury g gram(s) J, kj joule(s), kilojoule(s) nm nanometer(s) V volt(s) atm atmosphere(s) mol mole(s) ATOMIC STRUCTURE E hν c λν E energy ν frequency λ wavelength Planck s constant, h J s Speed of light, c ms 1 Avogadro s number mol 1 Electron charge, e coulomb EQUILIBRIUM c d [C] [D] K c a b, where a A + b B c C + d D [A] [B] c d ( P K p C)( PD ) a b ( PA) ( PB) + - [H ][A ] K a [HA] - + [OH ][HB ] K b [B] K w [H + ][OH ] at 25 C Equilibrium Constants K c (molar concentrations) K p (gas pressures) K a (weak acid) K b (weak base) K w (water) K a K b ph log[h + ],, poh log[oh ] 14 ph + poh ph pk a + log [A - ] [HA] pk a logk a, pk b logk b KINETICS ln[a] t ln[a] 0 kt 1-1 kt [ A] [ A] 0 t t ½ k k rate constant t time t ½ half-life
4 GASES, LIQUIDS, AND SOLUTIONS PV nrt P A P total X A, where X A P total P A + P B + P C +... n m M K C D m V KE per molecule 1 2 mv2 moles A total moles Molarity, M moles of solute per liter of solution A abc P V T n m M D KE Ã A a b c pressure volume temperature number of moles mass molar mass density kinetic energy velocity absorbance molarabsorptivity path length concentration -1-1 Gas constant, R J mol K L atm mol K L torr mol K 1 atm 760 mm Hg 760 torr STP 0.00 C and atm -1-1 THERMOCHEMISTRY/ ELECTROCHEMISTRY q mcdt DS ÂS products -ÂS reactants DH ÂDH products -ÂDH reactants f DG ÂDG products -ÂDG reactants DG DH - TDS -RT ln K -nfe q I t f f f q heat m mass c specific heat capacity T temperature S standard entropy H standard enthalpy G standard free energy n number of moles E I q t Faraday s constant, F standard reduction potential current (amperes) charge (coulombs) time (seconds) 96,485 coulombs per mole of electrons 1 joule 1volt 1coulomb
5 The Lab Based Questions Writing for a 5 What I Absolutely Have to Know to Survive the AP Exam If asked to do the following, it might indicate the question deals with laboratory questions: Design an experiment; list measurements needed; show setup of calculations needed, use sample data to do calculations; interpret or draw graphs; explain the affect of error on the calculated value; use qualitative observations Parts of a Lab Question You may not have done that exact experiment but you can use the knowledge gained doing other experiments to help account for certain observations. You may be asked to describe how to do an experiment or to design an experiment. Materials: If they give you a list of equipment, don t think you have to use all of it. Procedure: Be sure to include important techniques like rinsing the buret with the solution before a titration or heating to constant mass. Data needed: The data needed are values that can be measured like initial and final temperatures. Writing all the mathematical equations needed to do the calculations will help you determine what data is needed. Calculations: A calculation is using what was measured like temperature change. Show the set up of the mathematical equations required for the calculations. Use sample data when appropriate. Graphs: Be sure you label the axes and other important points on your graph. Error Analysis: State whether the quantity will be too high, too low, or no change. Use equations to help you determine what change will occur and to support your answer. Common Lab Procedure: Calorimetry Calorimetry is used to determine the heat released or absorbed in a chemical reaction. A calorimeter can determine the heat of a solution reaction at constant pressure. Techniques: Use a double Styrofoam cup with a plastic top and hole for the thermometer Determine the change in temperature accurately Measure solution volumes precisely Start with a dry calorimeter Information to know about calorimetry: Heat capacity (C) the amount of heat needed to raise the temperature of an object by one degree Celsius or Kelvin, J/ C or J/K. The heat capacity of 1 mol of a substance is called its molar heat capacity (Joules per mole per degree) J/mol C or J/mol K. Specific heat, c, also known as specific heat capacity, is defined as the amount of heat necessary to raise the temperature of 1.00 g of a substance by one degree. Units are (joules per gram per degree), J/g C or J/g K. You often use the specific heat capacity in analyzing gathered data then convert to molar heat capacity. Assumptions often made during calorimetry: (Be able to answer error analysis questions about each assumption below) The density of dilute solutions is the same for water. D 1.0 g/ml The specific heat of the solutions is the same as that for water. c J/g o C The solutions react in their stochiometric amounts. There is no loss of heat to the surroundings. Equations: q mcδt (c J/g C) q Δ H mcδ T, at constant pressure Use the density of the solution to convert from volume to mass for q mcδt Copyright 2014 National Math + Science Initiative, Dallas, Texas. All Rights Reserved. Visit us online at Page 1
6 Common Lab Procedure: Titration A titration is a laboratory procedure for quantitative analysis. In a titration two reagents are mixed, one with a known concentration & known volume [or a solid with a known mass] and one with an unknown concentration. There is some way to indicate when the two reagents have reacted completely (typically an indicator), and at the end of the titration the unknown solution's concentration can be calculated since you have accurately determined the volume of that solution required to complete the reaction. Terms to know: Titrant A solution of known concentration; it is often standardized Standardized solution A solution in which the exact concentration is known. Indicator - A weak acid or base used in a titration to indicate the endpoint has been reached. Equivalence point moles of acid moles of base; point at which enough titrant has been added to completely react with the solution being analyzed. End point the point at which the indicator changes color; important to pick an indicator with a pka very close to the ph at the equivalence point. Techniques: Preparing the Buret: 1. Rinse a clean buret with distilled water and then the titrant (the solution that will be added to the flask). 2. Allow the titrant to drain through the buret so that the tip gets rinsed with titrant as well. 3. Discard the rinse solution. Fill it with the titrant. Remove air bubbles from the tip of the buret by draining several milliliters of titrant. Preparing the Sample: 4. Pipet the desired volume of the solution to be analyzed into an Erlenmeyer flask. Record the exact volume. If the sample is a solid, weigh the desired mass, add the solid to an Erlenmeyer flask, and dissolve it in distilled water (the amount of water does matter since it doesn t change the moles of the solid). Be sure to record the exact mass of sample used. 5. Add the titrant to the flask until the equivalence point is reached. Calculate the volume of titrant added. 6. Change in color of a chemical indicator is usually used to signal the endpoint of the titration. The endpoint for this titration is reached when you reach a pale color that persists for several seconds. Measured Data Required: moles titrant moles of substance equivalence point mass of DRY substance analyzed OR accurately measured volume of solution analyzed [measure with a pipet OR buret ] initial volume of titrant (substance of known molarity) and final volume of titrant (required to reach end point) Molarity of titrant Calculation Hints: Substance analyzed is solution/liquid: M1 V1 equivalence point Volume of titrant used to reach end point [difference between final and initial volumes] M titrant V of titrant added moles of titrant moles of unknown moles of unknown Molarity of unknown Molarity of unknown Liters of unknown Copyright 2014 National Math + Science Initiative, Dallas, Texas. All Rights Reserved. Visit us online at Page 2
