REVIEW OF LITERATURE. Oral cavity is the foremost part of digestive system of human body because of its
|
|
|
- Alfred Hampton
- 5 years ago
- Views:
Transcription
1 REVIEW OF LITERATURE 4.1Introduction to Oral Cavity 4.1.1Oral Cavity Oral cavity is the foremost part of digestive system of human body because of its excellent accessibility and better patient compliance, oral mucosal cavity offers attractive route of drug administration for the local and systemic therapy [88] Overview of oral cavity Oral cavity is the area of mouth delineated by the lips, cheeks, hard palate, soft palate and floor of mouth. The oral cavity consists of two regions, 1. Outer oral vestibule, bounded by cheeks, lips, teeth and gingiva (gums). 2. Oral cavity proper, which extends from teeth and gums back to the faces (which lead to pharynx) with the roof comprising the hard and soft palate. The tongue projects from the floor of the cavity. Figure 1: Structure of oral cavity Dept of Pharmaceutics, JSSCP, Mysore Page 15
2 4.1.3 Anatomy of the Oral Mucosa The oral mucosa consists of three distinctive layers i.e., epithelium, basement membrane and connective tissues. The outer most layer is the epithelium, below which lies the supporting basement membrane. The basement membrane is, in turn, supported by connective tissues. Figure 2: Structure of oral mucosa The epithelium forms a protective layer for the tissues beneath and is divided into (a) non-keratinized epithelium in the mucosal lining of the soft palate, the ventral surface of the tongue, the floor of the mouth, alveolar mucosa, vestibule, lips, and cheeks, and (b) keratinized epithelium which is found in the hard palate and non-flexible regions of the oral cavity [89]. The epithelial cells increase in size and become flatter from the basal Dept of Pharmaceutics, JSSCP, Mysore Page 16
3 layers to the superficial layers. The oral mucosal thickness varies depending on the site, the buccal mucosa measuring about µm [90].The basement membrane forms a distinctive layer between the connective tissues and the epithelium. It provides the required adherence between the epithelium and the underlying connective tissues, and functions as a mechanical support for the epithelium. The underlying connective tissues provide many of the mechanical properties of oral mucosa. The buccal epithelium is penetrated by tall and conical-shaped connective tissues. These tissues, which are also referred to as the lamina propria, consist of collagen fibers, a supporting layer of connective tissues, blood vessels, and smooth muscles [91]. Keratinized layers of the orals mucosa epithelia form a protective surface which is mechanically tough and resistant to physical insult and penetration by any foreign substances. Thus epithelia contain neutral lipids like ceramides and acrylceramides which are associated with barrier function. The non-keratinized mucosa is more mobile and found in regions of the oral cavity where a more flexible mucosa is required for functional purposes e.g. in the buccal and sublingual regions where mastication is predominant [92]. These epithelia do not contain acrylceramides and only have small amounts of ceramides. They also contain small amounts of neutral polar lipids mainly cholesterol sulfate and glucosyl ceramides [93] Biochemistry of oral mucosa All the layers of the oral mucosal membranes contain a large amount of protein in the form of tonafilaments, consisting at least seven proteins called keratins with molecular sizes of kilodaltons. Both keratinized and nonkeratinized tissues of varying thickness and composition are found in oral cavity. The difference between keratinized Dept of Pharmaceutics, JSSCP, Mysore Page 17
4 and non-keratinized epithelia is merely the difference in the molecular size of existing keratins. Cells of non-keratinized epithelia contain lower molecular weight protein while those in keratinized epithelia contain mainly higher-molecular weight keratins. The lipid content of the cells varies between tissues [94] Routes of drug transport across the oral mucosa The epithelial cell membrane is lipophilic while the intercellular space is relatively hydrophilic. The entire epithelium therefore consists of hydrophilic and lipophilic regions, where the hydrophilic region is narrow and tortuous around the lipophilic cell membrane. Two pathways for passive diffusion of the drug across the buccal mucosa arise as a result of these regions i.e. paracellular and transcellular routes [95]. The hydrophilic compounds due to their low partition coefficient (low solubility) cannot penetrate the lipophilic cell membrane and hence paracellular route (intercellular spaces) is preferred route for these compounds. The major limitation encountered by hydrophilic molecules is the limited surface area of the intercellular space and the tortuosity of this pathway. For lipophilic compounds, transcellular pathway is preferred due to their high partition coefficients. Also, since the surface area for this route is large and the path length is relatively short, they can easily penetrate through the lipophilic cell membrane. However, in general drugs preferentially move through the route that offers least resistance and can traverse both routes of transport simultaneously [96, 97]. Although majority of the compounds diffuse through the buccal mucosa by passive diffusion, a carrier mediated process has also been reported. Dept of Pharmaceutics, JSSCP, Mysore Page 18
5 4.1.6 Barriers to drug transport across buccal mucosa The rate and extent of absorption of drugs through the buccal mucosa is affected by the presence of barriers such as mucus, basement membrane, membrane coating granules and saliva. The major barrier exists in the outer one third of the epithelium. Membrane Coating Granules: The permeability barrier in the oral mucosa is a result of intercellular material derived from membrane coating granules (MCG). As the cells undergo differentiation, MCG's start forming at the upper third of the epithelium and appear to fuse with the plasma membrane so as to extrude their contents into the intercellular space. The membrane-coating granules found in nonkeratinizing epithelia are spherical in shape, membrane-bound and measure about 0.2 um in diameter. Permeation studies performed using the tracers such as horseradish peroxidase and lanthanum nitrate indicated that these tracers penetrated only through outermost layer or two of the cells when applied to the outer surface of the epithelium. In both keratinized and non-keratinized epithelia, the limit of penetration coincided with the level where the MCG's could be seen adjacent to the superficial plasma membrane of the epithelial cells [98]. These results demonstrated that keratinization does not play a role in the barrier formation, while the MCGs serve as barriers to the transport of drug molecules through the epithelium. Basement Membrane: Although the superficial layers of the oral epithelium represent the primary barrier to the permeation of drugs, the basement membrane may also present some resistance to the passage of materials across the junction between epithelium and the connective tissue. Permeation studies suggested that the basal region of pig buccal Dept of Pharmaceutics, JSSCP, Mysore Page 19
6 epithelium may represent a barrier to small substances such as β-blocking agents [99]. The charge on the constituents of the basal lamina may limit the rate of penetration of lipophilic compounds that can traverse the superficial epithelial barrier relatively easily. Mucus: The epithelial cells of buccal mucosa are surrounded by the intercellular ground substance called mucus. The mucus has a thickness range of µm and consists principally of complexes made up of proteins and carbohydrates. In the oral mucosa, mucus is produced by major and minor salivary glands as a part of saliva. Minor salivary glands contribute approximately 70 % of the total mucin found in saliva. Mucus serves as an effective delivery vehicle by acting as a lubricant, allowing cells to move relative to one another and is believed to play a major role in adhesion of mucoadhesive drug delivery systems [100]. At physiological salivary ph ( ), mucus carries a negative charge and is capable of forming a strongly cohesive gel structure that binds to the epithelial cell surface as a gelatinous layer. Substances such as ions, protein chains, and enzymes can modify the interaction of the mucus molecules with the active substances. Saliva is mainly produced by the three pairs of salivary glands and is composed of 99% water and 1% organic and inorganic materials. It moistens the oral cavity and functions to protect all of the tissues of the oral cavity from abrasion by rough materials and chemicals. There is considerable variation in the salivary flow between individuals, with time of day and during disease conditions. The amount of saliva produced throughout the day varies from L, but can also be as low as L [101]. Salivary secretion can be stimulated by mechanical (pressure), chemical (swell, taste) and/or psychic stimuli. Acid stimuli can increase saliva secretion up to 2 to 3 fold its normal rate. The ph of the saliva ranges from depending on the flow rate. At higher flow rates, the higher Dept of Pharmaceutics, JSSCP, Mysore Page 20
7 sodium and bicarbonate concentrations lead to an increase in ph of saliva. The presence of saliva may be considered as a positive or negative factor for oral transmucosal delivery of drugs. For oral mucosal delivery saliva serves as the dissolution medium. The continuous secretion of saliva into the oral cavity provides the ample supply of solvent to dissolve the drug prior to its absorption. The mucin found in saliva forms a thin film that lines the membranes of oral cavity and provides an opportunity to retain the dosage form in contact with the mucosa for prolonged periods by incorporating mucoadhesive compounds in the formulation. Such a system would be in close contact with the absorbing membrane thus optimizing the drug concentration gradient across the membrane and reducing the diffusional pathway. However, excessive salivary flow can result in dilution and or accidental swallowing of the dissolved drug resulting in a decreased absorption. Also, pathological conditions such as xerostomia or drugs that patient may be taking concomitantly may decrease the salivary flow and decrease the drug absorption. 4.2 Buccal as a site for drug delivery The buccal route has been studied as a favorable site for the local and systemic delivery of drugs. Because of the rich blood supply and direct access to systemic circulation, the oral mucosa suitable for drugs, which are susceptible to acid hydrolysis in the stomach are extensively metabolized in the liver. The continuous secretions of saliva results in rapid removal of released drug and hence the oral cavity should be restricted to the delivery of drugs, which have a short systemic circulation. The buccal and gingival areas are associated with a smaller flow of saliva, thus the duration of adhesion of the delivery system would be longer at these areas than the sublingual region. The buccal or Dept of Pharmaceutics, JSSCP, Mysore Page 21
8 gingival membrane, with their accessible, smooth surface offers a platform for prolong delivery of drugs Advantages of mucoadhesive buccal drug delivery [102] Drug administration via the oral mucosa offers several advantages 1. Ease of administration and termination of therapy in emergency. 2. Permits localization of the drug to the oral cavity for a prolonged period of time. 3. Can be administered to unconscious and trauma patients. 4. Offers an excellent route for the systemic delivery of drug which bypasses first pass metabolism, thereby offering a greater bioavailability. 5. Significant reduction in dose can be achieved, thereby reducing dose, dose dependent side effects, and eliminates peak-valley profile. 6. Drugs which are unstable in acidic environment of stomach or are destroyed by the enzymatic or alkaline environment of the intestine can be administered by this route. 7. It offers a passive system for drug absorption and does not require any activation. 8. It can be made unidirectional to ensure only buccal absorption. 9. Drugs which show poor bioavailability via the oral route can be administered conveniently. 10. It allows for the local modification of tissue permeability, inhibition of protease activity or reduction in immunogenic response. Thus, selective use of therapeutic agents like peptides, proteins and ionized species can be achieved. 11. Flexibility in physical state, shape, size and surface. Dept of Pharmaceutics, JSSCP, Mysore Page 22
9 12. Maximized absorption rate due to intimate contact with the absorbing membrane and decreased diffusion barriers. 13. It satisfies several features of the controlled release system. 14. The buccal mucosa is highly perfused with blood vessels and offers a greater permeability than skin. 15. The oral mucosa lacks prominent mucus secreting goblet cells and therefore there is no problem of diffusion limited mucus buildup beneath the applied dosage form. 16. Rapid onset of action Limitation of buccal drug administration [103] 1. Drugs which are unstable at buccal ph cannot be administered. 2. Drugs which irritate the mucosa or have a bitter or unpleasant taste or an obnoxious odour cannot be administered by this route. 3. Only drug with small dose requirement can be administered. 4. Only those drugs which are absorbed by passive diffusion can be administered by this route. 5. Eating and drinking may become restricted. 6. There is an ever present possibility of the patient swallowing the dosage form. 7. Over hydration may lead to slippery surface and structural integrity of the formulation may get disrupted. Swelling and hydration of the bioadhesive polymers may occur. 8. Drugs contained in the swallowed saliva follows the peroral route and the advantages of buccal route are lost. Dept of Pharmaceutics, JSSCP, Mysore Page 23
10 4.3 FORMULATIONS: Buccal adhesive dosage forms are broadly classified in following dosage forms Solid buccal adhesive dosage forms Semi-solid buccal adhesive dosage forms Liquid buccal adhesive dosage forms Solid buccal adhesive dosage forms Tablets: Buccal tablets are small, flat, and oval, with a diameter of approximately 5 8 mm. Unlike conventional tablets, buccal mucoadhesive tablets allow for drinking and speaking without major discomfort. They soften, adhere to the mucosa, and are retained in position until dissolution and/or release is complete. These tablets can be applied to different sites in the oral cavity, including the palate, the mucosa lining the cheek, as well as between the lip and the gum. Buccal tablets are designed to release the drug either unidirectional targeting buccal mucosa or multidirectional in to the saliva. However, size is a limitation for tablets as they have to have intimate contact with the mucosal surface. Buccal tablets are usually prepared by direct compression, but wet granulation techniques can also be used. Multilayered tablet may be prepared by sequentially adding and compressing the ingredients layer by layer. Some newer approaches use tablets that melt at body temperature. Commercially available buccoadhesive buccal tablets are Buccastem (Prochlorperazine Maleate), Suscard (Nitroglycerine), Fentora (Fentanyl), Striant SR (30 mg testosterone), Oravig (miconazole), Corlan (2.5mg Hydrocortisone). Buccal Patch/Film: Patches are laminates consisting of an impermeable backing layer, a drug-containing reservoir layer from which the drug is released in a controlled manner, Dept of Pharmaceutics, JSSCP, Mysore Page 24
11 and a bioadhesive surface for mucosal attachment. Two methods used to prepare adhesive patches include solvent casting and direct milling. In the solvent casting method, the intermediate sheet from which patches are punched is prepared by casting the solution of the drug and polymer(s) onto a backing layer sheet and subsequently allowing the solvent(s) to evaporate. In the direct milling method, formulation constituents are homogenously mixed and compressed to the desired thickness, and patches of predetermined size and shape are then cut or punched out. An impermeable backing layer may also be applied to control the direction of drug release, prevent drug loss, and minimize deformation and disintegration of the device during the application period Semi-solid buccal adhesive dosage forms Buccal Gel/Ointment: Bioadhesive polymers include crosslinked polyacrylic acid that has been used to adhere to mucosal surfaces for extended periods of time and provide controlled release of drugs. These are widely used in the delivery of drugs to the oral cavity. They have the ability to form intimate contact with the mucosal membrane and release rapidly the drug at the absorption site. One of the oral mucosal adhesive delivery systems-"orabase R'' consists of finely ground pectin, gelatin and sodium carboxymethylcellulose ("Orahesive" Powder) dispersed in a poly(ethylene) and a mineral oil gel base, which can be maintained at its site of application for min. This has been used for the local application of steroids for the treatment of mucosal ulceration. Other commercially available bioadhesive gels are Daktarin oral gel (Miconazole), Pansoral gel buccal (Choline salicylate). Mucosal-adhesive ointment was a formulation containing polymethyl methacrylate in a base containing water, sodium hydroxide, glycerol and the active drug (tretinoin) which was used to treat lichen planus. Dept of Pharmaceutics, JSSCP, Mysore Page 25
12 4.3.3 Liquid dosage forms Liquid dosage forms like viscous liquids may be used to coat buccal surface either as protectants or as drug vehicles for delivery to the mucosal surface. Polymers were used to enhance the viscosity of products to aid their retention in the oral cavity. Eg.Buccolam contains Midazolam Hydrochloride 5 mg in 1ml and Epistatus contains Midazolam Maleate 10 mg in 1ml. 4.4 Introduction to Vagina Anatomy and histology of vagina Vagina is a fibromuscular tube that extends 6-12 cm from cervix of the uterus. The surface of the vagina is composed of numerous folds, often called rugae. These folds keep distensability, support and provide an increased surface area of the vaginal wall [104]. The vaginal wall is compromised of three layers: the epithelial layer, tunica adventia and the muscular coat. The epithelial layer creates the superficial layer, which is about 200 μm thick [105]. The smooth muscular fibres of the muscular coat are running along in both circular and longitudinal directions. This gives the vagina an excellent elastic character. Further, the connective tissue of the tunica adventia also increases the flexibility of the vagina. The vaginal wall compromises of a dense network of blood vessels and extends from internal iliac artery, uterine, middle rectal and internal pudental arteries [106]. The blood enters systemic circulation via rich venous plexus which empties primarily into internal iliac vein. The vagina lacks the direct release of mucus because it does not contain any goblet cells. But still it discharges a large amount of fluid. The fluid has its origin from transudates through the epithelium, cervical mucus, exfoliating cells, leukocytes, endometrial and tubal fluids [107]. The vagina s nerve Dept of Pharmaceutics, JSSCP, Mysore Page 26
13 supply comes from two sources. The peripheral, which primarily supplies the lower quarter of the vagina, makes it a highly sensitive area: the autonomic primarily supplies upper three quarters. Autonomic fibres respond to stretch and are not very sensitive to pain or temperature. The upper vagina is a very insensitive area because of few sensory fibres. This is why women rarely feel localized sensations or any discomfort when using vaginal rings, and are often unaware of the presence of such items in vagina [108]. The lactobacilli bacteria are an important component of the vaginal microflora. This bacteria converts glycogen from exfoliated epithelial cells into lactic acid, and as a result, maintains the ph around 4-5, with lowest being around the cervix. Body fluids such as menstrual blood, cervical and uterine secretions will all act as alkalizing agents and increase the vaginal ph. Figure 3: Schematic representation of vaginal wall Dept of Pharmaceutics, JSSCP, Mysore Page 27
14 4.4.2 Physiological changes and the influence on drug absorption Women in post-menopausal stage experiences many physiologically alterations. These changes manifests as reduction in production of estrogen through pre-and postmenopause. This leads to reduced glycogen content and elevated ph to due to less conversion of lactic acid from glycogen by lactobacillus. The increased vaginal ph could often result in frequent vaginal infections. Furthermore, reduced vaginal discharges and thinning of epithelial layer occurs. The reduction is estimated to 50 % compared to those produced by women of reproductive age. Environmental changes in fertile women are associated with hormonal events in menstrual cycle. The epithelial layer thickness is changed approximately μm during menstrual cycle. The ph is around and tends to be lowest when estrogen is highest (ovulation). In this period, glycogen and epithelial desquamation is at its maximum which causes an increased viscosity. The physiological changes in vagina would affect the absorption of drugs and must be taken into consideration in the development of formulations. The thickness of epithelial layer could affect the permeability, where thin epithelium causes increased absorption. Amount of fluid is important because drugs must be in solution before absorption. For bioadhesive systems, wetting of the tablet is crucial for bioadhesion and increasing the residence time [109]. Viscosity may pose as a barrier for drug absorption and continuous secretion can result in removal of the dosage form. The influence of ph is important for absorption, where the unionized form is more readily absorbed Vaginal secretions Vaginal discharge is a mixture of several components including transudates through the epithelium, cervical mucus, exfoliating epithelial cells, secretions of the Bartholin s and Dept of Pharmaceutics, JSSCP, Mysore Page 28
15 Skene s glands, leukocytes, endometrial and tubal fluids. The cervical mucus contains inorganic and organic salts, mucins, proteins, carbohydrates, urea and fatty acids (lactic and acetic acids). Estrogens and sexual stimulation increase vaginal fluid secretion Vaginal ph The vaginal ph of healthy women of reproductive age is acidic (ph 4 5); this value is maintained by lactobacilli that convert glycogen from exfoliated epithelial cells into lactic acid. The ph changes with age, stages of menstrual cycle, infections and sexual arousal. Menstrual, cervical and uterine secretions and semen act as alkalizing agents and increase ph Microflora The vaginal flora is a dynamic and closely interrelated system. The ecology of the vagina is influenced by factors such as the glycogen content of epithelial cells, glucose, ph, hormonal levels, and trauma during sexual intercourse, birth-control method, age, antimicrobial treatment and delivery. Lactobacillus (Doderlein s bacilli) is the most prevalent organism in the vaginal environment together with many other facultative and obligate aerobes and anaerobes Cyclic changes The changes in hormone levels (especially estrogen) during the menstrual cycle lead to alterations in the thickness of the epithelial cell layer, width of intercellular channels, ph and secretions. The variations in enzyme activity (endopeptidases and amino peptidases) with hormonal changes further complicate the problem of achieving consistent drug delivery. Dept of Pharmaceutics, JSSCP, Mysore Page 29