7 Graphs: Substance analyzed is solid: Same as process as above M titrant V of titrant added moles of titrant moles of unknown mass of solid dissolved Molecular weight of the unknown moles of unknown Common Lab Procedure: Gravimetric Analysis One method for determining the amount of a given substance in solution is to form a precipitate that includes the substance. The precipitate is then filtered and dried. This process is called gravimetric analysis. Techniques/Procedure: 1. Weigh sample 2. Form precipitate 3. Filter precipitate (A buchner funnel and aspirator can be used) 4. Dry precipitate (Be sure to dry to constant mass) 5. Weigh precipitate For example if we wanted to determine the amount of chloride ions present in a given solid, we would weigh the solid sample, dissolve the sample in water, add an excess of silver nitrate solution to form the precipitate silver chloride. This precipitant would be filtered, and dried to constant mass. From the mass of silver chloride formed, we can determine the moles of silver chloride and the moles of chloride ion in the original sample. Copyright 2014 National Math + Science Initiative, Dallas, Texas. All Rights Reserved. Visit us online at Page 3
8 Common Lab Procedure: Determining Molar Mass Organize answer around calculations paying special attention to what quantities are measured versus calculated! mass of sample in grams molar mass moles of sample Titration Data moles titrant moles of substance equivalence point Measured DATA required: o mass of substance o initial volume of titrant (substance of known molarity) and final volume of titrant (required to reach end point) o Molarity of titrant Calculations required: o Molarity of titrant o Substance analyzed is solid: o M titrant V titrant moles titrant o molar mass g of solid analzyed moles of titrant used Vaporization of a Volatile Liquid PV nrt used to determine moles Measured DATA Required: Pressure atmospheric pressure unless collected over water initial mass of flask final mass of flask Temperature of boiling water don t assume 100 C Volume of gas fill flask with water and measure the volume of water in a graduated cylinder Constants needed: If collected over water, the water vapor pressure at the experimental temperature. Calculations Required: If the gas was collected over water P vapor Patmospheric/baraometric- Pwater vapor at certain temperature mass of sample final mass of flask [includes vapors] initial mass of flask Common Lab Procedure: Colorimetric or Spectrophotometric Analysis Colorimetric analysis is a quantitative analysis of a solution using color based on Beer s Law. Colorimetric analysis can be used to determine the concentration of an unknown solution, the rate constant of a reaction, the order of a reaction, etc. Beer s Law is an expression than can be used to determine how much light passes through the solution. It also shows that concentration and absorbance are directly related. A εbc A absorbance (measured with a colorimeter) ε molar absorptivity (how much light will be absorbed by 1 cm of a 1 M solution of the chemical) b path length of the cuvette in cm c concentration in molarity Copyright 2014 National Math + Science Initiative, Dallas, Texas. All Rights Reserved. Visit us online at Page 4
9 Lab Based Questions Cheat Sheet Relationships Beer s Law Calorimetry q ΔH mcδt at constant pressure c specific heat ΔT change is temperature m mass q heat A εbc A absorbance ε molar absorptivity b path length of the cuvette in cm c concentration in molarity Ideal Gas Law PV nrt Other important relationships: V volume in liters n moles T temperature in Kelvin P pressure If collected using water displacement: P vapor P atmospheric/baraometric P water vapor at certain Equivalence point molar mass M V 1 1 moles of sample M2V mass of sample in grams accepted - experimental %error 100 accepted 2 Electrochemistry: draw diagram of galvanic or electrolytic cell, qualitative observations that can be made at the cathode and anode, etc. Connections Stoichiometry: empirical formula of a compound, percent of an element in a compound, etc. Thermodynamics: heat of reaction, molar heat capacity, etc. Qualitative Analysis: identifying a compound based on observations and tests Acids and Bases: standardizing a solution, drawing a titration curve, etc. Potential Pitfalls Be aware there are quantities you measure [such an initial temperature and final temperature or initial pressure and final pressure, etc.] and terms you calculate using what you measured such as Δ T or Δ P Be sure to include important steps in the procedure like heat to constant mass, rinse the buret with distilled water and then the solution before titrating, dissolve the solid in about 100 ml of water and then add water to the 500 ml mark on the volumetric flask, etc. Writing the mathematical equations may help determine how an error affects the results. Use the equations to justify your error analysis. If given a laboratory situation you have not specifically done, use the observations and the concepts you learned in your labs throughout the year to reason your way through the lab question. Copyright 2014 National Math + Science Initiative, Dallas, Texas. All Rights Reserved. Visit us online at Page 5
10 NMSI SUPER PROBLEM A sample of 6 M hydrochloric acid (about 5 ml) is placed at the bottom of the test tube and then carefully filled with distilled water so not to disturb the HCl. A piece of magnesium is placed on top of the distilled water and then the test tube is inverted into a beaker with distilled water. The magnesium is allowed to react completely. The gas is collected in the test tube at room temperature. Before the test tube with the gas is removed, the water levels inside and outside the test tube are the same. The chemicals and lab equipment used are listed below. 6 M hydrochloric acid Barometer Strip of magnesium metal 600 ml beaker Distilled water Balance Test tube Table of water vapor pressures Graduated cylinder Thermometer (a) Write the balanced net ionic reaction that occurs when hydrochloric acid reacts with magnesium. (b) List the measurements needed to calculate the molar volume of the gas produced? (c) Show the setup for the calculations needed to determine: (i) the moles of gas produced (ii) the volume of the gas produced
11 (iii) the molar volume of the gas (at STP) (d) What test can be done to prove which gas is produced? (e) What is the purpose of making the water level inside the test tube equal to the water level in the beaker? (f) If the vapor pressure of water is not used in the calculation, how will the molar volume of the gas at STP be affected? (higher, lower or the same) Explain.
12 Another sample of hydrochloric acid was used to titrate a solution of calcium hydroxide. (g) Describe the steps needed to make 50.0 ml of a 0.50 M solution from the 6 M solution of hydrochloric acid, using a dropper, 5.0 ml pipet, 50.0 ml volumetric flask, and distilled water. Be sure to mathematically justify your process. (h) Write the balanced net ionic equation for the dissociation of Ca(OH) 2 (s) in aqueous solution, and write the equilibrium-constant expression for the dissolving Ca(OH) 2. A 15.0 ml unknown solution of Ca(OH) 2 is titrated with a standardized 0.50 M solution of hydrochloric acid. It takes exactly 10.5 ml of hydrochloric acid to reach the equivalence point where the ph is (i) Sketch the titration curve that shows the ph change as the volume of hydrochloric acid added increases from 0 to 16.0 ml. Be sure to label the equivalence point of the titration.