16 4.5 Vagina as Site for Drug Therapy The effectiveness of the vagina as a site of drug administration for local effects has been well established [110]. It is an important route for local treatment of several gynecological conditions, such as infections and in hormonal therapy. This route provides advantages such as reducing or eliminating the incidence and severity of side effects, being easily and a non-invasive route of administration. These benefits could contribute to a better compliance, thus achieving improved therapeutic outcome [111]. Furthermore, the vagina possesses properties which include: large surface area of the vaginal wall, permeability, a rich blood supply and importantly, the ability to bypass first-pass liver metabolism [112]. These properties are considered to be advantageous in relation to drug absorption. Currently, there are varieties of pharmaceutical products available on the market designed for intravaginal therapy (tablets, creams, suppositories, pessaries, foams, solutions, ointments and gels). However, their efficacy is often limited by a poor retention at the site of action due to the self-cleansing action of the vaginal tract [113]. Furthermore, the vagina has unique features in terms of microflora, ph and cyclic changes, and these factors influence the performance of the formulations. Therefore, a successful delivery of drugs through the vagina represents a pharmaceutical challenge Advantages of vaginal drug delivery 1. Vaginal route can be used for local as well as systemic effect, attaining sustained therapeutic levels compared to conventional oral route. Dept of Pharmaceutics, JSSCP, Mysore Page 30
17 2. Vaginal administration permits use of prolonged dosing, with continuous release of medicaments, and hence, longer interval between doses, improving user compliance. 3. Avoiding the fluctuations resulting from daily intake may also lower the incidence of side effects. For example, vaginal delivery of bromocriptine reduces the incidence and severity of its gastrointestinal (GI) side-effects if administered orally. 4. Alleviating the inconvenience caused by pain, tissue damage and probable infection, it serves as a better alternative to parenteral route. 5. Some vaginal dosage forms allow self-insertion and removal of the dosage form like vaginal films, gel, pessaries, etc. 6. Unpredictable GI absorption consequent upon oral administration can be further complicated by vomiting, drug-drug interaction, or decreased intestinal absorption. 7. Avoiding metabolizing in liver and degradation in GI lumen for some compounds. For example, natural oestrogens are 95 percent metabolized by the liver when administered orally. Similarly, propranolol is more bioavailable when administered vaginally, compared to oral. 8. A number of compounds have been reported to exert greater effects when administered vaginally as against oral. For example, misoprostol, used for cervical ripening and labour induction, when administered vaginally has been shown to be more effective, having fewer side effects than when administered orally. Dept of Pharmaceutics, JSSCP, Mysore Page 31
18 4.5.2 Drawbacks of vaginal drug delivery 1. Unawareness and gender-specificity 2. Genital hygiene issues 3. Menstrual cycle-associated vaginal changes 4. Coitus interference 5. Local side effects 6. Variable drug permeability 4.6. Formulations The following types of dosage forms are available Vaginal semisolids: Vaginal semisolids dosage forms are vaginal creams, ointments and gels. These semisolid vaginal preparations are used mainly in conditions like infections, vaginitis, conditions of endometrial atrophy and for contraceptive purposes too. The vaginal topical preparations are mainly applied by special applicators. Anti-infective drugs (e.g. Nystatin, clotrimazole, miconazole, clindamycin, & sulfonamides); hormones (e.g. progesterone, dinesetrol) and contraceptives etc are applied by these dosage forms. Commercially available vaginal creams are Mycelex (Clotrimazole), AVC (Sulfanilamide), Cleocin (Clindamycin phosphate) and Terazol-7 (Terconazole) Vaginal Suppositories: Vaginal Suppositories are the most common dosage forms. Typically, these are torpedo-shaped dosage forms but in case of vagina, the oval shape is preferred. The composition is largely dictated by the physicochemical properties of the drug and the desired drug release profile. The most commonly used base for vaginal Dept of Pharmaceutics, JSSCP, Mysore Page 32
19 suppositories consist of combination of the various molecular weight polyethylene glycols & surfactants & preservatives. They are buffered to acidic ph of about 4.5. Commercially available vaginal suppositories are AVC suppositories (sulfanilamide) MONISTAT-7 (miconazole nitrate) Vaginal liquids: The vaginal douches and solutions are also available in market. They are used for irrigation cleansing of vagina. The unit dose douches are prepared which are mixed with warm water and applied by inserters in vagina Vaginal Aerosols: Aerosol foams containing estrogenic substances & contraceptive agents are available. The aerosol container has plunger which apply the foam in the vaginal cavity. Novel approach is use of bioadhesive foams. Marketed preparations like povidone-iodine vaginal foam and other contraceptive foams are available Vaginal rings: Medicated vaginal rings are fabricated from silastic 382 medical grade elastomer. Vaginal rings is revolution in contraceptive technology and hormone replacement therapy. This is due to their ease of administration, lack of gastrointestinal related symptoms, high efficacy and can be governed by the user. Marketed preparations like Estering vaginal ring (Estradiol) are available Vaginal inserts: These types of systems contains flat rectangular polymeric slab enclosed in a pouch of knitted polyester removal system. The buff colored semi transparent hydrogel slab contains drug. The retrieval system is in the shape of long knitted tape that is used to retrieve the slab. Marketed preparations like CERVIDIL are Dept of Pharmaceutics, JSSCP, Mysore Page 33
20 available. CERVIDIL is a vaginal insert of dinoprostone. It contains 10 mg of drug and release the drug at the rate of 0.3 mg/hr. It can give continuous release for up to 12 hrs In situ gelling: These systems are prepared by using temperature-sensitive and mucoadhesive polymers. The water insoluble polymers swells in vagina and form bioadhesive gel on vaginal layer. This allows continuous release up to 25 to 50 hrs. Commercially available in situ gelling vaginal formulation is Crinone gel containing progesterone Medicated Vaginal Tampons: A medicated vaginal tampon, approved as a medical device by the Food and Drug Administration (FDA) is available (Ela Tampon, Rostam, Israel). This bifunctional tampon contains a polymeric delivery system (strips) that absorb menstrual fluid while gradually releasing lactic acid and citric acid. Ex. Brilliant ph tampons Vaginal Films: Vaginal films are polymeric drug delivery systems shaped as thin sheets, usually ranging from 220 to 240 μm in thickness. These systems are often square (approximately 5 cm 5 cm) colorless and soft presenting a homogenous surface. Vaginal films are produced with polymers such as polyacrylates, polyethylene glycol, polyvinyl alcohol and cellulose derivatives. Ex. VCF vaginal contraceptive films. Dept of Pharmaceutics, JSSCP, Mysore Page 34
21 4.7 Introduction to Rectum Physiology and biopharmaceutical characteristics of rectum The colon consists of the ascending, transverse and descending colons which encircle the small intestine; the sigmoid colon, which turns medially and downward; the rectum; and the anal canal. The rectum is about cm long, and the anal canal is the last couple of centimetres of the colon that surrounds the anus. Clinically, the terminal end of the colon is usually referred to as the rectum [114]. The rectum has a good blood supply, and is characterized by absence of villi and a relatively small surface area ( ml 2 ). Rectum contains a small volume of viscous fluid ( ml) which spread over the surface, and which has a clearly limited buffer capacity. Based on these factors, many drugs which are well absorbed orally are poorly absorbed rectally, even when administered in solution. Even if well absorbed, it is quite common that blood levels may vary after rectal absorption [115,116] Permeability The effective permeability of drugs across the intestinal epithelium is influenced by several physico-chemical and physiological properties and may differ in various intestinal regions [117]. Studies report that the absorption of water occurred at a lower degree in the rectum when compared with other parts of the gastrointestinal tract. They estimated that it might be explained by a smaller pore radius, tighter epithelium, less fluidity in the rectal membrane, lower number of pores in the rectal region, and a decreased mucosal surface area [118,119]. It has been proposed that the electrolytes and water are transported transcellularly in the colon/rectum, which is different in a high permeable Dept of Pharmaceutics, JSSCP, Mysore Page 35
22 tissue in which the transport of electrolytes and water has been assumed to be by the paracellular route [120]. The tighter and less fluid rectal epithelium is probably due to a change in the lipid composition, such as increased cholesterol/phospholipid molar ratio and degree of saturation of fatty acid residues. Furthermore, Lennernas with his study group also suggest that an unstirred water layer is an essential factor since it might be thicker and more coherent in the colonic-rectal region [121] compared with what has been found in the jejunum in man [122]. The more pronounced effect of the absorption of drugs during an increased convective flow across the barrier is in agreement with indications that various absorption enhancers may be more effective in the colon/rectum compared with the small intestine [123]. Bile salts such as sodium glycocholate seem to bind with calcium ions and sodium caprate changes the pore size in the tight junctions of membranes. These promoters probably increase the permeability of membranes to hydrophilic macromolecules via the paracellular route. Sodium salicylate seems to increase transport by both paracellular and transcellular routes [124]. Nishihata [125] investigated the enhancement effect of salicylate on the rectal absorption of different types of drugs; theophylline as a neutral substance; lidocaine as a basic material; cefmetazole as acidic and levodopa which exists as a zwitterion in solution. Absorption of each drug was enhanced; particularly at ph values where the substances exist primarily in their ionic form. A requirement for the observed enhancement was that salicylate was present in the rectal membrane. Dept of Pharmaceutics, JSSCP, Mysore Page 36
23 4.7.3 Effect of ph The rectum as an absorption site has a higher ph (7-8) than that of the gastro-intestinal tract. It is not a favorable site for the absorption of most of the weak organic acids which have pka values lower than the ph of the rectum, because most of the drug molecules exist in an ionized form in the rectum. If the ph of the drug absorption site in the rectum was temporarily lowered below the pka value of the drug, increased rectal absorption of drug would be expected. Yagi and his study group [126] found that the mean areas under the plasma concentration-time curves (AUCs) following the administration of suppositories containing weak acids were larger than those of the suppositories containing bumetanide without weak acids (control) and those of an orally administered bumetanide suspension in rabbits. Moreover, the ph in the rectum decreased to between 2-4 for 30 minutes following the administration of the suppositories containing weak acids, like citric acid or tartaric acid. The ph of the rectal fluid is determined by the contents of the rectum, because of the lack of buffering capacity [127]. Consequently, the absorption of the drug could be improved by adding acids or bases to a formulation until the balance of ionized and unionized forms of the drug is optimal. In this way, it could be possible to improve the solubility of the drug in the rectal fluid and at the same time ensure sufficient permeability. On the other hand, the study of Crommelin et al [128] showed that the rectum is able to secrete neutralizing agents when the luminal ph deviates from the physiological ph. The degree of the secretion depends on the magnitude of the deviation. In general, the dissociation reaction of a drug is an equilibrium reaction. Thus, if some of the undissociated form of a drug is eliminated from the system, new undissociated drug will be formed to compensate Dept of Pharmaceutics, JSSCP, Mysore Page 37
24 [129]. Consequently, if the proportion of the undissociated drug is 1-2 % then it would be absorbed. 4.8 Rectal drug delivery Advantages of rectal drug delivery Useful in delivering drugs that cause nausea and vomiting. Irritation in stomach & small intestine associated with certain drugs can be avoided. Hepatic first pass elimination of high clearance drug may be avoided, also acidic & enzymatic degradation of the drug may be prevented. When oral intake is restricted, such as prior to x-ray studies or in patients with disease of upper GI tract or when the patient is unable to swallow. Useful in pediatric, geriatric & unconscious patients. Drug delivery can be stopped by removal of dosage form & drug absorption can be easily interrupted in case of accidental overdose or suicide attempts Disadvantages of rectal drug delivery Inconvenient for patients. The absorption of drugs is frequently irregular & difficult to predict. 4.9 Formulations The following rectal formulations are available Rectal semisolids: Rectal cream, gels and ointments are used for topical application to the perianal area for insertion within the anal canal. They largely are used to treat local conditions of anorectal pruritis, inflammation and pain and discomfort associated with hemorrhoids. The drugs include astringents (eg. Zinc oxide), protectants Dept of Pharmaceutics, JSSCP, Mysore Page 38
25 and lubricants (eg. Cocoa butter, lanolin), local anaestheics (eg. Pramoxine HCL), antipruritis and anti inflammatory agents (eg. Hydrocortisone). The bases used in anorectal creams and ointments includes combinations of polyethylene glycol 300 & 3350, emulsion cream bases using cetyl alcohol & cetyl esters wax, and white petroleum and mineral oil. The preservatives like methylparaben, propylparaben, benzlyacohol and butylated hydrocortisole (BHA) are also used. Several commercial rectal creams, ointments and gels available are - Anusol ointment (starch), Tronolane cream (pramoxine HCL), Analpram cream (Pramoxine), Diastat gel (Diazepam) Rectal suppositories: Rectal suppositories are the most common dosage form used for rectal drug administration and represent greater than 98 % of all rectal dosage forms. Typically, these are torpedo-shaped dosage forms composed of fatty bases (low-melting) or water-soluble bases (dissolving) which vary in weight from 1 g (children) to 2.5 g (adult). Lipophilic drugs are usually incorporated into water-soluble bases while hydrophilic drugs are formulated into the fatty base suppositories. For suppositories made from fatty bases, melting should occur rapidly near body temperature (37 C). Ideally the resultant melt would readily flow to provide thin, broad coverage of the rectal tissue, thereby minimizing lag time effects due to slow release of the drug from the suppository base. Water-soluble suppositories should likewise readily dissolve at 37 C to facilitate drug release and subsequent absorption. Commercially available suppository products are Dulcolax (Bisacodyl), Canasa (Mesalamine) Numorphan (Oxymorphane), Anusol HC (Hydrocortisone) Panadol (Paracetamol). Dept of Pharmaceutics, JSSCP, Mysore Page 39
26 4.9.3 Rectal liquids: Rectal suspensions, emulsions & solutions represent rectal dosage forms with very limited application, largely due to inconvenience of use and poor patient compliance. In many cases, these formulations are utilized to administer contrast media and imaging agents for lower GI roentgenography. Enema: These are used for local and systemic action. The drugs like hydrocortisone (local effect) or aminophylline (systemic effect) etc are used in this dosage form. Evacuation enema: These enemas are used for cleansing of bowel. The agents are sodium phosphate and sodium biphosphate, glycerin and doccusate potassium and light mineral oil. Rectal aerosols or foams: Rectal aerosol foam products are also accompanied by applicators to facilitate administration. The applicator is attached to the container and filled with a measured dose of product. Metered dose aerosols are available. The inserter is inserted in to the anus and the plunger is pushed to deliver the drug product. Marketed rectal aerosols are like Proctofoam HC, Cortifoam etc Bioadhesion Bioadhesion is an interfacial phenomenon in which two materials, at least one of which is biological, are held together by means of interfacial forces. The attachment could be between an artificial material and biological substrate, such as adhesion between polymer and / or co-polymer and a biological membrane. In case of polymer attached to the mucin layer of the mucosal tissue, the term mucoadhesion is employed. Bioadhesive is defined as a substance that is capable of interacting with biological material and being retained on them or holding them together for extended period of time. Dept of Pharmaceutics, JSSCP, Mysore Page 40
27 Bioadhesives are classified into three types based on phenomenological observation, rather than on the mechanism of bioadhesion [130]. Type-I: Bioadhesion is characterized by adhesion occurring between biological layers without involvement of artificial materials. Cell diffusion and cell aggregation are good examples. Type-II: Bioadhesion can be represented by cell adhesion onto culture dishes or adhesion to a variety of substances including metals, woods and other synthetic materials. Type-III: Bioadhesion can be defined as an adhesion of artificial substances to biological substrate such as adhesion of polymer to skin or other soft tissue Mechanism of bioadhesion Bioadhesion occur in 3 stages namely. Stage-1 Involves an intimate contact between a bioadhesive and a membrane either from a good wetting of the bioadhesive and a membrane or from the swelling of bioadhesive. Stage-2: After contact is established, penetration of the bio-adhesive into the surface of the tissue takes place. Stage-3: Inter penetration of the chains of the bioadhesive with those of the mucous takes place and the low chemical bonds settles down. Dept of Pharmaceutics, JSSCP, Mysore Page 41
28 The bond is established between the mucus and the biological substances mainly due to both physical and chemical interactions. Physical or mechanical bonds results from enlargement of the adhesive material and the extended mucus chains. Secondary chemical bonds may be due to electrostatic interaction, hydrophobic interactions, hydrogen bonding and dispersion forces Theories of bioadhesion / mucoadhesion Several theories have been proposed to explain the fundamental mechanism of adhesion. The surface characteristics, composition of the mucoadhesive materials as well as the substrate and the associated applied force to bring the adherence of substrate in contact, are important parameters to be considered in assessing mucoadhesion. The bonding occurs chiefly through both, physical and chemical interactions. a. Wetting theory Wetting theory is predominantly applicable to liquid bioadhesive systems and analyses adhesive and contact behavior in terms of a liquid or a paste to spread over a biological system. The work of adhesion [expressed in terms of surface and interfacial tension (Y)] is defined as energy per cm 2 released when an interface is formed. According to Dupres equation work of adhesion is given by: Wa = Y A + Y B - Y AB Where A & B refer to the biological membranes and the bioadhesive formulation respectively. The work of cohesion is given by: Wc = 2Y A or Y B Dept of Pharmaceutics, JSSCP, Mysore Page 42
29 For a bioadhesive material B spreading on a biological substrate A, the spreading coefficient is given by: S B/A = Y A - (Y B + Y AB ) S B/A should be positive for a bioadhesive material to adhere to a biological membrane. b. Diffusion theory According to this theory, the polymer chains and the mucus mix to a sufficient depth, to create a semipermanent adhesive bond. The bioadhesive material and the glycoprotein are brought into close contact. The polymer chains penetrate the mucous; the exact depth of penetration depends on the diffusion co-efficient, time of contact and other experimental variables. The diffusion co-efficient depends on the molecular weight and decreases rapidly as the cross-linking density increases as shown by Peppas. c. Electronic theory According to this theory, electronic transfer occurs upon contact of an adhesive polymer and the mucous glycoprotein network because of differences in their electronic structure. This result in the formation of an electronic double layer at the interface and adhesion occur due to attractive forces across the double layers. d. Fracture theory Fracture theory of adhesion is related to separation of two surfaces after adhesion. The fracture strength is equivalent to adhesive strength as given by G = (Eε/L)1/2 Dept of Pharmaceutics, JSSCP, Mysore Page 43
30 Where: E- Young's modules of elasticity ε - Fracture energy L - Critical crack length when two surfaces are separated e. Absorption theory According to this theory, after an initial contact between two surfaces, the materials adhere because of surface forces acting between the atoms in the two surfaces. Two types of chemical bonds resulting from these forces can be distinguished: (I) Primary chemical bonds covalent in nature, which are undesirable in as their high strength may result in permanent bonds. (II) Secondary chemical bonds having many different forces of attraction, including electrostatic forces, Vander Waals forces and hydrogen and hydrophobic bonds [131,132] Factors affecting muco / bioadhesion Structural and physicochemical properties of a potential bioadhesive material influence bioadhesion [133]. High molecular weight polymers are generally used for mucoadhesion and these polymers may be either natural or synthetic. Synthetic polymers are obtained by polymerization of monomers in presence or absence of cross-linking agents to give either a cross-linked water insoluble polymer or a linear polymer. Dept of Pharmaceutics, JSSCP, Mysore Page 44
31 Hydrogen bonding due to presence of hydrophilic groups such as -COOH or -OH plays a significant role in mucoadhesion. Studies have shown that strongly anionic polyelectrolytes particularly those with high charge density of -COOH or OH functionality are better candidates for bioadhesion than neutral molecules. The bioadhesive power of a polymer or of a series of polymers is affected by the nature of the polymer and also by the nature of the surrounding media. These factors are mainly divided in three classes: a. Polymer related factors b. Environment related factors c. Physiological variables Possible routes for drug transport across the oral mucosa The majority of drugs move across the epithelial membrane including the oral epithelia by passive mechanisms which are governed primarily by the laws of diffusion. In case of single diffusion, two potential routes of material transport across the epithelium are: 1. The paracellular pathway and 2. The transcellular pathway The paracellular route involves passage of molecules through the inter-cellular space, while transcellular route involving passage into and across the cell (intracellular). The most important property that determines whether a given non-electrolyte will pass rapidly across the oral mucosa seems to be its relative partition between lipid and water. Substances with a high solubility in lipid are expected to traverse the oral mucosa more Dept of Pharmaceutics, JSSCP, Mysore Page 45