13 (j) Calculate the concentration of [OH] from the titration. The Lab Based Question Writing for a 5
Experiment 6: STANDARDIZATION OF A BASE; MASS PERCENT OF AN ACID
 Experiment 6: STANDARDIZATION OF A BASE; MASS PERCENT OF AN ACID Introduction The reaction of an acid and a base to form a salt and water is known as neutralization. In this experiment; potassium acid
Experiment 6: STANDARDIZATION OF A BASE; MASS PERCENT OF AN ACID Introduction The reaction of an acid and a base to form a salt and water is known as neutralization. In this experiment; potassium acid
Thin Film PV Technologies CIGS PV Technology
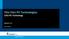 Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
Thin Film PV Technologies CIGS PV Technology Week 5.3 Arno Smets CIGS NiA1 MgF 2 TCO (ZnO:Al) TCO (intrinsic ZnO) CdS (n-type) CuInSe 2 (p-type) Mo Glass CIGS IV- semiconductors: III- V semiconductors:
Chelated & Essentials. For Your Pet Animals
 Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Chelated & Essentials For Your Pet Animals The Mineral Depletion In general the scientific community recognizes that our broad source of nourishment is at the present vastly depleted of its minerals and
Titration of Synthesized Aspirin A continuation of the aspirin synthesis lab
 Titration of Synthesized Aspirin A continuation of the aspirin synthesis lab In this lab, you will determine the percent purity of your product from the aspirin synthesis using an acid-base titration.
Titration of Synthesized Aspirin A continuation of the aspirin synthesis lab In this lab, you will determine the percent purity of your product from the aspirin synthesis using an acid-base titration.
GSJ Geochemical Reference Samples. Igneous Rock. Sedimentary Rock. For Instrumental analysis. Reference value for environmental analysis -1
 GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
GSJ Geochemical Reference Samples Igneous Rock Recommended and preferable (asterisked) values (major elements in % and minor and trace elements in ppm, unless otherwise noted) N. Imai, S. Terashima, S.
EXPERIMENT 2: ACID/BASE TITRATION. Each person will do this laboratory individually. Individual written reports are required.
 EXPERIMENT 2: ACID/BASE TITRATION Each person will do this laboratory individually. Individual written reports are required. OVERVIEW. Acid/base titration, relying on a color change of the indicator, is
EXPERIMENT 2: ACID/BASE TITRATION Each person will do this laboratory individually. Individual written reports are required. OVERVIEW. Acid/base titration, relying on a color change of the indicator, is
Take an initial volume reading and record it in your. 11/17/2014 ChemLab - Techniques - Titration
 Glassware Burets Flasks, Beakers, & Graduated Cylinders Pipets Repipets Volumetric Flasks Instruments Analytical Balance ph Meter: Analog ph Meter: Digital Spectrometer: Analog Spectrometer: Digital Spectrometer:
Glassware Burets Flasks, Beakers, & Graduated Cylinders Pipets Repipets Volumetric Flasks Instruments Analytical Balance ph Meter: Analog ph Meter: Digital Spectrometer: Analog Spectrometer: Digital Spectrometer:
Strength of Vinegar by Acid-Base Titration
 Strength of Vinegar by Acid-Base Titration Test Exercise 100 points 1? QUESTIONS? How are acid/base titrations conducted? What is standardization? How do you standardize a solution of a base? How, and
Strength of Vinegar by Acid-Base Titration Test Exercise 100 points 1? QUESTIONS? How are acid/base titrations conducted? What is standardization? How do you standardize a solution of a base? How, and
Chemistry 212. Experiment 3 ANALYSIS OF A SOLID MIXTURE LEARNING OBJECTIVES. - learn to analyze a solid unknown with volumetric techniques.
 Experiment 3 The objectives of this experiment are to LEARNING OBJECTIVES - learn to analyze a solid unknown with volumetric techniques. - use stoichiometry to determine the percentage of KHP in a solid
Experiment 3 The objectives of this experiment are to LEARNING OBJECTIVES - learn to analyze a solid unknown with volumetric techniques. - use stoichiometry to determine the percentage of KHP in a solid
2. is a set of principles intended to help sustain a habitable planet.
 Chapter 2 Tools of the Trade 1 Multiple Choice 2-1 Safe, Ethical Handling of Chemicals and Waste 1. Which of the following statements are TRUE? I Organic solvents, concentrated acids, and concentrated
Chapter 2 Tools of the Trade 1 Multiple Choice 2-1 Safe, Ethical Handling of Chemicals and Waste 1. Which of the following statements are TRUE? I Organic solvents, concentrated acids, and concentrated
Determining the Molecular Mass of an Unknown Acid by Titration
 Determining the Molecular Mass of an Unknown Acid by Titration Objectives: To perform an analytical titration. To standardize a basic solution. To determine the equivalent mass of an unknown acid. Background:
Determining the Molecular Mass of an Unknown Acid by Titration Objectives: To perform an analytical titration. To standardize a basic solution. To determine the equivalent mass of an unknown acid. Background:
EXPERIMENT. Titration of the Weak Acid Potassium Hydrogen Phthalate (KHP)
 INTRODUCTION EXPERIMENT Titration of the Weak Acid Potassium Hydrogen Phthalate (KHP) Materials generally considered to possess acidic and/or basic properties are widely distributed in nature and range
INTRODUCTION EXPERIMENT Titration of the Weak Acid Potassium Hydrogen Phthalate (KHP) Materials generally considered to possess acidic and/or basic properties are widely distributed in nature and range
TRATION: ANALYSIS OF SODIUM HYDROXIDE
 Experiment 8 Name: 22 Ti TRATION: ANALYSIS OF SODIUM HYDROXIDE In this experiment, you will learn the concept and technique of titration. You will use a chemical standard (potassium hydrogen phthalate)
Experiment 8 Name: 22 Ti TRATION: ANALYSIS OF SODIUM HYDROXIDE In this experiment, you will learn the concept and technique of titration. You will use a chemical standard (potassium hydrogen phthalate)