32 easily moving along or across the lipid rich plasma membranes of the epithelial cells while water soluble substances and ions probably move through the intercellular spaces. Passive diffusion is undoubtedly the major transport mechanism for drugs; the nutrients from the mouth are shown to be absorbed by carrier systems i.e. facilitated diffusion. Transmucosal permeation of polar molecules, such as peptide based pharmaceuticals, may be by the way of paracellular route. However, several barriers exist during the course of paracellular permeation: 1. Basal lamina whose barrier function is dependent upon the molecular weight of the permeant molecules and its reactivity with the barrier as well as the structural and functional factors of the barrier. 2. Membrane coating granules, which extrude into the intercellular region of both keratinized and non-keratinized oral epithelium. 3. The keratin layer whose barrier function in oral mucosa is not as well defined as in the skin, although rate of permeation of water was shown to be greater in nonkeratinized than keratinized oral epithelium [134] Permeation enhancers Permeation enhancers are substances added to pharmaceutical formulation in order to increase the membrane permeation rate or absorption rate of a co-administered drug. They are used to improve bioavailability of drugs with normally poor membrane permeation properties. The permeation enhancer to be clinically accepted must increase bioavailability or increase membrane permeability without damaging the membrane and causing toxicity. Membrane permeation can be a limiting factor for many drugs Dept of Pharmaceutics, JSSCP, Mysore Page 46
33 administered via the buccal or sublingual routes. For those compounds that penetrate the oral mucosal surface slowly or incompletely and for which effective systemic absorption cannot be attained, one strategy that can be used by those interested in their buccal or sublingual delivery is the co-administration of the permeation enhancing excipient. Enhancer efficacy depends on the physiochemical properties of the drug, administration site, nature of the vehicle and whether enhancer is used alone or in combination. Differences in the cellular morphology, membrane thickness, enzymatic activity, lipid composition and potential protein interactions, are the structural and functional factors that vary the effectiveness of the enhancer from site to site [135] Mechanism of Buccal Absorption Enhancer The mechanism by which enhancers act are been poorly understood. Surfactants such as sodium lauryl sulphate interact at either the polar head groups or the hydrophilic tail regions of the molecules comprising the lipid bilayer. Interactions at these sites will probably have the effect of disrupting the packing of the lipid molecules, increasing the fluidity of the bilayer and facilitating drug diffusion. Interaction of an enhancer with the polar head groups may also cause or permit the hydrophilic regions of adjacent bilayers to take up more water and move apart, thus opening the paracellular pathway. Non-ionic Surfactants, long chain fatty acids and alcohols also increase membrane fluidity and have the capacity to solubilize and extract membrane components, thereby increasing the permeability. Agents such as DMSO, polyethylene glycol, and ethanol can, if present in sufficiently high concentrations in the delivery vehicle, enter the aqueous phase of the Dept of Pharmaceutics, JSSCP, Mysore Page 47
34 stratum corneum and alter its solubilizing properties, thereby enhancing the partitioning of drugs from the vehicle into the skin. All these agents produce varying degree of enhancement depending on the characteristics of the permeant, the composition of the delivery vehicle, whether the tissue was pretreated with enhancer and other factors. An important issue to be considered is toxicity of penetration enhancers [135]. Mechanisms by which permeation enhancers are thought to improve mucosal absorption include the following: Changing mucus rheology. Increasing fluidity of lipid bilayer membrane. Affecting the components involved in the formation of intracellular junctions. Overcoming the enzymatic barrier. Increasing the thermodynamic activity of drugs. Protein denaturation or extraction. Dept of Pharmaceutics, JSSCP, Mysore Page 48
35 4.11 Review of Research Papers Fergany Mohammed A, et al., Prepared slow-release buccal bioadhesive tablets of miconazole nitrate and carried out in vitro and in vivo evaluation. The tablets were prepared by using polymer mixtures of buccoadhesive materials such as hydroxypropyl methylcellulose, sodium carboxy methylcellulose, carbopol 934P, and sodium alginate. The dissolution of miconazole from all the prepared tablets into phosphate buffer (ph 6.8) was controlled and followed non-fickian release mechanisms. All the prepared tablets gave reasonable buccoadhesion time ( h). They concluded that the prepared slow-release buccoadhesive tablets of miconazole would markedly prolong the duration of the antifungal activity with more patient convenience [136]. Noha Nafee A, et al., prepared mucoadhesive buccal patches of miconazole nitrate and studied in vitro/ in vivo performance and effect of ageing. The patches were prepared with ionic polymers, sodium carboxymethyl cellulose (SCMC) and chitosan, or non-ionic polymers, polyvinyl alcohol (PVA), hydroxyethyl cellulose (HEC) and hydroxypropylmethyl cellulose (HPMC). Patches containing 10 % w/v PVA and 5 % w/v PVP showed optimum drug release. Study of the in vivo release from this formulation revealed uniform and effective salivary levels with adequate comfort and compliance during at least 6 h. however, in vivo release of the commercial oral gel product resulted in a burst and transient release of miconazole, which diminished sharply after the first hour of application. They concluded that PVA patches exhibited uniform and effective miconazole levels in vitro and in vivo (>5 h) without being drastically being influenced by ageing [137]. Dept of Pharmaceutics, JSSCP, Mysore Page 49
36 Madgulkar, et al., developed buccal adhesive miconazole tablet with prolonged antifungal activity. The simplex centroid experimental design was used to arrive at optimum ratio of Carbopol 934P, hydroxypropylmethylcellulose K4M and polyvinylpyrollidone. Swelling index, mucoadhesive strength and in vitro drug release of the prepared tablets were determined. The optimized formulation was subjected to in vitro antifungal activity, transmucosal permeation, drug deposition in mucosa, residence time and bioadhesion studies. Dissolution of miconazole from tablets was sustained for 6 h. The prepared slow release buccoadhesive tablets of miconazole would markedly prolong the duration of antifungal activity. Comparison of in vitro antifungal activity of tablet with marketed gel showed that drug concentration above the minimum inhibitory concentration were achieved immediately from both formulations, but release from tablet was sustained upto 6 h, while the gel showed initially fast drug release. Drug permeation across buccal mucosa was minimum from the tablet as well as marketed gel, whereas in vitro residence time and bioadhesive strength of tablet was higher than gel. Thus, they concluded that buccoadhesive tablets of miconazole nitrate may offer better control of antifungal activity as compared to gel formulation [138]. Tarun, et al., studied Swelling-controlled release system of Miconazole nitrate for vaginal delivery. An aqueous solution of 15 % w/w poly (vinyl alcohol) was mixed with a specific amount of miconazole powder and cross-linked by freeze thawing. They studied the effect of the number of freeze thawing cycles at four different levels. The effect of the presence of PEG was studied by mixing different concentrations of two different PEG grades (PEG1000, PEG1450). They demonstrated that the drug release was upto 10 h and was independent of number of freeze thaw cycles. The drug release was Dept of Pharmaceutics, JSSCP, Mysore Page 50
37 lower from the batches containing PEG 1000 irrespective of concentrations when compared to PEG1450 [139]. Libero Italo Giannola, et al., Formulated 5-fluorouracil (5-FU) buccal tablets for locoregional chemotherapy of oral squamous cell carcinoma. The drug release and histological effects on reconstituted human oral epithelium and porcine buccal mucosa were studied. The study reported that the development of buccal tablets suitable for direct application of low doses of 5-FU on cancer lesions. The topical administration could be effective on tumor area while systemic undesired side effects are avoided. Matrix buccal tablets, were designed for 5-FU local delivery, developed, prepared and release tests showed a highly reproducible Higuchian drug discharge. After tablet administration on buccal tissue specimens, the occurrence of histomorphological effects of 5-FU was highlighted. Apoptotic events were registered in all samples treated while only negligible amounts of 5-FU permeated the buccal membrane and reached the simulated plasma. They concluded that loaded matrix tablets containing 5 % of 5-FU could be a useful means in topical treatment of oral squamous cell carcinoma [140]. Dhiman M, et al., formulated bioadhesive gels containing 5-fluorouracil (5-FU) for the treatment of oropharyngeal cancer. They investigated physicochemical interactions between FU and polymers by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectrophotometry and differential scanning calorimetry (DSC). The gel formulations containing FU were prepared by using Poloxamer 407, HPMC K 15 M, and Gantrez S-97 (polymethylvinylether-co-maleic anhydride). They concluded that with increase in proportion of HPMC K 15 M and Gantrez S-97, bioadhesiveness of the gels is also Dept of Pharmaceutics, JSSCP, Mysore Page 51
38 increased. In vitro release studies indicated that release could be sustained up to 8 h [141]. Munish, et al., studied the effect of permeation enhancers on the transbuccal delivery of 5-fluorouracil. Sodium deoxycholate (SDC), sodium dodecyl sulphate (SDS), Sodium tauroglycocholate (STGC) and oleic acid were the permeation enhancers and in vitro buccal permeability was assessed. The order of permeation enhancement was STGC > SDS > SDC > oleic acid. Histological investigations were also performed on buccal mucosa and indicated no major morphological changes. They also evaluated the enhancing effect of STGC on the buccal absorption of FU from the mucoadhesive gels in rabbits. The authors concluded that, STGC emerged as the most efficient enhancer among the enhancers investigated for buccal absorption of FU and at 3 % of concentration, STGC showed the highest K P and EF across the buccal mucosa. From the histological investigations, they revealed that increase in intercellular space and swelling, especially inside the cells may be responsible for the permeation enhancement [142]. Woolfson AD, et al., formulated bioadhesive cervical patch for the delivery of 5- fluorouracil for the treatment of cervical intraepithelial neoplasia (CIN). The patch was bilaminar design; with drug-loaded bioadhesive film cast from a gel containing 2 % (w/w) Carbopol 981 plasticized with 1 % (w/w) glycerin. Release of 5-fluorouracil from the bioadhesive layer into an aqueous sink was rapid but was controlled down to an undetectable level through the backing layer. It was observed that 5-fluorouracil is substantially released through human cervical tissue samples over approximately 20 h. They concluded that bioadhesive and drug release characteristics of the 5-fluorouracil Dept of Pharmaceutics, JSSCP, Mysore Page 52
39 cervical patch would be suitable for further clinical investigation as a drug treatment for CIN [143]. Galandiuk S, et al., studied suppository delivery of 5-fluorouracil in rectal cancer. They compared Suppository and intravenous 5-FU administration with respect to myelosuppression and tissue concentrations. White blood cell count, serum albumin, alkaline phosphatase, creatinine, and aspartate aminotransferase (AST) were determined before and serially after 5-FU administration in rats. Effect of 5-FU on Portal and systemic blood, rectal, iliac lymph node, liver, and lung tissue was also studied at 30 min, 1, 3, 6, and 12 h after drug administration. They observed that no toxicity was observed in 5-FU suppository animals, whereas 63 % of 5-FU i.v animals had diarrhea. Weight loss and myelosuppression occurred only in 5-FU i.v. animals. They concluded that 5-FU suppositories are associated with fewer systemic side effects and higher rectal 5-FU concentrations than with 5-FU i.v. administration [144]. Marija Glavas Dodov, et al., formulated Liposome gels bearing 5-fluorouracil, intended for topical application and evaluated. Different formulations of liposomes were prepared by the film hydration method by varying the lipid phase composition (PL 90H/cholesterol mass ratio) and hydration conditions of dry lipid film (drug/aqueous phase mass ratio). Topical liposome gels were prepared by incorporation of lyophilized liposomes into a structured vehicle (1 % m/m, chitosan gel base). Also, hydrogels containing different concentrations of 5-fluorouracil were prepared and drug release properties were investigated. The release rate of 5-FU from topical liposome gels was affected by the formulation variables. Comparing the liposome gels with hydrogel formulations, the release rate of liposome-entrapped drug was prolonged, while a steady-state release rate, Dept of Pharmaceutics, JSSCP, Mysore Page 53
40 established after 1.5 hours, suggests that the liposomes function as a reservoir system for continuous delivery of the encapsulated drug substance. They concluded that, developed drug delivery showed the suitability for prolonged action of topically applied antineoplastic agents, with fewer side effects than conventional formulations [145]. Erem Bilensoy, et al., formulated thermosensitive mucoadhesive gel formulation loaded with 5-FU: cyclodextrin complex for HPV-induced cervical cancer. 5-fluorouracil has been formulated in a vaginal gel using the thermosensitive polymer Pluronic F127 together with alternative mucoadhesive polymers e.g., hyaluronic acid, Carbopol 934 and hydroxypropylmethylcellulose. 5-fluorouracil was incorporated as its inclusion complex with 1:1 molar ratio with either β-cyclodextrin or hydroxypropyl-β-cyclodextrin. Following the characterization of drug: CD complexes, thermosensitive gel formulations containing different mucoadhesive polymers and the drug in free or complexed form were characterized. It was observed that complexation with cyclodextrin accelerated the release of 5-FU (except Carbopol containing formulation). They concluded that, formulation of an anticancer drug in free form or in complex with natural or synthetic cyclodextrins may exert favorable properties such as thermosensitive characteristics, controlled and prolonged release profile and high anticancer activity [146]. Shegokar Ranjita, et al., studied in-vitro release of paracetamol from suppocire suppositories and also studied role of additives. Effect of various additives such as sodium lauryl sulfate (SLS), dioctyl sulfosuccinate (DOSS), Labrasol, lecithin, Miglyol 812, aerosil, Capryol PGMC (CPGMC) and span 80 were studied on drug release. White coloured suppositories were formulated and evaluated for various parameters like appearance, melting range, uniformity of weight, disintegration time, % drug content and Dept of Pharmaceutics, JSSCP, Mysore Page 54
41 in vitro drug release. The Suppocire bases selected were evaluated for displacement value with respect to drug and noted to be 1.4, 1.46, 1.4 and 1.43 for Suppocire D, C, NCX and CP respectively. Paracetamol Suppocire suppository using amphiphilic base in combination with Labrasol showed optimal drug release as compared to other Suppocire bases. The developed paracetamol Suppocire suppositories gave controlled as well as fast release depending upon the additives added to the formulation and proved to be a good suppository base. Newly incorporated additives such as Labrasol and Capryol PGMC have promise to be used as adjuvants in suppository formulation [147]. Ofokansi KC, et al., formulated and evaluated microspheres based on gelatin-mucin admixtures for the rectal delivery of cefuroxime sodium. Cefuroxime sodium-loaded microspheres containing admixtures of gelatin and porcine mucin were prepared via an emulsification-crosslinking technique. The drug entrapment efficiency of the microspheres was evaluated in citrate/phosphate buffer (ph 7.4) while the swelling properties was evaluated in both simulated gastric fluid (SGF) without pepsin and simulated intestinal fluid (SIF) without pancreatin (ph 1.2). Release of cefuroxime sodium from the microspheres was evaluated in vitro in SIF and further evaluated in vivo after rectal administration to male Wistar rats. Microspheres formulated with equal portions of gelatin and mucin, showed high entrapment efficiency and led to a greater drug release (up to 85 %) and also a high bioavailability of the incorporated drug. Formulations based on varying portions of gelatin and mucin also showed high drug loading efficiency, resulted in high drug release in SIF within 3 h. Drug release from the different formulations was observed to be rapid and generally showed a biphasic pattern. They concluded that inclusion of S-mucin in the composition of the microspheres has an Dept of Pharmaceutics, JSSCP, Mysore Page 55
42 enhancer effect on the release and rectal bioavailability of cefuroxime sodium that may be exploited in the design of a rectal delivery system of the drug [148]. Singh S, et al., formulated buccal bioadhesive tablets containing clotrimazole and evaluated. Buccal bioadhesive tablets of clotrimazole (CTZ) and clotrimazole: hydroxypropyl-β-cyclodextrin (CTZ-HPβCD) complex were prepared by using polymer xanthan gum in combination with carbopol 974P. The prepared formulations were evaluated for physicochemical characteristics, swelling index, microenvironment ph, invitro drug release, bioadhesion strength, residence time and duration of antifungal activity (in-vitro). The dissolution of CTZ from the prepared tablets into phosphate buffer (ph 6.8) was controlled up to 8 h. All the prepared tablets gave reasonable in-vitro residence time. X-ray diffraction (XRD) studies of the CTZ-HPβCD complex, made by kneading and freeze-dried method, showed no CTZ crystal signals, demonstrating the inclusion of CTZ in the hydrophobic cavity of hydroxypropyl-β-cyclodextrin (HPβCD) and formation of amorphous inclusion complex. They concluded that the prepared buccal bioadhesive tablets of CTZ may increase the patient compliance by reducing the frequency of administration and improving overall therapy of oral candidiasis [149]. Lei Wang, et al., formulated and evaluated novel ketoconazole bioadhesive effervescent tablet for vaginal delivery. Carbomer (Carbopol 974P, Carbopol 934P), hydroxypropylmethylcellulose (HPMC) and hydroxypropyl cellulose (HPC) were used as candidate bioadhesive polymers. Effervescent was incorporated into the formulations as a disintegration agent. The swelling behavior and bioadhesive strength of the drug-free tablets were investigated. The formulation containing 100 mg of effervescent, with the Carbopol 934P: HPC ratio of 1:9 was considered as optimized formulation. In vivo drug Dept of Pharmaceutics, JSSCP, Mysore Page 56
43 residence tests were carried out by administering the formulation to female rats. They concluded that incorporation of effervescent into the bioadhesive tablets leads to the increase in the swellings and the rate of drug release and conversely decrease the tackiness. It is also observed that the KTZ release and bioadhesion properties of bioadhesive tablets can be controlled by changing the polymer type, polymer concentration and effervescent content [150]. Rakesh Kumar Dev, et al., formulated novel microbially triggered colon specific delivery system of 5-Fluorouracil and in vitro, in vivo cytotoxic study was also carried out. A 3 2 full factorial design was used for optimization. The independent variables employed were amount of pectin and amount of starch paste, each at three levels. The evaluated responses were hardness, percent cumulative drug release (% CDR) at 5 th h and t90%. Drug release studies were carried out using change over media [ph 1.2, 7.4 and 6.5 in presence of 4 % (w/v) rat caecal contents]. It was observed that optimized formulation consisting of pectin (66.67 %w/w) and starch paste (15 %w/w) released negligible amount of drug at ph 1.2 and ph 7.4 whereas significant (p < 0.05) drug release was observed at ph 6.5 in presence of 4 %(w/v) rat caecal contents. The optimized formulation was subjected to in vivo roentgenographic studies in New Zealand white rabbits. Roentgenographic studies corroborated the in vitro observations. They concluded that pectin-based coated matrix tablet to be a promising system for the colon specific delivery of 5-FU for colon carcinoma [151]. Dept of Pharmaceutics, JSSCP, Mysore Page 57
44 4.12 Drugs profile [152] Miconazole Nitrate Figure 4: Structure of Miconazole nitrate (MN) Molecular formula C 18 H 14 Cl 4 N 2 O.HNO 3 Molecular weight IUPAC name Description Solubility gm. 1-[(2RS)-2-[(2,4-dichlorobenzyl)oxy]-2-(2,4 Dichlorophenyl)ethyl]-1H-imidazole nitrate. A white or almost white powder. Miconazole nitrate is freely soluble in methanol, slightly soluble in ethanol (95%) and in chloroform, very slightly soluble in water and in ether. Storage Preparations Mechanism of action Miconazole must be stored in an airtight container, protected from light. Miconazole nitrate cream, gels and pessaries. Miconazole is an imidazole antifungal agent and acts by interfering with permeability of fungal cell membrane. It has wide antifungal spectrum and also possesses some antibacterial activity. Dept of Pharmaceutics, JSSCP, Mysore Page 58
45 Usual dose Absorption Elimination half life Therapeutic uses 100 mg per dose Miconazole nitrate is incompletely absorbed from GI tract. 24 hr. It is used in the treatment of oral candidiasis, vaginal candidiasis, caused by Dermatophytes and candida species. Fluorouracil Figure 5: Structure of 5-Fluorouracil (5-FU) Molecular formula C 4 H 3 FN 2 O 2 Molecular weight Melting point 282 to 283 C Description White to practically white, odourless, crystalline powder Dissociation Constant 8.0 Solubility Sparingly soluble in water, slightly soluble in alcohol, Storage Formulations practically insoluble in CHCl 3 Stored in air tight containers protected from light. Intravenous route, capsules. Dept of Pharmaceutics, JSSCP, Mysore Page 59
46 Identification- Light : absorption in the range nm of a 10 µg/ml solution in 7.4 ph buffer exhibits absorption maximum at 267 nm ph 4.5 to 5.0 Mechanism of action The nucleotide of 5-FU, 5-Fluoro-2-deoxy-thymidylate synthase blocks the conversion of deoxyuridic acid to deoxy thymidylic acid Pharmacokinetic profile of 5-FU Half life Volume of distribution Clearance 15 to 20 min 17.5 L/70kg 63 L/h Protein binding 94% Therapeutic uses Anti neoplastic and immunosuppressive agent Dept of Pharmaceutics, JSSCP, Mysore Page 60
47 4.13 Polymers profile [153] Chitosan Figure 6: Structure of chitosan Nonproprietary Names Synonyms Chemical Name Molecular Weight Functional Category Application Description Safety BP: Chitosan Hydrochloride, PhEur: Chitosan Hydrochloride 2-Amino-2-deoxy-(1,4)-b-D-glucopyranan; chitosani hydrochloridum; deacetylated chitin; deacetylchitin; b-1,4- poly-d-glucosamine; poly-d-glucosamine; poly-(1,4-b-dglucopyranosamine). Poly-β-(1, 4)-2-Amino-2-deoxy-D-glucose Chitosan is commercially available in several types and grades that vary in molecular weight by , and vary in degree of deacetylation and viscosity Coating agent, disintegrant, film-forming agent, mucoadhesive, tablet binder, viscosity increasing agent. Controlled drug delivery, mucoadhesive dosage forms, rapid release dosage forms, improved peptide delivery, colonic drug delivery systems, and gene delivery. Chitosan has been processed into several pharmaceutical forms including gels, films, beads, microspheres, tablets and coatings for liposomes. Chitosan occurs as odorless, white or creamy-white powder or flakes. Generally regarded as a nontoxic and nonirritant material biocompatible with both healthy and infected skin and biodegradable. Dept of Pharmaceutics, JSSCP, Mysore Page 61