3 To gain experience monitoring a titration with a ph electrode and determining the equivalence point.
 Titrations PURPOSE To determine the concentration of acetic acid in vinegar. GOALS 1 To perform an acid-base titration. 2 To gain experience titrating carefully to a visible endpoint. 3 To gain experience
Titrations PURPOSE To determine the concentration of acetic acid in vinegar. GOALS 1 To perform an acid-base titration. 2 To gain experience titrating carefully to a visible endpoint. 3 To gain experience
TRATION: ANALYSIS OF SODIUM HYDROXIDE
 Experiment 10 Name: 22 Ti TRATION: ANALYSIS OF SODIUM HYDROXIDE In this experiment, you will learn the concept and technique of titration. You will use a standard (potassium hydrogen phthalate) to determine
Experiment 10 Name: 22 Ti TRATION: ANALYSIS OF SODIUM HYDROXIDE In this experiment, you will learn the concept and technique of titration. You will use a standard (potassium hydrogen phthalate) to determine
EXPERIMENT 4 TITRATION OF AN UNKNOWN ACID
 EXPERIMENT 4 TITRATION OF AN UNKNOWN ACID The reaction of an acid and a base to form a salt and water is known as neutralization. In this experiment you will titrate an known amount of KHP with an unknown
EXPERIMENT 4 TITRATION OF AN UNKNOWN ACID The reaction of an acid and a base to form a salt and water is known as neutralization. In this experiment you will titrate an known amount of KHP with an unknown
Titration Lab 3/10/15. By Maya Parks. Partner: Colin Welch. Abstract:
 Titration Lab 3/10/15 By Maya Parks Partner: Colin Welch Abstract: In this lab, we used the technique of titration to determine the molarity of an acid. This was a concept learned in class, and this lab
Titration Lab 3/10/15 By Maya Parks Partner: Colin Welch Abstract: In this lab, we used the technique of titration to determine the molarity of an acid. This was a concept learned in class, and this lab
Standardization of a Base, Mass Percent of an Acid
 Experiment 7 Standardization of a Base, Mass Percent of an Acid Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Experiment 7 Standardization of a Base, Mass Percent of an Acid Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
Experiment 3. Potentiometric Titration Using a ph Electrode. information necessary for both purposes by monitoring the ph of the solution as the
 Experiment 3 Potentiometric Titration Using a Electrode Introduction Titrations are most commonly performed either to find out how much analyte is present or to measure equilibrium constants of the analyte.
Experiment 3 Potentiometric Titration Using a Electrode Introduction Titrations are most commonly performed either to find out how much analyte is present or to measure equilibrium constants of the analyte.
Experiment: Iodometric Titration Analysis of Ascorbic Acid Chem251 modified 09/2018
 Experiment: Iodometric Titration Analysis of Ascorbic Acid Chem251 modified 09/2018 Experiment. Iodometric Titration of Ascorbic Acid. Objective: The goal of this lab is to determine the concentration
Experiment: Iodometric Titration Analysis of Ascorbic Acid Chem251 modified 09/2018 Experiment. Iodometric Titration of Ascorbic Acid. Objective: The goal of this lab is to determine the concentration
Experiment 10 Acid-base Titrations: Part A Analysis of vinegar and Part B Analysis of a Carbonate/Bicarbonate mixture
 Chemistry 112 Section 201 Dates of Experiment: March 8 and March 22 Noah McNally Acid-base Titrations: Part A Analysis of vinegar and Part B Analysis of a Carbonate/Bicarbonate mixture Unknown Number:
Chemistry 112 Section 201 Dates of Experiment: March 8 and March 22 Noah McNally Acid-base Titrations: Part A Analysis of vinegar and Part B Analysis of a Carbonate/Bicarbonate mixture Unknown Number:
NEW DEVELOPMENTS IN CATALYSIS
 You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
You are now at www.wernerblank.com HOME NEWS PUBLICATIONS LECTURES PATENTS DOWNLOADS NEW DEVELOPMENTS IN CATALYSIS Werner J. Blank King Industries Inc. Science Road Norwalk, CT 06952 USA wblank@kingindustries.com
What Is the Relationship Between the Amount of Transmitted Light Through a Solution and Its Concentration?
 What Is the Relationship Between the Amount of Transmitted Light Through a and Its Concentration? Blue Food Dye Treats Spine Injury in Rats HTTP://WWW.WIRED.COM/WIREDSCIENCE/2009/07/BLUERATS/ BY HADLEY
What Is the Relationship Between the Amount of Transmitted Light Through a and Its Concentration? Blue Food Dye Treats Spine Injury in Rats HTTP://WWW.WIRED.COM/WIREDSCIENCE/2009/07/BLUERATS/ BY HADLEY
FIGURE 1. The structure of glucose and ascorbic acid (vitamin C). FIGURE 2. Reduced and oxidized forms of ascorbic acid.
 Chemistry Counts! Spring 2016 Lab 7: Copper from Malachite 2.0 and Titration of Vitamin C Adapted from Dr. Rebecca Sunderman and Dr. Peter Pessiki, TESC Purpose: Experiment 1: We will do the second roast
Chemistry Counts! Spring 2016 Lab 7: Copper from Malachite 2.0 and Titration of Vitamin C Adapted from Dr. Rebecca Sunderman and Dr. Peter Pessiki, TESC Purpose: Experiment 1: We will do the second roast
Chemistry Iodometric Determination of Vitamin C
 Chemistry 3200 Triiodide, I 3, is a mild oxidizing agent that is widely used in oxidation/reduction titrations. Triiodide is prepared by combining potassium iodide, KI, and potassium iodate, KI 3, in acidic
Chemistry 3200 Triiodide, I 3, is a mild oxidizing agent that is widely used in oxidation/reduction titrations. Triiodide is prepared by combining potassium iodide, KI, and potassium iodate, KI 3, in acidic
For Examiner s Use Total EMPA mark Surname. Candidate. Number. Other Names
 Centre Number Candidate Number For Examiner s Use Total EMPA mark Surname Other Names Notice to Candidate. The work you submit for assessment must be your own. If you copy from someone else or allow another
Centre Number Candidate Number For Examiner s Use Total EMPA mark Surname Other Names Notice to Candidate. The work you submit for assessment must be your own. If you copy from someone else or allow another
Chemistry 201 Laboratory Fall 2006 page 1 of 4
 Chemistry 201 Laboratory Fall 2006 page 1 of 4 Experiment: Determination of Iron in a Ferrous Ammonium Sulfate Sample (Fe) This experiment involves the determination of the percentage of ferrous iron in
Chemistry 201 Laboratory Fall 2006 page 1 of 4 Experiment: Determination of Iron in a Ferrous Ammonium Sulfate Sample (Fe) This experiment involves the determination of the percentage of ferrous iron in
Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria. Dr. Nesrine El Gohary 12 th lecture
 Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria Dr. Nesrine El Gohary 12 th lecture Revision lecture next week Next Saturday (21-5-2016) in the 1 st
Pharmaceutical Analytical Chemistry PHCM223 Lecture 12 Applications on different types of equilibria Dr. Nesrine El Gohary 12 th lecture Revision lecture next week Next Saturday (21-5-2016) in the 1 st