48 Carbopol 71G Synonyms Chemical Name Molecular weight Appearance Description Acritamer, acrylic acid polymer, Carbopol, carboxy Vinyl polymer. Carboxypolymethylene. 3.5 billions. Fluffy, white, mildly acidic polymer. Carbopol is a white- colored, fluffy, acidic, and hygroscopic powder with a slightly characteristic odor. The carboxyl groups provided by the acrylic acid backbone of the polymer are responsible for many of the product benefits. Carbopol polymers have an average equivalent weight of 76 per carboxyl group. Bulk Density Approximately 208 kg/m 3 (13 lbs. ft 3 ) Viscosity Specific gravity 1.41 Moisture content 2.0 % maximum Equilibrium moisture 8-10 % Content pka ph of 1.0% dispersion ph of 0.5% dispersion Equivalent weight Ash content ppm (average) Glass transition temp C ( F) Dept of Pharmaceutics, JSSCP, Mysore Page 62
49 Carboxymethyl tamarind Appearance Solubility Ionic characteristic yellowish powder Cold water soluble Anionic ph 9-11 Moisture Ash content Viscosity 10 % max 20 % max 60,000-65,000cps Degree of substitution 0.2 Dept of Pharmaceutics, JSSCP, Mysore Page 63
50 Noveon AA-1 Chemical nature Description Polycarbophil Polycarbophil occurs as fluffy, white to off- white, mildy acidic polymer powder with slightly acetic odor. Typical properties: Acidity / alkalinity ph Ash content 0.009ppm Density (bulk) g/cm3. Equilibrium moisture 8-10 % content Dissociation constant 6.0±0.5 Glass transition temp C Moisture content 2.0 %max Specific gravity 1.41 Application Thickening agent Used in bioadhesive drug delivery system. Dept of Pharmaceutics, JSSCP, Mysore Page 64
51 Sodium alginate Figure 7: Structure of sodium alginate Nonproprietary Names BP: Sodium Alginate, PhEur: Sodium Alginate, USP-NF: Sodium Alginate Synonyms Alginato sodico; algin; alginic acid, sodium salt; E401; Kelcosol; Keltone; natrii alginas; Protanal; sodium polymannuronate. Functional Category Applications Acidity/alkalinity Solubility Viscosity Safety Stabilizing agent; suspending agent; tablet and capsule disintegrant; tablet binder; viscosity increasing agent Oral and topical pharmaceutical formulations, binder and disintegrant in tablets, diluents in capsule, sustained-release polymer in oral formulations, creams, gels and as a stabilizing agent for oil-in-water emulsions ph ~ 7.2 (1% w/v aqueous solution) Practically insoluble in ethanol (95%), ether, chloroform, and ethanol/water mixtures, slowly soluble in water, form a viscous colloidal solution. Typically, a 1% w/v aqueous solution, at 20 C, will have a viscosity of mpa s ( cp). It is generally regarded as a nontoxic and nonirritant material, although excessive oral consumption may be harmful. Dept of Pharmaceutics, JSSCP, Mysore Page 65
52 4.14 Excipients profile [153] Microcrystalline Cellulose (MCC) Figure 8: Structure of Microcrystalline Cellulose Nonproprietary Name BP: Microcrystalline cellulose Ph Eur: Cellulosum microcrystalline USP NF: Microcrystalline cellulose Synonyms Avicel,cellulose gel,e460,emcocel,fibrocel,tabulose Empirical Formula (C 6 H 10 O 5 ) n Where n 220 Molecular weight Functional Category Adsorbent, suspending agent, tablet and capsule diluent, tablet disintegrant. Applications Description Safety Used as a diluent in oral tablet and capsules, where it is used in wet granulation and direct compression process. It also has some lubricant and disintegrant properties that make it useful in tabletting. White colourless, odorless, tasteless, crystalline powder. MCC is widely used in oral pharmaceutical formulations and food products and is generally regarded as non-toxic and non irritant material. It is not absorbed systemically followed by oral administration and thus has little toxic potential. Dept of Pharmaceutics, JSSCP, Mysore Page 66
53 Magnesium Stearate Nonproprietary Names BP: Magnesium stearate, JP: Magnesium stearate, PhEur: Magnesii stearas, USP: Magnesium stearate Synonyms Magnesium octadecanoate; stearic acid magnesium salt; octadecanoic acid, magnesium salt. Structural Formula [CH 3 (CH 2 ) 16 COO] 2 Mg Empirical Formula C 36 H 70 MgO 4 Molecular Weight Functional Category Tablet and capsule lubricant. Applications Description Safety Magnesium stearate is widely used in cosmetics, foods, and pharmaceutical formulations. It is primarily used as a lubricant in capsule and tablet manufacture at concentrations between %. It is also used in barrier creams. Magnesium stearate is a fine, white, precipitated or milled, impalpable powder of low bulk density, having a faint odor of stearic acid and a characteristic taste. The powder is greasy to touch and readily adheres to the skin. Magnesium stearate is widely used as a pharmaceutical excipient and is generally regarded as being nontoxic following oral administration. However, oral consumption of large quantities may result in some 1laxative effect or mucosal irritation. Dept of Pharmaceutics, JSSCP, Mysore Page 67
54 Talc Non proprietary Name Synonyms BP: Purified talc JP: Talc PhEur: Talcum USP: Talc Magsil Star; Powdered talc; Purified French chalk; Purtalc; soapstone; steatite. Empirical Formula Mg 6 (Si 2 O 5 ) 4 (OH) 4. Functional Category Description Applications Safety Anticaking agent; glidant; tablet and capsule diluent; tablet and capsule lubricant. Talc is a very fine, white to grayish-white, odorless, impalpable, unctuous, crystalline powder. It adheres readily to the skin and is soft to the touch and free from grittiness. Talc was once widely used in oral solid dosage formulations as a lubricant and diluents. However, it is widely used as a dissolution retardant in the development of controlled-release products. Talc is also used as a lubricant in tablet formulations and in a novel powder coating for extended-release pellets and as an adsorbant.. Talc is not absorbed systemically following oral ingestion and is therefore regarded as an essentially nontoxic material. However, intranasal or intravenous abuse of products containing talc can cause granulomas in body tissues, particularly the lungs. Contamination of wounds or body cavities with talc may also cause granulomas. Dept of Pharmaceutics, JSSCP, Mysore Page 68
55 Sodium deoxycholate Figure 9: Structure of Sodium deoxycholate Synonyms Formula Molecular Weight 7-Deoxycholic acidsodium salt; Desoxycholic acid sodium Salt; 3α, 12α-Dihydroxy-5β-cholanic acid sodium salt C 24 H 39 NaO 4 H 2 O 432, 57 g/mol ph at 43,3 g/l at 25 C Detergent Class Ionic (anionic) Purity (by HPLC) 98% Water solubility ca g/l at 20 C Functional Category Carcinogenicity Storage surfactant, permeation enhancer No component of this product presents at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Dept of Pharmaceutics, JSSCP, Mysore Page 69
BUCCAL DRUG DELIVERY SYSTEM
 BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester
 University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
The Digestive System. Chapter 25
 The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
Suppository Chapter Content
 10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
Routes of drug administration
 Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p.
 Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
D9G : Oro-Mucosal Dosage Forms Development Background Paper
 D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
ORGANS OF THE DIGESTIVE SYSTEM
 ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
The Digestive System. Chapter 16. Introduction. Overview of Digestive System. Histological Organization. Movement and Mixing of Digestive Materials
 The Digestive System Chapter 16 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Digestive System Chapter 16 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
OVERVIEW OF ORAL MUCOSA 1 : A] Structure:
![OVERVIEW OF ORAL MUCOSA 1 : A] Structure: OVERVIEW OF ORAL MUCOSA 1 : A] Structure:](/thumbs/95/123509578.jpg) INTRODUCTION Drug delivery systems that can precisely control the release rate of drug to specific body sites have an enormous potential in the healthcare world. The last two decades in the pharmaceutical
INTRODUCTION Drug delivery systems that can precisely control the release rate of drug to specific body sites have an enormous potential in the healthcare world. The last two decades in the pharmaceutical
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS. Pharmaceutical Manufacturing-4
 CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
Tissues. Definition. A group of similar cells and their intercellular substances specialized to perform a specific function.
 Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Includes mouth, pharynx, esophagus, stomach, small intestine, large intestine, rectum, anus. Salivary glands, liver, gallbladder, pancreas
 Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics Dr. Iman Lec. 3
 Pharmacokinetics r. Iman Lec. 3 Pharmacokinetics A dequate drug doses must be delivered to the target organ to get therapeutic but not toxic levels. So, pharmacokinetic examines the movement of drug over
Pharmacokinetics r. Iman Lec. 3 Pharmacokinetics A dequate drug doses must be delivered to the target organ to get therapeutic but not toxic levels. So, pharmacokinetic examines the movement of drug over
The Digestive System. What is the advantage of a one-way gut? If you swallow something, is it really inside you?
 The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
Digestive System. Digestive System. Digestion is the process of reducing food to small molecules that can be absorbed into the body.
 Digestive System Digestion is the process of reducing food to small molecules that can be absorbed into the body. 2 Types of Digestion Mechanical digestion physical breakdown of food into small particles
Digestive System Digestion is the process of reducing food to small molecules that can be absorbed into the body. 2 Types of Digestion Mechanical digestion physical breakdown of food into small particles
Tissues. tissue = many cells w/ same structure and function. cell shape aids its function tissue shape aids its function
 Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Fundamentals of Pharmacology for Veterinary Technicians Chapter 4
 (A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
(A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS. Mar 16 10:34 PM
 DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
Ch 7 Nutrition in humans
 Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Tissue: The Living Fabric
 PowerPoint Lecture Slide Presentation by Vince Austin Human Anatomy & Physiology FIFTH EDITION Elaine N. Marieb Chapter 4 Tissue: The Living Fabric Part A Tissues Groups of cells similar in structure and
PowerPoint Lecture Slide Presentation by Vince Austin Human Anatomy & Physiology FIFTH EDITION Elaine N. Marieb Chapter 4 Tissue: The Living Fabric Part A Tissues Groups of cells similar in structure and
Two main groups Alimentary canal continuous coiled hollow tube Accessory digestive organs
 Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
An overview of the digestive system. mouth pharynx esophagus stomach small intestine large intestine rectum anus
 An overview of the digestive system mouth pharynx esophagus stomach small intestine large intestine rectum anus Why GIT? What are the main steps in the digestive process? Ingestion intake of food via the
An overview of the digestive system mouth pharynx esophagus stomach small intestine large intestine rectum anus Why GIT? What are the main steps in the digestive process? Ingestion intake of food via the
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Histology Notes -Part 1: Epithelial Tissues
 Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Soft palate elevates, closing off the nasopharynx. Hard palate Tongue Bolus Epiglottis. Glottis Larynx moves up and forward.
 The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
The Digestive System. Prepares food for use by all body cells.