of human hair and nails. Part I. Analytical methodology, Sci. Tot. Environ. 2000, 250/1-3,
 Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Reference data Biomonitoring Trace elements in human biological material References cited below: 1. Rodushkin I., Ödman F., Branth S. Multielement analysis of whole blood by high resolution inductively
Lab 05 Introduction Reactions Pre Lab Problems (answer on separate paper)
 Lab 05 Introduction Among the many types of quantitative chemistry techniques, volumetric analysis is a timehonored classical method. The characteristic feature of volumetric analysis is measuring the
Lab 05 Introduction Among the many types of quantitative chemistry techniques, volumetric analysis is a timehonored classical method. The characteristic feature of volumetric analysis is measuring the
MODULE TOPIC: Percent Composition of Elements using EDTA titration. LESSON PLAN 1: EDTA titration of Calcium in a Citracal Tablet
 MODULE TOPIC: Percent Composition of Elements using EDTA titration LESSON PLAN 1: EDTA titration of Calcium in a Citracal Tablet LESSON PLAN 2: EDTA titration of Magnesium in a sample of Epsom Salt STANDARD
MODULE TOPIC: Percent Composition of Elements using EDTA titration LESSON PLAN 1: EDTA titration of Calcium in a Citracal Tablet LESSON PLAN 2: EDTA titration of Magnesium in a sample of Epsom Salt STANDARD
Lab #3 Potentiometric Titration of Soda Ash (after Christian, p , p ) (phenolphthalein)
 Lab #3 Potentiometric Titration of Soda Ash (after Christian, p.692-694, p.718-720) I: INTRODUCTION In this lab, an unknown sample of soda ash (a crude mixture of sodium carbonate) will be titrated with
Lab #3 Potentiometric Titration of Soda Ash (after Christian, p.692-694, p.718-720) I: INTRODUCTION In this lab, an unknown sample of soda ash (a crude mixture of sodium carbonate) will be titrated with
Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering
 Name Date Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering In this experiment, you will determine the amount (percent) of potassium hydrogen
Name Date Experiment 7, Analysis of KHP by titration with NaOH Wright College, Department of Physical Science and Engineering In this experiment, you will determine the amount (percent) of potassium hydrogen
Activity Sheet 1 Testing for Vitamin C- Part One
 Activity Sheet 1 Testing for - Part One Student Name: Purpose: To test the level of vitamin C in a variety of fruit juices. Procedure: Part 1 Testing Solution 1. Use the vitamin C solution for this experiment.
Activity Sheet 1 Testing for - Part One Student Name: Purpose: To test the level of vitamin C in a variety of fruit juices. Procedure: Part 1 Testing Solution 1. Use the vitamin C solution for this experiment.
LAB 04 Diffusion and Osmosis
 LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES. Consumables and Supplies Reference Guide
 AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES INTRODUCTION AND OVERVIEW Capable of handling even the most difficult, high-matrix samples without dilution, the Avio 200 brings a whole new level of performance
AVIO 200 ICP-OES CONSUMABLES AND SUPPLIES INTRODUCTION AND OVERVIEW Capable of handling even the most difficult, high-matrix samples without dilution, the Avio 200 brings a whole new level of performance
Stored Mechanical Gravitational Electrical. measured as. Forms of Energy
 Thermodynamics is the branch of science that studies the transformation of energy from one form to another. Thermochemistry specifically studies heat changes that accompany chemical reactions and phase
Thermodynamics is the branch of science that studies the transformation of energy from one form to another. Thermochemistry specifically studies heat changes that accompany chemical reactions and phase
The Energy in Food. Energy (J/g) = (final temperature start temperature) x mass of water (g) x 4.2 (J per o C) Mass of food burned (g)
 The Energy in Food NAME DATE Food supplies energy for all animals without it we could not live. The quantity of energy stored in food is of great interest to humans. The energy your body needs for running,
The Energy in Food NAME DATE Food supplies energy for all animals without it we could not live. The quantity of energy stored in food is of great interest to humans. The energy your body needs for running,
Name: Bio A.P. Lab Diffusion & Osmosis
 Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Complexometric Titration of Calcium in Antacids SUSB-017 Prepared by M. J. Akhtar and R. C. Kerber, SUNY at Stony Brook (Rev 1/13, RFS)
 61 SUSB- 017 017 exercise Complexometric Titration of Calcium in Antacids SUSB-017 Prepared by M. J. Akhtar and R. C. Kerber, SUNY at Stony Brook (Rev 1/13, RFS) purpose To determine the amount of calcium
61 SUSB- 017 017 exercise Complexometric Titration of Calcium in Antacids SUSB-017 Prepared by M. J. Akhtar and R. C. Kerber, SUNY at Stony Brook (Rev 1/13, RFS) purpose To determine the amount of calcium
Purity Tests for Modified Starches
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
» Croscarmellose Sodium is a cross linked polymer of carboxymethylcellulose sodium.
 BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
GB Translated English of Chinese Standard: GB NATIONAL STANDARD
 Translated English of Chinese Standard: GB5009.5-2016 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.5-2016 National food safety standard
Translated English of Chinese Standard: GB5009.5-2016 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.5-2016 National food safety standard
Petrolatum. Stage 4, Revision 1. Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum.
 1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series DESCRIPTION OF THE MOST IMPORTANT PROPERTIES AND THE POSSIBLE VARIATIONS AND TOLERANCES
 USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
Determination of Total Hardness in Water by Automatic Titration
 TECHNICAL NOTE Determination of Total Hardness in Water by Automatic Titration No. 7 Introduction Total hardness due to calcium and magnesium in water is determined using the preprogrammed method, T7 Total
TECHNICAL NOTE Determination of Total Hardness in Water by Automatic Titration No. 7 Introduction Total hardness due to calcium and magnesium in water is determined using the preprogrammed method, T7 Total
Organic Molecule Composition of Milk: Lab Investigation
 Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
Mike Hinds, Royal Canadian Mint
 Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
Experience in the Use of the LBMA Reference Materials Mike Hinds Royal Canadian Mint LBMA Assayer and Refiner March 2011 1 LBMA RM Project 2007-2010 2 Gold Reference Materials AuRM1 and AuRM2 Available
6.02 Uniformity of Dosage Units
 6.02 Uniformity of Dosage Units Change 1. Content Uniformity, 3. Criteria and Table 6.02-2 as follows: 1. Content Uniformity Select not less than 30 units, and proceed as follows for the dosage form designated.
6.02 Uniformity of Dosage Units Change 1. Content Uniformity, 3. Criteria and Table 6.02-2 as follows: 1. Content Uniformity Select not less than 30 units, and proceed as follows for the dosage form designated.
Serial No. 366,637. Filing Date 30 December 1994 NOTICE
 Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
Serial No. 366,637 Filing Date 30 December 1994 Inventor Geogre P. Chambers NOTICE The above identified patent application is available for licensing. Requests for information should be addressed to: Accesion
Sweet Calories: Energy Content of Marshmallows Lab
 Honors Chemistry Name: Date: Pd: Partner(s): Sweet Calories: Energy Content of Marshmallows Lab Introduction: All human activity requires burning food for energy. How much energy is released when food
Honors Chemistry Name: Date: Pd: Partner(s): Sweet Calories: Energy Content of Marshmallows Lab Introduction: All human activity requires burning food for energy. How much energy is released when food
Carbohydrates. Objectives. Background. Experiment 6
 1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
Catalytic Activity of Enzymes
 Catalytic Activity of Enzymes Introduction Enzymes are biological molecules that catalyze (speed up) chemical reactions. You could call enzymes the Builders and Do-ers in the cell; without them, life could
Catalytic Activity of Enzymes Introduction Enzymes are biological molecules that catalyze (speed up) chemical reactions. You could call enzymes the Builders and Do-ers in the cell; without them, life could
COLE-PARMER LABORATORY SURFACTANT ION ELECTRODE INSTRUCTION MANUAL
 COLE-PARMER LABORATORY SURFACTANT ION ELECTRODE INSTRUCTION MANUAL Cole-Parmer Instrument Company (800)323-4340 Fax:(847)247-2929 625 East Bunker Court, Vernon Hills, Illinois 60061 http://www.coleparmer.com
COLE-PARMER LABORATORY SURFACTANT ION ELECTRODE INSTRUCTION MANUAL Cole-Parmer Instrument Company (800)323-4340 Fax:(847)247-2929 625 East Bunker Court, Vernon Hills, Illinois 60061 http://www.coleparmer.com
Chemical Equations Part 1
 Chemical Equations Part 1 A Directed Learning Activity for Hartnell College Chemistry 1 Funded by the Title V STEM Grant #P031S090007 through Hartnell College For information contact lyee@hartnell.edu
Chemical Equations Part 1 A Directed Learning Activity for Hartnell College Chemistry 1 Funded by the Title V STEM Grant #P031S090007 through Hartnell College For information contact lyee@hartnell.edu
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION. READING: Please read pages & in your text prior to lab.
 BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
IODINE AFFINITY. 3. Extraction Shells: Paper, 80 x 22 mm (Note 1)
 IODIN.01-1 IODINE AFFINITY PRINCIPLE SCOPE Iodine complexes preferentially with the amylose (linear fraction) in corn starch. After defatting by solvent extraction, and drying, the sample is dispersed
IODIN.01-1 IODINE AFFINITY PRINCIPLE SCOPE Iodine complexes preferentially with the amylose (linear fraction) in corn starch. After defatting by solvent extraction, and drying, the sample is dispersed
Instruction Manual Updated 8/27/2013 Ver. 1.1
 Water Analysis Kit Part No. 144-95 Instruction Manual Updated 8/27/2013 Ver. 1.1 OFI Testing Equipment, Inc. 11302 Steeplecrest Dr. Houston, Texas 77065 U.S.A. Tele: 832.320.7300 Fax: 713.880.9886 www.ofite.com
Water Analysis Kit Part No. 144-95 Instruction Manual Updated 8/27/2013 Ver. 1.1 OFI Testing Equipment, Inc. 11302 Steeplecrest Dr. Houston, Texas 77065 U.S.A. Tele: 832.320.7300 Fax: 713.880.9886 www.ofite.com
ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET
 Activity 2.11 Student Sheet ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET To investigate how enzyme concentration can affect the initial rate of reaction. Wear eye protection, lab coats and
Activity 2.11 Student Sheet ENZYME CONCENTRATIONS AND ENZYME ACTIVITY: PLANNING SHEET To investigate how enzyme concentration can affect the initial rate of reaction. Wear eye protection, lab coats and
Engineering of Ministry of Education, Institute of Molecular Science, Shanxi University, Taiyuan , China.
 Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing in water and its application for the thiol detection in plasma Fang-Jun Huo, a Yu-Tao Yang, b Jing Su, b Yuan-Qiang
Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing in water and its application for the thiol detection in plasma Fang-Jun Huo, a Yu-Tao Yang, b Jing Su, b Yuan-Qiang
Hardness, Total, Sequential
 Hardness, Total, Sequential DOC316.53.01159 Titration Method with EDTA 1,2 Method 8338 0 25,000 mg/l as CaCO 3 Buret Titration Scope and application: For water, wastewater and seawater. 1 USEPA accepted
Hardness, Total, Sequential DOC316.53.01159 Titration Method with EDTA 1,2 Method 8338 0 25,000 mg/l as CaCO 3 Buret Titration Scope and application: For water, wastewater and seawater. 1 USEPA accepted
INVESTIGATION : Determining Osmolarity of Plant Tissue
 INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
Chapter MEMBRANE TRANSPORT
 Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE
 EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE This is a team experiment. Each team will prepare one set of reagents; each person will do an individual unknown and each team will submit a single report.
EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE This is a team experiment. Each team will prepare one set of reagents; each person will do an individual unknown and each team will submit a single report.
Most of the ethanol that is used as a biofuel in this country is produced from corn.