 The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
Digestive System. Why do we need to eat? Growth Maintenance (repair tissue) Energy
 Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION
 PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
Tissue: The Living Fabric: Part A
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Emulsions. Purpose of emulsions and of emulsification:
 Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Energy, Chemical Reactions and Enzymes
 Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Lecture Overview. Chapter 4 Epithelial Tissues Lecture 9. Introduction to Tissues. Epithelial Tissues. Glandular Epithelium
 Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
(b) Stomach s function 1. Dilution of food materials 2. Acidification of food (absorption of dietary Fe in small intestine) 3. Partial chemical digest
 (1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
(1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
Bio 12 Chapter 2 Test Review
 Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Compounding Options in Women s Health: Dosage Forms
 Compounding Options in Women s Health: Dosage Forms Ranel A. Larsen, RPh, PharmD HRT Symposium Las Vegas, NV February 16 18, 2017 Routes of Administration Common: Topical Oral Sublingual Vaginal Other:
Compounding Options in Women s Health: Dosage Forms Ranel A. Larsen, RPh, PharmD HRT Symposium Las Vegas, NV February 16 18, 2017 Routes of Administration Common: Topical Oral Sublingual Vaginal Other:
Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more
 Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
Enhanced delivery methods for greater efficacy
 On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 24 The Digestive System Introduction The purpose of this chapter is to Identify the anatomical components of the digestive system as well as their
Principles of Anatomy and Physiology 14 th Edition CHAPTER 24 The Digestive System Introduction The purpose of this chapter is to Identify the anatomical components of the digestive system as well as their
Ingestion Digestion- Absorption- Elimination
 DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
Chemical Level Of Organization
 Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Lecture Overview. Marieb s Human Anatomy and Physiology. Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9. Introduction to Tissues
 Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy
 Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
NOTES: CH 41 Animal Nutrition & Digestion
 NOTES: CH 41 Animal Nutrition & Digestion NUTRITION *Nutrition is the study of nutrients and how the body utilizes them! *ESSENTIAL NUTRIENTS: nutrients that human cells cannot synthesize (i.e. certain
NOTES: CH 41 Animal Nutrition & Digestion NUTRITION *Nutrition is the study of nutrients and how the body utilizes them! *ESSENTIAL NUTRIENTS: nutrients that human cells cannot synthesize (i.e. certain
3/16/2016. Food--mixture of carbohydrates, proteins, and lipids
 Food--mixture of carbohydrates, proteins, and lipids Food being broken down into small molecules Takes place in the alimentary canal Complete digestive system 4 layers of tissue (in book) Lumen 1) MECHANICAL/PHYSICAL--
Food--mixture of carbohydrates, proteins, and lipids Food being broken down into small molecules Takes place in the alimentary canal Complete digestive system 4 layers of tissue (in book) Lumen 1) MECHANICAL/PHYSICAL--
Human Nutrition (IGCSE Biology Syllabus )
 Human Nutrition (IGCSE Biology Syllabus 2016-2018) o Balanced diet: getting all the right nutrients in correct proportions o Diet related to: - Age - Gender - Activity - Pregnant women o Malnutrition:
Human Nutrition (IGCSE Biology Syllabus 2016-2018) o Balanced diet: getting all the right nutrients in correct proportions o Diet related to: - Age - Gender - Activity - Pregnant women o Malnutrition:
Digestive System. How your body obtains nutrients. Wednesday, March 2, 16
 Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Epithelial Tissue. Functions include: 1. Protection 4. Absorption 2. Secretion 5. Filtration 3. Sensory reception
 Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Determination of bioavailability
 Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
DIGESTIVE SYSTEM CLASS NOTES. tube along with several
 DIGESTIVE SYSTEM CLASS NOTES Digestion Breakdown of food and the of nutrients in the bloodstream. Metabolism Production of for and cellular activities. The digestive system is composed of the canal which
DIGESTIVE SYSTEM CLASS NOTES Digestion Breakdown of food and the of nutrients in the bloodstream. Metabolism Production of for and cellular activities. The digestive system is composed of the canal which
Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug
 Chapter 5 Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug absorption. It is the study of the kinetics of absorption,
Chapter 5 Is the science that study relation of physicochemical properties of drug, dosage form, & route of administration on rate and extent of drug absorption. It is the study of the kinetics of absorption,
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY. DIGESTIVE SYSTEM Dr.B.Jyothi
 KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
DIGESTION SBI 3C: NOVEMBER 2010
 DIGESTION SBI 3C: NOVEMBER 2010 DIAGRAM OF DIGESTIVE SYSTEM: Mouth Esophagus Liver Gallbladder Large Intestine Appendix Stomach Pancreas Small Intestine Rectum Anus STAGES OF DIGESTION: 1. INGESTION Taking
DIGESTION SBI 3C: NOVEMBER 2010 DIAGRAM OF DIGESTIVE SYSTEM: Mouth Esophagus Liver Gallbladder Large Intestine Appendix Stomach Pancreas Small Intestine Rectum Anus STAGES OF DIGESTION: 1. INGESTION Taking
Objective 4- Digestion
 Objective 4- Digestion 1. Describe why cells require nutrients Supply energy for metabolism (cell activities), matter for synthesis of new materials, cell reproduction, secretion and to regulate cell processes
Objective 4- Digestion 1. Describe why cells require nutrients Supply energy for metabolism (cell activities), matter for synthesis of new materials, cell reproduction, secretion and to regulate cell processes
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Human Biology. Digestive System
 Human Biology Digestive System Digestion - Defined Prepares food for use by all body cells The physical and/or chemical breakdown of food Did you know: the average person eats more than 500kg of food per
Human Biology Digestive System Digestion - Defined Prepares food for use by all body cells The physical and/or chemical breakdown of food Did you know: the average person eats more than 500kg of food per
Learning Targets. The Gastrointestinal (GI) Tract. Also known as the alimentary canal. Hollow series of organs that food passes through
 Digestion the multistep process of breaking down food into molecules the body can use Learning Targets Describe the path food takes through the digestive system. Identify the major organs of the digestive
Digestion the multistep process of breaking down food into molecules the body can use Learning Targets Describe the path food takes through the digestive system. Identify the major organs of the digestive
Tissues. How do cells form tissues?
 Tissues How do cells form tissues? Using cell junctions Tissues Epithelial tissue Connective tissue Muscle tissue Nervous tissue Epithelial Tissue Closely packed cells in continuous sheets connected by
Tissues How do cells form tissues? Using cell junctions Tissues Epithelial tissue Connective tissue Muscle tissue Nervous tissue Epithelial Tissue Closely packed cells in continuous sheets connected by
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
Membrane Structure and Function - 1
 Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY
 PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Excipients are inactive ingredients used as carriers for the active ingredients in a pharmaceutical product. These may
PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Excipients are inactive ingredients used as carriers for the active ingredients in a pharmaceutical product. These may
General Structure of Digestive Tract
 Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Digestive System. Unit 6.11 (6 th Edition) Chapter 7.11 (7 th Edition)
 Digestive System Unit 6.11 (6 th Edition) Chapter 7.11 (7 th Edition) 1 Learning Objectives Identify the major organs of the digestive system. Explain the locations and functions of three organs in the
Digestive System Unit 6.11 (6 th Edition) Chapter 7.11 (7 th Edition) 1 Learning Objectives Identify the major organs of the digestive system. Explain the locations and functions of three organs in the
Section 1.1: What is the function of digestion?
 Section 1.1: What is the function of digestion? When you have completed this section, you should be able to: Describe the overall function of the GI tract. Describe the processes involved in digestion.
Section 1.1: What is the function of digestion? When you have completed this section, you should be able to: Describe the overall function of the GI tract. Describe the processes involved in digestion.
13. SUPPOSITORY Suppository Bases. The active substance is prepared in a suitable bases. An ideal suppository bases should carry:
 13. SUPPOSITORY They are solid single-dose preparations whose shapes, volumes and consistencies are suitable for rectal administration. There are also such vaginal preparations in the treatment of local
13. SUPPOSITORY They are solid single-dose preparations whose shapes, volumes and consistencies are suitable for rectal administration. There are also such vaginal preparations in the treatment of local
For External Use Only. Should neither be swallowed. Nor injected
 For External Use Only Should neither be swallowed Nor injected Preparations which are applied to the skin. (Topical preparations) Preparations which are applied to the mucus membranes. Local effect Systemic
For External Use Only Should neither be swallowed Nor injected Preparations which are applied to the skin. (Topical preparations) Preparations which are applied to the mucus membranes. Local effect Systemic
Biology 12 - Digestion Notes
 Biology 12 - Digestion Notes Anatomy Physiology Functions of the Digestive System -------------------------------------------------------------------------------------- food (enzymes, bile, HCl) to assist
Biology 12 - Digestion Notes Anatomy Physiology Functions of the Digestive System -------------------------------------------------------------------------------------- food (enzymes, bile, HCl) to assist
Biopharmaceutics. Lec: 4
 64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
Lesson Overview The Digestive System
 30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
Pharmaceutical Preparation For Internal Use
 Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Chapter 8: Digestion. Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes
 Chapter 8: Digestion Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes What organisms need Digestion? Heterotrophs - rely on ingestion of organic molecules for production of
Chapter 8: Digestion Structure and Functions of Digestive Organs Macronutrients Digestive Enzymes What organisms need Digestion? Heterotrophs - rely on ingestion of organic molecules for production of
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
1. Three Main Functions. Chapter 19: 2. Two Groups of digestive organs. 2. Two Groups of digestive organs 6/1/2015. The Wall of the Digestive Tract
 1. Three Main Functions Chapter 19: General Structure and Function of the Digestive System Digestion-breakdown of food into small particles for transport to blood Absorption- into bloodstream to take to
1. Three Main Functions Chapter 19: General Structure and Function of the Digestive System Digestion-breakdown of food into small particles for transport to blood Absorption- into bloodstream to take to
INFORMATION TOPIC: II-5 OR DEMONSTRATION: II-5. DOSAGE, MEASUREMENTS, AND DRUG FORMS (Lesson Title) OBJECTIVES THE STUDENT WILL BE ABLE TO:
 LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
Tissues Review 4 type
 Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Right time, right place: bioactive delivery systems
 Right time, right place: bioactive delivery systems Zhigao Niu, Alejandra Acevedo-Fani & Ali Rashidinejad Science of Food Team Riddet Institute, Massey University Developing High-Value Foods Food Systems
Right time, right place: bioactive delivery systems Zhigao Niu, Alejandra Acevedo-Fani & Ali Rashidinejad Science of Food Team Riddet Institute, Massey University Developing High-Value Foods Food Systems
Chapter 3: Biochemistry Adapted from PPT by S. Edwards. By PresenterMedia.com
 Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
Gastrointestinal Anatomy and Physiology. Bio 219 Napa Valley College Dr. Adam Ross
 Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Digestion & The Alimentary Canal
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ igestion & The limentary anal Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ igestion & The limentary anal Question Paper Level Subject Exam oard Topic Sub Topic ooklet O Level iology ambridge International
PHAR 7632 Chapter 7. Table Market and Share of Pharmaceuticals by ROA Data from Viswanathan, 2004
 Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe various routes of drug administration including the concentration versus time
Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe various routes of drug administration including the concentration versus time
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Tissues 10/21/2016. Epithelial Tissue
 Tissues This is a generalized cell diagram. It shows the anatomy of a cell, but most cells do not actually look like this. Cells can have a wide variety of shapes and sizes, depending on their function.
Tissues This is a generalized cell diagram. It shows the anatomy of a cell, but most cells do not actually look like this. Cells can have a wide variety of shapes and sizes, depending on their function.
Digestive Tract. Also called alimentary canal or gastrointestinal tract. stomach small intestine large intestine - anus
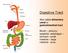 Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
Physical Pharmacy. Interfacial phenomena. Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department
 Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