 Chem 251 Ethanol from Corn Most of the ethanol that is used as a biofuel in this country is produced from corn. In this experiment you will make ethanol from frozen corn kernels using a process similar
Chem 251 Ethanol from Corn Most of the ethanol that is used as a biofuel in this country is produced from corn. In this experiment you will make ethanol from frozen corn kernels using a process similar
COLE-PARMER REPLACEABLE MEMBRANE SCIENTIFIC SURFACTANT ION ELECTRODES INSTRUCTION MANUAL
 COLE-PARMER REPLACEABLE MEMBRANE SCIENTIFIC SURFACTANT ION ELECTRODES INSTRUCTION MANUAL GENERAL INSTRUCTIONS Introduction The Cole-Parmer Surfactant Electrode indicates the potentiometric endpoint when
COLE-PARMER REPLACEABLE MEMBRANE SCIENTIFIC SURFACTANT ION ELECTRODES INSTRUCTION MANUAL GENERAL INSTRUCTIONS Introduction The Cole-Parmer Surfactant Electrode indicates the potentiometric endpoint when
Determination of Langelier Index in Water
 Determination of Langelier Index in Water Based on Hach method 8073 ISO 10523: 2008 ISO 6059: 1984 ISO 9963: 1994 DOC316.52.93108 Total Alkalinity and Ca Hardness: 10-1000 mg/l CaCO3 1. Introduction Langelier
Determination of Langelier Index in Water Based on Hach method 8073 ISO 10523: 2008 ISO 6059: 1984 ISO 9963: 1994 DOC316.52.93108 Total Alkalinity and Ca Hardness: 10-1000 mg/l CaCO3 1. Introduction Langelier
GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011
 GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011 Peter C. Webb 1 *, Michael Thompson 2, Philip J. Potts 1 and Stephen
GeoPT28 AN INTERNATIONAL PROFICIENCY TEST FOR ANALYTICAL GEOCHEMISTRY LABORATORIES REPORT ON ROUND 28 (Shale, SBC-1) / January 2011 Peter C. Webb 1 *, Michael Thompson 2, Philip J. Potts 1 and Stephen
Inspirational chemistry 97. Index sheets. Rhubarb contains oxalic acid, which has the formula C H 3. O + 2 Mn CO 2
 Inspirational chemistry 97 Rates and rhubarb Index 4.1.3 3 sheets Rhubarb contains oxalic acid, which has the formula C 2 H 2 O 4 : O OH C C HO Figure 1 Structural formula of oxalic acid Oxalic acid reacts
Inspirational chemistry 97 Rates and rhubarb Index 4.1.3 3 sheets Rhubarb contains oxalic acid, which has the formula C 2 H 2 O 4 : O OH C C HO Figure 1 Structural formula of oxalic acid Oxalic acid reacts
Biodiesel Fundamentals for High School Chemistry Classes. Laboratory 3: Determination of the Acid Number of Vegetable Oils by Titration
 Laboratory 3: Determination of the Acid Number of Vegetable Oils by Titration Topics Covered ph vs. acid number Acidity and acid values in organic solutions Titration techniques How to obtain acid values
Laboratory 3: Determination of the Acid Number of Vegetable Oils by Titration Topics Covered ph vs. acid number Acidity and acid values in organic solutions Titration techniques How to obtain acid values
Change to read: BRIEFING
 BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
CHY3F. (Jan11CHy3f01) General Certificate of Secondary Education Foundation Tier January Unit Chemistry C3. Written Paper TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier January 2011 1 2 Chemistry
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier January 2011 1 2 Chemistry
To understand osmosis, we must focus on the behavior of the solvent, not the solute.
 GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
Determination of sodium in margarine manufacture
 Titration Application Note H 124 Determination of sodium in margarine manufacture This Application Note describes the determination of the total sodium content of precursor solutions used in margarine
Titration Application Note H 124 Determination of sodium in margarine manufacture This Application Note describes the determination of the total sodium content of precursor solutions used in margarine
Determination of the total acid number in petroleum products
 Branch General analytical chemistry; petrochemistry Keywords Titration; nonaqueous titration; potentiometric titration; Solvotrode easyclean, Optrode; Thermoprobe; photometric titration; thermometric titration;
Branch General analytical chemistry; petrochemistry Keywords Titration; nonaqueous titration; potentiometric titration; Solvotrode easyclean, Optrode; Thermoprobe; photometric titration; thermometric titration;
Name: Date: AP Biology LAB : FACTORS INFLUENCING ENZYME ACTIVITY
 LAB : FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts is that the
LAB : FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts is that the
How would you prepare 455 grams of an aqueous solution that is 6.50% sodium sulfate by mass?
 62 How would you prepare 455 grams of an aqueous solution that is 6.50% sodium sulfate by mass? Start a concentration calculation by writing the definition of the unit(s) you're using! We know everything
62 How would you prepare 455 grams of an aqueous solution that is 6.50% sodium sulfate by mass? Start a concentration calculation by writing the definition of the unit(s) you're using! We know everything
1. ZOLLINGER - ELLISON SYNDROME
 1. ZOLLINGER - ELLISON SYNDROME Zollinger Ellison syndrome is characterized by gastric hypersecretion, ulceration (ulceration) and gastrin-producing tumor. Gastrin is a peptide hormone synthesized by the
1. ZOLLINGER - ELLISON SYNDROME Zollinger Ellison syndrome is characterized by gastric hypersecretion, ulceration (ulceration) and gastrin-producing tumor. Gastrin is a peptide hormone synthesized by the
It is recommended to use titanium coils. ph meter to control ph of the process. It is advised to use an automatic dosage for the maintenance products.
 CRODA III Decorative Trivalent Chrome Process CRODA III is a hexavalent free chrome process, allowing to obtain slightly blue and bright deposits, optimal for decorative applications. ADVANTAGES Depuration
CRODA III Decorative Trivalent Chrome Process CRODA III is a hexavalent free chrome process, allowing to obtain slightly blue and bright deposits, optimal for decorative applications. ADVANTAGES Depuration
Total Acid Number in petroleum products by automatic titration
 APPLICATION NOTE Total Acid Number in petroleum products by automatic titration No. T3 Water Analysis Instruments, Thermo Fisher Scientific Key words TAN, ASTM D664, ISO 6619, oil, used oil, lubricant,
APPLICATION NOTE Total Acid Number in petroleum products by automatic titration No. T3 Water Analysis Instruments, Thermo Fisher Scientific Key words TAN, ASTM D664, ISO 6619, oil, used oil, lubricant,
SECTIOn 3. Multi-Element ClP Standards for ICP & ICP-MS
 SECTIOn 3 Multi-Element for ICP & ICP-MS Your Science is Our Passion. Multi-Element for ICP & ICP-MS Every CLP standard allows you to Calibrate With Condfidence. The following standards are to be used
SECTIOn 3 Multi-Element for ICP & ICP-MS Your Science is Our Passion. Multi-Element for ICP & ICP-MS Every CLP standard allows you to Calibrate With Condfidence. The following standards are to be used
Laboratory: Patriotic Colors
 Laboratory: Patriotic Colors Report Requirement: Answer all of the questions/do all the computations requested in italics. Questions not in italics do NOT need to be answered. You do NOT have to write
Laboratory: Patriotic Colors Report Requirement: Answer all of the questions/do all the computations requested in italics. Questions not in italics do NOT need to be answered. You do NOT have to write
Experiment Optional #2: The Synthesis of Aspirin
 Experiment Optional #2: The Synthesis of Aspirin The natural world provides us with many of the medications in common use today. Taxol is the common name of a medication used in treating certain cancers;
Experiment Optional #2: The Synthesis of Aspirin The natural world provides us with many of the medications in common use today. Taxol is the common name of a medication used in treating certain cancers;
Copyright 2016 Dan Dill 1
 carbonate These solutions are mixed and a precipitate forms. After the precipitation, the solution 1. will be positively charged 2. will be electrically neutral 3. will be negatively charged 4. More information
carbonate These solutions are mixed and a precipitate forms. After the precipitation, the solution 1. will be positively charged 2. will be electrically neutral 3. will be negatively charged 4. More information
Matrix Interferences in ICP-MS: Causes, Effects, and Strategies to Reduce or Eliminate Them
 Matrix Interferences in ICP-MS: Causes, Effects, and Strategies to Reduce or Eliminate Them Ruth E. Wolf, Ph. D. and Daniel H. Jones HUMAN HEALTH ENVIRONMENTAL HEALTH 2017 2014 PerkinElmer Matrix Effects
Matrix Interferences in ICP-MS: Causes, Effects, and Strategies to Reduce or Eliminate Them Ruth E. Wolf, Ph. D. and Daniel H. Jones HUMAN HEALTH ENVIRONMENTAL HEALTH 2017 2014 PerkinElmer Matrix Effects
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation
 EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
Corn Starch Analysis B-47-1 PHOSPHORUS
 Corn Starch Analysis B-47-1 PHOSPHORUS PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized
Corn Starch Analysis B-47-1 PHOSPHORUS PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized
SOUTH AFRICAN NATIONAL STANDARD
 ISBN 978-0-626-31165-0 SOUTH AFRICAN NATIONAL STANDARD Water Sulfide content Published by SABS Standards Division 1 Dr Lategan Road Groenkloof Private Bag X191 Pretoria 0001 Tel: +27 12 428 7911 Fax: +27
ISBN 978-0-626-31165-0 SOUTH AFRICAN NATIONAL STANDARD Water Sulfide content Published by SABS Standards Division 1 Dr Lategan Road Groenkloof Private Bag X191 Pretoria 0001 Tel: +27 12 428 7911 Fax: +27
Fundamentals of Analytical Chemistry
 Homework Fundamentals of Analytical Chemistry Chapter Principles of Neutralization Titrations 3, 9-159 odd, 1, 19, 1, 5, 7, 3, 3, 3, 3,, 5 Problems in green may or may not be part of the assignment, depending
Homework Fundamentals of Analytical Chemistry Chapter Principles of Neutralization Titrations 3, 9-159 odd, 1, 19, 1, 5, 7, 3, 3, 3, 3,, 5 Problems in green may or may not be part of the assignment, depending
Qualitative test of protein-lab2
 1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
NAME: OPTION GROUP: WJEC
 NAME: OPTION GROUP: WJEC MATHS FOR AS BIOLOGY MAKING SOLUTIONS MATHS COVERED IN THIS BOOKLET ARE: 1. Revision of scientific notation. 2. Molar solutions. 3. Concentrations. 4. Volumes of liquids. 5. Dilutions.
NAME: OPTION GROUP: WJEC MATHS FOR AS BIOLOGY MAKING SOLUTIONS MATHS COVERED IN THIS BOOKLET ARE: 1. Revision of scientific notation. 2. Molar solutions. 3. Concentrations. 4. Volumes of liquids. 5. Dilutions.
Chemistry Thermodynamics Neatly answer all questions completely for credit. Show all work.
 Teacher Notes Time: 90 minutes (plus 30 minutes for teacher preparation) Student Difficult: moderate Purpose: Design and model processes, materials, and equipment that might be used to build a device that
Teacher Notes Time: 90 minutes (plus 30 minutes for teacher preparation) Student Difficult: moderate Purpose: Design and model processes, materials, and equipment that might be used to build a device that
Feedstuffs Analysis G-22-1 PROTEIN
 Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
Feedstuffs Analysis G-22-1 PROTEIN PRINCIPLE SCOPE Many modifications of the Kjeldahl method have been accepted for the estimation of protein in organic materials. It comprises sample oxidation and conversion
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A. Ac-ag...Au-Zr. Bearbeitet von B Predel
 Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology - New Series 12A Ac-ag...Au-Zr Bearbeitet von B Predel 1. Auflage 2006. Buch. XXX, 334 S. ISBN 978 3 540 43534 1
Used to pick up or hold hot objects Protects the eyes from flying objects or chemical splashes
 Chemistry Ms. Ye Name Date Block Do Now: Name that equipment! Label the piece of equipment appropriately. Not all names will be used. Object Name Used For Used to pick up or hold hot objects Protects the
Chemistry Ms. Ye Name Date Block Do Now: Name that equipment! Label the piece of equipment appropriately. Not all names will be used. Object Name Used For Used to pick up or hold hot objects Protects the
EXPT. 10 DETERMINATION OF ZINC BY PRECIPITATION WITH POTASSIUM FERROCYANIDE USING INTERNAL INDICATOR
 EXPT. 10 DETERINATION OF ZINC BY PRECIPITATION WITH POTASSIU FERROCYANIDE USING INTERNAL INDICATOR Structure 10.1 Introduction Objectives 10. Principle 10.3 Requirements 10. Solutions Provided 10.5 Procedure
EXPT. 10 DETERINATION OF ZINC BY PRECIPITATION WITH POTASSIU FERROCYANIDE USING INTERNAL INDICATOR Structure 10.1 Introduction Objectives 10. Principle 10.3 Requirements 10. Solutions Provided 10.5 Procedure
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB5009.85-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.85-2016
Translated English of Chinese Standard: GB5009.85-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.85-2016
REEF CARE PROGRAM / Reef Colors. Reef Foundation. Testing and Supplementing
 GB REEF CARE PROGRAM / Reef Colors Reef Foundation Testing and Supplementing Red Sea s Reef Care Program The complete Reef Care program is the result of years of research into the physiological demands
GB REEF CARE PROGRAM / Reef Colors Reef Foundation Testing and Supplementing Red Sea s Reef Care Program The complete Reef Care program is the result of years of research into the physiological demands
SAC 17 Queen Mary University of London
 East Anglia Region of the RSC Analytical Division National Schools Analyst Competition Regional Heat Queen Mary University of London, Friday 24 th February 2017 School of Biological and Chemical Sciences,
East Anglia Region of the RSC Analytical Division National Schools Analyst Competition Regional Heat Queen Mary University of London, Friday 24 th February 2017 School of Biological and Chemical Sciences,
Bottom Ash Data Week 37
 Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
Bottom Ash Data 2018 Week 37 The following analytical report was sent to the Ministry of Environment and Climate Change Strategy on October 2, 2018. The data represents bottom ash composite results for
